Published online Feb 21, 2022. doi: 10.3748/wjg.v28.i7.732
Peer-review started: September 7, 2021
First decision: November 8, 2021
Revised: November 18, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: February 21, 2022
Processing time: 163 Days and 0.9 Hours
Bifidobacterium longum 35624 has shown efficacy in improving irritable bowel syndrome (IBS) symptoms compared with placebo in double-blind randomized studies. However, few data are available from real-life clinical practice or from studies that used Rome IV criteria to diagnose IBS.
To assess the effect of B. longum 35624 on IBS severity and quality of life in a real-life setting.
From November 2018 to January 2020, 278 patients with IBS (according to Rome IV criteria) were enrolled in a prospective, open-label, multicenter observational study by private practice gastroenterologists to received one capsule of B. longum 35624 (109 colony-forming units) per day for 30 d. Participation in the study was independently proposed to patients during spontaneous consultations. Disease severity (assessed by the IBS severity scoring system) and patient quality of life (assessed by the IBS quality of life questionnaire) were compared between the inclusion visit (baseline) and the visit at the end of 30 d of treatment. The characteristics of patients were described at baseline. Continuous variables comparisons between inclusion and end-of-treatment visits were performed using the t-test and Kruskal-Wallis test. Categorical variables comparisons were performed using the χ2 test.
A total of 233 patients, with a mean age of 51.4 years and composed of 71.2% women, were included in the study. Of these patients, 48.1% had moderate IBS and 46.4% had severe IBS. After a 30-d treatment period with one B. longum 35624 capsule per day, a significant decrease in IBS severity was observed compared to baseline (mean ± SD, IBS severity scoring system scores: 208 ± 104 vs 303 ± 81, P < 0.001) and 57% of patients moved to lower severity categories or achieved remission. The quality of life of patients was also improved by the treatment (IBS Quality of Life questionnaire score: 68.8 ± 20.9 vs 60.2 ± 20.5; P < 0.001) and 63.8% of patients were satisfied with the treatment.
Thirty days of treatment with B. longum 35624 reduces disease severity and improves the quality of life of patients with IBS, particularly those with the most severe forms of IBS.
Core tip: Our observational study of 233 patients with moderate-to-severe irritable bowel syndrome demonstrated that 30 d of treatment with once-daily Bifidobacterium longum 35624 (a probiotic) resulted in a significant improvement in symptoms and disease severity for two thirds of the patients. Patient quality of life was also significantly improved, and the majority of patients expressed that they were satisfied with the treatment.
- Citation: Sabaté JM, Iglicki F. Effect of Bifidobacterium longum 35624 on disease severity and quality of life in patients with irritable bowel syndrome. World J Gastroenterol 2022; 28(7): 732-744
- URL: https://www.wjgnet.com/1007-9327/full/v28/i7/732.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i7.732
Irritable bowel syndrome (IBS) is a chronic functional bowel disorder, which combines abdominal pain and transit disorders and affects approximately 4% to 10% of the population, depending on whether the Rome III or Rome IV definition is used[1]. IBS may be responsible for impairment in quality of life, especially in the most severe forms, which represent approximately 25% of cases[2]. The patho
Probiotics are available over the counter and few have real recommendations from learned societies and/or claims substantiated by findings from properly conducted clinical trials. Few probiotics have shown efficacy in improving IBS symptoms relative to placebo in randomized double-blind studies[5], as it is the case with Bifidobacterium longum 35624 (formerly B. infantis) with two conclusive randomized clinical trials[6,7]. Studies of probiotics have been criticized for the criteria used to measure efficacy[8]. Few studies have thus far adopted the efficacy criteria for the assessment of treatments for IBS specified by the United States Food and Drug Administration or the European Medicines Agency (EMA)[9-11]. At a minimum, studies should demonstrate the ability of a probiotic to reduce disease severity, as is the case for fecal microbiota transplant studies[12], and to improve quality of life. However, in most clinical studies of probiotics, disease severity at baseline is often not reported, patients with mild or moderate forms of IBS are recruited and quality of life is not evaluated. The strengthening of the diagnostic criteria for IBS, with the deletion of the term “dis
The aim of our study was to assess the effect of B. longum 35624 treatment on IBS severity and patients’ quality of life in real-life clinical practice.
This was a prospective, open-label, multicenter study (FLORAVIE study). Patients over 18 years of age with IBS diagnosed according to the Rome IV criteria were enrolled from November 2018 to January 2020. Patients had to have had recurrent abdominal pain on average at least 1 d/wk in the last 3 mo, associated with two or more of the following criteria: Pain related to defecation or associated with a change in frequency of stool or associated with a change in form (appearance) of stool. IBS diagnosis criteria had to be fulfilled for the last 3 mo with symptom onset at least 6 mo prior to diagnosis. Participation in the study was proposed by private gastroenterologists during spontaneous consultations with new or former patients consulting for their IBS. The gastroenterologist was free to prescribe or not B. longum 35624 according to his appreciation if he tought it was a good therapeutic option for the patient. Participating patients were seen in consultation at a second visit after 30 d of treatment.
This study was conducted according to the guidelines of the Declaration of Helsinki and the Guidelines for Good Clinical Practice (EMA/Committee for Medicinal Products for Human Use/International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use/135/1995) and approved by the North-west III Protection of Persons Committee of the University Hospital of Caen, France. Study conduct was in full accordance with French applicable laws and regulations, including but not limited to current International Council on Harmonisation-Good Clinical Practices. All participants received an information note and were informed about the objectives, methodology and purpose of the study, and those who agreed to participate were required to provide oral non-opposition prior to entry, according to France regulation. The study was registered on clinicaltrials.gov under study number NCT04662502. To ensure that the patient population was representative of the country, participants were recruited by gastroenterologists working in private practice (with a maximum of 12 patients per investigator) throughout France.
Eligible patients were included only if they met all the inclusion and none of the exclusion criteria. Previous treatment with B. longum 35624 was an exclusion criterion. The use of other probiotics was not allowed within 2 wk from inclusion. Recent antibiotic treatment or treatment modification that could have an impact on microbiota, gut motility or digestive symptoms were prohibited. Participants with a history of abdominal surgery (except for appendectomy, cholecystectomy, surgery for hemorrhoids or cesarean section) were excluded.
After obtaining the patient's oral non-opposition to the study and verifying the inclusion and exclusion criteria, treatment with B. longum 35624 was initiated. Patients who were included in the study were to take one capsule per day containing 109 colony-forming units of B. longum 35624 for 30 d. Patients were advised not to change their diet during the study. Clear instructions were provided to patients and gastroenterologists on how to complete and review the study questionnaires.
During the first visit, patients’ demographics (sex, age), medical history, medical conditions other than IBS and concomitant treatments, disease characteristics (mode of onset, disease duration, transit sub-type, treatments, associated conditions) and the effect of IBS on their personal, professional, and social life were collected. The effect of IBS on the patient’s personal, professional, and social life was assessed using a six-point Likert scale, ranging from “no impact” to “very severe impact”. Patients recorded their treatment intake in a daily diary. Stool consistency was assessed using the Bristol stool scale[14] at baseline and every 10 d throughout the study and recorded in the diary. The severity of IBS was determined using the IBS severity scoring system (IBS-SSS), which consists of five domains assessing the intensity and frequency of abdominal pain, intensity of abdominal distension, satisfaction with transit and quality of life. IBS-SSS scores range from 0 to 500, with scores < 75 indicating remission, scores between 75 and 174 indicating mild severity, scores between 175 and 299 indicating moderate severity, and scores between 300 and 500 indicating severe disease[15]. Quality of life was assessed using the IBS quality of life questionnaire (IBS-QOL), which consists of 34 questions exploring eight dimensions, including dysphoria, interference with activity, body image, health worry, food avoidance, social reaction, sexual and relationships, with scores ranging from 0 to 100, 100 indicating the best quality of life[16]. IBS-SSS and IBS-QOL questionnaires were administered at baseline before treatment and after 30 d of treatment with B. longum 35624 (second visit). Satisfaction with the treatment was assessed by gastroenterologists and by patients independently using a five-point Likert scale. Adherence to treatment was assessed by recording daily medication intake in the patient's diary and based on the gastroenterologist's records at the follow-up visit. Adverse events (AEs) occurring throughout the study were recorded, as were any events that could have interfered with B. longum 35624 treatment, including changes in diet.
The main outcomes were the proportion of patients who had a decrease of > 50 points in the IBS-SSS score and the proportion of patients who had an increase of > 10 points in the IBS-QOL score after a 30-d treatment with B. longum 35624. Secondary outcomes were the change in IBS-SSS and IBS-QOL scores, the proportion of patients who had a shift from one severity category to another, and the proportion of patients and gastroenterologists who were satisfied with the treatment.
Considering a variation in the IBS-QOL score of 15, the total number of patients to be assessed was determined to be 203 to obtain a level of precision of 10%. To maintain this level of precision considering that a certain number of patients (about 10%) would not be eligible for primary endpoint analysis (missing data), 220 patients had to be included in the study.
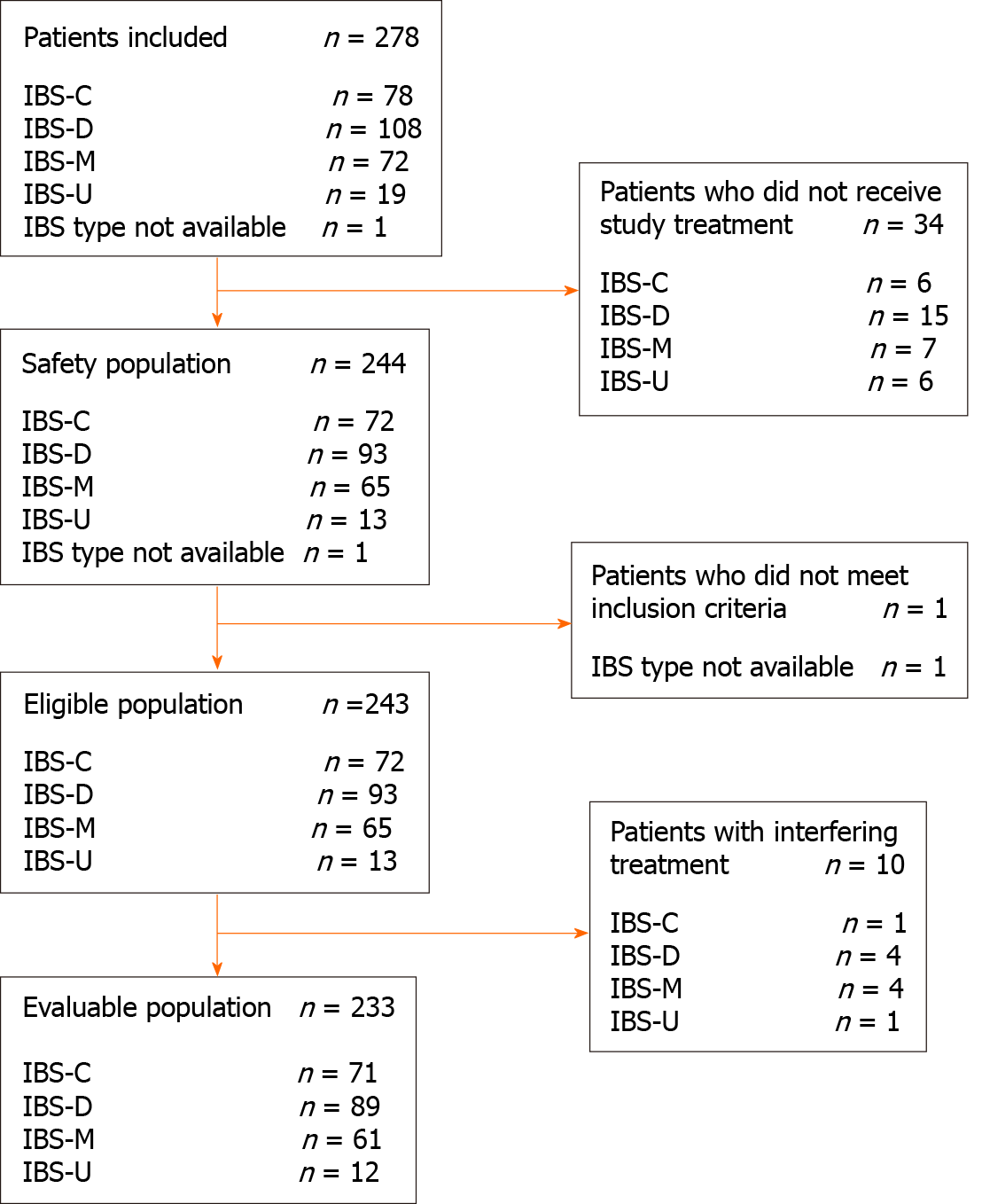
The safety population included all patients who received at least one dose of B. longum 35624. The evaluable population included all patients in the safety population with at least one post-baseline assessment who did not use any drugs that could have interfered with effect of B. longum 35624.
The statistical methods of this study were reviewed by a biomedical statistician from ICTA (International Clinical Trials Association, fontaine-les-Dijon, France). Statistical analyses were performed using SAS® software (version 9.2-SAS Institute, North Carolina, United States). Means and SD or medians and interquartile ranges (IQR) were calculated for continuous variables and comparisons were performed using the t-test and Kruskal-Wallis test. Frequencies were calculated for categorical variables and comparisons were performed using the χ2 test. Comparisons of IBS-QOL or IBS-SSS scores at baseline vs at the end of treatment were performed using the Wilcoxon test. Correlations between variables were evaluated using the Pearson correlation coefficient. Missing data were not replaced.
This observational study was proposed to 129 gastroenterologists throughout France, of whom, 86 were interested in participating and 61 recruited patients. They were representative of the profession with 80% being male (vs 70% nationally) and distributed all over the territory, in 12 of the 13 French regions, to be representative of the national practice (Supplementary Figure 1).
From November 2018 to January 2020, 278 patients were enrolled in the study, of which 233 were included in the evaluable population. The detailed flowchart of the population is shown in Figure 1.
Patient baseline characteristics are presented in Table 1. The patient cohort consisted primarily of middle-aged women, most were non-smokers (87.7%) and did not consume alcohol (63.9%). Body mass index was classified as normal in 51.2% of patients, as overweight in 33.5%, as obese in 9.4%, and as underweight in 5.2%. Thirty-two percent of patients (n = 75) had at least one pre-existing condition, the most common of which were gastrointestinal conditions other than IBS (7.3%, n = 17; gastroesophageal reflux, hiatal hernia, colonic diverticulum, hemorrhoids), endocrine (5.2%, n = 12) and vascular disorders (5.2%, n = 12). In addition, 21.0% of patients received at least one concomitant treatment that was unlikely to interfere with study treatment. At baseline, thirty-seven patients (15.8%) had already been on a specific diet [low fermentable oligo-, di-, mono-saccharides and polyols (FODMAP) diet, n = 7; gluten-free diet, n = 2; lactose-free diet, n = 4] for more than 1 mo, and did not change it during the study period.
| Characteristic | IBS group, n = 233 |
| Median age (IQR), yr | 51.0 (37–66) |
| Female sex, n (%) | 166 (71.2) |
| BMI (kg/m2) | 24.5 ± 4.3 |
| IBS sub-types (transit pattern), n (%) | |
| IBS-C | 71 (30.5) |
| IBS-D | 89 (38.2) |
| IBS-M | 61 (26.2) |
| IBS-U | 12 (5.2) |
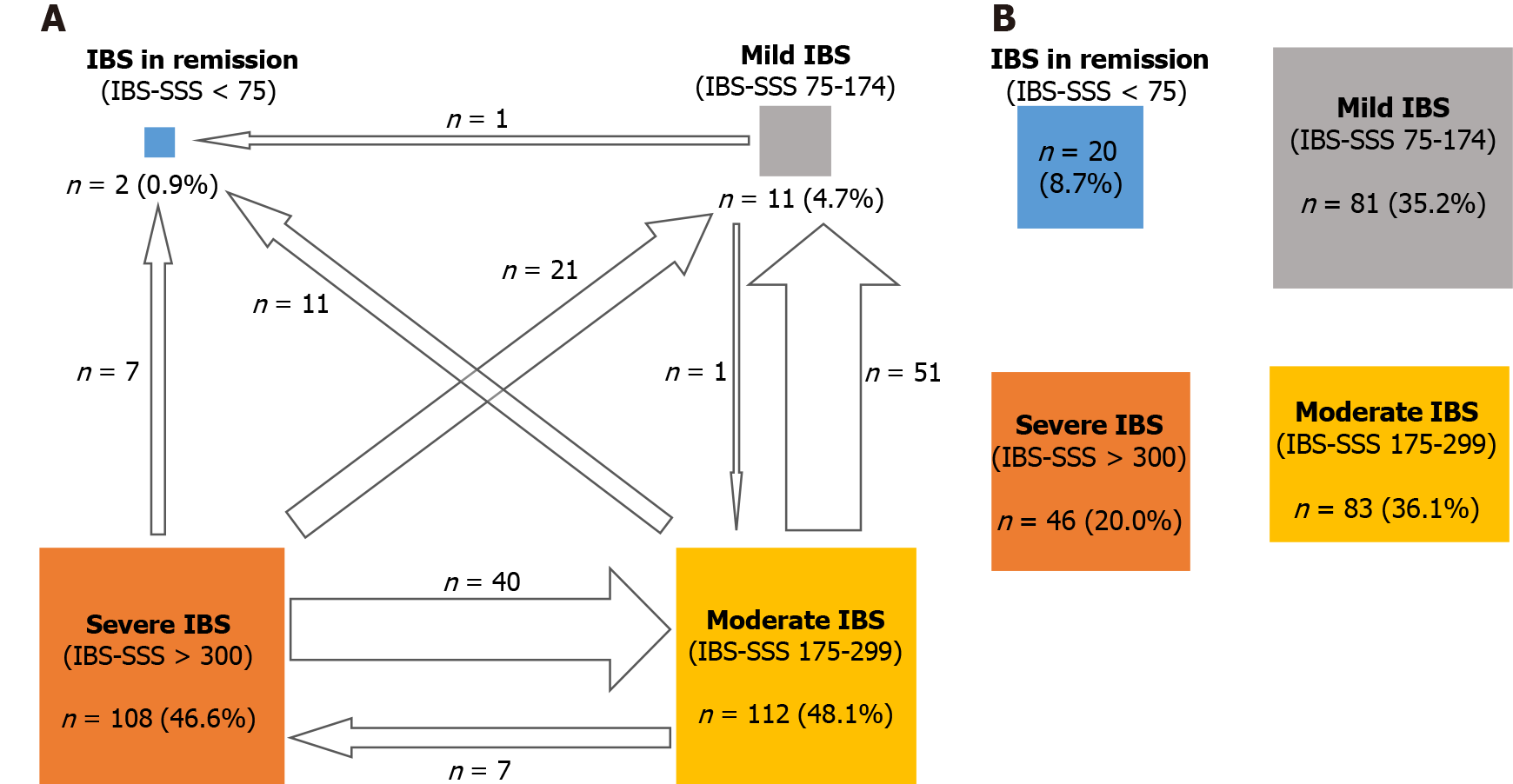
| IBS severity categories (IBS-SSS), n (%) | |
| Remission (0–74) | 2 (0.8) |
| Mild (75–174) | 11 (4.7) |
| Moderate (175–299) | 112 (48.1) |
| Severe (> 300) | 108 (46.4) |
| IBS-QOL (0–100) | 60.2 ± 20.5 |
IBS was triggered by an acute episode of gastroenteritis (post-infectious IBS) in nine patients (3.9%) and by a stressful event in 91 patients (34.8%), with sexual abuse reported in eight patients. No triggering factor for IBS was identified in 127 patients (54.5%). Median disease duration was 8.0 years (IQR: 3.0, 16.0). Diarrhea-predominant IBS (IBS-D) (38.2%) was the most common subtype, while IBS of unidentified subtype (IBS-U; 5.2%) was the least common; antispasmodics and transit modifiers had previously been prescribed respectively in 65.7% and 35.7% of IBS patients.
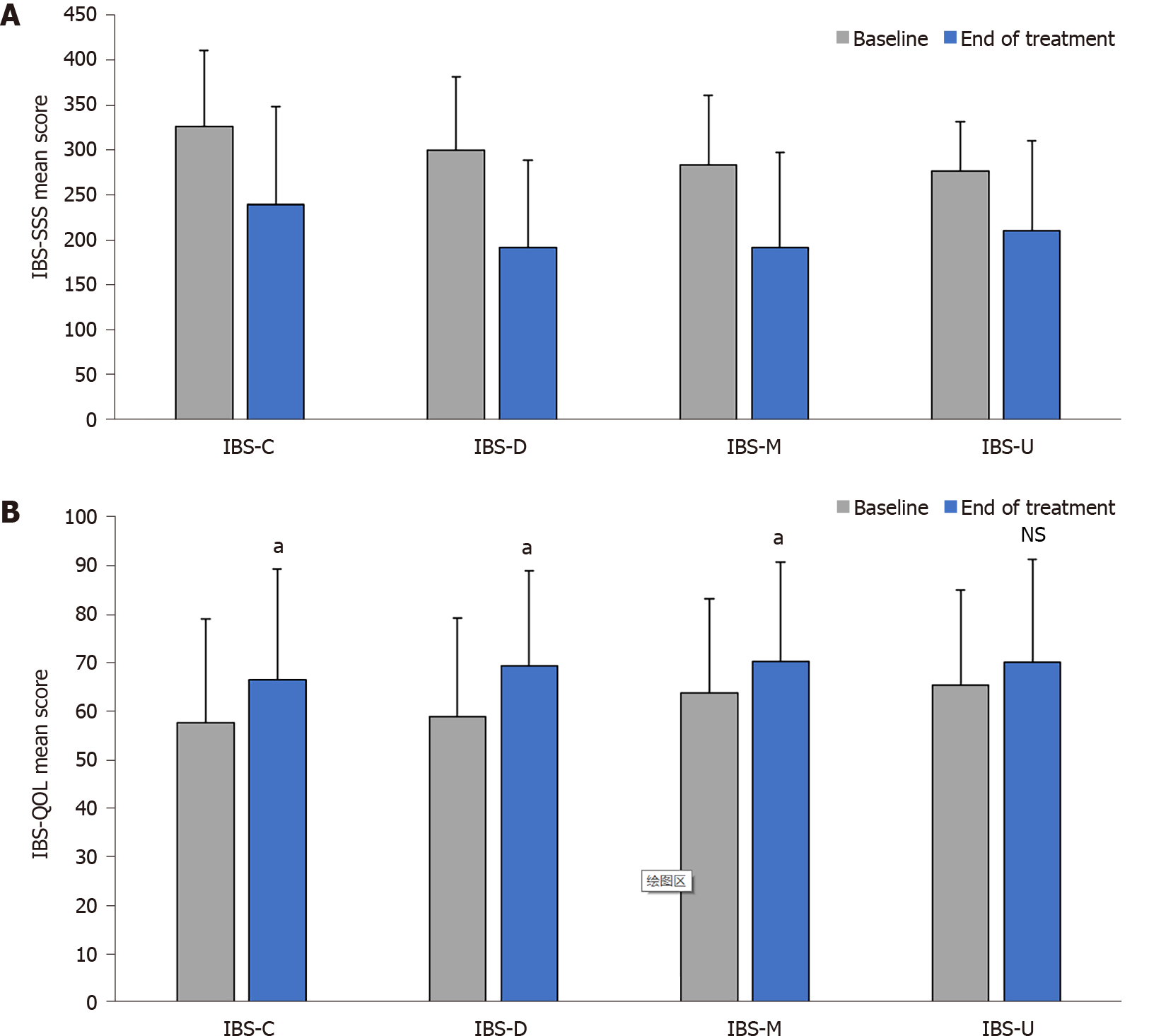
At baseline, the average IBS severity among the included patients was high (mean ± SD IBS-SSS score 303.0 ± 81.5), with the majority having either a severe or a moderate form of the disease, and fewer than 10% having mild disease severity or being in remission. IBS severity scores were different across transit pattern subtypes (Figure 2A) with constipation-predominant (IBS-C) and IBS-D having the highest scores (326.8 ± 84.2 and 300.8 ± 81.2, respectively) compared to mixed IBS (IBS-M) and IBS-U (284.1 ± 77.9 and 277.8 ± 54.3, respectively).
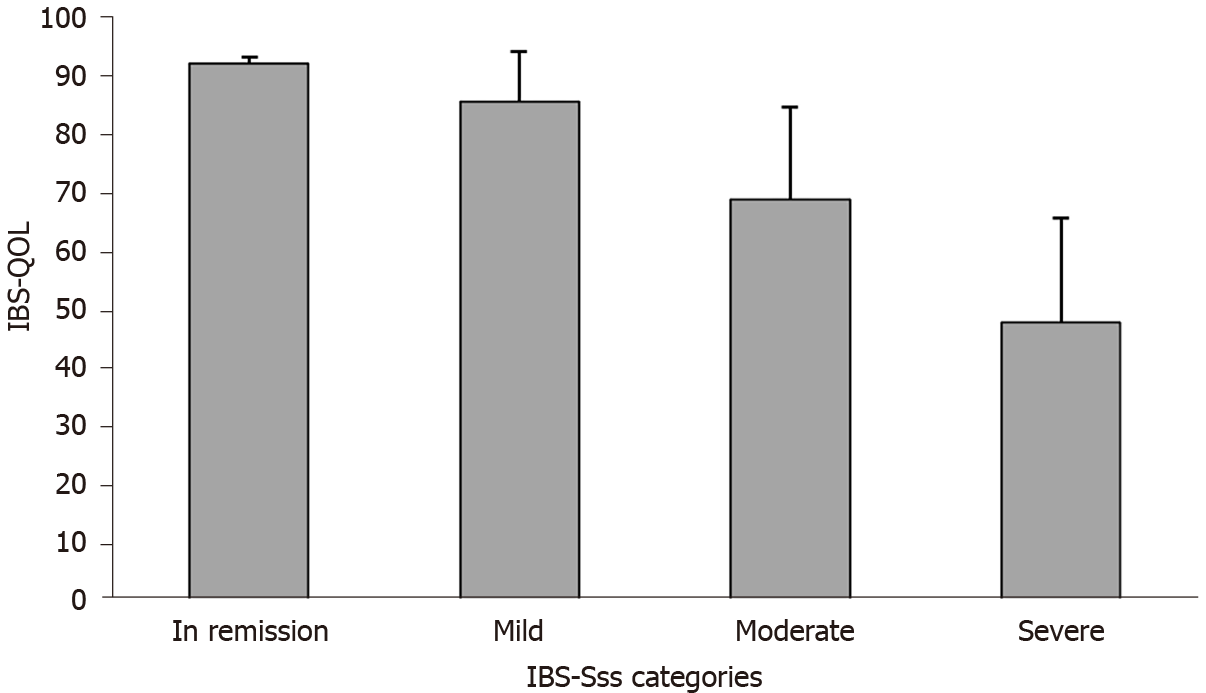
Quality of life was impaired in most patients (mean ± SD IBS-QOL score 60.2 ± 20.5). Quality of life scores were comparable across transit pattern subtypes (Figure 2B) and were correlated with disease severity (r = −0.66, P < 0.0001), with higher IBS severity associated with lower the IBS-QOL scores (i.e., lower quality of life) (Figure 3). In 96% of cases, IBS impaired the patient’s personal, professional, and social life (low or mild in 26.0% of patients, moderate in 37.7%, and severe or very severe in 36.3% of patients).
Duration of exposure to study treatment was 28.2 ± 3.4 d (mean ± SD). Patients’ adherence to treatment according to patients’ daily diary reports was excellent, with 94.1% adherence to capsule intake.
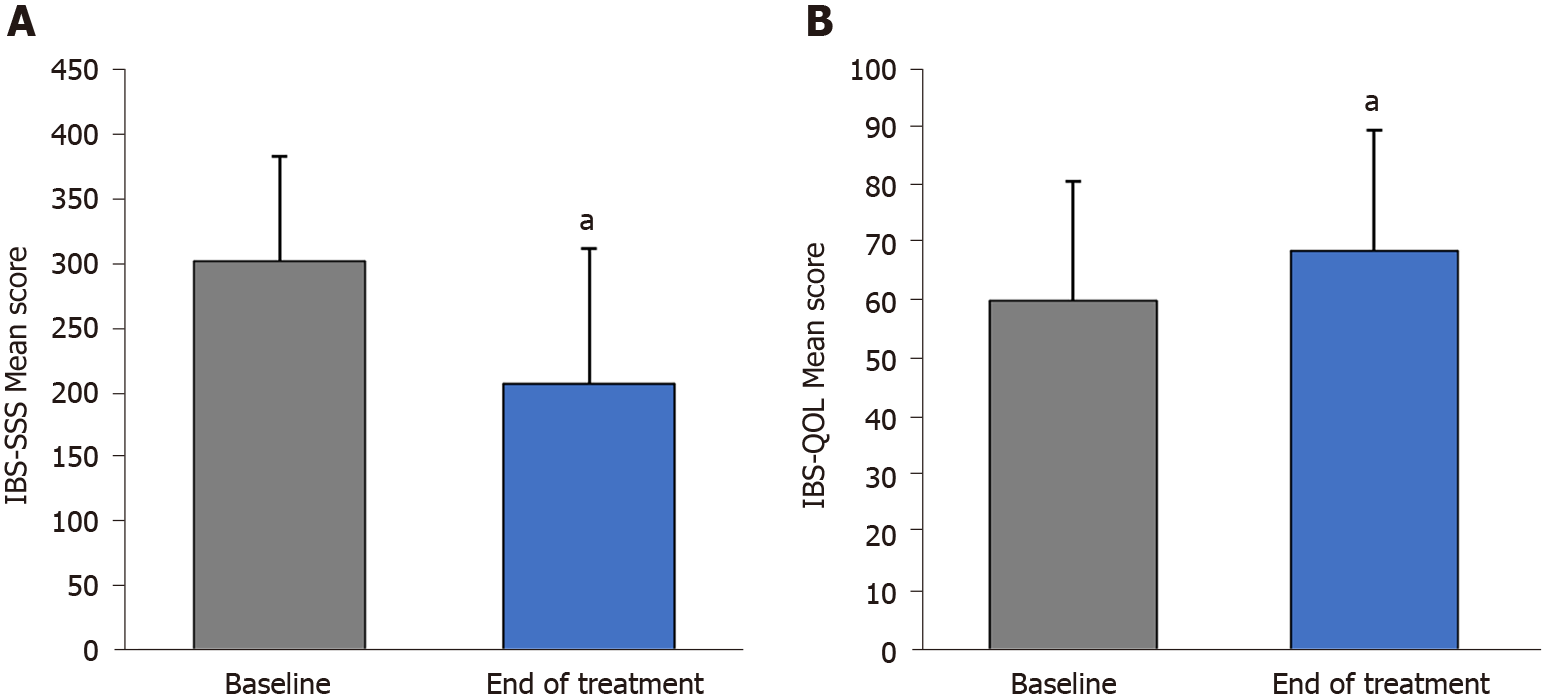
After 30 d of treatment, there was a significant reduction in IBS severity compared with baseline (mean ± SD overall IBS-SSS score: 208.1 ± 104.8 vs 303.2 ± 81.5, P < 0.001) (Figure 4A), and in all transit subtypes (Figure 2A). The evolution of IBS severity scores was similar and of the same magnitude in all IBS subtypes (P = 0.115) (Table 2). Hence, after treatment, IBS severity scores were different across transit pattern subtypes (Figure 2A) with IBS-C having the highest scores compared to IBS-D, IBS-M and IBS-U.
| IBS-C, n = 71 | IBS-D, n = 89 | IBS-M, n = 61 | IBS-U, n = 12 | P values1 | |
| IBS-SSS scores absolute evolution | -89.5 ± 105.9 | -107.4 ± 85.5 | -91.7 ± 85.6 | -66.0 ± 104.2 | 0.115 (NS) |
| IBS-QOL scores absolute evolution | 8.9 ± 14.1 | 10.4 ± 15.8 | 6.6 ± 14.8 | 4.7 ± 10.9 | 0.658 (NS) |
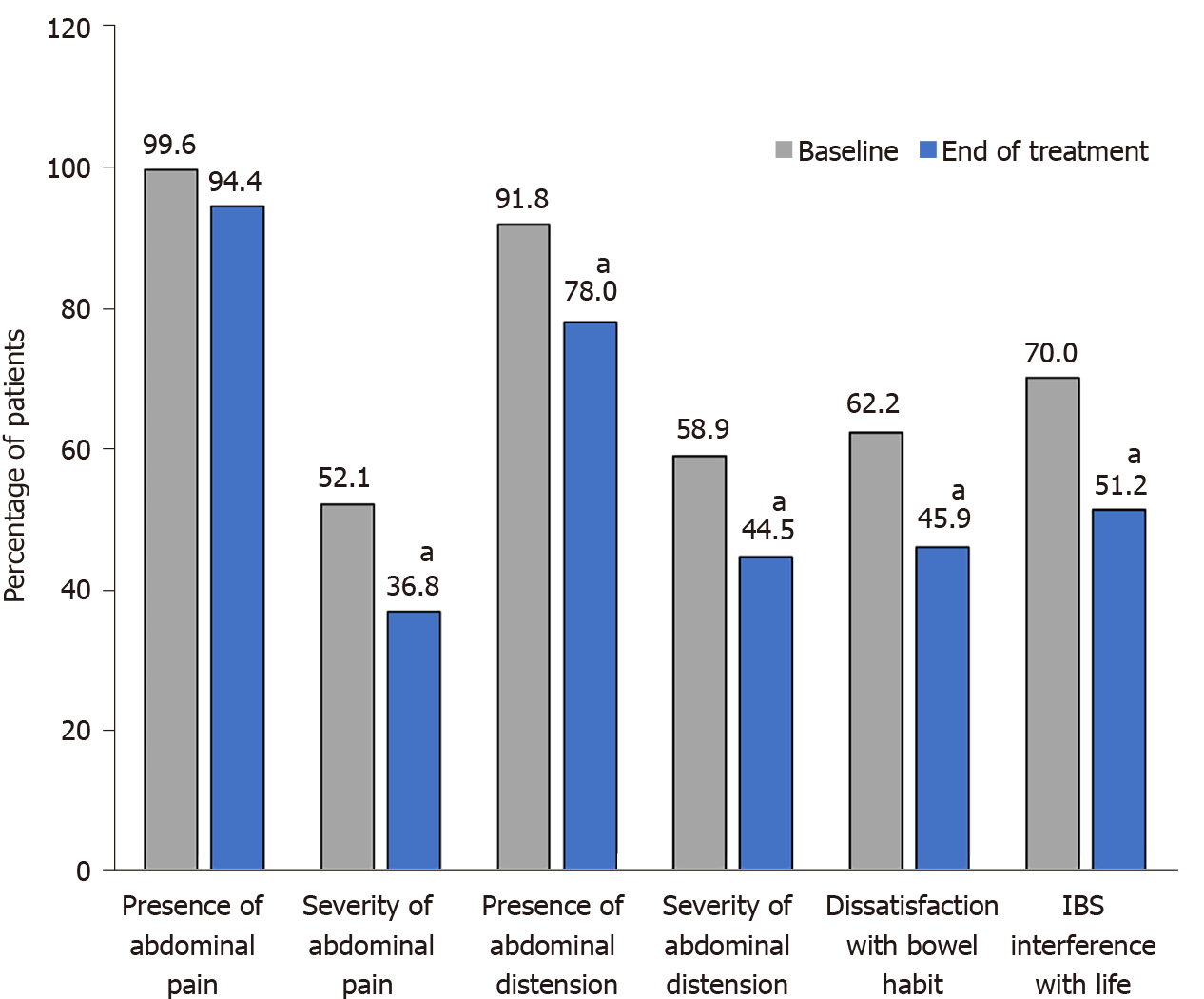
Each of the five IBS-SSS domains was improved (Figure 5 and Supplementary Figure 2). In addition, the proportion of patients who reported experiencing symptoms every day over a period of 10 d was reduced by half after treatment, dropping from 76 patients (32.9%) to 34 patients (15.6%). Moreover, the number of pain-free patients over a 10-d period, which was low at baseline (0.4%; one patient), increased to 10.1% (22 patients) after 30 d of treatment.
A change towards categories of lower IBS severity or remission occurred in 56.7% of patients (Figure 6). A significant improvement in disease severity (> 50-point decrease in the IBS-SSS score) was observed in 65.7% of patients, with significant improvement noted in all transit subtypes.
Compared with baseline, the overall quality of life was improved at the end of treatment in the entire patient population (mean ± SD IBS-QOL score 68.8 ± 20.9 vs 60.2 ± 20.5; P < 0.001) (Figure 4B) and in IBS-C, IBS-D and IBS-M subgroups (P < 0.001) without difference in absolute score evolution between transit pattern subgroups (P = 0.658) (Table 2 and Figure 2B). A clinically significant meaningful improvement in the overall IBS-QOL score (> 10-point increase) was observed in 36.9% of patients, and was more pronounced in patients with more severe disease at baseline (9.1% of patients with mild IBS, 28.6% of patients with moderate IBS, and 49.1% of patients with severe IBS). Each of the eight dimensions of IBS-QOL were improved at the end of treatment (P < 0.001, Supplementary Figure 3).
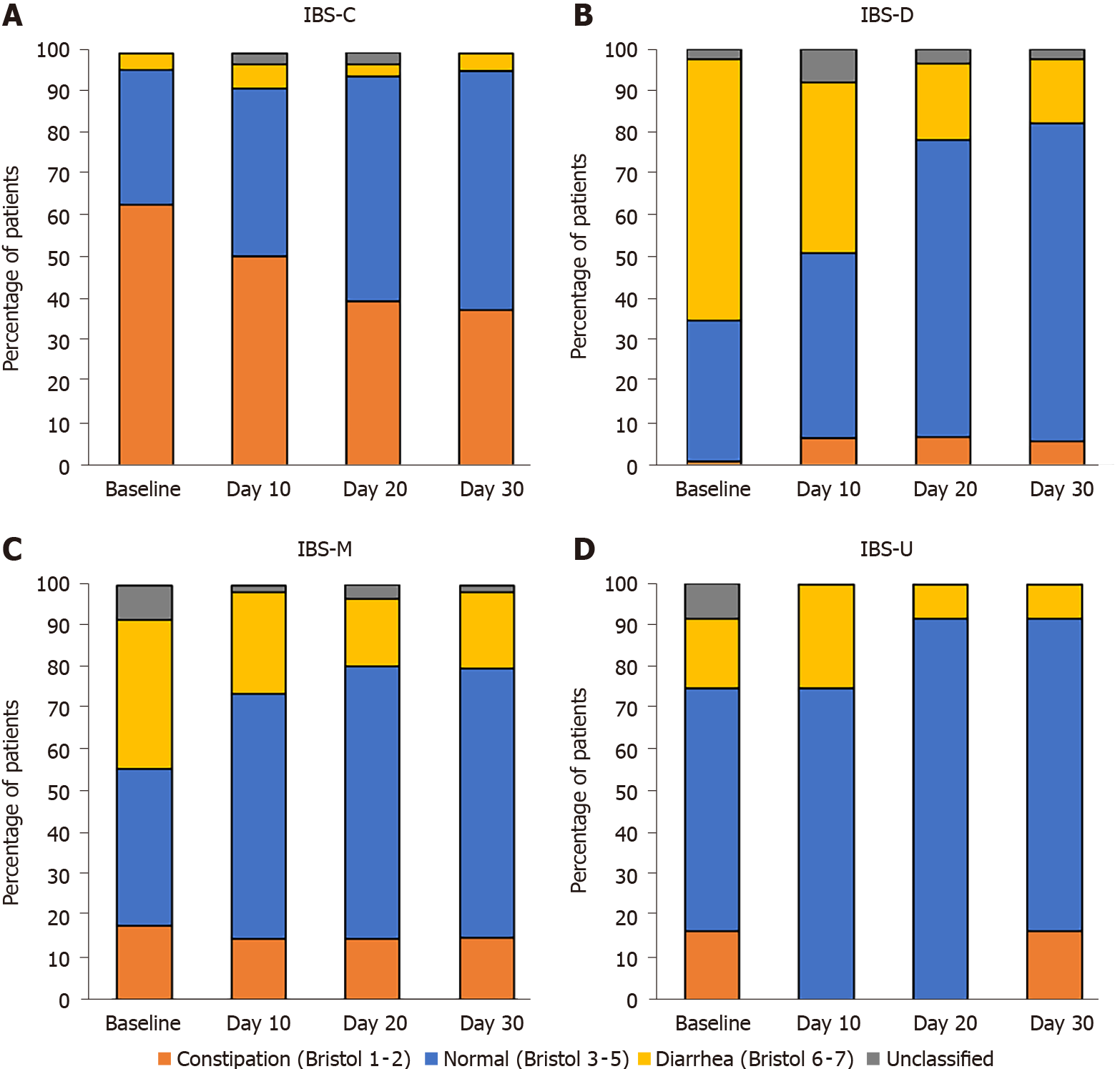
Over the course of the study, stool consistency tended to be normalized in all IBS transit subtypes (Figure 7) with a decrease in the percentage of patients reporting stool types 1–2 and 6–7 according to different transit patterns.
Approximately two-thirds of patients (63.8%) and gastroenterologists (63.8%) were satisfied with B. longum 35624 treatment at the end of the study.
During the study, 10 AEs possibly related to the use of B. longum 35624 were reported in 4.1% (n = 10) of patients in the safety population (n = 244 patients), including flatulence (n = 3), abdominal pain (n = 2), constipation (n = 1), abdominal distension (n = 1), upper abdominal pain (n = 1), gastrointestinal motor disorder (n = 1), and increased weight (n = 1).
While the new Rome criteria have decreased IBS prevalence and increased the percentage of patients with severe disease, few studies have evaluated the efficacy of probiotics with this new paradigm. In our study conducted in patients with IBS according to Rome IV definition with different transit subtypes and levels of severity, a 30-d treatment regimen with B. longum 35624 reduced disease severity and improved quality of life, especially in patients with the most severe forms of IBS.
The significant reduction in disease severity that we found in approximately two-thirds of patients in this study after 30 d of B. longum 35624 treatment is consistent with the literature reporting that two-thirds of patients with IBS have dysbiosis, further supporting the link between IBS severity and dysbiosis[17]. It is also consistent with the results of two previous randomized studies of the same probiotic strain, including a large 4-wk study performed in 362 women with IBS[7] and a smaller 8-wk study performed in 80 patients with IBS[6]. The effect on digestive symptoms and disease severity reduction could be secondary to an effect on pro-inflammatory cytokines as it was show previously by O’Mahony et al[6]. with a normalisation of an interleukin (IL)-10/IL-12 cytokine ratio that was impaired at baseline[6]. In a recent study[18], at baseline, similar IBS-SSS global scores, IBS severity distribution and IBS subtype distribution were observed for patients with IBS according to Rome IV criteria. Interestingly, in our study, the improvement in overall IBS-SSS score was observed in all its component items (intensity and frequency of abdominal pain, distension and its intensity; satisfaction with bowel habits) and regardless of the IBS subtype (IBS-C, IBS-D, or IBS-M), as also described in the study by Whorwell et al[7]. Few studies of probiotics conducted in patients with IBS[19] have included a large proportion of patients with severe forms of the disease, as in our present study (approximately half of the patients), and in many such studies, disease severity was not monitored. For a population of patients who are not being treated at tertiary centers, such a level of disease severity may appear surprising; however, a similar level of IBS severity has been reported in patients treated by private gastroenterologists in France[20] using Rome II criteria. Moreover, the selection of patients according to Rome IV criteria tends to increase the proportion of patients with severe IBS[13]. It should be noted that the magnitude of reduction in disease severity observed in our study is uncommon. Further, during the present study, the majority of patients shifted to lower IBS severity categories, some moving from a severe to a mild form of the disease, which has been associated in the literature with a decrease in health care seeking and an improvement of quality of life[2,21].
Quality of life is often impaired in patients with IBS, especially in those with severe disease. Therefore, the 2014 EMA “Guideline on the evaluation of medicinal products for the treatment of irritable bowel syndrome” recommended the use of validated quality of life scales in order to better understand the real impact of a treatment on the disease[10]. In the present study, overall quality of life measured by the IBS-QOL, a validated questionnaire, was significantly improved, as well as all its individual domains. Comparable levels of improvement in IBS-QOL scores (< 10 points at the group level vs placebo) were observed at 3 mo in the trials of drugs such as eluxadoline or linaclotide that have been approved for the treatment of IBS by health authorities in the United States or in Europe[22]. However, in our real-life study a “clinically meaningful” improvement (> 10 points)[23] was observed only in approximately one-third of patients (i.e., half as many as the number of patients who had a significant reduction in IBS severity). The link between disease severity and quality of life observed in this study has been previously described in IBS using different quality-of-life instruments[2,24]. The fact that a decrease in severity did not translate into a similar improvement in the quality of life could be because certain behaviors that affect quality of life may require more than 1 mo, which was the duration of the present study, to change (e.g., self-confidence, attitude at work, relationships with others). A similar lag in improvement in the quality of life compared with IBS severity was also observed in a recent study of two other probiotic strains, Lactobacillus acidophilus DDS-1 and B. lactis UABla-12[11]. Interestingly, a clinically significant improvement in quality of life was observed in our patients with the highest severity of IBS at baseline, suggesting that B. longum 35624 has also therapeutic potential in patients with severe IBS. The absence of improvement of quality of life in the study conducted by Whorwell et al[7] could be explained by the fact that their study probably included fewer patients with severe forms of IBS because it relied on the Rome II criteria for inclusion, and it has been shown that the Rome IV criteria, which are more stringent, increase the percentage of patients with severe forms of IBS[13]. Nevertheless, O'Mahony et al[6] have observed a significant improvement in quality of life over placebo in an 8-wk study using the same probiotic strain as in our study[6].
Most of the published studies on probiotics do not target a specific IBS subtype. In our study, the effect of treatment with B. longum 35624 over a 30-d period was analyzed according to IBS subtypes. Treatment was effective on each IBS subtype (IBS-C, IBS-D and IBS-M) in terms of disease severity and quality of life. During treatment, we observed the normalization of stool consistency, with a decrease in the frequency of extreme stool types according to the Bristol stool scale (type 1-2 for IBS-C or type 6-7 for IBS-D) and an increase of normal stool type (type 3-5), as it was the case using the same strain in the study of Whorwell et al[7].
We found that the incidence of AEs was low (5%), and that AEs were generally minor. This finding is in accordance with the results from a previous large randomized study of B. longum 35624[7], confirming its favorable tolerability profile.
This observational study, were only data concerning the patients who took treatment with B. longum 35624 are available, had several limitations, namely the absence of a placebo or comparator group and the relatively short treatment duration. Strong placebo effect and a tendency for spontaneous improvement are sometimes described in studies of IBS[25]. For example, in their study, Martoni et al[11] observed an average decrease of 30 points in the IBS-SSS score at 3 wk in the placebo group[11]. However, the magnitude of the reduction in severity, which is a relatively stable parameter[2], and the choice of the 50-point reduction in the IBS-SSS score as the threshold[15], which has been previously validated as a reliable indicator of improvement, provide some confidence in the robustness of our results. Even if short, the duration of the present study was sufficient to obtain positive results that are comparable to those of a large randomized study of B. longum 35624[7] and three recently published randomized studies of other probiotics[19,26,27]. It should also be noted that, to our knowledge, our study is one of the few[11] that simultaneously assessed severity and quality of life using validated instruments, and one of the few probiotic studies to include patients with IBS diagnosed using the Rome IV criteria. Stool analysis was not performed to correlate improvement of IBS patients' symptoms with a qualitative or quantitative improvement of the intestinal microbiota, but this is rarely done in clinical studies. Nevertheless, Charbonneau et al[28] using the same probiotic strain observed that after 4 and 8 wk of treatment, fecal levels of B. infantis 35624 from IBS subjects who received the probiotic rose significantly compared with those from subjects who received placebo. While in some diseases there may be variations in the microbiota according to ethnicity, this factor could also have influenced the results. However, in France, the legislation does not allow the collection of ethnic data for this type of analysis.
This study conducted in IBS patients diagnosed according to the Rome IV criteria and who had different transit pattern subtypes and different levels of symptom severity showed that 30 d of treatment with B. longum 35624, whose superiority to placebo has already been established, reduced IBS disease severity and improved patient quality of life in all subgroups of patients, and notably in those with the most severe form of IBS.
Some probiotics have been shown efficacy on irritable bowel syndrome (IBS) symptoms.
However, little data is available on the effectiveness of probiotics on IBS severity and quality of life.
To assess in real life settings efficacy of treatment with B. longum 35624 on IBS severity and quality of life.
To assess in an observational study on IBS patients defined according to Rome IV criteria, the effect of a 30 d of treatment with B. longum 35624 on the disease severity (IBS severity scoring system) and quality of life (IBS quality of life questionnaire).
After one month of treatment, the severity and quality of life improved in approximately two-thirds and one-third of patients respectively, especially in more severe patients with changes to lower severity categories in more than half of the patients. A gradual improvement in stool consistency was also observed in all transit sub-types.
In IBS patients defined according to Rome IV criteria, a 30 d treatment with B. longum 35624 reduces the disease severity and improves the quality of life even in patients with severe disease that were excluded of most published studies.
Future research should help to define predictors of good response to probiotic therapy and should study responses to prolonged therapy for this chronic disease.
Medical writing assistance in the preparation of this manuscript was provided by Georgii Filatov of Springer Healthcare Communications. Special thanks to the investigators of the study (last name, first name): Anacreon Nival Sophie; Audan Alain; Barbereau Didier; Bastid Christophe; Baudet Anne; Berry Pascal; Bion Eric; Blot Marie-Christine; Caumes Jean-Luc; Cazals Jean-Brice; Chambon Jacques; Chatrenet Philippe; Colonna Patrick; Constant Thierry; Courtial Philippe; D Abrigeon Gilles; Dalbies Pierre Adrien; Daude Mathieu; Delette Olivier; Dewaele François; Duchesne Charlène; Duval Gilles; Ecuer Stephane; Escartin Michel-Pierre; Etienney Isabelle; Geros Christos; Gilbert Thierry; Gorez Etienne; Helbert Thierry; Higuero Thierry; Hubert Jean; Jeandroz Madec Véronique; Juin De Faucal Boutet Dominique; Kerlirzin Anne; Lame Charles; Levy Jonathan; Luneau Fabrice; Maignan Philippe; Menat Jean Philippe; Pecriaux Olivier; Plegat Serge; Poggi Jean-Pierre; Pospait Dan; Pujol Pascale; Regensberg Michel; Remy André Jean; Renkes Pascal; Richard Mireille; Riot François; Rosenbaum Alain; Rouillon Jean-Michel; Rouquie Patrick; Rudelli Alain; Samak Valérie; Schneider Philippe; Stancu Feier Laura; Texier Frédéric; Thevenot Aldine; Vove Jean Paul; Wittersheim Christian; Zalar Alberto.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Nakaji K, Qin Z S-Editor: Fan JR L-Editor: A P-Editor: Wu RR
| 1. | Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, Whitehead WE, Dumitrascu DL, Fang X, Fukudo S, Kellow J, Okeke E, Quigley EMM, Schmulson M, Whorwell P, Archampong T, Adibi P, Andresen V, Benninga MA, Bonaz B, Bor S, Fernandez LB, Choi SC, Corazziari ES, Francisconi C, Hani A, Lazebnik L, Lee YY, Mulak A, Rahman MM, Santos J, Setshedi M, Syam AF, Vanner S, Wong RK, Lopez-Colombo A, Costa V, Dickman R, Kanazawa M, Keshteli AH, Khatun R, Maleki I, Poitras P, Pratap N, Stefanyuk O, Thomson S, Zeevenhooven J, Palsson OS. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021;160:99-114.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1196] [Article Influence: 299.0] [Reference Citation Analysis (0)] |
| 2. | Drossman DA, Chang L, Bellamy N, Gallo-Torres HE, Lembo A, Mearin F, Norton NJ, Whorwell P. Severity in irritable bowel syndrome: a Rome Foundation Working Team report. Am J Gastroenterol. 2011;106:1749-59; quiz 1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 3. | Ford AC, Lacy BE, Talley NJ. Irritable Bowel Syndrome. N Engl J Med. 2017;376:2566-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 4. | Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG; Rome Foundation Committee. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. 2013;62:159-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 655] [Cited by in RCA: 649] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 5. | Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:1044-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 354] [Article Influence: 50.6] [Reference Citation Analysis (1)] |
| 6. | O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, O'Sullivan GC, Kiely B, Collins JK, Shanahan F, Quigley EM. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1003] [Cited by in RCA: 959] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 7. | Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O'Mahony L, Kiely B, Shanahan F, Quigley EM. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 517] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 8. | Mazurak N, Broelz E, Storr M, Enck P. Probiotic Therapy of the Irritable Bowel Syndrome: Why Is the Evidence Still Poor and What Can Be Done About It? J Neurogastroenterol Motil. 2015;21:471-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | US Food and Drug Administration. Irritable bowel syndrome — clinical evaluation of products for treatment. 2012. [cited 10 August 2021]. Available from: https://www.fda.gov/media/78622/download. |
| 10. | European Medicines Agency. Guideline on the evaluation of medicinal products for the treatment of irritable bowel syndrome. 2014. [cited 10 August 2021]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-evaluation-medicinal-products-treatment-irritable-bowel-syndrome-revision-1_en.pdf. |
| 11. | Martoni CJ, Srivastava S, Leyer GJ. Lactobacillus acidophilus DDS-1 and Bifidobacterium lactis UABla-12 Improve Abdominal Pain Severity and Symptomology in Irritable Bowel Syndrome: Randomized Controlled Trial. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 12. | Ianiro G, Eusebi LH, Black CJ, Gasbarrini A, Cammarota G, Ford AC. Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2019;50:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 13. | Camilleri M. Irritable Bowel Syndrome: Straightening the road from the Rome criteria. Neurogastroenterol Motil. 2020;32:e13957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Heaton KW, Radvan J, Cripps H, Mountford RA, Braddon FE, Hughes AO. Defecation frequency and timing, and stool form in the general population: a prospective study. Gut. 1992;33:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 493] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 15. | Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 1226] [Article Influence: 43.8] [Reference Citation Analysis (1)] |
| 16. | Drossman DA, Patrick DL, Whitehead WE, Toner BB, Diamant NE, Hu Y, Jia H, Bangdiwala SI. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol. 2000;95:999-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 299] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 17. | Tap J, Derrien M, Törnblom H, Brazeilles R, Cools-Portier S, Doré J, Störsrud S, Le Nevé B, Öhman L, Simrén M. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology. 2017;152:111-123.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 444] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 18. | Black CJ, Yiannakou Y, Houghton LA, Ford AC. Epidemiological, Clinical, and Psychological Characteristics of Individuals with Self-reported Irritable Bowel Syndrome Based on the Rome IV vs Rome III Criteria. Clin Gastroenterol Hepatol. 2020;18:392-398.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 19. | Skrzydło-Radomańska B, Prozorow-Król B, Cichoż-Lach H, Majsiak E, Bierła JB, Kosikowski W, Szczerbiński M, Gantzel J, Cukrowska B. The Effectiveness of Synbiotic Preparation Containing Lactobacillus and Bifidobacterium Probiotic Strains and Short Chain Fructooligosaccharides in Patients with Diarrhea Predominant Irritable Bowel Syndrome-A Randomized Double-Blind, Placebo-Controlled Study. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 20. | Coffin B, Dapoigny M, Cloarec D, Comet D, Dyard F. Relationship between severity of symptoms and quality of life in 858 patients with irritable bowel syndrome. Gastroenterol Clin Biol. 2004;28:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Fan WJ, Xu D, Chang M, Zhu LM, Fei GJ, Li XQ, Fang XC. Predictors of healthcare-seeking behavior among Chinese patients with irritable bowel syndrome. World J Gastroenterol. 2017;23:7635-7643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Lembo AJ, Lacy BE, Zuckerman MJ, Schey R, Dove LS, Andrae DA, Davenport JM, McIntyre G, Lopez R, Turner L, Covington PS. Eluxadoline for Irritable Bowel Syndrome with Diarrhea. N Engl J Med. 2016;374:242-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 226] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 23. | Andrae DA, Patrick DL, Drossman DA, Covington PS. Evaluation of the Irritable Bowel Syndrome Quality of Life (IBS-QOL) questionnaire in diarrheal-predominant irritable bowel syndrome patients. Health Qual Life Outcomes. 2013;11:208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Sabaté JM, Ducrotté P, Piche T, Zerbib F, Dapoigny M, Bruley des Varannes S, Bonaz B, Mion F, Iglicki F, Denhez D, Façon S, Gourcerol G, Jouët P. Expectations of IBS patients concerning disease and healthcare providers: Results of a prospective survey among members of a French patients' association. Clin Res Hepatol Gastroenterol. 2020;44:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Weerts ZZRM, Vork L, Mujagic Z, Keszthelyi D, Hesselink MAM, Kruimel J, Leue C, Muris JWM, Jonkers DMAE, Masclee AAM. Reduction in IBS symptom severity is not paralleled by improvement in quality of life in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2019;31:e13629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Andresen V, Gschossmann J, Layer P. Heat-inactivated Bifidobacterium bifidum MIMBb75 (SYN-HI-001) in the treatment of irritable bowel syndrome: a multicentre, randomised, double-blind, placebo-controlled clinical trial. Lancet Gastroenterol Hepatol. 2020;5:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 27. | Oh JH, Jang YS, Kang D, Chang DK, Min YW. Efficacy and Safety of New Lactobacilli Probiotics for Unconstipated Irritable Bowel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Charbonneau D, Gibb RD, Quigley EM. Fecal excretion of Bifidobacterium infantis 35624 and changes in fecal microbiota after eight weeks of oral supplementation with encapsulated probiotic. Gut Microbes. 2013;4:201-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |