Published online Dec 14, 2022. doi: 10.3748/wjg.v28.i46.6564
Peer-review started: August 24, 2022
First decision: September 2, 2022
Revised: September 22, 2022
Accepted: November 22, 2022
Article in press: November 22, 2022
Published online: December 14, 2022
Processing time: 106 Days and 5.1 Hours
Endoscopic ultrasonography (EUS) has become a reliable method for predicting the invasion depth of early gastric cancer (EGC). However, diagnostic accuracy of EUS is affected by several factors. In particular, it is difficult to differentiate between T1a and T1b EGC through EUS.
To confirm whether submucosal saline injection (SSI) could improve the accuracy of EUS in distinguishing T1a and T1b lesions in EGC.
Twenty-four patients with EGC were examined by EUS and subsequently by SSI combined EUS to compare the degree of tumor invasion. Then, they underwent endoscopic or surgical resection within 7 d. The diagnostic accuracy of EUS and SSI combined EUS was evaluated based on the final pathological findings postoperatively. Saline injected into the submucosa acted as an echoic contrast enhancing agent and had the effect of distinguishing the mucosal and submucosal layers clearly.
Of total 24 patients, 23 were diagnosed with EGC (T1 cancer: 13 as T1a, and 10 as T1b). Standard EUS identified 6 of 13 T1a cancer patients and 3 of 10 T1b cancer patients. Whereas, EUS-SSI identified 12 of 13 T1a cancer patients and 6 of 10 T1b cancer patients. In this study, SSI combined EUS was more accurate than EUS alone in diagnosing T1a and T1b lesions of EGC (75.0% and 37.5%, respectively).
SSI improved the diagnostic accuracy of EUS in distinguishing between the T1a and T1b stages in EGC.
Core Tip: Submucosal saline injection improved the diagnostic accuracy of endoscopic ultrasonography in distinguishing between the T1a and T1b stages in early gastric cancer.
- Citation: Park JY, Jeon TJ. Diagnostic evaluation of endoscopic ultrasonography with submucosal saline injection for differentiating between T1a and T1b early gastric cancer. World J Gastroenterol 2022; 28(46): 6564-6572
- URL: https://www.wjgnet.com/1007-9327/full/v28/i46/6564.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i46.6564
Early gastric cancer (EGC) is a malignant lesion confined to the mucosa or submucosa (SM), regardless of lymph-node metastasis[1,2]. Endoscopic submucosal dissection (ESD) is widely used to treat EGC, and the indications for ESD are expanded in the cases assumed to have a low risk of lymph-node metastasis[3-5]. Even if the pathological depth of invasion is T1b (tumor invading the SM), ESD can be performed if the invasion is confined to SM1 (submucosal invasion to < 500 μm from the muscularis mucosae)[6,7]. However, an additional surgery is recommended for EGC when deep submucosal invasion is identified by pathological evaluation after ESD (more than SM2; depth of submucosal invasion, ≥ 500 μm) owing to the risk of lymph-node metastasis[8]. Therefore, the depth of invasion (T-stage) of gastric cancer is vital for determining the treatment strategy[3-7]. Endoscopic ultrasonography (EUS) has been used for T-staging of gastric cancer[9,10]. Although previous studies showed the clinical efficacy of EUS in T-staging of gastric cancer, the results have revealed a wide level of variability[1,2,11]. The diagnostic accuracy may be affected by endoscopic findings, lesion location, tumor size, and the skill of the examiner[1,12]. Specifically, EUS is difficult to distinguish between T1a (tumor invading the lamina propria and muscularis mucosae) and T1b lesions because the boundary between the mucosa and submucosa is thin and the difference in echogenicity is unclear[1,10].
Submucosal saline injection (SSI) is routinely administered prior to ESD to prevent damage to the surrounding tissue of the gastric wall and to avoid perforation during ESD[13]. SSI creates a cushion within the loose connective tissues of the submucosa, which has been reported as an effective medium and echoic contrast-enhancing agent for ultrasound transmission, enabling good distinction between the mucosal and submucosal layers[13-15]. Moreover, saline can increase the thickness of the submucosa[13-15]. According to previous studies, SSI improved the performance of EUS in characterizing the invasion depth of esophageal and colorectal cancers[13-15]. Therefore, this study was conducted to confirm whether SSI could be a method to improve the accuracy of EUS in distinguishing T1a and T1b lesions even in EGC and determine the feasibility of EUS for beginners.
Methods: During March–April 2019, 24 endoscopically diagnosed EGC lesions in 24 patients were examined by EUS. The macroscopic tumor classification was as follows: type I (protruded), type IIa (superficial elevated), type IIb (flat), type IIc (superficial depressed), and type III (excavated). Types I and IIa were classified as the elevated type, and IIb IIc, and III as the depressed type. All patients underwent standard EUS followed by EUS with SSI (EUS-SSI). EUS findings of T1a gastric cancers were defined as low-echoic lines of muscularis mucosae that were clearly demarcated from the submucosa, and T1b gastric cancers on EUS were defined as low-echoic line lesions that were not clearly distinguished from the boundary of submucosal layer. Subsequently, they underwent endoscopic or surgical resection within 7 d. Definitive classification was determined based on the postoperative pathology. All recruited patients agreed to be enrolled as participants in this clinical trial and were provided informed consent. This study was approved by the Institutional Review Board of the Inje University Sanggye Paik Hospital (SGPAIK2021-10-019).
EUS examination and staging were simultaneously conducted by one endoscopist with only 6 mo’ experience with EUS. The examiner performed EUS with a 12-MHz ultrasonic probe (Olympus GF-UE260-AL5 Endoscopic System, Olympus Co., Tokyo, Japan). SSI was thereafter conducted as follows: after the lesion was confirmed by conventional endoscopy and subsequently by iodine dye-enhanced endoscopy, the examiner injected 3–5 mL saline slowly into the submucosa using a single-use 22G mucosal needle (Endo-Flex Co., Voerde, Germany). The puncture points were located 0.5 cm beyond the edge of the lesion, and saline injection was stopped once the gastric mucosa had been elevated by approximately 1 cm. After SSI, the examiner determined the depth of the lesion using EUS.
All patients showed good tolerance of EUS-SSI without severe adverse events, such as significant bleeding, asphyxia, perforation, or problems related to anesthetics.
Of total 24 patients, 23 were diagnosed with EGC (T1 cancer: 13 as T1a, and 10 as T1b), except for one who was diagnosed with T2 cancer after the surgery. According to the macroscopic classification of tumors, there were 4 patients with elevated type lesions and 20 with depressed-type lesions. In 12 of the patients, the pathological T-stage was different between the standard EUS and EUS-SSI. Among them, EUS-SSI findings were consistent with the final pathological findings in 10 patients and standard EUS findings were consistent in one patient. The other patient was diagnosed with EGC stage-T2, which differed before and after the surgery (Table 1).
| Patient No. | Age | Sex | Location | Size (max, mm) | Endoscopic morphology (EGC type) | Ulcer | EUS-assessed preoperative stage | Final pathology | Type of resection | Differentiation | Regional LN invasion | Vascular invasion | |
| Before SSI (EUS-only) | After SSI (EUS-SSI) | ||||||||||||
| 1 | 72 | M | Lower third | 20 | 0-III | Y | T1a | T1b | T1b (sm3) | Surgery | Mod | ||
| 2 | 64 | M | Lower third | 20 | 0-IIc | T1b | T1b | T1b (sm1) | Surgery | Poor (signet ring) | Y | ||
| 3 | 59 | M | Lower third | 10 | 0-IIc | T1b | T1b | T1b (sm1) | Surgery | Poor | |||
| 4 | 53 | M | Upper third | 27 | 0-IIa | T1a | T1a | T1a | Surgery | Mod | |||
| 5 | 56 | F | Lower third | 38 | 0-IIc | T1b | T1a | T1a | Surgery | Poor | |||
| 6 | 73 | M | Upper third | 22 | 0-IIc | T1b | T1a | T1a | Surgery | Mod | |||
| 7 | 62 | M | Mid third | 65 | 0-IIc | T1b | T1a | T1a | Surgery | Mod | |||
| 8 | 68 | M | Upper third | 8 | 0-IIb | T1a | T1a | T1a | ESD | Well | |||
| 9 | 69 | M | Lower third | 15 | 0-IIa | T1a | T1a | T1a | ESD | Well | |||
| 10 | 71 | M | Lower third | 17 | 0-IIb | T1b | T1a | T1a | ESD | Poor | |||
| 11 | 54 | F | Lower third | 25 | 0-IIc | T1b | T1a | T1a | Surgery | Poor (signet ring) | |||
| 12 | 82 | M | Upper third | 15 | 0-IIb | T1a | T1a | T1b (sm1) | ESD | Poor | |||
| 13 | 71 | F | Lower third | 25 | 0-IIc | T1b | T1b | T1b (sm3) | Surgery | Poor | Y | Y | |
| 14 | 36 | F | Lower third | 20 | 0-IIc | T1a | T1b | T1b (sm3) | Surgery | Poor | Y | ||
| 15 | 60 | M | Lower third | 10 | 0-IIc | T1a | T1a | T1a | ESD | Mod | |||
| 16 | 62 | F | Mid third | 50 | 0-IIc | T2 | T1b | T1b (sm3) | Surgery | Poor | |||
| 17 | 74 | F | Upper third | 25 | 0-IIb | T1a | T1a | T1b (sm1) | Surgery | Poor | |||
| 18 | 60 | F | Upper third | 15 | 0-IIa | T1a | T1b | T2 | Surgery | Poor (signet ring) | |||
| 19 | 80 | F | Lower third | 15 | 0-IIb | T1a | T1b | T1a | ESD | Poor | |||
| 20 | 48 | F | Lower third | 45 | 0-IIc | T1a | T1a | T1b (sm3) | Surgery | Poor | |||
| 21 | 72 | F | Lower third | 10 | 0-IIc | T1a | T1a | T1b (sm1) | ESD | Mod | |||
| 22 | 50 | M | Lower third | 27 | 0-IIb | T1a | T1a | T1a | ESD | Well | |||
| 23 | 74 | M | Upper third | 15 | 0-IIc | T1b | T1a | T1a | Surgery | Mod | |||
| 24 | 76 | M | Upper third | 23 | 0-Is | T1b | T1a | T1a | Surgery | Well | |||
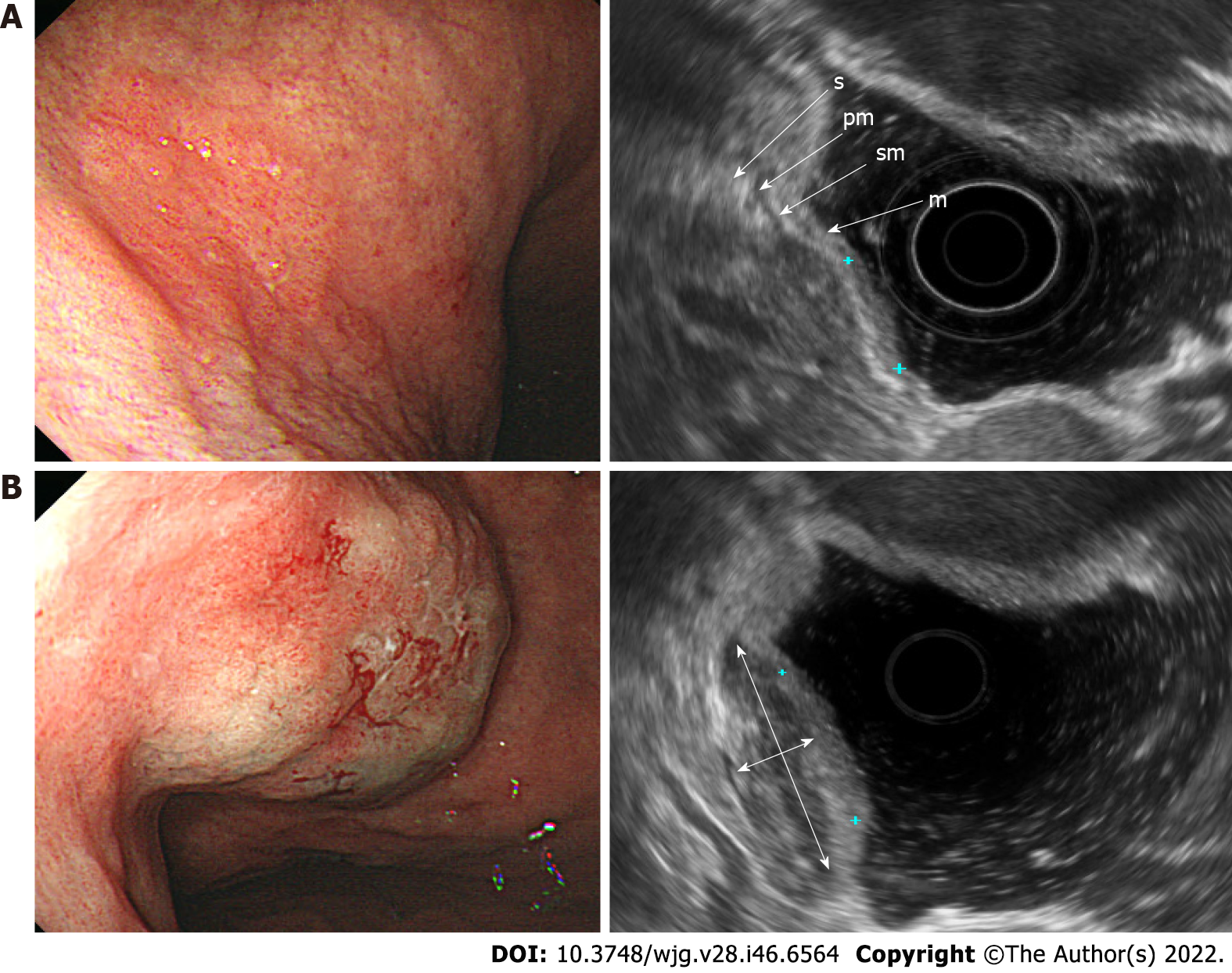
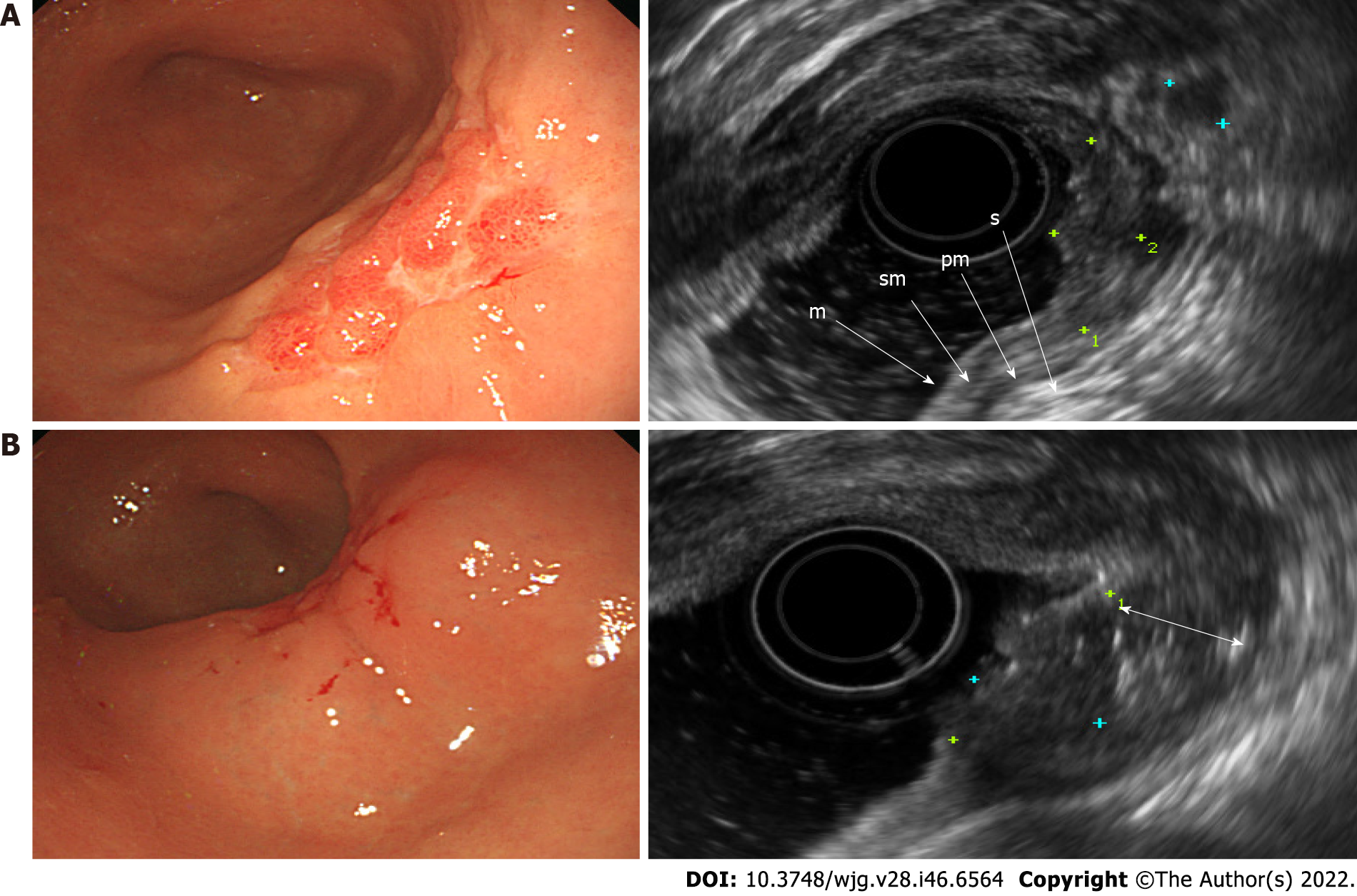
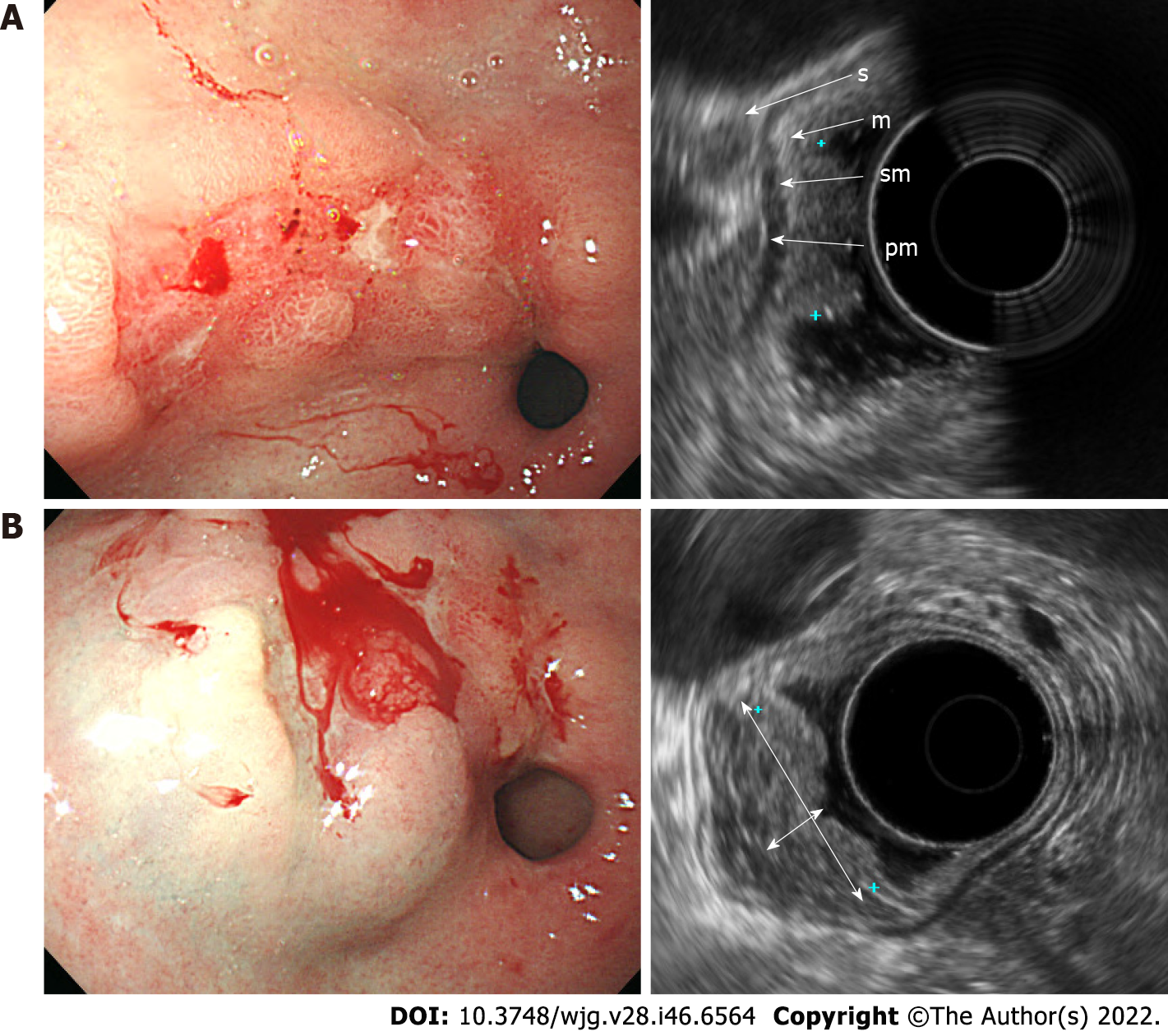
EGC was observed by using standard EUS as a localized thickening of the gastric mucosa or depression of the mucosal wall with a relatively low echogenicity. In patients with stage T1a disease, the muscularis mucosae was displayed as a low-echoic line between the mucosa and submucosa (Figure 1A). On the other hand, in patients with stage T1b, the muscularis mucosae was not clearly distinguished, and the boundary between the submucosal layer and the lower margin of the lesion was blurred, making it difficult to determine the degree of invasion of the submucosal layer on standard EUS (Figure 2A). After SSI, the mucosa had relatively enhanced echogenicity compared to the submucosa that was filled with saline. The boundary between the edge of the lesion and submucosa was apparent after SSI due to the saline-formed cushion in the submucosa (Figure 1B and 2B). Since the echoic difference between the lesion and the surrounding normal tissue became clear in EUS-SSI, the extent of tumor invasion was more distinct than that demonstrated by standard EUS (Figure 3).
Standard EUS identified 6 of 13 T1a cancer patients and 3 of 10 T1b cancer patients. Whereas, EUS-SSI identified 12 of 13 T1a cancer patients and 6 of 10 T1b cancer patients. The diagnostic accuracies of the standard EUS and EUS-SSI are shown in Table 2 (37.5% and 75.0%, respectively).
| Preoperative EUS reported stage | Postoperative pathologic stage | |
| T1a | T1b | |
| EUS-SSI, n (%) | ||
| T1a | 12 (92.3) | 4 (40) |
| T1b | 1 (7.7) | 6 (60) |
| EUS-only, n (%) | ||
| T1a | 6 (46.2) | 6 (66.7) |
| T1b | 7 (53.8) | 3 (33.3) |
EUS accurately characterizes the locoregional stage of gastric cancer and although the diagnostic accuracy of EUS in evaluating the invasion of depth of EGC has been reported, the results lack a consensus and have varying accuracy rates of 64.8%-92%[9-11]. Several studies also concluded that EUS has no significant advantage over conventional endoscopy in predicting the invasion depth[16]. Hence, it has been clarified that the accuracy of EUS can vary greatly depending on the experience of the endoscopist, macroscopic type of tumor, presence of ulceration, tumor located in the stomach, tumor size, and differentiation type[1,9,10,12]. Regarding ulcerative lesions, submucosal fibrosis occurs, which is observed on EUS as a hypoechoic lesion, similar to tumor invasion[2,10,16]. For lesions in the upper third of the stomach, the accuracy of EUS may decrease because of the different thicknesses of the stomach layer and presence of fibrosis or blood vessels surrounding the tumor[10,16]. In addition, it is difficult to fill the deaerated water and locate the EUS probe near the lesion because of the angulation of the EUS scope[10,16]. Previous studies have reported that a large tumor size is a risk factor for misdiagnosing the depth of invasion[10]. This is probably because the lesions might not extend even if the deaerated water is stored in cases of large tumors[11]. Undifferentiated-type tumors might have a diffuse or vesicular invasion of tumor cells to the submucosal layer of the gastric wall compared to differentiated-type tumors[11]. Thus, EUS cannot visualize these microinvasions and might underestimate the depth of invasion[11]. In our study, reviewing 15 patients with different results between final pathology and EUS-only findings, all patients had tumors located in the upper third of the stomach, sized ≥ 2 cm, ulcerative lesions, or undifferentiated type.
Regardless of the tumor characteristics, the diagnostic accuracy of EUS in predicting the T-stage of EGC in this study was 37.5%, which is low compared to that reported in previous studies. This study was conducted by a beginner endoscopist with approximately 6 mo’ experience. To increase the diagnostic accuracy of EUS for staging of gastric cancer, an endoscopist with a high experience and proficiency is required, but some techniques are also required for the classification of EGC. EUS may overestimate the depth of invasion due to underlying inflammation or fibrosis[10,11,16]. EUS-SSI showed improved results in reducing the overestimation and overall diagnostic accuracy (Table 3). By reducing over-staging, an unnecessary surgery can be avoided, surgery-related adverse events can be prevented, the recovery period can be further shortened, and the patient’s quality of life can be improved.
| EUS-only | EUS-SSI | |
| Overstaging, n (%) | 8 (33.3) | 1 (4.2) |
| Understaging, n (%) | 7 (29.2) | 5 (20.8) |
As limitations, we noted that EUS-SSI required a longer examination time than EUS-only, which may cause more patient discomfort. However, the patients in this study did not complain of discomfort and did not develop any adverse events related to SSI.
In our study, SSI improved the diagnostic accuracy of EUS in distinguishing between the T1a and T1b stages in EGC. This is probably because the saline injected into the submucosa serves as an echoic contrast-enhancing agent for the clear visualization of the boundary between the mucosa and the submucosa. However, our study is a clinical study conducted at a single institution, and the sample size is small, so there is a limit to interpreting the results. Therefore, a large-scale, prospective, randomized clinical trials for this are needed in the future. In particular, we suggest that beginners who are beginning EUS should try the EUS-SSI method when evaluating the depth of invasion of gastric cancer.
SSI improved the diagnostic accuracy of EUS in distinguishing between the T1a and T1b stages in EGC in this study. However, this needs to be confirmed in large-scale, prospective, randomized clinical trials in the future.
Although endoscopic ultrasound (EUS) is a method to predict the depth of invasion in early gastric cancer (EGC), it is still difficult to differentiate between T1a and T1b EGCs via EUS.
In particular, we considered a method to increase the accuracy of diagnosis for endoscopists who are beginning to perform EUS. It was thought that submucosal saline injection (SSI) during endoscopic mucosal resection may be helpful for examination because it can expand the submucosal layer.
The objectives of this study was to confirm whether SSI could be a method to improve the accuracy of EUS in distinguishing T1a and T1b lesions even in EGC and determine the feasibility of EUS for beginners.
During March-April 2019, 24 endoscopically diagnosed EGC lesions in 24 patients were examined by EUS. All patients underwent standard EUS followed by EUS with SSI (EUS-SSI). Thereafter, endoscopic or surgical resection was performed within 7 days. T1a and T1b lesions were diagnosed based on the final pathology results after treatment. The diagnostic accuracy of EUS and EUS-SSI for T stage was compared.
Standard EUS identified 6 of 13 T1a cancer patients and 3 of 10 T1b cancer patients. Whereas, EUS-SSI identified 12 of 13 T1a cancer patients and 6 of 10 T1b cancer patients. In this study, SSI combined EUS was more accurate than EUS alone in diagnosing T1a and T1b lesions of EGC (75.0% and 37.5%, respectively).
SSI improved the diagnostic accuracy of EUS in distinguishing between the T1a and T1b stages in EGC in this study. However, this needs to be confirmed in large-scale, prospective, randomized clinical trials in the future.
In our study, SSI improved the diagnostic accuracy of EUS in distinguishing between the T1a and T1b stages in EGC. In particular, we suggest that beginners who are beginning EUS should try the EUS-SSI method when evaluating the depth of invasion of gastric cancer. However, our study is a clinical study conducted at a single institution, and the sample size is small. Therefore, a large-scale, prospective, randomized clinical trials for this are needed in the future.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Arigami T, Japan; Ban T, Japan; Bestetti AM, Brazil S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Tsuzuki T, Okada H, Kawahara Y, Nasu J, Takenaka R, Inoue M, Kawano S, Kita M, Hori K, Yamamoto K. Usefulness and problems of endoscopic ultrasonography in prediction of the depth of tumor invasion in early gastric cancer. Acta Med Okayama. 2011;65:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 2. | Park JS, Kim H, Bang B, Kwon K, Shin Y. Accuracy of endoscopic ultrasonography for diagnosing ulcerative early gastric cancers. Medicine (Baltimore). 2016;95:e3955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Fukunaga S, Nagami Y, Shiba M, Ominami M, Tanigawa T, Yamagami H, Tanaka H, Muguruma K, Watanabe T, Tominaga K, Fujiwara Y, Ohira M, Hirakawa K, Arakawa T. Long-term prognosis of expanded-indication differentiated-type early gastric cancer treated with endoscopic submucosal dissection or surgery using propensity score analysis. Gastrointest Endosc. 2017;85:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Lee S, Choi KD, Han M, Na HK, Ahn JY, Jung KW, Lee JH, Kim DH, Song HJ, Lee GH, Yook JH, Kim BS, Jung HY. Long-term outcomes of endoscopic submucosal dissection vs surgery in early gastric cancer meeting expanded indication including undifferentiated-type tumors: a criteria-based analysis. Gastric Cancer. 2018;21:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 5. | Fujiya K, Takizawa K, Tokunaga M, Kawata N, Hikage M, Makuuchi R, Tanizawa Y, Bando E, Kawamura T, Tanaka M, Kakushima N, Ono H, Terashima M. The value of diagnostic endoscopic submucosal dissection for patients with clinical submucosal invasive early gastric cancer. Gastric Cancer. 2018;21:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Sanomura Y, Oka S, Tanaka S, Noda I, Higashiyama M, Imagawa H, Shishido T, Yoshida S, Hiyama T, Arihiro K, Chayama K. Clinical validity of endoscopic submucosal dissection for submucosal invasive gastric cancer: a single-center study. Gastric Cancer. 2012;15:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Ojima T, Takifuji K, Nakamura M, Nakamori M, Yamaue H. Feasibility of Endoscopic Submucosal Dissection for Submucosal-invasive Gastric Cancer and the Predictors of Residual or Recurrent Cancer. Surg Laparosc Endosc Percutan Tech. 2016;26:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1326] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 9. | Hwang SW, Lee DH. Is endoscopic ultrasonography still the modality of choice in preoperative staging of gastric cancer? World J Gastroenterol. 2014;20:13775-13782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Kuroki K, Oka S, Tanaka S, Yorita N, Hata K, Kotachi T, Boda T, Arihiro K, Chayama K. Clinical significance of endoscopic ultrasonography in diagnosing invasion depth of early gastric cancer prior to endoscopic submucosal dissection. Gastric Cancer. 2021;24:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Shi D, Xi XX. Factors Affecting the Accuracy of Endoscopic Ultrasonography in the Diagnosis of Early Gastric Cancer Invasion Depth: A Meta-analysis. Gastroenterol Res Pract. 2019;2019:8241381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Ahn HS, Lee HJ, Yoo MW, Kim SG, Im JP, Kim SH, Kim WH, Lee KU, Yang HK. Diagnostic accuracy of T and N stages with endoscopy, stomach protocol CT, and endoscopic ultrasonography in early gastric cancer. J Surg Oncol. 2009;99:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Li JJ, Shan HB, Gu MF, He L, He LJ, Chen LM, Luo GY, Xu GL. Endoscopic ultrasound combined with submucosal saline injection for differentiation of T1a and T1b esophageal squamous cell carcinoma: a novel technique. Endoscopy. 2013;45:667-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | He LJ, Xie C, Wang ZX, Li Y, Xiao YT, Gao XY, Shan HB, Luo LN, Chen LM, Luo GY, Yang P, Zeng SC, Xu GL, Li JJ. Submucosal Saline Injection Followed by Endoscopic Ultrasound vs Endoscopic Ultrasound Only for Distinguishing between T1a and T1b Esophageal Cancer. Clin Cancer Res. 2020;26:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Watanabe H, Miwa H, Terai T, Imai Y, Ogihara T, Sato N. Endoscopic ultrasonography for colorectal cancer using submucosal saline solution injection. Gastrointest Endosc. 1997;45:508-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Kim J, Kim SG, Chung H, Lim JH, Choi JM, Park JY, Yang HJ, Han SJ, Oh S, Kim MS, Kim HJ, Hong H, Lee HJ, Kim JL, Lee E, Jung HC. Clinical efficacy of endoscopic ultrasonography for decision of treatment strategy of gastric cancer. Surg Endosc. 2018;32:3789-3797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |