Published online Apr 14, 2023. doi: 10.3748/wjg.v29.i14.2202
Peer-review started: November 19, 2022
First decision: November 30, 2022
Revised: December 10, 2022
Accepted: March 14, 2023
Article in press: March 14, 2023
Published online: April 14, 2023
Processing time: 144 Days and 14.4 Hours
Gastric mucosa-associated lymphoid tissue (MALT) lymphoma (GML) is usually a low-grade B-cell neoplasia strongly associated with Helicobacter pylori (H. pylori)-induced chronic gastritis. Clinical practice guidelines currently recommend H. pylori eradication as the preferred initial treatment for early-stage GML. To determine the practical effect of bacterial eradication as the sole initial therapy for early-stage GML, an updated analysis and review of available evidence is imperative.
To perform a meta-analysis to assess the rate of complete remission (CR) of H. pylori-positive early-stage GML following bacterial eradication.
We performed independent, computer-assisted literature searches using the PubMed/MEDLINE, Embase, and Cochrane Central databases through September 2022. Prospective and retrospective observational studies evaluating the CR of early-stage GML following bacterial eradication in H. pylori-positive patients. The risk of bias was assessed using Joanna Briggs Institute (JBI) Critical Appraisal Tools. The pooled estimate of the complete histopathological remission rate and respective confidence intervals (95%CI) were calculated following the random-effects model. Heterogeneity and inconsistency were assessed using Cochran’s Q test and I2 statistic, and heterogeneity was defined as P < 0.01 and I² > 50%, respectively. Subgroup and meta-regression analyses were conducted to explore potential sources of heterogeneity.
The titles and abstracts of 1576 studies were screened; 96 articles were retrieved and selected for full-text reading. Finally, 61 studies were included in the proportional meta-analysis (P-MA). Forty-six were prospective and fifteen were retrospective uncontrolled, single-arm, observational studies. The overall risk of bias was low to moderate in all but a single report, with an average critical appraisal score across all studies of 79.02%. A total of 2936 H. pylori-positive early-stage GML patients, in whom H. pylori was successfully eradicated, were included in the analysis. The pooled CR of H. pylori-positive early-stage GML after bacterial eradication was 75.18% (95%CI: 70.45%-79.91%). P-MA indicated the substantial heterogeneity in CR reported across studies (I2 = 92%; P < 0.01). Meta-regression analysis identified statistically significant effect modifiers, including the proportion of patients with t(11;18)(q21;q21)-positive GML and the risk of bias in each study.
Comprehensive synthesis of available evidence suggests that H. pylori eradication is effective as the sole initial therapy for early-stage GML. Although the substantial heterogeneity observed across studies limits the interpretation of the pooled overall CR, the present study is a relevant to informing clinical practice.
Core Tip: Gastric mucosa-associated lymphoid tissue (MALT) lymphoma (GML) is usually a low-grade B-cell neoplasia strongly associated with Helicobacter pylori (H. pylori)-induced chronic gastritis. Clinical practice guidelines currently recommend H. pylori eradication as the preferred initial treatment for early-stage GML. Despite advances in determining the practical effect of bacterial eradication as sole initial therapy for early-stage GML, an updated meta-analysis of available evidence is imperative. We performed a systematic review with proportional meta-analysis to assess the complete remission rate of H. pylori-positive early-stage GML after eradication therapy.
- Citation: Lemos FFB, Castro CT, Calmon MS, Silva Luz M, Pinheiro SLR, Faria Souza Mendes dos Santos C, Correa Santos GL, Marques HS, Delgado HA, Teixeira KN, Souza CL, Oliveira MV, Freire de Melo F. Effectiveness of Helicobacter pylori eradication in the treatment of early-stage gastric mucosa-associated lymphoid tissue lymphoma: An up-to-date meta-analysis. World J Gastroenterol 2023; 29(14): 2202-2221
- URL: https://www.wjgnet.com/1007-9327/full/v29/i14/2202.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i14.2202
Marginal zone lymphomas (MZLs) are the third most common type of non-Hodgkin B-cell lymphoma following diffuse large B-cell lymphoma and follicular lymphoma[1]. The 5th edition of the World Health Organization Classification of Hematolymphoid Tumors - Lymphoid Neoplasms subdivides MZL into 4 subtypes: Extranodal MZL of mucosa-associated lymphoid tissue (MALT), primary cutaneous MZL, nodal MZL, and pediatric MZL[2].
Gastric MALT lymphoma (GML) is a low-grade B-cell neoplasia commonly associated with Helicobacter pylori (H. pylori)-induced chronic gastritis[3]. GML provides the best-characterized model of the antigen-induced transition from normal to malignant marginal-zone B-cells[4]. Despite the lack of lymphoid follicles in the normal gastric mucosa, MALT may appear as a result of inflammation. H. pylori chronic gastritis induces specific T helper cells and the subsequent expansion of polyclonal B cells, which can undergo malignant transformation[4,5]. Similar to that of gastric cancer, in advanced-stage GML, inflammatory signaling pathway and pro-oncogenic genetic changes allow a microenvironment-independent progression of the tumor, characterizing a “hit-and-run” mechanism[5,6]. The overwhelming evidence suggesting a causal relationship between H. pylori infection and GML is also supported by epidemiological data[7].
Although robust comparative studies such as randomized clinical trials have not been carried out, clinical practice guidelines currently recommend H. pylori eradication as the sole initial treatment for early-stage GML[8]. Triple-therapy, which comprises a proton pump inhibitor (PPI) for 4 wk and clarithromycin with either amoxicillin or metronidazole for 10-14 d, remains standard. However, given the increasing rate of bacterial clarithromycin resistance in many countries, international guidelines also recommend bismuth quadruple therapy (BQT) or concomitant non-BQT as possible alternatives[9-11]. Accordingly, a previous systematic review with pooled data analyses highlighted that, after a long-term follow-up period, lymphoma disappeared in more than 75% of low-grade, stage I or II1 gastric lymphoma patients treated with bacterial eradication[12]. This study also identified that when the neoplastic lesion is confined to the submucosa, the main lesion is localized in the distal stomach, and t(11;18)(q21;q21) translocation is absent, the effectiveness of H. pylori eradication is even greater.
Given the low incidence of GML and the small sample sizes and heterogeneity of available studies[12], there is a need for an updated statistical analysis of the current evidence regarding H. pylori eradication as the sole initial therapy. Here, we performed a systematic literature review with meta-analysis to assess the complete histopathologic remission rate of H. pylori-positive early-stage GML after bacterial eradication therapy.
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guideline, which consists of a 27-item checklist and a 3-phase flowchart. The checklist includes items considered critical to the transparent reporting of a systematic review[13].
The search strategy was designed following the Joanna Briggs Institute (JBI) Manual for Evidence Synthesis (https://synthesismanual.jbi.global). We performed independent, computer-assisted searches of the PubMed/MEDLINE, Embase, and Cochrane Central databases for studies published before September 2022. Medical Subject Headings and Embase Subject Headings (Emtree) index terms and free-text words were combined. Search terms included “Lymphoma, B-Cell, Marginal Zone,” “Mucosa-Associated Lymphoid Tissue Lymphoma,” “Marginal Zone B-Cell Lymphoma,” “MALT lymphoma,” “Stomach lymphoma,” “Helicobacter pylori,” “Therapeutics,” and “Eradication therapy.” Boolean operators (AND, OR) were also used to narrow or broaden the search as required. All citations were exported to the Rayyan (https://www.rayyan.ai/) tool and all duplicates were removed.
Two researchers independently assessed the articles according to predefined eligibility criteria. In the case of disagreement, a 3rd researcher was consulted. The titles and abstracts of the articles were analyzed and studies that did not fit the inclusion criteria were excluded. The full texts were then revised to select eligible studies for meta-analysis.
Studies that met the following criteria were included: (1) Prospective and retrospective observational studies (cohort, case-control, and case series) evaluating the complete remission (CR) rate of early-stage GML after bacterial eradication therapy in H. pylori-positive patients; and (2) Studies including H. pylori-positive patients exclusively treated with antibiotic eradication therapy. Also, only trials enrolling patients with either stage I or II1 GML according to Lugano classification were included[14].
Exclusion criteria were as follows: (1) Studies that did not report the CR rate of H. pylori-positive early-stage GML after bacterial eradication; (2) Studies investigating high-grade or diffuse large B cell lymphomas, except for those where it is possible to extrapolate data from a subgroup with early-stage GML; (3) Studies that included patients with non-gastric sites of MALT lymphoma or ineligible study subjects, such as animals or children; (4) Full-text article not available or article not available in English; (5) Case reports, reviews, meta-analyses, systematic reviews, editorials, conference abstracts; and (6) Studies with insufficient data regarding treatment outcome.
Two researchers independently assessed the risk of bias using the JBI checklists for cohort, case-control, and case series studies[15]. In cases of disagreement, a 3rd researcher was consulted. These tools include multiple questions to assess the methodological quality of a study and determine the extent to which a study has addressed the possibility of bias in its design, conduct, and analysis. The bias percentage risk was calculated by the number of “yes” (Y) answers selected in the checklist. Questions with “not applicable” (N/A) answers were not considered in the calculation. The risk of bias was classified using the following categories: High (scores up to 49.0%), moderate (scores between 50.0% and 70.0%), and low (scores above 70.0%).
Two investigators extracted data from the selected studies using a predefined data extraction worksheet. Any discrepancies were resolved by a 3rd reviewer. The primary outcome was the complete histopathologic remission of the lymphoma after bacterial eradication in H. pylori-positive early-stage GML patients. Data were extracted with respect to the following: (1) Included study-related information (1st author, year of publication, country of origin, study design, and study size); (2) Clinical characteristics of the study population (disease stage, diagnostic methods for H. pylori infection, and eradication schemes); (3) Number of H. pylori-positive early-stage GML patients treated only with bacterial eradication; (4) Number of patients in whom H. pylori was successfully eradicated (either provided or calculated); and (5) Number of patients who finally achieved complete remission of the lymphoma (either provided or calculated). The stage of the lymphoma was assessed using the Lugano classification system[13].
The pooled estimate of the complete histopathological remission rate and respective confidence intervals (95%CI) were calculated following the random-effects model. Forest plots were used to summarize the results. Heterogeneity and inconsistency were assessed using Cochran’s Q test and I2 statistic[16]; heterogeneity was defined as P < 0.01 and I² > 50%, respectively. A subgroup analysis by study design (prospective; retrospective) was conducted to create more homogenous groups. Furthermore, a meta-regression analysis was conducted to explore potential sources of heterogeneity, such as publication year (≤ 2015; > 2015), geographic region of the study (Asian; Western), the prevalence of the translocation t(11;18)(q21; q21), and risk of bias (low; moderate; high). Analysis of publication bias was not performed as this measure is inappropriate for proportional meta-analysis (P-MA)[17]. All analyses were performed using R software version 4.2.1 (R: A Language and Environment for Statistical Computing, Vienna, Austria), using the ‘Meta’ package, version 5.2-0.
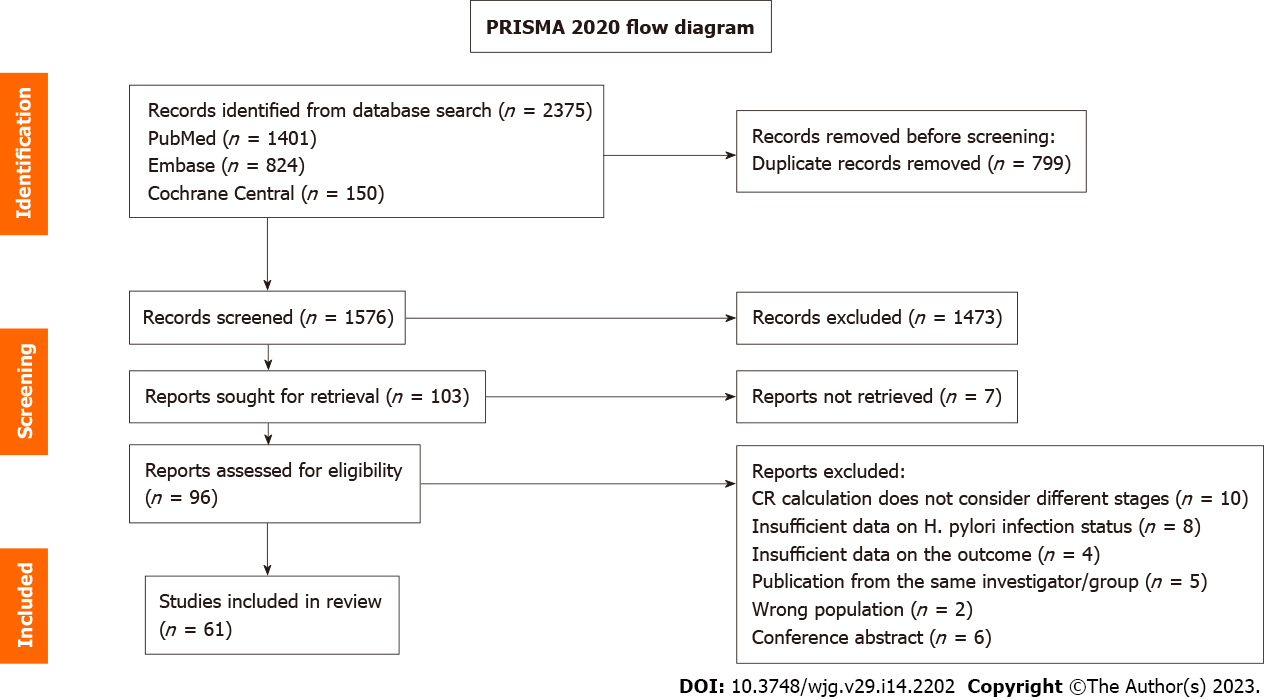
Figure 1 depicts the flow of information through the different phases of the systematic review. Database searches identified 2375 reports, and duplicates were removed. The titles and abstracts of 1576 studies were screened and 96 articles were retrieved and selected for full-text reading. Finally, 61 studies were included in the meta-analysis. Reasons for exclusion were as follows: (1) 10 reports did not consider different stages in CR calculation; (2) 8 had insufficient data on H. pylori infection status; (3) 6 were conference abstracts; (4) 5 were publications of the same investigator or group; (5) 4 had insufficient data on the outcome; and (6) 2 included ineligible study subjects.
Table 1 summarizes the characteristics of the studies included in the P-MA. The included reports were prospective and retrospective observational studies published between 1993 and 2021. A sample of 3315 patients with early-stage GML was obtained, of which 3003 were H. pylori-positive. A total of 2936 patients in whom H. pylori was successfully eradicated were included in the analysis. Twenty-nine of the included studies were conducted in Asian countries and 32 in Western countries. Concerning study design, 46 were prospective and 15 were retrospective uncontrolled, single-arm, observational studies. The median number of H. pylori-eradicated early-stage GML patients was 38 (ranging from 6-193). Multiple diagnostic tests for H. pylori infection and eradication were used, including histologic examination, H. pylori culture, rapid urease testing, 13C- or 14C-urea breath testing, serology, and H. pylori antigen stool testing. In most studies, at least 2 diagnostic tools were used to determine H. pylori infection status. Also in most studies eradication therapy consisted of a combination of 2 antibiotics, such as amoxicillin and clarithromycin, with a PPI. However, dual and quadruple therapies (2 antibiotics + PPI + bismuth or 3 antibiotics + PPI, respectively) were also used. Treatment duration ranged from 7 d to 21 d (Table 2).
| Ref. | Country | Design | Study period | Study population | ||||
| Early-stage gastric MALT lymphoma, n | Lugano stage | Median follow-up in mo | H. pylori-positive early-stage GML, n | Diagnosis of H. pylori infection | ||||
| Yang et al[18], 2021 | China | Retrospective | 2003-2015 | 70 | 70 | 30 | 52 | UBT; HE |
| Schmelz et al[19], 2019 | Germany | Prospective | 2001-2010 | 109 | 109 | 12 | 99 | HE |
| Sugizaki et al[20], 2018 | Japan | Prospective | 2010-2016 | 97 | 97 | 37.4 | 97 | HE; HpC; RUT; UBT; HpSA; S |
| Song et al[21], 2018 | China | Prospective | 2000-2013 | 122 | 122 | 38 | 47 | RUT; HE; UBT |
| Li et al[22], 2016 | China | Retrospective | 2001-2013 | 75 | 75 | 62.9 | 69 | HE; UBT |
| Kim et al[24], 2016 | Korea | Retrospective | 2001-2014 | 49 | 49 | 51 | 40 | HE; UBT; RUT |
| Moleiro et al[23], 2016 | Korea | Retrospective | 2005-2014 | 103 | 103 | 50.5 | 82 | RUT; UBT; HE |
| Park et al[25], 2016 | Portugal | Retrospective | 1993-2013 | 103 | 103 | 105 | 87 | HE; HpC; S; UBT |
| Grgov et al[26], 2015 | Serbia | Prospective | 2002-2012 | 20 | 20 | NR | 20 | RUT; HE |
| Nonaka et al[27], 2014 | Japan | Retrospective | 2007-2012 | 16 | 16 | NR | 12 | HE; S; UBT |
| Lima et al[28], 2014 | Brazil | Prospective | 2009-2010 | 8 | 8 | 24 | 7 | RUT; HE; UBT |
| Wündisch et al[29], 2012 | Germany | Prospective | 1993-1999 | 120 | 120 | 122 | 120 | HE |
| Choi et al[30], 2011 | Korea | Retrospective | 2003-2010 | 35 | 35 | 21.5 | 26 | HE; RUT; UBT |
| Ono et al[31], 2011 | Japan | Retrospective | 2003-2009 | 21 | 21 | 1 | 13 | RUT; UBT; HpC; HE; S |
| Andriani et al[32], 2009 | Italy | Retrospective | 1993-2006 | 60 | 60 | 65 | 60 | HE |
| Sumida et al[33], 2009 | Japan | Prospective | 1997-2007 | 66 | 66 | 40 | 57 | HE; S; UBT |
| Stathis et al[34], 2009 | Switzerland | Retrospective | 1990-2006 | 105 | 105 | 75.6 | 85 | HE; S; UBT |
| Terai et al[35], 2008 | Japan | Prospective | 1995-2006 | 74 | 74 | 46 | 70 | RUT; HE; S; UBT |
| Fischbach et al[36], 2007 | Germany | Retrospective | NR | 108 | 108 | 42.2 | 108 | HE; UBT |
| Kim et al[37], 2007 | Korea | Prospective | 1996-2006 | 99 | 99 | 41 | 99 | HE; RUT |
| El-Zahabi et al[38], 2007 | Lebanon | Retrospective | 1999-2005 | 22 | 22 | 12 | 19 | HE; S |
| Hong et al[39], 2006 | Korea | Prospective | 1996-2003 | 90 | 90 | 45 | 90 | HE; RUT; UBT |
| Wündisch et al[40], 2006 | Germany | Retrospective | 1993-2003 | 196 | 196 | 27 | 196 | HE |
| Akamatsu et al[41], 2006 | Japan | Prospective | 1993-2006 | 55 | 55 | 37.3 | 38 | HpC; HE |
| Wündisch et al[42], 2005 | Germany | Prospective | NR | 120 | 120 | 75 | 120 | HE |
| Montalban et al[43], 2005 | Spain | Prospective | 1993-2002 | 24 | 24 | 64 | 24 | HE; UBT |
| Chen et al[44], 2005 | Taiwan | Prospective | 1996-1999 | 34 | 34 | 70 | 31 | HE; RUT; S |
| Taji et al[45], 2005 | Japan | Prospective | 1995-2001 | 13 | 13 | 32.5 | 12 | HE; HpC; S; UBT; RUT |
| Takenaka et al[46], 2004 | Japan | Prospective | 1995-2002 | 33 | 33 | 5 | 33 | HpC; RUT |
| Fischbach et al[47], 2004 | Germany | Prospective | NR | 90 | 90 | 44.6 | 80 | RUT; HE; UBT |
| Sheu et al[48], 2003 | Taiwan | Prospective | NR | 15 | 15 | NR | 15 | RUT; HE |
| Caletti et al[49], 2002 | Italy | Prospective | 1997-1999 | 51 | 51 | 24 | 51 | HE; RUT; S |
| Nakamura et al[50], 2002 | Japan | Prospective | 1994-2001 | 21 | 21 | 14.5 | 17 | HpC; S |
| Liu et al[51], 2002 | France; Netherlands; Italy; Germany; England | Retrospective | NR | 111 | 111 | NR | 111 | HE; HpC |
| Bertoni et al[52], 2002 | England; Italy; Switzerland | Prospective | NR | 62 | 62 | 24 | 62 | HE; S |
| Ohashi et al[53], 2002 | Japan | Prospective | NR | 13 | 13 | NR | 13 | RUT; HE; HpC |
| Kim et al[54], 2002 | Korea | Prospective | NR | 20 | 20 | 18.3 | 20 | RUT; HE |
| Matsushima et al[55], 2002 | Japan | Prospective | 1995-1997 | 14 | 14 | 27.5 | 14 | RUT; HE; HpC; UBT |
| Kanda et al[56], 2001 | Japan | Prospective | 1994-1999 | 13 | 13 | 7 | 13 | HE |
| Raderer et al[57], 2001 | Austria | Retrospective | 1997-1999 | 22 | 22 | 25 | 22 | HE |
| Nakamura et al[58], 2001 | Japan | Prospective | 1994-1998 | 41 | 41 | 20.5 | 41 | HpC; S; HE |
| Ruskoné-Fourmestraux et al[59], 2001 | France | Prospective | 1995-1998 | 44 | 44 | 35 | 34 | HE; HpC; S; PCR |
| Thiede et al[60], 2001 | Germany | Prospective | NR | 97 | 97 | 20.8 | 97 | NR |
| de Jong et al[61], 2001 | Netherlands | Prospective | NR | 23 | 23 | 37 | 23 | HE; HpC |
| Urakami et al[62], 2000 | Japan | Prospective | NR | 47 | 47 | 20 | 47 | RUT; HE; HpC |
| Papa et al[63], 2000 | Italy | Prospective | 1995-1999 | 7 | 7 | 48 | 7 | HE; UBT |
| Yamashita et al[64], 2000 | Japan | Prospective | NR | 21 | 21 | NR | 21 | HE; RUT; HpC |
| Ohashi et al[65], 2000 | Japan | Prospective | NR | 11 | 11 | NR | 11 | RUT; HE; HpC |
| Nakamura et al[66], 2000 | Japan | Prospective | 1993-1998 | 30 | 30 | NR | 26 | HpC |
| Savio et al[67], 2000 | Italy | Prospective | 1991-1997 | 76 | 76 | NR | 76 | HE |
| Weston et al[68], 1999 | United States | Prospective | NR | 68 | 68 | NR | 65 | HE |
| Steinbach et al[69], 1999 | United States | Prospective | NR | 34 | 34 | NR | 28 | HE; RUT; S |
| Nobre-Leitão et al[70], 1998 | Portugal | Prospective | NR | 17 | 17 | 12 | 17 | HE; HpC |
| Thiede et al[71], 1997 | Germany | Prospective | NR | 84 | 84 | NR | 84 | NR |
| Sackmann et al[72], 1997 | Germany | Prospective | NR | 22 | 22 | 10 | 22 | HE; HpC |
| Neubauer et al[73], 1997 | Germany | Prospective | NR | 50 | 50 | 24 | 50 | HE |
| Pinotti et al[74], 1997 | Italy; Switzerland | Prospective | 1986-1995 | 86 | 86 | 23.3 | 45 | HE; S |
| Savio et al[75], 1996 | Italy; England | Prospective | 1991-1993 | 13 | 13 | NR | 13 | HE |
| Bayerdörffer et al[76], 1995 | Germany | Prospective | NR | 33 | 33 | 12.5 | 33 | HE |
| Roggero et al[77], 1995 | Switzerland; Italy | Prospective | NR | 26 | 26 | 12 | 26 | HE |
| Wotherspoon et al[78], 1993 | England; Italy | Prospective | NR | 6 | 6 | NR | 6 | HE |
| Ref. | Region | H. pylori-positive early-stage gastric MALT lymphoma, n | H. pylori-eradicated gastric MALT lymphoma patients, n | CR, n | t(11;18)(q21;q21)-investigated gastric MALT lymphoma, n | t(11;18)(q21;q21)-positive gastric MALT lymphoma, n | Eradication regimen |
| Yang et al[18] | Asian | 52 | 48 | 38 | NR | NR | 7-d-14-d triple therapy or 10-d quadruple therapy |
| Schmelz et al[19] | Western | 99 | 99 | 66 | 69 | 7 | 7-d triple therapy |
| Sugizaki et al[20] | Asian | 97 | 86 | 84 | 73 | 1 | 7-d triple therapy |
| Song et al[21] | Asian | 47 | 47 | 35 | NR | NR | 14-d triple therapy |
| Li et al[22] | Asian | 69 | 69 | 54 | NR | NR | ND-day quadruple therapy |
| Kim et al[24] | Asian | 40 | 35 | 35 | NR | NR | 7-d-14-d triple therapy or 7-d-14-d bismuth quadruple therapy |
| Moleiro et al[23] | Western | 82 | 81 | 77 | NR | NR | 7-d triple therapy or 7-d bismuth quadruple therapy |
| Park et al[25] | Asian | 87 | 81 | 73 | NR | NR | 7-d-14-d triple therapy |
| Grgov et al[26] | Western | 20 | 20 | 17 | NR | NR | 10-d triple therapy |
| Nonaka et al[27] | Asian | 12 | 12 | 9 | NR | NR | 7-d triple therapy |
| Lima et al[28] | Western | 7 | 7 | 5 | 8 | 4 | 7-d triple therapy or 10-d triple therapy |
| Wündisch et al[29] | Western | 120 | 120 | 96 | 66 | 10 | 14-d dual therapy or 10-d triple therapy |
| Choi et al[30] | Asian | 26 | 26 | 22 | NR | NR | ND-day triple therapy or ND-day bismuth quadruple therapy |
| Ono et al[31] | Asian | 13 | 13 | 13 | NR | NR | 7-d-triple therapy |
| Andriani et al[32] | Western | 60 | 53 | 42 | NR | NR | 7-d-14-d triple therapy or 10-d bismuth quadruple therapy |
| Sumida et al[33] | Asian | 57 | 57 | 47 | 66 | 7 | 7-d triple therapy |
| Stathis et al[34] | Western | 85 | 85 | 66 | NR | NR | ND-day triple therapy |
| Terai et al[35] | Asian | 70 | 70 | 56 | 22 | 0 | 7-d triple therapy |
| Fischbach et al[36] | Western | 108 | 108 | 35 | NR | NR | NR |
| Kim et al[37] | Asian | 99 | 99 | 84 | NR | NR | 7-d triple therapy or 7-d bismuth quadruple therapy |
| El-Zahabi et al[38] | Asian | 19 | 19 | 8 | NR | NR | ND-day quadruple therapy |
| Hong et al[39] | Asian | 90 | 90 | 85 | NR | NR | 14-d triple therapy or 14-d bismuth quadruple therapy |
| Wündisch et al[40] | Western | 196 | 193 | 146 | NR | NR | NR |
| Akamatsu et al[41] | Asian | 38 | 38 | 29 | 8 | 6 | 7-d triple therapy or ND-day quadruple therapy |
| Wündisch et al[42] | Western | 120 | 120 | 96 | 65 | 10 | 14-d dual therapy or 10-d triple therapy |
| Montalban et al[43] | Western | 24 | 24 | 22 | NR | NR | 14-d triple therapy |
| Chen et al[44] | Asian | 31 | 30 | 24 | NR | NR | 14-d triple therapy |
| Taji et al[45] | Asian | 12 | 12 | 7 | 13 | 4 | 14-d triple therapy |
| Takenaka et al[46] | Asian | 33 | 31 | 26 | NR | NR | ND-day triple therapy |
| Fischbach et al[47] | Western | 80 | 80 | 56 | NR | NR | 7-d triple therapy |
| Sheu et al[48] | Asian | 15 | 15 | 11 | NR | NR | 14-d triple therapy |
| Caletti et al[49] | Western | 51 | 45 | 25 | NR | NR | 7-d triple therapy |
| Nakamura et al[50] | Asian | 17 | 17 | 2 | 23 | 7 | 14-d triple therapy |
| Liu et al[51] | Western | 111 | 111 | 48 | 111 | 44 | 14-d dual therapy |
| Bertoni et al[52] | Western | 62 | 62 | 46 | NR | NR | 7-d triple therapy; 14-d triple therapy or 14-d bismuth quadruple therapy |
| Ohashi et al[53] | Asian | 13 | 13 | 11 | NR | NR | 14-d triple therapy |
| Kim et al[54] | Asian | 20 | 20 | 18 | NR | NR | 7-d triple therapy or 7-d bismuth quadruple therapy |
| Matsushima et al[55] | Asian | 14 | 14 | 10 | NR | NR | ND-day triple therapy |
| Kanda et al[56] | Asian | 13 | 12 | 9 | NR | NR | ND-day dual therapy or ND-day triple therapy |
| Raderer et al[57] | Western | 22 | 21 | 15 | NR | NR | ND-day dual therapy or ND-day triple therapy |
| Nakamura et al[58] | Asian | 41 | 41 | 29 | NR | NR | ND-day triple or ND-day quadruple therapy |
| Ruskoné-Fourmestraux et al[59] | Western | 34 | 34 | 19 | NR | NR | 14-d triple therapy |
| Thiede et al[60] | Western | 97 | 97 | 77 | NR | NR | 14-d dual therapy or 7-d triple therapy |
| de Jong et al[61] | Western | 23 | 23 | 13 | NR | NR | ND-day dual therapy; ND-day triple therapy or ND-day quadruple therapy |
| Urakami et al[62] | Asian | 47 | 44 | 42 | NR | NR | 7-d-14-d triple therapy |
| Papa et al[63] | Western | 7 | 7 | 7 | NR | NR | 7-d triple therapy |
| Yamashita et al[64] | Asian | 21 | 21 | 14 | NR | NR | 14-d triple therapy |
| Ohashi et al[65] | Asian | 11 | 11 | 9 | NR | NR | 14-d triple therapy |
| Nakamura et al[66] | Asian | 26 | 25 | 13 | NR | NR | 14-d dual therapy; 7-d triple therapy (14-d PPI); 14-d triple therapy |
| Savio et al[67] | Western | 76 | 76 | 71 | NR | NR | NR |
| Weston et al[68] | Western | 65 | 58 | 38 | NR | NR | ND-day triple or ND-day quadruple therapy |
| Steinbach et al[69] | Western | 28 | 28 | 14 | NR | NR | 21-d bismuth quadruple therapy |
| Nobre-Leitão et al[70] | Western | 17 | 17 | 17 | NR | NR | 14-d triple therapy |
| Thiede et al[71] | Western | 84 | 79 | 68 | NR | NR | NR-day-dual or 7-d-triple therapy |
| Sackmann et al[72] | Western | 22 | 22 | 12 | NR | NR | 14-d-dual therapy |
| Neubauer et al[73] | Western | 50 | 50 | 40 | NR | NR | 14-d-dual therapy or 7-d-triple therapy |
| Pinotti et al[74] | Western | 45 | 44 | 30 | NR | NR | 14-d-triple or quadruple therapy |
| Savio et al[75] | Western | 13 | 12 | 11 | NR | NR | NR-day-triple or quadruple therapy |
| Bayerdörffer et al[76] | Western | 33 | 33 | 23 | NR | NR | 14-d-dual therapy |
| Roggero et al[77] | Western | 26 | 25 | 5 | NR | NR | 14-d-triple therapy |
| Wotherspoon et al[78] | Western | 6 | 6 | 5 | NR | NR | NR-day-dual or triple therapy |
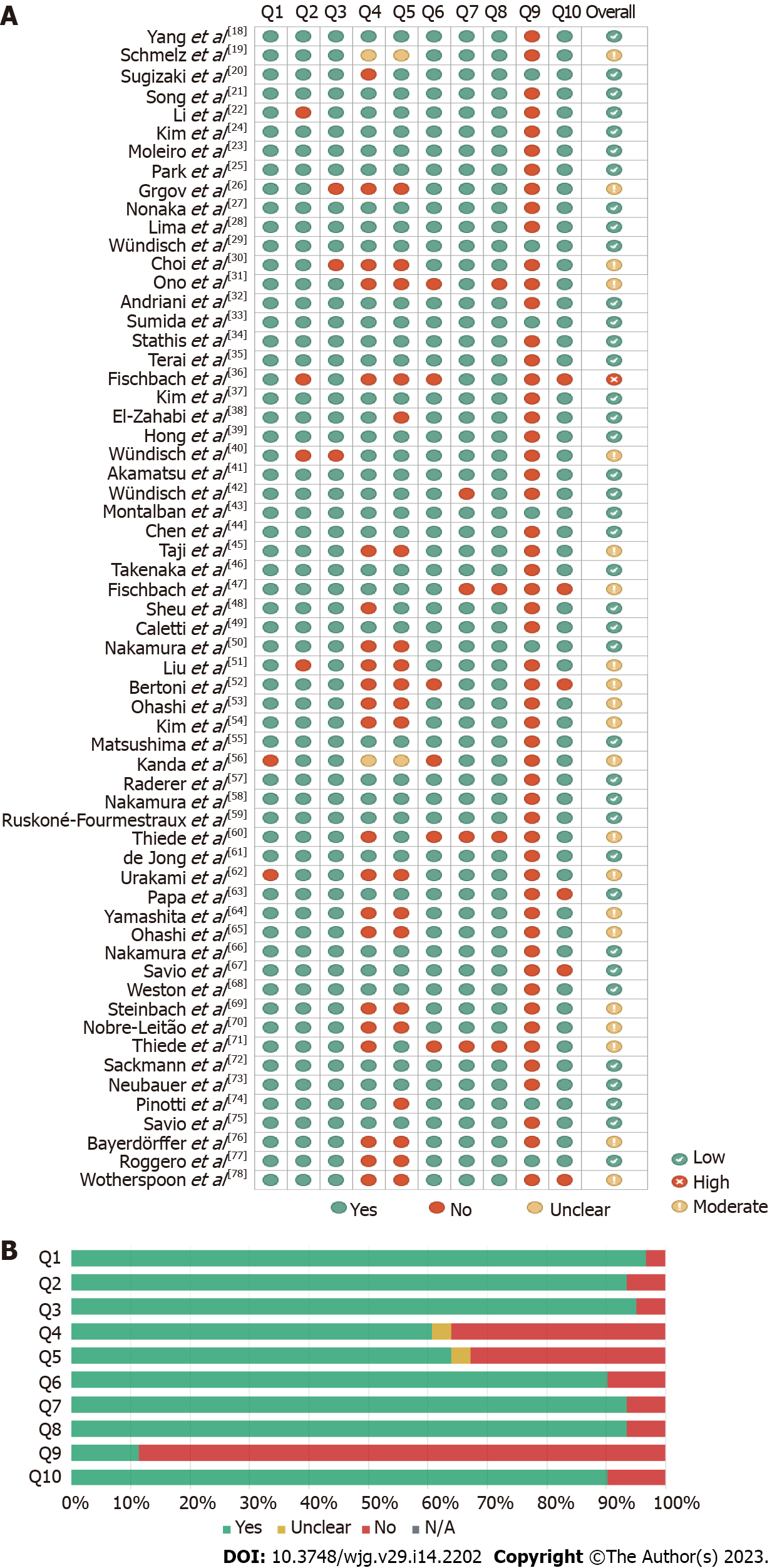
Risk of bias was assessed using JBI checklists (Figure 2). The included single-arm uncontrolled observational studies were classifiable and assessed as case series. The overall risk of bias was low to moderate in all but 1 study[36], with an average critical appraisal score across all studies of 79.02% (Figure 2A).
An increased risk of bias was due to “No” or “Unclear” answers to the following questions: (1) Was there clear reporting of the presenting site(s)/clinic(s) demographic information? (54/61 studies); (2) Did the case series have consecutive inclusion of participants (24/61 studies); (3) Did the case series have complete inclusion of participants? (22/61 studies); (4) Was there clear reporting of the demographics of the participants in the study? (6/61 studies); (5) Was statistical analysis appropriate? (6/61 studies); (6) Was the condition measured in a standard, reliable way for all participants included in the case series? (4/61 studies); (7) Was there clear reporting of clinical information of the participants? (4/61 studies); (8) Was there clear reporting of clinical information of the participants? (3/61 studies); (9) Were the outcomes or follow-up results of cases clearly reported? (8/61 studies); and (10) Were there clear criteria for inclusion in the case series? (2/61 studies). Figure 2B shows the discriminated assessments for each question across all studies.
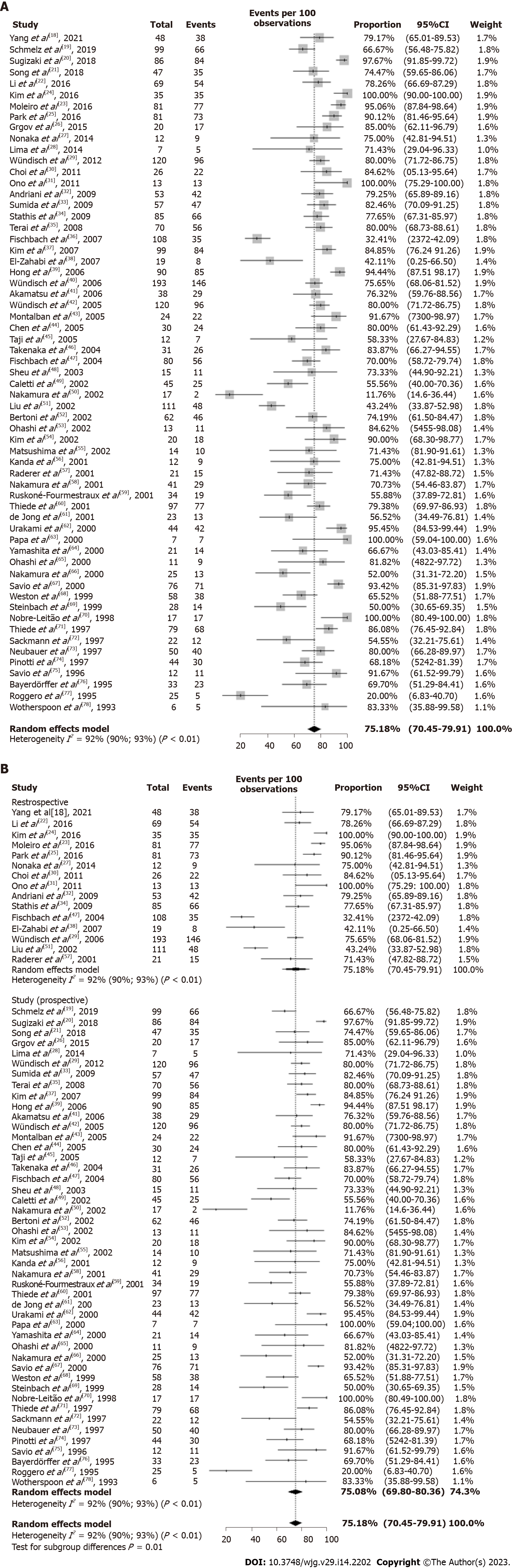
The overall CR of H. pylori-positive early-stage GML after bacterial eradication was 75.18% (95%CI: 70.45%-79.91%). P-MA highlighted substantial heterogeneity in CR rate reported across studies (I2 = 92%; P < 0.01) (Figure 3A).
Considering the high heterogeneity across studies (I2 = 92%; P < 0.01), a subgroup analysis by study design was conducted. The subgroup analysis revealed that retrospective and prospective studies presented similar overall CR rate after eradication therapy: 75.51% (95%CI: 64.96%-86.07%; I2 = 96%; P < 0.01) and 75.08% (95%CI: 69.80-80.36; I2 = 89%; P < 0.01), respectively (Figure 3B). The meta-regression analysis indicated that the proportion of patients with t(11;18)(q21;q21)-positive GML and study risk of bias were sources of heterogeneity. More precisely, studies with greater than 30% of patients with t(11;18)(q21;q21)-positive GML and high risk of bias showed the pooled estimate of the CR rate decreased to 0.40 (95%CI: -0.59 to -0.22; P < 0.0001) and 0.43 (95%CI: -0.77 to -0.09; P = 0.0139), respectively. There was no significant difference in outcomes with respect to geographic region (Table 3).
| Subgroup | Studies, n | Estimate | 95%CI | P value | I2, % |
| Year | |||||
| ≤ 2015 | 54 | - | - | - | 92.5 |
| > 2015 | 7 | 0.11 | -0.03 to 0.25 | 0.1188 | |
| Region | 92.8 | ||||
| Asian | 29 | - | - | - | |
| Western | 32 | -0.06 | -0.15 to 0.03 | 0.2145 | |
| Proportion of patients with t(11;18)(q21;q21)-positive gastric MALT lymphoma | |||||
| ≤ 30% | 7 | ||||
| > 30% | 4 | -0.40 | -0.59 to -0.22 | < 0.0001 | 88.6 |
| Risk of bias | 92.3 | ||||
| Low | 39 | - | - | - | |
| Moderate | 21 | 0.02 | -0.07 to 0.12 | 0.6190 | |
| High | 1 | -0.43 | -0.77 to -0.09 | 0.0139 | |
GML is rare and typically comprises a low-grade neoplasm[18]. H. pylori infection is predominant pathogenic mechanism underlying the development of GML[19], and international guidelines strongly recommend H. pylori eradication therapy for all patients irrespective of stage. In localized H. pylori-positive GML, bacterial eradication is the preferred initial treatment[79,80].
This study aimed to provide an up-to-date, comprehensive synthesis of evidence regarding H. pylori eradication as the sole initial therapy for early-stage GML. We identified prospective and retrospective uncontrolled, single-arm observational studies with a total of 3315 patients with early-stage GML, of which 3003 were H. pylori-positive. A total of 2936 patients in whom H. pylori was successfully eradicated were included in the analysis. The unavailability of robust comparative studies (e.g., prospective cohort studies) precluded pairwise meta-analysis (PW-MA); instead, a P-MA was conducted. In contrast to comparative PW-MA, which calculates a pooled estimate of effect over 2 groups, P-MA enables the calculation of a grouped overall proportion[81,82]. Though single-group analysis may not produce measures of relative association, it can be useful for estimating the impact of a treatment on a given condition in the absence of higher-quality evidence. This represents an alternative for informed decision making, especially in our field where robust comparative studies are scarce.
P-MA highlighted that the overall CR rate of H. pylori-positive early-stage GML after bacterial eradication was 75.18% (95%CI: 70.45%-79.91%), suggesting that H. pylori eradication as the sole initial therapy for early-stage GML is effective. These results are similar to those found in a pooled data analysis published in 2010 by Zullo et al[12] [77.5% (95%CI: 75.3%-79.7%)]. On the other hand, the substantial heterogeneity observed across studies (I2 = 92%; P < 0.01) limits, though does not preclude, the interpretation of the pooled overall CR rate. Subgroup analysis revealed that retrospective and prospective studies estimated similar overall CR rates after eradication therapy [75.51% (95%CI: 64.96%-86.07%; I2 = 96%; P < 0.01) and 75.08% (95%CI: 69.80%-80.36%; I2 = 89%; P < 0.01), respectively]. Nevertheless, meta-regression analysis indicated that the proportion of patients with t(11;18)(q21;q21)-positive GML and the studies’ risk of bias were sources of heterogeneity. More precisely, studies with greater than 30% of patients with t(11;18)(q21;q21)-positive GML or high risk of bias decrease in 0.40 (95%CI: -0.59 to -0.22; P < 0.0001) and 0.43 (95%CI: -0.77 to -0.09; P = 0.0139) the pooled estimate of the CR rate, respectively. In this sense, we reiterate the results of Zullo et al[12] which highlight the presence of the t(11;18)(q21; q21) translocation as a predictor of lymphoma remission after bacterial eradication. In contrast to the previous pooled analysis[12], our study did not observe significant differences in lymphoma remission between Western and Asian countries.
Hence, our results reaffirm that H. pylori eradication should be given as the first-line treatment for localized low-grade GML[8]. The anti-H. pylori regimen should be chosen based on regional microbial susceptibility; in many regions, BQT or high-dose PPI clarithromycin-containing triple therapy may be recommended as first-line empirical treatment[83]. In case of eradication failure, second-line treatment should be attempted following the currently recommended algorithm for empirical H. pylori eradication or as guided by individual antibiotic susceptibility testing. For patients with GML refractory to H. pylori eradication, irradiation and systemic oncological therapies should be used, depending on the stage of the disease. Radiotherapy (RT) is the first-line choice for the treatment of localized GML. Chemotherapy, immunotherapy, or combination chemoimmunotherapy are mainly considered if RT is not feasible or otherwise not indicated[84,85].
To our knowledge, our study is the first systematic review with meta-analysis to assess the CR rate of H. pylori-positive early-stage GML after H. pylori eradication. Our work has strengths in its design and execution, such as the use of random-effects meta-analysis to address heterogeneity between included studies, subgroup analyses by study design, and meta-regression to explore possible sources of heterogeneity. Nonetheless, the present analysis has several limitations inherent to the included studies and study design. Due to the unavailability of language resources (e.g., professional translators), we could not include studies in languages other than English. Although limiting study inclusion based on the language of publication is a common practice in systematic reviews, it introduces the risk of ignoring key data, referred to as language bias, which may limit the interpretation of our findings[86].
Moreover, discriminated assessments for each JBI Critical Appraisal Tool question across all reports showed that the included series had serious gaps in clinical and demographic information reporting. Thus, exploring possible sources of heterogeneity and identifying predictors of lymphoma remission was difficult. Furthermore, incomplete and non-consecutive inclusion of patients in several studies compromises the reliability of their results and increases the risk of bias. Another limitation was the failure to report the confirmation method for H. pylori eradication, which could be a covariate explaining the heterogeneity between studies. Inadequate reporting was an important reason for the exclusion of studies during screening and a complicating factor for data extraction. Observational studies evaluating the CR of GML after bacterial eradication should stratify the observed outcome according to H. pylori infection status. Furthermore, it is necessary to discriminate the lymphoma stage in H. pylori-positive patients undergoing treatment. In fields in which reliable and robust studies are scarce, proper reporting of the available evidence is vital to inform clinical practice. Therefore, this meta-analysis should be interpreted in the context of these limitations.
This comprehensive evidence synthesis suggests the effectiveness of H. pylori eradication as the sole initial therapy for early-stage GML. Although the substantial heterogeneity observed across studies limits the interpretation of the pooled overall CR rate, our study is a relevant alternative for informing clinical practice. Further robust comparative observational studies are needed to identify predictive factors for GML remission following H. pylori eradication and to provide more reliable evidence in our field.
Gastric mucosa-associated lymphoid tissue (MALT) lymphoma (GML) is usually a low-grade, B-cell neoplasia strongly associated with Helicobacter pylori (H. pylori)-induced chronic gastritis. As such, clinical practice guidelines currently recommend H. pylori eradication as the preferred initial treatment for early-stage GML.
Studies that aim to evaluate the effects of H. pylori eradication on early-stage GML are generally small and heterogenous single-arm uncontrolled observational studies. Hence, we recognized the need for an updated powerful statistical synthesis of the available evidence regarding the practical effect of H. pylori eradication as sole initial therapy for early-stage GML.
We aimed to perform a systematic review with an up-to-date proportional meta-analysis (P-MA) to assess the complete remission (CR) rate of H. pylori-positive early-stage GML after bacterial eradication therapy.
We performed independent computer-assisted searches of PubMed/MEDLINE, Embase and Cochrane Central databases culling reports published before September 2022. Prospective and retrospective observational studies evaluating the CR rate of early-stage GML after bacterial eradication therapy in H. pylori-positive patients were eligible for inclusion. The risk of bias was assessed using the JBI Critical Appraisal Tools. We followed the random-effects model to calculate the pooled estimate of the complete histopathological remission rate and respective confidence intervals (95%CI). We used Cochran’s Q test and I2 statistic to assess the heterogeneity and inconsistency, and we set the threshold for heterogeneity as P < 0.01 and I² > 50%, respectively. Subgroup and meta-regression analyses were conducted to explore potential sources of heterogeneity.
P-MA highlighted that the overall CR of H. pylori-positive early-stage GML after bacterial eradication was 75.18% (95%CI: 70.45%-79.91%). On the other hand, the substantial heterogeneity observed across studies (I2 = 92%; P < 0.01) limits, but does not preclude, the interpretation of the pooled overall CR rate. Subgroup analysis revealed that retrospective and prospective studies presented similar overall CR rate estimates after eradication therapy: 75.51% (95%CI: 64.96%-86.07%; I2 = 96%; P < 0.01) and 75.08% (95%CI: 69.80%-80.36%; I2 = 89%; P < 0.01), respectively. Nevertheless, meta-regression analysis indicated that the proportion of patients with t(11;18)(q21;q21)-positive GML and the studies’ risk of bias were sources of heterogeneity. More precisely, studies with greater than 30% of patients with t(11;18)(q21;q21)-positive GML and high risk of bias decrease in 0.40 (95%CI: -0.59 to -0.22; P < 0.0001) and 0.43 (95%CI: -0.77 to -0.09; P = 0.0139) the pooled estimate of the CR rate, respectively.
Comprehensive evidence synthesis suggests the effectiveness of H. pylori eradication as the sole initial therapy for early-stage GML. Although the substantial heterogeneity observed across studies limits the interpretation of the pooled overall CR rate, the present study is a relevant alternative for informing clinical practice.
Inadequate reporting was an important reason for the exclusion of studies during screening and a complicating factor for data extraction. As reliable and robust studies are scarce in our field, we emphasize that proper reporting of the available evidence is vital to inform clinical practice. Further robust comparative observational studies are needed to identify predictive factors for GML remission following H. pylori eradication and to provide more reliable evidence in our field.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haddadi S, Algeria; Viazis N, Greece S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Smith A, Crouch S, Lax S, Li J, Painter D, Howell D, Patmore R, Jack A, Roman E. Lymphoma incidence, survival and prevalence 2004-2014: sub-type analyses from the UK's Haematological Malignancy Research Network. Br J Cancer. 2015;112:1575-1584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 308] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 2. | Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D, Calaminici M, Chadburn A, Chan JKC, Cheuk W, Chng WJ, Choi JK, Chuang SS, Coupland SE, Czader M, Dave SS, de Jong D, Du MQ, Elenitoba-Johnson KS, Ferry J, Geyer J, Gratzinger D, Guitart J, Gujral S, Harris M, Harrison CJ, Hartmann S, Hochhaus A, Jansen PM, Karube K, Kempf W, Khoury J, Kimura H, Klapper W, Kovach AE, Kumar S, Lazar AJ, Lazzi S, Leoncini L, Leung N, Leventaki V, Li XQ, Lim MS, Liu WP, Louissaint A Jr, Marcogliese A, Medeiros LJ, Michal M, Miranda RN, Mitteldorf C, Montes-Moreno S, Morice W, Nardi V, Naresh KN, Natkunam Y, Ng SB, Oschlies I, Ott G, Parrens M, Pulitzer M, Rajkumar SV, Rawstron AC, Rech K, Rosenwald A, Said J, Sarkozy C, Sayed S, Saygin C, Schuh A, Sewell W, Siebert R, Sohani AR, Tooze R, Traverse-Glehen A, Vega F, Vergier B, Wechalekar AD, Wood B, Xerri L, Xiao W. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36:1720-1748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 1897] [Article Influence: 632.3] [Reference Citation Analysis (0)] |
| 3. | Zullo A, Hassan C, Ridola L, Repici A, Manta R, Andriani A. Gastric MALT lymphoma: old and new insights. Ann Gastroenterol. 2014;27:27-33. [PubMed] |
| 4. | Rossi D, Bertoni F, Zucca E. Marginal-Zone Lymphomas. N Engl J Med. 2022;386:568-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 5. | Troppan K, Wenzl K, Neumeister P, Deutsch A. Molecular Pathogenesis of MALT Lymphoma. Gastroenterol Res Pract. 2015;2015:102656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Hatakeyama M. Helicobacter pylori CagA and gastric cancer: a paradigm for hit-and-run carcinogenesis. Cell Host Microbe. 2014;15:306-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 386] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 7. | Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, Boot H, Du MQ, Megraud F, Montalban C, Raderer M, Savio A, Wotherspoon A; EGILS group. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011;60:747-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 8. | Zucca E, Arcaini L, Buske C, Johnson PW, Ponzoni M, Raderer M, Ricardi U, Salar A, Stamatopoulos K, Thieblemont C, Wotherspoon A, Ladetto M; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Marginal zone lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:17-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 213] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 9. | Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, Jones NL, Render C, Leontiadis GI, Moayyedi P, Marshall JK. The Toronto Consensus for the Treatment of Helicobacter pylori Infection in Adults. Gastroenterology. 2016;151:51-69.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 628] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 10. | Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am J Gastroenterol. 2017;112:212-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 1013] [Article Influence: 126.6] [Reference Citation Analysis (1)] |
| 11. | Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2220] [Cited by in RCA: 1970] [Article Influence: 246.3] [Reference Citation Analysis (1)] |
| 12. | Zullo A, Hassan C, Cristofari F, Andriani A, De Francesco V, Ierardi E, Tomao S, Stolte M, Morini S, Vaira D. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol. 2010;8:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 13. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 39313] [Article Influence: 9828.3] [Reference Citation Analysis (2)] |
| 14. | Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de Médula Ósea; German High-Grade Lymphoma Study Group; German Hodgkin's Study Group; Japanese Lymphorra Study Group; Lymphoma Study Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059-3068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2850] [Cited by in RCA: 3836] [Article Influence: 348.7] [Reference Citation Analysis (0)] |
| 15. | Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Chapter 5: Systematic reviews of prevalence and incidence. In: Aromataris E, Munn Z, editors. JBI Manual for Evidence Synthesis. Australia: JBI Collaboration, 2020. [DOI] [Full Text] |
| 16. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46253] [Article Influence: 2102.4] [Reference Citation Analysis (3)] |
| 17. | Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67:897-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 568] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 18. | Yang H, Jielili A, Cao Z, Yuan T. Clinical features & treatment of early-stage gastric mucosa-associated lymphoid tissue lymphoma. Indian J Med Res. 2021;154:504-508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 19. | Schmelz R, Miehlke S, Thiede C, Brueckner S, Dawel M, Kuhn M, Ruskoné-Formestraux A, Stolte M, Jentsch C, Hampe J, Morgner A. Sequential H. pylori eradication and radiation therapy with reduced dose compared to standard dose for gastric MALT lymphoma stages IE & II1E: a prospective randomized trial. J Gastroenterol. 2019;54:388-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Sugizaki K, Tari A, Kitadai Y, Oda I, Nakamura S, Yoshino T, Sugiyama T. Anti-Helicobacter pylori therapy in localized gastric mucosa-associated lymphoid tissue lymphoma: A prospective, nationwide, multicenter study in Japan. Helicobacter. 2018;23:e12474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Song Y, Jiang K, Su S, Wang B, Chen G. Clinical manifestations and epigenetic mechanisms of gastric mucosa associated lymphoid tissue lymphoma and long-term follow-up following Helicobacter pylori eradication. Exp Ther Med. 2018;15:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Li X, Wang X, Zhan Z, Zhang L, Sun B, Zhang Y. Evaluation of the clinical characteristics, management, and prognosis of 103 patients with gastric mucosa-associated lymphoid tissue lymphoma. Oncol Lett. 2016;11:1713-1718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Moleiro J, Ferreira S, Lage P, Dias Pereira A. Gastric malt lymphoma: Analysis of a series of consecutive patients over 20 years. United European Gastroenterol J. 2016;4:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Kim JS, Kang SH, Moon HS, Sung JK, Jeong HY. Clinical Outcome of Eradication Therapy for Gastric Mucosa-Associated Lymphoid Tissue Lymphoma according to H. pylori Infection Status. Gastroenterol Res Pract. 2016;2016:6794848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Park JY, Kim SG, Kim JS, Jung HC. Bone marrow involvement is rare in superficial gastric mucosa-associated lymphoid tissue lymphoma. Dig Liver Dis. 2016;48:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Grgov S, Katić V, Krstić M, Nagorni A, Radovanović-Dinić B, Tasić T. Treatment of low-grade gastric MALT lymphoma using Helicobacter pylori eradication. Vojnosanit Pregl. 2015;72:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Nonaka K, Ohata K, Matsuhashi N, Shimizu M, Arai S, Hiejima Y, Kita H. Is narrow-band imaging useful for histological evaluation of gastric mucosa-associated lymphoid tissue lymphoma after treatment? Dig Endosc. 2014;26:358-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Lima KS, Albuquerque W, Arantes VN, Drummond-Lage AP, Coelho LG. Helicobacter pylori and t(11;18)(q21;q21) translocation in gastric malt lymphoma. Arq Gastroenterol. 2014;51:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Wündisch T, Dieckhoff P, Greene B, Thiede C, Wilhelm C, Stolte M, Neubauer A. Second cancers and residual disease in patients treated for gastric mucosa-associated lymphoid tissue lymphoma by Helicobacter pylori eradication and followed for 10 years. Gastroenterology. 2012;143:936-42; quiz e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Choi YJ, Lee DH, Kim JY, Kwon JE, Jo HJ, Shin CM, Kim HY, Park YS, Kim N, Jung HC, Song IS. Low Grade Gastric Mucosa-associated Lymphoid Tissue Lymphoma: Clinicopathological Factors Associated with Helicobacter pylori Eradication and Tumor Regression. Clin Endosc. 2011;44:101-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Ono S, Kato M, Ono Y, Nishida U, Yamamoto K, Shimizu Y, Asaka M. Target biopsy using magnifying endoscopy in clinical management of gastric mucosa-associated lymphoid tissue lymphoma. J Gastroenterol Hepatol. 2011;26:1133-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Andriani A, Miedico A, Tedeschi L, Patti C, Di Raimondo F, Leone M, Schinocca L, Romanelli A, Bonanno G, Linea C, Giustini M, Hassan C, Cottone M, Zullo A. Management and long-term follow-up of early stage H. pylori-associated gastric MALT-lymphoma in clinical practice: an Italian, multicentre study. Dig Liver Dis. 2009;41:467-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Sumida T, Kitadai Y, Hiyama T, Shinagawa K, Tanaka M, Kodama M, Masuda H, Ito M, Tanaka S, Yoshihara M, Chayama K. Antibodies to Helicobacter pylori and CagA protein are associated with the response to antibacterial therapy in patients with H. pylori-positive API2-MALT1-negative gastric MALT lymphoma. Cancer Sci. 2009;100:1075-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Stathis A, Chini C, Bertoni F, Proserpio I, Capella C, Mazzucchelli L, Pedrinis E, Cavalli F, Pinotti G, Zucca E. Long-term outcome following Helicobacter pylori eradication in a retrospective study of 105 patients with localized gastric marginal zone B-cell lymphoma of MALT type. Ann Oncol. 2009;20:1086-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Terai S, Iijima K, Kato K, Dairaku N, Suzuki T, Yoshida M, Koike T, Kitagawa Y, Imatani A, Sekine H, Ohara S, Shimosegawa T. Long-term outcomes of gastric mucosa-associated lymphoid tissue lymphomas after Helicobacter pylori eradication therapy. Tohoku J Exp Med. 2008;214:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Fischbach W, Goebeler ME, Ruskone-Fourmestraux A, Wündisch T, Neubauer A, Raderer M, Savio A; EGILS (European Gastro-Intestinal Lymphoma Study) Group. Most patients with minimal histological residuals of gastric MALT lymphoma after successful eradication of Helicobacter pylori can be managed safely by a watch and wait strategy: experience from a large international series. Gut. 2007;56:1685-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 114] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Kim JS, Chung SJ, Choi YS, Cheon JH, Kim CW, Kim SG, Jung HC, Song IS. Helicobacter pylori eradication for low-grade gastric mucosa-associated lymphoid tissue lymphoma is more successful in inducing remission in distal compared to proximal disease. Br J Cancer. 2007;96:1324-1328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | El-Zahabi LM, Jamali FR, El-Hajj II, Naja M, Salem Z, Shamseddine A, El-Saghir NS, Zaatari G, Geara F, Soweid AM. The value of EUS in predicting the response of gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication. Gastrointest Endosc. 2007;65:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Hong SS, Jung HY, Choi KD, Song HJ, Lee GH, Oh TH, Jo JY, Kim KJ, Byeon JS, Myung SJ, Yang SK, Hong WS, Kim JH, Min YI. A prospective analysis of low-grade gastric malt lymphoma after Helicobacter pylori eradication. Helicobacter. 2006;11:569-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Wündisch T, Mösch C, Neubauer A, Stolte M. Helicobacter pylori eradication in gastric mucosa-associated lymphoid tissue lymphoma: Results of a 196-patient series. Leuk Lymphoma. 2006;47:2110-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Akamatsu T, Mochizuki T, Okiyama Y, Matsumoto A, Miyabayashi H, Ota H. Comparison of localized gastric mucosa-associated lymphoid tissue (MALT) lymphoma with and without Helicobacter pylori infection. Helicobacter. 2006;11:86-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Wündisch T, Thiede C, Morgner A, Dempfle A, Günther A, Liu H, Ye H, Du MQ, Kim TD, Bayerdörffer E, Stolte M, Neubauer A. Long-term follow-up of gastric MALT lymphoma after Helicobacter pylori eradication. J Clin Oncol. 2005;23:8018-8024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 217] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 43. | Montalban C, Santón A, Redondo C, García-Cosio M, Boixeda D, Vazquez-Sequeiros E, Norman F, de Argila CM, Alvarez I, Abraira V, Bellas C. Long-term persistence of molecular disease after histological remission in low-grade gastric MALT lymphoma treated with H. pylori eradication. Lack of association with translocation t(11;18): a 10-year updated follow-up of a prospective study. Ann Oncol. 2005;16:1539-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Chen LT, Lin JT, Tai JJ, Chen GH, Yeh HZ, Yang SS, Wang HP, Kuo SH, Sheu BS, Jan CM, Wang WM, Wang TE, Wu CW, Chen CL, Su IJ, Whang-Peng J, Cheng AL. Long-term results of anti-Helicobacter pylori therapy in early-stage gastric high-grade transformed MALT lymphoma. J Natl Cancer Inst. 2005;97:1345-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 132] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 45. | Taji S, Nomura K, Matsumoto Y, Sakabe H, Yoshida N, Mitsufuji S, Nishida K, Horiike S, Nakamura S, Morita M, Taniwaki M. Trisomy 3 may predict a poor response of gastric MALT lymphoma to Helicobacter pylori eradication therapy. World J Gastroenterol. 2005;11:89-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Takenaka R, Yokota K, Mizuno M, Okada H, Toyokawa T, Yamasaki R, Yoshino T, Sugiyama T, Asaka M, Shiratori Y, Oguma K. Serum antibodies to Helicobacter pylori and its heat-shock protein 60 correlate with the response of gastric mucosa-associated lymphoid tissue lymphoma to eradication of H. pylori. Helicobacter. 2004;9:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Fischbach W, Goebeler-Kolve ME, Dragosics B, Greiner A, Stolte M. Long term outcome of patients with gastric marginal zone B cell lymphoma of mucosa associated lymphoid tissue (MALT) following exclusive Helicobacter pylori eradication therapy: experience from a large prospective series. Gut. 2004;53:34-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 219] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 48. | Sheu BS, Shiesh SC, Wang JT, Yang HB, Lin ST, Wu JJ. Clinical application of 20 MHz endosonography and anti-Helicobacter pylori immunoblots to predict regression of low-grade gastric MALToma by H. pylori eradication. Helicobacter. 2003;8:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Caletti G, Zinzani PL, Fusaroli P, Buscarini E, Parente F, Federici T, Peyre S, De Angelis C, Bonanno G, Togliani T, Pileri S, Tura S; Italian Gastric Lymphona Study Group. The importance of endoscopic ultrasonography in the management of low-grade gastric mucosa-associated lymphoid tissue lymphoma. Aliment Pharmacol Ther. 2002;16:1715-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Nakamura T, Nakamura S, Yokoi T, Suzuki H, Ohashi K, Seto M. Clinicopathologic comparison between the API2-MALT1 chimeric transcript-positive and -negative gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type. Jpn J Cancer Res. 2002;93:677-684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Liu H, Ye H, Ruskone-Fourmestraux A, De Jong D, Pileri S, Thiede C, Lavergne A, Boot H, Caletti G, Wündisch T, Molina T, Taal BG, Elena S, Thomas T, Zinzani PL, Neubauer A, Stolte M, Hamoudi RA, Dogan A, Isaacson PG, Du MQ. T(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology. 2002;122:1286-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 272] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 52. | Bertoni F, Conconi A, Capella C, Motta T, Giardini R, Ponzoni M, Pedrinis E, Novero D, Rinaldi P, Cazzaniga G, Biondi A, Wotherspoon A, Hancock BW, Smith P, Souhami R, Cotter FE, Cavalli F, Zucca E; International Extranodal Lymphoma Study Group; United Kingdom Lymphoma Group. Molecular follow-up in gastric mucosa-associated lymphoid tissue lymphomas: early analysis of the LY03 cooperative trial. Blood. 2002;99:2541-2544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | Ohashi S, Segawa K, Okamura S, Urano F, Kanamori S, Hosoi T, Ishikawa H, Kanamori A, Kitabatake S, Sano H, Kobayashi T, Maeda M. Gastrin and Helicobacter pylori in low-grade MALT lymphoma patients. Scand J Gastroenterol. 2002;37:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Kim YS, Kim JS, Jung HC, Lee CH, Kim CW, Song IS, Kim CY. Regression of low-grade gastric mucosa-associated lymphoid tissue lymphoma after eradication of Helicobacter pylori: possible association with p16 hypermethylation. J Gastroenterol. 2002;37:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Matsushima Y, Kinoshita Y, Fukui H, Maekawa T, Yazumi S, Okada A, Nakase H, Kawanami C, Iwano M, Hashimoto K, Takeda Z, Okazaki K, Chiba T. Immunological and molecular analysis of B lymphocytes in low-grade MALT lymphoma of the stomach. Are there any useful markers for predicting outcome after Helicobacter pylori eradication? J Gastroenterol. 2002;37:428-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 56. | Kanda M, Suzumiya J, Ohshima K, Okada M, Tamura K, Kikuchi M. Changes in pattern of immunoglobulin heavy chain gene rearrangement and MIB-1 staining before and after eradication of Helicobacter pylori in gastric mucosa-associated lymphoid tissue (MALT) lymphoma. Leuk Lymphoma. 2001;42:639-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Raderer M, Osterreicher C, Machold K, Formanek M, Fiebiger W, Penz M, Dragosics B, Chott A. Impaired response of gastric MALT-lymphoma to Helicobacter pylori eradication in patients with autoimmune disease. Ann Oncol. 2001;12:937-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Nakamura S, Matsumoto T, Suekane H, Takeshita M, Hizawa K, Kawasaki M, Yao T, Tsuneyoshi M, Iida M, Fujishima M. Predictive value of endoscopic ultrasonography for regression of gastric low grade and high grade MALT lymphomas after eradication of Helicobacter pylori. Gut. 2001;48:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 59. | Ruskoné-Fourmestraux A, Lavergne A, Aegerter PH, Megraud F, Palazzo L, de Mascarel A, Molina T, Rambaud JL. Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment. Gut. 2001;48:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 189] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 60. | Thiede C, Wündisch T, Alpen B, Neubauer B, Morgner A, Schmitz M, Ehninger G, Stolte M, Bayerdörffer E, Neubauer A; German MALT Lymphoma Study Group. Long-term persistence of monoclonal B cells after cure of Helicobacter pylori infection and complete histologic remission in gastric mucosa-associated lymphoid tissue B-cell lymphoma. J Clin Oncol. 2001;19:1600-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 61. | de Jong D, Vyth-Dreese F, Dellemijn T, Verra N, Ruskoné-Fourmestraux A, Lavergne-Slove A, Hart G, Boot H. Histological and immunological parameters to predict treatment outcome of Helicobacter pylori eradication in low-grade gastric MALT lymphoma. J Pathol. 2001;193:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 62. | Urakami Y, Sano T, Begum S, Endo H, Kawamata H, Oki Y. Endoscopic characteristics of low-grade gastric mucosa-associated lymphoid tissue lymphoma after eradication of Helicobacter pylori. J Gastroenterol Hepatol. 2000;15:1113-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 63. | Papa A, Cammarota G, Tursi A, Gasbarrini A, Gasbarrini G. Helicobacter pylori eradication and remission of low-grade gastric mucosa-associated lymphoid tissue lymphoma: a long-term follow-up study. J Clin Gastroenterol. 2000;31:169-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 64. | Yamashita H, Watanabe H, Ajioka Y, Nishikura K, Maruta K, Fujino MA. When can complete regression of low-grade gastric lymphoma of mucosa-associated lymphoid tissue be predicted after helicobacter pylori eradication? Histopathology. 2000;37:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Ohashi S, Segawa K, Okamura S, Urano H, Kanamori S, Ishikawa H, Hara K, Hukutomi A, Shirai K, Maeda M. A clinicopathologic study of gastric mucosa-associated lymphoid tissue lymphoma. Cancer. 2000;88:2210-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 66. | Nakamura T, Nakamura S, Yonezumi M, Suzuki T, Matsuura A, Yatabe Y, Yokoi T, Ohashi K, Seto M. Helicobacter pylori and the t(11;18)(q21;q21) translocation in gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type. Jpn J Cancer Res. 2000;91:301-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Savio A, Zamboni G, Capelli P, Negrini R, Santandrea G, Scarpa A, Fuini A, Pasini F, Ambrosetti A, Paterlini A, Buffoli F, Angelini GP, Cesari P, Rolfi F, Graffeo M, Pascarella A, Valli M, Mombello A, Ederle A, Franzin G. Relapse of low-grade gastric MALT lymphoma after Helicobacter pylori eradication: true relapse or persistence? Recent Results Cancer Res. 2000;156:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Weston AP, Banerjee SK, Horvat RT, Zoubine MN, Campbell DR, Cherian R. Prospective long-term endoscopic and histologic follow-up of gastric lymphoproliferative disease of early stage IE low-grade B-cell mucosa-associated lymphoid tissue type following Helicobacter pylori eradication treatment. Int J Oncol. 1999;15:899-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 69. | Steinbach G, Ford R, Glober G, Sample D, Hagemeister FB, Lynch PM, McLaughlin PW, Rodriguez MA, Romaguera JE, Sarris AH, Younes A, Luthra R, Manning JT, Johnson CM, Lahoti S, Shen Y, Lee JE, Winn RJ, Genta RM, Graham DY, Cabanillas FF. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med. 1999;131:88-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 147] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 70. | Nobre-Leitão C, Lage P, Cravo M, Cabeçadas J, Chaves P, Alberto-Santos A, Correia J, Soares J, Costa-Mira F. Treatment of gastric MALT lymphoma by Helicobacter pylori eradication: a study controlled by endoscopic ultrasonography. Am J Gastroenterol. 1998;93:732-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Thiede C, Morgner A, Alpen B, Wündisch T, Herrmann J, Ritter M, Ehninger G, Stolte M, Bayerdörffer E, Neubauer A. What role does Helicobacter pylori eradication play in gastric MALT and gastric MALT lymphoma? Gastroenterology. 1997;113:S61-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Sackmann M, Morgner A, Rudolph B, Neubauer A, Thiede C, Schulz H, Kraemer W, Boersch G, Rohde P, Seifert E, Stolte M, Bayerdoerffer E. Regression of gastric MALT lymphoma after eradication of Helicobacter pylori is predicted by endosonographic staging. MALT Lymphoma Study Group. Gastroenterology. 1997;113:1087-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 160] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 73. | Neubauer A, Thiede C, Morgner A, Alpen B, Ritter M, Neubauer B, Wündisch T, Ehninger G, Stolte M, Bayerdörffer E. Cure of Helicobacter pylori infection and duration of remission of low-grade gastric mucosa-associated lymphoid tissue lymphoma. J Natl Cancer Inst. 1997;89:1350-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 181] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 74. | Pinotti G, Zucca E, Roggero E, Pascarella A, Bertoni F, Savio A, Savio E, Capella C, Pedrinis E, Saletti P, Morandi E, Santandrea G, Cavalli F. Clinical features, treatment and outcome in a series of 93 patients with low-grade gastric MALT lymphoma. Leuk Lymphoma. 1997;26:527-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 120] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 75. | Savio A, Franzin G, Wotherspoon AC, Zamboni G, Negrini R, Buffoli F, Diss TC, Pan L, Isaacson PG. Diagnosis and posttreatment follow-up of Helicobacter pylori-positive gastric lymphoma of mucosa-associated lymphoid tissue: histology, polymerase chain reaction, or both? Blood. 1996;87:1255-1260. [PubMed] |
| 76. | Bayerdörffer E, Neubauer A, Rudolph B, Thiede C, Lehn N, Eidt S, Stolte M. Regression of primary gastric lymphoma of mucosa-associated lymphoid tissue type after cure of Helicobacter pylori infection. MALT Lymphoma Study Group. Lancet. 1995;345:1591-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 594] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 77. | Roggero E, Zucca E, Pinotti G, Pascarella A, Capella C, Savio A, Pedrinis E, Paterlini A, Venco A, Cavalli F. Eradication of Helicobacter pylori infection in primary low-grade gastric lymphoma of mucosa-associated lymphoid tissue. Ann Intern Med. 1995;122:767-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 273] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 78. | Wotherspoon AC, Doglioni C, Diss TC, Pan L, Moschini A, de Boni M, Isaacson PG. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1564] [Cited by in RCA: 1385] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 79. | Violeta Filip P, Cuciureanu D, Sorina Diaconu L, Maria Vladareanu A, Silvia Pop C. MALT lymphoma: epidemiology, clinical diagnosis and treatment. J Med Life. 2018;11:187-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 80. | Floch P, Mégraud F, Lehours P. Helicobacter pylori Strains and Gastric MALT Lymphoma. Toxins (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 81. | Barker TH, Migliavaca CB, Stein C, Colpani V, Falavigna M, Aromataris E, Munn Z. Conducting proportional meta-analysis in different types of systematic reviews: a guide for synthesisers of evidence. BMC Med Res Methodol. 2021;21:189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 370] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 82. | Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1008] [Cited by in RCA: 1527] [Article Influence: 218.1] [Reference Citation Analysis (0)] |
| 83. | Malfertheiner P, Megraud F, Rokkas T, Gisbert JP, Liou JM, Schulz C, Gasbarrini A, Hunt RH, Leja M, O'Morain C, Rugge M, Suerbaum S, Tilg H, Sugano K, El-Omar EM; European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 640] [Article Influence: 213.3] [Reference Citation Analysis (0)] |
| 84. | Zucca E, Bertoni F. The spectrum of MALT lymphoma at different sites: biological and therapeutic relevance. Blood. 2016;127:2082-2092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 85. | Dreyling M, Thieblemont C, Gallamini A, Arcaini L, Campo E, Hermine O, Kluin-Nelemans JC, Ladetto M, Le Gouill S, Iannitto E, Pileri S, Rodriguez J, Schmitz N, Wotherspoon A, Zinzani P, Zucca E. ESMO Consensus conferences: guidelines on malignant lymphoma. part 2: marginal zone lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma. Ann Oncol. 2013;24:857-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 86. | Stern C, Kleijnen J. Language bias in systematic reviews: you only get out what you put in. JBI Evid Synth. 2020;18:1818-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |