Published online May 14, 2023. doi: 10.3748/wjg.v29.i18.2875
Peer-review started: January 29, 2023
First decision: February 22, 2023
Revised: March 8, 2023
Accepted: April 17, 2023
Article in press: April 17, 2023
Published online: May 14, 2023
Processing time: 101 Days and 23 Hours
Skeletal muscle abnormalities, such as muscle mass depletion (sarcopenia) and fatty infiltration of the muscle (myosteatosis), are frequent complications in cirrhotic patients scheduled for transjugular intrahepatic portosystemic shunt (TIPS).
To investigate the association and predictive value of sarcopenia and myosteatosis for overt hepatic encephalopathy (HE) and mortality after TIPS.
The records of cirrhotic patients who underwent the TIPS procedure at our hospital between January 2020 and June 2021 were retrospectively retrieved. The transversal psoas muscle thickness (TPMT) and psoas muscle attenuation (PMA) measured from the unenhanced abdominal computed tomography (CT) at the level of the third lumbar vertebrae were used to analyze the sarcopenia and myosteatosis, respectively. The area under curve (AUC) was used to evaluate the discriminative power of TPMT, PMA, and relevant clinical parameters. Fur-thermore, log-rank test was performed to compare the incidence of overt HE and survival between the different groups, and the association of risk factors with overt HE and mortality was analyzed using Cox proportional hazards regression models.
A total of 108 patients were collected. Among these patients, 45.4% of patients developed overt HE after TIPS treatment. Furthermore, 32.4% and 28.7% of these patients were identified to have myosteatosis and sarcopenia, respectively. Myosteatosis (51.0% vs 16.9%, P < 0.001) and sarcopenia (40.8 vs 18.6%, P = 0.011) were found to be more frequent in patients with overt HE, when compared to patients without overt HE. The receiver operating characteristics analysis indicated that the predictive power of TPMT and PMA in overt HE (AUC = 0.713 and 0.778, respectively) was higher when compared to the neutrophil lymphocyte ratio (AUC = 0.636). The cumulative incidence of overt HE was the highest in patients with concomitant sarcopenia and myosteatosis, followed by patients with myosteatosis or sarcopenia, while this was the lowest in patients without sarcopenia and myosteatosis. In addition, sarcopenia and myosteatosis were inde-pendently associated with overt HE and mortality after adjusting for confounding factors in post-TIPS patients.
CT-based estimations for sarcopenia and myosteatosis can be used as reliable predictors for the risk of developing overt HE and mortality in cirrhotic patients after TIPS.
Core Tip: Few studies have investigated the relationship among sarcopenia, myosteatosis, and overt hepatic encephalopathy (HE) after transjugular intrahepatic portosystemic shunt (TIPS). The present study revealed that the cumulative incidence of overt HE was the highest in patients with concomitant sarcopenia and myosteatosis, followed by patients with myosteatosis or sarcopenia, and the lowest incidence was found in patients without myosteatosis and sarcopenia. Sarcopenia and myosteatosis were the independent risk factors for overt HE and mortality in patients following TIPS. Therefore, identifying strategies for improving muscle mass (sarcopenia) and muscle fatty infiltration (myosteatosis) may help to reduce the incidence of HE after TIPS.
- Citation: Yin L, Chu SL, Lv WF, Zhou CZ, Liu KC, Zhu YJ, Zhang WY, Wang CX, Zhang YH, Lu D, Cheng DL. Contributory roles of sarcopenia and myosteatosis in development of overt hepatic encephalopathy and mortality after transjugular intrahepatic portosystemic shunt. World J Gastroenterol 2023; 29(18): 2875-2887
- URL: https://www.wjgnet.com/1007-9327/full/v29/i18/2875.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i18.2875
Transjugular intrahepatic portosystemic shunt (TIPS), which establishes an artificial shunt between the portal and hepatic vein, has been considered an effective method for treating portal hypertension, and can be used to manage refractory ascites and variceal hemorrhage in patients with liver cirrhosis[1]. However, the TIPS procedure leads to a significantly high incidence of overt hepatic encephalopathy (HE), which occurs in approximately 20%-50% of post-TIPS patients[2-4]. Previous studies have suggested that post-TIPS HE is associated with increased hospitalization rates, mortality, and poor quality of life[5]. The use of rifaximin and lactulose remains controversial in treating this pathological entity[6,7]. Thus, studies that can precisely predict post-procedure HE in cirrhotic patients would be beneficial in developing appropriate preventive measures.
More recent studies have revealed that abnormalities in the skeletal muscle, such as sarcopenia (muscle mass depletion) and myosteatosis (fatty infiltration of the muscle), are more prevalent[8,9], and that these are associated with the development of HE in cirrhotic patients[10]. Cirrhosis can lead to the excessive accumulation of ammonia in the skeletal muscle, in which a cascade of molecular alterations and metabolic disturbances may contribute to sarcopenia and myosteatosis. Consequently, both sarcopenia and myosteatosis may, in turn, further increase the circulating ammonia levels to induce overt HE. These two skeletal muscle abnormalities can hypothetically be targeted for preventing overt HE.
In clinic, both sarcopenia and myosteatosis can be reliably and objectively diagnosed through the computed tomography (CT) image-based measurement of body composition. Compared to the skeletal muscle index (SMI) and total psoas muscle volume, the CT-based measurement of transversal psoas muscle thickness (TPMT) has become an easy-to-use method to diagnose sarcopenia through gauging the psoas diameter, and this has become an independent risk factor for mortality in cirrhotic patients[11]. Furthermore, myosteatosis can be quantitatively graded by CT measurements, which shows that lower muscle radiodensity for designating muscle attenuation can be considered as evidence of fat infiltration in the skeletal muscle[12]. Previous studies have demonstrated that psoas muscle attenuation (PMA) is associated with postoperative complications and mortality in elderly patients[13,14]. The present study employed these CT-based methods to evaluate the association and predictive value of sarcopenia and myosteatosis for overt HE after TIPS.
The present study retrospectively analyzed the demographic, clinical, laboratory and radiological data of cirrhotic patients who underwent TIPS at our institution between January 2020 and June 2021. A total of 108 patients were included (Supplementary Figure 1). The inclusion criteria were, as follows: (1) Patients diagnosed with cirrhotic portal hypertension; and (2) patients with at least one episode of variceal hemorrhage or refractory ascites after treatment with vasoactive drugs, endoscopic treatment, or large-volume paracentesis. The exclusion criteria were, as follows: (1) Patients < 18 years old; (2) patients with a history of overt HE (grade ≥ 2, according to the West-Haven criteria[7]) within the past six months; (3) patients with hepatocellular carcinoma or other malignancies; (4) patients with no or poor quality preoperative abdominal CT scans; (5) patients with severe medical comorbidities, such as renal failure, pulmonary insufficiency, or extensive cardiovascular and cerebrovascular disease; and (6) patients who were lost to follow-up within three months.
Our institutional ethics review board approved the present retrospective study. According to the Declaration of Helsinki, all data of the study patients remained confidential throughout the study, and the informed consent for the study was waived due to the retrospective nature of the study.
The TIPS procedure was performed based on the following steps: (1) After successfully puncturing the internal jugular vein, a transjugular liver access set (RUPS-100; Cook Incorporated, Bloomington, IN, United States) was introduced over the guidewire into the right hepatic vein; (2) the bifurcation of the left and right branches of the portal vein was punctured from the right hepatic vein; (3) the pre-shunt portosystemic pressure gradient (PPG) was measured; (4) a balloon catheter of 6-7 mm in diameter was passed over the guidewire to dilate the puncture channel; (5) an 8-mm polytetrafluoroethylene-covered stent (Viatorr stent, W. L. Gore & Associates, Flagstaff, AZ, United States) was implanted to establish the portosystemic shunt; (6) an 8-mm balloon catheter was used to further dilate the in-place stent and ensure that the expansion of the stent reaches 8 mm; (7) after the stent insertion, portography was performed to visualize the left and right branches of the portal vein; (8) if the patient was complicated with variceal rebleeding, the esophageal and gastric varicose vessels were embolized at the same time; and (9) finally, the post-shunting PPG was measured again to determine whether the target PPG was ≤ 12 mmHg or reduced by more than 50% from baseline.
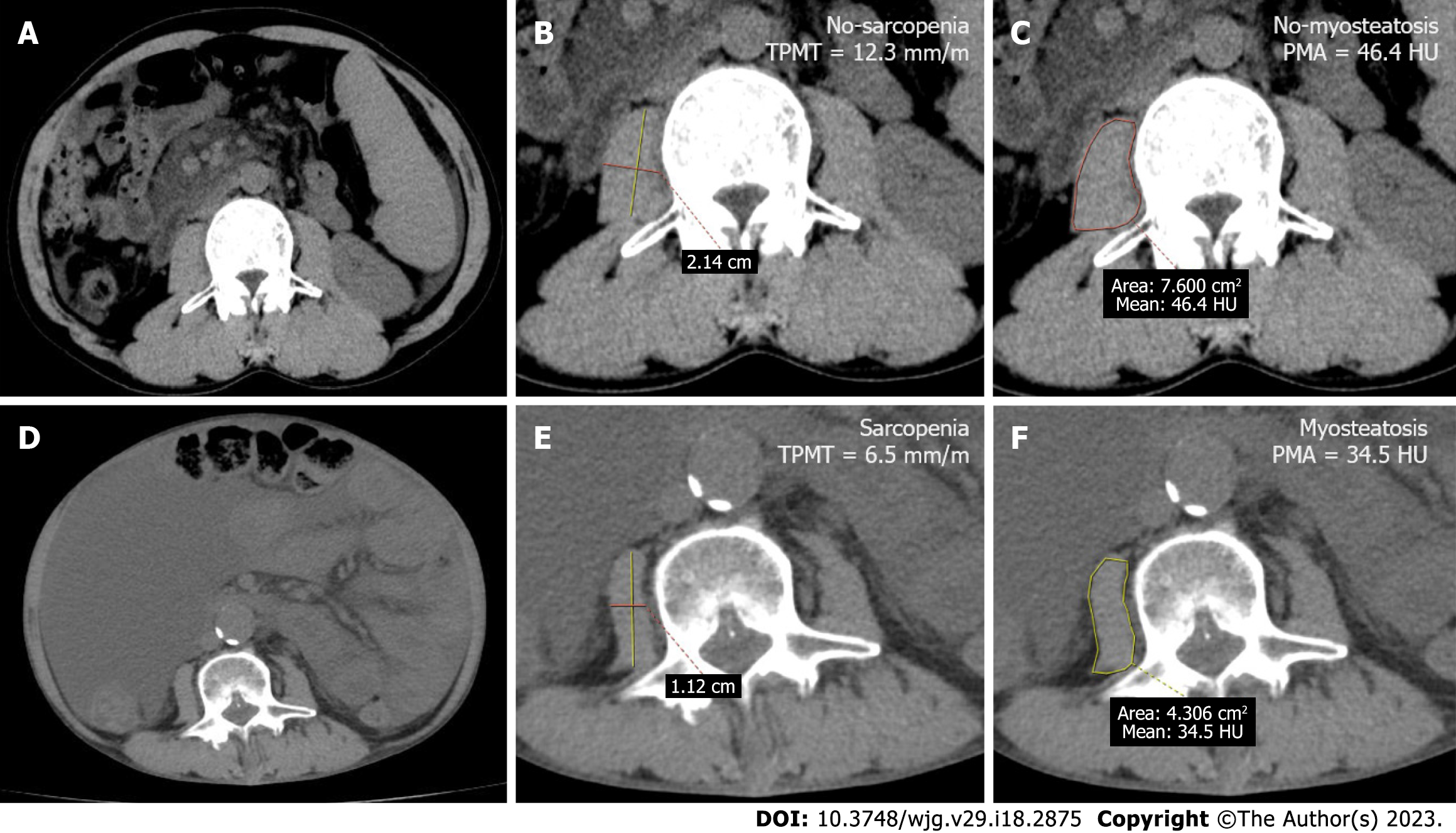
The abdominal CT scans of all studied patients were retrieved from our hospital’s Picture Archiving and Communication System (Carestream, Canada). Then, the PMA and TPMT were measured at the level of the third lumbar vertebrae (L3) using the ADW4.4 workstation. In order to standardize the measurement, TPMT was obtained by gauging the transversal diameters of the right psoas muscle, further normalizing these to the body height, and presenting the values in mm/m. PMA was determined as the mean muscle attenuation in Hounsfield unit (HU) of the right psoas muscle at the L3 level (Figure 1). For the present study, sarcopenia was defined as TPMT < 10.7 mm/m in males and TPMT < 7.8 mm/m in females[11], while myosteatosis was defined as PMA < 41 HU for body mass index (BMI) < 25 kg/m2 and PMA < 33 HU for BMI ≥ 25 kg/m2[15].
All patients were followed-up at 1, 3 and 6 mo after TIPS, and every six months thereafter, during the inpatient rounds and outpatient visits. The follow-up evaluation included the following: General clinical manifestations, physical examination, laboratory tests, shunt patency, and HE. The different stages of HE were assessed and classified based on the detailed neurological examination, and according to the clinical criteria (West-Haven criteria). Overt HE was clinically diagnosed according to the grade 2 West-Haven criteria or higher, or based on the evidence of asterixis and disorientation[5].
The statistical analysis was performed using SPSS v26.0 (IBM, United States). The data were presented as mean ± SE, or in frequency and percentage. Before the analysis, tests were performed to verify the normal distribution of the variables. The comparison of two groups for quantitative variables was performed using Student’s t-test or Mann-Whitney U-test, while the comparison of two groups with qualitative variables was analyzed using χ2 test. Receiver operating characteristic (ROC) analysis was performed to determine the prognostic significance and precision of various relevant parameters in predicting the development of overt HE. The Kaplan-Meier survival estimates were graphed to plot the survival trends of the patients, and log-rank test was conducted to determine the cumulative rates for overt HE. Univariate and multivariate Cox proportional hazards regression models were used to assess the association of risk factors with HE and mortality, and forward regression analysis was subsequently performed to select the appropriate variables in the multivariate model. A two-sided P value of < 0.05 was considered statistically significant for all statistical tests.
Among the 108 patients, 84 (77.8%) patients were male, with a mean age of 53.0 ± 10.8 years old. The detailed demographic data and clinical information of these 108 patients are presented in Table 1. The table shows that the most common etiology of cirrhosis was viral hepatitis (67.6%). Furthermore, 74 (68.5%) patients had ascites, and the mean Child-Pugh score, model for end-stage liver disease (MELD) score, and neutrophil-lymphocyte ratio (NLR) was 7.4 ± 1.5, 11.0 ± 4.4 and 4.4 ± 4.1, respectively. The mean levels for hepatic [total bilirubin (TBIL), 22.8 ± 12.9 umol/L; alanine aminotransferase, 27.5 ± 19.7 U/L; aspartate transaminase, 37.8 ± 28.6 U/L] and renal (creatinine, 67.8 ± 25.7 umol/L) function were within the normal range. The mean value for PMA and TPMT was 43.5 ± 6.9 HU and 11.6 ± 3.1 mm/m, respectively. In general, myosteatosis and sarcopenia were found in 35 (32.4%) and 31 (28.7%) patients, respectively, and overt HE was diagnosed in 49 (45.4%) post-TIPS patients.
| Variables | All patients (n = 108) | Non-HE (n = 59) | HE (n = 49) | P value |
| Age (years) | 53.0 ± 10.8 | 50.3 ± 9.6 | 56.2 ± 11.5 | 0.005a |
| Gender (male), n, % | 84 (77.8) | 47 (79.7) | 37 (75.5) | 0.605 |
| BMI (kg/m2) | 20.99 ± 2.59 | 21.00 ± 2.58 | 20.96 ± 2.63 | 0.856 |
| Etiology (n, %) | ||||
| Viral hepatitis | 74 (68.5) | 42 (71.2) | 32 (65.3) | 0.512 |
| Others | 34 (31.5) | 17 (28.8) | 17 (34.7) | |
| Ascites (n, %) | ||||
| Yes | 74 (68.5) | 41 (69.5) | 33 (67.3) | 0.811 |
| No | 34 (31.5) | 18 (30.5) | 16 (32.7) | |
| Child-Pugh score | 7.4 ± 1.5 | 6.9 ± 1.2 | 7.9 ± 1.8 | 0.002a |
| Child-Pugh class A/B/C (n) | 38/59/11 | 25/30/4 | 13/29/7 | 0.155 |
| MELD score | 11.0 ± 4.4 | 9.3 ± 3.8 | 13.0 ± 4.3 | < 0.001a |
| NLR | 4.4 ± 4.1 | 3.9 ± 4.5 | 4.9 ± 3.5 | 0.015a |
| TBIL (umol/L) | 22.8 ± 12.9 | 19.1 ± 9.4 | 27.3 ± 15.0 | 0.003a |
| ALT (U/L) | 27.5 ± 19.7 | 27.1 ± 16.9 | 28.0 ± 22.8 | 0.610 |
| AST (U/L) | 37.8 ± 28.6 | 32.0 ± 16.5 | 44.9 ± 37.4 | 0.071 |
| Albumin (g/L) | 32.6 ± 5.0 | 33.2 ± 5.3 | 31.9 ± 4.7 | 0.161 |
| Creatinine (umol/L) | 67.8 ± 25.7 | 66.6 ± 23.4 | 69.1 ± 28.5 | 0.858 |
| Reduction of PPG (%) | 53.7 ± 9.1 | 51.5 ± 9.1 | 56.5 ± 8.5 | 0.004a |
| PMA (HU) | 43.5 ± 6.9 | 46.3 ± 6.6 | 40.2 ± 5.7 | < 0.001a |
| Myosteatosis | 35 (32.4) | 10 (16.9) | 25 (51.0) | < 0.001a |
| No myosteatosis | 73 (67.6) | 49 (83.1) | 24 (49.0) | |
| TPMT (mm/m) | 11.6 ± 3.1 | 12.6 ± 2.9 | 10.4 ± 3.1 | < 0.001a |
| Sarcopenia | 31 (28.7) | 11 (18.6) | 20 (40.8) | 0.011a |
| No sarcopenia | 77 (71.3) | 48 (81.4) | 29 (59.2) |
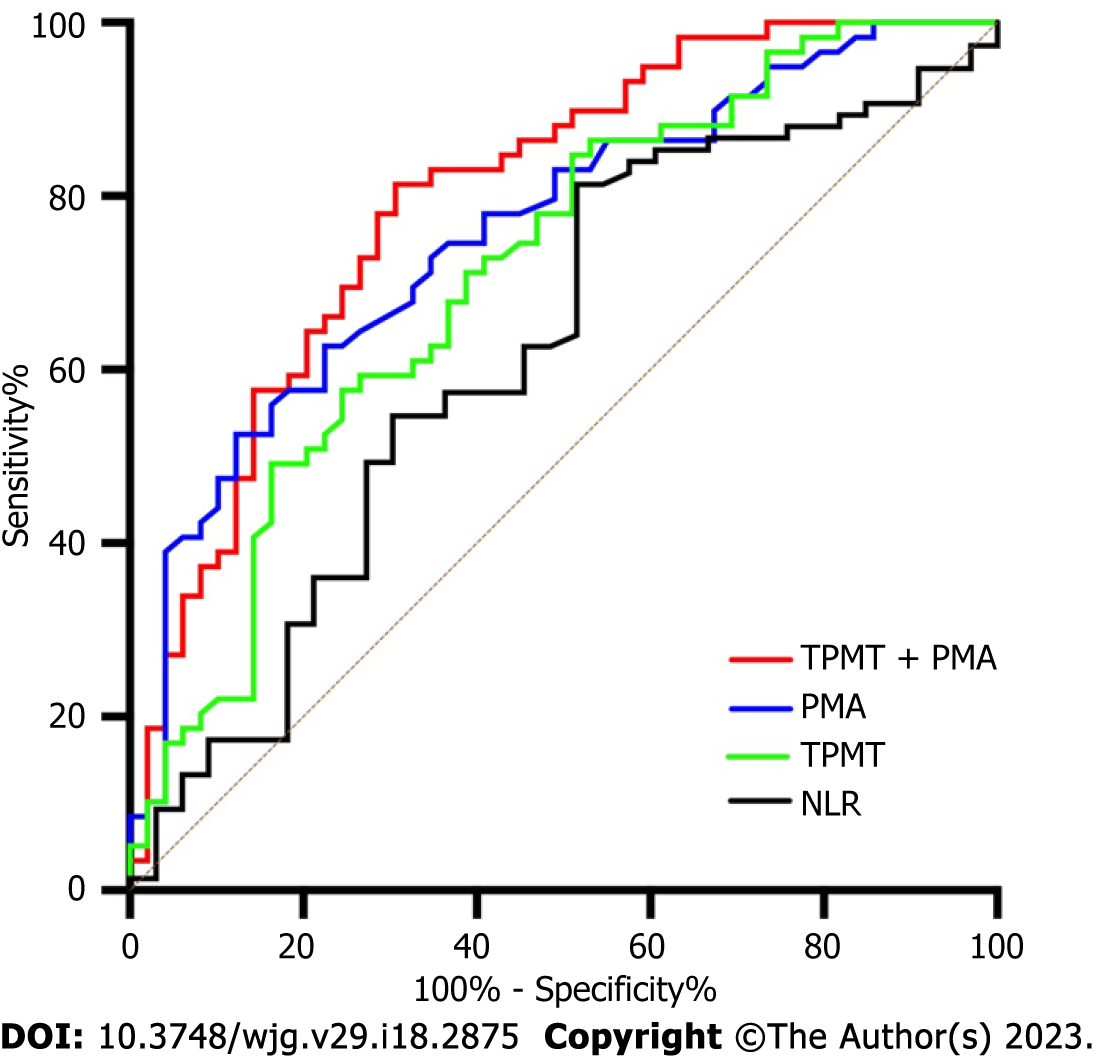
The comparison between patients with and without overt HE is presented in Table 1. The table shows that patients with overt HE were older, had higher TBIL, MELD, Child-Pugh scores and reduction of PPG, and had lower NLR, PMA and TPMT values, when compared to patients without overt HE (P < 0.05). Furthermore, both myosteatosis (51.0% vs 16.9%, P < 0.001) and sarcopenia (40.8 vs 18.6%, P = 0.011) were more common in patients with overt HE, when compared to patients without overt HE. ROC curves were further plotted to determine the area under curve (AUC) values for PMA (AUC = 0.759, 95%CI: 0.669-0.849) and TPMT (AUC = 0.7143, 95%CI: 0.615-0.811), which were similar to the MELD score (AUC = 0.737, 95%CI: 0.642-0.833), but were greater than the NLR (AUC = 0.636, 95%CI: 0.530-0.743). The diagnostic sensitivity and specificity for the combined TPMT and PMA were 81.4% and 69.4%, respectively, and the AUC value was 0.799 (95%CI: 0.715-0.884) (Figure 2). The univariate Cox proportional hazards regression analysis revealed that the following were potentially associated with overt HE: age (HR = 1.044, 95%CI: 1.016-1.071, P = 0.001), Child-Pugh score (HR = 1.292, 95%CI: 1.091-1.531, P = 0.003), MELD score (HR = 1.137, 95%CI: 1.074-1.203, P < 0.001), TBIL (HR = 1.033, 95%CI: 1.014-1.053, P = 0.001), reduction of PPG (HR = 1.039, 95%CI: 1.007-1.071, P = 0.017), PMA (HR = 0.901, 95%CI: 0.863-0.940; P < 0.001), and TPMT (HR = 0.853, 95%CI: 0.774-0.940, P < 0.001). The further multivariate Cox proportional hazards regression analysis identified the following as independent predictive variables for overt HE after TIPS: MELD score (HR = 1.083, 95%CI: 1.020-1.449, P = 0.009), PMA (HR = 0.930, 95%CI: 0.889-0.974, P = 0.002), and TPMT (HR = 0.895, 95%CI: 0.808-0.992, P = 0.035) (Table 2).
| Characteristics | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (years) | 1.044 | (1.016-1.071) | 0.001a | 1.043 | (1.017-1.069) | 0.330 |
| Gender (male) | 1.207 | (0.629-2.316) | 0.572 | |||
| BMI (kg/m2) | 1.012 | (0.902-1.134) | 0.844 | |||
| Etiology (viral hepatitis/others) | 1.277 | (0.706-2.310) | 0.418 | |||
| Ascites (yes/no) | 0.845 | (0.465-1.536) | 0.580 | |||
| Child-Pugh score | 1.292 | (1.091-1.531) | 0.003a | 1.078 | (0.832-1.397) | 0.740 |
| MELD score | 1.137 | (1.074-1.203) | < 0.001a | 1.083 | (1.020-1.449) | 0.009a |
| NLR | 1.041 | (0.984-1.101) | 0.161 | |||
| TBIL (umol/L) | 1.033 | (1.014-1.053) | 0.001a | 1.029 | (1.000-1.058) | 0.768 |
| ALT (U/L) | 1.003 | (0.989-1.018) | 0.681 | |||
| AST (U/L) | 1.010 | (0.998-1.023) | 0.107 | |||
| Albumin (g/L) | 0.956 | (0.903-1.013) | 0.128 | |||
| Creatinine (umol/L) | 1.004 | (0.993-1.015) | 0.454 | |||
| Reduction of PPG (%) | 1.039 | (1.007-1.071) | 0.017a | 1.050 | (1.014-1.087) | 0.082 |
| PMA (HU) | 0.901 | (0.863-0.940) | < 0.001a | 0.930 | (0.889-0.974) | 0.002a |
| TPMT (mm/m) | 0.853 | (0.774-0.940) | 0.001a | 0.895 | (0.808-0.992) | 0.035a |
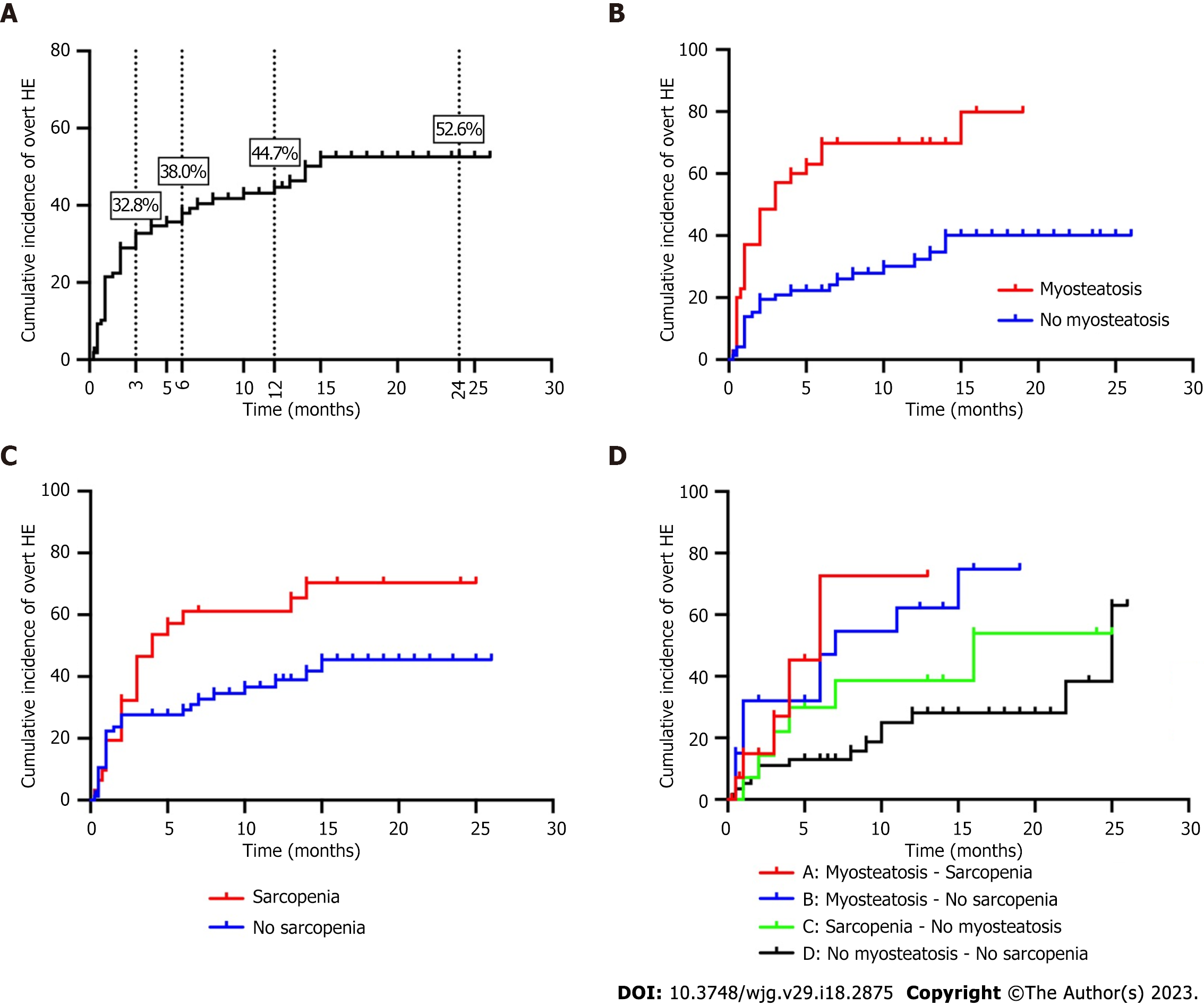
The 3-mo, 6-mo, 1-year, and 2-year cumulative incidence of overt HE after TIPS was 32.8%, 38.0%, 44.7% and 52.6%, respectively. The incidence of overt HE was significantly higher in patients with sarcopenia (log-rank P = 0.022) or myosteatosis (log-rank P < 0.001), when compared to those without either condition. Furthermore, the 108 studied patients were divided into the following groups: Concomitant sarcopenia and myosteatosis (13.9%, 15/108), myosteatosis alone (18.5%, 20/108), sarcopenia alone (13.0%, 14/108), and normal muscle (54.6%, 59/108) groups (Table 3). It was determined that the cumulative incidence of overt HE was the highest in the concomitant sarcopenia and myosteatosis group, followed by the myosteatosis alone and sarcopenia alone groups, while the incidence was the lowest in the non-myosteatosis and non-sarcopenia groups (Figure 3).
| Skeletal muscle abnormalities (n = 108) | PMA | ||
| Low (n = 35); BMI < 25 kg/m2: < 41 HU; BMI ≥ 25 kg/m2: < 33 HU | Normal (n = 73); BMI < 25 kg/m2: ≥ 41 HU; BMI ≥ 25 kg/m2: ≥ 33 HU | ||
| TPMT | Low (n = 31); Male: < 10.7 mm/m; Female: < 7.8 mm/m | Sarcopenia; Myosteatosis; (n = 15) | Sarcopenia; No myosteatosis; (n = 14) |
| Normal (n = 77); Male: ≥ 10.7 mm/m; Female: ≥ 7.8 mm/m | Myosteatosis; No sarcopenia; (n = 20) | No myosteatosis; No sarcopenia; (n = 59) | |
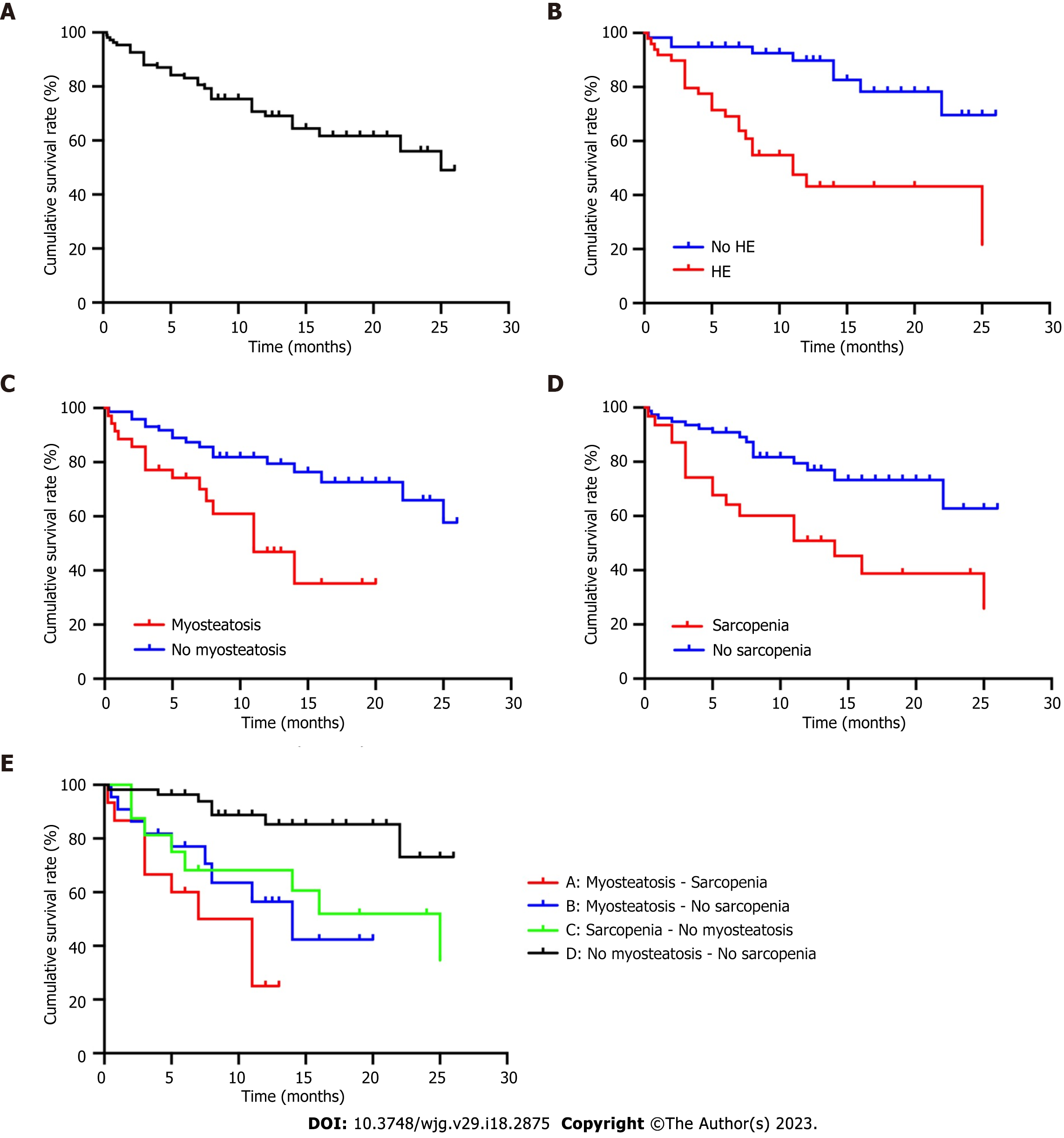
The univariate Cox proportional hazards regression analysis revealed that age (HR = 1.042, 95%CI: 1.008-1.077, P = 0.016), Child-Pugh score (HR = 1.317, 95%CI: 1.064-1.630, P = 0.011), MELD score (HR = 1.089, 95%CI: 1.013-1.172, P = 0.021), albumin (HR = 0.904, 95%CI: 0.836-0.977, P = 0.011), PMA (HR = 0.887; 95%CI: 0.839-0.938, P < 0.001), and TPMT (HR = 0.851, 95%CI: 0.759-0.954, P = 0.006) were potentially associated with overall mortality. When these above parameters were reprocessed using the multivariate Cox proportional hazards regression model, merely albumin (HR = 0.903, 95%CI: 0.827-0.986, P = 0.023), PMA (HR = 0.901, 95%CI: 0.853-0.951, P < 0.001), and TPMT (HR = 0.867, 95%CI: 0.760-0.988, P = 0.032) were identified as independent predictors for overall mortality (Table 4). Based on the Kaplan-Meier survival analysis, the cumulative survival rate was significantly lower in patients with overt HE (log-rank P < 0.001), sarcopenia (log-rank P = 0.001), and myosteatosis (log-rank P = 0.002), when compared to patients without overt HE. Furthermore, the cumulative survival rate was the lowest in patients with concomitant sarcopenia and myosteatosis, followed by patients with sarcopenia alone and myosteatosis alone, while the cumulative survival rate was the highest in patients without myosteatosis and sarcopenia (Figure 4).
| Characteristics | Univariate analysis | Multivariate analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age (years) | 1.042 | (1.008-1.077) | 0.016a | 1.047 | (1.012-1.084) | 0.278 |
| Gender (male) | 1.187 | (0.559-2.518) | 0.656 | |||
| BMI (kg/m2) | 1.000 | (0.871-1.148) | 0.997 | |||
| Etiology (Viral hepatitis/other) | 1.187 | (0.559-2.518) | 0.656 | |||
| Ascites (yes/no) | 1.652 | (0.714-3.823) | 0.241 | |||
| Child-Pugh score | 1.317 | (1.064-1.630) | 0.011a | 1.194 | (0.903-1.579) | 0.740 |
| MELD score | 1.089 | (1.013-1.172) | 0.021a | 1.050 | (0.963-1.146) | 0.845 |
| NLR | 1.060 | (0.994-1.130) | 0.075 | |||
| TBIL (umol/L) | 1.022 | (0.996-1.048) | 0.096 | |||
| ALT (U/L) | 1.003 | (0.986-1.020) | 0.745 | |||
| AST (U/L) | 1.007 | (0.998-1.016) | 0.109 | |||
| Reduction in PPG (%) | 1.030 | (0.992-1.069) | 0.120 | |||
| Albumin (g/L) | 0.904 | (0.836-0.977) | 0.011a | 0.903 | (0.827-0.986) | 0.023a |
| Creatinine (umol/L) | 1.012 | (1.000-1.024) | 0.044a | 1.005 | (0.993-1.018) | 0.342 |
| PMA (HU) | 0.887 | (0.839-0.938) | < 0.001a | 0.901 | (0.853-0.951) | < 0.001a |
| TPMT (mm/m) | 0.851 | (0.759-0.954) | 0.006a | 0.867 | (0.760-0.988) | 0.032a |
Skeletal muscle abnormalities are frequently observed in advanced cirrhosis and cancer, and are associated with unfavorable outcomes[16-18]. Few studies have investigated the relationship between sarcopenia and myosteatosis, and overt HE after TIPS. The present study revealed that the cumulative incidence of overt HE was the highest in patients with concomitant sarcopenia and myosteatosis, followed by patients with myosteatosis alone and sarcopenia alone, while the lowest incidence was identified in patients without myosteatosis and sarcopenia. Furthermore, both myosteatosis and sarcopenia were identified as independent risk factors for predicting the occurrence of overt HE and mortality in cirrhotic patients following TIPS.
Overt HE is a frequent complication of decompensated cirrhosis, which is eventually induced and/or aggravated by the TIPS procedure, and this can impact the quality of life and increase the mortality of patients[19]. The present study revealed that the overall incidence of overt HE after TIPS treatment was 45.4%, which is similar to the reports of previous studies[20,21]. However, merely the MELD score (HR = 1.083, 95%CI: 1.020-1.449, P = 0.009), myosteatosis (HR = 0.930, 95%CI: 0.889-0.974, P = 0.002) and sarcopenia (HR = 0.895, 95%CI: 0.808-0.992, P = 0.035) were identified as independent risk factors after adjusting for confounding variables in the present study. Two retrospective studies on 279 and 284 patients with TIPS reported that a unit of increase in the MELD score would lead to a 1.69 higher odds and a 1.06-fold increase in risk of post-TIPS HE development[22,23]. At the same time, a prospective study on 46 patients who underwent the TIPS procedure also reported that the MELD score and sarcopenia were independently associated with the development of HE after TIPS placement[24]. In addition, several studies reported that age, albumin, creatinine, Child-Pugh score, previous HE, PPG, proton pump inhibitors, and shunt size (> 8 mm vs 8 mm) are potential risks for developing post-TIPS HE[4,23,25-27]. Thus, correct patient selection and early intervention for TIPS based on these potential risk factors may contribute to preventing HE complications, and improving the life quality of patients.
The relationship among myosteatosis, sarcopenia and HE may have different pathophysiological mechanisms. Previous studies have suggested that sarcopenia is caused by impaired protein synthesis and reduced satellite cell function[28]. Furthermore, since the muscle has been considered as an alternative site for the detoxification of ammonia, the accumulation of ammonia may contribute to the development of sarcopenia in cirrhotic patients by interfering with the protein remodeling[29]. On the other hand, myosteatosis may reduce the detoxification of ammonia by inhibiting glutamine synthetase, and increasing inflammatory cytokines[30]. Previous systematic reviews and meta-analyses have revealed that sarcopenia and myosteatosis are highly associated with complications and poor overall survival in patients with various diseases[31,32]. In the present study, patients with sarcopenia (log-rank P = 0.022) or myosteatosis (log-rank P < 0.001) had a higher cumulative incidence of overt HE after TIPS. In addition, subgroup analysis revealed that the cumulative incidence of overt HE was the highest in patients with concomitant sarcopenia and myosteatosis, followed by patients with myosteatosis alone or sarcopenia alone, while the cumulative incidence was the lowest in patients with normal skeletal muscles. These findings are consistent with those reported by previous studies[10,33], showing that sarcopenia and myosteatosis are independent predictive factors for overt HE in patients with cirrhosis. Furthermore, the relationship between skeletal muscle abnormalities (sarcopenia and myosteatosis) and minimal HE in patients with cirrhosis was further suggested by the Firth’s bias-reduced multivariate analysis[33].
Similar to the present study, Liu et al[34] observed that the incidence of overt HE after TIPS was higher in patients with sarcopenia, when compared to patients without sarcopenia (29% and 16%, P = 0.04). However, that study could only identify sarcopenia as the possible risk factor for post-TIPS HE in the univariate analysis (HR = 1.86, 95%CI: 0.99-3.46, P = 0.004), and its significance in the multivariate analysis could not be verified. Another study conducted by Gioia et al[35] revealed that the psychometric HE score, ammonia, and occurrence of minimal and overt HE significantly improved in post-TIPS patients, with an amelioration SMI of > 10%, suggesting that the reversal of sarcopenia after TIPS can reduce the risk of occurrence of minimal or overt HE.
Furthermore, the present study revealed that patients with coexisting myosteatosis and sarcopenia had the lowest cumulative survival rate, and it was identified that hypoalbuminemia (HR = 0.903, 95%CI: 0.827-0.986, P = 0.023), sarcopenia (HR = 0.901, 95%CI: 0.853-0.951, P < 0.001) and myosteatosis (HR = 0.867, 95%CI: 0.760-0.988, P = 0.032) are independent predictors of post-TIPS morbidity. Although other relevant factors, including age, creatinine, Child-Pugh score and MELD score, were significant in the present univariate analysis, these lost its significance as independent predictors in the multivariate analysis. A possible explanation is the inherent complexity and heterogeneity of TIPS cohorts, in general, with a high number of factors potentially affecting the clinical outcomes. A retrospective cohort study on 855 patients with cirrhosis reported that myosteatosis and sarcopenia are associated with mortality after adjusting for factors, and it was even quantitatively extrapolated that one HU of increase in muscle radiodensity corresponds to a 2% decrease in mortality risk (HR = 0.98, 95%CI: 0.96-0.99, P < 0.001)[36]. Another recent retrospective study on 224 patients with TIPS also identified sarcopenia (determined by L3 SMI) as an independent risk factor for mortality after TIPS (HR = 3.0, 95%CI: 1.2-7.8), and that patients who converted from sarcopenic to non-sarcopenic had a higher cumulative survival rate, when compared to those who did not convert (96.4% vs 82.1%, log-rank P = 0.04)[34]. In the present study, the cumulative survival rates were significantly lower in patients with sarcopenia/myosteatosis, when compared to patients without sarcopenia/myosteatosis (log-rank P = 0.001 and log-rank P = 0.002, respectively), further confirming the predictive value of sarcopenia/myosteatosis in the unfavorable prognosis of cirrhotic patients with TIPS.
There were several limitations in the present study. First, the present study was a single-center retrospective cohort study with a limited number of patients, and the intrinsic limitation of the study design appeared to be inescapable. Second, the diagnostic values for sarcopenia and myosteatosis were heterogeneous across studies in the literature. Thus, more studies and external validation of data are needed to standardize the CT-derived diagnostic criteria for sarcopenia and myosteatosis. Third, sarcopenia and myosteatosis were merely evaluated through CT measurements at admission, and these were not re-evaluated through CT imaging during the post-TPS follow-up period[35]. Thus, further well-designed studies are needed to determine whether the improvement in muscle abnormalities after TIPS can reduce the risk of overt HE and variceal rebleeding in post-TIPS patients[34].
The present study revealed that patients with coexisting myosteatosis and sarcopenia had the highest incidence of overt HE, and the lowest cumulative survival rate after TIPS placement, when compared to patients with myosteatosis alone or sarcopenia alone, or patients without muscle abnormalities. Sarcopenia and myosteatosis are independent variables for the development of overt HE and mortality in post-TIPS patients.
Skeletal muscle abnormalities, such as muscle mass depletion (sarcopenia) and fatty infiltration of the muscle (myosteatosis), are frequent complications in cirrhotic patients scheduled for a transjugular intrahepatic portosystemic shunt (TIPS) procedure, leading to an incidence of approximately 20%-50% for overt hepatic encephalopathy (HE).
The motivation of the study was to provide computed tomography (CT) image-based methods for predicting overt HE and mortality after TIPS, based on the sarcopenia and myosteatosis.
The study aims to investigate the association and predictive volubility of sarcopenia and myosteatosis for overt HE, and mortality after TIPS.
The records of cirrhotic patients, who underwent the TIPS procedure at our hospital, were retrospectively reviewed. Transversal psoas muscle thickness and psoas muscle attenuation, which were measured by unenhanced abdominal CT at the level of the third lumbar vertebrae, were used to diagnose the sarcopenia and myosteatosis, respectively. Then, the incidence of overt HE and mortality were compared based on the sarcopenia and myosteatosis status.
A total of 108 patients were collected. Myosteatosis (51.0% vs 16.9%, P < 0.001) and sarcopenia (40.8 vs 18.6%, P = 0.011) were identified to be more frequent in patients with overt HE, when compared to patients without overt HE. The cumulative incidence of overt HE was the highest in patients with concomitant sarcopenia and myosteatosis, followed by patients with myosteatosis or sarcopenia, while this was the lowest in patients without sarcopenia and myosteatosis. In addition, sarcopenia and myosteatosis were independently associated with overt HE and mortality after adjusting for confounding factors in post-TIPS patients.
The CT-based diagnostic method of sarcopenia and myosteatosis can be used as a reliable predictor for the risk of developing overt HE and mortality in cirrhotic patients after TIPS.
In the future, more well-designated trials are required to standardize the CT-derived diagnostic criteria for sarcopenia and myosteatosis. In addition, more validation studies are needed to confirm the predictivities of sarcopenia and myosteatosis in post-TIPS overt HE.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barone M, Italy; Srpcic M, Slovenia S-Editor: Fan JR L-Editor: A P-Editor: Chen YX
| 1. | Lee HL, Lee SW. The role of transjugular intrahepatic portosystemic shunt in patients with portal hypertension: Advantages and pitfalls. Clin Mol Hepatol. 2022;28:121-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Dissegna D, Sponza M, Falleti E, Fabris C, Vit A, Angeli P, Piano S, Cussigh A, Cmet S, Toniutto P. Morbidity and mortality after transjugular intrahepatic portosystemic shunt placement in patients with cirrhosis. Eur J Gastroenterol Hepatol. 2019;31:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Fonio P, Discalzi A, Calandri M, Doriguzzi Breatta A, Bergamasco L, Martini S, Ottobrelli A, Righi D, Gandini G. Incidence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS) according to its severity and temporal grading classification. Radiol Med. 2017;122:713-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Riggio O, Angeloni S, Salvatori FM, De Santis A, Cerini F, Farcomeni A, Attili AF, Merli M. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol. 2008;103:2738-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Patidar KR, Bajaj JS. Covert and Overt Hepatic Encephalopathy: Diagnosis and Management. Clin Gastroenterol Hepatol. 2015;13:2048-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 6. | Bureau C, Thabut D, Jezequel C, Archambeaud I, D'Alteroche L, Dharancy S, Borentain P, Oberti F, Plessier A, De Ledinghen V, Ganne-Carrié N, Carbonell N, Rousseau V, Sommet A, Péron JM, Vinel JP. The Use of Rifaximin in the Prevention of Overt Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt : A Randomized Controlled Trial. Ann Intern Med. 2021;174:633-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 7. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1405] [Article Influence: 127.7] [Reference Citation Analysis (1)] |
| 8. | Ebadi M, Montano-Loza AJ. Clinical relevance of skeletal muscle abnormalities in patients with cirrhosis. Dig Liver Dis. 2019;51:1493-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Kim HY, Jang JW. Sarcopenia in the prognosis of cirrhosis: Going beyond the MELD score. World J Gastroenterol. 2015;21:7637-7647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 10. | Bhanji RA, Moctezuma-Velazquez C, Duarte-Rojo A, Ebadi M, Ghosh S, Rose C, Montano-Loza AJ. Myosteatosis and sarcopenia are associated with hepatic encephalopathy in patients with cirrhosis. Hepatol Int. 2018;12:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 11. | Paternostro R, Lampichler K, Bardach C, Asenbaum U, Landler C, Bauer D, Mandorfer M, Schwarzer R, Trauner M, Reiberger T, Ferlitsch A. The value of different CT-based methods for diagnosing low muscle mass and predicting mortality in patients with cirrhosis. Liver Int. 2019;39:2374-2385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Amini B, Boyle SP, Boutin RD, Lenchik L. Approaches to Assessment of Muscle Mass and Myosteatosis on Computed Tomography: A Systematic Review. J Gerontol A Biol Sci Med Sci. 2019;74:1671-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 235] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 13. | Margadant CC, Bruns ER, Sloothaak DA, van Duijvendijk P, van Raamt AF, van der Zaag HJ, Buskens CJ, van Munster BC, van der Zaag ES. Lower muscle density is associated with major postoperative complications in older patients after surgery for colorectal cancer. Eur J Surg Oncol. 2016;42:1654-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 14. | Wagner D, Büttner S, Kim Y, Gani F, Xu L, Margonis GA, Amini N, Kamel IR, Pawlik TM. Clinical and morphometric parameters of frailty for prediction of mortality following hepatopancreaticobiliary surgery in the elderly. Br J Surg. 2016;103:e83-e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 1512] [Article Influence: 126.0] [Reference Citation Analysis (0)] |
| 16. | Chang KV, Chen JD, Wu WT, Huang KC, Lin HY, Han DS. Is sarcopenia associated with hepatic encephalopathy in liver cirrhosis? J Formos Med Assoc. 2019;118:833-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Merli M, Giusto M, Gentili F, Novelli G, Ferretti G, Riggio O, Corradini SG, Siciliano M, Farcomeni A, Attili AF, Berloco P, Rossi M. Nutritional status: its influence on the outcome of patients undergoing liver transplantation. Liver Int. 2010;30:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 204] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 18. | Ryan AM, Power DG, Daly L, Cushen SJ, Ní Bhuachalla Ē, Prado CM. Cancer-associated malnutrition, cachexia and sarcopenia: the skeleton in the hospital closet 40 years later. Proc Nutr Soc. 2016;75:199-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 360] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 19. | Tripathi D, Stanley AJ, Hayes PC, Travis S, Armstrong MJ, Tsochatzis EA, Rowe IA, Roslund N, Ireland H, Lomax M, Leithead JA, Mehrzad H, Aspinall RJ, McDonagh J, Patch D. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut. 2020;69:1173-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 20. | Li J, Feng D, Pang N, Zhao C, Gao L, Liu S, Li L. Controlling nutritional status score as a new indicator of overt hepatic encephalopathy in cirrhotic patients following transjugular intrahepatic portosystemic shunt. Clin Nutr. 2022;41:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Schindler P, Heinzow H, Trebicka J, Wildgruber M. Shunt-Induced Hepatic Encephalopathy in TIPS: Current Approaches and Clinical Challenges. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Yao J, Zuo L, An G, Yue Z, Zhao H, Wang L, Liu F. Risk Factors for Hepatic Encephalopathy after Transjugular Intrahepatic Portosystemic Shunt in Patients with Hepatocellular Carcinoma and Portal Hypertension. J Gastrointestin Liver Dis. 2015;24:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Lewis DS, Lee TH, Konanur M, Ziegler C, Hall MD, Pabon-Ramos WM, Suhocki PV, Smith TP, Kim CY, Choi SS, Ronald J. Proton Pump Inhibitor Use Is Associated with an Increased Frequency of New or Worsening Hepatic Encephalopathy after Transjugular Intrahepatic Portosystemic Shunt Creation. J Vasc Interv Radiol. 2019;30:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Nardelli S, Lattanzi B, Torrisi S, Greco F, Farcomeni A, Gioia S, Merli M, Riggio O. Sarcopenia Is Risk Factor for Development of Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt Placement. Clin Gastroenterol Hepatol. 2017;15:934-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 25. | Nardelli S, Gioia S, Pasquale C, Pentassuglio I, Farcomeni A, Merli M, Salvatori FM, Nikolli L, Torrisi S, Greco F, Nicoletti V, Riggio O. Cognitive Impairment Predicts The Occurrence Of Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt. Am J Gastroenterol. 2016;111:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Elsaid MI, Rustgi VK. Epidemiology of Hepatic Encephalopathy. Clin Liver Dis. 2020;24:157-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 27. | Rowley MW, Choi M, Chen S, Hirsch K, Seetharam AB. Refractory Hepatic Encephalopathy After Elective Transjugular Intrahepatic Portosystemic Shunt: Risk Factors and Outcomes with Revision. Cardiovasc Intervent Radiol. 2018;41:1765-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 28. | Dasarathy S, McCullough AJ, Muc S, Schneyer A, Bennett CD, Dodig M, Kalhan SC. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J Hepatol. 2011;54:915-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65:1232-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 429] [Article Influence: 47.7] [Reference Citation Analysis (1)] |
| 30. | Aldridge DR, Tranah EJ, Shawcross DL. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol. 2015;5:S7-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 31. | Wijarnpreecha K, Werlang M, Panjawatanan P, Kroner PT, Cheungpasitporn W, Lukens FJ, Pungpapong S, Ungprasert P. Association between sarcopenia and hepatic encephalopathy: A systematic review and meta-analysis. Ann Hepatol. 2020;19:245-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Ahn H, Kim DW, Ko Y, Ha J, Shin YB, Lee J, Sung YS, Kim KW. Updated systematic review and meta-analysis on diagnostic issues and the prognostic impact of myosteatosis: A new paradigm beyond sarcopenia. Ageing Res Rev. 2021;70:101398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 33. | Nardelli S, Lattanzi B, Merli M, Farcomeni A, Gioia S, Ridola L, Riggio O. Muscle Alterations Are Associated With Minimal and Overt Hepatic Encephalopathy in Patients With Liver Cirrhosis. Hepatology. 2019;70:1704-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 34. | Liu J, Ma J, Yang C, Chen M, Shi Q, Zhou C, Huang S, Chen Y, Wang Y, Li T, Xiong B. Sarcopenia in Patients with Cirrhosis after Transjugular Intrahepatic Portosystemic Shunt Placement. Radiology. 2022;303:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 35. | Gioia S, Merli M, Nardelli S, Lattanzi B, Pitocchi F, Ridola L, Riggio O. The modification of quantity and quality of muscle mass improves the cognitive impairment after TIPS. Liver Int. 2019;39:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 36. | Ebadi M, Tsien C, Bhanji RA, Dunichand-Hoedl AR, Rider E, Motamedrad M, Mazurak VC, Baracos V, Montano-Loza AJ. Skeletal Muscle Pathological Fat Infiltration (Myosteatosis) Is Associated with Higher Mortality in Patients with Cirrhosis. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |