Published online Dec 21, 2022. doi: 10.3748/wjg.v28.i47.6732
Peer-review started: September 20, 2022
First decision: October 21, 2022
Revised: November 4, 2022
Accepted: November 25, 2022
Article in press: November 25, 2022
Published online: December 21, 2022
Processing time: 89 Days and 22 Hours
This review aimed to highlight the etiology, diagnosis, treatment, and prevention of obstructive and secretory complications associated with diverting ileostomy (DI). Obstructive complications at the stoma site are termed stoma outlet obstruction (SOO) or stoma-related obstruction (SRO). The incidence of SOO/SRO is 5.4%-27.3%, and the risk factors are multifactorial; however, the configuration of the stoma limb and the thickness of the rectus abdominis muscle (RAM) may be of particular concern. Trans-stomal tube decompression is initially attempted with a success rate of 33%-86%. A thick RAM may carry the risk of recurrence. Surgical refinement, including a wider incision of the anterior sheath and adequate stoma limb length, avoids tension and immobility and may decrease SOO/SRO. Secretory complications of DI are termed high output stoma (HOS). Persistent HOS lead to water and sodium depletion, and secondary hyperaldosteronism, resulting in electrolyte imbalances, such as hypomagnesemia. The incidence of HOS is 14%-24%, with an output of 1000-2000 mL/d lasting up to three days. Treatment of HOS is commenced after excluding postoperative complications or enteritis and includes fluid intake restriction, antimotility and antisecretory drug therapies, and magnesium supplementation. Intensive monitoring and surveillance programs have been successful in decreasing readmissions for dehydration.
Core Tip: This review highlights the etiology, diagnosis, treatment, and prevention of obstructive and secretory complications associated with diverting ileostomy (DI). Obstructive complications at the stoma site (stoma outlet obstruction/stoma-related obstruction, SOO/SRO) affect 5.4%-27.3% of patients with DI. Trans-stomal tube decompression is effective in most cases. Surgical refinement is important for reducing SOO/SRO. Secretory complications (high output stoma, HOS) lead to water and sodium depletion and secondary hyperaldosteronism with electrolyte imbalances. The incidence of HOS is 14%-24%, with an output of 1000-2000 mL/d. HOS treatment includes fluid intake restriction, antimotility and antisecretory drug therapies, and magnesium supplementation. Intensive monitoring and surveillance programs may decrease the readmission rates for dehydration.
- Citation: Tsujinaka S, Suzuki H, Miura T, Sato Y, Shibata C. Obstructive and secretory complications of diverting ileostomy. World J Gastroenterol 2022; 28(47): 6732-6742
- URL: https://www.wjgnet.com/1007-9327/full/v28/i47/6732.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i47.6732
Diverting ileostomy (DI) is often performed in patients undergoing low anterior resection for rectal cancer or restorative proctocolectomy with ileal-pouch anal anastomosis (IPAA) for ulcerative colitis. The purpose of DI creation is to protect the anastomosis from leakage and mitigate the severity of symptoms relative to anastomotic complications. However, the efficacy of DI is solely limited to a significant decrease in anastomotic leakage, and no other advantages may be found in the short term[1,2]. Moreover, stoma formation significantly increases the risk of small-bowel obstruction, postoperative ileus, dehydration, and electrolyte imbalance[2-4]. Some of these complications are specific to DI and can be classified into “obstructive” and “secretory” complications. The risk factors for developing these complications have been widely discussed, but the fundamental mechanisms and management of these complications are not fully understood. This review aimed to highlight the etiology, diagnosis, treatment, and prevention of obstructive and secretory complications associated with DI.
An electronic English literature search was performed on PubMed/MEDLINE database from the inception to September 15, 2022. The search items included “diverting ileostomy” or “covering ileostomy”, “small bowel obstruction”, “stoma outlet obstruction” (SOO) or “outlet obstruction”, “stoma-related obstruction” (SRO), “high output stoma” (HOS) or “high output syndrome”, and “dehydration”. Inclusion criteria for the article type were systematic review and meta-analyses, randomized controlled studies, retrospective observational studies, and narrative reviews for nursing aspects. Case reports were not included.
Small bowel obstruction (SBO) is one of the most common complications associated with colorectal surgery. The increased incidence of SBO when DI is created at the time of the initial surgery is of particular concern[4-8]. Since DI is brought up extracorporeally through the abdominal wall by splitting the rectus abdominis muscle (RAM), scar formation or tissue inflammation at the anterior rectus sheath (where the incision is made) may lead to stenosis of the stoma opening (outlet). Furthermore, the stoma outlet is physiologically vulnerable to the risk of obstruction, and its underlying mechanisms can be explained by the following speculations. First, the stoma is edematous, and the bowel lumen tends to be narrower in the early postoperative period. Second, the intraluminal pressure of the small bowel is lower than that of the colon, suggesting relative stenosis at the RAM level[9]. Third, if a high volume of bowel content flows into the lumen, the stoma outlet may have a change in caliber, leading to relative narrowing[10].
Obstructive complications at the stoma site have been termed SOO and SRO in the literature. Okita et al[11] proposed a definition of SBO at the stoma site that has the following criteria: (1) Radiographically confirmed dilatation of the oral stoma limb; (2) Increase in stomal output and relief of symptoms after trans-stomal tube decompression; and (3) Exclusion of SBO other than that at the stoma site. Some of the subsequent reports have followed these criteria for diagnosis[10,12-14], while others have used more simplified criteria, such as SBO at the stoma site with radiographic confirmation excluding paralytic ileus[15-17].
SOO/SRO occurs in 5.4%-27.3% of patients with DI[10-18]. Risk factors are classified into the following categories: patient characteristics[10,11,13,16,19] (young age, low body mass index, thick subcutaneous fat, thick RAM, and long distance between the superior mesenteric artery root and the bottom of the external anal sphincter), disease[15] (ulcerative colitis), surgical factors[12,14,20-22] (laparoscopic surgery, rotation of stoma limb, IPAA, two-stage surgery for ulcerative colitis, and short distance from the anastomosis to the stoma site), and stoma functions[10,17] (high output from stoma). The risk factors are summarized in Table 1.
| Risk factors |
| Patient characteristics |
| Young age (less than 16 yr old)[11] |
| Low body mass index (less than 21 kg/m2)[11] |
| Thick subcutaneous fat at the stoma marking site (20 mm or more) [16] |
| Thick rectus abdominis muscle at the stoma passage (10 mm or more)[10,13] |
| Long distance between the superior mesenteric artery root and the bottom of the external anal sphincter (height-adjusted, 191 mm/m)[19] |
| Disease |
| Ulcerative colitis (compared with colorectal cancer)[15] |
| Surgical factors |
| Laparoscopic surgery (compared with open surgery)[18,22] |
| Rotation of stoma limb (180-degree rotation, the oral limb situated on the caudal side)[21] |
| Ileal-pouch anal anastomosis (compared with low anterior resection or intersphincteric resection)[12] |
| Two-stage surgery for ulcerative colitis[20] |
| Short distance from the ileal pouch to the stoma site (< 30 cm)[12,18] |
| Stoma functions |
| High output from stoma (2000 mL or more per day)[10,17] |
Young age (less than 16 years old) and low body mass index (less than 21 kg/m2) in patients with ulcerative colitis were reported as risk factors by Okita et al[11]. The mechanisms and interpretation of the results were not clearly shown, although they assumed that the small abdominal cavity or small amount of mesenteric fat in these individuals allowed volvulus or kinking. A contradictory result was reported by Tamura et al[16], who found that increased subcutaneous fat [vertical distance of 20 mm or more at the stoma site marking on computed tomography (CT)] in obese patients was a significant predictor of SOO/SRO. However, the different pathologies in the study population (inflammatory disease vs colorectal cancer) make this comparison difficult. The authors assumed that the tension and twisting in the loop stoma might have been caused by the minimum size of stoma apertures at the skin and tight with a narrow subcutaneous cavity in obese patients, and thus surgeons should be aware of it. Mori et al[19] reported that SOO/SRO was more common in patients with a long distance between the superior mesenteric artery root and the bottom of the external anal sphincter (height-adjusted, 191 mm/m on CT), suggesting that the mesentery may be under tension. The authors encouraged surgeons to reduce tension in the mesentery using all applicable surgical techniques. Thick RAM at the stoma passage (vertical length of 10 mm or more on CT) has been shown to increase the risk of SOO/SRO[10,13], and it is speculated that the intraluminal pressure of the stoma may be overwhelmed by the increased pressure of the thick RAM[10]. The authors assumed that surgeons need to create a wider split of the RAM for these patients.
As a disease-related factor, ulcerative colitis has been reported as an independent risk factor for SOO/SRO, but the specific reason remains unclear[15]. It is speculated that the risk may not be due to the disease itself; rather, it refers to the surgical procedure of total proctocolectomy with IPAA with DI (also known as the first of the two-stage surgery)[12,20]. If the length of the diverted bowel (between the stoma and pouch) is too short, strong tension in the mesentery may occur, and it is difficult to revert if bowel twisting or mesenteric torsion occurs. If the length is too long, bowel twisting, kinking, or angulation may occur; however, a spontaneous resolution is expected in such cases[12,18]. Several studies have shown an increased risk of SOO/SRO in laparoscopic surgery[18,22]. Laparoscopic surgery essentially reduces adhesion formation and may induce torsion of the mesentery or bowel as a result of increased bowel mobility. Regarding rotation, the risk of SOO/SRO remains controversial. Forced stoma rotation by 180-degree with IPAA significantly increases the incidence of SBO; bowel kinking at or below the fascia is presumably responsible for the obstruction[21]. In contrast, recent reports have shown that stoma rotation did not increase the incidence of SOO/SRO[11,12,15,18,20]. The reason for the SOO/SRO associated with high output from the stoma has already been described in the Etiology section.
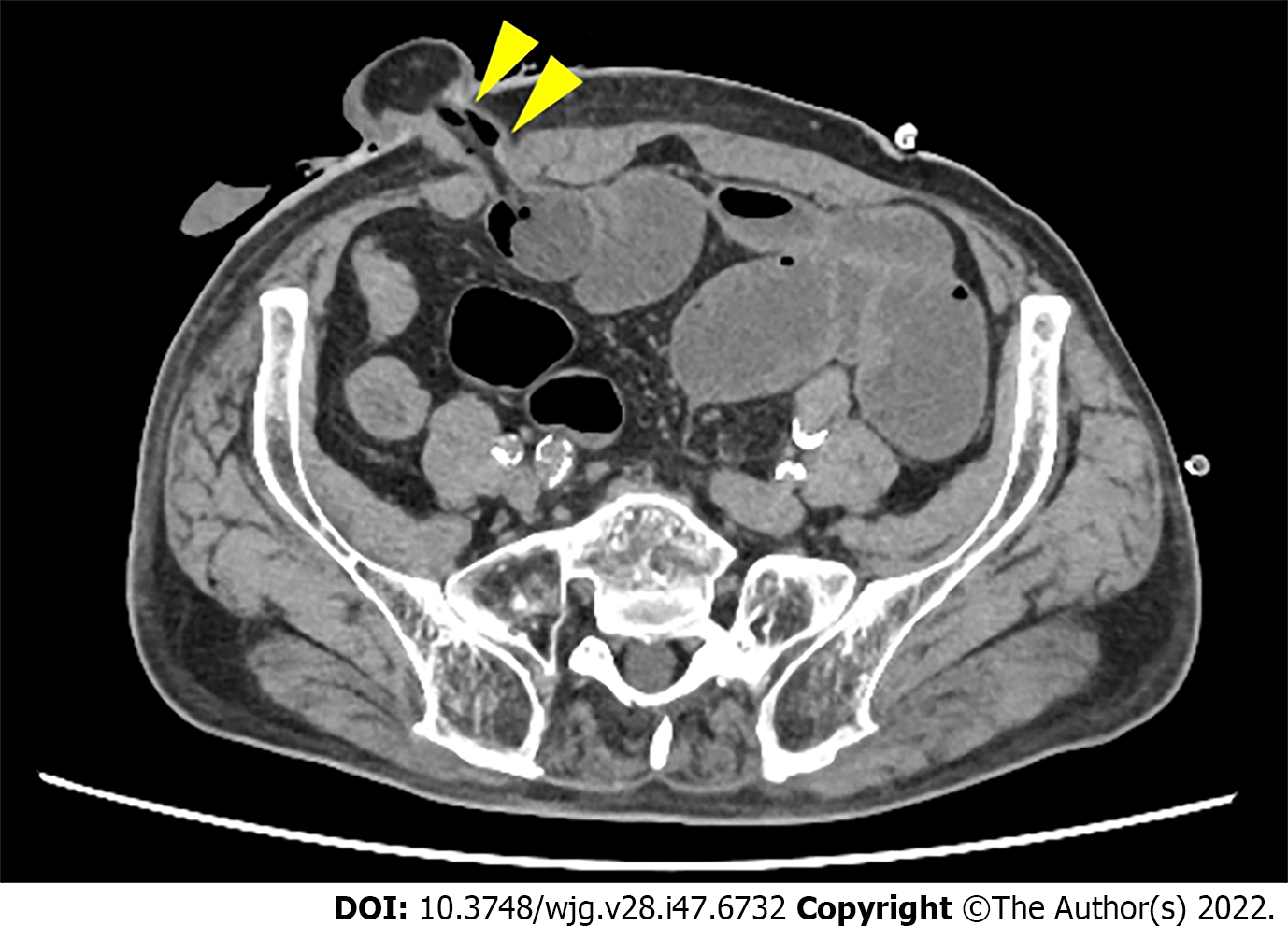
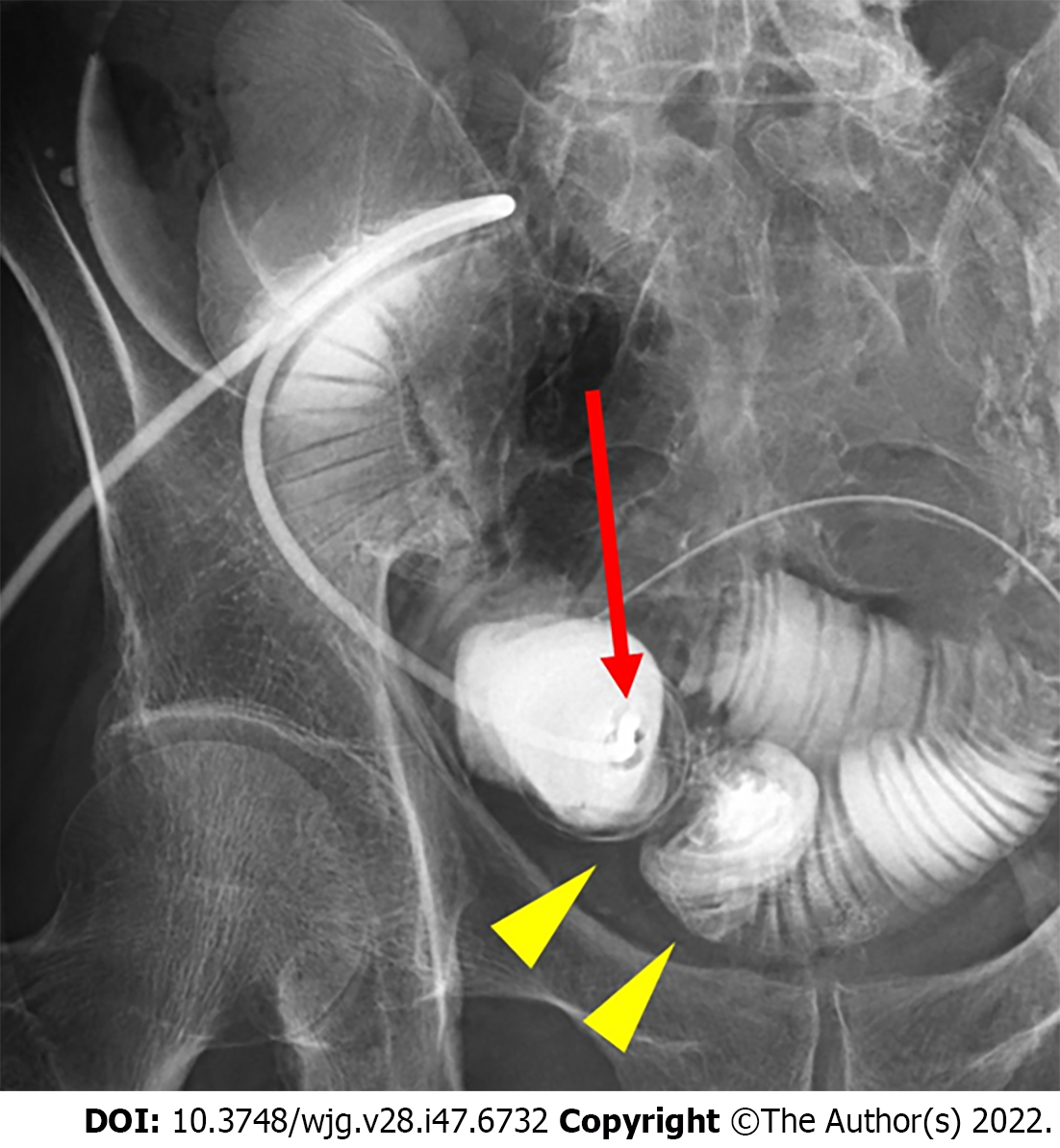
To examine whether the outlet is stenotic or obstructive, clinicians can simply insert their fingers into the stoma[23]. An abdominal CT scan or contrast enema study through the stoma may be useful for precise diagnosis, as they can demonstrate the location responsible for SOO/SRO and exclude other sites of SBO (Figures 1 and 2). Other pathologies causing similar symptoms, such as paralytic ileus, enteritis, volvulus, internal hernia, and parastomal hernia, should also be excluded.
In cases of SBO, fasting, bowel rest, and intravenous fluid administration are fundamental recommendations. When nausea, vomiting, or abdominal bloating/distention is present, a nasogastric tube may be placed. Although uncommon in Western societies, long nasointestinal tubes may be indicated to achieve quicker and more effective intraluminal decompression[11,24,25]. Trans-stomal tube decompression is attempted as an initial treatment for SOO/SRO. The success rate of local management using trans-stomal tubes ranges from 33%-86%[11,12,15,17]. Redo treatment may be indicated in cases of recurrent obstructions. It has been shown that thick RAM carries the risk of recurrent obstruction[17]. Importantly, adverse events related to trans-stomal tube decompression have also been reported. Bowel injury occurred in 3.7% of the intubated patients requiring emergency surgery. Stoma closure is the ultimate solution for SOO/SRO in patients for whom non-surgical, conservative management, or repeated tube decompressions were not successful.
Some of the risk factors for SOO/SRO, such as patient, disease, anatomical, and stoma function, may not be preventable, and surgical refinement must be fully considered. When creating DI, the anterior sheath is adequately incised, and the RAM is split and dilated to accommodate the stoma limbs. In the case of thick RAM, creating the tunnel by the conventional “two finger breadths rule” may not be sufficient; thus, wider dissection is necessary[13]. Moreover, care must be taken in laparoscopic surgery because the stoma is constructed under the effect of pneumoperitoneum with muscle relaxation[14]. In the case of IPAA, the distance between the ileal pouch and anastomosis must be longer than 30 cm to mitigate the risk of tension in the mesentery[12,18]. Rotation of the stoma limb is carefully performed if necessary. Although they did not advocate stoma rotation, Takehara et al[14] proposed that the oral stoma limb should be placed on the cranial side to avoid gravitational compression by the anal stoma limb. Some authors have recommended intra-abdominal suture fixation between the stoma limb and abdominal wall[26,27]; however, it might also carry the risk of immobility, tension, and fecal impaction in the stoma.
In healthy adults, approximately 1500 mL of intestinal fluid enters the colon from the ileum[28]. Theoretically, the same amount of fluid is drained from the newly established ileostomy; however, the normal output of ileostomy has been proposed as 600-1200 mL per day and may decrease over time[29-31]. This reduction in the quantity is called “ileostomy adaptation,” which suggests a compensatory increase in anti-diuretic hormones such as renin and aldosterone[28]. The consistency of the ileostomy output is generally watery when created and thickens in the next 2-3 mo[29]. Stool consistency, amount, and ileostomy output may be altered by the patient’s body weight, disease for the index surgery, liquid and food intake, and volume of gastrointestinal secretions[29,32].
Secretory complications of DI occur when the output exceeds the aforementioned normal limit. It is commonly termed “HOS” in the literature. This term may be considered similar to “dehydration’. Dehydration has been shown to be a cause of readmission in 9.3%-43% of the patients after ileostomy creation without preventive protocols[33-35]. The most recent meta-analysis showed that the pooled incidence of readmission due to dehydration was 6% regardless of HOS prevention and was accompanied by increased medical costs[36]. Moreover, HOS results in electrolyte imbalance and acute kidney injury in the early postoperative period and may lead to malnutrition and chronic renal impairment in the long term[30,37]. The prevalence of Clostridium difficile infection in the development of postoperative diarrhea/HOS was investigated, but it was found that the infection rate was low (1.6%) and that the patient outcomes were not affected by the infection[38]. Contradictory results have also been reported[39].
The ileostomy output includes large amounts of sodium (85-180 mmol/L per day)[40]. Persistent HOS can lead to sodium depletion, dehydration, and secondary hyperaldosteronism, which may cause sequential hypokalemia and hypomagnesemia, which are the main features of electrolyte imbalances. As the ileum and colon are the main sites of magnesium absorption, ileostomy patients may be susceptible to hypomagnesemia. Other reasons for hypomagnesemia include free fatty acid malabsorption and the use of proton pump inhibitors[31]. Baker et al[29] reported that nearly half of the patients with HOS exhibit hypomagnesemia.
The incidence of HOS varies from 14% to 24%[29,41-43] with various definitions. The majority of previous reports have defined using specific values of output volume with a timeframe, such as 2000 mL per day[38,44,45], 1500 mL per day for two days[41], 1000 mL per day for three days[42,46], and 2000 mL per day for three days[29,43]. Some authors have used a combination of output volume with laboratory findings of renal impairment and/or physical signs of dehydration[36,47].
HOS can occur in the early or late postoperative periods[29]. In a study, early HOS occurs within three weeks after stoma creation and resolves spontaneously in 49% of patients with HOS, while 7% receive persistent treatment. Late HOS occurs more than three weeks after stoma creation and resolves spontaneously in 15% of patients with HOS, while 47% receive persistent treatment. In that study, the diagnosis of cancer, followed by perforation and short bowel (less than 200 cm), correlated with the incidence of early HOS, whereas inflammatory bowel and obstruction were deemed to have an impact on late HOS[29]. Therefore, secretory complications of DI are an ongoing problem after stoma creation, and patients are always at risk, even after hospital discharge. This issue has become more relevant in the era of enhanced recovery after surgery.
The causes of HOS are multifactorial, including factors associated with patients[42,48], disease[29,42,43], anatomy[29], surgical procedure[29,42,43,48], medication[29,33], nutrition[29], enteritis/metabolism[29,39] and those related to postoperative complications[29,44,49]. Older age was identified as an independent risk factor of HOS in two studies: however, the cut-off values were not shown because the comparisons were made without dichotomization in their studies[42,48]. The reasons were not specified in either of the studies. Assaf et al[48] also showed that higher American Society of Anesthesiologists-physical status and elevated creatine levels were independently associated with HOS. Again, the cut-off values were not shown for the same reason as the older age. The authors assumed that impaired kidney functions resulted in less adaptation to fluids and electrolyte loss in these groups of patients.
Takeda et al[43] found that diabetes was one of the risk factors for HOS, with the following explanations. Diabetes may cause autonomic nervous system impairment that decreases the motor function of the bowel, followed by abnormal proliferation of intestinal bacteria and an augmented intestinal pressure with increased gas proliferation. Inflammatory bowel diseases, particularly Crohn’s disease, have been shown to be significantly associated with HOS[29,42,43]. Crohn’s disease presents an impaired intestinal permeability/barrier increased with altered gut microbiota and inflammatory tissue damage[42]. These factors can lead to HOS. Shortening of the small bowel with a more proximal ileostomy can also lead to HOS, and this may explain the reason why some surgical procedures (right-side colectomy, separate ileostomy, small bowel resection, and IPAA) were independent risk factors of HOS. Takeda et al[43] proposed another reason for HOS in patients who underwent IPAA: Inhibition of lipids absorption leads to hydroxylation or desaturation of unabsorbed long-chain acids, which triggers intestinal fluid and electrolyte secretion. Open surgery was significantly associated with HOS compared to laparoscopic surgery[42,48]. The possible explanation is that open surgery itself may not be the direct cause but patients undergoing more complex surgery with open laparotomy[48]. A short bowel (less than 200 cm) frequently causes HOS[29], but this may not be applicable in DI formation, where a stoma is created at the distal ileum.
Perioperative medication is an important underlying pathology for the development of HOS. The use of diuretics may easily result in dehydration because the body fluid balance is vulnerable in patients with ileostomy, even without any medications[33]. Administration of prokinetic drugs (e.g., metoclopramide) induces HOS; therefore, care must be taken when the patients suffer from nausea/vomiting with gastric stasis[31]. Sudden withdrawal of opiates and steroids induces reactive intestinal secretion[29-31]. Diarrhea with dehydration is commonly seen in patients who receive chemotherapy consisting of cytotoxic agents.
Regarding nutritional factors, intake of hypotonic fluids such as water, tea, coffee, fruit juice, and alcohol precipitates dehydration, and thus it is generally restricted or avoided[29-31]. Enteric infections, including Clostridium difficile or Salmonella, typically present with acute and severe diarrhea[29,50]. Chronic diarrhea may be caused by bacterial overgrowth from diverticula or blind loop fermentation[29,50].
Postoperative complications may induce secondary HOS, including prolonged ileus[44] (presented with nausea, vomiting, intolerance to oral feeding, abdominal distension, or failure to pass flatus or bowel movements within postoperative 7 d), SBO[29], SOO/SRO[49], or intra-abdominal sepsis/deep surgical site infection[29,49]. These postoperative complications must be excluded clinically and radiologically when suspicious of HOS. The details of the causes and risk factors of HOS are summarized in Table 2.
| Causes and risk factors of high output stoma |
| Patient |
| 1Older age[42,48] |
| 1Higher ASA-PS[48] |
| 1Elevated baseline creatine[48] |
| Disease |
| 1Diabetes[43] |
| Inflammatory bowel disease (i.e., ulcerative disease, Crohn’s disease)[29,42,43] |
| Anatomy |
| Short bowel (less than 200 cm)[29] |
| Surgical procedure |
| Open surgery (vs laparoscopic surgery)[42,48] |
| Total proctocolectomy (with ileal-pouch anal anastomosis)[42] |
| Right-side colectomy[42] |
| Separate ileostomy[42] |
| Small bowel resection[29,42,43] |
| Medication |
| Preoperative use of diuretics[31,33] |
| Prokinetic drugs (i.e., metoclopramide)[29,31] |
| Sudden withdrawal of corticosteroids or opiates[29,31] |
| Postoperative adjuvant chemotherapy[29] |
| Nutrition |
| Hypotonic liquids (low sodium): water, tea, coffee, fruit juice, alcohol[29,30,31] |
| Enteritis/metabolism |
| Clostridium difficile infection[29,50] |
| Salmonella infection[29,50] |
| Bacterial overgrowth from diverticula or blind loop fermentation[29,50] |
| Relative to postoperative complications |
| Postoperative ileus (symptoms of nausea, vomiting, intolerance to oral feeding, abdominal distension, or failure to pass flatus or bowel movements within postoperative 7 d)[44] |
| Intra-abdominal sepsis (pelvic sepsis, organ/space infection)[29,49] |
| Small bowel obstruction[29] |
| Stoma outlet obstruction[49] |
The key clinical symptoms of HOS are due to the loss of water and sodium and include thirst, cramps, muscle weakness, and faintness[31,50]. Patients may also present with loss of appetite, rapid weight loss, fall in postural blood pressure, or a decrease in urinary output[31]. The stomal output volume is measured every 8 h to facilitate early recognition of HOS. The stoma color and stool consistency should be inspected. Because stenosis or obstruction at the stoma outlet is one of the causes of HOS, a digital examination of the stoma is suitable for diagnosis. Laboratory findings may include elevated serum urea/creatinine ratio, hyponatremia, hypokalemia, and hypomagnesemia. A decrease in urinary sodium (less than 10 mmol/L) reflects sodium depletion more accurately than serum sodium level[31,50]. Long-lasting HOS may decrease the absorption of vitamin B12 and folic acid and increase the incidence of renal calculi and gallstones[40].
Exclusion of possible causes: As shown in Table 2, HOS occurs secondary to the underlying intra-abdominal complications. CT is useful for identifying these factors, and treatment of the identified cause must be prioritized. The administration or cessation of certain drugs is responsible for HOS and is corrected accordingly after the diagnosis. Enteritis induced by Clostridium difficile or Salmonella is excluded by taking stool cultures from the output.
Restriction of fluid intake: Since patients with dehydration complain of thirst in the early phase of HOS, increasing fluid intake may be inappropriately advised to relieve symptoms[30]. Consumption of excessive hypotonic fluids (less than 90 mmol/L of sodium) leads to worsening symptoms with sodium depletion caused by a net efflux from the plasma into the bowel lumen[29,50]. Hypertonic fluids containing glucose may also lead to increased stomal output[50]. Previous reports have suggested that hyper- and hypotonic fluid intake must be restricted to 0.5-1.0 mL per day[29,31,50]. Oral intake may not be possible when presenting with nausea/vomiting, and intravenous fluid rehydration containing 100-150 mmol/L of sodium (e.g., normal saline) is necessary to avoid acute kidney injury[31]. If oral intake is possible, it is recommended to sip 1 L or more of a glucose-saline solution containing at least 90 mmol/L sodium in a small quantity at intervals[31,50].
Drug therapies: Antimotility drugs include loperamide and codeine phosphate. Both drugs act against intestinal motility, and it has been shown that the ileotomy output reduces by 20%-30%[31]. Loperamide is preferred over codeine phosphate because it is not addictive. Loperamide can be prescribed at 4 mg/d to 16 mg/d, while codeine phosphate can be prescribed at 15 mg/d to 60 mg/d, and the effect becomes greater if both are taken together[31,41,50].
Antisecretory drugs include H2 antagonists and proton pump inhibitors that reduce gastric acid secretion. The somatostatin analog, octreotide, reduces gastric and pancreaticobiliary secretions and delays gastric emptying and small-bowel transit. Antisecretory drugs may be used when the stomal output exceeds 2 L/d, but octreotide is not preferred because the proton pump is as effective as octreotide in reducing the stomal output[29,31,41].
Hypomagnesemia in patients with HOS is caused by the chelation of magnesium with unabsorbed fatty acids and increased magnesium secretion due to secondary hyperaldosteronism[50] and reduced absorption of fatty acids. Magnesium is preferably supplemented orally; however, it can be slowly administered intravenously to avoid a flushing sensation.
Stoma closure: HOS may persist in 5.0%-15% of the affected patients until the stoma is reversed[29,35]. Stoma closure is planned for patients who do not respond to non-surgical conservative treatment. In a study, patients with readmission due to dehydration underwent stoma closure earlier than those without readmission[33]. Ihnát et al[5] reported that 3.8% of patients with DI underwent acute surgery for HOS.
Intensive monitoring and surveillance programs have been proposed recently. Most protocols consist of preoperative patient education, in-hospital monitoring and intervention protocols, post-discharge hospital visits, and surveillance using telephone or telemedicine platforms[51-54]. For example, Shaffer et al[54] proposed a pilot program for outpatient follow-ups using regular visits and telephone interviews. The triggers of intervention included tachycardia with > 100 beats/min, ileostomy output > 1200 mL/d, major changes in weight, fever, nausea, poor oral intake, dry mouth, and low urinary output. The response by the team included an assessment of the basic metabolic profile, intravenous hydration at home, and phone calls to the doctor’s office. This program successfully reduced the incidence of readmission from 21% to 8.7% and the cost of readmission by more than 80%.
These intense programs have been mostly successful in decreasing readmissions for dehydration (15%-21% before intervention vs 5.0%-8.8% after intervention)[52-54]; however, a conflicting result has also been reported, where the program failed to decrease readmissions for dehydration (8.2% vs 5.9%) despite obtaining better phone follow-up and an increase in outpatient intravenous fluid in the intervention group[51].
There are several limitations in this review. First, this is a narrative review without a systematic approach where a literature search was performed according to the authors’ experiences using their preferred keywords of choice. Furthermore, inclusion for the selection was purely based on the authors’ judgment considering their relevance to the topics. Second, the selected articles were mostly non-randomized, retrospective studies resulting in selection bias. Since the causes and risk factors of obstructive and secretory stoma complications are often multifactorial, it may be difficult to design prospective trials to investigate the efficacy of specific surgical or medical interventions. Instead, recent prospective studies have shown that postoperative monitoring and surveillance programs may facilitate early recognition and decrease the adverse events relative to the complications[51-54]. Third, the conclusions of the selected articles were based on their population-based analyses. The research findings may not be instantly applicable or comparable to the current practice of readers due to the differences in the definitions (diagnostic criteria), patient characteristics, disease, surgical settings, social background, and healthcare systems. The research findings must be carefully interpreted, considering these variables in the communities of interest.
Obstructive and secretory complications of DI occur both in the early and late postoperative periods and require intensive monitoring and intervention. Non-surgical conservative treatment is mostly effective; however, stoma closure may be considered in recurrent or refractory cases. To facilitate the diagnosis and treatment for patient safety, seamless communication and close collaboration in a multidisciplinary team are necessary.
Interestingly, obstructive and secretory complications may also occur simultaneously. However, the causative factors of these complex pathologies are various and inconsistent: the potential reason is that most evidence has been obtained by retrospective, observational studies with a small sample size. Further prospective randomized trials are needed to assess the efficacy of interventions or protocols to improve the outcomes of obstructive and secretory complications of ileostomy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Japanese Society of Gastroenterology, 035652.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pandit R, United States; Zharikov YO, Russia S-Editor: Gao CC L-Editor: A P-Editor: Chen YX
| 1. | Mu Y, Zhao L, He H, Zhao H, Li J. The efficacy of ileostomy after laparoscopic rectal cancer surgery: a meta-analysis. World J Surg Oncol. 2021;19:318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 2. | Ahmad NZ, Abbas MH, Khan SU, Parvaiz A. A meta-analysis of the role of diverting ileostomy after rectal cancer surgery. Int J Colorectal Dis. 2021;36:445-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 3. | Borucki JP, Schlaeger S, Crane J, Hernon JM, Stearns AT. Risk and consequences of dehydration following colorectal cancer resection with diverting ileostomy. A systematic review and meta-analysis. Colorectal Dis. 2021;23:1721-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Karjalainen EK, Renkonen-Sinisalo L, Mustonen HK, Lepistö AH. Morbidity related to diverting ileostomy after restorative proctocolectomy in patients with ulcerative colitis. Colorectal Dis. 2019;21:671-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Ihnát P, Guňková P, Peteja M, Vávra P, Pelikán A, Zonča P. Diverting ileostomy in laparoscopic rectal cancer surgery: high price of protection. Surg Endosc. 2016;30:4809-4816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Shimizu H, Yamaguchi S, Ishii T, Kondo H, Hara K, Takemoto K, Ishikawa S, Okada T, Suzuki A, Koyama I. Who needs diverting ileostomy following laparoscopic low anterior resection in rectal cancer patients? Surg Endosc. 2020;34:839-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Van Butsele J, Bislenghi G, D'Hoore A, Wolthuis AM. Readmission after rectal resection in the ERAS-era: is a loop ileostomy the Achilles heel? BMC Surg. 2021;21:267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Suwa K, Ushigome T, Ohtsu M, Narihiro S, Ryu S, Shimoyama Y, Okamoto T, Yanaga K. Risk Factors for Early Postoperative Small Bowel Obstruction After Anterior Resection for Rectal Cancer. World J Surg. 2018;42:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | FINK S. The intraluminal pressures in the intact human intestine. Gastroenterology. 1959;36:661-671. [PubMed] |
| 10. | Abe T, Nishimura J, Yasui M, Matsuda C, Haraguchi N, Nakai N, Wada H, Takahashi H, Omori T, Miyata H, Ohue M. Risk Factors for Outlet Obstruction in Patients with Diverting Ileostomy Following Rectal Surgery. J Anus Rectum Colon. 2021;5:254-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Okita Y, Araki T, Kondo S, Fujikawa H, Yoshiyama S, Hiro J, Inoue M, Toiyama Y, Kobayashi M, Ohi M, Inoue Y, Uchida K, Mohri Y, Kusunoki M. Clinical Characteristics of Stoma-Related Obstruction after Ileal Pouch-Anal Anastomosis for Ulcerative Colitis. J Gastrointest Surg. 2017;21:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Maemoto R, Tsujinaka S, Miyakura Y, Fukuda R, Kakizawa N, Takenami T, Machida E, Kikuchi N, Kanemitsu R, Tamaki S, Ishikawa H, Rikiyama T. Risk factors and management of stoma-related obstruction after laparoscopic colorectal surgery with diverting ileostomy. Asian J Surg. 2021;44:1037-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (2)] |
| 13. | Sasaki S, Nagasaki T, Oba K, Akiyoshi T, Mukai T, Yamaguchi T, Fukunaga Y, Fujimoto Y. Risk factors for outlet obstruction after laparoscopic surgery and diverting ileostomy for rectal cancer. Surg Today. 2021;51:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Takehara Y, Nakagawa M, Kobayashi H, Kakisako K, Takano Y, Seki J, Shimada S, Nakahara K, Mukai S, Enami Y, Sawada N, Ishida F, Kudo SE. A technique for constructing diverting loop ileostomy to prevent outlet obstruction after rectal resection and total colectomy: a retrospective single-center study. Surg Today. 2022;52:587-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Okada S, Hata K, Emoto S, Murono K, Kaneko M, Sasaki K, Otani K, Nishikawa T, Tanaka T, Kawai K, Nozawa H. Elevated risk of stoma outlet obstruction following colorectal surgery in patients undergoing ileal pouch-anal anastomosis: a retrospective cohort study. Surg Today. 2018;48:1060-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Tamura K, Matsuda K, Yokoyama S, Iwamoto H, Mizumoto Y, Murakami D, Nakamura Y, Yamaue H. Defunctioning loop ileostomy for rectal anastomoses: predictors of stoma outlet obstruction. Int J Colorectal Dis. 2019;34:1141-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Kitahara T, Sato Y, Oshiro T, Matsunaga R, Nagashima M, Okazumi S. Risk factors for postoperative stoma outlet obstruction in ulcerative colitis. World J Gastrointest Surg. 2020;12:507-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Mizushima T, Kameyama H, Watanabe K, Kurachi K, Fukushima K, Nezu R, Uchino M, Sugita A, Futami K. Risk factors of small bowel obstruction following total proctocolectomy and ileal pouch anal anastomosis with diverting loop-ileostomy for ulcerative colitis. Ann Gastroenterol Surg. 2017;1:122-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Mori R, Ogino T, Sekido Y, Hata T, Takahashi H, Miyoshi N, Uemura M, Doki Y, Eguchi H, Mizushima T. Long Distance Between the Superior Mesenteric Artery Root and Bottom of the External Anal Sphincter Is a Risk Factor for Stoma Outlet Obstruction After Total Proctocolectomy and Ileal-Pouch Anal Anastomosis for Ulcerative Colitis. Ann Gastroenterol Surg. 2022;6:249-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Kameyama H, Hashimoto Y, Shimada Y, Yamada S, Yagi R, Tajima Y, Okamura T, Nakano M, Miura K, Nagahashi M, Sakata J, Kobayashi T, Kosugi SI, Wakai T. Small Bowel Obstruction After Ileal Pouch-Anal Anastomosis With a Loop Ileostomy in Patients With Ulcerative Colitis. Ann Coloproctol. 2018;34:94-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Marcello PW, Roberts PL, Schoetz DJ Jr, Coller JA, Murray JJ, Veidenheimer MC. Obstruction after ileal pouch-anal anastomosis: a preventable complication? Dis Colon Rectum. 1993;36:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 58] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Dolejs S, Kennedy G, Heise CP. Small bowel obstruction following restorative proctocolectomy: affected by a laparoscopic approach? J Surg Res. 2011;170:202-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Babakhanlou R, Larkin K, Hita AG, Stroh J, Yeung SC. Stoma-related complications and emergencies. Int J Emerg Med. 2022;15:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 24. | Li de C, Li RH, Tian Q. Efficacy of intestinal decompression with long nasointestinal tube and selective contrast radiography in the treatment of small bowel obstruction in elderly patients. Minerva Chir. 2016;71:85-90. [PubMed] |
| 25. | Shi Y, Zhang XP, Qin H, Yu YJ. Naso-intestinal tube is more effective in treating postoperative ileus than naso-gastric tube in elderly colorectal cancer patients. Int J Colorectal Dis. 2017;32:1047-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Anderson DN, Driver CP, Park KG, Davidson AI, Keenan RA. Loop ileostomy fixation: a simple technique to minimise the risk of stomal volvulus. Int J Colorectal Dis. 1994;9:138-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Ng KH, Ng DC, Cheung HY, Wong JC, Yau KK, Chung CC, Li MK. Obstructive complications of laparoscopically created defunctioning ileostomy. Dis Colon Rectum. 2008;51:1664-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Kennedy HJ, Al-Dujaili EA, Edwards CR, Truelove SC. Water and electrolyte balance in subjects with a permanent ileostomy. Gut. 1983;24:702-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Baker ML, Williams RN, Nightingale JM. Causes and management of a high-output stoma. Colorectal Dis. 2011;13:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 30. | Goodey A, Colman S. Safe management of ileostomates with high-output stomas. Br J Nurs. 2016;25:S4-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Nightingale JMD. How to manage a high-output stoma. Frontline Gastroenterol. 2022;13:140-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 32. | Hill GL, Millward SF, King RF, Smith RC. Normal ileostomy output: close relation to body size. Br Med J. 1979;2:831-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Messaris E, Sehgal R, Deiling S, Koltun WA, Stewart D, McKenna K, Poritz LS. Dehydration is the most common indication for readmission after diverting ileostomy creation. Dis Colon Rectum. 2012;55:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 34. | Chan DKH, Ng J, Koh FH, Lim T, Yeo D, Tan KY, Tan KK. Journey for patients following ileostomy creation is not straightforward. Int J Colorectal Dis. 2019;34:2075-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Hayden DM, Pinzon MC, Francescatti AB, Edquist SC, Malczewski MR, Jolley JM, Brand MI, Saclarides TJ. Hospital readmission for fluid and electrolyte abnormalities following ileostomy construction: preventable or unpredictable? J Gastrointest Surg. 2013;17:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Vogel I, Shinkwin M, van der Storm SL, Torkington J, A Cornish J, Tanis PJ, Hompes R, Bemelman WA. Overall readmissions and readmissions related to dehydration after creation of an ileostomy: a systematic review and meta-analysis. Tech Coloproctol. 2022;26:333-349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 37. | Fielding A, Woods R, Moosvi SR, Wharton RQ, Speakman CTM, Kapur S, Shaikh I, Hernon JM, Lines SW, Stearns AT. Renal impairment after ileostomy formation: a frequent event with long-term consequences. Colorectal Dis. 2020;22:269-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 38. | Gaertner WB, Madoff RD, Mellgren A, Kwaan MR, Melton GB. Postoperative diarrhea and high ostomy output impact postoperative outcomes after elective colon and rectal operations regardless of Clostridium difficile infection. Am J Surg. 2015;210:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Williams RN, Hemingway D, Miller AS. Enteral Clostridium difficile, an emerging cause for high-output ileostomy. J Clin Pathol. 2009;62:951-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Shabbir J, Britton DC. Stoma complications: a literature overview. Colorectal Dis. 2010;12:958-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 275] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 41. | Arenas Villafranca JJ, López-Rodríguez C, Abilés J, Rivera R, Gándara Adán N, Utrilla Navarro P. Protocol for the detection and nutritional management of high-output stomas. Nutr J. 2015;14:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 42. | Seifarth C, Augustin LN, Lehmann KS, Stroux A, Lauscher JC, Kreis ME, Holmer C. Assessment of Risk Factors for the Occurrence of a High-Output Ileostomy. Front Surg. 2021;8:642288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | Takeda M, Takahashi H, Haraguchi N, Miyoshi N, Hata T, Yamamoto H, Matsuda C, Mizushima T, Doki Y, Mori M. Factors predictive of high-output ileostomy: a retrospective single-center comparative study. Surg Today. 2019;49:482-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 44. | Lee N, Lee SY, Kim CH, Kwak HD, Ju JK, Kim HR. The Relationship Between High-Output Stomas, Postoperative Ileus, and Readmission After Rectal Cancer Surgery With Diverting Ileostomy. Ann Coloproctol. 2021;37:44-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Ohta H, Miyake T, Ueki T, Kojima M, Kawasaki M, Tatsuta T, Iuchi T, Kamitani S, Shimizu T, Mekata E, Tani M. Predictors and clinical impact of postoperative diarrhea after colorectal cancer surgery: a prospective, multicenter, observational study (SHISA-1602). Int J Colorectal Dis. 2022;37:657-664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Fujino S, Miyoshi N, Ohue M, Takahashi Y, Yasui M, Sugimura K, Akita H, Takahashi H, Kobayashi S, Yano M, Sakon M. Prediction model and treatment of high-output ileostomy in colorectal cancer surgery. Mol Clin Oncol. 2017;7:468-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 47. | Li W, Stocchi L, Cherla D, Liu G, Agostinelli A, Delaney CP, Steele SR, Gorgun E. Factors associated with hospital readmission following diverting ileostomy creation. Tech Coloproctol. 2017;21:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 48. | Assaf D, Hazzan D, Ben-Yaacov A, Laks S, Zippel D, Segev L. Predisposing Factors for High Output Stoma in Patients With a Diverting Loop Ileostomy After Colorectal Surgeries. Ann Coloproctol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Hara Y, Miura T, Sakamoto Y, Morohashi H, Nagase H, Hakamada K. Organ/space infection is a common cause of high output stoma and outlet obstruction in diverting ileostomy. BMC Surg. 2020;20:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | Mountford CG, Manas DM, Thompson NP. A practical approach to the management of high-output stoma. Frontline Gastroenterol. 2014;5:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Grahn SW, Lowry AC, Osborne MC, Melton GB, Gaertner WB, Vogler SA, Madoff RD, Kwaan MR. System-Wide Improvement for Transitions After Ileostomy Surgery: Can Intensive Monitoring of Protocol Compliance Decrease Readmissions? Dis Colon Rectum. 2019;62:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Hsu AT, Crawford TC, Zhou X, Safar B, Efron J, Atallah C, Najjar PA, Girard AL, Glover JC, Warczynski T, Cowell NA, Cwik CL, Fang SH. Decreasing Readmissions After Ileostomy Creation Through a Perioperative Quality Improvement Program. Dis Colon Rectum. 2022;65:e797-e804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 53. | Nagle D, Pare T, Keenan E, Marcet K, Tizio S, Poylin V. Ileostomy pathway virtually eliminates readmissions for dehydration in new ostomates. Dis Colon Rectum. 2012;55:1266-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 145] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 54. | Shaffer VO, Owi T, Kumarusamy MA, Sullivan PS, Srinivasan JK, Maithel SK, Staley CA, Sweeney JF, Esper G. Decreasing Hospital Readmission in Ileostomy Patients: Results of Novel Pilot Program. J Am Coll Surg. 2017;224:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |