Published online Nov 28, 2022. doi: 10.3748/wjg.v28.i44.6249
Peer-review started: September 9, 2022
First decision: September 29, 2022
Revised: October 24, 2022
Accepted: November 9, 2022
Article in press: November 9, 2022
Published online: November 28, 2022
Processing time: 76 Days and 10.8 Hours
Epstein-Barr virus (EBV)-associated gastric cancer (EBVaGC) cells originate from a single-cell clone infected with EBV. However, more than 95% of patients with gastric cancer have a history of Helicobacter pylori (H. pylori) infection, and H. pylori is a major causative agent of gastric cancer. Therefore, it has long been argued that H. pylori infection may affect the development of EBVaGC, a subtype of gastric cancer. Atrophic gastrointestinal inflammation, a symptom of H. pylori infection, is observed in the gastric mucosa of EBVaGC. Therefore, it remains unclear whether H. pylori infection is a cofactor for gastric carcinogenesis caused by EBV infection or whether H. pylori and EBV infections act independently on gastric cancer formation. It has been reported that EBV infection assists in the onco-genesis of gastric cancer caused by H. pylori infection. In contrast, several studies have reported that H. pylori infection accelerates tumorigenesis initiated by EBV infection. By reviewing both clinical epidemiological and experimental data, we reorganized the role of H. pylori and EBV infections in gastric cancer formation.
Core Tip: Epstein-Barr virus (EBV)-associated gastric cancer (EBVaGC) tumor cells originate from a single cell clone infected with EBV. In contrast, it is reported that more than 95% of patients with gastric cancer have a history of Helicobacter pylori (H. pylori) infection. Accordingly, it has long been argued that H. pylori infection may have some effect on the development of EBVaGC, a subtype of gastric cancer. It is also a mystery that the number of gastric cancer patients is higher in Asia, South America, and the Middle East. We will reorganize the role of H. pylori and EBV infections in gastric cancer formation.
- Citation: Iizasa H, Kartika AV, Fekadu S, Okada S, Onomura D, Wadi AFAA, Khatun MM, Moe TM, Nishikawa J, Yoshiyama H. Development of Epstein-Barr virus-associated gastric cancer: Infection, inflammation, and oncogenesis. World J Gastroenterol 2022; 28(44): 6249-6257
- URL: https://www.wjgnet.com/1007-9327/full/v28/i44/6249.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i44.6249
Epstein-Barr virus (EBV)-associated gastric cancer (EBVaGC) accounts for 10% of all gastric cancers. At the same time, more than 95% of patients with gastric cancer have a history of Helicobacter pylori (H. pylori) infection. Thus, the question arises as to whether H. pylori and EBV infections promote gastric cancer formation in a dependent or independent manner. The high prevalence of gastric cancer in Asia, South America, and the Middle East is also intriguing.
EBV infects B lymphocytes and epithelial cells and is an oncogenic virus that assists in the proliferation of latently infected cells, resulting in the development of Burkitt lymphoma, Hodgkin lymphoma, nasopharyngeal carcinoma, and EBVaGC[1].
More than 90% of adults are latently infected with EBV; however, cytotoxic T lymphocytes that recognize EBV antigens suppress the proliferation of viral antigen-positive cells. When the local or systemic immune function is compromised, EBV-positive cells begin to proliferate. B lymphocytes that migrate to local areas where immune surveillance is weak often transition to lytic infection, resulting in viral production. Under such conditions, EBV appears to be transmitted to and infects gastric epithelial cells. The expression of EBV genes causes epithelial cells to acquire proliferative properties and resist apoptosis, and cells that escape immunological elimination may begin proliferating[2].
Classification of gastric cancer by molecular mechanism was performed through an exhaustive analysis of next-generation sequencing data from numerous cases. The results divided gastric cancer into the following four molecular subtypes: Microsatellite instability (MSI), chromosomal instability (CIN), genomically stable (GS), and EBV[3]. These classifications have facilitated the identification of specific therapeutic candidates for each subtype of gastric cancer, and have revealed that each of these four subtypes is driven by a specific developmental mechanism that needs to be clarified individually. In particular, the molecular biology of EBVaGC is characterized by frequent and extensive methylation of the promoter regions of tumor cell genes[4]. De novo EBV infection induces DNA methylation in more than 3000 gene promoter regions within 4 wk[4]. However, methylation of the promoter of the mismatch repair gene MLH1, which is frequently observed in MSI, is not observed in EBVaGC[5]. In addition to inactivation by DNA methylation, the EBV genome binds to heterochromatin, a region of inactivation that causes aberrant activation of the region (enhancer infestation) and increases the expression of surrounding proto-oncogenes[6].
In EBVaGC, which accounts for 5%–10% of all gastric cancers, all tumor cells are infected with EBV. Endoscopy is the most informative method for diagnosing gastric cancer. EBVaGC is observed as a superficial depressed lesion in the upper part of the stomach. Using endoscopic biopsy specimens, EBV-encoded RNA in situ hybridization (EBER-ISH), stains all gastric cancer cells positive for EBER, even in the intramucosal cancer stage[7]. The histological hallmark of EBVaGC is lymphoepithelioma-like carcinoma, in which a diffuse lymphocytic infiltrate is observed around EBER-positive epithelial tumor cells[8]. Furthermore, EBVaGC tumor cells are derived from the proliferation of a single EBV-infected epithelial cell[8,9].
Many studies have shown a male predominance (2-fold) of EBVaGC, suggesting that the risk may exist in male lifestyle and occupational factors[10]. The percentage of patients with EBVaGC to those with total gastric cancer is higher in younger patients. In men, the proportion of EBVaGC decreases with increasing age, especially in patients with pyloric gastric cancer. In women, the decrease in the proportion of EBVaGC with increasing age is unclear. Consumption of salty foods that cause mech-anical damage to the gastric epithelium as well as exposure to wood and iron filings are associated with a higher EBVaGC risk[11].
EBVaGC is a gastric cancer with a relatively good prognosis. A Dutch study reported that EBVaGC is characterized by fewer lymph node metastases, less residual disease, and younger patient age, which results in longer disease-free survival[12]. Cohort study data from TCGA also reported that EBVaGC has the best recurrence-free period and overall survival compared to MSI, GS, and CIN subtypes[13].
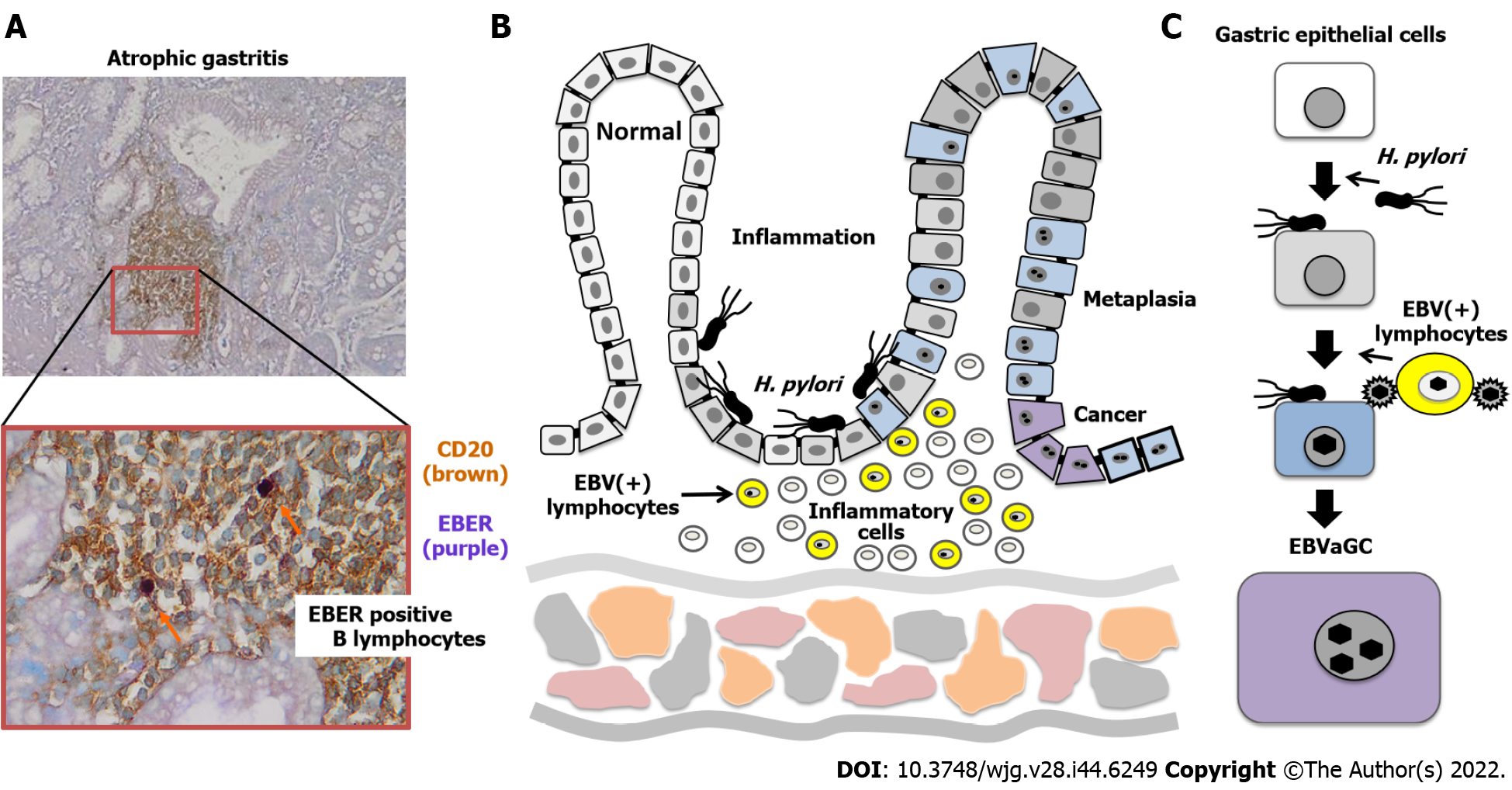
EBVaGC tumors are frequently found in non-antral parts of the stomach[10,14]. In contrast, H. pylori-associated gastric cancer mostly occurs in the antral region[10]. Because moderate to severe atrophic gastric mucosa due to H. pylori infection was characteristically observed surrounding early gastric cancers, gastritis may play an important role in the tumorigenesis of EBVaGC[14]. Development of gastric cancer is supposed to follow the "infection, inflammation, and carcinogenesis" route, which consists of H. pylori infection followed by chronic gastritis, intestinal metaplasia, and cancer. In contrast, in the case of EBVaGC, it is controversial whether tumor formation is initiated by EBV-infected normal mucosal cells or promoted by EBV-infected cells in precancerous lesions[15]. Abe et al[16] performed EBER-ISH on 1110 sections of non-neoplastic gastric mucosal tissue from 300 cases and found 2 (0.18%) ductal-level EBER-positive lesions.
The mutual contribution of EBV and H. pylori in the carcinogenesis will be discussed later in the chapter “Inflammation and carcinogenesis”.
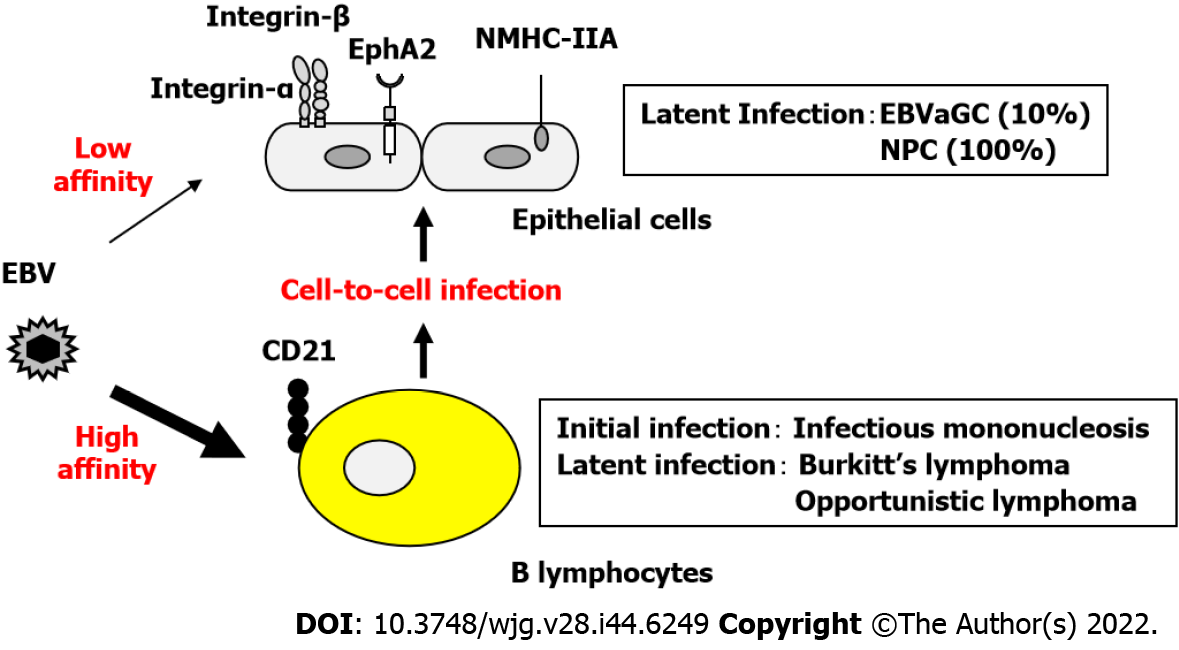
EBV infects B lymphocytes through the binding of the viral glycoprotein gp350 to the high-affinity receptor CD21, followed by binding of gp42 to HLA class II molecules, resulting in membrane fusion[17]. In contrast, when low-affinity co-receptors are used to infect CD21-negative epithelial cells, the infection efficiency is extremely low (Figure 1).
The CD21-independent routes of epithelial cell infection include the following: (1) The viral envelope glycoprotein gp350/220 binds to CD35; (2) Integrins αVβ5, αVβ6, and αVβ8 interact with the viral envelope glycoprotein gH/gL complex to fuse the viral envelope with the epithelial cell membrane; (3) The BMRF2 membrane protein expressed during EBV lytic infection binds to α3, α5, αV, and β1 integrins; and (4) EphA2 and NMHC-IIA bind to gH/gL produced by many herpesviruses and enhance infection efficiency.
A previous study reported that a boy with X-linked agammaglobulinemia who did not have mature B lymphocytes due to a genetic enzymatic deficiency did not develop an EBV infection[18]. EBV infection of epithelial cells was considered to occur after EBV infection of B lymphocytes because the epithelial cells of the affected boy were intact. EBV-infected B lymphocytes are believed to carry and deliver EBV to the epithelial cells via cell-to-cell transfer. In the case of CD21-independent infection, the efficiency of epithelial cell infection by cell-to-cell transfer is more than 1000 times higher than that of direct epithelial cell infection by EBV particles[19]. It is speculated that infection of epithelial cells via B lymphocytes is promoted when viral activation and lymphocyte infiltration are accompanied by inflammation (Figure 1).
In EBVaGC, all tumor cells are infected with EBV. However, cell lines established from gastric cancer tissues, similar to those in nasopharyngeal carcinoma, are almost entirely EBV-negative[20]. The EBV genome in EBVaGC tumor cells exists as a plasmid-like episome that does not integrate into the host chromosomes. However, the presence of the virus does not appear to favor cell growth in vitro. Rather, it may be more convenient for in vitro cell growth to avoid the use of extra energy to maintain the episomes. Alternatively, the expression of viral genes such as microRNAs may be crucial for tumor cells to evade elimination by the in vivo immune system. In fact, EBV-positive KT cells established from EBVaGC can only be passaged by transplantation into SCID mice and cannot be expanded in an in vitro culture system[21]. SNU-719, YCCEL1, and NCC-24 are rare cells established from EBVaGC and can be propagated in vitro. These cell lines appear to be unique because the presence of EBV episomes is essential for their growth. Experiments with hydroxyurea and EBNA1 siRNAs were not successful in shedding the EBV episome from SNU-719 cells[22].
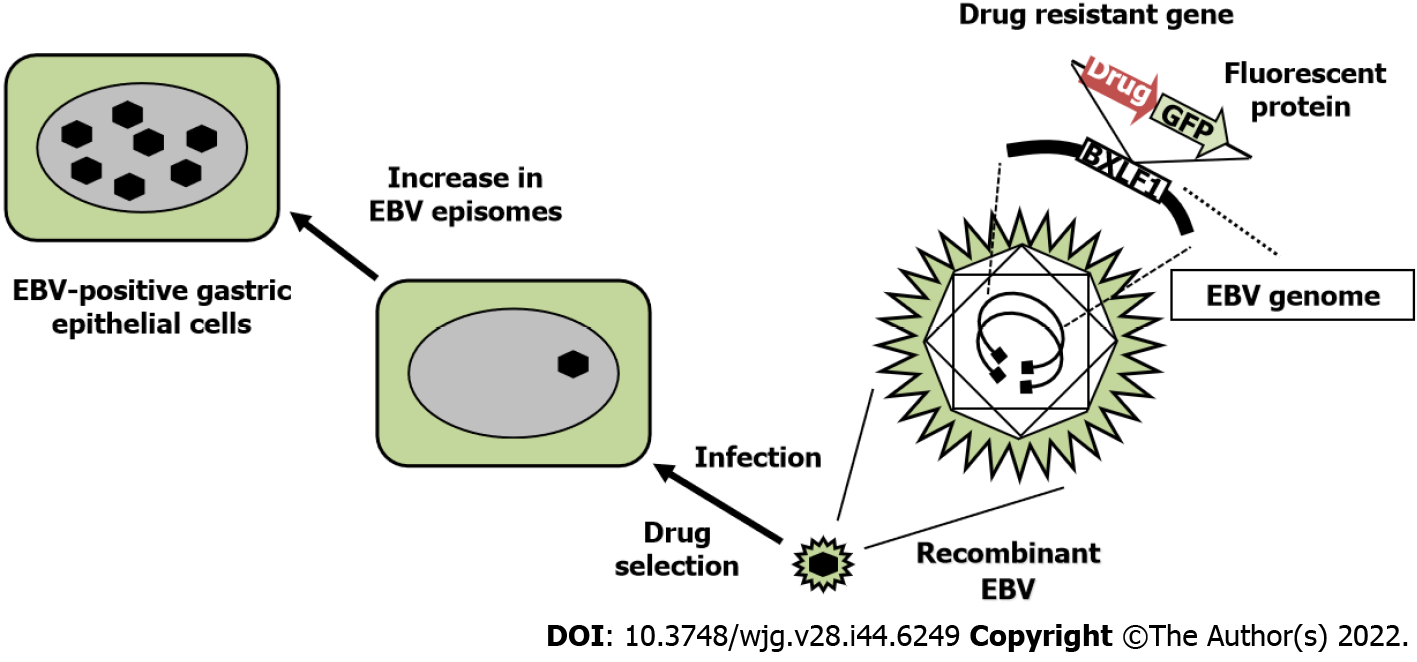
We established gastric epithelial cells infected with recombinant EBV, where a drug-resistant gene was inserted into the nonessential BXLF1 (thymidine kinase) gene (Figure 2). It is possible to elucidate the oncogenic molecular mechanism of EBV-infected epithelial cells by comparing EBV-positive cells with EBV-negative cells. EBV infection markedly promotes the proliferation of gastric epithelial cells[23].
EBV-infected gastric epithelial cells also exhibit type I latent infection that expresses EBNA1 and LMP2A, similar to that in EBVaGC in vivo. EBNA1 promotes tumorigenesis via p53 ubiquitination, suppresses transforming growth factor-β signaling, and enhances the transcription of the anti-apoptotic protein survivin[24]. In contrast, LMP2A activates PI3K/Akt signaling similar to that activated by B-cell receptor stimulation, increases survivin expression, and resists apoptosis[25]. LMP2A also induces DNA methyltransferases, resulting in epigenetic changes in infected cells[26]. BARF1 is strongly expressed as a latent gene in EBV-associated epithelial tumors[27]. Nasopharyngeal carcinoma-derived cells infected with recombinant EBV constitutively expressing BARF1 exhibit resistance to apoptosis[28].
In addition to the oncogenic activity of EBV proteins expressed in type I latent infections, non-coding RNAs (miRNAs and EBERs) that are not translated into proteins have been investigated. Multiple BART miRNAs cooperatively repress lytic replication[29]. BART miRNAs also downregulate pro- and anti-apoptotic mediators such as caspase 3[30]. EBERs bind to protein kinase R and disrupt innate immune function[31]. Elimination of EBER2 from the EBV genome reduces the efficiency of B lymphocyte transformation[32].
It is very difficult to collect EBVaGC cases without H. pylori infection, because most patients with gastric cancer are infected with H. pylori[33,34]. However, a clinical study was conducted to investigate the relationship between EBV infection, H. pylori infection, and atrophic gastritis in 468 patients with chronic gastritis[35]. This study confirmed that patients who were EBV-positive had a lower pepsinogen I/pepsinogen II ratio than patients who were EBV-negative. EBV infection significantly increases the risk of atrophic gastritis, especially in H. pylori-negative patients. However, a report from Mexico mentioned that EBER1 in situ hybridization showed that EBV infection of epithelial cells could be detected in gastric cancers as well as in one-third of non-atrophic gastritis samples[36]. This study showed that EBV infection affected early cancer precursor lesions. However, it is difficult to determine whether EBV causes cancer directly or indirectly by triggering inflammation.
Inflammation and initiation of innate immune mechanisms promote EBV activation, although it is difficult to assess the extent to which these mechanisms are involved in tumorigenesis of EBV-infected cells (Figure 3). EBV proliferation occurs at the early stage of EBVaGC formation because early antigens-immunoglobulin G (IgG) and viral capsid antigen-IgG antibodies against early viral antigens and capsids are elevated in the sera of patients with EBVaGC. In addition, while the incidence of EBVaGC is approximately 10% worldwide, the incidence of gastric cancer after surgical invasion by gastric anastomosis increases by three times (30%)[8].
Here, we investigated the relationship between H. pylori-associated gastritis and EBV propagation in the stomach. Gastric biopsy specimens were collected from patients with chronic atrophic gastritis and categorized into three histopathological stages: Mild, moderate, and severe. The specimens were subjected to DNA extraction and quantitative polymerase chain reaction to quantify EBV genome copy numbers[37]. More than 900 copies of the EBV genome have been frequently detected in patients with moderate atrophic gastritis. In other words, EBV frequently activates proliferation in patients with H. pylori infection with moderate chronic atrophic gastritis and strong histological inflammation.
In contrast, EBVaGC is significantly associated with marked mucosal atrophy and moderate to marked lymphocytic infiltration, but there is no direct association with intestinal metaplasia[7]. Although this appears to indicate that EBVaGC is not directly associated with H. pylori infection, this result is consistent with our findings. This is because the intestinal metaplastic epithelium resulting from prolonged gastritis is an unsuitable mucosal environment for the growth of both H. pylori and EBV[38].
Several studies have been investigated the interaction between EBV and H. pylori in gastric epithelial cell lines. Because it is difficult to infect the epithelial cells with the two microorganisms simultaneously, experiments have been conducted on sequential infection with EBV first and H. pylori second, or vice versa.
Persistent infection of the gastric mucosa by CagA-positive H. pylori strains causes gastric cancer. This is because the tyrosine-phosphorylated CagA protein binds to the tyrosine phosphatase SHP2 in gastric epithelial cells, activating Ras oncogene. In contrast, SHP1, which competes with SHP2 weakens the oncogenic activity of SHP2. Saju et al[39] showed that EBV infection of gastric epithelial cells activates host cell promoter methylation and decreases SHP1 expression[39]. In other words, SHP2 activity is relatively higher and EBV infection promotes carcinogenesis of H. pylori associated gastric carcinoma. The induction of DNA methylase by EBV infection in gastric epithelial cells also decreases the expression of tumor suppressor genes such as APC, breast cancer susceptibility gene 1, and phosphatase and tensin homolog deleted from chromosome 10 (PTEN)[40].
Furthermore, activation of innate immune signals by H. pylori attachment enhances the expression of the EBV co-receptor EPHA2 in gastric epithelial cells, thereby increasing the frequency of EBV infection in epithelial cells[41]. Another study demonstrated that organoids derived from gastric cancer cells were infected with EBV but did not infect those derived from the normal gastric epithelium[42]. The probable reason for this is that gastric organoids maintain cell polarity and express EPHA2 only between cells. Therefore, the localization of EPHA2 might change due to gastric epithelial cell injury caused by H. pylori infection or by a prior gene mutation, which subsequently facilitates EBV infection.
At present, it is difficult to infect primary gastric epithelial cells with EBV and immortalize them. Instead, gastric epithelial cell lines persistently infected with EBV have been used to elucidate the tumorigenic mechanisms of EBV genes during latent infections.
The EBV genome contains two miRNA clusters, consisting of four BHRF1 miRNAs and 40 BART miRNAs. Although BHRF1 miRNA is poorly expressed in epithelial cells, BART miRNAs are highly expressed in latently infected epithelial cells and play a substantial role in tumorigenesis[43].
Modification of gene expression via methylation is frequently observed in patients with EBVaGC. Tumor suppressor genes, such as p14, p16, p73, PTEN, APC, RASSF1A, and CXXC4, are repressed by promoter methylation. And the expression of molecules important for cell invasion, including THBS1, E-cadherin (CDH1), and TIMP2, is also repressed by promoter methylation. The decreased expression of these molecules may be involved in carcinogenic processes[44].
Multiple EBV episomal DNAs have been shown to approach enhancer sites in the genome, alter the surrounding chromatin structure (enhancer infestation), and activate genes such as transcription factors[6]. Although epigenetic analyses have been conducted to understand tumorigenesis, the overall mechanism remains unclear.
Viral gene products transcribed in cells latently infected with EBV confer resistance to apoptosis. EBV gene products also accumulate mutations in the genes of the infected cells. Genetic changes in infected cells further affect EBV gene expression and alter intercellular communication, including the cross-talk between EBV-infected epithelial cells and immune cells[45] or epithelial-mesenchymal transition[46]. In other words, changes induced by persistent EBV infection in host cell signaling and host immune responses advance the tumorigenic stage[47].
With the progress in research on EBER, miRNA, and long non-coding RNA, the functions of these molecules in latent EBV-infected cells are being elucidated. A highly tumorigenic B81 EBV strain was isolated from a patient with nasopharyngeal carcinoma[48]; however, an EBV strain unique to gastric cancer has not yet been isolated.
Host gene mutations frequently observed in EBVaGC, including changes in PIK3CA, ARID1A, PD-L1, and PD-L2[3] are considered to affect histological characteristics, clinical course, and response to treatment. EBV-induced tumorigenesis is believed to be affected by environmental factors such as previous infections; however, the molecular basis that characterizes EBVaGC remains to be elucidated.
Considering that EBVaGC most strongly expresses PD-L1 and PD-L2 among the four molecular subtypes of gastric cancer, immune checkpoint inhibitors are expected to be effective therapeutic agents for EBVaGC[49,50]. PIK3CA mutations and JAK2 amplification are frequently observed in EBVaGC. Therefore, PI3K and JAK2 inhibitors may be effective. Other EBNA-1 inhibitors are also expected to be EBV-specific therapeutic agents[51].
Several clinical and experimental data support the etiological role of H. pylori in EBV-associated gastric cancer.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Japanese Cancer Association, No. 19950.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng H, China; Liao J, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Stanfield BA, Luftig MA. Recent advances in understanding Epstein-Barr virus. F1000Res. 2017;6:386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Shannon-Lowe C, Rickinson A. The global landscape of EBV-associated tumors. Front Oncol. 2019;9:713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 309] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 3. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4845] [Article Influence: 440.5] [Reference Citation Analysis (2)] |
| 4. | Matsusaka K, Funata S, Fukuyo M, Seto Y, Aburatani H, Fukayama M, Kaneda A. Epstein-Barr virus infection induces genome-wide de novo DNA methylation in non-neoplastic gastric epithelial cells. J Pathol. 2017;242:391-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Matsusaka K, Kaneda A, Nagae G, Ushiku T, Kikuchi Y, Hino R, Uozaki H, Seto Y, Takada K, Aburatani H, Fukayama M. Classification of Epstein-Barr virus-positive gastric cancers by definition of DNA methylation epigenotypes. Cancer Res. 2011;71:7187-7197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 6. | Okabe A, Huang KK, Matsusaka K, Fukuyo M, Xing M, Ong X, Hoshii T, Usui G, Seki M, Mano Y, Rahmutulla B, Kanda T, Suzuki T, Rha SY, Ushiku T, Fukayama M, Tan P, Kaneda A. Cross-species chromatin interactions drive transcriptional rewiring in Epstein-Barr virus-positive gastric adenocarcinoma. Nat Genet. 2020;52:919-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 7. | Kaizaki Y, Sakurai S, Chong JM, Fukayama M. Atrophic gastritis, Epstein-Barr virus infection, and Epstein-Barr virus-associated gastric carcinoma. Gastric Cancer. 1999;2:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Iizasa H, Nanbo A, Nishikawa J, Jinushi M, Yoshiyama H. Epstein-Barr virus (EBV)-associated gastric carcinoma. Viruses. 2012;4:3420-3439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 9. | Imai S, Koizumi S, Sugiura M, Tokunaga M, Uemura Y, Yamamoto N, Tanaka S, Sato E, Osato T. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc Natl Acad Sci U S A. 1994;91:9131-9135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 337] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 10. | Akiba S, Koriyama C, Herrera-Goepfert R, Eizuru Y. Epstein-Barr virus associated gastric carcinoma: epidemiological and clinicopathological features. Cancer Sci. 2008;99:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Koriyama C, Akiba S, Minakami Y, Eizuru Y. Environmental factors related to Epstein-Barr virus-associated gastric cancer in Japan. J Exp Clin Cancer Res. 2005;24:547-553. [PubMed] |
| 12. | Camargo MC, Kim WH, Chiaravalli AM, Kim KM, Corvalan AH, Matsuo K, Yu J, Sung JJ, Herrera-Goepfert R, Meneses-Gonzalez F, Kijima Y, Natsugoe S, Liao LM, Lissowska J, Kim S, Hu N, Gonzalez CA, Yatabe Y, Koriyama C, Hewitt SM, Akiba S, Gulley ML, Taylor PR, Rabkin CS. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut. 2014;63:236-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 293] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 13. | Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC, Shim JJ, Lee KW, Kim EH, Yim SY, Lee SH, Cheong JH, Jeong W, Cho JY, Kim J, Chae J, Lee J, Kang WK, Kim S, Noh SH, Ajani JA, Lee JS. Clinical significance of four molecular subtypes of gastric cancer identified by The Cancer Genome Atlas project. Clin Cancer Res. 2017;23:4441-4449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 344] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 14. | Fukayama M, Hayashi Y, Iwasaki Y, Chong J, Ooba T, Takizawa T, Koike M, Mizutani S, Miyaki M, Hirai K. Epstein-Barr virus-associated gastric carcinoma and Epstein-Barr virus infection of the stomach. Lab Invest. 1994;71:73-81. [PubMed] |
| 15. | Zur Hausen A, van Rees BP, van Beek J, Craanen ME, Bloemena E, Offerhaus GJ, Meijer CJ, van den Brule AJ. Epstein-Barr virus in gastric carcinomas and gastric stump carcinomas: a late event in gastric carcinogenesis. J Clin Pathol. 2004;57:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Abe H, Kunita A, Otake Y, Kanda T, Kaneda A, Ushiku T, Fukayama M. Virus-host interactions in carcinogenesis of Epstein-Barr virus-associated gastric carcinoma: Potential roles of lost ARID1A expression in its early stage. PLoS One. 2021;16:e0256440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Rani A, Jakhmola S, Karnati S, Parmar HS, Chandra Jha H. Potential entry receptors for human γ-herpesvirus into epithelial cells: A plausible therapeutic target for viral infections. Tumour Virus Res. 2021;12:200227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 18. | Faulkner GC, Burrows SR, Khanna R, Moss DJ, Bird AG, Crawford DH. X-Linked agammaglobulinemia patients are not infected with Epstein-Barr virus: implications for the biology of the virus. J Virol. 1999;73:1555-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Shannon-Lowe CD, Neuhierl B, Baldwin G, Rickinson AB, Delecluse HJ. Resting B cells as a transfer vehicle for Epstein-Barr virus infection of epithelial cells. Proc Natl Acad Sci U S A. 2006;103:7065-7070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Dittmer DP, Hilscher CJ, Gulley ML, Yang EV, Chen M, Glaser R. Multiple pathways for Epstein-Barr virus episome loss from nasopharyngeal carcinoma. Int J Cancer. 2008;123:2105-2112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Iwasaki Y, Chong JM, Hayashi Y, Ikeno R, Arai K, Kitamura M, Koike M, Hirai K, Fukayama M. Establishment and characterization of a human Epstein-Barr virus-associated gastric carcinoma in SCID mice. J Virol. 1998;72:8321-8326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Oh ST, Kim M, Lee SK. Maintenance of the viral episome is essential for the cell survival of an Epstein-Barr virus positive gastric carcinoma cell line. Arch Pharm Res. 2009;32:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Nishikawa J, Imai S, Oda T, Kojima T, Okita K, Takada K. Epstein-Barr virus promotes epithelial cell growth in the absence of EBNA2 and LMP1 expression. J Virol. 1999;73:1286-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Frappier L. Contributions of Epstein-Barr nuclear antigen 1 (EBNA1) to cell immortalization and survival. Viruses. 2012;4:1537-1547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Hino R, Uozaki H, Inoue Y, Shintani Y, Ushiku T, Sakatani T, Takada K, Fukayama M. Survival advantage of EBV-associated gastric carcinoma: survivin up-regulation by viral latent membrane protein 2A. Cancer Res. 2008;68:1427-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Hino R, Uozaki H, Murakami N, Ushiku T, Shinozaki A, Ishikawa S, Morikawa T, Nakaya T, Sakatani T, Takada K, Fukayama M. Activation of DNA methyltransferase 1 by EBV latent membrane protein 2A leads to promoter hypermethylation of PTEN gene in gastric carcinoma. Cancer Res. 2009;69:2766-2774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 288] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 27. | Lo AK, Dawson CW, Lung HL, Wong KL, Young LS. The therapeutic potential of targeting BARF1 in EBV-associated malignancies. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Seto E, Yang L, Middeldorp J, Sheen TS, Chen JY, Fukayama M, Eizuru Y, Ooka T, Takada K. Epstein-Barr virus (EBV)-encoded BARF1 gene is expressed in nasopharyngeal carcinoma and EBV-associated gastric carcinoma tissues in the absence of lytic gene expression. J Med Virol. 2005;76:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Iizasa H, Kim H, Kartika AV, Kanehiro Y, Yoshiyama H. Role of viral and host microRNAs in immune regulation of Epstein-Barr virus-associated diseases. Front Immunol. 2020;11:367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | Lin X, Tsai MH, Shumilov A, Poirey R, Bannert H, Middeldorp JM, Feederle R, Delecluse HJ. The Epstein-Barr virus BART miRNA cluster of the M81 strain modulates multiple functions in primary B cells. PLoS Pathog. 2015;11:e1005344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | Nanbo A, Inoue K, Adachi-Takasawa K, Takada K. Epstein-Barr virus RNA confers resistance to interferon-alpha-induced apoptosis in Burkitt's lymphoma. EMBO J. 2002;21:954-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 209] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 32. | Wu Y, Maruo S, Yajima M, Kanda T, Takada K. Epstein-Barr virus (EBV)-encoded RNA 2 (EBER2) but not EBER1 plays a critical role in EBV-induced B-cell growth transformation. J Virol. 2007;81:11236-11245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3182] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 34. | Camargo MC, Kim KM, Matsuo K, Torres J, Liao LM, Morgan DR, Michel A, Waterboer T, Zabaleta J, Dominguez RL, Yatabe Y, Kim S, Rocha-Guevara ER, Lissowska J, Pawlita M, Rabkin CS. Anti-Helicobacter pylori antibody profiles in Epstein-Barr virus (EBV)-positive and EBV-negative gastric cancer. Helicobacter. 2016;21:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Zhao K, Zhang Y, Xia S, Feng L, Zhou W, Zhang M, Dong R, Tian D, Liu M, Liao J. Epstein-Barr virus is associated with gastric cancer precursor: Atrophic gastritis. Int J Med Sci. 2022;19:924-931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Martínez-López JL, Torres J, Camorlinga-Ponce M, Mantilla A, Leal YA, Fuentes-Pananá EM. Evidence of Epstein-Barr virus association with gastric cancer and non-atrophic gastritis. Viruses. 2014;6:301-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Kartika AV, Iizasa H, Ding D, Kanehiro Y, Tajima Y, Kaji S, Yanai H, Yoshiyama H. Application of biopsy samples used for Helicobacter pylori urease test to predict Epstein-Barr virus-associated cancer. Microorganisms. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Hirano A, Yanai H, Shimizu N, Okamoto T, Matsubara Y, Yamamoto K, Okita K. Evaluation of Epstein-Barr virus DNA load in gastric mucosa with chronic atrophic gastritis using a real-time quantitative PCR assay. Int J Gastrointest Cancer. 2003;34:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 39. | Saju P, Murata-Kamiya N, Hayashi T, Senda Y, Nagase L, Noda S, Matsusaka K, Funata S, Kunita A, Urabe M, Seto Y, Fukayama M, Kaneda A, Hatakeyama M. Host SHP1 phosphatase antagonizes Helicobacter pylori CagA and can be downregulated by Epstein-Barr virus. Nat Microbiol. 2016;1:16026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 40. | Pandey S, Jha HC, Shukla SK, Shirley MK, Robertson ES. Epigenetic regulation of tumor suppressors by Helicobacter pylori enhances EBV-induced proliferation of gastric epithelial cells. mBio. 2018;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 41. | Fekadu S, Kanehiro Y, Kartika AV, Hamada K, Sakurai N, Mizote T, Akada J, Yamaoka Y, Iizasa H, Yoshiyama H. Gastric epithelial attachment of Helicobacter pylori induces EphA2 and NMHC-IIA receptors for Epstein-Barr virus. Cancer Sci. 2021;112:4799-4811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Wallaschek N, Reuter S, Silkenat S, Wolf K, Niklas C, Kayisoglu Ö, Aguilar C, Wiegering A, Germer CT, Kircher S, Rosenwald A, Shannon-Lowe C, Bartfeld S. Ephrin receptor A2, the epithelial receptor for Epstein-Barr virus entry, is not available for efficient infection in human gastric organoids. PLoS Pathog. 2021;17:e1009210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 43. | Shinozaki-Ushiku A, Kunita A, Isogai M, Hibiya T, Ushiku T, Takada K, Fukayama M. Profiling of virus-encoded microRNAs in Epstein-Barr virus-associated gastric carcinoma and their roles in gastric carcinogenesis. J Virol. 2015;89:5581-5591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 44. | Chang MS, Uozaki H, Chong JM, Ushiku T, Sakuma K, Ishikawa S, Hino R, Barua RR, Iwasaki Y, Arai K, Fujii H, Nagai H, Fukayama M. CpG island methylation status in gastric carcinoma with and without infection of Epstein-Barr virus. Clin Cancer Res. 2006;12:2995-3002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 45. | Zhang G, Tsang CM, Deng W, Yip YL, Lui VW, Wong SC, Cheung AL, Hau PM, Zeng M, Lung ML, Chen H, Lo KW, Takada K, Tsao SW. Enhanced IL-6/IL-6R signaling promotes growth and malignant properties in EBV-infected premalignant and cancerous nasopharyngeal epithelial cells. PLoS One. 2013;8:e62284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 46. | Sides MD, Klingsberg RC, Shan B, Gordon KA, Nguyen HT, Lin Z, Takahashi T, Flemington EK, Lasky JA. The Epstein-Barr virus latent membrane protein 1 and transforming growth factor-β1 synergistically induce epithelial-mesenchymal transition in lung epithelial cells. Am J Respir Cell Mol Biol. 2011;44:852-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Tsao SW, Tsang CM, To KF, Lo KW. The role of Epstein-Barr virus in epithelial malignancies. J Pathol. 2015;235:323-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 250] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 48. | Tsai MH, Raykova A, Klinke O, Bernhardt K, Gärtner K, Leung CS, Geletneky K, Sertel S, Münz C, Feederle R, Delecluse HJ. Spontaneous lytic replication and epitheliotropism define an Epstein-Barr virus strain found in carcinomas. Cell Rep. 2013;5:458-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 173] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 49. | Sasaki S, Nishikawa J, Sakai K, Iizasa H, Yoshiyama H, Yanagihara M, Shuto T, Shimokuri K, Kanda T, Suehiro Y, Yamasaki T, Sakaida I. EBV-associated gastric cancer evades T-cell immunity by PD-1/PD-L1 interactions. Gastric Cancer. 2019;22:486-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 50. | Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, Liu XQ, Sher X, Jung H, Lee M, Lee S, Park SH, Park JO, Park YS, Lim HY, Lee H, Choi M, Talasaz A, Kang PS, Cheng J, Loboda A, Lee J, Kang WK. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 1162] [Article Influence: 166.0] [Reference Citation Analysis (0)] |
| 51. | Sivachandran N, Dawson CW, Young LS, Liu FF, Middeldorp J, Frappier L. Contributions of the Epstein-Barr virus EBNA1 protein to gastric carcinoma. J Virol. 2012;86:60-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 52. | Yoshiyama H, Imai S, Shimizu N, Takada K. Epstein-Barr virus infection of human gastric carcinoma cells: implication of the existence of a new virus receptor different from CD21. J Virol. 1997;71:5688-5691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 3.9] [Reference Citation Analysis (0)] |