INTRODUCTION
Acute pancreatitis (AP) is a common digestive disease that causes acute abdominal pain. A meta-analysis showed that the annual morbidity and mortality of AP all over the world were 33.74/100000 and 1.60/100000, respectively[1]. According to statistics, AP was the second leading cause of the total hospitalization rate, the largest contributor to the total cost, and the fifth leading cause of in-hospital mortality[2]. Many reports point out that the disease is increasing year by year, which is related to the incidence of biliary disease and the increase of alcoholism[3]. The clinical manifestations of AP vary greatly. Most of the patients who can recover after general treatment are considered to have mild AP, which is called a “self-limited disease”; some patients with transient organ failure (duration < 48 h) are considered to have moderately severe AP[4], with an approximately 20% developing severe AP (SAP). SAP has an aggressive onset with organ failure (duration > 48 h) and presence or absence of pancreatic or peripancreatic tissue necrosis, with a mortality rate of 20%-40%[5].
Acute respiratory distress syndrome (ARDS) is a life-threatening form of respiratory failure. Its main clinical manifestations are shortness of breath and refractory hypoxemia. ARDS is caused by ischemia and hypoxia injury secondary to pulmonary pathological changes under the influence of a variety of intrapulmonary and extrapulmonary factors, resulting in impaired lung function and serious condition of the life of patients[6]. The PaO2/FiO2 ratio (oxygenation index) is a component of the assessment of patients with ARDS[7], and refers to the ratio of the partial pressure of arterial oxygen to the concentration of oxygen in the inhaled air, with a normal value of 400-500 mmHg. The Berlin definition of ARDS is the most widely accepted criterion, which defines the specific time from clinical injury to the onset of new respiratory symptoms, specific chest X-ray findings, and the severity of ARDS based on the PaO2/FiO2 ratio. ARDS is defined as positive end-expiratory pressure ≥ 5 cm H2O, the ratio of atrial oxygen partial pressure to inhaled oxygen fraction ≤ 300 mmHg, and bilateral pulmonary infiltrative lesions that cannot be explained completely by fluid overload or heart failure. The Berlin definition also emphasizes that ARDS can be divided into three categories based on the severity of hypoxemia[8]: Mild (PaO2/FiO2 200-300), moderate (PaO2/FiO2 100-200), and severe (PaO2/FiO2 ≤ 100).
SAP is a clinical critical disease with rapid progression, many complications, and high mortality. In addition to causing local disorders, it can also cause damage to other organs, and its serious complications are the main factors leading to poor prognosis[5]. Previous studies have confirmed that the lung is the first damaged target organ in the early induction of systemic inflammatory response syndrome (SIRS) or multiple organ dysfunction syndrome by SAP, and respiratory failure is the most common organ failure in SAP[9-11]. ARDS is considered to be an important type of respiratory failure with high mortality[12]. It has been reported that 4%-15% of patients with AP have concomitant ARDS[13] , while this percentage may be up to a third in SAP[14]. ARDS is the most dangerous complication of AP, which usually occurs between 2 and 7 d after the onset of pancreatic inflammation. According to the literature data, SAP-related ARDS accounted for 60% of all deaths in SAP patients in the first week of the disease[15]. These data indicate that the more serious the condition of SAP, the worse the progression of the AP-related ARDS, which may further suggest that the severity of SAP is negatively correlated with PaO2/FiO2. If the occurrence of ARDS is not predicted early, the patient’s lung function will drop sharply, which may even lead to death during the acute reaction period. Thus, there is an urgent need for a simple and accurate new clinical prediction model combined with chest computed tomography (CT) findings of AP patients to diagnose and predict SAP-related ARDS at an early stage. And timely diagnosis and treatment can greatly improve the survival rate of SAP patients.
New prediction model compared with clinical scoring systems
In the recent issue of the World Journal of Gastroenterology, Li et al[16] published an interesting paper entitled “Development and external validation of models to predict acute respiratory distress syndrome related to severe acute pancreatitis”. This study constructed and validated a new simple and accurate prediction model for SAP-related ARDS. In this multicenter retrospective study, 597 patients diagnosed with AP from four hospitals in different regions of China from 2017 to 2021 were divided into two cohorts: The derivation cohort (n = 407) and the validation cohort (n = 190). Of these, 139 were diagnosed with SAP and 99 were diagnosed with ARDS. Multivariate logistic regression showed that four identical variables of SAP and ARDS were identified as independent risk factors, including heart rate, respiratory rate, serum calcium concentration, and blood urea nitrogen. In the derivation and validation cohorts, the area under the operating characteristic curve (AUC) for predicting SAP was 0.879 and 0.898, respectively, and that value for ARDS was 0.892 and 0.833, respectively. In the derivation cohort for SAP prediction, an AUC value of the new model was significantly better than that of the SIRS (AUC = 0.808) or quick sepsis-related organ failure assessment (qSOFA) (AUC = 0.730); with respect to ARDS prediction, a corresponding AUC value was better than that of the SIRS (AUC = 0.815) or qSOFA (AUC = 0.742). The study developed a novel predictive model for SAP-related ARDS in patients with AP. Furthermore, the results of the new model indicated that patients with AP who exhibited higher respiratory rate, heart rate, and blood urea nitrogen (BUN) concentration, and lower serum calcium concentration on admission might develop SAP and ARDS with a higher risk.
In another study by Ding et al[17], 779 AP patients were randomly assigned to the primary cohort (n = 560) and the validation cohort (n = 219), and AP patients in each cohort were further divided into an ARDS group and a non-ARDS group. The heart rate, BUN, and serum calcium in the ARDS group were higher than those in the non-ARDS group, and the heart rate was significantly different between the two groups. Comparing variables in the primary cohort, the heart rate and serum calcium were statistically significantly different between the two groups. Zhang et al[18] also divided SAP patients into ARDS and non-ARDS groups, and their results showed statistically significant differences in respiratory rate and heart rate between two groups. Respiratory rate > 30 /min (odds ratio = 2.405) was an independent risk factor for ARDS in patients with SAP. As far as we know, the BUN level has not been used as a direct predictor of ARDS. However, the marker can be used as a predictor of pathogenesis associated with other risk factors, such as AP[6]. BUN not only is selected in the new prediction model of Li et al[16], but also is participated in other prediction models of SAP, such as bedside index for severity in AP (BISAP). The results of Dai et al[19] showed that the only independent risk factor correlated with 30-d all-cause mortality was BUN level in AP patients. In addition, the validity of BUN as a prognostic marker was further verified using a receiver operating characteristic curve with an AUC of 0.803 for BUN and an optimal cut-off value of 12.01 mmol/L (sensitivity = 0.714, specificity = 0.810). Another study has shown that elevated BUN at admission and within 24 h after admission can predict AP mortality[20]. According to our knowledge, the study of Li et al[16] presented the first model to use serum calcium concentration to predict ARDS in SAP. Ye et al[21] showed that BISAP and serum calcium were independent predictors of AP severity. The results of the study showed that the model established by the combination of BISAP and serum calcium was remarkably better than that established by BISAP and serum calcium alone. Additionally, the study also found that serum calcium concentration was negatively correlated with the severity of AP, while BISAP was positively correlated with AP severity[21]. It is further suggested that there may be some relationship between serum calcium levels and SAP-related ARDS in patients with AP. A previous study showed that hypocalcemia was an independent risk factor for respiratory failure in SAP[22]. They believed that serum calcium concentration was a valuable tool for evaluating rapidly persistent organ failure in AP patients. Further studies need to be done in this respect.
Because of the high mortality rate of SAP patients, it is necessary to quickly identify patients with a more severe disease state and a higher risk of death at an early stage. For risk stratification, several pancreas-specific or general scoring systems have been established in the past. BISAP is a potential prognostic scoring system for identifying AP patients at high risk of death in hospital, with high specificity[23], but it needs to evaluate pleural exudation. And most hospitalized patients do not undergo the examination within 24 h, so the score cannot be completed[24]. Multi-parameter scores such as the acute physiology and chronic health evaluation II (APACHE II) and BISAP are very important for clinical trials, but the APACHE II score is not specific for AP. In addition, the method is too complex and time-consuming to calculate, and its application in practical clinical work is limited[25]. A clinical study has confirmed that SIRS can be used as an “early warning device” of the severity of AP[26]. At present, the commonly used AP scoring systems, such as the BISAP and the Japanese severe score systems, contain the relevant content of SIRS. The qSOFA score is also rapid and easy to obtain and can be used for rapid evaluation of preclinical patients or emergency patients, but its effect on the prognosis of the disease is limited[27]. Therefore, a simple model with a small number of parameters will be more practical. The novel prediction model reported by Li et al[16] involves only four routine parameters for SAP and ARDS prediction. This study confirms that the new model is not inferior to BISAP in predicting SAP-related ARDS, allowing early identification of patients at high risk of SAP and ARDS. It has clinical value for improving the prognosis of AP.
Chest CT findings of AP for early prediction of ARDS
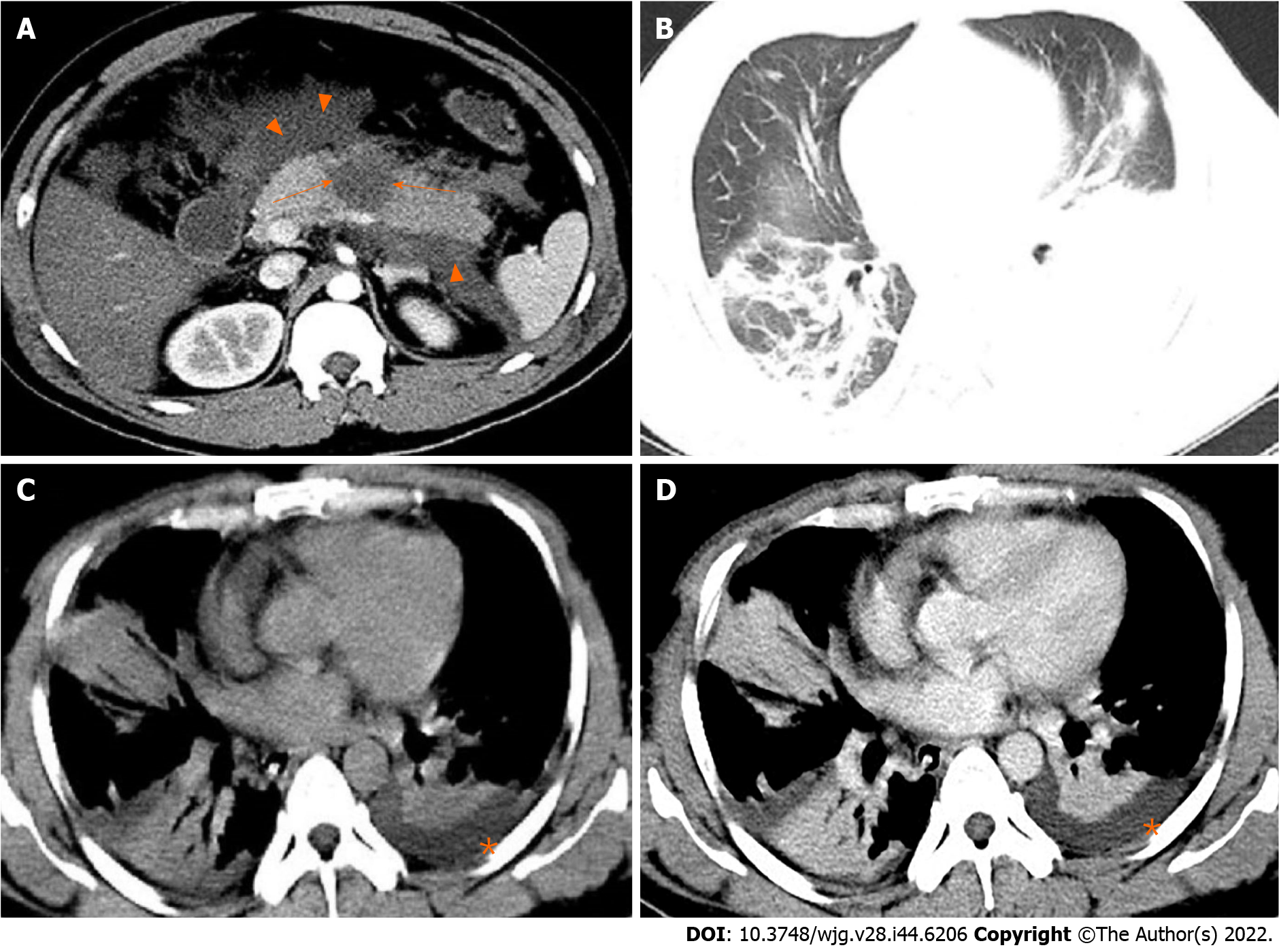
Based on clinical observations, we can evaluate SAP and related ARDS not only from the predictive new model but also from chest CT presentations of AP at the time of initial diagnosis. Up to 55% of AP patients may have abnormal chest CT findings, including pleural effusion, pulmonary atelectasis, pulmonary consolidation, and ARDS-related pulmonary edema[28]. Pulmonary consolidation and pleural effusion are common complications in patients with AP, which are closely related to the severity of AP. The imaging manifestations of pulmonary consolidation are patchy, segmental, and diffuse pulmonary changes, which may contain bronchial inflation signs. It is reported that the incidence of pleural effusion in AP was about 3%-17% in the earlier literature. But recent reports showed that, according to CT scan, the incidence was as high as 46%-50%[29,30]. The chest CT findings of SAP complicated with ARDS mainly include bilateral or left-sided pleural effusion[28,31], and solid changes in the basal segments of lower lobes of both lungs[32] (Figure 1).
Figure 1 A 31-year-old man with severe acute pancreatitis complicated with acute respiratory distress syndrome.
A: Axial contrast-enhanced computed tomography image in the arterial phase shows flake parenchymal necrosis (arrows) in the region of body of the pancreas, as well as extensive heterogeneous collections (acute necrotic collections) in the peripancreatic and the left pararenal anterior spaces (arrowheads); B: Lung window; C: Mediastinal window; D: Chest axial contrast-enhanced venous phase image. The three images show partial pulmonary consolidation in the middle and lower lobes of the right lung, in which bronchial inflation signs can be seen, and partial consolidation with partial atelectasis in the lower lobe of the left lung caused by external pressure of pleural effusion (asterisks).
Accordingly, we can semi-quantitatively evaluate the pleural effusion volume (PEV) and pulmonary consolidation score (based on the number of involved lobes) in patients with AP. A recent study showed that PEV and pulmonary consolidation lobes can provide early predictions of SAP and organ failure[32]. Peng et al[32] reported that PEV was strongly correlated with BISAP score and CT severity index (CTSI) score, but was weakly correlated with the length of hospital stay and APACHE II score. The lung consolidation score was moderately correlated with the BISAP score, CTSI score, and APACHE II score. On the contrary, Yan et al[33] showed that there was a strong correlation between PEV and length of hospitalization or APACHE II score. In addition, Peng et al[32] described that the accuracy of PEV in predicting SAP was similar to that of the BISAP score, APACHE II score, and CTSI score. As for predicting organ failure, the accuracy of PEV was also similar to that of the BISAP score, APACHE II score, and CTSI score. While Yan et al[33] predicted SAP, the accuracy of PEV was distinctly higher than that of the BISAP score and CTSI score, but was significantly lower than that of the APACHE II score. In the prediction of organ failure, the accuracy of PEV was distinctly higher than that of CTSI score, and its accuracy was similar to that from BISAP score or APACHE II score. Peng et al[32] also described that the accuracy of the lung consolidation score was similar to that of the BISAP score, APACHE II score, and CTSI score in predicting SAP and organ failure. Although some results of Peng et al[32] and Yan et al[33] were different, they both confirmed that chest imaging findings of AP patients could indicate the severity of AP and organ failure to some extent.
Previous studies have confirmed that respiratory failure is the most common type of organ failure in AP. Schepers et al[11] reported that the proportion of AP patients with respiratory failure was 92% (221/240). It was also found that this was the most common type of organ failure in the early and late stages of AP. Moreover, the distribution of different types of organ failure was different in this study, and the median duration of respiratory failure was 19 d. The mortality rate of respiratory failure was 37%, and the mortality rates of renal failure and circulatory failure was 47% and 40%, respectively. Raghu et al[28] claimed that the development of pulmonary consolidation in patients with AP was related to the occurrence of respiratory failure. As mentioned above, the incidence of ARDS was associated with respiratory failure. They also observed that pleural effusion was significantly associated with the severity of AP, the occurrence of respiratory failure, and poor prognosis. To sum up, chest CT findings of AP patients are potentially valuable for early prediction of possible subsequent respiratory failure in our daily clinical work.
Future trends and prospects
The 21st century is the era of big databases. Radiomics is an emerging technology that can extract a large number of parameters from images that are difficult for human eyes to observe and distinguish, and transform image data into high-dimensional and minable data through a variety of algorithms. Therefore, it can carry out a comprehensive quantitative analysis of the heterogeneity of the disease, and assist in clinical diagnosis, treatment, and other work[34]. Lin et al[35] first reported a radiomics model based on contrast-enhanced magnetic resonance imaging (MRI) to predict the clinical severity of AP. Their study showed that the prediction accuracy of the portal venous-phase MRI imaging radiomics model reached 85.6% and 81.0% in the training cohort and validation cohort, respectively. These results suggest that the portal venous-phase MRI radiomics model may be more accurate in early predicting the clinical severity of AP, and these findings may have a broad application prospect in the classification of AP severity. We believe that there is also great potential for predicting SAP-related ARDS in the future.