Published online Dec 28, 2022. doi: 10.3748/wjg.v28.i48.6846
Peer-review started: September 20, 2022
First decision: October 18, 2022
Revised: November 1, 2022
Accepted: December 5, 2022
Article in press: December 5, 2022
Published online: December 28, 2022
Processing time: 98 Days and 1.1 Hours
Bile acids (BAs) serve as physiological detergents that enable the intestinal absorption and transportation of nutrients, lipids and vitamins. BAs are primarily produced by humans to catabolize cholesterol and play crucial roles in gut metabolism, microbiota habitat regulation and cell signaling. BA-activated nuclear receptors regulate the enterohepatic circulation of BAs which play a role in energy, lipid, glucose, and drug metabolism. The gut microbiota plays an essential role in the biotransformation of BAs and regulates BAs composition and metabolism. Therefore, altered gut microbial and BAs activity can affect human metabolism and thus result in the alteration of metabolic pathways and the occurrence of metabolic diseases/syndromes, such as diabetes mellitus, obesity/hypercholesterolemia, and cardiovascular diseases. BAs and their metabolites are used to treat altered gut microbiota and metabolic diseases. This review explores the increasing body of evidence that links alterations of gut microbial activity and BAs with the pathogenesis of metabolic diseases. Moreover, we summarize existing research on gut microbes and BAs in relation to intracellular pathways pertinent to metabolic disorders. Finally, we discuss how therapeutic interventions using BAs can facilitate microbiome functioning and ease metabolic diseases.
Core Tip: Bile acids (BAs) in enterohepatic circulation regulate metabolism through interorgan communication between the gut and liver microbiota. BAs secreted from the liver contribute to glucose and lipid metabolism. Disruption of the BA-gut microbiome link contributes to the occurrence of metabolic diseases, such as obesity, type 2 diabetic mellitus, and dyslipidemia. BAs and their metabolites can be used as potential therapeutics for treating metabolic diseases.
- Citation: Sah DK, Arjunan A, Park SY, Jung YD. Bile acids and microbes in metabolic disease. World J Gastroenterol 2022; 28(48): 6846-6866
- URL: https://www.wjgnet.com/1007-9327/full/v28/i48/6846.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i48.6846
Bile acids (BAs) are unique amphipathic molecules that are primarily produced in the liver. They function as physiological detergents to facilitate bile flow and promote the transportation of nutrients, vitamins, and lipids via intestinal absorption[1]. Hepatic BA production accounts for a significant portion of the total cholesterol turnover in humans[2]. The principal constituents of bile are BAs, bilirubin, cholesterol, and phospholipids. BAs are mainly classified into primary and secondary types. Primary BAs (PBAs) include cholic acid (CA) and chenodeoxycholic acid (CDCA). Their corresponding secondary BAs are deoxycholic acid (DCA) and lithocholic acid (LCA), which are produced by microbial enzymes in the colon via deconjugation and 7α-dehydroxylation and are the most ubiquitous BAs in humans[3]. PBAs are formed by cholesterol in pericentral hepatocytes through a series of staged processes that are catalyzed by metabolic enzymes, particularly cytochrome P450 enzymes[4].
BAs synthesis is predominantly mediated by classic and alternative pathways in the liver. In the classic pathway, the rate-limiting enzyme CYP7A1 in the endoplasmic reticulum converts cholesterol into 7α-hydroxycholesterol (HOC). The intermediate 7α-hydroxy-4 cholesterin-3-one (C4) is converted by the sterol 12′α-hydroxylase (CYP8B1) to 7′α, 12′α-dihydroxy-4-cholesterin-3-one, which results in the production of CA. Without the 12α-hydroxylation of CYP8B1, C4 is ultimately transformed into CDCA. Both CA and CDCA syntheses use the mitochondrial enzyme CYP27A1 to catalyze the oxidation of the steroid side chains. In the alternative pathway, cholesterol is transformed by CYP27A1 into 27-HOC, which is in turn transformed into CDCA. Bacterial 7-dehydroxylase eliminates a hydroxyl group at C-7 in the large intestine, which converts CA into DCA and CDCA into LCA. The secondary BAs hyocholic acid, murideoxycholic acid, α-muricholic acid (ω-MCA), hyodeoxycholic acid (HDCA), and ursodeoxycholic acid (UDCA) are produced by CYP3A1 and epimerases from CDCA. The majority of LCA and ω-MCA are eliminated via feces[5].
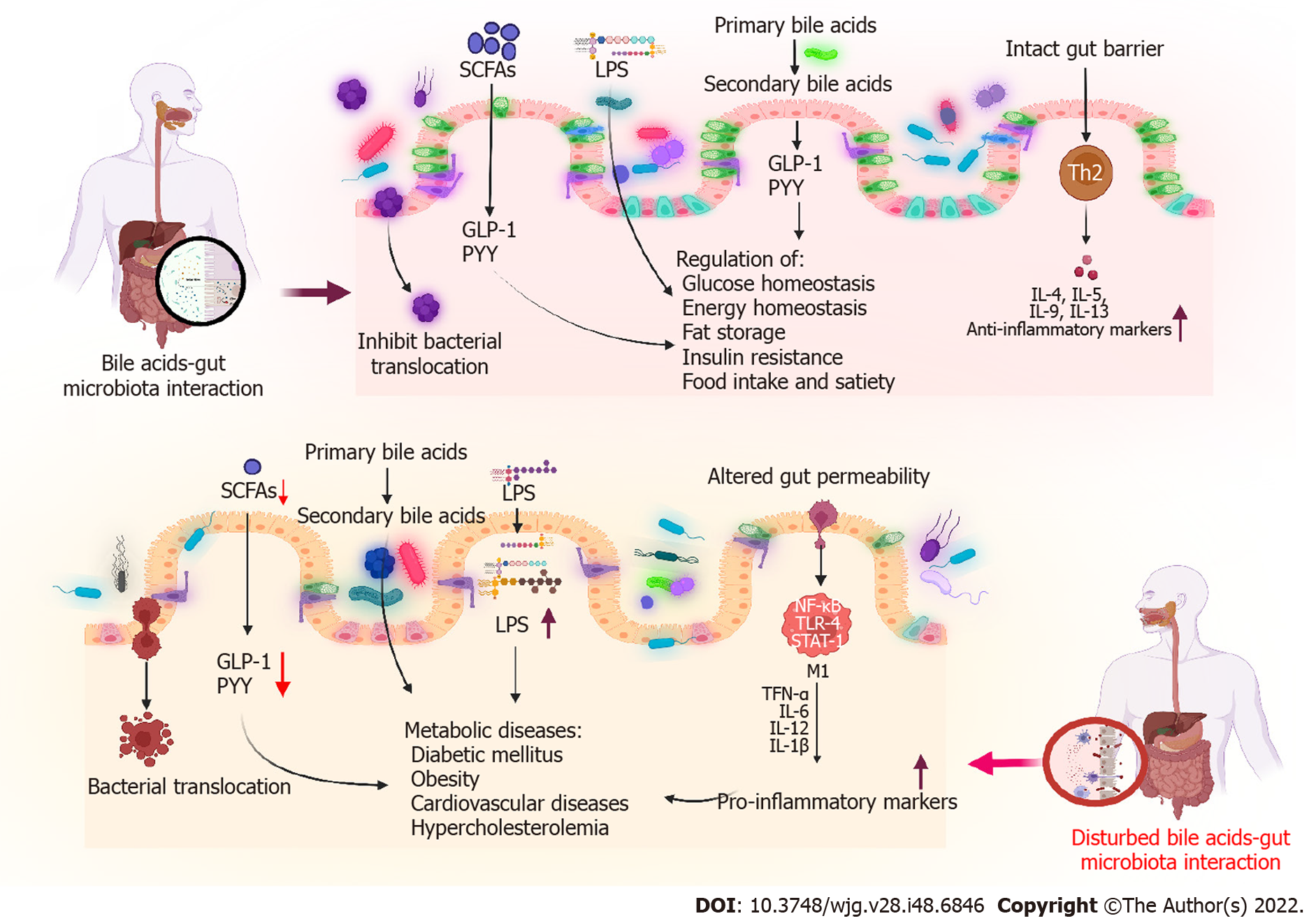
In addition to their involvement in the absorption of dietary lipids and cholesterol homeostasis, BAs play a versatile signaling role. Many signaling pathways can be activated by BAs. These include a wide range of metabolic pathways, such as those involved in glucose, lipid, drug, and energy metabolism[6]. During BAs metabolism, cholesterol is converted into BAs in the liver and is further metabolized by the gut microbiota. Moreover, dense populations of microorganisms inhabit the gut, making it one of the most complex ecosystems for health. For the past two decades, research has focused on the influence of the gut microbiome on health. BAs deconjugation occurs in the small intestine and is mediated by bile salt hydrolase (BSH)-active bacteria, resulting in the maintenance of normal circulating levels of deconjugated BAs and cholesterol. Through these bioconversions, BAs modulate diverse metabolic pathways in the host through signaling mediated by nuclear farnesoid X receptors (FXRs) and G-protein-coupled membrane receptors (GPCRs). Furthermore, BAs can influence the gut microbial composition both directly and indirectly by activating innate immune responses. Consequently, the host metabolism is affected by altered signaling via BA receptors (BARs) induced by microbial modification and by altered microbiota composition[7]. Therefore, the gut microbiota must be maintained for normal metabolic function and homeostasis. Altered gut microbiota composition may be related to metabolic diseases, such as diabetes mellitus (DM) and obesity[8]. Altered BAs synthesis and function are also associated with metabolic diseases. This review mainly focuses on the relationship between gut microbiota and BAs in metabolic diseases, emphasizing on the BA-mediated reversal of metabolic diseases (Figure 1).
The term microbiome refers to the entire genome of the gut microbiota, which is two orders of magnitude larger than the human nuclear genome. Humans inherit the vaginal microbiome of their mothers at the time of birth. Eventually, a mutualistic relationship between this microbiota and the host is developed[9]. The human gastrointestinal system is colonized by numerous microorganisms, collectively known as the gut microbiota; these include bacteria, viruses, archaea, fungi, and protozoa. The human gut microbiota contains up to 100 trillion microorganisms[10]. It plays an integral role in maintaining host health as it not only helps derive nutrients from food but also builds various metabolites that can regulate host metabolism[11]. One of these metabolites, BA, is produced in the liver through cholesterol and is additionally metabolized by the gut microbiota into secondary BAs[12]. A key physiological function of the gut microbiota is the modification of BAs composition. In addition to secondary BAs, different BAs species are produced in humans by the gut microbiota[13]. In the gut, branched-chain amino acids, short-chain fatty acids (SCFAs), indole, succinate, and imidazole are metabolites produced by gut microbes during anaerobic fermentation. These metabolites serve as key signaling components in the BA-gut microbe signaling pathways[14]. Various microbial genera produce these metabolites; these include Akkermansia, Bacteroides, Clostridium, Coprococcus, Eubacterium, Faecalibacterium, Fusobacterium, Lactobacillus, Prevotella, Propionibacterium, Ruminococcus, Roseburia, and Streptococcus[15]. The BAs composition is shaped by gut microbes that exhibit certain enzymatic activities, e.g., BSH or 7-dehydroxylation activity mediated by BA-inducible enzymatic reactions. BAs exert their effects by activating a class of receptors known as BARs. This receptor family comprises nuclear receptors, such as FXRs, vitamin D receptors, pregnane X receptor, and GPCRs (including GPBAR1)[13]. The gut microbiota modulates fibroblast growth factor 15 (FGF15) signaling through an FXR-dependent mechanism[16]. Recent research has linked gut microbe metabolism to the size of the BAs pool.
In germ-free (GF) and conventionally raised mice, the gut microbiota could not only regulate secondary BAs metabolism but also inhibit hepatic BA synthesis by suppressing FXR inhibition in the ileum[16]. Moreover, BAs can affect the gut bacterial composition by directly and indirectly activating genes associated with innate immunity in the small intestine[7]. Therefore, bacteria-induced changes in BAs may result in altered signaling of BARs and affect host metabolism. BAs and the gut microbiota can interact in various ways, and interruptions in these physiological interactions can cause several diseases. The composition of the intestinal microbiota and/or the intraluminal metabolome may be the cause or consequence of various disorders; however, their association remains unknown. Various recent studies have reported the association of dyslipidemia, insulin resistance (IR), and DM with the dysregulation of BAs metabolism and alteration of the gut microbiota. This review mainly focuses on altered BA-gut microbiome interactions in metabolic diseases.
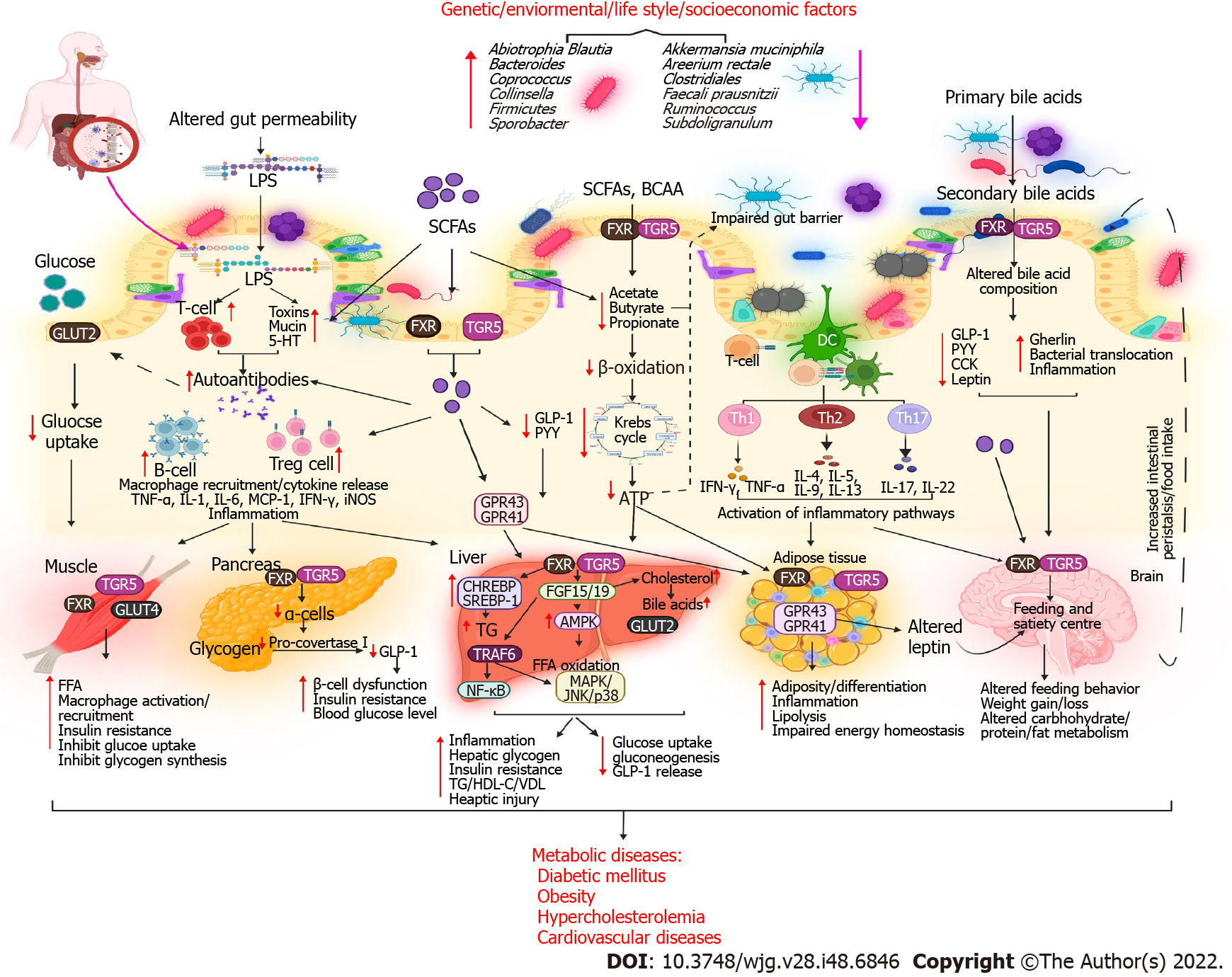
Research on BA has significantly enriched our understanding of BAs synthesis and metabolic syndrome over the last two decades. BAs play a crucial role in regulating glucose, lipid, and energy metabolism. Several metabolic diseases, including type 2 DM (T2DM), obesity, and nonalcoholic fatty liver disease (NAFLD), result from disrupted BA homeostasis[17]. The gut microbiota is a “metabolic organ” that regulates host metabolism[18]. Gut microbes, including Bacteroides, Bifidobacterium, Clostridium, Enterobacter, and Lactobacillus species, play an important role in the synthesis, modification, and signaling of BAs[15]. The gut microbiota has recently been reported to play a role in obesity, in addition to other widely acknowledged major causes, which include an increased caloric intake and decreased energy expenditure. These factors are also linked to T2DM, metabolic syndrome, and CADs[19]. Diverse mechanisms have been proposed by which gut microbes can modulate metabolic diseases. Disrupted BA-gut microbiome interactions can cause metabolic disease (Table 1 and Figure 2).
| No. | Model | Findings | Ref. |
| 1 | A T1DM clinical study | The abundance of Alistipes shahii, Asaccharobacter celatus, Blautia obeum, Coprococcus eutectic, Coprobacillus cateniforms, Clostridium symbiosum, and Eggerthella lenta significantly increased in adolescents with T1DM. Compared with healthy adolescents, the biosynthesis of vitamins, amino acids, electron carriers, and enzyme cofactors was downregulated, whereas fermentation pathways were upregulated in adolescents with T1D | [150] |
| 2 | An HFD-fed obese mouse model | Non-12-OH BA levels were higher in HF-OR mice. The levels of non-12-OH BASs, such as UDCA, CDCA, and LCA, decreased in HF-OP mice and were linked to changed gut flora. The abundance of C. scindens were reduced in HF-OP mice and positively correlated with UDCA and LCA. The administration of C. scindens to animals increased the levels of hepatic non-12-OH BAs and serum 7-hydroxy-4-cholesterin-3-one (C4). Changes in BA composition in HF-OP mice were associated with considerably lower GLP-1 expression levels in the ileum and PGC1 and UCP1 expression levels in brown adipose tissues | [18] |
| 3 | Patients with GDM and germ-free mice | The abundance of Bacteroides and Akkermansia decreased and that of Faecalibacterium increased with hyperglycemia | [151] |
| 4 | Women with GDM: A clinical study | The relative abundance of Streptococcus, Faecalibacterium, Veillonella, Prevotella, Haemophilus, and Actinomyces significantly increased with an increase in FBG levels and hyperlipidemia | [51] |
| 5 | A combination of BAs with dietary lard feeding in C57BL/6N mice | Impaired glucose tolerance; lower fasting insulin levels; lower counts of enteroendocrine cells; fatty liver; and elevated levels of hepatic TGs, cholesteryl esters, and monounsaturated fatty acids were noted. The relative abundance of Lachnospiraceae decreased and that of Desulfovibrionaceae, Clostridium lactatifermentans, and Flintibacter butyricus increased | [152] |
| 6 | A T2DM clinical study | Postprandial total BAs levels increased with an increase in the meal fat content and peaked after 1-2 h. Unconjugated and glycine-conjugated forms of DCA, CA, and UDCA were altered and FGF-19 levels were reduced in participants with T2DM | [153] |
| 7 | HFD-fed C57BL/6J mice with Enterobacter cloacae B29 | Obesity and IR were induced | [45] |
| 8 | A T2DM clinical study | BAs increased twofold, and more hydrophobicity and higher 12α-hydroxy/non-12α-hydroxy BAs ratios were linked with lower insulin sensitivity and higher plasma TG levels | [154] |
| 9 | C57BL/6J ob/ob mice, lean ob/+, and HFD-fed mice | The abundance of A. muciniphila decreased in mice who were obese and had T2DM | [32] |
| 10 | A clinical study | The postprandial total bile acid response decreased in obese participants | [155] |
| 11 | Pregnancy with obesity: A clinical study | The abundance of Bifidobacterium and Bacteroides decreased and that of Staphylococcus, Enterobacteriaceae, and E. coli increased in overweight pregnant women compared with that in normal-weight pregnant women. The abundance of E. coli was higher in women with excessive weight gain than in those with normal weight gain during pregnancy. Bifidobacterium and A. muciniphila showed an opposite trend. The abundance of total bacteria, Staphylococcus, Bacteroides, Bifidobacterium, Enterobacteriaceae, and E. coli increased and that of Bifidobacterium decreased | [22] |
| 12 | ApoA-1-knockout mice, HFD-fed mice, and wild-type mice | Gut barrier-protecting Bifidobacterium species were absent, and impaired glucose tolerance was significantly increased | [27] |
| 13 | Zucker rats (obese/lean) | Increased numbers of Halomonas and Sphingomonas species, plasma LDL and VLDL levels, and reduced urinary hippurate and creatinine levels were noted in obese rats | [156] |
| 14 | Overweight pregnant women: A clinical study | Increased numbers of Bacteroides and Staphylococcus species were noted in obese pregnant women | [29] |
| 15 | HFD-fed mice | The abundance of intestinal gram-negative and gram-positive bacteria and Bifidobacterium species significantly decreased and endotoxemia significantly increased | [146] |
| 16 | C57BL/6J ob/ob mice, lean ob/+, and wild-type mice | A 50% reduction was noted in the abundance of Bacteroidetes, and an increase was noted in the abundance of Firmicutes | [157] |
The global prevalence of various chronic diseases is increasing; obesity is the main cause and has been a serious concern for decades[20]. Obesity is linked to T2DM, NAFLD, hypertension, CAD, and cancer[21]. The prevalence of obesity is influenced by genetic and environmental factors, such as diet, culture, and socioeconomic status[22]. There is mounting evidence that the intestinal microbiota is inextricably related to general health, including obesity risk. Obesity-related metabolic diseases are defined by unique changes in the diversity and function of the human gut microbiome[23]. The human gut is home to trillions of microbes, which break down otherwise indigestible foods[24]. A study revealed that transferring the gut microbiota from healthy mice to GF recipients could increase body fat without a significant increase in food consumption and suggested that the composition of gut microbial communities could affect how much energy is derived from food[25]. In particular, the gut–brain axis indirectly affects commensal organisms, intestinal permeability, motility, and secretion and modifies the levels of plasma peptides, particularly glucagon-like peptide 1 (GLP-1) and peptide YY (PYY), by releasing signaling molecules into the gut lumen[26].
In humans, the gut microbiota has been linked to body weight and weight loss following a lifestyle change. Gram-negative opportunistic pathogens in the gut may play a significant role in obesity[27]. The gut microbiota of ectomorphs has more Bacteroidetes species, whereas that of obese individuals has more Firmicutes species, particularly Clostridium clusters[23]. Thus, the bacterial composition could enhance the capacity of the host to absorb energy from their diet and retain it in adipose tissues[28]. In lean as well as obese pregnant subjects, an increase in Bacteroides, Staphylococcus[29], and Bifidobacterium species was found to increase high-density lipoprotein cholesterol (HDL-C) and folic acid levels and reduce triglyceride (TG) levels[22]. On the other hand, the abundance of Akkermansia muciniphila (A. muciniphila), a mucoprotein-degrading bacterium present in the mucus layer[30], was negatively correlated with body weight[22]. A decrease in the abundance of A. muciniphila was noted in obese and diabetic mice[31]. Feeding high-fat diets with viable A. muciniphila can hinder the development of metabolic disorders, such as obesity, low-grade inflammation, and metabolic endotoxemia[32]. A metatranscriptomic analysis revealed that mice receiving the microbiome of obese twins had higher expression levels of microbial genes associated with detoxification and oxidative stress, amino acid metabolism, cobalamin biosynthesis, and the pentose phosphate pathway[13].
BA metabolism is altered in obese and diabetic individuals[33]. Patti et al[34] reported that patients who underwent Roux-en-Y gastric bypass (RYGB) had improved glucose and fat metabolism. This finding was attributed to the activation of GPCRs and subsequent stimulation of GPBAR1 (TGR5, a membrane-bound BAR) and increase in deiodinase (a type II thyroid hormone) levels. Although recent research has revealed a link between the gut microbiota and obesity, the precise molecular pathways remain unknown. In particular, the role of distinct gut microbial species and their metabolites in the regulation of obesity-related lipid metabolism and formation of the obese phenotype remains unknown. Mechanisms linking the gut microbiota to obesity are being revealed through a collaborative approach of translation-focused human and animal studies. Increasing evidence indicates that the gut microbiota mediates the effects of diet on host metabolism[35]. In BA metabolism, TGR5 signaling is regulated by the microbiota by generating agonists[36], whereas FXR signaling is regulated by metabolizing antagonists[3]. Both TGR5 and FXR have a significant influence on metabolism, and an altered microbiota may impact host physiology by modifying the signals transmitted through these receptors. The ability to metabolize TauroMCA, a naturally occurring FXR antagonist, is required for the microbiota to induce obesity, steatosis, and impaired glucose and insulin tolerance. An altered microbiota is responsible for these effects[37]. Taken together, these results indicate that targeting BAs, which function as microbiome-produced molecular regulators of energy homeostasis, can offer a substantial opportunity for treating obesity.
The prevalence of T2DM is increasing worldwide. By 2030, the incidence of T2DM is expected to be 360 million worldwide, with the estimated population being 8.5 billion[38]. BAs are involved in the alteration of glucose metabolism associated with obesity and T2DM. By stimulating GLP1 synthesis in the small bowel and colon, BAs contribute to carbohydrate and fat metabolism. In addition to inducing IR and T2DM, BAs exhibit an insulin-sensitizing effect[33]. They control glucose homeostasis by directly acting on FXR and TGR5 in the liver, intestine, and pancreas and by indirectly stimulating FXR-dependent intestinal FGF15/19 production[39]. Both FXR and TGR5 are abundant in pancreatic b cells, where they favorably control insulin production and glucose-induced insulin secretion. TGR5 activation in pancreatic α cells promotes the expression of proconvertase-1, which shifts the synthesis of glucagon to GLP-1, thereby enhancing β-cell density and functioning in a paracrine manner. In patients with DM, BAs may promote FXR activation in L cells in the ileum. In an animal model of obesity, Cipriani et al[40] found that 6E-CDCA activated FXR, reversed IR, and restored lipid metabolism. Moreover, the microbiota could downregulate FXR with the maximum efficiency by converting PBAs into secondary BAs[16].
External factors, such as diet, can alter the gut microbiota and cause dysregulation and secretory changes in intestinal microbial metabolites, triggering a series of possible mechanisms that lead to DM and insulin sensitivity[41]. In a metagenome-wide association study involving 345 Chinese participants with DM, gut microbial dysbiosis caused by opportunistic pathogens was moderate. Moreover, the reduction of butyrate-producing bacteria was associated with sulfate reduction and oxidative stress resistance[42]. Complex interactions between the immune system and gut microbiome have also been linked to both T1DM and T2DM. Aggarwal et al[43] reported that a combination of antidiabetic and antibiotic treatments reversed IR, hyperglycemia, and dyslipidemia and normalized blood glucose utilization in iNOS−/− mice. Duodenal-jejunal bypass liner (DJBL) replacement in obese patients with T2DM was found to increase unconjugated BA levels in a clinical study[44]. Fei et al[45] reported a high percentage of endobacteria (35%), pathogenic bacteria that produce endotoxin, in the gut microbiome of obese participants with hypertension, DM, and other severe metabolic complications. Patients with T2DM are particularly deficient in butyrate-producing microbes, such as Clostridiales species, Ruminococcus species, Subdoligranulum species, Areerium rectangle, Faecalibacterium prausnitzii, Roseburia intestinalis, and R. inulinivorans, and exhibit a high abundance of specific genera, such as Abiotrophia, Blautia, Coprococcus, Collinsella, Parasutterella, Peptostreptococcus, and Sporobacter[15].
Moreover, Lambeth et al[46] demonstrated that Actinobacteria, Bacteroidetes, Firmicutes, Proteobacteria, Pseudonocardiaceae, Verrucomicrobia, and Colorado species were significantly more prevalent in the pre-DM stage, whereas Enterobacteriaceae and Collinsella species were significantly more prevalent in patients with T2DM. Similarly, Larsen et al[47] reported a significant decrease in the prevalence of phylum Firmicutes, class Betaproteobacteria, and genus Clostridium in patients with T2DM. The Bacteroidetes: Firmicutes ratio and the Bacteroides-Prevotella group: C. coccoides-E. rectale group ratio showed a positive correlation and significantly increased the plasma glucose levels. During pregnancy, the abundance of the beneficial species R. intestinalis and F. prausnitzii decreases, whereas that of Proteobacteria and Actinobacteria phyla increases[48]. If these compositions are altered, pregnant women may experience an increase in adipose mass, blood sugar levels, IR, and circulating proinflammatory cytokines, resulting in gestational DM (GDM)[49]. In patients with GDM, obesity, and T2DM, the relative abundance of SCFA-producing bacteria belonging to the genera Faecalibacterium, Rubinococcus, Roseburia, Coprococcus, Akkermansia, Phascolarctobacterium, and Eubacterium was found to decrease[50]. Moreover, Liu et al[51] demonstrated increased hyperlipidemia and fasting blood glucose (FBG) levels and increased relative abundance of Streptococcus, Faecalibacterium, Veillonella, Prevotella, Haemophilus, and Actinomyces species in patients with GDM.
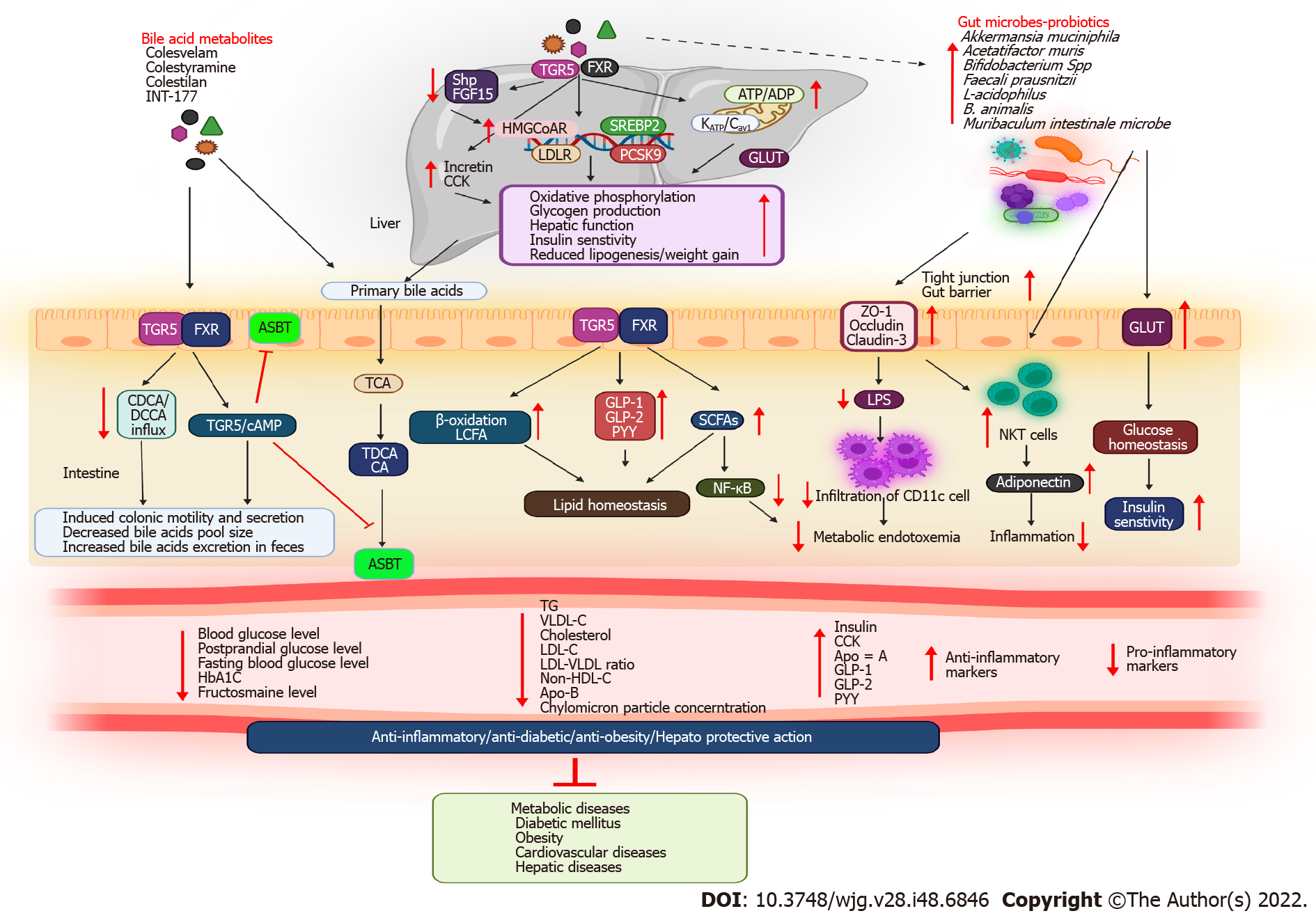
BAs play an important role in signaling and metabolism, reigniting interest in these molecules as potential therapeutic targets. Studies have revealed that drugs used to treat metabolic diseases can alter the gut microbial environment. Similarly, antidiabetic medications may alter the composition of the gut microbiota, plasma, and fecal BAs, which may improve metabolic health. Notably, patients with T2DM had better glycemic control when taking medications for preventing BAs absorption from the small intestine or limiting enterohepatic circulation. Hence, experimental and clinical studies have focused on the therapeutic applications of BAs in metabolic diseases. Furthermore, microbiota targeting could open novel research avenues. Table 2 and Figure 3 depict BAs and their metabolites used for treating metabolic diseases.
| No. | Drugs | Model | Findings | Ref. |
| 1 | A combination of GLP-1 and DMR | An insulin-dependent T2DM clinical study | Combined treatment allowed the discontinuation of insulin treatment in 69% of patients, increased postprandial unconjugated bile acid responses, induced an overall increase in the secondary bile acid response, induced an increase in the 12α-hydroxy: non-12α-hydroxy BA ratio, and improved the microbiome response | [158] |
| 2 | Colesevelam | Germ-free C57BL/6 mice with obesity, NAFLD, and NASH | Reduced body and liver weight gain were noted in microbiome-humanized mice, in addition to the amelioration of hepatic inflammation, steatosis, fibrosis, and IR. Colesevelam increased de novo bile acid synthesis and reduced the hepatic cholesterol content in microbiome-humanized mice, induced the expression of the antimicrobial genes Reg3g and Reg3b in the distal small intestine, and reduced plasma LPS levels | [159] |
| 3 | Vancomycin | iNOS−/− mice | Metabolic disturbances, dyslipidemia, and insulin resistance in iNOS−/− mice were improved by the vancomycin-mediated reduction of gram-positive bacteria | [160] |
| 4 | Sevelamer | Western diet-fed C57BL/6J mice with NASH | Interruption of intestinal reabsorption and reduction of circulating bile acid levels were noted. Microbiota complexity in the cecum was reversed by increasing the abundance of Lactobacillus and decreasing the abundance of Desulfovibrio. Hepatic injury was reversed, and the progression of NASH, including steatosis, inflammation, and fibrosis, was inhibited | [161] |
| 5 | Sevelamer | CDHF-fed C57BL/6J mice | Hepatic steatosis, macrophage infiltration, and pericellular fibrosis were prevented in CDHF-fed mice. The portal levels of total bile acid were reduced, and hepatic and intestinal FXR activation was inhibited. The α-diversity was decreased, and decreases in Lactobacillaceae and Clostridiaceae populations and increases in Desulfovibrionaceae and Enterobacteriaceae populations were prevented in CDHF-fed mice. Intestinal tight junction proteins were restored and portal LPS levels were reduced, resulting in the suppression of the hepatic toll-like receptor 4 signaling pathway | [162] |
| 6 | B. animalis 01 | A T2DM rat model | Treatment with B. animalis 01 improved OGTT, HOMA-IR, and lipid profiles; reduced hepatic tissue injury; increased glycogen levels; improved antioxidant levels; and modulated the expression of genes involved in hepatic glucose metabolism and the IRS/PI3K/AKT pathway. Moreover, it positively regulated the hepatic Keap1/Nrf2 pathway | [141] |
| 7 | A. muciniphila | Overweight/obese insulin-resistant volunteers | A. muciniphila improved insulin sensitivity; reduced insulinemia and plasma total cholesterol levels; and slightly reduced body weight, fat mass, and hip circumference. Three months after supplementation with A. muciniphila, liver dysfunction and inflammatory blood marker levels decreased without affecting the gut microbiome structure | [132] |
| 8 | Bacteroides transplantation | A clinical study on children with diabetes/germ-free NOD mice | Compared with germ-free NOD mice, the onset of diabetes was markedly delayed in all bacteriome-humanized participants | [163] |
| 9 | A. muciniphila | C57BL/6 mice/HFD-fed mice | A. muciniphila treatment reversed HFD-induced fat mass gain, metabolic endotoxemia, adipose tissue inflammation, and IR. A. muciniphila supplementation increased the intestinal levels of endocannabinoids that control inflammation, the gut barrier, and gut peptide secretion | [32] |
| 10 | Acarbose | A clinical study | The ratio of primary: secondary BAs and plasma levels of unconjugated BAs were increased. The relative abundance of Lactobacillus and Bifidobacterium species in the gut microbiota was increased and Bacteroides species were depleted in participants with T2DM | [164] |
| 11 | Metformin | A clinical study | Metformin-altered microbiota improved glucose tolerance, and a significant negative correlation was noted between unconjugated BAs and HbA1c levels | [165] |
| 12 | Lactobacillus acidophilus, L. casei, and Bifidobacterium bifidum for 6 wk | GDM: A clinical study | Significant reductions were noted in fasting plasma glucose, serum insulin, serum triglyceride, and VLDL cholesterol levels and a significant increase was noted in the quantitative insulin sensitivity check index in women with GDM | [119] |
| 13 | Probiotics | Cherry Valley Pekin ducks | The LXRα and CYP7α1 enzymatic activity increased and TG and TC concentrations decreased | [123] |
| 14 | Probiotics (Lactobacillus salivarius UCC118) | GDM: A clinical study | The body weight, FBG, and IR index significantly decreased and insulin sensitivity index increased in women with GDM | [166] |
| 15 | Probiotics (Lactobacillus salivarius UCC118) | Obese pregnant women: A clinical study | Significant alteration was noted in the BMI | [167] |
| 16 | Probiotic Lactobacillus sporogenes | Third-trimester pregnancy: a Clinical study | A significant decrease was noted in serum insulin levels and HOMA-IR, and a significant difference was noted in HOMA-B | [168] |
| 17 | Probiotics (VSL#3) | C57J/B6 male mice/HFD-fed mice | Probiotic supplementation reduced the body weight IR; modulated the gut microbe composition; and increased GLP-1 release, glucose tolerance, SCFA levels, and butyrate levels | [121] |
| 18 | Probiotics | Pregnant women: A clinical study | A significant reduction was noted in serum total LDL, HDL cholesterol, serum TG, and serum TC levels | [122] |
| 19 | Fecal microbiota transplantation | Male Caucasian obese participants | Improvement in peripheral and hepatic insulin sensitivity was noted, along with an increase in butyrate-producing intestinal microbiota | [126] |
| 20 | Probiotics | Obese (ob/ob) mice | An increase in the abundance of Bifidobacterium species reduced metabolic endotoxemia and inflammation. Intestinal permeability was lowered by altering GLP-2 levels | [147] |
| 21 | Probiotics (Lactobacillus rhamnosus GG and Bifidobacterium lactis Bb12; diet/probiotics) | First-trimester pregnancy: A clinical study | Reduced blood glucose and insulin levels, improved glucose tolerance, and the highest quantitative insulin sensitivity check index were noted | [169] |
For several years, BA sequestrants (BASs) have been utilized as therapeutics for patients with dyslipidemia and T2DM[52]. The BA-binding properties of BASs reduce the amount of BAs in enterohepatic circulation, thereby accelerating the conversion of cholesterol to BAs[53]. The effects of BASs on hyperglycemia have been demonstrated in both animal and clinical models of T2DM[54]. Furthermore, animal studies have revealed that BAs and BASs influence energy expenditure. A BAS molecule, also known as a resin, is a large, nonabsorbable polymeric molecule that binds negatively charged bile salts to the intestinal lining[55]. This promotes BA excretion through the feces by diverting the acids from the enterohepatic cycle. Consequently, BA synthesis increases and low-density lipoprotein (LDLR) receptors are upregulated. BASs can lower blood glucose as well as cholesterol levels, which may be beneficial for treating T2DM[54].
BASs can exert their metabolic effects beyond cholesterol-lowering effects through several mechanisms, including GLP-1, the FXR-small heterodimer partner-liver X receptor pathway, and TGR5[56]. BAS reduces intestinal FXR activity by trapping BAs in the lumen, resulting in decreased expression levels of ileal Shp and Fgf15[57]. The resulting decrease in the hepatic accessibility of BA and FGF15/19 leads to the deactivation of hepatic FXR and the CYP7A1-mediated conversion of cholesterol to BA, reducing LDL cholesterol (LDL-C) levels. Consequently, lipogenesis is attenuated by FXR. BAS raises plasma TG levels and accumulates hepatic lipids, while lowering LDL-C levels[58]. However, the exact mechanism by which BASs exert their metabolic effects beyond cholesterol-lowering effects remains unknown. Rectal administration of taurocholic acid (TCA) was found to increase GLP-1 and PYY production in obese participants and those with T2DM[59]. Similarly, CDCA and colesevelam increased glycogen and GLP-1 levels and delayed stomach emptying in patients with T2DM[60].
Cholestyramine is a polystyrene-based polymer that has been crosslinked with divinylbenzene and functionalized to quaternary ammonium units to produce a robust anion exchange resin and increase the secretion of the pancreatic exocrine hormone cholecystokinin (CCK)[61]. A study revealed that cholestyramine administration increases the expression levels of genes encoding HMGCoAR, LDLR, PCSK9, and SREBP2[62]. Cholestyramine stimulates hepatic BA synthesis from cholesterol, which activates SREBP2 by inhibiting BA absorption from the intestine. LDLR increases the transport of cholesterol from the plasma when SREBP2 is expressed. The upregulation of HMGCoAR compensates for the reduction in LDL-C levels in the plasma. In addition to activating SREBP2, PCSK9 gets upregulated, thereby degrading LDLR. By modulating PCSK9, cholestyramine-induced increases in LDLR expression can be modulated[58]. In addition to treatments using cholestyramine and inhibitors of ileal BA uptake, treatments aimed at reducing PCSK9 expression would be beneficial for reducing the enterohepatic circulation of BAs[63].
Similarly, several clinical and experimental models have revealed that cholestyramine improves BA-gut microbiome interactions, thereby facilitating glucose and fat metabolism. In clinical models of primary biliary cholangitis (PBC), two SCFA-producing Lachnospiraceae species were found to be enriched in the microbiome of the superior remission group after cholestyramine treatment. SCFAs derived from dietary fibers are produced by the gut microbiota, and SCFA signaling has anti-inflammatory, antiobesity, and antidiabetic properties[52]. This denotes the favorable effects of cholestyramine in treating PBC by enhancing BA-gut microbiome interactions[64]. Newman et al[57] reported that cholestyramine reduced hyperglycemia by increasing the ileal expression of glucagon through an increase in the prevalence of Acetatifactor Muris and Muribaculum intestinal. In another study, cholestyramine-treated ZDF rats showed reduced glycosylated hemoglobin A1c (HbA1c) levels, serum glucose levels, and FXR activation and increased PYY levels, GLP-1 Levels, and insulin release[65].
Colesevelam hydrochloride (HCl) is a polyallylamine that has been crosslinked with epichlorohydrin and alkylated with (6-bromohexyl)-trimethylammonium bromide and 1-bromodecane[66]. In clinical and animal studies on T2DM, obesity, and hyperlipidemia, colesevelam reduced blood glucose[67], FBG[68], mediator complex subunit 1, miR-182[69], HbA1c[70], hepatic TG, total LDL[71], very-low-density lipoprotein (VLDL), chylomicron particle[72], LDL-C[73], non-HDL-C, ApoB, TGR5/GLP-1-dependent glycogenolysis, FXR-dependent cholesterol, cytochrome P450, Cyp7a1[74], FGF-19[75], BA reabsorption[76], high-sensitivity C-reactive protein[77], and fructosamine levels[78-80] and increased glycolysis, postmeal glucose tolerance, insulin levels[81], splanchnic sequestration of meal-derived glucose[82], GLP-1/GIP levels[83], total HDL particle levels, miR-96/182/183 expression levels, β-cell function [as revealed by homeostatic model assessment (HOMA)][56], BA synthesis, ApoA-1 levels[54], and CCK levels[84]. As a molecularly engineered, second-generation BA sequestrant, colesevelam has been recommended for reducing LDL-C in patients with primary hypercholesterolemia by inhibiting b-hydroxymethylglutaryl coenzyme A reductase[85]. Colesevelam enhances glycemic control in patients with T2DM[86]. When metformin-based, sulfonylurea-based therapy fails to completely control T2DM, colesevelam can improve glycemic and lipid indices[54]. Moreover, colesevelam significantly alters BA metabolism. A non-absorbable complex of colesevelam in the gastrointestinal tract can stimulate the excretion of BAs through feces and their removal from enterohepatic circulation. Therefore, colesevelam treatment may reduce the total BA pool size[75]. Colesevelam reduces the influx of CDCA and DCA, two of the most potent FXR ligands, into ileal enterocytes. Therefore, plasma levels of FGF19 are likely to decrease when FXR is less activated[75].
Colestimide, an anion exchange resin, lowers serum cholesterol levels by binding to BAs in the intestinal tract[87]. Although colestimide is used to treat hyperlipidemia in Japanese patients, the mechanism by which it lowers blood glucose levels remains poorly understood[88]. CA reduces blood glucose levels and facilitates energy metabolism through the type 2 iodothyronine deiodinase (D2) enzyme. Various clinical and experimental studies have revealed that colestimide treatment reduced blood glucose, FBG, postprandial blood glucose, HbA1c, IR, and serum LDL-C levels and increased serum 1,5-AG and postprandial plasma GLP-1 Levels in patients with T2DM[89-91]. Another study revealed that colestimide altered BA composition and CA ratios, thereby reducing blood glucose levels via the TGR5-Camp-D2 pathway[92]. Similarly, elobixibat induced colonic motility and secretion by inhibiting an ileal BA transporter in a highly selective manner[93], reduced the LDL-C levels and LDL-C: HDL-C ratio, and increased the circulating GLP1 levels in a clinical study on dyslipidemia[94]. Colestilan is also a BAS that could reduce body weight and HbA1c, FBG, LDL-C, and total cholesterol levels and increase fecal lipid excretion in patients with T2DM[95].
Since BAs were initially considered lipid solubilizers, they have evolved into complex metabolic integrators. BAs can modulate their energy expenditure through the stimulation of TGR5- and FXR-mediated signaling pathways[36]. The metabolism-related protein TGR5 may be a novel promising target for treating metabolic disorders associated with obesity. Recently, TGR5 expression has been reported in enteroendocrine L cells, including STC-1 cells, which secrete GLP-1 upon calorie intake[96]. In preclinical studies, INT-177 (a semisynthetic BA derivative) and nonsteroidal TGR5 agonists promoted glucose homeostasis[97]. The activation of TGR5 by BAs reduced diet-induced obesity by increasing energy expenditure in brown adipose tissues and muscles[97]. Moreover, TGR5-mediated release of GLP-1 modulated the ATP/ADP ratio and oxidative phosphorylation in the mitochondria by activating the KATP/Cav channels. Thus, the TGR-5-mediated pathway is therapeutically beneficial, considering that incretin-based therapies are effective in treating DM[98]. Moreover, FXR activation has not yet been associated with significant weight loss[99]. FXR activation reduces hepatic glucose and fatty acid outputs by increasing glycogen production and decreasing lipogenesis and VLDL production, thereby increasing insulin sensitivity[99]. Similarly, 6E-CDCA was found to reduce blood glucose, insulin, TG, and plasma cholesterol levels and fatty acid synthesis and facilitate FXR activation in Zucker (fa/fa) obese rats with liver steatosis[40]. Moreover, tauroUDCA increased muscular and hepatic insulin signaling by phosphorylating the insulin receptor substrate Tyr and Akt at Ser473 in obese participants[100]. In summary, TGFR5 agonists activate the TGR5 signaling pathway by increasing mitochondrial function and enteroendocrine cell function, ultimately leading to increased incretin release. This has various metabolic effects, including reduction of weight gain and hepatic steatosis, improvement of liver function, and maintenance of insulin sensitivity and glucose homeostasis.
CDCA (3α,7α-dihydroxy-5β-cholan-24-oic acid) is a PBAs produced in the liver from cholesterol. CDCA is a potent inhibitor of CYP7A1, the enzyme responsible for BA synthesis. In addition to suppressing cholesterol synthesis, CDCA may inhibit HMGCoA reductase[101]. Mantovani et al[102] reported decreased plasma levels of CA and TCA but significantly increased plasma levels of TCDCA, TDCA, HDCA, GDCA, GLCA, and DCA in patients with T2DM. Moreover, Cariou et al[103] reported that plasma levels of CDCA, CA, and DCA were negatively correlated with insulin sensitivity in patients with T2DM. CDCA may inhibit high-fat diet (HFD)induced obesity and hyperglycemia through the activation of TGR5 and inhibition of PPARγ transcriptional activity[104]. Another study revealed that CDCA increased GLP-1 and glucagon secretion and delayed gastric emptying by activating GPBAR1 in patients with T2DM[60]. The activation of FXR and TGR5 through CDCA and DCA mimicked and suppressed SPX promoter activity induced by CDCA and DCA. SPX promoter activity was significantly increased by adenylate cyclase (AC)/cAMP activators and reduced by CDCA, DCA, and PKA pathway inhibitors. Through FXR- and TGR5-mediated AC/cAMP/PKA and MAPK cascades, CDCA and DCA could promote SPX expression at the hepatic level[105].
Obeticholic acid (OCA, 6E-CDCA) is a semisynthetic BAs with a 30-fold higher potency than that of CDCA for activating FXR. OCA-mediated inhibition of BAs synthesis increased the abundance of Firmicutes species and reduced nonalcoholic steatohepatitis in humans[106]. UDCA is commonly used for treating liver dysfunction. UDCA treatment reduced hyperinsulinemia and fasting hyperglycemia in a mouse model of T2DM with hepatic steatosis[107]. Moreover, Osorio et al[108] reported that UDCA inhibited sodium–glucose co-transporter overexpression, thereby reducing oxidative stress in mice with streptozotocin (STZ)-induced DM. A recent meta-analysis revealed that UDCA significantly reduced fasting plasma glucose, HbA1c, and insulin levels, indicating a positive impact on glucose homeostasis[109]. Another clinical trial demonstrated that UDCA treatment reduced HbA1c levels and increased early-phase GLP-1 secretion[110].
Bariatric surgery is effective in treating obesity, DM, and related complications. However, this surgery is not the only factor responsible for treating obesity. Bariatric surgery alters gut microbiota profiles and induces gut microbes to synthesize SCFAs. Gut microbes are crucial for improving the outcomes of bariatric surgery. Gut microbes are also important for reducing weight and lowering adverse events after bariatric surgery. Therefore, prebiotics, probiotics, and postbiotics are reco-mmended for patients who have undergone bariatric surgery in order to improve their clinical outcomes[111]. Bariatric surgery causes changes in the gut microbiota because of a malabsorptive status and changes in BA metabolism, gastric pH, and hormone metabolism[112]. It may also change the levels of hormones, such as leptin and ghrelin. Changes in hormones have been reported as a result of energy metabolism and the microbiota. Prebiotics modulate the intestinal microbiota and reduce the levels of ghrelin in the blood; however, the relationship between the two is not fully understood[113]. Similarly, postsurgical microbiomes were more different from lean microbiomes than obese microbiomes, whereas postsurgical microbiomes were less different from lean microbiomes than obese microbiomes. Body mass index loss following bariatric surgery could be predicted based on the presurgical microbiome. After surgery, the relative abundance of Proteobacteria and Fusobacteria increased, whereas that of Firmicutes decreased[114]. On the other hand, in patients with mild obesity, RYGB is an effective treatment option. It can also improve the metabolic and inflammatory status. Lau et al[115] reported that RYGB altered 29 rich bacterial genera in the gut microbiota of patients with T2DM. To better understand the weight-independent antidiabetic mechanisms of RYGB, researchers have developed DJB surgery. Han et al[116] demonstrated that DJB increased intraduodenal BAs levels and upregulated duodenal SIRT1 expression in rats with HFD- and STZ-induced DM. Patients with T2DM reported significant and long-lasting glycemic improvements after undergoing duodenal mucosal resurfacing, an endoscopic technique that involves circumferential hydrothermal ablation and subsequent regeneration of the duodenal mucosa[117].
Studies have revealed that obesity alters microbial composition and nature[23,28]. The development of metabolic illnesses, such as obesity and T2DM, has recently been linked to the gut microbiota. Increasing attention has been paid to altering the gut microbiota for treating metabolic diseases. Numerous microbial compositions (probiotics, symbiotics, and antibiotics) have been used to treat illnesses. Probiotics are live microorganisms that have positive effects on host health when administered in adequate concentrations[118].
The use of probiotic bacteria as prophylactics and therapeutics is receiving attention because of the potential effects of gut microbes in lowering IR and lipid levels. Increasing evidence suggests using probiotics to prevent metabolic diseases[119]. Probiotics are live microbes that provide the host with health benefits when administered in optimal concentrations[120]. Probiotic administration may lower TG and VLDL cholesterol (VLDL-C) levels by inhibiting the NF-κB pathway and the gut microbiota-SCFA-hormone axis[121]. Moreover, a substantial decrease in lipid levels was noted in healthy pregnant women without GDM after the administration of a two-strain probiotic containing L. acidophilus LA5 and B. animalis BB12 for 9 wk[122]. Probiotics can also increase the β-oxidation of long-chain fatty acids in muscle and liver tissues, modifying the energy pathways for fatty acid oxidation, lowering the formation of new TGs, and eventually reducing serum TG and VLDL-C levels[123]. Furthermore, probiotic consumption can increase the number of natural killer T cells in the liver[124], reduce inflammatory signaling, increase adiponectin levels, reduce inflammation, and prevent GLUT4 inhibition to improve glucose homeostasis. Probiotic dosages can also trigger enteroendocrine L cells to release GLP-1, thereby improving glucose metabolism, reducing glucotoxicity, and improving insulin sensitivity in the target tissue[125].
Recently, there have been many discussions on fecal microbiota transplantation. Vrieze et al[126] reported that participants with metabolic syndrome showed enhanced peripheral and hepatic insulin sensitivity in response to modest intestinal transfusions of fecal microbiota from allogenic lean donors, together with an upsurge of the gut microbiome. Various microbes are used as probiotics. Our review mainly focuses on A. muciniphila and Bifidobacterium species, which are closely associated with metabolic diseases.
In recent years, A. muciniphila, a commensal bacterium found in the intestine, has attracted increasing interest because of its health-promoting effects[127]. Interestingly, various clinical disorders in humans, including obesity, T2DM[128], inflammatory bowel disorder, hypertension, and liver disease, decrease the abundance of A. muciniphila[129]. Animal studies have demonstrated that A. muciniphila can alleviate obesity and related illnesses, such as steatosis, gut permeability, glucose intolerance, and IR[130,131]. Moreover, in one study, animals treated with live A. muciniphila did not exhibit IR or inflammatory cell (CD11c) infiltration in adipose tissues, which are crucial for the development of obesity, because of low inflammation[32]. A. muciniphila and F. prausnitzii can protect against the development of T2DM[132]. By activating tight junction proteins (occludin, claudin-3, and ZO-1) and preventing the accumulation of lipopolysaccharides (LPSs) and occurrence of metabolic endotoxemia, A. muciniphila can restore the thickness of the mucus layer[127]. Furthermore, A. muciniphila exhibits antibacterial and anti-inflammatory effects when administered endogenously and influences the endogenous synthesis of GLP-1 and GLP-2[133]. Notably, all these findings have now received backing from different firms and have been used for treating various disorders, including metabolic diseases, such as DM[134], obesity[135], atherosclerosis, hepatic inflammation, and hypercholesterolemia[136].
Probiotics, which are a component of the gut microbiome, successfully regulate the intestinal microbiota and have potential antidiabetic applications[137]. Bifidobacterium, one of the most significant probiotics found in the mammalian gut, exhibits positive effects on health[138]. Numerous studies have demonstrated that Bifidobacterium species improved insulin sensitivity in patients with T2DM[139,140]. In HFD-fed rats with T2DM, the administration of B. animalis 01 reduced food and water intake, blood glucose levels, HbA1c levels, and hepatic injuries and increased the antioxidant status, HOMA-IR, and lipid levels by affecting the IRS/PI3K/AKT and Keap1/Nrf2 signaling pathways[141]. Similarly, Le et al[142] reported that STZ-induced C57BL/6J mice treated with Bifidobacterium species exhibited significantly reduced blood glucose levels and significantly increased IR, IRS1, Akt/PKB, IKKα, and IκBα levels. Moreover, increased extracellular signal-regulated kinase 2 and adiponectin expression levels and decreased macrophage chemoattractant protein-1 and interleukin-6 expression levels were noted following the administration of Bifidobacterium species. Furthermore, in obese and DM models, treatment with B. animalis subsp. lactis GCL2505 reduced visceral fat accumulation, increased GLP-1 and acetate levels, and enhanced glucose tolerance[143].
Bifidobacterium, one of the most significant gut bacteria, diminishes intestinal endotoxin concentrations and enhances mucosal barrier function[144]. Recently, HFD-induced models verified an increase in intestinal inflammation. By lowering the levels of metabolic endotoxins and reducing intestinal inflammation, Bifidobacterium species may benefit patients with metabolic syndrome[145]. In an HFD-fed mouse model, Bifidobacterium species dramatically improved glucose-induced insulin secretion, increased glucose tolerance, and reduced endotoxemia and proinflammatory cytokine levels[146] by altering GLP-2 levels[147]. Thus, by lowering metabolic endotoxin levels and intestinal inflammation and increasing the expression level of intestinal Reg I, a growth factor regulator[148], Bifidobacterium supplementation could alleviate HFD-induced metabolic syndrome. Specific strategies for altering the gut microbiota in favor of Bifidobacterium species may be beneficial for mitigating the effect of HFD on the occurrence of metabolic syndrome.
In summary, A. muciniphila and Bifidobacterium species are highly viable and proliferative probiotics that can alleviate metabolic syndrome through increased glucose tolerance and reduced visceral fat accumulation by altering the overall bacterial composition of the gut microbiota. Moreover, they can increase the levels of SCFAs, which can activate several signaling pathways, including the AKT/ PKB/IRS/ERK/Nrf2 pathways.
Research on the synthesis of BAs and the pathogenesis of liver diseases and metabolic diseases has made significant progress in the last two decades. BAs exert several metabolic functions, and their physicochemical properties can affect their metabolic activities. Gut microbes can be modified by various factors, such as age, diseases, diet, and drugs. BAs play a significant role in regulating gut microbes. Moreover, the size of the BA pool has been shown to be affected by microbial metabolism in the intestines; however, most of these studies have been conducted on experimental animals. Therefore, further research is warranted to identify novel therapeutic targets for maintaining human intestinal health. Importantly, while increasing experimental evidence is available, clinical research on the importance of the human microbiota in relation to rodent metabolic functions is still in its inception. For example, BAS is not recommended for individuals who have a bowel obstruction or are pregnant. Cholestyramine and colestipol are classified as pregnancy category C, while colesevelam HCl is classified as pregnancy category B[149].
Clinical research has mainly been epidemiological in nature and has therefore failed to determine whether modifications in the intestinal microbiota play a molecular role in metabolic diseases. A better understanding of these aspects is required to determine whether BA-gut microbiota axes can promote human health and how these pathways can be used to design novel therapeutic interventions for metabolic diseases, such as obesity, DM, and hyperlipidemia, and CADs using BAs and its metabolites, probiotics, and microbial transplantation.
The major objective of this review was to assess the functional implications of gut microbes and BAs for metabolic diseases. In the past, the gut microbiota was considered a bystander in the intestinal tract. The role of these microbes in supporting intestinal function has become more widely recognized in recent years. BAs and the gut microbiota interact in a mutually beneficial manner. When the gut microbiota is disturbed in metabolic illnesses, inflammation occurs and the gut barrier is compromised. Modulating receptor-mediated transport, energy balance, gut permeability, and serum LPSs can impact BAs metabolism. The gut microbiota composition and the specific mechanisms in which the gut microbiota and BAs interact to alter the metabolism and functioning of the host-gut barrier remain somewhat unclear. Understanding the significance of the BAs-gut microbiota relationship in metabolic health could lead to revolutionary advances in the treatment of metabolic illnesses in the future.
The authors acknowledge Biorender.com for creating the figures.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Shalaby MN, Egypt; Yu W, China S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Chiang JY. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1034] [Cited by in RCA: 1244] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 2. | Li T, Chiang JY. Bile acid signaling in metabolic disease and drug therapy. Pharmacol Rev. 2014;66:948-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 694] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 3. | Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1044] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 4. | Chen I, Cassaro S. Physiology, Bile Acids. 2022 May 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. [PubMed] |
| 5. | Li T, Chiang JY. Bile acids as metabolic regulators. Curr Opin Gastroenterol. 2015;31:159-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 6. | Staels B, Fonseca VA. Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care. 2009;32 Suppl 2:S237-S245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 291] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 7. | Ramírez-Pérez O, Cruz-Ramón V, Chinchilla-López P, Méndez-Sánchez N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann Hepatol. 2017;16:s15-s20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 8. | Sze MA, Schloss PD. Looking for a Signal in the Noise: Revisiting Obesity and the Microbiome. mBio. 2016;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 392] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 9. | Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2162] [Cited by in RCA: 2278] [Article Influence: 119.9] [Reference Citation Analysis (0)] |
| 10. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Jian M, Zhou Y, Li Y, Zhang X, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 7837] [Article Influence: 522.5] [Reference Citation Analysis (4)] |
| 11. | Davis CD. The Gut Microbiome and Its Role in Obesity. Nutr Today. 2016;51:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 256] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 12. | Shiffka SJ, Kane MA, Swaan PW. Planar bile acids in health and disease. Biochim Biophys Acta Biomembr. 2017;1859:2269-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Fiorucci S, Distrutti E. Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol Med. 2015;21:702-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 368] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 14. | Roy CC, Kien CL, Bouthillier L, Levy E. Short-chain fatty acids: ready for prime time? Nutr Clin Pract. 2006;21:351-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 300] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 15. | Cunningham AL, Stephens JW, Harris DA. Gut microbiota influence in type 2 diabetes mellitus (T2DM). Gut Pathog. 2021;13:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 16. | Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1679] [Article Influence: 139.9] [Reference Citation Analysis (0)] |
| 17. | McGlone ER, Bloom SR. Bile acids and the metabolic syndrome. Ann Clin Biochem. 2019;56:326-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 18. | Wei M, Huang F, Zhao L, Zhang Y, Yang W, Wang S, Li M, Han X, Ge K, Qu C, Rajani C, Xie G, Zheng X, Zhao A, Bian Z, Jia W. A dysregulated bile acid-gut microbiota axis contributes to obesity susceptibility. EBioMedicine. 2020;55:102766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 174] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 19. | Surono IS, Wardana AA, Waspodo P, Saksono B, Verhoeven J, Venema K. Effect of functional food ingredients on gut microbiota in a rodent diabetes model. Nutr Metab (Lond). 2020;17:77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science. 2018;362:776-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 684] [Article Influence: 114.0] [Reference Citation Analysis (0)] |
| 21. | Castillo JJ, Orlando RA, Garver WS. Gene-nutrient interactions and susceptibility to human obesity. Genes Nutr. 2017;12:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Santacruz A, Collado MC, García-Valdés L, Segura MT, Martín-Lagos JA, Anjos T, Martí-Romero M, Lopez RM, Florido J, Campoy C, Sanz Y. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. 2010;104:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 631] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 23. | Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7222] [Cited by in RCA: 6407] [Article Influence: 337.2] [Reference Citation Analysis (0)] |
| 24. | Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, Gordon JI, Relman DA, Fraser-Liggett CM, Nelson KE. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355-1359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3709] [Cited by in RCA: 3191] [Article Influence: 167.9] [Reference Citation Analysis (0)] |
| 25. | Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718-15723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4530] [Cited by in RCA: 4412] [Article Influence: 210.1] [Reference Citation Analysis (4)] |
| 26. | Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6:306-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 919] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 27. | Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, Mao Y, Zhang X, Pang X, Wei C, Zhao G, Chen Y, Zhao L. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J. 2010;4:232-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 830] [Article Influence: 51.9] [Reference Citation Analysis (1)] |
| 28. | Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9796] [Cited by in RCA: 8753] [Article Influence: 460.7] [Reference Citation Analysis (1)] |
| 29. | Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr. 2008;88:894-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 602] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 30. | Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54:1469-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 1462] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 31. | Karlsson CL, Onnerfält J, Xu J, Molin G, Ahrné S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring). 2012;20:2257-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 400] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 32. | Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066-9071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2639] [Cited by in RCA: 3311] [Article Influence: 275.9] [Reference Citation Analysis (0)] |
| 33. | Tomkin GH, Owens D. Obesity diabetes and the role of bile acids in metabolism. J Transl Int Med. 2016;4:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 34. | Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, Auwerx J, Goldfine AB. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring). 2009;17:1671-1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 455] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 35. | Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1187] [Cited by in RCA: 1504] [Article Influence: 167.1] [Reference Citation Analysis (0)] |
| 36. | Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, Hinuma S, Fujisawa Y, Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435-9440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1060] [Cited by in RCA: 1229] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 37. | Parséus A, Sommer N, Sommer F, Caesar R, Molinaro A, Ståhlman M, Greiner TU, Perkins R, Bäckhed F. Microbiota-induced obesity requires farnesoid X receptor. Gut. 2017;66:429-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 353] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 38. | Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9344] [Cited by in RCA: 8964] [Article Influence: 426.9] [Reference Citation Analysis (1)] |
| 39. | Chávez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152:1679-1694.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 677] [Article Influence: 84.6] [Reference Citation Analysis (1)] |
| 40. | Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51:771-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 347] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 41. | Grigorescu I, Dumitrascu DL. IMPLICATION OF GUT MICROBIOTA IN DIABETES MELLITUS AND OBESITY. Acta Endocrinol (Buchar). 2016;12:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3971] [Cited by in RCA: 4828] [Article Influence: 371.4] [Reference Citation Analysis (1)] |
| 43. | Aggarwal H, Pathak P, Kumar Y, Jagavelu K, Dikshit M. Modulation of Insulin Resistance, Dyslipidemia and Serum Metabolome in iNOS Knockout Mice following Treatment with Nitrite, Metformin, Pioglitazone, and a Combination of Ampicillin and Neomycin. Int J Mol Sci. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | van Nierop FS, de Jonge C, Kulik W, Bouvy N, Schaap FG, Olde Damink SW, Rensen S, Romijn JA, Greve JWM, Soeters MR. Duodenal-jejunal lining increases postprandial unconjugated bile acid responses and disrupts the bile acid-FXR-FGF19 axis in humans. Metabolism. 2019;93:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013;7:880-884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 422] [Cited by in RCA: 541] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 46. | Lambeth SM, Carson T, Lowe J, Ramaraj T, Leff JW, Luo L, Bell CJ, Shah VO. Composition, Diversity and Abundance of Gut Microbiome in Prediabetes and Type 2 Diabetes. J Diabetes Obes. 2015;2:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 47. | Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sørensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1783] [Cited by in RCA: 2080] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 48. | Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, Gonzalez A, Werner JJ, Angenent LT, Knight R, Bäckhed F, Isolauri E, Salminen S, Ley RE. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1438] [Cited by in RCA: 1457] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 49. | Gohir W, Whelan FJ, Surette MG, Moore C, Schertzer JD, Sloboda DM. Pregnancy-related changes in the maternal gut microbiota are dependent upon the mother's periconceptional diet. Gut Microbes. 2015;6:310-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 154] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 50. | Obuchowska A, Gorczyca K, Standyło A, Obuchowska K, Kimber-Trojnar Ż, Wierzchowska-Opoka M, Leszczyńska-Gorzelak B. Effects of Probiotic Supplementation during Pregnancy on the Future Maternal Risk of Metabolic Syndrome. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 51. | Liu H, Pan LL, Lv S, Yang Q, Zhang H, Chen W, Lv Z, Sun J. Alterations of Gut Microbiota and Blood Lipidome in Gestational Diabetes Mellitus With Hyperlipidemia. Front Physiol. 2019;10:1015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 52. | Nishida S, Horinouchi A, Higashimura Y, Akahori R, Matsumoto K. Cholestyramine, a Bile Acid Sequestrant, Increases Cecal Short Chain Fatty Acids and Intestinal Immunoglobulin A in Mice. Biol Pharm Bull. 2020;43:565-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Sugimoto-Kawabata K, Shimada H, Sakai K, Suzuki K, Kelder T, Pieterman EJ, Cohen LH, Havekes LM, Princen HM, van den Hoek AM. Colestilan decreases weight gain by enhanced NEFA incorporation in biliary lipids and fecal lipid excretion. J Lipid Res. 2013;54:1255-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Bays HE, Goldberg RB, Truitt KE, Jones MR. Colesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: glucose and lipid effects. Arch Intern Med. 2008;168:1975-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 55. | Staels B, Kuipers F. Bile acid sequestrants and the treatment of type 2 diabetes mellitus. Drugs. 2007;67:1383-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 56. | Beysen C, Murphy EJ, Deines K, Chan M, Tsang E, Glass A, Turner SM, Protasio J, Riiff T, Hellerstein MK. Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: a randomised controlled study. Diabetologia. 2012;55:432-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 57. | Newman NK, Monnier PM, Rodrigues RR, Gurung M, Vasquez-perez S, Hioki KA, Greer RL, Brown K, Morgun A, Shulzhenko N. Host response to cholestyramine can be mediated by the gut microbiota. BioRxiv. 2020;. [DOI] [Full Text] |
| 58. | Nilsson LM, Abrahamsson A, Sahlin S, Gustafsson U, Angelin B, Parini P, Einarsson C. Bile acids and lipoprotein metabolism: effects of cholestyramine and chenodeoxycholic acid on human hepatic mRNA expression. Biochem Biophys Res Commun. 2007;357:707-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 59. | Wu T, Bound MJ, Standfield SD, Gedulin B, Jones KL, Horowitz M, Rayner CK. Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy humans. Diabetes Obes Metab. 2013;15:474-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 60. | Hansen M, Scheltema MJ, Sonne DP, Hansen JS, Sperling M, Rehfeld JF, Holst JJ, Vilsbøll T, Knop FK. Effect of chenodeoxycholic acid and the bile acid sequestrant colesevelam on glucagon-like peptide-1 secretion. Diabetes Obes Metab. 2016;18:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 61. | Hilmer AJ, Jeffrey RB, Park WG, Khosla C. Cholestyramine as a promising, strong anion exchange resin for direct capture of genetic biomarkers from raw pancreatic fluids. Biotechnol Bioeng. 2017;114:934-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 62. | Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci U S A. 2003;100:12027-12032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1021] [Cited by in RCA: 1123] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 63. | Lagace TA, Curtis DE, Garuti R, McNutt MC, Park SW, Prather HB, Anderson NN, Ho YK, Hammer RE, Horton JD. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J Clin Invest. 2006;116:2995-3005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 556] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 64. | Li B, Zhang J, Chen Y, Wang Q, Yan L, Wang R, Wei Y, You Z, Li Y, Miao Q, Xiao X, Lian M, Chen W, Qiu D, Fang J, Gershwin ME, Tang R, Ma X. Alterations in microbiota and their metabolites are associated with beneficial effects of bile acid sequestrant on icteric primary biliary Cholangitis. Gut Microbes. 2021;13:1946366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 65. | Chen L, McNulty J, Anderson D, Liu Y, Nystrom C, Bullard S, Collins J, Handlon AL, Klein R, Grimes A, Murray D, Brown R, Krull D, Benson B, Kleymenova E, Remlinger K, Young A, Yao X. Cholestyramine reverses hyperglycemia and enhances glucose-stimulated glucagon-like peptide 1 release in Zucker diabetic fatty rats. J Pharmacol Exp Ther. 2010;334:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 66. | Bays H, Dujovne C. Colesevelam HCl: a non-systemic lipid-altering drug. Expert Opin Pharmacother. 2003;4:779-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 67. | Henry RR, Aroda VR, Mudaliar S, Garvey WT, Chou HS, Jones MR. Effects of colesevelam on glucose absorption and hepatic/peripheral insulin sensitivity in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 68. | Bays HE. Colesevelam hydrochloride added to background metformin therapy in patients with type 2 diabetes mellitus: a pooled analysis from 3 clinical studies. Endocr Pract. 2011;17:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Sedgeman LR, Beysen C, Allen RM, Ramirez Solano MA, Turner SM, Vickers KC. Intestinal bile acid sequestration improves glucose control by stimulating hepatic miR-182-5p in type 2 diabetes. Am J Physiol Gastrointest Liver Physiol. 2018;315:G810-G823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 70. | Pavlou P, Koutroukas V, Lissett C, Smith JC. Colesevelam-induced hypoglycaemia in a patient with type 1 diabetes mellitus. Clin Case Rep. 2021;9:e04830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 71. | Vega GL, Dunn FL, Grundy SM. Effect of colesevelam hydrochloride on glycemia and insulin sensitivity in men with the metabolic syndrome. Am J Cardiol. 2011;108:1129-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Rosenson RS, Rigby SP, Jones MR, Chou HS. Effect of colesevelam HCl monotherapy on lipid particles in type 2 diabetes mellitus. Cardiovasc Drugs Ther. 2014;28:229-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 73. | Stein EA, Marais AD, Szamosi T, Raal FJ, Schurr D, Urbina EM, Hopkins PN, Karki S, Xu J, Misir S, Melino M. Colesevelam hydrochloride: efficacy and safety in pediatric subjects with heterozygous familial hypercholesterolemia. J Pediatr. 2010;156:231-6.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 74. | Cabré N, Duan Y, Llorente C, Conrad M, Stern P, Yamashita D, Schnabl B. Colesevelam Reduces Ethanol-Induced Liver Steatosis in Humanized Gnotobiotic Mice. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 75. | Brufau G, Stellaard F, Prado K, Bloks VW, Jonkers E, Boverhof R, Kuipers F, Murphy EJ. Improved glycemic control with colesevelam treatment in patients with type 2 diabetes is not directly associated with changes in bile acid metabolism. Hepatology. 2010;52:1455-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 76. | Herrema H, Meissner M, van Dijk TH, Brufau G, Boverhof R, Oosterveer MH, Reijngoud DJ, Müller M, Stellaard F, Groen AK, Kuipers F. Bile salt sequestration induces hepatic de novo lipogenesis through farnesoid X receptor- and liver X receptor alpha-controlled metabolic pathways in mice. Hepatology. 2010;51:806-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 77. | Jialal I, Abby SL, Misir S, Nagendran S. Concomitant reduction in low-density lipoprotein cholesterol and glycated hemoglobin with colesevelam hydrochloride in patients with type 2 diabetes: a pooled analysis. Metab Syndr Relat Disord. 2009;7:255-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 78. | Zieve FJ, Kalin MF, Schwartz SL, Jones MR, Bailey WL. Results of the glucose-lowering effect of WelChol study (GLOWS): a randomized, double-blind, placebo-controlled pilot study evaluating the effect of colesevelam hydrochloride on glycemic control in subjects with type 2 diabetes. Clin Ther. 2007;29:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 79. | Goldberg RB, Fonseca VA, Truitt KE, Jones MR. Efficacy and safety of colesevelam in patients with type 2 diabetes mellitus and inadequate glycemic control receiving insulin-based therapy. Arch Intern Med. 2008;168:1531-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 80. | Fonseca VA, Rosenstock J, Wang AC, Truitt KE, Jones MR. Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care. 2008;31:1479-1484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 187] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 81. | Schwartz SL, Lai YL, Xu J, Abby SL, Misir S, Jones MR, Nagendran S. The effect of colesevelam hydrochloride on insulin sensitivity and secretion in patients with type 2 diabetes: a pilot study. Metab Syndr Relat Disord. 2010;8:179-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 82. | Smushkin G, Sathananthan M, Piccinini F, Dalla Man C, Law JH, Cobelli C, Zinsmeister AR, Rizza RA, Vella A. The effect of a bile acid sequestrant on glucose metabolism in subjects with type 2 diabetes. Diabetes. 2013;62:1094-1101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 83. | Shang Q, Liu MK, Saumoy M, Holst JJ, Salen G, Xu G. The combination of colesevelam with sitagliptin enhances glycemic control in diabetic ZDF rat model. Am J Physiol Gastrointest Liver Physiol. 2012;302:G815-G823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 84. | Marina AL, Utzschneider KM, Wright LA, Montgomery BK, Marcovina SM, Kahn SE. Colesevelam improves oral but not intravenous glucose tolerance by a mechanism independent of insulin sensitivity and β-cell function. Diabetes Care. 2012;35:1119-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 85. | Zema MJ. Colesevelam hydrochloride: evidence for its use in the treatment of hypercholesterolemia and type 2 diabetes mellitus with insights into mechanism of action. Core Evid. 2012;7:61-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 86. | Handelsman Y. The role of colesevelam HCl in type 2 diabetes mellitus therapy. Postgrad Med. 2009;121:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 87. | Suzuki T, Oba K, Igari Y, Matsumura N, Watanabe K, Futami-Suda S, Yasuoka H, Ouchi M, Suzuki K, Kigawa Y, Nakano H. Colestimide lowers plasma glucose levels and increases plasma glucagon-like PEPTIDE-1 (7-36) levels in patients with type 2 diabetes mellitus complicated by hypercholesterolemia. J Nippon Med Sch. 2007;74:338-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 88. | Matsuzaki Y. Colestimide: the efficacy of a novel anion-exchange resin in cholestatic disorders. J Gastroenterol Hepatol. 2002;17:1133-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 89. | Suzuki T, Tsunoda-Kubota M, Aoyama J, Futami-Suda S, Hashimoto M, Igari Y, Watanabe K, Kigawa Y, Nakano H, Oba K. What characteristics at baseline are associated with the glucose-lowering effect of colestimide in patients with type 2 diabetes and hypercholesterolemia according to response to treatment? J Nippon Med Sch. 2013;80:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 90. | Suzuki T, Oba K, Igari Y, Watanabe K, Matsumura N, Futami-Suda S, Ouchi M, Suzuki K, Sekimizu K, Kigawa Y, Nakano H. Effects of bile-acid-binding resin (colestimide) on blood glucose and visceral fat in Japanese patients with type 2 diabetes mellitus and hypercholesterolemia: an open-label, randomized, case-control, crossover study. J Diabetes Complications. 2012;26:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 91. | Yamakawa T, Kaneko T, Shigematu E, Kawaguchi J, Kadonosono K, Morita S, Terauchi Y. Glucose-lowering effect of colestimide is associated with baseline HbA1c in type 2 diabetic patients with hypercholesterolemia. Endocr J. 2011;58:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 92. | Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1641] [Cited by in RCA: 1694] [Article Influence: 89.2] [Reference Citation Analysis (0)] |
| 93. | Kumagai Y, Amano H, Sasaki Y, Nakagawa C, Maeda M, Oikawa I, Furuie H. Effect of single and multiple doses of elobixibat, an ileal bile acid transporter inhibitor, on chronic constipation: A randomized controlled trial. Br J Clin Pharmacol. 2018;84:2393-2404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 94. | Rudling M, Camilleri M, Graffner H, Holst JJ, Rikner L. Specific inhibition of bile acid transport alters plasma lipids and GLP-1. BMC Cardiovasc Disord. 2015;15:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 95. | Kondo K, Kadowaki T. Colestilan monotherapy significantly improves glycaemic control and LDL cholesterol levels in patients with type 2 diabetes: a randomized double-blind placebo-controlled study. Diabetes Obes Metab. 2010;12:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 96. | Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008;8:532-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 612] [Cited by in RCA: 575] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 97. | Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1223] [Cited by in RCA: 1392] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 98. | Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2787] [Cited by in RCA: 2842] [Article Influence: 149.6] [Reference Citation Analysis (1)] |
| 99. | Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 697] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 100. | Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, Patterson BW, Horton JD, Mittendorfer B, Hotamisligil GS, Klein S. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59:1899-1905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 298] [Cited by in RCA: 320] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 101. | Einarsson C, Hillebrant CG, Axelson M. Effects of treatment with deoxycholic acid and chenodeoxycholic acid on the hepatic synthesis of cholesterol and bile acids in healthy subjects. Hepatology. 2001;33:1189-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 102. | Mantovani A, Dalbeni A, Peserico D, Cattazzo F, Bevilacqua M, Salvagno GL, Lippi G, Targher G, Danese E, Fava C. Plasma Bile Acid Profile in Patients with and without Type 2 Diabetes. Metabolites. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 103. | Cariou B, Chetiveaux M, Zaïr Y, Pouteau E, Disse E, Guyomarc'h-Delasalle B, Laville M, Krempf M. Fasting plasma chenodeoxycholic acid and cholic acid concentrations are inversely correlated with insulin sensitivity in adults. Nutr Metab (Lond). 2011;8:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 104. | Chen X, Yan L, Guo Z, Chen Y, Li M, Huang C, Chen Z, Meng X. Chenodeoxycholic acid attenuates high-fat diet-induced obesity and hyperglycemia via the G protein-coupled bile acid receptor 1 and proliferator-activated receptor γ pathway. Exp Ther Med. 2017;14:5305-5312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 105. | Lai Q, Ma Y, Bai J, Zhuang M, Pei S, He N, Yin J, Fan B, Bian Z, Zeng G, Lin C. Mechanisms for Bile Acids CDCA- and DCA-Stimulated Hepatic Spexin Expression. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 106. | Ferrell JM, Chiang JYL. Understanding Bile Acid Signaling in Diabetes: From Pathophysiology to Therapeutic Targets. Diabetes Metab J. 2019;43:257-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 107. | Tsuchida T, Shiraishi M, Ohta T, Sakai K, Ishii S. Ursodeoxycholic acid improves insulin sensitivity and hepatic steatosis by inducing the excretion of hepatic lipids in high-fat diet-fed KK-Ay mice. Metabolism. 2012;61:944-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 108. | Osorio H, Coronel I, Arellano A, Franco M, Escalante B, Bautista R. Ursodeoxycholic acid decreases sodium-glucose cotransporter (SGLT2) expression and oxidative stress in the kidney of diabetic rats. Diabetes Res Clin Pract. 2012;97:276-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 109. | Sánchez-García A, Sahebkar A, Simental-Mendía M, Simental-Mendía LE. Effect of ursodeoxycholic acid on glycemic markers: A systematic review and meta-analysis of clinical trials. Pharmacol Res. 2018;135:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 110. | Shima KR, Ota T, Kato KI, Takeshita Y, Misu H, Kaneko S, Takamura T. Ursodeoxycholic acid potentiates dipeptidyl peptidase-4 inhibitor sitagliptin by enhancing glucagon-like peptide-1 secretion in patients with type 2 diabetes and chronic liver disease: a pilot randomized controlled and add-on study. BMJ Open Diabetes Res Care. 2018;6:e000469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 111. | Gasmi A, Bjørklund G, Mujawdiya PK, Semenova Y, Dosa A, Piscopo S, Pen JJ, Gasmi Benahmed A, Costea DO. Gut microbiota in bariatric surgery. Crit Rev Food Sci Nutr. 2022;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 112. | Ulker İ, Yildiran H. The effects of bariatric surgery on gut microbiota in patients with obesity: a review of the literature. Biosci Microbiota Food Health. 2019;38:3-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 113. | Ravussin Y, Koren O, Spor A, LeDuc C, Gutman R, Stombaugh J, Knight R, Ley RE, Leibel RL. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity (Silver Spring). 2012;20:738-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 315] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 114. | Ben Izhak M, Eshel A, Cohen R, Madar-Shapiro L, Meiri H, Wachtel C, Leung C, Messick E, Jongkam N, Mavor E, Sapozhnikov S, Maharshak N, Abu-Abeid S, Alis A, Mahler I, Meoded A, Meron Eldar S, Koren O, Louzoun Y. Projection of Gut Microbiome Pre- and Post-Bariatric Surgery To Predict Surgery Outcome. mSystems. 2021;6:e0136720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 115. | Lau E, Belda E, Picq P, Carvalho D, Ferreira-Magalhães M, Silva MM, Barroso I, Correia F, Vaz CP, Miranda I, Barbosa A, Clément K, Doré J, Freitas P, Prifti E. Gut microbiota changes after metabolic surgery in adult diabetic patients with mild obesity: a randomised controlled trial. Diabetol Metab Syndr. 2021;13:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 116. | Han HF, Liu SZ, Zhang X, Wei M, Huang X, Yu WB. Duodenal-jejunal bypass increases intraduodenal bile acids and upregulates duodenal SIRT1 expression in high-fat diet and streptozotocin-induced diabetic rats. World J Gastroenterol. 2022;28:4338-4350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 117. | van Baar ACG, Holleman F, Crenier L, Haidry R, Magee C, Hopkins D, Rodriguez Grunert L, Galvao Neto M, Vignolo P, Hayee B, Mertens A, Bisschops R, Tijssen J, Nieuwdorp M, Guidone C, Costamagna G, Devière J, Bergman JJGHM. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: one year results from the first international, open-label, prospective, multicentre study. Gut. 2020;69:295-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 118. | Arora T, Singh S, Sharma RK. Probiotics: Interaction with gut microbiome and antiobesity potential. Nutrition. 2013;29:591-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 119. | Karamali M, Dadkhah F, Sadrkhanlou M, Jamilian M, Ahmadi S, Tajabadi-Ebrahimi M, Jafari P, Asemi Z. Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. 2016;42:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 118] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 120. | Salminen S, van Loveren H. Probiotics and prebiotics: health claim substantiation. Microb Ecol Health Dis. 2012;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 121. | Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288:25088-25097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 502] [Article Influence: 41.8] [Reference Citation Analysis (2)] |
| 122. | Asemi Z, Samimi M, Tabasi Z, Talebian P, Azarbad Z, Hydarzadeh Z, Esmaillzadeh A. Effect of daily consumption of probiotic yoghurt on lipid profiles in pregnant women: a randomized controlled clinical trial. J Matern Fetal Neonatal Med. 2012;25:1552-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 123. | Huang Z, Mu C, Chen Y, Zhu Z, Chen C, Lan L, Xu Q, Zhao W, Chen G. Effects of dietary probiotic supplementation on LXRα and CYP7α1 gene expression, liver enzyme activities and fat metabolism in ducks. Br Poult Sci. 2015;56:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 124. | Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821-830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 306] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 125. | Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2779] [Cited by in RCA: 3081] [Article Influence: 237.0] [Reference Citation Analysis (0)] |
| 126. | Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913-6.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1881] [Cited by in RCA: 2012] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 127. | Cani PD, de Vos WM. Next-Generation Beneficial Microbes: The Case of Akkermansia muciniphila. Front Microbiol. 2017;8:1765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 507] [Cited by in RCA: 698] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 128. | Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One. 2013;8:e71108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 481] [Cited by in RCA: 634] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 129. | Yassour M, Lim MY, Yun HS, Tickle TL, Sung J, Song YM, Lee K, Franzosa EA, Morgan XC, Gevers D, Lander ES, Xavier RJ, Birren BW, Ko G, Huttenhower C. Sub-clinical detection of gut microbial biomarkers of obesity and type 2 diabetes. Genome Med. 2016;8:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 204] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 130. | Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Doré J, Henegar C, Rizkalla S, Clément K. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049-3057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 898] [Cited by in RCA: 891] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 131. | de Goffau MC, Luopajärvi K, Knip M, Ilonen J, Ruohtula T, Härkönen T, Orivuori L, Hakala S, Welling GW, Harmsen HJ, Vaarala O. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes. 2013;62:1238-1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 433] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 132. | Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, Falony G, Raes J, Maiter D, Delzenne NM, de Barsy M, Loumaye A, Hermans MP, Thissen JP, de Vos WM, Cani PD. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25:1096-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 774] [Cited by in RCA: 1426] [Article Influence: 237.7] [Reference Citation Analysis (0)] |
| 133. | Cani PD, Everard A. Talking microbes: When gut bacteria interact with diet and host organs. Mol Nutr Food Res. 2016;60:58-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 134. | Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, Chilloux J, Ottman N, Duparc T, Lichtenstein L, Myridakis A, Delzenne NM, Klievink J, Bhattacharjee A, van der Ark KC, Aalvink S, Martinez LO, Dumas ME, Maiter D, Loumaye A, Hermans MP, Thissen JP, Belzer C, de Vos WM, Cani PD. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 967] [Cited by in RCA: 1461] [Article Influence: 162.3] [Reference Citation Analysis (0)] |
| 135. | Shen W, Shen M, Zhao X, Zhu H, Yang Y, Lu S, Tan Y, Li G, Li M, Wang J, Hu F, Le S. Anti-obesity Effect of Capsaicin in Mice Fed with High-Fat Diet Is Associated with an Increase in Population of the Gut Bacterium Akkermansia muciniphila. Front Microbiol. 2017;8:272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 136. | Grander C, Adolph TE, Wieser V, Lowe P, Wrzosek L, Gyongyosi B, Ward DV, Grabherr F, Gerner RR, Pfister A, Enrich B, Ciocan D, Macheiner S, Mayr L, Drach M, Moser P, Moschen AR, Perlemuter G, Szabo G, Cassard AM, Tilg H. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67:891-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 457] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 137. | Rad AH, Abbasalizadeh S, Vazifekhah S, Abbasalizadeh F, Hassanalilou T, Bastani P, Ejtahed HS, Soroush AR, Javadi M, Mortazavian AM, Khalili L. The Future of Diabetes Management by Healthy Probiotic Microorganisms. Curr Diabetes Rev. 2017;13:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 138. | Duncan SH, Flint HJ. Probiotics and prebiotics and health in ageing populations. Maturitas. 2013;75:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 122] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 139. | Stenman LK, Waget A, Garret C, Klopp P, Burcelin R, Lahtinen S. Potential probiotic Bifidobacterium animalis ssp. lactis 420 prevents weight gain and glucose intolerance in diet-induced obese mice. Benef Microbes. 2014;5:437-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 140. | Sharma P, Bhardwaj P, Singh R. Administration of Lactobacillus casei and Bifidobacterium bifidum Ameliorated Hyperglycemia, Dyslipidemia, and Oxidative Stress in Diabetic Rats. Int J Prev Med. 2016;7:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 141. | Zhang J, Wang S, Zeng Z, Qin Y, Shen Q, Li P. Anti-diabetic effects of Bifidobacterium animalis 01 through improving hepatic insulin sensitivity in type 2 diabetic rat model. J Funct Foods. 2020;67:103843. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 142. | Le TK, Hosaka T, Nguyen TT, Kassu A, Dang TO, Tran HB, Pham TP, Tran QB, Le TH, Pham XD. Bifidobacterium species lower serum glucose, increase expressions of insulin signaling proteins, and improve adipokine profile in diabetic mice. Biomed Res. 2015;36:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 143. | Aoki R, Kamikado K, Suda W, Takii H, Mikami Y, Suganuma N, Hattori M, Koga Y. A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Sci Rep. 2017;7:43522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 144. | Wang Z, Xiao G, Yao Y, Guo S, Lu K, Sheng Z. The role of bifidobacteria in gut barrier function after thermal injury in rats. J Trauma. 2006;61:650-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 145. | Brun P, Castagliuolo I, Di Leo V, Buda A, Pinzani M, Palù G, Martines D. Increased intestinal permeability in obese mice: new evidence in the pathogenesis of nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G518-G525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 646] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 146. | Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374-2383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1237] [Cited by in RCA: 1295] [Article Influence: 71.9] [Reference Citation Analysis (1)] |
| 147. | Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, Geurts L, Naslain D, Neyrinck A, Lambert DM, Muccioli GG, Delzenne NM. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1735] [Cited by in RCA: 1887] [Article Influence: 117.9] [Reference Citation Analysis (1)] |
| 148. | Chen JJ, Wang R, Li XF, Wang RL. Bifidobacterium longum supplementation improved high-fat-fed-induced metabolic syndrome and promoted intestinal Reg I gene expression. Exp Biol Med (Maywood). 2011;236:823-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 149. | Insull W Jr. Clinical utility of bile acid sequestrants in the treatment of dyslipidemia: a scientific review. South Med J. 2006;99:257-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 225] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 150. | Pon Velayutham AB, Mokhtari P, Metos JM, Jambal P, Shankar K. Gut Microbial and Metabolic Signatures are Altered in Adolescents with Type 1 Diabetes. FASEB J. 2022;36:R3170. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 151. | Liu Y, Qin S, Feng Y, Song Y, Lv N, Liu F, Zhang X, Wang S, Wei Y, Li S, Su S, Zhang W, Xue Y, Hao Y, Zhu B, Ma J, Yang H. Perturbations of gut microbiota in gestational diabetes mellitus patients induce hyperglycemia in germ-free mice. J Dev Orig Health Dis. 2020;11:580-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 152. | Just S, Mondot S, Ecker J, Wegner K, Rath E, Gau L, Streidl T, Hery-Arnaud G, Schmidt S, Lesker TR, Bieth V, Dunkel A, Strowig T, Hofmann T, Haller D, Liebisch G, Gérard P, Rohn S, Lepage P, Clavel T. The gut microbiota drives the impact of bile acids and fat source in diet on mouse metabolism. Microbiome. 2018;6:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 153. | Sonne DP, van Nierop FS, Kulik W, Soeters MR, Vilsbøll T, Knop FK. Postprandial Plasma Concentrations of Individual Bile Acids and FGF-19 in Patients With Type 2 Diabetes. J Clin Endocrinol Metab. 2016;101:3002-3009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 154. | Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes. 2013;62:4184-4191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 371] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 155. | Glicksman C, Pournaras DJ, Wright M, Roberts R, Mahon D, Welbourn R, Sherwood R, Alaghband-Zadeh J, le Roux CW. Postprandial plasma bile acid responses in normal weight and obese subjects. Ann Clin Biochem. 2010;47:482-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 156. | Waldram A, Holmes E, Wang Y, Rantalainen M, Wilson ID, Tuohy KM, McCartney AL, Gibson GR, Nicholson JK. Top-down systems biology modeling of host metabotype-microbiome associations in obese rodents. J Proteome Res. 2009;8:2361-2375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 192] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 157. | Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070-11075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4639] [Cited by in RCA: 4465] [Article Influence: 223.3] [Reference Citation Analysis (1)] |
| 158. | Meiring S, Meessen ECE, van Baar ACG, Holleman F, Nieuwdorp M, Olde Damink SW, Schaap FG, Vaz FM, Groen AK, Soeters MR, Bergman JJGHM. Duodenal mucosal resurfacing with a GLP-1 receptor agonist increases postprandial unconjugated bile acids in patients with insulin-dependent type 2 diabetes. Am J Physiol Endocrinol Metab. 2022;322:E132-E140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 159. | Hartmann P, Duan Y, Miyamoto Y, Demir M, Lang S, Hasa E, Stern P, Yamashita D, Conrad M, Eckmann L, Schnabl B. Colesevelam ameliorates non-alcoholic steatohepatitis and obesity in mice. Hepatol Int. 2022;16:359-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 160. | Aggarwal H, Pathak P, Singh V, Kumar Y, Shankar M, Das B, Jagavelu K, Dikshit M. Vancomycin-Induced Modulation of Gram-Positive Gut Bacteria and Metabolites Remediates Insulin Resistance in iNOS Knockout Mice. Front Cell Infect Microbiol. 2021;11:795333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 161. | Takahashi S, Luo Y, Ranjit S, Xie C, Libby AE, Orlicky DJ, Dvornikov A, Wang XX, Myakala K, Jones BA, Bhasin K, Wang D, McManaman JL, Krausz KW, Gratton E, Ir D, Robertson CE, Frank DN, Gonzalez FJ, Levi M. Bile acid sequestration reverses liver injury and prevents progression of nonalcoholic steatohepatitis in Western diet-fed mice. J Biol Chem. 2020;295:4733-4747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 162. | Tsuji Y, Kaji K, Kitade M, Kaya D, Kitagawa K, Ozutsumi T, Fujinaga Y, Takaya H, Kawaratani H, Namisaki T, Moriya K, Akahane T, Yoshiji H. Bile Acid Sequestrant, Sevelamer Ameliorates Hepatic Fibrosis with Reduced Overload of Endogenous Lipopolysaccharide in Experimental Nonalcoholic Steatohepatitis. Microorganisms. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 163. | Neuman V, Cinek O, Funda DP, Hudcovic T, Golias J, Kramna L, Petruzelkova L, Pruhova S, Sumnik Z. Human gut microbiota transferred to germ-free NOD mice modulate the progression towards type 1 diabetes regardless of the pace of beta cell function loss in the donor. Diabetologia. 2019;62:1291-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 164. | Gu Y, Wang X, Li J, Zhang Y, Zhong H, Liu R, Zhang D, Feng Q, Xie X, Hong J, Ren H, Liu W, Ma J, Su Q, Zhang H, Yang J, Zhao X, Gu W, Bi Y, Peng Y, Xu X, Xia H, Li F, Yang H, Xu G, Madsen L, Kristiansen K, Ning G, Wang W. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat Commun. 2017;8:1785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 298] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 165. | Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L, Ståhlman M, Olsson LM, Serino M, Planas-Fèlix M, Xifra G, Mercader JM, Torrents D, Burcelin R, Ricart W, Perkins R, Fernàndez-Real JM, Bäckhed F. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 824] [Cited by in RCA: 1135] [Article Influence: 141.9] [Reference Citation Analysis (0)] |
| 166. | Dolatkhah N, Hajifaraji M, Abbasalizadeh F, Aghamohammadzadeh N, Mehrabi Y, Abbasi MM. Is there a value for probiotic supplements in gestational diabetes mellitus? J Health Popul Nutr. 2015;33:25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 167. | Lindsay KL, Kennelly M, Culliton M, Smith T, Maguire OC, Shanahan F, Brennan L, McAuliffe FM. Probiotics in obese pregnancy do not reduce maternal fasting glucose: a double-blind, placebo-controlled, randomized trial (Probiotics in Pregnancy Study). Am J Clin Nutr. 2014;99:1432-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 168. | Taghizadeh M, Asemi Z. Editorial Expression of Concern for: Taghizadeh, M., Asemi, Z. Effects of synbiotic food consumption on glycemic status and serum hs-CRP in pregnant women: a randomized controlled clinical trial. Hormones (Athens). 2022;21:343-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 169. | Laitinen K, Poussa T, Isolauri E; Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota Group. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br J Nutr. 2009;101:1679-1687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 237] [Article Influence: 13.9] [Reference Citation Analysis (0)] |