Published online May 7, 2023. doi: 10.3748/wjg.v29.i17.2679
Peer-review started: December 19, 2022
First decision: February 8, 2023
Revised: February 24, 2023
Accepted: March 20, 2023
Article in press: March 20, 2023
Published online: May 7, 2023
Processing time: 138 Days and 21.2 Hours
Multiple studies investigating the relationship between intake of different types of fruit and colorectal cancer (CRC) risk have yielded inconsistent results.
To perform a meta-analysis of existing studies to assess the association between the intake of different kinds of fruit and the incidence of CRC.
We searched online literature databases including PubMed, Embase, WOS, and Cochrane Library for relevant articles available up to August 2022. With data extracted from observational studies, odds ratios (ORs) with 95% confidence intervals (CIs) were assessed using random-effects models. A funnel plot and Egger’s test were used to determine publication bias. Furthermore, subgroup analysis and dose-response analysis were performed. All analyses were conduc
Twenty-four eligible studies involving 1068158 participants were included in this review. The meta-analysis showed that compared to a low intake, a higher intake of citrus, apples, watermelon, and kiwi reduced the risk of CRC by 9% [OR (95%CI) = 0.91 (0.85-0.97)], 25% [OR (95%CI) = 0.75 (0.66-0.85)], 26% [OR (95%CI) = 0.74 (0.58-0.94)], 13% [OR (95%CI) = 0.87 (0.78-0.96)], respectively. No signifi
We found that a higher intake of citrus, apples, watermelon, and kiwi was negatively associated with the risk of CRC, while the intake of other types of fruits were not significantly associated with CRC. Citrus intake showed a non-linear dose-response relationship with the risk of CRC. This meta-analysis provides further evidence that a higher intake of specific types of fruit is effective in preventing the occurrence of CRC.
Core Tip: In this study, we summarized and analyzed existing studies on the association between the intake of different types of fruit and the risk of colorectal cancer (CRC). Some specific types of fruit, such as citrus and apples, were found to reduce the incidence of CRC. We also found a nonlinear association between citrus intake and CRC risk in the dose-response analysis. Finally, this study proposed that people should change their diets to lower the risk of CRC, thereby easing the heavy economic burden of cancer worldwide.
- Citation: Wu ZY, Chen JL, Li H, Su K, Han YW. Different types of fruit intake and colorectal cancer risk: A meta-analysis of observational studies. World J Gastroenterol 2023; 29(17): 2679-2700
- URL: https://www.wjgnet.com/1007-9327/full/v29/i17/2679.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i17.2679
As the third most common cancer, colorectal cancer (CRC) is the third leading cause of cancer death in both men and women, as well as the second leading cause of cancer death in the United States. when men and women are added together[1]. From 2000-2002 to 2014-2016, the incidence of CRC increased by nearly 15% among adults aged 40 to 49 years[2]. The prognosis of CRC varies which mainly depends on the cancer stage, with a 5-year survival rate of about 90% for stage I patients and only 0%-10% for stage IV patients, making the prevention of cancer of great potential and value.
Important risk factors for early-onset CRC include hyperlipidemia, obesity, alcohol consumption and a history of CRC in first-degree relatives[3], of which dietary habits are modifiable. Up to now, various phytochemicals with the potential to prevent cancer have been found in fruits, such as polysaccharides (modified apple polysaccharides, MAP), resveratrol, and flavonoids[4]. MAP inhibits the binding of galectin-3 to its ligand, which is considered to be the promoter of the inflammatory response[5-7], and this may be part of the mechanism by which MAP promotes apoptosis and prevents tumorigenesis[8]. As suggested by Liu et al[9], resveratrol regulates PTEN/PI3K/Akt and Wnt/β-catenin signaling pathways, respectively, and thus exhibits growth inhibitory effects in human colon cancer cells. Anticancer properties of flavonoids include modification or inactivation of enzymes that activate or detoxify carcinogens, free radical scavenging, inhibition of transcription factor induction (e.g., activator protein-1 activity), and induction of apoptosis[10]. Epidemiological studies have also highlighted the protective effect of chemicals present in plants and fruits on the risk of CRC[11-14]. For example, data from Jordan and Italy have shown that high intake of flavonoids can reduce CRC risk. Moreover, a considerable number of studies have demonstrated the association between higher intake of fruits and vegetables and lower mortality[15]. However, results from prospective cohorts began to show nonexistent or weak associations[16,17], and a pooled analysis of 14 studies[18] also showed a weak association. Wang et al[19] concluded that mortality was not further reduced in those who consumed five servings of fruits and vegetables daily. A meta-analysis showed that increased intake of vegetables, but not fruits, reduced the risk of liver cancer[20]. This finding was also questioned by a large prospective study[21]. Certain types of fruit may be more strongly associated with cancer risk compared with others due to their particular chemical composition and underlying molecular mechanisms, which may be hidden in epidemiological studies. Here, we systematically reviewed the existing evidence and explored potential sources of heterogeneity between study results and whether study results differ by gender, region, and tumor location in order to elucidate the association between intake of different types of fruits and CRC risk.
The effect of different types of fruit intake on the risk of CRC was reported in this study according to the Preferred Reporting Items for Systematic Reviews and meta-analyses (PRISMA) statement[22], and it was previously registered with Prospero (study number: CRD42022354620).
Two researchers (Zhen-Ying Wu and Jia-Li Chen) independently conducted a computerized literature search of PubMed, Cochrane, EMBASE, and Web of Science databases until August 2022 for literature on the association of different types of fruit consumption with CRC risk. Studies were identified with the following medical subject heading (MeSH) terms or keywords: (1) Fruit, berry, and plant; (2) cancer, neoplasm, colorectal tumor, CRC, and colorectal neoplasm; and (3) case-control, cohort, and prospective. Titles, abstracts and citations were exported to Endnote 20. The database search strategy is presented in Supplementary Table 1.
Two authors (Zhen-Ying Wu and Jia-Li Chen) independently evaluated the titles and abstracts of potentially eligible studies based on the following inclusion criteria: (1) Original articles; (2) human participants; (3) case-control or cohort design; and (4) studies examining the association between intake of different types of fruit and CRC risk. All full-text articles meeting the inclusion criteria were collected. The following exclusion criteria were applied: (1) Articles with confounding of fruits or other food sources; (2) no specific indication of fruit type; (3) no corresponding 95% confidence interval (95%CI) for the relative risk (RR), odds ratio (OR), or hazard ratio (HR) for estimating the highest to lowest levels of fruit consumption; and (4) systematic reviews, meta-analyses, and reviews. Differences between reviewers were resolved through discussion.
For each included potential study, selection evaluation, data extraction and quality assessment were performed independently by two researchers. We extracted the following data from the included studies: The surname of the first author, study area and design, year of publication, sample size (number of cases and controls; cohort size and incident cases), age, follow-up time of the cohort studies, dietary assessment methods, comparison of exposure levels, OR/RR/HR estimates corresponding to fruit intake, and 95%CIs for the highest and lowest fruit intake. We extracted the estimation models that adjusted the most for confounding factors when multiple estimates were reported in the article. If there were independent risk estimates for men and women in a study, or risk estimates for cancers at different sites such as the colon and rectum, we treated them as separate studies.
The number of cases and person-years or non-cases for each category of data are required to calculate the slope of the dose-response curve[23]. With citrus intake in each study divided into at least three groups, we took the mean or median consumption under each category and assigned it to the corresponding RR. The midpoint of the upper and lower boundaries was used as the dose for the corresponding category if the study only reported interval ranges for citrus consumption[24]. When the range of intake was unlimited, we assumed the same level as the adjacent category[25]. For instance, the median for the lowest group was 0, while the median for the highest group was 1.5 times the lower limit for that group. For most studies in the meta-analysis, we used 80 g/serving to calculate intake if the study reported intake in servings[26]. Discrepancies between researchers on included studies were resolved through discussion or consultation with the third author.
We assessed the quality of included studies and their potential risk of bias using the Risk of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool[27]. This assessment tool contains seven domains covering pre-intervention (Bias due to confounding, Bias due to selection of participants), at intervention (Bias in classification of interventions) and post-intervention (Bias due to deviations from intended interventions, Bias due to missing data, Bias in measurement of outcomes and Bias in selection of the reported result). The categories for risk of bias judgements are “low risk”, “moderate risk”, “serious risk”, “critical risk”, and “no information”. The risk of bias was determined independently by two reviewers, and their disagreements were resolved by mutual consensus.
The meta-analysis was conducted by comparing the risk of CRC reported in the highest and lowest fruit intake groups. Considering a risk of less than 10% for CRC and a small OR, the RR/HR we calculated was approximately equal to the OR[28]. For the overall estimation, the meta-analysis was performed according to the case where all types of rates were OR. The heterogeneity of the results across studies was evaluated with the I² test. Since observational study results are inevitably affected by various sources of heterogeneity such as statistical heterogeneity and conceptual heterogeneity in the real world, we followed the Cochrane Handbook for Systematic Reviews of Interventions and used combined results from random-effects models. The effect of individual studies on risk estimates was investigated through sensitivity analyses by omitting each study in turn. We also conducted sensitivity analyses based on quality assessment to improve the reliability of the results. When an outcome indicator was reported in more than 10 included studies, publication bias analysis was conducted using Egger’s linear regression test and funnel plots. Significant publication bias was considered to exist if the intercept of the Egger’s regression line deviated from zero and the P value < 0.05. In the present study, we performed pre-specified subgroup analyses based on study design type, location of CRC occurrence, geographic region, and gender. To check for possible non-linear relationships, we also carried out pre-specified dose-response analyses by calculating restrictive three-times sample bars for each study for three or more exposure categories[25]. All analyses were performed by R (version 4.1.3), with two-tailed P < 0.05 considered statistically significant.
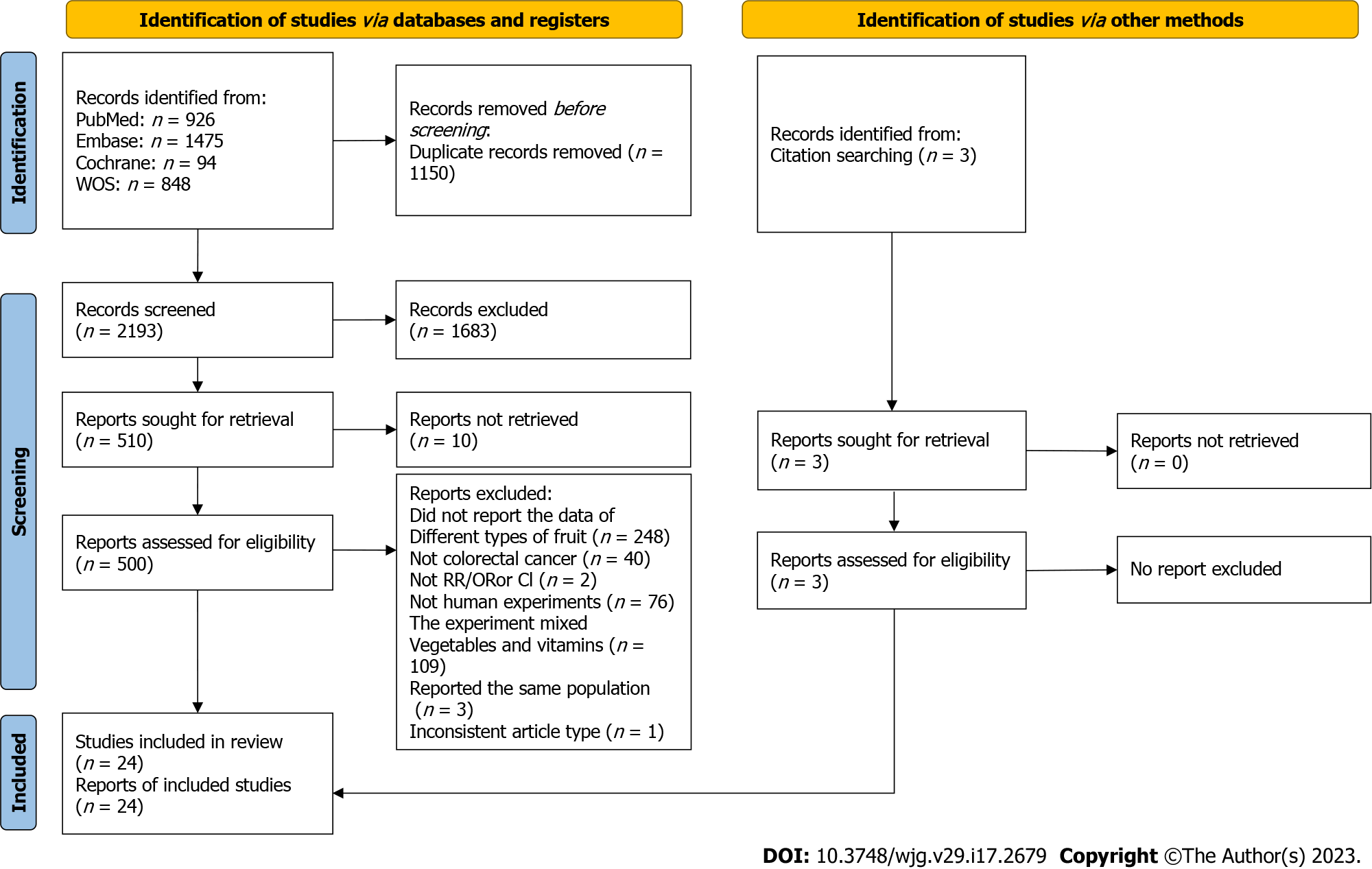
A total of 3343 articles were obtained by the initial literature screening, and after removing 1150 duplicate articles, we identified 2193 articles that were potentially eligible for review. Then 1683 irrelevant entries were eliminated by screening titles and abstracts. Of the remaining 510 articles, 486 were eliminated according to the exclusion criteria. In particular, three studies[29-31] were all from the same study, so only the one with the most complete data was included[29]. Another two studies[32,33] were also from the same study, and similarly the one with the most complete data was included[32]. The results of Tuyns et al[34] and Tajima et al[35] were removed due to lack of OR and corresponding 95%CI for citrus intake and CRC risk. Three additional[36-38] studies that met the inclusion criteria were identified by manually searching the reference list. The 24 articles were ultimately included in the current meta-analysis[29,32,36-57]. The flow chart for study selection is presented in Figure 1.
Detailed characteristics of the studies investigating the intake of different types of fruit and CRC risk are shown in Table 1. The final analysis included 16 case-control studies[29,32,37-39,41-49,56,57] and 8 cohort studies[36,40,50-55]. The articles were published between 1996 and 2017, with a total of 1068158 participants aged under 80 years. One study involved only men[50] and one study involved only women[55]. Seven articles distinguished between tumor locations such as rectum and colon (even into proximal and distal colon cancer)[29,38,39,44,50,53,54]. Five articles conducted the research with the classification of gender[36,38,39,52,56]. As for the regional distribution of the study population, nine studies were conducted in Europe[29,32,37,40,43,45,47,49,53], two studies in South America[38,57], seven studies in North America[36,46,48,52,54-56], five studies in Asia[39,41,42,50,51], and one study in Australia[44]. Most studies matched or adjusted for age and energy intake; several studies adjusted for BMI (body mass index), smoking status, alcohol use, history of disease associated with CRC, and physical activity; other adjusting factors included gender, education level, and red meat intake (Table 2). Based on the ROBINS-I tool, we identified all studies as having moderate risk of bias. Most of the problems found were regarding confounding and missing data. There was a moderate bias in the classification of interventions in five studies and a moderate bias in the selection of participants in five studies. Among all observational studies, Bias due to deviations from intended interventions, outcomes measurement bias and selection of reported results were considered low. Risk of bias assessment results are summarized in Table 3.
| Ref. | Country | No. of cases/controls (age) | Dietary assessment | Comparison of exposure level | Category, OR/RR (95%CI) | Confounding factors |
| Lee et al[39], 2017 | - | 923 (625 males, 298 females)/1846 (1250 males, 596 females) | SQFFQ, 106 food items | Orange/yellow fruits (g/d); males: T3 (≥ 47.9) vs T1 (< 15.9); females: T3 (≥ 90.6) vs T1 (< 32.5); proximal colon/distal colon/rectum: T3 (≥ 90.6) vs T1 (< 32.5) | Males: 0.98 (0.75-1.28); females: 0.64 (0.43-0.97); total: 0.85 (0.69-1.06); proximal colon: 0.79 (0.37-1.70); distal colon: 0.77 (0.44-1.35); rectum: 0.44 (0.25-0.80) | Age, education, alcohol consumption, BMI, regular exercise, red meat, processed meat, total EI |
| Leenders et al[40], 2015 | Ten European countries (Denmark, France, Germany, Greece, Italy, The Netherlands, Norway, Spain, Sweden, and United Kingdom | 442961 cohort; 3082 incident cases (2128 colon cancer (954 proximal, 965 distal), 1242 rectal cancer cases); 51.2 (38.3-63.0) years; follow-up 8 years | Center-specific dietary questionnaire | Medians of consumption per quartiles; berries: 21 g/d vs 1 g/d; citrus fruits: 110 g/d vs 7 g/d; grapes: 32 g/d vs 1 g/d; hard fruits: 153 g/d vs 10 g/d; stone fruits: 83 g/d vs 2 g/d | Colon cancer; berries: 1.04 (0.88-1.24); citrus fruits: 1.02 (0.88-1.17); grapes: 1.15 (0.97-1.37); stone fruits: 0.97 (0.81-1.15); rectal cancer: Berries: 1.04 (0.83-1.30); citrus fruits: 1.15 (0.95-1.38); grapes: 0.98 (0.78-1.25); stone fruits: 1.19 (0.94-1.50) | All other fruit and vegetable consumption, height, weight, dietary calcium consumption, dietary alcohol consumption, dietary cereal fiber consumption, smoking status, time since stopped smoking, duration of smoking, number of cigarettes smoked per day and PA |
| Abu Mweis et al[41], 2015 | Jordan | 167/240, NA | FFQ, 109 food items | ≥ 3 times/wk (high) vs ≥ 2 times/wk (low) | Apples: 0.915 (0.545-1.535); bananas: 1.167 (0.670-2.033); oranges: 0.999 (0.581-1.715) | Age, sex, total EI, metabolic equivalent, smoking, education level, marital status, work, income, and family history of CRC |
| Tayyem et al[42], 2014 | Jordan | 220/281 (248 males, 253 females); males: mean age 55.27 years; females: mean age 48.67 years | NA; 42 food items | Daily (high) vs ≤ rarely (low) | Apple: 0.73 (0.27-1.96); banana: 1.12 (0.34-3.67); orange: 0.90 (0.44-1.82); pear: 1.13 (0.56-2.29); peach: 0.64 (0.32-1.25); grape: 0.62 (0.27-1.40); melon: 0.82 (0.38-1.78); watermelon: 0.54 (0.26-1.11); strawberry: 0.75 (0.26-2.13); fig: 0.51 (0.28-0.92); kiwi: 1.14 (0.25-5.06); dried Fruit: 1.42 (0.55-3.67) | Age, sex, total EI, MET minutes/week, tobacco use, education level, marital status, work, income, PA, marital status, family history of CRC |
| Rosato et al[43], 2013 | Italia and Swiss | 329/1361, median age 40 yr | FFQ; 78 food items | High vs Low | Citrus fruit: 0.61 (0.45-0.84) | Age, sex, center, study, year of interview, education, family history, alcohol consumption, EI |
| Vogtmann et al[50], 2013 | China | 61274 male’s cohort (40-74 years); 398 incident cases (236 colon, 162 rectal); follow-up 2002-2006 to 2010 | Validated FFQ; 46 food items | Citrus fruit intake g/day: ≥ 12.61 (high) vs < 2.70 (low); watermelon intake g/day: ≥ 93.33 (high) vs < 33.33 (low) | Citrus fruit: Colorectal cancer: 0.82 (0.64-1.06); colon cancer: 0.86 (0.62-1.19); rectal cancer: 0.76 (0.51-1.14); watermelon: Colorectal cancer: 0.77 (0.59-0.99); colon cancer: 0.76 (0.55-1.06); rectal cancer 0.77 (0.51-1.15) | Age, total EI, red meat intake, total meat intake, education, income, occupation, smoking status, alcohol consumption, BMI, MET hours of exercise participation, history of diabetes mellitus, family history of CRC |
| Annema et al[44], 2011 | Western Australia | 834 (64.9 yr ± 8.9 yr)/939 (64.6 yr ± 9.4 yr) | FFQ; 74 food items | Servings/d) ≥ 0.50 (high) vs < 0.07 (low) | Total: Citrus fruit: 0.95 (0.72-1.25); apples: 0.74 (0.56-0.99); fruit juice: 1.38 (1.08-1.75); citrus fruit: Proximal Colon: 0.97 (0.65-1.45); distal Colon: 0.81 (0.53-1.24); rectum: 1.03 (0.71-1.49); apples: Proximal Colon: 1.13 (0.72-1.77); distal colon: 0.51 (0.34-0.77); rectum: 0.73 (0.49-1.08); fruit juice: Proximal Colon: 1.06 (0.74-1.49); distal colon: 1.41 (0.99-2.01); rectum: 1.74 (1.24-2.45) | Sex, age, BMI at age 20 yr, EI, multivitamin use, alcohol consumption, PA, smoking, diabetes, socioeconomic status |
| Foschi et al[45], 2010 | Italy and Switzerland | 3634 (median age 62 yr)/6804 (median age 57 yr) | Validated FFQ; 78 food items | Citrus fruit or citrus fruit juice intake: ≥ 4 portions/wk vs < 1 portion/wk | Citrus: 0.82 (0.72-0.93) | Age, sex, study center, tobacco smoking, alcohol, education, BMI, PA, EI |
| Li et al[51], 2010 | Japan | 42470 cohort (40-79 yr) (20222 males, 22248 females); 665 incident cases; follow-up 9 years | FFQ; 40 food items | Citrus consumption daily vs ≤ 2 times/wk | Citrus: 0.80 (0.61-1.06) | Age, sex, job status, years of education, BMI, time engaging in sports or exercise, time spent walking, cigarette smoking, alcohol drinking, history of hypertension, diabetes mellitus and gastric ulcer, family history of cancer, daily total EI, consumption of rice/miso soup/soybean products/total meat/total fish/dairy products/other fruits/total vegetables/oolong tea/black tea/coffee /green tea |
| Jedrychowski et al[32], 2010 | Poland | 592/765; NA | EPIC-FFQ148 food items | Apples, servings/d: > 1.50 (Q5; high) vs < 0.18 (Q1; low) | Apples: 0.53 (0.35-0.79) | Age, gender, place of residency, marital status, tobacco smoking, total EI, intake of vegetables, fruits excluding apples |
| Williams et al[46], 2009 | North Carolina | 945/959; 40-79 yr; whites (n = 1520); African-Americans (n = 384) | Diet history questionnaire; 124 food items | Citrus fruit (servings/wk): White: 16.4 Q4 (high) vs 1.89 Q1 (low); African-Americans: 21.7 Q4 (high) vs 2.3 Q1 (low) | Whites: 0.61 (0.43-0.86); African-Americans: 1.54 (0.71-3.35) | Age, sex, education, income, BMI 1 yr ago, PA, family history, nonsteroidal anti-inflammatory drug use, total EI |
| Nomura et al[52], 2008 | Hawaii and Los Angeles | 191011 cohort (85903 males, 105108 females); 2110 incident cases (1138 males, 972 females) (1571 of the colon, 515 of the rectum, 24 cases both sites) (45-75 yr); follow-up 7.3 years | Self-administered quantitative FFQ (QFFQ); 180 food items | Citrus fruit were quantified as g × 1000 kcal-1 × d-1; Q5 (high) vs Q1 (low) | Citrus fruit: Male: 0.85 (0.70-1.04); female: 1.04 (0.83-1.30) | Ethnicity, age, family history of CRC, history of colorectal polyp, pack-years of cigarette smoking, BMI, hours of vigorous activity, aspirin use, multivitamin use, replacement hormone use (women), log EI, alcohol, red meat, folate, vitamin D, calcium |
| Gallus et al[47], 2005 | Italy | 1953 (1225 of the colon, 728 of the rectum)/4154 | Validated FFQ 78 food items | Average consumption of apples per day ≥ 1 (high) vs < 1 (low) | Apples: 0.70 (0.62-0.79) | Age, sex, study center, education, BMI, tobacco smoking, alcohol drinking, total EI, vegetable consumption, PA, other fruit |
| Lin et al[55], 2005 | United States | 39876 female cohort (mean age 45 years); 240 incident cases; follow-up 10 years | FFQ; 131 food items | Citrus fruit (serving/day) Median intake; 1.6 (Q5) high vs 0.1 (Q1) low | Citrus fruit: 1.11 (0.71-1.74) | Age, randomized treatment assignment, BMI, family history of CRC in a first-degree relative, history of colon polyps, PA, smoking status, baseline aspirin use, red meat intake, alcohol consumption, total EI, menopausal status and baseline post-menopausal HT use, folate intake, multivitamin use |
| Satia-Abouta et al[48], 2004 | United States | 613 (337 Caucasians, 276 African-Americans)/996(596 Caucasians, 400 African-Americans) (40-80 years) | Validated FFQ; 100 food items | Citrus fruits and juices: Median g/d: Caucasians: 4th quartile (high) 168 g vs 1st quartile 0 g (low); African-Americans: 4th quartile (high) 173 g vs 1st quartile 0 g (low) | Caucasians: 1.0 (0.7-1.6); African-Americans: 1.0 (0.6-1.6) | Age, gender, total EI, education, BMI, smoking history, PA, family history of colon cancer, NSAID use, fat, carbohydrates, dietary fiber, vitamin C, vitamin E, beta-carotene, calcium, folate, fruits, vegetables |
| Voorrips et al[53], 2000 | Netherlands | 4087 cohort; 620 colon cancer cases (332 males, 288 females), 344 rectal cancer cases (217 males, 127 females); follow-up 6.3 years | Validated FFQ; 150 food items | Citrus fruit Median intake (g/d); male: Q5 (167 g/dk) (high) vs Q1 (0g/dk) (low); female: Q5 (187 g/dk) (high) vs Q1 (8 g/dk) (low) | Male: Colon cancer: 1.09 (0.75-1.59) Rectal cancer: 0.77 (0.49-1.20); female: Colon cancer: 1.00 (0.66-1.52); rectal cancer: 1.16 (0.63-2.12) | Age, family history of CRC, category of alcohol intake |
| Michels et al[54], 2000 | United States | 136089 cohort (88764 females (30-55 years), 47325 males (40-75years); 1181 incident cases (937 colon cancer, 244 rectal cancer); follow-up 16 years | Validated FFQs; 61 food items | Citrus fruit: Frequencies of intake ≥ 2 servings/d vs 1 serving/wk or fewer | Colon cancer: 1.05 (0.80-1.39); rectal cancer: 0.97 (0.58-1.64) | Age, family history of CRC, sigmoidoscopy, height, BMI, pack-years of smoking, alcohol intake, PA, menopausal status, postmenopausal hormone use, aspirin use, vitamin supplement intake, total caloric intake, red meat consumption |
| Franceschi et al[29], 1998 | Italy | 1953 (1225 colon cancer, 728 rectal cancer)/4154 (2073 males, 2081 females) | Validated FFQ; 78 food items | Mean weekly servings: Citrus fruit Q5 7.5 (high)/Q1 1.0 (low); apples/pears: Q5 15.0 (high)/Q1 3.0 (low); bananas: Q5 3.0 (high)/Q1 0.5 (low); kiwi: Q5 4.0 (high)/Q1 0.5 (low); peaches/apricots/prunes: Q5 5.0 (high)/Q1 0.8 (low); melon: Q5 0.5 (high)/Q1 0.1 (low); grapes: Q5 1.0 (high)/Q1 0.2 (low); Strawberries/cherries: Q5 0.4 (high)/Q1 0.1 (low) | Citrus: Total: 1.02 (0.85-1.22), colon: 1.0 (0.9-1.1), rectal: 0.8 (0.7-1.0); apples/pears: Colon: 0.9 (0.8-1.1) rectal: 0.8 (0.7-1.0); bananas: Colon 1.0 (0.9-1.1), rectal 1.0 (0.8-1.1); kiwi: colon 0.9 (0.8-1.0) rectal 0.8 (0.7-1.0); peaches/apricots/prunes: Colon 1.0 (0.9-1.1) rectal 0.8 (0.7-0.9); melon: Colon 1.0 (0.9-1.0) rectal 0.9 (0.8-1.0); grapes: colon 1.0 (0.9-1.0) rectal 0.9 (0.8-1.0); strawberries/cherries: Colon 1.0 (0.9-1.0) rectal 0.9 (0.9-1.0) | Age, sex, centre, education, PA, total EI |
| Levi et al[49], 1999 | Swiss | 223 (males 142, females 81) (119 colon cancer, 104 rectal cancer, median age 63 yr)/491 (211 males, 280 females, median age 58 yr) | FFQ; 79 food items | Citrus fruits (Servings per week): Q3 (> 3.5/wk) vs Q1 (1.5/wk) | Citrus fruits: 0.65 (0.40-1.05) | Age, sex, education, smoking, alcohol, BMI, PA, meat and vegetable consumption, total EI |
| Le Marchand et al[56], 1997 | Hawaii | 1192 (698 males, 494 females) (mean age 66 yr)/1192 (698 males, 494 females) (mean age 66 yr) | Validated FFQ; 282 food items | Bananas: Male ≥ 55 g/d (Q4 high) vs ≤ 9 g/d (Q1 low), female: ≥ 54 g/d (Q4 high) vs ≤ 11 g/d (Q1 low); citrus fruits: Male ≥ 52 g/d (Q4 high) vs ≤ 4 g/d (Q1 low), female: ≥ 58 g/d (Q4 high) vs ≤ 8 g/d (Q1 low) | Bananas: Male: 0.7 (0.5-1.1), female: 0.6 (0.4-0.9); citrus fruits: Male: 0.9 (0.6-1.3), female: 0.9 (0.6-1.4) | Age, family history of CRC, alcoholic drinks per week, pack-years of cigarette smoking, lifetime recreational activity, Quetelet index 5 years earlier, total calories, egg, and calcium |
| Deneo-Pellegrini et al[57], 1996 | Uruguay | 160 (71 rectal cancer, 89 colon cancer)/221 | FFQ; 61 food items | (T3; high) vs (T1; low) | Orange: 0.76 (0.47-1.19); apple: 0.40 (0.25-0.66); peach: 1.05 (0.65-1.69); pear: 1.06 (0.65-1.74); grape: 1.61 (0.94-2.74); fig: 1.36 (0.73-2.54); banana: 0.28 (0.16-0.50) | Age, sex, residence, education, BMI, total EI, alcohol intake |
| Lin et al[36], 2006 | United States (NHS and HPFS) | 71976 female cohort; 498 incident cases (30-55 yr); 35425 male cohort; 380 incident cases (40-75 yr); follow-up 10 yr | Validated FFQ; 131 food items | Apple: ≥ 2 servings/d (Q5; high) vs 0-2 servings/wk (Q1; low) | Total: 0.75 (0.52-1.08); NHS females: 0.64 (0.35-1.17); HPFS males: 0.82 (0.51-1.30) | Age, BMI, PA, history of CRC, previous colorectal polyps, prior screening sigmoidoscopy or colonoscopy, smoking, multivitamin use, current aspirin use, alcohol, EI, red meat, total Ca, total folate, total fibre |
| Theodoratou et al[37], 2007 | United Kingdom | 1456 (mean 63.9 yr ± 9.6 yr) yr)/1456 (64.7 yr ± 9.5 yr) | Validated FFQ; 150 food items | Apples: Q4 (high) vs Q1 (low) | Apples: 0.96 (0.62-1.50) | Age, sex, residence area, family history of CRC, total EI, fibre, alcohol, NSAID, smoking, BMI, PA |
| Deneo-Pellegrini et al[38], 2002 | Uruguay | 484 (260 colon cancer, 224 rectal cancer)/1452 | FFQ; 64 food items | Citrus fruits estimate: Q4 (high) vs Q1 (low); banana estimate: Q4 (high) vs Q1 (low) | Total: Citrus fruits: 0.8 (0.6-1.1), banana: 0.6 (0.4-0.8); citrus fruits: Male: 0.5 (0.3-0.8), female: 1.5 (0.9-2.5); colon: 0.9 (0.9-1.1); rectum: 0.9 (0.7-0.9); banana: male: 0.6 (0.4-0.9), female: 0.6 (0.3-0.9); colon: 0.8 (0.7-0.9); rectum: 0.9 (0.8-1.1) | Age, residence, urban/rural status, education, family history of colon cancer for first-degree relatives, BMI, total EI and red meat intakes |
| Ref. | Adjusted confounders | |||||||||
| Age | Sex | Energy intake | BMI | Family history of CRC | Alcohol use | Smoking status | Physical activity | Education level | Red meat | |
| Lee et al[39], 2017 | √ | √ | √ | √ | √ | √ | √ | |||
| Leenders et al[40], 2015 | √ | √ | √ | √ | ||||||
| Abu Mweis et al[41], 2015 | √ | √ | √ | √ | √ | √ | ||||
| Tayyem et al[42], 2014 | √ | √ | √ | √ | √ | √ | √ | |||
| Rosato et al[43], 2013 | √ | √ | √ | √ | √ | √ | ||||
| Vogtmann et al[50], 2013 | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Annema et al[44], 2011 | √ | √ | √ | √ | √ | √ | √ | |||
| Foschi et al[45], 2010 | √ | √ | √ | √ | √ | √ | √ | √ | ||
| Li et al[51], 2010 | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Jedrychowski et al[32], 2010 | √ | √ | √ | √ | ||||||
| Williams et al[46], 2009 | √ | √ | √ | √ | √ | √ | √ | |||
| Nomura et al[52], 2008 | √ | √ | √ | √ | √ | √ | √ | √ | ||
| Gallus et al[47], 2005 | √ | √ | √ | √ | √ | √ | √ | √ | ||
| Lin et al[55], 2005 | √ | √ | √ | √ | √ | √ | √ | √ | ||
| Satia-Abouta et al[48], 2004 | √ | √ | √ | √ | √ | √ | √ | √ | ||
| Voorrips et al[53], 2000 | √ | √ | √ | |||||||
| Michels et al[54], 2000 | √ | √ | √ | √ | √ | √ | √ | √ | ||
| Franceschi et al[29], 1998 | √ | √ | √ | √ | √ | |||||
| Levi et al[49], 1999 | √ | √ | √ | √ | √ | √ | √ | √ | ||
| Le Marchand et al[56], 1997 | √ | √ | √ | √ | √ | |||||
| Deneo-Pellegrini et al[57], 1996 | √ | √ | √ | √ | √ | √ | ||||
| Lin et al[55], 2005 | √ | √ | √ | √ | √ | √ | √ | √ | ||
| Theodoratou et al[37], 2007 | √ | √ | √ | √ | √ | √ | √ | √ | ||
| Deneo-Pellegrini et al[38], 2002 | √ | √ | √ | √ | √ | √ | ||||
| Ref. | Confounding | Selection of participants | Classification of interventions | Deviations from intended interventions | Bias due to missing data | Measurement of outcomes | Selection of reported result | Overall rating |
| Lee et al[39], 2017 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Abu Mweis et al[41], 2015 | Moderate | Low | Moderate | Low | Moderate | Low | Low | Moderate |
| Leenders et al[40], 2015 | Moderate | Moderate | Low | Low | Moderate | Low | Low | Moderate |
| Tayyem et al[42], 2014 | Moderate | Low | Low | Low | Low | Low | Low | Moderate |
| Rosato et al[43], 2013 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Vogtmann et al[50], 2013 | Low | Low | Low | Low | Moderate | Low | Low | Moderate |
| Annema et al[44], 2011 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Foschi et al[45], 2010 | Moderate | Moderate | Low | Low | Moderate | Low | Low | Moderate |
| Jedrychowski et al[32], 2010 | Moderate | Low | Moderate | Low | Moderate | Low | Low | Moderate |
| Williams et al[46], 2009 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Li et al[51], 2010 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Gallus et al[47], 2005 | Moderate | Low | Moderate | Low | Moderate | Low | Low | Moderate |
| Lin et al[36], 2006 | Low | Moderate | Low | Low | Moderate | Low | Low | Moderate |
| Satia-Abouta et al[48], 2004 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Voorrips et al[53], 2000 | Moderate | Moderate | Low | Low | Moderate | Low | Low | Moderate |
| Franceschi et al[29], 1998 | Moderate | Low | Moderate | Low | Moderate | Low | Low | Moderate |
| Levi et al[49], 1999 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Le Marchand et al[56], 1997 | Moderate | Low | Moderate | Low | Moderate | Low | Low | Moderate |
| Deneo-Pellegrini et al[57], 1996 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Theodoratou et al[37], 2007 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Deneo-Pellegrini et al[38], 2002 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Nomura et al[52], 2008 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Lin et al[55], 2005 | Moderate | Low | Low | Low | Moderate | Low | Low | Moderate |
| Michels et al[54], 2000 | Moderate | Moderate | Low | Low | Moderate | Low | Low | Moderate |
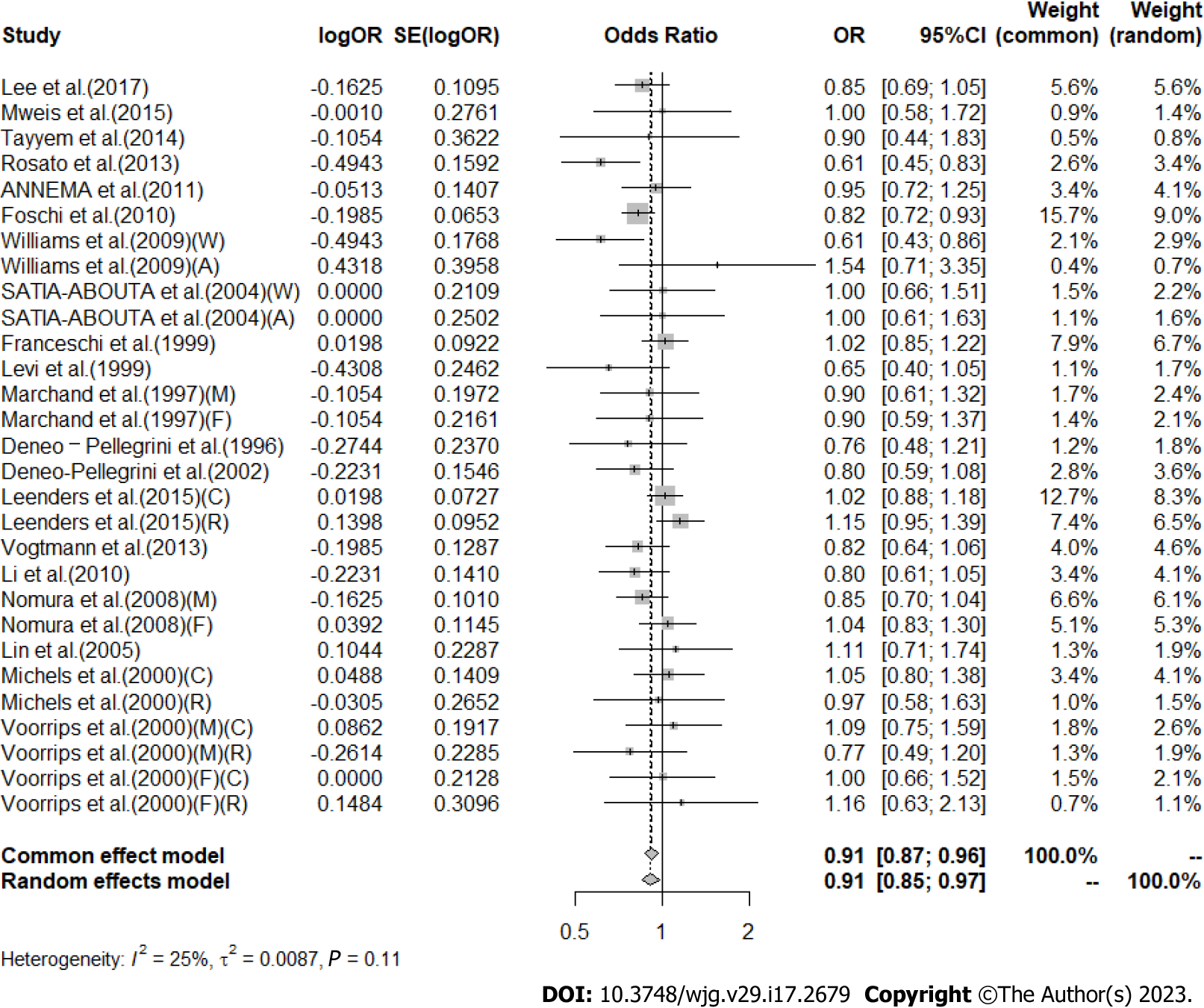
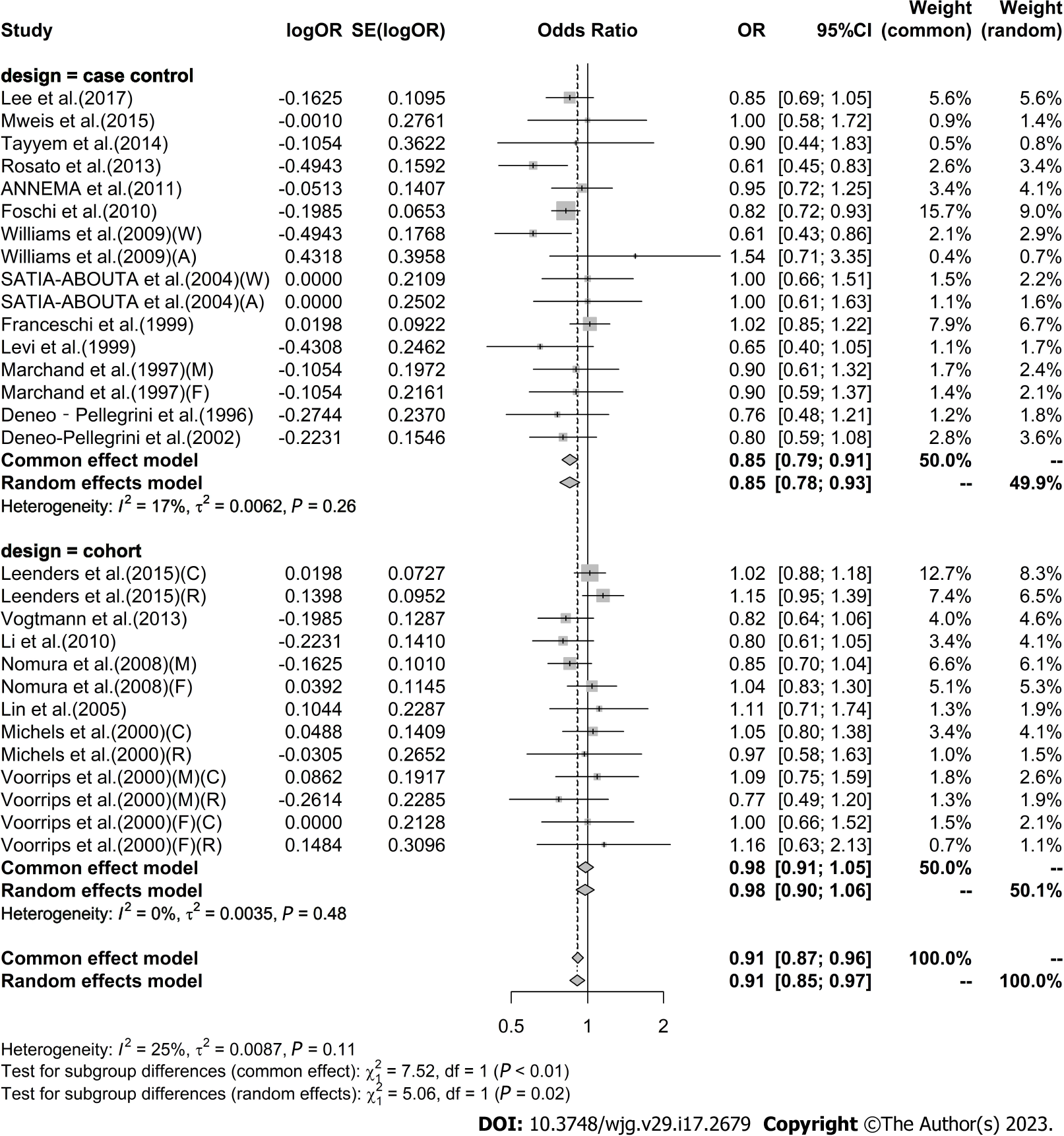
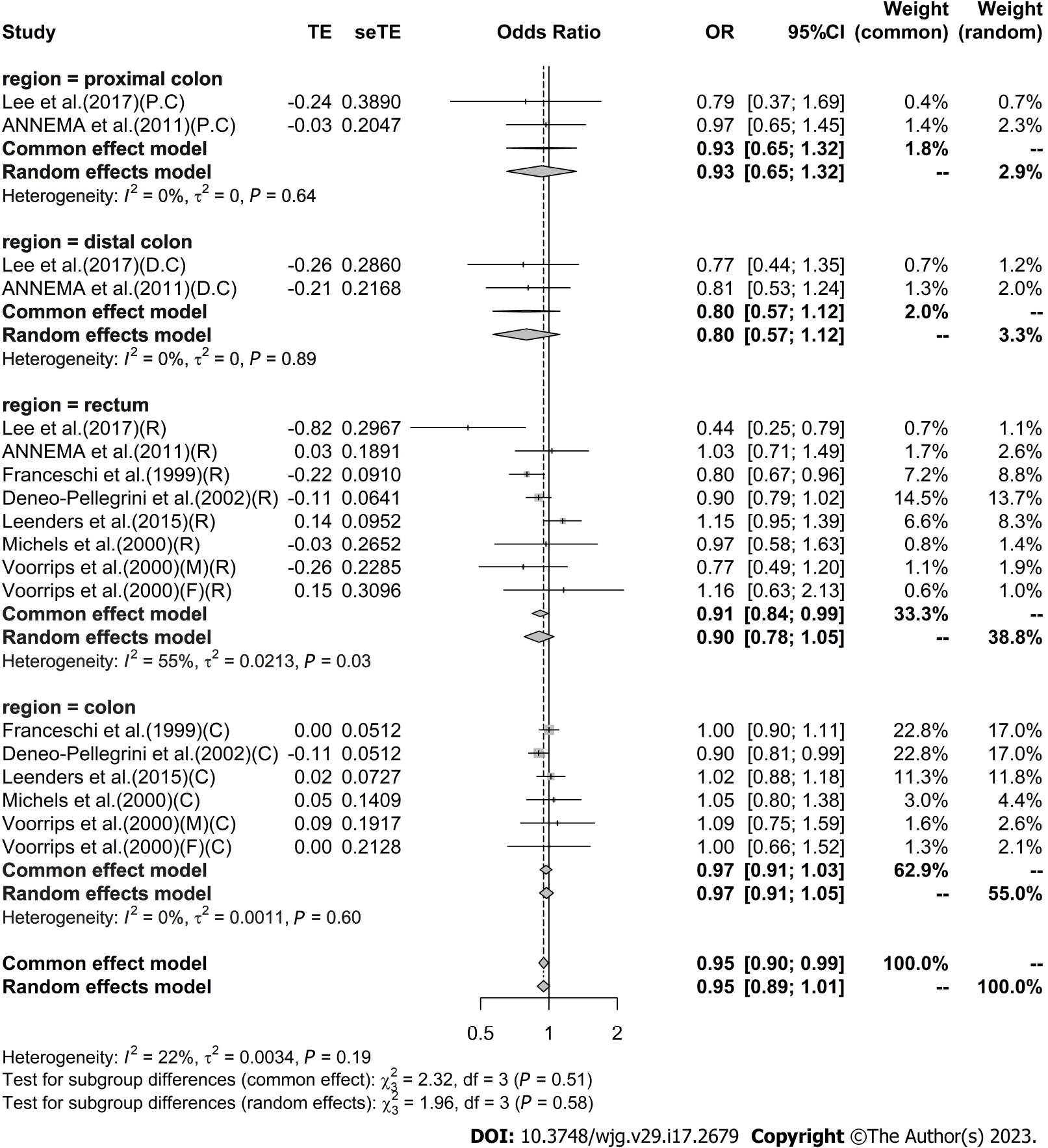
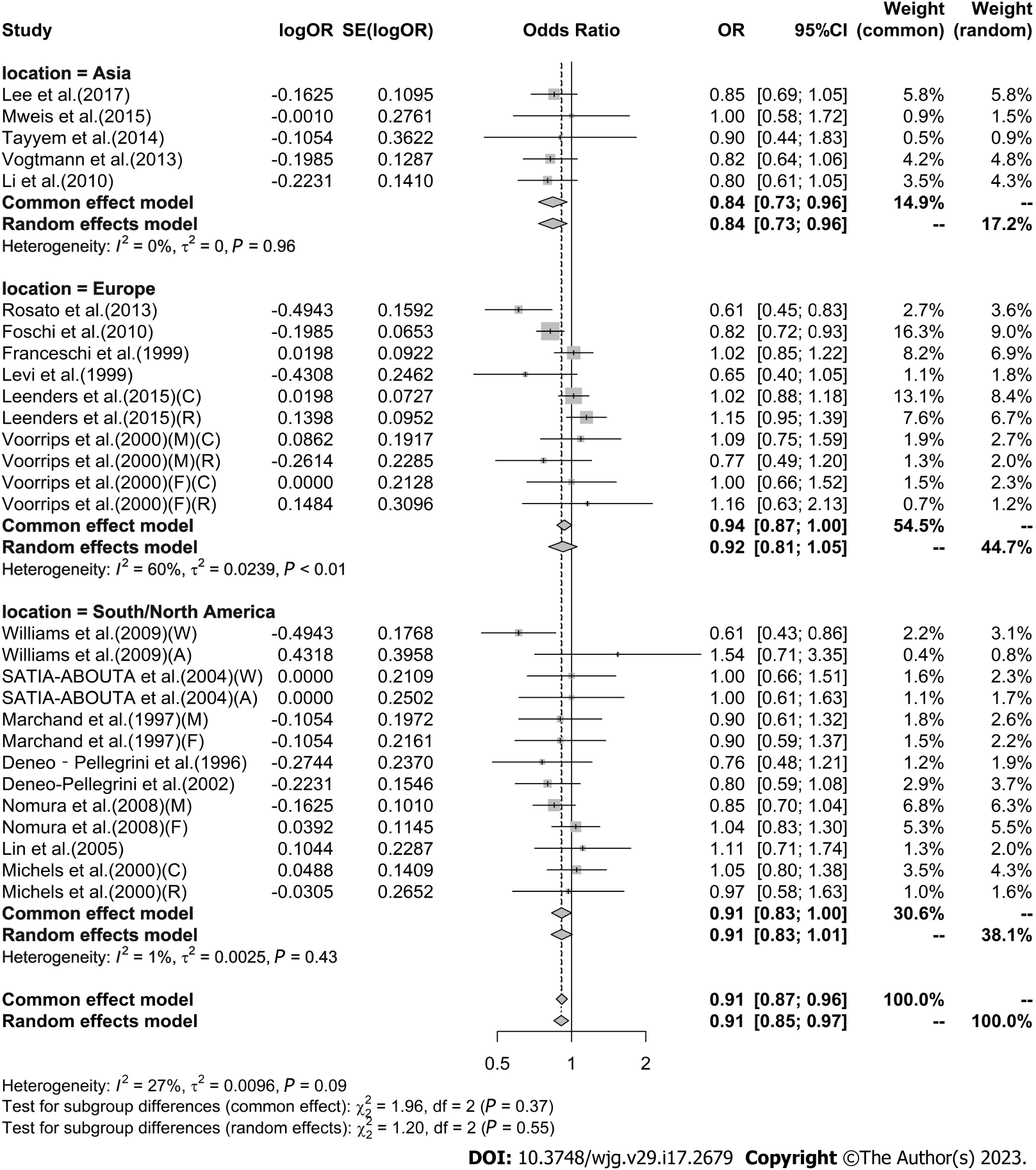
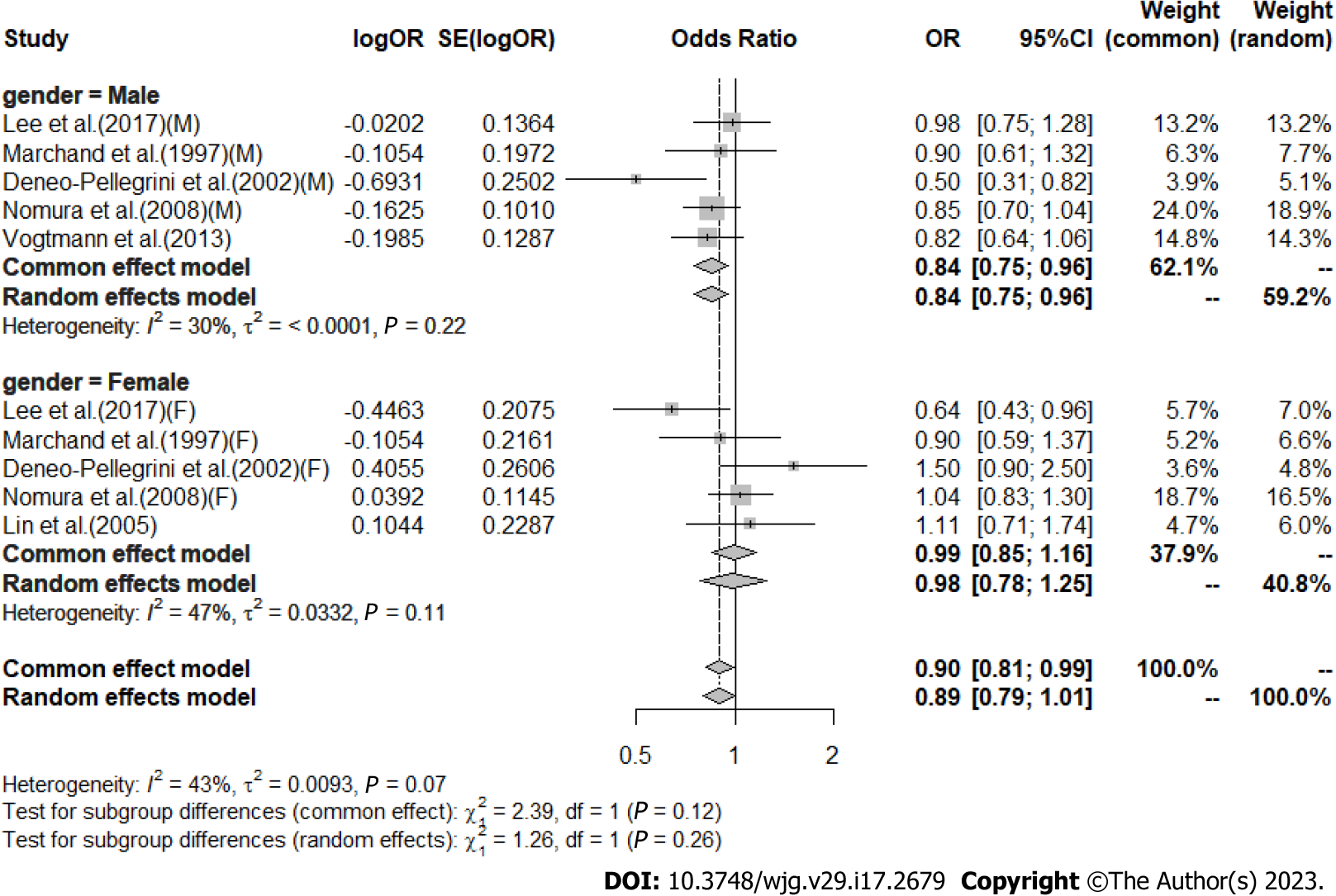
High vs low analysis: Citrus: For 20 included articles[29,38-46,48-57], the overall outcome analysis found that higher citrus intake was related to a lower risk of CRC [I2 = 25%, P = 0.11, REM; OR (95%CI) = 0.91 (0.85-0.97), P < 0.01] (Figure 2). Further subgroup analysis based on study design showed that citrus intake may reduce the risk of CRC by 15% in case-control studies [I2 = 17%, P = 0.26, REM; OR (95%CI) = 0.85 (0.78-0.93)], whereas a similar association was not found in cohort studies [I2 = 0%, P = 0.48, REM; OR (95%CI) = 0.98 (0.90-1.06)] (Figure 3). We also performed a subgroup analysis of 7 included articles based on the specific location of tumorigenesis, which were divided into a total of four locations, namely distal colon, proximal colon, colon, and rectum, but the results suggested no significant association between citrus intake and proximal colon [I2 = 0%, P = 0.64, REM; OR (95%CI) = 0.93 (0.65-1.32)], distal colon [I2 = 0%, P = 0.89, REM; OR (95%CI) = 0.80 (0.57-1.12)], colon [I2 = 0%, P = 0.60, REM; OR (95%CI) = 0.97 (0.91-1.05)], and rectum [I2 = 55%, P = 0.03, REM; OR (95%CI) = 0.90 (0.78-1.05)] (Figure 4). In the analysis stratified by region, an association between citrus consumption and lower CRC risk was demonstrated only in studies conducted in Asia [I2 = 0%, P = 0.96, REM; OR (95%CI) = 0.84 (0.73-0.96)], whereas no association was found in studies conducted in North/South America [I2 = 1%, P = 0.43, REM; OR (95%CI) = 0.91 (0.83-1.01)] and Europe [I2 = 60%, P < 0.01, REM; OR (95%CI) = 0.92 (0.81-1.05)] (Figure 5). Finally, our stratified analysis of gender in 6 included articles found the protective effect of citrus was only present in men [I2 = 30%, P = 0.22, REM; OR (95%CI) = 0.84 (0.75-0.96)] but not in women [I2 = 47%, P = 0.11, REM; OR (95%CI) = 0.98 (0.78-1.25)] (Figure 6).
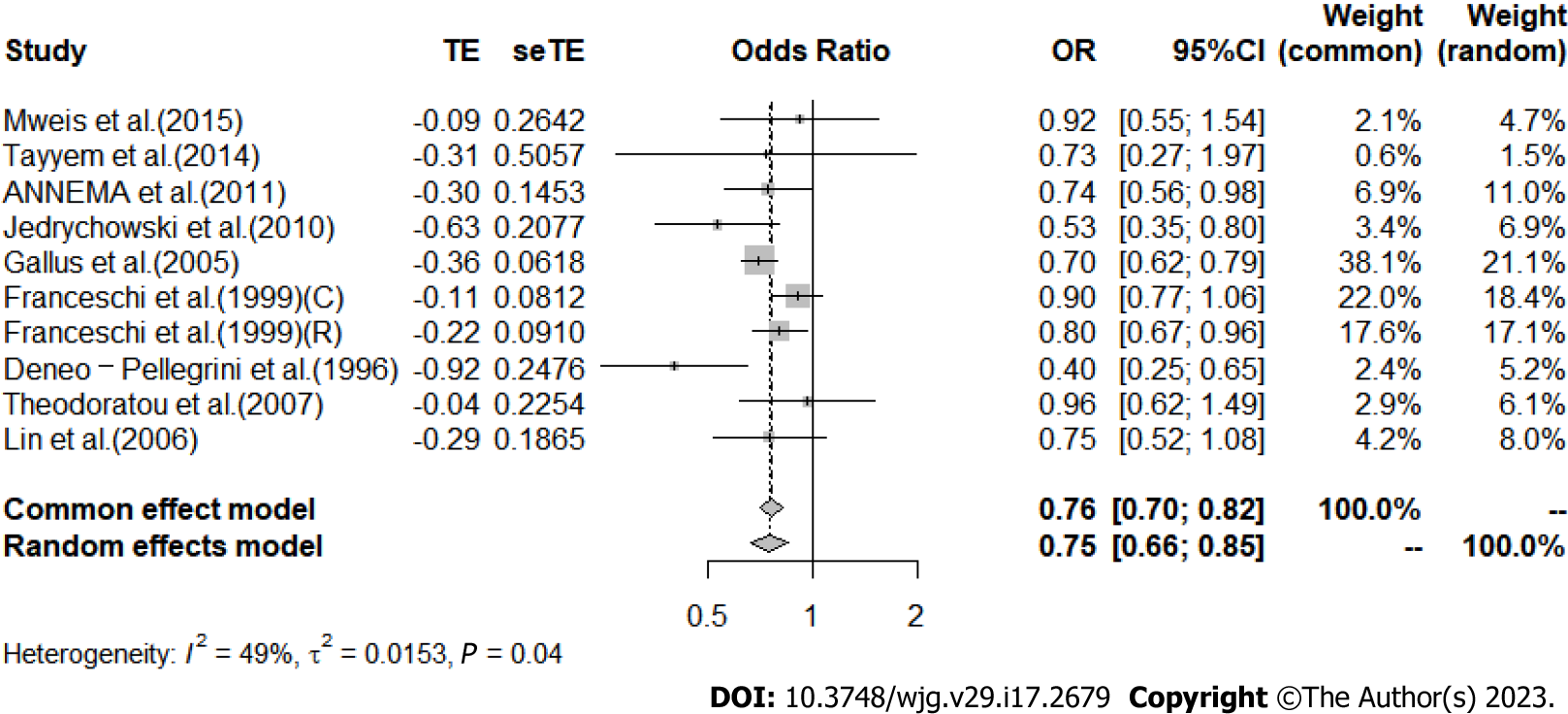
Apple: The analysis of the results in 9 included articles[29,32,36,37,41,42,44,47,57] showed that greater intake of apples led to a significant 25% reduction in CRC risk [I2 = 49%, P = 0.04, REM; OR (95%CI) = 0.75 (0.66-0.85), P < 0.01] (Figure 7). When studies were stratified by region, a significant association was found between apple intake and reduced risk of CRC in the European population [I2 = 62%, P = 0.03, REM; OR (95%CI) = 0.77 (0.67-0.90)], while no association was observed in the Asian population [I2 = 0%, P = 0.69, REM; OR (95%CI) = 0.87 (0.55-1.38)] and the North/South American population [I2 = 76%, P = 0.04, REM; OR (95%CI) = 0.56 (0.30-1.03)] (Supplementary Figure 1).
Banana: The analysis results of six included articles[29,38,41,42,56,57] demonstrated that consuming more bananas did not contribute to reduced risk of CRC [I2 = 79%, P < 0.01, REM; OR (95%CI) = 0.74 (0.55-1.00), P = 0.05] (Supplementary Figure 2). When stratified by region, banana intake was found to be related to a lower risk of CRC in North/South American populations [I2 = 58%, P = 0.07, REM; OR (95%CI) = 0.54 (0.39-0.76)], whereas no association was revealed in European populations [I2 = 0%, P = 1.00, REM; OR (95%CI) = 1.00 (0.92-1.09)] and Asian populations [I2 = 0%, P = 0.95, REM; OR (95%CI) = 1.16 (0.70-1.92)] (Supplementary Figure 3). When stratified by gender, we found a protective effect of Bananas for both men [I2 = 0%, P = 0.59, REM; OR (95%CI) = 0.65 (0.49-0.86)] and women [I2 = 0%, P = 1.00, REM; OR (95%CI) = 0.60 (0.43-0.83)] (Supplementary Figure 4). In a stratified analysis of tumor sites, high banana intake did not show the association with the risk of malignancy in either the colon [I2 = 86%, P < 0.01, REM; OR (95%CI) = 0.90 (0.72-1.12)] or the rectum [I2 = 0%, P = 0.36, REM; OR (95%CI) = 0.95 (0.85-1.06)] (Supplementary Figure 5).
Peach: For the four included articles[29,40,42,57], the total analysis results showed that consuming more peaches did not reduce the risk of CRC [I2 = 62%, P = 0.02, REM; OR (95%CI) = 0.95 (0.83-1.09), P = 0.50] (Supplementary Figure 6). When stratified by study type, both case-control studies [I2 = 86%, P = 0.03, REM; OR (95%CI) = 0.90 (0.75-1.07)] and cohort studies [I2 = 47%, P = 0.17, REM; OR (95%CI) = 1.06 (0.87-1.29)] indicated that peach intake was not related to CRC risk (Supplementary Figure 7). The subgroup analysis based on tumor sites revealed that greater peach intake was not associated with the risk of malignancy in the colon [I2 = 0%, P = 0.77, REM; OR (95%CI) = 0.99 (0.91-1.08)] and rectum [I2 = 88%, P < 0.01, REM; OR (95%CI) = 0.96 (0.65-1.42)] (Supplementary Figure 8).
Strawberry: With three articles included in the analysis[29,40,42], overall results demonstrated no reduction in CRC risk even with higher intake of strawberries [I2 = 58%, P = 0.05, REM; OR (95%CI) = 0.97 (0.90-1.05)], P = 0.42] (Supplementary Figure 9). In the stratified analysis of tumor sites, strawberry intake was not related to cancer risk in either the rectum [I2 = 34%, P = 0.22, REM; OR (95%CI) = 0.93 (0.83-1.04)] or colon [I2 = 0%, P = 0.67, REM; OR (95%CI) = 1.00 (0.95-1.06)] (Supplementary Figure 10). In the stratified analysis by study type, case-control studies [I2 = 75%, P = 0.02, REM; OR (95%CI) = 0.95 (0.86-1.05)] and cohort studies [I2 = 0%, P = 1.00, REM; OR (95%CI) = 1.04 (0.91-1.19)] showed that strawberry consumption was not associated with CRC risk (Supplementary Figure 11).
Grape: With four articles included[29,40,42,57], overall analysis results indicated that the intake of large amounts of grapes was not related to a reduced risk of CRC [I2 = 51%, P = 0.07, REM; OR (95%CI) = 1.00 (0.91-1.10), P = 0.97] (Supplementary Figure 12). Subgroup analysis by tumor site showed that grape intake was not significantly associated with malignancy in both the rectum [I2 = 0%, P = 0.52, REM; OR (95%CI) = 0.91 (0.83-1.01)] and colon [I2 = 57%, P = 0.13, REM; OR (95%CI) = 1.05 (0.92-1.19)] (Supplementary Figure 13). Stratified by study type, case-control studies [I2 = 59%, P = 0.06, REM; OR (95%CI) = 0.97 (0.87-1.08)] and cohort studies [I2 = 13%, P = 0.28, REM; OR (95%CI) = 1.08 (0.93-1.26)] revealed no reduction in the risk of CRC with grape consumption (Supplementary Figure 14).
Other fresh Fruits: Watermelon[42,50] [I2 = 0%, P = 0.37, REM; OR (95%CI) = 0.74 (0.58-0.94), P = 0.02] (Supplementary Figure 15) and kiwi[29,42] [I2 = 0%, P = 0.51, REM; OR (95%CI) = 0.87 (0.78-0.96), P < 0.01] (Supplementary Figure 16) were related to a reduced risk of CRC. Pears[42,57] [I2 = 0%, P = 0.88, REM; OR (95%CI) = 1.08 (0.72-1.62), P = 0.70] (Supplementary Figure 17), melons[29,42] [I2 = 34%, P = 0.22, REM; OR (95%CI) = 0.96 (0.87-1.06), P = 0.39] (Supplementary Figure 18), and figs[42,57] [I2 = 80%, P = 0.03, REM; OR (95%CI) = 0.83 (0.32-2.17), P = 0.70] (Supplementary Figure 19) were not associated with a reduced risk of CRC.
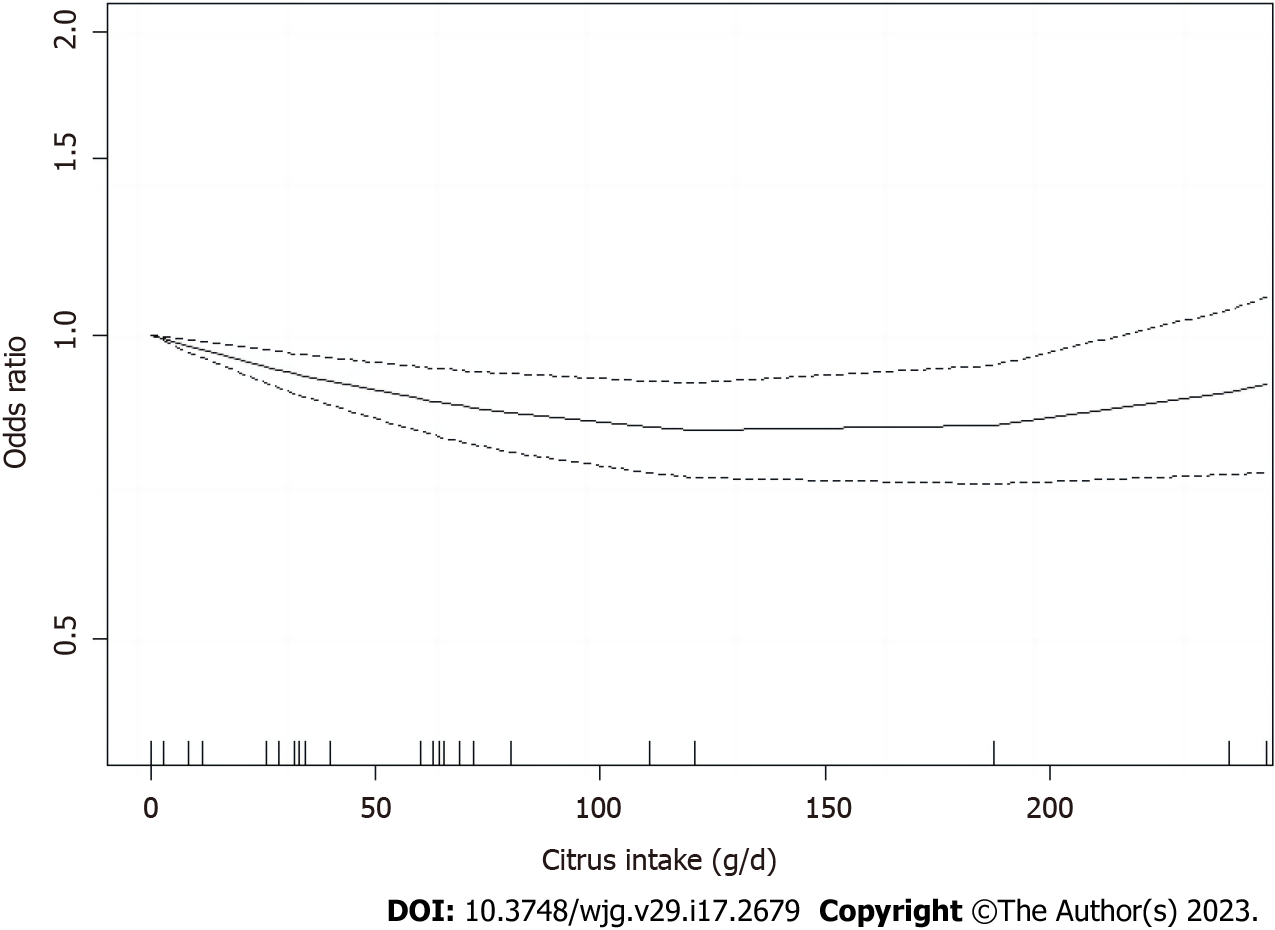
The dose-response analysis of citrus intake included seven articles[39,42,44-46,51,54] (Figure 8). A nonlinear relationship was observed between citrus intake and CRC risk [R (95%CI) = -0.0031 (-0.0047 to -0.0014), P < 0.001]. Based on the above meta-analyses results, a citrus intake of (0 g/d) was used as a reference group and the risk was minimized around 120 g/d (OR = 0.85), whereas no significant dose-response correlation was observed after continuing to increase intake, with correlations only assessed in the range of 0-248 g/d. Dose-response relationships between intake and CRC risk could not be calculated for other types of fruits due to the paucity of available data.
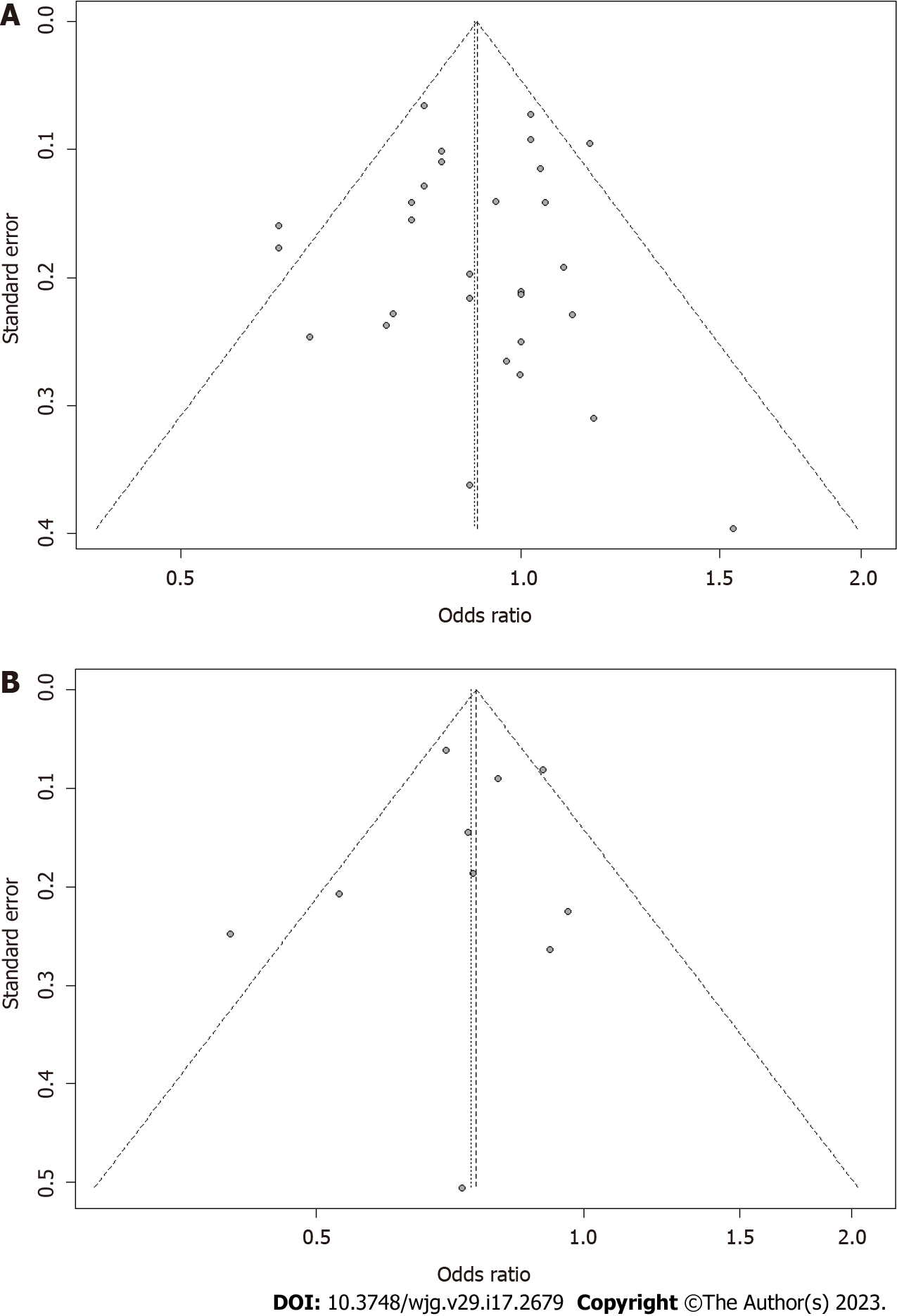
A sensitivity analysis was conducted for all outcome indicators with more than 50% heterogeneity and P < 0.05, and the results showed that the combined results were stable for heterogeneity. A sensitivity analysis based on quality assessment was also conducted. After articles with relatively low quality of evidence (three domains were graded as moderate risk) were excluded, the remaining data were pooled and analyzed again, and the outcome showed that our combined results were robust (SupplementaryFigures 20 and 21). No potential publication bias was found. For the analysis of high and low fruit intake, the P value of the citrus Egger’s test was 0.8467 (Figure 9A), and the P value for the apple Egger’s test was 0.6068 (Figure 9B). Other types of fruits were not tested for publication bias as the number of articles was less than 10.
The main findings of this meta-analysis demonstrated that compared to low intakes, higher intakes of citrus, apples, watermelon, and kiwi reduced the risk of CRC by 9%, 25%, 26%, and 13%, respectively, while bananas, grapes, strawberries, peaches, pears, figs, and other melons did not exhibit an association with CRC risk. A nonlinear dose-response relationship was observed between citrus and CRC risk in the present study. To our knowledge, this study is the first meta-analysis to investigate the association between different fruit intake and CRC risk, and the first to perform a dose-response analysis between citrus intake and CRC risk.
The results of this meta-analysis are supported by relevant biological theories. Citrus has many chemopreventive effects on CRC[58]. Nobiletin, a compound extracted from citrus, blocks the cell cycle, inhibits cell proliferation, induces apoptosis, prevents tumor formation, reduces inflammatory effects and limits angiogenesis[59]. Naringenin, which is rich in citrus, inhibits the proliferation of HT-29 colon cancer cells[60] and also reduces the severity of colorectal adenomas and colitis by inhibiting pro-inflammatory mediators GM-CSF/M-CSF, bone marrow-derived suppressor cells, IL-6 and TNF-α, and NF-κB/IL-6/STAT3 cascade in colorectal tissues[61]. Neohesperidin, derived from citrus fruits, has also been confirmed to prevent colorectal tumors by altering the intestinal microbiota[62]. Moreover, APs contained in apples have been verified to prevent AOM/dss-induced colitis-associated CRC (CACC) in ICR mice. APs modulate intestinal flora composition, reduce infiltration of neutrophils, macrophages and T cells in the colon, and more importantly, inhibit the entry of β-catenin into the nucleus, which in turn retards the Wnt/β-catenin pathway[63]. APs also induce apoptosis in colon cancer cells through microactivating the NF-κB pathway, and inhibit CRC cell migration and invasiveness by targeting the LPS/TLR4/NF-κB pathway[64,65]. It has also been shown that apple polyphenols and apple anthocyanin Cy3Gal inhibit and reduce the appearance of precancerous markers of CRC[66] as well as tumor lesions in AOM-induced CRC mice[67]. In addition, apple polyphenols affect the initiation of apoptosis in human colon cancer cells and the activity of protein kinase C[68]. That other fruits did not show protective effects may be owing to the small number of original studies, resulting in large heterogeneity and wide confidence intervals, which masked their anticancer effects. It could also be possible that the intake was too small to show a protective effect, and more research is needed for verification.
There are many reasons contributing to inconsistent results in several subgroup analyses. In citrus, case-control studies tended to show protective factors, while cohort studies did not. In other types of fruits, no correlation was seen in the subgroup analysis of the study type. Generally speaking, case-control studies have several weaknesses, such as a control group that may not be representative of the general population or more problems with reverse causality and recall bias. On the other side, dietary assessment questionnaires used in a prospective study setting may not be as accurate as those used in a retrospective case-control setting. Meanwhile, it is difficult for individuals to accurately report their fruit intake, and this low correlation has been confirmed in some studies (Spearman’s correlation coefficient of 0.6 for fruit consumption[69,70]), which may have weakened the estimates of the associated risk. Thus, the true association may be stronger than what we observed, reinforcing the conclusion of protective effect. In another subgroup analysis of studies’ geographic location, a negative association between citrus intake and CRC risk was observed in Asia but not in North/South America or Europe, and a negative association for bananas only in North/South America and apples in Europe. These results may be attributed to the varied consumption patterns of fruits and vegetables among countries, leading to errors in the measurement of dietary intake[71]. According to our pooled results, the specific sites of tumor occurrence, such as the distal colon, proximal colon, and rectum, were not significantly associated with the risk and benefit of fruit intake, indicating that fruits improve the function of the entire intestine or regulate the microbial flora of the entire digestive tract, but do not target specific sites, so there is no correlation with the specific location of tumors. No significant risk benefit was seen for men or women in the gender-based subgroup analysis either, possibly due to an insufficient number of included original studies or dietary measurement errors. From the dose-response analysis, the risk of CRC was found to be minimized at a citrus intake of 120 g/d, while the risk of CRC did not decrease further after continuing to increase intake. The underlying mechanism may be related to the availability and digestibility of nutrients from citrus fruits[72,73]. However, further studies are needed to validate our results.
Surgery, chemoradiotherapy and targeted drugs currently used to treat CRC are not only expensive but also highly toxic. Through this Meta-analysis, we can prioritize fruits with proven protective effects to prevent CRC. If cancer prevention can be achieved by changing dietary habits such as fruit supplementation, it will certainly reduce the huge economic burden and mortality of cancer in the world. As for future research directions, we hope to find the key components of anti-cancer through research and make element-specific nutritional preparations to help people better prevent cancer. More prospective studies are also expected to verify the anti-cancer effects of other kinds of fruits.
We observed low heterogeneity between studies. Despite moderate heterogeneity in the studies on bananas and peaches, further sensitivity analysis indicated robust primary outcome and the heterogeneity was acceptable. The funnel plots and Egger’s test we adopted produced consistent results, suggesting no publication bias. Moreover, the meta-analysis involved more than 1.06 million subjects, which makes it possible to explore associations between different subgroups, such as gender, geographic location and tumor location. Besides, a significant dose-response relationship was observed between citrus intake and CRC risk, further strengthening the association.
Nevertheless, there are some limitations of our study. First, synthetic results are limited due to the lack of research data on many types of fruits (e.g., grapes, pears, and figs, etc.). This is coupled with the fact that dietary assessments of the frequency/amount of fruit intake varies so much that the protective effect against cancer cannot be truly captured. Additional potential bias may exist due to the diversity of designs and inconsistency of adjustment factors in the studies we analyzed. Although we extracted data with the most comprehensive adjustment for confounders, a subset of studies still did not adjust for potential dietary confounding variables (e.g., meat, fiber, income status, and occupation). The limited range of citrus intake in the dose-response meta-analysis may have led to incomplete results. And limited available data for other fruits and the small number of original studies and made it impossible to investigate dose-response relationships between their intake and cancer risk.
Taken together, our results support the hypothesis that citrus, apple, watermelon and kiwi intake may contribute to a reduced risk of CRC. A nonlinear dose-response relationship was also observed between citrus intake and CRC risk within a certain range. However, the relationship between other types of fruit intake and CRC risk may be obscured by the various limitations mentioned above. Therefore, future prospective studies are required to further explore the effects of measurement error and control for important confounders, and thus reveal the true relationship between fruit and CRC.
Colorectal cancer (CRC) is the third most prevalent cancer, and the prevalence of CRC in adults aged 40-49 has increased by approximately 15% between 2000-2002 and 2014-2016. However, inconsistent findings have been reported in different studies on the association between the intake of different types of fruits and CRC. Given their different chemical compositions and underlying molecular mechanisms, some types of fruits may have a closer correlation with CRC risk than others. This meta-analysis provides more reliable evidence that a higher intake of certain fruits is more effective in CRC prevention.
The main topic of this study is exploring the association between intake of different types of fruits and CRC risk. The key problem to be solved is to compare the CRC risk in the highest and lowest intake groups and conduct a meta-analysis. The significance of this study is that we have found that certain types of fruits can effectively reduce CRC risk.
To help people improve their lifestyles and dietary habits to live a healthy life. The most important goal is to ease the CRC-related social and economic burden worldwide. In terms of goal realized, through this meta-analysis, we have found that eating more citrus, apple, watermelon and kiwi fruit can effectively reduce CRC risk. Implications of achieving the goals: Further analysis of specific types of fruit is needed to explore key anti-cancer components.
This meta-analysis was conducted by comparing the reported CRC risk between the highest and lowest fruit intake groups. Considering that CRC is rare, the risk is less than 10%, and the OR is small, the RR/HR we have calculated is approximately equal to the OR. Heterogeneity of results across studies was assessed by the I² test. Publication bias was determined using funnel plots and Egger’s linear regression test. A dose-response analysis of citrus fruits was also conducted to examine a possible nonlinear relationship. All analyses were performed using R (version 4.1.3). Characteristics and novelty of the research method: No research has used this method to explore this topic before. This research direction is very suitable for this research method.
Findings: High intakes of citrus, apple, watermelon, and kiwi reduced CRC risk by 9%, 25%, 26%, and 13%, respectively, compared with low intakes. However, other types of fruit did not show an association with CRC risk. A non-linear dose-response relationship was found between citrus and CRC risk. Contribution to the field: This study performed a meta-analysis of previous data in a scientific context and identified the fruit types most effective in reducing CRC risk. Unresolved issues: More prospective studies are needed in the future to further elucidate the association between fruit and CRC.
The results of this study underpin the hypothesis that certain types of fruit are effective in preventing CRC. This meta-analysis is based on the reported CRC risk of the highest and lowest fruit intake groups. It convincingly demonstrates the real association between fruit and CRC.
In future research, we hope to find out the key anti-cancer components of specific types of fruits, so as to help people prevent cancer more effectively.
We would like to thank the researchers and study participants for their contributions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chang YC, Taiwan; Mohamed SY, Egypt; Parthasarathy K, India; Rather AA, India S-Editor: Chen YL L-Editor: A P-Editor: Yuan YY
| 1. | El-Shami K, Oeffinger KC, Erb NL, Willis A, Bretsch JK, Pratt-Chapman ML, Cannady RS, Wong SL, Rose J, Barbour AL, Stein KD, Sharpe KB, Brooks DD, Cowens-Alvarado RL. American Cancer Society Colorectal Cancer Survivorship Care Guidelines. CA Cancer J Clin. 2015;65:428-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 327] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 2. | Haghighat S, Sussman DA, Deshpande A. US Preventive Services Task Force Recommendation Statement on Screening for Colorectal Cancer. JAMA. 2021;326:1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | O'Sullivan DE, Sutherland RL, Town S, Chow K, Fan J, Forbes N, Heitman SJ, Hilsden RJ, Brenner DR. Risk Factors for Early-Onset Colorectal Cancer: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:1229-1240.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 196] [Article Influence: 65.3] [Reference Citation Analysis (1)] |
| 4. | Li YH, Niu YB, Sun Y, Zhang F, Liu CX, Fan L, Mei QB. Role of phytochemicals in colorectal cancer prevention. World J Gastroenterol. 2015;21:9262-9272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 5. | François C, van Velthoven R, De Lathouwer O, Moreno C, Peltier A, Kaltner H, Salmon I, Gabius HJ, Danguy A, Decaestecker C, Kiss R. Galectin-1 and galectin-3 binding pattern expression in renal cell carcinomas. Am J Clin Pathol. 1999;112:194-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Piantelli M, Iacobelli S, Almadori G, Iezzi M, Tinari N, Natoli C, Cadoni G, Lauriola L, Ranelletti FO. Lack of expression of galectin-3 is associated with a poor outcome in node-negative patients with laryngeal squamous-cell carcinoma. J Clin Oncol. 2002;20:3850-3856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Nakahara S, Oka N, Raz A. On the role of galectin-3 in cancer apoptosis. Apoptosis. 2005;10:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 230] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 8. | Li Y, Liu L, Niu Y, Feng J, Sun Y, Kong X, Chen Y, Chen X, Gan H, Cao S, Mei Q. Modified apple polysaccharide prevents against tumorigenesis in a mouse model of colitis-associated colon cancer: role of galectin-3 and apoptosis in cancer prevention. Eur J Nutr. 2012;51:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Liu YZ, Wu K, Huang J, Liu Y, Wang X, Meng ZJ, Yuan SX, Wang DX, Luo JY, Zuo GW, Yin LJ, Chen L, Deng ZL, Yang JQ, Sun WJ, He BC. The PTEN/PI3K/Akt and Wnt/β-catenin signaling pathways are involved in the inhibitory effect of resveratrol on human colon cancer cell proliferation. Int J Oncol. 2014;45:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 10. | Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20:187-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 584] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 11. | Arafa MA, Waly MI, Jriesat S, Al Khafajei A, Sallam S. Dietary and lifestyle characteristics of colorectal cancer in Jordan: a case-control study. Asian Pac J Cancer Prev. 2011;12:1931-1936. [PubMed] |
| 12. | van Duijnhoven FJ, Bueno-De-Mesquita HB, Ferrari P, Jenab M, Boshuizen HC, Ros MM, Casagrande C, Tjønneland A, Olsen A, Overvad K, Thorlacius-Ussing O, Clavel-Chapelon F, Boutron-Ruault MC, Morois S, Kaaks R, Linseisen J, Boeing H, Nöthlings U, Trichopoulou A, Trichopoulos D, Misirli G, Palli D, Sieri S, Panico S, Tumino R, Vineis P, Peeters PH, van Gils CH, Ocké MC, Lund E, Engeset D, Skeie G, Suárez LR, González CA, Sánchez MJ, Dorronsoro M, Navarro C, Barricarte A, Berglund G, Manjer J, Hallmans G, Palmqvist R, Bingham SA, Khaw KT, Key TJ, Allen NE, Boffetta P, Slimani N, Rinaldi S, Gallo V, Norat T, Riboli E. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2009;89:1441-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 192] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Dahm CC, Keogh RH, Spencer EA, Greenwood DC, Key TJ, Fentiman IS, Shipley MJ, Brunner EJ, Cade JE, Burley VJ, Mishra G, Stephen AM, Kuh D, White IR, Luben R, Lentjes MA, Khaw KT, Rodwell Bingham SA. Dietary fiber and colorectal cancer risk: a nested case-control study using food diaries. J Natl Cancer Inst. 2010;102:614-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS, Giovannucci E. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr. 2010;92:1429-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 15. | Wang DD, Li Y, Bhupathiraju SN, Rosner BA, Sun Q, Giovannucci EL, Rimm EB, Manson JE, Willett WC, Stampfer MJ, Hu FB. Fruit and Vegetable Intake and Mortality: Results From 2 Prospective Cohort Studies of US Men and Women and a Meta-Analysis of 26 Cohort Studies. Circulation. 2021;143:1642-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 231] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 16. | Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Stampfer MJ, Rosner B, Speizer FE, Willett WC. Dietary fiber and the risk of colorectal cancer and adenoma in women. N Engl J Med. 1999;340:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 359] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 17. | Flood A, Velie EM, Chaterjee N, Subar AF, Thompson FE, Lacey JV Jr, Schairer C, Troisi R, Schatzkin A. Fruit and vegetable intakes and the risk of colorectal cancer in the Breast Cancer Detection Demonstration Project follow-up cohort. Am J Clin Nutr. 2002;75:936-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Koushik A, Hunter DJ, Spiegelman D, Beeson WL, van den Brandt PA, Buring JE, Calle EE, Cho E, Fraser GE, Freudenheim JL, Fuchs CS, Giovannucci EL, Goldbohm RA, Harnack L, Jacobs DR Jr, Kato I, Krogh V, Larsson SC, Leitzmann MF, Marshall JR, McCullough ML, Miller AB, Pietinen P, Rohan TE, Schatzkin A, Sieri S, Virtanen MJ, Wolk A, Zeleniuch-Jacquotte A, Zhang SM, Smith-Warner SA. Fruits, vegetables, and colon cancer risk in a pooled analysis of 14 cohort studies. J Natl Cancer Inst. 2007;99:1471-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 19. | Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1225] [Cited by in RCA: 1077] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 20. | Yang Y, Zhang D, Feng N, Chen G, Liu J, Zhu Y. Increased intake of vegetables, but not fruit, reduces risk for hepatocellular carcinoma: a meta-analysis. Gastroenterology. 2014;147:1031-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 21. | Norat T, Aune D, Chan D, Romaguera D. Fruits and vegetables: updating the epidemiologic evidence for the WCRF/AICR lifestyle recommendations for cancer prevention. Cancer Treat Res. 2014;159:35-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11206] [Cited by in RCA: 11041] [Article Influence: 690.1] [Reference Citation Analysis (0)] |
| 23. | Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 830] [Cited by in RCA: 721] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 24. | Rong Y, Chen L, Zhu T, Song Y, Yu M, Shan Z, Sands A, Hu FB, Liu L. Egg consumption and risk of coronary heart disease and stroke: dose-response meta-analysis of prospective cohort studies. BMJ. 2013;346:e8539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 252] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 25. | Vieira AR, Abar L, Vingeliene S, Chan DS, Aune D, Navarro-Rosenblatt D, Stevens C, Greenwood D, Norat T. Fruits, vegetables and lung cancer risk: a systematic review and meta-analysis. Ann Oncol. 2016;27:81-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 184] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 26. | Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78:559S-569S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 673] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 27. | Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7683] [Cited by in RCA: 10882] [Article Influence: 1209.1] [Reference Citation Analysis (2)] |
| 28. | Localio AR, Margolis DJ, Berlin JA. Relative risks and confidence intervals were easily computed indirectly from multivariable logistic regression. J Clin Epidemiol. 2007;60:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 29. | Franceschi S, Parpinel M, La Vecchia C, Favero A, Talamini R, Negri E. Role of different types of vegetables and fruit in the prevention of cancer of the colon, rectum, and breast. Epidemiology. 1998;9:338-341. [PubMed] |
| 30. | Franceschi S, Favero A. The role of energy and fat in cancers of the breast and colon-rectum in a southern European population. Ann Oncol. 1999;10 Suppl 6:61-63. [PubMed] |
| 31. | Franceschi S, Favero A, Parpinel M, Giacosa A, La Vecchia C. Italian study on colorectal cancer with emphasis on influence of cereals. Eur J Cancer Prev. 1998;7 Suppl 2:S19-S23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Jedrychowski W, Maugeri U, Popiela T, Kulig J, Sochacka-Tatara E, Pac A, Sowa A, Musial A. Case-control study on beneficial effect of regular consumption of apples on colorectal cancer risk in a population with relatively low intake of fruits and vegetables. Eur J Cancer Prev. 2010;19:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Jedrychowski W, Maugeri U, Pac A, Sochacka-Tatara E, Galas A. Reduced risk of colorectal cancer and regular consumption of apples: Hospital based case-control study in Poland. CEJ Med. 2009;4:320-326. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Tuyns AJ, Kaaks R, Haelterman M. Colorectal cancer and the consumption of foods: a case-control study in Belgium. Nutr Cancer. 1988;11:189-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 125] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Tajima K, Tominaga S. Dietary habits and gastro-intestinal cancers: a comparative case-control study of stomach and large intestinal cancers in Nagoya, Japan. Jpn J Cancer Res. 1985;76:705-716. [PubMed] |
| 36. | Lin J, Zhang SM, Wu K, Willett WC, Fuchs CS, Giovannucci E. Flavonoid intake and colorectal cancer risk in men and women. Am J Epidemiol. 2006;164:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Theodoratou E, Kyle J, Cetnarskyj R, Farrington SM, Tenesa A, Barnetson R, Porteous M, Dunlop M, Campbell H. Dietary flavonoids and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:684-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 38. | Deneo-Pellegrini H, Boffetta P, De Stefani E, Ronco A, Brennan P, Mendilaharsu M. Plant foods and differences between colon and rectal cancers. Eur J Cancer Prev. 2002;11:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Lee J, Shin A, Oh JH, Kim J. Colors of vegetables and fruits and the risks of colorectal cancer. World J Gastroenterol. 2017;23:2527-2538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Leenders M, Siersema PD, Overvad K, Tjønneland A, Olsen A, Boutron-Ruault MC, Bastide N, Fagherazzi G, Katzke V, Kühn T, Boeing H, Aleksandrova K, Trichopoulou A, Lagiou P, Klinaki E, Masala G, Grioni S, Santucci De Magistris M, Tumino R, Ricceri F, Peeters PH, Lund E, Skeie G, Weiderpass E, Quirós JR, Agudo A, Sánchez MJ, Dorronsoro M, Navarro C, Ardanaz E, Ohlsson B, Jirström K, Van Guelpen B, Wennberg M, Khaw KT, Wareham N, Key TJ, Romieu I, Huybrechts I, Cross AJ, Murphy N, Riboli E, Bueno-de-Mesquita HB. Subtypes of fruit and vegetables, variety in consumption and risk of colon and rectal cancer in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2015;137:2705-2714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 41. | Abu Mweis SS, Tayyem RF, Shehadah I, Bawadi HA, Agraib LM, Bani-Hani KE, Al-Jaberi T, Al-Nusairr M. Food groups and the risk of colorectal cancer: results from a Jordanian case-control study. Eur J Cancer Prev. 2015;24:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Tayyem RF, Shehadah I, Abu-Mweis SS, Bawadi HA, Bani-Hani KE, Al-Jaberi T, Al-Nusairr M, Heath DD. Fruit and vegetable intake among Jordanians: results from a case-control study of colorectal cancer. Cancer Control. 2014;21:350-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Rosato V, Bosetti C, Levi F, Polesel J, Zucchetto A, Negri E, La Vecchia C. Risk factors for young-onset colorectal cancer. Cancer Causes Control. 2013;24:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 44. | Annema N, Heyworth JS, McNaughton SA, Iacopetta B, Fritschi L. Fruit and vegetable consumption and the risk of proximal colon, distal colon, and rectal cancers in a case-control study in Western Australia. J Am Diet Assoc. 2011;111:1479-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Foschi R, Pelucchi C, Dal Maso L, Rossi M, Levi F, Talamini R, Bosetti C, Negri E, Serraino D, Giacosa A, Franceschi S, La Vecchia C. Citrus fruit and cancer risk in a network of case-control studies. Cancer Causes Control. 2010;21:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Williams CD, Satia JA, Adair LS, Stevens J, Galanko J, Keku TO, Sandler RS. Dietary patterns, food groups, and rectal cancer risk in Whites and African-Americans. Cancer Epidemiol Biomarkers Prev. 2009;18:1552-1561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Gallus S, Talamini R, Giacosa A, Montella M, Ramazzotti V, Franceschi S, Negri E, La Vecchia C. Does an apple a day keep the oncologist away? Ann Oncol. 2005;16:1841-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Satia-Abouta J, Galanko JA, Martin CF, Ammerman A, Sandler RS. Food groups and colon cancer risk in African-Americans and Caucasians. Int J Cancer. 2004;109:728-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Levi F, Pasche C, La Vecchia C, Lucchini F, Franceschi S. Food groups and colorectal cancer risk. Br J Cancer. 1999;79:1283-1287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 138] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Vogtmann E, Xiang YB, Li HL, Levitan EB, Yang G, Waterbor JW, Gao J, Cai H, Xie L, Wu QJ, Zhang B, Gao YT, Zheng W, Shu XO. Fruit and vegetable intake and the risk of colorectal cancer: results from the Shanghai Men's Health Study. Cancer Causes Control. 2013;24:1935-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Li WQ, Kuriyama S, Li Q, Nagai M, Hozawa A, Nishino Y, Tsuji I. Citrus consumption and cancer incidence: the Ohsaki cohort study. Int J Cancer. 2010;127:1913-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Nomura AM, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Pike MC, Kolonel LN. Association of vegetable, fruit, and grain intakes with colorectal cancer: the Multiethnic Cohort Study. Am J Clin Nutr. 2008;88:730-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | Voorrips LE, Goldbohm RA, van Poppel G, Sturmans F, Hermus RJ, van den Brandt PA. Vegetable and fruit consumption and risks of colon and rectal cancer in a prospective cohort study: The Netherlands Cohort Study on Diet and Cancer. Am J Epidemiol. 2000;152:1081-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 197] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 54. | Michels KB, Edward Giovannucci, Joshipura KJ, Rosner BA, Stampfer MJ, Fuchs CS, Colditz GA, Speizer FE, Willett WC. Prospective study of fruit and vegetable consumption and incidence of colon and rectal cancers. J Natl Cancer Inst. 2000;92:1740-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 260] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 55. | Lin J, Zhang SM, Cook NR, Rexrode KM, Liu S, Manson JE, Lee IM, Buring JE. Dietary intakes of fruit, vegetables, and fiber, and risk of colorectal cancer in a prospective cohort of women (United States). Cancer Causes Control. 2005;16:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 56. | Le Marchand L, Hankin JH, Wilkens LR, Kolonel LN, Englyst HN, Lyu LC. Dietary fiber and colorectal cancer risk. Epidemiology. 1997;8:658-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Deneo-Pellegrini H, De Stefani E, Ronco A. Vegetables, fruits, and risk of colorectal cancer: a case-control study from Uruguay. Nutr Cancer. 1996;25:297-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Jaganathan SK, Vellayappan MV, Narasimhan G, Supriyanto E. Role of pomegranate and citrus fruit juices in colon cancer prevention. World J Gastroenterol. 2014;20:4618-4625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 59. | Goh JXH, Tan LT, Goh JK, Chan KG, Pusparajah P, Lee LH, Goh BH. Nobiletin and Derivatives: Functional Compounds from Citrus Fruit Peel for Colon Cancer Chemoprevention. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 60. | Frydoonfar HR, McGrath DR, Spigelman AD. The variable effect on proliferation of a colon cancer cell line by the citrus fruit flavonoid Naringenin. Colorectal Dis. 2003;5:149-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 61. | Zhang YS, Wang F, Cui SX, Qu XJ. Natural dietary compound naringin prevents azoxymethane/dextran sodium sulfate-induced chronic colorectal inflammation and carcinogenesis in mice. Cancer Biol Ther. 2018;19:735-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 62. | Gong Y, Dong R, Gao X, Li J, Jiang L, Zheng J, Cui S, Ying M, Yang B, Cao J, He Q. Neohesperidin prevents colorectal tumorigenesis by altering the gut microbiota. Pharmacol Res. 2019;148:104460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 63. | Li Y, Wang S, Sun Y, Xu W, Zheng H, Wang Y, Tang Y, Gao X, Song C, Long Y, Liu J, Liu L, Mei Q. Apple polysaccharide protects ICR mice against colitis associated colorectal cancer through the regulation of microbial dysbiosis. Carbohydr Polym. 2020;230:115726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 64. | Zhang D, Sun Y, Yue Z, Li Q, Meng J, Liu J, Hekong X, Jiang F, Mi M, Liu L, Mei Q. Apple polysaccharides induce apoptosis in colorectal cancer cells. Int J Mol Med. 2012;30:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 65. | Zhang D, Li YH, Mi M, Jiang FL, Yue ZG, Sun Y, Fan L, Meng J, Zhang X, Liu L, Mei QB. Modified apple polysaccharides suppress the migration and invasion of colorectal cancer cells induced by lipopolysaccharide. Nutr Res. 2013;33:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 66. | Bars-Cortina D, Martínez-Bardají A, Macià A, Motilva MJ, Piñol-Felis C. Consumption evaluation of one apple flesh a day in the initial phases prior to adenoma/adenocarcinoma in an azoxymethane rat colon carcinogenesis model. J Nutr Biochem. 2020;83:108418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Marzo F, Milagro FI, Barrenetxe J, Díaz MT, Martínez JA. Azoxymethane-Induced Colorectal Cancer Mice Treated with a Polyphenol-Rich Apple Extract Show Less Neoplastic Lesions and Signs of Cachexia. Foods. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 68. | Kern M, Pahlke G, Balavenkatraman KK, Böhmer FD, Marko D. Apple polyphenols affect protein kinase C activity and the onset of apoptosis in human colon carcinoma cells. J Agric Food Chem. 2007;55:4999-5006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 69. | Steevens J, Schouten LJ, Goldbohm RA, van den Brandt PA. Vegetables and fruits consumption and risk of esophageal and gastric cancer subtypes in the Netherlands Cohort Study. Int J Cancer. 2011;129:2681-2693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 70. | Yamaji T, Inoue M, Sasazuki S, Iwasaki M, Kurahashi N, Shimazu T, Tsugane S; Japan Public Health Center-based Prospective Study Group. Fruit and vegetable consumption and squamous cell carcinoma of the esophagus in Japan: the JPHC study. Int J Cancer. 2008;123:1935-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 71. | Bae JM, Lee EJ, Guyatt G. Citrus fruit intake and stomach cancer risk: a quantitative systematic review. Gastric Cancer. 2008;11:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 72. | Holst B, Williamson G. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr Opin Biotechnol. 2008;19:73-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 299] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 73. | Oh H, Kim H, Lee DH, Lee A, Giovannucci EL, Kang SS, Keum N. Different dietary fibre sources and risks of colorectal cancer and adenoma: a dose-response meta-analysis of prospective studies. Br J Nutr. 2019;122:605-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |