Published online Feb 14, 2022. doi: 10.3748/wjg.v28.i6.665
Peer-review started: June 20, 2021
First decision: July 14, 2021
Revised: July 27, 2021
Accepted: January 19, 2021
Article in press: January 19, 2022
Published online: February 14, 2022
Processing time: 233 Days and 12.2 Hours
Several risk scores have been developed to predict hepatocellular carcinoma (HCC) risk in chronic hepatitis B (CHB) patients. The majority of risk scores are based on pretreatment variables that are no longer considered risk factors for HCC development due to the suppression of hepatitis B virus replication early in the course of potent antiviral treatment in most patients. The PAGE-B score, which is based on platelet levels, age and sex, has been shown to accurately predict HCC risk in CHB patients on antiviral treatment in various populations.
We aimed to evaluate the PAGE-B score in predicting HCC risk in Turkish CHB patients on antiviral treatment.
In this study, we recruited 742 CHB patients who had been treated with tenofovir disoproxil fumarate or entecavir for ≥ 1 year. Risk groups were determined according to the PAGE-B scores as follows: ≤ 9, low; 10-17, moderate and ≥ 18, high. The cumulative HCC incidences in each risk group were computed using Kaplan-Meier analysis and were compared using the log-rank test. The accuracy of the PAGE-B score in predicting HCC risk was evaluated using a time-dependent area under the receiver operating characteristic (AUROC) curve at all study time points. Univariate and multivariate logistic regression analyses were used to assess the risk factors for HCC development.
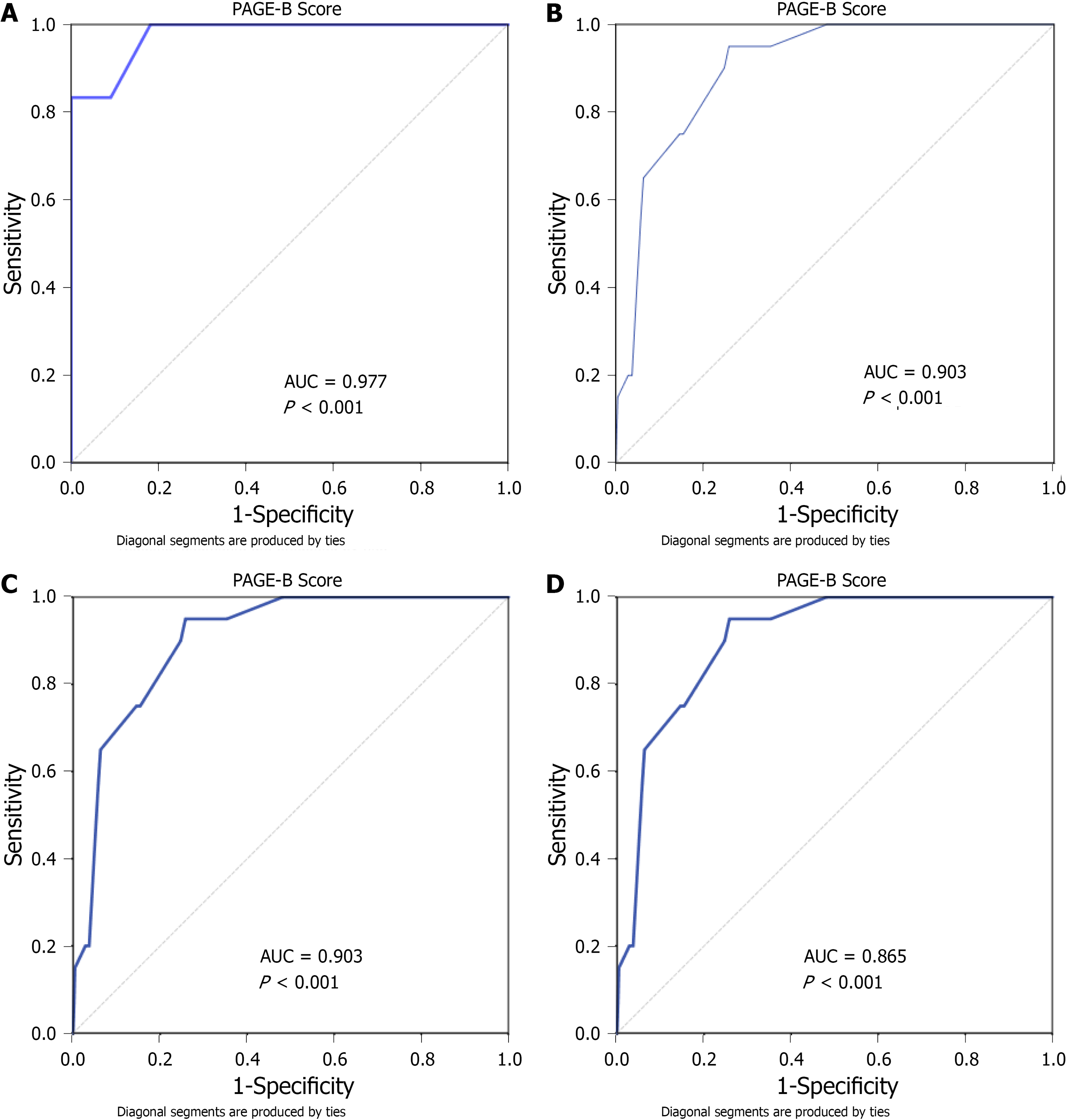
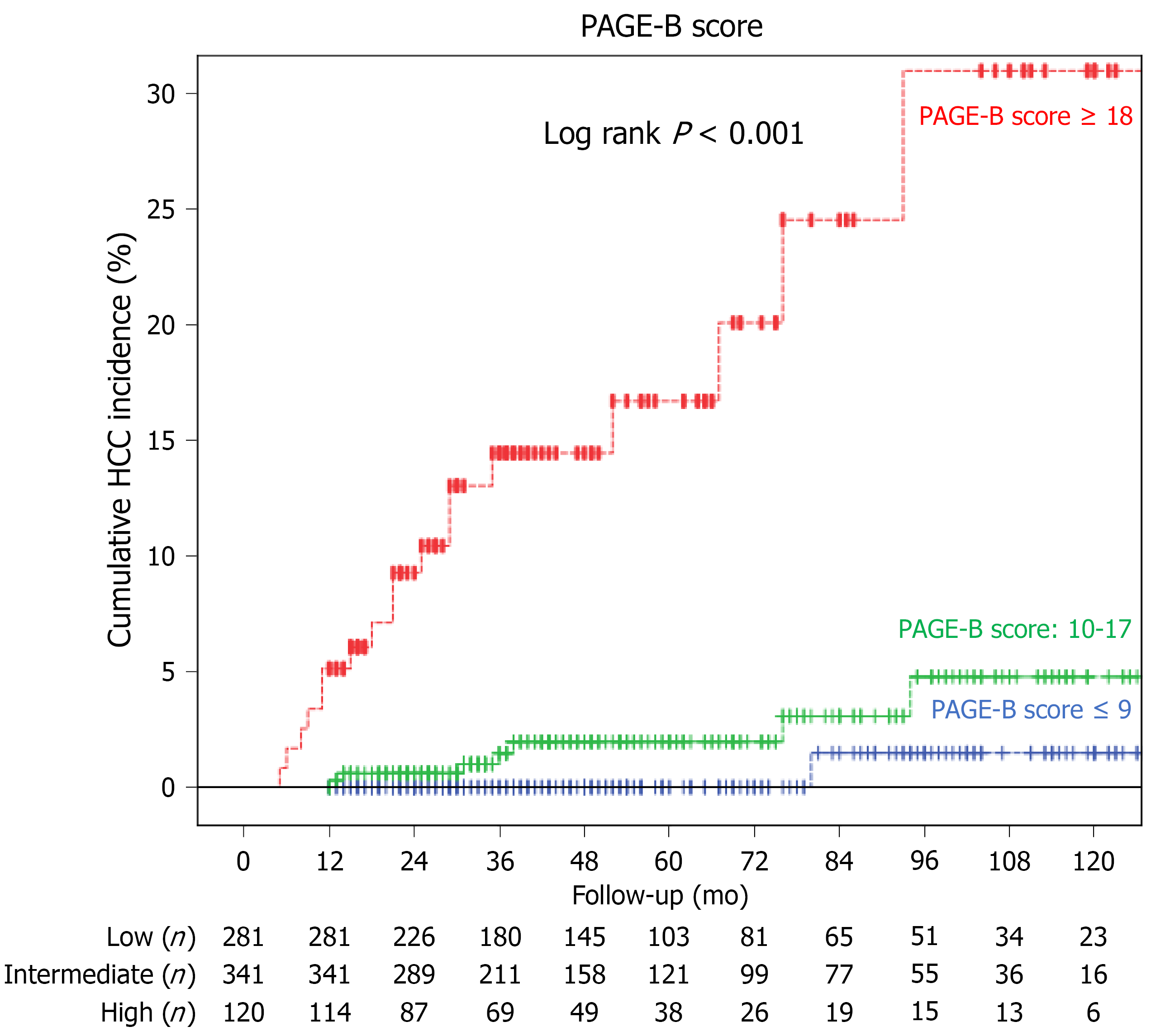
The mean follow-up time was 54.7 ± 1.2 mo. HCC was diagnosed in 26 patients (3.5%). The cumulative HCC incidences at 1, 3, 5 and 10 years were 0%, 0%, 0% and 0.4% in the PAGE-B low-risk group; 0%, 1.2%, 1.5% and 2.1% in the PAGE-B moderate-risk group; and 5%, 11.7%, 12.5%, and 15% in the PAGE-B high-risk group, respectively (log-rank P < 0.001). The AUROCs of the PAGE-B score in the prediction of HCC development at 1, 3, 5 and 10 years were 0.977, 0.903, 0.903 and 0.865, respectively. In the multivariable analysis, older age, male sex, lower platelet levels, presence of cirrhosis, and absence of alanine aminotransferase normalization at month 6 were associated with HCC development (all P < 0.05).
The PAGE-B score is a practical tool to predict HCC risk in Turkish patients with CHB and may be helpful to improve surveillance strategies.
Core Tip: We evaluated the accuracy of the PAGE-B score in predicting hepatocellular carcinoma (HCC) risk in Turkish patients with chronic hepatitis B on antiviral treatment. The cumulative HCC incidences at 5 and 10 years were 0% and 0.4%, 1.5% and 2.1%, and 12.5% and 15.0% in the low-, moderate- and high-risk groups based on the PAGE-B score, respectively. The area under the receiver operating characteristics of the PAGE-B score in the prediction of HCC risk at 5 and 10 years were 0.903 and 0.865, respectively. The PAGE-B score was found to be highly negative predictive and reliable for a cutoff value of ≤ 9 in predicting HCC development.
- Citation: Gokcen P, Guzelbulut F, Adali G, Degirmenci Salturk AG, Ozturk O, Bahadir O, Kanatsiz E, Kiyak M, Ozdil K, Doganay HL. Validation of the PAGE-B score to predict hepatocellular carcinoma risk in caucasian chronic hepatitis B patients on treatment. World J Gastroenterol 2022; 28(6): 665-674
- URL: https://www.wjgnet.com/1007-9327/full/v28/i6/665.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i6.665
Hepatocellular carcinoma (HCC) is the 7th most prevalent among all cancers and ranks 4th in cancer-related mortality[1]. Chronic hepatitis B (CHB) virus infection affects 257 million people worldwide and is one of the most common etiologies of HCC, accounting for 33% of HCC-related mortality[2]. Nucleos(t)ide analogs suppress hepatitis B virus (HBV) replication in most patients; however, the risk of HCC persists even in patients with suppressed viral replication. Treatment options for advanced-stage HCC are quite limited, and the 5-year survival rate is 18.1%[3]. Therefore, identifying patients who are at high risk for HCC development and detecting tumors at early stages are crucial. Recent guidelines recommend HCC surveillance with ultrasound (USG) twice a year in patients who are at high risk for HCC development[4]. However, not all patients with CHB have the same risk for HCC. There is an ongoing need for a scoring system to predict HCC risk that offers an easy application in clinical practice and a high predictive value to perform effective surveillance in high-risk patients and eliminate unnecessary surveillance in low-risk patients.
Various risk scores have been developed to identify CHB patients at high risk for HCC development. However, many of the risk scores for HCC have focused on untreated patients, and they are mostly based on pretreatment risk factors for HCC, such as hepatitis B e antigen (HBeAg) status, serum HBV DNA, alanine aminotransferase (ALT), albumin and bilirubin levels[5,6]. Most of the baseline virological factors are no longer considered risk factors for HCC, as HBV replication is suppressed early in the majority of patients receiving potent antiviral treatment. Therefore, risk scores that include parameters not easily modified by treatment are needed. Among these, the PAGE-B is a practical risk score that includes platelet count, age and sex and has been validated in various patient populations[7-9]. In this study, we aimed to evaluate the accuracy of the PAGE-B score in predicting HCC risk in Turkish CHB patients on tenofovir disoproxil fumarate (TDF) or entecavir (ETV) therapy.
Medical records of CHB patients who were on follow-up in hepatology outpatient clinics at Umraniye Training and Research Hospital (Istanbul, Turkey) and Haydarpaşa Numune Training and Research Hospital (Istanbul, Turkey) between January 2007 to December 2018 were retrospectively evaluated. The inclusion criteria were as follows: Age ≥ 16 years, HBsAg positivity for ≥ 6 mo, and treatment with TDF or ETV for at least 12 mo. The exclusion criteria were as follows: Age < 16 years, decompensated cirrhosis, having HCC diagnosis before or during the first 6 mo of therapy, history of liver transplantation, and coinfection with hepatitis C virus, hepatitis D virus or human immunodeficiency virus.
Laboratory tests, including HBeAg, anti-HBe, HBV DNA, aspartate aminotransferase, ALT, albumin, bilirubin and alpha-fetoprotein (AFP) levels, international normalized ratio, and complete blood count at the start of therapy and during follow-up at 3-6 mo intervals, were recorded. The results of imaging studies, e.g., USG, triphasic computed tomography (CT) and dynamic contrast-enhanced magnetic resonance imaging (MRI), at the start of therapy and during follow-up were recorded. The presence of comorbidities and liver biopsy results, if available, were also recorded. Virological response was defined as a serum HBV DNA level < 80 IU/mL. Maintained virological response was defined as serum HBV DNA negativity without subsequent positivity. A biochemical response was achieved when the serum ALT level dropped below 42 U/L. Hepatic flare was defined as an elevation of ALT ≥ 2 × upper limit of normal with subsequent HBV DNA positivity in patients with virological response. Liver biopsies were evaluated according to the ISHAK staging system[10]. Patients with pretreatment fibrosis scores between 0 and 4 (F0-4) were considered noncirrhotic. Patients with fibrosis scores 5 and 6 (F5-6) or those with radiological (nodular appearance of liver surface, parenchymal thickening, caudate lobe enlargement, portal vein diameter > 13 mm) or endoscopic (varices, portal gastropathy) findings of cirrhosis were considered compensated cirrhotic. Decompensated cirrhosis was defined as the presence of ascites, variceal bleeding or hepatic encephalopathy. Patients underwent HCC surveillance with abdominal USG at 6-12 mo intervals. In the presence of suspicious lesions on USG, cross-sectional imaging with triphasic CT and/or dynamic contrast-enhanced MRI were performed. A diagnosis of HCC was made following the current guidelines[5]. The PAGE-B score included the parameters of platelet count, age, and sex. Scoring was performed as follows: (1) For age 16-29 years, 0 points; 30-39 years, 2 points; 40-49 years, 4 points; 50-59 years, 6 points; 60-69 years, 8 points; ≥ 70 years, 10 points; (2) For female gender, 0 points and for male gender, 6 points; and (3) For platelet count (/mm³) ≥ 200000, 0 points; 100000-199999, 6 points; < 100000, 9 points. The score ranged from 0-25 points. Based on their PAGE-B scores, patients were classified as ≤ 9, low risk; 10-17, moderate risk; and ≥ 18, high risk[7].
Statistical data were analyzed using SPSS v.23.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics are presented as the mean ± standard error of the mean for continuous variables. Variables were tested for normality using the Kolmogorov-Smirnov test. Independent t-tests were used to compare parametric variables, and chi-squared tests, continuity correction, or Fisher’s exact tests were used to compare categorical variables. The cumulative effect of PAGE-B risk groups on survival was assessed using the log-rank test. Survival rates were computed by Kaplan-Meier survival analysis. Accuracy in predicting HCC occurrence was evaluated using a time-dependent area under the receiver operating characteristic (AUROC) curve at all study time points. Univariate and multivariate logistic regression analysis models were used to determine the effects of the variables on the risk of developing HCC. Cirrhosis and platelet count were analyzed separately in logistic regression model as they showed collinearity. Tests were interpreted at a 95% confidence interval. A P value ≤ 0.05 was considered statistically significant.
The study was approved by the local ethics committees of Umraniye Training and Research Hospital and Haydarpasa Numune Training and Research Hospital.
A total of 742 patients were enrolled in the study. The mean age was 45.0 ± 0.5 (17-93) years, and 472 (63.6%) patients were male. One hundred and sixty-one patients (21.7%) had cirrhosis. Of the total patients, 502 (67.7%) received TDF, and 240 (32.3%) received ETV. One hundred and sixty-two (21.8%) of patients were lamivudine-experienced. At month 12, 597 patients (85.4%) achieved virological response, and 620 patients (85.9%) achieved ALT normalization. Twenty-five (3.4%) patients had hepatic flare. The mean follow-up time was 54.7 ± 1.2 (5-145) mo. The demographic and clinical characteristics and follow-up data of the patients are presented in Table 1.
| n = 742 | |
| Age, yr ± SE | 45.0 ± 0.5 |
| Gender, male n (%) | 472 (63.6) |
| Follow-up, mo ± SE | 54.7 ± 1.2 |
| Diabetes mellitus, n (%) | 116 (15.7) |
| HBeAg positivity, n (%) | 171 (23.0) |
| Cirrhosis, n (%) | 161 (21.7) |
| NA(s) before ETV/TDF, n (%) | 162 (21.8) |
| Antiviral treatment (ETV/TDF), n (%) | 240 (32.3)/502(67.7) |
| MVR, n (%) | 633 (85.3) |
| Hepatic flare, n (%) | 25 (3.4) |
| ALT normalization at 6 mo, n (%) | 620 (85.9) |
| Virological response at 6 mo, n (%) | 597 (85.4) |
| PAGE-B score ± SE | 11.1 ± 0.2 |
| PAGE-B score-risk groups, n (%) | |
| Low | 281 (37.9) |
| Moderate | 341 (46) |
| High | 120 (16.2) |
| HCC cases during follow up, n (%) | 26 (3.5) |
During the follow-up period, 26 patients (3.5%) developed HCC. Patients who developed HCC were older, male predominant, had lower albumin and platelet levels, and had higher AFP levels than those who did not develop HCC (all P < 0.05). Cirrhosis and diabetes mellitus were more common in patients who developed HCC than in those who did not develop HCC (both P < 0.05) (Table 2).
| Patients with HCC | Patients without HCC | P value | |
| Age (yr) mean ± SE | 57.8 ± 2.3 | 44.5 ± 0.5 | < 0.001 |
| Male gender, n (%) | 24 (92.3) | 448 (62.6) | 0.004 |
| Cirrhosis, n (%) | 16 (61.5) | 145 (20.3) | < 0.001 |
| Diabetes mellitus, n (%) | 8 (32) | 108(15.1) | 0.043 |
| Antiviral treatment (ETV/TDF), n (%) | 10 (38.5)/16 (61.5) | 230 (32.1)/486 (67.9) | 0.642 |
| Laboratory (mean ± SE) | |||
| HBeAg positivity, n (%) | 6 (23.1) | 165 (23.0) | 1.000 |
| ALT (IU/L) | 92.9 ± 25.7 | 98.3 ± 5.7 | 0.856 |
| Albumin (g/dL) | 3.9 ± 0.1 | 4.1 ± 0.0 | 0.014 |
| Total bilirubin (mg/dL) | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.709 |
| AFP (ng/mL) | 23.3 ± 9.6 | 5.2 ± 0.4 | < 0.001 |
| INR | 1.1 ± 0.0 | 1.1 ± 0.0 | 0.143 |
| Platelet (10³/mL) | 128.8 ± 8.6 | 203.5 ± 2.5 | < 0.001 |
| HBV-DNA (log IU/mL) | 5.4 ± 0.3 | 5.5 ± 0.1 | 0.859 |
In the univariable analysis, older age, male sex, lower platelet levels, presence of diabetes mellitus, presence of cirrhosis, absence of ALT normalization at month 6, and pretreatment AFP levels were associated with HCC development (all P < 0.05). HCC was not detected in any patients with hepatic flare. In the multivariable analysis, older age [odd ratio (OR) = 1.1; 95% confidence interval (CI): 1.0-1.1], male sex (OR = 8.9; 95%CI: 1.1-70.7), lower platelet levels (OR = 1.0; 95%CI: 1.0-1.0), presence of cirrhosis (OR = 3.1; 95%CI: 1.1-8.2), and absence of ALT normalization at month 6 (OR = 0.2; 95%CI: 0.1-0.7) were associated with HCC occurrence (all P < 0.05) (Table 3).
| Univariate analysis, OR (95% CI) | P value | Multivariate analysis, OR (95% CI) | P value | |
| Age (per yr increase) | 1.1 (1.0-1.1) | < 0.001 | 1.1 (1.0-1.1) | < 0.001 |
| Gender (male vs female) | 7.2 (1.7-30.6) | 0.004 | 8.9 (1.1-70.7) | 0.038 |
| Platelet1 (103/mL) | 1.0 (1.0-1.0) | < 0.001 | 1.0 (1.0-1.0) | < 0.001 |
| AFP (ng/mL) | 1.0 (1.0-1.0) | < 0.001 | 1.0 (1.0-1.0) | 0.141 |
| HBeAg status (positive vs negative) | 1.0 (0.4-2.5) | 1.000 | ||
| Diabetes mellitus (yes vs no) | 2.7 (1.1-6.3) | 0.043 | 0.6 (0.2-1.7) | 0.308 |
| NA(s) before ETV/TDF (yes vs no) | 2.0 (0.9-4.5) | 0.143 | ||
| Cirrhosis1 (yes vs no) | 6.3 (2.8-14.2) | < 0.001 | 3.1 (1.1-8.2) | 0.026 |
| Antiviral treatment (ETV vs TDF) | 0.8 (0.3-1.7) | 0.642 | ||
| MVR (no vs yes) | 0.6 (0.2-1.4) | 0.253 | ||
| ALT normalization at month 6 (no vs yes) | 0.4 (0.2-0.9) | 0.043 | 0.2 (0.1-0.7) | 0.009 |
| ALT normalization at month 12 (no vs yes) | 0.4 (0.2-1.0) | 0.101 | ||
| Virological response at month 6 (no vs yes) | 0.8 (0.3-1.9) | 0.622 | ||
| Virological response at month 6 (no vs yes) | 0.7 (0.2-2.2) | 0.530 |
The mean PAGE-B score was 11.1 ± 0.2. According to the PAGE-B score, 281 (37.9%), 341 (46%) and 120 (16.2%) patients had low-risk, moderate-risk and high-risk of HCC development, respectively. Nineteen (6.8%), 78 (22.9%) and 64 (53.3%) patients had cirrhosis in the low-, moderate- and high-risk groups, respectively (P < 0.001). One (0.4%), 7 (2.1%) and 18 (15%) patients developed HCC in the low-, moderate- and high-risk groups, respectively (P < 0.001). For a PAGE-B score cutoff value ≤ 9, the sensitivity, specificity, positive and negative predictive values for the prediction of HCC were 96.2%, 39.1%, 5.4% and 99.6%, respectively. The AUROCs of the PAGE-B score in the prediction of HCC risk at 1, 3, 5 and 10 years were 0.977, 0.903, 0.903 and 0.865, respectively (Figure 1). The cumulative HCC incidences at 1, 3, 5 and 10 years were 0%, 0%, 0% and 0.4%, respectively, in the PAGE-B low-risk group; 0%, 1.2%, 1.5% and 2.1%, respectively, in the PAGE-B moderate-risk group; and 5.0%, 11.7%, 12.5%, and 15.0%, respectively, in the PAGE-B high-risk group (log-rank P < 0.001) (Figure 2).
The ultimate goal of CHB therapy is to extend the survival of patients by preventing progression to cirrhosis, HCC development and the need for transplantation. This objective has been achieved substantially with the widespread use of TDF and ETV, which have a high genetic barrier to resistance. However, the risk of HCC is not eliminated despite effective antiviral drugs. Various studies have aimed to evaluate the risk of HCC development in various populations using risk scores that include clinical or laboratory parameters. The REACH-B (age, sex, HBsAg status, and HBV DNA concentration) score was the first scoring system that did not include cirrhosis as a parameter, and it was associated with a 5-year HCC incidence of 2.6% in the low-risk group[11]. The GAG-HCC (age, sex, HBV DNA, core promoter mutations and cirrhosis) and CU-HCC (age, viral load, bilirubin, albumin, cirrhosis) scores have a negative predictive value of 98.3% for 5-year HCC incidence in treatment-naive Asian patients[12]. These scoring systems, which were validated in untreated patients, were less predictive when applied to patients on antiviral treatment[13]. For example, a high serum HBV DNA level, which is included in REACH-B, is no longer regarded as a risk factor for HCC with the use of potent antivirals[8]. Moreover, detection of core promoter mutations that are included in the GAG-HCC scoring system is not always possible. To solve these problems, novel scoring systems were developed in patients on treatment. The PAGE-B (age, sex and platelet count), CAGE-B (age, presence of baseline cirrhosis), SAGE-B (age, liver stiffness measurements), CAMD (cirrhosis, age, male sex, diabetes mellitus) and HCC-RESCUE (age, sex, cirrhosis) scoring systems all have high negative predictive values for HCC development in their low-risk groups[7,14-16]. Among current scoring systems, the PAGE-B is the only one that does not include cirrhosis as a parameter. The presence of cirrhosis is the most important risk factor for HCC development, and the annual risk of HCC in patients with cirrhosis is 2.5%-4%[17]. Liver biopsy is the gold standard method for the diagnosis of cirrhosis. However, biopsy is associated with certain disadvantages, such as being an invasive method that can lead to potential complications, requiring tissue samples of an appropriate amount and from an appropriate localization, and producing false-negative results in the early period. Meanwhile, noninvasive methods that assess fibrosis, such as transient elastography, can produce operator-dependent false-positive results. It should also be emphasized that liver biopsy is not performed in patients with lamivudine, adefovir or telbivudine resistance prior to the start of new antiviral agents with a high genetic barrier to resistance. Therefore, these nucleos(t)ide analog-experienced patients may not have cirrhosis at the start of rescue therapy due to the resolution of cirrhosis after years of therapy. Our cohort also included 162 patients (21.8%) who had received lamivudine and developed resistance prior to the start of TDF/ETV treatment. Therefore, definitive confirmation of cirrhosis for all patients is impractical. To that point, the PAGE-B score has an advantage compared to the other scoring systems that include cirrhosis as a parameter. Supporting this, implementing ISHAK stage in the PAGE-B score did not improve the prediction of HCC risk[18].
In the present study, the incidence of HCC was determined to be 3.5%, and the cumulative HCC incidences at 5 years in the low-, moderate-, and high-risk groups were 0%, 1.5% and 12.5%, respectively. These rates were lower than those in the PAGE-B database (0%, 3%, 17%) but higher than those in Spain’s CIBERHEP database (0%, 2.8%, 5%), which was used for the validation of the PAGE-B score. All three databases (ours, PAGE-B, CIBERHEP) had similar reliability of the PAGE-B score in the prediction of overall HCC development for a cutoff value ≥ 10[7,9]. It was thought that the lower HCC rates in CIBERHEP may be related to the relatively low total number of patients and the number of patients who completed the 5-year follow-up[9]. The present study included 267 (36%) patients who had completed the 5-year follow-up, which was similar to the PAGE-B database; however, patients were younger on average than the PAGE-B database (45 vs 52). This might be a reason for the lower HCC incidence seen in this study. Additionally, the present study included mainly genotype D patients who are known to have less risk for HCC development, while genotypes A, B, D predominated in the PAGE-B database and genotype B and D in the CIBERHEP database.
In this study, we also evaluated 10-year HCC incidence. Although we did not find any cases of HCC in the low-risk group during the first 5 years of follow-up, one patient developed HCC at month 80. This 37-year-old noncirrhotic male patient was treated with ETV, did not have comorbidities, and had a PAGE-B score of 8. The study by Brouwer et al[18] showed that the estimated HCC incidences at 10 years in PAGE-B low-, moderate-, and high-risk groups were < 1.5%, 1.5%-17.5% and ≥ 17.5%, respectively, supporting our results (0.4%, 2.1%, 15%).
Current international guidelines recommend that patients with cirrhosis should undergo HCC surveillance systematically. However, there is no consensus about noncirrhotic CHB patients[19-22]. Diverging from other guidelines, the European Association for the Study of the Liver suggests that only patients with a PAGE-B score ≥ 10 in the noncirrhotic group should be included in screening[19]. In the present study, unnecessary tests would be prevented in 281 (37.9%) patients with low-risk HCC by following this cost-effective approach.
The present study has some limitations. First, it is a retrospective study, so the effect of treatment non-compliance could not be determined precisely. Second, the patient number was relatively low. Third, only one-third of the patients completed the 5-year follow-up. Fourth, in Turkey, almost all patients are infected with genotype D virus (32), so no interpretation could be made for other genotypes.
PAGE–B successfully predicted patients who had a low risk for HCC during treatment with genetically high barrier antivirals. Ease of use without the need for biopsy or an impractical molecular test justifies implementing this score in clinical practice.
Chronic hepatitis B (CHB) infection is an important health issue worldwide. Novel antiviral treatments lead to complete suppression of the virus and maintained suppression of viral replication prevents cirrhosis, decompensation in already cirrhotic patients and hepatocellular carcinoma (HCC). However, HCC risk is not totally eliminated and in pursuance of detecting cancer in early stages comprehensive follow up is needed. It is critical to stratify patients for risk predictions, especially to prevent unnecessary tests in low-risk patients.
Various risk scores have been developed to predict the development of HCC in CHB patients. The majority of studies on the risk scores had focused on untreated patients. Currently, almost all patients with CHB are treated with antiviral agents and better risk scores for patients under treatment is needed. The PAGE-B is a risk scoring system that includes platelet count, age and sex and has been validated in patients treated with antivirals.
We aimed to evaluate the accuracy of the PAGE-B scoring system in the prediction of HCC risk in CHB patients receiving entecavir (ETV) or tenofovir disoproxil fumarat therapy.
We recruited 742 CHB patients who had been treated with tenofovir disoproxil fumarate or ETV for more than 1 year. Risk groups were determined according to the PAGE-B scores. We evaluated the accuracy of the PAGE-B score in predicting HCC.
HCC was diagnosed in 26 patients (3.5%) during 54.7 ± 1.2 mo mean follow up. The cumulative HCC incidences at 5 years were 0% in the PAGE-B low-risk group; 1.5% moderate-risk group; and 12.5%, in the high-risk group (log-rank p < 0.001). The AUROCs of the PAGE-B score in the prediction of HCC development at 5 years follow up was 0.903.
PAGE–B had successfully predicted the patients who had a low risk of HCC during treatment with genetically high barrier antivirals.
PAGE-B is a simple score that does not require biopsy or any impractical molecular test. The efficiency of PAGE-B justifies implementing this score in daily clinical practice.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Huang CF, Xu J S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018;4:1553-1568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1105] [Cited by in RCA: 1168] [Article Influence: 166.9] [Reference Citation Analysis (0)] |
| 2. | Global Burden of Disease Liver Cancer Collaboration. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol. 2017;3:1683-1691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1459] [Cited by in RCA: 1500] [Article Influence: 187.5] [Reference Citation Analysis (0)] |
| 3. | Jemal A, Ward EM, Johnson CJ, Cronin KA, Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, Anderson RN, Henley SJ, Kohler BA, Penberthy L, Feuer EJ, Weir HK. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 748] [Cited by in RCA: 1111] [Article Influence: 138.9] [Reference Citation Analysis (0)] |
| 4. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3243] [Article Influence: 463.3] [Reference Citation Analysis (1)] |
| 5. | Yang HI, Yuen MF, Chan HL, Han KH, Chen PJ, Kim DY, Ahn SH, Chen CJ, Wong VW, Seto WK; REACH-B Working Group. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol. 2011;12:568-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 521] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 6. | Wong VW, Chan SL, Mo F, Chan TC, Loong HH, Wong GL, Lui YY, Chan AT, Sung JJ, Yeo W, Chan HL, Mok TS. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clin Oncol. 2010;28:1660-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 403] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 7. | Papatheodoridis G, Dalekos G, Sypsa V, Yurdaydin C, Buti M, Goulis J, Calleja JL, Chi H, Manolakopoulos S, Mangia G, Gatselis N, Keskin O, Savvidou S, de la Revilla J, Hansen BE, Vlachogiannakos I, Galanis K, Idilman R, Colombo M, Esteban R, Janssen HL, Lampertico P. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64:800-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 393] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 8. | Kim MN, Hwang SG, Rim KS, Kim BK, Park JY, Kim DY, Ahn SH, Han KH, Kim SU. Validation of PAGE-B model in Asian chronic hepatitis B patients receiving entecavir or tenofovir. Liver Int. 2017;37:1788-1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Riveiro-Barciela M, Tabernero D, Calleja JL, Lens S, Manzano ML, Rodríguez FG, Crespo J, Piqueras B, Pascasio JM, Comas C, Gutierrez ML, Aguirre A, Suárez E, García-Samaniego J, Rivero M, Acero D, Fernandez-Bermejo M, Moreno D, Sánchez-Pobre P, de Cuenca B, Moreno-Palomares JJ, Esteban R, Buti M. Effectiveness and Safety of Entecavir or Tenofovir in a Spanish Cohort of Chronic Hepatitis B Patients: Validation of the Page-B Score to Predict Hepatocellular Carcinoma. Dig Dis Sci. 2017;62:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3521] [Cited by in RCA: 3784] [Article Influence: 126.1] [Reference Citation Analysis (1)] |
| 11. | Yang HI, Sherman M, Su J, Chen PJ, Liaw YF, Iloeje UH, Chen CJ. Nomograms for risk of hepatocellular carcinoma in patients with chronic hepatitis B virus infection. J Clin Oncol. 2010;28:2437-2444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 12. | Wong GL, Chan HL, Chan HY, Tse PC, Tse YK, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW, Leung JM, Wong VW. Accuracy of risk scores for patients with chronic hepatitis B receiving entecavir treatment. Gastroenterology. 2013;144:933-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 13. | Arends P, Sonneveld MJ, Zoutendijk R, Carey I, Brown A, Fasano M, Mutimer D, Deterding K, Reijnders JG, Oo Y, Petersen J, van Bömmel F, de Knegt RJ, Santantonio T, Berg T, Welzel TM, Wedemeyer H, Buti M, Pradat P, Zoulim F, Hansen B, Janssen HL; VIRGIL Surveillance Study Group. Entecavir treatment does not eliminate the risk of hepatocellular carcinoma in chronic hepatitis B: limited role for risk scores in Caucasians. Gut. 2015;64:1289-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 168] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 14. | Ji JH, Park SY, Son WJ, Shin HJ, Lee H, Lee HW, Lee JS, Kim SU, Park JY, Kim DY, Ahn SH, Kim BK. External validation of CAGE-B and SAGE-B scores for Asian chronic hepatitis B patients with well-controlled viremia by antivirals. J Viral Hepat. 2021;28:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Kim SU, Seo YS, Lee HA, Kim MN, Kim EH, Kim HY, Lee YR, Lee HW, Park JY, Kim DY, Ahn SH, Han KH, Hwang SG, Rim KS, Um SH, Tak WY, Kweon YO, Kim BK, Park SY. Validation of the CAMD Score in Patients With Chronic Hepatitis B Virus Infection Receiving Antiviral Therapy. Clin Gastroenterol Hepatol. 2020;18:693-699.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Güzelbulut F, Gökçen P, Can G, Adalı G, Değirmenci Saltürk AG, Bahadır Ö, Özdil K, Doğanay HL. Validation of the HCC-RESCUE score to predict hepatocellular carcinoma risk in Caucasian chronic hepatitis B patients under entecavir or tenofovir therapy. J Viral Hepat. 2021;28:826-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Mair RD, Valenzuela A, Ha NB, Ayoub WS, Daugherty T, Lutchman GA, Garcia G, Ahmed A, Nguyen MH. Incidence of hepatocellular carcinoma among US patients with cirrhosis of viral or nonviral etiologies. Clin Gastroenterol Hepatol. 2012;10:1412-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Brouwer WP, van der Meer AJP, Boonstra A, Plompen EPC, Pas SD, de Knegt RJ, de Man RA, Ten Kate FJW, Janssen HLA, Hansen BE. Prediction of long-term clinical outcome in a diverse chronic hepatitis B population: Role of the PAGE-B score. J Viral Hepat. 2017;24:1023-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6064] [Article Influence: 866.3] [Reference Citation Analysis (3)] |
| 20. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3031] [Article Influence: 433.0] [Reference Citation Analysis (3)] |
| 21. | Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1628] [Cited by in RCA: 1646] [Article Influence: 205.8] [Reference Citation Analysis (0)] |
| 22. | Kokudo N, Hasegawa K, Akahane M, Igaki H, Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y, Kudo M, Kubo S, Takayama T, Tateishi R, Fukuda T, Matsui O, Matsuyama Y, Murakami T, Arii S, Okazaki M, Makuuchi M. Evidence-based Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res. 2015;45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 320] [Article Influence: 32.0] [Reference Citation Analysis (0)] |