Published online Dec 14, 2022. doi: 10.3748/wjg.v28.i46.6433
Peer-review started: August 13, 2022
First decision: October 20, 2022
Revised: October 31, 2022
Accepted: November 21, 2022
Article in press: November 21, 2022
Published online: December 14, 2022
Processing time: 116 Days and 23.1 Hours
Hepatectomy is currently considered the most effective option for treating patients with early and intermediate hepatocellular carcinoma (HCC). Unfortunately, the postoperative prognosis of patients with HCC remains unsatisfactory, predominantly because of high postoperative metastasis and recurrence rates. Therefore, research on the molecular mechanisms of postoperative HCC metastasis and recurrence will help develop effective intervention measures to prevent or delay HCC metastasis and recurrence and to improve the long-term survival of HCC patients. Herein, we review the latest research progress on the molecular mechanisms underlying postoperative HCC metastasis and recurrence to lay a foundation for improving the understanding of HCC metastasis and recurrence and for developing more precise prevention and intervention strategies.
Core Tip: Surgical resection is currently a vital treatment option for patients with early and intermediate hepatocellular carcinoma (HCC). Unfortunately, the prognosis of HCC patients remains unsatisfactory due to high rates of postoperative metastasis and recurrence. Therefore, studies on the molecular mechanisms of postoperative HCC metastasis and recurrence will help develop effective intervention measures to prevent or delay HCC metastasis and recurrence and to improve the long-term survival of HCC patients. Herein, we review the latest research progress on the molecular mechanisms underlying postoperative HCC metastasis and recurrence to lay a foundation for improving the understanding of HCC metastasis and recurrence and for developing more precise prevention and intervention strategies.
- Citation: Niu ZS, Wang WH, Niu XJ. Recent progress in molecular mechanisms of postoperative recurrence and metastasis of hepatocellular carcinoma. World J Gastroenterol 2022; 28(46): 6433-6477
- URL: https://www.wjgnet.com/1007-9327/full/v28/i46/6433.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i46.6433
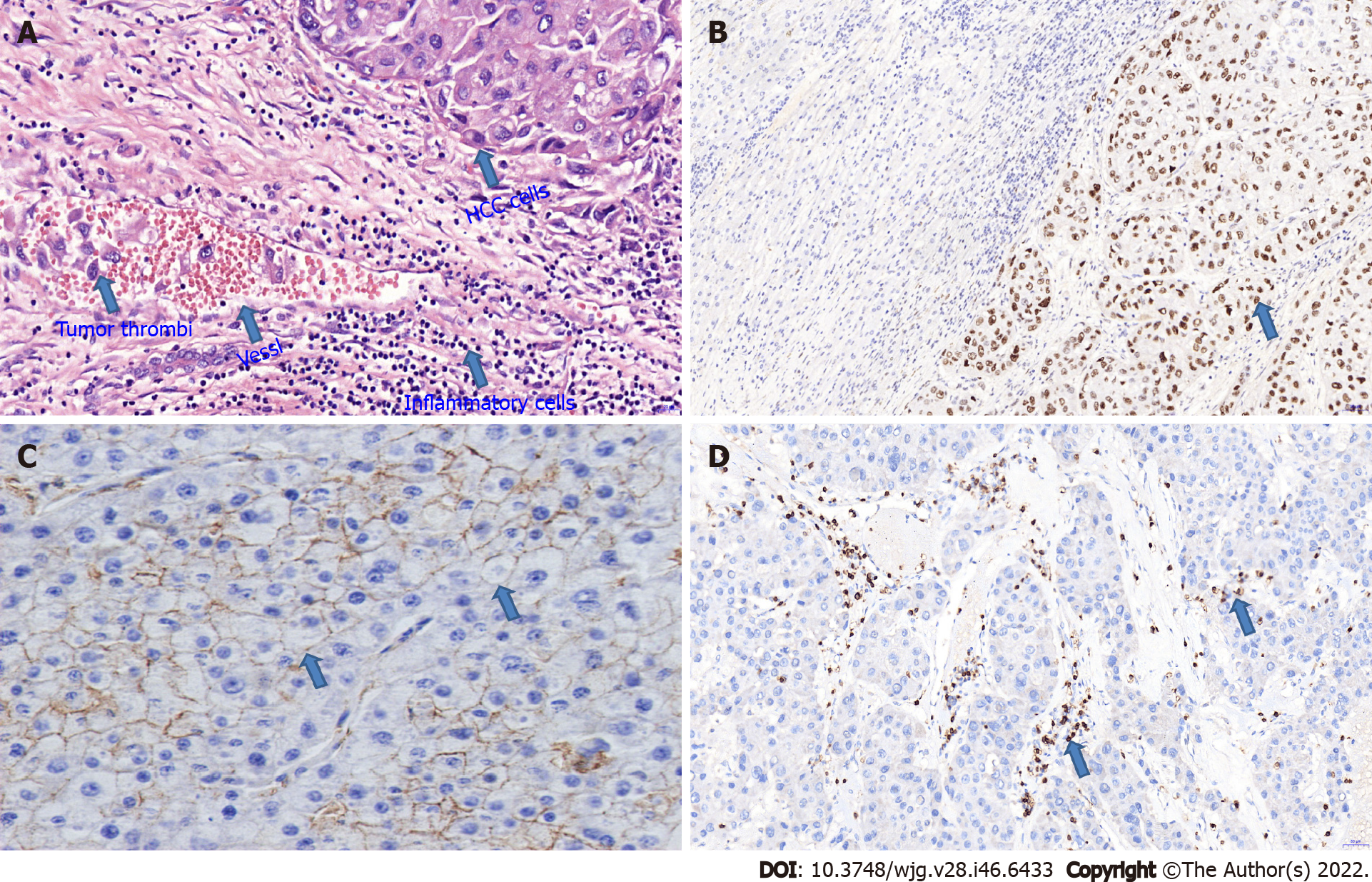
Hepatocellular carcinoma (HCC) is extremely metastatic and invasive, with postoperative metastasis and recurrence causing much of its high mortality. HCC metastasis can be roughly divided into intrahepatic and extrahepatic metastasis. Intrahepatic metastasis chiefly results from the direct invasion of cancer cells or tumor thrombus shedding to form lesions (Figure 1A), while extrahepatic metastasis includes hematogenous, lymphatic and implant metastases, which can reach many organs and tissues throughout the body. Surgical resection is currently the preferred therapy for patients with early- and mid-stage HCC, but the rate of postoperative HCC recurrence is high, and no effective preventive scheme is available. Thus, the prognosis of HCC remains poor. Numerous clinical studies have indicated that the 5-year recurrence rate of HCC after surgery is as high as 70%[1,2]. Moreover, the 5-year recurrence rate of small HCC tumors ranges from 50% to 60%[3]. Thus, HCC metastasis and recurrence have become the greatest obstacles to improving therapeutic efficacy and the long-term prognosis of HCC. Currently, the clinical detection and diagnosis of HCC metastasis and recurrence rely mainly on imaging examinations and the detection of traditional tumor markers, but the early detection of metastasis or recurrence is difficult. The molecular and biological characteristics of tumors are the key determinants of HCC metastasis and recurrence. Thus, elucidating the molecular mechanisms underlying HCC metastasis and recurrence and searching for early-warning molecules and metastasis-related intervention targets will have important implications for prolonging the survival of HCC patients after surgery.
HCC metastasis and recurrence are closely correlated with a series of molecular changes whose mechanisms may involve oncogenes, tumor suppressors, the tumor microenvironment (TME), epithelial-mesenchymal transition (EMT), liver cancer stem cells (LCSCs), circulating tumor cells (CTCs) and epigenetic modifications (Table 1). To date, the mechanisms of HCC metastasis and recurrence have not been completely elucidated. Therefore, research on the molecular mechanisms of postoperative HCC metastasis and recurrence will facilitate the development of effective intervention measures to prevent or delay HCC metastasis and recurrence and to improve the long-term survival of HCC patients. Herein, we review the latest research progress on the molecular mechanisms underlying postoperative HCC metastasis and recurrence to lay a foundation for improving the understanding of HCC metastasis and recurrence and for developing more precise prevention and intervention strategies.
| Molecular marker | Association with HCC metastasis and recurrence | Ref. |
| Oncogenes and tumor suppressors | ||
| Oncogens | ||
| H-ras | Increased expression | Ma et al[9] |
| OPN | Increased expression | Yu et al[22]; Zhu et al[25] |
| S100A9 | Increased expression | Chiou and Lee[29]; Liao et al[31] |
| Tumor suppressors | ||
| Mutated p53 | Increased expression | Nikolova et al[40]; Chaudhary et al[44] |
| Nm23 | Decreased expression | Khera et al[50] |
| KAI1 | Decreased or lost expression | Xu et al[55] |
| Tumor microenvironment | ||
| Tumor stromal cells | ||
| Immune cells | ||
| T lymphocytes | Decreased expression | Cai et al[68]; Gabrielson et al[70]; Li et al[71] |
| NK cells | Decreased expression | Wu et al[85]; Lee et al[90] |
| DCs | Decreased expression | Shi et al[108] |
| M2-TAMs | Increased expression | Yao et al[128]; Park et al[133]; Liao et al[134] |
| MDSCs | Increased expression | Kalathil and Thanavala[143]; Lu et al[147] |
| Tregs | Increased expression | Xiong et al[155]; Trehanpati and Vyas[159] |
| N2-TANs | Increased expression | Zhou et al[172]; Yang et al[175] |
| HSCs | Increased expression | Barry et al[198]; Cheng et al[200] |
| CAFs | Increased expression | Gao et al[217]; Zhang et al[220] |
| TECs | Increased expression | Tahmasebi et al[236]; Dong et al[239] |
| ECM | ||
| Adhesion enhancement in tumor cell-ECM | ||
| ICAM-1 | Increased expression | Chen et al[255] |
| E-Cadherin | Decreased or lost expression | Ren et al[267]; He et al[269] |
| Integrins | ||
| Oncogenic integrins | Increased expression | Zhang et al[276]; Huang et al[279] |
| Tumor-suppressive integrins | Decreased expression | Zhang et al[282] |
| Selectins | ||
| P-selectin | Increased expression | Xu et al[288] |
| CD44V6 | Increased expression | Chen et al[292] |
| ECM degradation | ||
| uPA | Increased expression | Song et al[293] |
| MMP-2 and MMP-9 | Increased expression | Liu et al[296]; Fu et al[297] |
| Cytokines | ||
| IFN-γ | Decreased expression | Li et al[302] |
| Interleukin | ||
| IL-6 | Increased expression | Jiang et al[315] |
| IL-8 | Increased expression | Zhang et al[327] |
| IL-1β | Increased expression | Duan et al[332] |
| TNF-α | Increased expression | Hammam et al[338] |
| Chemokines | ||
| CCL2/CCR2 axis | Increased expression | Avila and Berasain[348] |
| CXCL12-CXCR4 axis | Increased expression | Li et al[355] |
| Growth factors | ||
| VEGF | Increased expression | Zhang et al[365] |
| TGF-β | Increased expression | Sun et al[375] |
| Exosomes | Increased expression | Watanabe and Tanaka[385]; Luo et al[404] |
| EMT | ||
| E-cadherin | Decreased or lost expression | Xu et al[413]; Helal et al[417] |
| N-cadherin and Vimentin | Increased expression | Xu et al[413]; Helal et al[417] |
| LCSCs | ||
| CD133 | Increased expression | Liu et al[363] |
| EpCAM | Increased expression | Tsuchiya et al[435]; Krause et al[437] |
| CD90 | Increased expression | Yamashita and Kaneko[440]; Hwang et al[441] |
| CTCs | Increased expression | Schulze et al[463] |
| Epigenetic modifications | ||
| DNA methylation | ||
| Tumor suppressors | Hypermethylation | He et al[476]; Qian et al[478] |
| Oncogenes | Hypomethylation | Zhou et al[479]; Chen et al[480] |
| Noncoding RNAs | ||
| LncRNAs | ||
| Oncogenic lncRNAs | Increased expression | Shi et al[484] |
| Tumor-suppressive lncRNAs | Decreased expression | Song and Qiu[486] |
| CircRNAs | ||
| Oncogenic circRNAs | Increased expression | Gu et al[499] |
| Tumor-suppressive circRNAs | Decreased expression | Sun et al[501] |
| miRNAs | ||
| Tumor-suppressive miRNAs | Decreased expression | Yao et al[508] |
| Oncogenic miRNAs | Increased expression | Wang et al[510] |
Aberrant expression of cancer-related genes regulates tumor cell growth, differentiation, invasion and metastasis. The activation of oncogenes and the inactivation of tumor suppressor genes can lead to uncontrolled growth of cancer cells, enabling invasion and metastasis. In addition, the abnormal expression of certain cancer-related genes can lead to functional alterations in HCC cells and contribute to metastasis and recurrence. The main genes closely connected with HCC metastasis and recurrence are described below.
H-ras: H-ras proto-oncogenes encode G proteins. The 12th codon of Ras interacts with GTPase-activating protein. After binding with guanosine triphosphate (GTP), H-ras participates in intracellular information transmission. In normal cells, H-ras almost completely binds to guanosine diphosphate and is in an inactive state. However, H-ras is abnormally highly expressed in tumor tissues[4]. The abnormal activation of H-ras has been implicated in the invasiveness of multiple malignancies[5,6]. In HCC, H-ras mutations are closely associated with invasion and metastasis[7]. In addition, the EMT process is regulated by various signaling molecules, particularly transforming growth factor-β1 (TGF-β1)[8]. H-ras can induce EMT in HCC through the TGF-β1 pathway and thereby participate in HCC invasion and metastasis[9]. Thus, inhibition of H-ras activity may be a therapeutic target for HCC metastasis and recurrence.
Osteopontin: Osteopontin (OPN) is a glycoprotein and a secretory calcium-binding phosphoprotein with multiple biological activities encoded on chromosome 4q13. OPN can exert diverse functions by interacting with integrins or CD44 receptors to regulate cell adhesion and cell chemotaxis, regulate signal transduction, and facilitate tumor formation and progression[10]. OPN is also considered a metastasis-related gene given its high expression in metastatic cancer and tumors with high metastatic potential[11,12]. OPN overexpression is closely associated with tumor occurrence, metastasis and recurrence[13-15] and has become a molecular signature for the metastasis and recurrence of various malignancies.
OPN is overexpressed in HCC[16], in which it modulates overall signal transduction and performs a crucial role in invasion, metastasis and recurrence by binding to its receptors CD44 and integrin[17-20]. OPN promotes HCC metastasis and recurrence through several molecular mechanisms: (1) OPN inhibits apoptosis and promotes angiogenesis. Specific splice variants of OPN (OPN-b, OPN-c) inhibit tumor cell apoptosis, and OPN binding to the integrin receptors α9β1 and αvβ3 can induce angiogenesis and promote HCC metastasis[21]; (2) OPN induces EMT and increases the metastatic ability of HCC cells by directly upregulating the expression of Twist (an E-cadherin gene transcriptional regulator)[22]; (3) OPN increases the stemness of HCC cells. For example, it increases the stemness of CD133+/CD44+ cancer stem cells (CSCs) by regulating DNA methylation reorganization in HCC cells[23]; (4) OPN drives extracellular matrix (ECM) remodeling. OPN degrades the ECM by activating matrix metalloproteinase (MMP)-2 and urokinase-type plasminogen activator (uPA), resulting in HCC metastasis[24]; and (5) OPN mediates immune evasion. HCC cells secrete OPN, and serum OPN levels are elevated in HCC patients. OPN and programmed cell death ligand 1 (PD-L1) are linked in HCC, which implies that OPN is involved in immune evasion[25] and thus facilitates HCC cell infiltration and metastasis. Therefore, OPN may be a target for regulating HCC metastasis and recurrence.
S100A9: S100A9 belongs to the S100 family and contains an amino acid sequence that can bind calcium ions with high affinity and selectivity[26]. The gene encoding S100A9 is located on chromosome lq21. This region is easily affected by the internal and external environment, certain cytokines and other factors. Therefore, S100A9 exhibits poor stability and is prone to chromosomal deletion and translocation, which are connected to the growth, differentiation and metastasis potential of various malignancies[27,28]. High S100A9 levels in the preoperative serum of HCC patients are closely associated with metastasis and recurrence[29]. The mechanisms through which S100A9 mediates HCC metastasis are currently unclear. The possible mechanisms are as follows: (1) S100A9 in HCC tissues induces the migration and aggregation of bone marrow-derived myeloid-derived suppressor cells (MDSCs), a heterogeneous population composed of immature myeloid cells with immunosuppressive properties, and thereby the formation of a powerful immunosuppressive environment[30], which is conducive to HCC invasion and metastasis; (2) Immune neutrophils/macrophages in the HCC microenvironment induce cancer cells to produce S100A9, which acts as a proinflammatory factor associated with a high risk of HCC recurrence[31,32]; and (3) S100A9 is mainly expressed in epithelial cell adhesion molecule (EpCAM)-positive cells in HCC tissues, thereby promoting HCC recurrence[33].
p53: The tumor suppressor gene p53 encodes a transcription factor and stress sensor that can specifically bind to DNA sequences and is very important for maintaining genomic stability[34]. Compared with other tumor suppressor genes, which only lose their tumor suppressor function after mutation (nonsense mutation), p53 not only loses its original tumor suppressor function but also acquires a new oncogenic function after mutation (missense mutation)[35]. Mutated p53 facilitates cancer cell migration, invasion and metastasis through the activation of receptor tyrosine kinase signaling[36]. Mutant p53 is a valuable predictor of local recurrence in multiple malignancies[37,38].
TP53 alteration is the most common genetic alteration in HCC and participates in HCC metastasis (Figure 1B). The p53 gene has the potential to regulate HCC invasion and metastasis[39]; p53 gene mutations are closely related to vascular invasion and metastasis and serve as predictors of HCC metastasis, recurrence and poor prognosis[40-44]. Mounting evidence indicates that p53 gene mutation is related to loss of immune cells in the HCC microenvironment[45,46], which promotes the survival of HCC cells and their evasion of immune surveillance and thereby contributes to HCC metastasis. Thus, targeted therapy for p53 gene mutation may help improve the prognosis of HCC patients.
Nm23: Nm23 (synonym: NME) is a tumor metastasis-related suppressor gene located in the q22 region of chromosome 17. Nm23 has two isoforms, nm23-H1 and nm23-H2, which are regulated by two independent regulatory systems. Of the two, nm23-H1 is more closely related to tumor metastasis, with nucleoside diphosphate kinase and histidine protein kinase (PDK) activities[47]. Nm23-H1 has the potential to inhibit the metastasis of various malignancies[48,49]. In HCC, nm23-H1 participates in the regulation of metastasis: Low nm23-H1 expression reduces nm23-H1-mediated tumor suppression, which may be an important reason for HCC metastasis[50,51]. These findings suggest that reduced expression of nm23-H1 is related to HCC metastasis and recurrence and that nm23-H1 may be a therapeutic target for HCC.
KAI1: The KAI1 gene, also known as CD82, is a specific tumor suppressor gene[52]. The transmembrane domain of the KAI1 protein regulates tumor invasion and metastasis by relying on cell–cell or cell–ECM interactions or by regulating the expression of cell surface adhesion molecules[53,54]. The rate of KAI1/CD82-positive expression in HCC patients without intrahepatic metastasis is greater than that in patients with intrahepatic metastasis[55], which suggests that loss of KAI1/CD82 expression is linked to HCC local invasion and metastasis. In addition, KAI1 expression is downregulated or absent in HCC tissues (Figure 1C), resulting in decreased adhesion of tumor cells; this decreased adhesion allows the tumor to separate easily from the primary lesion, causing metastasis[55]. These findings suggest that the downregulation or absence of KAI1 expression may be a cause of HCC metastasis and that KAI1 may be a biomarker of HCC metastasis and recurrence. The regulation and activation of KAI1/CD82 expression may therefore be useful for HCC treatment.
The TME is a local steady-state environment that is required for tumor occurrence, progression and metastasis during the growth process. The HCC TME mainly consists of HCC cells, tumor stromal cells, ECM and exosomes[56]. In the TME, interactions occur at different levels, and links between tumor cells and intercellular substances are observed. Tumor cells can reshape the TME. The remodeled TME can affect the onset and progression of cancer. The interaction between tumor cells and TME promotes the proliferation, invasion, metastasis, angiogenesis, immune evasion of tumor cells[57,58]. Other nontumor components of the microenvironment also participate in HCC metastasis and recurrence. In recent years, the tumor immune microenvironment (TIME) has gradually been recognized as a decisive factor in HCC metastasis. Mounting evidence indicates that the gene expression profiles of nontumor cell components in peritumoral tissue can predict HCC metastasis and recurrence[59-61], and the HCC TME can regulate the TIME. Cytokine release and changes in angiogenesis and other pathways promote HCC cell growth, invasion and metastasis. These findings reveal that the microenvironment of HCC cell growth has an important influence on the potential for metastasis and recurrence. Therefore, research on the dynamic relationship between the TME and HCC metastasis and elucidation of the molecular mechanisms of different factors in the microenvironment during metastasis are key to inhibiting HCC metastasis.
Stromal cells are the major cellular components in the HCC microenvironment. HCC stromal cells mainly include immune cells, hepatic stellate cells (HSCs), cancer-associated fibroblasts (CAFs), and vascular endothelial cells (VECs). Interactions occur between tumor cells and stromal cells in the TME. Various stromal cells can secrete a variety of cytokines and chemokines, which act on nearby cancer cells in a paracrine manner to enhance tumor invasion and metastasis. In turn, the behavior patterns of stromal cells are influenced and modified by tumor cells.
The immune cells in the TME constitute the TIME and are mainly divided into effector immune cells and inhibitory immune cells. The former include T lymphocytes, natural killer (NK) cells, and dendritic cells (DCs), while the latter encompass tumor-associated macrophages (TAMs), MDSCs, regulatory T cells (Tregs), and tumor-associated neutrophils (TANs). The existing evidence indicates that the TME is in an immunosuppressive state and that the infiltrated immune effector cells show varying degrees of low immune function or even severe defects. Tumor cells establish immunosuppressive networks in the TME[62]. Under the severe metabolic and nutritional stress of the TME, immune cells are often in a disordered state, which impairs the antitumor immune response and thereby accelerates cancer metastasis and recurrence. Currently, considerable evidence indicates that the TIME is key to promoting immune evasion, metastasis and recurrence in HCC[63]. In brief, in addition to existing treatments, TIME regulation to delay postoperative HCC metastasis and recurrence warrants further consideration.
T lymphocytes: T lymphocytes are the main components of immune cells, and the detection of cellular immune-related indicators has important clinical value for evaluating the development and prognosis of tumors[64]. CD3+, CD4+, and CD8+ T cells are the main subsets of T lymphocytes, and their levels most strongly reflect the immune status of the human body. CD3+ T-cell numbers represent overall cellular immunity. CD4+ T cells can mediate the immune tolerance of tumor cells[65] and can differentiate into cytotoxic T lymphocytes (CTLs), which are stimulated by inflammatory factors and thus mediate a killing effect on tumor cells[66,67]. Therefore, dysregulation of the CD3+ (Figure 1D), CD4+ and CD8+ T-cell balance may participate in HCC cell growth and metastasis. The levels of T-cell infiltration decrease sequentially during the malignant progression of HCC, which suggests that decreased T-cell infiltration is associated with HCC occurrence and metastasis[68]. An increase in T-cell infiltration in HCC patients predicts a better prognosis, whereas a decrease implies a poorer prognosis[69]. Specifically, HCC patients with increased tumor-infiltrating CD3+ or CD4+ T cells are less likely to relapse[70,71]. A decreased number of CD8+ T cells in HCC tissues indicates a reduced killing effect, which contributes to HCC cell growth and metastasis[72]. Some studies have indicated that HCC patients with increased numbers of tumor-infiltrating CD8+ T cells are unlikely to relapse[71,73], suggesting that patients with elevated CD8+ T-cell numbers have a better prognosis[74,75]. However, other research has shown that HCC patients with increased CD8+ T-cell numbers are more likely to relapse[76,77]. Thus, the effects of CD8+ T cells on HCC metastasis and recurrence remain controversial, and the specific roles and mechanisms of these cells need to be further explored.
NK cells: NK cells are important innate immune cells that exert antiviral and antitumor effects in the body. Their cell surfaces contain activated receptors that stimulate NK cells to generate killing effects[78,79], directly kill tumor cells, activate downstream immune cells, and exert antitumor effects through immune clearance and surveillance[80]. Tumor cells can weaken the immune surveillance and clearance functions of NK cells by restraining the activation of activating receptors and facilitating the activation of inhibitory receptors[81,82]. NK-cell dysfunction, which enables tumor cells to evade the immune surveillance of NK cells, has been noted in many cancer patients[83,84].
The activation level of NK cells is strongly related to HCC metastasis and recurrence. Peripheral blood and HCC tissues often show reduced counts or inhibited functions of NK cells[85-87], which prevents the cells from performing their normal immune surveillance functions, resulting in the escape of HCC cells from immune surveillance and ultimately promoting HCC metastasis and recurrence[85,88-90]. Recent studies have found that the HCC microenvironment cannot recognize and kill tumor cells because of its functional inactivation by a variety of factors. For example, the upregulation of NK-cell immune checkpoint molecules, including programmed cell death protein 1 (PD-1), T lymphocyte immunoglobulin and mucin domain 3 (TIM-3), and cytotoxic T lymphocyte antigen 4, can restrain NK-cell function in the HCC microenvironment[91,92]. In addition, cytokines produced by HCC cells promote NK-cell dysfunction and suppress the killing function of NK cells[93], and interleukin (IL)-35 or IL-10 can inhibit NK-cell activity in the HCC microenvironment[94,95]. These factors negatively regulate NK-cell activity in HCC, which may be a significant mechanism through which NK cells participate in the immune evasion of HCC cells. Thus, blocking the regulation of NK cells mediated by these factors is an important strategy for improving NK-cell function.
Interestingly, liver-resident NK (LRNK) cells have been found to exert powerful immune killing effects. In early HCC, LRNK cells can effectively eliminate cancerous liver cells through surface receptors, such as NKG2D, TRAIL and FASL, and maintain the homeostasis of the normal liver microenvironment[96]. However, with the progression of HCC, the gradually formed HCC microenvironment affects the antitumor activity of LRNK cells. The expression of the immune checkpoint protein TIM-3 is significantly upregulated on the surface of tumor-infiltrating LRNK cells, decreasing cytokine secretion by LRNK cells and weakening cytotoxicity[97]. In addition to TIM-3, LRNK cells in HCC tissues also highly express PD-1, LAG-3, TIGIT and other immune checkpoint molecules, and HCC patients with higher proportions of immune checkpoint–expressing LRNK cells in tumors are more likely to develop tumor thrombi and to lack capsules[97,98], features that are closely related to HCC recurrence[99,100]. In summary, the immune function impairment caused by upregulated expression of immune checkpoint molecules on LRNK cells is conducive to HCC metastasis and recurrence. Thus, blocking the regulation of these immune checkpoint molecules, particularly inhibitory receptors, is an important strategy for improving the function of LRNK cells.
DCs: DCs typically function as antigen-presenting cells. After exposure to antigens in tumor patients, DCs present antigen information to T cells, stimulate T-cell proliferation and activation, and generate large amounts of tumor cell–killing cytotoxic T cells; these cells help completely remove some tiny cancer foci and thus delay and reduce tumor recurrence[101-103]. DCs that infiltrate tumor tissues are called tumor-infiltrating DCs (TIDCs). The immunosuppressive environment in the TME induces an abnormal state in TIDCs, including maturation disorder, functional abnormalities, and phenotypic changes. Thus, these cells cannot effectively present tumor antigens and activate T cells to kill tumor cells, resulting in tumor cell immune evasion[104,105]. Current evidence suggests that the expression of TIDCs is reduced in multiple malignancies[106,107], which indicates that cancer cells can achieve immune evasion by decreasing their TIDC levels.
In HCC patients, DCs are present in reduced numbers and exhibit abnormal differentiation and impaired maturation in the tumor regions and lymph nodes. These DCs fail to activate antigen-specific T-cell responses[108]. DC infiltration into tumors reflects the host’s immune defense mechanism. Greater levels of DC infiltration are associated with a lower tumor recurrence rate and a lower metastasis rate[109]. CD1a is typically considered a marker of immature DCs but is also expressed in mature DCs[110]. CD1a+ DC infiltration is closely associated with tumor metastasis and recurrence in a variety of malignancies, including HCC, and the degree of CD1a+ DC infiltration in tumors with distant metastasis is significantly lower than that in nonmetastatic tumors[110,111]. The potential mechanism involves DCs in tumors that ingest and present tumor antigens and subsequently induce the host’s antitumor immunity. This immune state is helpful for completely eliminating some residual tiny lesions, micrometastases and even early recurrent lesions after tumor resection, which ultimately delays and reduces tumor recurrence.
Plasmacytoid DCs (pDCs) are an interesting subgroup of DCs in human blood[112]. The infiltration of pDCs after HCC resection is a poor prognostic indicator. The infiltration of pDCs into tumors may induce the formation of an immune inflammatory TME via Tregs and IL-17. This condition may lead to a state of tolerance and promote tumor immune evasion[113,114], thereby promoting HCC invasion and metastasis.
TAMs: TAMs are the main infiltrating immune cells in the TME and exhibit phenotypes and functions that differ from those of other macrophages during inflammatory generation. Under the action of different stimulatory factors, TAMs may differentiate into M1-type TAMs with tumor-inhibitory activity or M2-type TAMs with tumor-promoting activity[115,116]. Most of the high-density TAMs located within or around tumors exhibit signatures of M2-type TAMs that promote tumor metastasis by regulating matrix remodeling, promoting neovascularization, and inhibiting local immunity[117-120].
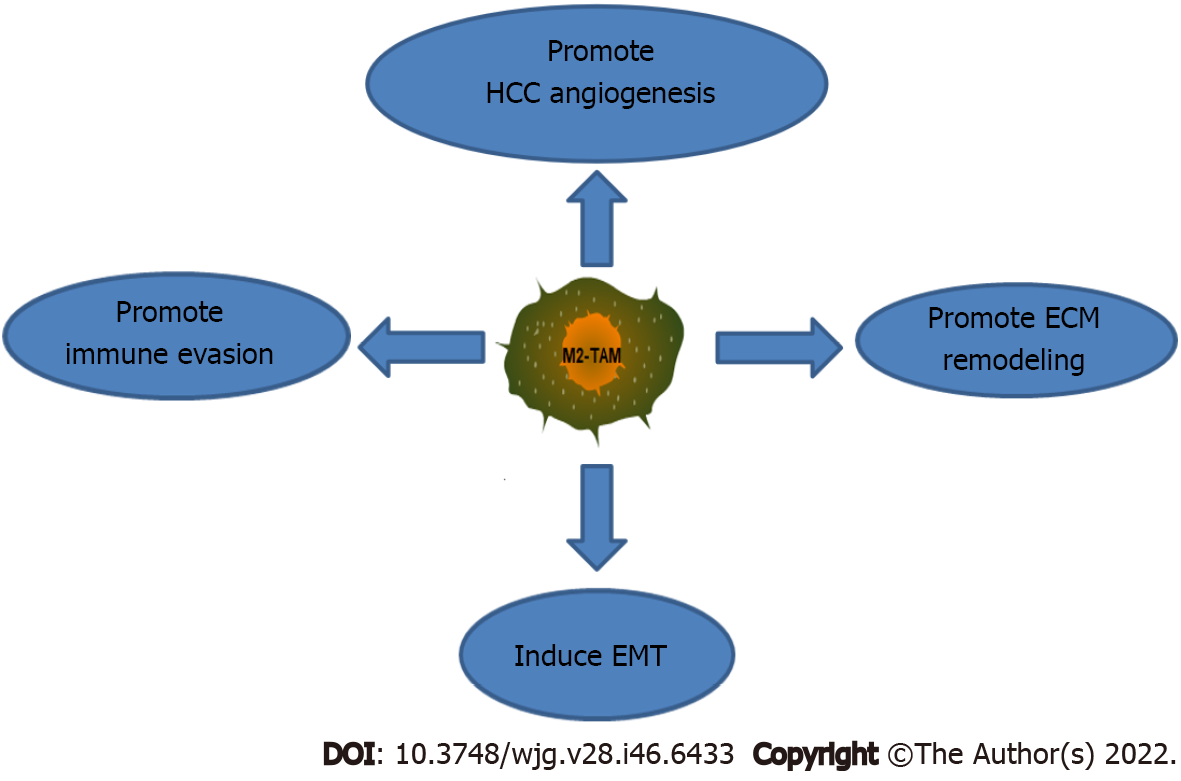
Current evidence suggests that M2-type TAMs act on HCC cells through complex signaling pathways, promoting HCC cell proliferation, angiogenesis, and intrahepatic metastasis and thus resulting in poor prognosis in HCC patients[121,122]. M2-type TAMs promote HCC metastasis and recurrence through several mechanisms (Figure 2): (1) M2-type TAMs promote tumor angiogenesis. TAMs are significantly correlated with angiogenesis in HCC patients[123]. TAMs secrete large amounts of vascular endothelial growth factor (VEGF), tumor necrosis factor-alpha (TNF-α), platelet-derived growth factor (PDGF), and TGF-β under stimulation by hypoxia or a lack of nutrients in tumor cells, ultimately inducing VEC proliferation and promoting HCC angiogenesis[124,125]. In addition, proteolytic enzymes and MMPs secreted by TAMs can promote chemotaxis to induce the abovementioned proangiogenic cytokines to travel to tumor tissues, accelerate the process of angiogenesis, and subsequently promote HCC invasion and metastasis[126]; (2) M2-type TAMs induce ECM remodeling. The ECM acts as a scaffold and barrier for tumor cell migration, and its degradation is a focus in the field of tumor metastasis research. TAMs can secrete a variety of proteolytic enzymes in HCC, particularly MMP-2 and MMP-9, which act on the ECM, degrade the basement membrane (BM) and promote tumor cell invasion and metastasis[127]; (3) M2-type TAMs induce EMT, a key event in HCC metastasis. TAMs are critical cells in the HCC microenvironment and promote EMT progression in HCC cells through various pathways. For example, M2-TAMs induce EMT by activating the Toll-like receptor 4 (TLR4)/STAT3 pathway and thus promote HCC metastasis[128]. In addition, TAMs secrete various factors, including IL-8, TNF-α and TGF-β1, that promote the activation of downstream signaling pathways and thereby induce EMT in HCC[129-131]. Furthermore, some proteases secreted by TAMs, such as MMPs, are also involved in the progression of EMT. For example, MMP17 secreted by TAMs induces EMT to facilitate HCC metastasis[132]; and (4) M2-type TAMs promote immune evasion. TAMs play a pivotal role in tumor-induced immunosuppression. TAMs in HCC can also express PD-1, an extremely important immune checkpoint target; these PD1+ TAMs show the characteristics of the M2 phenotype. PD1+ TAMs directly interact with PDL1+ cells to produce large amounts of IL-10, which inhibits CD8+ T-cell proliferation, destroys the CD8+ T-cell antitumor immune effect, and promotes HCC invasion and metastasis[133-135]. In addition, TAMs induce the HCC microenvironment to become immunosuppressive. TAM-enriched sites show decreased numbers and functional defects of infiltrated CD8+ T cells and inhibited function of NK cells and DCs among killer immune cells. Moreover, TAMs activate Tregs[136-138]. These phenomena all play key roles in tumor immune evasion. Some cytokines secreted by TAMs, particularly TGF-β and IL-10, are also significant factors in the formation of the immunosuppressive HCC microenvironment[139].
In summary, the above findings suggest that reducing the density or effects of M2-type TAMs can inhibit HCC metastasis and recurrence. Thus, M2-type TAMs are expected to be potential targets for HCC therapy.
MDSCs: MDSCs, one of the fundamental cell types in malignancies, can be recruited into the TME to help establish an immunosuppressive TME and promote tumor immune evasion[140]. In addition to immunosuppressive activity, MDSCs exhibit tumor immune evasion–supporting and nonimmunosuppressive effects, promoting tumor metastasis and recurrence via multiple molecular mechanisms[141].
Emerging evidence indicates that MDSCs in the TME are strongly connected to HCC invasion, metastasis and recurrence[142-144]. MDSCs accelerate HCC metastasis and recurrence through several main molecular mechanisms: (1) MDSCs promote the formation of a premetastatic niche (PMN). Tumors can recruit MDSCs to target organs by secreting cytokines or chemokines[144]. In addition, MDSCs secrete related inflammatory factors to form an immunosuppressive PMN, which can further promote HCC metastasis[140,145]; (2) MDSCs promote immunosuppression. MDSCs restrain T-cell proliferation or facilitate Treg expansion by secreting arginase-1, inducible nitric oxide synthase, reactive oxygen species (ROS), and IL-6 to exert immunosuppressive effects[114,143]. In addition, MDSCs effectively suppress the proliferation and function of CD8+ CTLs in the HCC microenvironment[146]. These changes allow tumor cells to escape immune surveillance and attack and ultimately promote HCC progression and metastasis; (3) MDSCs promote HCC angiogenesis. MDSCs can induce tumor angiogenesis and further modify the TME. For example, Bv8 produced by MDSCs has been shown to induce HCC angiogenesis[147] and thereby promote HCC growth and invasion; and (4) MDSCs induce ECM remodeling. MDSCs promote ECM degradation by secreting proteases, such as MMP9, this degradation enhances HCC cell invasion[148].
Taken together, these findings indicate that MDSCs are essential for the promotion of HCC metastasis and recurrence and may be potential targets for antitumor therapy. Notably, targeting MDSCs alone will be insufficient; a combination of different treatment strategies will be needed. However, combining therapies targeting MDSCs with existing treatments may be a new integrated strategy for HCC patients in the future.
Tregs: Tregs are a class of T lymphocytes with powerful negative immunomodulatory functions. Tregs restrain the activity of effector T and B cells, which are key to the maintenance of immune balance and tolerance. Massive infiltration of Tregs in the TME not only inhibits the immune system response to cancer cells, leading to tumor cell immune evasion and inhibiting the function of antitumor immunity[149], but also facilitates tumor cell proliferation, invasion and metastasis[150]. Treg infiltration in malignancies also predicts poor outcomes and is associated with postoperative local metastasis and recurrence[151].
The Treg density in the peripheral blood of HCC patients increases progressively during the malignant progression of HCC, which suggests that an increased density of Tregs is closely related to HCC invasiveness, metastasis and recurrence[152-154]. Tregs facilitate HCC invasion and metastasis through several molecular mechanisms: (1) An association exists between Treg infiltration and driver gene mutations. TP53 mutation is strongly associated with HCC metastasis and recurrence, and HCCs with TP53 mutations have higher Treg infiltration than those without TP53 mutations[155]; (2) Treg infiltration induces EMT. Tregs promote HCC invasion and migration by promoting TGF-β1 expression to induce EMT in HCC cells[156]; (3) Treg infiltration promotes immune evasion. Tregs promote HCC cell immune evasion through multiple pathways. (a) The recruitment of Tregs to the TME is related to tumor cell evasion of immune surveillance and clearance, and the powerful inhibitory function of Tregs promotes the immune tolerance of tumor cells and inhibits T-cell activity[157]; (b) Tregs upregulate the checkpoint inhibitors PD-L1 and PD-1 to suppress the production of T effector cells in the TME and thereby weaken the immune killing effect on HCC cells[158]; and (c) Tregs directly contact and/or secrete immunosuppressive factors, including IL-2, IL-10, TGF-β and IL-35. These immunosuppressive factors form an immunosuppressive microenvironment by inhibiting the activity of naïve T cells. The tumor cells then achieve immune evasion, resulting in HCC spread and even metastasis[159]; and (4) The increased numbers of Tregs may inhibit CD4+ and CD8+ T-cell activity, disrupting the immune balance in the TME and generating an immunosuppressive microenvironment; these effects enable HCC cells to escape attack by immune cells[160].
In summary, the Treg population is associated with HCC metastasis and recurrence. Thus, therapeutic strategies targeting Tregs or combined therapies can selectively inhibit Tregs on the premise of maintaining immune balance and can support antitumor immunity mediated by effector T cells to achieve effective treatment.
TANs: TANs are neutrophils that infiltrate the TME. As important inflammatory cells, TANs participate in the formation and maintenance of the tumor inflammatory microenvironment and regulate tumor progression[161]. Increasing amounts of evidence suggest that neutrophil plasticity and diversity underlie the dual potential of TANs in the TME. Depending on the different stimulating factors in the TME, TANs can be activated into the tumor-suppressive N1 type and the tumor-promoting N2 type. The N1 phenotype has cytotoxic and proinflammatory activities, whereas the N2 phenotype has strong immunosuppressive ability[161]. N2-TANs mainly promote tumor proliferation, invasion, angiogenesis and metastasis by releasing neutrophil extracellular traps (NETs), suppressing the immune response, and producing derivatives, such as cytokines and proteases[162-164].
Three types of granules (primary, secondary and tertiary granules) and secretory vesicles are present in neutrophils. These granules are composed of various proteases, including MMPs, cathepsins, and neutrophil elastase[165]. MMPs play various roles in tumor progression, not only mediating ECM degradation but also controlling tumor angiogenesis and affecting tumor cell growth. MMPs are principal regulators of tumor invasion and metastasis[166]. For example, MMPs induce HCC cells to break through the histological barrier composed of the BM and ECM, invade their neighboring tissues and metastasize to distant tissues by destroying the degradation balance of the matrix[167]. In addition, MMPs can induce and promote EMT in the HCC microenvironment, facilitating HCC metastasis[168]. Cathepsin is also closely related to HCC metastasis[169].
HCC cells can further activate chemokine ligand (CCL) 2 and CCL17 by activating TANs. These chemokines induce the migratory activity of tumor cells by affecting TAMs and Tregs[170]. These recruited immune cells promote tumor progression, facilitating HCC cell invasion and metastasis by releasing cytokines[171]. Moreover, TANs can accelerate HCC invasion and metastasis by inducing and enhancing the stemness traits of HCC cells and thus inducing increased secretion of CXC chemokine ligand 5 (CXCL5) to induce TAN intratumoral infiltration and form a positive feedback pathway consisting of TANs, HCC cells, and stem cell-like HCC cells[172]. Recent studies have confirmed that TANs form NETs by releasing chromatin structures from cells, blocking contact with immune cells by covering tumor cells, and protecting tumor cells from cytotoxicity mediated by CD8+ T cells and NK cells[173]. TANs also “wake up” and “trap” dormant cancer cells and serve as cancer cell adhesion substrates that promote tumor occurrence and metastasis[174]. The ability to form NETs is significantly enhanced in HCC patients, and NETs ultimately promote HCC metastasis by capturing CTCs and enhancing the migration and angiogenesis capabilities of CTCs[175]. In addition, NETs promote HCC metastasis by establishing a PMN[176]. In conclusion, numerous studies have demonstrated the potential mechanisms by which TANs promote HCC metastasis and recurrence. The phenotypic plasticity and functional diversity of TANs suggest that TANs are potential therapeutic targets for HCC.
HSCs are mainly distributed in the hepatic space of Disse[177] and have two phenotypes, quiescent and activated. Under physiological conditions, most HSCs are in a quiescent state and participate primarily in the dynamic balance of vitamin A metabolism. HSC activation is the central link in hepatic fibrosis caused by various etiologies, and activated HSCs play an important role in HCC metastasis and recurrence. Therefore, elucidating the molecular mechanisms of HSC activation is of great significance in preventing or reversing hepatic fibrosis and in preventing postoperative HCC recurrence and metastasis. The mechanisms of HSC activation are complicated. In addition to cytokines, transcription factors and oxidative stress can activate HSCs, and noncoding RNA (ncRNA) and exosomes have also been found to be involved in HSC activation in recent years. In brief, the mechanisms of HSC activation are as follows: (1) Cytokines participate in HSC activation. When the liver is injured, hepatocytes, Kupffer cells, sinusoidal endothelial cells, macrophages and platelets all secrete cytokines that play decisive roles in HSC activation, such as TGF-β and PDGF. The mechanisms of action by which cytokines activate HSCs are quite complex. Rather than acting in isolation, cytokines form a network through autocrine and paracrine interactions, thereby activating HSCs. TGF-β can activate HSCs by binding to specific receptors of HSCs and activating both small mother against decapentaplegic (SMAD)-dependent and non-SMAD pathways[178]. TGF-β can also activate HSCs through HAS2, TLR4he and Notch1[179]. PDGF is mainly produced by platelets, Kupffer cells and sinusoid endothelial cells in the liver. After PDGF binds to the receptor on the membrane of HSCs, it promotes HSC proliferation and activation mainly through phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT), Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3), and mitogen-activated protein kinase (MAPK)[180-182]; (2) Transcription factors participate in HSC activation. Transcription factors related to HSC activation mainly include nuclear factor-κB (NF-κB) and peroxisome proliferator-activated receptor γ (PPAR-γ). NF-κB is an important intracellular mediator of the liver inflammatory response. NF-κB activation can promote HSC activation by promoting the generation of proinflammatory factors such as IL-8, IL-1β, TNF-α and IL-6[183,184]. PPAR-γ is a class of ligand-activated nuclear receptor transcription factors that are members of the nuclear hormone receptor superfamily. PPAR-γ exerts anti-inflammatory effects by inhibiting the transcription of inflammatory cytokines and has a regulatory effect on maintaining HSC quiescence and inhibiting HSC activation. It has been shown that PPAR-γ inhibits HSC activation mainly by inhibiting the activity of the TGF-β1/Smad pathway or by downregulating the expression of potent fibrogenic factors such as PDGF, connective tissue growth factor (GF), and TGF-β1[185,186]; (3) Oxidative stress is an important factor in HSC activation. When oxidative stress occurs, excessive production of ROS in vivo not only directly damages hepatocytes but also activates various signaling pathways, such as nuclear factor-related factor-2, TGF-β, NF-κB and other related cytokines, signaling molecules and their downstream signaling pathways, that further aggravate liver tissue injury and activate the production of various inflammatory factors. This process further stimulates the continuous activation of NF-kB and regulates apoptosis-related proteins, IL-1β, TNF-α and IL-6, which play important roles in promoting HSC activation and proliferation[187,188]; (4) NcRNAs are also involved in HSC activation. NcRNAs include microRNAs (miRNAs), long noncoding RNAs (lncRNAs) and circular RNAs (circRNAs), which can regulate HSC activation through a variety of mechanisms. Among them, oncogenic ncRNAs facilitate HSC activation. For example, miRNA-503 overexpression can enhance HSC activation by activating the TGF-β/Smad pathway[189]; the upregulation of lncRNA SNHG7 promotes HSC activation by sponging miR-29b, which enhances DNA methyltransferase (DNMT) 3A expression[190]; and the upregulation of circUbe2k facilitates HSC activation by sponging miR-149-5p, which enhances TGF-β2 expression[191]. In contrast, tumor suppressive ncRNAs inhibit HSC activation. For example, overexpression of miR-489-3p and miR-122-5p suppresses HSC activation by restraining the uneven regulation of the jagged canonical Notch ligand 1/notch homolog protein 3 (NOTCH3) signaling pathway[192]; upregulation of lncRNA Meg8 inhibits HSC activation by suppressing the Notch pathway[193]; and increased expression of hsa_circ_0070963 suppresses HSC activation by enhancing LEM domain containing 3 expression by sponging miR-223-3p[194]; and (5) Exosomes regulate HSC activation through various mechanisms. For example, high expression of exosomal miR-192 derived from hepatitis C virus (HCV)-infected hepatocytes can stimulate TGF-β1 expression and thus induce HSC activation[195]. In contrast, NK-cell-derived exosomes can inhibit TGF-β1-induced HSC activation[196].
Activated HSCs subsequently secrete soluble proteins that cause interstitial changes, providing structural support to the microenvironment for HCC invasion and metastasis. HSCs play important roles in promoting HCC invasion and metastasis via multiple mechanisms: (1) The direct effects of activated HSCs on HCC cells promote HCC metastasis. Activated HSCs secrete numerous related proteins, such as vimentin, ECM protein and α-smooth muscle actin (α-SMA)[197], which subsequently promote the secretion of a variety of inflammatory factors and cytokines, aggravate the formation of the inflammatory microenvironment, and participate in various processes that affect the progression of HCC[198,199]. In addition, TGF-β1 promotes α-SMA expression in activated HSCs, upregulates the expression of IL-1 and VEGF in HCC, and thereby promotes HCC invasion and metastasis[200]; (2) HSCs also promote HCC invasion and metastasis by participating in ECM remodeling. When HSCs are activated, the secreted TGF-β1 can facilitate the degradation of MMPs and upregulate tissue inhibitor of metalloproteinase (TIMP) expression, breaking the balance between the two and resulting in reduced ECM degradation. The deposited ECM can promote HSC activation[201], forming an HSC–ECM–HSC positive feedback loop and promoting the occurrence and development of HCC. TGF-β1 can further encourage HSCs to synthesize large amounts of ECM and thus to promote HCC occurrence, development, invasion and metastasis[200]; (3) HSCs promote tumor angiogenesis by secreting cytokines and thus facilitating HCC metastasis. Activated HSCs can produce large amounts of proangiogenic factors, such as VEGF and angiopoietin, which promote tumor angiogenesis by binding with homologous receptors on the surfaces of endothelial cells[202] and facilitate HCC invasion and metastasis[203]; (4) Activated HSCs exert important immunosuppressive effects and thus promote HCC metastasis[203]; and (5) HSCs secrete hepatocyte GF to facilitate STMN1 expression in HCC cells, and the upregulation of this gene accelerates the activation of HSCs and results in the acquisition of the CAF phenotype[204].
In summary, HCC cells can induce the activation of HSCs, and activated HSCs can counteract HCC cells in the HCC microenvironment. The two-way interaction creates a cascade amplification effect that forms a microenvironment conducive to immune evasion and thus ultimately promotes tumor invasion and metastasis. Therefore, combining current HCC therapies with targeted therapies for HSCs in the tumor stroma is a promising strategy for the treatment of HCC.
Fibroblasts are abundant and widely distributed in the stroma. CAFs do not exist in healthy individuals, but once tumors develop in vivo, tumor cells can induce fibroblasts to transform into CAFs. CAFs further promote tumor cell proliferation and thereby form a vicious cycle[205-207]. CAFs are regulators of tumor cell metastasis to distant sites, which can generate PMNs in distant organs and trigger subsequent metastatic events. Thus, these cells are also known as metastasis-associated fibroblasts[208]. Numerous types of pro-invasion molecules and inflammatory mediators act in a paracrine manner in CAFs through cell-to-cell interactions and participate in the remodeling of the ECM, induction of EMT, and promotion of tumor angiogenesis, which increases tumor invasion and metastasis[209-213].
CAFs play a significant role in promoting HCC invasion and metastasis by secreting cytokines. In patients with high expression of CAF-specific molecular markers, HCC is prone to metastasis and recurrence[214]. Current evidence shows that CAFs may facilitate HCC metastasis through several mechanisms: (1) CAFs promote tumor angiogenesis. In HCC, CAFs promote angiogenesis by releasing proangiogenic factors[215]. For example, placental growth factor (PlGF) is highly expressed in CAFs, and PlGF overexpression is highly correlated with tumor angiogenesis. CAFs secrete PlGF to promote HCC angiogenesis[216]. In addition, CAFs form a network of cytokines with HCC cells, secreting GFs and MMPs that can induce tumor cell invasion through autocrine or paracrine signaling and secreting proangiogenic factors to recruit endothelial cells to stimulate tumor angiogenesis[217]. These findings further demonstrate the role of CAFs in HCC angiogenesis; (2) CAFs induce EMT. CAFs can enhance HCC invasion and distant metastasis by inducing EMT in HCC cells[218,219]; (3) CAFs induce ECM remodeling. CAFs alter the ECM structure, reshape the stroma, and subsequently affect HCC cell growth, migration and invasion[220,221]; (4) CAFs promote HCC metastasis by facilitating the stemness acquisition of tumor cells and maintaining the stemness of CSCs. CSCs are the “seeds” of tumor metastasis and recurrence, and maintaining the stemness of CSCs requires the support of the TME in which they are located. CAFs play a significant role in the transformation of the stemness phenotype of cancer cells, in the maintenance of the stemness of CSCs, and in increasing the number of CSCs[222,223]. CAFs enhance the stem cell-like characteristics of HCC cells by activating the IL-6/STAT3/Notch pathway[224]. Moreover, CAFs maintain and promote the stem-like biological characteristics of CD24+ HCC stem cells by secreting HGF and IL-6 to activate the STAT3 pathway[225]; (5) CAFs promote immune evasion. CAFs regulate peripheral immune cells by secreting chemokines and thus participate in tumor immune regulation. CAFs derived from HCC induce myeloid-derived immunosuppressive cells, such as TANs, to mediate immunosuppression and participate in HCC immune evasion[226]. CAFs in HCC recruit normal DCs and induce their transdifferentiation into regulatory DCs by activating the IL-6-mediated STAT3 pathway; these cells rarely present antigens and can also secrete inhibitory cytokines to form an immunosuppressive microenvironment[227]; (6) CAFs mediate HCC metastasis by secreting cytokines. Cytokines secreted by CAFs, such as CCL2, IL-6, and IL-8, promote HCC cell migration and metastasis[228,229]. CAFs also promote HCC metastasis via the chemokine-activated Hedgehog and TGF-β pathways; specifically, CCL2, CCL5, and CCL7 secreted by CAFs promote HCC metastasis by coactivating the Hedgehog and TGF-β pathways[230]; and (7) CAF-mediated HCC metastasis is partly related to the exosomes secreted by CAFs. CAFs produce numerous exosomes to regulate the microenvironment and biological characteristics of tumor cells and to facilitate tumor invasion and metastasis[231]. Dysregulated expression of exosomal miRNAs or lncRNAs secreted by CAFs is an important mechanism affecting tumor initiation, growth, invasion and metastasis; it is also an important feature of TME imbalance and an important cause of tumor metastasis and recurrence. Abnormal expression of exosomal miRNAs or lncRNAs secreted by CAFs may also occur in the HCC microenvironment. For example, miR-320a, miR-150-3p and miR-29b are miRNAs in CAF-secreted exosomes in HCC that act as tumor suppressors, and loss of their expression significantly facilitates HCC cell proliferation and metastasis and is an important risk factor for HCC recurrence[232-234].
In recent years, in-depth exploration of CAFs and their secreted cytokines has provided increasing amounts of evidence that CAFs should be new targets for anti-HCC therapy.
VECs control the entry of nutrients into tissues, maintain blood flow, and regulate leukocyte transport[235]. VECs in the TME can lead to endothelial dysfunction due to factors such as hypoxia and chronic GF stimulation, resulting in irregular shapes and sizes, wrinkled edges, and other abnormal morphologies in tumor endothelial cells (TECs)[236]. Various interactions exist between tumor cells and VECs. For example, tumor cells can induce endothelial cells to form neovascularization networks around tumor cells by secreting GFs[237]. Conversely, endothelial cells in the tumor neovasculature, as an important component of the TME, also synthesize and secrete certain cytokines to facilitate tumor cell migration and invasion and to promote tumor cell metastasis to distant tissues[238].
Tumor VECs promote the entire process of HCC metastasis through various mechanisms: (1) VECs promote HCC metastasis through interaction with cancer cells[236]; (2) VECs promote HCC metastasis by forming cancer nests. Unique small, spherical cancer cell nests surrounded by endothelial cells have been found in some cases of HCC. These spherical cancer nests can enter the blood circulation through fusion with microvessels and metastasize[239]. Unlike single HCC cells, cancer nests metastasize in small groups with higher metastasis efficiency; and (3) VECs promote HCC metastasis via rapid neovascularization. Neovascularization in tumors is an important channel for tumor cells to achieve metastasis. Secretory CXCL12 can rapidly induce neovascularization by regulating C-X-C chemokine receptor 4 (CXCR4) in endothelial cells and promote HCC cell metastasis[240]. Based on the above findings, TECs can be directly and selectively inhibited or killed to suppress or prevent HCC metastasis and recurrence.
The ECM is a glycoprotein network that constitutes the main component of the BM and forms a natural barrier to tumor metastasis[241]. Collagen protein, proteoglycans (glycosaminoglycan, proteoglycans and hyaluronic acid) and glycoproteins (such as elastin, fibronectin and laminin) are the major components of the ECM. The ECM connects the inside and outside of the cell as a whole through membrane integrins and thereby determines the cell shape and controls cell differentiation and migration[242,243]. Whether cancer cells infiltrate and metastasize is related to the cancer cells themselves and to the ECM. A series of dynamic changes occur between cancer cells and the surrounding ECM during cancer invasion and metastasis. The remodeled ECM creates a loose microenvironment for cancer cell proliferation and differentiation, leading to cancer cell infiltration and metastasis. Moreover, cancer cells adhere to the ECM and induce the production of proteases to degrade the ECM, which allows cancer cells to break away from their primary site, enter blood vessels or lymphatic vessels, circulate, survive, and then escape the BM and ECM to form secondary metastases[244-246].
The first step of tumor metastasis involves changes in cell adhesion characteristics. These changes weaken adhesion between tumor cells and enhance adhesion between tumor cells and the ECM involving cell adhesion molecules (CAMs)[247], which are transmembrane glycoproteins that mediate cell–cell and cell–ECM adhesion. The CAMs discovered thus far can be roughly divided into five categories: Immunoglobulin superfamily (IgSF) members, cadherin family members, integrin family members, selectin family members and CD44V6[248]. Abnormal expression or functional changes in CAMs decrease (homogeneous) adhesion between tumor cells and enhance (heterogeneous) the adhesion of ECM to nontumor cells, which is the initial step of tumor invasion and metastasis[249].
IgSF: IgSF-like cell adhesion molecules are a class of molecules that contain specific immunoglobulin-specific domains, which mediate both homogenous and heterogeneous adhesion. This superfamily mainly includes intercellular adhesion molecules (ICAMs; ICAM-1, 2, 3), vascular intercellular adhesion molecules-1 and nerve cell adhesion molecules[250]. Among the IgSF members, ICAM-1 is the most closely associated with HCC metastasis and recurrence. ICAM-1 is a glycoprotein expressed on the cell surface. High ICAM-1 expression reduces adhesion between tumor cells; thus, the tumor cells can detach, enter the blood and combine with lymphocytes to escape the killing effects of immune cells. The tumor cells easily become trapped and implant in capillaries or lymph sinuses, forming metastatic foci and completing tumor invasion and metastasis[251-253]. In addition to mediating adhesion, ICAM-1 exists in serum in a soluble form called sICAM-1. sICAM-1 retains the extracellular structure of ICAM-1, binds to ligands (lymphocyte function-associated antigen-1, very late antigen-4) on the surfaces of immune cells, and thus prevents immune cells from effectively recognizing and killing the tumor cells, enabling immune evasion[254], which is conducive to tumor cell invasion and metastasis.
HCC patients with high levels of sICAM-1 have higher distant metastasis and recurrence rates than HCC patients with normal or low levels of sICAM-1[255]. These findings suggest that the level of sICAM-1 in serum or plasma can be used as an important auxiliary indicator to predict and monitor HCC metastasis and recurrence. When combined with clinical and imaging examinations, sICAM-1 detection can improve the early diagnosis rate of HCC recurrence.
Cadherin family: A cadherin is a type of CAM containing an immunoglobulin domain that is dependent on calcium ions and can mediate signaling through a signal transduction pathway. The cadherin family contains E-cadherin, N-cadherin, P-cadherin, and T-cadherin[256]; among these, E-cadherin is most closely associated with tumor invasion and metastasis. E-cadherin is a tumor invasion suppressor that inhibits tumor metastasis in multiple malignancies[257,258], prevents the shedding of primary tumor cells and inhibits tumor metastasis. E-cadherin is a critical protein in the process of EMT, and its low expression or loss can lead to EMT. Tumor cell numbers significantly increase after the loss of contact inhibition features, resulting in local tumor cell detachment and eventually leading to tumor cell infiltration into the surrounding tissues and distant metastasis[259,260]. E-cadherin is the most important molecule in mediating homogeneous adhesion between cells of the same type and is the key negative regulatory adhesion factor for metastasis[261]. Abnormal expression of E-cadherin is associated with tumor metastasis and recurrence[262,263].
A reduction in or loss of E-cadherin expression is a significant factor leading to high aggression and metastatic ability of HCC cells. Loss of E-cadherin expression may lead to weakening or loss of adhesion between homologous cells, which allows cancer cells to spread and metastasize to other sites[264,265]. The loss of E-cadherin in HCC cells can induce EMT and thus facilitate HCC invasion and metastasis[266]. In addition, E-cadherin can be regulated by the Wnt/β-catenin or PI3K/AKT/mamma- lian/mechanistic target of rapamycin pathway, leading to participation in HCC invasion and metastasis[267,268]. In particular, HCC patients with metastasis have lower E-cadherin expression than patients without metastasis, which suggests that a decrease in or loss of E-cadherin expression is strongly associated with HCC metastasis and recurrence after radical resection[269]. Therefore, E-cadherin is expected to be a useful index for predicting HCC metastasis and recurrence.
Integrins: The integrins are a group of 24 heterodimers formed by the noncovalent combination of at least 18 α and 8 β subunits[270]. Integrins can preferentially recognize and bind to different ECM ligands, cell surface ligands and water-soluble ligands, and the Arg-Gly-Asp sequence in the ECM is a common binding motif of integrins[271]. The binding of integrins to the ECM generates bidirectional signals and triggers a variety of signal transduction pathways that mainly regulate cell adhesion, invasion, migration, proliferation, survival and apoptosis[272,273]. Integrins, which are highly expressed in numerous types of malignant cells, reduce the homogeneous adhesion between tumor cells and promote the shedding of tumor cells from the primary site into the blood circulation. Moreover, integrins can enhance the adhesion between tumor cells and heterogeneous cells or protein molecules and promote tumor invasion and metastasis[274,275]. Therefore, integrins play a major regulatory role in the TME, tumorigenesis, metastasis and recurrence.
Integrins also play a critical role in HCC cell proliferation, invasion and metastasis: They are target genes of miRNAs, and they regulate intracellular signaling pathways. For example, integrin-β1 (ITGB1), an oncogene of HCC, facilitates HCC cell growth, EMT and metastasis as the target gene of miR-3653 and accelerates HCC cell invasion as the target gene of miR-1226-3p[276,277]. ITGB5, another oncogene of HCC, accelerates HCC cell invasion and metastasis as the target gene of miR-221-5p and miR-134[278,279]. In addition, the HCC oncogenes ITGB1 and integrin alpha 7 (ITGA7) promote HCC cell migration, invasion, and stemness by regulating intracellular TGF-β/Smad and protein tyrosine kinase 2-PI3K-Akt signaling, respectively[280,281]. Conversely, the tumor-suppressive ITGA9 prevents HCC cell aggressiveness and metastasis via focal adhesion kinase/c-Src tyrosine kinase-Ras-related C3 botulinum toxin substrate 1/Ras homolog family member A signaling[282].
Selectins: Selectins are members of a class of Ca2+-dependent transmembrane glycoproteins with a lectin-like domain that are mainly expressed in bone marrow-derived and endothelial cells[283]. Under normal circumstances, selectins are in an inert state, and their main physiological function is to mediate the recruitment of leukocytes to inflammatory sites or lymphoid tissues[284]. Selectins facilitate the hematogenous metastasis of cancer cells, and cancer cells mimic the selectin-mediated exudation of leukocytes from VECs to aid cancer cell extravasation and even accelerate cancer cell metastasis via the formation of tumor thrombi through adhesion between cancer cells and platelets/leukocytes[285].
Selectins are mainly expressed on the surfaces of platelets and VECs. These proteins bind with ligands on the surfaces of tumor cells to modulate the adhesion of tumor cells to platelets and VECs and thus promote tumor metastasis[286,287]. Hematogenous metastasis is the main mode of HCC metastasis. P-selectin plays a major role in this process, and the most frequent sites of extrahepatic metastasis are the lungs. The expression of P-selectin in HCC and lung metastatic tissues is significantly higher than that in adjacent normal tissues, and the mechanism may involve the adhesion of CTCs to the surface of the vascular endothelium in a manner similar to that of inflammatory cells under the action of P-selectin. This regulatory mechanism of tumor cells is very conducive to HCC infiltration and metastasis[288]. This finding suggests that the clinical characteristics of HCC with easy infiltration and hematogenous metastasis may be tightly correlated with abundant P-selectin expression and that P-selectin can be used as an index to predict HCC extrahepatic hematogenous metastasis.
CD44V6: The CD44 gene, located on chromosome 11P13, encodes a highly glycosylated cell surface transmembrane protein. As a matrix hyaluronic acid receptor, CD44 has many biological functions; for example, it activates lymphocytes, participates in signal transduction and promotes specific cell-cell and cell-matrix adhesion. CD44 has a standard form (CD44s) and variant forms (CD44vs), and CD44V6 is one of the variant forms of CD44. CD44v6 overexpression has been implicated in the occurrence, development, invasion and metastasis of multiple malignancies[289,290]. CD44v6 can facilitate HCC progression by inducing the adhesion and infiltration of cancer cells and enhancing the ability of cancer cells to break through to BM tissue. High CD44V6 expression indicates highly invasive HCC. HCC patients with low differentiation exhibit higher levels of CD44V6 expression than patients with high differentiation[291]. In addition, CD44V6 expression is related to HCC invasion and metastasis[292]. These findings suggest that higher CD44V6 expression levels in HCC are associated with a higher degree of malignancy and with stronger HCC invasion and metastasis and that CD44V6 can be used as a marker for predicting HCC metastasis and recurrence.
The degradation of the ECM and BM is a crucial step in the process of tumor invasion and metastasis. Tumor cells adhere to various components of the ECM through their surface receptors and then degrade the ECM and BM by themselves or by inducing host cells to produce a variety of proteolytic enzymes. These effects lead to the formation of a local lytic area, which constitutes the metastatic pathway of tumor cells. Cancer cells pass through the ECM and BM and enter the blood circulation to form metastatic foci. The enzymes that are currently known to be most closely related to tumor invasion and metastasis include ECM-degrading enzymes, mainly uPA and MMPs.
uPA acts primarily by binding to uPA receptors located on the cell surface. After binding, uPA can hydrolyze and shear inactive serine plasminogen to transform it into active plasmin, which can degrade ECM proteins. High expression of uPA and its corresponding regulatory molecules has been observed in HCC, and these proteins are tightly linked to HCC metastasis and recurrence[293,294].
MMPs are a superfamily of Zn2+- and Ca2+-dependent endopeptidases that can specifically degrade the ECM and vascular BM and thereby enhance vascular permeability and promote tumor invasion and metastasis. In the MMP family, MMP-2 and MMP-9 exhibit the closest relationships with tumor invasion and metastasis. MMP-2 is a type IV collagenase in MMPs that can degrade structural proteins and the BM in the ECM as well as type IV collagen in the BM, which promotes the breakthrough of exfoliated HCC cells into the ECM and through the BM barrier to form metastases[295-297]. MMP-9 can degrade the ECM, promote cancer cell penetration of the BM, and participate in multiple sequential steps of HCC invasion and metastasis[298].
After deadhesion between tumor cells, adhesion between tumor cells and the ECM, and ECM degradation, tumor cells need to be activated by cytokines and move deeply into the site of metastasis. The cytokine family is mainly composed of a large class of soluble small-molecule peptides or proteins. This family of proteins participates in the development of diseases because of the leading roles of these proteins in cellular immunity. The major cytokines consist of interferons (IFNs), ILs, TNF, chemokines, and GFs. After the removal of lesions in HCC patients, the patients’ immune function is suppressed because cytokines accumulate in the TME, and malignancies interfere with the normal expression of various cytokines to inhibit normal immune function. Thus, the immune system cannot identify and kill tumor cells during hematogenous metastasis. Hence, immune evasion is achieved, and tumor growth and metastasis are promoted[299]. In addition, prolonged exposure to inflammatory cytokines may also induce angiogenesis, DNA damage and other conditions favorable for tumor occurrence and invasion[300].
IFN-γ: The IFN family of cytokines has antitumor effects that regulate immune function. Thus far, three types of cytokines have been found, among which IFN-γ exhibits the strongest immunostimulatory activity. In HCC, IFN-γ inhibits tumor occurrence and development by inhibiting tumor cell proliferation, inhibiting tumor neovascularization, affecting the TME and promoting tumor cell apoptosis[301]. The reduced serum IFN-γ levels in HCC patients are related to metastasis and recurrence[302]. One probable reason for this link is that a low level of IFN-γ facilitates HCC cell proliferation and inhibits HCC cell apoptosis, thus promoting HCC occurrence and development[303,304]. Another reason is that T helper (Th) cells are vital immunomodulatory cells. Th1 and Th2 cells are the main subsets of Th cells. Th1 cells are the main immune regulators of tumors and primarily mediate the cellular immune response by secreting cytokines such as TNF-γ and they play a role in tumor immune defense[305]. Th2 cells primarily mediate humoral immunity, which can inhibit the Th1-cellular immune response. It has been demonstrated that a Thl/Th2 imbalance (increase in Th2 cells and decrease in Thl cells) occurs in the HCC microenvironment. Thus, the HCC microenvironment is in an immunosuppressive state[306], which enables immune evasion by tumor cells, leading to HCC metastasis and recurrence.
ILs: ILs are a class of cytokines. Numerous types of ILs with extensive regulatory functions have been discovered, and among these, IL-6, IL-8, and IL-1β play important roles in HCC metastasis and recurrence.
IL-6 is a multipotent cytokine that is closely associated with the occurrence, progression, invasion and metastasis of various malignancies[307-310]. Mechanistically, IL-6 exerts its biological effects mainly by activating the IL-6/STAT3 and IL-6/JAK2/STAT3 signaling pathways[311]. In HCC, members of the abnormally activated IL-6/STAT3 and IL-6/JAK2/STAT3 pathways, namely, p-JAK2 and p-STAT3, can cause abnormally high expression of downstream related genes and thereby promote HCC angiogenesis, invasion and metastasis[312-315]. In addition, abnormal activation of IL-6/STAT3 signaling enhances immune evasion or establishes immune tolerance through various mechanisms, and the inflammatory microenvironment further promotes HCC angiogenesis, growth, invasion and metastasis[316,317]. High serum IL-6 levels in HCC patients are related to early postoperative recurrence[317,318]. We conclude that IL-6 acts as an activator of proinflammatory cytokines in the HCC microenvironment. With the progression of HCC, IL-6 is continuously activated, and the persistently high level of serum IL-6 may reflect the severe local inflammatory response of the tumor. Tumor inflammatory infiltration may cause tumor cells to break through and eventually spread to the outside of the liver capsule. The tumor is then in the expansion stage, which is closely associated with rapid progression, heightened metastasis and an increased risk of recurrence of HCC[319,320].
IL-8 is a chemotactic inflammatory cytokine that belongs to the CXC (C is cysteine; X is any amino acid) chemokine family. The binding of IL-8 to its two corresponding receptors, CXCR1 and CXCR2, causes biological effects through the G protein signal transduction chain and induces neutrophils and lymphocytes to aggregate at the injured site, which promotes tumor cell proliferation, differentiation, metastasis and angiogenesis[321,322]. High expression of IL-8 in the tissues and plasma of HCC patients is associated with HCC cell proliferation, migration, invasion and metastasis[323,324]. Moreover, the IL8 levels in nonmetastatic HCC are markedly lower than those in metastatic cancer[325]. This finding may be due to the “squeezing” of normal hepatocytes by invasively growing HCC cells, which results in liver injury, necrosis, and a strong inflammatory reaction in the liver and directly stimulates immune cells to secrete large amounts of IL-8. Another explanation is that most HCC cells synthesize and secrete IL-8, which may affect the biological behavior and metastasis of HCC through autocrine and paracrine signaling[326]. In addition, IL-8 plays major roles in the regulation of tissue cell homeostasis and immune regulation as well as in the stimulation of angiogenesis, which is closely related to HCC intrahepatic metastasis[327]. As an inflammatory factor, IL-8 activates a variety of signaling pathways to initiate or accelerate the occurrence of EMT and ultimately promote HCC invasion and metastasis[129]. In addition, IL-8 upregulates the expression of integrin to facilitate HCC invasion and metastasis[328].
IL-1β is generated by activated mononuclear macrophages, and its cleavage by IL-1β convertase yields a mature biologically active molecule that binds to the corresponding receptors and exerts biological effects. IL-1β not only promotes the inflammatory response but also participates in biological behaviors such as migration and invasion in a variety of cells[329]. IL-1β participates in tumorigenesis, angiogenesis, invasion and metastasis by mediating multiple pathways[330,331]. IL-1β expression is increased in HCC and is related to HCC metastasis[200,332]. IL-1β participates in HCC metastasis through a variety of mechanisms. For example, IL-1β promotes HCC metastasis by upregulating homeobox C10 expression in HCC cells[333]. In addition, IL-1β, as an inflammatory cytokine, can induce the aggregation of MDSCs and TAMs, which accounts for the finding that most IL-1β is located in the TME. Thus, IL-1β plays a significant role in inducing HCC cell angiogenesis, immune regulation and metastasis[334].
TNF-α: TNF-α is a polypeptide cytokine with multiple biological activities that is produced by activated mononuclear phagocytes and is tightly associated with the immune response of the body. TNF-α, as a proinflammatory cytokine, acts as an inflammatory mediator and immune regulator and is clearly expressed in multiple malignancies. TNF-α expression is closely correlated with tumor occurrence, development, invasion and metastasis[335,336].
TNF-α facilitates HCC invasion and metastasis through several main mechanisms: (1) TNF-α is related to tumor immunity. In HCC patients, the continuous stress response can cause immune disorders. These disorders lead to continuously increased TNF-α levels and thereby affect T lymphocyte differentiation[337]; (2) TNF-α promotes the production of multiple inflammatory cytokines and chemokines, directly participates in tumor angiogenesis, creates favorable conditions for tumor growth, and promotes HCC invasion and metastasis[338]; (3) TNF-α induces EMT and maintains the stemness of HCC stem cells. TNF-α, which is clearly expressed in M2 macrophages infiltrating HCC tissues, can induce EMT and cancer stemness of HCC cells and thus promote HCC invasion and metastasis[130,339]; and (4) In some cases, TNF-α promotes HCC metastasis by activating endothelial CAMs or transcription factors. For example, the expression of endothelial CAMs induced by TNF-α can promote adhesion between tumor cells and the vascular wall and infiltration into tissues at the early stage of HCC metastasis[340]. In addition, the proinflammatory effect of TNF-α occurs mainly through the activation of NF-κB to facilitate HCC metastasis and recurrence[315]. However, some studies have shown that high concentrations of TNF-α inhibit HCC metastasis and recurrence, whereas low concentrations of TNF-α promote HCC metastasis and recurrence[341]. Thus, the effects of TNF-α on HCC cells should be further studied.
In general, TNF-α, as an important inflammatory factor, maintains the inflammatory microenvironment by acting as the main triggering factor and master switch for the transformation from inflammation to cancer and thereby facilitates tumor invasion and metastasis. Exploring the mechanisms of HCC metastasis mediated by TNF-α will help to identify therapeutic and preventive targets.
Chemokines and their receptors: Chemokines are a series of protein signaling cytokines secreted by cells, so named due to their ability to induce reactive cells to move directionally via chemotaxis. Chemokines can be divided into four categories, CXC, CC, C, and CX3C, depending on the location of the N-terminal conserved cysteine residues. Chemokine receptors are G protein-coupled transmem
CCL2/CCR2 axis: CCL2 mainly binds to its receptor CCR2 and chemotactic CCR2-positive monocytes, macrophages and tumor cells through the CCR2-CCL2 axis. Numerous studies have shown that the CCR2-CCL2 axis facilitates tumor cell growth, survival, angiogenesis, invasion and metastasis[345,346]. CCL2/CCR2 promotes HCC metastasis through multiple main mechanisms: (1) CCL2/CCR2 promotes HCC metastasis directly or by recruiting TAMs. The chemokines CCL2 and CCR2 play significant roles in recruiting TAMs to participate in tumor invasion and metastasis[346]. High expression of the CCL2/CCR2 axis can promote HCC angiogenesis and metastasis, and the mechanism of action is related to the recruitment of TAMs by this axis[347,348]; and (2) EMT is induced by the activation of signaling pathways. EMT may be a key step in the promotion of tumor invasion and metastasis by the CCL2-CCR2 axis. The CCL2-CCR2 axis induces EMT by activating the Hedgehog pathway and promotes HCC metastasis[349].
CXCL12-CXCR4 axis: CXCR4, a specific G protein-coupled receptor (GPCR), has high affinity for its ligands. CXCL12 is the natural ligand of CXCR4. The CXCL12/CXCR4 axis can initiate a variety of signaling pathways, leading to gene transcription and mediating cell chemotaxis, cell survival and proliferation. The CXCL12-CXCR4 axis is related to the directional metastatic ability of malignancies[350-352] and is associated with neovascularization and distant metastasis in HCC[240,353]. High MMP-9 and MMP-2 expression levels are often used as indices to detect the invasion and metastasis of malignancies[354]. The interaction between CXCR4 and CXCL12 can mediate the secretion of MMP-9 and MMP-2, accelerate the degradation of the ECM, and promote HCC metastasis[355]. In addition, a synergistic effect has been noted between CXCR4 and MMP-9, and a positive correlation between the expression of these two proteins has been observed in HCC. High expression of both CXCR4 and MMP-9 indicates that HCC exhibits metastatic activity[356]. The combined detection of these molecules can be used as an important index for assessing the lymph node and distant metastasis of HCC.
The above findings indicate that the CCL2-CCR2 and CXCL12-CXCR4 axes are closely associated with HCC metastasis and recurrence and suggest that targeting these axes may be a new strategy for restraining HCC metastasis.
GFs: The HCC microenvironment contains many types of GFs; among these, VEGF and TGF-β are closely associated with HCC metastasis and recurrence.
Neovascularization promotes tumor growth, infiltration and metastasis and causes tumor recurrence[357]. VEGF can increase vascular permeability and facilitate VEC proliferation and angiogenesis[358]. The increased expression of VEGF in malignancies can result in degradation of the matrix of VECs, weakening of the vascular barrier, vascular infiltration of cancer cells, and spread of cancer cells through the bloodstream, which results in the induction of tumor metastasis[359,360]. The proliferation, invasion and metastasis of HCC cells depend on massive neovascularization within the tumor. VEGF has been confirmed to be related to postoperative HCC metastasis and recurrence[361,362]. VEGF, as a crucial regulatory factor in angiogenesis, plays a regulatory role in the proliferation, migration and vascular construction of HCC VECs. In addition to promoting angiogenesis, VEGF can increase the permeability of the vascular endothelium, making it easier for tumor cells to invade and penetrate blood vessels and consequently facilitating tumor invasion and metastasis. Therefore, the increased expression of VEGF in HCC tissues is an important reason for the high vascular invasion and the high incidence of postoperative recurrence and metastasis of HCC[363-365]. Of note, VEGF not only acts as an important proangiogenic GF but also affects the functions of various immunosuppressive cells and participates in tumor immune evasion. VEGF is involved in mediating the immunosuppressive HCC microenvironment[366,367]. Mechanistically, the concentration of VEGF in the serum of HCC is proportional to the number of MDSCs[368]. VEGF secreted by HCC cells can act as a chemokine, guiding MDSCs to accumulate and infiltrate locally into the tumor. Moreover, VEGF can affect the antigen presentation of DCs by inhibiting DC differentiation and maturation and subsequently influencing the activation and expansion of CTLs and the sensitivity of tumor cells to CTL-mediated killing[369,370]. HCC cell immune evasion is an important factor promoting metastasis[371]. Therefore, the above findings indicate that VEGF-mediated immune evasion can promote HCC metastasis.
TGF-β: TGF-β is a polypeptide cytokine with extensive biological effects. TGF-β plays two different roles in tumor occurrence and development. Specifically, TGF-β exhibits tumor-inhibitory or tumor-promotive activity that modulates the expression of oncogenes and tumor suppressor genes[372]. TGF-β can exert its antitumor role at the early stage of HCC by promoting hepatocyte cell cycle arrest and apoptosis. In the late stage of HCC, TGF-β induces changes in the microenvironment through various mechanisms, e.g., by acting as an autocrine or paracrine GF, and facilitates HCC metastasis and recurrence by promoting immune evasion, angiogenesis, and the acquisition of tumor stem cell-like characteristics by HCC cells and by inducing EMT. TGF-β can stimulate fibroblasts to transform into CAFs and can promote or induce HCC immune evasion by upregulating the expression of Tregs[373,374], providing a good internal environment for HCC metastasis and growth and thus promoting and accelerating HCC growth and metastasis. The TGF-β pathway is closely associated with tumor angiogenesis; specifically, the TGF-β/VEGF-related pathway is an important pathway of tumor angiogenesis. In addition, TGF-β can significantly increase VEGF/VEGF-R expression, promote capillary formation and the migration of endothelial cells, and lead to tumor cell angiogenesis, thereby promoting HCC invasion and metastasis[375]. TGF-β can lead to HCC metastasis by inducing tumor cells to undergo EMT[376,377]. TGF-β enhances the ability of HCC cells to acquire hepatic stem cell-like characteristics and thus promotes HCC growth and invasion[378]. In summary, TGF-β is closely related to HCC invasion and metastasis and may be a target for controlling metastasis in HCC.
Exosomes are membranous vesicles containing all types of bioactive substances, such as proteins, lipids, and nucleic acids, and they closely participate in the exchange of information and substances between cells[379,380]. Tumor-derived exosomes are important carriers for bidirectional communication between the TME and tumor cells and participate in TME remodeling processes, particularly in tumor angiogenesis and metastasis[381,382]. In addition, tumor-derived exosomes can induce metastatic target organs to form PMNs and promote tumor metastasis by stimulating neovascularization after the colonization of CTCs[383,384].
HCC-derived exosomes facilitate HCC metastasis and recurrence through the following mechanisms (Figure 3): (1) HCC-derived exosomes promote HCC angiogenesis. Tumor-derived exosomes participate in TME remodeling and release bioactive components such as cytokines, thereby inducing angiogenesis and facilitating tumor metastasis[385]. For example, HCC-derived exosome transfer lysyl oxidase-like 4 can accelerate HCC angiogenesis[386]. Vasculogenic mimicry (VM) is a newly discovered tumor microcirculation pattern that does not involve endothelial cells. Tumor cells interact with the ECM through their own morphological changes to form a pipeline system that can transport blood and connect with host blood vessels to remodel the tumor microcirculation[387,388]. VM occurs widely in multiple human malignancies and is closely related to tumor progression, invasion, metastasis and recurrence[389,390]. HCC-derived exosomes can promote HCC angiogenesis and VM and thus facilitate HCC metastasis[391]; (2) HCC-derived exosomes inhibit the host immune response to promote immune evasion. The 14-3-3ζ protein leads to T-cell exhaustion via HCC-derived exosomes[392]. In addition, endoplasmic reticulum stress can induce HCC cells to release exosomes that are rich in miRNA-23a-3p and enhance PD-L1 expression in macrophages by inhibiting the phosphatase and tensin homolog (PTEN) pathway and activating the AKT pathway, which results in the inhibition of T cells and mediation of immune evasion[393]; (3) HCC-derived exosomes promote EMT. Tumor-derived exosomes act as transporters of EMT initiation signals and transmit metastatic signals to metastatic tumor cells, resulting in EMT and metastasis[394]. For example, the expression of exosomal circ-0004277 secreted by HCC cells is upregulated, which induces EMT through cellular communication and promotes HCC intrahepatic metastasis[395]. HCC-derived exosomal miR-1307-5p also promotes HCC metastasis by inducing EMT[396]. Furthermore, the tumor metastasis induced by a hypoxic microenvironment is closely related to exosomes because exosomes produced by tumor cells under hypoxic conditions are enriched with EMT signaling molecules. For example, HCC-derived exosomes produced in a hypoxic environment are rich in miR-1273f, which can activate the Wnt/β-catenin pathway and induce EMT[397]. The mechanisms through which metastasis is promoted are relatively clear. However, whether all HCC-derived exosomes can promote EMT remains to be further explored; (4) HCC-derived exosomes promote ECM remodeling. The most important feature of ECM remodeling is the change in the molecular composition of the ECM. The remodeled ECM creates a permissive microenvironment for tumor cell proliferation and differentiation, which leads to tumor cell growth, infiltration, and metastasis[241,398]. In addition, CAFs are important cells in ECM remodeling and participate in the homeostatic maintenance of tissue ECM synthesis and degradation through cell–cell communication[399]. Exosomes produced by HCC cells participate in ECM remodeling by activating CAFs. For example, HCC cell-derived exosomal miR-1247-3p promotes fibroblast activation by directly acting on its target B4GALT3, and activated CAFs facilitate the lung metastasis of HCC by secreting proinflammatory cytokines, such as IL-6 and IL-8[229]. In addition, HCC-derived exosomal miRNA-21 directly targets PTEN, resulting in the activation of the inositol 3-phosphate dependent PDK1/serine threonine kinase (Akt) signaling pathway in normal HSCs, which can be transformed into CAFs. Activated CAFs can increase the density of blood vessels, remodel the ECM, and subsequently promote tumor metastasis[215]; and (5) HCC-derived exosomes promote the formation of PMNs before metastasis. The PMN is a microenvironment that is ready for tumor colonization and spread in distant organs. Studies have shown that the construction of specific microenvironments in potential metastatic target organs is an important reason why tumor metastasis occurs in specific organs[400]. Currently, distal cell–cell communication mediated by primary tumor cells through their exosomes is generally believed to be fundamental to the formation of PMNs in potential metastatic target organs[401]. Recent evidence has revealed that exosomes can also promote the establishment of PMNs in HCC[402,403]. For example, miR-103, an HCC cell-derived exosomal miRNA, enhances vascular permeability by targeting various endothelial connexins in endothelial cells, and changes in vascular changes promote the establishment of PMNs and thereby facilitate HCC metastasis[404].
Numerous clinical studies have confirmed the potential utility of exosomes as indicators for predicting HCC metastasis and recurrence. HCC patients with increased serum levels of exosomal miR-638 exhibit increased metastatic potential and an increased risk of recurrence[403]. Exosome-derived miR-1307-5p is highly expressed in preoperative plasma and can be indicative of HCC metastasis and recurrence[396]. In addition, exosome-derived differentiation-antagonizing nonprotein-coding RNAs are linked to postoperative HCC recurrence[405].
The specific mechanisms of exosomes in HCC invasion and metastasis have not been clearly defined to date. Future research should focus on the mechanisms of exosome generation and the specific signal transmission process of exosomes in HCC to obtain new findings regarding the roles of exosomes in HCC metastasis and recurrence. Exosome treatment may be an effective HCC treatment strategy in the future.
EMT is a process through which cells change from an epithelial state to a mesenchymal state, leading to migration and invasive behavior. As the initial step mediating tumor invasion and metastasis, EMT is often accompanied by changes in various marker proteins and transcription factors. Functional loss of E-cadherin and cytokeratins and upregulated expression of N-cadherin and vimentin are frequently observed in tumor cells undergoing EMT[406]. Cells undergoing EMT exhibit alterations in morphology and function, loss of epithelial–basal polarity and reductions in cell–cell connections, and these cells can penetrate the BM and interstitial tissue as well as the circulatory system to travel to distant tissues[407]. Therefore, EMT is an important cellular event during tumor invasion and metastasis. Increasing evidence indicates that EMT is strongly associated with tumor migration, invasion, metastasis and recurrence and that tumor cells can acquire the ability to invade and metastasize through EMT[408-411].
EMT plays a leading role in HCC metastasis and recurrence. Loss of E-cadherin expression and increases in N-cadherin and vimentin expression are closely related to HCC metastasis[412-414]. E-cadherin and vimentin form a complex between epithelial cells that can prevent tumor cell metastasis and invasion. In contrast, EMT occurs in malignant tumor cells, allowing the cells to separate from each other and thereby improving the migration ability of the cells[415,416]. A lack of E-cadherin expression and an increase in vimentin expression are the two most important markers of EMT that can predict the recurrence of HCV-associated HCC[417]. EMT is also an important mechanism through which many oncogenes promote HCC invasion, metastasis and recurrence. For example, the oncogenes vasodilator-stimulated phosphoprotein, thrombospondin 4, GPCR-kinase interacting protein-1, and lysyl oxidase promote HCC invasion, metastasis and recurrence by inducing EMT[418-420]. Studies have found that HCC cells with EMT are more likely to acquire stem cell characteristics and have stronger self-renewal and invasion capabilities than ordinary HCC cells and that these cells are closely associated with early HCC recurrence[421-423]. In addition, EMT can induce the release of CTCs from HCC cells, and CTCs are important indicators predicting HCC recurrence and poor prognosis[424,425].
In conclusion, EMT is a dynamic evolutionary process and an important initial stage of HCC metastasis. Elucidating the molecular mechanisms of EMT in HCC cells will be conducive to finding new targeted drugs to reverse EMT, which will provide new insights into the clinical targeted regulation of HCC.
CSCs are subsets of tumor cells with self-renewal ability and stem cell characteristics. CSCs have the ability to generate heterogeneous tumors, are highly invasive, and easily metastasize and develop multiple resistance properties, and they can accordingly drive tumor growth, metastasis and recurrence[426]. Increasing evidence indicates that LCSCs exist in HCC and drive HCC initiation, metastasis, drug resistance and recurrence. Currently, the molecules used to as LCSC markers include CD133, CD90, EpCAM, CD13, CD44, and acetaldehyde dehydrogenase[427]. Among them, CD133, EpCAM and CD90 are closely associated with HCC metastasis and recurrence. The expression of these markers indicates HCC with strong aggressiveness and metastasis[428].
CD133 is a transmembrane protein composed of five transmembrane regions and two large glycosylated cell outer rings located on the cell surface. CD133 is expressed on the surfaces of stem cells and various tumor stem cells[429,430]. CD133+ tumor cells are closely associated with tumor occurrence, invasion, metastasis, and recurrence[431,432]. Currently, CD133 is widely regarded as a specific surface marker of LCSCs. CD133+ LCSCs are strongly associated with HCC metastasis and early recurrence[55,363].
EpCAM, a transmembrane glycoprotein involved in cell adhesion, has the properties of an oncogene messenger and thus facilitates cell proliferation and tumor formation[433]. The expression level of EpCAM protein in HCC tissues, particularly in HCC metastases, is higher than that in normal liver tissues[434,435]. These findings suggest that EpCAM+ cells have strong characteristics of tumor stem cells, participate in HCC cell metastasis and invasion, and can be used as stem cell markers for the acquisition of metastatic ability by HCC cells. In addition, the positive expression rate of EpCAM protein in HCC patients with recurrence after surgery is significantly higher than that in patients without recurrence[436], and HCC patients with high EpCAM expression are more prone to relapse than those with low EpCAM expression[437]. These findings suggest that EpCAM is a predictor of postoperative HCC recurrence.
CD90, a surface marker of oval cells, is a glycosylphosphatidylinositol-anchored glycoprotein with a molecular weight of 25-37 kDa that is expressed in T cells, thymocytes, neurons, endothelial cells, fibroblasts and other cell types. In recent years, CD90 has been used as a marker of LCSCs. CD90 is associated with the high metastatic potential of HCC[438-440], and HCC patients with high CD90 expression have exacerbated pathological differentiation and an increased chance of postoperative recurrence[363,441], which suggests that CD90 may play a significant role in HCC metastasis and recurrence.
In summary, although studies have found that many LCSC-related markers are strongly associated with HCC metastasis and recurrence, the specificity remains low. In addition, although therapeutic methods targeting CSCs have great potential, specific markers are currently lacking. Therefore, improving the sorting and identification of LCSCs and continuing to identify and screen specific LCSCs represent future research directions in the field of LCSCs. In addition, given the complex and variable heterogeneity and plasticity of CSCs[442,443], obtaining a further understanding of the heterogeneity and plasticity of CSCs and the mechanisms through which they drive tumor metastasis and recurrence will greatly promote the development of new safe and effective therapeutic strategies, regimens and drugs targeting CSCs.
CTCs are tumor cells that are shed from primary or metastatic foci and enter the bloodstream to reach distant organs, where they may trigger metastasis. The number, morphology and phenotype of CTCs are closely and fundamentally related to tumor metastasis and recurrence[444,445]. The formation of metastatic tumors is a process of “natural selection”: Only a small proportion of potential cancer cells passively or actively detach from the primary tumor, enter the lymphatic or blood circulation to form CTCs, evade immune surveillance, and then reach target organs to form metastatic tumors. This process is complex, and although the mechanisms are not completely clear, the basic process has been outlined. First, an association between EMT and the TME exists. After cancer cells undergo EMT, upregulation of the expression of MMPs accelerates ECM degradation, resulting in the detachment of cancer cells from the lesion and the transformation of these cells into CTCs[446]. Then, CTCs typically penetrate the vascular system in the capillary bed and colonize the premetastatic microenvironment after they arrive at the target organ via the circulatory system. This process is also called metastatic dissemination, and the cells that colonize the premetastatic microenvironment are called disseminated tumor cells (DTCs)[447]. Under unfavorable conditions (such as when immune function remains intact), DTCs can be transferred and remain in a dormant state for a long time under the combined actions of many internal and external factors[448,449]. Dormant DTCs often exhibit CSC-like phenotypes and directly bind to other cells, such as CAFs and neutrophils. These cells then attach to the inner walls of blood vessels and not only resist mechanical damage or immune attack but also facilitate cancer cell dissemination through the vascular system and colonization of distal metastatic target organs at an appropriate time[450,451]. Under certain circumstances, dormant DTCs can be awakened and can rapidly and clonally grow to form metastatic tumors[452,453].
CTCs exhibit obvious phenotypic heterogeneity. Among the many phenotypes of CTCs, only a few have “seed” characteristics, that is, resistance to anoxic apoptosis, exudation, transformation into disseminated cells, high adaptability to PMNs, and the ability to induce angiogenesis and matrix formation during metastasis[454,455]. During the process of metastasis, CTCs undergo various phenotypic changes, among which EMT is the most important, to escape immune clearance or acquire the ability to resist anoxic apoptosis and adapt to the new living environment[456]. EMT is an important mechanism through which CTCs survive and acquire aggressive and metastatic abilities[457]. The EMT process can alter the tight connections and adhesion between tumor foci cells, and CTCs with invasive and metastatic potential often exhibit EMT characteristics[448]. CTCs with an EMT phenotype are closely related to HCC metastasis or recurrence[458,459]. Given the occurrence of EMT during HCC progression, EMT may be a crucial factor influencing the survival and metastatic ability of CTCs. Alternatively, CTCs, as cells with high activity and high metastatic potential that are present in the circulatory system, may possess CSC characteristics. CSCs persist in tumor tissues and are considered specific cell types that promote tumor metastasis and recurrence. The small fraction of tumor metastasis-initiating cells among CTCs that eventually lead to metastasis are a class of tumor stem cells or at least tumor cells with multiple stem cell characteristics[460]. Because CTCs exhibit EMT and CSC characteristics, interstitial CTCs are generally considered more capable of forming metastatic foci than other cell types. CTCs with CSC characteristics are closely related to HCC vascular invasion, metastasis and recurrence[461]. Given that CTCs have CSC characteristics during HCC progression, the CSC phenotype may be an important feature by which CTCs persist in the blood circulation for a long time and maintain high invasion and metastasis abilities. Hematogenous metastasis is a common form of HCC metastasis. The CTC levels in the blood of HCC patients are directly related to prognosis, and patients with large numbers of CTCs have a poor prognosis[462]. A total of 30.5%-93.3% of HCC patients have CTCs in the peripheral circulatory system that cause distant metastasis, which is the key event responsible for metastasis and recurrence[424,463,464]. Therefore, high levels of CTCs can be used to predict and evaluate the risk of HCC metastasis and recurrence.
The majority of previous studies on CTCs have concentrated on the characteristics of CTCs and ignored the overall relationships between CTCs and the primary tumor and between CTCs and the TME and internal environment. The regulation of the biological behavior of CTCs by an imbalance in the tumor environment may represent an important mechanism of HCC metastasis and recurrence. CTCs can survive in the blood circulation and eventually proliferate to form metastatic foci. This process is inseparable from the maintenance and regulation of GFs and corresponding signaling pathways, and research in this field may become key to further elucidating the mechanisms of HCC metastasis and recurrence.
Epigenetic modifications are heritable phenotypic changes unrelated to DNA mutations and are some of the key drivers of HCC metastasis and recurrence[465]. The main epigenetic regulatory mechanisms include DNA methylation, histone modification, genomic imprinting, chromatin remodeling, and ncRNA activity. Among these, DNA methylation and ncRNA-mediated modifications are the most studied epigenetic modifications associated with postoperative HCC metastasis and recurrence. These factors provide clues supporting an in-depth understanding of the biological functions of epigenetic events implicated in HCC metastasis and supporting the development of novel approaches and strategies for the treatment of HCC.
DNA methylation selectively adds methyl groups to cytosine residues in certain regions (CpG islands). Specifically, the cytosine 5' carbon position of the genomic CpG dinucleotide is covalently bonded to a one-carbon-unit methyl group provided by S-adenosylmethionine under catalysis by DNMTs[466,467]. An altered DNA methylation status characterized by a decrease in the overall methylation level and an increase in the local methylation level is prevalent in tumors. In tumor cells, oncogenes are activated in the state of hypomethylation, whereas tumor suppressor genes are inhibited in the state of hypermethylation[468]. In addition to point mutations and gene deletions, some tumor suppressor genes exhibit hypermethylation in the promoter region. This inhibits the transcription of tumor suppressor genes, resulting in inactivation of these genes and an inability to exert tumor-suppressive effects. Thus, hypermethylation has been implicated in tumor occurrence, invasion and metastasis[469,470]. The hypomethylation of specific genes can induce the activation of oncogenes and facilitate tumor growth and metastasis[471]. DNA hypermethylation and hypomethylation are strongly correlated with tumor development, invasion and metastasis.
During DNA methylation, DNMTs are needed to maintain the methylation state and function as key enzymes. DNMT expression is typically increased in malignancies; thus, DNMTs are important causes of the hypermethylation of a series of tumor suppressor genes[472,473]. The epigenetic alterations mediated by DNMTs regulate HCC invasion and metastasis[474,475]. Emerging evidence confirms that abnormal methylation of many genes is closely correlated with HCC metastasis and recurrence after surgery. For example, hypermethylation of the tumor suppressor genes SPG20, TERT and CCND2 is tightly associated with HCC metastasis and recurrence[476-478]. In contrast, hypomethylation of the oncogenes GTPBP4 and CTCFL is closely related to HCC metastasis and recurrence[479,480].
Collectively, the evidence from studies on the methylation of HCC-related genes will provide new insights to help predict HCC metastasis and recurrence and to develop targeted therapies.
LncRNAs: LncRNAs are a class of RNA molecules without protein-coding functions. LncRNAs can promote or inhibit tumor invasion and metastasis through multiple molecular mechanisms[481,482]. Tumor metastasis may alter lncRNA expression[482]. Therefore, lncRNAs that are dysregulated during tumor metastasis are potential molecular markers of tumor metastasis.
Mounting evidence suggests that lncRNAs are widely involved in HCC invasion and metastasis. LncRNAs can participate in regulating gene expression levels at various levels, such as epigenetic modification, transcription, posttranscription, and translation, and thereby affect HCC cell invasion and metastasis. LncRNAs play two different roles in HCC invasion and metastasis. Oncogenic lncRNAs promote invasion and metastasis[405], while tumor suppressor lncRNAs inhibit invasion and metastasis[483]. LncRNAs participate in HCC invasion and metastasis through multiple mechanisms (Figure 4): (1) LncRNAs regulate tumor angiogenesis, which is of great importance to tumor invasion and metastasis. For example, the oncogenic lncRNA OIP5-AS1 and the lncRNA noncoding RNA activated by DNA damage affect VEGF-A expression by competitively binding miR-3163 and miR-211-5p, respectively, ultimately inducing HCC angiogenesis and promoting HCC invasion and metastasis[484,485]. In contrast, the tumor suppressor lncRNA miR503HG suppresses angiogenesis and thereby inhibits HCC invasion and metastasis[486]; (2) LncRNAs regulate CSCs. Specific lncRNAs are abnormally expressed in LCSCs, and the stemness characteristics of these lncRNAs are regulated at different molecular levels. For example, the oncogenic lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) can bind to miR-124 via a competing endogenous RNA (ceRNA) mechanism, which means that ncRNA with a miRNA response element can relieve the miRNA-based inhibition of the target mRNA by competitively combining with the miRNA, and thereby regulate the characteristics of HCC stem cells induced by HBx through the PI3K/Akt pathway[487]. Moreover, the lncRNA MALAT1 is clearly expressed in LCSCs and facilitates the display of CSC characteristics by HCC cells by sponging miR-375 to enhance Yes-associated protein 1 expression[488]. Furthermore, the oncogenic lncRNA H19 facilitates the transformation of HCC stem cells by sponging miR-193b to enhance MAPK1 expression and thereby promotes HCC metastasis[489]; (3) LncRNAs regulate EMT, a vital step in initiating tumor metastasis. After undergoing EMT, HCC cells are primed to break through the BM and invade blood vessels or lymphatic vessels to achieve invasion and metastasis. LncRNAs are key regulators of EMT in HCC. The regulation of EMT by lncRNAs is mainly realized by ceRNAs, which are key elements among EMT-related miRNAs, or via regulation of the activity of EMT-related transcription factors. For example, the expression of the oncogenic lncRNA SNHG3 can induce EMT through miR-128/CD151 activation and thus promote HCC invasion and metastasis[490]. Conversely, the expression of the tumor suppressor lnc-Ma301 is reduced in HCC cells, which reduces the probability of HCC lung metastasis by participating in the suppression of EMT progression and even reversing EMT progression in HCC cells[491]; (4) LncRNAs participate in ECM remodeling. For example, the oncogenic lncRNA LINC01615 and the lncRNA SNHG16 promote HCC invasion and metastasis by participating in ECM remodeling[492,493]; and (5) LncRNAs participate in HCC cell immune evasion. Mounting evidence suggests that lncRNAs participate in TIME formation and thus affect tumorigenesis and metastasis[494,495]. For example, the oncogenic lncRNA lnc-epidermal growth factor receptor facilitates Treg differentiation, inhibits CTL activity, and thus promotes HCC cell immune escape and tumor metastasis[496].
Research on lncRNAs remains in its infancy, and a large number of lncRNAs associated with HCC metastasis have not yet been identified. In addition, although existing evidence reveals the important roles and some mechanisms of lncRNAs in HCC invasion and metastasis, research on the mechanisms of such lncRNAs remains relatively limited. Most studies have tended to investigate the effect of a single pathway on HCC invasion and metastasis, but these processes are complex and involve interactions among multiple factors. The next step should be to systematically study the involvement of lncRNAs in multiple pathways to jointly regulate HCC invasion and metastasis. This information will promote the use of lncRNAs as promising markers for the early prediction of HCC metastasis and recurrence. In addition, lncRNAs offer multiple effective therapeutic targets for HCC; thus, lncRNA research can provide a new direction and new treatments to prevent HCC metastasis and recurrence.
CircRNAs: CircRNAs are closed circular covalent RNA structures whose 5' and 3' ends are connected by nonclassical splicing. CircRNAs are highly conserved and exhibit tissue specificity that is not affected by RNA exonuclease. Recent studies have shown that circRNAs promote or inhibit tumor metastasis through multiple mechanisms[497,498].
In HCC, circRNAs facilitate or inhibit invasion and metastasis through several main mechanisms: (1) CircRNAs affect the stemness transition. The oncogenic circRNA circIPO11 facilitates the self-renewal of LCSCs through activation of the Hedgehog pathway and thus accelerates HCC invasion and metastasis[499]; (2) CircRNAs affect EMT. The oncogenic circRNA hsa_circ_0000092 binds miR-338-3p to upregulate downstream HN1 expression by reducing miR-338-3p expression and thus accelerating the EMT process of HCC and promoting HCC migration, invasion and metastasis[500]. The expression of the tumor-suppressive circRNA circSETD2 is reduced in HCC tissues, and overexpression of this circRNA restrains EMT occurrence in HCC cells and thereby inhibits HCC cell invasion and metastasis[501]; (3) CircRNAs affect ECM degradation. The tumor suppressor cSMARCA5 (hsa_circ_0001445) inhibits ECM degradation by affecting TIMP3 expression and thereby inhibits HCC cell invasion and ultimately tumor metastasis[502]; (4) CircRNAs affect tumor angiogenesis. For example, exosomal circ-100338 enhances the angiogenic capacity and permeability of HCC cells and promotes HCC metastasis[391]. In contrast, the tumor suppressor circ_0004018 inhibits HCC angiogenesis and thus HCC metastasis[503]; and (5) CircRNAs regulate tumor immune evasion. Oncogenic circRNAs can help tumor cells evade immune surveillance through multiple pathways. Hsa-circ-0003288, which is highly expressed in HCC cells, induces tumor immune evasion and promotes HCC metastasis by upregulating PD-L1 expression[504]. Hsa_circ_0048674 may lead to immunosuppression by inhibiting the cytotoxicity of NK cells toward HCC cells and thereby promote HCC metastasis[505].
In summary, research on circRNAs in the field of HCC metastasis remains in its infancy. Although the existing evidence indicates that circRNAs are associated with HCC metastasis, clinical research results remain lacking. Further in-depth investigation of the mechanisms of circRNAs in HCC metastasis will provide new strategies to combat HCC metastasis.
MiRNAs: MiRNAs are a group of endogenous ncRNAs with a length of 18 to 24 nucleotides. MiRNAs play significant regulatory roles in the expression of numerous protein-coding genes and participate in cancer cell proliferation, differentiation, angiogenesis and metastasis[506,507].
Emerging evidence suggests that miRNAs play significant roles in HCC metastasis and recurrence. MiRNAs mainly affect HCC metastasis via several mechanisms: (1) MiRNAs regulate EMT occurrence in HCC cells: miRNAs can either inhibit or promote EMT-mediated HCC invasion and metastasis by regulating signaling pathways and transcription factors. For example, the tumor suppressor miR-3194-3p inhibits the occurrence of EMT by inactivating Wnt/β-catenin signaling and thereby inhibits HCC metastasis and recurrence[508]. The tumor suppressor miR-509-3p prevents EMT by decreasing the expression of the transcription factor JDP2 to inhibit HCC invasion and metastasis[509]; (2) MiRNAs affect tumor angiogenesis. The oncogenic miRNA miR-1290 facilitates HCC angiogenesis and thus promotes HCC invasion and metastasis[510]. In contrast, the tumor suppressor miR-325-3p restrains HCC angiogenesis and thereby inhibits HCC invasion and metastasis[511]; and (3) MiRNAs modulate ECM degradation. The tumor suppressor miR-324-5p inhibits ECM degradation by reducing MMP2 and MMP9 activity, inhibiting invasion and metastasis in HCC[512].
Although miRNAs have been confirmed to participate in HCC metastasis and recurrence, the specific mechanisms of these functions need to be further studied. MiRNAs are expected to become new targets for the prediction and treatment of HCC metastasis and recurrence.
Postoperative HCC metastasis and recurrence are complicated processes involving multiple steps, links, factors, and molecules as well as interactions among the body, tumor tissues and the TME. Clinical studies have found that tumor size, number, vascular invasion, and disseminated foci are important prognostic factors of HCC, but clinical practice suggests that common clinicopathological features, such as the size and number of HCC tumors, cannot individually accurately predict the potential of tumor metastasis and recurrence. Rather, the biological characteristics of tumors may be the real key factors in metastasis and recurrence. Previous studies have reported numerous molecular markers associated with HCC metastasis and recurrence, but the current studies primarily involve single-factor and single-molecule research models and focus on the expression of a few genes and proteins. In addition, the sample sizes of these studies are limited, and accurately reflecting the molecular characteristics underlying HCC metastasis and recurrence is difficult. Thus far, there are no indicators that can be used to clinically predict HCC metastasis and recurrence. Therefore, additional experiments and clinical verification are needed to clarify the specific molecular mechanisms of HCC metastasis and recurrence. In the process of HCC treatment, some unavoidable objective factors, internal factors of the HCC tissue itself, and immunosuppression are encountered. These factors work together to make HCC metastasis and recurrence a bottleneck that severely restricts the efficacy of surgical resection. Detailed elucidation of the molecular mechanisms underlying HCC metastasis and recurrence will strengthen our understanding of HCC and help us to more accurately grasp the mechanisms of HCC metastasis and recurrence. Such knowledge will be crucial for the identification of new therapeutic targets.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ren G, United States; Vij M, India S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Byrd K, Alqahtani S, Yopp AC, Singal AG. Role of Multidisciplinary Care in the Management of Hepatocellular Carcinoma. Semin Liver Dis. 2021;41:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 3874] [Article Influence: 968.5] [Reference Citation Analysis (3)] |
| 3. | Sugawara Y, Hibi T. Surgical treatment of hepatocellular carcinoma. Biosci Trends. 2021;15:138-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 4. | Pan YH, Chen J, Sun C, Ma JF, Li X. Effect of Ras-guanine nucleotide release factor 1-mediated H-Ras/ERK signaling pathway on glioma. Brain Res. 2021;1754:147247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Qiu XY, Hu DX, Chen WQ, Chen RQ, Qian SR, Li CY, Li YJ, Xiong XX, Liu D, Pan F, Yu SB, Chen XQ. PD-L1 confers glioblastoma multiforme malignancy via Ras binding and Ras/Erk/EMT activation. Biochim Biophys Acta Mol Basis Dis. 2018;1864:1754-1769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 6. | Saeidi S, Kim SJ, Han HJ, Kim SH, Zheng J, Lee HB, Han W, Noh DY, Na HK, Surh YJ. H-Ras induces Nrf2-Pin1 interaction: Implications for breast cancer progression. Toxicol Appl Pharmacol. 2020;402:115121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Sui G, Ma X, Liu S, Niu H, Dong Q. Study of the correlation between H-ras mutation and primary hepatocellular carcinoma. Oncol Lett. 2012;4:779-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Deshmukh AP, Vasaikar SV, Tomczak K, Tripathi S, den Hollander P, Arslan E, Chakraborty P, Soundararajan R, Jolly MK, Rai K, Levine H, Mani SA. Identification of EMT signaling cross-talk and gene regulatory networks by single-cell RNA sequencing. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 9. | Ma C, Yang Y, Xu L, Tu W, Chen F, Wang J. Rce1 suppresses invasion and metastasis of hepatocellular carcinoma via epithelial-mesenchymal transition induced by the TGF-β1/H-Ras signaling pathway. J Cell Physiol. 2020;235:2506-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Icer MA, Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem. 2018;59:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 403] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 11. | Streuli I, Santulli P, Chouzenoux S, Chapron C, Batteux F. Serum Osteopontin Levels Are Decreased in Focal Adenomyosis. Reprod Sci. 2017;24:773-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Senbanjo LT, AlJohani H, Majumdar S, Chellaiah MA. Characterization of CD44 intracellular domain interaction with RUNX2 in PC3 human prostate cancer cells. Cell Commun Signal. 2019;17:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Shi L, Hou J, Wang L, Fu H, Zhang Y, Song Y, Wang X. Regulatory roles of osteopontin in human lung cancer cell epithelial-to-mesenchymal transitions and responses. Clin Transl Med. 2021;11:e486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Nazarizadeh A, Alizadeh-Fanalou S, Hosseini A, Mirzaei A, Salimi V, Keshipour H, Safizadeh B, Jamshidi K, Bahrabadi M, Tavakoli-Yaraki M. Evaluation of local and circulating osteopontin in malignant and benign primary bone tumors. J Bone Oncol. 2021;29:100377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Xu C, Yuan Q, Wang W, Chi C, Zhang Q, Li L, Yang R, Wang Y. Prognostic significance of serum osteopontin levels in small cell lung cancer. BMC Pulm Med. 2020;20:235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Desert R, Ge X, Song Z, Han H, Lantvit D, Chen W, Das S, Athavale D, Abraham-Enachescu I, Blajszczak C, Chen Y, Musso O, Guzman G, Hoshida Y, Nieto N. Role of Hepatocyte-Derived Osteopontin in Liver Carcinogenesis. Hepatol Commun. 2022;6:692-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Iqbal J, Sarkar-Dutta M, McRae S, Ramachandran A, Kumar B, Waris G. Osteopontin Regulates Hepatitis C Virus (HCV) Replication and Assembly by Interacting with HCV Proteins and Lipid Droplets and by Binding to Receptors αVβ3 and CD44. J Virol. 2018;92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Wang H, Guo D, Li J, Wei B, Zheng H. Increased expression of osteopontin indicates poor prognosis in hepatocellular carcinoma. Int J Clin Exp Pathol. 2018;11:5916-5922. [PubMed] |
| 19. | Rong W, Zhang Y, Yang L, Feng L, Wei B, Wu F, Wang L, Gao Y, Cheng S, Wu J, Xiao T. Post-surgical resection prognostic value of combined OPN, MMP7, and PSG9 plasma biomarkers in hepatocellular carcinoma. Front Med. 2019;13:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Zhang B, Shang L, Zhang Y, Li T, Fang Y. The effect of bone marrow mesenchymal stem cells on highly metastatic MHCC97-H hepatocellular carcinoma cells following OPN and TGFβ1 gene silencing. Exp Ther Med. 2020;20:3633-3642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 21. | Wang G, Chen S, Zhao C, Li X, Zhao W, Yang J, Chang C, Xu C. Comparative analysis of gene expression profiles of OPN signaling pathway in four kinds of liver diseases. J Genet. 2016;95:741-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Yu X, Zheng Y, Zhu X, Gao X, Wang C, Sheng Y, Cheng W, Qin L, Ren N, Jia H, Dong Q. Osteopontin promotes hepatocellular carcinoma progression via the PI3K/AKT/Twist signaling pathway. Oncol Lett. 2018;16:5299-5308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Gao X, Sheng Y, Yang J, Wang C, Zhang R, Zhu Y, Zhang Z, Zhang K, Yan S, Sun H, Wei J, Wang X, Yu X, Zhang Y, Luo Q, Zheng Y, Qiao P, Zhao Y, Dong Q, Qin L. Osteopontin alters DNA methylation through up-regulating DNMT1 and sensitizes CD133+/CD44+ cancer stem cells to 5 azacytidine in hepatocellular carcinoma. J Exp Clin Cancer Res. 2018;37:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 24. | Chen RX, Xia YH, Xue TC, Ye SL. Osteopontin promotes hepatocellular carcinoma invasion by up-regulating MMP-2 and uPA expression. Mol Biol Rep. 2011;38:3671-3677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Zhu Y, Yang J, Xu D, Gao XM, Zhang Z, Hsu JL, Li CW, Lim SO, Sheng YY, Zhang Y, Li JH, Luo Q, Zheng Y, Zhao Y, Lu L, Jia HL, Hung MC, Dong QZ, Qin LX. Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade. Gut. 2019;68:1653-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 303] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 26. | Liao WC, Huang BS, Yu YH, Yang HH, Chen PR, Huang CC, Huang HY, Wu MS, Chow LP. Galectin-3 and S100A9: Novel Diabetogenic Factors Mediating Pancreatic Cancer-Associated Diabetes. Diabetes Care. 2019;42:1752-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Chung YH, Park J, Cai H, Steinmetz NF. S100A9-Targeted Cowpea Mosaic Virus as a Prophylactic and Therapeutic Immunotherapy against Metastatic Breast Cancer and Melanoma. Adv Sci (Weinh). 2021;8:e2101796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 28. | Tomonobu N, Kinoshita R, Sakaguchi M. S100 Soil Sensor Receptors and Molecular Targeting Therapy Against Them in Cancer Metastasis. Transl Oncol. 2020;13:100753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Chiou SH, Lee KT. Proteomic analysis and translational perspective of hepatocellular carcinoma: Identification of diagnostic protein biomarkers by an onco-proteogenomics approach. Kaohsiung J Med Sci. 2016;32:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Ohta T, Makino I, Okazaki M, Miyashita T, Tajima H. [Intestinal Care Using L‒Glutamine Supplement and Probiotics Can Induce a Strong Anti‒Tumor Immune Response through the Induction of Mature Tertiary Lymphoid Structures in Pancreatic Cancer Patients Receiving Preoperative Chemotherapy]. Gan To Kagaku Ryoho. 2021;48:465-471. [PubMed] |
| 31. | Liao J, Li JZ, Xu J, Xu Y, Wen WP, Zheng L, Li L. High S100A9+ cell density predicts a poor prognosis in hepatocellular carcinoma patients after curative resection. Aging (Albany NY). 2021;13:16367-16380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Wei R, Zhu WW, Yu GY, Wang X, Gao C, Zhou X, Lin ZF, Shao WQ, Wang SH, Lu M, Qin LX. S100 calcium-binding protein A9 from tumor-associated macrophage enhances cancer stem cell-like properties of hepatocellular carcinoma. Int J Cancer. 2021;148:1233-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 33. | Zhong C, Niu Y, Liu W, Yuan Y, Li K, Shi Y, Qiu Z, Lin Z, Huang Z, Zuo D, Yang Z, Liao Y, Zhang Y, Wang C, Qiu J, He W, Li B. S100A9 Derived from Chemoembolization-Induced Hypoxia Governs Mitochondrial Function in Hepatocellular Carcinoma Progression. Adv Sci (Weinh). 2022;9:e2202206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 34. | Kastenhuber ER, Lowe SW. Putting p53 in Context. Cell. 2017;170:1062-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 1399] [Article Influence: 174.9] [Reference Citation Analysis (0)] |
| 35. | Syahruddin E, Zaini J, Sembiring R, Baginta R, Fadhillah MR, Noor DR. TP53 and EGFR Mutational Status in Thymoma: A Genetic Sequencing Study. Asian Pac J Cancer Prev. 2022;23:109-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Tang Q, Su Z, Gu W, Rustgi AK. Mutant p53 on the Path to Metastasis. Trends Cancer. 2020;6:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 37. | Kurnit KC, Kim GN, Fellman BM, Urbauer DL, Mills GB, Zhang W, Broaddus RR. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod Pathol. 2017;30:1032-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 234] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 38. | Zhou G, Gui X, Qu W. Clinical significance of CCN5 and mutant p53 in primary and recurrent lesions of breast cancer. Am J Transl Res. 2021;13:8433-8437. [PubMed] |
| 39. | Ghufran SM, Sharma S, Ghose S, Biswas S. Context dependent role of p53 during the interaction of hepatocellular carcinoma and endothelial cells. Microvasc Res. 2022;142:104374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 40. | Nikolova D, Chalovska-Ivanova V, Genadieva-Dimitrova M, Eftimov A, Jovanovik R, Janevska V. TP53 Mutation in Correlation to Immunohistochemical Expression of P53 Protein in Patients with Hepatocellular Carcinoma. Open Access Maced J Med Sci. 2018;6:1181-1186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Wang S, Shi H, Liu T, Li M, Zhou S, Qiu X, Wang Z, Hu W, Guo W, Chen X, Guo H, Shi X, Shi J, Zang Y, Cao J, Wu L. Mutation profile and its correlation with clinicopathology in Chinese hepatocellular carcinoma patients. Hepatobiliary Surg Nutr. 2021;10:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 42. | Wang W, Hu B, Qin JJ, Cheng JW, Li X, Rajaei M, Fan J, Yang XR, Zhang R. A novel inhibitor of MDM2 oncogene blocks metastasis of hepatocellular carcinoma and overcomes chemoresistance. Genes Dis. 2019;6:419-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 43. | Cancer Genome Atlas Research Network. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell. 2017;169:1327-1341.e23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1578] [Cited by in RCA: 1729] [Article Influence: 216.1] [Reference Citation Analysis (1)] |
| 44. | Chaudhary K, Poirion OB, Lu L, Garmire LX. Deep Learning-Based Multi-Omics Integration Robustly Predicts Survival in Liver Cancer. Clin Cancer Res. 2018;24:1248-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 588] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 45. | Long J, Wang A, Bai Y, Lin J, Yang X, Wang D, Jiang Y, Zhao H. Development and validation of a TP53-associated immune prognostic model for hepatocellular carcinoma. EBioMedicine. 2019;42:363-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 257] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 46. | Li Y, Zhang MC, Xu XK, Zhao Y, Mahanand C, Zhu T, Deng H, Nevo E, Du JZ, Chen XQ. Functional Diversity of p53 in Human and Wild Animals. Front Endocrinol (Lausanne). 2019;10:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Yu L, Wang X, Zhang W, Khan E, Lin C, Guo C. The multiple regulation of metastasis suppressor NM23-H1 in cancer. Life Sci. 2021;268:118995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 48. | Mátyási B, Farkas Z, Kopper L, Sebestyén A, Boissan M, Mehta A, Takács-Vellai K. The Function of NM23-H1/NME1 and Its Homologs in Major Processes Linked to Metastasis. Pathol Oncol Res. 2020;26:49-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Ozturk E, Aksoy SA, Ugras N, Tunca B, Ceylan S, Tezcan G, Yilmazlar T, Yerci O, Egeli U, Cecener G. Coexistence of MACC1 and NM23-H1 dysregulation and tumor budding promise early prognostic evidence for recurrence risk of early-stage colon cancer. APMIS. 2018;126:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Khera L, Paul C, Kaul R. Hepatitis C Virus E1 protein promotes cell migration and invasion by modulating cellular metastasis suppressor Nm23-H1. Virology. 2017;506:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Paul C, Khera L, Kaul R. Hepatitis C virus core protein interacts with cellular metastasis suppressor Nm23-H1 and promotes cell migration and invasion. Arch Virol. 2019;164:1271-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Zeng TD, Zheng B, Zheng W, Chen C. CD82/KAI1 inhibits invasion and metastasis of esophageal squamous cell carcinoma via TGF-β1. Eur Rev Med Pharmacol Sci. 2018;22:5928-5937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 53. | Yan W, Huang J, Zhang Q, Zhang J. Role of Metastasis Suppressor KAI1/CD82 in Different Cancers. J Oncol. 2021;2021:9924473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Al-Khater KM, Almofty S, Ravinayagam V, Alrushaid N, Rehman S. Role of a metastatic suppressor gene KAI1/CD82 in the diagnosis and prognosis of breast cancer. Saudi J Biol Sci. 2021;28:3391-3398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 55. | Xu J, Zhang Y, Wang Y, Tao X, Cheng L, Wu S, Tao Y. Correlation of KAI1, CD133 and vasculogenic mimicry with the prediction of metastasis and prognosis in hepatocellular carcinoma. Int J Clin Exp Pathol. 2018;11:3638-3646. [PubMed] |
| 56. | Xu R, Zhou X, Wang S, Trinkle C. Tumor organoid models in precision medicine and investigating cancer-stromal interactions. Pharmacol Ther. 2021;218:107668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 57. | Salemme V, Centonze G, Cavallo F, Defilippi P, Conti L. The Crosstalk Between Tumor Cells and the Immune Microenvironment in Breast Cancer: Implications for Immunotherapy. Front Oncol. 2021;11:610303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 167] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 58. | Elmusrati A, Wang J, Wang CY. Tumor microenvironment and immune evasion in head and neck squamous cell carcinoma. Int J Oral Sci. 2021;13:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 169] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 59. | Carone C, Olivani A, Dalla Valle R, Manuguerra R, Silini EM, Trenti T, Missale G, Cariani E. Immune Gene Expression Profile in Hepatocellular Carcinoma and Surrounding Tissue Predicts Time to Tumor Recurrence. Liver Cancer. 2018;7:277-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Li G, Ni A, Yu M. Pretumor microenvironment of hepatocellular carcinoma: Cancerization or anticancerization? Gene. 2019;701:46-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Li Q, Fan B, Ding J, Xiang X, Zhang J. A novel immune signature to predict the prognosis of patients with hepatocellular carcinoma. Medicine (Baltimore). 2021;100:e26948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Ugel S, Canè S, De Sanctis F, Bronte V. Monocytes in the Tumor Microenvironment. Annu Rev Pathol. 2021;16:93-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 63. | Chen X, Chi H, Zhao X, Pan R, Wei Y, Han Y. Role of Exosomes in Immune Microenvironment of Hepatocellular Carcinoma. J Oncol. 2022;2022:2521025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 64. | Landwehr LS, Altieri B, Schreiner J, Sbiera I, Weigand I, Kroiss M, Fassnacht M, Sbiera S. Interplay between glucocorticoids and tumor-infiltrating lymphocytes on the prognosis of adrenocortical carcinoma. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 65. | Dong C. Cytokine Regulation and Function in T Cells. Annu Rev Immunol. 2021;39:51-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 348] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 66. | Guo Y, Xie YQ, Gao M, Zhao Y, Franco F, Wenes M, Siddiqui I, Bevilacqua A, Wang H, Yang H, Feng B, Xie X, Sabatel CM, Tschumi B, Chaiboonchoe A, Wang Y, Li W, Xiao W, Held W, Romero P, Ho PC, Tang L. Metabolic reprogramming of terminally exhausted CD8+ T cells by IL-10 enhances anti-tumor immunity. Nat Immunol. 2021;22:746-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 262] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 67. | Lee HK, Shin HJ, Koo J, Kim TH, Kim CW, Go RE, Seong YH, Park JE, Choi KC. Blockade of transforming growth factor β2 by anti-sense oligonucleotide improves immunotherapeutic potential of IL-2 against melanoma in a humanized mouse model. Cytotherapy. 2021;23:599-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Cai Y, Tian Y, Wang J, Wei W, Tang Q, Lu L, Luo Z, Li W, Lu Y, Pu J, Yang Z. Identification of Driver Genes Regulating the T-Cell-Infiltrating Levels in Hepatocellular Carcinoma. Front Genet. 2020;11:560546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Pinato DJ, Guerra N, Fessas P, Murphy R, Mineo T, Mauri FA, Mukherjee SK, Thursz M, Wong CN, Sharma R, Rimassa L. Immune-based therapies for hepatocellular carcinoma. Oncogene. 2020;39:3620-3637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 70. | Gabrielson A, Wu Y, Wang H, Jiang J, Kallakury B, Gatalica Z, Reddy S, Kleiner D, Fishbein T, Johnson L, Island E, Satoskar R, Banovac F, Jha R, Kachhela J, Feng P, Zhang T, Tesfaye A, Prins P, Loffredo C, Marshall J, Weiner L, Atkins M, He AR. Intratumoral CD3 and CD8 T-cell Densities Associated with Relapse-Free Survival in HCC. Cancer Immunol Res. 2016;4:419-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 243] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 71. | Li J, Zhou J, Kai S, Wang C, Wang D, Jiang J. Functional and Clinical Characterization of Tumor-Infiltrating T Cell Subpopulations in Hepatocellular Carcinoma. Front Genet. 2020;11:586415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 72. | Guo M, Yuan F, Qi F, Sun J, Rao Q, Zhao Z, Huang P, Fang T, Yang B, Xia J. Expression and clinical significance of LAG-3, FGL1, PD-L1 and CD8+T cells in hepatocellular carcinoma using multiplex quantitative analysis. J Transl Med. 2020;18:306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 73. | Xu X, Tan Y, Qian Y, Xue W, Wang Y, Du J, Jin L, Ding W. Clinicopathologic and prognostic significance of tumor-infiltrating CD8+ T cells in patients with hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore). 2019;98:e13923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 74. | Ding H, Hu H, Tian F, Liang H. A dual immune signature of CD8+ T cells and MMP9 improves the survival of patients with hepatocellular carcinoma. Biosci Rep. 2021;41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 75. | Hsu CL, Ou DL, Bai LY, Chen CW, Lin L, Huang SF, Cheng AL, Jeng YM, Hsu C. Exploring Markers of Exhausted CD8 T Cells to Predict Response to Immune Checkpoint Inhibitor Therapy for Hepatocellular Carcinoma. Liver Cancer. 2021;10:346-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 76. | Ramzan M, Sturm N, Decaens T, Bioulac-Sage P, Bancel B, Merle P, Tran Van Nhieu J, Slama R, Letoublon C, Zarski JP, Jouvin-Marche E, Marche PN, Leroy V. Liver-infiltrating CD8(+) lymphocytes as prognostic factor for tumour recurrence in hepatitis C virus-related hepatocellular carcinoma. Liver Int. 2016;36:434-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 77. | Sun Y, Wu L, Zhong Y, Zhou K, Hou Y, Wang Z, Zhang Z, Xie J, Wang C, Chen D, Huang Y, Wei X, Shi Y, Zhao Z, Li Y, Guo Z, Yu Q, Xu L, Volpe G, Qiu S, Zhou J, Ward C, Sun H, Yin Y, Xu X, Wang X, Esteban MA, Yang H, Wang J, Dean M, Zhang Y, Liu S, Yang X, Fan J. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell. 2021;184:404-421.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 530] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 78. | Xu L, Zheng Y, Wang J, Xu Y, Xie Y, Yang ZP. IL33 activates CD8+T and NK cells through MyD88 pathway to suppress the lung cancer cell growth in mice. Biotechnol Lett. 2020;42:1113-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 79. | Ojo EO, Sharma AA, Liu R, Moreton S, Checkley-Luttge MA, Gupta K, Lee G, Lee DA, Otegbeye F, Sekaly RP, de Lima M, Wald DN. Membrane bound IL-21 based NK cell feeder cells drive robust expansion and metabolic activation of NK cells. Sci Rep. 2019;9:14916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 80. | Hu W, Wang G, Huang D, Sui M, Xu Y. Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities. Front Immunol. 2019;10:1205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 278] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 81. | Di Vito C, Mikulak J, Zaghi E, Pesce S, Marcenaro E, Mavilio D. NK cells to cure cancer. Semin Immunol. 2019;41:101272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 82. | Tan Q, Huang Q, Ma G, Lv Z, Mei P, Mao K, Wu F, Jin Y. Relationship between serum tumor markers and Anaplastic Lymphoma Kinase mutations in stage IV lung adenocarcinoma in Hubei province, Central China. J Clin Lab Anal. 2020;34:e23027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Ai L, Wang H. Effects of propofol and sevoflurane on tumor killing activity of peripheral blood natural killer cells in patients with gastric cancer. J Int Med Res. 2020;48:300060520904861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 84. | Guo S, Huang C, Han F, Chen B, Ding Y, Zhao Y, Chen Z, Wen S, Wang M, Shen B, Zhu W. Gastric Cancer Mesenchymal Stem Cells Inhibit NK Cell Function through mTOR Signalling to Promote Tumour Growth. Stem Cells Int. 2021;2021:9989790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 85. | Wu M, Mei F, Liu W, Jiang J. Comprehensive characterization of tumor infiltrating natural killer cells and clinical significance in hepatocellular carcinoma based on gene expression profiles. Biomed Pharmacother. 2020;121:109637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 86. | Tao L, Wang S, Yang L, Jiang L, Li J, Wang X. Reduced Siglec-7 expression on NK cells predicts NK cell dysfunction in primary hepatocellular carcinoma. Clin Exp Immunol. 2020;201:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 87. | Juengpanich S, Shi L, Iranmanesh Y, Chen J, Cheng Z, Khoo AK, Pan L, Wang Y, Cai X. The role of natural killer cells in hepatocellular carcinoma development and treatment: A narrative review. Transl Oncol. 2019;12:1092-1107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 88. | Han C, Jiang Y, Wang Z, Wang H. Natural killer cells involved in tumour immune escape of hepatocellular carcinomar. Int Immunopharmacol. 2019;73:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 89. | Zahran AM, Abdel-Meguid MM, Ashmawy AM, Rayan A, Elkady A, Elsherbiny NM, Hetta HF. Frequency and Implications of Natural Killer and Natural Killer T Cells in Hepatocellular Carcinoma. Egypt J Immunol. 2018;25:45-52. [PubMed] |
| 90. | Lee HA, Goh HG, Lee YS, Jung YK, Kim JH, Yim HJ, Lee MG, An H, Jeen YT, Yeon JE, Byun KS, Seo YS. Natural killer cell activity is a risk factor for the recurrence risk after curative treatment of hepatocellular carcinoma. BMC Gastroenterol. 2021;21:258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 91. | Zhang PF, Gao C, Huang XY, Lu JC, Guo XJ, Shi GM, Cai JB, Ke AW. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol Cancer. 2020;19:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 401] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 92. | Tao L, Wang S, Kang G, Jiang S, Yin W, Zong L, Li J, Wang X. PD-1 blockade improves the anti-tumor potency of exhausted CD3+CD56+ NKT-like cells in patients with primary hepatocellular carcinoma. Oncoimmunology. 2021;10:2002068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 93. | Mahgoub S, Abosalem H, Emara M, Kotb N, Maged A, Soror S. Restoring NK cells functionality via cytokine activation enhances cetuximab-mediated NK-cell ADCC: A promising therapeutic tool for HCC patients. Mol Immunol. 2021;137:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 94. | Liu S, Yang L, Jia S, Zhao R, Jin Z. Interleukin-35 suppresses the activity of natural killer-like B cells in patients with hepatocellular carcinoma. Int Immunopharmacol. 2021;100:108161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 95. | Lin D, Mei Y, Lei L, Binte Hanafi Z, Jin Z, Liu Y, Song Y, Zhang Y, Hu B, Liu C, Lu J, Liu H. Immune suppressive function of IL-1α release in the tumor microenvironment regulated by calpain 1. Oncoimmunology. 2022;11:2088467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 96. | Hwang S, Han J, Baek JS, Tak E, Song GW, Lee SG, Jung DH, Park GC, Ahn CS, Kim N. Cytotoxicity of Human Hepatic Intrasinusoidal CD56bright Natural Killer Cells against Hepatocellular Carcinoma Cells. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 97. | Tan S, Xu Y, Wang Z, Wang T, Du X, Song X, Guo X, Peng J, Zhang J, Liang Y, Lu J, Gao C, Wu Z, Li C, Li N, Gao L, Liang X, Ma C. Tim-3 Hampers Tumor Surveillance of Liver-Resident and Conventional NK Cells by Disrupting PI3K Signaling. Cancer Res. 2020;80:1130-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 98. | Sun H, Liu L, Huang Q, Liu H, Huang M, Wang J, Wen H, Lin R, Qu K, Li K, Wei H, Xiao W, Sun R, Tian Z, Sun C. Accumulation of Tumor-Infiltrating CD49a+ NK Cells Correlates with Poor Prognosis for Human Hepatocellular Carcinoma. Cancer Immunol Res. 2019;7:1535-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 99. | Ahn SJ, Kim JH, Park SJ, Kim ST, Han JK. Hepatocellular carcinoma: preoperative gadoxetic acid-enhanced MR imaging can predict early recurrence after curative resection using image features and texture analysis. Abdom Radiol (NY). 2019;44:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 100. | Yang X, Zhu Y, Zhao X, Li JH, Xu D, Jia HL, Zhang JB. The Prognostic Comparison Between Hepatocellular Carcinoma with Portal Vein Tumor Thrombus and Bile Duct Cancer Thrombus After Liver Resection. Cancer Manag Res. 2020;12:12077-12086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 101. | Obermajer N, Urban J, Wieckowski E, Muthuswamy R, Ravindranathan R, Bartlett DL, Kalinski P. Promoting the accumulation of tumor-specific T cells in tumor tissues by dendritic cell vaccines and chemokine-modulating agents. Nat Protoc. 2018;13:335-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 102. | Saxena M, Bhardwaj N. Re-Emergence of Dendritic Cell Vaccines for Cancer Treatment. Trends Cancer. 2018;4:119-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 226] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 103. | Herbert GS, Vreeland TJ, Clifton GT, Greene JM, Jackson DO, Hardin MO, Hale DF, Berry JS, Nichol P, Yin S, Yu X, Wagner TE, Peoples GE. Initial phase I/IIa trial results of an autologous tumor lysate, particle-loaded, dendritic cell (TLPLDC) vaccine in patients with solid tumors. Vaccine. 2018;36:3247-3253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 104. | Michea P, Noël F, Zakine E, Czerwinska U, Sirven P, Abouzid O, Goudot C, Scholer-Dahirel A, Vincent-Salomon A, Reyal F, Amigorena S, Guillot-Delost M, Segura E, Soumelis V. Adjustment of dendritic cells to the breast-cancer microenvironment is subset specific. Nat Immunol. 2018;19:885-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 105. | Jhunjhunwala S, Hammer C, Delamarre L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat Rev Cancer. 2021;21:298-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 844] [Article Influence: 211.0] [Reference Citation Analysis (0)] |
| 106. | Pei Y, Zhu Y, Wang X, Xu L. The expression and clinical value of tumor infiltrating dendritic cells in tumor tissues of patients with esophageal cancer. J Gastrointest Oncol. 2021;12:1996-2003. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 107. | Gerhard GM, Bill R, Messemaker M, Klein AM, Pittet MJ. Tumor-infiltrating dendritic cell states are conserved across solid human cancers. J Exp Med. 2021;218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 154] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 108. | Shi Y, Men X, Li X, Yang Z, Wen H. Research progress and clinical prospect of immunocytotherapy for the treatment of hepatocellular carcinoma. Int Immunopharmacol. 2020;82:106351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 109. | Truxova I, Kasikova L, Hensler M, Skapa P, Laco J, Pecen L, Belicova L, Praznovec I, Halaska MJ, Brtnicky T, Salkova E, Rob L, Kodet R, Goc J, Sautes-Fridman C, Fridman WH, Ryska A, Galluzzi L, Spisek R, Fucikova J. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J Immunother Cancer. 2018;6:139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 110. | Kai K, Tanaka T, Ide T, Kawaguchi A, Noshiro H, Aishima S. Immunohistochemical analysis of the aggregation of CD1a-positive dendritic cells in resected specimens and its association with surgical outcomes for patients with gallbladder cancer. Transl Oncol. 2021;14:100923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 111. | Giorello MB, Matas A, Marenco P, Davies KM, Borzone FR, Calcagno ML, García-Rivello H, Wernicke A, Martinez LM, Labovsky V, Chasseing NA. CD1a- and CD83-positive dendritic cells as prognostic markers of metastasis development in early breast cancer patients. Breast Cancer. 2021;28:1328-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 112. | Collin M, Bigley V. Human dendritic cell subsets: an update. Immunology. 2018;154:3-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 809] [Cited by in RCA: 905] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 113. | Huang C, Jiang X, Huang Y, Zhao L, Li P, Liu F. Identifying Dendritic Cell-Related Genes Through a Co-Expression Network to Construct a 12-Gene Risk-Scoring Model for Predicting Hepatocellular Carcinoma Prognosis. Front Mol Biosci. 2021;8:636991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 114. | Sachdeva M, Arora SK. Prognostic role of immune cells in hepatocellular carcinoma. EXCLI J. 2020;19:718-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 115. | Xiao H, Guo Y, Li B, Li X, Wang Y, Han S, Cheng D, Shuai X. M2-Like Tumor-Associated Macrophage-Targeted Codelivery of STAT6 Inhibitor and IKKβ siRNA Induces M2-to-M1 Repolarization for Cancer Immunotherapy with Low Immune Side Effects. ACS Cent Sci. 2020;6:1208-1222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 116. | Li H, Somiya M, Kuroda S. Enhancing antibody-dependent cellular phagocytosis by Re-education of tumor-associated macrophages with resiquimod-encapsulated liposomes. Biomaterials. 2021;268:120601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 117. | Aldawsari HM, Gorain B, Alhakamy NA, Md S. Role of therapeutic agents on repolarisation of tumour-associated macrophage to halt lung cancer progression. J Drug Target. 2020;28:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 118. | He Y, de Araújo Júnior RF, Cruz LJ, Eich C. Functionalized Nanoparticles Targeting Tumor-Associated Macrophages as Cancer Therapy. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 119. | Reichel D, Tripathi M, Perez JM. Biological Effects of Nanoparticles on Macrophage Polarization in the Tumor Microenvironment. Nanotheranostics. 2019;3:66-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 120. | Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 447] [Cited by in RCA: 980] [Article Influence: 163.3] [Reference Citation Analysis (0)] |
| 121. | Zhang Z, Zhu Y, Xu D, Li TE, Li JH, Xiao ZT, Chen M, Yang X, Jia HL, Dong QZ, Qin LX. IFN-α facilitates the effect of sorafenib via shifting the M2-like polarization of TAM in hepatocellular carcinoma. Am J Transl Res. 2021;13:301-313. [PubMed] |
| 122. | Xu W, Cheng Y, Guo Y, Yao W, Qian H. Targeting tumor associated macrophages in hepatocellular carcinoma. Biochem Pharmacol. 2022;199:114990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 123. | Bartneck M, Schrammen PL, Möckel D, Govaere O, Liepelt A, Krenkel O, Ergen C, McCain MV, Eulberg D, Luedde T, Trautwein C, Kiessling F, Reeves H, Lammers T, Tacke F. The CCR2+ Macrophage Subset Promotes Pathogenic Angiogenesis for Tumor Vascularization in Fibrotic Livers. Cell Mol Gastroenterol Hepatol. 2019;7:371-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 124. | Meng YM, Liang J, Wu C, Xu J, Zeng DN, Yu XJ, Ning H, Xu L, Zheng L. Monocytes/Macrophages promote vascular CXCR4 expression via the ERK pathway in hepatocellular carcinoma. Oncoimmunology. 2018;7:e1408745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 125. | Wei X, Pu J, Guo Z, Li T, Zhu D, Wu Z. [Tumor-associated macrophages promote the proliferation and migration as well as invasion of sorafenib-resistant hepatocellular carcinoma cells]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2017;33:617-622. [PubMed] |
| 126. | Wang B, Li Q, Wang J, Zhao S, Nashun B, Qin L, Chen X. Plasmodium infection inhibits tumor angiogenesis through effects on tumor-associated macrophages in a murine implanted hepatoma model. Cell Commun Signal. 2020;18:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 127. | Frión-Herrera Y, Gabbia D, Cuesta-Rubio O, De Martin S, Carrara M. Nemorosone inhibits the proliferation and migration of hepatocellular carcinoma cells. Life Sci. 2019;235:116817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 128. | Yao RR, Li JH, Zhang R, Chen RX, Wang YH. M2-polarized tumor-associated macrophages facilitated migration and epithelial-mesenchymal transition of HCC cells via the TLR4/STAT3 signaling pathway. World J Surg Oncol. 2018;16:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 129. | Peng S, Chen Y, Gong Y, Li Z, Xie R, Lin Y, Zou B, Li J, Zeng L. Predictive value of intratumour inflammatory cytokine mRNA levels of hepatocellular carcinoma patients and activation of two distinct pathways govern IL-8 induced epithelial-mesenchymal transition in human hepatic cancer cell lines. Cytokine. 2019;119:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 130. | Chen Y, Wen H, Zhou C, Su Q, Lin Y, Xie Y, Huang Y, Qiu Q, Lin J, Huang X, Tan W, Min C, Wang C. TNF-α derived from M2 tumor-associated macrophages promotes epithelial-mesenchymal transition and cancer stemness through the Wnt/β-catenin pathway in SMMC-7721 hepatocellular carcinoma cells. Exp Cell Res. 2019;378:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 131. | Zhang K, Fang T, Shao Y, Wu Y. TGF-β-MTA1-SMAD7-SMAD3-SOX4-EZH2 Signaling Axis Promotes Viability, Migration, Invasion and EMT of Hepatocellular Carcinoma Cells. Cancer Manag Res. 2021;13:7087-7099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 132. | Qi F, Li J, Guo M, Yang B, Xia J. MT4-MMP in tumor-associated macrophages is linked to hepatocellular carcinoma aggressiveness and recurrence. Clin Transl Med. 2020;10:e162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 133. | Park DJ, Sung PS, Lee GW, Cho S, Kim SM, Kang BY, Hur W, Yang H, Lee SK, Lee SH, Jung ES, Seo CH, Ahn J, Choi HJ, You YK, Jang JW, Bae SH, Choi JY, Yoon SK. Preferential Expression of Programmed Death Ligand 1 Protein in Tumor-Associated Macrophages and Its Potential Role in Immunotherapy for Hepatocellular Carcinoma. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 134. | Liao H, Chen W, Dai Y, Richardson JJ, Guo J, Yuan K, Zeng Y, Xie K. Expression of Programmed Cell Death-Ligands in Hepatocellular Carcinoma: Correlation With Immune Microenvironment and Survival Outcomes. Front Oncol. 2019;9:883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 135. | Chen J, Lin Z, Liu L, Zhang R, Geng Y, Fan M, Zhu W, Lu M, Lu L, Jia H, Zhang J, Qin LX. GOLM1 exacerbates CD8+ T cell suppression in hepatocellular carcinoma by promoting exosomal PD-L1 transport into tumor-associated macrophages. Signal Transduct Target Ther. 2021;6:397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 136. | Xiao N, Zhu X, Li K, Chen Y, Liu X, Xu B, Lei M, Xu J, Sun HC. Blocking siglec-10hi tumor-associated macrophages improves anti-tumor immunity and enhances immunotherapy for hepatocellular carcinoma. Exp Hematol Oncol. 2021;10:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 137. | Zhou Z, Hu Y, Wu Y, Qi Q, Wang J, Chen L, Wang F. The immunosuppressive tumor microenvironment in hepatocellular carcinoma-current situation and outlook. Mol Immunol. 2022;151:218-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 138. | Matsuda M, Seki E. Hepatic Stellate Cell-Macrophage Crosstalk in Liver Fibrosis and Carcinogenesis. Semin Liver Dis. 2020;40:307-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 116] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 139. | Yang J, Xing Z. Ligustilide counteracts carcinogenesis and hepatocellular carcinoma cell-evoked macrophage M2 polarization by regulating yes-associated protein-mediated interleukin-6 secretion. Exp Biol Med (Maywood). 2021;246:1928-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 140. | Cole K, Pravoverov K, Talmadge JE. Role of myeloid-derived suppressor cells in metastasis. Cancer Metastasis Rev. 2021;40:391-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 141. | Dysthe M, Parihar R. Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1224:117-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 142. | Novikova MV, Khromova NV, Kopnin PB. Components of the Hepatocellular Carcinoma Microenvironment and Their Role in Tumor Progression. Biochemistry (Mosc). 2017;82:861-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 143. | Kalathil SG, Thanavala Y. Importance of myeloid derived suppressor cells in cancer from a biomarker perspective. Cell Immunol. 2021;361:104280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 144. | Li R, Mukherjee MB, Lin J. Coordinated Regulation of Myeloid-Derived Suppressor Cells by Cytokines and Chemokines. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 145. | Zhu S, Zhao Y, Quan Y, Ma X. Targeting Myeloid-Derived Suppressor Cells Derived From Surgical Stress: The Key to Prevent Post-surgical Metastasis. Front Surg. 2021;8:783218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 146. | Zhou J, Liu M, Sun H, Feng Y, Xu L, Chan AWH, Tong JH, Wong J, Chong CCN, Lai PBS, Wang HK, Tsang SW, Goodwin T, Liu R, Huang L, Chen Z, Sung JJ, Chow KL, To KF, Cheng AS. Hepatoma-intrinsic CCRK inhibition diminishes myeloid-derived suppressor cell immunosuppression and enhances immune-checkpoint blockade efficacy. Gut. 2018;67:931-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 147. | Lu LC, Chang CJ, Hsu CH. Targeting myeloid-derived suppressor cells in the treatment of hepatocellular carcinoma: current state and future perspectives. J Hepatocell Carcinoma. 2019;6:71-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 148. | Shen P, Wang A, He M, Wang Q, Zheng S. Increased circulating Lin(-/low) CD33(+) HLA-DR(-) myeloid-derived suppressor cells in hepatocellular carcinoma patients. Hepatol Res. 2014;44:639-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 149. | Sarkar T, Dhar S, Sa G. Tumor-infiltrating T-regulatory cells adapt to altered metabolism to promote tumor-immune escape. Curr Res Immunol. 2021;2:132-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 150. | Chen JX, Yi XJ, Gao SX, Sun JX. The possible regulatory effect of the PD-1/PD-L1 signaling pathway on Tregs in ovarian cancer. Gen Physiol Biophys. 2020;39:319-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 151. | Stenström J, Hedenfalk I, Hagerling C. Regulatory T lymphocyte infiltration in metastatic breast cancer-an independent prognostic factor that changes with tumor progression. Breast Cancer Res. 2021;23:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 152. | Xu ZW. Gene mining of immune microenvironment in hepatocellular carcinoma. Medicine (Baltimore). 2022;101:e30453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 153. | Huang KW, Jayant K, Lee PH, Yang PC, Hsiao CY, Habib N, Sodergren MH. Positive Immuno-Modulation Following Radiofrequency Assisted Liver Resection in Hepatocellular Carcinoma. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 154. | Li C, Li S, Zhang J, Sun F. The increased ratio of Treg/Th2 in promoting metastasis of hepatocellular carcinoma. Cir Cir. 2022;90:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 155. | Xiong DD, Li JD, He RQ, Li MX, Pan YQ, He XL, Dang YW, Chen G. Highly expressed carbohydrate sulfotransferase 11 correlates with unfavorable prognosis and immune evasion of hepatocellular carcinoma. Cancer Med. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 156. | Shi C, Chen Y, Yang Y, Bing W, Qi J. CD4+ CD25+ regulatory T cells promote hepatocellular carcinoma invasion via TGF-β1-induced epithelial-mesenchymal transition. Onco Targets Ther. 2019;12:279-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 157. | Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 735] [Article Influence: 105.0] [Reference Citation Analysis (0)] |
| 158. | Langhans B, Nischalke HD, Krämer B, Dold L, Lutz P, Mohr R, Vogt A, Toma M, Eis-Hübinger AM, Nattermann J, Strassburg CP, Gonzalez-Carmona MA, Spengler U. Role of regulatory T cells and checkpoint inhibition in hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68:2055-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 159. | Trehanpati N, Vyas AK. Immune Regulation by T Regulatory Cells in Hepatitis B Virus-Related Inflammation and Cancer. Scand J Immunol. 2017;85:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 160. | Zhou L, Pan LC, Zheng YG, Zhang XX, Liu ZJ, Meng X, Shi HD, Du GS, He Q. Reduction of FoxP3+ Tregs by an immunosuppressive protocol of rapamycin plus Thymalfasin and Huaier extract predicts positive survival benefits in a rat model of hepatocellular carcinoma. Ann Transl Med. 2020;8:472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 161. | Shaul ME, Fridlender ZG. Neutrophils as active regulators of the immune system in the tumor microenvironment. J Leukoc Biol. 2017;102:343-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 162. | Shaul ME, Fridlender ZG. Tumour-associated neutrophils in patients with cancer. Nat Rev Clin Oncol. 2019;16:601-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 660] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 163. | Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. 2020;20:485-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 752] [Article Influence: 150.4] [Reference Citation Analysis (0)] |
| 164. | SenGupta S, Subramanian BC, Parent CA. Getting TANned: How the tumor microenvironment drives neutrophil recruitment. J Leukoc Biol. 2019;105:449-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 165. | Kim DG, Kwon YM, Kang IS, Kim C. Taurine chloramine selectively regulates neutrophil degranulation through the inhibition of myeloperoxidase and upregulation of lactoferrin. Amino Acids. 2020;52:1191-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 166. | Wątroba S, Wiśniowski T, Bryda J, Kurzepa J. The role of matrix metalloproteinases in pathogenesis of human bladder cancer. Acta Biochim Pol. 2021;68:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 167. | Scheau C, Badarau IA, Costache R, Caruntu C, Mihai GL, Didilescu AC, Constantin C, Neagu M. The Role of Matrix Metalloproteinases in the Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma. Anal Cell Pathol (Amst). 2019;2019:9423907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 234] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 168. | Yang X, Du X, Sun L, Zhao X, Zhu J, Li G, Tian J, Li X, Wang Z. SULT2B1b promotes epithelial-mesenchymal transition through activation of the β-catenin/MMP7 pathway in hepatocytes. Biochem Biophys Res Commun. 2019;510:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 169. | Zhang GP, Yue X, Li SQ. Cathepsin C Interacts with TNF-α/p38 MAPK Signaling Pathway to Promote Proliferation and Metastasis in Hepatocellular Carcinoma. Cancer Res Treat. 2020;52:10-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 170. | Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology. 2016;150:1646-1658.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 618] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 171. | Dhanasekaran R, Baylot V, Kim M, Kuruvilla S, Bellovin DI, Adeniji N, Rajan Kd A, Lai I, Gabay M, Tong L, Krishnan M, Park J, Hu T, Barbhuiya MA, Gentles AJ, Kannan K, Tran PT, Felsher DW. MYC and Twist1 cooperate to drive metastasis by eliciting crosstalk between cancer and innate immunity. Elife. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 172. | Zhou SL, Yin D, Hu ZQ, Luo CB, Zhou ZJ, Xin HY, Yang XR, Shi YH, Wang Z, Huang XW, Cao Y, Fan J, Zhou J. A Positive Feedback Loop Between Cancer Stem-Like Cells and Tumor-Associated Neutrophils Controls Hepatocellular Carcinoma Progression. Hepatology. 2019;70:1214-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 173. | Teijeira Á, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, de Andrea C, Ochoa MC, Otano I, Etxeberria I, Andueza MP, Nieto CP, Resano L, Azpilikueta A, Allegretti M, de Pizzol M, Ponz-Sarvisé M, Rouzaut A, Sanmamed MF, Schalper K, Carleton M, Mellado M, Rodriguez-Ruiz ME, Berraondo P, Perez-Gracia JL, Melero I. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity. 2020;52:856-871.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 497] [Article Influence: 99.4] [Reference Citation Analysis (0)] |
| 174. | Masucci MT, Minopoli M, Del Vecchio S, Carriero MV. The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis. Front Immunol. 2020;11:1749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 341] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 175. | Yang LY, Luo Q, Lu L, Zhu WW, Sun HT, Wei R, Lin ZF, Wang XY, Wang CQ, Lu M, Jia HL, Chen JH, Zhang JB, Qin LX. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol. 2020;13:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 243] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 176. | Guan X, Lu Y, Zhu H, Yu S, Zhao W, Chi X, Xie C, Yin Z. The Crosstalk Between Cancer Cells and Neutrophils Enhances Hepatocellular Carcinoma Metastasis via Neutrophil Extracellular Traps-Associated Cathepsin G Component: A Potential Therapeutic Target. J Hepatocell Carcinoma. 2021;8:451-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 177. | Urushima H, Yuasa H, Matsubara T, Kuroda N, Hara Y, Inoue K, Wake K, Sato T, Friedman SL, Ikeda K. Activation of Hepatic Stellate Cells Requires Dissociation of E-Cadherin-Containing Adherens Junctions with Hepatocytes. Am J Pathol. 2021;191:438-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 178. | Nair B, Nath LR. Inevitable role of TGF-β1 in progression of nonalcoholic fatty liver disease. J Recept Signal Transduct Res. 2020;40:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 179. | Yang YM, Noureddin M, Liu C, Ohashi K, Kim SY, Ramnath D, Powell EE, Sweet MJ, Roh YS, Hsin IF, Deng N, Liu Z, Liang J, Mena E, Shouhed D, Schwabe RF, Jiang D, Lu SC, Noble PW, Seki E. Hyaluronan synthase 2-mediated hyaluronan production mediates Notch1 activation and liver fibrosis. Sci Transl Med. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 180. | Zhang SL, Ma L, Zhao J, You SP, Ma XT, Ye XY, Liu T. The Phenylethanol Glycoside Liposome Inhibits PDGF-Induced HSC Activation via Regulation of the FAK/PI3K/Akt Signaling Pathway. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 181. | Li Y, Xia JY, Chen W, Deng CL. [Effects of Ling Qi Juan Gan capsule drug-containing serum on PDGF-induced proliferation and JAK/STAT signaling of HSC-T6 cells]. Zhonghua Gan Zang Bing Za Zhi. 2013;21:663-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 182. | Cai S, Wu L, Yuan S, Liu G, Wang Y, Fang L, Xu D. Carvacrol alleviates liver fibrosis by inhibiting TRPM7 and modulating the MAPK signaling pathway. Eur J Pharmacol. 2021;898:173982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 183. | Wang A, Zhang F, Xu H, Xu M, Cao Y, Wang C, Xu Y, Su M, Zhang M, Zhuge Y. TWEAK/Fn14 promotes pro-inflammatory cytokine secretion in hepatic stellate cells via NF-κB/STAT3 pathways. Mol Immunol. 2017;87:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 184. | Wang H, Che J, Cui K, Zhuang W, Li H, Sun J, Chen J, Wang C. Schisantherin A ameliorates liver fibrosis through TGF-β1mediated activation of TAK1/MAPK and NF-κB pathways in vitro and in vivo. Phytomedicine. 2021;88:153609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 185. | Ni XX, Li XY, Wang Q, Hua J. Regulation of peroxisome proliferator-activated receptor-gamma activity affects the hepatic stellate cell activation and the progression of NASH via TGF-β1/Smad signaling pathway. J Physiol Biochem. 2021;77:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 186. | Alatas FS, Matsuura T, Pudjiadi AH, Wijaya S, Taguchi T. Peroxisome Proliferator-Activated Receptor Gamma Agonist Attenuates Liver Fibrosis by Several Fibrogenic Pathways in an Animal Model of Cholestatic Fibrosis. Pediatr Gastroenterol Hepatol Nutr. 2020;23:346-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 187. | Ramos-Tovar E, Muriel P. Molecular Mechanisms That Link Oxidative Stress, Inflammation, and Fibrosis in the Liver. Antioxidants (Basel). 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 178] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 188. | Ge C, Tan J, Lou D, Zhu L, Zhong Z, Dai X, Sun Y, Kuang Q, Zhao J, Wang L, Liu J, Wang B, Xu M. Mulberrin confers protection against hepatic fibrosis by Trim31/Nrf2 signaling. Redox Biol. 2022;51:102274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 189. | Xie X, Dou CY, Zhou Y, Zhou Q, Tang HB. MicroRNA-503 Targets Mothers Against Decapentaplegic Homolog 7 Enhancing Hepatic Stellate Cell Activation and Hepatic Fibrosis. Dig Dis Sci. 2021;66:1928-1939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 190. | Xie Z, Wu Y, Liu S, Lai Y, Tang S. LncRNA-SNHG7/miR-29b/DNMT3A axis affects activation, autophagy and proliferation of hepatic stellate cells in liver fibrosis. Clin Res Hepatol Gastroenterol. 2021;45:101469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 191. | Zhu S, Chen X, Wang JN, Xu JJ, Wang A, Li JJ, Wu S, Wu YY, Li XF, Huang C, Li J. Circular RNA circUbe2k promotes hepatic fibrosis via sponging miR-149-5p/TGF-β2 axis. FASEB J. 2021;35:e21622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 192. | Li J, Dong S, Ye M, Peng G, Luo J, Wang C, Wang J, Zhao Q, Chang Y, Wang H. MicroRNA-489-3p Represses Hepatic Stellate Cells Activation by Negatively Regulating the JAG1/Notch3 Signaling Pathway. Dig Dis Sci. 2021;66:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 193. | Chen T, Lin H, Chen X, Li G, Zhao Y, Zheng L, Shi Z, Zhang K, Hong W, Han T. LncRNA Meg8 suppresses activation of hepatic stellate cells and epithelial-mesenchymal transition of hepatocytes via the Notch pathway. Biochem Biophys Res Commun. 2020;521:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 194. | Ji D, Chen GF, Wang JC, Ji SH, Wu XW, Lu XJ, Chen JL, Li JT. Hsa_circ_0070963 inhibits liver fibrosis via regulation of miR-223-3p and LEMD3. Aging (Albany NY). 2020;12:1643-1655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 195. | Kim JH, Lee CH, Lee SW. Exosomal Transmission of MicroRNA from HCV Replicating Cells Stimulates Transdifferentiation in Hepatic Stellate Cells. Mol Ther Nucleic Acids. 2019;14:483-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 196. | Wang L, Wang Y, Quan J. Exosomes derived from natural killer cells inhibit hepatic stellate cell activation and liver fibrosis. Hum Cell. 2020;33:582-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 197. | Shang L, Hosseini M, Liu X, Kisseleva T, Brenner DA. Human hepatic stellate cell isolation and characterization. J Gastroenterol. 2018;53:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 198. | Barry AE, Baldeosingh R, Lamm R, Patel K, Zhang K, Dominguez DA, Kirton KJ, Shah AP, Dang H. Hepatic Stellate Cells and Hepatocarcinogenesis. Front Cell Dev Biol. 2020;8:709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (1)] |
| 199. | Sällberg M, Pasetto A. Liver, Tumor and Viral Hepatitis: Key Players in the Complex Balance Between Tolerance and Immune Activation. Front Immunol. 2020;11:552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 200. | Cheng B, Huang Z, Wu L, Lin Y, Lin W, Zhu Q. Activation of human hepatic stellate cells enhances the metastatic ability of hepatocellular carcinoma cells via up-regulation of interleukin-1β. J BUON. 2021;26:435-443. [PubMed] |
| 201. | Geervliet E, Bansal R. Matrix Metalloproteinases as Potential Biomarkers and Therapeutic Targets in Liver Diseases. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 202. | Ruan Q, Wang H, Burke LJ, Bridle KR, Li X, Zhao CX, Crawford DHG, Roberts MS, Liang X. Therapeutic modulators of hepatic stellate cells for hepatocellular carcinoma. Int J Cancer. 2020;147:1519-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 203. | Wu M, Miao H, Fu R, Zhang J, Zheng W. Hepatic Stellate Cell: A Potential Target for Hepatocellular Carcinoma. Curr Mol Pharmacol. 2020;13:261-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 204. | Zhang R, Gao X, Zuo J, Hu B, Yang J, Zhao J, Chen J. STMN1 upregulation mediates hepatocellular carcinoma and hepatic stellate cell crosstalk to aggravate cancer by triggering the MET pathway. Cancer Sci. 2020;111:406-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 205. | Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18:99-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 1225] [Article Influence: 175.0] [Reference Citation Analysis (0)] |
| 206. | Ishii T, Suzuki A, Kuwata T, Hisamitsu S, Hashimoto H, Ohara Y, Yanagihara K, Mitsunaga S, Yoshino T, Kinoshita T, Ochiai A, Shitara K, Ishii G. Drug-exposed cancer-associated fibroblasts facilitate gastric cancer cell progression following chemotherapy. Gastric Cancer. 2021;24:810-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 207. | Dwyer BJ, Jarman EJ, Gogoi-Tiwari J, Ferreira-Gonzalez S, Boulter L, Guest RV, Kendall TJ, Kurian D, Kilpatrick AM, Robson AJ, O'Duibhir E, Man TY, Campana L, Starkey Lewis PJ, Wigmore SJ, Olynyk JK, Ramm GA, Tirnitz-Parker JEE, Forbes SJ. TWEAK/Fn14 signalling promotes cholangiocarcinoma niche formation and progression. J Hepatol. 2021;74:860-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 208. | Ganjre AP. Metastasis-associated fibroblasts in oral squamous cell carcinoma: An illusion or a reality. J Cancer Res Ther. 2019;15:690-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 209. | Cheteh EH, Sarne V, Ceder S, Bianchi J, Augsten M, Rundqvist H, Egevad L, Östman A, Wiman KG. Interleukin-6 derived from cancer-associated fibroblasts attenuates the p53 response to doxorubicin in prostate cancer cells. Cell Death Discov. 2020;6:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 210. | Ermakov MS, Nushtaeva AA, Richter VA, Koval OA. Cancer-associated fibroblasts and their role in tumor progression. Vavilovskii Zhurnal Genet Selektsii. 2022;26:14-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 211. | D'Arcangelo E, Wu NC, Cadavid JL, McGuigan AP. The life cycle of cancer-associated fibroblasts within the tumour stroma and its importance in disease outcome. Br J Cancer. 2020;122:931-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 212. | Kim BG, Sung JS, Jang Y, Cha YJ, Kang S, Han HH, Lee JH, Cho NH. Compression-induced expression of glycolysis genes in CAFs correlates with EMT and angiogenesis gene expression in breast cancer. Commun Biol. 2019;2:313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 213. | Fozzatti L, Cheng SY. Tumor Cells and Cancer-Associated Fibroblasts: A Synergistic Crosstalk to Promote Thyroid Cancer. Endocrinol Metab (Seoul). 2020;35:673-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 214. | Jia C, Wang G, Wang T, Fu B, Zhang Y, Huang L, Deng Y, Chen G, Wu X, Chen J, Pan Y, Tai Y, Liang J, Li X, Hu K, Xie B, Li S, Yang Y, Zhang Q, Liu W. Cancer-associated Fibroblasts induce epithelial-mesenchymal transition via the Transglutaminase 2-dependent IL-6/IL6R/STAT3 axis in Hepatocellular Carcinoma. Int J Biol Sci. 2020;16:2542-2558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 215. | Huang B, Huang M, Li Q. Cancer-Associated Fibroblasts Promote Angiogenesis of Hepatocellular Carcinoma by VEGF-Mediated EZH2/VASH1 Pathway. Technol Cancer Res Treat. 2019;18:1533033819879905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 216. | Liu Z, Chen M, Zhao R, Huang Y, Liu F, Li B, Qin Y. CAF-induced placental growth factor facilitates neoangiogenesis in hepatocellular carcinoma. Acta Biochim Biophys Sin (Shanghai). 2020;52:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 217. | Gao L, Morine Y, Yamada S, Saito Y, Ikemoto T, Tokuda K, Miyazaki K, Okikawa S, Takasu C, Shimada M. The BAFF/NFκB axis is crucial to interactions between sorafenib-resistant HCC cells and cancer-associated fibroblasts. Cancer Sci. 2021;112:3545-3554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 218. | Peng H, Zhu E, Zhang Y. Advances of cancer-associated fibroblasts in liver cancer. Biomark Res. 2022;10:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 219. | Guo J, Zeng H, Shi X, Han T, Liu Y, Liu C, Qu D, Chen Y. A CFH peptide-decorated liposomal oxymatrine inactivates cancer-associated fibroblasts of hepatocellular carcinoma through epithelial-mesenchymal transition reversion. J Nanobiotechnology. 2022;20:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 220. | Zhang J, Gu C, Song Q, Zhu M, Xu Y, Xiao M, Zheng W. Identifying cancer-associated fibroblasts as emerging targets for hepatocellular carcinoma. Cell Biosci. 2020;10:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 221. | Huang Y, Wang T, Yang J, Wu X, Fan W, Chen J. Current Strategies for the Treatment of Hepatocellular Carcinoma by Modulating the Tumor Microenvironment via Nano-Delivery Systems: A Review. Int J Nanomedicine. 2022;17:2335-2352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 222. | Huang TX, Guan XY, Fu L. Therapeutic targeting of the crosstalk between cancer-associated fibroblasts and cancer stem cells. Am J Cancer Res. 2019;9:1889-1904. [PubMed] |
| 223. | Du Y, Shao H, Moller M, Prokupets R, Tse YT, Liu ZJ. Intracellular Notch1 Signaling in Cancer-Associated Fibroblasts Dictates the Plasticity and Stemness of Melanoma Stem/Initiating Cells. Stem Cells. 2019;37:865-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 224. | Xiong S, Wang R, Chen Q, Luo J, Wang J, Zhao Z, Li Y, Wang Y, Wang X, Cheng B. Cancer-associated fibroblasts promote stem cell-like properties of hepatocellular carcinoma cells through IL-6/STAT3/Notch signaling. Am J Cancer Res. 2018;8:302-316. [PubMed] |
| 225. | Li Y, Wang R, Xiong S, Wang X, Zhao Z, Bai S, Wang Y, Zhao Y, Cheng B. Cancer-associated fibroblasts promote the stemness of CD24+ liver cells via paracrine signaling. J Mol Med (Berl). 2019;97:243-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 226. | Cheng Y, Li H, Deng Y, Tai Y, Zeng K, Zhang Y, Liu W, Zhang Q, Yang Y. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018;9:422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 289] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 227. | Cheng JT, Deng YN, Yi HM, Wang GY, Fu BS, Chen WJ, Liu W, Tai Y, Peng YW, Zhang Q. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis. 2016;5:e198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 228. | Mano Y, Yoshio S, Shoji H, Tomonari S, Aoki Y, Aoyanagi N, Okamoto T, Matsuura Y, Osawa Y, Kimura K, Yugawa K, Wang H, Oda Y, Yoshizumi T, Maehara Y, Kanto T. Bone morphogenetic protein 4 provides cancer-supportive phenotypes to liver fibroblasts in patients with hepatocellular carcinoma. J Gastroenterol. 2019;54:1007-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 229. | Fang T, Lv H, Lv G, Li T, Wang C, Han Q, Yu L, Su B, Guo L, Huang S, Cao D, Tang L, Tang S, Wu M, Yang W, Wang H. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun. 2018;9:191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 749] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 230. | Liu J, Chen S, Wang W, Ning BF, Chen F, Shen W, Ding J, Chen W, Xie WF, Zhang X. Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-β pathways. Cancer Lett. 2016;379:49-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 231. | Sun LP, Xu K, Cui J, Yuan DY, Zou B, Li J, Liu JL, Li KY, Meng Z, Zhang B. Cancerassociated fibroblastderived exosomal miR3825p promotes the migration and invasion of oral squamous cell carcinoma. Oncol Rep. 2019;42:1319-1328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 232. | Zhang Z, Li X, Sun W, Yue S, Yang J, Li J, Ma B, Wang J, Yang X, Pu M, Ruan B, Zhao G, Huang Q, Wang L, Tao K, Dou K. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 2017;397:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 240] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 233. | Yugawa K, Yoshizumi T, Mano Y, Itoh S, Harada N, Ikegami T, Kohashi K, Oda Y, Mori M. Cancer-associated fibroblasts promote hepatocellular carcinoma progression through downregulation of exosomal miR-150-3p. Eur J Surg Oncol. 2021;47:384-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 234. | Liu X, Wang H, Yang M, Hou Y, Chen Y, Bie P. Exosomal miR-29b from cancer-associated fibroblasts inhibits the migration and invasion of hepatocellular carcinoma cells. Transl Cancer Res. 2020;9:2576-2587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 235. | Zhou X, Nowicki M, Sun H, Hann SY, Cui H, Esworthy T, Lee JD, Plesniak M, Zhang LG. 3D Bioprinting-Tunable Small-Diameter Blood Vessels with Biomimetic Biphasic Cell Layers. ACS Appl Mater Interfaces. 2020;12:45904-45915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 236. | Tahmasebi Birgani M, Carloni V. Tumor Microenvironment, a Paradigm in Hepatocellular Carcinoma Progression and Therapy. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 237. | Lacal PM, Graziani G. Therapeutic implication of vascular endothelial growth factor receptor-1 (VEGFR-1) targeting in cancer cells and tumor microenvironment by competitive and non-competitive inhibitors. Pharmacol Res. 2018;136:97-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 238. | Tavora B, Mederer T, Wessel KJ, Ruffing S, Sadjadi M, Missmahl M, Ostendorf BN, Liu X, Kim JY, Olsen O, Welm AL, Goodarzi H, Tavazoie SF. Tumoural activation of TLR3-SLIT2 axis in endothelium drives metastasis. Nature. 2020;586:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 239. | Dong XF, Zhong JT, Liu TQ, Chen YY, Tang YT, Yang JR. [Angiopoietin-2 regulates vessels encapsulated by tumor clusters positive hepatocellular carcinoma nest-type metastasis via integrin α5β1]. Zhonghua Yi Xue Za Zhi. 2021;101:654-660. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 240. | Tsai CN, Yu SC, Lee CW, Pang JS, Wu CH, Lin SE, Chung YH, Tsai CL, Hsieh SY, Yu MC. SOX4 activates CXCL12 in hepatocellular carcinoma cells to modulate endothelial cell migration and angiogenesis in vivo. Oncogene. 2020;39:4695-4710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 241. | Pramanik D, Jolly MK, Bhat R. Matrix adhesion and remodeling diversifies modes of cancer invasion across spatial scales. J Theor Biol. 2021;524:110733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 242. | Dalton CJ, Lemmon CA. Fibronectin: Molecular Structure, Fibrillar Structure and Mechanochemical Signaling. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 146] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 243. | Schussler O, Chachques JC, Alifano M, Lecarpentier Y. Key Roles of RGD-Recognizing Integrins During Cardiac Development, on Cardiac Cells, and After Myocardial Infarction. J Cardiovasc Transl Res. 2022;15:179-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 244. | Skhinas JN, Cox TR. The interplay between extracellular matrix remodelling and kinase signalling in cancer progression and metastasis. Cell Adh Migr. 2018;12:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 245. | Mierke CT. The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells. Rep Prog Phys. 2019;82:064602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 246. | Ray SK, Mukherjee S. Consequences of Extracellular Matrix Remodeling in Headway and Metastasis of Cancer along with Novel Immunotherapies: A Great Promise for Future Endeavor. Anticancer Agents Med Chem. 2022;22:1257-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 247. | D'Arcy C, Kiel C. Cell Adhesion Molecules in Normal Skin and Melanoma. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 248. | Smart JA, Oleksak JE, Hartsough EJ. Cell Adhesion Molecules in Plasticity and Metastasis. Mol Cancer Res. 2021;19:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 249. | Janiszewska M, Primi MC, Izard T. Cell adhesion in cancer: Beyond the migration of single cells. J Biol Chem. 2020;295:2495-2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 416] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 250. | Ziliotto N, Zivadinov R, Jakimovski D, Baroni M, Tisato V, Secchiero P, Bergsland N, Ramasamy DP, Weinstock-Guttman B, Bernardi F, Ramanathan M, Marchetti G. Plasma levels of soluble NCAM in multiple sclerosis. J Neurol Sci. 2019;396:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 251. | Luo L, Xia L, Zha B, Zuo C, Deng D, Chen M, Hu L, He Y, Dai F, Wu J, Wang C, Wang Y, Zhang Q. miR-335-5p targeting ICAM-1 inhibits invasion and metastasis of thyroid cancer cells. Biomed Pharmacother. 2018;106:983-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 252. | Davis G, Lucero J, Fellers C, McDonald JD, Lund AK. The effects of subacute inhaled multi-walled carbon nanotube exposure on signaling pathways associated with cholesterol transport and inflammatory markers in the vasculature of wild-type mice. Toxicol Lett. 2018;296:48-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 253. | Pan B, Bu X, Cao M, Zhang X, Huo T, Li Z, Gao X, Jing L, Luo X, Feng H, Yuan F, Guo K. Inactivation of ICAM1 inhibits metastasis and improves the prognosis of Ewing's sarcoma. J Cancer Res Clin Oncol. 2021;147:393-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 254. | Kim SHJ, Hammer DA. Integrin cross-talk modulates stiffness-independent motility of CD4+ T lymphocytes. Mol Biol Cell. 2021;32:1749-1757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 255. | Chen VL, Le AK, Podlaha O, Estevez J, Li B, Vutien P, Chang ET, Rosenberg-Hasson Y, Pflanz S, Jiang Z, Ge D, Gaggar A, Nguyen MH. Soluble intercellular adhesion molecule-1 is associated with hepatocellular carcinoma risk: multiplex analysis of serum markers. Sci Rep. 2017;7:11169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 256. | Christgen M, Bartels S, van Luttikhuizen JL, Bublitz J, Rieger LU, Christgen H, Stark H, Sander B, Lehmann U, Steinemann D, Derksen PWB, Kreipe H. E-cadherin to P-cadherin switching in lobular breast cancer with tubular elements. Mod Pathol. 2020;33:2483-2498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 257. | Fearon ER. Cancer: Context Is Key for E-cadherin in Invasion and Metastasis. Curr Biol. 2019;29:R1140-R1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 258. | Biswas KH. Molecular Mobility-Mediated Regulation of E-Cadherin Adhesion. Trends Biochem Sci. 2020;45:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 259. | Zhu X, Wang X, Gong Y, Deng J. E-cadherin on epithelial-mesenchymal transition in thyroid cancer. Cancer Cell Int. 2021;21:695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 260. | Lourenco AR, Ban Y, Crowley MJ, Lee SB, Ramchandani D, Du W, Elemento O, George JT, Jolly MK, Levine H, Sheng J, Wong ST, Altorki NK, Gao D. Differential Contributions of Pre- and Post-EMT Tumor Cells in Breast Cancer Metastasis. Cancer Res. 2020;80:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 261. | Bruner HC, Derksen PWB. Loss of E-Cadherin-Dependent Cell-Cell Adhesion and the Development and Progression of Cancer. Cold Spring Harb Perspect Biol. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 262. | Mohanty SK, Tiwari A, Singh C, Walsh C, Chuang F, Kim E, Singh K, Dadmanesh F. High-grade ovarian serous carcinomas: Significant correlation of histologic patterns with IMP3 and E-Cadherin predicting disease recurrence and survival. Ann Diagn Pathol. 2019;40:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 263. | Ziaran S, Harsanyi S, Bevizova K, Varchulova Novakova Z, Trebaticky B, Bujdak P, Galbavy S, Danisovic L. Expression of E-cadherin, Ki-67, and p53 in urinary bladder cancer in relation to progression, survival, and recurrence. Eur J Histochem. 2020;64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 264. | Zhang HG, Pan YW, Feng J, Zeng CT, Zhao XQ, Liang B, Zhang WW. TRIM66 promotes malignant progression of hepatocellular carcinoma by inhibiting E-cadherin expression through the EMT pathway. Eur Rev Med Pharmacol Sci. 2019;23:2003-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 265. | Giovannini C, Fornari F, Dallo R, Gagliardi M, Nipoti E, Vasuri F, Coadă CA, Ravaioli M, Bolondi L, Gramantieri L. MiR-199-3p replacement affects E-cadherin expression through Notch1 targeting in hepatocellular carcinoma. Acta Histochem. 2018;120:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 266. | Han LL, Jia L, Wu F, Huang C. Sirtuin6 (SIRT6) Promotes the EMT of Hepatocellular Carcinoma by Stimulating Autophagic Degradation of E-Cadherin. Mol Cancer Res. 2019;17:2267-2280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 267. | Ren Y, Wang Y, Hao S, Yang Y, Xiong W, Qiu L, Tao J, Tang A. NFE2L3 promotes malignant behavior and EMT of human hepatocellular carcinoma (HepG2) cells via Wnt/βcatenin pathway. J Cancer. 2020;11:6939-6949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 268. | Xu M, Liu Z, Wang C, Yao B, Zheng X. EDG2 enhanced the progression of hepatocellular carcinoma by LPA/PI3K/AKT/ mTOR signaling. Oncotarget. 2017;8:66154-66168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 269. | He C, Zhou Z, Jiang H, Yin Z, Meng S, Zhang J, Huang P, Xu K, Bian L, Xiao Z, Wang J. Epithelial-Mesenchymal Transition is Superior to Vessels-Encapsulate Tumor Cluster in Promoting Metastasis of Hepatocellular Carcinoma: a Morphological Evidence. J Cancer. 2017;8:39-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 270. | Arun AS, Tepper CG, Lam KS. Identification of integrin drug targets for 17 solid tumor types. Oncotarget. 2018;9:30146-30162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 271. | Hamidi H, Ivaska J. Every step of the way: integrins in cancer progression and metastasis. Nat Rev Cancer. 2018;18:533-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 925] [Cited by in RCA: 1017] [Article Influence: 145.3] [Reference Citation Analysis (1)] |
| 272. | Bhatwadekar AD, Kansara V, Luo Q, Ciulla T. Anti-integrin therapy for retinovascular diseases. Expert Opin Investig Drugs. 2020;29:935-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 273. | Lino RLB, Dos Santos PK, Pisani GFD, Altei WF, Cominetti MR, Selistre-de-Araújo HS. Alphavbeta3 integrin blocking inhibits apoptosis and induces autophagy in murine breast tumor cells. Biochim Biophys Acta Mol Cell Res. 2019;1866:118536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 274. | El-Ghlban S, AbouElnour ES, El-Torgoman AEAE, Abu Elabas SMS. Gene expression of Epithelial Membrane Protein 2 gene and β1-Integrin gene in patients with breast cancer. Biochem Biophys Rep. 2020;22:100708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 275. | Wu Y, Chen J, Tan F, Wang B, Xu W, Yuan C. ITGA9: Potential Biomarkers and Therapeutic Targets in Different Tumors. Curr Pharm Des. 2022;28:1412-1418. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 276. | Zhang L, Zhang T, Deng Z, Sun L. MicroRNA3653 inhibits the growth and metastasis of hepatocellular carcinoma by inhibiting ITGB1. Oncol Rep. 2019;41:1669-1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 277. | Liu W, Zhang GQ, Zhu DY, Wang LJ, Li GT, Xu JG, Jin XL, Zhu YM, Yang XY. Long noncoding RNA ZFPM2-AS1 regulates ITGB1 by miR-1226-3p to promote cell proliferation and invasion in hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2020;24:7612-7620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 278. | Huang C, Li K, Huang R, Zhu J, Yang J. RNF185-AS1 promotes hepatocellular carcinoma progression through targeting miR-221-5p/integrin β5 axis. Life Sci. 2021;267:118928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 279. | Huang Y, Chu P, Bao G. Silencing of Long Non-coding RNA TTN-AS1 Inhibits Hepatocellular Carcinoma Progression by the MicroRNA-134/ITGB1 Axis. Dig Dis Sci. 2021;66:3916-3928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 280. | Huang W, Yu D, Wang M, Han Y, Lin J, Wei D, Cai J, Li B, Chen P, Zhang X. ITGBL1 promotes cell migration and invasion through stimulating the TGF-β signalling pathway in hepatocellular carcinoma. Cell Prolif. 2020;53:e12836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 281. | Ge JC, Wang YX, Chen ZB, Chen DF. Integrin alpha 7 correlates with poor clinical outcomes, and it regulates cell proliferation, apoptosis and stemness via PTK2-PI3K-Akt signaling pathway in hepatocellular carcinoma. Cell Signal. 2020;66:109465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 282. | Zhang YL, Xing X, Cai LB, Zhu L, Yang XM, Wang YH, Yang Q, Nie HZ, Zhang ZG, Li J, Zhang XL. Integrin α9 Suppresses Hepatocellular Carcinoma Metastasis by Rho GTPase Signaling. J Immunol Res. 2018;2018:4602570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 283. | Purdy M, Obi A, Myers D, Wakefield T. P- and E- selectin in venous thrombosis and non-venous pathologies. J Thromb Haemost. 2022;20:1056-1066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 284. | Tvaroška I, Selvaraj C, Koča J. Selectins-The Two Dr. Jekyll and Mr. Hyde Faces of Adhesion Molecules-A Review. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 285. | Borsig L. Selectins in cancer immunity. Glycobiology. 2018;28:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 286. | Fabricius HÅ, Starzonek S, Lange T. The Role of Platelet Cell Surface P-Selectin for the Direct Platelet-Tumor Cell Contact During Metastasis Formation in Human Tumors. Front Oncol. 2021;11:642761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 287. | Bergstrand J, Xu L, Miao X, Li N, Öktem O, Franzén B, Auer G, Lomnytska M, Widengren J. Super-resolution microscopy can identify specific protein distribution patterns in platelets incubated with cancer cells. Nanoscale. 2019;11:10023-10033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 288. | Xu J, Cheng X, Tan L, Fu C, Ahmed M, Tian J, Dou J, Zhou Q, Ren X, Wu Q, Tang S, Zhou H, Meng X, Yu J, Liang P. Microwave Responsive Nanoplatform via P-Selectin Mediated Drug Delivery for Treatment of Hepatocellular Carcinoma with Distant Metastasis. Nano Lett. 2019;19:2914-2927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 289. | Vega FM, Colmenero-Repiso A, Gómez-Muñoz MA, Rodríguez-Prieto I, Aguilar-Morante D, Ramírez G, Márquez C, Cabello R, Pardal R. CD44-high neural crest stem-like cells are associated with tumour aggressiveness and poor survival in neuroblastoma tumours. EBioMedicine. 2019;49:82-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 290. | Rustamadji P, Wiyarta E, Bethania KA. CD44 Variant Exon 6 Isoform Expression as a Potential Predictor of Lymph Node Metastasis in Invasive Breast Carcinoma of No Special Type. Int J Breast Cancer. 2021;2021:1586367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 291. | Wang X, Wang R, Bai S, Xiong S, Li Y, Liu M, Zhao Z, Wang Y, Zhao Y, Chen W, Billiar TR, Cheng B. Musashi2 contributes to the maintenance of CD44v6+ liver cancer stem cells via notch1 signaling pathway. J Exp Clin Cancer Res. 2019;38:505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 292. | Chen BL, Guo K, Liu YK. [Relationship between CD44 expression or glycosylation and hepatocellular carcinoma metastasis]. Zhonghua Gan Zang Bing Za Zhi. 2011;19:898-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 293. | Song Y, Li Z, Li L, Zhou H, Zeng TT, Jin C, Lin JR, Gao S, Li Y, Guan XY, Zhu YH. SERPINA11 Inhibits Metastasis in Hepatocellular Carcinoma by Suppressing MEK/ERK Signaling Pathway. J Hepatocell Carcinoma. 2021;8:759-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 294. | Niu FY, Jin C, Ma L, Shi YX, Li XS, Jiang P, Gao S, Lin JR, Song Y. Urokinase plasminogen activator predicts poor prognosis in hepatocellular carcinoma. J Gastrointest Oncol. 2021;12:1851-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 295. | Wu YJ, Wei WC, Dai GF, Su JH, Tseng YH, Tsai TC. Exploring the Mechanism of Flaccidoxide-13-Acetate in Suppressing Cell Metastasis of Hepatocellular Carcinoma. Mar Drugs. 2020;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 296. | Liu F, Zhu XT, Li Y, Wang CJ, Fu JL, Hui J, Xiao Y, Liu L, Yan R, Li XF, Liu Y. Magnesium demethylcantharidate inhibits hepatocellular carcinoma cell invasion and metastasis via activation transcription factor FOXO1. Eur J Pharmacol. 2021;911:174558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 297. | Fu X, Yang Y, Zhang D. Molecular mechanism of albumin in suppressing invasion and metastasis of hepatocellular carcinoma. Liver Int. 2022;42:696-709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 298. | Huang T, Chen QF, Chang BY, Shen LJ, Li W, Wu PH, Fan WJ. TFAP4 Promotes Hepatocellular Carcinoma Invasion and Metastasis via Activating the PI3K/AKT Signaling Pathway. Dis Markers. 2019;2019:7129214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 299. | Garner H, de Visser KE. Immune crosstalk in cancer progression and metastatic spread: a complex conversation. Nat Rev Immunol. 2020;20:483-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 287] [Article Influence: 57.4] [Reference Citation Analysis (0)] |
| 300. | Das D, Karthik N, Taneja R. Crosstalk Between Inflammatory Signaling and Methylation in Cancer. Front Cell Dev Biol. 2021;9:756458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 301. | Li J, Zeng M, Yan K, Yang Y, Li H, Xu X. IL-17 promotes hepatocellular carcinoma through inhibiting apoptosis induced by IFN-γ. Biochem Biophys Res Commun. 2020;522:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 302. | Li T, Zhang X, Lv Z, Gao L, Yan H. Increased Expression of Myeloid-Derived Suppressor Cells in Patients with HBV-Related Hepatocellular Carcinoma. Biomed Res Int. 2020;2020:6527192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 303. | He YF, Wang XH, Zhang GM, Chen HT, Zhang H, Feng ZH. Sustained low-level expression of interferon-gamma promotes tumor development: potential insights in tumor prevention and tumor immunotherapy. Cancer Immunol Immunother. 2005;54:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 304. | Aqbi HF, Wallace M, Sappal S, Payne KK, Manjili MH. IFN-γ orchestrates tumor elimination, tumor dormancy, tumor escape, and progression. J Leukoc Biol. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 305. | Gao S, Hsu TW, Li MO. Immunity beyond cancer cells: perspective from tumor tissue. Trends Cancer. 2021;7:1010-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 306. | Yao F, Yuan Q, Song X, Zhou L, Liang G, Jiang G, Zhang L. Yupingfeng Granule Improves Th2-Biased Immune State in Microenvironment of Hepatocellular Carcinoma through TSLP-DC-OX40L Pathway. Evid Based Complement Alternat Med. 2020;2020:1263053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 307. | Ene CV, Nicolae I, Geavlete B, Geavlete P, Ene CD. IL-6 Signaling Link between Inflammatory Tumor Microenvironment and Prostatic Tumorigenesis. Anal Cell Pathol (Amst). 2022;2022:5980387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 308. | Saito K, Mitsui A, Sumardika IW, Yokoyama Y, Sakaguchi M, Kondo E. PLOD2-driven IL-6/STAT3 signaling promotes the invasion and metastasis of oral squamous cell carcinoma via activation of integrin β1. Int J Oncol. 2021;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 309. | Lv B, Ma L, Tang W, Huang P, Yang B, Wang L, Chen S, Gao Q, Zhang S, Xia J. FXR Acts as a Metastasis Suppressor in Intrahepatic Cholangiocarcinoma by Inhibiting IL-6-Induced Epithelial-Mesenchymal Transition. Cell Physiol Biochem. 2018;48:158-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 310. | Daou HN. Exercise as an anti-inflammatory therapy for cancer cachexia: a focus on interleukin-6 regulation. Am J Physiol Regul Integr Comp Physiol. 2020;318:R296-R310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 311. | El-Sherbiny M, El-Sayed RM, Helal MA, Ibrahiem AT, Elmahdi HS, Eladl MA, Bilay SE, Alshahrani AM, Tawfik MK, Hamed ZE, Mohamed AO, Zaitone SA. Nifuroxazide Mitigates Angiogenesis in Ehlrich's Solid Carcinoma: Molecular Docking, Bioinformatic and Experimental Studies on Inhibition of Il-6/Jak2/Stat3 Signaling. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 312. | Ni JS, Zheng H, Ou YL, Tao YP, Wang ZG, Song LH, Yan HL, Zhou WP. miR-515-5p suppresses HCC migration and invasion via targeting IL6/JAK/STAT3 pathway. Surg Oncol. 2020;34:113-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 313. | Chen RY, Yen CJ, Liu YW, Guo CG, Weng CY, Lai CH, Wang JM, Lin YJ, Hung LY. CPAP promotes angiogenesis and metastasis by enhancing STAT3 activity. Cell Death Differ. 2020;27:1259-1273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 314. | Iwahasi S, Rui F, Morine Y, Yamada S, Saito YU, Ikemoto T, Imura S, Shimada M. Hepatic Stellate Cells Contribute to the Tumor Malignancy of Hepatocellular Carcinoma Through the IL-6 Pathway. Anticancer Res. 2020;40:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 315. | Jiang Y, Chen P, Hu K, Dai G, Li J, Zheng D, Yuan H, He L, Xie P, Tu M, Peng S, Qu C, Lin W, Chung RT, Hong J. Inflammatory microenvironment of fibrotic liver promotes hepatocellular carcinoma growth, metastasis and sorafenib resistance through STAT3 activation. J Cell Mol Med. 2021;25:1568-1582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 316. | Chan LC, Li CW, Xia W, Hsu JM, Lee HH, Cha JH, Wang HL, Yang WH, Yen EY, Chang WC, Zha Z, Lim SO, Lai YJ, Liu C, Liu J, Dong Q, Yang Y, Sun L, Wei Y, Nie L, Hsu JL, Li H, Ye Q, Hassan MM, Amin HM, Kaseb AO, Lin X, Wang SC, Hung MC. IL-6/JAK1 pathway drives PD-L1 Y112 phosphorylation to promote cancer immune evasion. J Clin Invest. 2019;129:3324-3338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 317. | Zhang W, Liu Y, Yan Z, Yang H, Sun W, Yao Y, Chen Y, Jiang R. IL-6 promotes PD-L1 expression in monocytes and macrophages by decreasing protein tyrosine phosphatase receptor type O expression in human hepatocellular carcinoma. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 318. | Pang XH, Zhang JP, Zhang YJ, Yan J, Pei XQ, Zhang YQ, Li JQ, Zheng L, Chen MS. Preoperative levels of serum interleukin-6 in patients with hepatocellular carcinoma. Hepatogastroenterology. 2011;58:1687-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 319. | Yang YM, Kim SY, Seki E. Inflammation and Liver Cancer: Molecular Mechanisms and Therapeutic Targets. Semin Liver Dis. 2019;39:26-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 308] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 320. | Han X, Gu Y, Li S, Chen M, Cai Q, Huang J. Inflammatory cytokines and alpha-fetoprotein concentrations for predicting survival in patients with hepatocellular carcinoma. Transl Cancer Res. 2019;8:1680-1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 321. | Teijeira A, Garasa S, Ochoa MC, Villalba M, Olivera I, Cirella A, Eguren-Santamaria I, Berraondo P, Schalper KA, de Andrea CE, Sanmamed MF, Melero I. IL8, Neutrophils, and NETs in a Collusion against Cancer Immunity and Immunotherapy. Clin Cancer Res. 2021;27:2383-2393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 322. | Matsushima K, Yang, Oppenheim JJ. Interleukin-8: An evolving chemokine. Cytokine. 2022;153:155828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 241] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 323. | Okimoto S, Tashiro H, Iwako H, Kuroda S, Kobayashi T, Hinoi T, Ohdan H. Antithrombin attenuates the progression of hepatocellular carcinoma by regulating neutrophil/interleukin-8 signaling. Hepatol Res. 2020;50:1284-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 324. | Iida-Ueno A, Enomoto M, Uchida-Kobayashi S, Hagihara A, Teranishi Y, Fujii H, Morikawa H, Murakami Y, Tamori A, Thuy LTT, Kawada N. Changes in plasma interleukin-8 and tumor necrosis factor-α levels during the early treatment period as a predictor of the response to sorafenib in patients with unresectable hepatocellular carcinoma. Cancer Chemother Pharmacol. 2018;82:857-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 325. | Wang Z, Si M, Yang N, Zhang H, Fu Y, Yan K, Zong Y, Zhu N, Wei Y. MicroRNA-506 suppresses invasiveness and metastasis of human hepatocellular carcinoma cells by targeting IL8. Am J Cancer Res. 2018;8:1586-1594. [PubMed] |
| 326. | Harimoto N, Shirabe K, Abe T, Kajiyama K, Nagaie T, Gion T, Kuroda Y, Maehara Y. Interleukin-8 producing hepatocellular carcinoma with pyrexia. HPB Surg. 2009;2009:461492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 327. | Zhang C, Gao Y, Du C, Markowitz GJ, Fu J, Zhang Z, Liu C, Qin W, Wang H, Wang F, Yang P. Hepatitis B-Induced IL8 Promotes Hepatocellular Carcinoma Venous Metastasis and Intrahepatic Treg Accumulation. Cancer Res. 2021;81:2386-2398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 328. | Sun F, Wang J, Sun Q, Li F, Gao H, Xu L, Zhang J, Sun X, Tian Y, Zhao Q, Shen H, Zhang K, Liu J. Interleukin-8 promotes integrin β3 upregulation and cell invasion through PI3K/Akt pathway in hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 108] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 329. | Tan Q, Duan L, Huang Q, Chen W, Yang Z, Chen J, Jin Y. Interleukin -1β Promotes Lung Adenocarcinoma Growth and Invasion Through Promoting Glycolysis via p38 Pathway. J Inflamm Res. 2021;14:6491-6509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 330. | Sangpairoj K, Vivithanaporn P, Apisawetakan S, Chongthammakun S, Sobhon P, Chaithirayanon K. RUNX1 Regulates Migration, Invasion, and Angiogenesis via p38 MAPK Pathway in Human Glioblastoma. Cell Mol Neurobiol. 2017;37:1243-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 331. | Wang YH, Huang JT, Chen WL, Wang RH, Kao MC, Pan YR, Chan SH, Tsai KW, Kung HJ, Lin KT, Wang LH. Dysregulation of cystathionine γ-lyase promotes prostate cancer progression and metastasis. EMBO Rep. 2019;20:e45986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 332. | Duan M, Wang ZC, Wang XY, Shi JY, Yang LX, Ding ZB, Gao Q, Zhou J, Fan J. TREM-1, an inflammatory modulator, is expressed in hepatocellular carcinoma cells and significantly promotes tumor progression. Ann Surg Oncol. 2015;22:3121-3129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 333. | Dang Y, Chen J, Feng W, Qiao C, Han W, Nie Y, Wu K, Fan D, Xia L. Interleukin 1β-mediated HOXC10 Overexpression Promotes Hepatocellular Carcinoma Metastasis by Upregulating PDPK1 and VASP. Theranostics. 2020;10:3833-3848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 334. | He Q, Liu M, Huang W, Chen X, Zhang B, Zhang T, Wang Y, Liu D, Xie M, Ji X, Sun M, Tian D, Xia L. IL-1β-Induced Elevation of Solute Carrier Family 7 Member 11 Promotes Hepatocellular Carcinoma Metastasis Through Up-regulating Programmed Death Ligand 1 and Colony-Stimulating Factor 1. Hepatology. 2021;74:3174-3193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 335. | Cruceriu D, Baldasici O, Balacescu O, Berindan-Neagoe I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: molecular insights and therapeutic approaches. Cell Oncol (Dordr). 2020;43:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 320] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 336. | Chattopadhyay I, Ambati R, Gundamaraju R. Exploring the Crosstalk between Inflammation and Epithelial-Mesenchymal Transition in Cancer. Mediators Inflamm. 2021;2021:9918379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 337. | Zhao L, Jin Y, Yang C, Li C. HBV-specific CD8 T cells present higher TNF-α expression but lower cytotoxicity in hepatocellular carcinoma. Clin Exp Immunol. 2020;201:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 338. | Hammam O, Mahmoud O, Zahran M, Sayed A, Salama R, Hosny K, Farghly A. A Possible Role for TNF-α in Coordinating Inflammation and Angiogenesis in Chronic Liver Disease and Hepatocellular Carcinoma. Gastrointest Cancer Res. 2013;6:107-114. [PubMed] |
| 339. | Wang X, Mao J, Zhou T, Chen X, Tu H, Ma J, Li Y, Ding Y, Yang Y, Wu H, Tang X. Hypoxia-induced myeloid derived growth factor promotes hepatocellular carcinoma progression through remodeling tumor microenvironment. Theranostics. 2021;11:209-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 340. | Chen H, Wu X, Zhou H, He Z, Li H, Wang Q. Epidermal growth factor upregulates the expression of A20 in hepatic cells via the MEK1/MSK1/p-p65 (Ser276) signaling pathway. Am J Transl Res. 2021;13:708-718. [PubMed] |
| 341. | Zhang M, Hu J, Li H, Zhang S, Hu W, Wu L, Han B. High TNF-α and/or p38MAPK expression predicts a favourable prognosis in patients with T1N0M0 hepatocellular carcinoma: An immunohistochemical study. Oncol Lett. 2019;17:4948-4956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 342. | Hughes CE, Nibbs RJB. A guide to chemokines and their receptors. FEBS J. 2018;285:2944-2971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 909] [Article Influence: 129.9] [Reference Citation Analysis (0)] |
| 343. | Strepkos D, Markouli M, Klonou A, Piperi C, Papavassiliou AG. Insights in the immunobiology of glioblastoma. J Mol Med (Berl). 2020;98:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 344. | Asokan S, Bandapalli OR. CXCL8 Signaling in the Tumor Microenvironment. Adv Exp Med Biol. 2021;1302:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 345. | Xu M, Wang Y, Xia R, Wei Y, Wei X. Role of the CCL2-CCR2 signalling axis in cancer: Mechanisms and therapeutic targeting. Cell Prolif. 2021;54:e13115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 204] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 346. | O'Connor T, Heikenwalder M. CCL2 in the Tumor Microenvironment. Adv Exp Med Biol. 2021;1302:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 347. | Li X, Yao W, Yuan Y, Chen P, Li B, Li J, Chu R, Song H, Xie D, Jiang X, Wang H. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 542] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 348. | Avila MA, Berasain C. Targeting CCL2/CCR2 in Tumor-Infiltrating Macrophages: A Tool Emerging Out of the Box Against Hepatocellular Carcinoma. Cell Mol Gastroenterol Hepatol. 2019;7:293-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 349. | Zhuang H, Cao G, Kou C, Liu T. CCL2/CCR2 axis induces hepatocellular carcinoma invasion and epithelial-mesenchymal transition in vitro through activation of the Hedgehog pathway. Oncol Rep. 2018;39:21-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 350. | Wu X, Zhang H, Sui Z, Wang Y, Yu Z. The biological role of the CXCL12/CXCR4 axis in esophageal squamous cell carcinoma. Cancer Biol Med. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 351. | Chen IX, Chauhan VP, Posada J, Ng MR, Wu MW, Adstamongkonkul P, Huang P, Lindeman N, Langer R, Jain RK. Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc Natl Acad Sci U S A. 2019;116:4558-4566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 295] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 352. | Yang P, Hu Y, Zhou Q. The CXCL12-CXCR4 Signaling Axis Plays a Key Role in Cancer Metastasis and is a Potential Target for Developing Novel Therapeutics against Metastatic Cancer. Curr Med Chem. 2020;27:5543-5561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 353. | Song JS, Chang CC, Wu CH, Dinh TK, Jan JJ, Huang KW, Chou MC, Shiue TY, Yeh KC, Ke YY, Yeh TK, Ta YN, Lee CJ, Huang JK, Sung YC, Shia KS, Chen Y. A highly selective and potent CXCR4 antagonist for hepatocellular carcinoma treatment. Proc Natl Acad Sci U S A. 2021;118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 354. | Song Z, Wang J, Su Q, Luan M, Chen X, Xu X. The role of MMP-2 and MMP-9 in the metastasis and development of hypopharyngeal carcinoma. Braz J Otorhinolaryngol. 2021;87:521-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 355. | Li M, Lu Y, Xu Y, Wang J, Zhang C, Du Y, Wang L, Li L, Wang B, Shen J, Tang J, Song B. Horizontal transfer of exosomal CXCR4 promotes murine hepatocarcinoma cell migration, invasion and lymphangiogenesis. Gene. 2018;676:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 356. | Saber S, Nasr M, Saad AS, Mourad AAE, Gobba NA, Shata A, Hafez AM, Elsergany RN, Elagamy HI, El-Ahwany E, Amin NA, Girgis S, Elewa YHA, Mahmoud MH, Batiha GE, El-Rous MA, Kamal I, Kaddah MMY, Khodir AE. Albendazole-loaded cubosomes interrupt the ERK1/2-HIF-1α-p300/CREB axis in mice intoxicated with diethylnitrosamine: A new paradigm in drug repurposing for the inhibition of hepatocellular carcinoma progression. Biomed Pharmacother. 2021;142:112029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 357. | Santoro A, Bufo P, Russo G, Cagiano S, Papagerakis S, Bucci P, Aquino G, Longo F, Feola A, Giordano A, Di Carlo A, Di Domenico M, Pannone G. Expression and clinical implication of cyclooxygenase-2 and E-cadherin in oral squamous cell carcinomas. Cancer Biol Ther. 2020;21:667-674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 358. | Melincovici CS, Boşca AB, Şuşman S, Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL, Mihu CM. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59:455-467. [PubMed] |
| 359. | Chen L, Lin G, Chen K, Liang R, Wan F, Zhang C, Tian G, Zhu X. VEGF promotes migration and invasion by regulating EMT and MMPs in nasopharyngeal carcinoma. J Cancer. 2020;11:7291-7301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 360. | Eguchi R, Kawabe JI, Wakabayashi I. VEGF-Independent Angiogenic Factors: Beyond VEGF/VEGFR2 Signaling. J Vasc Res. 2022;59:78-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 361. | Zhong C, Wei W, Su XK, Li HD, Xu FB, Guo RP. Serum and tissue vascular endothelial growth factor predicts prognosis in hepatocellular carcinoma patients after partial liver resection. Hepatogastroenterology. 2012;59:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 362. | Minata M, Harada KH, Kudo M, Ikai I, Nishida N. The prognostic value of vascular endothelial growth factor in hepatocellular carcinoma for predicting metastasis after curative resection. Oncology. 2013;84 Suppl 1:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 363. | Liu K, Hao M, Ouyang Y, Zheng J, Chen D. CD133+ cancer stem cells promoted by VEGF accelerate the recurrence of hepatocellular carcinoma. Sci Rep. 2017;7:41499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 364. | Zhang HH, Zhang Y, Cheng YN, Gong FL, Cao ZQ, Yu LG, Guo XL. Metformin incombination with curcumin inhibits the growth, metastasis, and angiogenesis of hepatocellular carcinoma in vitro and in vivo. Mol Carcinog. 2018;57:44-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 365. | Zhang T, Ding G, Wang H, Hu H, Wang Y, Li H, Zhang H, Wang L, Lin W, Hao L, Li B, Zheng C, Du Y, Song L. miR-497 targets VEGF signal pathway to regulate proliferation, invasion and migration of hepatocellular carcinoma cells: a primary study using DEC-MRI. J BUON. 2021;26:418-428. [PubMed] |
| 366. | Hack SP, Spahn J, Chen M, Cheng AL, Kaseb A, Kudo M, Lee HC, Yopp A, Chow P, Qin S. IMbrave 050: a Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020;16:975-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 367. | Ma L, Hernandez MO, Zhao Y, Mehta M, Tran B, Kelly M, Rae Z, Hernandez JM, Davis JL, Martin SP, Kleiner DE, Hewitt SM, Ylaya K, Wood BJ, Greten TF, Wang XW. Tumor Cell Biodiversity Drives Microenvironmental Reprogramming in Liver Cancer. Cancer Cell. 2019;36:418-430.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 545] [Article Influence: 90.8] [Reference Citation Analysis (0)] |
| 368. | Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, Nakamoto Y, Kaneko S. Increase in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother. 2013;62:1421-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 369. | Hato T, Zhu AX, Duda DG. Rationally combining anti-VEGF therapy with checkpoint inhibitors in hepatocellular carcinoma. Immunotherapy. 2016;8:299-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 370. | Dong S, Guo X, Han F, He Z, Wang Y. Emerging role of natural products in cancer immunotherapy. Acta Pharm Sin B. 2022;12:1163-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 154] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 371. | Gupta M, Akhtar J, Sarwat M. MicroRNAs: Regulators of immunological reactions in hepatocellular carcinoma. Semin Cell Dev Biol. 2022;124:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 372. | Katsuno Y, Derynck R. Epithelial plasticity, epithelial-mesenchymal transition, and the TGF-β family. Dev Cell. 2021;56:726-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 373. | Yang X, Ye X, Zhang L, Zhang X, Shu P. Disruption of LTBP4 Induced Activated TGFβ1, Immunosuppression Signal and Promoted Pulmonary Metastasis in Hepatocellular Carcinoma. Onco Targets Ther. 2020;13:7007-7017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 374. | Chen J, Gingold JA, Su X. Immunomodulatory TGF-β Signaling in Hepatocellular Carcinoma. Trends Mol Med. 2019;25:1010-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 375. | Sun L, Dong Z, Gu H, Guo Z, Yu Z. TINAGL1 promotes hepatocellular carcinogenesis through the activation of TGF-β signaling-medicated VEGF expression. Cancer Manag Res. 2019;11:767-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 376. | Modi SJ, Kulkarni VM. Discovery of VEGFR-2 inhibitors exerting significant anticancer activity against CD44+ and CD133+ cancer stem cells (CSCs): Reversal of TGF-β induced epithelial-mesenchymal transition (EMT) in hepatocellular carcinoma. Eur J Med Chem. 2020;207:112851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 377. | Cervello M, Augello G, Cusimano A, Emma MR, Balasus D, Azzolina A, McCubrey JA, Montalto G. Pivotal roles of glycogen synthase-3 in hepatocellular carcinoma. Adv Biol Regul. 2017;65:59-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 378. | Chen J, A Gingold J. Dysregulated PJA1-TGF-β signaling in cancer stem cell-associated liver cancers. Oncoscience. 2020;7:88-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 379. | Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 1820] [Article Influence: 364.0] [Reference Citation Analysis (0)] |
| 380. | Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 2625] [Article Influence: 437.5] [Reference Citation Analysis (0)] |
| 381. | Bae S, Brumbaugh J, Bonavida B. Exosomes derived from cancerous and non-cancerous cells regulate the anti-tumor response in the tumor microenvironment. Genes Cancer. 2018;9:87-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 382. | Cao Q, Liu Y, Wu Y, Hu C, Sun L, Wang J, Li C, Guo M, Liu X, Lv J, Huo X, Yue J, Du X, Chen Z. Profilin 2 promotes growth, metastasis, and angiogenesis of small cell lung cancer through cancer-derived exosomes. Aging (Albany NY). 2020;12:25981-25999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 383. | Kok VC, Yu CC. Cancer-Derived Exosomes: Their Role in Cancer Biology and Biomarker Development. Int J Nanomedicine. 2020;15:8019-8036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 384. | Yang X, Zhang Y, Zhang S, Qiu L, Zhuang Z, Wei M, Deng X, Wang Z, Han J. The Key Role of Exosomes on the Pre-metastatic Niche Formation in Tumors. Front Mol Biosci. 2021;8:703640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 385. | Watanabe T, Tanaka M. Exosomal circCMTM3 promotes the pathogenesis of hepatocellular carcinoma via angiogenesis. Hepatol Res. 2021;51:1100-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 386. | Li R, Wang Y, Zhang X, Feng M, Ma J, Li J, Yang X, Fang F, Xia Q, Zhang Z, Shang M, Jiang S. Exosome-mediated secretion of LOXL4 promotes hepatocellular carcinoma cell invasion and metastasis. Mol Cancer. 2019;18:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 183] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 387. | Hujanen R, Almahmoudi R, Karinen S, Nwaru BI, Salo T, Salem A. Vasculogenic Mimicry: A Promising Prognosticator in Head and Neck Squamous Cell Carcinoma and Esophageal Cancer? Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 388. | Li C, Chen Y, Zhang Q, Guo C, Chen F, Xi S, Zeng J, Ke C, Sharma HS, Chen Z. Expression of Twist associated to microcirculation patterns of human glioma correlated with progression and survival of the patient. Int Rev Neurobiol. 2020;151:201-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 389. | Bao Z, Cheng Z, Chai D. The expressions of CD133, ALDH1, and vasculogenic mimicry in osteosarcoma and their clinical significance. Int J Clin Exp Pathol. 2018;11:3656-3663. [PubMed] |
| 390. | Luo Q, Wang J, Zhao W, Peng Z, Liu X, Li B, Zhang H, Shan B, Zhang C, Duan C. Vasculogenic mimicry in carcinogenesis and clinical applications. J Hematol Oncol. 2020;13:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 391. | Xie JY, Wei JX, Lv LH, Han QF, Yang WB, Li GL, Wang PX, Wu SB, Duan JX, Zhuo WF, Liu PQ, Min J. Angiopoietin-2 induces angiogenesis via exosomes in human hepatocellular carcinoma. Cell Commun Signal. 2020;18:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 392. | Wang X, Shen H, Zhangyuan G, Huang R, Zhang W, He Q, Jin K, Zhuo H, Zhang Z, Wang J, Sun B, Lu X. 14-3-3ζ delivered by hepatocellular carcinoma-derived exosomes impaired anti-tumor function of tumor-infiltrating T lymphocytes. Cell Death Dis. 2018;9:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 393. | Liu J, Fan L, Yu H, Zhang J, He Y, Feng D, Wang F, Li X, Liu Q, Li Y, Guo Z, Gao B, Wei W, Wang H, Sun G. Endoplasmic Reticulum Stress Causes Liver Cancer Cells to Release Exosomal miR-23a-3p and Up-regulate Programmed Death Ligand 1 Expression in Macrophages. Hepatology. 2019;70:241-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 314] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 394. | Yang C, Dou R, Wei C, Liu K, Shi D, Zhang C, Liu Q, Wang S, Xiong B. Tumor-derived exosomal microRNA-106b-5p activates EMT-cancer cell and M2-subtype TAM interaction to facilitate CRC metastasis. Mol Ther. 2021;29:2088-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 153] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 395. | Zhu C, Su Y, Liu L, Wang S, Liu Y, Wu J. Circular RNA hsa_circ_0004277 Stimulates Malignant Phenotype of Hepatocellular Carcinoma and Epithelial-Mesenchymal Transition of Peripheral Cells. Front Cell Dev Biol. 2020;8:585565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 396. | Eun JW, Seo CW, Baek GO, Yoon MG, Ahn HR, Son JA, Sung S, Kim DW, Kim SS, Cho HJ, Cheong JY. Circulating Exosomal MicroRNA-1307-5p as a Predictor for Metastasis in Patients with Hepatocellular Carcinoma. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 397. | Yu Y, Min Z, Zhou Zhihang, Linhong M, Tao R, Yan L, Song H. Hypoxia-induced exosomes promote hepatocellular carcinoma proliferation and metastasis via miR-1273f transfer. Exp Cell Res. 2019;385:111649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 398. | Mohan V, Das A, Sagi I. Emerging roles of ECM remodeling processes in cancer. Semin Cancer Biol. 2020;62:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 223] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 399. | Eble JA, Niland S. The extracellular matrix in tumor progression and metastasis. Clin Exp Metastasis. 2019;36:171-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 397] [Article Influence: 66.2] [Reference Citation Analysis (0)] |
| 400. | Gao Y, Bado I, Wang H, Zhang W, Rosen JM, Zhang XH. Metastasis Organotropism: Redefining the Congenial Soil. Dev Cell. 2019;49:375-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 251] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 401. | Wortzel I, Dror S, Kenific CM, Lyden D. Exosome-Mediated Metastasis: Communication from a Distance. Dev Cell. 2019;49:347-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 901] [Article Influence: 180.2] [Reference Citation Analysis (0)] |
| 402. | Zeng Y, Yao X, Liu X, He X, Li L, Yan Z, Wu J, Fu BM. Anti-angiogenesis triggers exosomes release from endothelial cells to promote tumor vasculogenesis. J Extracell Vesicles. 2019;8:1629865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 403. | Yokota Y, Noda T, Okumura Y, Kobayashi S, Iwagami Y, Yamada D, Tomimaru Y, Akita H, Gotoh K, Takeda Y, Tanemura M, Murakami T, Umeshita K, Doki Y, Eguchi H. Serum exosomal miR-638 is a prognostic marker of HCC via downregulation of VE-cadherin and ZO-1 of endothelial cells. Cancer Sci. 2021;112:1275-1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 404. | Luo Y, Fu Y, Huang R, Gao M, Liu F, Gui R, Nie X. CircRNA_101505 sensitizes hepatocellular carcinoma cells to cisplatin by sponging miR-103 and promotes oxidored-nitro domain-containing protein 1 expression. Cell Death Discov. 2019;5:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 405. | Wang SC, Li CY, Chang WT, Cheng WC, Yen CH, Tu WY, Lin ZY, Lin CC, Yeh ML, Huang CF, Huang JF, Dai CY, Chuang WL, Chen YL, Yu ML. Exosome-derived differentiation antagonizing non-protein coding RNA with risk of hepatitis C virus-related hepatocellular carcinoma recurrence. Liver Int. 2021;41:956-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 406. | Liu L, Sun L, Zheng J, Cui L. Berberine modulates Keratin 17 to inhibit cervical cancer cell viability and metastasis. J Recept Signal Transduct Res. 2021;41:521-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 407. | Nieszporek A, Skrzypek K, Adamek G, Majka M. Molecular mechanisms of epithelial to mesenchymal transition in tumor metastasis. Acta Biochim Pol. 2019;66:509-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 408. | Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20:69-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1356] [Cited by in RCA: 2523] [Article Influence: 420.5] [Reference Citation Analysis (0)] |
| 409. | Menju T, Date H. Lung cancer and epithelial-mesenchymal transition. Gen Thorac Cardiovasc Surg. 2021;69:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 410. | Bacalini MG, Franceschi C, Gentilini D, Ravaioli F, Zhou X, Remondini D, Pirazzini C, Giuliani C, Marasco E, Gensous N, Di Blasio AM, Ellis E, Gramignoli R, Castellani G, Capri M, Strom S, Nardini C, Cescon M, Grazi GL, Garagnani P. Molecular Aging of Human Liver: An Epigenetic/Transcriptomic Signature. J Gerontol A Biol Sci Med Sci. 2019;74:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 411. | Wang Z, Xia F, Labib M, Ahmadi M, Chen H, Das J, Ahmed SU, Angers S, Sargent EH, Kelley SO. Nanostructured Architectures Promote the Mesenchymal-Epithelial Transition for Invasive Cells. ACS Nano. 2020;14:5324-5336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 412. | Yuan K, Xie K, Lan T, Xu L, Chen X, Li X, Liao M, Li J, Huang J, Zeng Y, Wu H. TXNDC12 promotes EMT and metastasis of hepatocellular carcinoma cells via activation of β-catenin. Cell Death Differ. 2020;27:1355-1368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 413. | Xu H, Han H, Tian G. High expression of WSB1 is associated with poor prognosis in hepatocellular carcinoma and affects epithelial-mesenchymal transition. J BUON. 2020;25:1890-1896. [PubMed] |
| 414. | Li W, Pei S, Zhang X, Qi D, Zhang W, Dou Y, Yang R, Yao X, Zhang Z, Xie S, Fang D, Sun H. Cinobufotalin inhibits the epithelial-mesenchymal transition of hepatocellular carcinoma cells through down-regulate β-catenin in vitro and in vivo. Eur J Pharmacol. 2022;922:174886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 415. | Takahashi K, Menju T, Nishikawa S, Miyata R, Tanaka S, Yutaka Y, Yamada Y, Nakajima D, Hamaji M, Ohsumi A, Chen-Yoshikawa TF, Sato T, Sonobe M, Date H. Tranilast Inhibits TGF-β1-induced Epithelial-mesenchymal Transition and Invasion/Metastasis via the Suppression of Smad4 in Human Lung Cancer Cell Lines. Anticancer Res. 2020;40:3287-3296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 416. | Jørgensen CLT, Forsare C, Bendahl PO, Falck AK, Fernö M, Lövgren K, Aaltonen K, Rydén L. Expression of epithelial-mesenchymal transition-related markers and phenotypes during breast cancer progression. Breast Cancer Res Treat. 2020;181:369-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 417. | Helal TE, Aref A, Gomaa AI, Nada O, Abd-Elghaffar M, Farouk K, Ehsan NA. Epithelial-Mesenchymal Transition Markers in HCVAssociated Hepatocellular Carcinoma: A Multivariate Follow Up Study. Asian Pac J Cancer Prev. 2022;23:839-849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 418. | Liu Z, Wang Y, Dou C, Xu M, Sun L, Wang L, Yao B, Li Q, Yang W, Tu K, Liu Q. Hypoxia-induced up-regulation of VASP promotes invasiveness and metastasis of hepatocellular carcinoma. Theranostics. 2018;8:4649-4663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 419. | Guo D, Zhang D, Ren M, Lu G, Zhang X, He S, Li Y. THBS4 promotes HCC progression by regulating ITGB1 via FAK/PI3K/AKT pathway. FASEB J. 2020;34:10668-10681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 420. | Wang G, Bai X, Jiang G, Jin S, Wang Q, Wang A, Peng R, Ke A, Bai D. GIT1 overexpression promotes epithelial-mesenchymal transition and predicts poor prognosis in hepatocellular carcinoma. Bioengineered. 2021;12:30-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 421. | Duan H, Liu Y, Gao Z, Huang W. Recent advances in drug delivery systems for targeting cancer stem cells. Acta Pharm Sin B. 2021;11:55-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 422. | Tiwari A, Modi SJ, Gabhe SY, Kulkarni VM. Evaluation of piperine against cancer stem cells (CSCs) of hepatocellular carcinoma: Insights into epithelial-mesenchymal transition (EMT). Bioorg Chem. 2021;110:104776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 423. | Yokota T, Nojima H, Kuboki S, Yoshitomi H, Furukawa K, Takayashiki T, Takano S, Ohtsuka M. Sphingosine-1-phosphate Receptor-1 Promotes Vascular Invasion and EMT in Hepatocellular Carcinoma. J Surg Res. 2021;259:200-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 424. | Zhang Q, Xing W, Zhang J, Hu J, Qi L, Xiang B. Circulating Tumor Cells Undergoing the Epithelial-Mesenchymal Transition: Influence on Prognosis in Cytokeratin 19-Positive Hepatocellular Carcinoma. Onco Targets Ther. 2021;14:1543-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 425. | Chen J, Cao SW, Cai Z, Zheng L, Wang Q. Epithelial-mesenchymal transition phenotypes of circulating tumor cells correlate with the clinical stages and cancer metastasis in hepatocellular carcinoma patients. Cancer Biomark. 2017;20:487-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 426. | Clarke MF. Clinical and Therapeutic Implications of Cancer Stem Cells. N Engl J Med. 2019;380:2237-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 255] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 427. | Wu Y, Zhang J, Zhang X, Zhou H, Liu G, Li Q. Cancer Stem Cells: A Potential Breakthrough in HCC-Targeted Therapy. Front Pharmacol. 2020;11:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 428. | Zarębska I, Gzil A, Durślewicz J, Jaworski D, Antosik P, Ahmadi N, Smolińska-Świtała M, Grzanka D, Szylberg Ł. The clinical, prognostic and therapeutic significance of liver cancer stem cells and their markers. Clin Res Hepatol Gastroenterol. 2021;45:101664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 429. | Itai S, Fujii Y, Nakamura T, Chang YW, Yanaka M, Saidoh N, Handa S, Suzuki H, Harada H, Yamada S, Kaneko MK, Kato Y. Establishment of CMab-43, a Sensitive and Specific Anti-CD133 Monoclonal Antibody, for Immunohistochemistry. Monoclon Antib Immunodiagn Immunother. 2017;36:231-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 430. | Barzegar Behrooz A, Syahir A, Ahmad S. CD133: beyond a cancer stem cell biomarker. J Drug Target. 2019;27:257-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 431. | Zhang L, Shen L, Wu D. Clinical significance of cancer stem cell markers in lung carcinoma. Acta Biochim Pol. 2021;68:187-191. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 432. | Rezaee M, Gheytanchi E, Madjd Z, Mehrazma M. Clinicopathological Significance of Tumor Stem Cell Markers ALDH1 and CD133 in Colorectal Carcinoma. Iran J Pathol. 2021;16:40-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 433. | Brown TC, Sankpal NV, Gillanders WE. Functional Implications of the Dynamic Regulation of EpCAM during Epithelial-to-Mesenchymal Transition. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 434. | Abdelgawad IA. Epithelial Cell Adhesion Molecule mRNA Can be a Potential Marker to Predict Metastasis in Hepatocellular Carcinoma Patients. Asian Pac J Cancer Prev. 2020;21:861-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 435. | Tsuchiya A, Suda T, Oda C, Kimura A, Hosaka K, Kimura N, Tominaga K, Hayashi K, Takamura M, Terai S. EpCAM- and/or NCAM-Expressing Hepatocellular Carcinoma in Which Behavior of Hepatic Progenitor Cell Marker-Positive Cells Are Followed. Case Rep Gastroenterol. 2019;13:118-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 436. | Noh CK, Wang HJ, Kim CM, Kim J, Yoon SY, Lee GH, Cho HJ, Yang MJ, Kim SS, Hwang JC, Cho SW, Roh J, Kim YB, Kim SJ, Kim BW, Cheong JY. EpCAM as a Predictive Marker of Tumor Recurrence and Survival in Patients Who Underwent Surgical Resection for Hepatocellular Carcinoma. Anticancer Res. 2018;38:4101-4109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 437. | Krause J, von Felden J, Casar C, Fründt TW, Galaski J, Schmidt C, Jung C, Ittrich H, Weidemann SA, Krech T, Heumann A, Li J, Fischer L, Sauter G, Lohse AW, Wege H, Schulze K. Hepatocellular carcinoma: Intratumoral EpCAM-positive cancer stem cell heterogeneity identifies high-risk tumor subtype. BMC Cancer. 2020;20:1130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 438. | Afify SM, Hassan G, Osman A, Calle AS, Nawara HM, Zahra MH, El-Ghlban S, Mansour H, Alam MJ, Abu Quora HA, Du J, Seno A, Iwasaki Y, Seno M. Metastasis of Cancer Stem Cells Developed in the Microenvironment of Hepatocellular Carcinoma. Bioengineering (Basel). 2019;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 439. | Yao Y, Li Y, Liu Q, Zhou K, Zhao W, Liu S, Yang J, Jiang Y, Sui G. Rapid detection of hepatocellular carcinoma metastasis using reverse transcription loop-mediated isothermal amplification. Talanta. 2020;208:120402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 440. | Yamashita T, Kaneko S. Liver cancer stem cells: Recent progress in basic and clinical research. Regen Ther. 2021;17:34-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 441. | Hwang HS, Yoo JE, Han DH, Choi JS, Lee JG, Joo DJ, Kim MS, Kim SI, Choi GH, Park YN. Circulating Cancer Stem Cells Expressing EpCAM/CD90 in Hepatocellular Carcinoma: A Pilot Study for Predicting Tumor Recurrence after Living Donor Liver Transplantation. Gut Liver. 2022;16:443-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 442. | Zhu P, Fan Z. Cancer stem cells and tumorigenesis. Biophys Rep. 2018;4:178-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 443. | Zhang R, Tu J, Liu S. Novel molecular regulators of breast cancer stem cell plasticity and heterogeneity. Semin Cancer Biol. 2022;82:11-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 444. | Crook T, Gaya A, Page R, Limaye S, Ranade A, Bhatt A, Patil S, Kumar P, Patil D, Akolkar D. Clinical utility of circulating tumor-associated cells to predict and monitor chemo-response in solid tumors. Cancer Chemother Pharmacol. 2021;87:197-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 445. | Ujiie D, Matsumoto T, Endo E, Okayama H, Fujita S, Kanke Y, Watanabe Y, Hanayama H, Hayase S, Saze Z, Ohki S, Kono K. Circulating tumor cells after neoadjuvant chemotherapy are related with recurrence in esophageal squamous cell carcinoma. Esophagus. 2021;18:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 446. | Franssen LC, Lorenzi T, Burgess AEF, Chaplain MAJ. A Mathematical Framework for Modelling the Metastatic Spread of Cancer. Bull Math Biol. 2019;81:1965-2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 447. | Hofbauer LC, Bozec A, Rauner M, Jakob F, Perner S, Pantel K. Novel approaches to target the microenvironment of bone metastasis. Nat Rev Clin Oncol. 2021;18:488-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 448. | Risson E, Nobre AR, Maguer-Satta V, Aguirre-Ghiso JA. The current paradigm and challenges ahead for the dormancy of disseminated tumor cells. Nat Cancer. 2020;1:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 154] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 449. | Akkoc Y, Gozuacik D. Autophagy and Hepatic Tumor Microenvironment Associated Dormancy. J Gastrointest Cancer. 2021;52:1277-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 450. | Hen O, Barkan D. Dormant disseminated tumor cells and cancer stem/progenitor-like cells: Similarities and opportunities. Semin Cancer Biol. 2020;60:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 451. | Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, Scheidmann MC, Donato C, Scherrer R, Singer J, Beisel C, Kurzeder C, Heinzelmann-Schwarz V, Rochlitz C, Weber WP, Beerenwinkel N, Aceto N. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature. 2019;566:553-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 856] [Article Influence: 142.7] [Reference Citation Analysis (0)] |
| 452. | Borgen E, Rypdal MC, Sosa MS, Renolen A, Schlichting E, Lønning PE, Synnestvedt M, Aguirre-Ghiso JA, Naume B. NR2F1 stratifies dormant disseminated tumor cells in breast cancer patients. Breast Cancer Res. 2018;20:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 453. | Talukdar S, Bhoopathi P, Emdad L, Das S, Sarkar D, Fisher PB. Dormancy and cancer stem cells: An enigma for cancer therapeutic targeting. Adv Cancer Res. 2019;141:43-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 454. | Majidpoor J, Mortezaee K. Steps in metastasis: an updated review. Med Oncol. 2021;38:3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 165] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 455. | Xu M, Hu K, Liu Y, Huang Y, Liu S, Chen Y, Wang D, Zhou S, Zhang Q, Mei N, Lu H, Li F, Gao X, Chen J. Systemic metastasis-targeted nanotherapeutic reinforces tumor surgical resection and chemotherapy. Nat Commun. 2021;12:3187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 456. | Genna A, Vanwynsberghe AM, Villard AV, Pottier C, Ancel J, Polette M, Gilles C. EMT-Associated Heterogeneity in Circulating Tumor Cells: Sticky Friends on the Road to Metastasis. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 457. | Zhang Z, Wuethrich A, Wang J, Korbie D, Lin LL, Trau M. Dynamic Monitoring of EMT in CTCs as an Indicator of Cancer Metastasis. Anal Chem. 2021;93:16787-16795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 458. | Yin LC, Luo ZC, Gao YX, Li Y, Peng Q, Gao Y. Twist Expression in Circulating Hepatocellular Carcinoma Cells Predicts Metastasis and Prognoses. Biomed Res Int. 2018;2018:3789613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 459. | Chen M, Xu R, Wu L, Chen X. Relationship between circulating tumor cells undergoing EMT and short-term efficacy following interventional treatment in patients with hepatocellular carcinoma. J Interv Med. 2020;3:146-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 460. | Ma Y, He J, Li Z. Specific study of biological tumor cytology: a narrative review. Transl Cancer Res. 2021;10:3843-3848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 461. | Zhang Q, Rong Y, Yi K, Huang L, Chen M, Wang F. Circulating tumor cells in hepatocellular carcinoma: single-cell based analysis, preclinical models, and clinical applications. Theranostics. 2020;10:12060-12071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 462. | Chen VL, Xu D, Wicha MS, Lok AS, Parikh ND. Utility of Liquid Biopsy Analysis in Detection of Hepatocellular Carcinoma, Determination of Prognosis, and Disease Monitoring: A Systematic Review. Clin Gastroenterol Hepatol. 2020;18:2879-2902.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 463. | Schulze K, Gasch C, Staufer K, Nashan B, Lohse AW, Pantel K, Riethdorf S, Wege H. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int J Cancer. 2013;133:2165-2171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 464. | Cui K, Ou Y, Shen Y, Li S, Sun Z. Clinical value of circulating tumor cells for the diagnosis and prognosis of hepatocellular carcinoma (HCC): A systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e22242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 465. | Shokoohian B, Negahdari B, Aboulkheyr Es H, Abedi-Valugerdi M, Baghaei K, Agarwal T, Maiti TK, Hassan M, Najimi M, Vosough M. Advanced therapeutic modalities in hepatocellular carcinoma: Novel insights. J Cell Mol Med. 2021;25:8602-8614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 466. | Hoang NM, Rui L. DNA methyltransferases in hematological malignancies. J Genet Genomics. 2020;47:361-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 467. | Vernier M, McGuirk S, Dufour CR, Wan L, Audet-Walsh E, St-Pierre J, Giguère V. Inhibition of DNMT1 and ERRα crosstalk suppresses breast cancer via derepression of IRF4. Oncogene. 2020;39:6406-6420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 468. | Casalino L, Verde P. Multifaceted Roles of DNA Methylation in Neoplastic Transformation, from Tumor Suppressors to EMT and Metastasis. Genes (Basel). 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 469. | Li JC, Chang X, Chen Y, Li XZ, Zhang XL, Yang SM, Hu CJ, Zhang H. Loss of the Tumor Suppressor HACE1 Contributes to Cancer Progression. Curr Drug Targets. 2019;20:1018-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 470. | Li Z, Guo Z. Comparison of CDH1 Gene Hypermethylation Status in Blood and Serum among Gastric Cancer Patients. Pathol Oncol Res. 2020;26:1057-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 471. | Xu J, Zhou Y, Yang J, Gu Y, Zhang E, Yuan W, Wang C, Jin G, Ma H, Hu Z. Hypomethylation-activated cancer-testis gene LIN28B promotes cell proliferation and metastasis in gastric cancer. Gene. 2022;813:146115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 472. | Wong KK. DNMT1 as a therapeutic target in pancreatic cancer: mechanisms and clinical implications. Cell Oncol (Dordr). 2020;43:779-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 473. | Nishiyama A, Nakanishi M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021;37:1012-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 516] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 474. | Wahid B, Ali A, Rafique S, Idrees M. New Insights into the Epigenetics of Hepatocellular Carcinoma. Biomed Res Int. 2017;2017:1609575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 475. | Li F, Qiao CY, Gao S, Fan YC, Chen LY, Wang K. Circulating cell-free DNA of methylated insulin-like growth factor-binding protein 7 predicts a poor prognosis in hepatitis B virus-associated hepatocellular carcinoma after hepatectomy. Free Radic Res. 2018;52:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 476. | He L, Fan X, Li Y, Cui B, Shi Z, Zhou D, Lin H. Aberrant methylation status of SPG20 promoter in hepatocellular carcinoma: A potential tumor metastasis biomarker. Cancer Genet. 2019;233-234:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 477. | Zhao J, Li L, Guo L, Wang R, Zhao Y, Li W, Liu Y, Ma Y, Jia J. Nano-Gold PCR in Detection of TERT Methylation and Its Correlation with Hepatitis B-Related Hepatocellular Carcinoma. J Biomed Nanotechnol. 2021;17:1284-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 478. | Qian Y, Wang H, Zhang Y, Wang JW, Fan YC, Gao S, Wang K. Hypermethylation of Cyclin D2 Predicts Poor Prognosis of Hepatitis B Virus-Associated Hepatocellular Carcinoma after Hepatectomy. Tohoku J Exp Med. 2021;254:233-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 479. | Zhou Q, Yin Y, Yu M, Gao D, Sun J, Yang Z, Weng J, Chen W, Atyah M, Shen Y, Ye Q, Li CW, Hung MC, Dong Q, Zhou C, Ren N. GTPBP4 promotes hepatocellular carcinoma progression and metastasis via the PKM2 dependent glucose metabolism. Redox Biol. 2022;56:102458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 480. | Chen MM, Zhao RC, Chen KF, Huang Y, Liu ZJ, Wei YG, Jian Y, Sun AM, Qin L, Li B, Qin Y. Hypomethylation of CTCFL promoters as a noninvasive biomarker in plasma from patients with hepatocellular carcinoma. Neoplasma. 2020;67:909-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 481. | Aalijahan H, Ghorbian S. Long non-coding RNAs and cervical cancer. Exp Mol Pathol. 2019;106:7-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 130] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 482. | Lv E, Sheng J, Yu C, Rao D, Huang W. LncRNA influence sequential steps of hepatocellular carcinoma metastasis. Biomed Pharmacother. 2021;136:111224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 483. | Pan T, Yu Z, Jin Z, Wu X, Wu A, Hou J, Chang X, Fan Z, Li J, Yu B, Li F, Yan C, Yang Z, Zhu Z, Liu B, Su L. Tumor suppressor lnc-CTSLP4 inhibits EMT and metastasis of gastric cancer by attenuating HNRNPAB-dependent Snail transcription. Mol Ther Nucleic Acids. 2021;23:1288-1303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 484. | Shi C, Yang Q, Pan S, Lin X, Xu G, Luo Y, Zheng B, Xie X, Yu M. LncRNA OIP5-AS1 promotes cell proliferation and migration and induces angiogenesis via regulating miR-3163/VEGFA in hepatocellular carcinoma. Cancer Biol Ther. 2020;21:604-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 485. | Sun DS, Guan CH, Wang WN, Hu ZT, Zhao YQ, Jiang XM. LncRNA NORAD promotes proliferation, migration and angiogenesis of hepatocellular carcinoma cells through targeting miR-211-5p/FOXD1/VEGF-A axis. Microvasc Res. 2021;134:104120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 486. | Song S, Qiu X. LncRNA miR503HG inhibits epithelial-mesenchymal transition and angiogenesis in hepatocellular carcinoma by enhancing PDCD4 via regulation of miR-15b. Dig Liver Dis. 2021;53:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 487. | He B, Peng F, Li W, Jiang Y. Interaction of lncRNA-MALAT1 and miR-124 regulates HBx-induced cancer stem cell properties in HepG2 through PI3K/Akt signaling. J Cell Biochem. 2019;120:2908-2918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 488. | Zhao L, Lou G, Li A, Liu Y. lncRNA MALAT1 modulates cancer stem cell properties of liver cancer cells by regulating YAP1 expression via miR375 sponging. Mol Med Rep. 2020;22:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 489. | Ye Y, Guo J, Xiao P, Ning J, Zhang R, Liu P, Yu W, Xu L, Zhao Y, Yu J. Macrophages-induced long noncoding RNA H19 up-regulation triggers and activates the miR-193b/MAPK1 axis and promotes cell aggressiveness in hepatocellular carcinoma. Cancer Lett. 2020;469:310-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 490. | Zhang PF, Wang F, Wu J, Wu Y, Huang W, Liu D, Huang XY, Zhang XM, Ke AW. LncRNA SNHG3 induces EMT and sorafenib resistance by modulating the miR-128/CD151 pathway in hepatocellular carcinoma. J Cell Physiol. 2019;234:2788-2794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 491. | Luo HL, Luo T, Liu JJ, Wu FX, Bai T, Ou C, Chen J, Li LQ, Zhong JH. Macrophage polarization-associated lnc-Ma301 interacts with caprin-1 to inhibit hepatocellular carcinoma metastasis through the Akt/Erk1 pathway. Cancer Cell Int. 2021;21:422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 492. | Ji D, Chen GF, Liu X, Zhu J, Sun JY, Zhang XY, Lu XJ. Identification of LINC01615 as potential metastasis-related long noncoding RNA in hepatocellular carcinoma. J Cell Physiol. 2019;234:12964-12970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 493. | Zhang QJ, Li DZ, Lin BY, Geng L, Yang Z, Zheng SS. SNHG16 promotes hepatocellular carcinoma development via activating ECM receptor interaction pathway. Hepatobiliary Pancreat Dis Int. 2022;21:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 494. | Park EG, Pyo SJ, Cui Y, Yoon SH, Nam JW. Tumor immune microenvironment lncRNAs. Brief Bioinform. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 149] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 495. | Xu S, Wang Q, Kang Y, Liu J, Yin Y, Liu L, Wu H, Li S, Sui S, Shen M, Zheng W, Pang D. Long Noncoding RNAs Control the Modulation of Immune Checkpoint Molecules in Cancer. Cancer Immunol Res. 2020;8:937-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 496. | Jiang R, Tang J, Chen Y, Deng L, Ji J, Xie Y, Wang K, Jia W, Chu WM, Sun B. The long noncoding RNA lnc-EGFR stimulates T-regulatory cells differentiation thus promoting hepatocellular carcinoma immune evasion. Nat Commun. 2017;8:15129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 274] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 497. | Chen Q, Wang H, Li Z, Li F, Liang L, Zou Y, Shen H, Li J, Xia Y, Cheng Z, Yang T, Wang K, Shen F. Circular RNA ACTN4 promotes intrahepatic cholangiocarcinoma progression by recruiting YBX1 to initiate FZD7 transcription. J Hepatol. 2022;76:135-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 146] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 498. | Xu X, Jing J. Advances on circRNAs Contribute to Carcinogenesis and Progression in Papillary Thyroid Carcinoma. Front Endocrinol (Lausanne). 2020;11:555243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 499. | Gu Y, Wang Y, He L, Zhang J, Zhu X, Liu N, Wang J, Lu T, Tian Y, Fan Z. Circular RNA circIPO11 drives self-renewal of liver cancer initiating cells via Hedgehog signaling. Mol Cancer. 2021;20:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 500. | Pu J, Wang J, Li W, Lu Y, Wu X, Long X, Luo C, Wei H. hsa_circ_0000092 promotes hepatocellular carcinoma progression through up-regulating HN1 expression by binding to microRNA-338-3p. J Cell Mol Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 501. | Sun K, Zhang L, Chen P, Qi D, Liu H, Bao H, Wang X, Li T. Circular RNA circ SET domain containing 2 (circSETD2) inhibits hepatocellular carcinoma cell proliferation and invasion in vivo and in vitro. Bioengineered. 2022;13:7293-7302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 502. | Yu J, Xu QG, Wang ZG, Yang Y, Zhang L, Ma JZ, Sun SH, Yang F, Zhou WP. Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma. J Hepatol. 2018;68:1214-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 561] [Article Influence: 80.1] [Reference Citation Analysis (0)] |
| 503. | Wu Y, Zhang M, Bi X, Hao L, Liu R, Zhang H. ESR1 mediated circ_0004018 suppresses angiogenesis in hepatocellular carcinoma via recruiting FUS and stabilizing TIMP2 expression. Exp Cell Res. 2021;408:112804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 504. | Xu G, Zhang P, Liang H, Xu Y, Shen J, Wang W, Li M, Huang J, Ni C, Zhang X, Zhu X. Circular RNA hsa_circ_0003288 induces EMT and invasion by regulating hsa_circ_0003288/miR-145/PD-L1 axis in hepatocellular carcinoma. Cancer Cell Int. 2021;21:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 505. | Li S, Chen Z, Zhou R, Wang S, Wang W, Liu, Li M, Guo T. Hsa_circ_0048674 facilitates hepatocellular carcinoma progression and natural killer cell exhaustion depending on the regulation of miR-223-3p/PDL1. Histol Histopathol. 2022;18440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 506. | Alwani A, Baj-Krzyworzeka M. MiRNAs - targets in cancer therapy. Postepy Biochem. 2021;67:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 507. | Mirzaei S, Saebfar H, Gholami MH, Hashemi F, Zarrabi A, Zabolian A, Entezari M, Hushmandi K, Samarghandian S, Aref AR, Ashrafizadeh M, Khan H. MicroRNAs regulating SOX2 in cancer progression and therapy response. Expert Rev Mol Med. 2021;23:e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 508. | Yao B, Li Y, Wang L, Chen T, Niu Y, Liu Q, Liu Z. MicroRNA-3194-3p inhibits metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by decreasing Wnt/β-catenin signaling through targeting BCL9. Artif Cells Nanomed Biotechnol. 2019;47:3885-3895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 509. | Zhang H, Liu S, Chen L, Sheng Y, Luo W, Zhao G. MicroRNA miR-509-3p inhibit metastasis and epithelial-mesenchymal transition in hepatocellular carcinoma. Bioengineered. 2021;12:2263-2273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 510. | Wang Q, Wang G, Niu L, Zhao S, Li J, Zhang Z, Jiang H, Zhang Q, Wang H, Sun P, Xiang R, Chang A, Yang S. Exosomal MiR-1290 Promotes Angiogenesis of Hepatocellular Carcinoma via Targeting SMEK1. J Oncol. 2021;2021:6617700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 511. | Li L, Ji Y, Chen YC, Zhen ZJ. MiR-325-3p mediate the CXCL17/CXCR8 axis to regulate angiogenesis in hepatocellular carcinoma. Cytokine. 2021;141:155436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 512. | Cao L, Xie B, Yang X, Liang H, Jiang X, Zhang D, Xue P, Chen D, Shao Z. MiR-324-5p Suppresses Hepatocellular Carcinoma Cell Invasion by Counteracting ECM Degradation through Post-Transcriptionally Downregulating ETS1 and SP1. PLoS One. 2015;10:e0133074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |