Published online Jan 14, 2023. doi: 10.3748/wjg.v29.i2.390
Peer-review started: August 12, 2022
First decision: September 2, 2022
Revised: September 12, 2022
Accepted: December 1, 2022
Article in press: December 1, 2022
Published online: January 14, 2023
Processing time: 146 Days and 12.5 Hours
Due to increasing resistance rates of Helicobacter pylori (H. pylori) to different antibiotics, failures in eradication therapies are becoming more frequent. Even though eradication criteria and treatment algorithms for first-line and second-line therapy against H. pylori infection are well-established, there is no clear recom
To perform a systematic review evaluating the efficacy and safety of rescue therapies against refractory H. pylori infection.
A systematic search of available rescue treatments for refractory H. pylori infection was conducted on the National Library of Medicine’s PubMed search platform based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Randomized or non-randomized clinical trials and observational studies evaluating the effectiveness of H. pylori infection rescue therapies were included.
Twenty-eight studies were included in the analysis of mean eradication rates as rescue therapy, and 21 of these were selected for analysis of mean eradication rate as third-line treatment. For rifabutin-, sitafloxacin-, levofloxacin-, or metroni
We recommend sitafloxacin-based triple therapy containing vonoprazan in regions with low macrolide resistance profile. In regions with known resistance to macrolides or unavailability of bismuth, rifabutin-based triple therapy is recommended.
Core Tip: The eradication of Helicobacter pylori is widely discussed given the high prevalence and incidence of its infection. Even with established criteria in the V Maastricht Consensus for the eradication of infection and treatment algorithms for choosing first-line and second-line therapeutic regimens, therapeutic failure is frequent. Therefore, establishing safe, effective, and accessible third-line and rescue therapies for patients in need of eradication is necessary in the management of such infection. Due to this need, the present systematic review performed a systematic review evaluating the efficacy and safety of rescue therapies against refractory Helicobacter pylori infection.
- Citation: de Moraes Andrade PV, Monteiro YM, Chehter EZ. Third-line and rescue therapy for refractory Helicobacter pylori infection: A systematic review. World J Gastroenterol 2023; 29(2): 390-409
- URL: https://www.wjgnet.com/1007-9327/full/v29/i2/390.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i2.390
Helicobacter pylori (H. pylori) is a Gram-negative microaerophilic bacterium with a wide genomic diversity, which are the product of mutations, recombination, migrations, and genetic drift that favored the emergence of multiple populations and subpopulations of this bacterium[1-3]. H. pylori is a microorganism of global relevance, infecting about 50% of the world population[4].
Exclusive or multifactorial infection by H. pylori is associated with the onset of multiple diseases. The exclusive action of H. pylori through its virulence factors is related to the development of peptic ulcer, duodenal ulcer, gastritis, and consequently, dyspepsia[5-7]. Although H. pylori infection is often the primary cause of gastric cancers, the development of this pathological process results from a multifactorial interaction between bacterial, host, and environmental factors[8]. Furthermore, H. pylori can also stimulate lymphocytic infiltration in the gastric mucosa, which combined with high-risk genotypes may be associated with a neoplastic transformation into mucosa-associated lymphoid tissue lymphoma[9,10].
According to the IV Brazilian Consensus on Infection by H. pylori and the Maastricht V/Florence Consensus, the eradication of H. pylori is recommended in cases of peptic ulcer, mucosa-associated lymphoid tissue lymphoma, atrophic gastritis, after gastric cancer resection and in patients with first-degree relatives with gastric cancer. However, in addition to its adverse effects, the eradication of H. pylori can result in changes in the stomach, intestine, pancreas, and other systems and allow the colonization of other bacteria. Therefore, the risk/benefit ratio of this therapy must be evaluated by the physician[11,12].
Treatment of H. pylori infection is based on a combination of antimicrobials and antisecretory agents that promote an increase in gastric pH, enabling the action of antimicrobials. The increasing rates of H. pylori resistance to the classes of antimicrobials commonly used in conventional therapeutic regimens has reduced the effectiveness of these drugs, and failures in eradication therapies have become increasingly frequent. In an attempt to combat the growing resistance to antimicrobials, new therapeutic regimens have been used as an alternative to conventional regimens. The association of bismuth, the use of new classes of antisecretory agents such as the competitive inhibitor of potassium channels, and the adoption of new antimicrobials have acted as an alternative to standard therapeutic regimens.
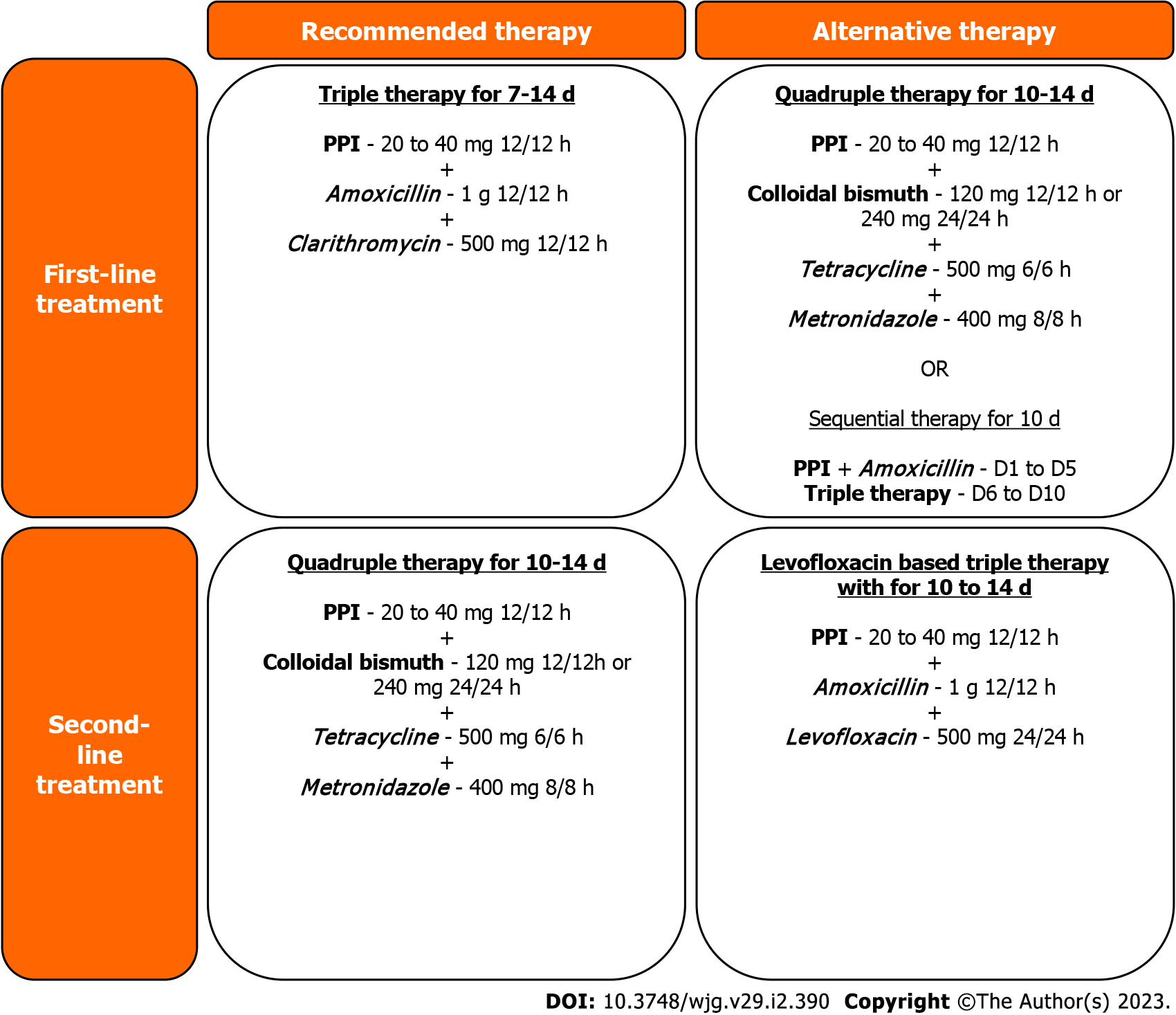
Although the Maastricht V/Florence Consensus presents very well-established criteria for the eradication of infection and treatment algorithms for the choice of first-line and second-line therapeutic regimens (Figure 1) against H. pylori infection, there is no clear recommendation for third-line and rescue regimens in refractory H. pylori infection. Given the need to establish safe, effective, and accessible therapies for patients, the aim of this study was to evaluate the efficacy and safety of third-line and rescue therapies in refractory H. pylori infection.
Although no review protocol was registered, the present review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 guideline, from a survey of available rescue treatments for refractory H. pylori infection in scientific articles on the PubMed search platform of the National Library of Medicine. The search was performed between April 22, 2021 and August 20, 2021. Different descriptors were used throughout the study for maximization of the database, namely: Helicobacter pylori multidrug resistance and rescue therapy; H. pylori multiresistant and rescue treatment; Helicobacter pylori multidrug resistance and rescue treatment; Helicobacter pylori rescue therapy; Helicobacter pylori and third line treatment; and fourth line therapy and Helicobacter pylori. After applying the inclusion and exclusion criteria, the selected articles were analyzed in two stages: first by two independent reviewers and later by the senior reviewer in order to minimize the possibility of errors and bias by the authors.
Information from articles selected and approved in both stages was extracted by reviewers independently to ensure reliable data detection and collection. A statistical analysis was performed from relevant data to the objective of this review to compare the results found in the studies. In addition to the analysis of eradication rates both by intention to treat (ITT) and per protocol (PP), a comparative analysis on adverse effects found in the different therapeutic approaches was also performed to assess their feasibility in clinical practice.
Due to the heterogeneity pool of objectives in the articles (most of them evaluated different classes or combinations of antibiotics), the level of evidence, grade rating, and bias analysis required in the PRISMA protocol could not be analyzed. Therefore, some items of the PRISMA checklist could not be applied. All articles selected according to our inclusion and exclusion criteria were included in this review, despite their PRISMA rating grade, evidence level, or bias. It was equally challenging to present their risk of bias, outcome level assessment, and strength of evidence, even with a two-phase analysis. Therefore, some of this information may be lacking in this review, but all articles included were analyzed in detail to minimize the inclusion of low evidence information.
The present review included randomized or non-randomized clinical trials and observational studies that evaluated the efficacy of rescue therapies in refractory H. pylori infection published from 2014 onwards in the search platforms defined by the authors.
Exclusion criteria adopted in the selection of articles of the present study were the following: Studies with pediatric patients; studies exclusively with patients who had only one failed eradication attempt; studies including patients with two or more previous failed eradication therapies, in which eradication rates for these patients were not specified; studies that did not fully discriminate the therapeutic approach used; studies without evidence of infection by H. pylori using methods of high sensitivity and specificity (13C-UBT and/or biopsy); and studies in which there was no subsequent follow-up of patients.
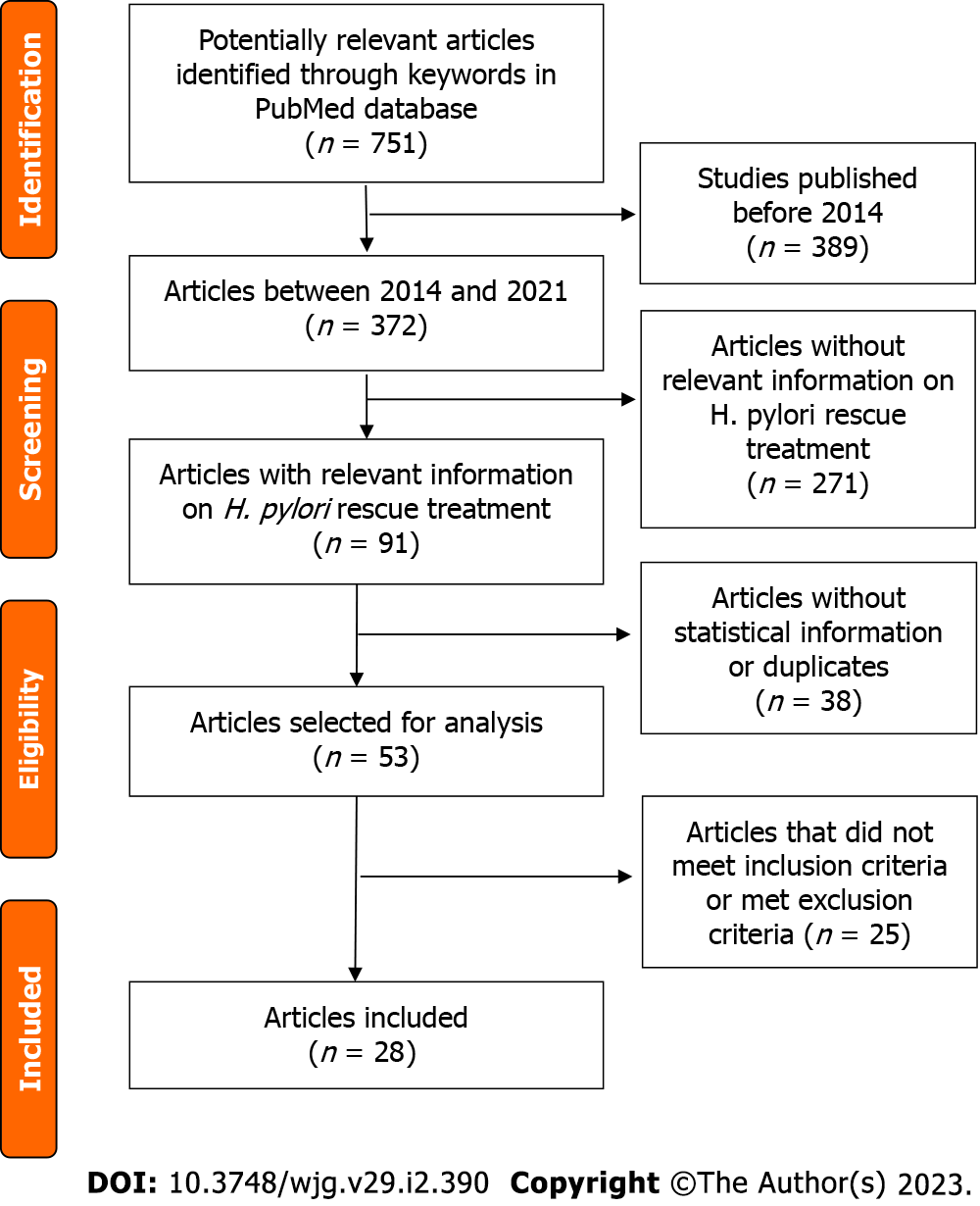
The initial search in the PubMed database resulted in 751 potential articles. After excluding those published before January 1, 2014, 362 articles remained. After temporal delimitation and reading the abstracts of the remaining articles, 271 articles did not contain relevant information about rescue treatment. Of the 91 remaining articles containing relevant information on rescue treatment, 38 were excluded after a double check with reviewers and the senior reviewer because they were duplicates and/or statistical information related to eradication rates of third-line and rescue therapies was lacking. Articles without data on adverse effects but with eradication rates were included in this review. At the end of this stage, 53 articles were selected for analysis and read in full by the authors. Finally, 25 articles were excluded in the final stage because they did not meet the inclusion criteria in full or met any of the exclusion criteria, leaving 28 articles for inclusion. The selection process is presented in the PRISMA diagram of included articles. PRISMA flow diagram reported in Figure 2.
The different approaches used in the selected articles and their eradication rates can be seen in Table 1[13-40].
| Year | Ref. | Type of study | Rescue therapy | Duration | Eradication rate |
| 2014 | Lim et al[13] | Randomized clinical trial | Group A: lansoprazole (30 mg, 12/12 h), amoxicillin (1 g, 8/8 h), and rifabutin 150 mg (12/12 h) | 7 d | ITT: 78.1%; PP: 80.6% |
| 2014 | Lim et al[13] | Randomized clinical trial | Group B: lansoprazole (60 mg, 12/12 h), amoxicillin (1 g, 8/8 h), and rifabutin 150 mg (12/12 h) | 7 d | ITT: 96.3%; PP: 100% |
| 2014 | Furuta et al[14] | Randomized clinical trial | RAS: rabeprazole (10 mg, 8/8 h or 12/12 h), amoxicillin (500 mg, 6/6 h), sitafloxacin (100 mg, 12/12 h) | 7 d | ITT: 84.1%; PP: 86.4% |
| 2014 | Furuta et al[14] | Randomized clinical trial | RAS: rabeprazole, amoxicillin (500 mg, 6/6 h), sitafloxacin (100 mg, 12/12 h) | 14 d | ITT: 88.9%; PP: 90.9% |
| 2014 | Furuta et al[14] | Randomized clinical trial | RMS: rabeprazole, metronidazole (250 mg, 12/12 h), sitafloxacin (100 mg, 12/12 h) | 7 d | ITT: 90.9%; PP: 90.9% |
| 2014 | Furuta et al[14] | Randomized clinical trial | RMS: rabeprazole, metronidazole (250 mg, 12/12 h), sitafloxacin (100 mg, 12/12 h) | 14 d | ITT: 87.2%; PP: 91.1% |
| 2014 | Gisbert et al[15] | Prospective multicenter observational study | PPI (standard dose, 12/12 h), bismuth subcitrate (120 mg 8/8 h or 240 mg, 12/12 h), tetracycline (250 mg, 6/6 h or 500 mg 8/8 h or 500 mg, 6/6 h), and metronidazole (250 mg, 8/8 h or 250 mg, 6/6 h or 500 mg, 8/8 h or 500 mg, 6/6 h) | 7-14 d | ITT: 65.0%; PP: 67.0% |
| 2014 | Okimoto et al[16] | Randomized clinical trial | RAL: rabeprazole (10 mg, 12/12 h), amoxicillin (750 mg, 12/12 h), levofloxacin (500 mg, 24/24 h) | 10 d | ITT: 45.8%; PP: 45.8% |
| 2014 | Okimoto et al[16] | Randomized clinical trial | RA: rabeprazole (10 mg, 6/6 h) and amoxicillin (500 mg, 6/6 h) | 14 d | ITT: 40.7%; PP: 45.8% |
| 2015 | Paoluzi et al[17] | Randomized clinical trial | Esomeprazole (20 mg, 12/12 h), levofloxacin (500 mg, 12/12 h), doxycycline (100 mg, 12/12 h) | 7 d | ITT: 40.0%; PP: 43.0% |
| 2015 | Paoluzi et al[17] | Randomized clinical trial | Esomeprazole (20 mg, 12/12 h), levofloxacin (500 mg, 12/12 h), doxycycline (100 mg, 12/12 h), Lactobacillus casei DG (24 billion units) | 7 d | ITT: 54%; PP: 55% |
| 2016 | Muller et al[18] | Non-randomized clinical trial | Pylera® (140 mg potassium bismuth subcitrate, 125 mg metronidazole, 125 mg tetracycline, 6/6 h), omeprazole (20 mg, 12/12 h) | 10 d | ITT: 83.0%; PP: 87.0% |
| 2016 | Mori et al[19] | Randomized clinical trial | Third-line: esomeprazole (20 mg, 6/6 h), amoxicillin (500 mg, 6/6 h), and rifabutin (300 mg, 24/24 h) | 10 d | ITT: 83.3%; PP: 81.8% |
| 2016 | Mori et al[19] | Randomized clinical trial | Third-line: esomeprazole (20 mg, 6/6 h), amoxicillin (500 mg, 6/6 h), and rifabutin (300 mg, 24/24 h) | 14 d | ITT: 94.1%; PP: 91.7% |
| 2016 | Mori et al[19] | Randomized clinical trial | Fourth-line: esomeprazole (20 mg, 6/6 h), amoxicillin (500 mg, 6/6 h), and rifabutin (300 mg, 24/24 h) | 10 d | ITT: 77.9%; PP: 77.9% |
| 2016 | Mori et al[19] | Randomized clinical trial | Fourth-line: esomeprazole (20 mg, 6/6 h), amoxicillin (500 mg, 6/6 h), and rifabutin (300 mg, 24/24 h) | 14 d | ITT:90.9%; PP: 90.9% |
| 2016 | Mori et al[20] | Randomized clinical trial | Esomeprazole (20 mg, 12/12 h), amoxicillin (500 mg, 6/6 h), and sitafloxacin (100 mg, 12/12 h) | 10 d | ITT: 81.0%; PP: 82.0% |
| 2016 | Mori et al[20] | Randomized clinical trial | Esomeprazole (20 mg, 12/12 h), metronidazole (250 mg, 12/12 h), and sitafloxacin (100 mg, 12/12 h) | 10 d | ITT: 72.4%; PP: 76.4% |
| 2016 | Chen et al[21] | Randomized clinical trial | Lansoprazole (30 mg, 12/12 h), potassium bismuth subcitrate (220 mg, 12/12 h), metronidazole (400 mg, 6/6 h), and amoxicillin (1 g, 8/8 h) | 14 d | ITT: 88.5%; PP: 93.7% |
| 2016 | Chen et al[21] | Randomized clinical trial | Lansoprazole (30 mg, 12/12 h), potassium bismuth subcitrate (220 mg, 12/12 h), metronidazole (400 mg, 6/6 h), and tetracycline (500 mg, 6/6 h) | 14 d | ITT: 87.2%; PP: 95.3% |
| 2016 | Noh et al[22] | Non-randomized clinical trial | PPI (standard dose, 12/12 h), levofloxacin (500 mg, 24/24 h), and amoxicillin (1 g, 12/12 h) | 7 d | ITT: 58.3%; PP: 58.3% |
| 2016 | Noh et al[22] | Non-randomized clinical trial | PPI (standard dose, 12/12 h), levofloxacin (500 mg, 24/24 h), and amoxicillin (1 g, 12/12 h) | 10 d | ITT: 62.5%; PP: 68.2% |
| 2016 | Noh et al[22] | Non-randomized clinical trial | PPI (standard dose, 12/12 h), levofloxacin (500 mg, 24/24 h), and amoxicillin (1 g, 12/12 h) | 14 d | ITT: 73.7%; PP: 93.3% |
| 2016 | Hirata et al[23] | Non-randomized clinical trial | Esomeprazole (20 mg, 12/12 h), amoxicillin (750 mg, 12/12 h), sitafloxacin (100 mg, 12/12 h) | 7 d | ITT: 83.0%; PP: 83.0% |
| 2017 | Rodríguez de Santiago et al[24] | Multicenter observational prospective study | Pylera® (140 mg potassium bismuth subcitrate, 125 mg metronidazole, 125 mg tetracycline, 3 capsules, 6/6 h) and esomeprazole (40 mg, 12/12 h) or omeprazole (40 mg, 12/12 h) | 10 d | ITT: 80.2%; PP: 84.4% |
| 2017 | Costa et al[25] | Single-center observational retrospective study | SGT | - | ITT: 59.5%; PP: 61.5% |
| 2017 | Puig et al[26] | Multicenter observational prospective study | Esomeprazole (40 mg, 12/12 h), amoxicillin (1 g, 8/8 h), and metronidazole (500 mg, 8/8 h) | 14 d | ITT: 62.0%; PP: 63.0% |
| 2018 | Fiorini et al[27] | Non-randomized clinical trial | Esomeprazole (40 mg, 12/12 h), amoxicillin (1 g, 12/12 h), rifabutin (150 mg, 24/24 h) | 12 d | PP: 87.9% |
| 2018 | Liou et al[28] | Randomized clinical trial | Clinical trial 1: sequential susceptibility-guided therapy: esomeprazole (40 mg, 12/12 h) and amoxicillin (1 g, 12/12 h), for the first 7 d followed by metronidazole (500 mg, 12/12 h) and levofloxacin (250 mg, 12/12 h) or clarithromycin (500 mg, 12/12 h) or doxycycline (100 mg, 12/12 h), for another 7 d. Sequential empirical therapy: esomeprazole (40 mg, 12/12 h) and amoxicillin (1 g, 12/12 h) for the first 7 d, followed by metronidazole (500 mg, 12/12 h) and doxycycline (100 mg, 12/12 h), for another 7 d | 14 d | SGT ITT: 81.0%, PP: 80.0%; Sequential empirical therapy ITT: 60.0%, PP: 60.0% |
| 2018 | Liou et al[28] | Randomized clinical trial | Clinical trial 2: sequential SGT: esomeprazole (40 mg, 12/12 h) and amoxicillin (1 g, 12/12 h) for the first 7 d followed by metronidazole (500 mg, 12/12 h) and levofloxacin (250 mg, 12/12 h) or clarithromycin (500 mg, 12/12 h) or tetracycline (500 mg, 12/12 h) for another 7 d. Sequential empirical therapy: esomeprazole (40 mg, 12/12 h) and amoxicillin (1 g, 12/12 h) for the first 7 d followed by metronidazole (500 mg, 12/12 h) and tetracycline (100 mg, 12/12 h) for another 7 d | 14 d | SGT ITT: 78.0%, PP: 78.4%; Sequential empirical therapy ITT: 72.2%, PP: 74.4% |
| 2018 | Huang et al[29] | Non-randomized clinical trial | SGT: esomeprazole (40 mg, 12/12 h), amoxicillin (1 g, 12/12 h) and tetracycline (500 mg, 6/6 h) or metronidazole (500 mg, 8/8 h) or levofloxacin (500 mg, 24/24 h) | 14 d | ITT: 81.4%; PP: 89.7% |
| 2018 | Huang et al[29] | Non-randomized clinical trial | Empirical quadruple therapy: esomeprazole (40 mg, 12/12 h), amoxicillin (1 g, 12/12 h), tetracycline (500 mg, 6/6 h), and metronidazole (500 mg, 8/8 h) | 14 d | ITT: 51.8%; PP: 58.3% |
| 2019 | Saito et al[30] | Non-randomized clinical trial | Esomeprazole (20 mg, 12/12 h), amoxicillin (750 mg, 12/12 h), and sitafloxacin (100 mg, 12/12 h) | 7 d | ITT: 54.2%; PP: 56.5% |
| 2019 | Saito et al[30] | Non-randomized clinical trial | Vonoprazan (20 mg, 12/12 h), amoxicillin (750 mg, 12/12 h), and sitafloxacin (100 mg, 12/12 h) | 7 d | ITT: 93.0%; PP: 93.0% |
| 2019 | Sue et al[31] | Randomized clinical trial | Vonoprazan (20 mg, 12/12 h) amoxicillin 750 mg, (12/12 h), and sitafloxacin (100 mg, 12/12 h) | 7 d | ITT: 75.8%; PP: 83.3% |
| 2019 | Sue et al[31] | Randomized clinical trial | Lansoprazole (30 mg, 12/12 h) or rabeprazole (10 mg, 12/12 h) or esomeprazole (20 mg, 12/12 h), amoxicillin (750 mg, 12/12 h), and sitafloxacin 100 mg, 12/12 h) | 7 d | ITT: 53.3%; PP: 57.1% |
| 2019 | Ribaldone et al[32] | Non-randomized clinical trial | Fifth-line: rifabutin (150 mg, 12/12 h), amoxicillin (1 g, 12/12 h), and omeprazole (20 mg, 12/12 h), esomeprazole (40 mg, 12/12 h), pantoprazole (40 mg, 12/12 h) rabeprazole (40 mg, 12/12 h), or lansoprazole (30 mg, 12/12 h) | 14 d | ITT: 71.5%; PP: 72.7% |
| 2020 | Liu et al[33] | Single center observational retrospective study | Lactobacilli acidophilus (1g, 8/8 h), esomeprazole (20mg, 12/12 h), potassium bismuth subcitrate (220 mg, 12/12 h), tetracycline (750 mg, 12/12 h), and furazolidone (100 mg, 12/12 h) | Lactobacilli acidophilus for 14 d and the others for 10 d | ITT: 92.0%; PP: 91.8% |
| 2020 | Sugimoto et al[34] | Single center observational retrospective study | Vonoprazan (20mg, 12/12 h), amoxicillin (500 mg, 6/6 h), and sitafloxacin (100 mg, 12/12 h) | 7 d | ITT: 87.5%; PP: 87.5% |
| 2020 | Saracino et al[35] | Single center observational retrospective study | Third-line: esomeprazole (40 mg, 12/12 h), amoxicillin (1 g, 12/12 h), and rifabutin (150 mg, 24/24 h) | 12 d | ITT: 56.1%; PP: 68.5% |
| 2020 | Saracino et al[35] | Single center observational retrospective study | Fourth-line: esomeprazole (40 mg, 12/12 h), amoxicillin (1 g, 12/12 h), and rifabutin (150 mg, 24/24 h) | 12 d | ITT: 54.5%; PP: 63.1% |
| 2020 | Saracino et al[35] | Single center observational retrospective study | Fifth-line or more: esomeprazole (40 mg, 12/12 h), amoxicillin (1 g, 12/12 h), and rifabutin (150 mg, 24/24 h) | 12 d | ITT: 24.4%; PP: 30.3% |
| 2020 | Saracino et al[35] | Single center observational retrospective study | Third-line: Pylera® (140 mg potassium bismuth subcitrate, 125 mg metronidazole, 125 mg tetracycline, 6/6 h) and esomeprazole (20 mg, 12/12 h) | 10 d | ITT: 87.5%; PP: 91.3% |
| 2020 | Saracino et al[35] | Single center observational retrospective study | Fourth-line: Pylera® (140 mg potassium bismuth subcitrate, 125 mg de metronidazole, 125 mg tetracycline, 3 capsules, 6/6 h) and esomeprazole (20 mg, 12/12 h) | 10 d | ITT: 83.9%; PP: 89.6% |
| 2020 | Saracino et al[35] | Single center observational retrospective study | Fifth-line or more: Pylera® (140 mg potassium bismuth subcitrate, 125 mg metronidazole, 125 mg tetracycline, 3 capsules, 6/6 h) and esomeprazole (20 mg, 12/12 h) | 10 d | ITT: 71.9%; PP: 74.2% |
| 2020 | Hirata et al[36] | Non-randomized clinical trial | Fourth-line: vonoprazan (20 mg, 12/12 h), amoxicillin (750 mg, 12/12 h), and rifabutin (150 mg, 12/12 h) | 10 d | ITT: 100.0%; PP: 100.0% |
| 2020 | Ji et al[37] | Randomized clinical trial | Susceptibility-guided quadruple therapy: rabeprazole (10 mg, 12/12 h), colloidal bismuth (200 mg, 12/12 h), 2 sensitive antibiotics | 14 d | PP: 86.49% |
| 2020 | Ji et al[37] | Randomized clinical trial | Rabeprazole (10 mg, 12/12 h), colloidal bismuth (200 mg, 12/12 h), amoxicillin (1 g, 12/12 h), levofloxacin (500 mg, 24/24 h), or furazolidone (100 mg, 12/12 h) | 14 d | PP: 82.4% |
| 2020 | Mori et al[38] | Non-randomized clinical trial | Esomeprazole (20 mg, 12/12 h), amoxicillin (500 mg, 6/6 h), and sitafloxacin (100 mg, 12/12 h) | 10 d | ITT: 81.6%; PP: 81.6% |
| 2020 | Nyssen et al[39] | Multicentric observational retrospective study | Bismuth and tetracycline-based quadruple therapy: PPI, bismuth salts (120 mg, 6/6 h or 240 mg, 12/12 h), metronidazole (500 mg, 8/8 h), and tetracycline (500 mg, 6/6 h) | 10 d | ITT: 66.0%; PP: 66.0% |
| 2020 | Nyssen et al[39] | Multicentric observational retrospective study | Bismuth and tetracycline-based quadruple therapy: PPI, bismuth salts (120 mg, 6/6 h or 240 mg, 12/12 h), metronidazole (500 mg, 8/8 h), and tetracycline (500 mg, 6/6 h) | 14 d | ITT: 82.0%; PP: 83.0% |
| 2020 | Nyssen et al[39] | Multicentric observational retrospective study | Bismuth and doxycycline-based quadruple therapy: PPI, bismuth salts (120 mg, 6/6 h or 240 mg, 12/12 h), metronidazole (500 mg, 8/8 h), and doxycycline (100 mg, 12/12 h) | 10 d | ITT: 63.0%; PP: 63.0% |
| 2020 | Nyssen et al[39] | Multicentric observational retrospective study | Bismuth and doxycycline-based quadruple therapy: PPI, bismuth salts (120 mg, 6/6 h or 240 mg, 12/12 h), metronidazole (500 mg, 8/8 h), and doxycycline (100 mg, 12/12 h) | 14 d | ITT: 70.0%; PP: 71.0% |
| 2020 | Nyssen et al[39] | Multicentric observational retrospective study | Bismuth-based quadruple therapy, three-in-one, Pylera®: PPI and Pylera® | 10 d | ITT: 88.0%; PP: 88.0% |
| 2020 | Nyssen et al[39] | Multicentric observational retrospective study | Bismuth-based quadruple therapy, three-in-one, Pylera®: PPI and Pylera® | 14 d | ITT: 100.0%; PP: 100.0% |
| 2020 | Kuo et al[40] | Non-randomized clinical trial | Rifabutin (150 mg, 12/12 h), amoxicillin (1 g, 12/12 h), and esomeprazole (40 mg, 12/12 h) | 10 d | ITT: 77.5%; PP: 79.5% |
Among the 28 selected articles, different active principles and therapeutic approaches were used as rescue treatment, achieving different eradication rates. Twenty-one studies were selected for analysis of the mean eradication rate as third-line treatment. Regarding the analysis of mean eradication rates of rescue therapies, studies containing patients with two or more previous failed eradications were included; the 28 studies presented in Table 1 were used. The analysis of eradication rates of regimens used as third-line treatment and rescue therapy were stratified into three subgroups based on the therapeutic regimens used, namely triple therapy, quadruple therapy, and susceptibility-guided therapy (SGT). Note that in the analysis of mean eradication rates of therapies performed in our study, therapeutic regimens were not discriminated based on the duration and dosage of the drugs used. In the absence of studies evaluating the effectiveness of therapeutic approaches as fourth-line or more, the third-line was considered as rescue therapy.
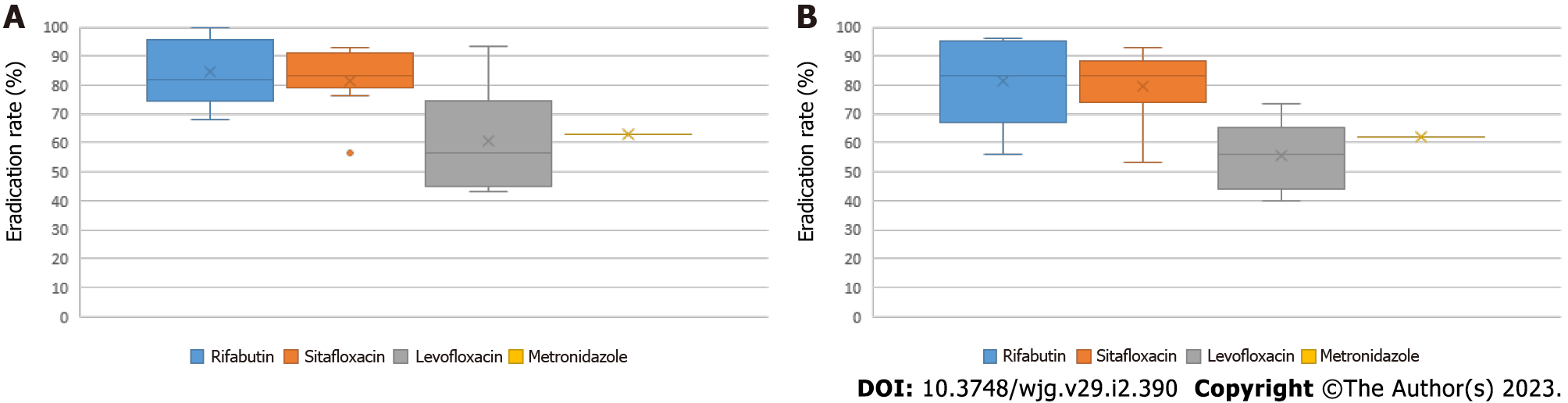
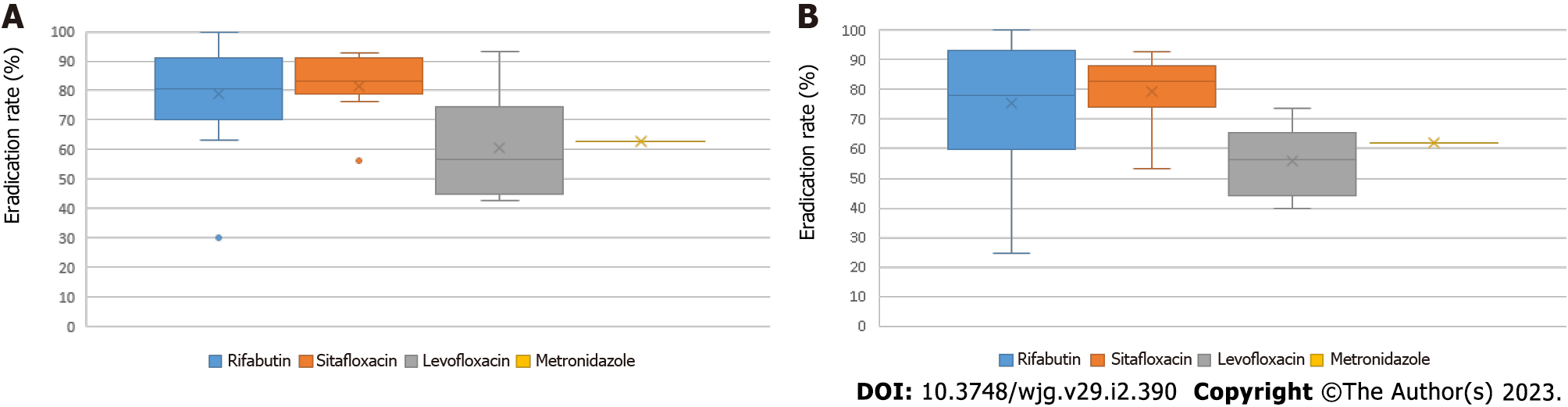
Triple therapy: Eradication rates found for triple therapy as third-line treatment were 81.6% and 84.4% for rifabutin-based regimens, 79.4% and 81.5% for sitafloxacin-based regimens, 55.7 % and 60.6% for levofloxacin-based regimens, and 62.0% and 63.0% for metronidazole-based regimen by ITT and PP, respectively (Figure 3). Regarding triple therapy as rescue treatment, mean eradication rates of 75.4% and 78.8% were found for rifabutin-based regimens, 79.4% and 81.5% for sitafloxacin-based regimens, 55.7% and 60.6% for levofloxacin-based regimens, and 62.0% and 63.0% for metronidazole-based regimen by ITT and PP, respectively (Figure 4).
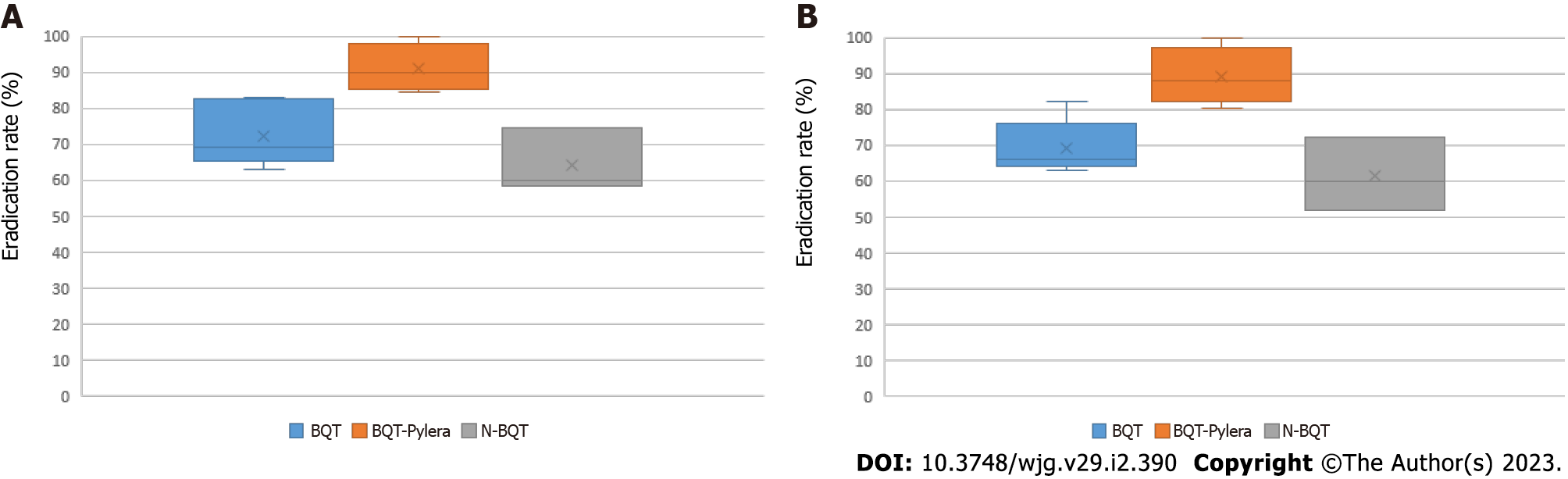
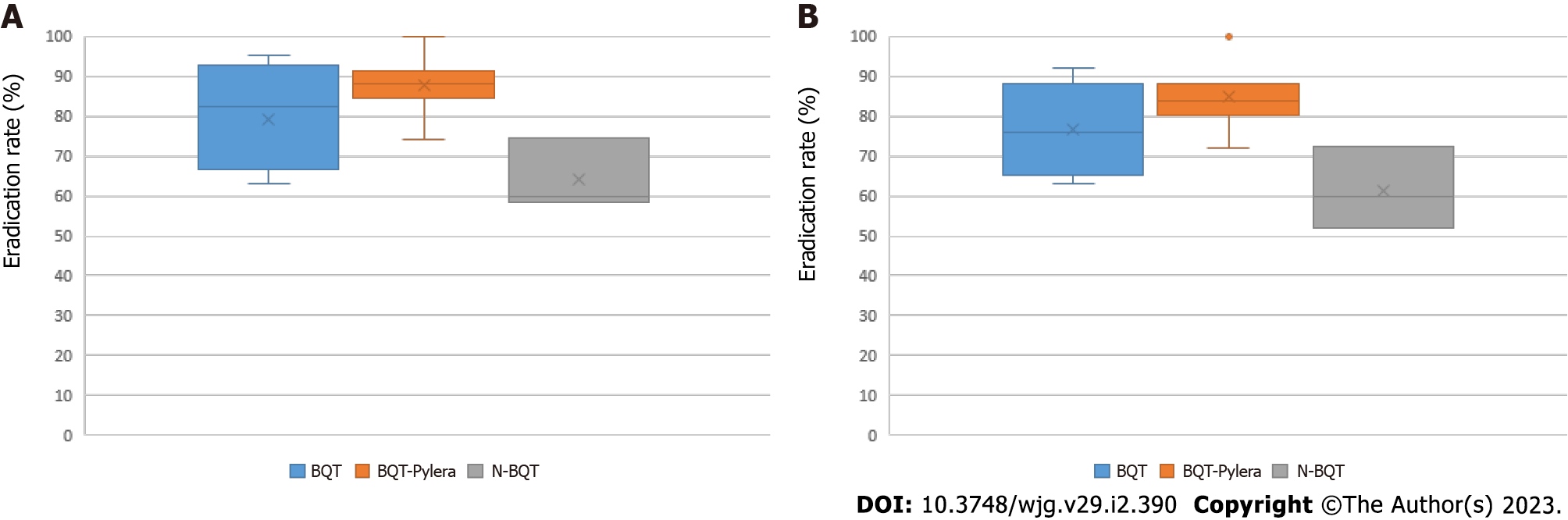
Quadruple therapy: Eradication rates found for quadruple therapy as third-line treatment were 69.2% and 72.1% for bismuth quadruple therapy (BQT), 88.9% and 90.9% for bismuth quadruple therapy, three-in-one Pylera® (BQT-Pylera®), and 61.3% and 64.2% for non-BQT by ITT and PP, respectively (Figure 5). Regarding quadruple therapy as rescue treatment, mean eradication rates of 76.7% and 79.2% were found for BQT, 84.9% and 87.8% for BQT-Pylera®, and 61.3% and 64.2% for non-BQT regimens by ITT and PP, respectively (Figure 6).
SGT: Eradication rates found for SGT as third-line treatment and rescue therapy were 75% and 79.2% by ITT and PP, respectively.
Adverse effects: From the reading of selected articles, information on adverse effects found in different therapeutic approaches was extracted, as shown in Table 2. The mean adverse effects rate for rifabutin-, sitafloxacin-, levofloxacin-, and metronidazole-based triple therapy in patients with two or more previous failed eradications was 53.70%, 52.36%, 13.93%, and 58.00%, respectively. With respect to adverse effects for BQT, BQT-Pylera, and non-BQT regimens, mean rates were 34.0%, 65.0%, and 45.0%, respectively. The safety of SGT was not evaluated in the present study since the choice of the therapeutic regimen was dependent on results obtained by susceptibility and genotypic resistance tests.
| Ref. | Therapeutic scheme | Adverse effects, n | Total rate |
| Okimoto et al[16], 2014 | Dual therapy: rabeprazole and amoxicillin | n = 24. Loose stools/diarrhea: 5 (20.8%); nausea: 1 (4.2%); skin rash: 0 (0%) | 25% |
| Okimoto et al[16], 2014 | Triple therapy: rabeprazole, amoxicillin, and levofloxacin | n = 24. Loose stools/diarrhea: 5 (20.8%); nausea: 0 (0%); skin rash: 1 (4.2%) | 25% |
| Lim et al[13], 2014 | Triple therapy: lansoprazole, amoxicillin, and rifabutin | Group A (n = 32). Epigastric pain: 3 (9.3%); epigastric discomfort: 2 (6.2%); asthenia: 1 (3.1%); nausea: 1 (3.1%); change in urine color: 1 (3.1%); drowsiness: 1 (3.1%); lip discomfort: 1 (3.1%); treatment was discontinued because of adverse effects: 1 (3.1%) | 15.5% |
| Lim et al[13], 2014 | Triple therapy: lansoprazole, amoxicillin, and rifabutin | Group B (n = 27). Epigastric pain: 1 (3.7); epigastric discomfort: 1 (3.7); asthenia: 0 (0%); nausea: 1 (3.7); urine color change: 0 (0%); drowsiness: 0 (0%), lip discomfort: 0 (0%) | |
| Furuta et al[14], 2014 | Triple therapy: rabeprazole, sitafloxacin, and amoxicillin or metronidazole | RAS, 7 d (n = 44). Diarrhea: 9 (20.4%); loose stools: 20 (45.4%) | 65.9% |
| Furuta et al[14], 2014 | Triple therapy: rabeprazole, sitafloxacin, and amoxicillin or metronidazole | RAS, 14 d (n = 45). Diarrhea: 12 (26.6%); loose stools: 17 (37.7%) | 64.4% |
| Furuta et al[14], 2014 | Triple therapy: rabeprazole, sitafloxacin, and amoxicillin or metronidazole | RMS, 7 d (n = 44). Diarrhea: 8 (18.2%); loose stools: 17 (38.6%) | 56.8% |
| Furuta et al[14], 2014 | Triple therapy: rabeprazole, sitafloxacin, and amoxicillin or metronidazole | RMS, 14 d (n = 47). Diarrhea: 12 (25.5%); loose stools: 26 (55.3%) | 86.3% |
| Paoluzi et al[17], 2015 | Triple therapy: esomeprazole, levofloxacin, and doxycycline | n = 142. Swelling:; flavor perversion; mild diarrhea; treatment was discontinued because of adverse effects: 1 (0.7%) | 7.7% |
| Mori et al[19], 2016 | Triple therapy: esomeprazole, amoxicillin, and rifabutin | 10-d group (n = 12). Fever: 2 (16.6%); diarrhea: 0 (0%); headache: 3 (25%); liver dysfunction: 2 (16.6%); loose stools: 2 (16.6%); urine discoloration: 1 (8.3%); allergy: 1 (8.3%); leukopenia: 1 (8.3%); stomatitis: 1 (8.3%); dysgeusia: 1 (8.3%); vertigo: 0 (0%); fatigue: 0 (0%); photophobia: 0 (0%); treatment was discontinued because of adverse effects: 1 (8.3%) | 75% |
| Mori et al[19], 2016 | Triple therapy: esomeprazole, amoxicillin, and rifabutin | 14-d group (n = 17). Fever: 6 (35%); diarrhea: 5 (29.4%); headache: 3 (17.7%); liver dysfunction: 3 (17.7%); loose stools: 2 (11.8%); urine discoloration: 3 (17.7%); allergy: 2 (11.8%); leukopenia: 1 (5.9%); stomatitis: 0 (0%); dysgeusia: 0 (0%); vertigo: 1 (5.9%); fatigue: 1 (5.9%); photophobia: 1 (5.9%); treatment was discontinued because of adverse effects: 5 (29.4%) | 94.1% |
| Mori et al[20], 2016 | Triple therapy: esomeprazole, amoxicillin, and sitafloxacin | EAS (n = 63). Diarrhea: 11 (17.5%); loose stools: 9 (14.2%); constipation: 1 (1.5%); abdominal pain: 3 (4.8%); dyspepsia: 2 (3.2%); dysgeusia: 7 (11.1%); stomatitis: 3 (4.8);; allergy: 2 (3.2%); pruritus: 1 (1.5%); headache: 0 (0%); fatigue: 1 (1.5%); fever: 0 (0%); treatment was discontinued because of adverse effects: 1 (1.5%) | 47.6% |
| Mori et al[20], 2016 | Triple therapy: esomeprazole, metronidazole, and sitafloxacin | EMS (n = 58). Diarrhea: 15 (25.8%); loose stools: 8 (13.8%); constipation: 2 (3.4%); abdominal pain: 2 (3.4%); dyspepsia: 1 (1.7%); dysgeusia: 5 (8.6%); stomatitis: 2 (3.4%); ; allergy: 1 (1.7%); pruritus: 1 (1.7%); headache: 2 (3.4%); fatigue: 0; fever: 1 (1.7%); treatment was discontinued because of adverse effects: 1 (1.7%) | 51.7% |
| Noh et al[22], 2016 | Triple therapy: PPI, amoxicillin, and levofloxacin | - | - |
| Hirata et al[23], 2016 | Triple therapy: esomeprazole, amoxicillin, and sitafloxacin | n = 30. Diarrhea: 5 (15.7%); rash: 1 (3.3%); asthma attack: 1 (3.3%); stomatitis: 1 (3.3%); cystitis: 1 (3.3%) | 26.6% |
| Puig et al[26], 2017 | Triple therapy: esomeprazole, amoxicillin, and metronidazole | n = 68. Diarrhea: 13 (20.0%); swelling: 3 (4.0%); dyspepsia: 14 (21.0%); taste disturbance: 23 (35.0%); nausea/vomiting: 10 (15.0%); asthenia: 4 (6.0%); others: 3 (4.0%); treatment was discontinued because of adverse effects: 2 (3.0%) | 58.0% |
| Fiorini et al[27], 2018 | Triple therapy: esomeprazole, amoxicillin, and rifabutin | n = 254. Nausea or vomiting: 6 (2.5%); abdominal pain: 13 (5.4%); mild diarrhea: 12 (5.1%); headache: 4 (1.6%); itching: 4 (1.6%); taste change: 4 (1.6%); myalgia: 1 (0.5%) | 18.3% |
| Saito et al[30], 2019 | Triple therapy: esomeprazole, amoxicillin, and sitafloxacin | - | - |
| Saito et al[30], 2019 | Triple therapy: vonoprazan, amoxicillin, and sitafloxacin | - | - |
| Sue et al[31], 2019 | Triple therapy: vonoprazan, amoxicillin, and sitafloxacin | n = 33. Diarrhea: 16 (50%); dysgeusia: 5 (15%); nausea: 1 (4%); anorexia: 3 (8%); abdominal pain: 5 (15%); heartburn: 6 (19%); headache: 4 (12%); eructations: 12 (35%); general malaise: 5 (16%); abdominal swelling: 12 (35%); urticaria: 3 (8%); treatment was suspended because of adverse effects: 2 (6.0%) | - |
| Sue et al[31], 2019 | Triple therapy: lansoprazole or rabeprazole or esomeprazole, amoxicillin, and sitafloxacin | n = 30. Diarrhea: 15 (51%); dysgeusia: 5 (17%); nausea: 5 (17%); anorexia: 4 (13%); abdominal pain: 6 (21%); heartburn: 4 (13%); headache: 2 (8%); eructations: 2 (8%); general malaise: 2 (8%); abdominal swelling: 6 (21%); urticaria: 2 (8%) | - |
| Ribaldone et al[32], 2019 | Triple therapy: omeprazole or esomeprazole or pantoprazole or rabeprazole or lansoprazole, amoxicillin, and rifabutin | n = 302. Abdominal/epigastric pain: 9 (3.0%); nausea/vomiting: 7 (2.3%); diarrhea: 2 (0.7%); fatigue: 1 (0.3%); headache: 1 (0.3%); oral candidiasis: 1 (0.3%); allergy: 1 (0.3%); treatment was discontinued because of adverse effects: 4 (1.3%) | 7.3% |
| Sugimoto et al[34], 2020 | Triple therapy: vonoprazan, amoxicillin, and sitafloxacin | n = 32. Diarrhea: 4 (12.5%); loose stools: 2 (6.2%); abdominal pain: 2 (6.2%); allergic reaction: 0 (0%); others: 1 (3.1%) | 28.1% |
| Saracino et al[35], 2020 | Triple therapy: esomeprazole, amoxicillin, and rifabutin | n = 270. Diarrhea: 21 (9.3%); abdominal pain: 20 (8.8%); nausea: 17 (7.7%); headache: 15 (6.6%); dyspepsia: 14 (6.0%); treatment was discontinued because of adverse effects: 3 (1.3%) | 46.4% |
| Saracino et al[35], 2020 | BQT-Pylera: Pylera® and esomeprazole | n = 153. Nausea: 43 (29.7); drowsiness: 35 (24.1%); asthenia: 33 (22.8%); dyspepsia: 28 (19.3%); diarrhea: 26 (17.9%); treatment was discontinued because of adverse effects: 8 (5.2%) | 65.5% |
| Hirata et al[36], 2020 | Triple therapy: vonoprazan, amoxicillin, and rifabutin | n = 19. Diarrhea: 4 (21.0%); headache: 1 (5.2%); taste change: 1 (5.2%); ear fullness: 1 (5.2%) | 42.0% |
| Gisbert et al[15], 2014 | BQT: Bismuth, PPI, tetracycline, and metronidazole | n = 192. Nausea: 24 (12%); abdominal pain: 22 (11%); metallic taste: 17 (8.5%); diarrhea: 16 (8%); asthenia: 15 (7.5%); vomiting: 13 (6.5%); headache: 2 (1%); oral injury: 1 (0.5%); dizziness: 1 (0.5%); myalgia: 1 (0.5%) | 22.0% |
| Chen et al[21], 2016 | BQT: bismuth, lansoprazole, metronidazole, and amoxicillin | n = 156. Flavor distortion: 2 (1.3%); dyspepsia: 2 (1.3%); nausea: 30 (19.2%); vomiting: 4 (2.6%); abdominal pain: 1 (0.7%); swelling: 8 (5.1%); diarrhea: 1 (0.7%); dizziness: 10 (6.4%); headache: 2 (1.3%); skin rash: 3 (1.9%); fatigue: 2 (1.3%); fever: 1 (0.7%); treatment was discontinued because of adverse effects: 8 (5.2%) | 34.0% |
| Rodríguez de Santiago et al[24], 2017 | BQT-Pylera: Pylera® and esomeprazole or omeprazole | n = 101. Dyspepsia: 43 (43.9%); asthenia: 35 (35.7%); dysgeusia: 34 (34.7%); nausea: 26 (26.5%); abdominal pain: 25 (25.5%); abdominal swelling: 20 (20.4%); hyporexia: 19 (19.4%); diarrhea: 14 (14.3%); headache: 13 (13.3%); myalgia: 13 (13.3%); heartburn: 7 (7.1%); flatulence: 8 (8.1%); urticaria/eczema: 5 (5.1%); paresthesia: 4 (4.1%); arthralgia: 4 (4.1%); drowsiness: 3 (3.1%); cough: 3 (3.1%); depression: 3 (3.1%); oral aphthous ulcers: 2 (2.7%); itching: 2 (2.7%); mucous candidiasis: 2 (2.7%); insomnia: 1 (1.4%); constipation: 1 (1.4%); hypertensive crisis: 1 (1.4%) | 67.3% |
| Huang et al[29], 2018 | N-BQT: esomeprazole, amoxicillin, tetracycline, and metronidazole | n = 24. Abdominal pain: 3 (12.5%); nausea/vomiting: 3 (12.5%); constipation: 1 (4.2%); dizziness: 1 (4.2%); headache: 1 (4.2%); skin rash: 0 (0%); diarrhea: 0 (0%) | 29.2% |
| Huang et al[29], 2018 | Susceptibility-guided therapy | n = 39, Abdominal pain: 3 (7.7%); nausea/vomiting: 3 (7.7%); constipation: 2 (5.1%); dizziness: 0 (0%); headache: 1 (2.6%); skin rash: 1 (2.6%); diarrhea: 0 (0%) | 25.6% |
| Liu et al[33], 2020 | BQT: bismuth, esomeprazole, tetracycline, furazolidone, and Lactobacillus acidophilus | n = 50. Loose stools: 1 (2.0%); dizziness: 4 (8.0%); skin rash: 2 (4.0%); foot joint pain: 1 (2.0%); dry mouth: 3 (6.0%) | 20.0% |
| Ji et al[37], 2020 | BQT: bismuth, rabeprazole, amoxicillin, levofloxacin or furazolidone | n = 191. Abdominal pain: 4 (2.09%); constipation: 2 (1.04%); nausea: 11 (5.7); diarrhea: 5 (2.6%); flatulence: 7 (3.6%); skin rash: 5 (2.6%); pruritus: 1 (0.5%); dysgeusia: 1 (0.5%); headache: 3 (1.6%); anorexia: 0 (0%); dizziness: 8 (4.1%); dyspepsia: 6 (3.1%); drowsiness: 0 (0%); Fever: 2 (1.0%); paresthesia: 1 (0.5%); tachycardia: 2 (1.0%); vomiting: 1 (0.5%); fatigue: 2 (1.0%); suspended treatment because of adverse effects: 6 (3.1%) | 20.4% |
| Ji et al[37], 2020 | Susceptibility-guided therapy | n = 163. Abdominal pain: 8 (4.9%); constipation: 2 (1.2%); nausea: 10 (6.2%); diarrhea: 3 (1.8%); flatulence: 9 (5.5%); skin rash: 2 (1.2%); pruritus: 3 (1.8%); dysgeusia: 6 (3.7%); headache: 2 (1.2%); anorexia: 1 (0.6%); dizziness: 3 (1.8%); dyspepsia: 1 (0.6%); drowsiness: 1 (0.6%); fever: 1 (0.6%); paresthesia: 0 (0%); tachycardia: 0 (0%); vomiting: 0 (0%); fatigue: 0 (0%); treatment was discontinued because of adverse effects: 2 (1.2%) | 23.3% |
| Nyssen et al[39], 2020 | BQT-Pylera: Pylera® and PPI | n = 275. Nausea: 45 (16.0%); metallic taste: 13 (4.7%); diarrhea: 44 (16.0%); vomiting: 27 (9.8%); fatigue: 33 (12.0%); abdominal pain: 22 (8.0%); anorexia: 32 (12.0%) | 42.0% |
| Nyssen et al[39], 2020 | BQT: bismuth, PPI, metronidazole, and tetracycline | n = 85. nausea: 35 (41.0%); metallic taste: 30 (35.0%); diarrhea: 22 (26.0%); vomiting: 15 (18.0%); fatigue: 10 (12.0%); abdominal pain: 5 (5.9%); anorexia: 6 (7.1%) | 52.0% |
| Nyssen et al[39], 2020 | BQT: bismuth, PPI, metronidazole, and doxycycline | n = 94. nausea: 12 (13.0%); metallic taste: 5 (5.3%); diarrhea: 3 (3.2%); vomiting: 3 (3.2%); fatigue: 4 (4.3%); abdominal pain: 4 (4.3%); anorexia: 0 (0%) | 30.0% |
| Costa et al[25], 2017 | Susceptibility-guided therapy | n = 42. Abdominal pain: 7 (16.7%); diarrhea: 5 (11.9%); nausea: 4 (9.5%); vomiting: 3 (7.1%); change in taste sensation: 1 (2.3%); treatment was discontinued because of adverse effects: 2 (4.7%) | 35.7% |
| Liou et al[28], 2018 | Susceptibility-guided therapy | Clinical Trial 1 (n = 21). Rash: 0 (0%); dizziness: 4 (19.0%); headache: 1 (4.8%); taste distortion: 0 (0%); swelling: 1 (4.8%); abdominal pain: 0 (0%); nausea: 1 (4.8%); vomiting: 0 (0.0%); diarrhea: 2 (9.5%); constipation: 0 (0.0%) | 42.9% |
| Liou et al[28], 2018 | Susceptibility-guided therapy | Clinical Trial 2 (n = 202). Skin rash: 5 (2.5%); dizziness: 25 (12.4%); headache: 8 (4.0%); taste distortion: 7 (3.5%); swelling: 22 (10.9%); abdominal pain: 9 (4.5%); nausea: 38 (18.8%); vomiting: 14 (6.9%); diarrhea: 4 (2.0%); constipation: 1 (0.5%) | 51.0% |
| Liou et al[28], 2018 | N-BQT: esomeprazole, amoxicillin, metronidazole, and tetracycline | Clinical Trial 2 (n = 202). Skin rash: 3 (1.5%); dizziness: 18 (8.9%); headache: 11 (5.5%); taste distortion: 9 (4.5%); swelling: 11 (5.5%); abdominal pain: 6 (3.0%); nausea: 30 (14.9%); vomiting: 6 (3.0%); diarrhea: 14 (6.9%); constipation: 4 (2.0%) | 50.5% |
| Liou et al[28], 2018 | N-BQT: esomeprazole, amoxicillin, metronidazole, and doxycycline | Clinical Trial 1 (n = 20). Rash: 0 (0%); dizziness: 3 (15.0%); headache: 2 (10.0%); taste distortion: 1 (5.0%); swelling: 0 (0%); abdominal pain: 2 (10.0%); nausea: 1 (5.0%); vomiting: 0 (0%); diarrhea: 3 (15.0%); constipation: 1 (5.0%) | 55.0% |
Given the high prevalence and incidence of H. pylori infection, its eradication is widely discussed in the current scenario. Even with very well-established criteria for eradicating the infection and treatment algorithms for choosing first-line and second-line regimens against H. pylori infection, therapeutic failure is still very frequent. Possible causes responsible for failure to eradicate H. pylori include factors related to the microorganism, host, or the treatment itself, such as poor adherence of patients because of adverse effects and complexity of therapeutic regimens[41-43]. Thus, it is necessary to establish safe, effective, and accessible third-line and rescue therapies for patients.
The Maastricht V/Florence Consensus states that after failure of a first-line therapy containing clarithromycin and BQT second-line, SGT or an empirical therapy based on fluoroquinolones should be used or a combination of different antibiotics with bismuth in regions with a profile of known fluoroquinolone resistance. In cases of failure of first-line treatment based on triple or quadruple therapy without bismuth and second-line treatment containing fluoroquinolones, the use of BQT as third-line is recommended. After failure to use BQT as first-line and therapy containing fluor
Rifabutin: Rifabutin-based triple therapy regimens have been widely discussed as an alternative for the rescue treatment of H. pylori infection. In the present review, most rifabutin-based triple therapy regimens used rifabutin 300 mg (150 mg twice daily or 300 mg once daily) plus amoxicillin (variable daily dosage) and a proton pump inhibitor (PPI) (variable daily dosage) lasting 7-14 d. The mean overall eradication rate of these third-line regimens was 81.6% and 84.4% by ITT and PP, respectively. Regarding the use of rifabutin-based triple therapy as a rescue regimen, i.e. in patients with two or more previous failed eradications, the mean overall eradication rate was 75.4% and 78.8% by ITT and PP, respectively.
In the prospective study conducted by Lim et al[13], the effectiveness of rifabutin-based triple therapy was evaluated according to the PPI dosage. In this study, patients who received a rifabutin-based triple therapy regimen with higher doses of PPIs had eradication rates of 96.3% and 100% by ITT and PP, respectively, whereas patients who received standard dose PPIs showed eradication rates of 78.1% and 80.6% by ITT and PP, respectively. In turn, Mori et al[19] performed a comparative analysis between the duration of rifabutin-based triple therapy regimens. In this study, longer duration regimens had higher eradication rates compared to shorter duration regimens, and eradication ranged from 83.3% to 94.1% and from 81.8% to 91.7% by ITT and PP, respectively. Both studies were in line with the review performed by Gisbert et al[42], which suggested increasing the dose of PPIs and the duration of the therapeutic regimen as a strategy for optimizing rifabutin-based treatment.
On the other hand, in studies conducted by Ribaldone et al[32] and Saracino et al[35], lower eradication rates than those of the other studies included in the present review were found, with values of 71.5% and 68.5% by ITT and 72.7% and 56.1% by PP, respectively. These results corroborate the mean eradication rate found in the same study conducted by Gisbert et al[42] in 2020, in which, based on an analysis of 678 patients using rifabutin-based triple therapy, an eradication rate of 69% was found for this regimen as third-line treatment. Regarding rifabutin-based triple therapy as fourth-line treatment, in the prospective study by Hirata et al[36] from 2020, an association between amoxicillin, rifabutin, and vonoprazan (a competitive inhibitor of potassium) was used in patients who used sitafloxacin-based third-line. The eradication rate found by Hirata et al[36] was 100% by ITT and PP.
Regarding adverse effects related to rifabutin-based triple therapy, the literature presents controversial consequences of this regimen. In our review, an average of 53.7% of patients using this approach had at least one adverse effect. Although most cases are related to mild and transient adverse effects, such as gastrointestinal discomfort, there is a lot of divergence between studies. In therapies with prolonged use of rifabutin, for example, Mori et al[19] reported a high rate of adverse effects, with 94.1% of patients having at least one effect and discontinuation of treatment by 29.4% of patients. On the other hand, Ribaldone et al[32] reported that only 7.3% of patients had at least one adverse effect, and treatment was discontinued by 1.3% of patients. In addition, the use of rifabutin is associated with serious adverse effects such as myelotoxicity[41]. However, only one of the studies included in this review[19] presented patients with myelotoxicity, and 6.8% of patients had transient leukopenia with recovery of hematological patterns after 1 wk of treatment.
The mean eradication rates of rifabutin-based triple therapies found in the present review are encouraging. However, the heterogeneity of studies, whether related to eradication rates or adverse effects, makes it difficult to assess the real efficacy and safety of using rifabutin-based triple therapy as third-line treatment and rescue regimen. In addition, rifabutin is used mainly for the treatment of tuberculosis and other mycobacteria, especially in the context of immunodeficiency or HIV infection, and a possible acquisition of resistance to rifabutin is a limitation to its widespread use. Resistance to rifabutin has been reported in patients with low CD4 lymphocyte counts and when intermittent dosages were used[44]. Although the use of rifabutin in the management of refractory H. pylori infection involves a risk, rifabutin-based therapies act as an important alternative for third-line treatment and rescue regimens, especially in regions of previously known resistance to quinolones.
Sitafloxacin: Sitafloxacin is a quinolone with low minimum inhibitory concentration for H. pylori that has been used as rescue therapy[14]. In the present study, most sitafloxacin-based triple therapy regimens had a treatment regimen with sitafloxacin 200 mg (100 mg twice daily) plus amoxicillin (750 mg twice daily or 500 mg four times daily) or metronidazole (250 mg twice daily) and a PPI (variable daily dose) or vonoprazan (20 mg twice daily) for 7-14 d. The mean overall eradication rate of these regimens as third-line treatment was 79.4% and 81.5% by ITT and PP, respectively.
Although eradication rates in the studies included in the present review showed satisfactory results for the use of sitafloxacin-based triple therapy as third-line treatment, results were not homogeneous between studies. In a retrospective study, Saito et al[30] compared the efficacy of using sitafloxacin-based therapy associated with amoxicillin and esomeprazole or vonoprazan for 7 d as third-line treatment. In this study, the eradication rate found in the sitafloxacin-esomeprazole association was 54.2% and 56.5% by ITT and PP, respectively. The same therapeutic regimen was used in two other studies showing discrepant eradication rates. While in the prospective study by Hirata et al[23], eradication rates of 83.0% by ITT and PP were found, in the randomized clinical trial performed by Sue et al[31], eradication rates were 53.3% by ITT and 57.1% by PP.
In a systematic review[45] from 2021, 12 clinical trials were analyzed. A mean eradication rate of 80.6% was found for sitafloxacin-based therapies containing PPIs or vonoprazan for a period of 7 d, corroborating the findings in the present study. Regarding the heterogeneity of studies included in our review, discrepancies may be based on the presence of bacterial strains with mutation in the gyrA gene. Mutations in this gene are responsible for conferring resistance to quinolones, leading to a lower eradication rate. The relevance of the gyrA gene mutation status in eradication rates of quinolone-containing regimens was expressed in the review by Mori et al[46]. Thus, it is recommended to identify the mutation in the gyrA gene before using regimens containing quinolones such as sitafloxacin, especially in regions of known previous resistance to quinolones.
Although equivalent therapeutic regimens between different studies present heterogeneous eradication rates, there is agreement regarding no statistically significant difference between the efficacy of the association of sitafloxacin with metronidazole or amoxicillin and between 7-d and 10-d duration regimens. In two studies, eradication rates between regimens containing sitafloxacin-amoxicillin and sitafloxacin-metronidazole as third-line treatment were compared, finding similar results. Furuta et al[14] found eradication rates for the use of amoxicillin and metronidazole, respectively, of 84.1% and 90.9% by ITT and 86.4% and 90.9% by PP for 7-d regimens and 88.9% and 87.2% by ITT and 90.9% and 91.1% by PP for 14-d regimens. Similarly, for a 10-d regimen, Mori et al[20] found eradication rates of 81% and 72.4% by ITT and 82% and 76.4% by PP for amoxicillin and metronidazole, respectively. Regarding the duration of therapeutic regimens, in the present study, eradication rates of 73.1%, 78.3%, and 88.0% by ITT and 74.8%, 80.0%, and 91.0% by PP were found in regimens of 7 d, 10 d, and 14 d, respectively. Both the results related to the duration of regimens and the results related to the association of sitafloxacin with amoxicillin or metronidazole were in agreement with data presented by Mori et al[46] in a review conducted in 2020. In this study, eradication rates of 82.0% and 76.4% were found for 10-d regimens containing amoxicillin or metronidazole, respectively, with no statistically significant difference between therapeutic regimens. In addition, as in the present review, no statistically significant difference was found between eradication rates of sitafloxacin-based treatments in regimens of 7-d and 10-d duration. Thus, the choice between the association of sitafloxacin with amoxicillin or metronidazole should be based on the availability of drugs, knowledge of previously used regimens, and the presence of penicillin allergy. The choice of therapeutic regimens with a 7-d duration is also recommended to obtain greater adherence to treatment.
The present review also showed that triple therapy based on sitafloxacin plus vonoprazan is more effective than regimens containing conventional PPIs. Two studies conducted in 2019 compared the efficacy between regimens containing vonoprazan and regimens containing PPIs. Among 63 patients involved in one of the studies[31], 33 used a regimen containing vonoprazan and 31 used a regimen containing PPIs, with eradication rates of 75.8% by ITT and 83.3% by PP with the use of vonoprazan and 53.3% by ITT and 57.1% by PP with the use of PPIs. The superiority of vonoprazan in relation to PPIs was also observed in the study by Saito et al[30], in which, among 81 patients with two previous failed therapies, 93.0% of those who used vonoprazan obtained successful eradication of H. pylori, while with the use of esomeprazole, eradication rates were 54.2% by ITT and 56.5% by PP. In a review[45] from 2021, a comparative analysis between therapies containing PPIs or vonoprazan was performed, finding eradication rates of 70.1% and 88.9%, respectively, demonstrating the superiority of regimens containing vonoprazan. Therefore, the association of sitafloxacin with vonoprazan is recommended for greater treatment efficacy when available.
Regarding adverse effects related to sitafloxacin-based triple therapy, an overall adverse event rate of 52.4% was found in our review. However, most adverse effects found were mild and transient gastrointestinal disorders. The intensity and duration of these adverse effects were also evaluated in two reviews[45,46] that reported mild and transient effects. Therefore, the use of sitafloxacin-based regimens as third-line treatment may act as a safe and effective alternative for the eradication of refractory H. pylori infection.
Levofloxacin: The mean eradication rate found for levofloxacin-based triple therapy as third-line treatment was 55.7% by ITT and 60.6% by PP. This unsatisfactory eradication rate was homogeneous among studies included in the present review, with the exception of a non-randomized clinical trial[22] that compared the efficacy of levofloxacin-based regimens with 7-d, 10-d, and 14-d duration as third-line treatment. In this clinical trial, from the use of a levofloxacin-based triple therapy for a 14-d period, an eradication rate of 73.7% by ITT and 93.3% by PP was reported. However, for 7-d and 10-d regimens, eradication rates were 58.3% and 62.5% by ITT and 58.3% and 68.2% by PP, respectively. These unsatisfactory rates were also found by Okimoto et al[16] in 2014 and by Paoluzi et al[17] in 2015 (see Table 1).
In a prospective observational study[47], 500 patients in third-line treatment were followed, reporting an eradication rate of 75.0% for levofloxacin-based triple therapy, which was different from the findings of the present review. However, this divergence can be explained by the increasing resistance to levofloxacin, which acts as an important factor in the failure of therapeutic regimens, as demonstrated by the meta-analysis performed by Chen et al[21]. The overall adverse effect rate related to levofloxacin-based triple therapy found in our review was 13.9%. Most adverse effects related to levofloxacin-based triple therapy regimens reported gastrointestinal tract disturbances of mild intensity and transient nature. As in one of the studies[22] in the present review, the follow-up of treatment-related adverse effects was not performed, and it was not included in the overall mean rate of adverse effects. Therefore, the safety of this therapeutic regimen in a 14-d regimen has not been evaluated.
Although BQT is recommended as second-line treatment by most guidelines, levofloxacin-based triple therapy is proposed as a potential alternative by the Maastricht V/Florence Consensus[12]. In addition to being associated with a wide incidence of adverse effects, BQT is also difficult to use because of the availability of bismuth in different regions. The association of these factors, together with the efficacy and safety of levofloxacin-based second-line therapies demonstrated in the systematic review by Gisbert et al[48], allow the use of these regimens as second-line treatment in regions with no bismuth availability or in regions with previously known resistance to clarithromycin regimens. Thus, the use of levofloxacin-based triple therapy as a third-line treatment and rescue therapy is not recommended in 7-d and 10-d regimens given the possibility of its use as a second-line treatment and low treatment efficacy. In turn, 14-d regimens require randomized clinical trials for a more accurate assessment of the efficacy and safety of this regimen as third-line treatment and rescue therapy.
BQT: In the present review, BQT regimens featured a treatment regimen with bismuth subcitrate (variable dose) plus a PPI (variable dose) and two antibiotics (amoxicillin, metronidazole, tetracycline, levofloxacin, furazolidone, and doxycycline, variable dose) with 7-14 d duration (see Table 1). The mean overall eradication rate of these third-line regimens was 69.2% by ITT and 72.1% by PP, with the mean overall rate of rescue treatment being 76.7% by ITT and 79.2% by PP.
In the multicenter observational study by Gisbert et al[15], the effectiveness and safety of BQT was investigated in 200 patients with two previous failed eradications with clarithromycin- and levofloxacin-based regimens. In this study, administration of a BQT regimen as third-line resulted in a common eradication rate of 65.0% by ITT and 67.0% by PP for regimens of 7 d, 10 d, and 14 d, with no increase in therapeutic efficacy with the extension of regimens. In contrast, in the study carried out by Nyssen et al[39], eradication rates of 66.0% by ITT and PP for a 10-d regimen and 82.0% by ITT and 83.0% by PP for a 14-d regimen were found, reporting an increase in therapeutic efficacy with prolonged regimens. An observational study by Hsu et al[49] reported eradication rates of 84.0% by ITT and PP for a 10-d BQT. Thus, the expansion of the effectiveness of therapeutic regimens based on their prolongation presents heterogeneous results among studies included in our review. More comparative studies should be performed with the objective of evaluating a possible optimization of regimens based on the increase in their duration.
In addition to this possible optimization of the quadruple therapy by increasing the regimen duration, the association of different antimicrobials, such as furazolidone proved to be effective, as demonstrated by Ji et al[37] and Liu et al[33] in 2020. Similarly, a non-inferiority randomized clinical trial[21] reported satisfactory and similar eradication rates between conventional BQT and an alternative BQT containing amoxicillin, although the alternative therapy reported better adherence and safety. These studies highlight the need to perform clinical trials comparing different combinations of antimicrobials in order to accurately assess the effectiveness of these regimens.
The evaluation of the efficacy of BQT regimens in our study showed heterogeneous results given the multiple antimicrobial combinations used in therapeutic regimens. The association of these different regimens, which were equivalently accounted to find the overall mean eradication rate of BQT, acts as a limitation of our study. Therefore, the use of BQT as third-line treatment and rescue therapy requires further investigation regarding the combination of antimicrobials and duration of regimens since in our review the mean eradication rates were unsatisfactory and heterogeneous.
In addition to the conventional use of BQT, the use of BQT-Pylera was also evaluated. In the present review, three-in-one quadruple therapy regimens featured a treatment regimen with Pylera® (140 mg potassium bismuth subcitrate, 125 mg metronidazole, 125 mg tetracycline, three capsules, 6/6 h) plus a PPI (variable dose) lasting 10-14 d. The mean overall eradication rate of this regimen as third-line was 88.9% and 90.9% by ITT and PP, respectively, while the mean overall rate of rescue treatment was 84.9% by ITT and 87.8% by PP.
In the study performed by Nyssen et al[39] in 2020, 222 patients with two previous failed eradications used BQT-Pylera for 10 d and 5 patients for 14 d, and eradication was observed in 88.0% of patients by ITT and PP, and 100% by ITT and PP, respectively. Both Rodríguez de Santiago et al[24] in 2017 and Saracino et al[35] in 2020 found similar eradication rates for the use of BQT-Pylera for a period of 10 d, with 80.2% and 87.5% by ITT and 84.4% and 91.3% by PP, respectively. Although eradication rates found in the present review are satisfactory, it is important to evaluate optimization strategies for these regimens.
The review performed by Liou et al[43] suggested the increase in the dose of PPIs and the prolongation of therapeutic approaches for 14-d regimens as optimization strategies for the treatment of refractory H. pylori infections. However, in our review, no statistical differences were observed in the use of these strategies for BQT-Pylera. The increase in the dose of PPI used was evaluated in the study by Rodríguez de Santiago et al[24], and it did not report results superior to those found by Saracino et al[35] in a regimen of equivalent duration. Regarding the prolongation of the therapeutic approach, even though the comparative evaluation performed by Nyssen et al[39] was encouraging, it had only 5 patients in the 14-d regimen. Hence, further comparative studies are needed to determine the most effective duration of this treatment regimen as triple and rescue therapy.
Regarding adverse effects of BQT, an overall rate of 34.0% was found in conventional therapy and a rate of 65.0% for BQT-Pylera. Among studies included in the present review, Rodríguez de Santiago et al[24] reported that 97.0% of patients had at least one adverse effect, and despite the high proportion, no impact was reported on treatment adherence related to these events. Note that most adverse effects found by the studies were mild and transient gastrointestinal disorders, which did not pose a significant limitation to the use of these therapeutic approaches. Thus, the use of BQT, either conventional or BQT-Pylera, is an effective and safe alternative for its use as rescue therapy. However, BQT is recommended by consensus[11,12] as second-line after failure of a first-line containing clarithromycin or as first-line in regions with clarithromycin resistance greater than 15%. Thus, the use of this approach as third-line, despite showing encouraging rates, is limited not only by the use of these therapies as first-line or second-line but also by the limited availability of bismuth salts in multiple regions.
Non-BQT: In the present review, non-BQT regimens featured a treatment regimen with a PPI (variable dose, see Table 1) plus amoxicillin (1 g, 12/12 h), metronidazole (variable dose, see Table 1), and tetracycline (variable dose, see Table 1) or doxycycline (100 mg, 12/12 h) for 14 d. The mean overall eradication rate of these regimens as third-line was 61.3% and 64.2% by ITT and PP, respectively.
In the clinical trial by Huang et al[29], the use of a non-BQT was analyzed in a sequential regimen with tetracycline in 27 patients, finding an eradication rate of 51.8% and 58.3% by ITT and PP, respectively. Ineffective eradication rates were also found by Liou et al[28] in a clinical trial from 2018. In this study, two clinical trials comparing quadruple therapies containing tetracycline or doxycycline were performed, resulting in eradication rates of 72.2% and 60.0% by ITT and 74.4% and 60.0% by PP, respectively. Regarding the safety of this therapeutic approach, the mean rate of adverse effects of 44.90% was found and most were mild and transient gastrointestinal effects.
Our results show that the use of non-BQT is safe but ineffective as third-line treatment. As only two studies with this therapeutic regimen were included in our review and none of them evaluated the use of this therapy in patients with three or more previous failed eradications, more clinical trials are needed for a more accurate assessment of the efficacy and safety of these regimens as third-line treatment and rescue therapy.
In the present review, SGT as third-line treatment had an overall mean eradication rate of 75.0% and 79.2% by ITT and PP, respectively, and these same values were found for the use of this therapy as rescue treatment. These findings, in turn, are in line with the systematic review performed by Puig et al[50], which found moderate results with a mean eradication rate of 72.0% by ITT and PP.
The reviews carried out by Liou et al[43] and Puig et al[50] agree with the recommendation of the Maastricht V/Florence Consensus, which suggests that SGT is recommended after failure of a second-line therapy whenever possible. However, both reviews have reservations regarding the use of this therapy as third-line treatment and as rescue therapy since the adoption of this regimen must account for the availability of tests, costs, and the patient’s preference. In addition to these limitations, comparative studies between SGT and empirical regimens are limited, acting as a further obstacle to assess the practical use of this therapeutic approach as third-line and rescue therapy.
Two studies[28,37] included in the present review concluded there is no superiority in the use of SGT compared to regimens based on drug history. In the randomized clinical trial performed by Liou et al[28], the effectiveness of an empirical quadruple therapy and an SGT was compared in two trials. In the first clinical trial, eradication rates of 81.0% and 60.0% by ITT and 80.0% and 60.0% by PP were found for SGT regimens and empirical therapy, respectively. In the second clinical trial, eradication rates were 78.0% and 72.2% by ITT and 78.4% and 74.4% by PP, for SGT regimens and empirical therapy, respectively, concluding the non-superiority of SGT compared to empirical therapy. In contrast, the comparative clinical trial developed by Huang et al[29] in 2018 found the superiority of SGT in relation to empirical therapy. In this study, the eradication rate of the group that performed susceptibility tests was 81.4% and 89.7% by ITT and PP, respectively, while for the empirical group, rates were 51.8% and 58.3% by ITT and PP, respectively.
Thus, although there are not enough comparative studies to determine the real effectiveness of SGT as rescue therapy and the results presented were heterogeneous, the present review agrees with the recommendation of the Maastricht V/Florence Consensus[12]. Even if results do not show superiority in relation to empirical therapy, the use of susceptibility and genotypic resistance tests should be performed whenever possible as they provide an alternative to the growing bacterial resistance to antimicrobials.
The present review highlighted the need to carry out a greater range of comparative studies on third-line treatment and rescue regimens in refractory H. pylori infection, given the increasing resistance to antimicrobials and reduction in eradication rates of therapeutic regimens. In view of recommendations of the Maastricht V/Florence Consensus, after two previous failed eradication attempts, our study also recommends performing susceptibility tests whenever possible. However, given the difficulties related to test availability, costs, and patient preference, this therapeutic approach is not always an option. Thus, the establishment of effective and safe empirical therapies is fundamental for the management of refractory H. pylori infection.
Among the therapeutic regimens evaluated as alternatives to third-line treatment and rescue therapy, rifabutin- or sitafloxacin-based triple therapies as well as BQT-Pylera were shown to be safe and effective. On the other hand, BQT or non-BQT and levofloxacin-based triple therapy did not present satisfactory eradication rates or presented limitations regarding their use. Therefore, although safe, their use in therapeutic management should be avoided. Note that studies related to BQT showed heterogeneous results, and further investigations regarding its use as third-line treatment and rescue therapy are necessary. Furthermore, it is also necessary to develop studies evaluating both the efficacy and the safety of regimens with levofloxacin for 14 d. As in the present review, encouraging results were found when using this regimen as third-line treatment.
From the comparison between therapeutic approaches that obtained satisfactory results as third-line treatment, the alternative with better eradication rates was the rifabutin-based triple therapy, with a mean overall eradication rate of 84.4% for third-line treatment. However, the use of rifabutin as third-line presents the risk of development of resistance by Mycobacterium tuberculosis as a possible limitation, and its use as third-line treatment and rescue therapy is encouraged in specific situations. Based on the encouraging results found in our study, triple therapy based on sitafloxacin containing vonoprazan is recommended as third-line treatment in regions with a low profile of macrolide resistance, and the association with amoxicillin or metronidazole should be based on availability of drugs, knowledge of previously used regimens, and the presence of allergy to penicillin since this approach had an eradication rate of at least 83.3%. Based on the promising results reported from the comparison between conventional PPIs and vonoprazan, it is important that new clinical trials are developed in order to assess the efficacy of regimens with different associations between antimicrobials and vonoprazan.
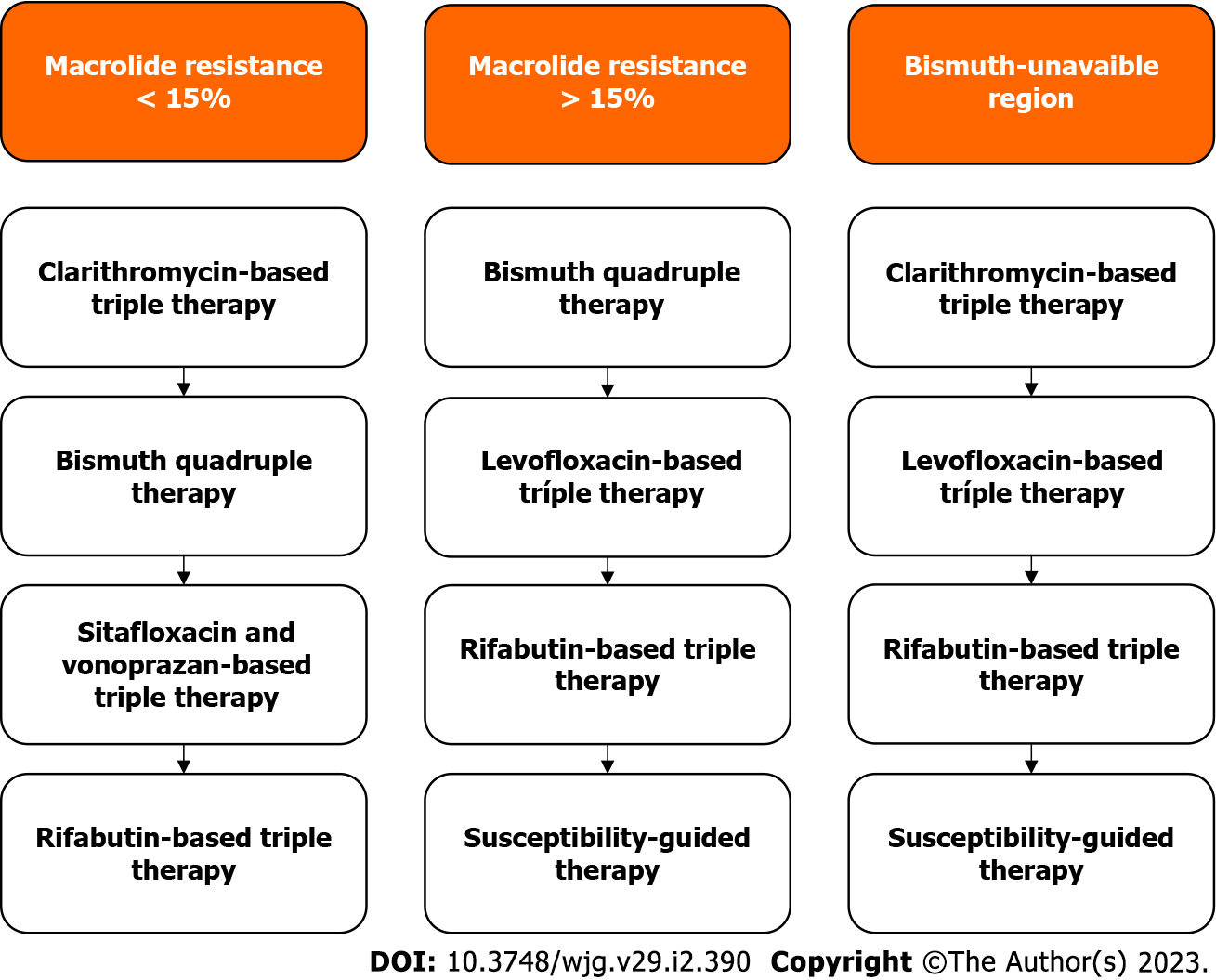
In regions with previously known resistance to macrolides or low availability of bismuth, quinolone-based therapies are used as second-line treatment, and the use of sitafloxacin-based therapies as third-line treatment and rescue therapy is not recommended. In these cases, rifabutin-based triple therapy should be used, and in cases of therapeutic failure, an evaluation of the susceptibility profile should be chosen. These recommendations can be seen in the recommendation diagram (Figure 7).
As a final consideration, despite the satisfactory mean eradication rates found with BQT-Pylera, BQTs are recommended by guidelines as second-line treatment after failure of a first-line containing clarithromycin or as first-line in regions with greater than 15% clarithromycin resistance, limiting its use as third-line treatment and rescue therapy. Note that the combination of three-in-one therapy drugs is related to the increase in positive outcomes in eradication, and this combination of BQT should be used instead of standardized BQTs as first- or second-line, when available.
The eradication of Helicobacter pylori (H. pylori) is widely discussed given the high prevalence and incidence of its infection and since therapeutic failure is frequent establishing safe, effective, and accessible third-line and rescue therapies for patients in need of eradication is necessary in the management of such infection.
Even though eradication criteria and treatment algorithms for first-line and second-line therapy against H. pylori infection are well-established, there is no clear recommendation for third-line and rescue therapy in refractory H. pylori infection.
To evaluate the efficacy and safety of rescue therapies against refractory H. pylori infection and to establish safe, effective, and accessible third-line and rescue therapies for patients in need of eradication.
A systematic search of available rescue treatments for refractory H. pylori infection was conducted on the National Library of Medicine’s PubMed search platform based on Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines. Different descriptors were used throughout the study for maximization of the database, namely: Helicobacter pylori multidrug resistance and rescue therapy; H. pylori multiresistant and rescue treatment; Helicobacter pylori multidrug resistance and rescue treatment; Helicobacter pylori rescue therapy; Helicobacter pylori and third line treatment; and fourth line therapy and Helicobacter pylori. Upon reliable data detection and collection, a statistical analysis was performed to compare eradication rates both by intention to treat and per protocol, and adverse effects found in the different therapeutic approaches to assess their feasibility in clinical practice.
Twenty-eight studies were included in the analysis of mean eradication rates as rescue therapy, and 21 of these were selected for mean eradication rate analysis as third-line treatment. Rifabutin-, sitafloxacin-, levofloxacin-, and metronidazole-based triple therapies, bismuth quadruple therapy (BQT), BQT, three-in-one, Pylera® (BQT-Pylera), non-BQT, and susceptibility-guided therapy were assessed. Furthermore, sitafloxacin-based and rifabutin-based triple therapies achieved higher efficacy than other therapeutic approaches.
We managed to create a recommendation flowchart regarding rescue therapies in different situations, such as regions with previously known resistance to macrolides and in areas where bismuth is unavailable. These results can aid the clinical management of the H. pylori infection and furthermore prevent an increase in resistance rates to different antibiotics.
New clinical trials should be developed in order to assess the efficacy of regimens with different associations between antimicrobials and vonoprazan, based on the promising results reported from the comparison between conventional proton pump inhibitors and vonoprazan.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng H, China; Nikolić M, Croatia S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Yuan YY
| 1. | Hanafiah A, Lopes BS. Genetic diversity and virulence characteristics of Helicobacter pylori isolates in different human ethnic groups. Infect Genet Evol. 2020;78:104135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Mobley HLT, Mendz GL, Hazell SL. Helicobacter pylori: Physiology and Genetics. ASM Press. 2001; Chapter 1. |
| 3. | Waskito LA, Yamaoka Y. The Story of Helicobacter pylori: Depicting Human Migrations from the Phylogeography. Adv Exp Med Biol. 2019;1149:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Kotilea K, Bontems P, Touati E. Epidemiology, Diagnosis and Risk Factors of Helicobacter pylori Infection. Adv Exp Med Biol. 2019;1149:17-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1322] [Cited by in RCA: 1183] [Article Influence: 118.3] [Reference Citation Analysis (0)] |
| 6. | Alakkari A, Zullo A, O'Connor HJ. Helicobacter pylori and nonmalignant diseases. Helicobacter. 2011;16 Suppl 1:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | den Hollander WJ, Sostres C, Kuipers EJ, Lanas A. Helicobacter pylori and nonmalignant diseases. Helicobacter. 2013;18 Suppl 1:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Correa P. Gastric cancer: overview. Gastroenterol Clin North Am. 2013;42:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 441] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 9. | Salar A. Gastric MALT lymphoma and Helicobacter pylori. Med Clin (Barc). 2019;152:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Floch P, Mégraud F, Lehours P. Helicobacter pylori Strains and Gastric MALT Lymphoma. Toxins (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Coelho LGV, Marinho JR, Genta R, Ribeiro LT, Passos MDCF, Zaterka S, Assumpção PP, Barbosa AJA, Barbuti R, Braga LL, Breyer H, Carvalhaes A, Chinzon D, Cury M, Domingues G, Jorge JL, Maguilnik I, Marinho FP, Moraes-Filho JP, Parente JML, Paula-E-Silva CM, Pedrazzoli-Júnior J, Ramos AFP, Seidler H, Spinelli JN, Zir JV. IVTH BRAZILIAN CONSENSUS CONFERENCE ON HELICOBACTER PYLORI INFECTION. Arq Gastroenterol. 2018;55:97-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2220] [Cited by in RCA: 1983] [Article Influence: 247.9] [Reference Citation Analysis (1)] |
| 13. | Lim HC, Lee YJ, An B, Lee SW, Lee YC, Moon BS. Rifabutin-based high-dose proton-pump inhibitor and amoxicillin triple regimen as the rescue treatment for Helicobacter pylori. Helicobacter. 2014;19:455-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Furuta T, Sugimoto M, Kodaira C, Nishino M, Yamade M, Uotani T, Sahara S, Ichikawa H, Yamada T, Osawa S, Sugimoto K, Watanabe H, Umemura K. Sitafloxacin-based third-line rescue regimens for Helicobacter pylori infection in Japan. J Gastroenterol Hepatol. 2014;29:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Gisbert JP, Perez-Aisa A, Rodrigo L, Molina-Infante J, Modolell I, Bermejo F, Castro-Fernández M, Antón R, Sacristán B, Cosme A, Barrio J, Harb Y, Gonzalez-Barcenas M, Fernandez-Bermejo M, Algaba A, Marín AC, McNicholl AG; H. pylori Study Group of the Spanish Gastroenterology Association. Third-line rescue therapy with bismuth-containing quadruple regimen after failure of two treatments (with clarithromycin and levofloxacin) for H. pylori infection. Dig Dis Sci. 2014;59:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Okimoto K, Arai M, Saito K, Minemura S, Maruoka D, Matsumura T, Nakagawa T, Katsuno T, Ishii C, Murata S, Watanabe M, Nomura F, Yokosuka O. Efficacy of Levofloxacin Based Triple and High-Dose PPI-Amoxicillin Dual Eradication Therapy for Helicobacter pylori after Failures of First- and Second-Line Therapies. Int Sch Res Notices. 2014;2014:631501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Paoluzi OA, Del Vecchio Blanco G, Visconti E, Coppola M, Fontana C, Favaro M, Pallone F. Low efficacy of levofloxacin-doxycycline-based third-line triple therapy for Helicobacter pylori eradication in Italy. World J Gastroenterol. 2015;21:6698-6705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Muller N, Amiot A, Le Thuaut A, Bastuji-Garin S, Deforges L, Delchier JC. Rescue therapy with bismuth-containing quadruple therapy in patients infected with metronidazole-resistant Helicobacter pylori strains. Clin Res Hepatol Gastroenterol. 2016;40:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Mori H, Suzuki H, Matsuzaki J, Tsugawa H, Fukuhara S, Miyoshi S, Hirata K, Seino T, Matsushita M, Nishizawa T, Masaoka T, Kanai T. Rifabutin-based 10-day and 14-day triple therapy as a third-line and fourth-line regimen for Helicobacter pylori eradication: A pilot study. United European Gastroenterol J. 2016;4:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Mori H, Suzuki H, Matsuzaki J, Tsugawa H, Fukuhara S, Miyoshi S, Hirata K, Seino T, Matsushita M, Masaoka T, Kanai T. Efficacy of 10-day Sitafloxacin-Containing Third-Line Rescue Therapies for Helicobacter pylori Strains Containing the gyrA Mutation. Helicobacter. 2016;21:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Chen Q, Zhang W, Fu Q, Liang X, Liu W, Xiao S, Lu H. Rescue Therapy for Helicobacter pylori Eradication: A Randomized Non-Inferiority Trial of Amoxicillin or Tetracycline in Bismuth Quadruple Therapy. Am J Gastroenterol. 2016;111:1736-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Noh HM, Hong SJ, Han JP, Park KW, Lee YN, Lee TH, Ko BM, Lee JS, Lee MS. Eradication Rate by Duration of Third-line Rescue Therapy with Levofloxacin after Helicobacter pylori Treatment Failure in Clinical Practice. Korean J Gastroenterol. 2016;68:260-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Hirata Y, Serizawa T, Shichijo S, Suzuki N, Sakitani K, Hayakawa Y, Yamada A, Koike K. Efficacy of triple therapy with esomeprazole, amoxicillin, and sitafloxacin as a third-line Helicobacter pylori eradication regimen. Int J Infect Dis. 2016;51:66-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Rodríguez de Santiago E, Martín de Argila de Prados C, Marcos Prieto HM, Jorge Turrión MÃ, Barreiro Alonso E, Flores de Miguel A, de la Coba Ortiz C, Rodríguez Escaja C, Pérez Álvarez G, Ferre Aracil C, Aguilera Castro L, García García de Paredes A, Rodríguez Pérez A, Albillos Martínez A. Limited effectiveness with a 10-day bismuth-containing quadruple therapy (Pylera®) in third-line recue treatment for Helicobacter pylori infection. A real-life multicenter study. Helicobacter. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Costa S, Soares JB, Gonçalves R. Efficacy and tolerability of culture-guided treatment for Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2017;29:1258-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Puig I, González-Santiago JM, Molina-Infante J, Barrio J, Herranz MT, Algaba A, Castro M, Gisbert JP, Calvet X. Fourteen-day high-dose esomeprazole, amoxicillin and metronidazole as third-line treatment for Helicobacter pylori infection. Int J Clin Pract. 2017;71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Fiorini G, Zullo A, Vakil N, Saracino IM, Ricci C, Castelli V, Gatta L, Vaira D. Rifabutin Triple Therapy is Effective in Patients With Multidrug-resistant Strains of Helicobacter pylori. J Clin Gastroenterol. 2018;52:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Liou JM, Chen PY, Luo JC, Lee JY, Chen CC, Fang YJ, Yang TH, Chang CY, Bair MJ, Chen MJ, Hsu YC, Hsu WF, Chang CC, Lin JT, Shun CT, El-Omar EM, Wu MS; Taiwan Gastrointestinal Disease and Helicobacter Consortium. Efficacies of Genotypic Resistance-Guided vs Empirical Therapy for Refractory Helicobacter pylori Infection. Gastroenterology. 2018;155:1109-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Huang HT, Wang HM, Yang SC, Tai WC, Liang CM, Wu KL, Lee CH, Chuah SK. Efficacy of a 14-day quadruple-therapy regimen for third-line Helicobacter pylori eradication. Infect Drug Resist. 2018;11:2073-2080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Saito Y, Konno K, Sato M, Nakano M, Kato Y, Saito H, Serizawa H. Vonoprazan-Based Third-Line Therapy Has a Higher Eradication Rate against Sitafloxacin-Resistant Helicobacter pylori. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Sue S, Shibata W, Sasaki T, Kaneko H, Irie K, Kondo M, Maeda S. Randomized trial of vonoprazan-based versus proton-pump inhibitor-based third-line triple therapy with sitafloxacin for Helicobacter pylori. J Gastroenterol Hepatol. 2019;34:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 32. | Ribaldone DG, Fagoonee S, Astegiano M, Durazzo M, Morgando A, Sprujevnik T, Giordanino C, Baronio M, De Angelis C, Saracco GM, Pellicano R. Rifabutin-Based Rescue Therapy for Helicobacter pylori Eradication: A Long-Term Prospective Study in a Large Cohort of Difficult-to-Treat Patients. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Liu A, Wang Y, Song Y, Du Y. Treatment with compound Lactobacillus acidophilus followed by a tetracycline- and furazolidone-containing quadruple regimen as a rescue therapy for Helicobacter pylori infection. Saudi J Gastroenterol. 2020;26:78-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Sugimoto M, Hira D, Murata M, Kawai T, Terada T. Effect of Antibiotic Susceptibility and CYP3A4/5 and CYP2C19 Genotype on the Outcome of Vonoprazan-Containing Helicobacter pylori Eradication Therapy. Antibiotics (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Saracino IM, Pavoni M, Zullo A, Fiorini G, Saccomanno L, Lazzarotto T, Antonelli G, Cavallo R, Borghi C, Vaira D. Rifabutin-Based Triple Therapy Or Bismuth-Based Quadruple Regimen As Rescue Therapies For Helicobacter pylori Infection. Eur J Intern Med. 2020;81:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Hirata Y, Yamada A, Niikura R, Shichijo S, Hayakawa Y, Koike K. Efficacy and safety of a new rifabutin-based triple therapy with vonoprazan for refractory Helicobacter pylori infection: A prospective single-arm study. Helicobacter. 2020;25:e12719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Ji CR, Liu J, Li YY, Qiao C, Qu JY, Hu JN, Lin MJ, Ji R, Li LX, Zuo XL, Li YQ. Susceptibility-guided quadruple therapy is not superior to medication history-guided therapy for the rescue treatment of Helicobacter pylori infection: A randomized controlled trial. J Dig Dis. 2020;21:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Mori H, Suzuki H, Matsuzaki J, Masaoka T, Kanai T. 10-Year Trends in Helicobacter pylori Eradication Rates by Sitafloxacin-Based Third-Line Rescue Therapy. Digestion. 2020;101:644-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Nyssen OP, Perez-Aisa A, Rodrigo L, Castro M, Mata Romero P, Ortuño J, Barrio J, Huguet JM, Modollel I, Alcaide N, Lucendo A, Calvet X, Perona M, Gomez B, Gomez Rodriguez BJ, Varela P, Jimenez-Moreno M, Dominguez-Cajal M, Pozzati L, Burgos D, Bujanda L, Hinojosa J, Molina-Infante J, Di Maira T, Ferrer L, Fernández-Salazar L, Figuerola A, Tito L, de la Coba C, Gomez-Camarero J, Fernandez N, Caldas M, Garre A, Resina E, Puig I, O'Morain C, Megraud F, Gisbert JP. Bismuth quadruple regimen with tetracycline or doxycycline versus three-in-one single capsule as third-line rescue therapy for Helicobacter pylori infection: Spanish data of the European Helicobacter pylori Registry (Hp-EuReg). Helicobacter. 2020;25:e12722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Kuo CJ, Lin CY, Le PH, Chang PY, Lai CH, Lin WR, Chang ML, Hsu JT, Cheng HT, Tseng CN, Lin CJ, Su MY, Hsieh SY, Chiu CT. Rescue therapy with rifabutin regimen for refractory Helicobacter pylori infection with dual drug-resistant strains. BMC Gastroenterol. 2020;20:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Choi JH, Yang YJ, Bang CS, Lee JJ, Baik GH. Current Status of the Third-Line Helicobacter pylori Eradication. Gastroenterol Res Pract. 2018;2018:6523653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Gisbert JP. Rifabutin for the Treatment of Helicobacter Pylori Infection: A Review. Pathogens. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 43. | Liou JM, Lee YC, Wu MS; Taiwan Gastrointestinal Disease and Helicobacter Consortium. Treatment of Refractory Helicobacter pylori Infection-Tailored or Empirical Therapy. Gut Liver. 2022;16:8-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Van der Poorten D, Katelaris PH. The effectiveness of rifabutin triple therapy for patients with difficult-to-eradicate Helicobacter pylori in clinical practice. Aliment Pharmacol Ther. 2007;26:1537-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Nishizawa T, Munkjargal M, Ebinuma H, Toyoshima O, Suzuki H. Sitafloxacin for Third-Line Helicobacter pylori Eradication: A Systematic Review. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | Mori H, Suzuki H. Update on quinolone-containing rescue therapies for Helicobacter pylori infection. World J Gastroenterol. 2020;26:1733-1744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 47. | Gisbert JP, Gisbert JL, Marcos S, Jimenez-Alonso I, Moreno-Otero R, Pajares JM. Empirical rescue therapy after Helicobacter pylori treatment failure: a 10-year single-centre study of 500 patients. Aliment Pharmacol Ther. 2008;27:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Gisbert JP, Morena F. Systematic review and meta-analysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Aliment Pharmacol Ther. 2006;23:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 243] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 49. | Hsu PI, Wu DC, Chen A, Peng NJ, Tseng HH, Tsay FW, Lo GH, Lu CY, Yu FJ, Lai KH. Quadruple rescue therapy for Helicobacter pylori infection after two treatment failures. Eur J Clin Invest. 2008;38:404-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Puig I, López-Góngora S, Calvet X, Villoria A, Baylina M, Sanchez-Delgado J, Suarez D, García-Hernando V, Gisbert JP. Systematic review: third-line susceptibility-guided treatment for Helicobacter pylori infection. Therap Adv Gastroenterol. 2016;9:437-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |