Published online Mar 21, 2023. doi: 10.3748/wjg.v29.i11.1669
Peer-review started: November 21, 2022
First decision: December 10, 2022
Revised: January 10, 2023
Accepted: March 2, 2023
Article in press: March 2, 2023
Published online: March 21, 2023
Processing time: 115 Days and 20.2 Hours
Since hepatocellular carcinoma (HCC) represents an important cause of mortality and morbidity all over the world. Currently, it is fundamental not only to achieve a curative treatment but also to manage in the best way any possible recurrence. Even if the latest update of the Barcelona Clinic Liver Cancer guidelines for HCC treatment has introduced new locoregional techniques and confirmed others as well-established clinical practices, there is still no consensus about the treatment of recurrent HCC (RHCC). Locoregional treatments and medical therapy repre
Core Tip: During the follow-up of patients affected by hepatocellular carcinoma (HCC), radiology is considered the key to the diagnosis of recurrence, by taking advantage of cross-sectional imaging with a special focus on computed tomography and magnetic resonance imaging. As in the case of active surveillance in a patient with mild to moderate risk for developing HCC, cross-section imaging can help in the quick identification of signs of recurrence. Moreover, radiology plays a key role in the evaluation of treatment response during medical therapy for HCC, recently approved in the revised version of the Barcelona Clinic Liver Cancer staging.
- Citation: Ippolito D, Maino C, Gatti M, Marra P, Faletti R, Cortese F, Inchingolo R, Sironi S. Radiological findings in non-surgical recurrent hepatocellular carcinoma: From locoregional treatments to immunotherapy. World J Gastroenterol 2023; 29(11): 1669-1684
- URL: https://www.wjgnet.com/1007-9327/full/v29/i11/1669.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i11.1669
Hepatocellular carcinoma (HCC) represents the sixth-leading cause of cancer-related deaths worldwide, and it is the most frequent primary liver tumor, accounting for about 85% of primary liver malignancies. Cirrhosis is the histological substrate on which 80% of HCCs arise[1]. According to the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases, all patients with a high risk of developing HCC should undergo a surveillance program[2,3]. Treatment options with curative intent are liver resection (LR), locoregional treatments (LRT), or orthotopic liver transplantation (OLT), and the choice of treatment is influenced by intrinsic features of the lesion, aspects related to the patient, and medical and economic resources available in each center[4,5].
Many HCCs are detected at an intermediate or advanced stage, which are not eligible, at least in the first instance, for curative treatment. In such cases, several treatment options are available, which can also be used in a combined or sequential manner including local termoablation [radiofrequency ablation (RFA), microwave ablation (MWA)], traditional transarterial embolization with traditional chemo
However, even if the primary goal is to have a curative intent, recurrence rate after transplantation is between 8% and 21% despite the use of new predictive models[8]. By contrast to OLT, both LRT and LR suffer from a high recurrence rate (60%-80%). When occurring, tumor recurrence may be considered non-transplantable if it exceeds the transplantation criteria such as those defined by the alpha-fetoprotein or Milan/up-to-seven criteria. Non-transplantable recurrence is a major cause of precluding salvage OLT, which showed comparable overall survival (OS) to primary OLT in patients with HCC with compensated cirrhosis[9].
Even if the latest update of the Barcelona Clinic Liver Cancer (BCLC) guidelines[10] for HCC treatment has introduced new locoregional techniques and confirmed others as well-established clinical practices, there is still no consensus about the treatment of recurrent HCC (RHCC)[11]. For these reasons, the multidisciplinary approach should be considered to define the best option for each RHCC patient[12]. On this basis, this review summarized the actual clinical practice by underlining the importance of the radiological approach both in the diagnosis and treatment of RHCC.
To date, the available options for RHCC were similar to naïve-HCC options and include LR, OLT, and LRT for patients with liver-only recurrence, TACE, TARE, and stereotactic ablative radiotherapy for patients with unresectable disease, and systemic therapies or enrollment in clinical trials for patients with extrahepatic disease recurrence[13-15].
Since only 15%-30% of patients with RHCC are suitable for an LR due to progressive liver dysfunction, presence of multiple nodules, tumor location, or donor shortage for LT, the ablative treatments play a crucial role in early-stage RHCC[16]. RFA for RHCC is a safe and feasible technique, offering no significant difference in OS compared to RFA for primary HCC[17]. As both RFA and LR are indicated in RHCC tumors with similar features, many studies have compared the two treatments.
Three interesting and recent meta-analyses[13,18] established that LR provided better outcomes than RFA, especially in long-term survival outcomes. RFA is associated with a decreased risk of major complications and requires shorter hospitalization time, a more cost-effective approach in comparison with LR. Moreover, in well-selected patients, RFA may be an optimal choice for RHCC with similar outcomes of LR, notably for a single lesion < 3 cm or in patients with three or fewer nodules, following the guidelines for primary HCC[10]. Also, other studies, including one randomized controlled trial[19], confirmed the same results[20-22].
RFA performances are found to be worse than LR in disease free-survival (DFS), because the LR may ensure removal of the tumor-bearing portal territory where micrometastases and microscopic vascular invasion are present and usually impossible to detect through external ultrasonography[13].
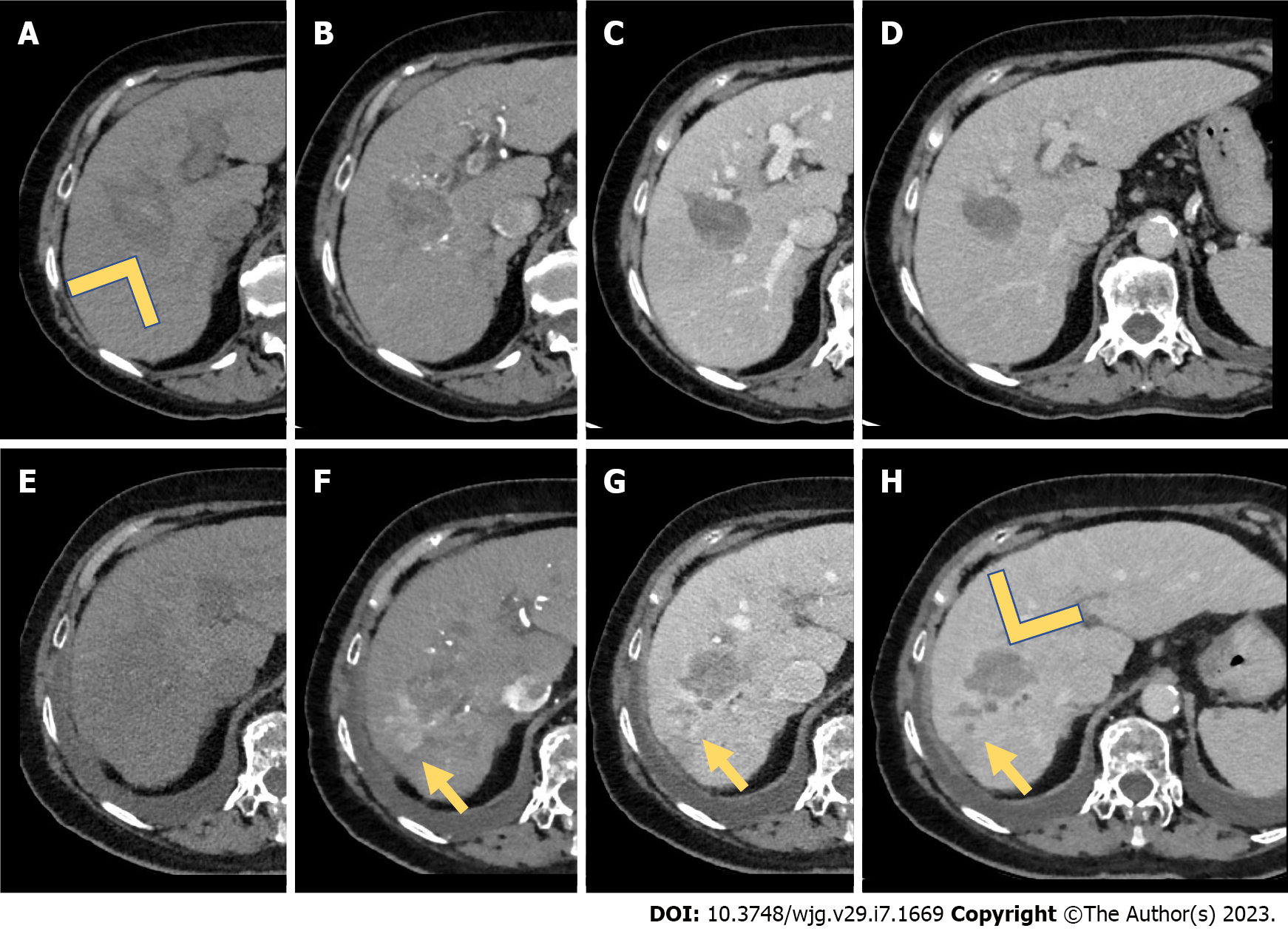
To overcome the shortcomings of RFA, MWA has been assessed in the treatment of HCC, as it produces significantly larger areas of necrosis, faster ablation times, higher intratumor temperature, less tumor seeding risk, and less susceptibility to heat-sink effect over RFA[15,23] (Figure 1). However, there are few studies about percutaneous MWA performance in RHCC. Only one has compared surgical MWA and LR for RHCC showing the safety and feasibility of surgical MWA for RHCC within 3 cm in size and no more than three nodules[24]. Nevertheless, MWA was proven to be superior to RFA[25] and competing with LR when the tumor is > 3 cm and < 5 cm and close to the large vessels[26]. During treatment of very early and early HCC, RFA, MWA, and cryoablation have substantially similar outcomes[23].
A multicentric randomized controlled trial comparing RFA with cryoablation in HCC < 4 cm reported no differences in terms of OS and DFS but found differences regarding local tumor control in favor of cryoablation (7.7% vs 18.2%, P = 0.04)[27]. While another study conducted on 3239 patients showed a significant advantage in liver cancer-specific survival for RFA[28]. Therefore, the results regarding cryoablation are still unclear[29]. However, data are currently lacking concerning outcomes following the use of cryoablation in RHCC, and future studies should be focused on these aspects.
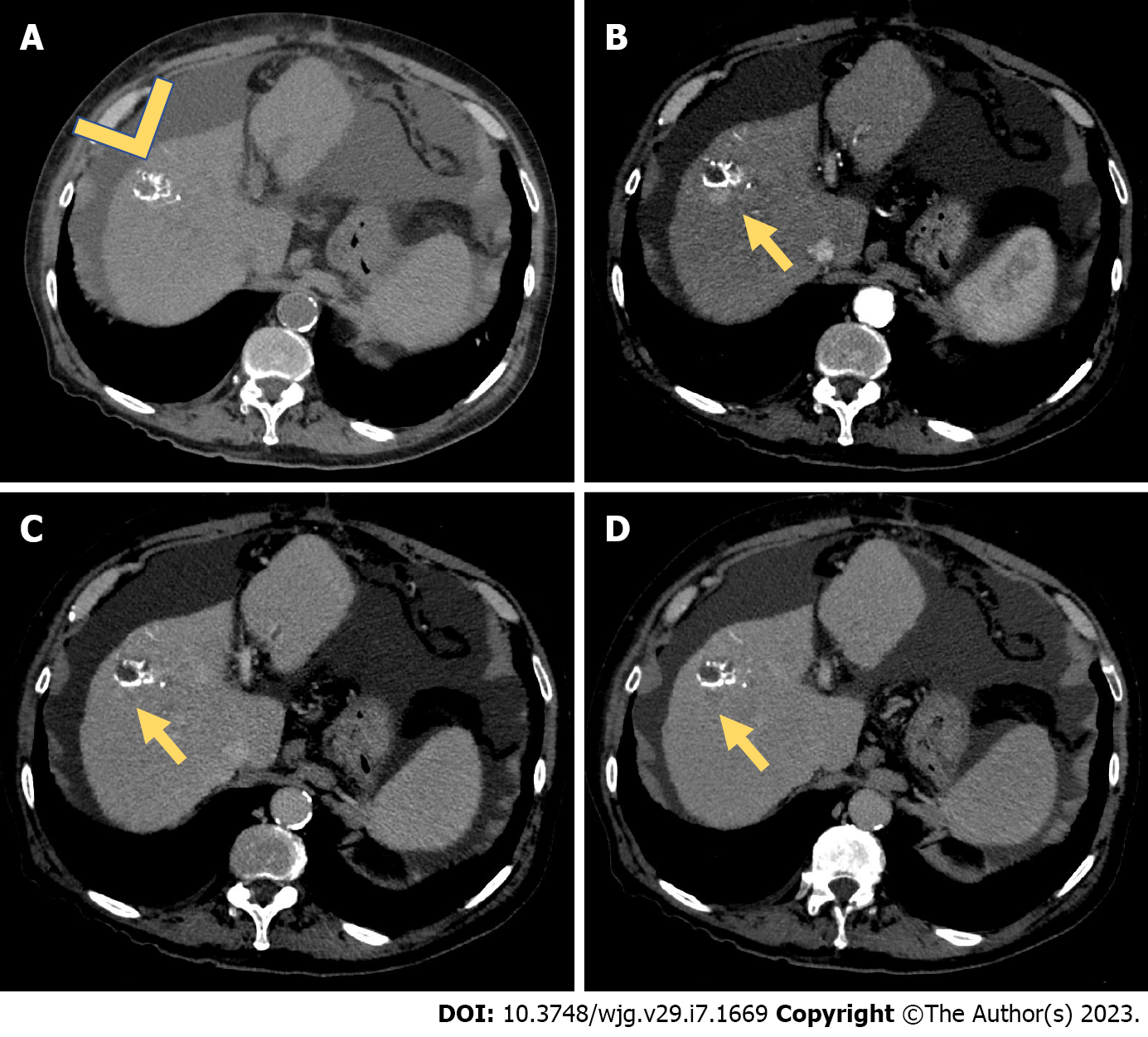
TACE is the most common treatment modality used for RHCC following initial resection[16,17]. However, as with LR, appropriate candidates for TACE should be carefully chosen based on their hepatic reserve[16,30] (Figure 2). However, there may exist a significant risk of worsened liver dysfunction following TACE among patients who have undergone prior hepatectomy[15,16,30]. Scores such as up-to-seven criteria or biomarkers such as Mac-2 binding protein glycosylation isomer to assess liver fibrosis can be used to identify patients who tolerate TACE less[16,30].
Regarding TACE in RHCC, Zu et al[31] demonstrated that the 1-, 2-, and 3-year OS rates after TACE were 73%, 52%, and 32%, respectively, while the number of resected HCC nodules (≥ 2), size (> 5 cm) of the RHCCs, and the number of TACE sessions (≤ 3) are independent risk factors for poor outcomes after TACE for recurrent HCC. Comparing TACE in naïve-HCC and RHCC, Liu et al[32] showed that RHCC treated with TACE accomplished acceptable results. After the propensity score matching analysis, there were no statistically significant differences between the naïve-HCC group and RHCC group in objective tumor regression and disease control rate. On the other side, the RHCC group had a shorter median OS (24 mo vs 33 mo) and PFS (10 mo vs 12 mo) in comparison with the naïve-HCC group.
Since it is a non-curative treatment, a recent meta-analysis demonstrated that TACE had worse outcomes (OS and DFS) than liver transplantation, LR, and RFA in RHCC patients[33]. Even comparing the two LRTs, Gou et al[34] showed that RFA had better short-term and long-term OS than TACE. Conversely, TACE may improve survival in patients with inoperable tumors, with large lesions or multifocal RHCC (beyond the Milan Criteria), and early (< 1 year) recurrence[35,36]. Interestingly, TACE proved to be a more effective option than LR/RFA in RHCC of BCLC stage 0 or A with microvascular invasion, especially in those that recur early after curative resection[37].
Among transarterial procedures, DEB-TACE, which uses doxorubicin, and TARE, using yttrium-90-labeled spheres, have been developed[12]. Even if it has been demonstrated that DEB-TACE facilitates higher concentrations of drugs within the target tumor and lower systemic concentrations with fewer adverse events than conventional-TACE in the management of HCC, especially on RHCC, there is no strong evidence showing the superiority of DEB-TACE over conventional TACE[38,39]. There are a lack of studies considering DEB-TACE as monotherapy for RHCC.
TARE may be an option for intermediate or advanced-stage HCC. It could also be used as an alternative to TACE especially for patients with portal vein thrombosis or for patients with earlier stages who are not eligible for curative procedures[16]. It is a safe and effective procedure for RHCC following LR, with satisfactory outcomes (median time-to-progression and OS were 11.3 mo and 22.1 mo, respectively)[40].
Since RHCC frequently requires aggressive treatment to reach good therapeutic outcomes, the combined approaches have been evaluated by several studies for RHCC[16]. It has been proven that TACE alone is unable to cause complete tumor necrosis[41] and that RFA cannot detect satellite lesions[13]. Therefore, combined therapies may have a synergistic effect and be beneficial for patients with RHCC. TACE-RFA combined treatment can cause tumor necrosis up to 7 cm in diameter in one session[42].
The combination of TACE and RFA leads to theoretical advantages over either monotherapy. TACE can reduce the heat sink effect of the RFA, thereby increasing the ablation range. On the other hand, satellite lesions can be detected through TACE[41]. Furthermore, TACE with the intralesional accumulation of radio-opaque iodized oil used or drug-eluting beads increases the echogenicity and conspicuity of small HCC, otherwise hardly visible on ultrasound (US) guidance during RFA[43].
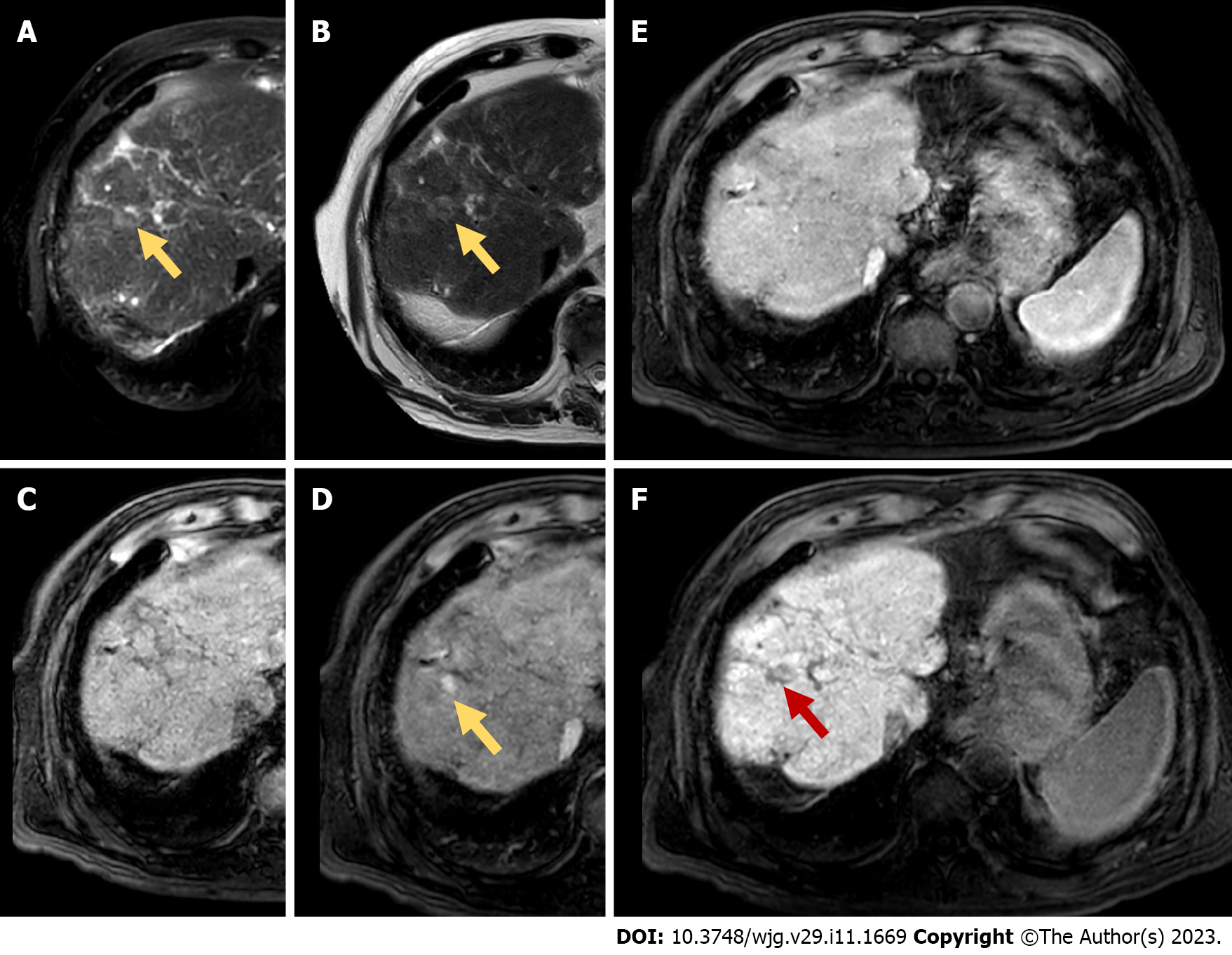
Song et al[44] showed that TACE-RFA had better DFS in comparison with TACE alone in patients with RHCC ≤ 5 cm. However, there were no significant differences between the two groups in OS and adverse events. Ascites is a frequent complication in the TACE-RFA group (Figure 3). Moreover, TACE-RFA provides comparable local efficacy and long-term survival results for patients with RHCC after hepatectomy, both for tumor size < 5 cm and > 5 cm. Furthermore, the TACE-RFA group has fewer complications[41,45] and lower hospitalization time in comparison with the LR group[45].
Zhang et al[46] demonstrated that DEB-TACE combined with RFA can increase the survival of patients with RHCC. Notably, OS rates were similar to primary HCC, while DFS rates were lower. A recent study[47] comparing MWA-TACE with TACE alone for small RHCC showed that the 5-year PFS of the combined therapy (37.5%) was higher than that of patients receiving TACE alone (18.7%), while the cumulative OS rates at 5 years were 61.1% for TACE-MWA and 50.3% for TACE alone, with no significant differences. Song et al[44] and Ji et al[47] demonstrated that combined therapies improve tumor control but not long-term survival outcomes.
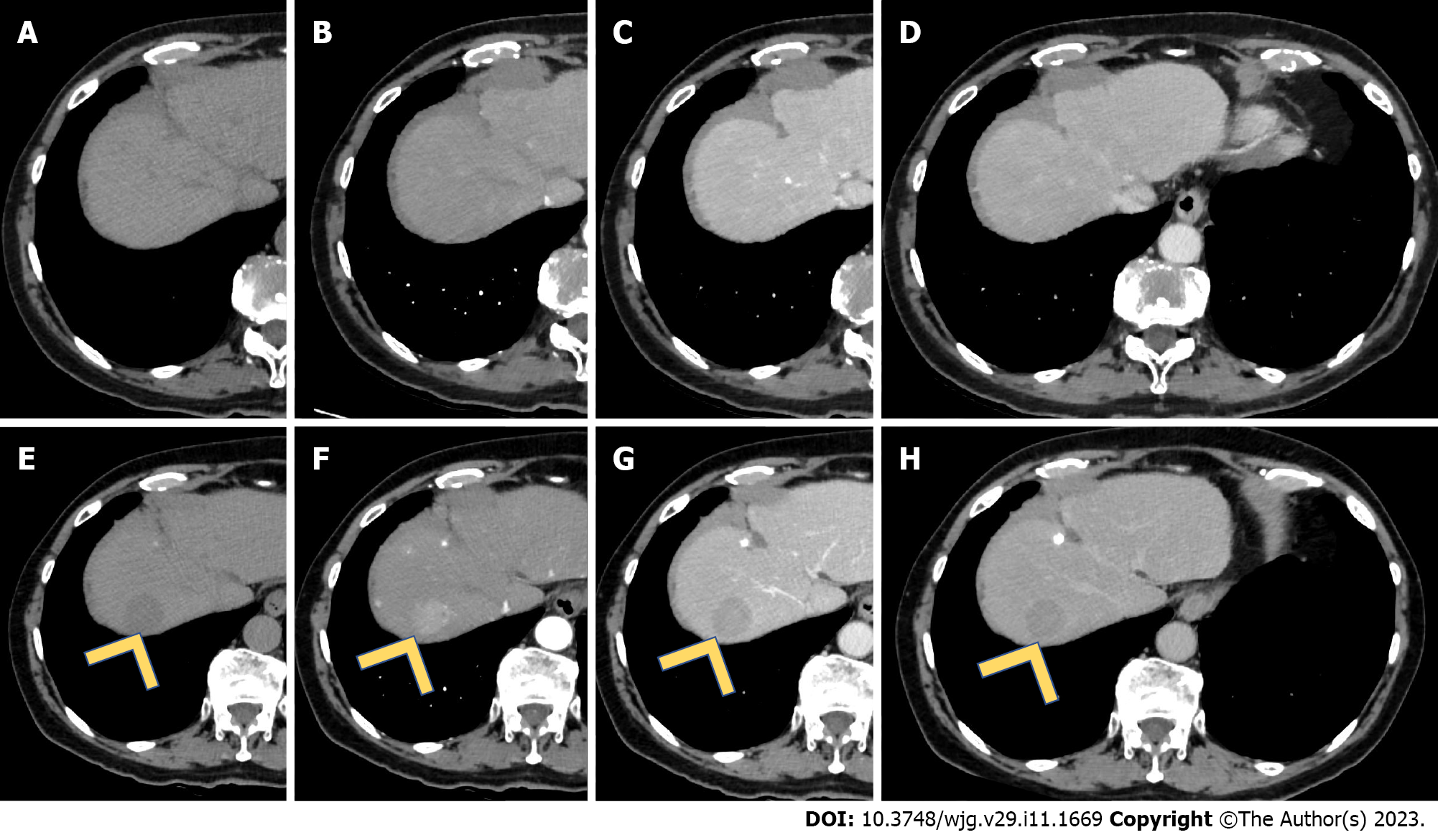
Since 2007, sorafenib represented the standard medical treatment of advanced HCC[48] (Figure 4). Sorafenib was the first multityrosine-kinase inhibitor, blocking different receptors, including Raf, the vascular endothelial growth factor, and platelet-derived growth factor, expressed by signaling pathways in HCC. Considering its large approval worldwide, sorafenib was employed not only for patients in an advanced stage of the disease but also as a bridging therapy to downstage the disease and include patients in the transplantation list[49].
Currently, the clinical landscape for patients with advanced liver cancer has changed quickly. Different agents were approved for clinical use, including lavatinib, cabozantinib, regorafenib, and ramucirumab, all addressed to the aforementioned pathways[50]. Moreover, different signs of progress have been made in immunotherapy, in particular with the advent of immune check-point blockers. Nivolumab (anti-PD-1 antibody), pembrolizumab (anti-PD-1 antibody), tremelimumab (anti-CTLA-4 antibody), and atezolizumab (anti-PD-L1 antibody) were tested for advanced HCC[51].
In 2022, Reig et al[10] refreshed the BCLC strategy for prognosis prediction and treatment recommendations. It has been established that the first line treatment of advanced HCC should be based on a combined approach. Atezolizumab with bevacizumab (anti-vascular endothelial growth factor antibody) is currently the first-choice first-line treatment. Finn et al[52], in a global, open-label, phase 3 trial, demonstrated the best OS and PFS of the combined therapy in comparison with sorafenib alone. Conversely, the atezolizumab-bevacizumab treatment can be used in patients with compensated Child-Pugh A cirrhosis and risk of upper gastrointestinal bleeding.
The second-line treatment is not well established yet. If patients underwent sorafenib treatment, then it is possible to evaluate the benefit from regorafenib[53], cabozatinib[54], or ramucirumab[55]. If the second-line treatment cannot add a clinical benefit or is not feasible due to patient contraindications, then the third-line treatment with cabozatinib can be considered to increase OS[56]. Finally, if all previously mentioned cases are not manageable, patients should be enrolled in clinical trials. Clinical and laboratory data used to choose the preferred medical treatment are out of the scope of the present review.
In this setting, patients who underwent LRTs should be followed up due to the risk of recurrence. In patients who underwent medical approaches it is important to monitor tumor response. All the above-mentioned medical strategies can determine apoptosis or necrosis of tumoral cells. One of the most important common findings to evaluate during follow-up is the change in tumor size. A significant increase in tumor volume or maximum axial diameter should be considered as a progression, according to the World Health Organization (WHO) criteria[56]. However, over time, different clinical studies were focused on the main issues related to the WHO classification. Consequently, RECIST 1.1 was introduced in clinical practice. However, RECIST 1.1 has some limitations, including the increase or decrease in size and necrosis, not being taken into account[57]. This last aspect is extremely important during medical treatments since the majority of drugs employed for HCC induce a reduction in tumor vascularization. For these reasons it is important to acquire images with complete protocols, to detect typical radiological findings of the primitive tumor, and to collect every significant change. First, increased dimensions of hypervascular areas or nodules should be considered as a main finding of tumor recurrence or progression[58,59]. To evaluate these, it is of utmost importance to acquire a correct arterial phase both on computed tomography (CT) and magnetic resonance imaging (MRI).
In 2014, Salvaggio et al[60] aimed to collect HCC enhancement changes after sorafenib treatment. The authors demonstrated that after medical treatment both arterial and portal venous enhancement was significantly reduced. In particular, the authors demonstrated that patients with partial response can manifest a greater decrease in arterial phase enhancement. However, they did not demonstrate the opposite. Patients with progressive disease did not show any statistically significant difference in arterial phase enhancement before and after treatment. To better understand the medical response, the international literature moved to the usefulness of MRI. Choi et al[61] reviewed the most common imaging findings of HCC during medical treatment by using MRI. The authors reported the importance of the hypervascular appearance during the arterial phase, as reported for CT. Moreover, MRI can help to detect early responders from non-responders by using diffusion-weighted imaging (DWI) and apparent diffusion coefficient maps, showing in the first group of patients an increase of DWI signal with correspondence on apparent diffusion coefficient map due to necrosis and reduced tumor cellularity. Finally, MR can benefit from the usefulness of hepatobiliary contrast agents, as demonstrated in the SORAMIC trial[62]. However, by searching PubMed and EMBASE no important studies have been published yet about this promising added value, and future studies should be focused on these aspects.
The advent of all the above-mentioned strategies, alone or combined, introduced a new class of response[52]. While about 8% can show a hyperprogression, a new atypical response is included in the iRECIST criteria[63]. However, no predictive biomarkers can help clinicians to determine the risk of atypical response during immunotherapy, and only the radiological approach, both with CT and MRI, can help follow patients during the treatment. Even if in the past medical treatment was considered the last useful medical treatment in advanced HCC, different ongoing studies are testing a combination of only medical drugs and in combination with LRTs, such as TACE, as reported by Pinter et al[64].
Combined strategies may be useful in advanced RHCC. Peng et al[65] showed that sorafenib combined with TACE-RFA was superior to therapy with sorafenib alone concerning time to progression and OS in patients with RHCC with one intrahepatic tumor size ≤ 7 cm or ≤ 5 cm intrahepatic nodules, with each tumor ≤ 3 cm.
Radiology plays a central role in the assessment of patient response LRT for RHCC. The identification of viable tumor treatment guides for further management, and it potentially affects transplantation eligibility. In these instances, it is often helpful to engage in a multidisciplinary discussion to determine how to best manage each patient. The Liver Imaging Reporting and Data System (LI-RADS) was developed in 2011 to relay the likelihood of HCC on CT or MRI in a standardized manner, in patients at risk for HCC. In 2017, the LI-RADS treatment response algorithm (LI-RADS TRA) was introduced for the assessment of lesions that have been previously treated with LRT[66]. Unlike the prior response criteria RECIST and WHO that focus on disease progression on a systemic level, LI-RADS TRA is based on enhancement features to predict viability on a lesion level[67]. Although modified RECIST (mRECIST) has historically been used for the evaluation of HCC after locoregional therapy, differences from LI-RADS TRA include a lack of equivocal category and a lack of additional features for diagnosing tumor viability[68]. mRECIST uses the presence of arterial enhancing components alone to diagnose viability while LI-RADS TRA includes additional imaging features such as washout during the portal venous or delayed phases and enhancement similar to pre-treatment to define viable tumors and encompass the equivocal category in addition to the binary evaluation[69].
Non-invasive imaging is superior to any other method for the surveillance of patients at risk of developing RHCC, either after OLT or other curative treatments. However, robust data lacks the optimal follow-up schedule of HCC-treated patients. Notably, international guidelines slightly differ in the recommended follow-up intervals, ranging from 3 mo to 6 mo, and duration of cross-sectional imaging after curative treatments. The National Comprehensive Cancer Network panel recommends ongoing total-body surveillance with multiphasic cross-sectional imaging (i.e. CT or MRI) every 3 mo to 6 mo for 2 years, then every 6 mo to 12 mo after curative therapies[70]. The 2018 Practice Guidance by the American Association for the Study of Liver Diseases suggests surveillance for HCC recurrence in posttransplant patients with abdominal and chest CT scan, though timing and duration as well as the impact of surveillance are not univocally defined[71]. After ablative therapies, the American Association for the Study of Liver Diseases recommends surveillance with contrast-enhanced CT or MRI every 3-6 mo[71].
The 2018 European Society for Medical Oncology Clinical Practice Guidelines were endorsed by the pan-Asian consensus conference, which included experts from several Asian societies. However, the Asian-adapted version slightly changed the follow-up timing after curative treatment, limiting the 3-mo interval by dynamic CT or MRI studies to the 1st year instead of 2[72,73]. Also, the European Association for the Study of the Liver recommends a follow-up after resection with curative intent with 3-4 mo intervals limited to the 1st year after treatment, with a return to regular surveillance thereafter[4].
Interestingly, Kim et al[74] found that HCC patients who undergo curative treatments with complete response and who present with increasing alpha-fetoprotein levels have a high probability of impending tumor recurrence even in the presence of a negative MRI. The follow-up schedule proposed within the European Society for Medical Oncology guidelines for patients treated with TACE or systemic therapies includes contrast-enhanced CT or MRI every 3 mo[74].
All the above-mentioned guidelines converge on the equivalent role of CT and MRI in clinical practice, given that the most important aspect for the diagnosis of HCC is the definition of criteria with the highest achievable accuracy, regardless of the imaging technique. Erkan et al[75] reviewed 3491 pathologically examined liver lesions, either studied by CT or MRI, comparing the diagnostic performance of different non-invasive diagnostic criteria of HCC. They found no statistically significant differences among criteria in diagnostic accuracy, with LI-RADS performing the best in terms of sensitivity and accuracy. Nevertheless, though CT and MRI have comparable performance in clinical practice, they present specific features to be considered.
CT has the advantage of being the most practical and widely available tool to perform surveillance in HCC-treated patients. Its main limitations consist of ionizing radiation exposure and iodinated contrast agents-related nephrotoxicity. The detection and characterization of liver nodules with conventional contrast-enhanced CT is substantially limited to the size, morphology, and enhancement pattern of the lesions, which are sufficient elements to reach a confident diagnosis according to LI-RADS. RHCC imaging findings are analogous to the primary lesion. In particular, the typical hallmarks in the imaging diagnosis of RHCC are the combination of hyperenhancement in the arterial phase and washout on the portal venous or delayed phases[4]. Several studies and meta-analyses have compared the performance of CT with other imaging techniques. In a multicenter prospective trial including 544 nodules in 381 patients, the sensitivity and specificity for the diagnosis of 10-20 mm HCC nodules were 67.9% and 76.8%, respectively, while for the 20-30 mm HCC nodules, the sensitivity and specificity were higher (71.6% and 93.6%, respectively)[76]. In a meta-analysis, CT had an overall sensitivity of 72% with a subgroup analysis revealing a sensitivity of 31% vs 82% for sub-centimetric lesions compared to ≥ 1 cm ones[77]. Of note, this data did not consider the prevalence of HCC diagnosis in HCC-naïve patients compared to previously treated patients, for whom the pre-test probability of disease is expected to be increased. A multicenter prospective study that enrolled patients scheduled for liver imaging before surgery showed a sensitivity of 70%[78].
The accuracy of MRI in detecting HCC, especially small nodules, is superior to that of CT as shown by several studies and meta-analyses, one of which reported a sensitivity of 82% compared with 66% of CT and a comparable specificity[4,79]. However, MRI is yet to be definitively recommended over CT, given that the quality of the available evidence is considered low[79]. Moreover, a distinction between extracellular contrast agents (ECA) and hepatobiliary contrast agents (HBCA) should be considered. Analogous to those used in CT, ECA detects and characterizes lesions through the enhancement pattern. Conversely, HBCA provides information on the hepatocellular function and bile excretion. Typical nodule hypointensity against a strongly enhanced background parenchyma in the hepatobiliary phase increases RHCC conspicuity and delineation, facilitating detection and consequently the diagnosis[80]. Despite this advantage, it must be pointed out that, if considered alone, hepatobiliary phase imaging is non-specific. Therefore, it always requires interpretation together with the dynamic study[81]. Martino et al[82] reported significantly higher diagnostic accuracy, sensitivity, and negative predictive value when dynamic and hepatobiliary phase MRI were combined compared to CT and dynamic phase MRI alone; a particular diagnostic benefit was obtained for lesions between 1 cm and 2 cm.
Nevertheless, although most HCC lesions are typically hypointense during the hepatobiliary phase, about 5%-12% HCC lesions can be hyperintense, owing to the overexpression of OATP[81]; conversely, some benign nodules may show no contrast uptake[83]. The knowledge of the pathological features of the originally treated nodules may predict the behavior of recurrent disease on hepatobiliary phase imaging, improving diagnostic confidence.
Different HBCA molecules have specific pharmacokinetic profiles. Gadoxetate disodium presents a 50% hepatic excretion, which contributes to an early liver parenchyma enhancement. Conversely, gadobenate-dimeglumine has a 3%-5% hepatic excretion that delays the hepatobiliary phase imaging onset. As a consequence, gadoxetate disodium does not provide a conventional delayed vascular phase but instead shows a transitional phase that lasts for several minutes, representing a transition from extracellular-dominant to intracellular-dominant enhancement[81]. Interestingly, Yim et al[84] recently observed that, in a retrospective cohort of patients who underwent both ECA and HBCA, RHCC was diagnosticated with higher accuracy using ECA.
DWI has been shown to improve the accuracy of RHCC detection, especially when combined with gadoxetic acid-enhanced imaging[85,86]. Finally, a recent meta-analysis confirmed that DWI may improve the ability to detect residual HCC or RHCC after TACE[87].
MRI likely has the highest accuracy compared to other imaging techniques in the detection of small recurrence after curative treatments[43]. However, results interpretation according to the standard LI-RADS may suffer from reduced sensitivity and specificity for disease recurrence detection. Wang et al[88] found that non-rim arterial phase hyperenhancement and three ancillary features (hepatobiliary phase hypointensity, mild-moderate T2 hyperintensity, and restriction of diffusion) were significantly related to RHCCs < 20 mm and concluded that the characterization of < 10 mm recurrence may show improved specificity compared with the LI-RADS 4 category combining at least two ancillary features. However, in patients treated with systemic therapies, according to the mRECIST criteria, new HCC lesions must measure at least 1 cm to define disease progression[58]. Despite the high sensitivity of MRI to detect recurrence after curative treatments, it has been shown that small viable RHCC may hide behind false-negative studies. This warrants regular short-term imaging surveillance[89].
However, in the absence of evidence to recommend a particular method or contrast agent over the other, practitioners are encouraged to base the choice on their judgment on an individual basis, considering the local availability of resources, personal experience, and imaging features of the previously-treated HCC[71].
The use of contrast-enhanced US (CEUS) is encouraged as it has been demonstrated that its specificity can be even superior compared to CT/MRI[76]. Although CEUS is inferior to both CT and MRI in terms of objectivity and panoramic view, it provides advantages in cases of renal dysfunction and iodine allergy. The current indications for CEUS are multiple, the most important of which are equivocal or inconclusive findings on CT or MRI studies and assessment of treatment response after TACE or ablation[90]. Bansal et al[91] proposed an algorithm with alternating MRI and CEUS for secondary surveillance following potentially curative therapy of HCC. In their prospective studies, the authors found similar diagnostic performance of the two techniques; of note, CEUS was able to confirm or disprove equivocal findings on MRI. The comparable diagnostic performance of CEUS, CT, and MRI was previously reported[92].
It has been reported that RHCC may differ from the initial tumor at imaging, and this may help to distinguish recurrence form residual diseases, which may have a prognostic relevance. Wu et al[93] recently described different CEUS patterns of RHCC compared to initial tumors: Among the others, more homogeneous enhancement, poorly defined borders, and marked washout were found to be typical features of recurrent disease.
The application of artificial intelligence and radiomics to preoperative CEUS has recently gained large interest, and it has been demonstrated to potentially predict the prognosis in terms of HCC recurrence and overall survival[94-97]. CEUS, added to other conventional US-based techniques, has also shown the ability to improve the prediction of microvascular invasion, which is probably the most important factor associated with a worse prognosis[98]. Finally, CEUS can be useful as guidance for ablative therapies, especially to target recurrence of previously treated lesions[99,100].
Perfusion imaging does not have a definite role in clinical practice, and it is mainly performed for investigative purposes. Although several authors have independently demonstrated that perfusion CT-derived parameters can discriminate between normal liver parenchyma, HCC, and hypervascular pseudolesions[101-103], they are yet to be included in clinical practice guidelines due to the absence of standardization among different centers[104]. However, perfusion imaging that provides quantitative parameters that could potentially be more reliable than qualitative/subjective parameters seems promising in the assessment of tumor response both to locoregional and systemic therapies[105-114].
Compared to CT, perfusion MRI has been investigated more regarding the possibility of predicting microvascular invasion of HCC before treatment. The microvascular invasion has been demonstrated to be correlated with poor outcomes of curative therapies due to higher rates of disease recurrence[115]. Perfusion MRI can be performed either with dynamic contrast-enhanced studies or with the intravoxel incoherent motion diffusion-weighted technique[116-119].
The role of nuclear medicine in the diagnosis and staging of HCC is debated. If on the one hand there is insufficient evidence to recommend the use of fluorodeoxyglucose positron emission tomography preoperatively, it has been demonstrated that nuclear medicine studies are able to predict tumor aggressiveness and may aid in identifying those patients at risk for HCC recurrence after liver transplantation, resection, or ablation for better treatment allocation[120,121]. Fluorodeoxyglucose positron emission tomography, with or without CT, has also been shown to present low sensitivity but high specificity for diagnosing extrahepatic metastases or local residual/recurrent HCC after treatment[121].
On the one hand, the assessment of the response to LRTs has been widely described[121-123]. On the other hand, histologic modifications induced by molecular therapies may explain different imaging findings of recurrent disease. Differentiation between treatment-induced tumor necrosis and viable tumor with reduced arterial perfusion may be challenging. After treatment with systemic targeted therapy, the tumor may show areas of necrosis without any contrast enhancement that must be distinguished from areas of reduced but still unequivocal arterial uptake consistent with viable tumor[44]. Even RHCC under systemic treatments may present with atypical enhancing patterns, especially lacking arterial hyperenhancement, which makes radiological assessment more difficult. All these aspects should be considered, and multimodal imaging evaluation combined with multidisciplinary framework can improve image interpretation. Conventional non-invasive imaging techniques provide robust criteria for HCC residual/recurrence detection, with high accuracy, representing the current standard of practice. Advanced imaging tools, either hardware- or software-based, have a double potential role: to predict HCC treatment response or the risk of recurrence, to increase sensitivity, specificity, and thus operator confidence in early RHCC detection.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen Q, China; Wei W, China; Kim BJ, South Korea S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YL
| 1. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4103] [Article Influence: 586.1] [Reference Citation Analysis (6)] |
| 2. | Sacco R, Gadaleta-Caldarola G, Galati G, Lombardi G, Mazza G, Cabibbo G. EASL HCC summit: liver cancer management. Future Oncol. 2014;10:1129-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Heimbach JK. Overview of the Updated AASLD Guidelines for the Management of HCC. Gastroenterol Hepatol (N Y). 2017;13:751-753. [PubMed] |
| 4. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6058] [Article Influence: 865.4] [Reference Citation Analysis (3)] |
| 5. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3027] [Article Influence: 432.4] [Reference Citation Analysis (3)] |
| 6. | Huang X, Liu Y, Xu L, Ma T, Yin X, Huang Z, Wang C, Bi X, Che X. Meta-analysis of Percutaneous vs. Surgical Approaches Radiofrequency Ablation in Hepatocellular Carcinoma. Front Surg. 2021;8:788771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int. 2019;13:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 379] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 8. | Sapisochin G, Bruix J. Liver transplantation for hepatocellular carcinoma: outcomes and novel surgical approaches. Nat Rev Gastroenterol Hepatol. 2017;14:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 333] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 9. | Gozzo C, Hermida M, Herrero A, Panaro F, Cassinotto C, Mohamad AM, Assenat E, Guillot C, Allimant C, Schembri V, Basile A, Dharancy S, Ursic-Bedoya J, Guiu B. Non-transplantable recurrence after percutaneous thermal ablation of ≤3-cm HCC: Predictors and implications for treatment allocation. Hepatol Commun. 2022;6:2975-2987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 10. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2606] [Article Influence: 868.7] [Reference Citation Analysis (59)] |
| 11. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3170] [Article Influence: 528.3] [Reference Citation Analysis (37)] |
| 12. | Kim KM. Nonsurgical multidisciplinary approach for recurrent hepatocellular carcinoma after surgical resection. Hepat Oncol. 2015;2:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 13. | Yang Y, Yu H, Tan X, You Y, Liu F, Zhao T, Qi J, Li J, Feng Y, Zhu Q. Liver resection versus radiofrequency ablation for recurrent hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2021;38:875-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Wen T, Jin C, Facciorusso A, Donadon M, Han HS, Mao Y, Dai C, Cheng S, Zhang B, Peng B, Du S, Jia C, Xu F, Shi J, Sun J, Zhu P, Nara S, Millis JM; MDT of West China Hospital*. Multidisciplinary management of recurrent and metastatic hepatocellular carcinoma after resection: an international expert consensus. Hepatobiliary Surg Nutr. 2018;7:353-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Aquina CT, Eskander MF, Pawlik TM. Liver-Directed Treatment Options Following Liver Tumor Recurrence: A Review of the Literature. Front Oncol. 2022;12:832405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Tampaki M, Papatheodoridis GV, Cholongitas E. Intrahepatic recurrence of hepatocellular carcinoma after resection: an update. Clin J Gastroenterol. 2021;14:699-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 17. | Fukuhara T, Aikata H, Hyogo H, Honda Y, Morio K, Morio R, Hatooka M, Kobayashi T, Naeshiro N, Kawaoka T, Tsuge M, Hiramatsu A, Imamura M, Kawakami Y, Chayama K. Efficacy of radiofrequency ablation for initial recurrent hepatocellular carcinoma after curative treatment: Comparison with primary cases. Eur J Radiol. 2015;84:1540-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Liu J, Zhao J, Gu HAO, Zhu Z. Repeat hepatic resection VS radiofrequency ablation for the treatment of recurrent hepatocellular carcinoma: an updated meta-analysis. Minim Invasive Ther Allied Technol. 2022;31:332-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Xia Y, Li J, Liu G, Wang K, Qian G, Lu Z, Yang T, Yan Z, Lei Z, Si A, Wan X, Zhang H, Gao C, Cheng Z, Pawlik TM, Wang H, Lau WY, Wu M, Shen F. Long-term Effects of Repeat Hepatectomy vs Percutaneous Radiofrequency Ablation Among Patients With Recurrent Hepatocellular Carcinoma: A Randomized Clinical Trial. JAMA Oncol. 2020;6:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 20. | Uhlig J, Sellers CM, Stein SM, Kim HS. Radiofrequency ablation versus surgical resection of hepatocellular carcinoma: contemporary treatment trends and outcomes from the United States National Cancer Database. EurRadiol. 2019;29:2679-2689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 21. | Feng Y, Wu H, Huang DQ, Xu C, Zheng H, Maeda M, Zhao X, Wang L, Xiao F, Lv H, Liu T, Qi J, Li J, Zhong N, Wang C, Feng H, Liang B, Ren W, Qin C, Nguyen MH, Zhu Q. Radiofrequency ablation versus repeat resection for recurrent hepatocellular carcinoma (≤ 5 cm) after initial curative resection. EurRadiol. 2020;30:6357-6368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Shi T, Xu C, Feng Y, Wei Y, Lv H, Zhu Q. Surgical resection versus radiofrequency ablation for early recurrent hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2022;34:844-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 23. | Gupta P, Maralakunte M, Kumar-M P, Chandel K, Chaluvashetty SB, Bhujade H, Kalra N, Sandhu MS. Overall survival and local recurrence following RFA, MWA, and cryoablation of very early and early HCC: a systematic review and Bayesian network meta-analysis. EurRadiol. 2021;31:5400-5408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 24. | Ryu T, Takami Y, Wada Y, Hara T, Sasaki S, Saitsu H. Efficacy of surgical microwave ablation for recurrent hepatocellular carcinoma after curative hepatectomy. HPB (Oxford). 2020;22:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, Carrafiello G, Azoulay D, Petrillo A, Curley SA. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist. 2019;24:e990-e1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 360] [Article Influence: 60.0] [Reference Citation Analysis (1)] |
| 26. | Wang Z, Liu M, Zhang DZ, Wu SS, Hong ZX, He GB, Yang H, Xiang BD, Li X, Jiang TA, Li K, Tang Z, Huang F, Lu M, Chen JA, Lin YC, Lu X, Wu YQ, Zhang XW, Zhang YF, Cheng C, Ye HL, Wang LT, Zhong HG, Zhong JH, Wang L, Chen M, Liang FF, Chen Y, Xu YS, Yu XL, Cheng ZG, Liu FY, Han ZY, Tang WZ, Yu J, Liang P. Microwave ablation versus laparoscopic resection as first-line therapy for solitary 3-5-cm HCC. Hepatology. 2022;76:66-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 60] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 27. | Wang C, Wang H, Yang W, Hu K, Xie H, Hu KQ, Bai W, Dong Z, Lu Y, Zeng Z, Lou M, Gao X, Chang X, An L, Qu J, Li J, Yang Y. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology. 2015;61:1579-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 28. | Xu J, Noda C, Erickson A, Mokkarala M, Charalel R, Ramaswamy R, Tao YU, Akinwande O. Radiofrequency Ablation vs. Cryoablation for Localized Hepatocellular Carcinoma: A Propensity-matched Population Study. Anticancer Res. 2018;38:6381-6386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, Lencioni R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 593] [Article Influence: 148.3] [Reference Citation Analysis (0)] |
| 30. | Eso Y, Takai A, Takahashi K, Ueda Y, Taura K, Marusawa H, Seno H. Combination of Mac-2 Binding Protein Glycosylation Isomer and Up-To-Seven Criteria as a Useful Predictor for Child-Pugh Grade Deterioration after Transarterial Chemoembolization for Hepatocellular Carcinoma. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Zu QQ, Liu S, Zhou CG, Yang ZQ, Xia JG, Zhao LB, Shi HB. Chemoembolization of recurrent hepatoma after curative resection: prognostic factors. AJR Am J Roentgenol. 2015;204:1322-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Liu Y, Ren Y, Ge S, Xiong B, Zhou G, Feng G, Song S, Zheng C. Transarterial Chemoembolization in Treatment-Naïve and Recurrent Hepatocellular Carcinoma: A Propensity-Matched Outcome and Risk Signature Analysis. Front Oncol. 2021;11:662408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Zheng J, Cai J, Tao L, Kirih MA, Shen Z, Xu J, Liang X. Comparison on the efficacy and prognosis of different strategies for intrahepatic recurrent hepatocellular carcinoma: A systematic review and Bayesian network meta-analysis. Int J Surg. 2020;83:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 34. | Gou H, Liu S, Zhu G, Peng Y, Li X, Yang X, He K. Effectiveness of radiofrequency ablation versus transarterial chemoembolization for recurrent hepatocellular carcinoma: A meta-analysis. Acta Radiol Open. 2022;11:20584601221085514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Cheng YC, Chen TW, Fan HL, Yu CY, Chang HC, Hsieh CB. Transarterial chemoembolization for intrahepatic multiple recurrent HCC after liver resection or transplantation. Ann Transplant. 2014;19:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Wang K, Liu G, Li J, Yan Z, Xia Y, Wan X, Ji Y, Lau WY, Wu M, Shen F. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol. 2015;41:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Jin YJ, Lee JW, Lee OH, Chung HJ, Kim YS, Lee JI, Cho SG, Jeon YS, Lee KY, Ahn SI, Shin WY. Transarterial chemoembolization versus surgery/radiofrequency ablation for recurrent hepatocellular carcinoma with or without microvascular invasion. J Gastroenterol Hepatol. 2014;29:1056-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Song JE, Kim DY. Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J Hepatol. 2017;9:808-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 39. | Bzeizi KI, Arabi M, Jamshidi N, Albenmousa A, Sanai FM, Al-Hamoudi W, Alghamdi S, Broering D, Alqahtani SA. Conventional Transarterial Chemoembolization Versus Drug-Eluting Beads in Patients with Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Ali R, Riaz A, Gabr A, Abouchaleh N, Mora R, Al Asadi A, Caicedo JC, Abecassis M, Katariya N, Maddur H, Kulik L, Lewandowski RJ, Salem R. Clinical outcomes of Y90 radioembolization for recurrent hepatocellular carcinoma following curative resection. Eur J Nucl Med Mol Imaging. 2017;44:2195-2202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 41. | Zheng X, Ren Y, Hu H, Qian K. Transarterial Chemoembolization Combined With Radiofrequency Ablation Versus Repeat Hepatectomy for Recurrent Hepatocellular Carcinoma After Curative Resection: A 10-Year Single-Center Comparative Study. Front Oncol. 2021;11:713432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Peng ZW, Zhang YJ, Chen MS, Xu L, Liang HH, Lin XJ, Guo RP, Zhang YQ, Lau WY. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol. 2013;31:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 394] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 43. | Lee MW, Lim HK. Management of sub-centimeter recurrent hepatocellular carcinoma after curative treatment: Current status and future. World J Gastroenterol. 2018;24:5215-5222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Song Q, Ren W, Fan L, Zhao M, Mao L, Jiang S, Zhao C, Cui Y. Long-Term Outcomes of Transarterial Chemoembolization Combined with Radiofrequency Ablation Versus Transarterial Chemoembolization Alone for Recurrent Hepatocellular Carcinoma After Surgical Resection. Dig Dis Sci. 2020;65:1266-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Peng Z, Wei M, Chen S, Lin M, Jiang C, Mei J, Li B, Wang Y, Li J, Xie X, Kuang M. Combined transcatheter arterial chemoembolization and radiofrequency ablation versus hepatectomy for recurrent hepatocellular carcinoma after initial surgery: a propensity score matching study. EurRadiol. 2018;28:3522-3531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 46. | Zhang Y, Zhang MW, Fan XX, Mao DF, Ding QH, Zhuang LH, Lv SY. Drug-eluting beads transarterial chemoembolization sequentially combined with radiofrequency ablation in the treatment of untreated and recurrent hepatocellular carcinoma. World J Gastrointest Surg. 2020;12:355-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Ji J, Yang W, Shi HB, Liu S, Zhou WZ. Transcatheter arterial chemoembolization alone versus combined with microwave ablation for recurrent small hepatocellular carcinoma after resection: a retrospective comparative study. BMC Gastroenterol. 2022;22:321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 48. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10266] [Article Influence: 603.9] [Reference Citation Analysis (2)] |
| 49. | Vitale A, Volk ML, Pastorelli D, Lonardi S, Farinati F, Burra P, Angeli P, Cillo U. Use of sorafenib in patients with hepatocellular carcinoma before liver transplantation: a cost-benefit analysis while awaiting data on sorafenib safety. Hepatology. 2010;51:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 50. | Akateh C, Black SM, Conteh L, Miller ED, Noonan A, Elliott E, Pawlik TM, Tsung A, Cloyd JM. Neoadjuvant and adjuvant treatment strategies for hepatocellular carcinoma. World J Gastroenterol. 2019;25:3704-3721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 112] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 51. | Ghavimi S, Apfel T, Azimi H, Persaud A, Pyrsopoulos NT. Management and Treatment of Hepatocellular Carcinoma with Immunotherapy: A Review of Current and Future Options. J Clin Transl Hepatol. 2020;8:168-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 52. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4691] [Article Influence: 938.2] [Reference Citation Analysis (2)] |
| 53. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G; RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 2710] [Article Influence: 338.8] [Reference Citation Analysis (0)] |
| 54. | Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1630] [Cited by in RCA: 1767] [Article Influence: 252.4] [Reference Citation Analysis (0)] |
| 55. | Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, Assenat E, Brandi G, Pracht M, Lim HY, Rau KM, Motomura K, Ohno I, Merle P, Daniele B, Shin DB, Gerken G, Borg C, Hiriart JB, Okusaka T, Morimoto M, Hsu Y, Abada PB, Kudo M; REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1027] [Cited by in RCA: 1247] [Article Influence: 207.8] [Reference Citation Analysis (0)] |
| 56. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12751] [Cited by in RCA: 13077] [Article Influence: 523.1] [Reference Citation Analysis (0)] |
| 57. | Gillmore R, Stuart S, Kirkwood A, Hameeduddin A, Woodward N, Burroughs AK, Meyer T. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55:1309-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 58. | Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol. 2020;72:288-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 437] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 59. | Ogunwobi OO, Harricharran T, Huaman J, Galuza A, Odumuwagun O, Tan Y, Ma GX, Nguyen MT. Mechanisms of hepatocellular carcinoma progression. World J Gastroenterol. 2019;25:2279-2293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 116] [Cited by in RCA: 169] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 60. | Salvaggio G, Furlan A, Agnello F, Cabibbo G, Marin D, Giannitrapani L, Genco C, Midiri M, Lagalla R, Brancatelli G. Hepatocellular carcinoma enhancement on contrast-enhanced CT and MR imaging: response assessment after treatment with sorafenib: preliminary results. Radiol Med. 2014;119:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Choi JI, Imagawa DK, Bhosale P, Bhargava P, Tirkes T, Seery TE, Lall C. Magnetic resonance imaging following treatment of advanced hepatocellular carcinoma with sorafenib. Clin Mol Hepatol. 2014;20:218-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | Öcal O, Rössler D, Gasbarrini A, Berg T, Klümpen HJ, Bargellini I, Peynircioglu B, van Delden O, Schulz C, Schütte K, Iezzi R, Pech M, Malfertheiner P, Sangro B, Ricke J, Seidensticker M. Gadoxetic acid uptake as a molecular imaging biomarker for sorafenib resistance in patients with hepatocellular carcinoma: a post hoc analysis of the SORAMIC trial. J Cancer Res Clin Oncol. 2022;148:2487-2496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 63. | Ippolito D, Maino C, Ragusi M, Porta M, Gandola D, Franzesi CT, Giandola TP, Sironi S. Immune response evaluation criteria in solid tumors for assessment of atypical responses after immunotherapy. World J Clin Oncol. 2021;12:323-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Pinter M, Jain RK, Duda DG. The Current Landscape of Immune Checkpoint Blockade in Hepatocellular Carcinoma: A Review. JAMA Oncol. 2021;7:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 252] [Article Influence: 63.0] [Reference Citation Analysis (1)] |
| 65. | Peng Z, Chen S, Wei M, Lin M, Jiang C, Mei J, Li B, Wang Y, Li J, Xie X, Chen M, Qian G, Kuang M. Advanced Recurrent Hepatocellular Carcinoma: Treatment with Sorafenib Alone or in Combination with Transarterial Chemoembolization and Radiofrequency Ablation. Radiology. 2018;287:705-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 66. | Kierans AS, Najjar M, Dutruel SP, Gavlin A, Chen C, Lee MJ, Askin G, Halazun KJ. Evaluation of the LI-RADS treatment response algorithm in hepatocellular carcinoma after trans-arterial chemoembolization. Clin Imaging. 2021;80:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Kim DH, Kim B, Choi JI, Oh SN, Rha SE. LI-RADS Treatment Response versus Modified RECIST for Diagnosing Viable Hepatocellular Carcinoma after Locoregional Therapy: A Systematic Review and Meta-Analysis of Comparative Studies. TaehanYongsangUihakhoe Chi. 2022;83:331-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 68. | Seo N, Joo DJ, Park MS, Kim SS, Shin HJ, Chung YE, Choi JY, Kim MS, Kim MJ. Optimal imaging criteria and modality to determine Milan criteria for the prediction of post-transplant HCC recurrence after locoregional treatment. EurRadiol. 2023;33:501-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 69. | Kielar A, Fowler KJ, Lewis S, Yaghmai V, Miller FH, Yarmohammadi H, Kim C, Chernyak V, Yokoo T, Meyer J, Newton I, Do RK. Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. AbdomRadiol (NY). 2018;43:218-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 70. | Benson AB, D'Angelica MI, Abbott DE, Anaya DA, Anders R, Are C, Bachini M, Borad M, Brown D, Burgoyne A, Chahal P, Chang DT, Cloyd J, Covey AM, Glazer ES, Goyal L, Hawkins WG, Iyer R, Jacob R, Kelley RK, Kim R, Levine M, Palta M, Park JO, Raman S, Reddy S, Sahai V, Schefter T, Singh G, Stein S, Vauthey JN, Venook AP, Yopp A, McMillian NR, Hochstetler C, Darlow SD. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl ComprCancNetw. 2021;19:541-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 575] [Article Influence: 143.8] [Reference Citation Analysis (0)] |
| 71. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3239] [Article Influence: 462.7] [Reference Citation Analysis (1)] |
| 72. | Chen LT, Martinelli E, Cheng AL, Pentheroudakis G, Qin S, Bhattacharyya GS, Ikeda M, Lim HY, Ho GF, Choo SP, Ren Z, Malhotra H, Ueno M, Ryoo BY, Kiang TC, Tai D, Vogel A, Cervantes A, Lu SN, Yen CJ, Huang YH, Chen SC, Hsu C, Shen YC, Tabernero J, Yen Y, Hsu CH, Yoshino T, Douillard JY. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with intermediate and advanced/relapsed hepatocellular carcinoma: a TOS-ESMO initiative endorsed by CSCO, ISMPO, JSMO, KSMO, MOS and SSO. Ann Oncol. 2020;31:334-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 73. | Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, Schirmacher P, Verslype C, Zech CJ, Arnold D, Martinelli E; ESMO Guidelines Committee. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv238-iv255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 722] [Article Influence: 103.1] [Reference Citation Analysis (0)] |
| 74. | Kim KE, Sinn DH, Choi MS, Kim H. Outcomes of patients presenting with elevated tumor marker levels but negative gadoxetic acid-enhanced liver MRI after a complete response to hepatocellular carcinoma treatment. PLoS One. 2022;17:e0262750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 75. | Erkan B, Meier J, Clark TJ, Kaplan J, Lambert JR, Chang S. Non-invasive diagnostic criteria of hepatocellular carcinoma: Comparison of diagnostic accuracy of updated LI-RADS with clinical practice guidelines of OPTN-UNOS, AASLD, NCCN, EASL-EORTC, and KLSCG-NCC. PLoS One. 2019;14:e0226291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 76. | Aubé C, Oberti F, Lonjon J, Pageaux G, Seror O, N'Kontchou G, Rode A, Radenne S, Cassinotto C, Vergniol J, Bricault I, Leroy V, Ronot M, Castera L, Michalak S, Esvan M, Vilgrain V; CHIC Group. EASL and AASLD recommendations for the diagnosis of HCC to the test of daily practice. Liver Int. 2017;37:1515-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 77. | Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, Han JK, Choi BI. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015;275:97-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 393] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 78. | Tsurusaki M, Sofue K, Isoda H, Okada M, Kitajima K, Murakami T. Comparison of gadoxetic acid-enhanced magnetic resonance imaging and contrast-enhanced computed tomography with histopathological examinations for the identification of hepatocellular carcinoma: a multicenter phase III study. J Gastroenterol. 2016;51:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 79. | Roberts LR, Sirlin CB, Zaiem F, Almasri J, Prokop LJ, Heimbach JK, Murad MH, Mohammed K. Imaging for the diagnosis of hepatocellular carcinoma: A systematic review and meta-analysis. Hepatology. 2018;67:401-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 342] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 80. | Ahn SS, Kim MJ, Lim JS, Hong HS, Chung YE, Choi JY. Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology. 2010;255:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 282] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 81. | Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014;273:30-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 392] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 82. | Di Martino M, De Filippis G, De Santis A, Geiger D, Del Monte M, Lombardo CV, Rossi M, Corradini SG, Mennini G, Catalano C. Hepatocellular carcinoma in cirrhotic patients: prospective comparison of US, CT and MR imaging. EurRadiol. 2013;23:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 83. | Kitao A, Zen Y, Matsui O, Gabata T, Kobayashi S, Koda W, Kozaka K, Yoneda N, Yamashita T, Kaneko S, Nakanuma Y. Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR Imaging--correlation with molecular transporters and histopathologic features. Radiology. 2010;256:817-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 281] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 84. | Yim JH, Kim YK, Min JH, Lee J, Kang TW, Lee SJ. Diagnosis of recurrent HCC: intraindividual comparison of gadoxetic acid MRI and extracellular contrast-enhanced MRI. AbdomRadiol (NY). 2019;44:2366-2376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Park MJ, Kim YK, Lee MW, Lee WJ, Kim YS, Kim SH, Choi D, Rhim H. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology. 2012;264:761-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 86. | Vandecaveye V, De Keyzer F, Verslype C, Op de Beeck K, Komuta M, Topal B, Roebben I, Bielen D, Roskams T, Nevens F, Dymarkowski S. Diffusion-weighted MRI provides additional value to conventional dynamic contrast-enhanced MRI for detection of hepatocellular carcinoma. EurRadiol. 2009;19:2456-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 87. | Liu Z, Fan JM, He C, Li ZF, Xu YS, Li Z, Liu HF, Lei JQ. Utility of diffusion weighted imaging with the quantitative apparent diffusion coefficient in diagnosing residual or recurrent hepatocellular carcinoma after transarterial chemoembolization: a meta-analysis. Cancer Imaging. 2020;20:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 88. | Wang W, Yang C, Zhu K, Yang L, Ding Y, Luo R, Zhu S, Chen C, Sun W, Zeng M, Rao SX. Recurrence After Curative Resection of Hepatitis B Virus-Related Hepatocellular Carcinoma: Diagnostic Algorithms on Gadoxetic Acid-Enhanced Magnetic Resonance Imaging. Liver Transpl. 2020;26:751-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 89. | Becker-Weidman D, Civan JM, Deshmukh SP, Roth CG, Herrine SK, Parker L, Mitchell DG. Hepatocellular carcinoma after locoregional therapy: Magnetic resonance imaging findings in falsely negative exams. World J Hepatol. 2016;8:685-690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 90. | Hai Y, Savsani E, Chong W, Eisenbrey J, Lyshchik A. Meta-analysis and systematic review of contrast-enhanced ultrasound in evaluating the treatment response after locoregional therapy of hepatocellular carcinoma. AbdomRadiol (NY). 2021;46:5162-5179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Bansal S, Lu F, Frehlich L, Wong JK, Burak KW, Wilson SR. A new proposal for secondary surveillance following potentially curative therapy of HCC: alternating MRI and CEUS. AbdomRadiol (NY). 2022;47:618-629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 92. | Frieser M, Kiesel J, Lindner A, Bernatik T, Haensler JM, Janka R, Hahn EG, Strobel D. Efficacy of contrast-enhanced US versus CT or MRI for the therapeutic control of percutaneous radiofrequency ablation in the case of hepatic malignancies. Ultraschall Med. 2011;32:148-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | Wu JY, Bai XM, Wang H, Xu Q, Wang S, Wu W, Yan K, Yang W. The Perfusion Features of Recurrent Hepatocellular Carcinoma After Radiofrequency Ablation Using Contrast-Enhanced Ultrasound and Pathological Stemness Evaluation: Compared to Initial Tumors. Front Oncol. 2020;10:1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 94. | Huang Z, Shu Z, Zhu RH, Xin JY, Wu LL, Wang HZ, Chen J, Zhang ZW, Luo HC, Li KY. Deep learning-based radiomics based on contrast-enhanced ultrasound predicts early recurrence and survival outcome in hepatocellular carcinoma. World J Gastrointest Oncol. 2022;14:2380-2392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 95. | Gu DY, Zhang Y, Hu JX, Qin HY, Lu X, He GB, Shang L. The value of contrast-enhanced ultrasound quantitative parameters in the prognosis prediction of hepatocellular carcinoma after thermal ablation: a retrospective cohort study. J Gastrointest Oncol. 2022;13:2522-2531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 96. | Zhang H, Huo F. Prediction of early recurrence of HCC after hepatectomy by contrast-enhanced ultrasound-based deep learning radiomics. Front Oncol. 2022;12:930458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 97. | Zhang Y, Wei Q, Huang Y, Yao Z, Yan C, Zou X, Han J, Li Q, Mao R, Liao Y, Cao L, Lin M, Zhou X, Tang X, Hu Y, Li L, Wang Y, Yu J, Zhou J. Deep Learning of Liver Contrast-Enhanced Ultrasound to Predict Microvascular Invasion and Prognosis in Hepatocellular Carcinoma. Front Oncol. 2022;12:878061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 98. | Zhong X, Peng J, Xie Y, Shi Y, Long H, Su L, Duan Y, Xie X, Lin M. A nomogram based on multi-modal ultrasound for prediction of microvascular invasion and recurrence of hepatocellular carcinoma. Eur J Radiol. 2022;151:110281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 99. | Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, Toyoda H, Imai Y, Hiraoka A, Ikeda M, Izumi N, Moriguchi M, Ogasawara S, Minami Y, Ueshima K, Murakami T, Miyayama S, Nakashima O, Yano H, Sakamoto M, Hatano E, Shimada M, Kokudo N, Mochida S, Takehara T. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer. 2021;10:181-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 451] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 100. | Bansal S, Gui J, Merrill C, Wong JK, Burak KW, Wilson SR. Contrast-enhanced US in Local Ablative Therapy and Secondary Surveillance for Hepatocellular Carcinoma. Radiographics. 2019;39:1302-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 101. | Fischer MA, Kartalis N, Grigoriadis A, Loizou L, Stål P, Leidner B, Aspelin P, Brismar TB. Perfusion computed tomography for detection of hepatocellular carcinoma in patients with liver cirrhosis. EurRadiol. 2015;25:3123-3132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 102. | Fischer MA, Marquez HP, Gordic S, Leidner B, Klotz E, Aspelin P, Alkadhi H, Brismar TB. Arterio-portal shunts in the cirrhotic liver: perfusion computed tomography for distinction of arterialized pseudolesions from hepatocellular carcinoma. EurRadiol. 2017;27:1074-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Hatzidakis A, Perisinakis K, Kalarakis G, Papadakis A, Savva E, Ippolito D, Karantanas A. Perfusion-CT analysis for assessment of hepatocellular carcinoma lesions: diagnostic value of different perfusion maps. Acta Radiol. 2019;60:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 104. | Nakamura Y, Higaki T, Honda Y, Tatsugami F, Tani C, Fukumoto W, Narita K, Kondo S, Akagi M, Awai K. Advanced CT techniques for assessing hepatocellular carcinoma. Radiol Med. 2021;126:925-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 105. | Ippolito D, Pecorelli A, Querques G, Drago SG, Maino C, Franzesi CT, Hatzidakis A, Sironi S. Dynamic Computed Tomography Perfusion Imaging: Complementary Diagnostic Tool in Hepatocellular Carcinoma Assessment From Diagnosis to Treatment Follow-up. AcadRadiol. 2019;26:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 106. | Ippolito D, Bonaffini PA, Capraro C, Leni D, Corso R, Sironi S. Viable residual tumor tissue after radiofrequency ablation treatment in hepatocellular carcinoma: evaluation with CT perfusion. Abdom Imaging. 2013;38:502-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 107. | Marquez HP, Puippe G, Mathew RP, Alkadhi H, Pfammatter T, Fischer MA. CT Perfusion for Early Response Evaluation of Radiofrequency Ablation of Focal Liver Lesions: First Experience. Cardiovasc InterventRadiol. 2017;40:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 108. | Yang L, Zhang XM, Tan BX, Liu M, Dong GL, Zhai ZH. Computed tomographic perfusion imaging for the therapeutic response of chemoembolization for hepatocellular carcinoma. J Comput Assist Tomogr. 2012;36:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 109. | Su TH, He W, Jin L, Chen G, Xiao GW. Early Response of Hepatocellular Carcinoma to Chemoembolization: Volume Computed Tomography Liver Perfusion Imaging as a Short-Term Response Predictor. J Comput Assist Tomogr. 2017;41:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 110. | Borgheresi A, Gonzalez-Aguirre A, Brown KT, Getrajdman GI, Erinjeri JP, Covey A, Yarmohammadi H, Ziv E, Sofocleous CT, Boas FE. Does Enhancement or Perfusion on Preprocedure CT Predict Outcomes After Embolization of Hepatocellular Carcinoma? AcadRadiol. 2018;25:1588-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 111. | Ippolito D, Querques G, Okolicsanyi S, Franzesi CT, Strazzabosco M, Sironi S. Diagnostic value of dynamic contrast-enhanced CT with perfusion imaging in the quantitative assessment of tumor response to sorafenib in patients with advanced hepatocellular carcinoma: A feasibility study. Eur J Radiol. 2017;90:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 112. | Ippolito D, Querques G, Pecorelli A, TaleiFranzesi C, Okolicsanyi S, Strazzabosco M, Sironi S. Diagnostic Value of Quantitative Perfusion Computed Tomography Technique in the Assessment of Tumor Response to Sorafenib in Patients With Advanced Hepatocellular Carcinoma. J Comput Assist Tomogr. 2019;43:206-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 113. | Nakamura Y, Kawaoka T, Higaki T, Fukumoto W, Honda Y, Iida M, Fujioka C, Kiguchi M, Aikata H, Chayama K, Awai K. Hepatocellular carcinoma treated with sorafenib: Arterial tumor perfusion in dynamic contrast-enhanced CT as early imaging biomarkers for survival. Eur J Radiol. 2018;98:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 114. | Kobe A, Kindler Y, Klotz E, Puippe G, Messmer F, Alkadhi H, Pfammatter T. Fusion of Preinterventional MR Imaging With Liver Perfusion CT After RFA of Hepatocellular Carcinoma: Early Quantitative Prediction of Local Recurrence. Invest Radiol. 2021;56:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 115. | Erstad DJ, Tanabe KK. Prognostic and Therapeutic Implications of Microvascular Invasion in Hepatocellular Carcinoma. Ann Surg Oncol. 2019;26:1474-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 288] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 116. | Wu L, Yang C, Halim A, Rao S, Xu P, Feng W, Chen C, Ji Y, Zhu J, Zeng M. Contrast-enhanced magnetic resonance imaging perfusion can predict microvascular invasion in patients with hepatocellular carcinoma (between 1 and 5 cm). AbdomRadiol (NY). 2022;47:3264-3275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 117. | Song Q, Guo Y, Yao X, Rao S, Qian C, Ye D, Zeng M. Comparative study of evaluating the microcirculatory function status of primary small HCC between the CE (DCE-MRI) and Non-CE (IVIM-DWI) MR Perfusion Imaging. AbdomRadiol (NY). 2021;46:2575-2583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 118. | Wang F, Yan CY, Wang CH, Yang Y, Zhang D. The Roles of Diffusion Kurtosis Imaging and Intravoxel Incoherent Motion Diffusion-Weighted Imaging Parameters in Preoperative Evaluation of Pathological Grades and Microvascular Invasion in Hepatocellular Carcinoma. Front Oncol. 2022;12:884854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 119. | Zhao W, Liu W, Liu H, Yi X, Hou J, Pei Y, Feng D, Liu L, Li W. Preoperative prediction of microvascular invasion of hepatocellular carcinoma with IVIM diffusion-weighted MR imaging and Gd-EOB-DTPA-enhanced MR imaging. PLoS One. 2018;13:e0197488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 120. | Asman Y, Evenson AR, Even-Sapir E, Shibolet O. [18F]fludeoxyglucose positron emission tomography and computed tomography as a prognostic tool before liver transplantation, resection, and loco-ablative therapies for hepatocellular carcinoma. Liver Transpl. 2015;21:572-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 121. | Kobayashi T, Aikata H, Honda F, Nakano N, Nakamura Y, Hatooka M, Morio K, Morio R, Fukuhara T, Masaki K, Nagaoki Y, Kawaoka T, Tsuge M, Hiramatsu A, Imamura M, Kawakami Y, Ohdan H, Awai K, Chayama K. Preoperative Fluorine 18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography for Prediction of Microvascular Invasion in Small Hepatocellular Carcinoma. J Comput Assist Tomogr. 2016;40:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 122. | Chiu RY, Yap WW, Patel R, Liu D, Klass D, Harris AC. Hepatocellular Carcinoma Post Embolotherapy: Imaging Appearances and Pitfalls on Computed Tomography and Magnetic Resonance Imaging. Can Assoc Radiol J. 2016;67:158-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 123. | Alnammi M, Wortman J, Therrien J, Afnan J. MRI features of treated hepatocellular carcinoma following locoregional therapy: a pictorial review. AbdomRadiol (NY). 2022;47:2299-2313. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |