Published online Feb 21, 2022. doi: 10.3748/wjg.v28.i7.755
Peer-review started: September 30, 2021
First decision: December 4, 2021
Revised: December 13, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: February 21, 2022
Processing time: 139 Days and 20.8 Hours
Extra-intestinal manifestations in inflammatory bowel diseases (IBD) are frequent and involve virtually all organs. Conversely, the clinical characteristics and course of inflammatory myopathies in IBD remain poorly described and mostly related to orbital myositis. Moreover, alternative therapeutic strategies in non-responder patients to corticosteroid therapy must still be clarified.
A 33-year-old woman with a history of unclassified colitis presented with acute bilateral calf pain. On admission, her clinical and biological examinations were non-specific. However, magnetic resonance imaging showed bilateral inflammatory changes in gastrocnemius muscles suggestive of myositis. Muscle biopsy confirmed the diagnosis of myositis and demonstrated an inflammatory infiltrate mainly located in the perimysial compartment including lympho-plasmocytic cells with the formation of several granulomatous structures while the endomysium was relatively spared. The combined clinical, biological and histomyopathological findings were concordant with the diagnosis of ‘gastrocnemius myalgia syndrome’ (GMS), a rare disorder associated with Crohn’s disease (CD). Ileocolonoscopy confirmed CD diagnosis and systemic corticosteroids (CS) therapy was started, resulting in a rapid clinical improvement. During CS tapering, however, she experienced a relapse of GMS together with a severe active ileocolitis. Infliximab was started and allowed a sustained remission of both conditions at the latest follow-up (20 mo).
The GMS represent a rare CD-associated inflammatory myopathy for which anti-tumour necrosis factor-α therapy might be considered as an effective therapeutic option.
Core Tip: Inflammatory myopathies are scarce in the setting of inflammatory bowel diseases (IBD) and could be wrongly attributed to IBD-related osteoarticular manifestations or to medications’ side effects. This case describes a very atypical presentation of myositis restricted to the legs called the ‘gastro
- Citation: Catherine J, Kadhim H, Lambot F, Liefferinckx C, Meurant V, Otero Sanchez L. Crohn’s disease-related ‘gastrocnemius myalgia syndrome’ successfully treated with infliximab: A case report. World J Gastroenterol 2022; 28(7): 755-762
- URL: https://www.wjgnet.com/1007-9327/full/v28/i7/755.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i7.755
The multi-system nature of inflammatory bowel diseases (IBD) is widely known. The extraintestinal manifestations (EIMs) of IBD are diverse and deferred from IBD diagnosis in a quarter of the cases. It has been estimated that 6% to 47% of patients with IBD experience at least one EIM[1]. The most frequently reported EIMs include peripheral and/or axial arthropathy, cutaneous lesions, ophthalmological immune-inflammatory manifestations and hepatobiliary affections[2]. However, EIMs can involve almost any organ or tissue, and some rare manifestations, such as specific muscular involvement can also occur and might even herald an hidden IBD[3].
The occurrence of muscular involvement in IBD is scarce, and mostly related to orbital myositis, a subtype of orbital pseudotumor principally reported during Crohn’s disease (CD)[4,5]. Myositis affecting the limbs is a much rarer entity, and is mostly localized in the lower extremities, involving the gastrocnemius muscles, a clinical entity designated as “gastrocnemius myalgia syndrome” (GMS)[3]. To date, very little is known about the full spectrum of myositis occurring during the course of IBD.
We hereby report the first CD-associated GMS in a Belgian patient that turned out to be “granulomatous myositis”, an exceptionally rare subtype. In addition, we performed a literature review by searching EMBASE, MEDLINE, Scopus, and the Cochrane Library databases for studies published between January 1, 1970, and July 7, 2021, using the following keywords or MeSH terms: ‘Inflammatory Bowel Diseases’, ‘Crohn’s Disease’, ‘Ulcerative colitis’, ‘Myositis’, ‘Gastrocnemius myalgia syndrome’ and ‘Granulomatous Myositis’. Finally, we discuss the role of anti-tumour necrosis factor (TNF)-α therapy in this specific context.
A 33-year-old woman presented at the emergency department with a 5-d history of isolated tenderness in both calves leading to walking difficulties.
She denied any traumatism but reported a colitis relapse 30 d before her admission which resolved with a short-course of modified-release beclomethasone therapy.
The patient had a history of allergic asthma, Heliobacter pylori gastritis and unclassified colitis. Previous investigations performed during colitis flares did not discriminate a specific IBD pattern concluding in an unclassified colitis. The patient was not taking any chronic medication. Her familial history was not contributive.
Clinical findings on admission included bilateral swelling of both calves which were warm and painful to palpation. Motricity and sensitive perception were preserved. Examination of other muscles, joints and the spine was unremarkable and her abdomen was soft and non-tender.
Initial laboratory investigations showed a C-reactive protein level at 106.6 mg/L (normal range, 0.4-12 mg/L) with mild neutrophilic leukocytosis (8770/mm3, normal range 1900-8000/mm3). Creatinine kinase (CK), aspartate aminotransferase, lactate dehydrogenase and D-dimer serum levels were within reference values. Hemocultures were negative as well as antinuclear, antineutrophil cytoplasmic and anti-saccharomyces cerevisiae antibodies.
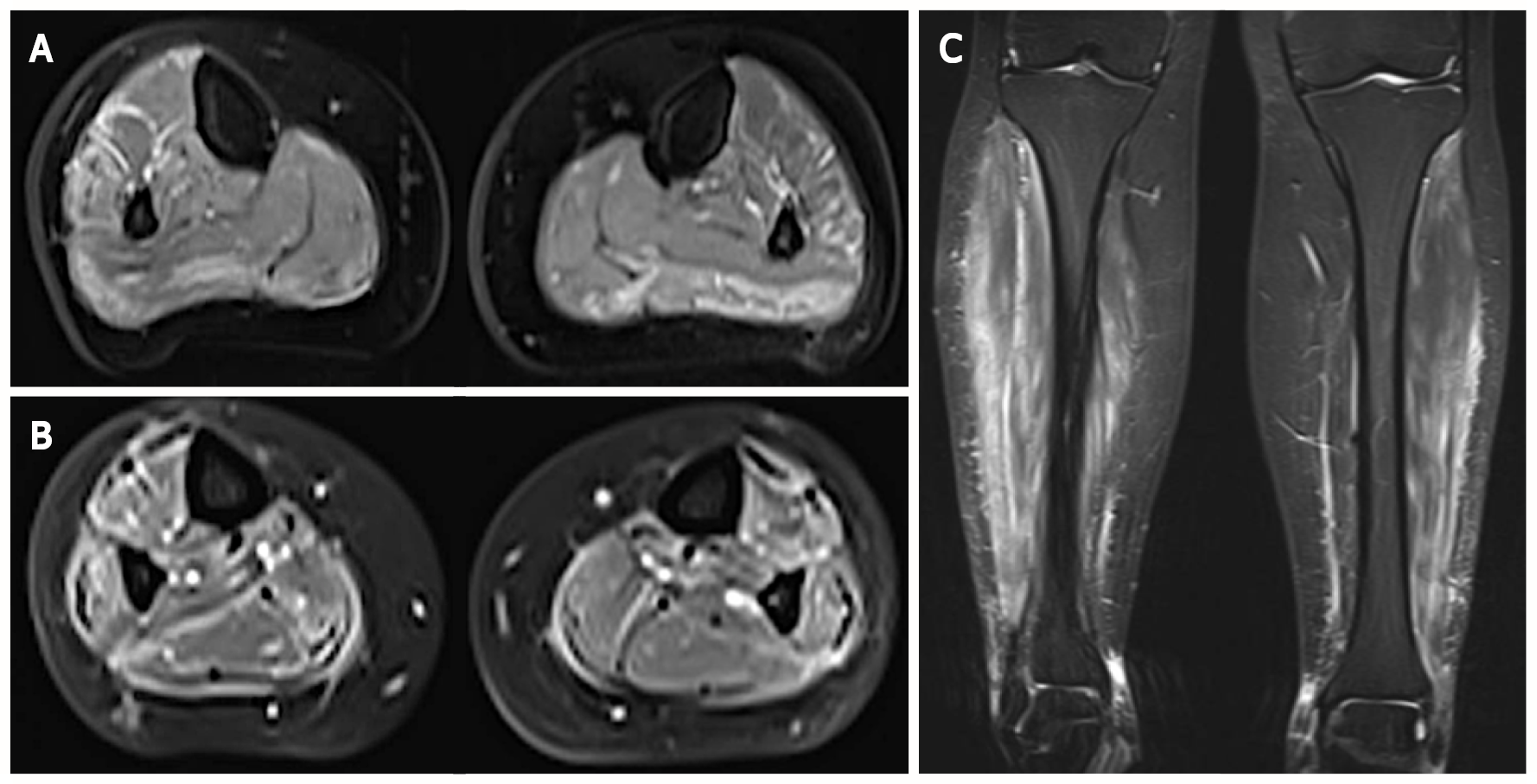
Ultrasonography of lower limbs revealed a bilateral 5-millimeter-thick edema surrounding gastrocnemius muscles in the absence of vascular abnormality while a magnetic resonance imaging showed marked inflammation of muscles and their fascia in both legs, suggested by a high signal on T2-weighted images and a strong gadolinium enhancement on fat-suppressed T1-weighted images (Figure 1).
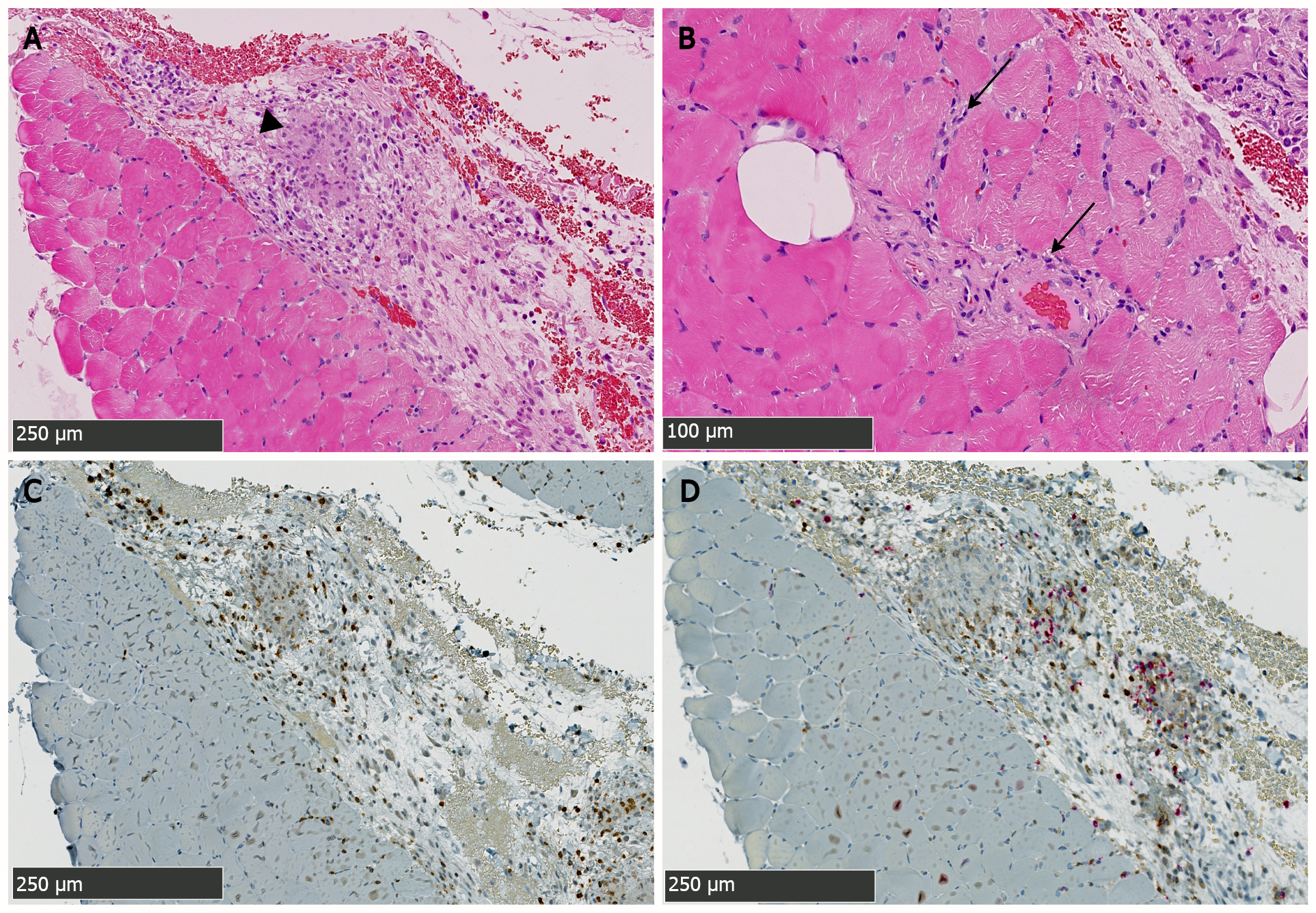
A muscular biopsy of the right gastrocnemius revealed a remarkable inflammatory reaction that particularly involved the perimysial compartment. The infiltrate mainly comprised dense lympho-plasmocytic cells with the formation of several granulomatous structures wherein several histiocytes, sometimes multinucleated, were observed (Figure 2A, C and D). Endomysial inflammatory infiltrate was however less remarkable and rather discrete and focal. There was besides a very mild and discrete perivascular affinity for the inflammatory infiltrate but there was no frank/convincing vasculitis in the examined sections (Figure 2B). A few muscle fibers showed suspected myofibrillary disintegration. Immunohistochemical analyses showed an outstanding cohort of CD3 positive cells (with a slight predominance of CD8+ over CD4+ cells) (Figure 2C and D). Electron microscopy was generally unremarkable and showed rare lipofuscin deposits. Moreover, rare mastocytes were observed.
This histomyopathological affection in this clinical context was concordant with the GMS, a rare disorder classically associated with CD[6]. Moreover, a new ileocolonoscopy was performed during the hospital stay and revealed endoscopic pattern in favor of CD diagnosis, despite the absence of intestinal granuloma on histopathologic examination.
Considering these results, the patient was started on a prednisone equivalent dose of 1 mg/kg, resulting in a rapid clinical and biochemical remission.
Two months later, the patient experienced intestinal symptoms associated with a recurrence of pain in both legs during corticosteroids (CS) tapering. The ileocolonoscopy performed at that time showed a very severe ileocolitis [CD Endoscopic Index of Severity (CDEIS) scored at 31] with no histopathologic evidence of granuloma for which intravenous methylprednisolone (1 mg/kg for 5 d) and infliximab (5 mg/kg at week 0, 1 and 4) therapies were started to target both muscular and intestinal disease leading to clinical remission of both ileocolitis- and myositis-related symptoms. Oral corticotherapy was then prescribed and slowly tapered while infliximab was continued (5 mg/kg, every 8 wk). Twenty months later, both ileocolitis and myositis were quiescent.
Inflammatory myopathies (IM) occurring in the setting of IBD are considered as rare EIMs and have been roughly described in the literature to date[6]. We report the first CD-associated GMS in a Belgian patient that besides turned out to be “granulomatous”. This represents a very exceptional subtype as there has been only two such reported cases. To the best of our knowledge, only 15 cases of GMS are described in the international literature and always occurred in patients with CD (Table 1). Apart from GMS, orbital myositis, which is currently considered as a subtype of idiopathic orbital inflammation, has also been reported in patients with IBD, mainly CD[5]. Few cases of dermatomyositis (DM) and polymyositis have also been described during the course of IBDs and a recent study showed that DM was more frequent in ulcerative colitis patients than CD patients and control subjects[7]. Nevertheless, the true incidence of IM occurring in this context is probably underestimated as myalgia can be confused with joint manifestations or with non-inflammatory muscle disorders (e.g., secondary to hypokalemia or medications) and by the fact that clinicians may rule out myositis’ diagnosis in the absence of CK elevation in serum.
| Ref. | Age (yr)/sex at GMS onset | Initial presentation, time-to-onset | Active CD at time of GMS dx | CK serum level | Biopsy | Treatment | Corticosteroids-dependence (d) or resistance (r) |
| Ménard et al[8], 1976 | 44/M | Muscular, 2 mo | No | N | Granulomatous myositis | PDS1 (80 mg/d) | No |
| Gilliam et al[13], 1981 | 19/M | Digestive, 6 mo | Yes | N | Necrotizing vasculitis | PDS1 (60 mg/d) | No |
| Hall et al[10], 1985 | 32/F | Muscular, 120 mo | Yes | N | Non-granulomatous myositis | 5-ASA1, PDS1 (25 mg/d) | No |
| Drabble and Gani[14], 1992 | 50/M | Digestive, 168 mo | Yes | N | Not performed | Hydrocortisone1 (400 mg/d), PDS1 | No |
| Disdier et al[11], 1997, Case 1 | 26/F | Muscular, 48 mo | Yes | N | Necrotizing vasculitis | PDS1 (60 mg/d), CYC1, 5-ASA1 | Yes (r) |
| Disdier et al[11], 1997, Case 2 | 21/F | Simultaneous | Yes | N | Vasculitis | PDS (1 mg/kg/d), AZA1 | Yes (d) |
| Christopoulos et al[3], 2003 | 19/F | Simultaneous | Yes | N | Granulomatous myositis | PDS1 (0.5 mg/kg/d) | No |
| Ullrich et al[15], 2009 | 25/F | Digestive, 84 mo | Yes | N | Vasculitis | PDS (50 mg/d), AZA, IFX1 | Yes (d) |
| Co et al[16], 2010 | 15/F | Digestive, 8 mo | Yes | N | Not performed | IFX1 | / |
| Mogul et al[17], 2010 | 15/M | Digestive, 60 mo | No | N | Non-granulomatous myositis | PDS1 (40 mg/d), MTX1 | No |
| Piette et al[18], 2010 | 45/M | Simultaneous | Yes | (3-13 × N) | Vasculitis | PDS1 (1 mg/kg/d) | No |
| Goldshmid et al[19], 2011 | 24/F | Simultaneous | Yes | (330 U/L) | Not performed | MPDS (100 mg/d), IFX1, AZA1 | Yes (d) |
| Vadala di Prampero et al[20], 2016 | 26/M | Digestive, 72 mo | Yes | N | Non-granulomatous myositis | PDS (60 mg/d), AZA1 (chronic), Adalimumab1 | Yes (d) |
| Saffar[12], 2017 | 33/F | Digestive, 120 mo | Yes | N | Not performed | PDS, IFX1 | Yes (r) |
| Osada et al[9], 2018 | 38/M | Digestive, 3 mo | Yes | N | Non-granulomatous myositis | PDS, AZA1, 5-ASA1 | Yes (d) |
| Current case | 33/F | Simultaneous | Yes | N | Granulomatous myositis | MDPS (0.8/kg/d), IFX1 | Yes (d) |
The first case of myositis affecting gastrocnemius muscles in CD was reported in 1979 by Ménard et al[8] who described a 44-year-old man with granulomatous myositis localized to the calf occurring two months before CD diagnosis[8]. Since then, several cases have been reported, sharing the classically following features: (1) Calf-limited myalgia revealing localized myositis; (2) Normal serum CK levels; and (3) A high early-response rate to CS therapy[9]. In 2003, this entity was denominated as “GMS” by Christopoulos et al[3], a term adopted in the literature ever since[10]. Most patients developed GMS months or years after the onset of CD but myositis could precede gastrointestinal manifestations by up to 10 years[11]. When CD had been diagnosed before GMS, the intestinal disease was active in most cases at myositis’ diagnosis (Table 1). Other EIMs were associated with GMS in 50% of patients which is in accordance with previous observations that patients who presented an EIM are at higher risk to develop another one[1].
While clinical and biological features in patients with GMS are generally characteristics, histomyopathological findings are rather heterogeneous. In fact, our case is only the third in which granulomatous lesions were observed while all remaining reported cases were characterized by non-granulomatous inflammation (4 cases) or localized vasculitis (5 cases) (Table 1). However, whatever the histopathologic image observed, the inflammatory infiltrate was more often localized in the perimysium and more discrete in the endomysium, probably explaining why most patients with GMS have normal CK values. GMS could therefore represent a form of localized “perimyositis” with no or limited myofiber injury. This observation also suggests that the immune response is directed against connective tissue components or vessels rather than against the myofibers themselves.
Finally, while GMS was initially controlled in this case by oral CS therapy, it ultimately relapsed during tapering. In this context, the presence of a concomitant severe active ileocolitis prompted us to start an anti-TNF-α agent. While CS were the first-line treatment prescribed in almost all cases of GMS, their efficacy, however, was not enduring as 6/16 patients (including ours) relapsed during dose de-escalation (Table 1). Moreover, two other patients were initially refractory to CS[12,13]. Various drugs were used in these corticosteroid-dependent or refractory cases. Anti-TNF-α agents were constantly associated with GMS remission in the six cases where they were introduced, suggesting that the muscular and intestinal affections in this context could share a common pathophysiological mechanism. Importantly, our case represents the first GMS with granulomatous inflammation treated with an anti-TNF-α agent. Further case descriptions and/or case series will be required to ascertain the role of such therapy in this specific context.
In conclusion, we report a rare extra-intestinal manifestation of CD, namely a GMS, characterized by a very rarely reported granulomatous perimyositis. The rapid relapse of GMS during CS tapering and the emergence of a severe active ileocolitis prompted the introduction of an anti-TNF-α agent that resulted in persistent clinical remission of both disorders. This might further suggest the effectiveness of such agents in CS-dependent or refractory cases. Finally, physicians should remain aware of the eventual emergence of a such complicating myositis when musculo-skeletal symptoms arise in IBD patients even in the absence of CK elevation.
We thank Anaïs Boisson (Laboratory of Molecular Immunology, Jules Bordet Institute, Brussels, 1000, Belgium) who performed immunohistochemistry stainings.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Belgium
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dhali A, Homan M S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm Bowel Dis. 2015;21:1982-1992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 472] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 2. | Ott C, Schölmerich J. Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol. 2013;10:585-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 242] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 3. | Christopoulos C, Savva S, Pylarinou S, Diakakis A, Papavassiliou E, Economopoulos P. Localised gastrocnemius myositis in Crohn's disease. Clin Rheumatol. 2003;22:143-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Bourikas LA, Roussomoustakaki M, Papadaki E, Valatas V, Koutroubakis IE, Papadakis KA, Kouroumalis EA. A case of orbital myositis preceding the intestinal symptoms of Crohn's disease. J Crohns Colitis. 2010;4:349-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Culver EL, Salmon JF, Frith P, Travis SP. Recurrent posterior scleritis and orbital myositis as extra-intestinal manifestations of Crohn's disease: Case report and systematic literature review. J Crohns Colitis. 2008;2:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Bourikas LA, Papadakis KA. Musculoskeletal manifestations of inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1915-1924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Tseng CC, Chang SJ, Liao WT, Chan YT, Tsai WC, Ou TT, Wu CC, Sung WY, Hsieh MC, Yen JH. Increased Cumulative Incidence of Dermatomyositis in Ulcerative Colitis: a Nationwide Cohort Study. Sci Rep. 2016;6:28175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Ménard DB, Haddad H, Blain JG, Beaudry R, Devroede G, Massé S. Granulomatous myositis and myopathy associated with crohn's colitis. N Engl J Med. 1976;295:818-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 61] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Osada A, Yamada H, Takehara S, Tozuka Y, Fukushima T, Oka H, Okazaki H, Nagaoka S. Gastrocnemius Myalgia as a Rare Initial Manifestation of Crohn's Disease. Intern Med. 2018;57:2001-2006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Hall MJ, Thomas WE, Cooper BT. Gastrocnemius myositis in a patient with inflammatory bowel disease. Digestion. 1985;32:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Disdier P, Swiader L, Harlé JR, Pellissier JF, Figarella-Branger D, Veit V, Gérolami A, Arlet Ph, Weiller PJ. Crohn's disease and gastrocnemius vasculitis: two new cases. Am J Gastroenterol. 1997;92:880-882. [PubMed] |
| 12. | Saffar H. An unusual case of gastrocnemius muscle syndrome in a patient with Crohn’s Disease. (e-pub ahead of print 2017; DOI: 10.1594/EURORAD/CASE.14254). [cited 10 August 2021]. Available from: https://journals.lww.com/pec-online/toc/9000/00000. |
| 13. | Gilliam JH 3rd, Challa VR, Agudelo CA, Albertson DA, Huntley CC. Vasculitis involving muscle associated with Crohn's colitis. Gastroenterology. 1981;81:787-790. [PubMed] |
| 14. | Drabble EM, Gani JS. Acute gastrocnemius myositis. Another extraintestinal manifestation of Crohn's disease. Med J Aust. 1992;157:318-320. [PubMed] |
| 15. | Ullrich S, Schinke S, Both M, Knop KC, Kirkiles-Smith NC, Gross WL, Lamprecht P. Refractory central nervous system vasculitis and gastrocnemius myalgia syndrome in Crohn's disease successfully treated with anti-tumor necrosis factor-alpha antibody. Semin Arthritis Rheum. 2009;38:337-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Co M, Batke M, Truding R. “Gastrocnemius Myalgia Syndrome” - First Description in a Pediatric Patient with Crohn’s Disease. Case Report And Literature Review: 970. Off J Am Coll Gastroenterol ACG. 2010;105:S351. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 17. | Mogul Z, Katz S, Bachman TR, Urmacher C. Isolated gastrocnemius myositis related to Crohn's disease. Gastroenterol Hepatol (N Y). 2010;6:453-455. [PubMed] |
| 18. | Piette AM, Beaudouin C, Charles P, Ackermann F, François D, Leport J, Guth A, Blétry O, Kahn JE, Geffray L. Sale temps pour une grenouille. Rev Médecine Interne. 2010;31:570-574. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Goldshmid O, Dovorish Z, Zehavi T, Eisen A, Bar-Dayan Y, Amital H. Coexistent pyoderma gangrenosum and tibialis anterior myositis as presenting manifestations of Crohn's disease: case report and review of the literature. Rheumatol Int. 2011;31:525-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Vadala di Prampero S, Marino M, Toso F, Avellini C, Nguyen V, Sorrentino D. Isolated Bilateral Gastrocnemius Myositis in Crohn Disease Successfully Treated with Adalimumab. Case Rep Gastroenterol. 2016;10:661-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |