Published online Dec 14, 2022. doi: 10.3748/wjg.v28.i46.6537
Peer-review started: September 30, 2022
First decision: October 21, 2022
Revised: October 29, 2022
Accepted: November 18, 2022
Article in press: November 18, 2022
Published online: December 14, 2022
Processing time: 68 Days and 23.4 Hours
Immune cells, including neutrophils, natural killer (NK) cells, T cells, NKT cells and macrophages, participate in the progression of acute liver injury and hepatic recovery. To date, there has been no systematic study on the quantitative changes in these different immune cells from initial injury to subsequent recovery.
To investigate the infiltration changes of various immune cells in acute liver injury models over time, and to study the relationship between the changes in leukocyte cell-derived chemotaxin 2 (LECT2) and the infiltration of several immune cells.
Carbon tetrachloride- and concanavalin A-induced acute liver injury models were employed to mimic toxin-induced and autoimmune-mediated liver injury respectively. The quantitative changes in various immune cells were monitored at different time points. Serum samples were collected, and liver tissues were harvested. Ly6G, CD161, CD4, CD8 and F4/80 staining were used to indicate neutrophils, NK/NKT cells, CD4+ T cells, CD8+ T cells and macrophages, respectively. Lect2-KO mice were used to detect the function of LECT2.
During the injury and repair process, different types of immune cells began to increase, reached their peaks and fell into decline at different time points. Furthermore, when the serum alanine transaminase (ALT) and aspartate transaminase (AST) indices reverted to normal levels 7 d after the injury, the infiltration of immune cells still existed even 14 d after the injury, showing an obvious lag effect. We found that the expression of LECT2 was upregulated in acute liver injury mouse models, and the liver injuries of Lect2-KO mice were less severe than those of wild-type mice. Compared with wild-type mice, Lect2-KO mice had different immune cell infiltration.
The recovery time of immune cells was far behind that of serum ALT and AST during the process of liver repair. LECT2 could regulate monocyte/macrophage chemotaxis and might be used as a therapeutic target for acute liver injury.
Core Tip: In our study, we systematically described the infiltration changes of various immune cells during the process of acute liver injury and repair. We found that the recovery time of immune cells was far behind that of serum alanine transaminase and aspartate transaminase during the process of liver repair. Moreover, we found that leukocyte cell-derived chemotaxin 2 (LECT2) was upregulated in acute liver injury mouse models and the changes in LECT2 were related to the changes in several immune cells. LECT2 could regulate monocyte/macrophage chemotaxis and might be used as a therapeutic target for acute liver injury.
- Citation: Xie Y, Zhong KB, Hu Y, Xi YL, Guan SX, Xu M, Lin Y, Liu FY, Zhou WJ, Gao Y. Liver infiltration of multiple immune cells during the process of acute liver injury and repair. World J Gastroenterol 2022; 28(46): 6537-6550
- URL: https://www.wjgnet.com/1007-9327/full/v28/i46/6537.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i46.6537
Acute liver injury is induced by a variety of causes, including viral infection, autoimmune diseases, alcohol or drug abuse and toxin intake, which can lead to liver failure and even death[1,2]. Various immune cells, including neutrophils, natural killer (NK) cells, T cells, NKT cells and macrophages, are directed and recruited to damaged sites and inflammatory lesions following acute injury[3]. Accumulated studies have indicated that these immune cells may participate in the progression of liver injury[4-6] and hepatic recovery[7-9]. A complete description of the changes in the number of various immune cells during the process of liver injury and repair could indicate the potential roles of these immune cells and may also help to treat acute liver injury by regulating these different immune cells at different time points. To date, there has been no systematic study on the quantitative changes in these different immune cells from initial injury to subsequent recovery. Here, we employed carbon tetra
Leukocyte cell-derived chemotaxin 2 (LECT2) is a 16-kDa protein mainly produced by hepatocytes, first isolated in PHA-activated human T-cell leukemia SKW-3 cells in 1996[10]. Increasing evidence suggests that LECT2 is a pleiotropic protein, it not only functions as a cytokine to exhibit chemotactic properties but also plays multifunctional roles in some physiological conditions and pathological abnor
C57BL6/J (wild-type) mice were purchased from Guangzhou University of Chinese Medicine. Lect2-KO mice were provided by Professor Jiong Chen from Ningbo University. The genomic DNA from the Lect2-KO mice was extracted for PCR amplification with the forward and reverse primers (Forward: 5’-CATAGCCAGGGGACTATGTTTTA-3’; Reverse: 5’-ATATAGTCATAGCTGCACACAGCA-3’). The PCR products were further used to confirm genotyping by Sanger sequencing. Mice were kept in a standard 12 h light–dark cycle under specific-pathogen-free conditions, and were allowed free access to water and food. All mice used for the study were male, healthy and immune-normal, weighing 22-25 g. Animal-related research protocols were consistent with the US Public Health Service Policy on Use of Laboratory Animals, and were approved by the Ethics Committee on Use and Care of Animals of Southern Medical University.
CCl4 (Maclin, C805332) and ConA (Solarbio, C8110) were separately used for the induction of acute liver injury model. For the CCl4-induced acute liver injury model, mice were injected intraperitoneally with a CCl4 mixture at a dose of 10 mL/kg body weight once (CCl4 dissolved in olive oil at a volume ratio of 1:4). For the ConA-induced acute liver injury model, mice were injected intravenously with ConA solution at a dose of 10 mg/kg body weight once (ConA dissolved in PBS at a concentration of 2 mg/mL). Serum samples and liver tissues were collected and stored at -80 ℃ refrigerator for subsequent analyses. There were five mice per group.
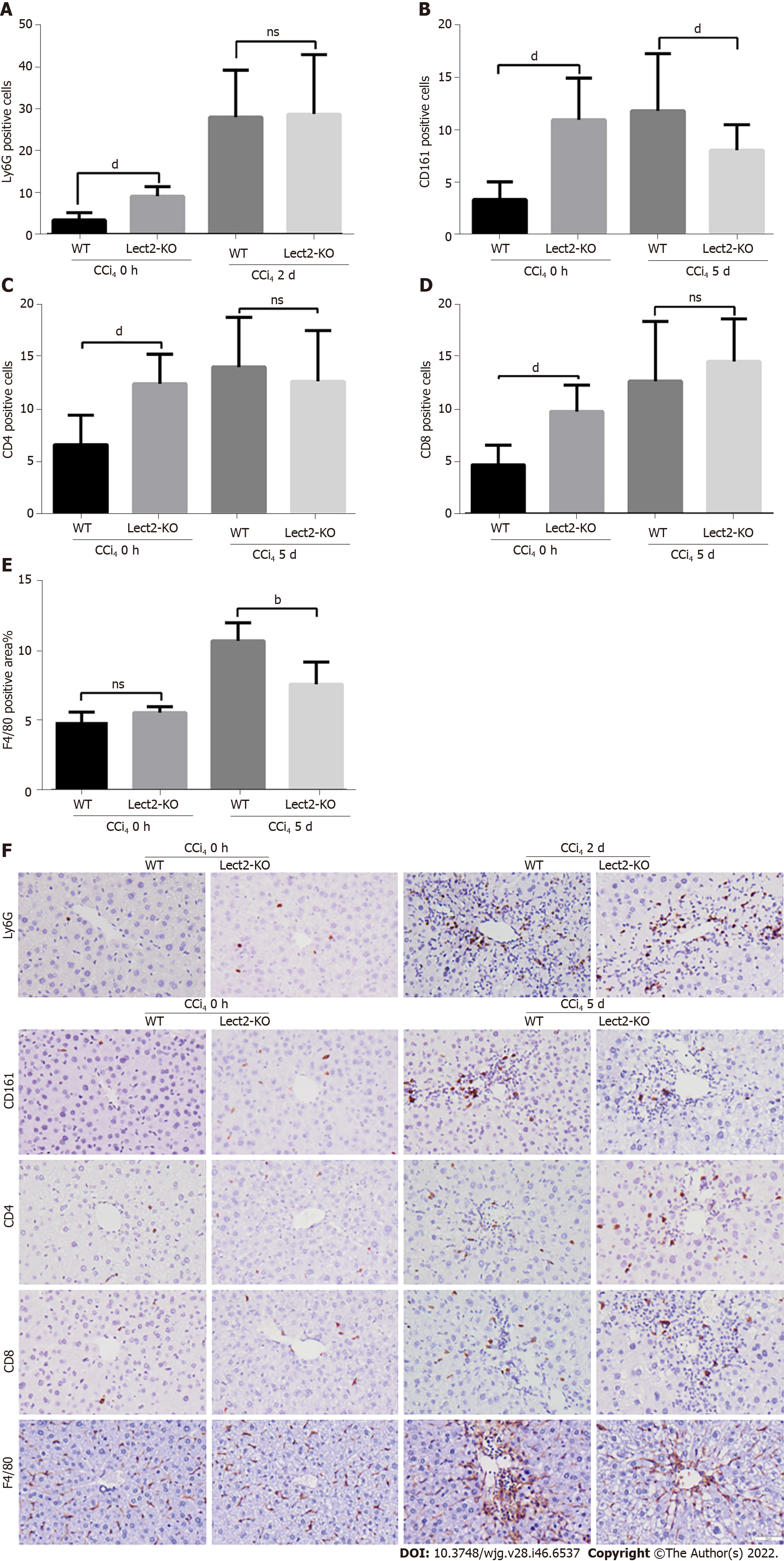
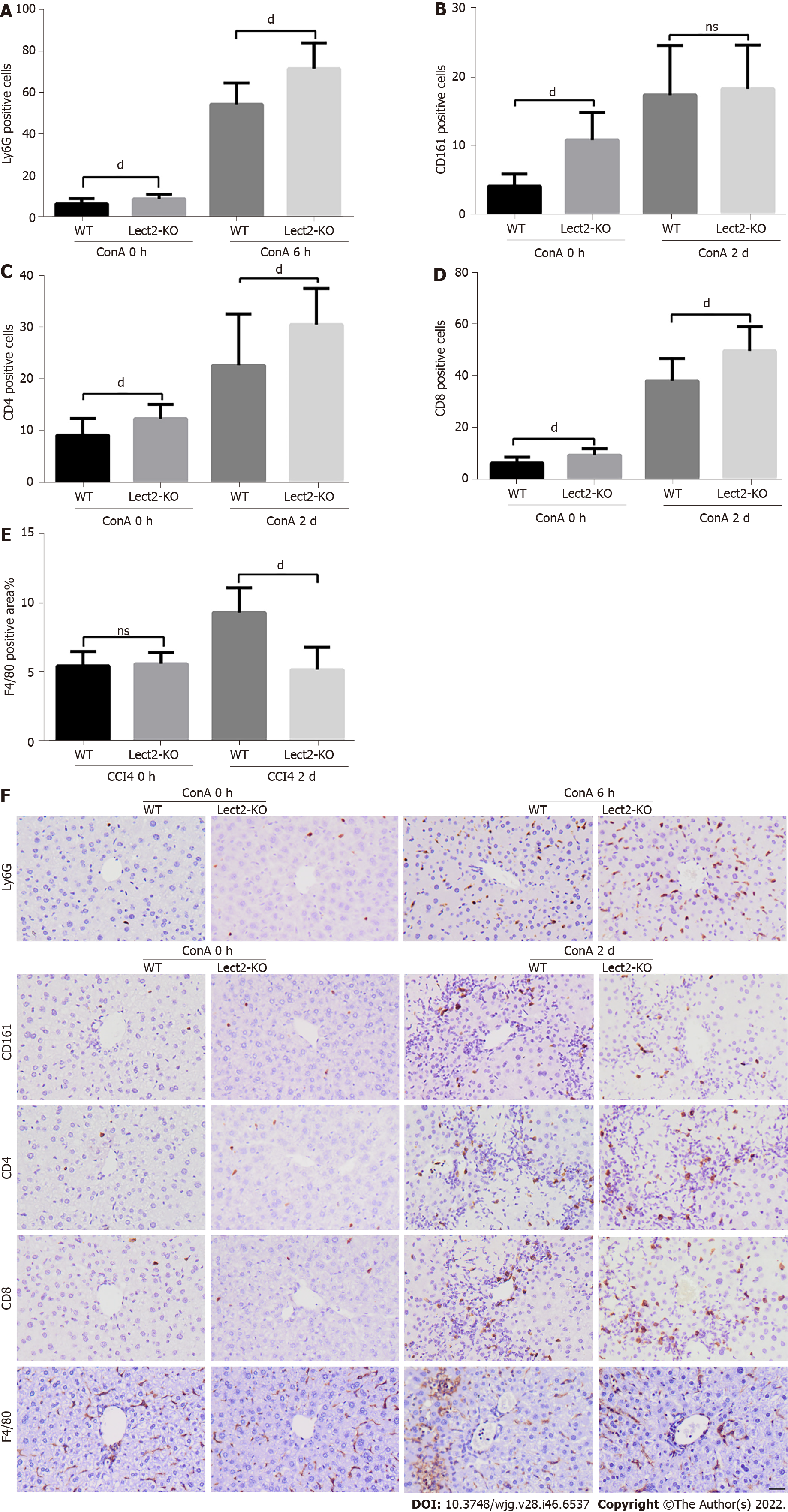
Harvested liver tissues were fixed in 4% neutral buffered formalin, and then dehydrated and embedded in paraffin. Sections of 3.5-mm thickness were dewaxed, and incubated in antigen retrieval solution for 5 min at 120 ℃, and then endogenous peroxidases were blocked with 3% H2O2 for 15 min. The slides were incubated with goat serum for 40 min at 37 ℃ to block nonspecific binding sites, followed by incubation with the appropriate primary antibodies overnight at 4 ℃, and then with HRP (horseradish peroxidase) anti-rabbit IgG for 30 min at 37 ℃. Color was developed by incubation with a DAB substrate kit (Zsbio, ZLI-9017). After washing with PBS, sections were counterstained with hematoxylin. Major primary antibodies used in the study included anti-Ly6G antibody (1:100, CST, 87048), anti-CD161 antibody (1:200, CST, 39197), anti-CD4 antibody (1:500, Abcam, ab183685), anti-CD8 antibody (1:1000, Abcam, ab209775) and anti-F4/80 antibody (1:200, CST, 70076). Images from ten random fields were taken for each section, under a 200 × light microscope. Cells positive for Ly6G, CD161, CD4 and CD8 were counted manually. The percentages of F4/80 positive areas were calculated automatically by ImageJ software.
Total RNA from mouse livers was extracted using TRIzol reagent (AG, 21102), and reverse transcribed using Evo M-MLV RT Premix (AG, 11706). Quantitative qRT-PCR was performed on an ABI 7500 Fast real-time PCR system with SYBR Green Master PCR Mix (AG, 11701), Rox Reference Dye (AG, 11710) and primers. The primers applied in qRT-PCR were as follows: Lect2 forward (mouse), 5’-GGACGTGTGACAGCTATGGC-3’; Lect2 reverse (mouse), 5’-TCCCAGTGAATGGTGCATACA-3’; GAPDH forward (mouse), 5’-AGAAGGTGGTGAAGCAGGCATC-3’, GAPDH reverse (mouse), 5’-CGAAGGTGGAAGAGTGGGAGTTG-3’.
The serum levels of ALT, AST and TBil were detected by an Automated Chemical Analyzer (Mindray, BS-240VET) with standard diagnostic kits. The serum levels of LECT2 were tested by an ELISA kit (Cloud-Clone, SEF541Mu).
The wild-type and Lect2-KO mice were injected with CCl4 or ConA, and then livers from these mice were harvested after 2 d. Livers were also obtained from wild-type mice without CCl4 and ConA treatment as standard controls. Total RNA was extracted from the tissue using TRIzol reagent according to the manufacturer’s instructions (Invitrogen) and genomic DNA was removed with DNaseI (TaKaRa). Then RNA quality was determined and only a high-quality RNA sample was used to construct a sequencing library. RNA-seq transcriptome libraries were prepared following the TruSeqTM RNA Sample Preparation Kit (Illumina, San Diego, CA, United States) using 1 μg of total RNA. Messenger RNA was isolated according to the poly (A) selection method by oligo (dT) beads and then fragmented by fragmentation buffer. Next, double-stranded cDNA was synthesized using a SuperScript double-stranded cDNA synthesis kit (Invitrogen, CA, United States) with random hexamer primers (Illumina). The synthesized cDNA was subjected to end-repair, phosphorylation and ‘A’ base addition according to Illumina’s library construction protocol. Libraries were size selected for cDNA target fragments of 300 bp, followed by PCR amplification with Phusion DNA polymerase (NEB) for 15 PCR cycles. After quantification by TBS380, the paired-end RNA-seq sequencing library was sequenced with an Illumina NovaSeq 6000 sequencer. All steps of RNA sequencing were performed by Majorbio BioPharm Technology Corporation, Shanghai.
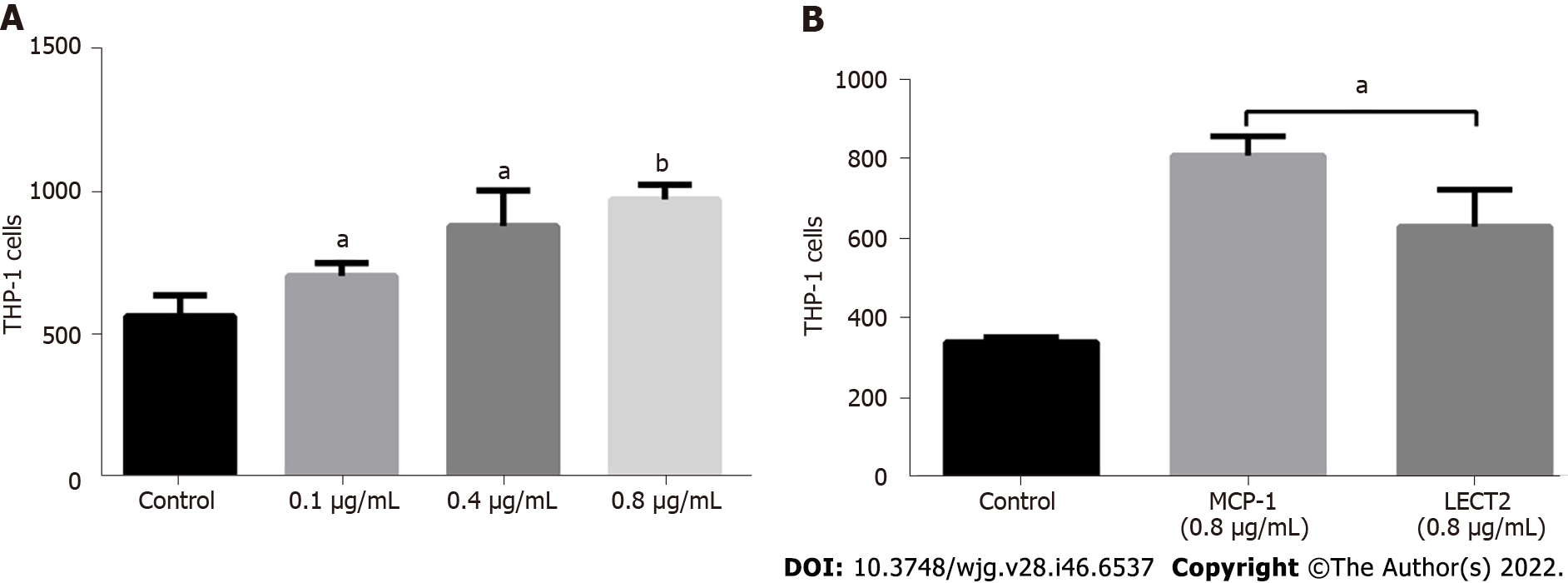
Transwell inserts with 8-μm pores were used for the chemotaxis assay. THP-1 cells (2 × 106/mL, 200 µL) were suspended in RPMI 1640 medium containing 10% FBS and 4000 nmol/L PMA (Bioss, D10289s), cells were seeded in each upper chamber. LECT2 (Abcam ab188467) and MCP-1 (Proteintech, Ag24085) were added to the lower chambers filled with 500 µl serum free medium containing 4000 nmol/L PMA. After incubation at 37 ℃ in 5% CO2 for 2 h, the Transwell inserts were removed. Six consecutive pictures were taken with 200 × light microscope for the cells that migrated to the lower chambers. The number of cells was counted by ImageJ software for each picture.
The results of the experiments are presented as the mean ± SD. The experimental data were analyzed by Student's t test. Statistical analysis and graph production were performed by GraphPad Prism software. A P value < 0.05 was considered to be statistically significant.
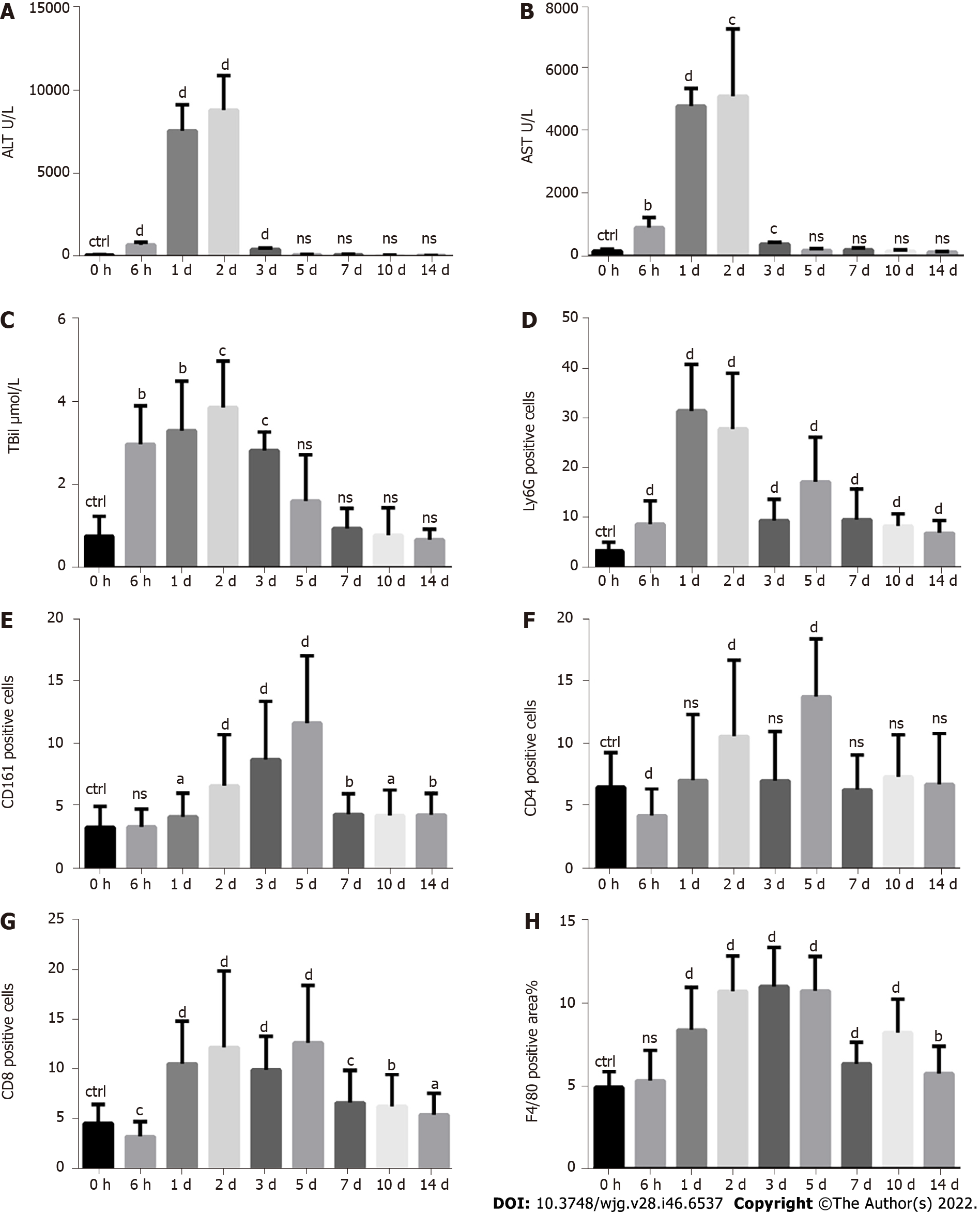
A single injection of CCl4 at a dose of 10 mL/kg body weight was used to mimic toxin-induced acute liver injury in mice. The levels of serum alanine transaminase (ALT), aspartate transaminase (AST) and total bilirubin (TBil) were measured to evaluate the degree of liver injury from 0 h to 14 d after CCl4 treatment. The levels of serum ALT, AST and TBil increased as early as 6 h, followed by a sharp rise and a peak on the 1st and 2nd days, respectively, and then decreased to normal on the 5th day (Figure 1A-C). Ly6G, CD161, CD4, CD8 and F4/80 staining were used to indicate neutrophils, NK/NKT cells, CD4+ T cells, CD8+ T cells and macrophages, respectively. Neutrophils increased as early as 6 h, followed by a sharp rise lasting for 2 d (Figure 1D and Supplementary Figure 1). NK/NKT cells increased as early as the 1st day, followed by a continuous rise with a peak on the 5th day (Figure 1E and Supplemen
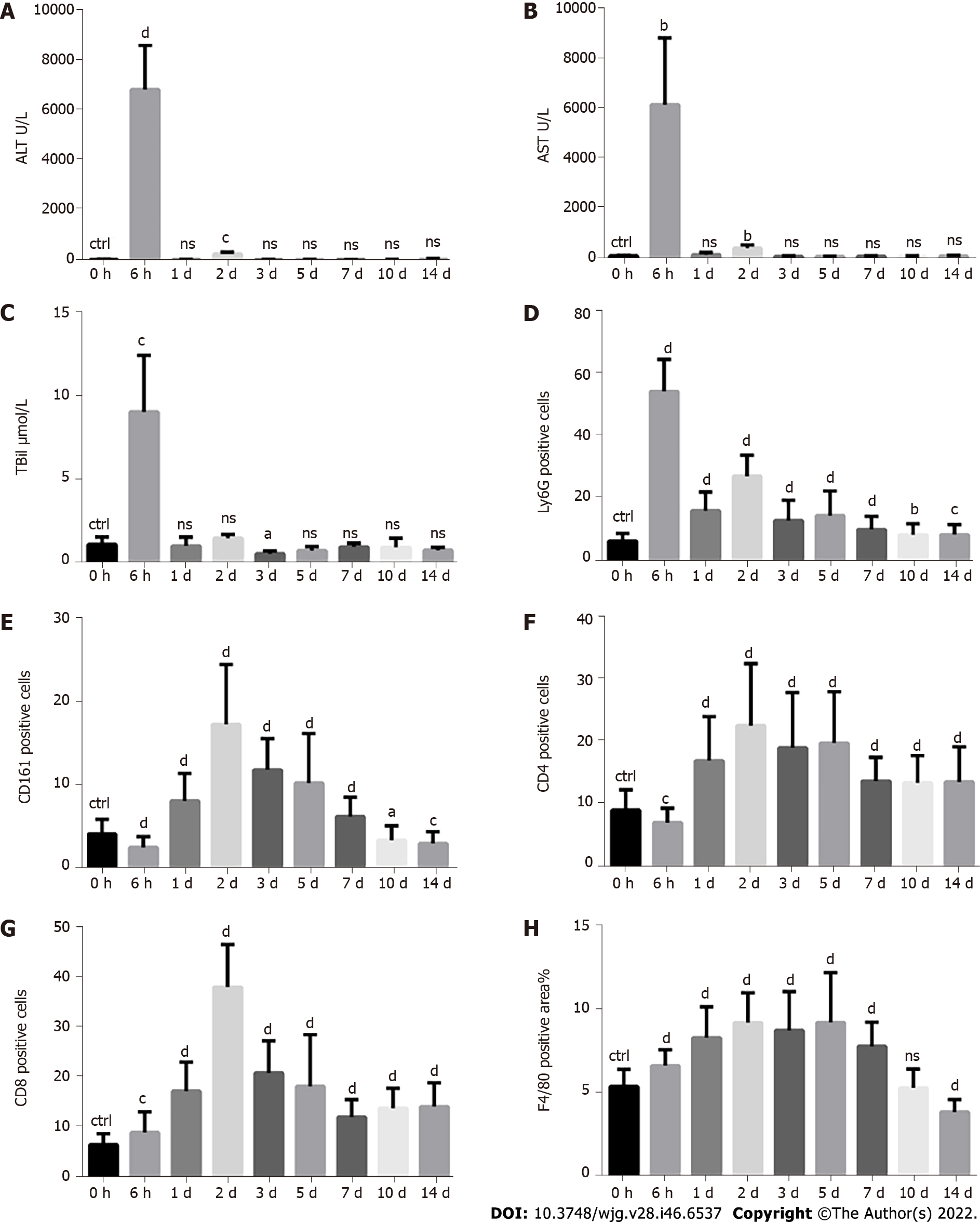
A single injection of ConA at a dose of 10 mg/kg body weight was used to mimic autoimmune-media
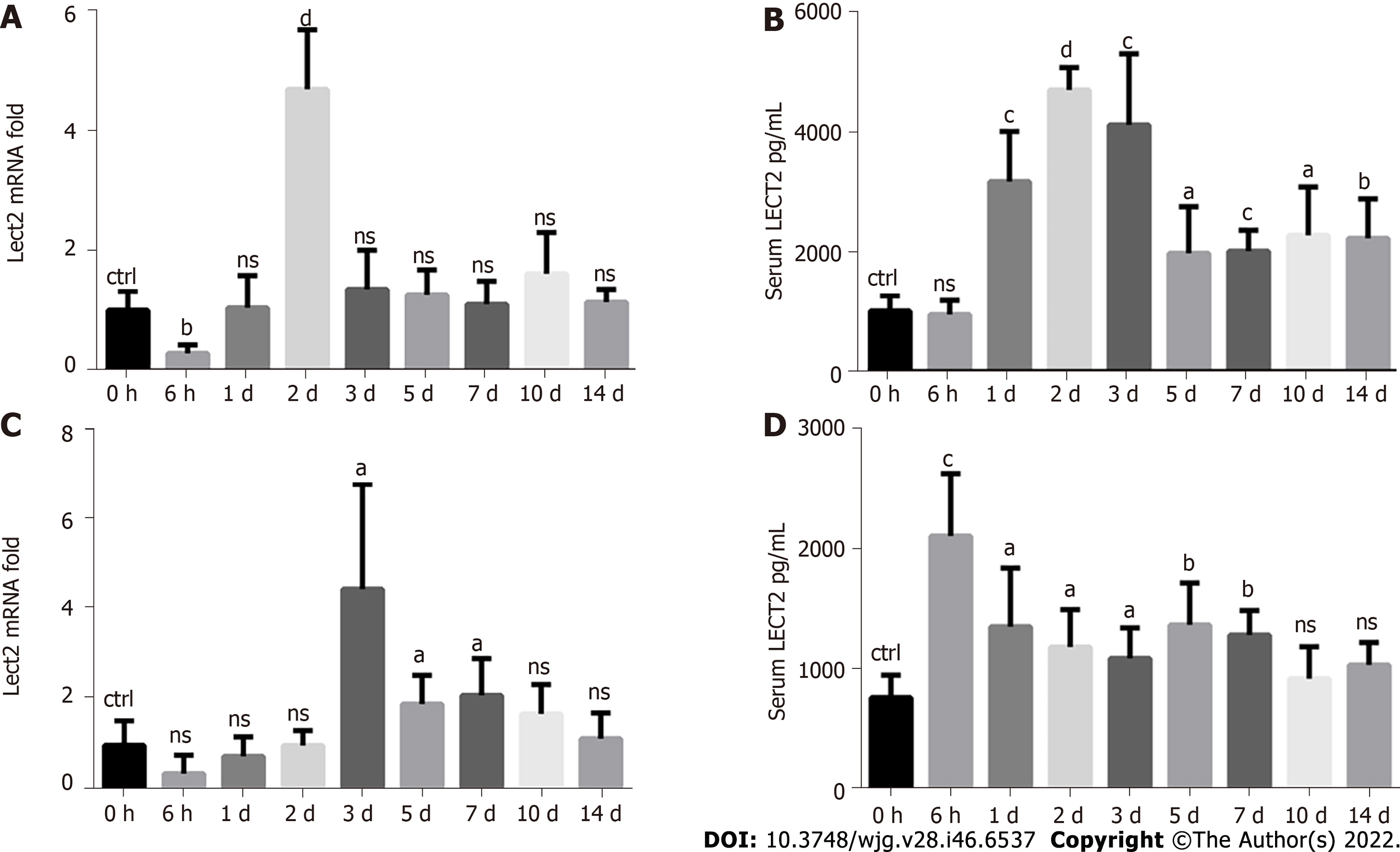
We detected the dynamic changes in LECT2 expression in liver tissues and serum from 0 h to 14 d after CCl4 and ConA treatment. We found that the expression of Lect2 mRNA was significantly increased on the 2nd day after CCl4 treatment (Figure 3A), while increased levels of serum LECT2 could be observed on the 1st day and lasted for 2 wk, with a peak on the 2nd day (Figure 3B). In the ConA-induced acute injury model, the expression of Lect2 mRNA was significantly increased on the 3rd, 5th and 7th days (Figure 3C), while increased levels of serum LECT2 could be observed at as early as 6 h and lasted for 1 wk, with a peak at 6 h (Figure 3D).
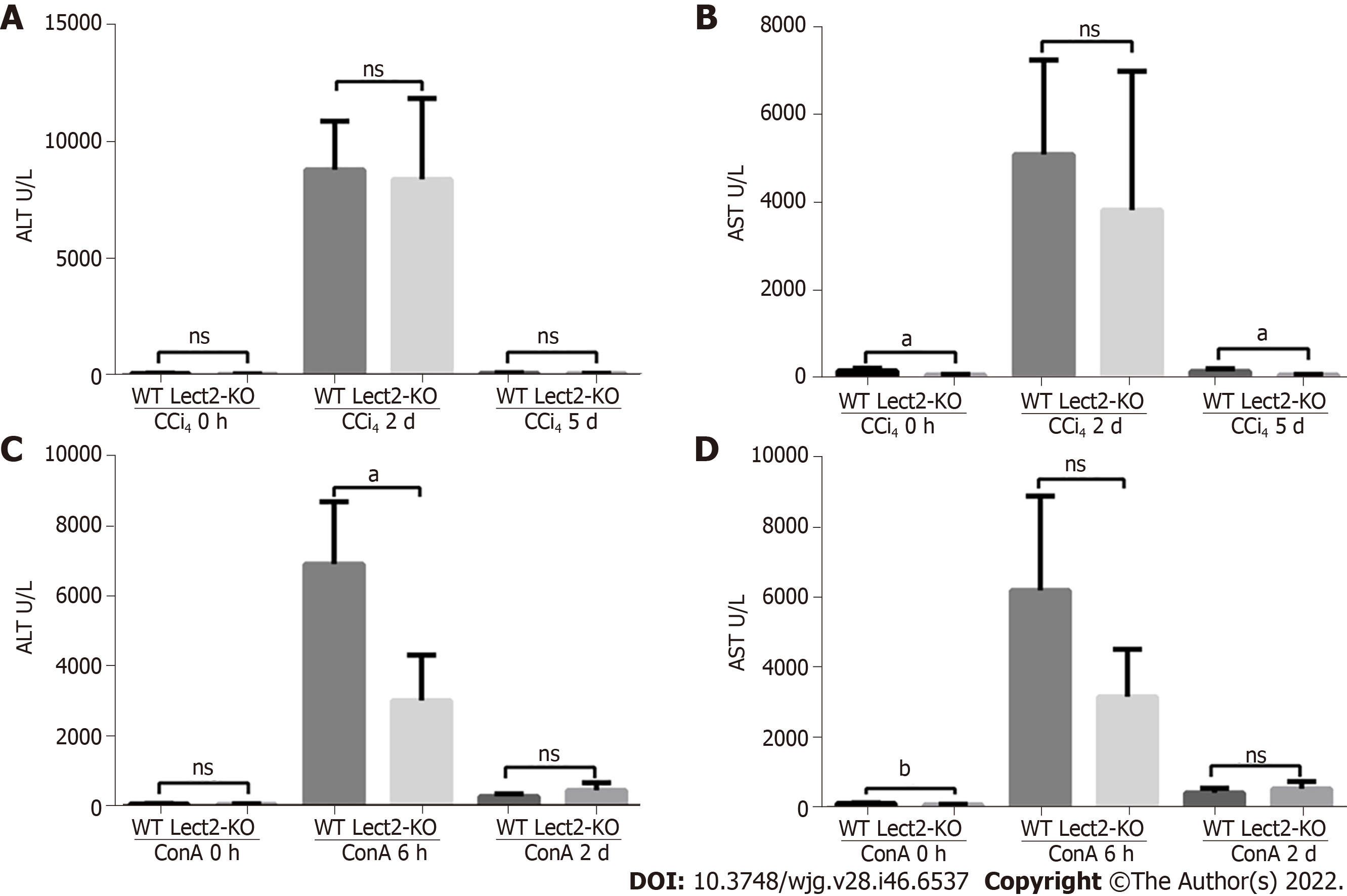
The levels of AST were significantly decreased in CCl4-treated Lect2-KO mice on the 5th day compared with their WT (wild-type) counterparts (Figure 4A and B). The levels of ALT were significantly decreased in ConA-treated Lect2-KO mice at 6 h compared with their WT counterparts (Figure 4C and D).
We selected the peak time for different types of immune cells in the CCl4 or ConA-induced acute injury model to observe the effect of LECT2 on the liver infiltration of various immune cells. WT and Lect2-KO mice were treated with CCl4 and ConA, respectively, and livers were harvested on the 2nd and 5th days after CCl4 treatment. For the mice treated with ConA, livers were harvested at the 6th hour and 2nd day. First, we compared the number of immune cells between Lect2-KO mice and WT controls at 0 h without CCl4 and ConA treatment. We found that more neutrophils, NK/NKT cells, CD4+ and CD8+ T cells emerged in Lect2-KO mice (Figure 5 and Figure 6). Next, we compared the number of immune cells between Lect2-KO mice and WT controls when treated with CCl4 or ConA. In the CCl4-induced acute injury model, we found that the livers of Lect2-KO mice had less infiltration of NK/NKT cells and macrophages than those of WT mice. There was no difference in the number of neutrophils, CD4+ T cells or CD8+ T cells (Figure 5A-F). In the ConA-induced acute injury model, the livers of Lect2-KO mice had more infiltrated neutrophils, CD4+ T cells and CD8+ T cells but fewer macrophages than those of WT mice. There was no difference in the number of NK/NKT cells (Figure 6A-F).
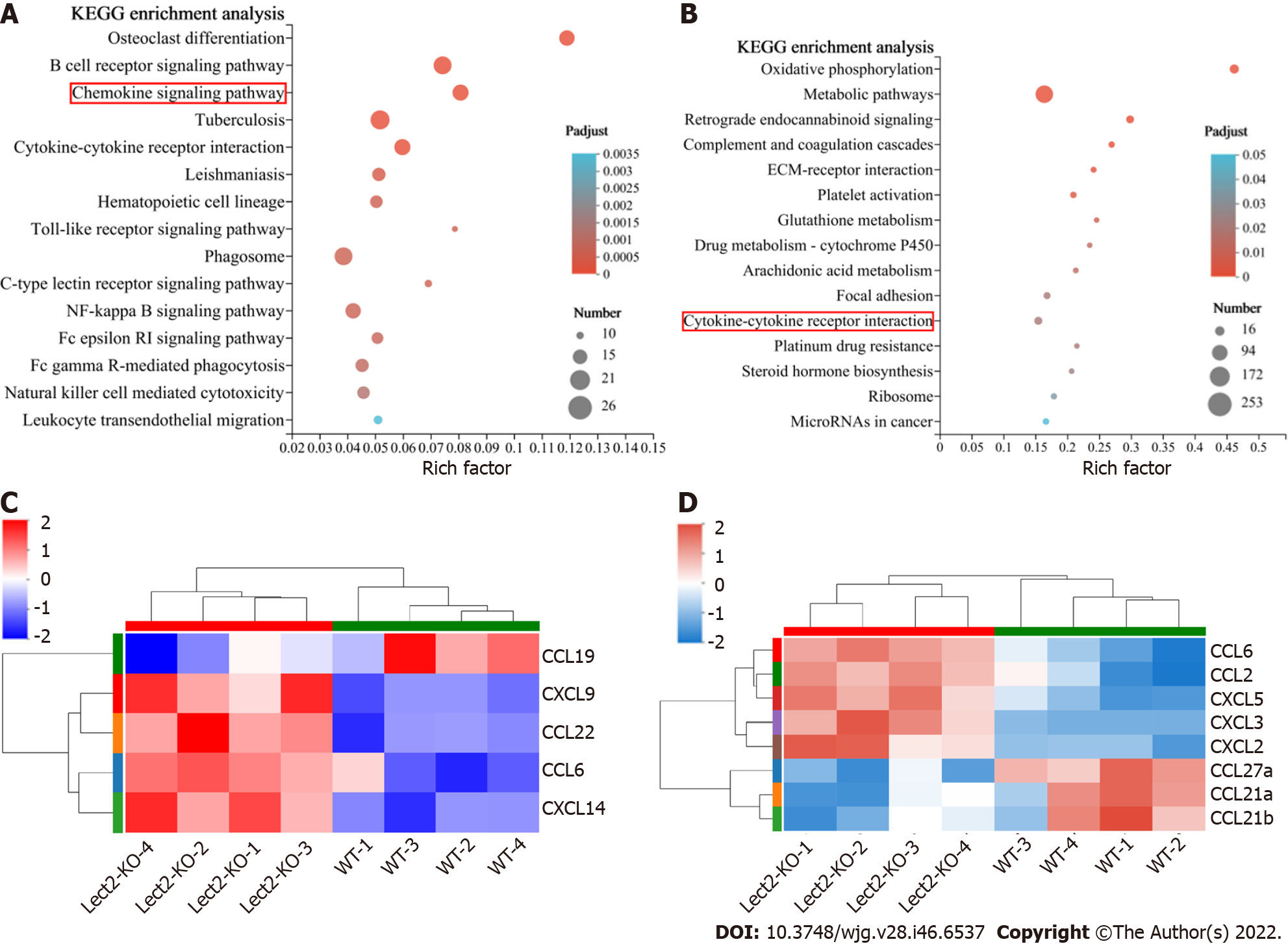
Chemokines are chemotactic cytokines that are capable of recruiting immune cells to sites of injury and inflammation. Next, we explored whether knockout of the Lect2 gene regulated the expression of chemokines in acute liver injury. RNA sequencing analysis was performed on injured liver samples derived from CCl4 or ConA-treated Lect2-KO and WT mice. Differentially expressed genes were identified with a p-adjust value < 0.05 and fold change > 2. By Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis, we found that these changed genes could be partly categorized into chemokine signaling pathways (Figure 7A) and cytokine-cytokine receptor interactions (Figure 7B). Knockout of the Lect2 gene upregulated the expression of CXCL9, CXCL14, CCL6 and CCL22, and downregulated the expression of CCL19 in the CCl4-treated mice (Figure 7C). In the ConA-treated mice, knockout of the Lect2 gene led to upregulated expression of CXCL2, CXCL3, CXCL5, CCL2 and CCL6 and downregulated expression of CCL21a, CCL21b and CCL27a (Figure 7D).
Since Lect2-KO mice displayed less macrophage infiltration than their WT counterparts in both CCl4- and ConA-induced acute injury models, we explored whether LECT2 promoted monocytes/ macrophage chemotaxis. The transwell assay showed that LECT2 could promote THP-1 monocytes chemotaxis (Figure 8A), but its chemotaxis was weaker than that of MCP-1, which is a well-recognized monocyte chemokine (Figure 8B).
The liver infiltration of various immune cell populations, including neutrophils, NK/NKT cells, T cells and monocytes/macrophages, is a critical pathological feature following acute liver injury and plays important roles in liver injury or repair[1-9]. Although there are many studies on the function of immune cells during liver injury or repair, there is still no study on the quantitative changes in immune cell infiltration at different time points from the beginning of injury to the end of repair. For the first time, employing CCl4- and ConA-induced acute liver injury models, we monitored the infiltration of immune cells over time and found that different types of immune cells began to increase, reached their peaks and fell into decline at different time points, indicating that they played different roles in different injuries or repair stages. Therefore, appropriate regulation of immune cells at different time points may be a better strategy for the treatment of acute liver injury.
We were surprised to find that during the repair process, when the serum ALT and AST indices had reverted to normal levels 7 d after the injury, the infiltration of immune cells still existed even 14 d after the injury, showing an obvious lag effect. This suggests that the liver does not completely recover to normal as quickly as we previously thought. The recovery of serum indicators (ALT, AST, etc.) does not mean that the liver has recovered to normal, and the corresponding treatment may need to be extended accordingly. Therefore, in addition to the serological indices, we suggest immunological indices to measure the repair of liver injury, which may have important value for the clinical treatment and prognosis of liver diseases. These remaining immune cells may continue to regulate liver repair and play a role in the response of the liver to rechallenges.
We previously reported that LECT2 regulated chronic liver fibrogenesis[15,16], while its role in acute liver injury is unclear. Here, we found that the expression of LECT2 was upregulated in acute liver injury models, and the liver injuries of Lect2-KO mice were less severe than those of wild-type mice. Compared with wild-type mice, Lect2-KO mice had different immune cell infiltration, suggesting that LECT2 may regulate acute liver injury and repair by regulating the infiltration of various immune cells. We demonstrated that LECT2 could indeed regulate monocyte/macrophage chemotaxis in vitro. Blocking LECT2 might be able to treat acute liver injury.
The limitation of this study is that we need further functional tests to verify the roles that different immune cells play in liver injury. In particular, when the liver is rechallenged, the roles of retained immune cells in the last injury need to be further studied. When the liver is rechallenged, these retained immune cells may cause greater damage to the liver, or may make the liver more tolerant to the same challenge.
In summary, we described the infiltration changes of various immune cells in acute liver injury models over time. We found that the recovery time of immune cells during the repair process was far behind that of serum ALT and AST. LECT2 could regulate monocyte/macrophage chemotaxis and might be used as a therapeutic target for acute liver injury.
Immune cells, including neutrophils, natural killer (NK) cells, T cells, NKT cells and macrophages, participate in the progression of acute liver injury and hepatic recovery. leukocyte cell-derived chemotaxin 2 (LECT2) is a pleiotropic protein that not only functions as a cytokine to exhibit chemotactic properties but also plays multifunctional roles in some physiological conditions and pathological abnormalities.
To date, there has been no systematic study on the quantitative changes in these different immune cells from initial injury to subsequent recovery. LECT2 regulates chronic liver fibrogenesis, but its role in acute liver injury is unclear.
To investigate the infiltration changes of various immune cells in acute liver injury mouse models over time and to study the relationship between the changes in LECT2 and the infiltration of several immune cells.
Carbon tetrachloride (CCl4)- and concanavalin A (ConA)-induced acute liver injury mouse models were employed to mimic toxin-induced and autoimmune-mediated liver injury, respectively. Immunohistochemical staining was used to indicate immune cells, and the quantitative changes in various immune cells were monitored at different time points. The levels of serum alanine transaminase (ALT), aspartate transaminase (AST) and total bilirubin (TBil) were measured by an automated chemical analyzer. The expression of LECT2 in liver tissues was detected by quantitative real-time polymerase chain reaction and the levels of LECT2 in serum were tested by enzyme-linked immunosorbent assay. Transwell assays were used to assess the chemotactic effect of LECT2 on THP-1 monocytes.
During the injury and repair process, different types of immune cells began to increase, reached their peaks and fell into decline at different time points. Furthermore, when the serum ALT and AST indices reverted to normal levels 7 d after injury, the infiltration of immune cells still existed even 14 d after injury, showing an obvious lag effect. The expression of LECT2 was up-regulated in acute liver injury models, and the liver injuries of Lect2-KO mice were less severe than those of wild-type mice. Compared with wild-type mice, Lect2-KO mice had different immune cell infiltration.
The recovery time of immune cells was far behind that of serum ALT and AST during the process of liver repair. LECT2 could regulate monocyte/macrophage chemotaxis and might be used as a thera
Our study indicated the potential roles of these immune cells and proposed a therapeutic concept that acute liver injury might be treated by regulating different immune cells at different time points.
We thank Central Laboratory, Southern Medical University for providing facilities and technical support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chieppa M, Italy; Siristatidis C, Greece S-Editor: Gong ZM L-Editor: A P-Editor: Yu HG
| 1. | Khoury T, Rmeileh AA, Yosha L, Benson AA, Daher S, Mizrahi M. Drug Induced Liver Injury: Review with a Focus on Genetic Factors, Tissue Diagnosis, and Treatment Options. J Clin Transl Hepatol. 2015;3:99-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Bernal W, Auzinger G, Dhawan A, Wendon J. Acute liver failure. Lancet. 2010;376:190-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 743] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 3. | Karlmark KR, Wasmuth HE, Trautwein C, Tacke F. Chemokine-directed immune cell infiltration in acute and chronic liver disease. Expert Rev Gastroenterol Hepatol. 2008;2:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Ayata CK, Ganal SC, Hockenjos B, Willim K, Vieira RP, Grimm M, Robaye B, Boeynaems JM, Di Virgilio F, Pellegatti P, Diefenbach A, Idzko M, Hasselblatt P. Purinergic P2Y₂ receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury. Gastroenterology. 2012;143:1620-1629.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Bi Y, Li J, Yang Y, Wang Q, Zhang X, Dong G, Wang Y, Duan Z, Shu Z, Liu T, Chen Y, Zhang K, Hong F. Human liver stem cells attenuate concanavalin A-induced acute liver injury by modulating myeloid-derived suppressor cells and CD4+ T cells in mice. Stem Cell Res Ther. 2019;10:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Geng J, Wang X, Wei H, Sun R, Tian Z. Efficient attenuation of NK cell-mediated liver injury through genetically manipulating multiple immunogenes by using a liver-directed vector. J Immunol. 2013;190:4821-4829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Yang W, Zhao X, Tao Y, Wu Y, He F, Tang L. Proteomic analysis reveals a protective role of specific macrophage subsets in liver repair. Sci Rep. 2019;9:2953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Miura A, Hosono T, Seki T. Macrophage potentiates the recovery of liver zonation and metabolic function after acute liver injury. Sci Rep. 2021;11:9730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 9. | Nguyen NT, Umbaugh DS, Sanchez-Guerrero G, Ramachandran A, Jaeschke H. Kupffer cells regulate liver recovery through induction of chemokine receptor CXCR2 on hepatocytes after acetaminophen overdose in mice. Arch Toxicol. 2022;96:305-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 10. | Yamagoe S, Yamakawa Y, Matsuo Y, Minowada J, Mizuno S, Suzuki K. Purification and primary amino acid sequence of a novel neutrophil chemotactic factor LECT2. Immunol Lett. 1996;52:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Xie Y, Fan KW, Guan SX, Hu Y, Gao Y, Zhou WJ. LECT2: A pleiotropic and promising hepatokine, from bench to bedside. J Cell Mol Med. 2022;26:3598-3607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Koshimizu Y, Ohtomi M. Regulation of neurite extension by expression of LECT2 and neurotrophins based on findings in LECT2-knockout mice. Brain Res. 2010;1311:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Ohtomi M, Nagai H, Ohtake H, Uchida T, Suzuki K. Dynamic change in expression of LECT2 during liver regeneration after partial hepatectomy in mice. Biomed Res. 2007;28:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Lu XJ, Chen Q, Rong YJ, Yang GJ, Li CH, Xu NY, Yu CH, Wang HY, Zhang S, Shi YH, Chen J. LECT2 drives haematopoietic stem cell expansion and mobilization via regulating the macrophages and osteolineage cells. Nat Commun. 2016;7:12719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Xu M, Xu HH, Lin Y, Sun X, Wang LJ, Fang ZP, Su XH, Liang XJ, Hu Y, Liu ZM, Cheng Y, Wei Y, Li J, Li L, Liu HJ, Cheng Z, Tang N, Peng C, Li T, Liu T, Qiao L, Wu D, Ding YQ, Zhou WJ. LECT2, a Ligand for Tie1, Plays a Crucial Role in Liver Fibrogenesis. Cell. 2019;178:1478-1492.e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 16. | Lin Y, Dong MQ, Liu ZM, Xu M, Huang ZH, Liu HJ, Gao Y, Zhou WJ. A strategy of vascular-targeted therapy for liver fibrosis. Hepatology. 2022;76:660-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 17. | Lan F, Misu H, Chikamoto K, Takayama H, Kikuchi A, Mohri K, Takata N, Hayashi H, Matsuzawa-Nagata N, Takeshita Y, Noda H, Matsumoto Y, Ota T, Nagano T, Nakagen M, Miyamoto K, Takatsuki K, Seo T, Iwayama K, Tokuyama K, Matsugo S, Tang H, Saito Y, Yamagoe S, Kaneko S, Takamura T. LECT2 functions as a hepatokine that links obesity to skeletal muscle insulin resistance. Diabetes. 2014;63:1649-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 18. | Kowalski A, Cabrera J, Nasr S, Lerma E. Renal LECT2 amyloidosis: a newly described disorder gaining greater recognition. Clin Nephrol. 2015;84:236-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |