Published online Oct 21, 2022. doi: 10.3748/wjg.v28.i39.5723
Peer-review started: July 16, 2022
First decision: August 19, 2022
Revised: August 24, 2022
Accepted: October 10, 2022
Article in press: October 10, 2022
Published online: October 21, 2022
Processing time: 94 Days and 4.4 Hours
The novel coronavirus disease 2019 is an infection caused by severe acute re
Core Tip: There are several reviews in the literature that discuss the pathophysiology, management, and outcomes of liver injury in coronavirus disease 2019 (COVID-19). Here, we reviewed the current understanding on various aspects of COVID-19-related liver injury, including the high-risk populations, the characteristic clinical manifestations, the possible pathogenic mechanism, the pathological changes, the current suggestions for clinical treatment for the spectrum of populations, and the prognosis of the condition.
- Citation: Payus AO, Mohd Noh M, Azizan N, Muthukaruppan Chettiar R. SARS-CoV-2-induced liver injury: A review article on the high-risk populations, manifestations, mechanisms, pathological changes, management, and outcomes. World J Gastroenterol 2022; 28(39): 5723-5730
- URL: https://www.wjgnet.com/1007-9327/full/v28/i39/5723.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i39.5723
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the name given to the newly emerged zoonotic virus that causes coronavirus disease 2019 (COVID-19)[1]. It was first reported in Wuhan, China on December 29, 2019 and was declared a global pandemic on March 11, 2020[2]. SARS-CoV-2 is an enveloped, single-stranded positive-sense RNA genome virus that harbors the largest genome among currently known RNA viruses, with a genome length of around 26 to 32 kb. It has an oval shape and an average size of 100 nm in diameter. Electron microscopy revealed large club-shaped spikes of glycoprotein membrane on the viral surface making the viral particles appear like a typical crown-like shape[3].
COVID-19 is a syndrome with various systemic and respiratory symptoms such as fever, fatigue, dry cough, and breathing difficulties. It can be critical, causing severe pneumonia and cardiorespiratory failure that requires specialized management in intensive care units[4]. SARS-CoV-2 can also affect other systems, namely the nervous system causing headache, anosmia, paresthesia, and altered consciousness[5]. Abnormal liver function parameters are commonly found in patients with SARS-CoV-2 infection, indicating that SARS-COV-2 infection is associated with liver injury and even failure. Apart from that, several studies suggested that liver injury has a significant role in determining the severity and mortality rate of the disease. Considering the ongoing global threat of SARS-CoV-2 infection and the necessity to improve the prognosis of the disease, the treating physicians need to be aware of the association and significance of SARS-CoV-2 infection-related liver injury not only for the severity of the disease but also for the mortality rate and prognosis as a whole. Therefore, this review aimed to elucidate the importance of hepatobiliary involvement in SARS-CoV-2 infections and provide helpful information for managing the condition and improving the overall prognosis of the disease.
Since the beginning of the pandemic, it was reported that patients with severe SARS-CoV-2 infection tended to develop liver injury compared to mild infection. Cai et al[6] reported that male patients of older age and higher body mass index have a higher tendency to develop liver injury during the course of the disease. A similar finding was seen in a study on 79 non-hospitalized SARS-CoV-2 patients by Xie et al[7], who reported that liver injury was more common among male patients. The authors also said that patients with an underlying severe chronic lung disease have a higher rate of liver injury, which was also reported by Zhang et al[8]. Cai et al[6] and Singh and Khan[9] both found that liver injury was more common among patients with underlying liver disease. According to Da et al[10], the common etiology of chronic liver disease that is prone to developing worsening liver injury during the infection is alcohol-related liver disease. Patients with nonalcoholic fatty liver disease and nonalcoholic steatohepatitis are usually associated with additional metabolic risk factors, such as obesity, that can increase the susceptibility to the infection and is commonly associated with a more severe presentation[11].
There has been significant concern about the increased susceptibility to SARS-CoV-2 infection among solid organ transplant recipients. In a systematic review by Piedade and Pereira[12], patients with liver transplant were not associated with an increased risk of SARS-CoV-2 infection. The risk is highly dependent on the sex, age, body mass index, history of hepatocellular carcinoma, and the immunosuppression drug dose of the patient. However, the prevalence of severe infection was higher among liver transplanted patients. A study by Becchetti et al[13] found that alterations in liver enzymes among liver transplanted patients with SARS-CoV-2 occurs more commonly among hospitalized patients. In addition, Ali Malekhosseini et al[14] showed that the admission rate of liver transplanted patients to the intensive care unit was as high as 33.3%.
No evidence shows that pregnancy increases susceptibility to SARS-CoV-2-induced liver injury. Nevertheless, a retrospective cohort study involving 122 pregnant patients with confirmed SARS-CoV-2 infection by Can et al[15] found that 13.9% developed an abnormal liver function that was generally mild, where most of them were critically ill and had a longer stay in the hospital compared to the normal liver function group.
The most common manifestations of SARS-CoV-2 induced liver injury was the elevation of liver enzymes, such as alanine transaminase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase, and alkaline phosphatase. In a meta-analysis completed in the first few months of the pandemic by Cai et al[6], about 25% of SARS-CoV-2 patients had increased liver enzyme levels, which showed a direct association with the disease activity. The prevalence of increased AST was higher than ALT levels and was positively correlated with the severity of cases, where the level was higher in patients with severe cases[7,8,15]. Lei et al[16] reported a significant association between inpatient mortality in SARS-CoV-2 infected patients and liver injury based on liver enzymes, specifically AST elevation.
In a study on 417 SARS-CoV-2 infected patients by Cai et al[6], 41.0% of patients had abnormal liver tests, and 5.0% had liver injury upon presentation to the hospital. Throughout hospitalization, 76.3% developed some form of abnormal liver function, and it was high enough to be considered liver injury in 21.5% of patients. A similar finding was reported by Fan et al[17], who conducted a retrospective single center study on 148 patients with SARS-CoV-2 infection, where 37.2% had an abnormal liver function at hospital admission. Patients with the abnormal liver function were also found to have an extended hospital stays. A retrospective study of 79 patients with SARS-CoV-2 by Xie et al[7] found that patients with an abnormal liver test had an extended stay in the hospital.
Phipps et al[18] reported that 21.0% of 2273 patients with SARS-CoV-2 infection had a moderate liver injury, which was defined as elevated liver enzymes two to five times above the upper limit of normal, and 6.4% had severe liver injury, which was defined as liver enzymes more than five times the upper limit of normal. In this study, 69% of the patients with liver injury required intensive care unit care. The reports also mentioned that severe liver injury was associated with elevated inflammation markers, including ferritin and interleukin 6 (IL-6).
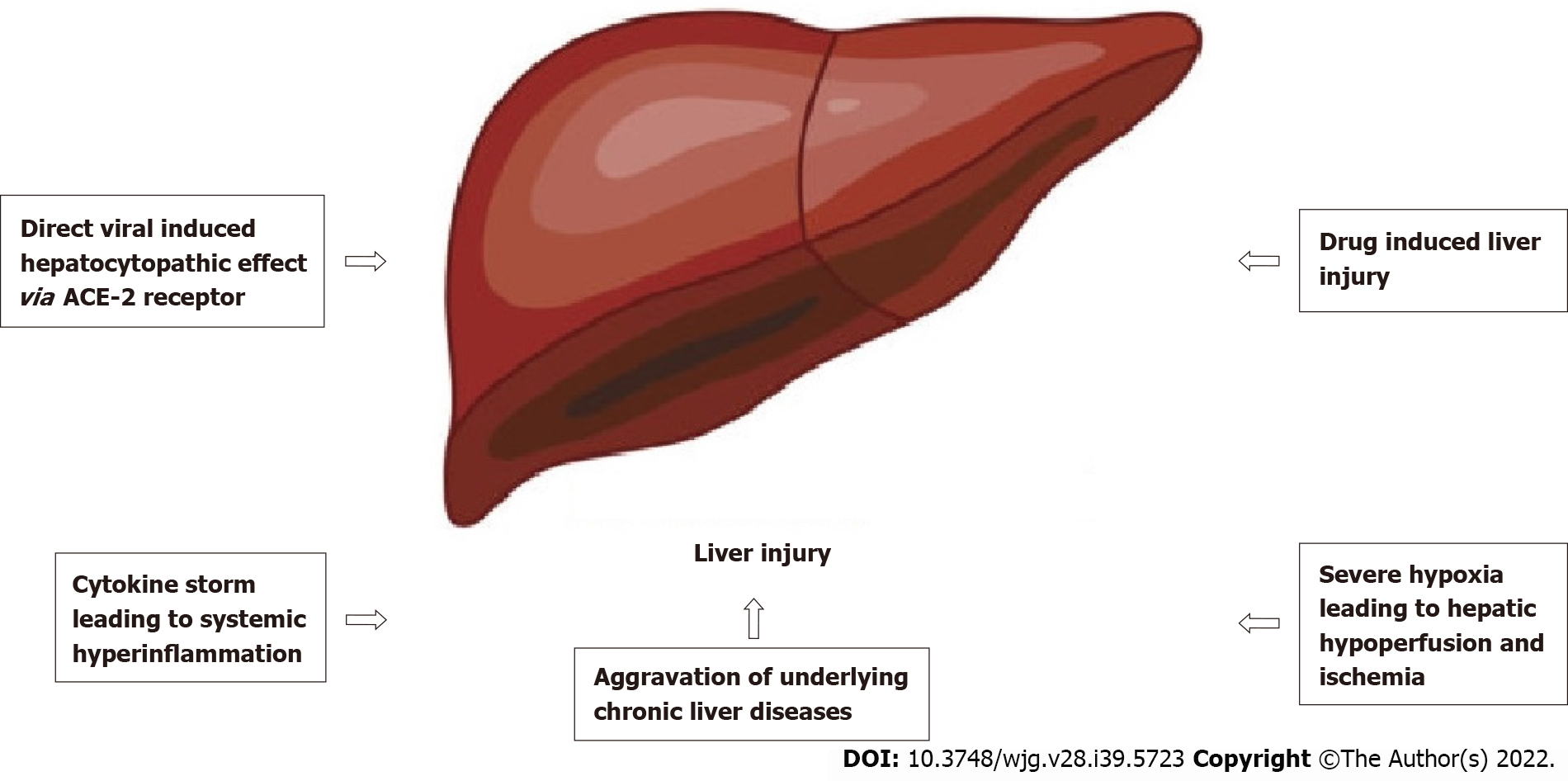
The exact pathophysiological mechanism of SARS-CoV-2-induced liver injury is still poorly understood, but evidence has shown it to be multifactorial (as shown in Figure 1). One of the factors is direct invasion of SARS-CoV-2, which has been suggested in several studies. The primary receptor for SARS-CoV-2 cellular entry is the angiotensin-converting enzyme 2 (ACE2) receptors, which are found not only in the lung parenchyma but also in other parts of the body[19], such as the brain[5], gastrointestinal tract, biliary tree, and liver epithelia[20]. Zhou et al[21] stated that SARS-CoV-2 patients with gastrointestinal symptoms had higher AST and ALT levels, which reflected that ACE2 receptor was expressed within the gastrointestinal tract and the biliary tree. However, even though the ACE2 receptor is expressed more within the biliary tree than the liver parenchyma, most studies showed a predominant pattern of parenchymal liver injury based on the elevated levels of AST and ALT rather than the damage to the bile ducts, which was reflected by increased gamma-glutamyl transferase and alkaline phosphatase[22].
Wu et al[23] found that almost 50% of SARS-CoV-2 infected patients who recovered from the disease had persistent virus shedding in their fecal specimens for more than 10 d after viral detection in respiratory tract samples became negative. This may further support the possibility of viral replication in the hepatobiliary system. Similarly, the previous SARS-CoV strains that caused an outbreak from 2002 to 2004 have also been shown to directly injure the liver parenchyma causing lobular inflammation and apoptosis of hepatocytes[24].
Apart from the direct viral-induced hepatocytopathic hypothesis, autoinflammatory mediated injury to the liver is another plausible explanation. Immune dysregulation can occur in severe SARS-CoV-2 infection, which the overactivation of the immune system will lead to systemic hyperinflammation in extreme conditions. This condition is called ‘cytokine storm syndrome’, which is a phenomenon that will not only cause pulmonary inflammation but also multiorgan involvement, including the nervous system causing encephalitis[25] and peripheral neuritis[26] and the liver causing acute hepatitis and even failure[27,28].
Drug-induced liver injury is also common in SARS-CoV-2 patients, as the medications used to treat the infection can be hepatotoxic. These include lopinavir/ritonavir, remdesivir, tocilizumab, and others[29]. A study of 148 cases of SARS-CoV-2 infected patients in Shanghai by Li et al[30] found that the utilization rate of lopinavir/ritonavir among patients with abnormal liver function was higher than the patients with normal liver function. There was no significant difference in the pre-hospital medication between the two groups of patients. The exact mechanism of how lopinavir/ritonavir induces liver injury is still uncertain, but there is evidence that it activates the endoplasmic reticulum stress pathway in the liver and induces hepatocytes apoptosis[31].
Ritonavir is also widely metabolized by the liver through the cytochrome P450 system, where the production of toxic intermediates of any drugs that are metabolized by the system will have the potential of causing liver injury[32]. Tocilizumab, which is an IL-6 inhibitor that is used to reduce overactive inflammation, has been reported to cause drug-induced liver injury and liver failure, which in some cases requires a liver transplant[33]. The exact mechanism is still unknown but may be due to its inhibitory effect on the IL-6 pathway, which is essential for liver regeneration.
SARS-CoV-2 patients with underlying chronic liver diseases are more likely to suffer from liver injury. This may suggest that SARS-CoV-2 infection can aggravate underlying liver diseases. In addition, there is a possibility that the liver damage may be caused by the viral reactivation of existing liver diseases in SARS-CoV-2 infection. Some biological medications such as tocilizumab and baricitinib may cause reactivation of viral hepatitis B, which causes deterioration of liver function[34].
Another simpler hypothesis is that prolonged hypoxia and tissue ischemia in critically ill SARS-CoV-2 patients who suffer from severe pneumonia and acute respiratory distress syndrome can also be one of the mechanisms of liver injury and even failure[35]. This occurs due to prolonged tissue hypoperfusion leading to ischemia, including in the liver. The anaerobic metabolism and lactic acidosis will further depress the cardiorespiratory effort, which will cause the continuation of the vicious circle[36].
The first post-mortem autopsy on a patient who succumbed to SARS-CoV-2 infection was reported by Xu et al[37]. The liver histology showed a moderate degree of microvesicular steatosis with mild lobular and portal vein activity in the study. Ji et al[38] reported overactivation of T cells, suggesting viral-induced cytotoxic T cell liver damage. Liu et al[39] described various hepatic lesions, including focal lobular necrosis, lobular lymphocytic and monocytic infiltration, ballooning degeneration of liver cells, and sinusoidal congestion with microthrombosis. A study on 48 liver autopsies by Sonzogni et al[40] reported focal portal and lobular lymphocytic infiltrates with multiple vascular changes, which are suggestive of hepatic vascular involvement.
Tian et al[41] also reported a similar autopsy finding of mild lobular lymphocytic infiltration, with sinusoidal expansion of the central lobule and patchy necrosis in the periportal and centrilobular areas. There was no significant inflammatory cell infiltration around the portal tracts, which is consistent with the mode of acute liver injury. Autopsy reports on 7 SARS-CoV-2 infected patients who died noted multiple platelet-fibrin microthrombi in the hepatic sinusoids[42]. Wang et al[43] and Wang et al[44] reported massive hepatic apoptosis, microvesicular steatosis, and inflammatory cell infiltration over the portal systems. In addition, there was a large amount of viral SARS-CoV-2 RNA titers detected in the liver via reverse transcriptase-polymerase chain reaction[41,45].
Liver injury is a severe complication of SARS-CoV-2 infection and can significantly affect the outcome of the patient. Multiple studies have suggested regular monitoring of liver function parameters in SARS-CoV-2-infected patients. Based on the consensus statement of the American Association for the Study of Liver Diseases[46], it is recommended to consider etiologies outside SARS-CoV-2, such as other viral hepatitis. This has been proven in a case reported by Hambali et al[47], where a patient with SARS-CoV-2 infection presented with abnormal liver function and high IL-6, which was due to hepatocellular carcinoma. It is also essential to consider other indirect causes of liver injury such as myositis, cardiac injury, ischemia, and cytokine release syndrome. Patients with liver enzymes more than five times the upper limit of normal may be excluded but not contraindicated from using medications such as remdesivir, tocilizumab, and hydroxychloroquine. Every patient receiving the medications, especially remdesivir and tocilizumab should be regularly monitored for liver biochemical indicators regardless of baseline values. It should not be assumed that patients with autoimmune hepatitis and liver transplantation have a sudden onset of disease or acute cellular rejection without biopsy confirmation. Patients who are immunocompromised or are treated with immunosuppressive drugs should be considered at increased risk for SARS-CoV-2 infection and should be prioritized for testing[46].
SARS-CoV-2-infected patients with ongoing antiviral treatment for hepatitis B or C should be continued, but the initiation of antiviral treatment for hepatitis C may need to be delayed. Patients with an underlying liver disease requiring immunosuppressants should be continued in cases of mild infection, but in moderate to severe infection, the treatment dosage of calcineurin inhibitors should be reduced. The position statement from the European Association for the Study of the Liver-European Society of Clinical Microbiology and Infectious Diseases recommended that the dose of immunosuppressant drugs can be adjusted according to antiviral treatment regimens because the drugs in both regimens will likely interact with each other[48].
The biomarkers of liver injury were significantly higher in severe cases of SARS-CoV-2 infection. In a meta-analysis by Henry et al[49], the severity and mortality rate of SARS-CoV-2 infection was related to the biomarkers of liver functions, which suggests that liver injury has a strong correlation with the severity of SARS-CoV-2 infection. A retrospective study that compared the clinical spectrum between patients with and without liver injury by Xie et al[7] found the hospitalization time was significantly longer in patients with liver injury. Lei et al[16] reported that abnormal AST in SARS-CoV-2 infection was associated with a higher risk of death during hospitalization than other indicators of liver injury.
Kulkarni et al[50] stated that the severity of elevated liver enzyme markers determined the outcome of SARS-CoV-2 infection, with the incidence of liver injury as high as 58%-78% among the death cases. A multicenter study involving 2780 SARS-CoV-2 infected patients by Singh and Khan[9] found that patients with underlying liver disease had higher mortality and hospitalization. In addition to the abnormal liver biochemistry, hypoalbuminemia during the illness is an important indicator of the severity of the SARS-CoV-2 infection. Both studies by Gong et al[51] and Huang et al[52] showed that hypoalbuminemia was associated with severe infection and an independent risk factor for death.
In conclusion, this review illuminated the significance of liver injury in SARS-CoV-2 infection based on the descriptions from the scientific literature. Although it is common and mild in the majority of cases, it is a strong predictor of the severity and a significant risk factor for the mortality rate of the disease, especially if it is associated with male sex, older age, the presence of other comorbidities or underlying chronic liver disease, and in severe respiratory symptoms. Therefore, it is prudent to monitor SARS-CoV-2-infected patients with liver injury and to individualize treatment for patients with an underlying disease who developed liver injury to improve the prognosis by delivering the appropriate mana
The authors would like to thank all who were involved in writing this review article.
Provenance and peer review: Invited article; Externally peer reviewed
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Universiti Malaysia Sabah.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Malaysia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Floreani A, Italy; Hernandez-Caballero A, Mexico; Nooripour R, Iran S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17641] [Article Influence: 3528.2] [Reference Citation Analysis (0)] |
| 2. | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11224] [Cited by in RCA: 9317] [Article Influence: 1863.4] [Reference Citation Analysis (0)] |
| 3. | Schoeman D, Fielding BC. Coronavirus envelope protein: current knowledge. Virol J. 2019;16:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1277] [Cited by in RCA: 1184] [Article Influence: 197.3] [Reference Citation Analysis (0)] |
| 4. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30112] [Article Influence: 6022.4] [Reference Citation Analysis (3)] |
| 5. | Payus AO, Liew Sat Lin C, Mohd Noh M, Jeffree MS, Ali RA. SARS-CoV-2 infection of the nervous system: A review of the literature on neurological involvement in novel coronavirus disease-(COVID-19). Bosn J Basic Med Sci. 2020;20:283-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 623] [Cited by in RCA: 661] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 7. | Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 213] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 8. | Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: A retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int. 2020;40:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 310] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 9. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768-771.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 10. | Da BL, Im GY, Schiano TD. Coronavirus Disease 2019 Hangover: A Rising Tide of Alcohol Use Disorder and Alcohol-Associated Liver Disease. Hepatology. 2020;72:1102-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 11. | Zheng KI, Gao F, Wang XB, Sun QF, Pan KH, Wang TY, Ma HL, Chen YP, Liu WY, George J, Zheng MH. Letter to the Editor: Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 12. | Piedade J, Pereira G. COVID-19 in liver transplant recipients. J Liver Transplant. 2021;3:100026. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Becchetti C, Zambelli MF, Pasulo L, Donato MF, Invernizzi F, Detry O, Dahlqvist G, Ciccarelli O, Morelli MC, Fraga M, Svegliati-Baroni G, van Vlierberghe H, Coenraad MJ, Romero MC, de Gottardi A, Toniutto P, Del Prete L, Abbati C, Samuel D, Pirenne J, Nevens F, Dufour JF; COVID-LT group. COVID-19 in an international European liver transplant recipient cohort. Gut. 2020;69:1832-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 14. | Ali Malekhosseini S, Nikoupour H, Gholami S, Shamsaeefar A, Arasteh P, Kazemi K, Dehghani M, Eghlimi H, Raeisi Shahraki H, Roozbeh J, Rezaianzadeh A, Nikeghbalian S. A Report of 85 Cases of COVID-19 and Abdominal Transplantation From a Single Center: What Are the Associated Factors With Death Among Organ Transplantation Patients. Transplantation. 2021;105:90-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Can E, Oğlak SC, Ölmez F. Abnormal liver function tests in pregnant patients with COVID-19 - a retrospective cohort study in a tertiary center. Ginekol Pol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 309] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 17. | Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18:1561-1566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 559] [Cited by in RCA: 556] [Article Influence: 111.2] [Reference Citation Analysis (0)] |
| 18. | Phipps MM, Barraza LH, LaSota ED, Sobieszczyk ME, Pereira MR, Zheng EX, Fox AN, Zucker J, Verna EC. Acute Liver Injury in COVID-19: Prevalence and Association with Clinical Outcomes in a Large U.S. Cohort. Hepatology. 2020;72:807-817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 272] [Article Influence: 54.4] [Reference Citation Analysis (2)] |
| 19. | Arima Y, Yoshimoto K, Namera A, Makita R, Murata K, Nagao M. The Sarin-like Organophosphorus Agent bis (isopropyl methyl)phosphonate Induces Apoptotic Cell Death and COX-2 Expression in SK-N-SH Cells. Hiroshima J Med Sci. 2016;65:1-8. [PubMed] |
| 20. | Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Lan F. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. 2020 Preprint. Available from: bioRxiv: 2020.02.03.931766. [DOI] [Full Text] |
| 21. | Zhou Z, Zhao N, Shu Y, Han S, Chen B, Shu X. Effect of Gastrointestinal Symptoms in Patients With COVID-19. Gastroenterology. 2020;158:2294-2297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 167] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 22. | Wijarnpreecha K, Ungprasert P, Panjawatanan P, Harnois DM, Zaver HB, Ahmed A, Kim D. COVID-19 and liver injury: a meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:990-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 23. | Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X, Yin H, Xiao Q, Tang Y, Qu X, Kuang L, Fang X, Mishra N, Lu J, Shan H, Jiang G, Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1050] [Cited by in RCA: 1150] [Article Influence: 230.0] [Reference Citation Analysis (0)] |
| 24. | Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, Choi KW, Tso YK, Lau T, Lai ST, Lai CL. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39:302-310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 307] [Cited by in RCA: 305] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 25. | Payus AO, Jeffree MS, Ohn MH, Tan HJ, Ibrahim A, Chia YK, Raymond AA. Immune-mediated neurological syndrome in SARS-CoV-2 infection: a review of literature on autoimmune encephalitis in COVID-19. Neurol Sci. 2022;43:1533-1547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Payus AO, Jan T, Ibrahim A, Raymond AA. Autoimmune polyradiculopathy In SARS-CoV-2: a narrative review of Guillain-Barre syndrome in novel coronavirus disease (COVID-19). Acta Med. 2020;. [DOI] [Full Text] |
| 27. | Liu J, Li S, Liu J, Liang B, Wang X, Wang H, Li W, Tong Q, Yi J, Zhao L, Xiong L, Guo C, Tian J, Luo J, Yao J, Pang R, Shen H, Peng C, Liu T, Zhang Q, Wu J, Xu L, Lu S, Wang B, Weng Z, Han C, Zhu H, Zhou R, Zhou H, Chen X, Ye P, Zhu B, Wang L, Zhou W, He S, He Y, Jie S, Wei P, Zhang J, Lu Y, Wang W, Zhang L, Li L, Zhou F, Wang J, Dittmer U, Lu M, Hu Y, Yang D, Zheng X. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1171] [Cited by in RCA: 1207] [Article Influence: 241.4] [Reference Citation Analysis (0)] |
| 28. | Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: The current evidence. United European Gastroenterol J. 2020;8:509-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 29. | Spearman CW, Aghemo A, Valenti L, Sonderup MW. COVID-19 and the liver: A 2021 update. Liver Int. 2021;41:1988-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Li J, Gong X, Wang Z, Chen R, Li T, Zeng D, Li M. Clinical features of familial clustering in patients infected with 2019 novel coronavirus in Wuhan, China. Virus Res. 2020;286:198043. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 31. | Zha BS, Wan X, Zhang X, Zha W, Zhou J, Wabitsch M, Wang G, Lyall V, Hylemon PB, Zhou H. HIV protease inhibitors disrupt lipid metabolism by activating endoplasmic reticulum stress and inhibiting autophagy activity in adipocytes. PLoS One. 2013;8:e59514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Cao R, Hu Y, Wang Y, Gurley EC, Studer EJ, Wang X, Hylemon PB, Pandak WM, Sanyal AJ, Zhang L, Zhou H. Prevention of HIV protease inhibitor-induced dysregulation of hepatic lipid metabolism by raltegravir via endoplasmic reticulum stress signaling pathways. J Pharmacol Exp Ther. 2010;334:530-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Ferrara F, Granata G, Pelliccia C, La Porta R, Vitiello A. The added value of pirfenidone to fight inflammation and fibrotic state induced by SARS-CoV-2 : Anti-inflammatory and anti-fibrotic therapy could solve the lung complications of the infection? Eur J Clin Pharmacol. 2020;76:1615-1618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int. 2020;40:1278-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 35. | Ghoda A, Ghoda M. Liver Injury in COVID-19 Infection: A Systematic Review. Cureus. 2020;12:e9487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Yang L, Wang W, Wang X, Zhao J, Xiao L, Gui W, Fan H, Xia J, Li Z, Yan J, Alasbahi A, Zhu Q, Hou X. Creg in Hepatocytes Ameliorates Liver Ischemia/Reperfusion Injury in a TAK1-Dependent Manner in Mice. Hepatology. 2019;69:294-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 37. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5781] [Article Influence: 1156.2] [Reference Citation Analysis (2)] |
| 38. | Ji D, Qin E, Xu J, Zhang D, Cheng G, Wang Y, Lau G. Non-alcoholic fatty liver diseases in patients with COVID-19: A retrospective study. J Hepatol. 2020;73:451-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (2)] |
| 39. | Liu Q, Wang RS, Qu GQ, Wang YY, Liu P, Zhu YZ, Fei G, Ren L, Zhou YW, Liu L. Gross examination report of a COVID-19 death autopsy. Fa Yi Xue Za Zhi. 2020;36:21-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 129] [Reference Citation Analysis (0)] |
| 40. | Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110-2116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 218] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 41. | Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 656] [Article Influence: 131.2] [Reference Citation Analysis (0)] |
| 42. | Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, Thomas S, Adler NM, Charytan DM, Gasmi B, Hochman JS, Reynolds HR. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. 2020;24:100434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 375] [Cited by in RCA: 404] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 43. | Wang XX, Shao C, Huang XJ, Sun L, Meng LJ, Liu H, Zhang SJ, Li HJ, Lv FD. Histopathological features of multiorgan percutaneous tissue core biopsy in patients with COVID-19. J Clin Pathol. 2021;74:522-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 44. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 45. | Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173:268-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1577] [Cited by in RCA: 1749] [Article Influence: 349.8] [Reference Citation Analysis (0)] |
| 46. | Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology. 2020;72:287-304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 47. | Hambali NL, Mohd Noh M, Paramasivam S, Chua TH, Hayati F, Payus AO, Tee TY, Rosli KT, Abd Rachman Isnadi MF, Manin BO. A Non-severe Coronavirus Disease 2019 Patient With Persistently High Interleukin-6 Level. Front Public Health. 2020;8:584552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Sahin TT, Akbulut S, Yilmaz S. COVID-19 pandemic: Its impact on liver disease and liver transplantation. World J Gastroenterol. 2020;26:2987-2999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 49. | Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 1165] [Article Influence: 233.0] [Reference Citation Analysis (0)] |
| 50. | Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 51. | Gong J, Ou J, Qiu X, Jie Y, Chen Y, Yuan L, Cao J, Tan M, Xu W, Zheng F, Shi Y, Hu B. A Tool for Early Prediction of Severe Coronavirus Disease 2019 (COVID-19): A Multicenter Study Using the Risk Nomogram in Wuhan and Guangdong, China. Clin Infect Dis. 2020;71:833-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 337] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 52. | Huang J, Cheng A, Kumar R, Fang Y, Chen G, Zhu Y, Lin S. Hypoalbuminemia predicts the outcome of COVID-19 independent of age and co-morbidity. J Med Virol. 2020;92:2152-2158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |