Published online Dec 21, 2022. doi: 10.3748/wjg.v28.i47.6769
Peer-review started: October 12, 2022
First decision: October 20, 2022
Revised: November 5, 2022
Accepted: November 30, 2022
Article in press: November 30, 2022
Published online: December 21, 2022
Processing time: 67 Days and 20.8 Hours
Gastric cancer (GC) is a common malignant tumor with high incidence and mortality rates globally, especially in East Asian countries. Helicobacter pylori (H. pylori) infection is a significant and independent risk factor for GC. However, its underlying mechanism of action is not fully understood. Dickkopf-related protein (DKK) 1 is a Wnt signaling antagonist, and cytoskeleton-associated protein (CKAP) 4 is a newly identified DKK1 receptor. Recent studies found that the binding of DKK1 to CAKP4 mediated the procancer signaling of DKK1 inde-pendent of Wnt signaling. We hypothesize that H. pylori-induced activation of DKK1/CKAP4 signaling contributes to the initiation and progression of GC.
To investigate the interaction of H. pylori infection, DKK1 and CAKP4 in GC, as well as the underlying molecular mechanisms.
RNA sequencing was used to identify differentially expressed genes (DEGs) between H. pylori-infected and uninfected primary GC cells. Gain- and loss-of-function experiments were performed to verify the H. pylori-induced upregulation of activator protein-1 (AP-1) in GC cells. A dual-luciferase reporter assay and co-immunoprecipitation were used to determine the binding of AP-1 to the DKK1 promoter and DKK1 to CKAP4. Western blotting and immunohistochemistry detected the expression of DKK1, CKAP4, and phos-phatidylinositol 3-kinase (PI3K) pathway-related proteins in GC cells and tissues. Functional experiments and tumorigenicity in nude mice detected malignant behavior of GC cells in vitro and in vivo.
We identified 32 DEGs between primary GC cells with and without H. pylori infection, including JUN, fos-like antigen-1 (FOSL1), and DKK1, and confirmed that the three proteins and CKAP4 were highly expressed in H. pylori-infected GC cells, H. pylori-infected gerbil gastric tissues, and human GC tissues. JUN and FOSL1 form AP-1 to transcriptionally activate DKK1 expression by binding to the DKK1 promoter. Activated DKK1 bound to CKAP4, but not the most common Wnt coreceptor low-density lipoprotein receptor-related protein 5/6, to promote GC cell growth, colony formation, migration, invasion, and xenograft tumor growth in nude mice. All these effects were driven by activation of the PI3K/AKT/mammalian target of rapamycin (mTOR) pathway. Targeting the PI3K signaling pathway by LY294002 inhibited DKK1-mediated CKAP4/PI3K signaling activity and the malignant behavior of GC cells.
H. pylori induces JUN and FOSL1 expression to form AP-1, which transcriptionally activates DKK1. Binding of DKK1 to KAKP4 contributes to gastric tumorigenesis via the PI3K/AKT/mTOR pathway.
Core Tip: Helicobacter pylori (H. pylori) infection is the most significant risk factor for gastric cancer (GC). More than half of the global population has H. pylori infection, and 1%-3% of the infected individuals develop GC, but the mechanism behind this link remains unclear. Here, we identified 32 highly expressed genes in H. pylori-infected GC cells and demonstrated that H. pylori-induced high expression of JUN and fos-like antigen-1 formed activator protein-1 to transcriptionally activate dickkopf-related protein (DKK) 1, which by binding to cytoskeleton-associated protein 4 (CKAP4) receptor activated the PI3K/AKT/mammalian target of rapamycin pathway and, consequently, gastric tumorigenesis. Targeting the DKK1/CKAP4 interaction may be a novel strategy to treat GC.
- Citation: Luo M, Chen YJ, Xie Y, Wang QR, Xiang YN, Long NY, Yang WX, Zhao Y, Zhou JJ. Dickkopf-related protein 1/cytoskeleton-associated protein 4 signaling activation by Helicobacter pylori-induced activator protein-1 promotes gastric tumorigenesis via the PI3K/AKT/mTOR pathway. World J Gastroenterol 2022; 28(47): 6769-6787
- URL: https://www.wjgnet.com/1007-9327/full/v28/i47/6769.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i47.6769
Gastric cancer (GC) is the fifth most prevalent tumor and fourth leading cause of tumor-related mortality worldwide[1]. Although the incidence and mortality rates of this malignancy have steadily decreased over the past several decades, GC remains a significant health problem in developing nations[2]. Most patients with GC present at an advanced stage when diagnosed, and metastasis and recurrence in individuals are commonly noted[3]. Helicobacter pylori (H. pylori) are Gram-negative and microaerophilic pathogenic bacteria. H. pylori infection is the most significant risk factor for GC. The International Agency for Research on Cancer designated it a Group I carcinogen for GC in 1994[4]. More than half of the global population has H. pylori infection, and 1%-3% of infected individuals develop GC[5]. Globally, an estimated 89.4% and 20% of new noncardia and cardia GC cases were attributable to H. pylori infection in 2018[6]. However, this figure was 78.5% for noncardia GC and 62.1% for cardia GC in China[7]. Although many studies have investigated the link between H. pylori infection and GC[8], the molecular mechanisms are not fully understood.
By RNA sequencing, we discovered that JUN (also known as c-JUN), fos-like antigen-1 (FOSL1, a FOS family member encoding FRA-1), and Dickkopf-related protein (DKK) 1 were elevated in primary GC cells infected with H. pylori. JUN and FOSL1 are the transcription factor activator protein (AP)-1 complex components and form the JUN::JUN homodimer and the JUN::FOSL1 heterodimer to regulate target gene transcription via binding to the promoters and enhancers of target genes[9]. In vitro and in vivo studies showed that activator protein-1 (AP-1) controlled tumor growth, progression and drug resistance[10,11]. DKK1 is a potent antagonist that suppresses oncogenic Wnt/β-catenin signaling and tumors by binding to the Wnt coreceptor: low-density lipoprotein receptor-related protein (LRP) 5/6[12,13]. However, recent studies demonstrated that DKK1 behaved like an oncogene in a variety of cancers[14,15]. Several studies consistently indicated that DKK1 levels were elevated in GC patient cancer tissues and serum, and higher DKK1 levels were significantly associated with worse outcomes[16-18]. However, little is known about the transcriptional regulation of DKK1, its relationship with H. pylori, and the mechanism of DKK1 promotion of gastric carcinogenesis.
Cytoskeleton-associated protein (CKAP) 4 is a newly identified DKK1 receptor[19]. The binding of DKK1 to CKAP4 recruits phosphatidylinositol 3-kinase (PI3K) via association between the proline-rich region of CKAP4 and the Src homology 3 domain of PI3K, which leads to the activation of serine/threonine-protein kinase AKT[20]. Although there is no evidence that the DKK1/CKAP4 axis exists in GC, the PI3K pathway involved in the malignant transformation of cells is activated in most GC patients[21]. AKT and p-AKT were overexpressed in > 74% of GC patients[22], and phosphorylated mammalian target of rapamycin (p-mTOR) expression was found in 60% of gastric adenocarcinoma specimens[23]. Therefore, we postulate that DKK1 contributes to GC through the PI3K pathway but not the Wnt pathway.
Here, we revealed that H. pylori infection induced high expression of JUN and FOSL1, which formed AP-1 to activate DKK1 transcriptionally. DKK1 binding to CKAP4, but not the most common Wnt coreceptor LRP5/6, promoted GC growth and invasion by triggering the PI3K/AKT/mTOR pathway. The results provide novel insight into the molecular mechanism underlying H. pylori-induced gastric carcinogenesis.
H. pylori GZ7 is a typical East Asian strain (cagA+) that was isolated from the gastric mucosa of a GC patient by our group[24]. H. pylori 26695 (ATCC 700392) is a typical western strain (cagA+) that was purchased from the American Type Culture Collection (ATCC, Manassas, VA, United States). Two strains were cultured on Columbia agar plates supplemented with H. pylori selective supplement (Oxoid, United Kingdom) and 10% sheep blood at 37 °C in a microaerobic environment (5% O2, 10%
The human GC cell lines AGS, NCI-N87 and SNU-16 cells were purchased from ATCC, and SGC-7901 and BGC823 cells were obtained from the tissue bank in Shanghai, China. Primary GC cells were separated and identified in our laboratory[25]. All cell lines were grown in 5% CO2 at 37 °C in RPMI-1640 with 10% FCS (Gibco, United States).
From June to December 2021, 12 pairs of GC and paracancer normal tissues were collected during surgery at the Affiliated Hospital of Guizhou Medical University in China. Tissues were immediately formalin-fixed and paraffin-embedded for pathological evaluation and immunohistochemistry. All subjects provided their written informed consent. The study was performed following the Declaration of Helsinki. The Guizhou Medical University Ethics Committee approved the study protocol, No. 2017(43).
In our earlier work, Mongolian gerbils were intragastrically infected with H. pylori NCTC 11637 (ATCC 43504, cagA+) for 2 years. At 3-24 mo after infection, gerbils developed erosion, atrophy, intestinal metaplasia, and well-differentiated GC in the gastric mucosa[26]. The stomach tissues of gerbils (n = 3 at each timepoint) were removed after decapitation and immediately fixed and embedded. The embedded tissues were sliced into sections (5 μm thick) and stained for immunohistochemistry.
Three primary GC cell lines (5 × 107) were cultured with 50 multiplicity of infection (MOI) H. pylori GZ7 for 6 h before being harvested. TRIzol (Invitrogen, Carlsbad, CA, United States) was used to extract overall RNA, which was then purified by DNase I treatment. After being enriched by Oligo (dT) beads, eukaryotic mRNA was interrupted into short fragments and synthesized into cDNA. Using the Illumina TruSeq library construction kit, purified cDNA was amplified by PCR to construct the RNA-sequencing (RNA-seq) library. After the library was tested for quality using the Agilent 2100 Bioanalyzer and ABI StepOnePlus Real-Time PCR System, the library was sequenced on the Illumina HiSeqX10 platform by Beijing Genomics Institute (China). Sequence reads were mapped to the reference human genome (hg19), and the gene and transcript expression levels were calculated. Differentially expressed genes (DEGs) with a fold-change > 2 and false discovery rate (FDR) < 0.05 were identified between H. pylori-infected and uninfected cells. The DEGs shared by three datasets were selected for further bioinformatics analysis. The R heatmap package (version 0.7.7) was used to generate a heatmap. Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG), and protein-protein interaction (PPI) analyses were performed using the Gene Ontology Consortium (http://geneontology.org/), KOBAS (http://kobas.cbi.pku.edu.cn/), and the STRING tool (https://string-db.org/), respectively. KEGG pathways and PPI networks were visualized using Cytoscape software (version 3.7.2). Gene set enrichment analysis (GSEA) was carried out using GSEA software (version 4.1.0). A significant gene set was defined as having an FDR < 0.25 and P < 0.01.
The expression vectors of JUN and FOSL1 were purchased from Hanheng Biotech (Shanghai, China). The human DKK1 promoter region from -1100 to + 1 bp was cloned into the luciferase reporter vector pGL4.29 (Promega, Charbonnières-les-Bains, France), which was cotransfected with pcDNA3.1-JUN and/or pcDNA3.1-FOSL1 into AGS cells for 48 h using Lipofectamine 2000 (Invitrogen). The activities of firefly luciferase and Renilla luciferase were determined using the dual luciferase reporter system (Promega). The ratio of firefly luciferase activities to Renilla luciferase activities (Fluc/Rluc) was used to describe the reporter activities.
Proteins (25 g) were resolved via SDS-PAGE and transferred by electroblotting onto the PVDF membrane (Millipore, Billerica, MA, United States). After blocking with 5% nonfat milk, primary antibodies were used to probe the membrane overnight at 4 °C. The membrane was incubated with horseradish-peroxidase-conjugated secondary antibodies. The bands were visualized using electrochemiluminescence. Details of the antibodies are provided in Supplementary Table 1.
RNA isolation was performed by TRIzol reagent. cDNA was synthesized through the process of reverse transcription (RT) using a standard protocol according to the manufacturer’s instructions. Quantitative PCR (qPCR) was performed using fluorogenic SYBR Green (BioRad, Hercules, CA, United States). The 2-ΔΔCT method was used for relative mRNA quantitation of target genes. GAPDH gene served as the loading control. RT-qPCR primers are listed in Supplementary Table 2.
Gerbil stomachs, nude mouse xenografts, and human GC tissues were cut into 5-μm thick sections. Immunohistochemistry was used to detect expression of JUN, DKK1, CKAP4 and Ki67 in these tissues, and immunofluorescence imaging was used to assess β-catenin expression and nuclear translocation in DKK1-silenced AGS cells, as described previously[25]. The quantification of immunohistochemical staining was performed by Image J software. Immunohistochemistry score was determined by multiplying the intensity score and the percentage score[27]. Details of the antibodies are provided in Supplementary Table 1.
Cell viability was determined using Cell Counting Kit-8 kit (CCK-8, Dojindo, Japan) in accordance with the kit instructions. Cells (1000 cells per well) were placed in a 96-well plate in sextuplicate per condition and cultured for 1-6 d. CCK-8 solution (10 μL) was added to each well at the corresponding time points, and the cells were cultured for an additional 2 h. CCK-8 was used to detect cell viability.
Cells (500 cells per well) were placed in a 6-well plate in triplicate per condition and cultured for 2 wk. The growth medium was changed once every 4 d. Two weeks later, 0.1% crystal violet (Solarbio, China) was used to stain the colonies after being fixed with 4% paraformaldehyde. Colony count was performed using a microscope.
Transwell migration and invasion assays were performed using a 24-well Transwell insert (pore size of 8 μm) with and without Matrigel (Biosciences, San Jose, CA, United States). A total of 1 × 104 cells in medium with 1% FBS was added to the upper chamber of the Transwell, and 800 μL of medium with 10% FBS was added in lower chamber. At 24 h and 72 h after culture, 4% paraformaldehyde was used to fix the inserts for 30 min at room temperature before being stained in a 0.1% crystal violet solution and imaged. Cells that migrated or invaded to the lower chamber were counted using a microscope.
Cell cycle and apoptosis analyses were performed using flow cytometry, as previously described[25]. Cell cycle distribution and apoptosis were analyzed using FloJo software.
Co-immunoprecipitation (Co-IP) was performed using an Absin Co-IP kit (Shanghai, China). BGC823 cells (3 × 106) with or without H. pylori infection were sonicated three times for 20 s each in ice-cold lysis buffer before being centrifuged at 14000 × g for 10 min at 4 °C. The supernatant was then collected as whole-cell lysates. Cell lysates were incubated overnight at 4 °C with a CKAP4 antibody (2 μg) and control IgG (1 μg). Protein A- and protein G-Sepharose (10 μL) (Sigma-Aldrich, St. Louis, MO, United States) were added to the cell lysates and left on for 8 h at 4 °C. After 1 min of centrifugation at 12000 × g, the beads were collected and washed three times with wash buffer. The beads were boiled in 2 × Laemmeli sample buffer for 5 min and centrifugated for 1 min at 14000 × g and 4 °C. SDS-PAGE was used to resolve proteins in the supernatants (15 μL) after being collected. Western blotting was used to detect DKK1 by an anti-DKK1 antibody (1:400).
The ARRIVE (Animal Research: Reporting In Vivo Experiments) guidelines (https://www.nc3rs.org.uk/arrive-guidelines) were followed for all animal experiments to minimize pain and discomfort for the animals. Guizhou Medical University Animal Care Welfare Committee approved the animal study (No. 1702155). Ten BALB/c nude mice (male, age 3-4 wk) were provided by Chongqing Tengxin Biotechnology (Chongqing, China). Mice were accustomed to the laboratory environment that was a 12-h light/12-h dark cycle at 23 °C with 50% humidity and were given water and food ad libitum. After 2 wk, BGC823 cells (2 × 106) stably overexpressing DKK1 and negative control cells were transplanted subcutaneously into the flanks of the mice (5 mice per group), and the tumor size was measured every 3 d. After 16 d, all mice were killed by intravenous injection of barbiturate overdose (150 mg/kg), and the tumors were removed. Tumor weights and volumes were determined using the formula (length × width2)/2.
The lentiviruses containing shJUN and shDKK1, overexpression lentiviruses for JUN and DKK1, and control lentiviruses were obtained from Shanghai Jikai Gene Co. Ltd. (China). These lentiviruses were used to infect AGS, SGC-7901, NCT-N87, SNU-16 and BGC823 cells (2 × 105) in a 12-well plate at MOI 10-30 for 48 h. Polybrene (8 μg/mL; Sigma) was added to the 12-well plate to increase the efficiency of lentivirus infection. After 24 h, the medium containing lentiviruses and polybrene was changed with fresh medium and cultured for a further 48 h. After that, the stable cell lines were selected for 2 wk using 2 μg/mL puromycin, in which the medium containing puromycin was changed every 2-3 d. The cells were maintained in 1 μg/mL puromycin. Western blotting and RT-qPCR were used to ensure that target genes were stably knocked down or overexpressed.
SPSS 22.0 (IBM, Armonk, NY, United States) was used for all statistical analyses. ImageJ software (National Institutes of Health, Bethesda, MD, United States) was used for the quantification of images from immunohistochemistry. GraphPad Prism 8.0 (La Jolla, CA, United States) was used to create statistical figures. All presented images were representative of three or more individual experiments. One-way or two-way analysis of variance was performed for comparisons between multiple groups. All results were expressed as mean ± SD. P < 0.05 indicated statistically significant differences.
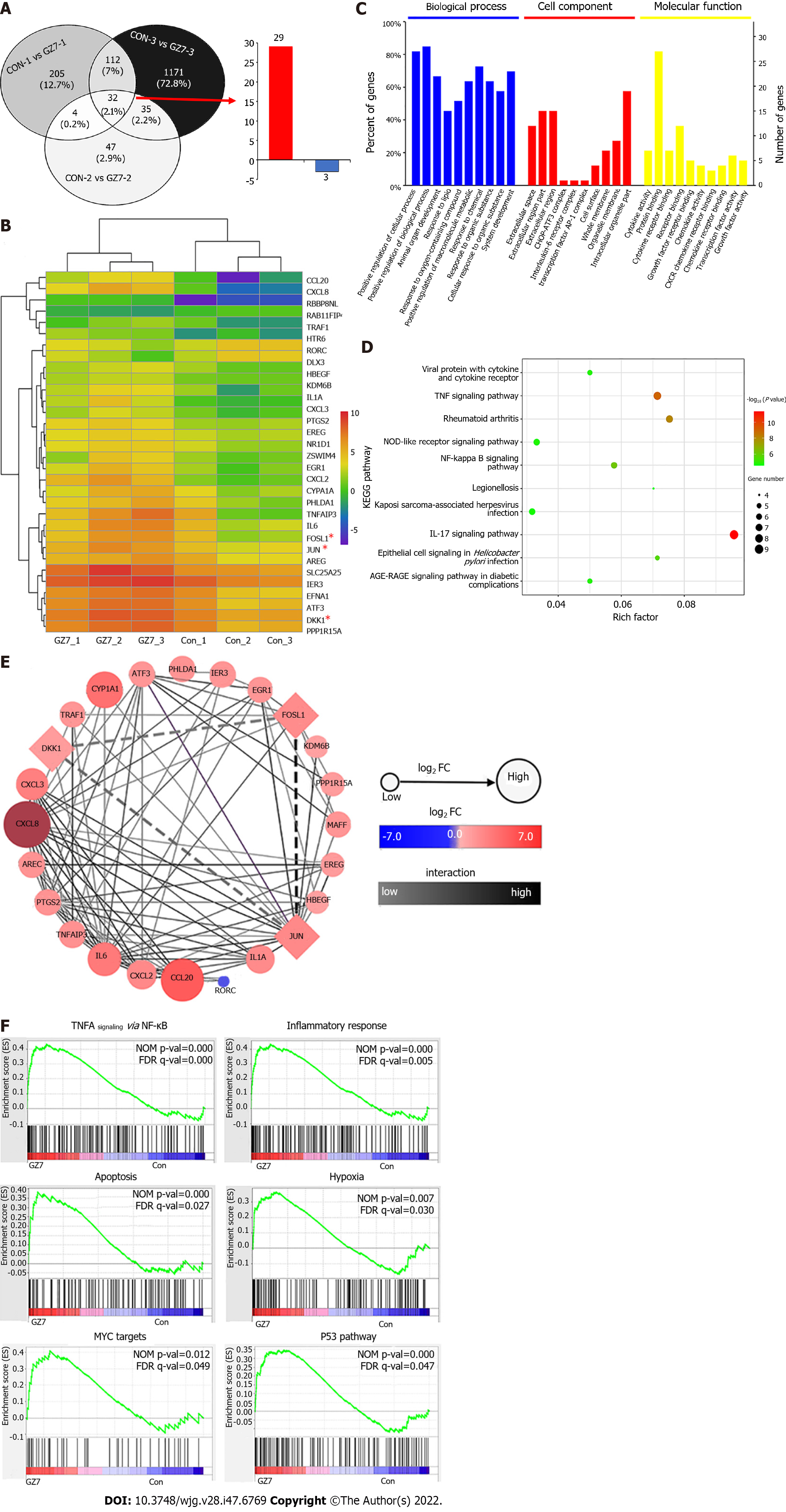
RNA-seq analysis identified 32 DEGs, including 29 upregulated and three downregulated genes that were shared by three pairs of primary GC cells infected with or without H. pylori (Figure 1A and B). These DEGs were mostly enriched in the regulation of biological processes, cytokine activity, protein binding, and tumor necrosis factor (TNF), nuclear factor (NF)-κB, and interleukin (IL)-17 pathways according to GO and KEGG pathway enrichment analysis (Figure 1C and D). The PPI network identified the hub genes JUN, CXCL8, CCL20, and FOSL1 and their interactions, and JUN and FOLS1 were directly connected to DKK1 (Figure 1E). Based on the GSEA of DEGs, TNFA signaling via NF-кB, the inflammatory response, apoptosis, hypoxia, MYC targets, and the P53 pathway were enriched in H. pylori-infected GC cells (Figure 1F). These molecular events are closely associated with GC development and progression. JUN, FOLS1, and DKK1 were selected for further investigation.
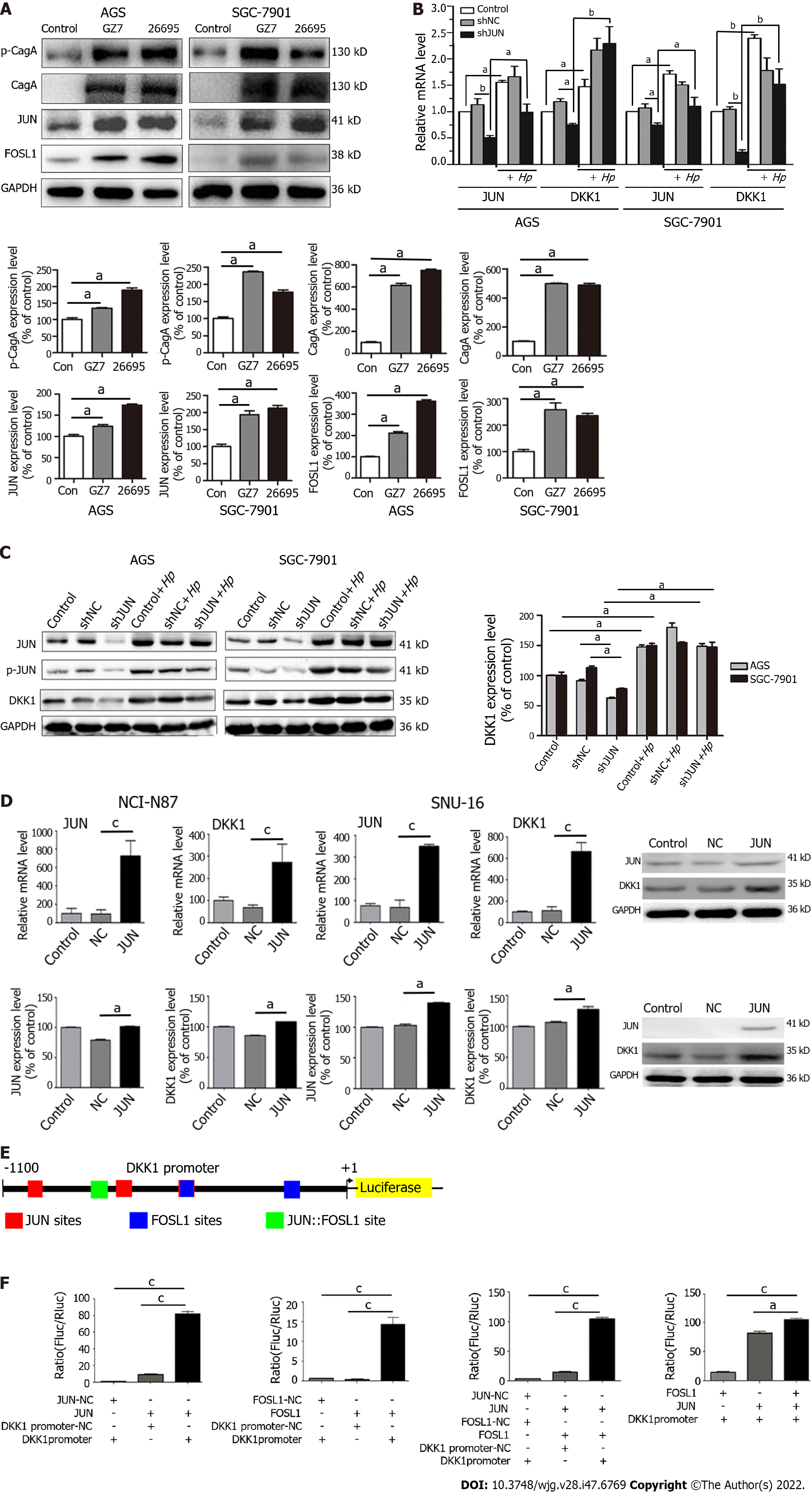
To confirm the results of RNA-seq, AGS and SGC-7901 cells were infected with H. pylori GZ7 and 26695. We discovered that JUN and FOSL1 proteins were highly expressed in H. pylori-infected cells compared to the control cells, which supported the RNA-seq resultss (Figure 2A). The H. pylori virulence factor CagA and its phosphorylation levels were dramatically elevated in H. pylori-infected cells (Figure 2A).
We determined JUN expression in eight GC cell lines. JUN was highly expressed in AGS and SGC-7901 cells but weakly expressed in NCI-N87 and SNU-16 cells (Supplementary Figure 1). JUN was knocked down using short hairpin RNA (shRNA) in AGS and SGC-7901 cells and overexpressed using lentivirus infection in NCI-N87 and SNU-16 cells. RT-qPCR and western blotting revealed a substantial decrease in DKK1 expression after JUN knockdown. When the JUN-knockdown cells were infected with H. pylori, the mRNA and protein levels of DKK1 recovered and JUN expression and phosphorylation increased (Figure 2B and C). In contrast, the mRNA and protein level of DKK1 was significantly increased in SNU-16 and NCI-N87 cells after JUN overexpression (Figure 2D). These findings revealed that H. pylori-induced JUN enhanced DKK1 expression.
JUN and FOSL1 comprise AP-1 via their basic leucine zipper domain[28]. AP-1-binding sites include the 5′-TGA(C/G) TCA-3′ (TRE motif), 5′-TCACGTCA-3′ (CRE motif), and their single-base variants[29]. To confirm that AP-1 transcriptionally regulated DKK1 expression, we predicted AP-1-binding sites in the promoter region from -1100 to + 1 bp upstream of the DKK1 transcription site, which is highly conserved in mammalian genomic DNA[30]. Three JUN sites, two FOSL1 sites, and a JUN::FOSL site were observed in the DKK1 promoter (Figure 2E). The DKK1 promoter region from -1100 to + 1 bp was inserted into a luciferase reporter plasmid, which was cotransfected into AGS cells with JUN and/or FOSL1 vectors. The dual-luciferase reporter assay indicated that transfection of AGS cells with JUN and FOSL1 markedly increased the luciferase activity, and the effect of the JUN vector was stronger. JUN and FOSL1 cotransfection exhibited the highest luciferase activity in AGS cells (Figure 2F). This result confirmed that AP-1, including the JUN::JUN and JUN::FOSL1 complexes, bound to the DKK1 promoter to initiate DKK1 transcription.
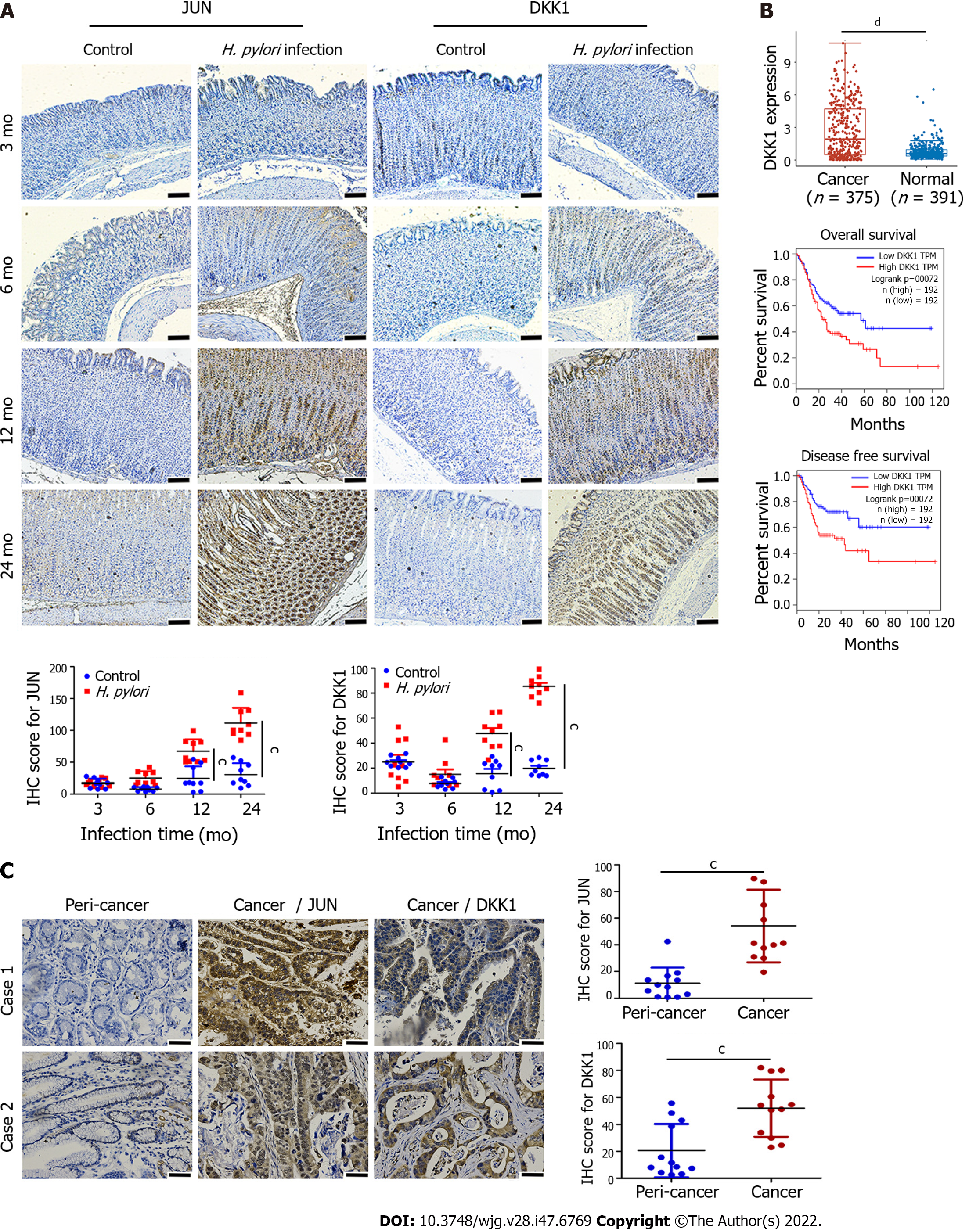
We successfully established GC models in Mongolian gerbils via H. pylori infection in a previous study, in which erosion, atrophy, intestinal metaplasia, and well-differentiated GC were progressively detected in the stomach mucosa[26]. Positive JUN and DKK1 staining increased progressively in gerbil gastric epithelium with H. pylori infection from 3 mo to 24 mo in the present study (Figure 3A), which suggested that the expression of JUN and DKK1 was linked to the pathological development of GC in gerbils. Human GC genomic data were extracted from the TCGA-Stomach Adenocarcinoma database (TCGA-STAD), which included 375 cancer and 391 normal tissues. The expression of DKK1 mRNA was significantly higher in GC tissues than normal tissues, and GC patients with a higher DKK1 expression had a shorter overall and disease-free survival (Figure 3B). Human GC tissues showed stronger positive staining for JUN and DKK1 than pericancer tissues (Figure 3C). However, no distinction was observed in the levels of JUN mRNA between GC and normal tissues in the TCGA-STAD database (Supplementary Figure 2).
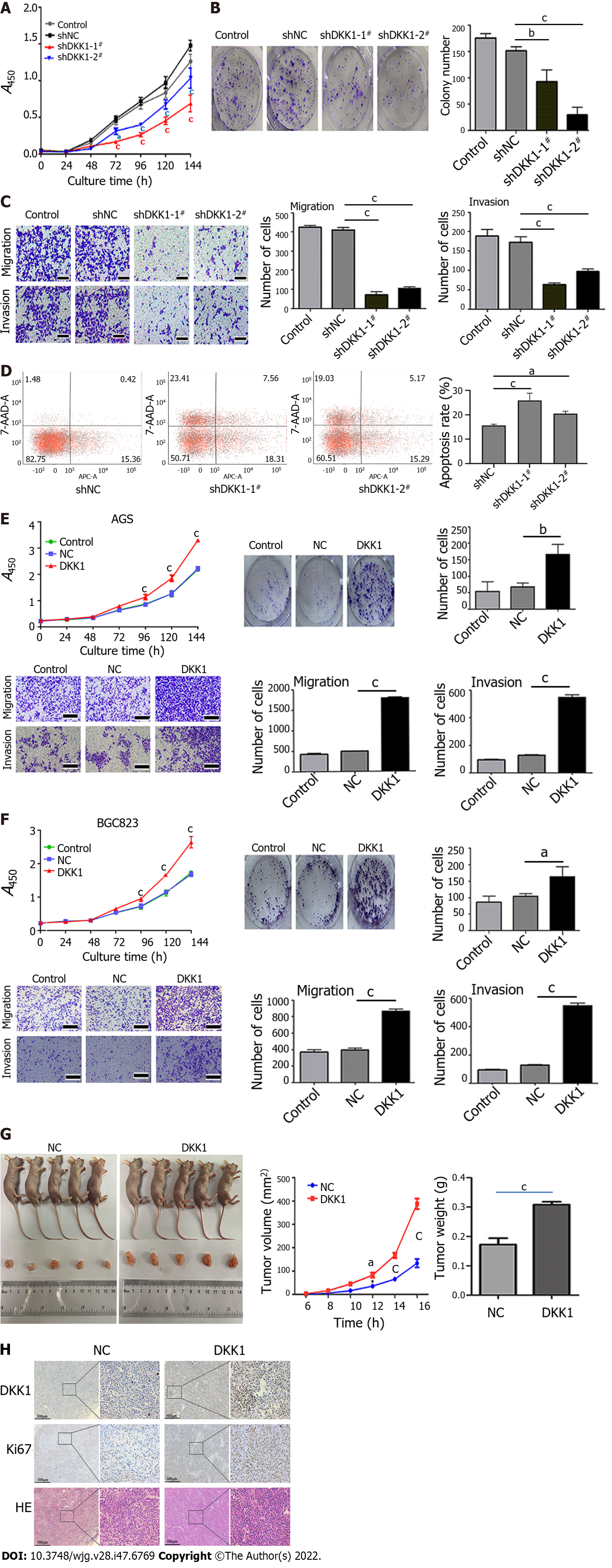
To investigate the function of DKK1 in GC, we first established DKK1-knockdown AGS cells using two independent shRNAs (Supplementary Figure 3A) and found that cell proliferation and colony formation were significantly inhibited in AGS cells (Figure 4A and B). DKK1 knockdown also suppressed AGS cell migration and invasion (Figure 4C). Flow cytometry showed that the knockdown of DKK1 greatly increased apoptosis in AGS cells, especially early apoptosis (Figure 4D).
We generated stable DKK1-overexpressing AGS and BGC823 cells (Supplementary Figure 3B). In contrast to DKK1 knockdown, DKK1 overexpression promoted AGS and BGC823 cell growth, colony formation, migration, and invasion (Figure 4E and F). We subcutaneously implanted the DKK1-overexpressing BGC823 cells into nude mice and found that DKK1 overexpression in cancer cells increased the growth of xenograft tumors in mice. The tumor weight and volume in DKK1-overexpressing nude mice were significantly greater than normal control mice (Figure 4G). The mice were killed, and the subcutaneous tumors were removed. Immunohistochemical staining confirmed that DKK1 and Ki67 (cell proliferation marker) were expressed at higher levels in DKK1-overexpressing than control tumors (Figure 4H). These findings verified the tumor-promoting effects of DKK1 in GC.
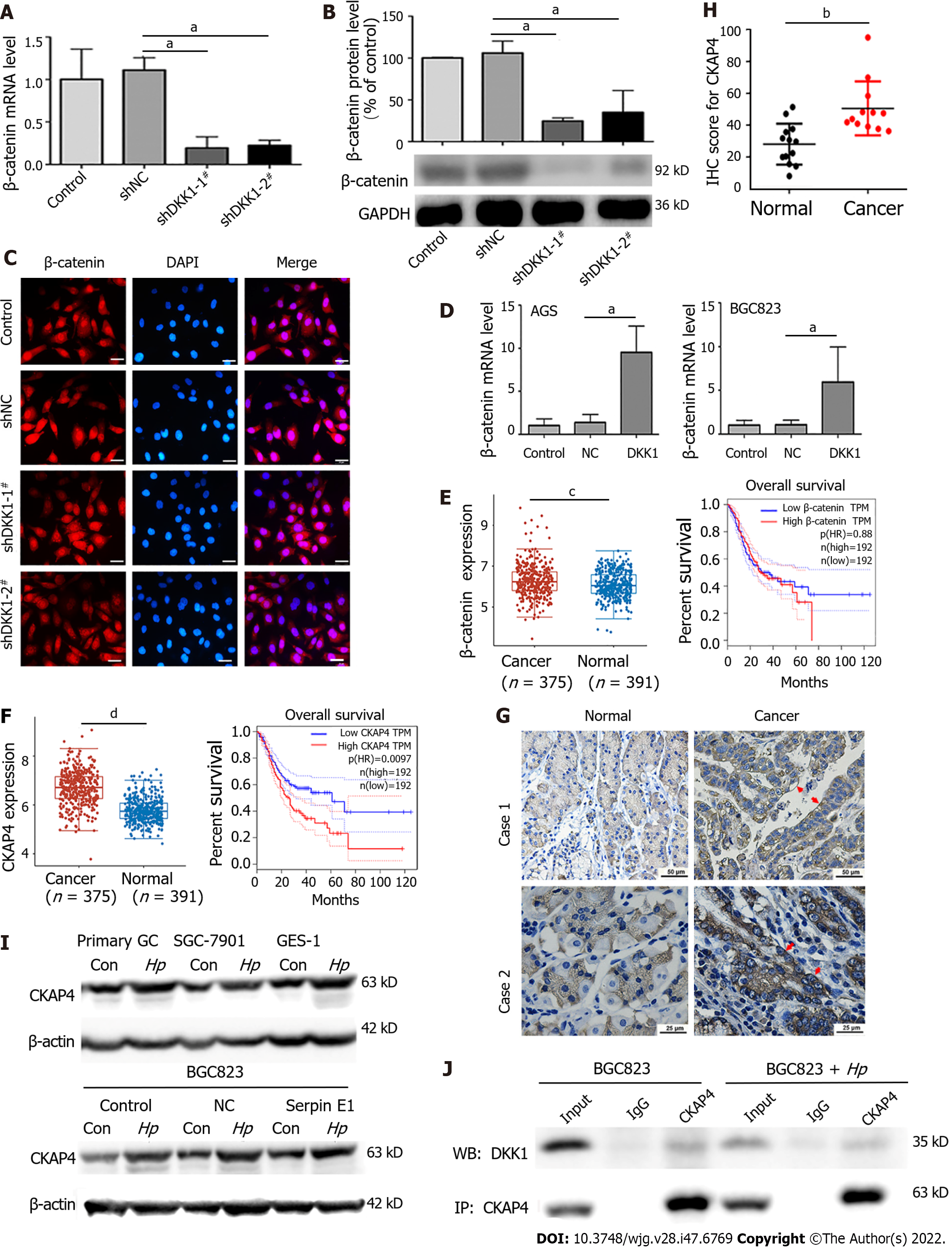
DKK1 suppresses the Wnt/β-catenin pathway by interacting with Wnt coreceptors LRP5/6. Therefore, we detected β-catenin expression and nuclear translocation in DKK1-knockdown AGS cells and discovered that the levels of β-catenin mRNA and protein were reduced by DKK1 knockdown without significant change in β-catenin nuclear translocation, which was even lower in shDKK1-2# cells (Figure 5A-C). In contrast, DKK1 overexpression enhanced β-catenin mRNA levels in AGS and BGC823 cells (Figure 5D). The expression of β-catenin in GC tissues was greater than in normal tissues, but there is no correlation between β-catenin expression and GC patients' survival in the TCGA-STAD dataset (Figure 5E). The findings suggest that the effects of DKK1 are independent of Wnt/β-catenin signaling in GC.
CKAP4 was recently discovered as a DKK1 receptor. We found that CKAP4 mRNA and protein expression was increased in 12 clinical GC samples, especially in the cell surface membrane (red arrow), and 375 GC specimens from the TCGA-STAD database (Figure 5F-H). Infection with H. pylori also upregulated CKAP4 expression in GC cells (Figure 5I). Higher CKAP4 expression was linked to lower overall survival in GC patients (Figure 5F). The binding of endogenous DKK1 to CKAP4 was detected in H. pylori-infected and uninfected BGC823 cells (Figure 5J).
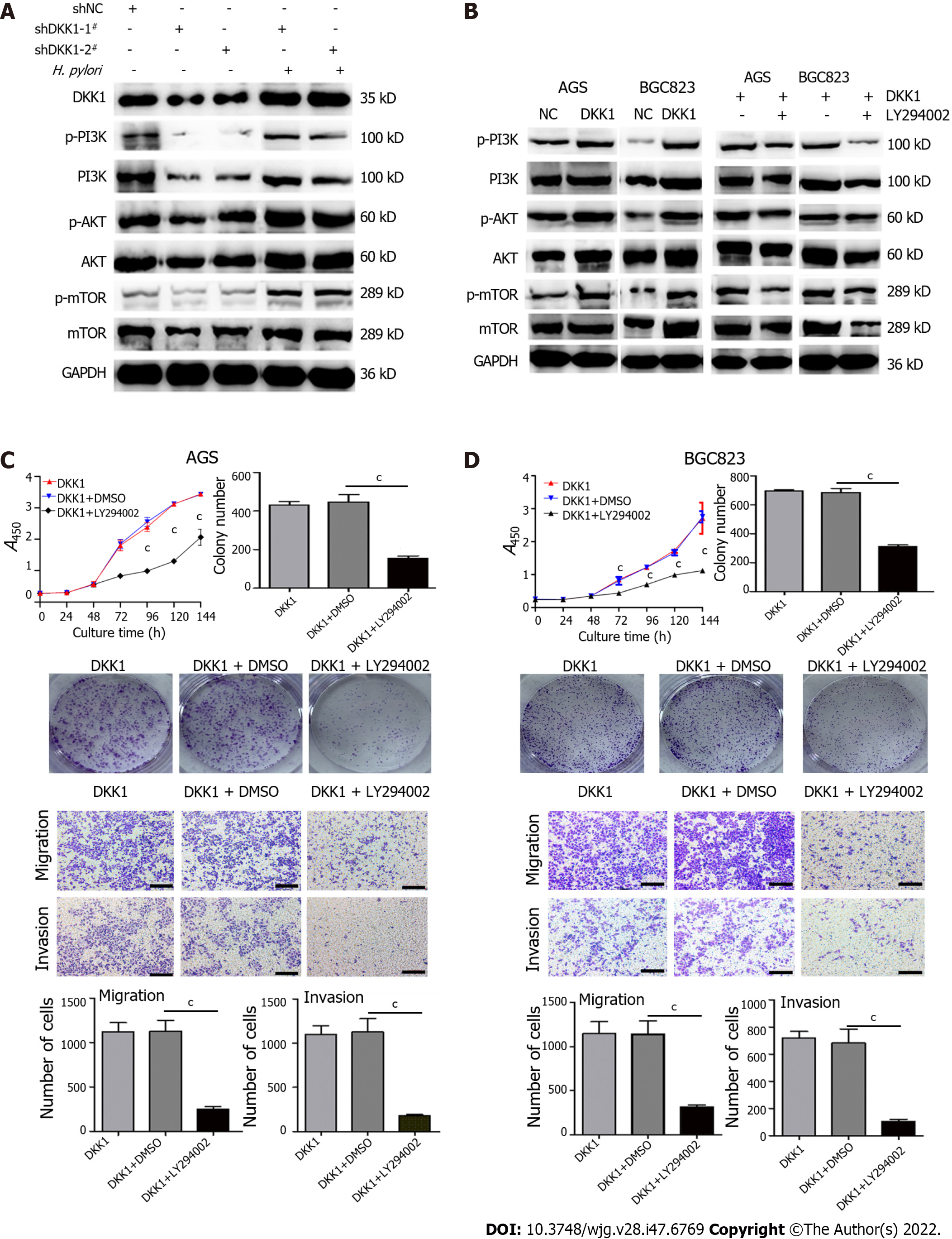
After confirming the DKK1/CKAP4 axis in GC cells, we evaluated whether DKK1 knockdown and overexpression affected the PI3K/AKT/mTOR pathway. DKK1 knockdown markedly inhibited the expression of p-PI3K, PI3K, p-AKT, AKT, p-mTOR, and mTOR, and p-PI3K, p-AKT, and p-mTOR were more inhibited. H. pylori infection restored the expression of DKK1 and increased PI3K/AKT/mTOR signaling in DKK1 knockdown cells (Figure 6A). Conversely, DKK1 overexpression increased PI3K, AKT and mTOR phosphorylation, and a specific inhibitor of PI3K, LY294002, inhibited the DKK1-induced increase in the three phosphorylated proteins in AGS and BGC823 cells (Figure 6B). These findings suggest that H. pylori-upregulated DKK1 activated the PI3K/AKT/mTOR pathway.
Next, we examined the effects of LY294002 in DKK1-overexpressing AGS and BGC823 cells and found that blockade of PI3K/AKT/mTOR signaling with LY294002 significantly decreased cell proliferation, colony formation, migration and invasion (Figure 6C and D), which further confirmed our hypothesis that DKK1 promoted the malignant phenotypes of GC cells via activation of the PI3K/AKT/mTOR pathway.
Our current study revealed that H. pylori infection upregulated the expression of JUN and FOSL1, which formed AP-1 to activate DKK1 transcription. Gain- and loss-of-function studies showed that DKK1 had important tumor-promoting functions in H. pylori-related GC via activation of the CKAP4/PI3-K/AKT/mTOR pathway.
The gene expression profiles obtained by independent research groups using RNA-seq were inconsistent due to the use of diverse cell lines, GC tissues, H. pylori strains, and whole-genome expression arrays[31-33]. The upregulated expression of JUN and FOLS1 was noted in H. pylori NCTC11639-infected AGS cells, but not experimentally verified[34]. DKK1 was also downregulated in H. pylori 26695-infected AGS cells, but this observation was not tested experimentally[35]. Using RNA-seq, we identified 32 DEGs that were shared by all three models of primary GC cells infected with H. pylori GZ7. Of the 32 DEGs, JUN, FOSL1 and DKK1 were highly expressed in H. pylori-infected cells, which was confirmed in GC tissues and H. pylori-infected gerbil stomach tissues. The enrichment analysis of KEGG pathways suggested that DEGs were primarily involved in inflammatory signalings such as TNF, NF-κB and IL-17. GESA also revealed that H. pylori infection activates the TNFA pathway, the P53 pathway, apoptosis, hypoxia, and the inflammatory response.
DKK1 was recently found to be epigenetically downregulated by promoter hypermethylation, which resulted in the nuclear translocation of β-catenin and activation of the Wnt/β-catenin pathway in gastric intestinal metaplasia, high-grade adenoma, and adenocarcinoma[36,37]. Conversely, Kikuchi et al[38] observed that DKK1 promoted the proliferation, invasion and metastasis of cancer cells independent of Wnt signaling. Other studies also suggested that DKK1 was often overexpressed in diverse tumor tissues, including GC, and DKK1 overexpression strongly correlated with poor cancer patient survival[39,40]. However, the mechanism underlying this association was not clear. Our study explained, for the first time, why DKK1 was upregulated in GC. H. pylori infection upregulates JUN and FOSL1 expression to form AP-1. AP-1 binds to the TRE and CRE motifs in the DKK1 promoter region to transcriptionally activate DKK1 expression in GC. This finding is contradictory to two earlier studies[36,37]. We speculate that decreased expression of DKK1 via promotor methylation, as reported by Lu et al[36], is more prevalent in gastric precancerous lesions and early-stage adenocarcinoma, and increased expression of DKK1 via promotor binding of AP-1, as observed in our study, is more likely to occur in H. pylori-infected and advanced GC. Two observations provide support for our assertion. First, we discovered that high expression of JUN and DKK1 was mostly observed in the stomach tissues of gerbils at least 12 mo after H. pylori infection. Second, DKK1 is frequently overexpressed in GC patients with lymph node invasion and distant metastasis[41].
We demonstrated that the cancer-promoting function of DKK1 in GC was independent of Wnt/β-catenin signaling by observing that β-catenin expression and nuclear translocation were inhibited in DKK1-knockdown cells. CKAP4 was initially identified as an endoplasmic reticulum protein that was trafficked to the cell surface membrane after palmitoylation[42]. The combination of CKAP4 and DKK1 on the cell surface triggers pro-oncogenic or tumor-suppressive DKK1 signaling in different tumors[43,44]. Although CKAP4 expression is high in many tumors[45,46], there is no information on CKAP4 expression in GC. We discovered that H. pylori infection simultaneously increased the expression of DKK1 and CKAP4 in GC cells, particularly CKAP4 expression on the cell membrane. High levels of DKK1 and CKAP4 expression were also consistently observed in GC tissues from the TCGA-STAD database and clinical GC specimens and were linked to a poor prognosis for GC patients. Notably, the interaction between DKK1 and CKAP4 was detected in GC cells. These results show that the DKK1/CKAP4 axis is present in H. pylori-infected GC cells and GC tissues.
There are two tandem cysteine-rich domains in the DKK1 promoter region, CRD1 and CRD2. DKK1 CRD1 domain binding to LPR6 inhibits Wnt signaling, and DKK1 CRD2 domain binding to CKAP4 activates PI3K/AKT signaling[38]. CKAP4 overexpression on the surface of cancer cells likely prevents DKK1 binding to LRP6, resulting in subsequent suppression of Wnt/β-catenin signaling[47]. This presumption is partially supported by our experimental results that H. pylori-induced DKK1 activated the CKAP4/PI3K/AKT/mTOR pathway rather than inhibiting LRP6/Wnt/β-catenin signaling in GC cells. Infection with H. pylori reversed the PI3K/AKT/mTOR signaling inhibition caused by DKK1 knockdown. PI3K inhibition with LY294002 also suppressed the DKK1-mediated activation of the PI3K pathway and the malignant phenotype of GC cells. Recent evidence has shown that the PI3K/ AKT/mTOR and Wnt/β-catenin signalings are commonly activated in GC[48,49]. However, the reason for DKK1 selective activation of the CKAP4/PI3K/AKT/mTOR pathway in H. pylori-related GC is not known and requires further study.
There were several limitations to this study. First, the number of clinical samples was small. Therefore, further study with larger sample sizes is required to determine the expression of the DKK1/CAKP4 axis in GC tissues and its association with H. pylori infection in cancer tissues. Second, only two strains of H. pylori were used in this study: An East Asian strain (H. pylori GZ7) and a western strain (H. pylori 26695). However, H. pylori exhibit intrastrain and interstrain heterogeneity. More H. pylori strains will be required to verify our findings.
H. pylori infection upregulated JUN and FOSL1 expression, which formed AP-1 to promote DKK1 transcription that resulted in gastric tumorigenesis via activation of the CKAP4/PI3K/AKT/mTOR pathway. DKK1/CKAP4 interaction could become an attractive target for H. pylori-related GC therapy. The identification of small compounds and drugs targeting the DKK1/CKAP4 axis will be a crucial aspect of future studies. We will also investigate this possibility further.
Gastric cancer (GC) is one of the most common malignant tumors with a high morbidity and mortality rate globally, especially in East Asian countries. Helicobacter pylori (H. pylori) infection is the most significant risk factor for GC. Studying their interaction can reveal the potential pathogenesis and therapeutic targets of GC.
Although substantial efforts have been done to link H. pylori infection and GC over the past decades, the molecular mechanisms of H. pylori-induced GC are not fully understood, which results in reduced treatment benefits.
The present study aimed to study the interaction of H. pylori infection, dickkopf-related protein (DKK) 1, and cytoskeleton-associated protein (CAKP) 4 in GC and the underlying molecular mechanisms.
RNA sequencing identified differentially expressed genes (DEGs) between H. pylori-infected and uninfected primary GC cells. Dual-luciferase reporter assay and co-immunoprecipitation determined the interaction of activator protein (AP)-1, DKK1 and CKAP4. Western blotting and immunohistochemistry detected the expression of DKK1, CKAP4 and phosphatidylinositol 3-kinase (PI3K) pathway-related proteins in GC cells and tissues. Functional experiments and tumorigenicity in nude mice detected the malignant behavior of GC cells in vitro and in vivo.
H. pylori infection upregulated JUN, FOSL1, DKK1 and CKAP4 expression in GC cells, H. pylori-infected gerbil gastric tissues, and human GC samples. JUN and FOSL1 formed activator protein-1 (AP-1) to transcriptionally activate DKK1. DKK1 bound to CKAP4, but not Wnt coreceptor, to promote GC cell growth, migration, invasion, and xenograft tumor growth in nude mice via activating the PI-3K/AKT/mammalian target of rapamycin (mTOR) pathway. Targeting PI3K inhibited DKK1-mediated CKAP4/PI3K signaling activity and the malignant behavior of GC cells.
H. pylori-induced AP-1 promotes the binding of DKK1 to CAKP4, which contributes to gastric tumorigenesis via the PI3K/AKT/mTOR pathway.
The findings suggest that the DKK1/CKAP4 interaction may be a therapeutic target for H. pylori-induced GC. The identification of small compounds and drugs targeting the DKK1/CKAP4 axis will be a crucial aspect of future studies. We will also investigate this possibility further.
We thank Mrs. Hai-Yan Liu from Guizhou Medical University for his expert review of statistical analysis. We also appreciate The Cancer Genome Atlas (TCGA) database.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gao YL, China; Keikha M, Iran S-Editor: Chen YL L-Editor: A P-Editor: Chen YX
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64442] [Article Influence: 16110.5] [Reference Citation Analysis (176)] |
| 2. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2838] [Article Influence: 567.6] [Reference Citation Analysis (5)] |
| 3. | Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric adenocarcinoma. Nat Rev Dis Primers. 2017;3:17036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 4. | Crowe SE. Helicobacter pylori Infection. N Engl J Med. 2019;380:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 273] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 5. | Wroblewski LE, Peek RM Jr, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 1012] [Article Influence: 67.5] [Reference Citation Analysis (1)] |
| 6. | de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180-e190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1299] [Article Influence: 259.8] [Reference Citation Analysis (0)] |
| 7. | Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1624] [Cited by in RCA: 1786] [Article Influence: 446.5] [Reference Citation Analysis (1)] |
| 8. | Sexton RE, Al Hallak MN, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020;39:1179-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 456] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 9. | Bejjani F, Evanno E, Zibara K, Piechaczyk M, Jariel-Encontre I. The AP-1 transcriptional complex: Local switch or remote command? Biochim Biophys Acta Rev Cancer. 2019;1872:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 10. | Atsaves V, Leventaki V, Rassidakis GZ, Claret FX. AP-1 Transcription Factors as Regulators of Immune Responses in Cancer. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 178] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 11. | Trop-Steinberg S, Azar Y. AP-1 Expression and its Clinical Relevance in Immune Disorders and Cancer. Am J Med Sci. 2017;353:474-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 12. | Jiang X, Hui F, Qin X, Wu Y, Liu H, Gao J, Li X, Xu Y, Zhang Y. Diagnosis Accuracy and Prognostic Significance of the Dickkopf-1 Protein in Gastrointestinal Carcinomas: Systematic Review and Network Meta-analysis. J Cancer. 2020;11:7091-7100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Ahn VE, Chu ML, Choi HJ, Tran D, Abo A, Weis WI. Structural basis of Wnt signaling inhibition by Dickkopf binding to LRP5/6. Dev Cell. 2011;21:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Menezes ME, Devine DJ, Shevde LA, Samant RS. Dickkopf1: a tumor suppressor or metastasis promoter? Int J Cancer. 2012;130:1477-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Jiang H, Zhang Z, Yu Y, Chu HY, Yu S, Yao S, Zhang G, Zhang BT. Drug Discovery of DKK1 Inhibitors. Front Pharmacol. 2022;13:847387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 16. | Gao C, Xie R, Ren C, Yang X. Dickkopf-1 expression is a novel prognostic marker for gastric cancer. J Biomed Biotechnol. 2012;2012:804592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Hong SA, Yoo SH, Lee HH, Sun S, Won HS, Kim O, Ko YH. Prognostic value of Dickkopf-1 and ß-catenin expression in advanced gastric cancer. BMC Cancer. 2018;18:506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Lee HS, Lee HE, Park DJ, Kim HH, Kim WH, Park KU. Clinical significance of serum and tissue Dickkopf-1 levels in patients with gastric cancer. Clin Chim Acta. 2012;413:1753-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Bhavanasi D, Speer KF, Klein PS. CKAP4 is identified as a receptor for Dickkopf in cancer cells. J Clin Invest. 2016;126:2419-2421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Kimura H, Fumoto K, Shojima K, Nojima S, Osugi Y, Tomihara H, Eguchi H, Shintani Y, Endo H, Inoue M, Doki Y, Okumura M, Morii E, Kikuchi A. CKAP4 is a Dickkopf1 receptor and is involved in tumor progression. J Clin Invest. 2016;126:2689-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 149] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 21. | Baghery Saghchy Khorasani A, Pourbagheri-Sigaroodi A, Pirsalehi A, Safaroghli-Azar A, Zali MR, Bashash D. The PI3K/Akt/mTOR signaling pathway in gastric cancer; from oncogenic variations to the possibilities for pharmacologic interventions. Eur J Pharmacol. 2021;898:173983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Nam SY, Lee HS, Jung GA, Choi J, Cho SJ, Kim MK, Kim WH, Lee BL. Akt/PKB activation in gastric carcinomas correlates with clinicopathologic variables and prognosis. APMIS. 2003;111:1105-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Lang SA, Gaumann A, Koehl GE, Seidel U, Bataille F, Klein D, Ellis LM, Bolder U, Hofstaedter F, Schlitt HJ, Geissler EK, Stoeltzing O. Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. Int J Cancer. 2007;120:1803-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 24. | Zeng X, Xiong L, Wang W, Zhao Y, Xie Y, Wang Q, Zhang Q, Li L, Jia C, Liao Y, Zhou J. Whole-genome sequencing and comparative analysis of Helicobacter pylori GZ7 strain isolated from China. Folia Microbiol (Praha). 2022;67:923-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 25. | Chen X, Chen W, Zhao Y, Wang Q, Wang W, Xiang Y, Yuan H, Xie Y, Zhou J. Interplay of Helicobacter pylori, fibroblasts, and cancer cells induces fibroblast activation and serpin E1 expression by cancer cells to promote gastric tumorigenesis. J Transl Med. 2022;20:322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 26. | Zhao Y, Xie Y, Chen X, Xu W, Wang Y, Zhou J. [Establishment of Mongolian gerbil model of gastric cancer induced by Helicobacter pylori infection and its proteomics analysis]. Zhonghua Bing Li Xue Za Zhi. 2014;43:820-826. [PubMed] |
| 27. | Varghese F, Bukhari AB, Malhotra R, De A. IHC Profiler: an open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 2014;9:e96801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 636] [Cited by in RCA: 986] [Article Influence: 89.6] [Reference Citation Analysis (0)] |
| 28. | Fan F, Podar K. The Role of AP-1 Transcription Factors in Plasma Cell Biology and Multiple Myeloma Pathophysiology. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Zhou H, Zarubin T, Ji Z, Min Z, Zhu W, Downey JS, Lin S, Han J. Frequency and distribution of AP-1 sites in the human genome. DNA Res. 2005;12:139-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Park SB, Seo KW, So AY, Seo MS, Yu KR, Kang SK, Kang KS. SOX2 has a crucial role in the lineage determination and proliferation of mesenchymal stem cells through Dickkopf-1 and c-MYC. Cell Death Differ. 2012;19:534-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Liu D, Zhu J, Ma X, Zhang L, Wu Y, Zhu W, Xing Y, Jia Y, Wang Y. Transcriptomic and Metabolomic Profiling in Helicobacter pylori-Induced Gastric Cancer Identified Prognosis- and Immunotherapy-Relevant Gene Signatures. Front Cell Dev Biol. 2021;9:769409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Sharma CM, Hoffmann S, Darfeuille F, Reignier J, Findeiss S, Sittka A, Chabas S, Reiche K, Hackermüller J, Reinhardt R, Stadler PF, Vogel J. The primary transcriptome of the major human pathogen Helicobacter pylori. Nature. 2010;464:250-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 908] [Cited by in RCA: 919] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 33. | Zhang J, Wei J, Wang Z, Feng Y, Wei Z, Hou X, Xu J, He Y, Yang D. Transcriptome hallmarks in Helicobacter pylori infection influence gastric cancer and MALT lymphoma. Epigenomics. 2020;12:661-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Sepulveda AR, Tao H, Carloni E, Sepulveda J, Graham DY, Peterson LE. Screening of gene expression profiles in gastric epithelial cells induced by Helicobacter pylori using microarray analysis. Aliment Pharmacol Ther. 2002;16 Suppl 2:145-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Kim SH, Sierra RA, McGee DJ, Zabaleta J. Transcriptional profiling of gastric epithelial cells infected with wild type or arginase-deficient Helicobacter pylori. BMC Microbiol. 2012;12:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Lu W, Ni Z, Tong M, Jiang S, Zhang J, Feng C, Han C, Yuan T, Wang N, Zhao J, Sun N, Liu C, Jia Q, Wu Q, Ning H, Shi Y. DKK1 is epigenetically downregulated by promoter methylation and inhibits bile acid-induced gastric intestinal metaplasia. Biochem Biophys Res Commun. 2020;523:780-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Wang Z, Ye Y, Liu D, Yang X, Wang F. Hypermethylation of multiple Wnt antagonist genes in gastric neoplasia: Is H pylori infection blasting fuse? Medicine (Baltimore). 2018;97:e13734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Kikuchi A, Matsumoto S, Sada R. Dickkopf signaling, beyond Wnt-mediated biology. Semin Cell Dev Biol. 2022;125:55-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Igbinigie E, Guo F, Jiang SW, Kelley C, Li J. Dkk1 involvement and its potential as a biomarker in pancreatic ductal adenocarcinoma. Clin Chim Acta. 2019;488:226-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 40. | Liu Y, Tang W, Xie L, Wang J, Deng Y, Peng Q, Zhai L, Li S, Qin X. Prognostic significance of dickkopf-1 overexpression in solid tumors: a meta-analysis. Tumour Biol. 2014;35:3145-3154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Liu QR, Li YF, Deng ZQ, Cao JQ. Prognostic Significance of Dickkopf-1 in Gastric Cancer Survival: A Meta-Analysis. Genet Test Mol Biomarkers. 2016;20:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Sada R, Kimura H, Fukata Y, Fukata M, Yamamoto H, Kikuchi A. Dynamic palmitoylation controls the microdomain localization of the DKK1 receptors CKAP4 and LRP6. Sci Signal. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 43. | Li SX, Liu LJ, Dong LW, Shi HG, Pan YF, Tan YX, Zhang J, Zhang B, Ding ZW, Jiang TY, Hu HP, Wang HY. CKAP4 inhibited growth and metastasis of hepatocellular carcinoma through regulating EGFR signaling. Tumour Biol. 2014;35:7999-8005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Li MH, Dong LW, Li SX, Tang GS, Pan YF, Zhang J, Wang H, Zhou HB, Tan YX, Hu HP, Wang HY. Expression of cytoskeleton-associated protein 4 is related to lymphatic metastasis and indicates prognosis of intrahepatic cholangiocarcinoma patients after surgery resection. Cancer Lett. 2013;337:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Li SX, Li J, Dong LW, Guo ZY. Cytoskeleton-Associated Protein 4, a Promising Biomarker for Tumor Diagnosis and Therapy. Front Mol Biosci. 2020;7:552056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Luo T, Ding K, Ji J, Zhang X, Yang X, Chen A, Huang B, Zhang D, Wang J, Li X. Cytoskeleton-associated protein 4 (CKAP4) promotes malignant progression of human gliomas through inhibition of the Hippo signaling pathway. J Neurooncol. 2021;154:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Kikuchi A, Fumoto K, Kimura H. The Dickkopf1-cytoskeleton-associated protein 4 axis creates a novel signalling pathway and may represent a molecular target for cancer therapy. Br J Pharmacol. 2017;174:4651-4665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Fattahi S, Amjadi-Moheb F, Tabaripour R, Ashrafi GH, Akhavan-Niaki H. PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci. 2020;262:118513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 247] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 49. | Wang Y, Zheng L, Shang W, Yang Z, Li T, Liu F, Shao W, Lv L, Chai L, Qu L, Xu Q, Du J, Liang X, Zeng J, Jia J. Wnt/beta-catenin signaling confers ferroptosis resistance by targeting GPX4 in gastric cancer. Cell Death Differ. 2022;29:2190-2202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 249] [Article Influence: 83.0] [Reference Citation Analysis (0)] |