Published online Mar 21, 2023. doi: 10.3748/wjg.v29.i11.1685
Peer-review started: December 1, 2022
First decision: February 1, 2023
Revised: February 2, 2023
Accepted: March 7, 2023
Article in press: March 7, 2023
Published online: March 21, 2023
Processing time: 105 Days and 15.6 Hours
Adequate bowel cleansing is critical for a high-quality colonoscopy because it affects diagnostic accuracy and adenoma detection. Nevertheless, almost a quarter of procedures are still carried out with suboptimal preparation, resulting in longer procedure times, higher risk of complications, and higher likelihood of missing lesions. Current guidelines recommend high-volume or low-volume polyethylene glycol (PEG)/non-PEG-based split-dose regimens. In patients who have had insufficient bowel cleansing, the colonoscopy should be repeated the same day or the next day with additional bowel cleansing as a salvage option. A strategy that includes a prolonged low-fiber diet, a split preparation regimen, and a colo
Core Tip: Almost a quarter of procedures are still performed with inadequate preparation. A strategy that includes a low-fiber diet for an extended period of time, a split preparation regimen, and a colonoscopy within 5 h of the end of preparation may improve cleansing success rates in the elderly. In addition, while no specific product is recommended for difficult-to-prepare patients, clinical evidence suggests that 1-L polyethylene glycol (PEG) plus ascorbic acid preparation is associated with higher cleansing success in hospitalized and inflammatory bowel disease patients. Isotonic high volume PEG solutions should be given to patients with severe renal failure.
- Citation: Shahini E, Sinagra E, Vitello A, Ranaldo R, Contaldo A, Facciorusso A, Maida M. Factors affecting the quality of bowel preparation for colonoscopy in hard-to-prepare patients: Evidence from the literature. World J Gastroenterol 2023; 29(11): 1685-1707
- URL: https://www.wjgnet.com/1007-9327/full/v29/i11/1685.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i11.1685
One of the most commonly used techniques for diagnosing colorectal diseases is colonoscopy. Furthermore, it is important in colorectal cancer (CRC) screening because early detection is linked to a long-term reduction in malignancy incidence and mortality[1,2].
As is well known, the quality of a colonoscopy is entirely dependent on adequate bowel cleansing, which can affect diagnostic accuracy and the rate of adenoma detection (ADR)[3]. Inadequate bowel preparation, on the other hand, leads to decreased sensitivity to colonoscopy, increased procedural time, a higher risk of adverse events, and a greater likelihood of having to repeat the exam at a higher cost[4-7].
This is a critical topic because data from the literature show that a quarter of procedures still have suboptimal preparation[8]. Similarly, a large Italian study discovered that poor preparation occurs in approximately 17% of colonoscopies[9], and results from the United Kingdom screening program confirmed that inadequate preparation accounts for more than 20% of incomplete procedures[10]. Finally, data from a recent survey of sixty-four Italian screening centers revealed that only 29% of centers meet the minimum standard of at least 90% colonoscopies with adequate cleansing[11].
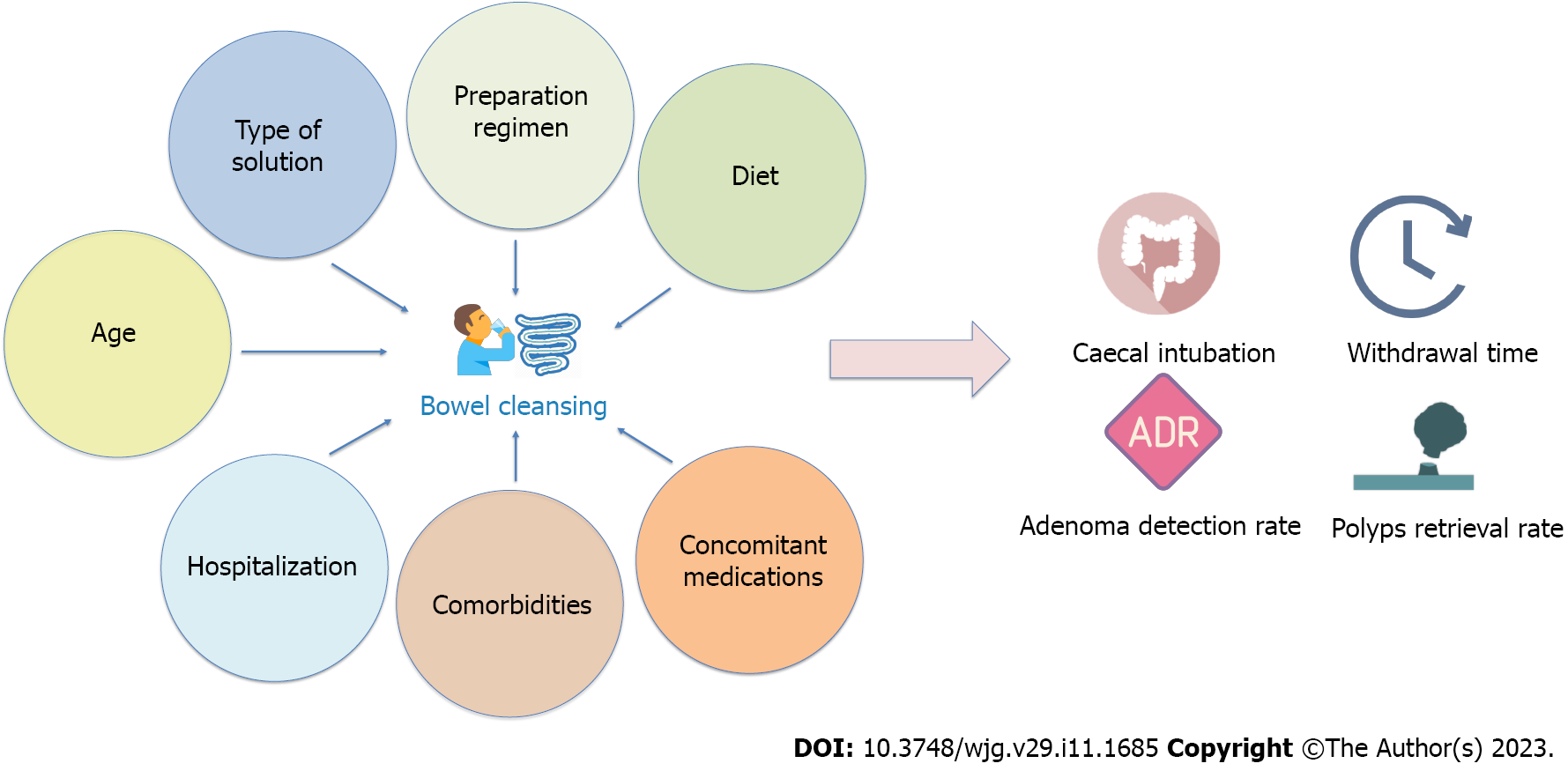
The type of bowel preparation, split-dose regimen, low-fiber diet, comorbidities, concomitant medications, inpatient status, and elderly age have all been found to affect the quality of bowel cleansing (Figure 1)[12]. Some of these variables, such as the type of solution, preparation regimen, and diet, are modifiable risk factors. In this regard, recent scientific advances have resulted in the introduction of new effective bowel cleansing solutions and reinforced the importance of specific preparation regimens.
On the other side, factors such as age or comorbidities are not modifiable and not susceptible to intervention. This makes it more difficult to intervene to increase the quality of bowel cleansing in specific conditions, also considering that guidelines do not provide specific recommendations for patients with multiple risk factors[13].
The purpose of this review is to summarize the evidence on the risk factors that influence the quality of cleansing in difficult-to-prepare patients, as well as strategies to improve colonoscopy preparation in these patients.
Several laxatives are currently available and have been studied, with varying efficacy and tolerability.
Among these solutions are polyethylene glycol plus ascorbate (PEG-ASC) (4-L, 2-L, and 1-L), 2-L PEG plus citrate, 2-L PEG plus Bisacodyl, magnesium citrate plus picosulphate (MCSP), and trisulfate (magnesium sulfate, sodium sulfate, and potassium sulfate), also known as oral sulfate solution (OSS).
A meta-analysis of PEG solutions discovered that split-dose high-volume PEG was more effective than other options, including low-volume regimens [odds ratio (OR) = 3.46, 95% confidence interval (95%CI) 2.45-4.89][14]. Another meta-analysis comparing split-dose high-volume PEG to split-dose low-volume PEG found that high-volume regimens were superior (OR = 1.89, 95%CI 1.01-3.46)[15]. Nonetheless, because high-volume solutions may reduce patient compliance, resulting in suboptimal preparation, new low-volume laxatives have been introduced in the last decade.
Following that, a 2-L PEG plus citrate and simethicone solution was added. Randomized controlled trials (RCTs) comparing this formulation 2-L PEG plus citrate vs 4-L PEG found similar cleansing efficacy (73.6% vs 72.3%, 95%CI difference -7.5 to 10.1), but with greater tolerability (25.4% vs 37.0%, P < 0.01) and acceptability (93.9% vs 82.2%, P < 0.001)[16].
Moreover, 2-L PEG plus citrate showed similar efficacy (78.3% vs 74.3%, P = 0.37) and acceptability (81.4% vs 80.8%, P = 0.74) also when compared to 2-L PEG-ASC[17].
Recently, a 1-L PEG-ASC solution with a very low volume has been introduced to improve patients’ experience during colonoscopy by reducing the total oral intake of liquids to be consumed. The development of this very low-volume solution was made possible by increasing the ascorbate content, which improves the laxative effect and allows the solution to be delivered in a smaller volume.
Three phase-3 RCTs comparing the effectiveness of 1-L PEG-ASC (Plenvu, Norgine, Harefield, United Kingdom) vs trisulfate[18], sodium picosulfate plus magnesium citrate[19], and 2-L PEG[20] revealed that 1-L PEG-ASC was non-inferior to the comparator.
Moreover, a multicenter prospective study was performed to assess the effectiveness of 1-L PEG-ASC compared to 2-L and 4-L PEG preparation in a real-life setting on a cohort of 1289 patients (n = 490 performing a 4-L PEG preparation, n = 566 a 2-L PEG and n = 233 a 1-L PEG)[21].
In this study, cleansing success was achieved in 72.4%, 74.1%, and 90.1% (P < 0.001), respectively, while high-quality cleansing of the right colon was achieved in 15.9%, 12.0%, and 41.4% (P < 0.001) for the 4-L, 2-L, and 1-L-PEG preparation groups, respectively. The 1-L PEG-ASC preparation was an independent predictor of overall cleansing success and high-quality cleansing of the right colon in multiple regression analysis. In that study, 44.8% of patients were over the age of 65, confirming the validity of the safety of 1-L PEG-ASC in the elderly.
A real-life study performed on a cohort of hospitalized subjects confirmed that, among all variables, the 1-L PEG-ASC solution (OR = 0.39, 95%CI 0.23-0.65) and a > 75% intake of bowel preparation (OR = 0.09, 95%CI 0.05-0.15) significantly reduced the risk of inadequate colon cleansing[22].
This information is undoubtedly valuable and should be considered even in the care of hospitalized patients.
Nonetheless, the same studies mentioned above found that 1-L PEG-ASC is associated with a higher incidence of adverse events, such as nausea and vomiting, even if none of these were serious enough to impair bowel cleansing quality[18-21].
Based on current data, guidelines recommend using high-volume or low-volume PEG-based regimens, and non-PEG-based validated products, in a split-dose fashion[13].
Even though no specific product is recommended for difficult-to-prepare patients, 1-L PEG-ASC appears to be promisingly superior in hospitalized patients and could be a reasonable choice in this setting; however, these data were obtained after the guidelines were issued.
The preparation regimen has a significant impact on the quality of bowel cleansing. A day before preparation was traditionally performed before a colonoscopy. Nonetheless, the most recent evidence suggests that dividing the preparation between the day before the exam and the day of the exam leads to better intestinal cleansing and a shorter time between the end of the preparation and the same colonoscopy.
A meta-analysis of 47 trials involving 13487 patients revealed that a split-dose regimen is associated with significantly better cleansing than day-before preparations (OR = 2.51, 95%CI 1.86-3.39), regardless of solution type and dose[15].
Furthermore, subgroup analysis by solution type confirmed that split was more effective than the day-before regimen for PEG, sodium phosphate, and picosulfate (OR = 2.60, 95%CI 1.46-4.63; OR = 9.34, 95%CI 2.12-41.11; OR = 3.54, 95%CI 1.95-6.45, respectively). Likewise, a higher proportion of patients (OR = 1.90, 95%CI 1.05-3.46) were willing to repeat preparation with split-dose vs day-before cleansing[15].
Subsequent RCTs evaluating the effectiveness of high-volume PEG[23,24], low-volume PEG[25,26], and sodium picosulfate[27-29] confirmed the split regimen’s superiority over the day-before regimen.
In terms of the effectiveness of a split regimen on the detection of neoplastic lesions, an RCT comparing 2-L PEG-ASC in a split vs day-before fashion found that a split regimen resulted in a higher detection rate of adenomas (53.0% vs 40.9%; RR = 1.22, 95%CI 1.03-1.46) and advanced adenomas (26.4% vs 20.0%; RR = 1.35, 95%CI 1.06-1.73)[26].
Another RCT comparing 2-L PEG-ASC in split dose vs split-dose sodium picosulfate/magnesium citrate (SPMC) in a day-before dose showed a non-significant higher polyp detection rate, and a significantly higher detection rate of right-sided polyps and adenomas (51.5% vs 44.0%, P = 0.14; 28.0% vs 16.6%, P = 0.007; 21.0% vs 11.9%, P = 0.015, respectively) in favor of split regimen[30].
Also, two meta-analyses (including 11 and 14 RCTs) compared split-dose bowel preparation with same-day bowel preparation and found similar results in terms of bowel preparation quality, patient willingness to repeat it, and overall tolerability[31,32].
In addition to the split preparation, patients who need to have a colonoscopy in the afternoon should consider same-day preparation. In this regard, data from two meta-analyses revealed that when split and same-day regimens were used, the quality of bowel preparation, tolerability, and willingness to repeat were similar.
As a result, for patients undergoing afternoon colonoscopy, current guidelines recommend split-dose bowel preparation and same-day bowel preparation[13].
Before a colonoscopy, a low-residue diet or clear liquids are usually advised. Patients should avoid foods high in fiber, such as fruits, vegetables, and whole grains, to achieve a low-residue diet. Colored liquids should be avoided because they can obscure proper mucosal visualization. Water, broth, coffee or tea, ice, gelatin, and fruit juices are the best clear liquid choices (apple, lemonade, and grapefruit).
On the day before the colonoscopy, two meta-analyses compared a low-residue diet to a clear liquid diet, with both arms using the same laxative. When compared to a clear liquid diet, Nguyen et al[33] discovered that a low residue diet was associated with better tolerability (OR = 1.92, 95%CI 1.36-2.70, P < 0.01) and higher willingness to repeat bowel preparation (OR = 1.86, 95%CI 1.34-2.59, P < 0.01). However, no differences in adequate bowel cleansing (OR = 1.21, 95%CI 0.64-2.28, P = 0.58) or adverse event incidence (OR = 0.88, 95%CI 0.58-1.35, P = 0.57) were found[33]. Another meta-analysis, this time by Avalos et al[34], compared a low-residue diet or a regular diet to a clear liquid diet. There were no differences in adequate cleansing (RR = 1.00, 95%CI 0.97-1.04). The low residue-regular diet was linked to a higher likelihood of repeating the procedure, better tolerability, and more frequent consumption of the solution (RR = 1.08, 95%CI 1.01-1.16; RR = 1.04, 95%CI 1.01-1.08; RR = 1.04, 95%CI 1.01-1.08, respectively). Except for more hungriness in the clear liquid diet group, there were no differences in ADR or adverse events between groups (RR = 1.93, 95%CI 1.13-3.30)[34]. In a third meta-analysis by Song et al[35] including 7 RCTs using different laxatives, a low residue diet showed higher tolerability (RR = 1.06, 95 %CI 1.02-1.11) and a higher likelihood of repeating the exam (RR = 1.17, 95%CI 1.09-1.26) compared with a clear liquid diet.
Concerning the duration of the diet, current guidelines recommend a low-residue diet or clear liquids for at least one day before the examination[13]. In this regard, two recent RCTs compared the 1-d vs 3-d diet, finding no significant difference after diet prolongation. The first RCT compared a 3-d vs 1-d low-fiber diet with a 4-L PEG split-dose preparation and found a similar rate of adequate bowel cleansing (91.7% for 3-d diet vs 94.7% for 1-d diet, P = 0.24)[36]. Similarly, the second RCT compared a 1-d vs 3-d low-residue diet with a 2-L PEG + ASC split-dose preparation (82.7% vs 85.6%), with no differences in adherence, satisfaction, or polyp or ADR[37].
However, it should be noted that fiber consumption varies greatly between people and regions of the world. As a result, in countries where fiber consumption is naturally low, a single day’s diet is more likely to suffice. On the contrary, in people or countries where fiber consumption is abundant, the diet should be tailored accordingly, particularly in hard-to-prepare patients.
The timing of colon preparation administration has a significant impact on both its tolerability and efficacy. Starting the preparation, the day before the colonoscopy improves the quality of colonic cleansing and the likelihood of finding flat lesions.
According to recent research, there is a negative correlation between the start of the colonoscopy, the interval since the last dose of bowel preparation, and the cleanliness of the mucosa. It is recommended to perform the colonoscopy sooner after the bowel preparation is complete to improve the quality of the examination[38,39].
Patients with intervals of 7 h or less between the start of PEG intake and the start of the colonoscopy had better bowel preparation than patients with intervals of more than 7 h (P = 0.03)[40].
Furthermore, bowel cleansing with the fractional regimen was superior within three hours of the last dose intake, declined gradually after 4-5 h, and became statistically insignificant at five hours, according to Bucci et al’s meta-analysis in 2014 including 29 RCTs comparing split vs day-before regimens[41].
Additionally, a subsequent multicenter, randomized, endoscopist-blinded study found that the best window of time for bowel preparation is the same for all preparations. Regardless of the preparation (PEG, PEG-ASC, ≤ 11.8, sodium picosulphate/magnesium citrate, ≤ 13.3 h), the ideal time before the colonoscopy was ≤ 11.8 h. The timing of the preparation did not affect the tolerability[42].
Anyway, the overall findings support the recommendation in the European Society of Gastrointestinal Endoscopy (ESGE) guidelines to begin the final dose of bowel preparation no later than 5 h before the colonoscopy and to finish it no later than 2 h before the procedure[13].
Of note, compared to other days of the week, Monday had the highest rate of inadequate preparation [Boston bowel preparation scale (BBPS) 6] of 16.5% in a 2022 retrospective review of 4279 colonoscopies. Notably, poor bowel preparation was not linked to post-holiday procedures[43].
However, in clinical practice, patients do not always adhere to the recommended timing due to a need related to the potential long distance to travel before having to perform the endoscopic exam, or as a result of the stress that they will have an urgent necessity for the public bathroom during the trip.
As a result of more accurate characterization of procedural and patient variables, bowel preparation may become more personalized.
Hospitalization is associated with a nearly twofold increase in the risk of unsuccessful bowel preparation before colonoscopy when compared to the ambulatory setting[44,45].
Furthermore, the percentage of inpatients with an adequately prepared colon does not exceed 50% because they are typically elderly, frail, and suffering from comorbidities that either prevent successful bowel prep ingestion or affect patients’ comprehension and compliance with the regimen’s instructions[44,45].
Inadequate bowel preparation increases the risk of missed adenomatous polyps or CRC, as well as a patient inconvenience; it also harms healthcare systems by causing procedures to be delayed or repeated, as well as prolonged hospital stays[45-47].
Several studies have evaluated the efficacy of various interventions, such as different laxatives, changes in preparation administration timing, and promotion of educational programs for physicians, nurses, and patients, to overcome these challenges. Lower socioeconomic class, opiate/tricyclic antidepressant use, afternoon colonoscopies, American Society of Anaesthesiologists (ASA) physical status classification system class 3, and pre-preparation nausea/vomiting were identified as the main potential predictors of inadequate inpatient preparation[46].
Furthermore, Gkolfakis et al[45] recently published a systematic review with meta-analysis to evaluate the efficacy of various interventions to improve the quality of colon preparation in inpatients.
In this study, which included 17 studies and 2733 inpatients, the authors concluded that, despite several interventions, only nearly two-thirds of inpatients achieve adequate colon preparation before colonoscopy, and that educational interventions significantly improve inpatients’ bowel preparation quality[45].
In the absence of standardized guidelines or recommendations, the ideal bowel preparation regimen for inpatients has yet to be determined[45].
In any preparation strategy, PEG-based regimens should be considered first because they are more likely to achieve adequate bowel cleansing while maintaining an optimal patient safety profile. Furthermore, case-by-case application of multiple and combined strategies (e.g., written educational material, nurse facilitation of the process, etc.) may have the potential to influence the outcome[45,48].
With the extension of life expectancy, the opportunities for medical care for elderly patients increase[49]. The rise in cancer patients, in particular, is a global challenge; the International Agency for Research on Cancer estimates that the number of cancer patients will rise dramatically[49]. Regardless of the procedure’s risks, bowel preparation can be problematic in terms of purgative effect and risk[50].
Patients must also go through a period of dietary restriction, fasting, and later fluid restriction, which can be difficult for people who have diabetes or chronic kidney disease (CKD)[50]. Furthermore, an elderly patient whose only access to the toilet is via stair-lift may be unable to prepare at home, necessitating in-patient admission for bowel preparation or alternative investigation to colonoscopy[50].
Despite the fact that little research has been conducted on bowel preparation in the elderly and very elderly population, a 2016 study discovered that diabetes, difficulty walking, or performing activities of daily living were associated with poor bowel preparation in patients over the age of 65[50,51]. Furthermore, poor bowel preparation is the leading cause of colonoscopy failure in patients aged 90 and up[50,52].
In five years, a British Patient Safety Agency alert reported one death and 218 patient safety incidents[53]. Although the vast majority of these incidents (93%) resulted in no or minor harm, 6% resulted in moderate harm, and one patient died[53].
Omitted medication (29%), incorrect drug (23%), and incorrect or unclear dose, strength, or frequency (11%) were all considered medicine errors. Side effects of bowel preparation include hypovolaemia, renal failure, and electrolyte disturbances[54]. An accurate bowel preparation prescription, as well as consideration of potential interactions and side effects, is especially important in older adults, who are more likely to have multiple comorbidities and polypharmacy, both of which may exacerbate these side effects. They may also have less physical reserve to deal with any unforeseen complications. In patients over the age of 65, serum albumin concentration before bowel preparation, for example, may predict hypovolaemia[55].
In elderly patients with pre-existing chronic renal failure, all oral laxatives should be used with caution, and patients undergoing dialysis or with advanced CKD should consult with the renal team. Sodium phosphate preparations should be avoided entirely in patients with renal failure[54]. Furthermore, any medications that, when combined with bowel preparation, may worsen renal failure, such as diuretics, angiotensin-converting enzyme inhibitors, and angiotensin II receptor antagonists, should be avoided[54].
Cleaning success was achieved in 70.3% of patients aged > 65 years with comorbidities undergoing colonoscopy after a 1-L PEG-ASC, 2-L PEG/PEG-ASC, or 4-L-PEG-based preparation, according to a recent retrospective analysis of a prospective cohort. Notably, elderly patients had a higher rate of inadequate colonoscopy cleansing than non-elderly patients (7.0% vs 3.8%, P = 0.012)[56]. Split regimen, adequate cleansing at last colonoscopy, tolerability score, a low-fiber diet for at least 3 days, and colonoscopy within 5 h after the end of preparation (OR = 2.43, P = 0.003; OR = 2.29, P = 0.02; OR = 1.29, P < 0.001; OR = 2.45, P = 0.001; OR = 2.67, P = 0.008, respectively) were predictors of bowel cleansing in the elderly[56]. Also, 1-L PEG had a higher tolerability score than 2-L and 4-L PEG in elderly (7.7 vs 7.2 and 7.2, P = 0.099). Interestingly, when compared to the other preparations, the 1-L PEG-ASC preparation was associated with higher quality cleansing of the right colon (39.6% vs 17.0% vs 9.4%, respectively, P < 0.001) and may thus be the preferred option for the elderly.
Methods for achieving safe and adequate bowel preparations in the elderly should include clear instructions, reminder calls, and case management for potential confounding patient-related factors[57].
Endoscopic techniques have become increasingly important in recent years, particularly in the treatment of colorectal flat lesions[58,59].
At the same time, the significance of accurate detection and assessment of such lesions in predicting malignancy has become clear. Indeed, proper bowel preparation is critical for colonoscopy because it allows for visualization of the entire colonic mucosa and improves the safety of therapeutic maneuvers[6,60].
In contrast, poor preparation lengthens the procedure, increases the risk of complications, and increases the likelihood of missing lesions[61].
The percentage of colonoscopies performed with inadequate bowel cleansing ranges between 5% and 35%[12,44,62-66].
Because a proper bowel cleansing regimen increases the likelihood of success, identifying risk factors for inadequate bowel cleansing is critical. Patient-related predictors of colonoscopy preparation failure include prior inadequate bowel cleansing, a history of constipation, increasing age (> 65 years), male gender, low health literacy (e.g., cognitive skills), inpatient status, obesity, diabetes mellitus (DM), inflammatory bowel disease (IBD), unexplained chronic diarrhea, megacolon, cirrhosis, stroke, dementia or Parkinson’s disease, patients at increased risk for electrolyte abnormalities (e.g., patients on diuretics), uncontrolled hypertension, severe congestive heart failure (New York Heart Association class III or IV), severe CKD (creatinine clearance < 30 mL/min/1.73 m), previous colorectal surgery, use of constipation-related medications (narcotics and tricyclic antidepressants), severe colonic stricture or obstructing tumor or perforation, dysphagia, gastroparesis, or gastric outlet obstruction, pregnancy or lactation[12,64,65,67-69].
Administration of the entire preparation the night before the colonoscopy, rather than split-dosing, and a later start time for the colonoscopy are procedure-related risk factors for inadequate bowel preparations[44]. The presence of one or more of these risk factors can influence bowel cleansing regimens and choices[70].
ESGE recommends the use of high-volume or low-volume PEG-based regimens, as well as non-PEG-based agents that have been clinically validated for routine bowel preparation. For elective colonoscopy, split-dose bowel preparation (with or without additional measures) should be used, as it has been linked to improved preparation quality[13].
In 2015, Dik et al[71] conducted a Dutch study that included only patients who who received a split-dose regimen. In total, 1331 colonoscopies were included in the study, with 12.9% having insufficient bowel preparation. Diabetes, chronic constipation, a history of abdominal or pelvic surgery, and recent hospitalization are all risk factors for poor bowel cleansing quality.
Gandhi et al[65] conducted a meta-analysis of independent risk factors in over 75,000 people receiving a split-dose bowel preparation. Constipation, diabetes, and medication use were identified as predictors of colonoscopy preparation failure despite the studies’ heterogeneity. In a 2018 meta-analysis by Mahmood et al[12], age, male sex, inpatient status, DM, hypertension, cirrhosis, narcotic use, constipation, stroke and tricyclic antidepressants were associated with inadequate bowel cleansing (OR = -1.20, OR = 0.85, OR = 0.57, OR = 0.58, OR = 0.58, OR = 0.49, OR = 0.59, OR = 0.61, OR = 0.51, respectively). Furthermore, in Western countries, diabetes, cirrhosis, male sex, stroke history, and tricyclic antidepressant use were found to be stronger risk factors for inadequate bowel preparation than in Asian countries.
In a 2022 United States retrospective study of 1029 patients, Agrawal et al[66] discovered the following factors to be associated with colonoscopy cancellations: Graduate school education, Hispanic ethnicity, a hemoglobin level of 10 g/dL, and if the colonoscopy was done for other indications (OR = 1.93, P = 0.04; OR = 0.47, P = 0.01; OR = 1.41, P = 0.05; OR = 0.53, P = 0.04, respectively). Dementia (OR = 2.44, P = 0.02) and gastroparesis (OR = 3.97, P = 0.01) were factors associated with poor bowel preparation in a multivariate analysis.
Ultimately, in a 2016 United States study of 2401 colonoscopies, African Americans were 70% more likely to have suboptimal preparation (95%CI 1.2-2.4); DM, tricyclic antidepressant use, narcotic use, and Miralax-Gatorade prep vs 4-L PEG 3350 were all associated with suboptimal preparation quality in a multivariable analysis (OR = 2.3, 95%CI 1.6-3.2; OR = 2.5, 95%CI 1.3-4.9; OR = 1.7, 95%CI 1.2-2.5; OR = 0.6, 95%CI 0.4-0.9, respectively)[72].
Obesity: Obesity, when combined with other risk factors, is an independent predictor of poor bowel preparation during a colonoscopy in practice.
In a 2013 retrospective study of 2163 consecutive patients, mostly men, who had colonoscopies in Indiana, one of the independent risk factors for inadequate preparation was a body mass index (BMI) of ≥ 30 Kg/m2 (OR = 1.46, 95%CI 1.21-1.75, P < 0.0001)[73].
Sharara et al[74] discovered that BMI was an independent risk factor for inadequate preparation in a 2016 Arabic study involving 541 patients. Obesity was associated with an OR of 5.3 (95%CI 1.4-19.8, P = 0.01) when compared to normal BMI. In a prospective study of 195 patients, obese patients had comparable rates of inadequate preparation to normal-weight individuals (OR = 0.7, 95%CI 1.10-3.96, P = 0.68). Patients who were underweight performed significantly worse than those with normal BMI (OR = 8.0, 95%CI 1.1-58.0, P = 0.04).
A high BMI had a significant difference in the effect of bowel cleansing between studies with mostly female patients (OR = 1.05) and studies with mostly male patients (OR = 1.30) (P = 0.013 for the difference), according to a 2018 systematic review and meta-analysis[65]. Inadequate bowel preparation was linked to diabetes (OR = 1.79) and hypertension (OR = 1.25), among other risk factors.
According to a recent study by Passi et al[75] in the United States, 49.4% of 27696 colonoscopies had insufficient bowel preparation, which was most common in the class III obesity group. When compared to the normal body mass index (BMI) group, a BMI of 30 kg/m2 and 40 kg/m2 was associated with an increased risk of an incomplete colonoscopy (P = 0.001 for overweight, P = 0.0004 for class I/II obesity), a longer procedure (P < 0.05 for all), and poorer tolerance (P < 0.0001 for class I/II obesity, P = 0.016 for class III obesity).
According to some studies, distinct bowel preparations are beneficial and safe for obese patients. In a 2012 prospective Australian study of 104 patients showing a similar bowel preparation quality after using sodium picosulphate, 90% of non-obese and 89% of obese patients had good bowel preparation (P > 0.99)[76].
Patients were randomized to receive split-dosing of either NER1006, 2-L PEG-ASC, or OSS in a recent (2021) two phases III Spanish trials[77]. Split-dose NER1006 (1-L-PEG-ASC) was associated with high levels of cleansing, ranging from 87% to 94% in a total of 551 patients, including those who were obese or diabetic. Obese males aged above 60 had significantly higher overall and high-quality bowel-cleansing success rates with 1-L-PEG-ASC, at 100.0% and 72.7%, respectively, compared to 86.7% and 50.0% in the control group (P = 0.015 and P = 0.033, respectively).
Diabetes mellitus: Due to the high prevalence of gastrointestinal symptoms and the increased risk of CRC, diabetic patients have a higher demand for colonoscopies than the general population[78-80].
As a result, adults with diabetes should be properly screened, and a longer bowel preparation may be necessary to ensure an adequate endoscopic examination[81].
Due to dietary/medication regimen changes, narcotic use, and diabetes-related complications/comorbidities such as hypoglycemia, electrolyte imbalance, acute renal failure, and ketoacidosis, diabetic patients are at risk of poor bowel preparation[82,83].
DM has been identified as an independent risk factor influencing bowel preparation quality by decreasing colonic motility[71,84-86].
The rate of insufficient bowel preparation in diabetic patients ranges from 9% to 30%[84,87,88], which should be significantly reduced by implementing a multifactorial strategy. Surprisingly, even though DM patients are notoriously difficult to prepare, few studies have looked into the best bowel preparation management strategy in this setting. In diabetic patients, taking 10 ounces of magnesium citrate two days before colonoscopies, in addition to a single 4-L PEG dose, improved colon cleansing (from 54% to 70%)[89].
Another single-blind prospective trial on DM patients discovered that adding lubiprostone, a highly selective locally-acting activator of chloride channels used in functional constipation, to a single 4-L PEG the day before the procedure improved colon cleansing; however, the improvement was statistically non-significant due to the small sample size[90]. A small trial in DM outpatients examined additional bowel cleansing strategies with 6-L PEG, but the results were not encouraging[84].
Current United States guidelines do not endorse any of these recommendations, instead recommending a split-dose bowel cleansing regimen for DM patients with no adjustments[8]. A subsequent European randomized, single-blind, superiority trial compared a conventional bowel preparation protocol with a diabetic-specific preparation protocol, which included a low-fiber diet for three days, a clear liquid diet for one day, and a 4-L split-dose PEG regimen[88].
The latter group was given a special education program that included diet, laxative intake, and blood glucose-lowering agent adjustment instructions. In the conventional protocol, inadequate bowel cleansing was statistically more common than in the diabetic-specific protocol (20% vs 7%; RR = 3.1, 95%CI 1.2-8.0, P = 0.014).
Chronic constipation: The most prevalent type of constipation, functional chronic constipation, frequently affects women and the elderly who undergo colonoscopies often and ranges in prevalence from 2% to 27% in Western countries[91-93]. Constipation has been identified as a risk factor for inadequate bowel preparation[13,94]. Currently, ESGE does not recommend any specific bowel preparation in patients suffering from constipation chronically[13].
In elderly patients, slow transit constipation, defined by decreased bowel movements, may result in insufficient laxative wash-out and bowel preparation. This hypothesis was confirmed in a 2015 Korean study[95], which discovered that colonic transit time of more than 30 h was associated with inadequate bowel preparation. Furthermore, slow-transit constipation, as determined by radiopaque marker colonic transit testing, was linked to a more than 2-fold increased risk of poor bowel preparation in a 2022 study of 274 American patients with chronic constipation (OR = 2.2, 95%CI 1.1-4.4)[96].
In patients with a history of constipation, additional bowel purgatives should be considered[8]. Numerous studies in recent years have suggested different bowel preparation regimens in patients with chronic constipation, with good results using a variety of laxatives.
In a double-blind 2008 United States trial, 200 CRC screening patients were randomly assigned to receive a 24 g dose of lubiprostone or placebo before a split-dose PEG with electrolytes bowel preparation in the absence of dietary restriction[97].
Split-dose PEG, electrolytes, and lubiprostone pretreatment was found to be more effective (P = 0.001) and tolerable (P = 0.003) than placebo, most likely due to a reduction in abdominal bloating (P = 0.049)[97].
In a 2015 Italian randomized, single-blind study, 400 constipated patients were enrolled and randomly assigned to one of two arms: Split 2-L PEG-citrate-simethicone plus 2-day bisacodyl or split 4-L PEG[98]. In a 2016 Chinese RCT[99], the addition of lactulose one day before colonoscopy in combination with 4-L split-dose PEG was shown to be significantly superior (P < 0.05) to the conventional preparation with oral PEG and electrolytes for colonoscopy bowel preparation.
In terms of ease of administration (P < 0.001), willingness to repeat (P < 0.001), and compliance (P = 0.002), the 2-L PEG-citrate-simethicone/bisacodyl solution was found to be significantly more acceptable[98]. According to a 2019 RCT[100], the optimal dose of crystalline lactulose for Japanese constipated patients is 26 g/day. A short therapy cycle of PEG plus electrolytes was effective and safe in improving bowel preparation in chronic constipation patients in a 2020 and 2021 Japanese study[101,102]. In 2016, a larger Asian population was studied in a randomized, double-blind, placebo-controlled trial[103]. Surprisingly, when lower doses of PEG were combined with lubiprostone, no significant difference in preparation quality was observed.
In a 2021 systematic review and meta-analysis of three RCTs, Dang et al[104] enrolled 225 chronically constipated patients, with 47.6% receiving sodium phosphate and 52.4% receiving PEG. Despite the low quality of evidence, patients who received sodium phosphate before their colonoscopy had cleaner colons than those who received PEG (OR = 1.87, 95%CI 1.06-3.32, P = 0.003).
IBD: Inadequate bowel preparation has also been linked to comorbidities such as IBD[105]. This was demonstrated in an Italian multicenter, randomized, single-blind study of 211 adult outpatients with ulcerative colitis (UC) undergoing colonoscopy and receiving either 2-L PEG plus bisacodyl or 4-L PEG[106]. Low-volume PEG was not inferior to 4-L PEG for bowel cleansing in UC (P = NS), but it was better tolerated (P < 0.0001) and accepted (P < 0.0001). The split dosage was associated with better cleansing regardless of preparation. A period of more than 6 h between the end of preparation and the colonoscopy predicted poor cleansing.
In a 2021 retrospective analysis of a prospective cohort, Maida et al[107] demonstrated the efficacy and safety of 1-L PEG-ASC in 45% of 411 patients.
IBD patients had higher cleansing success (92.9% vs 85.4%, P = 0.02) than controls, with a similar number of patients experiencing adverse events (22.2% vs 21.2%, P = 0.821) and treatment-emergent adverse events (51 vs 62%, P = 0.821). Furthermore, the presence of IBD (OR = 2.51, P = 0.019), lower age (OR = 0.98, P = 0.014), a split regimen (OR = 2.43, P = 0.033), the absence of diabetes (OR = 2.85, P = 0.015), and chronic constipation (OR = 3.35, P = 0.005) were all independently associated with cleansing success[107].
Endoscopic disease activity has recently been discovered to predict suboptimal bowel preparation, and biological therapy has been shown to protect IBD patients from it.
In a 2022 United States study by Kumar et al[108], the moderate-to-severe endoscopic disease was associated with higher odds of suboptimal bowel preparation vs mild or inactive disease [adjusted OR (aOR) 2.7; (95%CI 1.52-4.94)], whereas baseline biologic use was associated with a lower odds of suboptimal bowel preparation [aOR, 0.24 (0.09-0.65)] among the overall IBD cohort. Furthermore, age > 65 years and single-dose vs split-dose bowel preparation were independent predictors of suboptimal bowel preparation [aOR, 2.99 (1.19-7.54); aOR, 2.37 (1.43-3.95), respectively].
Liver cirrhosis: Liver cirrhosis predicts poor bowel preparation at screening colonoscopy[64,109].
This finding is most likely due to multiple factors impairing intestinal motility in cirrhotic patients[110,111]. The role of chronic liver disease in predisposing to inadequate bowel preparation in the absence of cirrhosis is unknown. In a 2016 United States study, Anam et al[112] compared 120 cirrhotics to 220 non-cirrhotics with chronic liver disease, and the first group performed significantly worse on bowel preparation. Cirrhotics had lower bowel preparation scores than non-cirrhotics (P = 0.0027), with cirrhotics having the lowest (48%) and non-cirrhotics having the highest (30%), with no effect of the MELD score.
The rate of failure to complete the bowel preparation and the incidence of side effects were comparable in 53 cirrhotics compared to 52 healthy subjects undergoing screening colonoscopy, according to an Italian 2015 study by Salso et al[113]. Despite this, nearly half of the cirrhotics (49% vs 5% control; P < 0.001) had poor bowel cleansing.
In a 2017 Chinese retrospective study, Lee et al[114] compared the safety of two bowel-cleansing agents in patients with liver cirrhosis (2-L PEG-ASC vs 4-L PEG). Patients preferred the 2-L PEG-ASC over the 4-L PEG group for acceptability and compliance. Finally, because both groups were successfully cleansed, the authors concluded that using 2-L PEG-ASC for colonoscopy in cirrhotics was a safe option.
Decompensated cirrhosis patients are more prone to frailty, cognitive abnormalities, and decreased ambulation. Clayton et al[115] discovered that patient educational video did not improve bowel preparations (split-prep) in the pre/post-intervention period in 121 patients with decompensated cirrhosis undergoing colonoscopy during the initial liver transplantation evaluation (29.8% vs 31.9%, respectively).
Furthermore, patients with moderate to severe ascites had a significantly higher rate of inadequate colonoscopy bowel preparation than non-ascites patients[115].
CKD: The use of cleansing agents in patients with CKD should be carefully evaluated due to the risk of electrolyte imbalance or worsening renal function[116]. No significant changes in vital or biochemical parameters have been linked to high volume osmotically balanced solutions containing PEG and electrolytes capable of maintaining bowel lumen isosmosis[13].
According to previous research[117,118], PEG is generally safe in CKD patients; however, adequate hydration and renal function monitoring should be ensured before and after colonoscopy in some cases to avoid acute kidney failure[119]. Individualized laxative choice is strongly advised for patients at risk of hydroelectrolyte disturbances (moderate quality evidence)[13].
Because of hyperosmolarity and the risk of magnesium toxicity, as well as acute phosphate nephropathy, magnesium-based preparations and sodium phosphate should be avoided in CKD patients[13,120,121].
Furthermore, due to the poor tolerability of high-volume PEG-based regimens, low-volume PEG (2-L) solutions with ascorbic acid (PEG-ASC) solutions have been proposed to reduce the patient’s excessive fluid intake. Ascorbic acid can act as an osmotic agent and enhance the laxative effect of PEG due to its hexose structure[122], and its pleasant taste makes it easier for patients to swallow. Ascorbic acid, on the other hand, has been linked to the formation of renal stones and acidosis, with contradictory results[123,124]. As a result, low-volume preparations continue to be a challenge for many CKD patients.
Notably, ESGE guidelines do not recommend aspartame and ascorbate-containing solutions (such as 2-L and 1-L PEG-ASC solutions) for patients with renal insufficiency and creatinine clearance less than 30 mL/min. A high rate of hypernatremia has been observed following the administration of 1-L PEG-ASC, owing primarily to the product’s sodium content[13]. If low volume PEG solutions combined with citrate and simethicone are administered to patients with creatinine clearance less than 30 mL/min, caution is advised[13].
In a 2016 retrospective study, a same-day 1-L low-volume PEG regimen with a previous-day low-residue diet and laxative was tested to improve tolerability[125]. The study included 5,427 patients who were instructed to consume a low-residue fiber diet with 10 mL sodium picosulfate one day before the colonoscopy, followed by 1-L low-volume PEG and 0.5-L water four hours before the exam. In 86 CKD patients (creatinine 1.1 mg/dL), the BBPS 6 success rate was 94.1%, and there were no serious complications[125]. Lee et al[123] found that the 2-L PEG-ASC was a safe choice for bowel preparation before colonoscopy in patients with impaired renal function in a 2016 study.
In one retrospective cohort, patients with a GFR of 60 mL/min were given either 4-L PEG or 2-L PEG-ASC solutions. Patients in the 2-L PEG-ASC group (n = 61) rated their tolerance and acceptability higher than those in the 4-L PEG group (n = 80)[123]. After either preparation, there was no statistically significant change in electrolytes, blood urea nitrogen, or creatinine. When the regimens were compared, 7.5% of 4-L PEG patients and 11.5% of 2-L PEG-ASC patients had a transient > 30% increase in creatinine levels, though the differences were not statistically significant[123]. Ohmiya et al[126] discovered that same-day conventional bowel preparation with PEG electrolyte lavage solution plus Ascorbate (PEG-ELS-ASC) was safe and effective in 56 CKD patients in the Japanese 2021 study.
Only retrospective cohorts have found PEG to be safer than other formulations in patients with impaired renal function[127].
The most severe kidney injury case reported reversible post-colonoscopy acute renal failure within a few weeks of oral sodium phosphate (OSP) intake, necessitating renal replacement therapy in 19% of patients[128]. Furthermore, during the 2006-2007 time period, the Food and Drug Administration received reports of 171 cases of renal failure caused by the use of OSP and 10 cases caused by the use of PEG[128]. A 2005 retrospective population-based Iceland study found that the risk of biopsy-proven acute phosphate nephropathy is about one in every 1000 OSP doses sold[128].
Three RCTs comparing OSS preparation to 4-L PEG found that split OSS was noninferior to split high-volume PEG in terms of efficacy, safety, and tolerability. Although real-world data on OSS in the setting of renal insufficiency are limited, and despite no significant differences in the frequency of acute renal failure reported with this preparation, European guidelines[13] recommend that it be avoided in patients with severe renal insufficiency (glomerular filtration rate 30 mL/min).
According to ESGE guidelines and current evidence, patients with severe renal insufficiency should be prepared with isotonic high volume PEG solutions rather than low volume PEG or non-PEG regimens.
Heart disease: Previously, it was thought that bowel preparation (particularly after administration of PEG-ELS solution) could worsen heart failure[129], as a result, except in urgent or emergency cases, exposing such patients to a colonoscopy was risky. Also, coronary heart disease has been identified as a risk factor for severe desaturation and relevant electrocardiographic changes during endoscopic sedation[130]. Furthermore, several studies have found that these solutions may be harmful to patients with heart disease due to the potential increase in plasma volume and their effects on electrolyte disturbances[131].
Thiazide diuretics and SSRIs, which have the potential to cause fluid and electrolyte imbalances, should be avoided in at-risk patients while undergoing bowel preparation[132].
Heart disease and CRC were the only predictors strongly associated with poor bowel cleansing in a 2019 Spanish single-center, endoscopist-blinded RCT of 136 patients (OR = 3.37, 95%CI 1.34-8.46, P = 0.010; OR = 3.82, 95%CI 1.26-11.61, P = 0.018, respectively)[133]. In a 2020 study, Poola et al[134] discovered that 44% of 315 inpatients’ bowel preparation was fair/poor. Poor bowel preparation was associated with elderly people who had a history of congestive heart failure.
Most medications can be taken with a small sip of water until the day of the colonoscopy. Because of the decreased oral intake prior to the procedure, some medications, such as diabetes medications or anticoagulants, may need to be adjusted. Because it causes residual feces, oral iron should be stopped at least five days before the colonoscopy[135]. Additionally, the procedure’s urgency and the availability of alternative tests must be taken into account.
In a 2017 Chinese systematic review and meta-analysis of RCTs (overall 3217 patients with chronic pain), Huang et al[136] discovered that treatment with a fixed-ratio combination of prolonged-release oxycodone/naloxone reduces the incidence of opioid-induced constipation and provides clinically significant intermediate-term bowel function improvement while maintaining pain relief. According to a 2019 Spanish study by Velázquez Rivera et al[137], tapentadol and oxycodone had better bowel function profiles with no differences in a cross-sectional observational study of 180 Spanish patients with opioid-induced constipation during long-term treatment.
Inadequate bowel cleansing is detrimental to the examination, resulting in lower ADR, longer procedural time, lower rates of cecal intubation, shorter intervals between examinations, and a 12%-22% increase in overall colonoscopy cost[4]. Previous colorectal surgery is a risk factor for patients who are difficult to prepare[64,138,139].
Previous colorectal surgery (along with diabetes, Parkinson’s disease, and liver cirrhosis) is a condition that significantly predicts inadequate colon preparation, according to a prospective study enrolling 2811 outpatients in 2012[64].
Another study published in 2015 discovered that diabetes, chronic constipation, a history of abdominal and/or pelvic surgery, and current hospitalization were all significant predictors of poor bowel cleansing in patients using a split-dose preparation scheme[71]. Improved bowel preparation strategies may be difficult to achieve in this patient population. The long-term effects of colonic surgery and anastomoses on colonic motor function are currently unknown[140]. Patients who have had colonic surgery are usually excluded from trials on the efficacy of bowel preparations due to this lack of knowledge. As a result, no evidence-based guidelines for preparing this subset of patients are now available, and the various bowel cleansing schemes rely on single-center experience or expert opinions recommending high-volume regimens[141]. Following surgery, some mechanisms affecting the enteric nervous system and autonomic innervation are known to be altered.
Indeed, Vather et al[142] discovered that distal colonic motor patterns traversed healed anastomosis sites regularly, indicating possible cellular regeneration. Some of the causes could be attributed to colorectal anastomoses’ effect on the enteric nervous system and autonomic innervation, which can result in changes in colonic retrograde and antegrade motor patterns[142-144]. Remarkably, patients who have had a previous colectomy appear to be the most difficult to prepare. While right colectomy reduces absorption and determines rapid transit, left colectomy may worsen peristalsis in moving luminal content outside the body[145].
Mussetto et al[139] found that a low-volume mixed preparation (15 mg bisacodyl plus 2-L PEG) was not inferior to 4-L PEG for adequate bowel cleansing during surveillance colonoscopy in a 2015 study of 120 patients who had prior colorectal resection for cancer (85.0% vs 81.7%, P = 0.624). Notably, the mixed low-volume regimen had a higher success rate and tolerability in patients who had previously undergone left colectomy vs right colectomy (P = 0.025 and P < 0.001, respectively). The only predictor of unsuccessful cleansing using logistic regression was previous left colectomy (P = 0.012). Similarly, Yoo et al[146] conducted a case-control study in Turkey in 2018, enrolling 200 patients who received either a low-volume or high-volume bowel preparation regimen. In terms of adequate cleansing (modified BBPS of 6-9), there was no statistical difference between the resection and control groups (88% vs 88%). Patients with a left colon resection had an OR of 0.27 (P = 0.003) for successful cleansing, according to the logistic regression analysis of the resection group, and low-volume preparation (OR = 3.09, P = 0.023) was the best predictor of an effective cleansing procedure.
According to Kim et al[147], 12.1% of the 12,881 participants in the National Cancer Center’s health screening cohort who underwent screening or surveillance colonoscopy had a history of abdominopelvic surgery. Poor bowel preparation was linked to gastric or minor intestinal surgery in a multivariate analysis (OR = 1.76, 95%CI 1.23-2.53, P = 0.002). Other types of surgery, on the other hand, did not affect bowel preparation quality.
Chung et al[148] looked at 247 patients who had previously had a colorectal resection and had a surveillance colonoscopy in a 2017 study. The right colon preservation group had a significant association with bowel cleansing quality in both univariate (22.3% vs 7.5%, P = 0.028) and multivariate (OR = 3.6, 95%CI 1.0-12.3, P = 0.038) analysis.
In contrast, Gandhi et al[65] discovered that a history of abdominal surgery (OR = 0.99) did not correlate with inadequate bowel preparation in a 2018 systematic review and meta-analysis of 67 studies involving 75818 patients.
A study (NCT02761317) is currently being conducted on patients who have had colorectal resection to compare the efficacy of bowel cleansing between the standard preparation (2-L PEG solution, 2-L PEG-ELS), low-volume preparation (10 mg bisacodyl plus 2-L PEG-ELS), and high-volume preparation (10 mg bisacodyl plus 2-L PEG-ELS) (4-L PEG-ELS).
The presence of one or more risk factors for inadequate preparation will influence preparation selection and regimen. Due to the high risk of missing clinically relevant lesions, patients with insufficient cleansing must have the colonoscopy repeated after a more thorough bowel cleansing attempt[13].
Patients who did not prepare adequately because they misinterpreted the instructions can be counseled and then directed to repeat the same bowel regimen. For patients who did not tolerate or respond adequately to the original preparation[149] alternative preparations should be tried.
Despite the lack of strong evidence from RCTs, the ESGE guidelines and some studies recommend repeating colonoscopy on the same or the next day with additional bowel cleansing (e.g., 500 mL PEG-ASC) or using enema as a salvage option in patients with insufficient bowel cleansing[72,149-152]. The bowel preparation regimen outlined below should be tailored to the potential causes of failure[13].
Some patients continue to have inadequate preparation despite following the regimen, switching regimens, and using a split-dose lavage. In such cases, two days of clear liquids are usually prescribed, followed by a morning procedure. If the patient’s preparation was extremely poor, options include adding a second laxative if contraindications exist (e.g., using 4-L PEG-ELS solution followed by a 1-L solution of magnesium citrate) or repeating the preparation administration over two days (except for sodium phosphate).
Within one year, 90% of patients who did not do adequate bowel cleansing require a repeat colonoscopy after one or more attempts at bowel cleansing[13,70]. Similarly, experts advise patients who did not tolerate or respond well to the first bowel cleansing to try another. Split-dosing should also be used for improved bowel cleansing[70]. Despite adherence to the regimen, switching to different regimens, and using a split-dose lavage, some patients continue to have insufficient bowel cleansing for the next exam, particularly those with severe comorbidities (e.g., stroke, dementia, DM, obesity), the elderly, men, and people taking psychotropic drugs, all of which are commonly associated with a chronic constipation condition[6,64,153]. The efficacy of a next-day or same-day colonoscopy after additional bowel preparation vs a later colonoscopy is limited and contradictory. In a 2009 Israelian single-center study of 235 patients with inadequate preparation, next-day colonoscopy was associated with a lower risk of secondary failure (OR = 0.31, 95%CI 0.1-0.92)[63]. In a 2013 retrospective study of 3047 procedures with inadequate cleansing, patients advised to have a next-day colonoscopy were more likely to follow the recommendation for a repeat colonoscopy[154]. In a larger 2016 United States single-center series of 397 patients with inadequate procedures, recurrent failure was observed in 30.0% of the next-day group and 23.5% of the non-next-day group (P = 0.48)[155].
In a single-center Korean prospective nonrandomized study conducted in 2014, 87 patients with insufficient preparation after an initial 4-L PEG were given either an additional 2-L PEG on the same day or a 4-L PEG plus bisacodyl one week later after 3 days of a low residue diet, with no difference found between the two regimens[149]. A 2017 Spanish randomized trial demonstrated the superiority of a high-volume PEG-based regimen over a low-volume PEG-based regimen when combined with an intensive preparation regimen[150]. In a 2018 observational study, 60 Turkish patients who had received inadequate preparation underwent a same-day repeat colonoscopy after receiving an additional laxative of 250 mL senna alkaloids with 1.5-L water, and 83% of patients had the repeat colonoscopy reach the cecum[151].
In a 2012 United States study, Sohn et al[152] found that direct administration of laxative enemas through the colonoscope into the right colon via the biopsy channel was effective in 21 patients with inadequate preparation after low-volume PEG and bisacodyl preparation. In a 2012 Japanese study, Horiuchi et al[156] published a study in which 26 patients with inadequate preparation after low-volume PEG were given 500 mL of PEG via the colonoscope biopsy channel, with 96.1% (25/26) of cases successfully prepared.
Yang et al[157] on the other hand, conducted a randomized trial in 125 patients with inadequate preparation, comparing administration of a 1-L PEG enema via colonoscope to additional oral ingestion of 2-L PEG, with 35 of 66 (53%) of the enema group and 53 of 67 (81%) of the oral group obtaining suitable preparation. 42 patients with inadequate preparation were randomly assigned to either pump irrigation or syringe irrigation in a 2012 Belgian monocenter study. In terms of pre-procedure preparation, pump irrigation outperformed hand irrigation, with a significant difference in the right colon[158].
Identifying risk factors for poor bowel cleansing can aid in determining which patients require more intensive bowel preparation. Six predictive models of inadequate bowel preparation have been developed to date based on patient potential risk factors[64,71,159-162].
Hassan et al[64] identified several factors that can significantly influence preparation quality in a 2012 Italian multicenter prospective study of 2811 consecutive outpatients, which were then used to develop an accurate predictive model. Overweight, male sex, having a high BMI, older age, previous colorectal surgery, cirrhosis, Parkinson’s disease, diabetes, and positive fecal occult test results were all associated with inadequate bowel preparation. With 60% sensitivity, 59% specificity, 41% positive predictive value, and 76% negative predictive value, these factors predicted which patients would have inadequate cleansing.
In the validation cohort, the scale’s discriminative ability was strong, with the area under the curve (AUC) of 0.77. An ASA score of 3, use of tricyclic antidepressants or narcotics, diabetes, constipation, previous abdominal and/or pelvic surgery, history of inadequate bowel preparation, and hospitalization were identified as independent predictors of inadequate bowel preparation in a 2015 Dutch study by Dik et al[71]. The scale’s discriminative ability was strong in the validation cohort, with an AUC of 0.77. Finally, in the multivariate analysis of 667 consecutive Spanish outpatients in 2017, antidepressants (OR = 4.25, 95%CI 1.91-9.47), co-morbidity (OR = 3.35, 95%CI 2.16-5.18), constipation (OR = 2.09, 95%CI 1.29-3.40), and abdominal/pelvic surgery (OR = 1.60, 95%CI 1.03-2.47) were independent predictors of inadequate cleansing. According to these findings, the model with all of these variables had an AUC of 0.72 in the development cohort and 0.70 in the validation cohort of 409 patients[150].
Berger et al[160] developed a predictive score called “Prepa-Co” in a recent French single-center study, allowing the identification of patients at high risk of inadequate bowel preparation. In total, 561 patients were included, with 25% having inadequate bowel preparation. In the prediction model of inadequate bowel preparation, the risk score includes seven variables: Diabetes or obesity, irregular physical activity, cirrhosis, use of antidepressants or neuroleptics, use of opiate medication, surgery history, and history of inadequate bowel preparation. With an AUC of 0.62, Prepa-Co correctly predicted bowel cleanliness in 68.3% of cases, with a specificity of 75.8% and a negative predictive value of 80.8%.
Sadeghi et al[161] conducted a population-based study on 2476 Iranian adults in 2022, and age, gender, ethnicity, BMI, abdominal circumference, fruit consumption, smoking, NSAIDs, SSRIs, education, constipation, physical activity, and diabetes were all factored into the predictive model, with the AUC reaching 0.70 in the final step.
Kurlander et al[162] developed prediction models for bowel preparation inadequacy in a retrospective cohort of 6885 United States veterans who underwent colonoscopy. The AUC for the validation cohort was 0.66 (95%CI 0.62-0.69), whereas the AUC for the validation cohort using random forest machine learning was 0.61 (95%CI 0.58-0.65).
So far, none of these predictive models have been tested outside of their validation cohorts, and no study has attempted to use a different regimen on patients who have risk factors for poor colon cleanliness. Furthermore, the ESGE concluded in 2019 that there was insufficient data to recommend the use of specific predictive models for inadequate bowel preparation in clinical practice[13].
To date, numerous efforts have been applied to increase the sensitivity of colonoscopy in the detection of polyps and advanced adenomas, reducing the risk of I-CRC[163,164]. These include the application of key-performance quality measures for colonoscopy[165], the use of distal attachment devices[166,167], and new intestinal preparations improving the quality of bowel cleansing[168].
Despite these efforts, adequate bowel cleansing remains a basic prerequisite for a quality colonoscopy, since it can affect other quality measures including ADR and cecal intubation rate.
Prior inadequate bowel cleansing, chronic constipation, elderly, male gender, low health literacy, obesity, diabetes, IBD, cirrhosis, neurologic disease, risk of electrolyte abnormalities, severe heart and renal failure, previous colorectal surgery, use of constipation-related medications, gastroparesis, and severe colonic stricture are the major patient-related predictors of colonoscopy preparation failure.
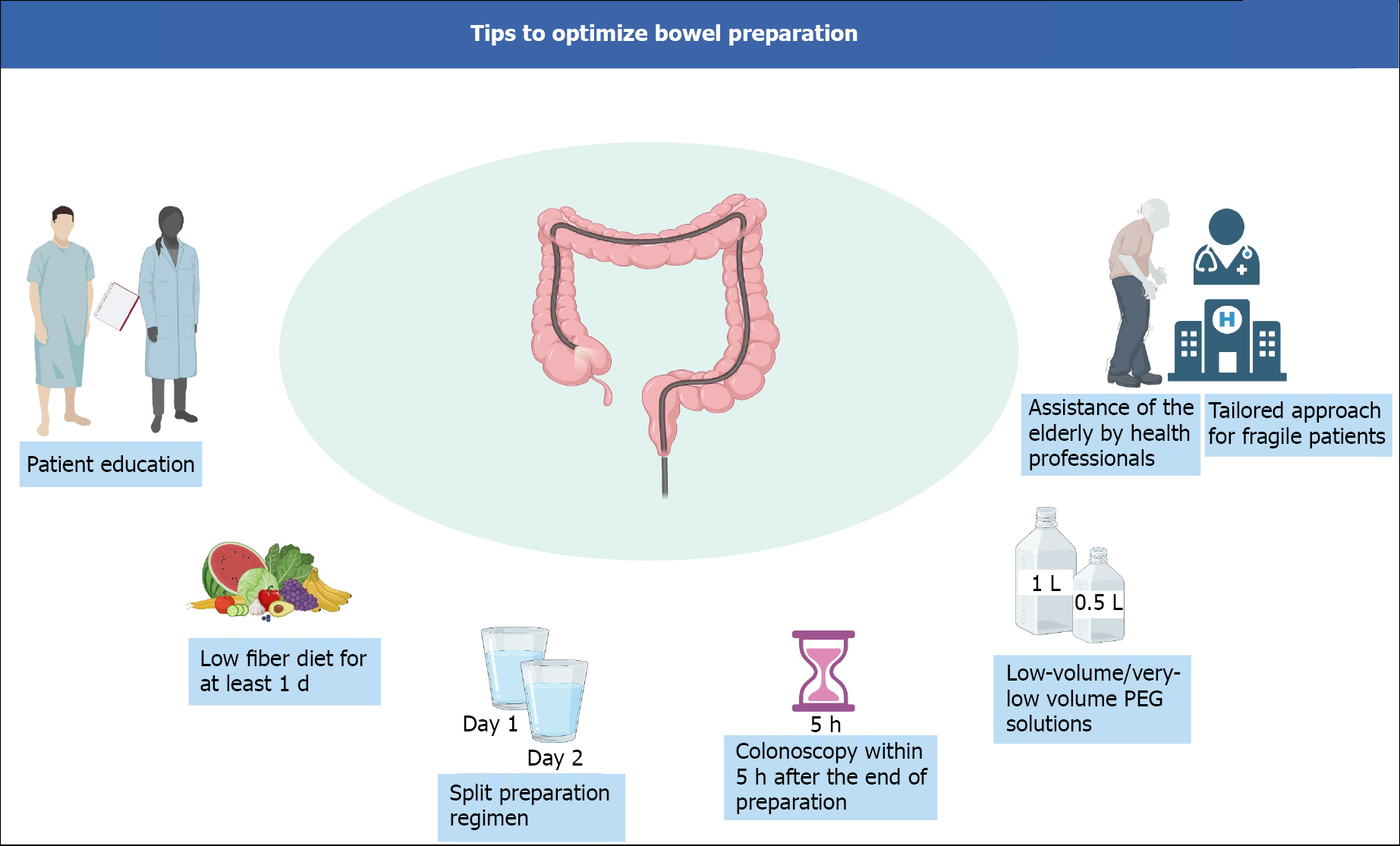
In the elderly, a strategy including a prolonged low-fiber diet, split preparation regimen, and colonoscopy within 5 h of the end of preparation, may increase the cleansing success rates. Furthermore, even though no specific product is specifically recommended in the other cases for difficult-to-prepare patients, recent evidence suggests that 1-L PEG-ASC preparation may be preferred in hospitalized and IBD patients. Figure 2 depicts a schematic view of the main bowel preparation tips to achieve a successful bowel cleansing.
According to current guidelines, patients with severe renal insufficiency (creatinine clearance less than 30mL/min) should be prepared with isotonic high volume PEG solutions, whereas low volume PEG plus adjuvants (e.g., 1-L/2-L-PEG-ASC, and 1-L PEG plus citrate) or non-PEG regimens (e.g., MCSP or OSS) are not advised.
To determine the most effective bowel preparation for difficult-to-prepare elderly, constipated, and cirrhotic patients, more high-quality research, including prospective studies with randomized designs, is required. Larger, multicenter, prospective studies are needed to determine the best bowel preparation for patients with prior abdominal-pelvic surgery, most importantly to improve ADR, especially for flat lesions in the right colon in patients who had a left colon resection.
Moreover, regardless of type of bowel solution, in very elderly patients with comorbidities, a careful assistance from family members or those who care for the elderly is necessary to guarantee the compliance to diet and preparation modalities.
In conclusion, an accurate characterization of procedural and patient variables may lead to a more personalized approach to bowel preparation (Table 1), reducing the risk of missed lesions and of I-CRC.
| Clinical scenario | Remarks |
| Hospitalization | PEG-based regimens should be considered first in any preparation strategy because they are more likely to achieve adequate bowel cleansing while maintaining an optimal patient safety profile[13,45,48] |
| Furthermore, multiple, combined strategies (e.g., written educational material, nurse facilitation of the process, etc.) based on a case-by-case decision could influence the outcome[45,48] | |
| Although no specific product is strongly recommended for difficult-to-prepare patients, clinical evidence suggests that a 1-L PEG-ASC preparation may be preferred in hospitalized patients[22] | |
| Elderly | A strategy that includes a low-fiber diet for an extended period of time, a split preparation regimen, and a colonoscopy within 5 h of the end of preparation may improve cleansing success rates in the elderly[57] |
| A 1-L PEG preparation may be preferred for the elderly due to higher cleansing quality and higher compliance due to lower volume[56,57,77] | |
| Obesity | ESGE recommends the use of high volume or low volume PEG-based regimens, as well as non-PEG-based agents that have been clinically validated for routine bowel preparation[13] |
| For elective colonoscopy, split-dose bowel preparation (with or without the additional measures) should be used, as it has been linked to improved preparation quality[13] | |
| Diabetes mellitus | Current US guidelines do not support assumption of lubiprostone or magnesium citrate, instead recommending a split-dose bowel cleansing regimen with no adjustments for DM patients[8] |
| Chronic constipation | ESGE does not recommend any specific bowel preparation in constipation patients[13] |
| Inflammatory bowel disease | Split dosage was associated with better cleansing regardless of preparation in some studies[106,108] |
| 1-L PEG-ASC is associate to higher cleansing success and good safety and should be preferred[107] | |
| Liver cirrhosis | The use of 2-L PEG-ASC for colonoscopy in liver cirrhosis to be a safe option[114] |
| Chronic kidney disease | All oral laxatives should be used with caution in patients with pre-existing chronic renal failure and liaison with the renal team is advised in patients undergoing dialysis or with advanced chronic kidney disease[54,106] |
| Individualized laxative selection is strongly recommended for patients at risk for hydroelectrolyte disturbances (moderate quality evidence)[13] | |
| Because of the risk of magnesium toxicity and acute phosphate nephropathy, magnesium-based preparations and sodium phosphate should be avoided in chronic kidney disease patients[54,120,121] | |
| Also, because high-volume PEG-based regimens are poorly tolerated, low-volume PEG (2-L) solutions with ascorbic acid (PEG-ASC) have been proposed to reduce the patient’s excessive fluid intake[122] | |
| According to ESGE guidelines, patients with severe renal insufficiency (creatinine clearance less than 30 mL/min) should be prepared with isotonic high volume PEG solutions, whereas low volume PEG plus adjuvants (e.g., 1L-, 2L-PEG-ASC, 1 L PEG plus citrate) or non-PEG regimens (e.g., MCSP or oral sulfate solution) are not advised[13] | |
| Heart disease | Bowel preparation (particularly after administration of PEG-ELS solution) could worsen heart failure[129] |
| Polypharmacy | Most medications can be taken up until the day of the colonoscopy and are taken with a small sip of water[135] |
| Some medications, such as diabetes medications or anticoagulants, may need to be adjusted due to decreased oral intake prior to the procedure[13] | |
| Oral iron should also be discontinued at least five days before the colonoscopy because it causes residual feces[135] | |
| History of colorectal surgery | In a study of 120 patients with prior colorectal resection for colorectal cancer, a low-volume mixed preparation (15 mg bisacodyl plus 2-L PEG) was not inferior to a high-volume regimen (4-L PEG) for adequate bowel cleansing during surveillance colonoscopy[139] |
| In patients who had previously undergone left colectomy vs right colectomy, the mixed low-volume regimen had a higher success rate and tolerability[139,146] | |
| History of poor bowel preparation | Despite a lack of strong evidence from randomized controlled trials, the ESGE guidelines and some studies recommend repeating colonoscopy using same-day or the next day with additional bowel cleansing (e.g., 500 mL PEG plus ascorbate) or using enema as a salvage option in patients with inadequate bowel cleansing[73,149-152,156] |
| The next bowel preparation regimen should be tailored to the potential causes of failure[13] |
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Imaeda H, Japan; Li GF, China; Lin WR, Taiwan S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Løberg M, Kalager M, Holme Ø, Hoff G, Adami HO, Bretthauer M. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med. 2014;371:799-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 2. | Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 920] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 3. | Sulz MC, Kröger A, Prakash M, Manser CN, Heinrich H, Misselwitz B. Meta-Analysis of the Effect of Bowel Preparation on Adenoma Detection: Early Adenomas Affected Stronger than Advanced Adenomas. PLoS One. 2016;11:e0154149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 4. | Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol. 2002;97:1696-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 471] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 5. | Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 700] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 6. | Wexner SD, Beck DE, Baron TH, Fanelli RD, Hyman N, Shen B, Wasco KE; American Society of Colon and Rectal Surgeons; American Society for Gastrointestinal Endoscopy; Society of American Gastrointestinal and Endoscopic Surgeons. A consensus document on bowel preparation before colonoscopy: prepared by a task force from the American Society of Colon and Rectal Surgeons (ASCRS), the American Society for Gastrointestinal Endoscopy (ASGE), and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Gastrointest Endosc. 2006;63:894-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 211] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 7. | Hillyer GC, Basch CH, Lebwohl B, Basch CE, Kastrinos F, Insel BJ, Neugut AI. Shortened surveillance intervals following suboptimal bowel preparation for colonoscopy: results of a national survey. Int J Colorectal Dis. 2013;28:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Johnson DA, Barkun AN, Cohen LB, Dominitz JA, Kaltenbach T, Martel M, Robertson DJ, Boland CR, Giardello FM, Lieberman DA, Levin TR, Rex DK; US Multi-Society Task Force on Colorectal Cancer. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the US multi-society task force on colorectal cancer. Gastroenterology. 2014;147:903-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 299] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 9. | Radaelli F, Meucci G, Sgroi G, Minoli G; Italian Association of Hospital Gastroenterologists (AIGO). Technical performance of colonoscopy: the key role of sedation/analgesia and other quality indicators. Am J Gastroenterol. 2008;103:1122-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Lee TJ, Rutter MD, Blanks RG, Moss SM, Goddard AF, Chilton A, Nickerson C, McNally RJ, Patnick J, Rees CJ. Colonoscopy quality measures: experience from the NHS Bowel Cancer Screening Programme. Gut. 2012;61:1050-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 11. | Maida M, Annibale B, Benedetti A, Burra P, Frulloni L, Ianiro G, Luzza F, Repici A, Savarino E, Sinagra E, Vecchi M, Ricciardiello L; Italian Society of Gastroenterology (SIGE). Quality of endoscopic screening for colorectal cancer in Italy: A national survey. Dig Liver Dis. 2022;54:1410-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Mahmood S, Farooqui SM, Madhoun MF. Predictors of inadequate bowel preparation for colonoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (1)] |
| 13. | Hassan C, East J, Radaelli F, Spada C, Benamouzig R, Bisschops R, Bretthauer M, Dekker E, Dinis-Ribeiro M, Ferlitsch M, Fuccio L, Awadie H, Gralnek I, Jover R, Kaminski MF, Pellisé M, Triantafyllou K, Vanella G, Mangas-Sanjuan C, Frazzoni L, Van Hooft JE, Dumonceau JM. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2019. Endoscopy. 2019;51:775-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 351] [Article Influence: 58.5] [Reference Citation Analysis (4)] |
| 14. | Enestvedt BK, Tofani C, Laine LA, Tierney A, Fennerty MB. 4-Liter split-dose polyethylene glycol is superior to other bowel preparations, based on systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2012;10:1225-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 15. | Martel M, Barkun AN, Menard C, Restellini S, Kherad O, Vanasse A. Split-Dose Preparations Are Superior to Day-Before Bowel Cleansing Regimens: A Meta-analysis. Gastroenterology. 2015;149:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 16. | Spada C, Cesaro P, Bazzoli F, Saracco GM, Cipolletta L, Buri L, Crosta C, Petruzziello L, Ceroni L, Fuccio L, Giordanino C, Elia C, Rotondano G, Bianco MA, Simeth C, Consalvo D, De Roberto G, Fiori G, Campanale M, Costamagna G. Evaluation of Clensia(®), a new low-volume PEG bowel preparation in colonoscopy: Multicentre randomized controlled trial versus 4L PEG. Dig Liver Dis. 2017;49:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Kump P, Hassan C, Spada C, Brownstone E, Datz C, Haefner M, Renner F, Schoefl R, Schreiber F. Efficacy and safety of a new low-volume PEG with citrate and simethicone bowel preparation for colonoscopy (Clensia): a multicenter randomized observer-blind clinical trial vs. a low-volume PEG with ascorbic acid (PEG-ASC). Endosc Int Open. 2018;6:E907-E913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | DeMicco MP, Clayton LB, Pilot J, Epstein MS; NOCT Study Group. Novel 1 L polyethylene glycol-based bowel preparation NER1006 for overall and right-sided colon cleansing: a randomized controlled phase 3 trial versus trisulfate. Gastrointest Endosc. 2018;87:677-687.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (1)] |
| 19. | Schreiber S, Baumgart DC, Drenth JPH, Filip RS, Clayton LB, Hylands K, Repici A, Hassan C; DAYB Study Group. Colon cleansing efficacy and safety with 1 L NER1006 versus sodium picosulfate with magnesium citrate: a randomized phase 3 trial. Endoscopy. 2019;51:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Bisschops R, Manning J, Clayton LB, Ng Kwet Shing R, Álvarez-González M; MORA Study Group. Colon cleansing efficacy and safety with 1 L NER1006 versus 2 L polyethylene glycol + ascorbate: a randomized phase 3 trial. Endoscopy. 2019;51:60-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 21. | Maida M, Sinagra E, Morreale GC, Sferrazza S, Scalisi G, Schillaci D, Ventimiglia M, Macaluso FS, Vettori G, Conoscenti G, Di Bartolo C, Garufi S, Catarella D, Manganaro M, Virgilio CM, Camilleri S. Effectiveness of very low-volume preparation for colonoscopy: A prospective, multicenter observational study. World J Gastroenterol. 2020;26:1950-1961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Fuccio L, Frazzoni L, Spada C, Mussetto A, Fabbri C, Manno M, Aragona G, Zagari RM, Rondonotti E, Manes G, Occhipinti P, Cadoni S, Bazzoli F, Hassan C, Radaelli F; QIPS study group. Factors That Affect Adequacy of Colon Cleansing for Colonoscopy in Hospitalized Patients. Clin Gastroenterol Hepatol. 2021;19:339-348.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 23. | Mohamed R, Hilsden RJ, Dube C, Rostom A. Split-Dose Polyethylene Glycol Is Superior to Single Dose for Colonoscopy Preparation: Results of a Randomized Controlled Trial. Can J Gastroenterol Hepatol. 2016;2016:3181459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Jung YS, Lee CK, Eun CS, Park DI, Han DS, Kim HJ. Low-Volume Polyethylene Glycol with Ascorbic Acid for Colonoscopy Preparation in Elderly Patients: A Randomized Multicenter Study. Digestion. 2016;94:82-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Horton N, Garber A, Hasson H, Lopez R, Burke CA. Impact of Single- vs. Split-Dose Low-Volume Bowel Preparations on Bowel Movement Kinetics, Patient Inconvenience, and Polyp Detection: A Prospective Trial. Am J Gastroenterol. 2016;111:1330-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Radaelli F, Paggi S, Hassan C, Senore C, Fasoli R, Anderloni A, Buffoli F, Savarese MF, Spinzi G, Rex DK, Repici A. Split-dose preparation for colonoscopy increases adenoma detection rate: a randomised controlled trial in an organised screening programme. Gut. 2017;66:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 27. | Manes G, Repici A, Hassan C; MAGIC-P study group. Randomized controlled trial comparing efficacy and acceptability of split- and standard-dose sodium picosulfate plus magnesium citrate for bowel cleansing prior to colonoscopy. Endoscopy. 2014;46:662-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Schulz C, Müller J, Sauter J, Miehlke S, Schmöcker C, Hartmann D, Malfertheiner P, Badiola C. Superiority of a Split-dose Regimen of Sodium Picosulfate/Magnesium Citrate (SPMC) in Comparison to a Prior-day Schedule (AM/PM) for Colonoscopy Preparation. A Randomized Single-blinded Study. J Gastrointestin Liver Dis. 2016;25:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Kiesslich R, Schubert S, Mross M, Klugmann T, Klemt-Kropp M, Behnken I, Bonnaud G, Keulen E, Groenen M, Blaker M, Ponchon T, Landry W, Stoltenberg M. Efficacy and safety of PICOPREP tailored dosing compared with PICOPREP day-before dosing for colon cleansing: a multi-centric randomised study. Endosc Int Open. 2017;5:E282-E290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Pohl J, Halphen M, Kloess HR, Fischbach W. Impact of the quality of bowel cleansing on the efficacy of colonic cancer screening: a prospective, randomized, blinded study. PLoS One. 2015;10:e0126067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Avalos DJ, Castro FJ, Zuckerman MJ, Keihanian T, Berry AC, Nutter B, Sussman DA. Bowel Preparations Administered the Morning of Colonoscopy Provide Similar Efficacy to a Split Dose Regimen: A Meta Analysis. J Clin Gastroenterol. 2018;52:859-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 32. | Pan H, Zheng XL, Fang CY, Liu LZ, Chen JS, Wang C, Chen YD, Huang JM, Zhou YS, He LP. Same-day single-dose vs large-volume split-dose regimens of polyethylene glycol for bowel preparation: A systematic review and meta-analysis. World J Clin Cases. 2022;10:7844-7858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Chen E, Chen L, Wang F, Zhang W, Cai X, Cao G. Low-residue versus clear liquid diet before colonoscopy: An updated meta-analysis of randomized, controlled trials. Medicine (Baltimore). 2020;99:e23541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Avalos DJ, Sussman DA, Lara LF, Sarkis FS, Castro FJ. Effect of Diet Liberalization on Bowel Preparation. South Med J. 2017;110:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Song GM, Tian X, Ma L, Yi LJ, Shuai T, Zeng Z, Zeng XT. Regime for Bowel Preparation in Patients Scheduled to Colonoscopy: Low-Residue Diet or Clear Liquid Diet? Medicine (Baltimore). 2016;95:e2432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Taveira F, Areia M, Elvas L, Alves S, Brito D, Saraiva S, Cadime AT. A 3-day low-fibre diet does not improve colonoscopy preparation results compared to a 1-day diet: A randomized, single-blind, controlled trial. United European Gastroenterol J. 2019;7:1321-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 37. | Gimeno-García AZ, de la Barreda Heuser R, Reygosa C, Hernandez A, Mascareño I, Nicolás-Pérez D, Jiménez A, Lara AJ, Alarcon-Fernández O, Hernandez-Guerra M, Romero R, Alonso I, González Y, Adrian Z, Hernandez G, Hernandez D, Delgado R, Quintero E. Impact of a 1-day versus 3-day low-residue diet on bowel cleansing quality before colonoscopy: a randomized controlled trial. Endoscopy. 2019;51:628-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 38. | Siddiqui AA, Yang K, Spechler SJ, Cryer B, Davila R, Cipher D, Harford WV. Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointest Endosc. 2009;69:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 152] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 39. | Seo EH, Kim TO, Park MJ, Joo HR, Heo NY, Park J, Park SH, Yang SY, Moon YS. Optimal preparation-to-colonoscopy interval in split-dose PEG bowel preparation determines satisfactory bowel preparation quality: an observational prospective study. Gastrointest Endosc. 2012;75:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Eun CS, Han DS, Hyun YS, Bae JH, Park HS, Kim TY, Jeon YC, Sohn JH. The timing of bowel preparation is more important than the timing of colonoscopy in determining the quality of bowel cleansing. Dig Dis Sci. 2011;56:539-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 41. | Bucci C, Rotondano G, Hassan C, Rea M, Bianco MA, Cipolletta L, Ciacci C, Marmo R. Optimal bowel cleansing for colonoscopy: split the dose! Gastrointest Endosc. 2014;80:566-576.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 42. | Kojecky V, Matous J, Keil R, Dastych M, Zadorova Z, Varga M, Kroupa R, Dolina J, Misurec M, Hep A, Griva M. The optimal bowel preparation intervals before colonoscopy: A randomized study comparing polyethylene glycol and low-volume solutions. Dig Liver Dis. 2018;50:271-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Rebhun J, Pagani W, Xia Y, Shuja A. Effect of the Weekend on Bowel Preparation Quality in Outpatient Colonoscopies. Dig Dis Sci. 2022;67:1231-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 44. | Ness RM, Manam R, Hoen H, Chalasani N. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96:1797-1802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 343] [Article Influence: 14.3] [Reference Citation Analysis (2)] |
| 45. | Gkolfakis P, Tziatzios G, Papanikolaou IS, Triantafyllou K. Strategies to Improve Inpatients' Quality of Bowel Preparation for Colonoscopy: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2019;2019:5147208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Yadlapati R, Johnston ER, Gregory DL, Ciolino JD, Cooper A, Keswani RN. Predictors of Inadequate Inpatient Colonoscopy Preparation and Its Association with Hospital Length of Stay and Costs. Dig Dis Sci. 2015;60:3482-3490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 47. | Gkolfakis P, Tziatzios G, Dimitriadis GD, Triantafyllou K. New endoscopes and add-on devices to improve colonoscopy performance. World J Gastroenterol. 2017;23:3784-3796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Argyropoulos SK, Mahmood SK, Campbell EJ, Richter JM. Improving the Quality of Inpatient Bowel Preparation for Colonoscopies. Dig Dis Sci. 2018;63:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 49. | Sinagra E, Luppino I, Messina M, Stasi E, Utzeri E, Messina C, Belletrutti P, Fabbri C, Anderloni A. Endoscopic approach to early gastric cancer in older adults. J Geriatr Oncol. 2021;12:160-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 50. | Neilson LJ, Thirugnanasothy S, Rees CJ. Colonoscopy in the very elderly. Br Med Bull. 2018;127:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 51. | Kumar A, Lin L, Bernheim O, Bagiella E, Jandorf L, Itzkowitz SH, Shah BJ. Effect of Functional Status on the Quality of Bowel Preparation in Elderly Patients Undergoing Screening and Surveillance Colonoscopy. Gut Liver. 2016;10:569-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 52. | Schmilovitz-Weiss H, Weiss A, Boaz M, Levin I, Chervinski A, Shemesh E. Predictors of failed colonoscopy in nonagenarians: a single-center experience. J Clin Gastroenterol. 2007;41:388-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | NPSA. Rapid Response Report: Reducing risk of harm from oral bowel cleansing solutions. Feb 19, 2009. [cited 20 Jan 2023]. Available from: https://www.cas.mhra.gov.uk/ViewandAcknowledgment/ViewAttachment.aspx?Attachment_id=100794. |
| 54. | Connor A, Tolan D, Hughes S, Carr N, Tomson C. Consensus guidelines for the safe prescription and administration of oral bowel-cleansing agents. Gut. 2012;61:1525-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 55. | Ogino N, Aridome G, Oshima J, Shibata M, Watanabe T, Kume K, Yoshikawa I, Harada M. Serum Albumin Concentrations Predict hypovolaemia Caused by Polyethylene Glycol Plus Ascorbic Acid Prior to Colonoscopy in Elderly Patients. Drugs Aging. 2016;33:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Maida M, Facciorusso A, Sinagra E, Morreale G, Sferrazza S, Scalisi G, Pallio S, Camilleri S. Predictive Factors of Adequate Bowel Cleansing for Colonoscopy in the Elderly: A Retrospective Analysis of a Prospective Cohort. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (4)] |
| 57. | Ho SB, Hovsepians R, Gupta S. Optimal Bowel Cleansing for Colonoscopy in the Elderly Patient. Drugs Aging. 2017;34:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 58. | Shahini E, Passera R, Lo Secco G, Arezzo A. A systematic review and meta-analysis of endoscopic mucosal resection vs endoscopic submucosal dissection for colorectal sessile/non-polypoid lesions. Minim Invasive Ther Allied Technol. 2022;31:835-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Shahini E, Libânio D, Lo Secco G, Pisani A, Arezzo A. Indications and outcomes of endoscopic resection for non-pedunculated colorectal lesions: A narrative review. World J Gastrointest Endosc. 2021;13:275-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (5)] |
| 60. | Landreneau SW, Di Palma JA. Update on preparation for colonoscopy. Curr Gastroenterol Rep. 2010;12:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 61. | Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest Endosc. 2012;75:1197-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 273] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 62. | Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther. 2007;25:373-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 151] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 63. | Ben-Horin S, Bar-Meir S, Avidan B. The outcome of a second preparation for colonoscopy after preparation failure in the first procedure. Gastrointest Endosc. 2009;69:626-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 64. | Hassan C, Fuccio L, Bruno M, Pagano N, Spada C, Carrara S, Giordanino C, Rondonotti E, Curcio G, Dulbecco P, Fabbri C, Della Casa D, Maiero S, Simone A, Iacopini F, Feliciangeli G, Manes G, Rinaldi A, Zullo A, Rogai F, Repici A. A predictive model identifies patients most likely to have inadequate bowel preparation for colonoscopy. Clin Gastroenterol Hepatol. 2012;10:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 65. | Gandhi K, Tofani C, Sokach C, Patel D, Kastenberg D, Daskalakis C. Patient Characteristics Associated With Quality of Colonoscopy Preparation: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:357-369.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 66. | Agrawal R, Majeed M, Attar BM, Flores E, Haque Z, Ba Aqeel S, Wang Y, Omar YA, Parajuli P, Demetria M, Gandhi S. Predictors of poor bowel preparations and colonoscopy cancellations in inpatient colonoscopies, a single center retrospective study. Transl Gastroenterol Hepatol. 2022;7:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 67. | Borg BB, Gupta NK, Zuckerman GR, Banerjee B, Gyawali CP. Impact of obesity on bowel preparation for colonoscopy. Clin Gastroenterol Hepatol. 2009;7:670-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 68. | Rex DK. Optimal bowel preparation--a practical guide for clinicians. Nat Rev Gastroenterol Hepatol. 2014;11:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 69. | Lebwohl B, Wang TC, Neugut AI. Socioeconomic and other predictors of colonoscopy preparation quality. Dig Dis Sci. 2010;55:2014-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 70. | Hassan C, Bretthauer M, Kaminski MF, Polkowski M, Rembacken B, Saunders B, Benamouzig R, Holme O, Green S, Kuiper T, Marmo R, Omar M, Petruzziello L, Spada C, Zullo A, Dumonceau JM; European Society of Gastrointestinal Endoscopy. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2013;45:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 311] [Article Influence: 25.9] [Reference Citation Analysis (1)] |
| 71. | Dik VK, Moons LM, Hüyük M, van der Schaar P, de Vos Tot Nederveen Cappel WH, Ter Borg PC, Meijssen MA, Ouwendijk RJ, Le Fèvre DM, Stouten M, van der Galiën O, Hiemstra TJ, Monkelbaan JF, van Oijen MG, Siersema PD; Colonoscopy Quality Initiative. Predicting inadequate bowel preparation for colonoscopy in participants receiving split-dose bowel preparation: development and validation of a prediction score. Gastrointest Endosc. 2015;81:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 72. | Govani SM, Elliott EE, Menees SB, Judd SL, Saini SD, Anastassiades CP, Urganus AL, Boyce SJ, Schoenfeld PS. Predictors of suboptimal bowel preparation in asymptomatic patients undergoing average-risk screening colonoscopy. World J Gastrointest Endosc. 2016;8:616-622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Fayad NF, Kahi CJ, Abd El-Jawad KH, Shin AS, Shah S, Lane KA, Imperiale TF. Association between body mass index and quality of split bowel preparation. Clin Gastroenterol Hepatol. 2013;11:1478-1485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 74. | Sharara AI, Harb AH, Sarkis FS, Chalhoub JM, Habib RH. Body mass index and quality of bowel preparation: Real life vs. clinical trials. Arab J Gastroenterol. 2016;17:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 75. | Passi M, Rahman F, Koh C, Kumar S. Efficacy and tolerability of colonoscopies in overweight and obese patients: Results from a national database on gastrointestinal endoscopic outcomes. Endosc Int Open. 2022;10:E311-E320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 76. | Hookey L, Bertiger G, Johnson KL 2nd, Boules M, Ando M, Dahdal DN. Efficacy, safety, and tolerability of a ready-to-drink bowel preparation in overweight and obese adults: subanalysis by body mass index from a phase III, assessor-blinded study. Therap Adv Gastroenterol. 2020;13:1756284820910050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 77. | Baile-Maxia S, Amlani B, Martínez RJ. Bowel-cleansing efficacy of the 1L polyethylene glycol-based bowel preparation NER1006 (PLENVU) in patient subgroups in two phase III trials. Therap Adv Gastroenterol. 2021;14:17562848211020286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 753] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 79. | Enck P, Rathmann W, Spiekermann M, Czerner D, Tschöpe D, Ziegler D, Strohmeyer G, Gries FA. Prevalence of gastrointestinal symptoms in diabetic patients and non-diabetic subjects. Z Gastroenterol. 1994;32:637-641. [PubMed] |
| 80. | Chen H, Zheng X, Zong X, Li Z, Li N, Hur J, Fritz CD, Chapman W Jr, Nickel KB, Tipping A, Colditz GA, Giovannucci EL, Olsen MA, Fields RC, Cao Y. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut. 2021;70:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 81. | Piper MS, Saad RJ. Diabetes Mellitus and the Colon. Curr Treat Options Gastroenterol. 2017;15:460-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 82. | Madhoun MF, Bitar H, Bhatti O, Zia H, Parava P, Bashir MH. Diabetics on Narcotics Are Less Likely to Achieve Excellent Bowel Preparation Than Are Patients with Either Condition. Dig Dis Sci. 2017;62:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 83. | Hochberg I, Segol O, Shental R, Shimoni P, Eldor R. Antihyperglycemic therapy during colonoscopy preparation: A review and suggestions for practical recommendations. United European Gastroenterol J. 2019;7:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 84. | Taylor C, Schubert ML. Decreased efficacy of polyethylene glycol lavage solution (golytely) in the preparation of diabetic patients for outpatient colonoscopy: a prospective and blinded study. Am J Gastroenterol. 2001;96:710-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 85. | Ozturk NA, Gokturk HS, Demir M, Erdogan D, Unler GK, Gur G, Yilmaz U. The effect of autonomous neuropathy on bowel preparation in type 2 diabetes mellitus. Int J Colorectal Dis. 2009;24:1407-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 86. | Romero RV, Mahadeva S. Factors influencing quality of bowel preparation for colonoscopy. World J Gastrointest Endosc. 2013;5:39-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 87. | Ozturk NA, Gokturk HS, Demir M, Unler GK, Gur G, Yilmaz U. Efficacy and safety of sodium phosphate for colon cleansing in type 2 diabetes mellitus. South Med J. 2010;103:1097-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 88. | Alvarez-Gonzalez MA, Flores-Le Roux JA, Seoane A, Pedro-Botet J, Carot L, Fernandez-Clotet A, Raga A, Pantaleon MA, Barranco L, Bory F, Lorenzo-Zuñiga V. Efficacy of a multifactorial strategy for bowel preparation in diabetic patients undergoing colonoscopy: a randomized trial. Endoscopy. 2016;48:1003-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 89. | Hayes A, Buffum M, Hughes J. Diabetic colon preparation comparison study. Gastroenterol Nurs. 2011;34:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 90. | Grigg E, Schubert MC, Hall J, Rahhal F, Raina D, Sridhar S, Chamberlain SM. Lubiprostone used with polyethylene glycol in diabetic patients enhances colonoscopy preparation quality. World J Gastrointest Endosc. 2010;2:263-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. | Panarese A, Pesce F, Porcelli P, Riezzo G, Iacovazzi PA, Leone CM, De Carne M, Rinaldi CM, Shahini E. Chronic functional constipation is strongly linked to vitamin D deficiency. World J Gastroenterol. 2019;25:1729-1740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 92. | Stewart WF, Liberman JN, Sandler RS, Woods MS, Stemhagen A, Chee E, Lipton RB, Farup CE. Epidemiology of constipation (EPOC) study in the United States: relation of clinical subtypes to sociodemographic features. Am J Gastroenterol. 1999;94:3530-3540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 303] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 93. | Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 676] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 94. | Fang J, Fu HY, Ma D, Wang D, Liu YP, Wang YF, Zhu CP, Qian W, Bai Y, Li ZS. Constipation, fiber intake and non-compliance contribute to inadequate colonoscopy bowel preparation: a prospective cohort study. J Dig Dis. 2016;17:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 95. | Park HJ, Chae MH, Kim HS, Kim JW, Kim MY, Baik SK, Kwon SO, Kim HM, Lee KJ. Colon Transit Time May Predict Inadequate Bowel Preparation in Patients With Chronic Constipation. Intest Res. 2015;13:339-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 96. | Pathipati MP, Silvernale CJ, Barshop KG, Ha JB, Richter JM, Staller KD. Rectal Evacuation Disorders are Associated With Poor Bowel Preparation in Patients With Chronic Constipation: Results From Two Centers. J Clin Gastroenterol. 2022;56:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 97. | Stengel JZ, Jones DP. Single-dose lubiprostone along with split-dose PEG solution without dietary restrictions for bowel cleansing prior to colonoscopy: a randomized, double-blind, placebo-controlled trial. Am J Gastroenterol. 2008;103:2224-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 98. | Parente F, Vailati C, Bargiggia S, Manes G, Fontana P, Masci E, Arena M, Spinzi G, Baccarin A, Mazzoleni G, Testoni PA. 2-Litre polyethylene glycol-citrate-simethicone plus bisacodyl versus 4-litre polyethylene glycol as preparation for colonoscopy in chronic constipation. Dig Liver Dis. 2015;47:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 99. | Lu J, Cao Q, Wang X, Pu J, Peng X. Application of Oral Lactulose in Combination With Polyethylene Glycol Electrolyte Powder for Colonoscopy Bowel Preparation in Patients With Constipation. Am J Ther. 2016;23:e1020-e1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 100. | Kasugai K, Iwai H, Kuboyama N, Yoshikawa A, Fukudo S. Efficacy and safety of a crystalline lactulose preparation (SK-1202) in Japanese patients with chronic constipation: a randomized, double-blind, placebo-controlled, dose-finding study. J Gastroenterol. 2019;54:530-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 101. | Yoshida N, Inagaki Y, Fukumoto K, Yoriki H, Inada Y, Murakami T, Tomita Y, Hashimoto H, Sugino S, Hirose R, Dohi O, Inoue K, Itoh Y. The Efficacy of Short-Duration Polyethylene Glycol plus Electrolytes for Improving Bowel Preparation of Colonoscopy in Patients with Chronic Constipation. Gastroenterol Res Pract. 2020;2020:8886073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 102. | Ichijima R, Suzuki S, Esaki M, Sugita T, Ogura K, Kusano C, Ikehara H, Gotoda T. Efficacy of macrogol 4000 plus electrolytes in bowel preparation for colonoscopy in patients with chronic constipation. BMC Gastroenterol. 2021;21:387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 103. | Banerjee R, Chaudhari H, Shah N, Saravanan A, Tandan M, Reddy DN. Addition of Lubiprostone to polyethylene glycol(PEG) enhances the quality & efficacy of colonoscopy preparation: a randomized, double-blind, placebo controlled trial. BMC Gastroenterol. 2016;16:133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 104. | Dang JT, Moolla M, Dang TT, Shaw A, Tian C, Karmali S, Sultanian R. Sodium phosphate is superior to polyethylene glycol in constipated patients undergoing colonoscopy: a systematic review and meta-analysis. Surg Endosc. 2021;35:900-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 105. | Martel M, Ménard C, Restellini S, Kherad O, Almadi M, Bouchard M, Barkun AN. Which Patient-Related Factors Determine Optimal Bowel Preparation? Curr Treat Options Gastroenterol. 2018;16:406-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 106. | Manes G, Fontana P, de Nucci G, Radaelli F, Hassan C, Ardizzone S. Colon Cleansing for Colonoscopy in Patients with Ulcerative Colitis: Efficacy and Acceptability of a 2-L PEG Plus Bisacodyl Versus 4-L PEG. Inflamm Bowel Dis. 2015;21:2137-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 107. | Maida M, Morreale GC, Sferrazza S, Sinagra E, Scalisi G, Vitello A, Vettori G, Rossi F, Catarella D, Di Bartolo CE, Schillaci D, Raimondo D, Camilleri S, Orlando A, Macaluso FS. Effectiveness and safety of 1L PEG-ASC preparation for colonoscopy in patients with inflammatory bowel diseases. Dig Liver Dis. 2021;53:1171-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 108. | Kumar A, Shenoy V, Buckley MC, Durbin L, Mackey J, Mone A, Swaminath A. Endoscopic Disease Activity and Biologic Therapy Are Independent Predictors of Suboptimal Bowel Preparation in Patients with Inflammatory Bowel Disease Undergoing Colonoscopy. Dig Dis Sci. 2022;67:4851-4865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 109. | Gu P, Lew D, Oh SJ, Vipani A, Ko J, Hsu K, Mirakhor E, Pattisapu V, Bullen T, Fuller G, Spiegel BMR, Almario CV. Comparing the Real-World Effectiveness of Competing Colonoscopy Preparations: Results of a Prospective Trial. Am J Gastroenterol. 2019;114:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 110. | Gupta A, Dhiman RK, Kumari S, Rana S, Agarwal R, Duseja A, Chawla Y. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol. 2010;53:849-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 111. | Maheshwari A, Thuluvath PJ. Autonomic neuropathy may be associated with delayed orocaecal transit time in patients with cirrhosis. Auton Neurosci. 2005;118:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 112. | Anam AK, Karia K, Jesudian AB, Bosworth BP. Cirrhotic Patients Have Worse Bowel Preparation at Screening Colonoscopy than Chronic Liver Disease Patients without Cirrhosis. J Clin Exp Hepatol. 2016;6:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 113. | Salso A, De Leonardis F, Lionetti R, Lenci I, Angelico M, Telese A, Baiocchi L. Standard bowel cleansing is highly ineffective in cirrhotic patients undergoing screening colonoscopy. Dig Liver Dis. 2015;47:523-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 114. | Lee JM, Lee JH, Kim ES, Lee JM, Yoo IK, Kim SH, Choi HS, Keum B, Seo YS, Jeen YT, Lee HS, Chun HJ, Um SH, Kim CD. The safety and effectiveness of 2-liter polyethylene glycol plus ascorbic acid in patients with liver cirrhosis: A retrospective observational study. Medicine (Baltimore). 2017;96:e9011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 115. | Clayton DB, Palmer WC, Robison SW, Heckman MG, Chimato NT, Harnois DM, Francis DL. Colonoscopy bowel preparation quality improvement for patients with decompensated cirrhosis undergoing evaluation for liver transplantation. Clin Transplant. 2016;30:1236-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 116. | Lien YH. Is bowel preparation before colonoscopy a risky business for the kidney? Nat Clin Pract Nephrol. 2008;4:606-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 117. | Russmann S, Lamerato L, Marfatia A, Motsko SP, Pezzullo JC, Olds G, Jones JK. Risk of impaired renal function after colonoscopy: a cohort study in patients receiving either oral sodium phosphate or polyethylene glycol. Am J Gastroenterol. 2007;102:2655-2663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 118. | Lim YJ, Hong SJ. What is the best strategy for successful bowel preparation under special conditions? World J Gastroenterol. 2014;20:2741-2745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 119. | Choi NK, Lee J, Chang Y, Jung SY, Kim YJ, Lee SM, Lee JH, Kim JY, Song HJ, Park BJ. Polyethylene glycol bowel preparation does not eliminate the risk of acute renal failure: a population-based case-crossover study. Endoscopy. 2013;45:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 120. | Kontani M, Hara A, Ohta S, Ikeda T. Hypermagnesemia induced by massive cathartic ingestion in an elderly woman without pre-existing renal dysfunction. Intern Med. 2005;44:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 121. | Desmeules S, Bergeron MJ, Isenring P. Acute phosphate nephropathy and renal failure. N Engl J Med. 2003;349:1006-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 122. | Mathus-Vliegen EM, van der Vliet K. Safety, patient's tolerance, and efficacy of a 2-liter vitamin C-enriched macrogol bowel preparation: a randomized, endoscopist-blinded prospective comparison with a 4-liter macrogol solution. Dis Colon Rectum. 2013;56:1002-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 123. | Lee JM, Keum B, Yoo IK, Kim SH, Choi HS, Kim ES, Seo YS, Jeen YT, Chun HJ, Lee HS, Um SH, Kim CD, Kim MG, Jo SK. Polyethylene glycol plus ascorbic acid for bowel preparation in chronic kidney disease. Medicine (Baltimore). 2016;95:e4755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 124. | Lee SP, Park E, Kim HV, Sung IK, Kim JH, Lee SY, Park HS, Shim CS. Does 2 L Polyethylene Glycol Plus Ascorbic Acid Increase the Risk of Renal Impairment Compared to 4 L Polyethylene Glycol? Dig Dis Sci. 2016;61:3207-3214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 125. | Yoshida N, Naito Y, Murakami T, Hirose R, Ogiso K, Inada Y, Dohi O, Okayama T, Kamada K, Uchiyama K, Ishikawa T, Handa O, Konishi H, Siah KT, Yagi N, Itoh Y. Safety and Efficacy of a Same-Day Low-Volume 1 L PEG Bowel Preparation in Colonoscopy for the Elderly People and People with Renal Dysfunction. Dig Dis Sci. 2016;61:3229-3235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 126. | Ohmiya N, Nakagawa Y, Horiguchi N, Omori T, Kamano T, Funasaka K, Nagasaka M, Shibata T. Safety of Polyethylene Glycol Solution plus Ascorbic Acid for Bowel Preparation for Colonoscopy in Patients with Chronic Kidney Disease. Gastroenterol Res Pract. 2021;2021:6696591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 127. | Russmann S, Lamerato L, Motsko SP, Pezzullo JC, Faber MD, Jones JK. Risk of further decline in renal function after the use of oral sodium phosphate or polyethylene glycol in patients with a preexisting glomerular filtration rate below 60 ml/min. Am J Gastroenterol. 2008;103:2707-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 128. | Markowitz GS, Stokes MB, Radhakrishnan J, D'Agati VD. Acute phosphate nephropathy following oral sodium phosphate bowel purgative: an underrecognized cause of chronic renal failure. J Am Soc Nephrol. 2005;16:3389-3396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 129. | Granberry MC, White LM, Gardner SF. Exacerbation of congestive heart failure after administration of polyethylene glycol-electrolyte lavage solution. Ann Pharmacother. 1995;29:1232-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 130. | Lazzaroni M, Bianchi Porro G. Preparation, premedication, and surveillance. Endoscopy. 2001;33:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 131. | Parikh K, Weitz H. Can a bowel preparation exacerbate heart failure? Cleve Clin J Med. 2011;78:157-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 132. | Samad N, Fraser I. Severe symptomatic hyponatremia associated with the use of polyethylene glycol-based bowel preparation. Endocrinol Diabetes Metab Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 133. | Guardiola-Arévalo A, Granja Navacerrada A, García-Alonso FJ, Bernal Checa P, Piqué Becerra R, Guerra I, Algaba A, de Andrés Esteban E, Bermejo F. Randomized clinical trial evaluating the effect of a visual educational leaflet on the preparation of colonoscopies in hospitalized patients. Rev Esp Enferm Dig. 2019;111:946-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 134. | Poola S, Jampala N, Tumin D, Ali E. Factors influencing inpatient colonoscopy bowel preparation quality. Minerva Gastroenterol Dietol. 2020;66:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 135. | ASGE Standards of Practice Committee; Anderson MA, Ben-Menachem T, Gan SI, Appalaneni V, Banerjee S, Cash BD, Fisher L, Harrison ME, Fanelli RD, Fukami N, Ikenberry SO, Jain R, Khan K, Krinsky ML, Lichtenstein DR, Maple JT, Shen B, Strohmeyer L, Baron T, Dominitz JA. Management of antithrombotic agents for endoscopic procedures. Gastrointest Endosc. 2009;70:1060-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 352] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 136. | Huang L, Zhou JG, Zhang Y, Wang F, Wang Y, Liu DH, Li XJ, Lv SP, Jin SH, Bai YJ, Ma H. Opioid-Induced Constipation Relief From Fixed-Ratio Combination Prolonged-Release Oxycodone/Naloxone Compared With Oxycodone and Morphine for Chronic Nonmalignant Pain: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Pain Symptom Manage. 2017;54:737-748.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 137. | Velázquez Rivera I, Velázquez Clavarana L, García Velasco P, Melero Ramos C. Opioid-induced constipation in chronic pain: Experience with 180 patients. J Opioid Manag. 2019;15:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 138. | Lim SW, Seo YW, Sinn DH, Kim JY, Chang DK, Kim JJ, Rhee JC, Shim SG, Kim YH. Impact of previous gastric or colonic resection on polyethylene glycol bowel preparation for colonoscopy. Surg Endosc. 2012;26:1554-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 139. | Mussetto A, Frazzoni L, Paggi S, Dari S, Laterza L, Radaelli F, Hassan C, Triossi O, Fuccio L. Split dosing with a low-volume preparation is not inferior to split dosing with a high-volume preparation for bowel cleansing in patients with a history of colorectal resection: a randomized trial. Endoscopy. 2015;47:917-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 140. | Bonavina L, Arini A, Ficano L, Iannuzziello D, Pasquale L, Aragona SE, Ciprandi G, On Digestive Disorders ISG. Post-surgical intestinal dysbiosis: use of an innovative mixture (Lactobacillus plantarum LP01, Lactobacillus lactis subspecies cremoris LLC02, Lactobacillus delbrueckii LDD01). Acta Biomed. 2019;90:18-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 141. | Rex DK. Bowel preparation for colonoscopy: entering an era of increased expectations for efficacy. Clin Gastroenterol Hepatol. 2014;12:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 142. | Vather R, O'Grady G, Arkwright JW, Rowbotham DS, Cheng LK, Dinning PG, Bissett IP. Restoration of normal colonic motor patterns and meal responses after distal colorectal resection. Br J Surg. 2016;103:451-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 143. | Huizinga JD, Lammers WJ. Gut peristalsis is governed by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1-G8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 257] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 144. | Mañé N, Jimenez M. Interplay between myogenic pacemakers and enteric neurons determine distinct motor patterns in the rat colon. Neurogastroenterol Motil. 2014;26:1508-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 145. | Phillips SF. Functions of the large bowel: an overview. Scand J Gastroenterol Suppl. 1984;93:1-12. [PubMed] |
| 146. | Yoo IK, Jeen YT, Choi SJ, Choi HS, Keum B, Kim ES, Chun HJ, Lee HS, Kim CD. Evaluation of bowel preparation quality in patients with a history of colorectal resection. Turk J Gastroenterol. 2019;30:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 147. | Kim B, Kim BC, Kim J, Oh HJ, Ryu KH, Park BJ, Sohn DK, Hong CW, Han KS. Quality of Bowel Preparation for Colonoscopy in Patients with a History of Abdomino-Pelvic Surgery: Retrospective Cohort Study. Yonsei Med J. 2019;60:73-78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 148. | Chung E, Kang J, Baik SH, Lee KY. Impact of Resected Colon Site on Quality of Bowel Preparation in Patients Who Underwent Prior Colorectal Resection. Surg Laparosc Endosc Percutan Tech. 2017;27:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 149. | Kim JW, Han JH, Boo SJ, Ko OB, Park SK, Park SH, Yang DH, Jung KW, Kim KJ, Ye BD, Myung SJ, Yang SK, Kim JH, Byeon JS. Rescue bowel preparation: same day 2 L polyethylene glycol addition, not superior to bisacodyl addition 7 days later. Dig Dis Sci. 2014;59:2215-2221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 150. | Gimeno-García AZ, Hernandez G, Aldea A, Nicolás-Pérez D, Jiménez A, Carrillo M, Felipe V, Alarcón-Fernández O, Hernandez-Guerra M, Romero R, Alonso I, Gonzalez Y, Adrian Z, Moreno M, Ramos L, Quintero E. Comparison of Two Intensive Bowel Cleansing Regimens in Patients With Previous Poor Bowel Preparation: A Randomized Controlled Study. Am J Gastroenterol. 2017;112:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 151. | Akgul G, Ozgur Yeniova A, Ozsoy Z, Yenidogan E, Kefeli A, Dasıran MF, Daldal E, Akbas A, Okan İ. Effect and Tolerability of Same-Day Repeat Colonoscopy. J Invest Surg. 2020;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 152. | Sohn N, Weinstein MA. Management of the poorly prepared colonoscopy patient: colonoscopic colon enemas as a preparation for colonoscopy. Dis Colon Rectum. 2008;51:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 153. | Nguyen DL, Wieland M. Risk factors predictive of poor quality preparation during average risk colonoscopy screening: the importance of health literacy. J Gastrointestin Liver Dis. 2010;19:369-372. [PubMed] |
| 154. | Chokshi RV, Hovis CE, Colditz GA, Early DS, Wang JS. Physician recommendations and patient adherence after inadequate bowel preparation on screening colonoscopy. Dig Dis Sci. 2013;58:2151-2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 155. | Murphy CJ, Jewel Samadder N, Cox K, Iqbal R, So B, Croxford D, Fang JC. Outcomes of Next-Day Versus Non-next-Day Colonoscopy After an Initial Inadequate Bowel Preparation. Dig Dis Sci. 2016;61:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 156. | Horiuchi A, Nakayama Y, Kajiyama M, Kato N, Kamijima T, Ichise Y, Tanaka N. Colonoscopic enema as rescue for inadequate bowel preparation before colonoscopy: a prospective, observational study. Colorectal Dis. 2012;14:e735-e739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 157. | Yang HJ, Park DI, Park SK, Kim S, Lee T, Jung Y, Eun CS, Han DS. A Randomized Controlled Trial Comparing Colonoscopic Enema With Additional Oral Preparation as a Salvage for Inadequate Bowel Cleansing Before Colonoscopy. J Clin Gastroenterol. 2019;53:e308-e315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 158. | Rigaux J, Juriens I, Devière J. A novel system for the improvement of colonic cleansing during colonoscopy. Endoscopy. 2012;44:703-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 159. | Gimeno-García AZ, Baute JL, Hernandez G, Morales D, Gonzalez-Pérez CD, Nicolás-Pérez D, Alarcon-Fernández O, Jiménez A, Hernandez-Guerra M, Romero R, Alonso I, Gonzalez Y, Adrian Z, Carrillo M, Ramos L, Quintero E. Risk factors for inadequate bowel preparation: a validated predictive score. Endoscopy. 2017;49:536-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 160. | Berger A, Cesbron-Métivier E, Bertrais S, Olivier A, Becq A, Boursier J, Lannes A, Luet D, Pateu E, Dib N, Caroli-Bosc FX, Vitellius C, Calès P. A predictive score of inadequate bowel preparation based on a self-administered questionnaire: PREPA-CO. Clin Res Hepatol Gastroenterol. 2021;45:101693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 161. | Sadeghi A, Rajabnia M, Bagheri M, Jamshidizadeh S, Saberi S, Shahnazi P, Pasharavesh L, Pourhoseingholi MA, Mirzaei M, Asadzadeh Aghdaei H, Zali MR. Predictive factors of inadequate bowel preparation for elective colonoscopy. Gastroenterol Hepatol Bed Bench. 2022;15:66-78. [PubMed] |
| 162. | Kurlander JE, Waljee AK, Menees SB, Lipson R, Kokaly AN, Read AJ, Shehadeh KS, Cohn A, Saini SD. Regression and Random Forest Machine Learning Have Limited Performance in Predicting Bowel Preparation in Veteran Population. Dig Dis Sci. 2022;67:2827-2841. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 163. | Samadder NJ, Curtin K, Tuohy TM, Pappas L, Boucher K, Provenzale D, Rowe KG, Mineau GP, Smith K, Pimentel R, Kirchhoff AC, Burt RW. Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology. 2014;146:950-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 164. | Facciorusso A, Di Maso M, Serviddio G, Vendemiale G, Spada C, Costamagna G, Muscatiello N. Factors Associated With Recurrence of Advanced Colorectal Adenoma After Endoscopic Resection. Clin Gastroenterol Hepatol. 2016;14:1148-1154.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 165. | Maida M, Morreale G, Sinagra E, Ianiro G, Margherita V, Cirrone Cipolla A, Camilleri S. Quality measures improving endoscopic screening of colorectal cancer: a review of the literature. Expert Rev Anticancer Ther. 2019;19:223-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 166. | Facciorusso A, Del Prete V, Buccino RV, Della Valle N, Nacchiero MC, Monica F, Cannizzaro R, Muscatiello N. Comparative Efficacy of Colonoscope Distal Attachment Devices in Increasing Rates of Adenoma Detection: A Network Meta-analysis. Clin Gastroenterol Hepatol. 2018;16:1209-1219.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 167. | Maida M, Camilleri S, Manganaro M, Garufi S, Scarpulla G. New endoscopy advances to refine adenoma detection rate for colorectal cancer screening: None is the winner. World J Gastrointest Oncol. 2017;9:402-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 168. | Maida M, Macaluso FS, Sferrazza S, Ventimiglia M, Sinagra E. Effectiveness and safety of NER1006 versus standard bowel preparations: A meta-analysis of randomized phase-3 clinical trials. Dig Liver Dis. 2020;52:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |