Published online May 14, 2023. doi: 10.3748/wjg.v29.i18.2717
Peer-review started: December 29, 2022
First decision: February 1, 2023
Revised: February 12, 2023
Accepted: April 14, 2023
Article in press: April 14, 2023
Published online: May 14, 2023
Processing time: 133 Days and 4.4 Hours
There has been a rapid expansion in the knowledge of paediatric gastroenterology over the recent decade, with a fast-growing repertoire of diagnostic techniques and management strategies for a wide spectrum of childhood gastrointestinal (GI) diseases. Paediatric GI endoscopy is a core competency every paediatric gastroenterologist should possess, and represents one of the most common procedures performed in children for both diagnostic and therapeutic purposes. Yet there remains a dearth of literature on the utility and outcomes of paediatric GI endoscopy in the Asia-Pacific region. Data on the diagnostic value of paediatric GI endoscopy would be an important aspect of discussion, with the emergence of inflammatory bowel disease (IBD) and eosinophilic GI disease as increasingly common endoscopic diagnoses. Time-based trends in paediatric GI endoscopy do point towards more IBD and gastroesophageal reflux disease-related complications being diagnosed, with a declining incidence of GI bleeding. However, the real-world diagnostic value of endoscopy in Asia must be contextualised to the region-specific prevalence of paediatric GI diseases. Helicobacter pylori infection, particularly that of multidrug-resistant strains, remains a highly prevalent problem in specific regions. Paediatric functional GI disorders still account for the majority of childhood GI complaints in most centres, hence the diagnostic yield of endoscopy should be critically evaluated in the absence of alarm symptoms. GI therapeutic endoscopy is also occasionally required for children with ingested foreign bodies, intestinal polyposis or oesophageal strictures requiring dilation. Endoscopic haemostasis is a potentially life-saving skill in cases of massive GI bleeding typically from varices or peptic ulcers. Advanced endoscopic techniques such as capsule endoscopy and balloon-assisted enteroscopy have found traction, particularly in East Asian centres, as invaluable diagnostic and therapeutic tools in the management of IBD, obscure GI bleeding and intestinal polyposis. State of the art endoscopic diagnostics and therapeutics, including the use of artificial intelligence-aided endoscopy algorithms, real-time confocal laser endomicroscopy and peroral endoscopic myotomy, are expected to gain more utility in paediatrics. As paediatric gastroenterology matures as a subspecialty in Asia, it is essential current paediatric endoscopists and future trainees adhere to minimum practice standards, and keep abreast of the evolving trends in the diagnostic and therapeutic value of endoscopy. This review discusses the available published literature on the utility of paediatric GI endoscopy in Asia Pacific, with the relevant clinical outcomes.
Core Tip: Paediatric gastrointestinal (GI) endoscopy has gained traction in Asia as an invaluable tool in the diagnosis and management of chronic GI diseases of current and emerging epidemiological importance. Yet the lack of consensus guidelines and heterogeneity in clinical practice, variability in the referral patterns, healthcare access and prevalence of diseases across the Asian continent, inevitably leads to a wide variance in outcomes for different endoscopic modalities. There is a need for comprehensive and accreditable paediatric endoscopy training in Asia, so that endoscopists adhere to a minimum practice standard and are adequately trained to apply diagnostic and therapeutic endoscopic techniques appropriately and competently.
- Citation: Huang JG, Tanpowpong P. Paediatric gastrointestinal endoscopy in the Asian-Pacific region: Recent advances in diagnostic and therapeutic techniques. World J Gastroenterol 2023; 29(18): 2717-2732
- URL: https://www.wjgnet.com/1007-9327/full/v29/i18/2717.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i18.2717
Gastrointestinal (GI) symptoms remain a common presenting feature of various ailments in childhood, and the advent of modern GI diagnostic and therapeutic techniques have allowed centres across Asia to utilise GI endoscopy early in the evaluation of a child with suspected GI disease. There remains a lack of consensus statements and guidelines on the utility of paediatric GI endoscopy in the Asian context, leading to a lack of consistency in clinical practice and highly variable clinical outcomes between centres[1]. The epidemiological situation in Asia is unique as rapidly evolving disease trends occur amidst changing lifestyle and environmental factors: Paediatric inflammatory bowel disease (IBD) is of rapidly emerging importance[2], yet its early diagnosis is often complicated by the relatively high prevalence of GI infections such as intestinal tuberculosis[3] and other infectious diseases in certain endemic regions. Helicobacter pylori (H. pylori) has fallen in prevalence in developed regions of Asia but remains a highly prevalent pathogen in association with peptic ulcer disease and gastric cancer in other regions[4]. There is increasing awareness and knowledge of the role of the gut-brain-axis in disorders of gut-brain interaction or functional GI disorders[5], prompting interest in the actual diagnostic yield of GI endoscopy in childhood abdominal pain syndromes. While Asia remains vastly heterogeneous in socioeconomic status, access to early endoscopy and advanced endoscopy techniques has greatly improved, and has enhanced both the diagnostics and therapeutics in various chronic paediatric GI ailments such as IBD, intestinal polyposis syndromes, varices in portal hypertension and GI strictures.
Improved healthcare access and the maturation of paediatric gastroenterology as a specialty has contributed to the rapid rise in endoscopies performed in children[6]. There are few paediatric endoscopy consortiums within Asia, with most of the published experience coming from East Asian centres. Data from the Japan Paediatric Endoscopy Society demonstrated a five-fold increase in the number of paediatric endoscopies performed in the latest survey in 2011-2016 vs 2000-2004, an increase in advanced endoscopies such as endoscopic retrograde cholangioscopies and balloon-assisted enteroscopies, with a slight rise in adverse events (0.25% from 0.03%) inevitably so from increased procedural complexity[7]. A retrospective review of children undergoing upper GI endoscopy at the Children’s Hospital of Philadelphia (CHOP) demonstrated a rapid rise of first-time endoscopes from 107 in 1985 to 1294 in 2005[8]. Interestingly this same study also showed a decline in the proportion of GI bleeding (34% to 5%), a decline in both the overall clinical severity of cases and corresponding endoscopic and histological abnormalities, and yet a rise in the proportion of upper GI endoscopies done for abdominal pain (23% to 43%)[8]. It begets the question if centres are performing more endoscopies in otherwise healthy children, hence the need to examine the diagnostic yield of paediatric GI endoscopies and the impact on clinical management.
There are very limited publications comparing trends in paediatric endoscopy within Asia, to contrast with the aforementioned North American data. In contrast to the significant fall in oesophageal histological abnormalities detected in the CHOP study, a Japanese paediatric study demonstrated a significant rise in the proportion of erosive oesophagitis (9.8% to 18.1%) or endoscopic Barrett’s oesophagus (2.5% to 9.6%) between eras 2005-2012 to 2013-2019[9]. It is postulated by the authors that this trend is related to dietary changes and the decreasing prevalence of H. pylori amongst the Japanese population. Published adult data from Japan[10] and Malaysia[11] also clearly demonstrates the decline in peptic ulcers and associated upper GI bleeding, with rates of gastroesophageal reflux disease (GERD) increasing on the contrary.
A single centre in Beijing shows a statistically significant rise in the number of colonoscopies performed amongst the 0-3 year age group, from the era 2005-2011 to 2012-2017 (3.0% to 14.1%, P < 0.001) but no significant change in diagnostic yield rates between the two eras (36.3% to 38.2%)[12]. The proportions of IBD and colonic polyps detected did not differ between the two eras. A paediatric South Korean cohort study in Busan, however did show a substantial rise in colonoscopies from 2001-2005 to 2011-2015 (200 to 746)[13], with trends in indications somewhat mirroring those in the CHOP study. The number of colonoscopies performed for abdominal pain increased from 27.5% to 43.7%, while those performed for haematochezia fell from 56.0% to 42.5%. There was an actual rise in diagnostic yield with the proportion of Crohn’s disease diagnosed doubling from 13.5% (2001-2005) to 26.8% (2011-2015). This is consistent with the rapid rise in paediatric IBD incidence observed in South Korea[14] and the rest of Asia[2].
Tables 1 and 2 describe the overall diagnostic yield in upper and lower endoscopies (follow-up endoscopies excluded unless stated otherwise). The positive endoscopic yield varies substantially between centres: Between 45%-93% for upper endoscopies (Table 1) and correspondingly 43%-85% (Table 2) for lower endoscopies. The large variability in the diagnostic yields is clearly multifactorial, from varying referral patterns and referral indications, regional differences in disease prevalence and healthcare resource allocation between centres. For instance, in single-centre studies out of Malaysia[15] and India[16], abdominal pain/dyspepsia were indications for just 13.4% and 17.4% of upper GI endoscopies respectively, while upper GI bleeding and variceal surveillance were more common indications. This is in contrast to other cohorts e.g., the North American CHOP[8] and the South Korean cohort[17], where abdominal pain/dyspepsia were the most common indications (43%-64% of upper GI endoscopies) and upper GI bleeding being far less prevalent. These differing trends in GI bleeding rates could be attributed to varying prevalence in H. pylori-associated gastroduodenal ulcer disease and upper GI haemorrhage[10], variability in healthcare access, timeliness of referrals for biliary atresia and other childhood liver diseases. Resource-scarce centres also likely prioritise allocation of endoscopy resources for GI emergencies, typically acute GI bleeding, rather than uncomplicated abdominal pain.
| Country | Year | Cohort size (n) | Indications (top three) (%) | Findings (%) | Ref. |
| China | 2018-2019 | 2268 | Abdominal pain (86.2). Vomiting (31.1). Weight loss (15.1) | 62.5% abnormal. Highest yield in dysphagia | [105] |
| Nepal | 2013-2016 | 270 | Abdominal pain (77.3). Vomiting/reflux (8.4). Failure to thrive (7.0) | 92.5% abnormal. Gastroduodenitis (28.1). Antral gastritis (18.5). Erosive gastritis (15.9) | [106] |
| India | 2013-2016 | 822 | Variceal surveillance (19.1). Dyspepsia (17.4). Upper GI bleed (16.5) | 45.8% abnormal. Duodenal ulcers/varices most common | [16] |
| Israel | 2014 | 407 | Suspected coeliac disease (28.2). Abdominal pain (15.0). Persistent H. pylori (10.3) | 59.2% abnormal. Coeliac disease (28), H. pylori (16.5), Crohn’s disease (5.4) | [107] |
| Jordan | 2014-2020 | 778 | Abdominal pain (45.1). Vomiting (21.1). Weight loss (10.3) | 47.2% abnormal. H. pylori (66.1). Coeliac disease (30.4). Eosinophilic GI disease (3.6) | [18] |
| Malaysia | 2008-2011 | 231 OGD. 44 OGD and Colonoscopies | Variceal surveillance (50.0). Upper GI bleed (26.0). Abdominal pain (13.4) | 79.0% abnormal | [15] |
| South Korea | 2008-2013 | 554 | Abdominal pain (64.1). Dysphagia (9.0). Vomiting (9.0) | 88.1% abnormal. Gastritis (53.1). Esophagitis (17.7) | [17] |
| Thailand | 2000-2002 | 38 | Recurrent abdominal pain | 45% abnormal. H. pylori (26.3) | [108] |
| United States | 2002-2005 | 454 | Recurrent abdominal pain | 38.1% abnormal. Reflux esophagitis (23.0). H. pylori (5.0). Peptic ulcers (3.0) | [37] |
| Country | Year | Cohort size (n) | Indications (top three) (%) | Findings (%) | Ref. |
| Australia | 2001-2010 | 999 colonoscopies (15.0% done as follow-up) | Suspected IBD (45.0). Haematochezia (20.0). Abdominal pain (5.0) | 61.0% abnormal. IBD (28.2). Polyp (3.9) | [109] |
| China | 2005-2017 | 326 | - | 62.6% abnormal. IBD (14.1). Nonspecific colitis (27.0). Polyp (12.0) | [12] |
| China | 2013-2016 | 229 | Abdominal pain (35.4). Haematochezia (27.9). Crissum abscess/anal fistula (17.5) | 64.2% abnormal. IBD (38.8). Polyp (27.2). Nonspecific colitis (26.5) | [110] |
| Hong Kong | 2003-2008 | 79 | Haematochezia (58.0). Suspected IBD (29.1) | 50.6% abnormal. IBD (16.5). Polyp (29.1) | [111] |
| Japan | 2011-2016 | 275 | Haematochezia (75.0). Diarrhoea (13.0). Abdominal pain (2.2) | 77.1% abnormal. IBD (18.5). Eosinophilic GI disease (23.0). Polyp (14.0) | [33] |
| Japan | 2007-2015 | 274 | Haematochezia (42.7). Abdominal pain (30.7). Diarrhoea (15.3) | 66.8% abnormal. IBD (43.4). Eosinophilic GI disease (2.2). Polyp (5.9). Nonspecific colitis (8.4) | [112] |
| Malaysia | 2010-2015 | 121 | Suspected IBD (30.0). Haematochezia (21.0). Change in bowel habits (17.0) | 85.0% abnormal. IBD (42.0). Polyp (7.0). Nonspecific/infective colitis (25.0) | [113] |
| Saudi Arabia | 1993-2002 | 183 | - | 44.0% abnormal. Nonspecific colitis or rectal ulcer (71.0). Polyp (20.0) | [114] |
| South Korea | 2008-2013 | 168 | Abdominal pain (37.5). Diarrhoea (28.0). Haematochezia (27.4) | 43.5% abnormal. IBD (19.6). Polyp (1.8). Nonspecific inflammation (14.3) | [17] |
| South Korea | 2011-2015 | 746 | Abdominal pain (43.7). Haematochezia (42.5). Diarrhoea (29.1) | 72.2% abnormal. IBD (33.9). Polyp (11.5) | [13] |
| Taiwan, China | 1998-2010 | 192 | Haematochezia (53.5). Abdominal pain (20.6). Iron deficiency anaemia (11.8) | 75% abnormal. IBD (8.3). Polyp (20.4). Nonspecific colitis (23.4) | [115] |
It must be emphasised that the vast Asian continent is both economically and culturally heterogenous: Peptic ulcerations and H. pylori still remain fairly common in certain regions. Jordanian data from 2014-2020 showed H. pylori accounted for 66.1% of all abnormal upper GI endoscopies in children[18]; an earlier Israeli paediatric study showed 22.5% of all upper GI endoscopies had peptic ulcerations, of which 66.3% of these were H. pylori positive[19]. The global trend of increasing antimicrobial resistance in H. pylori[20] hinders the implementation of eradication strategies in these regions with high H. pylori prevalence. Vietnam has a very high H. pylori prevalence rate (> 75%) associated with the highest prevalence of gastric cancer in Southeast Asia[4]. A study of 237 symptomatic Vietnamese children undergoing upper GI endoscopy for suspected H. pylori-associated gastroduodenal disease showed 80.6% and 71.7% of H. pylori isolates were resistant to clarithromycin and amoxicillin respectively[21]. These figures place Vietnam as one of the regions with the highest rates of H. pylori antimicrobial resistance, and emphasises the added importance of upper GI endoscopy to obtain biopsies for a culture and antimicrobial sensitivity-based eradication strategy. This has implications on the feasibility of healthcare access and early endoscopy, as H. pylori is typically most prevalent in regions of lower socioeconomic status.
While IBD was once considered uncommon in Asia, an emerging number of paediatric publications have documented its meteoric rise in prevalence[2,14] as alluded to earlier. Table 2 describes IBD detection rates between 14%-40% of all abnormal paediatric colonoscopies performed in Asia, emphasising the importance of IBD as an endoscopic diagnosis. Of note, ‘non-specific colitis’ accounts for a substantial 8%-27% of all abnormal colonoscopic findings across most published studies. It is highly plausible that these unspecified cases may evolve into a more definite diagnosis of IBD on follow-up investigations: These published IBD rates underestimate the true burden of IBD in Asia and may just represent the tip of the iceberg. Paediatric-onset IBD typically has less classical endoscopic features and more subtle histologic findings[22] compared to adult-onset disease, often prompting a diagnosis of ‘indeterminate colitis’[23] or ‘IBD-unclassified’.
Intestinal tuberculosis, while often considered as an important differential to IBD, was far less common a colonoscopic diagnosis than IBD, even in regions with high tuberculosis burden. Intestinal tuberculosis accounted for 1.5% cases vs 14.1% IBD and 27.0% non-specific colitis in a Mainland Chinese paediatric cohort[12]; a Kuwaiti study showed Intestinal tuberculosis in 1.2% vs IBD in 21.3% of children undergoing colonoscopy[24]. There is a paucity of epidemiologic data from the Indian subcontinent, where the world’s tuberculosis burden is the highest. An Indian publication in 1991 described a cohort of 72 Indian children undergoing colonoscopy: Tuberculous colitis was seen in 2.7%, ulcerative colitis in 5.5% and amoebic colitis in 1.3% of cases[25]. A more recent Bangladeshi study of 332 children undergoing colonoscopy showed intestinal tuberculosis in 1.5% vs IBD in 6% and nonspecific colitis in 13.6%[26]. It is not detailed in the aforementioned studies how intestinal tuberculosis is reliably distinguished from IBD, and this distinction remains a diagnostic challenge especially in India. A therapeutic trial of empirical anti-tuberculous therapy is still often practised in cases of diagnostic uncertainty[3].
There is little published data on the actual prevalence of eosinophilic GI diseases (EGIDs) in Asia, although it is believed the incidence of EGIDs will rise in tandem with the rise of allergic disorders[27]. Symptoms may mimic GERD especially in infants and young children, to recurrent dyspepsia, dysphagia and/or food impaction in the older child. The diagnosis of an EGID hinges greatly on histological findings of significant tissue eosinophilia (> 15 eosinophils per high power field) in the absence of other attributable causes. The increasingly widespread empirical use of proton-pump inhibitors in children[28,29] may reverse the tissue eosinophilia in a subset of patients with acid suppression-responsive EGID[30], further complicating the diagnostic process. Nevertheless, a short finite trial of acid-suppression is still deemed reasonable, with upper GI endoscopy and/or pH-impedance testing reserved for those refractory to empirical treatment or for those who cannot be weaned off acid-suppressive therapy[31].
In a fairly large study of 910 South Korean children presenting with symptoms of oesophageal dysfunction (vomiting, dysphagia, persistent reflux), 1.5% was diagnosed with eosinophilic oesophagitis (EoE) and 1.3% with eosinophilic gastroenteritis. 30.8% of patients with EGID had normal macroscopic findings, stating the importance of performing biopsies on even normal-appearing segments. The authors commented that the incidence of EoE was similar to a previous Japanese study but much lower than 10%-15% incidence in Western cohorts[32]. A multi-centre study in Japanese children undergoing colonoscopies by Nambu et al[33] showed EGIDs accounted for a substantially high proportion (23.0%) of the diagnoses followed by IBD (19.0%), but the authors had included food allergies and food protein-induced proctocolitis within the spectrum of EGIDs. Future studies are required to see if the incidence of EGIDs in Asia would mimic the rising trend seen with IBD.
While paediatric endoscopy has high value in the diagnosis and management of IBD, H. pylori and EGIDs, most children with GI complaints have a non-organic aetiology yet the prevalence of functional GI disorders in Asian children is largely unknown and seldom described. Functional constipation was the most common condition identified in otherwise healthy Vietnamese (5.6%) and Mainland Chinese (7.0%) children between 7-48 mo of age[34,35]. Recurrent abdominal pain and dyspepsia in children is far more likely to be of a functional aetiology than in adults, prompting one to question the necessity and cost-effectiveness of invasive investigations. El-Matary et al[36] evaluated a cohort of 103 British children fulfilling Apley’s original criteria of recurrent abdominal pain, via a series of blood, stool, imaging investigations with endoscopy done as clinically indicated. Approximately 70% of these children had a non-organic aetiology to their abdominal symptoms, with irritable bowel syndrome being the most common diagnosis. This study was conducted within a hospital setting, and it is likely that the actual prevalence of paediatric functional GI disorders would be much higher if the study were to be repeated in a community setting.
The diagnostic yield of paediatric GI endoscopy in childhood abdominal pain varies widely between published cohorts (Table 1), depending on the referral indication, presence of alarm symptoms[37] and/or index of suspicion guided by abnormal biochemistry (e.g., coeliac serology) or imaging pre-endoscopy. Moreover, the definition of a ‘positive diagnostic yield’ can be debatable as positive endoscopic pathology does not equate causality of symptoms e.g., H. pylori infection is often asymptomatic and may be an ‘innocent bystander’ in children with functional abdominal pain[38], especially in the high-prevalence areas. An Israeli study of 329 children undergoing endoscopy for various indications (abdominal pain, diarrhoea, failure to thrive, short stature and iron deficiency anaemia) showed only 36% of children with abdominal pain had a diagnostic finding on endoscopy; if the child had abdominal pain in association with an objective test e.g., positive coeliac serology and/or iron deficiency, the diagnostic yield would be more than 50% while those with subjective symptoms of nausea and constipation would have a positive yield in less than 25%[39]. A Hong Kong-based cohort of 80 children, fulfilling the Rome III criteria of functional dyspepsia and undergoing upper GI endoscopy, showed only 6.3% had ulcerations or erosions. There was a strongly positive correlation between alarm features e.g., nocturnal pain and endoscopic findings[40]. These findings suggest that a risk stratification strategy, combining a clinical assessment for alarm features and the use of non-invasive objective tests, would be potentially helpful in discerning those who would benefit most from diagnostic endoscopy. An example would be the stool calprotectin assay, which is now commonly used globally as a highly sensitive stool biomarker for gut inflammation, both in the diagnosis and monitoring of IBD[41,42].
Advanced endoscopy techniques such as video capsule endoscopy (VCE) and balloon-assisted enteroscopy (BAE) can enhance diagnostics especially for obscure small bowel pathology, which would otherwise be inaccessible with standard endoscopy. VCE has been approved in children as young as 2 years old since 2009[43] and remains one of the commonly used modalities to complement standard endoscopy findings or when standard endoscopy has been non-diagnostic. Much of the published Asian experience with VCE has been from large East Asian (China[44], Japan[45] and South Korea[46]) adult and paediatric cohorts. A large Mainland Chinese cohort of 825 children in a single paediatric IBD centre underwent VCE for the main indication of abdominal pain (61.2%) followed by anaemia (17.0%)[44]. The authors noted a much higher diagnostic yield (55.6%) primarily of Crohn’s disease-related small bowel pathology, compared to previous studies for similar indications quoting 20%-28%[47,48]; this was ascribed to a higher referral load of suspected IBD patients, further emphasising the strong influence of referral indications on published yields. Other typical VCE findings included idiopathic small bowel ulcers, intestinal polyps, lymphangiectasia and vascular malformations.
While the experience of VCE in adult cohorts has been for obscure GI bleeding, VCE has gained increasing utility for the pan-enteric evaluation of IBD[49]. VCE can be utilised as a non-invasive first-line modality in suspected IBD cases after a patency capsule test, followed by confirmatory endoscopy. VCE may also be advantageous in detecting early mucosal healing in IBD disease monitoring. A Korean paediatric study of Crohn’s disease found VCE to be more sensitive than magnetic resonance enterography in detecting mucosal healing and early therapeutic response in the first year post diagnosis[50]. Other indications for VCE include the surveillance of intestinal polyposis, particularly in Peutz-Jeghers’ syndrome where small bowel polyps have been known to form a lead point for small bowel intussusception. The use of VCE facilitates the planning of BAE for definitive small bowel polyp clearance[51].
As observed with studies of VCE, much of the published Asian paediatric experience with BAE are from East Asian cohorts[52-54]. Of note, these cohorts may include a varying number of patients undergoing balloon-assisted endoscopic retrograde cholangioscopes primarily for therapy of biliary stenoses. BAE complements the diagnostic value of VCE by providing the means to obtain histological samples and provide therapeutic intervention. Hagiwara et al[54] described a multi-centre study of 96 BAEs (both antegrade and retrograde) in 79 paediatric patients. The main indications were for follow-up of IBD, obscure GI bleeding, abdominal pain, and therapy of hereditary polyposis syndromes. The positive diagnostic yield in obscure GI bleeding and abdominal pain was 48%. There were higher reported diagnostic yields of approximately 77% for similar indications in the mainland Chinese paediatric cohorts[53,55], but this variation can be explained by differing referral indications as aforementioned.
While most of these techniques are not in mainstream use at most Asian-Pacific paediatric gastroenterology centres, they are worth discussing in brevity as recent publications have discussed their utility in enhancing current paediatric GI diagnostics.
Transnasal endoscopy: This technique would be useful especially in children with EoE as they commonly require multiple upper GI endoscopies for mucosal surveillance[56]. Transnasal endoscopy may be done as an unsedated office procedure with topical pharyngeal anaesthesia, and significantly save time, costs and endoscopy resources.
Mucosal impedance: This technique measures transmucosal conductivity and thus the mucosal integrity of the oesophageal mucosa, via a catheter containing very closely spaced impedance sensors resting close to the mucosa wall. This catheter is inserted through the working channel of a standard upper GI scope, such that the sensors rest directly on the oesophageal mucosa. This has advantages over standard 24-h pH impedance studies, by directly measuring the barrier function of the oesophageal mucosa in a matter of seconds while the child is sedated[57]. Mucosal impedance can be used to discern between GERD, EoE and non-GERD conditions, as well as to monitor treatment response/disease activity in GERD and EoE[58,59].
Endoluminal functional lumen imaging probe: Endoluminal functional lumen imaging probe (EndoFLIP) is another adjunct technique to measure oesophageal luminal dimensions, distensibility, pressure changes and motility[60], typically while the child is sedated for standard upper GI endoscopy. It involves insertion of a sensor balloon-mounted catheter transorally, and inflating the balloons within the oesophageal and gastric lumens. While high resolution oesophageal manometry is the current gold standard in the assessment of oesophageal function, it is potentially uncomfortable and requires the child to cooperate with swallowing during the manometry. EndoFLIP has utility in the assessment of patients with GERD, EoE[61] and achalasia, as well as patients experiencing persistent symptoms post fundoplication.
Confocal laser endomicroscopy: Confocal laser endomicroscopy is an endoscopic technique that allows for high-resolution histological examination of the GI mucosa at the cellular level. Besides its obvious utility in real-time detection of neoplastic lesions in adults, the group at Sheffield Children’s Hospital, United Kingdom has described its usefulness as a biopsy-free method of assessing mucosal pathology in enteropathies, EoE, ileo-colitis and polyposis[62]. This enhances the diagnostic accuracy of GI endoscopy, and reduces the time, risks and costs associated with multiple GI mucosal biopsies.
Artificial intelligence and machine learning in endoscopic diagnostics: Patel et al[63] recently reviewed the potential application of artificial intelligence (AI) in paediatric GI pathologies. The aim of utilising machine learning, a form of AI, is to at least semi-automate the process of macroscopic pathology and pattern recognition, which would otherwise be subject to the endoscopist’s individual expertise and experience. In paediatrics, AI could potentially automate the process of accurately classifying macroscopic disease severity and extent in IBD and celiac disease, as current endoscopic scoring systems are often time-consuming and subjective. Current AI research in adults is driven by the need for accurate polyp detection: Early published data of the computer-aided detection EYE system demonstrates its AI driven algorithm detects and classifies polyps with excellent sensitivity and specificity[64]. AI would play a major role in standardising endoscopy outcomes across different endoscopists of varying experience levels, especially in units with rotating trainees.
Foreign body ingestion is a common presentation in the ambulatory and emergency department settings. The exploratory behaviour and normal development of young infants and toddlers lead these kids to accidentally grasp and ingest foreign objects. The common age group usually ranges between 6 mo to 6 years[65]. The initial manifestation can range from asymptomatic to significant GI symptoms (drooling, dysphagia, vomiting, obstructive symptoms), coughing, choking, severe respiratory distress, or even death[66,67]. One of the largest cohorts from China reported 1265 children (aged 6 mo to 16 years) admitted with a history of foreign body ingestion, of which 552 (43%) children had detected foreign bodies from endoscopies. The two most common objects were coins (49%) and non-metallic sharp objects (31%)[66]. A systematic review also noted that the coin was the most frequently ingested object in the paediatric population[68]. In contrast to most reports, a medical record review in 105 Iranian children, the button battery was the most commonly found object in 41%[69]. The types of foreign body vary between different countries and geographic locations, that may be likely due to diverse sociocultural and dietary factors[70]. Another study from Khorana et al[67] from northern Thailand included 194 patients aged < 15 years (median age of 44 mo) found that most were symptomatic (56%), with vomiting as the commonest complaint. The most common location of impacted foreign bodies was in the oesophagus (37%), similar to most previous reports[68]. Plain radiography can usually confirm the location, size and shape of most radiopaque objects (such as coin, magnets, safety pin, etc.), but has little diagnostic value for radiolucent objects such as plastic toys, fishbone, or woods. Contrast studies or computed tomography scans may be needed to locate the radiolucent foreign bodies before deciding on the further management.
Timing of foreign body removal: As most of the ingested objects were coins, which were mostly considered as small and blunt objects, spontaneous passage usually occurred[67,71]. However, endoscopic intervention or even surgical exploration may be needed in some cases especially individuals with symptoms or complications. The timing of endoscopic intervention in paediatric foreign body ingestion has been proposed in various guidelines published from various scientific societies, namely the European Society for Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN)/European Society of Gastrointestinal Endoscopy (ESGE)[71], and the North American Society of Paediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Endoscopy Committee[72]. The proper timing of endoscopy is mainly based on 3 key factors: The type of ingested objects, the location, and the presence of symptoms. Types of common foreign bodies are button batteries, magnets, sharp objects, long objects, absorptive objects, drug packets, impacted food boluses, and coins.
Button batteries: During the past few decades, major concerns with button battery ingestion have been raised as the integrity of the strong alkali-containing battery can be degraded and cause severe caustic injury to the GI mucosa, especially the oesophagus (i.e., a hollow organ with small lumen). A cohort from the United States reviewing 8648 cases of button and cylindrical battery ingestions, occurring between 1990-2008, found 73 cases with major adverse outcomes (0.8%), including prolonged compromise of feeding and/or breathing that required surgical procedures, tube feedings, tracheostomies. There were 13 deaths (0.15%) related to damage to the oesophagus, major vessels, and/or the airway[73]. Huang et al[74] from China reported children with inhaled/ingested button batteries and found that 13 of 116 (11%) cases had button batteries either in the oesophagus or stomach (n = 6 and 7, respectively). One child developed an oesophageal stricture and another one died from sudden cardiac arrest during the perioperative period. A recent Position Paper from ESPGHAN proposed 2 major strategies in the diagnosis and management of button battery ingestion in children. This includes: (1) Computed tomography scan to evaluate injuries to the adjacent organs and blood vessels before endoscopic removal if ingested > 12 h even in asymptomatic children; and (2) Honey (in children > 1 year of age) and sucralfate can be considered in ingestions < 12 h while waiting for endoscopic removal[75].
Endoscopic techniques of foreign body removal: Conventional flexible endoscopy is a safe and effective tool for removing most foreign bodies from the GI tract. A high success rate is found when using retrieval nets, polypectomy snares, and the rat-tooth forceps[71]. Opasanon et al[76] reported 34 Thai patients with upper GI tract foreign bodies and found that removal was successfully performed in all cases with either rat-tooth forceps, snare, dormia basket or tripods with no procedure-related complications.
Device-assisted enteroscopy for foreign body removal: The challenging cases in paediatric foreign body ingestion are typically the ones with objects beyond the reach of the conventional endoscope, i.e., the depths of the small bowel in the jejunoileal area. Adult-based ESGE guidelines on small bowel endoscopy and device-assisted enteroscopy strongly recommend enteroscopy as an alternative to surgery for retrieving foreign bodies retained in the small bowel in patients without acute intestinal obstruction[77]. Device-assisted enteroscopy refers to any adjunct device used to assist endoscopic advancement into the small bowel (balloon, overtube, stiffening device). As with the use of BAE as an advanced diagnostic tool discussed earlier[54], age- and weight-appropriate device-assisted enteroscopy may be used for foreign body removal and other therapeutic applications within the small bowel.
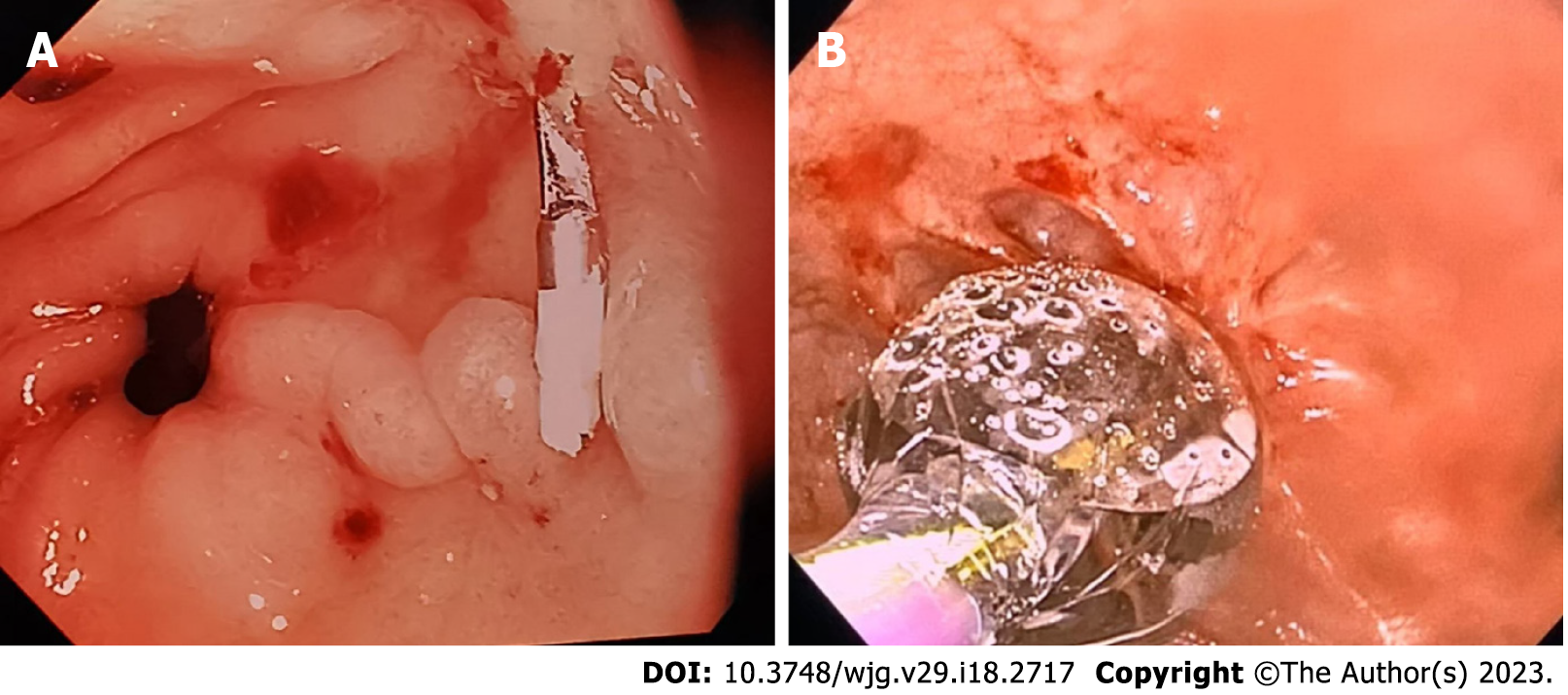
GI bleeding can be divided into upper GI and lower GI bleeding. Upper GI bleeding is defined as bleeding from the GI tract proximal to the ligament of Treitz, while lower GI bleeding is bleeding occurring distal to the aforementioned ligament. Upper GI bleeding can also be further divided into non-variceal bleeding and variceal bleeding which may (or usually) need haemostatic intervention (Figure 1A). On the other hand, for the paediatric population, lower GI bleeding rarely needs endoscopic intervention in the colon or distal small bowel mainly because of the 3 following reasons: (1) Most of the severe GI bleeding occurs up in the upper GI tract; (2) Acute colonic bleeding in children usually stops spontaneously; and (3) Various common aetiologies such as Meckel’s diverticulum, intussusception, colitis from infection, inflammation or allergy, or anal fissures rarely need endoscopic intervention to stop bleeding. One of the few exceptions being endoscopic polypectomy for juvenile (colonic) polyps which usually present in a non-urgent setting. After initial haemodynamic stabilization consisting of judicious fluid resuscitation and, if necessary, blood product replacement, endoscopic haemostatic intervention for the lesions causing GI bleeding would need to be justified based on the site and type of the lesion, patient’s underlying disease and resource availability/procedural feasibility in conjunction with the potential contraindications.
Haemostasis for acute upper GI bleeding: Non-variceal and variceal bleeding: ESPGHAN/ESGE guidelines suggest early oesophago-gastroduodenoscopy within 12 h in cases presenting with acute upper GI bleeding that require ongoing circulatory support, or those presenting with large volume haematemesis or melena (weak recommendation and low quality of evidence, but a strong reco
Endoscopic haemostatic techniques for non-variceal lesions: Overall, lesions causing non-variceal upper GI bleeding such as bleeding ulcers or Dieulafoy lesion should use either thermal techniques such as heater probe, bipolar probe or mechanical techniques such as haemostatic clips, with/without epinephrine injection for controlling bleeding[71,79]. Yabe et al[80] reported 36 Japanese children with upper GI bleeding from gastroduodenal ulcers (50%) or gastritis (26%), and 14/36 (39%) underwent haemostatic intervention [clips (n = 12), hypersaline and epinephrine injection and coagulation therapy (n = 1), pure ethanol injections (n = 1)] with 100% initial success rate. Rebleeding occurred only in one patient who was initially treated with ethanol injections.
Endoscopic haemostatic techniques for variceal bleeding: For oesophageal varices, Zargar et al[81] demonstrated that endoscopic variceal band ligation is more effective than sclerotherapy in 49 Indian children with extrahepatic portal vein obstruction and variceal bleeding. Band ligation required fewer endoscopic sessions [3.9 (SD 1.1) vs 6.1 (SD 1.7) times for sclerotherapy] and had lower rates of rebleeding (4% vs 25%)[81]. Since then, studies comparing the efficacy of banding with sclerotherapy in children are sparse. A recent Cochrane Review was initially planned to analyse randomized controlled trials (RCTs) but was eventually unable to find any RCTs comparing band ligation vs sclerotherapy as primary variceal prophylaxis (i.e., preventing the first variceal bleeding episode in children with oesophageal varices) in children with chronic liver disease or portal vein thrombosis[82].
The technique of polypectomy is based on the location, morphology, and size. The ESPGHAN guideline suggests using cold biopsy forceps for small polyps (< 3 mm), hot or cold snaring in polyps diameter 3-8 mm, and hot snaring in the larger polyps[71]. However, hot biopsy forceps induce larger histopathological lesions, increased necrotic depth and submucosal inflammation in a pig’s colon model[83], and more cytological artefacts[84]. A retrospective study in 91 Korean children, who underwent endoscopy to find polyps, found that polyp size was the one single factor associated with the presence of any polyps located proximal to the splenic flexure [odds ratio = 2.3, 95% confidence interval (CI): 1.3-4.3]. Polyps proximal to the splenic flexure and sessile morphology were associated with the presence of any adenomatous polyp. Therefore, the authors concluded that a full colonoscopy remains crucial before the occurrence of complications[85]. Another study from Thailand investigated 32 patients with symptoms of colorectal polyps such as haematochezia, rectal mass, or diarrhoea. Most (20/32, 63%) had a single polyp, 6/32 had 2-4 polyps, and a minority (6/32) was diagnosed with polyposis coli. Most had polyps in the rectosigmoid region and only 6 cases had polyps proximal to the splenic flexure. All had pathologically confirmed juvenile polyps without adenomatous changes, which demonstrated an absence of malignant potential[86].
Dilation of the oesophagus is indicated when symptoms of oesophageal stricture/stenosis occur. Various causes include congenital anomalies, post caustic ingestion, EoE, and GERD[87]. Symptoms of oesophageal stricture include dysphagia, odynophagia, food bolus impaction, vomiting and poor oral intake. Nowadays, the 2 main options for oesophageal dilatation in oesophageal stricture are balloon dilation and bougie dilation.
Balloon dilation (Figure 1B) can be safely performed under both the direct endoscopic and/or fluoroscopic examinations, while most of the bougie dilations are Savary-Gilliard bougies that could dilate up to 12 mm in children age < 5 years and 15 mm in older children. The “rule of 3” has been widely used as dilation to not more than 3 times the stricture diameter with a minimal period of 3 wk between dilation sessions and an average of 3 sessions in total[88,89]. Lan et al[90] reported 75 children from Hong Kong with oesophageal strictures [post-oesophageal atresia repair (n = 63), reflux esophagitis (n = 7), caustic ingestion (n = 3) and post-fundoplication (n = 2)], who underwent a total of 260 balloon dilations (mean number of 3.4 sessions per patient). Four oesophageal perforations (1.5%) were noted, with one child required surgical repair; all other patients were asymptomatic after the dilation sessions. Balloon dilation has been reported to be more effective and less traumatic than the bougie dilation, but a study from India reported comparable complications (perforations of 0.9%)[89], and a recent study from China reported a high perforation rate of 4.4% in children undergoing oesophageal balloon dilation[91]. The aforementioned study also reported only a 60% success rate, and found that stricture length was the main determining factor of treatment outcome. Therefore, a universally-agreed dilation choice remains controversial. Furthermore, oesophageal stent placement, intralesional mitomycin C or steroid injections have also been used in refractory oesophageal strictures in children[92].
Low volume bowel preparations preferred: Ileocolonoscopy is an established diagnostic and therapeutic tool in a variety of GI disorders. The optimal bowel preparation is an important key success factor in paediatric ileocolonoscopy[93]. While standardised bowel preparation protocols remain unavailable[94], more recent recommendations propose low-volume preparations using either polyethylene glycol (PEG)[95] along with ascorbate or sodium picosulfate magnesium citrate (SPMC)[71]. The preferable regimen in standard clinical practice should provide optimal colonic cleansing with small volumes of laxatives, acceptable palatability and drinkability and minimal side effects. In 2017, 15 RCTs (n = 1435) from 2124 studies with heterogeneity/bias risk compared PEG with other medications (sodium phosphate enema (n = 2 studies, relative risk = 1.27 with 95%CI: 0.66-2.44), SPMC (n = 3 studies, relative risk = 0.99 with 95%CI: 0.89-1.11), sennasoids (n = 3 studies, relative risk = 0.73 with 95%CI: 0.31-1.76) which showed no difference in the bowel preparation quality. Noninferior efficacy was also noted when comparing low volume PEG with SPMC vs standard volume PEG. Children who received PEG regimen also needed nasogastric tube insertions more often than those receiving the SPMC regimen (38% vs 1.6%)[96]. Later in 2022, four good quality RCTs (n = 390) showed higher tolerability and acceptability in the SPMC group when compared to the PEG group with comparable efficacy[97].
Split bowel preparation regimens: The newer studies also implement a ‘split regimen’ of the laxatives at different timepoints prior to GI endoscopy[98-100]. Sriphongphankul et al[98] performed an RCT in 45 children aged 2-18 years. The split dose group was given PEG in 2 split doses for 8-12 h apart and at least 6 h before the procedure, and the full single dose was given once the night before scope. Successful preparation (defined as Boston Bowel Preparation Scale ≥ 6) was superior in the split group (95% vs 72% in the standard high-volume PEG regimen). Willingness to repeat the same protocol was also much higher (83% vs 36%, P = 0.002), but nasogastric tube insertion rates were comparable (57% vs 68%). A later meta-analysis, including 4 paediatric studies, also found a trend of significantly higher efficacy in the split dose group (P = 0.07) but significant heterogeneity was noted among studies[99]. Therefore, further high-quality RCTs with low risk of bias are required. With regards to the recommended diet before the procedure, Jiao et al[101] studied 321 Chinese children and found that either 1-d or 2-d low residue diet had similar efficacy in bowel preparation but the 1-d low residue diet group had higher acceptability.
The endoscopic interventions for (esophageal) achalasia, a rare condition with incomplete or lack of normal lower esophageal sphincter relaxation, include pneumatic dilation and botulinum toxin injection. Recently, peroral endoscopic myotomy (POEM) has become another therapeutic intervention in both adults and children that demonstrates satisfactory success rate. The NASPGHAN Endoscopy Committee recently reviewed various aspects of POEM[102]. In brief, the myotomy is made starting from 8-10 cm above to 2-3 cm below the gastroesophageal junction along the cardia. Submucosal injection of the posterior wall is then performed to create a mucosotomy and submucosal tunnel, which would reveal the circular muscle fibers of the lower oesophagus. Complete myotomy of the circular muscle layer is made with longitudinal muscle layer underneath and the clip is finally deployed to close the mucosotomy. However, studies reporting efficacy and complications of POEM in children remain limited. Furthermore, appropriate training and adequate number of the performed procedures would also be required before implementing POEM as a standard of care in paediatric achalasia.
ESPGHAN launched a Position Paper on paediatric endoscopy training in 2020[103]. The main content on achievement of training milestones, with regards to competency and procedural numbers including ‘Train the trainers’ courses, have been mentioned. Educational material such as e-learning, simulator training would also be needed to train the trainees in paediatric endoscopy.
Different scientific societies recommend varying competency thresholds for lower and upper GI endoscopies. NASPGHAN, the Joint Advisory Group in GI Endoscopy Paediatric Certification from the United Kingdom, the Conjoint Committee for Recognition of Training in Gastrointestinal Endoscopy from Australia proposed a minimum of 100-120 lower GI endoscopies with a caecal intubation rate of ≥ 90% (ranges from 15-30 min). NASPGHAN and the Joint Advisory Group from the United Kingdom proposed a minimum of 100 upper GI endoscopies and the Australian Committee proposed a minimum of 200 upper GI endoscopies (≥ 100 in children). Interestingly, ESPGHAN did not specifically define any numbers for endoscopies. The number of therapeutic endoscopies such as foreign body removal, haemostatic intervention, and polypectomy vary greatly across different societies. One of the preprocedure objective outcomes is the rate of adequate bowel preparation before ileocolonoscopy, with a minimum standard of 90% and a target of 95%.
Another important point is that the group suggests endoscopic procedures in children be performed by endoscopists trained in paediatric gastroenterology with established procedure-specific competency. Special consideration must be made to a child requiring GI endoscopy. Physicians need to consider the size of the patient, indications and contraindication of the procedure, proper equipment, bowel preparation, anaesthesia and sedation as well as the psycho-emotional factors of the children and their caregivers throughout the process[104]. Therefore, before implementing training in paediatric endoscopy in the Asia-Pacific region, aforesaid aspects should be carefully considered and implemented in the formal standardized curriculum. The Asian Pan-Pacific Society for Paediatric Gastroenterology, Hepatology and Nutrition conducted its first Paediatric Endoscopy Masterclass on June 2 to 3, 2022 in Bangkok with the purpose of addressing gaps in paediatric endoscopy training within the Asia-Pacific region. The subsequent way forward would be a regionalised set of guidelines and consensus statements, to facilitate standardisation of indications and endoscopic terminology across different paediatric GI centres.
Paediatric GI endoscopy has undoubtedly gained utility in the Asia-Pacific region as an invaluable tool in the diagnostics and management of GI diseases of current and emerging epidemiological importance. This is the first article to comprehensively review the evolving epidemiologic trends in paediatric GI endoscopy within Asia-Pacific, and delve into the future directions for paediatric endoscopy training and the advent of state of the art endoscopic techniques which are increasingly applied in the adult population. Yet the lack of consensus guidelines and heterogeneity in clinical practice, variablity in the referral patterns, healthcare access and disease prevalence across the Asian continent, inevitably leads to a wide variance in outcomes for different endoscopic modalities. While it is crucial that early endoscopy is done for a prompt diagnosis and treatment, it must be balanced with avoiding un-necessarily invasive investigations in otherwise benign functional GI conditions. The maturation of paediatric gastroenterology as a subspecialty hence necessitates comprehensive and accreditable endoscopy training, so that paediatric endoscopists in Asia adhere to a minimum practice standard and are adequately trained to apply diagnostic and therapeutic endoscopic techniques appropriately and competently. The inauguration of regular endoscopy masterclasses and workshops by the regional society Asian Pan-Pacific Society for Paediatric Gastroenterology, Hepatology and Nutrition sets the stage for more uniformity in endoscopic practices and outcomes, as well as future inter-regional collaborative efforts in paediatric endoscopic research.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Singapore
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cabezuelo AS, Spain; Maldonado KE, Guatemala S-Editor: Wang JJ L-Editor: A P-Editor: Chen YX
| 1. | Jiang MZ. [Development and thoughts of digestive endoscopy in children]. Zhongguo Dang Dai Er Ke Za Zhi. 2022;24:350-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Huang JG, Wong YKY, Chew KS, Tanpowpong P, Calixto Mercado KS, Reodica A, Rajindrajith S, Chang KC, Ni YH, Treepongkaruna S, Lee WS, Aw MM. Epidemiological characteristics of Asian children with inflammatory bowel disease at diagnosis: Insights from an Asian-Pacific multi-centre registry network. World J Gastroenterol. 2022;28:1830-1844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Ma JY, Tong JL, Ran ZH. Intestinal tuberculosis and Crohn's disease: challenging differential diagnosis. J Dig Dis. 2016;17:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Quach DT, Vilaichone RK, Vu KV, Yamaoka Y, Sugano K, Mahachai V. Helicobacter pylori Infection and Related Gastrointestinal Diseases in Southeast Asian Countries: An Expert Opinion Survey. Asian Pac J Cancer Prev. 2018;19:3565-3569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Mukhtar K, Nawaz H, Abid S. Functional gastrointestinal disorders and gut-brain axis: What does the future hold? World J Gastroenterol. 2019;25:552-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 105] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (3)] |
| 6. | Friedt M, Welsch S. An update on pediatric endoscopy. Eur J Med Res. 2013;18:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Kudo T, Abukawa D, Nakayama Y, Segawa O, Uchida K, Jimbo K, Shimizu T. Nationwide survey of pediatric gastrointestinal endoscopy in Japan. J Gastroenterol Hepatol. 2021;36:1545-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Franciosi JP, Fiorino K, Ruchelli E, Shults J, Spergel J, Liacouras CA, Leonard M. Changing indications for upper endoscopy in children during a 20-year period. J Pediatr Gastroenterol Nutr. 2010;51:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Kusakari M, Nakayama Y, Horiuchi A, Nakazawa Y. Trends in gastroesophageal reflux disease in Japanese children and adolescents. Pediatr Int. 2020;62:1269-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Fujimoto S, Tsuruoka N, Esaki M, Takamori A, Sakata Y, Shimoda R, Akutagawa T, Node K, Anzai K, Sugisaki N, Iwakiri R, Takagi K, Yamanouchi K, Fujimoto K. Decline incidence in upper gastrointestinal bleeding in several recent years: data of the Japan claims database of 13 million accumulated patients. J Clin Biochem Nutr. 2021;68:95-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Leow AH, Lim YY, Liew WC, Goh KL. Time trends in upper gastrointestinal diseases and Helicobacter pylori infection in a multiracial Asian population--a 20-year experience over three time periods. Aliment Pharmacol Ther. 2016;43:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Li J, Gu F, Li ZL, Lu YM. [Pediatric colonoscopy findings and changing patterns from Beijing in one institutional experience over 12 years]. Beijing Da Xue Xue Bao Yi Xue Ban. 2019;51:819-823. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 13. | Park JH. Pediatric Colonoscopy: The Changing Patterns and Single Institutional Experience Over a Decade. Clin Endosc. 2018;51:137-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Kwak MS, Cha JM, Lee HH, Choi YS, Seo SI, Ko KJ, Park DI, Kim SH, Kim TJ. Emerging trends of inflammatory bowel disease in South Korea: A nationwide population-based study. J Gastroenterol Hepatol. 2019;34:1018-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Lee WS, Zainuddin H, Boey CC, Chai PF. Appropriateness, endoscopic findings and contributive yield of pediatric gastrointestinal endoscopy. World J Gastroenterol. 2013;19:9077-9083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 16. | Wani MA, Zargar SA, Yatoo GN, Haq I, Shah A, Sodhi JS, Gulzar GM, Khan M. Endoscopic Yield, Appropriateness, and Complications of Pediatric Upper Gastrointestinal Endoscopy in an Adult Suite: A Retrospective Study of 822 Children. Clin Endosc. 2020;53:436-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Lee YW, Chung WC, Sung HJ, Kang YG, Hong SL, Cho KW, Kang D, Lee IH, Jeon EJ. Current status and clinical impact of pediatric endoscopy in Korea. Korean J Gastroenterol. 2014;64:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Altamimi E, Odeh Y, Al-Quraan T, Mohamed E, Rawabdeh N. Diagnostic yield and appropriate indication of upper endoscopy in Jordanian children. BMC Pediatr. 2021;21:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Egbaria R, Levine A, Tamir A, Shaoul R. Peptic ulcers and erosions are common in Israeli children undergoing upper endoscopy. Helicobacter. 2008;13:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Ghotaslou R, Leylabadlo HE, Asl YM. Prevalence of antibiotic resistance in Helicobacter pylori: A recent literature review. World J Methodol. 2015;5:164-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 155] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 21. | Le LTT, Nguyen TA, Nguyen NA, Nguyen YTH, Nguyen HTB, Nguyen LT, Vi MT, Nguyen T. Helicobacter pylori Eradication Efficacy of Therapy Based on the Antimicrobial Susceptibility in Children with Gastritis and Peptic Ulcer in Mekong Delta, Vietnam. Children (Basel). 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 22. | Washington K, Greenson JK, Montgomery E, Shyr Y, Crissinger KD, Polk DB, Barnard J, Lauwers GY. Histopathology of ulcerative colitis in initial rectal biopsy in children. Am J Surg Pathol. 2002;26:1441-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Carvalho RS, Abadom V, Dilworth HP, Thompson R, Oliva-Hemker M, Cuffari C. Indeterminate colitis: a significant subgroup of pediatric IBD. Inflamm Bowel Dis. 2006;12:258-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Kalaoui M, Radhakrishnan S, al Shamali M, Hasan F, al-Nakib B. Findings of colonoscopy in children: experience from Kuwait. J Trop Pediatr. 1998;44:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Thapa BR, Mehta S. Diagnostic and therapeutic colonoscopy in children: experience from a pediatric gastroenterology centre in India. Indian Pediatr. 1991;28:383-389. [PubMed] |
| 26. | Begum F, Nahid KL, Islam F, Majumder W, Rukunuzzaman M, Karim AB. Paediatric Colonoscopy: Experience from Pediatric Gastroenterology and Nutrition Department, BSMMU. Bangladesh J Child Health. 2021;45:25-28. [DOI] [Full Text] |
| 27. | Gerez IF, Lee BW, van Bever HP, Shek LP. Allergies in Asia: differences in prevalence and management compared with western populations. Expert Rev Clin Immunol. 2010;6:279-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Yang S, Trinh NTH, Chalumeau M, Kaguelidou F, Ruemmele FM, Milic D, Lemaitre M, Cohen JF, Taine M. Pediatric Prescriptions of Proton Pump Inhibitors in France (2009-2019): A Time-Series Analysis of Trends and Practice Guidelines Impact. J Pediatr. 2022;245:158-164.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Hales CM, Kit BK, Gu Q, Ogden CL. Trends in Prescription Medication Use Among Children and Adolescents-United States, 1999-2014. JAMA. 2018;319:2009-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 30. | Franciosi JP, Mougey EB, Dellon ES, Gutierrez-Junquera C, Fernandez-Fernandez S, Venkatesh RD, Gupta SK. Proton Pump Inhibitor Therapy for Eosinophilic Esophagitis: History, Mechanisms, Efficacy, and Future Directions. J Asthma Allergy. 2022;15:281-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 31. | Rosen R, Vandenplas Y, Singendonk M, Cabana M, DiLorenzo C, Gottrand F, Gupta S, Langendam M, Staiano A, Thapar N, Tipnis N, Tabbers M. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:516-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 610] [Cited by in RCA: 529] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 32. | Lee K, Choe BH, Kang B, Kim S, Kim JY, Shim JO, Lee YM, Lee EH, Jang HJ, Ryoo E, Yang HR. Nationwide Multicenter Study of Eosinophilic Esophagitis in Korean Children. Pediatr Gastroenterol Hepatol Nutr. 2020;23:231-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Nambu R, Hagiwara SI, Kakuta F, Hara T, Shimizu H, Abukawa D, Iwama I, Kagimoto S, Arai K. Current role of colonoscopy in infants and young children: a multicenter study. BMC Gastroenterol. 2019;19:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Huang Y, Tan SY, Parikh P, Buthmanaban V, Rajindrajith S, Benninga MA. Prevalence of functional gastrointestinal disorders in infants and young children in China. BMC Pediatr. 2021;21:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Chia LW, Nguyen TVH, Phan VN, Luu TTN, Nguyen GK, Tan SY, Rajindrajith S, Benninga MA. Prevalence and risk factors of functional gastrointestinal disorders in Vietnamese infants and young children. BMC Pediatr. 2022;22:315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 36. | El-Matary W, Spray C, Sandhu B. Irritable bowel syndrome: the commonest cause of recurrent abdominal pain in children. Eur J Pediatr. 2004;163:584-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Thakkar K, Chen L, Tessier ME, Gilger MA. Outcomes of children after esophagogastroduodenoscopy for chronic abdominal pain. Clin Gastroenterol Hepatol. 2014;12:963-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | Jones NL, Koletzko S, Goodman K, Bontems P, Cadranel S, Casswall T, Czinn S, Gold BD, Guarner J, Elitsur Y, Homan M, Kalach N, Kori M, Madrazo A, Megraud F, Papadopoulou A, Rowland M; ESPGHAN, NASPGHAN. Joint ESPGHAN/NASPGHAN Guidelines for the Management of Helicobacter pylori in Children and Adolescents (Update 2016). J Pediatr Gastroenterol Nutr. 2017;64:991-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 294] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 39. | Fachler T, Shteyer E, Orlanski Meyer E, Shemasna I, Lev Tzion R, Rachman Y, Bergwerk A, Turner D, Ledder O. Pediatric Gastrointestinal Endoscopy: Diagnostic Yield and Appropriateness of Referral Based on Clinical Presentation: A Pilot Study. Front Pediatr. 2021;9:607418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 40. | Tam YH, Chan KW, To KF, Cheung ST, Mou JW, Pang KK, Wong YS, Sihoe JD, Lee KH. Impact of pediatric Rome III criteria of functional dyspepsia on the diagnostic yield of upper endoscopy and predictors for a positive endoscopic finding. J Pediatr Gastroenterol Nutr. 2011;52:387-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Lin WC, Wong JM, Tung CC, Lin CP, Chou JW, Wang HY, Shieh MJ, Chang CH, Liu HH, Wei SC; Taiwan Society of Inflammatory Bowel Disease Multicenter Study. Fecal calprotectin correlated with endoscopic remission for Asian inflammatory bowel disease patients. World J Gastroenterol. 2015;21:13566-13573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Samant H, Desai D, Abraham P, Joshi A, Gupta T, Dherai A, Ashavaid T. Fecal calprotectin and its correlation with inflammatory markers and endoscopy in patients from India with inflammatory bowel disease. Indian J Gastroenterol. 2015;34:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Fornaroli F, Gaiani F, Vincenzi F, Bizzarri B, Ghiselli A, Kayali S, Leandro G, Di Mario F, De' Angelis GL. Applications of wireless capsule endoscopy in pediatric age: an update. Acta Biomed. 2018;89:40-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 44. | Wu J, Huang Z, Wang Y, Tang Z, Lai L, Xue A, Huang Y. Clinical features of capsule endoscopy in 825 children: A single-center, retrospective cohort study. Medicine (Baltimore). 2020;99:e22864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Ohmiya N, Oka S, Nakayama Y, Iwama I, Nakamura M, Shimizu H, Sumioka A, Abe N, Kudo T, Osawa S, Honma H, Okuhira T, Mtsufuji S, Imaeda H, Ota K, Matsuoka R, Hotta N, Inoue M, Nakaji K, Takamaru H, Ozeki K, Kobayashi T, Hosoe N, Tajiri H, Tanaka S. Safety and efficacy of the endoscopic delivery of capsule endoscopes in adult and pediatric patients: Multicenter Japanese study (AdvanCE-J study). Dig Endosc. 2022;34:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Lim YJ, Lee OY, Jeen YT, Lim CY, Cheung DY, Cheon JH, Ye BD, Song HJ, Kim JS, Do JH, Lee KJ, Shim KN, Chang DK, Park CH, Jang BI, Moon JS, Chun HJ, Choi MG, Kim JO; Korean Gut Image Study Group. Indications for Detection, Completion, and Retention Rates of Small Bowel Capsule Endoscopy Based on the 10-Year Data from the Korean Capsule Endoscopy Registry. Clin Endosc. 2015;48:399-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Xue M, Chen X, Shi L, Si J, Wang L, Chen S. Small-bowel capsule endoscopy in patients with unexplained chronic abdominal pain: a systematic review. Gastrointest Endosc. 2015;81:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Huang L, Huang Z, Tai Y, Wang P, Hu B, Tang C. The small bowel diseases detected by capsule endoscopy in patients with chronic abdominal pain: A retrospective study. Medicine (Baltimore). 2018;97:e0025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Hilmi I, Kobayashi T. Capsule endoscopy in inflammatory bowel disease: when and how. Intest Res. 2020;18:265-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Hwang JY, Moon SW, Lee YJ, Park JH, Kim YW, Kim TU, Ryu H. Capsule Endoscopy versus Magnetic Resonance Enterography for Evaluation of Pediatric Small Bowel Crohn's Disease: Prospective Study. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 51. | Kirakosyan E, Lokhmatov M. High-Tech Diagnostic Methods and Enteroscopic Treatment of Children with Peutz-Jeghers Syndrome. Eur J Pediatr Surg. 2020;30:529-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Yokoyama K, Yano T, Kumagai H, Mizuta K, Ono S, Imagawa T, Yamamoto H, Yamagata T. Double-balloon Enteroscopy for Pediatric Patients: Evaluation of Safety and Efficacy in 257 Cases. J Pediatr Gastroenterol Nutr. 2016;63:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Chen H, Liu Y, Fu L, Lin X, Fan D, Li C. Clinical utility of double-balloon enteroscopy in children: A single-centre experience in South China. J Paediatr Child Health. 2019;55:188-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Hagiwara SI, Kudo T, Kakuta F, Inoue M, Yokoyama K, Umetsu S, Iwama I, Yodoshi T, Tatsuki M, Shimizu T, Nakayama Y. Clinical Safety and Utility of Pediatric Balloon-assisted Enteroscopy: A Multicenter Prospective Study in Japan. J Pediatr Gastroenterol Nutr. 2019;68:306-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Liu W, Xu C, Zhong J. The diagnostic value of double-balloon enteroscopy in children with small bowel disease: report of 31 cases. Can J Gastroenterol. 2009;23:635-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Nguyen N, Lavery WJ, Capocelli KE, Smith C, DeBoer EM, Deterding R, Prager JD, Leinwand K, Kobak GE, Kramer RE, Menard-Katcher C, Furuta GT, Atkins D, Fleischer D, Greenhawt M, Friedlander JA. Transnasal Endoscopy in Unsedated Children With Eosinophilic Esophagitis Using Virtual Reality Video Goggles. Clin Gastroenterol Hepatol. 2019;17:2455-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 57. | Vaezi MF, Choksi Y. Mucosal Impedance: A New Way To Diagnose Reflux Disease and How It Could Change Your Practice. Am J Gastroenterol. 2017;112:4-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Ates F, Yuksel ES, Higginbotham T, Slaughter JC, Mabary J, Kavitt RT, Garrett CG, Francis D, Vaezi MF. Mucosal impedance discriminates GERD from non-GERD conditions. Gastroenterology. 2015;148:334-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 59. | Lowry MA, Vaezi MF, Correa H, Higginbotham T, Slaughter JC, Acra S. Mucosal Impedance Measurements Differentiate Pediatric Patients With Active Versus Inactive Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2018;67:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Hoskins B, Almazan E, Mogul D, Ng K. Endoluminal Functional Lumen Imaging Probe Is Safe in Children Under Five Years Old. J Pediatr Gastroenterol Nutr. 2022;74:e148-e152. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 61. | Hoffmann NV, Keeley K, Wechsler JB. Esophageal Distensibility Defines Fibrostenotic Severity in Pediatric Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 62. | Venkatesh K, Cohen M, Evans C, Delaney P, Thomas S, Taylor C, Abou-Taleb A, Kiesslich R, Thomson M. Feasibility of confocal endomicroscopy in the diagnosis of pediatric gastrointestinal disorders. World J Gastroenterol. 2009;15:2214-2219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Patel V, Khan MN, Shrivastava A, Sadiq K, Ali SA, Moore SR, Brown DE, Syed S. Artificial Intelligence Applied to Gastrointestinal Diagnostics: A Review. J Pediatr Gastroenterol Nutr. 2020;70:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | Neumann H, Kreft A, Sivanathan V, Rahman F, Galle PR. Evaluation of novel LCI CAD EYE system for real time detection of colon polyps. PLoS One. 2021;16:e0255955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 65. | Reilly BK, Stool D, Chen X, Rider G, Stool SE, Reilly JS. Foreign body injury in children in the twentieth century: a modern comparison to the Jackson collection. Int J Pediatr Otorhinolaryngol. 2003;67 Suppl 1:S171-S174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 66. | Cheng W, Tam PK. Foreign-body ingestion in children: experience with 1,265 cases. J Pediatr Surg. 1999;34:1472-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 197] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 67. | Khorana J, Tantivit Y, Phiuphong C, Pattapong S, Siripan S. Foreign Body Ingestion in Pediatrics: Distribution, Management and Complications. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 68. | Jayachandra S, Eslick GD. A systematic review of paediatric foreign body ingestion: presentation, complications, and management. Int J Pediatr Otorhinolaryngol. 2013;77:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 69. | Jafari SA, Khalesi M, Partovi S, Kiani M, Ahanchian H, Kianifar H. Ingested Foreign Bodies Removed by flexible Endoscopy in Pediatric Patients: A 10-year Retrospective Study [corrected]. Iran J Otorhinolaryngol. 2014;26:175-179. [PubMed] |
| 70. | Chotigavanich C, Ballali S, Foltran F, Passali D, Bellussi L, Gregori D; ESFBI Study Group. Foreign bodies injuries in children: analysis of Thailand data. Int J Pediatr Otorhinolaryngol. 2012;76 Suppl 1:S80-S83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 71. | Thomson M, Tringali A, Dumonceau JM, Tavares M, Tabbers MM, Furlano R, Spaander M, Hassan C, Tzvinikos C, Ijsselstijn H, Viala J, Dall'Oglio L, Benninga M, Orel R, Vandenplas Y, Keil R, Romano C, Brownstone E, Hlava Š, Gerner P, Dolak W, Landi R, Huber WD, Everett S, Vecsei A, Aabakken L, Amil-Dias J, Zambelli A. Paediatric Gastrointestinal Endoscopy: European Society for Paediatric Gastroenterology Hepatology and Nutrition and European Society of Gastrointestinal Endoscopy Guidelines. J Pediatr Gastroenterol Nutr. 2017;64:133-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 72. | Kramer RE, Lerner DG, Lin T, Manfredi M, Shah M, Stephen TC, Gibbons TE, Pall H, Sahn B, McOmber M, Zacur G, Friedlander J, Quiros AJ, Fishman DS, Mamula P; North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Endoscopy Committee. Management of ingested foreign bodies in children: a clinical report of the NASPGHAN Endoscopy Committee. J Pediatr Gastroenterol Nutr. 2015;60:562-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 351] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 73. | Litovitz T, Whitaker N, Clark L. Preventing battery ingestions: an analysis of 8648 cases. Pediatrics. 2010;125:1178-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 74. | Huang T, Li WQ, Xia ZF, Li J, Rao KC, Xu EM. Characteristics and outcome of impacted button batteries among young children less than 7 years of age in China: a retrospective analysis of 116 cases. World J Pediatr. 2018;14:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Mubarak A, Benninga MA, Broekaert I, Dolinsek J, Homan M, Mas E, Miele E, Pienar C, Thapar N, Thomson M, Tzivinikos C, de Ridder L. Diagnosis, Management, and Prevention of Button Battery Ingestion in Childhood: A European Society for Paediatric Gastroenterology Hepatology and Nutrition Position Paper. J Pediatr Gastroenterol Nutr. 2021;73:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 76. | Opasanon S, Akaraviputh T, Methasate A, Sirikun J, Laohapensang M. Endoscopic management of foreign body in the upper gastrointestinal tract: a tertiary care center experience. J Med Assoc Thai. 2009;92:17-21. [PubMed] |
| 77. | Pennazio M, Rondonotti E, Despott EJ, Dray X, Keuchel M, Moreels T, Sanders DS, Spada C, Carretero C, Cortegoso Valdivia P, Elli L, Fuccio L, Gonzalez Suarez B, Koulaouzidis A, Kunovsky L, McNamara D, Neumann H, Perez-Cuadrado-Martinez E, Perez-Cuadrado-Robles E, Piccirelli S, Rosa B, Saurin JC, Sidhu R, Tacheci I, Vlachou E, Triantafyllou K. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2022. Endoscopy. 2023;55:58-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 150] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 78. | Thomson MA, Leton N, Belsha D. Acute upper gastrointestinal bleeding in childhood: development of the Sheffield scoring system to predict need for endoscopic therapy. J Pediatr Gastroenterol Nutr. 2015;60:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Valera JM, Pino RQ, Poniachik J, Gil LC, O'Brien M, Sáenz R, Quigley EM. Endoscopic band ligation of bleeding dieulafoy lesions: the best therapeutic strategy. Endoscopy. 2006;38:193-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 80. | Yabe K, Kouchi K, Takenouchi A, Matsuoka A, Kudou W, Nakata C. Current status and future challenges in the endoscopic management of non-variceal upper gastrointestinal bleeding in children. Pediatr Surg Int. 2020;36:1451-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 81. | Zargar SA, Javid G, Khan BA, Yattoo GN, Shah AH, Gulzar GM, Singh J, Rehman BU, Din Z. Endoscopic ligation compared with sclerotherapy for bleeding esophageal varices in children with extrahepatic portal venous obstruction. Hepatology. 2002;36:666-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 82. | Gana JC, Cifuentes LI, Gattini D, Torres-Robles R. Band ligation versus sclerotherapy for primary prophylaxis of oesophageal variceal bleeding in children with chronic liver disease or portal vein thrombosis. Cochrane Database Syst Rev. 2020;11:CD011803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 83. | Chino A, Karasawa T, Uragami N, Endo Y, Takahashi H, Fujita R. A comparison of depth of tissue injury caused by different modes of electrosurgical current in a pig colon model. Gastrointest Endosc. 2004;59:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Mönkemüller KE, Fry LC, Jones BH, Wells C, Mikolaenko I, Eloubeidi M. Histological quality of polyps resected using the cold versus hot biopsy technique. Endoscopy. 2004;36:432-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 85. | Kim JY, Kim YB, Choi S, Lee YM, Kim HJ, Kim SC, Jang HJ, Choi SY, Yi DY, Lee Y, Choi YJ, Kang Y, Lee KJ, Hong SJ, Hwang JH, Kwak S, Choe BH, Kang B. Associations of Polyp Characteristics in Children and Adolescents Presenting with Less Than Five Colorectal Polyps: A Full Colonoscopy Is Still Required. Gut Liver. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 86. | Ukarapol N, Singhavejakul J, Lertprasertsuk N, Wongsawasdi L. Juvenile polyp in Thai children--clinical and colonoscopic presentation. World J Surg. 2007;31:395-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Pearson EG, Downey EC, Barnhart DC, Scaife ER, Rollins MD, Black RE, Matlak ME, Johnson DG, Meyers RL. Reflux esophageal stricture--a review of 30 years' experience in children. J Pediatr Surg. 2010;45:2356-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 88. | Cakmak M, Boybeyi O, Gollu G, Kucuk G, Bingol-Kologlu M, Yagmurlu A, Aktug T, Dindar H. Endoscopic balloon dilatation of benign esophageal strictures in childhood: a 15-year experience. Dis Esophagus. 2016;29:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 89. | Poddar U, Thapa BR. Benign esophageal strictures in infants and children: results of Savary-Gilliard bougie dilation in 107 Indian children. Gastrointest Endosc. 2001;54:480-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Lan LC, Wong KK, Lin SC, Sprigg A, Clarke S, Johnson PR, Tam PK. Endoscopic balloon dilatation of esophageal strictures in infants and children: 17 years' experience and a literature review. J Pediatr Surg. 2003;38:1712-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 91. | Geng LL, Liang CP, Chen PY, Wu Q, Yang M, Li HW, Xu ZH, Ren L, Wang HL, Cheng S, Xu WF, Chen Y, Zhang C, Liu LY, Li DY, Gong ST. Long-Term Outcomes of Caustic Esophageal Stricture with Endoscopic Balloon Dilatation in Chinese Children. Gastroenterol Res Pract. 2018;2018:8352756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 92. | Angelino G, Tambucci R, Torroni F, De Angelis P, Dall'Oglio L. New therapies for esophageal strictures in children. Curr Opin Pediatr. 2021;33:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 93. | Pall H, Zacur GM, Kramer RE, Lirio RA, Manfredi M, Shah M, Stephen TC, Tucker N, Gibbons TE, Sahn B, McOmber M, Friedlander J, Quiros JA, Fishman DS, Mamula P. Bowel preparation for pediatric colonoscopy: report of the NASPGHAN endoscopy and procedures committee. J Pediatr Gastroenterol Nutr. 2014;59:409-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 94. | Hunter A, Mamula P. Bowel preparation for pediatric colonoscopy procedures. J Pediatr Gastroenterol Nutr. 2010;51:254-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 95. | Fang S, Song Y, Liu Y, Wang L. Randomized clinical trial: efficacy and tolerability of two different split dose of low-volume polyethylene glycol electrolytes for bowel preparation before colonoscopy in hospitalized children. Pediatr Res. 2021;90:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Gordon M, Karlsen F, Isaji S, Teck GO. Bowel preparation for elective procedures in children: a systematic review and meta-analysis. BMJ Paediatr Open. 2017;1:e000118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 97. | Dziechciarz P, Ruszczyński M, Horvath A. Sodium Picosulphate with Magnesium Citrate versus Polyethylene Glycol for Bowel Preparation in Children: A Systematic Review. Pediatr Gastroenterol Hepatol Nutr. 2022;25:228-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 98. | Sriphongphankul H, Tanpowpong P, Lertudomphonwanit C, Treepongkaruna S. Split dose versus full single-dose regimen of polyethylene glycol for bowel preparation in pediatric colonoscopy: a pilot study of randomized controlled trial. Eur J Gastroenterol Hepatol. 2019;31:1382-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 99. | Cao J, Zhang W, Hu J, Huang Y, Zhao L, Cai R, Bao Y, Li M. Single versus split dose polyethylene glycol for bowel preparation in children undergoing colonoscopy: a systematic review and meta-analysis. Ann Palliat Med. 2020;9:3028-3037. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 100. | Tripathi PR, Poddar U, Yachha SK, Sarma MS, Srivastava A. Efficacy of Single- Versus Split-dose Polyethylene Glycol for Colonic Preparation in Children: A Randomized Control Study. J Pediatr Gastroenterol Nutr. 2020;70:e1-e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 101. | Jiao L, Wang J, Zhao W, Zhu X, Meng X, Zhao L. Comparison of the effect of 1-day and 2-day low residue diets on the quality of bowel preparation before colonoscopy. Saudi J Gastroenterol. 2020;26:137-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 102. | Mencin AA, Sethi A, Barakat MT, Lerner DG. Peroral Endoscopic Myotomy (POEM) in Children: A State of the Art Review. J Pediatr Gastroenterol Nutr. 2022;75:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 103. | Broekaert I, Tzivinikos C, Narula P, Antunes H, Dias JA, van der Doef H, Isoldi S, Norsa L, Romano C, Scheers I, Silbermintz A, Tavares M, Torroni F, Urs A, Thomson M. European Society for Paediatric Gastroenterology, Hepatology and Nutrition Position Paper on Training in Paediatric Endoscopy. J Pediatr Gastroenterol Nutr. 2020;70:127-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 104. | ASGE Standards of Practice Committee; Lightdale JR, Acosta R, Shergill AK, Chandrasekhara V, Chathadi K, Early D, Evans JA, Fanelli RD, Fisher DA, Fonkalsrud L, Hwang JH, Kashab M, Muthusamy VR, Pasha S, Saltzman JR, Cash BD; American Society for Gastrointestinal Endoscopy. Modifications in endoscopic practice for pediatric patients. Gastrointest Endosc. 2014;79:699-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 105. | Wang S, Qiu X, Chen J, Mei H, Yan H, You J, Huang Y. Pediatric esophagogastroduodenoscopy in china: indications, diagnostic yield, and factors associated with findings. BMC Pediatr. 2022;22:522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 106. | Joshi BG, Ghimire M. Diagnostic Yield of Upper Gastrointestinal Endoscopy in Children: A Three Years Experience. J Nepal Paediat Society. 2020;40:87-92. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 107. | Berger TD, Soffer S, Vurzel-Harel T, Silbermintz A, Fleishaker H, Shamir R, Zevit N. The Yield of Upper Gastrointestinal Endoscopy at a Pediatric Tertiary Care Center. Isr Med Assoc J. 2020;22:164-168. [PubMed] |
| 108. | Ukarapol N, Lertprasertsuk N, Wongsawasdi L. Recurrent abdominal pain in children: the utility of upper endoscopy and histopathology. Singapore Med J. 2004;45:121-124. [PubMed] |
| 109. | Kawada PS, O'Loughlin EV, Stormon MO, Dutt S, Lee CH, Gaskin KJ. Are We Overdoing Pediatric Lower Gastrointestinal Endoscopy? J Pediatr Gastroenterol Nutr. 2017;64:898-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 110. | Chen J, Yu H, Zhong W, Chen H, Kong X, Sun J, Wang X, Li C. [Pediatric colonoscopy in South China: a single-center experience from 229 cases]. Zhonghua Wei Chang Wai Ke Za Zhi. 2017;20:1404-1408. [PubMed] |
| 111. | Tam YH, Lee KH, Chan KW, Sihoe JD, Cheung ST, Mou JW. Colonoscopy in Hong Kong Chinese children. World J Gastroenterol. 2010;16:1119-1122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 112. | Morita M, Takedatsu H, Yoshioka S, Mitsuyama K, Tsuruta K, Kuwaki K, Kato K, Yasuda R, Mizuochi T, Yamashita Y, Kawaguchi T. Utility of Diagnostic Colonoscopy in Pediatric Intestinal Disease. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 113. | Lee WS, Tee CW, Koay ZL, Wong TS, Zahraq F, Foo HW, Ong SY, Wong SY, Ng RT. Quality indicators in pediatric colonoscopy in a low-volume center: Implications for training. World J Gastroenterol. 2018;24:1013-1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 114. | El Mouzan MI, Al-Mofleh IA, Abdullah AM, Al Sanie AM, Al-Rashed RS. Colonoscopy in children. Saudi J Gastroenterol. 2005;11:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 115. | Wu CT, Chen CA, Yang YJ. Characteristics and Diagnostic Yield of Pediatric Colonoscopy in Taiwan. Pediatr Neonatol. 2015;56:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |