Published online May 21, 2023. doi: 10.3748/wjg.v29.i19.2950
Peer-review started: December 7, 2022
First decision: January 22, 2023
Revised: February 11, 2023
Accepted: April 25, 2023
Article in press: April 25, 2023
Published online: May 21, 2023
Processing time: 159 Days and 10.7 Hours
Helicobacter pylori (H. pylori) is a significant human pathogen that is responsible for a variety of illnesses, including mucosa-associated lymphoid tissue lymphoma, gastric cancer, peptic ulcers, and gastritis.
To investigate the frequency of H. pylori infection and its resistance patterns among Egyptian patients and to determine the influence of H. pylori virulence genetic determinants on the eradication success of 14-d triple therapy regimen.
H. pylori infections were investigated in 72 patients with gastroduodenal complications suggestive of H. pylori infection. The cagA and vacA genotypes of cultured strains were studied using polymerase chain reaction. The patients underwent 14 d of triple-therapy treatment. The treatment response was examined using histology and a rapid urease test 6 wk after therapy discontinuation.
The intention-to-treat eradication rate was 59.2% (95%CI: 48.2%–70.3%). Rates of H. pylori resistance to clarithromycin, amoxicillin, and metronidazole were 52.8%, 81.9%, and 100%, respectively. Successful eradication of H. pylori was more significantly associated with vacA s1-positive strains [adjusted odds ratio (aOR) = 0.507, 95%CI: 0.175–0.822]. A significant association was found between failed eradication rate and H. pylori strains resistant to clarithromycin (aOR = 0.204, 95%CI: –0.005 to 0.412) and amoxicillin (aOR = 0.223, 95%CI: 0.026–0.537).
This study’s low H. pylori eradication rate following 14-d triple therapy is concerning and worrying. H. pylori pan-resistance to metronidazole followed by the high resistance to ciprofloxacin, amoxicillin, and clarithromycin in this research is challenging and of great concern.
Core Tip: In this study, 72 patients with Helicobacter pylori infections were investigated. Half of the Helicobacter pylori strains had the cagA gene, and more than half of the strains were resistant to antibiotics except tetracycline and clarithromycin (CLR). However, CLR and tetracycline were effective at higher doses to achieve effective eradication by the CLR-based therapy. Most importantly, this study demonstrated that an alternative therapeutic regimen should be adopted to achieve effective infection eradication in Egypt.
- Citation: Asaad AM, El-Azab G, Abdelsameea E, Elbahr O, Kamal A, Abdel-Samiee M, Abdelfattah A, Abdallah H, Maher D, El-Refaie A, Ghanem SE, Ansari S, Awad SM. Susceptibility patterns and virulence genotypes of Helicobacter pylori affecting eradication therapy outcomes among Egyptian patients with gastroduodenal diseases. World J Gastroenterol 2023; 29(19): 2950-2960
- URL: https://www.wjgnet.com/1007-9327/full/v29/i19/2950.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i19.2950
Helicobacter pylori (H. pylori) is a prominent human pathogen and is responsible for a variety of diseases, such as mucosa-associated lymphoid tissue lymphoma, duodenal or peptic ulcer, gastritis, and gastric cancer[1,2]. As a result of its causative relationship to gastric adenocarcinoma, the World Health Organization has identified this pathogen as a class I carcinogen[3]. According to the global estimate, H. pylori infects roughly 4.4 billion people with prevalence rates ranging from 20%-90% in developed and underdeveloped nations, accordingly[2].
Pathologically, the existence of gastric mucosa inflammatory changes in areas with abundant H. pylori organisms together with the pathognomonic existence of either lymphoid aggregates and/or follicles with germinal centers and neutrophilic infiltration constitutes the definition of chronic H. pylori gastritis[2,3].
Previous epidemiological research has produced a long list of microbial virulence factors that have a crucial role in H. pylori colonization, persistence, serotype/genotype diversity, host immune responses, pathogenicity, and disease severity. These factors involve the outer inflammatory protein (oipA) gene, the cytotoxin-associated gene (cagA), the vacuolating cytotoxin gene (vacA), the babA2 adhesin gene, the epithelium gene A (iceA), and the duodenal ulcer-promoting gene (dupA)[4-7].
The variability of H. pylori strains is believed to be related to the genetic structural diversity with associated polymorphic arrangements of different virulence determinant genes[4]. For example, the vacA gene, which encodes a vacuolating toxin, is found in the vast majority of H. pylori strains and is an important virulence factor. Because of sequence variability in the middle region (m), m1 and m2 alleles, signal region (s), s1 or s2 alleles, and the intermediate region I subtypes 1 or 2, notable diversity in the vacuolating activity of different strains is found. iceA1 and iceA2 are two major alleles of the iceA gene, which is another example of microbial genetic variation[8].
Triple therapy for 14 d with proton pump inhibitors and a mixture of two antibiotics, clarithromycin (CLR) and amoxicillin (AMX) or metronidazole (MNZ) is the standard treatment for H. pylori infections[9,10]. CLR is the preferred antibiotic in areas in which resistance to this antibiotic is < 15%[10]. However, the continuous surge in antimicrobial resistance, including CLR-resistance, has been accompanied by a failure to eradicate H. pylori infections in a significant proportion of cases worldwide. The prevalence of H. pylori resistance to CLR ranges from 11.1% in Europe to 92.3% in Africa, reaching 18.9% in Asia and 29.3% in America as described in clinical reports[11-13].
Only a few studies addressing H. pylori infections, pathogenicity, and epidemiology among Egyptian patients are available. Besides, data regarding H. pylori resistance to CLR is scarce. Therefore, this research aimed to determine the H. pylori infection frequency and its resistance patterns among Egyptian patients and to determine the influence of H. pylori virulence genetic determinants on the eradication success of a 14-d triple therapy regimen.
This cross-sectional observational study was completed from August 2021 to June 2022 at the National Liver Institute (NLI), a 760-bed tertiary care hospital in Shebin El-Kom, Egypt. The research adhered to the Helsinki Declaration principles and received ethical approval from the ethics NLI research committee, No. 00308/2022. Written consent was obtained from all participants. The research followed the international principles of strengthening the reporting of observational studies in epidemiology[14]. During the research period, 86 adult cases with different dyspepsia symptoms (vomiting, epigastric, abdominal pain, and/or heartburn) and/or other symptoms indicative of H. pylori infection and likely to require H. pylori eradication therapy were enrolled in this research.
All cases provided a medical history and underwent a physical examination, a quick urease test, and inspection of the esophagus, stomach, and duodenum using an upper endoscopy.
Under topical lignocaine anesthesia, each patient underwent an upper gastrointestinal endoscopy (Olympus X Q40; Olympus Optical, Tokyo, Japan). Each patient’s antrum and stomach corpus were biopsied during the endoscopy to obtain two sets of biopsy samples. The first set of biopsies was utilized for a rapid urease test utilizing a rapid urease test kit (CLO test; Kimberly-Clark Ltd., Draper, UT, United States). Three hours later, the second set was transferred on ice to the laboratory for bacteriological culture after being packed in 3 mL of sterile normal saline. For histopathological analysis, the third specimen was immediately fixed in 10% formalin. The fourth section was added to a buffered solution (10 mmol/LTris, pH 8, 10 mmol/L ethylenediaminetetraacetic acid, and 0.5% sodium dodecyl sulfate) and then frozen at –80 °C for DNA extraction and polymerase chain reaction (PCR) assays. H. pylori was diagnosed using three techniques: (1) H. pylori culture; (2) Histopathology using hematoxylin and eosin and Giemsa staining; and (3) Rapid urease test. At baseline, a patient was considered H. pylori-positive if he had a positive culture or rapid urease test that was validated by histological features (foveolar-neutrophilic infiltration, lymphoid follicles and/or aggregates, and verified positive Giemsa stained I rods). Six weeks after the triple therapy cessation, a second gastrointestinal endoscopy was completed to determine and confirm the presence of H. pylori and whether or not the duodenal ulcer had been successfully cured. Eradication was defined as the absence of histological evidence and a negative result on the rapid urease test.
Using a tissue grinder, the biopsy specimen was homogenized and plated onto brain-heart infusion agar plates (Difco, Detroit, MI, United States) that were supplemented with vancomycin (6 mg/mL), amphotericin B (8 mg/mL), trimethoprim (5 mg/mL), and 10% glycerol. Under microaerophilic conditions (85% N2, 5% O2, and 10% CO2) in a humid atmosphere, the plates were incubated at 37 °C for 3-5 d. H. pylori was recognized based on Gram staining, helical shape, and biochemical assays that were positive for oxidase, catalase, and urease[15].
As suggested by the European committee on antimicrobial susceptibility testing, the E-test minimum inhibitory concentration technique was used to test for antimicrobial susceptibility. Tests were done on Mueller–Hinton agar plates enriched with 7% horse blood (bioMérieux Inc., Marcy-l'Étoile, France) using E-test strips (AB Biodisk, Slona, Sweden)[16]. Tetracycline (TET), CLR, AMX, rifampicin (RIF), and ciprofloxacin (CIP) were among the antibiotics that were evaluated. The isolate was considered resistant to AMX, CLR, and MNZ if minimal inhibitory concentrations (MICs) were > 0.125 mg/L, > 0.5 mg/L, and > 8 mg/L, respectively. Besides, the isolate was considered resistant to CIP, RIF, and TET if MICs were > 1 mg/L[16].
Each H. pylori isolate was sub-cultured and incubated for 72 h after which 7-10 colonies were pooled together. DNA was extracted according to the manufacturer’s instructions using the QIAamp DNA micro kit (Qiagen, Hilden, Germany) and then was eluted in 200 μL of 1x TE buffer (10 mM Tris-HCl, 1 mM ethylenediaminetetraacetic acid; pH 8.0) and stored at –20 °C until PCR amplification.
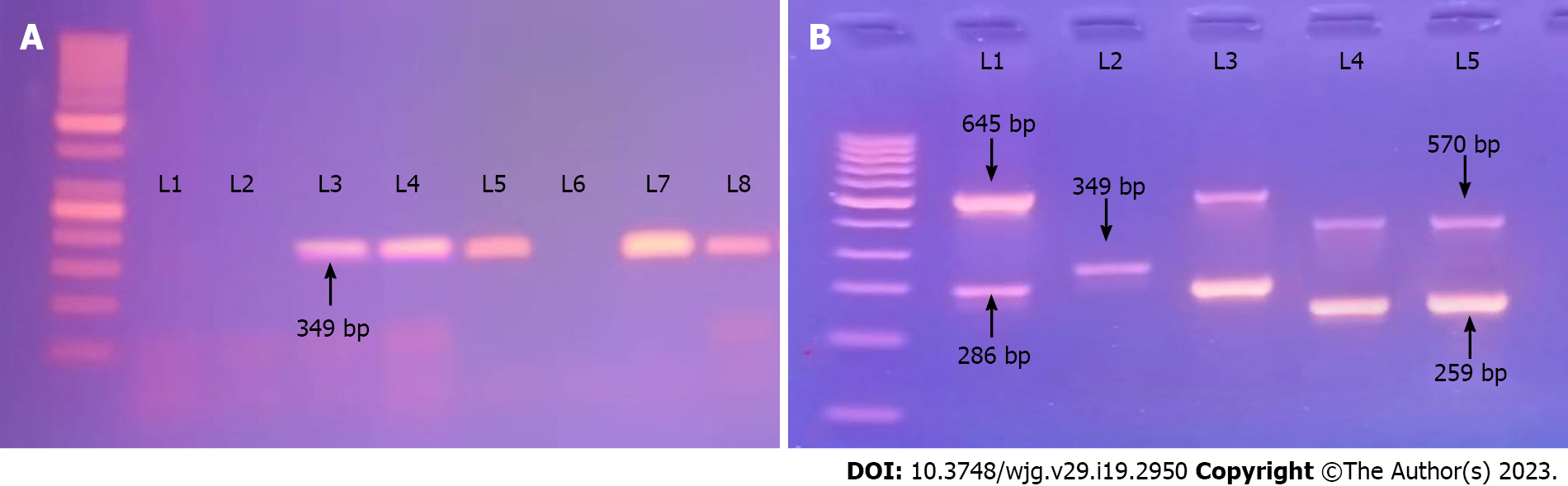
As previously disclosed, all isolates were subject to multiplex PCR to determine cagA and vacA genotypes[17-19]. Table 1 contains a list of all primers utilized for this research. Multiplex PCR was carried out in a thermocycler (Cyclogene; Bio-Techne, Minneapolis, MN, United Kingdom) in a reaction mixture volume of 50 μL. Each reaction contained 25 μL of 2 × multiplex PCR Master mix (Hot start DNA polymerase, multiplex buffer, dNTP mix MgCl2; ThermoScientific, Vilinus, Lithuania), 2 μL of each primer (40 pmol), and 200 ng of template DNA (6 µL) according to DNA concentration in yield. The reaction volume was brought to 50 µL with the addition of nuclease free water. PCR grade water and DNA from H. pylori strain American Type Culture Collection 43504 were used as negative and positive controls, respectively. Multiplex PCR was carried out by the simultaneous addition of primers in the same reaction mixture after test of each primer pair separately: (1) 35 cycles of 95 °C for 1 min; (2) Annealing at 54 °C for 1.5 min; (3) Extension at 72 °C for 1 min; and (4) A final extension at 72 °C for 10 min. The amplified PCR products were electrophoresed on 1.5% agarose gels using 1 × TBE after which the gel was stained with ethidium bromide using a 100 bp ladder as the molecular weight standard, visualized under an ultraviolet light source, and photographed using a BioRad Gel Doc device.
| Primer | Nucleotide sequence, 5'—3' | Gene | Size, bp |
| vacA | |||
| VA1-F | ATGGAAATACAACAAACACAC | vacA s1/s2 | 259/286 |
| VA1-R | CTGCTTGAATGCGCCAAAC | ||
| VA2-F | CAATCTGTCCAATCAAGCGAG | vacA m1/m2 | 570/645 |
| VA2-R | GCGTCAAAATAATTCCAAGG | ||
| Cag-F | GTTGATAACGCTGTCGCTTC | cagA | 349 |
| Cag-R | GGGTTGTATGATATTTTCCATAA | ||
Patients were given omeprazole (20 mg) and two antibiotics, AMX (1 g) and CLR (500 mg), twice daily for 14 d[9]. Clinical follow-up, including side-effect monitoring, was performed until the medication was completed. Using a rapid urease test, the therapeutic response was examined 6 wk following the termination of therapy. Successful eradication of H. pylori was indicated by a negative result from the rapid urease test and microbiological cultures and by the absence of neutrophilic infiltration with a decrease in lymphoid inflammatory changes in the biopsy tissue following treatment[9,20].
Coding, validating, and analyzing the data required the use of SPSS version 22 (IBM Corp., Armonk, NY, United States). Data were shown using average, median, and frequencies (%). A χ2 test or Fisher’s exact test was used to compare categorical data, and the Student’s t-test was used for numerical data. P values ≤ 0.05 based on a two-tail test were considered to be significant. Potential risk factors were identified using a binary logistic regression analysis that included an antecedent 95%CI and adjusted odds ratio (aOR).
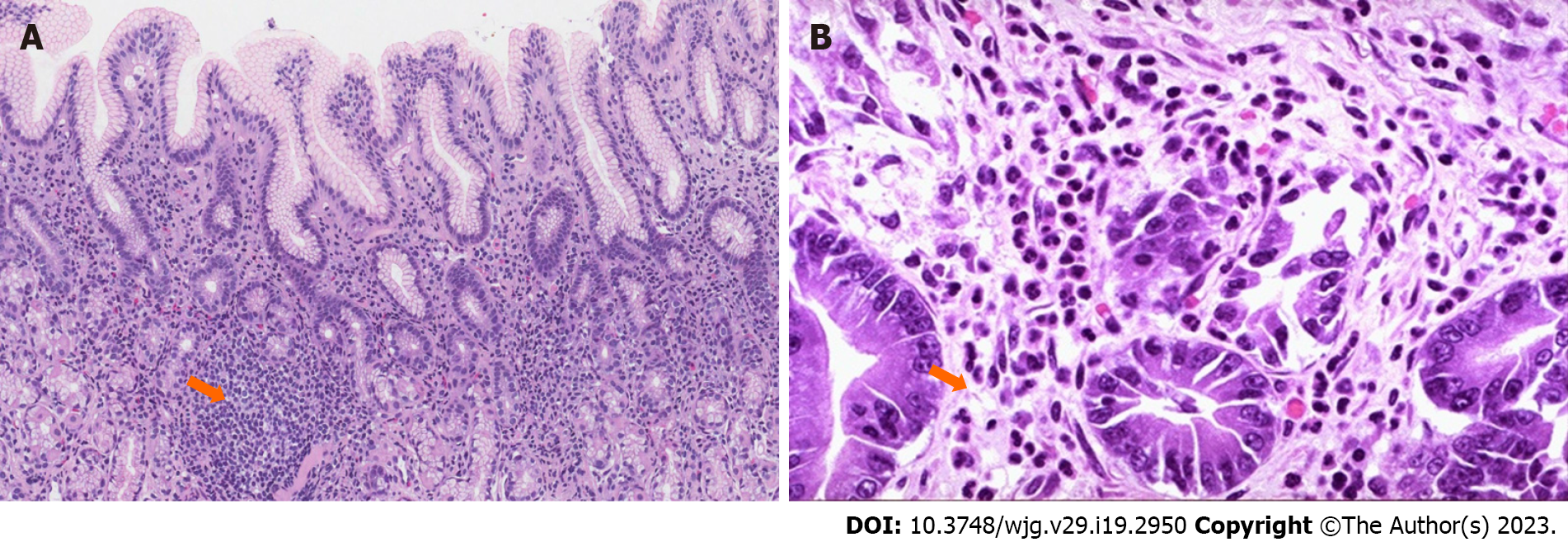
Table 2 shows the demographic and clinical characteristics of the patients. The age of the patients ranged from 19 years to 59 years (median: 39.5 years), and 57 (79.2%) were males. Based on endoscopic examination, more than half of the patients (54.2%) had gastritis, while 33.3% and 12.5% of patients had gastric ulcer and duodenitis, respectively. Based on histopathological examination of the tissue biopsy, features of chronic gastritis and rods of H. pylori were seen based on Giemsa stain (Figure 1).
| Variable | n (%) |
| Mean age in yr | 39.5 ± 12.9 |
| Male sex | 57 (79.2) |
| History of smoking | 22 (30.6) |
| History of upper abdominal pain | 48 (66.7) |
| Endoscopic finding | |
| Gastritis | 39 (54.2) |
| Gastric ulcer | 24 (33.3) |
| Duodenitis | 9 (12.5) |
| Co-morbid conditions | |
| Liver cirrhosis | 15 (20.8) |
| Diabetes mellitus | 9 (12.5) |
| Hypertension | 3 (4.2) |
| cagA | 36 (50.0) |
| vacA | 60 (83.3) |
| s1 | 42 (58.3) |
| s2 | 18 (25.0) |
| m1 | 27 (37.5) |
| m2 | 33 (45.8) |
Thirty-six (50%) strains were cagA-positive among 72 H. pylori isolates in this study, and in 50 (69.4%) of the H. pylori strains, the vacA gene was detected. For the vacA gene s and m region sub-typing, 42 and 18 strains were positive for s1 and s2, respectively, while 27 and 33 isolates were positive for m1 and m2, respectively (Figure 2).
During the research period, a total of 86 patients were recruited. Among them, 10 patients were negative for H. pylori infection, and 4 patients failed to complete treatment, leaving 72 patients eligible for the study protocol. Among the 72 H. pylori isolates, the resistance rates to MNZ, AMX, RIF, CLR, CIP, and TET were 100%, 81.9%, 62.5%, 52.8%, 41.7%, and 37.5%, respectively.
The eligible patients underwent 14-d triple therapy with two antibiotics: (1) AMX (1 g); (2) CLR (500 mg); and (3) Omeprazole (20 mg). In 45 individuals, the 14-d triple therapy was successful in completely eliminating H. pylori; however, in the other 27 patients, the infection persisted despite treatment. H. pylori eradication rates were 59.2% (95%CI: 48.2–70.3%) for intention-to-treat (ITT) and 62.5% (95%CI: 51.3%–73.7%) for per protocol treatment.
In patients with vacA s1-positive (P = 0.02), s2-positive (P = 0.03), or m1-positive (P = 0.01) strains, H. pylori eradication occurred more frequently. It was not surprising that the rates of resistance to AMX (P = 0.012) and CLR were higher in H. pylori isolates from patients who experienced unsuccessful eradication (P = 0.005). However, no significant association with eradication therapy and resistance rates to CIP, TET, and RIF was found (Table 3).
| Variable | Successful, n = 45 | Unsuccessful, n = 27 | P value |
| Mean age in yr | 43.13 ± 12.19 | 39.22 ± 11.91 | 0.188 |
| Male sex | 36 | 21 | 0.524 |
| Smoking | 15 | 12 | 0.349 |
| cagA | 21 (46.7) | 15 (55.6) | 0.313 |
| s1 | 31 (68.9) | 11 (40.7) | 0.022 |
| s2 | 15 (33.3) | 3 (11.1) | 0.031 |
| m1 | 22 (48.9) | 5 (18.5) | 0.014 |
| m2 | 21 (46.7) | 12 (44.4) | 0.525 |
| AMX | 33 (73.3) | 26 (96.3) | 0.012 |
| CIP | 18 (40.0) | 12 (44.4) | 0.45 |
| TET | 15 (33.3) | 12 (44.4) | 0.244 |
| CLR | 18 (40.0) | 20 (74.1) | 0.005 |
| RIF | 27 (60.0) | 18 (40.0) | 0.379 |
With 95%CI and aOR the multiple logistic regression analysis revealed possible risk factors associated with H. pylori eradication therapy (Table 4). Successful eradication of H. pylori was more significantly associated with strains harboring the vacA s1 genotype (aOR = 0.507, 95%CI: 0.175–0.822). In contrast, failed eradication rates were significantly associated with H. pylori strains resistant to AMX (aOR = 0.223, 95%CI: 0.026–0.537) and CLR (aOR = 0.204, 95%CI: –0.005 to –0.036).
| Variable | aOR (95%CI) | P value |
| s1 | 0.507 (0.175–0.822) | 0.003 |
| s2 | 0.074 (-0.227 to 0.393) | 0.595 |
| m1 | -0.028 (-0.291 to 0.234) | 0.83 |
| AMX | 0.223 (0.026–0.537) | 0.032 |
| CLR | 0.204 (-0.005 to 0.412) | 0.036 |
Eradication therapy of H. pylori infections has been deemed beneficial for cases with gastroduodenal disorders, such as gastric MALT lymphoma, peptic ulcer disease history, gastric cancer, dyspepsia, atrophic gastritis, and hyperplastic polyps, and for cases with certain extragastrointestinal disorders, such as unexplained iron-deficiency anemia, chronic idiopathic urticaria, and idiopathic thrombocytopenic purpura[1,21].
Despite establishment of multiple H. pylori eradication treatment regimens in different worldwide regions, the usual 14-d triple therapy [piperacillin/AMX/CLR (PAC)] produces adequate eradication rates for both adults and children in Egypt[21-23]. However, cure rates in this study were found to be unsatisfactory and disappointing. Our findings showed that the 14-d triple therapy efficacy of H. pylori eradication (59%) was lower than that reported from previous Egyptian studies (ITT range: 72%–83%)[22,23]. A previous meta-analysis investigated the global trend in eradication rates of two different first-line therapeutic regimens (PAC and piperacillin/AMX/MNZ) for 8061 patients infected with H. pylori from 30 countries[24]. In this report, the cure rate of PAC (77.1%, 95%CI = 75%–79%) was significantly higher than piperacillin /AMX/MNZ (70%, 95%CI = 67.7%–72.3%) (OR = 0.70, 95%CI = 0.56–0.88; P < 0.002). Previous clinical studies worldwide showed an unacceptable and continuous decrease in H. pylori triple eradication therapy-associated cure rates[13,25]. It is noteworthy that the overall global cure rates of these protocols are < 80%, which have recently been considered regimens with disappointing efficacy.
Inadequate treatment duration, antimicrobial resistance, inadequate stomach acid suppression, poor adherence to eradication regimens, and quick metabolism of proton pump inhibitors have all been implicated in the failure of the traditional triple therapy to eradicate a pathogen according to previous ecological research[26,27]. CLR resistance has been recognized as the primary cause of routine triple treatment failure. In a recent meta-analysis investigating 66142 patients from 65 countries, failure to achieve eradication was 7-fold higher in patients with CLR-resistant H. pylori infections (OR: 6.97; 95%CI: 5.23–9.01; P = 0.001) when treated with a CLR-containing regimen than patients with susceptible strains[13]. Therefore, in nations with a high prevalence of CLR resistance (> 15%–20%), bismuth quadruple treatment is recommended.
Pooled data from 25 randomized trials including 3990 patients showed that the ITT eradication rate of standard triple therapy (65.7%) was significantly lower than bismuth-containing regimens (74.9%; OR: 1.60; 95%CI: 1.07–2.39). In addition, in the per protocol analysis, the pooled eradication rate for bismuth-containing regimens was 86.7% vs 33.3% for the usual triple regimen (OR: 10.64; 95%CI: 2.96–39.53)[28]. It is noteworthy that all isolates in this study were MNZ-resistant, and more than half of the isolates were resistant to AMX and CLR. These findings are not surprising as MNZ has been abused by the public without prescription for various gastrointestinal infections and diarrhea, while both AMX and CLR have been included in empiric therapies for respiratory infections or non-tuberculous mycobacterial infections in our region. Therefore, continuous monitoring of susceptibility patterns of H. pylori to various antimicrobials seems crucial as multidrug resistant H. pylori strains undoubtedly induce failure of H. pylori eradication therapy.
H. pylori cagA and vacA genotypes are among the most important factors implicated in the pathogenesis of gastroduodenal diseases and in influencing the sequelae of treatment protocols. The association of cagA-positive strains with the H. pylori eradication therapy outcomes were demonstrated by former clinical and ecological investigations[4-9]. However, the results from these studies were inconsistent and controversial. In this study, strains with or without cagA had no effect on eradication rates, a finding that is similar to previous reports. In a previous meta-analysis including 25 studies, the influence of the virulence factors, vacA and cagA, on H. pylori eradication therapy in 2693 cases was investigated by Wang et al[29]. In their report, the pooled H. pylori eradication rate was 77% (95%CI: 70%–83%) for cagA-negative patients and 85% (95%CI: 81%–89%) for cagA-positive with an 8% higher eradication rate among cagA-positive strains. In addition, using subgroup analyses based on clinical presentations, eradication detection method, location, and therapeutic regimen types, the authors concluded that cagA-negative strains responded to successful H. pylori therapy eradication rates than cagA-positive strains with pooled risk ratios (RR) of 1.118 (95%CI: 1.051–1.189; P < 0.001) for Asia and 1.138 (95%CI: 1.000–1.295; P = 0.049) for Europe. In South America, cagA-positive strains and cagA-negative strains exhibited comparable H. pylori treatment rates (RR: 1.104, 95%CI: 0.953–1.279; P = 0.186).
The vacA s1-positive H. pylori strains are typically more virulent and more closely linked to progressive gastroduodenal disorders as reported in many previous studies[29]. An increase in blood flow to the site of infection and stronger inflammatory responses were stimulated by more virulent strains as reported by clinical and epidemiological-based evidence. In addition, the more virulent strains are usually more susceptible to antimicrobials because of faster replication. H. pylori strains containing vacA s1 were substantially related to a greater H. pylori eradication rate in the current investigation.
This result is consistent with earlier findings in which vacA s1-positive strains posed a significant risk for the development of gastric illnesses with easier eradication in diseased people. Wang et al[29] discovered that the pooled H. pylori eradication rate was 73% (95%CI: 61%–85%) for vacA s2 and 83% (95%CI: 75%–91%) for vacA s1 with a 10% improvement in eradication rates in the vacA s1 group compared to the vacA s2 group (95%CI: 1.040–1.303; P = 0.008). In their meta-analysis, vacA s1 status was associated with better eradication rates in the triple therapy subgroup according to an examination of subgroups based on different worldwide locations (RR: 1.175, 95%CI: 1.012–1.360)[29]. Brennan et al[7] reported that the incidence of the more virulent s1 genotype was substantially lower among previously treated individuals than among those who had never received therapy (58.3% vs 74.3%). A significant increase in the frequency of the least pathogenic s2/m2 genotype was seen in previously treated individuals (36.7% vs 21.0%)[7]. Our findings and results of other studies clearly demonstrated that the vacA s1 genotype would be useful for predicting successful outcomes of H. pylori eradication therapy.
This study had some limitations. First, this research may have been limited in its capability to investigate other virulence marker-associated pathogenic roles due to the lack of molecular analysis beyond PCR and genome sequencing. Second, this study was conducted within a single center. Therefore, our findings cannot be applied to other situations. To further understand the phenotypic and genotypic links between H. pylori virulence, antibiotic resistance, and the efficacy of eradication therapy, further molecular-based epidemiological multicenter investigations with longer monitoring durations are required.
This low H. pylori eradication rate following 14-d triple therapy is concerning and worrying. The pan-resistance of H. pylori to MNZ followed by the high resistance to AMX, CLR, and CIP in this research is challenging and of great concern. These findings draw attention to the urgent need for performing H. pylori antimicrobial susceptibility testing before starting eradication therapy in addition to continuous surveillance of H. pylori resistance patterns in our region to provide data that can guide empirical treatment. In addition, the vacA s1-positive H. pylori isolates are easier to eradicate and could be used as an indicator to predict the successful outcome of eradication therapy.
Helicobacter pylori (H. pylori) has been implicated in the development of gastric cancer and gastric mucosa-associated lymphoid tissue lymphoma. However, the bacterial eradication reduces the risk of theses gastric complications. The therapeutic regimens currently in use and the duration of therapy differ in different countries, which affects the therapy outcomes. The therapeutic outcomes have been found to be affected by the virulence characteristics of the infecting strains. The strains with more virulent characteristics possessing vacA s1 and m1 are eradicated more efficiently than the strains harboring less virulent characteristics.
To demonstrate that the infecting strains possessing more virulent characteristics are eradicated efficiently with a 14-d triple therapy. The vacA s1-positive H. pylori isolates are easier to eradicate and could be used as an indicator to predict the successful outcome of eradication therapy.
To evaluate the H. pylori infection frequency and its resistance patterns among Egyptian patients and to determine the H. pylori virulence characteristics influencing the eradication success of the 14-d triple therapy regimen.
The patients suggestive of H. pylori infections were subjected to endoscopy-based biopsy specimen collection. The collected biopsy specimens were used to evaluate the H. pylori infection by a combination of diagnostic tests that included urease test, bacterial culture, and histopathological investigation. The extracted DNA was subjected to PCR-based cagA and vacA genotype investigation. The H. pylori-infected patients received triple therapy for 14 d. Six weeks after completion of the therapy, the treatment response was examined utilizing histology and the rapid urease test.
Among the 86 recruited patients, infection was found in 76 individuals. All of the strains were resistant to metronidazole (MNZ), while 52.8% and 81.9% of the isolates were resistant to clarithromycin (CLR) and amoxicillin (AMX), respectively. Successful eradication of H. pylori was significantly associated with vacA s1-positive strains [adjusted odds ratio (aOR) = 0.507, 95%CI: 0.175–0.822]. H. pylori strains resistant to CLR (aOR = 0.204, 95%CI: -0.005 to 0.412) and AMX (aOR = 0.223, 95%CI: 0.026–0.537) were significantly associated with failed eradication rate.
The low eradication rate of 14-d triple therapy in this study is worrisome and indicates that an alternative therapy to achieve effective eradication must be identified. The findings of complete failure of MNZ and reduced efficacy of AMX, CLR, and ciprofloxacin draw attention to the urgent need of antimicrobial susceptibility testing-guided eradication therapy. In addition, the strains with virulent properties of vacA s1 are easier to eradicate and could be used as an indicator to predict the successful outcome of eradication therapy.
CLR, AMX, and MNZ-based 14-d eradication therapy is ineffective and discouraged in these populations. Extensive nationwide studies should be considered to document the efficacy and to find alternative therapeutic regimens in respect to the duration. Furthermore, antimicrobial susceptibility testing based therapy should be encouraged to help reduce the development of antimicrobial resistance.
The authors would like to thank the colleagues in the Department of Laboratory and Microbiology and Endoscopy and the patients in the National Liver Institute who aided this research.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Abadi ATB, Iran; Bordin DS, Russia; Kirkik D, Turkey; Koga Y, Japan; Machado NC, Brazil S-Editor: Li L L-Editor: Filipodia P-Editor: Fan JR
| 1. | Leja M, Axon A, Brenner H. Epidemiology of Helicobacter pylori infection. Helicobacter. 2016;21 Suppl 1:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | Atherton JC. The pathogenesis of Helicobacter pylori-induced gastro-duodenal diseases. Annu Rev Pathol. 2006;1:63-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 410] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 3. | Herrero R, Parsonnet J, Greenberg ER. Prevention of gastric cancer. JAMA. 2014;312:1197-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Sukri A, Hanafiah A, Mohamad Zin N, Kosai NR. Epidemiology and role of Helicobacter pylori virulence factors in gastric cancer carcinogenesis. APMIS. 2020;128:150-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 5. | Sugimoto M, Yamaoka Y. Virulence factor genotypes of Helicobacter pylori affect cure rates of eradication therapy. Arch Immunol Ther Exp (Warsz). 2009;57:45-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Oktem-Okullu S, Cekic-Kipritci Z, Kilic E, Seymen N, Mansur-Ozen N, Sezerman U, Gurol Y. Analysis of Correlation between the Seven Important Helicobacter pylori (H. pylori) Virulence Factors and Drug Resistance in Patients with Gastritis. Gastroenterol Res Pract. 2020;2020:3956838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Brennan DE, Dowd C, O’Morain C, McNamara D, Smith SM. Can bacterial virulence factors predict antibiotic resistant Helicobacter pylori infection? World J Gastroenterol. 2018;24:971-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Gangwer KA, Shaffer CL, Suerbaum S, Lacy DB, Cover TL, Bordenstein SR. Molecular evolution of the Helicobacter pylori vacuolating toxin gene vacA. J Bacteriol. 2010;192:6126-6135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, Gasbarrini A, Hunt RH, Leja M, O’Morain C, Rugge M, Suerbaum S, Tilg H, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 651] [Article Influence: 217.0] [Reference Citation Analysis (0)] |
| 10. | De Francesco V, Bellesia A, Ridola L, Manta R, Zullo A. First-line therapies for Helicobacter pylori eradication: a critical reappraisal of updated guidelines. Ann Gastroenterol. 2017;30:373-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Wu W, Yang Y, Sun G. Recent Insights into Antibiotic Resistance in Helicobacter pylori Eradication. Gastroenterol Res Pract. 2012;2012:723183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Papastergiou V, Georgopoulos SD, Karatapanis S. Treatment of Helicobacter pylori infection: meeting the challenge of antimicrobial resistance. World J Gastroenterol. 2014;20:9898-9911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 13. | Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology. 2018;155:1372-1382.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 815] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 14. | Strengthening the reporting of observational studies in epidemiology. STROBE checklist, version 4. 2007. [cited 22 October 2018]. Available from: http://www.strobe-statement.org/index.php?id=available-checklists. |
| 15. | Mégraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev. 2007;20:280-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 488] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 16. | European committee on antimicrobial susceptibility testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 9.0. 2019. [cited 1 January 2019]. Available from: http://www.eucast.org. |
| 17. | Tummuru MK, Cover TL, Blaser MJ. Cloning and expression of a high-molecular-mass major antigen of Helicobacter pylori: evidence of linkage to cytotoxin production. Infect Immun. 1993;61:1799-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 473] [Cited by in RCA: 493] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 18. | Atherton JC, Cao P, Peek RM Jr, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771-17777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1078] [Cited by in RCA: 1108] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 19. | Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999;37:2274-2279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 372] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 20. | Alboraie M, Elhossary W, Aly OA, Abbas B, Abdelsalam L, Ghaith D, Shady Z, Gaber Y, Adel E, Peura D, Armstrong D, Esmat G; special interest group; Egyptian Association for Study of Gastrointestinal Diseases and Liver (E A S G L D). Egyptian recommendations for management of Helicobacter pylori infection: 2018 report. Arab J Gastroenterol. 2019;20:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Kamboj AK, Cotter TG, Oxentenko AS. Helicobacter pylori: The Past, Present, and Future in Management. Mayo Clin Proc. 2017;92:599-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Ismail WA, Mostafa EF. A comparison between conventional triple therapy and sequential therapy on tolerance of treatment and eradication of Helicobacter pylori infection in Egyptian patients. Egy J Int Med. 2018;30:90-95. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Abd-Elsalam S, Kobtan A, El-Kalla F, Elkhalawany W, Nawasany SE, Saif SA, Yousef M, Ali LA, Soliman S, Mansour L, Habba E, Soliman H, Rizk F, Shehata MA. A 2-week Nitazoxanide-based quadruple treatment as a rescue therapy for Helicobacter pylori eradication: A single center experience. Medicine (Baltimore). 2016;95:e3879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Puig I, Baylina M, Sánchez-Delgado J, López-Gongora S, Suarez D, García-Iglesias P, Muñoz N, Gisbert JP, Dacoll C, Cohen H, Calvet X. Systematic review and meta-analysis: triple therapy combining a proton-pump inhibitor, amoxicillin and metronidazole for Helicobacter pylori first-line treatment. J Antimicrob Chemother. 2016;71:2740-2753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Li B, Lan X, Wang L, Zhao J, Ding J, Ding H, Lei J, Wei Y, Zhang W. Proton-pump inhibitor and amoxicillin-based triple therapy containing clarithromycin vs metronidazole for Helicobacter pylori: A meta-analysis. Microb Pathog. 2020;142:104075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Zullo A, De Francesco V, Hassan C. Predicting Helicobacter pylori eradication: how to teach an old dog new tricks! J Clin Gastroenterol. 2012;46:259-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Kotilea K, Mekhael J, Salame A, Mahler T, Miendje-Deyi VY, Cadranel S, Bontems P. Eradication rate of Helicobacter Pylori infection is directly influenced by adherence to therapy in children. Helicobacter. 2017;22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Ko SW, Kim YJ, Chung WC, Lee SJ. Bismuth supplements as the first-line regimen for Helicobacter pylori eradication therapy: Systemic review and meta-analysis. Helicobacter. 2019;24:e12565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 29. | Wang D, Li Q, Gong Y, Yuan Y. The association between vacA or cagA status and eradication outcome of Helicobacter pylori infection: A meta-analysis. PloS One. 2017;12:e0177455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |