Published online Feb 7, 2022. doi: 10.3748/wjg.v28.i5.570
Peer-review started: May 17, 2021
First decision: July 14, 2021
Revised: July 21, 2021
Accepted: January 20, 2022
Article in press: January 20, 2022
Published online: February 7, 2022
Processing time: 252 Days and 15.2 Hours
Abnormal liver chemistries are common findings in patients with Coronavirus Disease 2019 (COVID-19). However, the association of these abnormalities with the severity of COVID-19 and clinical outcomes is poorly understood
We aimed to assess the prevalence of elevated liver chemistries in hospitalized patients with COVID-19 and compare the serum liver chemistries to predict the severity and in-hospital mortality.
This retrospective, observational study included 3380 patients with COVID-19 who were hospitalized in the Johns Hopkins Health System (Baltimore, MD, United States). Demographic data, clinical characteristics, laboratory findings, treatment measures, and outcome data were collected. Cox regression modeling was used to explore variables associated with abnormal liver chemistries on admission with disease severity and prognosis
A total of 2698 (70.4%) had abnormal alanine aminotransferase (ALT) at the time of admission. Other more prevalent abnormal liver chemistries were aspartate aminotransferase (AST) (44.4%), alkaline phosphatase (ALP) (16.1%), and total bilirubin (T-Bil) (5.9%). Factors associated with liver injury were older age, Asian ethnicity, other race, being overweight, and obesity. Higher ALT, AST, T-Bil, and ALP levels were more commonly associated with disease severity. Multivariable adjusted Cox regression analysis revealed that abnormal AST and T-Bil were associated with the highest mortality risk than other liver injury indicators during hospitalization. Abnormal AST, T-Bil, and ALP were associated with a need for vasopressor drugs, whereas higher levels of AST, T-Bil, and a decreased albumin levels were associated with mechanical ventilation
Abnormal liver chemistries are common at the time of hospital admission in COVID-19 patients and can be closely related to the patient’s severity and prognosis. Elevated liver chemistries, specifically ALT, AST, ALP, and T-Bil levels, can be used to stratify risk and predict the need for advanced therapies in these patients.
Core Tip: Severe acute respiratory syndrome coronavirus-2 primarily infects the respiratory system. However, increasing evidence exists for the direct multiorgan effect. Liver injury in hospitalized patients is associated with a poor prognosis. We investigated whether abnormal liver chemistries in Coronavirus Disease 2019 (COVID-19) hospitalized patients can be of prognostic value. We show that abnormal liver chemistries were commonly observed on hospital admission and are associated with worse outcomes in COVID-19 patients, namely mortality, the need for vasopressor drugs, and mechanical ventilation. In hospitalized COVID-19 patients, elevated liver chemistries, specifically alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, and total bilirubin levels, can be used to stratify risk and predict the need for advanced therapies. These results strongly suggest that abnormal liver chemistries at the time of hospital admission are associated with worse outcomes in COVID-19 patients and should be closely followed in admitted patients.
- Citation: Krishnan A, Prichett L, Tao X, Alqahtani SA, Hamilton JP, Mezey E, Strauss AT, Kim A, Potter JJ, Chen PH, Woreta TA. Abnormal liver chemistries as a predictor of COVID-19 severity and clinical outcomes in hospitalized patients. World J Gastroenterol 2022; 28(5): 570-587
- URL: https://www.wjgnet.com/1007-9327/full/v28/i5/570.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i5.570
The Coronavirus Disease 2019 (COVID-19) infection is a global public health crisis that has spread rapidly throughout most of the world and has resulted in over 2 million deaths. Although it is primarily a respiratory disease, increasing evidence indicates that infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the virus that causes COVID-19, can affect multiple organ systems and cause long-term damage[1]. One possible explanation is the route of viral entry into cells via the angiotensin-converting enzyme 2 (ACE2) receptor, present on almost all human organs. The liver and the biliary system are no exception, and reports indicate that COVID-19 infection can induce varying degrees of liver injury, ranging from 19%-76% in reported cases[2-4]. The mechanism by which COVID-19 triggers liver injury remains poorly understood.
Direct infection of cholangiocytes via ACE2 is postulated as a potential mechanism for intrinsic liver injury from COVID-19[5]. The etiology of the abnormal liver chemistries frequently observed in patients with COVID-19 infection is multifactorial and associated with major adverse clinical outcomes[6]. The incidence of abnormal liver enzymes significantly increases during the course of the disease, which may indicate the effect of SARS-CoV-2 on the liver or the side effects of medications used to treat the infection[7]. Patients with severe COVID-19 infections have been shown to have higher rates of abnormal liver chemistries[8]. While some studies revealed abnormal liver chemistries are associated with increased disease severity and mortality[9,10], other studies did not find an association with disease progression[11], intensive care unit (ICU) admission[12], or the length of hospital stay[13] and mortality[14]. Thus, study results are inconsistent, with a high degree of heterogeneity.
To address some of these inconsistencies, we examine whether abnormal liver chemistries in COVID-19 hospitalized patients can be of prognostic value. We determined the prevalence of elevated liver chemistries in a large cohort of hospitalized patients with COVID-19 infection and identified whether an independent association exists between abnormal liver chemistries and clinical severity or the risk of in-hospital mortality.
In this observational, retrospective cohort study, we analyzed consecutive adult patients (> 18 years of age) who were admitted at the Johns Hopkins Health System (Baltimore, MD, United States) between March 01, 2020, and January 21, 2021, who tested positive for SARS-CoV-2. The diagnosis of COVID-19 was made by at least one positive SARS-CoV-2 real-time PCR test performed on nasopharyngeal swab samples[8]. Only laboratory-confirmed patients were included in this study. This study was approved by the Institutional Review Board (IRB00249001) of the Johns Hopkins University School of Medicine, and the informed consent was waived for a retrospective review of patient charts.
We obtained data from the COVID-19 Precision Medicine Analytics Platform Registry (JH-CROWN) database for this cohort study[15], which is a collection of data from the Johns Hopkins electronic health record (Epic) and available for analysis using an electronic database on the Precision Medicine Analystic Platform. Patients without any liver chemistry (n = 1874) results were excluded from the study. Demographic, clinical characteristics, laboratory tests, and treatment were retrieved from the medical records. Furthermore, the clinical outcomes were observed up to January 21, 2021, the final date of follow-up.
Elevated liver enzyme levels were defined as patients having alanine aminotransferase (ALT) levels greater than 25 U/L for women and 35 U/L for men, according to the American Association for the Study of Liver Diseases definitions[16]. Other liver chemistry abnormalities were characterized as using the upper limit of the normal range (ULN) for serum levels of aspartate aminotransferase (AST), total bilirubin (T-Bil), alkaline phosphatase (ALP), and gamma-glutamyl transpeptidase (GGT). Additionally, liver injury was categorized based on the degree of liver enzyme elevation as mild (1-2 times of ULN), moderate (> 2-5 times of ULN), and severe (> 5 times of ULN).
According to the World Health Organization interim guidance, patients in this study were classified based on COVID-19 disease severity[17]. Cases were defined as either: mild—if patients had mild symptoms of COVID-19 without abnormalities on chest imaging; moderate—if they had respiratory tract symptoms with no obvious hypoxemia and pneumonia manifestation by imaging; severe—if they had any of the following conditions: respiratory rate ≥ 30 breaths/minute; resting fingertip oxygen saturation <90%; a ratio of the arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) < 300mmHg or lung infiltrates critical—if they had respiratory failure requiring mechanical ventilation, or symptoms of shock, or respiratory failure combined with other organ dysfunction requiring intensive care. Identified COVID-19 patients were then stratified into two groups: non-severe (mild and moderate cases) and severe (severe and critical cases) disease, based on the above classification.
We defined mortality as the primary clinical outcome. Death was assessed at the end of the study period. We also examined the need for vasopressor drugs and mechanical ventilation as the secondary outcomes.
Categorical variables were summarized as frequencies (percentages). Chi-squared tests were used to compare categorical variables, and the Mann-Whitney-Wilcoxon test was used for continuous variables. The results are presented as median with interquartile range (IQR). Interaction analyzes were performed as needed. Missing data were not imputed, and only complete cases were included. Patients were considered right-censored if they were discharged from the hospital alive or remained in the hospital at the end of follow-up. We measured time to event in days from the date of hospital admission to the date of in-hospital mortality or hospital discharge alive. Cox regression modeling was used to explore the relationship between abnormal liver biochemistries and mechanical ventilation and risk of death using hazard ratios (HRs) and 95% confidence intervals (CIs). Univariate analyses were used to identify independent risk factors associated with mechanical ventilation and risk of death, and these were ultimately included in a multivariate model with the grade of liver chemistry elevation. Age, gender, ethnicity, race, body mass index, and all the preexisting comorbidities were adjusted as confounders in the Multivariable Cox proportional hazards model. Cox proportional hazards regression models were also used to estimate HRs for the grade of liver chemistry elevation associated with mortality after controlling for the empirical prognostic elements. Kaplan-Meier (KM) method was used to assess differences in mortality by the degree of liver chemistry elevation. The event-free survival rate was estimated using the KM method, and significance was evaluated with the log-rank test. All tests were two-tailed, and statistical significance was determined at P values < 0.05. All statistical data analyzes were conducted with Stata software (version SE16; StataCorp, College Station, TX, United States).
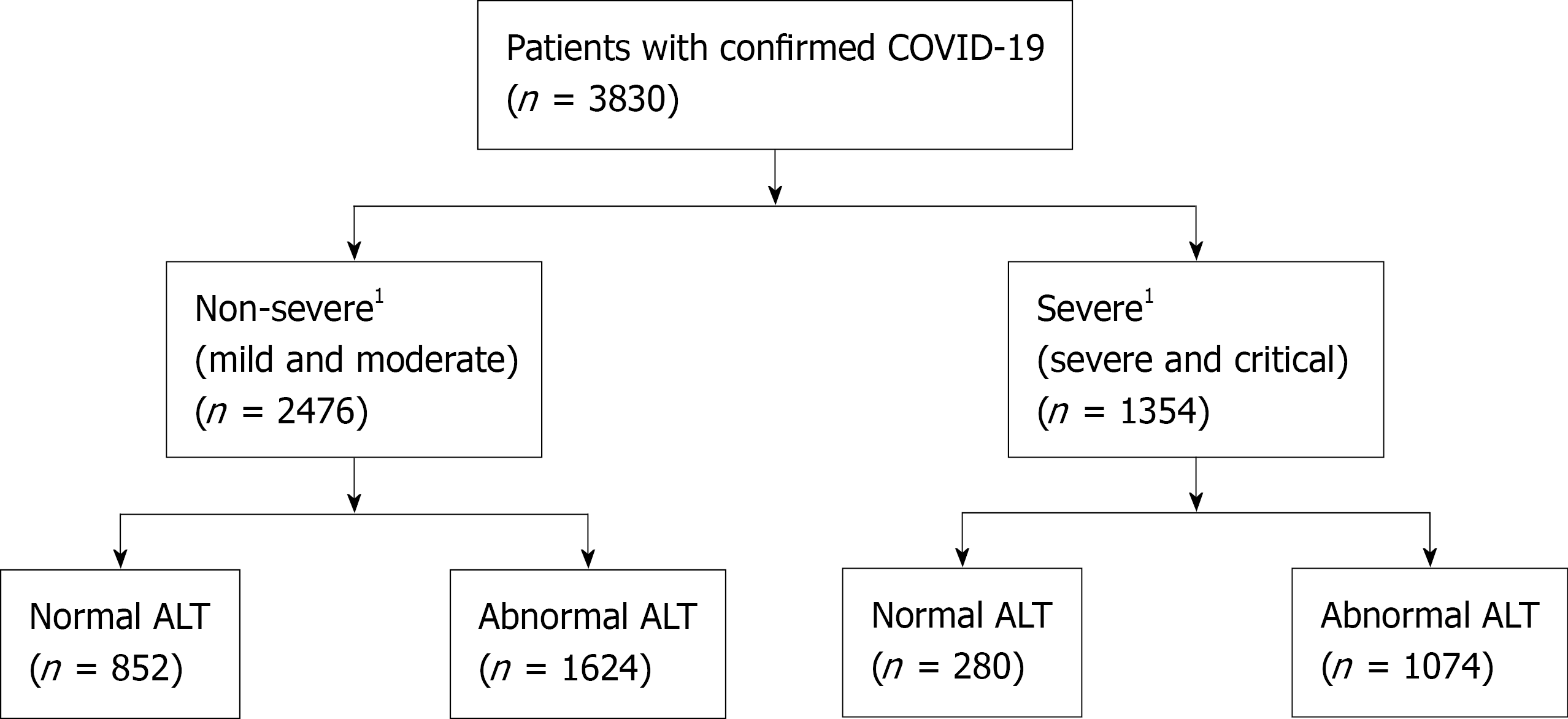
A total of 3830 hospitalized patients with confirmed SARS-CoV-2 infection were included in the analysis (Figure 1). Baseline clinical characteristics of the study cohort are summarized in Table 1. Among these patients, 2476 (64.6%) were non-severe cases, and 1354 (35.4%) were classified as severe cases during hospitalization. On hospital admission, abnormal liver chemistries were commonly seen (ALT 70.4%, AST 44.4%, T-Bil 5.9%, and ALP 16.1%) among all patients. The median age was 64.2 years (IQR 49.6-77.3), 51.1% were male, and 34.8% were African Americans. Obesity was present in 1494 (43.7%) of patients, and preexisting liver diseases in 392 (12.2%) patients. Severe disease was associated with older age and male sex. In patients with severe disease, the rate of coexisting diabetes mellitus without and with complications was significantly higher than in the non-severe group. In addition, these patients had higher cardiovascular disease, chronic respiratory disease, kidney disease, and neurological disease as comorbidities (P < 0.001), as well as significantly higher white blood cell and neutrophil counts and C-reactive protein (CRP), interleukin-6 (IL-6), fibrinogen, ferritin, prothrombin time (PT), international normalization ratio, D-dimer, lactate dehydrogenase (LDH), and cardiac troponin levels (P < 0.001). Levels of absolute lymphocyte, red blood cell, albumin, and total protein were lower (P < 0.001) in patients with severe disease
| Variables | All patients (n = 3830) | Non-severe1 (n = 2476) | Severe1 (n = 1354) | P value |
| Age in yr, median (IQR) | 64.2 (49.6- 77.3) | 62.1 (45.2-76) | 67.4 (55.6-79.1) | < 0.001 |
| Sex, n (%), Male | 1959 (51.1) | 1179 (47.6) | 780 (57.6) | < 0.001 |
| Ethnicity, n (%), Hispanic | 817 (21.5) | 565 (23) | 252 (18.8) | 0.003 |
| Race, n (%) | 0.12 | |||
| White | 1389 (36.6) | 877 (35.7) | 512 (38.2) | |
| Black | 1323 (34.8) | 848 (34.5) | 475 (35.4) | |
| Asian | 203 (5.3) | 133 (5.4) | 70 (5.2) | |
| Other | 883 (23.2) | 600 (24.4) | 283 (21.1) | |
| BMI (kg/m2), n (%) | 0.11 | |||
| ≤ 18.5 | 794 (23.2) | 519 (23.4) | 275 (22.8) | |
| 18.5-24.9 | 98 (2.9) | 56 (2.5) | 42 (3.5) | |
| 25-29.9 | 1036 (30.3) | 693 (31.3) | 343 (28.4) | |
| ≥ 30.0 | 1494 (43.7) | 946 (42.7) | 548 (45.4) | |
| Comorbidities, n (%) | ||||
| Chronic liver disease | 465 (12.1) | 295 (11.9) | 170 (12.6) | 0.56 |
| Cardiovascular disease | ||||
| Congestive heart failure | 869 (22.7) | 395 (16) | 474 (35) | < 0.001 |
| HT without complications | 2575 (67.2) | 1766 (71.3) | 717 (53) | < 0.001 |
| HT with complications | 1347 (35.2) | 710 (28.7) | 637 (47) | < 0.001 |
| Diabetes | ||||
| Diabetes without complications | 1459 (38.1) | 856 (34.6) | 603 (44.5) | 0.017 |
| Diabetes with complications | 1270 (33.2) | 679 (27.4) | 591 (43.6) | < 0.001 |
| Chronic respiratory disease | 1065 (27.8) | 618 (25) | 447 (33) | < 0.001 |
| Chronic neurological disease | 1033 (27.0) | 569 (23) | 464 (34.3) | < 0.001 |
| CKD of any stage | 973 (25.4) | 491 (19.8) | 482 (35.6) | < 0.001 |
| Anemia | 1655 (43.2) | 906 (36.6) | 749 (55.3) | < 0.001 |
| Hypothyroidism | 557 (14.5) | 330 (13.3) | 227 (16.8) | 0.004 |
| Malignancies | ||||
| Primary cancer | 458 (12) | 276 (11.1) | 182 (13.4) | 0.036 |
| Metastatic cancer | 277 (7.2) | 167 (6.7) | 110 (8.1) | 0.12 |
| Laboratory findings, median (IQR) | ||||
| Liver biochemistries: | ||||
| ALT, median (IQR) | 28 (18-47) | 27 (18-45) | 30 (19-49) | 0.003 |
| Normal, n (%) | 1132 (29.6) | 852 (34.4) | 280 (20.7) | |
| Abnormal, n (%) | 2698 (70.4) | 1624 (65.6) | 1074 (79.3) | < 0.001 |
| 1-2 ULN, n (%) | 1225 (32) | 829 (33.5) | 396 (29.2) | |
| > 2-5 ULN, n (%) | 1009 (26.3) | 583 (23.5) | 426 (31.5) | |
| > 5 ULN, n (%) | 464 (12.1) | 212 (8.6) | 252 (18.6) | |
| AST, median (IQR) | 36 (25-55) | 34 (24-51.5) | 42 (29-64) | < 0.001 |
| Normal, n (%) | 2046 (55.6) | 1443 (60.5) | 603 (46.4) | |
| Abnormal, n (%) | 1637 (44.4) | 941 (39.5) | 696 (53.6) | < 0.001 |
| 1-2 ULN, n (%) | 1187 (32.2) | 704 (29.5) | 483 (37.2) | |
| > 2-5 ULN, n (%) | 361 (9.8) | 194 (8.1) | 167 (12.9) | |
| > 5 ULN, n (%) | 89 (2.4) | 43 (1.8) | 46 (3.5) | |
| T.Bil, median (IQR) | 0.5 (0.3-6.1) | 0.4 (0.3-6.0) | 0.5 (0.4-7.0) | < 0.001 |
| Normal, n (%) | 3496 (94.1) | 2286 (95.3) | 1210 (91.7) | |
| Abnormal, n (%) | 221 (5.9) | 112 (4.7) | 109 (8.3) | < 0.001 |
| 1-2 ULN, n (%) | 177 (4.8) | 89 (3.7) | 88 (6.7) | |
| > 2-5 ULN, n (%) | 34 (0.9) | 17 (0.7) | 17 (1.3) | |
| > 5 ULN, n (%) | 10 (0.3) | 6 (0.3) | 4 (0.3) | |
| ALP, median (IQR) | 78 (61-103) | 77 (61-100) | 79 (61-108) | 0.014 |
| Normal, n (%) | 3183 (83.9) | 2101 (85.6) | 1082 (80.7) | |
| Abnormal, n (%) | 611 (16.1) | 353 (14.4) | 258 (19.3) | < 0.001 |
| 1-2 ULN, n (%) | 525 (13.8) | 311 (12.7) | 214 (16) | |
| > 2-5 ULN, n (%) | 78 (2.1) | 38 (1.5) | 40 (3) | |
| > 5 ULN, n (%) | 8 (0.2) | 4 (0.2) | 4 (0.3) | |
| GGT, median (IQR) | 119 (63-199) | 116 (80-161) | 144.5 (59-245) | 0.54 |
| Serum Albumin, g/dL median (IQR) | 3.8 (3.4- 4.1) | 3.9 (3.5-4.2) | 3.6 (3.1-3.9) | < 0.001 |
| Total protein (g/L), median (IQR) | 6.5 (5.9- 7.1) | 6.7 (6.1-7.2) | 6.3 (5.7-6.9) | < 0.001 |
| Coagulation test: median (IQR) | ||||
| PT (s) | 11 (10.5-11.9) | 10.9 (10.4-11.6) | 11.4 (10.8-12.4) | < 0.001 |
| INR | 1.07 (1-1.14) | 1.05 (1.0-1.1) | 1.1 (1.02-1.20) | < 0.001 |
| APTT (s) | 26.2 (1.2-32.2) | 25.9 (1.1-31) | 25.7 (1.3-33.6) | < 0.001 |
| D-Dimer | 1.0 (0.57, 2.06) | 0.83 (0.5-1.62) | 0.4 (0.8-3.0) | < 0.001 |
| Routine blood tests: median (IQR) | ||||
| Hemoglobin (g/dL) | 11.9 (10.1, 13.3) | 12.2 (10.7-13.6) | 10.9 (9-12.7) | < 0.001 |
| White blood cell (/mcL) | 7.4 (5.1-10.5) | 6.8 (4.7-9.3) | 9 (6.4-13) | < 0.001 |
| Red blood cells (/mcL) | 4.06 (3.34-4.63) | 4.19 (3.59-4.70) | 3.81 (3.02-4.46) | < 0.001 |
| Platelets (/mcL) | 229 (168-309) | 223 (166-298) | 238 (172-327) | < 0.001 |
| Neutrophils(/mcL) | 36 (5.23-73) | 36 (4.6-70.7) | 38.9 (6.75-77.9) | < 0.001 |
| Lymphocytes (/mcL) | 15.4 (9.2-23.6) | 17.8 (11.3-26.1) | 11.5 (6.3-18.4) | < 0.001 |
| Renal function tests: median (IQR) | ||||
| Creatinine (mg/dL) | 0.9 (0.7-1.3) | 0.85 (0.7-1.1) | 1.03 (0.7-1.8) | < 0.001 |
| Blood urea nitrogen, (mmol/L) | 17 (11-28) | 15 (10-22) | 25 (16-42) | < 0.001 |
| Sodium (mEq/L) | 138 (136-141) | 138 (136-140) | 139 (136-143) | < 0.001 |
| Potassium (mEq/L) | 4.1 (3.8-4.5) | 4.1 (3.8-4.4) | 4.2 (3.8-4.6) | < 0.001 |
| Inflammatory markers: median (IQR) | ||||
| Interleukin-6 (pg/mL) | 37 (14.5-90.8) | 24.7 (10.6-52.2) | 81.83 (31.2-181) | < 0.001 |
| Ferritin (ng/mL) | 587 (265-1090) | 482 (207-900) | 810 (413.5-1462.5) | < 0.001 |
| C reactive protein (mg/L) | 8.4 (3.4-21.4) | 6.4 (2.5-15.5) | 13.6 (6.6-33.2) | < 0.001 |
| Fibrinogen (mg/dL) | 495 (387-622) | 475 (374-579) | 524 (393-653) | < 0.001 |
| Lactate(mmol/L) | 1.4 (1.1-2.0) | 1.3 (1.0-1.7) | 1.6 (1.2-2.3) | < 0.001 |
| Cardiac markers: median (IQR) | ||||
| Cardiac troponin I (ng/L) | 0.07 (0.04-0.18) | 0.05 (0.03-0.1) | 0.09 (0.05-0.27) | < 0.001 |
| Lactate dehydrogenase (U/L) | 327 (245-460) | 303 (229-411) | 385 (290-533) | < 0.001 |
We compared the abnormal liver chemistries at different cut-off values as 1-2 ×, >2-5 ×, and > 5 × ULN, respectively, between the two groups (Table 1). On hospital admission, abnormal liver chemistries were commonly observed, and most patients had mild elevations within 1-2 × ULN. ALT, AST, T-Bil, and ALP levels were higher and more common in patients with severe disease. Overall, 2698 (70.4%) patients had an elevated ALT level, and the median ALT level was 28 U/L (IQR 18-47). A higher proportion of patients with severe disease had elevated ALT compared to the non-severe patients. The median ALT level was 27 U/L in non-severe disease compared to 30 U/L in severe cases (P = 0.003). In addition, there was a significant difference in the degree of ALT elevation between the two groups (P < 0.001). An elevated ALT at > 2-5 × ULN and > 5 × ULN were significantly more common among patients with severe disease than non-severe. The median AST was 34 U/L (IQR, 24-51.5) in non-severe cases vs 42 U/L (IQR, 29-64) in severe cases (P < 0.001). The elevated AST level of 1-2 × ULN (37.2% vs 29.5%), > 2-5 × ULN (12.9% vs 8.1%) and > 5 × ULN (3.5% vs 1.8%) were significantly more common among severe patients compared to non-severe (P < 0.001). Patients with severe COVID-19 also had a higher median T-Bil compared with non-severe patients. However, there was no difference in T-Bil distribution at different levels between the two severities (P = 0.056). Again, in patients with severe disease, the median ALP elevation was higher in the non-severe patients (P = 0.014). However, only four patients in both groups at >5 × ULN had elevated ALP levels. Nevertheless, there was a significant difference in ALP distribution levels between the two severity groups (P < 0.001). Finally, there was no variation in GGT levels at the three thresholds between the two groups (P = 0.540)
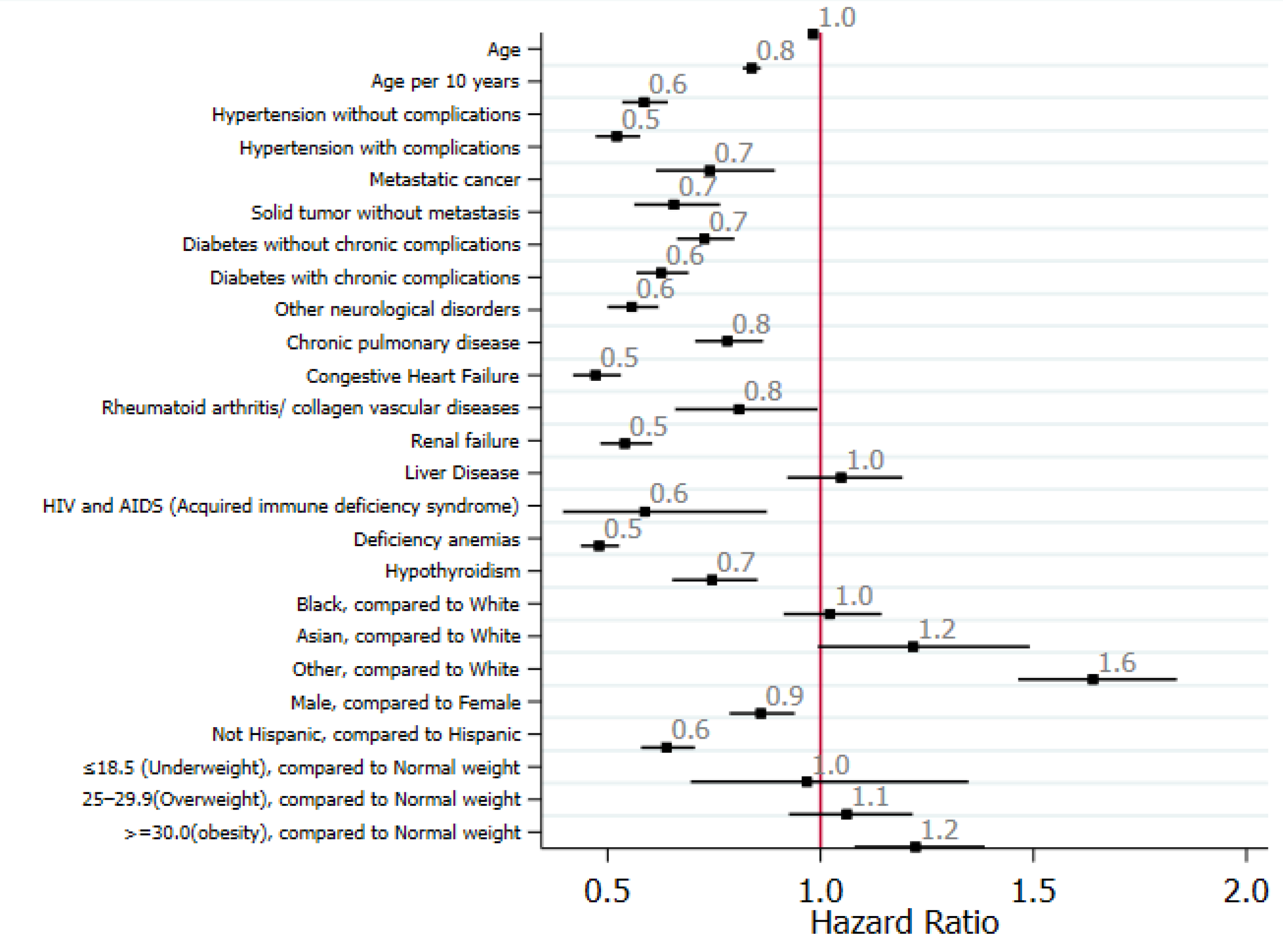
Risk factors and predictors of liver injury: Univariate analyses showed that older age, Asian ethnicity, and being overweight were associated with liver injury, whereas other races and obesity were associated with severe liver injury (Figure 2).
In-hospital management and clinical outcomes of patients with COVID-19 infection are shown in Table 2. Patients with severe diseases received more oxygen support and invasive ventilation (P < 0.001). Compared with the non-severe group, patients with the severe disease were more likely to receive antibacterial, antifungal, remdesivir, statins (P < 0.001), antiviral, and NSAID treatment (P = 0.005). The proportion of patients who received azithromycin and dexamethasone treatment was 24.7% and 40.7%, respectively. Moreover, 587 (40.7%) of the severe cases required vasopressor drugs for multiorgan failure (P < 0.001). Severe patients were more likely to develop kidney injury and need renal replacement therapy than non-severe patients. The median length of hospitalization prior to the death was 10.3 d (IQR, 4.5 to 18.8 d). The median length of stay was 6.3 d (IQR, 3.4 to 12.1 d), but patients with severe diseases had a significantly extended hospital stay compared with the non-severe group (median, 4.8 d vs 12.3 days, P < 0.001).
| Characteristics | All patients (n = 3830) | Non-severe1 (n = 2476) | Severe1 (n = 1354) | P value |
| Respiratory support, n (%) | ||||
| Non-rebreathing oxygen face mask | 3147 (82.2) | 1821 (73.5) | 1326 (97.9) | < 0.001 |
| High-flow nasal cannula oxygen therapy | 843 (22) | 18 (0.7) | 825 (60.9) | < 0.001 |
| Pharmacological treatment, n (%) | ||||
| NSAIDs | 3303 (86.2) | 2107 (85.1) | 1196 (88.3) | 0.005 |
| Antiviral therapy | 192 (5) | 106 (4.3) | 86 (6.4) | 0.005 |
| Antibacterial therapy | 2649 (69.2) | 1483 (59.9) | 1166 (86.1) | < 0.001 |
| Antifungal therapy | 234 (6.1) | 59 (2.4) | 175 (12.9) | < 0.001 |
| Azithromycin | 947 (24.7) | 530 (21.4) | 417 (30.8) | < 0.001 |
| Hydroxychloroquine | 423 (11) | 239 (9.7) | 184 (13.6) | < 0.001 |
| Oseltamivir | 10 (0.3) | 4 (0.2) | 6 (0.4) | 0.10 |
| Remdesivir | 1303 (34) | 741 (29.9) | 562 (41.5) | < 0.001 |
| Vitamin D | 391 (10.2) | 227 (9.2) | 164 (12.1) | 0.004 |
| Statins | 1457 (38) | 865 (34.9) | 592 (43.7) | < 0.001 |
| ACE inhibitors | 378 (9.9) | 215 (8.7) | 163 (12) | < 0.001 |
| ARB inhibitors | 414 (10.8) | 246 (9.9) | 168 (12.4) | 0.018 |
| Immunomodulatory therapy, n (%) | ||||
| Dexamethasone | 1560 (40.7) | 881 (35.6) | 679 (50.1) | < 0.001 |
| Tocilizumab | 95 (2.5) | 3 (0.1%) | 92 (6.8) | < 0.001 |
| Advanced therapies, n (%) | ||||
| Vasopressors | 587 (15.3) | 2 (0.1) | 585 (43.2) | < 0.001 |
| Renal replacement therapy/dialysis | 188 (4.9 ) | 6 (0.2) | 182 (13.4) | < 0.001 |
| Clinical outcome, n (%) | ||||
| Discharged alive from hospital | 3138 (87.2) | 2372 (100) | 766 (62.4) | < 0.001 |
| Median length of hospital stay (IQR) | 6.3 (3.4-12.1) | 4.8 (2.8-7.7) | 12.3 (7.1-22.3) | < 0.001 |
Mortality: Overall, 461 (12.1%) patients died, and all the patients belonged to the severe diseases group. 3138 (87.2%) were discharged at the time of data collection for this analysis. In addition, compared to survivors, non-survivors were older and had significantly higher rates of comorbidities (Table 3). In multivariable Cox proportional hazards analysis, increasing age, overweight, obesity, hypertension without complications, chronic neurological disease, and kidney disease were independently associated with an increased risk of in-hospital mortality after adjusting for confounders. Moreover, the results indicated that abnormal AST, T-Bil, ALP, and PT levels were significantly associated with all-cause mortality in all patients with COVID-19, but not the preexisting chronic liver disease, ALT, and albumin levels. Furthermore, a higher state of inflammation was also associated with mortality, with statistically significant increased levels of neutrophil count (P = 0.008), ferritin (P = 0.001), D-Dimer (P = 0.004), CRP, and IL-6 (P < 0.001).
| Liver function test | No mechanical ventilation ( n = 3205) | Mechanical ventilation (n = 625) | P value | Survivor (n = 3369) | Non-survivors (n = 461) | P value |
| Age in yr, median (IQR) | 63.8 (48.4-77.7) | 66.3 (54.9-75.2) | 0.030 | 62.3 (47.7-74.7) | 78.1 (66.9-87.6) | < 0.001 |
| Comorbidities, n (%) | ||||||
| Chronic liver disease | 385 (12) | 80 (12.8) | 0.58 | 419 (12.4) | 46 (10) | 0.13 |
| Cardiovascular disease | ||||||
| Congestive heart failure | 662 (20.7) | 207 (33.1) | < 0.001 | 693 (20.6) | 176 (38.2) | < 0.001 |
| HT without complications | 2112 (65.9) | 463 (74.1) | < 0.001 | 2214 (65.7) | 361 (78.3) | < 0.001 |
| HT with complications | 1059 (33) | 288 (46.1) | < 0.001 | 1097 (32.6) | 250 (54.2) | < 0.001 |
| Diabetes | ||||||
| Diabetes without complications | 1161 (36.2) | 298 (47.7) | < 0.001 | 1269 (37.7) | 190 (41.2) | 0.14 |
| Diabetes with complications | 979 (30.5) | 291 (46.6) | < 0.001 | 1076 (31.9) | 194 (42.1) | < 0.001 |
| Chronic respiratory disease | 865 (27) | 200 (32) | 0.011 | 910 (27) | 155 (33.6) | 0.03 |
| Chronic neurological disease | 810 (25.3) | 223 (35.7) | < 0.001 | 835 (24.8) | 198 (43) | < 0.001 |
| CKD of any stage | 764 (23.8) | 209 (33.4) | < 0.001 | 787 (23.4) | 186 (40.3) | < 0.001 |
| Anemia | 1281 (40) | 374 (59.8) | < 0.001 | 1379 (40.9) | 276 (59.9) | < 0.001 |
| ALT, median (IQR) | 27 (18-46) | 33 (21-54) | < 0.001 | 28 (18-47) | 27 (17-45) | 0.28 |
| Normal, n (%) | 1055 (32.9) | 77 (12.3) | 1011 (30) | 121 (26.2) | ||
| 1-2 ULN, n (%) | 1065 (33.2) | 160 (25.6) | < 0.001 | 1090 (32.4) | 135 (29.3) | < 0.001 |
| > 2-5 ULN, n (%) | 786 (24.5) | 223 (35.7) | 902 (26.8) | 107 (23.2) | ||
| > 5 ULN, n (%) | 299 (9.3) | 165 (26.4) | 366 (10.9) | 98 (21.3) | ||
| AST, median (IQR) | 35 (24-54) | 44.5 (31-70) | < 0.001 | 35 (25 -54) | 45 (29 -71) | < 0.001 |
| Normal, n (%) | 1794 (58.3) | 252 (41.7) | 1856 (57.2) | 190 (43.5) | ||
| 1-2 ULN, n (%) | 946 (30.7) | 241 (39.9) | < 0.001 | 1023 (31.5) | 164 (37.5) | < 0.001 |
| > 2-5 ULN, n (%) | 283 (9.2) | 78 (12.9) | 300 (9.2%) | 61 (14.0) | ||
| > 5 ULN, < 0.001 (%) | 56 (1.8) | 33 (5.5) | 67 (2.1%) | 22 (5) | ||
| Bilirubin, median (IQR) | 0.5 (0.3-0.6) | 0.5 (0.4-0.7) | < 0.001 | 0.5 (0.3-0.6) | 0.5 (0.4-0.8) | < 0.001 |
| Normal, n (%) | 2943 (94.7) | 553 (90.8) | 3102 (94.9) | 394 (88.1) | ||
| 1-2 ULN, n (%) | 133 (4.3) | 44 (7.2) | < 0.001 | 135 (4.1) | 42 (9.4) | < 0.001 |
| > 2-5 ULN, n (%) | 23 (0.7) | 11 (1.8) | 26 (0.8) | 8 (1.8) | ||
| > 5 ULN, n (%) | 9 (0.3) | 1 (0.2) | 7 (0.2) | 3 (0.7) | ||
| ALP, median (IQR)) | 77 (61-101) | 80 (62-110) | 0.062 | 77 (61-100) | 87 (64-118) | < 0.001 |
| Normal, n (%) | 2686 (84.5) | 497 (80.7) | 2837 (85) | 346 (75.7) | ||
| 1-2 ULN, n (%) | 420 (13.2) | 105 (17) | 0.091 | 435 (13) | 90 (19.7) | < 0.001 |
| > 2-5 ULN, n (%) | 65 (2) | 13 (2.1) | 58 (1.7) | 20 (4.4) | ||
| > 5 ULN, n (%) | 7 (0.2) | 1 (0.2) | 7 (0.2) | 1 (0.2) | ||
| GGT, median (IQR) | 116 (56-199) | 144.5 (106-235.5) | 0.483 | 117 (56-199) | 180 (116.5-447.5) | 0.28 |
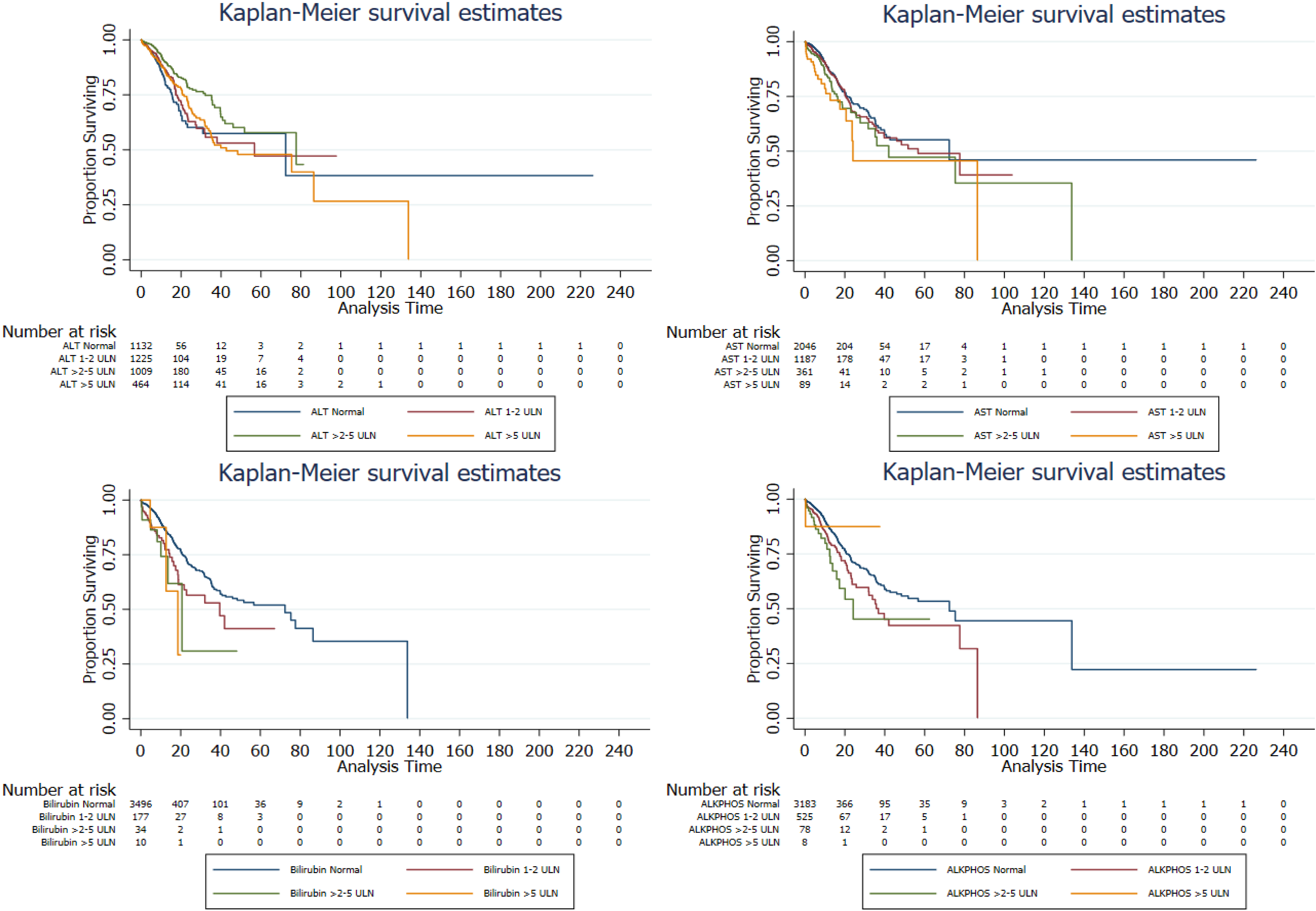
Determining the association of changes in liver chemistries and mortality: The associated distribution of liver chemistries at different levels with in-hospital mortality in patients with COVID-19 was explored using the Cox proportional hazards model (Tables 4 and Figure 3). Unadjusted models showed that a stepwise increase in liver chemistries levels conferred an incremental risk of in-hospital death. Patients with abnormal AST, T-Bil, and ALP levels during hospitalization had a higher mortality risk than patients with normal levels. Age, gender, ethnicity, race, BMI, and all the preexisting comorbidities were adjusted as confounders. Among these liver chemistries, elevated AST and T-Bil levels were associated with the highest risk of in-hospital mortality. Compared to patients with T-Bil in the normal level, all-cause mortality risk significantly increased 6-fold (95%CI, 2.90-12.41; P < 0.001) in patients with an elevated T-Bil level of >2-5 × ULN and increased 7.86-fold (95%CI, 1.88-32.96; P = 0.005) in patients with T-Bil > 5 × ULN (Table 5). A stepwise increase in the levels of AST was associated with a significant increased risk of all-cause mortality (HR, 1.49; 95%CI, 1.06-2.10; P < 0.001 for > 2-5 × ULN; HR, 2.19; 95%CI, 1.27-3.76; P = 0.005 for AST > 5 × ULN). The degree of ALT ranging from > 2-5 × ULN (adjusted HR, 0.68; 95%CI, 0.53-0.92; P = 0.013) was associated with a decreased risk of all-cause mortality; however, 1-2 × and > 5 × ULN were not significantly associated with all-cause mortality. Lastly, compared to the patients with ALP in the normal range, all-cause mortality risk significantly increased 1.42-fold (95%CI, 1.09-1.86; P = 0.009) in patients with ALP > 1-2 × ULN and increased 1.81-fold (95%CI, 1.05-3.10; P = 0.032) in patients with ALP > 2-5 × ULN after adjusting for confounders. Interestingly, the ALP levels > 5 × ULN were not significantly associated with mortality.
| Clinical predictors | Mechanical ventilation1 | Mortality1 | ||
| Multivariable HR (95%CI) | P Value | Multivariable HR (95%CI) | P Value | |
| Age | 1.00 (0.99-1.01) | 0.553 | 1.04 (1.03-1.05) | < 0.001 |
| Male Gender | 1.19 (0.99-1.43) | 0.067 | 1.11 (0.90-1.37) | 0.338 |
| Overweight | 0.93 (0.73-1.19) | 0.577 | 0.75 (0.59-0.97) | 0.030 |
| Obesity | 0.94 (0.74-1.19) | 0.609 | 0.58 (0.44-0.77) | < 0.001 |
| Liver diseases | 0.84 (0.65-1.09) | 0.198 | 0.78 (0.55-1.11) | 0.164 |
| Chronic respiratory disease | 1.15 (0.95-1.38) | 0.156 | 1.16 (0.94-1.45) | 0.167 |
| HT without complications | 0.85 (0.68-1.06) | 0.152 | 0.68 (0.53-0.89) | 0.005 |
| HT with complications | 1.29 (1.01-1.66) | 0.045 | 1.06 (0.78-1.430) | 0.727 |
| Congestive heart failure | 0.78 (0.64-0.95) | 0.014 | 1.00 (0.80-1.25) | 0.987 |
| Chronic neurological disease | 0.78 (0.65-0.95) | 0.012 | 1.44 (1.18-1.76) | < 0.001 |
| Chronic kidney disease | 0.94 (0.76-1.15) | 0.535 | 1.55 (1.26-1.88) | < 0.001 |
| ALT | 1.00 (1.00-1.00) | 0.420 | 1.00(1.00 - 1.00) | 0.892 |
| ALP | 1.00 (1.00-1.00) | 0.916 | 1.02 (1.02-1.03) | < 0.001 |
| AST | 1.00 (1.00-1.00) | 0.003 | 1.00 (1.00-1.01) | < 0.001 |
| T-Bil | 1.06 (0.99-1.14) | 0.008 | 1.21 (1.14-1.28) | < 0.001 |
| Albumin | 0.87 (0.76-1.01) | 0.071 | 0.84 (0.71-1.01) | 0.057 |
| INR | 0.91 (0.77-1.09) | 0.312 | 1.12 (0.99-1.26) | 0.071 |
| PT | 1.00 (0.98-1.02) | 0.814 | 1.03 (1.02-1.05) | < 0.001 |
| Neutrophil | 1.00 (1.00-1.00) | 0.858 | 1.00 (1.00-1.01) | 0.008 |
| BUN | 1.01 (1.00-1.01) | <0.001 | 1.01 (1.01-1.02) | < 0.001 |
| Creatinine | 1.07 (1.02-1.13) | 0.007 | 1.16 (1.11-1.22) | < 0.001 |
| Interleukin-6 | 1.00 (1.00-1.00) | <0.001 | 1.00 (1.00-1.00) | < 0.001 |
| CRP | 1.02 (1.01-1.03) | 0.001 | 1.04 (1.03-1.05) | < 0.001 |
| Ferritin | 1.00 (1.00-1.00) | 0.002 | 1.00 (1.00-1.00) | 0.001 |
| D-Dimer | 1.00 (0.98-1.02) | 0.721 | 1.03 (1.01-1.05) | 0.004 |
| LDH | 1.00 (1.00-1.00) | 0.063 | 1.00 (1.00-1.01) | < 0.001 |
| Parameters | Unadjusted, Cox regression | Adjusted1, Cox regression | Log-rank test | ||
| HR (95%CI) | P value | HR (95%CI) | P value | P value | |
| ALT, Abnormality type | |||||
| Normal, | Reference | Reference | |||
| 1-2 ULN | 0.83 (0.65-1.07) | 0.146 | 0.95 (0.72-1.25) | 0.719 | < 0.001 |
| > 2-5 ULN | 0.52 (0.40-0.68) | < 0.001 | 0.68 (0.53-0.92) | 0.013 | |
| > 5 ULN | 0.84 (0.64-1.11) | 0.219 | 1.31 (0.98-1.79) | 0.092 | |
| AST, Abnormality type | |||||
| Normal | Reference | Reference | |||
| 1-2 ULN | 1.16 (0.94-1.43) | 0.169 | 1.07 (0.84-1.35) | 0.584 | 0.001 |
| > 2-5 ULN | 1.48 (1.11-1.98) | 0.008 | 1.49 (1.06-2.10) | 0.021 | |
| > 5 ULN | 2.13 (1.37-3.32) | 0.001 | 2.19 (1.27-3.76) | 0.005 | |
| Bilirubin, Abnormality type | |||||
| Normal | Reference | Reference | |||
| 1-2 ULN | 1.74 (1.27-2.40) | 0.001 | 1.58 (1.04-2.22) | 0.032 | < 0.001 |
| > 2-5 ULN | 2.49 (1.24-5.02) | 0.011 | 6.00 (2.90-12.41) | < 0.001 | |
| > 5 ULN | 2.78 (0.89-8.65) | 0.078 | 7.86 (1.88-32.96) | 0.005 | |
| ALP, Abnormality type | |||||
| Normal | Reference | Reference | |||
| 1-2 ULN | 1.52 (1.20-1.92) | < 0.001 | 1.42 (1.09-1.86) | 0.009 | 0.001 |
| > 2-5 ULN | 2.13 (1.36-3.35) | 0.001 | 1.81 (1.05-3.10) | 0.032 | |
| > 5 ULN | 1.05 (0.15-7.45) | 0.964 | 1.84 (0.25-13.38) | 0.547 | |
Need for vasopressor support: Only two (0.1%) patients in the non-severe group required vasopressor drugs, whereas 585 (43.2%) patients with severe COVID-19 required vasopressors (P < 0.001). Multivariable adjusted Cox proportional hazard regression analysis revealed that patients with abnormal AST (HR, 1.00, 95%CI, 1.00-1.00; P < 0.001), T-Bil (HR, 1.09, 95%CI, 1.03-1.17; P = 0.003), ALP (HR, 1.02, 95%CI, 1.01-1.03; P < 0.001), blood urea nitrogen (BUN) (HR, 1.007, 95%CI, 1.00-1.01; P < 0.001), and PT (HR, 0.795, 95%CI, 0.72-0.88; P < 0.001) were associated with a need for vasopressor drugs. Similarly, a higher state of inflammation was also associated with this outcome, namely higher levels of ferritin (HR, 1.00, 95%CI, 1.00-1.00; P = 0.035), D-Dimer (HR, 1.02, 95%CI, 1.00-1.04; P = 0.04), and IL-6 (HR, 1.00, 95%CI, 1.00-1.00; P < 0.001). However, higher BMI and abnormal ALT were not independently associated with the increased risk for this outcome. Of note, chronic neurological disease (HR, 0.77, 95%CI, 0.63-0.93; P = 0.004) and albumin (HR, 0.804, 95%CI, 0.69-0.93; P = 0.004) were associated with lower vasopressor support hazards in multivariate analyses.
Need for mechanical ventilation: Patients who received invasive mechanical ventilation were older, more likely to have preexisting comorbidities than were patients who did not receive invasive mechanical ventilation (Table 3). However, preexisting liver diseases did not differ between these groups. Median levels of ALT, AST, and T-Bil were significantly increased for patients who received mechanical ventilation (P < 0.0001) compared to those who did not receive it. However, no difference in ALP and GGT levels were seen between these two groups (ventilated P = 0.091 vs non-ventilated patients P = 0.483). Furthermore, patients who received invasive mechanical ventilation had varying degrees of abnormal liver chemistries (1-2 ×, > 2-5 ×, and > 5 × ULN), but in general, ALT, AST, and T-Bil values were significantly higher in these patients (P < 0.001). Multivariable Cox regression analysis adjusted for age, gender, ethnicity, race, BMI, and preexisting comorbidities revealed that older age, HT with complications, and abnormal levels of AST, T-Bil, BUN, and creatinine were associated with mechanical ventilation. In addition, a higher state of inflammation was also associated with this outcome, namely higher levels of neutrophil, ferritin, CRP, and IL-6.
This retrospective cohort study is one of the largest and most comprehensive to evaluate liver chemistries and clinical outcomes of hospitalized patients with COVID-19. Overall, the results show that liver injury, assessed by elevated liver enzyme levels, is commonly seen in hospitalized patients with COVID-19 and is associated with the risk of in-hospital mortality and other adverse clinical outcomes, such as the need for vasopressor drugs and mechanical ventilation. The key findings of the study are: (1) There is a high prevalence of liver injury (70.4%), defined by an elevation in ALT levels, in hospitalized patients with COVID-19; (2) Abnormal liver chemistries during hospitalization are strongly associated with mortality (ALT, T-Bil, and ALP); (3) Liver injury measured in patients with COVID-19 on admission is associated with the need for vasopressor drugs (AST, T-Bil, and ALP), and mechanical ventilation (AST, and T-Bil); (4) A strong and independent association of AST, T-Bil, and ALP correlates with the severity of COVID-19 infection; and (5) Elevated inflammatory markers (CRP, IL-6, ferritin, D-dimer, and LDH) are associated with increased risk of disease severity.
The pathophysiology of SARS-CoV-2 infection is similar to other coronavirus infections [SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV)] and shares a large genome sequence homology with other pathogenic human coronaviruses. SARS-CoV and MERS-CoV cause abnormal liver chemistries, and various degrees of hepatic injury[18,19]. SARS-CoV viral RNA is detected in autopsied human liver, suggesting a direct liver involvement[20]. Thus, similar liver injury in COVID-19 patients is not surprising. In particular, the overall ALT elevations were observed in 70.4% of patients with COVID-19 on hospital admission, and 79.3% of patients in the severe group had elevations much higher than previous reports. Based on a recent systematic review and meta-analysis (17 studies, 2711 patients), abnormal ALT is estimated to occur in ~15% of COVID-19 patients[14]. However, in Chinese cohorts, the prevalence of abnormal ALT among patients with COVID-19 ranged between 4% and 53.1%, and recent studies from the United States reveal even higher estimates, ranging between 39% and 64.9%[21-23].
In our cohort, only a small percentage of patients in our study had underlying liver disease (12.2%), suggesting that liver damage in these patients is directly caused by the COVID-19 infection and not an underlying condition or drug-induced liver injury from medications. Furthermore, our study showed varying degrees of liver enzyme levels, ranging from mild to severe. A previous study reported that 9.2% of ALT elevations were > ULN at the time of hospital admission[11], while another study reported higher rates of 36.5% >2 × ULN on admission[24]. Overall, our study showed that 38.8% of patients had abnormal ALT elevations > 2 × ULN (2-5 × ULN 26.7% and > 5 × ULN 12.1%).
Another potential cause of liver injury in our cohort is drug-induced liver injury. Self-medication before hospital admission was not reported in the data and is an unlikely cause. However, during hospitalization, treatment consists of a combination of antibiotics, antivirals, systemic corticosteroids, antipyretics, and analgesics to treat the COVID-19 infection, which may promote liver injury. In this study, these combined drug treatments positively correlated in patients with severe disease. Thus, drug-related liver injury during the treatment of COVID-19 infection needs to be considered. Future studies are needed to evaluate the possible effects of drugs on liver chemistries in patients with COVID-19.
Various studies have shown that older patients with COVID-19 have a higher case fatality rate[25], and having certain comorbidities may contribute to worse outcomes[26]. Similarly, our analysis revealed that risk factors for severe infection and death included older age, chronic heart disease, elevated inflammatory response, more prolonged PT, elevated liver enzymes, and bilirubin. In addition to ALT elevation, AST, T-Bil, and ALP were independently associated with the severity of COVID-19 infection, with over half of severe patients exhibiting AST elevation. The current study is consistent with prior studies that the pattern of liver injury is primarily hepatocellular instead of cholestatic, while our study indicates that elevations in T-Bil and ALP may be more common than previously reported[27]. Upon hospitalization, the percentage of the patients with elevated ALP and T-Bil in severe cases was 18.6% and 6.8%, respectively, which was significantly higher than 14.6 % and 4.6% in patients with the non-severe disease.
COVID-19 causes severe respiratory distress and pneumonia, with the latter being independently correlated with the need for ICU care, mechanical ventilation, and death[28]. Our study complements this knowledge and reveals that elevated liver chemistries can predict the risk of major in-hospital outcomes, such as the need for vasopressor drugs, mechanical ventilation, and death. Moreover, significant hypoalbuminemia was observed, particularly among patients with severe disease, and was also a predictor for the need for vasopressor drugs and mechanical ventilation. In the present study, the elevated levels of LDH, creatinine, BUN, IL-6, CRP, and ferritin are independently associated with mechanical ventilation risk.
COVID-19 induces a release of inflammatory cytokines, leading to organ dysfunction[19]. Inflammatory cytokine storm during COVID-19 infections is not uncommon and can result in sudden patient clinical deterioration and multiorgan failure. Direct hepatocyte injury caused by the SARS-CoV-2 may be closely related to systemic inflammatory response syndrome, and overproduction of cytokines is linked to the lung-liver axis[20]. An increase in systemic immune mediators that cause inflammation, oxidative stress, and underlying hypoxia facilitates and exacerbates liver function[29]. Our findings indicate an association between elevated inflammatory markers (CRP, IL-6, ferritin, D-dimer, and LDH) with increased risk of disease severity. IL-6 values are increased in patients with both severe COVID-19 and significantly increased in patients with elevated liver chemistries compared to patients with non-severe COVID-19. IL-6 is the primary driver of cytokine release syndrome, and IL-6 inhibitors are effective in treating severe COVID-19 cases[30]. Moreover, the neutrophil levels and serum CRP are significantly increased in patients with liver injury from COVID-19. These data imply a potential association between liver injury and the inflammatory responses induced by SARS-CoV-2 infection. Clinical treatment against the cytokine storm might also reduce liver injury and liver injury-related mortality. We also found a reduction in red blood cells in severe patients, which coincides with the fact that SARS-CoV-2 destroys hemoglobin in red blood cells, dissociates deoxyhemoglobin and iron, and produces hypoxia and respiratory distress. Increased ferritin levels due to cytokine storm and secondary hemophagocytic lymphohistiocytosis have also been reported in severe COVID-19 patients[31]. A higher level of ferritin was observed in patients with severe disease on admission than patients with a non-severe disease in the present study. Hypoxia may also damage hepatocytes and induce liver injury; thus, elevated levels of serum ferritin and hypoxia are potential indicators of hepatocyte injury in patients with severe COVID-19 infection. In our study, abnormal levels of LDH were found in patients with severe COVID-19, which was also seen in patients with SARS and MERS and was an independent risk factor for severe disease[32]. LDH is an intracellular enzyme found in cells in almost all organ systems and can be released during tissue damage, and is involved in various pathophysiological processes. Abnormal levels of LDH seem to reflect that multiple organ injury and failure. Despite its lack of specificity, serum LDH can have great prognostic significance in patients with COVID-19.
Limitations: Despite analyzing a large cohort of patients, the study has some limitations. Data collection was a retrospective observational cohort study and used electronic health record extraction within a single health system. Our health system is a tertiary medical health system, potentially introducing referral bias. The analysis represents only patients who were hospitalized, i.e., more likely to be in severe cases. Therefore, it cannot be entirely excluded that abnormal liver chemistries at admission might represent a more severe course in patients with COVID-19 with multiorgan involvement, including hepatobiliary manifestations. However, irrespective of the cause of liver injury at the time of hospitalization, we show a strong association between severity and liver chemistries at hospital admission rather than peak values, which may help guide clinical decisions early in the disease course. Furthermore, it was not feasible to describe all the potential causes of liver injury and all the causes of liver injury in the patients progressing to liver injury, such as the use of hepatotoxic medications and self-medication before hospitalization. Our study’s data permit an initial evaluation of patients' clinical course and outcomes with COVID-19. The causes of death in COVID-19 patients may involve multiple organ injuries, and it is challenging to differentiate liver injury as the primary and direct cause of death. We were unable to obtain long-term outcomes due to a comparatively short observation period. Further studies with long-term periods are required to understand the long-term impact of COVID-19 on the liver and elucidate the pathogenic mechanisms.
This study found that abnormal liver chemistries (AST, ALT, T-Bil, and ALP) at the time of hospital admission are associated with worse outcomes in COVID-19 patients, namely mortality (ALT, T-Bil, and ALP), the need for vasopressor drugs (AST, T-Bil, and ALP), and mechanical ventilation (AST, and T-Bil). Consequently, in hospitalized COVID-19 patients, elevated liver chemistries, specifically ALT, AST, ALP, and T-Bil levels, can be used to stratify risk and predict the need for advanced therapies.
Severe acute respiratory syndrome coronavirus 2 primarily infects the respiratory system. Abnormal liver chemistries are common findings in patients with Coronavirus Disease 2019 (COVID-19). In addition, increasing evidence exists for the direct multiorgan effect. However, the association of these abnormalities with the severity of COVID-19 and clinical outcomes is poorly understood.
To explore the impact of abnormal liver chemistries in hospitalized patients with COVID-19 and whether it is associated with worse outcomes, namely mortality, the need for vasopressor drugs, and mechanical ventilation.
We examine whether abnormal liver chemistries in COVID-19 hospitalized patients can be of prognostic value. We determined the prevalence of elevated liver chemistries in a large cohort of hospitalized patients with COVID-19 infection and identified whether an independent association exists between abnormal liver chemistries and clinical severity or the risk of in-hospital mortality.
This retrospective, observational study included 3380 patients with COVID-19 who were hospitalized in the Johns Hopkins Health System. Demographic data, clinical characteristics, laboratory findings, treatment measures, and outcome data were collected. Cox regression modeling was used to explore variables associated with abnormal liver chemistries on admission with disease severity and prognosis.
A total of 2698 (70.4%) had abnormal ALT at the time of admission. Other more prevalent abnormal liver chemistries were AST (44.4%), ALP (16.1%), and T-Bil (5.9%). Factors associated with liver injury were older age, Asian ethnicity, other race, being overweight, and obesity. Higher ALT, AST, T-Bil, and ALP levels were more commonly associated with disease severity. Multivariable adjusted Cox regression analysis revealed that abnormal AST and T-Bil were associated with the highest mortality risk than other liver injury indicators during hospitalization. Abnormal AST, T-Bil and ALP were associated with a need for vasopressor drugs whereas, higher levels of AST, T-Bil, and a decreased albumin levels were associated with mechanical ventilation.
This study found that abnormal liver chemistries (AST, ALT, T-Bil, ALP, and albumin) at the time of hospital admission are associated with worse outcomes in COVID-19 patients, namely mortality (ALT, T-Bil, and ALP), the need for vasopressor drugs (AST, T-Bil, and ALP), and mechanical ventilation (AST, and T-Bil). Consequently, in hospitalized COVID-19 patients, elevated liver chemistries, specifically ALT, AST, ALP, and T-Bil levels, can be used to stratify risk and predict the need for advanced therapies.
Abnormal liver chemistries are common at the time of hospital admission are associated with worse outcomes in COVID-19 patients. In particular, abnormal levels of AST, T-Bil, ALP, and hypoalbuminemia correlate with the severity of COVID-19 infection, and abnormal liver chemistries (ALT, T-Bil, and ALP) during hospitalization are strongly associated with all-cause mortality in patients with COVID-19. Furthermore, liver injury measured in patients with COVID-19 on admission is associated with the need for vasopressor drugs (AST, T-Bil, and ALP) and mechanical ventilation (AST, and T-Bil).
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cai J, Tan JK S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Krishnan A, Hamilton JP, Alqahtani SA, A Woreta T. A narrative review of coronavirus disease 2019 (COVID-19): clinical, epidemiological characteristics, and systemic manifestations. Intern Emerg Med. 2021;16:815-830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 574] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 3. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 4. | Qi X, Liu C, Jiang Z, Gu Y, Zhang G, Shao C, Yue H, Chen Z, Ma B, Liu D, Zhang L, Wang J, Xu D, Lei J, Li X, Huang H, Wang Y, Liu H, Yang J, Pan H, Liu W, Wang W, Li F, Zou S, Zhang H, Dong J. Multicenter analysis of clinical characteristics and outcomes in patients with COVID-19 who develop liver injury. J Hepatol. 2020;73:455-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 5. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi GF, Fang N, Fan J, Cai J, Lan F. Specific ACE2 expression incholangiocytes may cause liver damage after 2019-nCoV infection. 2020 Preprint. Available from: bioRxiv:2020.02.03.931766. [DOI] [Full Text] |
| 6. | Mao R, Liang J, Shen J, Ghosh S, Zhu LR, Yang H, Wu KC, Chen MH; Chinese Society of IBD, Chinese Elite IBD Union; Chinese IBD Quality Care Evaluation Center Committee. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020;5:425-427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 7. | Centers for Disease Control and Prevention. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19) [cited 07 February 2020]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html. |
| 8. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 9. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18189] [Article Influence: 3637.8] [Reference Citation Analysis (0)] |
| 10. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 271] [Article Influence: 54.2] [Reference Citation Analysis (2)] |
| 11. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095-2103. [PubMed] [DOI] [Full Text] |
| 12. | Vespa E, Pugliese N, Piovani D, Capogreco A, Danese S, Aghemo A; Humanitas Covid-19 Task Force. Liver tests abnormalities in COVID-19: trick or treat? J Hepatol. 2020;73:1275-1276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 14. | Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, Falck-Ytter Y, El-Serag HB; AGA Institute. Electronic address: ewilson@gastro.org. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology. 2020;159:320-334.e27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (1)] |
| 15. | PMAP: The Johns Hopkins Precision Medicine Analytics Platform. [cited 07 February 2020]. Available from: https://pm.jh.edu/. |
| 16. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 2834] [Article Influence: 404.9] [Reference Citation Analysis (0)] |
| 17. | WHO. Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf. |
| 18. | Kong SL, Chui P, Lim B, Salto-Tellez M. Elucidating the molecular physiopathology of acute respiratory distress syndrome in severe acute respiratory syndrome patients. Virus Res. 2009;145:260-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Hilliard KL, Allen E, Traber KE, Yamamoto K, Stauffer NM, Wasserman GA, Jones MR, Mizgerd JP, Quinton LJ. The Lung-Liver Axis: A Requirement for Maximal Innate Immunity and Hepatoprotection during Pneumonia. Am J Respir Cell Mol Biol. 2015;53:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5781] [Article Influence: 1156.2] [Reference Citation Analysis (2)] |
| 21. | Lee IC, Huo TI, Huang YH. Gastrointestinal and liver manifestations in patients with COVID-19. J Chin Med Assoc. 2020;83:521-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (2)] |
| 22. | Hajifathalian K, Krisko T, Mehta A, Kumar S, Schwartz R, Fortune B, Sharaiha RZ; WCM-GI research group*. Gastrointestinal and Hepatic Manifestations of 2019 Novel Coronavirus Disease in a Large Cohort of Infected Patients From New York: Clinical Implications. Gastroenterology. 2020;159:1137-1140.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 23. | Cholankeril G, Podboy A, Aivaliotis VI, Tarlow B, Pham EA, Spencer SP, Kim D, Hsing A, Ahmed A. High Prevalence of Concurrent Gastrointestinal Manifestations in Patients With Severe Acute Respiratory Syndrome Coronavirus 2: Early Experience From California. Gastroenterology. 2020;159:775-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 165] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 24. | Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72:1169-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 25. | Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11409] [Cited by in RCA: 11504] [Article Influence: 2300.8] [Reference Citation Analysis (0)] |
| 26. | Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX; China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1711] [Cited by in RCA: 2188] [Article Influence: 437.6] [Reference Citation Analysis (0)] |
| 27. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 28. | Jain V, Yuan JM. Predictive symptoms and comorbidities for severe COVID-19 and intensive care unit admission: a systematic review and meta-analysis. Int J Public Health. 2020;65:533-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 248] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 29. | Portincasa P, Krawczyk M, Machill A, Lammert F, Di Ciaula A. Hepatic consequences of COVID-19 infection. Lapping or biting? Eur J Intern Med. 2020;77:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 30. | Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117:10970-10975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1811] [Cited by in RCA: 1748] [Article Influence: 349.6] [Reference Citation Analysis (0)] |
| 31. | Velavan TP, Meyer CG. Mild vs severe COVID-19: Laboratory markers. Int J Infect Dis. 2020;95:304-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 337] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 32. | Guo Y, Korteweg C, McNutt MA, Gu J. Pathogenetic mechanisms of severe acute respiratory syndrome. Virus Res. 2008;133:4-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |