Published online Feb 28, 2022. doi: 10.3748/wjg.v28.i8.840
Peer-review started: July 30, 2021
First decision: August 19, 2021
Revised: August 30, 2021
Accepted: January 27, 2022
Article in press: January 21, 2022
Published online: February 28, 2022
Processing time: 209 Days and 6.3 Hours
The clinical outcomes of endoscopic submucosal dissection (ESD) for undifferentiated (UD) intramucosal early gastric cancer (EGC) compared with those of surgery, regardless of lesion size, are not well known. Furthermore, there is a concern regarding the treatment plan before and after ESD in cases of UD intramucosal EGC within expanded indications.
To evaluate clinical outcomes of ESD compared with those of surgery in UD intramucosal EGC patients regardless of tumor size.
We enrolled patients with UD intramucosal EGC after ESD with complete resection or surgery from January 2005 to August 2020 who met the within or beyond expanded indications with lesion size > 2 cm (the only non-curative factor). Overall, 123 and 562 patients underwent ESD and surgery, respectively. After propensity-score matching, clinical and long-term outcomes, i.e., recurrence-free survival (RFS) and overall survival (OS), were analyzed. The multivariable Cox proportional hazard model with treatment modality and ESD indication was used to evaluate the recurrence risk.
After matching, 119 patients each were finally enrolled in the ESD and surgery groups. The median length of hospital stay was shorter in the ESD group than surgery group (4.0 vs 9.0 days, P < 0.001). Four cases of recurrence after ESD were local recurrences, all of which occurred within 1 year. Total recurrence was seven (5.9%) and two (1.7%) in the ESD and surgery groups, respectively. No difference was observed between the two groups with respect to OS (P = 0.948). However, the ESD group had inferior RFS compared with the surgery group (P = 0.031). ESD was associated with the risk of recurrence after initial treatment in all enrolled patients (hazard ratio, 5.2; 95% confidence interval: 1.0-25.8, P = 0.045).
Although OS was similar between the two groups, surveillance endoscopy was important for the ESD than for the surgery group because RFS was inferior and local recurrence was an issue.
Core Tip: This retrospective study evaluated the clinical outcomes of endoscopic submucosal dissection (ESD) compared with those of surgery in patients with undifferentiated (UD) intramucosal early gastric cancer (EGC) after propensity-score matching. No difference in overall survival was observed between two groups, although recurrence-free survival was inferior in the ESD group. Lymph node metastasis was not observed after ESD; however, local recurrence was higher after ESD than surgery. Surveillance endoscopy is important in ESD, even if complete resection is performed for UD intramucosal EGC. A short interval endoscopic follow-up is necessary when observing lesion sizes > 2 cm as the only non-curative factor.
- Citation: Lee GH, Lee E, Park B, Roh J, Lim SG, Shin SJ, Lee KM, Noh CK. Long-term outcomes of endoscopic submucosal dissection and surgery for undifferentiated intramucosal gastric cancer regardless of size. World J Gastroenterol 2022; 28(8): 840-852
- URL: https://www.wjgnet.com/1007-9327/full/v28/i8/840.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i8.840
Endoscopic submucosal dissection (ESD) is recommended as a treatment modality for early gastric cancer (EGC) because it allows curative en bloc resection and complete histopathological evaluation[1,2]. With the development of endoscopic instruments and techniques, the indication for ESD has expanded, and short- and long-term outcomes of ESD have been favorably reported in various studies[3-8]. Accordingly, ESD can be performed for patients with undifferentiated (UD) intramucosal EGC without lymphovascular invasion when the lesion size is ≤ 2 cm and there is no ulceration. Compared with surgery, ESD may be an alternative treatment option for UD intramucosal EGC within expanded indications[9-12]; however, concerns regarding lymph node (LN) metastasis in patients with UD intramucosal EGC remain[12].
Even if UD intramucosal EGC meets the criteria of expanded indications, additional surgical treatment is recommended if the lesion size alone is a non-curative factor (lesion diameter > 2 cm)[2]. In this case, physicians are concerned about determining the appropriate treatment modality and whether additional treatment should be performed. Various risk factors for LN metastasis have been reported in several surgical reports based on a lesion size of 2 cm[13-16]. Therefore, the role of ESD is limited in patients with UD intramucosal EGC because of the lesion size. However, patients may choose ESD for several reasons such as refusal of surgical treatment or older age. A recent multicenter study reported that mortality was not significantly higher in patients who underwent endoscopic resection for UD intramucosal EGC with tumor size > 2 cm as the only non-curative factor than in those who underwent additional surgery[17].
Compared with surgery, ESD can reduce the period of hospital stay after treatment and improve quality of life. To date, no study has compared long-term outcomes between ESD and surgical treatment based on propensity-score matching in patients with UD intramucosal EGC who are within or beyond expanded indications but satisfy the criteria of curative resection except for lesion size. Thus, this study aimed to evaluate the clinical outcomes and adverse events of ESD compared with those of surgery in patients with UD intramucosal EGC using propensity-score matching analysis. Furthermore, we compared long-term clinical outcomes of ESD and surgery after matching for patients with UD intramucosal EGC who are beyond the expanded indication but meet the criteria of curative resection, except for lesion sizes > 2 cm.
We retrospectively analyzed patients who underwent ESD (n = 212) or surgery (n = 1373) for UD intramucosal EGC at the Ajou University Medical Center (Suwon, Republic of Korea) between January 1, 2005 and August 31, 2020. Among those patients, patients with included expanded indications and curative resection[2] were enrolled. Patients who satisfied the condition of curative resection but had a lesion size > 2 cm (beyond expanded indications, tumor size > 2 cm as the only non-curative factor) were also included. The expanded indications with curative resection for UD intramucosal EGC were described as follows: intramucosal tumor; UD type; without ulceration; en bloc resection; tumor-free lateral and deep resection margin; without lymphovascular (or LN) invasion; and lesion size ≤ 2 cm[2]. Exclusion criteria were as follows: previous history of gastric cancer; previous history of other malignancy; or initial multiple gastric cancers. Additionally, we excluded patients who underwent additional surgery after ESD. The study protocol was approved by Ajou University Hospital Institutional Review Board and Ethics Committee (Approval No. AJIRB-MED-MDB-21-101). The requirement for informed patient consent was waived owing to the retrospective nature of the study. All co-authors had access to study data and reviewed and approved the final manuscript.
All ESD procedures were performed by expert endoscopists using single-channel (GIF-Q260J; Olympus, Tokyo, Japan) or two-channel (GIF-2TQ260M; Olympus) endoscopy. After identifying the lesion, circumferential marking was done 5 mm outside the tumor margin using a needle knife (Dual knife; Olympus) or argon plasma coagulation (Erbe Elektromedizin, Tübingen, Germany). Epinephrine mixed fluid (0.01 mg/mL) was injected into the submucosal layer to lift the lesion from the muscle layer, and dissection was performed using an insulated-tip knife (IT knife; Olympus). The resected specimen was retrieved using a Swirl Net (Olympus), and all samples were fixed in 10% buffered formalin solution and embedded in paraffin.
Patients underwent total or subtotal gastrectomy with LN dissection according to the treatment guidelines of the Japanese Gastric Cancer Association (2). Therefore, patients who were enrolled in the surgery group underwent laparoscopy-assisted or open gastrectomy with D1 or D1+β LN dissection. The surgeons decided on the extent of gastric resection according to the tumor location.
Tumor locations were categorized into upper, middle, or lower third of the stomach based on the longitudinal axis of the stomach. Endoscopic findings were classified into elevated, flat, and depressed according to the predominant type based on the Japanese Research Society for Gastric Cancer classification system[18]. A standard histopathological examination, including hematoxylin and eosin staining, was conducted. Tumor size, presence of ulceration, histologic type, depth of invasion, lymphatic and vascular invasions, and presence of tumor cells in the resection margin were assessed. Pathological diagnoses were made according to the Japanese Classification of Gastric Cancer[1].
Follow-up endoscopy was performed 3 mo after ESD. A subsequent endoscopy with abdominal computed tomography (CT) was performed every 6-12 mo for 2 years and annually thereafter for 5 years after the treatment. In surgically resected patients, follow-up endoscopy and abdominal CT scans were performed every 6 mo for the first 2-3 years and then annually until 5 years after the initial treatment.
The primary outcome of this study was overall survival (OS). The secondary outcomes were recurrence-free survival (RFS) and adverse events of short-term clinical outcomes. In this study, we defined OS as the duration between treatment and death owing to any cause; RFS was defined as the duration between treatment and first recurrence or death with evidence of recurrence. We collected data regarding survival status from the National Cancer Center (Goyang, South Korea); however, cause of death was not obtained for privacy after follow-up loss.
We defined local recurrence as a recurrence at the resection site after ESD or a recurrence at the anastomosis site after surgery. A synchronous lesion was defined as the occurrence of a new lesion detected at a different site from the previous treatment site within 1 year after gastric cancer resection. A metachronous lesion was defined as the occurrence of a new lesion detected at a different site from the previous treatment site more than 1 year after initial treatment. Distant metastasis was defined as a tumor metastasis in another organ.
We performed propensity-score matching analysis using the radius method to balance covariates across groups and reduce selection bias in the observational study. The propensity score was estimated using a logistic regression model with seven matching variables such as age, sex, comorbidities, lesion size, tumor location, gross morphology, histology appearance, and American Society of Anesthesiologists (ASA) physical status classification system score. Based on these propensity scores, the ESD and surgery groups were matched in a 1:1 ratio on an allowable absolute difference between exact propensity scores. The standardized mean differences were computed to measure the balance of covariates between groups before and after propensity-score matching.
We compared demographics and clinical characteristics, clinical outcomes, and adverse events between the ESD and surgery groups using the independent t-test or Wilcoxon rank-sum test for continuous variables and Pearson’s chi-square test or Fisher’s exact test for categorical variables, as appropriate. Survival curves were plotted, and 5-year survival rates with 95% confidence intervals (CIs) were estimated using the Kaplan-Meier method. Differences in OS and RFS were examined using the log-rank test between the ESD and surgery groups and within and beyond the expanded indication and between the ESD and surgery groups among patients beyond the expanded indication separately. The multivariable Cox proportional hazard model with treatment modality and ESD indication was used to estimate the hazard ratio (HR) with 95%CIs to assess the recurrence risk.
All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, United States), and R software, version 3.6.2 (R Project for Statistical Computing), and all P values < 0.05 were two sided and considered statistically significant.
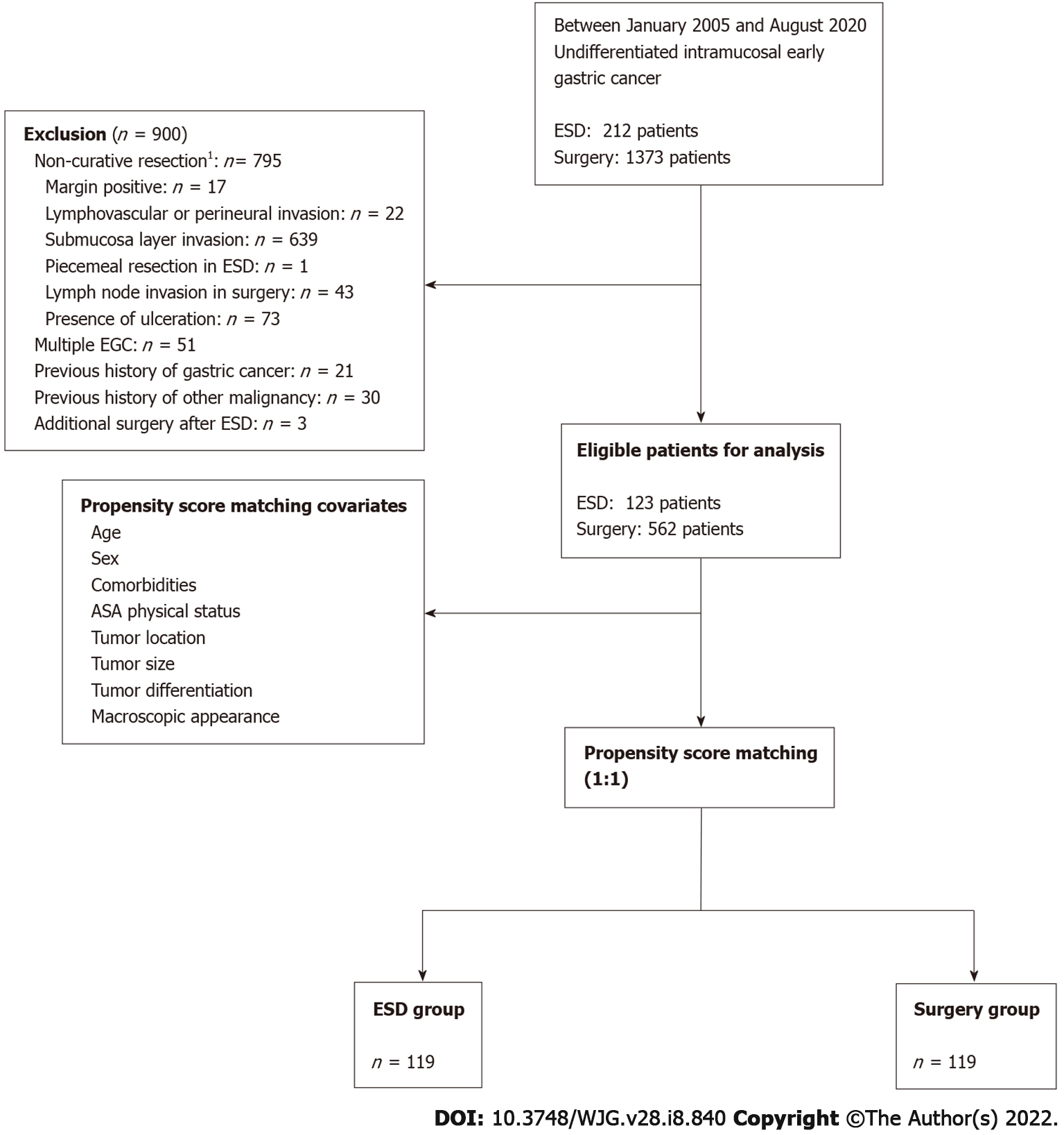
In our center, 212 and 1373 patients with UD intramucosal EGC underwent cancer resection via ESD and surgery, respectively. Patients who failed to meet the expanded ESD indication and curative resection criteria, except lesion size, were excluded. For long-term outcome analysis, we finally enrolled 123 and 562 patients in the ESD and surgery groups, respectively. Before matching, the mean age ± SD of the ESD group was older than that of the surgery group (55.3 ± 12.4 vs 53.0 ± 11.8, P < 0.001). The proportions of male patients were 55.3% and 50.2% in the ESD and surgery groups respectively (P = 0.305). These two groups showed differences with respect to hypertension history, ASA physical status, tumor location, and histology type. In particular, the proportion of patients with signet ring cell carcinoma was higher in the surgery group than in the ESD group (73.1% vs 54.5%, P < 0.001); however, there was no significant difference between the two groups with respect to lesion size and ESD indication. There was also no significant difference between the ESD and surgery groups [43 (35.0%) vs 233 (41.5%), P = 0.183] with respect to the proportion of beyond expanded indications with lesion size > 2 cm (the only non-curative factor). We performed propensity-score matching to compare long-term outcomes of the ESD and surgery groups on a one-to-one basis, and all differences in baseline characteristics after matching were eliminated (Table 1). The flow diagram of enrolled patients is shown in Figure 1, and the distribution of propensity scores is shown in Supplementary Figure 1.
| Variables | Before matching | After matching | ||||
| ESD (n = 123) | Surgery (n = 562) | P value | ESD (n = 119) | Surgery (n = 119) | P value | |
| Age, yr, mean SD | 55.3 12.4 | 53.0 11.8 | < 0.001 | 56.6 11.9 | 55.6 11.9 | 0.546 |
| Male, n (%) | 68 (55.3) | 282 (50.2) | 0.305 | 67 (56.3) | 57 (47.9) | 0.194 |
| Comorbidity, n (%) | ||||||
| Hypertension | 44 (35.8) | 143 (25.4) | 0.020 | 41 (34.5) | 40 (33.6) | 0.891 |
| Diabetes | 21 (17.1) | 65 (11.6) | 0.095 | 19 (16.0) | 15 (12.6) | 0.459 |
| Cerebrovascular disease | 8 (6.5) | 18 (3.2) | 0.113 | 7 (5.9) | 2 (1.7) | 0.171 |
| Respiratory disease | 7 (5.7) | 29 (5.2) | 0.811 | 6 (5.0) | 6 (5.0) | - |
| Liver disease | 3 (2.4) | 26 (4.6) | 0.275 | 3 (2.5) | 3 (2.5) | - |
| Renal disease | 2 (1.6) | 4 (0.7) | 0.294 | 1 (0.8) | 1 (0.8) | - |
| ASA physical status1, n (%) | 0.022 | 0.254 | ||||
| 1 | 101 (82.1) | 503 (89.5) | 100 (84.0) | 106 (89.1) | ||
| 2 | 22 (17.9) | 59 (10.5) | 19 (16.0) | 13 (10.9) | ||
| Tumor location, n (%) | < 0.001 | 0.822 | ||||
| Upper third | 8 (6.5) | 55 (9.8) | 8 (6.7) | 6 (5.0) | ||
| Middle third | 97 (78.9) | 321 (57.1) | 93 (78.2) | 93 (78.2) | ||
| Lower third | 18 (14.6) | 186 (33.1) | 18 (15.1) | 20 (16.8) | ||
| Lesion size, mm, n (%) | 0.430 | 0.418 | ||||
| 10 | 30 (24.4) | 105 (18.7) | 28 (23.5) | 19 (16.0) | ||
| 10-20 | 50 (40.7) | 224 (39.9) | 50 (42.0) | 49 (41.2) | ||
| 20-30 | 24 (19.5) | 133 (23.7) | 22 (18.5) | 28 (23.5) | ||
| > 30 | 19 (15.4) | 100 (17.8) | 19 (16.0) | 23 (19.3) | ||
| Gross morphology type1, n (%) | 0.315 | 0.760 | ||||
| Elevated | 33 (26.8) | 127 (22.6) | 29 (24.4) | 27 (22.7) | ||
| Flat or depressed | 90 (73.2) | 435 (77.4) | 90 (75.6) | 92 (77.3) | ||
| Helicobacter pylori infection, n (%) | 64 (51.2) | 316 (56.2) | 0.397 | 61 (51.3) | 60 (50.4) | 0.897 |
| ESD indication, n (%) | 0.183 | 0.183 | ||||
| Within expanded indication | 80 (65.0) | 329 (58.5) | 78 (65.5) | 68 (57.1) | ||
| Beyond expanded indication | 43 (35.0) | 233 (41.5) | 41 (34.5) | 51 (42.9) | ||
| Histology appearance, n (%) | < 0.001 | 0.794 | ||||
| Poorly differentiated carcinoma | 56 (45.5) | 151 (26.9) | 52 (43.7) | 54 (45.4) | ||
| Signet ring cell carcinoma | 67 (54.5) | 411 (73.1) | 67 (56.3) | 65 (54.6) | ||
The median hospital stay [interquartile range (IQR)] was shorter in the ESD group than in the surgery group [4.0 (4.0-5.0) vs 9.0 (8.0-10.0) d, P < 0.001]. Regarding intensive care unit admission related to treatment complications, treatment complications caused by severe bleeding was noted one patient in the ESD group, who eventually died. Although the incidence of all adverse events was not different between the two groups, five cases (4.2%) of perforations and eight cases (6.7%) of bleeding occurred in the ESD group, all of which were early complications within 30 days. Surgical complications, including anastomotic leakage (n = 1, 0.8%), bowel obstruction (n = 3, 2.5%), and hernia (n = 3, 2.5%), were mostly successfully treated with conservative treatment; however, three hernia cases required additional surgery. Late complications were not observed in the ESD group; however, four cases (3.4%) were observed in the surgery group (Table 2).
| Variables | ESD (n =119) | Surgery (n = 119) | P value |
| Median hospital stay, d (IQR) | 4.0 (4.0-5.0) | 9.0 (8.0-9.0) | < 0.001 |
| ICU admission, n (%) | 1 (0.8) | 0 (0.0) | - |
| 30-d readmission, n (%) | 3 (2.5)1 | 2 (1.7)2 | - |
| Operation-related death, n (%) | 1 (0.8) | 0 (0.0) | - |
| Complication, n (%) | 14 (11.8) | 7 (5.9) | 0.110 |
| Bleeding (early/late) | 8/0 | 0/0 | |
| Perforation (early/late) | 5/0 | N/A | |
| Pneumonia (early/late) | 1/0 | 0/0 | |
| Anastomosis site leakage (early/late) | N/A | 1/0 | |
| Adhesion or bowel obstruction (early/late) | 0/0 | 1/2 | |
| Hernia (early/late) | N/A | 1/2 |
The median follow-up period was 45 mo (IQR, 21-65 mo) and 59 mo (IQR, 36-81 mo) in the ESD and surgery groups, respectively. During the follow-up period, three (2.5%) and five (4.2%) patients died in the ESD and surgery groups, respectively. Among those patients, gastric cancer-related death was identified in one patient (0.8%) in each group. The incidence of total recurrence was higher in the ESD group (n = 7, 5.3%) than in the surgery group (n = 2, 1.7%) (Table 3). Local recurrence was identified in four patients (3.4%) who underwent ESD, and all whom had EGC. A synchronous lesion was identified in one patient (0.8%), who had EGC and was treated with surgery. A metachronous lesion was identified in three patients (2.5%) of the ESD group. Distant metastasis was identified in one patient (0.8%) who underwent surgery with peritoneal metastasis. The number of patients whose tumor size was ≤ 2 cm before ESD or surgery and therefore, satisfied the criteria for expanded-indication lesions but had a size of > 2 cm in the final pathology analysis was 22 (18.5%) and 20 (16.8%) in the ESD and surgery groups, respectively. Of these patients, one patient in the ESD group had a recurrence but no mortality in both groups. Clinical and tumor data for all recurrent patients are given in Table 4.
| Variables | ESD (n = 119) | Surgery (n = 119) | P value |
| Recurrence, n (%) | 7 (5.9) | 2 (1.7) | 0.171 |
| Local recurrence | 4 (3.4) | N/A | |
| Adenoma | 0 (0.0) | N/A | |
| Cancer | 4 (3.4) | N/A | |
| Differentiated | 3 (2.5) | N/A | |
| Undifferentiated | 1 (0.8) | N/A | |
| Synchronous lesion | 0 (0.0) | 1 (0.8) | |
| Adenoma | 0 (0.0) | 0 (0.0) | |
| Cancer | 0 (0.0) | 1 (0.8) | |
| Differentiated | 0 (0.0) | 0 (0.0) | |
| Undifferentiated | 0 (0.0) | 1 (0.8) | |
| Metachronous lesion | 3 (2.5) | 0 (0.0) | |
| Adenoma | 1 (0.8) | 0 (0.0) | |
| Cancer | 2 (1.7) | 0 (0.0) | |
| Differentiated | 1 (0.8) | 0 (0.0) | |
| Undifferentiated | 1 (0.8) | 0 (0.0) | |
| Distant metastasis | 0 (0.0) | 1 (0.8) |
| Age | Sex | Location | Size (mm) | Morphology | Histology | Initial treatment | Recurrence type | Pathology of recurred lesion | Recurrence location | Recurrence time (mo) | Treatment for recurred lesion |
| 61 | F | Middle 1/3 | 6 | Flat | SRC | ESD | Metachronous lesion | Undifferentiated cancer | Lower 1/3 | 70 | ESD |
| 62 | M | Middle 1/3 | 22 | Flat | SRC | ESD | Local recurrence | Undifferentiated cancer | Middle 1/3 | 6 | Surgery |
| 68 | F | Middle 1/3 | 68 | Flat | PDA | ESD | Metachronous lesion | Differentiated cancer | Upper 1/3 | 50 | ESD |
| 46 | M | Middle 1/3 | 60 | Flat | PDA | ESD | Local recurrence | Differentiated cancer | Middle 1/3 | 3 | Surgery |
| 50 | F | Middle 1/3 | 25 | Depressed | PDA | ESD | Local recurrence | Differentiated cancer | Middl1 1/3 | 12 | ESD |
| 62 | M | Middle 1/3 | 40 | Elevated | PDA | ESD | Local recurrence | Differentiated cancer | Middle 1/3 | 6 | Surgery |
| 56 | F | Lower 1/3 | 8 | Flat | PDA | ESD | Metachronous lesion | Adenoma | Middle 1/3 | 23 | ESD |
| 66 | M | Lower 1/3 | 15 | Flat | PDA | Surgery | Synchronous lesion | Undifferentiated cancer | Upper 1/3 | 5 | Surgery |
| 64 | M | Middle 1/3 | 10 | Elevated | SRC | Surgery | Distant metastasis | Undifferentiated cancer | Peritoneum | 122 | Conservative care |
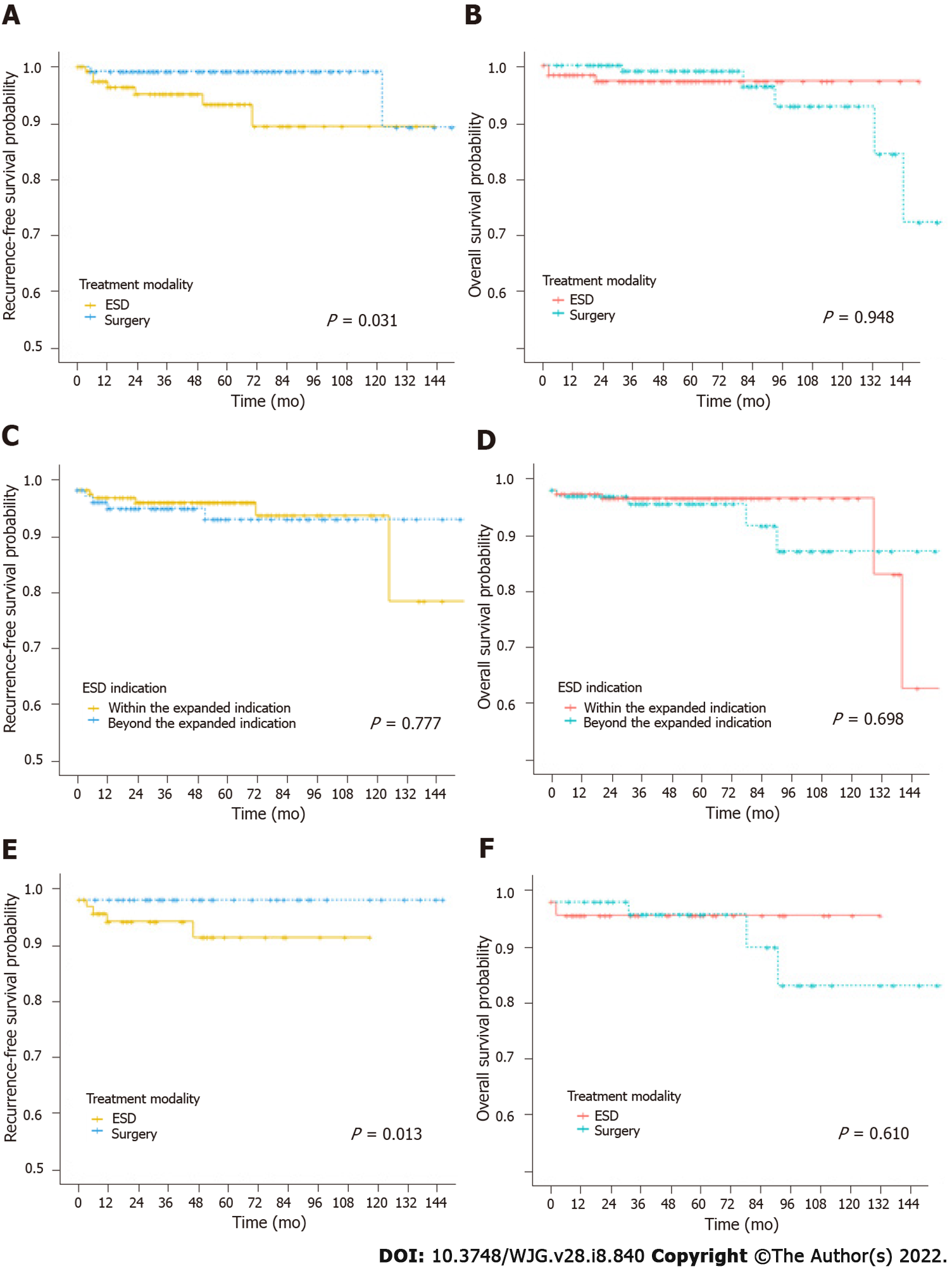
We analyzed RFS and OS using Kaplan-Meier survival plots (Figure 2). Regarding RFS, according to the treatment modality, the ESD group had inferior results compared with the surgery group (P = 0.031) (Figure 2A). The 5-year RFS rates were 93.3% (95%CI: 85.1-97.0) and 99.2% (95%CI: 94.2-99.9) in the ESD and surgery groups, respectively. However, there was no significant difference between the two groups with respect to OS (ESD vs surgery, 5-year OS, 97.2%; 95%CI: 91.6-99.1 vs 99.0%; 95%CI: 93.0-99.9, P = 0.948) (Figure 2B). Among non-curative factors, we analyzed RFS (within vs expanded, 5-year RFS, 97.7%; 95%CI: 93.0-99.3 vs 94.6%; 95%CI: 85.6-98.0, P = 0.777) and OS (within vs expanded, 5-year OS, 98.5%; 95%CI: 94.1-99.6 vs 97.4%; 95%CI: 90.1-99.4, P = 0.698) for patients with lesion size > 2 cm (beyond the expanded indication but meeting the criteria of curative resection except for lesion size > 2 cm) and within expanded indication, in which no difference according to the indication was observed (Figure 2C and D). While there was no difference in OS (ESD vs surgery, 5-year OS, 97.5%; 95%CI: 83.5-99.6 vs 97.7%; 95%CI: 84.9-99.7, P = 0.610) according to the treatment modality in patients with beyond expanded indication with lesion size > 2 cm only, the ESD group had a significantly lower RFS than the surgery group (5-year RFS, 86.2%; 95%CI: 64.9-95.0 vs 100.0%; 95%CI: 100.0-100.0, P = 0.013) (Figure 2E and F). In the multivariable analysis, ESD was a significant risk factor for recurrence after cancer resection in patients with UD intramucosal EGC (HR, 5.2; 95%CI: 1.0-25.8, P = 0.045). The lesion included in the beyond expanded indication was not associated with recurrence risk. Moreover, ESD as a treatment modality increased the HR for recurrence in the beyond expanded indication with lesion size > 2 cm compared with the within expanded indication; however, this result was not statistically significant (Table 5).
| Variables | Adjusted hazard ratio (95% confidence interval) | P value |
| Treatment modality | ||
| Surgery | 1.0 | |
| ESD | 5.2 (1.0-25.8) | 0.045 |
| Indication with any treatment modality | ||
| Within expanded indication | 1.0 | |
| Beyond expanded indication | 1.4 (0.4-5.4) | 0.585 |
| Indication with ESD | ||
| ESD for the lesion within expanded indication | 1.0 | |
| ESD for the lesion beyond expanded indication | 2.8 (0.6-12.4) | 0.183 |
We comparatively analyzed the long-term outcomes of patients who underwent ESD and surgery for UD intramucosal EGC with complete resection regardless of lesion size using propensity score-matched analysis. ESD was similar to surgery in terms of OS; however, RFS in the ESD group was lower than that in the surgery group. In both the groups, LN metastasis was absent during the follow-up period, and only one case of distant metastasis was observed in the surgery group. ESD was advantageous because of shorter hospital stays and fewer late complications compared with surgery. Although complete resection was performed for patients with UD intramucosal EGC, ESD was identified as a recurrence risk factor in terms of treatment modality compared with surgery. In patients with lesion size > 2 cm as the only non-curative factor, there was a difference in RFS depending on the treatment modality, with ESD having an inferior outcome.
In our study, there was a clear bias in the patient population depending on treatment modality. Before matching, patients in the ESD group were older and had a higher ASA physical status score, fewer lesions located in the upper third of the stomach, and lower signet ring cell carcinoma rate than those in the surgery group. While the expanded indication proposed by Gotoda et al[19] is based on the pathology of specimens, clinicians should select the treatment modality for EGC based on gross and pathological findings. However, this method sometimes makes it difficult to determine a treatment modality in clinical practice. Besides, there are cases in which patients beyond the expanded indication of ESD refuse surgery and thus undergo ESD in hopes of endoscopic resection instead of surgery. In contrast, there are a significant number of patients who meet the expanded indications and undergo surgery. As a result, patients and lesion characteristics are not the same between patients who undergo ESD and those who undergo surgery. Our results suggested that patients were hesitant to undergo surgery if they were older or if their ASA physical status score was high. For this reason, selection bias was inevitable in this study group. Therefore, we performed propensity-score matching analysis to reduce selection bias owing to treatment modality.
With the development of ESD techniques and instruments and the accumulation of various clinical outcomes, the scope of ESD has been gradually expanding. However, issues regarding the role of ESD in patients with UD intramucosal EGC are continuously raised. Currently, the ESD criteria for UD intramucosal EGC are strict. ESD is performed only when the lesion has no ulceration and is ≤ 2 cm and when it satisfies the complete resection conditions of negative resection margins and the absence of lymphovascular and perineural invasion in the final pathological examination[2]. Lesion size > 2 cm is a non-curative factor, and various studies have reported a difference in LN metastasis based on lesion size of 2 cm[13,14,15,20]. However, in cases of UD-EGC with a lesion size > 2 cm, LN metastasis was reported to be 0% (0/54) when neither ulceration nor lymphovascular invasion was present[16], all of which were from surgical studies. To our knowledge, only one study analyzed LN metastasis in patients who underwent endoscopic resection for UD intramucosal EGC with lesion size > 2 cm. Yang et al[17] reported an incidence of 1.1% (2/176), which showed no increase in mortality during observation after ESD based on Cox regression analysis. Similar results were also obtained in our study. LN metastasis was not observed among 119 patients who underwent ESD. However, 71.4% (5/7) of the total recurrence cases had a lesion size > 2 cm, which revealed a higher recurrence rate than that in patients with a lesion size ≤ 2 cm (11.9% vs 2.6%). Furthermore, patients with lesion size > 2 cm in the ESD group had a lower RFS than those in the surgery group.
The results of our analysis after matching for UD intramucosal EGC patients with complete resection, irrespective of lesion size, showed that RFS was inferior in the ESD group compared with the surgery group. However, no LN metastasis or distant metastasis was identified during the follow-up period. In other words, all recurrence cases occurred in the stomach, all were treated successfully after recurrence, and no further recurrences occurred during the follow-up period. However, taking ESD into account over surgery as the preferred treatment for UD intramucosal EGC irrespective of lesion size should be carefully considered. LN metastasis was not observed; however, local recurrence was found in four patients (4/119, 3.4%), all of whom had an initial tumor size > 2 cm that occurred less than a year after initial treatment, and surgery as a rescue treatment was performed because endoscopic treatment was impossible. In the study by Yang et al[17], the local recurrence rate was 2.3% (4/176), and one case had LN metastasis. Therefore, ESD must overcome the problem of local recurrence, which does not need to be considered in surgery. Furthermore, we should consider the why local recurrence occurs even after complete resection. The initial pathologic evaluation could have been incorrect or a new cancer may have occurred. We repeated the pathologic evaluation for these cases; however, the initial diagnosis did not change. Additional studies and data accumulation are required to examine why local recurrence occurs within a short period, although complete or curative resection is performed after the initial pathologic evaluation. In this study, in the 212 patients considered, the margin negative resection rate in the ESD group before matching was 92.0% (195/212). This was similar to previous studies where endoscopic resection was performed in UD EGC[21-26].
Therapeutic endoscopists always consider that a sufficient lateral margin can reduce the possibility of local recurrence. While it would be best to secure as much safety margin as possible, the operator should consider the duration of intervention, acute complications (bleeding or perforation), or delayed complications (bleeding, stricture). These considerations may be more prominent in UD EGC. A recently published study mentioned that local recurrence may be related to sequential molecular changes in various cancer-related proteins in histological margin-free endoscopically resected EGCs[27]. In this study, a tumor-free distance of 5.5 mm was considered insufficient as a safety margin. Besides, a subepithelial spread beneath the normal mucosa may exist in UD EGC, especially in signet ring cell cancer, and this subepithelial spread could reach up to 6 mm[28]. These studies suggest that securing sufficient margin in the endoscopic resection of UD EGC using the ESD method might reduce the rate of local recurrence. In the endoscopic resection of UD EGC using the ESD method, the endoscopically predicted and the actual size of the lesion is often different. In the Japanese algorithm, an additional biopsy was recommended from the surrounding mucosa of UD EGC to accurately evaluate the margin of the lesion[29]. In addition, other studies have reported that narrow-band imaging with magnifying endoscopy may help in accurately predicting the tumor extent in UD EGC[30]. Prospective randomized studies are required to evaluate whether the various attempts to accurately determine the tumor margin and resection with sufficient margin can reduce the rate of local recurrence.
In our study, all recurrence cases in the ESD group occurred in the lower or middle third of the stomach, except for one case (6/7, 85.7%). Although it is difficult to judge because of only a small number of recurrence cases, there is a report that incidence increased in the middle and lower third of the stomach in metachronous cancer after ESD[31]. This may be why fewer synchronous or metachronous recurrences were observed in the surgery group. In our study, 16.8% of patients underwent total gastrectomy after matching (20/119). Since the portion of the remnant stomach is small, even in patients who have undergone subtotal gastrectomy, surgery can be advantageous in terms of recurrence. In our study, only one recurrence occurred in the upper third of the stomach in the ESD and surgery groups. It is challenging to select ESD as the initial treatment option if surgical treatment is performed as a rescue treatment in a short period, given that ESD has a probability of local recurrence. Therefore, our results suggest that ESD should not be actively recommended to patients with UD intramucosal EGC with lesion size > 2 cm even without ulceration on preoperative workup.
This study had some limitations. First, this was a retrospective single-center study. A randomized study is required to compare long-term outcomes of ESD and surgery, but this is difficult to perform for UD intramucosal EGC. Second, baseline characteristics and tumor information were different between the groups; however, we analyzed the data after propensity-score matching to minimize the difference between baseline characteristics and reduce selection bias of treatment modality. Third, the number of patients with UD intramucosal EGC with lesion size > 2 cm was significantly lower in the pre-matching ESD group than in the surgery group (43 vs 233 patients). Although the data were corrected as much as possible with propensity-score matching, most ESD patients were assigned after matching; thus, selection bias could exist. Finally, although we confirmed survival based on data from the National Cancer Center registry, we did not check the cause of the death in all patients who died. Therefore, we did not evaluate gastric cancer-related deaths in both groups after follow-up loss.
In conclusion, ESD is a treatment modality for stomach preservation with fewer late complications and shorter hospital stays than surgery. For patients with UD intramucosal EGC, if the lesion size is the only non-curative factor, ESD may be an alternative treatment option when surgery is not possible. However, all cases of local recurrence were identified within 1 year in our study, all of which were cancer, although LN metastasis was not observed after ESD. Therefore, even for complete resection, endoscopic surveillance is essential. Especially in cases with lesion sizes > 2 cm, endoscopic surveillance should be more thoroughly performed.
Endoscopic submucosal dissection (ESD) is performed as an alternative treatment modality for undifferentiated (UD) intramucosal early gastric cancer (EGC) who are within the expanded indication. However, the ESD role for UD intramucosal EGC with lesion size > 2 cm (the only non-curative factor) is still controversial compared with surgery.
Several studies showed ESD could be performed for patients with UD intramucosal EGC within the expanded indication. However, the role of ESD is limited in these patients because of the lesion size. Even if UD intramucosal EGC meets the criteria of expanded indications, additional surgical treatment is recommended if the lesion size alone is a non-curative factor (lesion diameter > 2 cm).
In this study, the authors compared ESD with surgery in patients with UD intramucosal EGC who meet both the within expanded indications or beyond expanded indications with lesion size > 2 cm (the only non-curative factor).
The authors retrospectively analyzed patients with UD intramucosal EGC after ESD with complete resection or surgery. After propensity-score matching, clinical outcomes and long-term outcomes, i.e., recurrence-free survival (RFS) and overall survival (OS), were analyzed.
After propensity-scored matching, although ESD with complete resection was performed in UD intramucosal EGC regardless of lesion size, RFS increased, while there was no difference in OS compared to surgery. Especially, all cases of local recurrence were identified within 1 year in our study in the ESD group.
Although ESD may be an alternative treatment option when surgery is not possible for UD intramucosal EGC with lesion sizes > 2 cm, endoscopic surveillance should be carefully performed within one year for local recurrence.
Multicenter randomized studies with large cohorts are expected to evaluate ESD in patients with UD intramucosal EGC regardless of tumor size.
We would like to thank Cho W, Medical Information & Media Center, Ajou University School of Medicine for providing editing services for images and illustrations.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Korean Society of Gastroenterology, No. 1193012.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kinami S S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Onozato Y, Ishihara H, Iizuka H, Sohara N, Kakizaki S, Okamura S, Mori M. Endoscopic submucosal dissection for early gastric cancers and large flat adenomas. Endoscopy. 2006;38:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 2. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1338] [Article Influence: 334.5] [Reference Citation Analysis (2)] |
| 3. | Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 520] [Article Influence: 32.5] [Reference Citation Analysis (1)] |
| 4. | Liu Q, Ding L, Qiu X, Meng F. Updated evaluation of endoscopic submucosal dissection versus surgery for early gastric cancer: A systematic review and meta-analysis. Int J Surg. 2020;73:28-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 5. | Nishizawa T, Yahagi N. Long-Term Outcomes of Using Endoscopic Submucosal Dissection to Treat Early Gastric Cancer. Gut Liver. 2018;12:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 6. | Ahn JY, Jung HY. Long-term outcome of extended endoscopic submucosal dissection for early gastric cancer with differentiated histology. Clin Endosc. 2013;46:463-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Park CH, Shin S, Park JC, Shin SK, Lee SK, Lee YC, Lee H. Long-term outcome of early gastric cancer after endoscopic submucosal dissection: expanded indication is comparable to absolute indication. Dig Liver Dis. 2013;45:651-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Peng LJ, Tian SN, Lu L, Chen H, Ouyang YY, Wu YJ. Outcome of endoscopic submucosal dissection for early gastric cancer of conventional and expanded indications: systematic review and meta-analysis. J Dig Dis. 2015;16:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Park JC, Lee YK, Kim SY, Roh Y, Hahn KY, Shin SK, Lee SK, Lee YC, Kim HI, Cheong JH, Hyung WJ, Noh SH. Long-term outcomes of endoscopic submucosal dissection in comparison to surgery in undifferentiated-type intramucosal gastric cancer using propensity score analysis. Surg Endosc. 2018;32:2046-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Huh CW, Ma DW, Kim BW, Kim JS, Lee SJ. Endoscopic Submucosal Dissection versus Surgery for Undifferentiated-Type Early Gastric Cancer: A Systematic Review and Meta-Analysis. Clin Endosc. 2021;54:202-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Guo A, Du C, Tian S, Sun L, Guo M, Lu L, Peng L. Long-term outcomes of endoscopic submucosal dissection versus surgery for treating early gastric cancer of undifferentiated-type. Medicine (Baltimore). 2020;99:e20501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Nakamura R, Omori T, Mayanagi S, Irino T, Wada N, Kawakubo H, Kameyama K, Kitagawa Y. Risk of lymph node metastasis in undifferentiated-type mucosal gastric carcinoma. World J Surg Oncol. 2019;17:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Kunisaki C, Takahashi M, Nagahori Y, Fukushima T, Makino H, Takagawa R, Kosaka T, Ono HA, Akiyama H, Moriwaki Y, Nakano A. Risk factors for lymph node metastasis in histologically poorly differentiated type early gastric cancer. Endoscopy. 2009;41:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Chung JW, Jung HY, Choi KD, Song HJ, Lee GH, Jang SJ, Park YS, Yook JH, Oh ST, Kim BS, Kim JH. Extended indication of endoscopic resection for mucosal early gastric cancer: analysis of a single center experience. J Gastroenterol Hepatol. 2011;26:884-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Lee JH, Choi MG, Min BH, Noh JH, Sohn TS, Bae JM, Kim S. Predictive factors for lymph node metastasis in patients with poorly differentiated early gastric cancer. Br J Surg. 2012;99:1688-1692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Horiuchi Y, Ida S, Yamamoto N, Nunobe S, Ishizuka N, Yoshimizu S, Ishiyama A, Yoshio T, Hirasawa T, Tsuchida T, Kumagai K, Ohashi M, Sano T, Fujisaki J. Feasibility of further expansion of the indications for endoscopic submucosal dissection in undifferentiated-type early gastric cancer. Gastric Cancer. 2020;23:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Yang HJ, Nam SY, Min BH, Ahn JY, Jang JY, Kim J, Kim JH, Lee WS, Lee BE, Joo MK, Park JM, Shin WG, Lee HL, Gweon TG, Park MI, Choi J, Tae CH, Kim YI, Choi IJ. Clinical outcomes of endoscopic resection for undifferentiated intramucosal early gastric cancer larger than 2 cm. Gastric Cancer. 2021;24:435-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Kato M, Nishida T, Yamamoto K, Hayashi S, Kitamura S, Yabuta T, Yoshio T, Nakamura T, Komori M, Kawai N, Nishihara A, Nakanishi F, Nakahara M, Ogiyama H, Kinoshita K, Yamada T, Iijima H, Tsujii M, Takehara T. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut. 2013;62:1425-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 19. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 507] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 20. | Lee IS, Lee S, Park YS, Gong CS, Yook JH, Kim BS. Applicability of endoscopic submucosal dissection for undifferentiated early gastric cancer: Mixed histology of poorly differentiated adenocarcinoma and signet ring cell carcinoma is a worse predictive factor of nodal metastasis. Surg Oncol. 2017;26:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Yamamoto Y, Fujisaki J, Hirasawa T, Ishiyama A, Yoshimoto K, Ueki N, Chino A, Tsuchida T, Hoshino E, Hiki N, Fukunaga T, Sano T, Yamaguchi T, Takahashi H, Miyata S, Yamamoto N, Kato Y, Igarashi M. Therapeutic outcomes of endoscopic submucosal dissection of undifferentiated-type intramucosal gastric cancer without ulceration and preoperatively diagnosed as 20 millimetres or less in diameter. Dig Endosc. 2010;22:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Odagaki T, Taniguchi H, Kushima R, Saito Y. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy. 2013;45:703-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 23. | Oka S, Tanaka S, Higashiyama M, Numata N, Sanomura Y, Yoshida S, Arihiro K, Chayama K. Clinical validity of the expanded criteria for endoscopic resection of undifferentiated-type early gastric cancer based on long-term outcomes. Surg Endosc. 2014;28:639-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Jeon HK, Lee SJ, Kim GH, Park DY, Lee BE, Song GA. Endoscopic submucosal dissection for undifferentiated-type early gastric cancer: short- and long-term outcomes. Surg Endosc. 2018;32:1963-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Inokuchi Y, Kobayashi M, Kudo K, Yamada H, Inoue S, Nishimura K, Nakayama N, Motohashi O. Outcomes and precautions of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Therap Adv Gastroenterol. 2015;8:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Choi MH, Hong SJ, Han JP, Song JY, Kim DY, Seo SW, Ha JS, Lee YN, Ko BM, Lee MS. [Therapeutic outcomes of endoscopic submucosal dissection in undifferentiated-type early gastric cancer]. Korean J Gastroenterol. 2013;61:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Kang HS, Kwon MJ, Haynes P, Liang Y, Ren Y, Lim H, Soh JS, Kim NY, Lee HK. Molecular risk markers related to local tumor recurrence at histological margin-free endoscopically resected early gastric cancers: A pilot study. Pathol Res Pract. 2021;222:153434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Lee YM, Kang SH, Kim JS, Eun HS, Joo JS, Rou WS, Park JH, Moon HS, Lee ES, Kim SH, Sung JK, Lee BS, Jeong HY, Yeo MK, Song KS, Yoo HM. Subepithelial Spread of Early Gastric Signet Ring Cell Carcinoma: How Far They Can Reach? Dig Dis. 2020;38:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Yao K, Nagahama T, Matsui T, Iwashita A. Detection and characterization of early gastric cancer for curative endoscopic submucosal dissection. Dig Endosc. 2013;25 Suppl 1:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 30. | Okada K, Fujisaki J, Kasuga A, Omae M, Hirasawa T, Ishiyama A, Inamori M, Chino A, Yamamoto Y, Tsuchida T, Nakajima A, Hoshino E, Igarashi M. Diagnosis of undifferentiated type early gastric cancers by magnification endoscopy with narrow-band imaging. J Gastroenterol Hepatol. 2011;26:1262-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M; Japan Gast Study Group. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 935] [Article Influence: 55.0] [Reference Citation Analysis (0)] |