Published online May 21, 2023. doi: 10.3748/wjg.v29.i19.2979
Peer-review started: February 23, 2023
First decision: March 23, 2023
Revised: April 2, 2023
Accepted: April 25, 2023
Article in press: April 25, 2023
Published online: May 21, 2023
Processing time: 82 Days and 5.3 Hours
Low anterior resection syndrome (LARS) severely impairs patient postoperative quality of life, especially major LARS. However, there are few tools that can accurately predict major LARS in clinical practice.
To develop a machine learning model using preoperative and intraoperative factors for predicting major LARS following laparoscopic surgery of rectal cancer in Chinese populations.
Clinical data and follow-up information of patients who received laparoscopic anterior resection for rectal cancer from two medical centers (one discovery cohort and one external validation cohort) were included in this retrospective study. For the discovery cohort, the machine learning prediction algorithms were developed and internally validated. In the external validation cohort, we evaluated the trained model using various performance metrics. Further, the clinical utility of the model was tested by decision curve analysis.
Overall, 1651 patients were included in the present study. Anastomotic height, neoadjuvant therapy, diverting stoma, body mass index, clinical stage, specimen length, tumor size, and age were the risk factors associated with major LARS. They were used to construct the machine learning model to predict major LARS. The trained random forest (RF) model performed with an area under the curve of 0.852 and a sensitivity of 0.795 (95%CI: 0.681-0.877), a specificity of 0.758 (95%CI: 0.671-0.828), and Brier score of 0.166 in the external validation set. Compared to the previous preoperative LARS score model, the current model exhibited superior predictive performance in predicting major LARS in our cohort (accuracy of 0.772 for the RF model vs 0.355 for the preoperative LARS score model).
We developed and validated a robust tool for predicting major LARS. This model could potentially be used in the clinic to identify patients with a high risk of developing major LARS and then improve the quality of life.
Core Tip: We developed and externally validated a machine learning-based prediction model that integrated preoperative and intraoperative risk factors as input features and showed satisfactory predictive performance in Chinese patients. According to the decision curve analysis, patients with major low anterior resection syndrome (LARS) would have a net benefit superior to “treat all” or “treat none” with a range of threshold probabilities by using the model. This study provides a new tool for predicting major LARS, which can potentially be used for rectal cancer patients to acquire early postoperative consultation and strengthen self-management to improve their quality of life.
- Citation: Wang Z, Shao SL, Liu L, Lu QY, Mu L, Qin JC. Machine learning model for prediction of low anterior resection syndrome following laparoscopic anterior resection of rectal cancer: A multicenter study. World J Gastroenterol 2023; 29(19): 2979-2991
- URL: https://www.wjgnet.com/1007-9327/full/v29/i19/2979.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i19.2979
With advances in surgical techniques and the introduction of a multidisciplinary approach, the sphincter-saving procedure for rectal cancer has increased[1], with up to 50%-80% of rectal cancer patients undergoing this procedure[2] compared with only 25% before the circular stapling device was widely used[3]. However, low anterior resection syndrome (LARS), a postoperative complication that seriously impairs patient quality of life, has also increased[4,5], and 70%-90% of these patients undergoing sphincter-saving procedures have developed LARS[2]. The majority of LARS may go into remission within a variable interval of 6-18 mo following surgery[6,7]. However, beyond this point further improvements may be impossible, and the complication may become irreversible. It is reported that approximately 40% of patients with major LARS remain ‘toilet dependent,’ which results in a low quality of life[8,9].
Early management of major LARS, such as conservative drugs, transanal or transtomal irrigation, pelvic floor rehabilitation, biofeedback, and sacral nerve stimulation, can improve LARS symptoms[10-14]. Therefore, it is important to identify the patients who are at a high risk of developing major LARS after surgery. A recent study established a model based on preoperative risk factors to predict a LARS score for improving patient preoperative education and counseling[15]. However, it failed to achieve an accurate prediction when it was applied to other populations[16]. Furthermore, certain intraoperative factors that were previously reported as important contributors to LARS were not included in this aforementioned model[17,18].
Due to better vision and less surgical trauma[19], laparoscopic surgery has improved the post
Artificial intelligence (AI) is an innovative modeling technology and has produced promising results; our previous studies have shown that AI algorithms allow for good discrimination of anastomotic leakage and would be helpful in assisting surgeons’ decision-making[20,21]. Therefore, the present study aimed to develop a machine learning model based on AI technology using preoperative and intraoperative factors for predicting major LARS following laparoscopic surgery of rectal cancer in Chinese populations. This model was created to guide early postoperative management of medical intervention and improve patient postoperative consultation and quality of life.
The present study included a discovery cohort and an external validation cohort. To develop the machine learning model, clinical data of 2120 patients with rectal cancer who received laparoscopic anterior resection in the Department of Gastrointestinal Surgery, Tongji Hospital, Huazhong University of Science and Technology from January 1, 2012 to December 31, 2020 were reviewed and collected. For external validation, data from 289 patients from the Central Hospital of Enshi Tujia and Miao Autonomous Prefecture affiliated to Wuhan University between January 1, 2012 and December 31, 2020 were collected with the same criteria. The present study was performed according to the guidelines of the Declaration of Helsinki and approved by the ethics committees of Tongji Hospital, Huazhong University of Science and Technology and The Central Hospital of Enshi Tujia and Miao Autonomous Prefecture. The requirement for informed consent was waived due to the retrospective nature of the study.
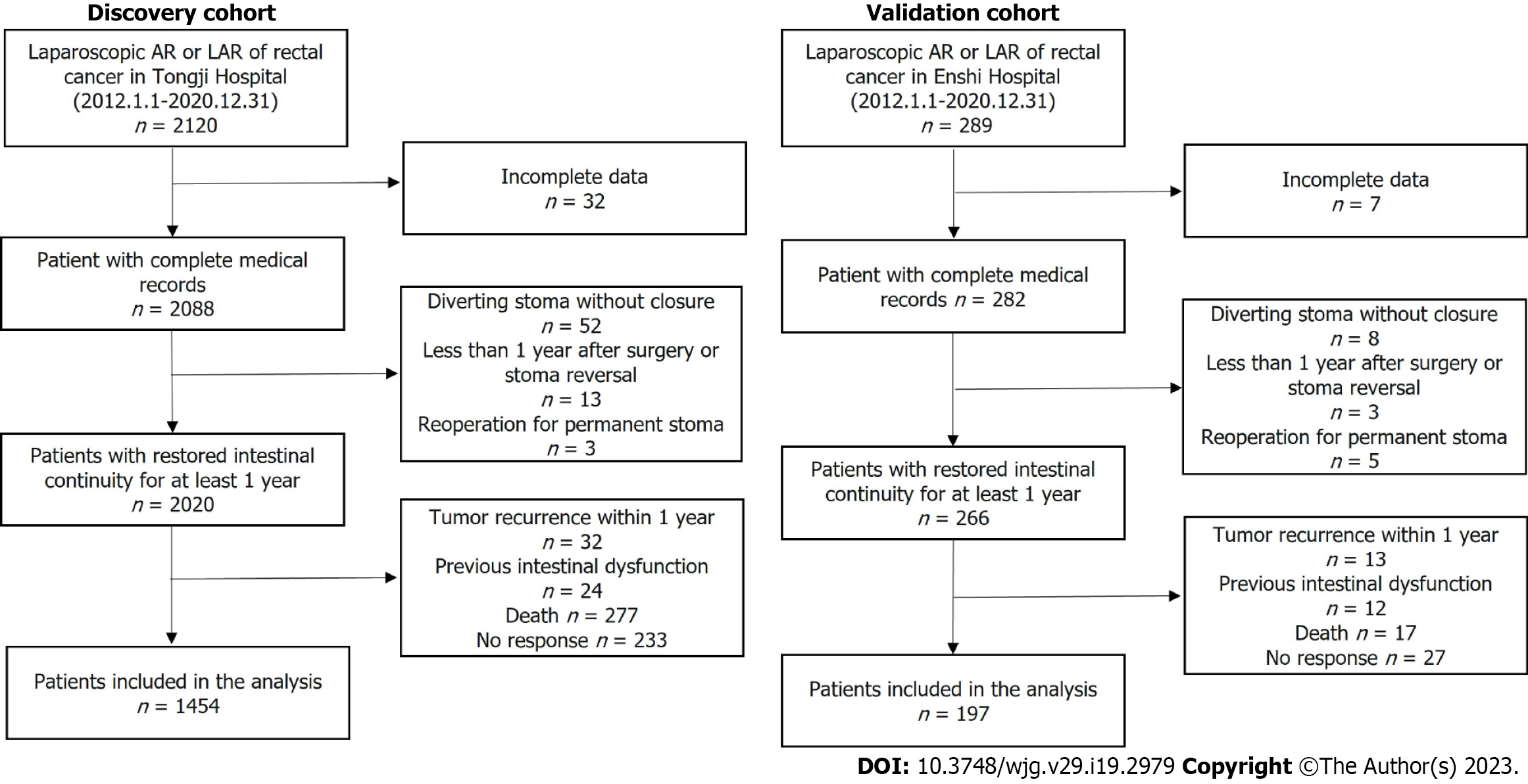
The inclusion criteria were as follows: (1) Age ≥ 18; (2) Primary rectal adenocarcinoma located 0-15 cm from the anal verge; and (3) Patients without communication difficulties. The exclusion criteria were as follows: (1) Patients who had their diverting stoma open; (2) Less than 1 year after laparoscopic anterior resection or after stoma reversal; (3) Patients with a history of abnormal bowel function, including drug-induced diarrhea, a chronic history of constipation, irritable bowel syndrome, and a history of pelvic injury; (4) Patients with local recurrence within 1 year after surgery; and (5) Missing data, death, or lost to follow-up.
In order to develop the early postoperative major LARS prediction model, only the clinical preoperative and intraoperative variables of each patient were included. The variables were as following: age at surgery; sex; body mass index (BMI); hypertension; diabetes; previous abdominal surgery; neoadjuvant therapy; American Society of Anesthesiologists (ASA) classification; tumor size (cm); clinical stages; anastomotic height (cm); diverting stoma; and specimen length (cm). Two authors independently completed the collection and collation of clinical data, and conflicting data were documented and confirmed by a final discussion. Anastomotic height was defined as the distance between anastomosis and anal verge measured using digital rectal examination, computed tomography, or magnetic resonance imaging. Specimen length was defined as the length of the bowel removed during surgery.
The Chinese version of the LARS score system was used to evaluate postoperative intestinal function[22], which is described by five questions concerning intestinal function. Each response was weighted and given a score according to the severity of the patient’s symptoms. Scores of 0-20 indicated no LARS, 21-29 indicated minor LARS, and 30-42 indicated major LARS. All the participants were followed up by telephone, short message service, and outpatient or inpatient visits using a LARS score questionnaire from November 1, 2021 to May 1, 2022. LARS scores of each participant at 1 year after anterior resection or after stoma reversal were obtained. To highlight major LARS, patients were classified into two groups according to LARS score, one with major LARS and another with no or minor LARS.
Excessive variables could lead to adverse predictions and be inconvenient in an application. The Boruta algorithm can address the minimal optimization problem of multidimensional clinical features in feature selection[23]. Thus, feature selection was conducted using the Boruta algorithm. The algorithm can screen out all the variables associated with the ground truth. The importance of the features was quantified by repeated iterations based on shadow feature creation, and some weakly correlated features were removed. Finally, the selected features, combined with clinical experience, were used as predictors. R software and Boruta packages (7.0.0) were used for feature selection (R version 4.1.2[2021-11-01]).
The one-in-ten rule is a generally accepted rule for estimating the minimum sample size[24]. According to at least ten events per variable, at least 325 to 667 patients were required in the discovery cohort for the 13 predictor variables, with an estimated event (major LARS) rate of 30%-50% and a lost follow-up rate of 20%-35%.
In the present study, four prevailing machine learning algorithms, including logistic regression (LR), random forest (RF), support vector machine (SVM), and extreme gradient boosting (XGBoost), were employed to develop the predictive models. Machine learning algorithms based on AI can overcome the limitations of traditional linear models by combining clinical nonlinear features. The participants from Tongji Hospital were randomly divided into a training set and a testing set at a ratio of 8:2. To gain high-performance models, hyperparameter adjustment was adopted using a grid search approach. To balance sensitivity and specificity, the optimal Youden index (cutoff value) was calculated via maximizing the value of sensitivity + specificity - 1[25]. The area under the curve (AUC) and Brier scores, which represent the discrimination and calibration power of the prediction model, were calculated. The Brier score measures the difference between the predicted probability and the ground truth[26], and a value of the Brier Score closer to 0 indicates a better calibration. In addition, to assess the clinical utility of the prediction model, decision curve analysis was used, which can determine whether patients benefit from using predictive models in clinical practice[27]. All machine learning algorithms were implemented using Python (version 3.9.7) with the scikit-learn (version 0.24.2) package.
The continuous variables were presented as mean ± SD and categorical variables as the count (%). A one-way analysis of variance with post hoc contrasts by the student-Newman-Keuls test was used to compare the differences between the continuous variables. For categorical variables, as appropriate, χ2 or Fisher’s exact test was used. All P values were reported as two-tailed, and P < 0.05 was considered as statistical significance. 95%CI for the AUC, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of the four models were calculated using IBM SPSS Statistics 20.0 (IBM Corp, Armonk, NY, United States) or Vassar Stats (online tool, http://vassarstats.net/index.html).
Figure 1 presents the patient flow chart. A total of 1651 eligible cases were included, with 1163 subjects included in the training set, 291 subjects included in the testing set, and another 197 subjects in the external validation set. Comparisons between the training, testing, and external validation sets are presented in Table 1. The mean age of the 1163 patients in the training set was 57.6 years, and 59.7% were males. For the testing and the external validation sets, the mean age was 57.6 and 59.7 years, and 56.0% and 53.8% were males, respectively. Major LARS was observed in 37.2% of patients in the training set, 35.1% in the testing set, and 37.1% in the external validation set.
| Variables | Training cohort, n = 1163 | Testing cohort, n = 291 | Validation cohort, n = 197 | P value |
| Age, yr | 57.60 ± 10.83 | 57.56 ± 11.23 | 59.72 ± 9.58 | 0.034 |
| Male | 694 (59.67) | 163 (56.01) | 106 (53.81) | 0.206 |
| BMI, kg/m2 | 22.79 ± 2.92 | 22.89 ± 2.75 | 22.61 ± 4.02 | 0.382 |
| Neoadjuvant | 67 (5.76) | 19 (6.53) | 9 (4.57) | 0.659 |
| Hypertension | 254 (21.84) | 66 (22.68) | 43 (21.83) | 0.952 |
| Diabetes | 83 (7.14) | 26 (8.93) | 9 (4.57) | 0.185 |
| Previous abdominal surgery | 141 (12.12) | 45 (15.46) | 22 (11.17) | 0.250 |
| ASA | < 0.001 | |||
| 1 | 178 (15.31) | 42 (14.43) | 55 (27.92) | |
| 2 | 893 (76.78) | 218 (74.91) | 89 (45.12) | |
| 3 | 90 (7.74) | 30 (10.31) | 50 (25.38) | |
| 4 | 2 (0.17) | 1 (0.34) | 3 (1.52) | |
| Anastomotic height, cm | 4.82 ± 2.37 | 4.57 ± 2.14 | 4.77 ± 2.56 | 0.298 |
| Specimen length, cm | 10.99 ± 3.01 | 10.88 ± 3.11 | 15.21 ± 4.49 | < 0.001 |
| Diverting ileostomy | 315 (27.09) | 81 (27.84) | 35 (17.77) | 0.017 |
| Tumor size, cm | 3.60 ± 1.29 | 3.53 ± 1.25 | 3.89 ± 1.35 | 0.546 |
| Stage | < 0.001 | |||
| 1 | 354 (30.44) | 91 (31.27) | 31 (15.74) | |
| 2 | 405 (34.82) | 94 (32.30) | 108 (54.82) | |
| 3 | 404 (34.74) | 106 (36.43) | 58 (29.44) | |
| LARS | 0.800 | |||
| Minor/no | 731 (62.85) | 189 (64.95) | 124 (62.94) | |
| Major | 432 (37.15) | 102 (35.05) | 73 (37.06) |
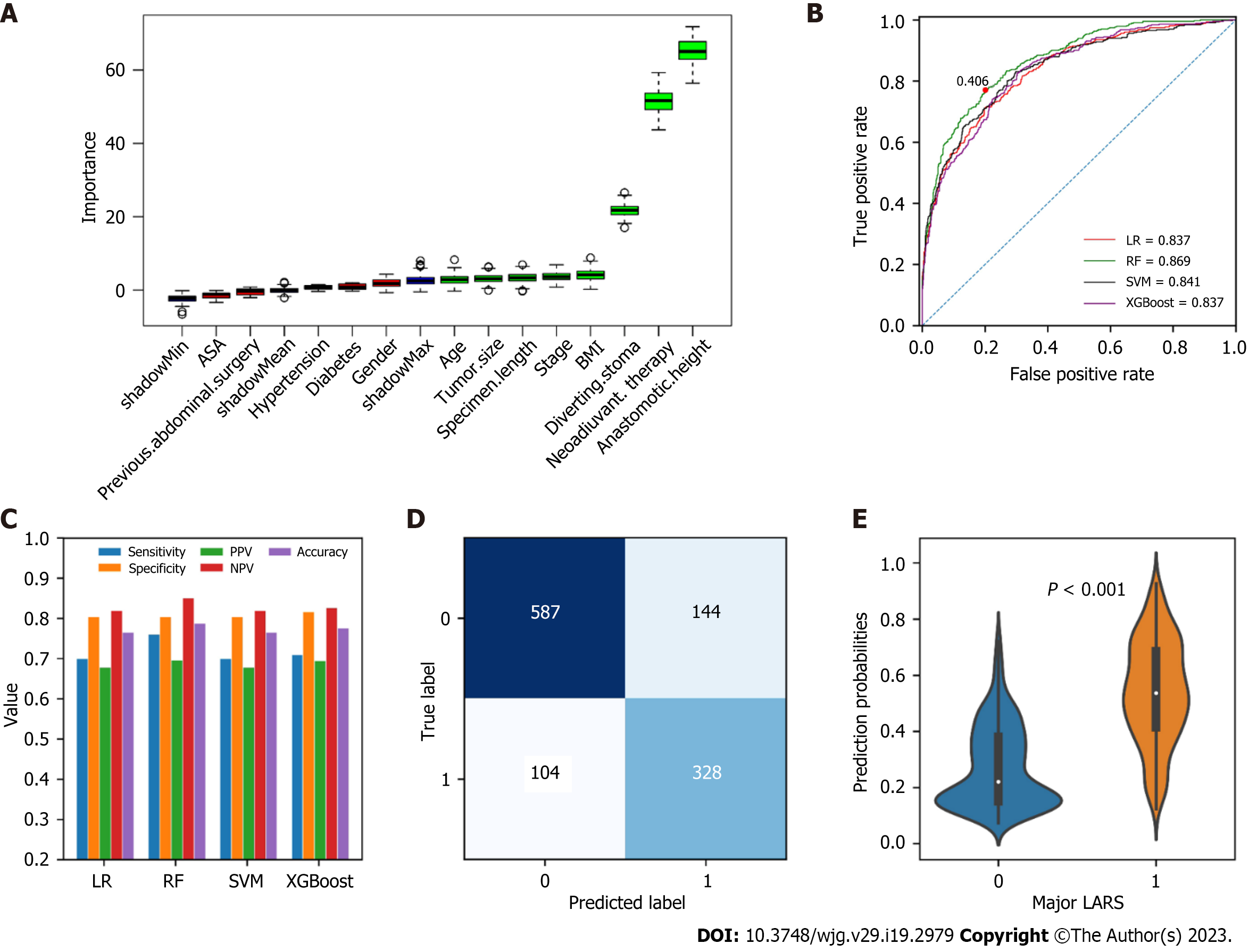
The importance of all the included variables calculated by the Boruta algorithm was shown in Figure 2A. Boruta calculates variables that are both strongly and weakly relevant to provide the best prediction accuracy. The blue boxes were shadow features automatically generated by the algorithm and were not included in the analysis. As the data indicated that anastomotic height, neoadjuvant therapy, diverting stoma, BMI, clinical stage, specimen length, tumor size, and age were selected as significantly relevant to major LARS.
The LR, RF, SVM and XGBoost algorithms were trained using the eight strongly related variables, and the AUCs, sensitivities, specificities, PPVs, NPVs, and accuracies were calculated (Figure 2B and C). The RF model exhibited optimal diagnostic performance (AUC = 0.869), and the optimal cutoff was 0.406. Therefore, the RF model was used for subsequent analysis. The details of the predictions generated by the RF model using the optimal threshold were shown in Figure 2D. Additionally, the predicted probabilities for major LARS were significantly relevant to the ground truth in the training set (P < 0.001) (Figure 2E).
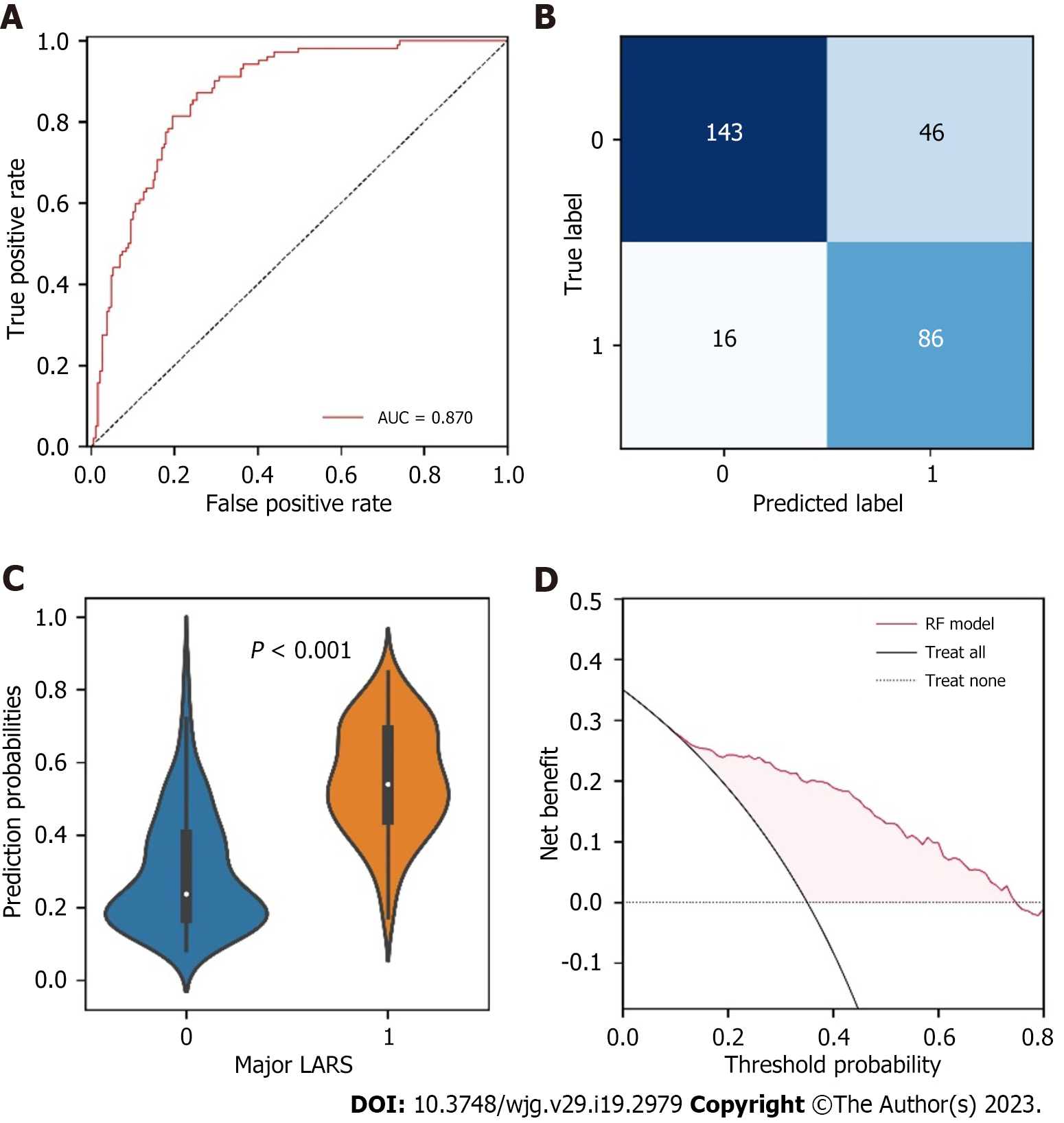
We tested the performance of the RF model in the testing set. The results demonstrated that the RF model performed with a favorable discrimination ability (AUC = 0.870, 95%CI: 0.833-0.901) (Figure 3A). The details of the predicted outcomes were presented in Figure 3B. Subsequently, the comparison of the predicted probabilities between the major LARS and no/minor LARS groups was conducted, and significant differences were observed (Figure 3C). Furthermore, a decision curve was plotted to evaluate whether using the RF model in the clinic would do better than harm[28]. According to the decision curve analysis, patients with major LARS would have a net benefit superior to “treat all” or “treat none” with a range of threshold probability in approximately 20%-75% (Figure 3D).
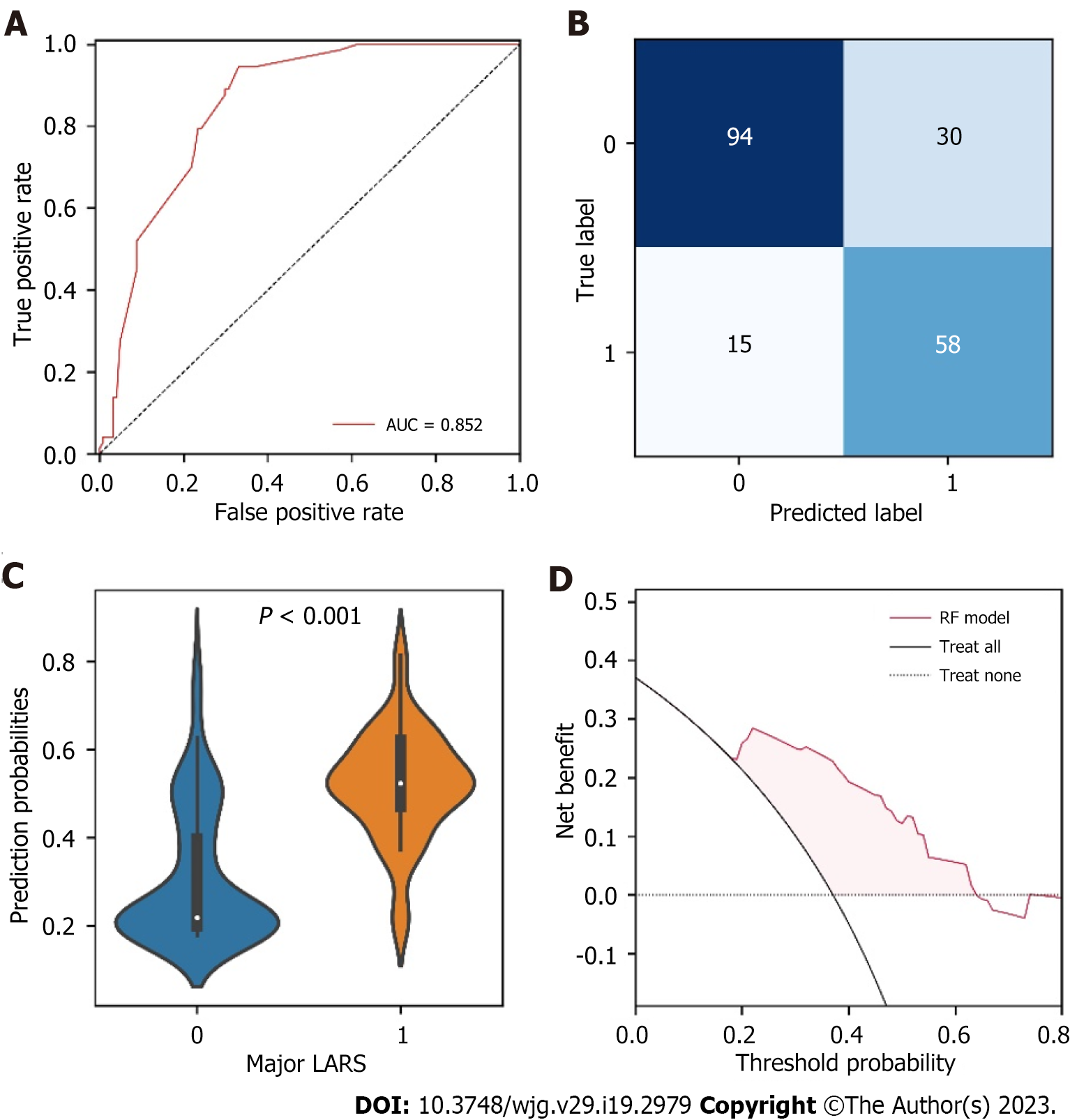
To assess the generalization capability of the RF model, an external validation based on 197 patients from another independent center was performed. The RF model identified patients with major LARS with an AUC of 0.852 (95%CI: 0.820-0.890) (Figure 4A). The confusion matrix presented the classification results generated by the RF model for identifying major LARS in the external validation set (Figure 4B). Figure 4C showed that the probabilities generated by the RF model for major LARS were significantly higher than those of no/minor LARS, suggesting that the predicted probabilities were significantly associated with the ground truth in the external validation set. Decision curve analysis also showed that patients would derive clinical benefits in a range of threshold probabilities (Figure 4D).
To assess the performance and calibration degree of the RF model in both the testing set and the external validation set, six performance metrics such as sensitivity, specificity, PPV, NPV, accuracy, and Brier score were applied. Their results calculated based on the optimal Youden index (cutoff) were summarized in Table 2. These results suggested that the RF model was determined to be capable and reliable in predicting major LARS, with satisfactory Brier score of 0.152 and 0.166 and accuracy of 0.787 and 0.772, in both the testing set and the external validation set, respectively. In addition, to highlight the advantages of the RF model, the sensitivity, specificity, PPV, NPV, and accuracy of the preoperative LARS score (POLARS) model were calculated in both our testing set and external validation set. Taken together, these values demonstrated that the performance of the RF model surpassed that of the POLARS score model, as shown in Table 3.
| Indicator (95%CI) | RF | |
| Testing set, n = 291 | Validation set, n = 197 | |
| Sensitivity | 0.843 (0.755-0.905) | 0.795 (0.681-0.877) |
| Specificity | 0.757 (0.688-0.815) | 0.758 (0.671-0.828) |
| PPV | 0.652 (0.563-0.731) | 0.659 (0.549-0.755) |
| NPV | 0.899 (0.839-0.940) | 0.862 (0.780-0.918) |
| Accuracy | 0.787 (0.736-0.830) | 0.772 (0.708-0.825) |
| Brier score | 0.152 | 0.166 |
| Indicators (95%CI) | Testing set, n = 291 | P value | Validation set, n = 197 | P value |
| Sensitivity | 0.931 (0.859-0.970) | 0.046 | 0.836 (0.727-0.909) | 0.522 |
| Specificity | 0.079 (0.047-0.130) | < 0.001 | 0.073 (0.036-0.137) | < 0.001 |
| PPV | 0.353 (0.297-0.414) | < 0.001 | 0.347 (0.278-0.422) | < 0.001 |
| NPV | 0.682 (0.451-0.853) | 0.004 | 0.429 (0.226-0.656) | < 0.001 |
| Accuracy | 0.378 (0.323-0.437) | < 0.001 | 0.355 (0.289-0.427) | < 0.001 |
LARS is the most common complication following rectal cancer surgery. It is a severe complication and seriously impairs patient quality of life[1]. A meta-analysis based on 11 studies indicated that the morbidity of major LARS was as high as 41% (95%CI: 34-48)[5]. Fortunately, surgeons are now paying more and more attention to the functional consequences of cancer treatment and the quality of life[1,4]. LARS is a time-dependent syndrome, and the symptoms of some patients with LARS are relieved partly or completely 1 year or more after surgery. However, the symptoms in approximately 40% of patients remain stable and cannot be further improved[6,9,29].
Due to the variable symptom spectrum of LARS, ranging from incontinence for gas and liquid fecal matter to evacuation dysfunctions, the complex etiology, and unknown pathophysiology, there is no standard treatment available at present[30]. However, if patients with a high-risk major LARS can be treated with a conservative method (e.g., pelvic floor rehabilitation, transanal irrigation), minimally invasive therapies (e.g., biofeedback therapy, sacral nerve stimulation), or multimodal treatments during the period of the first year after surgery, their intestinal dysfunction may be significantly improved[9]. Consequently, the negative impact of LARS on their quality of life could be minimized. In addition, since major LARS may counteract the relative benefits of anal sphincter-preserving surgery, the accurate prediction of major LARS may be helpful for patients and surgeons when deciding on temporary ileostomy, permanent colostomy, or sphincter-preserving surgery for low rectal cancer[31-33]. Therefore, it is crucial to perform risk stratification of rectal surgery cases to identify patients with a high risk of major LARS and to highlight patients who may require additional postoperative support.
Battersby et al[15] developed and validated the POLARS score for restorative sphincter-sparing surgery for rectal cancer to predict intestinal dysfunction. The POLARS score includes six risk factors, such as age at surgery, sex, tumor height, preoperative radiotherapy, total/partial mesorectal excision, and the presence of stoma, as predictors. The model performs with moderate discriminative accuracy with Harrell’s C statistic of 0.615 and 0.625 in their two datasets. Essangri et al[16] reported that the POLARS score was questionable, and it failed to successfully validate the model in another population. This previous study implied that the model predictions may be dependent on patient background, including treatment strategies and physical, lifestyle, and dietary habit differences. In the present study, all participants were Chinese and underwent laparoscopic sphincter-sparing surgery for rectal cancer without splenic flexure mobilization. Several previous studies have pointed out that routine splenic flexure mobilization is not necessary for anterior resection of rectal cancer[34-36]. Instead, no splenic flexure mobilization would result in a shorter operation time and lower morbidity of postoperative complications associated with intestinal function, such as anastomotic leakage[37]. Moreover, to date, there is no machine learning model for predicting major LARS in Asian patients undergoing laparoscopic anterior resection based on a multicenter study.
In the present study, four machine learning algorithms were used to develop the machine learning model for major LARS prediction. These data suggested that the RF model performed with an optimal AUC in the training set. As expected, the RF model also achieved favorable predictions when it was tested in the testing and external validation sets. To the best of our knowledge, this is the first multicentric study to develop a machine-learning model for predicting major LARS in Asian patients undergoing laparoscopic anterior resection of rectal cancer. More importantly, the model performed with a satisfactory prediction in an independent medical center (AUC = 0.852; 95%CI: 0.820-0.890). Moreover, compared with the POLARS score, the RF model achieved superior performance in predicting major LARS in our cohort (accuracy of 0.772 for the RF model vs 0.355 for the POLARS score). In addition, the decision curve analysis demonstrated the net benefit (benefit minus risk) by using the model for patients diagnosed with major LARS within a range of threshold probabilities.
Although the explicit pathophysiological mechanism of LARS is still unclear, numerous studies[38-40] agree that intestinal dysfunction in patients with rectal cancer who received restorative sphincter-sparing surgery is the result of a combination of multiple pathophysiological mechanisms. These include loss of rectal storage function, autonomic denervation, enhanced colonic movement, rectal-anus sensitivity reduction, anal resting pressure reduction, and diverting colitis[38]. Certain factors directly or indirectly related to these pathophysiological changes have been reported as important variables associated with LARS, such as low anastomosis, neoadjuvant therapy, postoperative chemoradiotherapy, anastomotic leakage, diverting stoma, and the time interval from the creation of diverting stoma to closure[5,18,41,42]. In order to identify major LARS in the early postoperative period, some postoperative factors were not included, such as chemoradiotherapy and the time interval from creating diverting stoma to its closure. Among the included factors, low anastomosis and neoadjuvant therapy have been unanimously considered as important predictors for major LARS[5,43]. For example, Filips et al[44] reported that LARS was negatively correlated with the distance from anastomosis to the anal verge (OR: -1.145, 95%CI: -2.149 to -1.141, P = 0.026). In the present study, our data also indicated that the anastomotic height was the most important factor in the development of major LARS. In addition, the specimen length was selected as a predictor for major LARS in the present study, and it may be caused by greater surgical trauma.
As with any retrospective observational study, the present study had some uncontrollable limitations. First, the model is based on the Chinese population and does not necessarily reflect the worldwide target population. Its generalizability needs to be further tested. Second, the influence of a patient’s socioeconomic and cultural background, self-management ability, and social support are difficult to control. Third, the data reflecting anal sphincter injury and its severity during surgery cannot be evaluated. Finally, the LARS score may be affected by a variety of biases, such as patient selective memory, exaggeration, or understatement. To overcome these limitations, a prospective study is proposed to assess the predictive ability of the model.
In the present study, a machine learning model based on preoperative and intraoperative risk factors for predicting LARS was developed. The model may be helpful for clinical medical staff to identify patients at an early stage with a high risk of developing major LARS within 1 year following laparoscopic surgery for rectal cancer. Moreover, it can potentially be used for patients to acquire early postoperative consultation and strengthen self-management to improve patient quality of life.
Low anterior resection syndrome (LARS) severely impairs patient postoperative quality of life, especially major LARS. However, there are few tools that can accurately predict major LARS in clinical practice.
To stratify patients with LARS and predict patients at high risk of developing major LARS, improve patient counseling, and highlight patients who may need additional support after surgery.
The study aimed to identify the risk factors associated with major LARS and develop a prediction model that helps improve patient counseling and highlight patients who may need additional support after surgery.
Clinical data and follow-up information of patients from two medical centers (one discovery cohort and one external validation cohort) were analyzed to identify independent factors associated with major LARS. For the discovery cohort, the machine learning prediction algorithms were developed and internally validated. In the external validation cohort, we evaluated the trained model using various performance metrics. Further, the clinical utility of the model was tested by decision curve analysis.
Eight factors, such as anastomotic height, neoadjuvant therapy, diverting stoma, body mass index, clinical stage, specimen length, tumor size, and age, were selected as significantly relevant to major LARS. A machine learning-based prediction model that integrated eight risk factors as input features was developed, externally validated, and demonstrated an acceptable predictive performance.
We have developed and validated a robust tool for predicting major LARS. This model could potentially be used in the clinic to identify patients with a high risk of developing major LARS and then improve their quality of life.
A prospective study including more medical centers is proposed to assess the model’s predictive ability.
I would like to thank all the medical staff of the Department of Gastrointestinal Surgery in Tongji Hospital and Enshi Central Hospital for providing convenient conditions for implementing this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Calabro F, Italy; Muneer A, Malaysia S-Editor: Zhang H L-Editor: Filipodia P-Editor: Chen YX
| 1. | Pieniowski EHA, Palmer GJ, Juul T, Lagergren P, Johar A, Emmertsen KJ, Nordenvall C, Abraham-Nordling M. Low Anterior Resection Syndrome and Quality of Life After Sphincter-Sparing Rectal Cancer Surgery: A Long-term Longitudinal Follow-up. Dis Colon Rectum. 2019;62:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 2. | Bryant CL, Lunniss PJ, Knowles CH, Thaha MA, Chan CL. Anterior resection syndrome. Lancet Oncol. 2012;13:e403-e408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 433] [Article Influence: 33.3] [Reference Citation Analysis (1)] |
| 3. | Ståhle E, Påhlman L, Enblad P. Double stapling technique in the management of rectal tumours. Acta Chir Scand. 1986;152:743-747. [PubMed] |
| 4. | Pieniowski EHA, Nordenvall C, Palmer G, Johar A, Tumlin Ekelund S, Lagergren P, Abraham-Nordling M. Prevalence of low anterior resection syndrome and impact on quality of life after rectal cancer surgery: population-based study. BJS Open. 2020;4:935-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 5. | Croese AD, Lonie JM, Trollope AF, Vangaveti VN, Ho YH. A meta-analysis of the prevalence of Low Anterior Resection Syndrome and systematic review of risk factors. Int J Surg. 2018;56:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 6. | Nicotera A, Falletto E, Arezzo A, Mistrangelo M, Passera R, Morino M. Risk factors for Low Anterior Resection Syndrome (LARS) in patients undergoing laparoscopic surgery for rectal cancer. Surg Endosc. 2022;36:6059-6066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Varghese C, Wells CI, O'Grady G, Christensen P, Bissett IP, Keane C; on behalf of the Longitudinal LARS Group. The Longitudinal Course of Low-anterior Resection Syndrome: An individual Patient Meta-analysis. Ann Surg. 2022;276:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 8. | Juul T, Ahlberg M, Biondo S, Espin E, Jimenez LM, Matzel KE, Palmer GJ, Sauermann A, Trenti L, Zhang W, Laurberg S, Christensen P. Low anterior resection syndrome and quality of life: an international multicenter study. Dis Colon Rectum. 2014;57:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 9. | Sturiale A, Martellucci J, Zurli L, Vaccaro C, Brusciano L, Limongelli P, Docimo L, Valeri A. Long-term functional follow-up after anterior rectal resection for cancer. Int J Colorectal Dis. 2017;32:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 10. | van der Heijden JAG, Kalkdijk-Dijkstra AJ, Pierie JPEN, van Westreenen HL, Broens PMA, Klarenbeek BR; FORCE trial group. Pelvic Floor Rehabilitation After Rectal Cancer Surgery: A Multicenter Randomized Clinical Trial (FORCE Trial). Ann Surg. 2022;276:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Li H, Guo C, Gao J, Yao H. Effectiveness of Biofeedback Therapy in Patients with Bowel Dysfunction Following Rectal Cancer Surgery: A Systemic Review with Meta-Analysis. Ther Clin Risk Manag. 2022;18:71-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Embleton R, Henderson M. Using transanal irrigation in the management of low anterior resection syndrome: a service audit. Br J Nurs. 2021;30:1226-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Itagaki R, Koda K, Yamazaki M, Shuto K, Kosugi C, Hirano A, Arimitsu H, Shiragami R, Yoshimura Y, Suzuki M. Serotonin (5-HT3) receptor antagonists for the reduction of symptoms of low anterior resection syndrome. Clin Exp Gastroenterol. 2014;7:47-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | De Meyere C, Nuytens F, Parmentier I, D'Hondt M. Five-year single center experience of sacral neuromodulation for isolated fecal incontinence or fecal incontinence combined with low anterior resection syndrome. Tech Coloproctol. 2020;24:947-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Battersby NJ, Bouliotis G, Emmertsen KJ, Juul T, Glynne-Jones R, Branagan G, Christensen P, Laurberg S, Moran BJ; UK and Danish LARS Study Groups. Development and external validation of a nomogram and online tool to predict bowel dysfunction following restorative rectal cancer resection: the POLARS score. Gut. 2018;67:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 16. | Essangri H, Majbar MA, Benkabbou A, Belkhadir Z, Amrani L, Mohsine R, Souadka A. Do we have enough Foreknowledge to predict the low anterior resection syndrome (LARS) score preoperatively? Colorectal Dis. 2020;22:1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Miacci FLC, Guetter CR, Moreira PH, Sartor MC, Savio MC, Baldin Júnior A, Nóbrega NL. Predictive factors of low anterior resection syndrome following anterior resection of the rectum. Rev Col Bras Cir. 2020;46:e20192361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Ekkarat P, Boonpipattanapong T, Tantiphlachiva K, Sangkhathat S. Factors determining low anterior resection syndrome after rectal cancer resection: A study in Thai patients. Asian J Surg. 2016;39:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Lourenco T, Murray A, Grant A, McKinley A, Krukowski Z, Vale L. Laparoscopic surgery for colorectal cancer: safe and effective? Surg Endosc. 2008;22:1146-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Shao S, Liu L, Zhao Y, Mu L, Lu Q, Qin J. Application of Machine Learning for Predicting Anastomotic Leakage in Patients with Gastric Adenocarcinoma Who Received Total or Proximal Gastrectomy. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Shao S, Zhao Y, Lu Q, Liu L, Mu L, Qin J. Artificial intelligence assists surgeons' decision-making of temporary ileostomy in patients with rectal cancer who have received anterior resection. Eur J Surg Oncol. 2023;49:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 22. | Hou XT, Pang D, Lu Q, Yang P, Jin SL, Zhou YJ, Tian SH. Validation of the Chinese version of the low anterior resection syndrome score for measuring bowel dysfunction after sphincter-preserving surgery among rectal cancer patients. Eur J Oncol Nurs. 2015;19:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Kursa MB, Rudnicki WR. Feature selection with the Boruta Package. J Stat Softw. 2010;36:1-13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1713] [Cited by in RCA: 1731] [Article Influence: 115.4] [Reference Citation Analysis (0)] |
| 24. | Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4758] [Cited by in RCA: 5369] [Article Influence: 185.1] [Reference Citation Analysis (0)] |
| 25. | Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005;47:458-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1260] [Cited by in RCA: 1741] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 26. | Roulston MS. Performance targets and the Brier score. Meteorological Applications. 2007;14:185-194. [DOI] [Full Text] |
| 27. | Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3515] [Cited by in RCA: 3464] [Article Influence: 182.3] [Reference Citation Analysis (1)] |
| 28. | Vickers AJ, van Calster B, Steyerberg EW. A simple, step-by-step guide to interpreting decision curve analysis. Diagn Progn Res. 2019;3:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 578] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 29. | Afshari K, Smedh K, Wagner P, Chabok A, Nikberg M. Risk factors for developing anorectal dysfunction after anterior resection. Int J Colorectal Dis. 2021;36:2697-2705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 30. | Dulskas A, Smolskas E, Kildusiene I, Samalavicius NE. Treatment possibilities for low anterior resection syndrome: a review of the literature. Int J Colorectal Dis. 2018;33:251-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 31. | Pachler J, Wille-Jørgensen P. Quality of life after rectal resection for cancer, with or without permanent colostomy. Cochrane Database Syst Rev. 2012;12:CD004323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 32. | Cornish JA, Tilney HS, Heriot AG, Lavery IC, Fazio VW, Tekkis PP. A meta-analysis of quality of life for abdominoperineal excision of rectum versus anterior resection for rectal cancer. Ann Surg Oncol. 2007;14:2056-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Trenti L, Galvez A, Biondo S, Solis A, Vallribera-Valls F, Espin-Basany E, Garcia-Granero A, Kreisler E. Quality of life and anterior resection syndrome after surgery for mid to low rectal cancer: A cross-sectional study. Eur J Surg Oncol. 2018;44:1031-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 34. | Park JS, Kang SB, Kim DW, Lee KH, Kim YH. Laparoscopic versus open resection without splenic flexure mobilization for the treatment of rectum and sigmoid cancer: a study from a single institution that selectively used splenic flexure mobilization. Surg Laparosc Endosc Percutan Tech. 2009;19:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Kim J, Choi DJ, Kim SH. Laparoscopic rectal resection without splenic flexure mobilization: a prospective study assessing anastomotic safety. Hepatogastroenterology. 2009;56:1354-1358. [PubMed] |
| 36. | Tian C, Li H, Meng WJ. Should splenic flexure be routinely mobilized during laparoscopic low anterior resection for rectal cancer? Surg Laparosc Endosc Percutan Tech. 2014;24:283-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Brennan DJ, Moynagh M, Brannigan AE, Gleeson F, Rowland M, O'Connell PR. Routine mobilization of the splenic flexure is not necessary during anterior resection for rectal cancer. Dis Colon Rectum. 2007;50:302-7; discussion 307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Buzatti KCLR, Petroianu A. Pathophysiological aspects of the low anterior resection syndrome for treatment of rectal cancer. Rev Col Bras Cir. 2017;44:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Ziv Y, Zbar A, Bar-Shavit Y, Igov I. Low anterior resection syndrome (LARS): cause and effect and reconstructive considerations. Tech Coloproctol. 2013;17:151-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 40. | van der Heijden JAG, van Heinsbergen M, Thomas G, Caers F, Slooter GD, Maaskant-Braat AJG. Implementation of a Postoperative Screening and Treatment Guidance for the Low Anterior Resection Syndrome: Preliminary Results. Dis Colon Rectum. 2019;62:1033-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 41. | Hughes DL, Cornish J, Morris C; LARRIS Trial Management Group. Functional outcome following rectal surgery-predisposing factors for low anterior resection syndrome. Int J Colorectal Dis. 2017;32:691-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 42. | Vogel I, Reeves N, Tanis PJ, Bemelman WA, Torkington J, Hompes R, Cornish JA. Impact of a defunctioning ileostomy and time to stoma closure on bowel function after low anterior resection for rectal cancer: a systematic review and meta-analysis. Tech Coloproctol. 2021;25:751-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 43. | Battersby NJ, Juul T, Christensen P, Janjua AZ, Branagan G, Emmertsen KJ, Norton C, Hughes R, Laurberg S, Moran BJ; United Kingdom Low Anterior Resection Syndrome Study Group. Predicting the Risk of Bowel-Related Quality-of-Life Impairment After Restorative Resection for Rectal Cancer: A Multicenter Cross-Sectional Study. Dis Colon Rectum. 2016;59:270-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 44. | Filips A, Haltmeier T, Kohler A, Candinas D, Brügger L, Studer P. LARS is Associated with Lower Anastomoses, but not with the Transanal Approach in Patients Undergoing Rectal Cancer Resection. World J Surg. 2021;45:873-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |