Published online Apr 7, 2023. doi: 10.3748/wjg.v29.i13.2015
Peer-review started: November 21, 2022
First decision: January 11, 2023
Revised: January 16, 2023
Accepted: March 20, 2023
Article in press: March 20, 2023
Published online: April 7, 2023
Processing time: 136 Days and 15.8 Hours
Nearly 290000 patients with chronic hepatitis C die annually from the most severe complications of the disease. One of them is liver cirrhosis, which occurs in about 20% of patients chronically infected with the hepatitis C virus (HCV). Direct-acting antivirals (DAAs), which replaced interferon (IFN)-based regimens, significantly improved the prognosis of this group of patients, increasing HCV eradication rates and tolerability of therapy. Our study is the first to assess changes in patient profile, effectiveness, and safety in the HCV-infected cirrhotic population in the IFN-free era.
To document changes in patient characteristics and treatment regimens along with their effectiveness and safety profile over the years.
The studied patients were selected from 14801 chronically HCV-infected individuals who started IFN-free therapy between July 2015 and December 2021 in 22 Polish hepatology centers. The retrospective analysis was conducted in real-world clinical practice based on the EpiTer-2 multicenter database. The measure of treatment effectiveness was the percentage of sustained virologic response (SVR) calculated after excluding patients lost to follow-up. Safety data collected during therapy and the 12-wk post-treatment period included information on adverse events, including serious ones, deaths, and treatment course.
The studied population (n = 3577) was balanced in terms of gender in 2015-2017, while the following years showed the dominance of men. The decline in the median age from 60 in 2015-2016 to 57 years in 2021 was accompanied by a decrease in the percentage of patients with comorbidities and comedications. Treatment-experienced patients dominated in 2015-2016, while treatment-naive individuals gained an advantage in 2017 and reached 93.2% in 2021. Genotype (GT)-specific options were more prevalent in treatment in 2015-2018 and were supplanted by pangenotypic combinations in subsequent years. The effectiveness of the therapy was comparable regardless of the period analyzed, and patients achieved an overall response rate of 95%, with an SVR range of 72.9%-100% for the different therapeutic regimens. Male gender, GT3 infection, and prior treatment failure were identified as independent negative predictors of therapeutic success.
We have documented changes in the profile of HCV-infected cirrhotic patients over the years of accessibility to changing DAA regimens, confirming the high effectiveness of IFN-free therapy in all analyzed periods.
Core Tip: Patients with cirrhosis in the course of chronic infection with the hepatitis C virus, in whom the risk of death due to advanced liver disease is the highest, seem to be the greatest beneficiaries of the introduction of therapies with direct-acting antiviral drugs. Our analysis tracking changes in the profile of these patients documents the very high effectiveness and good safety profile from the beginning of the interferon-free era to the present.
- Citation: Brzdęk M, Zarębska-Michaluk D, Rzymski P, Lorenc B, Kazek A, Tudrujek-Zdunek M, Janocha-Litwin J, Mazur W, Dybowska D, Berak H, Parfieniuk-Kowerda A, Klapaczyński J, Sitko M, Sobala-Szczygieł B, Piekarska A, Flisiak R. Changes in characteristics of patients with hepatitis C virus-related cirrhosis from the beginning of the interferon-free era. World J Gastroenterol 2023; 29(13): 2015-2033
- URL: https://www.wjgnet.com/1007-9327/full/v29/i13/2015.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i13.2015
Chronic infection with the hepatitis C virus (HCV) remains a global health problem, affecting 58 million people worldwide, according to the most recent estimates of the World Health Organization (WHO) published in 2022[1]. Despite its initially asymptomatic course, untreated chronic hepatitis C (CHC) can lead to progressive liver disease resulting in cirrhosis. Among HCV-infected patients, about 20% are at risk of developing cirrhosis after an average of about 20 years of infection[2], and 3%-6% of them develop decompensation of liver function each year[3]. Hepatic decompensation and hepatocellular carcinoma (HCC) are reported as the most severe complications of CHC, causing globally nearly 290000 deaths each year[1].
The antiviral treatment of patients with cirrhosis, as they are at risk of developing these most severe and potentially fatal complications, is crucial in achieving the WHO goal formulated in 2016, which assumes the reduction of mortality due to HCV by 65% by 2030[1]. In the era of interferon (IFN)-based antiviral regimens, therapeutic options were severely limited due to contraindications and poor safety profile in this patient population[4]. Even the introduction in 2011 of the first direct-acting antivirals (DAAs) and HCV protease inhibitors (telaprevir and boceprevir), which were registered for use with pegylated IFN, was not a breakthrough for this group of patients due to the limitations still associated with IFN[5]. Only the availability of IFN-free therapies based exclusively on a combination of DAA agents has significantly improved safety parameters and treatment efficacy, representing a real revolution in managing HCV-infected patients with cirrhosis, including those with decompensation[6]. As a result, at the beginning of the DAA era, cirrhotic patients were prioritized in access to treatment, with some countries even limiting therapy reimbursement to this group of patients[7]. The findings of a number of clinical trials and real-world experience (RWE) studies involving this particular patient population published to date indicate that, despite the lack of contraindications to DAA therapy, the presence of cirrhosis is still considered an unfavorable factor for achieving a sustained virologic response (SVR)[8,9].
Recently, several RWE studies have been conducted in different world parts, summarizing the effectiveness of DAA treatment and changes in demographic and clinical characteristics in HCV-infected patients treated with DAA regimens[10-14]. However, none of these have focused on cirrhotic patients, while filling key knowledge gaps regarding this population could support the WHO’s goal of eliminating HCV as a major public health burden by 2030.
Therefore, the present study aimed to track changes in the profile of HCV-infected patients with cirrhosis treated with DAA options, along with documenting the evolution of antiviral regimens in RWE practice over seven years of access to IFN-free therapy. To this end, a retrospective analysis of the Polish population of HCV-infected patients with cirrhosis who were treated between 2015 and 2021 was conducted.
The study population was selected from 14801 adult patients infected with HCV who started IFN-free antiviral treatment in 22 Polish hepatology centers from the beginning of the DAA availability between July 1, 2015 and December 31, 2021. The analysis was a part of the EpiTer-2 database, a retrospective national study evaluating antiviral therapy of HCV-infected patients in routine clinical practice, supported by the Polish Association of Epidemiologists and Infectiologists. The present study consisted of all consecutive CHC patients with liver cirrhosis treated with IFN-free therapy, reimbursed by the Polish National Health Fund (NFZ). Since the beginning of the availability of DAA regimens in Poland, there have been no restrictions related to the severity of liver disease or history of previous therapy in the qualification of patients for antiviral treatment. The choice of regimen, dose, and length of treatment course was at the discretion of the treating physician based on the available therapeutic options, and treatment was administered following the product characteristics, the protocol of the NFZ therapeutic program, and the recommendations of the Polish Group of Experts for HCV[15-19]. Before starting treatment, the patient signed a consent form as required by the current regulations of the therapeutic program.
The data were collected retrospectively with an online questionnaire operated by Tiba LLC based on the medical records. The study was carried out by comparison of six groups of patients diagnosed with liver cirrhosis who were divided based on the time of treatment initiation: 2015-2016, 2017, 2018, 2019, 2020, and 2021. Parameters gathered at baseline included demographic and clinical data: Gender, age, body mass index, HCV genotype (GT), comorbidities, concomitant medications, information on the severity of the liver disease, coinfections of the human immunodeficiency virus (HIV) and hepatitis B virus (HBV), and history of previous antiviral therapy. Baseline laboratory parameters were recorded, including serum alanine transaminase activity, bilirubin concentrations, albumin, creatinine, hemoglobin, platelet count, and HCV viral load. The patient groups were compared regarding the treatment regimens used and their efficacy and safety outcomes.
The advancement of liver disease was evaluated by assessing liver stiffness using real-time shear wave elastography with an Aixplorer (SuperSonic Imagine, Aix-en-Provence, France) or transient elastography with the usage of FibroScan (Echosens, France). Based on the METAVIR score, according to the European Association for the Study of the Liver guidelines, the cutoff value of 13 kPa was used for the prediction of individuals with F4 who were considered to be cirrhotic[20]. The patients were scored on Child-Pugh (CP) and model for end-stage liver disease, and data on the presence of esophageal varices, past or present hepatic decompensation and the history of HCC, and liver transplantation were collected. Patients who scored as B or C on the CP scale were considered decompensated.
The efficacy endpoint of the study was SVR. It was defined as undetectable HCV RNA at least 12 wk after completion of treatment. Patients with detectable HCV RNA at this time point were identified as virologic non-responders, whereas those with no HCV RNA assessment 12 wk after the end of treatment were considered lost to follow-up. Depending on local practices at the testing site, the concentration of HCV RNA was measured using COBAS TaqMan HCV v2.0 (Roche Molecular Diagnostics, Pleasanton, CA, United States), COBAS AmpliPrep HCV (Roche Molecular Diagnostics, Pleasanton, CA, United States), the m2000 Real-Time System (Abbott Molecular, Des Plaines, IL, United States), or the Xpert HCV Viral Load real-time assay (Cepheid, Sunnyvale, California, United States).
Through treatment and 12 wk after its completion, the following safety data were collected: The occurrence of adverse events (AEs), including severe AEs, and death, as well as the rates of modification or discontinuation of the therapy course. In addition, AEs of special interest related to the deterioration of liver function involving gastrointestinal bleeding, ascites, and encephalopathy were reported.
The data were originally collected not for scientific purposes but to evaluate treatment efficacy and safety in real-world settings with registered medications. Patients were not exposed to any experimental interventions. According to the local law (the Polish Pharmaceutical Law of 6 September 2001, art. 37al), noninterventional studies do not require ethics committee approval. Due to the retrospective design of the analysis, additional consent from patients was not required, but as mentioned, they signed a consent form to enter the therapeutic program. Data of patients were collected and analyzed following applicable data protection rules.
Categorical data are presented as numbers and percentages, whereas continuous data are expressed as the mean (SD) or median and interquartile ranges. The SVR was evaluated for all patients who initiated the treatment after the exclusion of those lost to follow-up as per protocol analysis. Statistical analyses were performed using Statistica v. 13 (StatSoft, Tulsa, OK, United States). Multiple logistic regression was used to predict the odds of no response to HCV treatment based on predictor variables selected through univariate analysis.
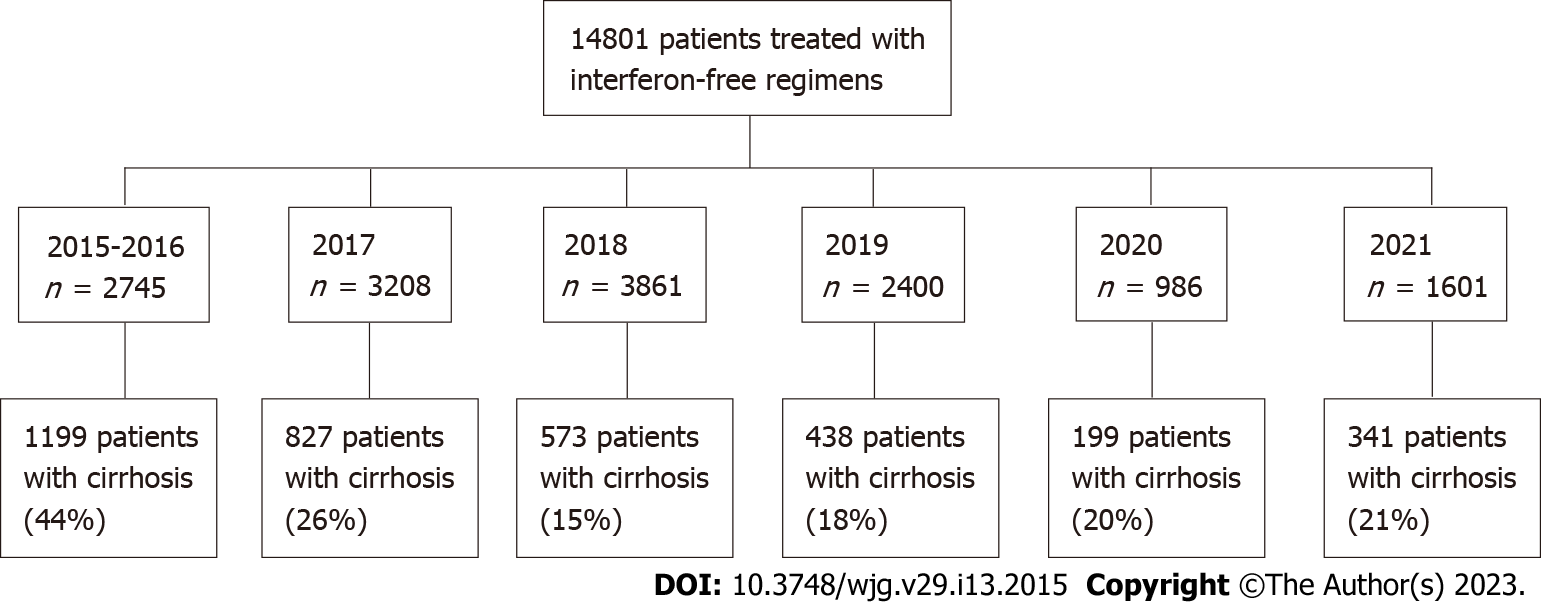
The studied population consisted of 3577 patients selected from 14801 individuals treated with IFN-free regimens included in the EpiTer-2 database. They were divided into six groups based on the date of treatment initiation: (1) July 1, 2015 to December 31, 2016; (2) January 1, 2017 to December 31, 2017; (3) January 1, 2018 to December 31, 2018; (4) January 1, 2019 to December 31, 2019; (5) January 1, 2020 to December 31, 2020; and (6) January 1, 2021 to December 31, 2021 (Figure 1).
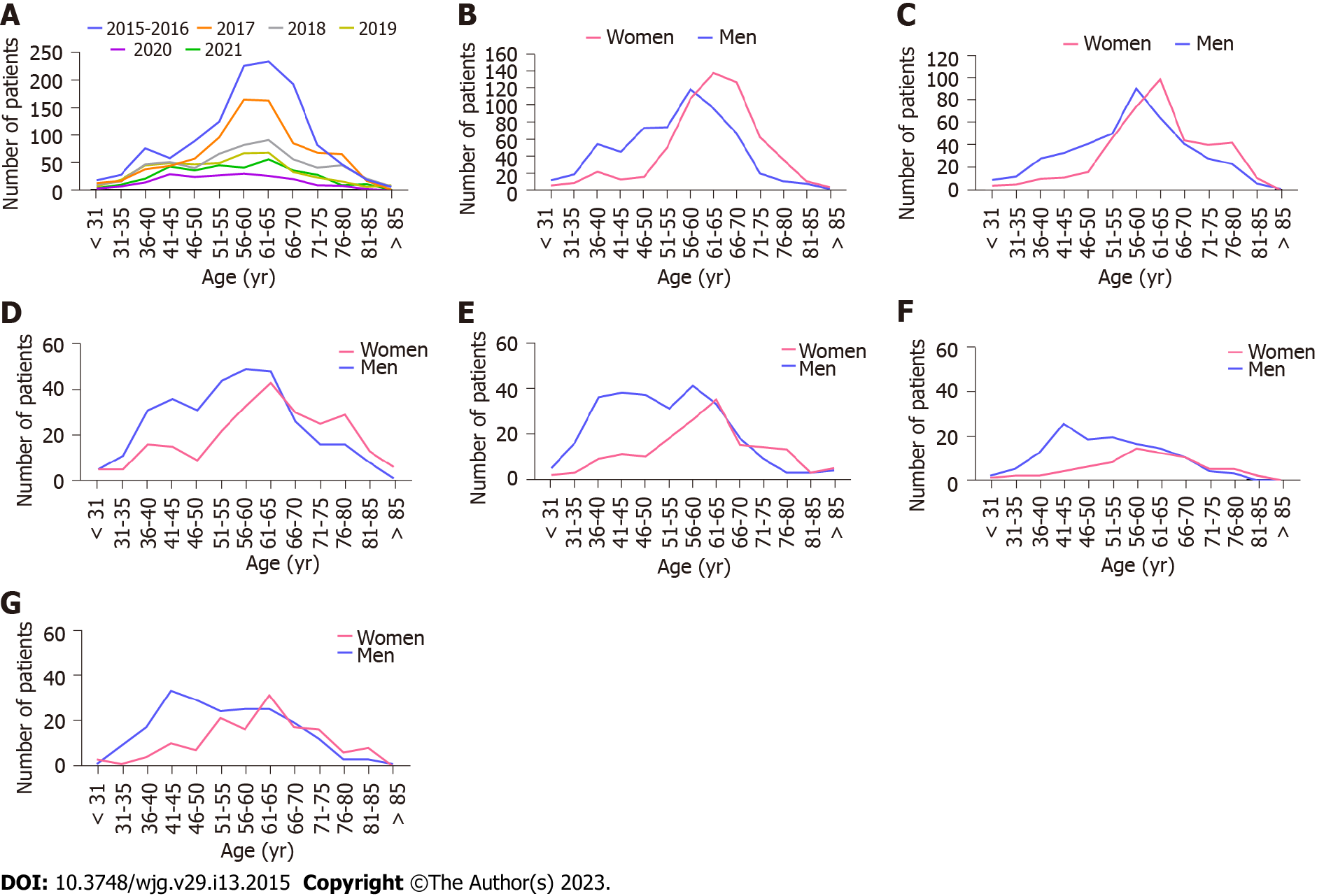
Between the first period analyzed and 2018, the percentage of cirrhotic patients treated with antiviral therapy significantly decreased from 43.7% to 14.8%, but they began to rise again in 2019 to a value of 21.3% in 2021. The analyzed population was sex-balanced until 2017, while men’s predominance was observed for consecutive years. Between the first and the last time interval, a minor reduction in the median (Q1, Q3) age was documented in women: From 63 (57, 69) in 2015-2016 to 61 (53, 68.5) years in 2021 and slightly greater reduction in men age: From 57 (47, 63) in 2015-2016 to 52 (44, 63) years in 2021 (Table 1). Women were older than men, with a peak of around 60-70 years in all the analyzed periods (Figure 2).
| Parameter | 2015-2016 | 2017 | 2018 | 2019 | 2020 | 2021 |
| Number of patients | 1199 | 827 | 573 | 438 | 199 | 341 |
| Gender, females/males, n (%) | 600 (50)/599 (50) | 401 (48.5)/426 (51.5) | 251 (43.8)/322 (56.2) | 164 (37.4)/274 (62.6) | 71 (35.7)/128 (64.3) | 140 (41.1)/201 (58.9) |
| Age (yr), mean ± SD; min-max | 58.6 ± 11.8; 21-97 | 59.4 ± 12.0; 22-87 | 58.2 ± 13.8; 21-89 | 55.2 ± 13.2; 26-91 | 54.6 ± 12.1; 28-83 | 56.6 ± 12.6; 25-86 |
| Females | 62.2 ± 10.8; 25-91 | 62.1 ± 11; 25-85 | 61.8 ± 14.2; 21-89 | 60.3 ± 12.8; 30-91 | 59.4 ± 12.2; 29-83 | 60.5 ± 12.3; 25-85 |
| Males | 55.1 ± 11.8; 21-97 | 56.8 ± 12.3; 22-87 | 55.4 ± 12.8; 27-88 | 52.1 ± 12.5; 26-89 | 51.9 ± 11.2; 28-80 | 53.9 ± 12.1; 30-86 |
| Median (Q1, Q3) | 60 (52, 67) | 60 (52, 67) | 59 (48, 68) | 56 (45, 63) | 55 (45, 63) | 57 (47, 65) |
| Females, median (Q1, Q3) | 63 (57, 69) | 62 (56, 70) | 62 (54, 73) | 61 (52.5, 68) | 60 (52, 68) | 61 (53, 68.5) |
| Males, median (Q1, Q3) | 57 (47, 63) | 57 (48, 65) | 56 (45, 64) | 51 (42, 61) | 52 (44, 60) | 52 (44, 63) |
| BMI, mean ± SD; min-max | 27.3 ± 4.5; 13.4-49.4 | 27.5 ± 4.8; 14.2-45.5 | 27.1 ± 4.7; 21-89 | 27.7 ± 5.2; 17-52.5 | 27.9 ± 5.6; 16-57.4 | 27.5 ± 4.9; 15.6-50 |
| Comorbidities, n (%) | ||||||
| Any comorbidity | 921 (76.8) | 682 (82.5) | 431 (75.2) | 303 (69.2) | 141 (70.9) | 252 (73.9) |
| Hypertension | 582 (48.5) | 384 (46.4) | 279 (48.7) | 179 (40.9) | 72 (36.2) | 158 (46.3) |
| Diabetes | 263 (21.9) | 212 (25.6) | 120 (20.9) | 76 (17.4) | 44 (22.1) | 136 (27) |
| Renal disease | 48 (4) | 39 (4.7) | 20 (3.5) | 10 (2.3) | 9 (4.5) | 12 (3.5) |
| Autoimmune diseases | 29 (2.4) | 14 (1.7) | 8 (1.4) | 9 (2.1) | 2 (1) | 3 (0.9) |
| Non-HCC tumors | 20 (1.7) | 15 (1.8) | 12 (2.1) | 12 (2.7) | 6 (3) | 15 (4.4) |
| Other | 628 (52.4) | 560 (67.7) | 352 (61.4) | 208 (47.5) | 147 (73.9) | 179 (52.5) |
| Concomitant medications, n (%) | 879 (73.3) | 635 (76.8) | 431 (75.2) | 305 (69.6) | 138 (69.3) | 245 (71.8) |
| ALT IU/L, mean ± SD | 97.3 ± 66.2 | 96.4 ± 69.9 | 96 ± 85 | 107.1 ± 91.7 | 114.5 ± 103.8 | 115.9 ± 101.2 |
| Bilirubin mg/dL, mean ± SD | 1.2 ± 0.9 | 1 ± 0.7 | 1.1 ± 0.9 | 1.1 ± 0.9 | 1.1 ± 1.6 | 1.1 ± 0.9 |
| Albumin g/dL, mean ± SD | 5.8 ± 12.8 | 3.8 ± 0.5 | 3.8 ± 0.5 | 3.9 ± 0.5 | 3.8 ± 0.5 | 3.9 ± 0.6 |
| Creatinine mg/dL, mean ± SD | 1.1 ± 3.8 | 0.8 ± 0.5 | 0.9 ± 0.5 | 0.9 ± 0.7 | 0.9 ± 0.3 | 0.9 ± 0.7 |
| Hemoglobin g/dL, mean ± SD | 14.1 ± 1.8 | 14.1 ± 1.8 | 14 ± 1.8 | 14.1 ± 1.9 | 14.2 ± 1.8 | 13.9 ± 1.9 |
| Platelets, × 1000/μL, mean ± SD | 118.7 ± 61.6 | 136.1 ± 67.9 | 135 ± 70 | 136.5 ± 61.9 | 145.8 ± 87.6 | 145.6 ± 76.9 |
| HCV RNA × 106 IU/mL, mean ± SD | 1.6 ± 4.6 | 2.5 ± 6.4 | 2 ± 4.8 | 3.3 ± 24 | 1.7 ± 2.6 | 2 ± 3.2 |
Age distribution in men demonstrated the first peak around age 56 to 65 and the second around age 41 to 45. The first peak was dominant until 2019, whereas the second peak appeared in 2018 and was higher in 2020 and 2021. The patient’s body mass index (BMI) was comparable irrespective of the analyzed time interval (Table 1). The prevalence of comorbidities initially decreased from 76.8% in 2015-2016 to 69.2% in 2019, but increased again in 2020 and 2021 (70.9% and 73.9%, respectively). The proportion of the most frequent comorbidities of hypertension and diabetes ranged from 36.2% in 2020 to 48.7% in 2018 and from 17.4% in 2019 to 27% in 2021, respectively. The rate of patients using concomitant medications decreased from 76.8% in 2017 to 69.3% in 2020, with a slight increase to 71.8% in 2021.
GT1b was the most common in all periods, but a reduction in the prevalence of infections with this GT from 89.2% to 62.8% between 2015 and 2020 was documented (Table 2). A renewed increase in the share of GT1b infection to 72.4% was noted in the last time frame analyzed. In parallel with the decrease in the percentage of GT1b, an increase in the share of GT3 was observed, ranging from 4.7% in 2015-2016 to 30.2% in 2020. Analogically to GT1b, a reversal of the earlier trend was documented in the last period. The percentage of patients classified as CP classes B and C, which was 10.5% in the first period analyzed, initially showed a downward trend, eventually increasing to 15.2% in the last time interval. No tendency was observed in the incidence of a history of hepatic decompensation, HCC, HBV, and HIV coinfection, as well as the presence of decompensation at baseline. Documented esophageal varices and a history of liver transplantation were more commonly observed at the beginning of the IFN-free era.
| Parameter | 2015-2016 | 2017 | 2018 | 2019 | 2020 | 2021 |
| Number of patients | 1199 | 827 | 573 | 438 | 199 | 341 |
| GT, n (%) | ||||||
| 1 | 17 (1.4) | 22 (2.7) | 15 (2.6) | 7 (1.6) | 1 (0.5) | 7 (2.1) |
| 1a | 21 (1.8) | 17 (2.1) | 10 (1.7) | 10 (2.3) | 9 (4.5) | 8 (2.3) |
| 1b | 1070 (89.2) | 652 (78.8) | 420 (73.3) | 291 (66.4) | 125 (62.8) | 247 (72.4) |
| 2 | 0 | 0 | 1 (0.2) | 3 (0.7) | 0 | 3 (0.9) |
| 3 | 56 (4.7) | 103 (12.4) | 109 (19.0) | 111 (25.3) | 60 (30.2) | 63 (18.5) |
| 4 | 35 (2.9) | 33 (4.0) | 17 (3) | 16 (3.7) | 4 (2) | 13 (3.8) |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 1 (0.2) | 0 | 0 | 0 |
| Child-Pugh class, n (%) | ||||||
| A | 1033 (86.2) | 724 (87.6) | 521 (90.9) | 397 (90.7) | 176 (88.4) | 285 (83.6) |
| B | 118 (9.8) | 77 (9.3) | 47 (8.2) | 36 (8.2) | 18 (9.1) | 49 (14.3) |
| C | 9 (0.7) | 1 (0.1) | 2 (0.4) | 1 (0.2) | 1 (0.5) | 3 (0.9) |
| No data | 39 (3.3) | 25 (3.0) | 3 (0.5) | 4 (0.9) | 4 (2.0) | 4 (1.2) |
| MELD score, n (%) | ||||||
| < 15 | 1072 (89.4) | 757 (91.5) | 529 (92.3) | 414 (94.5) | 188 (94.5) | 319 (93.5) |
| 15-18 | 45 (3.8) | 28 (3.4) | 29 (5.1) | 12 (2.8) | 3 (1.5) | 14 (4.1) |
| 19-20 | 11 (0.9) | 12 (1.5) | 5 (0.9) | 4 (0.9) | 2 (1) | 4 (1.2) |
| > 20 | 13 (1.1) | 4 (0.5) | 7 (1.2) | 3 (0.7) | 2 (1) | 2 (0.6) |
| No data | 58 (4.8) | 26 (3.1) | 3 (0.5) | 5 (1.1) | 4 (2) | 2 (0.6) |
| History of hepatic decompensation, n (%) | ||||||
| Ascites | 124 (10.3) | 65 (7.8) | 64 (11.2) | 26 (5.9) | 15 (7.5) | 47 (13.8) |
| Encephalopathy | 33 (2.8) | 15 (1.8) | 19 (3.3) | 4 (0.9) | 2 (1) | 7 (2.1) |
| Documented esophageal varices, n (%) | 375 (31.3) | 202 (24.4) | 127 (22.2) | 85 (19.4) | 38 (19.1) | 58 (17) |
| Hepatic decompensation at baseline, n (%) | ||||||
| Moderate ascites-responded to diuretics | 47 (3.9) | 30 (3.6) | 29 (5.1) | 22 (5) | 10 (5) | 29 (8.5) |
| Tense ascites-not responded to diuretics | 3 (0.3) | 0 | 2 (0.3) | 0 | 0 | 3 (0.9) |
| Encephalopathy | 30 (2.5) | 13 (1.6) | 7 (1.2) | 7 (1.6) | 2 (1) | 9 (2.6) |
| HCC history, n (%) | 57 (4.8) | 30 (3.6) | 19 (3.3) | 15 (3.4) | 7 (3.5) | 19 (5.6) |
| OLTx history, n (%) | 23 (1.9) | 4 (0.5) | 1 (0.2) | 1 (0.2) | 0 | 1 (0.3) |
| HBV coinfection (HBsAg+), n (%) | 12 (1) | 12 (1.5) | 12 (2.1) | 8 (1.8) | 3 (1.5) | 6 (1.8) |
| HIV coinfection, n (%) | 13 (1.1) | 16 (1.9) | 16 (2.8) | 28 (6.4) | 9 (4.5) | 10 (2.9) |
There has been a strong and steady trend of an increasing share of treatment-naïve patients over the years (Table 3). At the beginning of the IFN-free era, the majority of those with previous treatment failure had been previously treated with PegIFN ± ribavirin (RBV), and this tendency was observed until 2018. Since 2019, two-thirds of individuals were retreated after the failure of IFN-free treatment. From 2015 to 2018, the most common therapeutic options were GT-specific regimens, particularly ombitasvir (OBV)/paritaprevir (PTV)/[ritonavir (r) ± dasabuvir (DSV) ± RBV] in 2015-2017 and grazoprevir (GZR)/[elbasvir (EBR) ± RBV] in 2018. In subsequent years, these regimens were replaced by pangenotypic options, which were used to treat about 90% of patients as of 2020. In 2019 and 2020, the glecaprevir (GLE)/pibrentasvir (PIB) combination was the most commonly used regimen, while in the last interval, most patients were treated with sofosbuvir (SOF)/[velpatasvir (VEL) ± RBV].
| Parameter | 2015-2016 | 2017 | 2018 | 2019 | 2020 | 2021 |
| Number of patients | 1199 | 827 | 573 | 438 | 199 | 341 |
| History of previous therapy | ||||||
| Treatment-naïve | 543 (45.3) | 532 (64.3) | 470 (82.0) | 385 (87.9) | 171 (86) | 318 (93.2) |
| Nonresponder | 290 (24.2) | 103 (12.5) | 23 (4.0) | 7 (1.6) | 6 (3) | 11 (3.2) |
| Relapser | 173 (14.4) | 72 (8.7) | 49 (8.5) | 35 (8) | 17 (8.5) | 6 (1.8) |
| Discontinuation due to safety reason | 71 (5.9) | 40 (4.8) | 14 (2.5) | 4 (0.9) | 1 (0.5) | 3 (0.9) |
| Unknown type of response | 120 (10.0) | 76 (9.2) | 14 (2.5) | 7 (1.6) | 2 (1) | 3 (0.9) |
| No data | 2 (0.2) | 4 (0.5) | 3 (0.5) | 0 | 2 (1) | 0 |
| Number of patients with treatment failure | n = 654 | n = 291 | n = 100 | n = 53 | n = 26 | n = 23 |
| IFN ± RBV | 64 (9.8) | 36 (12.4) | 2 (2) | 0 | 0 | 0 |
| PegIFN ± RBV | 390 (59.6) | 197 (67.7) | 48 (48) | 14 (26.4) | 9 (34.6) | 9 (39.1) |
| PegIFN + RBV + DAA | 196 (30) | 47 (16.1) | 10 (10) | 3 (5.7) | 0 | 0 |
| IFN-free | 4 (0.6) | 11 (3.8) | 39 (39) | 35 (66) | 17 (65.4) | 14 (60.9) |
| No data | 0 | 0 | 1 (1) | 1 (1.9) | 0 | 0 |
| Current treatment regimen | ||||||
| Genotype-specific treatment regimens | ||||||
| ASV + DCV | 42 (3.5) | 13 (1.6) | 0 | 0 | 0 | 0 |
| LDV/(SOF ± RBV) | 350 (29.2) | 283 (34.2) | 154 (26.9) | 16 (3.7) | 5 (2.5) | 4 (1.2) |
| OBV/PTV/(r ± DSV ± RBV) | 745 (62.1) | 327 (39.5) | 32 (5.6) | 0 | 0 | 1 (0.3) |
| GZR/(EBR ± RBV) | 0 | 97 (11.7) | 205 (35.8) | 76 (17.3) | 16 (8) | 15 (4.4) |
| Other | 62 (5.2) | 104 (12.6) | 19 (3.3) | 0 | 0 | 0 |
| SOF ± SMV ± DCV ± RBV, SMV ± DCV ± RBV | ||||||
| Pangenotypic regimens | ||||||
| GLE/PIB | 0 | 1 (0.1) | 59 (10.3) | 178 (40.6) | 99 (49.8) | 117 (34.3) |
| GLE/(PIB + SOF + RBV) | 0 | 0 | 0 | 2 (0.5) | 0 | 0 |
| SOF/(VEL ± RBV) | 0 | 2 (0.3) | 104 (18.1) | 166 (37.9) | 79 (39.7) | 195 (57.2) |
| VOX/VEL/SOF | 0 | 0 | 0 | 0 | 0 | 9 (2.6) |
| Current-RBV-containing therapies | 855 (71.3) | 360 (43.5) | 163 (28.4) | 44 (10) | 12 (6) | 23 (6.7) |
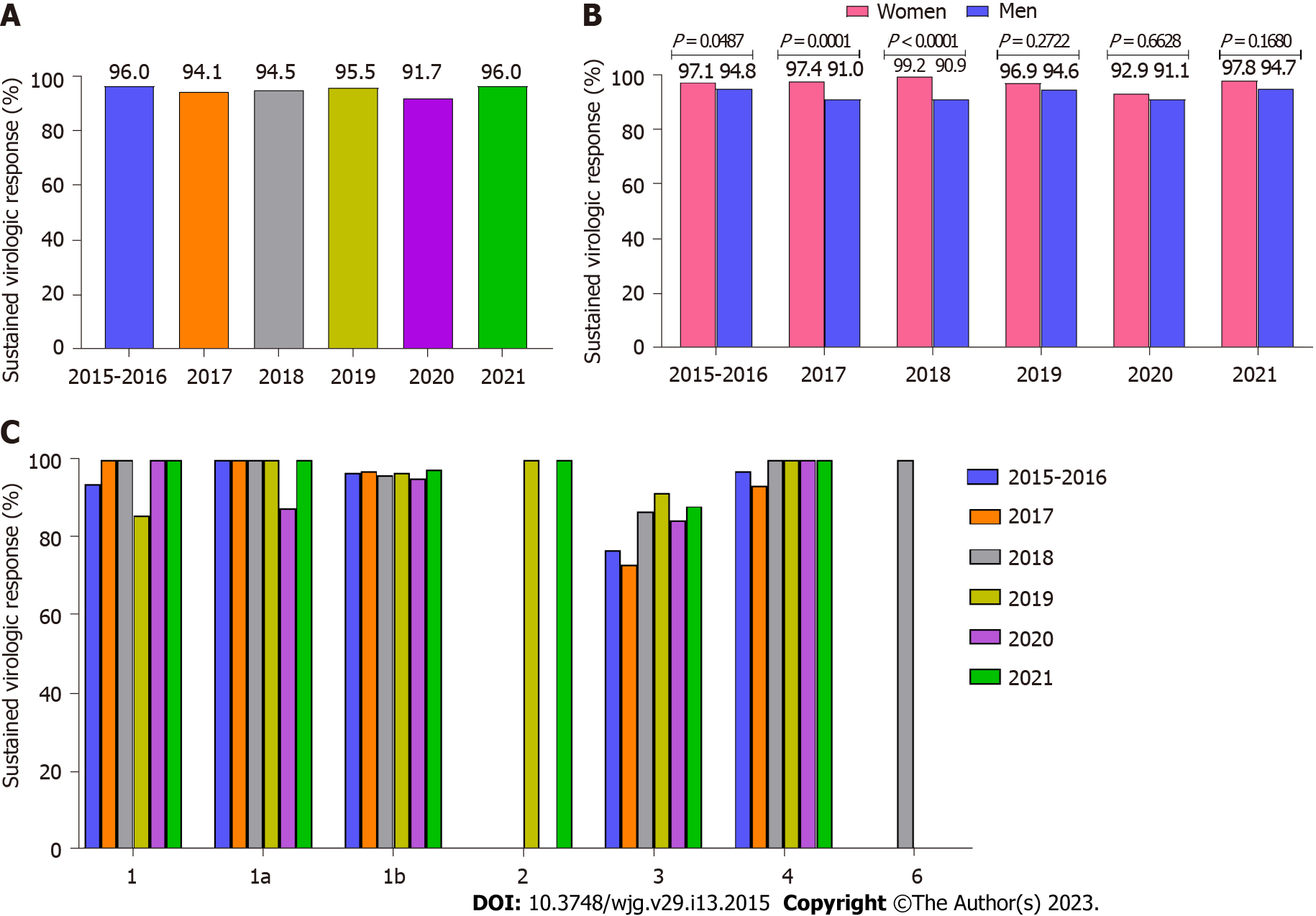
The overall SVR after excluding lost-to-follow-up patients was 95% (Table 4). A slightly higher effectiveness of 97.8% was noted in patients treated with the most common option of OBV/PTV/(r ± DSV ± RBV). A similar, high virologic response rate was achieved with other GT-specific regimens [LDV/(SOF ± RBV), GZR/(EBR ± RBV), and pangenotypic GLE/PIB combinations]. The administration of another pangenotypic option, VEL/(SOF ± RBV), resulted in a 91.6% cure rate. The lowest SVR rates were observed in those treated with SOF + RBV and asunaprevir (ASV) + daclatasvir (DCV) regimens - 72.9% and 85.5%, respectively. The smallest subgroups of patients treated with GLE/PIB/(SOF + RBV), SOF + (DCV ± RBV), SOF + (simeprevir ± RBV), and voxilaprevir (VOX)/VEL/SOF achieved a 100% treatment response.
| Regimen | SVR PP, n (%) |
| All regimens | 3271/3442 (95) |
| OBV/PTV/(r ± DSV ± RBV) | 1052/1076 (97.8) |
| LDV/(SOF ± RBV) | 748/778 (96.1) |
| GZR/(EBR ± RBV) | 387/398 (97.2) |
| VEL/(SOF ± RBV) | 477/521 (91.6) |
| GLE/PIB | 420/435 (96.6) |
| GLE/PIB/(SOF + RBV) | 2/2 (100) |
| ASV + DCV | 47/55 (85.5) |
| SOF + DCV ± RBV | 21/21 (100) |
| SOF + RBV | 105/144 (72.9) |
| SOF + SMV ± RBV | 5/5 (100) |
| VOX/VEL/SOF | 7/7 (100) |
Treatment effectiveness remained nearly similar in all six analyzed time intervals (Figure 3A). Analysis of SVR by GT showed comparable rates for all periods analyzed. The lowest response rates were obtained in GT3-infected patients (Figure 3C). A statistically significant higher SVR was observed in women between the first period analyzed and 2018 (Figure 3B). Univariate analysis showed that patients who did not achieve a virological response were significantly older, were more likely to be male, treatment-experienced, and had a higher BMI (Table 5).
| Parameter | Responders (n = 3271) | Non-responders (n = 171) | P value |
| Gender, females/males, n (%) | 1539 (47)/1732 (53) | 42 (24.6)/129 (75.4) | < 0.0001 |
| Age (yr), mean ± SD; min-max | 58.0 ± 12.6; 21-97 | 55.0 ± 10.5; 29-84 | 0.0002 |
| Females | 61.6 ± 11.8; 21-91 | 59.9 ± 10.6; 35-81 | 0.3173 |
| Males | 54.8 ± 12.4; 21-97 | 53.4 ± 10; 29-84 | 0.1045 |
| BMI, mean ± SD; min-max | 27.4 ± 5.0; 13.4-57.4 | 28.7 ± 4.6; 16-47.5 | 0.0001 |
| Current treatment regimen | |||
| Genotype-specific treatment regimens | |||
| ASV + DCV | 47 (1.4) | 8 (4.7) | 0.0001 |
| LDV/(SOF ± RBV) | 748 (22.9) | 30 (17.5) | 0.1047 |
| OBV/PTV/(r ± DSV ± RBV) | 1052 (32.2) | 24 (14) | < 0.0001 |
| GZR/(EBR ± RBV) | 387 (11.8) | 11 (6.4) | 0.0314 |
| Pangenotypic regimens | |||
| GLE/PIB | 420 (12.8) | 15 (8.8) | 0.1186 |
| GLE/(PIB + SOF + RBV) | 2 (0.1) | 0 | 0.7464 |
| SOF/(VEL ± RBV) | 477 (14.6) | 44 (25.7) | 0.0001 |
| VOX/VEL/SOF | 7 (0.2) | 0 | 0.5448 |
| GT, n (%) | < 0.0001 | ||
| 1 | 63 (1.9) | 2 (1.2) | |
| 1a | 70 (2.2) | 1 (0.6) | |
| 1b | 2624 (80.2) | 89 (52.0) | |
| 3 | 397 (12.1) | 76 (44.4) | |
| 4 | 110 (3.4) | 3 (1.8) | |
| Other | 7 (0.2) | 0 | |
| Comorbidities, n (%) | |||
| Any comorbidity | 2498 (76.4) | 131 (4) | 0.9425 |
| Hypertension | 1533 (46.9) | 66 (2) | 0.0345 |
| Diabetes | 731 (22.3) | 46 (1.4) | 0.1651 |
| Renal disease | 124 (3.8) | 8 (0.2) | 0.5558 |
| Autoimmune diseases | 63 (1.9) | 0 | 0.0670 |
| Non-HCC tumors | 68 (2.1) | 6 (0.2) | 0.1963 |
| Other | 1783 (54.5) | 96 (2.9) | 0.6762 |
| Concomitant medications, n (%) | 2389 (73.0) | 136 (79.5) | 0.0610 |
| Treatment experienced | 1039 (31.8) | 69 (40.4) | 0.0208 |
| History of hepatic decompensation, n (%) | |||
| Ascites | 297 (9.0) | 21 (12.2) | 0.1588 |
| Encephalopathy | 65 (2.0) | 7 (4.1) | 0.0606 |
| Documented esophageal varices, n (%) | 780 (23.8) | 64 (37.4) | 0.0001 |
| Hepatic decompensation at baseline, n (%) | 173 (5.3) | 18 (10.5) | 0.0035 |
| HCC history, n (%) | 117 (3.6) | 9 (5.3) | 0.2523 |
| OLTx history, n (%) | 28 (0.9) | 1 (0.6) | 0.7052 |
| Child-Pugh class, n (%) | |||
| B or C | 296 (9) | 29 (17) | 0.0006 |
| HBV coinfection (HBsAg+), n (%) | 50 (1.5) | 2 (1.2) | 0.7075 |
| HIV coinfection, n (%) | 79 (2.4) | 6 (3.5) | 0.3690 |
| ALT IU/L, mean ± SD | 101.2 ± 80.8 | 106.9 ± 82.0 | 0.3385 |
| Bilirubin mg/dL, mean ± SD | 1.1 ± 0.9 | 1.2 ± 0.7 | < 0.0001 |
| Albumin g/dL, mean ± SD | 4.5 ± 7.7 | 3.9 ± 3.1 | 0.0002 |
| Creatinine mg/dL, mean ± SD | 0.9 ± 2.3 | 0.8 ± 0.2 | 0.8562 |
| Hemoglobin g/dL, mean ± SD | 14.1 ± 1.8 | 13.9 ± 1.8 | 0.1319 |
| Platelets, × 1000/μL, mean ± SD | 132.6 ± 66.8 | 109.2 ± 59.4 | < 0.0001 |
| HCV RNA × 106 IU/ml, mean ± SD | 2.2 ± 10.0 | 2.0 ± 2.9 | 0.1123 |
The non-responding patient population also had a higher percentage of patients with GT3 infection, decompensated liver disease (CP classes B and C), and the presence of esophageal varices and liver decompensation in the form of encephalopathy at baseline. Those not responding virologically to treatment were significantly more likely to receive the ASV + DCV and SOF/(VEL ± RBV) regimens. A statistically significant difference in platelet count, bilirubin, and albumin levels between responders and virologic non-responders was also documented. Independent negative predictors of SVR in the logistic regression analysis were male gender [odds ratio (OR) = 2.28], GT3 infection (OR = 6.4), and prior failed treatment (OR = 1.66), while OBV/PTV/(r ± DSV ± RBV) therapy significantly increased the chance of response (OR = 0.58) (Table 6).
| Parameter | OR | 95%CI | P value |
| Age ≥ 75 yr | 0.81 | 0.38-1.72 | 0.5915 |
| Obesity (BMI ≥ 30 kg/m2) | 1.15 | 0.80-1.66 | 0.4473 |
| Male gender | 2.28 | 1.57-3.29 | < 0.0001 |
| Platelets < 100000/μL | 1.37 | 0.98-1.93 | 0.0694 |
| GT1b | 1.54 | 0.66-3.59 | 0.3145 |
| GT3 | 6.40 | 2.69-15.22 | < 0.0001 |
| Bilirubin > 3 mg/dL | 0.29 | 0.08-1.00 | 0.0506 |
| Albumin < 2.8 g/dL | 0.98 | 0.44-2.20 | 0.966 |
| Documented esophageal varices | 1.43 | 1.00-2.06 | 0.052 |
| Hepatic decompensation at baseline | 1.49 | 0.76-2.91 | 0.2496 |
| Hypertension | 0.93 | 0.67-1.30 | 0.6783 |
| Child-Pugh, B or C | 1.80 | 0.95-3.42 | 0.0716 |
| SOF/(VEL ± RBV) | 1.13 | 0.76-1.69 | 0.541 |
| GZR/(EBR ± RBV) | 0.91 | 0.46-1.80 | 0.7951 |
| OBV/PTV/(r ± DSV ± RBV) | 0.58 | 0.35-0.95 | 0.0319 |
| Treatment experienced | 1.66 | 1.18-2.33 | 0.0039 |
As shown in Table 7, increasing tolerability was observed over time. The higher percentage of patients who completed a course of therapy as scheduled, from 89.5% (in 2015-2016) to 97.9% (in 2021), was assisted by a decreasing incidence of AEs, including serious ones, those leading to treatment discontinuation, and deaths. The most commonly reported AEs were mild, and weakness or fatigue remained the most frequent in all subsequent time intervals. The improvement in safety profile was accompanied by a decrease in RBV use from 71.3% in 2015-2016 to 6.7% in 2021. None of the 45 deaths were reported by the treating physician as related to antiviral therapy.
| Parameter | 2015-2016 | 2017 | 2018 | 2019 | 2020 | 2021 |
| Number of patients | 1199 | 827 | 573 | 438 | 199 | 341 |
| Treatment course, n (%) | ||||||
| According to schedule | 1073 (89.5) | 776 (93.8) | 538 (93.9) | 428 (97.7) | 196 (98.5) | 334 (97.9) |
| Therapy modification | 71 (5.9) | 21 (2.6) | 26 (4.5) | 2 (0.5) | 0 | 0 |
| Therapy discontinuation | 44 (3.7) | 19 (2.3) | 9 (1.6) | 5 (1.1) | 1 (0.5) | 6 (1.8) |
| No data | 11 (0.9) | 11 (1.3) | 0 | 3 (0.7) | 2 (1.0) | 1 (0.3) |
| Patients with at least one AE, n (%) | 500 (41.7) | 212 (25.6) | 139 (24.3) | 80 (18.3) | 36 (18.1) | 66 (19.4) |
| Serious adverse events, n (%) | 48 (4) | 11 (1.3) | 16 (2.8) | 7 (1.6) | 4 (2) | 3 (0.9) |
| AEs leading to treatment discontinuation, n (%) | 35 (2.9) | 16 (1.9) | 4 (0.7) | 2 (0.5) | 1 (0.5) | 1 (0.3) |
| Most common AEs (≥ 2%), n (%) | ||||||
| Weakness/fatigue | 242 (20.2) | 78 (9.4) | 59 (10.3) | 28 (6.4) | 13 (6.5) | 16 (4.7) |
| Anemia | 63 (5.3) | 36 (4.4) | 17 (3) | 2 (0.5) | 0 | 2 (0.6) |
| Sleep disorders | 52 (4.3) | 20 (2.4) | 20 (3.5) | 4 (0.9) | 6 (3) | 6 (1.8) |
| Nausea | 32 (2.7) | 14 (1.7) | 3 (0.5) | 1 (0.2) | 5 (2.5) | 6 (1.8) |
| Abdominal pain | 21 (1.8) | 7 (0.8) | 4 (0.7) | 12 (2.7) | 5 (2.5) | 8 (2.3) |
| Headaches | 50 (4.2) | 14 (1.7) | 11 (1.9) | 7 (1.2) | 3 (0.5) | 8 (2.3) |
| Myalgia/arthralgia | 18 (1.5) | 18 (2.2) | 9 (1.6) | 6 (1.4) | 6 (3) | 8 (2.3) |
| Pruritis | 71 (5.9) | 19 (2.3) | 10 (1.7) | 10 (2.3) | 7 (3.5) | 7 (2.1) |
| Skin lesions | 26 (2.2) | 14 (1.7) | 7 (1.2) | 5 (1.1) | 2 (1) | 4 (1.2) |
| Bilirubin, > ULN | 44 (3.7) | 30 (3.6) | 3 (0.5) | 3 (0.7) | 0 | 0 |
| AEs of particular interest | ||||||
| Ascites | 30 (2.5) | 23 (2.8) | 12 (2.1) | 6 (1.4) | 0 | 13 (3.8) |
| Hepatic encephalopathy | 28 (2.3) | 12 (1.5) | 6 (1.0) | 2 (0.5) | 1 (0.5) | 6 (1.8) |
| Gastrointestinal bleeding | 9 (0.8) | 4 (0.5) | 5 (0.9) | 1 (0.2) | 1 (0.5) | 0 |
| Death, n (%) | 13 (1.1) | 10 (1.2) | 10 (1.7) | 3 (0.7) | 2 (1.0) | 7 (2.1) |
The WHO has established the goal of eliminating HCV as a significant public threat by 2030, defined as a 90% reduction in new chronic infections and a 65% reduction in mortality compared to 2015[1]. In Poland, in middle-2015, the introduction of a therapeutic regimen program of IFN-free regimens for patients with CHC gave hope that the WHO goal could be achieved in our country. The key to reducing mortality was using antiviral treatment in patients with the most advanced liver disease, as this population is most at risk of death[21]. It should be mentioned at this point that some countries initially reimbursed the therapy only for patients with advanced fibrosis and cirrhosis[22].
Despite the lack of restrictions in the Polish drug program, at the beginning of the IFN-free era, cirrhotic patients had priority in access to treatment due to long waiting lists. In the current study, they accounted for almost 44% of all treated patients in 2015-2016. This percentage decreased in consecutive periods, but the trend has been reversed since 2019. In the latter two intervals, not only were patients with cirrhosis reported more frequently, but this was also accompanied by an increase in the share of those with liver decompensation. One possible reason for the increase in cirrhosis rates is newly diagnosed cases in advanced stages of the disease in patients previously unaware of HCV infection[23]. This hypothesis is supported by the increasing percentage of patients previously untreated. At the same time, the total number of treated patients decreased. Other researchers point to the negative impact of the coronavirus disease 2019 (COVID-19) pandemic on the number of diagnosed and treated patients with chronic HCV infection[24,25].
To the best of our knowledge, this is the first study to track changes in patient profiles, therapeutic options used, and their efficacy in patients with cirrhosis in the IFN-free era. Our work summarizes 7 years of access to IFN-free therapy in Poland. In the current study, a slight decrease in the age of patients with cirrhosis was observed between the first and last time interval. This reduction was much less pronounced compared to analyzes of demographic changes in CHC patients regardless of liver fibrosis[11,13]. The differences can be explained by the fact that the cirrhotic patient population tended to be older compared to patients with non-advanced liver fibrosis. The age distribution was irregular and evolved throughout the entire analyzed period. Although the two-peak pattern was described previously not only in the Polish population with CHC, we noted only one peak at ages 60 to 70 in women in all six-time intervals. We observed a second peak around the age of 41 to 45, only in men in 2018-2021[11,13,26].
In addition to prioritizing cirrhotic patients at the beginning of the IFN-free era, those after previous treatment failure were also given priority in access to therapy. This explains the decreasing percentage of treatment-experienced patients over time, and it should be emphasized that despite the COVID-19 pandemic in 2020 and 2021, this trend continued. Patients after previous ineffective antiviral treatment up to 2018 were more likely to be on failed IFN-based regimens, while 2019-2021 documented a higher proportion of patients on failed DAA therapy, which is understandable, given the evolution and availability of antiviral regimens. An additional factor contributing to the growing proportion of treatment-naïve individuals was the detection of HCV infection in patients previously unaware of it. Since DAA therapy lasts much shorter than previously used IFN-based regimens, in the absence of restrictions on treatment reimbursement, the waiting list was shortened considerably and subsequently ceased to exist, while antiviral therapy is implemented immediately after the diagnosis is established[27]. This makes the characteristics of newly diagnosed patients quickly change the profile of the patients treated.
There was also a significant change in the share of HCV GTs in the treated patients. The most common HCV GT in Poland was GT1b[28,29]. Our study restricted to individuals with cirrhosis confirmed these findings, but we noted a changing distribution of GTs over time depending on the availability of treatment regimens in subsequent years. At the beginning of the IFN-free era, GT1- and GT4-infected patients had access to highly effective GT-specific regimens, while patients with GT3 infection in Poland were still treated with IFN-based options as the available SOF + RBV was considered suboptimal due to lower SVR[30,31]. The SOF + (DCV ± RBV) regimen with activity against GT3 was used incidentally in Poland due to problems with reimbursement. However, our analysis confirmed the high effectiveness of this option. For this reason, patients infected with GT, especially subtype 1b, definitely dominated in the initially analyzed time intervals. In 2018, new pangenotypic options, including GLE/PIB and SOF/(VEL ± RBV), which are highly effective for all HCV GTs, became available in Poland, thus enabling effective treatment of patients infected with GT3. This was reflected in the increase in the percentage of this population, despite the still visible dominance of GT1b resulting from the original distribution of GTs in the Polish population. Our findings are also supported by an RWE study conducted in Germany among patients with HCV infection, regardless of cirrhosis status treated with DAA[32].
Surprisingly, in the last analyzed period, we again recorded an increase in GT1b infections accompanied by a decrease in the percentage of GT3-infected patients. It seems that this change could have been influenced by both effective therapies of patients with GT3 infection, as well as the previously described phenomenon of detecting HCV infections in previously undiagnosed people, who, according to the original distribution of HCV GTs in the Polish population, are more likely to be infected with GT1b.
The change in the profile of patients with cirrhosis treated with DAA regimens in Poland was accompanied by changes in the options used related to the registration of new drugs and recommendations of the Polish Group of Experts for HCV[15-19]. At the beginning of the IFN-free era, GT-specific regimens were used, especially the OBV/PTV/(r + DSV ± RBV) combination, which was the first reimbursed IFN-free therapy in Poland. As mentioned above, as of 2018, pangenotypic regimens have become available in our country, and this has gradually displaced previous therapeutic options.
Regardless of the changing patient profile and therapeutic options, the effectiveness of therapy in this difficult-to-treat group of patients with advanced liver disease remained consistently high in all analyzed periods. The effectiveness of the administrated regimens was very high and exceeded 95% for most options. We documented the highest SVR for the regimen OBV/PTV/(r + DSV ± RBV), which was found to be an independent success factor in the multivariate analysis (OR = 0.58). This was an option used in the largest number of patients in the current study. Its efficacy of 99% in the population of patients with advanced liver disease was previously documented in the AMBER RWE study[33]. The high effectiveness achieved after treatment with other GT-specific regimens is also consistent with the results of the RWE GZR/(EBR ± RBV) and LDV/(SOF ± RBV) studies[34-36].
An excellent response rate of 97.6% was achieved in the GLE/PIB group, and these results were supported by a meta-analysis of 18 RWE studies[37]. Another pangenotypic regimen of VEL/(SOF ± RBV) was effective in 91.6%. Although the large meta-analysis from 12 cohorts documented a 97.9% SVR in 1078 patients with cirrhosis, it should be noted that only compensated patients were included in that analysis. In our cohort, 14% of patients receiving this option were decompensated, which may have affected the results obtained[38]. The lowest SVR rate of 72.9% was achieved by patients treated with SOF + RBV. This suboptimal therapy was received by individuals with GT3 infection who had contraindications or intolerance to PegIFN. Our results are lower than those demonstrated in the BOSTON study, where 24-wk SOF + RBV therapy in patients with GT3 cirrhosis was 79% effective[30]. On the other hand, the HCV-TARGET real-world study documented the effectiveness of this regimen in only 45% of patients with cirrhosis and GT3 infection[39].
GT3 infection was found in our study as an independent predictor of reduced SVR rate in multivariate analysis, in addition to male gender and history of prior therapy. Our observations are consistent with the results of a large retrospective RWE study involving 15720 United States veterans with a 40% share of cirrhotics, in whom GT3 infection significantly reduced the chances of responding to DAA[40]. However, it should be noted that in the above-cited study, SOF + RBV + PegIFN, LDV/(SOF + RBV) options were used in patients with GT3 infection, and only the introduction of highly effective pangenotypic options improved the response rate in this population[41].
Treatment experience has been documented as a factor associated with DAA therapy failure in the RWE study conducted by Chen et al[42]. In contrast, Berkan-Kawińska et al[43] did not find that it was a negative predictor of SVR among patients with cirrhosis. A specific subgroup of treatment-experienced patients is the population after the previous failure of DAA regimens. Despite other reports indicating a lower cure rate in DAA-experienced cirrhotic patients, the POLARIS-1 and POLARIS-4 clinical trials showed that these patients retreated with the pangenotypic SOF/VEL/VOX combination achieved high efficacy of 93%-98%[44-46]. Although in the current analysis, the pangenotypic rescue option of SOF/VEL/VOX or GLE/(PIB + SOF + RBV) was used in only 11 patients after failure of therapy with DAA regimens, all achieved a virological response.
Similar to our results, male gender was an independent negative predictor of achieving SVR in a large study from the United States Veterans Affairs[40]. Data from other RWE studies confirmed these results[34,47]. The high effectiveness of DAA regimens in our analysis was accompanied by a good safety profile with a low rate of discontinuation due to AEs, which supports findings from clinical trials and other RWE studies[34,40,48-50]. Importantly, we observed an improvement in the safety profile over time, which is explained by shortening the therapy and reducing the frequency of using regimens containing RBV. Weakness/fatigue was the most common AE regardless of the analyzed period. The percentage of patients with symptoms of liver decompensation during therapy and the 12-wk follow-up period did not exceed a few percent, which proves the good safety profile of DAA in patients with cirrhosis and is consistent with the observations of other authors[51]. This is also confirmed by the overall death rate of 1.3%, varying from 0.7% to 2.1% over time.
Our analysis has several limitations typical of retrospective studies, including possible physician bias due to incomplete data, inconsistent diagnosis or misclassification of data, underreporting of AEs, and possible data entry errors. In addition, the observational nature of the study may result in insufficient discipline during treatment; we did not capture data on adherence to the treatment, while available data suggest that lack of adherence to therapy may result in a lower chance of virologic response in cirrhotic patients treated with the SOF/LDV regimen[52,53].
Although the study was conducted in the same centers treating patients, and it seems that the decreasing overall number of patients and the changing percentage of patients with cirrhosis reflect the actual condition, distortion of the data by other factors cannot be excluded. One of these may be that the analysis also covered the COVID-19 pandemic period, when the availability of diagnostic tests and antiviral therapy was limited.
However, it is important to mention the main strength of our study, which is the collection of data from a truly geographically diverse population, representative of routine practice and a large number of patients enrolled from different centers in our country. This allows the generalization of the results. Another strong point is the large number of patients retained in post-treatment evaluation, with a rather low rate of those lost to follow-up (2.5% after the exclusion of deaths). Notably, the current analysis is the first study to directly compare changes in patient profile, antiviral treatment characteristics, efficacy, and treatment safety in patients with cirrhosis over time in the era of IFN-free therapy.
The current analysis documents changes in the characteristics of patients with HCV-infected cirrhosis that have occurred over the seven years of access to IFN-free therapies in the form of a decline in the median age of patients, the prevalence of comorbidities, and the use of concomitant medications. In addition, the evolution of the therapeutic regimens used is described, in the course of which GT-specific options were supplanted by pangenotypic regimens. Regardless of these changes, patients achieved consistently high efficacy in all analyzed periods. Male gender, GT3 infection, and prior treatment were identified as independent negative predictors of SVR.
Direct-acting antivirals (DAAs), which have replaced interferon (IFN)-based regimens, have significantly improved the prognosis of patients with cirrhosis, the population at highest risk for the most severe complications of infection with hepatitis C virus (HCV).
We aimed to track changes in the characteristics of HCV-infected patients with cirrhosis and document the evolving treatment regimens over the years, along with their efficacy and safety profile in this patient population.
Data of 3577 cirrhotics selected from 14801 HCV-infected patients treated between 2015 and 2021 with DAA regimens derived from the Epiter-2 database were analyzed.
The analysis used demographic, clinical, and laboratory data of the studied population collected retrospectively in the Epiter-2 database. The measure of treatment effectiveness was the percentage of sustained virologic response (SVR) calculated after excluding patients lost to follow-up. Safety data collected during therapy and the 12-wk post-treatment period included information on adverse events, including serious ones, deaths, and treatment course.
From 2015 to 2017, the study population was gender-balanced, while male dominance was evident in subsequent years. The decrease in the median age of patients documented during the study was accompanied by a decrease in the percentage of patients with comorbidities and comedications. A steady increase in the percentage of treatment-naïve patients was observed over the years. The genotype (GT)-specific options dominant in 2015-2018 were then replaced by pangenotypic regimens. The effectiveness of the therapy was comparable regardless of the period analyzed, and patients achieved an overall response rate of 95%, with an SVR range of 72.9%-100% for the different therapeutic regimens. Male gender, GT3 infection, and prior treatment failure were identified as independent negative predictors of therapeutic success.
The study documents changes in the profile of HCV-infected cirrhotic patients over the years of accessibility to changing DAA regimens, confirming the high effectiveness of IFN-free therapy in all analyzed periods.
Changes in the characteristics of patients, especially those with cirrhosis, may affect the expected change in the number of patients with liver cancer or at risk of decompensation. This knowledge is important from the point of view of planning the directions of health care development.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Huang J, China; IKram A, Pakistan; Masaki N, Japan S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | World Health Organization. Hepatitis C. [cited 15 November 2022]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. |
| 2. | Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58-S68. [PubMed] [DOI] [Full Text] |
| 3. | Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418-431. [PubMed] [DOI] [Full Text] |
| 4. | Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237-S244. [PubMed] [DOI] [Full Text] |
| 5. | Colombo M, Strasser S, Moreno C, Abrao Ferreira P, Urbanek P, Fernández I, Abdurakmonov D, Streinu-Cercel A, Verheyen A, Iraqi W, DeMasi R, Hill A, Lonjon-Domanec I, Wedemeyer H. Sustained virological response with telaprevir in 1,078 patients with advanced hepatitis C: the international telaprevir access program. J Hepatol. 2014;61:976-983. [PubMed] [DOI] [Full Text] |
| 6. | D'Ambrosio R, Degasperi E, Colombo M, Aghemo A. Direct-acting antivirals: the endgame for hepatitis C? Curr Opin Virol. 2017;24:31-37. [PubMed] [DOI] [Full Text] |
| 7. | Flisiak R, Urbánek P, Rokusz L, Oltman M, Makara M, Janicko M. New therapeutic options for HCV in Central Europe. Clin Exp Hepatol. 2016;2:7-11. [PubMed] [DOI] [Full Text] |
| 8. | Janczewska E, Kołek MF, Lorenc B, Klapaczyński J, Tudrujek-Zdunek M, Sitko M, Mazur W, Zarębska-Michaluk D, Buczyńska I, Dybowska D, Czauż-Andrzejuk A, Berak H, Krygier R, Jaroszewicz J, Citko J, Piekarska A, Dobracka B, Socha Ł, Deroń Z, Laurans Ł, Białkowska-Warzecha J, Tronina O, Adamek B, Tomasiewicz K, Simon K, Pawłowska M, Halota W, Flisiak R. Factors influencing the failure of interferon-free therapy for chronic hepatitis C: Data from the Polish EpiTer-2 cohort study. World J Gastroenterol. 2021;27:2177-2192. [PubMed] [DOI] [Full Text] |
| 9. | Xia H, Lu C, Wang Y, Zaongo SD, Hu Y, Wu Y, Yan Z, Ma P. Efficacy and Safety of Direct-Acting Antiviral Therapy in Patients With Chronic Hepatitis C Virus Infection: A Real-World Single-Center Experience in Tianjin, China. Front Pharmacol. 2020;11:710. [PubMed] [DOI] [Full Text] |
| 10. | Elsharkawy A, El-Raziky M, El-Akel W, El-Saeed K, Eletreby R, Hassany M, El-Sayed MH, Kabil K, Ismail SA, El-Serafy M, Abdelaziz AO, Shaker MK, Yosry A, Doss W, El-Shazly Y, Esmat G, Waked I. Planning and prioritizing direct-acting antivirals treatment for HCV patients in countries with limited resources: Lessons from the Egyptian experience. J Hepatol. 2018;68:691-698. [PubMed] [DOI] [Full Text] |
| 11. | Flisiak R, Zarębska-Michaluk D, Jaroszewicz J, Lorenc B, Klapaczyński J, Tudrujek-Zdunek M, Sitko M, Mazur W, Janczewska E, Pabjan P, Dybowska D, Buczyńska I, Czauż-Andrzejuk A, Belica-Wdowik T, Berak H, Krygier R, Piasecki M, Dobracka B, Citko J, Piekarska A, Socha Ł, Deroń Z, Tronina O, Laurans Ł, Białkowska J, Tomasiewicz K, Halota W, Simon K, Pawłowska M. Changes in patient profile, treatment effectiveness, and safety during 4 years of access to interferon-free therapy for hepatitis C virus infection. Pol Arch Intern Med. 2020;130:163-172. [PubMed] [DOI] [Full Text] |
| 12. | Reau N, Sulkowski MS, Thomas E, Sundaram V, Xu Q, Cheng WH, Marx SE, Hayes OA, Manthena SR, Chirikov V, Dylla DE, Brooks H, Carabino JM, Saab S. Epidemiology and Clinical Characteristics of Individuals with Hepatitis C Virus Infection in the United States, 2017-2019. Adv Ther. 2021;38:5777-5790. [PubMed] [DOI] [Full Text] |
| 13. | Hüppe D, Serfert Y, Buggisch P, Mauss S, Böker KHW, Müller T, Klinker H, Günther R, Berg T, Cornberg M, Niederau C, Sarrazin C, Simon KG, Zeuzem S, Manns MP, Wedemeyer H. [4 years of direct-acting antivirals (DAAs) in the German Hepatitis C-Registry (DHC-R)]. Z Gastroenterol. 2019;57:27-36. [PubMed] [DOI] [Full Text] |
| 14. | Yang J, Liu HX, Su YY, Liang ZS, Rao HY. Distribution and changes in hepatitis C virus genotype in China from 2010 to 2020. World J Clin Cases. 2022;10:4480-4493. [PubMed] [DOI] [Full Text] |
| 15. | Polish Group of HCV Experts; Halota W, Flisiak R, Boroń-Kaczmarska A, Juszczyk J, Małkowski P, Pawłowska M, Simon K, Tomasiewicz K. Recommendations for the treatment of hepatitis C issued by the Polish Group of HCV Experts - 2016. Clin Exp Hepatol. 2016;2:27-33. [PubMed] [DOI] [Full Text] |
| 16. | Polish Group of Experts for HCV; Halota W, Flisiak R, Juszczyk J, Małkowski P, Pawłowska M, Simon K, Tomasiewicz K. Recommendations for the treatment of hepatitis C in 2017. Clin Exp Hepatol. 2017;3:47-55. [PubMed] [DOI] [Full Text] |
| 17. | Halota W, Flisiak R, Juszczyk J, Małkowski P, Pawłowska M, Simon K, Tomasiewicz K. Recommendations for the treatment of viral hepatitis C in 2018 by Polish Group of Experts for HCV. Zakaż XXI wieku. 2018;. [DOI] [Full Text] |
| 18. | Halota W, Flisiak R, Juszczyk J, Małkowski P, Pawłowska M, Simon K, Tomasiewicz K. Recommendations for the treatment of viral hepatitis C in 2019 by Polish Group of Experts for HCV. Zakaż XXI wieku. 2019;. [DOI] [Full Text] |
| 19. | Halota W, Flisiak R, Juszczyk J, Małkowski P, Pawłowska M, Simon K, Tomasiewicz K. Recommendations of the Polish Group of Experts for HCV for the treatment of hepatitis C in 2020. Clin Exp Hepatol. 2020;6:163-169. [PubMed] [DOI] [Full Text] |
| 20. | European Association for the Study of the Liver. Clinical Practice Guidelines Panel: Chair:; EASL Governing Board representative:; Panel members:. EASL recommendations on treatment of hepatitis C: Final update of the series(☆). J Hepatol. 2020;73:1170-1218. [PubMed] [DOI] [Full Text] |
| 21. | Flisiak R, Zarębska-Michaluk D, Janczewska E, Staniaszek A, Gietka A, Mazur W, Tudrujek M, Tomasiewicz K, Belica-Wdowik T, Baka-Ćwierz B, Dybowska D, Halota W, Lorenc B, Sitko M, Garlicki A, Berak H, Horban A, Orłowska I, Simon K, Socha Ł, Wawrzynowicz-Syczewska M, Jaroszewicz J, Deroń Z, Czauż-Andrzejuk A, Citko J, Krygier R, Piekarska A, Laurans Ł, Dobracki W, Białkowska J, Tronina O, Pawłowska M. Treatment of HCV infection in Poland at the beginning of the interferon-free era-the EpiTer-2 study. J Viral Hepat. 2018;25:661-669. [PubMed] [DOI] [Full Text] |
| 22. | Herink MC, Geddes J, Vo K, Zaman A, Hartung DM. Effect of relaxing hepatitis C treatment restrictions on direct-acting antiviral use in a Medicaid program: an interrupted time series analysis. J Manag Care Spec Pharm. 2021;27:856-864. [PubMed] [DOI] [Full Text] |
| 23. | Piekarska A, Tomasiewicz K, Halota W, Jaroszewicz J, Krygier R, Małkowski P, Pawłowska M, Simon K, Tronina O, Zarębska-Michaluk D, Flisiak R. Searching for the optimal population for hepatitis C virus screening in Poland. Clin Exp Hepatol. 2020;6:74-76. [PubMed] [DOI] [Full Text] |
| 24. | Sagnelli C, Macera M, Camaioni C, Salvati A, Coppola N, Sagnelli E. SARS-CoV-2 infection: a hurricane that does not ignore chronic hepatitis. Infection. 2022;50:849-858. [PubMed] [DOI] [Full Text] |
| 25. | Kondili LA, Buti M, Riveiro-Barciela M, Maticic M, Negro F, Berg T, Craxì A. Impact of the COVID-19 pandemic on hepatitis B and C elimination: An EASL survey. JHEP Rep. 2022;4:100531. [PubMed] [DOI] [Full Text] |
| 26. | Brzdęk M, Dobrowolska K, Pabjan P, Zarębska-Michaluk D. Clinical characteristics and antiviral therapy in patients infected with hepatitis C virus in the interferonfree era. Pol Arch Intern Med. 2022;132. [PubMed] [DOI] [Full Text] |
| 27. | Flisiak R, Zarębska-Michaluk D, Frankova S, Grgurevic I, Hunyady B, Jarcuska P, Kupčinskas L, Makara M, Simonova M, Sperl J, Tolmane I, Vince A. Is elimination of HCV in 2030 realistic in Central Europe. Liver Int. 2021;41 Suppl 1:56-60. [PubMed] [DOI] [Full Text] |
| 28. | Panasiuk A, Flisiak R, Mozer-Lisewska I, Adamek A, Tyczyno M, Halota W, Pawłowska M, Stańczak J, Berak H, Wawrzynowicz-Syczewska M, Boroń-Kaczmarska A, Łapiński TW, Grzeszczuk A, Piekarska A, Tomasiewicz K, Jabłkowski M, Kryczka W, Zarebska-Michaluk D, Stepień P, Garlicki AM, Kozłowska J, Wiercińska-Drapało A, Zasik E, Mazur W, Dobracka B, Dobracki W, Simon K, Ryzko J, Pawłowska J, Dzierzanowska-Fangrat K, Januszkiewicz-Lewandowska D, Szenborn L, Zaleska I, Rokitka M, Strawińska E, Balinowska K, Smiatacz T, Stalke P, Sikorska K, Lakomy A, Zdrojewski M, Lachowicz A. Distribution of HCV genotypes in Poland. Przegl Epidemiol. 2013;67:11-16, 99. [PubMed] |
| 29. | Flisiak R, Pogorzelska J, Berak H, Horban A, Orłowska I, Simon K, Tuchendler E, Madej G, Piekarska A, Jabłkowski M, Deroń Z, Mazur W, Kaczmarczyk M, Janczewska E, Pisula A, Smykał J, Nowak K, Matukiewicz M, Halota W, Wernik J, Sikorska K, Mozer-Lisewska I, Rozpłochowski B, Garlicki A, Tomasiewicz K, Krzowska-Firych J, Baka-Ćwierz B, Kryczka W, Zarębska-Michaluk D, Olszok I, Boroń-Kaczmarska A, Sobala-Szczygieł B, Szlauer B, Korcz-Ondrzejek B, Sieklucki J, Pleśniak R, Ruszała A, Postawa-Kłosińska B, Citko J, Lachowicz-Wawrzyniak A, Musialik J, Jezierska E, Dobracki W, Dobracka B, Hałubiec J, Krygier R, Strokowska A, Chomczyk W, Witczak-Malinowska K. Prevalence of HCV genotypes in Poland - the EpiTer study. Clin Exp Hepatol. 2016;2:144-148. [PubMed] [DOI] [Full Text] |
| 30. | Foster GR, Pianko S, Brown A, Forton D, Nahass RG, George J, Barnes E, Brainard DM, Massetto B, Lin M, Han B, McHutchison JG, Subramanian GM, Cooper C, Agarwal K; BOSON Study Group. Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in patients with hepatitis C virus genotype 3 infection and treatment-experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology. 2015;149:1462-1470. [PubMed] [DOI] [Full Text] |
| 31. | Zarębska-Michaluk D, Flisiak R, Jaroszewicz J, Janczewska E, Czauż-Andrzejuk A, Berak H, Horban A, Staniaszek A, Gietka A, Tudrujek M, Tomasiewicz K, Dybowska D, Halota W, Piekarska A, Sitko M, Garlicki A, Orłowska I, Simon K, Belica-Wdowik T, Baka-Ćwierz B, Mazur W, Białkowska J, Socha Ł, Wawrzynowicz-Syczewska M, Laurans Ł, Deroń Z, Lorenc B, Dobracka B, Tronina O, Pawłowska M. Is Interferon-Based Treatment of Viral Hepatitis C Genotype 3 Infection Still of Value in the Era of Direct-Acting Antivirals? J Interferon Cytokine Res. 2018;38:93-100. [PubMed] [DOI] [Full Text] |
| 32. | Hüppe D, Stoehr A, Buggisch P, Mauss S, Klinker H, Teuber G, Hidde D, Lohmann K, Bondin M, Wedemeyer H. The changing characteristics of patients infected with chronic hepatitis C virus from 2014 to 2019: Real-world data from the German Hepatitis C-Registry (DHC-R). J Viral Hepat. 2021;28:1474-1483. [PubMed] [DOI] [Full Text] |
| 33. | Flisiak R, Janczewska E, Wawrzynowicz-Syczewska M, Jaroszewicz J, Zarębska-Michaluk D, Nazzal K, Bolewska B, Bialkowska J, Berak H, Fleischer-Stępniewska K, Tomasiewicz K, Karwowska K, Rostkowska K, Piekarska A, Tronina O, Madej G, Garlicki A, Lucejko M, Pisula A, Karpińska E, Kryczka W, Wiercińska-Drapało A, Mozer-Lisewska I, Jabłkowski M, Horban A, Knysz B, Tudrujek M, Halota W, Simon K. Real-world effectiveness and safety of ombitasvir/paritaprevir/ritonavir ± dasabuvir ± ribavirin in hepatitis C: AMBER study. Aliment Pharmacol Ther. 2016;44:946-956. [PubMed] [DOI] [Full Text] |
| 34. | Ioannou GN, Beste LA, Chang MF, Green PK, Lowy E, Tsui JI, Su F, Berry K. Effectiveness of Sofosbuvir, Ledipasvir/Sofosbuvir, or Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir Regimens for Treatment of Patients With Hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology. 2016;151:457-471.e5. [PubMed] [DOI] [Full Text] |
| 35. | Kramer JR, Puenpatom A, Erickson KF, Cao Y, Smith D, El-Serag HB, Kanwal F. Real-world effectiveness of elbasvir/grazoprevir In HCV-infected patients in the US veterans affairs healthcare system. J Viral Hepat. 2018;25:1270-1279. [PubMed] [DOI] [Full Text] |
| 36. | Zarębska-Michaluk D, Jaroszewicz J, Buczyńska I, Simon K, Lorenc B, Tudrujek-Zdunek M, Tomasiewicz K, Sitko M, Garlicki A, Janczewska E, Dybowska D, Halota W, Pawłowska M, Pabjan P, Mazur W, Czauż-Andrzejuk A, Berak H, Horban A, Socha Ł, Klapaczyński J, Piekarska A, Blaszkowska M, Belica-Wdowik T, Dobracka B, Tronina O, Deroń Z, Białkowska-Warzecha J, Laurans Ł, Flisiak R. Real-world experience with Grazoprevir/Elbasvir in the treatment of previously "difficult to treat" patients infected with hepatitis C virus genotype 1 and 4. J Gastroenterol Hepatol. 2020;35:1238-1246. [PubMed] [DOI] [Full Text] |
| 37. | Lampertico P, Carrión JA, Curry M, Turnes J, Cornberg M, Negro F, Brown A, Persico M, Wick N, Porcalla A, Pangerl A, Crown E, Larsen L, Yu Y, Wedemeyer H. Real-world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of patients with chronic HCV infection: A meta-analysis. J Hepatol. 2020;72:1112-1121. [PubMed] [DOI] [Full Text] |
| 38. | Mangia A, Milligan S, Khalili M, Fagiuoli S, Shafran SD, Carrat F, Ouzan D, Papatheodoridis G, Ramji A, Borgia SM, Wedemeyer H, Losappio R, Pérez-Hernandez F, Wick N, Brown RS Jr, Lampertico P, Doucette K, Ntalla I, Ramroth H, Mertens M, Vanstraelen K, Turnes J. Global real-world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: Analysis of 5552 patients from 12 cohorts. Liver Int. 2020;40:1841-1852. [PubMed] [DOI] [Full Text] |
| 39. | Feld JJ, Maan R, Zeuzem S, Kuo A, Nelson DR, Di Bisceglie AM, Manns MP, Sherman K, Frazier LM, Sterling R, Mailliard M, Schmidt M, Akushevich L, Vainorius M, Fried MW. Effectiveness and Safety of Sofosbuvir-Based Regimens for Chronic HCV Genotype 3 Infection: Results of the HCV-TARGET Study. Clin Infect Dis. 2016;63:776-783. [PubMed] [DOI] [Full Text] |
| 40. | Daniel KE, Saeian K, Rizvi S. Real-world experiences with direct-acting antiviral agents for chronic hepatitis C treatment. J Viral Hepat. 2020;27:195-204. [PubMed] [DOI] [Full Text] |
| 41. | Zarębska-Michaluk D, Jaroszewicz J, Parfieniuk-Kowerda A, Janczewska E, Dybowska D, Pawłowska M, Halota W, Mazur W, Lorenc B, Janocha-Litwin J, Simon K, Piekarska A, Berak H, Klapaczyński J, Stępień P, Sobala-Szczygieł B, Citko J, Socha Ł, Tudrujek-Zdunek M, Tomasiewicz K, Sitko M, Dobracka B, Krygier R, Białkowska-Warzecha J, Laurans Ł, Flisiak R. Effectiveness and Safety of Pangenotypic Regimens in the Most Difficult to Treat Population of Genotype 3 HCV Infected Cirrhotics. J Clin Med. 2021;10. [PubMed] [DOI] [Full Text] |
| 42. | Chen CY, Huang CF, Cheng PN, Tseng KC, Lo CC, Kuo HT, Huang YH, Tai CM, Peng CY, Bair MJ, Chen CH, Yeh ML, Lin CL, Lin CY, Lee PL, Chong LW, Hung CH, Huang JF, Yang CC, Hu JT, Lin CW, Chen CT, Wang CC, Su WW, Hsieh TY, Tsai WL, Lee TH, Chen GY, Wang SJ, Chang CC, Mo LR, Yang SS, Wu WC, Huang CS, Hsiung CK, Kao CN, Tsai PC, Liu CH, Lee MH, Liu CJ, Dai CY, Kao JH, Chuang WL, Lin HC, Yu ML. Factors associated with treatment failure of direct-acting antivirals for chronic hepatitis C: A real-world nationwide hepatitis C virus registry programme in Taiwan. Liver Int. 2021;41:1265-1277. [PubMed] [DOI] [Full Text] |
| 43. | Berkan-Kawińska A, Piekarska A, Janczewska E, Lorenc B, Tudrujek-Zdunek M, Tomasiewicz K, Berak H, Horban A, Zarębska-Michaluk D, Pabjan P, Buczyńska I, Pazgan-Simon M, Dybowska D, Halota W, Pawłowska M, Klapaczyński J, Mazur W, Czauż-Andrzejuk A, Socha Ł, Laurans Ł, Garlicki A, Sitko M, Jaroszewicz J, Citko J, Dobracka B, Krygier R, Białkowska-Warzecha J, Tronina O, Belica-Wdowik T, Baka-Ćwierz B, Flisiak R. Real-world effectiveness and safety of direct-acting antivirals in patients with cirrhosis and history of hepatic decompensation: Epi-Ter2 Study. Liver Int. 2021;41:1789-1801. [PubMed] [DOI] [Full Text] |
| 44. | Dietz J, Spengler U, Müllhaupt B, Schulze Zur Wiesch J, Piecha F, Mauss S, Seegers B, Hinrichsen H, Antoni C, Wietzke-Braun P, Peiffer KH, Berger A, Matschenz K, Buggisch P, Backhus J, Zizer E, Boettler T, Neumann-Haefelin C, Semela D, Stauber R, Berg T, Berg C, Zeuzem S, Vermehren J, Sarrazin C; European HCV Resistance Study Group. Efficacy of Retreatment After Failed Direct-acting Antiviral Therapy in Patients With HCV Genotype 1-3 Infections. Clin Gastroenterol Hepatol. 2021;19:195-198.e2. [PubMed] [DOI] [Full Text] |
| 45. | Schmitt A, Günther R, Mauss S, Boeker KHW, Buggisch P, Hillenbrand H, John C, Klinker H, Pathil A, Simon KG, Serfert Y, Niederau C, Vermehren J, Wedemeyer H, Sarrazin C. Treatment-failure to direct antiviral HCV regimens in real world: frequency, patient characteristics and rescue therapy - data from the German hepatitis C registry (DHC-R). Z Gastroenterol. 2020;58:341-351. [PubMed] [DOI] [Full Text] |
| 46. | Bourlière M, Gordon SC, Flamm SL, Cooper CL, Ramji A, Tong M, Ravendhran N, Vierling JM, Tran TT, Pianko S, Bansal MB, de Lédinghen V, Hyland RH, Stamm LM, Dvory-Sobol H, Svarovskaia E, Zhang J, Huang KC, Subramanian GM, Brainard DM, McHutchison JG, Verna EC, Buggisch P, Landis CS, Younes ZH, Curry MP, Strasser SI, Schiff ER, Reddy KR, Manns MP, Kowdley KV, Zeuzem S; POLARIS-1 and POLARIS-4 Investigators. Sofosbuvir, Velpatasvir, and Voxilaprevir for Previously Treated HCV Infection. N Engl J Med. 2017;376:2134-2146. [PubMed] [DOI] [Full Text] |
| 47. | Pabjan P, Brzdęk M, Chrapek M, Dziedzic K, Dobrowolska K, Paluch K, Garbat A, Błoniarczyk P, Reczko K, Stępień P, Zarębska-Michaluk D. Are There Still Difficult-to-Treat Patients with Chronic Hepatitis C in the Era of Direct-Acting Antivirals? Viruses. 2022;14. [PubMed] [DOI] [Full Text] |
| 48. | Zeuzem S, Foster GR, Wang S, Asatryan A, Gane E, Feld JJ, Asselah T, Bourlière M, Ruane PJ, Wedemeyer H, Pol S, Flisiak R, Poordad F, Chuang WL, Stedman CA, Flamm S, Kwo P, Dore GJ, Sepulveda-Arzola G, Roberts SK, Soto-Malave R, Kaita K, Puoti M, Vierling J, Tam E, Vargas HE, Bruck R, Fuster F, Paik SW, Felizarta F, Kort J, Fu B, Liu R, Ng TI, Pilot-Matias T, Lin CW, Trinh R, Mensa FJ. Glecaprevir-Pibrentasvir for 8 or 12 Weeks in HCV Genotype 1 or 3 Infection. N Engl J Med. 2018;378:354-369. [PubMed] [DOI] [Full Text] |
| 49. | Asselah T, Hézode C, Qaqish RB, ElKhashab M, Hassanein T, Papatheodoridis G, Feld JJ, Moreno C, Zeuzem S, Ferenci P, Yu Y, Redman R, Pilot-Matias T, Mobashery N. Ombitasvir, paritaprevir, and ritonavir plus ribavirin in adults with hepatitis C virus genotype 4 infection and cirrhosis (AGATE-I): a multicentre, phase 3, randomised open-label trial. Lancet Gastroenterol Hepatol. 2016;1:25-35. [PubMed] [DOI] [Full Text] |
| 50. | Welzel TM, Hinrichsen H, Sarrazin C, Buggisch P, Baumgarten A, Christensen S, Berg T, Mauss S, Teuber G, Stein K, Deterding K, van Bömmel F, Heyne R, John C, Zimmermann T, Lutz T, Schott E, Hettinger J, Kleine H, König B, Hüppe D, Wedemeyer H. Real-world experience with the all-oral, interferon-free regimen of ombitasvir/paritaprevir/ritonavir and dasabuvir for the treatment of chronic hepatitis C virus infection in the German Hepatitis C Registry. J Viral Hepat. 2017;24:840-849. [PubMed] [DOI] [Full Text] |
| 51. | Maan R, van Tilborg M, Deterding K, Ramji A, van der Meer AJ, Wong F, Fung S, Sherman M, Manns MP, Cornberg M, Hansen BE, Wedemeyer H, Janssen HL, de Knegt RJ, Feld JJ. Safety and Effectiveness of Direct-Acting Antiviral Agents for Treatment of Patients With Chronic Hepatitis C Virus Infection and Cirrhosis. Clin Gastroenterol Hepatol. 2016;14:1821-1830.e6. [PubMed] [DOI] [Full Text] |
| 52. | Tamai H, Shingaki N, Ida Y, Shimizu R, Maeshima S, Okamura J, Kawashima A, Nakao T, Hara T, Matsutani H, Nishikawa I, Higashi K. Real-world safety and efficacy of sofosbuvir and ledipasvir for elderly patients. JGH Open. 2018;2:300-306. [PubMed] [DOI] [Full Text] |
| 53. | Masaki N, Kawasaki Y, Nozaki Y, Yanase M. Characteristics of patients aged over 75 years with hepatitis C virus infection treated with direct-acting antivirals in Japan: Evidence based on the nationwide, real-world database in Japan. Hepatol Res. 2021;51:417-425. [PubMed] [DOI] [Full Text] |