Published online May 7, 2023. doi: 10.3748/wjg.v29.i17.2616
Peer-review started: November 4, 2022
First decision: February 18, 2023
Revised: February 28, 2023
Accepted: April 10, 2023
Article in press: April 10, 2023
Published online: May 7, 2023
Processing time: 183 Days and 19.2 Hours
Cryptotanshinone (CPT) has wide biological functions, including anti-oxidative, antifibrosis, and anti-inflammatory properties. However, the effect of CPT on hepatic fibrosis is unknown.
To investigate the effects of CPT treatment on hepatic fibrosis and its underlying mechanism of action.
Hepatic stellate cells (HSCs) and normal hepatocytes were treated with different concentrations of CPT and salubrinal. The CCK-8 assay was used to determine cell viability. Flow cytometry was used to measure apoptosis and cell cycle arrest. Reverse transcription polymerase chain reaction (RT-PCR) and Western blot analyses were used to measure mRNA levels and protein expression of endo
We found that CPT treatment significantly reduced fibrogenesis by modulating the synthesis and degradation of the extracellular matrix in vitro. CPT inhibited cell proliferation and induced cell cycle arrest at the G2/M phase in cultured HSCs. Furthermore, we found that CPT promoted apoptosis of activated HSCs by upregulating expression of ERS markers (CHOP and GRP78) and activating ERS pathway molecules (PERK, IRE1α, and ATF4), which were inhibited by salubrinal. Inhibition of ERS by salubrinal partially eliminated the therapeutic effect of CPT in our CCL4-induced hepatic fibrosis mouse model.
CPT can promote apoptosis of HSCs and alleviate hepatic fibrosis through modulating the ERS pathway, which represents a promising strategy for treating hepatic fibrosis.
Core Tip: Hepatic fibrosis is a necessary stage of liver cirrhosis, and there is currently no effective treatment. Cryptotanshinone (CPT), one of the extracts of Chinese herbal medicine Radix Salviae Miltiorrhizae, has a good anti-fibrosis effect. Through this study, we found that CPT can treat hepatic fibrosis by activating endoplasmic reticulum stress and leading to apoptosis of hepatic stellate cells, which provides a new method for the treatment of hepatic fibrosis.
- Citation: Hou XX, Li YW, Song JL, Zhang W, Liu R, Yuan H, Feng TT, Jiang ZY, Li WT, Zhu CL. Cryptotanshinone induces apoptosis of activated hepatic stellate cells via modulating endoplasmic reticulum stress. World J Gastroenterol 2023; 29(17): 2616-2627
- URL: https://www.wjgnet.com/1007-9327/full/v29/i17/2616.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i17.2616
Hepatic fibrosis is a reversible liver injury that occurs in response to viruses, drugs, inflammation, fat deposition, and other causes. The underlying pathological mechanism of hepatic fibrosis involves the deposition of extracellular matrix (ECM), which leads to the replacement of functional liver parenchyma by scar tissue, proliferation and activation of hepatic stellate cells (HSCs), and increased production of collagen and unfolded proteins[1-3]. The endoplasmic reticulum (ER) is an important organelle in eukaryotic cells that is involved in a variety of biological processes, including protein synthesis, processing, modification, folding, and transport, lipid and carbohydrate metabolism, and Ca2+ maintenance, among others[4-6]. In some cases, when the physiological function of the ER is disturbed, ER stress (ERS) will be activated to promote cell survival. However, persistent ERS can lead to apoptosis[7,8], which can either promote the occurrence and development of hepatic fibrosis or lead to the activation of HSCs, which can reverse the transformation of hepatic fibrosis[2,9-11].
Cryptotanshinone (CPT) is a diterpene quinone compound that is isolated from Salvia miltiorrhiza. CPT has been shown to have anti-oxidant, anti-inflammatory, and antibacterial properties[12]. Studies have also reported that CPT antifibrotic effects on cardiac and renal tissues[13-15]. These findings suggest that CPT plays a protective role in fibrotic disease. Therefore, we hypothesized that CPT might also protect against hepatic fibrosis. In this study, we treated LX2 cells with different concentrations of CPT and found that cell viability decreased and apoptosis increased in a dose-dependent manner. We also found that expression of proteins related to ERS increased, and inhibiting ERS decreased the apoptosis rate. Based on our findings, we speculate that CPT promotes apoptosis of HSCs through modulating the ERS pathway.
CPT (purity ≥ 98% purity by HPLC, Cat No. HY-N0174), were purchased from Med Chem Express LLC (Shanghai, China) (Figure 1A). Primary antibodies for α-SMA, Collagen I, eIF2α, p-eIF2α, p-PERK, PERK, IRE1-α, BAX, BCL2, CHOP, and GRP78/BIP and all secondary antibodies were purchased from Proteintech (China). ATF4, and XBP1 were purchased from Sigma (United States). The primers used in quantitative reverse transcription polymerase chain reaction (qRT-PCR) were purchased from Qingke Co. Ltd. (China) (Table 1).
| Primer | Sequence |
| GAPDH-F (human) | AAATCCCATCACCATCTTCCAG |
| GAPDH-R (human) | AGGGGCCATCCACAGTCTTCT |
| Bax-F (human) | TGAGCAGATCATGAAGACAGGG |
| Bax-R (human) | TGAGACACTCGCTCAGCTTC |
| BCL2-F (human) | TCACTTGTGGCCCAGATAGG |
| BCL2-R (human) | GATAACGGAGGCTGGGATGC |
| GRP78-F (human) | CATCACGCCGTCCTATGTCG |
| GRP78-R (human) | CGTCAAAGACCGTGTTCTCG |
| CHOP-F (human) | ACCTGAAAGCAGATGTGCT |
| CHOP-R (human) | GTCCTCATACCAGGCTTCC |
| α-SMA-F (human) | AAAAGACAGCTACGTGGGTGA |
| α-SMA-R (human) | GCCATGTTCTATCGGGTACTTC |
| Col1a1-F (human) | GAGCCAAGACGAAGACATC |
| Col1a1-R (human) | CAGATCACGTCATCGCACAAC |
| GAPDH-F (mouse) | TGTCGTGGAGTCTACTGGTG |
| GAPDH-R (mouse) | ACACCCATCACAAACATGG |
| α-SMA-F (mouse) | TGACGCTGAAGTATCCGATAGA |
| α-SMA-R (mouse) | CGAAGCTCGTTATAGAAAGAGTGG |
| Col1a1-F (mouse) | GATCCTGCCGATGTCGCTAT |
| Col1a1-R (mouse) | TGTAGGCTAGCTGTTCTTGCA |
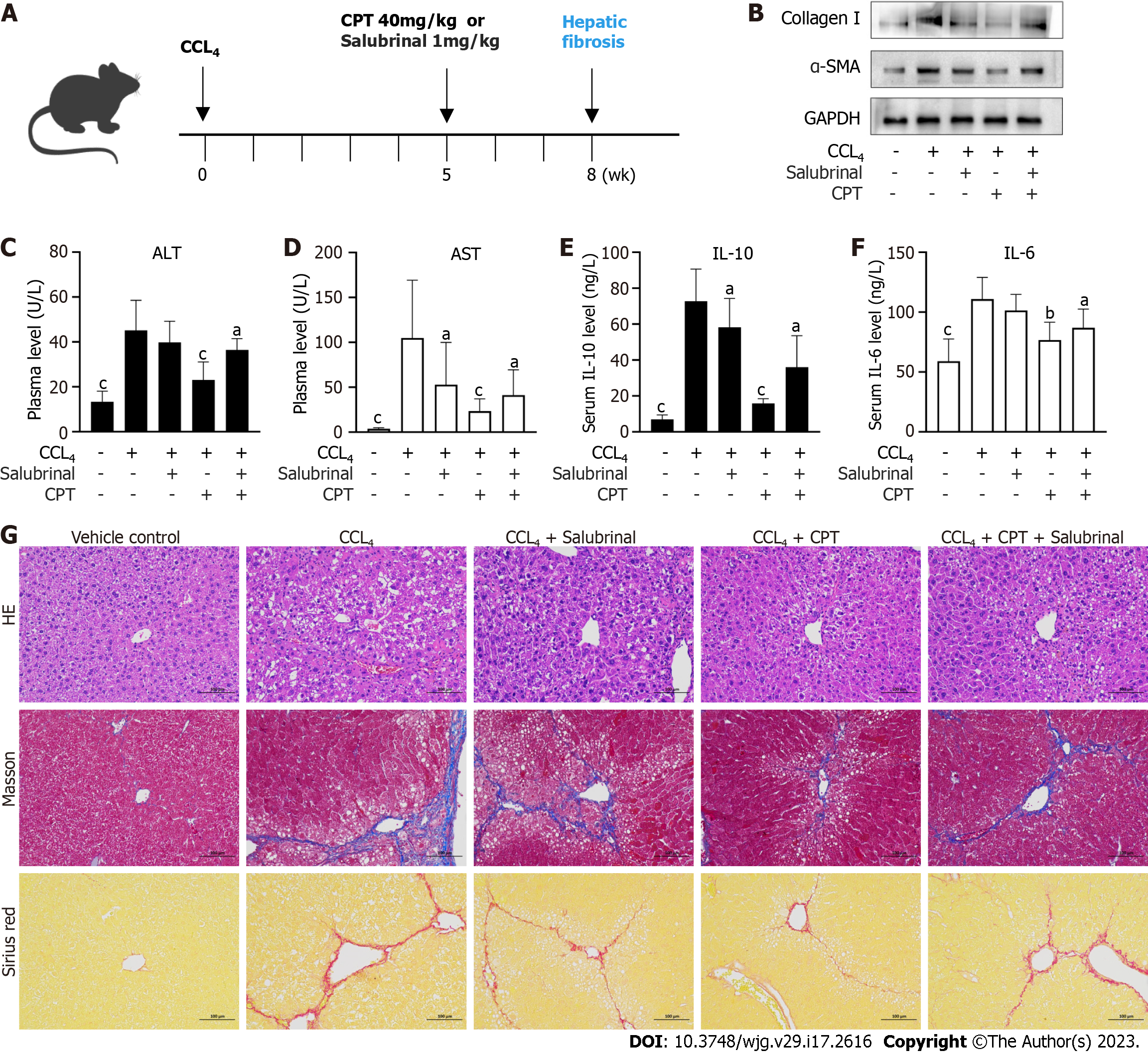
All experiments were approved by institutional and local committees. All mice were provided humane care according to the National Institutes of Health (NIH, United States) guidelines[16]. Male C57BL/6 mice (20-25 g) were randomly divided into five groups (n = 8 per group). Group 1 (vehicle control) did not receive carbon tetrachloride (CCL4) or CPT treatment. Group 2 (model group) received CCL4. Group 3 received intraperitoneal injections of salubrinal (1 mg/kg). Group 4 received CCL4 followed by intraperitoneal injections of CPT (40 mg/kg). Group 5 received simultaneous intraperitoneal injections of salubrinal and CPT. Groups 2, 3, 4, and 5 were administered CCL4 twice a week for 8 wk to induce hepatic fibrosis. Groups 3, 4, and 5 were intraperitoneally injected with CPT or salubrinal every day from 5 wk to 8 wk. CPT and salubrinal were dissolved in physiological saline. After 8 wk of treatment, blood samples and livers were collected. Livers were fixed in 4% buffered paraformaldehyde for histological (hematoxylin and eosin) and Western blot analyses.
The HSC LX2 cell line was donated by Professor Wenting Li. Cells were cultured in high-sugar DMEM medium with 10% fetal bovine serum (FBS) in an incubator at 37 °C[17].
RNA was extracted from the LX2 cells using an RNA extraction kit (Biyuntian, China) according to the kit’s instructions. The Stepone system was used for qRT-PCR, and the experiment was repeated three times to calculate the mRNA levels of the target genes.
RIPA buffer containing protease and phosphatase inhibitors was used to lyse cells and tissues on ice. Proteins were electrophoresed and transferred to PVDF membranes (Millipore, United States), which were blocked with 5% bovine serum albumin for 2 h. Subsequently, the membranes were incubated with primary antibodies at 4 °C overnight, followed by incubation with second antibodies at room temperature for 2 h. Finally, the PVDF membranes were washed using 1% TBST and detected by the Bio-Rad ChemiDoc Touch Imaging System.
Serum levels of interleukin (IL)-6 and IL-10 were determined using enzyme-linked immunosorbent assay (ELISA) kits (LinkTech Biotechnology, China) according to the manufacturer’s instructions. The absorbance was observed at 450 nm using a full-wavelength microplate reader.
LX2 cells were treated with CPT for 24 h, gently scraped with a cell scraper, washed with PBS three times, precipitated, centrifuged, and fixed overnight with an electron microscopy fixative. The fixed cells were imaged using a transmission electron microscope (Tokyo, Japan).
Serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured using a liver function analysis kit (Nanjing Chengjian Institute of Bioengineering, China) according to the kit’s instructions. The absorbance was measured using an enzyme-labeled instrument.
The Cell Counting Kit 8 (CCK-8) kit (Biyuntian, China) was used to assay cell proliferation. LX2 cells were inoculated into a 96-well plate at 3000 cells per well and cultured overnight at 37 °C. After 24 h cells were treated with CPT at different concentrations, and 10 μL of the CCK-8 solution was added to each well. The cells were cultured at 37 °C for 2 h. The absorbance of each well was measured at 450 nm using a microplate reader.
LX2 cells were inoculated into a 24-well plate. After the cells attached, DMSO, CPT, or salubrinal was added for 24 h. A TUNEL staining kit (Biyuntian, China) was used to detect apoptotic cells according to the kit’s instructions. Cell apoptosis was observed with a fluorescent microscope (Nikon).
LX2 cells were inoculated into a 6-well plate at a density of 2 × 104 cells/well. On the following day, the cells were treated with CPT at different concentrations for 24 h. Cell cycle was measured using a cell cycle kit (KeyGen Biotech). Cell apoptosis was determined using Annexin V and propidium iodide double staining with fluorescein isothiocyanate. The percentage of apoptotic cells was determined using flow cytometry (FACS Calibur)[17].
Experimental data were graphed using Prism 9.0 and statistically analyzed using SPSS26.0. Statistical significance of differences was determined using one-way analysis of variance with the post-hoc Dunnett’s test. A P value of <0.05 was considered statistically significant.
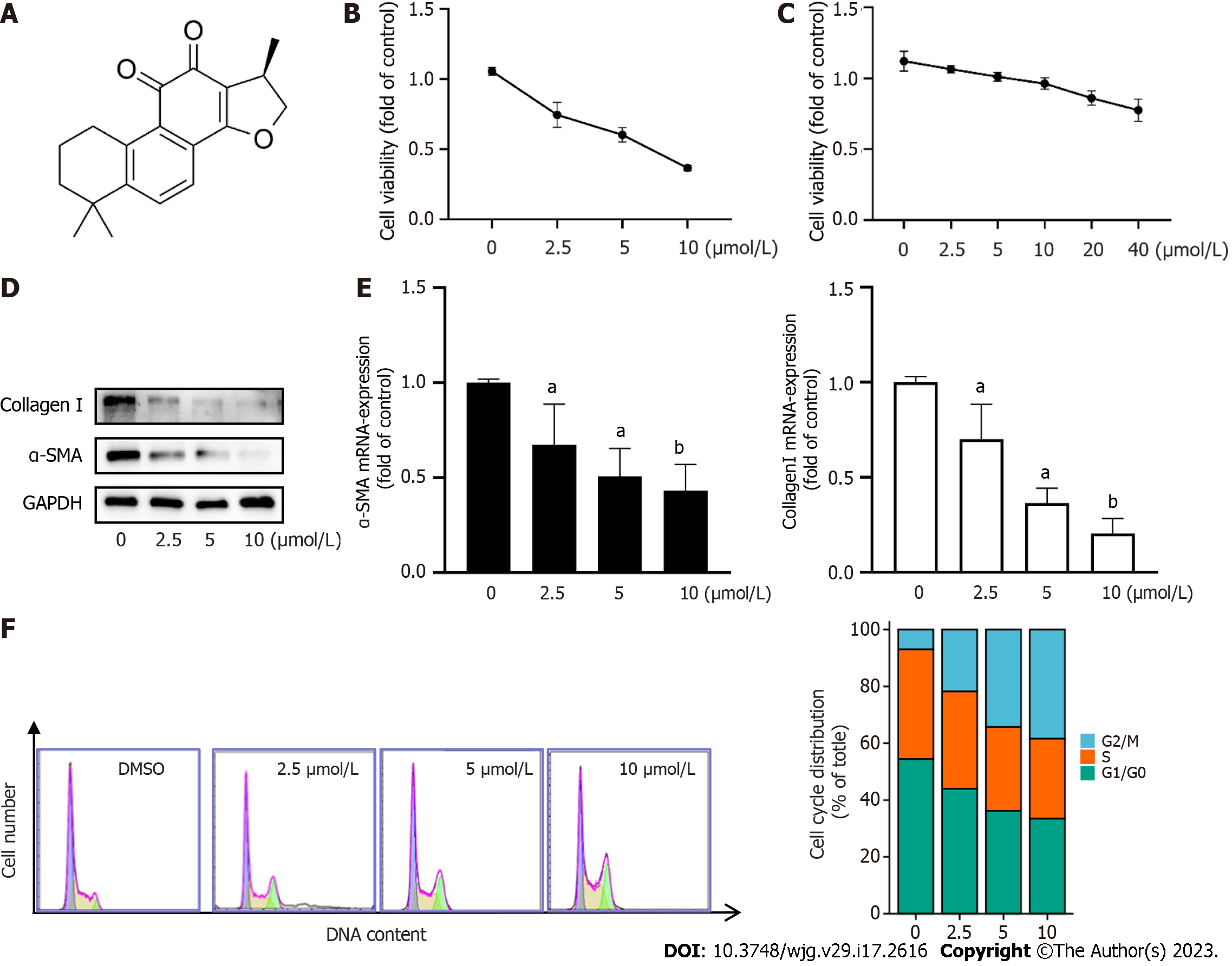
Hepatic fibrosis is characterized by progressive and excess accumulation of ECM, leading to disruption of normal liver structure and function. HSC activation is a key cellular event in the pathogenesis of hepatic fibrosis[18,19]. In this study, we sought to determine the effects of CPT on hepatic fibrosis. To do so we first investigated the effect of CPT on cell viability. We treated LX2 cells with varying concentrations of CPT (0, 2.5, 5, 10, and 20 μmol/L) for 24 h and assessed cell proliferation using the CCK-8 assay. The results showed that CPT significantly reduced cell viability in a dose-dependent manner (Figure 1B). We also observed that CPT inhibited the vitality of normal hepatocytes (L02) at 40 μm (Figure 1C), which was far in excess of the concentration that affected HSC viability. To further observe the inhibitory effect of CPT on activated LX2 cells in vitro, we examined ECM accumulation under different concentrations of CPT. We found that CPT reduced ECM deposition (Figure 1D and E). To further investigate the inhibitory effect of CPT on cell growth, we measured cell cycle distribution using flow cytometry. Cell cycle analysis showed that the number of cells in the G2/M phase significantly increased while those in the G1 phase significantly decreased with higher concentrations of CPT (Figure 1F). Collectively, these findings suggest that CPT can induce HSC growth.
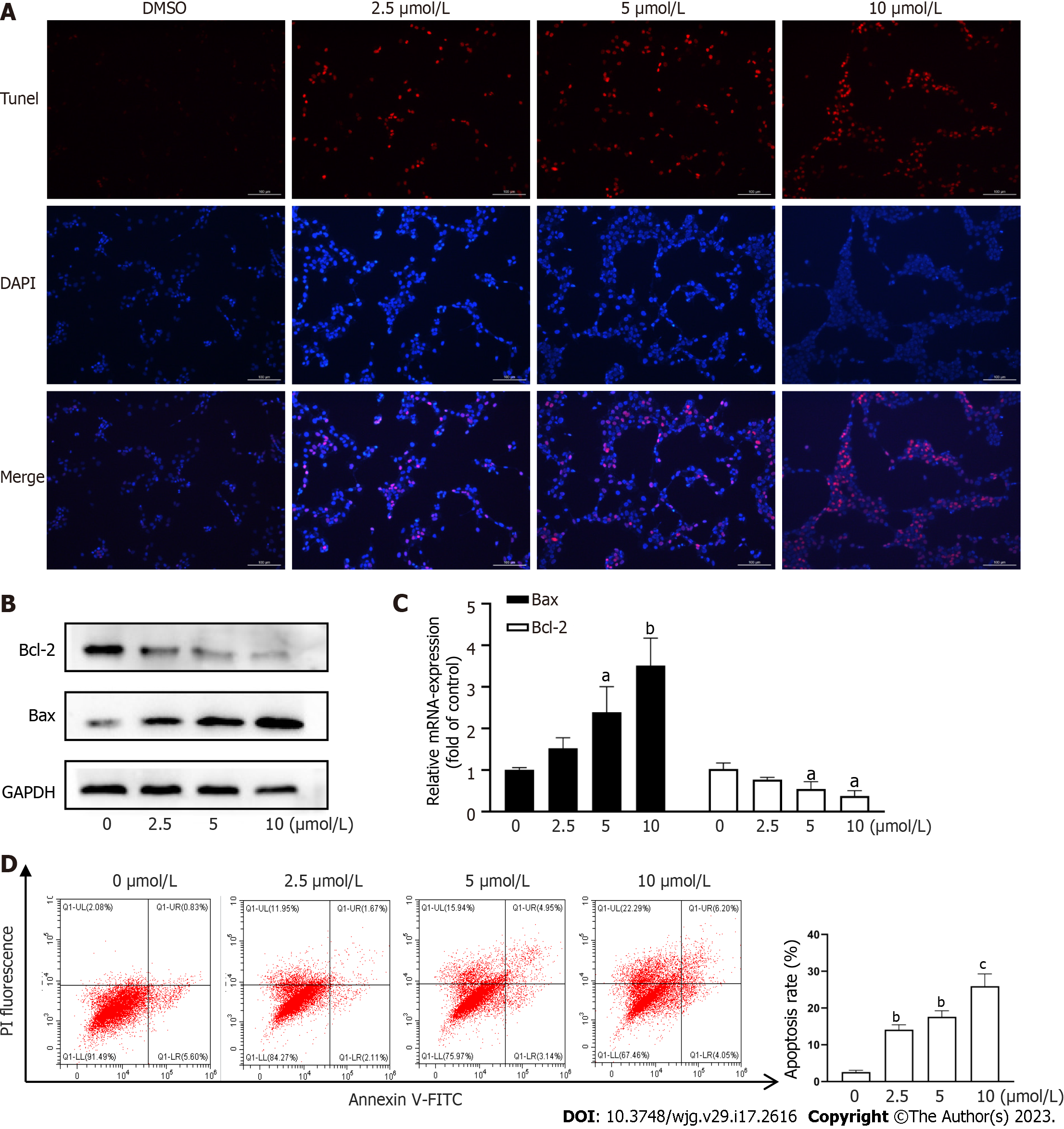
Apoptosis of activated HSCs has been shown to reduce the degree of hepatic fibrosis[2,3]. We assessed HSC apoptosis using TUNEL staining and found that CPT induced activated HSC apoptosis in a dose-dependent manner (Figure 2A). We next explored the pathway underlying CPT-induced HSC apoptosis. We observed that CPT dose-dependently reduced Bcl-2/Bax expression in HSCs (Figure 2B and C). We quantitatively evaluated the apoptotic rate using flow cytometry, which demonstrated that CPT dose-dependently increased the apoptotic rate in activated HSCs (Figure 2D). These data indicate that CPT activates HSC apoptosis.
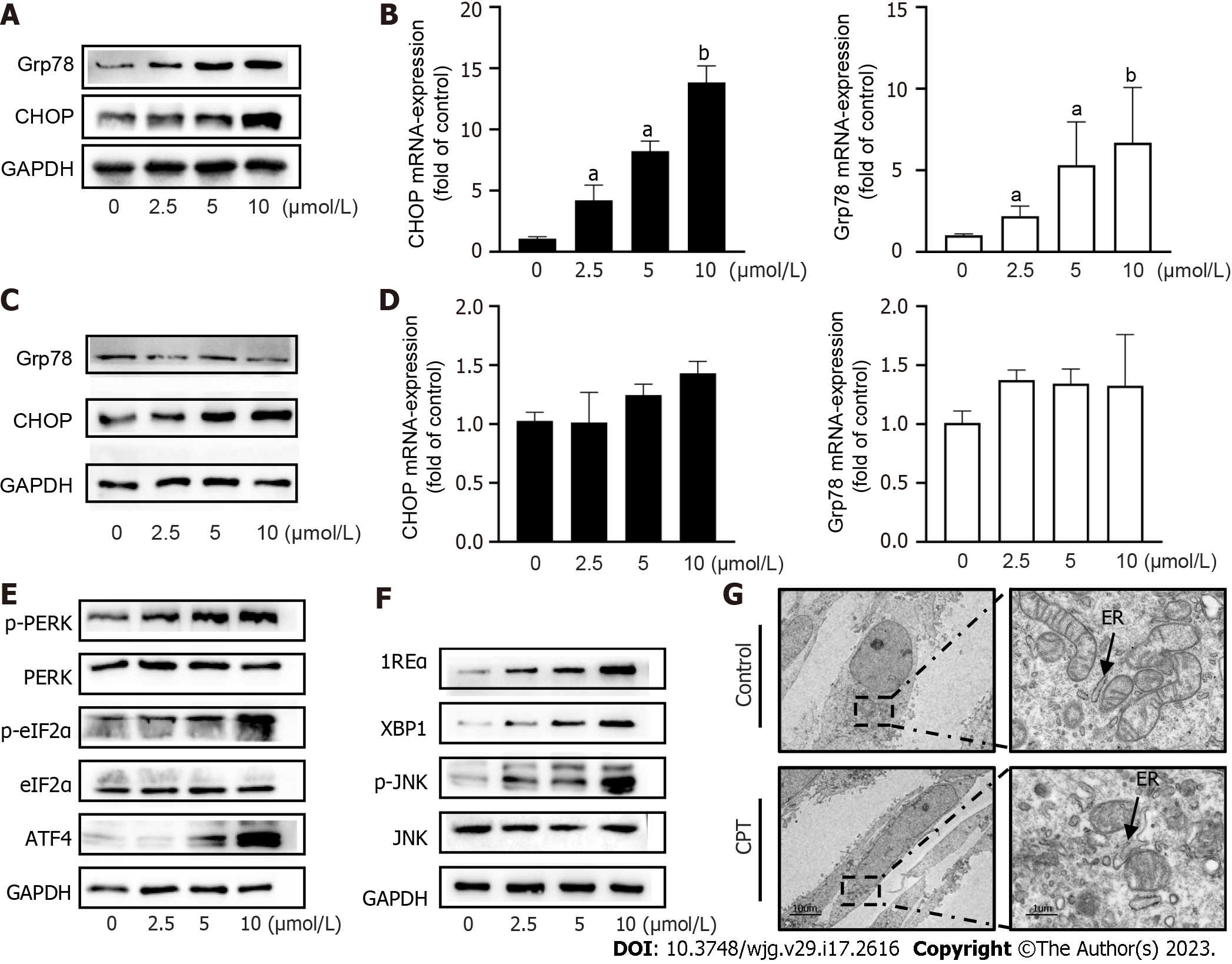
ERS is a signaling response pathway intended to protect against cell death. However, if the stress response is very strong or prolonged, it can cause cellular damage[20]. We investigated the underlying mechanism of CPT-induced HSC apoptosis by detecting ERS-related mediators. We found that CPT significantly upregulated the mRNA levels and protein expression of GFR78 and CHOP in LX2 cells, resepectively (Figure 3A and B). Subsequently, we examined the expression of GFR78 and CHOP in L02 cells and found that their expression was not significantly altered (Figure 3C and D). These results suggest that CPT promotes ERS in activated HSCs without triggering ERS in normal liver cells. Western blot analysis revealed that CPT significantly upregulated protein expression of ERS regulatory molecules (Figure 3E and F). We also observed the expansion and swelling of the ER lumen in CPT-treated HSCs using electron microscopy. More specifically, we observed a disappearance of the normal sheet-like folded structure and the formation of numerous vacuoles within the ER (Figure 3G).
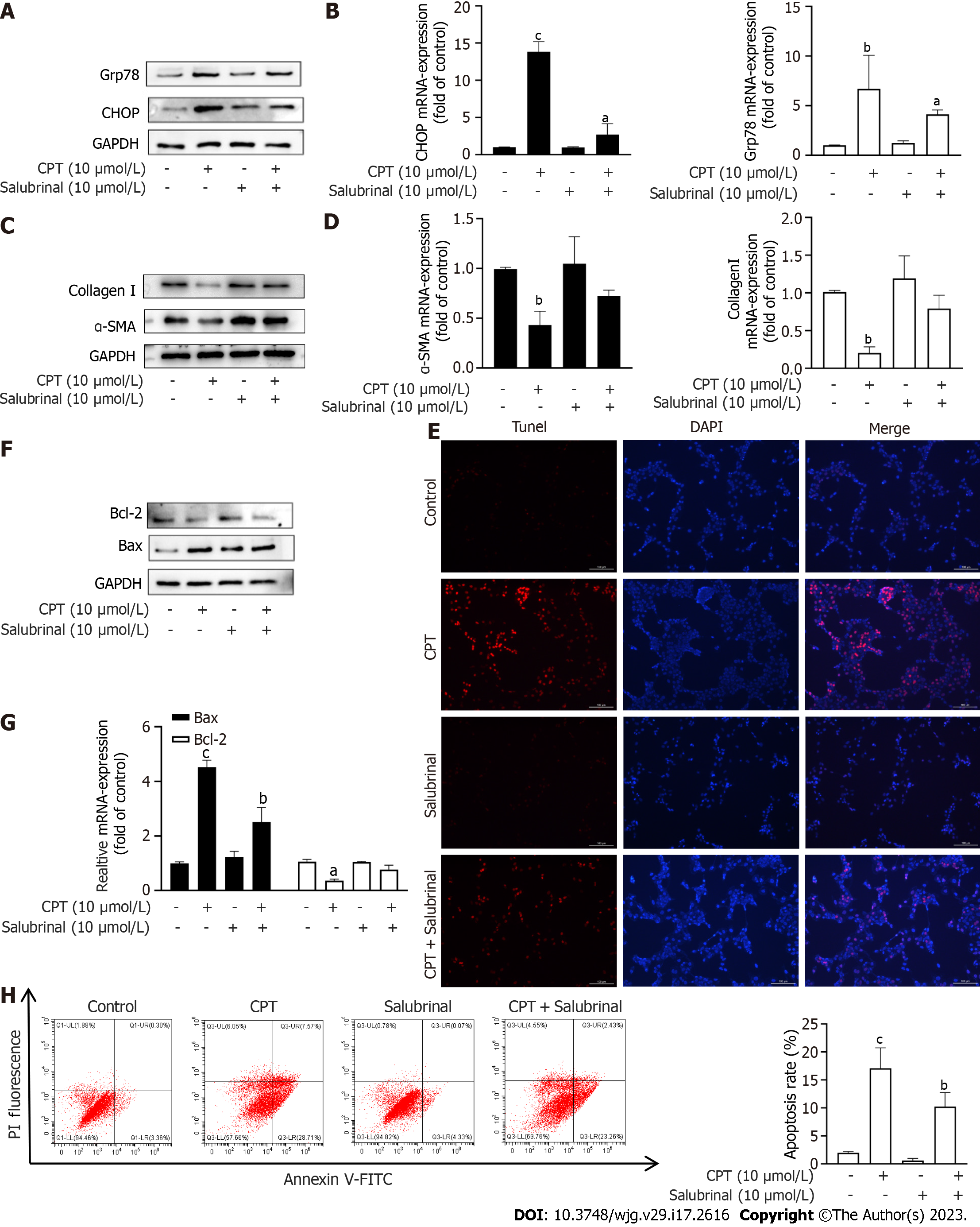
Salubrinal, an inhibitor of ERS, can protect HSCs from ERS-induced apoptosis[20]. Based on the above experimental results, we next investigated if activation of ERS by CPT leads to apoptosis of LX2 cells. To test this hypothesis, we treated LX2 cells with both CPT and salubrinal and found that CPT did not significantly upregulate the mRNA and Western blot levels of GFR78 and CHOP (Figure 4A and B). We also measured the expression of ECM components at the mRNA and protein levels. Interestingly, The decrease of ECM accumulation following CPT treatment was prevented by salubrinal (Figure 4C and D). These results suggest that CPT can activate ERS, leading to apoptosis of LX2 cells, which may be a mechanism to promote HSC clearance. We found that the apoptotic effect of CPT on activated LX2 cells could be reversed by salubrinal (Figure 4E-H). Taken together, these findings suggest that CPT can induce apoptosis of HSCs through modulating ERS, which may be a mechanism for promoting HSCs clearance.
CCl4-induced hepatic fibrosis is a well-established model to investigate hepatic fibrosis in vivo. We used this model to confirm the protective effect of CPT in mice(Figure 5A). To this end, we measured protein expression of major markers of hepatic fibrosis including α-SMA and collagen I. CPT effectively diminished expression of these proteins in the CCL4-induced model of hepatic fibrosis (Figure 5B). In addition, we analyzed biochemical markers of liver injury after 8 wk of CCL4 treatment. Levels of AST and ALT increased in the model group but were reduced in the CPT groups (Figure 5C and D). During chronic liver disease, hepatic fibrogenesis is commonly accompanied by inflammation. As demonstrated by ELISA, CPT decreased serum levels of IL-6 and IL-10 (Figure 5E and F). Furthermore, the inhibitory effect of CPT on the secretion of pro-inflammatory cytokines was diminished by salubrinal. Thus, we propose that CPT protects against CCL4-induced liver injury by suppressing inflammation. Next, we analyzed the pathological changes in the liver tissue after CPT treatment. Histological staining showed that treatment with CPT significantly improved morphological changes in liver tissue (Figure 5G). As fibrogenesis is accompanied by accumulation of collagen, liver tissue sections were stained with Masson’s reagent and Sirius red to detect collagen deposition. The results showed that collagen was markedly deposited in the CCL4-injured livers but reduced in the livers of mice in the CPT-treated groups, which was further suppressed by salubrinal (Figure 5G). Taken together, these data provide in vivo evidence that activation of ERS signaling can result in reduced collagen accumulation and attenuation of fibrotic damage in a hepatic fibrosis mouse model.
Due to their diverse sources and high safety profiles, there has recently been a widespread increase in the use of natural bioactive components from plants for the prevention and treatment of diseases. Salvia miltiorrhiza is a traditional Chinese medicine that has been used to treat many diseases. CPT is one of the main tanshinones extracted from Salvia miltiorrhiza Bunge and is considered an important compound with various pharmacological properties. Specifically, recent studies have shown that CPT has antifibrotic properties in the heart, lung, and kidney[14,15]. However, the relationship between CPT and hepatic fibrosis remains unclear.
Hepatic fibrosis involves inflammatory responses caused by various acute and chronic liver injuries. If left untreated, hepatic fibrosis can progress to liver cirrhosis or even liver cancer. The production of ECM by activated HSCs plays an important role in hepatic fibrosis and cirrhosis[21]. Hepatic fibrosis might be prevented or even reversed by inducing apoptosis of activated HSCs. In this study, we established that CPT induces apoptosis through ERS in activated HSCs, which may offer a new strategy for the treatment of hepatic fibrosis. We discovered that CPT decreased both mRNA levels and protein expression of α-SMA and type I collagen in activated HSCs, which resulted in reduced ECM deposition. Furthermore, flow cytometry revealed that CPT dose-dependently induced apoptosis of activated LX2 cells. Bax is considered an apoptotic factor, while Bcl2 is an anti-apoptotic molecule that inhibits the release of cytochrome C from the mitochondria and inhibits HSC apoptosis[22]. We found that CPT upregulated the expression of the pro-apoptotic protein Bax and downregulated the expression of the anti-apoptotic protein Bcl2 in activated HSCs, increased the Bax/Bcl ratio, and promoted HSC apoptosis. Interestingly, the pro-apoptotic effect of CPT was more specific for activated HSCs than for normal hepatocytes, which did not undergo apoptosis. This finding is important because it indicates that CPT does not affect normal liver cell activity. Thus, we found that CPT is an effective drug that can induce apoptosis of activated HSCs but have no effect on normal hepatocytes during the treatment of hepatic fibrosis.
Our results demonstrated that CPT upregulated expression of ER-resident chaperone proteins, such as CHOP and GRP78, which are significant markers of ERS[23,24]. In addition, the PERK, IRE1, and ATF6 signaling pathways were activated, all of which can phosphorylate the downstream molecules eIF2α, ATF4, and JNK, modulating the ERS response[25,26]. We also observed ultrastructure changes using electron microscopy. The results showed that the ER cavity of the HSCs was swollen, and the integrity of the mitochondrial membrane was damaged after CPT treatment. The ER and mitochondria are important organelles that cooperate to complete a variety of biological functions through their interactions with various proteins. In the early stage of ERS, GRP78 is translocated to the mitochondria, and the subsequent damage to the integrity of the mitochondrial membrane becomes an early feature of apoptosis[27,28]. Our results indicate that CPT could promote ERS in HSCs to promote apoptosis. Salubrinal is an ERS inhibitor that selectively induces eIF2α phosphorylation and inhibits its dephosphorylation, ultimately protecting cells from ERS-induced apoptosis[29]. To better understand if CPT-induced HSC apoptosis is regulated by activating the ERS pathway, we incubated HSCs with salubrinal to protect cells from ERS-induced apoptosis. In activated HSCs, salubrinal effectively attenuated CPT-induced apoptosis, reducing secretion of apoptotic proteins. Therefore, these results confirm that targeting ERS can trigger HSCs apoptosis.
To further verify the therapeutic effect of CPT on hepatic fibrosis, we conducted in vivo experiments using a CCL4-induced mouse model of hepatic fibrosis. We found that intraperitoneal injection of CPT reversed the development of hepatic fibrosis in mice, and the therapeutic effect of CPT was reduced after by salubrinal. Interestingly, CCL4 also induces experimental hepatocarcinoma, which shares common features with hepatic fibrosis, such as increased expression of matricellular proteins (SPARC, BM-40, and osteonectin)[30-32]. However, whether or not CPT also has a significant effect on the treatment of liver cancer requires further investigation.
In summary, we found that CPT limited HSC activation through an ERS-dependent pathway in vivo and in vitro, suggesting that CPT should be further investigated as a prospective therapeutic agent for hepatic fibrosis.
Cryptotanshinone (CPT) has been accepted to be an anti-inflammatory molecule.
Hepatic stellate cell (HSC) activation plays an indispensable role in hepatic fibrosis. Inducing apoptosis of activated HSCs can attenuate or reverse fibrogenesis.
This study investigated the effects of CPT treatment on hepatic fibrosis and its underlying mechanism of action.
In vitro, we used reverse transcription polymerase chain reaction, Western blot, TUNEL staining and flow cytometry, which demonstrated that CPT can induce HSC apoptosis through the ERS pathway. In vivo, we used liver function kit, enzyme-linked immunosorbent assay, pathological section staining a few columns of technical means to prove that CPT has a certain therapeutic effect on hepatic fibrosis.
In vitro, CPT was considered to activate HSC apoptosis by promoting endoplasmic reticulum stress (ERS) detrimental response to a certain extent. Main molecules came down to the unfolded protein response signaling pathway. In vivo, We found CPT protected the Carbon tetrachloride (CCL4)-induced hepatic fibrosis. Furthermore, CPT inhibited the levels of the downstream inflammatory cytokines, which were triggered by CCL4-induced hepatic fibrosis.
CPT can promote apoptosis of HSCs and alleviate hepatic fibrosis through modulating the ERS pathway, which represents a promising strategy for treating hepatic fibrosis.
Hepatic fibrosis, cirrhosis and liver cancer are typical "trilogy of liver diseases", which seriously endanger human health. Hepatic fibrosis is reversible in the early stage, but it is difficult to reverse in the late stage of liver cirrhosis. Early treatment is therefore essential. CPT is a diterpene quinone compound that is isolated from Salvia miltiorrhiza. CPT has been shown to have anti-oxidant. In this study, we found that CPT could induce apoptosis of activated HSCs and had good prospects for the treatment of hepatic fibrosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Maslennikov R, Russia; Perazzo JC, Argentina S-Editor: Gao CC L-Editor: A P-Editor: Zhao S
| 1. | Gan C, Cai Q, Tang C, Gao J. Inflammasomes and Pyroptosis of Liver Cells in Liver Fibrosis. Front Immunol. 2022;13:896473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 2. | Tsuchida T. [Mechanisms of hepatic stellate cell activation as a therapeutic target for the treatment of non-alcoholic steatohepatitis]. Nihon Yakurigaku Zasshi. 2019;154:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Yang F, Li H, Li Y, Hao Y, Wang C, Jia P, Chen X, Ma S, Xiao Z. Crosstalk between hepatic stellate cells and surrounding cells in hepatic fibrosis. Int Immunopharmacol. 2021;99:108051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 4. | Duwaerts CC, Maiers JL. ER Disposal Pathways in Chronic Liver Disease: Protective, Pathogenic, and Potential Therapeutic Targets. Front Mol Biosci. 2021;8:804097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 5. | Maiers JL, Malhi H. Endoplasmic Reticulum Stress in Metabolic Liver Diseases and Hepatic Fibrosis. Semin Liver Dis. 2019;39:235-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 6. | Bravo R, Parra V, Gatica D, Rodriguez AE, Torrealba N, Paredes F, Wang ZV, Zorzano A, Hill JA, Jaimovich E, Quest AF, Lavandero S. Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int Rev Cell Mol Biol. 2013;301:215-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 448] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 7. | Iurlaro R, Muñoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016;283:2640-2652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 794] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 8. | Demirtas L, Guclu A, Erdur FM, Akbas EM, Ozcicek A, Onk D, Turkmen K. Apoptosis, autophagy & endoplasmic reticulum stress in diabetes mellitus. Indian J Med Res. 2016;144:515-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 9. | Zhang CY, Yuan WG, He P, Lei JH, Wang CX. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol. 2016;22:10512-10522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 358] [Cited by in RCA: 450] [Article Influence: 50.0] [Reference Citation Analysis (4)] |
| 10. | Koo JH, Lee HJ, Kim W, Kim SG. Endoplasmic Reticulum Stress in Hepatic Stellate Cells Promotes Liver Fibrosis via PERK-Mediated Degradation of HNRNPA1 and Up-regulation of SMAD2. Gastroenterology. 2016;150:181-193.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 11. | Borkham-Kamphorst E, Steffen BT, Van de Leur E, Haas U, Tihaa L, Friedman SL, Weiskirchen R. CCN1/CYR61 overexpression in hepatic stellate cells induces ER stress-related apoptosis. Cell Signal. 2016;28:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Li H, Gao C, Liu C, Liu L, Zhuang J, Yang J, Zhou C, Feng F, Sun C, Wu J. A review of the biological activity and pharmacology of cryptotanshinone, an important active constituent in Danshen. Biomed Pharmacother. 2021;137:111332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 13. | Wang X, Wan W, Lu J, Zhang Y, Quan G, Pan X, Wu Z, Liu P. Inhalable cryptotanshinone spray-dried swellable microparticles for pulmonary fibrosis therapy by regulating TGF-β1/Smad3, STAT3 and SIRT3 pathways. Eur J Pharm Biopharm. 2022;172:177-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Wang W, Zhou PH, Hu W, Xu CG, Zhou XJ, Liang CZ, Zhang J. Cryptotanshinone hinders renal fibrosis and epithelial transdifferentiation in obstructive nephropathy by inhibiting TGF-β1/Smad3/integrin β1 signal. Oncotarget. 2018;9:26625-26637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Lo SH, Hsu CT, Niu HS, Niu CS, Cheng JT, Chen ZC. Cryptotanshinone Inhibits STAT3 Signaling to Alleviate Cardiac Fibrosis in Type 1-like Diabetic Rats. Phytother Res. 2017;31:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Xu W, Lu C, Zhang F, Shao J, Yao S, Zheng S. Dihydroartemisinin counteracts fibrotic portal hypertension via farnesoid X receptor-dependent inhibition of hepatic stellate cell contraction. FEBS J. 2017;284:114-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Bian M, Chen X, Zhang C, Jin H, Wang F, Shao J, Chen A, Zhang F, Zheng S. Magnesium isoglycyrrhizinate promotes the activated hepatic stellate cells apoptosis via endoplasmic reticulum stress and ameliorates fibrogenesis in vitro and in vivo. Biofactors. 2017;43:836-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Garbuzenko DV. Pathophysiological mechanisms of hepatic stellate cells activation in liver fibrosis. World J Clin Cases. 2022;10:3662-3676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (3)] |
| 19. | Kisseleva T, Brenner DA. Hepatic stellate cells and the reversal of fibrosis. J Gastroenterol Hepatol. 2006;21 Suppl 3:S84-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 20. | Li X, Wang Y, Wang H, Huang C, Huang Y, Li J. Endoplasmic reticulum stress is the crossroads of autophagy, inflammation, and apoptosis signaling pathways and participates in liver fibrosis. Inflamm Res. 2015;64:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 21. | Li X, Sun S, Appathurai S, Sundaram A, Plumb R, Mariappan M. A Molecular Mechanism for Turning Off IRE1α Signaling during Endoplasmic Reticulum Stress. Cell Rep. 2020;33:108563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Wolf P, Schoeniger A, Edlich F. Pro-apoptotic complexes of BAX and BAK on the outer mitochondrial membrane. Biochim Biophys Acta Mol Cell Res. 2022;1869:119317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 23. | Bian M, He J, Jin H, Lian N, Shao J, Guo Q, Wang S, Zhang F, Zheng S. Oroxylin A induces apoptosis of activated hepatic stellate cells through endoplasmic reticulum stress. Apoptosis. 2019;24:905-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Dubois V, Gheeraert C, Vankrunkelsven W, Dubois-Chevalier J, Dehondt H, Bobowski-Gerard M, Vinod M, Zummo FP, Güiza F, Ploton M, Dorchies E, Pineau L, Boulinguiez A, Vallez E, Woitrain E, Baugé E, Lalloyer F, Duhem C, Rabhi N, van Kesteren RE, Chiang CM, Lancel S, Duez H, Annicotte JS, Paumelle R, Vanhorebeek I, Van den Berghe G, Staels B, Lefebvre P, Eeckhoute J. Endoplasmic reticulum stress actively suppresses hepatic molecular identity in damaged liver. Mol Syst Biol. 2020;16:e9156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Mori K. Evolutionary Aspects of the Unfolded Protein Response. Cold Spring Harb Perspect Biol. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Kishino A, Hayashi K, Maeda M, Jike T, Hidai C, Nomura Y, Oshima T. Caspase-8 Regulates Endoplasmic Reticulum Stress-Induced Necroptosis Independent of the Apoptosis Pathway in Auditory Cells. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 27. | Liu Y, Pan X, Li S, Yu Y, Chen J, Yin J, Li G. Endoplasmic reticulum stress restrains hepatocyte growth factor expression in hepatic stellate cells and rat acute liver failure model. Chem Biol Interact. 2017;277:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Senft D, Ronai ZA. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem Sci. 2015;40:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 838] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 29. | Lan B, He Y, Sun H, Zheng X, Gao Y, Li N. The roles of mitochondria-associated membranes in mitochondrial quality control under endoplasmic reticulum stress. Life Sci. 2019;231:116587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Dhar D, Baglieri J, Kisseleva T, Brenner DA. Mechanisms of liver fibrosis and its role in liver cancer. Exp Biol Med (Maywood). 2020;245:96-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 31. | Zadorozhnii PV, Pokotylo IO, Kiselev VV, Okhtina OV, Kharchenko AV. Molecular docking studies of salubrinal and its analogs as inhibitors of the GADD34:PP1 enzyme. ADMET DMPK. 2019;7:140-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Madden E, Logue SE, Healy SJ, Manie S, Samali A. The role of the unfolded protein response in cancer progression: From oncogenesis to chemoresistance. Biol Cell. 2019;111:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 248] [Article Influence: 35.4] [Reference Citation Analysis (0)] |