Published online Dec 7, 2022. doi: 10.3748/wjg.v28.i45.6421
Peer-review started: August 14, 2022
First decision: October 20, 2022
Revised: November 2, 2022
Accepted: November 16, 2022
Article in press: November 16, 2022
Published online: December 7, 2022
Processing time: 109 Days and 21.5 Hours
Pancreatic acinar cell carcinoma (PACC) is a rare tumor. Up to 45% of PACCs have alterations in the DNA damage repair pathway and 23% harbor rearran
A 70-year-old male was diagnosed with advanced PACC. At presentation, he was cachectic with severe arthralgia despite prednisolone and a skin rash that was later confirmed to be panniculitis. He was treated with modified FOLFIRINOX (mFFX) with the knowledge of the germline BRCA2 LPV. Following 11 cycles of mFFX, a computed tomography (CT) scan demonstrated significant tumor response in the pancreatic primary and hepatic metastases, totaling 70% from baseline as per Response Evaluation Criteria in Solid Tumors. Resolution of the skin panniculitis was also noted. We identified two additional PACCs with druggable targets in our case series. Our data contribute to practical evidence for the value of germline and somatic profiling in the management of rare diseases like PACC.
This patient and others in our larger case series highlight the importance of genomic testing in PACC with potential utility in personalized treatment.
Core Tip: Pancreatic acinar cell carcinoma (PACC) is a rare tumor with distinct molecular features and a relatively high proportion of targetable mutations. In this article, we describe a case report of PACC with a germline BRCA2 likely pathogenic variant, with a series of 10 additional cases, along with an in-depth look at the patients’ therapeutic details. We aim to outline the advantages of genomic analysis and its outcome regarding treatment selection in this tumor type.
- Citation: Lee CL, Holter S, Borgida A, Dodd A, Ramotar S, Grant R, Wasson K, Elimova E, Jang RW, Moore M, Kim TK, Khalili K, Moulton CA, Gallinger S, O’Kane GM, Knox JJ. Germline BRCA2 variants in advanced pancreatic acinar cell carcinoma: A case report and review of literature. World J Gastroenterol 2022; 28(45): 6421-6432
- URL: https://www.wjgnet.com/1007-9327/full/v28/i45/6421.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i45.6421
Pancreatic acinar cell carcinoma (PACC) is a rare subtype of pancreatic cancer, accounting for 1%-2% of exocrine pancreatic neoplasms[1]. While there is some clinical and genotype difference, patients with PACC and pancreatic ductal adenocarcinoma (PDAC) are often treated as one disease entity. Evolving data has increased our understanding of this rare tumor’s biology, treatment, and prognosis. Due to the disease’s rarity, most data about PACC are limited to reviews, case reports, and case series. The tumor biology of PACC is not well characterized due to the lack of tissue availability for large-scale molecular analysis. Intriguingly, data obtained in recent years have indicated that PACC has a distinctive mutational landscape[2-4]. There is increasing interest in this area, particularly regarding the homologous repair deficiency (HRD) signature in PACC. Chmielecki et al[2] reported up to 45% deficiency of the DNA damage repair (DDR) pathway genes in the study population. Oncogenic therapeutic targets including RAF1 rearrangements and mismatch repair genes have proven elusive in a significant proportion of PACCs, and lack of tumor profiling probably contributes to low reporting[3,4].
Here, we present a PACC case to emphasize the clinical application of genomic profiling in the context of precision medicine for better patient outcomes. Although this patient was very unwell at the presentation, raising the question of suitability for modified FOLFIRINOX (mFFX), the knowledge of the BRCA2 likely pathogenic variant (LPV) as predictive for mFFX sensitivity guided our decision to use this regime. In the case series section, we describe the clinical characteristics, therapeutic outcomes, and mutational signatures of additional 10 patients with PACC treated in our center. As proof of concept, we describe the immediate clinical impact for the patients with distinct genomic alterations that have been associated with sensitivity to specific chemotherapeutic or targeted agents.
The patient was a 70 male smoker with recurrent lower limb joint pain and was generally unwell for the previous year.
He presented to a rheumatology service with joint pain, which was diagnosed as gout and treated with short courses of prednisolone; however, during the steroid treatment, he also experienced central abdominal discomfort, reduced appetite, and 10 kg weight loss. He had progressive lower joint pain with tender, warm skin nodules, which restricted mobility.
He had encephalitis and asthma as a child.
He had a history of transitional cell carcinoma of the renal pelvis at age 46 for which he underwent a left total nephrectomy and a non-small cell lung adenocarcinoma at age 51, which was treated by lung resection. His mother died of ovarian cancer at age 72.
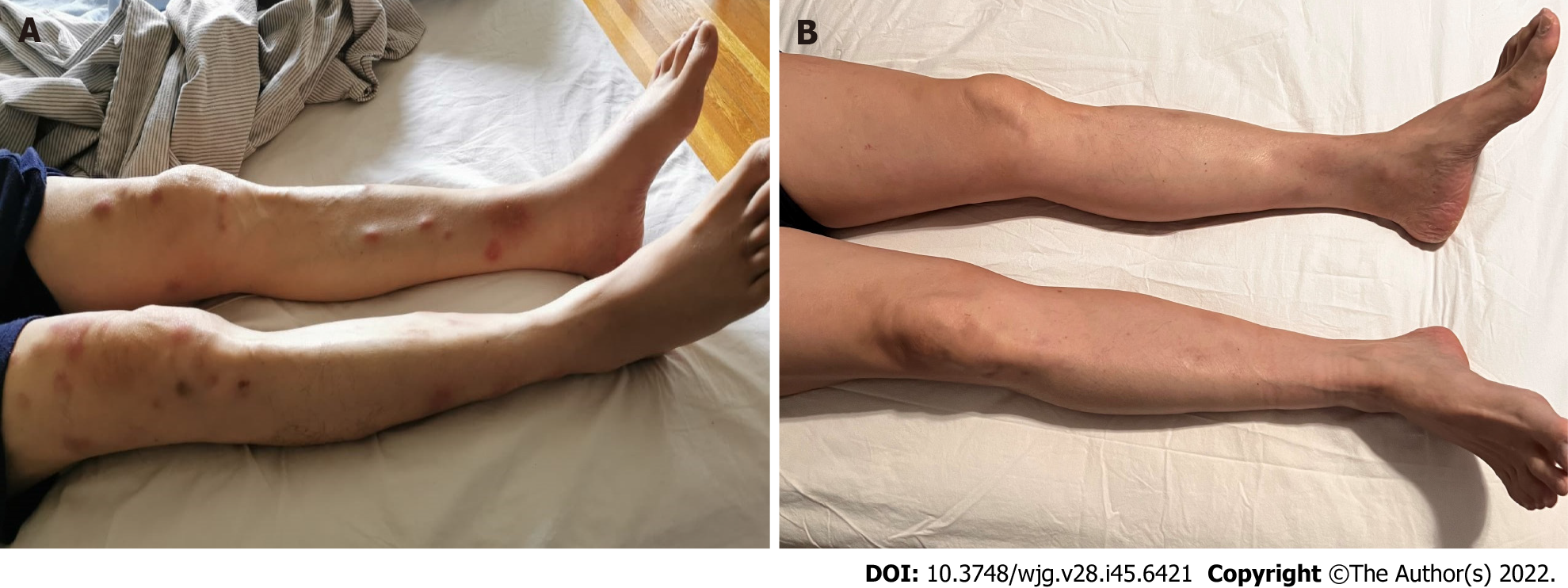
Physical examination revealed a cachectic man with a palpable liver edge and ill-defined widespread erythematous subcutaneous nodules on bilateral lower limbs (Figure 1A). Eastern Cooperative Oncology Group performance status (PS) was 2.
Initial blood tests demonstrated lipase > 6000 U/dL, elevated transaminases [alanine aminotransferase (ALT) 68 U/L and aspartate aminotransferase 58 U/L], total bilirubin 6 μmol/L, albumin 28 g/L and creatinine 120 μmol/L (estimated glomerular filtration rate 52 mL/min). Tumor markers were: Normal carbohydrate antigen 19-9 (CA19-9) 28 kU/L and raised alpha-fetoprotein 58 μg/L.
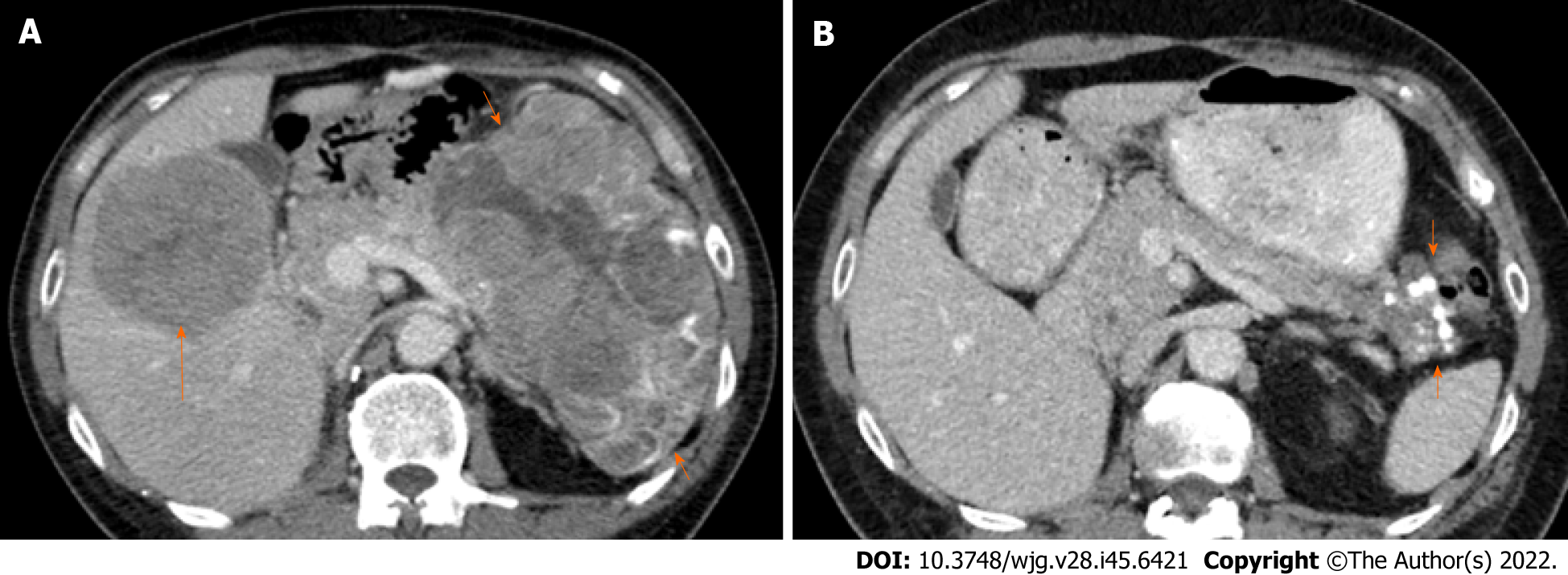
Initial computed tomography (CT) imaging showed a bulky pancreatic tumor measuring over 16 cm and multiple liver metastases. The largest liver lesion measured over 8 cm (Figure 2A). There was an ill-defined area of sclerosis in the right ischium which was suspicious of metastasis. Whole-body bone scintigraphy detected mild non-specific increased activity in the right ischium corresponding to the area of sclerosis but no significant bony abnormality. CT chest showed no evidence of thoracic metastases.
The patient underwent a liver biopsy that confirmed a poorly differentiated carcinoma staining positive for keratin 7, CAM5.2, claudin 4, glypican 3, and A1AT. Negative markers included keratin 20, arginase 1, hepPar1, synaptophysin, chromogranin, CD56, and TTF1. The tumor was mismatch repair proficient. An additional skin biopsy of one of the subcutaneous nodules confirmed pancreatic lobular panniculitis. Germline testing identified a BRCA2 LPV (c.4356delinsCA, p. Gln1452Hisfs*8).
We treated 11 PACC patients between August 2014 and July 2021 at Princess Margaret Cancer Centre (PMCC), Toronto. These comprised 6 (55%) pure and 5 (45%) mixed PACC. Approximately 2000 pancreatic carcinoma patients were managed at PMCC during this period. The median age at diagnosis of the PACC patients was 65 years (range 57-74) and all were male. At diagnosis, 2 (18%) were resectable, 2 (18%) locally advanced, and 7 (64%) metastatic. The full demographic features of all patients are summarized in Table 1.
| Characteristic | Number of patients (%) |
| Male | 11 (100) |
| Median age at diagnosis, yr | 65 (56.5-74.0) |
| Median tumor size, cm | 7.0 (2.7-16.4) |
| Histology | |
| Pure acinar | 6 (55) |
| Mixed acinar-neuroendocrine | 3 (27) |
| Mixed acinar-ductal | 2 (18) |
| Primary tumor site | |
| Head/uncinate | 4 (37) |
| Body | 2 (18) |
| Tail | 5 (45) |
| Stage | |
| Resectable | 2 (18) |
| Locally advanced | 2 (18) |
| Metastatic | 7 (64) |
| 1st line treatment | |
| Surgery only | 1 (9) |
| Surgery and chemotherapy | 2 (18) |
| Preoperative chemoradiotherapy and surgery | 1 (9) |
| Chemotherapy only | 7 (64) |
| Palliative chemotherapy | |
| Modified FOLFIRINOX | 6 (60) |
| Gemcitabine with Nab-paclitaxel | 3 (30) |
| Gemcitabine | 1 (10) |
Four (36%) patients had curative-intent surgery. Three of them developed systemic relapse and received subsequent treatment with palliative chemotherapy. All seven metastatic patients had chemotherapy. Altogether, ten patients received palliative chemotherapy: mFFX (6), Gemcitabine plus Nab-paclitaxel (GnP) (3), and Gemcitabine (1).
The median time to progression from the date of surgery to the first systemic relapse for the resected patients was 10.5 mo (1.5-10.6). After a follow-up period of 20.4 mo, 6 (55%) patients had died of the disease while five are still alive. The median overall survival (OS) of the cohort was 20.4 mo (range 4.6-36.0) but this variable is temporally immature. The median OS of the four resected patients was 30.3 mo (28.2-36.0).
Eligibility for germline genetic testing in Ontario has evolved with the advent of next-generation sequencing, newly identified genes, and the association of established genes with different cancer types. In April 2021, Ontario Health expanded the availability of germline testing to all individuals with pancreatic cancer regardless of age or family history[5]. Before this, germline testing for individuals with pancreatic cancer was based on personal and family history as well as the age of onset. The gene(s) or multi-gene panels performed for patients are based on the individuals’ personal and family history at the time of the initial genetic counseling.
Seven patients in our case series had clinical germline testing. Four patients did not have germline testing, as they did not meet eligibility criteria based on family history at the time of their diagnosis. Germline PV/LPV was identified in four patients [2 BRCA2 (18%), 1 ATM (9%), 1 CDKN2A (9%)]. Two (18%) patients had somatic testing with whole-genome sequencing and RNA sequencing as part of clinical trial participation. Identified somatic variants were SND1-BRAF fusion in one patient and KRAS, SMAD4, CDKN2A, ATM, TP53, TGFBR2, and KDM6A in another patient.
In terms of actionability, we identified two patients with BRCA2 PV/LPV (18%) and one SND1-BRAF fusion (9%). The first patient carrying a germline BRCA2 LPV is described in this case report. The second patient carrying a germline BRCA2 PV had advanced acinar neuroendocrine carcinoma. Briefly, he was commenced on a combination of 5 FU and Oxaliplatin with a dose reduction (30%) due to comorbidities. Despite this, the evaluation CT scan following 8 cycles of chemotherapy showed a partial response of the primary tumor (63% smaller than baseline) as per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. He continued additional 4 cycles with further tumor regression, followed by resection. The tumor was pathologic near complete treatment response.
The patient harboring an SND1-BRAF fusion was commenced on first line GnP. The evaluation CT scans following 6 cycles of chemotherapy showed a significant partial response (55% decrease than baseline) of the primary tumor and lymph nodes as per RECIST1.1. After 16 cycles of GnP, he developed progressive disease and was switched to single-agent Cobimetinib as part of clinical trial participation. Molecular profiling was negative for other key driver mutations KRAS, TP53, CDKN2A, SMAD4, and BRCA in this patient. Full mutational profiles of the patients and treatment history are outlined in Table 2.
| Year of diagnosis | Age at diagnosis | Germline testing | Somatic testing | Driver mutation | Personal or family history of malignancy | Disease staging | Surgery and perioperative chemotherapy | TTP after surgery (mo) | First line systemic therapy | Second line systemic therapy | Best treatment and clinical status | OS (mo) |
| 2014 | 65 | BRCA1, BRCA2 negative | Not performed | Nil | Family history of leukemia, colorectal, ovarian, and prostate cancers | T3N0M0 R1 | Neoadjuvant mFFX with radiotherapy followed by Whipple procedure | 1.5 | Gemcitabine for 4 cycles | BSC | PD, DOD | 28.2 |
| MMR IHC intact | ||||||||||||
| 2014 | 63 | Not performed | Not performed | Nil | Nil | T3N1bM0 R0 | Whipple procedure with perioperative mFFX for 12 cycles | 10.4 | BSC | Nil | PD, DOD | 23.2 |
| 2014 | 74 | CDKN2A pathogenic variant c.159G>C, p.Met53Ile | Not performed | Nil | Family history of melanoma and PDAC | Stage IV | Nil | NA | mFFX for 3 cycles | BSC | PD, DOD | 4.6 |
| Personal history of malignant melanoma | ||||||||||||
| 2015 | 60 | Not performed | Not performed | Nil | Thoracic cancer | T2N0M0 R0 | Distal pancreatectomy with adjuvant Gemcitabine for 2 cycles which were discontinued due to toxicities | 10.7 | GnP 18 mo | N/A | SD, DOD | 36.0 |
| 2017 | 66 | WGS and RNA seq | WGS and RNA seq | KRAS, SMAD4 | Nil | Stage IV | Nil | NA | mFFX for 8 cycles | BSC | SD, DOD | 13.4 |
| KRAS, SMAD4, CDKN2A TP53, ATM, TGFBR2, and KDM6A | ||||||||||||
| MMR IHC intact | ||||||||||||
| 2018 | 64 | Not performed | Not performed | Nil | Nil | T3N0M0 R0 | Whipple procedure. No adjuvant therapy | 10.6 | GnP for 4 cycles | BSC | PD, DOD | 32.3 |
| 2020 | 61 | 91 gene panel | WGS and RNA seq | BRAF | Family history of PDAC | Stage IV | Nil | NA | GnP for 16 cycles | Cobimetinib 60 mg OD PO (enrolled on CAPTUR trial | PR, AWD | 20.4 ongoing |
| SND1-BRAF fusion | ||||||||||||
| MMR IHC intact | ||||||||||||
| 2021 | 70 (case described) | 12 gene panel | Not performed | BRCA2 | Family history of ovarian cancer | Stage IV | Nil | NA | mFFX for 11 cycles | Maintenance Olaparib 150 mg twice daily | PR, AWD | 11.5 ongoing |
| BRCA2 | ||||||||||||
| Likely pathogenic variant | ||||||||||||
| c.4356delinsCA, p.Gln1452Hisfs*8 | MMR IHC intact | Personal history of renal cell cancer and NSCLA | ||||||||||
| 2021 | 65 | 91 gene panel | Not performed | Nil | Family history of head and neck cancer | Stage IV | Nil | NA | mFFX for 14 cycles, followed by maintenance FOLFIRI | RP-3500 in combination with Gemcitabine (enrolled on RP-3500-01 trial) | SD, ADW | 13.4 ongoing |
| ATM | ||||||||||||
| Pathogenic variant | ||||||||||||
| c.8418+5_8418+8del | ||||||||||||
| 2021 | 71 | BRCA1, BRCA 2 | Not performed | BRCA2 | Family history of breast cancers | Stage IV | Nil | NA | 5FU with Oxaliplatin for 12 cycles, downsized to Whipple procedure | N/A | PR, AWD | 12.1 ongoing |
| BRCA2 pathogenic variant c.8904delC, p.Val2969Cysfs*7 | ||||||||||||
| 2021 | 57 | 12 gene panel negative | Not performed | Nil | Family history of non-Hodgkins Lymphoma | Stage IV | Nil | NA | Ongoing mFFX; had 16 cycles | N/A | PR, AWD | 12.3 ongoing |
These findings were compatible with PACC with panniculitis, hepatic metastases, and indeterminate bony involvement. Histology revealed no concurrent existence of ductal adenocarcinoma, neuroendocrine or mixed tumor of the pancreas.
The initial plan was to treat the patient with GnP in consideration of his poor PS. However, this decision was changed to the mFFX regimen following the documentation of the germline BRCA2 LPV. mFFX was administered every 2-wk with an additional 20% dose reduction of Oxaliplatin and Irinotecan (Oxaliplatin 65 mg/m2, Irinotecan 120 mg/m2, Fluorouracil 4200 mg/m2 and Folinic acid 400 mg/m2). The chemotherapy calculations were based on a body surface area of 1.77 m2.
After the first cycle of mFFX, the patient was hospitalized due to fever, confusion, and worsening polyarthritis. A full septic screen revealed no clear infectious etiology. CT brain showed no brain abnormality. X-rays of several joint areas including sacroiliac joints showed no radiographic evidence of osteomyelitis or septic arthritis. A left knee joint aspiration revealed an inflammatory synovial fluid with elevated white blood cell count, but no growth of infectious organisms and negative for crystal arthropathy. Rheumatoid factor and anti-cyclic citrullinated peptide levels were negative. The rheumatology team believed the patient’s inflammatory seronegative arthritis was paraneoplastic in nature. The patient also displayed clinical adverse events consistent with steroid-induced psychosis, due to the concurrent prednisolone and dexamethasone use. He was started on Naproxen with a tapering dose of prednisolone (from 15 mg daily). His condition improved within a week time and chemo
After 11 cycles of mFFX, we decided to stop chemotherapy due to the accumulative neurotoxicity. Considering the germline BRCA2 LPV, we elected a therapeutic switch to Olaparib, a polyadenosine diphosphate-ribose polymerase inhibitor (PARPi), as maintenance therapy. He was started on Olaparib 150 mg twice daily dosing that was adjusted for his renal function. At the time of this writing, the patient experienced disease stability for 5 mo with Olaparib, which is ongoing. He tolerates Olaparib with grade 1 fatigue but has no major side effects. He is on monthly follow-ups.
PACC typically presents in the younger population with a median age of 62 years old. It is more frequent in males, with a male-to-female ratio of 2.3:1[6-8]. The majority (50%-60%) present at an advanced stage, with a median tumor size of 7 cm, and lesions smaller than 2 cm are rarely detected[6-10]. Some cases present with mixed differentiation including mixed acinar-ductal and mixed acinar-neuroendocrine subtypes. As the tumor is predominantly found in the tail of the pancreas, patients do not usually present with biliary obstruction, and elevation of CA19-9 is not typically seen[3,11]. However, there have been reports of elevated alpha-fetoprotein in younger patients[7,8]. In extreme cases, up to 10%, of patients have lipase hypersecretion which leads to systemic fat necrosis with eosinophilia, erythematous subcutaneous nodules, and polyarthralgia[6,7,9,10]. This paraneoplastic syndrome, also known as Schmid’s triad, is often associated with a poor prognosis[12-15]. The prognosis of PACC is slightly better than that for PDAC[6]. In comparison, 5-year OS for PACC was 42.8% vs PDAC 3.8%[16]. In this study, we analyzed the full clinical characteristics, therapeutic outcomes, and mutational signatures of 11 patients with PACC treated at our center. Based on our analysis, the median OS across all stages is 20.4 mo and 30.3 mo among the resected patients.
Available literature suggests that over one-third of PACC patients harbor potentially druggable alterations such as BRCA2, PALB2, ATM, BRAF, and JAK1[17]. We observed only one PACC with somatic KRAS mutation (9%). This result may be limited by the incomplete somatic testing rate in this study. In distinction to PDAC which is associated with KRAS driver mutations in more than 93% of cases, KRAS mutations occur at a much lower prevalence in the acinar/mixed neuroendocrine tumor (9%)[18-21]. While it is difficult to generalize as pancreatic carcinoma is a complex heterogeneous disease, a strong argument can be made that the lack of mutated KRAS identifies a cohort rich in targetable alterations including fusions, and should have access to integrative germline and somatic sequencing[22].
Multiple studies including a large series reported by Chmielecki et al[2] involving 44 PACCs reported a 45% deficiency of DDR pathway genes[3,4,23]. These are inclusive of deficiencies in the BRCA pathway and mismatch repair. Combined results suggested that approximately 23% of PACCs are enriched with fusion rearrangements involving BRAF or RAF1 genes[2,19]. It appears that PACC subgroups that are lacking RAF1 rearrangements (i.e., fusion-negative tumors) were significantly enriched for deficiency in HRD, and both tumor types are mutually exclusive[2]. Conceptually, these “fusion-negative” tumors can serve as a beneficial demarcation in over two-thirds of PACC patients who may be candidates for platinum-based chemotherapy. PACC with BRCA1/2 variants have greater sensitivity to platinum-based chemotherapy and demonstrate significantly better OS than when treated with non-platinum agents[24]. Platinum chemotherapy drugs exert their cytotoxic effect by binding directly to DNA, causing crosslinking of DNA strands and thereby inducing DNA double-strand breaks, which also are ineffectively repaired in cells lacking functioning BRCA1/2. Both the patients in our case series with germline BRCA2 PV/LPV had substantial radiographic regression despite dose reduced Oxaliplatin. Although our patient described in the case report was very unwell with poor PS at presentation, raising the question of suitability for mFFX, the knowledge of the BRCA2 LPV as predictive for platinum sensitivity guided our decision to use this regime and resulted in his improved outcome. The other patient was successfully downsized to enable the Whipple procedure for curative intent. Notably, we identified one patient with SND1-BRAF kinase fusion in our case series. Germline and somatic testing were negative for BRCA1 or BRCA2 in this patient. This particular variant fusion joins SND1 exons 1-10 with BRAF exons 11-18 and maintains the reading frame. It is worth noting that this particular configuration is the most prevalent gene fusion described in melanoma, thyroid, and lung cancers. It has also been reported in PACC[2]. This novel fusion is potentially targetable with MEK inhibitors, such as Trametinib and Cobimetinib[2,25].
Germline testing and tumor sequencing results are invaluable in identifying PACC patients for treatment regime determination and predictive biomarkers for investigational targeted therapies[14,22,23,26,27]. Newly diagnosed patients with PACC should undergo germline genetic testing and somatic profiling where appropriate, given the high frequency of pathogenic germline BRCA alterations in PACC. This should be made available to patients regardless of clinical presentation, the pattern of metastases, and pre-existing co-morbidities. This is also consistent with NCCN American Society of Clinical Oncology guidelines which recommend all PDAC patients have upfront germline testing as part of the evolving precision strategy and screening strategies[28]. Similar to numerous studies, our patients with pathogenic BRCA1/2 variants have an increased risk of pancreatic, ovarian, breast, and other cancers (Table 2). The lifetime risk for pancreatic cancer in BRCA1 and BRCA2 mutant carriers is 1% and 4.9%, respectively[29,30]. Unlike breast and ovarian cancers, germline BRCA1/2 mutations alone do not pose a significant risk of pancreatic cancers. Recent literature review shows BRCA2 confer to 5%-17% of familial pancreatic cancers (FPC) and BRCA1 is not as highly prevalent[31-34]. Studies show that germline susceptibility gene mutations were not found in 80% of pancreatic cancer individuals with strong family history[31,35]. Therefore, comprehensive genome sequencing is needed to identify new possible deleterious genes associated with FPC.
There are no current clinical practice algorithms for PACC, and it is treated in the same way as PDAC. Although FOLFIRINOX represents the standard treatment with the highest efficacy in PDAC, it is not well studied in PACC[36]. Since 2010, there is a recognized OS benefit to platinum-based agents compared to Gemcitabine or Capecitabine-based regimens, and current therapeutic approaches of metastatic PACCs utilize more FOLFOX or FOLFIRINOX. Furukawa et al[37] described a PACC patient with a BRCA2 PV who received Cisplatin after a recurrence of liver metastasis and had a complete remission of the recurring tumor. Ploquin et al[38] reported a PACC patient with a BRCA2 PV who experienced a 14-year complete remission following nine cycles of GEMOX, without surgical intervention. Therefore, Cisplatin and GEMOX may be alternatives in patients harboring deficiencies in DDR genes who are unfit for FOLFIRINOX.
Due to accumulative neurotoxicity after 11 cycles of mFFX, our patient decided to stop systemic chemotherapy completely and de-escalated to Olaparib as a maintenance approach. The use of PARPi in PACC patients with germline BRCA1 or BRCA2 PV/LPV is anecdotal[2,23,26]. Furthermore, the updated analysis of the POLO trial showed a lack of OS benefit and quality of life improvement in their Olaparib-treated patients compared to the placebo arm[39]. Despite the aforementioned, we believe that metastatic PACC patients with confirmed HRD phenotype and demonstrated strictly defined platinum sensitivity that involved exceptional response after 16 wk of chemotherapy should be considered for the benefit of PARPi, as the case described.
Like PDAC, surgery offers the best treatment approach for improved long-term survival[11,16,40]. The combination of surgical approach and perioperative chemotherapy in PACC is mainly adapted from the PDAC practice[40-42]. As mentioned, our patient with metastatic germline BRCA2 PV had remarkable tumor downstaging following mFFX, underwent curative surgery, and achieved a pathologic near complete treatment response. Optimizing treatment approaches from this standpoint, with growing access to germline and somatic profiling, should also be further explored in PACC.
Although it is a rare disease, it is important to identify both common and rare actionable variants in PACCs. In PACC patients with BRCA variants, the maintenance treatment of PARPi after effective platinum-based chemotherapy should be explored further. Surgical resection may provide the chance of cure after induction chemotherapy in very well-selected patients, particularly in patients with BRCA variants. Further large-scale studies are required to verify these therapeutic strategies for PACC patients.
We acknowledged Dr. Thiago Muniz’s contribution to reviewing this manuscript for grammar and syntax.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kitamura K, Japan; Pan Y, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Al-Hader A, Al-Rohil RN, Han H, Von Hoff D. Pancreatic acinar cell carcinoma: A review on molecular profiling of patient tumors. World J Gastroenterol. 2017;23:7945-7951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Chmielecki J, Hutchinson KE, Frampton GM, Chalmers ZR, Johnson A, Shi C, Elvin J, Ali SM, Ross JS, Basturk O, Balasubramanian S, Lipson D, Yelensky R, Pao W, Miller VA, Klimstra DS, Stephens PJ. Comprehensive genomic profiling of pancreatic acinar cell carcinomas identifies recurrent RAF fusions and frequent inactivation of DNA repair genes. Cancer Discov. 2014;4:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 140] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Thompson ED, Wood LD. Pancreatic Neoplasms With Acinar Differentiation: A Review of Pathologic and Molecular Features. Arch Pathol Lab Med. 2020;144:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 4. | Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K, Quinn MC, Robertson AJ, Fadlullah MZ, Bruxner TJ, Christ AN, Harliwong I, Idrisoglu S, Manning S, Nourse C, Nourbakhsh E, Wani S, Wilson PJ, Markham E, Cloonan N, Anderson MJ, Fink JL, Holmes O, Kazakoff SH, Leonard C, Newell F, Poudel B, Song S, Taylor D, Waddell N, Wood S, Xu Q, Wu J, Pinese M, Cowley MJ, Lee HC, Jones MD, Nagrial AM, Humphris J, Chantrill LA, Chin V, Steinmann AM, Mawson A, Humphrey ES, Colvin EK, Chou A, Scarlett CJ, Pinho AV, Giry-Laterriere M, Rooman I, Samra JS, Kench JG, Pettitt JA, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Jamieson NB, Graham JS, Niclou SP, Bjerkvig R, Grützmann R, Aust D, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Wolfgang CL, Morgan RA, Lawlor RT, Corbo V, Bassi C, Falconi M, Zamboni G, Tortora G, Tempero MA; Australian Pancreatic Cancer Genome Initiative, Gill AJ, Eshleman JR, Pilarsky C, Scarpa A, Musgrove EA, Pearson JV, Biankin AV, Grimmond SM. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2130] [Cited by in RCA: 1995] [Article Influence: 199.5] [Reference Citation Analysis (1)] |
| 5. | Cancer Care Ontario. 2021 Hered. Cancer Test. Eligibility Criteria Version 2. [cited 18 July 2022]. Available from: https://www.cancercareontario.ca/en/guidelines-advice/types-of-cancer/70161. |
| 6. | Hackeng WM, Hruban RH, Offerhaus GJ, Brosens LA. Surgical and molecular pathology of pancreatic neoplasms. Diagn Pathol. 2016;11:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Toll AD, Hruban RH, Ali SZ. Acinar cell carcinoma of the pancreas: clinical and cytomorphologic characteristics. Korean J Pathol. 2013;47:93-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | La Rosa S, Sessa F, Capella C. Acinar Cell Carcinoma of the Pancreas: Overview of Clinicopathologic Features and Insights into the Molecular Pathology. Front Med (Lausanne). 2015;2:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 9. | Kitagami H, Kondo S, Hirano S, Kawakami H, Egawa S, Tanaka M. Acinar cell carcinoma of the pancreas: clinical analysis of 115 patients from Pancreatic Cancer Registry of Japan Pancreas Society. Pancreas. 2007;35:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 10. | de Frutos Rosa D, Espinosa Taranilla L, González de Canales de Simón P, Vélez Velázquez MD, Guirado Koch C. Pancreatic panniculitis as a presentation symptom of acinar cell carcinoma. Rev Esp Enferm Dig. 2018;110:329-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Schmidt CM, Matos JM, Bentrem DJ, Talamonti MS, Lillemoe KD, Bilimoria KY. Acinar cell carcinoma of the pancreas in the United States: prognostic factors and comparison to ductal adenocarcinoma. J Gastrointest Surg. 2008;12:2078-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Borowicz J, Morrison M, Hogan D, Miller R. Subcutaneous fat necrosis/panniculitis and polyarthritis associated with acinar cell carcinoma of the pancreas: a rare presentation of pancreatitis, panniculitis and polyarthritis syndrome. J Drugs Dermatol. 2010;9:1145-1150. [PubMed] |
| 13. | Martin SK, Agarwal G, Lynch GR. Subcutaneous fat necrosis as the presenting feature of a pancreatic carcinoma: the challenge of differentiating endocrine and acinar pancreatic neoplasms. Pancreas. 2009;38:219-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Xing-Mao Z, Hong-Juan Z, Qing L, Qiang H. Pancreatic acinar cell carcinoma-case report and literature review. BMC Cancer. 2018;18:1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Dahl PR, Daniel Su WP, Cullimore KC, Dicken CH. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417. [RCA] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Wisnoski NC, Townsend CM Jr, Nealon WH, Freeman JL, Riall TS. 672 patients with acinar cell carcinoma of the pancreas: a population-based comparison to pancreatic adenocarcinoma. Surgery. 2008;144:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Jiao Y, Yonescu R, Offerhaus GJ, Klimstra DS, Maitra A, Eshleman JR, Herman JG, Poh W, Pelosof L, Wolfgang CL, Vogelstein B, Kinzler KW, Hruban RH, Papadopoulos N, Wood LD. Whole-exome sequencing of pancreatic neoplasms with acinar differentiation. J Pathol. 2014;232:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, Locasale JW, Son J, Zhang H, Coloff JL, Yan H, Wang W, Chen S, Viale A, Zheng H, Paik JH, Lim C, Guimaraes AR, Martin ES, Chang J, Hezel AF, Perry SR, Hu J, Gan B, Xiao Y, Asara JM, Weissleder R, Wang YA, Chin L, Cantley LC, DePinho RA. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1246] [Cited by in RCA: 1573] [Article Influence: 121.0] [Reference Citation Analysis (0)] |
| 19. | Aung KL, Fischer SE, Denroche RE, Jang GH, Dodd A, Creighton S, Southwood B, Liang SB, Chadwick D, Zhang A, O'Kane GM, Albaba H, Moura S, Grant RC, Miller JK, Mbabaali F, Pasternack D, Lungu IM, Bartlett JMS, Ghai S, Lemire M, Holter S, Connor AA, Moffitt RA, Yeh JJ, Timms L, Krzyzanowski PM, Dhani N, Hedley D, Notta F, Wilson JM, Moore MJ, Gallinger S, Knox JJ. Genomics-Driven Precision Medicine for Advanced Pancreatic Cancer: Early Results from the COMPASS Trial. Clin Cancer Res. 2018;24:1344-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 431] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 20. | Ma LX, Jang GH, Zhang A, Denroche RE, Dodd A, Ramotar S, Hutchinson S, Wang Y, Tehfe M, Ramjeesingh R, Biagi JJ, Lam B, Wilson J, Notta F, Fischer S, Grant RC, Zogopoulos G, Gallinger S, Knox JJ, O’Kane GM. Impact of KRAS mutational status on outcomes in patients with pancreatic cancer (PDAC). J Clin Oncol. 2021;39:4142. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2017;32:185-203.e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1247] [Cited by in RCA: 1390] [Article Influence: 173.8] [Reference Citation Analysis (0)] |
| 22. | O'Kane GM, Lowery MA. Moving the Needle on Precision Medicine in Pancreatic Cancer. J Clin Oncol. 2022;40:2693-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Lowery MA, Wong W, Jordan EJ, Lee JW, Kemel Y, Vijai J, Mandelker D, Zehir A, Capanu M, Salo-Mullen E, Arnold AG, Yu KH, Varghese AM, Kelsen DP, Brenner R, Kaufmann E, Ravichandran V, Mukherjee S, Berger MF, Hyman DM, Klimstra DS, Abou-Alfa GK, Tjan C, Covington C, Maynard H, Allen PJ, Askan G, Leach SD, Iacobuzio-Donahue CA, Robson ME, Offit K, Stadler ZK, O'Reilly EM. Prospective Evaluation of Germline Alterations in Patients With Exocrine Pancreatic Neoplasms. J Natl Cancer Inst. 2018;110:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 178] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 24. | Lowery MA, Kelsen DP, Stadler ZK, Yu KH, Janjigian YY, Ludwig E, D'Adamo DR, Salo-Mullen E, Robson ME, Allen PJ, Kurtz RC, O'Reilly EM. An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. Oncologist. 2011;16:1397-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 25. | Menzies AM, Yeh I, Botton T, Bastian BC, Scolyer RA, Long GV. Clinical activity of the MEK inhibitor trametinib in metastatic melanoma containing BRAF kinase fusion. Pigment Cell Melanoma Res. 2015;28:607-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Li M, Mou Y, Hou S, Cao D, Li A. Response of germline BRCA2-mutated advanced pancreatic acinar cell carcinoma to olaparib: A case report. Medicine (Baltimore). 2018;97:e13113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Béchade D, Desjardin M, Salmon E, Désolneux G, Bécouarn Y, Evrard S, Fonck M. Pancreatic Acinar Cell Carcinoma. Case Rep Gastroenterol. 2016;10:174-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 697] [Article Influence: 174.3] [Reference Citation Analysis (0)] |
| 29. | Thompson D, Easton DF; Breast Cancer Linkage Consortium. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 768] [Article Influence: 33.4] [Reference Citation Analysis (1)] |
| 30. | Ferrone CR, Levine DA, Tang LH, Allen PJ, Jarnagin W, Brennan MF, Offit K, Robson ME. BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. J Clin Oncol. 2009;27:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Kasuga A, Okamoto T, Udagawa S, Mori C, Mie T, Furukawa T, Yamada Y, Takeda T, Matsuyama M, Sasaki T, Ozaka M, Ueki A, Sasahira N. Molecular Features and Clinical Management of Hereditary Pancreatic Cancer Syndromes and Familial Pancreatic Cancer. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 32. | Axilbund JE, Argani P, Kamiyama M, Palmisano E, Raben M, Borges M, Brune KA, Goggins M, Hruban RH, Klein AP. Absence of germline BRCA1 mutations in familial pancreatic cancer patients. Cancer Biol Ther. 2009;8:131-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Couch FJ, Johnson MR, Rabe KG, Brune K, de Andrade M, Goggins M, Rothenmund H, Gallinger S, Klein A, Petersen GM, Hruban RH. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:342-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 34. | Hahn SA, Greenhalf B, Ellis I, Sina-Frey M, Rieder H, Korte B, Gerdes B, Kress R, Ziegler A, Raeburn JA, Campra D, Grützmann R, Rehder H, Rothmund M, Schmiegel W, Neoptolemos JP, Bartsch DK. BRCA2 germline mutations in familial pancreatic carcinoma. J Natl Cancer Inst. 2003;95:214-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 336] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 35. | Permuth-Wey J, Egan KM. Family history is a significant risk factor for pancreatic cancer: results from a systematic review and meta-analysis. Fam Cancer. 2009;8:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 36. | Sridharan V, Mino-Kenudson M, Cleary JM, Rahma OE, Perez K, Clark JW, Clancy TE, Rubinson DA, Goyal L, Bazerbachi F, Visrodia KH, Qadan M, Parikh A, Ferrone CR, Casey BW, Fernandez-Del Castillo C, Ryan DP, Lillemoe KD, Warshaw AL, Krishnan K, Hernandez-Barco YG. Pancreatic acinar cell carcinoma: A multi-center series on clinical characteristics and treatment outcomes. Pancreatology. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 37. | Furukawa T, Sakamoto H, Takeuchi S, Ameri M, Kuboki Y, Yamamoto T, Hatori T, Yamamoto M, Sugiyama M, Ohike N, Yamaguchi H, Shimizu M, Shibata N, Shimizu K, Shiratori K. Whole exome sequencing reveals recurrent mutations in BRCA2 and FAT genes in acinar cell carcinomas of the pancreas. Sci Rep. 2015;5:8829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 38. | Ploquin A, Baldini C, Vuagnat P, Makhloufi S, Desauw C, Hebbar M. Prolonged Survival in a Patient with a Pancreatic Acinar Cell Carcinoma. Case Rep Oncol. 2015;8:447-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Kindler HL, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, Bordia S, McGuinness D, Cui K, Locker GY, Golan T. Overall Survival Results From the POLO Trial: A Phase III Study of Active Maintenance Olaparib Versus Placebo for Germline BRCA-Mutated Metastatic Pancreatic Cancer. J Clin Oncol. 2022;JCO2101604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 120] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 40. | Wang Y, Wang S, Zhou X, Zhou H, Cui Y, Li Q, Zhang L. Acinar cell carcinoma: a report of 19 cases with a brief review of the literature. World J Surg Oncol. 2016;14:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Klimstra DS, Heffess CS, Oertel JE, Rosai J. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol. 1992;16:815-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 346] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 42. | Webb JN. Acinar cell neoplasms of the exocrine pancreas. J Clin Pathol. 1977;30:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 2.2] [Reference Citation Analysis (0)] |