Published online Apr 28, 2023. doi: 10.3748/wjg.v29.i16.2479
Peer-review started: January 31, 2023
First decision: February 23, 2023
Revised: March 5, 2023
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: April 28, 2023
Processing time: 82 Days and 21.5 Hours
Chronic hepatitis B virus (HBV) infection remains a major global public health problem. Chronic hepatitis B (CHB) patients can be divided into treatment indication and non-treatment indication individuals according to alanine transaminase (ALT), HBV DNA, serum hepatitis B e antigen status, disease status [liver cirrhosis, hepatocellular carcinoma (HCC), or liver failure], liver necroinflammation or fibrosis, patients’ age, and family history of HCC or cirrhosis. For example, normal ALT patients in ‘immune-tolerant’ phase with HBV DNA higher than 107 or 2 × 107 IU/mL, and those in ‘inactive-carrier’ phase with HBV DNA lower than 2 × 103 IU/mL do not require antiviral therapy. However, is it reas
To analyze the correlation of HBV DNA level and liver histopathological severity, and to explore the significance of HBV DNA for CHB with normal ALT.
From January 2017 to December 2021, a retrospective cross-sectional set of 1299 patients with chronic HBV infection (HBV DNA > 30 IU/mL) who underwent liver biopsy from four hospitals, including 634 with ALT less than 40 U/L. None of the patients had received anti-HBV treatment. The degrees of liver necroinflammatory activity and liver fibrosis were evaluated according to the Metavir system. On the basis of the HBV DNA level, patients were divided into two groups: Low/moderate replication group, HBV DNA ≤ 107 IU/mL [7.00 Log IU/mL, the European Association for the Study of the Liver (EASL) guidelines] or ≤ 2 × 107 IU/mL [7.30 Log IU/mL, the Chinese Medical Association (CMA) guidelines]; high replication group, HBV DNA > 107 IU/mL or > 2 × 107 IU/mL. Relevant factors (demographic characteristics, laboratory parameters and noninvasive models) for liver histopathological severity were analyzed by univariate analysis, logistics analysis and propensity score-matched analysis.
At entry, there were 21.45%, 24.29%, and 30.28% of the patients had liver histopathological severities with ≥ A2, ≥ F2, and ≥ A2 or/and ≥ F2, respectively. HBV DNA level (negative correlation) and noninvasive model liver fibrosis 5 value (positive correlation) were independent risk factors for liver histopathological severities (liver necroinflammation, liver fibrosis, and treatment indication). The AUROCs of the prediction probabilities (PRE_) of the models mentioned above (< A2 vs ≥ A2, < F2 vs ≥ F2, < A2 and < F2 vs ≥ A2 or/and ≥ F2) were 0.814 (95%CI: 0.770-0.859), 0.824 (95%CI: 0.785-0.863), and 0.799 (95%CI: 0.760-0.838), respectively. HBV DNA level (negative correlation) was still an independent risk factor when diagnostic models were excluded, the P values (< A2 vs ≥ A2, < F2 vs ≥ F2, < A2 and < F2 vs ≥ A2 or/and ≥ F2) were 0.011, 0.000, and 0.000, respectively. For the propensity score-matched pairs, whether based on EASL guidelines or CMA guidelines, the group with significant liver histology damage (≥ A2 or/and ≥ F2) showed much lower HBV DNA level than the group with non- significant liver histology damage (< A2 and < F2). Patients in the moderate replication group (with indeterminate phase) had the most serious liver disease pathologically and hematologically, followed by patients in the low replication group (with ‘inactive-carrier’ phase) and then the high replication group (with ‘immune-tolerant’ phase).
HBV DNA level is a negative risk factor for liver disease progression. The phase definition of CHB may be revised by whether the level of HBV DNA exceeds the detection low limit value. Patients who are in the indeterminate phase or ‘inactive carriers’ should receive antiviral therapy.
Core Tip: According to the guidelines, for patients with normal alanine transaminase (ALT), hepatitis B virus (HBV) DNA levels were defined as ≥ 107/2 × 107 and < 2 × 103 IU/mL in the ‘immune-tolerant’ and the ‘inactive-carrier’ phase, respectively. However, it is still controversial. In this study, we analyzed the liver histopathology and the risk factors in 634 cases with positive HBV DNA and normal ALT. We found that patients with low or moderate HBV DNA level had more severe liver diseases. HBV DNA level (negative correlation) was an independent risk factor for liver histopathological severity. Therefore, we consider that the phase definition of chronic hepatitis B may be revised based on whether the level of HBV DNA exceeds the detection low limit value.
- Citation: Jiang SW, Lian X, Hu AR, Lu JL, He ZY, Shi XJ, Zhu DD, Wang ZY, Huang GC. Liver histopathological lesions is severe in patients with normal alanine transaminase and low to moderate hepatitis B virus DNA replication. World J Gastroenterol 2023; 29(16): 2479-2494
- URL: https://www.wjgnet.com/1007-9327/full/v29/i16/2479.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i16.2479
With the promotion of the hepatitis B vaccine or combined hepatitis B immune globulin, new hepatitis B virus (HBV) infections are decreasing. However, there are still about 257 million people infected with HBV worldwide[1], and about 887000 people died from HBV infection each year, of which liver cirrhosis and hepatocellular carcinoma (HCC) deaths account for 52% and 38%, respectively[2]. It’s undeniable that chronic HBV infection is still a major global public health problem. Therefore, the World Health Organization (WHO) has proposed the global health sector strategy of ‘eliminating viral hepatitis as a major public health threat by 2030’. One of the goals is to achieve a diagnosis rate to 90% and a treatment rate to 80% of HBV infection by 2030[3]. China had made great progress in reducing HBV infections, but the challenges still remain. Currently, there are still 70 million chronic HBV infections in China[2].
The natural history of chronic HBV infection can be generally divided into four phases: Hepatitis B e antigen (HBeAg) positive chronic HBV infection/’immune-tolerant’ phase, HBeAg positive chronic hepatitis B (CHB)/immune-clearance phase, HBeAg negative chronic HBV infection/’inactive-carrier’ phase, and HBeAg negative CHB/reactivation phase[2,4,5].
The disease progression and treatment indications judgement is mainly based on serum HBeAg status, HBV DNA level, alanine transaminase (ALT) level, and severity of liver disease, combined with patients’ age, family history, and accompanying diseases[2,4-7]. In accordance with the European Association for the Study of the Liver (EASL) guidelines[4] and the CHB treatment algorithm in the United States[5], regardless of HBeAg status, patients with HBV DNA > 2 × 103 IU/mL, ALT > upper limit of normal (ULN), and/or at least moderate liver necrotic inflammation or liver fibrosis should be treated. However, if HBV DNA is less than 2 × 103 IU/mL, how to deal with it clinically becomes an issue. According to the EASL guidelines[4] and the CHB treatment algorithm in the United States[5], CHB patients with normal ALT in the immune tolerant phase refer to HBV DNA > 107 IU/mL, in the ‘inactive-carrier’ phase refer to HBV DNA < 2 × 103, and in the indeterminate phase refer to 2 × 103 ≤ HBV DNA ≤ 107 IU/mL. According to the Chinese Medical Association (CMA) guidelines[2], patients in the immune tolerant phase refer to HBV DNA > 2 × 107 IU/mL, in the ‘inactive-carrier’ phase refer to HBV DNA < 2 × 103, and in the indeterminate phase refer to 2 × 103 ≤ HBV DNA ≤ 2 × 107 IU/mL.
Although HBV DNA is an important indicator for judging disease progression and treatment indications, the reported conclusions about HBV DNA and disease severity remain controversial[8-14]. Moreover, the ‘gray-zone’ and/or the indeterminate phase population should not be ignored with the consideration of the guidelines. The aim of this study was to find the correlation of clinical and laboratory parameters with liver histopathological severity in 634 CHB patients with ALT < ULN who required liver biopsy to assess liver inflammation and fibrosis. Studies on liver pathological changes in the ‘gray-zone’ and/or the indeterminate phase population and the identification of the risk factors for disease progression might be of great significance.
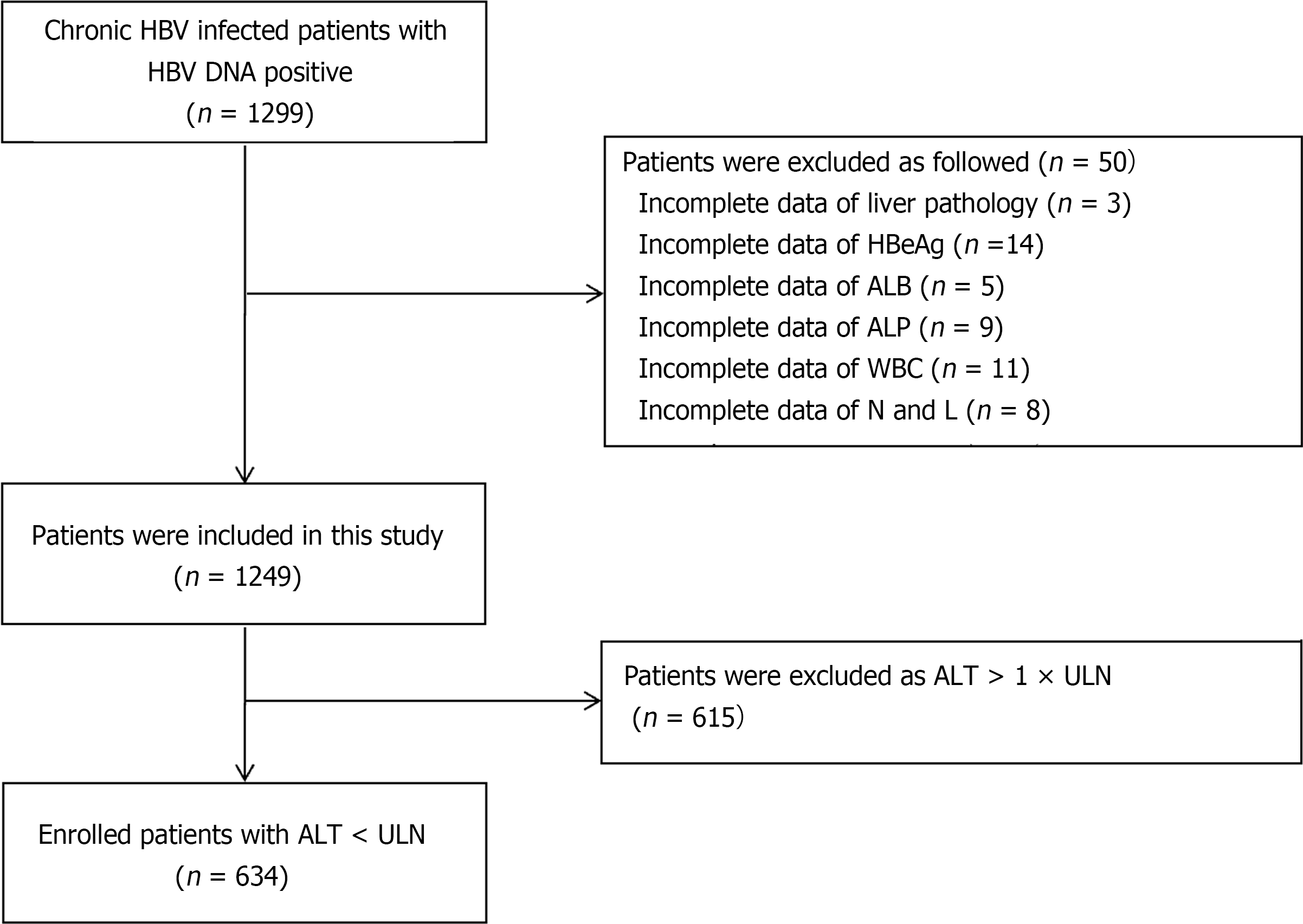
From January 2017 to December 2021, there were 1299 chronic HBV infections (including 634 with ALT < ULN) who underwent liver biopsy were included in this retrospective cross-sectional study conducted in four hospitals. The patients were hospitalized in the Department of Hepatology, Ningbo No. 2 Hospital; the Department of Infectious Diseases, Xiangshan Hospital Affiliated to Wenzhou Medical University; the Department of Infectious Diseases, The First Hospital of Ninghai County; and the Department of Infectious Diseases, the Affiliated Yangming Hospital of Ningbo University, Ningbo, China.
The inclusion criteria were as follows: Patients aged 13-78 years, HBsAg positivity for at least 6 mo, HBV DNA ≥ 30 IU/mL, and no previous anti-HBV treatment. The ULN of ALT was 40 U/L according to the WHO/EASL/Asian Pacific Association for the Study of the Liver guidelines[4,6,7]. The exclusion criteria were as follows: Co-infection with hepatitis C virus, hepatitis D virus, hepatitis E virus, and human immunodeficiency virus; autoimmune hepatitis; Wilson’s disease; nonalcoholic fatty liver disease; chronic alcohol consumption (> 30 g/d for men and > 20 g/d for women[15]); and incomplete data.
This study was approved by the ethics committee of Ningbo No. 2 Hospital (PJ-NBEY-KY-2017-069-01, PJ-NBEY-KY-2021-037-02, and PJ-NBEY-KY-2022-138-01). In this study, medical data was obtained from previous clinical diagnosis and treatment, and informed consent was exempted.
The clinical data was collected within one week before liver biopsy. Demographic characteristics and laboratory data, including age, sex, albumin (ALB), globulin (GLB), ALB–GLB ratio (AGR), ALT, aspartate aminotransferase (AST), alkaline phosphatase (ALP), gamma-glutamyl transpeptidase (GGT), white blood cell (WBC), neutrophil–lymphocyte ratio (NLR), platelet (PLT), HBeAg, HBV DNA, and noninvasive models such as aspartate transaminase to platelet ratio index (APRI)[16], fibrosis-4 (FIB-4)[17], liver inflammation and fibrosis-5 (LIF-5)[18], were recorded.
Blood routine was detected using Sysmex XN-1000 automated hematology analyzer (Sysmex Corporation, Japan). Serum liver function was detected with Simens Advia Chemistry XPT system analyzer (Siemens Healthcare, Germany). Serum HBV DNA was measured by real-time fluorescence quantitative PCR (ABI7500, Applied Biosystems, CA, USA) and HBV nucleic acid quantitative detection kit (DAAN Gene Co., Ltd. Sun Yat-sen University, China) with the lowest detection value of 30 IU/mL. According to the HBV DNA level, patients were divided into two groups: Low/moderate replication group, HBV DNA ≤ 107 IU/mL (7.00 Log IU/mL) or ≤ 2 × 107 IU/mL (7.30 Log IU/mL); and high replication group, HBV DNA > 107 IU/mL or > 2 × 107 IU/mL[2,4,5]. HBsAg and HBeAg were detected by chemiluminescence method (Abbott AxSYM System, IL, United States). In this study, HBeAg was presented as 1 for positive and 0 for negative. The same quality control standards were employed.
The biopsy device (BARD Magnum, United States) comprised a biopsy gun (with the tissue length of 22 mm) and a biopsy needle (18G). All patients had no liver biopsy contraindications and signed informed consent forms. Liver biopsy was performed under the guidance of color Doppler ultrasound. Liver tissue samples of more than 2 cm in length and more than 6 intact portal veins were required. The liver specimens were first assessed by two pathology experts from the hospital and then by a senior pathologist from the Department of Pathology, Fudan University, China. The degrees of liver necroinflammatory activity and liver fibrosis were evaluated according to the Metavir system[19]. A Metavir necroinflammatory activity score of ≥ 2 (A2) and ≥ 3 (A3) indicated significant and severe liver inflammation, respectively. A Metavir fibrosis score of ≥ 2 (F2), ≥ 3 (F3), and ≥ 4 (F4) indicated significant liver fibrosis, advanced fibrosis, and cirrhosis, respectively. In accordance with the guidelines, the treatment indications of patients with ALT < ULN were ≥ A2 or/and ≥ F2. Hence, these patients were divided into treatment indication group and nontreatment indication group (< A2 and < F2).
Propensity score-matched analysis was used to reduce the effect of selection bias and potential confounding between the two groups. According to the HBV DNA levels of EASL and CMA guidelines, the low/moderate replication group and high replication group were matched at a ratio of 1:1 (nearest neighbor matching within caliper) based on sex, age, ALB, GLB, AGR, ALT, AST, ALP, GGT, WBC, NLR, PLT, APRI, FIB-4, and LIF-5.
The data were analyzed via SPSS software version 22.0 (SPSS Inc., IL, United States). HBV DNA levels were expressed as logarithms. The normally distributed variables were presented as means with standard deviations analyzed by using independent-samples t test (two datasets). The non-normal distribution variables were expressed as medians (Q1-Q3) analyzed by using nonparametric tests (Mann-Whitney U test) for two datasets. The chi-square test was used for categorical data. Ridit analysis and Spearman’s rank correlation analysis were used for ranked data. The binary logistic regression analysis was performed taking liver histopathological severity (A and F) as the dependent variables and relevant factors (P value < 0.1) as independent variables. The dependent variables were < A2 vs ≥ A2, < F2 vs ≥ F2, and nontreatment indication vs treatment indication. The relevant factors were analyzed, and the diagnostic value was evaluated using receiver operating characteristic (ROC) curve and the area under the ROC curve (AUROC). All tests were two tailed, and statistical significance was set at P value < 0.05.
There were 50 patients were excluded due to incomplete data of liver pathology, HBeAg, ALB, ALP, WBC, neutrophils, and lymphocytes, and 615 patients were excluded as ALT > 1 × ULN. The flow diagram of the study population is shown in Figure 1. Finally, 634 patients were included in the study, among which 336 (EASL guidelines) and 377 (CMA guidelines) were classified into the low/moderate replication group, including 49 Low-replication (HBV DNA < 2 × 103); 298 (EASL guidelines) and 257 (CMA guidelines) were divided into the high replication group.
The baseline characteristics of 634 patients were divided according to liver pathology which are shown in Table 1. The mean age of the participants was 35.61 ± 10.30 years, the mean ALT, AST, and HBV DNA levels were 23.77 ± 8.58 U/L, 24.15 ± 8.91 U/L, and 6.18 ± 1.87 Log IU/mL, respectively. Among these patients, 349 (55.05%) were men and 432 (68.14%) were HBeAg positive.
| Parameters | All patients (n = 634) | HBV DNA levels (EASL guidelines) | HBV DNA levels (CMA guidelines) | ||||||
| Low/moderate replication (n = 336) | High replication (n = 298) | χ2/t /Z/u | P value | Low/moderate replication (n = 377) | High replication (n = 257) | χ2/t/Z/u | P value | ||
| Age, mean ± SD, yr | 35.61 ± 10.30 | 38.09 ± 9.99 | 32.82 ± 9.95 | 6.640 | < 0.001 | 37.34 ± 10.17 | 33.07 ± 9.99 | 5.222 | < 0.001 |
| Male, n (%) | 349 (55.05) | 192 (57.14) | 157 (52.68) | 1.269 | 0.260 | 209 (55.44) | 140 (54.47) | 0.057 | 0.811 |
| HBeAg positive, n (%) | 432 (68.14) | 142 (42.26) | 290 (97.31) | 220.486 | < 0.001 | 180 (47.74) | 252 (98.05) | 178.165 | < 0.001 |
| ALB, mean ± SD, g/L | 42.72 ± 4.45 | 42.91 ± 4.97 | 42.49 ± 3.78 | 1.189 | 0.235 | 42.75 ± 4.88 | 42.67 ± 3.74 | 0.220 | 0.826 |
| GLB, mean ± SD, g/L | 27.82 ± 4.20 | 27.96 ± 4.11 | 27.66 ± 4.30 | 0.893 | 0.372 | 28.11 ± 4.19 | 27.40 ± 4.18 | 2.105 | 0.036 |
| AGR, mean ± SD | 1.57 ± 0.29 | 1.57 ± 0.30 | 1.57 ± 0.28 | -0.148 | 0.882 | 1.56 ± 0.30 | 1.59 ± 0.28 | -1.581 | 0.114 |
| ALT, mean ± SD, U/L | 23.77 ± 8.58 | 24.84 ± 8.66 | 22.56 ± 8.34 | 3.372 | 0.001 | 24.38 ± 8.69 | 22.87 ± 8.35 | 2.186 | 0.029 |
| AST, mean ± SD, U/L | 24.15 ± 8.91 | 25.00 ± 7.52 | 23.20 ± 10.18 | 2.558 | 0.011 | 25.04 ± 10.28 | 22.85 ± 6.17 | 3.069 | 0.002 |
| ALP, mean ± SD, U/L | 71.26 ± 26.06 | 71.52 ± 24.85 | 70.96 ± 27.39 | 0.269 | 0.788 | 71.21 ± 24.99 | 71.32 ± 27.60 | -0.054 | 0.957 |
| GGT, median (Q1-Q3), U/L | 18.00 (13.00-25.00) | 20.00 (15.00-30.00) | 16.00 (13.00-23.00) | 4.966 | < 0.001 | 19.00 (14.00-29.00) | 16.00 (13.00-23.00) | 4.069 | < 0.001 |
| WBC count, mean ± SD, | 5.39 ± 1.42 | 5.33 ± 1.39 | 5.47 ± 1.45 | -1.208 | 0.228 | 5.33 ± 1.44 | 5.48 ± 1.39 | -1.321 | 0.187 |
| NLR, mean ± SD | 2.03 ± 1.24 | 2.05 ± 1.37 | 2.01 ± 1.08 | 0.443 | 0.658 | 2.04 ± 1.33 | 2.01 ± 1.09 | 0.298 | 0.766 |
| PLT count, mean ± SD, | 175.67 ± 48.83 | 163.93 ± 47.39 | 188.90 ± 47.09 | -6.641 | < 0.001 | 166.86 ± 47.55 | 188.58 ± 47.90 | -5.629 | < 0.001 |
| HBV DNA, mean ± SD, log IU/mL | 6.18 ± 1.87 | 4.68 ± 1.26 | 7.88 ± 0.50 | -41.111 | < 0.001 | 4.95 ± 1.41 | 7.99 ± 0.44 | -33.427 | < 0.001 |
| APRI, median (Q1–Q3) | 0.33 (0.25-0.44) | 0.36 (0.28-0.51) | 0.29 (0.23-0.38) | 6.936 | < 0.001 | 0.36 (0.27-0.48) | 0.30 (0.23-0.38) | 5.725 | < 0.001 |
| FIB-4, median (Q1-Q3) | 0.99 (0.69-1.38) | 1.13 (0.83-1.61) | 0.80 (0.58-1.13) | 8.109 | < 0.001 | 1.09 (0.79-1.56) | 0.81 (0.59-1.13) | 6.877 | < 0.001 |
| LIF-5, mean ± SD | 0.40 ± 0.15 | 0.45 ± 0.15 | 0.36 ± 0.14 | 7.832 | < 0.001 | 0.44 ± 0.15 | 0.35 ± 0.14 | 7.195 | < 0.001 |
| Liver inflammatory activity | |||||||||
| A0, n (%) | 117 (18.45) | 58 (17.26) | 59 (19.80) | 4.189 | < 0.001 | 61 (16.18) | 56 (21.79) | 4.426 | < 0.001 |
| A1, n (%) | 381 (60.10) | 174 (51.79) | 207 (69.46) | 206 (54.64) | 175 (68.09) | ||||
| A2, n (%) | 97 (15.30) | 73 (21.73) | 24 (8.05) | 78 (20.69) | 19 (7.39) | ||||
| A3, n (%) | 39 (6.15) | 31 (9.23) | 8 (2.68) | 32 (8.49) | 7 (2.72) | ||||
| ≥ A2, n (%) | 136 (21.45) | 104 (30.95) | 32 (10.74) | 38.299 | < 0.001 | 110 (29.18) | 26 (10.12) | 32.952 | < 0.001 |
| Liver fibrosis | |||||||||
| F0, n (%) | 148 (23.34) | 61 (18.15) | 87 (29.19) | 6.382 | < 0.001 | 70 (18.57) | 78 (30.35) | 6.053 | < 0.001 |
| F1, n (%) | 332 (52.37) | 153 (45.54) | 179 (60.07) | 179 (47.48) | 153 (59.53) | ||||
| F2, n (%) | 87 (13.72) | 65 (19.35) | 22 (7.38) | 70 (18.57) | 17 (6.61) | ||||
| F3, n (%) | 37 (5.84) | 29 (8.63) | 8 (2.68) | 30 (7.96) | 7 (2.72) | ||||
| F4, n (%) | 30 (4.73) | 28 (8.33) | 2 (0.67) | 28 (7.43) | 2 (0.78) | ||||
| ≥ F2, n (%) | 154 (24.29) | 122 (36.31) | 32 (10.74) | 56.155 | < 0.001 | 128 (33.95) | 26 (10.12) | 47.212 | < 0.001 |
| Treatment indication | |||||||||
| < A2 and < F2, n (%) | 442 (69.72) | 191 (56.85) | 251 (84.23) | 56.089 | < 0.001 | 224 (59.42) | 218 (84.82) | 46.730 | < 0.001 |
| ≥ A2 or/and ≥ F2, n (%) | 192 (30.28) | 145 (43.15) | 47 (15.77) | 153 (40.58) | 39 (15.18) | ||||
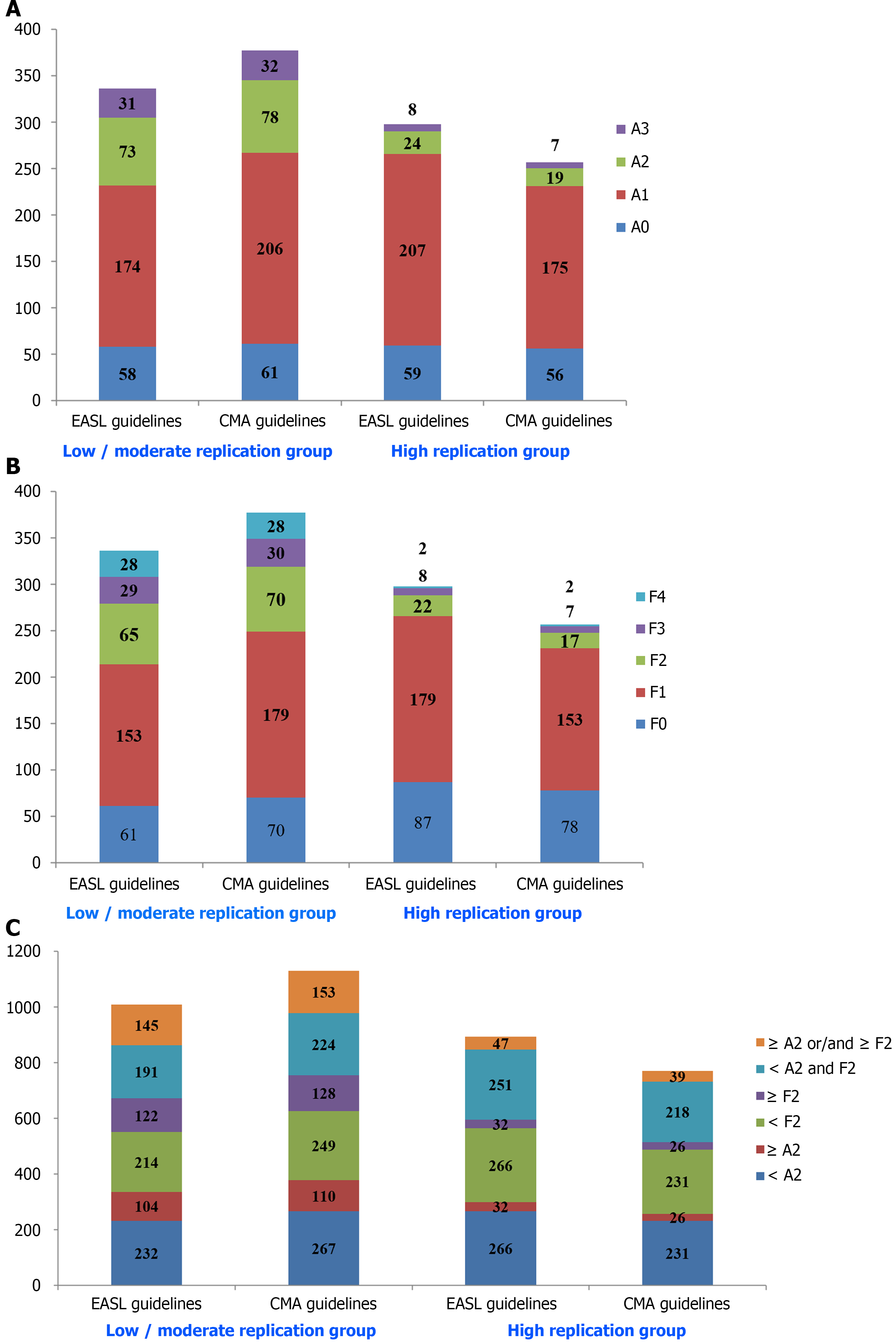
Patients with liver inflammation A0, A1, A2, and A3 were 117 (18.45%), 381 (60.10%), 97 (15.30%), and 39 (6.15%), with liver fibrosis F0, F1, F2, F3, and F4 were 148 (23.34%), 332 (52.37%), 87 (13.72%), 37 (5.84%), and 30 (4.73%), respectively. Patients with ≥ A2 accounted for 21.45% (136 patients), ≥F2 for 24.29% (154 patients), and treatment indication (≥ A2 or/and ≥ F2) for 30.28% (192 patients).
According to the EASL and CMA guidelines, the low/moderate replication group was comprised of 336 and 298 patients while the high replication group comprised 377 and 257 patients. Patients with high HBV DNA levels had a higher HBeAg-positive composition for both the two guidelines (χ2 = 220.486 and 178.165, P < 0.001). Compared with the low/moderate replication group, patients in the high replication group had lower age, ALT, AST, GGT, APRI, FIB-4, and LIF-5, and higher PLT. The results were detailed in Table 1.
In general, liver histopathological severity degree in the low/moderate replication group was of the most serious forms regardless of liver inflammation or liver fibrosis. The average Ridit values of necroinflammatory activity grading in the low/moderate replication group, and high replication group were 0.544 and 0.451 (EASL guidelines), 0.541 and 0.440 (CMA guidelines), respectively, with statistically significant differences (u = 4.189, 4.426; r = -0.183, -0.194; P < 0.001). The average Ridit values of liver fibrosis staging in the low/moderate replication group and high replication group were 0.567 and 0.424 (EASL guidelines), 0.556 and 0.418 (CMA guidelines), respectively, with statistically significant differences (u = 6.382, 6.053; r = -0.271, -0.257; P < 0.001). There were 104 (30.95%) and 32 (10.74%) (EASL guidelines), 110 (29.18%) and 26 (10.12%) (CMA guidelines) patients with liver inflammatory activity ≥ 2 (≥ A2) in the two groups, respectively (χ2 = 38.299, 32.952; P < 0.001). Patients with liver fibrosis ≥ 2 (≥ F2) in the two groups were 122 (36.31%) and 32 (10.74%) (EASL guidelines), 128 (33.95%) and 26 (10.12%) (CMA guidelines), respectively (χ2 = 56.155, 47.212; P < 0.001). Simultaneously, the number of patients with treatment indication (≥ A2 or/and ≥ F2) in the two groups were 145 (43.15%) and 47 (15.77%) by the EASL guidelines, or 153 (40.58%) and 39 (15.18%) by the CMA guidelines (χ2 = 56.089, 46.730; P < 0.001). The results are displayed in Table 1 and Figure 2.
In addition, there were 16 (32.65%), 20 (40.82%), and 22 (44.90%) patients with liver inflammatory activity ≥ 2 (≥ A2), liver fibrosis ≥ 2 (≥ F2), and treatment indication (≥ A2 or/and ≥ F2) in the low-replication (HBV DNA < 2 × 103, 49 cases), respectively.
The univariate analysis indicated that the statistically significant variables which could affect liver inflammation activity, liver fibrosis, and treatment indications were age, HBeAg, ALB, GLB, AGR, ALT, AST, GGT, PLT, HBV DNA, APRI, FIB-4, and LIF-5. The results are demonstrated in Table 2.
| Parameters | Liver inflammatory activity | Liver fibrosis | Treatment indication | P value | |||
| < A2 (n = 498) | ≥ A2 (n = 136) | < F2 (n = 480) | ≥ F2 (n = 154) | < A2 and < F2 (n = 442) | ≥ A2 or/and ≥ F2 (n = 192) | ||
| Age, mean ± SD, yr | 34.68 ± 9.88 | 39.02 ± 11.10 | 34.36 ± 9.86 | 39.52 ± 10.71 | 34.28 ± 9.84 | 38.69 ± 10.70 | < 0.001, < 0.001, < 0.001 |
| Male, n (%) | 268 (53.82) | 81 (59.56) | 253 (52.71) | 96 (62.34) | 234 (52.94) | 115 (59.90) | 0.232, 0.037, 0.105 |
| HBeAg positive, n (%) | 354 (71.08) | 78 (57.35) | 350 (72.92) | 82 (53.25) | 323 (73.08) | 109 (56.77) | 0.002, < 0.001, < 0.001 |
| ALB, mean ± SD, g/L | 43.06 ± 3.96 | 41.45 ± 5.75 | 42.96 ± 4.32 | 41.95 ± 4.75 | 43.06 ± 4.01 | 41.92 ± 5.24 | < 0.001, 0.014, 0.003 |
| GLB, mean ± SD, g/L | 27.46 ± 4.09 | 29.14 ± 4.35 | 27.54 ± 4.06 | 28.70 ± 4.51 | 27.49 ± 4.08 | 28.57 ± 4.38 | < 0.001, 0.003, 0.003 |
| AGR, mean ± SD | 1.60 ± 0.27 | 1.46 ± 0.33 | 1.59 ± 0.28 | 1.50 ± 0.31 | 1.60 ± 0.27 | 1.50 ± 0.32 | < 0.001, 0.001, < 0.001 |
| ALT, mean ± SD, U/L | 22.81 ± 8.48 | 27.26 ± 8.07 | 22.80 ± 8.48 | 26.80 ± 8.20 | 22.45 ± 8.43 | 26.80 ± 8.16 | < 0.001, < 0.001, < 0.001 |
| AST, mean ± SD, U/L | 22.93 ± 6.24 | 28.62 ± 14.26 | 22.79 ± 6.41 | 28.41 ± 13.26 | 22.64 ± 6.26 | 27.63 ± 12.45 | < 0.001, < 0.001, < 0.001 |
| ALP, mean ± SD, U/L | 68.68 ± 24.45 | 80.67 ± 29.48 | 68.62 ± 23.35 | 79.49 ± 31.80 | 68.58 ± 23.86 | 77.41 ± 29.68 | < 0.001, < 0.001, < 0.001 |
| GGT, mean ± SD, U/L | 20.24 ± 13.58 | 34.91 ± 33.78 | 19.87 ± 13.63 | 34.34 ± 31.87 | 19.91 ± 13.78 | 31.38 ± 29.60 | < 0.001, < 0.001, < 0.001 |
| WBC count, mean ± SD, × 109/L | 5.40 ± 1.41 | 5.33 ± 1.53 | 5.43 ± 1.44 | 5.26 ± 1.40 | 5.41 ± 1.42 | 5.33 ± 1.46 | 0.622, 0.210, 0.491 |
| NLR, mean ± SD | 2.05 ± 1.21 | 1.96 ± 1.35 | 2.07 ± 1.31 | 1.91 ± 0.99 | 2.04 ± 1.21 | 2.02 ± 1.32 | 0.447, 0.158, 0.824 |
| PLT count, mean ± SD, × 109/L | 184.72 ± 44.67 | 142.52 ± 49.26 | 186.16 ± 44.28 | 142.94 ± 47.99 | 187.73 ± 43.49 | 147.90 ± 49.24 | < 0.001, < 0.001, < 0.001 |
| HBV DNA, mean ± SD, log IU/mL | 6.35 ± 1.91 | 5.56 ± 1.59 | 6.44 ± 1.87 | 5.39 ± 1.64 | 6.47 ± 1.88 | 5.52 ± 1.67 | < 0.001, < 0.001, < 0.001 |
| APRI, mean ± SD | 0.33 ± 0.14 | 0.59 ± 0.42 | 0.32 ± 0.13 | 0.58 ± 0.39 | 0.32 ± 0.12 | 0.54 ± 0.37 | < 0.001, < 0.001, < 0.001 |
| FIB-4, mean ± SD | 1.01 0.53 | 1.81 ± 1.34 | 0.97 ± 0.49 | 1.81 ± 1.28 | 0.96 ± 0.49 | 1.67 ± 1.21 | < 0.001, < 0.001, < 0.001 |
| LIF-5, mean ± SD | 0.37 ± 0.13 | 0.54 ± 0.17 | 0.36 ± 0.12 | 0.53 ± 0.16 | 0.36 ± 0.12 | 0.51 ± 0.16 | < 0.001, < 0.001, < 0.001 |
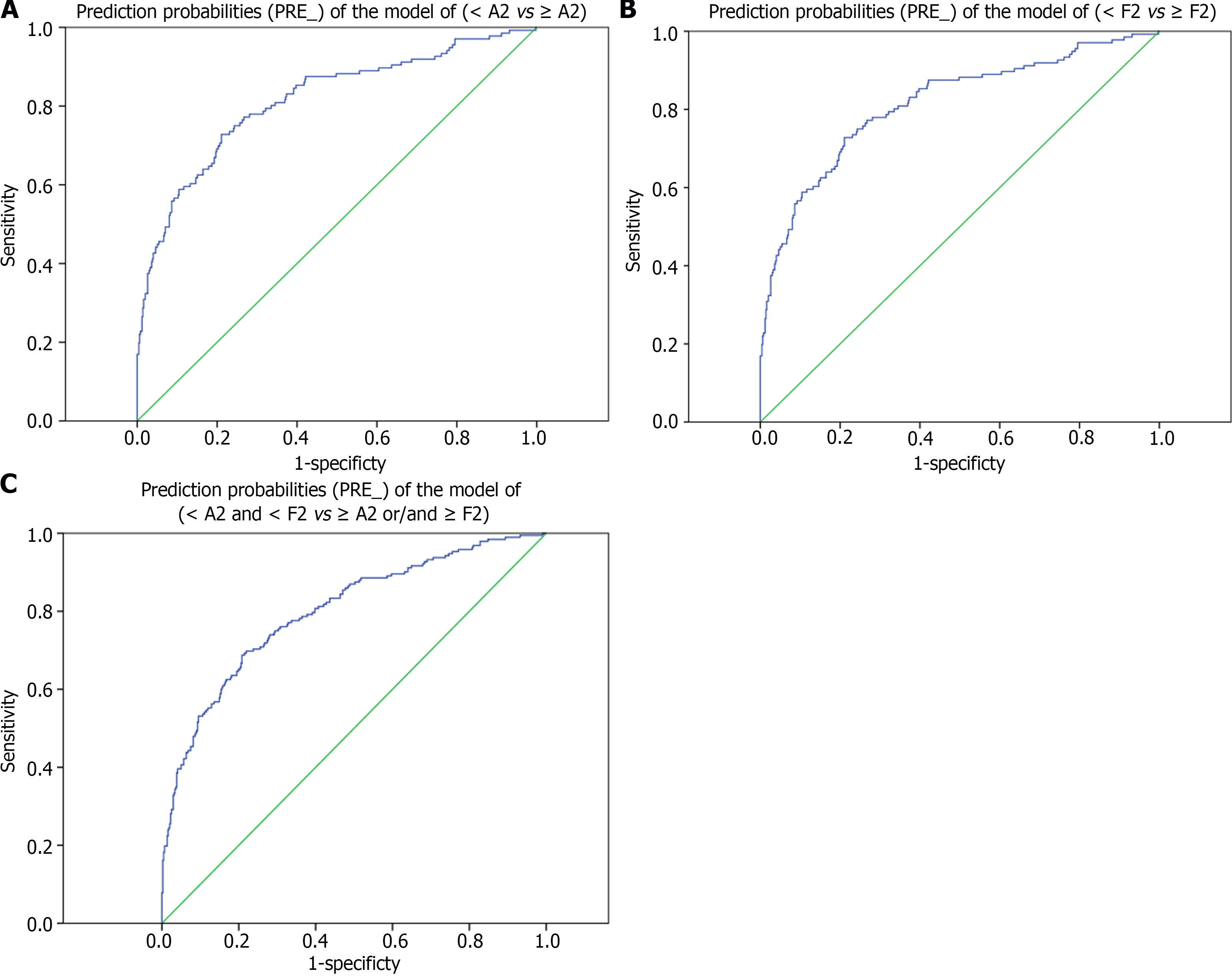
The logistics analysis showed that HBV DNA level (negative correlation), age (negative correlation), GGT level (positive correlation), and LIF-5 value (positive correlation) were independent risk factors for liver inflammation activity; HBV DNA level (negative correlation), GGT level (positive correlation), APRI value (positive correlation), and LIF-5 value (positive correlation) were independent risk factors for liver fibrosis; HBV DNA level (negative correlation), APRI value (positive correlation), and LIF-5 value (positive correlation) were independent risk factors for treatment indications. The AUROC of the prediction probabilities (PRE_) of the abovementioned models (< A2 vs ≥ A2, < F2 vs ≥ F2, < A2 and < F2 vs ≥ A2 or/and ≥ F2) was 0.814 (95%CI: 0.770-0.859), 0.824 (95%CI: 0.785-0.863), and 0.799 (95%CI: 0.760-0.838), respectively. Considering that the diagnostic models such as APRI, FIB-4, and LIF-5 contained some of the indices, the HBV DNA level (negative correlation) was still an independent risk factor for the dependent variables after the diagnostic models were eliminated. The results are listed in Table 3 and Figure 3.
| Variables | Regression coefficient | Standard error | Wald’s value | P value | OR | OR 95%CI | ||
| Upper limit | Lower limit | |||||||
| < A2 vs ≥ A2 | Age | -0.039 | 0.014 | 7.853 | 0.005 | 0.962 | 0.936 | 0.988 |
| GGT | 0.015 | 0.006 | 5.970 | 0.015 | 1.015 | 1.003 | 1.027 | |
| HBV DNA | -0.147 | 0.064 | 5.306 | 0.021 | 0.863 | 0.761 | 0.978 | |
| LIF-5 | 9.616 | 1.152 | 69.727 | 0.000 | 15002.994 | 1570.174 | 143353.477 | |
| Constant | -3.643 | 0.685 | 28.240 | 0.000 | 0.026 | |||
| Eliminate diagnostic models | HBV DNA | -0.161 | 0.063 | 6.429 | 0.011 | 0.851 | 0.752 | 0.964 |
| < F2 vs ≥ F2 | GGT | 0.016 | 0.006 | 5.968 | 0.015 | 1.016 | 1.003 | 1.029 |
| HBV DNA | -0.229 | 0.061 | 14.251 | 0.000 | 0.796 | 0.707 | 0.896 | |
| APRI | 2.747 | 0.921 | 8.890 | 0.003 | 15.593 | 2.563 | 94.859 | |
| LIF-5 | 4.759 | 1.285 | 13.708 | 0.000 | 116.591 | 9.390 | 1447.723 | |
| Constant | -3.392 | 0.577 | 34.608 | 0.000 | 0.034 | |||
| Eliminate diagnostic models | HBV DNA | -0.247 | 0.061 | 16.523 | 0.000 | 0.781 | 0.693 | 0.880 |
| < A2 and < F2 vs ≥ A2 or/and ≥ F2 | HBV DNA | -0.199 | 0.055 | 13.233 | 0.000 | 0.820 | 0.737 | 0.913 |
| APRI | 3.124 | 0.888 | 12.393 | 0.000 | 22.747 | 3.995 | 129.530 | |
| LIF-5 | 4.733 | 1.182 | 16.038 | 0.000 | 113.618 | 11.207 | 1151.897 | |
| Constant | -2.903 | 0.522 | 30.931 | 0.000 | 0.055 | |||
| Eliminate diagnostic models | HBV DNA | -0.221 | 0.056 | 15.782 | 0.000 | 0.801 | 0.718 | 0.894 |
To minimize the effect of potential confounders in the comparison of liver histology damages (< A2 and < F2 vs ≥ A2 or/and ≥ F2) between the low/moderate replication group and high replication group, we matched 316 pairs (EASL guidelines) and 277 pairs (CMA guidelines) of patients by propensity score-matching. In these pairs, there were no significant differences between the low/moderate replication and high replication groups (P > 0.05) in the baseline characteristics (sex, age, ALB, GLB, AGR, ALT, AST, ALP, GGT, WBC, NLR, PLT, APRI, FIB-4, and LIF-5) (Table 4), hence achieving covariate balance. For the propensity score-matched pairs, both EASL and CMA guidelines, the group of significant liver histology damage (≥ A2 or/and ≥ F2) had much lower HBV DNA levels than that of the non- significant liver histology damage group (< A2 and < F2) (EASL guidelines: 5.81 ± 1.23 Log IU/mL vs 7.90 ± 0.49 Log IU/mL, t = -27.967, P < 0.001; CMA guidelines: 5.78 ± 1.42 Log IU/mL vs 8.01 ± 0.43 Log IU/mL, t = -24.922, P < 0.001) (Table 4).
| Variables | EASL guidelines (316 pairs) | CMA guidelines (277 pairs) | ||||||
| ≥ A2 or/and ≥ F2 | < A2 and < F2 | χ2/t/Z | SMD | ≥ A2 or/and ≥ F2 | < A2 and < F2 | χ2/t/Z | SMD | |
| Male, n (%) | 171 (54.11) | 168 (53.16) | 0.057 | 0.811 | 138 (49.82) | 153 (55.23) | 1.629 | 0.202 |
| HBeAg positive, n (%) | 305 (96.52) | 306 (96.84) | 0.049 | 0.824 | 271 (97.83) | 271 (97.83) | 0 | 1.000 |
| Age, mean ± SD, yr | 32.92 ± 10.13 | 33.06 ± 9.96 | -0.178 | 0.859 | 32.54 ± 9.04 | 33.31 ± 10.07 | -0.937 | 0.349 |
| ALB, mean ± SD, g/L | 42.30 ± 4.59 | 42.65 ± 3.78 | -1.046 | 0.296 | 42.35 ± 4.81 | 42.79 ± 3.76 | -1.187 | 0.236 |
| GLB, mean ± SD, g/L | 27.74 ± 4.83 | 27.74 ± 4.26 | 0.019 | 0.985 | 27.23 ± 4.56 | 27.53 ± 4.25 | -0.809 | 0.419 |
| AGR, mean ± SD | 1.57 ± 0.33 | 1.57 ± 0.28 | -0.037 | 0.971 | 1.59 ± 0.29 | 1.59 ± 0.28 | 0.198 | 0.843 |
| ALT, mean ± SD, U/L | 23.81 ± 8.29 | 22.78 ± 8.29 | 1.569 | 0.117 | 23.18 ± 9.10 | 23.00 ± 8.38 | 0.235 | 0.814 |
| AST, mean ± SD, U/L | 22.13 ± 8.55 | 22.57 ± 5.76 | -0.769 | 0.442 | 21.87 ± 7.74 | 22.72 ± 5.70 | -1.465 | 0.143 |
| ALP, mean ± SD, U/L | 71.64 ± 27.61 | 70.56 ± 27.09 | 0.495 | 0.621 | 72.15 ± 25.92 | 71.37 ± 27.39 | 0.345 | 0.730 |
| GGT, median (Q1-Q3), U/L | 16.00 (13.00–23.00) | 16.00 (13.00–23.00) | 0.780 | 0.435 | 16.00 (13.00-24.00) | 17.00 (13.00-23.00) | 0.590 | 0.555 |
| WBC count, mean ± SD, | 5.44 ± 1.27 | 5.47 ± 1.38 | -0.276 | 0.783 | 5.51 ± 1.31 | 5.48 ± 1.30 | 0.274 | 0.784 |
| NLR, mean ± SD | 1.91 ± 0.80 | 2.01 ± 1.04 | -1.431 | 0.153 | 1.89 ± 1.01 | 2.00 ± 1.04 | -1.334 | 0.183 |
| PLT count, mean ± SD, | 183.43 ± 42.01 | 187.96 ± 47.38 | -1.272 | 0.204 | 184.38 ± 49.39 | 187.11 ± 48.08 | -0.659 | 0.510 |
| HBV DNA, mean ± SD, log IU/mL | 5.81 ± 1.23 | 7.90 ± 0.49 | -27.967 | < 0.001 | 5.78 ± 1.42 | 8.01 ± 0.43 | -24.922 | < 0.001 |
| APRI, median (Q1– Q3) | 0.31 (0.23-0.38) | 0.29 (0.23-0.38) | 0.507 | 0.612 | 0.32 (0.22–0.39) | 0.30 (0.23-0.38) | -0.147 | 0.883 |
| FIB-4, median (Q1–Q3) | 0.80 (0.49-1.11) | 0.80 (0.58-1.12) | 1.460 | 0.144 | 0.74 (0.50-1.17) | 0.82 (0.59-1.13) | -1.110 | 0.267 |
| LIF-5, mean ± SD | 0.37 ± 0.14 | 0.36 ± 0.14 | 0.903 | 0.367 | 0.35 ± 0.14 | 0.36± 0.14 | -0.187 | 0.852 |
At present, chronic HBV infections are classified by treatment indications which are based mainly on serum HBV DNA, ALT, and liver disease severity[2,4-7]. The treatment indications were easy to identify clinically. However, we should pay more attention to those not meeting treatment indications (the so-called gray-zone patients), and there is a considerable number of such people. One retrospective cohort study[20] involved 3366 CHB patients came from 5 clinical centers of America and 7 towns of Taiwan, China which were followed up for at least 1 year and the mean time was 12.5 years. Staging of the disease was determined according to the American Association for the Study of Liver Diseases (AASLD) 2018 hepatitis B guidance[21]. The result showed that patients in the indeterminate phase count for 50.9% in American cohort and 31.8% in Taiwan, China with an average of 38.7%. Yao et al[22] also adopted the same guidelines (ALT < ULN, male for 35 U/L and female for 25 U/L), and 4759 CHB patients in Nanjing, China were included among which 27.8% were in the indeterminate phase.
According to the guidelines/CHB treatment algorithm in the United States[2,4,5], the ‘gray-zone’ population is defined as the following: (1) ALT < ULN and HBV DNA < 2 × 103 IU/mL (most are HBeAg negative, that is, inactive CHB or HBeAg-negative HBV infection); (2) ALT continues to be normal, 2 × 103 ≤ HBV DNA ≤ 107 IU/mL (EASL guidelines ) or ≤ 2 × 107 IU/mL (CMA guidelines), that is, CHB in the indeterminate phase; and (3) immune-tolerant CHB (HBV DNA > 107 IU/mL or > 2 × 107 IU/mL).
Actually, it is an indisputable fact that a high proportion of the ‘gray-zone’ population still have disease progression[8,23-30]. A previous study found that in the ‘gray-zone’ population with ALT < 2 × ULN, 510 of 1148 patients (44.42%) had liver pathological changes ≥ A2 or/and ≥ F2, and in those with ALT < 1 × ULN, nearly 30% had liver pathological changes ≥ A2 or/and ≥ F2[31], regardless of the ULN cutoff of ALT (50 U/L or 30 U/L for men; 40 U/L or 19 U/L for women). In this study, among 634 patients with ALT < ULN, 136 (21.45%) had liver inflammation ≥ A2, 154 (24.29%) had liver fibrosis ≥ F2, and 192 (30.28%) had treatment indications (≥ A2 or/and ≥ F2). The judgment of treatment indications is not only based on ALT level, although ALT is the most commonly used surrogate indicator reflecting liver cell damage. In addition, other surrogate markers including non-invasive tests have been rapidly developed[32-35]. In current, APRI and FIB-4 are the most widely used diagnostic models, but they are not that accurate in assessing the degree of HBV-related liver fibrosis[36]. In a previous study, a linear diagnosis model LIF-5 [LIF-5 = 0.725 + 0.005 × age + 0.003 × ALT + 0.004 × AST - 0.201 × (A/G) - 0.002 × PLT (109/L)][18] was constructed for the treatment indication judgment (A≥ 2 and/or F ≥ 2) of CHB patients with ALT < 2 × ULN, which had higher diagnostic value than APRI and FIB-4. This study also confirmed that the LIF-5 value (positive correlation) was an independent risk factor for liver inflammation activity, liver fibrosis, and treatment indication in CHB patients with ALT < ULN.
The univariate analysis suggested that the above indexes or diagnostic models, such as gender male, HBeAg negativity, increase in age, GLB, ALT, AST, GGT, APRI, FIB-4, and LIF-5, and decrease in ALB, AGR and PLT were correlated with the liver histopathological severity. However, during the logistic regression analysis, only HBV DNA (negative correlation) and LIF-5 (positive correlation) were independent risk factors for liver histopathological severity (liver inflammatory activity, liver fibrosis, and treatment indication). After excluding the diagnostic models, HBV DNA (negative correlation) was still an independent risk factor for the dependent variables mentioned above. Regardless of ALT level, both entire cohort and propensity score-matched pairs, patients in the low/moderate replication group had more serious liver disease (including liver pathological changes and hematological indicators). And patients with A ≥ 2, F ≥ 2, and treatment indication (≥ A2 or/and ≥ F2) in the low-replication group (HBV DNA < 2 × 103) accounted for 32.65%, 40.82%, and 44.90%, respectively, while patients in the high replication group had relatively mild pathological changes. In the entire cohort, the mean HBV DNA levels were 6.35 ± 1.91 Log IU/mL and 5.56 ± 1.59 Log IU/mL for liver inflammatory activity <A2 and ≥ A2; 6.44 ± 1.87 Log IU/mL and 5.39 ± 1.64 Log IU/mL for liver fibrosis < F2 and ≥ F2; and 6.47±1.88 Log IU/mL and 5.52 ± 1.67 Log IU/mL at treatment indication (< A2 and < F2) and (≥ A2 or/and ≥ F2), respectively. In the propensity score-matched pairs, as treatment indication (< A2 and < F2) and (≥ A2 or/and ≥ F2), the mean HBV DNA levels were 7.90 ± 0.49 Log IU/mL and 5.81 ± 1.23 Log IU/mL (EASL guidelines), 8.01 ± 0.43 Log IU/mL and 5.78 ± 1.42 Log IU/mL (CMA guidelines), respectively.
Obviously, it is unreasonable to set a defined value of HBV DNA to judge the state of CHB disease (natural course) and to guide whether to start treatment. First, despite the correlation between the HBV DNA level and the severity of the disease, the results are not consistent[10-13,37-39]. In this study, regardless of ALT values, patients with HBV DNA low/moderate replication had more serious liver disease. The high level of HBV DNA replication causes the deficiency and dysfunction of the HBsAg specific cytotoxic T lymphocytes, leading to the consequent immune tolerance. However, during the prolonged reproduction, HBV interacts with the host immune system, which can induce a cumulative immune damage. The hepatocytes suffer occult and persistent pathological apoptosis, with HBV DNA decreases accordingly, while the liver damage continues[40].
Therefore, in the absence of liver pathology, can individuals with HBV DNA < 2 × 103 IU/mL be identified as the ‘inactive carriers’ when ALT or/and transient elastography (TE) and other indicators are normal? However, whether individuals with 2 × 103 ≤ HBV DNA ≤ 107 IU/mL can be identified as immune tolerant is still unclear (the indeterminate phase). Second, the correlation ship between the HBV DNA level and the progression of end-stage liver disease (such as HCC) is still controversial. Patients in the indeterminate phase without antiviral therapy had significantly higher risk of developing HCC than those in inactive phase[20,41]. Although patients with high HBV DNA level have a heighten risk of HCC progression in the immune tolerant phase, different studies have still held different views[42-44]. This study has shown the same option with the other studies that the proportion of patients with significant liver tissue damage and HCC progression in the immune tolerant phase is relatively low[43,44], and whether conduct antiviral therapy for them has always been a hot controversy topic on clinic. The patients with a low HBV DNA level (low-level viremia) still have high risk of disease progression[13,45-48]. Third, for CHB patients in the immune active phase, the defined value of HBV DNA exceeds 2 × 103 IU/mL when HBeAg is negative, while the value of HBV DNA exceeds 107 IU/mL or 2 × 107 IU/mL when HBeAg is positive, for which needs further exploration.
There were some limitations in this study. First, the ULN of ALT was 40 U/L, while the AASLD guidelines recommend 35 IU/L for men and 25 IU/L for women[6]. The lower ULN of ALT may help us find more suitable patients needed for treatment. Second, this study had a large time span, the patients enrolled earlier had no TE results due to the absence of the Fibroscan test. Third, it was a cross-sectional study, thus lacking follow-up data. The last, this study didn’t determine the HBV genotypes. The dominant genotypes in China are genotype B and C with higher incidence of mother to child transmission, and genotype C infections are more prone to progress to HCC earlier[2,40]. These limitations need to be addressed in the further studies.
In summary, this study analyzed the risk factors for liver histopathological severity in CHB patients with normal ALT. It found that HBV DNA (negative correlation) was an independent risk factor for liver disease progression. Because of the widespread use of first-line antiviral drugs and the underlying idea of ‘no virus, no disease’, it was presumed that the states of CHB disease (natural course) were not that suitable judged by the defined values of HBV DNA level. The classification of CHB may be revised based on whether HBV DNA exceeds the detection value. Patients who are in the indeterminate phase or regarded as the ‘inactive carriers’ (low HBV DNA, low-level viremia) should receive antiviral therapy.
Chronic hepatitis B (CHB) patients can be divided into treatment indication and non-treatment indication individuals. Normal alanine transaminase (ALT) patients in ‘immune-tolerant’ phase with hepatitis B virus (HBV) DNA higher than 107 or 2 × 107 IU/mL and in ‘inactive-carrier’ phase with HBV DNA lower than 2 × 103 IU/mL do not require antiviral therapy. In fact, we should pay more attention to those who do not match the treatment indications (gray-zone patients both in the indeterminate phase and in the ‘inactive-carrier’ phase).
In order to analyze the correlation of HBV DNA level and liver histopathological severity, and to explore the significance of HBV DNA for CHB with normal ALT. Patients who are in the indeterminate phase or regarded as the ‘inactive carriers’ (low HBV DNA, low-level viremia) may have severe liver disease pathologically and hematologically.
The states of CHB disease (natural course) were not that suitable judged by the defined values of HBV DNA level. The classification of CHB may be revised based on whether HBV DNA exceeds the detection value. Patients who are in the indeterminate phase or regarded as the ‘inactive carriers’ (low HBV DNA, low-level viremia) should receive antiviral therapy.
From January 2017 to December 2021, a retrospective cross-sectional set of 1299 patients with chronic HBV infection (HBV DNA > 30 IU/mL) who underwent liver biopsy from four hospitals, including 634 with ALT less than 40 U/L. The degrees of liver necroinflammatory activity and liver fibrosis were evaluated according to the Metavir system. Patients were divided into two groups: Low/moderate replication group, HBV DNA ≤ 107 IU/mL [the European Association for the Study of the Liver (EASL) guidelines] or ≤ 2 × 107 IU/mL [the Chinese Medical Association (CMA) guidelines]; high replication group, HBV DNA > 107 IU/mL or > 2 × 107 IU/mL. Relevant factors for liver histopathological severity were analyzed by univariate analysis, logistics analysis and propensity score-matched analysis.
At entry, there were 21.45%, 24.29%, and 30.28% of the patients had liver histopathological severities with ≥ A2, ≥ F2, and ≥ A2 or/and ≥ F2, respectively. HBV DNA level (negative correlation) and noninvasive model liver fibrosis 5 value (positive correlation) were independent risk factors for liver histopathological severities (liver necroinflammation, liver fibrosis, and treatment indication). HBV DNA level (negative correlation) was still an independent risk factor when diagnostic models were excluded. For the propensity score-matched pairs, whether based on EASL guidelines or CMA guidelines, the group with significant liver histology damage (≥ A2 or/and ≥ F2) showed much lower HBV DNA level than the group with non- significant liver histology damage (< A2 and < F2). Patients in the moderate replication group (with indeterminate phase) had the most serious liver disease pathologically and hematologically, followed by patients in the low replication group (with ‘inactive-carrier’ phase) and then the high replication group (with ‘immune-tolerant’ phase).
HBV DNA level is a negative risk factor for liver damage. The phase definition of CHB may be revised by whether the level of HBV DNA exceeds the detection low limit value. Normal ALT patients who are in the indeterminate phase or ‘inactive carriers’ should receive antiviral therapy.
How to define the natural history of chronic HBV infection and how to identify the patients with normal ALT who need treatment?
Thanks to Professor Liu LY of Ningbo University for her guidance and help on statistics in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fraga RS, Brazil; Reiche W, United States S-Editor: Yan JP L-Editor: A P-Editor: Cai YX
| 1. | Thomas DL. Global Elimination of Chronic Hepatitis. N Engl J Med. 2019;380:2041-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 2. | Chinese Society of Infectious Diseases; Chinese Medical Association; Chinese Society of Hepatology, Chinese Medical Association. [The guidelines of prevention and treatment for chronic hepatitis B (2019 version)]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:938-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 111] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. Global hepatitis report, 2017. April 19, 2017. [cited January 20, 2023]. Available from: https://www.who.int/publications-detail-redirect/9789241565455. |
| 4. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 3801] [Article Influence: 475.1] [Reference Citation Analysis (1)] |
| 5. | Martin P, Nguyen MH, Dieterich DT, Lau DT, Janssen HLA, Peters MG, Jacobson IM. Treatment Algorithm for Managing Chronic Hepatitis B Virus Infection in the United States: 2021 Update. Clin Gastroenterol Hepatol. 2022;20:1766-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 6. | World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. March 1, 2015. [cited January 20, 2023]. Available from: https://www.who.int/publications/i/item/9789241549059. |
| 7. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1959] [Article Influence: 217.7] [Reference Citation Analysis (0)] |
| 8. | Erratum to: Huang X-D, Li Y-C, Chen F-P, Zheng W-H, Zhou G-Q, Lin L, Hu J, He W-J, Zhang L-L, Kou J, Ma J, Zhang W-D, Qi Z-Y, and Sun Y. Evolution and Dosimetric Analysis of Magnetic Resonance Imaging-Detected Brain Stem Injury After Intensity Modulated Radiation Therapy in Nasopharyngeal Carcinoma. Int J Radiat Oncol Biol Phys 2019;105:124-131. Int J Radiat Oncol Biol Phys. 2020;106:651. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Kim JD, Choi JY, Bae SH, Yoon SK, Yang JM, Han NI, Choi SW, Lee CD, Lee YS, Jung ES. Hepatitis B virus load in serum does not reflect histologic activity in patients with decompensated cirrhosis. Clin Gastroenterol Hepatol. 2010;8:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Xing YF, Zhou DQ, He JS, Wei CS, Zhong WC, Han ZY, Peng DT, Shao MM, Sham TT, Mok DK, Chan CO, Tong GD. Clinical and histopathological features of chronic hepatitis B virus infected patients with high HBV-DNA viral load and normal alanine aminotransferase level: A multicentre-based study in China. PLoS One. 2018;13:e0203220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Shen J, Dai J, Zhang Y, Xie F, Yu Y, Li C, Wen T. Baseline HBV-DNA load plus AST/ALT ratio predicts prognosis of HBV-related hepatocellular carcinoma after hepatectomy: A multicentre study. J Viral Hepat. 2021;28:1587-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Zacharakis G, Koskinas J, Kotsiou S, Tzara F, Vafeiadis N, Papoutselis M, Maltezos E, Sivridis E, Papoutselis K. The role of serial measurement of serum HBV DNA levels in patients with chronic HBeAg(-) hepatitis B infection: association with liver disease progression. A prospective cohort study. J Hepatol. 2008;49:884-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Chen JD, Yang HI, Iloeje UH, You SL, Lu SN, Wang LY, Su J, Sun CA, Liaw YF, Chen CJ; Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer in HBV (REVEAL-HBV) Study Group. Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology. 2010;138:1747-1754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 14. | Praneenararat S, Chamroonkul N, Sripongpun P, Kanngurn S, Jarumanokul R, Piratvisuth T. HBV DNA level could predict significant liver fibrosis in HBeAg negative chronic hepatitis B patients with biopsy indication. BMC Gastroenterol. 2014;14:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J Hepatol. 2018;69:154-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 636] [Cited by in RCA: 588] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 16. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3246] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 17. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M; APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3566] [Article Influence: 187.7] [Reference Citation Analysis (0)] |
| 18. | Jiang SW, Hu A, Yan HD, Jin SS, Hu Y. Analysis on the judgment values of three non-invasive score systems (LIF-5, APRI and FIB-4) for treatment indication in chronic HBV infected patients with ALT less than two times of upper limits of normal. Zhonghua Quanke Yixue. 2017;15:558-561. |
| 19. | Pagliaro L. Lebrec D, Poynard T, Hillon P, Benhamou J-P. Propranolol for prevention of recurrent gastrointestinal bleeding in patients with cirrhosis. A controlled study [N Engl J Med 1981;305:1371-1374]. J Hepatol. 2002;36:148-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Huang DQ, Li X, Le MH, Le AK, Yeo YH, Trinh HN, Zhang J, Li J, Wong C, Cheung RC, Yang HI, Nguyen MH. Natural History and Hepatocellular Carcinoma Risk in Untreated Chronic Hepatitis B Patients With Indeterminate Phase. Clin Gastroenterol Hepatol. 2022;20:1803-1812.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (1)] |
| 21. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2844] [Article Influence: 406.3] [Reference Citation Analysis (0)] |
| 22. | Yao K, Liu J, Wang J, Yan X, Xia J, Yang Y, Wu W, Liu Y, Chen Y, Zhang Z, Li J, Huang R, Wu C. Distribution and clinical characteristics of patients with chronic hepatitis B virus infection in the grey zone. J Viral Hepat. 2021;28:1025-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 23. | Liu J, Wang J, Yan X, Xue R, Zhan J, Jiang S, Geng Y, Liu Y, Mao M, Xia J, Yin S, Tong X, Chen Y, Ding W, Huang R, Wu C. Presence of Liver Inflammation in Asian Patients With Chronic Hepatitis B With Normal ALT and Detectable HBV DNA in Absence of Liver Fibrosis. Hepatol Commun. 2022;6:855-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 24. | Kumar M, Sarin SK, Hissar S, Pande C, Sakhuja P, Sharma BC, Chauhan R, Bose S. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008;134:1376-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 320] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 25. | Xie Y, Yi W, Zhang L, Lu Y, Hao HX, Gao YJ, Ran CP, Lu HH, Chen QQ, Shen G, Wu SL, Chang M, Ping-Hu L, Liu RY, Sun L, Wan G, Li MH. Evaluation of a logistic regression model for predicting liver necroinflammation in hepatitis B e antigen-negative chronic hepatitis B patients with normal and minimally increased alanine aminotransferase levels. J Viral Hepat. 2019;26 Suppl 1:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Vlachogiannakos J, Papatheodoridis GV. HBV: Do I treat my immunotolerant patients? Liver Int. 2016;36 Suppl 1:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Oliveri F, Surace L, Cavallone D, Colombatto P, Ricco G, Salvati N, Coco B, Romagnoli V, Gattai R, Salvati A, Moriconi F, Yuan Q, Bonino F, Brunetto MR. Long-term outcome of inactive and active, low viraemic HBeAg-negative-hepatitis B virus infection: Benign course towards HBsAg clearance. Liver Int. 2017;37:1622-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Chao DT, Lim JK, Ayoub WS, Nguyen LH, Nguyen MH. Systematic review with meta-analysis: the proportion of chronic hepatitis B patients with normal alanine transaminase ≤ 40 IU/L and significant hepatic fibrosis. Aliment Pharmacol Ther. 2014;39:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Nguyen LH, Chao D, Lim JK, Ayoub W, Nguyen MH. Histologic changes in liver tissue from patients with chronic hepatitis B and minimal increases in levels of alanine aminotransferase: a meta-analysis and systematic review. Clin Gastroenterol Hepatol. 2014;12:1262-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Gill US, Pallett LJ, Kennedy PTF, Maini MK. Liver sampling: a vital window into HBV pathogenesis on the path to functional cure. Gut. 2018;67:767-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Jiang SW, Hu AR, Jin SS, Yan HD, Hu YR. [Pathological features of the liver in patients with chronic HBV infection and normal alanine aminotransferase]. Zhonghua Gan Zang Bing Za Zhi. 2016;24:927-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Shiha G, Ibrahim A, Helmy A, Sarin SK, Omata M, Kumar A, Bernstien D, Maruyama H, Saraswat V, Chawla Y, Hamid S, Abbas Z, Bedossa P, Sakhuja P, Elmahatab M, Lim SG, Lesmana L, Sollano J, Jia JD, Abbas B, Omar A, Sharma B, Payawal D, Abdallah A, Serwah A, Hamed A, Elsayed A, AbdelMaqsod A, Hassanein T, Ihab A, GHaziuan H, Zein N, Kumar M. Asian-Pacific Association for the Study of the Liver (APASL) consensus guidelines on invasive and non-invasive assessment of hepatic fibrosis: a 2016 update. Hepatol Int. 2017;11:1-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 33. | Lambrecht J, Verhulst S, Mannaerts I, Reynaert H, van Grunsven LA. Prospects in non-invasive assessment of liver fibrosis: Liquid biopsy as the future gold standard? Biochim Biophys Acta Mol Basis Dis. 2018;1864:1024-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Tanwar S, Rhodes F, Srivastava A, Trembling PM, Rosenberg WM. Inflammation and fibrosis in chronic liver diseases including non-alcoholic fatty liver disease and hepatitis C. World J Gastroenterol. 2020;26:109-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 137] [Article Influence: 27.4] [Reference Citation Analysis (4)] |
| 35. | Loomba R, Adams LA. Advances in non-invasive assessment of hepatic fibrosis. Gut. 2020;69:1343-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 36. | Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015;61:292-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 391] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 37. | McMahon BJ, Bulkow L, Simons B, Zhang Y, Negus S, Homan C, Spradling P, Teshale E, Lau D, Snowball M, Livingston SE. Relationship between level of hepatitis B virus DNA and liver disease: a population-based study of hepatitis B e antigen-negative persons with hepatitis B. Clin Gastroenterol Hepatol. 2014;12:701-6.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Yuen MF, Ng IO, Fan ST, Yuan HJ, Wong DK, Yuen JC, Sum SS, Chan AO, Lai CL. Significance of HBV DNA levels in liver histology of HBeAg and Anti-HBe positive patients with chronic hepatitis B. Am J Gastroenterol. 2004;99:2032-2037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Xie Q, Hu X, Zhang Y, Jiang X, Li X, Li J. Decreasing hepatitis B viral load is associated with a risk of significant liver fibrosis in hepatitis B e antigen positive chronic hepatitis B. J Med Virol. 2014;86:1828-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Chuang YC, Tsai KN, Ou JJ. Pathogenicity and virulence of Hepatitis B virus. Virulence. 2022;13:258-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 41. | Spradling PR, Xing J, Rupp LB, Moorman AC, Gordon SC, Teshale ET, Lu M, Boscarino JA, Schmidt MA, Trinacty CM, Holmberg SD; Chronic Hepatitis Cohort Study Investigators. Distribution of disease phase, treatment prescription and severe liver disease among 1598 patients with chronic hepatitis B in the Chronic Hepatitis Cohort Study, 2006-2013. Aliment Pharmacol Ther. 2016;44:1080-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 42. | Kim GA, Lim YS, Han S, Choi J, Shim JH, Kim KM, Lee HC, Lee YS. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut. 2018;67:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 43. | Lee HA, Lee HW, Kim IH, Park SY, Sinn DH, Yu JH, Seo YS, Um SH, Lee JI, Lee KS, Lee CH, Tak WY, Kweon YO, Kang W, Paik YH, Lee JW, Suh SJ, Jung YK, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, Yim HJ, Kim SU. Extremely low risk of hepatocellular carcinoma development in patients with chronic hepatitis B in immune-tolerant phase. Aliment Pharmacol Ther. 2020;52:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 44. | Jeon MY, Kim BK, Lee JS, Lee HW, Park JY, Kim DY, Ahn SH, Han KH, Kim SU. Negligible risks of hepatocellular carcinoma during biomarker-defined immune-tolerant phase for patients with chronic hepatitis B. Clin Mol Hepatol. 2021;27:295-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Fung J, Yuen MF, Yuen JC, Wong DK, Lai CL. Low serum HBV DNA levels and development of hepatocellular carcinoma in patients with chronic hepatitis B: a case-control study. Aliment Pharmacol Ther. 2007;26:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Tseng TC, Liu CJ, Hsu CY, Hong CM, Su TH, Yang WT, Chen CL, Yang HC, Huang YT, Fang-Tzu Kuo S, Liu CH, Chen PJ, Chen DS, Kao JH. High Level of Hepatitis B Core-Related Antigen Associated With Increased Risk of Hepatocellular Carcinoma in Patients With Chronic HBV Infection of Intermediate Viral Load. Gastroenterology. 2019;157:1518-1529.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 47. | Sinn DH, Lee J, Goo J, Kim K, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC, Yoo BC, Paik SW. Hepatocellular carcinoma risk in chronic hepatitis B virus-infected compensated cirrhosis patients with low viral load. Hepatology. 2015;62:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 48. | Tseng TC, Liu CJ, Yang HC, Su TH, Wang CC, Chen CL, Kuo SF, Liu CH, Chen PJ, Chen DS, Kao JH. High levels of hepatitis B surface antigen increase risk of hepatocellular carcinoma in patients with low HBV load. Gastroenterology. 2012;142:1140-1149.e3; quiz e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 437] [Article Influence: 33.6] [Reference Citation Analysis (0)] |