Published online Feb 28, 2022. doi: 10.3748/wjg.v28.i8.868
Peer-review started: August 29, 2021
First decision: September 29, 2021
Revised: October 7, 2021
Accepted: January 22, 2022
Article in press: January 22, 2022
Published online: February 28, 2022
Processing time: 179 Days and 0.3 Hours
During pancreaticoduodenectomy in patients with celiac axis (CA) stenosis due to compression by the median arcuate ligament (MAL), the MAL has to be divided to maintain hepatic blood flow in many cases. However, MAL division often fails, and success can only be determined intraoperatively. To overcome this problem, we performed endovascular CA stenting preoperatively, and thereafter safely performed pancreaticoduodenectomy. We present this case as a new preoperative treatment strategy that was successful.
A 77-year-old man with a diagnosis of pancreatic head cancer presented to our department for surgery. Preoperative assessment revealed CA stenosis caused by MAL. We performed endovascular stenting in the CA preoperatively because we knew that going into the operation without a strategy could lead to ischemic complications. Double-antiplatelet therapy (DAPT) – which is needed when a stent is inserted – was then administered in parallel with neoadjuvant chemothe
Preoperative endovascular stenting, with NAC and DAPT, is effective and safe prior to pancreaticoduodenectomy in potentially resectable pancreatic cancer.
Core Tip: Celiac axis stenosis (CAS), caused by the median arcuate ligament, is an anatomical anomaly that should be noted when performing pancreaticoduodenectomy. In this case, an endovascular stent was placed preoperatively to recanalize the stenotic celiac axis, allowing the patient to safely undergo radical surgery without concern for intraoperative organ perfusion. The point to be highlighted with this preoperative strategy, was the use of double-antiplatelet therapy following endovascular stenting, during the interval when for neoadjuvant chemotherapy for pancreatic cancer (PC), thus providing a rational and effective combination of the two preoperative treatments for patients with PC and CAS.
- Citation: Yoshida E, Kimura Y, Kyuno T, Kawagishi R, Sato K, Kono T, Chiba T, Kimura T, Yonezawa H, Funato O, Kobayashi M, Murakami K, Takagane A, Takemasa I. Treatment strategy for pancreatic head cancer with celiac axis stenosis in pancreaticoduodenectomy: A case report and review of literature. World J Gastroenterol 2022; 28(8): 868-877
- URL: https://www.wjgnet.com/1007-9327/full/v28/i8/868.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i8.868
Celiac axis stenosis (CAS) – caused by the median arcuate ligament (MAL) – often hinders blood flow to the upper abdominal organs, particularly the liver, during pancreaticoduodenectomy[1-4]. This is because the gastroduodenal artery (GDA), which is responsible for maintaining hepatic blood flow during the procedure, needs to be dissected. The most common intervention to maintain hepatic arterial flow is intraoperative MAL division[1,5,6], which fails to recanalize the arterial flow in 30%-40% of cases[1,4,7]. Additionally, the success of MAL division can only be determined intraoperatively and, in case of failure, surgeons must choose to either undertake a complex revascularization or abandon the resection intraoperatively.
Recently, neoadjuvant chemotherapy (NAC) has become a common preoperative treatment for pancreatic cancer (PC)[8,9]. NAC improves the overall survival of patients with PC and is usually required for 2-3 mo, or more, depending on the necessary treatment regimen. To eliminate concerns about MAL compression prior to the scheduled pancreaticoduodenectomy, we undertook preoperative endovascular celiac axis (CA) stenting. Double-antiplatelet therapy (DAPT) was subsequently administered to avoid stent obstruction, with the NAC for PC continuing concurrently. Herein, we report on a reasonable and effective preoperative strategy that combines NAC for PC and endovascular stenting for MAL compression.
A 77-year-old man was referred to our department with a progressively enlarging pancreatic head mass.
The patient had been followed-up for intraductal papillary mucinous neoplasm of the pancreas for 20 years, with no significant changes in the periodically scheduled examinations until half a year ago.
In the last six months, a tumor of approximately 30 mm became visible on the pancreatic head and diagnosed as cancer after further investigation.
The patient had undergone robot-assisted laparoscopic prostatectomy for prostate cancer six years prior, as well as endoscopic submucosal dissection for early gastric cancer two years prior. He had a pacemaker implanted for atrioventricular block two years prior.
The patient had no family history of PC or genetic disorders.
The patient’s appetite was normal, and he had no weight loss or abdominal pain. Stool was normal with no history of constipation or diarrhea. Furthermore, jaundice was not observed.
Laboratory results included an elevated carbohydrate antigen 19-9 (CA19-9) level of 308.5 U/mL, but the carcinoembryonic antigen level was normal. Other results, including diabetes indices such as HbA1c and glycoalbumin levels, were within normal limits. Bilirubin levels were also normal.
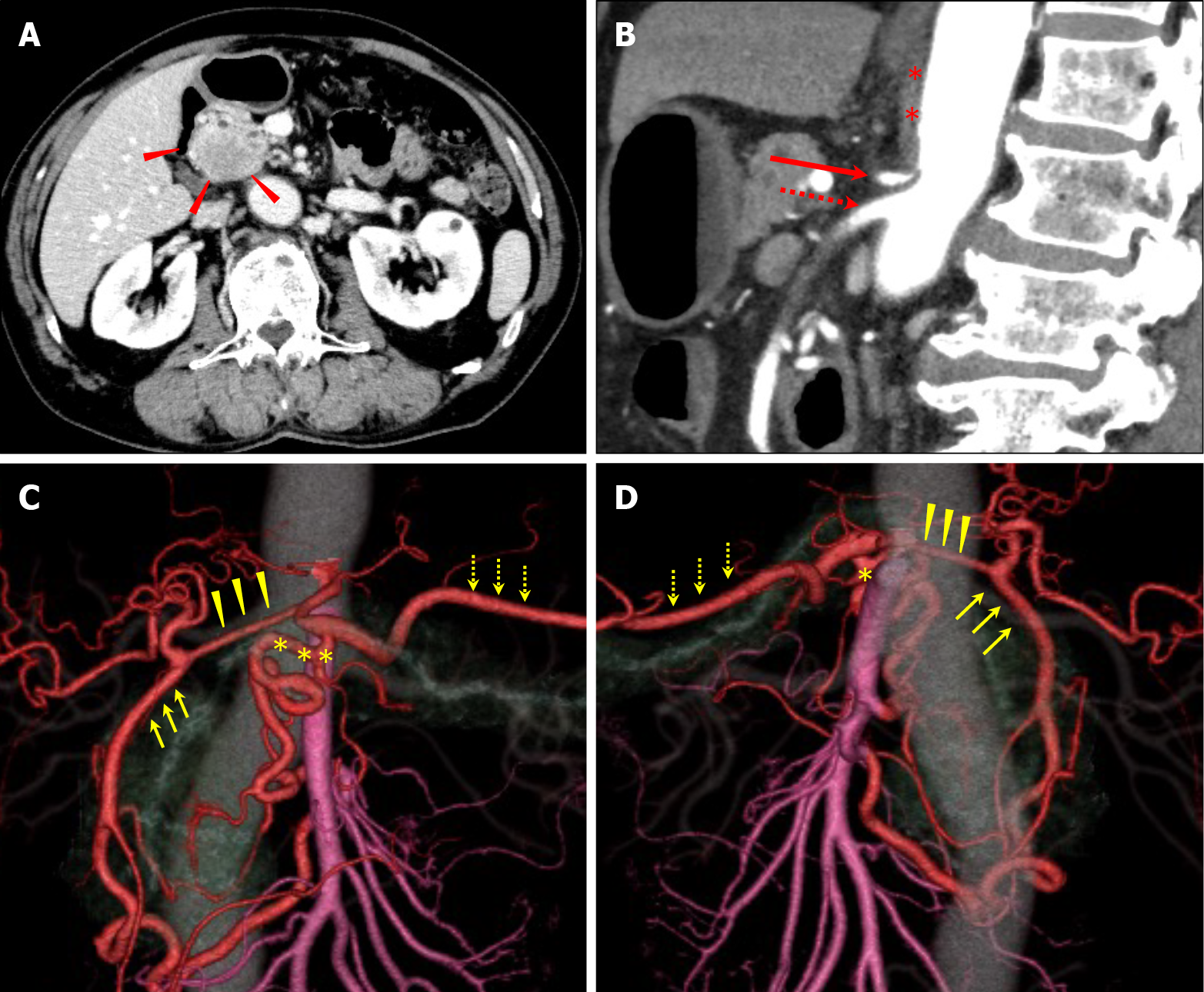
Computed tomography (CT) revealed a 30 mm pancreatic head tumor with poor enhancement in the early phase (Figure 1A). The tumor had not invaded the major vessels including the common hepatic artery, CA, and the superior mesenteric artery (SMA) and superior mesenteric vein. No metastatic lymph nodes and metastases to distant organs were apparent. Three-dimensional reconstruction images showed developed collateral pathways around the pancreatic head (Figure 1B and C). In the sagittal view, the CA was compressed by the MAL, which developed caudally (Figure 1D). Endoscopic retrograde cholangiopancreatography revealed stenosis in the main pancreatic duct (MPD) and biopsy from the stenotic site of the MPD detected adenocarcinoma.
He was diagnosed with cT2N0M0 (8th edition of the UICC-TNM classification) pancreatic head cancer comorbid with CAS caused by MAL.
A pancreaticoduodenectomy was planned due to the diagnosis of pancreatic head cancer. To secure hepatic blood flow, endovascular stenting of the CA was undertaken prior to the surgery. After successful endovascular stenting, DAPT was administered to avoid stent obstruction, with NAC for PC continuing concurrently.
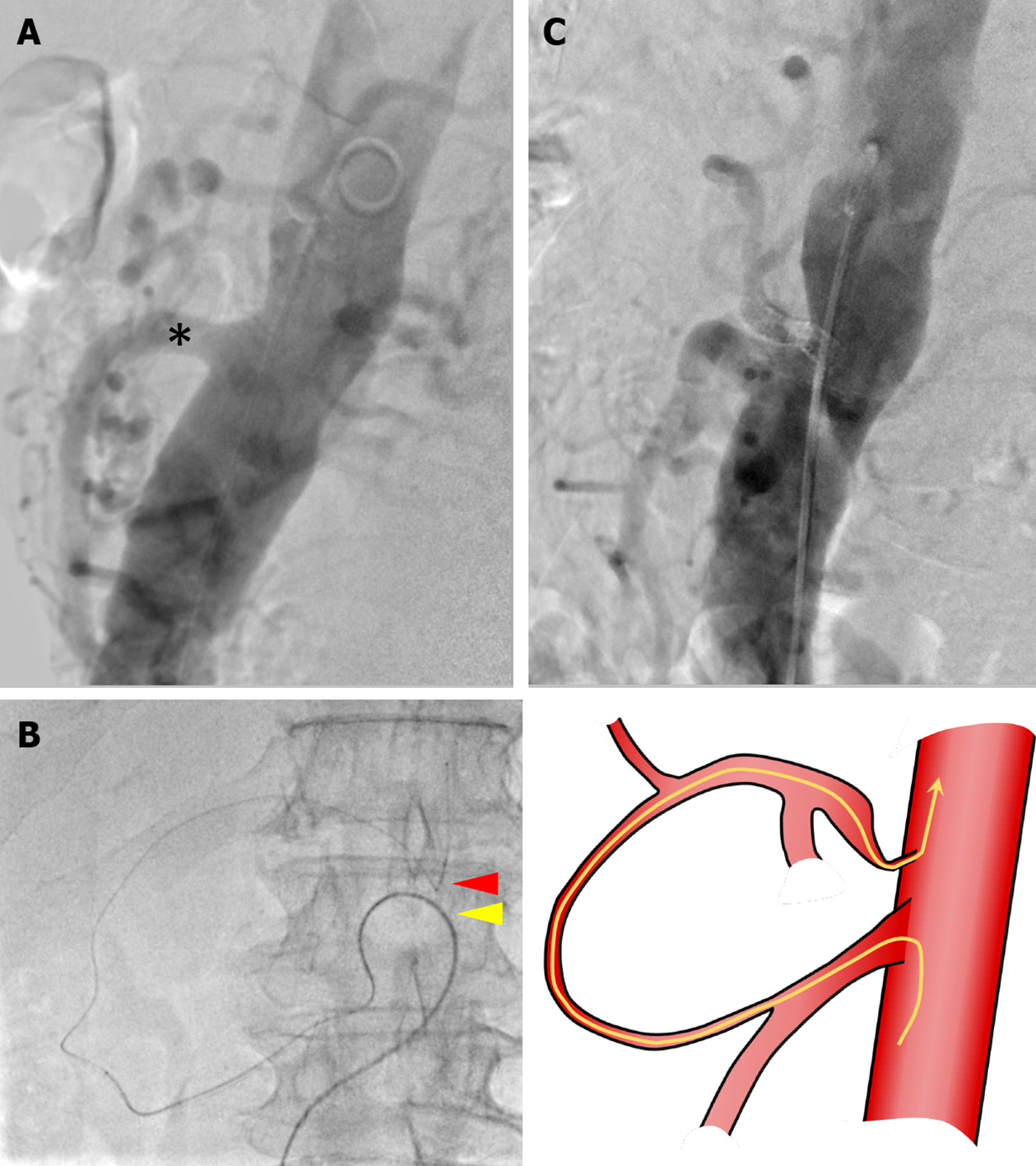
On aortography, the CA could not be visualized, and severe stenosis of the CA was suggested (Figure 2A). It was difficult to achieve an antegrade approach to the CA directly from the aorta because the root of the CA was severely stenotic, and the root was strongly bent. Therefore, we planned to approach the CA retrogradely from the SMA via the collateral pathway.
The procedure was initiated by placing sheaths in the right and left common femoral arteries. A 4Fr shepherd hook catheter (AngiomasterR, Terumo, Tokyo, Japan) was selectively inserted into the SMA through the left sheath. Next, we used a triple-coaxial system to reach the CA[10]. In the triple coaxial system, a 2.7 Fr high flow catheter (Bishop HF, Piolax Medical Devices, Yokohama, Japan), a 1.9 Fr micro catheter (CarnelianR MarvelR, Tokai Medical Products, Aichi, Japan), and a 0.014-inch micro guidewire (Jupiter™ FC, Boston Scientific, Marlborough, MA, United States) were used. Because the guidewire was as fine as 0.014-inch, it was possible to break through the stenosis of the CA (Figure 2B). The microguidewire that reached the aorta was grasped with a snare (Atrieve™ Vascular Snare Kit, Argon Medical Devices, TX, United States) inserted through the right sheath and pulled out of the body. Then, a guiding sheath (Parent PlusR60, Medikit, Tokyo, Japan) was inserted into the CA using the pull-through technique. A balloon expandable stent (Express™ LD vascular stent, Boston Scientific, Massachusetts, United States) was successfully deployed in the CA and an additional stent was used to reinforce the stenotic lesion. Finally, stent dilation was performed with a balloon (Mustang™ Balloon Dilatation Catheter, Boston Scientific, Massachusetts, United States). Aortography demonstrated CA patency and antegrade flow, and the procedure was completed (Figure 2C).
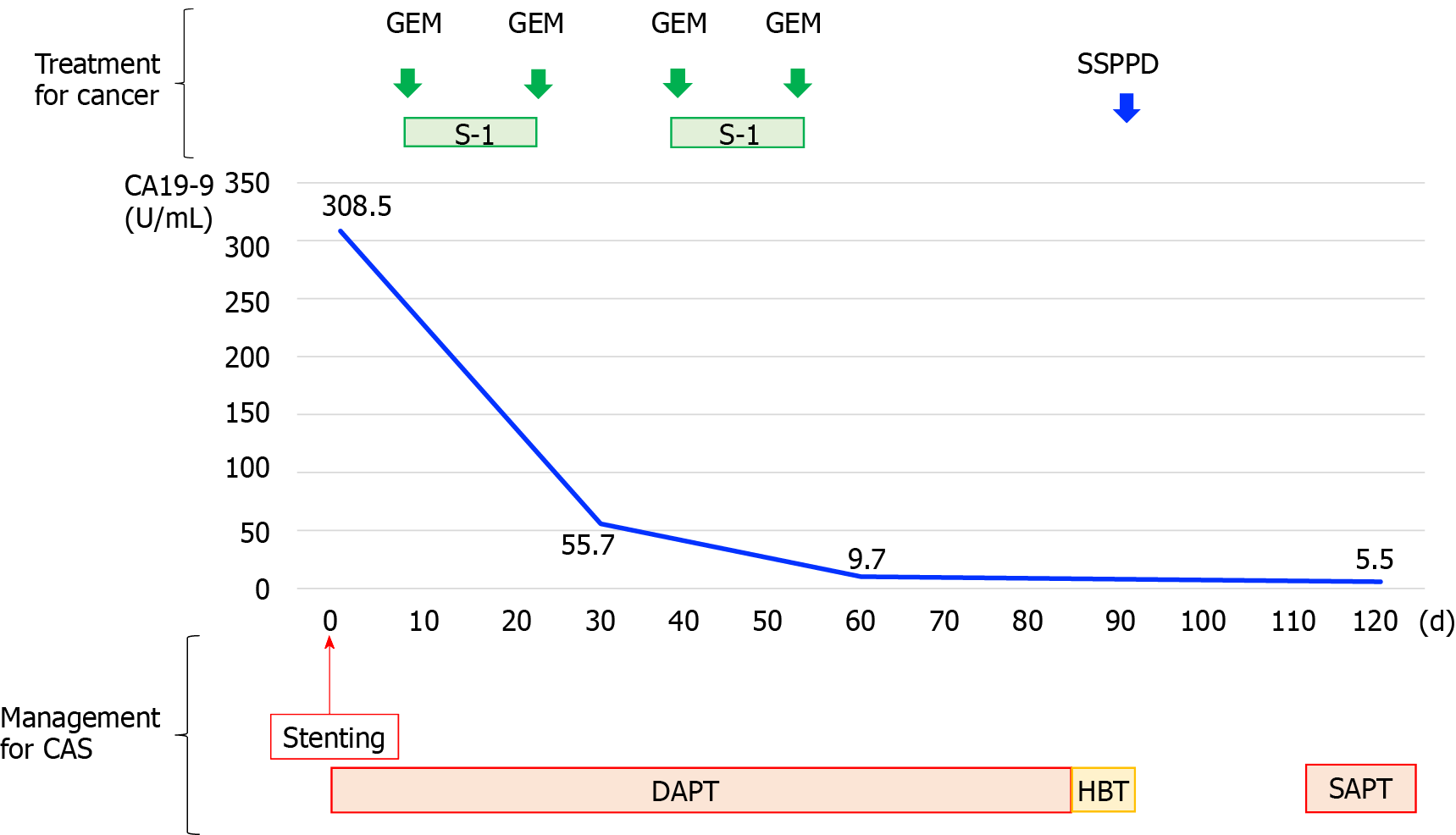
DAPT was started the day after stent insertion, and NAC was introduced on day 5. DAPT consisted of oral 100 mg aspirin and 75 mg P2Y12 inhibitor once daily. NAC regimen was gemcitabine plus S-1 therapy, which consisted of intravenous gemcitabine at a dose of 1000 mg/m2 on days 1 and 15 and S-1 orally at a dose of 60 mg twice daily on days 1-14 of a 28-d cycle. DAPT continued for 12 wk and two courses of NAC were administered. The preoperative course is shown in Figure 3.
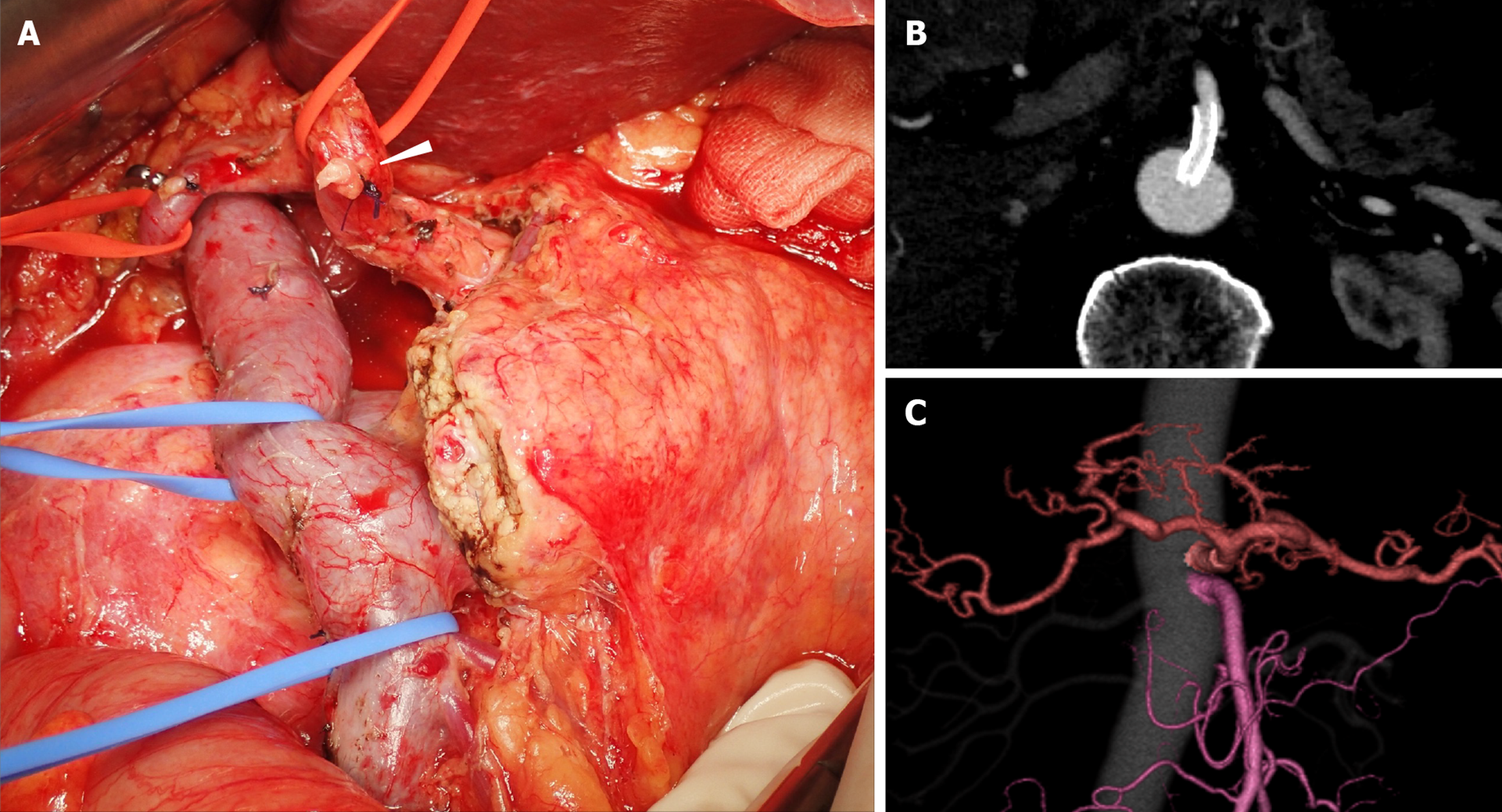
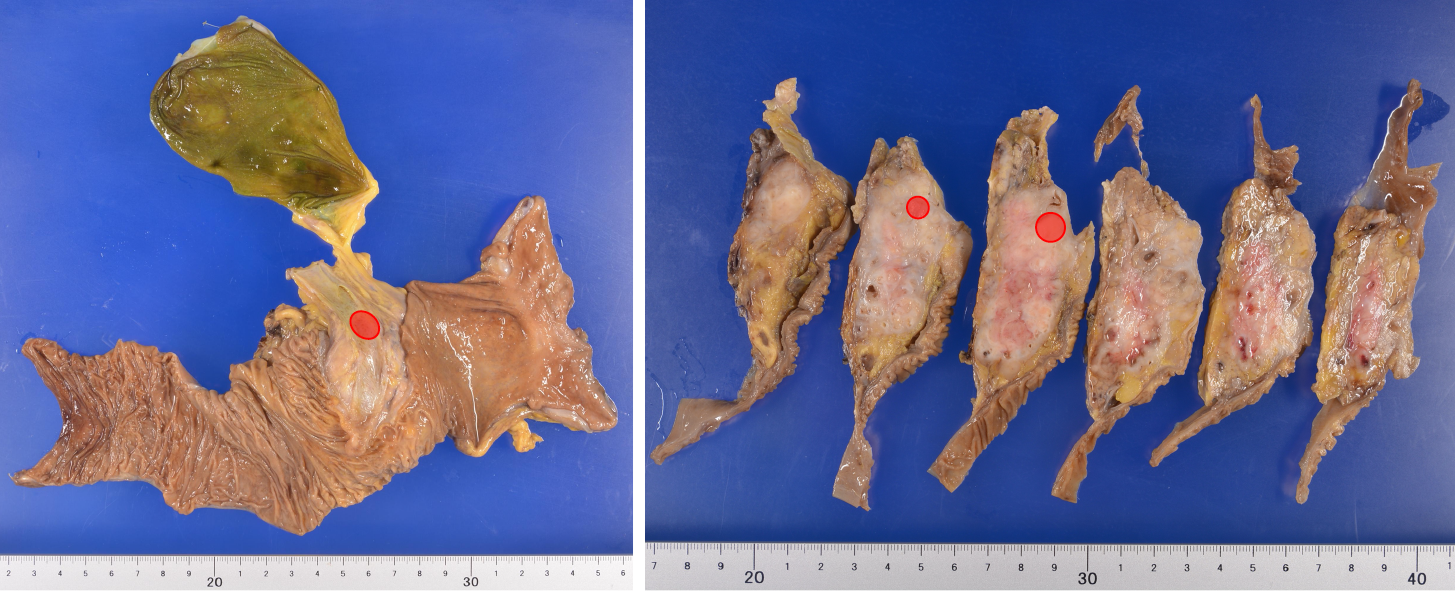
After completing preoperative treatment, subtotal stomach-preserving pancreaticoduodenectomy was performed (Figure 4A). Since the CA flow was sufficient before the surgery, the surgical procedure was routinely performed, without any special intraoperative techniques. After clamping the GDA, we confirmed good blood flow in the proper hepatic artery (PHA) by palpation, then dissected the GDA. There was no need to preserve the collateral pathways that continued to the DPA or GDA, so we dissected them and obtained an adequate SMA-margin. When dissecting these collateral pathways, we confirmed that the PHA flow remained pulsatile as an indicator that the blood flow in the CA was adequate. We did not apply the MAL division due to concerns regarding stent dislocation induced by dissection procedures near the stent.
Postoperatively, the transaminase level was transiently elevated which quickly improved, and there were no complications related to liver ischemia. No delayed gastric emptying due to decreased gastric blood flow occured, and the patient was able to resume eating four days post-surgery. The patient developed a Grade B postoperative pancreatic fistula (POPF), based on the International Study Group of Pancreatic Surgery grading, but no specific treatment other than drainage was needed[11]. The POPF did not cause any complications, such as hemorrhage and abscess, and the patient was discharged on postoperative day 39. Both the patient and his family were satisfied.
The pathological diagnosis was pancreatic adenocarcinoma, pT1cN0M0, the cancer was StageⅠA based on the Eighth edition of the UICC-TNM classification, and the pathological treatment effect was graded as IIb based on the Evans classification[12,13]. Since no viable cancer cells were observed in the resected margin, R0 resection was achieved (Figure 5).
Stent patency was confirmed by CT imaging up to six months postoperatively, and the patient had no complications related to liver ischemia (Figure 4) and no recurrence.
This case report introduces the procedure and outcome of a preoperative treatment strategy for pancreatic head cancer comorbid with CA stenosis due to compression by the MAL, which combined NAC for cancer and endovascular stenting for MAL compression.
CAS is often observed and reportedly present in approximately 4% to 10.5% of patients undergoing pancreaticoduodenectomy[7,14]. If the GDA is dissected while performing pancreaticoduodenectomy, hepatic blood flow disappears and hepatic ischemia may occur, leading to complications such as liver failure, liver abscess, and bile leakage[1-4]. There are various causes of stenosis, such as MAL compression, atherosclerosis, and arthritis; however, the most common cause is MAL compression[1,15]. In this case, MAL compression was diagnosed preoperatively, allowing us to intervene preoperatively to maintain hepatic blood flow.
In MAL compression, MAL division is often selected because it is easy and quick. However, while MAL division is simple, it is not reliable, because the CA often does not recanalize and hepatic blood flow is not secured even if the MAL is divided.
Through a comprehensive literature review using the MESH term “pancreat(ic)oduodenectomy and celiac axis stenosis”, we identified 108 cases in which the patients underwent pancreaticoduodenectomy and had MAL compression (Table 1). In a literature review of cases in which an intervention was performed for MAL compression, 90.4% underwent MAL division. However, hepatic arterial flow could not be restored in 21.2% of patients despite MAL division. The reason for the failure of MAL division in recanalizing the CA was scarring of the arterial wall caused by longstanding stenosis[16]. In this case, the CT images from approximately 10 years ago already showed signs of MAL compression, which suggested that blood flow might not resume even if the MAL was divided. Additionally, according to a previous report which classified MAL compression morphologically by stenosis rate, length of stenosis, and distance between the stenosis and the aorta, these factors were useful in predicting procedures that would be required during the operation. The authors found that MAL division was often ineffective, and revascularization or preservation of the collateral pathways were required, in cases of severe stenosis (stenosis rate of 80% or more) and with a small distance between the stenosis and the aorta[4].
| Factor | Subject | No. of cases (n = 108) |
| Age | yr, median (range) | 61 (38-91)1 |
| Sex | Male/female | 30/191 |
| Diagnosis | Ampullary cancer | 4 |
| Bile duct cancer | 10 | |
| Pancreatic head cancer | 33 | |
| Others | 4 | |
| None described | 57 | |
| Preoperative detection of CAS | Yes | 97 |
| No | 9 | |
| None described | 2 | |
| Procedure | MAL division | 66 |
| Revascularization | 5 | |
| Stenting | 1 | |
| Preservation of collateral pathway | 1 | |
| No | 35 | |
| Outcome | Success | 91 |
| Especially MAL division | 52 | |
| Failure | 17 | |
| Especially MAL division | 14 | |
| Additional procedures | Revascularization | 7 |
| Stenting | 5 | |
| Reoperation | 3 | |
| No | 32 | |
| Complications related to CAS | Liver abscess | 3 |
| Organ ischemia | 2 | |
| Anastomotic leakage | 32 |
The failure of MAL division can only be determined intraoperatively, and in these situations surgeons have to make a choice that is detrimental to the patient i.e., complex revascularization or abandonment of the surgery. Hepatic arterial revascularization during pancreaticoduodenectomy may result in substantial risk of postoperative hemorrhage – possibly related to POPF – and should be avoided. In previous reports, some cases of CAS had postoperative hemorrhage in the revascularized area[17,18].
NAC has become a common treatment for PC in recent years and is recommended by the NCCN guidelines. NAC is usually administered at an interval of 2-3 mo before radical surgery. With this background, we developed a preoperative strategy to eliminate concerns regarding MAL compression.
In our case, we applied stenting to the CA preoperatively, allowing CA recanalization and hepatic blood flow to be confirmed prior to the operation. Furthermore, DAPT was required to avoid stent obstruction, and could also be performed during the NAC treatment period. Clearly, the combination of these two treatments was reasonable. One of the aims in administering NAC is, primarily, to reduce postoperative recurrence. A recent study found that failure of CA19-9 to normalize from preoperatively elevated levels, by the time of surgery, is a predictor of early postoperative recurrence[19]. For our patient, the normalization of the initially very high levels of CA19-9 upon NAC during DAPT, and the preservation of stent patency during the same period, were helpful. The duration of DAPT was determined by referring to the antithrombotic therapy guidelines for patients with coronary artery disease, specifically antithrombotic therapy after percutaneous coronary intervention[20]. MAL division may also be an option to avoid stent obstruction, even after the CA stenting. However, we did not perform MAL division in this case because the risk of dislocating the stent during this procedure defeats the purpose of stenting.
Interventional radiology (IVR) for vascular stenosis is performed in various diseases. However, there are few reports regarding the adaptation of IVR for MAL compression, since MAL division is often performed. This case is the first report of the use of the combination of NAC, stent, and DAPT. There is no reason to perform MAL division in addition to this method, and there are no reports of MAL division performed after stenting in existing literature.
Clinical diagnosis of MAL compression is essential for preoperative intervention. In the literature review, a preoperative diagnosis was made in most of the cases (91.5%), indicating that preoperative diagnosis is relatively easy. This high proportion of preoperative diagnosis is thought to be due to the widespread use of multidetector CT and the high recognition of MAL compression[1]. Although the definitive diagnosis of blood flow disturbance by MAL compression can only be made intraoperatively, color doppler ultrasound is a useful diagnostic technique because it is easy and non-invasive[1,3,5].
Intraoperative procedures other than MAL division are used to preserve the collateral pathway, but these surgical techniques are complicated. Additionally, MAL division may impair the curativeness of cancer, so its indications are limited to benign tumors and inflammatory diseases[5,7], and it is not recommended for malignant PC, such as in our case. We were able to dissect the collateral pathway around the SMA and ensure the SMA-margin.
This method has two limitations. First, it requires a high level of skill for stenting in the case of MAL compression. There are previous reports of failure in cases of stenting[21-23]. It is hoped that IVR techniques will become more widespread and improve in the future. Second, the optimal time between stent insertion and radical surgery has not yet been investigated. In this case, since the patient had PC, NAC was administered and sufficient DAPT could be administered during the same period. However, in diseases where NAC is not a common treatment, such as biliary tract cancer, it may worsen the primary disease.
Thus, preoperative simulation and maintenance of skills, such as MAL division and revascularization, remain important for surgeons.
Preoperative stent insertion followed by a combination of NAC and DAPT is a safe way to perform pancreaticoduodenectomy; therefore, this procedure may be an effective preoperative treatment strategy.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hiep L, Mu PY S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Gaujoux S, Sauvanet A, Vullierme MP, Cortes A, Dokmak S, Sibert A, Vilgrain V, Belghiti J. Ischemic complications after pancreaticoduodenectomy: incidence, prevention, and management. Ann Surg. 2009;249:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Miyata T, Yamamoto Y, Sugiura T, Okamura Y, Ito T, Ashida R, Uemura S, Kato Y, Ohgi K, Kohga A, Uchida T, Sano S, Nakagawa M, Uesaka K. Pancreaticoduodenectomy with hepatic arterial revascularization for pancreatic head cancer with stenosis of the celiac axis due to compression by the median arcuate ligament: a case report. J Surg Case Rep. 2018;2018:rjy002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Berney T, Pretre R, Chassot G, Morel P. The role of revascularization in celiac occlusion and pancreatoduodenectomy. Am J Surg. 1998;176:352-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Sugae T, Fujii T, Kodera Y, Kanzaki A, Yamamura K, Yamada S, Sugimoto H, Nomoto S, Takeda S, Nakao A. Classification of the celiac axis stenosis owing to median arcuate ligament compression, based on severity of the stenosis with subsequent proposals for management during pancreatoduodenectomy. Surgery. 2012;151:543-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Sakorafas GH, Sarr MG, Peros G. Celiac artery stenosis: an underappreciated and unpleasant surprise in patients undergoing pancreaticoduodenectomy. J Am Coll Surg. 2008;206:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Miyazaki K, Morine Y, Saito Y, Yamada S, Tokuda K, Ikemoto T, Imura S, Shimada M. Pancreatoduodenectomy co-morbid with celiac axis compression syndrome: a report of three cases. Surg Case Rep. 2020;6:113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Kurosaki I, Hatakeyama K, Nihei KE, Oyamatsu M. Celiac axis stenosis in pancreaticoduodenectomy. J Hepatobiliary Pancreat Surg. 2004;11:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Motoi F, Kosuge T, Ueno H, Yamaue H, Satoi S, Sho M, Honda G, Matsumoto I, Wada K, Furuse J, Matsuyama Y, Unno M; Study Group of Preoperative Therapy for Pancreatic Cancer (Prep) and Japanese Study Group of Adjuvant Therapy for Pancreatic cancer (JSAP). Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 vs upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol. 2019;49:190-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 329] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 9. | Mokdad AA, Minter RM, Zhu H, Augustine MM, Porembka MR, Wang SC, Yopp AC, Mansour JC, Choti MA, Polanco PM. Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J Clin Oncol. 2017;35:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 305] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 10. | Shimohira M, Ohta K, Suzuki K, Goto T, Sawada Y, Shibamoto Y. Newly developed triaxial microcatheter for complicated interventions. Minim Invasive Ther Allied Technol. 2018;27:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 2947] [Article Influence: 368.4] [Reference Citation Analysis (35)] |
| 12. | Brierley JD, Gospodarowicz MK, Wittekind C. UICC: TNM classification of malignant tumors. 8th ed. Hoboken (NJ): Wiley Blackwell, 2016. |
| 13. | Evans DB, Rich TA, Byrd DR, Cleary KR, Connelly JH, Levin B, Charnsangavej C, Fenoglio CJ, Ames FC. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 541] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Thompson NW, Eckhauser FE, Talpos G, Cho KJ. Pancreaticoduodenectomy and celiac occlusive disease. Ann Surg. 1981;193:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Farma JM, Hoffman JP. Nonneoplastic celiac axis occlusion in patients undergoing pancreaticoduodenectomy. Am J Surg. 2007;193:341-4; discussion 344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Doyle AJ, Chandra A. Chronic mesenteric ischemia in a 26-year-old man: multivessel median arcuate ligament compression syndrome. Ann Vasc Surg. 2012;26:108.e5-108.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Shibuya K, Kamachi H, Orimo T, Nagatsu A, Shimada S, Wakayama K, Yokoo H, Kamiyama T, Taketomi A. Pancreaticoduodenectomy with Preservation of Collateral Circulation or Revascularization for Biliary Pancreatic Cancer with Celiac Axis Occlusion: A Report of 2 Cases. Am J Case Rep. 2018;19:413-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Beane JD, Schwarz RE. Vascular challenges from pancreatoduodenectomy in the setting of coeliac artery stenosis. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Imamura M, Nagayama M, Kyuno D, Ota S, Murakami T, Kimura A, Yamaguchi H, Kato T, Kimura Y, Takemasa I. Perioperative Predictors of Early Recurrence for Resectable and Borderline-Resectable Pancreatic Cancer. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Nakamura M, Kimura K, Kimura T, Ishihara M, Otsuka F, Kozuma K, Kosuge M, Shinke T, Nakagawa Y, Natsuaki M, Yasuda S, Akasaka T, Kohsaka S, Haze K, Hirayama A. JCS 2020 Guideline Focused Update on Antithrombotic Therapy in Patients With Coronary Artery Disease. Circ J. 2020;84:831-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 21. | Balakrishnan S, Kapoor S, Vijayanath P, Singh H, Nandhakumar A, Venkatesulu K, Shanmugam V. An innovative way of managing coeliac artery stenosis during pancreaticoduodenectomy. Ann R Coll Surg Engl. 2018;100:e168-e170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | McCracken E, Turley R, Cox M, Suhocki P, Blazer DG 3rd. Leveraging Aberrant Vasculature in Celiac Artery Stenosis: The Arc of Buhler in Pancreaticoduodenectomy. J Pancreat Cancer. 2018;4:4-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Celik S, Ringe KI, Boru CE, Constantinica V, Bektas H. A case of pancreatic cancer with concomitant median arcuate ligament syndrome treated successfully using an allograft arterial transposition. J Surg Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Fortner JG, Watson RC. Median arcuate ligament obstruction of celiac axis and pancreatic cancer. Ann Surg. 1981;194:698-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Kohler TR, Debas H, Crames M, Strandness DE Jr. Pancreaticoduodenectomy and the celiac artery compression syndrome. Ann Vasc Surg. 1990;4:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Okamura K, Hayakawa H, Kusagawa M, Takahashi H, Kosaka A, Katsuta K, Mizumoto R. Treatment of pancreas head carcinoma in a 91-yr-old man. Report of a case successfully treated with pylorus-preserving pancreatoduodenectomy. Int J Pancreatol. 1998;24:133-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Hasegawa K, Imamura H, Akahane M, Miura Y, Takayama T, Ohtomo K, Makuuchi M. Endovascular stenting for celiac axis stenosis before pancreaticoduodenectomy. Surgery. 2003;133:440-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Nara S, Sakamoto Y, Shimada K, Sano T, Kosuge T, Takahashi Y, Onaya H, Yamamoto J. Arterial reconstruction during pancreatoduodenectomy in patients with celiac axis stenosis--utility of Doppler ultrasonography. World J Surg. 2005;29:885-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Shima Y, Yagi T, Inagaki M, Sadamori H, Tanaka N, Horimi T, Hamazaki S. Intraductal oncocytic papillary neoplasm of the pancreas with celiac artery compression syndrome and a jejunal artery aneurysm: report of a case. Surg Today. 2005;35:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Nakano H, Yamamura T, Yamaguchi S, Otsubo T. Celiac axis occlusion of a patient undergoing pancreaticoduodenectomy after distal gastrectomy. Hepatogastroenterology. 2007;54:595-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Kabir S, Samra J. Median arcuate ligament: significance in pancreaticoduodenectomy. ANZ J Surg. 2013;83:686-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Turner KM, Majekodunmi K, Manejwala A, Neschis D, Novak Z, Boutros C. Image findings in celiac artery stenosis due to median arcuate ligament compression: a crucial diagnosis when planning for pancreaticoduodenectomy. J Gastrointest Surg. 2014;18:638-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Başkan Ö, Özdenkaya Y, Erol C, Dolay K. Problems with the Median Arcuate Ligament Should Be Recognized before Surgery; Its Importance in Pancreaticoduodenectomy. Balkan Med J. 2015;32:312-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Liang DH, Rosenberg WR, Martinez S. Bypass grafting between the supraceliac aorta and the common hepatic artery during pancreaticoduodenectomy. J Surg Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Park HM, Lee SD, Lee EC, Lee IJ, Han SS, Kim HB, Kim SH, Lee SA, Park SJ. Celiac axis stenosis as a rare but critical condition treated with pancreatoduodenectomy: report of 2 cases. Ann Surg Treat Res. 2016;91:149-153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Guilbaud T, Ewald J, Turrini O, Delpero JR. Pancreaticoduodenectomy: Secondary stenting of the celiac trunk after inefficient median arcuate ligament release and reoperation as an alternative to simultaneous hepatic artery reconstruction. World J Gastroenterol. 2017;23:919-925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Yamamoto M, Itamoto T, Oshita A, Matsugu Y. Celiac axis stenosis due to median arcuate ligament compression in a patient who underwent pancreatoduodenectomy; intraoperative assessment of hepatic arterial flow using Doppler ultrasonography: a case report. J Med Case Rep. 2018;12:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |