Published online Apr 28, 2023. doi: 10.3748/wjg.v29.i16.2397
Peer-review started: October 31, 2022
First decision: February 2, 2023
Revised: February 17, 2023
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: April 28, 2023
Processing time: 174 Days and 20.1 Hours
Liver is unlikely the key organ driving mortality in coronavirus disease 2019 (COVID-19) however, liver function tests (LFTs) abnormalities are widely observed mostly in moderate and severe cases. According to this review, the overall prevalence of abnormal LFTs in COVID-19 patients ranges from 2.5% to 96.8% worldwide. The geographical variability in the prevalence of underlying diseases is the determinant for the observed discrepancies between East and West. Multifactorial mechanisms are implicated in COVID-19-induced liver injury. Among them, hypercytokinemia with “bystander hepatitis”, cytokine storm syndrome with subsequent oxidative stress and endotheliopathy, hypercoagulable state and immuno-thromboinflammation are the most determinant mechanisms leading to tissue injury. Liver hypoxia may also contribute under specific conditions, while direct hepatocyte injury is an emerging mechanism. Except for initially observed severe acute respiratory distress syndrome corona virus-2 (SARS-CoV-2) tropism for cholangiocytes, more recent cumulative data show SARS-CoV-2 virions within hepatocytes and sinusoidal endothelial cells using electron microscopy (EM). The best evidence for hepatocellular invasion by the virus is the identification of replicating SARS-CoV-2 RNA, S protein RNA and viral nucleocapsid protein within hepatocytes using in-situ hybridization and immunostaining with observed intrahepatic presence of SARS-CoV-2 by EM and by in-situ hybridization. New data mostly derived from imaging findings indicate possible long-term sequelae for the liver months after recovery, suggesting a post-COVID-19 persistent live injury.
Core Tip: Following respiratory system, liver is the second most involved organ in coronavirus disease 2019 (COVID-19). Besides the well-observed cholangiocyte tropism, typical severe acute respiratory distress syndrome corona virus-2 (SARS-CoV-2) Lesions indicated by ultrastructural and histological evidence, identification of replicating SARS-CoV-2, S and nucleocapsid proteins RNAs within hepatocytes, as well as intrahepatic virus observation by electron microscopy and in-situ hybridization, converge to the conclusion that SARS-CoV-2 may also be hepatotropic. Most prevalent mechanisms of COVID-19-related liver injury are hypercytokinemia with “bystander hepatitis”, cytokine storm syndrome with subsequent oxidative stress, endotheliopathy and immuno-thromboinflammation. Depending on the grade of their abnormalities, increased serum aspartate aminotransferase, (mostly peak) alanine aminotransferase, alkaline phosphatase, total bilirubin, inflammatory markers (C-reactive protein, ferritin, interleukin-6, -10) and decreased albumin levels are independent discriminators of COVID-19 severity and mortality. Age, male gender, chronic liver disease, liver cirrhosis, obesity, diabetes, and non-alcoholic fatty liver disease are independent prognostic factors of unfavorable COVID-19 outcomes.
- Citation: Liatsos GD. SARS-CoV-2 induced liver injury: Incidence, risk factors, impact on COVID-19 severity and prognosis in different population groups. World J Gastroenterol 2023; 29(16): 2397-2432
- URL: https://www.wjgnet.com/1007-9327/full/v29/i16/2397.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i16.2397
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA determined by quantitative rt- polymerase chain reaction is widely spread outside the respiratory tract, including the liver[1]. Regardless of pre-existing chronic liver disease (CLD), coronavirus disease 2019 (COVID-19)-induced liver injury (LI) is mainly reflected by hypertransaminasemia, elevations of gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP) (less frequently), and hypoalbuminemia[2-8], with the later being a negative acute phase reactant rather than manifestation of liver failure and is one of the most prevalent abnormalities. COVID-19-induced LI is secondary than primary[9,10], mostly mild, transitory and self-limiting[11], it does not impact the majority of patients[12], and is common in absence of CLD[13]. In asymptomatic/subclinical cases randomly diagnosed by computed tomography (CT) scans a mild increase in transaminases (8.8%) is observed[14]. LI definition varies among values just above the upper limit of normal (ULN)[15,16] up to 2–5 × ULN[17,18]. Substantial transaminases increases are linked to unfavorable outcomes, such as death, invasive mechanical ventilation (IMV), and intensive care unit (ICU) admission[7,18-22]. The prognostic relevance of higher liver function tests (LFTs) may result from a more vigorous host immunological and inflammatory response to infection[12], particularly in younger individuals[12,23]. The pattern of LI is typically hepatocellular rather than cholestatic[24]. Severe LI (SLI) defined as alanine aminotransferase (ALT) elevations > 10–15 × ULN with or without jaundice, occurs in 2% of COVID-19[25], while acute liver failure (ALF) without underlying CLD is extremely rare and is typically associated with severe pneumonia and multiple organ dysfunction syndrome (MODS)[26]. SARS-CoV-2-induced ALF has been described in case reports[21].
The SARS-CoV-2 spike (S) protein is recognized by the angiotensin converting enzyme-2 (ACE2), whilst the androgen-induced transmembrane serine protease-2 (TMPRSS2) and paired basic amino acid cleaving enzyme (FURIN) are necessary for cell tropism and entry[27,28]. ACE2 cleaves the vasoconstrictor peptide angiotensin II to vasodilator angiotensin I[29]. S protein interacts with ACE2[30] and with an identified co-receptor neuropilin-1[31]. FURIN evades immune surveillance thus promoting transmission[28]. SARS-CoV-2 binding to ACE2 causes inflammation, oxidative stress, and pro-apoptotic reactions, ultimately leading to LI[30]. According to single-cell RNA sequencing studies of healthy livers, cholangiocytes exhibit the highest expression of ACE-2, with modest expression found in hepatocytes, sinusoidal endothelial cells, and resident Kupffer cells[32]. Luminal immunohistochemical staining for ACE2 is observed in the bile ducts[33]. A few hepatocytes co-express both TMPRSS2 and FURIN[34,35]. Liver ductal organoids that express ACE2 and TMPRSS2 have been shown to recapitulate SARS-CoV-2 infection[36], whereas liver organoids generated from pluripotent stem cells also express ACE2 and allow SARS-CoV-2 pseudoparticle entry[36,37]. A small population of TROP2+ liver epithelial progenitors express both ACE2 and TMPRSS2. In healthy livers vs cirrhotics, 1.8/10000 cells vs 10.6/10000 expressed ACE2 and 97.2/10000 vs 216/10000 expressed TMPRSS2 representing a significant (P < 0.001) increase in the number of TMPRSS2+ cells in cirrhotics[38]. In untreated hepatitis B virus (HBV) infected livers, only 1.4/10000 and 48.3/10000 cells expressed ACE2 and TMPRSS2 respectively, significantly fewer than both healthy and cirrhotics[38]. ACE2 expression is 30 times higher in hepatitis C virus (HCV)-related cirrhosis than in healthy liver[39]. As ACE2 has been identified as an interferon-inducible gene[40,41], LI and inflammation may therefore enhance SARS-CoV-2 hepatotropism by modifying viral receptor expression, which is consistent with the damage to the respiratory epithelia[41]. In non-infected individuals with obesity and Non-alcoholic Steatohepatitis (NASH), ACE2 and TMPRSS2 Liver mRNA co-expression is likewise upregulated[42]. Non-alcoholic fatty liver disease (NAFLD) and cirrhotic livers have much higher TMPRSS2+ progenitor cells indicating a susceptibility to SARS-CoV-2, findings consistent with the sc-RNA-seq results[38]. Given the recognized link between obesity and NAFLD[43], the finding of a larger abundance of TMPRSS2+ progenitor cells in NAFLD livers may offer a potential explanation for why obese people experience more severe COVID-19[38].
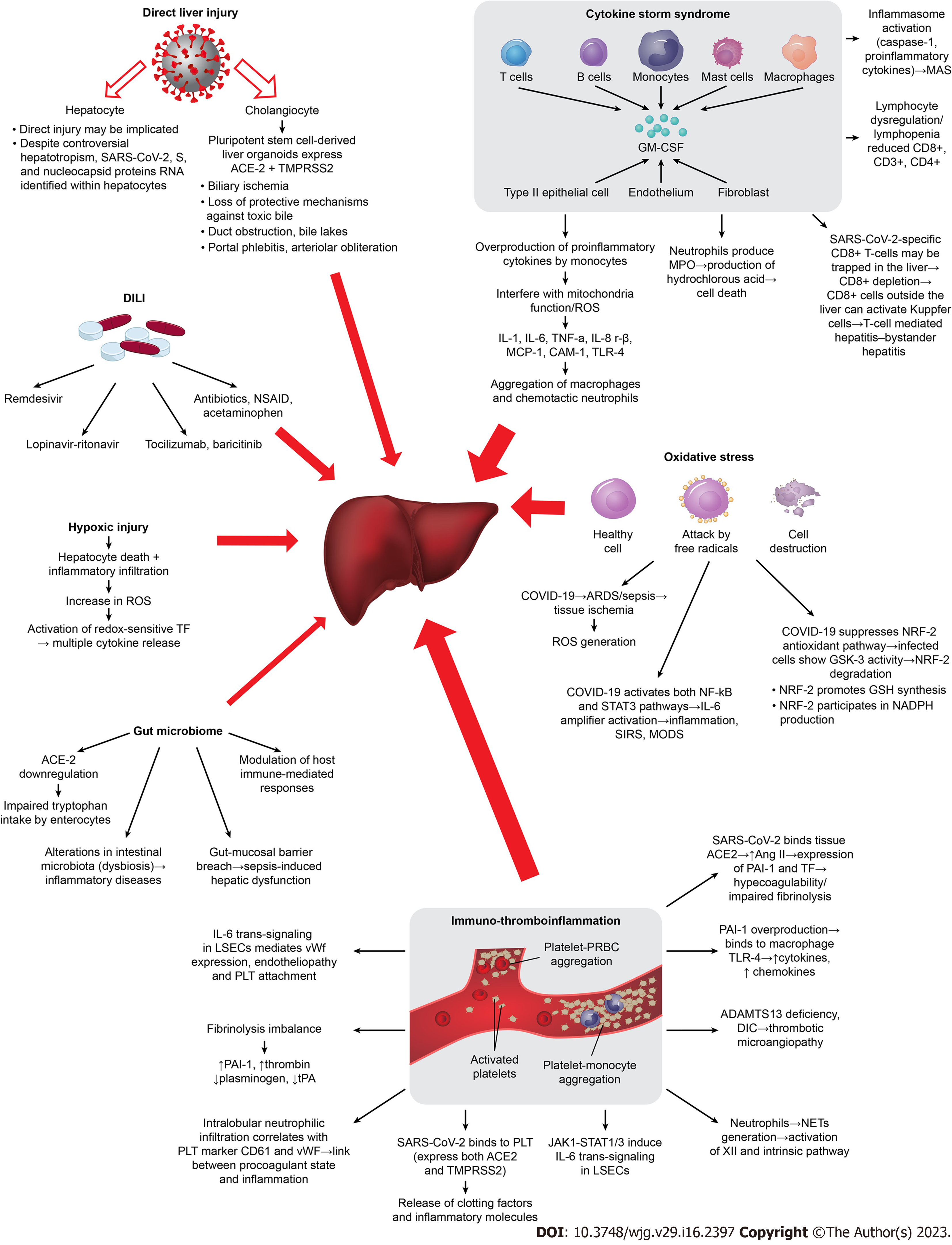
The variability in prevalence and severity of LI among COVID-19 patients suggests that the mechanisms of LI are multifactorial (Figure 1).
Many studies propose that SARS-CoV-2 hepatotropism and its direct liver function impairment is implicated in COVID-19-induced LI, while only a few speculate that definite evidence is lacking[44,45]. Liver progenitor cells, particularly those destined to become cholangiocytes, contain ACE2[46], in addition to virus isolation in bile[47] imply a direct invasion by SARS-CoV-2. Its infection triggers cell apoptosis factors resulting in cholangiocyte death[36] by lysis and/or by inducing necrosis and apoptosis[48-50]. SARS-CoV-2 virions have been seen within hepatocytes and sinusoidal endothelial cells using electron microscopy (EM)[51]. The best evidence is the identification of replicating SARS-CoV-2 RNA, S, and nucleocapsid proteins RNA within hepatocytes using in-situ hybridization and immunostaining[51,52]. In hepatocytes and sinusoidal endothelial cells, SARS-CoV-2 virions have been seen using EM[51]. The strongest supporting data were found employing in-situ hybridization and immunostaining to identify replicating SARS-CoV-2 RNA, S, and nucleocapsid proteins RNA within hepatocytes[51,52].Viral genomic RNA was also identified in postmortem COVID-19 Liver examinations[53,54], with observed intrahepatic presence of SARS-CoV-2 by EM and by in-situ hybridization[55-57], and viral replication within hepatocytes[58,59], thus reinforcing the role of direct SARS-CoV-2 hepatocyte injury. SARS-CoV-2 particles without membrane-bound vesicles were found in the hepatocyte cytoplasm of COVID-19 patients with aberrant LFTs[51], which is additional proof.
Hypoxia can cause hepatocytes inflammatory cells infiltration, lipid accumulation, an increase in reactive oxygen species (ROS), and death[60,61]. ROS peroxidation products act as a second messenger amplifying the release of multiple cytokines[62]. In COVID-19, hypoxia and cytokine storm syndrome (CSS) are considered as risk factors for LFT abnormalities[63]. Hypoxic hepatitis features (e.g. centrilobular necrosis) are widely shown in postmortem liver biopsies[64]. In severe COVID-19, IMV, positive end-expiratory pressure, and/or vasopressor support negatively impact hepatic perfusion by lowering cardiac output, raising hepatic vascular resistance, and increasing portal vein pressure, which obstructs venous drainage, leading to acute LI (ALI) and/or cholestasis[58,65,66]. Gut ischemic injury on the other hand, results in intestinal endotoxinaemia and activation of the sympathetic nervous and adrenocortical systems furthermore contributing to LI[58,66]. Additionally, Kupffer cells can stimulate cytokines due to ischemia[67], while mitochondrial damage by SARS-CoV-2 results in aspartate aminotransferase (AST) release[68-70]. Direct interaction of mitochondrial proteins with the virus nonstructural protein 5 provides a probable reason for the AST-dominant liver profile[71]. In addition, unlike the hepatic preponderance of ALT, zone 3 of the hepatic acini containing higher AST concentrations is more susceptible to hypoxic injury[72]. In COVID-19 aminotransferases elevations, typically mild, are incompatible with very high AST/ALT elevations of primary hypoxic hepatitis[73]. Secondary hypoxic LI owing to the presence of acute respiratory distress syndrome (ARDS) as well as to an overactive inflammatory response to SARS-CoV-2 and MODS[73] might be implicated. In addition, cell iron overload might play a role, and hepcidinmimetic action of S protein may induce ferroportin blockage[25].
In order to maintain homeostasis, the body activates the immunological defense system and the oxidative stress response with the release of many cytokines when activated by endogenous or external stimuli like viruses[74]. Severe COVID-19 exhibits a distinct immunological dysregulation with two essential characteristics: Lymphocyte dysregulation with lymphopenia and overproduction of pro-inflammatory cytokines by monocytes[75,76]. The relationship between lymphopenia and CSS in COVID-19 pathogenesis was described in previous coronaviruses outbreaks[77,78]. Severe hypercytokinemia results in a cascade of actions leading to tissue (especially liver) damage and MODS[79]. Lymphopenia, decreased CD4+, early and persistent elevation of cytokines [tumor necrosis factor-α (TNF-α), interleukin (IL)-2, -6, -7, -10, -18, granulocyte-colony stimulating factor, interferon gamma (IFN-γ), interferon gamma-induced protein 10 (IP-10), monocyte chemotactic protein-1 (MCP-1), and macrophage inflammatory-protein-1a, chemokines], lactate dehydrogenase (LDH), C-reactive protein (CRP), ferritin, D-dimer and of coagulopathy markers (thrombopoietin), are independent risk factors for SLI (Table 1), and are linked to unfavorable outcomes[23,37,50,59,80-87]. CSS in severe COVID-19 is also associated with reduced CD8+, CD3+ and CD4+ T-cells[88,89]. Depletion of circulating CD8+ T-cells, the main determinant of LI in viral infections (influenza, measles, and SARS), reflects their trapping in the liver[90,91]. The syndrome known as "bystander hepatitis," which is frequently seen in systemic viral infections[92] and in COVID-19[93], is caused by circulating cytokines activating hepatic immune cells without compromising liver function. By activating Kupffer cells in the absence of viral antigens in the liver, viral-specific CD8+ T-cells that are confined to locations outside the liver may cause T-cell-mediated hepatitis[94]. Also, T-cells depletion cannot control the viral infection, leading to macrophage activation and more secondary inflammatory reactions[95,96]. In severe situations, the SARS-CoV-2 virus may cause a hyperinflammatory disease known as macrophage activation syndrome (MAS) or secondary hemophagocytic lymphohistiocytosis[75,76]. This syndrome is characterized by CSS, cytopenias, disseminated intravascular coagulation (DIC) and MODS. The pathogenesis of cytokine-driven hyperinflammatory disorders is heavily dependent on IL-6 signaling[76], that strongly correlates with elevated transaminases[88]. Inflammasome, a complex intracellular protein that SARS-CoV-2 may produce, helps promote caspase-1's autocatalytic activation (apoptosis/pyrolysis) and the exudation of pro-inflammatory cytokines[97], triggering the expression of other genes involved in the immune process[98], therefore resulting in MODS[87]. However, patients with mild COVID-19 may experience LFT abnormalities regardless of their inflammatory condition, probably because the unique inflammation brought on by SARS-CoV-2 is more likely to do so than inflammation brought on by other pathogens[99].
| Author/yr | Type of study (n of patients) | Factor | Outcome - statistical significance (severity/mechanical ventilation/ICU/mortality) |
| Krishnan et al[321], 2022, United States | Retrospective (n = 3830) | TBIL1 | |
| 2–5 × ULN | Mortality risk significantly increased 6-fold (P < 0.001) | ||
| > 5 × ULN | Mortality risk increased 7.86-fold (P = 0.005) | ||
| AST1 | |||
| 2–5 × ULN | All-cause mortality HR, 1.49; P < 0.001 | ||
| > 5 × ULN | All-cause mortality HR, 2.19; P = 0.005 | ||
| ALP1 | |||
| 1–2 × ULN | All-cause mortality risk increased 1.42-fold (P = 0.009) | ||
| > 2–5 × ULN | All-cause mortality risk increased 1.81-fold (P = 0.032) | ||
| Inflammatory markers | |||
| CRP | aHR, 1.04 associated with mortality (P = 0.001) | ||
| Ferritin | aHR, 1.0 associated with mortality (P = 0.001) | ||
| IL-6 | aHR, 1.0 associated with mortality (P = 0.001) | ||
| neutrophil count | aHR, 1.0 associated with mortality (P = 0.008) | ||
| D-Dimer | aHR, 1.03 associated with mortality (P = 0.004) | ||
| LDH | aHR, 1.0 associated with mortality (P < 0.001) | ||
| AST, ALT, TBIL | Significantly increased for those who received MV (P < 0.0001) | ||
| Kodavoor et al[180], 2022, India | Retrospective (n = 708) | AST1 | aOR 1.007, per 1 IU/L increase for SD |
| AST1 | aHR 1.002 per 1 IU/L increase for mortality | ||
| Sensitivity/specificity | 90.6%/67% to predict mortality | ||
| PPV/NPV | 17.5%/95.73% to predict mortality | ||
| Albumin1 | aOR 0.217 per 1 g/dL increase for SD | ||
| aHR 0.396 per 1 g/dL increase for mortality | |||
| Lombardi et al[230], 2022, Italy | Retrospective (n = 382) | Transaminases1 | |
| > 2 × ULN | OR 2.6, 95%CI: 1.3–6.7 for SD | ||
| FIB-4 score < 1.451 | (OR 0.4; P = 0.04) protective factor for mortality | ||
| Hartl et al[326], 2022, Austria | Retrospective (n = 900) | AST1 | aHR: 1.47; P = 0.043 for mortality |
| TBIL1 | aHR: 2.20; P = 0.009 for mortality | ||
| Siddiqui et al[229], 2022, United States | Retrospective (n = 1935) | Abnormal LFTs | |
| Liver injury defined as: (AST/ALT > 3 × ULN or ALP/TBIL > 2 × ULN) | RR, 4.26; P < 0.0001 risk for mortality | ||
| Mild elevated enzymes | RR, 5.52; P < 0.0001 for ICU admission | ||
| (Levels lower than LI) | RR, 11.01; P < 0.0001 for MV | ||
| RR, 2.16; P < 0.0001 for mortality | |||
| RR, 2.48; P < 0.0001 ICU admission | |||
| Cirrhotics | RR, 3.76; P < 0.0001 for MV | ||
| RR, 2.19; P = 0.0022 for mortality | |||
| Cai et al[20], 2020, China | Retrospective (n = 417) | Hepatocellular LI | OR, 2.73; P = 0.02 for severe disease |
| Mixed LI | OR, 4.44; P < 0.001 for severe disease | ||
| LI1 | aOR, 9.04; P < 0.001 for severe disease | ||
| Huang et al[191], 2020, China | Retrospective (n = 675) | AST1 3-fold ULN | aOR, 19.27; P < 0.0001 for mortality |
| aOR, 116.72; P < 0.0001 for MV | |||
| Lei et al[192], 2020, China | Retrospective (n = 5771) | AST1 40-120 U/L | aOR, 4.81; P < 0.001 for all-cause mortality |
| AST1 > 120 U/L | aOR, 14.87; P < 0.001 for all-cause mortality | ||
| Ding et al[22], 2020, China | Retrospective (n = 2073) | Abnormal AST1 | aHR, 1.39; P = 0.027 for mortality |
| Abnormal DBIL1 | aHR, 1.66; P = 0.001 for mortality | ||
| LI during hospitalization1 | aHR, 4.63; P < 0.001 for in-hospital mortality | ||
| LI at admission1 | aHR 1.87; P = 0.003 for in-hospital mortality | ||
| Mixed LI1 | aHR, 4.77; P < 0.001 for in-hospital mortality | ||
| Cholestatic LI1 | aHR, 3.99; P = 0.008 for in-hospital mortality | ||
| Phipps et al[23], 2020, United States | Retrospective (n = 3381) | Ferritin1 | OR, 2.40; P < 0.001 for SLI |
| IL-61 | OR, 1.45; P = 0.009 for SLI | ||
| Peak ALT1 | OR, 1.14; P = 0.044 for mortality | ||
| Older age1 | OR, 1.07; P < 0.001 for mortality | ||
| DM1 | OR, 1.30; P = 0.045 for mortality | ||
| Medetalibeyoglu et al[221], 2020, Turkey | Retrospective (n = 554) | AST/ALT > 1 | AUC = 0.713, P = 0.001 marker of mortality risk |
| AUC = 0.636, P = 0.001 for ICU admission | |||
| Chen et al[199], 2020, China | Retrospective (n = 502) | Grade of Liver damage1 | aHR, 1.377; P = 0.049 risk factor for mortality |
| Mishra et al[200], 2021, United States | Retrospective (n = 348) | AST1 (1 unit increase) IU/L Peak AST1 (1 unit increase) | OR, 1.011; P = 0.006 for mortality |
| Peak ALT1 (1 unit increase) | OR, 1.007; P < 0.001 for mortality | ||
| TBIL1 (1 unit increase) mg/dL | OR, 1.005; P = 0.003 for mortality | ||
| Alb1 (1 unit increase) g/dL | OR, 1.997; P = 0.04 | ||
| Male1 | OR, 0.5; P = 0.01 | ||
| BMI > 40 kg/m2 | OR, 1.94; P = 0.001 | ||
| LI1 | OR, 2.17; P = 0.003 | ||
| OR, 1.79; P = 0.008 | |||
| Chew et al[190], 2021, United States | Retrospective (n = 834) | Ischemic disease state1 | OR, 2.4; P = 0.001 for mortality |
| Hypecoagulable1 | OR, 1.7; P = 0.02 for mortality | ||
| Hyperinflammatory1 | OR, 1.9; P = 0.02 for mortality | ||
| Ponziani et al[327], 2021, Italy | Retrospective (n = 515) | ALP1 peak value | aOR, 1.007; P = 0.005 for mortality |
| CRP1 | aOR, 1.007; P = 0.008 for mortality | ||
| Piano et al[246], 2020, Italy | Retrospective (n = 565) | Abnormal LFTs1 | OR, 3.53; P < 0.001 for ICU admission/death |
| Yip et al[287], 2021, China | Retrospective (n = 1040) | ALT/AST1 ≥ 2 × ULN | aOR, 7.92; P < 0.001 for ICU/MV/death |
| Marjot et al[237], 2021, multinational | Retrospective (n = 785) | Age1 | OR, 1.02; P = 0.011 for mortality |
| Cirrhotics CTP-A1 | OR, 1.90; P = 0.040 for mortality | ||
| Cirrhotics CTP-B1 | OR, 4.14; P < 0.001 for mortality | ||
| Cirrhotics CTP-C1 | OR, 9.32; P < 0.001 for mortality | ||
| ArLD1 | OR, 1.79; P = 0.040 for mortality | ||
| Lee et al[328], 2020, South Korea | Retrospective (n = 1005) | Age1 | aHR = 4.96; P < 0.001 for mortality |
| Liver cirrhosis1 | aHR = 2.86; P = 0.042 fro mortality | ||
| DM1 | aHR = 2.29; P < 0.001 for mortality | ||
| COPD1 | aHR = 4.52; P = 0.001 for mortality | ||
| Singh et al[236], 2020, United States | Retrospective (n = 2780) | CLD1 | RR, 2.8; P < 0.001 risk of mortality |
| propensity matching | RR, 3.0; P = 0.001 risk of mortality | ||
| Cirrhotics1 | RR, 4.6; P < 0.001 risk of mortality | ||
| Hashemi et al[232], 2020, United States | Retrospective (n = 363) | CLD1 | aOR 1.77; P = 0.04 for ICU admission |
| aOR, 2.08; P = 0.0092 for IMV | |||
| Cirrhotics1 | aOR, 12.5; P = 0.009 mortality risk | ||
| Sarin et al[235], 2020, Asian | Retrospective (n = 228 CLD) | Cirrhotics1 | |
| AST/ALT > 1.4 | HR = 1.4; P = 0.02 for mortality | ||
| Obesity | OR = 8.1; P = 0.002 for LI | ||
| Decompensated | OR = 2.5; P = 0.05 for mortality | ||
| CTP score > 8 | HR = 19.2; P < 0.001 for mortality | ||
| DM in CLD non-cirrhotics | OR = 2.1; P = 0.01 for LI | ||
| Wang et al[51], 2020, China | Retrospective (n = 657) | Male gender1 | OR, 2.038; P < 0.001 for LI |
| hsCRP ≥ 10 mg/L | OR, 1.733; p = 0.014 for LI | ||
| NLR ≥ 5 | OR, 2.154; P < 0.001 for LI | ||
| Zhang et al[183], 2020, China | Retrospective (n = 218) | Male1 | OR, 6.203; P < 0.001 risk for LI |
| Neutrophil percentage1 | OR, 1.004; P = 0.003 risk for LI | ||
| CRP1 | P < 0.001 in LI patients | ||
| D-dimer1 | OR, 1.486; P < 0.001 risk for LI | ||
| Shauly-Aharonov et al[329], 2021, Israel | Retrospective (n = 37121) | Age | OR = 1.1 for every year increase; P < 0.001) risk for severity |
| Male gender | OR = 1.34; P = 0.012 risk for severity | ||
| BMI | OR = 1.02 for 1 kg/m2 increase; P = 0.025 risk for severity | ||
| Kovalic et al[208], 2020, United States | Meta-analysis (n = 24299) | CLD1 | Pooled OR, 1.48; P = 0.001 for severity |
| Pooled OR, 1.78; P = 0.02 for mortality | |||
| Kulkarni et al[6], 2020, India | Meta-analysis Multinational (n = 20874) | Increased LFTs | OR, 3.46; P < 0.001 for mortality |
| OR, 2.87; P < 0.001 for severe disease | |||
| Sharma et al[207], 2021, United States | Meta-analysis (n = 12882) | AST1 | Pooled OR, 2.98; P < 0.00001 for poor outcomes |
| ALT1 | Pooled OR, 1.73; P < 0.0001 for poor outcomes | ||
| Del Zompo et al[323], 2020, Italy | Meta-analysis (n = 20724) | ALT1 | OR 1.54, 95%CI: 1.17-2.03 for severity |
| ALT1 | OR 1.48, 95%CI: 1.12-1.96 for mortality | ||
| AST1 | OR 3.17, 95%CI: 2.10-4.77 for severity | ||
| AST1 | OR 4.39, 95%CI: 2.68-7.18 for mortality | ||
| TBIL1 | OR 2.32, 95%CI: 1.18-4.58 for severity | ||
| TBIL1 | OR 7.75, 95%CI: 2.28-26.40 for mortality |
COVID-19 is considered to affect the endothelium, one of the largest organs in the human body[82]. SARS-CoV-2 may worsen microcirculation and encourage thrombus formation, tissue oedema, and organ ischemia by encouraging endothelial cell damage in the arteries, veins, arterioles, capillaries, and venules of all major organs[87,100-101]. Hepatic artery branches in the portal tract with endothelial enlargement and luminal constriction, as well as portal vein endophlebitis, and endotheliitis (leukocyte attachment to the vascular wall) with thrombotic material[102-105], are pathology findings indicative of endotheliopathy in COVID-19-related LI. The observed network of sinusoids decorated by CD34 suggests abnormal hepatic blood circulation[102]. In deceased patients with elevated ALT, significantly higher fibrinogen, factors VIII and II activity, and platelet marker CD61 liver staining was morphologically shown, in accordance with their serum levels (fibrinogen, D-dimer, von Willebrand factor (vWF) activity and antigen, and CRP[45,46,106,107]), thus resembling a microangiopathy thrombotic state[86,106,107]. Additionally, vWF-positive areas correlate with CD61-positive areas[60,101] and with intralobular neutrophil infiltration suggesting a link between the procoagulant state and liver inflammation[45,107]. Endotheliopathy, vWf expression on cell surfaces, and platelet adhesion are all mediated by IL-6 trans-signaling in liver sinusoidal endothelial cells (LSECs), which also plays a role in LSECs inflammation and activation of coagulation therefore being involved in COVID-19-related LI[45,107,108]. As LSECs are endothelial cells and do not express IL-6Ra, trans-signaling is thought to be the main method of IL-6 signaling to LSECs[109,110]. The Janus kinase-signal transducer and activator of transcription (JAK/STAT) pathway may also be used to promote IL-6 trans-signaling[45,107], which is essential for inducing a procoagulant and proinflammatory LSECs phenotype[111,112]. Activated neutrophils may generate neutrophil extracellular traps[113]. Decreased ADAMTS13 Levels, another typical finding in severe COVID-19 can induce increased platelet-endothelial interaction[111,114-118], while DIC may also result from CSS in critical/fatal COVID-19[119,120]. A significant imbalance between inhibitors and activators of fibrinolysis is also demonstrated. Reduced action of endogenous anticoagulants [antithrombin, tissue factor (TF) pathway inhibitor, and proteins C and S[121]] is a hallmark of hemostasis dysregulation. As the pulmonary inflammation worsens, hypofibrinolysis is caused by the consumption of plasminogen, high levels of plasminogen activator inhibitor-1 (PAI-1) and a decrease in tissue plasminogen activator, which prolongs the prothrombotic state[122,123].The pathological state involving platelet hyper-reactivity, hypercoagulability and hypofibrinolysis during COVID-19 is named “immuno-thromboinflammation”[123]. As platelets express both ACE2 and TMPRSS2 on their surface[124], it is intriguing that SARS-CoV-2 can attach to them directly and activate them, causing the release of clotting factors and inflammatory chemicals. Endothelial injury triggers the release of TF in circulation which may be also derived from macrophage/monocyte cells as a consequence of MAS[125]. When PAI-1 is overproduced, it binds to TLR4 on macrophages and triggers the release of cytokines and chemokines[126], which in turn promotes inflammation, steatosis, and microvascular thrombosis[127]. SARS-CoV-2 binds to the ACE2 receptor on tissues inceasing Ang II levels, favoring PAI-1 and TF expression thus promoting hypercoagulability and impairing fibrinolysis[115]. Extensive pericyte activation during LI contributes to the recruitment of inflammatory cells, and their conversion into cells that resemble myofibroblasts results in the creation of extracellular matrix proteins and the ensuing fibrosis of the vessel wall[128].
The expression of antioxidant proteins is regulated by nuclear factor erythroid 2-related factor 2 (NRF2), a transcription factor that is triggered by oxidative stress[129,130,131]. One of the most significant mechanisms of antioxidant and anti-inflammatory element signaling is the complex Kelch-like ECH-associated protein 1-NRF2-antioxidant response element[132]. The NRF2 antioxidant pathway is suppressed in COVID-19, while infected cells show a GSK-3 (conserved serine/threonine kinase) activity that degrades NRF2[133]. NRF2 interacts with NF-kB, a proinflammatory signal transduction pathway[134] driving the initial proinflammatory response[135], to reciprocally regulate redox metabolism[136-139]. When NRF2 activity reaches its maximum level, Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) activation is inhibited. In response to viral infection or other stimuli, inhibitor kappa B (IB) is phosphorylated, which releases and translocates NF-B to the nucleus, causing inflammatory cascades and the generation of inflammatory mediators[140]. Via the TNFR1-NF-B signaling axis, TNF- may activate NF-B[141], and NF-kB in turn enhances the release of inflammatory cytokines[135]. This creates a vicious loop that feeds CSS and exacerbates LI[142-144]. Oxidative stress is mostly caused by ROS. The NF-B pathway is activated by COVID-19, ARDS, and sepsis as they cause tissue ischemia and ROS production[145]. In the early stages of COVID-19, ACE2 is the most critical component, whereas the IL-6-STAT3 axis is crucial in the late stages and in CSS[146]. Indeed, both NF-κB and STAT3 pathways are activated in COVID-19 promoting inflammation by activating the IL-6 amplifier[147]. NRF2 promotes Glutathione synthesis[148], and participates in the tricarboxylic acid cycle by regulating the production of NADPH, a key co-factor of antioxidant reactions[149,150]. NRF2 inhibits liver fibrosis and promotes liver regeneration[151-153] therefore protecting liver cells in viral hepatitis[154], drug-induced LI (DILI)[155-157], cholestasis[144,158,159], and NAFLD[160,161], by reducing gluconeogenesis and fat deposition, restoring insulin resistance, and boosting the anti-inflammatory and antioxidant effects[162].
The large use of antiviral drugs may contribute to COVID-19-related LI especially in individuals with increased baseline ALT[163]. The pooled incidence of DILI in COVID-19 is reported 25.4%[6]. In DILI, AST usually peaks before ALT, a biochemical pattern also observed in severe COVID-19. In some cases, observed microvascular steatosis and mild hepatic inflammation are consistent with DILI[20,49]. In remdesivir-treated patients, 23%-35% show increased LFTs[164,165] indicating hepatotoxicity, while 2%–3% required treatment discontinuation[164]. Lopinavir/ritonavir incidence of DILI is 37.2%[9] with a significant increased risk (OR = 4.44) for severity[20]. Medicines with possible antiviral effects should only be administered on patients who have risk factors for severe illness[82] and early in the course of the disease[166]. In tocilizumab-treated patients, 15%-51% presented a transitory but not significant hypertransaminasemia between 9-13 d, some of which showed surprisingly higher mortality[167,168]. The liver's IL-6-mediated endotheliopathy should be improved by treatment with the JAK inhibitor baricitinib. A significant disadvantage of all those treatments those treatment clinical trials is the frequent exclusion of patients with AST/ALT > 5 × ULN[169]. Moreover, immunosuppressive drugs, such as tocilizumab, tofacitinib, and dexamethasone, can potentially induce LI via HBV reactivation in patients with occult infections[170,171], therefore antiviral prophylaxis should be administered. Dexamethasone may ameliorate endothelial injury[172] by dampening of endothelial IL-6 production[173]. The most typical contributors to DILI in the general population, antibiotics and nonsteroidal anti-inflammatory medications, may also cause LI, while acetaminophen can cause alterations in aminotransferases even at therapeutic doses[174].
In COVID-19, dysbiosis of the gut microbiota may have a significant impact on the clinical outcome of patients with comorbid conditions such diabetes, hypertension, and obesity and may lead to liver damage[175,176]. For example, older people often have less variety in their gut microbiota, and COVID-19 is more severe in this age group, supporting the possibility that microbes play a role in outcomes[177]. Hepatic dysfunction brought on by sepsis may result from disruption of the gut microbiota and a breach of the gut-mucosal barrier[178]. Moreover, the diversity of the gut microbiota influences how the host immune system responds[178]. It is hypothesized that changes in the gut-liver axis may contribute to the severity of COVID-19 seen in cirrhotics. Cirrhosis is characterized by changes to intestinal permeability, gut microbiota composition, and function[179].
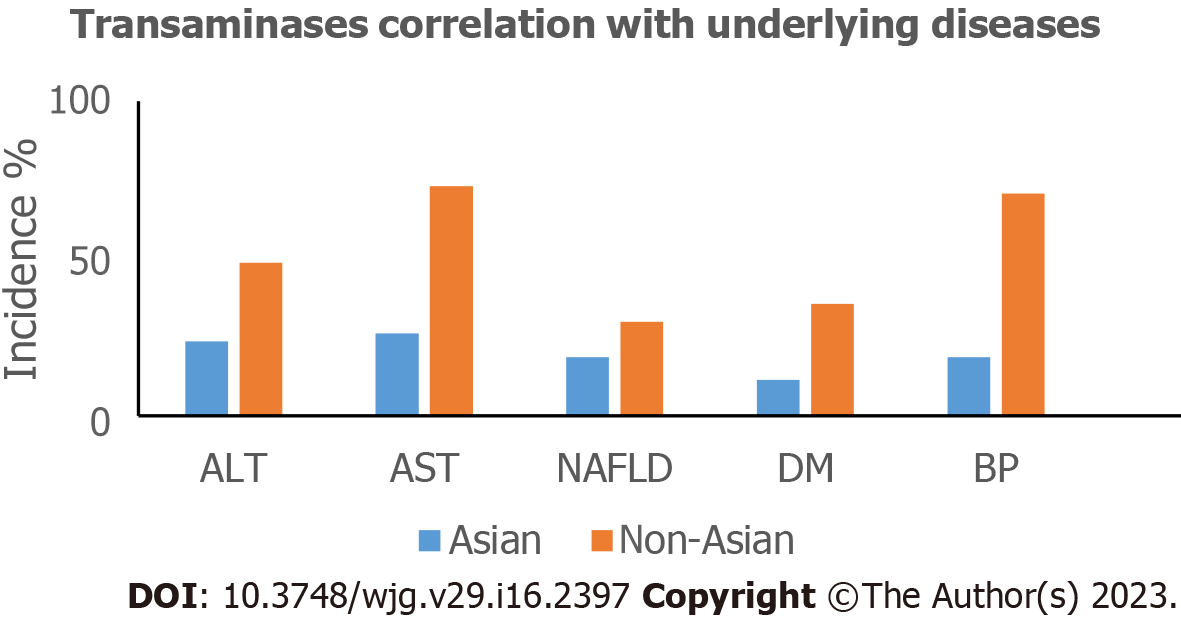
The earliest available epidemiological data of COVID-19 patients came from China. Abnormal LFTs were first reported in a cohort from Wuhan, China[9], making liver the most frequently damaged outside of the respiratory system. It's interesting to note that Wuhan, the COVID-19 epicenter, had a substantially greater incidence of elevated aminotransferases than the surrounding areas (21% vs 10%)[18,63] possibly because of higher SARS-CoV-2 doses exposure in Wuhan[80]. Western populations show abnormal LFTs more clearly than Eastern populations (Figure 2). The timing of LFT determination during disease course, different definitions, but mostly, the geographical variability in the prevalence of underlying diseases are the determinants for the observed discrepancies[18]. With respect to worldwide published data the overall prevalence of abnormal LFTs ranges from 2.5% to 96.8% (Table 2), while SLI accounts for 4.94%-21.8% of COVID-19 patients[7,20,22,23,38,80,180]. Patients with SLI are younger and more likely to be male[23]. Younger patients may exhibit a more robust immune response to infection, causing LI and determining its degree[23,38]. Aminotransferases are higher in severe COVID-19 cases, in accordance with the 2002-2004 SARS outbreak[181]. Concerning cholestatic enzymes, elevated GGT and ALP range between 15%-47.3% and 4%-58.5% respectively (Table 2). GGT, a surrogate marker for increased oxidative stress and chronic inflammation[182], usually increases in severe cases[58,59] implying cholangiocyte injury[183,184]. The GGT elevation without accompanied by ALP elevation[59] may also develop in DILI more frequently than obstruction. ALP elevation is rare, usually < 2 × ULN[185], and is mostly observed in MODS or death from COVID-19[186]. The joint trajectory of GGT, ALP, and bilirubin points towards a cholestatic LI seen in impaired survival[187]. The prevalence of total bilirubin (TBIL) elevations ranges between 3.1% and 52.1%. Concerning longitudinal changes, LFTs become more frequently, and more severely deranged during hospitalization[20,188,189]. The median time to peak AST levels is 3 d after admission, normalizing within 4.4 d[190]. ALT elevations peak between 4-17 hospital day[188]. In deceased patients, ALT levels are normal in the first week but subsequently rise rapidly along with AST at the third week. In survivors, slightly elevated ALT levels occur at 2-3 wk after symptoms onset when AST levels might remain normal[191]. A biphasic pattern with early aminotransferase onset, culminating around days 10-15 of hospitalization, and then gradual normalization accompanied by rising ALP is also suggested[187]. ALI (ALT > 3 × ULN) occurs between 17-18.5 d after symptoms onset[28,192]. AST is diffusely represented in many tissues while ALT is considered liver-specific[193]. Greater AST levels may be related to mitochondrial damage or damage to other organs[194]. In the liver, ALT is only found in the cellular cytoplasm[72,195] whereas AST is both cytosolic (20%) and mitochondrial (80%) localized, and is in higher concentrations in zone 3 of the hepatic acinus therefore ischemic or toxic damage to this zone may result in greater AST elevations.
| Author/citation LFTs performed | Type of study (n = participants) | Incidence (%) | Country/year of publication |
| Cai et al[20] | Study (n = 417) | China/2020 | |
| Abnormal LFTs | |||
| SLI (AST/ALT > 3 × ULN | 76.3 | ||
| or ALP/γGT > 2 × ULN) | 21.8 | ||
| ALT (> 3 × ULN) | 37 | ||
| GGT (> 3 × ULN) | 41 | ||
| AST (> 3 × ULN) | 20 | ||
| TBIL (> 3 × ULN) | 10 | ||
| MOF | 23.3 | ||
| Phipps et al[23] | Study (n = 2273) | United States/2020 | |
| Mild (peak ALT < 2 × ULN) | 45 | ||
| Moderate (peak ALT 2-5 × ULN) | 21 | ||
| SLI (peak ALT > 5 × ULN) | 6.4 | ||
| Huang et al[191] | Study (n = 675) | China/2020 | |
| Abnormal LFTs | 37.5 | ||
| SLI | 7.7 | ||
| Guan et al[284] | Study (n = 1099) | China/2020 | |
| AST/ALT | |||
| mild disease | 18.2–19.8 | ||
| severe disease | 28.1–39.4 | ||
| Hundt et al[185] | Study (n = 1827) | United States/2020 | |
| LFTs (on admission) | |||
| AST | 66.9 | ||
| ALT | 41.6 | ||
| TBIL | 4.3 | ||
| ALP | 13.5 | ||
| Wang et al[65] | Study (n = 657) | China/2020 | |
| Liver injury | 46.1 | ||
| ALT | 42.2 | ||
| GGT | 24.4 | ||
| TBIL | 4.9 | ||
| Chu et al[320] | Study (n = 838) | China/2020 | |
| Liver Injury | 51.2 | ||
| Yip et al[287] | Study (n = 1040) | China/2021 | |
| Aminotransferases | 22.5 | ||
| ALP | 58.5 | ||
| TBIL | 52.1 | ||
| Ding et al[22] | Study (n = 2073) | China/2021 | |
| Survivors | 90.3 | ||
| Any abnormal LFT | 61.8 | ||
| Mild abnormal LFT | 47.5 | ||
| SLI | 14.3 | ||
| LI type | |||
| Hepatocellular | 25.8 | ||
| Cholestatic | 6.7 | ||
| Mixed | 25.7 | ||
| Specific liver indices | |||
| ALT | 43.3 | ||
| AST | 38.9 | ||
| GGT | 31.8 | ||
| Shao et al[38] | Study (n = 1520) | China/2021 | |
| SLI | 17.9 | ||
| Mishra et al[200] | Study (n = 348) | United States/2021 | |
| New-onset LI | 52.8 | ||
| Sikkema et al[204] | Study (n = 382) | Netherlands/2021 | |
| LI | 41.6 | ||
| Moderate LI (ALT > 100 or ALP > 200) | 6.5 | ||
| Cholestatic LI | 9.2 | ||
| Chew et al[190] | Study (n = 834) | United States/2021 | |
| AST | 62.5 | ||
| ALT | 33.7 | ||
| ALP | 11.9 | ||
| TBIL | 3.1 | ||
| Richardson et al[25] | Study (n = 5700) | United States/2020 | |
| AST | 58.4 | ||
| ALT | 39 | ||
| Bernal-Monterde et al[187] | Study (n = 540) | Spain/2020 | |
| Abnormal LFTs | 64.3 | ||
| ALT | 28.6 | ||
| AST | 40.9 | ||
| GGT | 47.3 | ||
| Krishnan et al[321] | Study (n = 3830) | United States/2022 | |
| ALT | 70.4 | ||
| AST | 44.4 | ||
| ALP | 16.1 | ||
| TBIL | 5.9 | ||
| Kodavoor et al[180] | Study (n = 708) | India/2022 | |
| AST | 69.91 | ||
| < 1–2 times ULN | 42.51 | ||
| 2–3 times ULN | 14.26 | ||
| 3–5 times ULN | 8.19 | ||
| > 5 | 4.94 | ||
| ALT | 80.22 | ||
| < 1–2 times ULN | 42.93 | ||
| 2–3 times ULN | 17.93 | ||
| 3–5 times ULN | 12.14 | ||
| > 5 | 7.2 | ||
| Russo et al[234] | Study (n = 1641) | Italy/2022 | |
| AST | 27.7 | ||
| ALT | 23 | ||
| TBIL | 12.6 | ||
| Marjot et al[44] | Review | United Kingdom/2021 | |
| AST | 29–39 | ||
| ALT | 38–63 | ||
| Cai et al[82] | Review | China/2021 | |
| ALT | 11–56.3 | ||
| AST | 15–86.8 | ||
| TBIL | 2.7–30.6 | ||
| CLD | 2–11 | ||
| Ekpanyapong et al[322] | Review | Multinational/2022 | |
| Aminotransferases | 10–58 | ||
| ALP | 1–10 | ||
| TBIL | 3–23 | ||
| GGT | 13–54 | ||
| Esteban et al[209] | Review | United States/2022 | |
| Aminoransferases (admission) | 20–67 | ||
| Aminoransferases (hospitalization) | 61–83 | ||
| ALP | 23–30 | ||
| TBIL | 4–16 | ||
| Garrido et al[59] | Review | Portugal/2020 | |
| ALT | 2.5–50 | ||
| AST | 2.5–61.1 | ||
| TBIL | 0–35.3 | ||
| Kullar et al[2] | Meta-analysis (n = 3046) | United States/2020 | |
| ALT | 21 | ||
| AST | 24 | ||
| TBIL | 9 | ||
| Wijarnpreecha et al[198] | Meta-analysis (n = 64 studies) (n = 11245 pts) | United States/2021 | |
| AST | 23.2 | ||
| ALT | 21.2 | ||
| TBil | 9.7 | ||
| GGT | 15 | ||
| ALP | 4 | ||
| AST | |||
| Severe cases | 45.5 | ||
| Non-severe | 15 | ||
| Wu et al[253] | Meta-analysis (n = 45 studies) | Multinational/2018 | |
| Admission | |||
| Any abnormal LFT | 27.2 | ||
| ALT | 20.4 | ||
| AST | 21.8 | ||
| ALP | 4.7 | ||
| GGT | 35.8 | ||
| TBIL | 8.8 | ||
| Hospitalization | |||
| Any abnormal LFT | 36 | ||
| ALT | 38.4 | ||
| AST | 28.1 | ||
| TBIL | 23.2 | ||
| Del Zompo et al[323] | Meta-analysis (n = 36 studies) (n = 20724 patients) | Italy/2020 | |
| At admission (pooled prevalence) | |||
| Abnormal LFT | 46.9 | ||
| ALT | 22.8 | ||
| AST | 26.5 | ||
| GGT | 22.5 | ||
| ALP | 5.7 | ||
| TBIL | 8 | ||
| Zhu et al[262] | Meta-analysis (n = 38 studies) (n = 3063 pts) | China/2020 | |
| Abnormal LFTs | 29 | ||
| Mao et al[18] | Meta-analysis (n = 1267) | China/2020 | |
| Abnormal LFTs | 19 | ||
| Alqahtani et al[324] | Meta-analysis (n = 30 studies) | Multinational/2020 | |
| Abnormal LFTs | 61.1 | ||
| Sultan et al[325] | Meta-Analysis (n = 47 studies) (n = 10,890 pts) | United States/2020 | |
| Pooled prevalence | |||
| ALT | 15 | ||
| AST | 15 | ||
| TBIL | 16.7 | ||
| Kumar et al[210] | Meta-analysis (n = 128 studies) | India/2020 | |
| Pooled prevalence | |||
| TBIL | 13.71 | ||
| ALT | 31.1 | ||
| AST | 33.95 | ||
| ALP | 6.99 | ||
| GGT | 30.62 | ||
| ALB | 61.57 | ||
| Severe vs non-severe pts | |||
| TBIL | 18.80 vs 9.24 | ||
| ALT | 39.58 vs 24.15 | ||
| AST | 49.68 vs 19.40 | ||
| ALP | 11.33 vs 4.0 | ||
| GGT | 46.90 vs 18.66 | ||
| ALB | 75.91 vs 31.04 |
The median age of COVID-19-induced LI patients ranges between 51.5-56 years with male predominance[65,191]. The incidence of hypertension, diabetes, and coronary heart disease ranges between 23.08%-31,8%, 11.54%-15.3%, and 7.8%-11.54%, respectively[192]. Age, male sex, hypertension, and diabetes are negatively correlated with SLI in COVID-19[23]. The association of LI with hypertension and poorer prognosis is more significant in the absence of pre-existing CLD[38]. CLD prevalence varies widely with Chinese studies being reported between 1.4%-15.3%[9,184,196], lower than in Western countries (5%-37.6%)[197,198]).
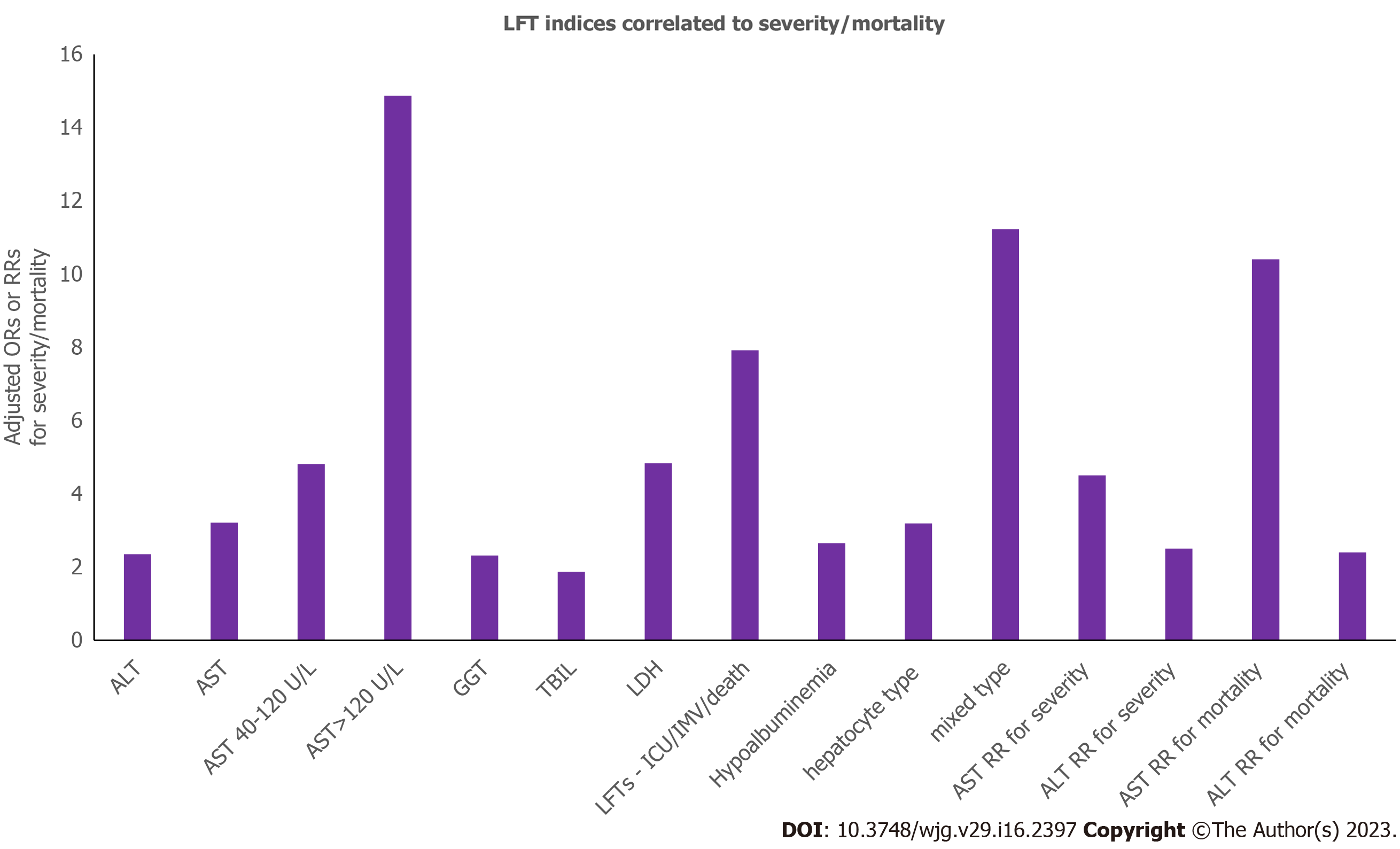
Risks of severity for specific LFTs indices are shown in Figure 3. Aminotransferases levels > 5 × ULN correlate to mortality[23,192], while incidence of LI is higher in ICU than non-ICU patients (61.5% vs 25.0%)[199]. Elevated LFTs on admission show a 3-fold greater risk of severe disease and 3.5-fold risk for mortality[6,200]. After adjustment, patients with LI are at a 9-fold greater risk of severe COVID-19[20], and at a 7.5-fold risk for mortality[201]. A few studies however, failed to show such an association with severity/mortality[202-205], disease progression[17], and ICU admission[202,206]. These differences could be attributed to the nature of the studies that were included and the various outcomes definitions[207,208]. It seems that LI portends the need for ICU care and IMV[209]. CLD is also associated with severity [odds ratio (OR)/relative risk (RR) = 1.48, 1.70][208,210-213], and mortality (OR/RR = 1.08, 2.65)[208,212,214] while in a few studies such an association was not observed[6,207,215-219] (Table 1). Age, male gender, higher body mass index (BMI), corticosteroids, antifungals, lymphocyte decrease, neutrophil increase, and CLD are factors positively associated with ALT/AST increase[6,18,20,192,194,220]. ALP levels are tightly associated with male gender, antifungals, neutrophil count increase, and CLD. Antifungals, antivirals, systemic corticosteroids, and platelet count reduction are positively correlated with increased TBIL levels.
Within five weeks, patients with SLI are significantly more likely to have been intubated, to require renal replacement therapy, or to decease compared to moderate/mild LI[23]. LFTs elevations during hospitalization correlate well with the severity of inflammatory indexes (CRP, procalcitonin, ferritin, LDH, GGT, lactate, and D-dimer)[16,200,221]. In essence, LFTs can be used as a surrogate for the monitoring of inflammation. ALT levels correlate well with the CSS inflammatory markers[317,222-225] (Table 3). This immunological response is consistent with that reported for other viral respiratory tract infections[226]. IL-6, -8, TNF-α are positively correlated with the increased AST, TBIL, and ALP, therefore, cytokines contribute to COVID-19-induced LI[65]. Significantly higher white blood cell (WBC) and neutrophils, and lowered lymphocytes are observed in LI[65,227,228].
| Parameters | Associated conditions |
| ALT | CSS inflammatory markers |
| Elevated serum IL-2R, IL-6, TNF-α | LI |
| IL-6, ferritin, CRP, ESR, Procalcitonin, hypoalbuminemia, low PLTs, low CD4+ T-cells and B-lymphocytes | Non-favorable course of LI |
| Simultaneous increase in IL-6 + ferritin + ALT + hypoalbuminaemia | Significant LI |
| On admission increased inflammatory markers + AST + GGT + LDH + lymphopenia+eosinopenia | More Severe clinical course |
| Lymphopenia, Thrombocytopenia | Disease severity |
| Thrombocytopenia | Consumptive coagulopathy |
| Low Hb | Controversial data |
In mixed-effect Cox model adjusted for age, gender, and comorbidities patients with AST > 3 × ULN compared to normal AST exhibited increased risks of death and IMV (19.27-fold and 116.72-fold, respectively)[191]. Risk of severity and all-cause mortality of LFTs abnormalities are shown in Figure 3. Patients with LI have a 4-fold higher rate of mortality, 7-fold higher rate of ICU admission, and 11-fold higher rate of intubation[20,229], while hepatocellular and cholestatic type LI increases the risk by 3-fold[20]. LI is suggested as an independent prognostic factor of COVID-19[200,211]. AST/ALT ratio > 1 predicts mortality, severe pneumonia and ICU admission[221]. While hepatic steatosis is considered to have no impact on disease course, fibrosis (FIB-4) score < 1.45 is a significant protective factor[230]. Vasopressor use (ischemia), and hyperinflammatory/hypercoagulable state are also independent predictors of death[190]. ALP peak value is a risk factor for in-hospital mortality[231]. All-cause adjusted mortality risk is 6-fold significantly increased in patients with an elevated TBIL > 2-5 × ULN and 1.42-fold in patients with ALP > 1-2 × ULN[232]. Serum albumin is negatively associated with severity. Hyperglycaemia at admission is associated with severity/mortality[233]. In fully models adjusted for confounders, increasing age, non-white and non-black race, hypertension, overweight/obesity, kidney disease, cardiovascular disease, diabetes, cancer, and dementia, are independently associated with an increased risk of in-hospital mortality[232-234]. Higher state of inflammation is also significantly associated with mortality[232], while peak ferritin and IL-6 Levels are associated with SLI (Table 1)[23].
In COVID-19 the presence of cirrhosis, mostly of decompensated, is an independent predictor of liver-related (OR = 3.24) and overall complications, as well as mortality (aOR; 11.3-12.5)[232,235]. In patients with CLD, the 30-d cumulative overall mortality is higher in cirrhotics (RR = 4.6)[236], with respiratory complications being the main cause of death[208,237-239].In terms of CLD etiology, more frequent is viral (60.5%), followed by NAFLD (32.6%), alcohol-related liver disease (ArLD) (4.7%), and autoimmune hepatitis (2.3%). ArLD, NAFLD and hepatocellular carcinoma (HCC) but neither viral nor autoimmune hepatitis are associated with increased mortality[237,240-242]. Cirrhosis-associated immune dysfunction (CAID), is a condition that affects patients with CLD, particularly cirrhosis, who display a variety of immune dysfunctional mechanisms that enhance their vulnerability to infection and abnormal inflammatory responses[44]. The CAID phenotypes represent a continuum of dynamic events that shift from being primarily pro-inflammatory to being primarily immunodeficient. Reduced bacterial opsonization, phagocytosis, protein C activity, antigen T-lymphocyte dependent responses, vaccination responses, hypoalbuminemia, hypocomplementaemia, and intestinal dysbiosis are some of the characteristics of this condition[44,243,244]. CAID is also associated with increased serum levels of IL-1β, -6, -17, -18, TNF-α, and IFN-γ[245], and predisposes to a variety of viral or fungal-related diseases[246]. Despite the increased risk of infection[247], cirrhotics exhibit a lower risk of acquiring SARS-CoV-2[209,248,249], whereas in large population studies patients with CLD are not over-represented[250]. In patients with CLD, a condition known as acute-on-chronic liver failure (ACLF) is characterized by abrupt hepatic decompensation and extrahepatic organ failures and is linked with a significant short-term mortality[251]. While being typically linked to bacterial infections, COVID-19, among other viruses may cause ACLF[251-253].The incidence of ACLF in COVID-19 patients with CLD ranges from 10% to 50%[235,237], with a reported 65% mortality rate[237]. ACLF may also occur in compensated cirrhotics with severe COVID-19[254]. Also, as the severity of cirrhosis is assessed by the Child-Turcotte-Pugh (CTP) score, there is a stepwise rise in morbidity and mortality[237]. After adjusting for baseline characteristics, COVID-19-related mortality is significant across the CTP stages (aORs): A = 1.90, B = 4.14 and C = 9.32[237], similar to non-COVID-19 hospitalized cirrhotics[235,238]. CTP score > 9 at presentation independently predicts mortality, in accordance with MELD and chronic liver failure consortium scores[235,238,239]. In cirrhotics LI is evident at presentation (OR = 6.2)[200]. The non-survivors cirrhotics have higher AST levels, AST/ALT ratio > 1.4 (aHR = 1.4), and a low R value that predicts mortality[235]. Development of liver decompensation during COVID-19 exhibits increased mortality compared to maintenance of hepatic compensation (63.2% vs 26.2%)[239].
Despite suffering higher mortality, those cirrhotics who survive the initial insult show re-admission/death rates at 90-d comparable to cirrhotics without COVID-19[254]. SARS-CoV-2 does not appear to accelerate the progression of liver disease beyond cirrhosis' normal course after acute infection. The interaction of severe COVID-19, pulmonary illness, and ACLF is probably mediated by CAID. Cirrhosis is linked to an increase in cytokine production and baseline endotoxinaemia, which may cause an increased inflammatory response to infection[44]. Hypercytokinemia, another characteristic of COVID-19, causes hepatocyte apoptosis and necrosis, which, in the presence of depleted liver reserves, may result in hepatic decompensation[244,255]. Therefore, cirrhosis and COVID-19 may have detrimental effects on each other. Cirrhosis decompensation in COVID-19 is characterized by deteriorating ascites, worsening of jaundice and hepatic encephalopathy more frequent than variceal bleeding or spontaneous bacterial peritonitis[235,240,241]. Despite compensated cirrhotics having greater problems, mortality among them and non-cirrhotics is comparable, supporting the idea that there is adequate hepatic reserve for recovery. The more significant predictor of death in COVID-19[237] is hepatic decompensation as opposed to cirrhosis per se. The data indicate that non-traditionally hepatotropic infections, such as SARS-CoV-2, may directly precipitate ACLF in cirrhotic patients[256] as also observed in influenza[252]. The median platelet (PLT) count and IFN-γ are significantly decreased in CLD than non-CLD patients[226,257], and cirrhotics are at higher risk for thrombotic events[105].
NAFLD has been advocated as a risk factor for severe COVID-19, it is present in the majority of COVID-19 patients with CLD worldwide, and shows longer viral shedding time[258]. Patients with NAFLD assessed by CT scan and with intermediate/high risk of FIB-4 have higher risk for severe COVID-19 (OR = 2.95) vs patients without NAFLD fibrosis score (NFS), suggesting a pathogenetic role for advanced liver fibrosis in severe COVID-19 with worse outcomes[232,250-260]. Increasing FIB-4 or NFS values when included as continuous measures in multivariable regression, they are significantly associated with COVID-19 severity (aORs = 1.90, and 2.57, respectively). NAFLD is epidemiologically associated with an increased risk of severe COVID-19[261,262], independently of BMI[263]. Hepatic steatosis is an interesting pathological characteristic that frequently occurs in COVID-19. It is possible that activation of coagulation, which is capable of causing hepatic steatosis in NAFLD, represents a unique mechanism connecting thrombosis and steatosis, two diseases that are both common in COVID-19[264]. Given that high PAI-1 has been linked to NAFLD and NASH[265,266], the involvement of PAI-1 in COVID-19 may be noteworthy. Lastly, obesity, a major risk factor for thrombosis owing to adipocytokine-mediated processes, increases inflammatory molecules, Ang II/Ang 1,7 imbalance, ROS-mediated endothelial dysfunction, and alterations in lipid/glucose metabolism[267-269]. Greater risk of severe COVID-19 is found in non-diabetics patients < 60 years with NAFLD (aOR = 4.07)[259,270]. Additionally, underlying diabetes with NAFLD shows a 2-fold higher risk of severe COVID-19, much higher when LI is present (OR = 6.4), or in obese NAFLD patients (aOR = 6.32)[271]. NAFLD is associated with an increased risk of severity when it coexists with obesity[272], in non-diabetics[270], in younger patients[259], and in individuals with high hepatic fibrosis scores[260]. Although it is unknown how obesity and NAFLD might worsen COVID-19, comparable mechanisms are frequently present in both disorders comprising alterations in the immune response, macrophage activation, and (low-grade) inflammation[260,273,274]. Obesity-related chronic inflammation impairs macrophage activation via antigen presentation and pro-inflammatory cytokine synthesis[275]. Moreover, obesity reduces B- and T-cell responses, which leads to greater susceptibility and delayed clearance of viral infections[275,276]. The advancement of COVID-19 is favored in NAFLD patients when hepatic macrophage polarization changes from M1 (which promotes inflammation) to M2 (which suppresses inflammation)[58,274,277]. The polarization states of macrophages may be unbalanced, influencing the host's inflammatory or tolerance response to SARS-CoV-2 signals provided via the gut-liver axis. ACE2 expression is elevated in CLD/NASH patients, and cytokine secretion is enhanced in association with COVID-19[42]. Conversely, in a few studies NASH was not associated with severe disease[278]. It is also demonstrated that COVID-19 patients exhibit increased serum levels of MCP-1, which exacerbates steatohepatitis[279]. Age, gender, obesity, and other comorbidities are thought to be less important risk factors for COVID-19 than NAFLD. NAFLD progression may also be hastened by COVID-19.
The secure-cirrhosis and COVID-Hep registries identified ArLD as a risk factor for COVID-19 mortality (aOR = 1.79) related to advanced disease and CAID[237]. Increased mortality is also seen in alcohol-related cirrhosis[238,239]. Immune dysregulation, particularly changes in the gut-liver axis, is accentuated in ArLD, with increased endotoxinaemia and Kupffer cell activation leading to proinflammatory cytokine transcription and superoxide production[44,178]. Moreover, alcohol impairs adaptive immunity, including both cell-mediated and humoral responses[179]. The inflammatory state caused by danger-associated molecular patterns is linked to ArLD leading to the production of pro-inflammatory cytokines[238,256]. It is postulated that CSS could aggravate the increased inflammatory process in ArLD, resulting in worse outcomes[119]. In the COVID-19 period, hospitalized patients with ArLD appear at more advanced disease stages, with acute decompensation, higher MELD scores, and greater rates of ICU and mortality[185].
Immunosuppressed patients exhibit higher SARS-CoV-2 viral titres, prolonged viral shedding but do not exhibit increased risk of complications[241-242,280,281]. Analysis of AIH vs non-CLD patients demonstrates increased risk of hospitalization, but equivalent risk of all other outcomes including death[281]. As a result, in stable patients, immunosuppression should not be lowered as a COVID-19 risk reduction strategy[11,282]. Immunosuppression may also reduce the risk of new-onset LI[242,281,282].
Persons with chronic HBV or HCV infection who do not have cirrhosis are not more likely to get COVID-19 or have a worse outcome[9,59]. Conversely, patients with SARS-CoV-2 and HBV co-infection are prone to a worse prognosis[283] and tend to have 2.2-fold more severe COVID-19[284]. The in-hospital mortality in COVID-19 patients with HBV is 6.0%[22] with ACLF precipitation < 1%, while LI prevalence in non-decompensated is comparable with non-CLD patients[22]. Significantly lower monocyte and WBC counts, higher levels of CD8+ T-cells and thrombocytopenia in HBV with COVID-19 are observed compared to COVID-19 alone[285]. The likelihood of HBV reactivation during SARS-CoV-2 infection is mentioned, however the risk appears to be low[286]. Those with HCV who have COVID-19 are more susceptible to hospitalization, but not at a higher risk of death[287]. Because recently treated HCV patients were less prone to contracting with SARS-CoV-2, HCV antibodies may be indicative of "protection" against COVID-19[288].
Liver transplant (LT) recipients are more frequently diagnosed with COVID-19 than general population. This might be attributable in part to more careful surveillance and a lower viral testing criterion in LT recipients[289]. The hospitalization rate for COVID-19-positive LT recipients is 50%–70%[290]. Immunocompromised individuals above the age of 60 are more prone to develop SARS-CoV-2 infection with protracted viral clearance[291,292]. LT recipients however, are not at increased risk of severity/death as compared to non-LT recipients[44,290]. LT can involve the donor-to-recipient transmission of SARS-CoV-2[293]. COVID-19 is linked to worse postoperative transplant outcomes, particularly in elderly and obese patients[294]. Most LT patients with COVID-19[295,209] should continue to receive systemic immunosuppression with stable dosages, except for immunomodulators[209], as mycophenolate treatment is considered an independent risk factor for severe COVID-19[289]. The case-fatality rates (17%-18%) are consistent with the expected mortality rates[296]. Those with underlying cancer may require particular consideration[297]. The mortality risk does not change between early (1year) and remote transplantation[296,297].The rate of graft dysfunction during COVID-19 infection is estimated 2.3%-5%[294,298]. The inflammatory reaction in COVID-19 solid organ transplant (SOT) patients is not more severe while IL-6 Levels and incidence of ARDS are lower in SOT suggesting that immunosuppressive medication might limit the COVID-19 hyperinflammation[92].
Data on HCC patients with COVID-19 infection are limited. COVID-19 in cancer patients is associated with poor outcomes especially if antitumor treatment was received within 14 d[299]. The all-cause mortality in the HCC subgroup is reported 52.4%, almost 7-fold higher than in patients without HCC[238]. COVID-19 patients with HCC may exhibit exacerbated progression with aggravation of existing liver disease[300].
Because neither the adenoviral vector nor the mRNA vaccines contain live or attenuated virus, it is unlikely that vaccination poses a special safety risk for CLD patients. Vaccine trials of both Pfizer-BioNTech[301] and Moderna[302] demonstrated consistent efficacy among subgroups with coexisting conditions, but small numbers of CLD patients were included. It is unknown if SARS-CoV-2 vaccinations are as effective in cirrhotic/transplanted/immunocompromised CLD patients as they are in the general population, especially against quickly evolving viral variations. Preliminary findings suggest that transplant patients are safe[303]. Whilst historically there have been anxieties that vaccination in SOT recipients might develop alloimmunity and graft rejection, no clinical evidence support this concern[44]. LT recipients should be prioritized for immunization since the advantages exceed the risks[44].
Patients who recovered from COVID-19 and followed for months post-infection show increased risk of LFTs abnormalities, suggesting some possible long-term sequelae for the liver[304].The likelihood of persisting liver inflammation and fat deposition (magnetic resonance imaging) following COVID-19[305] is discussed, as is the prospect of growing liver stiffness over time[306]. In a cohort, ninety randomly selected participants were enrolled three to nine months post-COVID-19 infection and were compared to healthy individuals (negative anti-SARS-CoV-2 immunoglobulin M/immunoglobulin G). The patterns of LI were defined using multiparametric ultrasound (mpUS)[307]. MpUS examination of post-COVID-19 hepatic parenchyma demonstrated higher liver stiffness and steatosis (attenuation imaging-ATI) scores suggestive of LI compared to healthy controls. The most notable change is increased liver stiffness, as measured by greater shear-wave elastography values[307]. Metabolomic analysis of individuals three months after COVID-19 infection reveals higher taurine concentrations, which may be suggestive of LI[308]. Additionally, persistent viral protein and RNA infection of enterocytes[309] in intestinal biopsies for several months after infection renders the small intestine a reservoir of long-term viral replication[34]. Despite mechanisms of long-term LI remain speculative, the sustained endotheliopathy following COVID-19[310] suggests endothelial-mediated inflammation as a possible mechanism. A new entity, post-COVID-19-cholangiopathy, resembling secondary sclerosing cholangitis has also been described in critical COVID-19 cases and typically presents several weeks (mean 118 d) after COVID-19 diagnosis, implying direct LI from COVID-19[311-313].
Increased liver stiffness correlates well with increased ALT/GGT values indicating underlying hepatocellular/cholangiocellular damage on a biochemical level[308]. Patients with increased liver echogenicity and increased ATI values have increased risk for severity (8-fold, 5-fold, respectively). Increased BMI and CRP levels are also associated with hepatic steatosis (ORs = 1.459, 1.387, respectively), while patients with higher steatosis scores present more severe disease[308]. Sonographic findings of ALI, including signs of acute hepatitis and vascular complications, appear in 37.3% of COVID-19 and in 48.7% of ICU COVID-19 patients[314]. CT scan findings are liver hypodensity (26%) and pericholecystic fat stranding (21%).
Most frequently observed liver-related histopathological findings (Table 4) are associated with underlying comorbidities (e.g., NAFLD), rather than of SARS-CoV-2 itself. In initial pathology reports some authors cautioned about the observed “spiked virions”, “corona-like” inclusions that could be artifacts of tissue autolysis or of an alternative origin (e.g., intrahepatic cholesterol crystals, lamellations, “crown-like” structures seen in NAFLD, multi-vesicular bodies, exosomes)[12,315,316]. Further investigations found evidence of apoptosis, an abundance of mitosis, mixed inflammatory infiltration in the portal region, and extensive bile duct damage. The hypothesis of direct cell damage was strengthened by identifiable viral particles, viral RNA in the liver, and hepatocytes, together with the intrahepatic detection of SARS-CoV-2 by EM and in-situ hybridization[55-57]. Hepatocytes' cytoplasm contained characteristic coronavirus particles with their spikes, according to ultrastructural analysis[317]. While EM reveals mitochondrial enlargement and apoptosis, in situ hybridization may identify SARS-CoV-2 in 68% of samples[36]. Mitochondrial swelling, endoplasmic reticulum dilatation, and cell membrane dysfunction, document SARS-CoV-2 ability to replicate within hepatocytes[102,317]. Proteomic analysis of autopsy tissue showed mitochondrial dysfunction with dysregulated fatty acid oxidation and oxidative phosphorylation[68]. Biopsies from LT recipients who tested positive for COVID-19 revealed endotheliitis, bile duct damage, and mixed inflammatory portal infiltrates, which are findings of T-cell-mediated rejection[318]. Steatosis is predominant in obesity and overweight patients[102], and its high prevalence is attributed to population’s baseline characteristics (severe COVID-19 and steatosis share common risk factors). DILI and CSS may also contribute to the development of hepatic steatosis. Direct vascular damage, portal endotheliitis, portal vein herniation, terminal vessel dilations, and thrombosis with luminal ectasia are examples of acute vascular alterations. Chronic alterations in the portal and sinusoidal vessels, characterized by fibrous thickening of the vascular wall (thrombotic bodies), are sinusoidal inclusions positive for the platelet marker CD61[56,102,108,319]. Lobular ductal pathology is common showing the presence of moderate nuclear pleomorphism in cholangiocytes and canalicular cholestasis[108].
| Findings | Frequency (%) |
| Portal and sinusoidal microthrombosis | 29.4–100 |
| Hepatic/macrovesicular steatosis | 50–75 |
| Mild portal inflammation | 13.2–66 |
| Centrilobular necrosis | 50 |
| Mild acute hepatitis | 50 |
| Congestion/dilation of hepatic sinuses | 34.7 |
| Portal fibrosis | 20.5 |
| Kupffer cell hyperplasia | 13.5 |
| Lobular inflammation | 11.6 |
| Inflamed cells within the sinusoids (neutrophils, plasmatocytes and Kupffer) | N/A |
| Panacinar hepatitis, zone 3 necrosis | N/A |
This review sheds light on issues raised by early COVID-19 studies concerning discrepancies in prevalence of LI and CLD, and the role of direct SARS-CoV-2 hepatocyte/cholangiocyte injury. The weighty implication of COVID-19-induced LI mechanisms comprising CSS, endotheliopathy/immuno-thromboinflammation, liver hypoxia, and oxidative stress with respect to histopathological and immunohistochemical findings is meticulously discussed. Finally, an emerging entity, long-COVID-19 persistent LI is also studied.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ait Addi R, Morocco; Kuznietsova H, Ukraine; Macedo G, Portugal S-Editor: LI L L-Editor: A P-Editor: Chen YX
| 1. | Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB. Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med. 2020;383:590-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1254] [Cited by in RCA: 1420] [Article Influence: 284.0] [Reference Citation Analysis (0)] |
| 2. | Kullar R, Patel AP, Saab S. Hepatic Injury in Patients With COVID-19. J Clin Gastroenterol. 2020;54:841-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Deidda S, Tora L, Firinu D, Del Giacco S, Campagna M, Meloni F, Orrù G, Chessa L, Carta MG, Melis A, Spolverato G, Littera R, Perra A, Onali S, Zorcolo L, Restivo A. Gastrointestinal coronavirus disease 2019: Epidemiology, clinical features, pathogenesis, prevention, and management. Expert Rev Gastroenterol Hepatol. 2021;15:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Fu Y, Zhu R, Bai T, Han P, He Q, Jing M, Xiong X, Zhao X, Quan R, Chen C, Zhang Y, Tao M, Yi J, Tian D, Yan W. Clinical Features of Patients Infected With Coronavirus Disease 2019 With Elevated Liver Biochemistries: A Multicenter, Retrospective Study. Hepatology. 2021;73:1509-1520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 5. | Hao SR, Zhang SY, Lian JS, Jin X, Ye CY, Cai H, Zhang XL, Hu JH, Zheng L, Zhang YM, Jia HY, Yu GD, Wang XY, Gu JQ, Lu YF, Yu XP, Yu L, Xiang DR, Jin CL, Qiu YQ, Li LJ, Sheng JF, Liang TB, Yang YD. Liver Enzyme Elevation in Coronavirus Disease 2019: A Multicenter, Retrospective, Cross-Sectional Study. Am J Gastroenterol. 2020;115:1075-1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: Liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 7. | Yip TC, Lui GC, Wong VW, Chow VC, Ho TH, Li TC, Tse YK, Hui DS, Chan HL, Wong GL. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2021;70:733-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 8. | Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2419] [Cited by in RCA: 2040] [Article Influence: 408.0] [Reference Citation Analysis (2)] |
| 9. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12961] [Article Influence: 2592.2] [Reference Citation Analysis (1)] |
| 10. | Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ. 2020;368:M1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2289] [Cited by in RCA: 2547] [Article Influence: 509.4] [Reference Citation Analysis (2)] |
| 11. | Spearman CW, Aghemo A, Valenti L, Sonderup MW. COVID-19 and the liver: A 2021 update. Liver Int. 2021;41:1988-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Bangash MN, Patel J, Parekh D. COVID-19 and the liver: Little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529-530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 357] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 13. | Choudhary NS, Dhampalwar S, Saraf N, Soin AS. Outcomes of COVID-19 in Patients with Cirrhosis or Liver Transplantation. J Clin Exp Hepatol. 2021;11:713-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect Dis. 2020;20:425-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2493] [Cited by in RCA: 2309] [Article Influence: 461.8] [Reference Citation Analysis (0)] |
| 15. | Zhong ZF, Huang J, Yang X, Peng JL, Zhang XY, Hu Y, Fu N, Lin HL, Jiang B, Tian YY, Yao HY, Deng LP, Tang XQ, Zhou JC, Tang J, Xie X, Liu Q, Liu J, Dou CY, Dai RJ, Yan B, Yang XF. Epidemiological and clinical characteristics of COVID-19 patients in Hengyang, Hunan Province, China. World J Clin Cases. 2020;8:2554-2565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 554] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 17. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 18. | Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 741] [Cited by in RCA: 748] [Article Influence: 149.6] [Reference Citation Analysis (0)] |
| 19. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5774] [Article Influence: 1154.8] [Reference Citation Analysis (2)] |
| 20. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 21. | Weber S, Mayerle J, Irlbeck M, Gerbes AL. Severe liver failure during SARS-CoV-2 infection. Gut. 2020;69:1365-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 22. | Ding ZY, Li GX, Chen L, Shu C, Song J, Wang W, Wang YW, Chen Q, Jin GN, Liu TT, Liang JN, Zhu P, Zhu W, Li Y, Zhang BH, Feng H, Zhang WG, Yin ZY, Yu WK, Yang Y, Zhang HQ, Tang ZP, Wang H, Hu JB, Liu JH, Yin P, Chen XP, Zhang B; Tongji Multidisciplinary Team for Treating COVID-19 (TTTC). Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol. 2021;74:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 23. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 271] [Article Influence: 54.2] [Reference Citation Analysis (2)] |
| 24. | Wu Y, Li H, Guo X, Yoshida EM, Mendez-Sanchez N, Levi Sandri GB, Teschke R, Romeiro FG, Shukla A, Qi X. Incidence, risk factors, and prognosis of abnormal liver biochemical tests in COVID-19 patients: A systematic review and meta-analysis. Hepatol Int. 2020;14:621-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 25. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;323:2052-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6503] [Article Influence: 1300.6] [Reference Citation Analysis (0)] |
| 26. | Lenti MV, Borrelli de Andreis F, Pellegrino I, Klersy C, Merli S, Miceli E, Aronico N, Mengoli C, Di Stefano M, Cococcia S, Santacroce G, Soriano S, Melazzini F, Delliponti M, Baldanti F, Triarico A, Corazza GR, Pinzani M, Di Sabatino A; Internal Medicine Covid-19 Team. Impact of COVID-19 on liver function: Results from an internal medicine unit in Northern Italy. Intern Emerg Med. 2020;15:1399-1407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1933] [Cited by in RCA: 2203] [Article Influence: 440.6] [Reference Citation Analysis (0)] |
| 28. | Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117:11727-11734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1930] [Cited by in RCA: 2368] [Article Influence: 473.6] [Reference Citation Analysis (0)] |
| 29. | Guy JL, Lambert DW, Warner FJ, Hooper NM, Turner AJ. Membrane-associated zinc peptidase families: Comparing ACE and ACE2. Biochim Biophys Acta. 2005;1751:2-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14218] [Article Influence: 2843.6] [Reference Citation Analysis (0)] |
| 31. | Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, van der Meer F, Kallio K, Kaya T, Anastasina M, Smura T, Levanov L, Szirovicza L, Tobi A, Kallio-Kokko H, Österlund P, Joensuu M, Meunier FA, Butcher SJ, Winkler MS, Mollenhauer B, Helenius A, Gokce O, Teesalu T, Hepojoki J, Vapalahti O, Stadelmann C, Balistreri G, Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1507] [Cited by in RCA: 1371] [Article Influence: 274.2] [Reference Citation Analysis (0)] |
| 32. | Song X, Hu W, Yu H, Zhao L, Zhao Y, Zhao X, Xue HH. Little to no expression of angiotensin-converting enzyme-2 on most human peripheral blood immune cells but highly expressed on tissue macrophages. Cytometry A. 2023;103:136-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 83] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 33. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4144] [Article Influence: 197.3] [Reference Citation Analysis (0)] |
| 34. | Gaebler C, Wang Z, Lorenzi JCC, Muecksch F, Finkin S, Tokuyama M, Cho A, Jankovic M, Schaefer-Babajew D, Oliveira TY, Cipolla M, Viant C, Barnes CO, Bram Y, Breton G, Hägglöf T, Mendoza P, Hurley A, Turroja M, Gordon K, Millard KG, Ramos V, Schmidt F, Weisblum Y, Jha D, Tankelevich M, Martinez-Delgado G, Yee J, Patel R, Dizon J, Unson-O'Brien C, Shimeliovich I, Robbiani DF, Zhao Z, Gazumyan A, Schwartz RE, Hatziioannou T, Bjorkman PJ, Mehandru S, Bieniasz PD, Caskey M, Nussenzweig MC. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1239] [Article Influence: 309.8] [Reference Citation Analysis (0)] |
| 35. | Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 740] [Article Influence: 148.0] [Reference Citation Analysis (0)] |
| 36. | Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 308] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 37. | Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, Tang X, Zhu J, Zhao Z, Jaffré F, Zhang T, Kim TW, Harschnitz O, Redmond D, Houghton S, Liu C, Naji A, Ciceri G, Guttikonda S, Bram Y, Nguyen DT, Cioffi M, Chandar V, Hoagland DA, Huang Y, Xiang J, Wang H, Lyden D, Borczuk A, Chen HJ, Studer L, Pan FC, Ho DD, tenOever BR, Evans T, Schwartz RE, Chen S. A Human Pluripotent Stem Cell-based Platform to Study SARS-CoV-2 Tropism and Model Virus Infection in Human Cells and Organoids. Cell Stem Cell. 2020;27:125-136.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 529] [Cited by in RCA: 510] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 38. | Shao J, Liang Y, Li Y, Ding R, Zhu M, You W, Wang Z, Huang B, Wu M, Zhang T, Li K, Wu W, Wu L, Wang Q, Xia X, Wang S, Lu L. Implications of liver injury in risk-stratification and management of patients with COVID-19. Hepatol Int. 2021;15:202-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Paizis G, Tikellis C, Cooper ME, Schembri JM, Lew RA, Smith AI, Shaw T, Warner FJ, Zuilli A, Burrell LM, Angus PW. Chronic liver injury in rats and humans upregulates the novel enzyme angiotensin converting enzyme 2. Gut. 2005;54:1790-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 281] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 40. | Chua RL, Lukassen S, Trump S, Hennig BP, Wendisch D, Pott F, Debnath O, Thürmann L, Kurth F, Völker MT, Kazmierski J, Timmermann B, Twardziok S, Schneider S, Machleidt F, Müller-Redetzky H, Maier M, Krannich A, Schmidt S, Balzer F, Liebig J, Loske J, Suttorp N, Eils J, Ishaque N, Liebert UG, von Kalle C, Hocke A, Witzenrath M, Goffinet C, Drosten C, Laudi S, Lehmann I, Conrad C, Sander LE, Eils R. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol. 2020;38:970-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 790] [Article Influence: 158.0] [Reference Citation Analysis (0)] |
| 41. | Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, Feldman J, Muus C, Wadsworth MH 2nd, Kazer SW, Hughes TK, Doran B, Gatter GJ, Vukovic M, Taliaferro F, Mead BE, Guo Z, Wang JP, Gras D, Plaisant M, Ansari M, Angelidis I, Adler H, Sucre JMS, Taylor CJ, Lin B, Waghray A, Mitsialis V, Dwyer DF, Buchheit KM, Boyce JA, Barrett NA, Laidlaw TM, Carroll SL, Colonna L, Tkachev V, Peterson CW, Yu A, Zheng HB, Gideon HP, Winchell CG, Lin PL, Bingle CD, Snapper SB, Kropski JA, Theis FJ, Schiller HB, Zaragosi LE, Barbry P, Leslie A, Kiem HP, Flynn JL, Fortune SM, Berger B, Finberg RW, Kean LS, Garber M, Schmidt AG, Lingwood D, Shalek AK, Ordovas-Montanes J; HCA Lung Biological Network. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181:1016-1035.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 1793] [Article Influence: 358.6] [Reference Citation Analysis (0)] |
| 42. | Fondevila MF, Mercado-Gómez M, Rodríguez A, Gonzalez-Rellan MJ, Iruzubieta P, Valentí V, Escalada J, Schwaninger M, Prevot V, Dieguez C, Crespo J, Frühbeck G, Martinez-Chantar ML, Nogueiras R. Obese patients with NASH have increased hepatic expression of SARS-CoV-2 critical entry points. J Hepatol. 2021;74:469-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 43. | Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: From pathophysiology to therapeutics. Metabolism. 2019;92:82-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 798] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 44. | Marjot T, Webb GJ, Barritt AS 4th, Moon AM, Stamataki Z, Wong VW, Barnes E. COVID-19 and liver disease: Mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 270] [Article Influence: 67.5] [Reference Citation Analysis (2)] |
| 45. | McConnell MJ, Kondo R, Kawaguchi N, Iwakiri Y. Covid-19 and Liver Injury: Role of Inflammatory Endotheliopathy, Platelet Dysfunction, and Thrombosis. Hepatol Commun. 2022;6:255-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 46. | Seow JJW, Pai R, Mishra A, Shepherdson E, Lim TKH, Goh BKP, Chan JKY, Chow PKH, Ginhoux F, DasGupta R, Sharma A. Single-Cell RNA-seq Reveals Angiotensin-Converting Enzyme 2 and Transmembrane Serine Protease 2 Expression in TROP2(+) Liver Progenitor Cells: Implications in Coronavirus Disease 2019-Associated Liver Dysfunction. Front Med (Lausanne). 2021;8:603374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 47. | Han D, Fang Q, Wang X. SARS-CoV-2 was found in the bile juice from a patient with severe COVID-19. J Med Virol. 2021;93:102-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors AA, Osterhaus AD, van der Voort PH, Mulder DJ, van Goor H. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol. 2020;251:228-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 736] [Article Influence: 147.2] [Reference Citation Analysis (0)] |
| 49. | Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol. 2020;8:13-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (2)] |
| 50. | Machhi J, Herskovitz J, Senan AM, Dutta D, Nath B, Oleynikov MD, Blomberg WR, Meigs DD, Hasan M, Patel M, Kline P, Chang RC, Chang L, Gendelman HE, Kevadiya BD. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J Neuroimmune Pharmacol. 2020;15:359-386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 357] [Cited by in RCA: 355] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 51. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 52. | Fiel MI, El Jamal SM, Paniz-Mondolfi A, Gordon RE, Reidy J, Bandovic J, Advani R, Kilaru S, Pourmand K, Ward S, Thung SN, Schiano T. Findings of Hepatic Severe Acute Respiratory Syndrome Coronavirus-2 Infection. Cell Mol Gastroenterol Hepatol. 2021;11:763-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 53. | Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, Thursz M, Manousou P, Corbett R, Goldin R, Al-Sarraj S, Abdolrasouli A, Swann OC, Baillon L, Penn R, Barclay WS, Viola P, Osborn M. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe. 2020;1:E245-e253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 399] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 54. | Remmelink M, De Mendonça R, D'Haene N, De Clercq S, Verocq C, Lebrun L, Lavis P, Racu ML, Trépant AL, Maris C, Rorive S, Goffard JC, De Witte O, Peluso L, Vincent JL, Decaestecker C, Taccone FS, Salmon I. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24:495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 55. | Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N, Marshall DA. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet. 2020;396:320-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 549] [Cited by in RCA: 620] [Article Influence: 124.0] [Reference Citation Analysis (0)] |
| 56. | Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, Del Portillo A, De Michele S, de Gonzalez AK, Saqi A, Khairallah P, Chong AM, Park H, Uhlemann AC, Lefkowitch JH, Verna EC. Hepatic pathology in patients dying of COVID-19: A series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147-2155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 57. | Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 655] [Article Influence: 131.0] [Reference Citation Analysis (0)] |
| 58. | Metawea MI, Yousif WI, Moheb I. COVID 19 and liver: An A-Z literature review. Dig Liver Dis. 2021;53:146-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (2)] |
| 59. | Garrido I, Liberal R, Macedo G. Review article: COVID-19 and liver disease-what we know on 1st May 2020. Aliment Pharmacol Ther. 2020;52:267-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 60. | Waseem N, Chen PH. Hypoxic Hepatitis: A Review and Clinical Update. J Clin Transl Hepatol. 2016;4:263-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 61. | Lightsey JM, Rockey DC. Current concepts in ischemic hepatitis. Curr Opin Gastroenterol. 2017;33:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 62. | Zhang XJ, Cheng X, Yan ZZ, Fang J, Wang X, Wang W, Liu ZY, Shen LJ, Zhang P, Wang PX, Liao R, Ji YX, Wang JY, Tian S, Zhu XY, Zhang Y, Tian RF, Wang L, Ma XL, Huang Z, She ZG, Li H. An ALOX12-12-HETE-GPR31 signaling axis is a key mediator of hepatic ischemia-reperfusion injury. Nat Med. 2018;24:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 63. | Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020;8:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 64. | Edler C, Schröder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F, Klein A, Langenwalder F, Lütgehetmann M, Meißner K, Püschel K, Schädler J, Steurer S, Mushumba H, Sperhake JP. Correction to: Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134:1977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Wang M, Yan W, Qi W, Wu D, Zhu L, Li W, Wang X, Ma K, Ni M, Xu D, Wang H, Chen G, Yu H, Ding H, Xing M, Han M, Luo X, Chen T, Guo W, Xi D, Ning Q. Clinical characteristics and risk factors of liver injury in COVID-19: A retrospective cohort study from Wuhan, China. Hepatol Int. 2020;14:723-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 66. | Chen P, Zhou B. Clinical characteristics of COVID-19 patients with abnormal liver tests. J Hepatol. 2020;73:712-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Rosser BG, Gores GJ. Liver cell necrosis: Cellular mechanisms and clinical implications. Gastroenterology. 1995;108:252-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 262] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 68. | Miller B, Silverstein A, Flores M, Cao K, Kumagai H, Mehta HH, Yen K, Kim SJ, Cohen P. Host mitochondrial transcriptome response to SARS-CoV-2 in multiple cell models and clinical samples. Sci Rep. 2021;11:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 69. | Caraceni P, Domenicali M, Vendemiale G, Grattagliano I, Pertosa A, Nardo B, Morselli-Labate AM, Trevisani F, Palasciano G, Altomare E, Bernardi M. The reduced tolerance of rat fatty liver to ischemia reperfusion is associated with mitochondrial oxidative injury. J Surg Res. 2005;124:160-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 70. | Nardo B, Grattagliano I, Domenicali M, Caraceni P, Catena F, Santoni B, Turi P, Cavallari G, Dall'Agata M, Trevisani F, Bernardi M, Cavallari A. Mitochondrial oxidative injury in rat fatty livers exposed to warm ischemia-reperfusion. Transplant Proc. 2000;32:51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 71. | Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, O'Meara MJ, Rezelj VV, Guo JZ, Swaney DL, Tummino TA, Hüttenhain R, Kaake RM, Richards AL, Tutuncuoglu B, Foussard H, Batra J, Haas K, Modak M, Kim M, Haas P, Polacco BJ, Braberg H, Fabius JM, Eckhardt M, Soucheray M, Bennett MJ, Cakir M, McGregor MJ, Li Q, Meyer B, Roesch F, Vallet T, Mac Kain A, Miorin L, Moreno E, Naing ZZC, Zhou Y, Peng S, Shi Y, Zhang Z, Shen W, Kirby IT, Melnyk JE, Chorba JS, Lou K, Dai SA, Barrio-Hernandez I, Memon D, Hernandez-Armenta C, Lyu J, Mathy CJP, Perica T, Pilla KB, Ganesan SJ, Saltzberg DJ, Rakesh R, Liu X, Rosenthal SB, Calviello L, Venkataramanan S, Liboy-Lugo J, Lin Y, Huang XP, Liu Y, Wankowicz SA, Bohn M, Safari M, Ugur FS, Koh C, Savar NS, Tran QD, Shengjuler D, Fletcher SJ, O'Neal MC, Cai Y, Chang JCJ, Broadhurst DJ, Klippsten S, Sharp PP, Wenzell NA, Kuzuoglu-Ozturk D, Wang HY, Trenker R, Young JM, Cavero DA, Hiatt J, Roth TL, Rathore U, Subramanian A, Noack J, Hubert M, Stroud RM, Frankel AD, Rosenberg OS, Verba KA, Agard DA, Ott M, Emerman M, Jura N, von Zastrow M, Verdin E, Ashworth A, Schwartz O, d'Enfert C, Mukherjee S, Jacobson M, Malik HS, Fujimori DG, Ideker T, Craik CS, Floor SN, Fraser JS, Gross JD, Sali A, Roth BL, Ruggero D, Taunton J, Kortemme T, Beltrao P, Vignuzzi M, García-Sastre A, Shokat KM, Shoichet BK, Krogan NJ. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459-468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3145] [Cited by in RCA: 3169] [Article Influence: 633.8] [Reference Citation Analysis (0)] |
| 72. | Giannini EG, Testa R, Savarino V. Liver enzyme alteration: A guide for clinicians. CMAJ. 2005;172:367-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1178] [Cited by in RCA: 1168] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 73. | Portincasa P, Krawczyk M, Machill A, Lammert F, Di Ciaula A. Hepatic consequences of COVID-19 infection. Lapping or biting? Eur J Intern Med. 2020;77:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 74. | Dinarello CA. Historical insights into cytokines. Eur J Immunol. 2007;37 Suppl 1:S34-S45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 674] [Cited by in RCA: 575] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 75. | Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P, Ntaganou M, Kyriakopoulou M, Dimopoulos G, Koutsodimitropoulos I, Velissaris D, Koufargyris P, Karageorgos A, Katrini K, Lekakis V, Lupse M, Kotsaki A, Renieris G, Theodoulou D, Panou V, Koukaki E, Koulouris N, Gogos C, Koutsoukou A. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27:992-1000.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1553] [Article Influence: 310.6] [Reference Citation Analysis (0)] |
| 76. | Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1371] [Article Influence: 274.2] [Reference Citation Analysis (0)] |
| 77. | Mahallawi WH, Khabour OF, Zhang Q, Makhdoum HM, Suliman BA. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. 2018;104:8-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 445] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 78. | Nicholls JM, Poon LL, Lee KC, Ng WF, Lai ST, Leung CY, Chu CM, Hui PK, Mak KL, Lim W, Yan KW, Chan KH, Tsang NC, Guan Y, Yuen KY, Peiris JS. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773-1778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 799] [Cited by in RCA: 872] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 79. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6740] [Article Influence: 1348.0] [Reference Citation Analysis (0)] |
| 80. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30056] [Article Influence: 6011.2] [Reference Citation Analysis (3)] |
| 81. | Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, Ellingson MK, Mao T, Oh JE, Israelow B, Takahashi T, Tokuyama M, Lu P, Venkataraman A, Park A, Mohanty S, Wang H, Wyllie AL, Vogels CBF, Earnest R, Lapidus S, Ott IM, Moore AJ, Muenker MC, Fournier JB, Campbell M, Odio CD, Casanovas-Massana A; Yale IMPACT Team, Herbst R, Shaw AC, Medzhitov R, Schulz WL, Grubaugh ND, Dela Cruz C, Farhadian S, Ko AI, Omer SB, Iwasaki A. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1791] [Cited by in RCA: 1573] [Article Influence: 314.6] [Reference Citation Analysis (0)] |
| 82. | Cai Y, Ye LP, Song YQ, Mao XL, Wang L, Jiang YZ, Que WT, Li SW. Liver injury in COVID-19: Detection, pathogenesis, and treatment. World J Gastroenterol. 2021;27:3022-3036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 83. | Zhan K, Liao S, Li J, Bai Y, Lv L, Yu K, Qiu L, Li C, Yuan G, Zhang A, Mei Z. Risk factors in patients with COVID-19 developing severe liver injury during hospitalisation. Gut. 2021;70:628-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 84. | Zhang H, Liao YS, Gong J, Liu J, Zhang H. Clinical characteristics and risk factors for liver injury in COVID-19 patients in Wuhan. World J Gastroenterol. 2020;26:4694-4702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 85. | Cichoż-Lach H, Michalak A. Liver injury in the era of COVID-19. World J Gastroenterol. 2021;27:377-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (2)] |
| 86. | Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 331] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 87. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4563] [Article Influence: 912.6] [Reference Citation Analysis (0)] |
| 88. | Effenberger M, Grander C, Grabherr F, Griesmacher A, Ploner T, Hartig F, Bellmann-Weiler R, Joannidis M, Zoller H, Weiss G, Adolph TE, Tilg H. Systemic inflammation as fuel for acute liver injury in COVID-19. Dig Liver Dis. 2021;53:158-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (2)] |
| 89. | Wiśniewska H, Skonieczna-Żydecka K, Parczewski M, Niścigorska-Olsen J, Karpińska E, Hornung M, Jurczyk K, Witak-Jędra M, Laurans Ł, Maciejewska K, Socha Ł, Leonciuk A, Bander D, Karasińska-Cieślak M, Aksak-Wąs B, Wawrzynowicz-Syczewska M. Hepatotropic Properties of SARS-CoV-2-Preliminary Results of Cross-Sectional Observational Study from the First Wave COVID-19 Pandemic. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Miarons M, Larrosa-García M, García-García S, Los-Arcos I, Moreso F, Berastegui C, Castells L, Pérez-Hoyos S, Varela J, Pau-Parra A, Varón-Galcera C, Parramon-Teixidó CJ, Martínez-Casanova J, Domènech L, García-Ortega P, Sánchez-Sancho P, Alonso-Martínez C, Gómez-Ganda L, Roch-Santed M, Gracia-Moya A, Del-Rio-Gutiérrez JM, Guillén-Del-Castillo A, Sans-Pola C, Antón A, Montoro B, Gorgas-Torner MQ; Vall d’Hebron COVID-19 Working Group. COVID-19 in Solid Organ Transplantation: A Matched Retrospective Cohort Study and Evaluation of Immunosuppression Management. Transplantation. 2021;105:138-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 91. | Polakos NK, Cornejo JC, Murray DA, Wright KO, Treanor JJ, Crispe IN, Topham DJ, Pierce RH. Kupffer cell-dependent hepatitis occurs during influenza infection. Am J Pathol. 2006;168:1169-78; quiz 1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 92. | Mahmoudi S, Rezaei M, Mansouri N, Marjani M, Mansouri D. Immunologic Features in Coronavirus Disease 2019: Functional Exhaustion of T Cells and Cytokine Storm. J Clin Immunol. 2020;40:974-976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 93. | Bowen DG, Warren A, Davis T, Hoffmann MW, McCaughan GW, Fazekas de St Groth B, Bertolino P. Cytokine-dependent bystander hepatitis due to intrahepatic murine CD8 T-cell activation by bone marrow-derived cells. Gastroenterology. 2002;123:1252-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 94. | Adams DH, Hubscher SG. Systemic viral infections and collateral damage in the liver. Am J Pathol. 2006;168:1057-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 95. | Tartey S, Takeuchi O. Pathogen recognition and Toll-like receptor targeted therapeutics in innate immune cells. Int Rev Immunol. 2017;36:57-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 96. | Klimstra WB, Ryman KD, Bernard KA, Nguyen KB, Biron CA, Johnston RE. Infection of neonatal mice with sindbis virus results in a systemic inflammatory response syndrome. J Virol. 1999;73:10387-10398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 97. | Shah A. Novel Coronavirus-Induced NLRP3 Inflammasome Activation: A Potential Drug Target in the Treatment of COVID-19. Front Immunol. 2020;11:1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 98. | Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 552] [Cited by in RCA: 776] [Article Influence: 155.2] [Reference Citation Analysis (0)] |
| 99. | Picchianti Diamanti A, Rosado MM, Pioli C, Sesti G, Laganà B. Cytokine Release Syndrome in COVID-19 Patients, A New Scenario for an Old Concern: The Fragile Balance between Infections and Autoimmunity. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 100. | Antoniak S, Mackman N. Multiple roles of the coagulation protease cascade during virus infection. Blood. 2014;123:2605-2613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 101. | Becker RC. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50:54-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 435] [Article Influence: 87.0] [Reference Citation Analysis (1)] |
| 102. | Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110-2116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 103. | Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP. COVID-19 and coagulation: Bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136:489-500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 924] [Article Influence: 184.8] [Reference Citation Analysis (0)] |
| 104. | Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, Bouman CCS, Beenen LFM, Kootte RS, Heijmans J, Smits LP, Bonta PI, van Es N. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18:1995-2002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1017] [Cited by in RCA: 1091] [Article Influence: 218.2] [Reference Citation Analysis (0)] |
| 105. | Díaz LA, Idalsoaga F, Cannistra M, Candia R, Cabrera D, Barrera F, Soza A, Graham R, Riquelme A, Arrese M, Leise MD, Arab JP. High prevalence of hepatic steatosis and vascular thrombosis in COVID-19: A systematic review and meta-analysis of autopsy data. World J Gastroenterol. 2020;26:7693-7706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 106. | Ladikou EE, Sivaloganathan H, Milne KM, Arter WE, Ramasamy R, Saad R, Stoneham SM, Philips B, Eziefula AC, Chevassut T. Von Willebrand factor (vWF): Marker of endothelial damage and thrombotic risk in COVID-19? Clin Med (Lond). 2020;20:E178-e182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 107. | Kondo R, Kawaguchi N, McConnell MJ, Sonzogni A, Licini L, Valle C, Bonaffini PA, Sironi S, Alessio MG, Previtali G, Seghezzi M, Zhang X, Sun Z, Utsumi T, Strazzabosco M, Iwakiri Y. Pathological characteristics of liver sinusoidal thrombosis in COVID-19 patients: A series of 43 cases. Hepatol Res. 2021;51:1000-1006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 108. | Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome : Results From a Prospective, Single-Center, Clinicopathologic Case Series. Ann Intern Med. 2020;173:350-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 600] [Cited by in RCA: 617] [Article Influence: 123.4] [Reference Citation Analysis (0)] |
| 109. | Schmidt-Arras D, Rose-John S. IL-6 pathway in the liver: From physiopathology to therapy. J Hepatol. 2016;64:1403-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 647] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 110. | Patra T, Meyer K, Geerling L, Isbell TS, Hoft DF, Brien J, Pinto AK, Ray RB, Ray R. SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells. PLoS Pathog. 2020;16:E1009128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 111. | Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:E438-e440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 878] [Cited by in RCA: 1058] [Article Influence: 211.6] [Reference Citation Analysis (0)] |
| 112. | Lippi G, Favaloro EJ. D-dimer is Associated with Severity of Coronavirus Disease 2019: A Pooled Analysis. Thromb Haemost. 2020;120:876-878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 394] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 113. | Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SH, Weitz JI, Liaw PC. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34:1977-1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 362] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 114. | Huisman A, Beun R, Sikma M, Westerink J, Kusadasi N. Involvement of ADAMTS13 and von Willebrand factor in thromboembolic events in patients infected with SARS-CoV-2. Int J Lab Hematol. 2020;42:E211-e212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 115. | Henry BM, Vikse J, Benoit S, Favaloro EJ, Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: A novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta. 2020;507:167-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 266] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 116. | Bazzan M, Montaruli B, Sciascia S, Cosseddu D, Norbiato C, Roccatello D. Low ADAMTS 13 plasma levels are predictors of mortality in COVID-19 patients. Intern Emerg Med. 2020;15:861-863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 117. | Martinelli N, Montagnana M, Pizzolo F, Friso S, Salvagno GL, Forni GL, Gianesin B, Morandi M, Lunardi C, Lippi G, Polati E, Olivieri O, De Franceschi L. A relative ADAMTS13 deficiency supports the presence of a secondary microangiopathy in COVID 19. Thromb Res. 2020;193:170-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 118. | Tiscia GL, Favuzzi G, De Laurenzo A, Cappucci F, Fischetti L, di Mauro L, Miscio G, Mirijello A, Chinni E, Grandone E; CSS COVID-19 Group. Reduction of ADAMTS13 Levels Predicts Mortality in SARS-CoV-2 Patients. TH Open. 2020;4:E203-e206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 119. | Mahmud N, Hubbard RA, Kaplan DE, Serper M. Declining Cirrhosis Hospitalizations in the Wake of the COVID-19 Pandemic: A National Cohort Study. Gastroenterology. 2020;159:1134-1136.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 120. | Xiong M, Liang X, Wei YD. Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID-19): A meta-analysis. Br J Haematol. 2020;189:1050-1052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 121. | Merad M, Martin JC. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1356] [Cited by in RCA: 1800] [Article Influence: 360.0] [Reference Citation Analysis (0)] |
| 122. | Lippi G, Henry BM, Sanchis-Gomar F. Plasma Antithrombin Values Are Significantly Decreased in Coronavirus Disease 2019 (COVID-19) Patients with Severe Illness. Semin Thromb Hemost. 2021;47:460-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 123. | Lippi G, Sanchis-Gomar F, Henry BM. Coronavirus disease 2019 (COVID-19): The portrait of a perfect storm. Ann Transl Med. 2020;8:497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 124. | Zhang S, Liu Y, Wang X, Yang L, Li H, Wang Y, Liu M, Zhao X, Xie Y, Yang Y, Zhang S, Fan Z, Dong J, Yuan Z, Ding Z, Zhang Y, Hu L. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 526] [Cited by in RCA: 471] [Article Influence: 94.2] [Reference Citation Analysis (0)] |
| 125. | Grover SP, Mackman N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler Thromb Vasc Biol. 2018;38:709-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 473] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 126. | Matsuyama T, Kubli SP, Yoshinaga SK, Pfeffer K, Mak TW. An aberrant STAT pathway is central to COVID-19. Cell Death Differ. 2020;27:3209-3225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 127. | Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: Designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17:395-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 462] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 128. | Guo H, Zhang Z, Zhang Y, Liu Y, Wang J, Qian Z, Zou Y, Lu H. Analysis of liver injury factors in 332 patients with COVID-19 in Shanghai, China. Aging (Albany NY). 2020;12:18844-18852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 129. | Tonelli C, Chio IIC, Tuveson DA. Transcriptional Regulation by Nrf2. Antioxid Redox Signal. 2018;29:1727-1745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 923] [Cited by in RCA: 1521] [Article Influence: 217.3] [Reference Citation Analysis (0)] |
| 130. | Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39:199-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1217] [Cited by in RCA: 1628] [Article Influence: 148.0] [Reference Citation Analysis (0)] |
| 131. | Zhu DD, Tan XM, Lu LQ, Yu SJ, Jian RL, Liang XF, Liao YX, Fan W, Barbier-Torres L, Yang A, Yang HP, Liu T. Interplay between nuclear factor erythroid 2-related factor 2 and inflammatory mediators in COVID-19-related liver injury. World J Gastroenterol. 2021;27:2944-2962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (2)] |
| 132. | Kopacz A, Kloska D, Forman HJ, Jozkowicz A, Grochot-Przeczek A. Beyond repression of Nrf2: An update on Keap1. Free Radic Biol Med. 2020;157:63-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 133. | Rana AK, Rahmatkar SN, Kumar A, Singh D. Glycogen synthase kinase-3: A putative target to combat severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. Cytokine Growth Factor Rev. 2021;58:92-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 134. | Tóbon-Velasco JC, Cuevas E, Torres-Ramos MA. Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS Neurol Disord Drug Targets. 2014;13:1615-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 135. | Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1654] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 136. | Sivandzade F, Prasad S, Bhalerao A, Cucullo L. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21:101059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 465] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 137. | Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865:721-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 1250] [Article Influence: 178.6] [Reference Citation Analysis (0)] |
| 138. | Sies H, Berndt C, Jones DP. Oxidative Stress. Annu Rev Biochem. 2017;86:715-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1456] [Cited by in RCA: 2240] [Article Influence: 280.0] [Reference Citation Analysis (0)] |
| 139. | Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans. 2015;43:621-626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 582] [Cited by in RCA: 874] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 140. | Hariharan A, Hakeem AR, Radhakrishnan S, Reddy MS, Rela M. The Role and Therapeutic Potential of NF-kappa-B Pathway in Severe COVID-19 Patients. Inflammopharmacology. 2021;29:91-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 209] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 141. | Zelová H, Hošek J. TNF-α signalling and inflammation: Interactions between old acquaintances. Inflamm Res. 2013;62:641-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 591] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 142. | Leist M, Gantner F, Bohlinger I, Tiegs G, Germann PG, Wendel A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol. 1995;146:1220-1234. [PubMed] |
| 143. | Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1429] [Cited by in RCA: 1429] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 144. | Aleksunes LM, Slitt AL, Maher JM, Dieter MZ, Knight TR, Goedken M, Cherrington NJ, Chan JY, Klaassen CD, Manautou JE. Nuclear factor-E2-related factor 2 expression in liver is critical for induction of NAD(P)H:quinone oxidoreductase 1 during cholestasis. Cell Stress Chaperones. 2006;11:356-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 145. | Cecchini R, Cecchini AL. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143:110102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 258] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 146. | Hirano T, Murakami M. COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity. 2020;52:731-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 436] [Cited by in RCA: 583] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 147. | Murakami M, Kamimura D, Hirano T. Pleiotropy and Specificity: Insights from the Interleukin 6 Family of Cytokines. Immunity. 2019;50:812-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 355] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 148. | Lu SC. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30:42-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1643] [Cited by in RCA: 1496] [Article Influence: 93.5] [Reference Citation Analysis (0)] |
| 149. | Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 947] [Cited by in RCA: 1106] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 150. | Wu KC, Cui JY, Klaassen CD. Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol Sci. 2011;123:590-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 151. | Probst BL, McCauley L, Trevino I, Wigley WC, Ferguson DA. Cancer Cell Growth Is Differentially Affected by Constitutive Activation of NRF2 by KEAP1 Deletion and Pharmacological Activation of NRF2 by the Synthetic Triterpenoid, RTA 405. PLoS One. 2015;10:E0135257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 152. | Duarte TL, Caldas C, Santos AG, Silva-Gomes S, Santos-Gonçalves A, Martins MJ, Porto G, Lopes JM. Genetic disruption of NRF2 promotes the development of necroinflammation and liver fibrosis in a mouse model of HFE-hereditary hemochromatosis. Redox Biol. 2017;11:157-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 153. | Luedde T, Schwabe RF. NF-κB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:108-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1094] [Cited by in RCA: 1082] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 154. | Ruan Y, Jiang Y, Wang W, Ma J, He B, Chen M. HBV down-regulates PTEN expression via Nrf2/GSK3β signaling pathway. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2020;45:1009-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 155. | Goldring CE, Kitteringham NR, Elsby R, Randle LE, Clement YN, Williams DP, McMahon M, Hayes JD, Itoh K, Yamamoto M, Park BK. Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology. 2004;39:1267-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 164] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 156. | Jadeja RN, Urrunaga NH, Dash S, Khurana S, Saxena NK. Withaferin-A Reduces Acetaminophen-Induced Liver Injury in Mice. Biochem Pharmacol. 2015;97:122-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 157. | Liu J, Wu KC, Lu YF, Ekuase E, Klaassen CD. Nrf2 protection against liver injury produced by various hepatotoxicants. Oxid Med Cell Longev. 2013;2013:305861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 158. | Tan KP, Wood GA, Yang M, Ito S. Participation of nuclear factor (erythroid 2-related), factor 2 in ameliorating lithocholic acid-induced cholestatic liver injury in mice. Br J Pharmacol. 2010;161:1111-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 159. | Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, Ohtsuki M, Goto N, Sugimoto H, Utsunomiya H, Oda K, Warabi E, Ishii T, Yamamoto M. Nrf2 counteracts cholestatic liver injury via stimulation of hepatic defense systems. Biochem Biophys Res Commun. 2009;389:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 160. | Sugimoto H, Okada K, Shoda J, Warabi E, Ishige K, Ueda T, Taguchi K, Yanagawa T, Nakahara A, Hyodo I, Ishii T, Yamamoto M. Deletion of nuclear factor-E2-related factor-2 leads to rapid onset and progression of nutritional steatohepatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G283-G294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 161. | Lamlé J, Marhenke S, Borlak J, von Wasielewski R, Eriksson CJ, Geffers R, Manns MP, Yamamoto M, Vogel A. Nuclear factor-eythroid 2-related factor 2 prevents alcohol-induced fulminant liver injury. Gastroenterology. 2008;134:1159-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 164] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 162. | Cuadrado A, Rojo AI, Wells G, Hayes JD, Cousin SP, Rumsey WL, Attucks OC, Franklin S, Levonen AL, Kensler TW, Dinkova-Kostova AT. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat Rev Drug Discov. 2019;18:295-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 801] [Cited by in RCA: 952] [Article Influence: 158.7] [Reference Citation Analysis (0)] |
| 163. | European Association for the Study of the Liver; Clinical Practice Guideline Panel: Chair; Panel members; EASL Governing Board representative. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol. 2019;70:1222-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 658] [Article Influence: 109.7] [Reference Citation Analysis (0)] |
| 164. | Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn MY, Nahass RG, Chen YS, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Muñoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A; GS-US-540-5773 Investigators. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020;383:1827-1837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 987] [Cited by in RCA: 985] [Article Influence: 197.0] [Reference Citation Analysis (0)] |
| 165. | Mahase E. Covid-19: Pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ. 2021;375:N2713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 276] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 166. | Liatsos GD. Controversies' clarification regarding ribavirin efficacy in measles and coronaviruses: Comprehensive therapeutic approach strictly tailored to COVID-19 disease stages. World J Clin Cases. 2021;9:5135-5178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 167. | Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, Tomelleri A, Baldissera E, Rovere-Querini P, Ruggeri A, Monti G, De Cobelli F, Zangrillo A, Tresoldi M, Castagna A, Dagna L; TOCI-RAF Study Group. Efficacy and safety of tocilizumab in severe COVID-19 patients: A single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 305] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 168. | Pettit NN, Nguyen CT, Mutlu GM, Wu D, Kimmig L, Pitrak D, Pursell K. Late onset infectious complications and safety of tocilizumab in the management of COVID-19. J Med Virol. 2021;93:1459-1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 169. | Kim MS, Jung SY, Lee SW, Li H, Koyanagi A, Kronbichler A, Dragioti E, Tizaoui K, Wasuwanich P, Hong SH, Ghayda RA, Yoo HW, Kim H, Jacob L, Salem JE, Kostev K, Shin YH, Kim SY, Gamerith G, Yon DK, Shin JI, Smith L. Hepatobiliary Adverse Drug Reactions Associated With Remdesivir: The WHO International Pharmacovigilance Study. Clin Gastroenterol Hepatol. 2021;19:1970-1972.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 170. | Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3386] [Cited by in RCA: 3625] [Article Influence: 725.0] [Reference Citation Analysis (0)] |
| 171. | Olry A, Meunier L, Délire B, Larrey D, Horsmans Y, Le Louët H. Drug-Induced Liver Injury and COVID-19 Infection: The Rules Remain the Same. Drug Saf. 2020;43:615-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 172. | Kim WY, Kweon OJ, Cha MJ, Baek MS, Choi SH. Dexamethasone may improve severe COVID-19 via ameliorating endothelial injury and inflammation: A preliminary pilot study. PLoS One. 2021;16:E0254167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 173. | Awasthi S, Wagner T, Venkatakrishnan AJ, Puranik A, Hurchik M, Agarwal V, Conrad I, Kirkup C, Arunachalam R, O'Horo J, Kremers W, Kashyap R, Morice W 2nd, Halamka J, Williams AW, Faubion WA Jr, Badley AD, Gores GJ, Soundararajan V. Plasma IL-6 levels following corticosteroid therapy as an indicator of ICU length of stay in critically ill COVID-19 patients. Cell Death Discov. 2021;7:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 174. | Bertolini A, van de Peppel IP, Bodewes FAJA, Moshage H, Fantin A, Farinati F, Fiorotto R, Jonker JW, Strazzabosco M, Verkade HJ, Peserico G. Abnormal Liver Function Tests in Patients With COVID-19: Relevance and Potential Pathogenesis. Hepatology. 2020;72:1864-1872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 175. | Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung ACK, Cheung CP, Chen N, Lai CKC, Chen Z, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chan FKL, Chan PKS, Ng SC. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology. 2020;159:944-955.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 739] [Cited by in RCA: 1057] [Article Influence: 211.4] [Reference Citation Analysis (0)] |
| 176. | Chattopadhyay I, Shankar EM. SARS-CoV-2-Indigenous Microbiota Nexus: Does Gut Microbiota Contribute to Inflammation and Disease Severity in COVID-19? Front Cell Infect Microbiol. 2021;11:590874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 177. | Dhar D, Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020;285:198018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 395] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 178. | Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS, Chan V, Ling L, Joynt G, Hui DS, Chow KM, Ng SSS, Li TC, Ng RW, Yip TC, Wong GL, Chan FK, Wong CK, Chan PK, Ng SC. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 995] [Cited by in RCA: 858] [Article Influence: 214.5] [Reference Citation Analysis (0)] |
| 179. | Szabo G, Saha B. Alcohol's Effect on Host Defense. Alcohol Res. 2015;37:159-170. [PubMed] |
| 180. | Kodavoor Vadiraj P, Thareja S, Raman N, Karantha SC, Jayaraman M, Vardhan V. Does Raised Transaminases Predict Severity and Mortality in Patients with COVID 19? J Clin Exp Hepatol. 2022;12:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 181. | Cui HJ, Tong XL, Li P, Hao YX, Chen XG, Li AG, Zhang ZY, Duan J, Zhen M, Zhang B, Hua CJ, Gong YW. Serum hepatic enzyme manifestations in patients with severe acute respiratory syndrome: Retrospective analysis. World J Gastroenterol. 2004;10:1652-1655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 182. | Yamada J, Tomiyama H, Yambe M, Koji Y, Motobe K, Shiina K, Yamamoto Y, Yamashina A. Elevated serum levels of alanine aminotransferase and gamma glutamyltransferase are markers of inflammation and oxidative stress independent of the metabolic syndrome. Atherosclerosis. 2006;189:198-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 183. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1293] [Article Influence: 258.6] [Reference Citation Analysis (4)] |
| 184. | Shao T, Tong Y, Lu S, Jeyarajan AJ, Su F, Dai J, Shi J, Huang J, Hu C, Wu L, Dai X, Cheng Z, Yan J, Huang P, Tian Y, Li S, Chung RT, Chen D. Gamma-Glutamyltransferase Elevation Is Frequent in Patients With COVID-19: A Clinical Epidemiologic Study. Hepatol Commun. 2020;4:1744-1750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 185. | Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72:1169-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 186. | Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK; Washington State 2019-nCoV Case Investigation Team. First Case of 2019 Novel Coronavirus in the United States. N Engl J Med. 2020;382:929-936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4155] [Cited by in RCA: 3819] [Article Influence: 763.8] [Reference Citation Analysis (1)] |
| 187. | Bernal-Monterde V, Casas-Deza D, Letona-Giménez L, de la Llama-Celis N, Calmarza P, Sierra-Gabarda O, Betoré-Glaria E, Martínez-de Lagos M, Martínez-Barredo L, Espinosa-Pérez M, M Arbones-Mainar J. SARS-CoV-2 Infection Induces a Dual Response in Liver Function Tests: Association with Mortality during Hospitalization. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 188. | Wang Q, Zhao H, Liu LG, Wang YB, Zhang T, Li MH, Xu YL, Gao GJ, Xiong HF, Fan Y, Cao Y, Ding R, Wang JJ, Cheng C, Xie W. Pattern of liver injury in adult patients with COVID-19: A retrospective analysis of 105 patients. Mil Med Res. 2020;7:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 189. | Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, Schaefer E, Chung RT. Liver Biochemistries in Hospitalized Patients With COVID-19. Hepatology. 2021;73:890-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 147] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 190. | Chew M, Tang Z, Radcliffe C, Caruana D, Doilicho N, Ciarleglio MM, Deng Y, Garcia-Tsao G. Significant Liver Injury During Hospitalization for COVID-19 Is Not Associated With Liver Insufficiency or Death. Clin Gastroenterol Hepatol. 2021;19:2182-2191.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 191. | Huang H, Chen S, Li H, Zhou XL, Dai Y, Wu J, Zhang J, Shao L, Yan R, Wang M, Wang J, Tu Y, Ge M. The association between markers of liver injury and clinical outcomes in patients with COVID-19 in Wuhan. Aliment Pharmacol Ther. 2020;52:1051-1059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 192. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 193. | WROBLEWSKI F. The clinical significance of alterations in transaminase activities of serum and other body fluids. Adv Clin Chem. 1958;1:313-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 194. | Khateri S, Mohammadi H, Khateri R, Moradi Y. The Prevalence of Underlying Diseases and Comorbidities in COVID-19 Patients; an Updated Systematic Review and Meta-analysis. Arch Acad Emerg Med. 2020;8:E72. [PubMed] |
| 195. | Rej R. Aminotransferases in disease. Clin Lab Med. 1989;9:667-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 196. | Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, Li SB, Wang HY, Zhang S, Gao HN, Sheng JF, Cai HL, Qiu YQ, Li LJ. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series. BMJ. 2020;368:M606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 1263] [Article Influence: 252.6] [Reference Citation Analysis (0)] |
| 197. | Leo M, Galante A, Pagnamenta A, Ruinelli L, Ponziani FR, Gasbarrini A, De Gottardi A. Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points. Dig Liver Dis. 2022;54:565-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 198. | Wijarnpreecha K, Ungprasert P, Panjawatanan P, Harnois DM, Zaver HB, Ahmed A, Kim D. COVID-19 and liver injury: A meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:990-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 199. | Chen LY, Chu HK, Bai T, Tu SJ, Wei Y, Li ZL, Hu LL, Zhu R, Zhang L, Han CQ, Xiao L, He Q, Song J, Liu WH, Zhu QJ, Chen H, Yang L, Hou XH. Liver damage at admission is an independent prognostic factor for COVID-19. J Dig Dis. 2020;21:512-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 200. | Mishra K, Naffouj S, Gorgis S, Ibrahim H, Gill S, Fadel R, Chatfield A, Tang A, Salgia R. Liver Injury as a Surrogate for Inflammation and Predictor of Outcomes in COVID-19. Hepatol Commun. 2021;5:24-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 201. | Váncsa S, Hegyi PJ, Zádori N, Szakó L, Vörhendi N, Ocskay K, Földi M, Dembrovszky F, Dömötör ZR, Jánosi K, Rakonczay Z Jr, Hartmann P, Horváth T, Erőss B, Kiss S, Szakács Z, Németh D, Hegyi P, Pár G. Pre-existing Liver Diseases and On-Admission Liver-Related Laboratory Tests in COVID-19: A Prognostic Accuracy Meta-Analysis With Systematic Review. Front Med (Lausanne). 2020;7:572115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 202. | Vespa E, Pugliese N, Piovani D, Capogreco A, Danese S, Aghemo A; Humanitas Covid-19 Task Force. Liver tests abnormalities in COVID-19: Trick or treat? J Hepatol. 2020;73:1275-1276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 203. | Kunutsor SK, Laukkanen JA. Markers of liver injury and clinical outcomes in COVID-19 patients: A systematic review and meta-analysis. J Infect. 2021;82:159-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 204. | Sikkema BJB, Sint Nicolaas JJ, van Wijngaarden PP. No association between COVID-19 related liver injury and the course of disease: A retrospective study. Scand J Gastroenterol. 2021;56:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 205. | Kaushik A, Wani SN, Baba MA, Agarwal AK. Prevalence of Abnormal Liver Function Tests in COVID-19 Patients at a Tertiary Care Centre. J Assoc Physicians India. 2020;68:73-75. [PubMed] |
| 206. | Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, Li F, Xu Q, Zhang Y, Xu S, Song Z, Zeng Y, Shen Y, Shi Y, Zhu T, Lu H. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80:E1-e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 536] [Cited by in RCA: 521] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 207. | Sharma A, Jaiswal P, Kerakhan Y, Saravanan L, Murtaza Z, Zergham A, Honganur NS, Akbar A, Deol A, Francis B, Patel S, Mehta D, Jaiswal R, Singh J, Patel U, Malik P. Liver disease and outcomes among COVID-19 hospitalized patients - A systematic review and meta-analysis. Ann Hepatol. 2021;21:100273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 208. | Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: A systematic review and meta-analysis. Hepatol Int. 2020;14:612-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 209. | Esteban JP, Sobotka L, Rockey DC. Coronavirus disease 2019 and the liver. Curr Opin Gastroenterol. 2022;38:191-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 210. | Kumar-M P, Mishra S, Jha DK, Shukla J, Choudhury A, Mohindra R, Mandavdhare HS, Dutta U, Sharma V. Coronavirus disease (COVID-19) and the liver: A comprehensive systematic review and meta-analysis. Hepatol Int. 2020;14:711-722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 211. | Wu T, Zuo Z, Kang S, Jiang L, Luo X, Xia Z, Liu J, Xiao X, Ye M, Deng M. Multi-organ Dysfunction in Patients with COVID-19: A Systematic Review and Meta-analysis. Aging Dis. 2020;11:874-894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 212. | Dorjee K, Kim H, Bonomo E, Dolma R. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: A comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS One. 2020;15:E0243191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 213. | Barek MA, Aziz MA, Islam MS. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID-19 cases: A meta-analysis with 55 studies and 10014 cases. Heliyon. 2020;6:E05684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 214. | Patel U, Malik P, Usman MS, Mehta D, Sharma A, Malik FA, Khan N, Siddiqi TJ, Ahmed J, Patel A, Sacks H. Age-Adjusted Risk Factors Associated with Mortality and Mechanical Ventilation Utilization Amongst COVID-19 Hospitalizations-a Systematic Review and Meta-Analysis. SN Compr Clin Med. 2020;2:1740-1749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 215. | Dong ZY, Xiang BJ, Jiang M, Sun MJ, Dai C. The Prevalence of Gastrointestinal Symptoms, Abnormal Liver Function, Digestive System Disease and Liver Disease in COVID-19 Infection: A Systematic Review and Meta-Analysis. J Clin Gastroenterol. 2021;55:67-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 216. | Zhou Y, Yang Q, Chi J, Dong B, Lv W, Shen L, Wang Y. Comorbidities and the risk of severe or fatal outcomes associated with coronavirus disease 2019: A systematic review and meta-analysis. Int J Infect Dis. 2020;99:47-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 254] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 217. | Liu C, Yang J, Wang W, Zheng P, Tang Y. Liver injury could be associated with severe disease in COVID-19 patients: A meta-analysis. Eur J Gastroenterol Hepatol. 2022;34:237-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 218. | Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS One. 2020;15:E0238215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 348] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 219. | Biswas M, Rahaman S, Biswas TK, Haque Z, Ibrahim B. Association of Sex, Age, and Comorbidities with Mortality in COVID-19 Patients: A Systematic Review and Meta-Analysis. Intervirology. 2020;1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 220. | Shokri Afra H, Amiri-Dashatan N, Ghorbani F, Maleki I, Rezaei-Tavirani M. Positive association between severity of COVID-19 infection and liver damage: A systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. 2020;13:292-304. [PubMed] |
| 221. | Medetalibeyoglu A, Catma Y, Senkal N, Ormeci A, Cavus B, Kose M, Bayramlar OF, Yildiz G, Akyuz F, Kaymakoglu S, Tukek T. The effect of liver test abnormalities on the prognosis of COVID-19. Ann Hepatol. 2020;19:614-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 222. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18175] [Article Influence: 3635.0] [Reference Citation Analysis (0)] |
| 223. | Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1171] [Cited by in RCA: 1206] [Article Influence: 241.2] [Reference Citation Analysis (0)] |
| 224. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5510] [Article Influence: 1102.0] [Reference Citation Analysis (1)] |
| 225. | Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, Gulick RM, Safford MM. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372-2374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1644] [Cited by in RCA: 1614] [Article Influence: 322.8] [Reference Citation Analysis (0)] |
| 226. | Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, Zhang HY, Sun W, Wang Y. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92:577-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 886] [Cited by in RCA: 846] [Article Influence: 169.2] [Reference Citation Analysis (0)] |
| 227. | Anurag A, Jha PK, Kumar A. Differential white blood cell count in the COVID-19: A cross-sectional study of 148 patients. Diabetes Metab Syndr. 2020;14:2099-2102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 228. | Yuan J, Zou R, Zeng L, Kou S, Lan J, Li X, Liang Y, Ding X, Tan G, Tang S, Liu L, Liu Y, Pan Y, Wang Z. The correlation between viral clearance and biochemical outcomes of 94 COVID-19 infected discharged patients. Inflamm Res. 2020;69:599-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 229. | Siddiqui MA, Suresh S, Simmer S, Abu-Ghanimeh M, Karrick M, Nimri F, Musleh M, Mediratta V, Al-Shammari M, Russell S, Jou J, Dang D, Salgia R, Zuchelli T. Increased Morbidity and Mortality in COVID-19 Patients with Liver Injury. Dig Dis Sci. 2022;67:2577-2583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 230. | Lombardi R, Mura V, Cespiati A, Iuculano F, Sigon G, Pallini G, Proietti M, Motta I, Montinaro B, Fiorelli E, Cesari M, Bandera A, Valenti L, Peyvandi F, Montano N, Baldini M, Fracanzani AL. Usefulness of fibrosis-4 (FIB-4) score and metabolic alterations in the prediction of SARS-CoV-2 severity. Intern Emerg Med. 2022;17:1739-1749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 231. | Aghemo A, Piovani D, Parigi TL, Brunetta E, Pugliese N, Vespa E, Omodei PD, Preatoni P, Lleo A, Repici A, Voza A, Cecconi M, Malesci A, Bonovas S, Danese S; Humanitas COVID-19 Task Force. COVID-19 Digestive System Involvement and Clinical Outcomes in a Large Academic Hospital in Milan, Italy. Clin Gastroenterol Hepatol. 2020;18:2366-2368.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 232. | Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, Hathorn KE, Wong D, Njie C, Shen L, Chan WW. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicentre United States experience. Liver Int. 2020;40:2515-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (2)] |
| 233. | Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1450] [Cited by in RCA: 1437] [Article Influence: 287.4] [Reference Citation Analysis (0)] |
| 234. | Russo A, Pisaturo M, Palladino R, Maggi P, Numis FG, Gentile I, Sangiovanni V, Esposito V, Punzi R, Calabria G, Rescigno C, Salomone Megna A, Masullo A, Manzillo E, Russo G, Parrella R, Dell'Aquila G, Gambardella M, Ponticiello A, Coppola N; On Behalf Of CoviCam Group. Prognostic Value of Transaminases and Bilirubin Levels at Admission to Hospital on Disease Progression and Mortality in Patients with COVID-19-An Observational Retrospective Study. Pathogens. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 235. | Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M; APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (1)] |
| 236. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768-771.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 269] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 237. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 383] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 238. | Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 239. | Moon AM, Webb GJ, Aloman C, Armstrong MJ, Cargill T, Dhanasekaran R, Genescà J, Gill US, James TW, Jones PD, Marshall A, Mells G, Perumalswami PV, Qi X, Su F, Ufere NN, Barnes E, Barritt AS, Marjot T. High mortality rates for SARS-CoV-2 infection in patients with pre-existing chronic liver disease and cirrhosis: Preliminary results from an international registry. J Hepatol. 2020;73:705-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 240. | Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen VL, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin KD, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch AD, Viveiros K, Chan W, Chascsa DM, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients With Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2021;19:1469-1479.e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 241. | Kushner T, Cafardi J. Chronic Liver Disease and COVID-19: Alcohol Use Disorder/Alcohol-Associated Liver Disease, Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis, Autoimmune Liver Disease, and Compensated Cirrhosis. Clin Liver Dis (Hoboken). 2020;15:195-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 242. | Efe C, Dhanasekaran R, Lammert C, Ebik B, Higuera-de la Tijera F, Aloman C, Rıza Calışkan A, Peralta M, Gerussi A, Massoumi H, Catana AM, Torgutalp M, Purnak T, Rigamonti C, Gomez Aldana AJ, Khakoo N, Kacmaz H, Nazal L, Frager S, Demir N, Irak K, Ellik ZM, Balaban Y, Atay K, Eren F, Cristoferi L, Batıbay E, Urzua Á, Snijders R, Kıyıcı M, Akyıldız M, Ekin N, Carr RM, Harputluoğlu M, Hatemi I, Mendizabal M, Silva M, Idilman R, Silveira M, Drenth JPH, Assis DN, Björnsson E, Boyer JL, Invernizzi P, Levy C, Schiano TD, Ridruejo E, Wahlin S. Outcome of COVID-19 in Patients With Autoimmune Hepatitis: An International Multicenter Study. Hepatology. 2021;73:2099-2109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 243. | Noor MT, Manoria P. Immune Dysfunction in Cirrhosis. J Clin Transl Hepatol. 2017;5:50-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 244. | Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:727-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 278] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 245. | Sun HQ, Zhang JY, Zhang H, Zou ZS, Wang FS, Jia JH. Increased Th17 cells contribute to disease progression in patients with HBV-associated liver cirrhosis. J Viral Hepat. 2012;19:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 246. | Piano S, Dalbeni A, Vettore E, Benfaremo D, Mattioli M, Gambino CG, Framba V, Cerruti L, Mantovani A, Martini A, Luchetti MM, Serra R, Cattelan A, Vettor R, Angeli P; COVID-LIVER study group. Abnormal liver function tests predict transfer to intensive care unit and death in COVID-19. Liver Int. 2020;40:2394-2406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 247. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 844] [Article Influence: 76.7] [Reference Citation Analysis (1)] |
| 248. | Fan VS, Dominitz JA, Eastment MC, Locke ER, Green P, Berry K, O'Hare AM, Shah JA, Crothers K, Ioannou GN. Risk Factors for Testing Positive for Severe Acute Respiratory Syndrome Coronavirus 2 in a National United States Healthcare System. Clin Infect Dis. 2021;73:E3085-e3094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 249. | Ioannou GN, Liang PS, Locke E, Green P, Berry K, O'Hare AM, Shah JA, Crothers K, Eastment MC, Fan VS, Dominitz JA. Cirrhosis and Severe Acute Respiratory Syndrome Coronavirus 2 Infection in US Veterans: Risk of Infection, Hospitalization, Ventilation, and Mortality. Hepatology. 2021;74:322-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 250. | Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4343] [Cited by in RCA: 4189] [Article Influence: 837.8] [Reference Citation Analysis (0)] |
| 251. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M, Gerbes A, Wendon J, Alessandria C, Laleman W, Zeuzem S, Trebicka J, Bernardi M, Arroyo V; CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-1437, 1437.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1720] [Cited by in RCA: 2159] [Article Influence: 179.9] [Reference Citation Analysis (5)] |
| 252. | Schütte A, Ciesek S, Wedemeyer H, Lange CM. Influenza virus infection as precipitating event of acute-on-chronic liver failure. J Hepatol. 2019;70:797-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 253. | Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, Shi D, Jiang J, Sun S, Jin L, Ye P, Yang L, Lu Y, Li T, Huang J, Xu X, Chen J, Hao S, Chen Y, Xin S, Gao Z, Duan Z, Han T, Wang Y, Gan J, Feng T, Pan C, Li H, Huang Y, Xie Q, Lin S, Li L; Chinese Group on the Study of Severe Hepatitis B (COSSH). Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut. 2018;67:2181-2191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 293] [Article Influence: 41.9] [Reference Citation Analysis (2)] |
| 254. | Bajaj JS, Garcia-Tsao G, Wong F, Biggins SW, Kamath PS, McGeorge S, Chew M, Pearson M, Shaw J, Kalluri A, Fagan A, Olofson A, Moini M, de la Rosa Rodriguez R, Reddy KR. Cirrhosis Is Associated With High Mortality and Readmissions Over 90 Days Regardless of COVID-19: A Multicenter Cohort. Liver Transpl. 2021;27:1343-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 255. | Strnad P, Tacke F, Koch A, Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 440] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 256. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Correction to: Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver (APASL): An update. Hepatol Int. 2019;13:826-828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 257. | Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 3408] [Article Influence: 681.6] [Reference Citation Analysis (0)] |
| 258. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 259. | Zhou YJ, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, George J, Zheng MH. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: A multicenter preliminary analysis. J Hepatol. 2020;73:719-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 260. | Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 261. | Shelton JF, Shastri AJ, Ye C, Weldon CH, Filshtein-Sonmez T, Coker D, Symons A, Esparza-Gordillo J; 23andMe COVID-19 Team, Aslibekyan S, Auton A. Trans-ancestry analysis reveals genetic and nongenetic associations with COVID-19 susceptibility and severity. Nat Genet. 2021;53:801-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 172] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 262. | Zhu Z, Hasegawa K, Ma B, Fujiogi M, Camargo CA Jr, Liang L. Association of obesity and its genetic predisposition with the risk of severe COVID-19: Analysis of population-based cohort data. Metabolism. 2020;112:154345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 263. | Sachdeva S, Khandait H, Kopel J, Aloysius MM, Desai R, Goyal H. NAFLD and COVID-19: A Pooled Analysis. SN Compr Clin Med. 2020;2:2726-2729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 264. | Kopec AK, Abrahams SR, Thornton S, Palumbo JS, Mullins ES, Divanovic S, Weiler H, Owens AP 3rd, Mackman N, Goss A, van Ryn J, Luyendyk JP, Flick MJ. Thrombin promotes diet-induced obesity through fibrin-driven inflammation. J Clin Invest. 2017;127:3152-3166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 265. | Targher G, Bertolini L, Scala L, Zenari L, Lippi G, Franchini M, Arcaro G. Plasma PAI-1 levels are increased in patients with nonalcoholic steatohepatitis. Diabetes Care. 2007;30:E31-e32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 266. | Chang ML, Hsu CM, Tseng JH, Tsou YK, Chen SC, Shiau SS, Yeh CT, Chiu CT. Plasminogen activator inhibitor-1 is independently associated with non-alcoholic fatty liver disease whereas leptin and adiponectin vary between genders. J Gastroenterol Hepatol. 2015;30:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 267. | Reaven GM, Scott EM, Grant PJ, Lowe GD, Rumley A, Wannamethee SG, Stratmann B, Tschoepe D, Blann A, Juhan-Vague I, Alessi MC, Bailey C. Hemostatic abnormalities associated with obesity and the metabolic syndrome. J Thromb Haemost. 2005;3:1074-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 268. | Mertens I, Van Gaal LF. Obesity, haemostasis and the fibrinolytic system. Obes Rev. 2002;3:85-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 241] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 269. | Samad F, Ruf W. Inflammation, obesity, and thrombosis. Blood. 2013;122:3415-3422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 287] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 270. | Gao F, Zheng KI, Wang XB, Yan HD, Sun QF, Pan KH, Wang TY, Chen YP, George J, Zheng MH. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J Gastroenterol Hepatol. 2021;36:204-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 271. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2792] [Article Influence: 558.4] [Reference Citation Analysis (1)] |
| 272. | Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, Liu WY, George J, Zheng MH. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 273. | Michalakis K, Ilias I. SARS-CoV-2 infection and obesity: Common inflammatory and metabolic aspects. Diabetes Metab Syndr. 2020;14:469-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 274. | Lefere S, Tacke F. Macrophages in obesity and non-alcoholic fatty liver disease: Crosstalk with metabolism. JHEP Rep. 2019;1:30-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 275. | Luzi L, Radaelli MG. Influenza and obesity: Its odd relationship and the lessons for COVID-19 pandemic. Acta Diabetol. 2020;57:759-764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 230] [Article Influence: 46.0] [Reference Citation Analysis (1)] |
| 276. | Ahn SY, Sohn SH, Lee SY, Park HL, Park YW, Kim H, Nam JH. The effect of lipopolysaccharide-induced obesity and its chronic inflammation on influenza virus-related pathology. Environ Toxicol Pharmacol. 2015;40:924-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 277. | Sharma P, Kumar A. Metabolic dysfunction associated fatty liver disease increases risk of severe Covid-19. Diabetes Metab Syndr. 2020;14:825-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 278. | Severe Covid-19 GWAS Group; Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, Invernizzi P, Fernández J, Prati D, Baselli G, Asselta R, Grimsrud MM, Milani C, Aziz F, Kässens J, May S, Wendorff M, Wienbrandt L, Uellendahl-Werth F, Zheng T, Yi X, de Pablo R, Chercoles AG, Palom A, Garcia-Fernandez AE, Rodriguez-Frias F, Zanella A, Bandera A, Protti A, Aghemo A, Lleo A, Biondi A, Caballero-Garralda A, Gori A, Tanck A, Carreras Nolla A, Latiano A, Fracanzani AL, Peschuck A, Julià A, Pesenti A, Voza A, Jiménez D, Mateos B, Nafria Jimenez B, Quereda C, Paccapelo C, Gassner C, Angelini C, Cea C, Solier A, Pestaña D, Muñiz-Diaz E, Sandoval E, Paraboschi EM, Navas E, García Sánchez F, Ceriotti F, Martinelli-Boneschi F, Peyvandi F, Blasi F, Téllez L, Blanco-Grau A, Hemmrich-Stanisak G, Grasselli G, Costantino G, Cardamone G, Foti G, Aneli S, Kurihara H, ElAbd H, My I, Galván-Femenia I, Martín J, Erdmann J, Ferrusquía-Acosta J, Garcia-Etxebarria K, Izquierdo-Sanchez L, Bettini LR, Sumoy L, Terranova L, Moreira L, Santoro L, Scudeller L, Mesonero F, Roade L, Rühlemann MC, Schaefer M, Carrabba M, Riveiro-Barciela M, Figuera Basso ME, Valsecchi MG, Hernandez-Tejero M, Acosta-Herrera M, D'Angiò M, Baldini M, Cazzaniga M, Schulzky M, Cecconi M, Wittig M, Ciccarelli M, Rodríguez-Gandía M, Bocciolone M, Miozzo M, Montano N, Braun N, Sacchi N, Martínez N, Özer O, Palmieri O, Faverio P, Preatoni P, Bonfanti P, Omodei P, Tentorio P, Castro P, Rodrigues PM, Blandino Ortiz A, de Cid R, Ferrer R, Gualtierotti R, Nieto R, Goerg S, Badalamenti S, Marsal S, Matullo G, Pelusi S, Juzenas S, Aliberti S, Monzani V, Moreno V, Wesse T, Lenz TL, Pumarola T, Rimoldi V, Bosari S, Albrecht W, Peter W, Romero-Gómez M, D'Amato M, Duga S, Banales JM, Hov JR, Folseraas T, Valenti L, Franke A, Karlsen TH. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N Engl J Med. 2020;383:1522-1534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1512] [Cited by in RCA: 1386] [Article Influence: 277.2] [Reference Citation Analysis (0)] |
| 279. | Lopez-Mendez I, Aquino-Matus J, Gall SM, Prieto-Nava JD, Juarez-Hernandez E, Uribe M, Castro-Narro G. Association of liver steatosis and fibrosis with clinical outcomes in patients with SARS-CoV-2 infection (COVID-19). Ann Hepatol. 2021;20:100271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 280. | Jose RJ, Manuel A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir Med. 2020;8:E46-e47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 789] [Cited by in RCA: 876] [Article Influence: 175.2] [Reference Citation Analysis (0)] |
| 281. | Marjot T, Buescher G, Sebode M, Barnes E, Barritt AS 4th, Armstrong MJ, Baldelli L, Kennedy J, Mercer C, Ozga AK, Casar C, Schramm C; contributing Members and Collaborators of ERN RARE-LIVER/COVID-Hep/SECURE-Cirrhosis, Moon AM, Webb GJ, Lohse AW. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 282. | APASL Covid-19 Task Force, Lau G, Sharma M. Clinical practice guidance for hepatology and liver transplant providers during the COVID-19 pandemic: APASL expert panel consensus recommendations. Hepatol Int. 2020;14:415-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 283. | Zou X, Fang M, Huang J. Liver Function Should Be Monitored When Treating COVID-19 in Chronic HBV-Infected Patients. Clin Gastroenterol Hepatol. 2020;18:3056-3057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 284. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18843] [Article Influence: 3768.6] [Reference Citation Analysis (7)] |
| 285. | Sarkar S, Khanna P, Singh AK. Impact of COVID-19 in patients with concurrent co-infections: A systematic review and meta-analyses. J Med Virol. 2021;93:2385-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 286. | Rodríguez-Tajes S, Miralpeix A, Costa J, López-Suñé E, Laguno M, Pocurull A, Lens S, Mariño Z, Forns X. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J Viral Hepat. 2021;28:89-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 287. | Yip TC, Wong VW, Lui GC, Chow VC, Tse YK, Hui VW, Liang LY, Chan HL, Hui DS, Wong GL. Current and Past Infections of HBV Do Not Increase Mortality in Patients With COVID-19. Hepatology. 2021;74:1750-1765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 288. | Mangia A, Cenderello G, Verucchi G, Ciancio A, Fontana A, Piazzolla V, Minerva N, Squillante MM, Copetti M. Is positivity for hepatitis C virus antibody predictive of lower risk of death in COVID-19 patients with cirrhosis? World J Clin Cases. 2020;8:5831-5834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 289. | Colmenero J, Rodríguez-Perálvarez M, Salcedo M, Arias-Milla A, Muñoz-Serrano A, Graus J, Nuño J, Gastaca M, Bustamante-Schneider J, Cachero A, Lladó L, Caballero A, Fernández-Yunquera A, Loinaz C, Fernández I, Fondevila C, Navasa M, Iñarrairaegui M, Castells L, Pascual S, Ramírez P, Vinaixa C, González-Dieguez ML, González-Grande R, Hierro L, Nogueras F, Otero A, Álamo JM, Blanco-Fernández G, Fábrega E, García-Pajares F, Montero JL, Tomé S, De la Rosa G, Pons JA. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 270] [Article Influence: 67.5] [Reference Citation Analysis (0)] |
| 290. | Webb GJ, Moon AM, Barnes E, Barritt AS, Marjot T. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol. 2020;5:643-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 291. | Liu H, He X, Wang Y, Zhou S, Zhang D, Zhu J, He Q, Zhu Z, Li G, Sun L, Wang J, Cheng G, Liu Z, Lau G. Management of COVID-19 in patients after liver transplantation: Beijing working party for liver transplantation. Hepatol Int. 2020;14:432-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 292. | Qin J, Wang H, Qin X, Zhang P, Zhu L, Cai J, Yuan Y, Li H. Perioperative Presentation of COVID-19 Disease in a Liver Transplant Recipient. Hepatology. 2020;72:1491-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 293. | Michaels MG, La Hoz RM, Danziger-Isakov L, Blumberg EA, Kumar D, Green M, Pruett TL, Wolfe CR. Coronavirus disease 2019: Implications of emerging infections for transplantation. Am J Transplant. 2020;20:1768-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 294. | Waisberg DR, Abdala E, Nacif LS, Haddad LB, Ducatti L, Santos VR, Gouveia LN, Lazari CS, Martino RB, Pinheiro RS, Arantes RM, Terrabuio DR, Malbouisson LM, Galvao FH, Andraus W, Carneiro-D'Albuquerque LA. Liver transplant recipients infected with SARS-CoV-2 in the early postoperative period: Lessons from a single center in the epicenter of the pandemic. Transpl Infect Dis. 2021;23:E13418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 295. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 296. | Becchetti C, Zambelli MF, Pasulo L, Donato MF, Invernizzi F, Detry O, Dahlqvist G, Ciccarelli O, Morelli MC, Fraga M, Svegliati-Baroni G, van Vlierberghe H, Coenraad MJ, Romero MC, de Gottardi A, Toniutto P, Del Prete L, Abbati C, Samuel D, Pirenne J, Nevens F, Dufour JF; COVID-LT group. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020;69:1832-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 297. | Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State. JAMA. 2020;323:1612-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1511] [Cited by in RCA: 1623] [Article Influence: 324.6] [Reference Citation Analysis (0)] |
| 298. | Belli LS, Fondevila C, Cortesi PA, Conti S, Karam V, Adam R, Coilly A, Ericzon BG, Loinaz C, Cuervas-Mons V, Zambelli M, Llado L, Diaz-Fontenla F, Invernizzi F, Patrono D, Faitot F, Bhooori S, Pirenne J, Perricone G, Magini G, Castells L, Detry O, Cruchaga PM, Colmenero J, Berrevoet F, Rodriguez G, Ysebaert D, Radenne S, Metselaar H, Morelli C, De Carlis LG, Polak WG, Duvoux C; ELITA-ELTR COVID-19 Registry. Protective Role of Tacrolimus, Deleterious Role of Age and Comorbidities in Liver Transplant Recipients With Covid-19: Results From the ELITA/ELTR Multi-center European Study. Gastroenterology. 2021;160:1151-1163.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 299. | Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, Jia P, Guan HQ, Peng L, Chen Y, Peng P, Zhang P, Chu Q, Shen Q, Wang Y, Xu SY, Zhao JP, Zhou M. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann Oncol. 2020;31:894-901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 819] [Cited by in RCA: 1015] [Article Influence: 203.0] [Reference Citation Analysis (0)] |
| 300. | Chan SL, Kudo M. Impacts of COVID-19 on Liver Cancers: During and after the Pandemic. Liver Cancer. 2020;9:491-502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 301. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 10651] [Article Influence: 2130.2] [Reference Citation Analysis (1)] |
| 302. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7073] [Cited by in RCA: 7520] [Article Influence: 1880.0] [Reference Citation Analysis (1)] |
| 303. | Ou MT, Boyarsky BJ, Motter JD, Greenberg RS, Teles AT, Ruddy JA, Krach MR, Jain VS, Werbel WA, Avery RK, Massie AB, Segev DL, Garonzik-Wang JM. Safety and Reactogenicity of 2 Doses of SARS-CoV-2 Vaccination in Solid Organ Transplant Recipients. Transplantation. 2021;105:2170-2174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 304. | Daugherty SE, Guo Y, Heath K, Dasmariñas MC, Jubilo KG, Samranvedhya J, Lipsitch M, Cohen K. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ. 2021;373:N1098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 209] [Cited by in RCA: 269] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 305. | Dennis A, Wamil M, Alberts J, Oben J, Cuthbertson DJ, Wootton D, Crooks M, Gabbay M, Brady M, Hishmeh L, Attree E, Heightman M, Banerjee R, Banerjee A; COVERSCAN study investigators. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: A prospective, community-based study. BMJ Open. 2021;11:E048391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 266] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 306. | Bende F, Tudoran C, Sporea I, Fofiu R, Bâldea V, Cotrău R, Popescu A, Sirli R, Ungureanu BS, Tudoran M. A Multidisciplinary Approach to Evaluate the Presence of Hepatic and Cardiac Abnormalities in Patients with Post-Acute COVID-19 Syndrome-A Pilot Study. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 307. | Radzina M, Putrins DS, Micena A, Vanaga I, Kolesova O, Platkajis A, Viksna L. Post-COVID-19 Liver Injury: Comprehensive Imaging With Multiparametric Ultrasound. J Ultrasound Med. 2022;41:935-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 308. | Holmes E, Wist J, Masuda R, Lodge S, Nitschke P, Kimhofer T, Loo RL, Begum S, Boughton B, Yang R, Morillon AC, Chin ST, Hall D, Ryan M, Bong SH, Gay M, Edgar DW, Lindon JC, Richards T, Yeap BB, Pettersson S, Spraul M, Schaefer H, Lawler NG, Gray N, Whiley L, Nicholson JK. Incomplete Systemic Recovery and Metabolic Phenoreversion in Post-Acute-Phase Nonhospitalized COVID-19 Patients: Implications for Assessment of Post-Acute COVID-19 Syndrome. J Proteome Res. 2021;20:3315-3329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 309. | Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, Ravelli RBG, Paul van Schayck J, Mykytyn AZ, Duimel HQ, van Donselaar E, Riesebosch S, Kuijpers HJH, Schipper D, van de Wetering WJ, de Graaf M, Koopmans M, Cuppen E, Peters PJ, Haagmans BL, Clevers H. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1245] [Cited by in RCA: 1307] [Article Influence: 261.4] [Reference Citation Analysis (0)] |
| 310. | Fogarty H, Townsend L, Morrin H, Ahmad A, Comerford C, Karampini E, Englert H, Byrne M, Bergin C, O'Sullivan JM, Martin-Loeches I, Nadarajan P, Bannan C, Mallon PW, Curley GF, Preston RJS, Rehill AM, McGonagle D, Ni Cheallaigh C, Baker RI, Renné T, Ward SE, O'Donnell JS; Irish COVID-19 Vasculopathy Study (iCVS) investigators. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost. 2021;19:2546-2553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 211] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 311. | Faruqui S, Okoli FC, Olsen SK, Feldman DM, Kalia HS, Park JS, Stanca CM, Figueroa Diaz V, Yuan S, Dagher NN, Sarkar SA, Theise ND, Kim S, Shanbhogue K, Jacobson IM. Cholangiopathy After Severe COVID-19: Clinical Features and Prognostic Implications. Am J Gastroenterol. 2021;116:1414-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 312. | Roth NC, Kim A, Vitkovski T, Xia J, Ramirez G, Bernstein D, Crawford JM. Post-COVID-19 Cholangiopathy: A Novel Entity. Am J Gastroenterol. 2021;116:1077-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 313. | Durazo FA, Nicholas AA, Mahaffey JJ, Sova S, Evans JJ, Trivella JP, Loy V, Kim J, Zimmerman MA, Hong JC. Post-Covid-19 Cholangiopathy-A New Indication for Liver Transplantation: A Case Report. Transplant Proc. 2021;53:1132-1137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 314. | Brito CA, Barros FM, Lopes EP. Mechanisms and consequences of COVID-19 associated liver injury: What can we affirm? World J Hepatol. 2020;12:413-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 315. | Philips CA, Ahamed R, Augustine P. SARS-CoV-2 related liver impairment - perception may not be the reality. J Hepatol. 2020;73:991-992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 316. | Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-Cell RNA Expression Profiling of ACE2, the Receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;202:756-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 361] [Cited by in RCA: 599] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 317. | Bangash MN, Patel JM, Parekh D, Murphy N, Brown RM, Elsharkawy AM, Mehta G, Armstrong MJ, Neil D. SARS-CoV-2: Is the liver merely a bystander to severe disease? J Hepatol. 2020;73:995-996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 318. | Lagana SM, De Michele S, Lee MJ, Emond JC, Griesemer AD, Tulin-Silver SA, Verna EC, Martinez M, Lefkowitch JH. COVID-19 Associated Hepatitis Complicating Recent Living Donor Liver Transplantation. Arch Pathol Lab Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 319. | Idalsoaga F, Ayares G, Arab JP, Díaz LA. COVID-19 and Indirect Liver Injury: A Narrative Synthesis of the Evidence. J Clin Transl Hepatol. 2021;9:760-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 320. | Chu H, Bai T, Chen L, Hu L, Xiao L, Yao L, Zhu R, Niu X, Li Z, Zhang L, Han C, Song S, He Q, Zhao Y, Zhu Q, Chen H, Schnabl B, Yang L, Hou X. Multicenter Analysis of Liver Injury Patterns and Mortality in COVID-19. Front Med (Lausanne). 2020;7:584342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 321. | Krishnan A, Prichett L, Tao X, Alqahtani SA, Hamilton JP, Mezey E, Strauss AT, Kim A, Potter JJ, Chen PH, Woreta TA. Abnormal liver chemistries as a predictor of COVID-19 severity and clinical outcomes in hospitalized patients. World J Gastroenterol. 2022;28:570-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 322. | Ekpanyapong S, Bunchorntavakul C, Reddy KR. COVID-19 and the Liver: Lessons Learnt from the EAST and the WEST, A Year Later. J Viral Hepat. 2022;29:4-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 323. | Del Zompo F, De Siena M, Ianiro G, Gasbarrini A, Pompili M, Ponziani FR. Prevalence of liver injury and correlation with clinical outcomes in patients with COVID-19: Systematic review with meta-analysis. Eur Rev Med Pharmacol Sci. 2020;24:13072-13088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 324. | Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020;8:509-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 325. | Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, Falck-Ytter Y, El-Serag HB; AGA Institute. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology. 2020;159:320-334.e27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 292] [Article Influence: 58.4] [Reference Citation Analysis (1)] |
| 326. | Hartl L, Haslinger K, Angerer M, Jachs M, Simbrunner B, Bauer DJM, Semmler G, Scheiner B, Eigenbauer E, Strassl R, Breuer M, Kimberger O, Laxar D, Trauner M, Mandorfer M, Reiberger T. Age-adjusted mortality and predictive value of liver chemistries in a Viennese cohort of COVID-19 patients. Liver Int. 2022;42:1297-1307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 327. | Ponziani FR, Nesci A, Del Zompo F, Santopaolo F, Pompili M, Gasbarrini A. Correlation Between Liver Function Tests Abnormalities and Interleukin-6 Serum Levels in Patients With SARS-CoV-2 Infection. Gastroenterology. 2021;160:1891-1893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 328. | Lee YR, Kang MK, Song JE, Kim HJ, Kweon YO, Tak WY, Jang SY, Park JG, Lee C, Hwang JS, Jang BK, Suh JI, Chung WJ, Kim BS, Park SY. Clinical outcomes of coronavirus disease 2019 in patients with pre-existing liver diseases: A multicenter study in South Korea. Clin Mol Hepatol. 2020;26:562-576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 329. | Shauly-Aharonov M, Shafrir A, Paltiel O, Calderon-Margalit R, Safadi R, Bicher R, Barenholz-Goultschin O, Stokar J. Both high and low pre-infection glucose levels associated with increased risk for severe COVID-19: New insights from a population-based study. PLoS One. 2021;16:E0254847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (1)] |