Published online Dec 28, 2022. doi: 10.3748/wjg.v28.i48.6827
Peer-review started: September 17, 2022
First decision: October 30, 2022
Revised: November 4, 2022
Accepted: November 28, 2022
Article in press: November 28, 2022
Published online: December 28, 2022
Processing time: 100 Days and 16.3 Hours
Pancreatic cancer (PC) is the third-leading cause of cancer deaths. The overall 5-year survival rate of PC is 9%, and this rate for metastatic PC is below 3%. However, the PC-induced death cases will increase about 2-fold by 2060. Many factors such as genetic and environmental factors and metabolic diseases can drive PC development and progression. The most common type of PC in the clinic is pancreatic ductal adenocarcinoma, comprising approximately 90% of PC cases. Multiple pathogenic processes including but not limited to inflammation, fibrosis, angiogenesis, epithelial-mesenchymal transition, and proliferation of cancer stem cells are involved in the initiation and progression of PC. Early diagnosis is essential for curable therapy, for which a combined panel of serum markers is very helpful. Although some mono or combined therapies have been approved by the United States Food and Drug Administration for PC treatment, current therapies have not shown promising outcomes. Fortunately, the development of novel immunotherapies, such as oncolytic viruses-mediated treatments and chimeric antigen receptor-T cells, combined with therapies such as neoadjuvant therapy plus surgery, and advanced delivery systems of immunotherapy will improve therapeutic outcomes and combat drug resistance in PC patients. Herein, the pathogenesis, molecular signaling pathways, diagnostic markers, prognosis, and potential treatments in completed, ongoing, and recruiting clinical trials for PC were reviewed.
Core Tip: Pancreatic cancer (PC) is the third-leading cause of cancer deaths. Pancreatic ductal adenocarcinoma is the most common type of PC in the clinic. Multiple pathogenic processes including inflammation, fibrosis, angiogenesis, epithelial-mesenchymal transition, and proliferation of cancer stem cells are involved in PC initiation and progression. Although some therapies have been approved for PC treatment, the overall 5-year survival rate is still very low. A combined panel of serum markers is very helpful for PC diagnosis. New treatments and more clinical trials are required to search for new potent therapeutic agents and to evaluate their efficacy in PC treatment.
- Citation: Zhang CY, Liu S, Yang M. Clinical diagnosis and management of pancreatic cancer: Markers, molecular mechanisms, and treatment options. World J Gastroenterol 2022; 28(48): 6827-6845
- URL: https://www.wjgnet.com/1007-9327/full/v28/i48/6827.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i48.6827
Pancreatic cancer (PC) accounts for 7% of all cancer-related deaths[1]. The overall 5-year survival rate of PC is 9%, with only 3% for metastatic PC[2]. However, the number of PC-induced death cases will increase about 2-fold by 2060[3,4]. Many factors are involved in the development of PC[5-7], including genetic mutations, environmental factors, and metabolic diseases such as obesity and diabetes. The most commonly diagnosed PC in the clinic is pancreatic ductal adenocarcinoma (PDAC), which accounts for more than 90% of all PC cases[4]. The rest of the PC cases are pancreatic neuroendocrine neoplasms. Pancreatic neuroendocrine neoplasms originate from precursor cells in the pancreatic ductal epithelium with neuroendocrine differentiation, which can be divided into well-differentiated pancreatic neuroendocrine tumors and poorly differentiated pancreatic neuroendocrine carcinomas[8].
PC is a heterogenous and desmoplastic cancer. Genetic variants of tumor cells, immunosuppressive tumor microenvironment (TME), high metastatic rate, and limited therapeutic outcomes cause challenges for current therapies[9-11]. Early diagnosis of PC is critically important for longer survival outcomes. Serum biomarkers can be applied for PC diagnosis, including microRNAs[12] and cancer antigens such as carbohydrate antigen 19-9 (CA 19-9)[13]. In addition, some of these markers such as CA 19-9 can be applied to predict tumor recurrence and survival of PC patients[14,15].
Herein, this review first summarized the pathogenic factors and their associated molecular signaling pathways that are involved in PC development and progression. Then, diagnostic and prognostic markers were reviewed, especially serum biomarkers. Based on the pathogenic factors, corresponding treatments were discussed, and currently completed, ongoing, and recruiting clinical trials for PC treatment were summarized.
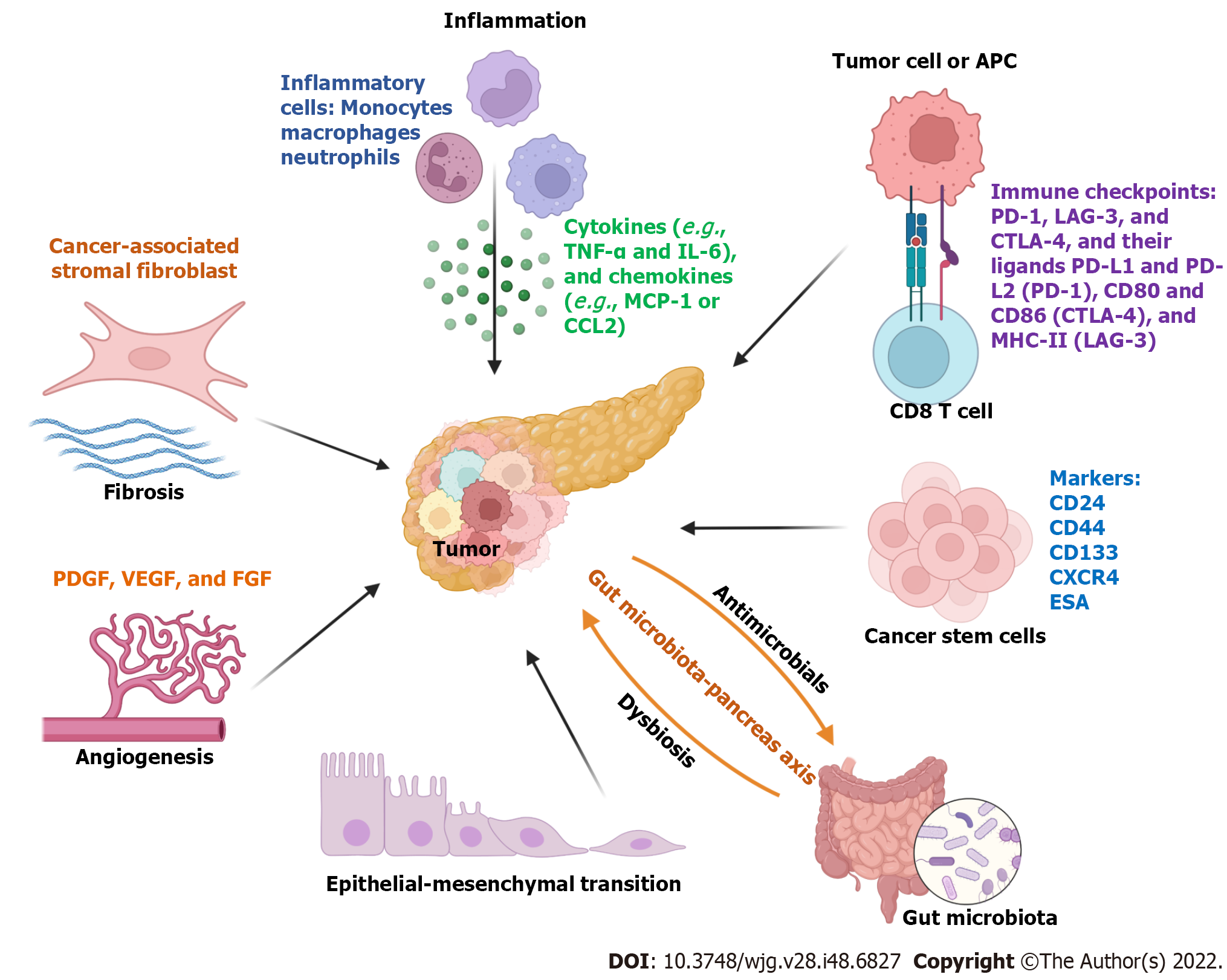
The initiation and progression of PC are impacted by many factors, including chronic inflammation or pancreatitis, fibrosis, immunosuppressive TME, epithelial-mesenchymal transition (EMT), proliferation and differentiation of cancer stem cells, and alteration of gut microbiota. In this section, we discussed each of these factors in PC pathogenesis.
Inflammation is a key mediator for the initiation and progression of PC[16]. In TME during PC development, tumor growth accompanies the infiltration of innate and adaptive immune cells. For example, tumor-associated macrophages are one of the major immune cells in the TME, which have been shown to play an essential role in the initiation, progression, and metastasis of PC as well as chemotherapeutic resistance[17]. Tumor necrosis factor alpha-expressing macrophages are recruited by monocyte chemoattractive protein-1 or CCL2, which can induce the reprogramming of classical neoplastic cells into an aggressive phenotype via the bromodomain-containing protein 4-mediated signaling pathway[18]. In addition, other infiltrating immune cells including monocytes, myeloid-derived suppressor cells, natural killer cells, neutrophils, and CD4+ and CD8+ T cells interplay with cancer cells by secreting cytokines. Molecular signaling pathways such as nuclear factor-κB, reactive oxygen species, and toll-like receptors (TLRs) are involved in the inflammatory condition in the TME of PC[16].
Both local and systemic inflammation contributes to PC development and progression. A clinical study showed that PDAC patients with systemic inflammation characterized by a neutro-phil/lymphocyte ratio > 3.1 have a lower median overall survival compared to patients with a neutrophil/lymphocyte ratio < 3.1 in response to the treatment of anti-CD40 monoclonal antibody in combination with gemcitabine[19]. Obesity can induce systemic inflammation and contribute to cancer progression, including PDAC[20]. The chronic low-grade inflammation in white adipose tissues of obese patients plays an important role in PDAC progression[21]. Some important adipokines such as lipocalin 2, proinflammatory cytokines [e.g., tumor necrosis factor alpha and interleukin (IL)-6], and chemokines (e.g., monocyte chemoattractive protein-1 or CCL2) drive the progression of PDACs[21,22].
Chronic pancreatitis characterized by redness and swelling inflammation in the pancreas can be induced by factors such as heavy alcohol consumption[23], smoking, and gallstones[24]. It is a risk factor for the initiation of the progression of PDAC[25] or PC[26]. In the United States, 1.04% of patients with chronic pancreatitis were diagnosed with PDAC, which was higher than the rate in the control group (0.2%)[27]. Therefore, targeting inflammation is a therapeutic strategy for PC treatments.
Chronic pancreatitis and PC are commonly associated with desmoplastic tissue proliferation, which is mainly caused by activated pancreatic stellate cells[28]. Fibrotic stroma is formed by fibroblasts and their secreted extracellular matrix proteins that contribute to cancer cell proliferation and invasion, an immunosuppressive environment, and therapeutic resistance[29]. Some molecules including epithelium-specific E-twenty six factor 3[30], galectin-1[28], β-catenin[31], and transforming growth factor-β1 (TGF-β1)[32] are essential drivers for pancreatic stellate cell activation in PC. In addition, activated pancreatic stellate cells can secrete many molecules, such as IL-6, TGF-β1, stromal cell-derived factor-1, hepatocyte growth factor, and galectin-1, to induce PC cell proliferation, migration, and chemotherapeutic resistance[33].
Cancer-associated stromal fibroblasts (CAFs) are a heterogenous cell population, which can secrete many factors to regulate inflammation, cancer development, progression, metastasis, recurrence, and drug resistance[34]. For instance, CAFs and tumor cells can crosstalk through extracellular vesicles (EVs). Annexin A6-enriched EVs secreted by CAFs can increase the aggressiveness of PDACs[35,36]. CD9 is an important component of these EVs[36]. In addition, the uptake of CD9+ANXA6+ EVs secreted by CAFs can activate the mitogen-activated protein kinase signaling pathway to promote PC cell migration and EMT[36].
Overexpression of immune checkpoints, such as programmed cell death protein 1 (PD-1), lymphocyte-activation gene 3, and cytotoxic T-lymphocyte-associated antigen 4, and their ligands programmed death-ligand 1 (PD-L1) and PD-L2, CD80 and CD86 (cytotoxic T-lymphocyte-associated antigen 4), and major histocompatibility complex molecule II or major histocompatibility complex-II (lymphocyte-activation gene 3) mediate the immunosuppression in TME[37]. Therefore, targeting these molecular signaling axes provide novel therapeutic options. For example, PD-L1 is overexpressed by tumor cells and some immunosuppressive cells in PDAC, which can be targeted by PD-1-expressing chimeric antigen receptor (CAR) T cells to mediate anti-tumor activity by PD-1/PD-L1 interaction[38].
Additionally, other proteins, such as fibroblast activation protein, CD73, and inhibitor of DNA binding 1[39], can mediate the immunosuppressive environment in the TME. In a mouse PDAC model, elevated expression of CD73, a cell surface-localized ecto-5’-nucleotidase, is positively associated with the infiltration of myeloid-derived suppressor cells and expression of granulocyte-macrophage colony-stimulating factor, which causes suppression of interferon-gamma production in intratumoral T cells. The CD73-mediated suppressive effect on T cells can be abolished by genetic knockdown in PDAC cells[40]. In human PDAC cells, the elevated expression of CD73 causes cancer cell resistance to gemcitabine by activating the protein kinase B (AKT) signaling pathway[41].
EMT plays a pivotal role in PC progression and metastasis, which is defined by cell phenotypic transition from an epithelial to a mesenchymal state[42]. Dermokine genes regulate the oncogenesis of PC. Overexpression of dermokine-α can promote PC cell proliferation, EMT, migration, and invasion by regulating the phosphorylation of signal transducer and activator of transcription 3 (STAT3)[43,44]. Methylsterol monooxygenase 1 as a tumor suppressor can inhibit PC progression by suppressing the phosphoinositide 3-kinase-AKT-mammalian target of rapamycin signaling pathway and EMT[45]. EMT of PC stem cells also plays a critical role in PC initiation and progression, which is discussed in the following section.
Angiogenesis plays a key role in PC development, progression, and metastasis, which is commonly associated with the activation of proangiogenic and angiogenic molecules. For example, the epidermal growth factor receptor is overexpressed in PC cells, which is associated with angiogenesis and cancer cell metastasis[46]. A high density of macrovessels, impaired integrity of microvessels, and poorly perfused vessels are the characterizations of vascularization in TME of PC[29]. Many proteins are involved in the angiogenesis of PC, including vascular endothelial growth factor (VEGF), platelet-derived growth factor, fibroblast growth factor, and their receptors such as VEGF receptors (VEGFR1-3)[29]. In inflammatory conditions, the expression of fibroblast growth factor 1 on PC cells is stimulated by inflammatory product prostaglandin E2, resulting in the proliferation of CAFs and an increased VEGFA expression to maintain angiogenesis[47].
Cancer stem cells (CSCs) are a small part of the TME, where they play a vital role in chemotherapy resistance. Pancreatic CSCs express several surface markers such as CD24, CD44, CD133, C-X-C chemokine receptor type 4, tyrosine-protein kinase Met, epithelial cell adhesion molecule, and doublecortin like kinase 1 as well as intracellular markers such as aldehyde dehydrogenase 1 and RNA polymerase II-associated factor 1[48]. One study showed that miR-497 can resensitize pancreatic CSCs to gemcitabine treatment by inhibiting nuclear factor-κB expression[49]. Exosomes can horizontally transfer drug-resistant traits from gemcitabine-resistant pancreatic CSCs to gemcitabine-sensitive PC cells by delivering miR-210[50]. Small nucleolar RNAs such as SNORD35A play an important role in the proliferation, migration, invasion, and EMT of pancreatic CSCs through regulating the hepatocyte growth factor/tyrosine-protein kinase Met signaling pathway, which is a prognostic biomarker and therapeutic target for PC treatment[51]. In addition, CSCs are involved in drug resistance. For instance, a hypoxic niche can further activate AKT/Notch1 signaling pathway to enhance gemcitabine-induced stemness to cause chemoresistance[52].
Gut microbiomes, including bacteria, viruses, archaea, and fungal species, play important roles in energy digestion, synthesis of secondary bile acids, vitamins and proteins, and immune regulation. Most of the microbes reside (> 95%) within the gut and maintain intestinal homeostasis[53]. Dysbiosis of gut microbiota can regulate inflammation and immune response in the TME to promote cancer progression[54,55], including PC[56,57]. A preclinical study showed that gut microbiota can promote PDAC progression by regulating the infiltration and anti-cancer activity of natural killer cells in the TME[58]. Cohort studies in three different countries from Asia (Japan) and Europe (Spanish and German) showed that Streptococcus and Veillonella spp were significantly enriched and Faecalibacterium prausnitzii was depleted in gut microbial profiles of PDAC patients[59]. A comprehensive analysis of microbiota in several tumors including PC showed that most intratumor bacteria were present intracellularly in both cancer and immune cells[60].
Recent studies also showed that intratumor bacteria were associated with the survival time of PDAC patients[61,62]. In addition, bacteria-mediated treatment (e.g., a bacterium Megasphaera sp. XA511) can enhance the anti-tumor effect of anti-PD-1 treatment[61]. Another study also revealed that the alpha diversity of tumor microbiota was higher in long-term survival patients compared to that in short-term survival patients. Fecal microbiota transplantation from humans into mice can regulate tumor growth and anti-tumor immune response[62]. Moreover, pancreatic secretions (e.g., antimicrobial peptides) influence the change of gut microbiota profiles, and the pancreas-bacteria interplay forms a gut-microbiota-pancreas axis[63].
Oral microbiota may also impact the development of PC. One study showed that the presence of oral Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans was positively associated with a high PC risk, whereas genus Leptotrichia (phylum Fusobacteria) was related to a low PC risk[64]. In addition, other oral bacterial species including Clostridium difficile, Campylobacter jejuni, Escherichia coli, Enterococcus faecalis, Helicobacter pylori, Fusobacterium nucleatum, Vibrio cholera, and Porphyromonas gingivalis have been reported to be associated with PC development, which may regulate anti-tumor immunity by signaling pathways such as the miR-21/phosphatase and tensin homolog axis[65].
Overall, multiple factors influence the initiation and progression of PC, which is summarized in Figure 1.
The tumors in PC patients can be classified into resectable, borderline resectable, locally advanced, and metastatic tumors[66]. Early diagnosis ensures curable treatment by surgical resection[67]. An accurate diagnosis of PC can improve therapeutic outcomes. Although there is an advanced improvement in new diagnostic technologies, biopsy is still the gold standard for PC diagnosis[68]. Imaging methods including computed tomography, positron emission tomography, magnetic resonance imaging, and endoscopic ultrasound are commonly applied in PC diagnosis and staging[68,69]. In addition, several serum markers have promising diagnostic and prognostic values for PC.
Serum markers including carcinoembryonic antigen and CAs can be applied in PC diagnosis and prognosis[70,71]. Currently, CA 19-9 is the most broadly used serum biomarker for PC diagnosis. A meta-analysis showed that the average sensitivity and specificity of CA 19-9 in PC diagnosis were 72% [95% confidence interval (CI): 71%-73%] and 86% (95%CI: 85%-86%), respectively, with an area under the curve (AUC) of 0.8474 (95%CI: 0.8272-0.8676)[72]. It has been reported that CA 19-9 serum levels are significantly associated with positive lymph nodes and positive margin status in patients with resectable PDAC, which is important for the decision of neoadjuvant treatments[73]. In addition, the preoperative levels of CA 19-9 are negatively associated with the overall survival, nodal involvement, and margin status positivity in resectable PC. However, some limitations impair the role of CA 19-9 in PDAC preoperative staging and management[74], including up to 50% of PDAC patients without CA 19-9 secretion.
In combination with other markers, the diagnostic value of CA 19-9 can be amplified or increased. For example, a combination of CA19-9 with serum mucin 5AC, a heavily glycosylated protein of the mucin family, improves both sensitivity (73.8%) and specificity (88.6%) as well as the AUC (0.894; 95%CI: 0.844-0.943) for PC diagnosis in patients, better than the values of each individual marker[75]. There are many other serum markers that can be used for PC or PDAC diagnosis, including macrophage inhibitory cytokine-1[76], keratin 8[77], protein induced by vitamin K absence II[78], and gremlin 1 (GREM1)[79] (Table 1). Meanwhile, combining different markers in a panel could increase the values of sensitivity, specificity, and AUC. For example, a panel including CA 19-9, factor VIII, fibrinogen, albumin, and alkaline phosphatase increase the AUC value to 0.95 (95%CI: 0.89-0.99) when compared to 0.80 (0.71-0.88) for CA 19-9 alone in distinguishing PDAC from intraductal papillary mucinous neoplasm, a benign tumor[80]. Another study also showed that a panel of four biomarkers including S100 calcium-binding protein A2, S100 calcium-binding protein A4, CA 125, and CA 19-9 increased AUC to 0.913[81].
MicroRNAs are small non-coding RNAs with about 22 nucleotides, which regulate the expression of their target mRNAs through degradation or translational repression. Serum expression profiles of microRNAs in PC patients are significantly changed compared to healthy controls. Among them, a panel model including miR-125a-3p, miR-5100, and miR-642b-3p showed the most promising value for PC diagnosis with an AUC of 0.95, sensitivity of 0.98, and specificity of 0.97[82]. Another study also showed that serum microRNAs such as miR-25-3p, miR-19a/b-3p, miR-192-5p, miR-223-3p, and let-7b-5p were upregulated in PC patients and can be used as diagnostic markers as a panel[83].
Multi-omics profiling studies can provide new markers for PC diagnosis. Proteomic analysis of EVs derived from co-cultured epithelial and stromal cells in the condition mimicking TME showed that kinesin family member 5B and secreted frizzled related protein 2 had promising values as early PC biomarkers[84]. PancRISK score evaluated by three urine markers, including lymphatic vessel endothelial hyaluronan receptor 1, regenerating family member 1 beta, and trefoil factor 1, showed reasonable sensitivity and specificity for PDAC detection compared to CA 19-9[85]. Another study also showed that a panel of three urine markers with CA 19-9 had pre-diagnostic values before PDAC diagnosis, with a sensitivity of 72% at 90% specificity up to 1 year and 60% sensitivity with 80% specificity up to 2 years[86].
Bioinformatics analysis showed that gremlin 1, a bone morphogenetic protein signaling regulator, was overexpressed in PDAC and predicted a poorer prognosis for patients with PDAC[79]. In addition, serum gremlin 1 is increased in PDAC patients compared to healthy controls and has a diagnostic value. In combination with CA 19-9, the AUC value increases from 0.718 to 0.914[79].
One study displayed that PC patients with low lymphocyte-C-reactive protein ratio have significantly low recurrence-free survival and overall survival values[87]. Another study showed that the systemic immune-inflammation index, which is calculated using the absolute platelet, neutrophil, and lymphocyte counts [systemic immune-inflammation index = platelet × (neutrophil/lymphocyte)], can be used as an independent negative prognostic marker of overall survival of PDAC patients receiving neoadjuvant therapy[88]. A meta-analysis showed that mucin 4 (hazard ratio = 2.04, 95%CI: 1.21-3.45) and mucin 16 (hazard ratio = 2.10, 95%CI: 1.31-3.37) had predictive values for the prognosis of PC patients[89]. The expression of aquaporin-5, a water channel protein, is increased in pancreatic adenocarcinoma (PAAD), which is positively associated with the infiltration of different immune cells (e.g., macrophages) in tumors and poor prognosis of PAAD patients[90]. A bioinformatics study showed that overexpression of matrix metallopeptidase 14 and collagen XII alpha 1 is significantly related to the poor prognosis of PAAD patients[91]. Analysis of RNA sequencing data from the Gene Expression Omnibus and the Cancer Genome Atlas databases gives us some conclusion that some biomarkers such as transmembrane protein 170B (TMEM170B) can be applied to predict cancer progression[92]. Overall, improvement in bioinformatics and new technologies accelerates the development of new diagnostic and prognostic markers for PC, which will result in good outcomes for PC therapy.
Surgical resection is a curable therapy for patients with early stages of PC and good health conditions[66,68]. There are some Food and Drug Administration (FDA)-approved drugs for PC treatment (Table 2), including belzutifan[93,94], erlotinib hydrochloride[95,96], everolimus[97,98], fluorouracil, also known as 5-fluorouracil[99,100], gemcitabine hydrochloride[101,102], irinotecan hydrochloride liposome[103,104], mitomycin[105,106], olaparib[107,108], paclitaxel albumin-stabilized nanoparticle formulation[109,110], and sunitinib malate[111,112]. In addition, combined treatments including leucovorin calcium (folinic acid) + fluorouracil + irinotecan hydrochloride + oxaliplatin, gemcitabine hydrochloride + cisplatin, gemcitabine hydrochloride + oxaliplatin, and oxaliplatin + fluorouracil + leucovorin calcium (folinic acid) have been also approved by the United States FDA for PC treatment[113-115].
| Drug names | Conditions | Targets | Ref. |
| Belzutifan | Pancreatic neuroendocrine tumors | An inhibitor of hypoxia-inducible factor-2α | [93,94] |
| Erlotinib hydrochloride | Gemcitabine hydrochloride-treated PC, not removable with surgery, with metastasis or local progression | An EGFR inhibitor | [95,96] |
| Everolimus | Progressive pancreatic neuroendocrine tumors, not removable with surgery, with metastasis or local advance | A mammalian target of rapamycin inhibitor | [97,98] |
| Fluorouracil, also called 5-FU | Pancreatic cancer | An anti-metabolite drug with multiple functions such as inhibition of cellular thymidylate synthase to prevent DNA replication and inhibit RNA synthesis | [99,100] |
| Gemcitabine hydrochloride | PC with metastasis, local advance, or fluorouracil treatment | An antimetabolite drug and an inhibitor of DNA synthesis | [101,102] |
| Irinotecan hydrochloride liposome | Metastatic PC or gemcitabine hydrochloride-treated PC with precision | An inhibitor of topoisomerase I | [103,104] |
| Mitomycin | Pancreatic adenocarcinoma with local advance or metastasis to other parts of the body, which has no approvement with other types of treatment | An inhibitor of DNA synthesis and thioredoxin reductase | [105,106] |
| Olaparib | Metastatic PC after first-line therapy with platinum chemotherapy and with certain germline mutations in the breast cancer 1 or BRCA2 gene | A poly ADP-ribose polymerase inhibitor | [107,108] |
| Paclitaxel albumin-stabilized nanoparticle formulation | PC with metastasis | It prevents cell mitosis and inhibits the growth of cancer cells | [109,110] |
| Sunitinib malate | Progressive neuroendocrine tumors that are not removable with surgery, with metastasis to other parts of the body or local advance | An antiangiogenic tyrosine kinase inhibitor | [111,112] |
In this section, we discussed some treatments targeting the driving factors of PC, including immune checkpoint inhibitors, antifibrosis, anti-inflammation, anti-angiogenesis, growth factor inhibitors, anti-cancer peptides, alteration of gut microbiota, T cell therapy, and oncolytic viruses as well as combined therapies.
Immunotherapy by targeting immune checkpoints such as PD-1 and PD-L1 has achieved big success in the treatment of many different tumors[116,117]. Currently, pembrolizumab (anti-PD-1) is the only FDA-approved immune checkpoint inhibitor for the treatment of patients who have advanced PDACs with mismatch repair deficiency or microsatellite instability high[118]. There are many ongoing clinical trials for the evaluation of synergistic effects of ipilimumab or tremelimumab (anti-cytotoxic T-lymphocyte-associated antigen 4 antibody), nivolumab (anti-PD-1 antibody), and durvalumab (anti-PD-L1 antibody) with other chemotherapy, vaccines, or radiotherapy[119].
CAFs are one of the most abundant stromal cells in PDAC and contribute to cancer progression and chemoresistance[120]. Therefore, reshaping the fibrotic stroma is a strategy to treat PC. The integrin-mediated signaling pathway plays a critical role in remodeling and induction of pancreatic tissue stiffness during PC development and progression, promoting chemoresistance. The phosphorylation of tyrosine397 in focal adhesion kinase (FAK) of CAFs is significantly increased compared to that in fibroblasts of the normal pancreas. Therefore, inhibiting FAK activity can dramatically suppress CAF migration and extracellular matrix deposition[120]. Meanwhile, FAK inhibition can also resensitize PDAC cells to chemotherapy[121]. Some tyrosine kinase inhibitors such as cabozantinib, pazopanib, lenvatinib, and surufatinib are under clinical evaluation for the treatment of pancreatic neuroendocrine tumors[122]. A study in a murine PC model also showed that stromal hyaluronan degradation by PEGylated recombinant human hyaluronidase in combination with FAK inhibitor could improve anti-PD-1 antibody efficacy on the survival of PDAC-bearing mice by increasing T cell infiltration and efficacy[123].
The anti-inflammatory drug aspirin can inhibit cell proliferation of PC cell lines by suppressing cyclin D1 expression to induce G0/G1 cell cycle arrest. Aspirin can also inactivate the glycogen synthase kinase-3β signaling pathway and regulate the expression of microRNAs in PC cells[124]. A phase I trial (https://clinicaltrials.gov, registration number NCT03207724) showed that treatment with bermekimab (anti-IL-1α antibody) can decrease inflammatory cytokines and endothelial growth factor, which is associated with an increase in healthy gut microbiota Akkermansia compared to the baseline[125]. A phase 3 clinical trial (NCT02923921) showed that adding pegilodecakin (PEG, a pegylated recombinant human IL-10) to folinic acid, fluorouracil, and oxaliplatin increased the expression of total IL-18, interferon-gamma, and granzyme B and decreased TGF-β in patients with post-gemcitabine metastatic PDACs[126].
The expression of mitofusin-2 in PC tissues is significantly decreased, which is negatively associated with VEGFA expression. A molecular study showed that overexpression of mitofusin-2 could inhibit the expression of VEGFA, VEGFR2, angiopoietin-1 gene, and tissue inhibitor of metalloproteinase 1 in human umbilical vein endothelial cells[127]. Another study showed that escin, a pentacyclic triterpenoid isolated from the horse chestnut, can inhibit angiogenesis by suppressing the expression of IL-8 and VEGF in PC cells through the blockade of nuclear factor-κB[128]. Treatment with apatinib, a small molecule targeting VEGFR2, can inhibit the proliferation, migration, and invasion of PC cells (ASPC-1 and PANC-1 cells) and the growth of their xenografted tumors by inhibiting cancer cell growth and angiogenesis via suppression of the phosphorylation of VEGFR2, AKT, and ERK1/2[129].
Growth factors play essential roles in PC cell survival, progression, and drug resistance[130]. For example, TGF-β contributes to PDAC progression by inducing N-glycomic changes in SMA-related and MAD-related protein 4-deficient PDAC cell line PaTu-8955S cells[131].
Anti-cancer peptides are short peptides with direct and indirect anti-cancer properties. Anti-cancer peptides can be classified into natural and synthetic peptides. For example, human cathelicidin peptide LL-37 can suppress PC growth in vitro and in vivo by activating the mammalian target of rapamycin signaling pathway to suppress autophagy of PC cells and induce reactive oxygen species production to cause DNA damage and cell cycle arrest[132]. KS-58, a derivative of KRpep-2d that is an artificial cyclic peptide that can selectively inhibit K-Ras (G12D), can suppress the human PC cell line PANC-1 proliferation in vitro. In addition, KS-58 also displays anti-tumor activity in subcutaneous and orthotropic PANC-1 cell xenografted tumors, which shows a synergistic effect with gemcitabine[133].
Patients with high levels of antibodies against a pathogenic periodontal bacterium Porphyomonas gigivalis have a double risk of developing PC compared to subjects with low levels of antibodies. In contrast, individuals who have high levels of antibodies against commensal oral bacteria reduce the risk of PC development[134]. The microbiome in the cancerous pancreas can induce immune tolerance by causing macrophage-mediated suppression of T cell functions through TLR signaling pathways (e.g., TLR2 and TLR5)[135]. Depletion of gut microbiota by antibiotics can inhibit tumor progression and metastasis via upregulation of interferon-gamma-producing T cells and downregulation of IL-10 and IL-17A-producing T cells[136]. Therefore, the alteration of microbial components in the gut and mouth, as well as the TME, can effectively inhibit PC progression.
CAR-engineered T cell (CAR-T) therapy shows potential for many tumors. To date, six CAR-T products have been approved by the United States FDA for the treatment of hematopoietic cancers, such as B-cell acute lymphoblastic leukemia (tisagenlecleucel), mantle cell lymphoma (brexucabtagene autoleucel), and multiple myeloma (idecabtagene vicleucel)[137]. However, current clinical trials of CAR-T therapy in PC patients have not shown significance in the improvement of survival and other outcomes[138]. CAF-derived extracellular matrix proteins, enzymes, and growth factors impact the infiltration and efficacy of CAR-T. Enhancing the expression of chemokine ligands such as chemokine (C-C motif) ligand 19 (CCL19) can increase the infiltration of the CAR-T to promote their anti-PDAC efficacy[139].
Virus-mediated delivery of cytokines and shRNAs shows promising effects in PC treatment. Oncolytic viruses are designed to directly target tumor cells or to activate anti-tumor immune responses. However, treatment of oncolytic viruses alone is not sufficient to eliminate PC to date[140]. For example, oncolytic adenoviruses loading CD55-ST13 (suppression of tumorigenicity 13)-tumor necrosis factor-related apoptosis-inducing ligand can significantly inhibit but not delete tumor development in a murine xenografted PDAC tumor model by inducing cancer cell apoptosis[141].
The combined therapy of MEK inhibitor (trametinib) and STAT3 inhibitor (ruxolitinib) inhibits the phenotype of proinflammatory and myofibroblastic IL6+CXCL1+LRRC15+CAFs and increases mesenchymal stem cell-like Ly6a+Cd34+CAFs in a murine model detected by single-cell RNA sequencing[142]. The CAF phenotype change is associated with M2-to-M1 reprogramming of tumor-associated macrophages s and effector CD8+ T cell infiltration. In addition, treatment of MEK and STAT3 inhibitors together with a PD-1 inhibitor (nivolumab) shows clinical benefit in patients with chemotherapy-refractory metastatic PDACs[142]. In contrast, a pharmacodynamic separation treatment for erlotinib plus gemcitabine can improve their treatment efficacy, especially for patients with detected plasma Kirsten rat sarcoma virus mutation[143].
Four-week intraperitoneal injection of gemcitabine together with oral administration of probiotic cocktails (Lactobacillus paracasei GMNL-133 and Lactobacillus reuteri GMNL-89) can inhibit pancreatic intraepithelial neoplasia formation and suppress serum levels of aspartate aminotransferase and alanine aminotransferase and the expression of vimentin and Ki-67 in pancreatic sections[144].
Some treatments are under clinical investigation, see Table 3 (https://clinicaltrials.gov, accessed on September 6, 2022). These treatments include galunisertib plus gemcitabine[145], Janus kinase 1/2 inhibitor ruxolitinib[146], adoptive transfer of T cells[147], gemcitabine and trastuzumab plus erlotinib[148], erlotinib plus gemcitabine[149], pegilodecakin plus folinic acid, fluorouracil, and oxaliplatin[126], liposomal irinotecan plus 5-fluorouracil/leucovorin[150], bevacizumab[151], and sunitinib[152].
| Trial number | Phase | Treatment | Condition |
| NCT01373164 | 1b/2 | Galunisertib, a TGF-β receptor inhibitor, or placebo plus gemcitabine | Unresectable PC |
| NCT01423604 | 2 | Ruxolitinib, a Janus kinase 1/2 inhibitor or placebo plus capecitabine | PC |
| NCT00965718 | 2 | Activated T lymphocyte (ex vivo-expanded, CIK cells cultured with anti-CD3 monoclonal antibody and IL-2) | PC |
| NCT01204372 | 2 | Gemcitabine, trastuzumab plus erlotinib | Metastatic PC |
| CONKO-005 | 3 | Erlotinib (inhibits the intracellular phosphorylation of tyrosine kinase associated with the EGFR) or placebo plus gemcitabine | Primarily resectable PDAC after R0 resection |
| NCT02923921 | 3 | Pegilodecakin (a pegylated recombinant human IL-10) plus folinic acid, fluorouracil, and oxaliplatin | Metastatic PDAC |
| NCT01494506 | 3 | Liposomal irinotecan (it prevents the religation of the DNA strand by binding to the topoisomerase I-DNA complex.) or placebo plus 5-FU/LV | Metastatic PC |
| NCT01214720 | 3 | Bevacizumab that acts by selectively binding circulating VEGF, thereby inhibiting the binding of VEGF to its cell surface receptors plus chemotherapy | Metastatic PC |
| NCT00428597 | 3 | Sunitinib, an inhibitor of multiple receptor tyrosine kinases | Metastatic pancreatic neuroendocrine tumors |
| NCT01525550 | 4 |
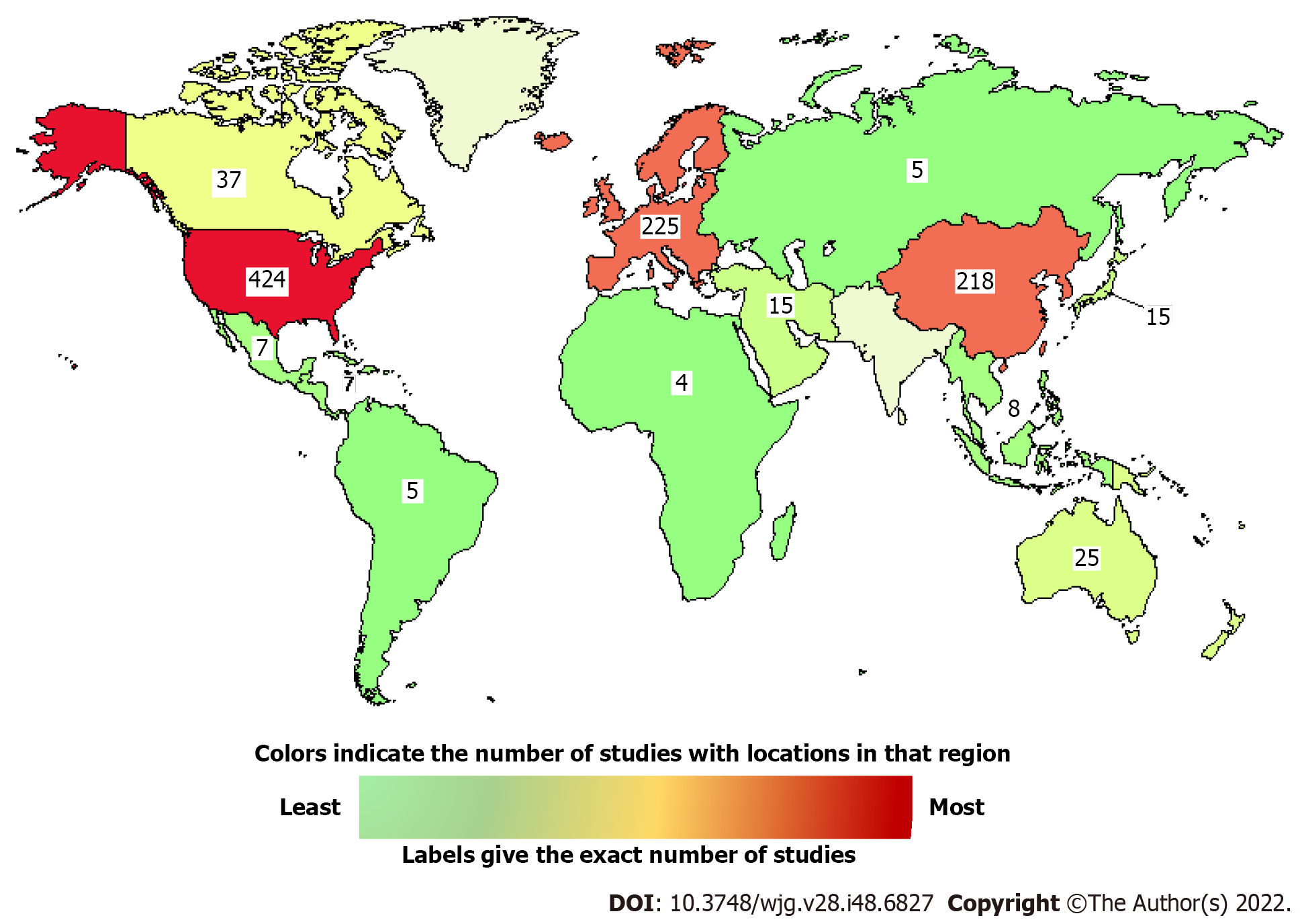
In addition, more than 900 studies in the world are recruiting or enrolling by invitation, and some have a ‘not yet recruiting’ status for evaluating PC treatments (https://clinicaltrials.gov, accessed on September 17, 2022). A graphic map shows the number of studies at different locations (Figure 2), and some examples are listed in Table 4.
| Trial number | Phase | Treatments | Conditions |
| NCT03192462 | 1 or 2 | Intravenous infusions of TAA-specific cytotoxic T lymphocytes | Pancreas cancer with metastatic, locally advanced unresectable, or resectable disease |
| NCT04637698 | 1 or 2 | Oncolytic viral therapy (Type 2 Herpes simplex virus) expressing GM-CSF | Pancreatic cancer |
| NCT04247165 | 1 or 2 | Dual checkpoint inhibition (nivolumab and ipilimumab) in combination with gemcitabine and nab-paclitaxel followed by immune-chemoradiation | Borderline resectable, locally advanced, or metastatic pancreatic cancer |
| NCT05141149 | 1 or 2 | Anti-PAUF monoclonal antibody PBP1510 or in combination with gemcitabine | Advanced/metastatic pancreatic cancer |
| NCT04825288 | 1 or 2 | Anti-IL-1α true human antibody XB2001 or in combination with ONIVYDE + leucovorin + 5-FU chemotherapy | Advanced pancreatic cancer |
| NCT03662412 | 1 or 2 | Sirolimus, a selective inhibitor of mTOR | Advanced pancreatic cancer |
| NCT05131776 | 2 or 3 | Concurrent EUS-guided intratumor injection of P-32 microparticles (OncoSil) | Locally advanced pancreatic carcinoma |
| NCT03941093 | 3 | Neoadjuvant treatment with pamrevlumab or placebo in combination with either gemcitabine plus nab-paclitaxel or FOLFIRINOX | Locally advanced pancreatic cancer |
| NCT05529940 | 3 | FOLFIRINOX | Resectable pancreatic cancer |
| NCT04969731 | 3 | Adjuvant Immuncell-LC (Cytokine-induced killer cells) therapy combined with gemcitabine | Resectable pancreatic cancer |
| NCT04025840 | 4 | Perioperative epidural block and/or dexamethasone | Resectable pancreatic cancer |
| NCT04217096 | 4 | Paclitaxel liposome plus S-1, an oral anticancer drug that consists of tegafur, gimeracil, and potassium oteracil in a molar ratio of 1.0:0.4:1.0 | Advanced metastatic pancreatic cancer as the first-line therapy |
A synergistic treatment is a good option for killing drug-resistant PC cells. For example, astaxanthin can resensitize human PC cells to gemcitabine by activating the hypoxia-inducible factor 1α/STAT3 signaling pathway to promote gemcitabine-induced cell apoptosis and inhibit gemcitabine-induced EMT of PC cells[153]. Guadecitabine, an effective inhibitor of DNA methyltransferase 1, has the potential to resensitize PDAC cells to chemotherapy and immune checkpoint blockade therapy (e.g., anti-PD-L1)[154,155].
Neoadjuvant therapy has been applied in clinical trials for patients with resectable PDACs, which include neoadjuvant chemotherapy, neoadjuvant radiotherapy, and neoadjuvant chemoradiotherapy[156]. The results from three randomized controlled trials with a total of 130 patients (56 receiving neoadjuvant therapy and 74 in the control group) indicated that neoadjuvant therapy (chemotherapy or chemoradiation + surgery followed by adjuvant therapy) increased the disease-free survival time compared to upfront surgery followed by adjuvant therapy[157]. Another single-center long-term study also showed that PDAC patients with treatment of neoadjuvant therapy, consisting of folinic acid + fluorouracil + irinotecan + oxaliplatin, single gemcitabine, or combined with cisplatin, nab-paclitaxel, or capecitabine with or without radiation had longer median disease-specific survival and disease-free survival than those receiving treatment with upfront surgery[158]. The benefit of neoadjuvant therapy could be a stage-dependent manner. A retrospective cohort study showed that neoadjuvant therapy was positively associated with an improved survival benefit compared with conventional upfront surgery, especially in clinical stage III PC after propensity score matching within each stage[159]. Overall, it can benefit surgical treatment.
Delivery systems can be applied to enhance the efficacy of anti-cancer treatments. For example, arginine glycine peptide-human serum albumin-mediated drug nanoparticles show tumor-targeting effects and increase the cytotoxicity of gemcitabine and curcumin[160]. Administration of VG161, the first recombinant oncolytic herpes simplex virus type 1 that delivers multiple synergistic antitumor immunomodulatory factors, can systematically activate both innate and adaptive immunity and improve the anti-tumor function of the tumor immune microenvironment[161]. Another study showed that using gold nanoparticles could enhance the intracellular delivery of oncolytic adenoviruses into PC cell lines[162]. Radiofrequency hyperthermia also can enhance the local delivery of oncolytic immuno-virotherapy for PAAD in vitro and in vivo[163].
Furthermore, radiofrequency ablation may be applied to treat patients with PC who are unfit for surgery and includes endoscopic ultrasound-guided radiofrequency ablation[164] and endoluminal biliary radiofrequency ablation[165].
PDAC is the most common type of PC in the clinic. Multiple factors induce PC development and progression, including but not limited to inflammation, fibrosis, angiogenesis, EMT, and proliferation of CSCs. A combined panel of serum markers is very helpful for PC diagnosis, which is essential for curable therapy. Although several mono or combined therapies have been approved by the FDA for PC treatment, the overall 5-year survival rate is still not promising. The development of novel immunotherapies such as oncolytic viruses-mediated treatments and CAR-T, combined therapies (neoadjuvant therapy plus surgery), and advanced delivery systems of immunotherapy will improve therapeutic outcomes and combat drug resistance in PC patients. More clinical trials are required to evaluate the efficacy of existing treatments and to find new potent therapies.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng W, China; Farolfi T, Italy S-Editor: Chen YL L-Editor: Filipodia P-Editor: Chen YL
| 1. | American Cancer Society. Key Statistics for Pancreatic Cancer. 2022. [cited 3 November 2022]. Available from: https://www.cancer.org/cancer/pancreatic-cancer/about/key-statistics.html. |
| 2. | Krebs N, Klein L, Wegwitz F, Espinet E, Maurer HC, Tu M, Penz F, Küffer S, Xu X, Bohnenberger H, Cameron S, Brunner M, Neesse A, Kishore U, Hessmann E, Trumpp A, Ströbel P, Brekken RA, Ellenrieder V, Singh SK. Axon guidance receptor ROBO3 modulates subtype identity and prognosis via AXL-associated inflammatory network in pancreatic cancer. JCI Insight. 2022;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 3. | Lippi G, Mattiuzzi C. The global burden of pancreatic cancer. Arch Med Sci. 2020;16:820-824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 4. | Yang M, Zhang CY. Diagnostic biomarkers for pancreatic cancer: An update. World J Gastroenterol. 2021;27:7862-7865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 5. | McCubrey JA, Yang LV, Abrams SL, Steelman LS, Follo MY, Cocco L, Ratti S, Martelli AM, Augello G, Cervello M. Effects of TP53 Mutations and miRs on Immune Responses in the Tumor Microenvironment Important in Pancreatic Cancer Progression. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Poman DS, Motwani L, Asif N, Patel A, Vedantam D. Pancreatic Cancer and the Obesity Epidemic: A Narrative Review. Cureus. 2022;14:e26654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Bennett C, Suguitan M, Abad J, Chawla A. Identification of high-risk germline variants for the development of pancreatic cancer: Common characteristics and potential guidance to screening guidelines. Pancreatology. 2022;22:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Khanna L, Prasad SR, Sunnapwar A, Kondapaneni S, Dasyam A, Tammisetti VS, Salman U, Nazarullah A, Katabathina VS. Pancreatic Neuroendocrine Neoplasms: 2020 Update on Pathologic and Imaging Findings and Classification. Radiographics. 2020;40:1240-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Sivapalan L, Kocher HM, Ross-Adams H, Chelala C. The molecular landscape of pancreatic ductal adenocarcinoma. Pancreatology. 2022;22:925-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 10. | Joiner JB, Kren NP, Durham PG, McRee AJ, Dayton PA, Pylayeva-Gupta Y. Low-Intensity Focused Ultrasound Produces Immune Response in Pancreatic Cancer. Ultrasound Med Biol. 2022;48:2344-2353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Botrus G, Kosirorek H, Sonbol MB, Kusne Y, Uson Junior PLS, Borad MJ, Ahn DH, Kasi PM, Drusbosky LM, Dada H, Surapaneni PK, Starr J, Ritter A, McMillan J, Wylie N, Mody K, Bekaii-Saab TS. Circulating Tumor DNA-Based Testing and Actionable Findings in Patients with Advanced and Metastatic Pancreatic Adenocarcinoma. Oncologist. 2021;26:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Zhou X, Lu Z, Wang T, Huang Z, Zhu W, Miao Y. Plasma miRNAs in diagnosis and prognosis of pancreatic cancer: A miRNA expression analysis. Gene. 2018;673:181-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 13. | Hartlapp I, Valta-Seufzer D, Siveke JT, Algül H, Goekkurt E, Siegler G, Martens UM, Waldschmidt D, Pelzer U, Fuchs M, Kullmann F, Boeck S, Ettrich TJ, Held S, Keller R, Anger F, Germer CT, Stang A, Kimmel B, Heinemann V, Kunzmann V; German Pancreatic Cancer Group (AIO-PAK) and NEOLAP investigators. Prognostic and predictive value of CA 19-9 in locally advanced pancreatic cancer treated with multiagent induction chemotherapy: results from a prospective, multicenter phase II trial (NEOLAP-AIO-PAK-0113). ESMO Open. 2022;7:100552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Wu Z, Zhao P, Wang Z, Huang X, Wu C, Li M, Wang L, Tian B. Adjusting CA19-9 values with clinical stage and bilirubin to better predict survival of resectable pancreatic cancer patients: 5-year-follow-up of a single center. Front Oncol. 2022;12:966256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Amburn T, Davenport D, Patel R, Moss J, Pandalai P, Kim J, Cavnar M. New Cancer-Related Symptoms Predict Recurrence in CA19-9 Non-Expressers After Resection of Pancreatic Ductal Adenocarcinoma. Am Surg. 2022;31348221117031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Shadhu K, Xi C. Inflammation and pancreatic cancer: An updated review. Saudi J Gastroenterol. 2019;25:3-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 17. | Yang S, Liu Q, Liao Q. Tumor-Associated Macrophages in Pancreatic Ductal Adenocarcinoma: Origin, Polarization, Function, and Reprogramming. Front Cell Dev Biol. 2020;8:607209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 18. | Tu M, Klein L, Espinet E, Georgomanolis T, Wegwitz F, Li X, Urbach L, Danieli-Mackay A, Küffer S, Bojarczuk K, Mizi A, Günesdogan U, Chapuy B, Gu Z, Neesse A, Kishore U, Ströbel P, Hessmann E, Hahn SA, Trumpp A, Papantonis A, Ellenrieder V, Singh SK. TNF-α-producing macrophages determine subtype identity and prognosis via AP1 enhancer reprogramming in pancreatic cancer. Nat Cancer. 2021;2:1185-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 19. | Wattenberg MM, Herrera VM, Giannone MA, Gladney WL, Carpenter EL, Beatty GL. Systemic inflammation is a determinant of outcomes of CD40 agonist-based therapy in pancreatic cancer patients. JCI Insight. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Yang M, Liu S, Zhang C. The Related Metabolic Diseases and Treatments of Obesity. Healthcare (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 21. | Chang HH, Eibl G. Obesity-Induced Adipose Tissue Inflammation as a Strong Promotional Factor for Pancreatic Ductal Adenocarcinoma. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Gomez-Chou SB, Swidnicka-Siergiejko AK, Badi N, Chavez-Tomar M, Lesinski GB, Bekaii-Saab T, Farren MR, Mace TA, Schmidt C, Liu Y, Deng D, Hwang RF, Zhou L, Moore T, Chatterjee D, Wang H, Leng X, Arlinghaus RB, Logsdon CD, Cruz-Monserrate Z. Lipocalin-2 Promotes Pancreatic Ductal Adenocarcinoma by Regulating Inflammation in the Tumor Microenvironment. Cancer Res. 2017;77:2647-2660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 23. | Mehra S, Srinivasan S, Singh S, Zhou Z, Garrido V, Silva IC, Totiger TM, Dosch AR, Dai X, Dawra RK, Jala VR, Shi C, Datta J, VanSaun M, Merchant N, Nagathihalli N. Urolithin A attenuates severity of chronic pancreatitis associated with continued alcohol intake by inhibiting PI3K/AKT/mTOR signaling. Am J Physiol Gastrointest Liver Physiol. 2022;323:G375-G386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Spagnolo DM, Greer PJ, Ohlsen CS, Mance S, Ellison M, Breze C, Busby B, Whitcomb DC, Haupt M. Acute and Chronic Pancreatitis Disease Prevalence, Classification, and Comorbidities: A Cohort Study of the UK BioBank. Clin Transl Gastroenterol. 2022;13:e00455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 25. | Gandhi S, de la Fuente J, Murad MH, Majumder S. Chronic Pancreatitis Is a Risk Factor for Pancreatic Cancer, and Incidence Increases With Duration of Disease: A Systematic Review and Meta-analysis. Clin Transl Gastroenterol. 2022;13:e00463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 26. | Kirkegård J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am J Gastroenterol. 2017;112:1366-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 346] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 27. | Munigala S, Subramaniam DS, Subramaniam DP, Burroughs TE, Conwell DL, Sheth SG. Incidence and Risk of Pancreatic Cancer in Patients with a New Diagnosis of Chronic Pancreatitis. Dig Dis Sci. 2022;67:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Tang D, Wu Q, Zhang J, Zhang H, Yuan Z, Xu J, Chong Y, Huang Y, Xiong Q, Wang S, Tian Y, Lu Y, Ge X, Shen W, Wang D. Galectin-1 expression in activated pancreatic satellite cells promotes fibrosis in chronic pancreatitis/pancreatic cancer via the TGF-β1/Smad pathway. Oncol Rep. 2018;39:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Annese T, Tamma R, Ruggieri S, Ribatti D. Angiogenesis in Pancreatic Cancer: Pre-Clinical and Clinical Studies. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 30. | Zhao T, Xiao D, Jin F, Sun X, Yu J, Wang H, Liu J, Cai W, Huang C, Wang X, Gao S, Liu Z, Yang S, Gao C, Hao J. ESE3-positive PSCs drive pancreatic cancer fibrosis, chemoresistance and poor prognosis via tumour-stromal IL-1β/NF-κB/ESE3 signalling axis. Br J Cancer. 2022;127:1461-1472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Che M, Kweon SM, Teo JL, Yuan YC, Melstrom LG, Waldron RT, Lugea A, Urrutia RA, Pandol SJ, Lai KKY. Targeting the CBP/β-Catenin Interaction to Suppress Activation of Cancer-Promoting Pancreatic Stellate Cells. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Xu XF, Liu F, Xin JQ, Fan JW, Wu N, Zhu LJ, Duan LF, Li YY, Zhang H. Respective roles of the mitogen-activated protein kinase (MAPK) family members in pancreatic stellate cell activation induced by transforming growth factor-β1 (TGF-β1). Biochem Biophys Res Commun. 2018;501:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Wu Q, Tian Y, Zhang J, Zhang H, Gu F, Lu Y, Zou S, Chen Y, Sun P, Xu M, Sun X, Xia C, Chi H, Ying Zhu A, Tang D, Wang D. Functions of pancreatic stellate cell-derived soluble factors in the microenvironment of pancreatic ductal carcinoma. Oncotarget. 2017;8:102721-102738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Czekay RP, Cheon DJ, Samarakoon R, Kutz SM, Higgins PJ. Cancer-Associated Fibroblasts: Mechanisms of Tumor Progression and Novel Therapeutic Targets. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 35. | Leca J, Martinez S, Lac S, Nigri J, Secq V, Rubis M, Bressy C, Sergé A, Lavaut MN, Dusetti N, Loncle C, Roques J, Pietrasz D, Bousquet C, Garcia S, Granjeaud S, Ouaissi M, Bachet JB, Brun C, Iovanna JL, Zimmermann P, Vasseur S, Tomasini R. Cancer-associated fibroblast-derived annexin A6+ extracellular vesicles support pancreatic cancer aggressiveness. J Clin Invest. 2016;126:4140-4156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 36. | Nigri J, Leca J, Tubiana SS, Finetti P, Guillaumond F, Martinez S, Lac S, Iovanna JL, Audebert S, Camoin L, Vasseur S, Bertucci F, Tomasini R. CD9 mediates the uptake of extracellular vesicles from cancer-associated fibroblasts that promote pancreatic cancer cell aggressiveness. Sci Signal. 2022;15:eabg8191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 37. | Kiaie SH, Sanaei MJ, Heshmati M, Asadzadeh Z, Azimi I, Hadidi S, Jafari R, Baradaran B. Immune checkpoints in targeted-immunotherapy of pancreatic cancer: New hope for clinical development. Acta Pharm Sin B. 2021;11:1083-1097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Yang CY, Fan MH, Miao CH, Liao YJ, Yuan RH, Liu CL. Engineering Chimeric Antigen Receptor T Cells against Immune Checkpoint Inhibitors PD-1/PD-L1 for Treating Pancreatic Cancer. Mol Ther Oncolytics. 2020;17:571-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 39. | Liu S, Ren J, Ten Dijke P. Targeting TGFβ signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 237] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 40. | King RJ, Shukla SK, He C, Vernucci E, Thakur R, Attri KS, Dasgupta A, Chaika NV, Mulder SE, Abrego J, Murthy D, Gunda V, Pacheco CG, Grandgenett PM, Lazenby AJ, Hollingsworth MA, Yu F, Mehla K, Singh PK. CD73 induces GM-CSF/MDSC-mediated suppression of T cells to accelerate pancreatic cancer pathogenesis. Oncogene. 2022;41:971-982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 41. | Yu X, Liu W, Wang Z, Wang H, Liu J, Huang C, Zhao T, Wang X, Gao S, Ma Y, Wu L, Li X, Yang S, Hao J. CD73 induces gemcitabine resistance in pancreatic ductal adenocarcinoma: A promising target with non-canonical mechanisms. Cancer Lett. 2021;519:289-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Wang S, Huang S, Sun YL. Epithelial-Mesenchymal Transition in Pancreatic Cancer: A Review. Biomed Res Int. 2017;2017:2646148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 43. | Zhang Y, Zhang HW, Zhu XD, Wang YQ, Wang XW, Zheng BS, Chen BC, Chen ZJ. Overexpression of Dermokine-α enhances the proliferation and epithelial-mesenchymal transition of pancreatic tumor cells. Cell Signal. 2022;99:110439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 44. | Huang C, Xiang Y, Chen S, Yu H, Wen Z, Ye T, Sun H, Kong H, Li D, Yu D, Chen B, Zhou M. Dermokine contributes to epithelial-mesenchymal transition through increased activation of signal transducer and activator of transcription 3 in pancreatic cancer. Cancer Sci. 2017;108:2130-2141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Cao R, Zhang Z, Tian C, Sheng W, Dong Q, Dong M. Down-regulation of MSMO1 promotes the development and progression of pancreatic cancer. J Cancer. 2022;13:3013-3021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 46. | Oliveira-Cunha M, Newman WG, Siriwardena AK. Epidermal growth factor receptor in pancreatic cancer. Cancers (Basel). 2011;3:1513-1526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 47. | Bu L, Yonemura A, Yasuda-Yoshihara N, Uchihara T, Ismagulov G, Takasugi S, Yasuda T, Okamoto Y, Kitamura F, Akiyama T, Arima K, Itoyama R, Zhang J, Fu L, Hu X, Wei F, Arima Y, Moroishi T, Nishiyama K, Sheng G, Mukunoki T, Otani J, Baba H, Ishimoto T. Tumor microenvironmental 15-PGDH depletion promotes fibrotic tumor formation and angiogenesis in pancreatic cancer. Cancer Sci. 2022;113:3579-3592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 48. | Vaz AP, Ponnusamy MP, Seshacharyulu P, Batra SK. A concise review on the current understanding of pancreatic cancer stem cells. J Cancer Stem Cell Res. 2014;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Yu Q, Xiu Z, Jian Y, Zhou J, Chen X, Chen C, Chen H, Yang S, Yin L, Zeng W. microRNA-497 prevents pancreatic cancer stem cell gemcitabine resistance, migration, and invasion by directly targeting nuclear factor kappa B 1. Aging (Albany NY). 2022;14:5908-5924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 50. | Yang Z, Zhao N, Cui J, Wu H, Xiong J, Peng T. Exosomes derived from cancer stem cells of gemcitabine-resistant pancreatic cancer cells enhance drug resistance by delivering miR-210. Cell Oncol (Dordr). 2020;43:123-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 51. | Chen F, Zheng Y, Zhou H, Li C. The Regulatory Role of SNORD35A in Pancreatic Cancer Involves the HGF/C-Met Pathway. Cancer Biother Radiopharm. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Zhang Z, Han H, Rong Y, Zhu K, Zhu Z, Tang Z, Xiong C, Tao J. Hypoxia potentiates gemcitabine-induced stemness in pancreatic cancer cells through AKT/Notch1 signaling. J Exp Clin Cancer Res. 2018;37:291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 53. | Panebianco C, Ciardiello D, Villani A, Maiorano BA, Latiano TP, Maiello E, Perri F, Pazienza V. Insights into the role of gut and intratumor microbiota in pancreatic ductal adenocarcinoma as new key players in preventive, diagnostic and therapeutic perspective. Semin Cancer Biol. 2022;86:997-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 54. | Zhang CY, Liu S, Yang M. Crosstalk between gut microbiota and COVID-19 impacts pancreatic cancer progression. World J Gastrointest Oncol. 2022;14:1456-1468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 55. | Zhang C, Yang M. The Emerging Factors and Treatment Options for NAFLD-Related Hepatocellular Carcinoma. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 56. | Scheithauer TPM, Herrema H, Yu H, Bakker GJ, Winkelmeijer M, Soukhatcheva G, Dai D, Ma C, Havik SR, Balvers M, Davids M, Meijnikman AS, Aydin Ö, van den Born BH, Besselink MG, Busch OR, de Brauw M, van de Laar A, Belzer C, Stahl M, de Vos WM, Vallance BA, Nieuwdorp M, Verchere CB, van Raalte DH. Gut-derived bacterial flagellin induces beta-cell inflammation and dysfunction. Gut Microbes. 2022;14:2111951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 57. | Hashimoto S, Tochio T, Funasaka K, Funahashi K, Hartanto T, Togashi Y, Saito M, Nishimoto Y, Yoshinori M, Nakaoka K, Watanabe A, Nagasaka M, Nakagawa Y, Miyahara R, Shibata T, Hirooka Y. Changes in intestinal bacteria and imbalances of metabolites induced in the intestines of pancreatic ductal adenocarcinoma patients in a Japanese population: a preliminary result. Scand J Gastroenterol. 2022;1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 58. | Yu Q, Newsome RC, Beveridge M, Hernandez MC, Gharaibeh RZ, Jobin C, Thomas RM. Intestinal microbiota modulates pancreatic carcinogenesis through intratumoral natural killer cells. Gut Microbes. 2022;14:2112881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 59. | Nagata N, Nishijima S, Kojima Y, Hisada Y, Imbe K, Miyoshi-Akiyama T, Suda W, Kimura M, Aoki R, Sekine K, Ohsugi M, Miki K, Osawa T, Ueki K, Oka S, Mizokami M, Kartal E, Schmidt TSB, Molina-Montes E, Estudillo L, Malats N, Trebicka J, Kersting S, Langheinrich M, Bork P, Uemura N, Itoi T, Kawai T. Metagenomic Identification of Microbial Signatures Predicting Pancreatic Cancer From a Multinational Study. Gastroenterology. 2022;163:222-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 110] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 60. | Nejman D, Livyatan I, Fuks G, Gavert N, Zwang Y, Geller LT, Rotter-Maskowitz A, Weiser R, Mallel G, Gigi E, Meltser A, Douglas GM, Kamer I, Gopalakrishnan V, Dadosh T, Levin-Zaidman S, Avnet S, Atlan T, Cooper ZA, Arora R, Cogdill AP, Khan MAW, Ologun G, Bussi Y, Weinberger A, Lotan-Pompan M, Golani O, Perry G, Rokah M, Bahar-Shany K, Rozeman EA, Blank CU, Ronai A, Shaoul R, Amit A, Dorfman T, Kremer R, Cohen ZR, Harnof S, Siegal T, Yehuda-Shnaidman E, Gal-Yam EN, Shapira H, Baldini N, Langille MGI, Ben-Nun A, Kaufman B, Nissan A, Golan T, Dadiani M, Levanon K, Bar J, Yust-Katz S, Barshack I, Peeper DS, Raz DJ, Segal E, Wargo JA, Sandbank J, Shental N, Straussman R. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368:973-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 1436] [Article Influence: 287.2] [Reference Citation Analysis (0)] |
| 61. | Huang Y, Zhu N, Zheng X, Liu Y, Lu H, Yin X, Hao H, Tan Y, Wang D, Hu H, Liang Y, Li X, Hu Z, Yin Y. Intratumor Microbiome Analysis Identifies Positive Association Between Megasphaera and Survival of Chinese Patients With Pancreatic Ductal Adenocarcinomas. Front Immunol. 2022;13:785422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 62. | Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, Scheet P, Xu H, Hanash SM, Feng L, Burks JK, Do KA, Peterson CB, Nejman D, Tzeng CD, Kim MP, Sears CL, Ajami N, Petrosino J, Wood LD, Maitra A, Straussman R, Katz M, White JR, Jenq R, Wargo J, McAllister F. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell. 2019;178:795-806.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 1024] [Article Influence: 204.8] [Reference Citation Analysis (0)] |
| 63. | Schepis T, De Lucia SS, Nista EC, Manilla V, Pignataro G, Ojetti V, Piccioni A, Gasbarrini A, Franceschi F, Candelli M. Microbiota in Pancreatic Diseases: A Review of the Literature. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 64. | Fan X, Alekseyenko AV, Wu J, Peters BA, Jacobs EJ, Gapstur SM, Purdue MP, Abnet CC, Stolzenberg-Solomon R, Miller G, Ravel J, Hayes RB, Ahn J. Human oral microbiome and prospective risk for pancreatic cancer: a population-based nested case-control study. Gut. 2018;67:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 550] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 65. | Li R, Hu Y, Hou S. An Exploration of Oral-Gut Pathogens Mediating Immune Escape of Pancreatic Cancer via miR-21/PTEN Axis. Front Microbiol. 2022;13:928846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 66. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1650] [Article Influence: 330.0] [Reference Citation Analysis (1)] |
| 67. | Ansari D, Gustafsson A, Andersson R. Update on the management of pancreatic cancer: surgery is not enough. World J Gastroenterol. 2015;21:3157-3165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 138] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 68. | Zhang L, Sanagapalli S, Stoita A. Challenges in diagnosis of pancreatic cancer. World J Gastroenterol. 2018;24:2047-2060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 272] [Cited by in RCA: 369] [Article Influence: 52.7] [Reference Citation Analysis (12)] |
| 69. | Gao JF, Pan Y, Lin XC, Lu FC, Qiu DS, Liu JJ, Huang HG. Prognostic value of preoperative enhanced computed tomography as a quantitative imaging biomarker in pancreatic cancer. World J Gastroenterol. 2022;28:2468-2481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (2)] |
| 70. | Jiang XT, Tao HQ, Zou SC. Detection of serum tumor markers in the diagnosis and treatment of patients with pancreatic cancer. Hepatobiliary Pancreat Dis Int. 2004;3:464-468. [PubMed] |
| 71. | Ni XG, Bai XF, Mao YL, Shao YF, Wu JX, Shan Y, Wang CF, Wang J, Tian YT, Liu Q, Xu DK, Zhao P. The clinical value of serum CEA, CA19-9, and CA242 in the diagnosis and prognosis of pancreatic cancer. Eur J Surg Oncol. 2005;31:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 218] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 72. | Zhao B, Zhao B, Chen F. Diagnostic value of serum carbohydrate antigen 19-9 in pancreatic cancer: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2022;34:891-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 73. | Coppola A, La Vaccara V, Fiore M, Farolfi T, Ramella S, Angeletti S, Coppola R, Caputo D. CA19.9 Serum Level Predicts Lymph-Nodes Status in Resectable Pancreatic Ductal Adenocarcinoma: A Retrospective Single-Center Analysis. Front Oncol. 2021;11:690580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 74. | Coppola A, La Vaccara V, Farolfi T, Fiore M, Cammarata R, Ramella S, Coppola R, Caputo D. Role of CA 19.9 in the Management of Resectable Pancreatic Cancer: State of the Art and Future Perspectives. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 75. | Zhang J, Wang Y, Zhao T, Li Y, Tian L, Zhao J, Zhang J. Evaluation of serum MUC5AC in combination with CA19-9 for the diagnosis of pancreatic cancer. World J Surg Oncol. 2020;18:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 76. | Yang Y, Yan S, Tian H, Bao Y. Macrophage inhibitory cytokine-1 versus carbohydrate antigen 19-9 as a biomarker for diagnosis of pancreatic cancer: A PRISMA-compliant meta-analysis of diagnostic accuracy studies. Medicine (Baltimore). 2018;97:e9994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Xiong F, Guo T, Wang X, Wu G, Liu W, Wang Q, Wang B, Chen Y. Keratin 8 Is an Inflammation-Induced and Prognosis-Related Marker for Pancreatic Adenocarcinoma. Dis Markers. 2022;2022:8159537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 78. | Tartaglione S, Mancini P, Viggiani V, Chirletti P, Angeloni A, Anastasi E. PIVKA-II: A biomarker for diagnosing and monitoring patients with pancreatic adenocarcinoma. PLoS One. 2021;16:e0251656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 79. | Yang S, Zhang Y, Hua Y, Cui M, Wang M, Gao J, Liu Q, Liao Q. GREM1 is a novel serum diagnostic marker and potential therapeutic target for pancreatic ductal adenocarcinoma. Front Oncol. 2022;12:968610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 80. | Mattila N, Seppänen H, Mustonen H, Przybyla B, Haglund C, Lassila R. Preoperative Biomarker Panel, Including Fibrinogen and FVIII, Improves Diagnostic Accuracy for Pancreatic Ductal Adenocarcinoma. Clin Appl Thromb Hemost. 2018;24:1267-1275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 81. | Mehta S, Bhimani N, Gill AJ, Samra JS, Sahni S, Mittal A. Serum Biomarker Panel for Diagnosis and Prognosis of Pancreatic Ductal Adenocarcinomas. Front Oncol. 2021;11:708963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 82. | Shams R, Saberi S, Zali M, Sadeghi A, Ghafouri-Fard S, Aghdaei HA. Identification of potential microRNA panels for pancreatic cancer diagnosis using microarray datasets and bioinformatics methods. Sci Rep. 2020;10:7559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 83. | Zou X, Wei J, Huang Z, Zhou X, Lu Z, Zhu W, Miao Y. Identification of a six-miRNA panel in serum benefiting pancreatic cancer diagnosis. Cancer Med. 2019;8:2810-2822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 84. | Charles Jacob HK, Signorelli R, Charles Richard JL, Kashuv T, Lavania S, Middleton A, Gomez BA, Ferrantella A, Amirian H, Tao J, Ergonul AB, Boone MM, Hadisurya M, Tao WA, Iliuk A, Kashyap MK, Garcia-Buitrago M, Dawra R, Saluja AK. Identification of novel early pancreatic cancer biomarkers KIF5B and SFRP2 from "first contact" interactions in the tumor microenvironment. J Exp Clin Cancer Res. 2022;41:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 85. | Blyuss O, Zaikin A, Cherepanova V, Munblit D, Kiseleva EM, Prytomanova OM, Duffy SW, Crnogorac-Jurcevic T. Development of PancRISK, a urine biomarker-based risk score for stratified screening of pancreatic cancer patients. Br J Cancer. 2020;122:692-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 86. | Debernardi S, Blyuss O, Rycyk D, Srivastava K, Jeon CY, Cai H, Cai Q, Shu XO, Crnogorac-Jurcevic T. Urine biomarkers enable pancreatic cancer detection up to 2 years before diagnosis. Int J Cancer. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 87. | Iseda N, Iguchi T, Hirose K, Itoh S, Honboh T, Sadanaga N, Matsuura H. Prognostic Impact of Lymphocyte-to-C-Reactive Protein Ratio in Patients Who Underwent Surgical Resection for Pancreatic Cancer. Am Surg. 2022;31348221117034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 88. | Murthy P, Zenati MS, Al Abbas AI, Rieser CJ, Bahary N, Lotze MT, Zeh HJ 3rd, Zureikat AH, Boone BA. Prognostic Value of the Systemic Immune-Inflammation Index (SII) After Neoadjuvant Therapy for Patients with Resected Pancreatic Cancer. Ann Surg Oncol. 2020;27:898-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 89. | Xu W, Zhang M, Liu L, Yin M, Xu C, Weng Z. Association of mucin family members with prognostic significance in pancreatic cancer patients: A meta-analysis. PLoS One. 2022;17:e0269612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 90. | Chen G, Song H, Yang Z, Du T, Zheng Y, Lu Z, Zhang K, Wei D. AQP5 Is a Novel Prognostic Biomarker in Pancreatic Adenocarcinoma. Front Oncol. 2022;12:890193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 91. | Li Y, Su Z, Wei B, Qin M, Liang Z. Bioinformatics analysis identified MMP14 and COL12A1 as immune-related biomarkers associated with pancreatic adenocarcinoma prognosis. Math Biosci Eng. 2021;18:5921-5942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 92. | Zhang Z, Shang J, Dai Z, Yao Y, Shi Y, Zhong D, Liang Y, Lai C, Yang Q, Feng T, Huang X. Transmembrane Protein 170B is a Prognostic Biomarker and Associated With Immune Infiltrates in Pancreatic Adenocarcinoma. Front Genet. 2022;13:848391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | FDA OK's HIF2α Inhibitor Belzutifan. Cancer Discov. 2021;11:2360-2361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 94. | Deeks ED. Belzutifan: First Approval. Drugs. 2021;81:1921-1927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 95. | Abdelgalil AA, Al-Kahtani HM, Al-Jenoobi FI. Erlotinib. Profiles Drug Subst Excip Relat Methodol. 2020;45:93-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 96. | Abdel-Wahab R, Varadhachary GR, Bhosale PR, Wang X, Fogelman DR, Shroff RT, Overman MJ, Wolff RA, Javle M. Randomized, phase I/II study of gemcitabine plus IGF-1R antagonist (MK-0646) versus gemcitabine plus erlotinib with and without MK-0646 for advanced pancreatic adenocarcinoma. J Hematol Oncol. 2018;11:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 97. | Peng T, Dou QP. Everolimus Inhibits Growth of Gemcitabine-Resistant Pancreatic Cancer Cells via Induction of Caspase-Dependent Apoptosis and G(2) /M Arrest. J Cell Biochem. 2017;118:2722-2730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 98. | Cui J, Guo Y, Wu H, Xiong J, Peng T. Everolimus regulates the activity of gemcitabine-resistant pancreatic cancer cells by targeting the Warburg effect via PI3K/AKT/mTOR signaling. Mol Med. 2021;27:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 99. | Meneses-Medina MI, Gervaso L, Cella CA, Pellicori S, Gandini S, Sousa MJ, Fazio N. Chemotherapy in pancreatic ductal adenocarcinoma: When cytoreduction is the aim. A systematic review and meta-analysis. Cancer Treat Rev. 2022;104:102338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 100. | Ghafouri-Fard S, Abak A, Tondro Anamag F, Shoorei H, Fattahi F, Javadinia SA, Basiri A, Taheri M. 5-Fluorouracil: A Narrative Review on the Role of Regulatory Mechanisms in Driving Resistance to This Chemotherapeutic Agent. Front Oncol. 2021;11:658636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 101. | Namima D, Fujihara S, Iwama H, Fujita K, Matsui T, Nakahara M, Okamura M, Hirata M, Kono T, Fujita N, Yamana H, Kato K, Kamada H, Morishita A, Kobara H, Tsutsui K, Masaki T. The Effect of Gemcitabine on Cell Cycle Arrest and microRNA Signatures in Pancreatic Cancer Cells. In Vivo. 2020;34:3195-3203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 102. | Wachtel MS, Xu KT, Zhang Y, Chiriva-Internati M, Frezza EE. Pancreas cancer survival in the gemcitabine era. Clin Med Oncol. 2008;2:405-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 103. | Ghosh S, Sun B, Jahagirdar D, Luo D, Ortega J, Straubinger RM, Lovell JF. Single-treatment tumor ablation with photodynamic liposomal irinotecan sucrosulfate. Transl Oncol. 2022;19:101390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 104. | Reyhanoglu G, Smith T. Irinotecan. 2022 Jul 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. [PubMed] |
| 105. | Doll DC, Weiss RB, Issell BF. Mitomycin: ten years after approval for marketing. J Clin Oncol. 1985;3:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 63] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 106. | Paz MM, Zhang X, Lu J, Holmgren A. A new mechanism of action for the anticancer drug mitomycin C: mechanism-based inhibition of thioredoxin reductase. Chem Res Toxicol. 2012;25:1502-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 107. | Shu Y, He X, Liu Y, Wu P, Zhang Q. A Real-World Disproportionality Analysis of Olaparib: Data Mining of the Public Version of FDA Adverse Event Reporting System. Clin Epidemiol. 2022;14:789-802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 108. | Chi J, Chung SY, Prasad S, Saif MW. The Role of Olaparib in Metastatic Pancreatic Cancer. Cancer Med J. 2021;4:89-91. [PubMed] |
| 109. | Zhu L, Chen L. Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett. 2019;24:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 335] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 110. | Kim G. nab-Paclitaxel for the treatment of pancreatic cancer. Cancer Manag Res. 2017;9:85-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 111. | Wiedmer T, Blank A, Pantasis S, Normand L, Bill R, Krebs P, Tschan MP, Marinoni I, Perren A. Autophagy Inhibition Improves Sunitinib Efficacy in Pancreatic Neuroendocrine Tumors via a Lysosome-dependent Mechanism. Mol Cancer Ther. 2017;16:2502-2515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 112. | Blumenthal GM, Cortazar P, Zhang JJ, Tang S, Sridhara R, Murgo A, Justice R, Pazdur R. FDA approval summary: sunitinib for the treatment of progressive well-differentiated locally advanced or metastatic pancreatic neuroendocrine tumors. Oncologist. 2012;17:1108-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 113. | Ay S, Atcı MM, Arıkan R, Dülgar Ö, Özyükseler DT, Paksoy N, Doğan İ, Öztosun B, Taştekin D, Öven BB, Gümüş M. FOLFIRINOX versus gemcitabine plus nab-paclitaxel as the first-line chemotherapy in metastatic pancreatic cancer. J Chemother. 2022;34:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 114. | Otsu T, Inokawa Y, Takami H, Hayashi M, Kurimoto K, Tanaka N, Tanaka H, Shimizu D, Hattori N, Kanda M, Tanaka C, Nakayama G, Kodera Y. Comparison Between FOLFIRINOX and nal-IRI/FL as Second-line Treatment After Gemcitabine Plus Nab-paclitaxel for Pancreatic Cancer. Anticancer Res. 2022;42:3889-3894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 115. | National Cancer Institute. Drugs Approved for Pancreatic Cancer. 2021. [cited 3 November 2022]. Available from: https://www.cancer.gov/about-cancer/treatment/drugs/pancreatic. |
| 116. | Shalhout SZ, Emerick KS, Kaufman HL, Silk AW, Thakuria M, Miller DM. A Retrospective Study of Ipilimumab Plus Nivolumab in Anti-PD-L1/PD-1 Refractory Merkel Cell Carcinoma. J Immunother. 2022;45:299-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 117. | O'Connor JM, Fessele KL, Steiner J, Seidl-Rathkopf K, Carson KR, Nussbaum NC, Yin ES, Adelson KB, Presley CJ, Chiang AC, Ross JS, Abernethy AP, Gross CP. Speed of Adoption of Immune Checkpoint Inhibitors of Programmed Cell Death 1 Protein and Comparison of Patient Ages in Clinical Practice vs Pivotal Clinical Trials. JAMA Oncol. 2018;4:e180798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 118. | Bian J, Almhanna K. Pancreatic cancer and immune checkpoint inhibitors-still a long way to go. Transl Gastroenterol Hepatol. 2021;6:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 119. | Kabacaoglu D, Ciecielski KJ, Ruess DA, Algül H. Immune Checkpoint Inhibition for Pancreatic Ductal Adenocarcinoma: Current Limitations and Future Options. Front Immunol. 2018;9:1878. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 120. | Zaghdoudi S, Decaup E, Belhabib I, Samain R, Cassant-Sourdy S, Rochotte J, Brunel A, Schlaepfer D, Cros J, Neuzillet C, Strehaiano M, Alard A, Tomasini R, Rajeeve V, Perraud A, Mathonnet M, Pearce OM, Martineau Y, Pyronnet S, Bousquet C, Jean C. FAK activity in cancer-associated fibroblasts is a prognostic marker and a druggable key metastatic player in pancreatic cancer. EMBO Mol Med. 2020;12:e12010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 121. | Murphy KJ, Zhu J, Trpceski M, Pereira BA, Timpson P, Herrmann D. Focal adhesion kinase priming in pancreatic cancer, altering biomechanics to improve chemotherapy. Biochem Soc Trans. 2022;50:1129-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 122. | Fazio N, Cella CA, Del Re M, Laffi A, Rubino M, Zagami P, Spada F. Pharmacodynamics, clinical findings and approval status of current and emerging tyrosine-kinase inhibitors for pancreatic neuroendocrine tumors. Expert Opin Drug Metab Toxicol. 2019;15:993-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 123. | Blair AB, Wang J, Davelaar J, Baker A, Li K, Niu N, Shao Y, Funes V, Li P, Pachter JA, Maneval DC, Dezem F, Plummer J, Chan KS, Gong J, Hendifar AE, Pandol SJ, Burkhart R, Zhang Y, Zheng L, Osipov A. Dual Stromal Targeting Sensitizes Pancreatic Adenocarcinoma for Anti-Programmed Cell Death Protein 1 Therapy. Gastroenterology. 2022;163:1267-1280.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 124. | Nakabayashi R, Fujihara S, Iwama H, Hamaya S, Mizuo T, Hirata M, Fujita K, Kono T, Namima D, Fujita N, Yamana H, Kobayashi K, Kamada H, Morishita A, Kobara H, Ono M, Okano K, Masaki T. Effect of Aspirin on G(0)/G(1) Cell Cycle Arrest and microRNA Signatures in Pancreatic Adenocarcinoma Cells. Anticancer Res. 2022;42:4037-4048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 125. | Gong J, Thomassian S, Kim S, Gresham G, Moshayedi N, Ye JY, Yang JC, Jacobs JP, Lo S, Nissen N, Gaddam S, Tighiouart M, Osipov A, Hendifar A. Phase I trial of Bermekimab with nanoliposomal irinotecan and 5-fluorouracil/folinic acid in advanced pancreatic ductal adenocarcinoma. Sci Rep. 2022;12:15013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 126. | Hecht JR, Lonardi S, Bendell J, Sim HW, Macarulla T, Lopez CD, Van Cutsem E, Muñoz Martin AJ, Park JO, Greil R, Wang H, Hozak RR, Gueorguieva I, Lin Y, Rao S, Ryoo BY. Randomized Phase III Study of FOLFOX Alone or With Pegilodecakin as Second-Line Therapy in Patients With Metastatic Pancreatic Cancer That Progressed After Gemcitabine (SEQUOIA). J Clin Oncol. 2021;39:1108-1118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 127. | Lin Z, Lin X, Chen J, Huang G, Chen T, Zheng L. Mitofusin-2 is a novel anti-angiogenic factor in pancreatic cancer. J Gastrointest Oncol. 2021;12:484-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 128. | Omi K, Matsuo Y, Ueda G, Aoyama Y, Kato T, Hayashi Y, Imafuji H, Saito K, Tsuboi K, Morimoto M, Ogawa R, Takahashi H, Takiguchi S. Escin inhibits angiogenesis by suppressing interleukin8 and vascular endothelial growth factor production by blocking nuclear factorκB activation in pancreatic cancer cell lines. Oncol Rep. 2021;45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 129. | Hu Y, Jing J, Shi Y, Zhang P, Dong D, Wu Y, Dong X, Li E, Fan Y. Apatinib inhibits pancreatic cancer growth, migration and invasion through the PI3K/AKT and ERK1/2/MAPK pathways. Transl Cancer Res. 2021;10:3306-3316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 130. | Xelwa N, Candy GP, Devar J, Omoshoro-Jones J, Smith M, Nweke EE. Targeting Growth Factor Signaling Pathways in Pancreatic Cancer: Towards Inhibiting Chemoresistance. Front Oncol. 2021;11:683788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 131. | Zhang J, Zhang Z, Holst S, Blöchl C, Madunic K, Wuhrer M, Ten Dijke P, Zhang T. Transforming growth factor-β challenge alters the N-, O-, and glycosphingolipid glycomes in PaTu-S pancreatic adenocarcinoma cells. J Biol Chem. 2022;298:101717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 132. | Zhang Z, Chen WQ, Zhang SQ, Bai JX, Lau CL, Sze SC, Yung KK, Ko JK. The human cathelicidin peptide LL-37 inhibits pancreatic cancer growth by suppressing autophagy and reprogramming of the tumor immune microenvironment. Front Pharmacol. 2022;13:906625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 133. | Sakamoto K, Masutani T, Hirokawa T. Generation of KS-58 as the first K-Ras(G12D)-inhibitory peptide presenting anti-cancer activity in vivo. Sci Rep. 2020;10:21671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 134. | Michaud DS, Izard J, Wilhelm-Benartzi CS, You DH, Grote VA, Tjønneland A, Dahm CC, Overvad K, Jenab M, Fedirko V, Boutron-Ruault MC, Clavel-Chapelon F, Racine A, Kaaks R, Boeing H, Foerster J, Trichopoulou A, Lagiou P, Trichopoulos D, Sacerdote C, Sieri S, Palli D, Tumino R, Panico S, Siersema PD, Peeters PH, Lund E, Barricarte A, Huerta JM, Molina-Montes E, Dorronsoro M, Quirós JR, Duell EJ, Ye W, Sund M, Lindkvist B, Johansen D, Khaw KT, Wareham N, Travis RC, Vineis P, Bueno-de-Mesquita HB, Riboli E. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. 2013;62:1764-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 310] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 135. | Pushalkar S, Hundeyin M, Daley D, Zambirinis CP, Kurz E, Mishra A, Mohan N, Aykut B, Usyk M, Torres LE, Werba G, Zhang K, Guo Y, Li Q, Akkad N, Lall S, Wadowski B, Gutierrez J, Kochen Rossi JA, Herzog JW, Diskin B, Torres-Hernandez A, Leinwand J, Wang W, Taunk PS, Savadkar S, Janal M, Saxena A, Li X, Cohen D, Sartor RB, Saxena D, Miller G. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018;8:403-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 958] [Article Influence: 136.9] [Reference Citation Analysis (0)] |
| 136. | Sethi V, Kurtom S, Tarique M, Lavania S, Malchiodi Z, Hellmund L, Zhang L, Sharma U, Giri B, Garg B, Ferrantella A, Vickers SM, Banerjee S, Dawra R, Roy S, Ramakrishnan S, Saluja A, Dudeja V. Gut Microbiota Promotes Tumor Growth in Mice by Modulating Immune Response. Gastroenterology. 2018;155:33-37.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 296] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 137. | National Cancer Institute. CAR T Cells: Engineering Patients’ Immune Cells to Treat Their Cancers. 2022. [cited 3 November 2022]. Available from: https://www.cancer.gov/about-cancer/treatment/research/car-t-cells. |
| 138. | Henze J, Tacke F, Hardt O, Alves F, Al Rawashdeh W. Enhancing the Efficacy of CAR T Cells in the Tumor Microenvironment of Pancreatic Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 139. | Hu JF, Wang ZW, Liao CY, Chen ZW, Kang FP, Lin CF, Lin TS, Huang L, Tian YF, Chen S. Induced expression of CCL19 promotes the anti-tumor ability of CAR-T cells by increasing their infiltration ability. Front Immunol. 2022;13:958960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 140. | Hamidi-Sofiani V, Rakhshi R, Moradi N, Zeynali P, Nakhaie M, Behboudi E. Oncolytic viruses and pancreatic cancer. Cancer Treat Res Commun. 2022;31:100563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 141. | Zhang Y, Ye M, Huang F, Wang S, Wang H, Mou X, Wang Y. Oncolytic Adenovirus Expressing ST13 Increases Antitumor Effect of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Against Pancreatic Ductal Adenocarcinoma. Hum Gene Ther. 2020;31:891-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 142. | Datta J, Dai X, Bianchi A, De Castro Silva I, Mehra S, Garrido VT, Lamichhane P, Singh SP, Zhou Z, Dosch AR, Messaggio F, Ban Y, Umland O, Hosein PJ, Nagathihalli NS, Merchant NB. Combined MEK and STAT3 Inhibition Uncovers Stromal Plasticity by Enriching for Cancer-Associated Fibroblasts With Mesenchymal Stem Cell-Like Features to Overcome Immunotherapy Resistance in Pancreatic Cancer. Gastroenterology. 2022;163:1593-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 94] [Article Influence: 31.3] [Reference Citation Analysis (7)] |
| 143. | Semrad T, Barzi A, Lenz HJ, Hutchins IM, Kim EJ, Gong IY, Tanaka M, Beckett L, Holland W, Burich RA, Snyder-Solis L, Mack P, Lara PN Jr. Pharmacodynamic separation of gemcitabine and erlotinib in locally advanced or metastatic pancreatic cancer: therapeutic and biomarker results. Int J Clin Oncol. 2015;20:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 144. | Chen SM, Chieng WW, Huang SW, Hsu LJ, Jan MS. The synergistic tumor growth-inhibitory effect of probiotic Lactobacillus on transgenic mouse model of pancreatic cancer treated with gemcitabine. Sci Rep. 2020;10:20319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 145. | Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M, Trojan J, Kozloff M, Simionato F, Cleverly A, Smith C, Wang S, Man M, Driscoll KE, Estrem ST, Lahn MMF, Benhadji KA, Tabernero J. TGFβ receptor inhibitor galunisertib is linked to inflammation- and remodeling-related proteins in patients with pancreatic cancer. Cancer Chemother Pharmacol. 2019;83:975-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 146. | Hurwitz HI, Uppal N, Wagner SA, Bendell JC, Beck JT, Wade SM 3rd, Nemunaitis JJ, Stella PJ, Pipas JM, Wainberg ZA, Manges R, Garrett WM, Hunter DS, Clark J, Leopold L, Sandor V, Levy RS. Randomized, Double-Blind, Phase II Study of Ruxolitinib or Placebo in Combination With Capecitabine in Patients With Metastatic Pancreatic Cancer for Whom Therapy With Gemcitabine Has Failed. J Clin Oncol. 2015;33:4039-4047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 226] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 147. | Chung MJ, Park JY, Bang S, Park SW, Song SY. Phase II clinical trial of ex vivo-expanded cytokine-induced killer cells therapy in advanced pancreatic cancer. Cancer Immunol Immunother. 2014;63:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 148. | Assenat E, Mineur L, Mollevi C, Lopez-Crapez E, Lombard-Bohas C, Samalin E, Portales F, Walter T, de Forges H, Dupuy M, Boissière-Michot F, Ho-Pun-Cheung A, Ychou M, Mazard T. Phase II study evaluating the association of gemcitabine, trastuzumab and erlotinib as first-line treatment in patients with metastatic pancreatic adenocarcinoma (GATE 1). Int J Cancer. 2021;148:682-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 149. | Sinn M, Bahra M, Liersch T, Gellert K, Messmann H, Bechstein W, Waldschmidt D, Jacobasch L, Wilhelm M, Rau BM, Grützmann R, Weinmann A, Maschmeyer G, Pelzer U, Stieler JM, Striefler JK, Ghadimi M, Bischoff S, Dörken B, Oettle H, Riess H. CONKO-005: Adjuvant Chemotherapy With Gemcitabine Plus Erlotinib Versus Gemcitabine Alone in Patients After R0 Resection of Pancreatic Cancer: A Multicenter Randomized Phase III Trial. J Clin Oncol. 2017;35:3330-3337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 223] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 150. | Macarulla Mercadé T, Chen LT, Li CP, Siveke JT, Cunningham D, Bodoky G, Blanc JF, Lee KH, Dean A, Belanger B, Wang-Gillam A. Liposomal Irinotecan + 5-FU/LV in Metastatic Pancreatic Cancer: Subgroup Analyses of Patient, Tumor, and Previous Treatment Characteristics in the Pivotal NAPOLI-1 Trial. Pancreas. 2020;49:62-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 151. | Rebelo R, Polónia B, Santos LL, Vasconcelos MH, Xavier CPR. Drug Repurposing Opportunities in Pancreatic Ductal Adenocarcinoma. Pharmaceuticals (Basel). 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 152. | Fazio N, Kulke M, Rosbrook B, Fernandez K, Raymond E. Updated Efficacy and Safety Outcomes for Patients with Well-Differentiated Pancreatic Neuroendocrine Tumors Treated with Sunitinib. Target Oncol. 2021;16:27-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 153. | Yan T, Li HY, Wu JS, Niu Q, Duan WH, Han QZ, Ji WM, Zhang T, Lv W. Astaxanthin inhibits gemcitabine-resistant human pancreatic cancer progression through EMT inhibition and gemcitabine resensitization. Oncol Lett. 2017;14:5400-5408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 154. | Thakar M, Hu Y, Morreale M, Lerner L, Ying Lin W, Sen R, Cai Y, Karunasena E, Thakar M, Saggi S, Keer H, Ahuja N. A novel epigenetic modulating agent sensitizes pancreatic cells to a chemotherapy agent. PLoS One. 2018;13:e0199130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 155. | Wong KK. DNMT1 as a therapeutic target in pancreatic cancer: mechanisms and clinical implications. Cell Oncol (Dordr). 2020;43:779-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 156. | Zhang HQ, Li J, Tan CL, Chen YH, Zheng ZJ, Liu XB. Neoadjuvant therapy in resectable pancreatic cancer: A promising curative method to improve prognosis. World J Gastrointest Oncol. 2022;14:1903-1917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 157. | Birrer DL, Golcher H, Casadei R, Haile SR, Fritsch R, Hussung S, Brunner TB, Fietkau R, Meyer T, Grützmann R, Merkel S, Ricci C, Ingaldi C, Di Marco M, Guido A, Serra C, Minni F, Pestalozzi B, Petrowsky H, DeOliveira M, Bechstein WO, Bruns CJ, Oberkofler CE, Puhan M, Lesurtel M, Heinrich S, Clavien PA. Neoadjuvant Therapy for Resectable Pancreatic Cancer: A New Standard of Care. Pooled Data From 3 Randomized Controlled Trials. Ann Surg. 2021;274:713-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 158. | Nurmi A, Mustonen H, Parviainen H, Peltola K, Haglund C, Seppänen H. Neoadjuvant therapy offers longer survival than upfront surgery for poorly differentiated and higher stage pancreatic cancer. Acta Oncol. 2018;57:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 159. | de Geus SW, Eskander MF, Bliss LA, Kasumova GG, Ng SC, Callery MP, Tseng JF. Neoadjuvant therapy versus upfront surgery for resected pancreatic adenocarcinoma: A nationwide propensity score matched analysis. Surgery. 2017;161:592-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 160. | Ma T, Jiang JL, Qi WX, Chen JY, Xu HP. A Novel Delivery System of RGD-HSA Loaded GEM/CUR Nanoparticles for the Treatment of Pancreatic Cancer Therapy. Drug Des Devel Ther. 2022;16:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 161. | Shen Y, Song W, Lin D, Zhang X, Wang M, Li Y, Yang Z, Guo S, Wang Z, Sheng J, Murad Y, Ding J, Lou Y, Pan X, Wu Z, Zhao R, Jia W, Bai X, Liang T. VG161 activates systemic antitumor immunity in pancreatic cancer models as a novel oncolytic herpesvirus expressing multiple immunomodulatory transgenes. J Med Virol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 162. | Man YKS, Aguirre-Hernandez C, Fernandez A, Martin-Duque P, González-Pastor R, Halldén G. Complexing the Oncolytic Adenoviruses Ad∆∆ and Ad-3∆-A20T with Cationic Nanoparticles Enhances Viral Infection and Spread in Prostate and Pancreatic Cancer Models. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 163. | Li Q, Zhou Y, Zhang F, McGregor H, Yang X. Radiofrequency Hyperthermia Enhances Locally Delivered Oncolytic Immuno-Virotherapy for Pancreatic Adenocarcinoma. Cardiovasc Intervent Radiol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 164. | Rossi G, Petrone MC, Capurso G, Partelli S, Falconi M, Arcidiacono PG. Endoscopic ultrasound radiofrequency ablation of pancreatic insulinoma in elderly patients: Three case reports. World J Clin Cases. 2022;10:6514-6519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 165. | Jarosova J, Macinga P, Krupickova L, Fialova M, Hujova A, Mares J, Urban O, Hajer J, Spicak J, Striz I, Hucl T. Impact of Endoluminal Radiofrequency Ablation on Immunity in Pancreatic Cancer and Cholangiocarcinoma. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |