Published online Oct 21, 2022. doi: 10.3748/wjg.v28.i39.5750
Peer-review started: July 2, 2022
First decision: August 1, 2022
Revised: September 24, 2022
Accepted: October 10, 2022
Article in press: October 10, 2022
Published online: October 21, 2022
Processing time: 107 Days and 14.2 Hours
Immune checkpoint inhibitor-mediated colitis (IMC) is a common adverse event following immune checkpoint inhibitor (ICI) therapy for cancer. IMC has been associated with improved overall survival (OS) and progression-free survival (PFS), but data are limited to a single site and predominantly for melanoma pat
To determine the association of IMC with OS and PFS and identify clinical pre
We performed a retrospective case-control study including 64 ICI users who dev
IMC was significantly associated with a higher OS (mean 24.3 mo vs 17.7 mo, P = 0.05) but not PFS (mean 13.7 mo vs 11.9 mo, P = 0.524). IMC was significantly associated with OS greater than 12 mo [Odds ratio (OR) 2.81, 95% confidence interval (CI) 1.17-6.77]. Vitamin D supplementation was significantly associated with increased risk of IMC (OR 2.48, 95%CI 1.01-6.07).
IMC was significantly associated with OS greater than 12 mo. In contrast to prior work, we found that vitamin D use may be a risk factor for IMC.
Core Tip: Immune checkpoint inhibitor-mediated colitis (IMC) is a common adverse event following immune checkpoint inhibitor (ICI) therapy for cancer. We sought to determine the association of IMC with overall survival (OS) and progression-free survival (PFS) among cancer patients treated with ICI and identify clinical predictors of IMC. We performed a retrospective case-control study including 64 ICI users who developed IMC. In multivariate logistic regression analysis, IMC was significantly associated with a higher OS but not PFS. IMC was significantly associated with OS greater than 12 mo. Vitamin D supplementation was associated with increased risk of IMC.
- Citation: Weingarden AR, Gubatan J, Singh S, Balabanis TC, Patel A, Sharma A, Habtezion A. Immune checkpoint inhibitor-mediated colitis is associated with cancer overall survival. World J Gastroenterol 2022; 28(39): 5750-5763
- URL: https://www.wjgnet.com/1007-9327/full/v28/i39/5750.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i39.5750
Immune checkpoint inhibitors (ICI) have dramatically changed the landscape of cancer therapy. Early studies showed significantly prolonged survival in patients with metastatic melanoma compared to standard chemotherapy[1], and evidence now exists for improved outcomes in a variety of tumors ranging from lung cancers to urothelial carcinoma to breast cancer[2-5]. Although these are powerful treatments in our armamentarium against malignancy, ICI can cause immune-related adverse events (irAE) characterized by autoimmune-like inflammation in a variety of non-tumor organs, leading to in
One of the most common irAE is immune checkpoint inhibitor-mediated colitis (IMC). IMC may occur in up to 40% of patients treated with ipilimumab, an antibody targeting CTLA-4, 11%-17% of patients treated with antibodies against anti-PD-1 or anti-PD-L1, such as nivolumab, pembrolizumab, or atezolizumab, and around 32% of patients treated with a combination of anti-CTLA-4 and anti-PD-1[7]. Prior retrospective analyses of patients with IMC have attempted to identify characteristics associated with development of IMC, including type of malignancy, ICI class, dose of ICI, cancer stage, and vitamin D use[8-11]. Intriguingly, two prior studies have suggested that development of IMC may positively correlate with improved progression-free survival (PFS) and overall survival (OS)[9,10]. One of these studies controlled for confounding effects of ICI class via frequency matching, but was limited to patients with melanoma, hindering wider applicability of their findings[10]. These findings also conflict with data suggesting that use of steroids and the anti-TNF antibody infliximab in patients treated with ICI are associated with worse cancer outcomes[12,13]. These discrepancies represent a significant knowledge gap that impedes our ability to evaluate and manage IMC and ICI use.
Here we present data from a retrospective study of patients treated with ICI at our institution who developed IMC across malignancy types. We compare this cohort to a matched control cohort to determine whether IMC was associated with improved progression-free survival and overall survival. We also evaluate which clinical characteristics increase the risk of developing IMC, including severe IMC.
We conducted a retrospective case-control single-center study after obtaining approval from the Institutional Review Board at Stanford University (IRB 57125, approved 6/30/2020). Our primary aim was to determine the association of presence and severity of IMC on OS and PFS in ICI users. Our secondary aim was to identify clinical variables which predicted development of IMC in ICI users. We evaluated all patients over the age of 18 who had been treated with immune checkpoint inhibitors (ICI) for malignancy at Stanford Health Care from May 2011 to May 2020, including anti-CTLA-4 (ipilimumab), anti-PD-1 (nivolumab, pembrolizumab), and anti-PD-L1 (atezolizumab, avelumab, durvalumab), with follow up through October 2020. Using the Stanford Research Repository tool, we screened patients treated with ICI who were assigned International Classification of Diseases (ICD) 9 and ICD 10 codes associated with non-infectious colitis and diarrhea (Supplementary Table 1). Each chart which passed the initial screen was further screened by review of clinic notes to confirm diagnosis of immune checkpoint inhibitor-related colitis by oncology providers. Any patient found to have other explanations for their clinical presentation was excluded from the study.
Control patients were matched one to one with each IMC patient for sex, age, malignancy, type of ICI used, prior ICI exposure, and duration of ICI exposure (matched to number of doses from initiation of ICI to development of colitis in study cohort). Control patients were initially screened by those lacking the above ICD codes and were confirmed via direct evaluation of each chart to lack diarrhea and/or colitis ascribable to ICI per their treating oncologist.
We extracted clinical data on IMC and control patient charts including demographics (age at time of ICI initiation, sex, body mass index, race per patient report), medical history (presence of prior non-liver and non-upper gastrointestinal disease, personal history of autoimmune disease, family history of autoimmune disease), and cancer history (type of malignancy, tumor stage at ICI initiation, prior chemotherapy, prior radiation therapy, type of ICI used, duration of ICI use, OS and PFS) (Supplementary Table 2). OS was determined as time from initiation of ICI to death, while PFS was determined as time from initiation of ICI to death or progression of disease as determined by oncology providers, based on radiographic evidence of progression. IMC severity was graded using commonly accepted determinants of IMC and irAE grading[14]. We specifically noted prior use of therapies designed to increase immune responses [interleukin (IL)-2, interferon (IFN)-γ, toll-like receptor (TLR)-9 agonist, tebentafusp, or anti-CD47 antibody]. Vitamin D and non-steroidal anti-inflammatory (NSAID) use were defined as vitamin D supplement or NSAID medication, respectively, noted in the history of present illness or on the patient’s medication list at the clinic visit closest to their date of ICI initiation.
We collected data on IMC diagnosis including number of patients who received endoscopy (flexible sigmoidoscopy or colonoscopy), findings on endoscopy, and fecal calprotectin (Supplementary Table 3). Data on management of IMC included treatment with anti-diarrheal medications, mesalamine, steroids (prednisone, budesonide, dexamethasone), infliximab, and vedolizumab.
The rate of the primary outcomes (OS > 12 mo and PFS > 6 mo among all ICI users, OS > 12 mo and PFS > 6 mo in patients with IMC) and secondary outcomes (risks of IMC among patients with malignancy using ICI, IMC severity), predictive value of clinical variables on primary and secondary outcomes, odds ratio (OR) with its 95% confidence interval (CI), and P values were calculated using Statistics/Data Analysis (Stata/IC 15.1 for Windows, College Station, TX, United States). Dichotomous variables were analyzed for outcomes using the chi-squared test or the Fisher’s exact test where appropriate, and continuous variables were analyzed using Student’s t-tests if normally distributed, or the Wilcoxon signed-rank test for non-normal data. For our multivariate analyses, model building was based on forward stepwise logistic regression, with a P value of 0.05 required for entry, and known predictors were also included. We constructed Kaplan Meier curves for the outcomes of OS and PFS between patients with and without IMC and patients with mild vs severe IMC using GraphPad Prism (version 8.3; GraphPad Software, Inc., La Jolla, CA, United States). All authors had access to the study data and reviewed and approved the final manuscript.
We identified a total of 314 patients treated with ICI at Stanford Health Care from May 2011 to May 2020 who had ICD codes matching our query (Supplementary Table 1). Of these, 64 had a diagnosis of IMC per review of Oncology providers’ notes, after excluding patients with alternative diagnoses for their symptoms. 24 (37.5%) of these IMC patients underwent an endoscopy (colonoscopy or flexible sigmoidoscopy) during workup, of which seven (29.2%) had a normal endoscopic appearance, consistent with prior reports demonstrating that approximately one third of patients with IMC related to anti-PD-1 therapy have microscopic colitis[15] (Supplementary Table 3). An additional 14 patients (21.9%) had imaging findings suggestive of IMC while 3 patients (4.69%) without imaging or endoscopy had an elevated calprotectin or fecal lactoferrin.
These 64 patients were manually matched 1:1 with control patients based on age, sex, malignancy, type of ICI, whether or not the patient had prior ICI exposure, and duration of ICI use. We compared clinical characteristics of patients from the IMC cohort and the control cohort (Table 1). None of the matched characteristics were significantly different between the two cohorts. The mean age across the combined cohorts was 66.6 years, with an average age of 67.4 in the cohort with IMC compared with 65.8 in the control cohort (P = 0.42). 57.81% of patients in each group were male (P = 1.00). Patients were predominantly white in both groups, with 52 (81.25%) white individuals in the IMC cohort compared to 50 (78.13%) in the control group (P = 0.66). The most common malignancy in each group was melanoma [33 (51.56%) in both cohorts], followed by renal cell carcinoma [8 (12.5%) in the IMC cohort and 7 (10.94%) in the control cohort] and non-small cell lung cancer [6 (9.38%) in both cohorts]. Both groups had similar numbers of patients with stage IV malignancy [56 (87.5%) in the IMC cohort and 58 (90.63%) in the control cohort, P = 0.778]. Combination ipilimumab and nivolumab was the most commonly used checkpoint therapy [24 (37.5%) of patients in each cohort], followed by nivolumab monotherapy [19 (29.69%) of each cohort] and ipilimumab monotherapy [11 (17.19%) of each cohort].
| Clinical variables | All patients (n = 128) | Patients with IMC (n = 64) | Patients without IMC (n = 64) | P value | |||
| Age, yr (mean ± SD)1 | 66.6 (± 11.5) | 67.4 (± 11.7) | 65.8 (± 11.3) | 0.420 | |||
| Sex1 | |||||||
| Male, n (%) | 74 | 57.81% | 37 | 57.81% | 37 | 57.81% | 1.000 |
| Female, n (%) | 54 | 42.19% | 27 | 42.19% | 27 | 42.19% | |
| Race | |||||||
| White, n (%) | 102 | 79.69% | 52 | 81.25% | 50 | 78.13% | 0.660 |
| Black, n (%) | 4 | 3.13% | 2 | 3.13% | 2 | 3.13% | 1.000 |
| Asian, n (%) | 9 | 7.03% | 4 | 6.25% | 5 | 7.81% | 0.730 |
| Type of malignancy1 | |||||||
| Melanoma, n (%) | 66 | 51.56% | 33 | 51.56% | 33 | 51.56% | 1.000 |
| RCC, n (%) | 15 | 11.72% | 8 | 12.50% | 7 | 10.94% | 0.783 |
| NSCLC, n (%) | 12 | 9.38% | 6 | 9.38% | 6 | 9.38% | 1.000 |
| Sarcoma, n (%) | 11 | 8.59% | 5 | 7.81% | 6 | 9.38% | 0.752 |
| Head and neck SCC, n (%) | 7 | 5.47% | 3 | 4.69% | 4 | 6.25% | 0.697 |
| Other, n (%) | 17 | 13.28% | 9 | 14.06% | 8 | 12.50% | 0.795 |
| Stage IV malignancy, n (%) | 114 | 89.07% | 56 | 87.50% | 58 | 90.63% | 0.778 |
| Type of immune checkpoint inhibitor1 | |||||||
| Ipilimumab plus nivolumab, n (%) | 48 | 37.50% | 24 | 37.50% | 24 | 37.50% | 1.000 |
| Ipilimumab, n (%) | 22 | 17.19% | 11 | 17.19% | 11 | 17.19% | 1.000 |
| Nivolumab, n (%) | 12 | 9.38% | 6 | 9.38% | 6 | 9.38% | 1.000 |
| Pembrolizumab, n (%) | 38 | 29.69% | 19 | 29.69% | 19 | 29.69% | 1.000 |
| Atezolizumab, n (%) | 8 | 6.25% | 4 | 6.25% | 4 | 6.25% | 1.000 |
| Number of Infusionsa (mean ± SD)1 | 6.91 (± 8.40) | 6.09 (± 7.20) | 7.73 (± 9.40) | 0.268 | |||
| Dose of ICI (mg/kg) (mean ± SD) | 2.47 (± 1.30) | 2.63 (± 1.60) | 2.31 (± 1.00) | 0.318 | |||
| Prior ICI use1 | 19 | 14.84% | 10 | 15.63% | 9 | 14.06% | 0.500 |
| Medical history, n (%) | |||||||
| Non-liver, non-upper GI diseaseb, n (%) | 28 | 21.88% | 18 | 28.13% | 10 | 15.63% | 0.087 |
| Personal history of autoimmune diseaseb, n (%) | 30 | 23.44% | 20 | 31.25% | 10 | 15.63% | 0.037 |
| Prior irAEb, n (%) | 8 | 12.50% | 7 | 10.90% | 1 | 1.56% | 0.062 |
| Family history of autoimmune diseaseb, n (%) | 10 | 7.81% | 8 | 12.50% | 2 | 3.13% | 0.048 |
| Prior immune-enhancing therapyb, n (%) | 11 | 8.59% | 2 | 3.13% | 9 | 14.06% | 0.027 |
| Prior interferon-γ therapy, n (%) | 7 | 5.47% | 1 | 1.56% | 6 | 9.38% | 0.115 |
| Vitamin D use, n (%) | 38 | 29.69% | 25 | 39.06% | 13 | 20.31% | 0.020 |
| Smoking (current or prior), n (%) | 61 | 47.66% | 33 | 51.56% | 28 | 43.75% | 0.376 |
| NSAID use, n (%) | 21 | 16.41% | 10 | 15.63% | 11 | 17.19% | 0.811 |
| Any vaccine, n (%) | 25 | 19.53% | 9 | 14.06% | 16 | 25.00% | 0.119 |
| Flu vaccine, n (%) | 19 | 14.84% | 7 | 10.94% | 12 | 18.75% | 0.214 |
| Pneumonia vaccine, n (%) | 11 | 8.59% | 4 | 6.25% | 7 | 10.94% | 0.344 |
| Other vaccine, n (%) | 2 | 1.56% | 1 | 1.56% | 1 | 1.56% | 1.000 |
| Weight at start of ICI (kg) (mean ± SD) | 78.1 (± 17.4) | 79.4 (± 16.9) | 76.8 (± 17.9) | 0.396 | |||
| Medications | |||||||
| Steroid at start of ICI, n (%) | 20 | 15.63% | 11 | 17.19% | 9 | 14.06% | 0.626 |
| Steroid duration (d) | N/A | 107.7 (± 164.2) | N/A | ||||
| Infliximab use, n (%) | N/A | 10 | 15.63% | N/A | |||
| Vedolizumab use, n (%) | N/A | 1 | 1.56% | N/A | |||
| Malignancy outcomes | |||||||
| Mean PFS (mo) | 12.8 (± 15.3) | 13.7 (± 14.9) | 11.9 (± 15.8) | 0.524 | |||
| PFS > 6 mo, n (%) | 63 | 49.22% | 35 | 54.69% | 28 | 43.75% | 0.216 |
| OS (mo) | 21.0 (± 18.9) | 24.3 (± 19.4) | 17.7 (± 18.0) | 0.050 | |||
| OS > 12 mo, n (%) | 72.0 | 56.25% | 42 | 65.63% | 30 | 46.88% | 0.025 |
| Death, n (%) | 20 | 15.63% | 6 | 9.38% | 14 | 21.88% | 0.051 |
Among the remainder of the clinical characteristics evaluated, personal history of autoimmune disease (including prior irAE) and family history of autoimmune disease were significantly more common in patients with IMC (P = 0.037 and 0.048, respectively). Intriguingly, prior use of a therapy designed to increase immune responses was more common in the control cohort without IMC (P = 0.027). In contrast to prior data[11], use of vitamin D supplementation at the time of first dose of ICI was significantly more prevalent in patients with IMC (P = 0.020). Neither smoking status, NSAID use at time of ICI initiation, steroid use at the time of ICI initiation, nor recent vaccination were significantly more common in IMC patients compared to controls.
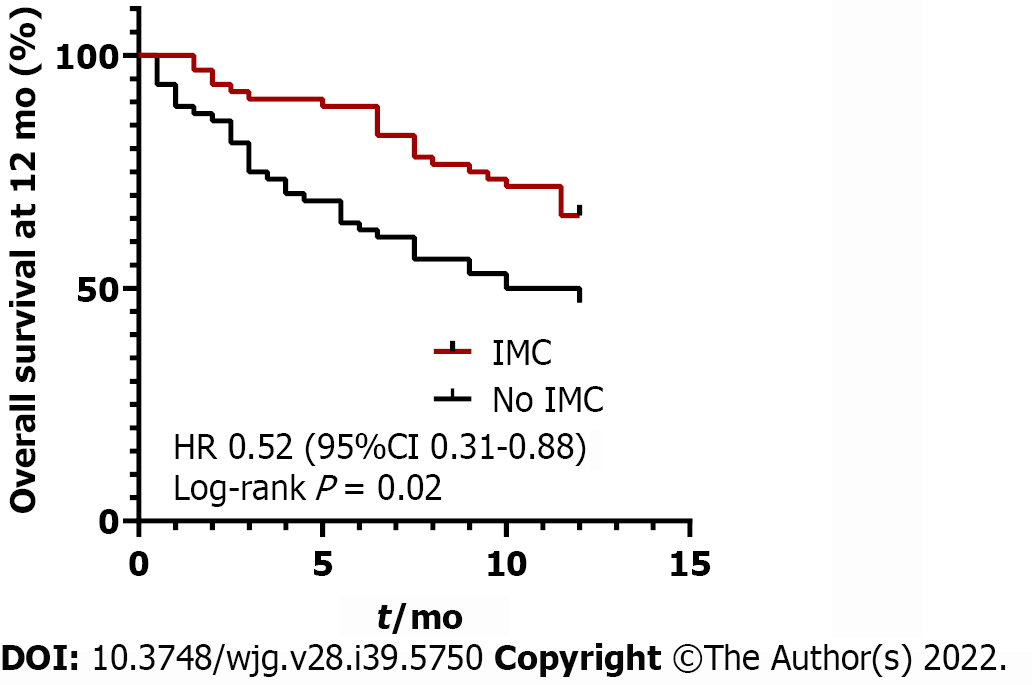
As IMC has previously been associated with increased OS and PFS in cancer patients[9,10], we evaluated whether this association was seen in our study. We found that OS was significantly longer in patients who developed IMC compared to those who did not, with a mean OS of 24.3 mo in patients with IMC and 17.7 mo in control (P = 0.05, Table 1). OS at 12 mo following ICI initiation was significantly higher in patients who developed IMC compared to those who did not (P = 0.02, Figure 1). However, in contrast to prior findings, our study did not find a significant difference in PFS between IMC patients and controls, with a mean PFS 13.7 mo in IMC patients and 11.9 mo in controls (P = 0.524) (Table 1). PFS also did not differ between patients who developed mild vs severe IMC (P = 0.690, Supplementary Table 5).
Across both cohorts, we identified clinical characteristics significantly associated with OS greater than 12 mo and PFS greater than 6 mo, which are correlated with cancer outcomes in patients treated with ICI[16] (Tables 2 and 3) (Supplementary Tables 4 and 5). IMC was significantly and independently associated with OS > 12 mo in the multivariate model (OR 2.81, 95%CI 1.17-6.77, P = 0.021) (Table 2). Number of ICI infusions was also positively associated with OS > 12 mo (OR 1.23, 95%CI 1.09-1.40), while sarcoma as underlying malignancy was significantly associated with OS < 12 mo (OR 0.17, 95%CI 0.029-0.947). Within the IMC cohort, nivolumab use was associated with OS < 12 mo in the univariate analysis (OR 0.09, 95%CI 0.01-0.83), while only age was associated with OS < 12 mo in multivariate analysis (OR 0.93, 95%CI 0.88-0.99) (Table 3). No individual malignancy was significantly associated with OS > 12 mo within the IMC cohort (Table 3).
| Clinical variables | Univariate predictors | Multivariate predictors | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Demographics | ||||||
| Age (yr) | 1.00 | 0.97-1.03 | 0.970 | |||
| Male | 0.92 | 0.45-1.87 | 0.822 | |||
| Female | 1.08 | 0.53-2.20 | 0.822 | |||
| Race | ||||||
| White | 1.37 | 0.58-3.25 | 0.473 | |||
| Black | 2.39 | 0.24-23.6 | 0.456 | |||
| Asian | 0.97 | 0.25-3.79 | 0.965 | |||
| Other | 0.45 | 0.14-1.45 | 0.181 | |||
| Type of malignancy | ||||||
| Melanoma | 0.87 | 0.43-1.74 | 0.688 | |||
| RCC | 1.65 | 0.53-5.12 | 0.390 | |||
| NSCLC | 2.52 | 0.65-9.80 | 0.181 | |||
| Sarcoma | 0.15 | 0.03-0.72 | 0.018 | 0.17 | 0.03-0.95 | 0.043 |
| Head and neck SCC | 1.04 | 0.22-4.84 | 0.961 | |||
| Other | 1.50 | 0.52-4.35 | 0.453 | |||
| Presence of IMC | 2.16 | 1.06-4.41 | 0.034 | 2.81 | 1.17-6.77 | 0.021 |
| Presence of high grade IMC | 0.47 | 0.16-1.38 | 0.167 | |||
| Stage IV malignancy | 0.48 | 0.14-1.61 | 0.233 | |||
| Type of Immune Checkpoint Inhibitor | ||||||
| Ipilimumab plus nivolumab | 1.32 | 0.30-5.77 | 0.714 | |||
| Ipilimumab | 0.74 | 0.29-1.85 | 0.517 | |||
| Nivolumab | 1.63 | 0.46-5.70 | 0.448 | |||
| Pembrolizumab | 2.93 | 1.27-6.73 | 0.011 | 1.06 | 0.38-2.98 | 0.911 |
| Atezolizumab | 1.32 | 0.30-5.77 | 0.714 | |||
| Number of ICI infusionsa | 1.19 | 1.08-1.32 | 0.001 | 1.23 | 1.09-1.40 | 0.001 |
| Dose of ICI (mg/kg) | 1.33 | 0.86-2.05 | 0.198 | |||
| Prior ICI use | 0.51 | 0.19-1.37 | 0.183 | |||
| Medical history | ||||||
| Non-liver, non-upper GI diseaseb | 0.87 | 0.38-2.02 | 0.747 | |||
| Personal history of autoimmune diseaseb | 1.47 | 0.63-3.40 | 0.373 | |||
| Family history of autoimmune diseaseb | 1.03 | 0.32-4.41 | 0.804 | |||
| Prior irAE | 2.84 | 0.31 - 25.9 | 0.356 | |||
| Prior immune-enhancing therapyb | 0.62 | 0.18-2.15 | 0.454 | |||
| Vitamin D use | 0.60 | 0.28-1.29 | 0.190 | |||
| Smoking (current or prior) | 0.74 | 0.37-1.50 | 0.410 | |||
| NSAID use | 1.04 | 0.41-2.69 | 0.928 | |||
| Any vaccine | 0.36 | 0.14-0.89 | 0.026 | 1.03 | 0.16-6.70 | 0.972 |
| Flu vaccine | 0.22 | 0.08-0.67 | 0.007 | 0.30 | 0.04-2.31 | 0.248 |
| Pneumonia vaccine | 0.41 | 0.11-1.48 | 0.175 | |||
| Other vaccine | 0.77 | 0.05-12.66 | 0.858 | |||
| Weight at start of ICI (kg) | 0.99 | 0.97-1.01 | 0.207 | |||
| Medications | ||||||
| Steroid at start of ICI | 0.74 | 0.29-1.93 | 0.541 | |||
| Steroid duration (d) | 1.00 | 0.997-1.01 | 0.368 | |||
| Infliximab use | 0.76 | 0.21-2.77 | 0.226 | |||
| Vedolizumab use | 1.00 | 0.99-1.01 | 1.000 | |||
| Clinical variables | Univariate predictors | Multivariate predictors | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Demographics | ||||||
| Age (yr) | 0.96 | 0.92-1.01 | 0.103 | 0.93 | 0.88-0.99 | 0.023 |
| Male | 0.82 | 0.29-2.32 | 0.711 | |||
| Female | 1.22 | 0.43-3.44 | 0.711 | |||
| Race | ||||||
| White | 0.87 | 0.23-3.27 | 0.835 | |||
| Black | 1.00 | 0.90-1.34 | 0.996 | |||
| Asian | 0.54 | 0.07-4.10 | 0.550 | |||
| Other | 1.07 | 0.97-1.11 | 0.912 | |||
| Type of malignancy | ||||||
| Melanoma | 1.26 | 0.45-3.51 | 0.654 | |||
| RCC | 0.51 | 0.12-2.28 | 0.381 | |||
| NSCLC | 0.53 | 0.10-2.85 | 0.456 | |||
| Sarcoma | 2.38 | 0.25-22.65 | 0.451 | |||
| Head and neck SCC | 1.05 | 0.89-1.10 | 0.865 | |||
| Other | 5.33 | 0.62-45.68 | 0.127 | |||
| Stage IV malignancy | 0.60 | 0.11-3.26 | 0.554 | |||
| Presence of high grade IMC | 0.91 | 0.32-2.57 | 0.855 | |||
| Type of immune checkpoint inhibitor | ||||||
| Ipilimumab plus nivolumab | 0.95 | 0.31-2.88 | 0.922 | |||
| Ipilimumab | 0.98 | 0.25-3.77 | 0.974 | |||
| Nivolumab | 0.09 | 0.01-0.83 | 0.033 | 0.13 | 0.01-1.43 | 0.096 |
| Pembrolizumab | 2.74 | 0.78-9.58 | 0.114 | 3.46 | 0.84-14.19 | 0.084 |
| Atezolizumab | 1.74 | 0.17-17.73 | 0.641 | |||
| Number of ICI infusionsa | 0.28 | 0.04-1.82 | 0.183 | |||
| Dose of ICI (mg/kg) | 1.88 | 0.36-9.83 | 0.457 | |||
| Prior ICI use | 0.46 | 0.12-1.80 | 0.265 | |||
| Medical history | ||||||
| Non-liver, non-upper GI diseaseb | 1.67 | 0.51-5.49 | 0.397 | |||
| Personal history of autoimmune diseaseb | 0.78 | 0.26-2.31 | 0.648 | |||
| Family history of autoimmune diseaseb | 0.93 | 0.20-4.29 | 0.922 | |||
| Prior immune-enhancing therapyb | 0.55 | 0.03-9.23 | 0.678 | |||
| Prior interferon-g therapy | 1.00 | 0.99-1.10 | 0.976 | |||
| Vitamin D use | 2.45 | 0.80-7.46 | 0.116 | 2.77 | 0.75-10.20 | 0.124 |
| Smoking (current or prior) | 1.66 | 0.59-5.65 | 0.334 | |||
| NSAID use | 2.55 | 0.49-13.16 | 0.265 | |||
| Any vaccine | 5.33 | 0.62-45.68 | 0.127 | |||
| Flu vaccine | 1.46 | 0.26-8.19 | 0.668 | |||
| Pneumonia vaccine | 1.00 | 0.99-1.05 | 0.995 | |||
| Other vaccine | 1.00 | 1.00-1.01 | 0.941 | |||
| Weight at start of ICI (kg) | 1.02 | 0.98-1.05 | 0.329 | |||
| Medications | ||||||
| Steroid at start of ICI | 0.98 | 0.25-3.77 | 0.974 | |||
| Steroid duration (d) | 1.00 | 1.00-1.01 | 0.736 | |||
| Infliximab use | 2.55 | 0.49-13.16 | 0.265 | |||
| Vedolizumab use | 1.00 | 1.00-1.01 | 0.936 | |||
As certain clinical characteristics were significantly more common in patients with IMC compared to controls, we evaluated whether any of these clinical characteristics were associated with risk of developing IMC (Table 4). In univariate analysis, history of autoimmune disease and vitamin D use were both significantly associated with increased risk of IMC (OR 2.45, 95%CI 1.04-5.78, P = 0.040 for autoimmune disease; OR 2.51, 95%CI 1.14-5.54, P = 0.022 for vitamin D use). Interestingly, the use of vitamin D supplementation has previously been associated with a decreased risk of IMC, in contrast to our findings here[11]. Prior use of an immune-enhancing therapy (Supplementary Table 2) was associated with a significantly decreased risk of IMC (OR 0.20, 95%CI 0.04-0.95, P = 0.043). In the multivariate model which incorporated these characteristics, only the use of immune-enhancing therapy remained significantly associated with decreased risk of IMC, with an OR of 0.20 (95%CI 0.04-1.00, P = 0.050).
| Clinical variables | Univariate predictors | Multivariate predictors | |||||
| OR | 95%CI | P value | OR | 95%CI | P value | ||
| Demographics | |||||||
| Age (yr) | 1.01 | 0.98-1.04 | 0.417 | ||||
| Male | 1.00 | 0.50-2.02 | 1.000 | ||||
| Female | 1.00 | 0.50-2.02 | 1.000 | ||||
| Race | |||||||
| White | 1.21 | 0.51-2.88 | 0.661 | ||||
| Black | 1.00 | 0.14-7.33 | 1.000 | ||||
| Asian | 0.79 | 0.20-3.07 | 0.730 | ||||
| Other | 0.84 | 0.27-2.66 | 0.770 | ||||
| Type of malignancy | |||||||
| Melanoma | 1.00 | 0.50-2.00 | 1.000 | ||||
| RCC | 1.16 | 0.40-3.42 | 0.784 | ||||
| NSCLC | 1.00 | 0.30-3.28 | 1.000 | ||||
| Sarcoma | 0.82 | 0.24-2.83 | 0.753 | ||||
| Head and neck SCC | 0.74 | 0.16-3.44 | 0.698 | ||||
| Other | 1.15 | 0.41-3.18 | 0.795 | ||||
| Stage IV malignancy | 0.72 | 0.24-2.22 | 0.572 | ||||
| Type of Immune Checkpoint Inhibitor | |||||||
| Ipilimumab plus nivolumab | 1.00 | 0.49-2.05 | 1.000 | ||||
| Ipilimumab | 1.00 | 0.40-2.51 | 1.000 | ||||
| Nivolumab | 1.00 | 0.30-3.28 | 1.000 | ||||
| Pembrolizumab | 1.00 | 0.47-2.13 | 1.000 | ||||
| Atezolizumab | 1.00 | 0.24-4.18 | 1.000 | ||||
| Number of Infusionsa | 0.98 | 0.93-1.02 | 0.273 | ||||
| Dose of ICI (mg/kg) | 1.23 | 0.82-1.84 | 0.327 | ||||
| Medical History | |||||||
| Non-liver, non-upper GIb | 2.11 | 0.89-5.03 | 0.091 | ||||
| Autoimmune diseaseb | 2.45 | 1.04-5.78 | 0.040 | 1.87 | 0.74-4.74 | 0.186 | |
| Prior irAE | 7.74 | 0.92-64.82 | 0.059 | ||||
| Family history of autoimmune diseaseb | 4.43 | 0.90-21.74 | 0.067 | 3.98 | 0.74-21.38 | 0.107 | |
| Prior immune-enhancing therapyb | 0.20 | 0.04-0.95 | 0.043 | 0.19 | 0.04-1.01 | 0.052 | |
| Prior interferon-γ therapy | 0.15 | 0.018-1.31 | 0.087 | ||||
| Vitamin D use | 2.51 | 1.14-5.54 | 0.022 | 2.48 | 1.01-6.07 | 0.047 | |
| Smoking (current or prior) | 1.37 | 0.68-2.74 | 0.377 | ||||
| NSAID use | 0.89 | 0.35-2.28 | 0.811 | ||||
| Any vaccine | 0.49 | 0.20-1.21 | 0.123 | ||||
| Flu vaccine | 0.53 | 0.19-1.45 | 0.219 | ||||
| Pneumonia vaccine | 0.54 | 0.15-1.95 | 0.350 | ||||
| Other vaccine | 1.00 | 0.06-16.34 | 1.000 | ||||
| Weight at start of ICI (kg) | 1.01 | 0.99-1.03 | 0.393 | ||||
We next determined if any variables were associated with an increased risk of severe IMC. Consistent with prior studies of irAE in ICI[17-19], we defined grade 1-2 IMC as mild and grade 3 or higher IMC as severe. In our study, 38 of the 64 patients (59.4%) had severe IMC (Supplementary Table 3). In the univariate model, ipilimumab and vitamin D supplementation were significantly associated with development of severe IMC (OR 8.93, 95%CI 1.07-74.8, P = 0.043 for ipilimumab; OR 3.33, 95%CI 1.10-10.14, P = 0.034 for vitamin D) (Supplementary Table 6). Combination therapy (ipilimumab plus nivolumab) trended towards an increased risk of severe IMC but did not reach significance (P = 0.053). In contrast, pembrolizumab was significantly associated with a decreased risk of severe IMC (OR 0.26, 95%CI 0.09-0.81, P = 0.020). In the multivariate model no characteristic reached significance for association with severe IMC, although both combination therapy and ipilimumab monotherapy approached significance for increased risk of severe IMC (P = 0.058 and 0.060, respectively).
In our study, development of IMC following ICI use was associated with improved overall survival, although not improved progression-free survival, compared to ICI users without IMC. This is similar to findings at another center demonstrating both improved OS and PFS in patients with IMC[9,10]. We also found that vitamin D supplementation at the start of ICI treatment is a risk factor for developing IMC, in contrast to other research suggesting vitamin D use is associated with lower risk of IMC[11]. Our results, therefore, provide critical additional information on these previous associations and present a need for prospective studies.
Both publications showing improved survival in patients with IMC were retrospective analyses performed at the same center[9,10]. One study noted that ICI class was significantly associated with development of IMC[9], a finding that has been demonstrated several times in retrospective work[8,17,18,20-23]. However, unlike our work, this study did not match control patients to account for this likely confounder, as ICI class has been associated with differences in PFS in some malignancies[24,25]. The second study at this center examined survival in melanoma patients with IMC, compared to our work across multiple malignancies, although frequency matching was performed to account for use of different ICI classes[10]. Since our study is the first to examine survival in patients with IMC at a different center, our work here reinforces that IMC may be associated with increased overall survival and prompts a need for prospective studies.
The only other independent factor in our study positively associated with OS > 12 mo was number of ICI doses. This finding may be due to trivial length-time bias, as patients who survive longer are more likely to receive more doses of ICI. It is also possible that patients who required cessation of ICI due to IMC had worse outcomes, although prior work has suggested that patients still derive equivalent long-term benefit from ICI even if stopped due to irAE[26]. Type of underlying malignancy (sarcoma) was independently associated with OS < 12 mo in our study. These findings are not unexpected, as most advanced soft tissue sarcomas have a median OS of less than one year[27].
In contrast to prior work, we found a positive association between vitamin D supplementation and development of IMC[11]. It is unclear if this is related to low serum vitamin D levels or negative impact of the supplementation itself, as vitamin D levels near the time of ICI initiation were not recorded in most patients. Additionally, the prior report on vitamin D in IMC was in melanoma patients only, which may partially account for discrepancies with our study. As this association did not remain significant in our multivariate analysis, it is possible that another confounding factor may explain the association between vitamin D supplementation and IMC in our study.
In addition to challenging existing findings, we report here on additional novel risk factors for IMC. We are the first to report that prior use of immune-enhancing medications prior to ICI, such as IL-2 or interferon-γ, is significantly and independently associated with decreased risk of IMC. Much more work should be done to evaluate the relationship between these medications and future risk of IMC.
Finally, our study is the first to examine risk factors for severe IMC. In addition to increasing risk for IMC overall, we find that vitamin D supplementation may also be a risk factor for severe IMC. Similarly, our results suggest that the use of ipilimumab may be associated with increased risk of severe IMC, while pembrolizumab may be associated with decreased risk of severe IMC in patients who develop this syndrome. As ipilimumab has previously been associated with increased risk of IMC overall, while anti-PD-1, including pembrolizumab, are associated with lower risk of IMC overall[8,9], these findings emphasize that ICI class may affect severity of IMC.
Our findings may significantly impact clinical practice by identifying novel risks for IMC and severe IMC that clinicians, including oncologists and gastroenterologists, should be aware of, while also potentially providing reassurance to physicians and patients that development of IMC may be a positive prognosticator for cancer survival. Neither prior work nor ours found that treatment of IMC, including steroids or infliximab, negatively impacts OS[9,10], and therefore appropriate treatment of IMC should be pursued early on to minimize morbidity and mortality. Both steroid and infliximab use have been suggested to worsen survival in ICI users[12,13], but all current evidence suggests that use of these medications for IMC specifically does not impair cancer outcomes. Our work also cautions against supplementation with vitamin D in ICI users, as this may increase risk of IMC and severe IMC, although carefully designed studies with vitamin D measurements should be performed.
Our work has several strengths. We performed robust cohort matching to minimize confounding effects of ICI class and malignancy. This is also the first study to explore risk factors associated with severe IMC. However, there are limitations to our work. As a retrospective, observational study, it is subject to recall bias and cannot evaluate causation, and may also be subject to immortal time bias (ITB). Patients may have longer exposure to checkpoint inhibitors before developing IMC, compared to patients who do not manifest this irAE, leading to a period where they must survive for long enough to develop IMC and are therefore “immortal”[28]. We found that OS > 12 mo was significantly associated with greater numbers of ICI infusions (Table 2), which is likely due to ITB. However, greater numbers of infusions were not associated with IMC (Table 4). This suggests that the association between OS > 12 mo and IMC is likely independent of the number of ICI infusions, limiting this as a source of ITB in our study.
Other weaknesses of our work include selection of patients based on clinical criteria for IMC, including those who did not undergo endoscopy or other objective testing for intestinal inflammation, and therefore may not have had a true colitis. Like prior work, this is also a single-center study, and our results may not be widely generalizable, particularly since we identified fewer patients compared to prior work and our patient population is highly variable, including individuals with several different underlying malignancies. We did not exclude patients with prior non-GI irAEs in either group, although the presence of these was not independently associated with increased OS in our study. We also have not accounted for other factors which may be potential predictors of ICI response, including tumor PD-L1 expression burden, tumor mutational burden, gut microbial composition, proton pump inhibitor use, and combination treatment with tyrosine kinase inhibitors[29-34].
In conclusion, our findings suggest presence of IMC is associated with improved OS in cancer patients when cases were matched closely to controls. We also found that vitamin D supplementation was significantly associated with development of both IMC and severe IMC, while immune-enhancing medications were significantly associated with decreased risk of IMC. Future work should focus on broader populations to resolve the discrepancies raised in our work, and to confirm the association between IMC and increased cancer survival. Closely involving gastroenterologists with the workup and management of IMC will be crucial to ensuring the best care possible for these patients.
Immune checkpoint inhibitor-mediated colitis (IMC) is a common immune-related side effect (irAE) of checkpoint inhibitor treatment for cancer. Prior work has suggested that IMC may be associated with increased survival from cancer.
We sought to determine if IMC was associated with increased overall survival (OS) in a cohort of patients at our institution. These findings could expand existing data on IMC and cancer outcomes and might suggest a common immunological underpinning between the efficacy of checkpoint inhibitors and certain irAEs.
We performed a retrospective case-control study of individuals treated with immune checkpoint inhibitors at our institution who developed IMC, closely matched to a cohort of patients treated with checkpoint inhibitors without IMC. Using univariate and multivariate logistic regression, we determined significant clinical predictors of IMC and the association of presence of IMC on OS.
We found that IMC was significantly associated with a higher OS as well as OS greater than 12 mo. In contrast to previous findings, vitamin D supplementation was significantly associated with development of both IMC and severe IMC. However, prior treatment with immune-enhancing medications was significantly associated with decreased risk of IMC.
In multivariate logistic regression analysis, IMC was significantly associated with a higher OS but not PFS. IMC was significantly associated with OS greater than 12 mo. Vitamin D supplementation was associated with increased risk of IMC.
Our findings lend strength to the idea that IMC is associated with improved cancer outcomes with checkpoint inhibitor treatment. This may suggest common immunologic underpinnings between IMC and the anti-tumor effects of checkpoint inhibitors. These results also emphasize the importance of involving gastroenterologists with the management of IMC.
Future research in this area should seek to expand current knowledge of the relationship between IMC and cancer survival. In particular, future work should focus on broadening the type and number of patients treated with immune checkpoint inhibitors and on tracking patients prior to initiating checkpoint inhibitors to determine if this relationship remains significant prospectively.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen YH, China; Rizzo A, Italy; Zhang XJ, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10799] [Cited by in RCA: 11786] [Article Influence: 785.7] [Reference Citation Analysis (0)] |
| 2. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7247] [Article Influence: 724.7] [Reference Citation Analysis (0)] |
| 3. | Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhäufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crinò L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab vs Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6945] [Cited by in RCA: 7536] [Article Influence: 753.6] [Reference Citation Analysis (0)] |
| 4. | Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi A, Gerritsen W, Gurney H, Quinn DI, Culine S, Sternberg CN, Mai Y, Poehlein CH, Perini RF, Bajorin DF; KEYNOTE-045 Investigators. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med. 2017;376:1015-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2051] [Cited by in RCA: 2627] [Article Influence: 328.4] [Reference Citation Analysis (0)] |
| 5. | Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Foukakis T, Fasching PA, Cardoso F, Untch M, Jia L, Karantza V, Zhao J, Aktan G, Dent R, O'Shaughnessy J; KEYNOTE-522 Investigators. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med. 2020;382:810-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1006] [Cited by in RCA: 1899] [Article Influence: 379.8] [Reference Citation Analysis (0)] |
| 6. | Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 3158] [Article Influence: 451.1] [Reference Citation Analysis (0)] |
| 7. | Collins M, Soularue E, Marthey L, Carbonnel F. Management of Patients With Immune Checkpoint Inhibitor-Induced Enterocolitis: A Systematic Review. Clin Gastroenterol Hepatol. 2020;18:1393-1403.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Khoja L, Day D, Wei-Wu Chen T, Siu LL, Hansen AR. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28:2377-2385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 647] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS, Qiao W, Lum P, Raju G, Shuttlesworth G, Stroehlein J, Diab A. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer. 2018;6:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 184] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 10. | Abu-Sbeih H, Ali FS, Qiao W, Lu Y, Patel S, Diab A, Wang Y. Immune checkpoint inhibitor-induced colitis as a predictor of survival in metastatic melanoma. Cancer Immunol Immunother. 2019;68:553-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Grover S, Dougan M, Tyan K, Giobbie-Hurder A, Blum SM, Ishizuka J, Qazi T, Elias R, Vora KB, Ruan AB, Martin-Doyle W, Manos M, Eastman L, Davis M, Gargano M, Haq R, Buchbinder EI, Sullivan RJ, Ott PA, Hodi FS, Rahma OE. Vitamin D intake is associated with decreased risk of immune checkpoint inhibitor-induced colitis. Cancer. 2020;126:3758-3767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, Massard C, Fuerea A, Ribrag V, Gazzah A, Armand JP, Amellal N, Angevin E, Noel N, Boutros C, Mateus C, Robert C, Soria JC, Marabelle A, Lambotte O. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1248] [Cited by in RCA: 1572] [Article Influence: 174.7] [Reference Citation Analysis (0)] |
| 13. | Verheijden RJ, May AM, Blank CU, Aarts MJB, van den Berkmortel FWPJ, van den Eertwegh AJM, de Groot JWB, Boers-Sonderen MJ, van der Hoeven JJM, Hospers GA, Piersma D, van Rijn RS, Ten Tije AJ, van der Veldt AAM, Vreugdenhil G, van Zeijl MCT, Wouters MWJM, Haanen JBAG, Kapiteijn E, Suijkerbuijk KPM. Association of Anti-TNF with Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1-Treated Patients in the Dutch Melanoma Treatment Registry. Clin Cancer Res. 2020;26:2268-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 14. | National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available from: https://ctep.cancer.gov/20protocoldevelopment/electronic_applications/%20docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. [DOI] [Full Text] |
| 15. | Collins M, Michot JM, Danlos FX, Mussini C, Soularue E, Mateus C, Loirat D, Buisson A, Rosa I, Lambotte O, Laghouati S, Chaput N, Coutzac C, Voisin AL, Soria JC, Marabelle A, Champiat S, Robert C, Carbonnel F. Inflammatory gastrointestinal diseases associated with PD-1 blockade antibodies. Ann Oncol. 2017;28:2860-2865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 120] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 16. | Kok PS, Cho D, Yoon WH, Ritchie G, Marschner I, Lord S, Friedlander M, Simes J, Lee CK. Validation of Progression-Free Survival Rate at 6 Months and Objective Response for Estimating Overall Survival in Immune Checkpoint Inhibitor Trials: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3:e2011809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 17. | Wang DY, Ye F, Zhao S, Johnson DB. Incidence of immune checkpoint inhibitor-related colitis in solid tumor patients: A systematic review and meta-analysis. Oncoimmunology. 2017;6:e1344805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 18. | Bishay K, Tandon P, Bourassa-Blanchette S, Laurie SA, McCurdy JD. The risk of diarrhea and colitis in patients with lung cancer treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Curr Oncol. 2020;27:e486-e494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | De Velasco G, Je Y, Bossé D, Awad MM, Ott PA, Moreira RB, Schutz F, Bellmunt J, Sonpavde GP, Hodi FS, Choueiri TK. Comprehensive Meta-analysis of Key Immune-Related Adverse Events from CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol Res. 2017;5:312-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 359] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 20. | Soularue E, Lepage P, Colombel JF, Coutzac C, Faleck D, Marthey L, Collins M, Chaput N, Robert C, Carbonnel F. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut. 2018;67:2056-2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 21. | Tandon P, Bourassa-Blanchette S, Bishay K, Parlow S, Laurie SA, McCurdy JD. The Risk of Diarrhea and Colitis in Patients With Advanced Melanoma Undergoing Immune Checkpoint Inhibitor Therapy: A Systematic Review and Meta-Analysis. J Immunother. 2018;41:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Komaki Y, Komaki F, Yamada A, Micic D, Ido A, Sakuraba A. Meta-Analysis of the Risk of Immune-Related Adverse Events With Anticytotoxic T-Lymphocyte-Associated Antigen 4 and Antiprogrammed Death 1 Therapies. Clin Pharmacol Ther. 2018;103:318-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Yao J, Li M, Zhang H, Ge Y, Weygant N, An G. Differential risks of immune-related colitis among various immune checkpoint inhibitor regimens. Int Immunopharmacol. 2020;87:106770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, Larkin J, Lorigan P, Neyns B, Blank C, Petrella TM, Hamid O, Zhou H, Ebbinghaus S, Ibrahim N, Robert C. Pembrolizumab vs ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet. 2017;390:1853-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 922] [Article Influence: 115.3] [Reference Citation Analysis (0)] |
| 25. | Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil CM, Lotem M, Larkin JMG, Lorigan P, Neyns B, Blank CU, Petrella TM, Hamid O, Su SC, Krepler C, Ibrahim N, Long GV. Pembrolizumab vs ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20:1239-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 839] [Article Influence: 139.8] [Reference Citation Analysis (0)] |
| 26. | Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hogg D, Hill A, Márquez-Rodas I, Haanen J, Guidoboni M, Maio M, Schöffski P, Carlino MS, Lebbé C, McArthur G, Ascierto PA, Daniels GA, Long GV, Bastholt L, Rizzo JI, Balogh A, Moshyk A, Hodi FS, Wolchok JD. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381:1535-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1826] [Cited by in RCA: 2640] [Article Influence: 440.0] [Reference Citation Analysis (0)] |
| 27. | Savina M, Le Cesne A, Blay JY, Ray-Coquard I, Mir O, Toulmonde M, Cousin S, Terrier P, Ranchere-Vince D, Meeus P, Stoeckle E, Honoré C, Sargos P, Sunyach MP, Le Péchoux C, Giraud A, Bellera C, Le Loarer F, Italiano A. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa in a real-life setting: the METASARC observational study. BMC Med. 2017;15:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 146] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 28. | Yadav K, Lewis RJ. Immortal Time Bias in Observational Studies. JAMA. 2021;325:686-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 244] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 29. | Rizzo A, Mollica V, Santoni M, Ricci AD, Rosellini M, Marchetti A, Montironi R, Ardizzoni A, Massari F. Impact of Clinicopathological Features on Survival in Patients Treated with First-line Immune Checkpoint Inhibitors Plus Tyrosine Kinase Inhibitors for Renal Cell Carcinoma: A Meta-analysis of Randomized Clinical Trials. Eur Urol Focus. 2022;8:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 30. | Mollica V, Santoni M, Matrana MR, Basso U, De Giorgi U, Rizzo A, Maruzzo M, Marchetti A, Rosellini M, Bleve S, Maslov D, Tawagi K, Philon E, Blake Z, Massari F. Concomitant Proton Pump Inhibitors and Outcome of Patients Treated with Nivolumab Alone or Plus Ipilimumab for Advanced Renal Cell Carcinoma. Target Oncol. 2022;17:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 31. | Rizzo A, Ricci AD. PD-L1, TMB, and other potential predictors of response to immunotherapy for hepatocellular carcinoma: how can they assist drug clinical trials? Expert Opin Investig Drugs. 2022;31:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 32. | Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, Fidelle M, Flament C, Poirier-Colame V, Opolon P, Klein C, Iribarren K, Mondragón L, Jacquelot N, Qu B, Ferrere G, Clémenson C, Mezquita L, Masip JR, Naltet C, Brosseau S, Kaderbhai C, Richard C, Rizvi H, Levenez F, Galleron N, Quinquis B, Pons N, Ryffel B, Minard-Colin V, Gonin P, Soria JC, Deutsch E, Loriot Y, Ghiringhelli F, Zalcman G, Goldwasser F, Escudier B, Hellmann MD, Eggermont A, Raoult D, Albiges L, Kroemer G, Zitvogel L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2493] [Cited by in RCA: 3802] [Article Influence: 475.3] [Reference Citation Analysis (0)] |
| 33. | Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre ML, Luke JJ, Gajewski TF. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 2132] [Article Influence: 304.6] [Reference Citation Analysis (1)] |
| 34. | Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, Cogdill AP, Zhao L, Hudgens CW, Hutchinson DS, Manzo T, Petaccia de Macedo M, Cotechini T, Kumar T, Chen WS, Reddy SM, Szczepaniak Sloane R, Galloway-Pena J, Jiang H, Chen PL, Shpall EJ, Rezvani K, Alousi AM, Chemaly RF, Shelburne S, Vence LM, Okhuysen PC, Jensen VB, Swennes AG, McAllister F, Marcelo Riquelme Sanchez E, Zhang Y, Le Chatelier E, Zitvogel L, Pons N, Austin-Breneman JL, Haydu LE, Burton EM, Gardner JM, Sirmans E, Hu J, Lazar AJ, Tsujikawa T, Diab A, Tawbi H, Glitza IC, Hwu WJ, Patel SP, Woodman SE, Amaria RN, Davies MA, Gershenwald JE, Hwu P, Lee JE, Zhang J, Coussens LM, Cooper ZA, Futreal PA, Daniel CR, Ajami NJ, Petrosino JF, Tetzlaff MT, Sharma P, Allison JP, Jenq RR, Wargo JA. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2999] [Cited by in RCA: 3310] [Article Influence: 472.9] [Reference Citation Analysis (0)] |