Published online Oct 7, 2022. doi: 10.3748/wjg.v28.i37.5444
Peer-review started: June 22, 2022
First decision: August 1, 2022
Revised: August 16, 2022
Accepted: September 13, 2022
Article in press: September 13, 2022
Published online: October 7, 2022
Processing time: 98 Days and 13.1 Hours
Metabolic associated fatty liver disease (MAFLD) is associated with complications and mortality in patients with coronavirus disease 2019 (COVID-19). However, there are no prognostic scores aimed to evaluate the risk of severe disease speci
To evaluate the prognostic performance of an index based on lactate dehydrogenase and transaminases (aspartate aminotransferase/alanine aminotransferase) in patients with COVID-19 and MAFLD [liver fibrosis and nutrition (LNF)-COVID-19 index].
In this retrospective cohort study, two cohorts from two different tertiary centers were included. The first was the derivation cohort to obtain the score cutoffs, and the second was the validation cohort. We included hospitalized patients with severe COVID-19 and MAFLD. Liver steatosis was evaluated by computed tomography scan. Area under the receiver operating characteristic (ROC) curve analysis and survival analysis were used.
In the derivation cohort, 44.6% had MAFLD; ROC curve analysis yielded a LFN-COVID-19 index > 1.67 as the best cutoff, with a sensitivity of 78%, specificity of 63%, negative predictive value of 91% and an area under the ROC curve of 0.77. In the multivariate analysis, the LFN-COVID-19 index > 1.67 was independently associated with the development of acute kidney injury (odds ratio: 1.8, 95% confidence interval: 1.3-2.5, P < 0.001), orotracheal intubation (odds ratio: 1.9, 95% confidence interval: 1.4-2.4, P < 0.001), and death (odds ratio: 2.86, 95% confidence interval: 1.6-4.5, P < 0.001) in both cohorts.
LFN-COVID-19 index has a good performance to predict prognosis in patients with MAFLD and COVID-19, which could be useful for the MAFLD population.
Core Tip: The liver fibrosis and nutrition-coronavirus disease 2019 (LFN-COVID-19) index that includes lactate dehydrogenase and transaminases is a new prognostic index for patients with metabolic associated fatty liver disease and COVID-19; it was developed to specifically predict adverse clinical outcomes, including mortality, in this population with both conditions. The variables included in this index allow an easy, quick and reliable risk assessment in this population with simple markers, allowing for broad implementation.
- Citation: Macías-Rodríguez RU, Solís-Ortega AA, Ornelas-Arroyo VJ, Ruiz-Margáin A, González-Huezo MS, Urdiales-Morán NA, Román-Calleja BM, Mayorquín-Aguilar JM, González-Regueiro JA, Campos-Murguía A, Toledo-Coronado IV, Chapa-Ibargüengoitia M, Valencia-Peña B, Martínez-Cabrera CF, Flores-García NC. Prognostic performance of an index based on lactic dehydrogenase and transaminases for patients with liver steatosis and COVID-19. World J Gastroenterol 2022; 28(37): 5444-5456
- URL: https://www.wjgnet.com/1007-9327/full/v28/i37/5444.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i37.5444
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic (coronavirus disease 2019, COVID-19) still affects the entire world. As of June 15, 2022, 536720870 people have been infected, of whom 6312601 have died[1].
Different risk factors associated with the development of complications and mortality in patients with COVID-19 have been identified, including age > 60 years, the presence of cirrhosis, diabetes, immuno
Acute respiratory distress syndrome is the major complication in patients with severe COVID-19; other complications such as cardiac or cardiovascular, renal and secondary infections may occur[7]. These patients, mainly those admitted to the intensive care unit, may present with laboratory abnormalities, such as leukopenia, lymphopenia (< 800 mm3 at admission), elevated prothrombin time, elevated serum levels of D-dimer (> 1000 ng/mL), elevated inflammatory markers (ferritin > 300 μg/L), elevated lactate dehydrogenase (LDH), elevated liver enzymes, elevated creatine phosphokinase (twice the upper limit of normal) and elevated troponin I[7-9].
In patients with pneumonia associated with SARS-CoV-2 infection, high LDH levels correlate with lung damage, severe disease and mortality at day 30[10-13]. In the study by Yan et al[14], LDH (> 365 U/L), lymphocyte count (< 14.7%) and C-reactive protein (> 41.2 mg/L) were identified as the three laboratory abnormalities that predicted mortality risk with 90% accuracy, which represented a simple way to promptly recognize severe illness.
Likewise, in patients with acute liver injury (non-COVID-19 related), an increase in LDH levels has been reported, secondary to endothelial damage induced by macrophages during acute inflammation, conditioning microcirculation alterations and hypoxia. Thus, it has been suggested that LDH may have a discriminatory role in identifying the etiology of liver damage. As a marker of damage due to liver ischemia, it must be taken into account that LDH has a shorter half-life. Therefore, a faster fall occurs when the damage disappears, and it has been suggested as a parameter to monitor the evolution of patients with acute liver injury. The ubiquitous nature of LHD in the human body makes it a nonspecific but sensitive biomarker, which in the context of organ damage can provide information with diagnostic and prognostic potential. In the same way, increase in alanine aminotransferase (ALT), aspartate aminotransferase (AST) and the AST/ALT ratio has been associated to adverse clinical outcomes including mortality in patients with COVID-19[10-13,15].
Identifying factors associated with poor prognosis that may be related to a pathophysiological mechanism is ideal in patients with COVID-19 since those patients at risk of progressing to a severe illness could be identified promptly. Therefore, measures could be taken to influence the outcomes of those patients. In this sense, having a prognostic index specific for patients with MAFLD who develop COVID-19 may be useful to identify individuals at risk of developing adverse clinical outcomes.
Therefore, the aim of this study was to evaluate the performance of a prognostic index based on LDH, AST and ALT in patients with MAFLD and COVID-19 and its association with the development of adverse clinical outcomes and mortality.
This was a retrospective cohort study performed at two third-level hospitals in Mexico, (INCMNSZ and ISSEMYM) from March 2020 to July 2020, during the first phase of the COVID-19 pandemic and before steroids became a standard of care for severe COVID-19. The study was carried out according to the Declaration of Helsinki and was approved by the institutional Ethics Committee, ref. No. 3777).
This study consisted of two phases. Phase 1, a derivation/training cohort, (its methods are described below) used to create and evaluate the newly proposed prognostic index. This cohort was derived from a tertiary care center hospital in Mexico City (INCMNSZ). Phase 2, the validation cohort aimed to evaluate the diagnostic performance of the proposed index in patients with COVID-19 and liver steatosis at a different center. This cohort was derived from a tertiary care center hospital in Toluca, in the center of Mexico (ISSEMYM).
All patients admitted during the period of study, > 18 years of age, either sex and with a confirmed diagnosis of SARS-CoV-2 infection by RT-PCR and with severe disease (pneumonia + respiratory rate > 30/respiratory distress/SaO2 < 90%), were included in the study[19]. Patients without an adequate follow-up were excluded from the analysis (e.g., those requiring referral to another hospital, those with insufficient information in the clinical records, etc). Follow-up and evaluation of the clinical outcomes were conducted through revision of electronic clinical records.
Upon admission, a blood sample was drawn for determination of the following tests: complete blood count, glucose, creatinine, electrolytes, ferritin, C-reactive protein, LDH, liver chemistry, creatine phosphokinase, arterial blood gases, D-dimer, troponin I and fibrinogen. HIV (human Immunodeficiency virus) and viral hepatitis panel (HCV and HBV) were performed in all participants. All the tests met the quality standards from our central laboratory, accredited by the College of American Pathologists.
In order to evaluate the severity of pulmonary involvement, all patients underwent a pulmonary computed tomography scan, where a portion of the liver was also evaluated for the presence of steatosis. The methodology was previously described from our group[16]. Briefly, an expert radiologist blinded to the patient´s clinical status evaluated computed tomography scans to detect liver steatosis, according to the following criteria: (1) Attenuation coefficient ≤ 40 Hounsfield units in the liver (segments VII and VIII); and (2) Attenuation coefficient ≥ 10 Hounsfield units between the splenic and liver parenchyma.
The liver fibrosis and nutrition (LNF)-COVID-19 index was calculated according to the following formula:
LFN-COVID-19 index = (AST/ALT) × (LDH/LDHULN).
Where AST/ALT ratio included transaminase levels expressed in U/L and was multiplied by the times above the upper limit of normal value for LDH (U/L). The final value was included in the statistical analysis for characterization of clinical outcomes.
Sample size estimation considered a hypothetical area under the receiver operating characteristic curve (AUROC) of 0.8 for LFN-COVID-19 index and 0.7 as null hypothesis. Considering an alpha error of 0.05 and beta 0.20 and a negative/positive ratio of 1/1, estimation yielded 81 negative/positive cases (162 patients in total).
Normality of the data was evaluated with Kolmogorov-Smirnov test. Data was presented as mean ± standard deviation, median (P25-P75) or absolute frequencies. Comparisons between the groups were made through Mann-Whitney U or Student’s t test. ROC curve analysis was performed to obtain the best cutoff from the LFN-COVID-19 index for mortality, through the Youden index as well as sensitivity, specificity, positive and negative predictive values and likelihood ratios.
Clinical outcomes were evaluated by logistic regression and a time-dependent survival analysis, including Kaplan-Meier and Cox regression (Cox proportional-hazards model) for 28-d mortality and general mortality. Statistical analysis was carried out with the statistics software SPSS version 20.0 (IBM, Armonk, NY, United States) and ROC analysis with MedCalc Statistical Software version 19.4.1 (MedCalc Software Ltd, Ostend, Belgium).
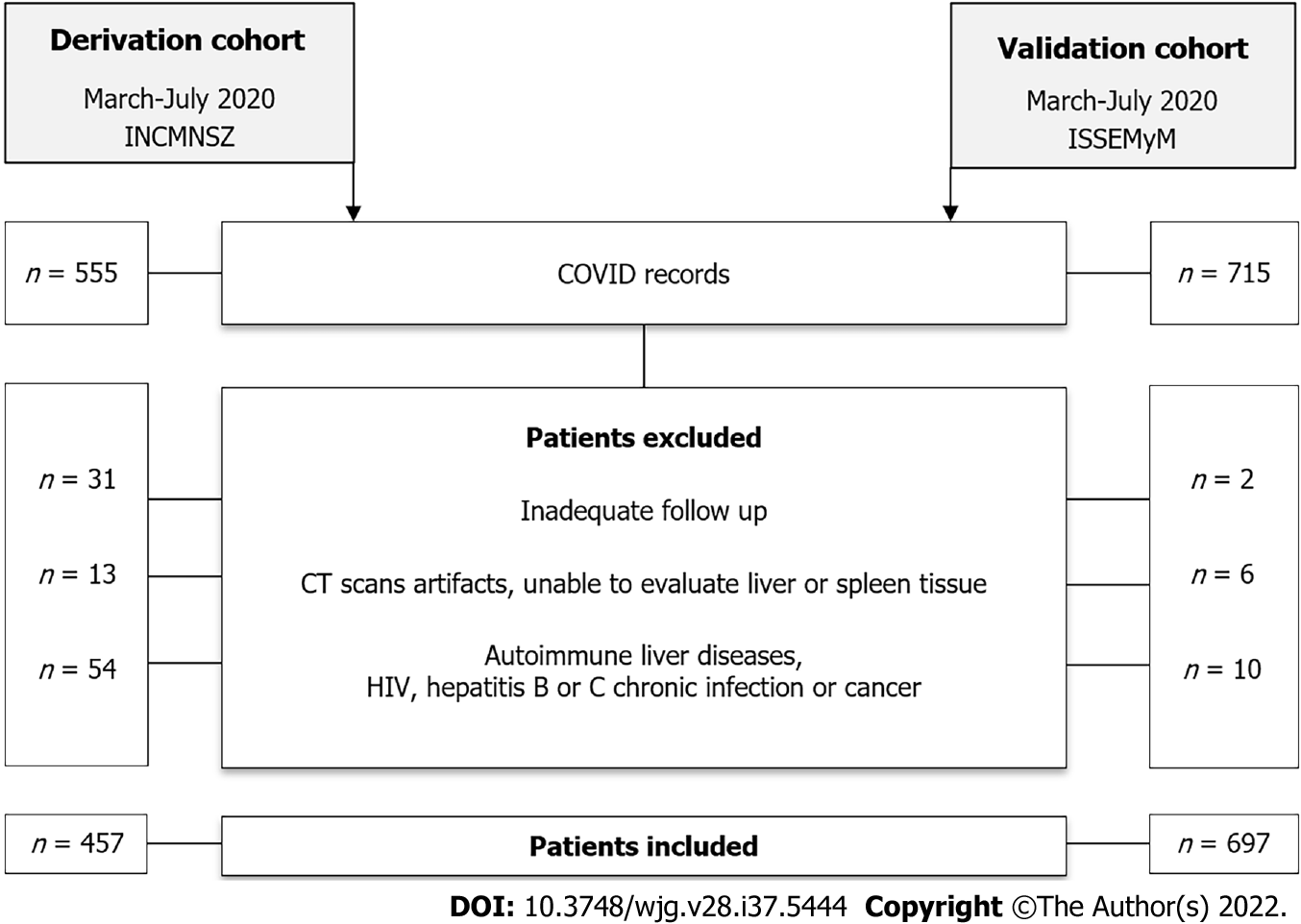
In the validation cohort a total of 457 patients were included in the final analysis (Figure 1), after excluding those without an adequate follow-up, those with computed tomography scan issues (artifacts, unable to evaluate liver or spleen tissue, post-surgical changes) or those with known autoimmune liver diseases, HIV, hepatitis C or B chronic infection or cancer.
General characteristics of the study population, with and without MAFLD are presented in Table 1. Mean age in the total population was 50.4 ± 13.3 years, most of the patients were male (65.2%), and the mean body mass index (BMI) was 30.1 ± 5.6 kg/m2. In general, in the group of patients with MAFLD there was a higher prevalence of overweight and obese patients, they were younger than those without MAFLD, and they had a higher prevalence of diabetes and metabolic syndrome.
| Characteristics | All, n = 457 | No MAFLD, n = 253 | MAFLD, n = 204 | P value |
| Demographic features | ||||
| Sex as male/female, % | 65.2/34.8 | 63.6/36.4 | 67.2/32.8 | 0.432 |
| Age in yr | 50.4 ± 13.3 | 52.4 ± 14.0 | 47.8 ± 11.8 | < 0.0001 |
| BMI in kg/m2 | 30.1 ± 5.6 | 28.7 ± 4.9 | 31.8 ± 5.8 | < 0.0001 |
| Comorbidities, n (%) | ||||
| Malnutrition | 10 (2.8) | 7 (3.4) | 3 (1.9) | < 0.0001 |
| Normal weight | 49 (13.6) | 43 (21.0) | 6 (3.9) | |
| Overweight | 136 (37.9) | 82 (40.0) | 54 (35.1) | |
| Obesity G1 | 110 (30.6) | 51 (24.9) | 59 (38.3) | |
| Obesity G2 | 36 (10.0) | 16 (7.8) | 20 (13.0) | |
| Obesity G3 | 18 (5.0) | 6 (2.9) | 12 (7.8) | |
| T2DM | 107 (23.5) | 47 (18.7) | 60 (29.6) | 0.006 |
| Hypertension | 122 (26.8) | 60 (23.8) | 62 (30.5) | 0.107 |
| Chronic kidney disease | 8 (1.8) | 6 (2.4) | 2 (1.0) | 0.225 |
| Pulmonary obstructive disease | 4 (0.9) | 1 (0.4) | 3 (1.5) | 0.235 |
| Autoimmune disease | 6 (1.3) | 3 (1.2) | 3 (1.5) | 0.551 |
| Immunosuppression | 3 (0.7) | 3 (1.2) | 0 (0) | 0.169 |
| Metabolic syndrome | 155 (36.0) | 61 (25.5) | 94 (49.0) | < 0.0001 |
| Prognostic scores | ||||
| qSOFA | 1 (0-1) | 1 (0-1) | 1 (0-1) | 0.800 |
| SOFA | 2 (1-2) | 2 (1-2) | 2 (1-2) | 0.034 |
| NEWS | 6.7 ± 2.3 | 6.6 ± 2.3 | 6.8 ± 2.2 | 0.190 |
| PSI/PORT | 62 (50-80) | 62 (50-82) | 61 (49-77) | 0.316 |
| SMART COP | 3 (2-4) | 3 (2-4) | 3 (2-4) | 0.091 |
| Biochemical values | ||||
| CRP, ref: 0-1 mg/dL | 13.2 (6.6-20.7) | 13.1 (6.6-20.0) | 13.7 (6.2-21.5) | 0.286 |
| Ferritin, ref: 11.0-306.8 ng/mL | 747.8 ± 665.0 | 717.2 ± 662.0 | 784.0 ± 668.0 | 0.290 |
| D-dimer, ref: 0-500 ng/mL | 707 (426-1146) | 699 (413-1138) | 721 (451-1182) | 0.418 |
| LDH, ref: 120-246 U/L | 388 ± 160 | 374 ± 149 | 406 ± 173 | 0.032 |
| Troponin, ref: < 15 pg/mL | 4.7 (3.2-8.2) | 4.7 (3.1-10.4) | 4.6 (3.2-7.1) | 0.525 |
| CPK, ref: 30-233 U/L | 110 (59-242) | 98 (55-210) | 133 (66-311) | 0.006 |
| Bilirubin, ref: 0/3-1 mg/dL | 0.68 ± 0.49 | 0.66 ± 0.54 | 0.69 ± 0.43 | 0.593 |
| ALT, ref: 7-52 U/L | 37.5 (25.0-56.0) | 33.0 (23.8-54.7) | 41.0 (28.0-59.0) | 0.004 |
| AST, ref: 13-39 U/L | 42.0 (30.0-62.0) | 40.0 (29.0-58.0) | 43.9 (32.9-64.3) | 0.051 |
| Globulins, ref: 1.9-3.7 g/dL | 3.2 ± 0.4 | 3.2 ± 0.4 | 3.2 ± 0.4 | 0.560 |
| Albumin, ref: 3.5-5.7 g/dL | 3.7 ± 0.4 | 3.6 ± 0.4 | 3.7 ± 0.4 | 0.051 |
| ALP, ref: 34-104 U/L | 86 (70-111) | 86 (70-113) | 85 (69-109) | 0.505 |
| Creatinine, ref: 0.6-1.2 mg/dL | 0.9 (0.8-1.1) | 0.9 (0.8-1.1) | 0.9 (0.7-1.1) | 0.877 |
| Glucose, ref: 70-99 mg/dL | 116 (102-144) | 110 (99-131) | 124 (105-184) | < 0.0001 |
| Leukocytes, ref: 4-12 × 103/μL | 7.6 (5.6-10.0) | 7.2 (5.4-9.8) | 7.9 (5.7-10.3) | 0.191 |
| Lymphocytes, ref: 1-3.9 × 103/μL | 881.6 ± 509.0 | 835.0 ± 352.0 | 938.0 ± 649.0 | 0.043 |
| Platelets, ref: 150-450 K/μL | 239 ± 88 | 248 ± 95 | 227 ± 78 | 0.012 |
| 25 (HO) vitamin D, ref: 30-100 ng/mL | 21.5 ± 8.0 | 21.6 ± 8.1 | 21.5 ± 8.0 | 0.917 |
| Triglycerides, ref: < 150 mg/dL | 159 ± 85 | 155 ± 60 | 165 ± 110 | 0.264 |
| CT scan results, pulmonary involvement | ||||
| Mild, < 20% | 91 (20.0) | 51 (20.3) | 40 (19.6) | 0.281 |
| Moderate, 20%-50% | 172 (37.8) | 102 (40.6) | 70 (34.3) | |
| Severe, > 50% | 192 (42.2) | 98 (39.0) | 94 (46.1) | |
| Treatment, n (%) | ||||
| Antibiotics | 402 (88.4) | 228 (90.8) | 174 (85.3) | 0.096 |
| Antimalarials | 132 (28.9) | 72 (28.5) | 60 (29.4) | 0.823 |
| Tocilizumab | 51 (11.2) | 26 (10.3) | 25 (12.3) | 0.504 |
| Remdesivir | 9 (2.0) | 7 (2.8) | 2 (1.0) | 0.152 |
| PaO2/FiO2 ratio | 233.9 ± 109.9 | 239.0 ± 91.0 | 227.0 ± 130.0 | 0.011 |
| Neutrophil/lymphocyte ratio | 7.0 (4.4-11.6) | 7.2 (4.5-12.0) | 6.7 (4.0-10.8) | 0.860 |
| Days between the beginning of symptoms and hospitalization | 8.2 ± 4.4 | 8.6 ± 4.6 | 7.8 ± 4.0 | 0.110 |
Biochemical tests related to proinflammatory status, such as LDH, creatine phosphokinase, lymphocytes and neutrophil/lymphocyte ratio, were higher in the MAFLD group, as well as liver chemistry abnormalities, glucose, triglycerides and prognostic scores (SOFA: sequential organ failure assessment;).
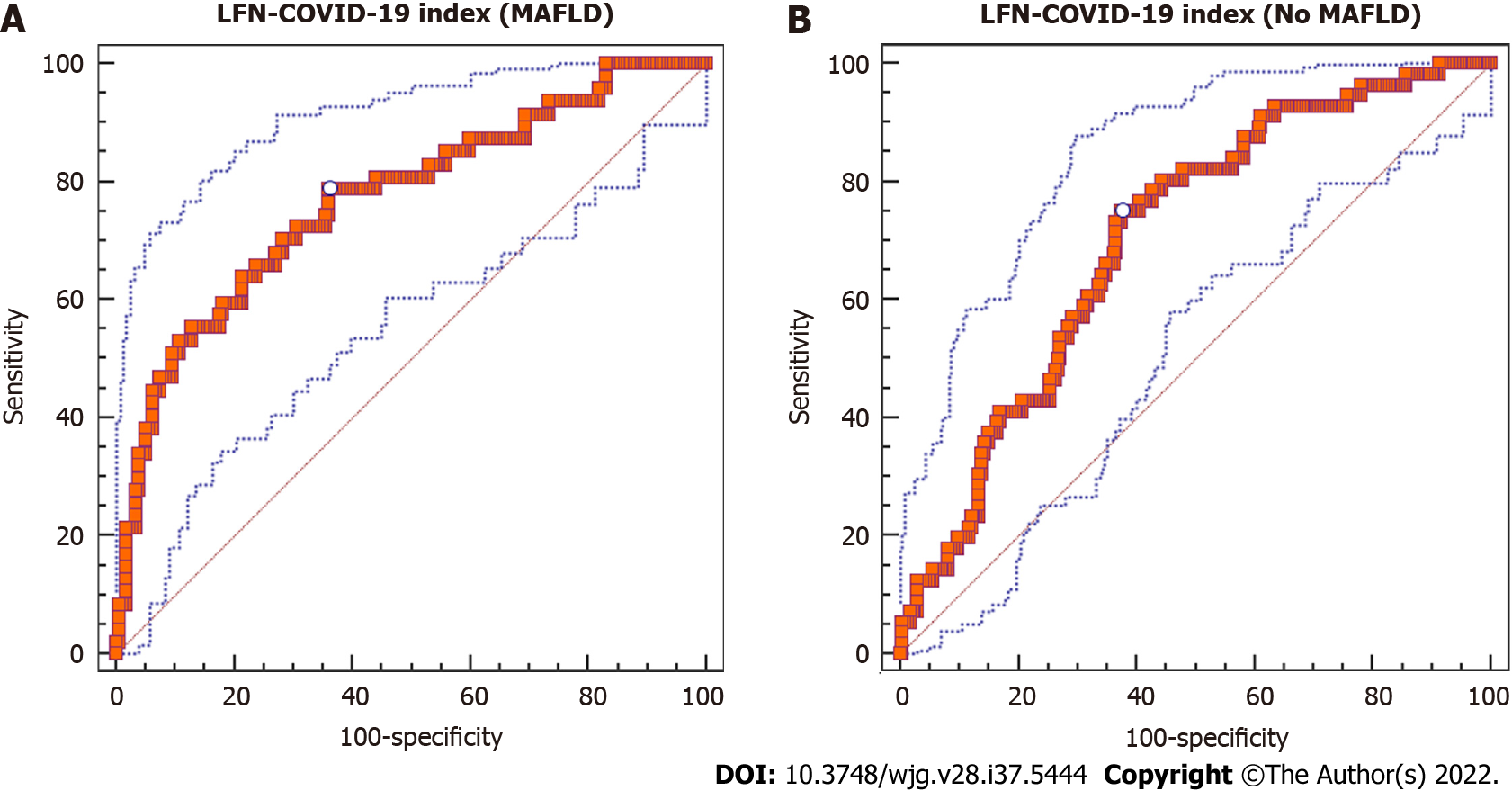
In the group of patients with MAFLD, diagnostic yield of the LFN-COVID-19 index [(AST/ALT) × (LDH/LDHULN)] was investigated through the AUROC analysis to determine the prognostic value of the index as a prognostic marker in patients with COVID-19. Characteristics related to diagnostic yield of the LFN-COVID-19 index are shown in Table 2. According to Youden’s index, the best cutoff value of the LFN-COVID-19 index for mortality in patients with MAFLD was > 1.67. This cutoff value showed an AUROC of 0.77 [95% confidence interval (CI): 0.709-0.823, P < 0.0001], with a sensitivity of 78.7% and specificity of 63.8% (Figure 2A). In general, the AUROC in this group of patients was better than in patients without MAFLD (AUROC: 0.703, 95%CI: 0.647-0.755, P < 0.0001) (Figure 2B).
| Diagnostic yield | |
| Sensitivity | 0.787 (0.643-0.893) |
| Specificity | 0.638 (0.563-0.709) |
| Positive predictive value, % | 0.360 (0.273-0.468) |
| Negative predictive value, % | 0.910 (0.855-0.960) |
| + Likelihood ratio | 2.18 (1.70-2.80) |
| - Likelihood ratio | 0.33 (0.20-0.60) |
| AUROC | 0.770 (0.709-0.823), P < 0.0001 |
| Youden index | 0.4257 |
Table 3 shows the characteristics of patients with MAFLD according to the LFN-COVID-19 index. Similitudes in both groups regarding metabolic syndrome and BMI were observed, while other variables including age, prognostic scores, and biomarkers related to proinflammatory and prothrombotic status, severe COVID-19 (PaO2/FiO2 < 100 mmHg), orotracheal intubation and other clinical outcomes, including mortality, were higher in the > 1.67 group.
| < 1.67, n = 115 | > 1.67, n = 89 | P value | |
| Demographic features | |||
| Sex as male/female, % | 63.5/36.5 | 71.9/28.1 | 0.203 |
| Age in yr | 46 ± 10 | 50 ± 12 | 0.011 |
| BMI in kg/m2 | 31.1 ± 4.8 | 32.5 ± 6.9 | 0.111 |
| Prognostic scores | |||
| qSOFA | 1 (0-1) | 1 (1-1) | 0.007 |
| SOFA | 2 (1-2) | 2 (2-3) | 0.004 |
| NEWS | 7 (5-8) | 7 (6-9) | 0.035 |
| PSI/PORT | 56 (47-69) | 66 (53-85) | < 0.0001 |
| SMART COP | 3 (2-4) | 4 (3-4) | 0.012 |
| Biochemical values | |||
| CRP, ref: 0-1 mg/dL | 8.5 (4.2-18.1) | 17.2 (11.6-23.8) | < 0.0001 |
| Ferritin, ref: 11.0-306.8 ng/mL | 503 (266-970) | 795 (412-1114) | 0.003 |
| D-dimer, ref: 0-500 ng/mL | 587 (399-962) | 967 (606-1549) | < 0.0001 |
| LDH, ref: 120-246 μ/L | 312 ± 86 | 529 ± 180 | < 0.0001 |
| Troponin, ref: < 15 pg/mL | 3.7 (2.9-5.7) | 6.1 (3.8-10.9) | < 0.0001 |
| CPK, ref: 30-223 μ/L | 107 (58-222) | 190 (78-414) | 0.001 |
| Bilirubin, ref: 0/3-1 mg/dL | 0.62 ± 0.30 | 0.78 ± 0.54 | 0.017 |
| ALT, ref: 7-52 U/L | 43.2 (31.0-61.2) | 37.0 (26.3-52.8) | 0.026 |
| AST, ref: 13-39 U/L | 38.3 (27.8-52.2) | 52.4 (42.0-73.7) | < 0.0001 |
| Globulins, ref: 1.9-3.7 g/dL | 3.22 ± 0.39 | 3.29 ± 0.43 | 0.259 |
| Albumin, ref: 3.5-5.7 g/dL | 3.90 ± 0.42 | 3.50 ± 0.40 | < 0.0001 |
| ALP, ref: 34-104 μ/L | 85 (70-109) | 85 (67-110.5) | 0.786 |
| Creatinine, ref: 0.6-1.2 mg/dL | 0.85 (0.69-1.00) | 0.95 (0.79-1.16) | 0.005 |
| Glucose, ref: 70-99 mg/dL | 118 (102-180) | 135 (114-187) | 0.03 |
| Leukocytes, ref: 4-12 × 103/μL | 7.6 (5.6-9.9) | 8.3 (6.3-10.8) | 0.089 |
| Lymphocytes, ref: 1-3.9 × 103/μL | 937 (693-1210) | 715 (510-967) | < 0.0001 |
| Platelets, ref: 150-450 K/μL | 228 ± 78 | 226 ± 79 | 0.827 |
| 25 (HO) vitamin D, ref: 30-100 ng/mL | 21.9 ± 7.8 | 20.9 ± 8.3 | 0.488 |
| Triglycerides, ref: < 150 mg/dL | 151 (118-187) | 137 (111-184) | 0.13 |
| PaO2/FiO2 ratio | 240 (161-287) | 159 (96-245) | < 0.0001 |
| Neutrophil/lymphocyte ratio | 5.9 (3.5-9.9) | 9.6 (6.4-13.7) | < 0.0001 |
| Other, n (%) | |||
| Metabolic syndrome | 49.0 (46.2) | 45.0 (52.3) | 0.401 |
| Severe COVID-19, PaO2/FiO2 < 100 mmHg | 9 (8.2) | 23 (26.7) | < 0.0001 |
| Orotracheal intubation | 13 (11.3) | 36 (40.9) | < 0.0001 |
| Acute kidney injury | 11 (11) | 26 (34.7) | < 0.0001 |
| Thrombotic event | 1 (1.0) | 2 (2.7) | 0.576 |
| Death | 6 (5.3) | 25 (29.8) | < 0.0001 |
| Days between the beginning of symptoms and hospitalization | 7.2 ± 3.4 | 8.6 ± 4.9 | 0.027 |
| Length of hospital stay in days | 7 (4-10) | 8 (6-10) | 0.131 |
| Days in ICU | 7 (5-12) | 12 (6-13) | 0.395 |
| Days between ICU requirement and death | 7 (6-7) | 5 (3-7) | 0.203 |
In order to determine if the LFN-COVID-19 index was independently associated with the presence of acute kidney injury or orotracheal intubation during hospitalization, a logistic regression was performed, observing that a value of > 1.67 was associated to adverse clinical outcomes, independently of metabolic factors, severity scores and demographic variables (Table 4).
| Orotracheal intubation | Acute kidney injury | |||||||
| OR | 95%CI | B coefficient | P value | OR | 95%CI | B coefficient | P value | |
| LFN-COVID-19 index | 1.900 | 1.481-2.437 | 0.642 | 0.000 | 1.849 | 1.366-2.504 | 0.615 | 0.000 |
| Sex | 0.605 | 0.288-1.271 | -0.502 | 0.185 | 0.280 | 0.103-0.765 | -1.272 | 0.013 |
| Age | 0.966 | 0.939-0.993 | -0.035 | 0.015 | 1.021 | 0.988-1.054 | 0.021 | 0.209 |
| BMI | 1.054 | 0.997-1.114 | 0.053 | 0.061 | 1.085 | 1.011-1.164 | 0.081 | 0.023 |
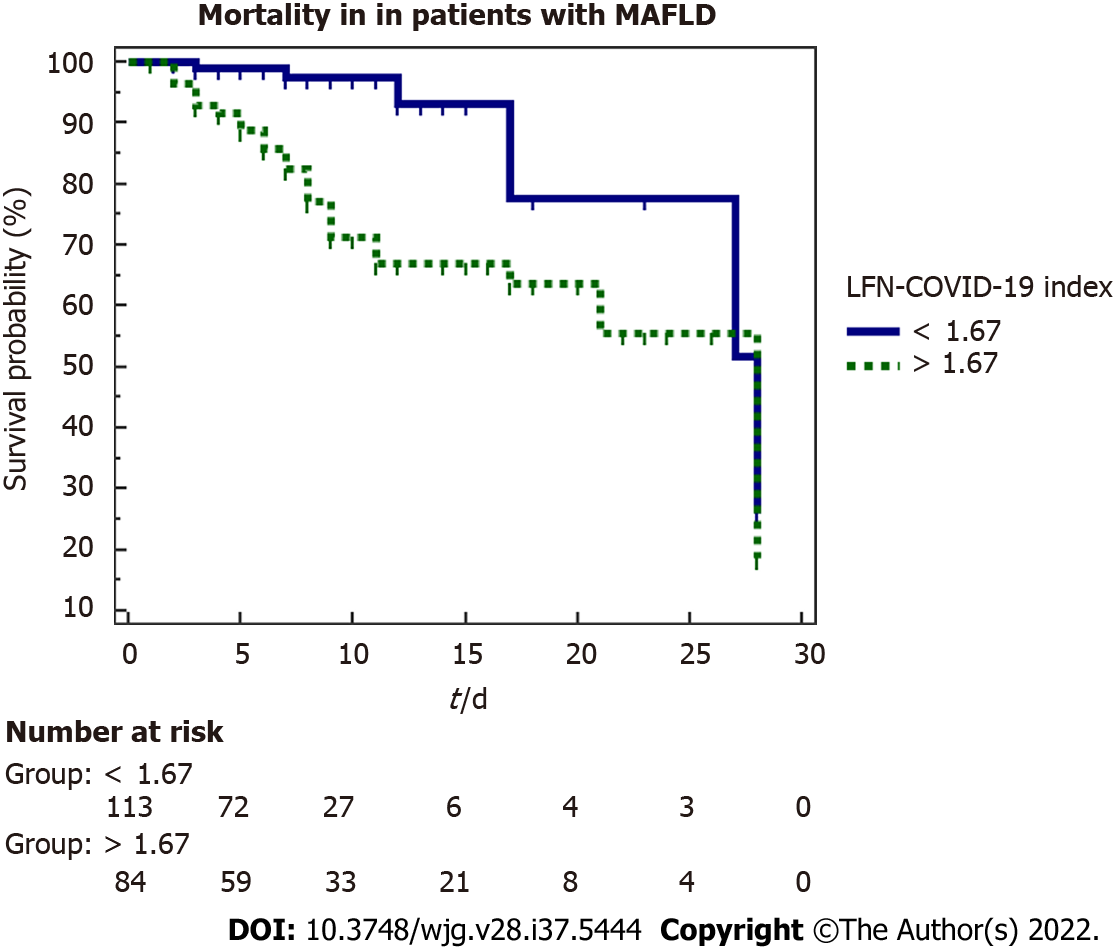
A marker of mortality was studied by a 28-d Kaplan-Meier survival analysis (Figure 3), observing that patients with a value > 1.67 have a lower survival than those with a value < 1.67 (P < 0.001). The influence of other variables on mortality was evaluated through univariate and multivariate Cox proportional hazard analysis. Table 5 summarized the variables that were significant in the univariate analysis, with the results subjected to the multivariate analysis where the variables that were independently associated with mortality were the LFN-COVID-19 index, the neutrophil/lymphocyte ratio and BMI.
| OR | B coefficient | P value | 95%CI | |
| LFN-COVID-19 index | 0.241 | -1.422 | 0.013 | 0.079-0.741 |
| PaO2/FiO2 ratio | 1.000 | 0.000 | 0.877 | 0.996-1.004 |
| Neutrophil/lymphocyte ratio | 1.043 | 0.042 | 0.030 | 1.004-1.083 |
| Creatine phosphokinase | 1.001 | 0.001 | 0.340 | 0.999-1.002 |
| Body mass index in kg/m2 | 1.093 | 0.089 | 0.002 | 1.033-1.157 |
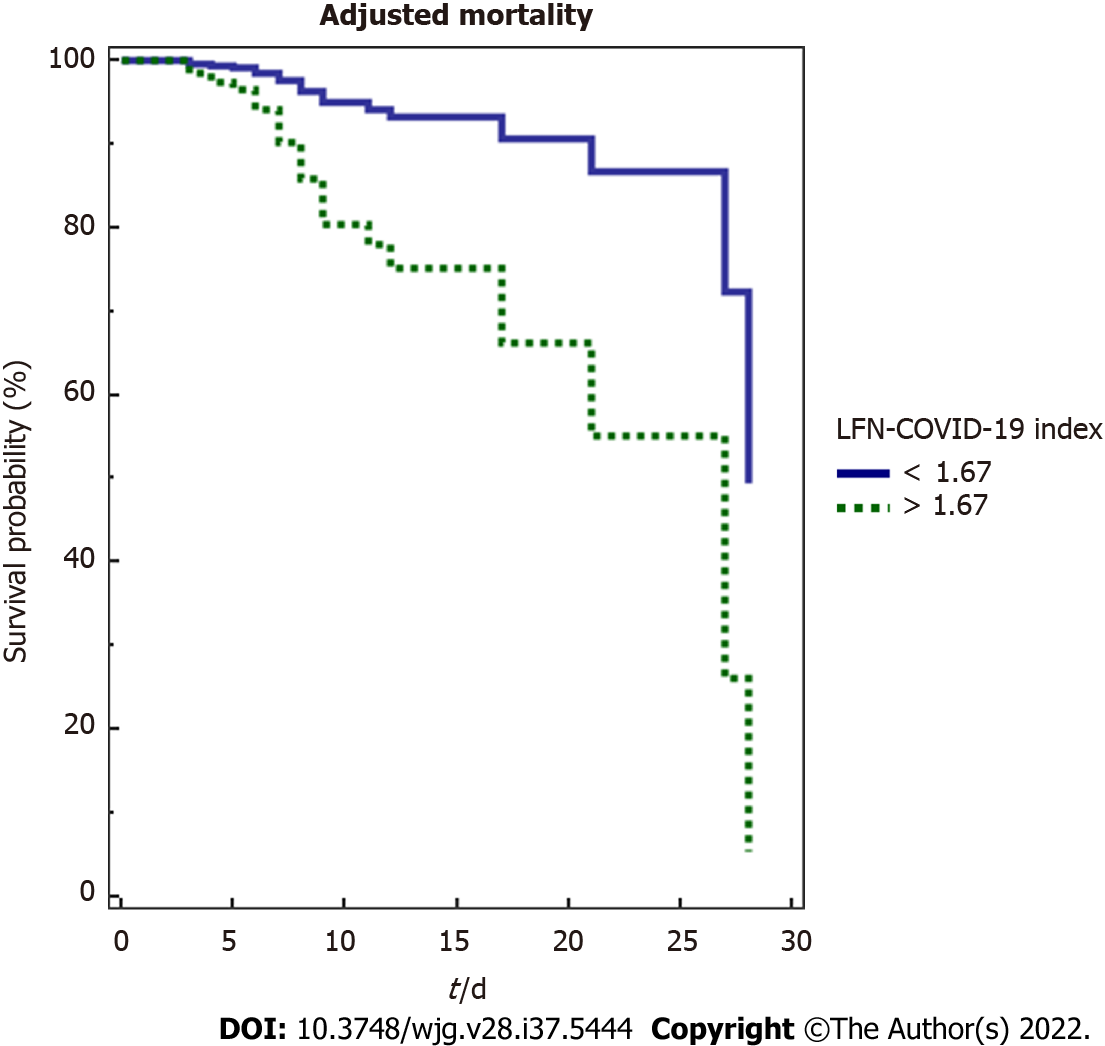
In this analysis, a LFN-COVID-19 index > 1.67 was associated independently to other variables of mortality, including severity markers, prognostic scores and general characteristics (Figure 4).
From the 697 patients included in the validation cohort, 104 had MAFLD (15.0%). In general, patients with MAFLD were younger and had higher degrees of obesity and mild abnormalities in liver chemistry (Supplementary Table 1). The MAFLD group was further analyzed according to the LFN-COVID-19 index, finding higher levels of C-reactive protein and D-dimer in the group > 1.67, with little changes in the rest of the variables (Supplementary Table 2). Interestingly, mechanical intubation and clinical outcomes including mortality, were more frequent in the > 1.67 group, as was found in the initial cohort (Supplementary Table 3). These same findings in another cohort and in a different hospital highlight the validity of the LFN-COVID-19 index.
MAFLD is currently the main etiology of chronic liver disease in the world. The main associated risk factors are obesity, type 2 diabetes, dyslipidemia and metabolic syndrome, all factors with a growing incidence. Both risk factors for MAFLD and MAFLD itself have also been shown to have prognostic value in COVID-19, associating their presence with higher severity and mortality. However, it remains controversial whether all patients within the spectrum of MAFLD have a worse prognosis or only those who, in addition to steatosis, have fibrosis[16].
Evidence pointing to MAFLD as a prognostic factor emerged from different studies around the world. A retrospective study in patients with COVID-19 found an association of MAFLD with higher intensive care unit admittance (OR: 2.3, 95%CI: 1.27-4.17), mechanical ventilation (2.08, 95%CI: 1.2-3.6) and in patients with cirrhosis with higher mortality (12.5, 95%CI: 2.16-72.5)[6]. In a cohort study in patients with COVID-19 and chronic liver disease (42% MAFLD), the authors observed a relative risk of 2.8 (95%CI: 1.9-4.0) for death in this group of patients, regardless of age, race, BMI, presence of hypertension or diabetes[12]. Another study conducted in Zhejiang, China found that hospitalized COVID-19 patients who had MAFLD with fibrosis (evaluated through FIB-4 and NFS - nonalcoholic fatty liver disease fibrosis score) were at increased risk of severe disease, regardless of other comorbidities[5]. Lastly, a study conducted by Lucifora et al[17] showed that patients with COVID-19 and MAFLD had a higher prevalence of alterations in the liver biochemistry test as well as a longer viral clearance time compared with patients without MAFLD.
Considering the evidence mentioned above, it is possible that the synergism between the baseline proinflammatory state of patients with MAFLD together with the body’s inflammatory response to COVID-19 could be the pathophysiological support that explains greater severity and worse prognosis in these patients. Another important component in multiorgan damage in COVID-19, is the state of hypoxemia, cell death and hypoperfusion reflected by biomarkers such as LDH, which correlates positively with worse clinical outcomes (including mortality). Although it is not specific for liver damage, it can be a sensitive and dynamic marker of hypoxic tissue damage due to its short half-life, together with other well-known markers of liver damage, such as AST, ALT and the AST/ALT ratio[10].
Due to the link between MAFLD and COVID-19 and the higher risk of mortality and adverse clinical outcomes, we conceived a prognostic index intended to be used in patients with MAFLD, including variables reflecting the pathophysiology of liver damage, mainly hepatocyte cell death induced by the factors previously mentioned, and associating it with hard clinical outcomes, including mortality[18]. The LFN-COVID-19 index includes the AST/ALT ratio as well as LDH levels normalized by the laboratory’s upper limit of normal, facilitating the implementation of the index by non-restricting its usefulness to a specific cutoff value (AST, ALT or LDH). This overcomes the problem of regional variations in laboratory values. The use of this index has potential implications in clinical practice establishing a prognosis of patients. On the other hand, the simplicity of the index allows easy calculation and includes widely available, cheap and reliable laboratory tests.
In the present study, we found a good diagnostic performance of the LFN-COVID-19 index in hospitalized patients with MAFLD and COVID-19. In the ROC curve analysis, a cutoff value of > 1.67 was associated with adverse clinical outcomes including the need for mechanical ventilation, acute kidney injury and higher mortality. This was reproduced in the validation cohort performed at a different center finding this cutoff point as the best for predicting these outcomes[19].
An interesting finding was that there were no differences in the days of stay in the intensive care unit based on this cutoff point. The same length of stay in the intensive care unit could be explained by the severity of the disease, where those with an index below 1.67 were discharged from the critical care area earlier and those with an index above 1.67 present earlier mortality.
Among the weaknesses of this study was the fact that the diagnosis of hepatic steatosis was made with computed tomography. However, given the high risk of transmission of SARS-CoV-2 to healthcare workers, this safer approach was chosen in order to reduce the exposure involved in carrying out a study such as transient hepatic elastography or magnetic resonance imaging requiring more time to perform it. Another aspect to highlight is that patients with COVID-19 usually present with elevated transaminases and LDH from multifactorial causes. Nevertheless, both biomarkers have been widely used as markers of hepatocyte cell death and may reflect liver damage occurring during SARS-CoV-2 infection and exacerbated in patients with MAFLD.
This study has several strengths. The sample size was adequate and sufficient due to the fact that the study was carried out in a center fully converted for the care of COVID-19 patients and included the general population in a country with a highest prevalence of MAFLD and a genetic profile that predisposes the population to the development of metabolic diseases such as type 2 diabetes mellitus, obesity and metabolic syndrome. In addition, we included an external validation cohort, where the results were replicated, enhancing the validity of the LFN-COVID-19 index.
Based on the findings of this study, we propose a new prognostic index based on markers of liver damage and severity in patients with MAFLD and COVID-19, which can be used in clinical practice to stratify the risk of adverse outcomes in MAFLD patients. Timely actions to reduce the associated morbidity and mortality in this population could be achieved through the implementation of this index.
This article was conceived considering the high prevalence of metabolic associated fatty liver disease (MAFLD) in the general population amid the coronavirus disease 2019 (COVID-19) pandemic and the risk of these patients in clinical settings with limited resources.
The growing evidence showing worse clinical outcomes in patients with metabolic diseases and COVID-19, including those with fatty liver disease, and the lack of a specific index to specifically stratify patients with both conditions motivated the creation of an index capable of discriminating those patients with an unfavorable outcome.
To evaluate the diagnostic yield of the liver fibrosis and nutrition (LNF)-COVID-19 index (includes lactate dehydrogenase, aspartate aminotransferase and alanine aminotransferase values), to predict adverse clinical outcomes, including mortality, in patients with both COVID-19 and MAFLD.
Data from a derivation cohort, including patients admitted with a diagnosis of severe COVID-19 and meeting the MAFLD criteria identified the best LFN-COVID-19 index cutoff value for risk stratification. The results were evaluated using a validation cohort.
The LFN-COVID-19 index with a cutoff point > 1.67 was associated with higher mortality (P < 0.001) with an area under the curve of 0.77 (95% confidence interval: 0.709-0.823), sensitivity of 78.7% and specificity of 63.8%. It was independently associated with worse outcomes such as higher mortality, intubation rate and acute kidney injury in both cohorts.
The LFN-COVID-19 index with a cutoff point > 1.67 showed good discrimination capability in patients with severe COVID-19 and MAFLD, identifying patients with an unfavorable prognosis associated with the need for mechanical ventilation, acute kidney injury and higher mortality.
The use of this prognostic index will allow timely identification of patients with MAFLD and COVID-19 at higher risk of adverse clinical outcomes, leading to better therapeutic decision-making and resource allocation.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Asociación Mexicana de Gastroenterología; American Association for the Study of Liver Diseases; European Association for the Study of the Liver; Asociación Mexicana de Hepatología.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hasabo EA, Sudan; Mamom J, Thailand S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li X
| 1. | Johns Hopkins University. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). JHU. 2022;1-2. [DOI] [Full Text] |
| 2. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18866] [Article Influence: 3773.2] [Reference Citation Analysis (7)] |
| 3. | Santoso A, Pranata R, Wibowo A, Al-Farabi MJ, Huang I, Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: A meta-analysis. Am J Emerg Med. 2021;44:352-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 4. | Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2428] [Cited by in RCA: 3008] [Article Influence: 601.6] [Reference Citation Analysis (1)] |
| 5. | Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 6. | Hashemi N, Viveiros K, Redd WD, Zhou JC, McCarty TR, Bazarbashi AN, Hathorn KE, Wong D, Njie C, Shen L, Chan WW. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: A multicentre United States experience. Liver Int. 2020;40:2515-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 117] [Article Influence: 23.4] [Reference Citation Analysis (2)] |
| 7. | Zhang G, Hu C, Luo L, Fang F, Chen Y, Li J, Peng Z, Pan H. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J Clin Virol. 2020;127:104364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 441] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 8. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30097] [Article Influence: 6019.4] [Reference Citation Analysis (3)] |
| 9. | Siordia JA Jr. Epidemiology and clinical features of COVID-19: A review of current literature. J Clin Virol. 2020;127:104357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 10. | Chaudhary A, Chauhan V. Lactate dehydrogenase as an indicator of liver diseases. J Adv Med Dental Sci Res. 2015;3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Kotoh K, Enjoji M, Kato M, Kohjima M, Nakamuta M, Takayanagi R. A new parameter using serum lactate dehydrogenase and alanine aminotransferase level is useful for predicting the prognosis of patients at an early stage of acute liver injury: a retrospective study. Comp Hepatol. 2008;7:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Singh S, Khan A. Clinical Characteristics and Outcomes of Coronavirus Disease 2019 Among Patients With Preexisting Liver Disease in the United States: A Multicenter Research Network Study. Gastroenterology. 2020;159:768-771.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 205] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 13. | Cassidy WM, Reynolds TB. Serum lactic dehydrogenase in the differential diagnosis of acute hepatocellular injury. J Clin Gastroenterol. 1994;19:118-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 14. | Yan L, Zhang HT, Xiao Y, Wang M, Guo Y, Sun C, Liang J, Li S, Zhang M, Tang X, Cao H, Tan X, Huang NN, Jiao B, Luo A, Cao Z, Xu H, Yuan Y. Prediction of criticality in patients with severe Covid-19 infection using three clinical features: a machine learning-based prognostic model with clinical data in Wuhan. 2020 Preprint. Available from: medRxiv:20028027. [DOI] [Full Text] |
| 15. | Li X, Xu S, Yu M, Wang K, Tao Y, Zhou Y, Shi J, Zhou M, Wu B, Yang Z, Zhang C, Yue J, Zhang Z, Renz H, Liu X, Xie J, Xie M, Zhao J. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1450] [Cited by in RCA: 1437] [Article Influence: 287.4] [Reference Citation Analysis (0)] |
| 16. | Ruiz-Margáin A, Campos-Murguía A, González-Regueiro JA, Román-Calleja BM, Delint DK, Macías-Rodríguez RU. Reply to: "Liver fibrosis and adverse outcomes in COVID-19". Dig Liver Dis. 2021;53:800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Lucifora J, Michelet M, Rivoire M, Protzer U, Durantel D, Zoulim F. Two-dimensional-cultures of primary human hepatocytes allow efficient HBV infection: Old tricks still work! J Hepatol. 2020;73:449-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, Bonten MMJ, Dahly DL, Damen JAA, Debray TPA, de Jong VMT, De Vos M, Dhiman P, Haller MC, Harhay MO, Henckaerts L, Heus P, Kammer M, Kreuzberger N, Lohmann A, Luijken K, Ma J, Martin GP, McLernon DJ, Andaur Navarro CL, Reitsma JB, Sergeant JC, Shi C, Skoetz N, Smits LJM, Snell KIE, Sperrin M, Spijker R, Steyerberg EW, Takada T, Tzoulaki I, van Kuijk SMJ, van Bussel B, van der Horst ICC, van Royen FS, Verbakel JY, Wallisch C, Wilkinson J, Wolff R, Hooft L, Moons KGM, van Smeden M. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ. 2020;369:m1328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1833] [Cited by in RCA: 1746] [Article Influence: 349.2] [Reference Citation Analysis (0)] |
| 19. | World Health Organization. Clinical management of COVID-19: evolutionary guidelines, January 25, 2021. [cited 16 Jun 2022]. In: World Health Organization [Internet]. Available from: https://apps.who.int/iris/handle/10665/338882. |