Published online Nov 14, 2022. doi: 10.3748/wjg.v28.i42.6056
Peer-review started: June 27, 2022
First decision: August 1, 2022
Revised: August 15, 2022
Accepted: October 26, 2022
Article in press: October 26, 2022
Published online: November 14, 2022
Processing time: 136 Days and 7.4 Hours
Chylous ascites (CA) presents a challenge as a relatively common postoperative complication in gastric cancer (GC). Primary conservative therapy involved total parenteral nutrition, continuous low-pressure drainage, somatostatin, and a low-fat diet. Drainage tube (DT) clamping has been presented as a potential alternative conservative treatment for GC patients with CA.
To propose novel conservative treatment strategies for CA following GC surgery.
The data of patients with CA after GC surgery performed at the Fudan University Shanghai Cancer Center between 2006 and 2021 were evaluated retrospectively.
53 patients underwent surgery for GC and exhibited postoperative CA during the study period. Postoperative hospitalization and time of DT removal showed a significant positive association (R2 = 0.979, P < 0.001). We further observed that delayed DT removal significantly extended the total and postoperative hospitalization, antibiotic usage duration, and hospitalization cost (postoperative hospitalization: 25.8 d vs 15.5 d, P < 0.001; total hospitalization: 33.2 d vs 24.7 d, P < 0.01; antibiotic usage duration: 10.8 d vs 6.2 d, P < 0.01; hospitalization cost: ¥9.2 × 104vs ¥6.5 × 104, P < 0.01). Multivariate analysis demonstrated that postoperative infection and antibiotic usage were independent factors for delayed DT removal. Furthermore, DT removal times were shorter in seven patients who underwent DT clamping (clamped DT vs normal group, 11.8 d vs 13.6 d, P = 0.047; clamped DT vs delayed group, 13.6 d vs 27.4 d, P < 0.001). In addition, our results indicated that removal of the DT may be possible after three consecutive days of drainage volumes less than 300 mL in GC patients with CA.
Infection and antibiotic usage were vital independent factors that influenced delayed DT removal in patients with CA. Appropriate standards for DT removal can significantly reduce the duration of hospitalization. Furthermore, DT clamping might be a recommended option for conservative treatment of postoperative CA.
Core Tip: Chylous ascites (CA) is one of uncommon postoperative complication in the patients received gastric cancer (GC) surgery. Previously, the primary treatment for CA was conservative therapy, which mainly involved total parenteral nutrition, continuous low-pressure drainage, somatostatin, and a low-fat diet. Therefore, we retrospectively analyzed the patients with CA after GC surgery in our center, aiming to explore the vital factors that influence CA treatment and recommend novel conservative treatment strategies for postoperative CA in GC.
- Citation: Kong PF, Xu YH, Lai ZH, Ma MZ, Duan YT, Sun B, Xu DZ. Novel management indications for conservative treatment of chylous ascites after gastric cancer surgery. World J Gastroenterol 2022; 28(42): 6056-6067
- URL: https://www.wjgnet.com/1007-9327/full/v28/i42/6056.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i42.6056
Chylous ascites (CA) was first reported by Morton in 1691 and is defined as the leakage of milk-like fluid that contains high level of triglyceride (TG)[1,2]. Gastric cancer (GC) is one of the most common malignant tumors worldwide, and a standardized protocol for radical surgical resection has been widely accepted as a safe and effective treatment[3,4]. CA generally occurs following abdominal surgery, the incidence of postoperative CA ranges from 2.06% to 11.80% in GC patients[5-7], as a result of disturbance of the cisterna chyli or its major tributaries[8,9]. The increased incidence of CA is considered to be likely due to the increased number of cancer patients undergoing more aggressive surgical interventions in addition to laparoscopic surgery[10]. CA presents a challenge as a relatively common postoperative complication and impacts subsequent adjuvant treatments in GC. In addition, massive and prolonged CA may lead to infection, malnutrition and immunodeficiency[11].
To date, treatment options for CA have included dietary measures, use of pharmacological agents and surgical or percutaneous interventions. A high-protein and low-fat diet with medium-chain triglycerides is often recommended for patients with CA[12]. Patients who do not respond to dietary restriction should receive total parenteral nutrition (TPN), which bypasses the bowel and may thus reduce lymph flow[13]. Continuous low-pressure drainage and somatostatin also represent effective conservative treatment for postoperative CA[6,14]. CA can be cured by lymphangiography and adjunctive embolization techniques that include direct percutaneous injection of glue into the leakage site or into the surrounding lymphoid tissue[15]. Furthermore, the use of surgical measures to successfully treat CA has been reported in patients with cirrhosis and CA that is resistant to conservative therapy[1].
In this study, we retrospectively analyzed 53 patients with CA after GC surgery, aiming to explore the vital factors that influence CA treatment and recommend novel conservative treatment strategies for postoperative CA in GC.
We retrospectively reviewed all patients with CA who had undergone surgery for GC at our institution from 1 March 2006 to 31 May 2021. Three investigators performed a thorough review of all available data from the Fudan University Shanghai Cancer Center (FUSCC) medical record system, using RED Cap electronic data capture tools. In this cohort, 53 patients were admitted for gastric resection and lymphadenectomy: 2 underwent palliative resection and 51 underwent radical gastric resection with curative intent. This study was approved by the FUSCC review board in accordance with Chinese bioethical regulations, and all enrolled patients signed informed consent forms.
CA was defined as the presence of milky or creamy peritoneal fluid in the drainage tubes, at a volume of ≥ 200 mL/d and a TG levels ≥ 110 mg/dL[1,11]. Additionally, the chyle test was routinely performed if the milky peritoneal fluid was suspected to be CA[16]. Clinical and pathological data, including the age, gender, AJCC (American Joint Committee on Cancer) stage, surgical procedure, lymph node dissection, drainage tube (DT) removal, time of oral feeding, time to CA onset, drainage duration, and hospitalization duration were collected and analyzed. All patients with CA were managed conservatively; the conservative treatments included TPN, continuous low-pressure drainage, somatostatin, DT clamping, and a low-fat diet. The time to CA onset was defined as the interval between the surgical procedure and the appearance of CA. Delay DT removal was defined as a DT removal time > 16 d after surgery for all patients or patients discharge with DT. Additionally, white blood cell counts, body temperature measurement, and germiculture were performed to diagnose CA combined with infection. DT clamping is defined as physical closing of the abdominal DT, with a daily open drainage time of about 2 h.
Categorical variable analysis was performed using the χ2 test or Fisher’s exact test, and continuous variables were compared using Student’s t test. We used univariate logistic regression models to evaluate the risk factors of delayed DT removal in GC patients with postoperative CA, and a Cox regression model was used to perform multivariable analysis to calculate relative risk. All values were categorized into groups according to medians. All results were considered clinically significant at a P value < 0.05. Statistical analyses were performed using SPSS software version 19.0.
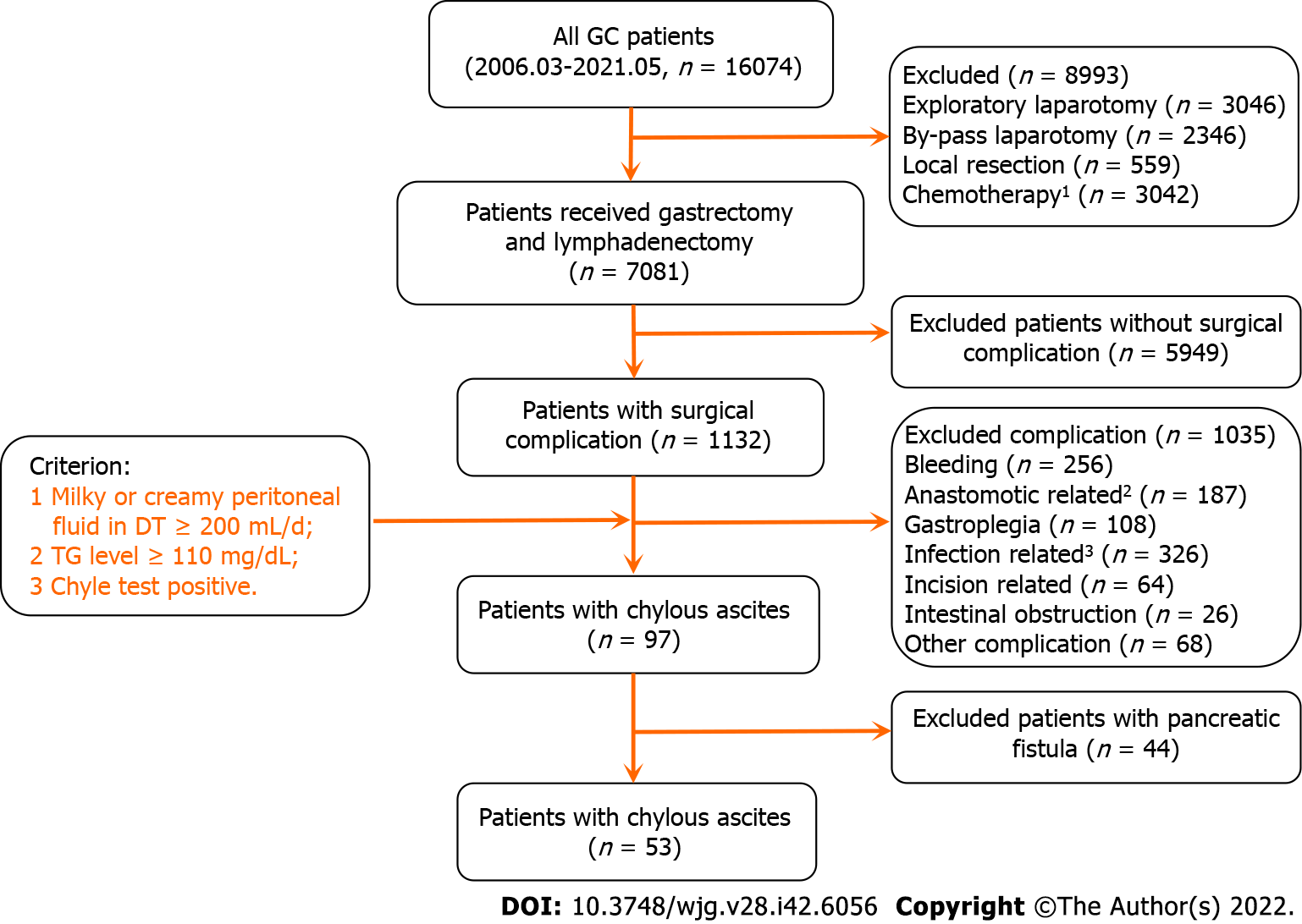
Between 1 March 2006 and 31 May 2021, 16074 GC patients were hospitalized in our department and 7081 patients underwent gastrectomy and lymphadenectomy. Of these patients, 53 underwent surgical resection for GC and developed CA. The main characteristics and patient selection are shown in Table 1 and Figure 1. The patients had an average age of 61.0 ± 11.3 years, a high ratio of male and advanced stage of disease (Male vs female: 77.40% vs 22.60%, early stage vs advanced stage: 39.6% vs 60.4%), 51 underwent radical surgery, 43 underwent D2 lymph node dissection, and 13 were discharged with DT. The average oral feeding and CA onset times after surgery were 3.8 and 7.5 d, respectively. The average durations of DT drainage and postoperative hospitalization were 14.3 and 21.9 d, respectively.
| Characteristics | Cases |
| Age, yr | 61.0 ± 11.3 |
| Gender, n (%) | |
| Male | 41 (77.4) |
| Female | 12 (22.6) |
| Tumor location, n (%) | |
| Upper | 18 (40.0) |
| Middle | 11 (20.6) |
| Bottom | 24 (45.3) |
| AJCC 8th stage, n (%) | |
| I | 21 (39.6) |
| II | 11 (20.8) |
| III | 18 (34.0) |
| IV | 3 (5.7) |
| Type of surgery, n (%) | |
| Radical | 51 (96.2) |
| Non-radical | 2 (3.8) |
| LN dissection, n (%) | |
| D1 | 8 (15.1) |
| D2 | 43 (81.1) |
| D3 | 2 (3.8) |
| Discharged without DT, n (%) | |
| Yes | 40 (75.5) |
| No | 13 (24.5) |
| Postoperative time of oral feeding (d) | 3.8 ± 1.0 |
| Postoperative time of CA appearance (d) | 7.5 ± 2.4 |
| DT removal duration (d) | 14.3 ± 12.6 |
| Postoperative hospitalization duration (d) | 21.9 ± 11.1 |
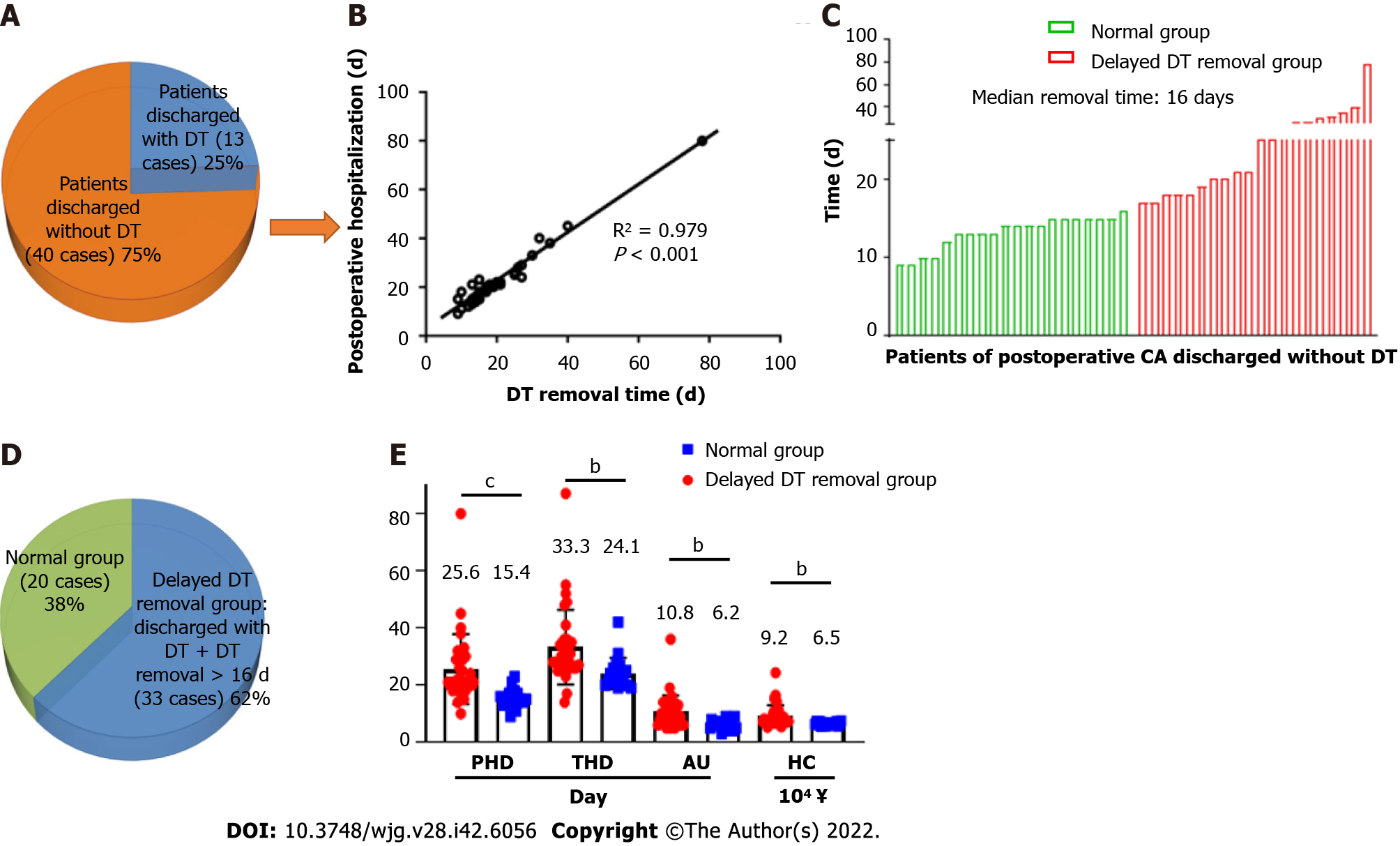
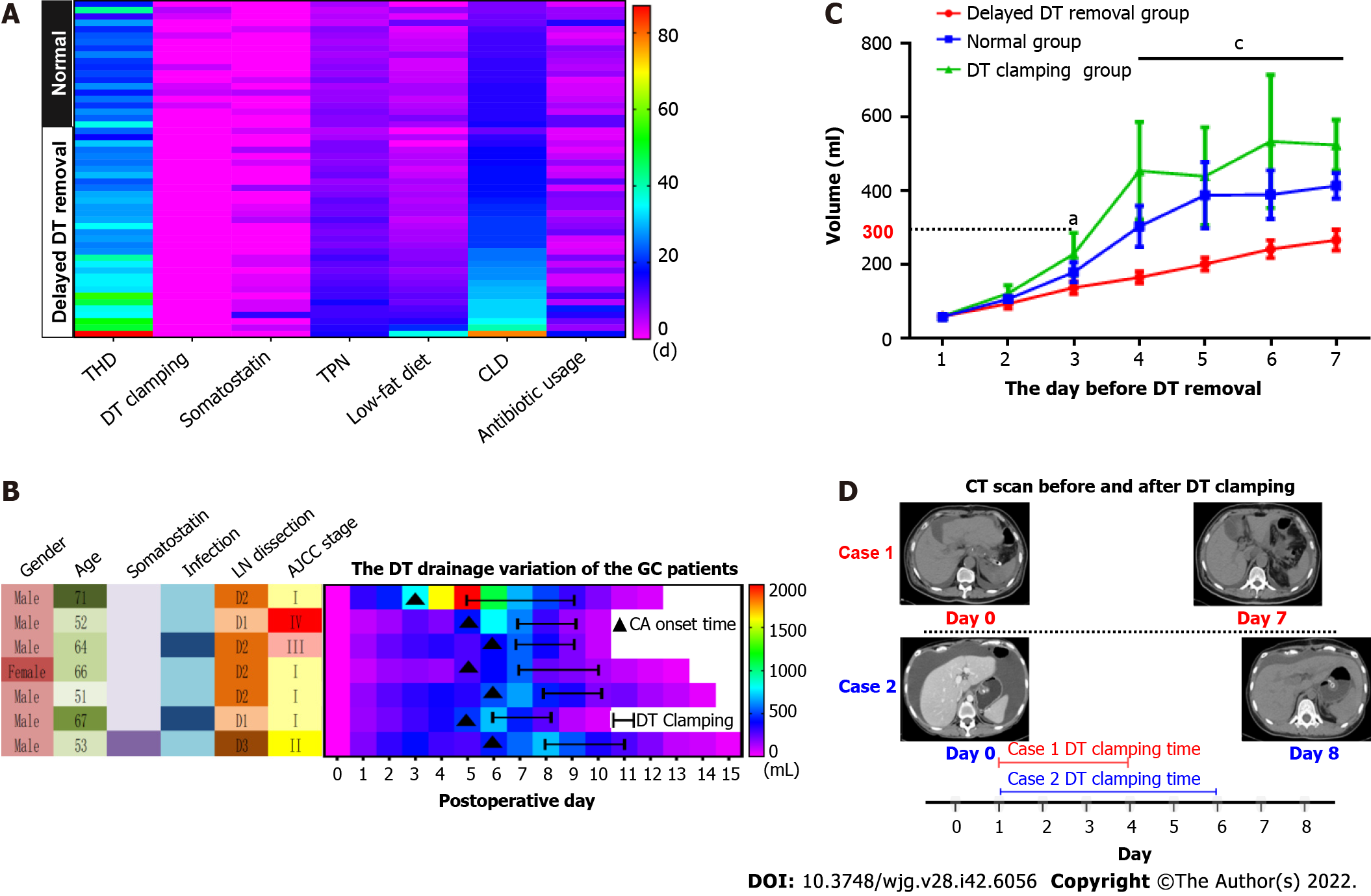
In our data, 40 patients (75%) had their DTs removed during the hospitalization period, and 13 patients (25%) were discharged with DT (Figure 2A). As shown in Figure 2B and Supplementary Figure 1, both postoperative (R2 = 0.979, P < 0.001) and total hospitalization time (R2 = 0.791, P < 0.001) had a significant positive association with DT removal time. Moreover, the median postoperative DT removal time of the patients discharged with or without DT was 30 and 16 d, respectively (Figure 2C and Supplementary Figure 2). We defined the patients’ DT removal time > the median time (16 d) or the patients discharged with DT as delayed DT removal, and the patients were categorized into either the delayed DT removal or normal group (Figure 2D). Comparing the delayed and normal groups, delayed DT removal significantly extended the total and postoperative hospitalization times, duration of antibiotic usage, and hospitalization costs in the GC patients (postoperative hospitalization duration: 25.8 d vs 15.5 d, P < 0.001; total hospitalization duration: 33.2 d vs 24.7 d, P < 0.01; antibiotic usage: 10.8 d vs 6.2 d, P < 0.01; hospitalization cost: ¥9.2 × 104vs ¥6.5 × 104, P < 0.01) (Figure 2E).
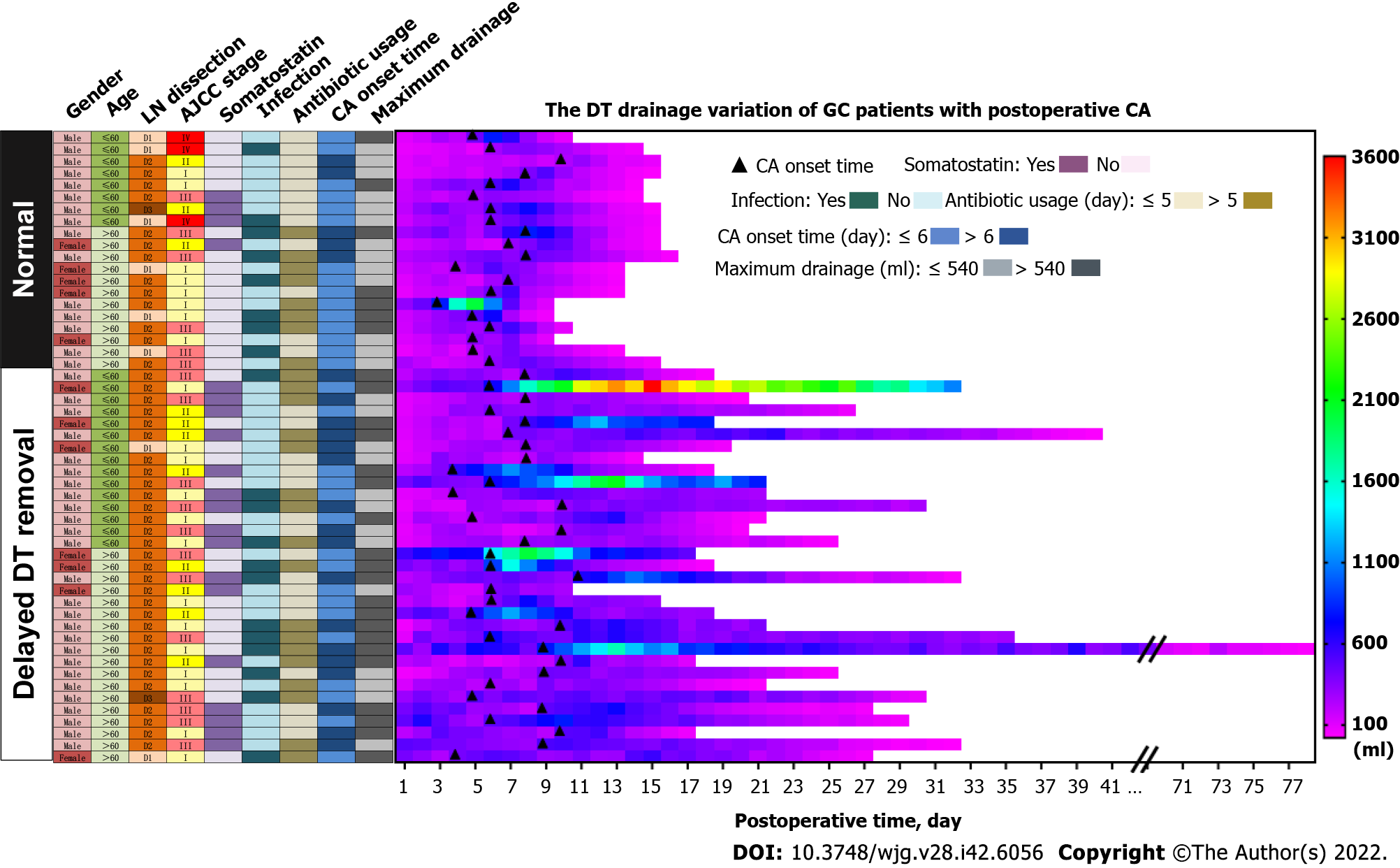
We present the characteristic differences between the normal and delayed DT removal groups in Figure 3, Table 2 and Supplementary Table 1. First, we evaluated the clinical characteristics and detected that there were no differences between the two groups regarding gender, age, tumor size or location, lymphadenectomy, and AJCC stage. Second, the treatment-related features were further explored. Of note, the patients in the normal group were more likely to undergo DT clamping than the delayed DT removal group (35.0% vs 0%, P < 0.001). In addition, compared with the patients in the delayed group, a shorter duration of low-fat diet were slightly shared in the normal group patients (40.0% vs 63.6%, P = 0.082). Third, we estimated the DT drainage variation between the two groups. Obviously, the delayed DT removal group generally had a longer duration of DT drainage than the normal group; however, the CA onset time and maximum drainage volumes were not significantly different between the two groups.
| Subgroup | No. of patients | |
| Normal (n = 20) | Delayed DT removal (n = 33) | |
| Clamp DT | ||
| Yes | 7 | 0 |
| No | 13 | 0 |
| Preoperative HGB, g/L | ||
| ≤ 130 | 12 | 15 |
| > 130 | 8 | 18 |
| Preoperative ALB, g/L | ||
| ≤ 41 | 9 | 17 |
| > 41 | 11 | 16 |
| Maximum drainage, mL | ||
| ≤ 540 | 13 | 13 |
| > 540 | 7 | 20 |
| Postoperative intake1, d | ||
| ≤ 3 | 10 | 17 |
| > 3 | 10 | 16 |
| CA onset time, d | ||
| ≤ 7 | 15 | 16 |
| > 7 | 5 | 17 |
| Antibiotic usage, d | ||
| ≤ 5 | 12 | 16 |
| > 5 | 8 | 17 |
| Postoperative infection | ||
| Yes | 8 | 12 |
| No | 12 | 21 |
| AJCC stage | ||
| Early | 8 | 13 |
| Advanced | 12 | 20 |
| LN dissection | ||
| D1 | 6 | 2 |
| D2+ | 14 | 31 |
| Age, yr | ||
| ≤ 60 | 8 | 15 |
| > 60 | 12 | 18 |
| Gender | ||
| Male | 15 | 26 |
| Female | 5 | 7 |
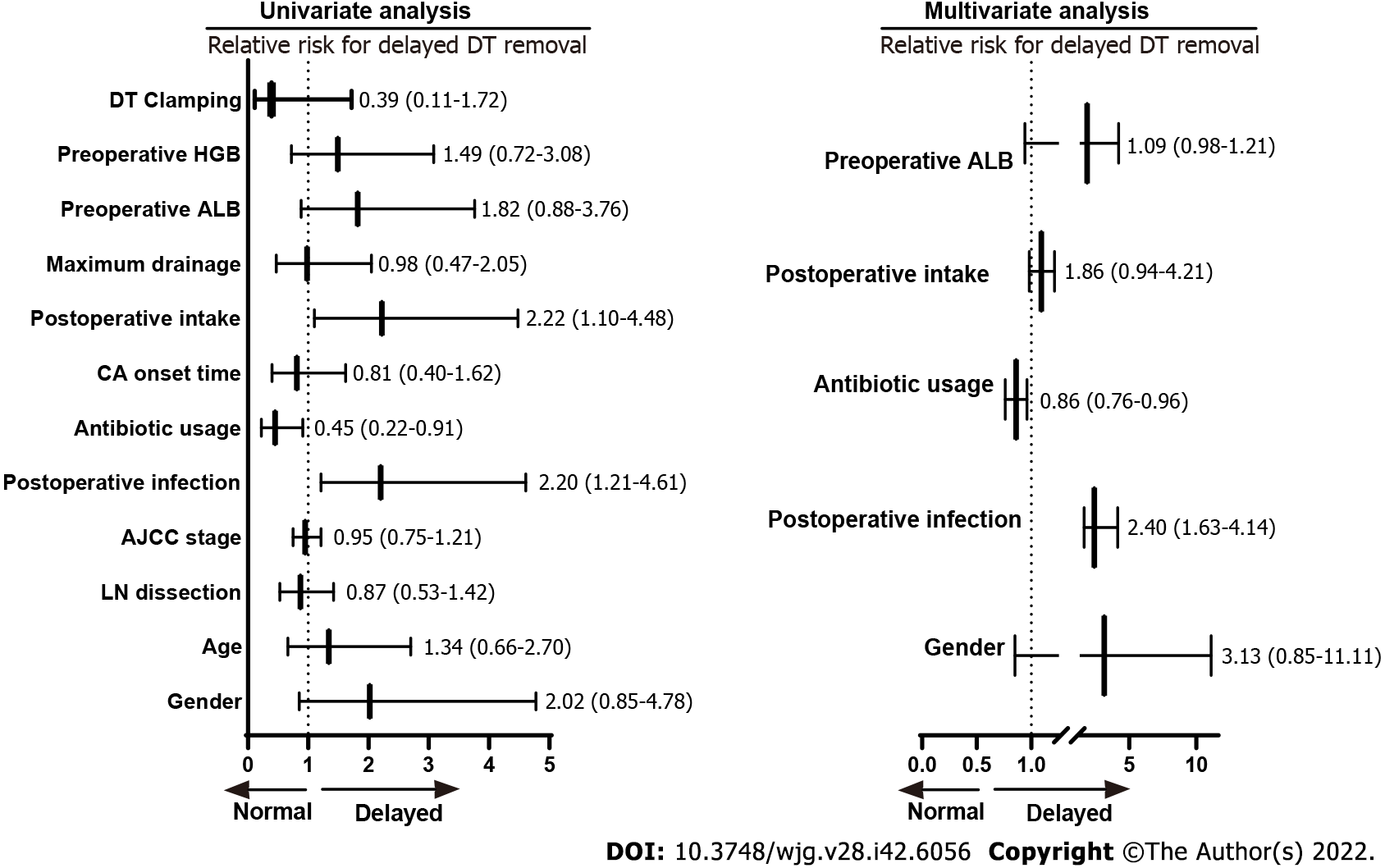
As shown in Table 3 and Figure 4, the univariate analysis revealed that early postoperative intake (RR: 2.22, 1.10–4.48, P = 0.031), postoperative infection (RR: 2.20, 1.21-4.61, P = 0.003), and antibiotic usage (RR: 0.45, 0.22–0.91, P = 0.009) were significantly associated with delayed DT removal in GC patients with CA. However, the baseline characteristics (age, gender, and AJCC stage), lymph node dissection, CA onset time, maximum drainage volume, postoperative albumin, postoperative hemoglobin, and DT clamping were not significantly associated with delayed DT removal (all P > 0.05). Furthermore, multivariate analysis demonstrated that postoperative infection (HR: 2.40, 1.63-4.14, P = 0.007) and antibiotic usage (HR: 0.86, 0.76-0.96, P = 0.009) were independent factors that influenced delayed DT removal in GC patients with postoperative CA.
| Univariate analysis | Multivariate analysis | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| DT clamping | 0.39 (0.11-1.72) | 0.281 | - | - |
| Postoperative HGB | 1.49 (0.72-3.08) | 0.283 | - | - |
| Postoperative ALB | 1.82 (0.88-3.76) | 0.303 | 1.09 (0.98-1.21) | 0.127 |
| Maximum drainage | 0.98 (0.47-2.05) | 0.367 | - | - |
| Postoperative intake time | 2.22 (1.10-4.48) | 0.031 | 1.86 (0.94-4.21) | 0.234 |
| CA onset time | 0.81 (0.40-1.62) | 0.486 | - | - |
| Duration of antibiotic usage | 0.45 (0.22-0.91) | 0.009 | 0.86 (0.76-0.96) | 0.009 |
| Postoperative infection | 2.20 (1.21-4.61) | 0.003 | 2.40 (1.63-4.14) | 0.007 |
| AJCC Stage | 0.95 (0.75-1.21) | 0.676 | - | - |
| LN dissection | 0.87 (0.53-1.42) | 0.595 | - | - |
| Age | 1.34 (0.66-2.70) | 0.471 | - | - |
| Gender | 2.02 (0.85-4.78) | 0.141 | 3.13 (0.85-11.1) | 0.187 |
In Figure 5A, we describe comprehensive treatment for GC patients with postoperative CA; the the
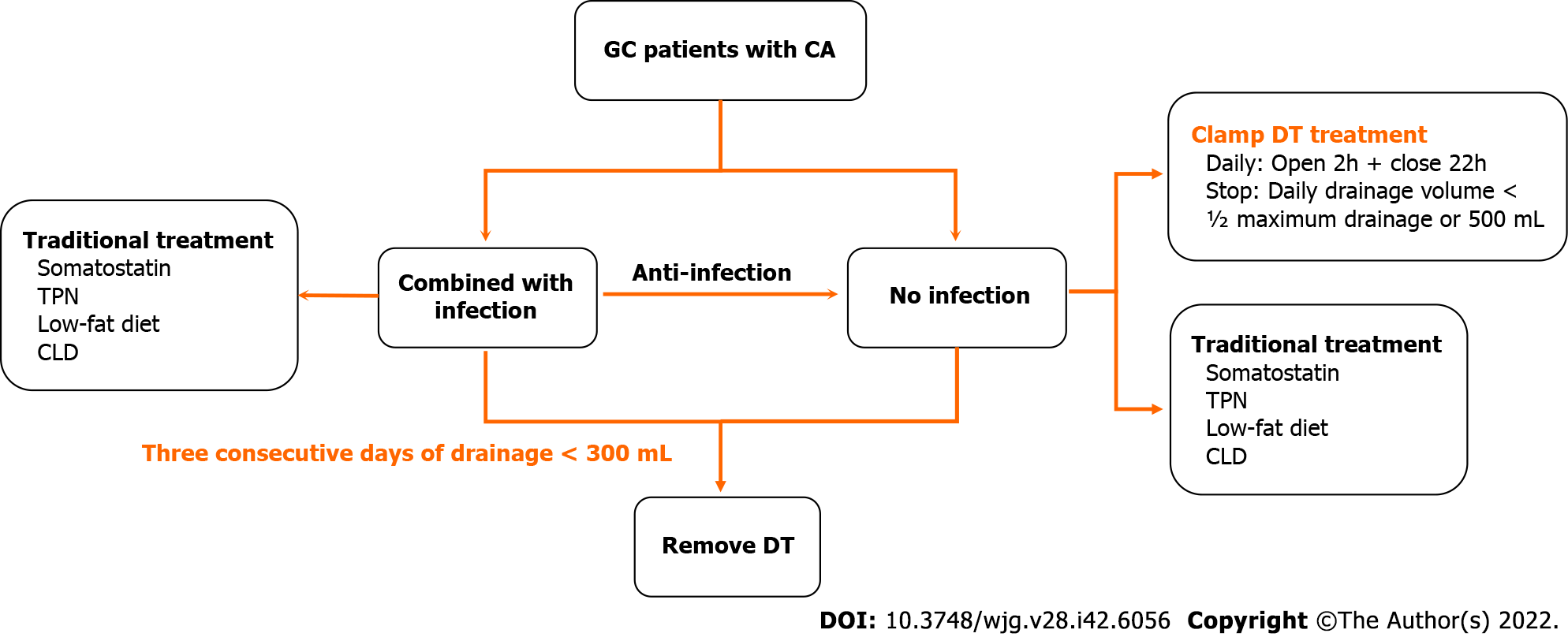
As the results mentioned above, we subsequently summarized the experiences of the GC patients with postoperative CA treatment in our department (Figure 6). First, the CA patients were divided into two sub-groups according to their postoperative infection status. Second, in the patients with infection, based on traditional treatments, antibiotic therapy was a vital supplement. Third, in the patients without infection, DT clamping was a viable option. Finally, for patients with 3 consecutive days of drainage less than 300 mL, DT removal might be the appropriate management.
In this study, we retrospectively analyzed 53 cases of GC with postoperative CA at the FUSCC. Our results indicated that hospitalization duration was closely associated with DT removal time. Furthermore, postoperative infection and antibiotic usage were important independent factors that influenced delayed DT removal in GC patients with postoperative CA. Our study also implied that DT clamping was an appropriate method of postoperative CA treatment for patients without postoperative infection. More importantly, appropriate and lenient indications for DT removal can significantly reduce the duration of hospitalization.
In most of the GC patients, postoperative CA cannot be discharged at a routine time and have a significant impact on subsequent adjuvant treatment[6,7]. Normally, patients are discharged within 7 d after undergoing GC surgery and most start adjuvant treatment within 30 d at our center. However, in the 53 patients with postoperative CA in our study, the average postoperative hospitalization duration was 21.9 d, and 8 patients’ postoperative hospital stays were longer than 30 d. As previously reported, lymphadenectomy was a key influencing factor in GC patients with postoperative CA[5,9]. As shown in Supplementary Table 3, the clinical characteristics of patients with CA tended to be consistent among those who underwent variety of lymphadenectomy, similar with previous studies that CA was found to be a rare complication even for gastric carcinoma patients undergoing D3 dissection[5,17].
Our results clearly indicated that hospitalization duration is mainly dependent on the time of DT removal in GC patients with CA. A multi-center prospective study recommended the criterion for DT removal be drainage flow between 500 and 1000 mL/d[6]. In fact, we previous performed relatively rigorous standards for DT removal in the patients with CA. Usually, while the volume of drainage less than 100 mL/d, the DT removal will be truly considered. Although all the patients’ DTs were removed until the flow volume less than 300 mL/d, and the delayed removal group preferred to perform a significantly high criterion. Therefore, we have a sufficient reason to conclude that, after excluding the influence of postoperative infection, early DT removal is a better choice in GC patients with CA. Moreover, our study found that postoperative infection and antibiotic usage were vital independent factors that influenced delayed DT removal in patients with CA, and clearly clarified anti-infection were an effective supplemental therapy for conservative treatment of postoperative CA. Similarly, Lu et al[7] reported the patients with CA had a certain higher level of postoperative white blood cell counts than the other patients in GC.
Previously, the primary treatment for CA was conservative therapy, which mainly involved TPN, continuous low-pressure drainage, somatostatin, and a low-fat diet[18]. Recently, DT clamping has been presented as a potential alternative for patients with CA in other malignancies[19]. In this study, the DTs of 7 patients were clamped until the daily drainage was less than 500 mL/d (or ½ the maximum drainage). After DT clamping, the flow amount was significantly reduced, and the patients were successfully discharged without DT. For the reason of clamping DT facilitates DT removal, previous research has demonstrated absorption and lymphatic drainage increase along with the interstitial hydrostatic pressure[20]. Furthermore, DT clamping could help to evaluate the feasibility of DT removal by conveniently simulating the removal and conversion back to drainage[21]. Several studies have suggested DT clamping as an important alternative, and the detailed suggestion was daily drainage ranging from 1000 to 1500 mL[6,22]. However, a consensus on the threshold of drainage volume for DT clamping has not yet been reached. Therefore, determination of an appropriate criterion for DT removal and DT clamping is urgently needed for GC patients with postoperative CA.
There are certain limitations to our study. First, due to the retrospective study design, it was difficult to individually balance a variety of influencing factors; thus, various biases were unavoidable. Second, despite routine chyle test and TG were measured, the definition of CA is slightly less rigorous. In particular, CA with co-infection cannot fully rule-out the influence of pancreatic and anastomotic leakage, and other infection-related complications. In addition, small-volume CA (i.e., daily drainage volume ranging from 30 to 200 mL) was not considered in this study.
In conclusion, postoperative infection and antibiotic usage were vital independent factors that influenced delayed DT removal in GC patients with CA. Appropriate and lenient standards for DT removal can significantly reduce the duration of hospitalization. Furthermore, DT clamping might be a recommend alternative for conservative treatment of postoperative CA.
Chylous ascites (CA) is relatively common postoperative complication in patients undergoing received gastric cancer (GC) surgery that obviously prolongs hospitalization and has a major impacts on subsequent adjuvant treatments.
Drainage tube (DT) clamping has been presented as a potential alternative conservative treatment for GC patients with CA.
This study aimed to explore key factors influencing CA treatment and recommend novel conservative treatment strategies for postoperative CA in GC patients.
Data from patients with CA after GC surgery performed at the Fudan University Shanghai Cancer Center between 2006 and 2021 were retrospectively evaluated. Patients were classified into two distinct groups with respect to DT removal time. We further explored the differences in clinical-pathological features of the different DT removal groups.
Fifty-three patients underwent surgery for GC and experienced postoperative CA during the study period. Postoperative hospitalization and DT removal time showed a significant positive association (R2 = 0.979, P < 0.001), while delayed DT removal significantly extended total and postoperative hospitalization times, antibiotic usage duration, and hospitalization cost. In addition, postoperative infection and antibiotic usage were independent factors for delayed DT removal.
Postoperative infection and antibiotic usage were vital independent factors that influenced delayed DT removal in GC patients with CA. Appropriate and lenient standards for DT removal may significantly reduce the duration of hospitalization.
DT clamping could be recommended as an alternative for conservative treatment of postoperative CA.
We thank Dr. Xuan Li for statistical advising and review of the manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Food Science and technology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aoki H, Japan; Ilhan E, Turkey S-Editor: Gong ZM L-Editor: A P-Editor: Yuan YY
| 1. | Cárdenas A, Chopra S. Chylous ascites. Am J Gastroenterol. 2002;97:1896-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 195] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Huang Q, Jiang ZW, Jiang J, Li N, Li JS. Chylous ascites: treated with total parenteral nutrition and somatostatin. World J Gastroenterol. 2004;10:2588-2591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70:145-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2268] [Cited by in RCA: 3272] [Article Influence: 654.4] [Reference Citation Analysis (2)] |
| 4. | Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, Okajima K; Japan Clinical Oncology Group. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 754] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 5. | Yol S, Bostanci EB, Ozogul Y, Ulas M, Akoglu M. A rare complication of D3 dissection for gastric carcinoma: chyloperitoneum. Gastric Cancer. 2005;8:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Ilhan E, Demir U, Alemdar A, Ureyen O, Eryavuz Y, Mihmanli M. Management of high-output chylous ascites after D2-lymphadenectomy in patients with gastric cancer: a multi-center study. J Gastrointest Oncol. 2016;7:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Lu J, Wei ZQ, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M. Small-volume chylous ascites after laparoscopic radical gastrectomy for gastric cancer: results from a large population-based sample. World J Gastroenterol. 2015;21:2425-2432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Pabst TS 3rd, McIntyre KE Jr, Schilling JD, Hunter GC, Bernhard VM. Management of chyloperitoneum after abdominal aortic surgery. Am J Surg. 1993;166:194-8; discussion 198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Barchi LC, Charruf AZ, de Oliveira RJ, Jacob CE, Cecconello I, Zilberstein B. Management of postoperative complications of lymphadenectomy. Transl Gastroenterol Hepatol. 2016;1:92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Bhardwaj R, Vaziri H, Gautam A, Ballesteros E, Karimeddini D, Wu GY. Chylous Ascites: A Review of Pathogenesis, Diagnosis and Treatment. J Clin Transl Hepatol. 2018;6:105-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 11. | Aalami OO, Allen DB, Organ CH Jr. Chylous ascites: a collective review. Surgery. 2000;128:761-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 279] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | Lizaola B, Bonder A, Trivedi HD, Tapper EB, Cardenas A. Review article: the diagnostic approach and current management of chylous ascites. Aliment Pharmacol Ther. 2017;46:816-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 13. | Lopez-Gutierrez JC, Tovar JA. Chylothorax and chylous ascites: management and pitfalls. Semin Pediatr Surg. 2014;23:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Shibuya Y, Asano K, Hayasaka A, Shima T, Akagi K, Ozawa N, Wada Y. A novel therapeutic strategy for chylous ascites after gynecological cancer surgery: a continuous low-pressure drainage system. Arch Gynecol Obstet. 2013;287:1005-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Kim J, Won JH. Percutaneous Treatment of Chylous Ascites. Tech Vasc Interv Radiol. 2016;19:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Kaas R, Rustman LD, Zoetmulder FA. Chylous ascites after oncological abdominal surgery: incidence and treatment. Eur J Surg Oncol. 2001;27:187-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Kim DW, Kim MH, Kim CG. Lymphoscintigraphy revealed chyloperitoneum after gastrectomy for gastric cancer. Clin Nucl Med. 2015;40:41-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Adler E, Bloyd C, Wlodarczyk S. Chylous Ascites. J Gen Intern Med. 2020;35:1586-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Thiel FC, Parvanta P, Hein A, Mehlhorn G, Lux MP, Renner SP, Preisner A, Beckmann MW, Schrauder MG. Chylous ascites after lymphadenectomy for gynecological malignancies. J Surg Oncol. 2016;114:613-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 1997;10:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 160] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Yan S, Wang X, Wang Y, Lv C, Wang J, Yang Y, Wu N. Intermittent chest tube clamping may shorten chest tube drainage and postoperative hospital stay after lung cancer surgery: a propensity score matching analysis. J Thorac Dis. 2017;9:5061-5067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Scaletta G, Quagliozzi L, Cianci S, Vargiu V, Mele MC, Scambia G, Fagotti A. Management of postoperative chylous ascites after surgery for ovarian cancer: a single-institution experience. Updates Surg. 2019;71:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |