Published online May 21, 2023. doi: 10.3748/wjg.v29.i19.2992
Peer-review started: February 6, 2023
First decision: March 20, 2023
Revised: April 3, 2023
Accepted: April 20, 2023
Article in press: April 20, 2023
Published online: May 21, 2023
Processing time: 98 Days and 19.4 Hours
Since Heald proposed the total mesorectal excision (TME) procedure, the prognosis of patients with rectal cancer has been significantly improved. But Heald did not specifically describe the anterior surgical plane in female patients. And the surgical plane for mobilizing the anterior rectal wall during TME surgery in female patients remains controversial.
To investigate the anatomy of the female pelvis and identify the optimal plane for mobilizing the anterior rectal wall.
We retrospectively collected surgical procedure videos and clinical data of female patients diagnosed with middle or low rectal cancer who underwent the TME procedure between January 2020 and October 2022 across six hospitals. The patients were divided into two groups based on the surgical approach used to mobilize the anterior rectal wall: The experimental group was to open the peritoneum at the lowest point of the peritonea reflection and enter the plane for mobilizing, while the control group was cut at 0.5-1 cm above the peritoneal reflection and enter another plan. Then, we compared the preoperative and postoperative information between the two groups. We also dissected and observed ten adult female pelvises to analyze the anatomic structure and compare the entry plane between the two approaches. Finally, we researched the pathological structure between the rectum and the vagina.
Finally, 77 cases that met the criteria were included in our study. Our observations revealed that the experimental group underwent a smooth procedure, entering the plane amidst the mesorectal fascia and adventitia of the vagina, whereas the control group entered the plane between the vaginal adventitia and muscle layers. Compared to the control group, the experimental group showed a significant decrease in intraoperative bleeding [22.5 (19.5-50) mL vs 17 (5-20) mL, P = 0.01], as well as a shorter duration of hospitalization [9 (7-11.25) d vs 7 (6-10) d, P = 0.03]. Through the examination of surgical videos and cadaveric studies, we discovered that Denonvilliers' fascia is absent in females. Additionally, pathological sections further revealed the absence of Denonvilliers' fascia in females, with only loose connective tissue present between the mesorectal fascia and adventitia of the vagina.
The plane amidst the mesorectal fascia and vaginal adventitia is the optimal surgical plane to mobilize the anterior rectal wall for female patients undergoing the TME procedure.
Core Tip: In combination with the macroscopic and microscopic perspectives, we discovered that liberating the anterior rectal wall within a certain plane not only guarantees negative perirectal margins but also mitigates the potential for hemorrhage. This plane, situated amidst the mesorectal fascia and vaginal adventitia, proves to be the most advantageous approach for female patients undergoing total mesorectal excision.
- Citation: Jin W, Yang J, Li XY, Wang WC, Meng WJ, Li Y, Liang YC, Zhou YM, Yang XD, Li YY, Li ST. Where is the optimal plane to mobilize the anterior rectal wall in female patients undergoing total mesorectal excision? World J Gastroenterol 2023; 29(19): 2992-3002
- URL: https://www.wjgnet.com/1007-9327/full/v29/i19/2992.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i19.2992
In 1982, Heald proposed the total mesorectal excision (TME) procedure[1], which significantly improved the prognosis of patients with rectal cancer[2]. The local recurrence of rectal cancer in 5 years decreased from 25% to 5%, as compared to the traditional operation plus radiotherapy group in the TME operation group[3]. TME soon became a classical operation method and was widely accepted by colorectal surgeons. Heald subsequently described the bloodless planes of TME surgery, known as the "holy plane," as follows: “If we cut straight on to the vesicles we find an essentially bloodless plane between them and the fascia of Denonvilliers and we can proceed down the enemy to this until it comes forward to become somewhat should come to the state, when we must cut through it to liberate the lower third of the correction enemy”[4].
Professor Heald believes that in male patients, the anterior plane of TME should be anterior to Denonvilliers' fascia, and the anterior aspect of the specimen should include the complete Denonvilliers' fascia and peritoneal folds[3]. However, he did not specifically describe the anterior surgical plane in female patients[1], and the presence of Denonvilliers’ fascia in females (rectovaginal septum[5]) remains controversial[6,7]. We also haven’t found any research describing the plane of TME for female patients when the front rectal wall performs mobilization till now[8-10]. Many colorectal surgeons struggle to find an ideal plane for mobilizing the anterior rectal wall during TME, which can result in intraoperative bleeding or vaginal damage.
Therefore, we conducted this study to explore the optimal plane to mobilize the anterior rectal wall in female patients. We retrospectively analyzed rectal cancer surgery videos collected from different medical centers and studied the anatomy of the female pelvis for the accurate determination of the optimal plane to mobilize the anterior rectal wall in female patients with rectal cancer.
We retrospectively collected clinical data and surgical videos from six hospitals between January 2020 and October 2022. The surgeries were performed by experienced colorectal surgeons, each of whom performed more than 100 colorectal cancer operations annually. Our study initially included female patients with middle or low rectal cancer who underwent laparoscopic TME. Patients who had preoperative magnetic resonance imaging assessment indicating invasion of the anterior rectal wall, a history of rectal surgery, absence of surgical videos or relevant clinical data, and the presence of distant metastases were excluded. And they were divided into two groups based on the surgical approaches used. In the experimental group, the peritoneum was incised at the lowest point of peritoneal reflection to access the mobilization plane, while in the control group, the peritoneum was incised 0.5-1 cm above the peritoneal reflection, accessing a different plane. The surgical procedures were reviewed by two experienced colorectal surgeons separately.
The patient's pre- and postoperative data were obtained from medical records, while intraoperative bleeding was measured as the total amount of blood loss recorded in surgical records. Postoperative complications were classified into postoperative bleeding, anastomotic leakage, and other complications (such as pleural effusion, fever, etc.). Anastomotic leakage was defined as a communication between the intra- and extraluminal compartments due to a defect in the integrity of the intestinal wall at the anastomosis between the colon and rectum or colon and anus[11], diagnosis through computed tomography (CT) imaging. Pleural effusion was confirmed through CT imaging to establish the diagnosis. Complications were graded by the Clavien-Dindo classification[12].
Ten female cadavers were dissected in the anatomy laboratory of Wenzhou Medical University, which had been donated to the Department of Anatomy following ethical guidelines. The cadavers underwent arterial perfusion with 8% formalin and preservation with 30% alcohol. The corpses were well-preserved, without tissue decay and structural damage. All female cadavers were sourced from young adult females without a history of pelvic diseases. After separating the pelvis from the body, the pelvises were cut in the midsagittal position to expose the rectum and vagina. The pelvises were divided into two to show the rectum and vagina. After clearly exposing the rectal and vaginal structures, a skilled colorectal surgeon and anatomist performed the subsequent operations in accordance with the two different operation procedures of TME, on the same pelvis. At first, the peritoneum was cut at the lowest point of peritoneal reflection and entered the plane to mobilize as the procedure of the experimental group. Then, the surgeon incised the peritoneum at approximately 0.5–1 cm above the peritoneal reflection and started the separation as the procedure of the control group. Photos and records were taken during the dissection.
A pathologist participated in and supervised the pathological research. We separated the rectovaginal tissue from the other half of the complete pelvis and preserved it in 8% formalin. Then, the remaining tissue was used for pathology and immunohistochemistry analysis. Sections were stained with hematoxylin-eosin and observed under an electron microscope with a magnification of ten times. Microscopic examination of the rectal and vaginal structures enabled us to determine the presence of Denonvilliers’ fascia. Immunohistochemical and pathological studies were completed by the Pathology Department of Wenzhou Medical University.
IBM SPSS Statistics 25 software was used for the analysis of clinical data, Two-sided P < 0.05 indicated significance. Continuous variables with normal distribution were summarized as mean (SD) and two independent samples t-test was used for the statistics. For continuous variables with non-normal distribution were summarized as median (IQR) and Mann-Whitney U test were used for the statistics. Categorical variables were summarized as numbers (percentages) and analyzed using the chi-square test, while the Mann-Whitney U test was used for the Statistics of ordered classification variables. The statistical review of this study was performed by a biomedical statistician.
Seventy-seven patients who met the criteria were included in our study, with 35 in the experimental group and 42 in the control group. There were no significant differences in the general information between the two groups. The patients' general information is summarized in Table 1.
| Control group (n = 42) | Experimental group (n = 35) | P value | |
| Height, mean (SD), cm | 157.64 (6.03) | 157.17 (5.29) | 0.72 |
| Weight, mean (SD), kg | 57.02 (8.82) | 57.37 (10.22) | 0.87 |
| BSA, mean (SD) | 1.62 (0.14) | 1.67 (0.15) | 0.17 |
| Age, mean (SD), y | 60.50 (10.94) | 63.86 (12.77) | 0.22 |
| Preoperative chemoradiotherapy | 6 (14.3) | 4 (11.4) | 0.98 |
In the experimental group, the peritoneum was cut at the lowest point of the peritoneal reflection to enter the anterior rectal space (as shown in Figure 1), in which the anterior rectal wall can be easily dissociated from the posterior wall of the vagina. The rectum was light yellow due to the light-yellow adipose tissue surrounded by the mesorectal fascia. Blood vessels could be seen in the mesorectum, and the mesorectum was dissected completely. It can be dissociated through blunt separation combined with sharp separation. In this process, the operation field could remain bloodless, and the vaginal structure and rectal structure were easy to distinguish from each other. The mesorectal fascia and vaginal were complete after dissection, and no Denonvilliers’ fascia-like structure was present between them. In contrast, the control group cut the peritoneum at approximately 0.5–1 cm above the peritoneal reflection and freed the rectal wall between the vaginal muscular and the vaginal adventitia (as shown in Figure 2). Although a structure similar to Denonvilliers’ fascia was found, it was closely connected to the vaginal muscular and could only be torn off through sharp separation. The muscular structure of the vagina was revealed after the operation, which often caused bleeding. Furthermore, the vaginal structure was no longer intact after dissection since the vaginal adventitia was separated from the muscular layer. After reviewing all the surgical procedures, finding an obvious membrane structure between the mesorectal fascia and vaginal adventitia in females was difficult. The fascial structure that we found during the operation was the vaginal adventitia, while the so-called female Denonvilliers’ fascia does not exist.
Compared with the control group, the experimental group had less intraoperative bleeding [22.5 (19.5-50) mL vs 17 (5-20) mL; P = 0.01], and shorter length of hospitalization [9 (7-11.25) d vs 7 (6-10) d; P = 0.03]. Although the incidence of postoperative complications was lower than that of the control group, the results were not statistically significant. No deaths occurred in either group in the first 30 d after surgery. All Pathological specimens’ Circumferential Resection Margins (CRM) were negative after the operation. Statistical result is summarized in Table 2.
| Control group (n = 42) | Experimental group (n = 35) | P value | |
| Operation time, mean (SD), min | 233.14 (75.12) | 228.66 (80.32) | 0.8 |
| IB, median (IQR), mL | 22.5 (19.5-50) | 17 (5-20) | 0.01 |
| NRL, median (IQR) | 13.5 (10-16.5) | 15 (13-19) | 0.24 |
| Hospital stays, median (IQR), days | 9 (7-11.25) | 7 (6-10) | 0.03 |
| Pathology stage | 0.52 | ||
| I | 21 (50.0) | 15 (42.8) | |
| II | 10 (23.8) | 9 (25.7) | |
| III | 11 (26.2) | 11 (31.5) | |
| T | 0.62 | ||
| 1 | 12 (28.6) | 8 (22.9) | |
| 2 | 12 (28.6) | 12 (34.3) | |
| 3 | 15 (35.7) | 10 (28.6) | |
| 4 | 3 (7.1) | 5 (14.3) | |
| N | 0.75 | ||
| 0 | 32 (76.2) | 24 (68.6) | |
| 1 | 8 (19.0) | 9 (25.7) | |
| 2 | 2 (4.8) | 2 (5.7) | |
| CDC | 0.84 | ||
| I | 8 (19.0) | 3 (8.6) | |
| II | 2 (4.8) | 1 (2.9) | |
| Postoperative complications | 10 (23.81) | 4 (11.43) | 0.16 |
| Postoperative bleeding | 1 | 0 | |
| Anastomotic leakage | 3 | 2 | |
| Other complications | 8 | 4 |
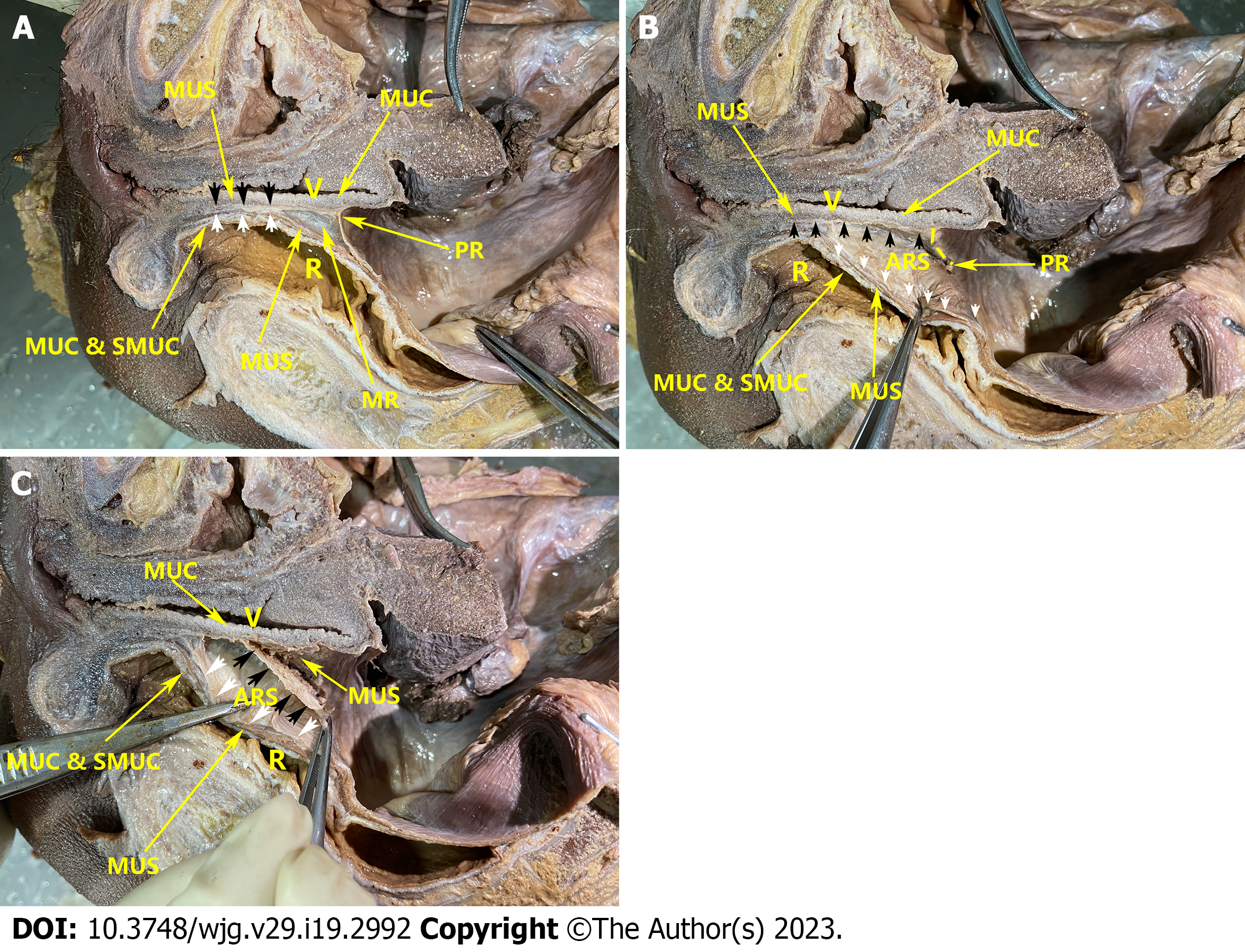
As depicted in Figure 3A, the layers of the rectum and vagina were displayed, and the main structures could be distinguished. The vaginal muscular layer was brown, and the mucosal layer was gray. The mucosa of the rectum was yellowish, the submucosa was white, and the muscular layer was brown. However, distinguishing between the vaginal adventitia and the mesorectal fascia with the naked eye was difficult.
The dissociation process of the experimental group proceeded seamlessly. As depicted in Figure 3B, the mobilization plane traversed the interface between the vaginal adventitia and the fascia of the mesorectum. A discernible space between the vaginal adventitia and the mesorectal fascia was observed, which could be adroitly separated through blunt dissection. The vaginal adventitia and the mesorectal fascia were generally white without fascia-like tissues or blood vessels between them. Occasionally, blood vessels were visible beneath the vaginal adventitia. The boundary between the muscular layer and the adventitia was clear, with the muscular layer appearing dark and the adventitia appearing white.
As shown in Figure 3C, the mobilization plane entered the plane between the vaginal adventitia and the muscular layer by the procedure of the control group. When the vaginal adventitia was separated from the muscular layer, we found that the attachment between the vaginal adventitia and the muscular layer was stronger than the attachment between the vaginal adventitia and the mesorectal fascia, which could not be easily mobilized by blunt separation and can only be mobilized by sharp separation. After dissociation, the vaginal adventitia could be seen to be a single-layer fascia-like structure with a white color that was different and easy to distinguish from the dark muscle layer. No distinct or separate Denonvilliers' fascia was identified. At the same time, after the vaginal adventitia was forcibly torn off from the muscular layer, the muscular layer structure was damaged. Some residual muscle fiber tissue could be seen on the vaginal adventitia.
As shown in Figure 4, the rectal and vaginal tissues showed clear stratification: The rectum was divided into the mucosa, submucosa, inner ring muscle layer, outer longitudinal muscle layer, mesorectum, and the mesorectal fascia surrounding the mesorectum. The vaginal wall was divided into three layers: Mucosa, muscular, and adventitia layers. The adventitia layer was composed of dense connective tissue with scattered loose connective tissue among it. A gap was present between the mesorectal fascia and the vaginal adventitia. Numerous loose connective tissue, but no obvious fascia-like structures, were present inside this gap. Occasionally, some scattered dense connective tissue and fragment-like fascial structure could be seen but were difficult to identify as fascial structures.
In 1836, after observing the anatomy of the male pelvis, Denonvillier found a single-layer membrane-like structure between the rectum and prostate that he called the “prostatoperitoneal membrane”[13]. Later, in memory of Denonvillier, people called this fascial structure Denonvilliers’ fascia, which is considered a structure that starts from the peritoneal reflection and ends at the perineum. The prostate and seminal vesicle glands are located in front of this structure. The rectum locates behind it, and vascular and nerve bundles are located on both sides of this structure. Undoubtedly, colorectal doctors have reached a consensus on the existence of Denonvilliers’ fascia in the male pelvis. However, whether this fascia exists in females remains highly debated. Denonvillier elaborated on this structure by dissecting the male pelvis but did not mention its relevance in females. Later researchers also encountered many contradictions and disputes regarding the description of this structure.
In 1969, after dissecting 143 bodies, Milley and Nichols[14] confirmed the presence of a rectovaginal septum. They found a rectovaginal septum in 23 of 25 adult females and all dissected female infants. Despite the individual differences, the existence of this structure appears to be unaffected by age and hormones. At the same time, a close adhesion existed between this diaphragm and the fascia surrounding the vagina, they believed that this close adhesion may be a major reason why some anatomists deny the existence of the vaginal rectal septum[14].
Zhai et al[5] proved the existence of the female rectovaginal septum by studying the whole pelvic viscera embedded in celloidin (25 female pelvic visceral organs). He pointed out that the rectovaginal septum could be divided into two layers: Denonvilliers’ fascia and the mesorectal fascia from the traditional point of view. Denonvilliers’ fascia tightly surrounds the posterior and lateral walls of the vagina, and the Denonvilliers’ fascia and the mesorectal fascia are not attached to the rectouterine pouch. Instead, they extend upward along the peritoneum. In this anatomy, the anterior layer integrates into the uterus, and the posterior layer gradually thins and disappears. The rectovaginal septum plays an important role in preventing the spread of malignant tumors[5].
Bertrand et al[6] found that Denonvilliers’ fascia is an independent structure of the mesorectal fascia and the cervix as well as the vagina by studying female fetuses’ anatomy[6]. However, they were likely to mistake the vaginal adventitia for Denonvilliers’ fascia (rectovaginal septum). In our study on adult females, we only found loose connective tissue in the plane between the mesorectal fascia and the adventitia of the vagina. No other fascia-like structures were found between these structures. Due to the absence of other structures in this plane, we were able to easily separate the rectum and vagina without causing damage to the vaginal structure during the complete removal of the mesorectum. And the vaginal adventitia seems like Denonvilliers’ fascia if it was separated from the vaginal muscle. Recognizing the adventitia of the vagina as Denonvilliers' fascia and forcefully separating it from the muscular layer could often cause bleeding.
At the same time, some people proposed the opposite opinion. Zhang et al[15] found only some membrane-like fascial fragments in the adipose tissue between the rectum and the vagina in the frozen sections of the corpses of three adult females aged 58–86 years old. They believed that previous studies may have regarded these fascial fragments as Denonvilliers’ fascia; however, considering the age limit of their samples, their results still need further research[15]. An analysis of surgical and pathological samples from three females showed that the so-called fascia and vaginal wall had the same histological manifestations under pathology and were not distinguishable[16]. Therefore, Farrell et al[16] believed that the so-called fascia was an artificial surgical separation from the vagina that occurred when separating the vagina and surrounding organs[16]. In 2005, through the analysis of four female anatomical samples, Kleeman et al[17] also obtained a similar conclusion, that is, no fascia exists between the rectum and vagina. The rectovaginal septum used to repair the rectocele is an artificial surgical separation of tears from the vagina[17]. Meanwhile, after dissecting twenty-five female cadavers, García-Gausí et al[7] came to the same conclusion as Farrell et al[16]: That an independent rectovaginal septum could only be produced by tearing the vaginal adventitia. They found only a layer of loose connective tissue between the vagina and the rectum[7]. This is consistent with the findings of our study.
Based on the anatomy of four cadavers, Fang proposed that for surgery in early rectal cancer, mobilizing the rectum behind Denonvilliers’ fascia can not only ensure the integrity of the mesorectum but also control related postoperative complications[18]. Although his viewpoint on whether Denonvilliers’ fascia exists in females is different from ours, his conclusion on the female surgical approach is similar to ours. Simultaneously, some researchers have discovered that in the case of early rectal cancer, mobilizing the rectal wall behind Denonvilliers' fascia results in similar 5-year local recurrence rates as the traditional TME approach, while also reducing the incidence of complications[19]. Nevertheless, we believe that these researchers may have misidentified the vaginal adventitia as Denonvilliers' fascia in female patients.
We found that in females, the plane between the vaginal adventitia and the mesorectal fascia is suitable for rectal cancer surgery. By dissecting the rectum along the plane between the mesorectal fascia and the vaginal adventitia, not only can the risk of bleeding and damage to physiological structures be minimized, resulting in a faster recovery and shorter hospitalization, but it can also guarantee complete removal of the mesorectum and negative CRM status. Thus, this plane is the optimal plane in female patients with rectal cancer undergoing TME. Our research focused on the macro- and microlevels, combined with clinical data to explore the anatomy of the rectum and the vagina from multiple angles to obtain a highly scientific conclusion. Not only did we provide stronger evidence supporting the absence of Denonvilliers' fascia as an independent structure in females, but we also discovered that the anterior rectal space is the optimal plane to mobilize the anterior rectal wall for female patients undergoing TME.
However, as this study was retrospective and had a limited sample size, the observed differences in complication rates between the two procedures were not statistically significant. Future studies with larger sample sizes may yield more conclusive results. Additionally, due to the relatively short follow-up period since the patients' surgeries, long-term prognostic and sexual function outcomes have not been thoroughly investigated. Nonetheless, we intend to address this limitation in our subsequent studies.
In adult females, Denonvilliers’ fascia is absent, we could only find loose connective tissue between the mesorectal fascia and vaginal adventitia. The vaginal adventitia is tightly adherent to the vaginal muscular layer and was difficult to separate from the muscle layer. By incising the peritoneum at the lowest point of peritoneal reflection, a plane between the mesorectal fascia and vaginal adventitia can be accessed. Mobilizing the anterior rectal wall in this plane could not only ensures the integrity of the mesorectum but also reduces intraoperative bleeding and hospital stay. Dissecting in this plane follows a natural avascular space without damaging the vaginal structure and simplifies the surgical procedure. Therefore, this is the optimal plane for mobilizing the anterior rectal wall for female patients undergoing TME procedures.
Currently, there are no comprehensive descriptions available regarding the approach for dissecting the anterior wall of the female rectum. Many surgeons encounter intraoperative bleeding due to the lack of an appropriate dissection plane.
The surgical approach for mobilizing the anterior rectal wall during total mesorectal excision surgery in female patients remains controversial. However, with a more profound comprehension of the pelvic anatomy, we can identify the avascular plane, reducing intraoperative bleeding and preventing harm to physiological structures.
We aim to gain a better understanding of the female pelvic anatomy to identify an optimal approach for dissecting the anterior wall of the rectum. This will facilitate improved surgical outcomes for female patients with middle or low rectal cancer.
Firstly, we retrospectively grouped patients based on different approaches after reviewing surgical videos. Clinical information was collected and pre-and post-operative data were compared, along with reviewing surgical videos to understand the anatomy and intraoperative situation. Subsequently, the female pelvic structure was studied through cadaveric dissection and histological sections.
We discovered that opening the peritoneum at the lowest point of peritoneal reflection allows access to the plane between the vaginal adventitia and mesorectal fascia. Opening the peritoneum 0.5-1 cm above the peritoneal reflection enters another plane located between the vaginal adventitia and vaginal muscle layer. The first approach has lower intraoperative bleeding and shorter hospital stay compared to the second approach. Neither cadaveric dissection nor pathological examination revealed the existence of Denonvilliers' fascia. Only loose connective tissue exists between the rectosacral fascia and the vaginal adventitia.
Denonvilliers' fascia is absent in females. The plane amidst the mesorectal fascia and vaginal adventitia is the optimal surgical plane to mobilize the anterior rectal wall for female patients.
In future studies, we will explore the long-term prognosis of the two approaches for women, as well as the impact on postoperative sexual and vaginal function.
Thanks to Xin-Yu Li, Yang-Yang Li, and Xin-Dong Yang’s great contributions to our work.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bae SU, South Korea; Brisinda G, Italy; Luglio G, Italy; Martínez-Pérez A, Spain; M'Koma AE, United States; Tonelli F, Italy S-Editor: Li L L-Editor: A P-Editor: Cai YX
| 1. | Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 1933] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 2. | Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 1913] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 3. | MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet. 1993;341:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1220] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 4. | Heald RJ. The 'Holy Plane' of rectal surgery. J R Soc Med. 1988;81:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 492] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Zhai LD, Liu J, Li YS, Yuan W, He L. Denonvilliers' fascia in women and its relationship with the fascia propria of the rectum examined by successive slices of celloidin-embedded pelvic viscera. Dis Colon Rectum. 2009;52:1564-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Bertrand MM, Alsaid B, Droupy S, Benoit G, Prudhomme M. Biomechanical origin of the Denonvilliers' fascia. Surg Radiol Anat. 2014;36:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | García-Gausí M, García-Armengol J, Mulas Fernández C, Pellino G, Roig JV, García-Granero A, Pla-Marti V, Martínez-Soriano F. Surgical Anatomy of the Rectovaginal Space: Does a Standalone Rectovaginal Septum or Denonvilliers Fascia Exist in Women? Dis Colon Rectum. 2021;64:576-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W, Simes J; ALaCaRT Investigators. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA. 2015;314:1356-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 762] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 9. | Fleshman J, Branda ME, Sargent DJ, Boller AM, George VV, Abbas MA, Peters WR Jr, Maun DC, Chang GJ, Herline A, Fichera A, Mutch MG, Wexner SD, Whiteford MH, Marks J, Birnbaum E, Margolin DA, Larson DW, Marcello PW, Posner MC, Read TE, Monson JRT, Wren SM, Pisters PWT, Nelson H. Disease-free Survival and Local Recurrence for Laparoscopic Resection Compared With Open Resection of Stage II to III Rectal Cancer: Follow-up Results of the ACOSOG Z6051 Randomized Controlled Trial. Ann Surg. 2019;269:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 271] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 10. | Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR Jr, Maun D, Chang G, Herline A, Fichera A, Mutch M, Wexner S, Whiteford M, Marks J, Birnbaum E, Margolin D, Larson D, Marcello P, Posner M, Read T, Monson J, Wren SM, Pisters PW, Nelson H. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA. 2015;314:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 823] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 11. | Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Büchler MW. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 1029] [Article Influence: 68.6] [Reference Citation Analysis (4)] |
| 12. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24750] [Article Influence: 1178.6] [Reference Citation Analysis (0)] |
| 13. | Denonvilliers C. Anatomie du perinee. Bull Soc Anat Paris. 1836;10:105-107. |
| 14. | Milley PS, Nichols DH. A correlative investigation of the human rectovaginal septum. Anat Rec. 1969;163:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 65] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Zhang M, Kaw A, Chapuis PH, Bokey L. Does Denonvilliers' fascia exist in women? Am J Obstet Gynecol. 2016;214:663-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Farrell SA, Dempsey T, Geldenhuys L. Histologic examination of "fascia" used in colporrhaphy. Obstet Gynecol. 2001;98:794-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Kleeman SD, Westermann C, Karram MM. Rectoceles and the anatomy of the posteriorvaginal wall: revisited. Am J Obstet Gynecol. 2005;193:2050-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Fang J, Zheng Z, Wei H. Reconsideration of the Anterior Surgical Plane of Total Mesorectal Excision for Rectal Cancer. Dis Colon Rectum. 2019;62:639-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Killingback M, Barron P, Dent OF. Local recurrence after curative resection of cancer of the rectum without total mesorectal excision. Dis Colon Rectum. 2001;44:473-83; discussion 483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |