Published online Dec 7, 2022. doi: 10.3748/wjg.v28.i45.6397
Peer-review started: July 30, 2022
First decision: September 2, 2022
Revised: September 14, 2022
Accepted: October 19, 2022
Article in press: October 19, 2022
Published online: December 7, 2022
Processing time: 125 Days and 0.9 Hours
The optimal method to remove sessile colorectal lesions sized 10-20 mm remains uncertain. Piecemeal and incomplete resection are major limitations in current practice, such as endoscopic mucosal resection (EMR) and cold or hot snare polypectomy. Recently, EMR with circumferential precutting (EMR-P) has emerged as an effective technique, but the quality of current evidence in comparative studies of conventional EMR (CEMR) and EMR-P is limited.
To investigate whether EMR-P is superior to CEMR in removing sessile colorectal polyps.
This multicenter randomized controlled trial involved seven medical institutions in China. Patients with colorectal polyps sized 10-20 mm were enrolled and randomly assigned to undergo EMR-P or CEMR. EMR-P was performed following submucosal injection, and a circumferential mucosa incision (precutting) was conducted using a snare tip. Primary outcomes included a comparison of the rates of en bloc and R0 resection, defined as one-piece resection and one-piece resection with histologically assessed clear margins, respectively.
A total of 110 patients in the EMR-P group and 110 patients in the CEMR group were finally evaluated. In the per-protocol analysis, the proportion of en bloc resections was 94.3% [95% confidence interval (CI): 88.2%-97.4%] in the EMR-P group and 86% (95%CI: 78.2%-91.3%) in the CEMR group (P = 0.041), while subgroup analysis showed that for lesions > 15 mm, EMR-P also resulted in a higher en bloc resection rate (92.0% vs 58.8% P = 0.029). The proportion of R0 resections was 81.1% (95%CI: 72.6%-87.4%) in the EMR-P group and 76.6% (95%CI: 68.8%-84.4%) in the CEMR group (P = 0.521). The EMR-P group showed a longer median procedure time (6.4 vs 3.0 min; P < 0.001). No significant difference was found in the proportion of patients with adverse events (EMR-P: 9.1%; CEMR: 6.4%; P = 0.449).
In this study, EMR-P served as an alternative to CEMR for removing nonpedunculated colorectal polyps sized 10-20 mm, particularly polyps > 15 mm in diameter, with higher R0 and en bloc resection rates and without increasing adverse events. However, EMR-P required a relatively longer procedure time than CEMR. Considering its potential benefits for en bloc and R0 resection, EMR-P may be a promising technique in colorectal polyp resection.
Core Tip: The optimal method for removal of sessile colorectal lesions sized 10-20 mm remains uncertain. Piecemeal and incomplete resection are major limitations of conventional endoscopic mucosal resection (CEMR) in removing sessile colorectal polyps. In this study, we found that endoscopic mucosal resection with circumferential precutting achieved better en bloc resection (94.3% vs 86.0%; P = 0.041) with no increase in adverse events, and can serve as an alternative technique to CEMR in the removal of sessile colorectal lesions sized 10-20 mm.
- Citation: Zhang XQ, Sang JZ, Xu L, Mao XL, Li B, Zhu WL, Yang XY, Yu CH. Endoscopic mucosal resection-precutting vs conventional endoscopic mucosal resection for sessile colorectal polyps sized 10-20 mm. World J Gastroenterol 2022; 28(45): 6397-6409
- URL: https://www.wjgnet.com/1007-9327/full/v28/i45/6397.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i45.6397
Colorectal cancer (CRC) is the third most common cancer in men and second most common cancer in women[1]. In China, the incidence and mortality rates of CRC showed an increasing trend in 2000-2011; approximately 376.3 thousand new CRC cases and 191.0 thousand CRC deaths occurred in 2015[2], posing a severe threat to people’s health. Endoscopic resection of colorectal polyps has proven effective in reducing CRC incidence and mortality[2,3]. Currently, all polyps must be resected except for diminutive (≤ 5 mm) rectal and rectosigmoid polyps as they are predicted with high confidence to be hyperplastic[4].
Endoscopic mucosal resection (EMR) is a well-established method for removing sessile colorectal polyps. However, as the polyp size increases, the piecemeal resection rate increases[5,6], which is a well-known risk factor for local recurrence after EMR[7-9]. Although endoscopic submucosal dissection (ESD) has been increasingly used to overcome the disadvantage of EMR, the technically challenging, time-consuming practice and longer hospital stay required limit its wider use[10]. Recently, EMR with circumferential precutting (EMR-P), an alternative to conventional EMR (CEMR), has emerged as an effective method[11,12]. However, most comparative studies were retrospective, with a small sample size, involving large (≥ 20 mm) or difficult lesions (where en bloc resection could not be achieved by CEMR), and the quality of evidence was relatively poor.
To date, no high-quality evidence or specific recommendation is available in recent guidelines for the optimal resection of 10-20 mm sessile lesions. Therefore, a prospective comparative randomized study was conducted to compare the efficacy of EMR-P with that of CEMR in medium-sized (10-20 mm) colorectal polyps.
This prospective multicenter randomized controlled trial involved seven Chinese institutions: the First Affiliated Hospital, School of Medicine, Zhejiang University (Institution A); Renmin Hospital of Yuyao City, Zhejiang (Institution B); Jinhua Municipal Central Hospital, Zhejiang (Institution C); Taizhou Hospital, Zhejiang (Institution D); the Central Hospital of Lishui City, Zhejiang (Institution E); Ningbo Medical Center, Lihuili Hospital, Zhejiang (Institution F); and Ningbo First Hospital, Zhejiang (Institution G). The trial complied with the Declaration of Helsinki, and the study protocol was approved by the Institutional Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University (No. 20191477); Ningbo First Hospital, Zhejiang (No. 2020-R013) and other participating institutions. This study was registered at ClinicalTrials.gov (NCT04191473).
Patients aged ≥ 18 years undergoing endoscopic resection for colorectal mucosal lesions (adenoma, intramucosal adenocarcinoma, or sessile serrated adenoma/polyps) 10-20 mm in size were included. Endoscopic diagnosis of mucosal lesions was based on macroscopic appearance[13], narrow-band imaging (NBI) findings, or classification of pit patterns on magnifying chromoendoscopy (MCE).
The exclusion criteria were as follows: (1) Pedunculated lesions; (2) residual lesions after endoscopic resection; (3) lesions with submucosal infiltration judged by advanced endoscopic imaging; (4) lesions in patients with inflammatory bowel disease; (5) familial polyposis; (6) electrolyte abnormality; (7) coagulation disorders; (8) severe organ failure; and (9) patients who were pregnant or nursing and who were taking NSAID drugs or anticoagulant medications. All the patients were informed of the research aims and endoscopy procedures and provided written informed consent for research participation.
Colonoscopy was performed after bowel preparation with polyethylene glycol solution, magnesium sulfate, or sodium phosphate subject to the common practice of each center (the detailed information of used materials in each institution was listed in Supplementary Table 1). In both polypectomy proce
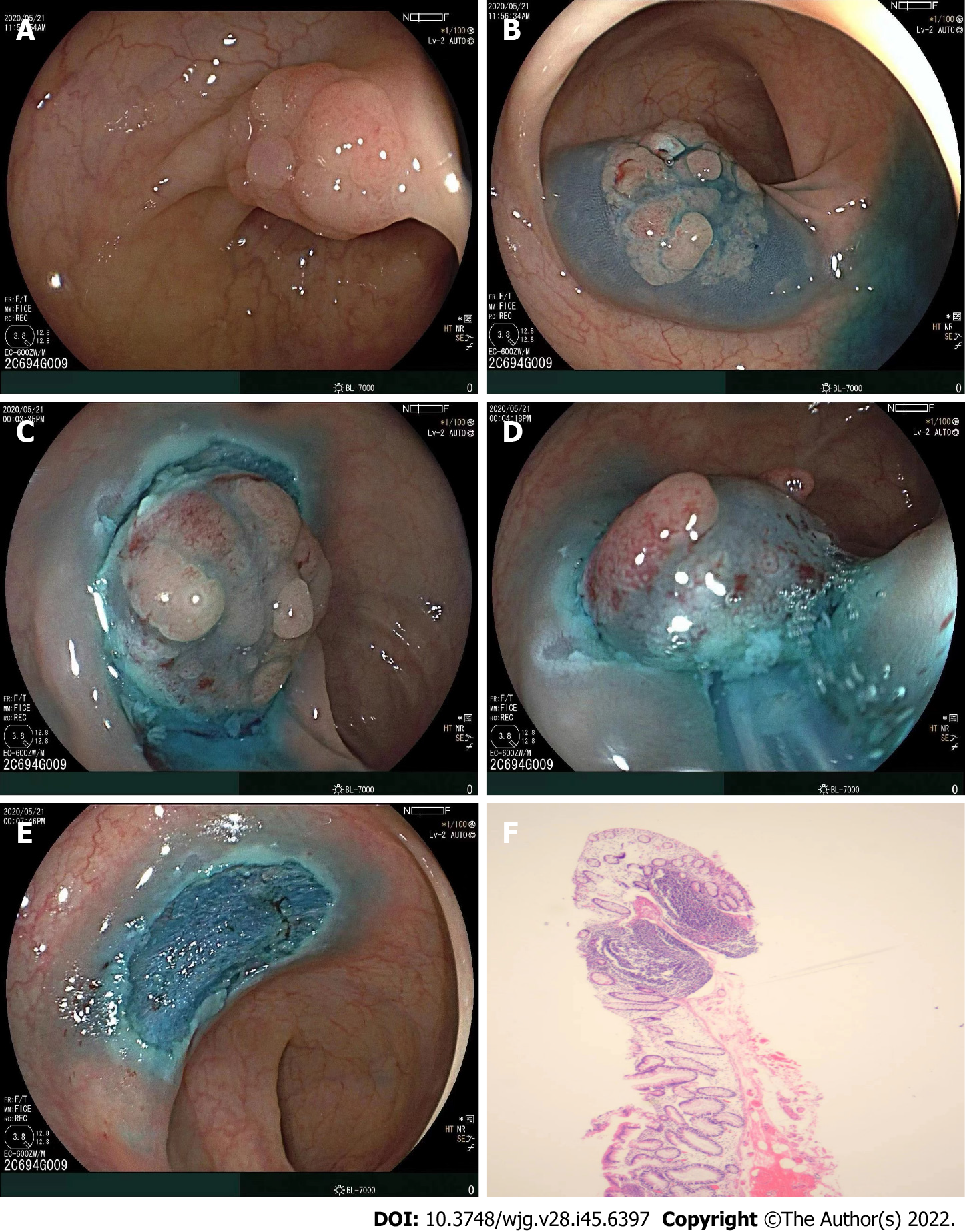
The EMR-P procedure included the following steps: (1) Submucosal solution injection; (2) a fully circumferential mucosa incision (precutting) using a snare tip to separate the lesion from the surrounding non-neoplastic mucosa (the polypectomy snares were chosen at the discretion of each institution); and (3) snaring the circumferential lesion and removing the tumor in the same manner as for CEMR. Following resection of the lesion, hemostatic forceps (with or without argon plasma coagulation) were used to prevent postoperative bleeding (Figure 1).
The Paris classification was used to classify the morphology of polyps with superficial appearance (category 0): Polypoid type [lesions 2.5 mm above the mucosal layer: Pedunculated (0-Ip), sessile (0-Is), or mixed (0-Isp)], nonpolypoid [lesions less than 2.5 mm (0-IIa), flat (0-IIb), or slightly depressed (0-IIc)], and mixed types[14].
Regarding patients with multiple lesions, only the lesion closest to the anus and sized 10-20 mm was registered to remove the subjective bias of researchers. All operations were performed by certain endoscopists in each center. Experienced endoscopists were defined as those who had performed > 1000 colonoscopies, > 300 EMRs, and > 10 ESDs. Compared with the size of open (approximately 7 mm) or closed (approximately 2 mm) biopsy forceps according to its endoscopic appearance, the size of the lesion was initially estimated and then was confirmed by comparison with an opened snare (20-30 mm) during treatment.
The SPSS™ Statistics (version 21; Chicago, IL, United States) software was used to generate random numbers, and simple randomization was adopted to determine whether the EMR-P or CEMR grouping depended on a 1:1 ratio. The operation method corresponding to the random numbers was sent to each center within a closed envelope by the research coordinator. A randomized competitive enrollment mode was applied at each center until the envelopes were allocated completely. Every enrolled patient received an envelope, but the grouping information was blinded for them during the endoscopic procedures. Only immediately before starting the colonoscopy was the operator informed of the treatment allocated by a research assistant.
The primary study outcomes were a comparison of the R0 and en bloc resection rates between the groups. The secondary outcomes included procedure time and adverse events (intraprocedural bleeding, postoperative bleeding, or perforation). En bloc resection was defined as one-piece resection, and R0 resection was defined as one-piece resection with histologically clear margins of lesions. Non-R0 resection included a positive resection margin (R1) or an unclear resection margin (RX). The procedure time of both polypectomy techniques was measured from the start of submucosal injection to complete removal of the polyp. Intraprocedural bleeding was defined as bleeding occurring during the procedure that persisted for more than 60 s and required any form of endoscopic hemostasis (e.g., endoscopic coagulation or mechanical therapy, with and without adrenaline injection)[4]. Postoperative bleeding was defined as, within 14 d after EMR-P or CEMR, hematochezia, with a > 2 g/dL decrease in hemoglobin or requiring endoscopic hemostasis. All the patients were followed up within two weeks after the operation to assess the presence of any adverse events.
It was hypothesized that EMR-P would be superior to CEMR for endoscopic R0 resection of colorectal polyps sized 10-20 mm. As previous studies reported that the CEMR R0 resection rate for colorectal polyps 10-20 mm in size was 40%-60%[15], it was preliminarily estimated that the R0 resection rate of CEMR for intermediate-sized (10-20 mm) colorectal polyps in the present study would be 50%. Next, an assumption was made that EMR-P could increase the R0 resection rate to 70%. The enrollment of 100 patients in each group provided 80% statistical power (α = 0.05) to detect a 20% difference between the groups. Considering a possibility of a dropout rate of 10%, it was planned to increase the sample size to 220 patients in total.
The primary endpoints were analyzed according to the principle of intention-to-treat (ITT). The chi-squared test and Fisher’s exact test were used to compare categorical variables, while the Mann-Whitney U-test and the unpaired two-sample t-test were used for continuous variables. Additionally, logistic regression analysis was applied to calculate the odds ratios. All the analyses were two-sided, and P values < 0.05 were considered statistically significant. If the primary outcomes of a patient were missing or abnormal, the case data were discarded directly; if the secondary outcomes were missing or abnormal, the mean value of each group was used as a replacement. All statistical analyses were performed using SPSS™ Statistics (version 21; Chicago, IL, United States) and R (version 4.1.2) software.
After resection, the specimens were immersed in 10% formalin and sent to the pathology department of each institution for histological evaluation. All the retrieved specimens were evaluated in terms of histologic types and involvement of the resection margin. Histological diagnosis of the lesion and involvement of the resection margin were evaluated according to the 2019 World Health Organization Classification of Tumors of the Digestive System.
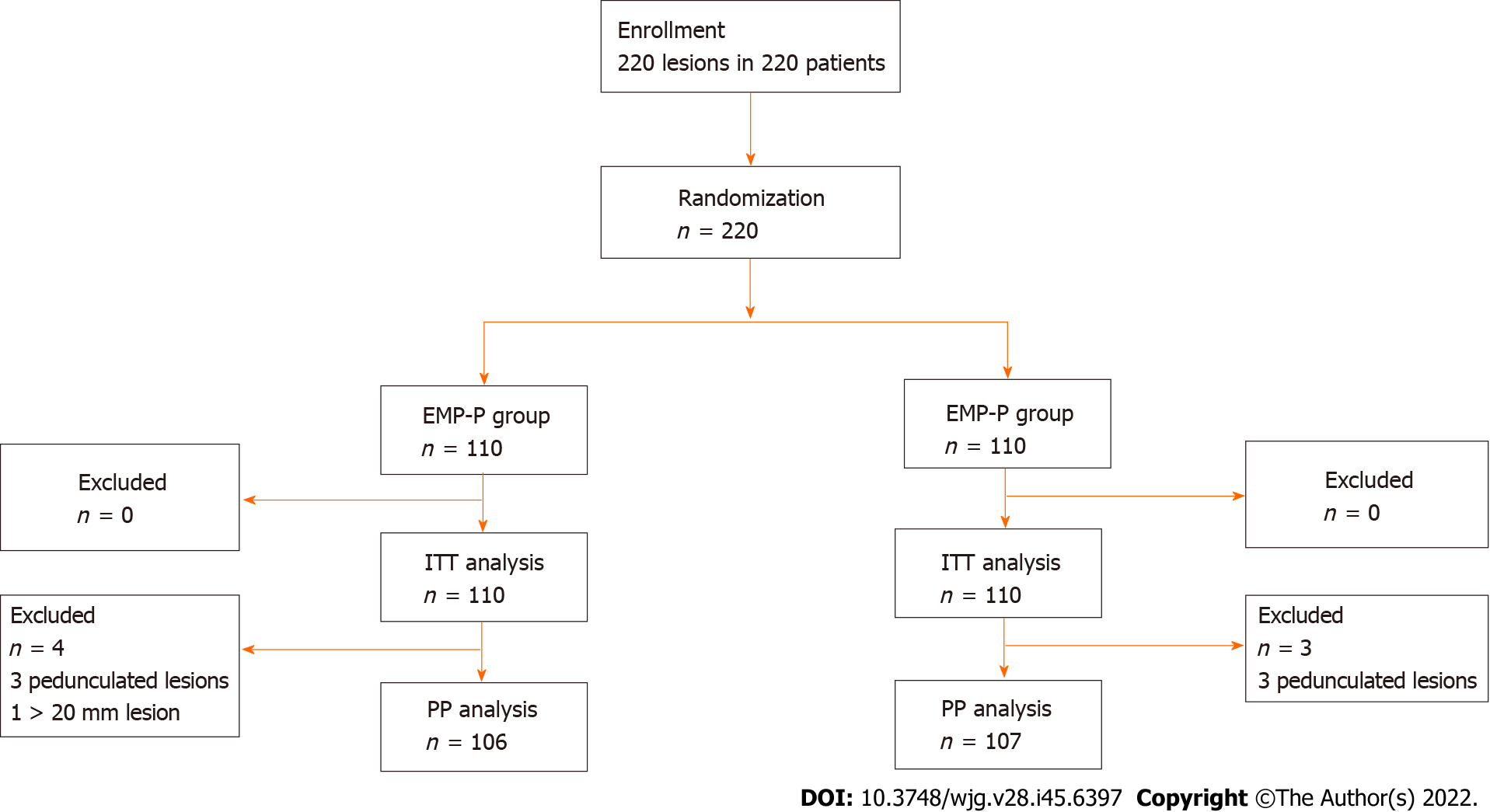
A total of 220 patients with 220 polyps were included in the study and were randomly assigned to two groups (the CEMR Group, n = 110; and the EMR-P Group, n = 110). These patients with 220 colorectal lesions were included in the ITT analysis for the primary endpoint. Three patients with pedunculated lesions in each group were excluded, while in the EMR-P group, one patient was excluded as the polyp was > 20 mm in size, leaving 213 patients (106 patients in the EMR-P group and 107 patients in the CEMR group) in the per-protocol analysis (PP analysis) (Figure 2).
No significant difference was observed in age, sex, location or morphology of lesions, polyp size or endoscopist experience between the groups. In this study, 97.0% of patients in the EMR-P group and 99.0% of patients in the CEMR group received prophylactic clipping of resected wounds. All 220 patients were hospitalized for endoscopic treatment (Table 1).
| Characteristic | EMR-P (n = 110), % | CEMR (n = 110), % | P value |
| Male (%) | 67 (60.9) | 70 (63.6) | 0.676 |
| Mean age (yr, ± SD) | 60.4 ± 10.4 | 59.9 ± 11.5 | 0.735 |
| Height (cm) | 164.4 ± 7.7 | 165.0 ± 7.5 | 0.566 |
| Weight (kg) | 61.4 ± 10.5 | 63.5 ± 10.7 | 0.151 |
| Location, n (%) | 0.725 | ||
| Cecum | 6 (5.5) | 5 (4.5) | |
| Ascending | 19 (17.3) | 18 (16.4) | |
| Transverse | 24 (21.8) | 28 (25.5) | |
| Descending | 7 (6.4) | 9 (8.2) | |
| Sigmoid | 26 (23.6) | 31 (28.2) | |
| Rectum | 28 (25.5) | 19 (17.3) | |
| Morphology, n (%) | 0.858 | ||
| 0-Ip | 3 (2.7) | 3 (2.7) | |
| 0-Is | 36 (32.7) | 42 (38.2) | |
| 0-IIa | 68 (61.8) | 62 (56.4) | |
| 0-IIb | 2 (1.8) | 3 (2.7) | |
| 0-IIc | 1 (0.9) | 0 (0.0) | |
| Institution, n (%) | 0.570 | ||
| A | 16 (14.5) | 9 (8.2) | |
| B | 31 (28.2) | 28 (25.5) | |
| C | 6 (5.5) | 5 (4.5) | |
| D | 19 (17.3) | 16 (14.5) | |
| E | 9 (8.2) | 14 (12.7) | |
| F | 13 (11.8) | 18 (16.4) | |
| G | 16 (14.5) | 20 (18.2) | |
| Tumor size (mm) | 14.0 ± 3.7 | 13.1 ± 3.1 | 0.055 |
| Operator experience, n (%) | 0.072 | ||
| Experienced endoscopist | 109 (99.1) | 103 (93.6) | |
| Non-experienced endoscopist | 1 (0.9) | 7 (6.4) |
The en bloc resection rate in the EMR-P group was higher than that in the CEMR group in both ITT and PP analyses (Table 1). However, only PP analysis showed a significant difference (94.3% vs 86.0%; P = 0.041). No significant difference was found in the R0, R1, and RX resection rates between the groups, though EMR-P showed a slightly but not significantly higher R0 resection rate. Histologically, most of the polyps in the two groups were adenomas (74.5% in the EMR-P group and 77.3% in the CEMR group), without a significant difference between the groups. The median operation times in the EMR-P and CEMR groups were 6.4 and 3.0 min (P < 0.001), respectively (Table 2).
| Parameter | EMR-P group, % | CEMR group, % | P value |
| ITT analysis | n = 110 | n = 110 | |
| En bloc resection | 103 (93.6) [87.5-96.9] | 95 (86.4) [78.7-91.6] | 0.072 |
| R0 resection | 89 (80.9) [72.6-87.2] | 86 (78.2) [69.6-84.9] | 0.616 |
| R1 resection | 9 (8.2) [4.4-14.8] | 9 (8.2) [4.4-14.8] | > 0.999 |
| RX resection | 6 (5.5) [2.5-11.4] | 6 (5.5) [2.5-11.4] | > 0.999 |
| PP analysis | n = 106 | n = 107 | |
| En bloc resection | 100 (94.3) [88.2-97.4] | 92 (86.0) [78.2-91.3] | 0.041 |
| R0 resection | 86 (81.1) [72.6-87.4] | 83 (77.6) [68.8-84.4] | 0.521 |
| R1 resection | 9 (8.4) [4.5-15.4] | 9 (8.5) [4.5-15.2] | 0.983 |
| RX resection | 6 (5.7) [2.6-11.8] | 6 (5.6) [2.6-11.7] | 0.987 |
| Histological type, n (%) | n = 110 | n = 110 | 0.354 |
| Adenoma | 82 (74.5) | 85 (77.3) | |
| Tubular adenoma with low-grade dysplasia | 62 (56.4) | 63 (57.3) | |
| Tubular adenoma with high-grade dysplasia | 6 (5.5) | 7 (6.4) | |
| Villous adenoma with low-grade dysplasia | 2 (1.8) | 1 (0.9) | |
| Villous adenoma with high-grade dysplasia | 0 (0.0) | 0 (0.0) | |
| Tubular villous adenoma with low-grade dysplasia | 10 (9.1) | 12 (10.9) | |
| Tubular villous adenoma with high-grade dysplasia | 2 (1.8) | 2 (1.8) | |
| Hyperplastic | 11 (10.0) | 14 (12.7) | |
| Serrated lesions | 9 (8.2) | 7 (6.4) | |
| Cancer | 4 (3.6) | 4 (3.6) | |
| Intramucosal adenocarcinoma | 3 (2.7) | 4 (3.6) | |
| Submucosal invasive adenocarcinoma | 1 (0.9) | 0 (0.0) | |
| Other | 4 (3.6) | 0 (0.0) | |
| Procedure time (min) | 6.4 (4.5, 10.3) | 3.0 (1.9, 6.2) | < 0.001 |
In the EMR-P group, four cases had bleeding during precutting while six cases had bleeding during the snaring and removal procedure; however, the occurrence of intraprocedural bleeding was not significantly different between the groups during the whole operation. One case of intraprocedural perforation occurred in the EMR-P group but did not result in a significant difference compared with the CEMR group (Table 3). These adverse events were successfully addressed by hemostatic clip placement. During the two-week follow-up period, no postprocedural bleeding or perforation occurred in either group, except for one patient who was required to undergo additional surgery due to the pathological diagnosis of rectal cancer with submucosal infiltration above 3500 µm.
| Parameter | EMR-P group (n = 110) | CEMR group (n = 110) | P value |
| Intraprocedural bleeding | 10 (9.1) | 7 (6.3) | 0.449 |
| Postoperative bleeding | 0 | 0 | / |
| Intraprocedural perforation | 1 | 0 | / |
| Postoperative perforation | 0 | 0 | / |
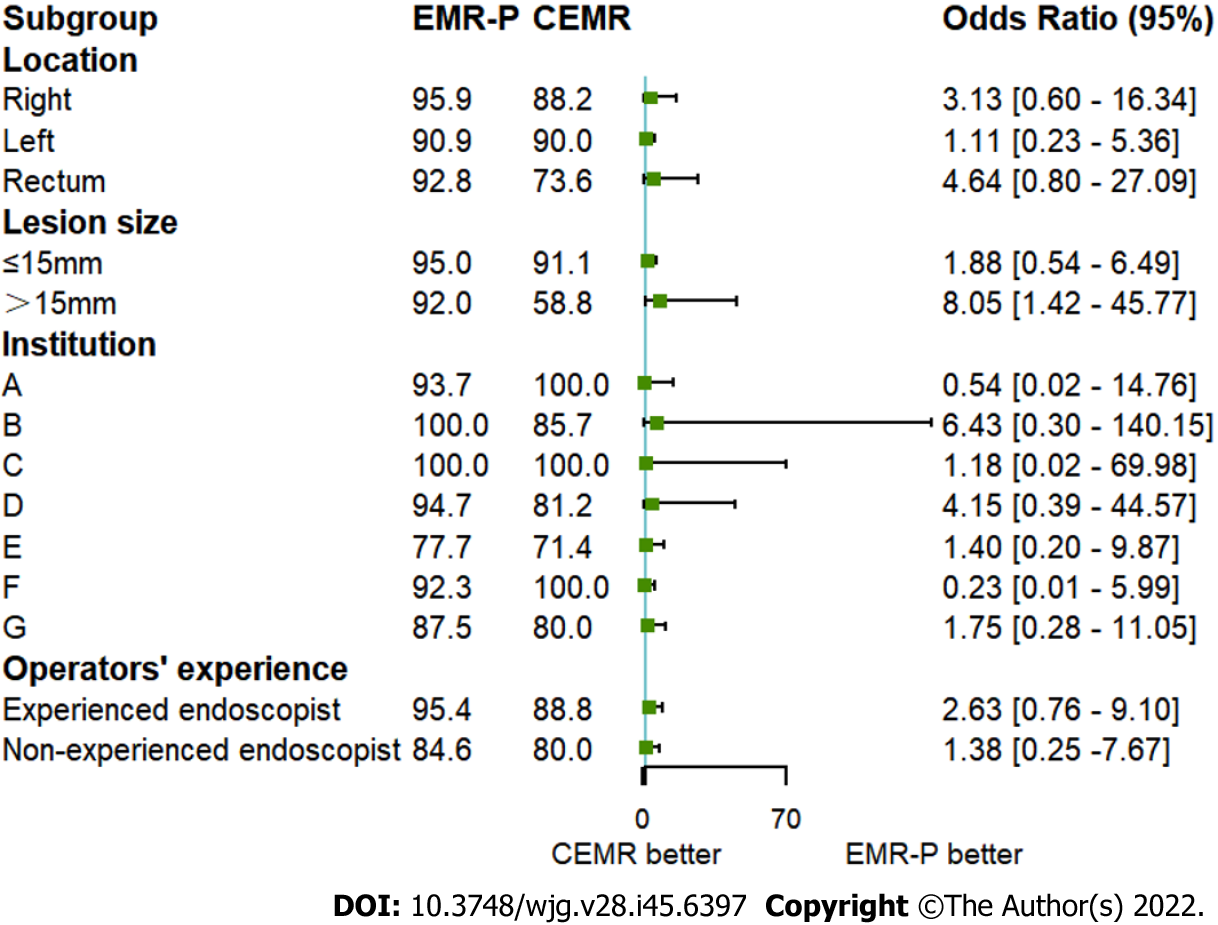
An exploratory subgroup analysis was conducted and found that in lesions > 15 mm, EMR-P was better for en bloc resection (92.0% vs 58.8%; P = 0.029). However, in lesions ≤ 15 mm, no significant difference was detected in the en bloc resection rate (95.0% vs 91.1%; P = 0.313). Overall, EMR-P showed a trend toward a higher en bloc resection rate than CEMR, regardless of location, lesion size, institution, or operator experience (Figure 3).
The present multicenter randomized controlled trial demonstrated that for medium-sized (10-20 mm) sessile colorectal polyps, the en bloc resection rate of EMR-P (94.3%) was significantly higher than that of CEMR (86%), particularly in lesions > 15 mm. Additionally, compared with CEMR, EMR-P did not increase the incidence of adverse events.
Although hot snare polypectomy (HSP) has been recommended by the European Society of Gastrointestinal Endoscopy Clinical Guidelines as a predominant technique to remove polyps sized 10-19 mm[4], incomplete resection remains an unavoidable issue. The Complete Adenoma Resection (“CARE”) study reported a high incomplete resection rate of 17.3% after HSP in polyps sized 10-20 mm[16]. Additionally, incompletely resected lesions may lead to 10%-30% of post-colonoscopy CRC[17].
Currently, EMR was proven to be superior to HSP in terms of the complete resection rate (89% vs 73%; P = 0.02)[18], a finding also supported by another pooled study[19]. However, the increased lesion size was accompanied by an augmented piecemeal EMR resection rate[6], an important risk factor for local recurrence and metachronous neoplasia[20-23]. Notably, Oka et al[23] revealed that piecemeal resection and histologically positive margins were both risk factors for local recurrence in univariate analysis; however, only piecemeal resection was an independent risk factor in multivariate logistic regression analysis. Furthermore, as previously reported, piecemeal-resected lesions reduced the quality and reliability of histological evaluation[24], possibly leading to the inability to provide proper additional treatment and recommendations of appropriate surveillance intervals[4,25]. To improve the effectiveness and safety of endoscopic colorectal lesion resection, several improved EMR techniques have been developed, such as EMR-P, underwater EMR (UEMR), anchored EMR, and cap-shaped EMR[4,26-28].
Of these, EMR-P was first described by Hirao et al[29] in 1986 and has been reported as EMR with a small or circumferential incision or simplified ESD[12,30,31]. The application of this method for gastric neoplasms has also been reported[32-34]. In 2007, Repici et al[35] first performed a single-center nonrandomized trial to evaluate the safety and efficacy of EMR-P (an it-knife was used for precutting) in the en bloc resection of large colonic polyps. Although only 55.1% (16 of 29 patients) of the lesions (> 30 mm) achieved en bloc resection, the authors suggested EMR-P was a promising technique.
Although some studies have confirmed the superiority of EMR-P to CEMR for large polyps (> 20 mm)[12,36], even noninferior to ESD[37,38], only one other study has directly compared the efficiency of EMR-P with CEMR in medium-sized polyps[11]. That study reported that EMR-P had a higher complete resection rate (87.8% vs 67.3%; P < 0.001) and en bloc resection rate (98.0% vs 85.7%; P < 0.004) than CEMR. However, that study was retrospective with a small number of follow-up cases and an objective to analyze the efficacy of EMR-P for lesions that were challenging for standard EMR[11]. Thus, the guiding role of that study for clinical practice to tackle normal nonpedunculated lesions was limited.
In accordance with the results of PP analysis in the present study, EMR-P was superior to CEMR regarding the en bloc resection rate (94.3% vs 86%; P = 0.041), particularly in lesions > 15 mm (92.0% vs 58.8%; P = 0.029). However, these significant differences were not found in the ITT analysis.
Although EMR-P also showed a higher R0 resection rate, no significant difference was found (ITT analysis: EMR-P vs EMR, 80.9% vs 78.2%; PP analysis: EMR-P vs EMR, 81.1% vs 77.6%). Notably, the results in the present study showed an overall higher R0 removal rate than in previous studies, regardless of the method applied. In a trial by Yamashina et al[15], only a 50% R0 resection rate was achieved by CEMR for nonpedunculated colorectal polyps (median size: 13.5 mm); and UEMR, another alternative technique for medium-sized colorectal polyps, only resulted in a 75% R0 resection rate. For polyps sized 15-20 mm, Imai et al[27] also indicated that only a 65.3% R0 resection rate was achieved by CEMR with added tip-in EMR in the whole group.
Despite a lack of direct comparisons, these data implied that the similar R0 resection rates between the groups might be due to the high R0 removal rate by CEMR in the present study, leaving less room for improvement by EMR-P. Theoretically, EMR-P does have some advantages over the above two alternative treatments of CEMR. As it does not need to fill the entire lumen only with fluid, compared with UEMR, EMR-P saves a lot of time in deflating the lumen completely; moreover, its visual field during operation is less affected by poor intestinal preparation or intraprocedural bleeding. In comparison with tip-in EMR, circumferential incision of EMR-P allows the snare to be closer to the vertical margins of the lesions when snaring the polyps, which may be conducive to a better R0 resection rate.
Owing to the additional precutting step, EMR-P required a slightly longer total procedure time (EMR-P vs EMR: 6.4 vs 3.0 min, respectively; P < 0.001). However, considering the potential cost of retreatment caused by the higher risk of recurrence, spending some three more minutes in clinical operation, with no additional devices needed, seems more cost-effective. Additionally, despite including circumferential incision, more intraprocedural or postoperative bleeding cases in the EMR-P group were not observed. In the present study, only one patient in the EMR-P group experienced intraprocedural perforation, which was resolved through defect closure with endoscopic clips and antibiotic use. However, as most of the endoscopists were experienced, confirming the safety of EMR-P was challenging, particularly for endoscopists with less experience. This finding requires further clarification with follow-up research.
Theoretically, EMR-P is more difficult than CEMR but simpler than ESD, and EMR-P could also become an intermediate link to ESD training programs in the future. In the present study, the better performance of EMR-P was because of the circumferential incision. This additional step could facilitate the snare to be placed along the incision and then grasp and remove the lesions more reliably. However, during the snaring process, the benefit for vertical resection margins of EMR-P seems limited, likely explaining why it is difficult for all improved EMR techniques to achieve a 100% R0 resection rate. Additionally, circumferential incision may lead to unfavorable injected submucosal fluid maintenance, resulting in poor uplifting of mucosa and affecting the visual field. Fortunately, this weakness could be resolved by using thicker submucosal injectates to provide a sustained lift, such as succinylated gelatin, hydroxyethyl starch, or glycerol[4,39].
The present study has several limitations. First, the recurrence rate was not evaluated because follow-up colonoscopies were not performed. Thus, in accordance with the European guideline, surveillance colonoscopy three years after complete endoscopic resection is recommended[25]. Second, the single-blind study design may cause better outcomes of the new operation method, an inevitable problem of randomized controlled trials concerning endoscopy. Third, biopsy of the resected site after EMR has been recommended as the gold standard for evaluating the completeness of resection[40,41]. In the present study, en bloc and histologically negative margins of the sample were used to define R0 resection, a strategy that might be prone to sampling error because marginal biopsies only represent part of the lesion margin.
The present randomized study revealed that EMR-P successfully achieved a > 90% en bloc resection rate in 10-20 mm nonpedunculated polyps without increasing the incidence of adverse events. Although EMR-P may take slightly longer and result in a similar R0 resection rate than CEMR, its potential benefits are promising in clinical practice, particularly in lesions > 15 mm.
The optimal method for removing sessile colorectal lesions sized 10-20 mm remains uncertain. Piecemeal and incomplete resection are major limitations in current practice, including conventional endoscopic mucosal resection (CEMR). Recently, EMR with circumferential precutting (EMR-P) has emerged as an effective technique to overcome the deficiency of EMR. However, the current quality of evidence in comparative studies concerning about CEMR and EMR-P is limited.
To investigate a better method for removing sessile colorectal lesions sized 10-20 mm.
To investigate if EMR-P is superior to CEMR in colorectal lesions sized 10-20 mm.
This is a single-blind multicenter randomized controlled trial which involved seven medical institutions in China. The EMR-P was performed following submucosal injection, and a circumferential mucosa incision (precutting) was conducted using a snare tip. The en bloc and R0 resection rate were the primary outcomes analyzed, defined as one-piece resection and one-piece resection with histologically assessed clear margins, respectively.
In the per-protocol analysis, the en bloc resection rate of EMR-P was significantly higher than CEMR (94.3% vs 86%, P = 0.041), while in subgroup analysis, EMR-P also resulted in a higher en bloc resection rate (92.0% vs 58.8% P = 0.029) for lesions > 15 mm. The R0 resection rate was 81.1% (95%CI: 72.6%-87.4%) in the EMR-P group, higher than in CEMR group (76.6%; 95%CI: 68.8%-84.4%), but without a significant difference between the groups. The EMR-P group had a longer median procedure time (6.4 vs 3.0 min; P < 0.001). No significant difference in the incidence of adverse events was found between the groups (EMR-P: 9.1%; CEMR: 6.4%; P = 0.449).
EMR-P could serve as an alternative treatment for sessile colorectal polyps sized 10-20 mm.
The factors affecting the en bloc or R0 resection rate of EMR-P should be further studied, such as the experience of endoscopists, the depth of circumferential incision, the size of the polyp and so on.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cho YS, South Korea; Murakami T, Japan S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21363] [Article Influence: 2136.3] [Reference Citation Analysis (3)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13205] [Article Influence: 1467.2] [Reference Citation Analysis (3)] |
| 3. | Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3126] [Article Influence: 97.7] [Reference Citation Analysis (1)] |
| 4. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 765] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 5. | Serrano M, Mão de Ferro S, Fidalgo P, Lage P, Chaves P, Dias Pereira A. Endoscopic mucosal resection of superficial colorectal neoplasms: review of 140 procedures. Acta Med Port. 2012;25:288-296. [PubMed] |
| 6. | Hotta K, Fujii T, Saito Y, Matsuda T. Local recurrence after endoscopic resection of colorectal tumors. Int J Colorectal Dis. 2009;24:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Knabe M, Pohl J, Gerges C, Ell C, Neuhaus H, Schumacher B. Standardized long-term follow-up after endoscopic resection of large, nonpedunculated colorectal lesions: a prospective two-center study. Am J Gastroenterol. 2014;109:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Mannath J, Subramanian V, Singh R, Telakis E, Ragunath K. Polyp recurrence after endoscopic mucosal resection of sessile and flat colonic adenomas. Dig Dis Sci. 2011;56:2389-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Komeda Y, Watanabe T, Sakurai T, Kono M, Okamoto K, Nagai T, Takenaka M, Hagiwara S, Matsui S, Nishida N, Tsuji N, Kashida H, Kudo M. Risk factors for local recurrence and appropriate surveillance interval after endoscopic resection. World J Gastroenterol. 2019;25:1502-1512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 10. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 927] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 11. | Yoshida N, Inoue K, Dohi O, Yasuda R, Hirose R, Naito Y, Murakami T, Ogiso K, Inada Y, Inagaki Y, Morinaga Y, Kishimoto M, Itoh Y. Efficacy of precutting endoscopic mucosal resection with full or partial circumferential incision using a snare tip for difficult colorectal lesions. Endoscopy. 2019;51:871-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Sakamoto T, Matsuda T, Nakajima T, Saito Y. Efficacy of endoscopic mucosal resection with circumferential incision for patients with large colorectal tumors. Clin Gastroenterol Hepatol. 2012;10:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Rex DK, Ponugoti P, Kahi C. The "valley sign" in small and diminutive adenomas: prevalence, interobserver agreement, and validation as an adenoma marker. Gastrointest Endosc. 2017;85:614-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Holt BA, Bourke MJ. Wide field endoscopic resection for advanced colonic mucosal neoplasia: current status and future directions. Clin Gastroenterol Hepatol. 2012;10:969-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Yamashina T, Uedo N, Akasaka T, Iwatsubo T, Nakatani Y, Akamatsu T, Kawamura T, Takeuchi Y, Fujii S, Kusaka T, Shimokawa T. Comparison of Underwater vs Conventional Endoscopic Mucosal Resection of Intermediate-Size Colorectal Polyps. Gastroenterology. 2019;157:451-461.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 16. | Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, Levy LC, Toor A, Mackenzie TA, Rosch T, Robertson DJ. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74-80.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 553] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 17. | Adler J, Robertson DJ. Interval Colorectal Cancer After Colonoscopy: Exploring Explanations and Solutions. Am J Gastroenterol. 2015;110:1657-64; quiz 1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Horiuchi A, Makino T, Kajiyama M, Tanaka N, Sano K, Graham DY. Comparison between endoscopic mucosal resection and hot snare resection of large nonpedunculated colorectal polyps: a randomized trial. Endoscopy. 2016;48:646-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 19. | Yuan X, Gao H, Liu C, Cui H, Zhang Z, Xie J, Lu H, Xu L. Effectiveness and safety of the different endoscopic resection methods for 10- to 20-mm nonpedunculated colorectal polyps: A systematic review and pooled analysis. Saudi J Gastroenterol. 2021;27:331-341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T, Fujii T. Clinical outcome of endoscopic submucosal dissection vs endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 428] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 21. | Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy. 2014;46:388-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 275] [Article Influence: 25.0] [Reference Citation Analysis (2)] |
| 22. | Adler J, Toy D, Anderson JC, Robertson DJ, Pohl H. Metachronous Neoplasias Arise in a Higher Proportion of Colon Segments From Which Large Polyps Were Previously Removed, and Can be Used to Estimate Incomplete Resection of 10-20 mm Colorectal Polyps. Clin Gastroenterol Hepatol. 2019;17:2277-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Oka S, Tanaka S, Saito Y, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K, Hisabe T, Tsuruta O, Sano Y, Yamano H, Shimizu S, Yahagi N, Watanabe T, Nakamura H, Fujii T, Ishikawa H, Sugihara K; Colorectal Endoscopic Resection Standardization Implementation Working Group of the Japanese Society for Cancer of the Colon and Rectum, Tokyo, Japan. Local recurrence after endoscopic resection for large colorectal neoplasia: a multicenter prospective study in Japan. Am J Gastroenterol. 2015;110:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 24. | Conio M, Repici A, Demarquay JF, Blanchi S, Dumas R, Filiberti R. EMR of large sessile colorectal polyps. Gastrointest Endosc. 2004;60:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 25. | Hassan C, Antonelli G, Dumonceau JM, Regula J, Bretthauer M, Chaussade S, Dekker E, Ferlitsch M, Gimeno-Garcia A, Jover R, Kalager M, Pellisé M, Pox C, Ricciardiello L, Rutter M, Helsingen LM, Bleijenberg A, Senore C, van Hooft JE, Dinis-Ribeiro M, Quintero E. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2020. Endoscopy. 2020;52:687-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 311] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 26. | Nagl S, Ebigbo A, Goelder SK, Roemmele C, Neuhaus L, Weber T, Braun G, Probst A, Schnoy E, Kafel AJ, Muzalyova A, Messmann H. Underwater vs Conventional Endoscopic Mucosal Resection of Large Sessile or Flat Colorectal Polyps: A Prospective Randomized Controlled Trial. Gastroenterology. 2021;161:1460-1474.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 27. | Imai K, Hotta K, Ito S, Yamaguchi Y, Kishida Y, Yabuuchi Y, Yoshida M, Kawata N, Tanaka M, Kakushima N, Takizawa K, Ishiwatari H, Matsubayashi H, Mori K, Oishi T, Ono H. Tip-in Endoscopic Mucosal Resection for 15- to 25-mm Colorectal Adenomas: A Single-Center, Randomized Controlled Trial (STAR Trial). Am J Gastroenterol. 2021;116:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Liu Y, Shi M, Ren J, Zhou XL, Liu S. Effectiveness of underwater endoscopic mucosal resection versus conventional endoscopic mucosal resection for 10 to 20 mm colorectal polyps: A protocol of systematic review and meta-analysis. Medicine (Baltimore). 2020;99:e23041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Hirao M, Masuda K, Nakamura M. Endoscopic resection with local injection of HSE (ERHSE) in early gastric carcinomas. Gan No Rinsho. 1986;32:1180-1184. [PubMed] |
| 30. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Kudo SE, Tsuruta O, Sugihara KI, Watanabe T, Saitoh Y, Igarashi M, Toyonaga T, Ajioka Y, Ichinose M, Matsui T, Sugita A, Sugano K, Fujimoto K, Tajiri H. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 435] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 31. | Toyonaga T, Man-I M, Morita Y, Sanuki T, Yoshida M, Kutsumi H, Inokuchi H, Azuma T. The new resources of treatment for early stage colorectal tumors: EMR with small incision and simplified endoscopic submucosal dissection. Dig Endosc. 2009;21 Suppl 1:S31-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Muto M, Miyamoto S, Hosokawa A, Doi T, Ohtsu A, Yoshida S, Endo Y, Hosokawa K, Saito D, Shim CS, Gossner L. Endoscopic mucosal resection in the stomach using the insulated-tip needle-knife. Endoscopy. 2005;37:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001;33:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 309] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 34. | Miyamoto S, Muto M, Hamamoto Y, Boku N, Ohtsu A, Baba S, Yoshida M, Ohkuwa M, Hosokawa K, Tajiri H, Yoshida S. A new technique for endoscopic mucosal resection with an insulated-tip electrosurgical knife improves the completeness of resection of intramucosal gastric neoplasms. Gastrointest Endosc. 2002;55:576-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 35. | Repici A, Conio M, De Angelis C, Sapino A, Malesci A, Arezzo A, Hervoso C, Pellicano R, Comunale S, Rizzetto M. Insulated-tip knife endoscopic mucosal resection of large colorectal polyps unsuitable for standard polypectomy. Am J Gastroenterol. 2007;102:1617-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Lee EJ, Lee JB, Lee SH, Youk EG. Endoscopic treatment of large colorectal tumors: comparison of endoscopic mucosal resection, endoscopic mucosal resection-precutting, and endoscopic submucosal dissection. Surg Endosc. 2012;26:2220-2230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 37. | Kim YJ, Kim ES, Cho KB, Park KS, Jang BK, Chung WJ, Hwang JS. Comparison of clinical outcomes among different endoscopic resection methods for treating colorectal neoplasia. Dig Dis Sci. 2013;58:1727-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 38. | Oh CK, Cho YW, Choi IH, Lee HH, Lim CH, Kim JS, Lee BI, Cho YS. Comparison of precutting endoscopic mucosal resection and endoscopic submucosal dissection for large (20-30 mm) flat colorectal lesions. J Gastroenterol Hepatol. 2022;37:568-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | ASGE Technology Committee; Hwang JH, Konda V, Abu Dayyeh BK, Chauhan SS, Enestvedt BK, Fujii-Lau LL, Komanduri S, Maple JT, Murad FM, Pannala R, Thosani NC, Banerjee S. Endoscopic mucosal resection. Gastrointest Endosc. 2015;82:215-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 40. | Kim JS, Lee BI, Choi H, Jun SY, Park ES, Park JM, Lee IS, Kim BW, Kim SW, Choi MG. Cold snare polypectomy vs cold forceps polypectomy for diminutive and small colorectal polyps: a randomized controlled trial. Gastrointest Endosc. 2015;81:741-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 41. | Efthymiou M, Taylor AC, Desmond PV, Allen PB, Chen RY. Biopsy forceps is inadequate for the resection of diminutive polyps. Endoscopy. 2011;43:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |