Published online Nov 28, 2022. doi: 10.3748/wjg.v28.i44.6213
Peer-review started: August 7, 2022
First decision: October 20, 2022
Revised: October 31, 2022
Accepted: November 7, 2022
Article in press: November 7, 2022
Published online: November 28, 2022
Processing time: 109 Days and 12.1 Hours
Primary sclerosing cholangitis (PSC) is an autoimmune disease characterized by chronic cholestasis, a persistent inflammation of the bile ducts that leads to sclerotic occlusion and cholestasis. Gut microbes, consisting of microorganisms colonized in the human gut, play an important role in nutrient intake, metabolic homeostasis, immune regulation, and immune regulation; however, their presence might aid PSC development. Studies have found that gut-liver axis interactions also play an important role in the pathogenesis of PSC. Patients with PSC have considerably reduced intestinal flora diversity and increased abundance of potentially pathogenic bacteria. Dysbiosis of the intestinal flora leads to increased intestinal permeability, homing of intestinal lymphocytes, entry of bacteria and their associated metabolites, such as bile acids, into the liver, stimulation of hepatic immune activation, and promotion of PSC. Currently, PSC effective treatment is lacking. However, a number of studies have recently investigated the targeted modulation of gut microbes for the treatment of various liver diseases (alcoholic liver disease, metabolic fatty liver, cirrhosis, and autoimmune liver disease). In addition, antibiotics, fecal microbiota transplantation, and probiotics have been reported as successful PSC therapies as well as for the treatment of gut dysbiosis, suggesting their effectiveness for PSC treatment. Therefore, this review briefly summarizes the role of intestinal flora in PSC with the aim of providing new insights into PSC treatment.
Core Tip: Primary sclerosing cholangitis (PSC) is an autoimmune disease that currently lacks treatment. The intestinal flora comprises microorganisms that colonize the human gut and play essential roles in nutrient intake, metabolic homeostasis, immune regulation, and PSC development. Thus, the intestinal flora may be a potential therapeutic target for PSC, and many recent studies have attempted to regulate it. In this review, we have reviewed the role of the intestinal flora in PSC. We believe that our study makes a significant contribution to the literature because our paper demonstrated the great potential of the gut flora as a therapeutic target for PSC treatment.
- Citation: Li ZJ, Gou HZ, Zhang YL, Song XJ, Zhang L. Role of intestinal flora in primary sclerosing cholangitis and its potential therapeutic value. World J Gastroenterol 2022; 28(44): 6213-6229
- URL: https://www.wjgnet.com/1007-9327/full/v28/i44/6213.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i44.6213
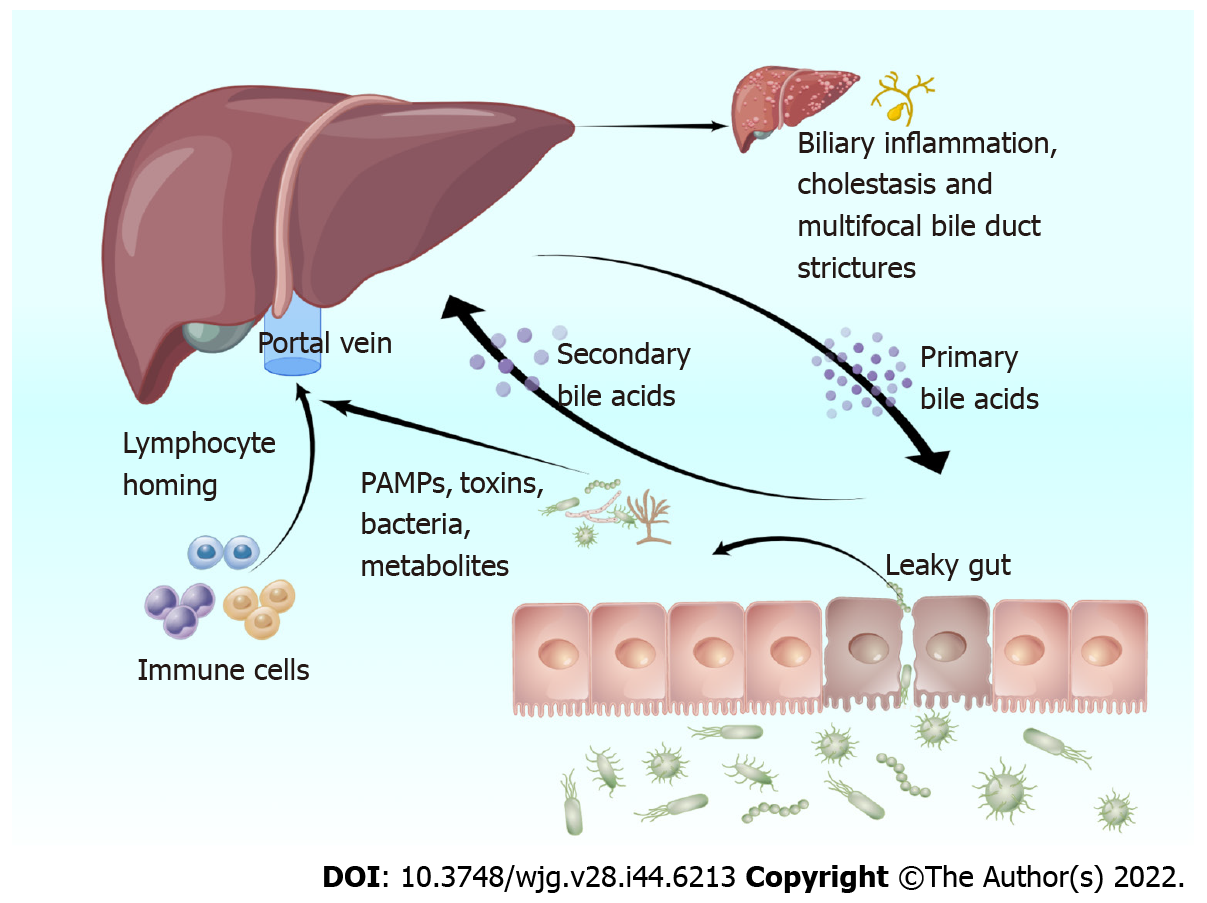
Primary sclerosing cholangitis (PSC) is an autoimmune-mediated chronic cholestatic liver disease characterized by progressive bile duct inflammation, leading to intra- and extrahepatic bile duct stenosis and occlusion and cholestatic cirrhosis[1]. Patients with PSC have a greatly increased risk of developing cancers (cholangiocarcinoma, gallbladder cancer, hepatocellular carcinoma, and colorectal cancer); approximately half of patients with PSC develop cancer, ultimately leading to death[2]. Although the etiology of PSC remains unclear, it is generally accepted that interactions between genetics and the environment determine PSC development[3]. Owing to the close anatomical and physiological connection between the intestine and the liver, the intestinal flora is closely related to liver disease[4]. Several studies have suggested that the intestinal flora is involved in PSC development through the gut-liver axis[5,6]. Moreover, patients with PSC have significantly reduced intestinal flora diversity and an increased abundance of potentially pathogenic bacteria[7,8]. Intestinal flora dysbiosis leads to increased intestinal permeability, intestinal lymphocyte homing, entry of bacteria and their associated metabolites [e.g. bile acids (BAs)] into the liver, activation of the hepatic immune response, and bile duct inflammation and fibrosis[9].
The incidence of PSC is increasing, but an effective treatment does not exist currently. PSC can eventually progress to cirrhosis or liver failure, but these conditions are still symptomatically treated[10,11]. For patients with end-stage PSC, liver transplantation is the sole option; however, transplantations are not universally available owing to high costs and potential transplant rejection. Furthermore, approximately 30%-50% of patients experience PSC recurrence within 10 years of transplantation[12].
Many studies have reported that the gut flora is a promising therapeutic target for PSC, and that antimicrobial therapy based on gut flora modulation, fecal microbiota transplantation (FMT), and probiotics is an emerging therapeutic options[13,14]. Thus, in this review, we discuss the latest research findings on the role of intestinal flora in PSC and provide important insights into potential microbe-altering interventions.
PSC is a rare disease with an incidence of 0.91-1.30/100000. The incidence of small bile duct PSC is approximately 0.15/100000, with the highest prevalence in the Nordic countries, reaching an incidence of 16.2/100000[15]. PSC mostly occurs in men aged 40-50 years, with age of diagnosis at 30-40 years and a male to female ratio of approximately 2:1[1]. The pathogenesis of PSC is complex, but it is currently believed that interactions between genetic susceptibility factors and environmental promoters plays a role in the occurrence and development of PSC[16]. Human leukocyte antigen is strongly associated with PSC pathogenesis[17]. However, the risk ratio for first-degree relatives is approximately 11, suggesting that environmental factors play a more critical role in the pathogenesis of PSC[18]. In addition, the geographic distribution of PSC pathogenesis may indicate that the disease is influenced by the environment[19]. Interactions of the gut-liver axis, such as damage to the intestinal mucosal barrier, dysbiosis, and immune interactions, also play a role in the pathogenesis of PSC[1].
The gut-liver axis refers to the bidirectional relationship between the intestine, its microbiota, and the liver, resulting from the interaction of dietary, genetic, and environmental factors[20]. The close association between the intestine and the liver begins during embryonic development, with both organs originating together in the ventral foregut endoderm. From a physiological point of view, the gut-liver axis is one of the most important links between the intestinal flora and the liver[21]. The liver, which receives approximately 70% of its blood from the portal vein, is a receiver and filter of nutrients and bacterially produced toxins and their metabolites. The secretion of substances such as BAs and antibodies into the intestine acts as a feedback mechanism that affects intestinal homeostasis[22].
Approximately 70% of patients with PSC have concomitant inflammatory bowel disease (IBD) and more commonly ulcerative colitis (UC)[23-25]. Patients with PSC have a reduced risk of death after a colectomy, and after receiving liver transplantation, colectomy significantly reduces the risk of PSC recurrence. This close association between PSC and IBD suggests that intestinal flora may play a key role in the pathogenesis of PSC[26,27] through the gut-liver axis[28].
Under normal physiological conditions, the human body contains a large and diverse community of intestinal microorganisms, reaching up to 1014 organisms that comprise more than 1000 species; this is collectively known as the intestinal flora[29]. A normal intestinal flora is primarily composed of Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, which promote nutrient digestion and absorption, defend against foreign pathogens, regulate immunity, and participate in metabolic processes[30].
In healthy populations, the intestinal microenvironment is in homeostasis due to the mutual regulation of various flora. Intestinal flora dysbiosis is a disruption of the dynamic balance between intestinal flora when various factors cause disturbances in the human body environment, and changes in the number, species, and ratio of favorable, conditionally pathogenic, and harmful bacteria[31,32]. The manifestations of intestinal flora dysbiosis include intestinal flora translocation (transfer of the original colonizing bacteria from the intestine to the deep mucosa or from the intestine to other sites) and intestinal flora imbalance (decrease in the abundance of the original beneficial intestinal flora and increase in the abundance of pathogenic flora). Dysbiosis of the intestinal flora leads to impairment of the intestinal barrier, increased endotoxemia, and a breakdown of the liver's immune tolerance to the intestinal flora and its metabolites, which in turn causes a strong immune response in the liver[33].
It was found that patients with PSC have a marked dysbiosis of the intestinal flora compared with the healthy population. Rossen et al[34] performed the first 16S rRNA analysis of the microbiota of the intestinal mucosa in the ileocecal region of patients with PSC and found that, at the genus level, the relative abundance of uncultured Clostridium II was notably lower in patients with PSC compared with patients with UC and non-inflammatory controls. In addition, the mucosal adherent microbiota at the level of the ileocecal region in patients with PSC showed considerably reduced diversity and abundance. Torres et al[8], using bacterial 16S rRNA next-generation sequencing, reported that patients with PCS had similar overall microbiome characteristics at different locations in the gut, exhibiting enrichment in Blautia and Barnesiellaceae spp. A more in-depth taxon analysis at the operational taxonomic unit (OTU) level revealed the most significant changes in Clostridiales, with 3 decreases and 66 OTU enrichments. Sabino’s study found that species richness (defined as the number of different OTUs observed in the samples) was reduced in patients with PSC compared with healthy controls, that Enterococcus, Clostridium, Lactobacillus, and Streptococcus were enriched, and that an operational taxonomic unit belonging to the Enterococcus genus is positively correlated with the levels of alkaline phosphatase (ALP) levels (a marker of disease severity)[35]. In addition, PSC has its own unique gut microbial profile that is not associated with IBD co-morbidity. Subsequently, a study by Kummen et al[36] also confirmed the unique gut microbiota of PSC independent of the receipt of antibiotics and ursodeoxycholic acid (UDCA) treatment, with a marked reduction in bacterial diversity in patients with PSC. Furthermore, Quraishi et al[37] explored the intestinal mucosal flora of PSC-IBD patients, further complementing the study by Kummen et al[36] Escherichia, Lachnospiraceae, and Megasphera were markedly increased, whereas Prevotella and Roseburia (butyrate producers) were decreased in abundance in PSC-IBD patients compared with controls. They hypothesized that intestinal microecological dysregulation in patients with PSC may prompt dysregulation associated with mucosal immunity by modulating abnormal homing of intestinal-specific lymphocytes and intestinal permeability. This is consistent with the hypothesis of Kummen et al[36], Rühlemann et al[38,39] also showed that PSC itself drives the observed changes in fecal microbiota and that the findings of Kummen et al[36] regarding microbiota as a diagnostic marker are promising.
In the last two years, studies on the PSC gut flora have gained more interest. Quraishi et al[40] tried to unravel the PSC disease mechanism by integrating mucosal transcriptomics, immunophenotyping, and mucosal microbial analysis; their study reported that PSC-IBD patients had considerably higher abundance of Bacteroides fragilis, Roseburia spp., Shewanella sp., and Clostridium ramosum species, which was associated with changes in the BA metabolic pathway. In addition, the amine oxidase-expressing bacterium Sphingomonas sp. is upregulated in PSC-IBD. Amine oxidase is associated with abnormal homing of intestinal lymphocytes to the liver[41]. The upper gastrointestinal tract and bile ducts of patients with PSC are equally affected by microbial ecological dysbiosis. Liwinski et al[42] showed that the biliary microbiome of patients with PSC exhibited the most extensive changes, including reduced biodiversity and expansion of pathogenic bacteria, with the marked increase of Enterococcus spp. directly causing epithelial barrier damage and mucosal inflammation. Lapidot et al[43] found that microbial alterations in PSC were consistent in saliva and gut, with 27 bacterial species present in both the salivary and gut microbiomes, including Clostridium perfringens XlVa, Veillonella, Lachnospiraceae, Streptococcus, and Blautia. Of these, Lactobacillus, Ruminococcus gnavus, and Streptococcus salivarius were extensively enriched. The study by Lemoinne et al[44] confirmed previous findings of altered bacterial microbiota composition in patients with PSC, such as Faecalibacterium and Ruminococcus in reduced proportions, and reported for the first time the occurrence of fungal ecological dysbiosis in patients with PSC. PSC-associated fungal ecological dysbiosis is characterized by increased biodiversity (alpha diversity) and altered composition compared with in healthy subjects or patients with IBD, including a marked increase in the abundance of Exophiala spp. In some patients. Kummen et al[45] provided a detailed functional analysis of microbial genes encoding enzymes and metabolic pathways by using metagenomic shotgun sequencing. Clostridium spp. increased in the intestinal flora of patients with PSC, while Eubacterium spp. and Ruminococcus obeum decreased. Targeted metabolomics revealed reduced concentrations of vitamin B6 and branched-chain amino acids in PSC. Microbial metabolism of essential nutrients and circulating metabolites associated with the disease process were considerably altered in patients with PSC compared with in healthy individuals, suggesting that microbial function may be related to the disease process in PSC.
Most of these studies used 16S gene sequencing to examine the microbiota in the intestinal mucosa and feces of patients with PSC. Although these studies came from all over the world, some of them had relatively small sample sizes, and dietary and lifestyle habits varied between the samples of each study, possibly affecting the final gut floral composition of patients with PSC and limiting the generalization of the results. However, these studies also yielded some common findings that reveal to some extent the gut microbiota characteristics of patients with PSC[46]. Patients with PSC suffer intestinal dysbiosis, which has its own unique biological characteristics, as evidenced by a decrease in gut bacterial α-diversity (average species diversity of the ecosystem) and marked changes in β-diversity (spatial variation in species composition), a decrease in specialized anaerobic bacteria, and an increase in the abundance of potentially pathogenic bacteria (Table 1)[7,35-39,42-44,47]. Among which, Veillonella, Enterococcus, Streptococcus, Clostridium, and Lactobacillus spp. were markedly elevated[36,38,42-44]. An increase in Veillonella species, a potential pathogen in humans, can serve as a biomarker of the severity of certain diseases, such as autoimmune liver disease and cirrhosis[48,49].
| Methods | Increased | Decreased | Ref. |
| 16S RNA gene sequencing: ileum, colon, and rectal samples | Actinobacillus, Bifidobacterium, Fusobacterium, Haemophilus, Roseburia | Bacteroides | [135] |
| qPCR: fecal samples | Enterobacteriaceae | Bifidobacterium, Lactobacillus | [136] |
| 16S RNA gene sequencing: fecal samples | Clostridium, Lactobacillus, Ruminococcus gnavus, Veillonella | Coprococcus, Faecalibacterium, Phascolarctobacterium, Ruminococcus | [137] |
| 16S RNA gene sequencing: duodenal fluid, saliva, duodenal mucosa, and bile samples | Duodenal mucosa biopsy: Escherichia coli, Veillonella dispar; Bile fluid: Enterococcus, Neisseria, Proteobacteria, Staphylococcus, Veillonella dispar | [42] | |
| Metagenomic shotgun sequencing | Clostridiales | Eubacterium, Ruminococcus obeum | [45] |
| 16S RNA gene sequencing: fecal and saliva samples | Bacteroides fragilis, Blautia, Clostridium spp. Enterococcus, Enterobacteriaceae, Lactobacillus, Ruminococcus gnavus, Streptococcus salivarius Veillonella dispar | Bacteroides thetaiotaomicron, Faecalibacterium prausnitzii | [43] |
| Sigmoid mucosal biopsies and 16S RNA gene sequencing | Haemophilus parainfluenzae, Pseudomonas, Streptococcus | Lachnospiraceae | [40] |
| 16S RNA gene sequencing: fecal samples | Veillonella | Blautia, Faecalibacterium, Lachnoclostridium, Ruminococcus | [44] |
| 16S RNA gene sequencing: fecal samples | Enterococcus, Lactobacillus, Streptococcus, Veillonella | Clostridium cluster IV, Coprococcus, Desulfovibrio, Faecalibacterium, Holdemanella | [38] |
| 16S RNA gene sequencing: fecal samples | Veillonella | Coprococcus, Desulfovibrio, Phascolarctobacterium, Succinivibrio | [36] |
| 16S RNA gene sequencing: fecal samples | Clostridium, Enterococcus, Haemophilus, Rothia, Streptococcus, Veillonella | Adlercreutzia equolifaciens, Coprococcus catus, Faecalibacterium prausnitzii, Prevotella copri, Ruminococcus gnavus | [47] |
| Colonic mucosal biopsies and 16S RNA gene sequencing | Escherichia, Lachnospiraceae, Megasphaera | Bacteroides, Prevotella, Roseburia | [37] |
| 16S RNA gene sequencing: fecal samples | Veillonella | [39] | |
| 16S RNA gene sequencing: fecal samples | Enterococcus, Streptococcus, Veillonella | [7] | |
| 16S RNA gene sequencing: fecal samples | Bacteroidetes, Enterococcus, Fusobacterium, Lactobacillus, Morganella, Streptococcus, Veillonella | Anaerostipes | [35] |
| Colonic mucosal biopsies and 16S RNA gene sequencing: ileal samples | Barnesiellaceae, Blautia | [8] | |
| Mucosal biopsy of the ileocecal region and 16S RNA gene sequencing | Clostridium clusters IVand XIVa, Akkermansia sp. (Verrucomicrobia), Uncultured Clostridiales II | [34] |
The leaky gut hypothesis was first proposed by Bjarnason et al[50] in 1984, providing theoretical support for the involvement of the intestinal flora in the development of PSC. Normal intestinal flora plays the role of maintaining the balance of the intestinal environment and preventing pathogenic bacteria and toxins from entering the blood circulation[51]. The germ-free (GF) multidrug resistance 2 knockout (Mdr2-/-) mice is a well-studied PSC model that shows a lack of microbial regulation, which is direct evidence that intestinal flora has a key role in PSC development[52]. Intestinal flora dysbiosis damages the intestinal barrier in patients with PSC, allowing bacteria and enteric-derived endotoxins to enter the liver via the portal vein, triggering an immune response[53]. Simultaneously, when liver function is impaired, Kupffer cells cannot inactivate endotoxins as efficiently, impairing bile excretion. Furthermore, this increases intestinal permeability, intestinal lymphocyte nesting, and the entry of bacteria and their metabolites [i.e. pathogen-associated molecular patterns (PAMPs)] into the liver, impairs normal BA metabolism, and promotes bile duct inflammation and fibrosis (Figure 1)[54,55].
Intestinal flora dysbiosis damages the intestinal barrier: Lapidot et al[43] found that in patients with PSC, a decrease in the relative abundance of commensal bacteria in the intestinal flora, including Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii, and bacterial diversity led to decreased short-chain fatty acids (SCFAs) with anti-inflammatory effects, such as acetate and butyric acid. This decrease caused intestinal barrier dysfunction and lack of antimicrobial peptides, exacerbating the leaky gut syndrome. When intestinal flora dysregulation occurs in patients with PSC, PAMPs in the gut bind to Toll-like receptors (TLRs) and NOD-like receptors (NLRPs) on the surface of dendritic cells. This event activates the cytoplasmic downstream nuclear transcription factor κB (NF-κB), causing the production and secretion of inflammatory cytokines and chemokines. Disruption of intestinal epithelium tight junctions and the normal intestinal barrier leads to increased intestinal permeability[31,56]. Furthermore, Enterococcus faecalis (E. faecalis), which increased the most in the intestinal flora of patients with PSC, produces gelatinase, which damages the intestinal epithelium and causes impaired intestinal barrier function[35]. Nakamoto et al[57] also found that increased Klebsiella pneumoniae during PSC forms pores by disrupting the intestinal epithelium, leading to increased intestinal permeability, thus prompting other bacteria (e.g. Proteus mirabilis and Enterococcus gallinarum) to cross the intestinal barrier. In turn, a Th17 cell-mediated inflammatory response initiates in the liver. Finally, Manfredo et al[58] demonstrated that Enterococcus gallinarum could reach several organs, such as the mesentery, mesenteric lymph nodes, liver, and spleen, after crossing the damaged intestinal epithelium, causing autoimmune diseases such as PSC.
In addition, PSC recurrence in patients who had undergone liver transplantation was associated with specific intestinal flora changes before transplantation. The rate of PSC recurrence was decreased in patients with a higher abundance of Shigella spp. in the intestinal flora before transplantation, suggesting that Shigella spp. may reduce bacterial translocation and endotoxemia by improving the intestinal mucus layer function and repairing the intestinal barrier[59].
Intestinal bacterial translocation induces liver inflammation: Secondary bacterial overgrowth in the small intestine of rats, achieved by using a blind jejunal loop, led to the translocation of intestinal flora and its metabolite. Consequently, the intestines exhibited characteristic pathological changes of PSC, such as irregular dilatation and bead-like changes in the intra- and extrahepatic bile ducts[60]. Furthermore, Tedesco et al[61] found elevated serum interleukin (IL)-17 levels in PSC mice; enriched Lactobacillus gasseri, peribiliary collagen deposition, and periportal fibrosis; and increased numbers of IL-17A+ and γδTCR+ cells in mouse liver tissues, which are characteristic inflammatory responses. Additionally, Liao et al[62] used Mdr2-/- mice to investigate the role of intestinal flora in PSC, reporting that Mdr2-/- mice had intestinal flora dysbiosis. This caused the NLRP3-mediated innate immune response in the liver, amplified by intestinal barrier failure and enhanced bacterial translocation. Finally, Dhillon et al[63] compared the serum soluble cluster of differentiation 14 (sCD14) and lipopolysaccharide-binding protein (LBP) levels of patients with PSC and healthy controls, finding that patients with PSC had elevated levels of sCD14 and LBP. The sCD14 and LBP bind to lipopolysaccharides (typical bacterial translocation markers in humans) in response to significant intestinal flora translocation in patients with PSC[64].
The liver contains many immune cells, including Kupffer cells, natural killer (NK) cells, NK T cells, T cells, and B cells, and is a vital immune organ. In healthy individuals, only a few translocated bacterial products make it to the liver. The liver immune system tolerates these bacterial products to avoid harmful reactions, known as hepatic immune tolerance[65]. The intestinal flora dysbiosis in PSC impairs the intestinal barrier function, allowing bacteria and their products to enter the liver continuously. Thus, the hepatic immune tolerance breaks, inducing local inflammation and immune responses by activating TLR-based pattern recognition receptors on hepatic immune cells. Gram-positive bacteria mainly mediate TLR2 activation, endotoxins mediate TLR4 activation, bacterial flagella mediate TLR5 activation, and unmethylated CpG DNA mediates TLR9 activation[66]. TLR activation promotes a downstream inflammatory cascade that activates the MyD88-mediated NF-κB pathway to induce liver fibrosis[67]. Simultaneously, inflammatory cytokines and chemokines [e.g. IL-6 and tumor necrosis factor-α (TNF-α)] are overexpressed, inflammatory cells infiltrate, and oxidative stress and endoplasmic reticulum stress occur in the bile duct epithelium. Eventually, bile duct sclerosis and occlusion, cholestasis, and bile duct fibrosis develop[54,68].
Up to 70% of patients with PSC also develop IBD, suggesting a correlation between the intestine and the liver in patients with PSC and IBD. The discovery of reciprocal transport pathways of lymphocytes to target tissues, as well as the expression of gut-specific adhesion molecules and chemokines in the liver, suggest the homing of intestinal lymphocytes as a contributing factor to PSC pathogenesis[69,70]. Endothelial cells in the hepatic sinusoids of patients with PSC overexpress mucosal vascular addressin cell adhesion molecule 1 (an endothelial adhesion molecule) and C-C motif chemokine ligand 25 (a chemokine), which bind to α4β7 integrin and C-C motif chemokine receptor expressed by intestinal mucosal lymphocytes. This event prompts the recruitment of lymphocytes of an intestinal origin into the liver, which then recognizes the corresponding antigen and triggers an autoimmune response, causing liver injury[71,72]. Trivedi et al[41] suggested that this mechanism is associated with hepatic vascular adhesion protein-1 (VAP-1) overexpression. Increased Veillonella in the gut of patients with PSC results in primary amine metabolism, which participates in VAP-1 synthesis (as a VAP-1 substrate). Furthermore, hepatic interstitial cells express VAP-1, which recruits intestine-derived T cells to the liver, promoting liver inflammation and fibrosis[73]. Moro-Sibilot et al[74] found that elevated levels of sVAP-1 were associated with poor disease outcomes in PSC. High sVAP-1 Levels correlate with the expression of mucosal addressin cell adhesion molecule 1 in the liver, which contributes to the homing of intestinally activated T cells to the hepatobiliary tract[75]. Meanwhile, sVAP-1 triggers oxidative stress in hepatocytes and aggravates liver injury[76]. B cells in the liver are also derived from intestine-associated lymphoid tissue. B cells are activated by intestinal bacteria and enter the liver, producing antibacterial molecules, such as immunoglobin A, that aggravate liver damage.
It has been established that several intestinal bacterial genera produce BA hydrolases, such as Lactobacillus, Clostridium, Enterococcus, and Bifidobacterium. Normal microbial metabolism increases BA diversity as well as hydrophobicity, which facilitates BA excretion[77,78]. Intestinal flora plays a key role in the pathogenesis of PSC by mediating BA biosynthesis and farnesol X receptor (FXR) signaling. FXR regulates BA synthesis through a negative feedback loop thereby affecting the intestinal flora[79]. BAs can directly damage intestinal bacterial cell membranes and indirectly affect the intestinal flora composition by binding to FXR and enhancing the action of antimicrobial peptides. Intestinal flora can also alter BA metabolism by affecting the ab initio synthesis of BAs and enterohepatic circulation[80]. Liwinski et al[42] found that patients with PSC had increased taurolithocholic acid concentrations in their bile, which causes inflammation; the levels were closely related to the abundance of Enterococcus. BA hydrolase expression, which catalyzes the conversion of primary BAs to secondary BAs, is highest when the human intestinal flora contains E. faecalis. Thus, a significant increase in E. faecalis in the bile of patients with PSC may affect BA metabolism and cause excessive accumulation of secondary BAs in the body, exacerbating PSC[7,42,81]. Tabibian et al[82] found that Mdr2-/- mice produced similar biochemical and histological features of PSC (confirmed by liver pathology and hydroxyproline assays) compared to conventionally reared Mdr2-/- mice; these mice were deficient in secondary BAs due to lack of intestinal flora. Further studies showed that GF-Mdr2-/- mice and antibiotic-induced specific pathogen-free Mdr2-/- mice showed imbalance in BA homeostasis, increased BA reuptake, and accelerated accumulation of harmful BAs in the liver due to dysregulation of intestinal microecology[83].
A recent study showed that Prevotella copri in the human gut regulates BA metabolism and transport pathways through gut microbiota interactions, especially the FXR signaling pathway, significantly improving chlorosis and liver fibrosis in 3,5-diethoxy-carbonyl-1,4-dihydropyridine-induced PSC mice[84]. Another study showed that intestinal flora attenuates liver damage by promoting UDCA production. The mechanism of UDCA, which has antioxidant, immunomodulatory, hepatocyte-protective, and membrane-maintaining functions, includes re-establishing the intestinal flora, and is widely used to treat PSC[85]. Lee et al[86] found that Ruminococcus gnavus N53 and Collinsella aerofaciens in normal human intestinal flora catalyze the conversion of goose deoxycholic acid to UDCA by expressing the 7β-hydroxysteroid dehydrogenase gene, which increases UDCA acid, thereby reducing liver damage in pathological conditions.
There are no clear and effective options for treating PSC. Pharmacological and endoscopic treatments exist; however, these treatments primarily target the symptoms, and the only effective treatment for end-stage PSC is liver transplantation[16]. In recent years, the incidence of PSC has increased, but intestinal flora research has also expanded, resulting in antimicrobial therapy based on intestinal flora modulation and FMT as potential PSC treatment options[87]. Studies have found that antibiotics, probiotics, and FMT improve intestinal flora disorders, thereby treating PSC (Table 2)[88,89].
| Treatment | Results | Ref. | ||
| Oral Antibiotics | Vancomycin | 125 mg four times daily for 90 d | Fecal calprotectin and serum GGT levels returned to normal | [94] |
| 125 mg four times daily for 12 wk | Significantly decreased ALP, MRS, ESR, and GGT levels. Fatigue, pruritus, diarrhea, and anorexia significantly improved | [91] | ||
| 50 mg/kg/d for 30 to 118 mo | Decreased ALT, AST, GGT, and ESR levels. Jaundice improved. The overall rate of positive serum autoantibodies decreased after 3.5 mo | [138] | ||
| Vancomycin and metronidazole | Vancomycin: 125 mg or 250 mg four times daily for 12 wk; Metronidazole: 250 mg or 500 mg three times daily for 12 wk | Decreased ALP and MRS levels. The decrease in ALP level was more pronounced following vancomycin administration | [90] | |
| Metronidazole with ursodeoxycholic acid | Taken together for 36 mo | Significantly decreased ALP and MRS levels | [98] | |
| Azithromycin | 500 mg three days per week for 6 wk | Decreased ALP and TBIL levels and cholestasis-related symptoms. The urine was turned dark in color again | [139] | |
| Rifaximin | 550 mg twice daily for 12 wk | Decreased GGT and CRP levels; Improved pruritus symptoms | [95] | |
| Minocycline | 100 mg twice daily for one year | Significantly decreased ALP and MRS levels | [100] | |
| Fecal microbiota transplantation at 24 wk | Significantly decreased ALP levels; Reduced AST levels (by at least 30%) | [107] | ||
| Probiotics | Lactobacilli and Bifidobacteria | Three months of: Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus salivarius, and Lactococcus lactis, Bifidobacterium bifidum, Bifidobacterium | Reduced ALP levels (by 15%) | [113] |
| Combined | Prednisolone (30 mg/d), salazosulfapyridine (3000 mg/d), and Lactobacillus casei Shirota (3 g/d) for 2 wk | Decreased ALP, ALT, AST, and GGT levels | [114] | |
Studies have shown that patients with PSC treated with vancomycin had significant reductions in their serum ALP and bilirubin levels and Mayo PSC risk scores (MRSs) and significant improvements in clinical symptoms, such as fatigue and pruritus[90,91]. An open-label prospective therapeutic clinical trial study showed that oral vancomycin was well tolerated in patients with PSC, with peripheral blood γ-gamma-glutamyl transpeptidase (GGT), alanine aminotransferase (ALT) concentrations, white blood cell counts, and neutrophil counts returning to normal from pre-treatment elevated levels within 3 mo of oral administration. Cholangiography, histological, and liver stiffness assessment at the end of follow-up showed improved results, and the trial also showed that that peripheral blood levels of CD4 + CD25hiCD127 Lo and CD4 + FoxP3 + regulatory T cells were also elevated in PSC-IBD patients treated with oral vancomycin[92,93]. Furthermore, Britto et al[94] found fewer potentially pathogenic bacteria, such as Fusobacterium, Haemophilus, and Neisseria, in the intestinal flora of patients with PSC after oral vancomycin treatment. A significant recovery in flora diversity was also observed, suggesting that vancomycin treatment indirectly leads to a secondary increase in bacterial diversity by prompting the intestinal flora to suppress mucosal inflammation. The efficacy of vancomycin for PSC may be related to its selectivity for Clostridium perfringens[95]. Shah et al[96] reported that vancomycin has a relatively narrow antibiotic spectrum and specifically targets Clostridiales. Consequently, vancomycin affects the abundance of Clostridiales in the intestinal flora of the distal small intestine and colon by reducing primary BA dehydroxylation and preventing excessive secondary BA accumulation, thereby reducing PSC activity. In addition, Davies et al[97] demonstrated that vancomycin directly attenuates the inflammatory response to periportal inflammation and liver injury during PSC.
Studies in animal models have demonstrated that metronidazole also has a therapeutic effect on liver injury in PSC[60]. For example, Karvonen et al[98] found that treating patients with PSC with both UDCA and metronidazole significantly reduces the serum glutamyl transpeptidase and ALP levels, and significantly improves the MRS and pathological staging compared with those treated with only UDCA. Furthermore, Krehmeier et al[99] reported that metronidazole reduced intestinal permeability, decreased bacterial endotoxin entry into the blood, inhibited endotoxin-induced TNF-α production, inhibited hepatic Kupffer cells and macrophage activation, reduced chemokine and cytokine secretion by biliary epithelial cells, attenuated liver inflammation, and prevented PSC-like bead-like liver injury. Finally, Silveira et al[100] showed that minocycline is a safe and effective PSC treatment, significantly reducing the ALP level and MRS after one year of oral minocycline administration.
FMT is the transplantation of fecal flora from healthy individuals into a patient’s intestine to replenish or restore normal intestinal flora. This procedure aims to reverse intestinal dysbiosis, regulate product metabolism, and improve clinical symptoms to treat the disease (Clostridium difficile infection, IBD, diabetes mellitus, cancer, liver cirrhosis, gut-brain disease and others)[101,102]. FMT restores the health of the intestinal flora, further reducing the transport of harmful metabolites, such as endotoxins to the liver, and reducing the damage caused by metabolites to the liver[103]. FMT uses the principle of bacterial therapy to restore the health of the intestinal flora. The transplanted beneficial bacteria (Bifidobacteria, etc.) are involved in the conversion of polysaccharides to monosaccharides, producing SCFAs such as acetate, propionate, and butyrate[104]. These metabolites regulate normalization of the intestinal flora and reduce intestinal permeability in patients with liver disease, to further reduce the transport of metabolites such as endogenous ethanol and endotoxins to the liver, thus, reducing the damage to the liver[103,105,106]. Studies have shown intestinal flora normalization, a significant improvement in intestinal flora diversity, reduced cholestasis, and decreased ALP levels in PSC patients after FMT. Allegretti et al[107] performed the first human FMT trial in ten patients with PSC who had ALP levels more than three times the normal upper limit. After FMT, 30% of the patients had decreased ALP levels by 50%, and 70% had a 30% reduction in the levels of serum liver transaminases (ALT and aspartate aminotransferase). One week after FMT, the recipients’ intestinal flora diversities were higher than the baseline level of all patients and continued increasing for 24 wk. Furthermore, Philips et al[108] found that fecal flora diversity improved in patients with PSC after FMT, with a decrease in the relative abundance of Proteobacteria and an increase in the abundances of Bacteroidetes and Firmicutes; this intestinal flora composition was more similar to that of healthy individuals. The blood biochemistry and total BA indicators also significantly improved.
Probiotic is a general term for a group of active microorganisms that have beneficial roles by regulating intestinal flora growth and improving the host’s intestinal microecology. They regulate the intestinal microenvironment metabolism, increase SCFAs production, and reduce the permeability of the intestinal barrier[109,110]. Additionally, probiotics upregulate intestinal epithelial tight junction protein expression, improve intestinal motility[110,111], increase adhesion and colonization of intestinal flora, reduce TNF-α production, and maintain tissue homeostasis[112]. One study demonstrated that oral administration of probiotic preparations (consisting of six strains of viable and freeze-dried bacteria: Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus salivarius, Lactococcus lactis, Bifidobacterium bifidum, and Bifidobacterium lactis) decreased the serum alkaline phosphatase level by 15 % in patients with PSC compared to healthy individuals[113]. Furthermore, Shimizu et al[114] treated a patient with PSC with a combination of prednisolone, salazosulfapyridine, and probiotics, and reported that the patient’s symptoms and tests improved after two weeks. Additionally, repeat pathological biopsies at 30 mo showed significant improvements in liver inflammatory cell infiltration and peribiliary fibrosis. Lactobacillus plantarum Lp2 has the potential to ameliorate liver injury by inhibiting the activation of LPS-induced inflammatory pathways in the liver, reducing inflammation, and decreasing oxidative damage and apoptosis[115]. Therefore, probiotics have a therapeutic effect on PSC by suppressing intestinal inflammation and maintaining intestinal flora homeostasis.
Compared to conventional mice, germ-free mice show higher concentrations of BA in the plasma and significantly reduced concentrations in the feces. Additionally, FXR signaling is significantly inhibited, resulting in reduced BA synthesis in germ-free mice[116,117]. Colonization of germ-free mice with human feces activates the expression of FXR target genes and increases the levels of BAs in the liver and ileal tissue[118]. FXR agonists inhibit cholesterol 7α-hydroxylase activity and, thus, intracellular BA synthesis. These agonists can activate transcription of the bile salt export pump on the hepatocyte membrane, enhancing the transport of BAs from hepatocytes to bile ducts and promoting BA excretion. Simultaneously, These agonists can inhibit the expression of extracellular matrix proteins in hepatic astrocytes and, thus, prevent the transformation of liver fibrosis in patients with PSC[119]. Obeticholic acid (OCA) is one of FXR agonists representative drugs that alleviates the cholestatic symptoms of PSC by reducing the BA pool[120]. OCA is also approved for the treatment of PSC[121,122]. In fact, there are phase II clinical trials demonstrating the efficacy and safety of OCA in patients with PSC[123].
In addition to the above-mentioned studies, there are currently several relevant clinical trials demonstrating the efficacy of treatments targeting intestinal flora and its metabolites in PSC (Table 3). From these clinical studies, we found that oral vancomycin is the most established for the treatment of PSC, and all phase IV clinical trials using vancomycin have been completed. Vancomycin can significantly reduce biochemical indexes such as ALP and ALT and reduce MRS in patients with PSC[92,124]. One case study also described a decrease in serum γ-GGT, which reached normal levels at 195 d, in pediatric patients with PSC-UC who were administered vancomycin[94]. Fusobacterium, Haemophilus, and Neisseria, which generally have a significantly high abundance in PSC, showed decreased abundance in the saliva and feces of these patients[40,42,43,47]. Results of meta-analyses have also shown vancomycin to be beneficial in patients with PSC[96]. Currently, there are clinical guidelines recommending the use of antimicrobial agents and FXR agonists for the treatment of PSC[125,126]. Clinical trials of UDCA for PSC are also well established[127]. UDCA is a hydrophilic dihydroxy BA, and pharmacological studies have confirmed that UDCA has a strong affinity in bile, promoting bile secretion, protecting bile duct cells from the cytotoxicity of hydrophobic BAs, and protecting hepatocytes from BA-induced apoptosis[128]. It promotes the formation of liquid cholesterol crystal complexes, accelerates cholesterol excretion and clearance to the intestine, acts as a cholagogue, and competitively inhibits endogenous hepatic BA absorption in the small intestine, reducing serum BA levels[129]. 24-norUDCA is a side chain shortened congener of C23UDCA, which makes a bile hepatic shunt possible. Based on its pharmacological properties of relative amidation resistance and reduced secondary BA production, norUDCA is a promising drug for a range of cholestatic liver and bile duct diseases[130]. Some clinical trials have shown that norUDCA improved cholestasis and significantly reduced serum alkaline phosphatase levels in patients after 12 wk in a dose-dependent manner. Importantly, norUDCA treatment has shown a good safety profile[131]. OCA is a potent FXR agonist that affects the hepatic transport of conjugated BAs in humans and reduces duration of hepatocyte exposure to potentially cytotoxic BAs[132,133]. Clinical trials have demonstrated the efficacy and safety of OCA in patients with PSC; Treatment with OCA 5-10 mg resulted in a significant reduction in ALP in patients with PSC after 24 wk[123]. In addition, clinical studies of probiotics, FMT, and other approaches targeting intestinal flora for the treatment of PSC are ongoing to highlight their efficacy and safety in PSC and demonstrate their therapeutic potential[108,134].
| Study Phase | Study title | ClinicalTrials.gov identifier | Actual enrollment | Status | Interventions |
| Phase 1 | A Pilot Study of Vancomycin or Metronidazole in Patients with Primary Sclerosing Cholangitis (PSC) | NCT01085760 | 35 participants | Completed | Drug: vancomycin; Drug: metronidazole |
| Minocycline in Primary Sclerosing Cholangitis (PSC) | NCT00630942 | 16 participants | Completed | Drug: minocycline | |
| Treating Primary Sclerosing Cholangitis and Biliary Atresia with Vancomycin | NCT02137668 | 200 participants | Recruiting | Drug: oral vancomycin | |
| A Pilot Study to Characterize Bile Acid Metabolism and Dysbiosis in Primary Sclerosing Cholangitis | NCT02464020 | 8 participants | Completed | Drug: vancomycin | |
| Gastrointestinal Microbiota in Primary Sclerosing Cholangitis and Biliary Atresia with Vancomycin (PSC) | NCT01322386 | 32 participants | Completed | Drug: vancomycin | |
| Fecal Microbiota Transplantation for the Treatment of Primary Sclerosing Cholangitis. | NCT02424175 | 10 participants | Completed | Biological: Fecal microbiota transplantation | |
| Phase 2 | Norursodeoxycholic Acid in the Treatment of Primary Sclerosing Cholangitis (NUC-3) | NCT01755507 | 159 participants | Completed | Drug: norursodeoxycholic acid; Drug: placebo |
| Obeticholic Acid (OCA) in Primary Sclerosing Cholangitis (PSC) (AESOP) | NCT02177136 | 77 participants | Completed | Drug: OCA; Drug: placebo | |
| Vancomycin for Primary Sclerosing Cholangitis | NCT03710122 | 102 participants | Recruiting | Drug: vancomycin; Other: placebo | |
| Trial of High-dose Urso in Primary Sclerosing Cholangitis | NCT00059202 | 150 participants | Completed | Drug: ursodeoxycholic acid; Drug: placebo | |
| Fecal Microbiota Transplantation for the Treatment of Primary Sclerosing Cholangitis. | NCT02424175 | 10 participants | Completed | Biological: fecal microbiota transplantation | |
| Phase 3 | Primary Sclerosing Cholangitis with Oral Vancomycin by the Study of Its Antimicrobial and Immunomodulating Effects (PSC) | NCT01802073 | 34 participants | Completed | Drug: oral vancomycin |
| Probiotics in Patients with Primary Sclerosing Cholangitis | NCT00161148 | 12 participants | Unknown1 | Drug: probiotics | |
| Norursodeoxycholic Acid vs Placebo in PSC | NCT03872921 | 300 participants | Recruiting | Drug: norursodeoxycholic acid | |
| Vancomycin for Primary Sclerosing Cholangitis | NCT03710122 | 102 participants | Recruiting | Drug: vancomycin; Other: placebo | |
| Trial of High-dose Urso in Primary Sclerosing Cholangitis | NCT00059202 | 150 participants | Completed | Drug: ursodeoxycholic acid; Drug: placebo | |
| Phase 4 | Effect and Safety of Oral Vancomycin in Primary Sclerosing Cholangitis Patients | NCT02605213 | 30 participants | Unknown1 | Drug: vancomycin; Drug: placebo |
PSC is a chronic progressive autoimmune disease that can develop into cirrhosis or liver failure, thereby severely affecting the patient’s quality of life if not actively and effectively treated. Intestinal flora dysbiosis is crucial in the occurrence and development of PSC, as it destroys the intestinal barrier and prompts intestinal lymphocyte homing and translocation of bacteria and their metabolites, thus aggravating the immune damage to the liver. The intestinal flora also interacts with BAs and participates in PSC development.
Our understanding of the gut flora has expanded with the development of genomics, metabolomics, and high-throughput sequencing technologies. These research approaches help elucidate the complex role of the gut flora in diseases, such as PSC. Technological advances have also provided individualized treatment options for patients with PSC that target the intestinal flora with good clinical results. Treatments, including antibiotics, FMT, and probiotics, have offered new ideas for managing PSC. More precise therapies, such as probiotics, synbiotics, and phages, have shown promising results in PSC patients. However, there remain some challenges in the use of intestinal flora for PSC treatment. The intestinal flora regulation mechanisms for PSC are not fully understood, and the optimal method and timing have not been standardized. Future prospective studies with a large sample size or multi-center studies are warranted to provide direct evidence of the role of the intestinal flora in PSC and establish a therapeutic protocol for the use of the intestinal flora. If these issues are resolved, targeted regulation of the intestinal flora will become a new option for PSC treatment.
We would like to thank Prof. Long-Fei Ren for providing critical revisions to the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cossiga V, Italy; Ker CG, Taiwan S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Dyson JK, Beuers U, Jones DEJ, Lohse AW, Hudson M. Primary sclerosing cholangitis. Lancet. 2018;391:2547-2559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 286] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 2. | Fung BM, Lindor KD, Tabibian JH. Cancer risk in primary sclerosing cholangitis: Epidemiology, prevention, and surveillance strategies. World J Gastroenterol. 2019;25:659-671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 88] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 3. | Rabiee A, Silveira MG. Primary sclerosing cholangitis. Transl Gastroenterol Hepatol. 2021;6:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Bruneau A, Hundertmark J, Guillot A, Tacke F. Molecular and Cellular Mediators of the Gut-Liver Axis in the Progression of Liver Diseases. Front Med (Lausanne). 2021;8:725390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH, Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology. 2020;72:671-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 571] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 6. | Cao RR, He P, Lei SF. Novel microbiota-related gene set enrichment analysis identified osteoporosis associated gut microbiota from autoimmune diseases. J Bone Miner Metab. 2021;39:984-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Iwasawa K, Suda W, Tsunoda T, Oikawa-Kawamoto M, Umetsu S, Inui A, Fujisawa T, Morita H, Sogo T, Hattori M. Characterisation of the faecal microbiota in Japanese patients with paediatric-onset primary sclerosing cholangitis. Gut. 2017;66:1344-1346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Torres J, Bao X, Goel A, Colombel JF, Pekow J, Jabri B, Williams KM, Castillo A, Odin JA, Meckel K, Fasihuddin F, Peter I, Itzkowitz S, Hu J. The features of mucosa-associated microbiota in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2016;43:790-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 119] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 9. | Tornai T, Palyu E, Vitalis Z, Tornai I, Tornai D, Antal-Szalmas P, Norman GL, Shums Z, Veres G, Dezsofi A, Par G, Par A, Orosz P, Szalay F, Lakatos PL, Papp M. Gut barrier failure biomarkers are associated with poor disease outcome in patients with primary sclerosing cholangitis. World J Gastroenterol. 2017;23:5412-5421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 10. | Mehta TI, Weissman S, Fung BM, Sotiriadis J, Lindor KD, Tabibian JH. Global incidence, prevalence and features of primary sclerosing cholangitis: A systematic review and meta-analysis. Liver Int. 2021;41:2418-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (38)] |
| 11. | Floreani A, De Martin S. Treatment of primary sclerosing cholangitis. Dig Liver Dis. 2021;53:1531-1538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Karlsen TH, Folseraas T, Thorburn D, Vesterhus M. Primary sclerosing cholangitis - a comprehensive review. J Hepatol. 2017;67:1298-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 567] [Article Influence: 70.9] [Reference Citation Analysis (35)] |
| 13. | Liu C, Wang YL, Yang YY, Zhang NP, Niu C, Shen XZ, Wu J. Novel approaches to intervene gut microbiota in the treatment of chronic liver diseases. FASEB J. 2021;35:e21871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Gallo C, Howardson BO, Cristoferi L, Carbone M, Gershwin ME, Invernizzi P. An update on novel pharmacological agents for primary sclerosing cholangitis. Expert Opin Ther Targets. 2022;26:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 15. | Nicoletti A, Maurice JB, Thorburn D. Guideline review: British Society of Gastroenterology/UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Frontline Gastroenterol. 2021;12:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Eaton JE, Talwalkar JA, Lazaridis KN, Gores GJ, Lindor KD. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology. 2013;145:521-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 294] [Article Influence: 24.5] [Reference Citation Analysis (36)] |
| 17. | Liu JZ, Hov JR, Folseraas T, Ellinghaus E, Rushbrook SM, Doncheva NT, Andreassen OA, Weersma RK, Weismüller TJ, Eksteen B, Invernizzi P, Hirschfield GM, Gotthardt DN, Pares A, Ellinghaus D, Shah T, Juran BD, Milkiewicz P, Rust C, Schramm C, Müller T, Srivastava B, Dalekos G, Nöthen MM, Herms S, Winkelmann J, Mitrovic M, Braun F, Ponsioen CY, Croucher PJ, Sterneck M, Teufel A, Mason AL, Saarela J, Leppa V, Dorfman R, Alvaro D, Floreani A, Onengut-Gumuscu S, Rich SS, Thompson WK, Schork AJ, Næss S, Thomsen I, Mayr G, König IR, Hveem K, Cleynen I, Gutierrez-Achury J, Ricaño-Ponce I, van Heel D, Björnsson E, Sandford RN, Durie PR, Melum E, Vatn MH, Silverberg MS, Duerr RH, Padyukov L, Brand S, Sans M, Annese V, Achkar JP, Boberg KM, Marschall HU, Chazouillères O, Bowlus CL, Wijmenga C, Schrumpf E, Vermeire S, Albrecht M; UK-PSCSC Consortium, Rioux JD, Alexander G, Bergquist A, Cho J, Schreiber S, Manns MP, Färkkilä M, Dale AM, Chapman RW, Lazaridis KN; International PSC Study Group, Franke A, Anderson CA, Karlsen TH; International IBD Genetics Consortium. Dense genotyping of immune-related disease regions identifies nine new risk loci for primary sclerosing cholangitis. Nat Genet. 2013;45:670-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 335] [Cited by in RCA: 301] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 18. | Park JW, Kim JH, Kim SE, Jung JH, Jang MK, Park SH, Lee MS, Kim HS, Suk KT, Kim DJ. Primary Biliary Cholangitis and Primary Sclerosing Cholangitis: Current Knowledge of Pathogenesis and Therapeutics. Biomedicines. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | de Vries AB, Janse M, Blokzijl H, Weersma RK. Distinctive inflammatory bowel disease phenotype in primary sclerosing cholangitis. World J Gastroenterol. 2015;21:1956-1971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 120] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 20. | Albillos A, de Gottardi A, Rescigno M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J Hepatol. 2020;72:558-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 542] [Cited by in RCA: 1254] [Article Influence: 250.8] [Reference Citation Analysis (1)] |
| 21. | Wang Y, Liu Y. Gut-liver-axis: Barrier function of liver sinusoidal endothelial cell. J Gastroenterol Hepatol. 2021;36:2706-2714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | Wang R, Tang R, Li B, Ma X, Schnabl B, Tilg H. Gut microbiome, liver immunology, and liver diseases. Cell Mol Immunol. 2021;18:4-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 260] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 23. | Bambha K, Kim WR, Talwalkar J, Torgerson H, Benson JT, Therneau TM, Loftus EV Jr, Yawn BP, Dickson ER, Melton LJ 3rd. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003;125:1364-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 301] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 24. | Weismüller TJ, Trivedi PJ, Bergquist A, Imam M, Lenzen H, Ponsioen CY, Holm K, Gotthardt D, Färkkilä MA, Marschall HU, Thorburn D, Weersma RK, Fevery J, Mueller T, Chazouillères O, Schulze K, Lazaridis KN, Almer S, Pereira SP, Levy C, Mason A, Naess S, Bowlus CL, Floreani A, Halilbasic E, Yimam KK, Milkiewicz P, Beuers U, Huynh DK, Pares A, Manser CN, Dalekos GN, Eksteen B, Invernizzi P, Berg CP, Kirchner GI, Sarrazin C, Zimmer V, Fabris L, Braun F, Marzioni M, Juran BD, Said K, Rupp C, Jokelainen K, Benito de Valle M, Saffioti F, Cheung A, Trauner M, Schramm C, Chapman RW, Karlsen TH, Schrumpf E, Strassburg CP, Manns MP, Lindor KD, Hirschfield GM, Hansen BE, Boberg KM; International PSC Study Group. Patient Age, Sex, and Inflammatory Bowel Disease Phenotype Associate With Course of Primary Sclerosing Cholangitis. Gastroenterology. 2017;152:1975-1984.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 362] [Article Influence: 45.3] [Reference Citation Analysis (1)] |
| 25. | Liang H, Manne S, Shick J, Lissoos T, Dolin P. Incidence, prevalence, and natural history of primary sclerosing cholangitis in the United Kingdom. Medicine (Baltimore). 2017;96:e7116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Palmela C, Peerani F, Castaneda D, Torres J, Itzkowitz SH. Inflammatory Bowel Disease and Primary Sclerosing Cholangitis: A Review of the Phenotype and Associated Specific Features. Gut Liver. 2018;12:17-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 27. | Da Cunha T, Vaziri H, Wu GY. Primary Sclerosing Cholangitis and Inflammatory Bowel Disease: A Review. J Clin Transl Hepatol. 2022;10:531-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Lazaridis KN, LaRusso NF. The Cholangiopathies. Mayo Clin Proc. 2015;90:791-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (37)] |
| 29. | Yu D, Meng X, de Vos WM, Wu H, Fang X, Maiti AK. Implications of Gut Microbiota in Complex Human Diseases. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 30. | Weinstock GM. Genomic approaches to studying the human microbiota. Nature. 2012;489:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 359] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 31. | Kho ZY, Lal SK. The Human Gut Microbiome - A Potential Controller of Wellness and Disease. Front Microbiol. 2018;9:1835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 451] [Cited by in RCA: 646] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 32. | Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, Zhu D, Koya JB, Wei L, Li J, Chen ZS. Microbiota in health and diseases. Signal Transduct Target Ther. 2022;7:135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 1323] [Article Influence: 441.0] [Reference Citation Analysis (0)] |
| 33. | Tranah TH, Edwards LA, Schnabl B, Shawcross DL. Targeting the gut-liver-immune axis to treat cirrhosis. Gut. 2021;70:982-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 116] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 34. | Rossen NG, Fuentes S, Boonstra K, D'Haens GR, Heilig HG, Zoetendal EG, de Vos WM, Ponsioen CY. The mucosa-associated microbiota of PSC patients is characterized by low diversity and low abundance of uncultured Clostridiales II. J Crohns Colitis. 2015;9:342-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 35. | Sabino J, Vieira-Silva S, Machiels K, Joossens M, Falony G, Ballet V, Ferrante M, Van Assche G, Van der Merwe S, Vermeire S, Raes J. Primary sclerosing cholangitis is characterised by intestinal dysbiosis independent from IBD. Gut. 2016;65:1681-1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 312] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 36. | Kummen M, Holm K, Anmarkrud JA, Nygård S, Vesterhus M, Høivik ML, Trøseid M, Marschall HU, Schrumpf E, Moum B, Røsjø H, Aukrust P, Karlsen TH, Hov JR. The gut microbial profile in patients with primary sclerosing cholangitis is distinct from patients with ulcerative colitis without biliary disease and healthy controls. Gut. 2017;66:611-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 309] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 37. | Quraishi MN, Sergeant M, Kay G, Iqbal T, Chan J, Constantinidou C, Trivedi P, Ferguson J, Adams DH, Pallen M, Hirschfield GM. The gut-adherent microbiota of PSC-IBD is distinct to that of IBD. Gut. 2017;66:386-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 133] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 38. | Rühlemann M, Liwinski T, Heinsen FA, Bang C, Zenouzi R, Kummen M, Thingholm L, Tempel M, Lieb W, Karlsen T, Lohse A, Hov J, Denk G, Lammert F, Krawczyk M, Schramm C, Franke A. Consistent alterations in faecal microbiomes of patients with primary sclerosing cholangitis independent of associated colitis. Aliment Pharmacol Ther. 2019;50:580-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 39. | Rühlemann MC, Heinsen FA, Zenouzi R, Lieb W, Franke A, Schramm C. Faecal microbiota profiles as diagnostic biomarkers in primary sclerosing cholangitis. Gut. 2017;66:753-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Quraishi MN, Acharjee A, Beggs AD, Horniblow R, Tselepis C, Gkoutos G, Ghosh S, Rossiter AE, Loman N, van Schaik W, Withers D, Walters JRF, Hirschfield GM, Iqbal TH. A Pilot Integrative Analysis of Colonic Gene Expression, Gut Microbiota, and Immune Infiltration in Primary Sclerosing Cholangitis-Inflammatory Bowel Disease: Association of Disease With Bile Acid Pathways. J Crohns Colitis. 2020;14:935-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 41. | Trivedi PJ, Tickle J, Vesterhus MN, Eddowes PJ, Bruns T, Vainio J, Parker R, Smith D, Liaskou E, Thorbjørnsen LW, Hirschfield GM, Auvinen K, Hubscher SG, Salmi M, Adams DH, Weston CJ. Vascular adhesion protein-1 is elevated in primary sclerosing cholangitis, is predictive of clinical outcome and facilitates recruitment of gut-tropic lymphocytes to liver in a substrate-dependent manner. Gut. 2018;67:1135-1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 42. | Liwinski T, Zenouzi R, John C, Ehlken H, Rühlemann MC, Bang C, Groth S, Lieb W, Kantowski M, Andersen N, Schachschal G, Karlsen TH, Hov JR, Rösch T, Lohse AW, Heeren J, Franke A, Schramm C. Alterations of the bile microbiome in primary sclerosing cholangitis. Gut. 2020;69:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 104] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 43. | Lapidot Y, Amir A, Ben-Simon S, Veitsman E, Cohen-Ezra O, Davidov Y, Weiss P, Bradichevski T, Segev S, Koren O, Ben-Ari Z, Safran M. Alterations of the salivary and fecal microbiome in patients with primary sclerosing cholangitis. Hepatol Int. 2021;15:191-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 44. | Lemoinne S, Kemgang A, Ben Belkacem K, Straube M, Jegou S, Corpechot C; Saint-Antoine IBD Network, Chazouillères O, Housset C, Sokol H. Fungi participate in the dysbiosis of gut microbiota in patients with primary sclerosing cholangitis. Gut. 2020;69:92-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 45. | Kummen M, Thingholm LB, Rühlemann MC, Holm K, Hansen SH, Moitinho-Silva L, Liwinski T, Zenouzi R, Storm-Larsen C, Midttun Ø, McCann A, Ueland PM, Høivik ML, Vesterhus M, Trøseid M, Laudes M, Lieb W, Karlsen TH, Bang C, Schramm C, Franke A, Hov JR. Altered Gut Microbial Metabolism of Essential Nutrients in Primary Sclerosing Cholangitis. Gastroenterology. 2021;160:1784-1798.e0. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 46. | Kummen M, Hov JR. The gut microbial influence on cholestatic liver disease. Liver Int. 2019;39:1186-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 47. | Bajer L, Kverka M, Kostovcik M, Macinga P, Dvorak J, Stehlikova Z, Brezina J, Wohl P, Spicak J, Drastich P. Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J Gastroenterol. 2017;23:4548-4558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 215] [Cited by in RCA: 262] [Article Influence: 32.8] [Reference Citation Analysis (4)] |
| 48. | Wei Y, Li Y, Yan L, Sun C, Miao Q, Wang Q, Xiao X, Lian M, Li B, Chen Y, Zhang J, Huang B, Cao Q, Fan Z, Chen X, Fang JY, Gershwin ME, Tang R, Ma X. Alterations of gut microbiome in autoimmune hepatitis. Gut. 2020;69:569-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 217] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 49. | Lang S, Fairfied B, Gao B, Duan Y, Zhang X, Fouts DE, Schnabl B. Changes in the fecal bacterial microbiota associated with disease severity in alcoholic hepatitis patients. Gut Microbes. 2020;12:1785251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 50. | Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1:179-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 294] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 51. | Milosevic I, Vujovic A, Barac A, Djelic M, Korac M, Radovanovic Spurnic A, Gmizic I, Stevanovic O, Djordjevic V, Lekic N, Russo E, Amedei A. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 355] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 52. | Li M, Ping J, Xu LM. [Application of Mdr2 gene knockout mice in liver disease research]. Zhonghua Gan Zang Bing Za Zhi. 2021;29:585-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Zheng Y, Ran Y, Zhang H, Wang B, Zhou L. The Microbiome in Autoimmune Liver Diseases: Metagenomic and Metabolomic Changes. Front Physiol. 2021;12:715852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 54. | Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15:397-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 872] [Cited by in RCA: 970] [Article Influence: 138.6] [Reference Citation Analysis (0)] |
| 55. | Bozward AG, Ronca V, Osei-Bordom D, Oo YH. Gut-Liver Immune Traffic: Deciphering Immune-Pathogenesis to Underpin Translational Therapy. Front Immunol. 2021;12:711217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 56. | Maroni L, Ninfole E, Pinto C, Benedetti A, Marzioni M. Gut-Liver Axis and Inflammasome Activation in Cholangiocyte Pathophysiology. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 57. | Nakamoto N, Sasaki N, Aoki R, Miyamoto K, Suda W, Teratani T, Suzuki T, Koda Y, Chu PS, Taniki N, Yamaguchi A, Kanamori M, Kamada N, Hattori M, Ashida H, Sakamoto M, Atarashi K, Narushima S, Yoshimura A, Honda K, Sato T, Kanai T. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. Nat Microbiol. 2019;4:492-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 306] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 58. | Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, Costa FRC, Tiniakou E, Greiling T, Ruff W, Barbieri A, Kriegel C, Mehta SS, Knight JR, Jain D, Goodman AL, Kriegel MA. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359:1156-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 596] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 59. | Visseren T, Fuhler GM, Erler NS, Nossent YRA, Metselaar HJ, IJzermans JNM, Darwish Murad S, Peppelenbosch MP. Recurrence of primary sclerosing cholangitis after liver transplantation is associated with specific changes in the gut microbiome pretransplant - a pilot study. Transpl Int. 2020;33:1424-1436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 60. | Lichtman SN, Keku J, Schwab JH, Sartor RB. Hepatic injury associated with small bowel bacterial overgrowth in rats is prevented by metronidazole and tetracycline. Gastroenterology. 1991;100:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 114] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 61. | Tedesco D, Thapa M, Chin CY, Ge Y, Gong M, Li J, Gumber S, Speck P, Elrod EJ, Burd EM, Kitchens WH, Magliocca JF, Adams AB, Weiss DS, Mohamadzadeh M, Grakoui A. Alterations in Intestinal Microbiota Lead to Production of Interleukin 17 by Intrahepatic γδ T-Cell Receptor-Positive Cells and Pathogenesis of Cholestatic Liver Disease. Gastroenterology. 2018;154:2178-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 62. | Liao L, Schneider KM, Galvez EJC, Frissen M, Marschall HU, Su H, Hatting M, Wahlström A, Haybaeck J, Puchas P, Mohs A, Peng J, Bergheim I, Nier A, Hennings J, Reißing J, Zimmermann HW, Longerich T, Strowig T, Liedtke C, Cubero FJ, Trautwein C. Intestinal dysbiosis augments liver disease progression via NLRP3 in a murine model of primary sclerosing cholangitis. Gut. 2019;68:1477-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 63. | Dhillon AK, Kummen M, Trøseid M, Åkra S, Liaskou E, Moum B, Vesterhus M, Karlsen TH, Seljeflot I, Hov JR. Circulating markers of gut barrier function associated with disease severity in primary sclerosing cholangitis. Liver Int. 2019;39:371-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 64. | Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2839] [Cited by in RCA: 2907] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 65. | Doherty DG. Immunity, tolerance and autoimmunity in the liver: A comprehensive review. J Autoimmun. 2016;66:60-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 229] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 66. | Yamamoto M, Takeda K. Current views of toll-like receptor signaling pathways. Gastroenterol Res Pract. 2010;2010:240365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 67. | Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol. 2012;590:447-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 339] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 68. | Anand G, Zarrinpar A, Loomba R. Targeting Dysbiosis for the Treatment of Liver Disease. Semin Liver Dis. 2016;36:37-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 69. | de Krijger M, Visseren T, Wildenberg ME, Hooijer GKJ, Verstegen MMA, van der Laan LJW, de Jonge WJ, Verheij J, Ponsioen CY. Characterization of gut-homing molecules in non-endstage livers of patients with primary sclerosing cholangitis and inflammatory bowel disease. J Transl Autoimmun. 2020;3:100054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | de Krijger M, Wildenberg ME, de Jonge WJ, Ponsioen CY. Return to sender: Lymphocyte trafficking mechanisms as contributors to primary sclerosing cholangitis. J Hepatol. 2019;71:603-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 71. | Trivedi PJ, Bruns T, Ward S, Mai M, Schmidt C, Hirschfield GM, Weston CJ, Adams DH. Intestinal CCL25 expression is increased in colitis and correlates with inflammatory activity. J Autoimmun. 2016;68:98-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 72. | Schippers A, Hübel J, Heymann F, Clahsen T, Eswaran S, Schlepütz S, Püllen R, Gaßler N, Tenbrock K, Tacke F, Wagner N. MAdCAM-1/α4β7 Integrin-Mediated Lymphocyte/Endothelium Interactions Exacerbate Acute Immune-Mediated Hepatitis in Mice. Cell Mol Gastroenterol Hepatol. 2021;11:1227-1250.e1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | UniProt Consortium T. UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2018;46:2699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1090] [Cited by in RCA: 1254] [Article Influence: 179.1] [Reference Citation Analysis (0)] |
| 74. | Moro-Sibilot L, Blanc P, Taillardet M, Bardel E, Couillault C, Boschetti G, Traverse-Glehen A, Defrance T, Kaiserlian D, Dubois B. Mouse and Human Liver Contain Immunoglobulin A-Secreting Cells Originating From Peyer's Patches and Directed Against Intestinal Antigens. Gastroenterology. 2016;151:311-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 75. | Tornai D, Ven PL, Lakatos PL, Papp M. Serological biomarkers for management of primary sclerosing cholangitis. World J Gastroenterol. 2022;28:2291-2301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 76. | Weston CJ, Shepherd EL, Claridge LC, Rantakari P, Curbishley SM, Tomlinson JW, Hubscher SG, Reynolds GM, Aalto K, Anstee QM, Jalkanen S, Salmi M, Smith DJ, Day CP, Adams DH. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic fibrosis. J Clin Invest. 2015;125:501-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 77. | Horáčková Š, Plocková M, Demnerová K. Importance of microbial defence systems to bile salts and mechanisms of serum cholesterol reduction. Biotechnol Adv. 2018;36:682-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 78. | Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016;24:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1317] [Cited by in RCA: 1844] [Article Influence: 204.9] [Reference Citation Analysis (0)] |
| 79. | Fickert P, Wagner M. Biliary bile acids in hepatobiliary injury - What is the link? J Hepatol. 2017;67:619-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 80. | Schneider KM, Candels LS, Hov JR, Myllys M, Hassan R, Schneider CV, Wahlström A, Mohs A, Zühlke S, Liao L, Elfers C, Kilic K, Henricsson M, Molinaro A, Hatting M, Zaza A, Drasdo D, Frissen M, Devlin AS, Gálvez EJC, Strowig T, Karlsen TH, Hengstler JG, Marschall HU, Ghallab A, Trautwein C. Gut microbiota depletion exacerbates cholestatic liver injury via loss of FXR signalling. Nat Metab. 2021;3:1228-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 81. | Chand D, Panigrahi P, Varshney N, Ramasamy S, Suresh CG. Structure and function of a highly active Bile Salt Hydrolase (BSH) from Enterococcus faecalis and post-translational processing of BSH enzymes. Biochim Biophys Acta Proteins Proteom. 2018;1866:507-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 82. | Tabibian JH, O'Hara SP, Trussoni CE, Tietz PS, Splinter PL, Mounajjed T, Hagey LR, LaRusso NF. Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis. Hepatology. 2016;63:185-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 165] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 83. | Awoniyi M, Wang J, Ngo B, Meadows V, Tam J, Viswanathan A, Lai Y, Montgomery S, Farmer M, Kummen M, Thingholm L, Schramm C, Bang C, Franke A, Lu K, Zhou H, Bajaj JS, Hylemon PB, Ting J, Popov YV, Hov JR, Francis HL, Sartor RB. Protective and aggressive bacterial subsets and metabolites modify hepatobiliary inflammation and fibrosis in a murine model of PSC. Gut. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 63] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 84. | Jiang B, Yuan G, Wu J, Wu Q, Li L, Jiang P. Prevotella copri ameliorates cholestasis and liver fibrosis in primary sclerosing cholangitis by enhancing the FXR signalling pathway. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 85. | Poupon R, Poupon RE. Ursodeoxycholic acid therapy of chronic cholestatic conditions in adults and children. Pharmacol Ther. 1995;66:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 86. | Lee JY, Arai H, Nakamura Y, Fukiya S, Wada M, Yokota A. Contribution of the 7β-hydroxysteroid dehydrogenase from Ruminococcus gnavus N53 to ursodeoxycholic acid formation in the human colon. J Lipid Res. 2013;54:3062-3069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 87. | Shah A, Macdonald GA, Morrison M, Holtmann G. Targeting the Gut Microbiome as a Treatment for Primary Sclerosing Cholangitis: A Conceptional Framework. Am J Gastroenterol. 2020;115:814-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 88. | Kanmani P, Suganya K, Kim H. The Gut Microbiota: How Does It Influence the Development and Progression of Liver Diseases. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 89. | Assis DN, Levy C. Oral Vancomycin or Ursodeoxycholic Acid for Pediatric Primary Sclerosing Cholangitis? Hepatology. 2021;73:887-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 90. | Tabibian JH, Weeding E, Jorgensen RA, Petz JL, Keach JC, Talwalkar JA, Lindor KD. Randomised clinical trial: vancomycin or metronidazole in patients with primary sclerosing cholangitis - a pilot study. Aliment Pharmacol Ther. 2013;37:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 199] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 91. | Rahimpour S, Nasiri-Toosi M, Khalili H, Ebrahimi-Daryani N, Nouri-Taromlou MK, Azizi Z. A Triple Blinded, Randomized, Placebo-Controlled Clinical Trial to Evaluate the Efficacy and Safety of Oral Vancomycin in Primary Sclerosing Cholangitis: a Pilot Study. J Gastrointestin Liver Dis. 2016;25:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 92. | Ali AH, Damman J, Shah SB, Davies Y, Hurwitz M, Stephen M, Lemos LM, Carey EJ, Lindor KD, Buness CW, Alrabadi L, Berquist WE, Cox KL. Open-label prospective therapeutic clinical trials: oral vancomycin in children and adults with primary sclerosing cholangitis. Scand J Gastroenterol. 2020;55:941-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 93. | Abarbanel DN, Seki SM, Davies Y, Marlen N, Benavides JA, Cox K, Nadeau KC, Cox KL. Immunomodulatory effect of vancomycin on Treg in pediatric inflammatory bowel disease and primary sclerosing cholangitis. J Clin Immunol. 2013;33:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 94. | Britto SL, Hoffman KL, Tessier ME, Petrosino J, Miloh T, Kellermayer R. Microbiome Responses to Vancomycin Treatment in a Child With Primary Sclerosing Cholangitis and Ulcerative Colitis. ACG Case Rep J. 2021;8:e00577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 95. | Tabibian JH, Gossard A, El-Youssef M, Eaton JE, Petz J, Jorgensen R, Enders FB, Tabibian A, Lindor KD. Prospective Clinical Trial of Rifaximin Therapy for Patients With Primary Sclerosing Cholangitis. Am J Ther. 2017;24:e56-e63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 96. | Shah A, Crawford D, Burger D, Martin N, Walker M, Talley NJ, Tallis C, Jones M, Stuart K, Keely S, Lewindon P, Macdonald GA, Morrison M, Holtmann GJ. Effects of Antibiotic Therapy in Primary Sclerosing Cholangitis with and without Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. Semin Liver Dis. 2019;39:432-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 97. | Davies YK, Tsay CJ, Caccamo DV, Cox KM, Castillo RO, Cox KL. Successful treatment of recurrent primary sclerosing cholangitis after orthotopic liver transplantation with oral vancomycin. Case Rep Transplant. 2013;2013:314292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 98. | Färkkilä M, Karvonen AL, Nurmi H, Nuutinen H, Taavitsainen M, Pikkarainen P, Kärkkäinen P. Metronidazole and ursodeoxycholic acid for primary sclerosing cholangitis: a randomized placebo-controlled trial. Hepatology. 2004;40:1379-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 99. | Krehmeier U, Bardenheuer M, Voggenreiter G, Obertacke U, Schade FU, Majetschak M. Effects of antimicrobial agents on spontaneous and endotoxin-induced cytokine release of human peripheral blood mononuclear cells. J Infect Chemother. 2002;8:194-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 100. | Silveira MG, Torok NJ, Gossard AA, Keach JC, Jorgensen RA, Petz JL, Lindor KD. Minocycline in the treatment of patients with primary sclerosing cholangitis: results of a pilot study. Am J Gastroenterol. 2009;104:83-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 101. | Bakker GJ, Nieuwdorp M. Fecal Microbiota Transplantation: Therapeutic Potential for a Multitude of Diseases beyond Clostridium difficile. Microbiol Spectr. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 102. | Zhang F, Cui B, He X, Nie Y, Wu K, Fan D; FMT-standardization Study Group. Microbiota transplantation: concept, methodology and strategy for its modernization. Protein Cell. 2018;9:462-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 229] [Cited by in RCA: 206] [Article Influence: 29.4] [Reference Citation Analysis (1)] |
| 103. | Xu HM, Huang HL, Zhou YL, Zhao HL, Xu J, Shou DW, Liu YD, Zhou YJ, Nie YQ. Fecal Microbiota Transplantation: A New Therapeutic Attempt from the Gut to the Brain. Gastroenterol Res Pract. 2021;2021:6699268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 104. | Gupta M, Krishan P, Kaur A, Arora S, Trehanpati N, Singh TG, Bedi O. Mechanistic and physiological approaches of fecal microbiota transplantation in the management of NAFLD. Inflamm Res. 2021;70:765-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 105. | Craven L, Rahman A, Nair Parvathy S, Beaton M, Silverman J, Qumosani K, Hramiak I, Hegele R, Joy T, Meddings J, Urquhart B, Harvie R, McKenzie C, Summers K, Reid G, Burton JP, Silverman M. Allogenic Fecal Microbiota Transplantation in Patients With Nonalcoholic Fatty Liver Disease Improves Abnormal Small Intestinal Permeability: A Randomized Control Trial. Am J Gastroenterol. 2020;115:1055-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 227] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 106. | Gu X, Lu Q, Zhang C, Tang Z, Chu L. Clinical Application and Progress of Fecal Microbiota Transplantation in Liver Diseases: A Review. Semin Liver Dis. 2021;41:495-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 107. | Allegretti JR, Kassam Z, Carrellas M, Mullish BH, Marchesi JR, Pechlivanis A, Smith M, Gerardin Y, Timberlake S, Pratt DS, Korzenik JR. Fecal Microbiota Transplantation in Patients With Primary Sclerosing Cholangitis: A Pilot Clinical Trial. Am J Gastroenterol. 2019;114:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 169] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 108. | Philips CA, Augustine P, Phadke N. Healthy Donor Fecal Microbiota Transplantation for Recurrent Bacterial Cholangitis in Primary Sclerosing Cholangitis - A Single Case Report. J Clin Transl Hepatol. 2018;6:438-441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 109. | Wieërs G, Belkhir L, Enaud R, Leclercq S, Philippart de Foy JM, Dequenne I, de Timary P, Cani PD. How Probiotics Affect the Microbiota. Front Cell Infect Microbiol. 2019;9:454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 312] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 110. | Maslennikov R, Ivashkin V, Efremova I, Poluektova E, Shirokova E. Probiotics in hepatology: An update. World J Hepatol. 2021;13:1154-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 111. | Gou HZ, Zhang YL, Ren LF, Li ZJ, Zhang L. How do intestinal probiotics restore the intestinal barrier? Front Microbiol. 2022;13:929346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 113] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 112. | Rodríguez-Pastén A, Pérez-Hernández N, Añorve-Morga J, Jiménez-Alvarado R, Cariño-Cortés R, Sosa-Lozada T, Fernández-Martínez E. The Activity of Prebiotics and Probiotics in Hepatogastrointestinal Disorders and Diseases Associated with Metabolic Syndrome. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 113. | Vleggaar FP, Monkelbaan JF, van Erpecum KJ. Probiotics in primary sclerosing cholangitis: a randomized placebo-controlled crossover pilot study. Eur J Gastroenterol Hepatol. 2008;20:688-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 114. | Shimizu M, Iwasaki H, Mase S, Yachie A. Successful treatment of primary sclerosing cholangitis with a steroid and a probiotic. Case Rep Gastroenterol. 2012;6:249-253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 115. | Chen Y, Guan W, Zhang N, Wang Y, Tian Y, Sun H, Li X, Liu J. Lactobacillus plantarum Lp2 improved LPS-induced liver injury through the TLR-4/MAPK/NFκB and Nrf2-HO-1/CYP2E1 pathways in mice. Food Nutr Res. 2022;66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 116. | Out C, Patankar JV, Doktorova M, Boesjes M, Bos T, de Boer S, Havinga R, Wolters H, Boverhof R, van Dijk TH, Smoczek A, Bleich A, Sachdev V, Kratky D, Kuipers F, Verkade HJ, Groen AK. Gut microbiota inhibit Asbt-dependent intestinal bile acid reabsorption via Gata4. J Hepatol. 2015;63:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 117. | Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, Cai J, Qi Y, Fang ZZ, Takahashi S, Tanaka N, Desai D, Amin SG, Albert I, Patterson AD, Gonzalez FJ. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 539] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 118. | Wahlström A, Kovatcheva-Datchary P, Ståhlman M, Khan MT, Bäckhed F, Marschall HU. Induction of farnesoid X receptor signaling in germ-free mice colonized with a human microbiota. J Lipid Res. 2017;58:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 119. | Tanaka N, Aoyama T, Kimura S, Gonzalez FJ. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol Ther. 2017;179:142-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 120. | Adorini L, Pruzanski M, Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov Today. 2012;17:988-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 121. | Hirschfield GM, Mason A, Luketic V, Lindor K, Gordon SC, Mayo M, Kowdley KV, Vincent C, Bodhenheimer HC Jr, Parés A, Trauner M, Marschall HU, Adorini L, Sciacca C, Beecher-Jones T, Castelloe E, Böhm O, Shapiro D. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751-61.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 430] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 122. | Nevens F, Andreone P, Mazzella G, Strasser SI, Bowlus C, Invernizzi P, Drenth JP, Pockros PJ, Regula J, Beuers U, Trauner M, Jones DE, Floreani A, Hohenester S, Luketic V, Shiffman M, van Erpecum KJ, Vargas V, Vincent C, Hirschfield GM, Shah H, Hansen B, Lindor KD, Marschall HU, Kowdley KV, Hooshmand-Rad R, Marmon T, Sheeron S, Pencek R, MacConell L, Pruzanski M, Shapiro D; POISE Study Group. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016;375:631-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 873] [Cited by in RCA: 799] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 123. | Kowdley KV, Vuppalanchi R, Levy C, Floreani A, Andreone P, LaRusso NF, Shrestha R, Trotter J, Goldberg D, Rushbrook S, Hirschfield GM, Schiano T, Jin Y, Pencek R, MacConell L, Shapiro D, Bowlus CL; AESOP Study Investigators. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J Hepatol. 2020;73:94-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 124. | de Chambrun GP, Nachury M, Funakoshi N, Gerard R, Bismuth M, Valats JC, Panaro F, Navarro F, Desreumaux P, Pariente B, Blanc P. Oral vancomycin induces sustained deep remission in adult patients with ulcerative colitis and primary sclerosing cholangitis. Eur J Gastroenterol Hepatol. 2018;30:1247-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 125. | Chinese Society of Hepatology; Chinese Medical Association. [Guidelines on the diagnosis and management of primary sclerosing cholangitis (2021)]. Zhonghua Gan Zang Bing Za Zhi. 2022;30:169-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 126. | Lindor KD, Kowdley KV, Harrison ME; American College of Gastroenterology. ACG Clinical Guideline: Primary Sclerosing Cholangitis. Am J Gastroenterol. 2015;110:646-59; quiz 660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 337] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 127. | Lindor KD, Kowdley KV, Luketic VA, Harrison ME, McCashland T, Befeler AS, Harnois D, Jorgensen R, Petz J, Keach J, Mooney J, Sargeant C, Braaten J, Bernard T, King D, Miceli E, Schmoll J, Hoskin T, Thapa P, Enders F. High-dose ursodeoxycholic acid for the treatment of primary sclerosing cholangitis. Hepatology. 2009;50:808-814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 572] [Cited by in RCA: 490] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 128. | Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 475] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 129. | Shah RA, Kowdley KV. Current and potential treatments for primary biliary cholangitis. Lancet Gastroenterol Hepatol. 2020;5:306-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 130. | Halilbasic E, Steinacher D, Trauner M. Nor-Ursodeoxycholic Acid as a Novel Therapeutic Approach for Cholestatic and Metabolic Liver Diseases. Dig Dis. 2017;35:288-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 131. | Fickert P, Hirschfield GM, Denk G, Marschall HU, Altorjay I, Färkkilä M, Schramm C, Spengler U, Chapman R, Bergquist A, Schrumpf E, Nevens F, Trivedi P, Reiter FP, Tornai I, Halilbasic E, Greinwald R, Pröls M, Manns MP, Trauner M; European PSC norUDCA Study Group. norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis. J Hepatol. 2017;67:549-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 201] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 132. | Kjærgaard K, Frisch K, Sørensen M, Munk OL, Hofmann AF, Horsager J, Schacht AC, Erickson M, Shapiro D, Keiding S. Obeticholic acid improves hepatic bile acid excretion in patients with primary biliary cholangitis. J Hepatol. 2021;74:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 133. | Gulamhusein AF, Hirschfield GM. Primary biliary cholangitis: pathogenesis and therapeutic opportunities. Nat Rev Gastroenterol Hepatol. 2020;17:93-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 186] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 134. | Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 454] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 135. | Denoth L, Juillerat P, Kremer AE, Rogler G, Scharl M, Yilmaz B, Bluemel S, On Behalf Of The Swiss Ibd Cohort Study. Modulation of the Mucosa-Associated Microbiome Linked to the PTPN2 Risk Gene in Patients with Primary Sclerosing Cholangitis and Ulcerative Colitis. Microorganisms. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 136. | Ostadmohammadi S, Azimirad M, Houri H, Naseri K, Javanmard E, Mirjalali H, Yadegar A, Sadeghi A, Asadzadeh Aghdaei H, Zali MR. Characterization of the gut microbiota in patients with primary sclerosing cholangitis compared to inflammatory bowel disease and healthy controls. Mol Biol Rep. 2021;48:5519-5529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 137. | Liu Q, Li B, Li Y, Wei Y, Huang B, Liang J, You Z, Qian Q, Wang R, Zhang J, Chen R, Lyu Z, Chen Y, Shi M, Xiao X, Wang Q, Miao Q, Fang JY, Gershwin ME, Lian M, Ma X, Tang R. Altered faecal microbiome and metabolome in IgG4-related sclerosing cholangitis and primary sclerosing cholangitis. Gut. 2022;71:899-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 138. | Davies YK, Cox KM, Abdullah BA, Safta A, Terry AB, Cox KL. Long-term treatment of primary sclerosing cholangitis in children with oral vancomycin: an immunomodulating antibiotic. J Pediatr Gastroenterol Nutr. 2008;47:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 139. | Boner AL, Peroni D, Bodini A, Delaini G, Piacentini G. Azithromycin may reduce cholestasis in primary sclerosing cholangitis: a case report and serendipitous observation. Int J Immunopathol Pharmacol. 2007;20:847-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |