Published online Jan 14, 2023. doi: 10.3748/wjg.v29.i2.378
Peer-review started: September 11, 2022
First decision: October 22, 2022
Revised: November 4, 2022
Accepted: December 23, 2022
Article in press: December 23, 2022
Published online: January 14, 2023
Processing time: 116 Days and 12.6 Hours
Histological remission is increasingly accepted as a treatment endpoint in the management of ulcerative colitis (UC). However, the knowledge of histology guidelines and the attitudes towards their use in clinical practice by gastroenterologists and pathologists is unknown.
To evaluate the knowledge of histology guidelines and attitudes towards the use of histology in UC by gastroenterologists and pathologists.
A prospective, cross-sectional nationwide survey of gastroenterologists and pathologists who analyse UC specimens was conducted. The survey consisted of 34 questions to assess gastroenterologists’ and pathologists’ knowledge (score out of 19) and attitudes towards histological assessment in UC. Survey questions were formulated using the European Crohn’s and Colitis position paper on histopathology and the British Society of Gastroenterology biopsy reporting guidelines. It included knowledge of histological assessment of disease activity and dysplasia, knowledge of histological scoring systems for ulcerative colitis, uptake of histology scoring systems in routine practice, attitudes towards the role of histological activity, and the use of histological activity in clinical scenarios.
Of 89 responders (77 gastroenterologists, 12 pathologists), there was almost universal acceptance that histological assessment should form part of UC evaluation [95% gastroenterologists, 92% pathologists]. However, gastroenterologists reported that 92% of their pathologists do not use a histological scoring system. Utilisation of a formal histological scoring system was preferred by 77% of gastroenterologists and 58% of pathologists. Both groups lacked awareness of the Geboes Score, Nancy Index and Robarts Histopathological Index scoring systems with 91%, 87%, and 92% of gastroenterologists respectively; and 83%, 83%, and 92% pathologists respectively, being uncertain of scoring systems’ remission definitions. Histology knowledge score was not significantly different between gastroenterologists and pathologists [9/19 (IQR: 8-11) vs 8/19 (IQR: 7-10), P = 0.54]. Higher knowledge scores were predicted by hospital attending gastroenterologists (P = 0.004), participation in inflammatory bowel disease (IBD) multidisciplinary teams (P = 0.009), and self-declared IBD sub-specialist (P = 0.03).
Histological remission is a recognised target for both gastroenterologists and pathologists. Despite this, knowledge of histological scoring systems and their utilisation is poor.
Core Tip: This manuscript describes, for the first time, the knowledge and attitudes of gastroenterologists and pathologists towards the use of histology in clinical practice. Given the increasing literature and use of histology in trials, there is a need to understand the current perceptions of using histology in the real-world. Using a novel Inflammatory Bowel Disease Knowledge score, we demonstrate that although histology is an accepted endpoint, knowledge is poor, particularly relating to histological scoring systems. As such, these results illustrate a pressing need and opportunity to improve knowledge around histology scores amongst gastroenterologists and pathologists and develop consensus agreements on a reporting approach.
- Citation: Pudipeddi A, Fung C, Christensen B, Bryant RV, Subramaniam K, Chetwood J, Paramsothy S, Leong RW. Knowledge and attitudes towards the use of histological assessments in ulcerative colitis by gastroenterologists vs pathologists. World J Gastroenterol 2023; 29(2): 378-389
- URL: https://www.wjgnet.com/1007-9327/full/v29/i2/378.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i2.378
Ulcerative colitis (UC) is a chronic inflammatory disease characterised by a relapsing and remitting course[1]. Disease activity is typically evaluated using clinical, biochemical and endoscopic assessments. Treatment goals have evolved over time, and current consensus guidelines from the Selecting Therapeutic Targets in Inflammatory Bowel Disease initiative (STRIDE-II) recommend achieving clinical and endoscopic remission[2]. However, up to 40% of patients who achieve these therapeutic endpoints may have persistent histological inflammatory activity[3,4].
Despite endoscopic normalization, ongoing active histological activity may be associated with poorer clinical outcomes including higher clinical relapse rates, corticosteroid requirement, hospitalization, colectomy and development of colorectal neoplasia[3-7]. Although histological remission is currently not a formal treatment target by consensus expert-opinion, STRIDE-II guidelines do recommend that formal histological assessment take place to determine the depth of remission and help prognosticate patient outcomes. Further, it is increasingly incorporated into clinical drug trials, with central reading to reduce bias, to provide objective scoring of inflammatory activity[2]. Standardized histological scoring systems with varying levels of validity have been developed to quantify the degree of microscopic inflammatory activity and provide a more accurate assessment of mucosal inflammation[8-12]. The three most commonly used are the Geboes score, Nancy index and Robarts histopathology index due to evidence of their content validity and reliability in evaluating histological features[13].
Although accepted in modern clinical drug trials and research settings, histological disease activity and scoring systems have not been incorporated in routine clinical practice. It is not known whether gastroenterologists understand these scoring systems or if they welcome their incorporation into routine clinical care. Achieving consensus in a formal reporting scoring system will require agreement by pathologists, but their knowledge of these scoring systems and willingness to use them is also unknown. Many pathologists use written descriptions of UC activity in their reports. Whether this translates to a numerical value, if they favour a particular scoring system, or their attitude towards synaptic reporting of histological activity, is not known. This cross-sectional survey study evaluated gastroenterologist and pathologist knowledge of histological findings and scoring systems, together with their attitudes towards the role of histology in UC management. We hypothesised that based on their dedicated training, knowledge of histological scoring systems would be significantly higher in pathologists than gastroenterologists.
This was a prospective cross-sectional survey of Australian gastroenterologists and pathologists from July 2021 to January 2022. Gastroenterologists were contacted by proxy through the Gastroenterological Society of Australia, and pathologists who review UC specimens were contacted by their associated gastroenterologists to participate in the survey.
A survey was developed to explore the knowledge and attitudes towards the use of histology in inflammatory bowel disease (IBD) for both gastroenterologists and pathologists. The European Crohn’s and Colitis Organisation (ECCO) position paper on histopathology and the British Society of Gastroenterology (BSG) biopsy reporting guidelines were utilised to formulate questions and quantify knowledge[14,15]. The structured survey was designed by a focus group of three gastroenterologists and comprised of 34 questions. It included knowledge of histological assessment of disease activity and dysplasia, knowledge of histological scoring systems for ulcerative colitis, uptake of histology scoring systems in routine practice, attitudes towards the role of histological activity, and the use of histological activity in clinical scenarios (Supplementary Data 1). Questionnaire language and ambiguity were evaluated by the focus group. A novel IBD Histology Knowledge Score was created that was derived from the survey as a tool to measure overall performance and tested for construct validity and discriminant ability (Supplementary Table 1). The IBD Histology Knowledge Score was calculated as the sum of correct responses to survey questions that aligned with the ECCO position paper on histopathology and the BSG reporting guidelines on IBD biopsies[14,15]. The maximum possible score was nineteen. For construct validity, a high-performance score had to represent a good understanding of histological findings. During the development phase, the survey was administered to senior gastroenterologists and pathologists not directly involved in designing the study, and they were deemed as criterion standards. The survey was then administered to gastroenterology fellows, junior resident medical officers and non-medical staff. Senior staff scored significantly higher (P = 0.001) than junior doctors, establishing content validity. Discriminant validity compared the knowledge scores of those who followed published guidelines vs those who did not.
The IBD Histology Knowledge Score was analysed as a non-parametric continuous variable, described as medians with interquartile ranges and compared using Mann-Whitney U-test and Kruskal-Wallis test. Parametric continuous variables were described as means and compared using the t-test and ANOVA test. Predictors of the IBD histology knowledge score were determined using linear regression with backward elimination regression modelling. A P-value of < 0.05 was deemed statistically significant. Statistical analyses were performed with SPSS version 27 (SPSS Inc, Chicago, IL, United States).
The study was approved by the Sydney Local Health District Human Research Ethics Committee (HREC CH62/6/2021-055).
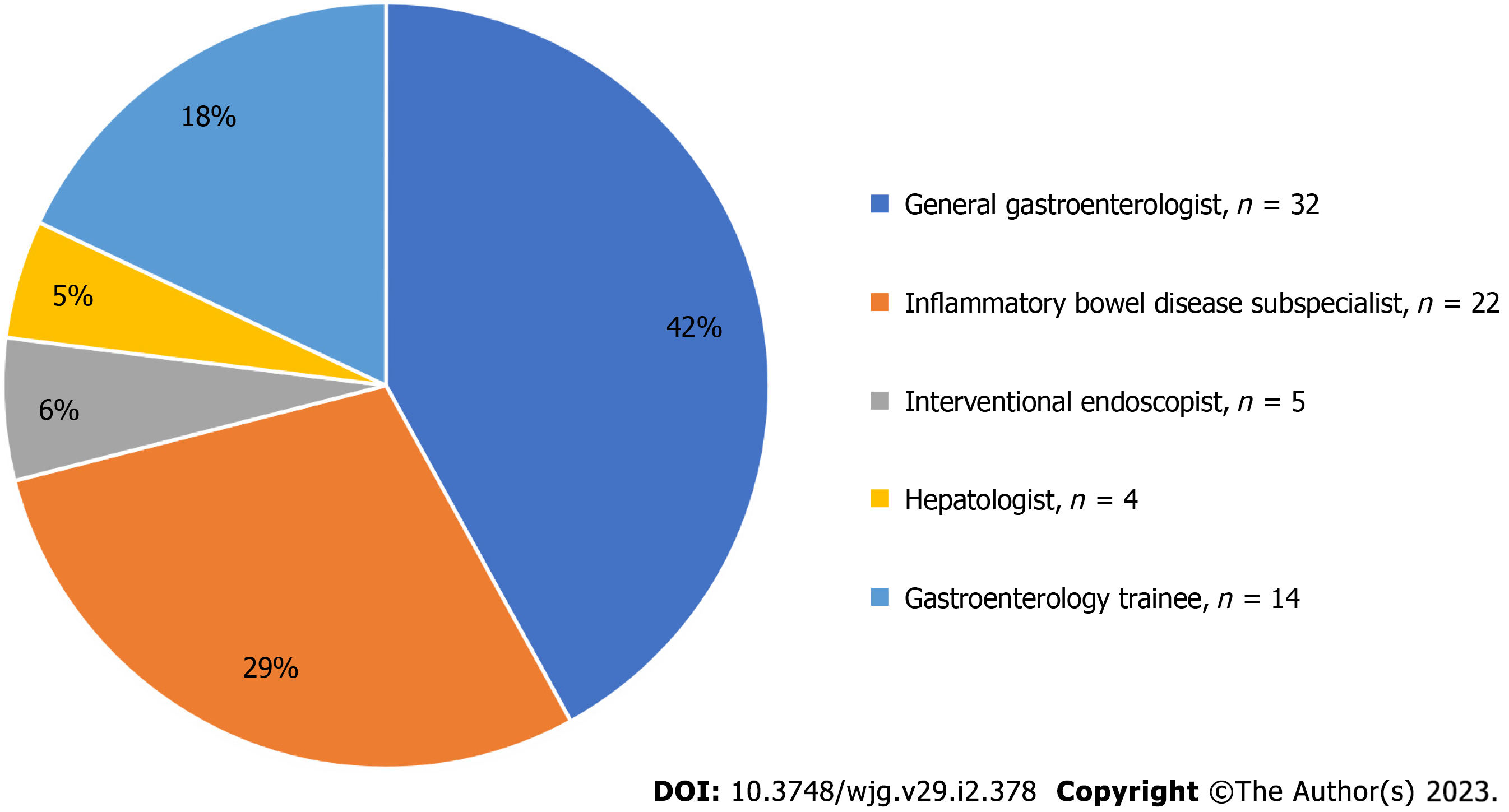
A total of 89 responses were obtained, comprising 77 gastroenterologists and 12 pathologists. The response rate for gastroenterologists was 25% (n = 77/310). Subspecialty breakdown of gastroenterologists is shown in Figure 1. Gastroenterologists listed their predominant work as 31% public hospital staff specialists, 30% private practice, 21% trainee gastroenterologists, 17% visiting medical officers and 1% research-based gastroenterologist. Ninety-four percent of respondents saw > 2 IBD patients each week and 30% saw > 10 patients each week. Forty-five percent of gastroenterologists were involved in a regular IBD multidisciplinary team. Full study cohort characteristics are shown in Table 1.
| Gastroenterologists (n = 77) | Pathologists (n = 12) | |
| Age (yr) | ||
| < 30 | 4 (5.2) | 0 (0.0) |
| 30-40 | 30 (39.0) | 1 (8.3) |
| 41-50 | 15 (19.5) | 4 (33.3) |
| 51-60 | 19 (24.7) | 4 (33.3) |
| > 60 | 9 (11.7) | 3 (25.0) |
| Location | ||
| New South Wales | 46 (59.7) | 8 (66.7) |
| Victoria | 11 (14.3) | 2 (16.7) |
| Queensland | 11 (14.3) | 2 (16.7) |
| Western Australia | 8 (10.4) | 0 (0.0) |
| Australian Capital Territory | 1 (1.3) | 0 (0.0) |
| Highest level of education | ||
| Bachelor of medicine/bachelor of surgery | 51 (66.2) | 11 (91.7) |
| Masters | 10 (13.0) | 0 (0.0) |
| PhD | 16 (20.8) | 1 (8.3) |
| What is your predominant practice | ||
| Staff specialist | 24 (31.2) | 10 (83.3) |
| University academic work | 1 (1.3) | 0 (0.0) |
| Visiting medical officer | 13 (16.9) | 0 (0.0) |
| Private practice | 23 (29.9) | 2 (16.7) |
| In training program | 16 (20.8) | 0 (0.0) |
| How many IBD patients do you see each week | ||
| 0-1 | 5 (6.5) | N/A |
| 2-5 | 31 (40.3) | N/A |
| 6-10 | 18 (23.4) | N/A |
| > 10 | 23 (29.9) | N/A |
| Involved in regular IBD multidisciplinary meeting | ||
| Yes | 35 (45.5) | 6 (50.0) |
| No | 42 (54.5) | 6 (50.0) |
Of the 12 surveyed pathologists, 83% worked in tertiary teaching hospitals and 17% were solely in private practice. Half of all pathologists were involved in regular IBD multidisciplinary meetings. Full study cohort characteristics are shown in Table 1.
Histological activity was considered to have an ‘emerging’ or ‘established’ role in UC by 40% and 55% of gastroenterologists respectively. Proportions for pathologists were 33% and 58% respectively. Histological remission was considered more important to achieve than endoscopic remission by 65% of gastroenterologists (‘somewhat agree’ and ‘agree’) (Table 2).
| Gastroenterologists (n = 77) | Pathologists (n = 12) | |
| The role of histological activity in IBD is | ||
| Not established | 3 (3.9) | 1 (8.3) |
| Preliminary | 1 (1.3) | 0 (0.0) |
| Emerging | 31 (40.3) | 4 (33.3) |
| Established | 42 (54.5) | 7 (58.3) |
| Histological remission is more important to achieve than endoscopic remission | ||
| Disagree | 4 (5.2) | N/A |
| Somewhat disagree | 13 (16.9) | N/A |
| Neither agree nor disagree | 10 (13.0) | N/A |
| Somewhat agree | 36 (46.8) | N/A |
| Agree | 14 (18.2) | N/A |
| What histological scoring system does your pathologist routinely or frequently use in their reports | ||
| Geboes | 2 (2.6) | 0 (0.0) |
| Nancy index | 3 (3.9) | 1 (8.3) |
| RHI | 1 (1.3) | 0 (0.0) |
| They do not routinely use a scoring system | 71 (92.2) | 10 (83.3) |
| Other | IBD-DCA score (n = 1) | |
| I would like to use a histological scoring system for my IBD patients | ||
| Never | 8 (10.4) | 4 (33.3) |
| Rarely | 10 (13.0) | 1 (8.3) |
| Occasionally | 14 (18.2) | 1 (8.3) |
| Sometimes | 23 (29.9) | 3 (25.0) |
| Always | 22 (28.6) | 3 (25.0) |
| Which scoring systems have undergone the most validation | ||
| Modified Riley score | 1 (1.3) | 1 (8.3) |
| Geboes score | 13 (16.9) | 3 (25.0) |
| Nancy index | 20 (26.0) | 5 (41.7) |
| RHI | 9 (11.7) | 3 (25.0) |
| Truelove and Richards score | 5 (6.5) | 0 (0.0) |
| Not sure | 49 (63.6) | 7 (58.3) |
| What Geboes score is considered histological remission | ||
| < 1.1 | 2 (2.6) | 1 (8.3) |
| < 2.1 | 7 (9.1) | 2 (16.7) |
| < 3.1 | 4 (5.2) | 0 (0.0) |
| < 4.1 | 1 (1.3) | 0 (0.0) |
| Not sure | 63 (81.8) | 9 (75.0) |
| What Nancy index is considered histological remission | ||
| 0 | 10 (13.0) | 2 (16.7) |
| ≤ 1 | 4 (5.2) | 3 (25.0) |
| ≤ 2 | 0 (0.0) | 0 (0.0) |
| ≤ 3 | 0 (0.0) | 0 (0.0) |
| Not sure | 63 (81.8) | 7 (58.3) |
| What Robarts histopathology index is considered histological remission | ||
| ≤ 2 | 4 (5.2) | 1 (8.3) |
| ≤ 3 | 6 (7.8) | 1 (8.3) |
| ≤ 4 | 0 (0.0) | 0 (0.0) |
| ≤ 5 | 0 (0.0) | 1 (8.3) |
| Not sure | 67 (87.0) | 9 (75.0) |
The proportion of gastroenterologists who want to use a histological scoring system at least ‘sometimes’ or ‘always’ was 59%, and 50% for pathologists. Gastroenterologists reported that 92% of their pathologists do not routinely use a histological scoring system, whilst 83% pathologists report not routinely using a scoring system. More than half of gastroenterologists (64%) and pathologists (58%) did not know which scoring systems had undergone the most validation (Table 2).
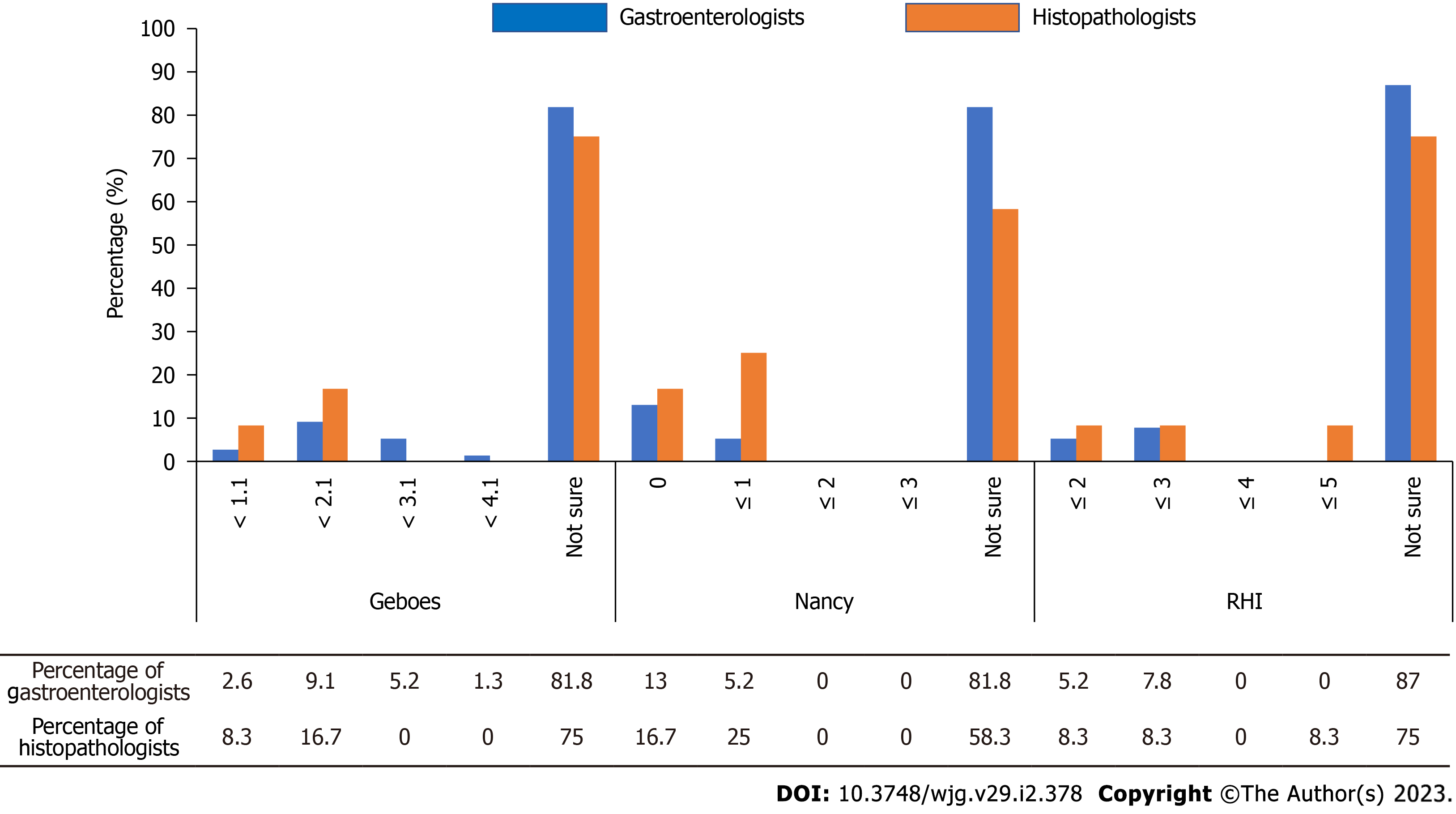
For the Geboes score, 91% of gastroenterologists and 83% of pathologists did not know the defined histological remission score of ‘< 2.1’[14]. For the Nancy index, 87% of gastroenterologists and 83% of pathologists did not know the defined histological remission score of ‘0’[14]. For the Robarts histopathology index (RHI), 92% of gastroenterologists and pathologists did not know the defined histological remission score of ‘≤ 3’[14] (Table 2 and Figure 2).
The impact of histological disease activity on gastroenterologists’ decisions to escalate treatment or de-escalate in particular scenarios is summarized in Table 3. In the setting of clinical and endoscopic remission, but histological activity alone, 10% of gastroenterologists would escalate therapy (‘often’ or ‘always’). When combined with an elevated faecal calprotectin, 30% of gastroenterologists would escalate treatment. A greater proportion of gastroenterologists would de-escalate treatment if two consecutive colonoscopies showed endoscopic and histological remission, compared with a single episode of endoscopic and histological remission (53% vs 19% respectively). A greater proportion of gastroenterologists would aim for histological remission if a patient with UC had other risk factors for colon cancer (71%).
| Scenario | Never | Not often | Sometimes | Often | Always |
| If a patient is in clinical and endoscopic remission, but has histological activity, then I will escalate medical therapy | 14 (18.2) | 35 (45.5) | 20 (26.0) | 5 (6.5) | 3 (3.9) |
| If a patient is in clinical and endoscopic remission, but has an elevated faecal calprotectin (> 100 μg/g) and histological activity, then I will escalate medical therapy | 4 (5.2) | 18 (23.4) | 31 (40.3) | 19 (24.7) | 5 (6.5) |
| If a patient is in clinical, endoscopic and histological remission, (but prior colonoscopy showed Mayo 1 endoscopic disease), then I will de-escalate medical therapy | 7 (9.1) | 19 (24.7) | 36 (46.8) | 15 (19.5) | 0 (0.0) |
| If a patient is in clinical remission, with their last 2 colonoscopies showing endoscopic and histological remission, then I will de-escalate medical therapy | 2 (2.6) | 2 (2.6) | 31 (40.3) | 38 (49.4) | 4 (5.2) |
| If a patient with ulcerative colitis has other risk factors for colon cancer, then I will aim to achieve histological remission | 0 (0.0) | 7 (9.1) | 14 (18.2) | 27 (35.1) | 29 (37.7) |
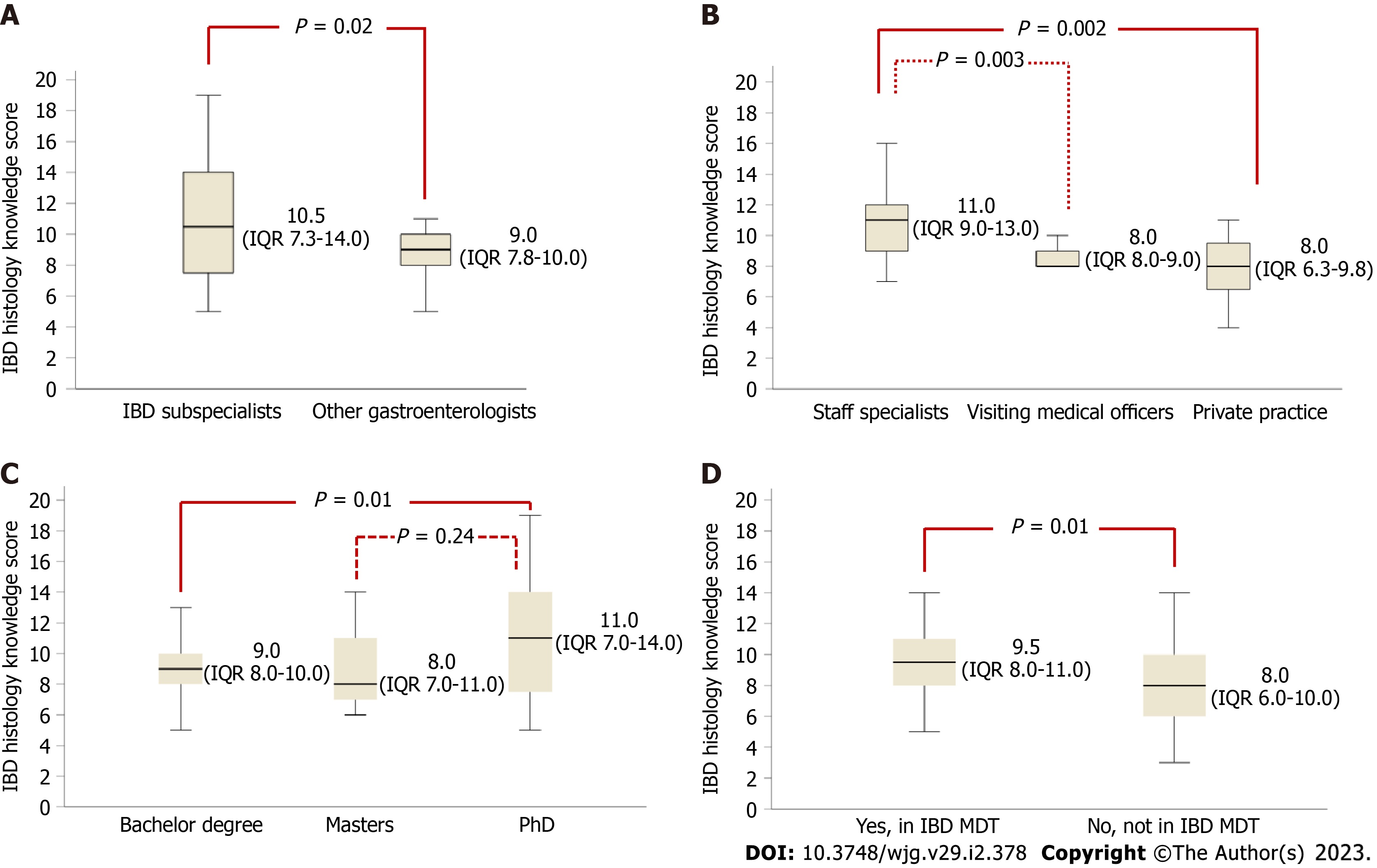
Gastroenterologists and pathologists had similar IBD histology knowledge scores [8.0 (IQR: 6.5-10.0) vs 9.0 (IQR: 7.8-11.0), P = 0.54] (Table 4). Within gastroenterologists, IBD sub-specialists had higher knowledge scores compared with other gastroenterologists [10.5 (IQR: 7.3-14) vs 9.0 (IQR: 7.8-10.0), P = 0.02] (Figure 3A). Public hospital staff specialists had higher knowledge scores than visiting medical officers [11.0 (IQR: 9.0-13.0) vs 8.0 (IQR: 8.0-9.0), P = 0.003] and those in private practice [11.0 (IQR: 9.0-13.0) vs 8.0 (IQR: 6.3-9.8), P = 0.002] (Figure 3B). Gastroenterologists with a PhD had higher knowledge scores than those whose highest level of education was a bachelor degree [11.0 (IQR: 7.0-14.0) vs 9.0 (IQR: 8.0-10.0), P = 0.01] (Figure 3C). Involvement in an IBD multidisciplinary team was associated with a higher knowledge score [9.5 (IQR: 8.0-11.0) vs 8.0 (IQR: 6.0-10.0), P = 0.002] (Figure 3D).
| Gastroenterologists (n = 77) | Pathologists (n = 12) | |
| IBD histology knowledge score [median (IQR)] | 9.0 (7.8-11.0) | 8.0 (6.5-10.0) |
| Type of subspecialist | ||
| General gastroenterologist | 8.0 (7.0-9.0) | N/A |
| IBD subspecialist | 10.5 (7.3-14) | N/A |
| Interventional endoscopist | 9.0 (4.5-9.8) | N/A |
| Hepatologist | 10.5 (8.5-11) | N/A |
| Gastroenterology trainee | 8.5 (6.0-10.0) | N/A |
| Predominant practice | ||
| Staff specialist | 11.0 (9.0-13.0) | N/A |
| Visiting medical officer | 8.0 (8.0-9.0) | N/A |
| Private practice | 8.0 (6.3-9.8) | N/A |
| In training program | 8.5 (6.0-10.0) | N/A |
| Highest level of education | ||
| Bachelor degree | 9.0 (8.0-10.0) | N/A |
| Masters | 8.0 (7.0-11.0) | N/A |
| PhD | 11.0 (7.0-14.0) | N/A |
| Involved in regular IBD multidisciplinary meeting | 35 (45.5%) | 6 (50.0%) |
| Yes | 9.5 (8.0-11.0) | N/A |
| No | 8.0 (6.0-10.0) | N/A |
On univariate analysis, subspecialty type (P = 0.005), predominant practice (p=0.004), involvement in an IBD multidisciplinary team (P = 0.002) and a higher level of education (P = 0.02) were all significantly associated with higher IBD histology knowledge scores (Table 5). On multivariate analysis, subspecialty type (P = 0.03), predominant practice (P = 0.005) and involvement in an IBD multidisciplinary team (P = 0.009) remained significant predictors for higher IBD histology knowledge scores (Table 5).
| Univariate analysis P value | Multivariate analysis P value | |
| Type of subspecialty | 0.005 | 0.03 |
| Predominant practice | 0.004 | 0.005 |
| Involvement in IBD MDT | 0.002 | 0.009 |
| Highest level of education | 0.02 |
Therapeutic goals in UC have evolved from achieving clinical response to attaining objective targets of resolution of inflammation beyond symptoms such as biochemical and endoscopic remission. However, histological remission outside of the research setting has yet to be adopted by gastroenterologists and pathologists. Our study revealed firstly that histological activity is a recognised treatment goal for gastroenterologists who wish to use histology results in combination with other endpoints to guide management decisions. Secondly and conversely, despite this awareness and use of histology, there is a poor knowledge of histological scoring systems in UC not only by gastroenterologists, but by pathologists as well. As such there is an opportunity to develop consensus guidelines incorporating gastroenterologists and pathologists that are adopted by the respective societies to further this evolving field.
Our study showed 95% of gastroenterologists believe histological activity plays a role in the management of UC, with 76% wanting to use a histological scoring system in clinical practice. Further evidence on the role of UC histological activity scores is required as only a small proportion of gastroenterologists currently make treatment decisions based solely on histological activity. In UC patients with clinical and endoscopic remission but ongoing histological disease activity, 10% of gastroenterologists would escalate medical therapy. However, when histological activity coincides with elevated faecal calprotectin, 30% were prepared to escalate treatment. These decisions match the current STRIDE-II guidelines given that histological activity is not currently an accepted target, but shows that gastroenterologists are prepared to include this endpoint as a treatment target[2]. Histological remission becomes even more important if a patient with UC had other risk factors for colon cancer, with 72% prepared to escalate treatment, given that histological activity increases the risk of colorectal neoplasia (odds ratio 3.0, 95%CI: 1.4-6.3)[5]. Therefore, when UC subjects have greater colonic disease extent, more prolonged duration of UC, presence of primary sclerosing cholangitis, or presence of a family history of colorectal cancer, gastroenterologists might escalate treatment in the presence of histological disease activity irrespective of symptoms.
Despite the awareness of the importance of histology in UC, our survey demonstrated a lack of knowledge of histological scoring systems by gastroenterologists. Clinical trials have used Nancy index, RHI and the Geboes score but recent European Crohn’s and Colitis Organisation (ECCO) guidelines recommended the use of the Nancy index and RHI for randomised clinical trials, and the Nancy index for clinical practice given its ease of use[14]. Gastroenterologists did not know which scoring systems had undergone the most validation, or were unaware of the histological remission scores for the Geboes score (91%), Nancy index (87%) and RHI (92%). Despite the increasing interest and evolving role of histological scoring systems in UC, there is an opportunity to educate gastroenterologists about these scoring systems and how to apply them in clinical practice. Predictors for higher knowledge included employment as a public hospital staff specialist and involvement in an IBD multidisciplinary team. As such, it is likely working in public hospitals within an IBD team would lead to increased exposure to the understanding of common histological scoring systems in UC. Conversely, gastroenterologists working in private practice would have less exposure to these scoring systems and their utility in UC management, contributing to lower knowledge scores.
Few studies have evaluated pathologists’ views on histological activity, but most believe that they have a role in evaluating UC. However, pathologists’ knowledge of UC histology was comparable to gastroenterologists [median knowledge score 8.0 (IQR: 6.5-10.0) vs 9.0 (IQR: 7.8-11.0) P = 0.54]. Similar to gastroenterologists, they also lacked knowledge of histological scoring systems and their remission definitions. There is an opportunity, therefore, to improve the utilisation of histological activity scoring for both pathologists and gastroenterologists. A harmonised approach to histological assessment in UC is lacking[16]. Future directions should include the development of histology consensus guidelines in consultation with pathologists to ensure homogeneity in reporting across hospitals to permit comparability of mucosal biopsies across different sites.
This study has several limitations. First, responder bias may have played a role, whereby responders having greater knowledge were more likely to take part on the survey. However, this would indicate a greater unawareness of histological activity scoring in the assessment of UC and a greater need for education and a harmonized approach towards the adoption of a scoring system. Secondly, a smaller respondent number for pathologists was surveyed. However, we demonstrated statistically that pathologists did not differ in their knowledge of histological scoring systems in UC despite expertise in reading biopsy histology. Thirdly, the results may lack worldwide generalisability given the survey was sent to Australian health professionals.
Strengths of this study included: (1) Being the first to report gastroenterologists’ knowledge and attitudes towards the use of histology in UC; (2) recruitment of pathologists to compare their awareness against gastroenterologists; and (3) to target respondents nationwide to demonstrate generalisability.
The study highlights that while there is an acknowledgment of the importance of histological assessment in UC, there is a lack of knowledge of histological scoring systems. It indicates areas of educational need in the field of UC histology, and the importance of including pathologists in developing future consensus guidelines on the use of histology in clinical practice.
The role of histology in ulcerative colitis has evolved over time. Histological activity despite endoscopic remission is associated with poorer clinical outcomes, and various histological scoring systems have been developed. However, the knowledge and attitudes towards the use of histology in the management of ulcerative colitis by gastroenterologists and pathologists is unknown.
Although there has been an increasing literature into the use of histology in ulcerative colitis, it is unknown whether this has translated into knowledge and use by gastroenterologists and pathologists in clinical practice.
The main objective was to evaluate the knowledge of histology guidelines and attitudes towards the use of histology in ulcerative colitis by gastroenterologists and pathologists.
A prospective, cross-sectional survey of gastroenterologists and pathologists was conducted in Australia. The survey was formulated by using peer-reviewed guidelines.
Of 89 responders (77 gastroenterologists, 12 pathologists), there was almost complete acceptance that histological assessment should form part of ulcerative colitis evaluation (95% gastroenterologists, 92% pathologists). However, the majority of both groups lacked awareness of the Geboes score, Nancy index and Robarts histopathological index. Higher knowledge scores were predicted by public hospital attending gastroenterologists and involvement in an inflammatory bowel disease meeting.
Histological remission is a recognised target for both gastroenterologists and pathologists. However knowledge of histological scoring systems was poor.
Future research should involve the development of consensus guidelines in consultation with pathologists on the use of histology in ulcerative colitis management. This should include an agreement on a standardised scoring system to ensure homogenity in reporting across hospitals to permit comparability of biopsies.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Exbrayat JM, France; Iizuka M, Japan; Xing HC, China S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2480] [Article Influence: 310.0] [Reference Citation Analysis (2)] |
| 2. | Turner D, Ricciuto A, Lewis A, D'Amico F, Dhaliwal J, Griffiths AM, Bettenworth D, Sandborn WJ, Sands BE, Reinisch W, Schölmerich J, Bemelman W, Danese S, Mary JY, Rubin D, Colombel JF, Peyrin-Biroulet L, Dotan I, Abreu MT, Dignass A; International Organization for the Study of IBD. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology. 2021;160:1570-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 1633] [Article Influence: 408.3] [Reference Citation Analysis (1)] |
| 3. | Park S, Abdi T, Gentry M, Laine L. Histological Disease Activity as a Predictor of Clinical Relapse Among Patients With Ulcerative Colitis: Systematic Review and Meta-Analysis. Am J Gastroenterol. 2016;111:1692-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 4. | Christensen B, Hanauer SB, Erlich J, Kassim O, Gibson PR, Turner JR, Hart J, Rubin DT. Histologic Normalization Occurs in Ulcerative Colitis and Is Associated With Improved Clinical Outcomes. Clin Gastroenterol Hepatol. 2017;15:1557-1564.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 5. | Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, Bodian C, Ullman T. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099-105; quiz 1340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 570] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 6. | Hefti MM, Chessin DB, Harpaz NH, Steinhagen RM, Ullman TA. Severity of inflammation as a predictor of colectomy in patients with chronic ulcerative colitis. Dis Colon Rectum. 2009;52:193-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Wang H, Fewings I, Bornman L, Shadbolt B, Fadia M, Subramaniam K. Histologic Remission (NANCY Index) is Superior to Endoscopic Mucosal Healing in Predicting Relapse Free Survival in Patients With Ulcerative Colitis in Clinical and Endoscopic Remission. J Clin Gastroenterol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Löfberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 691] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 9. | Riley SA, Mani V, Goodman MJ, Dutt S, Herd ME. Microscopic activity in ulcerative colitis: what does it mean? Gut. 1991;32:174-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 396] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Marchal-Bressenot A, Salleron J, Boulagnon-Rombi C, Bastien C, Cahn V, Cadiot G, Diebold MD, Danese S, Reinisch W, Schreiber S, Travis S, Peyrin-Biroulet L. Development and validation of the Nancy histological index for UC. Gut. 2017;66:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 325] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 11. | Mosli MH, Feagan BG, Zou G, Sandborn WJ, D'Haens G, Khanna R, Shackelton LM, Walker CW, Nelson S, Vandervoort MK, Frisbie V, Samaan MA, Jairath V, Driman DK, Geboes K, Valasek MA, Pai RK, Lauwers GY, Riddell R, Stitt LW, Levesque BG. Development and validation of a histological index for UC. Gut. 2017;66:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 263] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 12. | Chateau T, Feakins R, Marchal-Bressenot A, Magro F, Danese S, Peyrin-Biroulet L. Histological Remission in Ulcerative Colitis: Under the Microscope Is the Cure. Am J Gastroenterol. 2020;115:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 13. | Mosli MH, Parker CE, Nelson SA, Baker KA, MacDonald JK, Zou GY, Feagan BG, Khanna R, Levesque BG, Jairath V. Histologic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev. 2017;5:CD011256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Magro F, Doherty G, Peyrin-Biroulet L, Svrcek M, Borralho P, Walsh A, Carneiro F, Rosini F, de Hertogh G, Biedermann L, Pouillon L, Scharl M, Tripathi M, Danese S, Villanacci V, Feakins R. ECCO Position Paper: Harmonization of the Approach to Ulcerative Colitis Histopathology. J Crohns Colitis. 2020;14:1503-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 15. | Feakins RM; British Society of Gastroenterology. Inflammatory bowel disease biopsies: updated British Society of Gastroenterology reporting guidelines. J Clin Pathol. 2013;66:1005-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Vespa E, D'Amico F, Sollai M, Allocca M, Furfaro F, Zilli A, Dal Buono A, Gabbiadini R, Danese S, Fiorino G. Histological Scores in Patients with Inflammatory Bowel Diseases: The State of the Art. J Clin Med. 2022;11:939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |