Published online Dec 28, 2022. doi: 10.3748/wjg.v28.i48.6791
Peer-review started: September 2, 2022
First decision: October 20, 2022
Revised: November 7, 2022
Accepted: December 6, 2022
Article in press: December 6, 2022
Published online: December 28, 2022
Processing time: 116 Days and 1.6 Hours
Various vaccines against severe acute respiratory syndrome coronavirus 2 have been developed in response to the coronavirus disease 2019 (COVID-19) global pandemic, several of which are highly effective in preventing COVID-19 in the general population. Patients with chronic liver diseases (CLDs), particularly those with liver cirrhosis, are considered to be at a high risk for severe COVID-19 and death. Given the increased rates of disease severity and mortality in patients with liver disease, there is an urgent need to understand the efficacy of vaccination in this population. However, the data regarding efficacy and safety of COVID-19 vaccination in patients with CLDs is limited. Indeed, several organ-specific or systemic immune-mediated side effects following COVID-19 vaccination, including liver injury similar to autoimmune hepatitis, have been recently reported. Although the number of cases of vaccine-related liver injury is increasing, its frequency, clinical course, and mechanism remain unclear. Here, we review the current findings on COVID-19 vaccination and liver disease, focusing on: (1) The impact of COVID-19 in patients with CLD; (2) The efficacy, safety, and risk-benefit profiles of COVID-19 vaccines in patients with CLD; and (3) Liver injury following COVID-19 vaccination.
Core Tip: Patients with chronic liver disease (CLD), including cirrhosis, are a high-risk group for severe coronavirus disease 2019 (COVID-19). Presently, the results of several clinical trials for measuring the efficacy and safety of the available COVID-19 vaccines in patients with CLD have been reported. Given the increased rates of severity and mortality of COVID-19 in patients with CLD, the importance of aggressive vaccination in the effective management of severe acute respiratory syndrome coronavirus 2 infection should be emphasized. Although liver injury following COVID-19 vaccination has also been reported, it is infrequent and is not a factor in vaccine hesitancy.
- Citation: Ozaka S, Kobayashi T, Mizukami K, Murakami K. COVID-19 vaccination and liver disease. World J Gastroenterol 2022; 28(48): 6791-6810
- URL: https://www.wjgnet.com/1007-9327/full/v28/i48/6791.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i48.6791
The December 2019 outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) rapidly spread worldwide and became a global health threat[1,2]. COVID-19 is a respiratory disease caused by SARS-CoV-2, which can damage not only the lungs, but also other organs, including the cardiovascular system, liver, and gastrointestinal tract[3-5]. Vaccines are the most effective prophylaxis against COVID-19, and several vaccines against SARS-CoV-2 have been developed in response to the COVID-19 pandemic, among which vaccines produced mainly by Pfizer-BioNTech, Moderna, and Oxford-AstraZeneca are now widely used[6]. These vaccines are of great importance in controlling severe COVID-19 not only in healthy individuals, but also in high-risk populations, including those with chronic diseases. Chronic liver disease (CLD) is characterized by the gradual destruction of liver tissue over time, and includes liver diseases that are caused by chronic inflammation [chronic viral hepatitis, non-alcoholic fatty liver disease (NAFLD), alcoholic liver disease, and autoimmune hepatitis (AIH)] with or without cirrhosis and hepatocellular carcinoma (HCC). Patients with CLD, particularly those with cirrhosis, and liver transplantation (LT) recipients infected with SARS-CoV-2 have been reported to have a higher risk of adverse outcomes than the general population. For example, among 2780 individuals with COVID-19 in the United States, comparison between 250 individuals with liver diseases vs the rest of the cases indicated a significantly higher mortality rate in those with liver disease [hazard ratio (HR), 2.8; 95% confidence interval (CI): 1.9-4.0] and a higher mortality risk in those with cirrhosis (HR: 3.0; 95%CI: 1.5-6.0)[7]. In a recent study, the mortality rate of cirrhotic patients with COVID-19 was significantly higher than that of non-cirrhotic patients (HR: 2.38; 95%CI: 2.18-2.59), and was also increased in cirrhotic patients with underlying CLD (HR: 3.31; 95%CI: 2.91-3.77)[8]. Therefore, patients with CLD are considered to be at an increased risk of SARS-CoV-2 infection and worse outcomes. Given the increased rates of severity and mortality in patients with liver disease, there is an urgent need to understand the efficacy and safety of vaccination, as well as the importance of aggressive vaccination in this population. However, since most of the phase 2/3 trials of COVID-19 vaccines mainly recruited healthy individuals, data regarding the efficacy and safety in patients with liver diseases is limited. Presently, the results of several clinical trials for measuring the efficacy and safety of the available COVID-19 vaccines in patients with CLD have been reported.
The safety profiles of each vaccine have also been extensively studied. Common side effects of COVID-19 vaccines include injection site pain, transient fever, headache and fatigue[9,10]. Recently, however, several organ specific or systemic immune-mediated side effects following COVID-19 vaccination have also been reported[11]. These side effects include immune-mediated liver injury resembling AIH.
In this review, we summarized the current knowledge, focusing on the impact of COVID-19 in patients with liver disease, as well as the efficacy and safety of COVID-19 vaccination in these patients. In addition, we analyzed case reports of acute liver injury following COVID-19 vaccination.
Vaccines against SARS-CoV-2 can be categorized based on the platform they are developed on into mRNA, viral vector, inactivated virus, attenuated virus, protein subunit, and recombinant DNA vaccines. The major COVID-19 vaccines currently used worldwide include the vaccines produced by Pfizer-BioNTech, Moderna, and Oxford-AstraZeneca[6]. These, plus the CoronaVac vaccine (Sinovac Biotech) and Janssen-Ad26.COV2.S vaccine (Johnson & Johnson), are the five vaccines registered in the WHO Emergency Use Listing of Qualified Vaccines (Table 1)[12]. Various COVID-19 vaccines have been proven to be highly effective and have good safety profiles in healthy populations. The efficacy of vaccines is evaluated based on their: (1) Immunogenicity; (2) Efficacy rate in clinical trials; and (3) Real-world efficacy rate.
| Corporation | Vaccine | Mechanism | Vaccination Group: Number of cases/number of vaccinations (%) | Invaccination Group: Number of cases/number of vaccinations (%) | Efficacy % (95%CI) |
| Pfizer-BioNTech[18] | BNT162b2 | mRNA | 8/21720 (0.037) | 162/21728 (0.746) | 95.0 (90.3-97.6) |
| Moderna[20] | mRNA-1273 | mRNA | 11/15210 (0.072) | 185/15210 (1.216) | 94.1 (89.3-96.8) |
| Oxford-AstraZeneca[25] | ChAdOx1 nCoV-19 (AZD122) | Vector | 27/4440 (0.608) | 71/4455 (1.594) | 62.1 (41.0-75.7) |
| Johnson & Johnson[26] | Ad26.COV2.S | Vector | 433/19113 (2.265) | 883/18924 (4.666) | 52.9 (47.1-58.1) |
| Sinovac Life Science[29] | Corona Vac | Inacctivated | 9/6559 (0.137) | 32/3470 (0.922) | 83.5 (65.4–92.1) |
The BNT162b2 mRNA (Pfizer-BioNTech) and mRNA-1273 mRNA (Moderna) vaccines are based on mRNA encoding SARS-CoV-2 spike proteins[13]. The injected mRNA is internalized into local host cells and translated, resulting in the production of antigen proteins and antigen-specific immune responses[14,15]. mRNA based vaccines have been shown to be safe and well tolerated in clinical trials. The BNT162b2 mRNA vaccine was administered to healthy adults (18-55 and 65-85 years old) at doses of 10 μg, 30 μg, or 100 μg in a phase 1/2 trial and showed immunogenicity, tolerability, and safety profiles consistent with these doses[16,17]. A phase 3 study of 43548 individuals also showed an efficacy rate of 95% [95%CI: 90.3-97.6; 8 cases of COVID-19 (0.04%) of 21720 in the BNT162b2 group vs 162 cases of COVID-19 (0.75%) of 21,728 in the placebo group][18], and a 6-mo follow-up of 44616 adults aged 16 years and older and 2264 participants aged 12 to 15 years showed an efficacy rate of 91.3% (95%CI: 89.0-93.2)[19]. The mRNA-1273 vaccine also showed an efficacy of 94.1% [95%CI: 89.3-96.8; 11 cases of COVID-19 (0.07%) of 15210 in the mRNA-1273 group vs 185 cases of COVID-19 (1.22%) of 15210 in the placebo group] in a phase 3 study of 30420 healthy individuals aged 18 or above[20]. It should be noted, however, that the efficacy against the recent epidemic Omicron strain is reduced for both these mRNA vaccines. Neutralizing antibody titers 3 wk after two doses of the BNT162b2 vaccine were significantly lower for the Omicron strain compared with the Wuhan strain[21], and even with the mRNA-1273 vaccine, neutralizing antibody titers against the Omicron strain after two doses of the vaccine were 1/41 to 1/84 of those against the European strain[22]. On the other hand, it was also shown that the neutralizing antibody titer against the Omicron strain increased significantly after the third dose of both vaccines and was almost equivalent to that after two doses[21,22], and the booster dose of mRNA vaccines was also effective against the Omicron strain, with a good efficacy rate of 60 to 70%[23].
The ChAdOx1 nCoV-19 vaccine (AZD1222), which was developed by Oxford-AstraZeneca, is a viral vector vaccine. Immunity to the spike protein is induced by administration of a chimpanzee adenovirus vector containing the SARS-CoV-2 spike protein gene[24]. A clinical trial of ChAdOx1 nCoV-19 reported an efficacy of 62.1% [95%CI: 41.0-75.7; 27 cases of COVID-19 (0.6%) of 4440 in the ChAdOx1 nCoV-19 group vs 71 cases of COVID-19 (1.6%) of 4455 in the control group][25]. The efficacy of Ad26.COV2.S, a single-dose viral vector vaccine produced by Johnson & Johnson, has also been reported. In a phase 3 study, vaccine efficacy after 28 d was 52.9% [95%CI: 47.1-58.1; 433 cases of COVID-19 of 19113 (2.3%) in the Ad26.COV2.S group vs 883 cases of COVID-19 of 18924 (4.7%) in the placebo group][26,27].
Sinovac-CoronaVac is an inactivated vaccine developed by Sinovac Life Sciences and is being used in several countries. Its efficacy and safety were demonstrated in a phase 1/2 study by Zhang et al[28] and the phase 3 study of Tanriover et al[29]. In a population aged 18-59 years, CoronaVac was shown to be highly effective in preventing symptomatic COVID-19 (83.5% vs placebo) and COVID-19-related hospitalizations (100%) at least 14 d after the second dose[29].
During the COVID-19 pandemic, many cases have been accumulated and the clinical course of COVID-19 in patients with CLD has been characterized. Patients with CLD, including those with cirrhosis or HCC, and LT recipients, are a high-risk group for severe COVID-19[30]. In a large cohort study using electronic health record data from more than 17 million patients in the United Kingdom, which included more than 0.1 million patients with CLD, CLD was a risk factor for death from COVID-19 (HR: 2.39; 95%CI: 2.06-2.77)[31]. A particularly high mortality rate due to COVID-19 has been reported in patients with cirrhosis[7,8,32], and recent prospective data from a multicenter study reported a high mortality rate of 32% among 729 patients with CLD from 29 countries. In particular, patients with decompensated cirrhosis were found to be at a higher risk of hospitalization, mechanical ventilation and death (overall mortality: Child-Pugh-A: 19%, Child-Pugh-B: 32%, Child-Pugh-C: 51%, non-cirrhosis: 8%)[33]. Similarly, in a North American multicenter cohort study, compensated cirrhosis had no effect on mortality in COVID-19, while mortality was increased in patients with decompensated cirrhosis[34]. Furthermore, cirrhotic patients with COVID-19 had a significantly higher mortality rate than patients with COVID-19 alone (30% vs 13%, P = 0.03) in a multicenter cohort study in the United States and Canada[35]. Patients with CLD, particularly those with cirrhosis, have multiple mechanisms of immune dysfunction that can lead to increased susceptibility to SARS-CoV-2 infection and an abnormal inflammatory response during infection. Thus, it has become clear that liver cirrhosis patients are at an increased risk of adverse COVID-19 outcomes, including death, as has been established by large observational cohorts and population-level data and international registry findings.
In addition to cirrhosis, NAFLD, alcoholic liver injury, and HCC are known to be factors affecting COVID-19 severity. Several observational cohort studies revealed a significant increase in the risk of severe COVID-19 in patients with NAFLD. In a Chinese study analyzing 202 COVID-19 cases, NAFLD complications were significantly more frequent in 39 patients whose disease progressed after hospitalization than in non-progressors (87.2% vs 25.8%), and the rate of severe disease was significantly higher in patients with NAFLD than in those without NAFLD (44.7% vs 6.6%)[36]. A meta-analysis by Pan et al[37] showed that NAFLD was associated with more severe COVID-19 [odds ratio (OR): 2.93; 95%CI: 1.87-4.60][37]. Two other meta-analyses have also been reported, both of which showed that NAFLD is a severity factor for COVID-19[38,39]. Patients with NAFLD have decreased hepatic innate immunity with skewed M1/M2 macrophage polarization, as well as increased levels of inflammatory mediators and cytokines. This underlying inflammatory status associated with NAFLD might lead to further exacerbation of the SARS-CoV-2 infection and can lead to a cytokine storm, which greatly increases the mortality rate[37]. Furthermore, it should be noted that diabetes mellitus, obesity, and cardiovascular diseases are also frequently present in the background of NAFLD, and these metabolic disorders might also be factors related to the increased mortality of COVID-19. Regarding alcoholic liver disease, Marjot et al[33] reported that it is an independent risk factor for COVID-19 related death (OR: 1.79; 95%CI: 1.03-3.13)[33], and Kim et al[34] also reported a 2.4-fold increase in COVID-19 mortality (HR: 2.42; 95%CI: 1.29-4.55) in these patients[34]. Their report also showed that HCC is a risk factor for death from COVID-19 (HR: 3.96; 95%CI: 1.74-8.98)[34]. Since HCC often occurs secondary to cirrhosis, the greater severity of COVID-19 in this patient group is thought to be a result of reduced immunity. Since LT recipients are required to use immunosuppressive agents for a long period of time, they are also considered to be a high-risk group for severe COVID-19. However, many reports from actual cohort studies concluded that LT is not an independent risk factor for COVID-19 related death[40-42]. Webb et al[43] compared mortality in 151 COVID-19 patients who underwent LT with 627 healthy subjects without LT, and reported no difference in overall mortality between the two groups (absolute risk difference 1.4%; 95%CI: 7.7-10.4)[43]. On the other hand, their study also reported that gastrointestinal symptoms, such as diarrhea, but not respiratory symptoms, were significantly increased in post-LT patients affected by COVID-19 (30% vs 12%, P < 0.0001)[43]. In addition, a report from the European Liver Transplant Association of 103 LT recipients affected by COVID-19 showed a significantly higher mortality rate in recipients older than 60 years[44].
Thus, since patients with CLD, especially those with cirrhosis, are a high-risk group for severe COVID-19, aggressive vaccination of this patient group is most important for the effective management of SARS-CoV-2 infection.
Given the increased severity and mortality rates of COVID-19 in patients with liver disease, we need to understand the efficacy and safety of vaccination in this population. However, data regarding the efficacy and safety of COVID-19 vaccination in patients with CLD is limited. Previous studies on vaccine efficacy and safety in patients with liver disease that have been reported to date are shown in Table 2[45-65]. It should be noted that most of the reports in Table 2 do not take into account the influence of the omicron variant, which is a limitation of using previous data in the current clinical environment of omicron variant predominance.
| Ref. | Design | Vaccine | Country/region | Number of participants | Value | Major findings (efficacy) | Major findings (safety) |
| Cirrhosis | |||||||
| John et al[45], 2021 | Multicentre retrospective cohort study | BNT162b2 and mRNA-1273 | United States | Cirrhosis group (n = 20037); Control | Efficacy | 64.8% decrease in the development of COVID-19 infection after the first dose and a 78.6% decrease after the second dose | NA |
| Thuluvath et al[46], 2021 | Prospective cohort study | BNT162b2, mRNA-1273, and AZD1222 | United States | LT (n = 62); Cirrhosis (n = 79); CLD (n = 92) | Immunogenicity | Antibody was detectable in 82.2% of LT recipients, 96.2% of cirrhosis and 95.7% of CLD without cirrhosis. 61.3% of LT recipients and 24% CLD with/without cirrhosis had poor antibody responses | No patient had any serious AEs |
| Ruether et al[47], 2022 | Prospective cohort study | BNT162b2, mRNA-1273, and AZD1222 | Germany | LT (n = 138); Cirrhosis (n = 48); Control (n = 52) | Immunogenicity | Immunological response rates were 36.6%, 65.4%, and 100% in LT, cirrhosis, and controls, respectively | NA |
| Willuweit et al[48], 2022 | Prospective cohort study | BNT162b2 | Germany | Cirrhosis (n = 166); Control (n = 79) | Immunogenicity | Antibody was detectable in 96% of cirrhosis and 99% of controls. The median SARS-CoV-2 IgG titer was significantly lower in cirrhosis compared to the controls (939 vs 1905 BAU/mL, P = 0.0001) | NA |
| Wang et al[49], 2022 | Multicentre retrospective cohort study | Inactivated vaccine | China | Compensated-cirrhosis (n = 388); Decompensated cirrhosis (n = 165) | Immunogenicity | Antibodies were detectable in 71.6% and 66.1% in compensated-cirrhosis and decompensated-cirrhosis | The vaccines were well tolerated, most AEs were mild and transient |
| Ai et al[50], 2022 | Multicentre prospective cohort study | Inactivated vaccine | China | CLD (n = 284); Compensated cirrhosis (n = 123); Decompensated cirrhosis (n = 30) | Immunogenicity | Antibody detection rates were 76.8% in noncirrhotic CLD group, 78.9% in compensated cirrhotic group, 76.7% in decompensated cirrhotic group, and 90.3% in controls (P = 0.008 vs CLD) | There was no significant difference in AE among subgroups |
| Liver transplant recipients | |||||||
| Rabinowich et al[51], 2021 | Multicentre retrospective cohort study | BNT162b2 mRNA vaccine | Israel | LT patients (n = 80); Control (n = 25) | Immunogenicity | Immunogenicity among LT recipients was significantly lower [47.5% (LT) vs 100% (control), P < 0.001] | No patient had any serious AEs |
| Herrera et al[52], 2021 | Multicentre prospective cohort study | mRNA-1273 | Spain | LT recipients (n = 58) | Immunogenicity | 93% of patients developed immunologic responses to mRNA-1273 vaccine | No serious AEs were reported in LT recipients |
| Strauss et al[53], 2021 | Multicentre retrospective cohort study | BNT162b2 and mRNA-1273 | United States | LT recipients (n = 161) | Immunogenicity | Antibody was detectable in 34% (95%CI: 27%-42%) of participants after first dose, and in 81% (95%CI: 74%-87%) after second dose | NA |
| Nazaruk et al[54], 2021 | Retrospective cohort study | BNT162b2 mRNA vaccine | Poland | LT recipients (n = 65) | Immunogenicity | Antibody detection rate was 88.9% in LT recipients after the second dose | NA |
| Timmermann et al[55], 2021 | Retrospective cohort study | mRNA vaccines | Germany | LT recipients (n = 118) | Immunogenicity | The seroconversion rate was 78.0% in LT recipients. MMF for immunosuppression was risk factors for seronegativity | NA |
| D'Offizi et al[56], 2022 | Retrospective cohort study | BNT162b2 and mRNA-1273 | Italy | LT patients (n = 61); Control (n = 51) | Immunogenicity | Immunological response rates 2 wk after 2nd dose were 47.5% (LT) and 100% (control) (P < 0.001) | NA |
| John et al[57], 2022 | Multicentre retrospective cohort study | BNT162b2 and mRNA-1273 | United States | LT patients (n = 1133); Control (n = 791) | Efficacy | Vaccination with 2 doses of an mRNA vaccine was associated with a 64% decrease in COVID-19 infection and 87% decrease in COVID-19–related death in LT recipients | NA |
| Davidov et al[58], 2022 | Retrospective cohort study | BNT162b2 mRNA vaccine | Israel | LT patients (n = 76); Control (n = 174) | Immunogenicity | Immunological response rates 2 wk after 2nd dose were 72.0% (LT) and 94.2% (control) (P < 0.001) | AEs were reported by 51% LT recipients. No serious events were reported |
| Sakai et al[59], 2022 | Retrospective cohort study | BNT162b2 | Japan | LT patients (n = 56); Control (n = 42) | Immunogenicity | LT recipients showed a lower seroconversion rate (44/56; 78.6%) than healthy controls (41/42; 97.6%) | NA |
| Calleri et al[60], 2022 | Retrospective cohort study | BNT162b2 and mRNA-1273 | Italy | Pre-LT patients (n = 89) | Immunogenicity | In the 89 pre-LT patients, seroconversion rate was 94.4% (23 d after vaccination), and 92.0% (68 d after vaccination) | No serious AEs were reported in participants |
| Viral hepatitis and NAFLD | |||||||
| Xiang et al[61], 2021 | Retrospective cohort study | Inactivated vaccine | China | CHB patients (n = 149) | Immunogenicity | The seroconversion rate was 87.2% in CHB | No serious AEs were reported in participants |
| He et al[62], 2022 | Cross-sectional observational study | Inactivated vaccine | China | CHB patients (n = 362); Control (n = 87) | Immunogenicity | The seroconversion rates of SARS-CoV-2 antibodies were similar between CHB patients and healthy controls | The incidence was similar between CHB patients and controls. No serious AE |
| Wang et al[63], 2021 | Multicentre retrospective cohort study | Inactivated vaccine | China | NAFLD patients (n = 381) | Immunogenicity | The inactivated COVID-19 vaccine was good immunogenicity (95.5%) in patients with NAFLD | AEs within 7 d and within 28 d totaled 95 (24.9%) and 112 (29.4%), respectively. No serious AEs were recorded |
| Autoimmune liver disease | |||||||
| Duengelhoef et al[64], 2022 | Prospective cohort study | BNT162b2, mRNA-1273, and AZD1222 | Germany | AIH (n = 103); PSC (n = 64); PBC (n = 61); Control (n = 95) | Immunogenicity | Seroconversion was measurable in 97% of AIH and 99% of PBC/PSC patients, respectively. In 14% of AIH patients antibody levels were lower compared to PBC/PSC or controls | NA |
| Schneider et al[65], 2022 | Prospective cohort study | BNT162b2 mRNA vaccine | Austria | AIH (n = 12); Control (n = 24) | Immunogenicity | Patients of AIH and healty controls acquired sufficient antibodies after third vaccination | NA |
Given the higher COVID-19 related mortality in individuals with decompensated cirrhosis, it is important to prioritize vaccination in this group. Patients with cirrhosis have previously shown hyporesponsiveness to hepatitis B virus and pneumococcal vaccines[66,67], and were also considered to be less responsive to COVID-19 vaccines[68]. Indeed, cirrhosis has been reported to be a high-risk factor (3.0-fold) for COVID-19 related deaths after COVID-19 mRNA vaccination in a large United Kingdom cohort study. A defect in acquired immunity in patients with cirrhosis probably predicts a low response to vaccination in this patient population[69]. However, a recent study in the United States found that 75% of CLD patients without cirrhosis and 77% of those with cirrhosis had adequate antibody responses to COVID-19 vaccination[46]. In that study, antibody responses to the BNT162b2 mRNA vaccine and mRNA-1273 mRNA vaccine were favorable (64.4% and 76.4%, respectively), whereas those to the Ad26.COV2.S vaccine (Johnson & Johnson) were low (15.8%). In a study analyzing the immune response of 110 patients with cirrhosis after two doses of the BNT162b2 mRNA vaccine, while the antibody initial acquisition rate was 96%, which was not significantly different from that in the control group (99%) (P = 0.04), the antibody titer showed a rapid and significant reduction in patients with cirrhosis[48]. This suggests that although the initial results after vaccination with the COVID-19 BNT162b2 vaccine are favorable in patients with cirrhosis, it should be noted that the antibody response deteriorates rapidly over time, and, hence, the timing of the booster shot needs to be addressed in the near future.
The immunogenicity of COVID-19 vaccines in patients with cirrhosis appears to be inferior to that in healthy individuals, although real-world cohort studies showed that BNT162b2 mRNA and mRNA-1273 vaccines reduce the development of COVID-19 infection by 64.8% after the first dose and 78.6% after the second dose[45]. Furthermore, a recent report on BNT162b2 mRNA, mRNA-1273, and Ad26.COV2.S vaccines has shown significantly reduced COVID-19-related mortality in vaccinated patients with cirrhosis (HR: 0.21; 95%CI: 0.11-0.42)[70]. In the report on the Sinovac-CoronaVac vaccine, the immunogenicity in patients with CLD was lower (77.3%) compared to that in healthy controls (90.3%), although the presence or absence of cirrhosis in CLD patients did not affect the antibody retention rate [non-cirrhotic CLD (76.8%), compensated cirrhosis (78.9%), and decompensated cirrhosis (76.7%)]. The safety in each group was as good as in healthy controls in that report [healthy controls (16.0%), non-cirrhotic CLD (15.5%), compensated cirrhosis (16.3%), and decompensated cirrhosis (20.0%)][50]. In a study examining the efficacy of the Ad26.COV2.S vaccine, the vaccine efficacy in patients with cirrhosis was 64% (OR: 0.36; 95%CI: 0.20-0.62, P = 0.005), which was non-inferior to the results of a phase 3 trial[71].
Regarding safety, Cao et al[72] reported that of 85 patients with decompensated cirrhosis who received at least one dose of a SARS-CoV-2 vaccine, only one patient (1.2%) had an adverse reaction requiring hospitalization[72]. Bakasis et al[73] also reported that there was no significant difference in the safety of mRNA vaccines given to 87 patients with liver diseases including cirrhosis, and 40 controls[73]. The limitations of these studies[50,71-73], however, include a relatively small sample size of participants who received the vaccines and a short follow-up period. Research with larger sample sizes is, thus, required in the future.
Hence, although each vaccine is slightly inferior in immunogenicity in patients with cirrhosis compared with healthy individuals, they can be safely used with adequate efficacy in patients with cirrhosis. However, there is insufficient data to allow recommendation of one vaccine over another.
COVID-19 outcomes in LT recipients are not necessarily worse than those in the general population, although they have a higher rate of admission to the intensive care unit. Thus, COVID-19 vaccination should be prioritized for these patients, since the benefits far outweigh the potential risks. Vaccination for LT recipients is an interesting area of research, with a series of reports on its efficacy and safety.
Several reports of low humoral and cellular responses after COVID-19 vaccination in LT recipients suggest that vaccine-induced immunity in this patient subgroup is lower than in the general population. As shown in Table 2, LT recipients exhibit significantly attenuated humoral and cellular immunity 2 wk after mRNA vaccination compared to healthy individuals[56,58], lower seroconversion rates[59] and significantly lower immunogenicity[51]. Indeed, combination immunosuppressive therapy, including mofetil mycophenolate, is reportedly a predictor of reduced responsiveness to vaccination[55,58,59]. The results of such vaccine responses in LT recipients were similar to the results of other studies in kidney transplant recipients[74], lung transplant recipients[75], and allogeneic hematopoietic stem-cell transplant recipients[76]. On the other hand, a recent multicenter cohort study showed that mRNA vaccines reduce SARS-CoV-2 infection rate, occurrence of symptomatic COVID-19, and mortality in LT recipients as well as in patients with cirrhosis[70]. While the immunogenicity of mRNA vaccines in LT recipients appears to be attenuated compared with that in healthy individuals, the disease status of COVID-19 is greatly attenuated in vaccinated LT recipients compared to unvaccinated recipients. Additionally, the value and safety of routine immunization in liver transplant recipients has been well established, and current guidelines recommend pre-transplant vaccination whenever possible[77]. However, since LT recipients also show a rapid decrease in antibody titers after mRNA vaccination[59], and because booster doses are reportedly extremely safe in post-organ transplant patients [78], it is recommended that all LT recipients receive a third or fourth booster dose if they have low or insufficient antibody titers[47].
HCC patients are considered a high risk group for severe COVID-19[34]. Nevertheless, there are no cohort studies investigating the immunogenicity and safety of COVID-19 vaccines in patients with HCC. According to the American Association for the Study of Liver Diseases, patients with HCC receiving locoregional or systemic therapy should also be considered for vaccination without interruption of treatment[79]. Most patients treated for solid tumors, including HCC, show an adequate humoral response against SARS-CoV-2 after two vaccine doses. However, vaccination during chemotherapy tends to be associated with lower antibody levels, resulting in a suboptimal response in a small percentage of patients[80,81]. Furthermore, the concentration of neutralizing antibodies decreases over time, further reducing immunity[82]. Based on these reports, many countries are prioritizing a third vaccine dose in patients with solid tumors.
With regard to viral hepatitis and NAFLD, several cohort studies evaluating the efficacy of inactivated vaccines have been reported from China. Xiang et al[61] analyzed the seroprevalence of anti-spike protein antibodies and neutralizing antibodies in chronic hepatitis B (CHB) patients who received two doses of inactivated vaccines, and reported high seropositivity rates of 87.2% and 74.5%, respectively[61]. This report showed that nucleotide analog therapy has no effect on vaccine-induced immune responses, suggesting that vaccination should be performed even during treatment of CHB. He et al[62] also reported that seroconversion rates of SARS-CoV-2 antibodies after 1, 2, and 3 mo in patients with CHB who received an inactivated vaccine were comparable to those in healthy controls[62]. No serious adverse reactions were reported in either study.Next, in a multicenter study of the safety and immunogenicity of inactivated vaccines in 381 individuals with NAFLD, the incidence of adverse reactions within 7 d and 28 d after vaccination was 24.9% and 29.4%, respectively. Neutralizing antibodies were detectable in 364 (95.5%) patients, and titers of neutralizing antibodies were shown to persist for a long time[63]. Given that NAFLD is a risk factor for severe COVID-19[37-39], active vaccination of this patient population would be ideal.
There have been two reports on the immunogenicity of COVID-19 vaccines in patients with AIH. Duengelhoef et al[64] reported that 91 of 94 (97%) AIH patients who received the second dose of a vaccine achieved seroconversion, although AIH patients showed impaired spike-specific T-cell responses and lower antibody levels at 7 mo compared with healthy individuals or patients with primary biliary cholangitis (PBC)/primary sclerosing cholangitis (PSC) (641 vs 1020 vs 1200 BAU/mL, respectively). These results were not related to the use of immunosuppressive medications, suggesting that the underlying immune abnormality of AIH might be involved in this diminished response[64]. In addition, a study that followed the antibody responses after mRNA vaccination in patients with AIH and healthy controls showed that anti-SARS-CoV-2 antibodies were sufficiently produced after the second and third vaccinations in both groups[65]. Therefore, early booster doses of vaccines should be considered in patients with AIH. Although none of the patients in these reports had serious adverse reactions, it is noteworthy that several case reports have been published reporting an increased risk of developing AIH-like syndromes after SARS-CoV-2 vaccination.
Vaccination in patients with liver diseases is generally considered safe and effective and should be strongly recommended. The common side effects of COVID-19 vaccination include injection site pain, fever, headaches, and fatigue. All these side effects are mild and typically resolve 1-3 d after vaccination. However, recent worldwide dissemination of COVID-19 vaccines and post-marketing surveillance have led to an increasing number of reports of several organ-specific immune-related diseases, including myocarditis, immune thrombotic thrombocytopenia, Guillain-Barré syndrome, and pancreatitis, among others[11,83-85]. It is speculated that an abnormal immune response following vaccination is involved in the development of such diseases. Acute liver injury was not previously reported in clinical trials on COVID-19 vaccination because the sample size was insufficient to detect rare adverse events after vaccination. Recently, a large-scale population-based study on acute liver injury occurring after COVID-19 vaccination was reported from Hong Kong. In that study, among 2343288 COVID-19 vaccine recipients, acute liver injury within 56 d after the first and second vaccine dose occurred in 307 and 521 (335 and 334 per 100000 person-years) individuals vaccinated with BNT162b2, and 304 and 474 (358 and 403 per 100000 person-years) of those who received CoronaVac. The incidence of acute liver injury within 56 d of SARS-CoV-2 infection, on the other hand, was 32997 cases per 100000 person-years, indicating that the incidence of acute liver injury after COVID-19 vaccination was much lower than that after SARS-CoV-2 infection. It was also concluded that compared to the non-exposure period, no increased risk of acute liver injury was observed in the 56-d risk period following the first (IRR: 0.800; 95%CI: 0.680-0.942) and second (IRR: 0.944; 95%CI: 0.816-1.091) BNT162b2 dose, and the first (IRR: 0.689; 95%CI: 0.588-0.807) and second (IRR: 0.905, 95%CI: 0.781-1.048) CoronaVac dose. Thus, COVID-19 vaccines do not seem to increase the risk of acute liver injury[86]. However, since there have been case reports of acute liver injury requiring hospitalization after COVID-19 vaccination, this is an adverse effect that should not be overlooked. The current review aimed to increase awareness of this rare adverse effect to promote its early recognition.Review of case reports on liver injury after COVID-19 vaccinationSince the summer of 2021, several case reports of liver injury after COVID-19 vaccination have been reported. The clinical and histological findings of most patients resembled AIH and the reported cases responded well to corticosteroid therapy. Previous cases of AIH-like acute liver injury after COVID-19 vaccinations are shown in Table 3[87-109]. Twenty-three reports (28 cases) of acute liver injury secondary to COVID-19 vaccines have been published in the PubMed database as of July 2022 (Table 3). The median age at the time of diagnosis was 61 (range 27-80) years, with a predominance of women (79%, females: 22, males: 6). Eight patients (29%) had no underlying disease, whereas nine patients (32%) had been diagnosed with other immune disorders (Hashimoto’s disease: 5, PSC: 2, PBC: 1, sarcoidosis: 1). One patient was three months postpartum, and another patient was taking hormonal therapy due to menstrual irregularities. Liver injury occurred after vaccination following the BNT162b2 vaccine in 11 (39%) of the cases, mRNA-1273 vaccine in nine (32%), ChAdOx1 nCoV-19 vaccine in seven (25%), and CoronaVac vaccine in one case (4%). Seventeen patients (61%) developed liver injury after the first dose, 10 patients (36%) after the second dose, and two patients (7%) after the booster shot. The median time from vaccination to the onset of acute liver injury was 11 (range 3-35) d. The most common symptom was jaundice, while other symptoms, such as fatigue and anorexia, were also frequently observed. The most common pattern of liver injury was hepatocellular injury, with transaminase levels exceeding 1000 U/L in many cases. The mean alanine aminotransferase level was 848.2 (± 465.0) U/L, mean aspartate aminotransferase level was 1031.5 (± 578.3) U/L, and mean total bilirubin level was 9.08 (± 5.76) mg/dL. Serum immunoglobulin G (IgG) levels were measured in 25 patients and were elevated in 22 patients (88%). Anti-nuclear antibodies (ANA) were positive in 22 (82%) of 27 patients, while anti-smooth muscle antibodies and anti-mitochondrial antibodies (AMA) were elevated in some cases. Liver biopsy was performed in all cases. According to the simplified international diagnostic criteria published by the international AIH-group, seven cases were “typical” of AIH and 20 cases were “compatible with” AIH. Only one patient had poor findings of typical AIH and was diagnosed with drug-induced liver injury. Steroids as first-line treatment were used in 26 of the patients (93%), of whom 10 received prednisone, 14 received prednisolone, one received budesonide, and one was given methyl-prednisolone intravenously. Azathioprine was concomitantly used in five patients. The overall outcomes with corticosteroid therapy were favorable, and improvement was seen in 27 patients (96%). Only one male patient reported from India progressed to liver failure and died despite five cycles of plasma exchange[99].
| Ref. | Age/sex | Past history | Vaccine | Onset | AST/ALT (U/ L) | Total bilirubin (mg/ dL) | IgG | Antibody | Biopsy | Diagnose | Treatment | Outcome |
| Bril et al[87] | 35/F | Third month postpartum | BNT162b2 | 7 d after the1st dose | 754/2001 | 4.8 | Normal | ANA: 1:1280 | Interface hepatitis, rosette formation, eosinophil infiltration | Typical for AIH | Prednisone (20 mg/d) | Improved |
| Lodato et al[88] | 43/F | Dyslipidemia | BNT162b2 | 15 d after the 1st dose | 52/51 | 17.54 | Normal | ANA: negative | Moderate portal inflammatory infiltrate with interface hepatitis, biliary injury | Compatible with AIH | Methyl-prednisolone (1 mg/kg/d) | Improved |
| Vuille-Lessard et al[89] | 76/F | Hashimoto's disease, urothelial carcinoma | mRNA-1273 | 3 d after the 1st dose | 811/579 | 3.8 | Increased | ANA: 1:1280, AMA: 1:1280 | Interface hepatitis, plasma cells infiltration, pseudorosettes | Compatible with AIH | Prednisolone (40 mg/d) + azathioprine | Improved |
| Londoño et al[90] | 41/F | Premature ovarian failure | mRNA-1273 | 7 d after the 2nd dose | 993/1312 | 2.3 | Increased | ANA: 1:80 | Interface hepatitis with a lymphoplasmacytic infiltrate | Typical for AIH | Prednisone (1 mg/kg) | Improved |
| Rocco et al[91] | 80/F | Hashimoto's disease | BNT162b2 | 7 d after the 3rd dose | 1401/1186 | 10.5 | Increased | ANA: 1:160 | Interface hepatitis with a lymphoplasmacytic infiltrate | Typical for AIH | Prednisone | Improved |
| McShane et al[92] | 71/F | Osteoarthritis | mRNA-1273 | 4 d after the 1st dose | -/1067 | 15.7 | Increased | ASMA: 1:2560 | Interface hepatitis, eosinophil infiltration | Compatible with AIH | Prednisolone (40 mg/d) | Improved |
| Clayton-Chubb et al[93] | 36/M | Hypertension | AZD1222 | 26 d after the 1st dose | 633/1774 | 9.9 | Normal | ANA: 1:160 | Interface hepatitis | Compatible with AIH | Prednisolone (60 mg/d) | Improved |
| Tan et al[94] | 56/F | None | mRNA-1273 | 35 d after the 1st dose | 1124/1701 | 5.9 | Increased | ANA: positive, ASMA: Positive | Interface hepatitis, rosette formation, eosinophil infiltration | Compatible with AIH | Budesonide | Improved |
| Ghielmetti et al[95] | 63/M | Type 2 diabetes, ischemic heart disease | mRNA-1273 | 7 d after the 1st dose | 1127/1038 | 11.9 | Increased | ANA: 1:640 | Inflammatory portal infiltrate with interface hepatitis | Typical for AIH | Prednisone (40 mg/d) | Improved |
| Zhou et al[96] | 36/F | Ulcerative colitis, primary sclerosing cholangitis | mRNA-1273 | 11 d after the 1st dose | 581/588 | 1.4 | Increased | ANA: 1:2560 | Interface hepatitis with a lymphoplasmacytic infiltrate, rosette, eosinophil | Compatible with AIH | Prednisone (50 mg/d) | Improved |
| Garrido et al[97] | 65/F | JAK2 V617F-positive polycythemia | mRNA-1273 | 14 d after the 1st dose | 1056/1092 | 1.1 | Increased | ANA: 1:100 | Interface hepatitis | Compatible with AIH | Prednisolone (60 mg/d) | Improved |
| Goulas et al[98] | 52/F | None | mRNA-1273 | 14 d after the 1st dose | 350/936 | 9.06 | Increased | ANA: 1:320, ASMA: positive | Portal, periportal inflammation, rosette formation | Typical for AIH | Prednisolone (50 mg/d) + azathioprine | Improved |
| Rela et al[99] | 38/F | Hypothyroidism | AZD1222 | 20 d after the 1st dose | 1101/1025 | 14.9 | Increased | ANA: positive | Multiacnar hepatic necrosis and periportal neocholangiolar proliferation | Compatible with AIH | Prednisolone (30 mg/d) | Improved |
| Rela et al[99] | 62/M | None | AZD1222 | 13 d after the 2nd dose | 1361/1094 | 19.2 | NA | ANA: negative | Portal neocholangiolar proliferation and mild to moderate inflammation | Compatible with AIH | Prednisolone (30 mg/d) + plasma exchange | Death |
| Palla et al[100] | 40/F | Sarcoidosis | BNT162b2 | 28 d after the 2nd dose | 4 times upper limit of normal | NA | Increased | ANA: 1:640 | Interface hepatitis with plasma cells infiltration | Compatible with AIH | Prednisolone (40 mg/d) | Improved |
| Mann et al[101] | 61/F | Irritable bowel disease, cholecystectomy | BNT162b2 | 9 d after the 2nd dose | 37/37 | 6.2 | NA | ANA: negative | Scattered inflammatory cells consisting of lymphocyte and few eosinophils | Drug induced liver injury | Conservative treatment | Improved |
| Avci et al[102] | 61/F | Hashimoto's disease, hypertension | BNT162b2 | 28 d after the 1st dose | 455/913 | 11.8 | Increased | ANA: 1:100, ASMA: 1:100 | Interface hepatitis | Compatible with AIH | Prednisolone (40 mg/d) + azathioprine | Improved |
| Torrente et al[103] | 46/F | Hypothyroidism, anemia | AZD1222 | 21 d after the 1st dose | 241/353 | Normal | Increased | ANA: 1:160 | Lymphoplasmacytic portal infiltrate | Compatible with AIH | Prednisone (30 mg/d) + azathioprine | Improved |
| Ghorbani et al[104] | 62/M | None | Corona Vac | 3 d after the 2nd dose | 722/435 | 8 | NA | ANA: negative, ASMA: negative | Interface hepatitis, infiltration of lymphocytes and eosinophils in portal tract | Compatible with AIH | Ursodeoxycholic acid | Improved |
| Kang et al[105] | 27/F | None | BNT162b2 | 7 d after the 2nd dose | 1004/1478 | 8.6 | Increased | ANA: 1:80 | Interface hepatitis with a lymphoplasmacytic infiltrate, rosette, eosinophil | Typical for AIH | Prednisolone (40 mg/d) | Improved |
| Camacho-Domínguez et al[106] | 79/M | None | AZD1222 | 15 d after the 1st dose | 2003/1994 | 11.9 | Increased | ANA: 1:80 | Interface hepatitis with a lymphocytic infiltrate, eosinophil | Compatible with AIH | Prednisone (30 mg/d) + azathioprine | Improved |
| Shahrani et al[107] | 59/F | Dyslipidemia | AZD1222 | 12 d after the 2nd dose | 962/1178 | 7.5 | Increased | NA | Lympho-plasmacellular portal infiltrate | Compatible with AIH | Prednisolone (40 mg/d) | Improved |
| Shahrani et al[107] | 63/F | Ulcerative colitis, primary sclerosing cholangitis | AZD1222 | 14 d after the 1st dose | 505/354 | 18.6 | Increased | ANA: positive | Interface hepatitis | Compatible with AIH | Prednisolone (40 mg/d) | Improved |
| Shahrani et al[107] | 72/F | None | BNT162b2 | 10 d after boostershot | 1452/2280 | 1.7 | Increased | ANA: negative, AMA: positive | Infiltration of lymphocytes and plasma cells in portal tract | Compatible with AIH | Prednisolone (40 mg/d) | Improved |
| Zin Tun et al[108] | 47/M | None | mRNA-1273 | 3 d after the 1st dose; A few days after the 2nd dose | NA/1048 | 11.3 | Increased | ANA: positive | Interface hepatitis with a lymphoplasmatic infiltrate, rosette, emperipolesis | Typical for AIH | Prednisolone (40 mg/d) | Improved |
| Suzuki et al[109] | 80/F | Gastroesophageal reflux esophagitis | BNT162b2 | 10 d after the 2nd dose | 995/974 | 3.5 | Increased | ANA: 1:40 | Lymphoplasmacytic infiltration in the portal area, interface hepatitis | Compatible with AIH | Prednisone (0.8 mg/kg/d) | Improved |
| Suzuki et al[109] | 75/F | Dyslipidemia | BNT162b2 | 4 d after the 2nd dose | 1085/820 | 17.7 | Increased | ANA: 1:80 | Lymphoplasmacytic infiltration in the portal area, interface hepatitis | Compatible with AIH | Prednisone (1.0 mg/kg/d) | Improved |
| Suzuki et al[109] | 78/F | Primary biliary cholangitis | BNT162b2 | 7 d after the 1st dose | 401/542 | 1.3 | Increased | ANA: 1:80 | Lymphoplasmacytic infiltration in the portal area, interface hepatitis | Compatible with AIH | Prednisone (0.6 mg/kg/d) | Improved |
All of the patients developed acute liver injury soon after COVID-19 vaccination, with findings consistent with AIH on liver biopsy, and responded well to corticosteroids. These case reports strongly suggest that the association between vaccination and the onset of AIH-like liver injury might be more than coincidental. However, it is difficult to establish a causal relationship between vaccination and liver injury with certainty. Indeed, post-pregnancy status, use of statins, and concomitant history of autoimmune diseases included in the reported cases are likely major confounding factors. It should be noted that almost all the reported cases lacked pre-vaccination laboratory data, and hence, the presence of pre-existing hepatitis cannot be ruled out. Indeed, Cao et al[110] reported that a patient with vaccine-induced AIH had advanced fibrosis on liver biopsy, suggesting the presence of CLD prior to vaccination[110].
Furthermore, a large international case series that provided evidence for the hepatotoxic potential of COVID-19 vaccines has recently been reported[111]. In that study, data from 18 countries on 87 patients who developed liver injury after COVID-19 vaccination were retrospectively collected. The median age at diagnosis was 48 (range 18-79) years and 63% were female. The median time from vaccination to the onset of liver injury was 15 (range 3-65) d. Liver injury occurred after vaccination with BNT162b2 in 59% of the cases, mRNA-1273 in 18% and ChAdOx1 nCoV-19 in 23%. When elevated IgG and autoantibody positivity were used to define immune-mediated hepatitis, 57% of the patients had immune-mediated hepatitis. Corticosteroids were mainly used in cases of severe liver injury and immune-mediated hepatitis (53%). In this study, there was one case of liver failure requiring LT, while the remaining cases had a good prognosis. There were no differences in the severity of liver injury, the rate of immune-mediated hepatitis, or the rate of steroid usage depending on the type of vaccine. Responses to treatment and outcomes were favorable in all groups. These results were generally consistent with the characteristics of the previous case reports shown in Table 3.
There is also a concern that hepatitis might be exacerbated by vaccination of patients originally diagnosed with AIH. Shroff et al[112] reported that six of 16 patients who developed vaccine-induced liver injury previously had AIH. They concluded that hepatotoxicity could be induced after vaccination by autoimmune induction[112]. However, the number of patients described in previous reports[87-109] (Table 3) is extremely small compared with the overall vaccinated population worldwide. The current review, thus, aimed to increase awareness about this rare adverse effect in order to promote its early recognition.
The mechanisms underlying the development of liver injury following COVID-19 vaccination remain unclear. As shown in Table 3, liver injury following COVID-19 vaccination is clinically and pathologically similar to AIH, suggesting that immune abnormalities associated with vaccination contribute to its development. However, the association between vaccines and the development of autoimmune diseases is controversial and most studies related to this have been inconclusive[113].
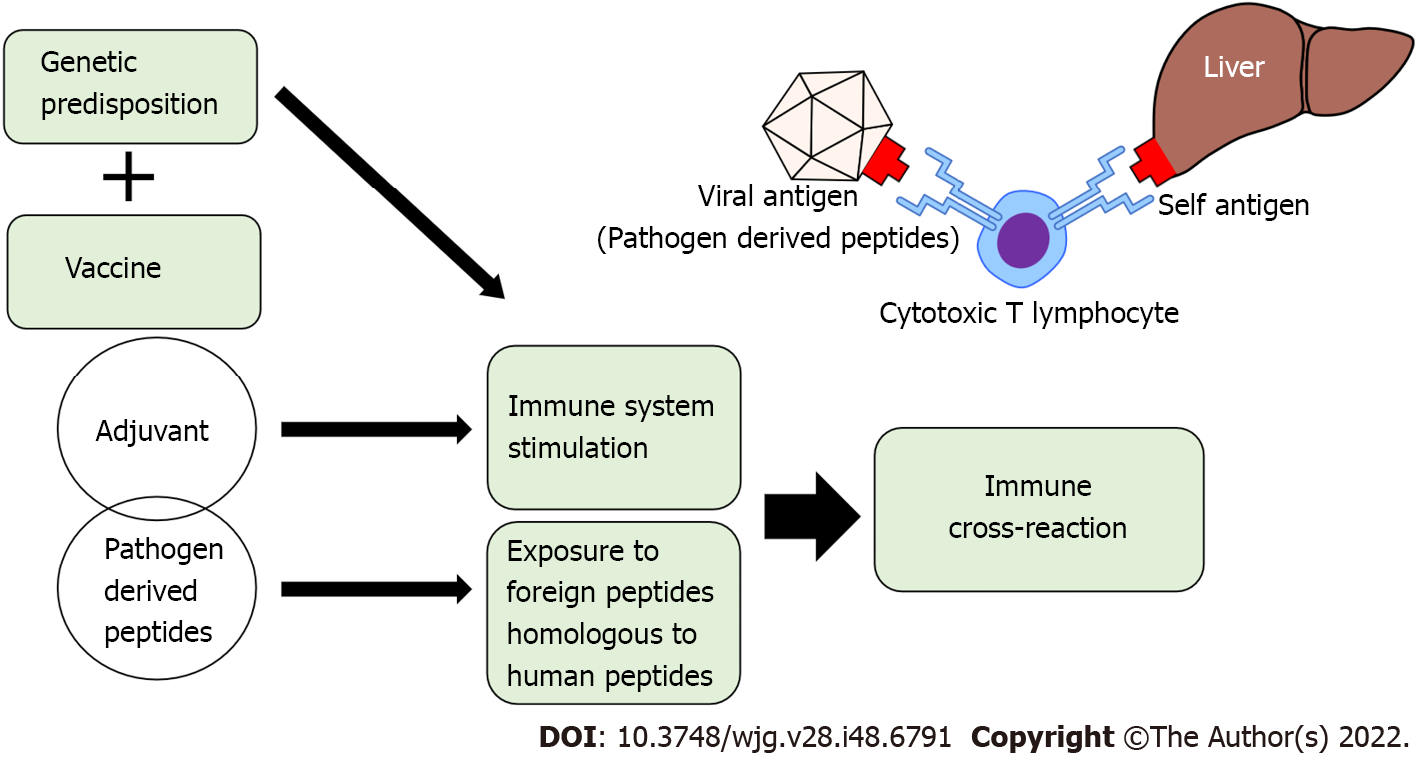
Molecular mimicry and bystander activation have been hypothesized as possible mechanisms by which vaccines can trigger autoimmune reactions. In the antigen-specific mechanism of molecular mimicry, it is hypothesized that similarities between certain pathogenic elements contained in the vaccine and specific human proteins cause cross-reactions. It is believed that the injurious antibodies produced by this mechanism destroy human proteins and cause organ damage[114]. In support of this hypothesis, it has been reported that antibodies against the spike protein of SARS-CoV-2 have cross-reactivity against many human tissue antigens[115]. Although the target antigen of the autoimmune response in hepatocytes and specific autoantibodies in AIH have not yet been identified, Vojdani et al[115] reported that anti-SARS-CoV-2 protein antibodies cross-react with liver microsomal antigen, pyruvate dehydrogenase peptide E2, and mitochondrial M2 antigen[115]. It has also been shown that anti-SARS-CoV-2 spike protein antibodies cross-react with human tissue antigens, resulting in a marked increase in autoimmune markers such as ANA and AMA[116]. This suggests that COVID-19 vaccination might induce autoimmune reactions based on molecular mimicry in liver tissues, resulting in AIH-like liver injury (Figure 1). Bystander activation, on the other hand, is an antigen non-specific mechanism, in which self-antigens are released extracellularly by vaccination and are taken up by antigen-presenting cells. Then, autoreactive T cells are activated by type I interferon (IFN) and recognize the presented self-antigen, which is hypothesized to attack normal cells[117]. Bystander activation has also been proposed as one of the mechanisms of autoimmune disease development after vaccination. Furthermore, there is a rapid increase in type I IFN expression and oxidative stress coupled with effective anti-SARS-CoV-2 neutralizing antibody production after vaccination in healthy individuals[118]. Therefore, the side effects of COVID-19 vaccines are thought to be only a by-product of a transient burst of type I IFN generation with induction of an effective immune response[119]. It has also recently been hypothesized that the COVID-19 vaccine triggers autoimmune diseases via induction of age-associated B cells (ABCs)[120]. The number of ABCs, a rare subset of B cells that express CD11c and T-bet, increases with age in healthy individuals, and is increased early in patients with infectious diseases and autoimmune disorders[121]. In the pathogenesis of autoimmune diseases, ABCs are implicated in generating IgG, in increasing antigen presentation to T cells, and in germinal center formation. Moreover, ABCs are hyper-responsive to Toll-like receptor (TLR) 7 signaling, and are capable of generating autoreactive antibody-secreting plasmablasts. COVID-19 vaccines use TLR7/8 agonists as adjuvants, which might stimulate ABCs, leading to the triggering of post-vaccination autoimmune syndromes[122]. Activation of TLR7 can lead to the production of type I IFN, which is an important cytokine in the development of autoimmune disorders, such as rheumatic diseases and systemic lupus erythematosus[123]. It was previously shown in a mouse model that lipid nanoparticles, which are one of the potent adjuvants of mRNA vaccines, could trigger inflammatory responses. This is characterized by activation of diverse inflammatory pathways, massive neutrophil infiltration, and production of various inflammatory cytokines, including the secretion of interleukin (IL)-1β and IL-6[11,124].
Hence, although several hypotheses have been considered for the mechanism of vaccine-induced autoimmune disease, the exact mechanism remains to be elucidated. In any event, only a very small percentage of vaccinated subjects subsequently developed autoimmune phenomena, suggesting a genetic predisposition to vaccine-induced autoimmune disorders. Further research into the direct associations between vaccines and autoimmune diseases, as well as the biological mechanisms behind them, is warranted.
Since COVID-19 is an infectious disease with a high burden of morbidity and mortality, and that has resulted in a global pandemic, vaccination against COVID-19 is our best strategy for its control. For this, highly effective and safe vaccines are desperately needed. Although various COVID-19 vaccines have been proven to be highly effective and have good safety profiles in healthy populations, data regarding the efficacy and safety of vaccination in special population groups is limited. Thus, we believe it is worthwhile to summarize the efficacy and safety of the COVID-19 vaccines in patients with CLD. Based on the evidence from real-world studies, this review shows that vaccination in patients with CLD is effective and safe, and should be strongly recommended.
As shown in Table 3, although a number of case reports of acute liver injury after COVID-19 vaccination have been reported, their frequency is extremely low. Thus, given the serious health sequalae from COVID-19 in patients with liver disease, the potential benefits of vaccination appear to outweigh the risk of vaccine-related liver injury. However, it is important to remember that most of the studies referred to in this review were conducted in the era before the emergence of new viral variants. Since new SARS-CoV-2 variants are still emerging all over the world, COVID-19 remains a global public health problem. In addition, since vaccines against the mutant viruses are still being developed, it will be necessary to continue evaluating the efficacy and safety of these new vaccines.
Given the increased rates of severity and mortality of COVID-19 in patients with CLD, especially those with cirrhosis, the importance of aggressive vaccination in the effective management of SARS-CoV-2 infection should be emphasized. However, there is insufficient evidence about the immunogenicity and safety of COVID-19 vaccines in patients with CLD. According to the accumulated real-world data on each vaccine, the safety of COVID-19 vaccines in patients with CLD appears to be comparable to that in healthy individuals. Regarding efficacy, the disease behavior of COVID-19 is known to be attenuated in vaccinated compared with unvaccinated patients, including in those with CLD+ADs- however, vaccine-induced immunity seems lower in CLD patients as compared with the general population. Since a rapid decrease in acquired antibodies has also been observed in this patient population, an effective booster shot is desirable, particularly in patients with cirrhosis, LT recipients, and those with HCC.
On the other hand, acute liver injury following COVID-19 vaccination has also been frequently reported. However, recent large cohort studies found no increased risk of liver injury after COVID-19 vaccines. Since acute liver injury after SARS-CoV-2 infection is much more common than after COVID-19 vaccination, the benefits of vaccination might outweigh the risk of liver injury during this pandemic. The reported rare immune-mediated liver injury after COVID-19 vaccination is clinically and pathologically similar to AIH. Although the involvement of abnormalities in the immune system, including molecular mimicry, bystander activation, and induction of ABCs in the pathogenesis of this condition have been pointed out, the relationship between vaccination and acute liver injury is an issue that remains to be clarified in the future. Finally, clinicians should consider the possibility of AIH-like liver injury in patients who present with elevated liver enzymes following COVID-19 vaccination, and treat it with corticosteroids.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Janczewska E, Poland; Khidir KA, Iraq; Kim J, Korea; Li SW, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18875] [Article Influence: 3775.0] [Reference Citation Analysis (7)] |
| 2. | Mahase E. Covid-19: WHO declares pandemic because of "alarming levels" of spread, severity, and inaction. BMJ. 2020;368:m1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 387] [Article Influence: 77.4] [Reference Citation Analysis (0)] |
| 3. | Zhong P, Xu J, Yang D, Shen Y, Wang L, Feng Y, Du C, Song Y, Wu C, Hu X, Sun Y. COVID-19-associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct Target Ther. 2020;5:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (1)] |
| 4. | Chang WT, Toh HS, Liao CT, Yu WL. Cardiac Involvement of COVID-19: A Comprehensive Review. Am J Med Sci. 2021;361:14-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 5. | Dufour JF, Marjot T, Becchetti C, Tilg H. COVID-19 and liver disease. Gut. 2022;71:2350-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 6. | Khandker SS, Godman B, Jawad MI, Meghla BA, Tisha TA, Khondoker MU, Haq MA, Charan J, Talukder AA, Azmuda N, Sharmin S, Jamiruddin MR, Haque M, Adnan N. A Systematic Review on COVID-19 Vaccine Strategies, Their Effectiveness, and Issues. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768-771.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 8. | Ge J, Pletcher MJ, Lai JC; N3C Consortium. Outcomes of SARS-CoV-2 Infection in Patients With Chronic Liver Disease and Cirrhosis: A National COVID Cohort Collaborative Study. Gastroenterology. 2021;161:1487-1501.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 9. | Ossato A, Tessari R, Trabucchi C, Zuppini T, Realdon N, Marchesini F. Comparison of medium-term adverse reactions induced by the first and second dose of mRNA BNT162b2 (Comirnaty, Pfizer-BioNTech) vaccine: a post-marketing Italian study conducted between 1 January and 28 February 2021. Eur J Hosp Pharm. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 10. | Amodio E, Minutolo G, Casuccio A, Costantino C, Graziano G, Mazzucco W, Pieri A, Vitale F, Zarcone M, Restivo V. Adverse Reactions to Anti-SARS-CoV-2 Vaccine: A Prospective Cohort Study Based on an Active Surveillance System. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Chen Y, Xu Z, Wang P, Li XM, Shuai ZW, Ye DQ, Pan HF. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165:386-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 322] [Article Influence: 80.5] [Reference Citation Analysis (0)] |
| 12. | Zhou Z, Zhu Y, Chu M. Role of COVID-19 Vaccines in SARS-CoV-2 Variants. Front Immunol. 2022;13:898192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 13. | Teo SP. Review of COVID-19 mRNA Vaccines: BNT162b2 and mRNA-1273. J Pharm Pract. 2022;35:947-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 110] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 14. | Wadhwa A, Aljabbari A, Lokras A, Foged C, Thakur A. Opportunities and Challenges in the Delivery of mRNA-based Vaccines. Pharmaceutics. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 355] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 15. | Versteeg L, Almutairi MM, Hotez PJ, Pollet J. Enlisting the mRNA Vaccine Platform to Combat Parasitic Infections. Vaccines (Basel). 2019;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Türeci Ö, Tompkins KR, Walsh EE, Frenck R, Falsey AR, Dormitzer PR, Gruber WC, Şahin U, Jansen KU. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1003] [Cited by in RCA: 1054] [Article Influence: 210.8] [Reference Citation Analysis (0)] |
| 17. | Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R, Swanson KA, Li P, Koury K, Kalina W, Cooper D, Fontes-Garfias C, Shi PY, Türeci Ö, Tompkins KR, Lyke KE, Raabe V, Dormitzer PR, Jansen KU, Şahin U, Gruber WC. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med. 2020;383:2439-2450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1747] [Cited by in RCA: 1902] [Article Influence: 380.4] [Reference Citation Analysis (0)] |
| 18. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 10698] [Article Influence: 2139.6] [Reference Citation Analysis (1)] |
| 19. | Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, Bailey R, Swanson KA, Xu X, Roychoudhury S, Koury K, Bouguermouh S, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Yang Q, Liberator P, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Gruber WC, Jansen KU; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N Engl J Med. 2021;385:1761-1773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 937] [Cited by in RCA: 1037] [Article Influence: 259.3] [Reference Citation Analysis (0)] |
| 20. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7073] [Cited by in RCA: 7565] [Article Influence: 1891.3] [Reference Citation Analysis (1)] |
| 21. | Muik A, Lui BG, Wallisch AK, Bacher M, Mühl J, Reinholz J, Ozhelvaci O, Beckmann N, Güimil Garcia RC, Poran A, Shpyro S, Finlayson A, Cai H, Yang Q, Swanson KA, Türeci Ö, Şahin U. Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science. 2022;375:678-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 281] [Article Influence: 93.7] [Reference Citation Analysis (0)] |
| 22. | Doria-Rose NA, Shen X, Schmidt SD, O'Dell S, McDanal C, Feng W, Tong J, Eaton A, Maglinao M, Tang H, Manning KE, Edara VV, Lai L, Ellis M, Moore K, Floyd K, Foster SL, Atmar RL, Lyke KE, Zhou T, Wang L, Zhang Y, Gaudinski MR, Black WP, Gordon I, Guech M, Ledgerwood JE, Misasi JN, Widge A, Roberts PC, Beigel J, Korber B, Pajon R, Mascola JR, Suthar MS, Montefiori DC. Booster of mRNA-1273 Strengthens SARS-CoV-2 Omicron Neutralization. medRxiv. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 23. | Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O'Connell AM, Simons D, Blomquist PB, Zaidi A, Nash S, Iwani Binti Abdul Aziz N, Thelwall S, Dabrera G, Myers R, Amirthalingam G, Gharbia S, Barrett JC, Elson R, Ladhani SN, Ferguson N, Zambon M, Campbell CNJ, Brown K, Hopkins S, Chand M, Ramsay M, Lopez Bernal J. Covid-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N Engl J Med. 2022;386:1532-1546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1597] [Cited by in RCA: 1607] [Article Influence: 535.7] [Reference Citation Analysis (1)] |
| 24. | Zhao J, Zhao S, Ou J, Zhang J, Lan W, Guan W, Wu X, Yan Y, Zhao W, Wu J, Chodosh J, Zhang Q. COVID-19: Coronavirus Vaccine Development Updates. Front Immunol. 2020;11:602256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 25. | Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O'Reilly PJ, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3551] [Cited by in RCA: 3438] [Article Influence: 859.5] [Reference Citation Analysis (0)] |
| 26. | Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, Goepfert PA, Truyers C, Van Dromme I, Spiessens B, Vingerhoets J, Custers J, Scheper G, Robb ML, Treanor J, Ryser MF, Barouch DH, Swann E, Marovich MA, Neuzil KM, Corey L, Stoddard J, Hardt K, Ruiz-Guiñazú J, Le Gars M, Schuitemaker H, Van Hoof J, Struyf F, Douoguih M; ENSEMBLE Study Group. Final Analysis of Efficacy and Safety of Single-Dose Ad26.COV2.S. N Engl J Med. 2022;386:847-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 163] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 27. | Le Gars M, Hendriks J, Sadoff J, Ryser M, Struyf F, Douoguih M, Schuitemaker H. Immunogenicity and efficacy of Ad26.COV2.S: An adenoviral vector-based COVID-19 vaccine. Immunol Rev. 2022;310:47-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, Chen X, Liu X, Jiang C, Li J, Yang M, Song Y, Wang X, Gao Q, Zhu F. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 743] [Cited by in RCA: 965] [Article Influence: 193.0] [Reference Citation Analysis (0)] |
| 29. | Tanriover MD, Doğanay HL, Akova M, Güner HR, Azap A, Akhan S, Köse Ş, Erdinç FŞ, Akalın EH, Tabak ÖF, Pullukçu H, Batum Ö, Şimşek Yavuz S, Turhan Ö, Yıldırmak MT, Köksal İ, Taşova Y, Korten V, Yılmaz G, Çelen MK, Altın S, Çelik İ, Bayındır Y, Karaoğlan İ, Yılmaz A, Özkul A, Gür H, Unal S; CoronaVac Study Group. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 740] [Cited by in RCA: 649] [Article Influence: 162.3] [Reference Citation Analysis (0)] |
| 30. | Sahin TT, Akbulut S, Yilmaz S. COVID-19 pandemic: Its impact on liver disease and liver transplantation. World J Gastroenterol. 2020;26:2987-2999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 31. | Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4343] [Cited by in RCA: 4207] [Article Influence: 841.4] [Reference Citation Analysis (0)] |
| 32. | Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 33. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 34. | Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen VL, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin KD, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch AD, Viveiros K, Chan W, Chascsa DM, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients With Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2021;19:1469-1479.e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 35. | Bajaj JS, Garcia-Tsao G, Biggins SW, Kamath PS, Wong F, McGeorge S, Shaw J, Pearson M, Chew M, Fagan A, de la Rosa Rodriguez R, Worthington J, Olofson A, Weir V, Trisolini C, Dwyer S, Reddy KR. Comparison of mortality risk in patients with cirrhosis and COVID-19 compared with patients with cirrhosis alone and COVID-19 alone: multicentre matched cohort. Gut. 2021;70:531-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 176] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 36. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 37. | Pan L, Huang P, Xie X, Xu J, Guo D, Jiang Y. Metabolic associated fatty liver disease increases the severity of COVID-19: A meta-analysis. Dig Liver Dis. 2021;53:153-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 38. | Hegyi PJ, Váncsa S, Ocskay K, Dembrovszky F, Kiss S, Farkas N, Erőss B, Szakács Z, Hegyi P, Pár G. Metabolic Associated Fatty Liver Disease Is Associated With an Increased Risk of Severe COVID-19: A Systematic Review With Meta-Analysis. Front Med (Lausanne). 2021;8:626425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 39. | Singh A, Hussain S, Antony B. Non-alcoholic fatty liver disease and clinical outcomes in patients with COVID-19: A comprehensive systematic review and meta-analysis. Diabetes Metab Syndr. 2021;15:813-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 40. | Kates OS, Haydel BM, Florman SS, Rana MM, Chaudhry ZS, Ramesh MS, Safa K, Kotton CN, Blumberg EA, Besharatian BD, Tanna SD, Ison MG, Malinis M, Azar MM, Rakita RM, Morilla JA, Majeed A, Sait AS, Spaggiari M, Hemmige V, Mehta SA, Neumann H, Badami A, Goldman JD, Lala A, Hemmersbach-Miller M, McCort ME, Bajrovic V, Ortiz-Bautista C, Friedman-Moraco R, Sehgal S, Lease ED, Fisher CE, Limaye AP; UW COVID-19 SOT Study Team. Coronavirus Disease 2019 in Solid Organ Transplant: A Multicenter Cohort Study. Clin Infect Dis. 2021;73:e4090-e4099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 295] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 41. | Rabiee A, Sadowski B, Adeniji N, Perumalswami PV, Nguyen V, Moghe A, Latt NL, Kumar S, Aloman C, Catana AM, Bloom PP, Chavin KD, Carr RM, Dunn W, Chen VL, Aby ES, Debes JD, Dhanasekaran R; COLD Consortium. Liver Injury in Liver Transplant Recipients With Coronavirus Disease 2019 (COVID-19): U.S. Multicenter Experience. Hepatology. 2020;72:1900-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 42. | Ravanan R, Callaghan CJ, Mumford L, Ushiro-Lumb I, Thorburn D, Casey J, Friend P, Parameshwar J, Currie I, Burnapp L, Baker R, Dudley J, Oniscu GC, Berman M, Asher J, Harvey D, Manara A, Manas D, Gardiner D, Forsythe JLR. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: A national cohort study. Am J Transplant. 2020;20:3008-3018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 43. | Webb GJ, Marjot T, Cook JA, Aloman C, Armstrong MJ, Brenner EJ, Catana MA, Cargill T, Dhanasekaran R, García-Juárez I, Hagström H, Kennedy JM, Marshall A, Masson S, Mercer CJ, Perumalswami PV, Ruiz I, Thaker S, Ufere NN, Barnes E, Barritt AS 4th, Moon AM. Outcomes following SARS-CoV-2 infection in liver transplant recipients: an international registry study. Lancet Gastroenterol Hepatol. 2020;5:1008-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 44. | Belli LS, Duvoux C, Karam V, Adam R, Cuervas-Mons V, Pasulo L, Loinaz C, Invernizzi F, Patrono D, Bhoori S, Ciccarelli O, Morelli MC, Castells L, Lopez-Lopez V, Conti S, Fondevila C, Polak W. COVID-19 in liver transplant recipients: preliminary data from the ELITA/ELTR registry. Lancet Gastroenterol Hepatol. 2020;5:724-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 45. | John BV, Deng Y, Scheinberg A, Mahmud N, Taddei TH, Kaplan D, Labrada M, Baracco G, Dahman B. Association of BNT162b2 mRNA and mRNA-1273 Vaccines With COVID-19 Infection and Hospitalization Among Patients With Cirrhosis. JAMA Intern Med. 2021;181:1306-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 46. | Thuluvath PJ, Robarts P, Chauhan M. Analysis of antibody responses after COVID-19 vaccination in liver transplant recipients and those with chronic liver diseases. J Hepatol. 2021;75:1434-1439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 47. | Ruether DF, Schaub GM, Duengelhoef PM, Haag F, Brehm TT, Fathi A, Wehmeyer M, Jahnke-Triankowski J, Mayer L, Hoffmann A, Fischer L, Addo MM, Lütgehetmann M, Lohse AW, Schulze Zur Wiesch J, Sterneck M. SARS-CoV2-specific Humoral and T-cell Immune Response After Second Vaccination in Liver Cirrhosis and Transplant Patients. Clin Gastroenterol Hepatol. 2022;20:162-172.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 116] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 48. | Willuweit K, Frey A, Passenberg M, Korth J, Saka N, Anastasiou OE, Möhlendick B, Schütte A, Schmidt H, Rashidi-Alavijeh J. Patients with Liver Cirrhosis Show High Immunogenicity upon COVID-19 Vaccination but Develop Premature Deterioration of Antibody Titers. Vaccines (Basel). 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 49. | Wang J, Zhang Q, Ai J, Liu D, Liu C, Xiang H, Gu Y, Guo Y, Lv J, Huang Y, Liu Y, Xu D, Chen S, Li J, Li Q, Liang J, Bian L, Zhang Z, Guo X, Feng Y, Liu L, Zhang X, Zhang Y, Xie F, Jiang S, Qin W, Wang X, Rao W, Tian Q, Zhu Y, Cong Q, Xu J, Hou Z, Zhang N, Zhang A, Zu H, Wang Y, Yan Z, Du X, Hou A, Yan Y, Qiu Y, Wu H, Hu S, Deng Y, Ji J, Yang J, Huang J, Zhao Z, Zou S, Ji H, Ge G, Zhong L, He S, Yan X, Yangzhen BB, Qu C, Zhang L, Yang S, Gao X, Lv M, Zhu Q, Xu X, Zeng QL, Qi X, Zhang W. Safety and immunogenicity of SARS-CoV-2 vaccines in Chinese patients with cirrhosis: a prospective multicenter study. Hepatol Int. 2022;16:691-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 50. | Ai J, Wang J, Liu D, Xiang H, Guo Y, Lv J, Zhang Q, Li J, Zhang X, Li Q, Liang J, Guo X, Feng Y, Liu L, Qin W, Wang X, Rao W, Tian Q, Zhang Y, Xie F, Jiang S, Yan Y, Qiu Y, Wu H, Hou Z, Zhang N, Zhang A, Ji J, Yang J, Huang J, Zhao Z, Gu Y, Bian L, Zhang Z, Zou S, Ji H, Ge G, Du X, Hou A, Zhu Y, Cong Q, Xu J, Zu H, Wang Y, Yan Z, Yan X, BianBa Y, Ci Q, Zhang L, Yang S, Gao X, Zhong L, He S, Liu C, Huang Y, Liu Y, Xu D, Zhu Q, Xu X, Lv M, Zhang W, Qi X. Safety and Immunogenicity of SARS-CoV-2 Vaccines in Patients With Chronic Liver Diseases (CHESS-NMCID 2101): A Multicenter Study. Clin Gastroenterol Hepatol. 2022;20:1516-1524.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 51. | Rabinowich L, Grupper A, Baruch R, Ben-Yehoyada M, Halperin T, Turner D, Katchman E, Levi S, Houri I, Lubezky N, Shibolet O, Katchman H. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021;75:435-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 272] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 52. | Herrera S, Colmenero J, Pascal M, Escobedo M, Castel MA, Sole-González E, Palou E, Egri N, Ruiz P, Mosquera M, Moreno A, Juan M, Vilella A, Soriano A, Farrero M, Bodro M. Cellular and humoral immune response after mRNA-1273 SARS-CoV-2 vaccine in liver and heart transplant recipients. Am J Transplant. 2021;21:3971-3979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 53. | Strauss AT, Hallett AM, Boyarsky BJ, Ou MT, Werbel WA, Avery RK, Tobian AAR, Massie AB, Hamilton JPA, Garonzik-Wang JM, Segev DL. Antibody Response to Severe Acute Respiratory Syndrome-Coronavirus-2 Messenger RNA Vaccines in Liver Transplant Recipients. Liver Transpl. 2021;27:1852-1856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 54. | Nazaruk P, Monticolo M, Jędrzejczak AM, Krata N, Moszczuk B, Sańko-Resmer J, Pilecki T, Urbanowicz A, Florczak M, Pączek L, Foroncewicz B, Mucha K. Unexpectedly High Efficacy of SARS-CoV-2 BNT162b2 Vaccine in Liver versus Kidney Transplant Recipients-Is It Related to Immunosuppression Only? Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 55. | Timmermann L, Globke B, Lurje G, Schmelzle M, Schöning W, Öllinger R, Pratschke J, Eberspächer B, Drosten C, Hofmann J, Eurich D. Humoral Immune Response following SARS-CoV-2 Vaccination in Liver Transplant Recipients. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 56. | D'Offizi G, Agrati C, Visco-Comandini U, Castilletti C, Puro V, Piccolo P, Montalbano M, Meschi S, Tartaglia E, Sorace C, Leone S, Lapa D, Grassi G, Goletti D, Ippolito G, Vaia F, Ettorre GM, Lionetti R. Coordinated cellular and humoral immune responses after two-dose SARS-CoV2 mRNA vaccination in liver transplant recipients. Liver Int. 2022;42:180-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 57. | John BV, Deng Y, Khakoo NS, Taddei TH, Kaplan DE, Dahman B. Coronavirus Disease 2019 Vaccination Is Associated With Reduced Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Death in Liver Transplant Recipients. Gastroenterology. 2022;162:645-647.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 58. | Davidov Y, Tsaraf K, Cohen-Ezra O, Likhter M, Ben Yakov G, Levy I, Levin EG, Lustig Y, Mor O, Rahav G, Ben Ari Z. Immunogenicity and Adverse Effects of the 2-Dose BNT162b2 Messenger RNA Vaccine Among Liver Transplantation Recipients. Liver Transpl. 2022;28:215-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 59. | Sakai A, Morishita T, Matsunami H. Antibody Response After a Second Dose of the BNT162b2 mRNA COVID-19 Vaccine in Liver Transplant Recipients. Transpl Int. 2022;35:10321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Calleri A, Saracco M, Pittaluga F, Cavallo R, Romagnoli R, Martini S. Seroconversion After Coronavirus Disease 2019 Vaccination in Patients Awaiting Liver Transplantation: Fact or Fancy? Liver Transpl. 2022;28:180-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 61. | Xiang T, Liang B, Wang H, Quan X, He S, Zhou H, He Y, Yang D, Wang B, Zheng X. Safety and immunogenicity of a SARS-CoV-2 inactivated vaccine in patients with chronic hepatitis B virus infection. Cell Mol Immunol. 2021;18:2679-2681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 62. | He T, Zhou Y, Xu P, Ling N, Chen M, Huang T, Zhang B, Yang Z, Ao L, Li H, Chen Z, Zhang D, Shi X, Lei Y, Wang Z, Zeng W, Hu P, Lan Y, Zhou Z, Kang J, Huang Y, Shi T, Pan Q, Zhu Q, Ran X, Zhang Y, Song R, Xiang D, Xiao S, Zhang G, Shen W, Peng M, Cai D, Ren H. Safety and antibody response to inactivated COVID-19 vaccine in patients with chronic hepatitis B virus infection. Liver Int. 2022;42:1287-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 63. | Wang J, Hou Z, Liu J, Gu Y, Wu Y, Chen Z, Ji J, Diao S, Qiu Y, Zou S, Zhang A, Zhang N, Wang F, Li X, Wang Y, Liu X, Lv C, Chen S, Liu D, Ji X, Liu C, Ren T, Sun J, Zhao Z, Wu F, Li F, Wang R, Yan Y, Zhang S, Ge G, Shao J, Yang S, Huang Y, Xu D, Ai J, He Q, Zheng MH, Zhang L, Xie Q, Rockey DC, Fallowfield JA, Zhang W, Qi X. Safety and immunogenicity of COVID-19 vaccination in patients with non-alcoholic fatty liver disease (CHESS2101): A multicenter study. J Hepatol. 2021;75:439-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 64. | Duengelhoef P, Hartl J, Rüther D, Steinmann S, Brehm TT, Weltzsch JP, Glaser F, Schaub GM, Sterneck M, Sebode M, Weiler-Normann C, Addo MM, Lütgehetmann M, Haag F, Schramm C, Schulze Zur Wiesch J, Lohse AW. SARS-CoV-2 vaccination response in patients with autoimmune hepatitis and autoimmune cholestatic liver disease. United European Gastroenterol J. 2022;10:319-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 65. | Schneider L, Schubert L, Winkler F, Munda P, Winkler S, Tobudic S. SARS-CoV-2 Vaccine Response in Patients With Autoimmune Hepatitis. Clin Gastroenterol Hepatol. 2022;20:2145-2147.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 66. | Aggeletopoulou I, Davoulou P, Konstantakis C, Thomopoulos K, Triantos C. Response to hepatitis B vaccination in patients with liver cirrhosis. Rev Med Virol. 2017;27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 67. | McCashland TM, Preheim LC, Gentry MJ. Pneumococcal vaccine response in cirrhosis and liver transplantation. J Infect Dis. 2000;181:757-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 125] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 68. | Hippisley-Cox J, Coupland CA, Mehta N, Keogh RH, Diaz-Ordaz K, Khunti K, Lyons RA, Kee F, Sheikh A, Rahman S, Valabhji J, Harrison EM, Sellen P, Haq N, Semple MG, Johnson PWM, Hayward A, Nguyen-Van-Tam JS. Risk prediction of covid-19 related death and hospital admission in adults after covid-19 vaccination: national prospective cohort study. BMJ. 2021;374:n2244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 201] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 69. | Albillos A, Lario M, Álvarez-Mon M. Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J Hepatol. 2014;61:1385-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 847] [Article Influence: 77.0] [Reference Citation Analysis (1)] |
| 70. | John BV, Deng Y, Schwartz KB, Taddei TH, Kaplan DE, Martin P, Chao HH, Dahman B. Postvaccination COVID-19 infection is associated with reduced mortality in patients with cirrhosis. Hepatology. 2022;76:126-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 71. | John BV, Sidney Barritt A 4th, Moon A, Taddei TH, Kaplan DE, Dahman B, Doshi A, Deng Y, Mansour N, Ioannou G, Martin P, Chao HH. Effectiveness of COVID-19 Viral Vector Ad.26.COV2.S Vaccine and Comparison with mRNA Vaccines in Cirrhosis. Clin Gastroenterol Hepatol. 2022;20:2405-2408.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 72. | Cao Z, Zhang C, Zhao S, Sheng Z, Xiang X, Li R, Qian Z, Wang Y, Chen B, Li Z, Liu Y, An B, Zhou H, Cai W, Wang H, Gui H, Xin H, Xie Q. COVID-19 vaccines in patients with decompensated cirrhosis: a retrospective cohort on safety data and risk factors associated with unvaccinated status. Infect Dis Poverty. 2022;11:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 73. | Bakasis AD, Bitzogli K, Mouziouras D, Pouliakis A, Roumpoutsou M, Goules AV, Androutsakos T. Antibody Responses after SARS-CoV-2 Vaccination in Patients with Liver Diseases. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 74. | Benotmane I, Gautier G, Perrin P, Olagne J, Cognard N, Fafi-Kremer S, Caillard S. Antibody Response After a Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients With Minimal Serologic Response to 2 Doses. JAMA. 2021;326:1063-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 269] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 75. | Narasimhan M, Mahimainathan L, Clark AE, Usmani A, Cao J, Araj E, Torres F, Sarode R, Kaza V, Lacelle C, Muthukumar A. Serological Response in Lung Transplant Recipients after Two Doses of SARS-CoV-2 mRNA Vaccines. Vaccines (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 76. | Redjoul R, Le Bouter A, Beckerich F, Fourati S, Maury S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet. 2021;398:298-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 77. | Danziger-Isakov L, Kumar D; AST ID Community of Practice. Vaccination of solid organ transplant candidates and recipients: Guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33:e13563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 293] [Article Influence: 48.8] [Reference Citation Analysis (35)] |
| 78. | Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three Doses of an mRNA Covid-19 Vaccine in Solid-Organ Transplant Recipients. N Engl J Med. 2021;385:661-662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 609] [Cited by in RCA: 665] [Article Influence: 166.3] [Reference Citation Analysis (0)] |
| 79. | Fix OK, Blumberg EA, Chang KM, Chu J, Chung RT, Goacher EK, Hameed B, Kaul DR, Kulik LM, Kwok RM, McGuire BM, Mulligan DC, Price JC, Reau NS, Reddy KR, Reynolds A, Rosen HR, Russo MW, Schilsky ML, Verna EC, Ward JW, Fontana RJ; AASLD COVID-19 Vaccine Working Group. American Association for the Study of Liver Diseases Expert Panel Consensus Statement: Vaccines to Prevent Coronavirus Disease 2019 Infection in Patients With Liver Disease. Hepatology. 2021;74:1049-1064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 80. | Oosting SF, van der Veldt AAM, Fehrmann RSN, GeurtsvanKessel CH, van Binnendijk RS, Dingemans AC, Smit EF, Hiltermann TJN, den Hartog G, Jalving M, Westphal TT, Bhattacharya A, de Wilt F, Boerma A, van Zijl L, Rimmelzwaan GF, Kvistborg P, van Els CACM, Rots NY, van Baarle D, Haanen JBAG, de Vries EGE. Immunogenicity after second and third mRNA-1273 vaccination doses in patients receiving chemotherapy, immunotherapy, or both for solid tumours. Lancet Oncol. 2022;23:833-835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 81. | Peeters M, Verbruggen L, Teuwen L, Vanhoutte G, Vande Kerckhove S, Peeters B, Raats S, Van der Massen I, De Keersmaecker S, Debie Y, Huizing M, Pannus P, Neven K, Ariën KK, Martens GA, Van Den Bulcke M, Roelant E, Desombere I, Anguille S, Goossens M, Vandamme T, van Dam P. Reduced humoral immune response after BNT162b2 coronavirus disease 2019 messenger RNA vaccination in cancer patients under antineoplastic treatment. ESMO Open. 2021;6:100274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 82. | Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, Doolman R, Asraf K, Mendelson E, Ziv A, Rubin C, Freedman L, Kreiss Y, Regev-Yochay G. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N Engl J Med. 2021;385:e84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1282] [Cited by in RCA: 1268] [Article Influence: 317.0] [Reference Citation Analysis (0)] |
| 83. | Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, Loran D, Hrncir D, Herring K, Platzer M, Adams N, Sanou A, Cooper LT Jr. Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021;6:1202-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 398] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 84. | Arepally GM, Ortel TL. Vaccine-induced immune thrombotic thrombocytopenia: what we know and do not know. Blood. 2021;138:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 85. | Ozaka S, Kodera T, Ariki S, Kobayashi T, Murakami K. Acute pancreatitis soon after COVID-19 vaccination: A case report. Medicine (Baltimore). 2022;101:e28471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 86. | Wong CKH, Mak LY, Au ICH, Lai FTT, Li X, Wan EYF, Chui CSL, Chan EWY, Cheng WY, Cheng FWT, Yuen MF, Wong ICK. Risk of acute liver injury following the mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccines. J Hepatol. 2022;77:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 87. | Bril F, Al Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? J Hepatol. 2021;75:222-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 167] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 88. | Lodato F, Larocca A, D'Errico A, Cennamo V. An unusual case of acute cholestatic hepatitis after m-RNABNT162b2 (Comirnaty) SARS-CoV-2 vaccine: Coincidence, autoimmunity or drug-related liver injury. J Hepatol. 2021;75:1254-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 89. | Vuille-Lessard É, Montani M, Bosch J, Semmo N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J Autoimmun. 2021;123:102710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 87] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 90. | Londoño MC, Gratacós-Ginès J, Sáez-Peñataro J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination - still casualty? J Hepatol. 2021;75:1248-1249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 91. | Rocco A, Sgamato C, Compare D, Nardone G. Autoimmune hepatitis following SARS-CoV-2 vaccine: May not be a casuality. J Hepatol. 2021;75:728-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 92. | McShane C, Kiat C, Rigby J, Crosbie Ó. The mRNA COVID-19 vaccine - A rare trigger of autoimmune hepatitis? J Hepatol. 2021;75:1252-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 93. | Clayton-Chubb D, Schneider D, Freeman E, Kemp W, Roberts SK. Autoimmune hepatitis developing after the ChAdOx1 nCoV-19 (Oxford-AstraZeneca) vaccine. J Hepatol. 2021;75:1249-1250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 94. | Tan CK, Wong YJ, Wang LM, Ang TL, Kumar R. Autoimmune hepatitis following COVID-19 vaccination: True causality or mere association? J Hepatol. 2021;75:1250-1252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 95. | Ghielmetti M, Schaufelberger HD, Mieli-Vergani G, Cerny A, Dayer E, Vergani D, Terziroli Beretta-Piccoli B. Acute autoimmune-like hepatitis with atypical anti-mitochondrial antibody after mRNA COVID-19 vaccination: A novel clinical entity? J Autoimmun. 2021;123:102706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 96. | Zhou T, Fronhoffs F, Dold L, Strassburg CP, Weismüller TJ. New-onset autoimmune hepatitis following mRNA COVID-19 vaccination in a 36-year-old woman with primary sclerosing cholangitis - should we be more vigilant? J Hepatol. 2022;76:218-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 97. | Garrido I, Lopes S, Simões MS, Liberal R, Lopes J, Carneiro F, Macedo G. Autoimmune hepatitis after COVID-19 vaccine - more than a coincidence. J Autoimmun. 2021;125:102741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 98. | Goulas A, Kafiri G, Kranidioti H, Manolakopoulos S. A typical autoimmune hepatitis (AIH) case following Covid-19 mRNA vaccination. More than a coincidence? Liver Int. 2022;42:254-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 99. | Rela M, Jothimani D, Vij M, Rajakumar A, Rammohan A. Auto-immune hepatitis following COVID vaccination. J Autoimmun. 2021;123:102688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 100. | Palla P, Vergadis C, Sakellariou S, Androutsakos T. Letter to the editor: Autoimmune hepatitis after COVID-19 vaccination: A rare adverse effect? Hepatology. 2022;75:489-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 101. | Mann R, Sekhon S. Drug-Induced Liver Injury After COVID-19 Vaccine. Cureus. 2021;13:e16491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 102. | Avci E, Abasiyanik F. Autoimmune hepatitis after SARS-CoV-2 vaccine: New-onset or flare-up? J Autoimmun. 2021;125:102745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 103. | Torrente S, Castiella A, Garmendia M, Zapata E. Probable autoimmune hepatitis reactivated after COVID-19 vaccination. Gastroenterol Hepatol. 2022;45 Suppl 1:115-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 104. | Ghorbani H, Rouhi T, Vosough Z, Shokri-Shirvani J. Drug-induced hepatitis after Sinopharm COVID-19 vaccination: A case study of a 62-year-old patient. Int J Surg Case Rep. 2022;93:106926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 105. | Kang SH, Kim MY, Cho MY, Baik SK. Autoimmune Hepatitis Following Vaccination for SARS-CoV-2 in Korea: Coincidence or Autoimmunity? J Korean Med Sci. 2022;37:e116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 106. | Camacho-Domínguez L, Rodríguez Y, Polo F, Restrepo Gutierrez JC, Zapata E, Rojas M, Anaya JM. COVID-19 vaccine and autoimmunity. A new case of autoimmune hepatitis and review of the literature. J Transl Autoimmun. 2022;5:100140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 107. | Shahrani S, Sooi CY, Hilmi IN, Mahadeva S. Autoimmune hepatitis (AIH) following coronavirus (COVID-19) vaccine-No longer exclusive to mRNA vaccine? Liver Int. 2022;42:2344-2345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 108. | Zin Tun GS, Gleeson D, Al-Joudeh A, Dube A. Immune-mediated hepatitis with the Moderna vaccine, no longer a coincidence but confirmed. J Hepatol. 2022;76:747-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 109. | Suzuki Y, Kakisaka K, Takikawa Y. Letter to the editor: Autoimmune hepatitis after COVID-19 vaccination: Need for population-based epidemiological study. Hepatology. 2022;75:759-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 110. | Cao Z, Gui H, Sheng Z, Xin H, Xie Q. Letter to the editor: Exacerbation of autoimmune hepatitis after COVID-19 vaccination. Hepatology. 2022;75:757-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 111. | Efe C, Kulkarni AV, Terziroli Beretta-Piccoli B, Magro B, Stättermayer A, Cengiz M, Clayton-Chubb D, Lammert C, Bernsmeier C, Gül Ö, la Tijera FH, Anders M, Lytvyak E, Akın M, Purnak T, Liberal R, Peralta M, Ebik B, Duman S, Demir N, Balaban Y, Urzua Á, Contreras F, Venturelli MG, Bilgiç Y, Medina A, Girala M, Günşar F, Londoño MC, Androutsakos T, Kisch A, Yurci A, Güzelbulut F, Çağın YF, Avcı E, Akyıldız M, Dindar-Demiray EK, Harputluoğlu M, Kumar R, Satapathy SK, Mendizabal M, Silva M, Fagiuoli S, Roberts SK, Soylu NK, Idilman R, Yoshida EM, Montano-Loza AJ, Dalekos GN, Ridruejo E, Schiano TD, Wahlin S. Liver injury after SARS-CoV-2 vaccination: Features of immune-mediated hepatitis, role of corticosteroid therapy and outcome. Hepatology. 2022;76:1576-1586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 112. | Shroff H, Satapathy SK, Crawford JM, Todd NJ, VanWagner LB. Liver injury following SARS-CoV-2 vaccination: A multicenter case series. J Hepatol. 2022;76:211-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 113. | Vadalà M, Poddighe D, Laurino C, Palmieri B. Vaccination and autoimmune diseases: is prevention of adverse health effects on the horizon? EPMA J. 2017;8:295-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 114. | Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 288] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 115. | Vojdani A, Vojdani E, Kharrazian D. Reaction of Human Monoclonal Antibodies to SARS-CoV-2 Proteins With Tissue Antigens: Implications for Autoimmune Diseases. Front Immunol. 2020;11:617089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 233] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 116. | Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 424] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 117. | Kim TS, Shin EC. The activation of bystander CD8(+) T cells and their roles in viral infection. Exp Mol Med. 2019;51:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 118. | Ntouros PA, Vlachogiannis NI, Pappa M, Nezos A, Mavragani CP, Tektonidou MG, Souliotis VL, Sfikakis PP. Effective DNA damage response after acute but not chronic immune challenge: SARS-CoV-2 vaccine versus Systemic Lupus Erythematosus. Clin Immunol. 2021;229:108765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 119. | Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 532] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 120. | Jara LJ, Vera-Lastra O, Mahroum N, Pineda C, Shoenfeld Y. Autoimmune post-COVID vaccine syndromes: does the spectrum of autoimmune/inflammatory syndrome expand? Clin Rheumatol. 2022;41:1603-1609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 121. | Manni M, Gupta S, Ricker E, Chinenov Y, Park SH, Shi M, Pannellini T, Jessberger R, Ivashkiv LB, Pernis AB. Regulation of age-associated B cells by IRF5 in systemic autoimmunity. Nat Immunol. 2018;19:407-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 122. | Sachinidis A, Garyfallos A. COVID-19 vaccination can occasionally trigger autoimmune phenomena, probably via inducing age-associated B cells. Int J Rheum Dis. 2022;25:83-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 123. | Muskardin TLW, Niewold TB. Type I interferon in rheumatic diseases. Nat Rev Rheumatol. 2018;14:214-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 220] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 124. | Ndeupen S, Qin Z, Jacobsen S, Bouteau A, Estanbouli H, Igyártó BZ. The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021;24:103479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 341] [Article Influence: 85.3] [Reference Citation Analysis (0)] |