Published online Dec 28, 2022. doi: 10.3748/wjg.v28.i48.6811
Peer-review started: September 9, 2022
First decision: October 19, 2022
Revised: November 1, 2022
Accepted: December 6, 2022
Article in press: December 6, 2022
Published online: December 28, 2022
Processing time: 108 Days and 18.1 Hours
The global coronavirus disease 2019 (COVID-19) has become one of the biggest threats to the world since 2019. The respiratory and gastrointestinal tracts are the main targets for severe acute respiratory syndrome coronavirus 2 infection for they highly express angiotensin-converting enzyme-2 and transmembrane protease serine 2. In patients suffering from COVID-19, gastrointestinal symptoms have ranged from 12% to 61%. Anorexia, nausea and/or vomiting, diarrhea, and abdominal pain are considered to be the main gastrointestinal symptoms of COVID-19. It has been reported that the direct damage of intestinal mucosal epithelial cells, malnutrition, and intestinal flora disorders are involved in COVID-19. However, the underlying mechanisms remain unclear. Thus, in this study, we reviewed and discussed the correlated mechanisms that cause gastrointestinal symptoms in order to help to develop the treatment strategy and build an appropriate guideline for medical workers.
Core Tip: Gastrointestinal symptoms in coronavirus disease 2019 patients have ranged from 12% to 61%, which include anorexia, nausea and/or vomiting, diarrhea, abdominal pain, and so on. However, the underlying mechanisms remain unclear. This study reviewed and discussed the correlated mechanisms that cause gastrointestinal symptoms in order to help to develop the treatment strategy and build an appropriate guideline for medical workers.
- Citation: Yao Y, Liu ZJ, Zhang YK, Sun HJ. Mechanism and potential treatments for gastrointestinal dysfunction in patients with COVID-19. World J Gastroenterol 2022; 28(48): 6811-6826
- URL: https://www.wjgnet.com/1007-9327/full/v28/i48/6811.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i48.6811
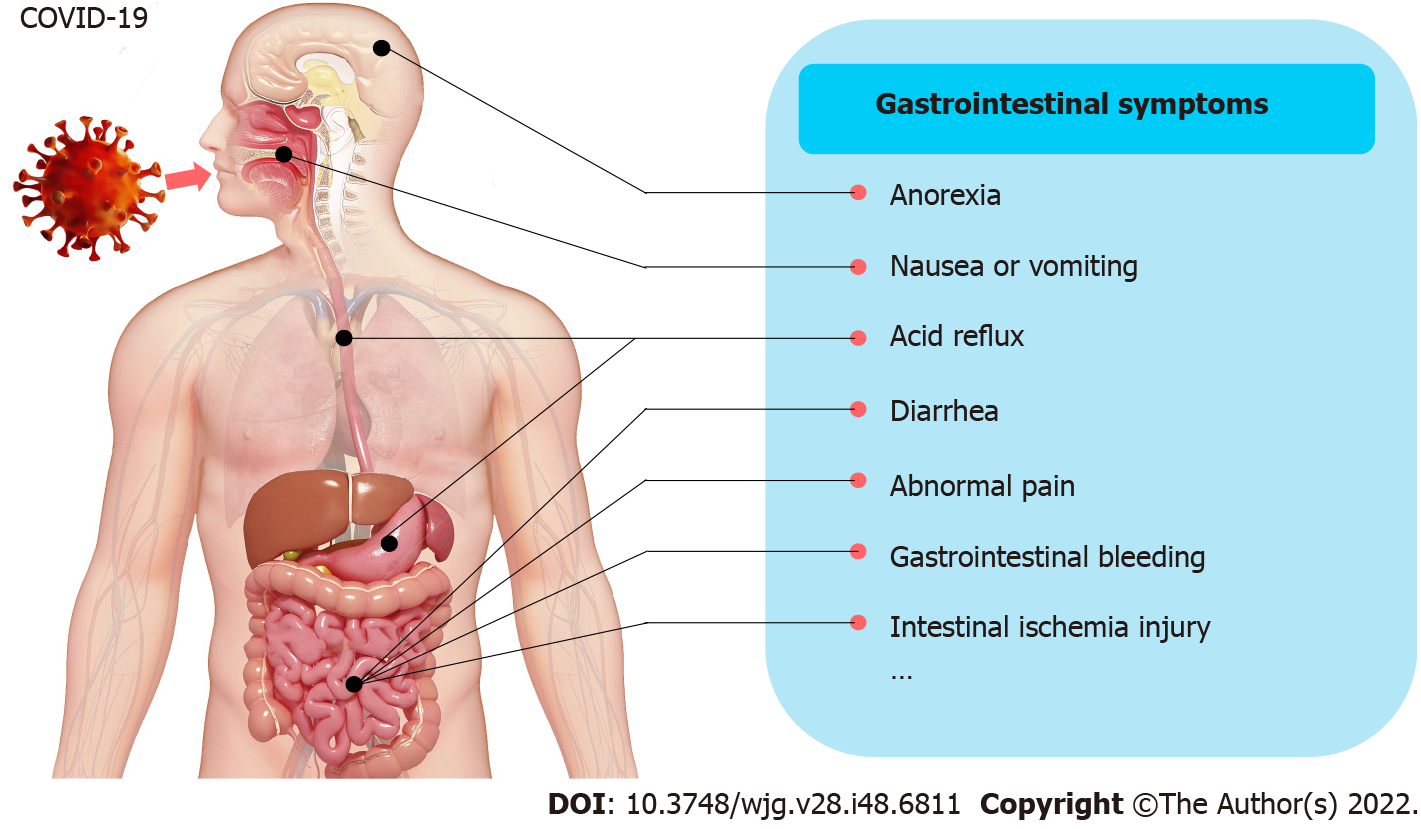
Since 2019, coronavirus disease 2019 (COVID-19) has become one of the world’s most serious threats. In the past, much attention has been given to the respiratory symptoms of patients, but the occurrence of extrapulmonary symptoms has been ignored. The occurrence of gastrointestinal symptoms has ranged from 12% to 61% in patients suffering from COVID-19[1-5], which may result in a longer duration of illness but not increased mortality[2,3]. In a recent meta-analysis from China, the main gastrointestinal symptoms in COVID-19 patients were reported to include anorexia (21%), nausea and/or vomiting (7%), diarrhea (9%), and abdominal pain (3%)[2]. Moreover, gastrointestinal bleeding was rarely observed[6]. A study from the United States reported a higher prevalence of gastrointestinal symptoms (anorexia, 34.8%; diarrhea, 33.7%; and nausea, 26.4%)[4] (Figure 1). Thus, diarrhea, nausea and/or vomiting, abdominal pain, and anorexia are considered to be the main gastrointestinal symptoms.
Currently, after struggling with the Omicron variant, China has found that a large number of COVID-19 patients, especially elderly patients in critical condition, are more likely to suffer from gastrointestinal dysfunction. Over 85% of patients showed symptoms such as intestinal barrier dysfunction, digestive and absorption dysfunction, or gastrointestinal motility dysfunction due to the direct damage the virus caused to the intestinal mucosal epithelial cells. In addition, malnutrition and intestinal flora disorders occurred next. Imaging studies show that COVID-19 patients with gastrointestinal symptoms presented thickening of the bowel wall, sometimes with hyperemia and thickening of the mesentery, and large bowel fluid[7]. However, the underlying mechanisms remain unclear. Thus, in this study, we reviewed and discussed the correlated mechanisms of gastrointestinal symptoms and damage in order to help build an appropriate guideline for medical workers.
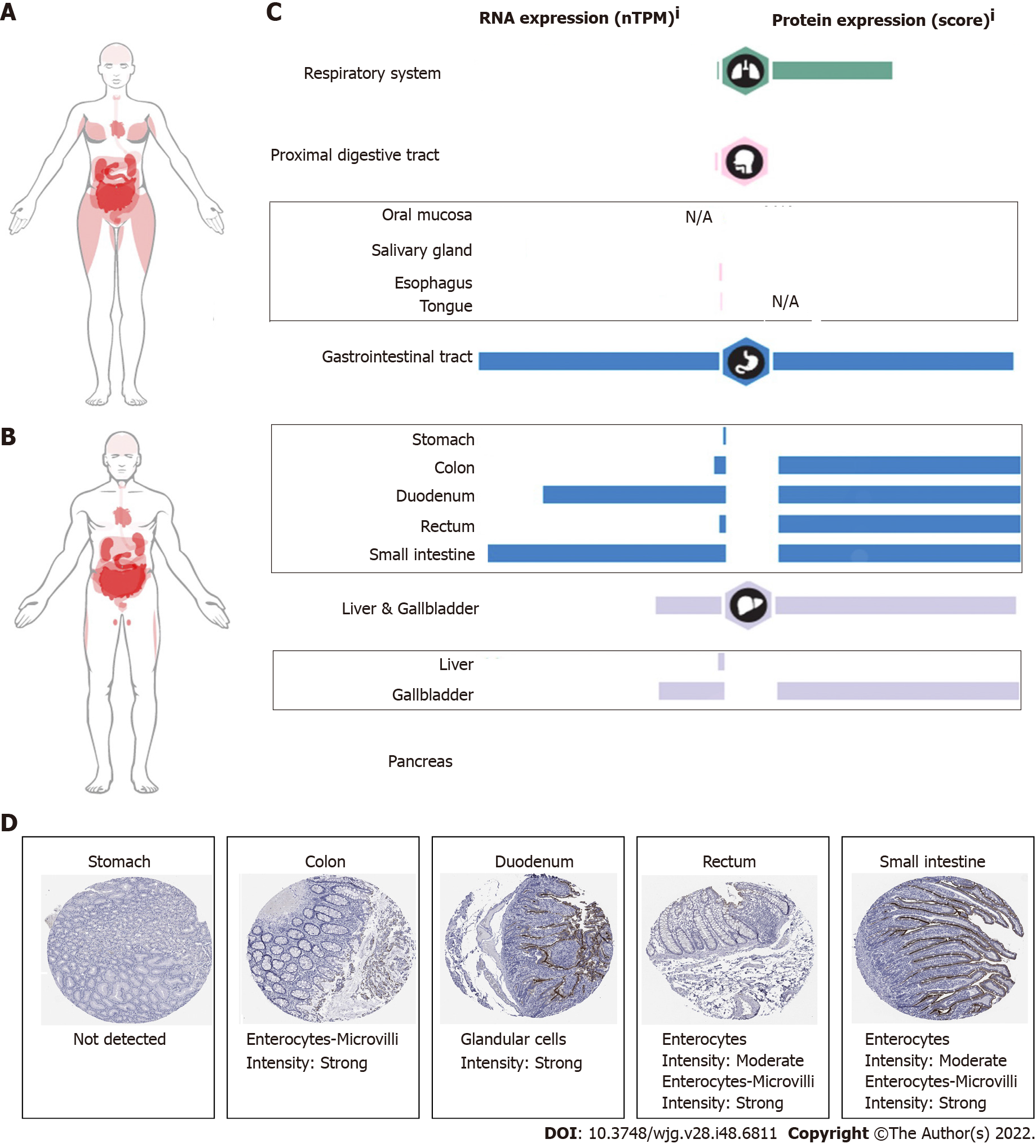
The pathophysiology of gastrointestinal damage in COVID-19 is probably multifactorial. The most important factor is due to the direct infection of the virus. High titers of viral RNA from COVID-19 have been isolated from fecal samples[8,9]. Live viral shedding of infectious virions in fecal matter has been reported even after the resolution of symptoms, which may be a potential source of transmission[10]. Angiotensin-converting enzyme-2 (ACE2), as the entry receptor for the causative coronavirus of COVID-19, is expressed in multiple extrapulmonary tissues, including gastrointestinal tissue[11]. A study based on single-cell sequencing also showed that ACE2 and transmembrane protease serine 2 (TMPRS2) are expressed in cholangiocytes, colonocytes, esophageal keratinocytes, gastrointestinal epithelial cells, and so on[12-14]. The expression profile of ACE2 in the digestive system is shown in Figure 2. Histopathological studies also indicated that gastrointestinal tissue is the target organ of COVID-19[15]. This finding indicates that direct viral-induced tissue damage is a plausible mechanism of COVID-19 injury. Here, however, we hold the idea that the expression of ACE2 is related to the virus entering the body, but the expression level of ACE2 does not appear to be directly proportional to the severity of the disease[16].
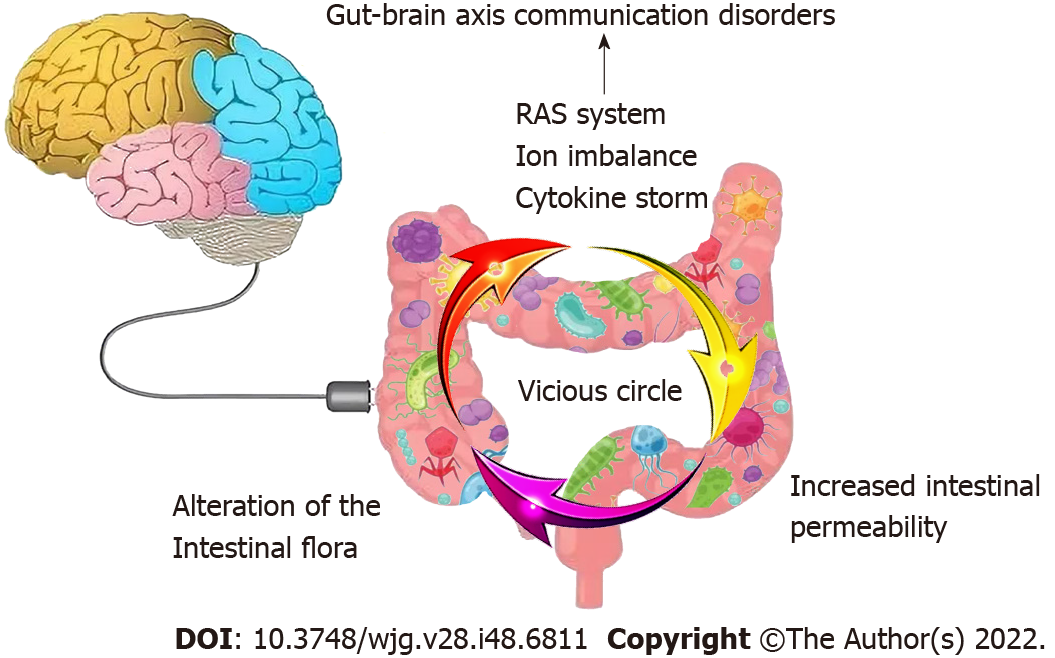
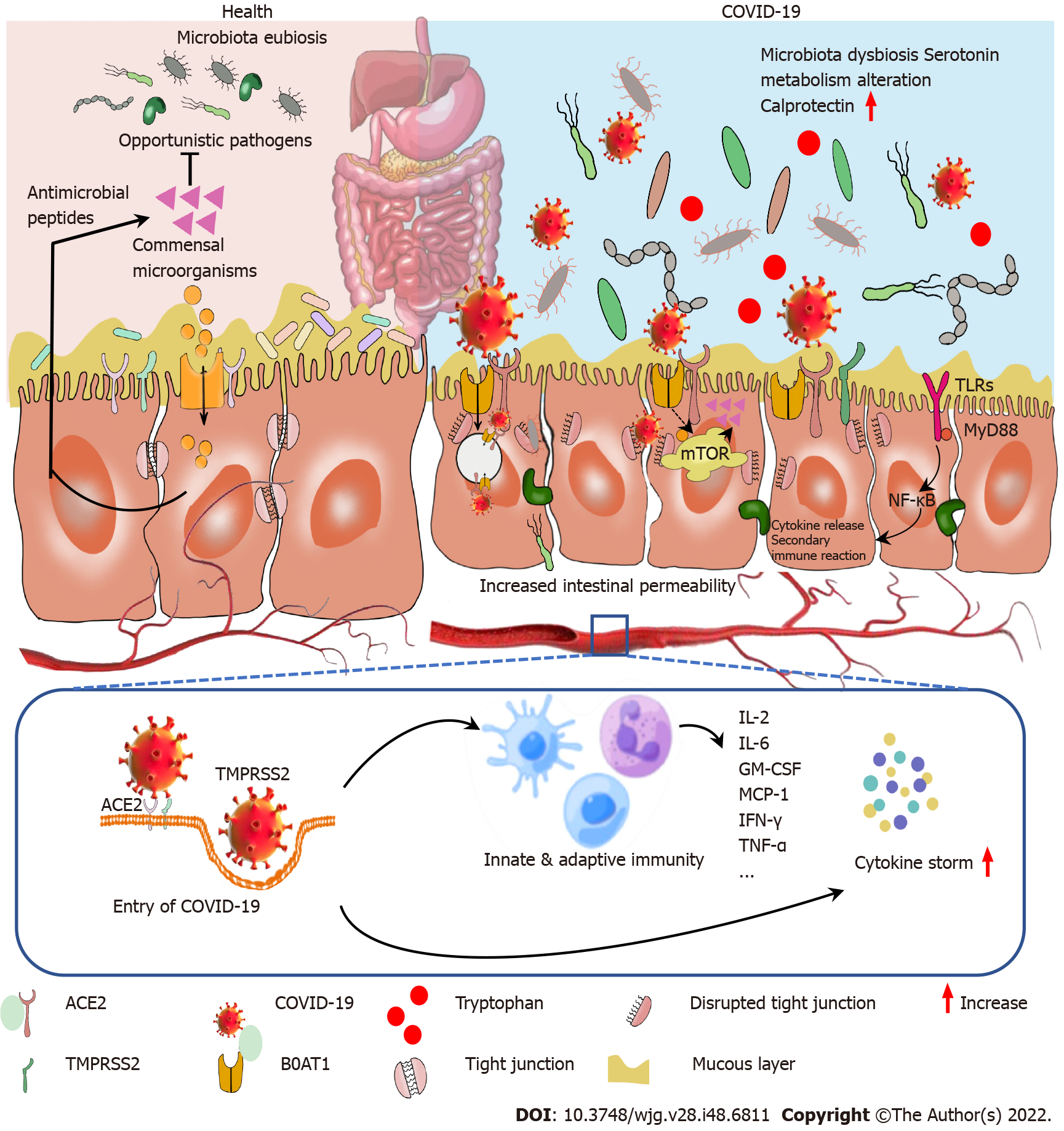
In addition, responses following ACE2 activation are closely related to gastrointestinal side effects. ACE2 is also a key enzyme in the renin-angiotensin system (RAS)[17]. RAS dysregulation may exacerbate ion imbalance and inflammation, potentially affecting cellular metabolic status, microbial composition, and cell viability, leading to progressive bowel function and diarrhea[18]. Histopathological evidence also shows diffuse endothelial inflammation and mesenteric ischemia in the submucosal vessels of the small intestine in patients with COVID-19[19]. Furthermore, infiltrating plasma cells, lymphocytes, and interstitial edema have been found in the lamina propria of COVID-19 patients’ stomachs, duodenums and rectums. Virus-induced cytokine storms, as well as inflammatory responses, may also contribute to enhanced permeability of the mucosal barrier, damaged enteric nervous system, altered intestinal flora[20], and gut-brain axis communication disorders and then form a vicious circle (Figure 3). The syndrome-correlated underlying mechanism is discussed in the following sections.
A literature review was conducted using a keyword search in PubMed from 2019 to 2022. Keyword search items included “COVID-19”, “severe acute respiratory syndrome coronavirus 2”, “gas-trointestinal disorders”, “nausea”, “vomiting”, “diarrhea”, “ACE2”, “abdominal pain”, “anorexia”, and “combinations thereof”. The inclusion criteria included articles with randomized or blinded studies, case-control studies, descriptive research, and studies with objective outcomes. Exclusion criteria included articles of primary opinion papers with no reference to available data and industry-sponsored publications.
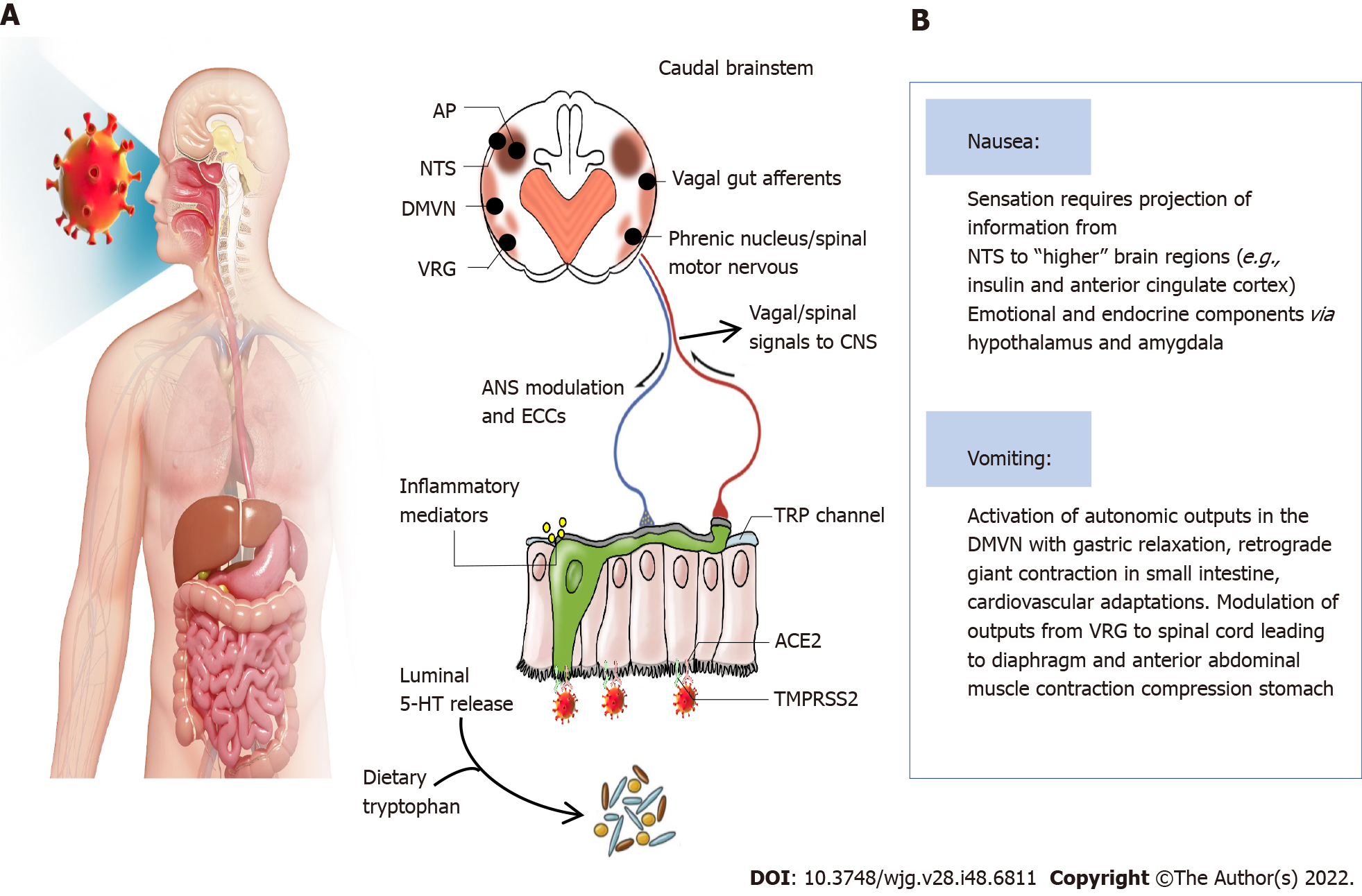
Nausea and/or vomiting is an early alerting symptom of a challenge (toxic food and chemicals, bacterial toxin, and virus) to the upper digestive tract, which can also be the early presenting symptom in COVID-19 patients[21]. The reported incidences of vomiting and nausea were 1.0%-12.5% and 1.0%-27.5%, respectively[4,22]. Some of the patients may eject aerosolized, virally contaminated vomit, which also occurs in patients infected with norovirus[23]. Importantly, nausea and vomiting can be the early presenting symptoms of COVID-19[21].
The virus enters the digestive tract with the air during swallowing and binds to ACE2 receptors, which are highly expressed in the airways and digestive tract. COVID-19 could increase the release of neuroactive agents from enteroendocrine cells and inflammatory mediations, which act by stimulating/sensitizing abdominal vagal afferent terminals and/or act on the area postrema in the dorsal medulla where the blood-brain and blood-cerebrospinal fluid barriers are relatively permeable[21]. The consequences of vagal afferent and area postrema activation induce nausea and vomiting by the projection of information to higher brain regions (nausea and anorexia) and vomiting by motor pathways in the ventral brainstem and spinal cord[21]. Some researchers also indicated that the interaction between transient receptor potential (TRP) channels and food intake might be associated with anorexia due to COVID-19[24,25]. Some TRP channels are broadly expressed in the gastrointestinal tract and play important roles in noxious irritants[26]. TRPV1 expression in esophageal sensory neurons, stomach-labeled vagal nodose neurons and colon-labeled afferent neurons has been well described[27-29]. A TRPV1 agonist, capsaicin, can evoke nausea[30]. Moreover, TRPV1 and TRPA1 are co-expressed in the esophagus, stomach, intestine, and colon[31-33]. TRPV1 can participate in appetite regulation by affecting hormones and gastrointestinal vagal afferent nerves[25]. In vitro activation of TRPA1 by allyl isothiocyanates can increase serotonin release, leading to the stimulation of vomiting[34]. These results suggest that TRP channel activation is involved in COVID-19-induced nausea and vomiting. However, whether inhibiting TRP channels can alleviate the gastrointestinal symptoms caused by COVID-19 needs further investigation. The underlying mechanisms are summarized in Figure 4.
Diarrhea is a frequent presenting symptom in patients suffering from COVID-19. In the clinical case analysis, the incidence of diarrhea is between 2% and 50%[35]. It may precede or trail respiratory symptoms. Although the specific mechanisms involved in COVID-19-related diarrhea are not entirely known, we hold the idea that the targeting of intestinal ACE2 by the virus, virus infection-induced cytokine storms, increased intestinal barrier permeability, and even changes in microbiome are all considered to be the main causes of gastrointestinal symptoms. Moreover, hepatic and pancreatic injuries may also cause diarrhea. Antibiotic-induced iatrogenic diarrhea caused by the activation of Clostridium spp. should also be taken into consideration.
The direct effect of binding ACE2: ACE2 mRNA appears to increase with age and to display higher levels in patients taking ACE inhibitors. This may be one of the causes of gastrointestinal symptoms such as diarrhea being more common in elderly patients or those with hypertension. ACE2 controls the production of antimicrobial peptides and participates in the uptake of dietary amino acids[36], which promote the homeostasis of the gut flora. Additionally, ACE2 expression is positively correlated with the severity of colitis, suggesting that virus activity may lead to changes in the way certain enzymes function, making people more susceptible to developing diarrhea and intestinal inflammation[37].
Altered serotonin metabolism: Altered serotonin metabolism has been found in COVID-19 patients[38]. Serotonin, known as the mood neurotransmitter, is important in certain bodily processes, such as sleep, libido, and body temperature. Studies have reported that enterohemorrhagic Escherichia coli O157 can significantly reduce the expression of a group of genes that cause infection after exposure to serotonin[39,40]. In addition, a study in mice showed that increased serotonin levels in the gastrointestinal tract could reduce the ability of murine Citrobacter to infect the host and cause disease. Intervention with fluoxetine, a selective serotonin reuptake inhibitor, produced similar results[40]. Activation of intestinal serotonin receptors may also lead to diarrhea by modulating the enteric nervous system and intestinal motility[41,42]. The authors believe that COVID-19-related diarrhea is correlated with increased serotonin levels and that elevated serotonin levels may be a protective regulatory mechanism that accelerates the excretion of enteroviruses.
Changes in fecal calprotectin: Increased levels of fecal calprotectin expression have also been detected in COVID-19 patients[43,44]. Fecal calprotectin is a reliable fecal marker for the detection of inflammatory bowel disease and infectious colitis[45]. The calprotectin value in stool is elevated in patients with acute or bloody diarrhea[46]. Calprotectin is a calcium-containing protein derived from neutrophils and macrophages that serves as a marker of inflammatory cell activation[47]. Therefore, diarrhea and increased fecal calprotectin levels could be related to the immune activation and inflammatory responses caused by COVID-19.
Cytokine storms and their induced inflammatory cascade: Cytokine release syndrome caused by a dysregulated immune response is also one of the important factors causing multiple organ dysfunction, especially diarrhea, in patients with COVID-19. Severe COVID-19 manifests as acute respiratory distress syndrome (ARDS) with elevated plasma proinflammatory cytokines, including interleukin (IL)-1β, IL-6, tumor necrosis factor α (TNF-α), C-X-C motif chemokine ligand 10, macrophage inflammatory protein 1α, and chemokine (C-C motif) ligand 2, with low levels of interferon type I (IFN-I) in the early stage and elevated levels of IFN-I during the advanced stage of COVID-19[48]. Changes in these proinflammatory cytokines can also be found in clinical and experimental colitis[49,50], accompanied by increased intestinal permeability via activation of the inflammatory-related cascade. High levels of circulating cytokines and mediators of the toxic response, including IL-6, TNF-α, nitric oxide, and activity modulation of the calcium channel, have been described[51]. Toll-like receptors (TLRs) are important sensors that interact with COVID-19. Previous studies indicated that COVID-19 interacts with TLRs in the host cell membrane and increases gene 88 of the primary response to myeloid differentiation (MyD88), following active nuclear transcription factor-κB (NF-κB), promoting an inflammatory cascade[52], which in turn aggravates the inflammatory response and increases intestinal permeability. Additionally, severe COVID-19 individuals have been found to have significant levels of indicators for tight junction permeability as well as the translocation of bacterial and fungal products into the blood[53,54]. Thus, virus infection-induced cytokine storms and their induced inflammatory response may be other factors that cause diarrhea.
Increased intestinal barrier permeability and microbiome change: Accumulating evidence shows that the intestinal microbiome is broadly altered in COVID-19 patients, which may be followed by increased intestinal permeability. The incidence of sepsis and ARDS, two high-mortality risks in COVID-19, may be minimized by the intestinal microbiota[55]. Intestinal ACE2 functions as a chaperone for the amino acid transporter B0AT1. The B0AT1/ACE2 complex within the intestinal epithelium acts as a regulator of gut microbiota composition and function[56], which can also be considered a marker of inflammation and disease severity[57]. Changes in ACE2 by COVID-19 can impair intestinal uptake of certain dietary amino acids, such as tryptophan, which is involved in enteritis[58-60]. ACE2 knockout mice also exhibit microbiome dysbiosis[61-63]. Through shotgun sequencing of total DNA extracted from stool, researchers found that the gut microbial ecological network was markedly weakened and became sparse, which combined with a decrease in gut microbiome diversity[64], could reflect the disease’s severity[65]. The infection causes intestinal microbiome disturbance and reduction, which may activate immune cells and provoke the release of inflammatory cytokines that increase systemic inflammation[66,67]. In addition, probiotics may help enhance the host immune system, improve the gut microbiome and gut barrier function, and reduce COVID-19-related diarrhea[68].
More significantly, B0AT1 substrates such as tryptophan and glutamine operate as signals to reduce lymphoid proinflammatory cytokines, activate antimicrobial release peptides, and control mucosal autophagy as a defensive mechanism[69]. Downregulated intestinal ACE2-B0AT1 on the cellular surface leads to a series of downstream sequelae to promote leaky gut and dysbiosis of gut flora[69]. Gut microbiomes also play important roles in gut inflammatory regulation. Butyric acid from gut flora was reported to inhibit cytokine storms[27]. This indicates the disrupted composition of intestinal microbiota and impaired gut permeability, followed by the creation of a destructive cycle. In summary, we propose the important role of intestinal microbiota in preventing and decreasing COVID-19 complications. The underlying mechanism involved in COVID-19-correlated diarrhea is summarized in Figure 5.
Abdominal pain is uncommon and extremely rare in patients with COVID-19. Clinical data indicate that abdominal pain is more common in intensive care unit (ICU) patients than in non-ICU patients[41]. The aggregative presence rate of RNA from COVID-19 in stool samples from COVID-19 patients was 54%[2]. In previous clinical research based on COVID-19 patients who underwent emergency abdominal surgery due to different conditions, the peritoneal samples from 5 patients were sufficient for reverse transcription polymerase chain reaction analysis, and no intraperitoneal viral RNA was observed in these 5 patients[70]. Although the number of cases is rare, we can speculate that abdominal pain is linked to gastrointestinal but not intraperitoneal viral infection. The possible mechanisms are summarized as follows.
Immune and inflammatory regulation: The most common cause of pain is the inflammation-induced release of many cytokines and chemokines. Cytokine storms are a potentially fatal immune disease characterized by high levels of activation of immune cells and excessive production of a large number of inflammatory cytokines and chemical mediators, and they have been reported to be associated with the exacerbation of a number of infectious diseases, including severe acute respiratory syndrome[71] and Middle East respiratory syndrome. They are also considered to be the main cause of disease severity and death in patients with COVID-19[72,73]. T cells play an important role in antiviral immunity. A rise in T cell activation and differentiation was found in early COVID-19-infected patients, resulting in immune rebalancing between IFN and NF-κB activity and restoration of cell homeostasis. Two major intracellular transduction antigen-activating signals, the phosphatidylinositol pathway and the MAP kinase-related pathway, are activated. However, the number of T cells is significantly decreased with increasing infective time in COVID-19 patients, accompanied by an increase in the T cell exhaustion marker programmed death 1[74]. The T cell count is negatively correlated with serum cytokine levels in patients with COVID-19[74]. Despite the lack of direct evidence for a relationship between T cell status and abdominal pain in COVID-19 patients, we hypothesized that abdominal pain is related to T cells. Moreover, COVID-19 can rapidly activate pathogenic Th1 cells to secrete proinflammatory cytokines, such as granulocyte-macrophage colony-stimulating factor and IL-6. Increased cytokines, chemokines, and other compounds can simultaneously cause a secondary pain response by activating pain-sensing neurons[75]. The COVID-19 cytokine storm is characterized by high expression of IL-6 and TNF-α[76]. Elevated IL-6 levels also increase mortality. Therefore, we hypothesized that the abdominal pain of COVID-19 patients may be associated with the high expression of IL-6 and TNF-α.
Eosinophils are circulating and tissue-resident white blood cells that have a powerful proinflammatory effect in many diseases. Recently, eosinophils have been shown to have a variety of other functions, including immunomodulatory and antiviral activity. Peripheral eosinophilia is hypothesized to play a protective role in COVID-19 patients[77]. In the United States, a review of administrative data compared the hospitalization rates, ventilator dependence, and death of patients with and without eosinophilic gastrointestinal disorders from an extensive central medical system. The results indicated that eosinophilic gastrointestinal disorder might provide a protective immune response[78]. The mechanism may be related to the upregulation of IL-13 in eosinophilic gastrointestinal disorder and the decreased expression of ACE2 and TMPRS2 on epithelial cells of eosinophilic gastrointestinal disorder patients. In addition, eosinophil-derived neurotoxins may exert direct antiviral effects[79]. Moreover, eosinophilia-related disorders such as eosinophilic gastroenteritis[80] and eosinophilic esophagitis[81] are often accompanied by symptoms of abdominal pain and vomiting, suggesting that abdominal pain in patients with COVID-19 may also be caused by increased eosinophils.
Regulation of the enteric and central nervous systems: Intestinal pain perception can be coregulated by the central and enteric nervous systems. A balance between neuronal excitatory and inhibitory signaling pathways maintains the physiological pain threshold in the intestine. Altered neurotransmitter levels may be closely linked to abdominal pain. A variety of receptors and their endogenous ligands are involved in pain signaling, including receptors responsible for nociceptive sensations [e.g., 5-hydroxytryptamine (5-HT) receptors, TRP channels, IL receptors] and antinociceptive sensations (e.g., opioid and cannabinoid receptors). In COVID-19 patients, intestinal inflammatory infiltration increases intestinal mucosal permeability, and the direct effect of viruses can aggravate dysbiosis and cause changes in tryptophan metabolism. Studies have shown that tryptophan is a precursor of 5-HT. 5-HT plays an important role in gastrointestinal, nervous, and liver diseases. 5-HT acts on 5-HT receptors to initiate peristaltic and secretory reflexes in the viscera[82,83]. Research also indicated that 5-HT might be the key to exacerbating inflammatory bowel disease symptoms, including diarrhea and abdominal pain[84]. Intraperitoneal injection of 5-HT can significantly increase the expression of IL-1β and IL-6 and the activity of myeloperoxidase by activating 5-HT3 and 5-HT4 receptors in the colonic mucosa of mice with colitis and block the signal of pain relief[85]. Further studies have reported that elevated 5-HT can increase the expression of IL-6 and IL-8 and the production of monocyte chemoattractant protein-1, thereby leading to the initial event of intestinal inflammation[86]. However, the plasma 5-HT level is increased in hospitalized patients with COVID-19. The change in 5-HT in patients with COVID-19 may be an important cause of abdominal pain[87]. Therefore, we speculate that the abdominal pain in COVID-19 may be related to 5-HT. Moreover, regulating the level of 5-HT may be a therapeutic modality for the treatment of patients with abdominal pain due to COVID-19.
Ion channel: Pain signals are detected in response to harmful stimuli and release nerve impulses that encode pain. Many of these nociceptive neurons are equipped with a large number of specific ion channels that act as nociceptors. Stretching, inflammation, ischemia, pH, bacterial products, immune mediators, and neurotransmitters have all been implicated in visceral pain[88]. Studies have reported multiple electrolyte abnormalities in patients with COVID-19 infection[89]. COVID-19 infection is associated with decreased serum concentrations of sodium, potassium, magnesium, and calcium. Thus, we speculate that ion channels may play important roles in COVID-19-induced abdominal pain. As TRP channels are widely expressed in COVID-19-infected tissues, TRP channels and TRPML2 are also involved in the fusion of viral envelopes with endolysosomal membranes[90,91]. Thus, TRP channels may be valuable targets for disrupting the COVID-19 life cycle. A report indicated that TRP channels were involved in abdominal pain caused by COVID-19. TRPV1 and TRPA1 induce inflammation, increase sensory or vagal secretions, and cause pain[92]. It has also been suggested that afferent neuronal TRPV1 desensitization (via RTX) can reduce pain-related complications in COVID-19 patients[93]. Because TRP channels are widely expressed in the gastrointestinal tract, we speculate that the abdominal pain caused by COVID-19 is related to TRP channel activation.
Social pressure: Anorexia nervosa is an eating disorder characterized by restrictive eating and an intense fear of gaining weight. A study in 2020 evaluated the early effects of COVID-19 on patients with eating disorders and reported an increase in anxiety and alarming eating behaviors during the pandemic. This report shows that 69% of individuals had anorexia nervosa and experienced worries about their dietary schedules, while subjects with bulimia nervosa or binge eating disorders reported more episodes of binging[94]. This may be due to new living conditions, social distancing, self-isolation, changes in food access, daily habits, and so on; in addition, more difficult access to health care practitioners is also an essential factor leading to an increased incidence of anorexia[95].
Neuromodulation: Researchers have reported that neuroendocrine pathways are disrupted by miscommunication between brain-gut-adipose tissue in patients suffering from COVID-19[96]. Studies have shown that dopamine neurons in the ventral tegmental area of the midbrain and serotonin neurons in the dorsal raphe nucleus are involved in the regulation of motivational behaviors, including feeding[22,97], and increased serotonin levels have been observed in COVID-19 patients[38]. However, there is no direct evidence of whether it is related to anorexia nervosa. Changes in the microbiota-gut-brain axis from COVID-19 as well as gender differences may also be responsible for anorexia nervosa[98]. Moreover, changes in brain serotonin and tryptophan concentrations have been reported to be critical mechanisms in the regulation of eating behavior both in mice and humans[99]. Coincidentally, post-COVID-19 infection was also accompanied by changes in serotonin and tryptophan levels. We speculate that COVID-19-induced anorexia is at least partly correlated with increased serotonin and tryptophan levels. In addition, COVID-19 infection of nonneuronal cells can lead to anosmia and related odor perception impairment[100], which may be associated with the development and aggravation of anorexia[101,102].
In a retrospective study of poor prognosis of gastrointestinal symptoms in patients with COVID-19, 12 (n = 1077, 1.1%) patients developed acid reflux[103], and the incidence of acid reflux was relatively low compared with other gastrointestinal symptoms. Generally, the main causes of gastric acid reflux include: (1) Relaxation of the lower esophageal sphincter; (2) Gastric and duodenal dysfunction leading to obstruction of gastric emptying; and (3) Esophageal mucosal barrier damage. In a clinical study, the prevalence of acid reflux was associated with poorer clinical outcomes in COVID-19, and the mechanism was related to damage to the upper esophageal sphincter[104]. We believe that increased serotonin levels and mucosal barrier damage caused by cytokine storms may also be risk factors for acid reflux. Antacid therapy is generally used, but some studies have noted that the use of proton pump inhibitors may increase the risk of achlorhydria-related intestinal infections in patients with COVID-19[105], while histamine H2 receptor antagonists do not increase this risk. Some studies have suggested that high-dose famotidine may be clinically beneficial to COVID-19 patients[106]. Therefore, the clinical use of histamine receptor antagonists may be more beneficial; however, due to low incidence and insufficient samples, there has been no systematic clinical evaluation of COVID-19 patients accompanied by acid reflux.
A case-control study noted that the main causes of gastrointestinal bleeding in COVID-19 patients were peptic and rectal ulcers. Among the many potential predictors of upper gastrointestinal bleeding, a history of upper gastrointestinal bleeding was the only significant risk factor[107,108]. Gastrointestinal bleeding may be the direct impact of COVID-19 on gastrointestinal mucosa integrity. However, in a case of a COVID-19 patient who showed vomiting coffee crumbs and esophageal mucosal injury by esophagogastroscopy and duodenoscopy, there was no mucosal injury[107]. This may exist in other mechanisms that have not been elucidated. Compared with patients in the initial presentation, most bleeding occurred during hospitalization. The results suggested that bleeding may be one of the treatment-related or secondary factors related to critical illness.
It has been reported in the literature that exposure to COVID-19 may lead to an increased risk of ischemia associated with extremity venous thrombosis, pulmonary embolism, and mesenteric ischemia[109,110]. The overall mortality in COVID-19 patients with gastrointestinal ischemia and imaging-diagnosed mesenteric ischemia was 38.7% and 40%, respectively[111]. Mesenteric ischemia can be a fatal clinical emergency with high mortality[112]. Existing studies also have shown that the incidence of intestinal obstruction and intestinal ischemia is positively correlated with elevated aminotransferase levels[113].
Abdominal distension and loss of taste also have been indicated in the previous investigation[114], however, the specific underlying mechanism needs to be further explored. Another important issue we should be concerned about is that some adverse gastrointestinal events in COVID-19 patients are related to medication[115]. An observation line cohort study among patients with moderate severity of COVID-19 pointed out that patients receiving hydroxychloroquine or chloroquine treatment may occur serious gastrointestinal adverse practices (diarrhea, nausea, abdominal pain, etc.) which is an important reason make the patients are forced to withdrawal[115].
Patients suffering from COVID-19 are often accompanied by various types of gastrointestinal symptoms. Gastrointestinal symptoms may be accompanied by or precede respiratory symptoms. This suggests that gastrointestinal symptoms may indicate the possibility of a new COVID-19 infection in the context of this unmanageable pandemic trend. During COVID-19 infection, the main mechanisms of gastrointestinal symptoms include the interaction between virus and gastrointestinal ACE2, inflammatory factor storm, intestinal mucosal barrier damage, and composition and metabolites of intestinal flora change. A vicious cycle is formed between the above factors in COVID-19 patients, which prolongs the recovery time. This also suggests that focusing on gut symptoms and alterations in gut microbes or their metabolites may be a beneficial option for coping with COVID-19. Adjunctive treatment with probiotics may help break this vicious cycle and help the patients recover. However, this review has some limitations. The assumptions in this review are mostly based on clinical observations. To clearly elucidate the mechanism of gastrointestinal symptoms caused by COVID-19 and find appropriate treatments still needs a lot of basic research.
We would like to thank the participants involved in this study. The figures were created with Procreate.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mishra AK, United States; Tanabe S, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med. 2020;382:1787-1799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3386] [Cited by in RCA: 3627] [Article Influence: 725.4] [Reference Citation Analysis (0)] |
| 2. | Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 741] [Cited by in RCA: 754] [Article Influence: 150.8] [Reference Citation Analysis (0)] |
| 3. | Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1160] [Cited by in RCA: 1205] [Article Influence: 241.0] [Reference Citation Analysis (0)] |
| 4. | Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Thompson CC, Shen L, Chan WW. Prevalence and Characteristics of Gastrointestinal Symptoms in Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection in the United States: A Multicenter Cohort Study. Gastroenterology. 2020;159:765-767.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 241] [Cited by in RCA: 283] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 5. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5517] [Article Influence: 1103.4] [Reference Citation Analysis (1)] |
| 6. | Nobel YR, Phipps M, Zucker J, Lebwohl B, Wang TC, Sobieszczyk ME, Freedberg DE. Gastrointestinal Symptoms and Coronavirus Disease 2019: A Case-Control Study From the United States. Gastroenterology. 2020;159:373-375.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 265] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 7. | Boraschi P, Giugliano L, Mercogliano G, Donati F, Romano S, Neri E. Abdominal and gastrointestinal manifestations in COVID-19 patients: Is imaging useful? World J Gastroenterol. 2021;27:4143-4159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 8. | Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, Xiong Y, Cheng Z, Gao S, Liang K, Luo M, Chen T, Song S, Ma Z, Chen X, Zheng R, Cao Q, Wang F, Zhang Y. Clinical Characteristics of Refractory Coronavirus Disease 2019 in Wuhan, China. Clin Infect Dis. 2021;73:e4208-e4213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 486] [Cited by in RCA: 601] [Article Influence: 120.2] [Reference Citation Analysis (0)] |
| 9. | Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, Niemeyer D, Jones TC, Vollmar P, Rothe C, Hoelscher M, Bleicker T, Brünink S, Schneider J, Ehmann R, Zwirglmaier K, Drosten C, Wendtner C. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4682] [Cited by in RCA: 4809] [Article Influence: 961.8] [Reference Citation Analysis (0)] |
| 10. | Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831-1833.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1994] [Article Influence: 398.8] [Reference Citation Analysis (1)] |
| 11. | Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2419] [Cited by in RCA: 2048] [Article Influence: 409.6] [Reference Citation Analysis (2)] |
| 12. | Qi F, Qian S, Zhang S, Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 741] [Article Influence: 148.2] [Reference Citation Analysis (0)] |
| 13. | Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 2020;46:1114-1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 425] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 14. | Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, Feldman J, Muus C, Wadsworth MH 2nd, Kazer SW, Hughes TK, Doran B, Gatter GJ, Vukovic M, Taliaferro F, Mead BE, Guo Z, Wang JP, Gras D, Plaisant M, Ansari M, Angelidis I, Adler H, Sucre JMS, Taylor CJ, Lin B, Waghray A, Mitsialis V, Dwyer DF, Buchheit KM, Boyce JA, Barrett NA, Laidlaw TM, Carroll SL, Colonna L, Tkachev V, Peterson CW, Yu A, Zheng HB, Gideon HP, Winchell CG, Lin PL, Bingle CD, Snapper SB, Kropski JA, Theis FJ, Schiller HB, Zaragosi LE, Barbry P, Leslie A, Kiem HP, Flynn JL, Fortune SM, Berger B, Finberg RW, Kean LS, Garber M, Schmidt AG, Lingwood D, Shalek AK, Ordovas-Montanes J; HCA Lung Biological Network. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181:1016-1035.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 1794] [Article Influence: 358.8] [Reference Citation Analysis (0)] |
| 15. | Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323:1843-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1894] [Cited by in RCA: 2658] [Article Influence: 531.6] [Reference Citation Analysis (0)] |
| 16. | Mishra AK, Sahu KK, Lal A. Reporting of all cardiac medications and their outcome in COVID-19. J Med Virol. 2020;92:1419-1420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2710] [Cited by in RCA: 2645] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 18. | Delorme-Axford E, Klionsky DJ. Highlights in the fight against COVID-19: does autophagy play a role in SARS-CoV-2 infection? Autophagy. 2020;16:2123-2127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4579] [Article Influence: 915.8] [Reference Citation Analysis (0)] |
| 20. | Kesavelu D, Franklyn N, Sreedharan L. Can Nutrition Play a Role as a Stimulant for COVID 19 in Children? Rev Recent Clin Trials. 2021;16:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Andrews PLR, Cai W, Rudd JA, Sanger GJ. COVID-19, nausea, and vomiting. J Gastroenterol Hepatol. 2021;36:646-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1039] [Cited by in RCA: 907] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 23. | Tung-Thompson G, Libera DA, Koch KL, de Los Reyes FL 3rd, Jaykus LA. Aerosolization of a Human Norovirus Surrogate, Bacteriophage MS2, during Simulated Vomiting. PLoS One. 2015;10:e0134277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Wu W, Zhou HR, Pestka JJ. Potential roles for calcium-sensing receptor (CaSR) and transient receptor potential ankyrin-1 (TRPA1) in murine anorectic response to deoxynivalenol (vomitoxin). Arch Toxicol. 2017;91:495-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Christie S, Wittert GA, Li H, Page AJ. Involvement of TRPV1 Channels in Energy Homeostasis. Front Endocrinol (Lausanne). 2018;9:420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 26. | Yu X, Yu M, Liu Y, Yu S. TRP channel functions in the gastrointestinal tract. Semin Immunopathol. 2016;38:385-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Zhang M, Zhou Q, Dorfman RG, Huang X, Fan T, Zhang H, Zhang J, Yu C. Butyrate inhibits interleukin-17 and generates Tregs to ameliorate colorectal colitis in rats. BMC Gastroenterol. 2016;16:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 162] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 28. | Matthews PJ, Aziz Q, Facer P, Davis JB, Thompson DG, Anand P. Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur J Gastroenterol Hepatol. 2004;16:897-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 197] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 29. | Zhang L, Jones S, Brody K, Costa M, Brookes SJ. Thermosensitive transient receptor potential channels in vagal afferent neurons of the mouse. Am J Physiol Gastrointest Liver Physiol. 2004;286:G983-G991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Hammer J, Vogelsang H. Characterization of sensations induced by capsaicin in the upper gastrointestinal tract. Neurogastroenterol Motil. 2007;19:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Yu S, Gao G, Peterson BZ, Ouyang A. TRPA1 in mast cell activation-induced long-lasting mechanical hypersensitivity of vagal afferent C-fibers in guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol. 2009;297:G34-G42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Zhao H, Sprunger LK, Simasko SM. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am J Physiol Gastrointest Liver Physiol. 2010;298:G212-G221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Mitrovic M, Shahbazian A, Bock E, Pabst MA, Holzer P. Chemo-nociceptive signalling from the colon is enhanced by mild colitis and blocked by inhibition of transient receptor potential ankyrin 1 channels. Br J Pharmacol. 2010;160:1430-1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Doihara H, Nozawa K, Kawabata-Shoda E, Kojima R, Yokoyama T, Ito H. Molecular cloning and characterization of dog TRPA1 and AITC stimulate the gastrointestinal motility through TRPA1 in conscious dogs. Eur J Pharmacol. 2009;617:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | D'Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L. Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin Gastroenterol Hepatol. 2020;18:1663-1672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 378] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 36. | Camargo SMR, Vuille-Dit-Bille RN, Meier CF, Verrey F. ACE2 and gut amino acid transport. Clin Sci (Lond). 2020;134:2823-2833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 37. | Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477-481. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 787] [Cited by in RCA: 989] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 38. | Ramli FF, Cowen PJ, Godlewska BR. The Potential Use of Ebselen in Treatment-Resistant Depression. Pharmaceuticals (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 39. | Dejene H, Abunna F, Tuffa AC, Gebresenbet G. Epidemiology and Antimicrobial Susceptibility Pattern of E. coli O157:H7 Along Dairy Milk Supply Chain in Central Ethiopia. Vet Med (Auckl). 2022;13:131-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 40. | Kumar A, Russell RM, Pifer R, Menezes-Garcia Z, Cuesta S, Narayanan S, MacMillan JB, Sperandio V. The Serotonin Neurotransmitter Modulates Virulence of Enteric Pathogens. Cell Host Microbe. 2020;28:41-53.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 41. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14766] [Article Influence: 2953.2] [Reference Citation Analysis (0)] |
| 42. | Gu Y, Wang C, Qin X, Zhou B, Liu X, Liu T, Xie R, Liu J, Wang B, Cao H. Saccharomyces boulardii, a yeast probiotic, inhibits gut motility through upregulating intestinal serotonin transporter and modulating gut microbiota. Pharmacol Res. 2022;181:106291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 43. | O'Moráin N, Stack R, Doherty J, Tosetto M, Garcia Leon A, Mallon P, Doherty G; All Ireland Infectious Diseases (AIID) Study Group. Faecal calprotectin as a potential biomarker of disease severity in SARS-CoV-2 infection. J Infect. 2022;85:436-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 44. | Pokryszka J, Wagner A, Wiedermann U, Tobudic S, Herkner H, Winkler S, Brehovsky S, Reinisch W, Novacek G. Course of Fecal Calprotectin after mRNA SARS-CoV-2 Vaccination in Patients with Inflammatory Bowel Diseases. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 45. | Lin S, Chanchlani N, Carbery I, Janjua M, Nice R, McDonald TJ, Bewshea C, Kennedy NA, Ahmad T, Selinger CP, Goodhand JR; PANTS consortium. Understanding anti-TNF treatment failure: does serum triiodothyronine-to-thyroxine (T3/T4) ratio predict therapeutic outcome to anti-TNF therapies in biologic-naïve patients with active luminal Crohn's disease? Aliment Pharmacol Ther. 2022;56:783-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 46. | Shao S, Huang M, Zhang H, Peng G, Song M, Liu J, Xu D. A Retrospective Analysis of Clinical Features and Treatment of the Inflammatory Bowel Disease in China. J Inflamm Res. 2022;15:3587-3597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 47. | Rayment JH, Asfour F, Rosenfeld M, Higgins M, Liu L, Mascia M, Paz-Diaz H, Tian S, Zahigian R, McColley SA. A Phase 3, Open-Label Study of Lumacaftor/Ivacaftor in Children 1 to Less Than 2 Years of Age with Cystic Fibrosis Homozygous for F508del-CFTR. Am J Respir Crit Care Med. 2022;206:1239-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 48. | Ramasamy S, Subbian S. Critical Determinants of Cytokine Storm and Type I Interferon Response in COVID-19 Pathogenesis. Clin Microbiol Rev. 2021;34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 49. | Islam D, Lombardini E, Ruamsap N, Imerbsin R, Khantapura P, Teo I, Neesanant P, Gonwong S, Yongvanitchit K, Swierczewski BE, Mason CJ, Shaunak S. Controlling the cytokine storm in severe bacterial diarrhoea with an oral Toll-like receptor 4 antagonist. Immunology. 2016;147:178-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Sweeny KF, Zhang YJ, Crume B, Martz CA, Blessing MM, Kahn SA. Inflammatory Bowel Disease Presenting With Concurrent COVID-19 Multisystem Inflammatory Syndrome. Pediatrics. 2021;147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762-768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2495] [Cited by in RCA: 3338] [Article Influence: 667.6] [Reference Citation Analysis (0)] |
| 52. | Chansrichavala P, Chantharaksri U, Sritara P, Chaiyaroj SC. Atorvastatin attenuates TLR4-mediated NF-kappaB activation in a MyD88-dependent pathway. Asian Pac J Allergy Immunol. 2009;27:49-57. [PubMed] |
| 53. | Giron LB, Dweep H, Yin X, Wang H, Damra M, Goldman AR, Gorman N, Palmer CS, Tang HY, Shaikh MW, Forsyth CB, Balk RA, Zilberstein NF, Liu Q, Kossenkov A, Keshavarzian A, Landay A, Abdel-Mohsen M. Plasma Markers of Disrupted Gut Permeability in Severe COVID-19 Patients. Front Immunol. 2021;12:686240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 54. | Yonker LM, Gilboa T, Ogata AF, Senussi Y, Lazarovits R, Boribong BP, Bartsch YC, Loiselle M, Rivas MN, Porritt RA, Lima R, Davis JP, Farkas EJ, Burns MD, Young N, Mahajan VS, Hajizadeh S, Lopez XIH, Kreuzer J, Morris R, Martinez EE, Han I, Griswold K Jr, Barry NC, Thompson DB, Church G, Edlow AG, Haas W, Pillai S, Arditi M, Alter G, Walt DR, Fasano A. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 185] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 55. | Haak BW, Wiersinga WJ. The role of the gut microbiota in sepsis. Lancet Gastroenterol Hepatol. 2017;2:135-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 223] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 56. | Jin B, Singh R, Ha SE, Zogg H, Park PJ, Ro S. Pathophysiological mechanisms underlying gastrointestinal symptoms in patients with COVID-19. World J Gastroenterol. 2021;27:2341-2352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 57. | Chakraborty C, Sharma AR, Bhattacharya M, Dhama K, Lee SS. Altered gut microbiota patterns in COVID-19: Markers for inflammation and disease severity. World J Gastroenterol. 2022;28:2802-2822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (5)] |
| 58. | Takeshita H, Yamamoto K. Tryptophan Metabolism and COVID-19-Induced Skeletal Muscle Damage: Is ACE2 a Key Regulator? Front Nutr. 2022;9:868845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 59. | Guarnieri T. Hypothesis: Emerging Roles for Aryl Hydrocarbon Receptor in Orchestrating CoV-2-Related Inflammation. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 60. | Qin WH, Liu CL, Jiang YH, Hu B, Wang HY, Fu J. Gut ACE2 Expression, Tryptophan Deficiency, and Inflammatory Responses The Potential Connection That Should Not Be Ignored During SARS-CoV-2 Infection. Cell Mol Gastroenterol Hepatol. 2021;12:1514-1516.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Koester ST, Li N, Lachance DM, Morella NM, Dey N. Variability in digestive and respiratory tract Ace2 expression is associated with the microbiome. PLoS One. 2021;16:e0248730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 62. | Patankar JV, Chiriac MT, Lehmann M, Kühl AA, Atreya R, Becker C; Callaborators, Gonzalez-Acera M, Schmitt H, Gamez-Belmonte R, Mahapatro M, Diemand L, Hartmann L, Mascia F, Hracsko Z, Thonn V, Schödel L, Zielinska M, Yu Y, Erkert L, Li W, Zeitler M, Ruder B, Ganzleben I, Günther C, Voehringer D, Zundler S, Neurath MF, Siegmund B. Severe Acute Respiratory Syndrome Coronavirus 2 Attachment Receptor Angiotensin-Converting Enzyme 2 Is Decreased in Crohn's Disease and Regulated By Microbial and Inflammatory Signaling. Gastroenterology. 2021;160:925-928.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 63. | Oliveira LP, Guimarães VHD, Oliveira JR, Guimarães ALS, de Paula AMB, Bader M, Santos RASD, Santos SHS. Genetic deletion of the angiotensin-(1-7) receptor Mas leads to alterations in gut villi length modulating TLR4/PI3K/AKT and produces microbiome dysbiosis. Neuropeptides. 2020;82:102056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 64. | Zuo T, Wu X, Wen W, Lan P. Gut Microbiome Alterations in COVID-19. Genomics Proteomics Bioinformatics. 2021;19:679-688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 65. | Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS, Chan V, Ling L, Joynt G, Hui DS, Chow KM, Ng SSS, Li TC, Ng RW, Yip TC, Wong GL, Chan FK, Wong CK, Chan PK, Ng SC. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;70:698-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 995] [Cited by in RCA: 863] [Article Influence: 215.8] [Reference Citation Analysis (0)] |
| 66. | Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl Res. 2020;226:57-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 217] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 67. | Ye Q, Wang B, Zhang T, Xu J, Shang S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am J Physiol Gastrointest Liver Physiol. 2020;319:G245-G252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (2)] |
| 68. | Din AU, Mazhar M, Waseem M, Ahmad W, Bibi A, Hassan A, Ali N, Gang W, Qian G, Ullah R, Shah T, Ullah M, Khan I, Nisar MF, Wu J. SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed Pharmacother. 2021;133:110947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 69. | Saakre M, Mathew D, Ravisankar V. Perspectives on plant flavonoid quercetin-based drugs for novel SARS-CoV-2. Beni Suef Univ J Basic Appl Sci. 2021;10:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 70. | Seeliger B, Philouze G, Benotmane I, Mutter D, Pessaux P. Is the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) present intraperitoneally in patients with coronavirus disease 2019 (COVID-19) infection undergoing emergency operations? Surgery. 2020;168:220-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 71. | Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, Lei HY. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol. 2005;75:185-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 547] [Cited by in RCA: 586] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 72. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6751] [Article Influence: 1350.2] [Reference Citation Analysis (0)] |
| 73. | Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1356] [Cited by in RCA: 1805] [Article Influence: 361.0] [Reference Citation Analysis (0)] |
| 74. | Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Wang G, Yuan Z, Feng Z, Zhang Y, Wu Y, Chen Y. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front Immunol. 2020;11:827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1687] [Cited by in RCA: 1758] [Article Influence: 351.6] [Reference Citation Analysis (0)] |
| 75. | van Thiel IAM, Botschuijver S, de Jonge WJ, Seppen J. Painful interactions: Microbial compounds and visceral pain. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 76. | Hirano T, Murakami M. COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome. Immunity. 2020;52:731-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 436] [Cited by in RCA: 584] [Article Influence: 116.8] [Reference Citation Analysis (0)] |
| 77. | Ferastraoaru D, Hudes G, Jerschow E, Jariwala S, Karagic M, de Vos G, Rosenstreich D, Ramesh M. Eosinophilia in Asthma Patients Is Protective Against Severe COVID-19 Illness. J Allergy Clin Immunol Pract. 2021;9:1152-1162.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 78. | Qeadan F, Chehade M, Tingey B, Egbert J, Dellon ES, Peterson KA. Patients with eosinophilic gastrointestinal disorders have lower in-hospital mortality rates related to COVID-19. J Allergy Clin Immunol Pract. 2021;9:4473-4476.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 79. | Perlini S, Ciprandi G, Castagnoli R, Licari A, Marseglia GL. Eosinopenia could be a relevant prognostic biomarker in patients with coronavirus disease 2019. Allergy Asthma Proc. 2020;41:e80-e82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Sequeira M, Cruz D, Abecasis F, Santos H, Delerue F. Eosinophilic Ascites: An Infrequent Presentation of Eosinophilic Gastroenteritis. Cureus. 2022;14:e24303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 81. | Muñoz FV, Almeida PH, Carrión-Jaramillo E, Montalvo AV. Clinical Features of Eosinophilic Esophagitis: A Single Center Experience in Ecuador. Pediatr Gastroenterol Hepatol Nutr. 2022;25:293-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 82. | Stasi C, Sadalla S, Milani S. The Relationship Between the Serotonin Metabolism, Gut-Microbiota and the Gut-Brain Axis. Curr Drug Metab. 2019;20:646-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 83. | Kim DY, Camilleri M. Serotonin: a mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2698-2709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 303] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 84. | Minderhoud IM, Oldenburg B, Schipper ME, ter Linde JJ, Samsom M. Serotonin synthesis and uptake in symptomatic patients with Crohn's disease in remission. Clin Gastroenterol Hepatol. 2007;5:714-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 85. | Salaga M, Binienda A, Piscitelli F, Mokrowiecka A, Cygankiewicz AI, Verde R, Malecka-Panas E, Kordek R, Krajewska WM, Di Marzo V, Fichna J. Systemic administration of serotonin exacerbates abdominal pain and colitis via interaction with the endocannabinoid system. Biochem Pharmacol. 2019;161:37-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 86. | Regmi SC, Park SY, Ku SK, Kim JA. Serotonin regulates innate immune responses of colon epithelial cells through Nox2-derived reactive oxygen species. Free Radic Biol Med. 2014;69:377-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 87. | Martínez-Force E, Lakhe-Reddy S, Wise JA. Systematic mutagenesis of the fission yeast Srp54 protein. Curr Genet. 1999;35:88-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 88. | Sengupta JN. Visceral pain: the neurophysiological mechanism. Handb Exp Pharmacol. 2009;31-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 89. | Mabillard H, Sayer JA. Electrolyte Disturbances in SARS-CoV-2 Infection. F1000Res. 2020;9:587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 90. | Rinkenberger N, Schoggins JW. Mucolipin-2 Cation Channel Increases Trafficking Efficiency of Endocytosed Viruses. mBio. 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 91. | Zhao Z, Qin P, Huang YW. Lysosomal ion channels involved in cellular entry and uncoating of enveloped viruses: Implications for therapeutic strategies against SARS-CoV-2. Cell Calcium. 2021;94:102360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 92. | Bousquet J, Czarlewski W, Zuberbier T, Mullol J, Blain H, Cristol JP, De La Torre R, Pizarro Lozano N, Le Moing V, Bedbrook A, Agache I, Akdis CA, Canonica GW, Cruz AA, Fiocchi A, Fonseca JA, Fonseca S, Gemicioğlu B, Haahtela T, Iaccarino G, Ivancevich JC, Jutel M, Klimek L, Kraxner H, Kuna P, Larenas-Linnemann DE, Martineau A, Melén E, Okamoto Y, Papadopoulos NG, Pfaar O, Regateiro FS, Reynes J, Rolland Y, Rouadi PW, Samolinski B, Sheikh A, Toppila-Salmi S, Valiulis A, Choi HJ, Kim HJ, Anto JM. Potential Interplay between Nrf2, TRPA1, and TRPV1 in Nutrients for the Control of COVID-19. Int Arch Allergy Immunol. 2021;182:324-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 93. | Nahama A, Ramachandran R, Cisternas AF, Ji H. The role of afferent pulmonary innervation in ARDS associated with COVID-19 and potential use of resiniferatoxin to improve prognosis: A review. Med Drug Discov. 2020;5:100033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 94. | Termorshuizen JD, Watson HJ, Thornton LM, Borg S, Flatt RE, MacDermod CM, Harper LE, van Furth EF, Peat CM, Bulik CM. Early impact of COVID-19 on individuals with self-reported eating disorders: A survey of ~1,000 individuals in the United States and the Netherlands. Int J Eat Disord. 2020;53:1780-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 174] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 95. | Dumitrașcu MC, Șandru F, Carsote M, Petca RC, Gheorghisan-Galateanu AA, Petca A, Valea A. Anorexia nervosa: COVID-19 pandemic period (Review). Exp Ther Med. 2021;22:804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 96. | Baranowska B, Kochanowski J. Neuroendocrine aspects of anorexia nervosa and bulimia nervosa. Neuro Endocrinol Lett. 2018;39:172-178. [PubMed] |
| 97. | He Y, Cai X, Liu H, Conde KM, Xu P, Li Y, Wang C, Yu M, He Y, Liang C, Yang T, Yang Y, Yu K, Wang J, Zheng R, Liu F, Sun Z, Heisler L, Wu Q, Tong Q, Zhu C, Shu G, Xu Y. 5-HT recruits distinct neurocircuits to inhibit hunger-driven and non-hunger-driven feeding. Mol Psychiatry. 2021;26:7211-7224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 98. | Shobeiri P, Kalantari A, Teixeira AL, Rezaei N. Shedding light on biological sex differences and microbiota-gut-brain axis: a comprehensive review of its roles in neuropsychiatric disorders. Biol Sex Differ. 2022;13:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 99. | Rossi-Fanelli F, Cangiano C. Increased availability of tryptophan in brain as common pathogenic mechanism for anorexia associated with different diseases. Nutrition. 1991;7:364-367. [PubMed] |
| 100. | Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, Chance R, Macaulay IC, Chou HJ, Fletcher RB, Das D, Street K, de Bezieux HR, Choi YG, Risso D, Dudoit S, Purdom E, Mill J, Hachem RA, Matsunami H, Logan DW, Goldstein BJ, Grubb MS, Ngai J, Datta SR. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. 2020;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 708] [Cited by in RCA: 727] [Article Influence: 145.4] [Reference Citation Analysis (0)] |
| 101. | Perisetti A, Goyal H, Gajendran M, Boregowda U, Mann R, Sharma N. Prevalence, Mechanisms, and Implications of Gastrointestinal Symptoms in COVID-19. Front Med (Lausanne). 2020;7:588711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 102. | Aziz M, Perisetti A, Lee-Smith WM, Gajendran M, Bansal P, Goyal H. Taste Changes (Dysgeusia) in COVID-19: A Systematic Review and Meta-analysis. Gastroenterology. 2020;159:1132-1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 103. | Chen R, Yu YL, Li W, Liu Y, Lu JX, Chen F, Zhou Q, Xia ZY, Gao L, Meng QT, Ma D. Gastrointestinal Symptoms Associated With Unfavorable Prognosis of COVID-19 Patients: A Retrospective Study. Front Med (Lausanne). 2020;7:608259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 104. | Jiang G, Cai Y, Yi X, Li Y, Lin Y, Li Q, Xu J, Ke M, Xue K. The impact of laryngopharyngeal reflux disease on 95 hospitalized patients with COVID-19 in Wuhan, China: A retrospective study. J Med Virol. 2020;92:2124-2129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 105. | Almario CV, Chey WD, Spiegel BMR. Increased Risk of COVID-19 Among Users of Proton Pump Inhibitors. Am J Gastroenterol. 2020;115:1707-1715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 106. | Malone RW. More Than Just Heartburn: Does Famotidine Effectively Treat Patients with COVID-19? Dig Dis Sci. 2021;66:3672-3673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 107. | Martin TA, Wan DW, Hajifathalian K, Tewani S, Shah SL, Mehta A, Kaplan A, Ghosh G, Choi AJ, Krisko TI, Fortune BE, Crawford CV, Sharaiha RZ. Gastrointestinal Bleeding in Patients With Coronavirus Disease 2019: A Matched Case-Control Study. Am J Gastroenterol. 2020;115:1609-1616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 108. | Chen H, Tong Z, Ma Z, Luo L, Tang Y, Teng Y, Yu H, Meng H, Peng C, Zhang Q, Zhu T, Zhao H, Chu G, Li H, Lu H, Qi X. Gastrointestinal Bleeding, but Not Other Gastrointestinal Symptoms, Is Associated With Worse Outcomes in COVID-19 Patients. Front Med (Lausanne). 2021;8:759152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 109. | Salehi S, Gholamrezanezhad A. Reply to "Segmental Pulmonary Vascular Changes in COVID-19 Pneumonia". AJR Am J Roentgenol. 2020;215:W34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 110. | Eslambolchi A, Aghaghazvini L, Gholamrezanezhad A, Kavosi H, Radmard AR. Coronavirus disease 2019 (COVID-19) in patients with systemic autoimmune diseases or vasculitis: radiologic presentation. J Thromb Thrombolysis. 2021;51:339-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 111. | Keshavarz P, Rafiee F, Kavandi H, Goudarzi S, Heidari F, Gholamrezanezhad A. Ischemic gastrointestinal complications of COVID-19: a systematic review on imaging presentation. Clin Imaging. 2021;73:86-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 112. | Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) Outbreak: What the Department of Radiology Should Know. J Am Coll Radiol. 2020;17:447-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 280] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 113. | El Moheb M, Naar L, Christensen MA, Kapoen C, Maurer LR, Farhat M, Kaafarani HMA. Gastrointestinal Complications in Critically Ill Patients With and Without COVID-19. JAMA. 2020;324:1899-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 114. | Chen TH, Hsu MT, Lee MY, Chou CK. Gastrointestinal Involvement in SARS-CoV-2 Infection. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 115. | Verheijen S, van Luin M, Brüggemann RJ, de Mast Q, Hassing RJ, Burger DM. More gastro-intestinal adverse events in non-ICU hospitalised COVID-19 patients treated with chloroquine versus hydroxychloroquine. Int J Infect Dis. 2021;103:402-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |