Published online May 7, 2023. doi: 10.3748/wjg.v29.i17.2571
Peer-review started: December 27, 2022
First decision: January 11, 2023
Revised: January 19, 2023
Accepted: April 11, 2023
Article in press: April 11, 2023
Published online: May 7, 2023
Processing time: 130 Days and 11.7 Hours
Hepatocellular carcinoma (HCC) is one of the most lethal malignant tumours worldwide. The mortality-to-incidence ratio is up to 91.6% in many countries, representing the third leading cause of cancer-related deaths. Systemic drugs, including the multikinase inhibitors sorafenib and lenvatinib, are first-line drugs used in HCC treatment. Unfortunately, these therapies are ineffective in most cases due to late diagnosis and the development of tumour resistance. Thus, novel pharmacological alternatives are urgently needed. For instance, immune check
Core Tip: Hepatocellular carcinoma (HCC) is one of the most lethal malignant tumours worldwide. Unfortunately, most HCC cases are diagnosed at an advanced stage, and "curative" options are not suggested for these patients. The best option is to start with drug therapy, with sorafenib and lenvatinib as the first-choice drugs. However, most patients do not respond to these treatments; therefore, new therapeutic strategies are urgently needed. Here, we review current potential and novel pharmacological approaches, including immunotherapy, drug combination, and drug repositioning, that should help to improve the prognosis of HCC patients.
- Citation: Villarruel-Melquiades F, Mendoza-Garrido ME, García-Cuellar CM, Sánchez-Pérez Y, Pérez-Carreón JI, Camacho J. Current and novel approaches in the pharmacological treatment of hepatocellular carcinoma. World J Gastroenterol 2023; 29(17): 2571-2599
- URL: https://www.wjgnet.com/1007-9327/full/v29/i17/2571.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i17.2571
Liver cancer ranks seventh in incidence and fourth in mortality worldwide. It is one of the malignancies with the highest mortality-to-incidence ratio, reaching up to 91.6%, according to the World Health Organization[1]. This cancer frequently occurs in association with chronic liver disease and is classified according to the cells of origin of the tumour. Hepatocellular carcinoma (HCC) is the most common, originating in hepatocytes and accounting for 75%-85% of all cases[2].
The main risk factors for developing HCC are chronic liver disease, such as non-alcoholic fatty liver disease and non-alcoholic steatohepatitis, as well as hepatitis B (HBV) and C virus (HCV) infections[3]. In addition, some habits, including excessive alcohol consumption and smoking, are also considered major risk factors for developing HCC[2].
The most appropriate management in clinical practice depends on the stage of the disease. At an early or even intermediate stage, the treatment options currently available are surgical methods (liver resection and transplantation), locoregional therapy (radiofrequency ablation), and transarterial chemoembolization therapy[4]. The 5-year survival rate for patients at these stages is 14%, and only 30% can be subjected to curative treatment. Unfortunately, most diagnoses are made when HCC is at an advanced stage, and treatment options are no longer viable[5,6]; pharmacological therapy is suggested in these cases. Chemotherapy is a potential treatment for these patients, but the main disadvantage is that such agents target both cancer and healthy cells, leading to unwanted events that can even endanger the life of the patient. The use of chemotherapeutic agents in monotherapy is ineffective; therefore, more effective and directed drugs are urgently needed. A new generation of treatments called “targeted therapy” (also known as “systemic therapy”) aims to specifically target some molecular features that provide malignant advantages to cancer cells while having low toxicity to non-cancerous cells[7,8]. Table 1 summarizes the recommended HCC management based on the Barcelona Clinic Liver Cancer strategy (BCLC), the most widely used liver cancer staging system. This system has five stages depending on disease extension, liver function, and performance status (Table 1)[9-11].
| Stage | Very early stage (0) | Early stage (A) | Intermediate stage (B) | Advanced stage (C) | Terminal stage (D) |
| Characteristics | Single nodule < 2 cm, preserved liver function, ECOG PS 0 | Single or 2-3 nodules < 3 cm, preserved liver function, ECOG PS 0 | Multinodular, unresectable, preserved liver function, ECOG PS 0 | Portal invasion/extrahepatic spread, preserved liver function, ECOG PS 1-2 | Not transplantable HCC, end-stage liver function, ECOG PS 3-4 |
| Treatment | Ablation, resection, transplant | Chemoembolization | Systemic therapy | Best supportive care | |
| Survival | > 5 yr | > 2.5 yr | > 2 yr | 3 mo | |
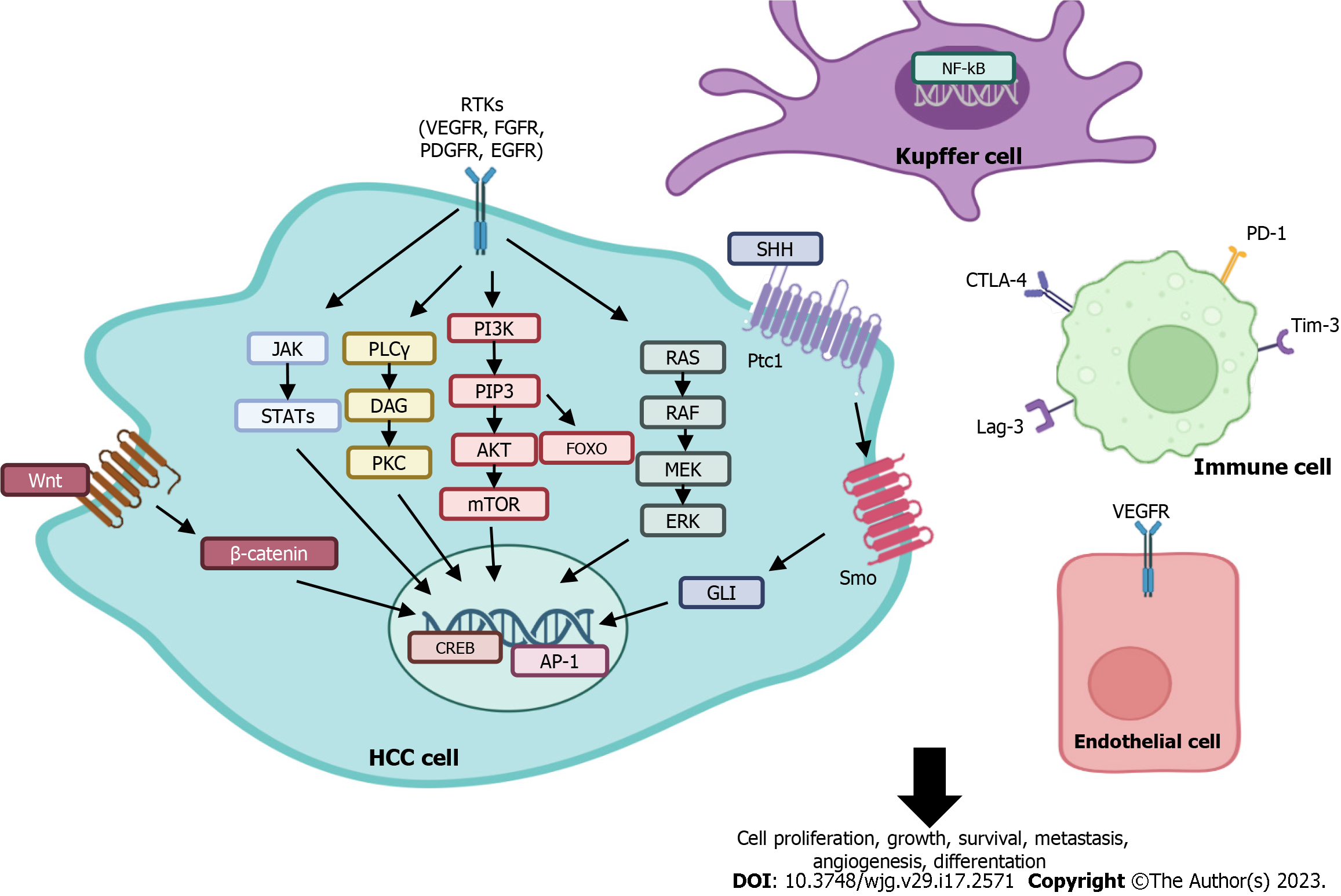
Systemic therapy is the standard treatment for advanced-stage disease (BCLC stage C). This type of treatment is classified into first- and second-line therapies, and its use in clinical practice depends on the individual patient characteristics. To date, many potential drug targets for the treatment of HCC have been investigated; the most critical targets are listed below and represented in Figure 1[8].
Growth factors and their receptors include vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), mesenchymal-epithelial transition factor (c-Met), insulin-like growth factor, and transforming growth factor α (TGF-α).
Intracellular signalling pathways include phosphoinositide 3-kinase (PI3K)/Akt/mechanistic target of rapamycin (mTOR), RAS/RAF/MEK/ERK, Janus kinase (JAK)/signal transducer and activator of transcription (STAT), and Wnt/β-catenin and the Hedgehog pathway. There are also cell cycle regulators such as CDKs. Transcription factors include nuclear factor kB (NF-kB), activating protein-1 and cyclic AMP response element binding (CREB).
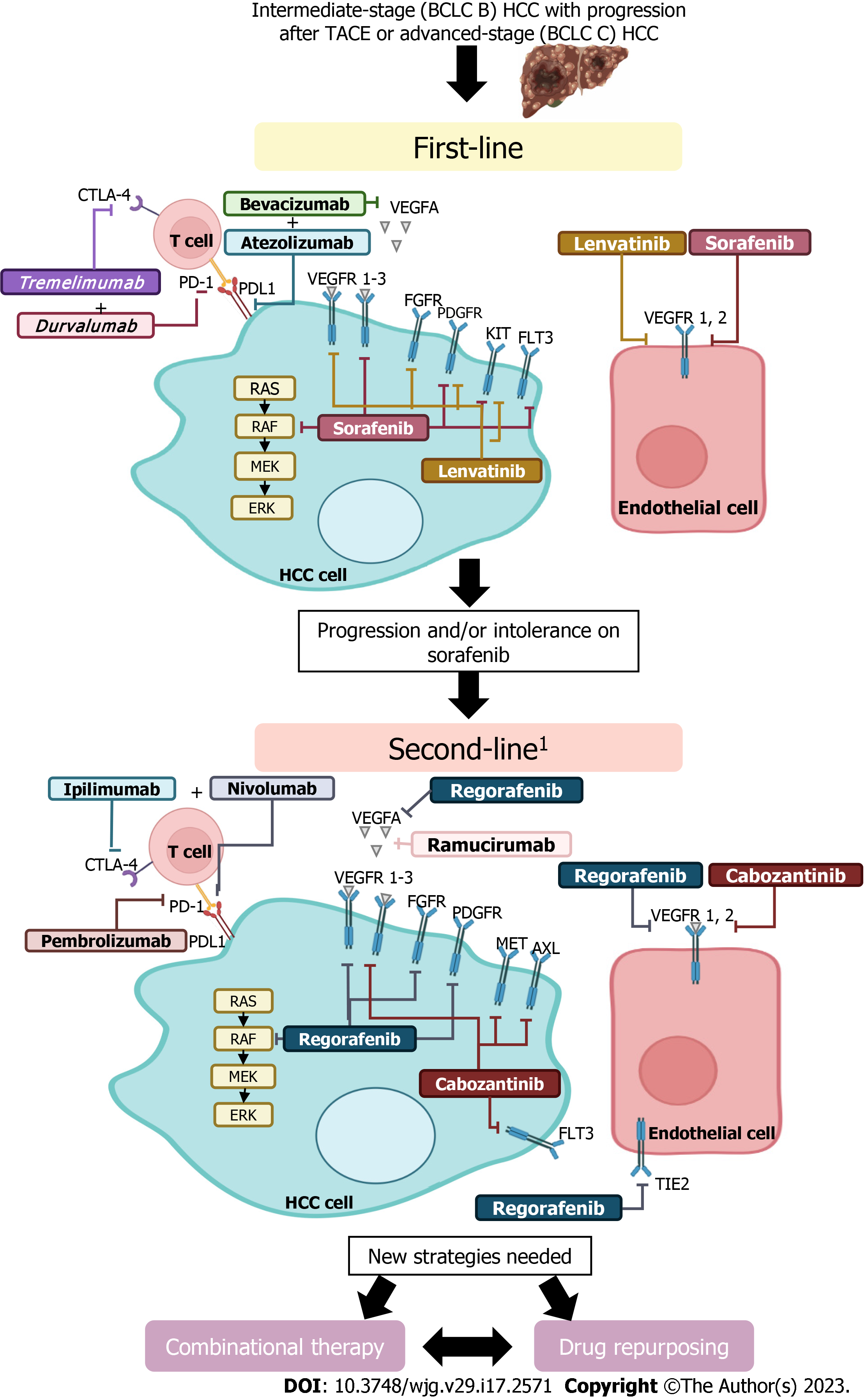
Next, first- and second-line systemic therapies approved for advanced HCC, as well as those drugs and targets under investigation for treating the disease, are described and discussed in detail and summarized in Table 2.
| Drug | Pharmacological target | Trial (NCT) | Treatment arm | Control arm | |
| First-line | |||||
| Systemic therapy | |||||
| Sorafenib | VEGF 1-3, PDGF, KIT, FLT3, BRAF, RAF | SHARP (NCT00105443) | Sorafenib (400 mg twice daily) | Placebo | |
| Lenvatinib | VEGFR1-3, FGFR 1-4, PDGR, RET and KIT | REFLECT (NCT01761266) | Lenvatinib (12 mg/day for bodyweight ≥ 60 kg or 8 mg/day for bodyweight < 60 kg) | Sorafenib (400 mg twice-daily in 28-d cycles) | |
| Immunotherapy | |||||
| Atezolizumab plus bevacizumab | PD-L1, vEGF | IMbrave150 (NCT03434379) | 1200 mg of atezolizumab plus 15 mg per kilogram of body weight of bevacizumab intravenously every 3 wk | Sorafenib (400 mg orally twice daily) | |
| Tremelimumab plus durvalumab | CTLA-4, PD-L1 | HIMALAYA (NCT03298451) | STRIDE: Tremelimumab plus durvalumab or durvalumab alone (300 mg, one dose of tremelimumab plus 1500 mg every 4 wk for durvalumab) | Sorafenib (400 mg orally twice daily) | |
| Second-line1 | |||||
| Systemic therapy | |||||
| Regorafenib | VEGFR 1-3, PDGFR, FGFR 1-2, RET, RAF | RESORCE (NCT01774344) | Regorafenib (160 mg once daily during weeks 1-3 of each 4-wk cycle) | Placebo | |
| Cabozantinib | VEGFR 1-3, MET and AXL | CELESTIAL (NCT01908426) | Cabozantinib (60 mg once daily) | Placebo | |
| Ramucirumab | VEGFR | REACH-2 (NCT02435433) | Ramucirumab 8 mg/kg intravenous ramucirumab every 2 wk | Placebo | |
| Immunotherapy | |||||
| Nivolumab | PD-1 | CheckMate-459 (NCT02576509) | Nivolumab (240 mg intravenously every 2 wk) | Sorafenib(400 mg orally twice daily) | |
| Nivolumab plus ipilimumab | PD-1, CTLA-4 | CheckMate-040 (NCT01658878) | Nivolumab 1 mg/kg plus ipilimumab 3 mg/kg, administered every 3 wk (4 doses), followed by nivolumab 240 mg every 2 wk (arm A); nivolumab 3 mg/kg plus ipilimumab 1 mg/kg, administered every 3 wk (4 doses), followed by nivolumab 240 mg every 2 wk (arm B); or nivolumab 3 mg/kg every 2 wk plus ipilimumab 1 mg/kg every 6 wk (arm C) | Placebo | |
| Pembrolizumab | PD-1 | KEYNOTE-240 (NCT02702401) | Pembrolizumab (200 mg intravenously every 3 wk for at least 35 cycles during approximately 2 yr) | Placebo | |
The need for new and better treatments for patients in advanced stages has led researchers to develop molecules to specifically target components of the carcinogenesis process. Sorafenib was the first oral multikinase inhibitor drug approved by the Food and Drug Administration (FDA) in 2007 to treat advanced HCC. In vitro experiments found that this drug inhibited HCC cell line growth and angiogenesis by inhibiting the RAF/MEK/ERK signalling pathway, as well as tyrosine and serine/threonine kinase receptors, including those for VEGF, PDGF, c-KIT, FLT3 and BRAF[12]. This drug is indicated in patients with preserved liver function who are not candidates for surgical or locoregional therapies and those with advanced tumours according to the BCLC classification and the Child-Pugh scale[9].
Sorafenib was the first drug to significantly improve the survival of patients with advanced HCC in the Asia-Pacific region. The median overall survival (OS) was 6.5 mo [95% confidence interval (CI): 5.56-7.56] in patients treated with sorafenib compared to 4.2 mo (3.75-5.46) in patients treated with placebo[13]. Similar results in terms of improved survival were observed in the SHARP clinical trial (ClinicalTrials.gov identifier: NCT00105443), a phase III, double-blind, placebo-controlled study that evaluated the effect of sorafenib on OS and time to symptomatic progression in patients diagnosed with advanced HCC. Here, the median OS was 10.7 mo in the sorafenib group and 7.9 mo in the placebo group. The most frequent adverse events in the sorafenib group were weight loss, diarrhoea, hypophosphataemia, and hand-foot skin reactions. Based on these trials, sorafenib became the first targeted therapy drug to be approved for the treatment of advanced HCC and has been the first-choice drug since its approval. Currently, the treatment of patients with sorafenib until significant radiographic progression and simultaneous treatment with regorafenib (discussed below) are recommended[9]. Unfortunately, only 30% of sorafenib users benefit, and within a short period of time, resistance to sorafenib often develops, rendering further use ineffective[14].
Following the approval of sorafenib, research led to the recognition of lenvatinib as another drug targeting important receptors and pathways in HCC. Lenvatinib is an oral receptor tyrosine kinase inhibitor and was approved in 2018 by the FDA as a first-line treatment for unresectable HCC[15]. VEGF receptor (VEGFR), FGF receptor (FGFR), PDGRα, RET, and KIT[16,17] are among its therapeutic targets. In preclinical models, lenvatinib was shown to selectively inhibit the proliferation of human HCC cell lines and in vivo tumour growth in xenograft models[18]. The approval of this molecule was based on the REFLECT clinical trial (ClinicalTrials.gov identifier: NCT01761266), a phase III, multicentre, open-label, non-inferiority trial that evaluated the OS of patients diagnosed with advanced HCC treated with lenvatinib vs patients treated with sorafenib. Lenvatinib met non-inferiority criteria against sorafenib, as the median survival for lenvatinib was 13.6 mo (95%CI: 12.1-14.9) vs 12.3 mo for the sorafenib group [12.3 mo, 10.4-13.9; hazard ratio (HR): 0.92, 95%CI: 0.79-1.06][19]. The most common adverse events in the lenvatinib group in this study were diarrhoea, loss of appetite, and weight loss.
Atezolizumab is a monoclonal antibody that selectively targets programmed death ligand-1 (PD-L1) to reverse the suppression of T-cell activity[20]. Its activity was assessed through an assay that determined predictive correlates of response to this antibody in cancer patients. According to Herbst et al[20], who studied various types of cancer, responses were observed in patients with tumours expressing high levels of PD-L1, mainly when it was expressed on tumour-infiltrating immune cells[20].
Conversely, the monoclonal antibody bevacizumab interferes with angiogenesis and tumour growth by inhibiting VEGF activity[21]. Finn et al[22] demonstrated the potential of bevacizumab as a promising anti-VEGF treatment for liver cancer in preclinical trials. They evaluated the effect of this humanized antibody in an orthotopic mouse model of HCC using the Hep3B cell line and found that bevacizumab treatment significantly reduced tumour microvessel density and alpha-fetoprotein (AFP) levels and prolonged time to progression compared to the control group[22].
The combination of atezolizumab and bevacizumab is a therapeutic strategy aiming to simultaneously inhibit PD-L1 (atezolizumab) and VEGF (bevacizumab) signalling in patients with advanced HCC. This combination was evaluated in the Imbrave150 clinical trial (ClinicalTrials.gov identifier: NCT03434379), a global, open-label, phase III study that evaluated the effect of the combination of these two antibodies in patients with unresectable HCC against the effect of sorafenib as a single drug, resulting in improved OS and progression-free survival (PFS) for the combination group [67. 2% (95%CI: 61.3-73.1) and 54.6% for the sorafenib group (95%CI: 45.2-64.0)][23]. A few years later, a longer follow-up of these patients was performed, reporting that the combination maintained the improvement in patients over the effect of sorafenib[24]. Accordingly, atezolizumab plus bevacizumab combination therapy was very recently approved by the FDA (2020) as the initial treatment for advanced HCC, adding a targeted drug combination to the first-line treatment strategies for these patients[25].
In October 2022, the FDA approved the combination of tremelimumab plus durvalumab for treating patients with unresectable HCC, which could form part of the first-line treatment for this cancer[26].
Tremelimumab is a fully human immunoglobulin G (IgG)2 monoclonal antibody against cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), a receptor that inhibits T-cell activity[27]. Duffy et al[28] subjected patients diagnosed with HCC to a study evaluating the efficacy of treatment with tremelimumab plus an ablative procedure performed during week 6 (ClinicalTrials.gov identifier: NCT01853618). The authors observed that such a combination led to the accumulation of intratumoural CD8+ T-cells and suggested it as a potential new treatment for patients with advanced HCC[28]. In contrast, dirvalumab is another human monoclonal antibody that binds to the PD-L1 protein and has been shown to be effective in liver cancer and in small-cell lung cancer, especially when used in combination with another therapeutic agent[29,30].
A phase I/II clinical trial (ClinicalTrials.gov identifier: NCT02519348) evaluated the safety and efficacy of monotherapy and combination treatment of tremelimumab plus durvalumab (300 mg and 1500 mg, respectively) in patients with HCC who had progressed on, were intolerant to, or refused sorafenib. The objective response rates (ORRs) were 24.0% (95%CI: 14.9-35.3) for the combination, 10.6% for durvalumab (5.4-18.1), and 7.2% (2.4-16.1) for tremelimumab, while the median OS was 18.7 (10.8-27.3), 13.6 (8.7-17.6), and 15.1 (11.3-20.5) months, respectively[31]. However, the clinical study that led to the approval of this combination was HIMALAYA (ClinicalTrials.gov identifier: NCT03298451), a phase III and global study with a heterogeneous population representative of HCC patients and no previous systemic treatment, which also evaluated the effect of durvalumab monotherapy and compared it to sorafenib. Here, the median OS was 16.43 mo for the combination (95%CI: 14.16-19.58), 16.56 mo (95%CI: 14.06-19.12) with durvalumab, and 13.77 mo (95%CI: 12.25-16.13) with sorafenib. The combination significantly improved OS over sorafenib, and durvalumab was not inferior to sorafenib in these patients, suggesting that it could be used as a first-line treatment[32].
Although approximately 30% of patients receiving sorafenib show improvement, resistance to treatment can develop after prolonged use (approximately 6 mo), rendering it ineffective after this period[14]. Therefore, second-line treatments are indicated for patients who either have progressed on previous sorafenib or do not respond to it. Notably, to date, no clinical trials have evaluated second-line therapy after lenvatinib[33].
Regorafenib is another oral multikinase inhibitor that primarily targets VEGFR, PDGF receptors, FGFR, RET, and RAF and, in preclinical trials, was shown to significantly inhibit liver tumour development[34]. In addition, it activates proteins involved in MAPK signalling, apoptosis, and autophagy[35]. The FDA approved this drug as a second-line treatment for patients who have already been treated with sorafenib. The safety and efficacy of this drug in humans were tested in a multinational, randomized, double-blind, placebo-controlled, phase III clinical trial (RESORCE, ClinicalTrials.gov identifier: NCT01774344), in which the median OS was 10.6 mo (95%CI: 9.1-12.1) for the regorafenib group vs 7.8 mo (6.3-8.8) in the placebo group[36]. The most common adverse events reported in this study were hypertension, hand-foot skin reactions, fatigue, and diarrhoea. Thus, because of the survival benefit of regorafenib in HCC patients unresponsive to sorafenib, it was approved by health agencies in several countries, including the United States, Japan, and China.
Cabozantinib is a multikinase inhibitor suppressor of tumour growth, metastasis, and angiogenesis that primarily targets VEGFR but also targets MET and AXL, which in addition to being implicated in HCC progression are involved in sorafenib resistance[37]. Initially, this drug showed clinical activity in a phase II study in previously untreated HCC patients and those with progression or no response to sorafenib, resulting in a median OS of 5.5 mo with no significant radiographic responses[38]. However, its approval was based on the results of the CELESTIAL clinical trial (ClinicalTrials.gov identifier: NCT01908426), a randomized, double-blind, phase III trial evaluating cabozantinib vs placebo in patients diagnosed with advanced HCC, in which cabozantinib demonstrated significant benefit over placebo in OS and tumour PFS. The median OS was 10.2 mo with cabozantinib and 8.0 mo with placebo (95%CI: 0.63-0.92). The median PFS was 5.2 mo with cabozantinib and 1.9 mo with placebo (95%CI: 0.36-0.52)[39]. In 2019, the FDA approved cabozantinib for treating patients with advanced HCC who were previously treated with sorafenib[40].
Interestingly, this drug is involved in tyrosine kinase inhibition and has also been reported to be an immunomodulator since some of its targets are involved in the immune response. Indeed, in combination with anti-programmed cell death protein 1 (PD-1) therapy, cabozantinib showed a greater antitumour effects than monotherapy or placebo in animal models of HCC. On its own, cabozantinib significantly increased neutrophil infiltration and reduced intratumoural CD8+ PD-1+ T-cell ratios, while the combination further stimulated this effect[41].
Ramucirumab is an antiangiogenic anti-VEGFR2 monoclonal antibody[42,43]. The efficacy of this drug was tested in the REACH clinical trial, a randomized, placebo-controlled, double-blind, multicentre, phase III trial (ClinicalTrials.gov identifier: NCT01140347). Here, ramucirumab was tested against placebo in patients diagnosed with advanced HCC who were previously treated with sorafenib and who experienced either progression or intolerance. The median OS for the ramucirumab group was 9.2 mo (95%CI: 8.0-10.6) vs 7.6 mo (6.0-9.3) for the placebo group (HR: 0.87; 95%CI: 0.72-1.05; P = 0.14). Consistent with these results, second-line treatment with ramucirumab did not significantly improve survival over placebo in patients with advanced HCC[44]. A follow-up trial, REACH-2, was a randomized, double-blind, placebo-controlled, phase III trial comparing ramucirumab vs placebo at a 2:1 ratio in the same population, but this time for a preselected population with AFP concentrations ≥ 400 ng/mL. The median OS was 8.5 mo (95%CI: 7.0-10.6) vs 7.3 mo (5.4-9.1); HR: 0.71 (95%CI: 0.53-0.95; P = 0.0199) and PFS [2.8 mo (2.8-4.1) vs 1.6 mo (1.5-2.7); 0.452 (0.33-0.60); P < 0.0001] were significantly improved in the ramucirumab group compared with the placebo group. This trial showed improved OS with ramucirumab compared to placebo in patients with HCC and AFP concentrations of at least 400 ng/mL who had previously received sorafenib[45].
Very recently, the clinical relevance of rechallenge treatment with previously administered drugs was evaluated, with five consecutive patients with advanced HCC who received rechallenge treatment with lenvatinib and with failure after treatment with ramucirumab. Here, the radiological findings using the modified Response Evaluation Criteria in Solid Tumours showed stable disease in four patients and a partial response in one. This trial demonstrated that re-exposure to lenvatinib treatment after ramucirumab might be effective for treating advanced HCC[46].
Unfortunately, cancer cells can develop resistance to systemic therapies. Thus, more and better drug treatments must be devised for these patients. Potential strategies include combination approaches and immunological therapy. In fact, immunotherapy has now been postulated as a possible therapeutic strategy to treat different types of cancer.
Cancer cells can escape the defence mechanisms of immune cells in the tumour microenvironment, thus avoiding detection and elimination by host lymphocytes by downregulating stimulatory immunoreceptors and stimulating inhibitory immunoreceptors. For example, in the case of T cells, tumour cells can modulate stimulatory activity by downregulating MHC-I on the surface. Conversely, inhibitory activity can be modulated by these cells through upregulation of PD-L1 on the surface[47-49]. Such molecules are known as immune checkpoints, which act as modulators of immune responses. These molecules can be used as pharmacological targets to generate various monoclonal antibodies that modulate their activity. Among the most studied immune receptors are PD-1, CTLA-4, LAG3, TIM3, TIGIT, and BTLA[49].
Immunotherapy has been shown to have significant efficacy in the treatment of different types of cancer, including HCC[50], making it an excellent option for patients with cancer progression or for whom systemic therapy with sorafenib was ineffective. Several immune checkpoint inhibitors that primarily target CTLA-4, PD-1, and its ligand PD-L1 have been tested to date. Some ICIs have also been approved by the FDA for the treatment of HCC. PD-L1, also called B7-H1 or CD274, is expressed in many cancer and immune cells and plays an essential role in blocking the "cancer immunity cycle" by binding to PD-1 and B7.1 (CD80), both of which are negative regulators of T-cell activation[51,52].
Antibody-based therapy is the main strategy designed to modulate the tumour immune response, but other cancer immunotherapeutic strategies, such as adoptive cell therapy, chimeric antigen receptor-modified immune cells, engineered cytokines, and therapeutic cancer vaccines, are still under development[53,54].
Nivolumab is a human IgG4 monoclonal antibody that binds to the PD-1 receptor, inhibiting its interaction with PD-L1 and PD-L2 and restoring T-cell activity[55]. Nivolumab has been shown to be effective in several malignancies, including melanoma, renal Hodgki’'s lymphoma, lung cancer, and gastric cancer[56-60].
Nivolumab was evaluated in HCC patients in a study called CheckMate-040 (ClinicalTrials.gov identifier: NCT01658878), a phase I/II, open-label, non-comparative, multicentre trial that included patients with advanced HCC who either had also reported progression after treatment with sorafenib or were sorafenib naive. Patients received intravenous nivolumab 240 mg every 2 wk until unacceptable toxicity or disease progression occurred. In this study, nivolumab showed favourable clinical activity and safety with manageable toxicities, suggesting that it could be suitable for patients with advanced HCC[61].
Subsequently, the activity of nivolumab (240 mg intravenously every 2 wk) was compared to that of sorafenib (400 mg orally twice daily) in a randomized, open-label, phase III trial (CheckMate 459, ClinicalTrials.gov identifier: NCT02576509) until disease progression or unacceptable toxicity. OS was improved from 14.7 mo for sorafenib to 16.4 mo with nivolumab but did not reach statistical significance (P = 0.0752). In addition, there was no significant difference in PFS (3.7 vs 3.8 mo). The ORRs for nivolumab and sorafenib were 15% and 7%, respectively. Nivolumab showed relevant clinical activity and a favourable safety profile for patients with advanced HCC. However, nivolumab administered for the first time in these patients was not significantly better than sorafenib in terms of OS[62]. Thus, in September 2017, nivolumab was approved by the FDA as a second-line treatment for patients with advanced HCC. It is worth mentioning that this antibody has only been approved by this institution[63].
Recently, the effect of the combination of nivolumab plus ipilimumab, a fully human IgG1 monoclonal antibody that binds to CTLA-4 on T cells[64], was evaluated. The efficacy of this combination was tested in the CheckMate 040 study (ClinicalTrials.gov identifier: NCT01658878), a multicentre, open-label, multicohort, phase I/II study in which it was shown that the combination of nivolumab plus ipilimumab led to high OS rates and had a manageable safety profile[65]. Based on the results of this trial, in March 2020, the FDA approved the combination of nivolumab plus ipilimumab as a second-line treatment for patients with HCC who had been previously treated with sorafenib[66].
Pembrolizumab is an anti-PD-1 monoclonal antibody that demonstrated clinical efficacy in patients with advanced HCC. This antibody was evaluated in a non-randomized, multicentre, open-label, phase II study in patients with BCLC B-C HCC pre-treated with sorafenib (ClinicalTrials.gov identifier: NCT02702414), and it showed antineoplastic activity with an ORR of 17% and a manageable safety profile[67]. Based on these results, in November 2018, the FDA granted accelerated approval to pembrolizumab for patients with HCC who had been previously treated with sorafenib[68]. A subsequent study was then conducted evaluating pembrolizumab in a randomized, double-blind, phase III study in patients with advanced HCC previously treated with sorafenib (KEYNOTE-240, ClinicalTrials.gov identifier: NCT02702401). Here, the median OS was 13.9 mo (95%CI: 11.6-16.0 mo) for pembrolizumab vs 10.6 mo (95%CI: 8.3-13.5 mo) for placebo (HR: 0.781; 95%CI: 0.611-0.998; P = 0.0238). The median PFS for pembrolizumab was 3.0 mo (95%CI: 2.8-4.1 mo) vs 2.8 mo (95%CI: 1.6 to 3.0 mo) for placebo[69].
Additionally, the KEYNOTE-394 trial (ClinicalTrials.gov identifier: NCT03062358), a randomized, double-blind, phase III study, evaluated the efficacy of pembrolizumab plus best supportive care vs placebo in Asian HCC patients as second-line therapy. Overall, the results were consistent with those seen in previous trials, further supporting the use of pembrolizumab in patients with advanced HCC. Here, the median OS was 14.6 mo (12.6-18.0) for pembrolizumab and 13.0 mo (10.5-15.1) for placebo (95%CI); furthermore, pembrolizumab showed significant improvement of PFS (HR: 0.74, 95%CI: 0.60-0.92, P = 0.0032)[70].
Currently, monotherapies are ineffective in fighting cancer mainly due to the development of resistance of cancer cells to available drugs[71], and preclinical findings may not be replicated in patients. For instance, the monoclonal antibody bortezomib demonstrated promising antineoplastic activity in preclinical assays, but in humans, it did not show notable single-agent activity compared to sorafenib[72]. Thus, more and better treatment options are needed for these patients, and drug combinations are promising options. This strategy consists of simultaneously administering two or more drugs aimed at different cancer-specific drug targets and has shown significant benefits compared to monotherapy[73]. However, combining drugs must become a rational strategy that guarantees significant pharmacological responses, especially for those patients who either did not respond to current therapy or developed resistance.
Several tools have been developed for the identification of potentially useful drug combinations; these tools include dose-response matrices, RNA interference technology, and the wide adaptation of clustered regularly interspaced short palindromic repeats (CRISPR) systems, as well as more novel techniques such as patient-derived xenograft (PDX) models and ex vivo primary cell and organoid models[74]. For instance, Lim et al[75] used PDXs of HCC, PDX-derived organoids and a hybrid experimental-computational approach–namely, the quadratic phenotypic optimization platform–and found that the combination of the second-generation proteasome inhibitor ixazomib and the CDK inhibitor dinaciclib (Dina), which they tested in vitro and in vivo, is effective against HCC[75]. Another example of the usefulness of these strategies is CRISPR-Cas9 combinatorial screening, a technique that accelerated the discovery of combination treatment with the approved drug ifenprodil (an NMDA receptor antagonist) and sorafenib as a new therapeutic alternative for advanced HCC[76].
Therefore, implementing the abovementioned strategies should aid the discovery of potentially useful drug combinations. In this manner, medical staff may have different choices and establish a selection order more suitable for each HCC patient in a personalized manner[77,78].
Conversely, it is well known that the discovery of new molecules with pharmacological activity is a process with two main disadvantages: Time and cost. It takes at least 10 years to bring a new drug to the market, and the approximate cost ranges from $314 million to $2.8 billion[79]. Furthermore, in emergency situations such as cancer, the demand for effective treatments is extremely high, and additional strategies are required to obtain novel therapies in an expedited manner. Thanks to advances in pharmacology and genomics, several non-cancer drugs have been shown to have great potential for use in treating multiple cancers. Drug repurposing is a strategy that involves the discovery of already known drugs initially used to treat other diseases but with the potential to treat various malignancies. The main advantage of this approach is that it allows an accelerated and less costly process to identify new cancer treatments since it focuses on the selection of drugs already approved by relevant health institutions[80,81]. Some non-cancer drugs showing antineoplastic activity against HCC and with high repurposing potential are described below and summarized in Table 3.
| Drug | Original therapeutic indication | Molecular targets in HCC | Ref. |
| Antihistamines | Allergy | H1R-H4R, Eag1, CYP2J2, TRPV2, AP-1, NF-B | [88,91,96,98,102,103,105,109,111,113,115,116] |
| Raloxifene, bazedoxifene | Breast cancer (raloxifene), osteoporosis | ER-, ER-, GPER, IL-6R, aHR, NF-B, STAT3, PI3K/AKT, MAPK | [135-137,141,142] |
| Disulfiram | Alcoholism | NF-B, TGF-β, ROS-JNK | [149-151,157] |
| Clofazimine | Antimycobacterial used to treat leprosy | Wnt/-catenin pathway | [166-178] |
| Albendazole | Anthelmintic | Tubulin, ERK1/2-HIF-1α-p300/CREB | [175] |
| Pimozide | Antipsychotic used to manage Tourette's Disorder | STAT3, Wnt/-catenin | [185,188] |
| Natamycin | Macrolide antifungal | PRDX1 | [191] |
| Metformin | Glycemic control in type 2 diabetes mellitus | PI3K-mTOR pathway, AMPK | [200-202,206,207] |
| Valproate | Anticonvulsant | HDAC, Notch-1, MAPK pathway, -catenin pathway | [220,221,223] |
| Atorvastatin | Lower lipid levels and reduce the risk of cardiovascular disease | Mevalonate pathway | [231] |
| Celebrex | NSAID | COX-2, PNO1 | [237,243] |
| Hydroxychloroquine | Antimalarial | Autophagy inhibition, TLR9 pathway | [247] |
One of the differences between repositioned drugs and existing drugs approved to treat HCC is that the latter target relatively few signalling pathways; in contrast, repositioned drugs have the enormous advantage of targeting a surprisingly wide variety of signalling pathways involved in liver carcinogenesis (Table 3). For instance, Nair et al[82] identified CDC20 as a marker of poor prognosis during the development of early and advanced HCC. Through molecular docking studies, it was determined that labetalol, a beta blocker, binds with high affinity to CDC20[82], suggesting that the effect of labetalol against the development of HCC should be tested. The same research group further investigated this possibility by in vitro cytotoxicity studies, in which labetalol significantly inhibited the growth of the HepG2 cell line[83]. Subsequent application of bioinformatics analysis tools to repositioned drugs provides an incredible advantage for the identification of unknown drug targets and signalling pathways potentially involved in liver carcinogenesis.
Histamine exerts a variety of physiological activities via its G protein-coupled receptors, the activation of which has been associated with the progression of different types of cancer[84,85]. It is well known that histamine favours the development of different types of cancer, including liver cancer[86]. Accordingly, overexpression of the histamine H1 receptor (H1HR) is associated with HCC cell proliferation and metastasis by inducing cell cycle progression, lamellipodia production, matrix metalloprotease 2 (MMP2) production and inhibition of apoptosis. Furthermore, suppressing H1HR activity significantly inhibited tumour growth and metastasis in mouse xenograft models[86]. Interestingly, very recently, it was shown that antihistamine consumption is associated with a significant decrease in the developing of liver cancer in patients diagnosed with HBV, HCV or both viruses[87].
Ellegaard et al[88] analysed cohort studies and reported that the use of cationic amphiphilic antihistamines, including astemizole (an H1-antihistamine), was associated with a significant reduction in mortality in lung cancer patients[88]. It is worth mentioning that astemizole might affect cancer cell proliferation via different molecular mechanisms, including ion channels.
The ether à-go-go-1 (Eag1) potassium channel has been reported to play an important role in the development of several types of cancer, such as liver, cervical, breast, lung and colon cancer[89-93], suggesting that this channel is a potential early biomarker and drug target for these tumours[94]. Eag1 overexpression has been implicated in cell cycle progression and cancer cell proliferation[95], and inhibition of this channel has reduced tumour progression in both in vitro and in vivo assays in leukaemia and gastric cancer[86-97]. De Guadalupe Chávez-López et al[98] found that astemizole inhibited cell proliferation and induced apoptosis in human HCC cell lines[98]. In the same study using an HCC model induced by diethylnitrosamine (DEN) in mice, they found that astemizole inhibited tumour development and decreased Eag1 mRNA and protein levels[98].
Cytochrome P450 2J2 (CYP2J2) is implicated in the development of different types of cancer. In HCC, CYP2J2 is overexpressed, and its activity promotes cell proliferation[99-101]. Interestingly, astemizole and loratadine inhibit CYP2J2 activity[102]. Ellegaard et al[88] proposed the possibility that this drug targets this protein, which would partially explain the effect of reduced mortality in lung cancer patients[88].
TRPV2 is a calcium channel expressed in and associated with several types of cancer, including HCC progression. Both mRNA and protein levels of this channel are increased in well-differentiated HCC tumour tissue compared to undifferentiated tissue. There is a strong association between its expression and portal vein invasion. Van den Eynde et al[103] reported that TRPV2 is related to endometrial cancer progression and identified astemizole, loratadine, and clemizole as TRPV2 blockers, with loratadine being the most potent antagonist, leading to inhibition of cell proliferation and migration in in vitro assays in HEK293 cells[103].
Loratadine is a second-generation antihistamine indicated for the treatment of allergic rhinitis and urticaria. It is a selective H1HR receptor antagonist[104]. Fritz et al[105,106] evaluated the effect of loratadine through retrospective studies in patients diagnosed with breast cancer and melanoma and found that loratadine use was associated with improved OS[105,106]. In patients diagnosed with lung cancer, a cohort study found that the use of loratadine or its metabolite desloratadine was associated with a significant reduction in mortality; the authors proposed that cancer-related changes in lysosomal membranes could favour the entry of these antihistamines[88,107]. To date, there has been scarce evidence studying the effect of loratadine in HCC. In a thesis from a few years ago, the cytotoxic effect of the combination of loratadine and cisplatin in HCC cell lines was evaluated; loratadine alone had a concentration-dependent cytotoxic effect on liver cancer cell lines, and the combination of loratadine with cisplatin had a synergistic effect[108]. The same thesis showed that loratadine alone and in combination also induced apoptosis and cell cycle arrest in the G2/M phase. Loratadine targets proteins that have been suggested to be involved in the development of HCC. As in the case of astemizole, loratadine might exert antineoplastic effects through H1HR antagonism, lysosomal membrane sensitization, TRPV2 calcium channel blockade and inhibition of CYP2J2 activity[86,88,102,103]. Furthermore, it has been reported that loratadine could exert anti-inflammatory activity by inhibiting the inflammatory response triggered by NF-kB signalling[109], an effect that reduces the levels of proinflammatory components, including interleukin (IL)-6 and tumour necrosis factor (TNF)-α. The effect of loratadine on HCC and its potential mechanisms of action deserve further investigation.
Deptropine is a first-generation H1HR antihistamine indicated to treat asthma[110]. This drug has activity against cancer cells. For example, in vitro assays in human liver cancer cells showed that, compared to the activity of other first- and second-generation antihistamines, deptropine was more potent in inhibiting cell proliferation and inducing autophagosome formation by significantly increasing the expression of light chain 3B-II. In mouse xenograft models, deptropine potently inhibited the tumour effect[111].
This antihistamine is a potential anti-HCC agent. Feng et al[112] evaluated the activity of cypro
A cohort study evaluated the efficacy of the combination of cyproheptadine with sorafenib compared to sorafenib alone in patients with advanced HCC. The median OS was 11 mo in the combination group (95%CI: 6.8-15.1 mo) and 4.8 mo in the sorafenib group (95%CI: 3.1-6.6 mo), while the median PFS time was 7.5 mo (95%CI: 5.1-10.0 mo) in the combination group compared with 1.7 mo (95%CI: 1.4-2.1 mo) in the sorafenib group[116].
Histamine 2 receptor: Histamine receptor 2 (H2HR) activation has an inhibitory effect on tumour progression in colorectal cancer; gene expression profiling studies in tumour samples from colorectal cancer patients described the elevated expression of this receptor, associated with improved OS outcomes[117]. In human liver cancer cells, H2HR activation leads to the inhibition of IL-6 expression and signalling, arresting cell proliferation[118]. In contrast, there is evidence that H2HR activation in HCC favours the expression of β-catenin and survivin, leading to cell survival[119]. Cimetidine, an H2 antihistamine, decreases intracellular cAMP concentrations, as well as EGF-induced cell proliferation and migration, and it has been suggested as an HCC chemopreventive agent[120]. Additionally, cimetidine treatment was shown to inhibit liver carcinogenesis in rats with DEN-induced HCC[121]. Recently, Crouchet et al[122] developed a system in human liver cells that models a clinical prognostic liver signature predicting long-term liver disease progression to HCC, and they identified nizatidine, an H2HR antihistamine, for the treatment of advanced liver disease and prevention of HCC[122].
Histamine 3 receptor: The H3HR receptor has been described to participate in the carcinogenesis of different types of cancer, including colorectal and pancreatic cancer[123,124]. In tumour tissues from HCC patients, H3HR was overexpressed and associated with poor prognosis, and its activation promoted the growth and metastasis of HCC cell lines by inducing lamellipodia formation[125,126]. Zhang et al[126] reported that H3HR activation favours HCC progression through an acceleration of the G1-S phase transition, inhibition of apoptosis, and activation of the AMPc/PKA/CREB signalling pathway to downregulate the expression of CDKN1A, a cyclin-dependent kinase inhibitor that has anti-oncogene activity[126]. Thus, the oncogenic role of H3HR might be antagonized as a potential therapy in HCC.
Histamine 4 receptor: Analysis of cancer genomic data from The Cancer Genome Atlas showed that the histamine H4 receptor (H4HR) is slightly but significantly overexpressed in human HCC tumour tissues compared to healthy tissue[84]. Furthermore, patients with increased H4HR protein expression in tumour cells also had increased tumour sizes and more metastasis compared to those with lower receptor expression, suggesting that H4HR levels could be used as a prognostic marker for liver cancer[127]. However, to date, there have been insufficient studies demonstrating the role of H4HR in HCC and its clinical relevance, so it is crucial to investigate its association with this cancer.
According to the Global Cancer Observatory, liver cancer ranks fifth in incidence in men, while in women, it ranks ninth[1]. This fact has attracted the attention of researchers, who have argued that oestrogens explain this difference. Accordingly, oestrogens play a protective role against liver damage and prevent the development of HCC[128-130]. Epidemiological data have indicated that oestrogen deficiency in peri- and postmenopausal women increases the risk of developing liver damage and increases HCC incidence in postmenopausal women; in concordance, oestrogen treatment suppresses this phenomenon[131,132].
Both isoforms of the nuclear oestrogen receptor (ER), ER-α and ER-β, are involved in the development of liver cancer; however, the functions of ER-β have not yet been fully described. Both ER-α and ER-β are expressed in the liver under normal conditions, but their expression is modified during inflammatory processes. Both are decreased in HCC patients compared to healthy tissue samples[133] and are believed to lose their function during disease progression; indeed, the ER-α isoform might even be considered a predictor of poor prognosis in HCC[128,134]. Conversely, Matsushima et al[135] reported that the selective oestrogen receptor modulators (SERMs) raloxifene and bazedoxifene inhibited HCC progression through their specific interaction with ER-β. They proposed that both drugs could activate the ER-β receptor in the liver, which through downstream signal transduction suppresses TGF-induced HCC cell migration via inhibition of Akt[135].
Raloxifene is indicated for the treatment of osteoporosis and is used for the treatment and prevention of breast cancer[136]. Raloxifene is a potent inhibitor of the IL-6/GP130 signalling pathway, which is involved in the process of oncogenesis of various cancers, including HCC[137]. This research group observed that raloxifene inhibited cell viability in human liver cancer cell lines. Furthermore, using an in vivo model, they also demonstrated that it could inhibit tumour growth.
Because liver cancer frequently develops in the context of chronic inflammatory liver disease, proinflammatory cytokines and immune cells play important roles in carcinogenesis. One of the most relevant cytokines in the development of HCC is IL-6; when overproduced, it has a strong effect on liver carcinogenesis, and its high expression is related to a high rate of metastasis and poor prognosis in HCC[138-140]. Naugler et al[141] reported in an animal model that oestrogen administration inhibited IL-6 secretion and significantly reduced DEN-induced injury in males[141]. When IL-6 binds to its receptor, it recruits JAK, leading to activation of STAT3, a transcription factor that favours proliferative processes, angiogenesis, invasion, etc[142]. In addition, ER-α could interact directly with NF-kB and inhibit IL-6 secretion, and raloxifene could interact with ER-α and inhibit IL-6 secretion and thus tumour progression[142].
Disulfiram is an FDA-approved drug from several years ago and has been extensively used in the treatment of alcoholism[143]. This drug has potential anticancer activity in different types of cancer, including lymphoma[144], breast cancer[145,146], and pancreatic cancer[147]. A phase II, multicentre, randomized, double-blind trial (ClinicalTrials.gov identifier: NCT00312819) evaluated the safety and efficacy of the combination of disulfiram with cisplatin and vinorelbine in patients diagnosed with lung cancer. Interestingly, a significant increase in survival was observed in patients given this combined treatment[148]. Intracellular copper (Cu) levels are significantly elevated in HCC cells and are associated with poor patient prognoses[149,150]. Surprisingly, the increase in Cu concentration might be harnessed for therapeutic use since disulfiram has Cu-dependent anticancer properties. Li et al[151] found that disulfiram inhibited the proliferation, migration, and invasion of liver cancer cells; interestingly, Cu enhanced this activity when combined with disulfiram; however, Cu alone did not[151]. In this regard, a phase I clinical trial determined the maximum tolerated dose of Cu administered with disulfiram in patients with liver cancer and found that 250 mg of daily Cu gluconate were well tolerated by these patients[152]. In the same study, temporary disease stabilization was observed in some patients, but there were no objective responses. Disulfiram can penetrate cancer cells and chelate intracellular Cu because Cu levels are elevated in many cancers. This action provides the advantage of specificity for cancer cells compared to healthy cells. Disulfiram might work as a Cu ionophore that induces oxidative stress by promoting reactive oxygen species (ROS) production, resulting in the inhibition of NF-kB[151], a transcription factor involved in the regulation of inflammatory processes and the development of liver injury, as well as HCC progression[153,154]. Blocking NF-kB signalling leads to an increase in ROS-induced toxicity and consequent cell apoptosis[155]. Furthermore, Thiery[156] found that inhibition of NF-kB signalling also resulted in inhibition of liver cancer cell metastasis by reversing the epithelial-to-mesenchymal transition (EMT), an important process in cancer metastasis in which NF-kB and TGF-β are important components[156]. Indeed, in this same study, disulfiram was found to inhibit TGF-kβ signalling. Interestingly, disulfiram plus Cu reversed EMT more effectively than disulfiram alone[151].
Most recently, Zhang et al[157] reported that disulfiram plus copper in combination with sorafenib resulted in increased anticancer activity against HCC under in vitro and in vivo conditions. Moreover, this combination synergistically inhibited the proliferation of human HCC cell lines and significantly increased autophagy and apoptosis compared to sorafenib alone. In addition, in a mouse orthotopic HCC xenograft model, the combination effectively inhibited tumour growth compared to the effect of sorafenib alone[157].
The canonical Wnt/β-catenin signalling pathway is a crucial component during embryonic development and normal adult homeostasis because it participates in processes such as cell differentiation, polarity, migration, and apoptosis[158]. However, abnormal activation of this pathway (especially of the transcription factor β-catenin) has been linked to cellular malignant transformation and promotion of carcinogenesis, and it is present in many types of cancer, including HCC[159-161]. Notably, mutations in the CTNNB1 gene, which codes for β-catenin, are the most frequent mutations during HCC[162,163]. Interestingly, clofazimine, an anti-leprosy agent, could be useful for treating Wnt-dependent cancers. For instance, it has been shown to be effective against triple-negative breast cancer, both in vitro and in vivo, through inhibition of Wnt/β-catenin signalling[164,165]. Furthermore, Xu et al[166] demonstrated that clofazimine could effectively suppress HCC cell growth, inhibiting Wnt/β-catenin canonical signalling[166]. This drug has been evaluated for some years, and the results have suggested that it might work successfully as an antitumour agent. For example, Van Rensburg et al[167] found that it inhibited HCC cell line proliferation in vitro[167]. In addition, in a phase II clinical trial in patients with unresectable or metastatic liver cancer, 600 mg of this drug were administered daily for two weeks, followed by a dose reduction to 400 mg until progression or death. In this trial, 13 of 30 treated patients had disease stability for up to 20 mo, and the median OS was 13 wk[168]. Furthermore, a phase II clinical trial evaluated the combination of clofazimine plus doxorubicin in patients diagnosed with HCC. Although no patients showed complete or partial response, this combination showed only mild toxic effects, and the authors recommended further studies involving this antileprosy agent[169]. Overall, these trials provided strong evidence to suggest that clofazimine might be useful in treating HCC.
Albendazole is an antiparasitic agent used to treat parenchymal neurocysticercosis and other helminth infections by blocking parasite microtubules, leading to the inhibition of glucose uptake and transport and, ultimately, cell death[170]. Interestingly, this drug has been reported to possess antitumour activity and has been studied in different malignancies, including liver, lung, breast, prostate, and colorectal cancers and melanoma[171-175]. Pourgholami et al[176] evaluated the effect of this drug in several liver cancer cell lines and in mouse xenograft models (human SKHEP-1 tumour growth in nude mice), reporting that the drug induced dose-dependent inhibition of [3H] thymidine incorporation in all the cell lines studied and a significant decrease in the number of SKHEP-1 cells significantly inhibiting tumour growth[176].
Pimozide is a dopamine receptor antagonist neuroleptic drug[177] that was approved by the FDA for the treatment of Tourette’s syndrome and schizophrenia[178] and it has shown efficacy for the treatment of different types of cancer, such as breast cancer[179,180], prostate cancer[181], brain tumours[182], colorectal cancer[183], and chronic myelogenous leukaemia[184]. In liver cancer, pimozide effectively inhibited cell proliferation of HCC cell lines through disruption of Wnt/β-catenin signalling and reduction of epithelial cell adhesion molecule expression, a marker of both liver stem cells[185] and HCC tumour-initiating cells[186,187]. Furthermore, Chen et al[188] found that pimozide was able to inhibit cell proliferation, migration, colony formation, and sphere formation in vitro in HCC cell lines and stem-like cells by suppressing STAT3 activity. Additionally, pimozide reduced the tumour burden in a xenograft model in nude mice[188]. Moreover, the same research group found that the antiproliferative effects of pimozide on HCC cell lines were reversible and in line with the involvement of cell quiescence and ROS production. Interestingly, pimozide combined with sorafenib synergistically inhibited HCC cell proliferation in vitro[188].
Natamycin is a natural polyene amphoteric macrolide antibiotic with antifungal properties[189]. It has been reported to significantly inhibit the proliferation of prostate cancer cells[190]. Conversely, An et al[191] found that natamycin induced apoptosis and inhibited the proliferation of HCC cells by triggering excessive ROS production through the downregulation of peroxiredoxin 1 (PRDX-1). Additionally, they found that the combination of natamycin plus sorafenib exerted a synergistic effect on cell growth suppression compared to the effect of monotherapy[191].
Dysregulation of cellular redox systems is a critical feature of many types of cancer. Increased ROS play a fundamental role in the tumour microenvironment, activating important signalling pathways in carcinogenesis, such as MAPK/ERK, JNK, and PI3K/AKT, and in turn activating NF-kB, MMPs, and VEGF, consequently affecting angiogenesis, metastasis and cell survival in many types of cancer[192-194]. However, at significantly elevated ROS concentrations, cancer cells are able to develop antioxidant defence systems to maintain redox homeostasis and survive[195]. An example of this situation is the participation of the peroxiredoxin family, which consists of peroxidases that break down hydrogen peroxide, protecting the cancer cell from oxidative stress and consequently providing a survival advantage; thus, this family of enzymes are potential targets for tumour growth arrest and cancer therapy[196,197]. It is worth mentioning that the PRDX-1 isoform is the most abundant and positively regulated protein in different types of cancer, and its expression is associated with poor prognosis[198].
Valproic acid is a drug that possesses anticonvulsant activity and is primarily indicated for the treatment of epilepsy. However, it is also useful for treating migraine, bipolar disorder, anxiety, and psychiatric disorders[199]. The interest in testing the activity of this drug as an antineoplastic agent came from findings in human neuroblastoma models. This molecule was able to inhibit proliferation and induce differentiation of primitive neuroectodermal tumour cells in vivo, providing evidence for using valproic acid as a treatment for neuroblastoma patients[200]. Machado et al[201] reported the effect of this drug on human liver cancer cells both in vitro and in vivo. Valproic acid significantly inhibited cell proliferation in a dose-dependent manner, while in mouse xenograft models, it reduced tumour growth, in addition to negatively regulating Notch-1 mRNA levels[201]. Very recently, Bai et al[202] evaluated the effect of valproate in animal models of HCC in rats treated with DEN and found that this drug significantly reduced liver nodules and AFP levels, as well as other important liver enzymes, compared to rats treated with DEN alone. Additionally, valproate reduced inflammatory cytokines, such as TNF-α, IL-6, IL-1β, NF-kB and TGF-β1, in liver tissue[202].
Lee et al[203] took advantage of the benefits of combination therapy to evaluate the effects of cytokine-induced killer (CIK) cells with valproic acid. CIK cells are ex vivo expanded T lymphocytes expressing natural killer and T-cell markers that are used as adjuvant therapy to reduce HCC recurrence, yet CIK cell monotherapy is insufficient to treat advanced HCC[203-205]. Therefore, this research group determined whether treatment with CIK cells and valproic acid synergized to inhibit tumour growth in mouse models of HCC. After seven days of the combined treatment, there was a synergistic effect on relative tumour volume in the animals since the relative tumour volume in control animals was significantly increased[206].
Additionally, Yu et al[207] implemented a therapeutic strategy in HCC cells in vitro and in vivo that consisted of testing the combined effect of valproic acid with proton and photon irradiation. Histone deacetylase (HDAC) inhibitors, including valproic acid, have shown promising results in the treatment of different cancers[208-210]. However, their use as monotherapy has not been satisfactory, so using them in combination with another therapy is an appealing strategy. HDAC inhibitors can sensitize human cancer cells to ionizing radiation[211], which is a therapeutic strategy for cancer[212]. In a study by Yu et al[207], valproic acid prolonged DNA damage and increased proton-induced apoptosis and ROS formation while suppressing the expression of nuclear factor erythroid 2–related factor 2, a transcription factor involved in cellular antioxidant regulation. In tumour xenograft models, valproic acid significantly enhanced tumour growth retardation[207]. In addition, An et al[213] reported that valproic acid could induce cellular senescence in HCC cells through its role as an HDAC inhibitor[213].
Table 4 summarizes the ongoing clinical trials for HCC patients. Immunotherapy is currently positioned as the most innovative pharmacological strategy to treat different types of cancer, including liver cancer. In addition, it is interesting to note that most of the ongoing HCC clinical trials are evaluating the effects of combination therapy and that drug repurposing is gaining tremendous interest, as non-oncology molecules are now being tested. Next, the non-oncology drugs used in ongoing clinical trials (Tables 3 and 4) are discussed.
| Study title | NTC number | Study design | Drugs | Status |
| Monotherapy | ||||
| Study of Pembrolizumab (MK-3475) as Monotherapy in Participants With Advanced Hepatocellular Carcinoma (MK-3475-224/KEYNOTE-224) | NCT02702414 | Phase II, non-randomized, parallel assignment, open label | Pembrolizumab | Active, not recruiting |
| An Investigational Immuno-therapy Study of Nivolumab Compared to Sorafenib as a First Treatment in Patients With Advanced Hepatocellular Carcinoma | NCT02576509 | Phase III, randomized, parallel assignment, open label | Nivolumab. Sorafenib | Active, not recruiting |
| Exploratory Study on Combined Conversion Immunotherapy for Liver Metastasis of MSS Type Initial Unresectable Colorectal Cancer Based on Gene Status | NCT05409417 | Phase II, III, single group assignment, open label | Experimental drug | Recruiting |
| First-in-Human Safety, Tolerability and Antitumour Activity Study of MTL-CEBPA in Patients With Advanced Liver Cancer | NCT02716012 | Phase I, non-randomized, parallel assignment, open label | MLT-CEBPA. Sorafenib (200 mg) | Active, not recruiting |
| Drug combination | ||||
| A Phase III, Open-Label, Randomized Study of Atezolizumab in Combination With Bevacizumab Compared With Sorafenib in Patients With Untreated Locally Advanced or Metastatic Hepatocellular Carcinoma (IMbrave150) | NCT03434379 | Phase III, randomized, parallel assignment, open label | Atezolizumab. Bevacizumab. Sorafenib | Active, not recruiting |
| A Trial of Lenvatinib Plus Pembrolizumab in Participants With Hepatocellular Carcinoma | NCT03006926 | Phase I, single group, open label | Lenvatinib. Pembrolizumab (200 mg) | Active, not recruiting |
| A Study of Durvalumab or Tremelimumab Monotherapy, or Durvalumab in Combination With Tremelimumab or Bevacizumab in Advanced Hepatocellular Carcinoma | NCT02519348 | Phase II, randomized, parallel assignment, open label | Tremelimumab. Durvalumab. Bevacizumab | Active, not recruiting |
| Pembrolizumab With or Without Elbasvir/Grazoprevir and Ribavirin in Treating Patients With Advanced Refractory Liver Cancer | NCT02940496 | Phase II, non-randomized, parallel assignment, open label | Elbasvir/Grazoprevir. Pembrolizumab. Ribavirin | Active, not recruiting |
| Clinical Recruitment of Patients With First-line Targeted Drug Resistance or Intolerance to Hepatocellular Cancer With PD-1 Inhibitor (Toripalimab, JS001) Detected on the NGS Platform Combined With Anlotinib | NCT05453383 | Phase II, single group assignment, open label | Anlotinib. Toripalimab | Recruiting |
| TACE Combined With Camrelizumab and Apatinib in the Treatment of Advanced Liver Cancer | NCT05550025 | Phase II, single group assignment, open label | Camrelizumab. Apatinib | Recruiting |
| IBR900 Cell Injection Combined With Lenvatinib or Bevacizumab in the Treatment of Advanced Primary Liver Cancer | NCT05411757 | Phase I, single group assignment, open label | IBR900. Lenvatinib. Bevacizumab | Not recruiting yet |
| Trial to Evaluate the Safety of Talimogene Laherparepvec Injected Into Tumors Alone and in Combination With Systemic Pembrolizumab MK-3475-611/Keynote-611 | NCT02509507 | Phase I, II, non-randomized, sequential assignment, open label | Talimogene. Laherparepvec. Pembrolizumab | Active, not recruiting |
| HAIC Sequential TAE Combined With Lenvatinib and Tislelizumab in Unresectable HCC | NCT05532319 | Phase II, single group assignment, open label | HAIC sequential TAE. Lenvatinib. Tislelizumab | Not recruiting yet |
| A Study of E7386 in Combination With Other Anticancer Drug in Participants With Solid Tumor | NCT04008797 | Phase I, non-randomized, sequential assignment, open label | E7386. Lenvatinib | Recruiting |
| An Immuno-therapy Study to Evaluate the Effectiveness, Safety and Tolerability of Nivolumab or Nivolumab in Combination With Other Agents in Patients With Advanced Liver Cancer | NCT01658878 | Phase I, II, parallel assignment, open label | Nivolumab. Sorafenib. Ipilimumab. Cabozantinib | Active, not recruiting |
| A Phase I Clinical Study of Recombinant Humanized Anti-BTLA Monoclonal Antibody (JS004) Injection Combined With Toripalimab Injection in Patients With Advanced Solid Tumors | NCT05427396 | Phase I, single group assignment, open label | JS004. Toripalimab | Recruiting |
| mFOLFOX7 Plus Camrelizumab and Apatinib for Advanced HCC | NCT05412589 | Phase II, single group assignment, open label | mFOLFOX7. Camrelizumab. Apatinib | Recruiting |
| Trial of PXS-5505 Combined With First Line Atezolizumab Plus Bevacizumab For Treating Patients With Unresectable Hepatocellular Carcinoma | NCT05109052 | Phase II, III, single group assignment, open label | PXS-5505. Atezolizumab. Bevacizumab | Not recruiting yet |
| Combination of Regorafenib and Nivolumab in Unresectable Hepatocellular Carcinoma | NCT04310709 | Phase II, single group assignment, open label | Regorafenib. Nivolumab | Recruiting |
| Phase Ib Trial of Infigratinib In Combination With Atezolizumab And Bevacizumab for The Second-Line Treatment of Advanced Cholangiocarcinoma With FGFR2 Fusion/Amplification | NCT05510427 | Phase I, randomized, single group assignment, open label | Infigratinib. Atezolizumab. Bevacizumab | Recruiting |
| A Study of TAK-500 With or Without Pembrolizumab in Adults With Select Locally Advanced or Metastatic Solid Tumors | NCT05070247 | Phase I, non-randomized, parallel assignment, open label | TAK-500. Pembrolizumab | Recruiting |
| A Study of Nivolumab and Relatlimab in Combination With Bevacizumab in Advanced Liver Cancer | NCT05337137 | Phase I, II, randomized, parallel assignment, quadruple (participant care, provider, investigator, outcomes assessor) | Relatlimab. Nivolumab. Bevacizumab | Recruiting |
| Drug repurposing | ||||
| High Dose Vitamin C Combined With Metformin in the Treatment of Malignant Tumors | NCT04033107 | Phase II, single group assignment, open label | Vitamin C. Metformin | Recruiting |
| Statin Combination Therapy in Patients Receiving Sorafenib for Advanced Hepatocellular Carcinoma | NCT03275376 | Phase II, randomized, parallel assignment, Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) | Atorvastatin | Terminated |
| The Combination Effect of Statin Plus Metformin on Relapse-free | NCT02819869 | Phase II, randomized, parallel assignment | Statin. Metfotmin | Terminated |
| Statin for Preventing Hepatocellular Carcinoma Recurrence After Curative Treatment | NCT03024684 | Phase IV, randomized, parallel assignment, triple masking (Participant, Care Provider, Investigator) | Atorvastatin | Recruiting |
| Meclizine for Hepatocellular Carcinoma | NCT03253289 | Phase I, single group assignment, open label | Meclizine | Recruiting |
| Celebrex and Metformin for Postoperative Hepatocellular Carcinoma | NCT03184493 | Phase III, non- randomized, parallel assignment | Celebrex plus metformin | Recruiting |
| Sorafenib Induced Autophagy Using Hydroxychloroquine in Hepatocellular Cancer | NCT03037437 | Phase II, non-randomized, parallel assignment, open label | Sorafenib. Hydroxychloroquine | Recruiting |
This drug is commonly used for the treatment of type 2 diabetes and was approved by the FDA in 1994. Metformin lowers glucose levels and improves insulin sensitivity[214]. Surprisingly, metformin has been shown to have antineoplastic activity in different types of cancer[83,215]. It is one of the most successful non-cancer drugs used in oncology. Several clinical trials are currently investigating the therapeutic potential of this drug in various cancers, including breast, prostate, endometrial, and colorectal cancer[216-219]. In the case of liver cancer, metformin has gained interest as an antineoplastic agent, given the increased risk of developing liver cancer in diabetic patients. Meta-analyses have reported that metformin has a beneficial effect on the incidence and/or survival of patients with liver cancer. For example, Ma et al[220] reported the association between metformin use and improved survival in diabetic patients with liver cancer[220]. Afterwards, the same research group reported a meta-analysis of 19 studies in diabetic subjects and suggested that metformin use reduced the proportion of liver cancer by 48% compared to non-users[221]. At the molecular level, metformin reduces insulin levels, activating the PI3K-mTOR signalling pathway and inhibiting cell proliferation in cancers expressing the insulin receptor[222]. Other mechanisms include negative regulation of mTOR via AMPK activation[223].
Ongoing clinical trials in HCC patients are evaluating the use of metformin in combination with other molecules, such as vitamin C (ClinicalTrials.gov identifier: NCT04033107), statins (ClinicalTrials.gov identifier: NCT02819869) and Celebrex (ClinicalTrials.gov identifier: NCT03184493).
Statins are agents that decrease the level of low-density lipoprotein cholesterol in the blood. They are specific inhibitors of the mevalonate pathway through inhibition of the conversion of 3-hydroxy-3-methylglutaryl coenzyme A into mevalonate, which is responsible for cholesterol synthesis[224]. Interestingly, mevalonate signalling is deregulated in several types of cancer and is also involved in the process of tumorigenesis[225,226], making it a potentially useful target for cancer treatment. Preclinical trials have demonstrated that statins can be used as antitumour agents in colorectal cancer[227-229]. It was suggested that statins might reduce the risk of developing HCC[230]. Kim et al[231] reported that atorvastatin inhibited the activation of YAP (via the mevalonate pathway) and AKT (via stabilization of the truncated retinoid X receptor alpha pathway), which are involved in cancer development[231].
Ongoing clinical trials are evaluating the effects of statins in patients with advanced HCC, such as a trial evaluating atorvastatin in patients receiving treatment with sorafenib (ClinicalTrials.gov identifier: NCT03275376) and a clinical trial studying two non-oncology drugs-statins and metformin-either alone or in combination (ClinicalTrials.gov identifier: NCT03024684).
Celebrex (celecoxib) is a cyclooxygenase-2 (COX-2) selective non-steroidal anti-inflammatory drug indicated for the treatment of pain and inflammation caused by osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis[232]. Interestingly, celecoxib anticancer activity is presumed to occur by inhibiting COX-2[233-235] because this cyclooxygenase isoform is frequently expressed in many types of cancer and promotes carcinogenesis and resistance of cancer cells to chemotherapy[236]. Dai et al[237] found that celecoxib also targets the RNA-binding protein "“partner of NOB1"” (PNO1) and exerts antitumour activity through the AKT/mTOR pathway[237]. In addition, PNO1 has been reported to participate in the progression of lung, oesophageal, breast, bladder, and colorectal cancer[238-242]. Targeting PNO1 (which is overexpressed in HCC tissues) can inhibit cell apoptosis by promoting autophagy through the ERK/MAPK signalling pathway[243].
A clinical trial is currently ongoing to compare the effect of Celebrex alone, metformin alone, and the combination of both drugs in preventing HCC recurrence after hepatic resection (ClinicalTrials.gov identifier: NCT03184493). In preclinical trials, it was shown that the combination of the two in vitro and in vivo inhibited HCC proliferation more effectively than the effect of each drug alone[244].
Hydroxychloroquine is an antimalarial drug that has been evaluated as an antitumour agent in HCC and has even been used for the treatment of other cancers, either alone or in combination with other therapeutic agents[215,245]. This drug targets cancer cells and the tumour microenvironment; among its molecular mechanisms of action, it inhibits autophagosome-lysosome fusion and Toll-like receptor 9 (TLR9) signalling, along with TLR7, which are overexpressed in HCC and are involved in cell proliferation and inhibition of apoptosis[246]. Furthermore, Chen et al[247] reported that hydroxychloroquine and miRNA (hsa-miR-30a-5p) target and resensitize sorafenib-resistant HCC cells to sorafenib through impairment of autophagy and DNA damage by oxidative stress via the TLR9/SOD1/hsa-miR-30a-5p/Beclin-1 pathway[247]. Since one of the mechanisms of sorafenib resistance is the induction of autophagy, a prospective, phase II clinical trial is currently under way to evaluate the efficacy of sorafenib and hydroxychloroquine treatment in patients with advanced HCC (ClinicalTrials.gov identifier: NCT03037437).
The high mortality caused by liver cancer remains an important concern in oncology. Therefore, there is an urgent need to implement new therapeutic strategies to provide significant benefits in patients with advanced HCC.
Immunotherapy is a tool that has shown great promise in treating HCC. Nevertheless, it is necessary to continue developing immunotherapy agents, which increase understanding of the role of the immune response in the tumour and take advantage of this process.
In contrast, the use of combination therapy has shown very favourable results compared to the effect of monotherapy, reflected by the diversity of current clinical trials evaluating the impact of the combination of two or more agents in HCC. Drug combinations simultaneously targeting relevant signalling pathways in liver carcinogenesis provide at least four potential advantages: (1) Anticancer synergistic effects; (2) minimization of treatment resistance; (3) reduction of individual drug doses; and (4) the occurrence of minimal adverse events. These advantages should facilitate HCC treatment, making it extremely important to consider possible future drug combinations to achieve greater benefits for HCC patients.
Furthermore, growing evidence has supported that non-cancer drugs possess antineoplastic activity. In emergency situations such as cancer, drug repurposing can be a very useful strategy. Compared to the traditional process of developing new drugs, drug repurposing allows for the rapid and less costly discovery of new treatments, increasing the likelihood of success with the advantage that safety issues in humans have already been described. It is crucial to mention that drug repurposing does not replace the traditional process; both are extremely important. However, repurposing non-oncology drugs is an attractive strategy to obtain more treatment options for advanced HCC.
Simultaneously, implementing non-oncology drug repurposing and proposing combinations of non-oncology drugs with systemic therapy or immunotherapy are very attractive strategies to generate significant benefits in patients with unresectable HCC. In addition, new relevant signalling pathways, critical drug targets, and biomarkers of this cancer might be identified along the way, providing significant advantages for understanding liver carcinogenesis.
Currently, approved drug options for treating advanced HCC are limited, and the likelihood of generating resistance is high, making the use of novel pharmacological approaches urgent (Figure 2). Compared to monotherapy, this review demonstrated that combining therapies has resulted in more significant benefits for HCC patients. Furthermore, evidence has been provided indicating that several non-oncology drugs are potentially useful for the treatment of this cancer. In addition, immunotherapy has significant effects in some cases compared to current systemic therapy, making this approach, along with repositioning and combination therapy, promising for the pharmacological treatment of advanced liver cancer.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bao YZ, China; Han J, China; Wang C, China S-Editor: Fan JR L-Editor: A P-Editor: Zhao S
| 1. | Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F (2022). Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. [cited 14 October 2022]. Available from: https://gco.iarc.fr/today. |
| 2. | Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017;24:1073274817729245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 437] [Article Influence: 54.6] [Reference Citation Analysis (1)] |
| 3. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64698] [Article Influence: 16174.5] [Reference Citation Analysis (177)] |
| 4. | Bruix J, Han KH, Gores G, Llovet JM, Mazzaferro V. Liver cancer: Approaching a personalized care. J Hepatol. 2015;62:S144-S156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 5. | Khalaf N, Ying J, Mittal S, Temple S, Kanwal F, Davila J, El-Serag HB. Natural History of Untreated Hepatocellular Carcinoma in a US Cohort and the Role of Cancer Surveillance. Clin Gastroenterol Hepatol. 2017;15:273-281.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Zhu XD, Li KS, Sun HC. Adjuvant therapies after curative treatments for hepatocellular carcinoma: Current status and prospects. Genes Dis. 2020;7:359-369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Baudino TA. Targeted Cancer Therapy: The Next Generation of Cancer Treatment. Curr Drug Discov Technol. 2015;12:3-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 381] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 8. | Chow AK, Yau SW, Ng L. Novel molecular targets in hepatocellular carcinoma. World J Clin Oncol. 2020;11:589-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 9. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6065] [Article Influence: 866.4] [Reference Citation Analysis (3)] |
| 10. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2618] [Article Influence: 872.7] [Reference Citation Analysis (59)] |
| 11. | Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 409] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 12. | Abdelgalil AA, Alkahtani HM, Al-Jenoobi FI. Sorafenib. Profiles Drug Subst Excip Relat Methodol. 2019;44:239-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 13. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4653] [Article Influence: 273.7] [Reference Citation Analysis (0)] |
| 14. | Tang W, Chen Z, Zhang W, Cheng Y, Zhang B, Wu F, Wang Q, Wang S, Rong D, Reiter FP, De Toni EN, Wang X. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther. 2020;5:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 701] [Article Influence: 140.2] [Reference Citation Analysis (0)] |
| 15. | U.S. Food & Drug Administration. FDA approves lenvatinib for unresectable hepatocellular carcinoma. 2018 Ago 16 [Internet]. [cited 30 October 2022]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lenvatinib-unresectable-hepatocellular-carcinoma. |
| 16. | Al-Salama ZT, Syed YY, Scott LJ. Lenvatinib: A Review in Hepatocellular Carcinoma. Drugs. 2019;79:665-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 17. | Vogel A, Bathon M, Saborowski A. Advances in systemic therapy for the first-line treatment of unresectable HCC. Expert Rev Anticancer Ther. 2021;21:621-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Matsuki M, Hoshi T, Yamamoto Y, Ikemori-Kawada M, Minoshima Y, Funahashi Y, Matsui J. Lenvatinib inhibits angiogenesis and tumor fibroblast growth factor signaling pathways in human hepatocellular carcinoma models. Cancer Med. 2018;7:2641-2653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (1)] |
| 19. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3832] [Article Influence: 547.4] [Reference Citation Analysis (1)] |
| 20. | Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3965] [Cited by in RCA: 4189] [Article Influence: 380.8] [Reference Citation Analysis (0)] |
| 21. | Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 704] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 22. | Finn RS, Bentley G, Britten CD, Amado R, Busuttil RW. Targeting vascular endothelial growth factor with the monoclonal antibody bevacizumab inhibits human hepatocellular carcinoma cells growing in an orthotopic mouse model. Liver Int. 2009;29:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; Imbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4707] [Article Influence: 941.4] [Reference Citation Analysis (2)] |
| 24. | Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from Imbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 963] [Article Influence: 321.0] [Reference Citation Analysis (0)] |
| 25. | U.S. Food & Drug Administration. FDA approves atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. 2020 Jun 01 [Internet]. [cited 30 October 2022]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-plus-bevacizumab-unresectable-hepatocellular-carcinoma. |
| 26. | U.S. Food & Drug Administration. FDA approves tremelimumab in combination with durvalumab for unresectable hepatocellular carcinoma. 2022 Oct 10 [Internet]. [cited 30 October 2022]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tremelimumab-combination-durvalumab-unresectable-hepatocellular-carcinoma. |
| 27. | Tarhini AA. Tremelimumab: a review of development to date in solid tumors. Immunotherapy. 2013;5:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, Davis JL, Hughes MS, Heller T, ElGindi M, Uppala A, Korangy F, Kleiner DE, Figg WD, Venzon D, Steinberg SM, Venkatesan AM, Krishnasamy V, Abi-Jaoudeh N, Levy E, Wood BJ, Greten TF. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 640] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 29. | Al-Salama ZT. Durvalumab: A Review in Extensive-Stage SCLC. Target Oncol. 2021;16:857-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeño J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Özgüroğlu M; PACIFIC Investigators. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2750] [Cited by in RCA: 3244] [Article Influence: 405.5] [Reference Citation Analysis (0)] |
| 31. | Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, Qin S, Tai DW, Lim HY, Yau T, Yong WP, Cheng AL, Gasbarrini A, Damian S, Bruix J, Borad M, Bendell J, Kim TY, Standifer N, He P, Makowsky M, Negro A, Kudo M, Abou-Alfa GK. Safety, Efficacy, and Pharmacodynamics of Tremelimumab Plus Durvalumab for Patients With Unresectable Hepatocellular Carcinoma: Randomized Expansion of a Phase I/II Study. J Clin Oncol. 2021;39:2991-3001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 330] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 32. | Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, Sukeepaisarnjaroen W, Kang YK, Van Dao T, De Toni EN, Rimassa L, Breder V, Vasilyev A, Heurgué A, Tam VC, Mody K, Thungappa SC, Ostapenko Y, Yau T, Azevedo S, Varela M, Chrng AL, Qin S, Galle PR, Ali S, Marcovitz M, Makowsky M, He P, Kurland JF, Negro A, Sangro B. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evidence. 2022;1. [RCA] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 662] [Article Influence: 220.7] [Reference Citation Analysis (0)] |
| 33. | Frenette C, Blanc JF, Cheng AL, Finn R, Galle, P, Pfiffer T, Sun HC. The importance of systemic treatment sequencing in improving survival for patients with hepatocellular carcinoma. Bayer. [cited 10 September 2020]. Available from: https://www.bayer.com/sites/default/files/Bayer_HCC_Expert_Statement_1.pdf. |
| 34. | Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schütz G, Thierauch KH, Zopf D. Regorafenib (BAY 73-4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer. 2011;129:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 1022] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 35. | Carr BI, Cavallini A, Lippolis C, D’Alessandro R, Messa C, Refolo MG, Tafaro A. Fluoro-Sorafenib (Regorafenib) effects on hepatoma cells: growth inhibition, quiescence, and recovery. J Cell Physiol. 2013;228:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 36. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2718] [Article Influence: 339.8] [Reference Citation Analysis (0)] |
| 37. | Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, Qian F, Chu F, Bentzien F, Cancilla B, Orf J, You A, Laird AD, Engst S, Lee L, Lesch J, Chou YC, Joly AH. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10:2298-2308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 1021] [Article Influence: 72.9] [Reference Citation Analysis (0)] |
| 38. | Kelley RK, Verslype C, Cohn AL, Yang TS, Su WC, Burris H, Braiteh F, Vogelzang N, Spira A, Foster P, Lee Y, Van Cutsem E. Cabozantinib in hepatocellular carcinoma: results of a phase 2 placebo-controlled randomized discontinuation study. Ann Oncol. 2017;28:528-534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 39. | Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1630] [Cited by in RCA: 1771] [Article Influence: 253.0] [Reference Citation Analysis (0)] |
| 40. | U.S. Food & Drug Administration. FDA approves cabozantinib for hepatocellular carcinoma. 2019 Mar 03 [Internet]. [cited 30 October 2022]. Available from: https://www.fda.gov/drugs/fda-approves-cabozantinib-hepatocellular-carcinoma. |
| 41. | Esteban-Fabró R, Willoughby CE, Piqué-Gili M, Montironi C, Abril-Fornaguera J, Peix J, Torrens L, Mesropian A, Balaseviciute U, Miró-Mur F, Mazzaferro V, Pinyol R, Llovet JM. Cabozantinib Enhances Anti-PD1 Activity and Elicits a Neutrophil-Based Immune Response in Hepatocellular Carcinoma. Clin Cancer Res. 2022;28:2449-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 42. | Syed YY. Ramucirumab: A Review in Hepatocellular Carcinoma. Drugs. 2020;80:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Turkes F, Chau I. Ramucirumab and its use in the treatment of hepatocellular carcinoma. Future Oncol. 2019;15:979-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE, Okusaka T, Kubackova K, Trojan J, Sastre J, Chau I, Chang SC, Abada PB, Yang L, Schwartz JD, Kudo M; REACH Trial Investigators. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 655] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 45. | Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1251] [Article Influence: 208.5] [Reference Citation Analysis (0)] |
| 46. | Komatsu S, Yano Y, Kido M, Kuramitsu K, Gon H, Fukushima K, Urade T, So S, Yanagimoto H, Toyama H, Kodama Y, Fukumoto T. Lenvatinib Rechallenge After Ramucirumab Treatment Failure for Hepatocellular Carcinoma. Anticancer Res. 2021;41:4555-4562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 783] [Article Influence: 130.5] [Reference Citation Analysis (0)] |
| 48. | Franzin R, Netti GS, Spadaccino F, Porta C, Gesualdo L, Stallone G, Castellano G, Ranieri E. The Use of Immune Checkpoint Inhibitors in Oncology and the Occurrence of AKI: Where Do We Stand? Front Immunol. 2020;11:574271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 49. | He X, Xu C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 2020;30:660-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 856] [Article Influence: 171.2] [Reference Citation Analysis (0)] |
| 50. | Keilson JM, Knochelmann HM, Paulos CM, Kudchadkar RR, Lowe MC. The evolving landscape of immunotherapy in solid tumors. J Surg Oncol. 2021;123:798-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 51. | Onuma AE, Zhang H, Huang H, Williams TM, Noonan A, Tsung A. Immune Checkpoint Inhibitors in Hepatocellular Cancer: Current Understanding on Mechanisms of Resistance and Biomarkers of Response to Treatment. Gene Expr. 2020;20:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 52. | Xu F, Jin T, Zhu Y, Dai C. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 284] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 53. | Lai E, Astara G, Ziranu P, Pretta A, Migliari M, Dubois M, Donisi C, Mariani S, Liscia N, Impera V, Persano M, Tolu S, Balconi F, Pinna G, Spanu D, Pireddu A, Saba G, Camera S, Musio F, Puzzoni M, Pusceddu V, Madeddu C, Casadei Gardini A, Scartozzi M. Introducing immunotherapy for advanced hepatocellular carcinoma patients: Too early or too fast? Crit Rev Oncol Hematol. 2021;157:103167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 54. | Liu Z, Liu X, Liang J, Liu Y, Hou X, Zhang M, Li Y, Jiang X. Immunotherapy for Hepatocellular Carcinoma: Current Status and Future Prospects. Front Immunol. 2021;12:765101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 55. | Finkelmeier F, Waidmann O, Trojan J. Nivolumab for the treatment of hepatocellular carcinoma. Expert Rev Anticancer Ther. 2018;18:1169-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 56. | Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2598] [Cited by in RCA: 2805] [Article Influence: 280.5] [Reference Citation Analysis (0)] |
| 57. | Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinó L, Blumenschein GR, Antonia SJ, Dorange C, Harbison CT, Finckenstein FG, Brahmer JR. Nivolumab vs Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6945] [Cited by in RCA: 7537] [Article Influence: 753.7] [Reference Citation Analysis (0)] |
| 58. | Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, Yoshikawa T, Oh SC, Bai LY, Tamura T, Lee KW, Hamamoto Y, Kim JG, Chin K, Oh DY, Minashi K, Cho JY, Tsuda M, Chen LT. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1283] [Cited by in RCA: 1716] [Article Influence: 214.5] [Reference Citation Analysis (0)] |
| 59. | Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, Castellano D, Choueiri TK, Gurney H, Donskov F, Bono P, Wagstaff J, Gauler TC, Ueda T, Tomita Y, Schutz FA, Kollmannsberger C, Larkin J, Ravaud A, Simon JS, Xu LA, Waxman IM, Sharma P; CheckMate 025 Investigators. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1803-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4375] [Cited by in RCA: 4605] [Article Influence: 460.5] [Reference Citation Analysis (0)] |
| 60. | Weber JS, D’Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD, Linette GP, Thomas L, Lorigan P, Grossmann KF, Hassel JC, Maio M, Sznol M, Ascierto PA, Mohr P, Chmielowski B, Bryce A, Svane IM, Grob JJ, Krackhardt AM, Horak C, Lambert A, Yang AS, Larkin J. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1853] [Cited by in RCA: 2106] [Article Influence: 210.6] [Reference Citation Analysis (0)] |
| 61. | Kudo M, Matilla A, Santoro A, Melero I, Gracián AC, Acosta-Rivera M, Choo SP, El-Khoueiry AB, Kuromatsu R, El-Rayes B, Numata K, Itoh Y, Di Costanzo F, Crysler O, Reig M, Shen Y, Neely J, Tschaika M, Wisniewski T, Sangro B. CheckMate 040 cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J Hepatol. 2021;75:600-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 62. | Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Sieghart W, Assenat E, Zaucha R, Furuse J, Abou-Alfa GK, El-Khoueiry AB, Melero I, Begic D, Chen G, Neely J, Wisniewski T, Tschaika M, Sangro B. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 746] [Article Influence: 186.5] [Reference Citation Analysis (0)] |
| 63. | U.S. Food & Drug Administration. FDA grants accelerated approval to nivolumab for HCC previously treated with sorafenib. 2017 Sep 25 [Internet]. [cited 30 October 2022]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-hcc-previously-treated-sorafenib. |
| 64. | Tsang J, Wong JSL, Kwok GGW, Li BCW, Leung R, Chiu J, Cheung TT, Yau T. Nivolumab + Ipilimumab for patients with hepatocellular carcinoma previously treated with Sorafenib. Expert Rev Gastroenterol Hepatol. 2021;15:589-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, Kudo M, Hou MM, Matilla A, Tovoli F, Knox JJ, Ruth He A, El-Rayes BF, Acosta-Rivera M, Lim HY, Neely J, Shen Y, Wisniewski T, Anderson J, Hsu C. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020;6:e204564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 908] [Cited by in RCA: 971] [Article Influence: 194.2] [Reference Citation Analysis (0)] |
| 66. | U.S. Food & Drug Administration. FDA grants accelerated approval to nivolumab and ipilimumab combination for hepatocellular carcinoma. 2020 Mar 03 [Internet]. [cited 31 October 2022]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-nivolumab-and-ipilimumab-combination-hepatocellular-carcinoma. |
| 67. | Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A, Sarker D, Verset G, Chan SL, Knox J, Daniele B, Webber AL, Ebbinghaus SW, Ma J, Siegel AB, Cheng AL, Kudo M; KEYNOTE-224 investigators. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1902] [Article Influence: 271.7] [Reference Citation Analysis (0)] |
| 68. | U.S. Food & Drug Administration. FDA grants accelerated approval to pembrolizumab for hepatocellular carcinoma. 2018 Dic 14 [Internet]. [cited 31 October 2022]. Available from: https://www.fda.gov/drugs/fda-grants-accelerated-approval-pembrolizumab-hepatocellular-carcinoma. |
| 69. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL; KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1343] [Article Influence: 268.6] [Reference Citation Analysis (0)] |
| 70. | Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo BY, Meng Z, Bai Y, Chen X, Liu X, Xiao J, Ho GF, Mao Y, Ye X, Ying J, Li J, Zhong WY, Zhou Y, Siegel AB, Hao C. Pembrolizumab plus best supportive care vs placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase 3 KEYNOTE-394 study. J Clin Oncol. 2022;40 Suppl 4:383-383. [RCA] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 71. | Bayat Mokhtari R, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, Yeger H. Combination therapy in combating cancer. Oncotarget. 2017;8:38022-38043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 890] [Cited by in RCA: 1568] [Article Influence: 224.0] [Reference Citation Analysis (0)] |
| 72. | Kim GP, Mahoney MR, Szydlo D, Mok TS, Marshke R, Holen K, Picus J, Boyer M, Pitot HC, Rubin J, Philip PA, Nowak A, Wright JJ, Erlichman C. An international, multicenter phase II trial of bortezomib in patients with hepatocellular carcinoma. Invest New Drugs. 2012;30:387-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 73. | He B, Lu C, Zheng G, He X, Wang M, Chen G, Zhang G, Lu A. Combination therapeutics in complex diseases. J Cell Mol Med. 2016;20:2231-2240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 74. | Pemovska T, Bigenzahn JW, Superti-Furga G. Recent advances in combinatorial drug screening and synergy scoring. Curr Opin Pharmacol. 2018;42:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 75. | Lim JJ, Hooi L, Dan YY, Bonney GK, Zhou L, Chow PK, Chee CE, Toh TB, Chow EK. Rational drug combination design in patient-derived avatars reveals effective inhibition of hepatocellular carcinoma with proteasome and CDK inhibitors. J Exp Clin Cancer Res. 2022;41:249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 76. | Xu F, Tong M, Tong CSW, Chan BKC, Chu HY, Wong TL, Fong JHC, Cheung MSH, Mak KH, Pardeshi L, Huang Y, Wong KH, Choi GCG, Ma S, Wong ASL. A Combinatorial CRISPR-Cas9 Screen Identifies Ifenprodil as an Adjunct to Sorafenib for Liver Cancer Treatment. Cancer Res. 2021;81:6219-6232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 77. | Canzoneri R, Lacunza E, Abba MC. Genomics and bioinformatics as pillars of precision medicine in oncology. Medicina (B Aires). 2019;79:587-592. [PubMed] |
| 78. | Suwinski P, Ong C, Ling MHT, Poh YM, Khan AM, Ong HS. Advancing Personalized Medicine Through the Application of Whole Exome Sequencing and Big Data Analytics. Front Genet. 2019;10:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 79. | Wouters OJ, McKee M, Luyten J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA. 2020;323:844-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 808] [Cited by in RCA: 749] [Article Influence: 149.8] [Reference Citation Analysis (0)] |
| 80. | Corsello SM, Nagari RT, Spangler RD, Rossen J, Kocak M, Bryan JG, Humeidi R, Peck D, Wu X, Tang AA, Wang VM, Bender SA, Lemire E, Narayan R, Montgomery P, Ben-David U, Garvie CW, Chen Y, Rees MG, Lyons NJ, McFarland JM, Wong BT, Wang L, Dumont N, O’Hearn PJ, Stefan E, Doench JG, Harrington CN, Greulich H, Meyerson M, Vazquez F, Subramanian A, Roth JA, Bittker JA, Boehm JS, Mader CC, Tsherniak A, Golub TR. Discovering the anti-cancer potential of non-oncology drugs by systematic viability profiling. Nat Cancer. 2020;1:235-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 511] [Article Influence: 102.2] [Reference Citation Analysis (0)] |
| 81. | Shah RR, Stonier PD. Repurposing old drugs in oncology: Opportunities with clinical and regulatory challenges ahead. J Clin Pharm Ther. 2019;44:6-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 82. | Nair G, Saraswathy GR, Hema Sree GNS. 48P Target mining and drug repurposing for hepatocellular carcinoma via bioinformatic and computational approaches. Ann Oncol. 2021;32:S19. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 83. | Nair G, Hema Sree GNS, Saraswathy GR, Marise VLP, Krishna Murthy TP. Application of comprehensive bioinformatics approaches to reconnoiter crucial genes and pathways underpinning hepatocellular carcinoma: a drug repurposing endeavor. Med Oncol. 2021;38:145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 84. | Massari NA, Nicoud MB, Medina VA. Histamine receptors and cancer pharmacology: an update. Br J Pharmacol. 2020;177:516-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 85. | Nguyen PL, Cho J. Pathophysiological Roles of Histamine Receptors in Cancer Progression: Implications and Perspectives as Potential Molecular Targets. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 86. | Zhao J, Hou Y, Yin C, Hu J, Gao T, Huang X, Zhang X, Xing J, An J, Wan S, Li J. Upregulation of histamine receptor H1 promotes tumor progression and contributes to poor prognosis in hepatocellular carcinoma. Oncogene. 2020;39:1724-1738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 87. | Shen YC, Hsu HC, Lin TM, Chang YS, Hu LF, Chen LF, Lin SH, Kuo PI, Chen WS, Lin YC, Chen JH, Liang YC, Chang CC. H1-Antihistamines Reduce the Risk of Hepatocellular Carcinoma in Patients With Hepatitis B Virus, Hepatitis C Virus, or Dual Hepatitis B Virus-Hepatitis C Virus Infection. J Clin Oncol. 2022;40:1206-1219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 88. | Ellegaard AM, Dehlendorff C, Vind AC, Anand A, Cederkvist L, Petersen NHT, Nylandsted J, Stenvang J, Mellemgaard A, Østerlind K, Friis S, Jäättelä M. Repurposing Cationic Amphiphilic Antihistamines for Cancer Treatment. EbioMedicine. 2016;9:130-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 89. | Ortiz CS, Montante-Montes D, Saqui-Salces M, Hinojosa LM, Gamboa-Dominguez A, Hernández-Gallegos E, Martínez-Benítez B, Del Rosario Solís-Pancoatl M, Garcia-Villa E, Ramírez A, Aguilar-Guadarrama R, Gariglio P, Pardo LA, Stühmer W, Camacho J. Eag1 potassium channels as markers of cervical dysplasia. Oncol Rep. 2011;26:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 90. | Chen J, Xuan Z, Song W, Han W, Chen H, Du Y, Xie H, Zhao Y, Zheng S, Song P. EAG1 enhances hepatocellular carcinoma proliferation by modulating SKP2 and metastasis through pseudopod formation. Oncogene. 2021;40:163-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 91. | García-Quiroz J, Camacho J. Astemizole: an old anti-histamine as a new promising anti-cancer drug. Anticancer Agents Med Chem. 2011;11:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 92. | Hemmerlein B, Weseloh RM, Mello de Queiroz F, Knötgen H, Sánchez A, Rubio ME, Martin S, Schliephacke T, Jenke M, Heinz-Joachim-Radzun, Stühmer W, Pardo LA. Overexpression of Eag1 potassium channels in clinical tumours. Mol Cancer. 2006;5:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 93. | Toplak Ž, Hendrickx LA, Abdelaziz R, Shi X, Peigneur S, Tomašič T, Tytgat J, Peterlin-Mašič L, Pardo LA. Overcoming challenges of HERG potassium channel liability through rational design: Eag1 inhibitors for cancer treatment. Med Res Rev. 2022;42:183-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 94. | Rodríguez-Rasgado JA, Acuña-Macías I, Camacho J. Eag1 channels as potential cancer biomarkers. Sensors (Basel). 2012;12:5986-5995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 95. | Asher V, Sowter H, Shaw R, Bali A, Khan R. Eag and HERG potassium channels as novel therapeutic targets in cancer. World J Surg Oncol. 2010;8:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Smith GA, Tsui HW, Newell EW, Jiang X, Zhu XP, Tsui FW, Schlichter LC. Functional up-regulation of HERG K+ channels in neoplastic hematopoietic cells. J Biol Chem. 2002;277:18528-18534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 97. | Shao XD, Wu KC, Hao ZM, Hong L, Zhang J, Fan DM. The potent inhibitory effects of cisapride, a specific blocker for human ether-a-go-go-related gene (HERG) channel, on gastric cancer cells. Cancer Biol Ther. 2005;4:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 98. | de Guadalupe Chávez-López M, Pérez-Carreón JI, Zuñiga-García V, Díaz-Chávez J, Herrera LA, Caro-Sánchez CH, Acuña-Macías I, Gariglio P, Hernández-Gallegos E, Chiliquinga AJ, Camacho J. Astemizole-based anticancer therapy for hepatocellular carcinoma (HCC), and Eag1 channels as potential early-stage markers of HCC. Tumour Biol. 2015;36:6149-6158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Gui L, Xu Q, Huang J, Wu G, Tang H, Hui L, Hua P, Zhang L, Zhu Y. CYP2J2 promotes the development of hepatocellular carcinoma by increasing the EETs production to improve HIF-1α stability. Am J Transl Res. 2020;12:7923-7937. [PubMed] |
| 100. | Hwang GH, Park SM, Han HJ, Baek KM, Kim JS, Chang W, Lee HJ, Yun SP, Ryu JM, Lee MY. Role of cytochrome P450 2J2 on cell proliferation and resistance to an anticancer agent in hepatocellular carcinoma HepG2 cells. Oncol Lett. 2017;14:5484-5490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 101. | Jeon YJ, Kim JS, Hwang GH, Wu Z, Han HJ, Park SH, Chang W, Kim LK, Lee YM, Liu KH, Lee MY. Inhibition of cytochrome P450 2J2 by tanshinone IIA induces apoptotic cell death in hepatocellular carcinoma HepG2 cells. Eur J Pharmacol. 2015;764:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 102. | Lee CA, Jones JP 3rd, Katayama J, Kaspera R, Jiang Y, Freiwald S, Smith E, Walker GS, Totah RA. Identifying a selective substrate and inhibitor pair for the evaluation of CYP2J2 activity. Drug Metab Dispos. 2012;40:943-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 103. | Van den Eynde C, Held K, Ciprietti M, De Clercq K, Kerselaers S, Marchand A, Chaltin P, Voets T, Vriens J. Loratadine, an antihistaminic drug, suppresses the proliferation of endometrial stromal cells by inhibition of TRPV2. Eur J Pharmacol. 2022;928:175086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 104. | Sidhu G, Akhondi H. Loratadine. In StatPearls. StatPearls Publishing 2022. [cited 31 October 2022]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK542278/. |
| 105. | Fritz I, Wagner P, Broberg P, Einefors R, Olsson H. Desloratadine and loratadine stand out among common H(1)-antihistamines for association with improved breast cancer survival. Acta Oncol. 2020;59:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 106. | Fritz I, Wagner P, Olsson H. Improved survival in several cancers with use of H(1)-antihistamines desloratadine and loratadine. Transl Oncol. 2021;14:101029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 107. | Kallunki T, Olsen OD, Jäättelä M. Cancer-associated lysosomal changes: friends or foes? Oncogene. 2013;32:1995-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 108. | Adly N. Evaluation of cytotoxic potential of loratadine and the combination of loratadine and cisplatin on hepatocellular carcinoma cell lines [American University in Cairo (AUC)] 2018. [cited 31 October 2022]. Available from: https://fount.aucegypt.edu/etds/418. |
| 109. | Hunto ST, Kim HG, Baek KS, Jeong D, Kim E, Kim JH, Cho JY. Loratadine, an antihistamine drug, exhibits anti-inflammatory activity through suppression of the NF-(k)B pathway. Biochem Pharmacol. 2020;177:113949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 110. | de Vries TW, Tobi H, Schirm E, van den Berg P, Duiverman EJ, de Jong-van den Berg LT. The gap between evidence-based medicine and daily practice in the management of paediatric asthma. A pharmacy-based population study from The Netherlands. Eur J Clin Pharmacol. 2006;62:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Liang YC, Chang CC, Sheu MT, Lin SY, Chung CC, Teng CT, Suk FM. The Antihistamine Deptropine Induces Hepatoma Cell Death through Blocking Autophagosome-Lysosome Fusion. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 112. | Feng YM, Feng CW, Chen SY, Hsieh HY, Chen YH, Hsu CD. Cyproheptadine, an antihistaminic drug, inhibits proliferation of hepatocellular carcinoma cells by blocking cell cycle progression through the activation of P38 MAP kinase. BMC Cancer. 2015;15:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 113. | Hsieh MC, Lee WH, Wu AT, Chow JM, Chang CL, Yuan KS, Wu SY. Cyproheptadine use in hepatocellular carcinoma. Am J Cancer Res. 2017;7:584-602. [PubMed] |
| 114. | Feng YM, Chen TH, Berman D, Chou CK, Liao KS, Hsieh MC, Chen CY. Efficacy of Cyproheptadine Monotherapy in Hepatocellular Carcinoma With Bone Metastasis: A Case Report. Front Oncol. 2021;11:620212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 115. | Feng YM, Feng CW, Chen SC, Hsu CD. Unexpected remission of hepatocellular carcinoma (HCC) with lung metastasis to the combination therapy of thalidomide and cyproheptadine: report of two cases and a preliminary HCC cell line study. BMJ Case Rep. 2012;2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 116. | Feng YM, Feng CW, Lu CL, Lee MY, Chen CY, Chen SC. Cyproheptadine significantly improves the overall and progression-free survival of sorafenib-treated advanced HCC patients. Jpn J Clin Oncol. 2015;45:336-342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 117. | Shi Z, Fultz RS, Engevik MA, Gao C, Hall A, Major A, Mori-Akiyama Y, Versalovic J. Distinct roles of histamine H1- and H2-receptor signaling pathways in inflammation-associated colonic tumorigenesis. Am J Physiol Gastrointest Liver Physiol. 2019;316:G205-G216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 118. | Merétey K, Falus A, Taga T, Kishimoto T. Histamine influences the expression of the interleukin-6 receptor on human lymphoid, monocytoid and hepatoma cell lines. Agents Actions. 1991;33:189-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 119. | Lampiasi N, Azzolina A, Montalto G, Cervello M. Histamine and spontaneously released mast cell granules affect the cell growth of human hepatocellular carcinoma cells. Exp Mol Med. 2007;39:284-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 120. | Fujikawa T, Shiraha H, Nakanishi Y, Takaoka N, Ueda N, Suzuki M, Shiratori Y. Cimetidine inhibits epidermal growth factor-induced cell signaling. J Gastroenterol Hepatol. 2007;22:436-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 121. | Furuta K, Sato S, Miyake T, Okamoto E, Ishine J, Ishihara S, Amano Y, Adachi K, Kinoshita Y. Anti-tumor effects of cimetidine on hepatocellular carcinomas in diethylnitrosamine-treated rats. Oncol Rep. 2008;19:361-368. [PubMed] |
| 122. | Crouchet E, Bandiera S, Fujiwara N, Li S, El Saghire H, Fernández-Vaquero M, Riedl T, Sun X, Hirschfield H, Jühling F, Zhu S, Roehlen N, Ponsolles C, Heydmann L, Saviano A, Qian T, Venkatesh A, Lupberger J, Verrier ER, Sojoodi M, Oudot MA, Duong FHT, Masia R, Wei L, Thumann C, Durand SC, González-Motos V, Heide D, Hetzer J, Nakagawa S, Ono A, Song WM, Higashi T, Sanchez R, Kim RS, Bian CB, Kiani K, Croonenborghs T, Subramanian A, Chung RT, Straub BK, Schuppan D, Ankavay M, Cocquerel L, Schaeffer E, Goossens N, Koh AP, Mahajan M, Nair VD, Gunasekaran G, Schwartz ME, Bardeesy N, Shalek AK, Rozenblatt-Rosen O, Regev A, Felli E, Pessaux P, Tanabe KK, Heikenwälder M, Schuster C, Pochet N, Zeisel MB, Fuchs BC, Hoshida Y, Baumert TF. A human liver cell-based system modeling a clinical prognostic liver signature for therapeutic discovery. Nat Commun. 2021;12:5525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 123. | Tanaka T, Kochi T, Shirakami Y, Mori T, Kurata A, Watanabe N, Moriwaki H, Shimizu M. Cimetidine and Clobenpropit Attenuate Inflammation-Associated Colorectal Carcinogenesis in Male ICR Mice. Cancers (Basel). 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 124. | Cricco GP, Mohamad NA, Sambuco LA, Genre F, Croci M, Gutiérrez AS, Medina VA, Bergoc RM, Rivera ES, Martín GA. Histamine regulates pancreatic carcinoma cell growth through H3 and H4 receptors. Inflamm Res. 2008;57 Suppl 1:S23-S24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 125. | Yu D, Zhao J, Wang Y, Hu J, Zhao Q, Li J, Zhu J. Upregulated histamine receptor H3 promotes tumor growth and metastasis in hepatocellular carcinoma. Oncol Rep. 2019;41:3347-3354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 126. | Zhang C, Yu Y, Ma L, Fu P. Histamine H3 Receptor Promotes Cell Survival via Regulating PKA/CREB/CDKN1A Signal Pathway in Hepatocellular Carcinoma. Onco Targets Ther. 2020;13:3765-3776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 127. | Nicoud MB, Formoso K, Medina VA. Pathophysiological Role of Histamine H4 Receptor in Cancer: Therapeutic Implications. Front Pharmacol. 2019;10:556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 128. | Shi L, Feng Y, Lin H, Ma R, Cai X. Role of estrogen in hepatocellular carcinoma: is inflammation the key? J Transl Med. 2014;12:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 129. | Villa E. Role of estrogen in liver cancer. Womens Health (Lond). 2008;4:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 130. | Wei Q, Guo P, Mu K, Zhang Y, Zhao W, Huai W, Qiu Y, Li T, Ma X, Liu Y, Chen X, Han L. Estrogen suppresses hepatocellular carcinoma cells through Erβ-mediated upregulation of the NLRP3 inflammasome. Lab Invest. 2015;95:804-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 131. | Brady CW. Liver disease in menopause. World J Gastroenterol. 2015;21:7613-7620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 87] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 132. | Shimizu I. Impact of oestrogens on the progression of liver disease. Liver Int. 2003;23:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 133. | Sukocheva OA. Estrogen, estrogen receptors, and hepatocellular carcinoma: Are we there yet? World J Gastroenterol. 2018;24:1-4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 134. | Villa E, Colantoni A, Cammà C, Grottola A, Buttafoco P, Gelmini R, Ferretti I, Manenti F. Estrogen receptor classification for hepatocellular carcinoma: comparison with clinical staging systems. J Clin Oncol. 2003;21:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 135. | Matsushima-Nishiwaki R, Yamada N, Hattori Y, Hosokawa Y, Tachi J, Hori T, Kozawa O. SERMs (selective estrogen receptor modulator), acting as estrogen receptor β agonists in hepatocellular carcinoma cells, inhibit the transforming growth factor-α-induced migration via specific inhibition of AKT signaling pathway. PloS One. 2022;17:e0262485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 136. | Pinkerton JV. Selective Estrogen Receptor Modulators in Gynecology Practice. Clin Obstet Gynecol. 2021;64:803-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 137. | Wang Y, Ma H, Zhao C, Liu T, Yan D, Jou D, Li H, Zhang C, Lü J, Li C, Lin J, Li S, Lin L. Growth-suppressive activity of raloxifene on liver cancer cells by targeting IL-6/GP130 signaling. Oncotarget. 2017;8:33683-33693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 138. | Hsia CY, Huo TI, Chiang SY, Lu MF, Sun CL, Wu JC, Lee PC, Chi CW, Lui WY, Lee SD. Evaluation of interleukin-6, interleukin-10 and human hepatocyte growth factor as tumor markers for hepatocellular carcinoma. Eur J Surg Oncol. 2007;33:208-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 139. | Nakagawa H, Maeda S, Yoshida H, Tateishi R, Masuzaki R, Ohki T, Hayakawa Y, Kinoshita H, Yamakado M, Kato N, Shiina S, Omata M. Serum IL-6 levels and the risk for hepatocarcinogenesis in chronic hepatitis C patients: an analysis based on gender differences. Int J Cancer. 2009;125:2264-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 140. | Xu J, Lin H, Wu G, Zhu M, Li M. IL-6/STAT3 Is a Promising Therapeutic Target for Hepatocellular Carcinoma. Front Oncol. 2021;11:760971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 141. | Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1362] [Cited by in RCA: 1490] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 142. | Svinka J, Mikulits W, Eferl R. STAT3 in hepatocellular carcinoma: new perspectives. Hepat Oncol. 2014;1:107-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 143. | Mutschler J, Grosshans M, Soyka M, Rösner S. Current Findings and Mechanisms of Action of Disulfiram in the Treatment of Alcohol Dependence. Pharmacopsychiatry. 2016;49:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 144. | Zha J, Chen F, Dong H, Shi P, Yao Y, Zhang Y, Li R, Wang S, Li P, Wang W, Xu B. Disulfiram targeting lymphoid malignant cell lines via ROS-JNK activation as well as Nrf2 and NF-kB pathway inhibition. J Transl Med. 2014;12:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 145. | Liu P, Kumar IS, Brown S, Kannappan V, Tawari PE, Tang JZ, Jiang W, Armesilla AL, Darling JL, Wang W. Disulfiram targets cancer stem-like cells and reverses resistance and cross-resistance in acquired paclitaxel-resistant triple-negative breast cancer cells. Br J Cancer. 2013;109:1876-1885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 146. | Wang Y, Li W, Patel SS, Cong J, Zhang N, Sabbatino F, Liu X, Qi Y, Huang P, Lee H, Taghian A, Li JJ, DeLeo AB, Ferrone S, Epperly MW, Ferrone CR, Ly A, Brachtel EF, Wang X. Blocking the formation of radiation-induced breast cancer stem cells. Oncotarget. 2014;5:3743-3755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 91] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 147. | Cong J, Wang Y, Zhang X, Zhang N, Liu L, Soukup K, Michelakos T, Hong T, DeLeo A, Cai L, Sabbatino F, Ferrone S, Lee H, Levina V, Fuchs B, Tanabe K, Lillemoe K, Ferrone C, Wang X. A novel chemoradiation targeting stem and nonstem pancreatic cancer cells by repurposing disulfiram. Cancer Lett. 2017;409:9-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 148. | Nechushtan H, Hamamreh Y, Nidal S, Gotfried M, Baron A, Shalev YI, Nisman B, Peretz T, Peylan-Ramu N. A phase Iib trial assessing the addition of disulfiram to chemotherapy for the treatment of metastatic non-small cell lung cancer. Oncologist. 2015;20:366-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 149. | Davis CI, Gu X, Kiefer RM, Ralle M, Gade TP, Brady DC. Altered copper homeostasis underlies sensitivity of hepatocellular carcinoma to copper chelation. Metallomics. 2020;12:1995-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 150. | Denoyer D, Masaldan S, La Fontaine S, Cater MA. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics. 2015;7:1459-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 579] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 151. | Li Y, Wang LH, Zhang HT, Wang YT, Liu S, Zhou WL, Yuan XZ, Li TY, Wu CF, Yang JY. Disulfiram combined with copper inhibits metastasis and epithelial-mesenchymal transition in hepatocellular carcinoma through the NF-κB and TGF-β pathways. J Cell Mol Med. 2018;22:439-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 152. | Kelley KC, Grossman KF, Brittain-Blankenship M, Thorne KM, Akerley WL, Terrazas MC, Kosak KM, Boucher KM, Buys SS, McGregor KA, Werner TL, Agarwal N, Weis JR, Sharma S, Ward JH, Kennedy TP, Sborov DW, Shami PJ. A Phase 1 dose-escalation study of disulfiram and copper gluconate in patients with advanced solid tumors involving the liver using S-glutathionylation as a biomarker. BMC Cancer. 2021;21:510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 153. | Czauderna C, Castven D, Mahn FL, Marquardt JU. Context-Dependent Role of NF-κB Signaling in Primary Liver Cancer-from Tumor Development to Therapeutic Implications. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 154. | Luedde T, Schwabe RF. NF-κB in the liver–linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1094] [Cited by in RCA: 1089] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 155. | Yip NC, Fombon IS, Liu P, Brown S, Kannappan V, Armesilla AL, Xu B, Cassidy J, Darling JL, Wang W. Disulfiram modulated ROS-MAPK and NFκB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br J Cancer. 2011;104:1564-1574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 347] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 156. | Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4877] [Cited by in RCA: 5118] [Article Influence: 222.5] [Reference Citation Analysis (0)] |
| 157. | Zhang G, Wang Y, Fuchs BC, Guo W, Drum DL, Erstad DJ, Shi B, DeLeo AB, Zheng H, Cai L, Zhang L, Tanabe KK, Wang X. Improving the Therapeutic Efficacy of Sorafenib for Hepatocellular Carcinoma by Repurposing Disulfiram. Front Oncol. 2022;12:913736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 158. | Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1164] [Cited by in RCA: 1072] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 159. | Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol. 2016;99:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 410] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 160. | Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461-1473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1646] [Cited by in RCA: 1894] [Article Influence: 236.8] [Reference Citation Analysis (0)] |
| 161. | Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 917] [Article Influence: 183.4] [Reference Citation Analysis (0)] |
| 162. | Arzumanian VA, Kiseleva OI, Poverennaya EV. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 180] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 163. | He S, Tang S. WNT/β-catenin signaling in the development of liver cancers. Biomed Pharmacother. 2020;132:110851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 164. | Ahmed K, Koval A, Xu J, Bodmer A, Katanaev VL. Towards the first targeted therapy for triple-negative breast cancer: Repositioning of clofazimine as a chemotherapy-compatible selective Wnt pathway inhibitor. Cancer Lett. 2019;449:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 165. | Koval AV, Vlasov P, Shichkova P, Khunderyakova S, Markov Y, Panchenko J, Volodina A, Kondrashov FA, Katanaev VL. Anti-leprosy drug clofazimine inhibits growth of triple-negative breast cancer cells via inhibition of canonical Wnt signaling. Biochem Pharmacol. 2014;87:571-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 166. | Xu J, Koval A, Katanaev VL. Beyond TNBC: Repositioning of Clofazimine Against a Broad Range of Wnt-Dependent Cancers. Front Oncol. 2020;10:602817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 167. | Van Rensburg CE, Van Staden AM, Anderson R. The riminophenazine agents clofazimine and B669 inhibit the proliferation of cancer cell lines in vitro by phospholipase A2-mediated oxidative and nonoxidative mechanisms. Cancer Res. 1993;53:318-323. [PubMed] |
| 168. | Ruff P, Chasen MR, Long JE, van Rensburg CE. A phase II study of oral clofazimine in unresectable and metastatic hepatocellular carcinoma. Ann Oncol. 1998;9:217-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 169. | Sato B. Can an autocrine loop explain sex-hormone-dependent tumor growth? Oncology. 1999;57 Suppl 2:3-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 170. | Venkatesan P. Albendazole. J Antimicrob Chemother. 1998;41:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 171. | Kim U, Shin C, Kim CY, Ryu B, Kim J, Bang J, Park JH. Albendazole exerts antiproliferative effects on prostate cancer cells by inducing reactive oxygen species generation. Oncol Lett. 2021;21:395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 172. | Patel K, Doudican NA, Schiff PB, Orlow SJ. Albendazole sensitizes cancer cells to ionizing radiation. Radiat Oncol. 2011;6:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 173. | Petersen JSSM, Baird SK. Treatment of breast and colon cancer cell lines with anti-helmintic benzimidazoles mebendazole or albendazole results in selective apoptotic cell death. J Cancer Res Clin Oncol. 2021;147:2945-2953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 174. | Zhou F, Du J, Wang J. Albendazole inhibits HIF-1α-dependent glycolysis and VEGF expression in non-small cell lung cancer cells. Mol Cell Biochem. 2017;428:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 175. | Zhu L, Yang Q, Hu R, Li Y, Peng Y, Liu H, Ye M, Zhang B, Zhang P, Liu-Smith F, Li H, Liu J. Novel therapeutic strategy for melanoma based on albendazole and the CDK4/6 inhibitor palbociclib. Sci Rep. 2022;12:5706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 176. | Pourgholami MH, Woon L, Almajd R, Akhter J, Bowery P, Morris DL. In vitro and in vivo suppression of growth of hepatocellular carcinoma cells by albendazole. Cancer Lett. 2001;165:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 177. | Smyj R, Wang XP, Han F. Pimozide. Profiles Drug Subst Excip Relat Methodol. 2012;37:287-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 178. | Egolf A, Coffey BJ. Current pharmacotherapeutic approaches for the treatment of Tourette syndrome. Drugs Today (Barc). 2014;50:159-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 179. | Dakir el-H, Pickard A, Srivastava K, McCrudden CM, Gross SR, Lloyd S, Zhang SD, Margariti A, Morgan R, Rudland PS, El-Tanani M. The anti-psychotic drug pimozide is a novel chemotherapeutic for breast cancer. Oncotarget. 2018;9:34889-34910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 180. | Li J, Qu P, Zhou XZ, Ji YX, Yuan S, Liu SP, Zhang QG. Pimozide inhibits the growth of breast cancer cells by alleviating the Warburg effect through the P53 signaling pathway. Biomed Pharmacother. 2022;150:113063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 181. | Kim U, Kim CY, Lee JM, Ryu B, Kim J, Shin C, Park JH. Pimozide Inhibits the Human Prostate Cancer Cells Through the Generation of Reactive Oxygen Species. Front Pharmacol. 2019;10:1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 182. | Ranjan A, Kaushik I, Srivastava SK. Pimozide Suppresses the Growth of Brain Tumors by Targeting STAT3-Mediated Autophagy. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 183. | Ren Y, Tao J, Jiang Z, Guo D, Tang J. Pimozide suppresses colorectal cancer via inhibition of Wnt/β-catenin signaling pathway. Life Sci. 2018;209:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 184. | Nelson EA, Walker SR, Weisberg E, Bar-Natan M, Barrett R, Gashin LB, Terrell S, Klitgaard JL, Santo L, Addorio MR, Ebert BL, Griffin JD, Frank DA. The STAT5 inhibitor pimozide decreases survival of chronic myelogenous leukemia cells resistant to kinase inhibitors. Blood. 2011;117:3421-3429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 185. | Fako V, Yu Z, Henrich CJ, Ransom T, Budhu AS, Wang XW. Inhibition of wnt/β-catenin Signaling in Hepatocellular Carcinoma by an Antipsychotic Drug Pimozide. Int J Biol Sci. 2016;12:768-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 186. | Noh CK, Wang HJ, Kim CM, Kim J, Yoon SY, Lee GH, Cho HJ, Yang MJ, Kim SS, Hwang JC, Cho SW, Roh J, Kim YB, Kim SJ, Kim BW, Cheong JY. EpCAM as a Predictive Marker of Tumor Recurrence and Survival in Patients Who Underwent Surgical Resection for Hepatocellular Carcinoma. Anticancer Res. 2018;38:4101-4109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 187. | Vasanthakumar S, Sasikala P, Padma M, Balachandar V, Venkatesh B, Ganesan S. EpCAM as a novel therapeutic target for hepatocellular carcinoma. J Oncol Sci. 2017;3:71-76. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 188. | Chen JJ, Zhang LN, Cai N, Zhang Z, Ji K. Antipsychotic agent pimozide promotes reversible proliferative suppression by inducing cellular quiescence in liver cancer. Oncol Rep. 2019;42:1101-1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 189. | National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 5284447, Natamycin. [cited 9 November 2022]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Natamycin. |
| 190. | Vasquez JL, Lai Y, Annamalai T, Jiang Z, Zhang M, Lei R, Zhang Z, Liu Y, Tse-Dinh YC, Agoulnik IU. Inhibition of base excision repair by natamycin suppresses prostate cancer cell proliferation. Biochimie. 2020;168:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 191. | An Y, Jiang J, Zhou L, Shi J, Jin P, Li L, Peng L, He S, Zhang W, Huang C, Zou B, Xie N. Peroxiredoxin 1 is essential for natamycin-triggered apoptosis and protective autophagy in hepatocellular carcinoma. Cancer Lett. 2021;521:210-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 192. | Aggarwal V, Tuli HS, Varol A, Thakral F, Yerer MB, Sak K, Varol M, Jain A, Khan MA, Sethi G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 915] [Cited by in RCA: 746] [Article Influence: 124.3] [Reference Citation Analysis (0)] |
| 193. | Reczek CR, Chandel NS. The Two Faces of Reactive Oxygen Species in Cancer. Annu Rev Cancer Biol. 2017;1:79-98. [RCA] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 329] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 194. | Singh R, Manna PP. Reactive oxygen species in cancer progression and its role in therapeutics. Explor Med. 2022;43-57. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 195. | Harris IS, DeNicola GM. The Complex Interplay between Antioxidants and ROS in Cancer. Trends Cell Biol. 2020;30:440-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 196. | Hampton MB, Vick KA, Skoko JJ, Neumann CA. Peroxiredoxin Involvement in the Initiation and Progression of Human Cancer. Antioxid Redox Signal. 2018;28:591-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 197. | Kim Y, Jang HH. The Role of Peroxiredoxin Family in Cancer Signaling. J Cancer Prev. 2019;24:65-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 198. | Sun YL, Cai JQ, Liu F, Bi XY, Zhou LP, Zhao XH. Aberrant expression of peroxiredoxin 1 and its clinical implications in liver cancer. World J Gastroenterol. 2015;21:10840-10852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 199. | Ghodke-Puranik Y, Thorn CF, Lamba JK, Leeder JS, Song W, Birnbaum AK, Altman RB, Klein TE. Valproic acid pathway: pharmacokinetics and pharmacodynamics. Pharmacogenet Genomics. 2013;23:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 272] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 200. | Cinatl J Jr, Cinatl J, Driever PH, Kotchetkov R, Pouckova P, Kornhuber B, Schwabe D. Sodium valproate inhibits in vivo growth of human neuroblastoma cells. Anticancer Drugs. 1997;8:958-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 201. | Machado MC, Bellodi-Privato M, Kubrusly MS, Molan NA, Tharcisio T Jr, de Oliveira ER, D’Albuquerque LA. Valproic acid inhibits human hepatocellular cancer cells growth in vitro and in vivo. J Exp Ther Oncol. 2011;9:85-92. [PubMed] |
| 202. | Bai X, Liu L, Wang Y. Valproate Ameliorates Diethylnitrosamine/Phenobarbital- Induced Hepatic Cancer via the Role of TNF-α and TGF-β1. Int J Pharmacol. 2021;17:156-168. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 203. | Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383-91.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 388] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 204. | Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Sustained efficacy of adjuvant immunotherapy with cytokine-induced killer cells for hepatocellular carcinoma: an extended 5-year follow-up. Cancer Immunol Immunother. 2019;68:23-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 205. | Ma Y, Xu YC, Tang L, Zhang Z, Wang J, Wang HX. Cytokine-induced killer (CIK) cell therapy for patients with hepatocellular carcinoma: efficacy and safety. Exp Hematol Oncol. 2012;1:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 206. | Lee DH, Nam JY, Chang Y, Cho H, Kang SH, Cho YY, Cho E, Lee JH, Yu SJ, Kim YJ, Yoon JH. Synergistic effect of cytokine-induced killer cell with valproate inhibits growth of hepatocellular carcinoma cell in a mouse model. Cancer Biol Ther. 2017;18:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 207. | Yu JI, Choi C, Shin SW, Son A, Lee GH, Kim SY, Park HC. Valproic Acid Sensitizes Hepatocellular Carcinoma Cells to Proton Therapy by Suppressing NRF2 Activation. Sci Rep. 2017;7:14986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 208. | Lane AA, Chabner BA. Histone Deacetylase Inhibitors in Cancer Therapy. J Clin Oncol. 2009;27:5459-5468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 688] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 209. | Li G, Tian Y, Zhu WG. The Roles of Histone Deacetylases and Their Inhibitors in Cancer Therapy. Front Cell Dev Biol. 2020;8:576946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 192] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 210. | West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 943] [Cited by in RCA: 1096] [Article Influence: 99.6] [Reference Citation Analysis (0)] |
| 211. | Munshi A, Tanaka T, Hobbs ML, Tucker SL, Richon VM, Meyn RE. Vorinostat, a histone deacetylase inhibitor, enhances the response of human tumor cells to ionizing radiation through prolongation of gamma-H2AX foci. Mol Cancer Ther. 2006;5:1967-1974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 175] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 212. | Chen W, Chiang CL, Dawson LA. Efficacy and safety of radiotherapy for primary liver cancer. Chin Clin Oncol. 2021;10:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 213. | An HM, Xue YF, Shen YL, Du Q, Hu B. Sodium valproate induces cell senescence in human hepatocarcinoma cells. Molecules. 2013;18:14935-14947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 214. | Pernicova I, Korbonits M. Metformin–mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 942] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 215. | Zhang Z, Zhou L, Xie N, Nice EC, Zhang T, Cui Y, Huang C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct Target Ther. 2020;5:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 315] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 216. | Akce M, Rupji M, Switchenko JM, Shaib WL, Wu C, Alese OB, Diab M, Lesinski GB, El-Rayes BF. Phase II trial of nivolumab and metformin in patients with treatment refractory microsatellite stable metastatic colorectal cancer. J Clin Oncol. 2021;39 Suppl 3:95. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 217. | Goodwin PJ, Chen BE, Gelmon KA, Whelan TJ, Ennis M, Lemieux J, Ligibel JA, Hershman DL, Mayer IA, Hobday TJ, Bliss JM, Rastogi P, Rabaglio-Poretti M, Mukherjee SD, Mackey JR, Abramson VG, Oja C, Wesolowski R, Thompson AM, Rea DW, Stos PM, Shepherd LE, Stambolic V, Parulekar WR. Effect of Metformin vs Placebo on Invasive Disease-Free Survival in Patients With Breast Cancer: The MA.32 Randomized Clinical Trial. JAMA. 2022;327:1963-1973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 140] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 218. | Soliman PT, Westin SN, Iglesias DA, Fellman BM, Yuan Y, Zhang Q, Yates MS, Broaddus RR, Slomovitz BM, Lu KH, Coleman RL. Everolimus, Letrozole, and Metformin in Women with Advanced or Recurrent Endometrioid Endometrial Cancer: A Multi-Center, Single Arm, Phase II Study. Clin Cancer Res. 2020;26:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 219. | Wilson BE, Armstrong AJ, de Bono J, Sternberg CN, Ryan CJ, Scher HI, Smith MR, Rathkopf D, Logothetis CJ, Chi KN, Jones RJ, Saad F, De Porre P, Tran N, Hu P, Gillessen S, Carles J, Fizazi K, Joshua AM. Effects of metformin and statins on outcomes in men with castration-resistant metastatic prostate cancer: Secondary analysis of COU-AA-301 and COU-AA-302. Eur J Cancer. 2022;170:296-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 220. | Ma SJ, Zheng YX, Zhou PC, Xiao YN, Tan HZ. Metformin use improves survival of diabetic liver cancer patients: systematic review and meta-analysis. Oncotarget. 2016;7:66202-66211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 221. | Ma S, Zheng Y, Xiao Y, Zhou P, Tan H. Meta-analysis of studies using metformin as a reducer for liver cancer risk in diabetic patients. Medicine (Baltimore). 2017;96:e6888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 222. | Pollak M. Overcoming Drug Development Bottlenecks With Repurposing: Repurposing biguanides to target energy metabolism for cancer treatment. Nat Med. 2014;20:591-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 223. | Howell JJ, Hellberg K, Turner M, Talbott G, Kolar MJ, Ross DS, Hoxhaj G, Saghatelian A, Shaw RJ, Manning BD. Metformin Inhibits Hepatic mTORC1 Signaling via Dose-Dependent Mechanisms Involving AMPK and the TSC Complex. Cell Metab. 2017;25:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 311] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 224. | Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5:378-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 593] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 225. | Clendening JW, Pandyra A, Boutros PC, El Ghamrasni S, Khosravi F, Trentin GA, Martirosyan A, Hakem A, Hakem R, Jurisica I, Penn LZ. Dysregulation of the mevalonate pathway promotes transformation. Proc Natl Acad Sci U S A. 2010;107:15051-15056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 311] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 226. | Mullen PJ, Yu R, Longo J, Archer MC, Penn LZ. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer. 2016;16:718-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 488] [Article Influence: 54.2] [Reference Citation Analysis (2)] |
| 227. | Jiang W, Hu JW, He XR, Jin WL, He XY. Statins: a repurposed drug to fight cancer. J Exp Clin Cancer Res. 2021;40:241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 240] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 228. | Kodach LL, Jacobs RJ, Voorneveld PW, Wildenberg ME, Verspaget HW, van Wezel T, Morreau H, Hommes DW, Peppelenbosch MP, van den Brink GR, Hardwick JC. Statins augment the chemosensitivity of colorectal cancer cells inducing epigenetic reprogramming and reducing colorectal cancer cell ‘stemness’ via the bone morphogenetic protein pathway. Gut. 2011;60:1544-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 229. | Longo J, van Leeuwen JE, Elbaz M, Branchard E, Penn LZ. Statins as Anticancer Agents in the Era of Precision Medicine. Clin Cancer Res. 2020;26:5791-5800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 230. | Mansourian PG, Yoneda M, Krishna Rao M, Martinez FJ, Thomas E, Schiff ER. Effects of statins on the risk of hepatocellular carcinoma. Gastroenterol Hepatol (N Y). 2014;10:417-426. [PubMed] |
| 231. | Kim MH, Kim MY, Salloum S, Qian T, Wong LP, Xu M, Lee Y, Shroff SG, Sadreyev RI, Corey KE, Baumert TF, Hoshida Y, Chung RT. Atorvastatin favorably modulates a clinical hepatocellular carcinoma risk gene signature. Hepatol Commun. 2022;6:2581-2593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 232. | Krasselt M, Baerwald C. Celecoxib for the treatment of musculoskeletal arthritis. Expert Opin Pharmacother. 2019;20:1689-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 233. | Khafaga AF, Shamma RN, Abdeen A, Barakat AM, Noreldin AE, Elzoghby AO, Sallam MA. Celecoxib repurposing in cancer therapy: molecular mechanisms and nanomedicine-based delivery technologies. Nanomedicine (Lond). 2021;16:1691-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 234. | Sobolewski C, Legrand N. Celecoxib Analogues for Cancer Treatment: An Update on OSU-03012 and 2,5-Dimethyl-Celecoxib. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 235. | Tołoczko-Iwaniuk N, Dziemiańczyk-Pakieła D, Nowaszewska BK, Celińska-Janowicz K, Miltyk W. Celecoxib in Cancer Therapy and Prevention – Review. Curr Drug Targets. 2019;20:302-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 236. | Hashemi Goradel N, Najafi M, Salehi E, Farhood B, Mortezaee K. Cyclooxygenase-2 in cancer: A review. J Cell Physiol. 2019;234:5683-5699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 526] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 237. | Dai H, Zhang S, Ma R, Pan L. Celecoxib Inhibits Hepatocellular Carcinoma Cell Growth and Migration by Targeting PNO1. Med Sci Monit. 2019;25:7351-7360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 238. | Li J, Liu L, Chen Y, Wu M, Lin X, Shen Z, Cheng Y, Chen X, Weygant N, Wu X, Wei L, Sferra TJ, Han Y, Shen A, Peng J. Ribosome assembly factor PNO1 is associated with progression and promotes tumorigenesis in triplenegative breast cancer. Oncol Rep. 2022;47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 239. | Lin C, Yuan H, Wang W, Zhu Z, Lu Y, Wang J, Feng F, Wu J. Importance of PNO1 for growth and survival of urinary bladder carcinoma: Role in core-regulatory circuitry. J Cell Mol Med. 2020;24:1504-1515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 240. | Liu D, Lin L, Wang Y, Chen L, He Y, Luo Y, Qi L, Guo Y, Han Z, Li G, Li Q, Liu Z, Chen P, Guo H. PNO1, which is negatively regulated by miR-340-5p, promotes lung adenocarcinoma progression through Notch signaling pathway. Oncogenesis. 2020;9:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 241. | Shen Z, Chen Y, Li L, Liu L, Peng M, Chen X, Wu X, Sferra TJ, Wu M, Lin X, Cheng Y, Chu J, Shen A, Peng J. Transcription Factor EBF1 Over-Expression Suppresses Tumor Growth in vivo and in vitro via Modulation of the PNO1/p53 Pathway in Colorectal Cancer. Front Oncol. 2020;10:1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 242. | Wang G, Li Q, Li C, Duan G, Sang H, Dong H, Yang Y, Ma C, Tao T. Knockdown of PNO1 inhibits esophageal cancer progression. Oncol Rep. 2021;45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 243. | Han Z, Liu D, Chen L, He Y, Tian X, Qi L, Luo Y, Chen Z, Hu X, Li G, Zhan L, Wang Y, Li Q, Chen P, Liu Z, Guo H. PNO1 regulates autophagy and apoptosis of hepatocellular carcinoma via the MAPK signaling pathway. Cell Death Dis. 2021;12:552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 244. | Hu JW, Chen B, Zhang J, Qi YP, Liang JH, Zhong JH, Xiang BD. Novel combination of celecoxib and metformin improves the antitumor effect by inhibiting the growth of Hepatocellular Carcinoma. J Cancer. 2020;11:6437-6444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 245. | Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1382] [Cited by in RCA: 1948] [Article Influence: 243.5] [Reference Citation Analysis (0)] |
| 246. | Mohamed FE, Al-Jehani RM, Minogue SS, Andreola F, Winstanley A, Olde Damink SW, Habtesion A, Malagó M, Davies N, Luong TV, Dhillon AP, Mookerjee RP, Dhar DK, Jalan R. Effect of toll-like receptor 7 and 9 targeted therapy to prevent the development of hepatocellular carcinoma. Liver Int. 2015;35:1063-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 247. | Chen MY, Yadav VK, Chu YC, Ong JR, Huang TY, Lee KF, Lee KH, Yeh CT, Lee WH. Hydroxychloroquine (HCQ) Modulates Autophagy and Oxidative DNA Damage Stress in Hepatocellular Carcinoma to Overcome Sorafenib Resistance via TLR9/SOD1/hsa-miR-30a-5p/Beclin-1 Axis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |