Published online Feb 21, 2022. doi: 10.3748/wjg.v28.i7.715
Peer-review started: September 14, 2021
First decision: November 7, 2021
Revised: November 20, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: February 21, 2022
Processing time: 156 Days and 4.1 Hours
Methods for predicting the prognosis of patients undergoing surgery for recurrent hepatolithiasis after biliary surgery are currently lacking.
To establish a nomogram to predict the prognosis of patients with recurrent hepatolithiasis after biliary surgery.
In this multicenter, retrospective study, data of consecutive patients in four large medical centers who underwent surgery for recurrent hepatolithiasis after biliary surgery were retrospectively analyzed. We constructed a nomogram to predict the prognosis of recurrent hepatolithiasis in a training cohort of 299 patients, following which we independently tested the nomogram in an external validation cohort of 142 patients. Finally, we used the concordance index (C-index), calibra-tion, area under curve, decision curve analysis, clinical impact curves, and visual fit indices to evaluate the accuracy of the nomogram.
Multiple previous surgeries [2 surgeries: Odds ratio (95% confidence interval), 1.451 (0.719-2.932); 3 surgeries: 4.573 (2.015-10.378); ≥ 4 surgeries: 5.741 (1.347-24.470)], bilateral hepatolithiasis [1.965 (1.039-3.717)], absence of immediate clearance [2.398 (1.304-4.409)], neutrophil-to-lymphocyte ratio ≥ 2.462 [1.915 (1.099-3.337)], and albumin-to-globulin ratio ≤ 1.5 [1.949 (1.056-3.595)] were found to be independent factors influencing the prognosis. The nomogram constructed on the basis of these variables showed good reliability in the training (C-index: 0.748) and validation (C-index: 0.743) cohorts. Compared with predictions using traditional classification models, those using our nomogram showed better agreement with actual observations in the calibration curve for the probability of endpoints and the receiver operating characteristic curve. Dichloroacetate and clinical impact curves showed a larger net benefit of the nomogram.
The nomogram developed in this study demonstrated superior performance and discriminative power compared to the three traditional classifications. It is easy to use, highly accurate, and shows excellent calibration.
Core Tip: This is a multicenter, retrospective study to establish a nomogram to predict the prognosis of patients with recurrent hepatolithiasis after biliary surgery. After this, an online calculator was developed, and the nomograms are freely available on the internet. We used the concordance index, calibration, area under curve, decision curve analysis, clinical impact curves, and visual fit indices to evaluate the accuracy of the nomogram. Compared with predictions using traditional classification models, those using our nomogram showed better agreement with actual observations.
- Citation: Pu T, Chen JM, Li ZH, Jiang D, Guo Q, Li AQ, Cai M, Chen ZX, Xie K, Zhao YJ, Wang C, Hou H, Lu Z, Geng XP, Liu FB. Clinical online nomogram for predicting prognosis in recurrent hepatolithiasis after biliary surgery: A multicenter, retrospective study. World J Gastroenterol 2022; 28(7): 715-731
- URL: https://www.wjgnet.com/1007-9327/full/v28/i7/715.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i7.715
Hepatolithiasis is mostly prevalent in East and Southeast Asia, and the incidence in China is the highest in the world[1]. Although treatment strategies for hepatolithiasis have been improving, the overall treatment rate and prognosis remain poor because of the long course, complex pathological changes, high incidence of postoperative complications, and high recurrence rate. Sporadic cases of hepatolithiasis have been reported in Western countries, and with the increasing immigration from Asian countries, hepatolithiasis has become increasingly prevalent in the West[2-4]. The prevalence of this disease is 30%-50% in East Asia and 0.6%-1.3% in Western countries[5].
The treatment of hepatolithiasis involves pharmacologic, endoscopic, and surgical approaches. Surgery is the most effective treatment. However, the course of the disease varies, and stones can easily remain in the surgery and recur at a later date. Patients with recurrent hepatolithiasis who undergo multiple biliary surgeries often experience varying degrees of abdominal adhesions, causing greater difficulties and increasing the risks for surgery. Thus, surgeons should focus on improving the prognosis and quality of life of patients.
Recurrent hepatolithiasis is defined as hepatolithiasis with a history of biliary tract surgery for different reasons. Some classification systems for hepatolithiasis have been established, such as classification based on clinical manifestations[3], Nakayama[6]’s classification based on the distribution of stones, Tsunoda et al[7]’s classification based on dilatation or stenosis, and the Chinese Classification model proposed by the Biliary Study Group of the Chinese Medical Association[8]. Despite their wide acceptance, these classification models are too complicated to implement in guiding clinical treatment. Thus, clinical classification of hepatolithiasis has practical significance in guiding treatment decisions and predicting patient prognoses. Nomograms are statistical tools that enable simultaneous consideration of various factors to facilitate visualization of prognoses. Moreover, nomograms offer many advantages, including personalized evaluation, user-friendliness, and ease of comprehension[9]. Considering the absence of a prediction model for the quality of life of patients with recurrent hepatolithiasis, this study aimed to establish a nomogram for predicting the prognosis of patients with recurrent hepatolithiasis after biliary surgery.
Clinical and prognostic data of patients with recurrent hepatolithiasis who underwent surgery between January 2015 and December 2020 at the Departments of Hepatopancreatobiliary Surgery in four medical centers were retrospectively analyzed for evaluating patients’ quality of life. The patients from the First Affiliated Hospital of Anhui Medical University constituted the training cohort, while those from the Second Affiliated Hospital of Anhui Medical University, the First Affiliated Hospital of University of Science and Technology of China and the First Affiliated Hospital of Bengbu Medical College served as the validation cohort. All hospitals are high-volume surgical centers employing similar therapeutic approaches for hepatolithiasis.
The diagnosis of hepatolithiasis was confirmed by preoperative imaging examinations and intraoperative findings. Recurrent hepatolithiasis was diagnosed when the patient had a history of biliary surgery. These patients had already undergone at least one bile duct surgery for hepatolithiasis. Patients from the four centers were included if they met the following inclusion criteria: (1) A history of at least one biliary surgery; (2) Confirmation of stones by preoperative imaging examination; (3) Confirmation of intrahepatic cholangiolithiasis during the procedure; and (4) A preoperative liver function of Child-Pugh grade A or initial grade B that improved to grade A. The exclusion criteria were as follows: (1) Hepatolithiasis occurring within 6 mo after the last biliary tract surgery; (2) No history of surgical treatment; (3) History of abdominal surgery not involving the biliary tract system; (4) Malignant tumor on postoperative pathological evaluation; and (5) Patchy clinical or follow-up data. All clinical data were screened and collected in a computerized database by a specialized research assistant. This retrospective study was conducted in accordance with the declaration of Helsinki and was approved by the institutional ethics committees (Quick-PJ2021-08-19). All included patients or their relatives provided written informed consent before their data were analyzed.
Under the same preoperative evaluation protocol across all centers, the patients underwent blood tests, including routine blood counts and analysis of blood biochemistry, hemostatic function, immunological markers, and tumor markers. All patients underwent at least two imaging examinations, including ultrasound (US), computed tomography (CT), magnetic resonance imaging, or magnetic resonance cholangiopancreatography (MRCP), which provided information on the location of stones, biliary strictures, or liver atrophy. Definitive planning of the procedure was performed according to the findings of imaging studies. For patients with complex bilateral hepatolithiasis or those expected to undergo extensive liver resection, the ratio of the future remnant liver to total functional liver volume was calculated by volumetric CT scans or three-dimensional visualization techniques, and the indocyanine green 15-min retention rate was measured to evaluate the safety of surgeries.
Patients who failed to reach Child-Pugh grade A before surgery underwent liver protection and supportive treatment. All surgeries were performed by experienced hepatobiliary surgeons. Patients with a history of repeated surgeries often have abdominal adhesions. After relieving the abdominal adhesions, a detailed surgical plan was created on the basis of the intraoperatively confirmed stone location, bile duct stenosis, liver atrophy, and function of the sphincter of Oddi (SO). The main surgical objective was to remove as many stones as possible and choose the appropriate method for biliary drainage. Routine intraoperative flexible choledo-choscopy was performed after longitudinal incision of the common bile duct and removal of visible stones to determine the stone distribution and identify residual stones, which were directly extracted with a stone basket when needed. Then, SO function was evaluated, and the biliary drainage method was chosen based on SO function and the presence of residual stones; external T tube drainage was chosen for normal SO without residual stones, and cholangioenterostomy was chosen for SO laxity without residual stones. If residual stones could not be prevented, cholangioenterostomy and T-tube drainage were performed simultaneously[10]. Hepatectomy should be performed when bile duct stones are located within one liver lobe accompanied by atrophy or fibrosis, multiple liver abscesses secondary to bile duct infection, and suspected malignant masses. We applied the Pringle maneuver to occlude the blood inflow to the liver if necessary. Choledochoscopy was performed again after hepatic lobe resection to check for residual stones and to assess whether stones were cleared immediately. Bile acid was collected during the surgery and sent for bacterial culture and drug sensitivity testing in all patients.
Standardized and meticulous postoperative patient management was performed in all patients at an early stage, including monitoring of vital signs, proper tissue perfusion, and nutritional support. Gastric acid secretion inhibitors and broad-spectrum antibiotics were administered immediately after the surgery, and antibiotics were adjusted according to the results of bile acid culture. Liver function tests were performed at 1, 3, and 7 d after the surgery. According to the Clavien-Dindo classification system, complications occurring within 90 d postoperatively were classified as grades I-V. Before discharge, all patients underwent abdominal CT examination again to further confirm whether the stone was removed immediately during the surgery. Patients who undergo external drainage of the T tube should undergo cholangiography or choledochoscopy after discharge to confirm or remove residual stones. In the present study, for patients with immediate stone residue, we usually performed choledochoscopy through the sinus of the T tube at 6-8 wk after the surgery; this was performed several times until the stone was removed or could not be removed by any means. For patients with immediate clearance, we performed T-tube cholangiography at 2 wk after surgery. In case a residual stone was observed, we performed choledo-choscopy as described above.
All patients underwent regular postoperative follow-up by the same team of surgeons in the hepatobiliary outpatient clinics or through telephone interviews at 2-3 mo after discharge. Follow-up evaluations included assessment of clinical symptoms and signs, routine blood tests, liver-function assessments, and US, CT, or MRCP to observe residual or recurrent stones. Postoperative residual stones were defined as stones that could not be removed by any method and were confirmed by US, CT, or MRCP 3 mo postoperatively[11]. Prognosis was evaluated according to the Terblanche criteria[12]. The patients were evaluated from 30 d to the end of the follow-up: (1) Grade I, no bile duct-related symptoms; (2) Grade II, occasional bile duct-related symptoms requiring no treatment; (3) Grade III, obvious bile duct-related symptoms requiring treatment; or (4) Grade IV, presence of anastomotic stricture or formation of bile duct stones requiring surgical intervention and causing disease-related cancer or death. Ter-blanche grades III and IV were considered to indicate a poor prognosis, which was the study endpoint.
Continuous variables are expressed as mean ± SD for normally distributed variables or median (interquartile range) for non-normally distributed variables, and appropriate statistical tests (the independent samples t-test or the Mann-Whitney U test) were used. Categorical variables are expressed as number (n) or proportion (%) and compared using the χ2 test or Fisher’s exact test, as appropriate. Assigned cutoff values for continuous variables were derived from the Youden index[13]. Univariate logistic regression was used to determine the independent risk factors related to the prognosis of patients with recurrent hepatolithiasis after multiple biliary surgeries in the training cohort. Multivariate logistic regression was conducted using variables with clinical meaning or statistical significance in the univariate analyses. A nomogram for the prognosis of patients with recurrent hepatolithiasis after biliary surgery was created based on a multivariate logistic regression model. The performance of the nomogram was evaluated using the concordance index (C-index) and calibration plots with bootstrap samples. The C-index is a numerical measure of discriminative ability, and calibration plots are graphical evaluations of predictive ability that compare observed probabilities with nomogram-predicted probabilities. The area under the curve (AUC) of the receiver operating characteristic (ROC) curves and quality indices of models[14] in the training cohort and the external validation cohort were used to assess the predictive accuracy of the model in comparison with the three traditional classifications. The clinical usefulness of the nomogram was examined by determining the net benefit by using decision curve analysis (DCA)[15]. Clinical impact curves were also analyzed to demonstrate the predictive accuracy and clinical usefulness of the nomogram. The accuracy of the optimal cutoff value was assessed based on sensitivity, specificity, and predictive values. Statistical analyses were performed using R version 4.0.5, and SPSS version 23.0. Tests were 2-sided, and statistical significance was set at P < 0.05.
Data of 943 consecutive patients who underwent surgical treatment for hepatolithiasis at the First Affiliated Hospital of Anhui Medical University between January 2015 and December 2020 were collected continuously. Among them, 363 patients (38.5%) with a history of biliary tract surgeries were classified as having recurrent hepatolithiasis. Of these 363 patients, 64 (17.6%) who did not fulfill the inclusion criteria were excluded: 28 were admitted to the hospital with a malignant tumor, 9 had a history of other abdominal surgery, 1 died in the perioperative period, 22 had incomplete clinical or follow-up data, and 4 died of other causes after surgery. Ultimately, 299 (82.4%) patients were identified as the training cohort. Using the same criteria, 142 patients from the Second Affiliated Hospital of Anhui Medical University (57 cases), the First Affiliated Hospital of the University of Science and Technology of China (51 cases), and the First Affiliated Hospital of Bengbu Medical College (34 cases) were included in the external validation cohort. The two cohorts showed significant differences in the pre-, intra-, and postoperative clinical characteristics, as shown in Tables 1 and 2.
| Characteristic | Training cohort | Validation cohort | P value |
| N = 299 | N = 142 | ||
| Sex | 0.785 | ||
| Male | 93 (31.1%) | 46 (32.4%) | |
| Female | 206 (68.9%) | 96 (67.6%) | |
| Age (yr) | 0.613 | ||
| < 60 | 153 (51.2%) | 69 (48.6%) | |
| ≥ 60 | 146 (48.8%) | 73 (51.4%) | |
| BMI | 21.72 ± 2.87 | 22.19 ± 2.96 | 0.317 |
| Abdominal pain | 0.204 | ||
| No | 39 (13.0%) | 25 (17.6%) | |
| Yes | 260 (87.0%) | 117 (82.4%) | |
| Fever | 0.162 | ||
| No | 175 (58.5%) | 93 (65.5%) | |
| Yes | 124 (41.5%) | 49 (34.5%) | |
| Jaundice | 0.060 | ||
| No | 227 (75.9%) | 119 (83.8%) | |
| Yes | 72 (24.1%) | 23 (16.2%) | |
| Number of previous surgeries | 0.374 | ||
| 1 | 199 (66.6%) | 101 (71.1%) | |
| 2 | 57 (19.1%) | 29 (20.4%) | |
| 3 | 33 (11.0%) | 9 (6.3%) | |
| ≥ 4 | 10 (3.3%) | 3 (2.1%) | |
| Previous hepatectomy | 0.293 | ||
| No | 238 (79.6%) | 119 (83.8%) | |
| Yes | 61 (20.4%) | 23 (16.2%) | |
| Previous cholangioenterostomy | 0.057 | ||
| No | 257 (86.0%) | 131 (92.3%) | |
| Yes | 42 (14.0%) | 11 (7.7%) | |
| NLR | 0.453 | ||
| < 2.462 | 188 (62.9%) | 84 (59.2%) | |
| ≥ 2.462 | 111 (37.1%) | 58 (40.8%) | |
| < PLR | 0.804 | ||
| 173.74 | 237 (79.3%) | 114 (80.3%) | |
| ≥ 173.74 | 62 (20.7%) | 28 (19.7%) | |
| AGR | 0.430 | ||
| > 1.5 | 104 (34.8%) | 44 (31.0%) | |
| ≤ 1.5 | 195 (65.2%) | 98 (69.0%) | |
| TB (μmol/L) | 0.262 | ||
| < 34.2 | 249 (83.3%) | 112 (78.9%) | |
| ≥ 34.2 | 50 (16.7%) | 30 (21.1%) | |
| ALT (IU/L) | 0.474 | ||
| < 50 | 181 (60.5%) | 91 (64.1%) | |
| ≥ 50 | 118 (39.5%) | 51 (35.9%) | |
| AST (IU/L) | 0.522 | ||
| < 40 | 180 (60.2%) | 90 (63.4%) | |
| ≥ 40 | 119 (39.8%) | 52 (36.6%) | |
| ALP (IU/L) | 0.053 | ||
| < 200 | 180 (60.2%) | 99 (69.7%) | |
| ≥ 200 | 119 (39.8%) | 43 (30.3%) | |
| GGT (IU/L) | 0.182 | ||
| < 150 | 146 (48.8%) | 79 (55.6%) | |
| ≥ 150 | 153 (51.2%) | 63 (44.4%) | |
| HBsAg | 0.107 | ||
| Negative | 271 (90.6%) | 135 (95.1%) | |
| Positive | 28 (9.4%) | 7 (4.9%) | |
| CA19-9 (U/mL) | 0.428 | ||
| < 34 | 208 (69.6%) | 104 (73.2%) | |
| ≥ 34 | 91 (30.4%) | 38 (26.8%) | |
| Hepatolithiasis research group | 0.089 | ||
| I | 19 (6.4%) | 18 (12.7%) | |
| II | 152 (50.8%) | 67 (47.2%) | |
| III | 100 (33.4%) | 49 (34.5%) | |
| IV | 28 (9.4%) | 8 (5.6%) | |
| Tsunoda classification | 0.052 | ||
| I | 17 (5.7%) | 19 (13.4%) | |
| II | 166 (55.5%) | 74 (52.1%) | |
| III | 100 (33.4%) | 43 (30.3%) | |
| IV | 16 (5.4%) | 6 (4.2%) | |
| Chinese medical association | 0.295 | ||
| I | 238 (79.6%) | 123 (86.6%) | |
| IIa | 30 (10.0%) | 8 (5.6%) | |
| IIb | 25 (8.4%) | 8 (5.6%) | |
| IIc | 6 (2.0%) | 3 (2.1%) |
| Characteristic | Training cohort | Validation cohort | P value |
| N = 299 | N = 142 | ||
| Operation duration (h) | 0.168 | ||
| ≤ 2 | 66 (22.1%) | 38 (26.8%) | |
| 2-4 | 122 (40.8%) | 64 (45.1%) | |
| > 4 | 111 (37.1%) | 40 (28.2%) | |
| Liver cirrhosis | 0.690 | ||
| No | 261 (87.3%) | 122 (85.9%) | |
| Yes | 38 (12.7%) | 20 (14.1%) | |
| Hepatic atrophy | 0.068 | ||
| No | 124 (41.5%) | 72 (50.7%) | |
| Yes | 175 (58.5%) | 70 (49.3%) | |
| Intrahepatic stenosis | 0.182 | ||
| No | 238 (79.6%) | 105 (73.9%) | |
| Yes | 61 (20.4%) | 37 (26.1%) | |
| Extrahepatic stones | 0.813 | ||
| No | 64 (21.4%) | 29 (20.4%) | |
| Yes | 235 (78.6%) | 113 (79.6%) | |
| Hepatectomy | 0.084 | ||
| No | 101 (33.8%) | 60 (42.3%) | |
| Yes | 198 (66.2%) | 82 (57.7%) | |
| Bilateral hepatolithiasis | 0.074 | ||
| No | 238 (79.6%) | 123 (86.6%) | |
| Yes | 61 (20.4%) | 19 (13.4%) | |
| Drainage mode | 0.125 | ||
| External T tube drainage | 205 (68.6%) | 109 (76.8%) | |
| Cholangioenterostomy | 44 (14.7%) | 19 (13.4%) | |
| Combined drainage | 50 (16.7%) | 14 (9.9%) | |
| Function of the SO | 0.521 | ||
| Normal | 130 (43.5%) | 72 (50.7%) | |
| Dysfunction | 62 (20.7%) | 27 (19.0%) | |
| Nonfunctional | 83 (27.8%) | 32 (22.5%) | |
| Resected | 24 (8.0%) | 11 (7.7%) | |
| Intraoperative bleeding (mL) | 0.465 | ||
| < 400 | 285 (95.3%) | 133 (93.7%) | |
| ≥ 400 | 14 (4.7%) | 9 (6.3%) | |
| Blood transfusion | 0.112 | ||
| No | 258 (86.3%) | 130 (91.5%) | |
| Yes | 41 (13.7%) | 12 (8.5%) | |
| TB after operation (μmol/L) | 0.131 | ||
| < 34.2 | 232 (77.6%) | 119 (83.8%) | |
| ≥ 34.2 | 67 (22.4%) | 23 (16.2%) | |
| Bile culture | 0.471 | ||
| Negative | 196 (65.6%) | 98 (69.0%) | |
| Positive | 103 (34.4%) | 44 (31.0%) | |
| Clavien-Dindo classification | 0.784 | ||
| < III | 286 (95.7%) | 135 (95.1%) | |
| ≥ III | 13 (4.3%) | 7 (4.9%) | |
| Hospitalization expenses | 48272 ± 22537 | 44933 ± 25354 | 0.164 |
| Immediate clearance | 0.298 | ||
| Yes | 229 (76.6%) | 115 (81.0%) | |
| No | 70 (23.4%) | 27 (19.0%) | |
| Final clearance | 0.399 | ||
| Yes | 283 (94.6%) | 137 (96.5%) | |
| No | 16 (5.4%) | 5 (3.5%) |
All patients had a history of multiple (1-6) biliary tract surgeries 1-55 years before this surgery. The training cohort included 199 (66.6%), 57 (19.1%), 33 (11.0%), and 10 (3.3%) patients who underwent 1, 2, 3, and ≥ 4 surgeries, respectively. In total, 167 (55.9%) patients had a history of cholecystectomy only, without hepatectomy or biliary drainage, while 61 (20.4%) and 42 (14.0%) patients had a history of hepatectomy and cholangioenterostomy, respectively. The validation cohort included 101 (71.1%), 29 (20.4%), 9 (6.3%), and 3 (2.1%) patients who underwent 1, 2, 3, and ≥ 4 surgeries, respectively; 167 (55.9%) patients had previously undergone only cholecystectomy, while 23 (16.2%) and 11 (7.7%) patients had a history of hepatectomy and cholangioenterostomy, respectively. Gall bladder removal was performed in the first (93.0%) or second (7.0%) surgeries for all patients. The appropriate surgical method was selected on the basis of preoperative evaluation and intraoperative conditions, and anastomosis reconstruction was performed depending on the presence of stenosis. In the training cohort, 229 (76.6%) and 283 (94.6%) patients achieved immediate and final clearance, respectively, and 129 (43.1%), 78 (26.1%), 66 (22.1%), and 26 (8.7%) patients showed Terblanche grades I-IV, respectively. In the validation cohort, 115 (81.0%) and 137 (96.5%) patients showed immediate and final clearance, respectively, and 53 (37.3%), 43 (30.3%), 34 (23.9%), and 12 (8.5%) patients showed Terblanche grades I-IV, respectively.
The results of univariate and multivariate analyses of prognosis based on common variables, including demographic data, clinical symptoms, surgical histories, serologic data, and operative data, in the training cohort are listed in Table 3. The optimal cutoff values of the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio were determined as described above. Factors that significantly affected prognosis in the univariate analysis were subjected to multivariate analysis, which demonstrated that more previous surgeries, bilateral hepatolithiasis, lack of immediate clearance, NLR ≥ 2.462, and albumin-to-globulin ratio (AGR) ≤ 1.5 were independent risk factors for a poor prognosis in patients with recurrent hepatolithiasis.
| Variable | Univariable analysis | Multivariable analysis | ||
| P value | OR (95%CI) | P value | OR (95%CI) | |
| Sex: Female/Male | 0.328 | 1.311 (0.762-2.257) | ||
| Age (yr): ≥ 60/< 60 | 0.603 | 1.139 (0.697-1.862) | ||
| BMI | 0.068 | 0.921 (0.843-1.006 | ||
| Abdominal pain: Yes/No | 0.066 | 0.525 (0.264-1.043) | ||
| Fever: Yes/No | 0.083 | 1.551 (0.945-2.547) | ||
| Jaundice: Yes/No | 0.406 | 1.270 (0.724-2.230) | ||
| Previous operation times | ||||
| 2 times/1 time | 0.384 | 1.337 (0.695-2.571) | 0.299 | 1.451 (0.719-2.932) |
| 3 times/1 time | < 0.001 | 4.840 (2.241-10.454) | < 0.001 | 4.573 (2.015-10.378) |
| ≥ 4 times/1 time | 0.005 | 7.340 (1.827-29.498) | 0.018 | 5.741 (1.347-24.470) |
| Previous hepatectomy: Yes/No | 0.026 | 1.936 (1.082-3.463) | 0.144 | 1.642 (0.845-3.190) |
| Previous cholangioenterostomy: Yes/No | 0.455 | 1.299 (0.654-2.577) | ||
| NLR: ≥ 2.462/< 2.462 | 0.001 | 2.334 (1.410-3.863) | 0.022 | 1.915 (1.099-3.337) |
| PLR: ≥ 173.74/< 173.74 | 0.069 | 1.714 (0.959-3.065) | ||
| AGR: ≤ 1.5/> 1.5 | 0.002 | 2.459 (1.393-4.338) | 0.033 | 1.949 (1.056-3.595) |
| TB (μmol/L): ≥ 34.2/< 34.2 | 0.381 | 1.330 (0.703-2.518) | ||
| ALT (IU/L): ≥ 50/< 50 | 0.664 | 1.117 (0.677-1.843) | ||
| AST (IU/L): ≥ 40/< 40 | 0.169 | 1.418 (0.862-2.333) | ||
| ALP (IU/L): ≥ 200/< 200 | 0.060 | 1.613 (0.981-2.654) | ||
| GGT (IU/L): ≥ 150/< 150 | 0.464 | 1.202 (0.735-1.967) | ||
| HBsAg: Positive/Negative | 0.791 | 0.890 (0.377-2.103) | ||
| CA19-9 (U/mL): ≥ 34/< 34 | 0.058 | 1.656 (0.984-2.787) | ||
| Operation duration (h) | ||||
| 2-4/≤ 2 | 0.803 | 1.085 (0.572-2.057) | ||
| > 4/≤ 2 | 0.497 | 0.794 (0.408-1.545) | ||
| Liver cirrhosis: Yes/No | 0.049 | 2.008 (1.004-4.016) | 0.478 | 1.343 (0.595-3.034) |
| Hepatic atrophy: Yes/No | 0.469 | 0.833 (0.507-1.368) | ||
| Intrahepatic stenosis: Yes/No | 0.054 | 1.772 (0.989-3.176) | ||
| Extrahepatic stones: Yes/No | 0.481 | 0.810 (0.450-1.456) | ||
| Hepatectomy: Yes/No | 0.019 | 0.543 (0.326-0.904) | 0.211 | 0.692 (0.389-1.232) |
| Bilateral hepatolithiasis: Yes/No | 0.011 | 2.114 (1.183-3.775) | 0.038 | 1.965 (1.039-3.717) |
| Drainage mode | ||||
| Cholangioenterostomy/External T tube drainage | 0.292 | 0.663 (0.308-1.425) | ||
| Combined drainage/External T tube drainage | 0.325 | 1.381 (0.726-2.629) | ||
| Function of the SO | ||||
| Dysfunction/Normal | 0.760 | 1.110 (0.567-2.173) | ||
| Nonfunctional/Normal | 0.051 | 1.791 (0.997-3.219) | ||
| Resected/Normal | 0.845 | 0.905 (0.332-2.464) | ||
| Intraoperative bleeding (mL): ≥ 400/< 400 | 0.682 | 1.264 (0.412-3.883) | ||
| Blood transfusion: Yes/No | 0.053 | 1.946 (0.993-3.815) | ||
| TB after operation (μmol/L): ≥ 34.2/< 34.2 | 0.908 | 1.035 (0.576-1.862) | ||
| Bile culture: Positive/Negative | 0.384 | 1.255 (0.753-2.093) | ||
| Clavien-Dindo classification: ≥ III/< III | 0.541 | 1.430 (0.455-4.494) | ||
| Hospitalization expenses | 0.913 | 1.000 (1.000-1.000) | ||
| Immediate clearance: No/Yes | < 0.001 | 3.271 (1.874-5.711) | 0.005 | 2.398 (1.304-4.409) |
| Final clearance: No/Yes | 0.030 | 3.098 (1.117-8.595) | 0.558 | 1.448 (0.420-4.996) |
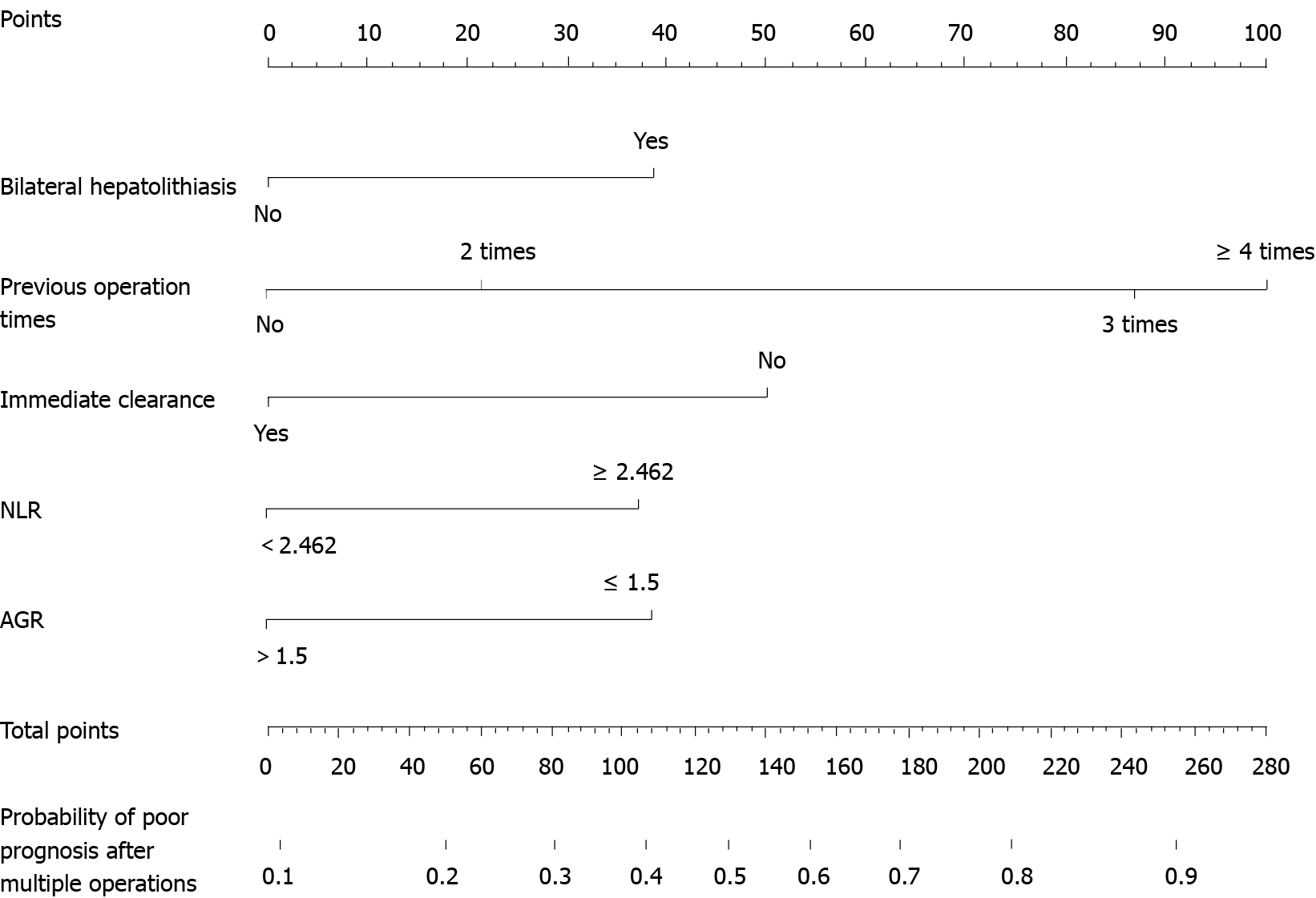
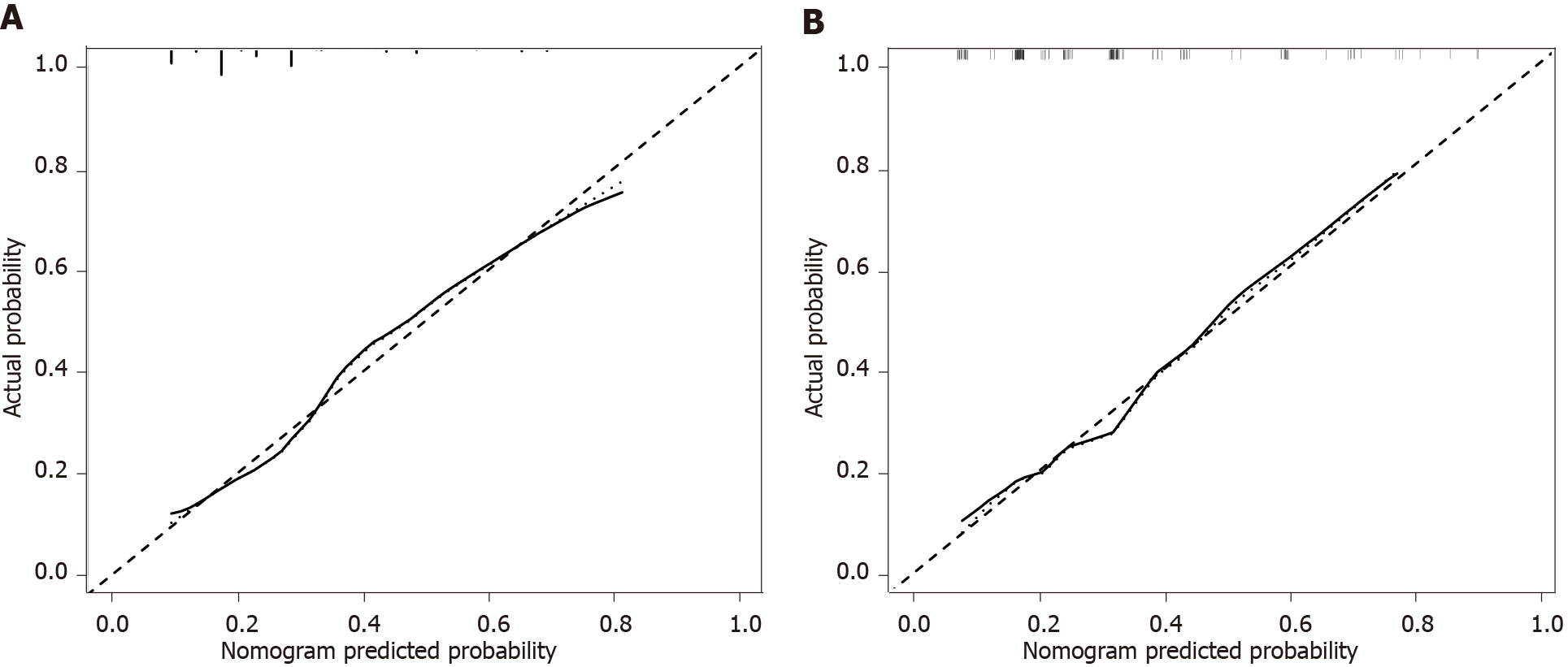
The independent risk factors associated with prognosis were then used to construct a nomogram for estimating the risk of poor prognosis (Figure 1). The nomogram demonstrated good predictive performance in estimating the risk of poor prognosis after reoperation for recurrent hepatolithiasis [C-index, 0.748; 95% confidence interval (CI): 0.687-0.810] in the training cohort and 0.743 (95%CI: 0.654-0.832 in the validation cohort). The constructed model was internally validated using the bootstrap validation method (n = 1000) to reduce the overfitting bias, and the calibration plots in the internal and external validations demonstrated good consistency between the observed and predicted probabilities. The predicted curves approximately overlapped with the reference curves, indicating good performance of the nomograms in both cohorts (Figure 2). The Brier score for overall performance, which assesses the difference between the observed and predicted values, was 0.175 (values closer to 0 indicated better predictive ability). The calibration slope, which assesses the agreement between the observed and predicted values, was 1.0 (values closer to 1 indicate better performance)[16]. An online calculator was developed, and the nomograms are freely available at https://ahmuptt.shinyapps.io/DynNomapp/ for prognosis.
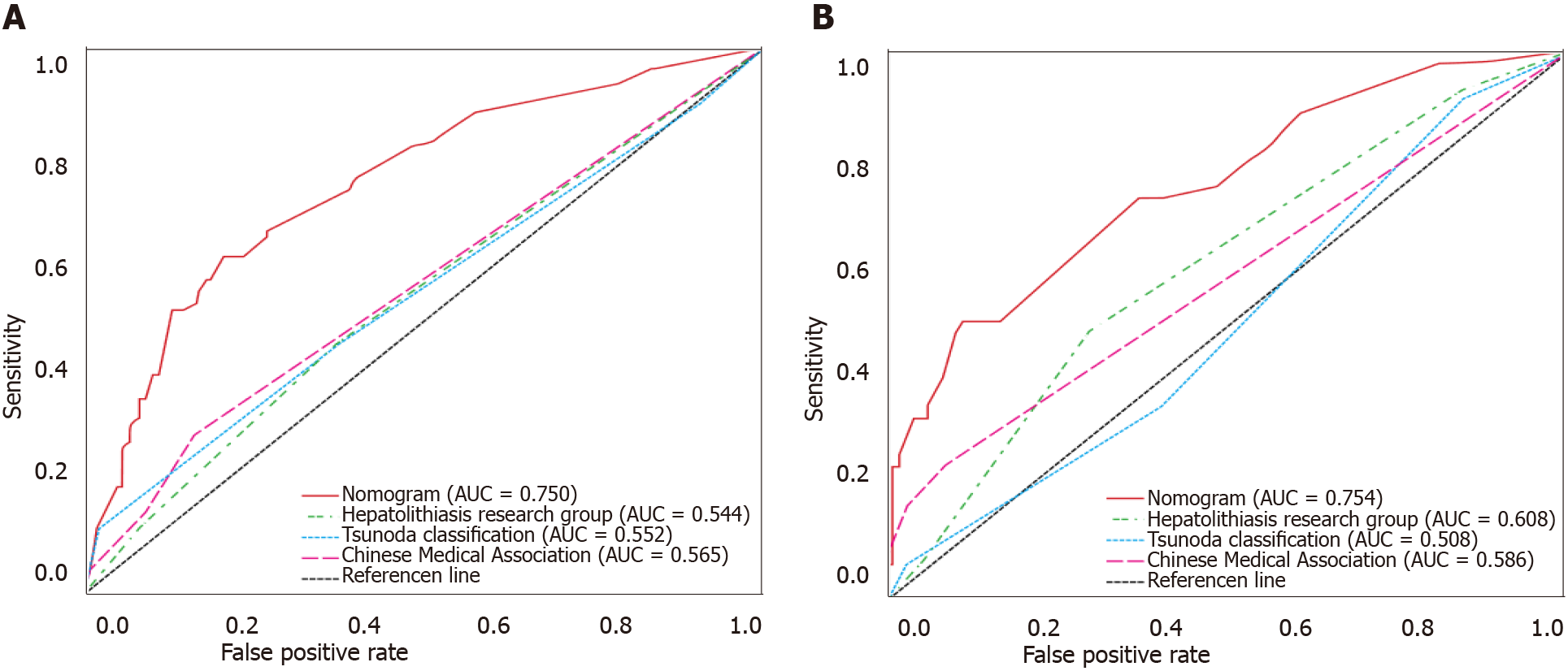
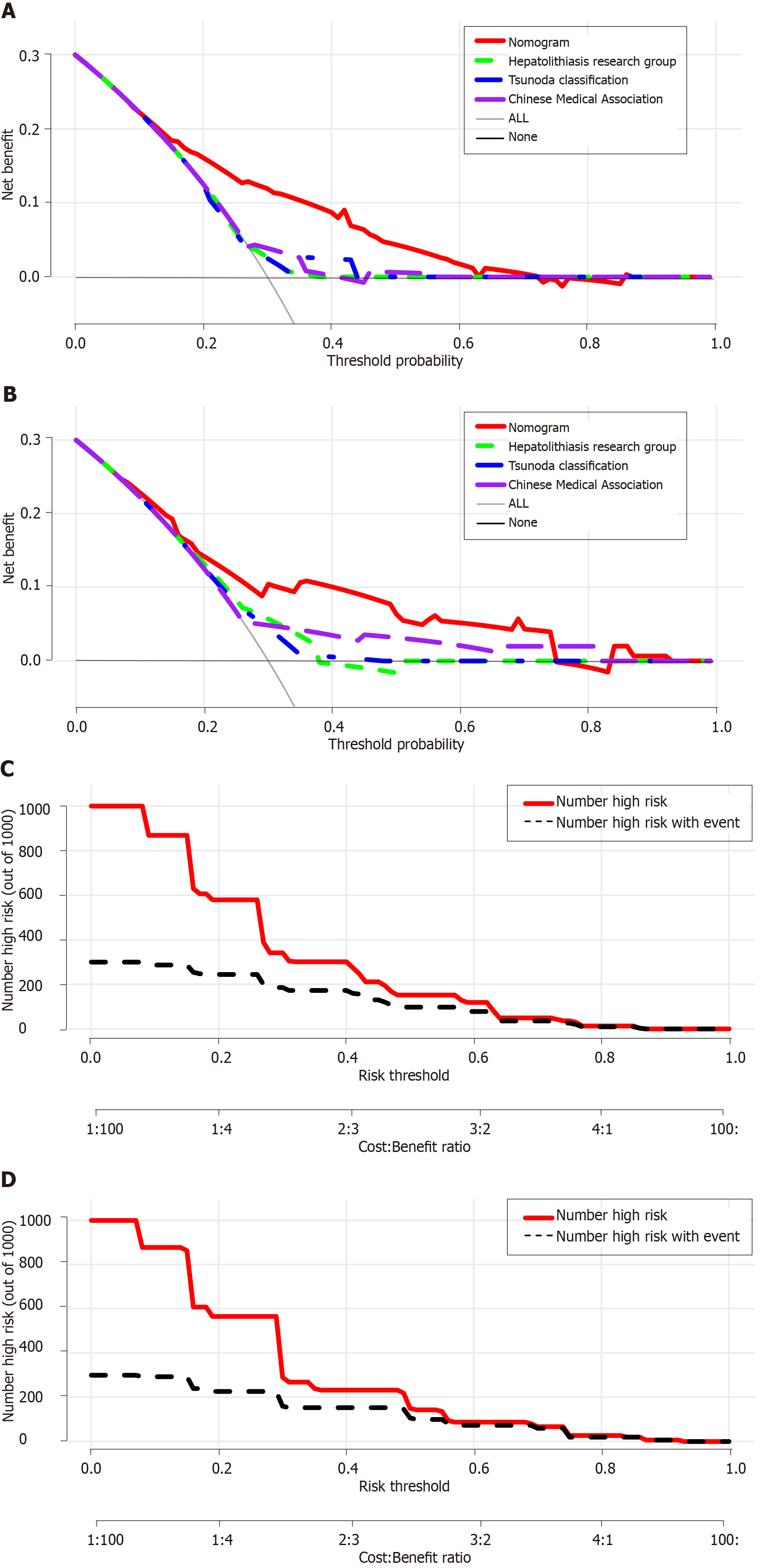
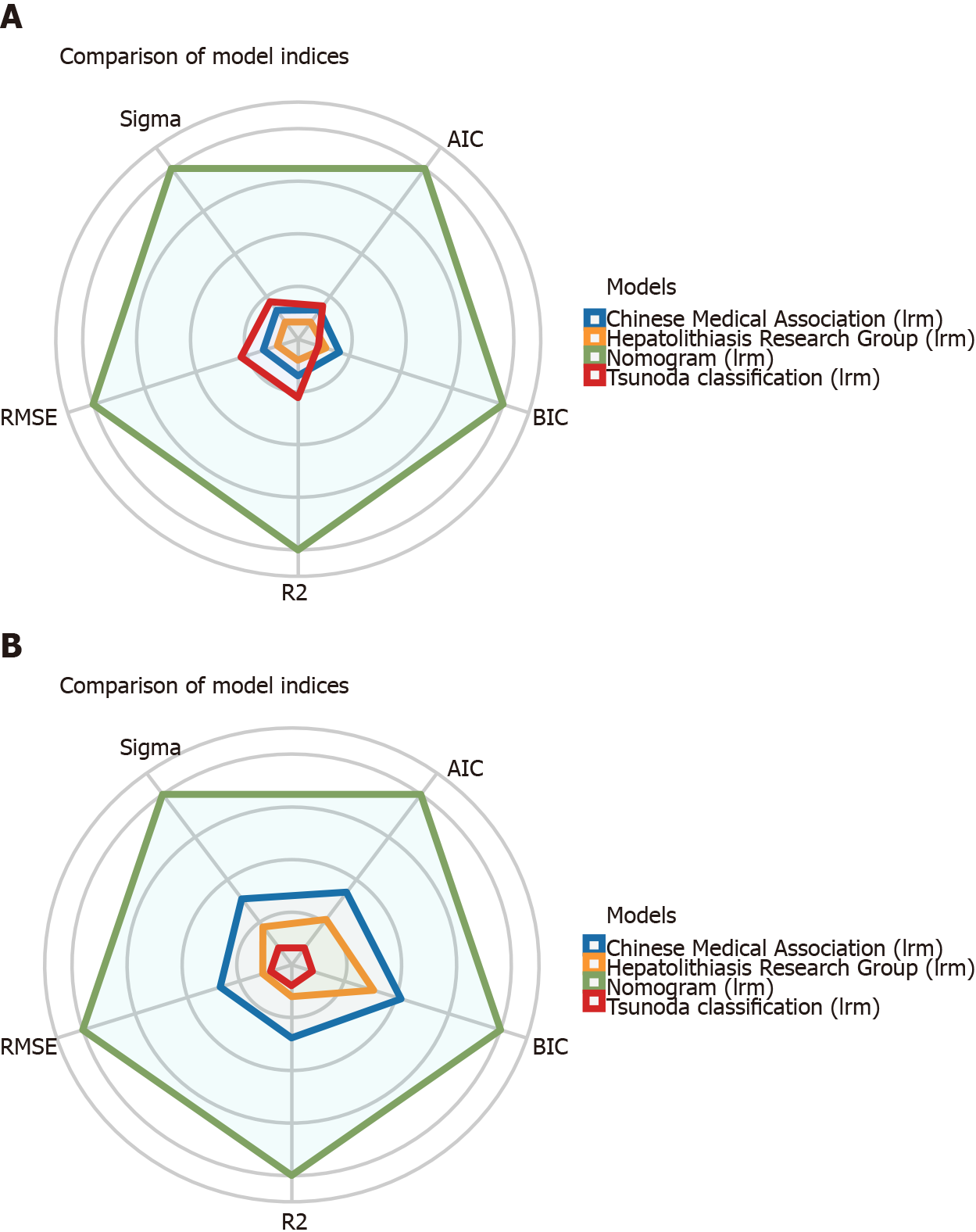
The discriminative performance of the three traditional classification models (Hepatolithiasis Research Group, Tsunoda classification, and Chinese Medical Association) were compared with that of the nomogram established in this study through ROC analyses (Figure 3). The AUC of the nomogram and the three classification models were 0.750, 0.544, 0.552, and 0.565 in the training cohort and 0.754, 0.608, 0.508, and 0.586 in the validation cohort, respectively. Thus, the nomogram showed better accuracy in predicting the prognosis for recurrent hepatolithiasis after reoperation. The optimal cutoff value of the nomogram total score was 77.5 in the ROC curve considering the maximum Youden index value, and the sensitivity and specificity for differentiating between good and poor prognoses were 62.0% and 79.2%, respectively. Using this cutoff value, patients with total nomogram scores of < 77.5 points or ≥ 77.5 points were classified as having a low or high risk of poor prognosis. In Figure 4, DCA graphically shows that the use of the nomogram to predict prognosis when the threshold probability ranged from 0.2 to 0.6 added more net benefit than the other three traditional classifications. The clinical impact curves of the nomogram indicated that the models had remarkable predictive power. Finally, in comparison with the other three traditional classifications, the fit indices of the nomogram are also visually reported in Figure 5.
Our study indicated that for patients with recurrent hepatolithiasis following multiple biliary tract surgeries, multiple previous surgeries, bilateral hepatolithiasis, failure to clear the stones immediately, preoperative NLR ≥ 2.462, and preoperative AGR ≤ 1.5 were significant predictors. These factors combined the patients’ medical history, preoperative imaging and serological data, and intraoperative outcomes to comprehensively quantify the prognosis of patients in a concise and intuitive manner. Multiple validation methods also indicated that the model had sufficient statistical power to predict the prognosis. Moreover, considering the inconvenience of traditional nomograms for clinical use, an online version of the nomogram was built, which could be easily accessed using computers, smartphones, or other mobile devices, thereby greatly improving clinical practicability.
Reoperation remains the preferred treatment for patients with recurrent hepatolithiasis. Satisfactory stone-clearance rates can be achieved through comprehensive preoperative evaluation, meticulous intraoperative exploration, and postoperative T-tube angiography with or without choledochoscopy. All patients in this study showed recurrent hepatolithiasis after biliary tract surgery. The immediate and final clearance rates were 78.0% and 95.2%, respectively, which were lower than those reported in previous studies that did not distinguish between primary and recurrent hepatolithiasis[17-19].
In addition to requiring traumatic wounds, repeated surgeries impose an enormous psychological and economic burden on patients and their families. Moreover, consi-dering the difficulty in guaranteeing the prognosis, patients will inevitably blame the surgeon, raising the possibility of doctor-patient conflict. Thus, accurate evaluation of the condition of patients with recurrent hepatolithiasis and provision of references for clinical efficacy are essential. However, the existing classification models for hepatolithiasis cannot describe the curative effect of prospective evaluation. In this study, we collected clinical and follow-up data of patients who underwent reoperation for recurrent hepatolithiasis at four large hepatobiliary centers and established and validated a nomogram model based on multicenter data. To the best of our know-ledge, this is the first study to establish a predictive model for prognosis follow-ing multiple biliary tract surgeries in patients with recurrent hepatolithiasis after initial biliary surgery.
The nomogram clearly showed that the risk of a worse prognosis increased with the number of previous surgeries. The serious abdominal adhesions caused by repeated surgeries and the resultant disconnection and anastomosis of the tube will lead to complicated intraoperative conditions, making it difficult to excise the lesion and clear the stones accurately and increasing the possibility of a poor prognosis. Moreover, the nomogram showed that the model score for cases with three previous surgeries (67 points) was much higher than that for cases with two previous surgeries (33 points), suggesting that three previous surgeries significantly increased the possibility of a poor prognosis. Thus, in patients with an extended surgical history for the treatment of hepatolithiasis, a curative procedure and good quality of life are difficult to achieve, and such patients may experience prolonged disease in addition to the tremendous economic pressure caused by the repeated surgeries. Therefore, the benefits and disadvantages of repeat surgeries for patients should be weighed with care. We propose that conservative or non-open surgical treatment should be considered as the first choice of treatment, with conventional open surgery considered the second choice, for patients with a total nomogram score of > 77.5 or patients who meet the following criteria: (1) ≥ 3 previous surgeries; (2) No obvious bile duct stenosis on preoperative imaging examinations; (3) No suspicious malignant liver-occupying sites; and (4) No obvious jaundice or cholangitis.
In recent years, newer interventional therapies such as percutaneous transhepatic choledochoscopic lithotripsy (PTCSL) have been attempted by an increasing number of surgeons. Since its development in the 1970s[20], PTCSL has undergone major advancements and shows an ideal effect when combined with 3D visualization techno-logy[21,22]. One study reported that PTCSL could be performed in patients with biliary strictures and yielded an optimal effect[23]. However, since most hepatobiliary surgeons have not gained expertise in this new technique, patients undergoing PTCSL were not included in this study for comparison. Through continuous learning, we hope to conduct prospective studies in the future to verify and enrich the pre-diction model of the nomogram in our study.
The treatment of bilateral hepatolithiasis is more complicated and difficult than that of unilateral hepatolithiasis: Intraoperative lithotomy is more difficult, and the postoperative residual stone rate is higher[19]. Moreover, even if liver resection is performed, it is difficult to avoid residual stones on the opposite side of the liver, resulting in a higher probability of poor prognosis or recurrence. Many studies have confirmed these outcomes[18,24,25]. Hepatectomy on the severe side combined with choledochoscopic lithotripsy is a better treatment for bilateral hepatolithiasis with or without intrahepatic biliary strictures[18,19]. Due to the repeated stone stimulation and attacks of cholangitis, the affected hepatic segments are usually damaged, atrophied, or narrowed, while the unaffected hepatic segments may show compen-satory hyperplasia. Anatomical hepatectomy is a crucial factor in the treatment of hepatolithiasis[18,26,27]. In addition, for bilateral hepatolithiasis, the use of three-dimensional reconstruction has been shown to improve the immediate clearance rate (96.1% vs 81%) and the final clearance rate (100% vs 90.5%)[28].
The nomogram also included two laboratory indicators, NLR and AGR. As common indicators of immune function and inflammation, NLR and AGR have been used to determine the prognosis of various benign and malignant diseases[29-32]. AGR reflects the degree of inflammation as well as the nutritional status of the human body, which can form a vicious cycle and promote disease development[33]. A reduction or inversion of AGR, which also appears in cirrhosis and chronic hepatitis, indicates serious liver damage. A previous study indicated that elevated NLR is an independent risk factor for secondary intrahepatic cholangiocarcinoma (ICC) after surgery for hepatolithiasis[34], but the association between elevated NLR and ICC remains to be elucidated. Inflammation and subtle alterations in immune regulation may play important roles in this process[35]. We found that NLR and AGR independently affected the prognosis of patients with recurrent hepatolithiasis. Therefore, we included both in the nomogram and validated them in the validation group.
This study highlights the problem of concomitant ICC, which has been reported to occur in 2.5% of patients with intrahepatic stones[19]. To avoid the influence of malignant tumors on the prognosis of patients with hepatolithiasis during the follow-up period, patients in the training and validation groups who showed ICC at the time of hospitalization were excluded (7.7%). Moreover, cancer was regarded as one of the long-term complications of hepatolithiasis in the follow-up, and was classified as a follow-up endpoint (15 patients, 3.4%), in agreement with previous studies[19,25,34,36,37].
Our study has some limitations that merit discussion. First, although laparoscopic treatment of biliary tract stones has gradually attracted research attention, some studies have suggested that laparoscopic treatment of biliary tract stones in patients with a history of biliary tract surgery is feasible and has advantages[38,39]. In our study, only 35 and 19 patients in the training and validation groups, respectively, were completely operated by laparoscopy. We did not use laparoscopic surgery as a routine procedure because all patients included in this study had a history of biliary tract surgery, unlike the previous studies. During the actual surgeries, the abdominal cavity adhesions of patients with different surgical durations were very different. Converse-ly, a previous study reported that laparoscopic surgery and open surgery for patients with a history of biliary system surgery showed no statistically significant differences in the surgical duration, blood loss, the postoperative hospitalization duration, postoperative complications, and the calculi clearance rate[40]. Moreover, considering the majority of rural patients, economic affordability also needs to be considered. Thus, since many patients had undergone multiple biliary tract surgeries, we chose the most suitable surgical mode according to the individual patient characteristics.
Moreover, this was a retrospective study with inherent defects as a result of potential biases, and prospective validation is required to confirm the value of the findings. Since the aim of this study was to establish prognosis prediction in surgically treated recurrent hepatolithiasis patients, subsequent treatment and prognosis of patients who progressed to ICC after reoperation were not analyzed further. Lastly, the present algorithm considered only patients who underwent surgery; therefore, a selection bias is likely.
In conclusion, our study is the first to develop and validate a novel online nomogram based on independent risk factors to dynamically predict the prognosis of patients with recurrent hepatolithiasis after reoperation. The nomogram is easy to use, highly accurate, and shows excellent calibration. The nomogram demonstrated superior performance and discriminative power compared to the three traditional classifications, which can help clinicians alert people at a higher risk of poor prognosis as early as possible and provide information for designing personalized clinical treatment of different patients.
Hepatolithiasis is a refractory benign disease with high recurrence rate. Many patients have poor prognosis.
There have been no large studies of patients with hepatolithiasis, and there are no clear risk factors for prognosis in these patients.
We aimed to find the risk factors affecting the prognosis of these patients and establish a prediction model which is conducive to clinical surgical decision-making.
We collected data of hepatolithiasis patients in four large medical centers, identified independent risk factors and established nomogram. And then we used the concor-dance index, calibration, area under curve, decision curve analysis, clinical impact curves, and visual fit indices to evaluate the accuracy of the nomogram.
Multiple previous surgeries, bilateral hepatolithiasis, absence of immediate clearance, neutrophil-to-lymphocyte ratio ≥ 2.462, and albumin-to-globulin ratio ≤ 1.5 were found to be independent factors influencing the prognosis. And our nomogram has a higher predictive value than traditional classifications.
A nomogram for predicting the prognosis of patients with recurrent hepatolithiasis was established for the first time, and an online calculator was set up to help surgeons make clinical decisions.
More medical centers included, more data collection, and application of “Artificial Intelligence”.
We would like to thank Prof. Faming Pan (Department of Epidemiology and Biostatistics, School of Public Health, Anhui Medical University), who had full access to all the data in the present study, for ensuring the integrity and accuracy of the data analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dilek ON, Ker CG, Tsujinaka S S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Geng XP. Treatment strategies of hepatolithiasis based on clinical classification. Chin J Dig Surg. 2020;19:804-807. [DOI] [Full Text] |
| 2. | Harris HW, Kumwenda ZL, Sheen-Chen SM, Shah A, Schecter WP. Recurrent pyogenic cholangitis. Am J Surg. 1998;176:34-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Sakpal SV, Babel N, Chamberlain RS. Surgical management of hepatolithiasis. HPB (Oxford). 2009;11:194-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 4. | Kim HJ, Kim JS, Joo MK, Lee BJ, Kim JH, Yeon JE, Park JJ, Byun KS, Bak YT. Hepatolithiasis and intrahepatic cholangiocarcinoma: A review. World J Gastroenterol. 2015;21:13418-13431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (3)] |
| 5. | Feng X, Zheng S, Xia F, Ma K, Wang S, Bie P, Dong J. Classification and management of hepatolithiasis: A high-volume, single-center's experience. Intractable Rare Dis Res. 2012;1:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (2)] |
| 6. | Nakayama F. Intrahepatic calculi: a special problem in East Asia. World J Surg. 1982;6:802-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 7. | Tsunoda T, Tsuchiya R, Harada N, Yoshino R, Noda T, Izawa K, Yamaguchi T, Yamamoto K. Long-term results of surgical treatment for intrahepatic stones. Jpn J Surg. 1985;15:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 8. | Dong JH, Zheng SG, Chen P, Han DB. Biliary surgery group of surgery branch of the Chinese Medical Association. The guideline of diagnosis and treatment of hepatolithiasis. Chin J Dig Surg. 2007;6:156-161.. [DOI] [Full Text] |
| 9. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 2397] [Article Influence: 239.7] [Reference Citation Analysis (0)] |
| 10. | Liu FB, Wang GB, Luo YZ, Zhao YJ, Chen JM, Xie K, Mao CK, Geng XP. Clinical efficacy of precision liver surgery in the management of hepatolithiasis. Chin J Dig Surg. 2015;13:447-451. [DOI] [Full Text] |
| 11. | Fang CH, Liu J, Fan YF, Yang J, Xiang N, Zeng N. Outcomes of hepatectomy for hepatolithiasis based on 3-dimensional reconstruction technique. J Am Coll Surg. 2013;217:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Terblanche J, Worthley CS, Spence RA, Krige JE. High or low hepaticojejunostomy for bile duct strictures? Surgery. 1990;108:828-834. [PubMed] |
| 13. | YOUDEN WJ. Index for rating diagnostic tests. Cancer. 1950;3:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 126] [Reference Citation Analysis (0)] |
| 14. | Lüdecke D, Ben-Shachar MS, Patil I, Waggoner P, Makowski D. Performance: an R package for Assessment, Comparison and Testing of Statistical Models. J Open Source Softw. 2021;6. [DOI] [Full Text] |
| 15. | Vickers AJ, Cronin AM, Elkin EB, Gonen M. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inform Decis Mak. 2008;8:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 596] [Cited by in RCA: 993] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 16. | Semenkovich TR, Yan Y, Subramanian M, Meyers BF, Kozower BD, Nava R, Patterson GA, Kreisel D, Puri V. A Clinical Nomogram for Predicting Node-positive Disease in Esophageal Cancer. Ann Surg. 2021;273:e214-e221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 17. | Uenishi T, Hamba H, Takemura S, Oba K, Ogawa M, Yamamoto T, Tanaka S, Kubo S. Outcomes of hepatic resection for hepatolithiasis. Am J Surg. 2009;198:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Li SQ, Liang LJ, Peng BG, Hua YP, Lv MD, Fu SJ, Chen D. Outcomes of liver resection for intrahepatic stones: a comparative study of unilateral vs bilateral disease. Ann Surg. 2012;255:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Yang T, Lau WY, Lai EC, Yang LQ, Zhang J, Yang GS, Lu JH, Wu MC. Hepatectomy for bilateral primary hepatolithiasis: a cohort study. Ann Surg. 2010;251:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 20. | Takada T, Suzuki S, Nakamura M, Uchida Y, Takemoto T. Percutaneous transhepatic cholangioscopy as a new approach to the diagnosis of the biliary diseases. Gastroenterol Endosc. 2011;16. [DOI] [Full Text] |
| 21. | Fang CH, Li G, Wang P, Fan YF, Zhong SZ. Computer-aided rigid choledochoscopy lithotripsy for hepatolithiasis. J Surg Res. 2015;195:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Guan T, Fang C, Mo Z, Xiang N, Yang J, Zeng N. Long-Term Outcomes of Hepatectomy for Bilateral Hepatolithiasis with Three-Dimensional Reconstruction: A Propensity Score Matching Analysis. J Laparoendosc Adv Surg Tech A. 2016;26:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Huang MH, Chen CH, Yang JC, Yang CC, Yeh YH, Chou DA, Mo LR, Yueh SK, Nien CK. Long-term outcome of percutaneous transhepatic cholangioscopic lithotomy for hepatolithiasis. Am J Gastroenterol. 2003;98:2655-2662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Otani K, Shimizu S, Chijiiwa K, Ogawa T, Morisaki T, Sugitani A, Yamaguchi K, Tanaka M. Comparison of treatments for hepatolithiasis: hepatic resection vs cholangioscopic lithotomy. J Am Coll Surg. 1999;189:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Chen DW, Tung-Ping Poon R, Liu CL, Fan ST, Wong J. Immediate and long-term outcomes of hepatectomy for hepatolithiasis. Surgery. 2004;135:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Jarufe N, Figueroa E, Muñoz C, Moisan F, Varas J, Valbuena JR, Bambs C, Martínez J, Pimentel F. Anatomic hepatectomy as a definitive treatment for hepatolithiasis: a cohort study. HPB (Oxford). 2012;14:604-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Jiang H, Wu H, Xu YL, Wang JZ, Zeng Y. An appraisal of anatomical and limited hepatectomy for regional hepatolithiasis. HPB Surg. 2010;2010:791625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Fang CH. Outcomes of hepatectomy for hepatolithiasis based on a medical image three-dimensional visualization system. HPB. 2016;18:e88. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 851] [Article Influence: 77.4] [Reference Citation Analysis (1)] |
| 30. | Zucker A, Winter A, Lumley D, Karwowski P, Jung MK, Kao J. Prognostic role of baseline neutrophil-to-lymphocyte ratio in metastatic solid tumors. Mol Clin Oncol. 2020;13:25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Yang H, Wang J, Li Z, Yang Y, Yang L, Zhang Y, Shi Y, Cao Y, Zhou J, Wang Z, Chen Q. Risk Factors and Outcomes of Early Relapse After Curative Resection of Intrahepatic Cholangiocarcinoma. Front Oncol. 2019;9:854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 32. | Shimizu T, Ishizuka M, Suzuki T, Tanaka G, Park KH, Matsumoto T, Shiraki T, Sakuraoka Y, Kato M, Aoki T, Kubota K. The preoperative globulin-to-albumin ratio, a novel inflammation-based prognostic system, predicts survival after potentially curative liver resection for patients with hepatocellular carcinoma. J Surg Oncol. 2017;116:1166-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Xiao S, Feng F, Liu N, Liu Z, Guo Y, Lian X, Zhang H. Preoperative Albumin Level Is Superior To Albumin-Globulin Ratio As A Predicting Indicator In Gastric Cancer Patients Who Underwent Curative Resection. Cancer Manag Res. 2019;11:9931-9938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Shen H, Zhang S, Xia Y, Chen C, Huo L, Gan L, Li J, Wang K, Pawlik TM, Lau WY, Wu M, Shen F. A Nomogram in Predicting Risks of Intrahepatic Cholangiocarcinoma After Partial Hepatectomy for Hepatolithiasis. J Gastrointest Surg. 2021;25:2258-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Grenader T, Waddell T, Peckitt C, Oates J, Starling N, Cunningham D, Bridgewater J. Prognostic value of neutrophil-to-lymphocyte ratio in advanced oesophago-gastric cancer: exploratory analysis of the REAL-2 trial. Ann Oncol. 2016;27:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 36. | Nuzzo G, Clemente G, Giovannini I, De Rose AM, Vellone M, Sarno G, Marchi D, Giuliante F. Liver resection for primary intrahepatic stones: a single-center experience. Arch Surg. 2008;143:570-3; discussion 574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Lee TY, Chen YL, Chang HC, Chan CP, Kuo SJ. Outcomes of hepatectomy for hepatolithiasis. World J Surg. 2007;31:479-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Chen B, Hu SY, Wang L, Wang KX, Zhang GY, Zhang HF. Reoperation of biliary tract by laparoscopy: a consecutive series of 26 cases. Acta Chir Belg. 2007;107:292-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Li L, Cai X, Mou Y, Wei Q. Reoperation of the biliary tract by laparoscopy: an analysis of 39 cases. J Laparoendosc Adv Surg Tech A. 2008;18:687-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Tian J, Li JW, Chen J, Fan YD, Bie P, Wang SG, Zheng SG. The safety and feasibility of reoperation for the treatment of hepatolithiasis by laparoscopic approach. Surg Endosc. 2013;27:1315-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |