Published online May 7, 2023. doi: 10.3748/wjg.v29.i17.2642
Peer-review started: December 26, 2022
First decision: February 8, 2023
Revised: February 17, 2023
Accepted: April 13, 2023
Article in press: April 13, 2023
Published online: May 7, 2023
Processing time: 132 Days and 2.8 Hours
An in-depth study of the pathogenesis and biological characteristics of ampullary carcinoma is necessary to identify appropriate treatment strategies. To date, only eight ampullary cancer cell lines have been reported, and a mixed-type ampullary carcinoma cell line has not yet been reported.
To establish a stable mixed-type ampullary carcinoma cell line originating from Chinese.
Fresh ampullary cancer tissue samples were used for primary culture and subculture. The cell line was evaluated by cell proliferation assays, clonal formation assays, karyotype analysis, short tandem repeat (STR) analysis and transmission electron microscopy. Drug resistances against oxaliplatin, paclitaxel, gemcitabine and 5-FU were evaluated by cell counting kit-8 assay. Subcutaneous injection 1 × 106 cells to three BALB/c nude mice for xenograft studies. The hematoxylin-eosin staining was used to detect the pathological status of the cell line. The expression of biomarkers cytokeratin 7 (CK7), cytokeratin 20 (CK20), cytokeratin low molecular weight (CKL), Ki67 and carcinoembryonic antigen (CEA) were determined by immunocytochemistry assay.
DPC-X1 was continuously cultivated for over a year and stably passaged for more than 80 generations; its population doubling time was 48 h. STR analysis demonstrated that the characteristics of DPC-X1 were highly consistent with those of the patient’s primary tumor. Furthermore, karyotype analysis revealed its abnormal sub-tetraploid karyotype. DPC-X1 could efficiently form organoids in suspension culture. Under the transmission electron microscope, microvilli and pseudopods were observed on the cell surface, and desmosomes were visible between the cells. DPC-X1 cells inoculated into BALB/C nude mice quickly formed transplanted tumors, with a tumor formation rate of 100%. Their pathological characteristics were similar to those of the primary tumor. Moreover, DPC-X1 was sensitive to oxaliplatin and paclitaxel and resistant to gemcitabine and 5-FU. Immunohistochemistry showed that the DPC-X1 cells were strongly positive for CK7, CK20, and CKL; the Ki67 was 50%, and CEA was focally expressed.
Here, we have constructed a mixed-type ampullary carcinoma cell line that can be used as an effective model for studying the pathogenesis of ampullary carcinoma and drug development.
Core Tip: A new ampullary carcinoma cell line has been established, making up for the shortage of Chinese ampullary carcinoma cell lines. And this is the the first study to report a mixed-type ampullary carcinoma cell line. The cell line inoculated into BALB/c nude mice can quickly form xenograft tumors. It is an excellent model for studying the mechanisms of invasion, metastasis and other mechanisms of ampullary carcinoma. The cell line is sensitive to oxaliplatin and paclitaxel but is naturally resistant to gemcitabine and fluorouracil, which can be used for drug resistance mechanism research and new drug development.
- Citation: Xu H, Chai CP, Miao X, Tang H, Hu JJ, Zhang H, Zhou WC. Establishment and characterization of a new human ampullary carcinoma cell line, DPC-X1. World J Gastroenterol 2023; 29(17): 2642-2656
- URL: https://www.wjgnet.com/1007-9327/full/v29/i17/2642.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i17.2642
Ampullary carcinoma is a relatively rare tumor, accounting for 0.2% of gastrointestinal cancers but 20% of all periampullary cancers[1,2]. Recently, the incidence rate of ampullary cancer has gradually increased[3,4]. Ampullary carcinoma can occur at any age; however, it is more common between 60 and 65 years of age, and the sex ratio is approximately 3:2[1,2]. As biliary obstruction symptoms appear early, the surgical resection rate of ampullary carcinoma is higher than that of other periampullary malignant tumors, and approximately 50% of patients are eligible for surgery on diagnosis[1,5,6]. Its operation amount is over one-third of the total operation amount of pancreatoduodenectomy. The prognosis is good, and the 5-year survival rate exceeds 52.8%[7]. Despite a high potential curative resection rate, most patients with ampullary cancer eventually succumb to tumor recurrence[8,9].
In 1994, Kimura et al[10] first classified ampullary carcinoma into intestinal and pancreaticobiliary types according to their histological characteristics. The prevalence of the pancreaticobiliary type was 72%, which is much higher than that of the intestinal type[10]. In 2010, according to the morphological and immunohistochemical characteristics, WHO revised the pathological diagnosis criteria of ampullary carcinoma into three different histopathological subtypes: Intestinal-, pancreaticobiliary-, and mixed-type ampullary carcinoma[11,12].
To date, only eight ampullary cancer cell lines have been included in the official databases of ATCC, JCRB, RIKEN, and DSMZ. One is American, one is Italian, two are Korean, and four are Japanese (three of them are from different lesions of the same patient)[13-17] (Table 1). Among them, SNU-869 is an intestinal-type cell line, whereas the remaining are pancreaticobiliary-type cell lines[18]. No mixed-type ampullary carcinoma cell line has yet been reported.
| Cell line | Age (years) | Gender | Source of culture | Race | Differentiation | Primary culture | Ref. |
| MDAAmp-7 | 40 | Male | Abdominal metastases | American | Well differentiated | 1989 | [13] |
| RCB1169/TGBC18TKB | 79 | Female | Primary tumor | Japanese | - | 1995 | [14] |
| RCB1280/TGBC50TKB | 52 | Male | Liver metastasis | Japanese | Poorly differentiatedadenosquamous carcinoma | 1996 | [15] |
| RCB1280/TGBC51TKB | 525 | Male | Ascites | Japanese | Poorly differentiatedadenosquamous carcinoma | 1996 | [15] |
| RCB1280/TGBC52TKB | 52 | Male | Lymph node | Japanese | Poorly differentiatedadenosquamous carcinoma | 1996 | [15] |
| SNU478 | - | - | - | Korean | Poorly differentiated with signet ring cell | - | [16] |
| SNU869 | - | - | - | Korean | Well differentiated | - | [16] |
| AVC1 | 71 | Female | Primary tumor | Italian | Moderately differentiated | 1997 | [17] |
No randomized clinical trial for adjuvant chemotherapy of ampullary carcinoma currently exists, and the data based on which doctors choose treatment methods are limited. Presently, adjuvant therapy for ampullary carcinoma is being tailored according to the histological subtypes. Pancreatic biliary ampullary carcinoma is typically treated similarly to pancreatic adenocarcinoma or biliary tract carcinoma. In contrast, patients with intestinal ampulla receive the usual protocol for colorectal cancer. Based on these premises, the optimal treatment plan for ampullary carcinoma in adjuvant therapy and chemotherapy for advanced patients remains to be determined[19,20].
Based on the aforementioned factors, establishing an ampullary carcinoma cell line, conducting in-depth research on the pathogenesis and biological characteristics of ampullary carcinoma, and then investigating therapeutic targets to formulate effective treatment strategies for patients with ampullary carcinoma are currently required.
In this study, we establish a stable mixed-type ampullary carcinoma cell line named DPC-X1 from the tumor tissue of a patient with ampullary carcinoma. Our study findings underscore the effectiveness of DPC-X1 as an experimental model that can be used to explore the molecular mechanism of ampullary carcinoma and develop therapeutic schemes for disease control.
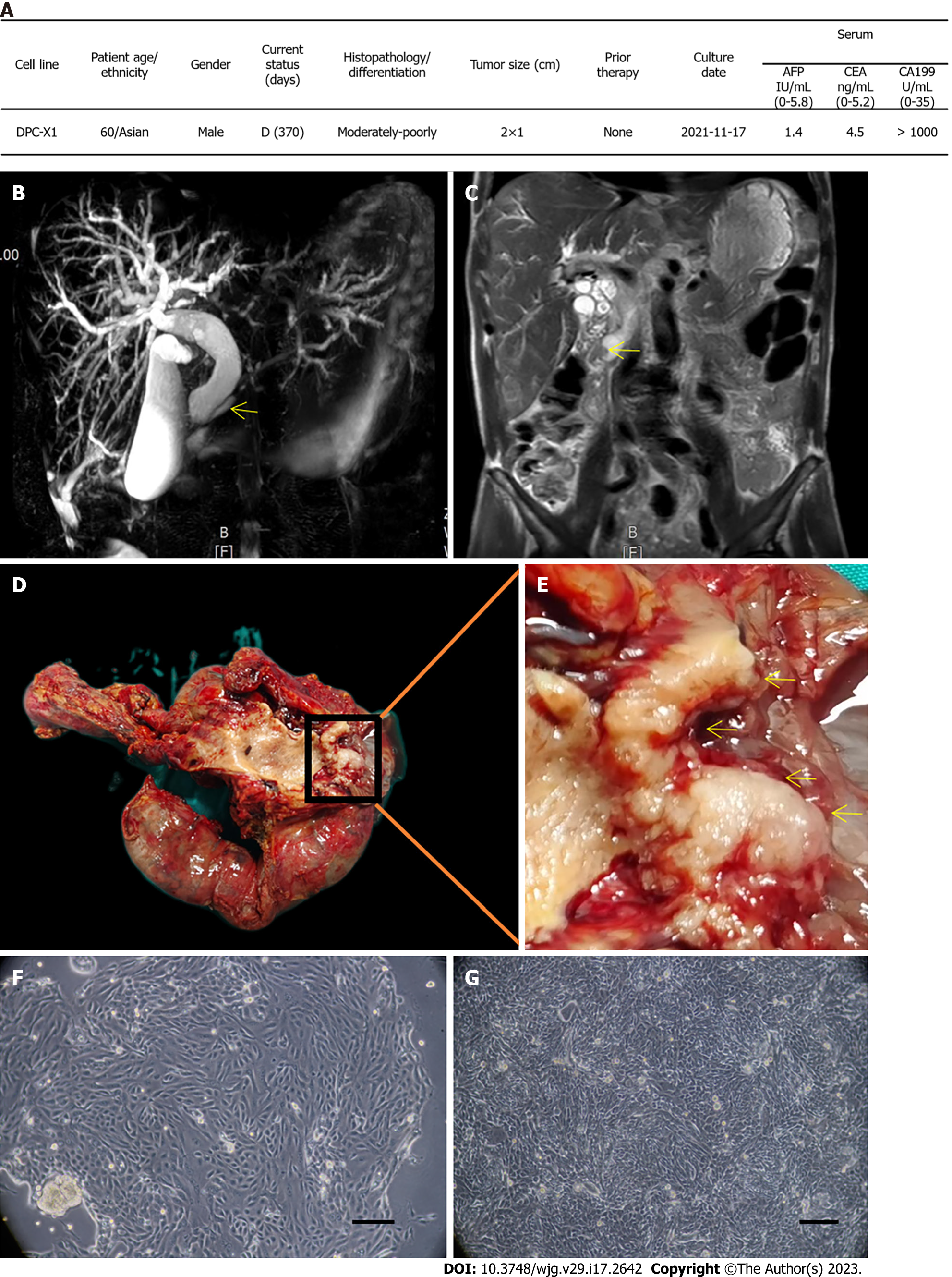
The tissue samples were collected from a patient with ampullary cancer who was admitted to the First Hospital of Lanzhou University on November 17, 2021, for pancreatoduodenectomy. The patient was a 60-year-old man who presented with jaundice, had a long history of smoking and drinking, and had no history of carrying hepatitis B or C. His carbohydrate antigen 199 levels exceeded 1000 U/mL (reference range: 0–35 U/mL; Figure 1A), and preoperative magnetic resonance imaging + magnetic resonance cholangiopancreatography indicated ampullary carcinoma (Figure 1B and C). The new organisms growing around the ampulla were grayish-white and were taken from the primary focus for primary culture and subculture (Figure 1D and E).
This study was approved by the Medical Ethics Committee of the First Hospital of Lanzhou University (LDYYLL-2022-487), and signed informed consent was obtained from the patient.
Gemcitabine was obtained from Jiangsu Haosen Pharmaceutical Group Co., Ltd., oxaliplatin from Jiangsu Hengrui Pharmaceutical Co., Ltd., 5-FU from Tianjin Jinyao Pharmaceutical Co., Ltd., and paclitaxel from Jiangsu Aosaikang Pharmaceutical Co., Ltd.
In this study, experiments were conducted on 4–6-week-old BALB/c nude female mice, weighing 16–20 g. They were obtained from Changzhou Kavens Experimental Animal Co., Ltd. and raised in the SPF laboratory of the Animal Experiment Center of Lanzhou University. The animal protocol was designed to minimize pain or discomfort to the animals. The animals were acclimatized to laboratory conditions (23 ℃, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for 2 wk prior to experimentation. All animals were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection.
All animal experiments were reviewed and approved by the Medical Animal Experiment Ethics Committee of the First Hospital of Lanzhou University (LDYYLL-2022-487). The methods followed in this study were similar or identical to those employed in previous studies[21-23].
The tumor tissue samples were immersed in sterile phosphate buffered saline (PBS) (Gibco) 3–5 times and sectioned into small pieces. The small woven pieces were mixed with type II collagenase (Gibco) and neutral protease (Invitrogen) and digested in a shaking table at 37 ℃. On digestion of half the tissue block, the supernatant was absorbed, filtered with a 100 mesh filter screen, and centrifuged at 300 × g for 3 min. Thereafter, the supernatant was discarded; the sample was resuspended in PBS and centrifugated at 300 × g for 3 min. The precipitate was added to a complete medium [RPMI-1640 + 10% fetal bovine serum (FBS) + 1% penicillin–streptomycin, Biological industries (BI)] and uniformly inoculated on a six-well plate (NEST). The medium was refreshed after 48 h. The mixed fibroblasts in the primary culture were removed using differential digestion with 0.25% trypsin. When the cells reached 70% confluency, they were digested and passed on. The cell growth was regularly observed under a light microscope. From the fifth generation, the cells were subcultured in a ratio of 1:2 and frozen with Serum-free rapid cell cryopreservation solution (Mei5 Biotechnology Co., Ltd.).
The cell density of DPC-X1 cells in the logarithmic growth phase (P20) was adjusted to 1 × 104/mL after trypsin digestion. After mixing, 0.1 mL of sample was inoculated in each hole in a 96-hole plate cell counting kit-8 (CCK-8) reagent (Dojindo) was added at the same time for 4 consecutive days after inoculation and allowed to react for 2 h. The UV absorbance value at 450 nm wavelength was measured with a microplate reader. The cell doubling time was calculated using the following formula: Td = t × Lg2/Lg (N1/N0). The cell growth curve was plotted with time as the horizontal axis and absorbance value as the vertical axis.
DPC-X1 cells in the logarithmic growth stage (P10) were collected after trypsin digestion. Together with the primary tumor tissue, they were sent to Suzhou Jianda Biotechnology Company for short tandem repeat (STR) analysis to determine the correlation between the cells and primary tumor tissue.
DPC-X1 cells in the logarithmic growth phase (P40) were treated with 0.25 μg/mL colchicine for 6 h overnight at 37 ℃. The metaphase cells were collected and fixed with methanol glacial acetic acid (3:1). After trypsin digestion, the specimens were stained using Giemsa dye and observed under a microscope. The mitotic phase cells with good dispersion and moderate staining were selected for karyotype analysis.
DPC-X1 cells in the logarithmic growth phase (P35) were digested, centrifuged, washed twice with PBS, resuspended in a complete culture medium (RPMI-1640 + 10% FBS + 1% penicillin–streptomycin, BI), and inoculated on an ultra-low adsorption cell culture plate (Corning). A thousand cells were added to each well, and 2 mL of culture medium was added for 14 d. The state and number of organ-like cultures were observed under a light microscope.
DPC-X1 cells in the logarithmic growth phase (P45) were digested, centrifuged, fixed with an electron microscope fixative, and stored and transported at 4 ℃. Embedding, ultrathin sectioning, and dyeing were performed at Wuhan Servicebio Co., Ltd. Finally, the images were observed under the transmission electron microscope.
The logarithmically grown DPC-X1 cells (P50) were digested using trypsin to prepare a single-cell suspension. We inoculated 10000 cells/100 μL per well into 96-well plates, and 6 wells in each group were repeated. After the cells adhered to the wall, different concentrations of anti-tumor drugs were added. After 72 h of drug action, 100% X containing 10% (v/v) CCK-8 μL serum-free medium was used to replace the complete medium. The optical density value was measured after 2 h at 450 nm.
The cell density of cells in the logarithmic growth phase (P45) was adjusted to 1 × 107/mL after trypsin digestion. Thereafter, three BALB/c nude mice each were inoculated with 0.1 mL of this sample. We observed and recorded the tumor growth of the nude mice every alternate day. The mice carrying the tumor were killed after four weeks, and the tumor tissue was excised and fixed with 10% formalin. The surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering. Thereafter, routine histopathological and immunohistochemical examinations were performed.
Cells of the 40th generation were digested and inoculated onto sterile slides. After 48 h, the slides were washed with PBS, fixed with 4% paraformaldehyde for 15 min, dried, and treated with 0.5% Triton X-100 for 20 min.
The paraffin sections of primary tumors, transplanted tumors, and organ-like tissues were prepared and incubated at 60 ℃ overnight. The dewaxing, gradient alcohol hydration, and antigen repair processes were completed using Dako's Autostainer Link 48 instrument. Subsequently, 3% hydrogen peroxide solution was incubated at 37 ℃ for 15 min to block the activity of peroxidase; 100 μL of normal goat serum was dripped and then kept sealed at 37 ℃ for 15 min. The first antibody was incubated at 37 ℃ for 1 h with Fuzhou Maxin ready-to-use antibodies [cytokeratin 7 (CK7), cytokeratin 20 (CK20), cytokeratin low molecular weight (CKL), Ki67, and carcinoembryonic antigen (CEA)]. The DAB dye kit (Dako) was used for color development, followed by rinsing with running water for 5 min. Dehydration and xylene transparency were carried out after hematoxylin counterstaining, and the samples were observed under the microscope after neutral resin sealing.
All statistical analyses were performed using the SPSS 22.0 software. The data are presented as mean ± SD. Student’s t-tests and ANOVA were used for group comparisons. Statistical significance was set at P < 0.05.
Through primary culture and subculture of primary tumor tissue of ampullary carcinoma, an ampullary carcinoma cell line, named DPC-X1, was successfully established. Using light microscopy, we found that DPC-X1 cells adhered to the wall and grew like typical epithelial cells. The cells were mainly short spindle-shaped, with large nuclei and visible nucleoli. The polykaryocytes and megakaryocytes were visible. Loss of contact inhibition between cells likely led to accumulation growth as the cells were closely adherent and grew in clumps and sheets (Figure 1F and Video). The cell morphology and growth mode remained unaltered even though it passed to the 80th generation (Figure 1G).
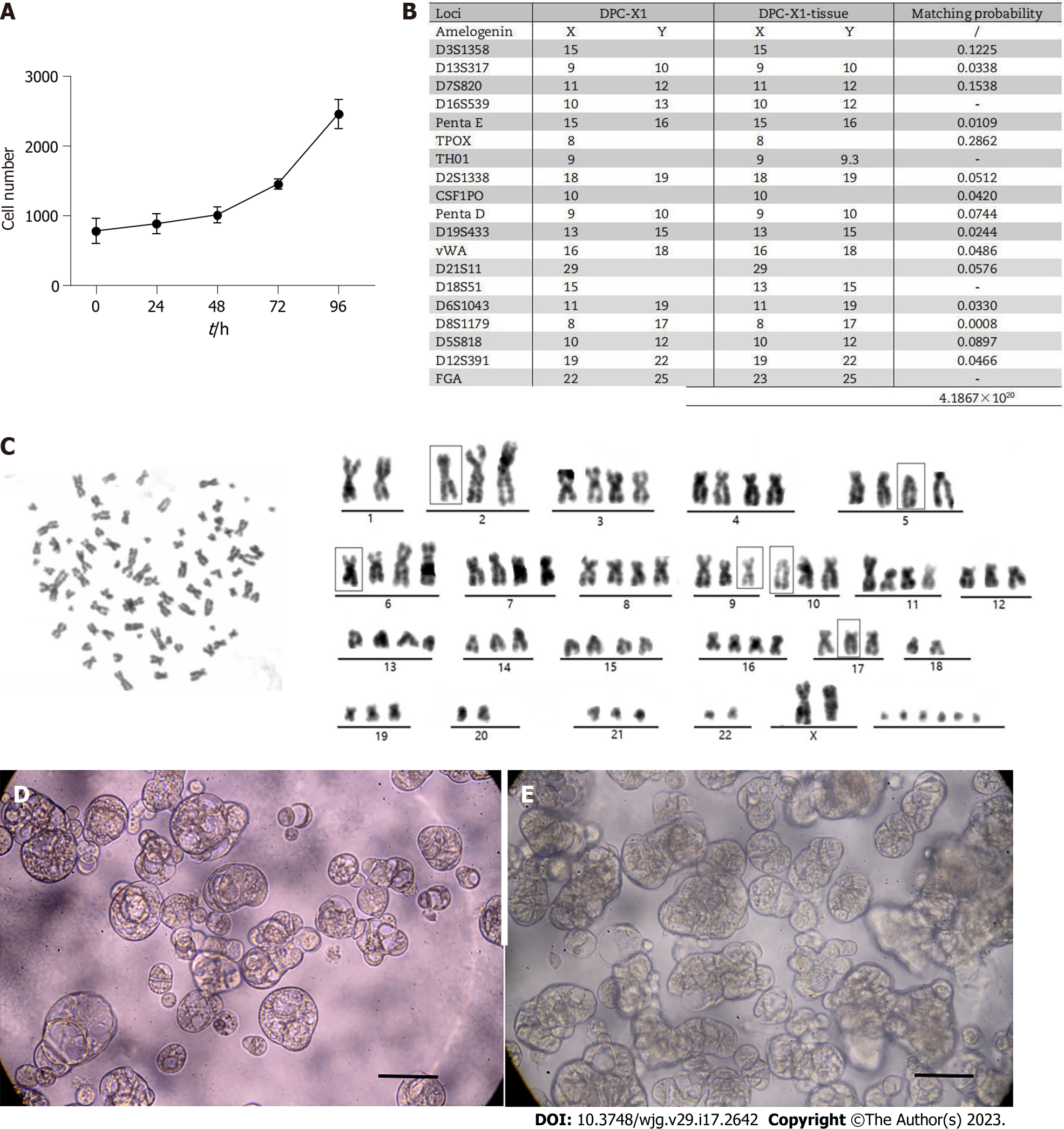
DPC-X1 cells proliferated vigorously and grew stably in RPMI-1640 medium with 10% FBS. Through the CCK-8 method, the doubling time of the DPC-X1 cell population was found to be 48 h using the CCK-8 method. The cell growth curve was plotted using culture time as the abscissa and absorbance values as the ordinate (Figure 2A).
The DNA typing results demonstrated that the two submitted samples were from the same individual with a likelihood ratio = 4.1867 × 1020 (Figure 2B and Supplementary material). The results indicate that DPC-X1 is a new human ampullary carcinoma cell line with the same origin as that of the primary tumor tissue and is not contaminated by the existing cell line.
Karyotype analysis showed that DPC-X1 cells were mainly sub-tetraploid, with large differences in chromosome number and morphology. The representative karyotype was 80, XX del (2) (q32) del (5) (p12) del (6) (q24) inv (9) del (10) p (13) del (17) p (12) (Figure 2C).
After inoculating the DPC-X1 cells on the ultra-low attachment culture plate, the cells were observed to proliferate well and gradually form spherical or cystic organs (Figure 2D). With time, the number of organoid organs gradually increased and appeared spore-like or branch-like (Figure 2E).
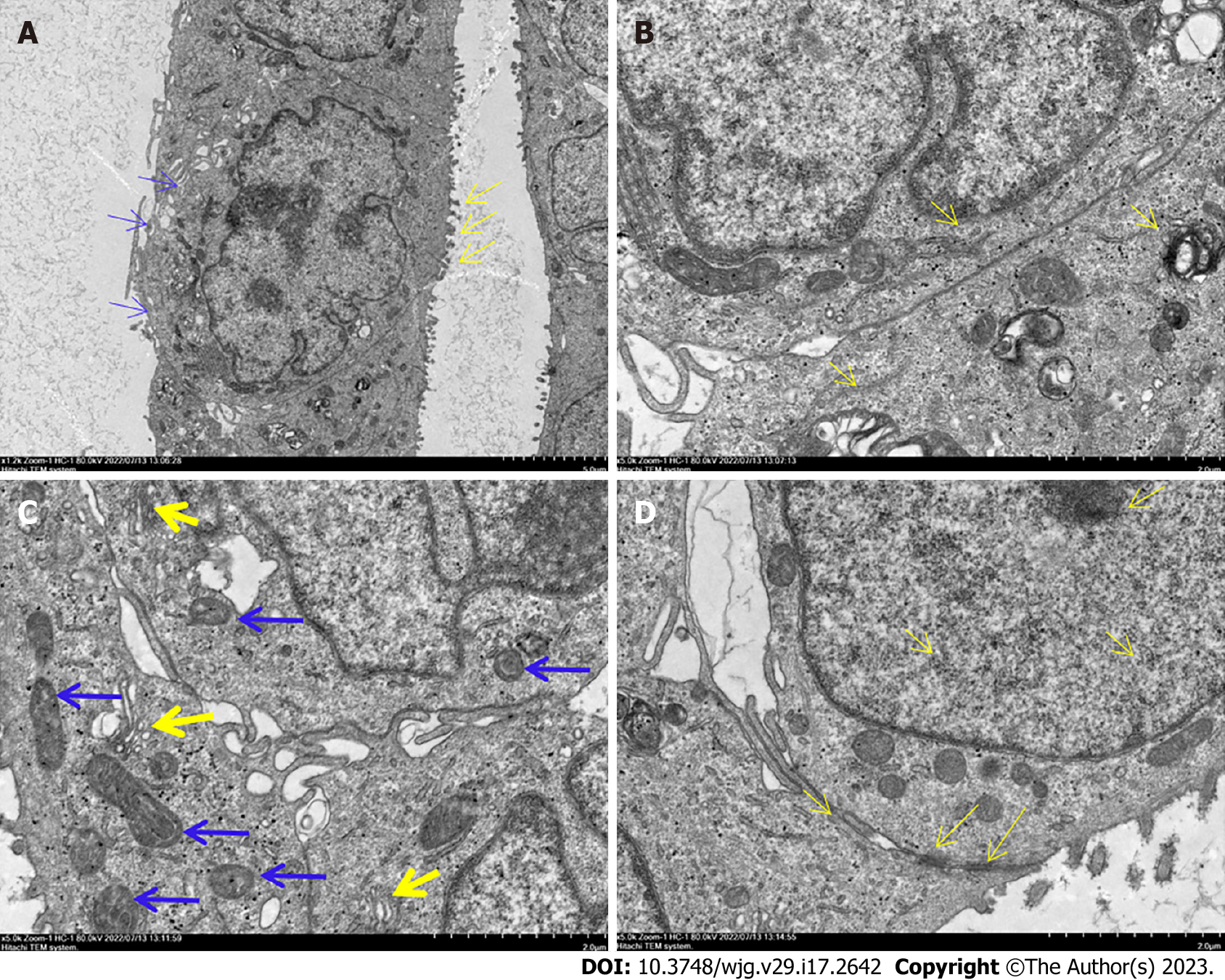
Under the transmission electron microscope, the DPC-X1 nucleus appeared large and deformed, and its number was increased. The nucleolus was clustered in the nuclear membrane, and the cytoplasm was less. Microvilli and pseudopodia were visible on the cell surface (Figure 3A). The endoplasmic reticulum and ribosome were abundantly present in the cells (Figure 3B). Furthermore, the Golgi apparatus was developed, and the mitochondria differed in size and shape (Figure 3C). The desmosome structure was observed between the cells (Figure 3D).
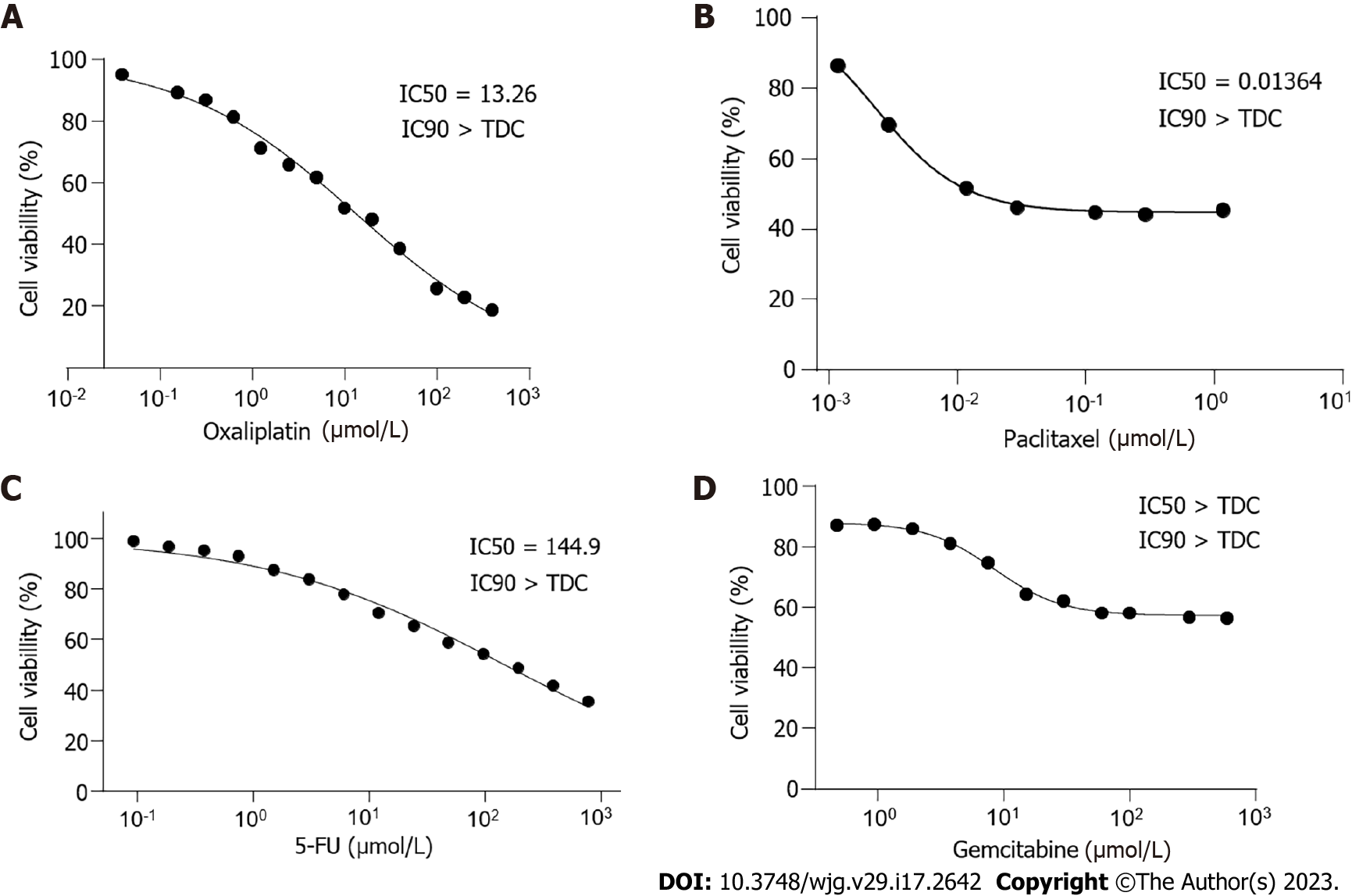
The chemotherapy drugs gemcitabine and paclitaxel are commonly used for biliary and pancreatic tumors, whereas 5-FU and oxaliplatin are commonly used for gastrointestinal tumors. Our drug sensitivity test revealed that DPC-X1 was sensitive to oxaliplatin [half maximal inhibitory concentration (IC50) = 13.26 μmol/L; Figure 4A] and paclitaxel (IC50 = 0.014 μmol/L; Figure 4B) and resistant to fluorouracil (IC50 = 144.9 μmol/L; Figure 4C) and gemcitabine (IC50 > 600 μmol/L; Figure 4D).
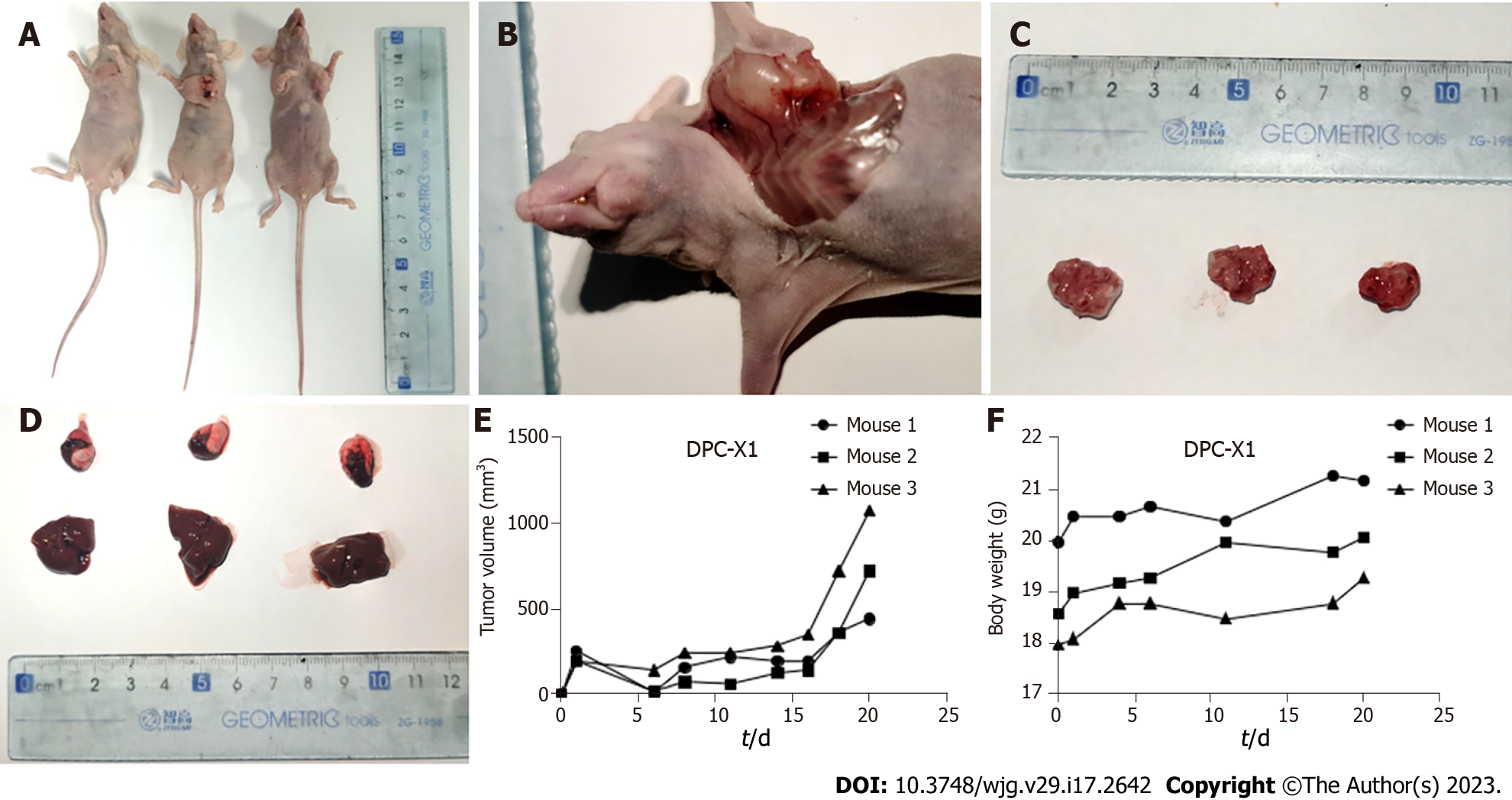
To verify the ability of DPC-X1 to form xenograft tumors in vivo, 1 × 106 DPC-X1 cells were inoculated subcutaneously in three BALB/c nude mice. The results showed that DPC-X1 was able to rapidly form xenograft tumors under the skin of nude mice, and the tumor formation rate was 100% (Figure 5A). The xenograft tumor grew rapidly and showed invasive growth. New blood vessels were visible on the surface of the xenograft tumor (Figure 5B). At four weeks, the diameter of the tumor body exceeded 1 cm (Figure 5C). No metastatic lesions were found in the liver and lungs of the dissected mice (Figure 5D). Tumor growth curve and mouse weight curve are shown in the figure (Figure 5E and F).
Hematoxylin-eosin staining showed that DPC-X1 cells were of different shapes, primarily short fusiform, with enlarged nuclei, apparent nucleoli, and less cytoplasm. The polykaryocytes and megakaryocytes were visible, exhibiting typical malignant tumor characteristics (Figure 6A).
The DPC-X1 organoids were observed to form irregular gland-like structures; the cells were closely connected, with a gland cavity-like structure inside. The cells in the organoid organs were highly heterotypic and varied in size (Figure 6B), which is highly similar to the histomorphology of the primary tumor tissue.
The xenograft tumor formed an irregular gland-like structure, and its histological morphology was similar to that of the primary tumor. The nucleus of the tumor was large and hyperchromatic, and the megakaryocytes were visible. The mitotic images depicted the vigorous proliferation of the DPC-X1 cells (Figure 6C); this observation is consistent with the characteristics of malignant tumors.
The postoperative pathological diagnosis of the patient’s primary tumor showed moderately to poorly differentiated, and the tumor cells were arranged in irregular glandular tubes, strips, and nests (Figure 6D).
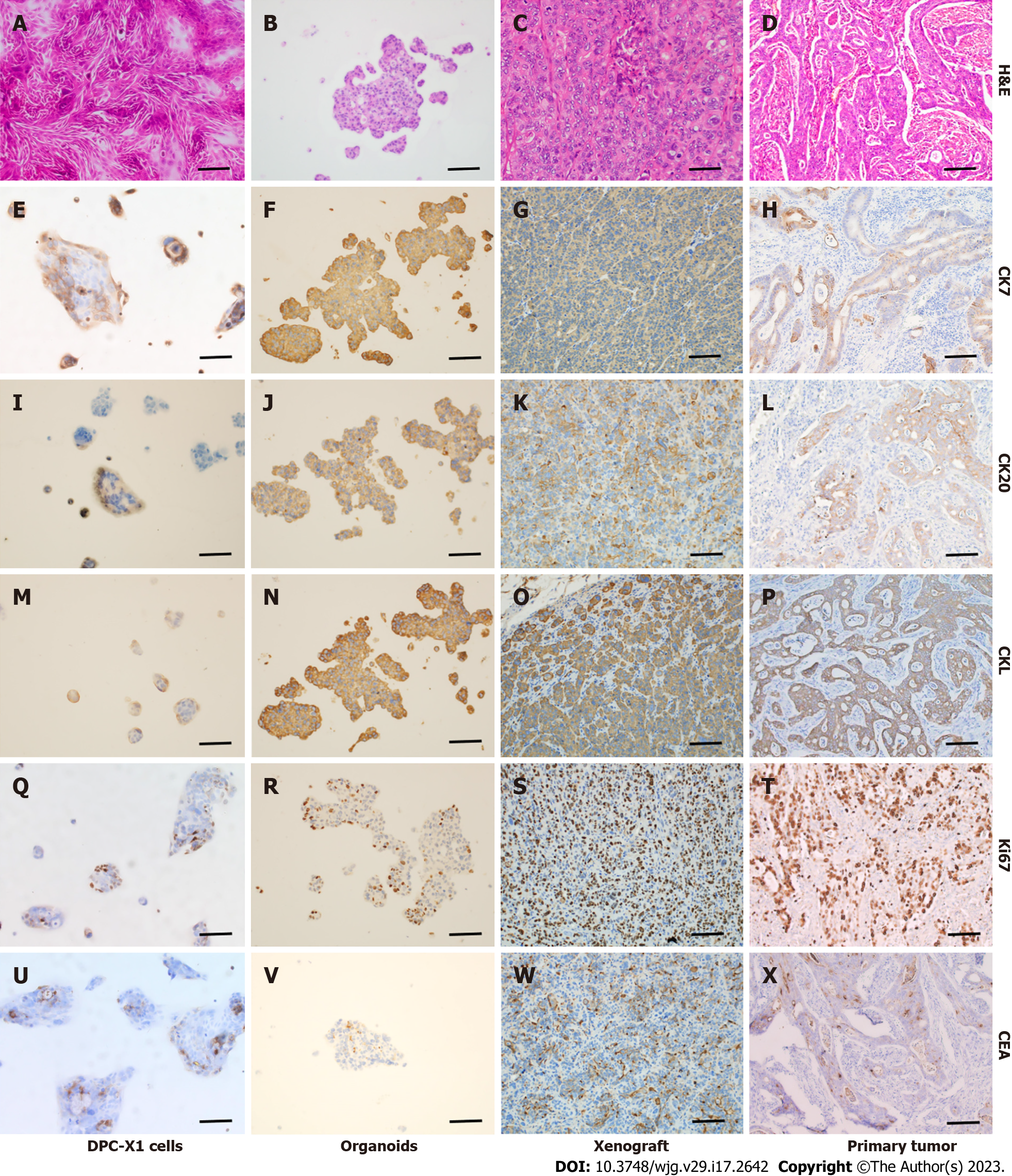
Immunohistochemical examination of DPC-X1 cells, organoids, xenograft tumor, and primary tumor showed that CK7 (Figure 6E–H), CK20 (Figure 6I–L), and CKL (Figure 6M–P) were strongly expressed among the four groups, indicating that they had the same origin. Moreover, DPC-X1 cells were found to exhibit both intestinal and pancreaticobiliary characteristics; thus, they were a mixed ampullary carcinoma cell line. Ki67 (Figure 6Q–T) was highly expressed, consistent with the rapid proliferation of the tumor, and CEA (Figure 6U–X) showed focal expression. These observations are consistent with the characteristics of malignant tumors.
In recent years, with development in endoscopy, imaging equipment, and technological progress, an increasing number of ampullary cancer cases have been diagnosed[24-26]. Owing to the lack of cell lines and related research models of ampullary carcinoma, research on the pathogenesis and biological characteristics of ampullary carcinoma remains limited.
In this study, we successfully established a novel ampullary carcinoma cell line named DPC-X1 from the primary tumor tissue of patients with ampullary carcinoma through primary culture and subculture. This cell line was continuously cultured for more than a year and stably passaged for over 80 generations. DPC-X1 cells adhered to the wall and grew like typical epithelial cells. The cells were chiefly short spindle-shaped, with large nuclei and visible nucleoli, and multinucleated cells and megakaryocytes were visible. The cells were closely adherent and grew in clumps and sheets. The characteristics of DPC-X1 were highly consistent with those of the patient’s primary tumor, and it was not contaminated by other cell lines and microorganisms. DPC-X1 could efficiently form organoids in suspension culture. The Ki67 of DPC-X1 was 50%, indicating that DPC-X1 has a vigorous proliferation, which is consistent with its population doubling time of 48 h.
CK7 is typically used as a tumor marker of biliary and pancreatic origin, whereas CK20 and villin are used as tumor markers of gastrointestinal origin[18,20,24,27,28]. In this study, DPC-X1 immunohistochemistry showed that CK7, CK20, and CKL were strongly positive, indicating that DPC-X1 was a mixed ampullary carcinoma cell line. To the best of our knowledge, this is the first study to report a mixed-type ampullary carcinoma cell line. Thus, DPC-X1 may provide a useful model for studying mixed-type ampullary carcinoma.
Chromosome instability and chromosomal aneuploidy are common in human cancers and are important characteristics of tumor cells[29-32]. Aneuploid karyotype of tumor cells is closely related to poor prognosis of patients[33,34]. Triploid karyotype may be related to endogenous drug resistance of tumors, whereas tetraploidy of tumors is associated with acquired drug resistance[35]. Spontaneous chromosomal missegregation events in aneuploid cells promote chromosomal instability, thereby increasing the risk of tumor recurrence[36]. Karyotype analysis showed that DPC-X1 cells had a sub-tetraploid abnormal karyotype, and the representative karyotype was 80, XX del (2) (q32) del (5) (p12) del (6) (q24) inv (9) del (10) p (13) del (17) p (12). The results of drug sensitivity showed that DPC-X1 was sensitive to oxaliplatin and paclitaxel but was naturally resistant to gemcitabine and fluorouracil. The patient underwent four cycles of chemotherapy with the XELOX regimen and two cycles with the AG regimen after surgery; however, he succumbed to tumor recurrence one year after surgery. Our results are highly consistent with that of earlier studies.
Desmosomes are intercellular junction complexes that anchor the intermediate filaments of adjacent cells and provide them with strong cell adhesion; thus, they are essential in maintaining the organizational structure and structural integrity[37]. Much evidence has substantiated the importance of desmosomes and their components in cancer. Desmosome expression is downregulated in poorly differentiated head and neck transitional and squamous cell carcinoma. In these tissues, desmosomes may have the function of inhibiting invasion and metastasis. In colon cancer, no downregulation has been found[38,39]. The change in desmosome component expression may promote tumor progression by altering the intracellular signal transduction pathway or leading to reduced cell adhesion. The loss of desmosome function is a prerequisite for epithelial-mesenchymal transformation, which is related to the transformation of early tumors to invasive cancer[40-44]. When DPC-X1 cells were subcultured, most of them grew in clumps and sheets, and digesting them into single cells in conventional digestion time was difficult, indicating the strong adhesion between the cells. The presence of intercellular desmosomes under transmission electron microscopy provides some ultrastructural evidence for the aforementioned phenomenon. DPC-X1 cells can be used to further study the relationship between tumors and desmosomes.
Animal models are an intermediate step between cell experiments and human clinical trials. They are a powerful tool for studying canceration and tumor progression and for testing the efficacy and toxicity of therapeutic compounds[45]. Suitable animal models not only help to explore the mechanism of ampullary carcinoma occurrence and development but also provide a good platform for exploring new strategies for early clinical diagnosis and precise treatment[46]. DPC-X1 cells inoculated into BALB/C nude mice could quickly form transplanted tumors, with a tumor formation rate of 100%, and the histology of the xenografts resembled that of the original tumor.
In summary, we report a novel human mixed-type ampullary carcinoma cell line DPC-X1 developed from a Chinese patient’s tumor. This cell line provides a new experimental model for studying the biological and molecular mechanisms of ampullary carcinoma and developing new therapeutic drugs.
To date, only eight ampullary cancer cell lines have been reported, and a mixed-type ampullary carcinoma cell line has not yet been reported.
There is no ampullary cancer cell line of Chinese origin, and there is no report of mixed ampullary cancer cell line.
To establish a stable mixed-type ampullary carcinoma cell line originating from Chinese.
Establish cell lines through primary culture and subculture, and identify their biological characteristics.
In this study, we successfully established and characterized of a mixed-type ampullary carcinoma cell line, DPC-X1, from the primary tumor of a patient with ampullary carcinoma.
DPC-X1 can be used as an effective model for studying the pathogenesis of ampullary carcinoma and drug development.
The establishment of ampullary carcinoma cell lines provides suitable experimental models for further study of ampullary carcinoma.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kode JA, India; Lai SW, Taiwan S-Editor: Fan JR L-Editor: A P-Editor: Yu HG
| 1. | Ahn DH, Bekaii-Saab T. Ampullary cancer: an overview. Am Soc Clin Oncol Educ Book. 2014;112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 2. | Zheng-Pywell R, Reddy S. Ampullary Cancer. Surg Clin North Am. 2019;99:357-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Saha SK, Zhu AX, Fuchs CS, Brooks GA. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist. 2016;21:594-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 561] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 4. | Fischer HP, Zhou H. Pathogenesis of carcinoma of the papilla of Vater. J Hepatobiliary Pancreat Surg. 2004;11:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | de Castro SM, van Heek NT, Kuhlmann KF, Busch OR, Offerhaus GJ, van Gulik TM, Obertop H, Gouma DJ. Surgical management of neoplasms of the ampulla of Vater: local resection or pancreatoduodenectomy and prognostic factors for survival. Surgery. 2004;136:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 83] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Yeh CC, Jeng YM, Ho CM, Hu RH, Chang HP, Tien YW. Survival after pancreaticoduodenectomy for ampullary cancer is not affected by age. World J Surg. 2010;34:2945-2952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Miyakawa S, Ishihara S, Horiguchi A, Takada T, Miyazaki M, Nagakawa T. Biliary tract cancer treatment: 5,584 results from the Biliary Tract Cancer Statistics Registry from 1998 to 2004 in Japan. J Hepatobiliary Pancreat Surg. 2009;16:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 163] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 8. | O'Connell JB, Maggard MA, Manunga J Jr, Tomlinson JS, Reber HA, Ko CY, Hines OJ. Survival after resection of ampullary carcinoma: a national population-based study. Ann Surg Oncol. 2008;15:1820-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Partelli S, Crippa S, Capelli P, Neri A, Bassi C, Zamboni G, Barugola G, Falconi M. Adequacy of lymph node retrieval for ampullary cancer and its association with improved staging and survival. World J Surg. 2013;37:1397-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Kimura W, Futakawa N, Yamagata S, Wada Y, Kuroda A, Muto T, Esaki Y. Different clinicopathologic findings in two histologic types of carcinoma of papilla of Vater. Jpn J Cancer Res. 1994;85:161-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 163] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 11. | Bosman FT, Carnerio F, Hruban RH, Theise ND. WHO classifcation of tumors of the digestive system. 4th ed. Lyon: IARC, 2010. |
| 12. | Sobin LH, Gospodarowicz MK, Ch W. International Union Against Cancer (UICC). TNM classifcation of malignant tumours, 7th edn. Chichester: Wiley-Blackwell, 2010. |
| 13. | Frazier ML, Brown N, Pathak S, Mackay B, Cleary K, Olive M, Byrd DR, Evans DB, Levin B. Human cell line from an adenocarcinoma of the ampulla of Vater. In Vitro Cell Dev Biol. 1992;28A:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Liu SQ, Liu LS, Ohno T. Growth stimulation of tumor-specific cytotoxic T lymphocytes on concanavalin a-immobilized carrier beads. Cytotechnology. 1998;26:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Ghosh M, Koike N, Tsunoda S, Hirano T, Kaul S, Kashiwagi H, Kawamoto T, Ohkohchi N, Saijo K, Ohno T, Miwa M, Todoroki T. Characterization and genetic analysis in the newly established human bile duct cancer cell lines. Int J Oncol. 2005;26:449-456. [PubMed] |
| 16. | Ku JL, Yoon KA, Kim IJ, Kim WH, Jang JY, Suh KS, Kim SW, Park YH, Hwang JH, Yoon YB, Park JG. Establishment and characterisation of six human biliary tract cancer cell lines. Br J Cancer. 2002;87:187-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Sorio C, Moore PS, Ennas MG, Tecchio C, Bonora A, Sartoris S, Balzarini P, Grigolato P, Scarpa A. A novel cell line and xenograft model of ampulla of Vater adenocarcinoma. Virchows Arch. 2004;444:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Lai ZW, Bolm L, Fuellgraf H, Biniossek ML, Makowiec F, Hopt UT, Werner M, Keck T, Bausch D, Sorio C, Scarpa A, Schilling O, Bronsert P, Wellner UF. Characterization of various cell lines from different ampullary cancer subtypes and cancer associated fibroblast-mediated responses. BMC Cancer. 2016;16:195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Ecker BL, Vollmer CM Jr, Behrman SW, Allegrini V, Aversa J, Ball CG, Barrows CE, Berger AC, Cagigas MN, Christein JD, Dixon E, Fisher WE, Freedman-Weiss M, Guzman-Pruneda F, Hollis RH, House MG, Kent TS, Kowalsky SJ, Malleo G, Salem RR, Salvia R, Schmidt CR, Seykora TF, Zheng R, Zureikat AH, Dickson PV. Role of Adjuvant Multimodality Therapy After Curative-Intent Resection of Ampullary Carcinoma. JAMA Surg. 2019;154:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Rizzo A, Dadduzio V, Lombardi L, Ricci AD, Gadaleta-Caldarola G. Ampullary Carcinoma: An Overview of a Rare Entity and Discussion of Current and Future Therapeutic Challenges. Curr Oncol. 2021;28:3393-3402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Xu H, Miao X, Chai C, Tang H, Hu J, Zhao Z, Luo W, Zhu K, Zhou W. Establishment and characterization of a new Chinese hepatocellular carcinoma cell line, Hep-X1. Hum Cell. 2023;36:434-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Peng J, Xu H, Cai J. Establishment and characterization of a new gastric cancer cell line, XGC-1. Cancer Cell Int. 2020;20:437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Xu H, Peng JG, Zhuang YF, Chen JJ, Luo QC, Huang WF, Lin CD, Cai JC. Establishment and characterization of an expanding-type gastric cancer cell line by Ming's classification. Oncol Rep. 2016;36:3030-3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Okano K, Oshima M, Suto H, Ando Y, Asano E, Kamada H, Kobara H, Masaki T, Suzuki Y. Ampullary carcinoma of the duodenum: current clinical issues and genomic overview. Surg Today. 2022;52:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Albores-Saavedra J, Schwartz AM, Batich K, Henson DE. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. J Surg Oncol. 2009;100:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 26. | Talamini MA, Moesinger RC, Pitt HA, Sohn TA, Hruban RH, Lillemoe KD, Yeo CJ, Cameron JL. Adenocarcinoma of the ampulla of Vater. A 28-year experience. Ann Surg. 1997;225:590-9; discussion 599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 197] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Perysinakis I, Minaidou E, Leontara V, Mantas D, Sotiropoulos GC, Tsipras H, Zografos GN, Margaris I, Kouraklis G. Differential Expression of β-Catenin, EGFR, CK7, CK20, MUC1, MUC2, and CDX2 in Intestinal and Pancreatobiliary-Type Ampullary Carcinomas. Int J Surg Pathol. 2017;25:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Luchini C, Veronese N, Nottegar A, Riva G, Pilati C, Mafficini A, Stubbs B, Simbolo M, Mombello A, Corbo V, Cheng L, Yachida S, Wood LD, Lawlor RT, Salvia R, Scarpa A. Perineural Invasion is a Strong Prognostic Moderator in Ampulla of Vater Carcinoma: A Meta-analysis. Pancreas. 2019;48:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, Mc Henry KT, Pinchback RM, Ligon AH, Cho YJ, Haery L, Greulich H, Reich M, Winckler W, Lawrence MS, Weir BA, Tanaka KE, Chiang DY, Bass AJ, Loo A, Hoffman C, Prensner J, Liefeld T, Gao Q, Yecies D, Signoretti S, Maher E, Kaye FJ, Sasaki H, Tepper JE, Fletcher JA, Tabernero J, Baselga J, Tsao MS, Demichelis F, Rubin MA, Janne PA, Daly MJ, Nucera C, Levine RL, Ebert BL, Gabriel S, Rustgi AK, Antonescu CR, Ladanyi M, Letai A, Garraway LA, Loda M, Beer DG, True LD, Okamoto A, Pomeroy SL, Singer S, Golub TR, Lander ES, Getz G, Sellers WR, Meyerson M. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899-905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3271] [Cited by in RCA: 3057] [Article Influence: 203.8] [Reference Citation Analysis (0)] |
| 30. | Gemble S, Wardenaar R, Keuper K, Srivastava N, Nano M, Macé AS, Tijhuis AE, Bernhard SV, Spierings DCJ, Simon A, Goundiam O, Hochegger H, Piel M, Foijer F, Storchová Z, Basto R. Genetic instability from a single S phase after whole-genome duplication. Nature. 2022;604:146-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 31. | López S, Lim EL, Horswell S, Haase K, Huebner A, Dietzen M, Mourikis TP, Watkins TBK, Rowan A, Dewhurst SM, Birkbak NJ, Wilson GA, Van Loo P, Jamal-Hanjani M; TRACERx Consortium, Swanton C, McGranahan N. Interplay between whole-genome doubling and the accumulation of deleterious alterations in cancer evolution. Nat Genet. 2020;52:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 168] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 32. | Bielski CM, Zehir A, Penson AV, Donoghue MTA, Chatila W, Armenia J, Chang MT, Schram AM, Jonsson P, Bandlamudi C, Razavi P, Iyer G, Robson ME, Stadler ZK, Schultz N, Baselga J, Solit DB, Hyman DM, Berger MF, Taylor BS. Genome doubling shapes the evolution and prognosis of advanced cancers. Nat Genet. 2018;50:1189-1195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 460] [Cited by in RCA: 428] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 33. | Chen Y, Yang Z, Wang Y, Wang J, Wang C. Karyotyping of circulating tumor cells for predicting chemotherapeutic sensitivity and efficacy in patients with esophageal cancer. BMC Cancer. 2019;19:651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Wang Y, Liu Y, Zhang L, Tong L, Gao Y, Hu F, Lin PP, Li B, Zhang T. Vimentin expression in circulating tumor cells (CTCs) associated with liver metastases predicts poor progression-free survival in patients with advanced lung cancer. J Cancer Res Clin Oncol. 2019;145:2911-2920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Huang M, Ma Y, Lv C, Li S, Lu F, Zhang S, Wang DD, Lin PP, Yang Y. Aneuploid Circulating Tumor Cells as a Predictor of Response to Neoadjuvant Chemotherapy in Non-Small Cell Lung Cancer. Int J Gen Med. 2021;14:6609-6620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Lee AJ, Endesfelder D, Rowan AJ, Walther A, Birkbak NJ, Futreal PA, Downward J, Szallasi Z, Tomlinson IP, Howell M, Kschischo M, Swanton C. Chromosomal instability confers intrinsic multidrug resistance. Cancer Res. 2011;71:1858-1870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 367] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 37. | Brown L, Wan H. Desmoglein 3: a help or a hindrance in cancer progression? Cancers (Basel). 2015;7:266-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Garrod DR. Desmosomes and cancer. Cancer Surv. 1995;24:97-111. [PubMed] |
| 39. | Okegawa T, Li Y, Pong RC, Hsieh JT. Cell adhesion proteins as tumor suppressors. J Urol. 2002;167:1836-1843. [PubMed] |
| 40. | Chidgey M, Dawson C. Desmosomes: a role in cancer? Br J Cancer. 2007;96:1783-1787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 41. | Dusek RL, Attardi LD. Desmosomes: new perpetrators in tumour suppression. Nat Rev Cancer. 2011;11:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 42. | Moh MC, Shen S. The roles of cell adhesion molecules in tumor suppression and cell migration: a new paradox. Cell Adh Migr. 2009;3:334-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4715] [Cited by in RCA: 6227] [Article Influence: 566.1] [Reference Citation Analysis (0)] |
| 44. | Davies M, Robinson M, Smith E, Huntley S, Prime S, Paterson I. Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-beta1 involves MAPK, Smad and AP-1 signalling pathways. J Cell Biochem. 2005;95:918-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 193] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 45. | Massa A, Varamo C, Vita F, Tavolari S, Peraldo-Neia C, Brandi G, Rizzo A, Cavalloni G, Aglietta M. Evolution of the Experimental Models of Cholangiocarcinoma. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 46. | Li M, Zhou X, Wang W, Ji B, Shao Y, Du Q, Yao J, Yang Y. Selecting an Appropriate Experimental Animal Model for Cholangiocarcinoma Research. J Clin Transl Hepatol. 2022;10:700-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |