Published online Apr 21, 2023. doi: 10.3748/wjg.v29.i15.2310
Peer-review started: November 4, 2022
First decision: January 3, 2023
Revised: January 21, 2023
Accepted: March 20, 2023
Article in press: March 20, 2023
Published online: April 21, 2023
Processing time: 160 Days and 22.3 Hours
The preoperative prediction of peritoneal metastasis (PM) in gastric cancer would prevent unnecessary surgery and promptly indicate an appropriate treatment plan.
To explore the predictive value of visceral fat (VF) parameters obtained from preoperative computed tomography (CT) images for occult PM and to develop an individualized model for predicting occult PM in patients with gastric carcinoma (GC).
A total of 128 confirmed GC cases (84 male and 44 female patients) that underwent CT scans were analyzed and categorized into PM-positive (n = 43) and PM-negative (n = 85) groups. The clinical characteristics and VF parameters of two regions of interest (ROIs) were collected. Univariate and stratified analyses based on VF volume were performed to screen for predictive characteristics for occult PM. Prediction models with and without VF parameters were established by multivariable logistic regression analysis.
The mean attenuations of VFROI 1 and VFROI 2 varied significantly between the PM-positive and PM-negative groups (P = 0.044 and 0.001, respectively). The areas under the receiver operating characteristic curves (AUCs) of VFROI 1 and VFROI 2 were 0.599 and 0.657, respectively. The mean attenuation of VFROI 2 was included in the final prediction combined model, but not an independent risk factor of PM (P = 0.068). No significant difference was observed between the models with and without mean attenuation of VF (AUC: 0.749 vs 0.730, P = 0.339).
The mean attenuation of VF is a potential auxiliary parameter for predicting occult PM in patients with GC.
Core Tip: The preoperative prediction of peritoneal metastasis (PM) in gastric cancer would prevent unnecessary surgery and promptly indicate an appropriate treatment plan. We therefore aimed to explore the predictive value of visceral fat (VF) parameters obtained from preoperative computed tomography images for occult PM and to develop an individualized model for predicting occult PM in patients with gastric carcinoma (GC). Consequently, mean attenuation of VF is a potential auxiliary parameter to predict occult PM in patients with GC.
- Citation: Li LM, Feng LY, Liu CC, Huang WP, Yu Y, Cheng PY, Gao JB. Can visceral fat parameters based on computed tomography be used to predict occult peritoneal metastasis in gastric cancer? World J Gastroenterol 2023; 29(15): 2310-2321
- URL: https://www.wjgnet.com/1007-9327/full/v29/i15/2310.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i15.2310
Peritoneal metastasis (PM) is the major cause of postoperative distant metastasis and occurs in more than 10% of patients with newly diagnosed gastric cancer[1]. Gastric carcinoma (GC) is the third leading cause of cancer-related deaths worldwide. In gastric cancer with PM, the survival time is only a few months[2]. Many recent studies[3] have shown that the combination of complete cell reduction and intraperitoneal thermal perfusion chemotherapy can significantly prolong the median survival time in gastric cancer with PM. Thus, the preoperative prediction of PM in gastric cancer would prevent unnecessary surgery and promptly indicate an appropriate treatment plan. For example, laparoscopic exploration may be the preferred choice. Additionally, intraperitoneal hyperthermic chemotherapy may be prepared, and open exploration may be the final choice for a patient with occult PM.
Traditional preoperative imaging methods, such as computed tomography (CT) and magnetic resonance imaging, demonstrate high specificity and low sensitivity in the detection of PM[4]. Therefore, occult PM has attracted extensive attention; it refers to metastasis not indicated on preoperative CT imaging but detected during surgery and pathologic examination[5]. In the last few years, many studies have demonstrated the great potential of radiomics in the prediction of occult PM in gastric cancer[6]. However, most studies have used manual delineation of regions of interest (ROIs) in PM, which reduces repeatability and increases subjective dependence. Therefore, the urgent exploration for new parameters for predicting occult PM is warranted.
Tumor infiltration of the peritoneum alters the characteristics of the surrounding visceral fat (VF) on CT, and these changes have been investigated in previous radiomics studies[7]. In addition, the feature referred to as “smudge-like ground-glass opacity” in the abdominal cavity, which presents an increased CT attenuation of VF, is a common but easily overlooked sign of early-stage omental metastasis[8]. In addition, the adipose microenvironment has a subtle relationship with tumor progression[9].
We therefore aimed to explore the predictive value of VF parameters based on preoperative CT imaging for occult PM in GC and to develop an individualized model for predicting occult PM preoperatively.
This retrospective study was approved by the local institutional review board, and the requirement for informed consent was waived.
Patients at our hospital who met the inclusion/exclusion criteria were consecutively enrolled between July 2012 and July 2021. The inclusion criteria were as follows: (1) Diagnosis of gastric cancer by endoscopy and biopsy, with complete biopsy information; (2) Diagnosis of cT3 and cT4 gastric cancer by CT without obvious PM characteristics; and (3) PM status confirmed by exploratory surgery and pathological biopsy. The exclusion criteria were follows: (1) A history of abdominal tumors, cirrhosis, or other inflammatory diseases; (2) A history of abdominal surgery; (3) An interval between CT and surgical exploration longer than 2 wk; and (4) The presence of artifacts that affect the evaluation of VF parameters.
Clinical patient data (e.g., sex and age), blood test information [e.g., carcinoembryonic antigen (CEA), carbohydrate antigen 199 (CA199), and carbohydrate antigen 125 (CA125)], and biopsy information (e.g., pathological type and differentiation) were collected for further analysis.
CT examinations were performed using SOMATOM Definition Flash CT or SOMATOM Force (Siemens, Germany). All patients fasted for 6 h, and drank 800-1000 mL water 5-15 min before CT examination. Following an unenhanced scan, dual-phase enhanced CT images were obtained at 30- and 70-s delays. Approximately 1.3 mL/kg iodinated contrast agent (Ultravist 370, Bayer Schering Pharma) was injected intravenously at a rate of 3.0 mL/s. The parameters of the unenhanced scan were as follows: 120 kV tube voltage, 250–350 mAs, and 5 mm slice thickness and slice interval. The parameters of the enhanced scan were as follows: 120 kV tube voltage, automatic tube current modulation techniques, and 5- or 1.25-mm slice thickness and slice interval.
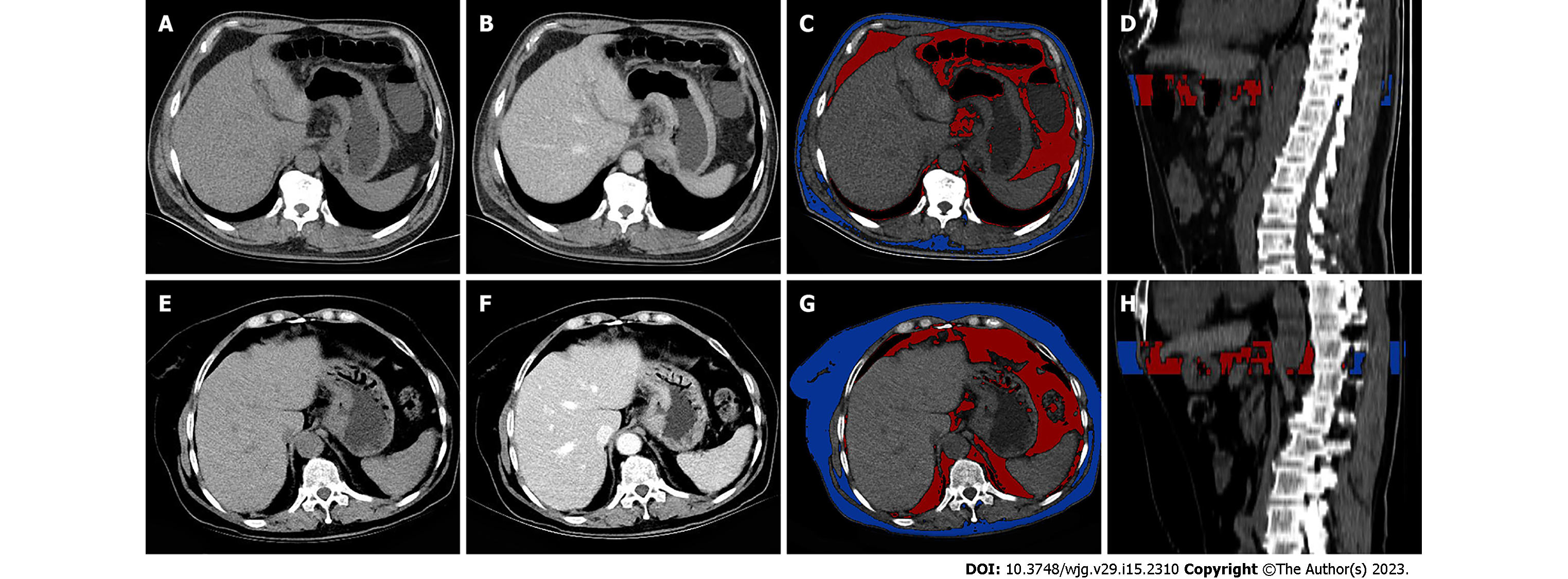
The volume and mean CT attenuation of VF and subcutaneous fat (SF) were measured using unenhanced scan CT images on a workstation (MM Research Frontier SyngoVia, VB2.0, Siemens Healthineers, Forchheim, Germany). VF was defined as fat within the abdominal cavity, whereas SF was defined as fat below the skin. ROIs were manually drawn by tracing the medial edge of the abdominal muscle using a semiautomated technique, starting from the maximal axial section of the tumor and extending 15 mm (ROI 1) and 25 mm (ROI 2) downward. The contour of each layer can be adjusted manually as necessary. A primary radiologist (with 6 years of experience) was responsible for outlining, while another senior radiologist (with 25 years of experience) was responsible for supervising each operation (Figure 1). Twenty cases were randomly chosen for outlining by another radiologist (with 3 years of experience) to verify the consistency.
CT attenuation between −150 and −50 Hounsfield units (HU) was performed to evaluate voxels containing adipose tissue[10]. Adipose tissue volume was measured in cubic centimeters (cm3), while mean CT attenuation was measured in HU. The mean CT attenuation and volume of VF were obtained directly on the workstation simultaneously with a table including the CT attenuation of each voxel. The 10th, 30th, 50th, 70th, 90th, 25th, and 75th percentiles of CT attenuation were obtained during postprocessing and calculation.
Two radiologists blinded to the pathologic data analyzed the CT characteristics. When disagreements occurred, a senior radiologist made the final decision. The cT and cN stages were analyzed according to the 8th American Joint Committee staging classification[11,12]. Mild ascites refers to ascites that could not be clearly determined to be caused by metastasis[5]. The GC thickness was measured at the maximal axial section[13].
Statistical analyses were conducted using SPSS software (version 22.0) and MedCalc software (version 15.2). Interobserver reliability was assessed using the intraclass correlation coefficient (ICC), where ICC ≥ 0.80 is excellent, 0.61-0.80 is good, and < 0.60 is the difference. The discrimination capability of various VF characteristics was evaluated using the receiver operating characteristic (ROC) curve and compared using Delong’s test. The relationship between volume and mean attenuation was evaluated using Pearson correlation analysis. Stratified analysis based on VF volume was performed. The differences in continuous variables were compared using the two-tailed t test, Mann–Whitney U test, Kruskal-Wallis H test, and one-way ANOVA. The differences in categorical variables were compared using the χ2 test. Significant variables were analyzed using multifactorial logistic regression analysis (backward: Conditional). P < 0.05 was considered to suggest a statistically significant difference.
A total of 43 PM-positive patients were included in the study, and 85 PM-negative patients treated between January 2021 and July 2021 were included as control subjects. Significant differences in age, CA125, tumor location, thickness, mild ascites, and cT (P < 0.05 for all) were found between the PM-positive and PM-negative groups (Table 1). No significant differences were found for sex, pathological type, differentiation, CEA, CA199, or cN stage (P > 0.05 for all).
| Variable | PM (-) (n = 85) | PM (+) (n = 43) | P value | |
| Sex | Male | 60 (70.6) | 24 (55.8) | 0.096 |
| Female | 25 (29.4) | 19 (44.2) | ||
| Age (yr) (mean ± SD) | 56.44±12.63 | 61.25±11.11 | 0.0291 | |
| Pathological type | Adenocarcinoma | 83 (97.6) | 38 (88.4) | 0.077 |
| Non-adenocarcinoma | 2 (2.4) | 5 (11.6) | ||
| Differentiation | Poorly differentiated | 50 (58.8) | 30 (69.8) | 0.227 |
| Moderately and well differentiated | 35 (41.2) | 13 (30.2) | ||
| CEA | Normal | 74 (87.1) | 37 (86.0) | 0.873 |
| Elevated | 11 (12.9) | 6 (14.0) | ||
| CA199 | Normal | 74 (87.1) | 32 (74.4) | 0.073 |
| Elevated | 11 (12.9) | 11 (25.6) | ||
| CA125 | Normal | 83 (97.6) | 37 (86.0) | 0.0301 |
| Elevated | 2 (2.4) | 6 (14.0) | ||
| Location | Cardia | 44 (51.8) | 7 (16.3) | 0.0011 |
| Body | 12 (14.1) | 14 (32.6) | ||
| Antrum | 20 (23.5) | 13 (30.2) | ||
| ≥ 2 parts | 9 (10.6) | 9 (20.9) | ||
| Mild ascites | (-) | 75 (88.2) | 24 (55.8) | 0.0011 |
| (+) | 10 (11.8) | 29 (44.2) | ||
| cN stage | N0 | 26 (30.6) | 11 (25.6) | 0.150 |
| N1-2 | 41 (48.2) | 16 (37.2) | ||
| N3-4 | 18 (21.2) | 16 (37.2) | ||
| cT stage | ≤ T3 | 57 (67.1) | 14 (32.6) | 0.0011 |
| ≥ T4 | 28 (32.9) | 29 (67.4) | ||
| Thickness (mm), median (IQR) | 15.11 (13.02, 18.85) | 13.43 (11.28, 17.83) | 0.0231 |
Significant differences in the mean attenuations of VFROI 1 and VFROI 2 (P = 0.044 and 0.001, respectively) were found between the PM-positive and PM-negative groups (Table 2). No significant differences were found for the mean attenuations of SFROI 1 and SFROI 2, the volumes of VFROI 1, VFROI 2, SFROI 1, and SFROI 2, or the volume ratio VF/SF (P > 0.05 for all).
| Variable | PM (+) (n = 43) | PM (-) (n = 85) | P value | ICC |
| VFROI 1 | ||||
| Volume (cc) | 84.45 (36.70, 161.04) | 87.92 (49.89, 160.75) | 0.412 | 0.913 |
| Mean attenuation (HU) | -82.07 ± 6.06 | -85.33 ± 5.89 | 0.0441 | 0.810 |
| SD attenuation (HU) | 18.82 (17.78, 21.25) | 19.51 (18.63, 21.11) | 0.133 | 0.926 |
| SFROI 1 | ||||
| Volume (cc) | 108.79 (55.10, 170.90) | 115.96 (62.70, 178.72) | 0.760 | 0.979 |
| Mean attenuation (HU) | -85.33 ± 5.89 | -86.05 ± 8.23 | 0.934 | 0.955 |
| SD attenuation (HU) | 17.94 (16.60, 19.37) | 18.58 (17.27, 20.16) | 0.180 | 0.981 |
| VFROI 2 | ||||
| Volume (cc) | 132.63 (58.85, 273.65) | 145.48 (82.58, 277.42) | 0.302 | 0.932 |
| Mean attenuation (HU) | -82.19 ± 5.35 | -85.70 ± 5.65 | 0.0011 | 0.974 |
| SD attenuation (HU) | 19.86 (17.58, 20.46) | 19.13 (18.47, 20.66) | 0.317 | 0.950 |
| SFROI 2 | ||||
| Volume (cc) | 163.24 (82.57, 261.17) | 175.86 (97.86, 267.52) | 0.76 | 0.991 |
| Mean attenuation (HU) | -86.28 ± 8.61 | -86.41 ± 8.08 | 0.938 | 0.980 |
| SD attenuation (HU) | 17.80 (16.42, 19.33) | 18.45 (17.29, 19.91) | 0.124 | 0.993 |
| Volume ratio | ||||
| VF/SFROI 1 | 0.92 (0.44, 1.50) | 1.00 (0.61, 1.65) | 0.317 | 0.804 |
| VF/SFROI 2 | 0.98 (0.47, 1.41) | 1.00 (0.63, 1.68) | 0.279 | 0.884 |
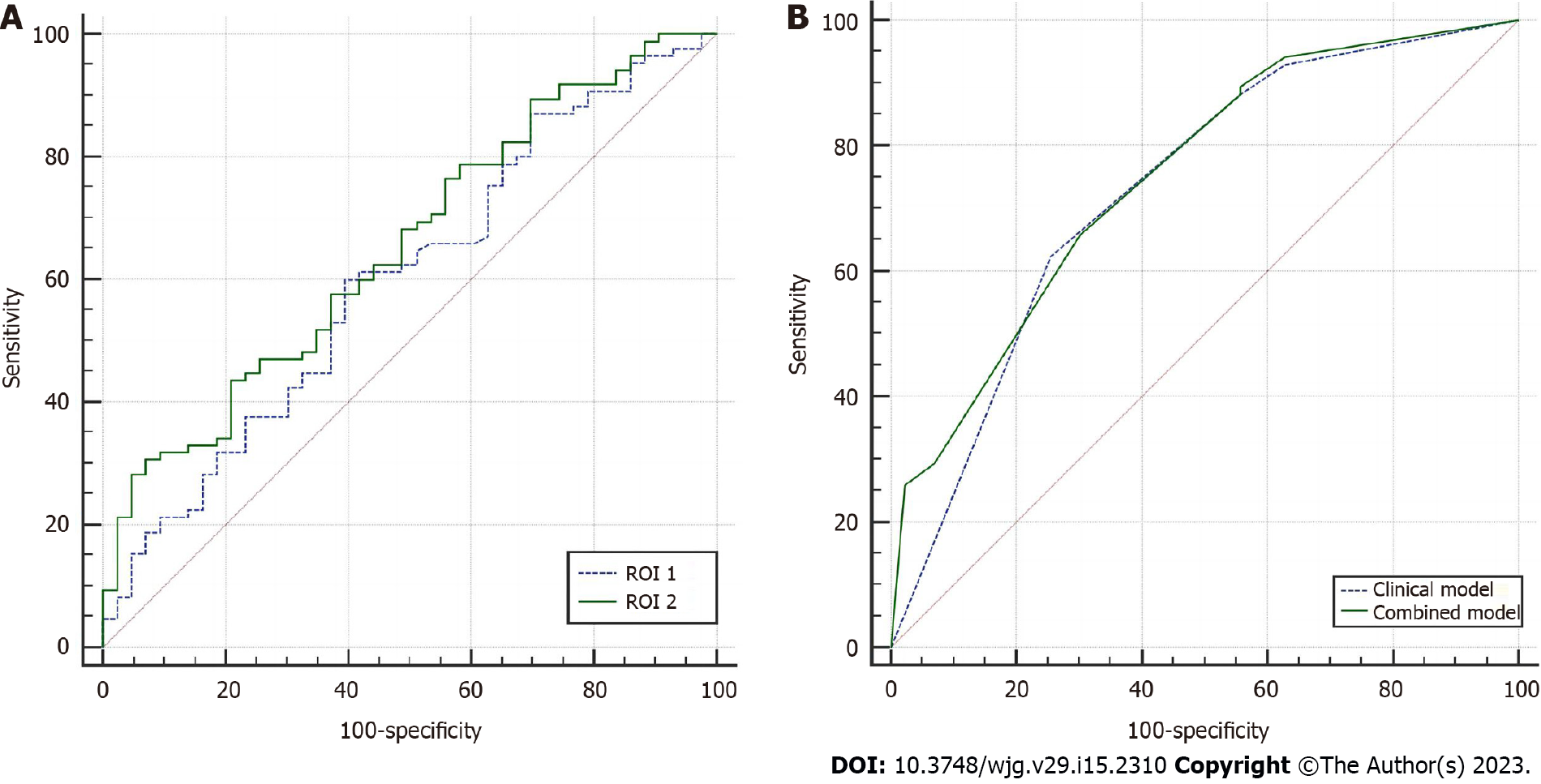
The areas under the receiver operating characteristic curves (AUCs) of VFROI 1 and VFROI 2 for predicting PM in GC were 0.599 [95% confidence interval (CI): 0.509-0.685] and 0.657 (95%CI: 0.568-0.738), respectively (Figure 2A). The comparison of the ROC curves of the VF characteristics suggested that VFROI 2 significantly outperformed VFROI 1 (P = 0.002). Most of the interobserver agreements between the two operators were excellent (Table 2).
Significant differences in the 30th, 50th, 70th, 25th, and 75th percentiles of CT attenuation of VFROI 2 were found between the PM-positive and PM-negative groups (Supplementary Table 1). The AUCs of the 10th, 30th, 50th, 70th, 90th, 25th, and 75th percentiles of CT attenuation for predicting PM in GC were 0.612, 0.642, 0.641, 0.619, 0.608, 0.629, and 0.619, respectively. The comparison of ROC curves suggested no significant difference between the 30th percentile and the mean CT attenuation of VFROI 2 (P = 0.664) (Supplementary Figure 1).
Significant differences in the mean attenuation of VFROI 2 were observed between the different cN and cT stage classifications (P = 0.030 and 0.003, respectively). The mean attenuation of VFROI 2 in patients with elevated CA125, elevated CA199, and mild ascites was significantly higher than that in normal conditions (P = 0.002, 0.039, and 0.001, respectively) (Table 3).
| Variable | Mean attenuation of VF ROI 2 | P value | |
| Sex | Male | -84.90 ± 6.19 | 0.306 |
| Female | -83.83 ± 4.88 | ||
| Age (yr) | ≤ 61 | -84.48 ± 5.11 | 0.937 |
| > 61 | -84.46 ± 5.14 | ||
| Pathological type | Adenocarcinoma | -84.64 ± 5.81 | 0.339 |
| Non-adenocarcinoma | -82.49 ± 4.99 | ||
| Differentiation | Poorly differentiated | -84.18 ± 5.91 | 0.377 |
| Moderately and well differentiated | -85.11 ± 5.56 | ||
| CEA | Normal | -84.64 ± 5.92 | 0.583 |
| Elevated | -83.81 ± 4.86 | ||
| CA199 | Normal | -85.04 ± 6.04 | 0.0021 |
| Elevated | -82.07 ± 3.40 | ||
| CA125 | Normal | -84.80 ± 5.81 | 0.0391 |
| Elevated | -80.46 ± 3.41 | ||
| Location | Cardia/Body/Antrum | -84.93 ± 5.86 | 0.0491 |
| ≥ 2 parts | -82.05 ± 4.67 | ||
| Mild ascites | (-) | -85.41 ± 5.79 | 0.0011 |
| (+) | -81.52 ± 4.69 | ||
| cN stage | N0 | -85.48 (-89.85, -82.95) | 0.0301 |
| N1-2 | -83.08 (-86.68, -79.43) | ||
| N3-4 | -84.54 (-87.51, -80.60) | ||
| cT stage | ≤ T3 | -85.88 ± 5.83 | 0.0031 |
| ≥ T4 | -82.84 ± 5.29 | ||
| Thickness (mm) | ≤ 14.5 | -84.82 ± 6.36 | 0.517 |
| > 14.5 | -84.15 ± 4.98 |
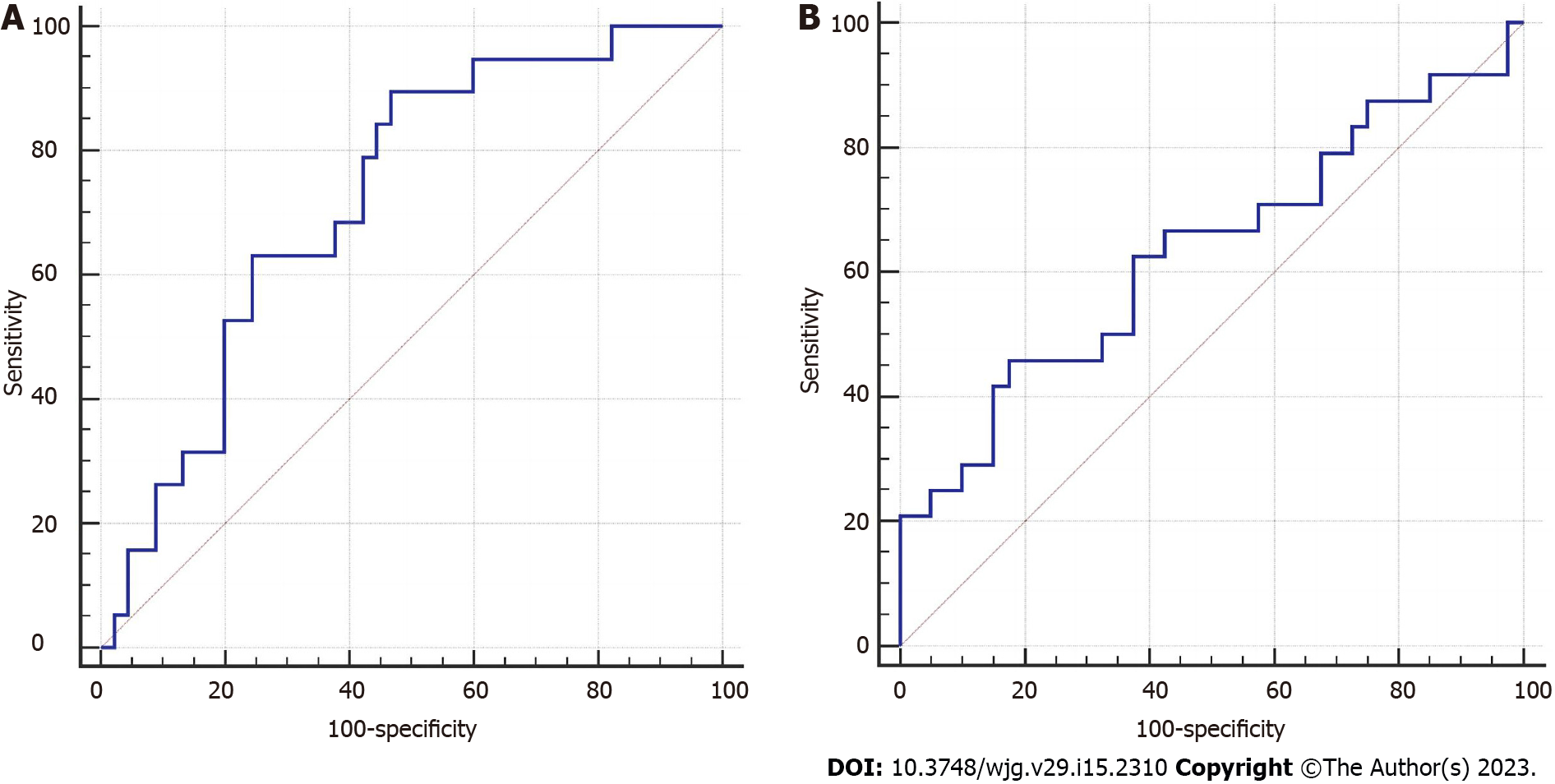
The mean attenuation was negatively correlated with the volume of VFROI 2 (Pearson correlation coefficient = -0.688; P = 0.001) (Supplementary Figure 2). Patients were divided into two groups according to the median volume of VF ROI 2 (median = 141.58 cm3). Stratified analysis suggested that the mean attenuation of VF ROI 2 was more effective in predicting occult PM in the high-volume group than in the low-volume group (Table 4, Figure 3).
Multiple logistic regression analysis based on clinical characteristics showed that mild ascites and cT stage (≥ T4) were independent risk factors for occult PM (P = 0.003 and 0.010, respectively) (Table 5). The Hosmer-Lemeshow test revealed that the model had a good fit (P = 0.977). Furthermore, the clinical model showed a moderate prediction performance (AUC = 0.730, 95%CI: 0.644-0.804).
| Variable | OR | 95%CI | P value |
| Based on all characteristics | |||
| Mild ascites (+ vs -) | 3.552 | (1.375-9.175) | 0.0091 |
| Mean attenuations of VFROI 2 | 3.437 | (0.914-12.914) | 0.068 |
| cT stage (≥ T4 vs ≤ T3) | 2.631 | (1.126-6.144) | 0.0251 |
| Intercept | 0.009 | 0.0011 | |
| Based on clinical characteristics | |||
| Mild ascites (+ vs -) | 4.188 | (1.636-10.722) | 0.0031 |
| cT stage (≥ T4 vs ≤ T3) | 2.992 | (1.301-6.881) | 0.0101 |
| Intercept | 0.068 | 0.0011 |
Multiple logistic regression analyses of both clinical characteristics and VF parameters showed that the mean attenuation of VFROI 2, mild ascites, and cT stage (≥ T4) were included in the final prediction combined model, but the mean attenuation of VFROI 2 was not an independent risk factor for PM (P = 0.068) (Table 5). The Hosmer-Lemeshow test indicated that the model had a good fit (P = 0.523). The combined model showed a moderate prediction performance (AUC = 0.749, 95%CI: 0.665-0.821). No significant difference was found between the prediction performance of the two models (P = 0.339) (Figure 2B).
We explored the value of VF parameters in predicting occult PM in GC and subsequently developed a preoperative prediction model based on clinical features and VF parameters. A significant difference in the mean attenuation of VF was found between the PM-positive and PM-negative groups. The mean attenuation of VF was included in the final prediction combined model but was not an independent predictor of occult PM in GC.
Previous studies on VF have mostly focused on the effect of volume or area on postoperative prognosis, and few studies have investigated the CT attenuation of VF[14,15]. CT attenuation of pericoronary adipose tissue has been shown to be strongly correlated with cardiovascular disease[16]. Xiang et al[9] investigated the relationship between adipocytes and gastric cancer cells and found that adipocytes may promote cancer cell invasion through a specific signaling pathway. CT attenuation is an index reflecting changes in the VF microenvironment, and its increase may be closely related to tumor progression[17]. Given all of that, we believe that certain changes occur in VF with PM. The indicators used to quantify such changes in our study included the mean attenuation, volume, and volume ratio. Previous studies[5,18] have suggested that the radiomics features of the adjacent peritoneum are helpful in predicting PM in GC. Therefore, two nearby ROIs that delineated from the maximal axial section of the tumor were adopted in our study, and these ROIs were considered to be the most likely sites of metastasis.
The regression analysis indicated that the mean attenuation of VFROI 2 was not an independent risk factor for PM, and it had a lower predictive ability than ascites and cT stage. The changes in the adipose microenvironment and the increase in the mean attenuation of VF may be mainly concentrated around the tumor, and the ROI of VF on the whole axial level may dilute this change and reduce the predictive sensitivity. However, the results confirmed that the mean attenuation of VF was significantly different between the PM-positive and PM-negative groups in both ROI 1 and ROI 2. The 30th, 50th, 70th, 25th, and 75th percentiles of attenuation of VFROI 2 were also significantly different between the two groups. Moreover, the mean attenuation of VF was related to clinical features representing tumor invasiveness. Therefore, we have reasons to believe that the mean attenuation of VF is a potential auxiliary parameter for predicting occult PM in patients with GC, especially for those with only nonenhanced CT images or upper abdominal CT images.
The mean attenuation was negatively correlated with the volume of VF; however, noticeably, no significant difference in the VF volume was found between the PM-positive and PM-negative groups. Considering the relationship between the mean attenuation and volume of VF, volume-stratified analysis was applied to minimize the deviations in volume variation. The mean attenuation of VFROI 2 showed a higher predictive potential in the high-volume group than in the low-volume group. This result suggests that patients with high-volume VF may have more hypoxic or disordered adipocytes that secrete proinflammatory cytokines and angiogenic factors and promote tumor metastasis than patients with low-volume VF[19]. The attenuation of VF was significantly correlated with cT stage, cN stage, CA125, and CA199, indicating that increased VF attenuation is highly correlated with tumor progression.
Mild ascites and cT stage are independent predictive factors for occult PM. Liu et al[7] reported that cT stage was significantly correlated with occult PM, which reflects tumor aggressiveness. Mild ascites was also incorporated into a clinical model for predicting occult PM in a multicenter study[5]. Significant differences were observed in the mean attenuations of VF between the different ascites statuses and cT stages.
Compared with previous radiomics studies, the extraction of VF parameters in our study was based on unenhanced CT images through semiautomated sketching technology, which would reduce the selection differences between operators. The consistency between the operators of VF parameters was excellent. The ROIs in the peritoneum used in most previous studies on occult PM were manually sketched[5,18]. Thus, evaluating the consistency among different operators in defining the location and area of ROIs selected was difficult. In addition, three-dimensional ROIs were defined in our study, which remain applicable in patients with lesser VF.
This study has certain limitations. First, the VF parameters were measured using data obtained from a single center with a small sample size, and multicenter studies with a large sample size are thus needed to validate the results. Second, the parameters used to quantify VF changes were obtained only from unenhanced CT images, which warrants the need to consider more parameters from multiphase enhanced images.
In conclusion, our study demonstrates the great potential of VF parameters in predicting occult PM in GC and presents a noninvasive preoperative model that combines the mean attenuation of VF and clinical factors for predicting occult PM in GC.
The preoperative prediction of peritoneal metastasis (PM) in gastric cancer (GC) would prevent unnecessary surgery and promptly indicate an appropriate treatment plan.
Tumor infiltration of the peritoneum alters the characteristics of the surrounding VF on computed tomography (CT), and these changes have been investigated in previous studies.
We therefore aimed to explore the predictive value of VF parameters obtained from preoperative CT images for occult PM and to develop an individualized model for predicting occult PM in patients with GC.
A total of 128 confirmed GC cases that underwent CT scans were analyzed and categorized into PM-positive and PM-negative groups. The clinical characteristics and VF parameters of two regions of interest (ROIs) were collected. Univariate and stratified analyses based on VF volume were performed to screen for predictive characteristics for occult PM. Prediction models with and without VF parameters were established by multivariable logistic regression analysis.
The mean attenuations of VFROI 1 and VFROI 2 varied significantly between the PM-positive and PM-negative groups (P = 0.044 and 0.001, respectively). The mean attenuation of VFROI 2 was included in the final prediction combined model, but not an independent risk factor of PM (P = 0.068). No significant difference was observed between the models with and without mean attenuation of VF (area under the curve: 0.749 vs 0.730, P = 0.339).
The mean attenuation of VF is a potential auxiliary parameter for predicting occult PM in patients with GC.
Our study demonstrates the great potential of VF parameters in predicting occult PM in GC and presents a noninvasive preoperative model that combines the mean attenuation of VF and clinical factors for predicting occult PM in GC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hori T, Japan; Koganti SB, United States; Sun D, China; Tanabe H, Japan S-Editor: Liu GL L-Editor: Wang TQ P-Editor: Liu GL
| 1. | Maehara Y, Hasuda S, Koga T, Tokunaga E, Kakeji Y, Sugimachi K. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg. 2000;87:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 209] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 2. | GLOBOCAN 2012: Stomach cancer: estimated incidence, mortality and prevalence worldwide in 2012. [Internet] [accessed 5 November 2021]. Available from: http://globocan.iarc.fr/old/FactSheets/cancers/stomach-new.asp. |
| 3. | Rau B, Brandl A, Piso P, Pelz J, Busch P, Demtröder C, Schüle S, Schlitt HJ, Roitman M, Tepel J, Sulkowski U, Uzunoglu F, Hünerbein M, Hörbelt R, Ströhlein M, Beckert S, Königsrainer I, Königsrainer A; Peritoneum Surface Oncology Group and members of the StuDoQ|Peritoneum Registry of the German Society for General and Visceral Surgery (DGAV). Peritoneal metastasis in gastric cancer: results from the German database. Gastric Cancer. 2020;23:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 4. | Kim SJ, Kim HH, Kim YH, Hwang SH, Lee HS, Park DJ, Kim SY, Lee KH. Peritoneal metastasis: detection with 16- or 64-detector row CT in patients undergoing surgery for gastric cancer. Radiology. 2009;253:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Dong D, Tang L, Li ZY, Fang MJ, Gao JB, Shan XH, Ying XJ, Sun YS, Fu J, Wang XX, Li LM, Li ZH, Zhang DF, Zhang Y, Li ZM, Shan F, Bu ZD, Tian J, Ji JF. Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann Oncol. 2019;30:431-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 315] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 6. | Huang W, Zhou K, Jiang Y, Chen C, Yuan Q, Han Z, Xie J, Yu S, Sun Z, Hu Y, Yu J, Liu H, Xiao R, Xu Y, Zhou Z, Li G. Radiomics Nomogram for Prediction of Peritoneal Metastasis in Patients With Gastric Cancer. Front Oncol. 2020;10:1416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Liu S, He J, Liu S, Ji C, Guan W, Chen L, Guan Y, Yang X, Zhou Z. Radiomics analysis using contrast-enhanced CT for preoperative prediction of occult peritoneal metastasis in advanced gastric cancer. Eur Radiol. 2020;30:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 8. | Li ZY, Tang L, Li ZM, Li YL, Fu J, Zhang Y, Li XT, Ying XJ, Ji JF. Four-Point Computed Tomography Scores for Evaluation of Occult Peritoneal Metastasis in Patients with Gastric Cancer: A Region-to-Region Comparison with Staging Laparoscopy. Ann Surg Oncol. 2020;27:1103-1109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Xiang F, Wu K, Liu Y, Shi L, Wang D, Li G, Tao K, Wang G. Omental adipocytes enhance the invasiveness of gastric cancer cells by oleic acid-induced activation of the PI3K-Akt signaling pathway. Int J Biochem Cell Biol. 2017;84:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Chen Y, Xi W, Yao W, Wang L, Xu Z, Wels M, Yuan F, Yan C, Zhang H. Dual-Energy Computed Tomography-Based Radiomics to Predict Peritoneal Metastasis in Gastric Cancer. Front Oncol. 2021;11:659981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Tsoukalas N, Tsapakidis K, Kamposioras K. AJCC-8 TNM Staging System for Gastric Cancer. Is There a Scope for Improvement? J Invest Surg. 2020;33:939-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Marano L, D'Ignazio A, Cammillini F, Angotti R, Messina M, Marrelli D, Roviello F. Comparison between 7th and 8th edition of AJCC TNM staging system for gastric cancer: old problems and new perspectives. Transl Gastroenterol Hepatol. 2019;4:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Li J, Fang M, Wang R, Dong D, Tian J, Liang P, Liu J, Gao J. Diagnostic accuracy of dual-energy CT-based nomograms to predict lymph node metastasis in gastric cancer. Eur Radiol. 2018;28:5241-5249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 14. | Lin YC, Lin G, Yeh TS. Visceral-to-subcutaneous fat ratio independently predicts the prognosis of locally advanced gastric cancer-----highlighting the role of adiponectin receptors and PPARα, β/δ, ɤ. Eur J Surg Oncol. 2021;47:3064-3073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 15. | Taniguchi Y, Kurokawa Y, Takahashi T, Saito T, Yamashita K, Tanaka K, Makino T, Yamasaki M, Nakajima K, Eguchi H, Doki Y. Impacts of Preoperative Psoas Muscle Mass and Visceral Fat Area on Postoperative Short- and Long-Term Outcomes in Patients with Gastric Cancer. World J Surg. 2021;45:815-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Ichikawa K, Miyoshi T, Osawa K, Nakashima M, Miki T, Nishihara T, Toda H, Yoshida M, Ito H. High pericoronary adipose tissue attenuation on computed tomography angiography predicts cardiovascular events in patients with type 2 diabetes mellitus: post-hoc analysis from a prospective cohort study. Cardiovasc Diabetol. 2022;21:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 17. | Lee JW, Son MW, Chung IK, Cho YS, Lee MS, Lee SM. Significance of CT attenuation and F-18 fluorodeoxyglucose uptake of visceral adipose tissue for predicting survival in gastric cancer patients after curative surgical resection. Gastric Cancer. 2020;23:273-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 18. | Kim HY, Kim YH, Yun G, Chang W, Lee YJ, Kim B. Could texture features from preoperative CT image be used for predicting occult peritoneal carcinomatosis in patients with advanced gastric cancer? PLoS One. 2018;13:e0194755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Divella R, De Luca R, Abbate I, Naglieri E, Daniele A. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J Cancer. 2016;7:2346-2359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 215] [Article Influence: 23.9] [Reference Citation Analysis (0)] |