Published online Nov 14, 2022. doi: 10.3748/wjg.v28.i42.6017
Peer-review started: September 12, 2022
First decision: October 19, 2022
Revised: October 24, 2022
Accepted: October 27, 2022
Article in press: October 27, 2022
Published online: November 14, 2022
Processing time: 59 Days and 1.2 Hours
Liver injury is an increasingly recognized extra-pulmonary manifestation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Corona
Core Tip: Liver injury is an increasingly recognized extra-pulmonary manifestation of coronavirus disease 2019 (COVID-19). COVID-19 associated liver injury (COVALI) is a clinical syndrome encompassing all patients with COVID-19 infection and biochemical liver injury. Unfortunately, most information on COVALI is derived from the general population and may not be applicable to individuals under-represented in research, including pregnant individuals. In this review we summarize clinical features of COVALI and the leading theories of pathophysiology and present existing literature on COVALI during pregnancy, a topic not widely explored in the literature.
- Citation: Cooper KM, Colletta A, Asirwatham AM, Moore Simas TA, Devuni D. COVID-19 associated liver injury: A general review with special consideration of pregnancy and obstetric outcomes. World J Gastroenterol 2022; 28(42): 6017-6033
- URL: https://www.wjgnet.com/1007-9327/full/v28/i42/6017.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i42.6017
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) disease pandemic (COVID-19) is responsible for and upwards of 6.3 million fatalities worldwide[1]. The SARS-CoV-2 virus is a member of the Coronaviridae family, a diverse family of single-stranded positive RNA viruses[2]. Coronaviruses are frequently implicated in mild upper respiratory infections and cause 15%-30% of cases of the “common cold”[3,4]. However, Coronaviridae viruses have also demonstrated an ability to infect the lower respiratory tract and cause severe lung disease associated with substantial mortality[5,6].
Mortality associated with COVID-19 is usually secondary to lung pathology that causes severe respiratory distress syndrome[7-9]. However, patients infected with SARS-CoV-2 often suffer other devastating end-organ injuries[10], suggesting the virus causes systemic infection and inflammation. These observations have prompted interest in the extra-pulmonary manifestations of COVID-19[11], including those in the heart[12,13], intestines[14,15], kidney[16], reproductive system[17,18], and the liver, where the effect of SARS-CoV-2 is poorly understood[19,20].
COVID-19 associated liver injury (COVALI) is a clinical entity encompassing any abnormal liver function test present in individuals positive for SARS-CoV-2[20]. Currently there are no specific or unique diagnostic criteria for COVALI relative to other causes of transaminitis[21] which complicates the process of synthesizing evidence from clinical studies. This is most salient when applying available data to those underrepresented in the literature, such as pregnant and birthing persons.
In the first section of this review, we will summarize clinical features of COVALI and the leading theories on the mechanism of liver damage in the general population. In the second section, we present existing literature on liver injury in SARS-CoV-2 positive pregnant persons, a topic not widely explored in the literature despite significant clinical relevance. Ultimately, we aim to synthesize data on COVALI in the general and perinatal populations and offer perspective on approaching this problem in the obstetric setting.
At the present time, COVALI is an umbrella term that applies to all patients with SARS-CoV-2 infection and transaminitis. Meta-analyses estimate that one in four patients with COVID-19 develop acute liver injury[22-24], but this figure is variable across studies and ranges from 14%-74%[25-27]. There seem to be no demographic factors to account for this variability, which may ultimately be due to differences in the study timeline or definition of liver injury[28]. Interestingly, only one of the three cited meta-analyses on COVALI included a study involving pregnant patients (n = 9)[29]. In the following sections we will review clinical and pathophysiologic considerations for COVALI in the general population.
COVID-19 associated liver injury is a hepatocellular or mixed pattern liver injury with aspartate aminotransferase (AST) predominant transaminitis[28,30-33]. Most studies report mild liver injury with liver enzymes that peak at values less than five times the upper limit of normal[34-38]. Conversely, some reports suggest up to 25% of patients’ aminotransaminases exceed this threshold[39,40] and there is mounting evidence that liver enzymes can increase to the thousands (U/L) in patients with severe COVID-19[26,38,41-45]. The timeline of developing liver injury is not fully elucidated and has varied between studies[32,33].
Non-transaminase laboratory evidence of liver damage has also been identified, but is reported less consistently in the literature. Specifically, total bilirubin and alkaline phosphatase have been reported to be elevated in 1%-53%[46-48] and 0.3%-80.0%[48,49] of patients, respectively. This variability may be due to study timeline relative to the temporal course of laboratory changes in patients with COVALI. For example, it has been shown that alkaline phosphatase elevations begin and peak later in the disease course than aminotransaminases and may not be captured by studies that don’t follow laboratory data for extended periods[50,51].
The interest in COVALI is rooted in its association with disease severity and negative patient outcomes. First, patients with elevated liver enzymes at presentation or at hospital admission are more likely to develop severe COVID-19 lung disease[5,52-54]. Additionally, a large study by Guan et al[55] reported laboratory data from patients at over 500 hospitals and found patients with severe COVID-19 were more likely to have transaminitis compared to patients with non-severe COVID-19. Going further, Bloom et al[31] studied trends in aminotransaminase levels from time of admission to peak in patients hospitalized for COVID-19 and found that in addition to higher mean AST and alanine aminotransferase (ALT), there was a greater change from baseline to peak transaminases in patients with severe compared to non-severe COVID-19. A small single center study found that elevated AST was observed more often in patients who required intensive level care compared to those who did not require intensive care[56]. Further, in a cohort of 1611 hospitalized patients across 11 Latin American countries, abnormal liver enzymes conferred a 2.6-fold risk for severe COVID-19 pneumonia and a 1.5-fold risk of death[37].
Within the umbrella of COVALI, AST has been shown to have specific prognostic value[29,37,57]. For example, the numeric value for serum AST has been incorporated in clinical calculators created to predict progression from mild or moderate to severe COVID-19 disease[57]. Moreover, elevated AST has been found to be independently associated with increased risk of death, apart from other markers of hepatic dysfunction[29,34,50,58,59]. In a study including 206 patients across 26 institutions in Brazil, AST level greater than twice the upper limit of normal significantly increased the risk of in-hospital mortality when adjusted for age and biologic sex[29]. However, is important to note that when elevated, bilirubin may be a stronger predictor of death than AST in some cohorts[34].
Given the association with poor patient outcomes, identifying potential risk factors for COVALI is imperative. We found one meta-analysis that sought to define predictors for the development of COVALI. In this study Harapan et al[60] pooled data from 16 studies (n = 6253) to assess whether any of the following were associated with development of severe liver injury in patients infected with SARS-CoV-2: Age, biologic sex, body mass index (BMI), diabetes mellitus, coronary artery disease, hypertension, underling liver disease, white blood cell count, lymphocyte count, and neutrophil count. They observed significant association between male sex, higher BMI, presence underlying liver disease, elevated white blood cell, and elevated lymphocyte counts with development of acute liver injury. After controlling for bias introduced by the meta-analysis, they concluded male sex and lymphocyte count were found to be independent risk factors for COVALI[60]. Not evaluated in this meta-analysis, inflammatory markers have also been shown to be a risk factor associated with liver injury[61-64]. For example, multiple studies have inflammatory markers directly correlate with liver enzymes[64] and that liver injury can be predicted using inflammatory markers such as ferritin and C-reactive protein[61,62].
Professional societies recommend clinically relevant work up for other causes of liver injury in patients who develop COVALI[21,65-68]. The American and Asian Pacific Association(s) for the Study of Liver Diseases suggest ruling out other causes of viral and toxin-mediated hepatitis in all COVID-19 patients with liver injury[21,66]. More nuanced suggestions include considering cytokine-syndrome, myositis, or cardiac injury in patients with disproportionally elevated AST, and primary sclerosing cholangitis in critically ill patients with cholestatic liver injury[21,65,66]. They advise trending liver enzymes of patients hospitalized with COVID-19, those with known chronic liver disease diagnosed with COVID-19 and of those receiving anti-retroviral medications for treatment of COVID-19 pneumonia[66]. Patients with chronic hepatitis B may be at particularly high risk both due to risk of severe infection and viral reactivation when receiving immunosuppressive therapy[66,69]. However, they do not recommend changing management and offer no specific intervention for liver injury in most cases of COVALI. They endorse targeting the viral illness in the acute setting is sufficient for liver injury and encourage work up for chronic liver disease when illness is resolved.
The underlying mechanism(s) of liver injury in COVID-19 are not fully understood. While there is increasing literature on this topic, the absence of explicit diagnostic criteria has resulted in heterogeneity in clinical studies and has impeded recognition of specific mechanisms of injury. There is consensus that COVALI is likely multifactorial and due to a combination of exacerbation of underlying liver disease, direct cytotoxicity, hypoxic liver injury, drug induced injury and systemic inflammation with immune dysregulation[28,70].
Early theories focused on exacerbation of underlying liver disease and toxicity from pharmacologic agents used to treat severe COVID-19 infection as a sources of liver injury. It is true that patients with cirrhosis are at risk for developing severe pneumonia and hepatic decompensation during SARS-CoV-2 infection[21]. Likewise, some antiviral medications used to treat COVID-19 have hepatotoxic properties and have been associated with abnormal liver function during the pandemic (e.g., lopinavir/ritonavir)[27,40,61,71]. In a combination of these, SARS-CoV2 infection treated with corticosteroids or tocilizumab has been showed facilitate reactivation and accelerate liver injury in patients with chronic hepatitis B[72]. However, these two factors are unable to explain most of this phenomena as: (1) Over 90% of patients with COVALI have no evidence of underlying liver disease; and (2) transaminitis is often present at baseline prior to administration of medications[73]. While it is possible that liver injury during SARS-CoV-2 infection may be exacerbated by these factors, COVALI is likely a distinct clinical entity.
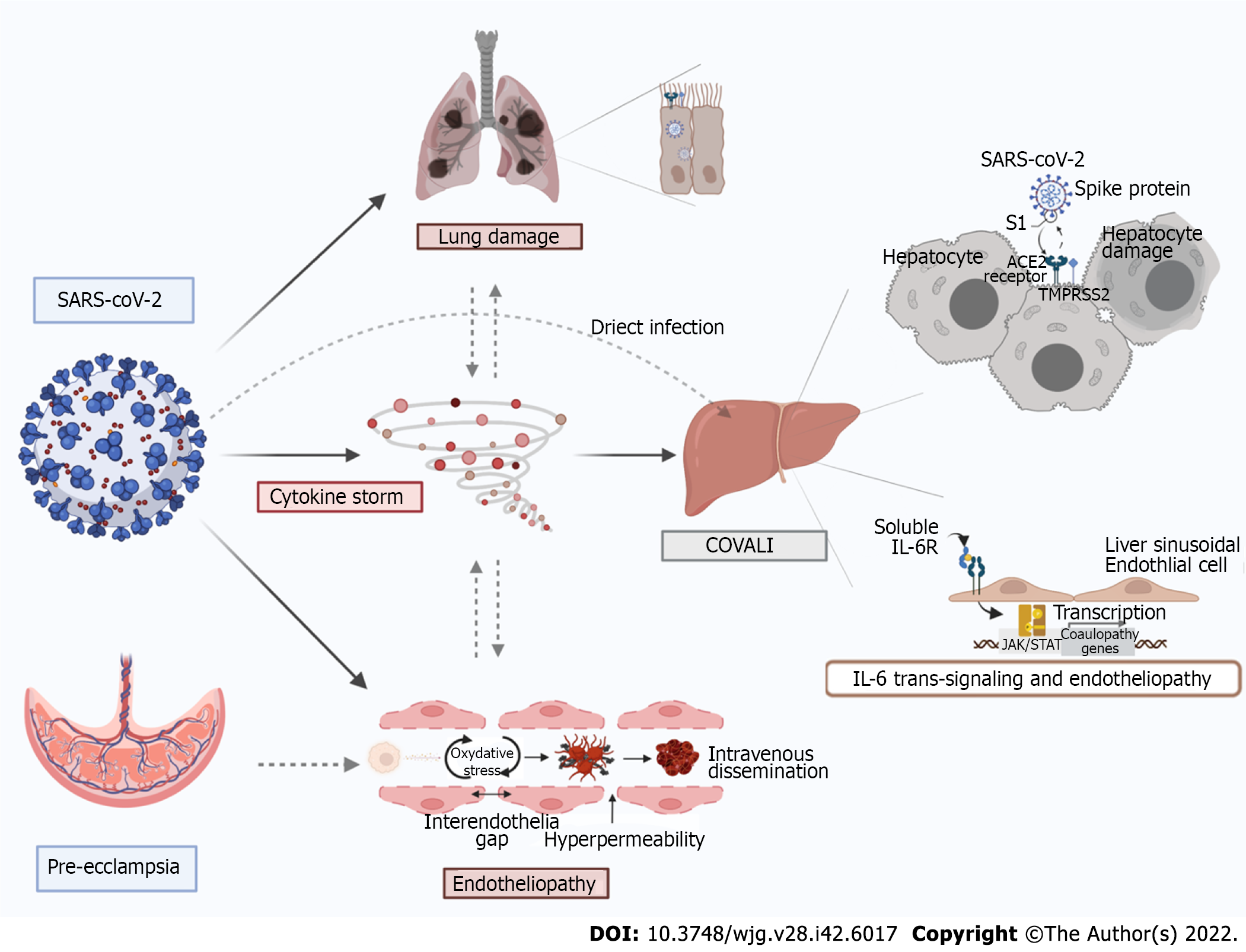
Diverse studies have demonstrated direct viral infection of the liver can occur during COVID-19 infection. In a study including 156 autopsy samples, postmortem hepatic tissue evaluation revealed typical coronavirus particles in hepatocyte cytoplasm with associated mitochondrial swelling and endoplasmic reticulum dilatation in patients who died with COVID-19[74]. Other reports have shown SARS-CoV-2 nuclear material in liver tissue, including RNA in hepatocytes of live patients who underwent liver biopsy during SARS-CoV-2 infection[75]. Some of the most convincing data comes from a recent paper by Wanner et al[76] who demonstrated SARS-CoV-2 can be detected in up to three-fourths of post-mortem liver biopsies using reverse transcriptase-polymerase chain reaction. Ultimately, there is irrefutable histologic evidence that SARS-CoV-2 directly infects hepatocytes, providing strong evidence that SARS-CoV-2-mediated cytopathy plays a role in COVALI. It is thought that angiotensin converting enzyme 2 and/or its receptor (ACE-2) may mediate cytopathy by enabling viral access to the liver[76,77]. However, the understanding of SARS-CoV-2 hepatotropism of is still evolving.
Epidemiology-based correlates support direct ACE-2 mediated entry into hepatocytes based on data that shows groups at increased risk of COVALI also have increased hepatic ACE-2 expression. For example, ACE-2 Levels are higher in males than females[78] and ACE-2 is upregulated in decom
Reduced blood oxygen, which can negatively affect the liver, occurs in up to 50% of patients with COVID-19 infection[84]. However, only a small percentage of patients have transaminitis to the degree expected in ischemic hepatitis[34-38]. While ischemia from low blood oxygen seems to have a limited direct role in COVALI pathophysiology, the relationship between hypoxia and inflammatory pathways is significant. Specifically, hypoxia can trigger and amplify immune dysregulation via inflammatory pathways mediated by hypoxia inducible factor and tumor necrosis factor[85]. This may explain the link between severity of lung disease with liver injury and provide support for and transition to the inflammatory hypotheses of COVALI[86].
There is substantial data suggesting systemic inflammation and associated immune dysregulation, endotheliopathy and thrombosis are central to the pathophysiology of COVALI[87,88]. It is well established that severe COVID-19 infection induces systemic inflammation and that concentrations of several clinically evaluated inflammatory markers are increased in patients with COVID-19, such as D-dimer, C-reactive protein, procalcitonin, ferritin, and interleukin-6 (IL-6)[89-92]. Inflammatory markers are also higher in COVID-19 positive patients with biochemical liver derangements compared to COVID-19 positive patients without such derangements across, suggesting a link between liver injury and inflammation[61,62,92-94]. For example, a large retrospective analysis (n = 800) showed patients with COVID-19 complicated by COVALI had higher levels of C-reactive protein, procalcitonin, D-dimer, and serum ferritin compared to patients without COVALI[61]. In a unique study, Diaz-Louzao et al[86] used joint regression modeling to evaluate the temporal relationship between increases in markers of liver injury and inflammation. They found that elevation of inflammatory markers precedes elevation of liver enzymes. Ultimately they created a statistical model that implicates inflammation in causation of liver injury. The specific inflammatory markers increased during COVALI are known to be involved in in vivo endotheliopathy and hypercoagulability[95,96], as has been visualized in hepatic tissue of patients with liver injury secondary to COVID-19. Further, histologic findings of macrovesicular steatosis, mild acute hepatitis, portal inflammation and portal/sinusoidal thrombosis in hepatic tissue of patients who have direct viral infection of the liver support that even with direct cytopathy, inflammation may have a preceding role[83,97-100].
Interleukin-6 is an inflammatory cytokine associated with endotheliopathy and a hallmark indicator of severe COVID-19. It has been shown that IL-6 can activate platelets and precipitate endothelitis in multiple organs during systemic COVID-19 infection, particularly those with a predilection for intravascular clot formation (e.g., the liver)[95]. Due to its association with biochemical liver injury[85,101-103] and known function[85,101,102], IL-6 has received interest as a likely active contributor to development of liver injury in COVID-19[103]. Recent work by McConnell et al[102] found a potential mechanism for this in that activating a soluble form of the IL-6 receptor triggers downstream pro-inflammatory and pro-coagulation pathways in the liver[102,104]. Further, that IL-6 signaling induces a hypercoagulable state in liver sinusoidal cells[85,104], which may contribute to the known endothelitis and thrombosis in hepatic tissue of patients with COVALI. Similarly, increased staining of a well-known platelet marker (CD-61) has been identified within dilated sinusoids in COVID-19 patients with elevated liver enzymes, suggesting activated platelets and endotheliopathy are critical in liver injury during COVID-19[85]. These findings are consistent with studies showing portal or sinusoidal vascular thrombosis is present in hepatic tissue of up to 50% of patients with COVID-19[83]. In context of literature on inflammatory markers in COVALI, Il-6 shows true mechanistic potential and bolsters the theory that inflammation, endotheliopathy and thrombosis are at the crux of this clinical syndrome.
Liver injury is a rare and potentially serious complication of pregnancy that is estimated to affect 3%-5% of birthing persons[105]. The differential diagnosis for hepatic dysfunction in this population includes specific pregnancy related (perinatal) liver diseases[106], such as pre-eclampsia/eclampsia, hemolysis elevated liver enzymes and low platelet (HELLP) syndrome, acute fatty liver of pregnancy and intrahepatic cholestasis of pregnancy (obstetric cholestasis), and non-pregnancy related liver diseases, such as auto-immune hepatitis, viral hepatitis, non-alcoholic steatohepatitis, and now COVALI[107-109]. Perinatal liver diseases are associated with significant mortality and often require prompt delivery of the fetus for safety of the mother (summarized in Table 1). Because liver injury can strongly influence decisions regarding delivery[107], COVALI during pregnancy is of serious clinical significance.
| COVID-19 | PEC/severe PEC | HELLP | ICHP | AFLP | |
| Epidemiology | - | 5.0%-7.5% | 1% | 0.3%-5.6% | 0.005%-0.010% |
| Symptoms | Respiratory +/- GI symptoms | Variable: Headache, swelling, vision changes (or none) | Variable: Headache, nausea, vomiting, RUQ pain (or none) | Pruritis; starting at palms + soles (can be diffuse) | Nausea, vomiting, abdominal pain |
| Pathophysiology of liver disease | SARS-CoV-2 infection and systemic inflammation | Inflammation and imbalanced endothelial activity | Thrombotic micro-angiopathy | Hormonal cholangiopathy | Mitochondrial dysfunction + fatty acid accumulation in hepatocytes |
| Increased transaminases | 13%-42%, 2-5 × ULN | Approximately 50%, > 2 × ULN | Typical, > 2 × ULN | Typical, > 2 × ULN | Always, < 10 × ULN |
| Jaundice | Rare (unclear%) | Rare | Rare (< 5%) | Uncommon (< 25%) | Mostly (> 70%) |
| Other findings | Radiographic lung disease | HTN, ↑ sFLT-1/PIGF | ↓ PLC; ↓ haptoglobin; ↑ LDH ↑ D-dimer | ↑ ALP; ↑ bile acids | Coagulopathy; hypoglycemia |
| Diagnosis | Viral antigen PCR or Nucleic acid amplification test (NAAT) | HTN ≥ 140/90 + organ dysfunction (proteinuria not required) | Tennessee or Mississippi classification | Bile acids (BA); > 10 umol/L | Swansea criteria; biopsy if unclear |
| Management | Anti-virals +/- Steroids Mono-clonal antibodies | HTN control; delivery if > 37 wk GA or > 34 wk if severe | Delivery after 34 wk GA | Ursodiol; delivery at 36 wk GA if BA > 100 or 36-39 wk if BA < 100 | Prompt delivery |
| Complications | ↑ Risk of post-partum hemorrhage; ↑ multi-systemic organ failure | ↑ Complications; mortality: 1%-5%; ↑ Neonatal respiratory distress + mortality | ↑ Complications Mortality: 1%-3% | ↑ Neonatal complications | ↑ Maternal + neonatal complications; morality: 20% (mother); 6%-77% (neonate) |
General clinical course: Clinical characteristics of COVID-19 during pregnancy given current knowledge are well represented in the literature, but there is limited data specific to the course of liver injury. In the obstetric setting, COVALI is an AST-predominant transaminitis that affects 13%-42% of COVID-19 positive pregnant patients[108,110-112]. While these statistics are comparable to the general population, a meta-analysis that included pregnant patients reported key differences. They found (1) higher prevalence of COVALI in pregnant patients compared to non-pregnant patients; and (2) more severely elevated liver enzymes in pregnant patients with COVALI compared to non-pregnant patients with COVALI[113]. This was confirmed in a study that directly compared laboratory values of COVID-19 positive pregnant patients with non-pregnant counterparts and found COVALI was more common in pregnancy[110]. The authors of this study cautioned that many of their observations were likely related to physiological changes of pregnancy, but they concluded the rate of COVID-19 positive pregnant individuals with acute liver injury was out of proportion to expected physiologic changes. This may indicate that COVID-19 confers an increased risk of liver injury specific to pregnancy.
Clinical Cases: Obstetric providers are tasked with differentiating liver disease that necessitates urgent delivery for the health and safety of the pregnant person vs that which can be managed expectantly and will be stable or resolve without delivery. Multiple reports illustrate this dilemma through cases of pregnant patients with acute liver injury who are COVID-19 positive and have concurrent features of high-risk perinatal liver diseases[114-120]. We identified seven cases and classified them according to the pattern in which liver enzymes improved throughout the clinical course: A, improved without delivery; B, improved with delivery; C, other (no improvement within 72 h of delivery, no timeline of COVID-19 symptoms) (Table 2). We will discuss cases that improved without delivery and highlight features that favored COVALI relative to perinatal liver diseases.
| Ref. | Patient | Case information | Laboratory data | Clinical course | |
| Anness and Siddiqui[114], 2020 | 35 y/o G2P1, GA 285w, PMH: IHCP | CC: Progressive dyspnea and cough; vitals: HR 133, RR 42, O2 96%; chest CT: Patchy peri-hilar inflammatory changes; differential: ICHP vs ICHP + COVID-19 vs COVID-19 | AST | Normal bile acids @ GA 20 | |
| ALT | 571 | ↑ Bile acids and NO itch | |||
| Bilirubin | 0.76 | Conservative management | |||
| PLC | 135 | LFTs resolved with COVID-10 | |||
| CRP | 60 | Discharged home | |||
| LDH | 194 | Healthy delivery at GA 391 | |||
| Ferritin | |||||
| Azimi et al[115], 2021 | 27 y/o G2P1, GA 30 wk | CC: Headache and lower limb pain; vitals: BP 100/70, HR 90-100; chest CT: Peripheral GGO’s + consolidation; differential: HELLP vs systemic lupus vs COVID-19 | AST | 126 | No delivery |
| ALT | 89 | LFTs resolved with COVID-19 | |||
| Bilirubin | 2.3 | Discharged at GA 33 | |||
| PLC | 220 | Healthy delivery at GA 39 | |||
| CRP | 114 | ||||
| LDH | 1036 | ||||
| Ferritin | 1360 | ||||
| Naeh et al[116], 2022 | 39 y/o G5P1, GA 264 wk | CC: Dry cough and dyspnea; vitals: BP 152/132, HR 141, RR 20, SpO2 96%; chest CT: Patchy multi-focal GGO’s; differential: PEC with severe features vs COVID-19 | AST | 1154 | Evaluated for PEC with PIGF |
| ALT | 864 | PIGF 158 (high)→No delivery | |||
| Bilirubin | LFTs resolving with COVID-10 | ||||
| PLC | WNL | Discharged HD13; AST 331 | |||
| CRP | Healthy delivery at GA 392 | ||||
| LDH | 1018 | ||||
| Ferritin | |||||
| Improved with delivery | |||||
| Ronnje et al[117], 2020 | 26 y/o, G2P1, GA 321 wk | CC: Cough, fever. Dyspnea, abdominal pain; vitals: BP 116/71, HR 113, RR 22, SpO2 95; chest CT bilateral diffuse GGO; differential: aHELLP vs COVID-19 | AST | 1687 | 5 d earlier normal labs |
| ALT | 348 | Delivery on HD2 @ GA 326 | |||
| Bilirubin | 1.23 | LFTs trend down after delivery | |||
| PLC | 122 | ||||
| CRP | 136 | ||||
| LDH | 2039 | ||||
| Ferritin | 875 | ||||
| Arslan et al[118], 2022 | 30 y/o G3P2, GA 32 wk | CC: 6 d of chills, cough, dyspnea; vitals: RR 26, SpO2 84%; chest CT bilateral GGO’s + peripheral thickening; differential: HELLP vs PEC vs AFLP vs SLE vs COVID-19 | AST | 146 | HD 2: BP 185/120, + proteinuria, intubated, IV nitroprusside |
| ALT | 102 | HD 3: Cardiac injury, ↓ PLC, ↑ fetal distress→ Cesarean section | |||
| Bilirubin | 2.54 | HD4: LFTs improved | |||
| PLC | 59 | Patient + child died | |||
| CRP | 215 | ||||
| LDH | 697 | ||||
| Ferritin | |||||
| Delivery without improvement in 24-72 h of delivery (or other) | |||||
| Madaan et al[119], 2022 | 26 y/o G1P0, GA 39w | CC: RUQ pain and headache; vitals: BP 160/100, HR 98, SpO2 95%; chest CT: Bilateral diffuse GGO’s; differential: Not given | AST | 589 | Suspicion of HELLP→ Cesarean section |
| ALT | 300 | Improved over hospitalization and LFTs trended down (no timeline given) | |||
| Bilirubin | 9.4 | ||||
| PLC | 90 | ||||
| CRP | 78.5 | ||||
| LDH | 3100 | ||||
| Ferritin | 734 | ||||
| Choudhary et al[120], 2021 | 27 y/o G1P0, GA 35 wk, di-di twins | CC: Cough, fever, abdominal pain; vitals: BP 142/94, HR 88, RR 20. SpO2 98%; chest X-ray: Bilateral basal opacities; differential: aHELLP vs PEC vs AFLP vs COVID 19 | AST | 728.5 | Suspicion of aHELLP→Cesarean-section |
| ALT | 473.2 | POD 0: Hypo-glycemia, altered mentation, ↑ bilirubin→AFLP | |||
| Bilirubin | 4.9 | Transfer to ICU + IV labetalol | |||
| PLC | 162 | POD 8 discharged, normal LFT’s | |||
| CRP | 22 | ||||
| LDH | 96.9 | ||||
| Ferritin | 120 | ||||
In a case described by Azimi et al[115], a 27-year-old Gravida (G) 2 Para (P) 1 woman presented at 30-wk’ gestation with a headache and was found to have abnormal liver enzymes, low platelets, increased inflammatory markers (LDH, ferritin D-dimer), and chest radiograph showing diffuse ground glass opacities, concerning for autoimmune disease vs HELLP vs systemic COVID-19. Pending extensive laboratory evaluation that was negative for autoantibodies and signs of hemolysis, the patient was noted to be improving with only supportive care. She was discharged at 33-wk’ gestation and underwent normal delivery at 39-wk’ gestation. The next case was that of a 35-year-old G2 P1 with prior obstetric cholestasis presenting at 28-wk’ gestation with progressive fever and cough who was found to have high ALT and elevated serum bile acids[114]. The patient denied pruritis and had normal labs at her 20-wk appointment which reduced the likelihood of obstetric cholestasis; she subsequently tested positive for COVID-19 which was then thought to be the source of her liver injury. The final case is that of a 39-year-old G5P1 presenting at 26 wk’ gestation with progressive dry cough and dyspnea. She was found to have new hypertension (BP 152/132), severe transaminitis (AST 1154 U/L, ALT 864 U/L), and PCR proven COVID-19 infection, concerning for pre-eclampsia with severe features vs systemic COVID-19[116]. Based on high suspicion for pre-eclampsia, the patient received betamethasone and dexamethasone to assist fetal lung maturation. Surprisingly, the patient’s blood pressure was noted to be improving, inconsistent with pre-eclampsia which requires delivery to return to normotension. To further evaluate this, serum maternal placental growth factor was tested and normal. Normal maternal placental growth factor effectively ruled out pre-eclampsia and favored a diagnosis of COVID-19 with COVALI. This patient went on to deliver a healthy full-term fetus. In each case hypertension and liver injury improved with conservative management for COVID-19 and did not require delivery as is the case with perinatal liver diseases.
Cases in the latter two sections demonstrate complicated cases that are difficult to parse out based on clinical course. For example, in the case by Arslan et al[118], the patient’s proteinuria was concerning for pre-eclampsia and liver enzymes trended down as expected after cesarean delivery, though both maternal and neonatal outcomes were poor which complicates interpretation of the case. Similarly, in the case by Choudhary et al[111], hypoglycemia and elevated bilirubin were highly suspicious for AFLP, but liver enzymes remained elevated for multiple days after delivery.
Outcomes: COVID-19 associated liver injury correlates with worse clinical outcomes and increased mortality in the obstetric setting. A retrospective cohort study of 122 COVID-19 positive pregnant patients in Istanbul found acute liver injury conferred a 3.5-fold risk of becoming critically ill during hospitalization[112]. Maternal mortality is reportedly more common in pregnant patients who delivered while COVID-19 positive with acute liver injury than COVID-19 positive without liver injury[111].
The largest published study evaluating COVALI in pregnancy is a 249-patient prospective cohort study performed at large tertiary care hospital in eastern India[111]. Unlike in previous studies, patients with hypertensive disorders, diabetic disorders, or concern for intrahepatic cholestasis were not excluded. Overall, 107 (42.1%) had evidence of hepatic dysfunction, but liver injury was more common in patients with perinatal hypertensive, diabetic, or cholestatic disorders (47/87, 54%) compared to those without (60/162, 37%). Although no statistical metric of significance was provided by the study, it appears that COVID-19 may increase risk of or exacerbate underlying obstetric conditions associated with liver injury. The primary aim of the study was to evaluate the relationship between liver injury in COVID-19 and obstetric outcomes. While no associations between liver injury and mode of delivery or neonatal outcomes were identified, those with liver injury tended to deliver pre-term and/or require cesarean delivery more often, both of which increase morbidity. Their key finding was that obstetric complications were significantly higher in COVID-19 positive pregnant patients with liver injury, despite no differences in maternal or gestational age[111]. Specifically, pregnant persons with COVALI were less likely to have a normal vaginal delivery than those without liver injury (18.7% vs 30.3%). Further, postpartum hemorrhage, sepsis, and death were more common in those who delivered while COVID-19 positive with acute liver injury[111].
The pathophysiology COVID-19 is not well studied outside the general population and thus the pathophysiology of COVALI in pregnancy is not well understood. Studies comparing COVID-19 positive pregnant individuals with acute liver injury and COVID-19 positive pregnant individuals with normal liver enzymes are crucial to build understanding of disease mechanisms in this cohort. However, there are only a handful of published studies on this to date[111,112,121]. We first use these studies to establish that relationships relevant to pathophysiology in the general population also exist in the obstetric population.
Specifically: (1) What is the relationship between severe COVID-19 disease and COVALI in pregnant patients? Patients with severe COVID-19 are more likely to develop COVALI. A prospective cohort study found that 87.5% of pregnant patients with severe COVID-19 pneumonia during hospitalization developed abnormal liver enzymes after having normal liver enzymes at baseline[122]. A later study demonstrated pregnant patients with liver injury had more severe disease and two thirds of this cohort ultimately died due to COVID-19 lung disease[111]; (2) what is the relationship between COVALI and markers of inflammation in pregnant patients? COVALI during pregnancy has been associated with elevated markers of inflammation. COVID-19 positive pregnant patients with liver injury have higher serum ferritin than expected in normal pregnancy, where a state of physiologic anemia is to be expected[112]. Furthermore, a study by Deng et al[121] evaluating liver chemistries in 37 COVID-19 positive pregnant patients found those with liver injury had higher inflammatory markers, such as procalcitonin and IL-6; and (3) what is the relationship between COVALI and systemic inflammatory manifestations of COVID-19? Research by Choudhary et al[111] showed that obstetric complications were found to be more common in patients with COVALI. Most of these complications were related to inflammation, endotheliopathy, and coagulopathy. For example, they found pregnant persons with liver injury had higher prothrombin time and were more likely to experience postpartum hemorrhage requiring blood transfusion. Further, systemic inflammation was more common in those who delivered while COVID-19 positive with acute liver injury, as evidence by increased risk of sepsis with multi-organ failure[111].
Overall, these studies suggest the relationships between liver injury and disease severity, patient outcomes, and inflammation identified in the general population persist in the obstetric population. While pathophysiology is likely stable across cohorts, considering the increased risk of COVALI during pregnancy could help further elucidate pathophysiology.
One potential link to the increased risk is the upregulation of ACE-2 to increased plasma levels above non-pregnant individuals, secondary to increase in estrogen production[123,124]. During pregnancy ACE-2 is highly expressed in the placenta and helps regulate blood pressure via systemic vascular resistance. This suggests there is increased activity of ACE-2 in the endothelium of pregnant patients[125] leading to the placenta as a potential target for COVID-19 infection. The interruption of the physiologic function of ACE-2 in pregnancy has been postulated to be a major contributing factor to the development of complications[126]. Lower levels of ACE-2 have been detected in the placentas from COVID-19 positive patients, suggesting that COVID-19 infection may alter ACE-2 expression and its biologic function in both the placenta and more widely in maternal circulation[124], potentially causing endothelial dysregulation as seen in COVALI.
Based on clinical manifestations, it is also reasonable to consider that pathophysiology of COVALI resembles or amplifies that of obstetric hepatobiliary pathology. This is exhibited in the case reports narrating the difficulty of differentiating COVALI from obstetric disorders that cause transaminitis in the clinical setting. Overall, the greatest overlap occurs between severe pre-eclampsia and the extra-pulmonary manifestations of COVID-19, and pre-eclampsia has been diagnosed more often in pregnant persons with COVID-19 compared to pregnant persons without COVID-19[127,128]. A potential link to the increased risk is alpha-1-antitrypsin, an enzyme that can inhibit SARS-CoV-2 infection and protects endothelial cells from oxidative stress during pregnancy, which is reduced in seen in pregnant patients with pre-eclampsia[129,130].
Work by Mendoza et al[122] sought to determine the prevalence of “pre-eclampsia findings” in 42 COVID-19 positive pregnant women. Eight women had severe pneumonia secondary to COVID-19 of which seven (87.5%) had elevated liver enzymes consistent with COVALI and five (62.5%) had hypertension meeting criteria for pre-eclampsia. However, sonographic evidence of placental hypoperfusion was only found in one patient who ultimately required delivery to prompt resolution of hypertension and liver injury. The remaining patients did not require delivery and instead, liver injury and hypertension improved in parallel with symptoms of pneumonia due to COVID-19. They measured ratio of soluble fms-like tyrosine kinase-1 (sFlt-1) ad serum placental growth factor (PIGF), which has been shown to be predictive of pre-eclampsia[131], and found sFlt-1/PIGF normal ratio in patients who did not require delivery compared to an elevated sFlt-1/PIGF ratio in the patient with evidence of placental hypoperfusion who required delivery. These findings suggest severe COVID-19 complicated by COVALI can mimic hypertensive disease of pregnancy and may represent shared disease mechanisms (Figure 1).
Literature that was published during the writing of this review directly compared the patho
In this paper we reviewed COVID-19 associated liver injury with a special focus on pregnancy. We demonstrated COVALI to be an inflammatory mediated AST-predominant transaminitis associated with COVID-19 disease severity and poorer patient outcomes. Emerging research in the general and obstetric populations supports inflammation and endothelial dysfunction as central to pathophysiology in systemic COVID-19 and COVALI. Figure 1 summarizes proposed mechanisms of COVALI and illustrates how some physiologic changes in pregnancy can pre-dispose or exacerbate processes of liver injury during COVID-19. There is significant opportunity to improve understanding of COVALI during pregnancy. At present COVALI appears to be independently associated with worse post-partum outcomes, though this has not been fully parsed on in the literature. Further research should be done to elucidate the relationship between post-partum outcomes and COVALI, relevant to short and long-term outcomes. There is also data supporting the use of specific of circulating biomarkers to differentiate systemic COVID-19 from other causes of transaminitis in pregnancy, but further research is required to define criteria that can guide management.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Naem AA, Germany; Rotondo JC, Italy; Zhang XQ, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | World Health Organization. WHO Coronavirus (COVID-19) Dashboard. July 9, 2022. [cited 23 October 2022]. Available from: https://covid19.who.int. |
| 2. | V'kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19:155-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2164] [Cited by in RCA: 2020] [Article Influence: 505.0] [Reference Citation Analysis (0)] |
| 3. | Mesel-Lemoine M, Millet J, Vidalain PO, Law H, Vabret A, Lorin V, Escriou N, Albert ML, Nal B, Tangy F. A human coronavirus responsible for the common cold massively kills dendritic cells but not monocytes. J Virol. 2012;86:7577-7587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Abbasi J. COVID-19 and the Common Cold-Preexisting Coronavirus Antibodies May Hinder SARS-CoV-2 Immunity. JAMA. 2022;327:609-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1348] [Cited by in RCA: 1295] [Article Influence: 259.0] [Reference Citation Analysis (4)] |
| 6. | Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5202] [Cited by in RCA: 4633] [Article Influence: 926.6] [Reference Citation Analysis (0)] |
| 7. | Wang FS, Zhang C. What to do next to control the 2019-nCoV epidemic? Lancet. 2020;395:391-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 8. | Slater TA, Straw S, Drozd M, Kamalathasan S, Cowley A, Witte KK. Dying 'due to' or 'with' COVID-19: a cause of death analysis in hospitalised patients. Clin Med (Lond). 2020;20:e189-e190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Ketcham SW, Bolig TC, Molling DJ, Sjoding MW, Flanders SA, Prescott HC. Causes and Circumstances of Death among Patients Hospitalized with COVID-19: A Retrospective Cohort Study. Ann Am Thorac Soc. 2021;18:1076-1079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2419] [Cited by in RCA: 2049] [Article Influence: 409.8] [Reference Citation Analysis (2)] |
| 11. | Louis TJ, Qasem A, Abdelli LS, Naser SA. Extra-Pulmonary Complications in SARS-CoV-2 Infection: A Comprehensive Multi Organ-System Review. Microorganisms. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Zaccone G, Tomasoni D, Italia L, Lombardi CM, Metra M. Myocardial Involvement in COVID-19: an Interaction Between Comorbidities and Heart Failure with Preserved Ejection Fraction. A Further Indication of the Role of Inflammation. Curr Heart Fail Rep. 2021;18:99-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Haddadin FI, Mahdawi TE, Hattar L, Beydoun H, Fram F, Homoud M. A case of complete heart block in a COVID-19 infected patient. J Cardiol Cases. 2021;23:27-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Tao W, Wang X, Zhang G, Guo M, Ma H, Zhao D, Sun Y, He J, Liu L, Zhang K, Wang Y, Weng J, Ma X, Jin T, Zhu S. Re-detectable positive SARS-CoV-2 RNA tests in patients who recovered from COVID-19 with intestinal infection. Protein Cell. 2021;12:230-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Lehmann M, Allers K, Heldt C, Meinhardt J, Schmidt F, Rodriguez-Sillke Y, Kunkel D, Schumann M, Böttcher C, Stahl-Hennig C, Elezkurtaj S, Bojarski C, Radbruch H, Corman VM, Schneider T, Loddenkemper C, Moos V, Weidinger C, Kühl AA, Siegmund B. Human small intestinal infection by SARS-CoV-2 is characterized by a mucosal infiltration with activated CD8+ T cells. Mucosal Immunol. 2021;14:1381-1392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 16. | Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, Chilla S, Heinemann A, Wanner N, Liu S, Braun F, Lu S, Pfefferle S, Schröder AS, Edler C, Gross O, Glatzel M, Wichmann D, Wiech T, Kluge S, Pueschel K, Aepfelbacher M, Huber TB. Multiorgan and Renal Tropism of SARS-CoV-2. N Engl J Med. 2020;383:590-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1254] [Cited by in RCA: 1424] [Article Influence: 284.8] [Reference Citation Analysis (0)] |
| 17. | Wang N, Qin L, Ma L, Yan H. Effect of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) on reproductive system. Stem Cell Res. 2021;52:102189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Liu C, Mu C, Zhang Q, Yang X, Yan H, Jiao H. Effects of Infection with SARS-CoV-2 on the Male and Female Reproductive Systems: A Review. Med Sci Monit. 2021;27:e930168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Marjot T, Webb GJ, Barritt AS 4th, Moon AM, Stamataki Z, Wong VW, Barnes E. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18:348-364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 272] [Article Influence: 68.0] [Reference Citation Analysis (2)] |
| 20. | Mohammed SA, Eid KM, Anyiam FE, Wadaaallah H, Muhamed MAM, Morsi MH, Dahman NBH. Liver injury with COVID-19: laboratory and histopathological outcome-systematic review and meta-analysis. Egypt Liver J. 2022;12:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | APASL Covid-19 Task Force, Lau G, Sharma M. Clinical practice guidance for hepatology and liver transplant providers during the COVID-19 pandemic: APASL expert panel consensus recommendations. Hepatol Int. 2020;14:415-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 22. | Wijarnpreecha K, Ungprasert P, Panjawatanan P, Harnois DM, Zaver HB, Ahmed A, Kim D. COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:990-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 23. | Alimentary Pharmacology and Therapeutics in 2018 - big changes but much the same. Aliment Pharmacol Ther. 2018;47:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Labenz C, Toenges G, Wörns MA, Sprinzl MF, Galle PR, Schattenberg JM. Liver injury in patients with severe acute respiratory syndrome coronavirus-2 infection: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:1194-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75:1742-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 26. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 27. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 28. | Yu D, Du Q, Yan S, Guo XG, He Y, Zhu G, Zhao K, Ouyang S. Liver injury in COVID-19: clinical features and treatment management. Virol J. 2021;18:121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 29. | Pozzobon FM, Perazzo H, Bozza FA, Rodrigues RS, de Mello Perez R, Chindamo MC. Liver injury predicts overall mortality in severe COVID-19: a prospective multicenter study in Brazil. Hepatol Int. 2021;15:493-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Pazgan-Simon M, Serafińska S, Kukla M, Kucharska M, Zuwała-Jagiełło J, Buczyńska I, Zielińska K, Simon K. Liver Injury in Patients with COVID-19 without Underlying Liver Disease. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, Schaefer E, Chung RT. Liver Biochemistries in Hospitalized Patients With COVID-19. Hepatology. 2021;73:890-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 147] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 32. | Gomi K, Ito T, Yamaguchi F, Kamio Y, Sato Y, Mori H, Endo K, Abe T, Sakakura S, Kobayashi K, Shimada K, Noda J, Hibiki T, Ohta S, Sagara H, Tanaka A, Jinno M, Yamawaki M, Nishimoto F, Inoue K, Nagahama M. Clinical features and mechanism of liver injury in patients with mild or moderate coronavirus disease 2019. JGH Open. 2021;5:888-895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Chew M, Tang Z, Radcliffe C, Caruana D, Doilicho N, Ciarleglio MM. Significant liver injury during hospitalization for COVID-19 is not associated with liver insufficiency or death. Clin Gastroenterol Hepatol. 2021;19:2182-91. e7. |
| 34. | Zhang SS, Dong L, Wang GM, Tian Y, Ye XF, Zhao Y, Liu ZY, Zhai JY, Zhao ZL, Wang JH, Zhang HM, Li XL, Wu CX, Yang CT, Yang LJ, Du HX, Wang H, Ge QG, Xiu DR, Shen N. Progressive liver injury and increased mortality risk in COVID-19 patients: A retrospective cohort study in China. World J Gastroenterol. 2021;27:835-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Wong YJ, Tan M, Zheng Q, Li JW, Kumar R, Fock KM, Teo EK, Ang TL. A systematic review and meta-analysis of the COVID-19 associated liver injury. Ann Hepatol. 2020;19:627-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 36. | Zhang Q, Li J, Zhang Y, Gao J, Wang P, Ai M, Ding W, Tan X. Differences in clinical characteristics and liver injury between suspected and confirmed COVID-19 patients in Jingzhou, Hubei Province of China. Medicine (Baltimore). 2021;100:e25913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Mendizabal M, Piñero F, Ridruejo E, Anders M, Silveyra MD, Torre A, Montes P, Urzúa A, Pages J, Toro LG, Díaz J, Gonzalez Ballerga E, Miranda-Zazueta G, Peralta M, Gutiérrez I, Michelato D, Venturelli MG, Varón A, Vera-Pozo E, Tagle M, García M, Tassara A, Brutti J, Ruiz García S, Bustios C, Escajadillo N, Macias Y, Higuera-de la Tijera F, Gómez AJ, Dominguez A, Castillo-Barradas M, Contreras F, Scarpin A, Schinoni MI, Toledo C, Girala M, Mainardi V, Sanchez A, Bessone F, Rubinstein F, Silva MO. Prospective Latin American cohort evaluating outcomes of patients with COVID-19 and abnormal liver tests on admission. Ann Hepatol. 2021;21:100298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 38. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12977] [Article Influence: 2595.4] [Reference Citation Analysis (1)] |
| 39. | Serviddio G, Villani R, Stallone G, Scioscia G, Foschino-Barbaro MP, Lacedonia D. Tocilizumab and liver injury in patients with COVID-19. Therap Adv Gastroenterol. 2020;13:1756284820959183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 40. | Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1,827 Patients in a Major U.S. Hospital Network. Hepatology. 2020;72:1169-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 41. | Orandi BJ, Li G, Dhall D, Bajpai P, Manne U, Arora N. Acute liver failure in a healthy young female with COVID-19. JPGN Reports. 2021;2:e108. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Wander P, Epstein M, Bernstein D. COVID-19 Presenting as Acute Hepatitis. Am J Gastroenterol. 2020;115:941-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 43. | Dehghani S, Teimouri A. Severe Acute Hepatitis in a COVID-19 patient: A Case Report. Clin Case Rep. 2021;9:e04869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Gurala D, Al Moussawi H, Philipose J, Abergel JR. Acute Liver Failure in a COVID-19 Patient Without any Preexisting Liver Disease. Cureus. 2020;12:e10045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Elmunzer BJ, Spitzer RL, Foster LD, Merchant AA, Howard EF, Patel VA, West MK, Qayed E, Nustas R, Zakaria A, Piper MS, Taylor JR, Jaza L, Forbes N, Chau M, Lara LF, Papachristou GI, Volk ML, Hilson LG, Zhou S, Kushnir VM, Lenyo AM, McLeod CG, Amin S, Kuftinec GN, Yadav D, Fox C, Kolb JM, Pawa S, Pawa R, Canakis A, Huang C, Jamil LH, Aneese AM, Glamour BK, Smith ZL, Hanley KA, Wood J, Patel HK, Shah JN, Agarunov E, Sethi A, Fogel EL, McNulty G, Haseeb A, Trieu JA, Dixon RE, Yang JY, Mendelsohn RB, Calo D, Aroniadis OC, LaComb JF, Scheiman JM, Sauer BG, Dang DT, Piraka CR, Shah ED, Pohl H, Tierney WM, Mitchell S, Condon A, Lenhart A, Dua KS, Kanagala VS, Kamal A, Singh VK, Pinto-Sanchez MI, Hutchinson JM, Kwon RS, Korsnes SJ, Singh H, Solati Z, Willingham FF, Yachimski PS, Conwell DL, Mosier E, Azab M, Patel A, Buxbaum J, Wani S, Chak A, Hosmer AE, Keswani RN, DiMaio CJ, Bronze MS, Muthusamy R, Canto MI, Gjeorgjievski VM, Imam Z, Odish F, Edhi AI, Orosey M, Tiwari A, Patwardhan S, Brown NG, Patel AA, Ordiah CO, Sloan IP, Cruz L, Koza CL, Okafor U, Hollander T, Furey N, Reykhart O, Zbib NH, Damianos JA, Esteban J, Hajidiacos N, Saul M, Mays M, Anderson G, Wood K, Mathews L, Diakova G, Caisse M, Wakefield L, Nitchie H, Waljee AK, Tang W, Zhang Y, Zhu J, Deshpande AR, Rockey DC, Alford TB, Durkalski V; North American Alliance for the Study of Digestive Manifestations of COVID-19. Digestive Manifestations in Patients Hospitalized With Coronavirus Disease 2019. Clin Gastroenterol Hepatol. 2021;19:1355-1365.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 46. | Kumar A, Kumar P, Dungdung A, Kumar Gupta A, Anurag A, Kumar A. Pattern of liver function and clinical profile in COVID-19: A cross-sectional study of 91 patients. Diabetes Metab Syndr. 2020;14:1951-1954. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Hwaiz R, Merza M, Hamad B, HamaSalih S, Mohammed M, Hama H. Evaluation of hepatic enzymes activities in COVID-19 patients. Int Immunopharmacol. 2021;97:107701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Bzeizi K, Abdulla M, Mohammed N, Alqamish J, Jamshidi N, Broering D. Effect of COVID-19 on liver abnormalities: a systematic review and meta-analysis. Sci Rep. 2021;11:10599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 49. | Sivandzadeh GR, Askari H, Safarpour AR, Ejtehadi F, Raeis-Abdollahi E, Vaez Lari A, Abazari MF, Tarkesh F, Bagheri Lankarani K. COVID-19 infection and liver injury: Clinical features, biomarkers, potential mechanisms, treatment, and management challenges. World J Clin Cases. 2021;9:6178-6200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 50. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 51. | Xu W, Huang C, Fei L, Li Q, Chen L. Dynamic Changes in Liver Function Tests and Their Correlation with Illness Severity and Mortality in Patients with COVID-19: A Retrospective Cohort Study. Clin Interv Aging. 2021;16:675-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Kunutsor SK, Laukkanen JA. Markers of liver injury and clinical outcomes in COVID-19 patients: A systematic review and meta-analysis. J Infect. 2021;82:159-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 53. | Chen Y, Zhou X, Yan H, Huang H, Li S, Jiang Z, Zhao J, Meng Z. CANPT Score: A Tool to Predict Severe COVID-19 on Admission. Front Med (Lausanne). 2021;8:608107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Piano S, Dalbeni A, Vettore E, Benfaremo D, Mattioli M, Gambino CG, Framba V, Cerruti L, Mantovani A, Martini A, Luchetti MM, Serra R, Cattelan A, Vettor R, Angeli P; COVID-LIVER study group. Abnormal liver function tests predict transfer to intensive care unit and death in COVID-19. Liver Int. 2020;40:2394-2406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 55. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX. Clinical characteristics of 2019 novel coronavirus infection in China. MedRxiv. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 349] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 56. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30123] [Article Influence: 6024.6] [Reference Citation Analysis (3)] |
| 57. | Wang M, Wu D, Liu CH, Li Y, Hu J, Wang W, Jiang W, Zhang Q, Huang Z, Bai L, Tang H. Predicting progression to severe COVID-19 using the PAINT score. BMC Infect Dis. 2022;22:498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Madian A, Eliwa A, Abdalla H, Aly HAA. Hepatocellular injury and the mortality risk among patients with COVID-19: A retrospective cohort study. World J Hepatol. 2021;13:939-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (4)] |
| 59. | Ding ZY, Li GX, Chen L, Shu C, Song J, Wang W, Wang YW, Chen Q, Jin GN, Liu TT, Liang JN, Zhu P, Zhu W, Li Y, Zhang BH, Feng H, Zhang WG, Yin ZY, Yu WK, Yang Y, Zhang HQ, Tang ZP, Wang H, Hu JB, Liu JH, Yin P, Chen XP, Zhang B; Tongji Multidisciplinary Team for Treating COVID-19 (TTTC). Association of liver abnormalities with in-hospital mortality in patients with COVID-19. J Hepatol. 2021;74:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 60. | Harapan H, Fajar JK, Supriono S, Soegiarto G, Wulandari L, Seratin F, Prayudi NG, Dewi DP, Monica Elsina MT, Atamou L, Wiranata S, Aprianto DP, Friska E, Sari Firdaus DF, Alaidin M, Wardhani FA, Husnah M, Hidayati NW, Hendriyanti Y, Wardani K, Evatta A, Manugan RA, Pradipto W, Rahmawati A, Tamara F, Mahendra AI, Nainu F, Santoso B, Irawan Primasatya CA, Tjionganata N, Budiman HA. The prevalence, predictors and outcomes of acute liver injury among patients with COVID-19: A systematic review and meta-analysis. Rev Med Virol. 2022;32:e2304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | Chu H, Bai T, Chen L, Hu L, Xiao L, Yao L, Zhu R, Niu X, Li Z, Zhang L, Han C, Song S, He Q, Zhao Y, Zhu Q, Chen H, Schnabl B, Yang L, Hou X. Multicenter Analysis of Liver Injury Patterns and Mortality in COVID-19. Front Med (Lausanne). 2020;7:584342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 62. | Cao P, Wu Y, Wu S, Wu T, Zhang Q, Zhang R, Wang Z, Zhang Y. Elevated serum ferritin level effectively discriminates severity illness and liver injury of coronavirus disease 2019 pneumonia. Biomarkers. 2021;26:207-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 63. | Goel H, Harmouch F, Garg K, Saraiya P, Daly T, Kumar A, Hippen JT. The liver in COVID-19: prevalence, patterns, predictors, and impact on outcomes of liver test abnormalities. Eur J Gastroenterol Hepatol. 2021;33:e274-e281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 64. | Mishra K, Naffouj S, Gorgis S, Ibrahim H, Gill S, Fadel R, Chatfield A, Tang A, Salgia R. Liver Injury as a Surrogate for Inflammation and Predictor of Outcomes in COVID-19. Hepatol Commun. 2021;5:24-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Hartl L, Haslinger K, Angerer M, Semmler G, Schneeweiss-Gleixner M, Jachs M, Simbrunner B, Bauer DJM, Eigenbauer E, Strassl R, Breuer M, Kimberger O, Laxar D, Lampichler K, Halilbasic E, Stättermayer AF, Ba-Ssalamah A, Mandorfer M, Scheiner B, Reiberger T, Trauner M. Progressive cholestasis and associated sclerosing cholangitis are frequent complications of COVID-19 in patients with chronic liver disease. Hepatology. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 66. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 67. | Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1374] [Article Influence: 274.8] [Reference Citation Analysis (0)] |
| 68. | Boettler T, Marjot T, Newsome PN, Mondelli MU, Maticic M, Cordero E, Jalan R, Moreau R, Cornberg M, Berg T. Impact of COVID-19 on the care of patients with liver disease: EASL-ESCMID position paper after 6 months of the pandemic. JHEP Rep. 2020;2:100169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 69. | Alqahtani SA, Buti M. COVID-19 and hepatitis B infection. Antivir Ther. 2020;25:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 70. | Gracia-Ramos AE, Jaquez-Quintana JO, Contreras-Omaña R, Auron M. Liver dysfunction and SARS-CoV-2 infection. World J Gastroenterol. 2021;27:3951-3970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 71. | Yip TC, Lui GC, Wong VW, Chow VC, Ho TH, Li TC, Tse YK, Hui DS, Chan HL, Wong GL. Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19. Gut. 2021;70:733-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 72. | Sagnelli C, Montella L, Grimaldi P, Pisaturo M, Alessio L, De Pascalis S, Sagnelli E, Coppola N. COVID-19 as Another Trigger for HBV Reactivation: Clinical Case and Review of Literature. Pathogens. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 73. | Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020;40:1316-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 74. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 75. | Fiel MI, El Jamal SM, Paniz-Mondolfi A, Gordon RE, Reidy J, Bandovic J, Advani R, Kilaru S, Pourmand K, Ward S, Thung SN, Schiano T. Findings of Hepatic Severe Acute Respiratory Syndrome Coronavirus-2 Infection. Cell Mol Gastroenterol Hepatol. 2021;11:763-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 76. | Wanner N, Andrieux G, Badia-I-Mompel P, Edler C, Pfefferle S, Lindenmeyer MT, Schmidt-Lauber C, Czogalla J, Wong MN, Okabayashi Y, Braun F, Lütgehetmann M, Meister E, Lu S, Noriega MLM, Günther T, Grundhoff A, Fischer N, Bräuninger H, Lindner D, Westermann D, Haas F, Roedl K, Kluge S, Addo MM, Huber S, Lohse AW, Reiser J, Ondruschka B, Sperhake JP, Saez-Rodriguez J, Boerries M, Hayek SS, Aepfelbacher M, Scaturro P, Puelles VG, Huber TB. Molecular consequences of SARS-CoV-2 liver tropism. Nat Metab. 2022;4:310-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 119] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 77. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14274] [Article Influence: 2854.8] [Reference Citation Analysis (0)] |
| 78. | Patel SK, Velkoska E, Burrell LM. Emerging markers in cardiovascular disease: where does angiotensin-converting enzyme 2 fit in? Clin Exp Pharmacol Physiol. 2013;40:551-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 79. | Gao F, Zheng KI, Fan YC, Targher G, Byrne CD, Zheng MH. ACE2: A Linkage for the Interplay Between COVID-19 and Decompensated Cirrhosis. Am J Gastroenterol. 2020;115:1544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. Biorxiv. 2020;. [DOI] [Full Text] |
| 81. | Zhao B, Ni C, Gao R, Wang Y, Yang L, Wei J, Lv T, Liang J, Zhang Q, Xu W, Xie Y, Wang X, Yuan Z, Zhang R, Lin X. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11:771-775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 312] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 82. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 83. | Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110-2116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 84. | Rai DK, Thakur S. Study to identify predictor of hypoxia in COVID-19 infection: A single-center, retrospective study. J Family Med Prim Care. 2021;10:1852-1855. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 85. | McConnell MJ, Kawaguchi N, Kondo R, Sonzogni A, Licini L, Valle C, Bonaffini PA, Sironi S, Alessio MG, Previtali G, Seghezzi M, Zhang X, Lee AI, Pine AB, Chun HJ, Fernandez-Hernando C, Qing H, Wang A, Price C, Sun Z, Utsumi T, Hwa J, Strazzabosco M, Iwakiri Y. Liver injury in COVID-19 and IL-6 trans-signaling-induced endotheliopathy. J Hepatol. 2021;75:647-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 86. | Diaz-Louzao C, Barrera-Lopez L, Lopez-Rodriguez M, Casar C, Vazquez-Agra N, Pernas-Pardavila H, Marques-Afonso A, Vidal-Vazquez M, Montoya JG, Andrade AH, Fernandez-Castro I, Varela P, Gonzalez-Quintela A, Otero E, Gude F, Cadarso-Suarez C, Tome S. Longitudinal relationship of liver injury with inflammation biomarkers in COVID-19 hospitalized patients using a joint modeling approach. Sci Rep. 2022;12:5547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 87. | Saviano A, Wrensch F, Ghany MG, Baumert TF. Liver Disease and Coronavirus Disease 2019: From Pathogenesis to Clinical Care. Hepatology. 2021;74:1088-1100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 88. | Liao D, Zhou F, Luo L, Xu M, Wang H, Xia J, Gao Y, Cai L, Wang Z, Yin P, Wang Y, Tang L, Deng J, Mei H, Hu Y. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. Lancet Haematol. 2020;7:e671-e678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 336] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 89. | Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID-19 - A systematic review. Life Sci. 2020;254:117788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 420] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 90. | Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020;92:2409-2411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 91. | Ghahramani S, Tabrizi R, Lankarani KB, Kashani SMA, Rezaei S, Zeidi N, Akbari M, Heydari ST, Akbari H, Nowrouzi-Sohrabi P, Ahmadizar F. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res. 2020;25:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 194] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 92. | Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, Marron TU, Xie H, Patel M, Tuballes K, Van Oekelen O, Rahman A, Kovatch P, Aberg JA, Schadt E, Jagannath S, Mazumdar M, Charney AW, Firpo-Betancourt A, Mendu DR, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M, Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1894] [Cited by in RCA: 1798] [Article Influence: 359.6] [Reference Citation Analysis (0)] |
| 93. | Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, Poucke SV, Liu WY, Zheng MH. COVID-19 and Liver Dysfunction: Current Insights and Emergent Therapeutic Strategies. J Clin Transl Hepatol. 2020;8:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 289] [Cited by in RCA: 278] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 94. | Amiri-Dashatan N, Koushki M, Ghorbani F, Naderi N. Increased inflammatory markers correlate with liver damage and predict severe COVID-19: a systematic review and meta-analysis. Gastroenterol Hepatol Bed Bench. 2020;13:282-291. [PubMed] |
| 95. | Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res. 2020;69:1181-1189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 282] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 96. | Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, Dentali F, Montecucco F, Massberg S, Levi M, Abbate A. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 726] [Cited by in RCA: 640] [Article Influence: 160.0] [Reference Citation Analysis (0)] |
| 97. | Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, Hochman JS, Reynolds HR. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. 2020;24:100434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 404] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 98. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5786] [Article Influence: 1157.2] [Reference Citation Analysis (2)] |
| 99. | Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, Del Portillo A, De Michele S, de Gonzalez AK, Saqi A, Khairallah P, Chong AM, Park H, Uhlemann AC, Lefkowitch JH, Verna EC. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147-2155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 181] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 100. | Zhao CL, Rapkiewicz A, Maghsoodi-Deerwester M, Gupta M, Cao W, Palaia T, Zhou J, Ram B, Vo D, Rafiee B, Hossein-Zadeh Z, Dabiri B, Hanna I. Pathological findings in the postmortem liver of patients with coronavirus disease 2019 (COVID-19). Hum Pathol. 2021;109:59-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 101. | Da BL, Kushner T, El Halabi M, Paka P, Khalid M, Uberoi A, Lee BT, Perumalswami PV, Rutledge SM, Schiano TD, Friedman SL, Saberi B. Liver Injury in Patients Hospitalized with Coronavirus Disease 2019 Correlates with Hyperinflammatory Response and Elevated Interleukin-6. Hepatol Commun. 2021;5:177-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 102. | McConnell MJ, Kondo R, Kawaguchi N, Iwakiri Y. Covid-19 and Liver Injury: Role of Inflammatory Endotheliopathy, Platelet Dysfunction, and Thrombosis. Hepatol Commun. 2022;6:255-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 103. | Effenberger M, Grander C, Grabherr F, Griesmacher A, Ploner T, Hartig F, Bellmann-Weiler R, Joannidis M, Zoller H, Weiss G, Adolph TE, Tilg H. Systemic inflammation as fuel for acute liver injury in COVID-19. Dig Liver Dis. 2021;53:158-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (2)] |
| 104. | Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, Baluha A, Bar N, Bona RD, Burns AJ, Dela Cruz CS, Dumont A, Halene S, Hwa J, Koff J, Menninger H, Neparidze N, Price C, Siner JM, Tormey C, Rinder HM, Chun HJ, Lee AI. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575-e582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 605] [Cited by in RCA: 805] [Article Influence: 161.0] [Reference Citation Analysis (0)] |
| 105. | Tran TT, Ahn J, Reau NS. ACG clinical guideline: liver disease and pregnancy. Am J Gastroenterol. 2016;111:176-94. |
| 106. | Brady CW. Liver Disease in Pregnancy: What's New. Hepatol Commun. 2020;4:145-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 107. | Westbrook RH, Dusheiko G, Williamson C. Pregnancy and liver disease. J Hepatol. 2016;64:933-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 108. | Raju S, Ziemann S, Mayigegowda KK, Nevah MI, Reddy PM, Machicao VI. Su323 The patterns of liver injury in covid-19 positive pregnant females: A Case series. Gastroenterology. 2021;160:S-849. |
| 109. | Klyachman L, Advani R, Sun E. S2425 Pregnancy and Transaminitis in the COVID-19 Era. Am J Gastroenterol. 2020;115:S1286. |
| 110. | Li Q, Chen L, Jiang H, Zheng D, Wang Y, Mei J. Clinical characteristics of pregnant women infected with Coronavirus Disease 2019 in China: a nationwide case-control study. MedRxiv. 2021;. [DOI] [Full Text] |
| 111. | Choudhary A, Singh V, Bharadwaj M. Maternal and Neonatal Outcomes in Pregnant Women With SARS-CoV-2 Infection Complicated by Hepatic Dysfunction. Cureus. 2022;14:e25347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 112. | Can E, Oğlak SC, Ölmez F. Abnormal liver function tests in pregnant patients with COVID-19 - a retrospective cohort study in a tertiary center. Ginekol Pol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 113. | Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809-815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2400] [Cited by in RCA: 2273] [Article Influence: 454.6] [Reference Citation Analysis (0)] |
| 114. | Anness A, Siddiqui F. COVID-19 complicated by hepatic dysfunction in a 28-week pregnant woman. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 115. | Azimi H, Saghafi N, Tara F, Mirzaeian S, Hatamian Z, Afshar Delkhah F. COVID-19 Mimicking Hemolysis, Elevated Liver Enzymes and Low Platelets (HELLP) Syndrome: A Case Report. J Midwifery Womens Health. 2021;9:3050-4. [DOI] [Full Text] |
| 116. | Naeh A, Berezowsky A, Yudin MH, Dhalla IA, Berger H. Preeclampsia-Like Syndrome in a Pregnant Patient With Coronavirus Disease 2019 (COVID-19). J Obstet Gynaecol Can. 2022;44:193-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 117. | Ronnje L, Länsberg JK, Vikhareva O, Hansson SR, Herbst A, Zaigham M. Complicated COVID-19 in pregnancy: a case report with severe liver and coagulation dysfunction promptly improved by delivery. BMC Pregnancy Childbirth. 2020;20:511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 118. | Arslan E. COVID-19: A Cause of HELLP Syndrome? Int J Womens Health. 2022;14:617-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 119. | Madaan S, Talwar D, Kumar S, Jaiswal A, Acharya N, Acharya S. HELLP Syndrome and COVID-19; association or accident: A case series. J Family Med Prim Care. 2022;11:802-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 120. | Choudhary A, Singh V, Bharadwaj M, Barik A. Pregnancy With SARS-CoV-2 Infection Complicated by Preeclampsia and Acute Fatty Liver of Pregnancy. Cureus. 2021;13:e15645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 121. | Deng G, Zeng F, Zhang L, Chen H, Chen X, Yin M. Characteristics of pregnant patients with COVID-19 and liver injury. J Hepatol. 2020;73:989-991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 122. | Mendoza M, Garcia-Ruiz I, Maiz N, Rodo C, Garcia-Manau P, Serrano B, Lopez-Martinez RM, Balcells J, Fernandez-Hidalgo N, Carreras E, Suy A. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020;127:1374-1380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 228] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 123. | Levy A, Yagil Y, Bursztyn M, Barkalifa R, Scharf S, Yagil C. ACE2 expression and activity are enhanced during pregnancy. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1953-R1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 124. | Azinheira Nobrega Cruz N, Stoll D, Casarini DE, Bertagnolli M. Role of ACE2 in pregnancy and potential implications for COVID-19 susceptibility. Clin Sci (Lond). 2021;135:1805-1824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 125. | Dhaundiyal A, Kumari P, Jawalekar SS, Chauhan G, Kalra S, Navik U. Is highly expressed ACE 2 in pregnant women "a curse" in times of COVID-19 pandemic? Life Sci. 2021;264:118676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 126. | Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130:1003-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 654] [Article Influence: 59.5] [Reference Citation Analysis (1)] |
| 127. | Papageorghiou AT, Deruelle P, Gunier RB, Rauch S, García-May PK, Mhatre M, Usman MA, Abd-Elsalam S, Etuk S, Simmons LE, Napolitano R, Deantoni S, Liu B, Prefumo F, Savasi V, do Vale MS, Baafi E, Zainab G, Nieto R, Maiz N, Aminu MB, Cardona-Perez JA, Craik R, Winsey A, Tavchioska G, Bako B, Oros D, Rego A, Benski AC, Hassan-Hanga F, Savorani M, Giuliani F, Sentilhes L, Risso M, Takahashi K, Vecchiarelli C, Ikenoue S, Thiruvengadam R, Soto Conti CP, Ferrazzi E, Cetin I, Nachinab VB, Ernawati E, Duro EA, Kholin A, Firlit ML, Easter SR, Sichitiu J, Bowale A, Casale R, Cerbo RM, Cavoretto PI, Eskenazi B, Thornton JG, Bhutta ZA, Kennedy SH, Villar J. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021;225:289.e1-289.e17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 188] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 128. | Conde-Agudelo A, Romero R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2022;226:68-89.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 171] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 129. | Feng Y, Xu J, Zhou Q, Wang R, Liu N, Wu Y, Yuan H, Che H. Alpha-1 Antitrypsin Prevents the Development of Preeclampsia Through Suppression of Oxidative Stress. Front Physiol. 2016;7:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 130. | Azouz NP, Klingler AM, Callahan V, Akhrymuk IV, Elez K, Raich L, Henry BM, Benoit JL, Benoit SW, Noé F, Kehn-Hall K, Rothenberg ME. Alpha 1 Antitrypsin is an Inhibitor of the SARS-CoV-2-Priming Protease TMPRSS2. bioRxiv. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 131. | Moore Simas TA, Crawford SL, Solitro MJ, Frost SC, Meyer BA, Maynard SE. Angiogenic factors for the prediction of preeclampsia in high-risk women. Am J Obstet Gynecol. 2007;197:244.e1-244.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 132. | Palomo M, Youssef L, Ramos A, Torramade-Moix S, Moreno-Castaño AB, Martinez-Sanchez J, Bonastre L, Pino M, Gomez-Ramirez P, Martin L, Garcia Mateos E, Sanchez P, Fernandez S, Crovetto F, Escolar G, Carreras E, Castro P, Gratacos E, Crispi F, Diaz-Ricart M. Differences and similarities in endothelial and angiogenic profiles of preeclampsia and COVID-19 in pregnancy. Am J Obstet Gynecol. 2022;227:277.e1-277.e16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 133. | Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstet Gynecol. 2020;135:1492-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 344] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 134. | Morrison MA, Chung Y, Heneghan MA. Managing hepatic complications of pregnancy: practical strategies for clinicians. BMJ Open Gastroenterol. 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 135. | Goel A, Jamwal KD, Ramachandran A, Balasubramanian KA, Eapen CE. Pregnancy-related liver disorders. J Clin Exp Hepatol. 2014;4:151-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 136. | Terrault NA, Williamson C. Pregnancy-Associated Liver Diseases. Gastroenterology. 2022;163:97-117.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 137. | Hammoud GM, Ibdah JA. Preeclampsia-induced Liver Dysfunction, HELLP syndrome, and acute fatty liver of pregnancy. Clin Liver Dis (Hoboken). 2014;4:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 138. | Kongwattanakul K, Saksiriwuttho P, Chaiyarach S, Thepsuthammarat K. Incidence, characteristics, maternal complications, and perinatal outcomes associated with preeclampsia with severe features and HELLP syndrome. Int J Womens Health. 2018;10:371-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |