Published online Nov 21, 2022. doi: 10.3748/wjg.v28.i43.6099
Peer-review started: September 24, 2022
First decision: October 18, 2022
Revised: October 20, 2022
Accepted: November 6, 2022
Article in press: November 6, 2022
Published online: November 21, 2022
Processing time: 53 Days and 2.1 Hours
Nonalcoholic fatty liver disease (NAFLD) is strongly associated with sleep apnea syndrome (SAS). Many NAFLD patients have SAS, and obstructive sleep apnea hypopnea syndrome is also considered to be an independent risk factor for NAFLD, as it contributes to the progression of NAFLD via oxidative stress, lipid peroxidation, inflammation, and insulin resistance. This review aims to provide some recommendations for the management of NAFLD patients with SAS, including diet, exercise, weight loss, and continuous positive airway pressure. This review also highlights the importance of effective strategies in NAFLD pre
Core Tip: Nonalcoholic fatty liver disease (NAFLD) is strongly associated with sleep apnea syndrome (SAS). This minireview presents the relationship between NAFLD and SAS; addresses the role of obesity, insulin resistance, and oxidative stress, and emphasizes the management of NAFLD with SAS, which mainly includes lifestyle interventions and continuous positive airway pressure therapy. This review also highlights the importance of effective strategies in NAFLD prevention and treatment.
- Citation: Sheng W, Ji G, Zhang L. Management of non-alcoholic fatty liver disease patients with sleep apnea syndrome. World J Gastroenterol 2022; 28(43): 6099-6108
- URL: https://www.wjgnet.com/1007-9327/full/v28/i43/6099.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i43.6099
Nonalcoholic fatty liver disease (NAFLD) is defined as hepatic steatosis greater than 5% and excludes causes such as alcohol, viral infection and hereditary factors[1]. Due to numerous studies that have characterized the association of NAFLD with metabolic syndromes, such as obesity and type 2 diabetes (T2DM), an international consensus in 2020 proposed renaming NAFLD as metabolic-associated fatty liver disease (MAFLD)[2]. NAFLD can progress into nonalcoholic steatohepatitis (NASH) with or without fibrosis. Currently, there are two main hypotheses about the pathogenesis of NASH. One is the “two-hit” hypothesis proposed by James and Day[3] in 1998: The first strike is induced by insulin resistance and excessive accumulation of hepatic lipids, while oxidative stress and inflammation are considered to compose the second strike. As research in this field continues to advance, the “parallel multihits” hypothesis is thought to more accurately explain the complex mechanisms of NAFLD progression, which involves genetic and epigenetic factors, insulin resistance, endoplasmic reticulum stress, oxidative stress, and gut dysbiosis[3-5].
NAFLD patients are prone to sleep apnea syndrome (SAS), a common respiratory disease. It is estimated that nearly 1 billion adults aged 30-69 years worldwide have SAS. A real-world study of uncomplicated NAFLD patients in South Korea showed that during a median follow-up of 5.3 years, 1351 patients (0.4%, total 334334) were diagnosed with SAS; among patients with a fatty liver index > 31.0, SAS occurred in 0.8% of patients[6]. Based on the differences in their pathogenesis, SAS is broadly categorized into three types: Obstructive sleep apnea hypopnea syndrome (OSAHS), central SAS and mixed SAS. OSAHS is the most common type of SAS, and obesity is a strong risk factor for OSAHS. Approximately 40% of individuals with obesity are reported to have OSAHS, and 70% of OSAHS patients have obesity[7]. The etiology of OSAHS is complex, with the main causes including anatomical narrowing of the upper airway and local soft tissue collapse. Obesity exacerbates upper respiratory obstruction due to compression of the pharyngeal cavity and airway by neck fat[8,9]. It has been reported that central obesity and neck circumference thickening are significantly and positively associated with the apnea hypoventilation index (AHI), and the AHI is also positively associated with insulin resistance[10]. In addition, the incidence of metabolic syndrome is elevated in patients with OSAHS compared to non-OSAHS; in particular, components of metabolic syndrome, such as T2DM, obesity, and dyslipidemia, are more strongly associated with OSAHS[11]. A German observational cohort study also found a higher incidence of hepatic steatosis in patients with moderate-to-severe OSAHS, and the assessment of a snoring index may help to identify the risks of associated liver disease in OSAHS patients[12]. More importantly, a previous meta-analysis found that OSAHS is associated with an increased risk of advanced fibrosis in NAFLD patients, independent of age, sex and body mass index (BMI)[13]. Another Italian observational cohort study suggested that evaluating the mean oxyhemoglobin percentage in obese patients with OSAHS is of great clinical value in identifying the risks of NAFLD progression[14]. However, because comorbidities coexist and are independently associated with systemic inflammation, it is very difficult to clarify the effects of OSAHS on the development and progression of NAFLD[15,16]. The exact mechanisms of NAFLD and SAS are still largely unknown, but some biological processes are considered to be integrated in NAFLD patients with SAS.
Lipid accumulation and subsequent oxidative stress in the liver are considered the initial causes of NAFLD progression and the development of NASH. The accumulation of free fatty acids in hepatocytes due to insulin resistance, increased de novo lipogenesis or excessive lipid uptake from dietary sources can disrupt the mitochondrial microstructure, leading to impaired fatty acid β-oxidation and the production of reactive oxygen species (ROS)[17,18]. Overwhelmed ROS can trigger oxidative stress, which is a stressful state involving an imbalance between oxidative and antioxidant actions, usually due to excess ROS interfering with endogenous antioxidant defense system[5]. Oxidative stress can exacerbate lipid accumulation in hepatocytes, disrupt the structure and function of mitochondria; activate Kupffer cells; and promote the release of inflammatory factors such as tumor necrosis factor-alpha (TNF-α), interleukin (IL)-6, and IL-8, thereby exacerbating the inflammatory response of hepatocytes[19].
OSAHS is characterized by chronic intermittent hypoxia (CIH) that occurs at night, and prolonged CIH can lead to tissue hypoxia and promote oxidative stress, lipid peroxidation, and systemic inflammation. The disturbed lipid metabolism and excessive oxidative stress in the liver during NAFLD progression may further affect OSAHS. Accordingly, the combination of OSAHS aggravates hepatic impairment, promotes lipid metabolism disorder and increases insulin resistance in patients with NAFLD[20-22]. This was also demonstrated by the positive correlation between the severity of CIH in OSAHS patients and the severity of liver fibrosis measured by liver elastography[23]. Evidence from clinical investigations suggests an increased incidence of NAFLD in patients with OSAHS even in the absence of obesity or metabolic syndrome, while the severity of NAFLD is parallelly associated with the severity of OSAHS in NAFLD patients with OSAHS[24]. More importantly, even in children with NAFLD, nocturnal hypoxia-induced oxidative stress promotes disease progression[25]. In addition, patients with OSAHS are at risk of hypoxemia and even hypercapnia due to the collapse of the pharynx during sleep, resulting in upper airway obstruction and airflow restriction, as well as respiratory distress and even interruption of breathing, leading to a decrease in oxygen saturation.
CIH, insulin resistance and disorders of lipid metabolism caused by OSAHS may exacerbate NAFLD in patients with obesity and lead to an increased risk of NASH and more serious diseases[26,27]. It has been reported that polymorphisms of proinflammatory cytokine genes including the highly sensitive C-reactive protein, IL-6 and leptin receptor genes are associated with increased risk of OSA and NAFLD in Asian Indians[28]. Moreover, Asian Indian subjects carrying the Gly972Arg polymorphism of insulin receptor substrate 1 are predisposed to developing OSA and NAFLD[29]. However, as mentioned previously, we do not yet fully understand the association between OSAHS and NAFLD. Current evidence suggests that OSAHS-related CIH triggers excessive lipolysis, manifesting as an increase in plasma free fatty acids[30,31]. Excessive free fatty acids lead to ectopic fat accumulation, insulin resistance, vascular dysfunction and dyslipidemia[32,33], which may be one of the mechanisms by which OSAHS promotes the progression of NAFLD[34]. Furthermore, CIH has also been hypothesized to promote oxidative stress through increased ROS production and angiogenesis, increased sympathetic activation with elevated blood pressure, and systemic and vascular inflammation with endothelial dysfunction[35].
The diagnosis of NAFLD is primarily an exclusionary diagnosis, which is different from the diagnostic criteria for MAFLD. Secondary causes of hepatic steatosis and causes of liver complications due to other diseases must be excluded to make the diagnosis of NAFLD, including alcohol and drug use, hepatic viral infections and autoimmune liver diseases, Wilson’s disease and lipodystrophy[1,36,37]. Currently, the gold standard for NAFLD diagnosis (e.g., assessment of steatosis, steatohepatitis and the stage of liver fibrosis) remains liver biopsy and histological staining; however, it is inappropriate to routinely perform liver biopsy; because it is invasive and may pose a risk of complications, sampling errors and expert interpretation inconsistencies. Therefore, many noninvasive assessment and clinical scoring systems are used to diagnose and assess the degree of steatosis and fibrosis in patients with NAFLD. For example, abdominal ultrasonography, as a noninvasive and convenient method, and controlled attenuation parameter measurement based on transient elastography for ultrasonography attenuation are commonly used to detect the extent of liver steatosis[38]. Magnetic resonance imaging (MRI) is also applied in the noninvasive diagnosis and grading of NAFLD, and is considered to be efficient in quantifying liver fat, stiffness, and visceral adipose tissue[39]. The further developed MRI-proton density fat fraction and proton magnetic resonance spectroscopy are reported to be more concise technologies for NAFLD diagnosis[40]. Furthermore, hepatologists from the American Gastroenterology Association and Chronic Liver Disease Foundation summarized several commonly used noninvasive scores[41]. Among them, the fibrosis-4 index is the most widely studied and preferred simple noninvasive algorithm and it is used to evaluate the degree of liver fibrosis based on age and levels of platelet, aspartate aminotransferase (AST) and alanine aminotransferase (ALT)[42].
The recommended treatment for NAFLD focuses on lifestyle interventions, including dieting, exercise, weight loss, and promotion of energy expenditure. In 2020, the Asia Pacific Association for the Study of the Liver proposed clinical practice guidelines for NAFLD. This guideline points out that conservative management of lifestyle changes remains the best choice for the treatment of NAFLD[37]. Notably, when NAFLD patients are combined with SAS, supplemental treatment with continuous positive airway pressure (CPAP) is the most appropriate approach. CPAP therapy has been reported to help improve sleep apnea and hypoventilation, as well as stabilize or delay the progression of NAFLD[24]. Inflammation, a hypoxic environment and oxidative stress are key pathogenic mechanisms in patients with NAFLD and OSAHS. Therefore, antioxidants such as vitamin E may be effective, and anti-obesity medications have also shown considerable potential in the treatment of NAFLD and OSAHS[43,44]. The glucagon-like peptide-1 receptor agonist, liraglutide and semaglutide may improve AHI and protect against NASH and obesity, especially in relation to T2DM[45,46]. Notably, liraglutide at a dose of 3 mg is well tolerated and is effective at improving NAFLD and OSAHS[47,48].
A reasonable dietary structure and appropriate dietary intake are beneficial for obesity, especially for NAFLD patients with OSAHS. Severely obese patients with NAFLD should strictly limit calorice intake, and if necessary, scientific recipes should be prescribed by nutritional experts to help alter the metabolic pattern. A Mediterranean diet is recommended for patients with NAFLD and NASH. Based on high amounts of monounsaturated fatty acids derived from olive oil, fruits, grains, whole grains and low-fat dairy products, the Mediterranean dietary pattern can reduce the incidence of metabolic syndrome, obesity, T2DM and cardiovascular disease, as well as certain cancers[49-51]. Remarkably, the Mediterranean diet may potentially counteract the inflammation and oxidative stress that occur in OSAHS and improve upper-airway neuromuscular control and muscle force-generating capacity[52]. In an interdisciplinary weight loss and lifestyle intervention trial, participants who adhered to the Mediterranean diet demonstrated greater weight loss as well as more significant improvement in OSAHS[53].
In addition, the diversity of foods should be increased for patients with NAFLD, and the foods need to be enriched for antioxidants and high-quality proteins, which can contribute to NAFLD and OSAHS improvement. However, NAFLD patients should reduce the consumption of specific foods, including red meat and overprocessed foods[54]. Moderate coffee consumption is allowed for NAFLD patients, but the intake of alcohol, fructose and sugar-containing drinks should be avoided because alcohol or sugar consumption can increase lipid synthesis, visceral fat accumulation and insulin resistance, and increase the risk of liver fibrosis in patients with NAFLD.
Exercise is beneficial for NAFLD and SAS. Previous studies have shown that sedentary and reduced physical activity is associated with the progression of NAFLD and the development of moderate to severe OSAHS[55,56]. There is growing evidence showing that aerobic exercise training is beneficial for patients with SAS[57]. Active physical activity may reduce body weight, improve blood circulation, contribute to sleep quality and address excessive daytime sleepiness. Therefore, regular exercise facilitates weight loss and improves NAFLD and associated complications[58,59]. The recommended minimum level of exercise is 5 d per week (> 30 min each day) of moderate intensity aerobic exercise or 3 d per week (> 20 min each day) of vigorous exercise.
For NAFLD patients with severe obesity, weight loss is very difficult due to their large weight base and may require metabolic bariatric surgery (MBS) intervention. Over the past few decades, MBS has evolved from the simple gastric volume-limiting procedure laparoscopic adjustable gastric banding to surgical options with hormonal effects, including vertical sleeve gastrectomy (VSG) and Roux-en-Y gastric bypass (RYGB), both of which induce appetite changes by modulating intestinal and central hormones, thereby reducing food consumption and increasing satiety[60]. Usually, MBS is the most effective treatment for obesity and often results in dramatic improvements in glycemic control, insulin resistance and NAFLD[61,62].
SAS is also one of the comorbidities with the greatest response rate to MBS. The 6th IFSO Global Registry Report shows that MBS leads to a reduction of SAS ranging from 58%-65%, depending on the type of operation (LSG, RYGB, OAGB). Evidence from clinical studies has confirmed the efficacy of MBS for patients with obesity and OSAHS[63,64]. A retrospective study comparing the effect of VSG and RYGB on weight loss and their comorbidity remission also showed that both MBSs are effective in improving OSAHS symptoms[65]. Most surprisingly, in a study observing the effect of VSG in patients with a BMI ≥ 50 kg/m², complete remission was observed in all 13 patients with comorbid OSAHS[66]. MBS can effectively improve sleep apnea and nocturnal hypoxia, as well as hepatic steatosis and fibrosis[67,68]. More importantly, the severity of OSAHS determines the risk of NAFLD before MBS, and after surgery, it also determines the improvement of NAFLD[67].
CPAP is the first-line recommendation for the treatment of OSAHS. For NAFLD patients with OSAHS, early intervention is even more important. Additionally, for obesity and OSAHS patients scheduled for MBS, guidelines recommend preoperative CPAP for at least 4 wk[69]. CPAP acts in multiple pathways to prevent airway collapse, reduce pharyngeal edema and upper airway resistance, increase the action of upper airway opening muscles through vagal reflexes, and restore chemoreceptor sensitivity and respiratory center drive. CPAP treatment has been reported to significantly reduce sleep apnea and hypoventilation in patients with OSAHS, improve quality of life; reduce daytime sleepiness, and decrease the occurrence of hypertension, diabetes, cardiovascular and cerebrovascular complications[70]. This may be because CPAP treatment reduces markers of oxidative stress and thus improves metabolic syndrome[71]. In addition, a randomized controlled trial showed that patients with OSAHS had increased markers of liver injury and atherogenic risk[72], whereas CPAP treatment helped stabilize or delay the progression of NAFLD and demonstrated improvements in metabolic and cardiovascular function[24]. A meta-analysis of 192 obesity patients combined with OSAHS showed a significant reduction in serum AST and ALT levels after CPAP treatment for 3 mo[73]. Another meta-analysis of six randomized controlled trials involving 699 subjects also showed that CPAP treatment reduces total cholesterol and triacylglycerol levels[74]. More importantly, CPAP treatment of patients with OSAHS significantly reduces serum inflammatory markers, including CRP and TNF-α[75]. More interestingly, in another randomized controlled trial, Sundaram et al[76] found that CPAP treatment reverses the parameters of liver injury, reduces oxidative stress in children with NAFLD can also be used to predict NAFLD progression in obese children with OSAHS.
Although these reports point to a beneficial effect of CPAP on NAFLD-associated parameters[73,74,77,78], short-term (usually 4-12 wk) CPAP treatment may not be as effective[72,79,80]. The most significant result of CPAP treatment is a case report published in 2018 that reported the reversal of NAFLD and the normalization of liver enzymes and the associated lipidome in a patient with both NAFLD and severe OSAHS after 6 years of treatment[81]. In addition, long-term follow-up data based on a Taiwanese population also showed a lower cumulative incidence of NAFLD and cirrhosis in CPAP-treated patients than in nontreated patients[82].
Therefore, the beneficial effects of short-term CPAP therapy on OSAHS and NAFLD may be elusive, and although long-term and effective CPAP treatment can show significant improvements in both OSAHS and NAFLD, most patients are not amenable to long-term oral and nasal mask therapy. In addition, CPAP therapy is not yet commonly used due to the high cost of the device.
Obesity is a high-risk factor for NAFLD and SAS. Most patients living with obesity and metabolic syndrome are prone to SAS, and the severity of disease in patients with SAS is significantly associated with NAFLD progression. It is important to note that, even in nonobese patients, there is a relationship between NAFLD progression and SAS severity. SAS is usually one of the complications of NAFLD. However, at present, we can only confirm that lipid disorders, hypoxia, oxidative stress, and inflammation are involved, and a deeper understanding of the pathogenesis remains unclear. More in-depth studies are needed to elucidate the causal relationship between them. More importantly, an under
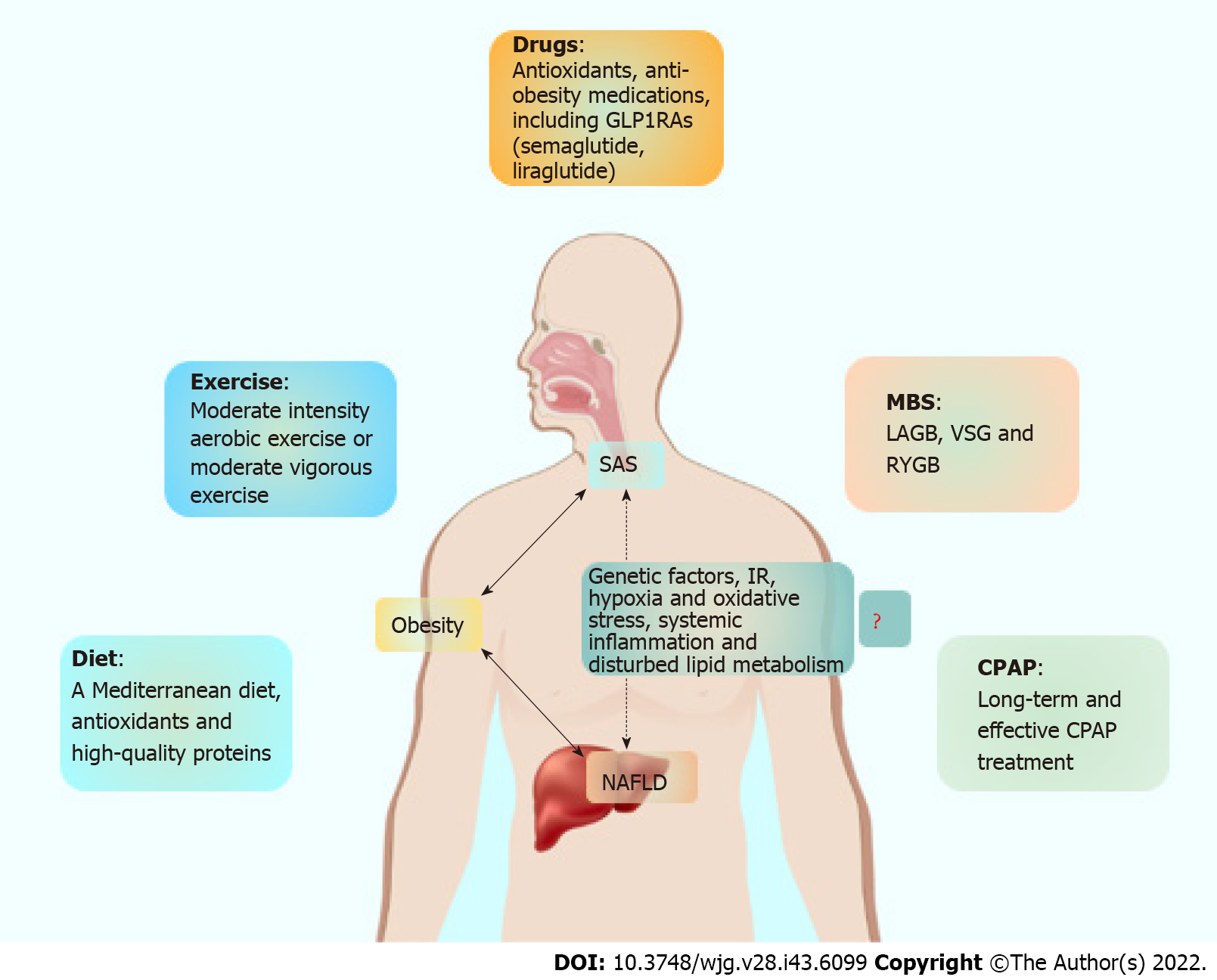
Collectively, healthy lifestyle management remains the most important strategy for the treatment of NAFLD, including diet, exercise and weight loss. Furthermore, the use of antioxidants such as vitamin E and the insulin-sensitizing drug pioglitazone is also necessary. It needs to be emphasized again that timely CPAP intervention is also important for NAFLD patients with SAS. We have summarized our recommendations for NAFLD combined with SAS management in Figure 1, and the improvements in SAS and NAFLD after intervention with lifestyle measures, medications and MBS are summarized in Table 1.
| Category | Effects | Ref. | |
| Lifestyle measures | Mediterranean diet | Inhibition of inflammation and oxidative stress that occur in OSAHS and improvement of upper-airway neuromuscular control and muscle force-generating capacity | [51] |
| Dietary behavior change, moderate-intensity aerobic exercise, sleep hygiene, and tobacco and alcohol avoidance | Increases in adherence to the Mediterranean diet | [52] | |
| Reduced AHI and oxygen desaturation index | |||
| Increased sleep quality | |||
| Decease of body weight, fat mass, visceral adipose tissue, and neck, chest, and waist circumferences | |||
| Aerobic exercise training | Reduced body weight improved blood circulation, better sleep quality and less daytime sleepiness | [56-58] | |
| Medications | Phentermine plus extended-release topiramate | Significant improvements in overnight oxygen saturation and reduction in blood pressure | [42] |
| Liraglutide and semaglutide | Histological resolution of NASH and improved metabolic control | [43-48] | |
| Decreased AHI, body weight, SBP and HbA1c | |||
| MBS | VSG or other MBS | Reduced AHI | [62-64] |
| VSG | 100% remission rate in patients with OSAHS who also underwent hiatal hernia repair | [65] | |
| MBS | Improved sleep apnea and nocturnal hypoxia, as well as liver steatosis and fibrosis | [66] | |
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pantelis AG, Greece; Villela-Nogueira CA, Brazil S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3544] [Cited by in RCA: 4949] [Article Influence: 707.0] [Reference Citation Analysis (9)] |
| 2. | Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Järvinen H, Fan JG, Grønbæk H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020;73:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2883] [Cited by in RCA: 2832] [Article Influence: 566.4] [Reference Citation Analysis (1)] |
| 3. | James OF, Day CP. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol. 1998;29:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 266] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism. 2016;65:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 2119] [Article Influence: 235.4] [Reference Citation Analysis (1)] |
| 5. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1820] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 6. | Kim N, Roh JH, Lee H, Kim D, Heo SJ. The impact of non-alcoholic fatty liver disease on sleep apnea in healthy adults: A nationwide study of Korea. PLoS One. 2022;17:e0271021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Xanthopoulos MS, Berkowitz RI, Tapia IE. Effects of obesity therapies on sleep disorders. Metabolism. 2018;84:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 560] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 9. | Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993;148:462-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 260] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Liu Y, Zou J, Li X, Zhao X, Liu S, Meng L, Qian Y, Xu H, Yi H, Guan J, Yin S. Effect of the Interaction between Obstructive Sleep Apnea and Lipoprotein(a) on Insulin Resistance: A Large-Scale Cross-Sectional Study. J Diabetes Res. 2019;2019:9583286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Wang F, Xiong X, Xu H, Huang H, Shi Y, Li X, Qian Y, Zou J, Yi H, Guan J, Yin S. The association between obstructive sleep apnea syndrome and metabolic syndrome: a confirmatory factor analysis. Sleep Breath. 2019;23:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Bahr K, Simon P, Leggewie B, Gouveris H, Schattenberg J. The Snoring Index Identifies Risk of Non-Alcoholic Fatty Liver Disease in Patients with Obstructive Sleep Apnea Syndrome. Biology (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 13. | Musso G, Cassader M, Olivetti C, Rosina F, Carbone G, Gambino R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14:417-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 185] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 14. | Bettini S, Serra R, Fabris R, Dal Prà C, Favaretto F, Dassie F, Duso C, Vettor R, Busetto L. Association of obstructive sleep apnea with non-alcoholic fatty liver disease in patients with obesity: an observational study. Eat Weight Disord. 2022;27:335-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, Kales A, Chrousos GP. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 573] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 16. | Ong CW, O'Driscoll DM, Truby H, Naughton MT, Hamilton GS. The reciprocal interaction between obesity and obstructive sleep apnoea. Sleep Med Rev. 2013;17:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Serviddio G, Sastre J, Bellanti F, Viña J, Vendemiale G, Altomare E. Mitochondrial involvement in non-alcoholic steatohepatitis. Mol Aspects Med. 2008;29:22-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Fromenty B, Robin MA, Igoudjil A, Mansouri A, Pessayre D. The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab. 2004;30:121-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 190] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Masarone M, Rosato V, Dallio M, Gravina AG, Aglitti A, Loguercio C, Federico A, Persico M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid Med Cell Longev. 2018;2018:9547613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 496] [Cited by in RCA: 482] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 20. | Hernández A, Geng Y, Sepúlveda R, Solís N, Torres J, Arab JP, Barrera F, Cabrera D, Moshage H, Arrese M. Chemical hypoxia induces pro-inflammatory signals in fat-laden hepatocytes and contributes to cellular crosstalk with Kupffer cells through extracellular vesicles. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. 2016;65:1124-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | Parikh MP, Gupta NM, McCullough AJ. Obstructive Sleep Apnea and the Liver. Clin Liver Dis. 2019;23:363-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Agrawal S, Duseja A, Aggarwal A, Das A, Mehta M, Dhiman RK, Chawla Y. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int. 2015;9:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 24. | Umbro I, Fabiani V, Fabiani M, Angelico F, Del Ben M. Association between non-alcoholic fatty liver disease and obstructive sleep apnea. World J Gastroenterol. 2020;26:2669-2681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 25. | Sundaram SS, Halbower A, Pan Z, Robbins K, Capocelli KE, Klawitter J, Shearn CT, Sokol RJ. Nocturnal hypoxia-induced oxidative stress promotes progression of pediatric non-alcoholic fatty liver disease. J Hepatol. 2016;65:560-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 26. | Fu Y, Zhang N, Tang W, Bi Y, Zhu D, Chu X, Shan X, Shen Y, Sun X, Feng W. Chronic intermittent hypoxia contributes to non-alcoholic steatohepatitis progression in patients with obesity. Hepatol Int. 2022;16:824-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 27. | Ahmed MH, Byrne CD. Obstructive sleep apnea syndrome and fatty liver: association or causal link? World J Gastroenterol. 2010;16:4243-4252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Bhatt SP, Guleria R, Vikram NK, Vivekanandhan S, Singh Y, Gupta AK. Correction: Association of inflammatory genes in obstructive sleep apnea and non alcoholic fatty liver disease in Asian Indians residing in north India. PLoS One. 2018;13:e0203182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Bhatt SP, Guleria R. Association of IRS1 (Gly972Arg) and IRS2 (Gly1057Asp) genes polymorphisms with OSA and NAFLD in Asian Indians. PLoS One. 2021;16:e0245408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 30. | Barceló A, Piérola J, de la Peña M, Esquinas C, Fuster A, Sanchez-de-la-Torre M, Carrera M, Alonso-Fernandez A, Ladaria A, Bosch M, Barbé F. Free fatty acids and the metabolic syndrome in patients with obstructive sleep apnoea. Eur Respir J. 2011;37:1418-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Jun JC, Drager LF, Najjar SS, Gottlieb SS, Brown CD, Smith PL, Schwartz AR, Polotsky VY. Effects of sleep apnea on nocturnal free fatty acids in subjects with heart failure. Sleep. 2011;34:1207-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Tumova J, Andel M, Trnka J. Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiol Res. 2016;65:193-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 33. | Boden G, Lebed B, Schatz M, Homko C, Lemieux S. Effects of acute changes of plasma free fatty acids on intramyocellular fat content and insulin resistance in healthy subjects. Diabetes. 2001;50:1612-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 411] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 34. | Gu C, Younas H, Jun JC. Sleep apnea: An overlooked cause of lipotoxicity? Med Hypotheses. 2017;108:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest. 2015;147:266-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 464] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 36. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3180] [Article Influence: 353.3] [Reference Citation Analysis (4)] |
| 37. | Eslam M, Sarin SK, Wong VW, Fan JG, Kawaguchi T, Ahn SH, Zheng MH, Shiha G, Yilmaz Y, Gani R, Alam S, Dan YY, Kao JH, Hamid S, Cua IH, Chan WK, Payawal D, Tan SS, Tanwandee T, Adams LA, Kumar M, Omata M, George J. The Asian Pacific Association for the Study of the Liver clinical practice guidelines for the diagnosis and management of metabolic associated fatty liver disease. Hepatol Int. 2020;14:889-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 552] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 38. | Lee HW, Wong GL, Kwok R, Choi KC, Chan CK, Shu SS, Leung JK, Chim AM, Luk AO, Ma RC, Chan HL, Chan JC, Kong AP, Wong VW. Serial Transient Elastography Examinations to Monitor Patients With Type 2 Diabetes: A Prospective Cohort Study. Hepatology. 2020;72:1230-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 39. | Cunha GM, Villela-Nogueira CA, Bergman A, Lobo Lopes FPP. Abbreviated mpMRI protocol for diffuse liver disease: a practical approach for evaluation and follow-up of NAFLD. Abdom Radiol (NY). 2018;43:2340-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Tang A, Tan J, Sun M, Hamilton G, Bydder M, Wolfson T, Gamst AC, Middleton M, Brunt EM, Loomba R, Lavine JE, Schwimmer JB, Sirlin CB. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 422] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 41. | Younossi ZM, Noureddin M, Bernstein D, Kwo P, Russo M, Shiffman ML, Younes Z, Abdelmalek M. Role of Noninvasive Tests in Clinical Gastroenterology Practices to Identify Patients With Nonalcoholic Steatohepatitis at High Risk of Adverse Outcomes: Expert Panel Recommendations. Am J Gastroenterol. 2021;116:254-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 42. | Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: Clinical prediction rules and blood-based biomarkers. J Hepatol. 2018;68:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 447] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 43. | Winslow DH, Bowden CH, DiDonato KP, McCullough PA. A randomized, double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults. Sleep. 2012;35:1529-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 44. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K; LEAN trial team, Abouda G, Aldersley MA, Stocken D, Gough SC, Tomlinson JW, Brown RM, Hübscher SG, Newsome PN. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1470] [Article Influence: 163.3] [Reference Citation Analysis (1)] |
| 45. | Pi-Sunyer X, Astrup A, Fujioka K, Greenway F, Halpern A, Krempf M, Lau DC, le Roux CW, Violante Ortiz R, Jensen CB, Wilding JP; SCALE Obesity and Prediabetes NN8022-1839 Study Group. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N Engl J Med. 2015;373:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1103] [Cited by in RCA: 1576] [Article Influence: 157.6] [Reference Citation Analysis (0)] |
| 46. | Iqbal J, Wu HX, Hu N, Zhou YH, Li L, Xiao F, Wang T, Jiang HL, Xu SN, Huang BL, Zhou HD. Effect of glucagon-like peptide-1 receptor agonists on body weight in adults with obesity without diabetes mellitus-a systematic review and meta-analysis of randomized control trials. Obes Rev. 2022;23:e13435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 101] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 47. | Khoo J, Hsiang J, Taneja R, Law NM, Ang TL. Comparative effects of liraglutide 3 mg vs structured lifestyle modification on body weight, liver fat and liver function in obese patients with non-alcoholic fatty liver disease: A pilot randomized trial. Diabetes Obes Metab. 2017;19:1814-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 48. | Blackman A, Foster GD, Zammit G, Rosenberg R, Aronne L, Wadden T, Claudius B, Jensen CB, Mignot E. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond). 2016;40:1310-1319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 299] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 49. | Sofi F, Macchi C, Abbate R, Gensini GF, Casini A. Mediterranean diet and health status: an updated meta-analysis and a proposal for a literature-based adherence score. Public Health Nutr. 2014;17:2769-2782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 708] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 50. | Bach-Faig A, Berry EM, Lairon D, Reguant J, Trichopoulou A, Dernini S, Medina FX, Battino M, Belahsen R, Miranda G, Serra-Majem L; Mediterranean Diet Foundation Expert Group. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14:2274-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1356] [Cited by in RCA: 1087] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 51. | Franco I, Bianco A, Mirizzi A, Campanella A, Bonfiglio C, Sorino P, Notarnicola M, Tutino V, Cozzolongo R, Giannuzzi V, Aballay LR, Buongiorno C, Bruno I, Osella AR. Physical Activity and Low Glycemic Index Mediterranean Diet: Main and Modification Effects on NAFLD Score. Results from a Randomized Clinical Trial. Nutrients. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 52. | Dobrosielski DA, Papandreou C, Patil SP, Salas-Salvadó J. Diet and exercise in the management of obstructive sleep apnoea and cardiovascular disease risk. Eur Respir Rev. 2017;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 53. | Carneiro-Barrera A, Amaro-Gahete FJ, Jurado-Fasoli L, Sáez-Roca G, Martín-Carrasco C, Tinahones FJ, Ruiz JR. Effect of a Weight Loss and Lifestyle Intervention on Dietary Behavior in Men with Obstructive Sleep Apnea: The INTERAPNEA Trial. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Zheng J, Zhao L, Dong J, Chen H, Li D, Zhang X, Hassan MM, Steck SE, Li X, Xiang YB, Wang H. The role of dietary factors in nonalcoholic fatty liver disease to hepatocellular carcinoma progression: A systematic review. Clin Nutr. 2022;41:2295-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 55. | Simpson L, McArdle N, Eastwood PR, Ward KL, Cooper MN, Wilson AC, Hillman DR, Palmer LJ, Mukherjee S. Physical Inactivity Is Associated with Moderate-Severe Obstructive Sleep Apnea. J Clin Sleep Med. 2015;11:1091-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 56. | Verwimp J, Ameye L, Bruyneel M. Correlation between sleep parameters, physical activity and quality of life in somnolent moderate to severe obstructive sleep apnea adult patients. Sleep Breath. 2013;17:1039-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Przybyłowski T, Bielicki P, Kumor M, Hildebrand K, Maskey-Warzechowska M, Korczyński P, Chazan R. Exercise capacity in patients with obstructive sleep apnea syndrome. J Physiol Pharmacol. 2007;58 Suppl 5:563-574. [PubMed] |
| 58. | Mascaró CM, Bouzas C, Montemayor S, Casares M, Llompart I, Ugarriza L, Borràs PA, Martínez JA, Tur JA. Effect of a Six-Month Lifestyle Intervention on the Physical Activity and Fitness Status of Adults with NAFLD and Metabolic Syndrome. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 59. | Cho K, Park S, Koyanagi A, Jacob L, Yon DK, Lee SW, Kim MS, Kim SU, Kim BK, Shin JI, Smith L. The effect of pharmacological treatment and lifestyle modification in patients with nonalcoholic fatty liver disease: An umbrella review of meta-analyses of randomized controlled trials. Obes Rev. 2022;23:e13464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Albaugh VL, Flynn CR, Tamboli RA, Abumrad NN. Recent advances in metabolic and bariatric surgery. F1000Res. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 61. | Pories WJ, Swanson MS, MacDonald KG, Long SB, Morris PG, Brown BM, Barakat HA, deRamon RA, Israel G, Dolezal JM. Who would have thought it? Ann Surg. 1995;222:339-50; discussion 350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1610] [Cited by in RCA: 1457] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 62. | Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2014;2:152-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 214] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 63. | Del Genio G, Limongelli P, Del Genio F, Motta G, Docimo L, Testa D. Sleeve gastrectomy improves obstructive sleep apnea syndrome (OSAS): 5 year longitudinal study. Surg Obes Relat Dis. 2016;12:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 64. | Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 65. | Arapis K, Macrina N, Kadouch D, Ribeiro Parenti L, Marmuse JP, Hansel B. Outcomes of Roux-en-Y gastric bypass versus sleeve gastrectomy in super-super-obese patients (BMI ≥60 kg/m2): 6-year follow-up at a single university. Surg Obes Relat Dis. 2019;15:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 66. | Ozturk A, Celik Y. A Single-Center Experience: What is the Effect of Sleeve Gastrectomy in Patients With a BMI ≥ 50 kg/m²? Cureus. 2022;14:e27992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 67. | Zhang YX, Yang L, Yang CC, Wang WY, Shen JH, Shi ML, Yu Y, Dai QC, Gu Y, Yang JJ, Yu WW, Yao K, Hu M, Ni J, Sun JL, Zhang L, Sun HX, Lu XF, Wang B. Correlation between Obstructive Sleep Apnea and Non-Alcoholic Fatty Liver Disease before and after Metabolic Bariatric Surgery. Obes Surg. 2020;30:3803-3812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 68. | Lassailly G, Caiazzo R, Buob D, Pigeyre M, Verkindt H, Labreuche J, Raverdy V, Leteurtre E, Dharancy S, Louvet A, Romon M, Duhamel A, Pattou F, Mathurin P. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology. 2015;149:379-88; quiz e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 551] [Article Influence: 55.1] [Reference Citation Analysis (3)] |
| 69. | Schiavo L, Pierro R, Asteria C, Calabrese P, Di Biasio A, Coluzzi I, Severino L, Giovanelli A, Pilone V, Silecchia G. Low-Calorie Ketogenic Diet with Continuous Positive Airway Pressure to Alleviate Severe Obstructive Sleep Apnea Syndrome in Patients with Obesity Scheduled for Bariatric/Metabolic Surgery: a Pilot, Prospective, Randomized Multicenter Comparative Study. Obes Surg. 2022;32:634-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 70. | Libman E, Bailes S, Fichten CS, Rizzo D, Creti L, Baltzan M, Grad R, Pavilanis A, Tran DL, Conrod K, Amsel R. CPAP Treatment Adherence in Women with Obstructive Sleep Apnea. Sleep Disord. 2017;2017:2760650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 71. | Oyama J, Yamamoto H, Maeda T, Ito A, Node K, Makino N. Continuous positive airway pressure therapy improves vascular dysfunction and decreases oxidative stress in patients with the metabolic syndrome and obstructive sleep apnea syndrome. Clin Cardiol. 2012;35:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 72. | Jullian-Desayes I, Tamisier R, Zarski JP, Aron-Wisnewsky J, Launois-Rollinat SH, Trocme C, Levy P, Joyeux-Faure M, Pepin JL. Impact of effective versus sham continuous positive airway pressure on liver injury in obstructive sleep apnoea: Data from randomized trials. Respirology. 2016;21:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 73. | Chen LD, Lin L, Zhang LJ, Zeng HX, Wu QY, Hu MF, Xie JJ, Liu JN. Effect of continuous positive airway pressure on liver enzymes in obstructive sleep apnea: A meta-analysis. Clin Respir J. 2018;12:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 74. | Lin MT, Lin HH, Lee PL, Weng PH, Lee CC, Lai TC, Liu W, Chen CL. Beneficial effect of continuous positive airway pressure on lipid profiles in obstructive sleep apnea: a meta-analysis. Sleep Breath. 2015;19:809-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 75. | Xie X, Pan L, Ren D, Du C, Guo Y. Effects of continuous positive airway pressure therapy on systemic inflammation in obstructive sleep apnea: a meta-analysis. Sleep Med. 2013;14:1139-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 117] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 76. | Sundaram SS, Halbower AC, Klawitter J, Pan Z, Robbins K, Capocelli KE, Sokol RJ. Treating Obstructive Sleep Apnea and Chronic Intermittent Hypoxia Improves the Severity of Nonalcoholic Fatty Liver Disease in Children. J Pediatr. 2018;198:67-75.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 77. | Chin K, Nakamura T, Takahashi K, Sumi K, Ogawa Y, Masuzaki H, Muro S, Hattori N, Matsumoto H, Niimi A, Chiba T, Nakao K, Mishima M, Ohi M. Effects of obstructive sleep apnea syndrome on serum aminotransferase levels in obese patients. Am J Med. 2003;114:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 78. | Kim D, Ahmed A, Kushida C. Continuous Positive Airway Pressure Therapy on Nonalcoholic Fatty Liver Disease in Patients With Obstructive Sleep Apnea. J Clin Sleep Med. 2018;14:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Kohler M, Pepperell JC, Davies RJ, Stradling JR. Continuous positive airway pressure and liver enzymes in obstructive sleep apnoea: data from a randomized controlled trial. Respiration. 2009;78:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 80. | Ng SSS, Wong VWS, Wong GLH, Chu WCW, Chan TO, To KW, Ko FWS, Chan KP, Hui DS. Continuous Positive Airway Pressure Does Not Improve Nonalcoholic Fatty Liver Disease in Patients with Obstructive Sleep Apnea. A Randomized Clinical Trial. Am J Respir Crit Care Med. 2021;203:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 81. | Bajantri B, Lvovsky D. A Case of Concomitant Obstructive Sleep Apnea and Non-Alcoholic Steatohepatitis Treated With CPAP Therapy. Gastroenterology Res. 2018;11:252-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 82. | Hang LW, Chen CF, Wang CB, Wu TN, Liang WM, Chou TC. The association between continuous positive airway pressure therapy and liver disease development in obstructive sleep apnea/hypopnea syndrome patients: a nationwide population-based cohort study in Taiwan. Sleep Breath. 2017;21:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |