Published online Apr 14, 2023. doi: 10.3748/wjg.v29.i14.2101
Peer-review started: December 17, 2022
First decision: February 8, 2023
Revised: February 21, 2023
Accepted: March 23, 2023
Article in press: March 23, 2023
Published online: April 14, 2023
Processing time: 117 Days and 4.3 Hours
Chronic pancreatitis is a debilitating pancreatic inflammatory disease characterized by intractable pain resulting in poor quality of life. Conventional management of pancreatic pain consists of a step-up approach with medications and lifestyle modifications followed by endoscopic intervention. Traditionally surgery is reserved for patients who do not improve with other interventions. However, recent studies suggest that early surgical intervention is more beneficial as it can mitigate the progression of the pathological process and prevent loss of pancreatic function. Despite the widespread adoption of minimally invasive approaches in various gastrointestinal surgical disorders, minimally invasive surgery for chronic pancreatitis is slow to evolve. Technical difficulty due to severe inflammatory changes has been the major impediment to the widespread usage of minimally invasive surgery in chronic pancreatitis. With this back
Core Tip: Pain in chronic pancreatitis is a significant symptom that demands utmost attention as it compromises the quality of life and inherently risks narcotic addiction. Surgical management for chronic pancreatitis began with various open surgical drainage and resection procedures. Since pain is the primary indication for intervention, a minimally invasive approach is an attractive proposition in chronic pancreatitis. Despite the slow adoption of laparoscopic and robotic surgery in chronic pancreatitis, safety, and feasibility have been documented in recent studies. The challenges and limitations highlighted in the present review could guide future research on minimally invasive surgery in chronic pancreatitis.
- Citation: Kalayarasan R, Shukla A. Changing trends in the minimally invasive surgery for chronic pancreatitis. World J Gastroenterol 2023; 29(14): 2101-2113
- URL: https://www.wjgnet.com/1007-9327/full/v29/i14/2101.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i14.2101
Chronic pancreatitis is a progressive pancreatic inflammatory disease that leads to fibrosis and parenchymal tissue loss resulting in impaired endocrine and exocrine function. While consumption of alcohol is the leading cause of chronic pancreatitis worldwide, idiopathic pancreatitis remains common in India and China[1,2]. The most common and dominant symptom of chronic pancreatitis is pain, which can be persistent, severe, or recurrent episodes with pain-free intervals significantly impacting the quality of life[2,3].
Traditionally, pain in chronic pancreatitis is managed initially with analgesics, pancreatic enzyme replacement therapy, and lifestyle modifications followed by endoscopic or surgical intervention. Almost half of the patients who do not respond to medical or endoscopic management are referred for surgical intervention[4]. The most common indication for surgery is intractable pain. Studies have reported that early surgical management has better outcomes than intervention in the advanced disease stage[3,5]. While the minimally invasive approach is widely used for various gastrointestinal and pancreatobiliary disorders, its application in chronic pancreatitis is disproportionately low. As chronic pancreatitis is a benign disorder with pain as the primary indication for intervention, a minimally invasive approach is an attractive proposition. The technical difficulty, combined with the potential for vascular injury and bleeding associated with pancreatic inflammation and fibrosis, is the primary reason for the slow adoption of minimally invasive techniques in chronic pancreatitis. However, recent studies have shown the feasibility of laparoscopic and robotic surgery for chronic pancreatitis. Also, with advancements in endoscopic treatment, there is a trend towards the less invasive treatment of chronic pancreatitis. The present review focuses on the challenges, evolution, and changing trends in the minimally invasive management of chronic pancreatitis.
All the authors did a PubMed search of relevant articles. Further, the articles’ reference lists were also searched for additional appropriate studies. The keywords and combinations included in the search were: “Pancreatitis”; ”chronic pancreatitis”; “idiopathic pancreatitis”; endoscopic management” and “chronic pancreatitis”; “Frey’s procedure” and “Laparoscopic”; “Frey’s procedure” and “robotic”; “Puestow procedure” and “Laparoscopic”; “Puestow procedure” and “ robotic”; “Beger procedure” and “Laparoscopic”; “Beger procedure” and “robotic”; “chronic pancreatitis” and “total pancreatectomy” and “ laparoscopic”; “chronic pancreatitis” and “total pancreatectomy”, and “robotic”. The search was limited to publications in English literature. All the authors agreed that the articles selected for review were relevant.
Endoscopic intervention is recommended as a minimally invasive alternative to surgery in patients who do not improve with medical management. The journey of stone removal from the pancreatic duct dates back to 1891 when Alfred Pearce Gould retrieved calculi from the Wirsung duct in a London hospital[6]. Berkeley G Moynihan performed transduodenal removal of pancreatic stones in 1902, followed by transpancreatic stone removal in 1908 by Mayo Robson. The development of the fiberoptic endoscope for diagnosis in 1958 by Basil Isaac Hirschowitz changed the trends of endoscopic management[7]. Watson et al[8] succeeded in developing the technique for papillotomy with an energy source leading to the endoscopic extraction of calculi and subsequent stent placement. Pathogenesis of pain in chronic pancreatitis is multifactorial and includes anatomical and neuropathic factors. Anatomical alterations include ductal hypertension, raised pancreatic parenchymal pressure, acute inflammation, and pancreatic ischemia[9-11]. The lack of correlation between pain severity and anatomical changes suggests neurological factors’ role. Neuropathophysiology of pain in chronic pancreatitis includes peripheral sensitization-induced pain, neuropathic remodeling, and central sensitization of pancreatic pain[10,11]. Endoscopic therapy aims to relieve pain by clearance of intraductal stones, thereby decompressing the pancreatic duct. Proponents of endoscopic therapy suggest that patients with complete ductal clearance by endoscopic approach have shown similar pain relief compared to the surgical group[12]. At the same time, the ability to modify the disease progression and prevent loss of pancreatic function with early surgical intervention was proposed in favor of surgical management[13,14].
Traditionally chronic pancreatitis patients were managed initially with analgesics and pancreatic enzyme replacement therapy, followed by endoscopic intervention with or without extracorporeal shockwave therapy[15,16]. Patients with an inadequate clinical response following endoscopic treatment should be discussed in the multidisciplinary meeting and considered for surgical management[10,16]. The potential benefit of this approach is that surgery could be avoided in some patients with successful endoscopic management. However, various studies comparing the efficacy of endoscopic management with surgery have shown better pain control with the early surgical intervention[17-19]. Randomized trials comparing the endoscopic and surgical approaches have shown a better quality of life and pain relief in the surgical group, especially with early surgery[12,20,21] (Table 1). Also, patients with inflammatory pancreatic head mass, distal pancreatic duct stricture, and extensive parenchyma calcifications of the pancreatic head might be difficult to treat by endoscopy. Despite the available evidence favoring surgical treatment, advancements in endoscopic lithotripsy techniques and extracorporeal shockwave lithotripsy (ESWL) are continuously improving the ductal clearance rate, and the endoscopic approach is helpful in a subset of chronic pancreatitis patients[16]. Patients with dominant stricture in the pancreatic head with upstream dilatation and those with intraductal calculi in the pancreatic head or proximal body are ideal candidates for endoscopic intervention.
| Ref. | Country | No. of patients (n), surgery/endoscopy | Complete and partial pain relief (%), surgery/endoscopy | Complication (%), surgery/endoscopy | Mortality (n), surgery/endoscopy | Follow up (mo), surgery/endoscopy |
| Díte et al[12], 2003 | Czech Republic | 36/36 | 86/61 | Not reported | 0/0 | 60 |
| Cahen et al[20], 2011 | Netherlands | 16/15 | 80/38 | 0/25 | 0/1 | 79 |
| Hong et al[19], 2011 | China | 27/35 | 77/47 | 14/22 | 1/0 | 60 |
| Kawashima et al[17], 2018 | Japan | 41/10 | 100/100 | 20/27 | 0/0 | - |
| Jiang et al[18], 2018 | China | 40/46 | 83/80 | 26/8 | 0/0 | 63.5/57.3 |
| Issa et al[21], 2020 | Netherlands | 44/44 | 58/39 | 27/25 | 0/0 | 18 |
Worldwide, ESWL is commonly used for pancreatic lithotripsy, especially in Asia and Europe. While recent advancements like intraductal endoscopic laser or electrohydraulic lithotripsy might improve ductal clearance, they are not widely available. Hence, most of the available data are for ESWL[22]. A meta-analysis of various studies has reported a 70% stone clearance rate with ESWL[23]. With ESWL, stone clearance is more favorable for solitary calculus in the head of the pancreas. However, recurrence of stones after ESWL was seen in 14% to 23% of patients mandating further intervention[24]. ESWL is combined with pancreatic duct stenting in patients with associated pancreatic duct stricture[25]. European Society of Gastrointestinal Endoscopy (ESGE) recommends ESWL for the removal of radiopaque obstructive main pancreatic duct calculi greater than 5 mm found in the head or body of the pancreas and endoscopic retrograde cholangiopancreatography (ERCP) for main pancreatic duct calculi that are radiolucent or smaller than 5 mm[26].
Main pancreatic duct strictures are often seen in half of the chronic pancreatitis patients and are usually located in the pancreatic head region. The standard management of these strictures is balloon dilatation and placement of a temporary stent for at least a year[27]. Pain relief after a long-term follow-up of 5 years has been seen in almost half of patients after stent withdrawal[28,29]. However, stricture recurrence has been reported in up to 38% of patients after two years[28,30]. Complications related to stenting include stent migration and occlusion. Distal stent migration toward the pancreatic tail and proximal stent migration to the duodenum was reported in 7.5% and 5.2% of patients, respectively[31]. Stent migration can be prevented with large-winged or pigtail catheters[31,32]. The ESGE recommends managing painful pain pancreatic duct strictures with the help of a single 10 Fr stent for one uninterrupted year if symptoms improve after placement[26]. The stent should be exchanged based on symptoms or signs of stent dysfunction on imaging at least six months intervals.
Endoscopic ultrasound (EUS) guided drainage of the main pancreatic duct is used as a second-line procedure after the failure of ERCP. Despite high success rates of 68% to 75%, the complications like perforation, bleeding, and pancreatitis reported in 5% to 43% of patients were key drawbacks for EUS-guided drainage[33,34]. Another EUS-guided procedure is the celiac plexus block, whereby a steroid with a local anesthetic agent is injected at the celiac plexus to block the pancreatic nerve fibers. EUS-guided celiac plexus block is preferred over the traditional percutaneous method. However, despite the high technical success and short-term pain relief in 55%-70% of patients, long-term outcomes are discouraging[35-37].
The systemic reviews and meta-analyses of the available studies comparing surgical management with endoscopic intervention continue to support the superiority of surgical treatment in chronic pancreatitis[38-40]. The intriguing voyage of surgical management of chronic pancreatitis has witnessed a huge transformation from surgical decompression of the obstructed duct and denervation of the pancreas to pancreatic head resection, total pancreatectomy, and various other hybrid procedures. In 1911, Link[41], a gynecologist from Indiana, was the first to describe external tube drainage of the pancreatic duct for ductal calculi or stricture with good long-term pain relief. Desjardins and Coffey, around similar times, proposed drainage of the pancreas using the intestine after conducting animal studies[42,43]. This empiricism was materialized by Duval[44] and separately by Zollinger et al[45] in 1954 by describing the first caudal end-to-end pancreaticojejunostomy using a Roux-en-Y jejunal loop for chronic pancreatitis. Further, modification in the decompressive procedure was done by Puestow and Gillesby[46], in 1958, by invaginating caudal pancreaticojejunostomy after longitudinally opening the pancreatic duct from the body to the tail region of the pancreas. Their procedure was further modified by Partington and Rochelle[47], in 1960, by creating side-to-side Roux-en-Y pancreaticojejunostomy, commonly called the Puestow procedure. Though the Puestow procedure was considered standard drainage procedure for almost 40 years, the long-term benefits were not befitting. Despite short-term pain relief in 80% of patients, the pain recurred in 30% on long-term follow-up[48-51]. The foremost reason for recurrent pain was undealt nidus of inflammation and persistent ductal disease in the head of the pancreas. To tackle the head disease, German surgeon Hans Beger performed the first duodenum-preserving pancreatic head resection in 1971 and reported postoperative outcomes of 52 patients in 1980[52]. In 1984, Warren described splenopancreatic flap, a denervation procedure for chronic pancreatitis[53]. However, the long-term results of this complex procedure were never published or replicated by other surgeons[9,53].
In 1987, Frey and Smith[54] described a hybrid operation consisting of pancreatic head resection and longitudinal pancreaticojejunostomy, also known as Frey’s procedure. Izbicki et al[55] modified Frey’s procedure by doing a more extensive excavation of the pancreatic head and duct and named it Hamburg modification. Similarly, Gloor et al[56] from Berne modified the duodenum-preserving pancreatic head resection by omitting the challenging step of pancreatic neck transection. To minimize the risk of penetrating the posterior pancreatic capsule, Ho and Frey[57], and Frey and Amikura[58] recommended limiting the posterior extent of head coring to the back wall of opened Wirsung and the uncinate duct, also known as modified Frey’s procedure. Sakata et al[59] described the minimum Frey procedure in which a small spindle-shaped anterior resection of the pancreatic head was performed and reported an equivalent outcome. However, a retrospective study by Tan et al[60] reported superior long-term pain relief and quality of life with the original Frey’s procedure compared to the modified Frey’s. A systematic review and meta-analysis of trials comparing various resectional and hybrid procedures reported similar postoperative pain relief. However, quality of life and other perioperative outcomes favor duodenum-preserving pancreatic head resection procedures[61].
There is a trend towards minimally invasive procedures for various surgical disorders, and the change is inevitable for chronic pancreatitis[62-64]. Though the surgical procedures described for chronic pancreatitis are complex and challenging due to inflamed gland they can be accomplished in selected patients[63-65]. In high-volume centers with expertise in advanced laparoscopic procedures, complex pancreatic procedures can be safely performed with comparable postoperative outcomes[63-69]. Also, with its ergonomic advantages, robotic surgery could overcome some of the technical limitations of laparoscopic surgery and potentially widen the use of a minimally invasive approach in chronic pancreatitis[70,71].
Like open surgical procedures, minimally invasive surgery for chronic pancreatitis began with a modified Puestow procedure. Kurian and Gagner[72], in 1999, reported the first series of five patients who underwent a laparoscopic Puestow procedure. Subsequently, two series with 17 and 12 patients were published from India[73,74]. The first small case series of 5 patients from the United Kingdom was published by Khaled et al[75] in 2014. The feasibility and favorable short-term outcomes of the laparoscopic Puestow procedure were documented in multiple case series[76-81] (Table 2). In most series, the procedure was performed with five laparoscopic trocars. The initial entry to the lesser sac and exposure of the anterior surface of the pancreas can be technically challenging in patients with recent or recurrent acute episodes of pancreatitis. Hence, those patients should be avoided during the early phase of the minimally invasive Puestow procedure. In most laparoscopic series, two to three gastric retraction sutures are used to lift the stomach away from the pancreas and improve exposure. Needle aspiration is commonly used to identify the pancreatic duct, and intraoperative ultrasound is helpful in patients with undilated duct[74]. Extraction of all intraductal calculi, especially those in the head and tail region, is critical for long-term pain relief. Sahoo et al[76] reported the usefulness of cystoscope and endoscopic basket in clearing residual intraductal stones. Proficient intracorporal suturing skill is critical to accomplish safe pancreaticojejunostomy. Kim et al[77] reported the benefits of using barbed sutures for laparoscopic pancreaticojejunostomy. Bhandarwar et al[78] used endostaplers for laparoscopic pancreaticojejunostomy anastomosis with the anvil part placed within the pancreatic duct. However, as highlighted by the authors, the technique was feasible only in seven out of 17 patients with pancreatic duct diameter of more than 10 mm[78]. Alternatively, a robotic platform can minimize the challenges associated with intracorporeal suturing. After the initial case reports of the robotic Puestow procedure documented its usefulness in a series of seven patients[82-87]. However, with the emerging evidence supporting some form of head resection to achieve long-term pain relief, the minimally invasive Puestow procedure is recommended only in a subset of chronic pancreatitis patients with an atrophic pancreas and dilated pancreatic duct with predominant intraductal calculi.
| Ref. | Country | No. of patients (n) | Mean operative time (min) | Mean hospital stay (d) | Conversion (n) | Mortality | Follow up, (mo) | Complete pain relief (%) |
| Kurian et al[72], 1999 | United States | 5 | 240.0 | 3-7 | Nil | Nil | 30.0 | 80.0 |
| Tantia et al[73], 2004 | India | 17 | 277.0 | 5.2 | 4 | Nil | 12.0 | 82.3 |
| Palanivelu et al[74], 2006 | India | 12 | 172.0 | 5.0 | Nil | Nil | 52.8 | 83.3 |
| Khaled et al[75], 2014 | United Kingdom | 6 | 278.0 | 7.0 | Not reported | Nil | 14.2 | 66.7 |
| Sahoo et al[76], 2014 | India | 12 | 265.5 | 5.8 | Nil | Nil | 16.5 | 100 (follow up reported for 8 patients) |
| Kim and Hong[77], 2016 | Korea | 11 | 200.0 | 7.0 | Nil | Nil | 21.0 | 100 |
| Bhandarwar, et al[78], 2019 | India | 28 | 189.7 | 5.8 | 4 | Nil | 12.0 | 87.5 |
| Rege et al[79], 2019 | India | 32 | 131.2 | 5.2 | 1 | Nil | 14.2 | 75.0 |
| Javed et al[80], 2020 | India | 41 | 180.0 | 5.0 | Excluded | Nil | 43.6 | 91.0 |
| Nag et al[81], 2022 | India | 33 | 300.0 | 7.0 | Nil | Nil | 25.0 | 71.0 |
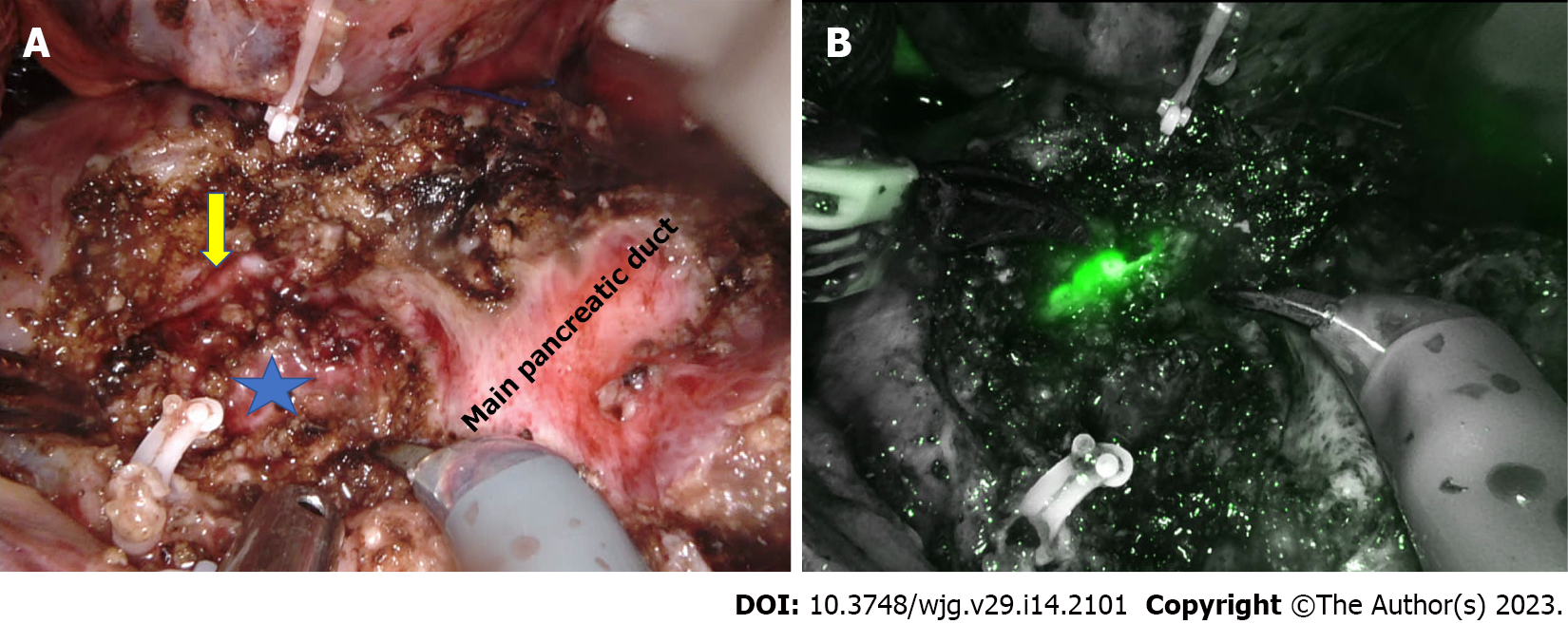
Frey’s procedure is one of the most commonly performed surgeries for chronic pancreatitis. Pancreatic head coring is the technically challenging step of this hybrid procedure, especially during minimally invasive surgery. The first series of laparoscopic Frey’s procedure was published by Tan et al[88] in 2015. Subsequently, a small series of four patients reported the feasibility and short-term outcomes of laparoscopic Frey’s procedure[89]. The largest published series to date had 15 patients in the laparoscopic Frey’s group[90]. The relatively small number of studies with fewer patients highlight the technical challenges of laparoscopic Frey’s procedure (Table 3). In open Frey’s procedure, the surgeon’s left hand, kept under the posterior surface of the pancreas head, guides the extent of posterior head coring. In the absence of a definite landmark, pancreatic head coring until the level of the posterior pancreatic capsule is challenging during laparoscopic surgery. Hence, in all the laparoscopic series, only modified Frey’s procedure was performed using the main pancreatic duct as the landmark and coring to the posterior wall of the duct[88-90]. In the laparoscopic approach, ultrasonic shears and bipolar vessel sealing devices are commonly used for head coring. Lack of articulation and difficulty securing precise hemostatic sutures further increase the difficulty of laparoscopic pancreatic head coring, especially along uncinate ducts[90]. With its articulating instruments, the robotic platform could potentially overcome technical difficulties during head coring and pancreaticojejunostomy. Hamad et al[62] highlighted the usefulness of the robotic approach in bleeding control during head coring in their series of four patients. However, similar to the laparoscopic approach, due to the lack of a definite landmark, coring was limited to the posterior wall of the duct in the robotic modified Frey’s procedure (Figure 1). The median operative time and blood loss were 372 min and 163 mL, respectively[62]. The parenchyma posterior to the main pancreatic duct in the pancreatic head was preserved to prevent injury to the superior mesenteric vein[62,91].
| Ref. | Country | Patients (n) | Mean operative time (min) | Mean hospital stay (d) | Conversion (n) | Mortality | Follow up (mo) | Complete and partial pain relief |
| Tan et al[88], 2015 | China | 9 | 323 | 7 | 2 | Nil | 3 | Not reported |
| Kilburn et al[89], 2017 | Australia | 4 | 130 | 7 | Nil | Nil | 26 | 100% |
| Senthilnathan et al[90], 2019 | India | 15 | 271 | 6.4 | 10 out of 57 patients in different arms | Nil | 60 | 88% |
Shukla et al[92] reported the feasibility of robot assisted Frey’s procedure in nine patients with chronic pancreatitis. Robotic approach is associated with less blood loss and shorter hospital stay compared to open Frey’s procedure. Tile Pro technology in the robotic platform allows the surgeon to view ultrasound images in the console, thereby avoiding damage to the common bile duct and portal vein during dissection[62]. Bleeding is one of the common causes of conversion in minimally invasive Frey’s procedure. Inflammatory pancreatic head mass and preoperative acute exacerbation of pancreatitis were identified as significant risk factors for intraoperative blood loss. Hence, minimally invasive Frey’s procedure is recommended in patients with dilated pancreatic duct and enlarged pancreatic head on imaging without inflammatory mass, recent acute exacerbation, and pancreatitis-related complications.
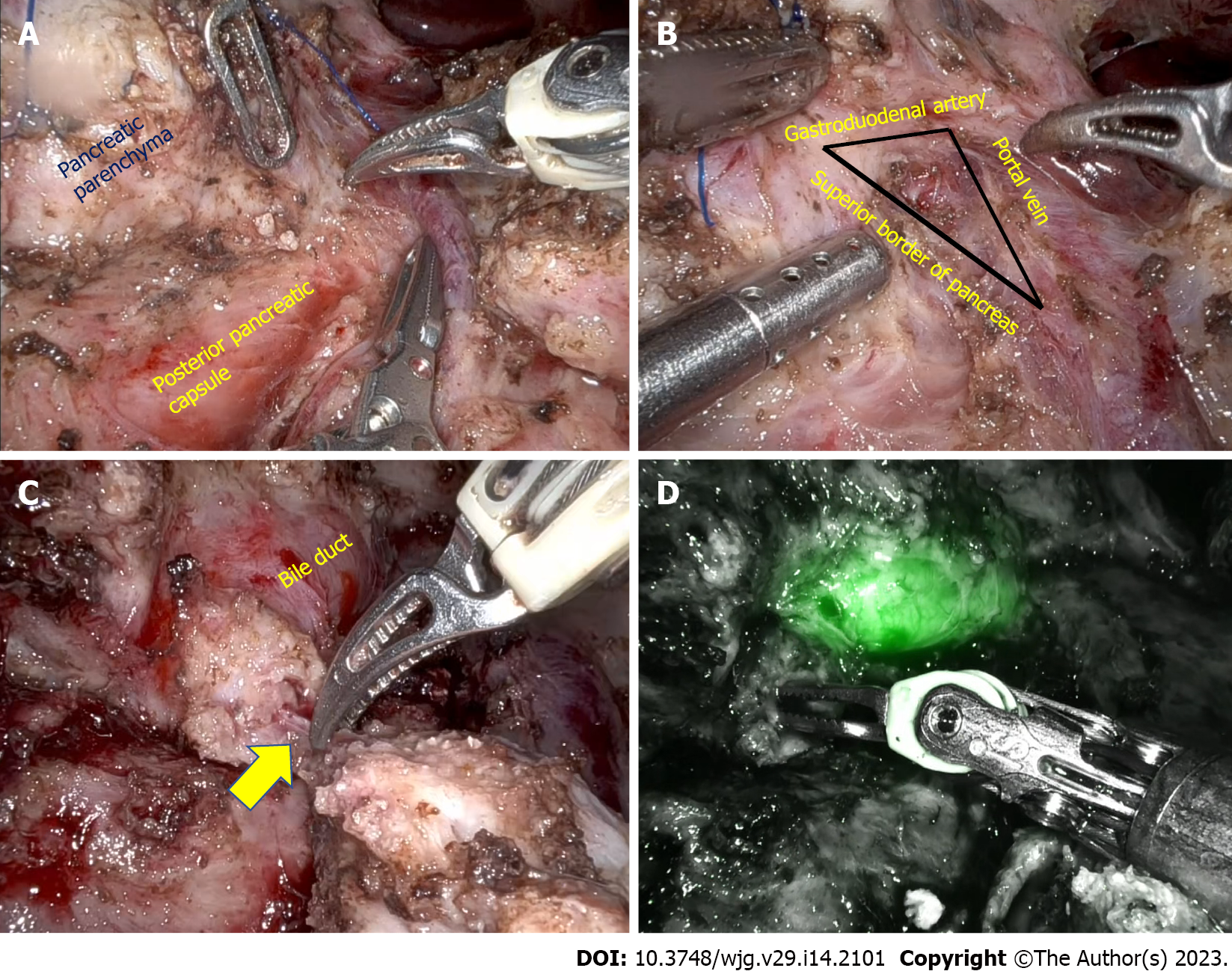
In patients with inflammatory head mass, duodenum preserving pancreatic head resection (DPHR) is preferred over pancreatoduodenectomy because of favorable long-term outcomes. As previously highlighted, lack of definite landmarks precludes pancreatic head coring until the posterior pancreatic capsule in minimally invasive Frey’s procedure. Minimally invasive duodenum-preserving pancreatic head resection could potentially overcome that limitation. After the initial case reports, it is reported that the feasibility of laparoscopic DPHR procedure in 5 patients with chronic pancreatitis[93-95]. The mean operative time and hospital stay were 275 min and 11 d, respectively. One patient had grade B postoperative pancreatic fistula, and pancreaticojejunostomy anastomotic site bleed in one patient[95]. In laparoscopic DPHR after the pancreatic neck transection, the pancreatic head is retracted to identify the plane between the pancreatic parenchyma and posterior pancreatic capsule. As the dissection proceeds along the superior border of the pancreas, the bile duct should be identified and preserved. Identifying the intrapancreatic duct is a significant technical challenge with minimally invasive DPHR. Indocyanine green (ICG) fluorescence facilitates bile duct identification in the triangle formed by the gastroduodenal artery, portal vein, and superior border of the pancreas (Figure 2). Energy sources should be judiciously used around the bile duct to prevent thermal damage[96-98]. Also, ischemia of the bile duct can be prevented by preserving the posterosuperior pancreaticoduodenal artery, a proximal branch of the gastroduodenal artery, and preserving pancreatic tissue medial to the bile duct. To prevent duodenal ischemia and delayed gastric emptying, the pancreaticoduodenal arcade along the medial border of the duodenum should be preserved. Hong et al[95] reported the usefulness of ICG in assessing vascular arcade and identifying common bile duct in a series of 22 patients with different pancreatic pathology. The mean operative time and blood loss of five patients with chronic pancreatitis included in their series were 264 min and 215 mL, respectively. The mean postoperative hospital stay was 7.5 d, and there was no conversion to open surgery or postoperative mortality[96].
Inflammation and tissue adhesion in chronic pancreatitis can distort the anatomy of pancreaticoduodenal vessels resulting in vascular injury and significant bleeding during DPHR. A 3D reconstruction of preoperative cross-sectional imaging could help to better understand the anatomy of pancreaticoduodenal vessels and the relationship of the intrapancreatic common bile duct. Also, 3D printing technology can be helpful for surgical training and preoperative planning in patients undergoing minimally invasive DPHR[96-98]. As with other minimally invasive procedures for chronic pancreatitis, a robotic platform could minimize the technical challenges associated with DPHR. Peng et al[98] first reported the feasibility of robotic DPHR. However, in a recent series of 68 patients undergoing robotic DPHR for various pancreatic diseases, only three patients had chronic pancreatitis[99]. Different published series on minimally invasive DPHR highlight technical development and challenges of the procedure in chronic pancreatitis. Minimally invasive DPHR is the preferred procedure in patients with inflammatory head mass and those with enlarged pancreatic head and extensive parenchymal calcifications. However, if the inflammatory changes preclude the safe creation of a retropancreatic tunnel over the portal vein alternative surgical procedure should be considered.
Total pancreatectomy is primarily indicated in chronic pancreatitis patients with debilitating pain in whom all other measures are unsuccessful and those with recurrent acute pancreatitis[100]. However, total pancreatectomy should be combined with islet cell autotransplantation to minimize the risk of brittle diabetes. Some centers recommend total pancreatectomy early in the disease course before activation of neuropathic pain circuits, especially in patients with small duct disease or genetic etiology[101]. However, selecting suitable patients is critical as, despite islet cell autotransplantation, more than 50% of patients might require lifelong exogenous insulin. Literature on minimally invasive total pancreatectomy is sparse, with variations in technique[102-105]. Blair et al[103], in 2016, reported the feasibility and safety of laparoscopic total pancreatectomy with islet cell autotransplantation in 20 patients with chronic pancreatitis. The mean operative time and hospital stay were 430 min and 11 d, respectively, with no postoperative mortality. Similarly, Fan et al[101] reported the feasibility of laparoscopic total pancreatectomy with islet cell autotransplantation in 22 patients with two conversions. In both the laparoscopic series, the pancreatic neck was transected, and two-stage retrieval was used, with the pancreatic head and body retrieved separately[102,104]. However, studies have shown the importance of preserving pancreatic arterial and venous flow until retrieval to reduce warm ischemia time during the pancreatic dissection phase and improve islet yield[105]. In the robotic series reported by Galvani et al[102] and Zureikat et al[104] the feasibility of total pancreatectomy without pancreatic neck transection and preserving vascular flow till the final step to reduce warm ischemia was documented. Another technical challenge is dense retroperitoneal adhesions due to recurrent pancreatic inflammation. Although laparoscopic and robotic total pancreatectomy with islet autotransplantation is safe and feasible, appropriate patient selection is critical for deriving the benefit of a minimally invasive approach.
The available evidence suggests that minimally invasive surgery for chronic pancreatitis is feasible in selected patients. However, the poor quality of available evidence precludes definite conclusions. Also, the surgeon should adhere to surgery principles for chronic pancreatitis, irrespective of the approach. As the pancreatic head is the pacemaker of pain in most patients, adequate resection and decompression of the pancreatic head are critical. However, most of the reported series on minimally invasive approaches for chronic pancreatitis have focused on the feasibility of lateral pancreaticojejunostomy or modified Frey’s procedure which may be appropriate only in a minority of chronic pancreatitis patients. Recent series have shown the feasibility of minimally invasive duodenal preserving pancreas head resection, which may be the ideal procedure for most chronic pancreatitis patients. However, a minimally invasive approach is feasible only in patients without extensive inflammatory adhesions or recent acute exacerbation. Preoperative cross-sectional imaging and biochemical parameters like serum amylase and lipase are not sensitive to predict inflammatory changes. Also, studies evaluating the predictive value of markers of systemic inflammation like white blood cell count, IL-6, and C reactive protein yielded disappointing results[88]. Future studies should focus on identifying reliable markers that can accurately predict ongoing pancreatic inflammation, thereby aiding patient selection for a minimally invasive approach. With recent evidence supporting early surgical intervention before the development of extensive fibrosis or local complications, more patients may be suitable for minimally invasive surgery. Also, the main problem with the existing procedures is they are primarily focused on pancreatic ductal and parenchymal decompression. However, it is well-documented that anatomical factors alone do not contribute to pancreatic pain in all patients. In a subgroup of chronic pancreatitis patients, neurological pathways of pain play a dominant role, which is not addressed by the commonly performed surgical procedures. Also, future studies should compare laparoscopic and robotic procedures for chronic pancreatitis to document the advantages of the robotic platform.
As pain is the primary indication for intervention in chronic pancreatitis use of a minimally invasive approach is an attractive proposition. However, due to technical challenges, both endoscopic intervention and minimally invasive surgery for chronic pancreatitis have lagged compared to other benign gastrointestinal orders. With improvements in laparoscopic instrumentation and technological advances like ICG fluorescence, minimally invasive surgery for chronic pancreatitis is gaining momentum. Also, with its distinct advantages, the robotic platform can widen the adoption of minimally invasive surgery in chronic pancreatitis. However, well-designed trials with long-term follow-ups are required to document the superiority of minimally invasive surgery for chronic pancreatitis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: International Hepato-Pancreato-Biliary Association (IHPBA); Asia-Pacific Hepato-Pancreato-Biliary Association (A-PHPBA); Society for Surgery of the Alimentary Tract (SSAT).
Specialty type: Surgery
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hassan MA, Pakistan; Liu H, China; Wang ZX, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Garg PK, Tandon RK. Survey on chronic pancreatitis in the Asia-Pacific region. J Gastroenterol Hepatol. 2004;19:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in pathogenesis, genetics, diagnosis, and therapy. Gastroenterology. 2007;132:1557-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 346] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 3. | Yang CJ, Bliss LA, Freedman SD, Sheth S, Vollmer CM, Ng SC, Callery MP, Tseng JF. Surgery for chronic pancreatitis: the role of early surgery in pain management. Pancreas. 2015;44:819-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Ray S, Basu C, Dhali A, Dhali GK. Frey procedure for chronic pancreatitis: A narrative review. Ann Med Surg (Lond). 2022;80:104229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Nitesh PNB, Pottakkat B. Bile duct preserving pancreatic head resection (BDPPHR): Can we conclusively define the extent of head resection in surgery for chronic pancreatitis? Ann Hepatobiliary Pancreat Surg. 2020;24:309-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Howard JM, Hess W. History of the pancreas: mysteries of a hidden organ. Nueva York: Kluwer Academic/Plenum Publishers, 2002. [DOI] [Full Text] |
| 7. | Navarro S. The art of pancreatic surgery. Past, present and future. The history of pancreatic surgery. Gastroenterol Hepatol. 2017;40:648.e1-648.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Watson WC. Direct vision of the ampulla of Vater through the gastroduodenal fiberscope. Lancet. 1966;1:902-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Andersen DK, Frey CF. The evolution of the surgical treatment of chronic pancreatitis. Ann Surg. 2010;251:18-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Dumonceau JM. Endoscopic therapy for chronic pancreatitis. Gastrointest Endosc Clin N Am. 2013;23:821-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Drewes AM, Krarup AL, Detlefsen S, Malmstrøm ML, Dimcevski G, Funch-Jensen P. Pain in chronic pancreatitis: the role of neuropathic pain mechanisms. Gut. 2008;57:1616-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Díte P, Ruzicka M, Zboril V, Novotný I. A prospective, randomized trial comparing endoscopic and surgical therapy for chronic pancreatitis. Endoscopy. 2003;35:553-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 322] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 13. | Maartense S, Ledeboer M, Bemelman WA, Ringers J, Frolich M, Masclee AA. Effect of surgery for chronic pancreatitis on pancreatic function: pancreatico-jejunostomy and duodenum-preserving resection of the head of the pancreas. Surgery. 2004;135:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Lamme B, Boermeester MA, Straatsburg IH, van Buijtenen JM, Boerma D, Offerhaus GJ, Gouma DJ, van Gulik TM. Early versus late surgical drainage for obstructive pancreatitis in an experimental model. Br J Surg. 2007;94:849-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Dumonceau JM, Kapral C, Aabakken L, Papanikolaou IS, Tringali A, Vanbiervliet G, Beyna T, Dinis-Ribeiro M, Hritz I, Mariani A, Paspatis G, Radaelli F, Lakhtakia S, Veitch AM, van Hooft JE. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2020;52:127-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 494] [Article Influence: 98.8] [Reference Citation Analysis (1)] |
| 16. | Drewes AM, Bouwense SAW, Campbell CM, Ceyhan GO, Delhaye M, Demir IE, Garg PK, van Goor H, Halloran C, Isaji S, Neoptolemos JP, Olesen SS, Palermo T, Pasricha PJ, Sheel A, Shimosegawa T, Szigethy E, Whitcomb DC, Yadav D; Working group for the International (IAP – APA – JPS – EPC) Consensus Guidelines for Chronic Pancreatitis. Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology. 2017;17:720-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 17. | Kawashima Y, Kawaguchi Y, Kawanishi A, Ogawa M, Hirabayashi K, Nakagohri T, Mine T. Comparison between Endoscopic Treatment and Surgical Drainage of the Pancreatic Duct in Chronic Pancreatitis. Tokai J Exp Clin Med. 2018;43:117-121. [PubMed] |
| 18. | Jiang L, Ning D, Cheng Q, Chen XP. Endoscopic versus surgical drainage treatment of calcific chronic pancreatitis. Int J Surg. 2018;54:242-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Hong J, Wang J, Keleman AM, Imagawa DK, Xu K, Wang W, Liu E, Niu W, Sun Q, Peng C, Zhao W, Niu J. Endoscopic versus surgical treatment of downstream pancreatic duct stones in chronic pancreatitis. Am Surg. 2011;77:1531-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Cahen DL, Gouma DJ, Laramée P, Nio Y, Rauws EA, Boermeester MA, Busch OR, Fockens P, Kuipers EJ, Pereira SP, Wonderling D, Dijkgraaf MG, Bruno MJ. Long-term outcomes of endoscopic vs surgical drainage of the pancreatic duct in patients with chronic pancreatitis. Gastroenterology. 2011;141:1690-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 21. | Issa Y, Kempeneers MA, Bruno MJ, Fockens P, Poley JW, Ahmed Ali U, Bollen TL, Busch OR, Dejong CH, van Duijvendijk P, van Dullemen HM, van Eijck CH, van Goor H, Hadithi M, Haveman JW, Keulemans Y, Nieuwenhuijs VB, Poen AC, Rauws EA, Tan AC, Thijs W, Timmer R, Witteman BJ, Besselink MG, van Hooft JE, van Santvoort HC, Dijkgraaf MG, Boermeester MA; Dutch Pancreatitis Study Group. Effect of Early Surgery vs Endoscopy-First Approach on Pain in Patients With Chronic Pancreatitis: The ESCAPE Randomized Clinical Trial. JAMA. 2020;323:237-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 22. | Fishman DS, Tarnasky PR, Patel SN, Raijman I. Management of pancreaticobiliary disease using a new intra-ductal endoscope: the Texas experience. World J Gastroenterol. 2009;15:1353-1358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 70] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Guda NM, Partington S, Freeman ML. Extracorporeal shock wave lithotripsy in the management of chronic calcific pancreatitis: a meta-analysis. JOP. 2005;6:6-12. [PubMed] |
| 24. | Tandan M, Reddy DN, Santosh D, Vinod K, Ramchandani M, Rajesh G, Rama K, Lakhtakia S, Banerjee R, Pratap N, Venkat Rao G. Extracorporeal shock wave lithotripsy and endotherapy for pancreatic calculi-a large single center experience. Indian J Gastroenterol. 2010;29:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Tandan M, Nageshwar Reddy D. Endotherapy in chronic pancreatitis. World J Gastroenterol. 2013;19:6156-6164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 26. | Dumonceau JM, Delhaye M, Tringali A, Arvanitakis M, Sanchez-Yague A, Vaysse T, Aithal GP, Anderloni A, Bruno M, Cantú P, Devière J, Domínguez-Muñoz JE, Lekkerkerker S, Poley JW, Ramchandani M, Reddy N, van Hooft JE. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Updated August 2018. Endoscopy. 2019;51:179-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 246] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 27. | Ponchon T, Bory RM, Hedelius F, Roubein LD, Paliard P, Napoleon B, Chavaillon A. Endoscopic stenting for pain relief in chronic pancreatitis: results of a standardized protocol. Gastrointest Endosc. 1995;42:452-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 153] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Binmoeller KF, Jue P, Seifert H, Nam WC, Izbicki J, Soehendra N. Endoscopic pancreatic stent drainage in chronic pancreatitis and a dominant stricture: long-term results. Endoscopy. 1995;27:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 174] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Costamagna G, Bulajic M, Tringali A, Pandolfi M, Gabbrielli A, Spada C, Petruzziello L, Familiari P, Mutignani M. Multiple stenting of refractory pancreatic duct strictures in severe chronic pancreatitis: long-term results. Endoscopy. 2006;38:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 147] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Moon SH, Kim MH, Park DH, Song TJ, Eum J, Lee SS, Seo DW, Lee SK. Modified fully covered self-expandable metal stents with antimigration features for benign pancreatic-duct strictures in advanced chronic pancreatitis, with a focus on the safety profile and reducing migration. Gastrointest Endosc. 2010;72:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 31. | Johanson JF, Schmalz MJ, Geenen JE. Incidence and risk factors for biliary and pancreatic stent migration. Gastrointest Endosc. 1992;38:341-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 235] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 32. | Sherman S, Hawes RH, Savides TJ, Gress FG, Ikenberry SO, Smith MT, Zaidi S, Lehman GA. Stent-induced pancreatic ductal and parenchymal changes: correlation of endoscopic ultrasound with ERCP. Gastrointest Endosc. 1996;44:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 76] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Shah JN, Marson F, Weilert F, Bhat YM, Nguyen-Tang T, Shaw RE, Binmoeller KF. Single-operator, single-session EUS-guided anterograde cholangiopancreatography in failed ERCP or inaccessible papilla. Gastrointest Endosc. 2012;75:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 34. | Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. EUS-guided pancreaticogastrostomy: analysis of its efficacy to drain inaccessible pancreatic ducts. Gastrointest Endosc. 2007;65:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Stevens T, Costanzo A, Lopez R, Kapural L, Parsi MA, Vargo JJ. Adding triamcinolone to endoscopic ultrasound-guided celiac plexus blockade does not reduce pain in patients with chronic pancreatitis. Clin Gastroenterol Hepatol. 2012;10:186-191, 191.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Santosh D, Lakhtakia S, Gupta R, Reddy DN, Rao GV, Tandan M, Ramchandani M, Guda NM. Clinical trial: a randomized trial comparing fluoroscopy guided percutaneous technique vs. endoscopic ultrasound guided technique of coeliac plexus block for treatment of pain in chronic pancreatitis. Aliment Pharmacol Ther. 2009;29:979-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Sahai AV, Lemelin V, Lam E, Paquin SC. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: a comparative study of short-term effectiveness. Am J Gastroenterol. 2009;104:326-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 38. | Ahmed Ali U, Pahlplatz JM, Nealon WH, van Goor H, Gooszen HG, Boermeester MA. Endoscopic or surgical intervention for painful obstructive chronic pancreatitis. Cochrane Database Syst Rev. 2015;CD007884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Jawad ZAR, Kyriakides C, Pai M, Wadsworth C, Westaby D, Vlavianos P, Jiao LR. Surgery remains the best option for the management of pain in patients with chronic pancreatitis: A systematic review and meta-analysis. Asian J Surg. 2017;40:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Ma KW, So H, Shin E, Mok JHM, Yuen KHK, Cheung TT, Park DH. Endoscopic versus Surgical Intervention for Painful Obstructive Chronic Pancreatitis: A Systematic Review and Meta-Analysis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (2)] |
| 41. | Link G. V. The Treatment of Chronic Pancreatitis by Pancreatostomy: A New Operation. Ann Surg. 1911;53:768-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Desjardins A. Technique de la pancreatectomy. Rev Chir Paris. 1907;35:945-957. |
| 43. | Coffey RC. XVII. Pancreato-enterostomy and Pancreatectomy: A Preliminary Report. Ann Surg. 1909;50:1238-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Duval MK Jr. Caudal pancreatico-jejunostomy for chronic relapsing pancreatitis. Ann Surg. 1954;140:775-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 118] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Zollinger RM, KEITH LM Jr, ELLISON EH. Pancreatitis. N Engl J Med. 1954;251:497-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Puestow CB, GILLESBY WJ. Retrograde surgical drainage of pancreas for chronic relapsing pancreatitis. AMA Arch Surg. 1958;76:898-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 193] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Partington PF, ROCHELLE RE. Modified Puestow procedure for retrograde drainage of the pancreatic duct. Ann Surg. 1960;152:1037-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 217] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 48. | Bradley EL 3rd. Long-term results of pancreatojejunostomy in patients with chronic pancreatitis. Am J Surg. 1987;153:207-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 161] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 49. | Mannell A, Adson MA, McIlrath DC, Ilstrup DM. Surgical management of chronic pancreatitis: long-term results in 141 patients. Br J Surg. 1988;75:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 50. | Taylor RH, Bagley FH, Braasch JW, Warren KW. Ductal drainage or resection for chronic pancreatitis. Am J Surg. 1981;141:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Fry WJ, Child CG 3rd. Ninety-five per cent distal pancreatectomy for chronic pancreatitis. Ann Surg. 1965;162:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 52. | Beger HG, Witte C, Krautzberger W, Bittner R. [Experiences with duodenum-sparing pancreas head resection in chronic pancreatitis]. Chirurg. 1980;51:303-307. [PubMed] |
| 53. | Warren WD, Millikan WJ Jr, Henderson JM, Hersh T. A denervated pancreatic flap for control of chronic pain in pancreatitis. Surg Gynecol Obstet. 1984;159:581-583. [PubMed] |
| 54. | Frey CF, Smith GJ. Description and rationale of a new operation for chronic pancreatitis. Pancreas. 1987;2:701-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 267] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 55. | Izbicki JR, Yekebas EF. Chronic pancreatitis--lessons learned. Br J Surg. 2005;92:1185-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 56. | Gloor B, Friess H, Uhl W, Büchler MW. A modified technique of the Beger and Frey procedure in patients with chronic pancreatitis. Dig Surg. 2001;18:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Ho HS, Frey CF. The Frey procedure: local resection of pancreatic head combined with lateral pancreaticojejunostomy. Arch Surg. 2001;136:1353-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Frey CF, Amikura K. Local resection of the head of the pancreas combined with longitudinal pancreaticojejunostomy in the management of patients with chronic pancreatitis. Ann Surg. 1994;220:492-504; discussion 504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 59. | Sakata N, Egawa S, Motoi F, Goto M, Matsuno S, Katayose Y, Unno M. How much of the pancreatic head should we resect in Frey's procedure? Surg Today. 2009;39:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 60. | Tan CL, Zhang H, Yang M, Li SJ, Liu XB, Li KZ. Role of original and modified Frey's procedures in chronic pancreatitis. World J Gastroenterol. 2016;22:10415-10423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Zhao X, Cui N, Wang X, Cui Y. Surgical strategies in the treatment of chronic pancreatitis: An updated systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2017;96:e6220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Hamad A, Zenati MS, Nguyen TK, Hogg ME, Zeh HJ 3rd, Zureikat AH. Safety and feasibility of the robotic platform in the management of surgical sequelae of chronic pancreatitis. Surg Endosc. 2018;32:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Galvez D, Sorber R, Javed AA, He J. Technical considerations for the fully robotic pancreaticoduodenectomy. J Vis Surg. 2017;3:81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Giannini A, D'Oria O, Chiantera V, Margioula-Siarkou C, Di Donna MC, Terzic S, Sleiman Z, Laganà AS. Minimally Invasive Surgery for Cervical Cancer: Should We Look beyond Squamous Cell Carcinoma? J Invest Surg. 2022;35:1602-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 65. | Cuschieri A. Laparoscopy for pancreatic cancer: does it benefit the patient? Eur J Surg Oncol. 1988;14:41-44. [PubMed] |
| 66. | Pietrabissa A, Caramella D, Di Candio G, Carobbi A, Boggi U, Rossi G, Mosca F. Laparoscopy and laparoscopic ultrasonography for staging pancreatic cancer: critical appraisal. World J Surg. 1999;23:998-1002; discussion 1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 67. | Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8:408-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 656] [Article Influence: 21.2] [Reference Citation Analysis (1)] |
| 68. | Cuschieri A. Laparoscopic surgery of the pancreas. J R Coll Surg Edinb. 1994;39:178-184. [PubMed] |
| 69. | Cuschieri A, Jakimowicz JJ, van Spreeuwel J. Laparoscopic distal 70% pancreatectomy and splenectomy for chronic pancreatitis. Ann Surg. 1996;223:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 154] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 70. | Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 770] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 71. | Zureikat AH, Moser AJ, Boone BA, Bartlett DL, Zenati M, Zeh HJ 3rd. 250 robotic pancreatic resections: safety and feasibility. Ann Surg. 2013;258:554-9; discussion 559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 268] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 72. | Kurian MS, Gagner M. Laparoscopic side-to-side pancreaticojejunostomy (Partington-Rochelle) for chronic pancreatitis. J Hepatobiliary Pancreat Surg. 1999;6:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 73. | Tantia O, Jindal MK, Khanna S, Sen B. Laparoscopic lateral pancreaticojejunostomy: our experience of 17 cases. Surg Endosc. 2004;18:1054-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 74. | Palanivelu C, Shetty R, Jani K, Rajan PS, Sendhilkumar K, Parthasarthi R, Malladi V. Laparoscopic lateral pancreaticojejunostomy: a new remedy for an old ailment. Surg Endosc. 2006;20:458-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 75. | Khaled YS, Ammori BJ. Laparoscopic lateral pancreaticojejunostomy and laparoscopic Berne modification of Beger procedure for the treatment of chronic pancreatitis: the first UK experience. Surg Laparosc Endosc Percutan Tech. 2014;24:e178-e182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 76. | Sahoo MR, Kumar A. Laparoscopic longitudinal pancreaticojejunostomy using cystoscope and endoscopic basket for clearance of head and tail stones. Surg Endosc. 2014;28:2499-2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 77. | Kim EY, Hong TH. Laparoscopic Longitudinal Pancreaticojejunostomy Using Barbed Sutures: an Efficient and Secure Solution for Pancreatic Duct Obstructions in Patients with Chronic Pancreatitis. J Gastrointest Surg. 2016;20:861-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 78. | Bhandarwar A, Arora E, Gajbhiye R, Gandhi S, Patel C, Wagh A, Kothari P, Jadhav S. Laparoscopic lateral pancreaticojejunostomy: an evolution to endostapled technique. Surg Endosc. 2019;33:1749-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | Rege S, Singh A, Rewatkar A, Murugan J, Menezes R, Surpam S, Chiranjeev R. Laparoscopic parastomal hernia repair: A modified technique of mesh placement in Sugarbaker procedure. J Minim Access Surg. 2019;15:224-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 80. | Javed A, Kiran SBD, Aravinda PS, Saravanan MN, Agarwal AK. Laparoscopic lateral pancreatico-jejunostomy: an experience from a tertiary care center. Trop Gastroenterol. 2020;41:66-72. [DOI] [Full Text] |
| 81. | Nag HH, Nekarakanti PK, Arvinda PS, Sharma A. Laparoscopic versus open surgical management of patients with chronic pancreatitis: Amatched case-control study. J Minim Access Surg. 2022;18:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 82. | Criss CN, Gadepalli SK. Sponsoring surgeons: An investigation on the influence of the da Vinci robot. Am J Surg. 2018;216:84-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 83. | Meehan JJ, Sawin R. Robotic lateral pancreaticojejunostomy (Puestow). J Pediatr Surg. 2011;46:e5-e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Eid GM, Entabi F, Watson AR, Zuckerbraun BS, Wilson MA. Robotic-assisted laparoscopic side-to-side lateral pancreaticojejunostomy. J Gastrointest Surg. 2011;15:1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 85. | Kirks RC, Lorimer PD, Fruscione M, Cochran A, Baker EH, Iannitti DA, Vrochides D, Martinie JB. Robotic longitudinal pancreaticojejunostomy for chronic pancreatitis: Comparison of clinical outcomes and cost to the open approach. Int J Med Robot. 2017;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Khan AS, Siddiqui I, Vrochides D, Martinie JB. Robotic pancreas drainage procedure for chronic pancreatitis: robotic lateral pancreaticojejunostomy (Puestow procedure). J Vis Surg. 2018;4:72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 87. | Balduzzi A, Zwart MJW, Kempeneers RMA, Boermeester MA, Busch OR, Besselink MG. Robotic Lateral Pancreaticojejunostomy for Chronic Pancreatitis. J Vis Exp. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 88. | Tan CL, Zhang H, Li KZ. Single center experience in selecting the laparoscopic Frey procedure for chronic pancreatitis. World J Gastroenterol. 2015;21:12644-12652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Kilburn DJ, Chiow AKH, Leung U, Siriwardhane M, Cavallucci DJ, Bryant R, O'Rourke NA. Early Experience with Laparoscopic Frey Procedure for Chronic Pancreatitis: a Case Series and Review of Literature. J Gastrointest Surg. 2017;21:904-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 90. | Senthilnathan P, Subrahmaneswara Babu N, Vikram A, Sabnis SC, Srivatsan Gurumurthy S, Anand Vijai N, Nalankilli VP, Palanivelu C. Laparoscopic longitudinal pancreatojejunostomy and modified Frey's operation for chronic calcific pancreatitis. BJS Open. 2019;3:666-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 91. | Zureikat AH, Nguyen KT, Bartlett DL, Zeh HJ, Moser AJ. Robotic-assisted major pancreatic resection and reconstruction. Arch Surg. 2011;146:256-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 92. | Shukla A, Gnanasekaran S, Kalayarasan R, Pottakkat B. Early experience with robot-assisted Frey's procedure surgical outcome and technique: Indian perspective. J Minim Invasive Surg. 2022;25:145-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (2)] |
| 94. | Cai H, Cai Y, Wang X, Peng B. Laparoscopic Beger procedure for the treatment of chronic pancreatitis: a single-centre first experience. BMC Surg. 2020;20:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 95. | Hong D, Cheng J, Wu W, Liu X, Zheng X. How to Perform Total Laparoscopic Duodenum-Preserving Pancreatic Head Resection Safely and Efficiently with Innovative Techniques. Ann Surg Oncol. 2021;28:3209-3216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 96. | Strobel O, Büchler MW, Werner J. [Duodenum-preserving pancreatic head resection: technique according to Beger, technique according to Frey and Berne modifications]. Chirurg. 2009;80:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 97. | Cai Y, Zheng Z, Gao P, Li Y, Peng B. Laparoscopic duodenum-preserving total pancreatic head resection using real-time indocyanine green fluorescence imaging. Surg Endosc. 2021;35:1355-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 98. | Peng CH, Shen BY, Deng XX, Zhan Q, Han B, Li HW. Early experience for the robotic duodenum-preserving pancreatic head resection. World J Surg. 2012;36:1136-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Jiang Y, Jin JB, Zhan Q, Deng XX, Peng CH, Shen BY. Robot-assisted duodenum-preserving pancreatic head resection with pancreaticogastrostomy for benign or premalignant pancreatic head lesions: a single-centre experience. Int J Med Robot. 2018;14:e1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 100. | Abu-El-Haija M, Anazawa T, Beilman GJ, Besselink MG, Del Chiaro M, Demir IE, Dennison AR, Dudeja V, Freeman ML, Friess H, Hackert T, Kleeff J, Laukkarinen J, Levy MF, Nathan JD, Werner J, Windsor JA, Neoptolemos JP, Sheel ARG, Shimosegawa T, Whitcomb DC, Bellin MD. The role of total pancreatectomy with islet autotransplantation in the treatment of chronic pancreatitis: A report from the International Consensus Guidelines in chronic pancreatitis. Pancreatology. 2020;20:762-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 101. | Fan CJ, Hirose K, Walsh CM, Quartuccio M, Desai NM, Singh VK, Kalyani RR, Warren DS, Sun Z, Hanna MN, Makary MA. Laparoscopic Total Pancreatectomy With Islet Autotransplantation and Intraoperative Islet Separation as a Treatment for Patients With Chronic Pancreatitis. JAMA Surg. 2017;152:550-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 102. | Galvani CA, Rodriguez Rilo H, Samamé J, Porubsky M, Rana A, Gruessner RW. Fully robotic-assisted technique for total pancreatectomy with an autologous islet transplant in chronic pancreatitis patients: results of a first series. J Am Coll Surg. 2014;218:e73-e78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 103. | Blair AB, Burkhart RA, Hirose K, Makary MA. Laparoscopic total pancreatectomy with islet autotransplantation for chronic pancreatitis. J Vis Surg. 2016;2:121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 104. | Zureikat AH, Nguyen T, Boone BA, Wijkstrom M, Hogg ME, Humar A, Zeh H 3rd. Robotic total pancreatectomy with or without autologous islet cell transplantation: replication of an open technique through a minimal access approach. Surg Endosc. 2015;29:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 105. | Nathan JD, Yang Y, Eaton A, Witkowski P, Wijkstrom M, Walsh M, Trikudanathan G, Singh VK, Schwarzenberg SJ, Pruett TL, Posselt A, Naziruddin B, Mokshagundam SP, Morgan K, Lara LF, Kirchner V, He J, Gardner TB, Freeman ML, Ellery K, Conwell DL, Chinnakotla S, Beilman GJ, Ahmad S, Abu-El-Haija M, Hodges JS, Bellin MD. Surgical approach and short-term outcomes in adults and children undergoing total pancreatectomy with islet autotransplantation: A report from the Prospective Observational Study of TPIAT. Pancreatology. 2022;22:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |