Published online Feb 7, 2022. doi: 10.3748/wjg.v28.i5.594
Peer-review started: October 2, 2021
First decision: November 7, 2021
Revised: December 3, 2021
Accepted: January 19, 2022
Article in press: January 19, 2022
Published online: February 7, 2022
Processing time: 114 Days and 17.9 Hours
Adult-onset Ménétrier’s disease is strongly associated with Helicobacter pylori (H. pylori) infection and an elevated risk of carcinogenesis. Cases of early-stage gastric cancer developed in H. pylori-negative Ménétrier’s disease are extremely rare. We report a case of early gastric cancer in H. pylori-negative Ménétrier’s disease that was curatively resected with endoscopic submucosal dissection (ESD).
A 60-year-old woman was referred to our hospital after her medical examination detected anemia. Contrast-enhanced upper gastrointestinal (UGI) radiography revealed translucency of the nodule-aggregating surface with giant rugae. Blood tests showed hypoproteinemia and were negative for serum H. pylori immunoglobulin G antibodies. The 99mTc-DTPA-human serum albumin scintigraphy showed protein loss from the stomach. UGI endoscopy showed a 40-mm protruding erythematous lesion on giant rugae of the greater curvature of lower gastric body, suggesting early-stage gastric cancer due to Ménétrier’s disease. En bloc resection with ESD was performed for diagnosis and treatment. Histology of ESD showed well-differentiated tubular adenocarcinoma. The cancer was confined to the mucosa, and complete curative resection was achieved. Foveolar hyperplasia and atrophy of the gastric glands were observed in non-tumor areas, histologically corresponding to Ménétrier’s disease. Three years after ESD, gastric cancer had not recurred, and Ménétrier’s disease remained in remission with spontaneous regression of giant gastric rugae.
Complete curative resection was achieved through ESD in a patient with early-stage gastric cancer and H. pylori-negative Ménétrier’s disease.
Core Tip: We reported an extremely rare case of gastric cancer as a complication of Helicobacter pylori-negative Ménétrier’s disease. The cancer was early-stage, and a minimally invasive curative resection was achieved with endoscopic submucosal dissection (ESD). There was no recurrence, and the patient’s course was extremely good. This case also highlights that when endoscopic biopsy tissue from a cancer-suspected lesion showed atypical glands in Ménétrier’s disease, total biopsy with ESD can be useful for diagnosis as well as treatment. ESD seems to be a beneficial therapy for early-stage gastric cancer in Ménétrier’s disease because of its low invasiveness and high complete curative resection rate.
- Citation: Fukushi K, Goda K, Kino H, Kondo M, Kanazawa M, Kashima K, Kanamori A, Abe K, Suzuki T, Tominaga K, Yamagishi H, Irisawa A. Curative resection with endoscopic submucosal dissection of early gastric cancer in Helicobacter pylori-negative Ménétrier’s disease: A case report. World J Gastroenterol 2022; 28(5): 594-601
- URL: https://www.wjgnet.com/1007-9327/full/v28/i5/594.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i5.594
Ménétrier’s disease (discovered in 1888) is a relatively rare gastric disease[1] characterized by giant rugae in the stomach accompanied by hypoproteinemia. Adult-onset Ménétrier’s disease is strongly associated with Helicobacter pylori (H. pylori) infection and an elevated risk of carcinogenesis[2]. Our literature review suggests that H. pylori-negative cases of early-stage gastric cancer are extremely rare[3,4].
We report the first case of H. pylori-negative Ménétrier’s disease with early gastric cancer in which complete curative resection was achieved through endoscopic submucosal dissection (ESD).
A 60-year-old woman was referred to our hospital for examination and treatment of anemia.
After observing transient melena, the patient’s previous doctor conducted a medical examination and detected severe anemia (blood hemoglobin 6.1 g/dL).
The patient had undergone successful treatment of chronic hepatitis C with interferon therapy performed by a previous doctor.
The patient had no history of smoking or drinking alcohol. There was no relevant family history.
On admission, the patient’s temperature was 36.7 °C, heart rate was 81 bpm, blood pressure was 128/80 mmHg. There was conjunctival pallor and no spontaneous abdominal pain or no tenderness.
The blood test revealed hypoproteinemia (serum albumin, 3.4 g/dL), and the serum H. pylori immunoglobulin G antibody level was negative (< 3).
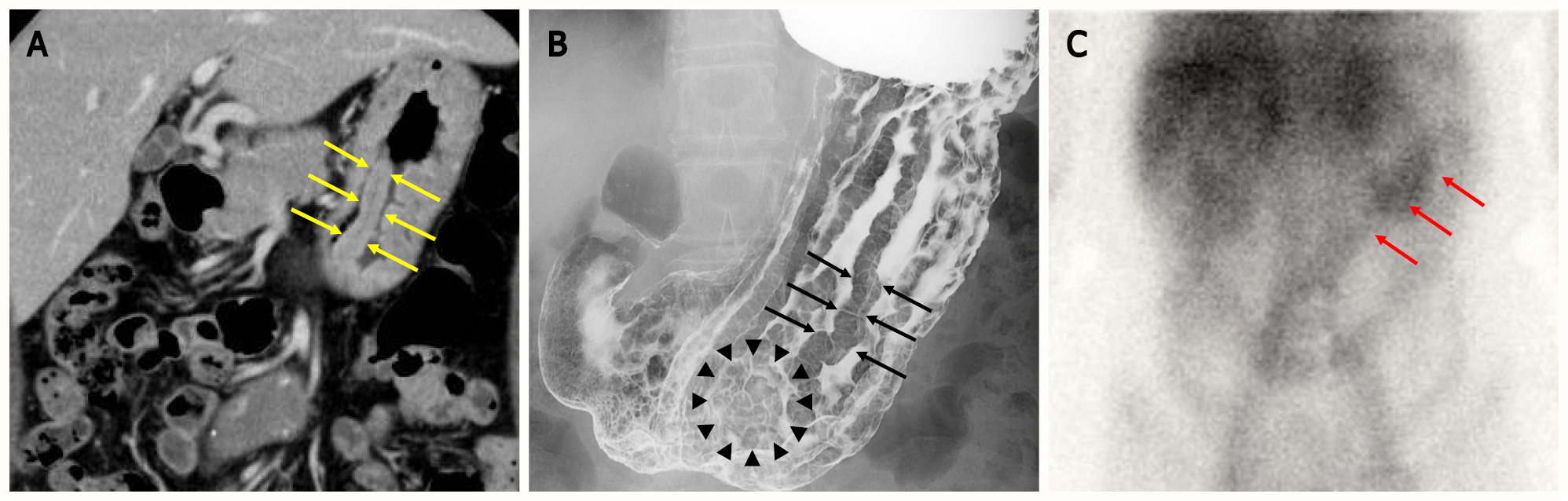
Computed tomography (CT) of the abdomen revealed marked mucosal thickening of the body of the stomach, with no enlarged lymph nodes around the stomach (Figure 1A). Upper gastrointestinal (UGI) radiograph showed giant gastric rugae[5] and translucency of the nodule-aggregating surface in the greater curvature of the lower gastric body (Figure 1B). The 99mTc-DTPA-human serum albumin (HSA-D) scintigraphy showed protein loss from the stomach after 24 h (Figure 1C).
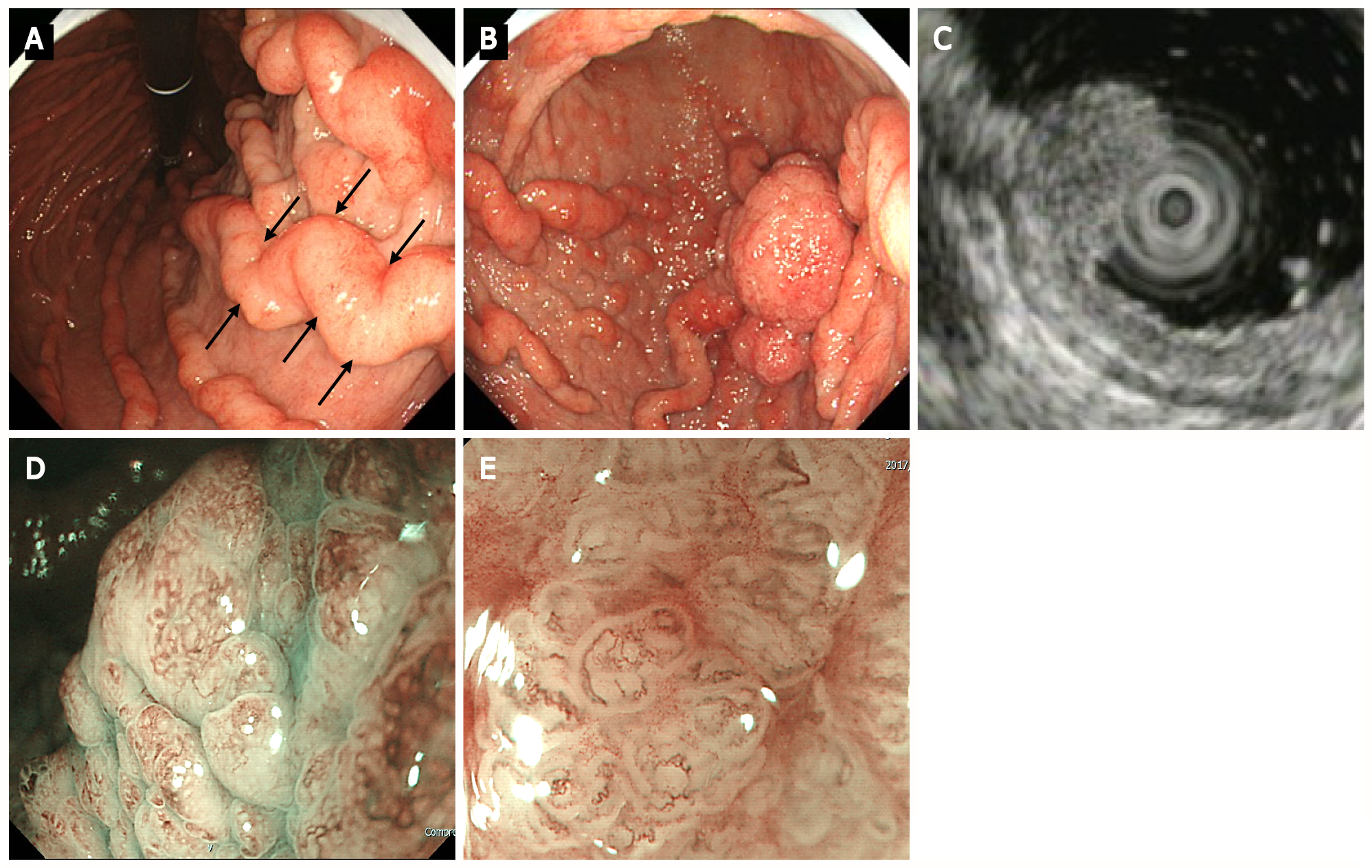
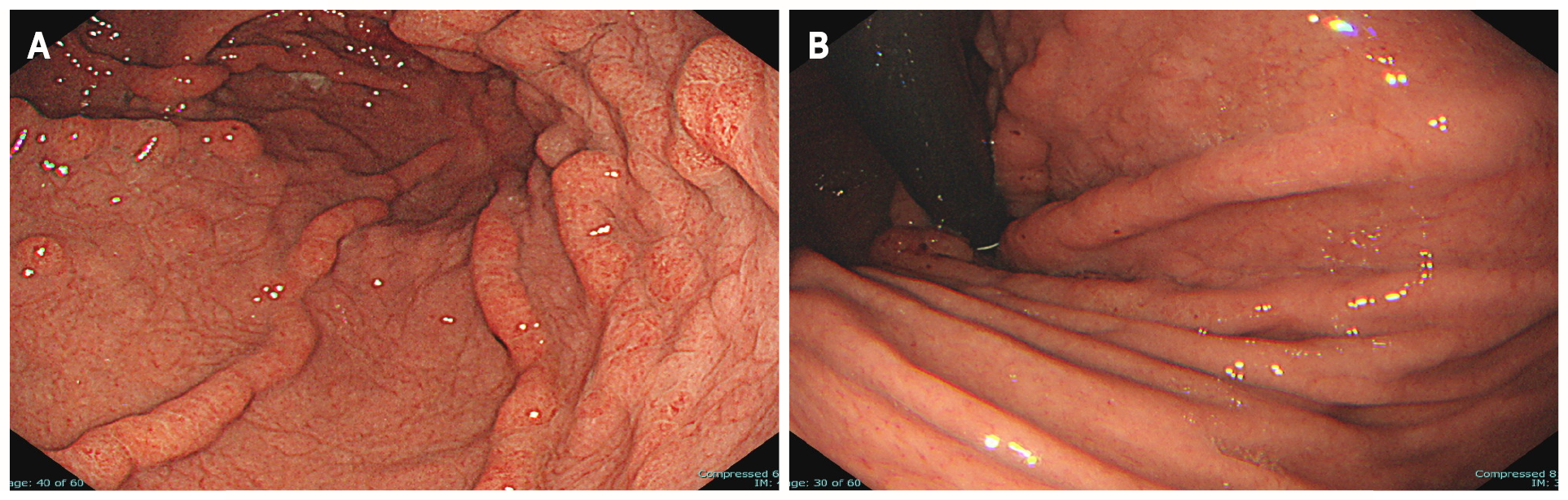
UGI endoscopy revealed giant rugae in the gastric body, similar to contrast-enhanced radiographic findings (Figure 2A). A 40-mm broad-based, protruding erythematous lesion with lobular surface was observed on the giant rugae of the gastric body (Figure 2B). UGI endoscopy showed no evidence of atrophic gastritis and intestinal metaplasia.
Endoscopic ultrasonography (EUS) demonstrated the five layers of the gastric wall. In the lesion area, hypertrophy was observed in the first two layers (equivalent to the mucosa and the muscularis mucosae); however, no noticeable structural changes were evident in the third layer (equivalent to the submucosal layer) or deeper (Figure 2C). These findings were consistent with early tumor confined to the mucosal layer.
Low-magnification narrow-band imaging (NBI) showed granular surfaces with various sizes/forms and dilated vessels (Figure 2D). High-magnification NBI demonstrated irregular microstructures of various forms and tortuous microvessels with changes in caliber (Figure 2E).
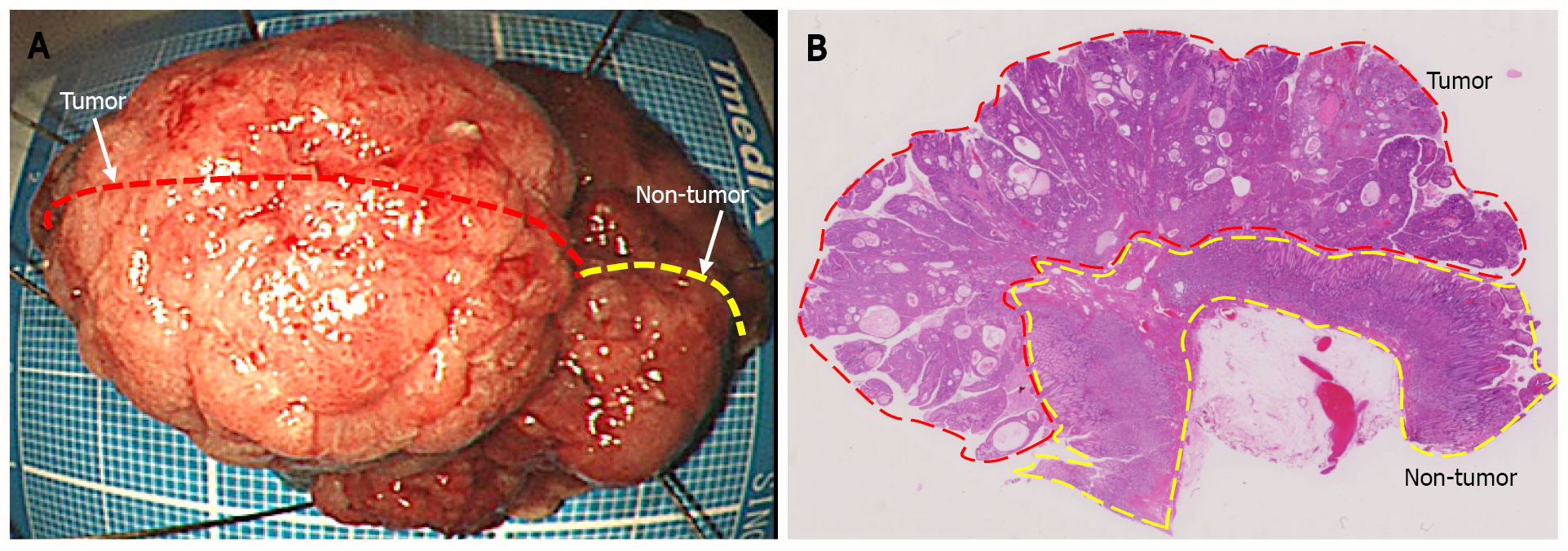
Histological diagnosis based on endoscopic biopsy was Group 2 (non-tumorous changes suspected, though tumorous lesions cannot be ruled out)[6]. Considering the tumor size and high probability of a cancerous lesion based on the endoscopic images, en bloc resection with ESD was performed for definitive diagnosis and treatment (Figure 3A). Loupe image showed that the well-differentiated tubular adenocarcinoma was confined to the mucosal layer, with no lymphovascular invasion (ly0/v0) or ulceration (UL0), and both the lateral and vertical margins were negative (Figure 3B).
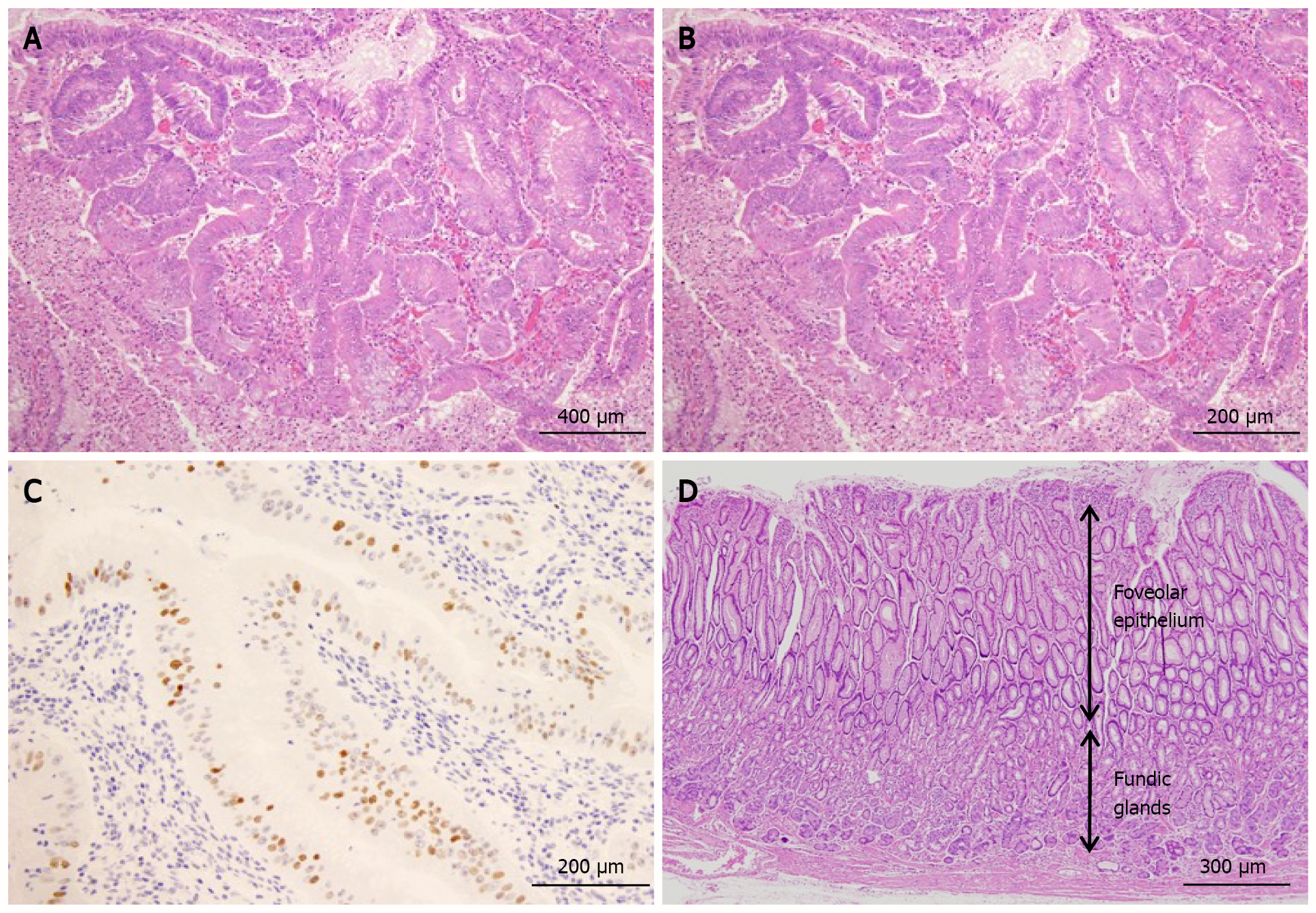
Histology of ESD specimens also showed proliferation of atypical cells with an irregular glandular structure (Figure 4A and B). Additionally, scattered p53 protein-positive cells were observed (Figure 4C). Based on these findings, the atypical glands were diagnosed with early gastric cancer of a well-differentiated tubular adenocarcinoma. Foveolar hyperplasia and atrophy of the proper gastric glands were observed in the non-tumor areas, a histological image corresponding to Ménétrier’s disease (Figure 4D).
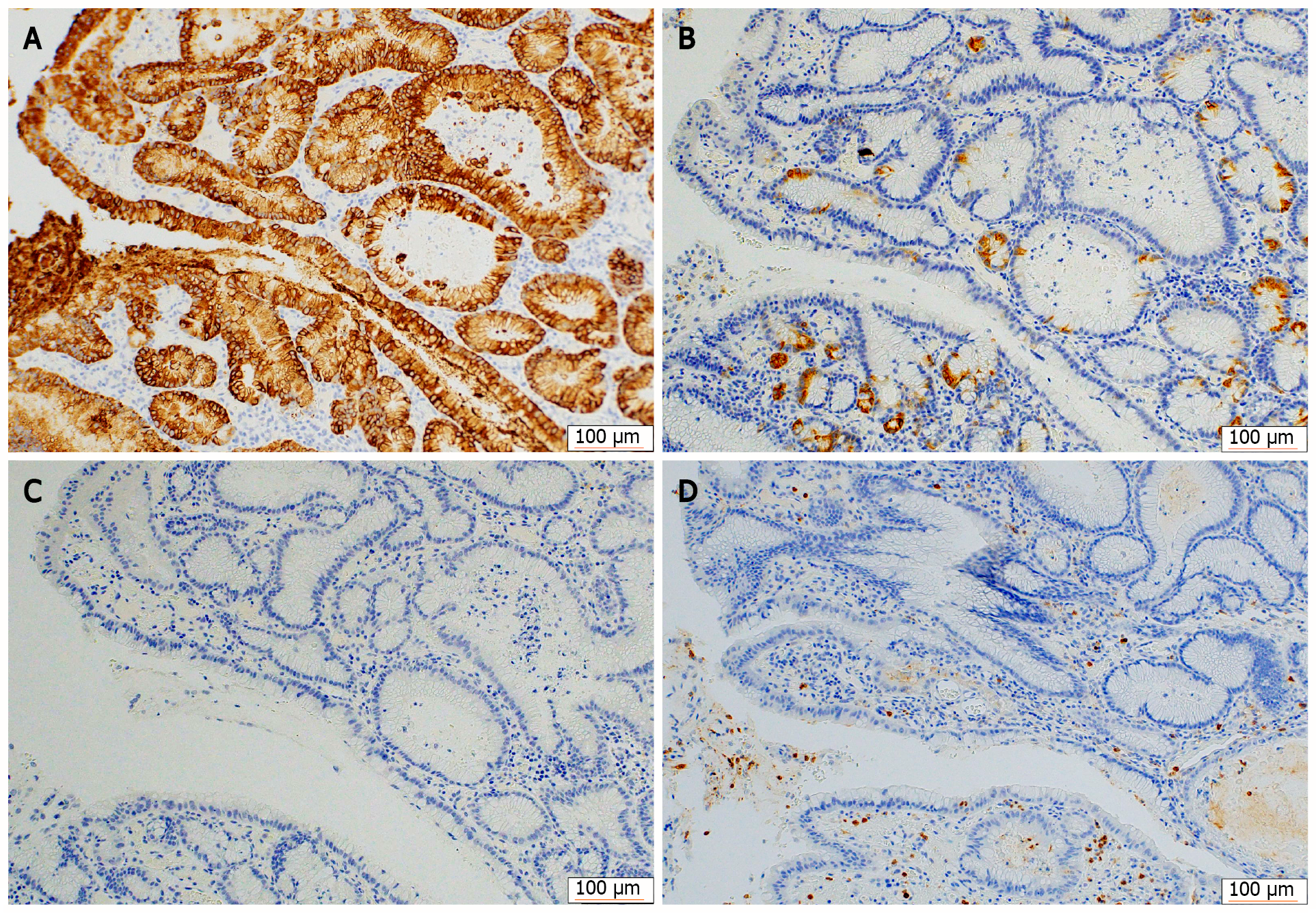
Immunohistochemical staining showed that the tumor cells were diffusely positive for MUC5AC (Figure 5A) and partially positive for MUC6 (Figure 5B), and negative for MUC2 (Figure 5C) and CD10 (Figure 5D). H. pylori were not observed in any biopsy or ESD specimens.
The final diagnosis of the presented case was early-stage gastric cancer and H. pylori-negative Ménétrier’s disease.
The patient underwent ESD.
Histology of ESD specimens showed complete curative resection. Endoscopic follow-up was performed twice a year for 3 years after ESD, and no recurrence was detected (Figure 6). Even though no therapeutic agents were administered specifically for Ménétrier’s disease, the giant rugae regressed spontaneously, hypoproteinemia and anemia improved gradually, and the remission was maintained until the last surveillance endoscopy.
We reported an extremely rare case of early gastric cancer as a complication of H. pylori-negative Ménétrier’s disease. We succeeded in curative resection of the cancerous lesion with ESD. Three years after the ESD, there was no recurrence of gastric cancer and Ménétrier’s disease regressed spontaneously and had maintained regression for about 30 mo.
Although the present case was H. pylori-negative, there have been two reports of close associations between adult-onset Ménétrier’s disease and H. pylori infection[7,8]. It has been reported that Ménétrier’s disease with hypertrophic gastropathy increases the risk of gastric carcinogenesis[9]; however, the association between gastric carcinogenesis and H. pylori infection in Ménétrier’s disease remains unclear.
There have been about 80 case reports on gastric cancer (including advanced cancer) in adult-onset Ménétrier’s disease, though few have reported early-stage cancers. Our literature search found four case reports of Ménétrier’s disease with early gastric cancer and recorded endoscopic findings and H. pylori infection status (Table 1); two of these cases were H. pylori-positive[10,11], and three cases, including the present one, were H. pylori-negative[3,4]. Our case was the second early gastric cancer to undergo endoscopic resection and the first resection with ESD instead of conventional endoscopic mucosal resection. Macroscopically, all the lesions of early gastric cancer were of protruding-type[12] and located on the oral side of the ventricular angle (corpus/fundus region)[13]. Hypoalbuminemia was observed in three cases and was unclear in one case.
| Ref. | Age | Gender | Hypoalbuminemia1 (g/L) | H. pylori | Differentiation | Tumor invasion depth | Macroscopic types | Tumor location | Therapy | Outcome |
| Raderer et al[10] | 79 | F | Present (1.5) | Positive (Hp IgG-Ab) | Well | M | 0-Ⅰs | Fundus | EMR | No recurrence for 3 yr |
| Johnson et al[11] | 73 | M | Present (2.4) | Positive (histology) | Well | SM | 0-Ⅱa + Ⅱc | Corpus | TG | Unknown |
| Ozawa et al[3] | 48 | F | Present (1.5) | Negative (Hp IgG-Ab) | Poor | M | 0-Ⅰs | Corpus to Antrum | TG | No recurrence for 2 yr |
| Charton-Bain et al[4] | 62 | F | Unknown | Negative (histology) | Well | M | 0-Ⅰs | Corpus | TG | Death on the 7th day post-operation2 |
| Present case | 60 | F | Present (3.4) | Negative (Hp IgG-Ab) | Well | M | 0-Ⅰs | Corpus | ESD | No recurrence for 3 yr |
These findings (protruding lesions located in the corpus or fundus region and hypoalbuminemia) may depict the clinicopathological features of Ménétrier’s disease-associated early gastric cancer.
Ménétrier’s disease generally presents with hypertrophic gastropathy, primarily in the fundic gland region, which may explain the tendency for Ménétrier’s disease-associated early-stage gastric cancer to occur in this region[14]. Endoscopic gastric cancer screening of patients with Ménétrier’s disease should focus on two characteristics: The location (corpus or fundus) and the macroscopic type.
In the present case, cancer could not be confirmed by preoperative biopsy; therefore, a definitive diagnosis of cancer was made using the specimen from the endoscopic resection specimen. Based on histopathological findings, Ménétrier’s disease is considered a chronic inflammatory disease[15], making it difficult to differentiate between inflammatory atypia and neoplastic atypia via small tissue biopsy alone, as in the present case. When endoscopic biopsy tissue from a superficial tumor-like lesion exhibits atypical glands in patients with Ménétrier’s disease, total biopsy with en bloc ESD can be useful for definitive diagnosis as well as treatment including complete curative resection, such as in this case.
Severe Ménétrier’s disease cases with clinical symptoms such as abdominal pain and vomiting require total gastrectomy to control severe hypoalbuminemia[16,17]. Besides the present patient, the two other patients with H. pylori-negative early-stage cancer underwent total gastrectomy. There was one case of surgery-related death. Therefore, if Ménétrier’s disease is not severe and the stomach can be preserved, ESD seems a beneficial therapy for early-stage gastric cancer, especially for mucosal cancer, because of its low invasiveness and high complete curative resection rate.
The mucin phenotype of the early gastric cancer in this case was gastric type with strongly positive for MUC5AC and low expression of MUC6. These suggest the gastric-type mucin of foveolar-dominant type. In the non-tumor area involved by Ménétrier’s disease, although p53 staining was negative and Ki-67 index was low, significant hyperplasia of foveolar epithelium was shown in the fundic gland region. We deduced that early cancer in the present case could be developed from a polypoid lesion with foveolar hyperplasia along hyperplasia–dysplasia–carcinoma sequence[18], even though the mechanism of gastric cancer development in Ménétrier’s disease is unknown.
To our knowledge, this is the first report of an extremely rare case of early-stage gastric cancer detected in a patient with H. pylori-negative Ménétrier’s disease in which complete curative resection was achieved with ESD.
We express our sincere thanks to Prof. Takashi Yao (the Department of Human Pathology, Juntendo Graduate University School of Medicine) for the helpful advice on histopathology of this case.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He C, Li XB, Ohnita K S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Ménétrier P. Des polyadenomes gastriques et de leurs rapports avec le cancer de l’estomac. Arch Physiol Norm Pathol. 1888;32:236-262. [DOI] [Full Text] |
| 2. | Hsu CT, Ito M, Kawase Y, Sekine I, Ohmagari T, Hashimoto S. Early gastric cancer arising from localized Ménétrier's disease. Gastroenterol Jpn. 1991;26:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Ozawa T, Wachi E, Yamashita N. [A case of juvenile polyposis limited to the stomach accompanied by double gastric cancers and Ménétrier's disease]. Nihon Shokakibyo Gakkai Zasshi. 2010;107:1641-1650. [PubMed] |
| 4. | Charton-Bain MC, Paraf F, Bruneval P. Superficial gastric carcinoma developed on localized hypertrophic lymphocytic gastritis: a variant of localized Ménétrier's disease? Pathol Res Pract. 2000;196:125-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Umegaki E, Sanomura M. Giant rugae. (in Japanese) Stomach and Intestine, Tokyo: Igakushoin, 2017; 52: 573. |
| 6. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, Hattori T, Hirota T, Itabashi M, Iwafuchi M, Iwashita A, Kim YI, Kirchner T, Klimpfinger M, Koike M, Lauwers GY, Lewin KJ, Oberhuber G, Offner F, Price AB, Rubio CA, Shimizu M, Shimoda T, Sipponen P, Solcia E, Stolte M, Watanabe H, Yamabe H. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1463] [Cited by in RCA: 1549] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 7. | Bayerdörffer E, Ritter MM, Hatz R, Brooks W, Stolte M. Ménétrier's disease and Helicobacter pylori. N Engl J Med. 1993;329:60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Stolte M, Bätz CH, Bayerdörffer E, Eidt S. Helicobacter pylori eradication in the treatment and differential diagnosis of giant folds in the corpus and fundus of the stomach. Z Gastroenterol. 1995;33:198-201. [PubMed] |
| 9. | Wood MG, Bates C, Brown RC, Losowsky MS. Intramucosal carcinoma of the gastric antrum complicating Menetrier's disease. J Clin Pathol. 1983;36:1071-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Raderer M, Oberhuber G, Templ E, Wagner L, Pötzi R, Wrba F, Hejna M, Base W. Successful symptomatic management of a patient with Ménétrier's disease with long-term antibiotic treatment. Digestion. 1999;60:358-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Johnson MI, Spark JI, Ambrose NS, Wyatt JI. Early gastric cancer in a patient with Menetrier's disease, lymphocytic gastritis and Helicobacter pylori. Eur J Gastroenterol Hepatol. 1995;7:187-190. [PubMed] |
| 12. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1327] [Article Influence: 60.3] [Reference Citation Analysis (4)] |
| 13. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2873] [Article Influence: 205.2] [Reference Citation Analysis (0)] |
| 14. | Greenson JK, Lauwers GY, Owens SR, Montgomery EA, Polydorides AD, Srivastava A. Diagnostic Pathology Gastrointestinal 2nd edition, Philadelphia, Pennsylvania: AMIRSYS (Elsevier), 2015: 156-157. |
| 15. | Toubia N, Schubert ML. Menetrier's Disease. Curr Treat Options Gastroenterol. 2008;11:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Scott HW Jr, Shull HJ, Law DH 4th, Burko H, Page DL. Surgical management of Menetrier's disease with protein-losing gastropathy. Ann Surg. 1975;181:765-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Remes-Troche JM, Zapata-Colindres JC, Starkman I, De Anda J, Arista-Nasr J, Valdovinos-Diaz MA. Early gastric cancer in Menetrier's disease. BMJ Case Rep. 2009;2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Terada T. Malignant transformation of foveolar hyperplastic polyp of the stomach: a histopathological study. Med Oncol. 2011;28:941-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |