Published online Dec 21, 2022. doi: 10.3748/wjg.v28.i47.6752
Peer-review started: August 29, 2022
First decision: October 20, 2022
Revised: November 2, 2022
Accepted: November 22, 2022
Article in press: November 22, 2022
Published online: December 21, 2022
Processing time: 111 Days and 20.1 Hours
Although expression of interleukin (IL)-34 is upregulated in active ulcerative colitis (UC), the molecular function and underlying mechanism are largely un
To investigate the function of IL-34 in acute colitis, in a wound healing model and in colitis-associated cancer in IL-34-deficient mice.
Colitis was induced by administration of dextran sodium sulfate (DSS), and carcinogenesis was induced by azoxymethane (AOM). Whether the impact of IL-34 on colitis was dependent on macrophages was validated by depletion of macrophages in a murine model. The association between IL-34 expression and epithelial proliferation was studied in patients with active UC.
IL-34 deficiency aggravated murine colitis in acute colitis and in wound healing phase. The effect of IL-34 on experimental colitis was not dependent on macrophage differentiation and polarization. IL-34-deficient mice developed more tumors than wild-type mice following administration of AOM and DSS. No significant difference was shown in degree of cellular differentiation in tumors between wild-type and IL-34-deficient mice. IL-34 was dramatically increased in the active UC patients as previously reported. More importantly, expression of IL-34 was positively correlated with epithelial cell proliferation in patients with UC.
IL-34 deficiency exacerbates colonic inflammation and accelerates colitis-associated carcinogenesis in mice. It might be served as a potential therapeutic target in UC.
Core Tip: This study highlights the role of interleukin (IL)-34 in acute experimental colitis and wound healing phase in mice. We found that IL-34 did not drive inflammatory response and tissue destruction in physiological conditions, but protects the host from inflammatory injury and reduces the risk of colitis-associated cancer. IL-34 might serve as a potential therapeutic target for inducing mucosal healing in treatment of ulcerative colitis (UC) and reducing colitis-associated cancer in UC.
- Citation: Liu ZX, Chen WJ, Wang Y, Chen BQ, Liu YC, Cheng TC, Luo LL, Chen L, Ju LL, Liu Y, Li M, Feng N, Shao JG, Bian ZL. Interleukin-34 deficiency aggravates development of colitis and colitis-associated cancer in mice. World J Gastroenterol 2022; 28(47): 6752-6768
- URL: https://www.wjgnet.com/1007-9327/full/v28/i47/6752.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i47.6752
Ulcerative colitis (UC) is a chronic progressive recurrent intestinal inflammatory disorder characterized by bloody diarrhea and abdominal pain[1]. UC is an important promoter of colorectal cancer[2]. The exact pathogenesis of UC remains unclear. It is currently assumed that it involves immunological derangement of the gut microbiota in genetically predisposed individuals after exposure to environmental factors[1]. Cytokines exert multiple effects and participate in the pathogenesis of colitis[3]. Identification of distinctive cytokines involved in immunopathogenesis of UC has become a hot spot for the development of biological therapies[4].
Colony-stimulating factor-1 receptor (CSF-1R) signaling regulates intestinal and colon development, gut homeostasis and inflammatory reaction[5]. Interleukin (IL)-34 was discovered in 2008 as a specific ligand for CSF-1R independent of CSF-1[6,7]. Human IL-34 is a homodimeric glycoprotein that consists of 242 amino acids produced by various cell types including epithelial, endothelial and immune cells, and fibroblasts[8]. Secreted IL-34 binds to the extracellular domains of CSF-1R and protein-tyrosine phosphatase, the other receptor for IL-34, which is regulated by syndecan-1, which results in autophosphorylation of its intracellular tyrosine residues and activates several signaling pathways controlling cell biological function[8,9]. IL-34 functions as a pivotal regulator in cell differentiation, proliferation and survival in the mononuclear phagocyte system[10]. Additionally, IL-34 mediates the crosstalk between the innate and adaptive immune systems during inflammation[8,11].
Under normal circumstances, IL-34 is primarily expressed in a tissue-specific manner in keratinocytes and neurons[8]. In pathological status, the expression pattern is changed. Increased IL-34 expression positively associates with disease progression, severity and chronicity in autoimmune diseases such as rheumatoid arthritis and Sjogren’s syndrome[12]. In contrast, IL-34 has demonstrated beneficial effects in other diseases. IL-34 has been identified as a new mediator to induce transplant tolerance by targeting suppressive T regulatory cells[13]. Therefore, the multiple function of IL-34 in different diseases is complex, disputable, and context-dependent[8].
The involvement of IL-34 in inflammatory bowel disease (IBD) has also drawn attention recently. Higher expression of IL-34 in human ileum compared with colon has been revealed[14]. In human and experimental colitis, the mRNA and protein level of IL-34 is markedly increased in inflamed mucosa compared to that in the matched normal mucosa and in healthy controls[14]. Recently, it has been proposed that IL-34 plays a prominent role in intestinal fibrogenesis via a p38 mitogen-activated-protein-kinase-dependent mechanism in Crohn’s disease[15]. In vitro, tumor necrosis factor (TNF)-α significantly upregulates expression of IL-34 in lamina propria mononuclear cells (LPMCs) via NF-kB signaling. Intriguingly, LPMCs treated with IL-34 increase expression of proinflammatory cytokines such as IL-6, TNF-α and chemokine CC ligand 20[14]. Accordingly, IL-34 neutralization decreases synthesis of TNF-α and IL-6 in IBD mucosal explants[14]. Therefore, it has been noted that IL-34 sustains gut inflammation via regulating positive feedback of proinflammatory cytokine production in vitro[8,14]. On the contrary, IL-34 has previously demonstrated immunosuppressive characteristics that contribute to inflammation improvement by inducing differentiation of monocytes into M2 phenotype[16]. M2 macrophages mediate immunotolerance and promote wound healing in experimental colitis in vivo[17,18]. IL-34 is upregulated in colon cancer and sustains the protumorigenic signals by inducing proliferation of cancer cells via extracellular signal-regulated kinase (ERK) 1/2 or cancer-associated fibroblasts[19,20]. Whether IL-34 is “friend or foe” in the pathogenesis of UC remains to be explored. In this study, we investigated the potential role of IL-34 in experimental colitis, colitis-associated carcinogenesis and UC.
IL-34-deficient mice were generated in C57BL/6J mice by using CRISPR/Cas9 technology (Beijing Cas Gene Biotech, Beijing, China). Gene targeting technology was applied to delete exons 3-5 in the IL-34 gene and led to frameshift mutation of IL-34 gene. IL-34-deficient and C57BL/6J wild-type mice were bred and maintained in a specific-pathogen-free animal facility in the Laboratory Animal Center of Nantong University. All animal experiments were approved by the Institutional Ethics Committee of Nantong University (Date: 21/12/2015, Number: S20151221-908).
Acute colitis was induced by oral administration of 3% (M/V) dextran sulfate sodium (DSS, MW: 36 000-50 000; MP Biologicals, LLC, California, United States) in drinking water for 7 d. The mice were killed to obtain colon tissues at the indicated time or until day 16 to record the mortality. The murine wound healing model was established by oral administration of 3% DSS for 5 d and then switched to normal water for the following 5 d. The mice were killed on day 8 or 10. Colitis-associated cancer was induced by administration of the carcinogen azoxymethane (AOM; Sigma, Darmstadt, Germany) and repeated administration of DSS. The mice were given a single injection of AOM (10 mg/kg). Seven days later, mice were administrated 1.25% DSS (w/v) in drinking water for seven consecutive days, and fresh drinking water for 14 d. Seven days of DSS and 14 d of fresh water was repeated four times as a cycle[21]. The mice were killed, and the incidence rate of tumors was analyzed. Macrophage depletion from murine colons in vivo was performed as described previously[22]. In brief, 200 μL clodronate liposomes (Liposoma Research, Amsterdam, Netherlands) were intraperitoneally injected into mice 2 d prior to onset of experimental colitis and every 2 d during the process.
The percentage of body weight change of each mouse was recorded throughout the duration of DSS administration. Fresh feces from mice were collected for occult blood tests using a fecal occult blood kit (Nanjing Jiancheng Bioengineering, Jiangsu, China). The clinical score index of the murine model consisted of stool consistency and fecal occult blood, as previously described[23].
Human tissue specimens were obtained from 40 adult patients with active UC and 20 healthy controls during colonoscopy in Nantong Third People’s Hospital Affiliated to Nantong University and Affiliated Hospital of Nantong University between January 2014 and August 2015. Histopathology was evaluated by an experienced pathologist. This study was approved by the Ethics Committee of Nantong Third People’s Hospital Affiliated to Nantong University and Affiliated Hospital of Nantong University, and all people signed an informed consent form. All patients met the diagnostic criteria that were in line with the Consensus on Diagnosis and Management of Inflammatory Bowel Disease (2018, Beijing, China). The patients did not receive any therapy before colonoscopy. More details on the patients’ characteristics can be found in Supplementary Table 1.
The entire colon was harvested for measuring the length. The colon was washed in phosphate-buffer saline and fixed in 10% formaldehyde solution for 24 h. Hematoxylin-eosin (H&E) staining was performed on the tissue sections. The inflammatory score was as follows: Presence of occasional inflammatory cells in the lamina propria was scored as 0; increased numbers of inflammatory cells in the lamina propria was scored as 1; confluence of inflammatory cells extending into the submucosa was scored as 2; and transmural extension of the infiltrate was scored as 3. For tissue damage score, no mucosal damage was scored as 0; lymphoepithelial lesions were scored as 1; surface mucosal erosion or focal ulceration was scored as 2; and extensive mucosal damage and extension into deeper structures of the bowel wall was scored as 3. The combined histological score was calculated by inflammatory and tissue damage score, and ranged from 0 (no changes) to 6 (extensive infiltration and tissue damage).
The colonic tissues were sectioned into 4-μm slices following fixation with 10% formaldehyde and embedded in paraffin. Immunohistochemical staining was conducted as in our previous study[24]. Tissue slices were incubated with 3% H2O2 for 15 min after deparaffinization. Antigen was retrieved in citrate buffer (pH 6.0; Maxim Biotechnologies, Fuzhou, China) in a microwave for 10 min. Nonimmune 10% goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA, United States) was used to block nonspecific reactions. The sections were incubated with primary antibodies: rabbit anti human/mouse IL34, 1:100 (Abcam, Cambridge, MA, United States); rabbit anti human/mouse Ki-67 antibody, 1:200 (Abcam); rabbit anti mouse CSF1-R 1:1000 (Abcam); and rabbit anti-mouse CD68, 1:100 (Boster Biological Technology, Wuhan, China) overnight at 4 °C. On the second day, the sections were incubated with horseradish-peroxidase-conjugated secondary antibody (Shanghai Long Island Biotechnology, Shanghai, China) for 30 min at room temperature. 3, 3’-diaminobenzidine (Maxim Biotechnologies) was applied for 30 s. Hematoxylin was applied for contrast staining. Proliferation index of Ki-67 was defined as the percentage of Ki-67-positive cells in crypts (CK4) within the random visual scope. Positive index of CSF1-R was defined as the percentage of CSF1-R-positive cells in colonic mucosa within the random visual scope. All the tissue sections were analyzed by optical microscopy (IX73; Olympus, Tokyo, Japan) by experienced pathologists.
Colonic tissue sections were prepared as described above. Apoptosis was analyzed by fluorescence microscopy according to standard procedure using in situ cell death detection (Roche, Basel, Switzerland). Five randomly optical fields were chosen for further analysis.
Total RNA was extracted from the indicated murine colonic tissues by Trizol Reagent (TaKaRa Bio, Dalian, China). RNA samples were reverse-transcribed into cDNA with a PrimeScript™RT Master Mix kit (TaKaRa Bio). cDNA samples were detected using a SYBR®Premix Ex Taq™II kit (TaKaRa Bio) on CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, United States). PCR was performed at 95 °C for 30 s, and the samples were subjected to 40 cycles of amplification at 95 °C for 5 s and 60 °C for 30 s. Expression of target genes was normalized to β-actin. Gene expression was calculated using the 2-ΔΔCt method. All the primers are listed in Supplementary Table 2.
Data are presented as mean ± SD. The difference between more than two groups was analyzed using one-way analysis of variance and comparison of two groups was carried out using a t-test. Differences in survival between two groups were analyzed by Kaplan-Meier test. All statistical analyses were performed using GraphPad Prism 6.0 (San Diego, CA, United States). P < 0.05 was considered statistically significantly.
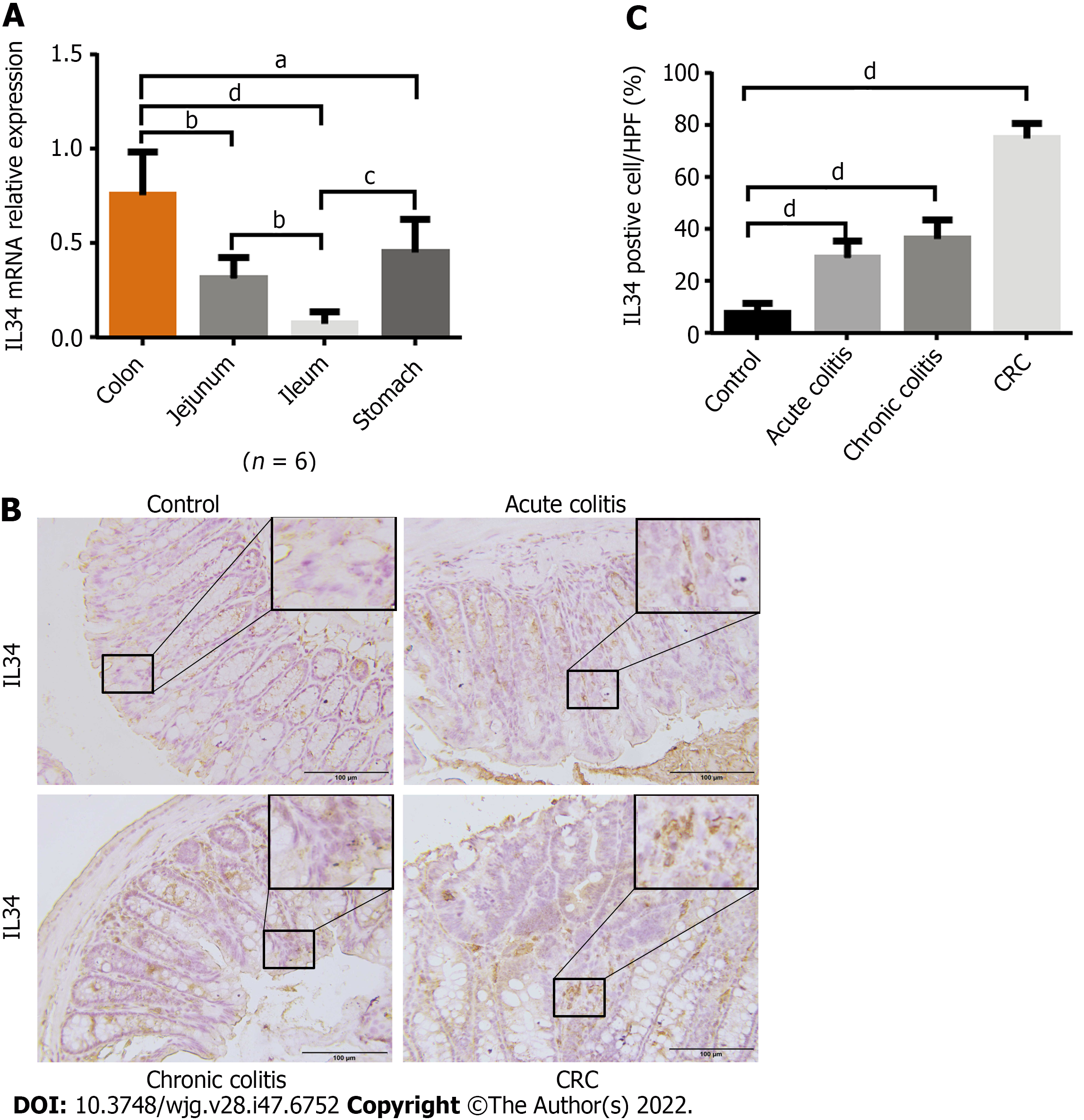
We analyzed IL-34 mRNA expression in gastrointestinal mucosal epithelium in wild-type C57BL/6J mice. Expression of IL-34 was highest in the colon compared with other parts of the digestive tract (Figure 1A). We investigated expression of IL-34 in DSS-induced colitis and AOM-DSS-induced colitis-associated cancer in wild-type mice. Immunohistochemical staining of murine colon tissue revealed that expression of IL-34 was elevated in both acute and chronic colitis, with the highest expression in colitis-associated cancer (Figure 1B and C).
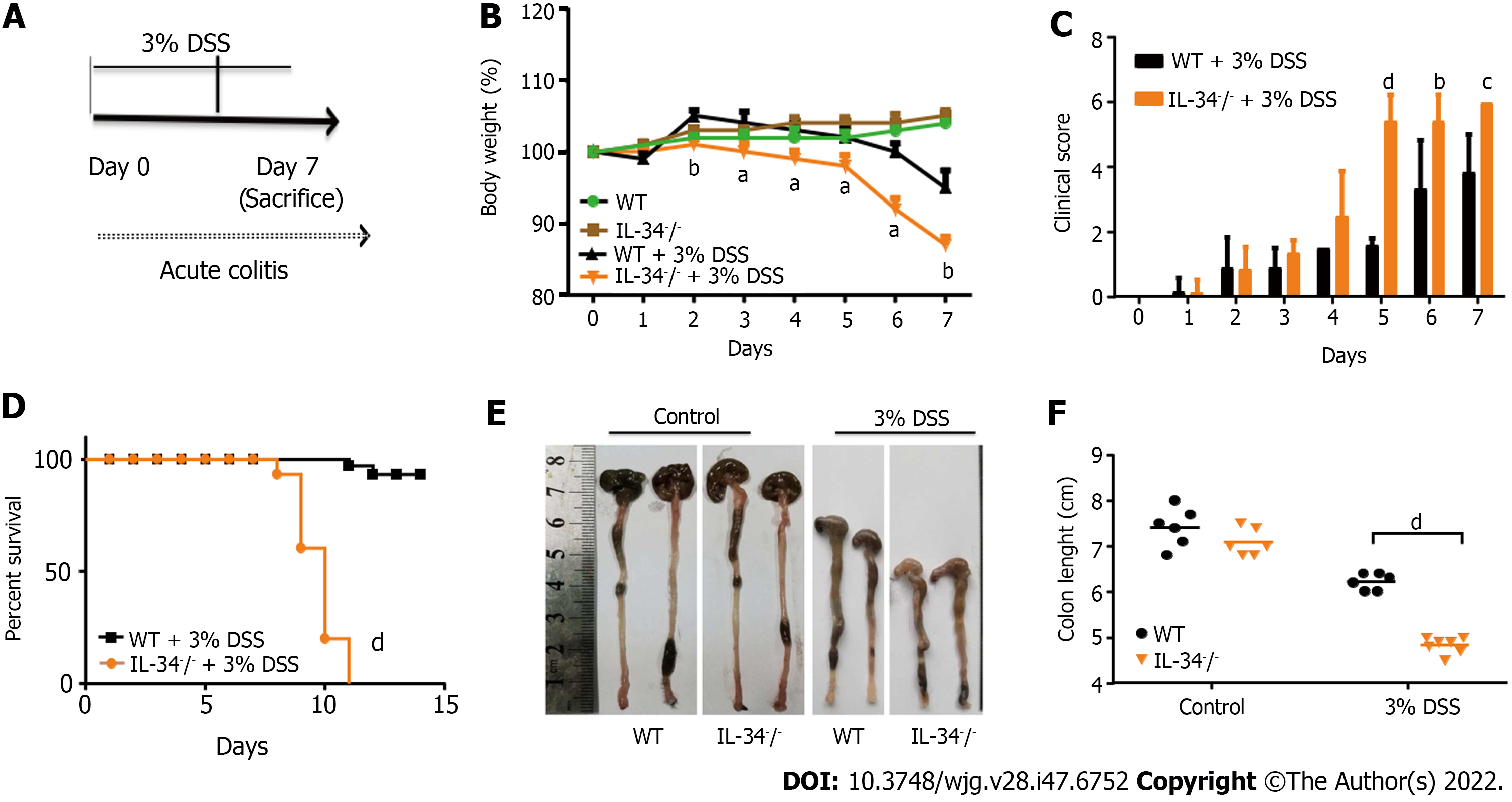
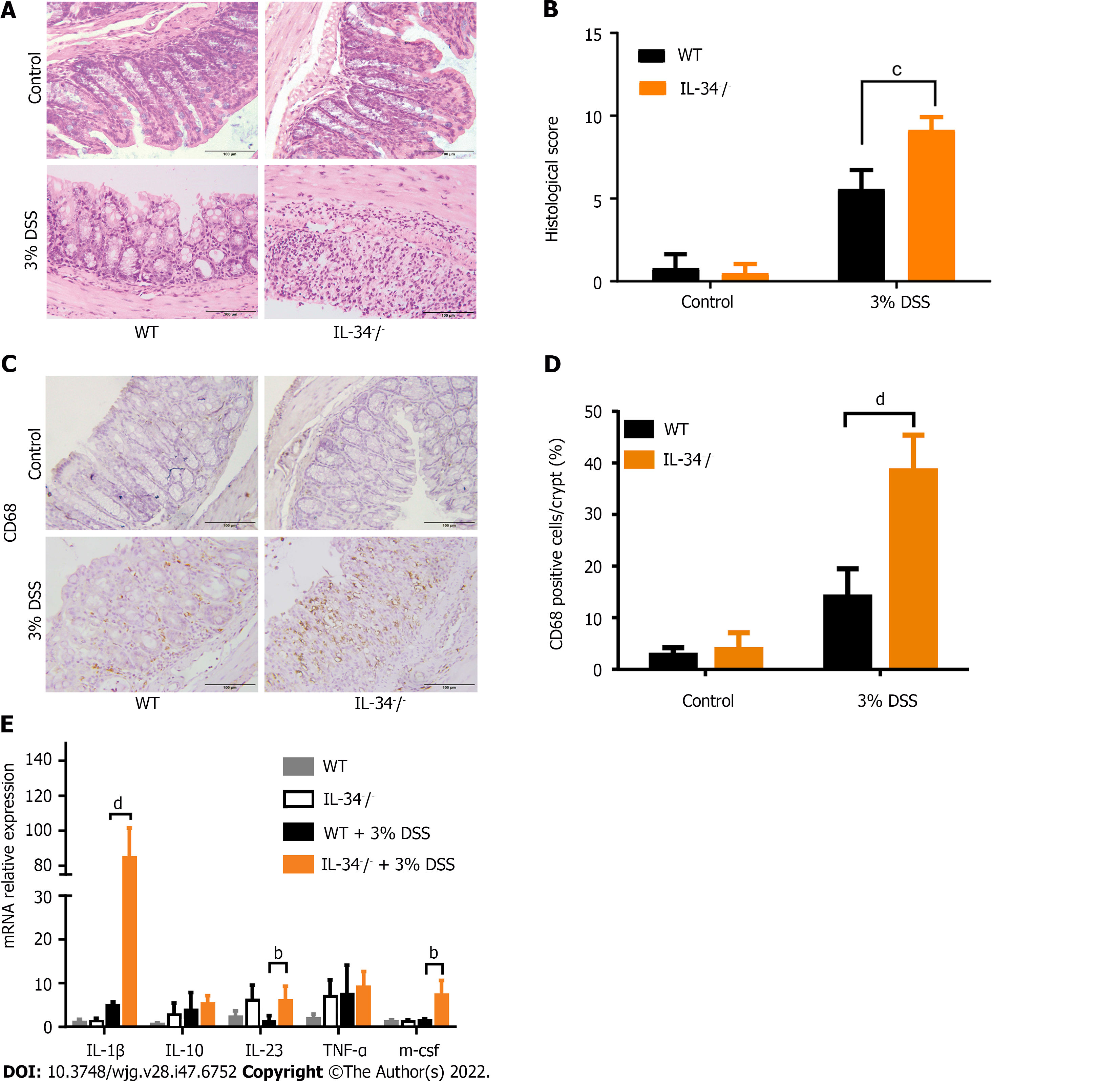
To determine the role of IL-34 in murine acute experimental colitis, IL-34-/- and wild-type mice were fed with 3% DSS in drinking water for 7 d and then killed (Figure 2A). DSS-fed IL-34-/- mice showed significantly greater body weight loss compared to DSS-fed wild-type mice. The average weight loss in DSS-fed IL-34-/- mice was approximately double than that in the wild-type mice (Figure 2B). IL-34-/- mice displayed a significantly higher clinical score compared to wild-type mice (Figure 2C). Strikingly, the mortality of IL-34-/- mice was 100% (10/10), whereas only 20% (2/10) of the wild-type mice died during the experiment, as illustrated by Kaplan-Meier curves (Figure 2D, P < 0.001). Following DSS administration, IL-34-/- mice showed remarkably shorter colon compared to wild-type mice (4.83 cm ± 0.13 cm vs 6.27 cm ± 0.14 cm, P < 0.001), suggesting more severe colitis in IL-34-/- mice (Figure 2E and F). DSS-fed IL-34-/- mice exhibited more severe inflammation with ulceration and necrotic lesions compared to wild-type mice, while there was no difference in the wild-type or IL-34-/- mice fed with normal water. More inflammatory cells infiltrated the lamina propria and submucosa in DSS-fed IL-34-/- mice. More importantly, the normal tissue architecture damage was more severe in DSS-fed IL-34-/- mice compared with wild-type mice (Figure 3A). Semiquantitative score of histopathology confirmed more severe colitis in DSS-fed IL-34-/- mice compared to DSS-fed wild-type mice (9.17 ± 0.31 vs 5.60 ± 1.10, P < 0.001) (Figure 3B). The expression of CD68, which serves as a characteristic marker for macrophages, was indistinguishable between IL-34-/- and wild-type mice untreated with DSS (Figure 3C and D). CD68 expression was significantly increased in DSS-fed IL-34-/- mice compared to DSS-fed wild-type controls (Figure 3C and D), which indicated worsening colitis. IL-1β, IL-23, and macrophage colony-stimulating factor levels were significantly upregulated in DSS-treated IL-34-/- mice compared to wild-type mice treated with DSS (Figure 3E).
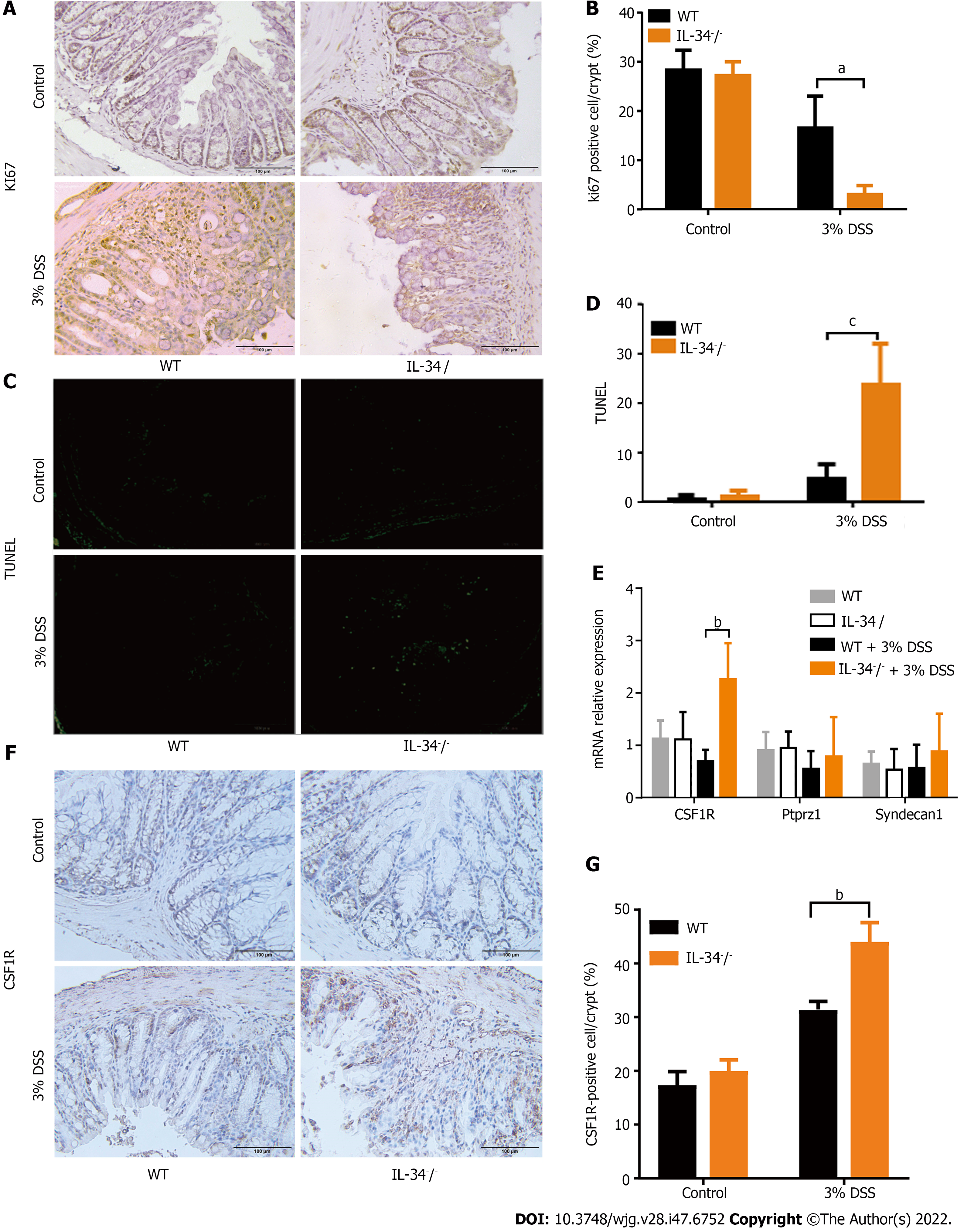
Prior to DSS administration, the levels of colonic epithelium proliferation and apoptosis were both comparable between wild-type and IL-34-/- mice (Figure 4A-D). A marked reduction in colonic epithelial cells stained positive for Ki-67 was detected in DSS-fed IL-34-/- mice compared to DSS-fed wild-type mice (Figure 4A and B). A marked increased in colonic epithelial cell apoptosis was noted in DSS-fed IL-34-/- mice compared to the DSS-fed wild-type mice (Figure 4C and D). We detected expression of three receptors of IL-34 in mouse colonic tissue by real-time quantitative PCR. In DSS-fed IL-34-/- mice, we only observed an increase in the expression of CSF1-R, but the other two receptors of IL34, ptprz1, and syndecan-1, did not change significantly (Figure 4E). By immunohistochemical staining of the mouse colonic tissues, we found that CSF1-R was expressed in epithelial and mesenchymal cells. Compared to the control group, the mice in the DSS-fed group showed an increasing positive index of CSF1-R. Expression of CSF1-R did not show a significant difference between IL-34-/- and wild-type mice untreated with DSS (P > 0.05, Figure 4F and G). DSS-fed IL-34-/- mice exhibited a higher index of CSF1-R positivity compared to DSS-fed wild-type mice (P < 0.01, Figure 4F and G). CSF1-R acts as a receptor for IL-34 and was compensatorily increased when colitis developed and IL-34 was absent. The results were consistent with the results of quantitative real-time PCR of CSF1R.
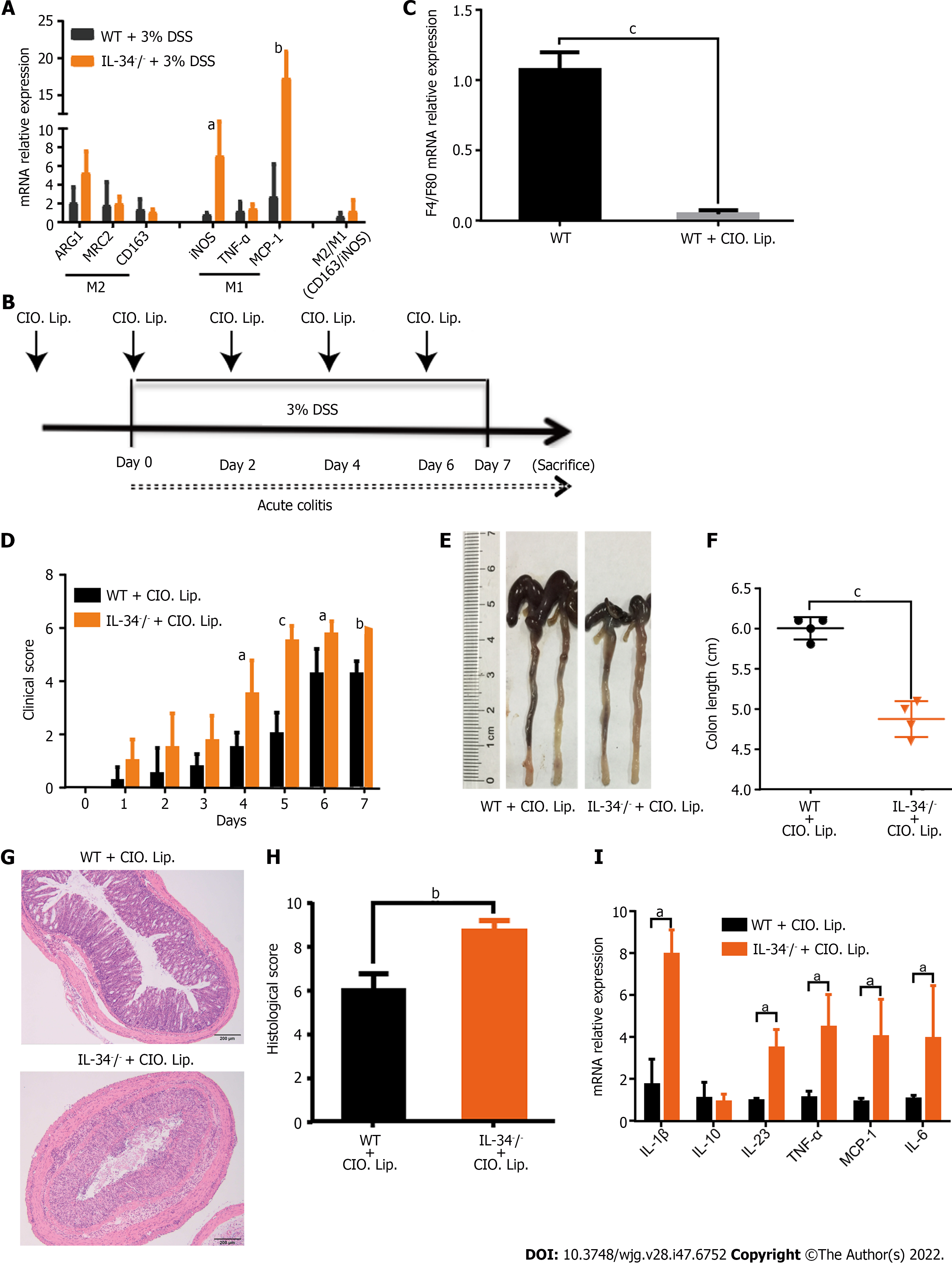
Macrophages were increased in the IL-34-/- mice treated with 3% DSS compared with the wild-type mice. We investigated whether the protective effect of IL-34 in colitis was dependent on macrophages. It is known that IL-34 plays a role in macrophage polarization. We studied the potential role of IL-34 in macrophage polarization by examining the selected markers for M1 and M2 macrophages. Most importantly, there was no difference in the ratio of colonic M2/M1 macrophage markers between DSS-treated wild-type and IL-34-/- mice (Figure 5A). It was speculated that the effect of IL-34 on experimental colitis was not attributed to macrophage polarization. In order to confirm that the protective effect of IL-34 in colitis was not dependent on macrophages, we depleted macrophages by intraperitoneal injection of clodronate liposomes (Clo-lips) in IL-34-/- and wild-type mice (Figure 5B). The macrophage cell marker F4/80 was significantly reduced in colonic mucosa of mice treated with Clo-lips, which confirmed that the liposomes effectively depleted macrophages in the colon (Figure 5C). Despite macrophage depletion, DSS-fed IL-34-/- mice still showed significantly higher clinical score and shorter colon length compared with DSS-fed wild-type mice (Figure 5D-F). Similarly, the histopathological manifestation showed that colitis severity in DSS-fed IL-34-/- mice was more pronounced compared to that in DSS-fed wild-type mice (Figure 5G and H). The inflammatory cytokines remained significantly higher in colonic mucosa of DSS-fed IL-34-/- mice compared with DSS-fed wild-type mice (Figure 5I).
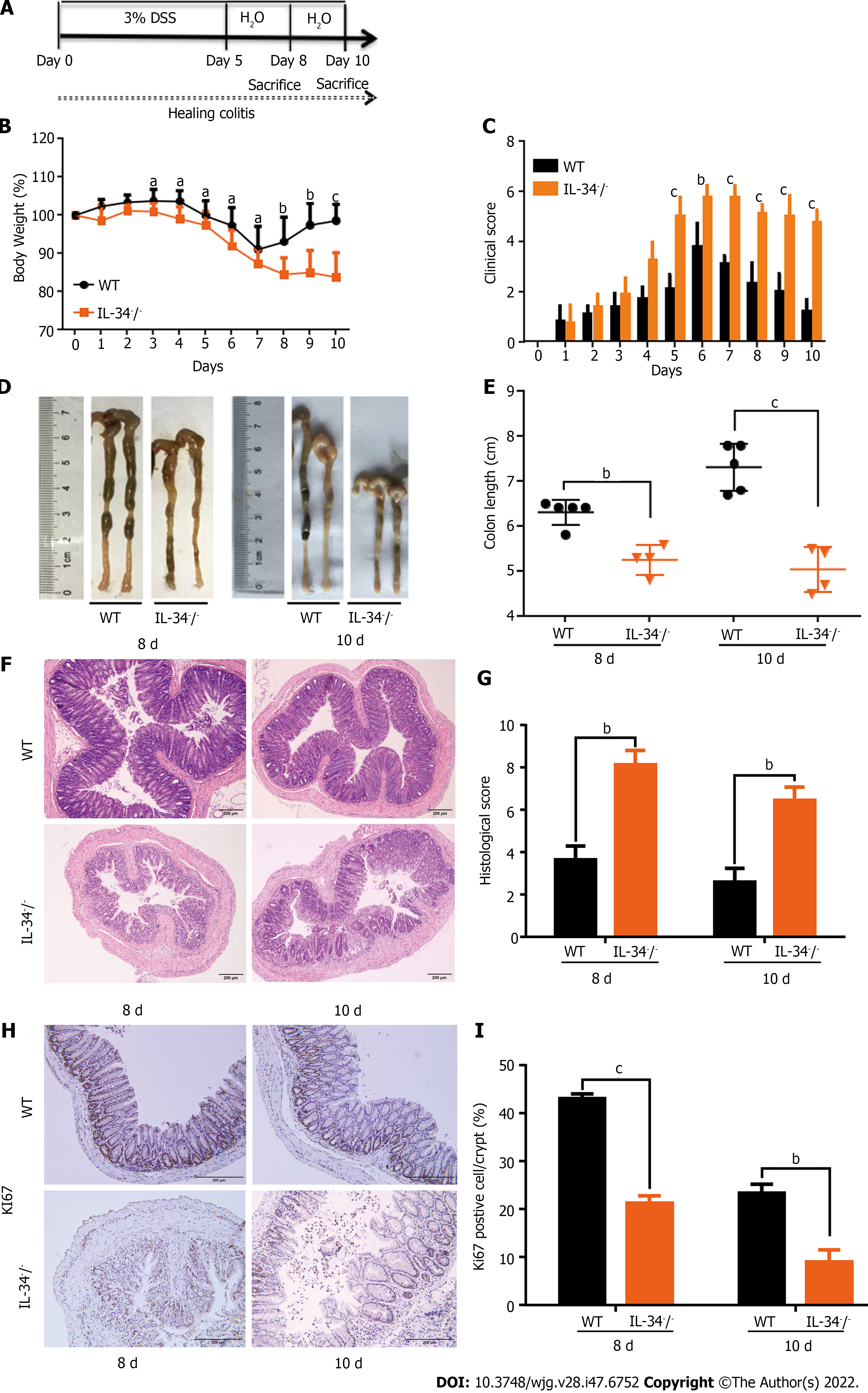
IL-34-/- and wild-type mice were fed with 3% DSS for 5 d and then switched to normal water for the following 5 d to establish a mucosal healing model. The mice in both groups were killed on day 8 or 10 (Figure 6A). The body weight loss was significantly greater in IL-34-/- mice compared to the wild-type control mice. The body weight increased quickly on day 8 in wild-type mice, while it did not recover until day 10 in IL-34-/- mice (Figure 6B). The clinical score was higher in IL-34-/- mice compared to wild-type mice from day 5. The clinical score decreased rapidly from day 7 in wild-type mice, while it was falling slowly from day 8 in IL-34-/- mice (Figure 6C). IL-34-/- mice showed remarkably shorter colon length compared to wild-type controls on day 8 (5.25 cm ± 0.32 cm vs 6.30 cm ± 0.25 cm, P < 0.01) and day 10 (5.03 cm ± 0.49 cm vs 7.30 cm ± 0.47 cm, P < 0.005) (Figure 6D and E). Histopathological analysis showed that IL-34-/- mice exhibited more severe inflammation and tissue damage compared to wild-type mice on days 8 and 10, respectively (Figure 6F). Semiquantitative scoring of histopathology showed that colitis severity in IL-34-/- mice was significantly higher than that in wild-type mice on day 8 (8.13 ± 0.66 vs 3.63 ± 0.58, P < 0.01) and day 10 (8.45 ± 0.61 vs 2.60 ± 0.58, P < 0.01), respectively (Figure 6G). The number of colonic epithelial cells positive for Ki-67 was markedly decreased in IL-34-/- mice compared to wild-type mice on days 8 and 10 (Figure 6H and I).
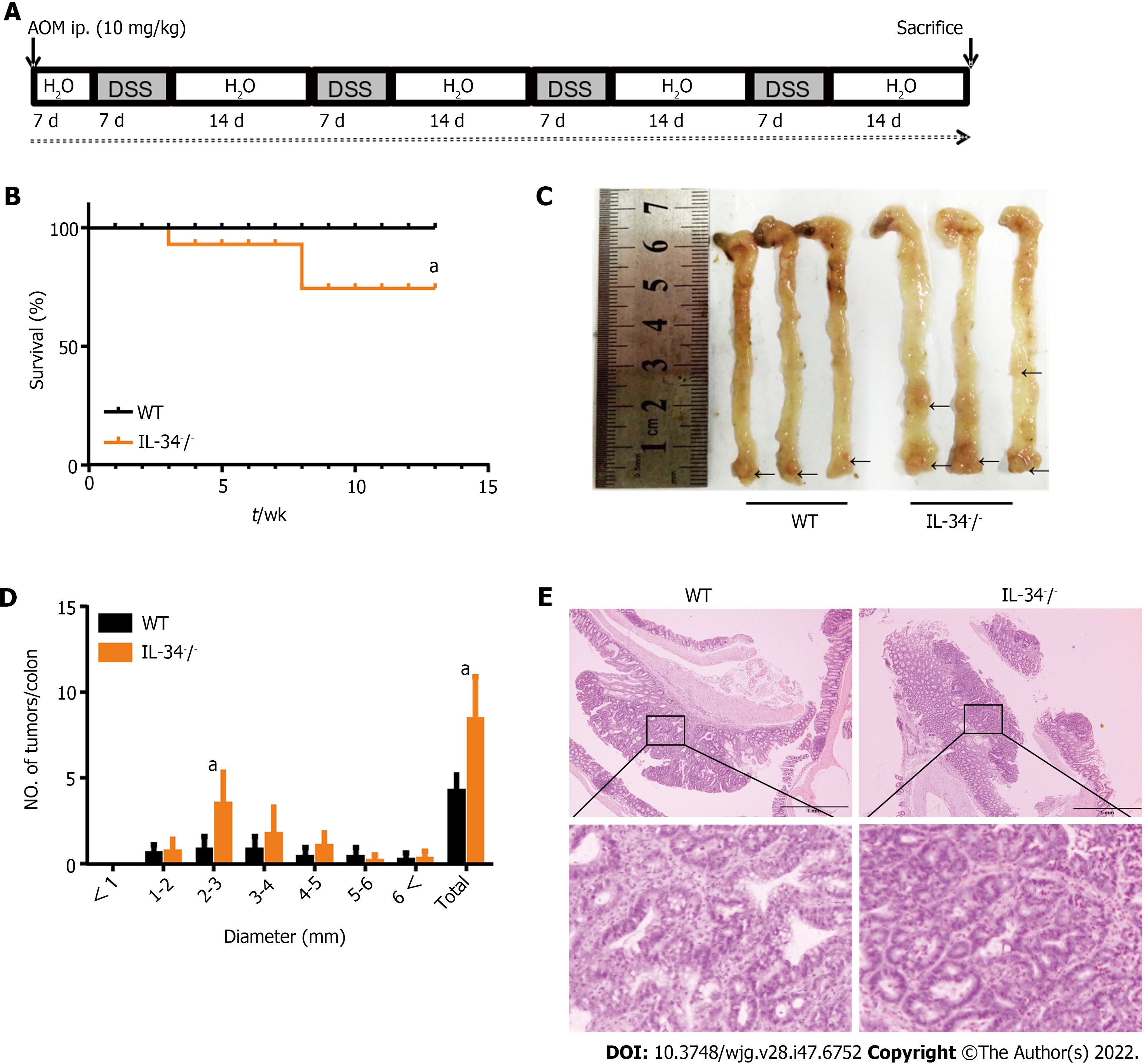
To investigate the role of IL-34 in colitis-associated tumorigenesis, wild-type and IL-34-/- mice were treated with AOM and DSS (Figure 7A). No wild-type mice died, whereas a mortality rate of 30% was noted in IL-34-/- mice (Figure 7B). More importantly, a marked difference in tumor number was observed between the two groups. IL-34-/- mice developed a greater number of colon tumors than wild-type mice (Figure 7C and D). Representative H&E staining of colitis-associated cancer in wild-type and IL-34-/- mice is shown in Figure 7E.
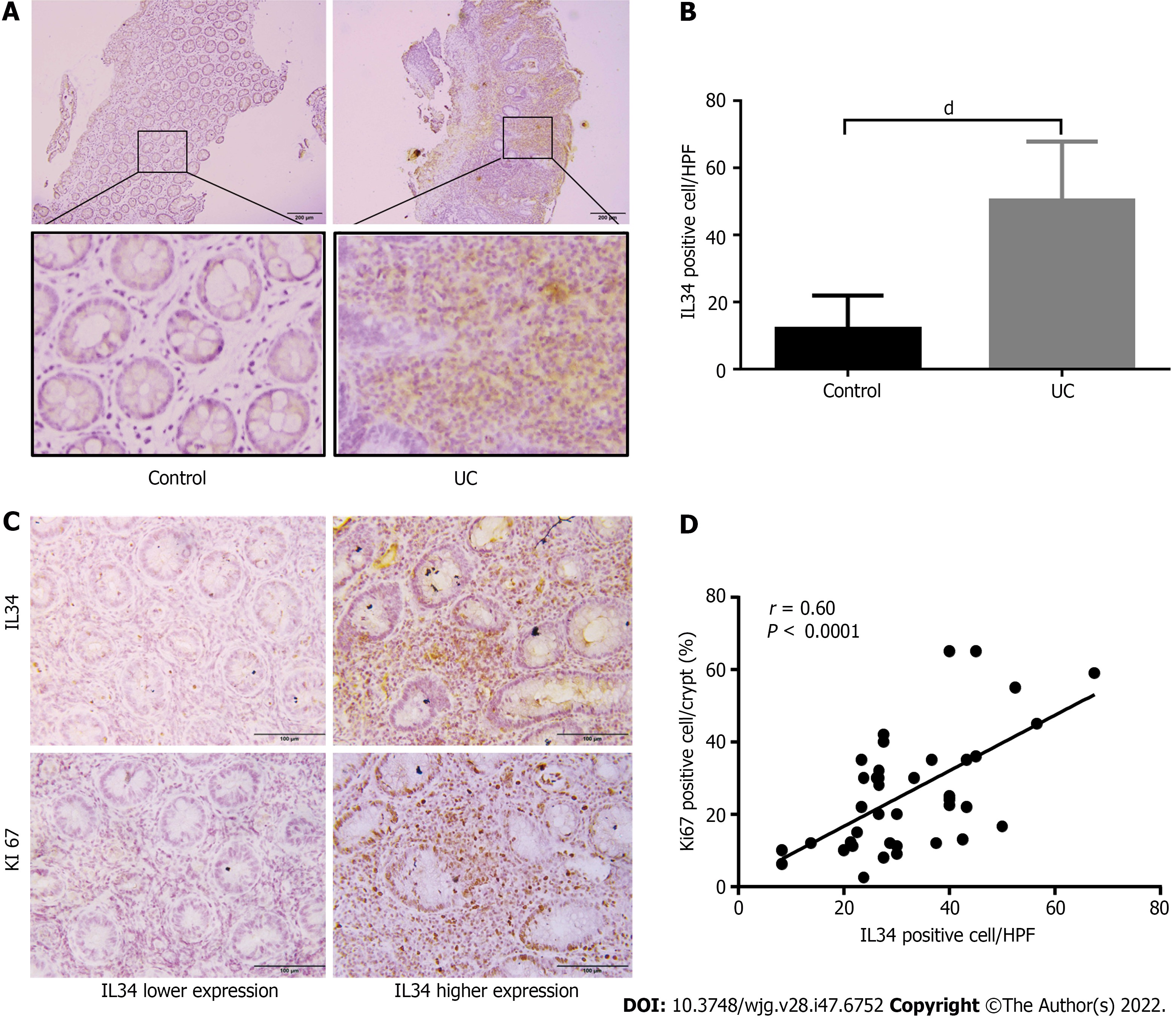
We investigated the IL-34 expression pattern and its relationship with colonic epithelium proliferation in active UC patients. There was no age or sex difference between the groups (Supplementary Table 1). Consistent with previous studies, we also observed elevated expression of IL-34 in diseased mucosa of UC patients compared with the normal controls as detected by immunohistochemistry. Importantly, IL-34 was suspected to be predominantly expressed in the colonic stromal tissue (Figure 8A and B). Significantly enhanced colonic expression of proliferation index Ki-67 was detected in UC patients with higher IL-34 expression in colonic mucosa (Figure 8C). There was a positive correlation between IL-34 and Ki-67 expression in UC-inflamed mucosa with a correlation coefficient of 0.60 (P < 0.0001) (Figure 8D). No correlation was found between IL-34-positive and TUNEL-positive cells (data not shown).
Mucosal healing has become the goal of therapy because of its association with better prognosis and improved quality of life[25]. However, the underlying molecular mechanism is unclear. Here, we revealed that IL-34 deficiency strongly increased susceptibility to acute DSS-induced colitis and intestinal wounding. Additionally, IL-34 deficiency enhanced AOM/DSS-induced colitis-associated tumorigenesis. Different from the previous reports, our findings suggest the protective role of IL-34 in UC- and colitis-associated cancer and may help to establish a new potential approach for management of UC.
The IL-34 knockout mice used in our study facilitated us to illustrate the role of IL-34 in pathogenesis of UC. Prior to DSS administration, no difference in colon morphological and histological manifestations was observed between IL-34-deficient and wild-type control mice. The results suggest that IL-34 is dispensable for colon homoeostasis under steady state.
In past studies, overexpression of IL-34 stimulated by inflammatory cytokines augmented the production of proinflammatory cytokines in vitro, probably raising IL-34 as a pathogenic contributor to sustained colon inflammation[14]. However, the increased expression of IL-34 might be a consequence of inflammation as a protective mechanism rather than a disease cause[16]. Indeed, IL-34 transgenic mice do not display aggravating inflammatory responses in the colon[26]. The precise mechanistic role of IL-34 during colon inflammation has not been clarified yet. Until now, there has been a lack of research on IL-34 involved in UC in vivo. A recent study has reported that IL-34 is critical for the suppressive function of CD4+ T regulatory cells, and its deficiency leads to increased susceptibility to autoimmunity[27]. We demonstrated for the first time that IL-34 deficiency exacerbates DSS-induced colitis during acute and delayed mucosal healing process and is associated with high colitis-related mortality. We assumed that significantly increased expression of IL-34 in mucosa from patients with active UC might act as an urgent requirement for alleviating inflammation and promoting mucosal healing. Our findings support the protective characteristic of IL-34 during DSS-induced colitis and early healing stage.
Macrophages are critical to mucosal homeostasis in orchestrating innate and adaptive immunity[28]. Depending on the local microenvironment, macrophages differentiated from the peripheral blood monocytes develop towards M1-like or M2-like phenotype macrophages[29]. M1 macrophages secreting proinflammatory cytokines such as TNF-α or IL-12 are considered to amplify the inflammatory response, whereas M2 macrophages producing anti-inflammatory mediators such transforming growth factor-β or IL-10 suppress the inflammatory response[29]. IL-34 has been identified to trigger CSF-1R signaling and induce macrophage differentiation to M2 phenotype via activation of the ERK1/ 2/AKT/AMPK signaling pathway[8], which contributes to mucosal homeostasis maintenance and tissue remodeling[17]. Regulation of M1 to M2 phenotype switch has been confirmed to promote the proliferative phase of wound healing and ameliorates DSS-induced colitis, indicating a possible role of IL-34 in alleviation of colonic inflammation[17,18]. In our study, IL-34-deficient mice treated with DSS presented with worse colitis with significant accumulation of macrophages compared to DSS-treated wild-type control mice. It is speculated that downregulation of the M1 to M2 macrophage phenotypic switch results in more severe colitis. However, M2/M1 macrophage ratio was not significantly decreased in DSS-treated IL-34-deficient mice. We subsequently depleted the macrophages in the murine colitis model by administration of Clo-lips, which is a well-established method for macrophage depletion[30], showing that IL-34 deficiency aggravated murine colitis whether or not macrophages were depleted. Our results suggested that the protective effect of IL-34 on experimental colitis was not primarily dependent on macrophage polarization.
Mucosal healing largely relies on controlled colonic epithelial proliferation and apoptosis, which are manipulated by multiple growth factors, gut peptides, cytokines, as well as signaling pathways[25]. Given different DSS-induced injury levels in normal and IL-34-deficient colonic mucosa, we should be cautious to draw the conclusion that IL-34 deficiency inhibits the mucosal healing process in vivo. Based upon immunohistochemical staining, IL-34 was predominantly localized in the colon stromal tissue in our study. The specific cellular source of IL-34 needs to be further explored. In the acute colitis model, epithelial cell proliferation was inhibited but epithelial cell apoptosis was induced in IL-34-/- mice. CSF-1R was compensatorily increased in the IL-34-/- mice treated with DSS. Furthermore, it has been proved that IL-34 expression is positively correlated with epithelial proliferation in mucosal biopsies from UC patients. No correlation was shown between IL-34 expression level and apoptosis in UC patients. It is speculated that IL-34 might be produced from colon stromal tissue and promote colonic epithelial cell growth and wound closure by binding to CSF-1R in inflammatory conditions. Further studies are warranted to investigate the potential function of IL-34 in colonic epithelial cells.
Emerging data have demonstrated the multidimensional role of IL-34 in tumor progression and metastasis. The function of IL-34 in cancer may vary among different types of tumors[31]. A recent study on the relationship between IL-34 and gastric cancer proposed that the reduction of IL-34 in gastric cancer was inversely related to the degree of tumor differentiation and was closely related to the poor survival rate of patients[32]. However, the relationship of IL-34 to colorectal cancer remains controversial. It has been recently reported that IL-34 stimulates colorectal adenocarcinoma cell proliferation via an ERK1/2-dependent pathway. The connection between chronic colon inflammation and colitis-associated cancer has been documented in UC patients. Repeated mucosal damage and repair in the inflamed microenvironment can result in uncontrolled epithelial cell proliferation and induce cancer events[33]. In a cohort study, Wang et al[34] showed that low expression of IL-34 is associated with poor survival in colorectal cancer. Accumulating evidence suggests that chronic inflammation enhances the development of colitis-associated cancer through multiple mechanisms, including oxidative stress, DNA damage, abnormal immune response, and gut microbiome dysbiosis[35]. In our study, tumor burden was markedly enhanced in IL-34-/- mice treated with AOM/DSS compared to wild-type control mice. We assumed that IL-34 deficiency worsened the colonic inflammation response and tissue damage, and thus sustained inflammatory injury increased the risk of colitis-associated cancer.
In summary, our work has highlighted the role of IL-34 during acute experimental colitis and wound healing. We found that IL-34 does not drive the inflammatory response and tissue destruction under physiological conditions, but protects the host from inflammatory injury and reduces the risk of colitis-associated cancer. IL-34 might serve as a potential therapeutic target for inducing mucosal healing in treatment for UC and reducing colitis-associated cancer in UC.
The exact pathogenesis of ulcerative colitis (UC) remains unclear. Identification of distinctive cytokines involved in immunopathogenesis of UC has become a hot spot for the development of biological therapies. The expression of interleukin (IL)-34 is upregulated in active UC but the molecular function and underlying mechanism are largely unknown.
Whether IL-34 is a “friend or foe” in the pathogenesis of UC remains be explored. In this study, we investigated the potential role of IL-34 in experimental colitis, colitis-associated carcinogenesis and UC.
To investigate the function of IL-34 in acute colitis, in a wound healing model and in colitis-associated cancer, and the IL-34 expression pattern and its relationship with colonic epithelium proliferation in active UC patients.
We conducted a controlled study using IL-34 knockout mice and wild-type mice (C57BL/6J). Colitis was induced by administration of dextran sodium sulfate, and carcinogenesis was induced by azo
IL-34 deficiency in vivo exacerbated colitis in mice during acute and wound healing phases and increased tumor susceptibility in the mouse colon. The effect was independent of macrophage differentiation and polarization. IL-34 was markedly increased in patients with active UC and the expression was positively correlated with epithelial cell proliferation in UC.
IL-34 deficiency exacerbates colonic inflammation and accelerates colitis-associated carcinogenesis in mice.
IL-34 might serve as a potential therapeutic target for inducing mucosal healing in treatment of UC and reducing colitis-associated cancer in UC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sahin Y, Turkey; Sitkin S, Russia S-Editor: Chen YL L-Editor: A P-Editor: Cai YX
| 1. | Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2199] [Cited by in RCA: 2449] [Article Influence: 306.1] [Reference Citation Analysis (2)] |
| 2. | Tang B, Zhu J, Fang S, Wang Y, Vinothkumar R, Li M, Weng Q, Zheng L, Yang Y, Qiu R, Xu M, Zhao Z, Ji J. Pharmacological inhibition of MELK restricts ferroptosis and the inflammatory response in colitis and colitis-propelled carcinogenesis. Free Radic Biol Med. 2021;172:312-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 3. | Scheibe K, Backert I, Wirtz S, Hueber A, Schett G, Vieth M, Probst HC, Bopp T, Neurath MF, Neufert C. IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut. 2017;66:823-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 148] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 4. | Colombel JF, D'haens G, Lee WJ, Petersson J, Panaccione R. Outcomes and Strategies to Support a Treat-to-target Approach in Inflammatory Bowel Disease: A Systematic Review. J Crohns Colitis. 2020;14:254-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 199] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 5. | Freuchet A, Salama A, Remy S, Guillonneau C, Anegon I. IL-34 and CSF-1, deciphering similarities and differences at steady state and in diseases. J Leukoc Biol. 2021;110:771-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, Sylvestre V, Stanley ER. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 864] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 7. | Lin H, Lee E, Hestir K, Leo C, Huang M, Bosch E, Halenbeck R, Wu G, Zhou A, Behrens D, Hollenbaugh D, Linnemann T, Qin M, Wong J, Chu K, Doberstein SK, Williams LT. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 614] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 8. | Baghdadi M, Umeyama Y, Hama N, Kobayashi T, Han N, Wada H, Seino KI. Interleukin-34, a comprehensive review. J Leukoc Biol. 2018;104:931-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 9. | Monteleone G, Franzè E, Troncone E, Maresca C, Marafini I. Interleukin-34 Mediates Cross-Talk Between Stromal Cells and Immune Cells in the Gut. Front Immunol. 2022;13:873332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Muñoz-Garcia J, Cochonneau D, Télétchéa S, Moranton E, Lanoe D, Brion R, Lézot F, Heymann MF, Heymann D. The twin cytokines interleukin-34 and CSF-1: masterful conductors of macrophage homeostasis. Theranostics. 2021;11:1568-1593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 11. | Ge Y, Huang M, Yao YM. Immunomodulation of Interleukin-34 and its Potential Significance as a Disease Biomarker and Therapeutic Target. Int J Biol Sci. 2019;15:1835-1845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Xu WD, Huang AF, Fu L, Liu XY, Su LC. Targeting IL-34 in inflammatory autoimmune diseases. J Cell Physiol. 2019;234:21810-21816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Bézie S, Picarda E, Ossart J, Tesson L, Usal C, Renaudin K, Anegon I, Guillonneau C. IL-34 is a Treg-specific cytokine and mediates transplant tolerance. J Clin Invest. 2015;125:3952-3964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | Zwicker S, Martinez GL, Bosma M, Gerling M, Clark R, Majster M, Söderman J, Almer S, Boström EA. Interleukin 34: a new modulator of human and experimental inflammatory bowel disease. Clin Sci (Lond). 2015;129:281-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Franzè E, Dinallo V, Laudisi F, Di Grazia A, Di Fusco D, Colantoni A, Ortenzi A, Giuffrida P, Di Carlo S, Sica GS, Di Sabatino A, Monteleone G. Interleukin-34 Stimulates Gut Fibroblasts to Produce Collagen Synthesis. J Crohns Colitis. 2020;14:1436-1445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Guillonneau C, Bézie S, Anegon I. Immunoregulatory properties of the cytokine IL-34. Cell Mol Life Sci. 2017;74:2569-2586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 17. | Cosín-Roger J, Ortiz-Masiá D, Calatayud S, Hernández C, Esplugues JV, Barrachina MD. The activation of Wnt signaling by a STAT6-dependent macrophage phenotype promotes mucosal repair in murine IBD. Mucosal Immunol. 2016;9:986-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 18. | Song WJ, Li Q, Ryu MO, Ahn JO, Ha Bhang D, Chan Jung Y, Youn HY. TSG-6 Secreted by Human Adipose Tissue-derived Mesenchymal Stem Cells Ameliorates DSS-induced colitis by Inducing M2 Macrophage Polarization in Mice. Sci Rep. 2017;7:5187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 19. | Franzè E, Dinallo V, Rizzo A, Di Giovangiulio M, Bevivino G, Stolfi C, Caprioli F, Colantoni A, Ortenzi A, Grazia AD, Sica G, Sileri PP, Rossi P, Monteleone G. Interleukin-34 sustains pro-tumorigenic signals in colon cancer tissue. Oncotarget. 2018;9:3432-3445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Franzè E, Di Grazia A, Sica GS, Biancone L, Laudisi F, Monteleone G. Interleukin-34 Enhances the Tumor Promoting Function of Colorectal Cancer-Associated Fibroblasts. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Li J, Qu C, Li F, Chen Y, Zheng J, Xiao Y, Jin Q, Jin G, Huang X, Jin D. Inonotus obliquus Polysaccharide Ameliorates Azoxymethane/Dextran Sulfate Sodium-Induced Colitis-Associated Cancer in Mice via Activation of the NLRP3 Inflammasome. Front Pharmacol. 2020;11:621835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Weisser SB, Brugger HK, Voglmaier NS, McLarren KW, van Rooijen N, Sly LM. SHIP-deficient, alternatively activated macrophages protect mice during DSS-induced colitis. J Leukoc Biol. 2011;90:483-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Su L, Nalle SC, Shen L, Turner ES, Singh G, Breskin LA, Khramtsova EA, Khramtsova G, Tsai PY, Fu YX, Abraham C, Turner JR. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology. 2013;145:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 295] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 24. | Li YK, Li YM, Li Y, Wei YR, Zhang J, Li B, You ZR, Chen Y, Huang BY, Miao Q, Wang QX, Peng YS, Gershwin ME, Tang RQ, Bian ZL, Ma X. CTHRC1 expression in primary biliary cholangitis. J Dig Dis. 2019;20:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 656] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Colonna M. Interkeukin-34, a cytokine crucial for the differentiation and maintenance of tissue resident macrophages and Langerhans cells. Eur J Immunol. 2014;44:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Freuchet A, Salama A, Bézie S, Tesson L, Rémy S, Humeau R, Règue H, Sérazin C, Flippe L, Peterson P, Vimond N, Usal C, Ménoret S, Heslan JM, Duteille F, Blanchard F, Giral M, Colonna M, Anegon I, Guillonneau C. IL-34 deficiency impairs FOXP3+ Treg function in a model of autoimmune colitis and decreases immune tolerance homeostasis. Clin Transl Med. 2022;12:e988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Huang Y, Chen Z. Inflammatory bowel disease related innate immunity and adaptive immunity. Am J Transl Res. 2016;8:2490-2497. [PubMed] |
| 29. | Daskalaki MG, Vyrla D, Harizani M, Doxaki C, Eliopoulos AG, Roussis V, Ioannou E, Tsatsanis C, Kampranis SC. Neorogioltriol and Related Diterpenes from the Red Alga Laurencia Inhibit Inflammatory Bowel Disease in Mice by Suppressing M1 and Promoting M2-Like Macrophage Responses. Mar Drugs. 2019;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Bader JE, Enos RT, Velázquez KT, Carson MS, Nagarkatti M, Nagarkatti PS, Chatzistamou I, Davis JM, Carson JA, Robinson CM, Murphy EA. Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. Am J Physiol Gastrointest Liver Physiol. 2018;314:G22-G31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 31. | Lelios I, Cansever D, Utz SG, Mildenberger W, Stifter SA, Greter M. Emerging roles of IL-34 in health and disease. J Exp Med. 2020;217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 32. | Liu Q, Zhang Y, Zhang J, Tao K, Hambly BD, Bao S. Inverse correlation between Interleukin-34 and gastric cancer, a potential biomarker for prognosis. Cell Biosci. 2020;10:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S, Lozano G, Pikarsky E, Forshew T, Rosenfeld N, Harpaz N, Itzkowitz S, Harris CC, Rotter V, Gorgoulis VG, Oren M. Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell. 2013;23:634-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 388] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 34. | Wang B, Xu W, Tan M, Xiao Y, Yang H, Xia TS. Integrative genomic analyses of a novel cytokine, interleukin-34 and its potential role in cancer prediction. Int J Mol Med. 2015;35:92-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Nagao-Kitamoto H, Kitamoto S, Kamada N. Inflammatory bowel disease and carcinogenesis. Cancer Metastasis Rev. 2022;41:301-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |