Published online Dec 14, 2022. doi: 10.3748/wjg.v28.i46.6512
Peer-review started: September 14, 2022
First decision: October 19, 2022
Revised: October 27, 2022
Accepted: November 21, 2022
Article in press: November 21, 2022
Published online: December 14, 2022
Processing time: 84 Days and 19.4 Hours
Shear wave elastography (SWE) is now becoming an indispensable diagnostic tool in the routine examination of liver diseases. In particular, accuracy is required for shear wave propagation velocity measurement, which is directly related to diagnostic accuracy. It is generally accepted that the liver shear wave propagation velocity reflects the degree of fibrosis, but there are still few reports on other factors that increase the shear wave propagation velocity. In this study, we reviewed such factors in the literature and examined their mechanisms. Current SWE measures propagation velocity based on the assumption that the medium has a homogeneous structure, uniform density, and is purely elastic. Otherwise, the measurement is subject to error. The other (confounding) factors that we routinely experience are primarily: (1) Conditions that appear to increase the viscous component; and (2) Conditions that appear to increase tissue density. Clinically, the former includes acute hepatitis, congested liver, biliary obstruction, etc, and the latter includes diffuse infiltration of malignant cells, various storage diseases, tissue necrosis, etc. In any case, it is important to evaluate SWE in the context of the entire clinical picture.
Core Tip: Shear wave elastography (SWE) has become an indispensable diagnostic tool for diagnosing liver disease patients. The shear wave propagation velocity usually reflects the degree of fibrosis, but we must keep in mind other (confounding) factors. The confounding factors due to viscosity include acute hepatitis, congestive liver, and biliary stasis. The other confounding factors due to an increase of tissue density include diffuse infiltration of malignant cells, various storage diseases, and tissue necrosis. It is important to judge SWE results in the context of the entire clinical picture.
- Citation: Naganuma H, Ishida H. Factors other than fibrosis that increase measured shear wave velocity. World J Gastroenterol 2022; 28(46): 6512-6521
- URL: https://www.wjgnet.com/1007-9327/full/v28/i46/6512.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i46.6512
Percussion examination has been historically performed as part of physical examinations and is clinically important[1]. It is a method of investigating the mechanical characteristics of internal organs through the changes in reflected sound during percussion to recognize how internal organs respond to vibrations. This phenomenon indicates that the sound of reflection is related to tissue stiffness, and it provides useful diagnostic information of tissues. Elastography (stiffness measurement technique) scientifically confirmed this subjective experience-based phenomenon. In recent years, various ultrasound (US) and magnetic resonance elastography techniques for evaluating tissue elasticity have been developed and applied in the clinical setting[2,3]. The basic principle of these techniques is to use external vibration (or focused US) to create shear waves (SWs) in the tissue and to measure the SW propagation velocity as a function of time and distance[4,5]. Many algorithms then calculate the SW propagation velocity, based on the assumption that the tissue is unstressed, homogeneous, isotropic, and purely elastic. Under these conditions, the SW velocity is directly related to the elastic modulus of the tissue[6,7].
Presently, magnetic resonance elastography[2,3] and US elastography[8,9] are primarily used. The former is used for detailed examination due to its excellent stability and reproducibility[2,3]. However, it is bulky and costly. US elastography has attained preferential use in the clinical setting because of its lower cost and easier manipulability of the instrument[10,11].
Currently, there are three kinds of US-based SW elastography (SWE), namely transient elastography[12], point SWE[13], and two-dimensional SWE (2DSWE)[14,15]. Among these methods, 2DSWE has emerged as the most frequently used diagnostic technique due to its ability to sample a large area in the liver, change the sampling area quickly under B-mode observation, and display color mapping of SW values over the B-mode image, which gives the operator a sense of security. Recently, various 2DSWE devices have been developed by a number of US companies[16,17], and there have been many studies on the relationship between 2DSWE and liver histology that have shown a high degree of agreement, with the area under the receiver operating characteristic curve of 2DSWE performance of more than 0.9 in the prediction of fibrosis staging[18,19]. This indicates that 2DSWE can be a useful and accurate tool for evaluating liver stiffness. The precise definition of “increased SW propagation velocity” is not strictly determined, but the reported optimal cutoff values to differentiate liver cirrhosis are approximately 9 kPa (1.7 m/s) in most cases[20]. Thus, measurements above this threshold are considered increased values.
There are two ways to quantify relative tissue stiffness as SW propagation velocity expressed as m/s and as kPa. The SW propagation velocity is converted automatically to kPa, using the equation 1 kPa = 3 × P × (SW propagation velocity)2, with the assumption that the examined tissue is always homogeneous, and P (tissue density) is defined as 1.00 kg/m3.
Existing studies regarding 2DSWE of the liver included various manufacturers and models[21]. A detailed explanation of the complex working principles of recent SWE devices in each company is beyond the scope of this review. Despite variations in employed algorithm, the fundamental operating principles of these devices are similar[21].
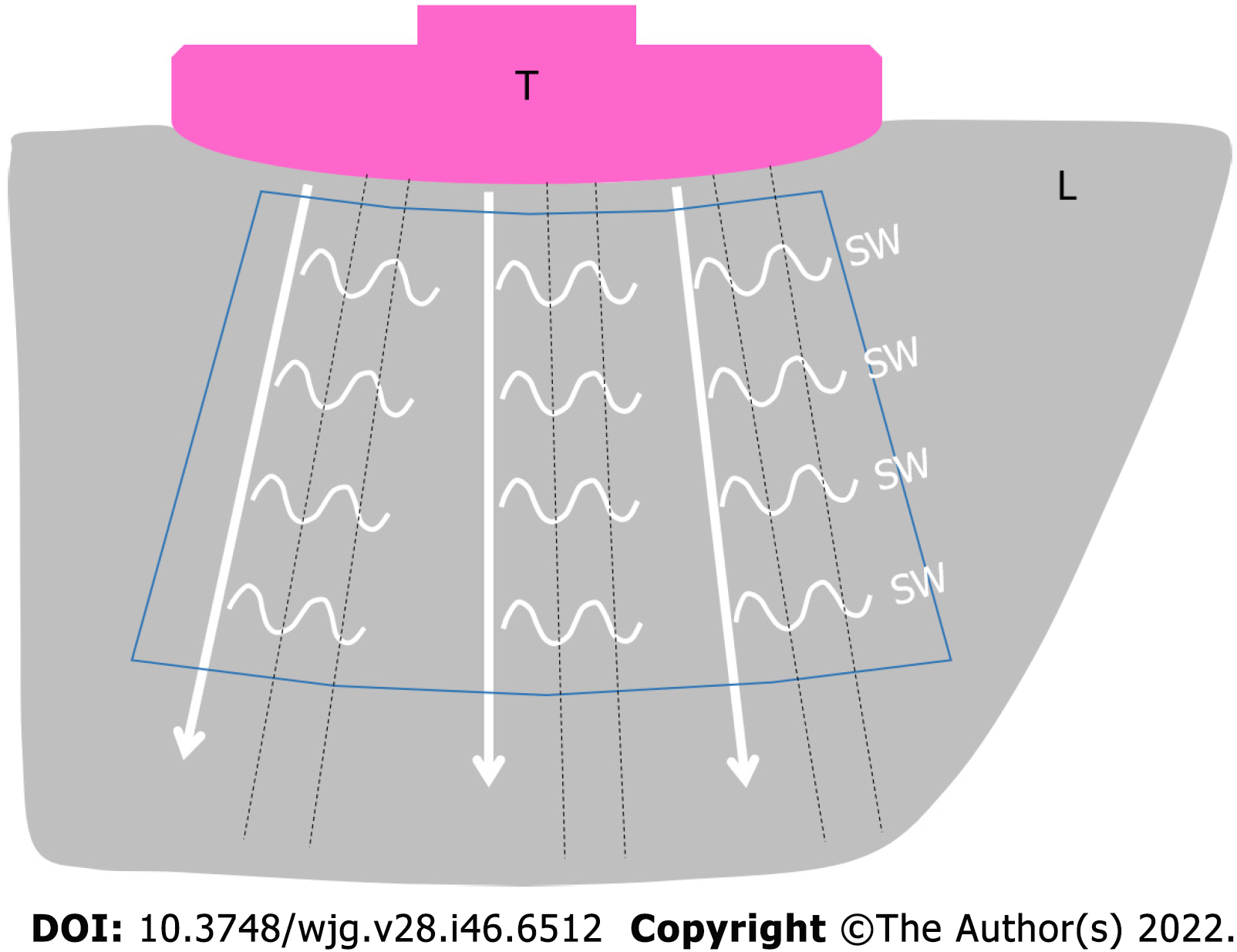
The push-pulse produces small tissue movements in the push-pulse plane. These tissue movements produce SWs that propagate and produce minimal tissue movements in the horizontal plane of the push-pulse. The tissue movements in the horizontal plane are called “SWs” and further propagate through the tissue in a sideway direction, away from the push-pulse. The SW movements are tracked by the regular-interval tracking conventional US pulses, which are used to measure the arrival time of SWs (Figure 1). The simple formula to determine the SW velocity is arrival time of SW/distance from the push-pulse. This measurement is possible because the SW propagation velocity is very low (1-10 m/s) compared to the velocity of US pulses (1540 m/s)[22].
For SW propagation velocity measurements, assumptions are made that the tissue is homogeneous, perfectly elastic (no viscous component), and the SW propagates in a straight direction[23,24]. The normal liver is the closest to this condition of all organs, and it is not surprising that a large number of papers have been focused on liver disease[9]. Generally speaking, the factors that increase the SW propagation velocity include an increase in cell density and heterogeneous tissue structure[23,24].
Reports on fiber components and SW propagation in muscles have observed that the SW propagation velocity increases when SWs propagate along the fiber running in the muscle[25,26]. This is an important factor in the increase in SW propagation velocity due to SW propagation of fibrosis in the liver tissue.
In SWE, tissue stiffness is estimated by measuring the SW propagation velocity[23,27]. Fibrotic tissues are typically stiffer than normal tissues. Therefore, the SW propagation velocity in fibrotic tissue is naturally faster than in normal tissue. Presently, there are many successful demonstrations of SWE in assessing the severity of liver fibrosis showing a significantly high correlation between the progress of fibrosis and increase in SW propagation velocity in patients with chronic viral hepatitis[9]. Although there are a relatively small number of publications, increased hepatic SW propagation velocity has been reported due to factors other than fibrosis, including acute, hepatitis[28], cholestasis[29,30], hepatic congestion[31], storage disease[32], hepatic necrosis[33], and diffuse infiltration of malignant tumors[34].
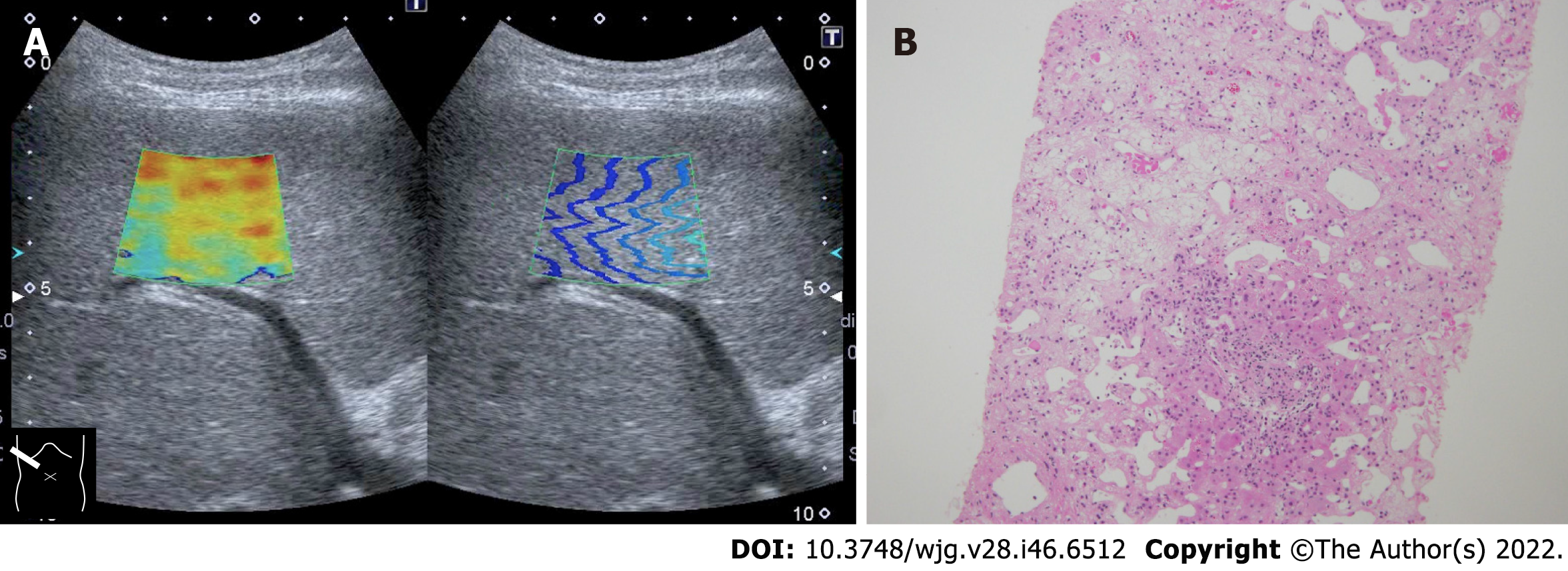
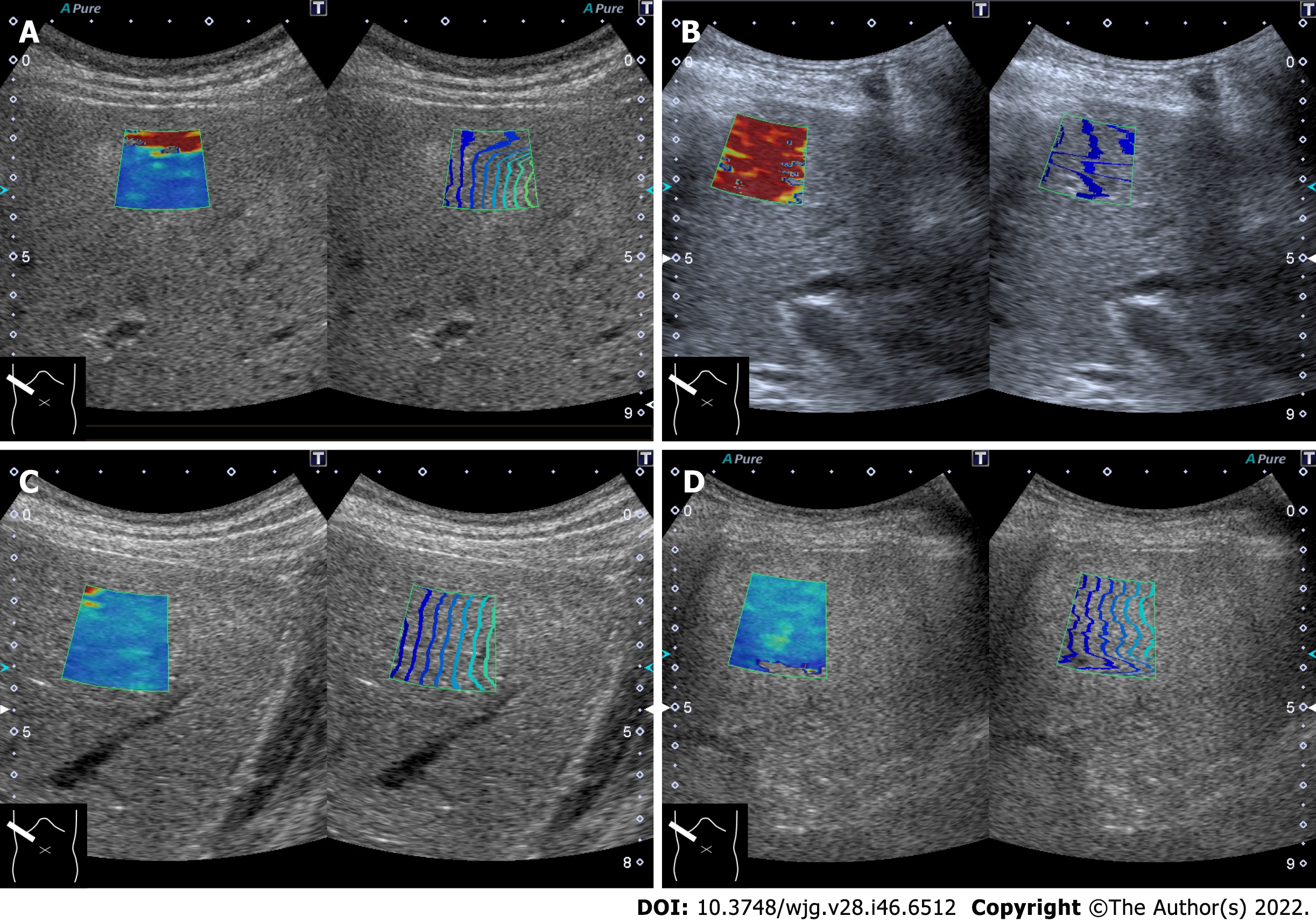
As mentioned in the previous section, the current SW propagation velocity measurement is performed on the assumption that the tissue to be measured is a perfect elastic body. However, the liver is indeed “viscoelastic,” and current SWE measures elastic components and viscous components without distinction. This is thought to be the main cause of increased SW propagation velocity seen in acute hepatitis, hepatic congestion, and biliary congestion[28,30,31]. On the other hand, increased SW propagation velocity in storage disease, necrosis (Figure 2), or diffuse infiltration of malignant tumors are related to increased tissue density[34].
The main target of current hepatic SWE is various chronic hepatic diseases, particularly viral hepatitis[9]. Presence of parenchymal fibrosis is considered to be the main cause of increased SW propagation velocity. However, it indicates that similar phenomena can be observed in other pathologies with increased fibrosis within the liver parenchyma.
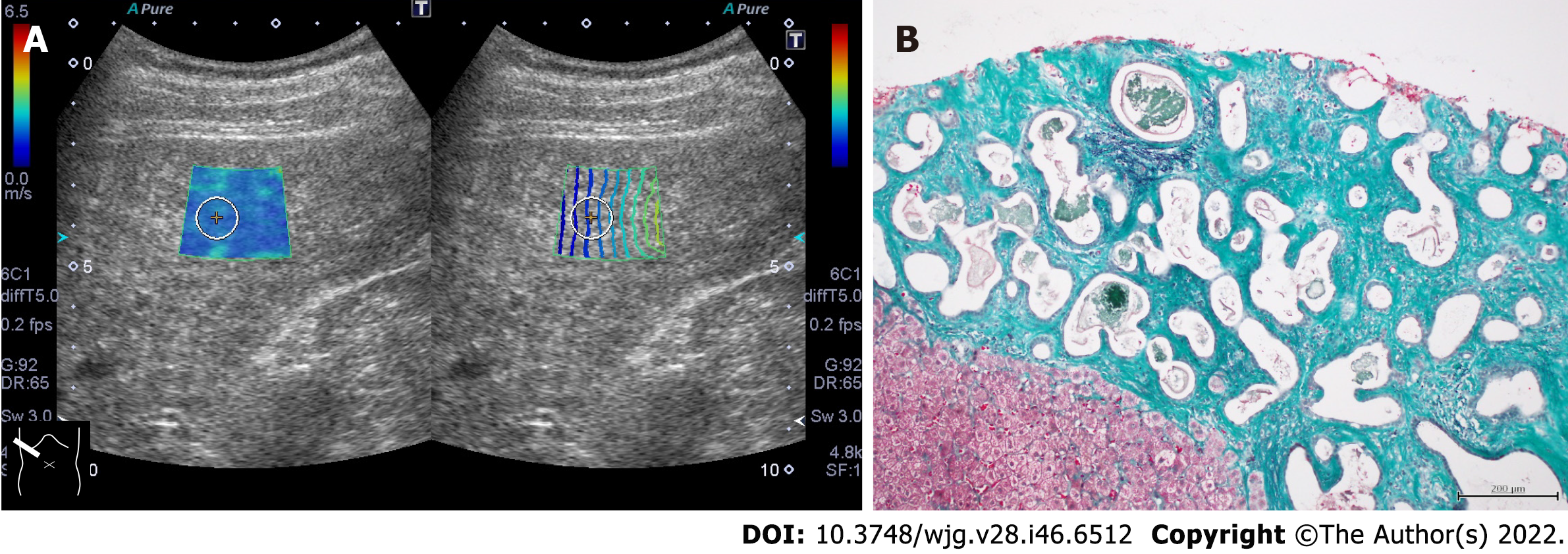
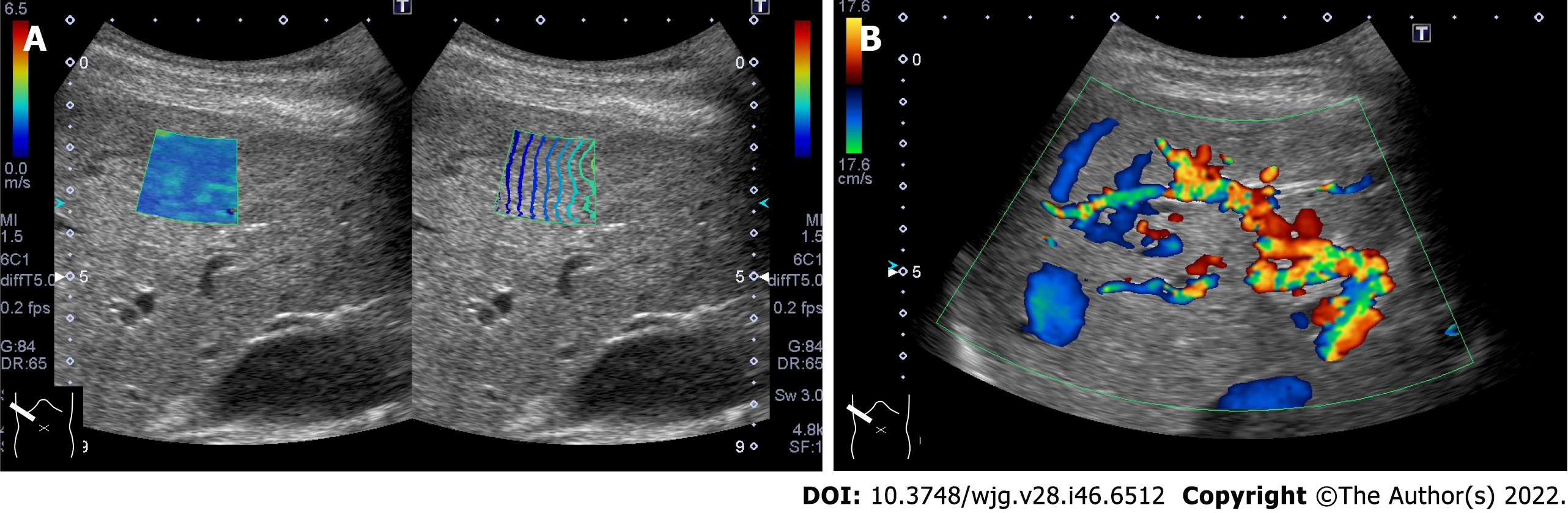
In patients with chronic cholangitis, it has been reported that the SW propagation velocity in the peripheral liver tissue, in which the tubular structure is invisible on B-mode imaging, is increased[35,36]. It is presumed that fibrotic peripheral bile ducts are distributed throughout the liver and form an environment similar to fibrosis of chronic hepatitis for SW propagation. Increased SW propagation velocity can be observed in patients with von Meyenburg complex (Figure 3). Von Meyenburg complex is a benign developmental ductal plate malformation affecting the small peripheral bile ducts[37]. In addition, cases of thickened-wall peripheral small vessels can increase SW propagation velocity in patients with hereditary hemorrhagic telangiectasia (also known as Rendu-Osler-Weber disease)[38] (Figure 4). Hepatic involvement in hereditary hemorrhagic telangiectasia is characterized by diffuse development of small vascular shunts in the hepatic periphery, which likely increases SW propagation velocity[38].
Measurement of SW propagation velocity can be easily performed using 2DSWE, but it is well known that the results change according to the level of expertise of the ultrasound operator[39]. The literature includes reports of artifactual factors that give rise to pseudo-increased SW propagation velocity, including a reverberation artifact (Figure 5A), motion artifact due to cardiac motion (seen in the left lobe of the liver) (Figure 5B), and excessive probe compression during subcostal scanning (Figure 5C). However, the possibility of misinterpretation of these artifacts by an experienced SWE practitioner is extremely rare because the patterns produced by the artifacts are characteristic and recognizable.
For example, there are some studies in the literature comparing SWE results of the left lobe and the right lobe of the liver[40]. However, current guidelines, including the World Federation for Ultrasound in Medicine and Biology and the European Federation for Ultrasound in Medicine and Biology, recommend SW measurement through the right intercostal space only because SW measurements obtained in the left lobe are usually affected by cardiac movement[8,41].
Recent clinical studies have used SWE to evaluate fatty liver (FL) stiffness. Unfortunately, there is a lack of understanding of the biomechanics of SWE to assess patients with FL due to the complexity of the structure of FL. Unlike the normal liver whose structure may be modeled as isotropic, FL has a heterogeneous structure. The complex structure and mechanical properties present a challenge in obtaining accurate results of SW propagation velocity[42]. It is not so rare to encounter artifactually (pseudo) increased SW propagation velocity when observing FL (Figure 5D). The various factors that affect propagation of SW waves in FL need to be properly understood before SWE can be used for accurately assess the severity of FL.
Although the precise mechanisms are undetermined, studies have shown that a reduction in blood flow typically results in a decrease in SW propagation velocity in the liver[43]. In contrast, SW propagation velocity tends to increase with increasing organ perfusion. Other reported factors that change SW propagation velocity include water intake[44], breathing phases[45], eccentric exercise, patient positioning[46], and high calorie meal intake[47]. These factors can potentially contribute to minimal fluctuations in SW measurements, and care should be taken to account for these fluctuations to reduce the degree of uncertainty.
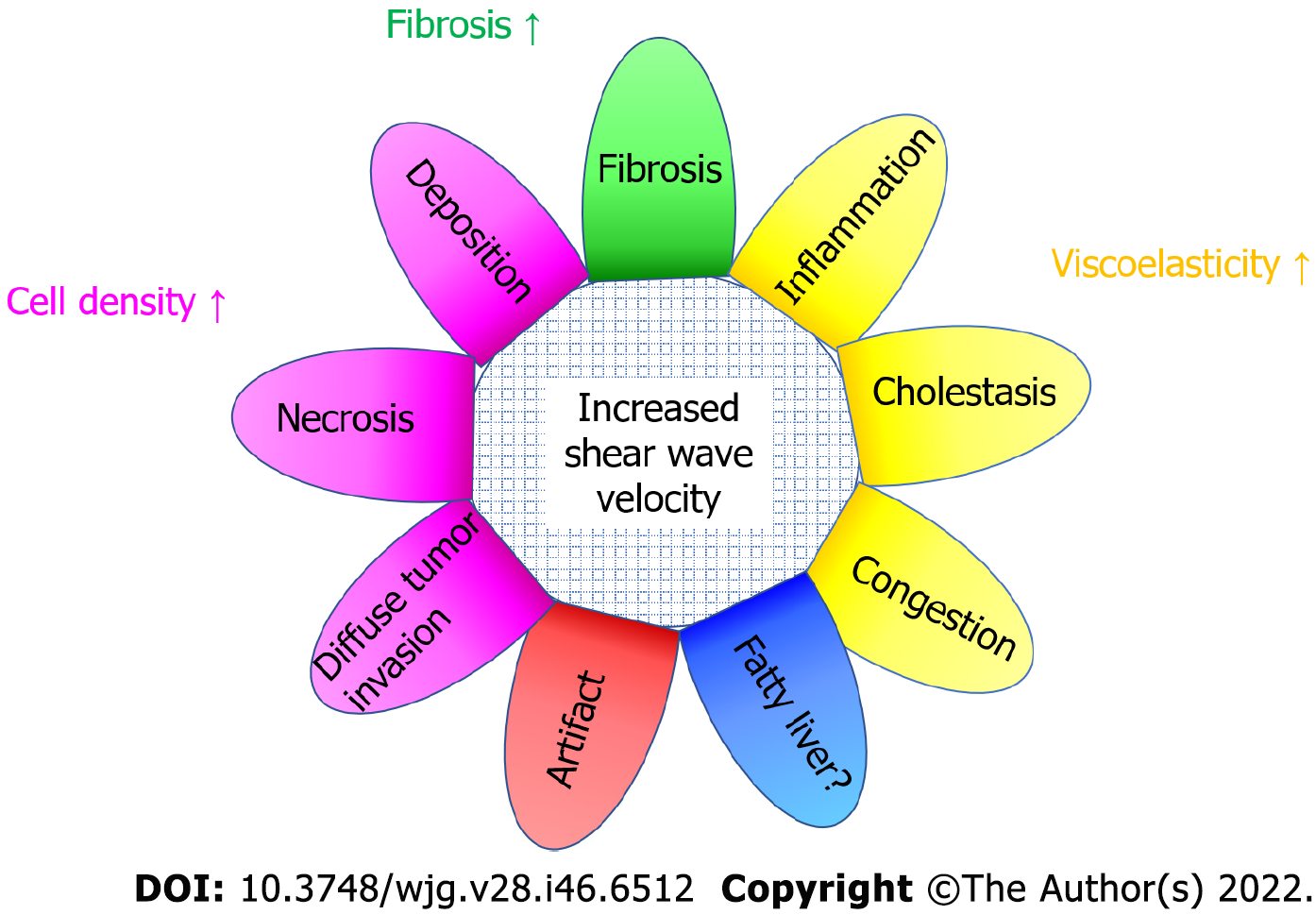
Presently, SWE is routinely used for providing a quantitative evaluation of liver elasticity[7]. The SW propagation velocity measured is reflective of the biomechanical properties of liver tissue where a higher value usually reflects stiffer (i.e., fibrotic) parenchyma[8,9]. SWE algorithms assume that the liver is purely elastic and homogeneously structured. In most cases these assumptions are true. However, other factors may increase SW propagation velocity. They include acute hepatitis, cholestasis, hepatic congestion, storage disease, hepatic necrosis, and diffuse infiltration of malignant cells and others (Figure 6). Among them, the problem of viscosity (related to acute hepatitis, cholestasis, and hepatic congestion) is the most important because most commercial SWE systems assume soft tissues to be purely elastic and neglect the effects of viscosity when evaluating liver tissue stiffness.
However, the liver tissue is a viscoelastic environment that results in a counterbalance of liver tissue stiffening[48,49]. When the liver tissue is modeled as a viscoelastic material, the SWs experience frequency dispersion. The SW propagation velocity and SW attenuation increases with increasing frequency rather than remaining constant, and the rate of change (slope) is positively correlated with viscosity[50-52]. As a result, modeling a viscoelastic material as linear elastic tends to overestimate the SW propagation velocity. When the effects of viscosity are considered, the scope of the diagnostic technique can be further broadened, and SW dispersion provides an estimation of liver viscosity, which may provide additional information of the underlying liver parenchyma.
Recent studies have reported that viscosity alone can serve as a parameter to diagnose several medical conditions such as inflammation and congestion[48,49]. Although only one company has developed this type of SW dispersion device, it is expected to be an area of great potential for further development. An increase in tissue density is another confounding factor that often poses a challenge in obtaining reliable and accurate SWE readings. However, many problems remain that must be clarified in future studies.
Currently, automated diagnostics incorporating artificial intelligence are spreading rapidly worldwide[53-55]. SWE is a likely area in which artificial intelligence could be easily utilized. The adoption of artificial intelligence for SWE measurements is expected to correct slight variances in reference values (normal range) among manufacturers and enable prediction of liver fibrosis with higher probability. Artificial intelligence may also be able to detect when the probability of contamination by a confounding factor is high.
Although many confounding factors are recognized, our review emphasized that the interpretation of 2DSWE results must incorporate knowledge of these factors. As the use of 2DSWE becomes widespread, the problems related to confounding factors need clarification and solutions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Japanese Society of Gastroenterology, No. 020968; and The Japan Society of Ultrasonics in Medicine, No. 19891027.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ariyachet C, Thailand; Zhang HP, China S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Rastogi V, Singh D, Tekiner H, Ye F, Mazza JJ, Yale SH. Abdominal Physical Signs and Medical Eponyms: Part II. Percussion and Auscultation, 1924-1980. Clin Med Res. 2020;18:102-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 2. | Serai SD, Obuchowski NA, Venkatesh SK, Sirlin CB, Miller FH, Ashton E, Cole PE, Ehman RL. Repeatability of MR Elastography of Liver: A Meta-Analysis. Radiology. 2017;285:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Idilman IS, Li J, Yin M, Venkatesh SK. MR elastography of liver: current status and future perspectives. Abdom Radiol (NY). 2020;45:3444-3462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 4. | Ozturk A, Grajo JR, Dhyani M, Anthony BW, Samir AE. Principles of ultrasound elastography. Abdom Radiol (NY). 2018;43:773-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 5. | Srinivasa Babu A, Wells ML, Teytelboym OM, Mackey JE, Miller FH, Yeh BM, Ehman RL, Venkatesh SK. Elastography in Chronic Liver Disease: Modalities, Techniques, Limitations, and Future Directions. Radiographics. 2016;36:1987-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 6. | Nenadic IZ, Qiang B, Urban MW, Zhao H, Sanchez W, Greenleaf JF, Chen S. Attenuation measuring ultrasound shearwave elastography and in vivo application in post-transplant liver patients. Phys Med Biol. 2017;62:484-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Ferraioli G, Filice C, Castera L, Choi BI, Sporea I, Wilson SR, Cosgrove D, Dietrich CF, Amy D, Bamber JC, Barr R, Chou YH, Ding H, Farrokh A, Friedrich-Rust M, Hall TJ, Nakashima K, Nightingale KR, Palmeri ML, Schafer F, Shiina T, Suzuki S, Kudo M. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol. 2015;41:1161-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 483] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 8. | Ferraioli G, Wong VW, Castera L, Berzigotti A, Sporea I, Dietrich CF, Choi BI, Wilson SR, Kudo M, Barr RG. Liver Ultrasound Elastography: An Update to the World Federation for Ultrasound in Medicine and Biology Guidelines and Recommendations. Ultrasound Med Biol. 2018;44:2419-2440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 373] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 9. | Herrmann E, de Lédinghen V, Cassinotto C, Chu WC, Leung VY, Ferraioli G, Filice C, Castera L, Vilgrain V, Ronot M, Dumortier J, Guibal A, Pol S, Trebicka J, Jansen C, Strassburg C, Zheng R, Zheng J, Francque S, Vanwolleghem T, Vonghia L, Manesis EK, Zoumpoulis P, Sporea I, Thiele M, Krag A, Cohen-Bacrie C, Criton A, Gay J, Deffieux T, Friedrich-Rust M. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology. 2018;67:260-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 343] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 10. | Yin L, Cheng L, Wang F, Zhu X, Hua Y, He W. Application of intraoperative B-mode ultrasound and shear wave elastography for glioma grading. Quant Imaging Med Surg. 2021;11:2733-2743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Ferraioli G, Barr RG. Ultrasound liver elastography beyond liver fibrosis assessment. World J Gastroenterol. 2020;26:3413-3420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Castera L. Non-invasive diagnosis of steatosis and fibrosis. Diabetes Metab. 2008;34:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Ferraioli G, Tinelli C, Lissandrin R, Zicchetti M, Dal Bello B, Filice G, Filice C. Point shear wave elastography method for assessing liver stiffness. World J Gastroenterol. 2014;20:4787-4796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 116] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Wu M, Wu L, Jin J, Wang J, Li S, Zeng J, Guo H, Zheng J, Chen S, Zheng R. Liver Stiffness Measured with Two-dimensional Shear-Wave Elastography Is Predictive of Liver-related Events in Patients with Chronic Liver Disease Due to Hepatis B Viral Infection. Radiology. 2020;295:353-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Zeng J, Zheng J, Huang Z, Chen S, Liu J, Wu T, Zheng R, Lu M. Comparison of 2-D Shear Wave Elastography and Transient Elastography for Assessing Liver Fibrosis in Chronic Hepatitis B. Ultrasound Med Biol. 2017;43:1563-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Ferraioli G, Barr RG, Farrokh A, Radzina M, Cui XW, Dong Y, Rocher L, Cantisani V, Polito E, D'Onofrio M, Roccarina D, Yamashita Y, Dighe MK, Dietrich CF. How to perform shear wave elastography. Part I. Med Ultrason. 2022;24:95-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 17. | Ferraioli G, Barr RG, Farrokh A, Radzina M, Cui XW, Dong Y, Rocher L, Cantisani V, Polito E, D'Onofrio M, Roccarina D, Yamashita Y, Dighe MK, Fodor D, Dietrich CF. How to perform shear wave elastography. Part II. Med Ultrason. 2022;24:196-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Abe T, Kuroda H, Fujiwara Y, Yoshida Y, Miyasaka A, Kamiyama N, Takikawa Y. Accuracy of 2D shear wave elastography in the diagnosis of liver fibrosis in patients with chronic hepatitis C. J Clin Ultrasound. 2018;46:319-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Furlan A, Tublin ME, Yu L, Chopra KB, Lippello A, Behari J. Comparison of 2D Shear Wave Elastography, Transient Elastography, and MR Elastography for the Diagnosis of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. AJR Am J Roentgenol. 2020;214:W20-W26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 20. | Yoo JJ, Kim SG, Kim YS. The Diagnostic Accuracy of LOGIQ S8 and E9 Shear Wave Elastography for Staging Hepatic Fibrosis, in Comparison with Transient Elastography. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Iijima H, Tada T, Kumada T, Kobayashi N, Yoshida M, Aoki T, Nishimura T, Nakano C, Ishii A, Takashima T, Sakai Y, Aizawa N, Nishikawa H, Ikeda N, Iwata Y, Enomoto H, Ide YH, Hirota S, Fujimoto J, Nishiguchi S. Comparison of liver stiffness assessment by transient elastography and shear wave elastography using six ultrasound devices. Hepatol Res. 2019;49:676-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Naganuma H, Ishida H, Uno A, Nagai H, Kuroda H, Ogawa M. Diagnostic problems in two-dimensional shear wave elastography of the liver. World J Radiol. 2020;12:76-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Shiina T, Nightingale KR, Palmeri ML, Hall TJ, Bamber JC, Barr RG, Castera L, Choi BI, Chou YH, Cosgrove D, Dietrich CF, Ding H, Amy D, Farrokh A, Ferraioli G, Filice C, Friedrich-Rust M, Nakashima K, Schafer F, Sporea I, Suzuki S, Wilson S, Kudo M. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41:1126-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 646] [Article Influence: 64.6] [Reference Citation Analysis (1)] |
| 24. | Nitta N, Yamakawa M, Hachiya H, Shiina T. A review of physical and engineering factors potentially affecting shear wave elastography. J Med Ultrason (2001). 2021;48:403-414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Andonian P, Viallon M, Le Goff C, de Bourguignon C, Tourel C, Morel J, Giardini G, Gergelé L, Millet GP, Croisille P. Shear-Wave Elastography Assessments of Quadriceps Stiffness Changes prior to, during and after Prolonged Exercise: A Longitudinal Study during an Extreme Mountain Ultra-Marathon. PLoS One. 2016;11:e0161855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 26. | Romano A, Staber D, Grimm A, Kronlage C, Marquetand J. Limitations of Muscle Ultrasound Shear Wave Elastography for Clinical Routine-Positioning and Muscle Selection. Sensors (Basel). 2021;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Bamber J, Cosgrove D, Dietrich CF, Fromageau J, Bojunga J, Calliada F, Cantisani V, Correas JM, D'Onofrio M, Drakonaki EE, Fink M, Friedrich-Rust M, Gilja OH, Havre RF, Jenssen C, Klauser AS, Ohlinger R, Saftoiu A, Schaefer F, Sporea I, Piscaglia F. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 772] [Article Influence: 64.3] [Reference Citation Analysis (1)] |
| 28. | Jin JY, Zheng YB, Zheng J, Liu J, Mao YJ, Chen SG, Gao ZL, Zheng RQ. 2D shear wave elastography combined with MELD improved prognostic accuracy in patients with acute-on-chronic hepatitis B liver failure. Eur Radiol. 2018;28:4465-4474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Sirisomboonlarp K, Udomsinprasert W, McConachie E, Woraruthai T, Poovorawan Y, Honsawek S. Increased serum glypican-3 is associated with liver stiffness and hepatic dysfunction in children with biliary atresia. Clin Exp Hepatol. 2019;5:48-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Kubo K, Kawakami H, Kuwatani M, Nishida M, Kawakubo K, Kawahata S, Taya Y, Kubota Y, Amano T, Shirato H, Sakamoto N. Liver elasticity measurement before and after biliary drainage in patients with obstructive jaundice: a prospective cohort studya prospective cohort study. BMC Gastroenterol. 2016;16:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Wang HW, Shi HN, Cheng J, Xie F, Luo YK, Tang J. Real-time shear wave elastography (SWE) assessment of short- and long-term treatment outcome in Budd-Chiari syndrome: A pilot study. PLoS One. 2018;13:e0197550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Wang J, Hu M, Zhu Q, Sun L. Liver stiffness assessed by real-time two-dimensional shear wave elastography predicts hypersplenism in patients with Wilson's disease: a prospective study. BMC Med Imaging. 2022;22:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Bo XW, Li XL, Xu HX, Guo le H, Li DD, Liu BJ, Wang D, He YP, Xu XH. 2D shear-wave ultrasound elastography (SWE) evaluation of ablation zone following radiofrequency ablation of liver lesions: is it more accurate? Br J Radiol. 2016;89:20150852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Ichikawa K, Narita Y, Ota Y, Komatsu N, Koike M. Transient elastography-derived liver stiffness measurements were found to be useful for predicting liver infiltration in a case of mature T-cell neoplasm involving liver dysfunction. Int J Clin Exp Pathol. 2015;8:4220-4226. [PubMed] |
| 35. | Fossdal G, Mjelle AB, Wiencke K, Bjørk I, Gilja OH, Folseraas T, Karlsen TH, Rosenberg W, Giil LM, Vesterhus M. Fluctuating biomarkers in primary sclerosing cholangitis: A longitudinal comparison of alkaline phosphatase, liver stiffness, and ELF. JHEP Rep. 2021;3:100328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 36. | Reiter R, Shahryari M, Tzschätzsch H, Klatt D, Siegmund B, Hamm B, Braun J, Sack I, Asbach P. Spatial heterogeneity of hepatic fibrosis in primary sclerosing cholangitis vs. viral hepatitis assessed by MR elastography. Sci Rep. 2021;11:9820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Tohmé-Noun C, Cazals D, Noun R, Menassa L, Valla D, Vilgrain V. Multiple biliary hamartomas: magnetic resonance features with histopathologic correlation. Eur Radiol. 2008;18:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Naganuma H, Ishida H, Kuroda H, Suzuki Y, Ogawa M. Hereditary hemorrhagic telangiectasia: how to efficiently detect hepatic abnormalities using ultrasonography. J Med Ultrason (2001). 2020;47:421-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Bruce M, Kolokythas O, Ferraioli G, Filice C, O'Donnell M. Limitations and artifacts in shear-wave elastography of the liver. Biomed Eng Lett. 2017;7:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 40. | Yokoo T, Kanefuji T, Suda T, Nagayama I, Hoshi T, Abe S, Morita S, Kamimura H, Kamimura K, Tsuchiya A, Takamura M, Yagi K, Terai S. Rational arrangement of measuring shear wave speed in the liver. World J Gastroenterol. 2019;25:2503-2513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, Cosgrove D, Ferraioli G, Friedrich-Rust M, Gilja OH, Goertz RS, Karlas T, de Knegt R, de Ledinghen V, Piscaglia F, Procopet B, Saftoiu A, Sidhu PS, Sporea I, Thiele M. EFSUMB Guidelines and Recommendations on the Clinical Use of Liver Ultrasound Elastography, Update 2017 (Long Version). Ultraschall Med. 2017;38:e48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 42. | Piscaglia F, Salvatore V, Mulazzani L, Cantisani V, Schiavone C. Ultrasound Shear Wave Elastography for Liver Disease. A Critical Appraisal of the Many Actors on the Stage. Ultraschall Med. 2016;37:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 43. | Ipek-Ugay S, Tzschätzsch H, Braun J, Fischer T, Sack I. Physiologic Reduction of Hepatic Venous Blood Flow by the Valsalva Maneuver Decreases Liver Stiffness. J Ultrasound Med. 2017;36:1305-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Lee J, Lee R, Erpelding T, Siddoway RL, Gao J. The effect of water intake on ultrasound tissue characteristics and hemodynamics of adult livers. Clin Exp Hepatol. 2021;7:223-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 45. | Cha J, Kim J, Ko J, Eom K. Effects of Confounding Factors on Liver Stiffness in Two-Dimensional Shear Wave Elastography in Beagle Dogs. Front Vet Sci. 2022;9:827599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Zelesco M, Abbott S, O’Hara S. Pitfalls and source of variability in two dimensional shear wave elastography of the liver: as overview. Sonography. 2018;5:20-28. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Kjærgaard M, Thiele M, Jansen C, Stæhr Madsen B, Görtzen J, Strassburg C, Trebicka J, Krag A. High risk of misinterpreting liver and spleen stiffness using 2D shear-wave and transient elastography after a moderate or high calorie meal. PLoS One. 2017;12:e0173992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 48. | Sugimoto K, Moriyasu F, Oshiro H, Takeuchi H, Abe M, Yoshimasu Y, Kasai Y, Sakamaki K, Hara T, Itoi T. The Role of Multiparametric US of the Liver for the Evaluation of Nonalcoholic Steatohepatitis. Radiology. 2020;296:532-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 49. | Sugimoto K, Lee DH, Lee JY, Yu SJ, Moriyasu F, Sakamaki K, Oshiro H, Takahashi H, Kakegawa T, Tomita Y, Abe M, Yoshimasu Y, Takeuchi H, Choi BI, Itoi T. Multiparametric US for Identifying Patients with High-Risk NASH: A Derivation and Validation Study. Radiology. 2021;301:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 50. | Chen S, Sanchez W, Callstrom MR, Gorman B, Lewis JT, Sanderson SO, Greenleaf JF, Xie H, Shi Y, Pashley M, Shamdasani V, Lachman M, Metz S. Assessment of liver viscoelasticity by using shear waves induced by ultrasound radiation force. Radiology. 2013;266:964-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 51. | Cetinic I, de Lange C, Simrén Y, Ekvall N, Östling M, Stén L, Boström H, Lagerstrand K, Hebelka H. Ultrasound Shear Wave Elastography, Shear Wave Dispersion and Attenuation Imaging of Pediatric Liver Disease with Histological Correlation. Children (Basel). 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 52. | Ormachea J, Parker KJ, Barr RG. An initial study of complete 2D shear wave dispersion images using a reverberant shear wave field. Phys Med Biol. 2019;64:145009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 53. | Secasan CC, Onchis D, Bardan R, Cumpanas A, Novacescu D, Botoca C, Dema A, Sporea I. Artificial Intelligence System for Predicting Prostate Cancer Lesions from Shear Wave Elastography Measurements. Curr Oncol. 2022;29:4212-4223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 54. | Lu X, Zhou H, Wang K, Jin J, Meng F, Mu X, Li S, Zheng R, Tian J. Comparing radiomics models with different inputs for accurate diagnosis of significant fibrosis in chronic liver disease. Eur Radiol. 2021;31:8743-8754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 55. | Wang K, Lu X, Zhou H, Gao Y, Zheng J, Tong M, Wu C, Liu C, Huang L, Jiang T, Meng F, Lu Y, Ai H, Xie XY, Yin LP, Liang P, Tian J, Zheng R. Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: a prospective multicentre study. Gut. 2019;68:729-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 354] [Article Influence: 59.0] [Reference Citation Analysis (1)] |