Published online May 28, 2023. doi: 10.3748/wjg.v29.i20.3048
Peer-review started: March 3, 2023
First decision: March 21, 2023
Revised: March 22, 2023
Accepted: April 21, 2023
Article in press: April 21, 2023
Published online: May 28, 2023
Processing time: 83 Days and 14.2 Hours
Hericium erinaceus is an edible and medicinal mushroom commonly used in traditional Chinese medicine for centuries. Several studies have highlighted its therapeutic potential for gastrointestinal disorders such as gastritis and inflammatory bowel diseases. In addition, some components of this mushroom appear to possess strong antineoplastic capabilities against gastric and colorectal cancer. This review aims to analyse all available evidence on the digestive therapeutic potential of this fungus as well as the possible underlying molecular mechanisms.
Core Tip: Various natural and non-pharmacological principles have been used to treat gastrointestinal disorders. Hericium erinaceus is a Chinese mushroom with a centuries-old medicinal tradition. Several preclinical studies have demonstrated their anti-inflammatory and antineoplastic potential. The therapeutic activity of this mushroom also targets inflammatory bowel diseases, as demonstrated in several animal experiments. However, evidence from in vivo studies is not generally available for patients with gastrointestinal disorders. It is also unclear which component of this mushroom has the greatest potency and the best safety profile.
- Citation: Gravina AG, Pellegrino R, Auletta S, Palladino G, Brandimarte G, D’Onofrio R, Arboretto G, Imperio G, Ventura A, Cipullo M, Romano M, Federico A. Hericium erinaceus, a medicinal fungus with a centuries-old history: Evidence in gastrointestinal diseases. World J Gastroenterol 2023; 29(20): 3048-3065
- URL: https://www.wjgnet.com/1007-9327/full/v29/i20/3048.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i20.3048
Gastrointestinal disorders are one of the most prevalent diseases in the general population. They are associated with a significant epidemiological and economic burden, with an estimated annual cost of over a hundred and thirty billion in the United States alone[1]. Many gastrointestinal disorders require a pharmacological approach; however, the possibility of adopting naturally derived complementary therapies whenever possible is emerging[2,3].
Among the abundant natural compounds studied, there is a Chinese mushroom, Hericium erinaceus (H. erinaceus) that has shown the potential to prevent and treat digestive diseases, such as gastric ulcers[4]. Furthermore, its therapeutic potential has been demonstrated in several conditions, including diabetes, hyperlipidaemia, neurodegenerative disorders, and cancer[5-8]. In addition, mild cognitive impairment is another disorder in which H. erinaceus has shown encouraging results in randomised clinical trials[9,10].
Therefore, H. erinaceus has traditionally and historically been used as a natural remedy for epigastric pain caused by chronic gastritis, gastric ulcers, or even atrophic gastritis[8].
Despite the strong need for clinical studies, several experiments, mainly preclinical and mouse model-based, have been conducted on the beneficial effects of many H. erinaceus extracts and components on gastrointestinal diseases. Therefore, this narrative review aimed to provide overall evidence of the therapeutic potential of H. erinaceus in gastrointestinal tract diseases.
H. erinaceus, also known as Yamabushitake (in the Japanese language), Houtou (in the Chinese language), or also as “lion’s mane” is a fungus that belongs to the class Basidiomycetes, subclass Holobasidiomycetidae, order Hericiales, and family Hericiaceae[4]. This fungus is mainly distributed in European, Asian, and American regions[11]. It is a saprophytic fungus or weak parasite that typically grows on hardwoods, such as beech, chestnut, and cherry[12].
Many active metabolites of H. erinaceus that are structurally different from each other and potentially bioactive have been discovered[13].
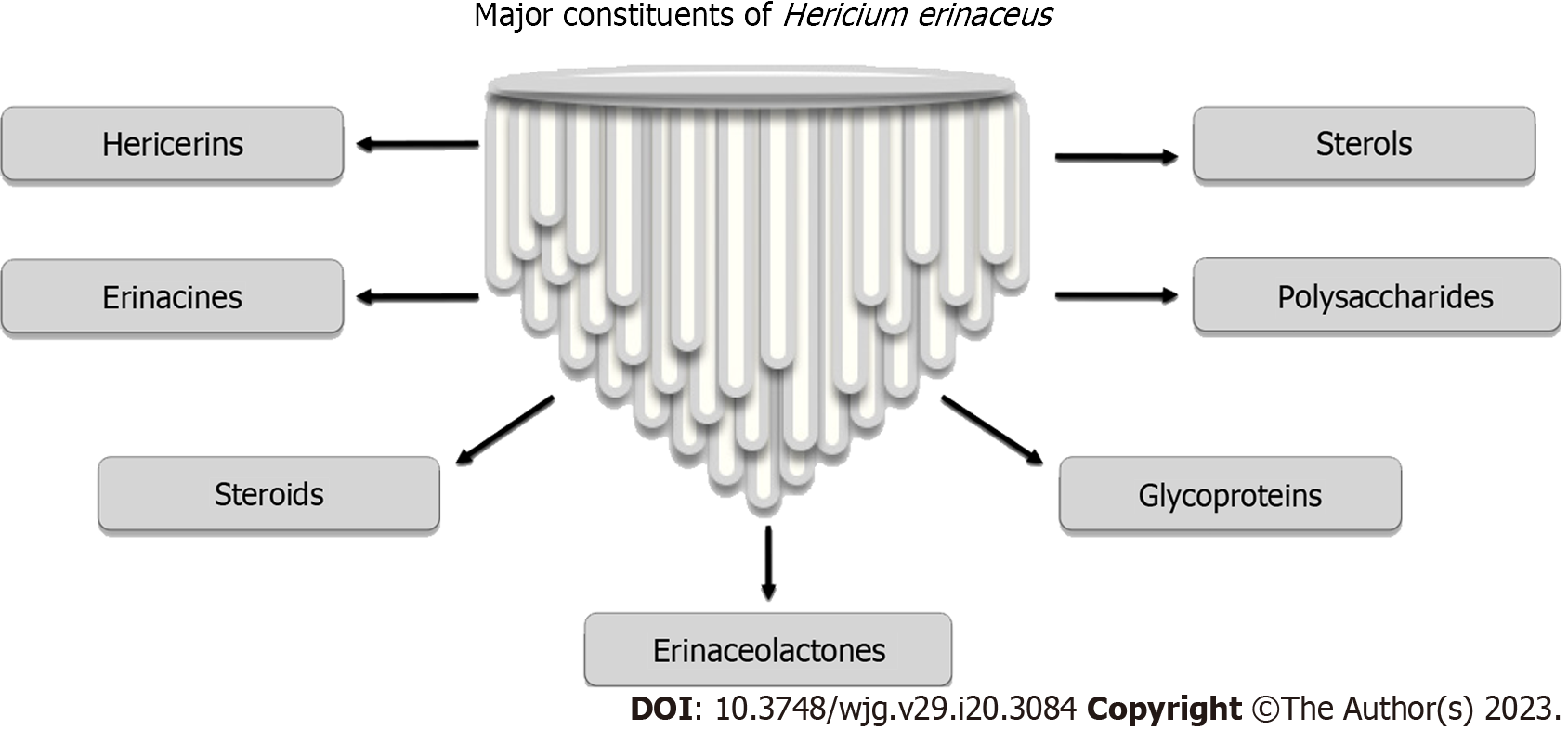
The main constituents of H. erinaceus are erinacines (cyathane-type diterpenoid aromatic compounds as erinacines A-I), steroids (such as ergosterol, erinarols A-F, and ergostane-type steroids), alkaloids (such as hericirine, 12β-hydroxyverruculogenTR-2, fumitremorgin, methylthioglioto, pseurotin A, and FD-838), and lactones such as vitamin B12-c-lactone (Figure 1)[13].
In addition, each 100 g of dried H. erinaceus contains approximately 61.3-77.5 g of total sugars, of which β-glucans, α-glucans, and glucan-protein complexes are the most abundant[14,15]. Among these, the β-glucans in the fungal cell wall have known and marked anti-inflammatory and anti-cancer potency and can positively modify the gut microbiota[16].
Much of the research devoted to the chemical characterisation of H. erinaceus has focused on its polysaccharide components, which are generally obtained from its fruiting body, and various extraction methods have been developed[17-19].
Gastric ulcers are a significant epidemiological burden[20]. Among the most common forms of gastric ulcers, those caused by non-steroidal anti-inflammatory drugs are also included. This is due to the pharmacological inhibition of cyclooxygenases 1 and 2, which are responsible for producing proinflammatory cytokines and prostaglandins, which help maintain the integrity of the gastric mucosal barrier[21]. An adequate balance between proinflammatory and anti-inflammatory cytokines is necessary for maintaining gastric mucosal integrity, such that polymorphisms in genes encoding proinflammatory cytokines can increase the risk of peptic ulcer and gastric cancer[22].
As anticipated, H. erinaceus has shown various anti-inflammatory, antioxidative, and gastroprotective properties. Boddy et al[23] showed, for example, that the action of several polysaccharides of H. erinaceus inhibits the secretion of proinflammatory cytokines interleukin 6 (IL-6), IL-8, and IL-12 and promotes the secretion of the anti-inflammatory cytokine IL-10 in a co-culture system of Cancer coli 2 (Caco-2) cells and Caco-2/RAW264.7 cells under bacterial lipopolysaccharide stimulation. This emphasises how this fungus can intervene in cytokine imbalance in an inflamed environment by shifting the balance toward an anti-inflammatory cytokine pattern.
To evaluate the gastroprotective, antioxidant, and anti-inflammatory activities in vivo, Wang et al[24] conducted experiments in a mouse model in which ethanol or ligation of the pylorus induced gastric ulcers. The study involved two polysaccharides, namely the crude polysaccharide of H. erinaceus, [i.e., crude polysaccharide (HECP)] and the refined polysaccharide of H. erinaceus [i.e., refined polysaccharide (HERP)], obtained from the fruiting body using water extraction and ethanol precipitation methods[25]. The mice were divided into several groups, including control groups and those receiving H. erinaceus polysaccharides at different dosages (100 mg/kg, 200 mg/kg, and 400 mg/kg). In the ethanol-induced gastric ulcer model, there was a reduction in the severity of the ulcers in a dose-dependent manner in the HERP/HECP-treated groups, with a significant reduction when pre-treatment with 400 mg/kg of HERP/HECP was performed. In contrast, in the pylorus-ligation-induced ulcer model, significant ulcer-inhibiting power was achieved when mice were administered HERP or HECP in a 200 mg/kg dosage. Nevertheless, the ulcers appeared to be more mitigated by HECP polysaccharide than HERP.
These results generally indicate a marked gastroprotective effect of HERP/HECP polysaccharides in ethanol-induced and pylorus-ligated gastric ulcers. However, the authors also showed results related to the control of gastric secretions. HERP/HECP administration provided a regulatory advantage over the imbalance in acid secretion induced by pylorus ligation.
Once the gastric mucosa has been damaged, the inflammatory process is activated, thereby increasing the mediators of inflammation, including tumor necrosis factor α (TNF-α), IL-1β, and IL-6[26]. TNF-α stimulates neutrophil infiltration and apoptosis of epithelial cells, reduces gastric microcirculation around the ulcer region, and delays its healing[27]. Leucocyte infarction in the gastric mucosa is generally assessed using myeloperoxidase (MPO) activity[28]. IL-1β significantly promotes ulcer formation[29]. Another defensive element that protects against gastric ulceration is the mucus-bicarbonate barrier. The mucus is a gel that adheres to the mucosa, preventing gastric acid penetration and injury. Mucus typically works in conjunction with nitric oxide (NO), prostaglandin E2 (PGE2), and epidermal growth factor (EGF) to maintain mucosal integrity[30]. NO protects the mucosal barrier and integrity of the gastric epithelium by inducing the inactivation of gastric parietal cells that secrete hydrochloric acid, thereby reducing acidity[31]. PEG2 increases mucus and bicarbonate production, leading to a decrease in gastric epithelial permeability[32]. EGF induces the proliferation of epithelial cells, thereby promoting tissue healing[30].
Wang et al[24] discovered that rats administered with HERP or HECP had lower serum TNF-α and IL-1β levels and lower gastric tissue MPO activity than in the control group, indicating that these polysaccharides reduced the inflammatory response. In addition, the mucus content in the stomach was higher in the H. erinaceus polysaccharides-treated group than in the control group, suggesting that polysaccharides may protect the integrity of the gastric mucosa. The latter was also promoted by the increased release of NO, PGE2, and EGF in the H. erinaceus polysaccharides-treated group. HERP/HECP also showed scavenging effects for 2,2-diphenyl-1-picrylhydrazyl, chelating capacity for Fe2+ and OH in vitro, antioxidant activity, and increased superoxide dismutase (SOD) and glutathione peroxidase (GPx) activities. It is known that SOD can rapidly convert peroxyl radicals into biologically safe and inactive substances[33].
Furthermore, GPx protects the gastric mucosa from reactive oxygen species (ROS)-induced injury and reduces lipid peroxidation[34]. Phenolic compounds appear to be the main contributors to the antioxidant capacity of H. erinaceus[35]. The antioxidant and scavenger properties of H. erinaceus exerted through its polysaccharide component have also been confirmed by other studies, in which it was shown to prevent H2O2-induced apoptotic cell death in gastric epithelial cell lines (i.e., GES-1 cells)[25].
In general, there are several studies on the use of H. erinaceus in ethanol-induced ulcers[36-38], with some focusing on acetic acid-induced ulcers[39]. Mao et al[36] also highlighted a possible therapeutic mechanism for ethanol-induced ulceration in mice via epidermal differentiation by studying the differences in the expression of several keratins, including 16, 6b, and transglutaminase E, in mucosa treated with H. erinaceus and untreated mucosa.
In addition to its multidimensional gastroprotective properties, H. erinaceus can regulate chaperonins, including HSP70. For example, in a model of ethanol-induced ulcers in Sprague Dawley mice, immunohistochemical studies have demonstrated an increased presence of HSP70 and downregulation of pro-apoptotic Bcl-2-associated X proteins[40]. Heat shock proteins (as, for example, HSP70) have a well-defined role in the pathogenesis of gastric ulcers. They are among the key players in the intracellular defence mechanisms of gastric cells. Some maintain protein integrity under homeostatic and non-stressful conditions, while others are activated after noxious stimuli[41].
However, the literature on this fungus has focused on both the erosive and atrophic patterns of gastric mucosal damage. Wang et al[42] examined the EP-1 fraction obtained from H. erinaceus mycelium in chronic atrophic gastritis. They found the potential to reduce the proliferation of MC cells (a model of atrophic gastritis) by arresting them in the G0/G1 phase of the cell cycle. However, there is a clinical, double-blinded, preliminary Chinese report for atrophic gastritis, although it was conducted in 1985 on 25 patients with atrophic gastritis who were administered H. erinaceus orally for three months. Clinical and histological improvements were observed in 63% and 52% of treated patients[43].
Although there is a considerable amount of preclinical experience, there is a substantial lack of clinical trials that have evaluated this mushroom as a pharmacological intervention in erosive gastritis, gastric ulcers, and atrophic gastritis.
Helicobacter pylori (H. pylori) is a gram-negative spiral-shaped bacillus that contributes to several gastrointestinal disorders, including chronic gastritis, peptic ulcer, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue lymphoma[44]. In addition, it is associated (to varying degrees) with several extra-gastric disorders, including vitamin B12 deficiency anemia, primary immune thrombocytopenia, as well as ophthalmic conditions (such as glaucoma and central serous chorioretinopathy), dermatological disorders (such as rosacea and psoriasis), inflammatory bowel diseases (IBD), metabolic and neurological disorders[45]. The International Agency for Research on Cancer has designated H. pylori as a Group I carcinogen for gastric cancer[46]. Therefore, eradication is imperative when an infection is diagnosed[47]. However, we frequently encounter this bacterium’s substantial antibiotic resistance; therefore, the guidelines suggest an algorithm based on several successive lines of treatment until eradication is achieved[48,49]. In addition to standard drug therapy, probiotics have been proposed to reduce adverse events associated with drug therapy[48]. H. erinaceus components (obtained by various extraction techniques) have shown marked antimicrobial properties against H. pylori[50-54]; however, the significant available evidence is preclinical. The minimum inhibitory concentrations (MIC) of the various components of H. erinaceus against H. pylori are shown in Table 1. MIC values fluctuate by varying the extractive, qualitative, and quantitative characteristics of the extracted components while reaching interesting values in some cases, as in the experience of Liu et al[52].
| Ref. | H. erinaceus fraction employed | Extraction method | Anti-H.pylori MIC (µg/mL) |
| Shang et al[50], 2013 | Ethyl acetate fractions | 62.5-250.01 | |
| Zhu et al[51], 2014 | HEP25 (197 kDa) | Ethanol precipitation (ethanol concentration 25%) | 3202 |
| HEP75 (20 kDa) | Ethanol precipitation (ethanol concentration 75%) | 1602 | |
| Bi3+plus HEP25 | Complexation of peptides with bismuth[54] | 202 | |
| Bi3+plus HEP75 | 202 | ||
| Liu et al[52], 2016 | PE2s (4 g) | Petroleum ether extract | 250-5003 |
| II-14-18 (311.000 mg) | Methyl alcohol elution from PE2s | 12.5-503 | |
| II-19-30 (355.100 mg) | 12.5-253 | ||
| II-10-13 (306.100 mg) | 25-1003 | ||
| II-54-58 (96.000 mg) | 100-400 +3 | ||
| II-31-45 (363.900 mg) | 25-503 | ||
| II-46-53 (184.500 mg) | 25-1003 | ||
| II-59-63 (78.100 mg) | 50-1003 | ||
| II-64-78 (425.400 mg) | 100-400 +3 | ||
| II-1-6 (215.700 mg) | 50-2003 | ||
| II-7-9 (319.900 mg) | 25-2003 | ||
| 1-(5-chloro-2-hydroxyphenyl)-3-methyl-1-butanone) | Recrystallized from II-10-13 and II-54-58 | 12.5-503 | |
| 2,5-bis(methoxycarbonyl)terephthalic acid | Recrystallized from II-10-13 and II-54-58 | 6.25-253 | |
| Thi My Ngan et al[53], 2021 | fEtOAc (11.040 g) | Culture filtrate-derived ethyl acetate fraction | 1.254 |
| mEtOAc (0.091 g) | Mycelium-derived Ethyl acetate fraction | 1.54 | |
| mHexane (0.162 g) | Mycelium-derived hexane fraction | 7.54 | |
| PS (26.400 g) | Culture filtrate-derived polysaccharide | 7.54 | |
| fHexane (0.120 g) | Culture filtrate-derived hexane fraction | 104 | |
| mWater (0.509 g) | Mycelium-derived water fraction | 10 +4 | |
| fWater (72.480 g) | Culture filtrate-derived water fraction | 10 +4 | |
Therefore, it would be desirable to determine through clinical trials whether supplementation with H. erinaceus can have an additive effect on the anti-H. pylori efficacy of available antibiotic therapies, and whether such supplementation can reduce the adverse events associated with these antibiotic therapies.
Gastric cancer is now the fourth leading cause of cancer-related deaths, based on its incidence and prevalence[55]. Surgical and medical therapy take the lead in managing this neoplasm[56]; however, while not changing this premise, several natural substances have been studied as complementary treatments[57-59].
Potential applications of H. erinaceus also extend in this context with a specific component of this fungus named in connection with these properties (i.e., erinacines). They are diterpenoids with known neuroprotective properties, of which erinacine A is obtained from the ethanol extract of H. erinaceus mycelium[13]. With its exact origin, it is possible to obtain another extract, a sesterterpene, erinacine S[60].
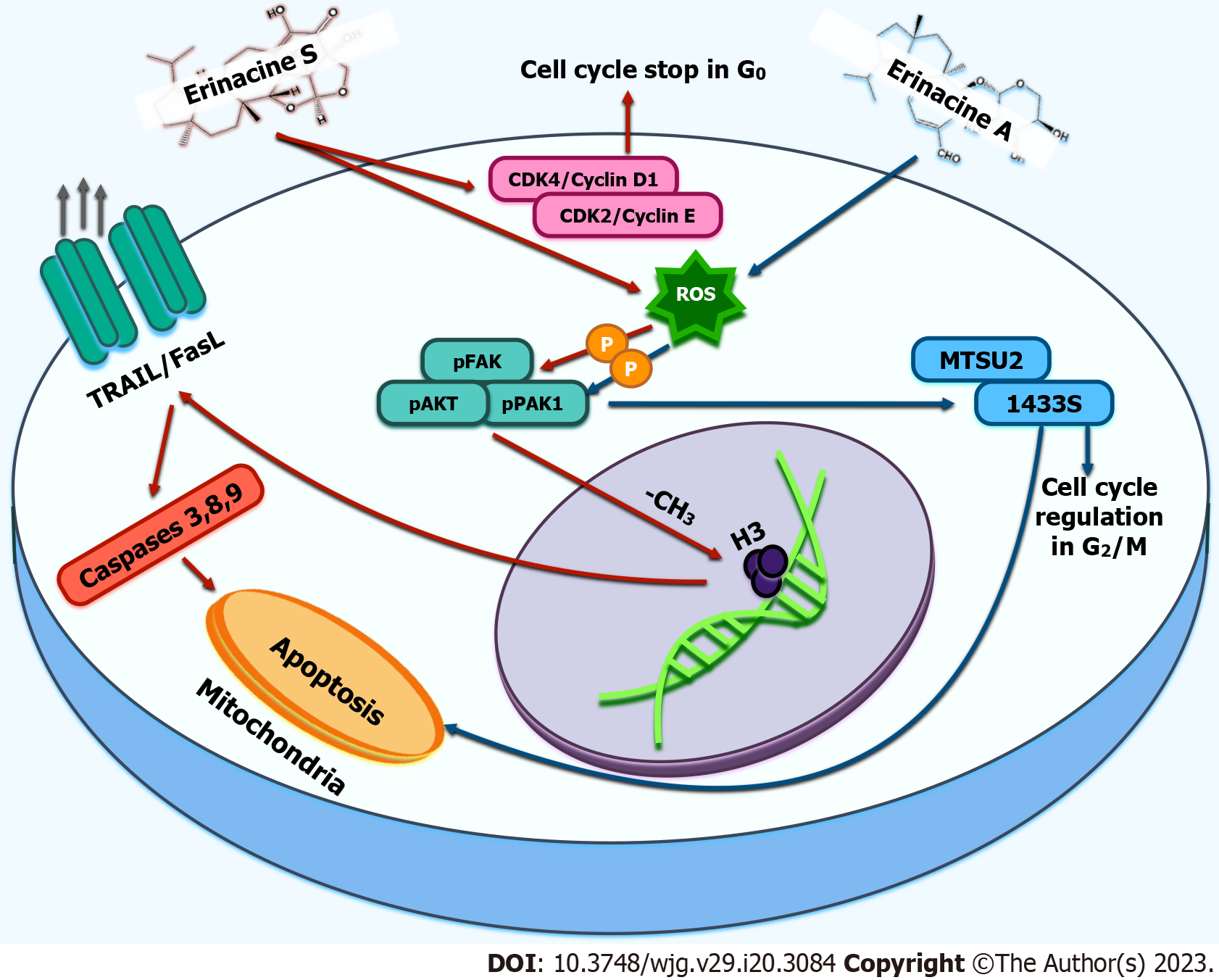
Tung et al[61] demonstrated a unique mechanism by which erinacine S could intervene in gastric carcinogenesis through epigenetic regulation. This molecule can induce selective apoptosis in gastric cancer cell lines (i.e., AGS) mediated by ROS toxicity while sparing normal cells. A mouse model of AGS-xenografts in which erinacine S suppressed tumour growth also confirmed this phenomenon[61]. In general, erinacine S may promote apoptosis in gastric carcinoma cells by inducing a specific pathway involving several molecules, such as TNF-related apoptosis-inducing ligand T (TRAIL), Fas ligand (Fas-L), and caspases 3,8,9 which are known to be involved in apoptotic death. At the same time, erinacine S suppressed the expression of anti-apoptotic molecules (i.e., Bcl-2 and Bcl-XL). In addition, cell arrest is promoted in the G1 phase of the cell cycle by the inactivation of specific cyclins and cyclin-dependent kinases[61]. Furthermore, erinacine S promotes the expression of Fas-L and TRAIL in gastric cancer cells undergoing apoptosis by trimethylation of histone H3 in the promoter regions of the Fas-L and TRAIL genes[61].
Erinacine A exhibits characteristics similar to erinacine S with respect to apoptosis induction. Several studies have shown that erinacine S can inhibit the growth of colorectal cancer both in vitro and in vivo, which could be attributed to the inhibition of proliferation and induction of the apoptosis signalling pathway, such as the generation of ROS via the phosphatidylinositol 3-kinase (PI3K)/mechanistic target of rapamycin (mTOR)/ribosomal protein S6 kinase beta-1 (p70S6K) pathway[62,63].
Proteomic analyses have confirmed that erinacine A reduces the growth and invasiveness of TSGH9201 gastric cancer cells via ROS-mediated phosphorylation of focal adhesion kinase (FAK)/ protein kinase B (also known as AKT)/p70S6K and p21-activated kinase 1 (PAK1)[64]. Previous studies have shown that erinacine A-mediated apoptosis involves the actin depolymerisation pathway[65]. Furthermore, several PAK partners can phosphorylate or activate mitogen-activated protein kinases. The kinases PI3-kinase/AKT and LIM are involved in the regulation of the cytoskeleton[66,67]. In addition, erinacine A is believed to induce upregulation of the onco-suppressive proteins microtubule-associated scaffold protein 2 (MTUS2) and 14-3-3 protein sigma (1433S), associated with antitumour activity in gastric cancer cells[63]. Recent studies have shown that the 1433S protein appears to intervene in gastric cancer by exerting G2/M checkpoint regulation in the cell cycle[68,69].
Furthermore, the MTUS2 gene plays a central role in controlling microtubule plus-end-tracking proteins (also known as + TIPs) by regulating cell division and migration through its mitotic kinesin-associated centromere, a microtubule depolymerase[70,71]. Moreover, the cytoskeleton depolymerisation pathway has been recognised as a critical cellular response that controls apoptosis and inhibits Rho GTPase-activated cell migration through its effector kinases, Rho-associated coiled-coil containing protein kinases 1 and 2[72]. These findings are significant and imply that phosphorylation of the FAK/AKT/p70S6K and PAK1 pathways determines the downstream expression of the MTUS2 and 1433S genes, the execution of cancer cell apoptosis, and the role of erinacine A as an anti-invasive agent. This effect most likely reflects cytoskeleton rearrangement, reducing erinacine A-dependent cell motility[63,73,74]. Figure 2 summarises the primary antineoplastic mechanisms of erinacines.
An additional polysaccharide protein extracted from the fermented mycelia of H. erinaceus (HEG-5) was studied in SGC-7901 gastric cancer lines. Again, positive regulation of apoptosis and the cell cycle appears to be the mechanisms underlying this antineoplastic action. Indeed, it seems that HEG-5 blocks the development of SGC-7901 cells in the S phase of the cell cycle by promoting the opposite regulation of anti- and pro-apoptotic genes. That is, predictably, the downregulation of anti-apoptotic molecules (such as Bcl-2, PI3K, and AKT) and, conversely, by upregulating caspases 3,8, p53, the bcl-2-associated X-protein, and the bcl-2-associated death promoter. Thus, caspase 3,8-dependent, p53-dependent, and PI3K/AKT-mediated apoptotic pathways are activated[75].
A synergy between doxorubicin and H. erinaceus was also observed in their pro-apoptotic action toward SGC-7901 cells via ROS-induced stress and caspase activation[76].
Moreover, two extracts of H. erinaceus (i.e., HTH5 and HTJ5A) have been shown in an experiment conducted in NCI-87 gastric carcinoma cells to possess both in vitro and in vivo (in xenograft models including severe combined immunodeficient bearing mice) concentration-dependent cytotoxicity toward such cells, lower toxicity, and more efficacy than 5-fluorouracil[77].
Finally, H. erinaceus (via the EP-1 polysaccharide) targets not only cancer cells but also precancerous cell lines by promoting their arrest in the G0/G1 phase of the cell cycle[78].
IBD is a chronic digestive disease that results in sustained gastrointestinal inflammation and consists mainly of ulcerative colitis (UC) and Crohn’s disease[79].
Available evidence related to H. erinaceus primarily focuses on UC. Wang et al[80] evaluated three polysaccharides (i.e., wHEP-1, wHEP-2, and wHEP-3) and proposed the third as the one showing the greatest anti-inflammatory action in a UC-like model in Caco-2 cells inflamed by bacterial lipopolysaccharide.
A more complete in vivo model has been reported by Diling et al[81]. The authors experimentally induced UC-like colitis in mice using trinitrobenzene-sulfonic acid enemas. They were then treated with mixed extracts of H. erinaceus (polysaccharide, alcoholic, and cumulative fractions) for 14 d. Significant clinical improvements were observed in the treated mice compared with the untreated control mice. Also, histologically, the treated group had significantly less severe lesions. They recorded reduced MPO levels in the treated mice to verify tissue infiltration of neutrophils. This was accompanied by a modulation of cytokines in the treated group with the restoration of proinflammatory and anti-inflammatory cytokines to pre-treatment levels with trinitro-benzene-sulfonic acid.
Further study has confirmed the anti-UC properties of ethanolic extracts of H. erinaceus in C57BL/6 mice exposed to dextran sulphate sodium orally to induce experimental UC-like colitis. The dosage used by the authors was 250/500 mg/kg/d[82]. This study showed as much clinical improvement as histologic (including neutrophil infiltration by MPO dosing) and cytokine improvement. However, these authors also stigmatised antioxidant potential by upregulating NO, malondialdehyde, and SOD. Wang et al[83] also focused on the antioxidant potential of H. erinaceus polysaccharides as a therapeutic mechanism in UC experimental colitis and discovered the positive regulation of SOD and reduced ROS production. It is no coincidence that combating oxidative stress is part of the therapeutic proposals for IBD[84].
UC pathogenesis remains largely unclear, but bowel inflammation and oxidative stress are considered fundamental mechanisms underlying its pathophysiology. During the active phase of UC, activated leukocytes generate many proinflammatory cytokines and pro-oxidative stress reactions. The joint deterioration caused by inflammation and oxidative stress significantly alters the redox balance within the intestinal mucosa, which accelerates the apoptosis of intestinal epithelial cells[85,86]. Excessive ROS production directly leads to tissue damage and induces an inflammatory cascade[87]. When mitochondria are damaged by oxidative stress, they enter a vicious cycle in which the loss of respiration disrupts redox homeostasis and, in turn, increases intracellular oxygen availability, resulting in increased ROS formation and subsequent oxidative damage to DNA[88]. Several studies have shown that UC onset and course are related to changes in mitochondrial structure and function[89,90].
Finally, in the context of H. erinaceus polysaccharides, Ren et al[91] confirmed this finding in C57BL/6 mice with experimental UC-like colitis induced by dextran sulphate sodium. Furthermore, the authors recorded (as also done by Diling et al[81]) an anti-inflammatory downregulation of nuclear factor kappa B (NF-κB).
NF-κB is part of several pathways (i.e., the canonical and noncanonical pathways) that have been extensively studied in IBD, upon which the mainstay of biological therapy for IBD, namely anti-TNF-α agents, has been built[92].
However, another mechanism by which the anti-IBD effect of H. erinaceus has been studied is the modulation of the gut microbiota, as described in the last section of this review.
Diverticular disease has acquired several modifications of its nomenclature over time, including the concept of symptomatic uncomplicated diverticular disease (SUDD) in its gnoseological entity. SUDD is characterised by colonic diverticulosis associated with chronic abdominal pain without signs, symptoms, or evidence of underlying diverticulitis or colitis[93]. Several pathogenetic mechanisms have been implicated, including visceral hypersensitivity and a reduction in the interstitial Cajal cells, resulting in slowed colonic motility[94]. SUDD therapy includes poorly absorbable antibiotics (such as rifaximin[95]), mesalamine[96], or probiotics[97], as well as modification of habits with increased physical activity[94]. However, definitive medical therapy for SUDD has not yet been defined.
Paradoxically, in the case of diverticular disease, the H. erinaceus research trend was reversed with the availability of clinical studies and the absence of preclinical studies.
Brandimarte et al[98], in a single study, evaluated a combination nutraceutical compound mainly consisting of polysaccharide extracts of H. erinaceus in 305 patients with SUDD. The authors recorded clinical remission rates (defined by them as the disappearance of all symptoms) of 9.34% and 17.64% at three and six months of treatment, respectively. Beyond clinical remission, it is interesting to note that the clinical response rate (defined as symptom reduction) was > 90% at three months and approximately 85% at six months. Furthermore, at three and six months, the authors recorded a significant decrease in faecal calprotectin values from baseline. However, these data should be interpreted within the limitations of a single study and the lack of clarification regarding the actual mechanism underlying this clinical improvement.
Nevertheless, it is clear how the inflammatory process plays a role in the pathogenesis of diverticular disease[99]. In addition, TNF-α levels appear to increase progressively with the severity of diverticular disease in both diverticulitis and SUDD[100]. Therefore, as in the other gastrointestinal disorders already discussed in this review, H. erinaceus might potentially intervene in diverticular disease through the regulation of the local inflammatory load; however, as already mentioned, there is currently no evidence.
Unlike IBD, where H. erinaceus has been extensively studied in preclinical models, no evidence is available regarding its role in irritable bowel syndrome (IBS). However, it is becoming increasingly clear that IBS is coded within functional gastrointestinal disorders and how ROME IV has now defined these disorders as “disorders of brain-gut interaction”, stigmatising the decisive role that the gut-brain axis has acquired in the pathogenesis and clinical features of IBS and other similar functional disorders[101]. Indeed, it is also clear how many brain-derived factors (from neurotransmitters to psychological disorders) are directly involved in IBS pathogenesis[101]. Patients with IBS experience a notably higher prevalence of anxiety-depressive disorders than the healthy population[102]. H. erinaceus has been widely studied in clinical settings in patients with anxiety and depression. One randomised controlled trial provided results in favour of positive regulation of psychiatric disorders[103]. Moreover, several pathogenic mechanisms have been suggested in studies on mood disorders. In mice, H. erinaceus appears to exert anti-inflammatory effects (negative regulation of proinflammatory cytokines and positive regulation of anti-inflammatory cytokines), stimulate hippocampal neurogenesis, and increase neurotransmitters such as 5-hydroxytryptamine, dopamine, and noradrenaline. However, in humans, it appears to increase salivary levels of free 3-methoxy-4-hydroxyphenethyleneglycol and circulating levels of pro-brain-derived neurotrophic factor. These molecular changes are associated with an improved anxiety-depressive effect[104-107].
Beyond that, the potential of H. erinaceus to intervene in the gut-brain axis could also be explored in patients with IBD, where the prevalence and impact on the disease course of anxiety-depressive disorders are not negligible[108,109]. In addition, factors leading to anxiety-depressive disorders can impact therapeutic adherence, as observed during the COVID-19 pandemic[110,111].
IBS therapy is challenging, and much more needs to be added to the research field[112]. In addition, naturally derived substances have repeatedly been considered possible therapies for IBS[113-115]. Ultimately, despite the interesting prospect of the impact of H. erinaceus on the dysregulation of the gut-brain axis in IBS, studies evaluating the effects of this fungus on both the gastrointestinal clinical features and the impact of modulation of anxiety and depression on the latter are still awaited.
As observed for gastric cancer, even in the case of colorectal cancer, studies have been conducted regarding the antineoplastic potential of H. erinaceus. Table 2 summarises the main pathophysiological mechanisms identified.
| Ref. | H. erinaceus fraction employed | Colonic cancer model | Identified mechanism |
| Kim et al[119], 2011 | Hot water/ microwave ethanol extraction extracts | CT-26 cancer cells graft in mice | NK cells activity ↑; macrophages activity ↑; angiogenesis ↓ |
| Li et al[77], 2014 | Polysaccharides | HT-29 cancer cells graft in mice | - |
| Lee et al[65], 2017 | Erinacine A | Cancer cells (HCT-116, DLD1) | PI3K/AKT/mTOR/; p70S6K pathway; ROS ↑ |
| Sharif et al[120], 2018 | Ethanolic and methanolic extracts | Cancer cells (HT-29) | α-glucosidase activity ↑; anti-tyrosinase activity ↓ |
| Liu et al[116], 2020 | Polysaccharides | Cancer cells (HCT-116) | CDK1 ↓; CDK2 ↓; Cyclin A2 ↓; MCM5 ↓ |
| Hou et al[118], 2020 | Polysaccharides | Cancer cells (HCT-116, DLD1) | Clived caspases 3,9 ↑; ROS ↑; Bax ↑; Bcl-2 ↓ |
Liu et al[116] focused on two polysaccharides from the fruiting body of H. erinaceus extracted by hot water and ferrocyanide-zinc acetate (HEFP-1 and HEFP-2). These polysaccharides showed the ability in their assay to selectively inhibit the growth of colonic cancer cells (i.e., HCT-116) while sparing normal colonic cells. Furthermore, the HEFP polysaccharide 2b fraction (HEFP-2b) was determined to be responsible for this action. In other words, HEFP-2b induced S-phase cell cycle arrest of such cells through the downregulation of CDK1,2 and cyclin A2 and concomitant inhibition of mini-chromosomal maintenance protein 5 (MCM5), a protein essential for the transition from the S-phase to the M-phase[117].
Using the exact extraction mechanism, Hou et al[118] obtained and characterised another polysaccharide fraction of the mushroom fruiting body with antineoplastic properties in colonic cancer. The model was similar to the cellular model employing both the same cells as the previous authors (i.e., HCT-116) but with the addition of the DLD1 cell group. They showed an upregulation of cleaved caspase-9 and cleaved caspase-3 without a change in the cleavage of caspase-8, confirming that the apoptotic mechanism was mitochondrial and not extrinsic with relative inhibition of the Bcl-2 protein and stimulation of the pro-apoptotic Bax protein. Confirming this evidence, the authors identified that ROS production might be one of the triggers of this apoptotic phenomenon.
Another study examined fungal extracts obtained by boiling water, microwave extraction in ethanol, and acid or alkaline extracts with hydrochloric acid or sodium hydroxide, respectively[119]. These extracts specifically demonstrated inhibitory effects on implanted tumours in mice (using CT-26 murine cancer cells). Furthermore, intraperitoneal administration of the extracts obtained by boiling water and microwaving in ethanol reduced tumour growth by 38% and 41%, respectively. These extracts increased the cytolytic activity of natural killer cells and phagocytic activity of macrophages and blocked tumour angiogenesis.
In addition, as in gastric cancer, HTJ5 and HTJ5A extracts were shown to block the growth of HT-29 colon cancer cell implants in mice with severe combined immunodeficiency[77]. Another study also confirmed the antineoplastic action of H. erinaceus in HT-29 cells by evaluating its anti-tyrosinase and α-glucosidase activities[120].
As previously described, erinacines are the principal antineoplastic components of H. erinaceus in gastric cancer. However, erinacine A showed marked antineoplastic effects against colon cancer. In detail, Lee et al[65] highlighted this in HCT-116 and DLD-1 cells by demonstrating how erinacine A was able to exert its cytotoxic action similar to that observed in gastric cancer by increasing ROS production and decreasing cancer cell proliferation through upregulation of the PI3K/mTOR/p70S6K pathway.
The gut microbiota, although not fully detailed and understood, plays a crucial role in the development, progression, and treatment of several gastrointestinal pathological conditions, including IBS and IBD[121].
H. erinaceus is closely related to the modulation of the gut microbiota. In general, it seems to be able to change the gut microbiota’s quantitative and qualitative phenotypes in a health-promoting manner. Therefore, it has often been defined as a prebiotic or probiotic[81,122-124]. It appears that H. erinaceus selects certain beneficial bacterial strains from the gut microbiota at the expense of pathogenic strains. For example, Xie et al[124] studied the fourteen days administration of 1 g of H. erinaceus dry powder in submerged cultures in 13 healthy young volunteers and recorded an increase in the alpha diversity of the gut microbiota. They recorded an increase in Bifidobacterium and Bacteroides and an increase in short-chain fatty acid (SCFAs) production (i.e., Roseburia faecis, Faecalibacterium prausnitzii, Eubacterium rectale, Fusicatenibacter saccharivorans, Kineothrix alysoides, Gemmiger formicilis, and Dorea longicatena). Confirming the modulation of the microbiota, in addition to this whole series of beneficial bacterial species, H. erinaceus resulted in a reduction in the relative abundance of pathogenic bacteria (Streptococcus thermophilus, Roseburia intestinalis, Bacteroides caccae, and Anaerostipes hadrus).
SCFA-producing bacteria may intervene in immune homeostasis through the regulation of lymphocyte chemotaxis and phagocytosis and possess anti-inflammatory and anti-tumourigenic properties[125]. In addition, SCFAs produced mainly in the colon from indigestible polysaccharides are associated with a reduced risk of IBD and IBD-associated dysbiosis[126]. Not surprisingly, they regulate the immune response by suppressing TNF-α production in neutrophils, contributing to intestinal barrier integrity by inducing secretion of IL-18, mucin, and antimicrobial peptides by intestinal epithelial cells and impacting the ability of dendritic cells to bind to T lymphocytes[126].
Moreover, the authors posited the impact of such changes in the gut microbiota with a shift in several hematochemical parameters by observing a beneficial correlation with several analytes (i.e., alkaline phosphatase, low-density lipoprotein, creatinine, and uric acid). These data suggest a possible clinical impact of H. erinaceus-driven modulation of the gut microbiota.
A further study recreated some experimental conditions of digestion to evaluate whether some polysaccharides of H. erinaceus could overcome the digestive barrier of the upper digestive tract and influence gut microbiota composition.
Following this experimental model, several H. erinaceus polysaccharides obtained by alcohol precipitation (i.e., HEP30, HEP50, and HEP70) increased the relative abundance of SCFA-producing bacteria and reduced pathobiont concentrations (i.e., Escherichia-Shigella, Klebsiella, and Enterobacter in this experience), stigmatised the role of such polysaccharides as possible functional foods[127]. Therefore, they set up an experimental in vitro digestion model, as previously described. First, they suggested the likely passage of polysaccharides through the gastrointestinal tract without being digested by the saliva of healthy donors or gastric and small intestinal juices (simulated in the laboratory). Therefore, they may reach the distal tract of the intestine. Second, at that level, the authors demonstrated how the gut microbiota utilised HEP50 for fermentation by increasing the levels of SCFAs and decreasing the pH of the faecal fermentation broth.
Furthermore, Yang et al[127] examined the impact of a polysaccharide from the mycelium of H. erinaceus on the quality of murine gut microbiota. The authors observed a change in the relative abundance of different bacteria depending on the age of the mice used for the microbiota analysis. In both the control and experimental groups of adult and middle-aged mice, there was an increase in the relative abundance of Lachnospiraceae, Ruminococcaceae, and Akkermansiaceae and a decrease in the relative abundance of Muribaculaceae, Rikenellaceae, Lactobacillaceae, and Bacteroidaceae. On the other hand, only the treated adult mice showed an increase in Erysipelotrichaceae, Enterobacteriaceae, Christensenellaceae, and Coriobacteriaceae and a decrease in Bifidobacteriaceae and Peptostreptococcaceae. Finally, in the group of middle-aged and old mice, the increased bacterial species were Rhizobiaceae, Desulfovibrionaceae, and Lachnospiraceae, while the decreased species were Corynebbacteraceae and Rikenellaceae. Among the many modified families of bacteria, the relevant ones are the butyrate-producing bacteria (i.e., Lachnospiraceae and Ruminococcaceae). Butyrate is an SCFA used as an energy source by the intestinal mucosa to promote gut health and protect against colorectal cancer[128-130]. These two species of bacteria are among the leading producers of butyrate[131]. Further in vitro studies have shown the beneficial effects of H. erinaceus in modulating SCFA-producing bacteria[132]. Positive H. erinaceus-driven modulation of the gut microbiota has also been confirmed in elderly dogs, with ameliorative effects on immunity and obesity[133].
Cancer therapy is associated with significant adverse events, including gastrointestinal complications. The latter includes dysbiosis induced by antineoplastic treatments[134]. However, while the microbiota may be impacted by antineoplastic therapy, it is also true that several reports suggest an opposite mechanism whereby the gut microbiota may modulate the response to treatment, specifically immunotherapy[135]. In this context, H. erinaceus showed some preclinical results, demonstrating its potential in cancer therapy-induced toxicity. For this purpose, an investigation based on polysaccharides was conducted in mice treated with cyclophosphamide[136]. This brought the composition of the gut microbiota of chemotherapy-treated mice closer to that of control and healthy mice through increased alpha and beta diversity. Similar results were reported in another study[123]. Moreover, these data are also available for 5-fluorouracil toxicity. Wang et al[137] examined the proteins of H. erinaceus in a xenograft cancer model in mice successfully treated with 5-fluorouracil and revealed an anti-dysbiosis action.
Although a therapy based on the direct modification of the gut microbiota is not yet recommended in the current guidelines for managing IBD, it is clear that the potential of this option has been extensively studied and is currently under investigation[138-141].
Ren et al[142] studied whether the administration of H. erinaceus to Cynomolgus monkeys affected the clinical features of spontaneous UC by exerting an anti-inflammatory effect through modulation of the gut microbiota. They recorded an increase in the abundance of bacteria, such as Lactobacillus reuteri (already implicated in improving the clinical features of IBS, acute gastrointestinal infections, and IBD in children and adults). In contrast, Streptococcus lutetiensis is negatively modulated and is known to cause sepsis in newborns[143].
In addition, Diling et al[81], in the above cited model of murine colitis induced by trinitro-benzene-sulfonic acid, demonstrated how the administration of extracts (i.e., polysaccharide, alcoholic extracts, and whole extracts) of H. erinaceus improved both the clinical and histological picture but, more importantly, the gut microbiota by promoting a switch to a microbial composition similar to that of the controls. In other words, a reduction in proinflammatory strains (Corynebacterium, Staphylococcus, Ruminococcus, Roseburia, Dorea, and Sutterella) and an increase in anti-inflammatory strains (Bacteroides, Bifidobacterium, Prevotella, Parabacteroides, Coprococcus, Desulfovibrio, and Lactobacillus) were observed.
In a similar study, in an acetic acid-induced murine colitis model, the mycelium polysaccharide EP-1 drastically improved the gut microbiota of mice by increasing SCFA-producing populations while suppressing the expression of G protein-coupled receptor 41 (GPR41) and GPR43[144]. SCFAs can bind to GPR41 and GPR43 and increase the production of inflammatory cytokines and chemokines in the intestine[145].
Finally, positive microbiota modulation was observed in mice with dextran sulphate sodium-induced colitis[91].
Despite the possibility that H. erinaceus may have intervened in the pathogenesis of IBD through the gut microbiota, studies conducted in humans as well as those exploring the clinical impact of such microbiota modification, are still awaited, especially with clinical tools and scores that are widely validated and used in clinical and IBD research practise[146,147].
H. erinaceus is a mushroom with a long tradition of use as a medicinal product. Numerous preclinical studies have probed its gastrointestinal anti-inflammatory and antineoplastic properties and its impact on the composition of the intestinal microbiota (Figure 3). In the face of a large body of evidence, there is a strong need for clinical studies conducted on humans, especially considering the promising results of previous studies. Furthermore, it is necessary to determine whether this fungus can represent an excellent nutritional supplement in gastrointestinal pathologies, the patients who may benefit from it, and whether there is a possible therapeutic role for the compounds extracted from H. erinaceus. Finally, various technical processes for such fungi yield many extracts and fractions. Therefore, it is essential to understand which of these presents the best safety and efficacy profiles.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu J, China; Sun SY, China S-Editor: Chen YL L-Editor: A P-Editor: Zhao S
| 1. | Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, Jensen ET, Shaheen NJ, Barritt AS, Lieber SR, Kochar B, Barnes EL, Fan YC, Pate V, Galanko J, Baron TH, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology. 2019;156:254-272.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 1073] [Article Influence: 178.8] [Reference Citation Analysis (1)] |
| 2. | Deutsch JK, Levitt J, Hass DJ. Complementary and Alternative Medicine for Functional Gastrointestinal Disorders. Am J Gastroenterol. 2020;115:350-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 3. | Cheifetz AS, Gianotti R, Luber R, Gibson PR. Complementary and Alternative Medicines Used by Patients With Inflammatory Bowel Diseases. Gastroenterology. 2017;152:415-429.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 4. | Thongbai B, Rapior S, Hyde KD, Wittstein K, Stadler M. Hericium erinaceus, an amazing medicinal mushroom. Mycol Progress. 2015;14:91. [RCA] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (1)] |
| 5. | Cheng JH, Tsai CL, Lien YY, Lee MS, Sheu SC. High molecular weight of polysaccharides from Hericium erinaceus against amyloid beta-induced neurotoxicity. BMC Complement Altern Med. 2016;16:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 6. | Lee JS, Hong EK. Hericium erinaceus enhances doxorubicin-induced apoptosis in human hepatocellular carcinoma cells. Cancer Lett. 2010;297:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 7. | Shang HM, Song H, Xing YL, Niu SL, Ding GD, Jiang YY, Liang F. Effects of dietary fermentation concentrate of Hericium caput-medusae (Bull.:Fr.) Pers. on growth performance, digestibility, and intestinal microbiology and morphology in broiler chickens. J Sci Food Agric. 2016;96:215-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 8. | Zhang Z, Lv G, Pan H, Pandey A, He W, Fan L. Antioxidant and hepatoprotective potential of endo-polysaccharides from Hericium erinaceus grown on tofu whey. Int J Biol Macromol. 2012;51:1140-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 9. | Saitsu Y, Nishide A, Kikushima K, Shimizu K, Ohnuki K. Improvement of cognitive functions by oral intake of Hericium erinaceus. Biomed Res. 2019;40:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Mori K, Inatomi S, Ouchi K, Azumi Y, Tuchida T. Improving effects of the mushroom Yamabushitake (Hericium erinaceus) on mild cognitive impairment: a double-blind placebo-controlled clinical trial. Phytother Res. 2009;23:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Miles PG, Chang S-T. Mushrooms. 0 ed. CRC Press, Taylor & Francis Group, 2004. [DOI] [Full Text] |
| 12. | Boddy L, Crockatt ME, Ainsworth AM. Ecology of Hericium cirrhatum, H. coralloides and H. erinaceus in the UK. Fungal Ecology. 2011;4:163-173. [RCA] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (2)] |
| 13. | Friedman M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion's Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J Agric Food Chem. 2015;63:7108-7123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (2)] |
| 14. | Li W, Gu Z, Yang Y, Zhou S, Liu Y, Zhang J. Non-volatile taste components of several cultivated mushrooms. Food Chem. 2014;143:427-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 15. | Rodrigues DMF, Freitas AC, Rocha-Santos TAP, Vasconcelos MW, Roriz M, Rodríguez-Alcalá LM, Gomes AMP, Duarte AC. Chemical composition and nutritive value of Pleurotus citrinopileatus var cornucopiae, P. eryngii, P. salmoneo stramineus, Pholiota nameko and Hericium erinaceus. J Food Sci Technol. 2015;52:6927-6939. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Jayachandran M, Chen J, Chung SSM, Xu B. A critical review on the impacts of β-glucans on gut microbiota and human health. J Nutr Biochem. 2018;61:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 209] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 17. | Zhu F, Du B, Xu B. Preparation and Characterization of Polysaccharides from Mushrooms. In: Ramawat KG, Mérillon J-M, editors. Polysaccharides. Cham: Springer International Publishing, 2014: 1-16. |
| 18. | Liu J, Wang W, Hu Q, Wu X, Xu H, Su A, Xie M, Yang W. Bioactivities and molecular mechanisms of polysaccharides from Hericium erinaceus. J Future Foods. 2022;2:103-111. [DOI] [Full Text] |
| 19. | Łubek-Nguyen A, Ziemichód W, Olech M. Application of Enzyme-Assisted Extraction for the Recovery of Natural Bioactive Compounds for Nutraceutical and Pharmaceutical Applications. Appl Sci. 2022;12:3232. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Lanas A, Chan FKL. Peptic ulcer disease. Lancet. 2017;390:613-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 559] [Article Influence: 69.9] [Reference Citation Analysis (37)] |
| 21. | Takeuchi K. Pathogenesis of NSAID-induced gastric damage: importance of cyclooxygenase inhibition and gastric hypermotility. World J Gastroenterol. 2012;18:2147-2160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 147] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 22. | Sugimoto M, Yamaoka Y, Furuta T. Influence of interleukin polymorphisms on development of gastric cancer and peptic ulcer. World J Gastroenterol. 2010;16:1188-1200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 92] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Boddy L, Wald P. Creolophus (Hericium) cirrhatus, Hericium erinaceus and H. coralloides in England (English nature research report 492). English Nature. 2003;168-169. |
| 24. | Wang XY, Yin JY, Zhao MM, Liu SY, Nie SP, Xie MY. Gastroprotective activity of polysaccharide from Hericium erinaceus against ethanol-induced gastric mucosal lesion and pylorus ligation-induced gastric ulcer, and its antioxidant activities. Carbohydr Polym. 2018;186:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 25. | Wang M, Kanako N, Zhang Y, Xiao X, Gao Q, Tetsuya K. A unique polysaccharide purified from Hericium erinaceus mycelium prevents oxidative stress induced by H2O2 in human gastric mucosa epithelium cell. PLoS One. 2017;12:e0181546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Liu J, Wang F, Luo H, Liu A, Li K, Li C, Jiang Y. Protective effect of butyrate against ethanol-induced gastric ulcers in mice by promoting the anti-inflammatory, anti-oxidant and mucosal defense mechanisms. Int Immunopharmacol. 2016;30:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Rozza AL, Meira de Faria F, Souza Brito AR, Pellizzon CH. The gastroprotective effect of menthol: involvement of anti-apoptotic, antioxidant and anti-inflammatory activities. PLoS One. 2014;9:e86686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Amirshahrokhi K, Khalili AR. The effect of thalidomide on ethanol-induced gastric mucosal damage in mice: involvement of inflammatory cytokines and nitric oxide. Chem Biol Interact. 2015;225:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Moezi L, Heidari R, Amirghofran Z, Nekooeian AA, Monabati A, Dehpour AR. Enhanced anti-ulcer effect of pioglitazone on gastric ulcers in cirrhotic rats: the role of nitric oxide and IL-1β. Pharmacol Rep. 2013;65:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Bi WP, Man HB, Man MQ. Efficacy and safety of herbal medicines in treating gastric ulcer: a review. World J Gastroenterol. 2014;20:17020-17028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (1)] |
| 31. | Yandrapu H, Sarosiek J. Protective Factors of the Gastric and Duodenal Mucosa: An Overview. Curr Gastroenterol Rep. 2015;17:24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 32. | Sofidiya MO, Orisaremi CO, Sansaliyu I, Adetunde TO. Gastroprotective and antioxidant potentials of ethanolic stem bark extract of Margaritaria discoidea (Euphorbiaceae) in rats. J Ethnopharmacol. 2015;171:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | deFoneska A, Kaunitz JD. Gastroduodenal mucosal defense. Curr Opin Gastroenterol. 2010;26:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Sidahmed HM, Hashim NM, Amir J, Abdulla MA, Hadi AH, Abdelwahab SI, Taha MM, Hassandarvish P, Teh X, Loke MF, Vadivelu J, Rahmani M, Mohan S. Pyranocycloartobiloxanthone A, a novel gastroprotective compound from Artocarpus obtusus Jarret, against ethanol-induced acute gastric ulcer in vivo. Phytomedicine. 2013;20:834-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Guo YJ, Deng GF, Xu XR, Wu S, Li S, Xia EQ, Li F, Chen F, Ling WH, Li HB. Antioxidant capacities, phenolic compounds and polysaccharide contents of 49 edible macro-fungi. Food Funct. 2012;3:1195-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Mao X, Lu ZM, Gong TT, Wang KL, Geng Y, Xu HY, Xu GH, Shi JS, Xu ZH. Therapeutic Effect and Potential Mechanisms of Lion's Mane Medicinal Mushroom, Hericium erinaceus (Agaricomycetes), Mycelia in Submerged Culture on Ethanol-Induced Chronic Gastric Injury. Int J Med Mushrooms. 2019;21:1137-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 37. | Chen W, Wu D, Jin Y, Li Q, Liu Y, Qiao X, Zhang J, Dong G, Li Z, Li T, Yang Y. Pre-protective effect of polysaccharides purified from Hericium erinaceus against ethanol-induced gastric mucosal injury in rats. Int J Biol Macromol. 2020;159:948-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Hou C, Liu L, Ren J, Huang M, Yuan E. Structural characterization of two Hericium erinaceus polysaccharides and their protective effects on the alcohol-induced gastric mucosal injury. Food Chem. 2022;375:131896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 39. | Wang XY, Wang M, Yin JY, Song YH, Wang YX, Nie SP, Xie MY. Gastroprotective activity of polysaccharide from the fruiting body of Hericium erinaceus against acetic acid-induced gastric ulcer in rats and structure of one bioactive fraction. Int J Biol Macromol. 2022;210:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 40. | Wong JY, Abdulla MA, Raman J, Phan CW, Kuppusamy UR, Golbabapour S, Sabaratnam V. Gastroprotective Effects of Lion's Mane Mushroom Hericium erinaceus (Bull.:Fr.) Pers. (Aphyllophoromycetideae) Extract against Ethanol-Induced Ulcer in Rats. Evid Based Complement Alternat Med. 2013;2013:492976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Choi SR, Lee SA, Kim YJ, Ok CY, Lee HJ, Hahm KB. Role of heat shock proteins in gastric inflammation and ulcer healing. J Physiol Pharmacol. 2009;60 Suppl 7:5-17. [PubMed] |
| 42. | Wang M, Gao Y, Xu D, Gao Q. A polysaccharide from cultured mycelium of Hericium erinaceus and its anti-chronic atrophic gastritis activity. Int J Biol Macromol. 2015;81:656-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 43. | Xu CP, Liu WW, Liu FX, Chen SS, Liao FQ, Xu Z, Jiang LG, Wang CA, Lu XH. A double-blind study of effectiveness of hericium erinaceus pers therapy on chronic atrophic gastritis. A preliminary report. Chin Med J (Engl). 1985;98:455-456. [PubMed] |
| 44. | Gravina AG, Zagari RM, De Musis C, Romano L, Loguercio C, Romano M. Helicobacter pylori and extragastric diseases: A review. World J Gastroenterol. 2018;24:3204-3221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 244] [Cited by in RCA: 196] [Article Influence: 28.0] [Reference Citation Analysis (6)] |
| 45. | Gravina AG, Priadko K, Ciamarra P, Granata L, Facchiano A, Miranda A, Dallio M, Federico A, Romano M. Extra-Gastric Manifestations of Helicobacter pylori Infection. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 46. | Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 569] [Article Influence: 51.7] [Reference Citation Analysis (1)] |
| 47. | Flores-Treviño S, Mendoza-Olazarán S, Bocanegra-Ibarias P, Maldonado-Garza HJ, Garza-González E. Helicobacter pylori drug resistance: therapy changes and challenges. Expert Rev Gastroenterol Hepatol. 2018;12:819-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 48. | Romano M, Gravina AG, Eusebi LH, Pellegrino R, Palladino G, Frazzoni L, Dajti E, Gasbarrini A, Di Mario F, Zagari RM; Members of SIGE; Members of SIED National Council. Management of Helicobacter pylori infection: Guidelines of the Italian Society of Gastroenterology (SIGE) and the Italian Society of Digestive Endoscopy (SIED). Dig Liver Dis. 2022;54:1153-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 49. | Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, Gasbarrini A, Hunt RH, Leja M, O'Morain C, Rugge M, Suerbaum S, Tilg H, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 647] [Article Influence: 215.7] [Reference Citation Analysis (0)] |
| 50. | Shang X, Tan Q, Liu R, Yu K, Li P, Zhao GP. In vitro anti-Helicobacter pylori effects of medicinal mushroom extracts, with special emphasis on the Lion's Mane mushroom, Hericium erinaceus (higher Basidiomycetes). Int J Med Mushrooms. 2013;15:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Zhu Y, Chen Y, Li Q, Zhao T, Zhang M, Feng W, Takase M, Wu X, Zhou Z, Yang L. Preparation, characterization, and anti-Helicobacter pylori activity of Bi3+-Hericium erinaceus polysaccharide complex. Carbohydr Polym. 2014;110:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 52. | Liu JH, Li L, Shang XD, Zhang JL, Tan Q. Anti-Helicobacter pylori activity of bioactive components isolated from Hericium erinaceus. J Ethnopharmacol. 2016;183:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 53. | Thi My Ngan L, Thien Vi N, Thi Mong Tham D, Thi Thanh Loan L, Thanh Ho P, Trung Hieu T. Antioxidant and anti-Helicobacter pylori activities of Hericium erinaceus mycelium and culture filtrate. Biomed Res Ther. 2021;8:4266-4275. [RCA] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 54. | Jin Y, Ling P, He Y, Chen L, Chen J, Zhang T. Preparation, characterization and anti-Helicobacter pylori activity of Bi3+-hyaluronate complex. Carbohydr Polym. 2008;74:50-58. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 844] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 56. | Sexton RE, Al Hallak MN, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020;39:1179-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 452] [Article Influence: 90.4] [Reference Citation Analysis (0)] |
| 57. | Ju HM, Yu KW, Cho SD, Cheong SH, Kwon KH. Anti-cancer effects of traditional Korean wild vegetables in complementary and alternative medicine. Complement Ther Med. 2016;24:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Jain A, Tiwari A, Verma A, Jain SK. Vitamins for Cancer Prevention and Treatment: An Insight. Curr Mol Med. 2017;17:321-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Hassanalilou T, Ghavamzadeh S, Khalili L. Curcumin and Gastric Cancer: a Review on Mechanisms of Action. J Gastrointest Cancer. 2019;50:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 60. | Chen CC, Tzeng TT, Chen CC, Ni CL, Lee LY, Chen WP, Shiao YJ, Shen CC. Erinacine S, a Rare Sesterterpene from the Mycelia of Hericium erinaceus. J Nat Prod. 2016;79:438-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 61. | Tung SY, Lee KC, Lee KF, Yang YL, Huang WS, Lee LY, Chen WP, Chen CC, Teng CC, Shen CH, Hsieh MC, Huang CY, Sheen JM, Kuo HC. Apoptotic mechanisms of gastric cancer cells induced by isolated erinacine S through epigenetic histone H3 methylation of FasL and TRAIL. Food Funct. 2021;12:3455-3468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 62. | Kallergi G, Agelaki S, Markomanolaki H, Georgoulias V, Stournaras C. Activation of FAK/PI3K/Rac1 signaling controls actin reorganization and inhibits cell motility in human cancer cells. Cell Physiol Biochem. 2007;20:977-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 63. | Kuo HC, Kuo YR, Lee KF, Hsieh MC, Huang CY, Hsieh YY, Lee KC, Kuo HL, Lee LY, Chen WP, Chen CC, Tung SY. A Comparative Proteomic Analysis of Erinacine A's Inhibition of Gastric Cancer Cell Viability and Invasiveness. Cell Physiol Biochem. 2017;43:195-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Loo G. Redox-sensitive mechanisms of phytochemical-mediated inhibition of cancer cell proliferation (review). J Nutr Biochem. 2003;14:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 65. | Lee KC, Kuo HC, Shen CH, Lu CC, Huang WS, Hsieh MC, Huang CY, Kuo YH, Hsieh YY, Teng CC, Lee LY, Tung SY. A proteomics approach to identifying novel protein targets involved in erinacine A-mediated inhibition of colorectal cancer cells' aggressiveness. J Cell Mol Med. 2017;21:588-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 66. | Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1034] [Cited by in RCA: 1148] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 67. | Johannessen M, Delghandi MP, Moens U. What turns CREB on? Cell Signal. 2004;16:1211-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 493] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 68. | Luk SC, Siu SW, Lai CK, Wu YJ, Pang SF. Cell Cycle Arrest by a Natural Product via G2/M Checkpoint. Int J Med Sci. 2005;2:64-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 69. | Tsukamoto Y, Nakada C, Noguchi T, Tanigawa M, Nguyen LT, Uchida T, Hijiya N, Matsuura K, Fujioka T, Seto M, Moriyama M. MicroRNA-375 is downregulated in gastric carcinomas and regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer Res. 2010;70:2339-2349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 346] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 70. | Adams G Jr, Zhou J, Wang W, Wu H, Quan J, Liu Y, Xia P, Wang Z, Zhou S, Jiang J, Mo F, Zhuang X, Thomas K, Hill DL, Aikhionbare FO, He P, Liu X, Ding X, Yao X. The Microtubule Plus End Tracking Protein TIP150 Interacts with Cortactin to Steer Directional Cell Migration. J Biol Chem. 2016;291:20692-20706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Jiang K, Wang J, Liu J, Ward T, Wordeman L, Davidson A, Wang F, Yao X. TIP150 interacts with and targets MCAK at the microtubule plus ends. EMBO Rep. 2009;10:857-865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 72. | Croft DR, Olson MF. Transcriptional regulation of Rho GTPase signaling. Transcription. 2011;2:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 73. | Gu S, Kounenidakis M, Schmidt EM, Deshpande D, Alkahtani S, Alarifi S, Föller M, Alevizopoulos K, Lang F, Stournaras C. Rapid activation of FAK/mTOR/p70S6K/PAK1-signaling controls the early testosterone-induced actin reorganization in colon cancer cells. Cell Signal. 2013;25:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Papakonstanti EA, Kampa M, Castanas E, Stournaras C. A rapid, nongenomic, signaling pathway regulates the actin reorganization induced by activation of membrane testosterone receptors. Mol Endocrinol. 2003;17:870-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 75. | Zan X, Cui F, Li Y, Yang Y, Wu D, Sun W, Ping L. Hericium erinaceus polysaccharide-protein HEG-5 inhibits SGC-7901 cell growth via cell cycle arrest and apoptosis. Int J Biol Macromol. 2015;76:242-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 76. | Yang W, Han D, Wu L, Huang Y, Li J, Guo H, Liu Y. Hericium erinaceus synergizing with doxorubicin induced SGC7901 cell apoptosis. Int J Clin Exp Med. 2016;9:1447-1457. |
| 77. | Li G, Yu K, Li F, Xu K, Li J, He S, Cao S, Tan G. Anticancer potential of Hericium erinaceus extracts against human gastrointestinal cancers. J Ethnopharmacol. 2014;153:521-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 78. | Wang M, Zhang Y, Xiao X, Xu D, Gao Y, Gao Q. A Polysaccharide Isolated from Mycelia of the Lion's Mane Medicinal Mushroom Hericium erinaceus (Agaricomycetes) Induced Apoptosis in Precancerous Human Gastric Cells. Int J Med Mushrooms. 2017;19:1053-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 79. | Chang JT. Pathophysiology of Inflammatory Bowel Diseases. N Engl J Med. 2020;383:2652-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 759] [Article Influence: 151.8] [Reference Citation Analysis (0)] |
| 80. | Wang D, Xu D, Zhao D, Wang M. Screening and Comparison of Anti-Intestinal Inflammatory Activities of Three Polysaccharides from the Mycelium of Lion's Mane Culinary-Medicinal Mushroom, Hericium erinaceus (Agaricomycetes). Int J Med Mushrooms. 2021;23:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 81. | Diling C, Xin Y, Chaoqun Z, Jian Y, Xiaocui T, Jun C, Ou S, Yizhen X. Extracts from Hericium erinaceus relieve inflammatory bowel disease by regulating immunity and gut microbiota. Oncotarget. 2017;8:85838-85857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (1)] |
| 82. | Qin M, Geng Y, Lu Z, Xu H, Shi JS, Xu X, Xu ZH. Anti-Inflammatory Effects of Ethanol Extract of Lion's Mane Medicinal Mushroom, Hericium erinaceus (Agaricomycetes), in Mice with Ulcerative Colitis. Int J Med Mushrooms. 2016;18:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 83. | Wang D, Zhang Y, Yang S, Zhao D, Wang M. A polysaccharide from cultured mycelium of Hericium erinaceus relieves ulcerative colitis by counteracting oxidative stress and improving mitochondrial function. Int J Biol Macromol. 2019;125:572-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 84. | Moura FA, de Andrade KQ, Dos Santos JCF, Araújo ORP, Goulart MOF. Antioxidant therapy for treatment of inflammatory bowel disease: Does it work? Redox Biol. 2015;6:617-639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 272] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 85. | Watt DG, Horgan PG, McMillan DC. Routine clinical markers of the magnitude of the systemic inflammatory response after elective operation: a systematic review. Surgery. 2015;157:362-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 244] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 86. | Biasi F, Leonarduzzi G, Oteiza PI, Poli G. Inflammatory bowel disease: mechanisms, redox considerations, and therapeutic targets. Antioxid Redox Signal. 2013;19:1711-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 229] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 87. | Morcillo EJ, Estrela J, Cortijo J. Oxidative stress and pulmonary inflammation: pharmacological intervention with antioxidants. Pharmacol Res. 1999;40:393-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 88. | Sung HJ, Ma W, Wang PY, Hynes J, O'Riordan TC, Combs CA, McCoy JP Jr, Bunz F, Kang JG, Hwang PM. Mitochondrial respiration protects against oxygen-associated DNA damage. Nat Commun. 2010;1:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 89. | Roediger WE. The colonic epithelium in ulcerative colitis: an energy-deficiency disease? Lancet. 1980;2:712-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 471] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 90. | Santhanam S, Rajamanickam S, Motamarry A, Ramakrishna BS, Amirtharaj JG, Ramachandran A, Pulimood A, Venkatraman A. Mitochondrial electron transport chain complex dysfunction in the colonic mucosa in ulcerative colitis. Inflamm Bowel Dis. 2012;18:2158-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 91. | Ren Y, Geng Y, Du Y, Li W, Lu ZM, Xu HY, Xu GH, Shi JS, Xu ZH. Polysaccharide of Hericium erinaceus attenuates colitis in C57BL/6 mice via regulation of oxidative stress, inflammation-related signaling pathways and modulating the composition of the gut microbiota. J Nutr Biochem. 2018;57:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 92. | Wang B, Shen J. NF-κB Inducing Kinase Regulates Intestinal Immunity and Homeostasis. Front Immunol. 2022;13:895636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 93. | Piscopo N, Ellul P. Diverticular Disease: A Review on Pathophysiology and Recent Evidence. Ulster Med J. 2020;89:83-88. [PubMed] |
| 94. | Rezapour M, Ali S, Stollman N. Diverticular Disease: An Update on Pathogenesis and Management. Gut Liver. 2018;12:125-132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 95. | Colecchia A, Vestito A, Pasqui F, Mazzella G, Roda E, Pistoia F, Brandimarte G, Festi D. Efficacy of long term cyclic administration of the poorly absorbed antibiotic Rifaximin in symptomatic, uncomplicated colonic diverticular disease. World J Gastroenterol. 2007;13:264-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 96. | Floch MH, White JA. Management of diverticular disease is changing. World J Gastroenterol. 2006;12:3225-3228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Lahner E, Esposito G, Zullo A, Hassan C, Cannaviello C, Paolo MC, Pallotta L, Garbagna N, Grossi E, Annibale B. High-fibre diet and Lactobacillus paracasei B21060 in symptomatic uncomplicated diverticular disease. World J Gastroenterol. 2012;18:5918-5924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | Brandimarte G, Frajese GV, Bargiggia S, Castellani D, Cocco A, Colucci R, Evangelista E, Gravina AG, Napoletano D, Nardi E, Maisto T, Morabito A, Pianese G, Romano A, Sacco R, Sediari L, Sinnona N, Tifi L, D'Avino A, Elisei W, Tursi A. Performance of a multicompounds nutraceutical formulation in patients with symptomatic uncomplicated diverticular disease. Minerva Gastroenterol (Torino). 2022;68:216-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 99. | Kupcinskas J, Strate LL, Bassotti G, Torti G, Herszènyi L, Malfertheiner P, Cassieri C, Walker MM, Tursi A. Pathogenesis of Diverticulosis and Diverticular Disease. J Gastrointestin Liver Dis. 2019;28:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 100. | Tursi A, Elisei W, Brandimarte G, Giorgetti GM, Inchingolo CD, Nenna R, Picchio M, Giorgio F, Ierardi E. Musosal tumour necrosis factor α in diverticular disease of the colon is overexpressed with disease severity. Colorectal Dis. 2012;14:e258-e263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 101. | Fukudo S, Okumura T, Inamori M, Okuyama Y, Kanazawa M, Kamiya T, Sato K, Shiotani A, Naito Y, Fujikawa Y, Hokari R, Masaoka T, Fujimoto K, Kaneko H, Torii A, Matsueda K, Miwa H, Enomoto N, Shimosegawa T, Koike K. Evidence-based clinical practice guidelines for irritable bowel syndrome 2020. J Gastroenterol. 2021;56:193-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 102. | Fond G, Loundou A, Hamdani N, Boukouaci W, Dargel A, Oliveira J, Roger M, Tamouza R, Leboyer M, Boyer L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2014;264:651-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 404] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 103. | Nagano M, Shimizu K, Kondo R, Hayashi C, Sato D, Kitagawa K, Ohnuki K. Reduction of depression and anxiety by 4 weeks Hericium erinaceus intake. Biomed Res. 2010;31:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 104. | Chong PS, Fung ML, Wong KH, Lim LW. Therapeutic Potential of Hericium erinaceus for Depressive Disorder. Int J Mol Sci. 2019;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 105. | Fijałkowska A, Jędrejko K, Sułkowska-Ziaja K, Ziaja M, Kała K, Muszyńska B. Edible Mushrooms as a Potential Component of Dietary Interventions for Major Depressive Disorder. Foods. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 106. | Rodriguez MN, Lippi SLP. Lion's Mane (Hericium erinaceus) Exerts Anxiolytic Effects in the rTg4510 Tau Mouse Model. Behav Sci (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 107. | Chou MY, Ho JH, Huang MJ, Chen YJ, Yang MD, Lin LH, Chi CH, Yeh CH, Tsao TY, Tzeng JK, Hsu RJ, Huang PH, Lu WC, Li PH, Wang MF. Potential antidepressant effects of a dietary supplement from the chlorella and lion's mane mushroom complex in aged SAMP8 mice. Front Nutr. 2022;9:977287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 108. | Spina A, Mazzarella C, Dallio M, Romeo M, Pellegrino R, Durante T, Romano M, Loguercio C, Di Mauro M, Federico A, Gravina AG. The Lesson from the First Italian Lockdown: Impacts on Anxiety and Depressive Symptoms and Sleep Quality in Patients with Remission of Inflammatory Bowel Disease. Rev Recent Clin Trials. 2022;17:109-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (2)] |
| 109. | Barberio B, Zamani M, Black CJ, Savarino EV, Ford AC. Prevalence of symptoms of anxiety and depression in patients with inflammatory bowel disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:359-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 393] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 110. | Ryu JE, Kang SG, Jung SH, Lee SH, Kang SB. Psychological Effects and Medication Adherence among Korean Patients with Inflammatory Bowel Disease during the Coronavirus Disease 2019 Pandemic: A Single-Center Survey. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 111. | Pellegrino R, Pellino G, Selvaggi F, Federico A, Romano M, Gravina AG. Therapeutic adherence recorded in the outpatient follow-up of inflammatory bowel diseases in a referral center: Damages of COVID-19. Dig Liver Dis. 2022;54:1449-1451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (5)] |
| 112. | Barbara G, Cremon C, Bellini M, Corsetti M, Di Nardo G, Falangone F, Fuccio L, Galeazzi F, Iovino P, Sarnelli G, Savarino EV, Stanghellini V, Staiano A, Stasi C, Tosetti C, Turco R, Ubaldi E, Zagari RM, Zenzeri L, Marasco G. Italian guidelines for the management of irritable bowel syndrome: Joint Consensus from the Italian Societies of: Gastroenterology and Endoscopy (SIGE), Neurogastroenterology and Motility (SINGEM), Hospital Gastroenterologists and Endoscopists (AIGO), Digestive Endoscopy (SIED), General Medicine (SIMG), Gastroenterology, Hepatology and Pediatric Nutrition (SIGENP) and Pediatrics (SIP). Dig Liver Dis. 2023;55:187-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 113. | Chumpitazi BP, Kearns GL, Shulman RJ. Review article: the physiological effects and safety of peppermint oil and its efficacy in irritable bowel syndrome and other functional disorders. Aliment Pharmacol Ther. 2018;47:738-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 114. | Currò D, Ianiro G, Pecere S, Bibbò S, Cammarota G. Probiotics, fibre and herbal medicinal products for functional and inflammatory bowel disorders. Br J Pharmacol. 2017;174:1426-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 115. | Lu Q, Tan D, Luo J, Ye Y, Zuo M, Wang S, Li C. Potential of natural products in the treatment of irritable bowel syndrome. Phytomedicine. 2022;106:154419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 116. | Liu JY, Hou XX, Li ZY, Shan SH, Chang MC, Feng CP, Wei Y. Isolation and structural characterization of a novel polysaccharide from Hericium erinaceus fruiting bodies and its arrest of cell cycle at S-phage in colon cancer cells. Int J Biol Macromol. 2020;157:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 117. | Giaginis C, Georgiadou M, Dimakopoulou K, Tsourouflis G, Gatzidou E, Kouraklis G, Theocharis S. Clinical significance of MCM-2 and MCM-5 expression in colon cancer: association with clinicopathological parameters and tumor proliferative capacity. Dig Dis Sci. 2009;54:282-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 118. | Hou XX, Liu JY, Li ZY, Chang MC, Guo M, Feng CP, Shi JY. Fruiting body polysaccharides of Hericium erinaceus induce apoptosis in human colorectal cancer cells via ROS generation mediating caspase-9-dependent signaling pathways. Food Funct. 2020;11:6128-6138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 119. | Kim SP, Kang MY, Kim JH, Nam SH, Friedman M. Composition and mechanism of antitumor effects of Hericium erinaceus mushroom extracts in tumor-bearing mice. J Agric Food Chem. 2011;59:9861-9869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 120. | Sharif S, Atta A, Huma T, Shah AA, Afzal G, Rashid S, Shahid M, Mustafa G. Anticancer, antithrombotic, antityrosinase, and anti-α-glucosidase activities of selected wild and commercial mushrooms from Pakistan. Food Sci Nutr. 2018;6:2170-2176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 121. | Hills RD Jr, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 669] [Article Influence: 111.5] [Reference Citation Analysis (0)] |
| 122. | Yang Y, Zhao C, Diao M, Zhong S, Sun M, Sun B, Ye H, Zhang T. The Prebiotic Activity of Simulated Gastric and Intestinal Digesta of Polysaccharides from the Hericium erinaceus. Molecules. 2018;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 123. | Diling C, Chaoqun Z, Jian Y, Jian L, Jiyan S, Yizhen X, Guoxiao L. Immunomodulatory Activities of a Fungal Protein Extracted from Hericium erinaceus through Regulating the Gut Microbiota. Front Immunol. 2017;8:666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 124. | Xie XQ, Geng Y, Guan Q, Ren Y, Guo L, Lv Q, Lu ZM, Shi JS, Xu ZH. Influence of Short-Term Consumption of Hericium erinaceus on Serum Biochemical Markers and the Changes of the Gut Microbiota: A Pilot Study. Nutrients. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 125. | Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1076] [Cited by in RCA: 1610] [Article Influence: 146.4] [Reference Citation Analysis (0)] |
| 126. | Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. 2017;52:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 691] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 127. | Yang Y, Ye H, Zhao C, Ren L, Wang C, Georgiev MI, Xiao J, Zhang T. Value added immunoregulatory polysaccharides of Hericium erinaceus and their effect on the gut microbiota. Carbohydr Polym. 2021;262:117668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 128. | Dmitrieva-Posocco O, Wong AC, Lundgren P, Golos AM, Descamps HC, Dohnalová L, Cramer Z, Tian Y, Yueh B, Eskiocak O, Egervari G, Lan Y, Liu J, Fan J, Kim J, Madhu B, Schneider KM, Khoziainova S, Andreeva N, Wang Q, Li N, Furth EE, Bailis W, Kelsen JR, Hamilton KE, Kaestner KH, Berger SL, Epstein JA, Jain R, Li M, Beyaz S, Lengner CJ, Katona BW, Grivennikov SI, Thaiss CA, Levy M. β-Hydroxybutyrate suppresses colorectal cancer. Nature. 2022;605:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 189] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 129. | Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1596] [Cited by in RCA: 1949] [Article Influence: 177.2] [Reference Citation Analysis (0)] |
| 130. | Bultman SJ. Interplay between diet, gut microbiota, epigenetic events, and colorectal cancer. Mol Nutr Food Res. 2017;61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 131. | Louis P, Flint HJ. Formation of propionate and butyrate by the human colonic microbiota. Environ Microbiol. 2017;19:29-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 998] [Cited by in RCA: 1703] [Article Influence: 189.2] [Reference Citation Analysis (0)] |
| 132. | Mitsou EK, Saxami G, Stamoulou E, Kerezoudi E, Terzi E, Koutrotsios G, Bekiaris G, Zervakis GI, Mountzouris KC, Pletsa V, Kyriacou A. Effects of Rich in Β-Glucans Edible Mushrooms on Aging Gut Microbiota Characteristics: An In Vitro Study. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 133. | Cho HW, Choi S, Seo K, Kim KH, Jeon JH, Kim CH, Lim S, Jeong S, Chun JL. Gut microbiota profiling in aged dogs after feeding pet food contained Hericium erinaceus. J Anim Sci Technol. 2022;64:937-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 134. | Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25:377-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 774] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 135. | Khan MAW, Ologun G, Arora R, McQuade JL, Wargo JA. Gut Microbiome Modulates Response to Cancer Immunotherapy. Dig Dis Sci. 2020;65:885-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 136. | Tian B, Liu R, Xu T, Cai M, Mao R, Huang L, Yang K, Zeng X, Peilong S. Modulating effects of Hericium erinaceus polysaccharides on the immune response by regulating gut microbiota in cyclophosphamide-treated mice. J Sci Food Agric. 2023;103:3050-3064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 137. | Wang D, Zhu X, Tang X, Li H, Yizhen X, Chen D. Auxiliary antitumor effects of fungal proteins from Hericium erinaceus by target on the gut microbiota. J Food Sci. 2020;85:1872-1890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 138. | Larussa T, Abenavoli L, Fabiano G, Mancuso MA, Polimeni N, Dumitrascu DL, Luzza F. Gut microbiota in inflammatory bowel disease: a target for therapy not to be missed. Minerva Gastroenterol (Torino). 2021;67:357-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 139. | Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 356] [Cited by in RCA: 419] [Article Influence: 38.1] [Reference Citation Analysis (2)] |
| 140. | Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787-8803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1421] [Cited by in RCA: 1824] [Article Influence: 182.4] [Reference Citation Analysis (57)] |
| 141. | Wang WL, Xu SY, Ren ZG, Tao L, Jiang JW, Zheng SS. Application of metagenomics in the human gut microbiome. World J Gastroenterol. 2015;21:803-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 318] [Cited by in RCA: 238] [Article Influence: 23.8] [Reference Citation Analysis (5)] |
| 142. | Ren Z, Xu Z, Amakye WK, Liu W, Zhao Z, Gao L, Wang M, Ren J. Hericium erinaceus mycelium-Derived Polysaccharide Alleviates Ulcerative Colitis and Modulates Gut Microbiota in Cynomolgus Monkeys. Mol Nutr Food Res. 2023;67:e2200450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 143. | Yu AT, Shapiro K, Beneri CA, Wilks-Gallo LS. Streptococcus lutetiensis neonatal meningitis with empyema. Access Microbiol. 2021;3:000264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 144. | Shao S, Wang D, Zheng W, Li X, Zhang H, Zhao D, Wang M. A unique polysaccharide from Hericium erinaceus mycelium ameliorates acetic acid-induced ulcerative colitis rats by modulating the composition of the gut microbiota, short chain fatty acids levels and GPR41/43 respectors. Int Immunopharmacol. 2019;71:411-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 145. | Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396-406.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 762] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 146. | Kishi M, Hirai F, Takatsu N, Hisabe T, Takada Y, Beppu T, Takeuchi K, Naganuma M, Ohtsuka K, Watanabe K, Matsumoto T, Esaki M, Koganei K, Sugita A, Hata K, Futami K, Ajioka Y, Tanabe H, Iwashita A, Shimizu H, Arai K, Suzuki Y, Hisamatsu T. A review on the current status and definitions of activity indices in inflammatory bowel disease: how to use indices for precise evaluation. J Gastroenterol. 2022;57:246-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 147. | Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, Hayee B, Lomer MCE, Parkes GC, Selinger C, Barrett KJ, Davies RJ, Bennett C, Gittens S, Dunlop MG, Faiz O, Fraser A, Garrick V, Johnston PD, Parkes M, Sanderson J, Terry H; IBD guidelines eDelphi consensus group, Gaya DR, Iqbal TH, Taylor SA, Smith M, Brookes M, Hansen R, Hawthorne AB. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1-s106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1402] [Cited by in RCA: 1547] [Article Influence: 257.8] [Reference Citation Analysis (0)] |